User login
Study questions canagliflozin amputation risk, but concerns remain
ORLANDO – but clinicians should still favor other options in patients at risk for amputations, according to investigator John Buse, MD, PhD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill.
Canagliflozin is the only sodium-glucose transporter 2 (SGLT2) inhibitor that carries a black box warning of “lower limb amputations, most frequently of the toe and midfoot” but also the leg. The drug doubled the risk versus placebo in its approval trials, particularly in patients with baseline histories of prior amputations, peripheral vascular disease, neuropathy, or diabetic foot ulcers.
One trial, for instance, reported 7.5 amputations per 1,000 patient years versus 4.2 with placebo, according to labeling.
The new, observational study, which was funded by canagliflozin’s maker Johnson & Johnson and, with the exception of Dr. Buse, conducted by its employees, found no such connection. Investigators reviewed claims data from 142,800 new users of canagliflozin, 110,897 new users of the competing SGLT2 inhibitors empagliflozin (Jardiance) and dapagliflozin (Farxiga), and 460,885 new users of other diabetes drugs except for metformin, Dr. Buse said when he presented the results at the annual scientific sessions of the American Diabetes Association.
The hazard ratio for below-knee amputations with canagliflozin versus non-SGLT2 inhibitors was 0.75 (95% confidence interval, 0.40-1.41; P = 0.30). The ratio versus other SGLT2 inhibitors was 1.14 (95% CI, 0.67-1.93; P = 0.53). Overall, there were 1-5 amputations per 1,000 patient years with the drug.
However, the median follow-up was a few months, far shorter than the median follow-up of over 2 years in the randomized trials. “Therefore, the current study had limited statistical power to detect differences in the 6-12 month time period, the time at which amputation risk began to emerge” in the trials, the study report noted. Also, the investigators didn’t parse out results according to baseline amputation risk. Overall, “none of the analyses were sufficiently powered to rule out the possibility of a modest effect” on amputation rates (Diabetes Obes Metab. 2018 Jun 25. doi: 10.1111/dom.13424).
When moderator Robert H. Eckel, MD, a professor in the division of endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora, asked the 150 or so people who heard the presentation if they use SGLT2 inhibitors in their practices, only a small number raised their hands. Few, if any, raised their hands when he asked if the new results would make them more comfortable prescribing canagliflozin.
“I find [the study] somewhat informative,” Dr. Eckel said in an interview afterwards, “but I think the issue is that the prescribing label still demands that patients be informed of the black box warning. I think we are going to have to wait for the longer term outcomes to determine if [amputation] is a molecule effect or a class effect.”
Dr. Buse later said that “I think for the general population of patients with diabetes, they are at low risk for an amputation,” but “if you are at high risk for having an amputation, we really have to take this risk very seriously. [Canagliflozin] may increase your risk for amputation.
“If I have a patient who has had an amputation and I want to use an SGLT2 inhibitor, I wouldn’t use canagliflozin because of the label. I would use empagliflozin because [amputation] is not in the label, and there was no evidence” of it in trials, he added.
The new study, meanwhile, confirmed the cardiac benefits of SGLT2 inhibitors in type 2 patients. Canagliflozin, for instance, reduced the risk of hospitalization for heart failure by about 60%, compared with non-SGLT2 inhibitors in patients with cardiovascular disease, but it offered no statistically significant heart benefit over other members of its class.
Dr. Buse is an investigator for Johnson and Johnson.
ORLANDO – but clinicians should still favor other options in patients at risk for amputations, according to investigator John Buse, MD, PhD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill.
Canagliflozin is the only sodium-glucose transporter 2 (SGLT2) inhibitor that carries a black box warning of “lower limb amputations, most frequently of the toe and midfoot” but also the leg. The drug doubled the risk versus placebo in its approval trials, particularly in patients with baseline histories of prior amputations, peripheral vascular disease, neuropathy, or diabetic foot ulcers.
One trial, for instance, reported 7.5 amputations per 1,000 patient years versus 4.2 with placebo, according to labeling.
The new, observational study, which was funded by canagliflozin’s maker Johnson & Johnson and, with the exception of Dr. Buse, conducted by its employees, found no such connection. Investigators reviewed claims data from 142,800 new users of canagliflozin, 110,897 new users of the competing SGLT2 inhibitors empagliflozin (Jardiance) and dapagliflozin (Farxiga), and 460,885 new users of other diabetes drugs except for metformin, Dr. Buse said when he presented the results at the annual scientific sessions of the American Diabetes Association.
The hazard ratio for below-knee amputations with canagliflozin versus non-SGLT2 inhibitors was 0.75 (95% confidence interval, 0.40-1.41; P = 0.30). The ratio versus other SGLT2 inhibitors was 1.14 (95% CI, 0.67-1.93; P = 0.53). Overall, there were 1-5 amputations per 1,000 patient years with the drug.
However, the median follow-up was a few months, far shorter than the median follow-up of over 2 years in the randomized trials. “Therefore, the current study had limited statistical power to detect differences in the 6-12 month time period, the time at which amputation risk began to emerge” in the trials, the study report noted. Also, the investigators didn’t parse out results according to baseline amputation risk. Overall, “none of the analyses were sufficiently powered to rule out the possibility of a modest effect” on amputation rates (Diabetes Obes Metab. 2018 Jun 25. doi: 10.1111/dom.13424).
When moderator Robert H. Eckel, MD, a professor in the division of endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora, asked the 150 or so people who heard the presentation if they use SGLT2 inhibitors in their practices, only a small number raised their hands. Few, if any, raised their hands when he asked if the new results would make them more comfortable prescribing canagliflozin.
“I find [the study] somewhat informative,” Dr. Eckel said in an interview afterwards, “but I think the issue is that the prescribing label still demands that patients be informed of the black box warning. I think we are going to have to wait for the longer term outcomes to determine if [amputation] is a molecule effect or a class effect.”
Dr. Buse later said that “I think for the general population of patients with diabetes, they are at low risk for an amputation,” but “if you are at high risk for having an amputation, we really have to take this risk very seriously. [Canagliflozin] may increase your risk for amputation.
“If I have a patient who has had an amputation and I want to use an SGLT2 inhibitor, I wouldn’t use canagliflozin because of the label. I would use empagliflozin because [amputation] is not in the label, and there was no evidence” of it in trials, he added.
The new study, meanwhile, confirmed the cardiac benefits of SGLT2 inhibitors in type 2 patients. Canagliflozin, for instance, reduced the risk of hospitalization for heart failure by about 60%, compared with non-SGLT2 inhibitors in patients with cardiovascular disease, but it offered no statistically significant heart benefit over other members of its class.
Dr. Buse is an investigator for Johnson and Johnson.
ORLANDO – but clinicians should still favor other options in patients at risk for amputations, according to investigator John Buse, MD, PhD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill.
Canagliflozin is the only sodium-glucose transporter 2 (SGLT2) inhibitor that carries a black box warning of “lower limb amputations, most frequently of the toe and midfoot” but also the leg. The drug doubled the risk versus placebo in its approval trials, particularly in patients with baseline histories of prior amputations, peripheral vascular disease, neuropathy, or diabetic foot ulcers.
One trial, for instance, reported 7.5 amputations per 1,000 patient years versus 4.2 with placebo, according to labeling.
The new, observational study, which was funded by canagliflozin’s maker Johnson & Johnson and, with the exception of Dr. Buse, conducted by its employees, found no such connection. Investigators reviewed claims data from 142,800 new users of canagliflozin, 110,897 new users of the competing SGLT2 inhibitors empagliflozin (Jardiance) and dapagliflozin (Farxiga), and 460,885 new users of other diabetes drugs except for metformin, Dr. Buse said when he presented the results at the annual scientific sessions of the American Diabetes Association.
The hazard ratio for below-knee amputations with canagliflozin versus non-SGLT2 inhibitors was 0.75 (95% confidence interval, 0.40-1.41; P = 0.30). The ratio versus other SGLT2 inhibitors was 1.14 (95% CI, 0.67-1.93; P = 0.53). Overall, there were 1-5 amputations per 1,000 patient years with the drug.
However, the median follow-up was a few months, far shorter than the median follow-up of over 2 years in the randomized trials. “Therefore, the current study had limited statistical power to detect differences in the 6-12 month time period, the time at which amputation risk began to emerge” in the trials, the study report noted. Also, the investigators didn’t parse out results according to baseline amputation risk. Overall, “none of the analyses were sufficiently powered to rule out the possibility of a modest effect” on amputation rates (Diabetes Obes Metab. 2018 Jun 25. doi: 10.1111/dom.13424).
When moderator Robert H. Eckel, MD, a professor in the division of endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora, asked the 150 or so people who heard the presentation if they use SGLT2 inhibitors in their practices, only a small number raised their hands. Few, if any, raised their hands when he asked if the new results would make them more comfortable prescribing canagliflozin.
“I find [the study] somewhat informative,” Dr. Eckel said in an interview afterwards, “but I think the issue is that the prescribing label still demands that patients be informed of the black box warning. I think we are going to have to wait for the longer term outcomes to determine if [amputation] is a molecule effect or a class effect.”
