User login
Fluoroquinolones can cause fatal hypoglycemia, FDA warns
Fluoroquinolones have caused at least 67 cases of life-threatening hypoglycemic coma, including 13 deaths and 9 permanent and disabling injuries, according to an internal safety review by the Food and Drug Administration. Most cases (44) were associated with levofloxacin.
The review also found new neuropsychiatric side effects associated with fluoroquinolones, including disturbances in attention, memory impairment, and delirium.
Considering these findings, the agency will strengthen warning labels on all fluoroquinolones, which already warn that the antibiotics may cause hypoglycemia and mental health issues, especially in older people, the FDA said in a press statement.
“Health care professionals should be aware of the potential risk of hypoglycemia, sometimes resulting in coma, occurring more frequently in the elderly and those with diabetes taking an oral hypoglycemic medicine or insulin,” the statement said. “Alert patients of the symptoms of hypoglycemia and carefully monitor blood glucose levels in these patients and discuss with them how to treat themselves if they have symptoms of hypoglycemia. Inform patients about the risk of psychiatric adverse reactions that can occur after just one dose. Stop fluoroquinolone treatment immediately if a patient reports any central nervous system side effects, including psychiatric adverse reactions, or blood glucose disturbances and switch to a non–fluoroquinolone antibiotic if possible. Stop fluoroquinolone treatment immediately if a patient reports serious side effects involving the tendons, muscles, joints, or nerves, and switch to a non–fluoroquinolone antibiotic to complete the patient’s treatment course.”
The statement also warned not to prescribe fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections because the risks outweigh the benefits in these patients.
The FDA conducted the postmarketing review on all five of the fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, moxifloxacin, and ofloxacin). The newest fluoroquinolone, delafloxacin, approved a year ago, was not included in the class review. However, the agency expects that similar adverse events will be associated with delafloxacin and labeling on that drug will include the new warnings.
The agency reviewed cases in the FDA Adverse Event Reporting System, and in published medical literature, during 1987-2017. Most of the incidents (56) were in the system; 11 additional cases were published. Levofloxacin caused most of the incidents (44), followed by ciprofloxacin (12), moxifloxacin (9), and ofloxacin (2). Four of the fluoroquinolones have a labeled drug interaction with sulfonylurea agents, which can cause hypoglycemia.
Some of those who died were getting the antibiotics for complicated infections, including urinary tract and upper respiratory tract infections, and postoperative antibiotic prophylaxis. Others had renal insufficiency – a risk factor for hypoglycemia.
Of the 54 patients who survived, 9 never fully recovered and had permanent disabilities. Four patients remained in a coma for at least 1 month, despite blood sugar normalization. Five experienced some type of neurologic injury.
The new label changes will also fortify the existing warning about mental health side effects, after the review found new reactions that are not listed in the current warning, including the new reports of disturbance in attention, memory impairment, and delirium.
The FDA statement did not include the number of cases found or the associated drugs. Again, the safety review was based on reports in the FAERS database and published medical literature.
“We found that psychiatric adverse reactions were not consistent in the drug labels. The labels of fluoroquinolones currently include many psychiatric adverse reactions in the Warnings and Precautions section, for example, hallucination, psychoses, confusion, depression, anxiety, and paranoia. In an effort to harmonize the psychiatric adverse reactions described in the drug labels across the class of fluoroquinolones, we are requiring that all fluoroquinolones include six psychiatric adverse reactions (disturbance in attention, memory impairment, delirium, nervousness, agitation, and disorientation) in the Central Nervous System Effects of the Warnings and Precautions section of the labels. Disturbance in attention, memory impairment, and delirium are new adverse reactions to be added to the labels of the entire class of fluoroquinolones. Nervousness, agitation, and disorientation had been previously listed in the fluoroquinolone drug labels and will now be added to the Warnings and Precautions section of each drug label to harmonize labels across the fluoroquinolone drug class. The new label changes will make the psychiatric adverse reactions more prominent and more consistent.”
The FDA has previously warned about other adverse events associated with fluoroquinolones in May 2016, restricting use for certain uncomplicated infections; July 2016, for disabling side effects; August 2013, for peripheral neuropathy, and July 2008, for tendinitis and tendon rupture.