Dr. Buse later said that “I think for the general population of patients with diabetes, they are at low risk for an amputation,” but “if you are at high risk for having an amputation, we really have to take this risk very seriously. [Canagliflozin] may increase your risk for amputation.
“If I have a patient who has had an amputation and I want to use an SGLT2 inhibitor, I wouldn’t use canagliflozin because of the label. I would use empagliflozin because [amputation] is not in the label, and there was no evidence” of it in trials, he added.
The new study, meanwhile, confirmed the cardiac benefits of SGLT2 inhibitors in type 2 patients. Canagliflozin, for instance, reduced the risk of hospitalization for heart failure by about 60%, compared with non-SGLT2 inhibitors in patients with cardiovascular disease, but it offered no statistically significant heart benefit over other members of its class.
Dr. Buse is an investigator for Johnson and Johnson.
REPORTING FROM ADA 2018
Key clinical point: A large, observational study found no increased risk of below-the-knee amputations with canagliflozin for type 2 diabetes, but clinicians should still favor other options in patients at risk for amputations.
Major finding: The hazard ratio for below-knee amputations with canagliflozin versus non-SGLT2 inhibitors was 0.75 (95% confidence interval, 0.40-1.41; P = 0.30).
Study details: An observational study of over 700,000 patients with type 2 diabetes.
Disclosures: The work was funded by canagliflozin’s maker Johnson & Johnson and, with the exception of the presenter, conducted by its employees.
Navigating travel with diabetes
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
KEY POINTS
- Patients should pack all diabetes medications and supplies in a carry-on bag and keep it in their possession at all times.
- A travel letter will facilitate easy transfer through security and customs.
- Patients should always take more supplies than needed to accommodate changes in travel plans.
- If patients will cross multiple time zones during their travel, they will likely need to adjust their medication and food schedules.
Osmotic demyelination syndrome due to hyperosmolar hyperglycemia
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
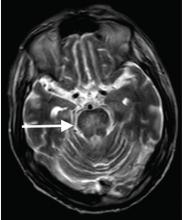
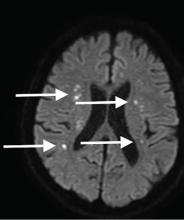
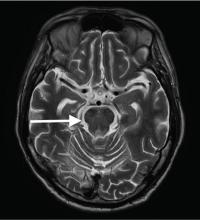
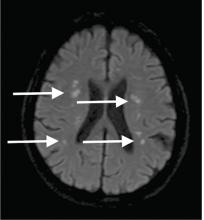
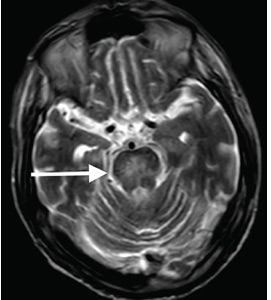
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
Dietary recommendations for patients with diabetes
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2
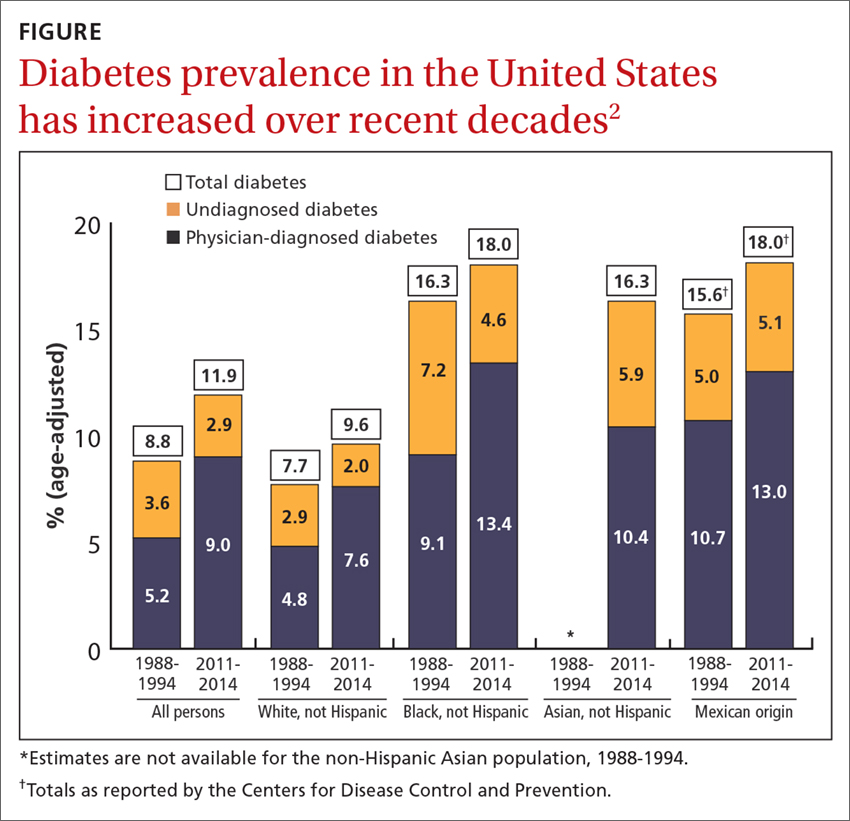
Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes in the elderly: Matching meds to needs
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2
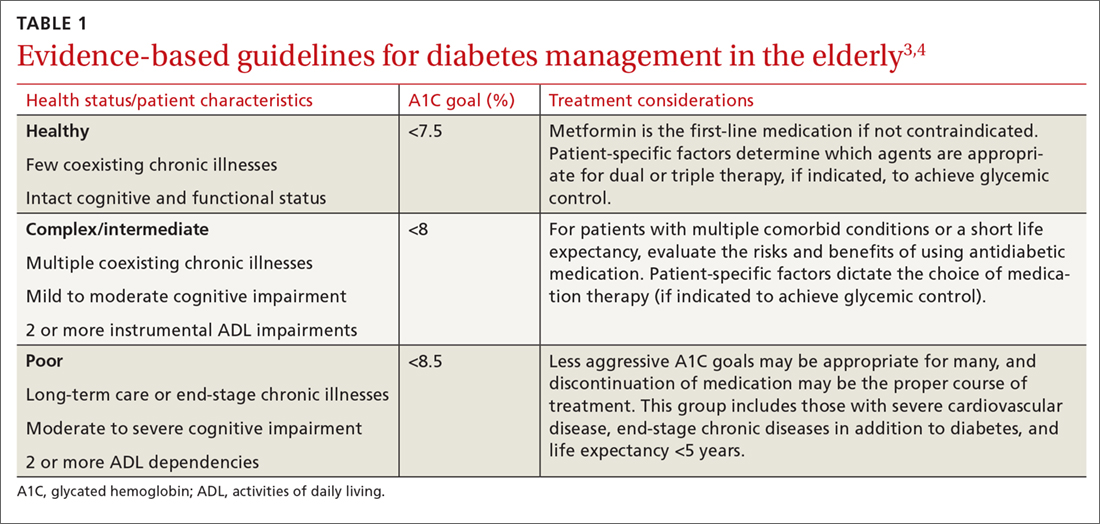
Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; bkeber@northwell.edu.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; bkeber@northwell.edu.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; bkeber@northwell.edu.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
From The Journal of Family Practice | 2018;67(7):408-410,412-415.
PRACTICE RECOMMENDATIONS
› Allow higher A1C goals for elderly patients who have such comorbid conditions as cognitive dysfunction, dementia, or cardiovascular or renal disease. B
› Look to metformin first in most instances if there are no contraindications. Monitor renal function frequently and vitamin B12 levels periodically. B
› Consider glucagon-like peptide-1 receptor agonists for patients who also have established cardiovascular disease, or consider starting basal insulin instead of using multiple oral agents. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Blood pressure targets: How low should you go (and for whom)?
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.
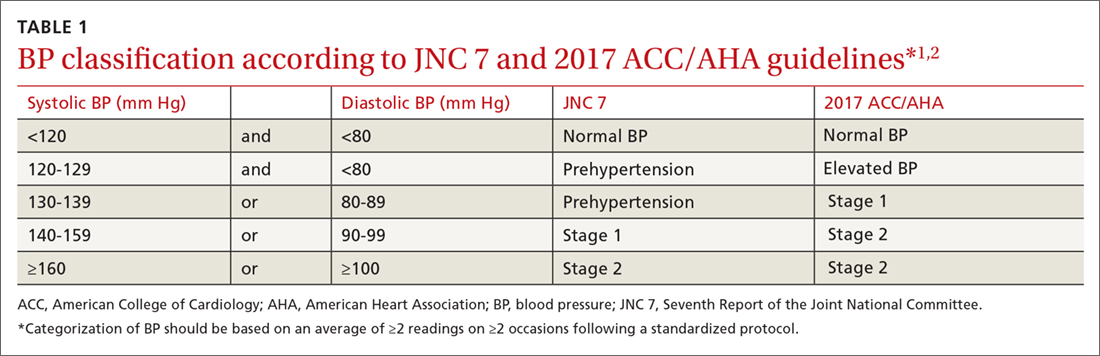
Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.
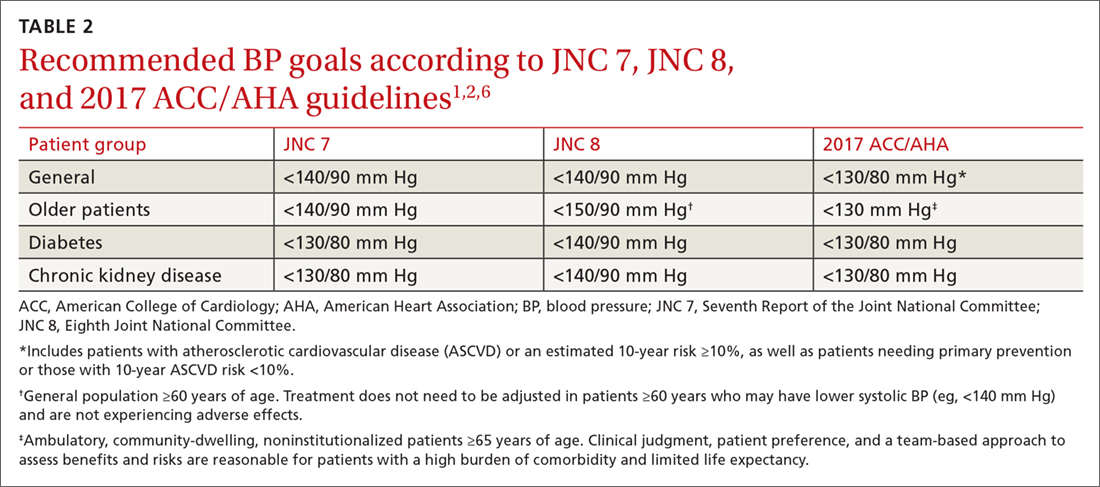
SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.
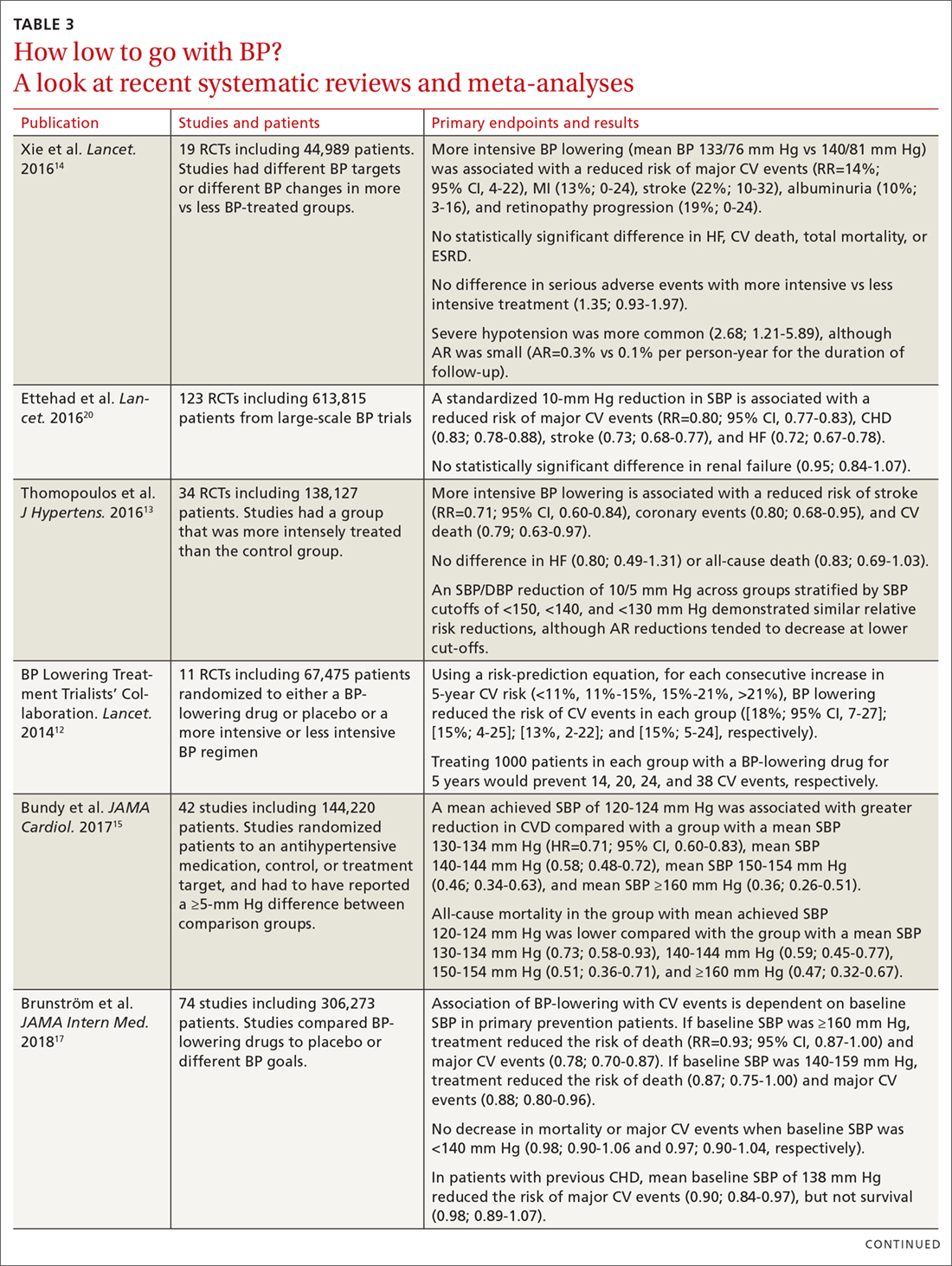
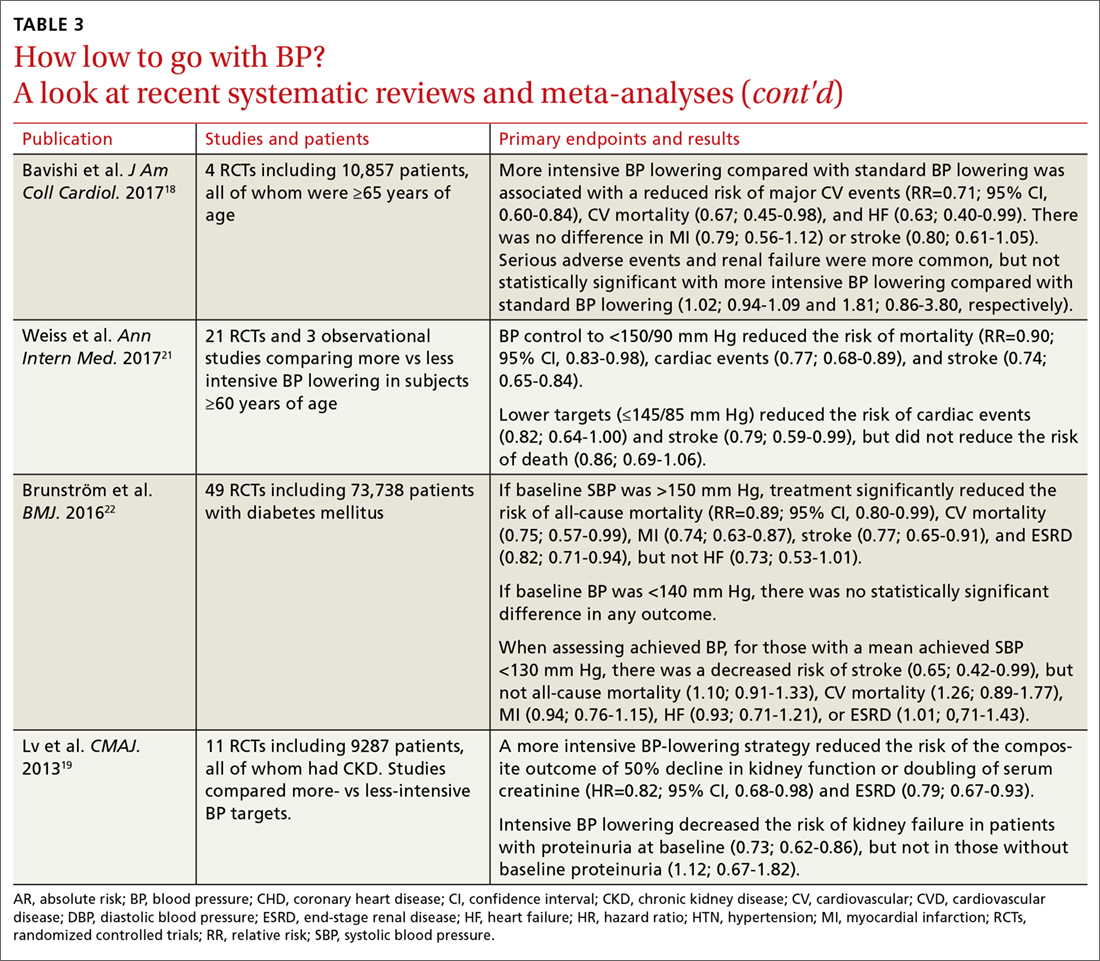
Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.
Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; Eric.MacLaughlin@ttuhsc.edu.
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; Eric.MacLaughlin@ttuhsc.edu.
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
For more than a century, clinicians have pondered the significance of elevated blood pressure (BP) and its contribution to cardiovascular disease (CVD). While it is widely understood that high BP increases CVD events, and that treatment lowers that risk, the most appropriate BP goal continues to be a subject of debate.
This article briefly summarizes the evidence to support lower BP goals for patients with hypertension who are commonly seen in family practice, including those needing primary prevention, as well as those with, or at high risk for, atherosclerotic cardiovascular disease (ASCVD), patients with diabetes, and those with chronic kidney disease (CKD). Detailed information regarding specific lifestyle and medication treatment recommendations and thresholds for drug therapy is beyond the scope of this review.
A brief history: ACC/AHA guidelines vs JNC 7 and 8
The most recent comprehensive, evidence-based guideline on the prevention, detection, evaluation, and management of high BP in adults was released in late 2017 by the American College of Cardiology (ACC) and the American Heart Association (AHA).1 It was the first comprehensive BP guideline since the Seventh Report of the Joint National Committee (JNC 7) in 2003.2 The new guideline includes several changes, notably in how BP is classified, the threshold for initiation of antihypertensive drug therapy, and target BP.
While widely viewed as positive, the changes in classification, thresholds, and targets for BP therapy have generated controversy and disagreement. Common reasons cited include concern about the data supporting lower thresholds for treatment, the applicability of trial findings to broad patient populations, and the risk of harm with lower BP goals.3 The American Academy of Family Physicians (AAFP) declined to endorse the ACC/AHA guidelines and continues to support the 2014 report by the panel members appointed to the Eighth Joint National Committee (JNC 8) by the National Heart Lung and Blood Institute (NHLBI).4 A primary reason cited for the lack of support for the 2017 guideline is that the majority of recommendations made in the ACC/AHA guideline were not “based on a systematic evidence review.”4 However, there are significant differences in purpose, structure, and scope between the ACC/AHA and JNC 8.
In 2013, the NHLBI announced that it would cease involvement in creating guidelines and transferred responsibility for development to professional organizations.5 Of the 5 guidelines that were in the process of creation (cholesterol, lifestyle intervention, obesity, risk assessment, and high BP), all but the high BP guideline were transferred to the ACC/AHA for completion. The panel members appointed to the JNC 8 elected to publish their recommendations independently and focused only on 3 “critical questions” related to hypertension therapy (eg, therapy initiation, BP goals, and choice of initial agent).6
[polldaddy:10041785]
The JNC 8 report generated significant controversy with the recommendation to relax the BP goal for patients ≥60 years of age to <150/90 mm Hg. Members of the JNC 8 panel who disagreed with this goal published a "minority view" citing concerns about the negative impact the goal would have on CVD and public health, and the "insufficient and inconsistent" evidence supporting relaxed goals.7 The dissenting group cited additional drawbacks of the recommendation, noting that it was highly focused, included data only from randomized controlled trials (RCTs; no meta-analyses or observational data), and did not address or provide guidance on numerous other issues of importance in the care of hypertension.
While the 2017 ACC/AHA guideline also includes formal systematic evidence reviews on major critical questions (ie, optimal BP targets, preferred antihypertensives, the role of home and ambulatory BP monitoring),8 it was designed to be comprehensive and useful for clinicians, providing 106 graded recommendations on commonly encountered questions. It would have been unrealistic to do a formal systematic evidence review and meta-analysis on all clinically relevant questions seen in practice. However, available systematic reviews, meta-analyses, and observational data were scrutinized and used to support the recommendations wherever possible.
Continue to: Say "goodbye" to prehypertension; say "hello" to elevated BP
Say “goodbye” to prehypertension; say “hello” to elevated BP
The 2017 ACC/AHA guideline changed the BP classification for adults (TABLE 11,2). While “normal” remained respectively.1 Removal of the “prehypertension” category and use of the term “elevated” instead was meant to better convey the importance of lifestyle interventions to forestall the development of hypertension.

Don’t underestimate the power of BP measurement technique
The importance of appropriate BP measurement technique to confirm the diagnosis of hypertension and assist with medication titration was also emphasized.1 BP measurement technique in usual clinical practice is frequently suboptimal, most commonly resulting in falsely elevated readings.9,10 The guideline recommends the use of out-of-office measurements to confirm elevated clinic readings, screen for white-coat and masked hypertension, and assist in medication adjustment decisions. It is critically important that appropriate BP measurement technique is used, which in many cases, will avoid inappropriate treatment. (See “Getting the hypertension Dx right: Patient positioning matters,” JFP. 2018;67:199-207.)
A look at the evidence supporting lower BP goals
The 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for adults with hypertension commonly seen in clinical practice, including those with CVD or an elevated ASCVD risk (10-year risk ≥10% using the Pooled Cohort Equations11), those with hypertension and low ASCVD risk (10-year risk <10%), and those with hypertension who have concomitant diabetes or CKD.1 The guideline also recommends an SBP goal <130 mm Hg for independently-living, ambulatory older adults (≥65 years) with hypertension.1 TABLE 21,2,6 compares the BP goals in the new 2017 ACC/AHA guidelines to previous recommendations.

SPRINT. Significant new literature has been generated since the publication of JNC 8 that supports these lower BP goals, particularly in patients with CVD or who are at high ASCVD risk.8,12-15 For example, the Systolic Blood Pressure Intervention Trial (SPRINT) was the largest RCT to assess whether lower BP goals decrease the risk of adverse CVD outcomes.16 In SPRINT, 9361 patients with an SBP ≥130 mm Hg and an increased risk of CVD, but without diabetes or a history of stroke, were randomized to intensive BP treatment (SBP goal <120 mm Hg) or standard treatment (SBP goal <140 mm Hg). After a median follow-up of 3.26 years, the study was stopped early due to a decreased risk in the primary composite outcome of myocardial infarction (MI), other acute coronary syndromes (ACS), stroke, heart failure, or death from CV causes (number needed to treat [NNT] to prevent one event=61).
Intensive treatment was also associated with a lower risk of all-cause mortality (NNT=90), heart failure (NNT=123), death from CV causes (NNT=172), and the primary outcome or death (NNT=52
Continue to: Meta-analyses that have been conducted since SPRINT...
Meta-analyses that have been conducted since SPRINT, and that have incorporated SPRINT data, also support lower BP goals. In the systematic review performed for the 2017 ACC/AHA guideline, an SBP <130 mm Hg compared to a higher BP target was associated with a reduced risk of major CV events, stroke, MI, and heart failure, although not all-cause mortality.8 These findings were largely consistent with other recent meta-analyses.12-15 For example, Bundy et al15 reported significant CV benefit with more vs less intensive BP lowering, whether or not the data from SPRINT were included, with the greatest reduction in risk seen in the groups with highest baseline BP.
It is important to consider a patient’s baseline level of risk when evaluating the absolute benefit of lower BP targets on CV outcomes. For patients with higher CV risk, the absolute benefit of treatment is greater.12-14 These findings support the 2017 ACC/AHA guideline, which recommends initiating drug therapy, in addition to lifestyle modification, in adults with hypertension and high ASCVD risk when the average BP is >130/80 mm Hg, with a goal of <130/80 mm Hg. TABLE 312-15,17-22 summarizes recent systematic reviews and meta-analyses conducted since the publication of JNC 8 that assess the association between intensity of BP lowering and adverse CV and related outcomes.


Treating patients with low CV risk
The evidence supporting a lower BP goal in patients with low CV risk is less than for patients at elevated risk. There are no large RCTs for this group that have assessed whether an intensive BP lowering strategy decreases CV outcomes more than a standard BP strategy (eg, <140/90 mm Hg). It is likely that absolute benefit is much smaller than for patients with, or at high risk for, ASCVD.
However, epidemiologic observational studies have indicated a significant log-linear increase in CV mortality starting at an SBP of 115 mm Hg.23 A 20-mm Hg increase in SBP above 115 mm Hg is associated with an approximate doubling of stroke and ischemic heart disease mortality risk.23 Decades worth of exposure to “elevated” BP levels would likely result in significant vascular damage, and attenuation of this process would likely be beneficial.24,25 An RCT specifically designed to test this hypothesis, however, would not be pragmatic considering the substantial number of patient-years that would be required.
Due to insufficient data documenting the value of antihypertensive drug therapy for primary prevention in adults with “elevated” BP and stage 1 hypertension at low risk for CVD, the 2017 ACC/AHA guideline recommends that drug therapy be initiated for all adults only when their BP average is ≥140/90 mm Hg.1 In contrast, for patients needing secondary prevention and for those with elevated CVD risk, the guideline recommends medication in addition to lifestyle modifications once the average BP is ≥130/80 mm Hg. The recommendation to withhold drug therapy until the BP is ≥140/90 mm Hg in patients needing primary prevention is supported by a new meta-analysis of 74 trials with 306,273 participants that aimed to assess the association between BP-lowering treatment and death and CVD at various BP levels.17 In this analysis, pharmacologic treatment was associated with a reduced risk of all-cause mortality, major CVD events, and coronary heart disease if the SBP was ≥140 mm Hg.