Fluoroquinolones have caused at least 67 cases of life-threatening hypoglycemic coma, including 13 deaths and 9 permanent and disabling injuries, according to an internal safety review by the Food and Drug Administration. Most cases (44) were associated with levofloxacin.
The review also found new neuropsychiatric side effects associated with fluoroquinolones, including disturbances in attention, memory impairment, and delirium.
Considering these findings, the agency will strengthen warning labels on all fluoroquinolones, which already warn that the antibiotics may cause hypoglycemia and mental health issues, especially in older people, the FDA said in a press statement.
“Health care professionals should be aware of the potential risk of hypoglycemia, sometimes resulting in coma, occurring more frequently in the elderly and those with diabetes taking an oral hypoglycemic medicine or insulin,” the statement said. “Alert patients of the symptoms of hypoglycemia and carefully monitor blood glucose levels in these patients and discuss with them how to treat themselves if they have symptoms of hypoglycemia. Inform patients about the risk of psychiatric adverse reactions that can occur after just one dose. Stop fluoroquinolone treatment immediately if a patient reports any central nervous system side effects, including psychiatric adverse reactions, or blood glucose disturbances and switch to a non–fluoroquinolone antibiotic if possible. Stop fluoroquinolone treatment immediately if a patient reports serious side effects involving the tendons, muscles, joints, or nerves, and switch to a non–fluoroquinolone antibiotic to complete the patient’s treatment course.”
The statement also warned not to prescribe fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections because the risks outweigh the benefits in these patients.
The FDA conducted the postmarketing review on all five of the fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, moxifloxacin, and ofloxacin). The newest fluoroquinolone, delafloxacin, approved a year ago, was not included in the class review. However, the agency expects that similar adverse events will be associated with delafloxacin and labeling on that drug will include the new warnings.
The agency reviewed cases in the FDA Adverse Event Reporting System, and in published medical literature, during 1987-2017. Most of the incidents (56) were in the system; 11 additional cases were published. Levofloxacin caused most of the incidents (44), followed by ciprofloxacin (12), moxifloxacin (9), and ofloxacin (2). Four of the fluoroquinolones have a labeled drug interaction with sulfonylurea agents, which can cause hypoglycemia.
Some of those who died were getting the antibiotics for complicated infections, including urinary tract and upper respiratory tract infections, and postoperative antibiotic prophylaxis. Others had renal insufficiency – a risk factor for hypoglycemia.
Of the 54 patients who survived, 9 never fully recovered and had permanent disabilities. Four patients remained in a coma for at least 1 month, despite blood sugar normalization. Five experienced some type of neurologic injury.
The new label changes will also fortify the existing warning about mental health side effects, after the review found new reactions that are not listed in the current warning, including the new reports of disturbance in attention, memory impairment, and delirium.
The FDA statement did not include the number of cases found or the associated drugs. Again, the safety review was based on reports in the FAERS database and published medical literature.
“We found that psychiatric adverse reactions were not consistent in the drug labels. The labels of fluoroquinolones currently include many psychiatric adverse reactions in the Warnings and Precautions section, for example, hallucination, psychoses, confusion, depression, anxiety, and paranoia. In an effort to harmonize the psychiatric adverse reactions described in the drug labels across the class of fluoroquinolones, we are requiring that all fluoroquinolones include six psychiatric adverse reactions (disturbance in attention, memory impairment, delirium, nervousness, agitation, and disorientation) in the Central Nervous System Effects of the Warnings and Precautions section of the labels. Disturbance in attention, memory impairment, and delirium are new adverse reactions to be added to the labels of the entire class of fluoroquinolones. Nervousness, agitation, and disorientation had been previously listed in the fluoroquinolone drug labels and will now be added to the Warnings and Precautions section of each drug label to harmonize labels across the fluoroquinolone drug class. The new label changes will make the psychiatric adverse reactions more prominent and more consistent.”
The FDA has previously warned about other adverse events associated with fluoroquinolones in May 2016, restricting use for certain uncomplicated infections; July 2016, for disabling side effects; August 2013, for peripheral neuropathy, and July 2008, for tendinitis and tendon rupture.