Continue to: Treating older patients
Treating older patients
Significant controversy has existed regarding the optimal BP goal in older patients, particularly once the JNC 8 recommended relaxing the SBP goal to <150 mm Hg for pateints ≥60 years of age.6,7 This recommendation was consistent with the guideline from the American College of Physicians (ACP)/AAFP,26 which also recommended a lower SBP of <140 mm Hg in patients with a history of stroke or transient ischemic attack and those at high CV risk.26
Evidence is available, however, supporting more intensive BP goals in older independently-living ambulatory adults. A pre-planned subgroup analysis was conducted in 2636 SPRINT participants ≥75 years of age.27 Similar to the overall experience in SPRINT, lower SBP goals were associated with significant reductions in CV events, including the composite CVD primary outcome (NNT=27), heart failure (NNT=63), nonfatal heart failure (NNT=66), and all-cause mortality (NNT=41). In addition, the relative benefits were approximately equal whether the patients were the most fit, non-fit, or frail, with the absolute benefit being greatest in those who were frail (recognizing that the SPRINT participants were independently-living ambulatory adults). While the absolute rate of serious adverse events was higher in the more intensive BP goal group, there was no statistically significant difference in the incidence of hypotension, orthostatic hypotension, syncope, electrolyte abnormalities, or acute kidney injury or renal failure.
Use of lower BP goals than recommended by JNC 8 was also supported by another recent meta-analysis that compared the outcomes of intensive BP lowering (SBP <140 mm Hg) to a standard BP-lowering strategy (SBP <150 mm Hg).18 Using a random-effects model, more intensive BP lowering was associated with a significant reduction in major adverse CV events (29%), CV mortality (33%), and heart failure (37%), with no increase in serious adverse events or renal failure. Findings with the fixed-effects model used to confirm results were largely consistent, with the exception of a possible increase in renal failure.
Although the evidence supporting lower BP goals in older, ambulatory, noninstitutionalized patients is sound, it is important to consider a patient’s overall disease burden. For older adults with multiple comorbidities and limited life expectancy, as well as those who are nonambulatory or institutionalized, decisions on the intensity of BP lowering should be made using a team-based approach, weighing the risks and benefits.1
Continue to: Treating patients with diabetes
Treating patients with diabetes
The most appropriate BP goal for patients with diabetes has been the subject of much debate, with different goals recommended in different guidelines (TABLE 21,2,6). The most recent American Diabetes Association guideline recommends a BP goal <140/90 mm Hg for most patients, with lower targets (<130/80 mm Hg) for patients at high CV risk if it is achievable without undue treatment burden,28 whereas the 2017 ACC/AHA guideline recommends a BP goal <130/80 mm Hg for all adults with diabetes.1
The ACCORD trial. There is limited evidence to suggest which BP goal is most appropriate for patients with diabetes. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial is the only RCT specifically designed to assess the impact of intensive vs standard BP goals in patients with diabetes.29 In ACCORD, 4733 patients with type 2 diabetes were randomized to either an intensive BP-lowering group (SBP <120 mm Hg) or a standard BP-lowering group (SBP <140 mm Hg). After a mean follow-up of 4.7 years, there was no difference in the primary composite endpoint of nonfatal MI, nonfatal stroke, or death from CV causes. However, the risk of stroke was reduced (NNT=89). Interpretation of ACCORD is limited due to its factorial design and because the trial was significantly underpowered.

Systematic reviews and meta-analyses. Literature supporting lower BP goals in patients with diabetes primarily comes from systematic reviews and meta-analyses.30 In the evidence-based review performed for the 2017 ACC/AHA guidelines, more intensive treatment was associated with a decrease in fatal or nonfatal stroke.8 The results from the ACCORD trial and SPRINT are consistent,31 and a sub-study of SPRINT patients with pre-diabetes showed preservation of CV benefit.32 Also, a meta-analysis of subgroups of trial participants with diabetes showed that more intensive BP lowering in patients is associated with a decrease in major CV events.14
Treating patients with chronic kidney disease
As with diabetes and older patients, recommended goals for patients with CKD have varied (TABLE 21,2,6). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guideline recommended the same target BP as JNC 7 and the 2017 ACC/AHA guideline: ≤130/80 mm Hg in patients with CKD and urine albumin excretion ≥30 mg/24 hours (or equivalent).1,2,33 KDIGO recommended a more relaxed target (≤140/90 mm Hg), however, for patients with CKD and urine albumin excretion <30 mg/24 hours.1,33
Scant data exist from RCTs designed to assess the CV effects of intensive BP targets in patients with CKD. In SPRINT, where 28% of patients had stage 3 or 4 CKD, benefits of more intensive therapy were similar to those observed in the overall cohort.16,34 While some RCTs have assessed the effect of more intensive BP lowering on progression of CKD, they were not specifically designed or powered to address CV outcomes.35,36
Continue to: In recent meta-analyses assessing the effects...
In recent meta-analyses assessing the effects of intensive BP lowering on renal and CV events in patients with CKD, a lower BP strategy was not associated with a decrease in CV events.8,14,19 However, more intensive therapy was associated with a 17% reduced risk of composite kidney failure events and an 18% reduction in end-stage kidney disease.19 The risk of kidney failure with lower BP goals was 27% lower in patients with baseline proteinuria, but was not significant in patients who did not have proteinuria.19
Evidence supports lower BP goals, but guidelines should guide
The lower BP goals advised in the 2017 ACC/AHA guideline are supported by substantial new high-quality evidence that was not available at the time of the JNC 8 report.1 The strongest evidence for lower goals is found in patients with, or at high risk for, CVD, but other patients commonly seen by primary care providers, including those at lower CVD risk, older patients, and those with diabetes or CKD are also likely to benefit.1
Despite the debates, it is important to remember that guidelines are intended to “guide.” As stated in the guideline, “Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.”1 They should be easy to understand and apply, and a consistent, evidence-based BP goal of <130/80 mm Hg for most patients facilitates implementation.
Although more of the US population is categorized as hypertensive under the new guideline (46% now vs 32% before), only 1.9% more require drug therapy, as the vast majority of the newly classified hypertensives are primary prevention patients for whom only lifestyle modification is recommended.37 However, to attain these goals, greater emphasis will be needed on utilizing team-based care, health information technology including electronic medical records and telehealth, performance measures, quality improvement strategies, and financial incentives.1
Finally, as emphasized in the guidelines, BP monitoring technique matters. Clinicians should not accept flawed BP measurement techniques any more than they would accept flawed results from studies performed incorrectly.
CORRESPONDENCE
Eric J. MacLaughlin, PharmD, BCPS, FASHP, FCCP, Texas Tech University Health Sciences Center,1300 S. Coulter Dr., Amarillo, TX 79106; Eric.MacLaughlin@ttuhsc.edu.
ACKNOWLEDGEMENTS
The authors thank Paul K. Whelton, MB, MD, MSc, FAHA, and Robert M. Carey, MD, FAHA, for their review of this manuscript.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
3. Wilt TJ, Kansagara D, Qaseem A; Clinical Guidelines Committee of the American College of Physicians. Hypertension limbo: balancing benefits, harms, and patient preferences before we lower the bar on blood pressure. Ann Intern Med. 2018;168:369-370.
4. American Academy of Family Physicians. AAFP decides to not endorse AHA/ACC hypertension guideline. Available at: https://www.aafp.org/news/health-of-the-public/20171212notendorseaha-accgdlne.html. Accessed January 9, 2018.
5. Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation. 2013;128:1713-1715.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Wright JT Jr., Fine LJ, Lackland DT, et al. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499-503.
8. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e116-e135.
9. Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904-905.
10. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
11. American College of Cardiology. ASCVD Risk Estimator Plus. Available at: http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/. Accessed January 9, 2018.
12. The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591-598.
13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613-622.
14. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435-443.
15. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775-781.
16. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
17. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:28-36.
18. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol. 2017;69:486-493.
19. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
20. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967.
21. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
22. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
23. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
24. Guo X, Zhang X, Guo L, et al. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703-716.
25. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153-1161.
26. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
27. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
28. American Diabetes Association. 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S86-S104.
29. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
30. Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253-1269.
31. Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med. 2015;373:2175-2178.
32. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017 Aug 9.
33. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337-414.
34. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812-2823.
35. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939-946.
36. Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
37. Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109-188.
PRACTICE RECOMMENDATIONS
› Treat adults with hypertension and cardiovascular disease or those at high risk (≥10%) of an atherosclerotic cardiovascular disease (ASCVD) event to a blood pressure (BP) goal <130/80 mm Hg. A for systolic BP goal; C for diastolic BP goal.
› Treat adults with hypertension and a low risk of a cardiovascular event (ie, primary prevention and ASCVD <10%) to a BP goal <130/80 mm Hg. B for systolic BP goal; C for diastolic BP goal.
› Treat ambulatory, community-dwelling, noninstitutionalized older patients to a systolic BP goal <130 mm Hg. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
High-bleeding-risk AF patients cut stroke risk with Amplatzer Amulet
Left atrial appendage closure slashes stroke risk in high-risk atrial fibrillation patients with Amplatzer Amulet; how diabetes patients fared with alirocumab in ODYSSEY Outcomes; the new word in stents is “abluminal”; and cheerful news about midlife fitness. Listen to Cardiocast for the week’s top news.
Left atrial appendage closure slashes stroke risk in high-risk atrial fibrillation patients with Amplatzer Amulet; how diabetes patients fared with alirocumab in ODYSSEY Outcomes; the new word in stents is “abluminal”; and cheerful news about midlife fitness. Listen to Cardiocast for the week’s top news.
Left atrial appendage closure slashes stroke risk in high-risk atrial fibrillation patients with Amplatzer Amulet; how diabetes patients fared with alirocumab in ODYSSEY Outcomes; the new word in stents is “abluminal”; and cheerful news about midlife fitness. Listen to Cardiocast for the week’s top news.
Pancreas volume studies may offer insight into T1DM
ORLANDO – The pancreas is drawing wider interest among scientists: In one study, researchers report that already-shrunken organs keep atrophying in young people with recently diagnosed type 1 diabetes mellitus (T1DM) even as they go through adolescent growth. And another study finds that the pancreas is smaller in first-degree relatives of people with T1DM, especially relatives with pre-T1DM.
“Pancreas volume is a really exciting area of research because it may tell us something about diabetes that we just didn’t understand before,” said pediatric endocrinologist Michael J. Haller, MD, of the University of Florida, Gainesville, in an interview. Dr. Haller is coauthor of the study into pancreas volume in first-degree relatives. That study’s findings, along with the results of a Vanderbilt University/University of Texas study into pancreas volume in early T1DM, were presented at the annual meeting of the American Diabetes Association.
Scientists already know that the pancreas is 40% smaller in people who have lived with T1DM, Dr. Haller said, and “we now know that occurs long before the clinical diagnosis has been made.”
The pancreas volume mystery, he said, revolves around the fact that only 2%-3% of the organ is made up of beta cells, which make insulin. So why is there a 40% reduction in the pancreas overall? “It tells you the other parts of the pancreas are experiencing considerable damage because of the process,” he said.
In the Vanderbilt/UT study, led by Jack Virostko, PhD, at Vanderbilt University, Nashville, Tenn., researchers examined pancreas volume via MRI in patients with recent-onset T1DM (n = 51; mean age = 14 years, range 8-24 years).
The patients had a smaller median pancreas volume (29.6 mL) than similarly aged controls (49.6 mL; n = 51; P less than .001), and the gap held up after adjustment for factors like age, weight, and body mass index.
Pancreas volumes increased in controls over time as the subjects grew, but shrank even more in those with T1DM over the next year (P less than .001).
“Their research suggests there is a bit of ongoing reduction in volume,” Dr. Haller said. “That’s an area of debate in the literature, and additional larger datasets are being collected to prove or disprove that.”
For his study, Dr. Haller and University of Florida colleagues examined pancreas volume in 223 subjects (average age = 20 years; 45% males).
They found that relative pancreas volume was significantly lower vs. age-matched controls in subjects with recent-onset T1DM and in their first-degree relatives, regardless of their T1DM-related autoantibody status. First-degree relatives whose T1DM-related autoantibody status was positive – a sign of pre-T1DM – had smaller pancreas volumes than controls.
“This suggests there’s a stepwise reduction in volume that may help us understand risk better for certain patients,” Dr. Haller said.
In the future, he said, physicians may be able to use pancreas volume to distinguish between patients who otherwise appear to face the same T1DM risk. As a result, he said, they could adjust treatment accordingly.
“The datasets still need to become more robust, but it’s a really translatable kind of research,” he said, especially since it relies on MRIs “that you could use at any hospital.”
Funding for the Vanderbilt/UT study is not reported, and its authors report no relevant disclosures. Funding for the University of Florida study is not reported, and its authors report no disclosures except for one who reports a patent issued.
SOURCE: Campbell-Thompson ML et al. ADA 2018. Abstract 1816-P; Virostko J et al. ADA 2018. Abstract 233-OR.
ORLANDO – The pancreas is drawing wider interest among scientists: In one study, researchers report that already-shrunken organs keep atrophying in young people with recently diagnosed type 1 diabetes mellitus (T1DM) even as they go through adolescent growth. And another study finds that the pancreas is smaller in first-degree relatives of people with T1DM, especially relatives with pre-T1DM.
“Pancreas volume is a really exciting area of research because it may tell us something about diabetes that we just didn’t understand before,” said pediatric endocrinologist Michael J. Haller, MD, of the University of Florida, Gainesville, in an interview. Dr. Haller is coauthor of the study into pancreas volume in first-degree relatives. That study’s findings, along with the results of a Vanderbilt University/University of Texas study into pancreas volume in early T1DM, were presented at the annual meeting of the American Diabetes Association.
Scientists already know that the pancreas is 40% smaller in people who have lived with T1DM, Dr. Haller said, and “we now know that occurs long before the clinical diagnosis has been made.”
The pancreas volume mystery, he said, revolves around the fact that only 2%-3% of the organ is made up of beta cells, which make insulin. So why is there a 40% reduction in the pancreas overall? “It tells you the other parts of the pancreas are experiencing considerable damage because of the process,” he said.
In the Vanderbilt/UT study, led by Jack Virostko, PhD, at Vanderbilt University, Nashville, Tenn., researchers examined pancreas volume via MRI in patients with recent-onset T1DM (n = 51; mean age = 14 years, range 8-24 years).
The patients had a smaller median pancreas volume (29.6 mL) than similarly aged controls (49.6 mL; n = 51; P less than .001), and the gap held up after adjustment for factors like age, weight, and body mass index.
Pancreas volumes increased in controls over time as the subjects grew, but shrank even more in those with T1DM over the next year (P less than .001).
“Their research suggests there is a bit of ongoing reduction in volume,” Dr. Haller said. “That’s an area of debate in the literature, and additional larger datasets are being collected to prove or disprove that.”
For his study, Dr. Haller and University of Florida colleagues examined pancreas volume in 223 subjects (average age = 20 years; 45% males).
They found that relative pancreas volume was significantly lower vs. age-matched controls in subjects with recent-onset T1DM and in their first-degree relatives, regardless of their T1DM-related autoantibody status. First-degree relatives whose T1DM-related autoantibody status was positive – a sign of pre-T1DM – had smaller pancreas volumes than controls.
“This suggests there’s a stepwise reduction in volume that may help us understand risk better for certain patients,” Dr. Haller said.
In the future, he said, physicians may be able to use pancreas volume to distinguish between patients who otherwise appear to face the same T1DM risk. As a result, he said, they could adjust treatment accordingly.
“The datasets still need to become more robust, but it’s a really translatable kind of research,” he said, especially since it relies on MRIs “that you could use at any hospital.”
Funding for the Vanderbilt/UT study is not reported, and its authors report no relevant disclosures. Funding for the University of Florida study is not reported, and its authors report no disclosures except for one who reports a patent issued.
SOURCE: Campbell-Thompson ML et al. ADA 2018. Abstract 1816-P; Virostko J et al. ADA 2018. Abstract 233-OR.
ORLANDO – The pancreas is drawing wider interest among scientists: In one study, researchers report that already-shrunken organs keep atrophying in young people with recently diagnosed type 1 diabetes mellitus (T1DM) even as they go through adolescent growth. And another study finds that the pancreas is smaller in first-degree relatives of people with T1DM, especially relatives with pre-T1DM.
“Pancreas volume is a really exciting area of research because it may tell us something about diabetes that we just didn’t understand before,” said pediatric endocrinologist Michael J. Haller, MD, of the University of Florida, Gainesville, in an interview. Dr. Haller is coauthor of the study into pancreas volume in first-degree relatives. That study’s findings, along with the results of a Vanderbilt University/University of Texas study into pancreas volume in early T1DM, were presented at the annual meeting of the American Diabetes Association.
Scientists already know that the pancreas is 40% smaller in people who have lived with T1DM, Dr. Haller said, and “we now know that occurs long before the clinical diagnosis has been made.”
The pancreas volume mystery, he said, revolves around the fact that only 2%-3% of the organ is made up of beta cells, which make insulin. So why is there a 40% reduction in the pancreas overall? “It tells you the other parts of the pancreas are experiencing considerable damage because of the process,” he said.
In the Vanderbilt/UT study, led by Jack Virostko, PhD, at Vanderbilt University, Nashville, Tenn., researchers examined pancreas volume via MRI in patients with recent-onset T1DM (n = 51; mean age = 14 years, range 8-24 years).
The patients had a smaller median pancreas volume (29.6 mL) than similarly aged controls (49.6 mL; n = 51; P less than .001), and the gap held up after adjustment for factors like age, weight, and body mass index.
Pancreas volumes increased in controls over time as the subjects grew, but shrank even more in those with T1DM over the next year (P less than .001).
“Their research suggests there is a bit of ongoing reduction in volume,” Dr. Haller said. “That’s an area of debate in the literature, and additional larger datasets are being collected to prove or disprove that.”