Fluoroquinolones have caused at least 67 cases of life-threatening hypoglycemic coma, including 13 deaths and 9 permanent and disabling injuries, according to an internal safety review by the Food and Drug Administration. Most cases (44) were associated with levofloxacin.
The review also found new neuropsychiatric side effects associated with fluoroquinolones, including disturbances in attention, memory impairment, and delirium.
Considering these findings, the agency will strengthen warning labels on all fluoroquinolones, which already warn that the antibiotics may cause hypoglycemia and mental health issues, especially in older people, the FDA said in a press statement.
“Health care professionals should be aware of the potential risk of hypoglycemia, sometimes resulting in coma, occurring more frequently in the elderly and those with diabetes taking an oral hypoglycemic medicine or insulin,” the statement said. “Alert patients of the symptoms of hypoglycemia and carefully monitor blood glucose levels in these patients and discuss with them how to treat themselves if they have symptoms of hypoglycemia. Inform patients about the risk of psychiatric adverse reactions that can occur after just one dose. Stop fluoroquinolone treatment immediately if a patient reports any central nervous system side effects, including psychiatric adverse reactions, or blood glucose disturbances and switch to a non–fluoroquinolone antibiotic if possible. Stop fluoroquinolone treatment immediately if a patient reports serious side effects involving the tendons, muscles, joints, or nerves, and switch to a non–fluoroquinolone antibiotic to complete the patient’s treatment course.”
The statement also warned not to prescribe fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections because the risks outweigh the benefits in these patients.
The FDA conducted the postmarketing review on all five of the fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, moxifloxacin, and ofloxacin). The newest fluoroquinolone, delafloxacin, approved a year ago, was not included in the class review. However, the agency expects that similar adverse events will be associated with delafloxacin and labeling on that drug will include the new warnings.
The agency reviewed cases in the FDA Adverse Event Reporting System, and in published medical literature, during 1987-2017. Most of the incidents (56) were in the system; 11 additional cases were published. Levofloxacin caused most of the incidents (44), followed by ciprofloxacin (12), moxifloxacin (9), and ofloxacin (2). Four of the fluoroquinolones have a labeled drug interaction with sulfonylurea agents, which can cause hypoglycemia.
Some of those who died were getting the antibiotics for complicated infections, including urinary tract and upper respiratory tract infections, and postoperative antibiotic prophylaxis. Others had renal insufficiency – a risk factor for hypoglycemia.
Of the 54 patients who survived, 9 never fully recovered and had permanent disabilities. Four patients remained in a coma for at least 1 month, despite blood sugar normalization. Five experienced some type of neurologic injury.
The new label changes will also fortify the existing warning about mental health side effects, after the review found new reactions that are not listed in the current warning, including the new reports of disturbance in attention, memory impairment, and delirium.
The FDA statement did not include the number of cases found or the associated drugs. Again, the safety review was based on reports in the FAERS database and published medical literature.
“We found that psychiatric adverse reactions were not consistent in the drug labels. The labels of fluoroquinolones currently include many psychiatric adverse reactions in the Warnings and Precautions section, for example, hallucination, psychoses, confusion, depression, anxiety, and paranoia. In an effort to harmonize the psychiatric adverse reactions described in the drug labels across the class of fluoroquinolones, we are requiring that all fluoroquinolones include six psychiatric adverse reactions (disturbance in attention, memory impairment, delirium, nervousness, agitation, and disorientation) in the Central Nervous System Effects of the Warnings and Precautions section of the labels. Disturbance in attention, memory impairment, and delirium are new adverse reactions to be added to the labels of the entire class of fluoroquinolones. Nervousness, agitation, and disorientation had been previously listed in the fluoroquinolone drug labels and will now be added to the Warnings and Precautions section of each drug label to harmonize labels across the fluoroquinolone drug class. The new label changes will make the psychiatric adverse reactions more prominent and more consistent.”
The FDA has previously warned about other adverse events associated with fluoroquinolones in May 2016, restricting use for certain uncomplicated infections; July 2016, for disabling side effects; August 2013, for peripheral neuropathy, and July 2008, for tendinitis and tendon rupture.
RISE: Insulin glargine, metformin offer no beta cell function benefit in youth
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
ORLANDO – in the pediatric medication portion of the Restoring Insulin Secretion (RISE) study.
The treatments, including either metformin for 12 months in 47 participants or insulin glargine for 3 months followed by metformin for 9 months in 44 participants, were not associated with improvement in beta cell function at 12 months, compared with baseline, according to reports from members of the RISE Consortium at the annual scientific sessions of the American Diabetes Association.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Furthermore, measures of beta cell function worsened in both groups at 15-month follow-up, and the same was true for participants with impaired glucose tolerance only; the outcomes in that subset of patients were similar to the entire group, including patients with early T2DM.
“Beta cell failure progressed despite that intervention, and though both [metformin and insulin glargine] were effective for lowering glucose – and metformin for lowering weight ... it had nothing to do with the natural history of the disease, and that’s really quite disappointing,” John B. Buse, MD, said in a video interview.
But that’s not to say the findings weren’t of value.
“The exciting bit was our greater understanding of what’s different about diabetes in youth, and basically [the findings] showed that, both in the setting of impaired glucose tolerance and early diabetes, youth have more insulin resistance than adults, they have relatively more well-preserved beta cell function – they’re secreting more insulin at both impaired glucose tolerance and diabetes, and they have lesser hepatic insulin clearance,” said Dr. Buse, professor, chief of the division of endocrinology, and director of the Diabetes Center at the University of North Carolina, Chapel Hill.
Dr. Buse provided invited commentary on the findings at the ADA scientific sessions and elaborated on those comments in this interview, noting that, in addition to identifying important differences between children and adults with impaired glucose tolerance and diabetes, the RISE study demonstrated that the numerous challenges associated with conducting a major study involving children with impaired glucose tolerance or T2DM can be overcome.
“It’s a really heartwarming story,” he said of the efforts and successes of the RISE investigators in completing the pediatric medication portion of the study. “It at least gives us hope that, even if we haven’t found a cure for type 2 diabetes in children, we at least know we can do the studies.”
Dr. Buse also provided his take on what the future holds for both parts of the RISE study (findings from the adult medication and adult surgery portions are expected to be reported within the next year) and for other studies in children and youth with diabetes; he noted that the current findings and successes in enrolling and completing the pediatric portion of the study highlight multiple opportunities for future research.
Dr. Buse reported financial relationships with Adocia, AstraZeneca, Dexcom, Elcelyx, Eli Lilly, Fractyl Laboratories, Intarcia Therapeutics, Lexicon Pharmaceuticals, Metavention, NovaTarg Therapeutics, Novo Nordisk, Sanofi, VTV Therapeutics, Boehringer Ingelheim, Johnson & Johnson Services, Theracos, Shenzhen Hightide Biopharmaceutical, National Heart Lung and Blood Institute, National Center for Advancing Translational Sciences, National Institute of Diabetes and Digestive and Kidney Diseases, American Diabetes Association, Patient-Centered Outcomes Research Institute, and the National Institute of Environmental Health Sciences.
EXPERT ANALYSIS AT ADA 2018
CANVAS data: Canagliflozin generally well tolerated up to 6.5 years
ORLANDO – according to the latest safety data from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program.
CANVAS included two studies (CANVAS and CANVAS-R) involving a total of 10,142 patients, which established the superiority of canagliflozin (Invokana) over placebo for reducing the risk of a three-point major adverse cardiac event endpoint, including cardiovascular death, nonfatal MI, and nonfatal stroke. The sodium-glucose cotransporter-2 (SGLT2) inhibitor also improved other cardiovascular outcomes.
For the current analysis, outcomes in the CANVAS participants were compared with those from a general population of 8,114 patients with type 2 diabetes mellitus (T2DM) who participated in 12 non-CANVAS studies of canagliflozin. As previously reported, the risks for fracture or amputation were novel safety findings associated with canagliflozin in the CANVAS program and, in the current analysis, the incidence of fractures per 1,000 patient-years in CANVAS was 15.4 vs. 11.9 with treatment vs. placebo, whereas no significant difference was seen in the non-CANVAS studies (incidence rate of 11.8 vs. 10.8 per 1,000 patient-years for treatment vs. placebo), Priscilla Hollander, MD, reported at the annual meeting of the American Diabetes Association.