For his study, Dr. Haller and University of Florida colleagues examined pancreas volume in 223 subjects (average age = 20 years; 45% males).
They found that relative pancreas volume was significantly lower vs. age-matched controls in subjects with recent-onset T1DM and in their first-degree relatives, regardless of their T1DM-related autoantibody status. First-degree relatives whose T1DM-related autoantibody status was positive – a sign of pre-T1DM – had smaller pancreas volumes than controls.
“This suggests there’s a stepwise reduction in volume that may help us understand risk better for certain patients,” Dr. Haller said.
In the future, he said, physicians may be able to use pancreas volume to distinguish between patients who otherwise appear to face the same T1DM risk. As a result, he said, they could adjust treatment accordingly.
“The datasets still need to become more robust, but it’s a really translatable kind of research,” he said, especially since it relies on MRIs “that you could use at any hospital.”
Funding for the Vanderbilt/UT study is not reported, and its authors report no relevant disclosures. Funding for the University of Florida study is not reported, and its authors report no disclosures except for one who reports a patent issued.
SOURCE: Campbell-Thompson ML et al. ADA 2018. Abstract 1816-P; Virostko J et al. ADA 2018. Abstract 233-OR.
REPORTING FROM ADA 2018
How to Manage Diabetes While Keeping Costs Down
A cost-effective community program at Pennsylvania State University helped most participants change their behavior and significantly improve their HbA1c and blood pressure, according to a report in Preventing Chronic Disease.
The researchers for the extension program, Dining with Diabetes, collected data on 2,738 adults with type 2 diabetes or prediabetes and adult family members without diabetes. The program consisted of 4 weekly 2-hour classes and a follow-up class conducted 3 months later. The classes included hands-on food preparation, food tastings, and physical activity.
At the follow-up class, participants who completed the program had significant improvements in diabetes-related biomarkers. A greater percentage said they were confident they could keep their diabetes under control, compared with the number at baseline (67% v 58%). At baseline, most participants were adhering to medications; the researchers found no significant change in adherence.
Participants also increased the number of days per week on which they exercised for ≥ 20 minutes (from 2.9 to 3.4 days), and slightly increased the number of days on which they ate a variety of fruits and vegetables.
Nearly half of participants with baseline and follow-up measurements had a drop in HbA1c. At follow-up, 21% had a reduction large enough to lower their diabetes status. The changes translated to a 5.9% decrease in HbA1c for 27% of those who had uncontrolled diabetes at baseline. More than half (59%) had a drop in blood pressure, including 60% of those with uncontrolled diabetes.
The program, which was free to participants, cost Penn State Extension $407 per person. The researchers estimate that extending the program to half of the 1.3 million people with diabetes in Pennsylvania would save the state approximately $195 million at 1 year.
A cost-effective community program at Pennsylvania State University helped most participants change their behavior and significantly improve their HbA1c and blood pressure, according to a report in Preventing Chronic Disease.
The researchers for the extension program, Dining with Diabetes, collected data on 2,738 adults with type 2 diabetes or prediabetes and adult family members without diabetes. The program consisted of 4 weekly 2-hour classes and a follow-up class conducted 3 months later. The classes included hands-on food preparation, food tastings, and physical activity.
At the follow-up class, participants who completed the program had significant improvements in diabetes-related biomarkers. A greater percentage said they were confident they could keep their diabetes under control, compared with the number at baseline (67% v 58%). At baseline, most participants were adhering to medications; the researchers found no significant change in adherence.
Participants also increased the number of days per week on which they exercised for ≥ 20 minutes (from 2.9 to 3.4 days), and slightly increased the number of days on which they ate a variety of fruits and vegetables.
Nearly half of participants with baseline and follow-up measurements had a drop in HbA1c. At follow-up, 21% had a reduction large enough to lower their diabetes status. The changes translated to a 5.9% decrease in HbA1c for 27% of those who had uncontrolled diabetes at baseline. More than half (59%) had a drop in blood pressure, including 60% of those with uncontrolled diabetes.
The program, which was free to participants, cost Penn State Extension $407 per person. The researchers estimate that extending the program to half of the 1.3 million people with diabetes in Pennsylvania would save the state approximately $195 million at 1 year.
A cost-effective community program at Pennsylvania State University helped most participants change their behavior and significantly improve their HbA1c and blood pressure, according to a report in Preventing Chronic Disease.
The researchers for the extension program, Dining with Diabetes, collected data on 2,738 adults with type 2 diabetes or prediabetes and adult family members without diabetes. The program consisted of 4 weekly 2-hour classes and a follow-up class conducted 3 months later. The classes included hands-on food preparation, food tastings, and physical activity.
At the follow-up class, participants who completed the program had significant improvements in diabetes-related biomarkers. A greater percentage said they were confident they could keep their diabetes under control, compared with the number at baseline (67% v 58%). At baseline, most participants were adhering to medications; the researchers found no significant change in adherence.
Participants also increased the number of days per week on which they exercised for ≥ 20 minutes (from 2.9 to 3.4 days), and slightly increased the number of days on which they ate a variety of fruits and vegetables.
Nearly half of participants with baseline and follow-up measurements had a drop in HbA1c. At follow-up, 21% had a reduction large enough to lower their diabetes status. The changes translated to a 5.9% decrease in HbA1c for 27% of those who had uncontrolled diabetes at baseline. More than half (59%) had a drop in blood pressure, including 60% of those with uncontrolled diabetes.
The program, which was free to participants, cost Penn State Extension $407 per person. The researchers estimate that extending the program to half of the 1.3 million people with diabetes in Pennsylvania would save the state approximately $195 million at 1 year.
Alirocumab’s benefit greater in diabetes patients: ODYSSEY Outcomes
ORLANDO – Higher risk translates to higher benefits. That’s the message of a new analysis of the ODYSSEY Outcomes trial in the PCSK9-inhibitor alirocumab that finds people with diabetes gained about twice the reduction in risk of major adverse cardiac events as their non-diabetic counterparts.
“Patients with diabetes and a recent heart attack are at double the risk of a cardiovascular event in the next 3 years as are nondiabetics, despite guideline-based care,” said study presenting author Kausik Ray, MD, ChB, of the School of Public Health of Imperial College London, in an interview. “These patients in our study had LDL of around 89 mg/dL despite high-intensity statins. Current guidelines recommend a goal of LDL of 55 mg/dL in this group. We brought LDL down to around 38 mg/ dL, and showed that by doing this, diabetics derived a greater reduction in the risk of major cardiovascular events. A greater absolute benefit was observed, and a smaller number needed to treat.”
Dr. Ray presented the study findings, a prespecified analysis of results of ODYSSEY Outcomes, at the annual scientific sessions of the American Diabetes Association.
The trial randomly assigned 18,924 patients with recent acute coronary syndrome and LDL cholesterol of at least 70 mg/dL, despite maximum statin therapy, to 75 mg of alirocumab every 2 weeks or placebo. Doses of alirocumab were increased blindly, to 150 mg, to reach LDL cholesterol levels of 25-50 mg/dL.
During a median 2.8 years of follow-up, the overall cumulative rate of major cardiac adverse events (coronary heart disease death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina) occurred in 9.5% of the overall population randomized to alirocumab and 11.1% of those on placebo, for an absolute risk reduction of 1.6% and a statistically significant and clinically meaningful 15% reduction in relative risk. The results were presented at the annual scientific sessions of the American College of Cardiology in March.
In the current analysis, in patients with diabetes, the cumulative rate of incidents was 14.1% (380 of 2,693) with alirocumab and 16.4% (452 of 2,751) with placebo, for an ARR of 2.3%.
The ARRs for the prediabetes and normoglycemia groups were both 1.2%.
Dr. Ray noted that there’s no sign that the drug works differently in patients with diabetes. “The drug works in the same way and as effectively in everyone: LDL came down by 64% at 16 weeks in everyone. But absolute risk depends upon absolute risk to start with. So, in higher-risk patients, the absolute benefit is greater.”
According to Dr. Ray, the number needed to treat is 43 over 30 months for people with diabetes and 73 over 30 months for people without diabetes.
Prediman K. Shah, MD, director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles, questioned the cost effectiveness of the medication in an interview.
“Even among the diabetics, the absolute risk reduction is about 2%, which is underwhelming considering the high cost,” he said. “If the cost were to drop to levels closer to cost of statins, such a small risk reduction may be worth the expense.”
Insurers have been skeptical of covering alirocumab because of its $14,000/year cost. However, Sanofi and Regeneron, which jointly market alirocumab, announced in March 2018 that they “will offer U.S. payers that agree to reduce burdensome access barriers for high-risk patients a further reduced net price for Praluent Injection (alirocumab) in alignment with a new value assessment for high-risk patients from the [United States].”
In response, Dr. Ray said “the benefits quoted are time-to-first-event, and these are modest. But if you look at recurrent events, which represent the natural course of disease, then the benefits and absolute benefits are greater. These are add-on therapies and will never be used in every single patient at current cost.”