Of note, when the CANVAS and CANVAS-R studies were compared, the imbalance was seen only in CANVAS (incidence rates of 16.9 vs. 10.9 for treatment vs. placebo [hazard ratio, 1.55], compared with incidence rates of 11.3 and 13.2 , respectively, in CANVAS-R [HR, 0.86]), said Dr. Hollander, Baylor Scott & White Endocrine Center in Dallas.
“Ongoing analyses are trying to determine why there is a difference between the two studies,” she noted.
For the novel safety finding of increased amputation risk with canagliflozin, an excess of three events per 1,000 patient years was seen in both CANVAS (incidence of 6.3 vs. 3.4; HR, 1.97) and CANVAS-R (incidence of 5.9 vs. 2.8; HR, 2.12). No difference in risk was seen among the non-CANVAS population (incidence of 0.5 and 2.2 with treatment vs. placebo; HR, 0.23).
“Amputations were primarily at the level of the toe or the metatarsal. Patients with a history of amputation or peripheral vascular disease had the highest risk of amputation,” she said, adding that this was true in both treatment and placebo groups.
“Again, ongoing analyses are being done to look at the mechanism in this regard,” she said.
For safety outcomes known to be related to the mechanism of SGLT2 inhibition, including osmotic diuresis, volume depletion, and genital mycotic infection (GMI), similar differences between canagliflozin and placebo groups were seen in the CANVAS and non-CANVAS studies at 6.5 years, Dr. Hollander said.
Hazard ratios in the canagliflozin vs. placebo groups for the CANVAS and non-CANVAS studies, respectively, were 2.80 and 2.66 for osmotic diuresis, 1.44 and 1.35 for volume depletion, 4.37 and 4.32 for female GMI, and 3.76 and 6.26 for male GMI.
No imbalances were observed in other AEs of interest – including hypoglycemia, urinary tract infections, or hypersensitivity reactions – in either the CANVAS or the non-CANVAS studies.
“The point estimate for [diabetic ketoacidosis] was 2.3, but with very wide confidence intervals due to a very low number of events, so it really did not reach significance,” Dr. Hollander noted. “Again, due to the mechanism of action of canagliflozin, and the warning for acute kidney injury on the label, renal adverse events were also of interest, but there was no imbalance observed in the renal-related AEs between the CANVAS program and the non-CANVAS program.”
A closer look at renal-related adverse events (AEs) of interest in the CANVAS program only (not in comparison with the non-CANVAS findings) also showed no significant difference with canagliflozin vs. placebo in blood creatinine increase, blood urea increase, glomerular filtration rate decrease, acute kidney injury, renal impairment, renal failure, oliguria, acute prerenal failure, hypercreatininemia, nephritis, or prerenal failure, she said.
Furthermore, although hyperkalemia is noted as a risk with canagliflozin in patients with moderate renal impairment who are taking medications that interfere with potassium excretion, no significant differences were observed between the treatment and placebo groups over 6.5 years in the CANVAS program, she added, noting that “this was also supported by the lack of imbalance between the laboratory changes for serum potassium in the two groups.”
There also were no differences seen between the treatment and placebo groups in the rates of all serious AEs or in the rates of AEs leading to discontinuation, she said.
Canagliflozin has been generally well tolerated in both placebo-controlled trials and trials in which the SGLT2 inhibitor was compared with other active treatments. The non-CANVAS studies used for comparison in the current analysis included phase 3/4 canagliflozin clinical development program studies lasting up to 104 weeks and involving a general T2DM patient population, Dr. Hollander noted.
The CANVAS program, which was launched in 2009, included patients with T2DM and established cardiovascular disease or high cardiovascular disease risk who received a 2-week placebo run-in followed by placebo or either 100- or 300-mg doses of canagliflozin. CANVAS participants had hemoglobin A1c of 7%-10.5%; estimated glomerular filtration rate of 30 mL/min per 1.72m2 or greater; age of 30 years or greater plus a history of a prior cardiovascular event, or age of 50 years or greater with at least 2 cardiovascular risk factors, including diabetes for 10 years or more; systolic blood pressure greater than 140 mm Hg on at least one medication; current smoking status; micro- or macroalbuminuria; and an HDL cholesterol level less than 1 mmol/L.