Glen J. Pearson, PharmD, of the University of Alberta, Edmonton, said in an interview that, “while these absolute numbers do seem relatively small, it must be remembered that these patients are already receiving very effective therapies to reduce their risk of future cardiovascular outcomes.”
ODYSSEY Outcomes was funded by Sanofi and Regeneron. The presenter reports various disclosures including consulting and research support relationships with Sanofi and Regeneron. The other study authors report various disclosures. Dr. Pearson reports no relevant disclosures. Dr. Shah reports receiving grant support from Sanofi Regeneron.
SOURCE: Ray K et al. ADA 2018, Abstract 6-LB.
ORLANDO – Higher risk translates to higher benefits. That’s the message of a new analysis of the ODYSSEY Outcomes trial in the PCSK9-inhibitor alirocumab that finds people with diabetes gained about twice the reduction in risk of major adverse cardiac events as their non-diabetic counterparts.
“Patients with diabetes and a recent heart attack are at double the risk of a cardiovascular event in the next 3 years as are nondiabetics, despite guideline-based care,” said study presenting author Kausik Ray, MD, ChB, of the School of Public Health of Imperial College London, in an interview. “These patients in our study had LDL of around 89 mg/dL despite high-intensity statins. Current guidelines recommend a goal of LDL of 55 mg/dL in this group. We brought LDL down to around 38 mg/ dL, and showed that by doing this, diabetics derived a greater reduction in the risk of major cardiovascular events. A greater absolute benefit was observed, and a smaller number needed to treat.”
Dr. Ray presented the study findings, a prespecified analysis of results of ODYSSEY Outcomes, at the annual scientific sessions of the American Diabetes Association.
The trial randomly assigned 18,924 patients with recent acute coronary syndrome and LDL cholesterol of at least 70 mg/dL, despite maximum statin therapy, to 75 mg of alirocumab every 2 weeks or placebo. Doses of alirocumab were increased blindly, to 150 mg, to reach LDL cholesterol levels of 25-50 mg/dL.
During a median 2.8 years of follow-up, the overall cumulative rate of major cardiac adverse events (coronary heart disease death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina) occurred in 9.5% of the overall population randomized to alirocumab and 11.1% of those on placebo, for an absolute risk reduction of 1.6% and a statistically significant and clinically meaningful 15% reduction in relative risk. The results were presented at the annual scientific sessions of the American College of Cardiology in March.
In the current analysis, in patients with diabetes, the cumulative rate of incidents was 14.1% (380 of 2,693) with alirocumab and 16.4% (452 of 2,751) with placebo, for an ARR of 2.3%.
The ARRs for the prediabetes and normoglycemia groups were both 1.2%.
Dr. Ray noted that there’s no sign that the drug works differently in patients with diabetes. “The drug works in the same way and as effectively in everyone: LDL came down by 64% at 16 weeks in everyone. But absolute risk depends upon absolute risk to start with. So, in higher-risk patients, the absolute benefit is greater.”
According to Dr. Ray, the number needed to treat is 43 over 30 months for people with diabetes and 73 over 30 months for people without diabetes.
Prediman K. Shah, MD, director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles, questioned the cost effectiveness of the medication in an interview.
“Even among the diabetics, the absolute risk reduction is about 2%, which is underwhelming considering the high cost,” he said. “If the cost were to drop to levels closer to cost of statins, such a small risk reduction may be worth the expense.”
Insurers have been skeptical of covering alirocumab because of its $14,000/year cost. However, Sanofi and Regeneron, which jointly market alirocumab, announced in March 2018 that they “will offer U.S. payers that agree to reduce burdensome access barriers for high-risk patients a further reduced net price for Praluent Injection (alirocumab) in alignment with a new value assessment for high-risk patients from the [United States].”
In response, Dr. Ray said “the benefits quoted are time-to-first-event, and these are modest. But if you look at recurrent events, which represent the natural course of disease, then the benefits and absolute benefits are greater. These are add-on therapies and will never be used in every single patient at current cost.”
Glen J. Pearson, PharmD, of the University of Alberta, Edmonton, said in an interview that, “while these absolute numbers do seem relatively small, it must be remembered that these patients are already receiving very effective therapies to reduce their risk of future cardiovascular outcomes.”
ODYSSEY Outcomes was funded by Sanofi and Regeneron. The presenter reports various disclosures including consulting and research support relationships with Sanofi and Regeneron. The other study authors report various disclosures. Dr. Pearson reports no relevant disclosures. Dr. Shah reports receiving grant support from Sanofi Regeneron.
SOURCE: Ray K et al. ADA 2018, Abstract 6-LB.
ORLANDO – Higher risk translates to higher benefits. That’s the message of a new analysis of the ODYSSEY Outcomes trial in the PCSK9-inhibitor alirocumab that finds people with diabetes gained about twice the reduction in risk of major adverse cardiac events as their non-diabetic counterparts.
“Patients with diabetes and a recent heart attack are at double the risk of a cardiovascular event in the next 3 years as are nondiabetics, despite guideline-based care,” said study presenting author Kausik Ray, MD, ChB, of the School of Public Health of Imperial College London, in an interview. “These patients in our study had LDL of around 89 mg/dL despite high-intensity statins. Current guidelines recommend a goal of LDL of 55 mg/dL in this group. We brought LDL down to around 38 mg/ dL, and showed that by doing this, diabetics derived a greater reduction in the risk of major cardiovascular events. A greater absolute benefit was observed, and a smaller number needed to treat.”
Dr. Ray presented the study findings, a prespecified analysis of results of ODYSSEY Outcomes, at the annual scientific sessions of the American Diabetes Association.
The trial randomly assigned 18,924 patients with recent acute coronary syndrome and LDL cholesterol of at least 70 mg/dL, despite maximum statin therapy, to 75 mg of alirocumab every 2 weeks or placebo. Doses of alirocumab were increased blindly, to 150 mg, to reach LDL cholesterol levels of 25-50 mg/dL.
During a median 2.8 years of follow-up, the overall cumulative rate of major cardiac adverse events (coronary heart disease death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina) occurred in 9.5% of the overall population randomized to alirocumab and 11.1% of those on placebo, for an absolute risk reduction of 1.6% and a statistically significant and clinically meaningful 15% reduction in relative risk. The results were presented at the annual scientific sessions of the American College of Cardiology in March.
In the current analysis, in patients with diabetes, the cumulative rate of incidents was 14.1% (380 of 2,693) with alirocumab and 16.4% (452 of 2,751) with placebo, for an ARR of 2.3%.
The ARRs for the prediabetes and normoglycemia groups were both 1.2%.
Dr. Ray noted that there’s no sign that the drug works differently in patients with diabetes. “The drug works in the same way and as effectively in everyone: LDL came down by 64% at 16 weeks in everyone. But absolute risk depends upon absolute risk to start with. So, in higher-risk patients, the absolute benefit is greater.”
According to Dr. Ray, the number needed to treat is 43 over 30 months for people with diabetes and 73 over 30 months for people without diabetes.
Prediman K. Shah, MD, director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center and professor of medicine at the University of California, Los Angeles, questioned the cost effectiveness of the medication in an interview.
“Even among the diabetics, the absolute risk reduction is about 2%, which is underwhelming considering the high cost,” he said. “If the cost were to drop to levels closer to cost of statins, such a small risk reduction may be worth the expense.”
Insurers have been skeptical of covering alirocumab because of its $14,000/year cost. However, Sanofi and Regeneron, which jointly market alirocumab, announced in March 2018 that they “will offer U.S. payers that agree to reduce burdensome access barriers for high-risk patients a further reduced net price for Praluent Injection (alirocumab) in alignment with a new value assessment for high-risk patients from the [United States].”
In response, Dr. Ray said “the benefits quoted are time-to-first-event, and these are modest. But if you look at recurrent events, which represent the natural course of disease, then the benefits and absolute benefits are greater. These are add-on therapies and will never be used in every single patient at current cost.”
Glen J. Pearson, PharmD, of the University of Alberta, Edmonton, said in an interview that, “while these absolute numbers do seem relatively small, it must be remembered that these patients are already receiving very effective therapies to reduce their risk of future cardiovascular outcomes.”
ODYSSEY Outcomes was funded by Sanofi and Regeneron. The presenter reports various disclosures including consulting and research support relationships with Sanofi and Regeneron. The other study authors report various disclosures. Dr. Pearson reports no relevant disclosures. Dr. Shah reports receiving grant support from Sanofi Regeneron.
SOURCE: Ray K et al. ADA 2018, Abstract 6-LB.
REPORTING FROM ADA 2018
Key clinical point:
Major finding: Over a median 34-month period, patients with diabetes who took alirocumab had a 2.3% absolute risk reduction in major cardiac adverse events incidents. Counterparts without diabetes had an ARR of 1.2%.
Study details: ODYSSEY Outcomes, a double-blind, randomized trial of nearly 19,000 patients with a recent acute coronary syndrome and an LDL cholesterol level of 70 mg/dL or more despite intensive statin therapy.
Disclosures: The study was funded by Sanofi and Regeneron Pharmaceuticals, and many study authors disclose financial relationships with the companies.
Source: Ray K et al. ADA 2018, Abstract 6-LB.