The current analysis provides the longest-term safety data to date for the program, Dr. Hollander said.
The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
SOURCE: Hollander P et al. ADA 2018, Abstract 259-OR.
ORLANDO – according to the latest safety data from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program.
CANVAS included two studies (CANVAS and CANVAS-R) involving a total of 10,142 patients, which established the superiority of canagliflozin (Invokana) over placebo for reducing the risk of a three-point major adverse cardiac event endpoint, including cardiovascular death, nonfatal MI, and nonfatal stroke. The sodium-glucose cotransporter-2 (SGLT2) inhibitor also improved other cardiovascular outcomes.
For the current analysis, outcomes in the CANVAS participants were compared with those from a general population of 8,114 patients with type 2 diabetes mellitus (T2DM) who participated in 12 non-CANVAS studies of canagliflozin. As previously reported, the risks for fracture or amputation were novel safety findings associated with canagliflozin in the CANVAS program and, in the current analysis, the incidence of fractures per 1,000 patient-years in CANVAS was 15.4 vs. 11.9 with treatment vs. placebo, whereas no significant difference was seen in the non-CANVAS studies (incidence rate of 11.8 vs. 10.8 per 1,000 patient-years for treatment vs. placebo), Priscilla Hollander, MD, reported at the annual meeting of the American Diabetes Association.
Of note, when the CANVAS and CANVAS-R studies were compared, the imbalance was seen only in CANVAS (incidence rates of 16.9 vs. 10.9 for treatment vs. placebo [hazard ratio, 1.55], compared with incidence rates of 11.3 and 13.2 , respectively, in CANVAS-R [HR, 0.86]), said Dr. Hollander, Baylor Scott & White Endocrine Center in Dallas.
“Ongoing analyses are trying to determine why there is a difference between the two studies,” she noted.
For the novel safety finding of increased amputation risk with canagliflozin, an excess of three events per 1,000 patient years was seen in both CANVAS (incidence of 6.3 vs. 3.4; HR, 1.97) and CANVAS-R (incidence of 5.9 vs. 2.8; HR, 2.12). No difference in risk was seen among the non-CANVAS population (incidence of 0.5 and 2.2 with treatment vs. placebo; HR, 0.23).
“Amputations were primarily at the level of the toe or the metatarsal. Patients with a history of amputation or peripheral vascular disease had the highest risk of amputation,” she said, adding that this was true in both treatment and placebo groups.
“Again, ongoing analyses are being done to look at the mechanism in this regard,” she said.
For safety outcomes known to be related to the mechanism of SGLT2 inhibition, including osmotic diuresis, volume depletion, and genital mycotic infection (GMI), similar differences between canagliflozin and placebo groups were seen in the CANVAS and non-CANVAS studies at 6.5 years, Dr. Hollander said.
Hazard ratios in the canagliflozin vs. placebo groups for the CANVAS and non-CANVAS studies, respectively, were 2.80 and 2.66 for osmotic diuresis, 1.44 and 1.35 for volume depletion, 4.37 and 4.32 for female GMI, and 3.76 and 6.26 for male GMI.
No imbalances were observed in other AEs of interest – including hypoglycemia, urinary tract infections, or hypersensitivity reactions – in either the CANVAS or the non-CANVAS studies.
“The point estimate for [diabetic ketoacidosis] was 2.3, but with very wide confidence intervals due to a very low number of events, so it really did not reach significance,” Dr. Hollander noted. “Again, due to the mechanism of action of canagliflozin, and the warning for acute kidney injury on the label, renal adverse events were also of interest, but there was no imbalance observed in the renal-related AEs between the CANVAS program and the non-CANVAS program.”
A closer look at renal-related adverse events (AEs) of interest in the CANVAS program only (not in comparison with the non-CANVAS findings) also showed no significant difference with canagliflozin vs. placebo in blood creatinine increase, blood urea increase, glomerular filtration rate decrease, acute kidney injury, renal impairment, renal failure, oliguria, acute prerenal failure, hypercreatininemia, nephritis, or prerenal failure, she said.
Furthermore, although hyperkalemia is noted as a risk with canagliflozin in patients with moderate renal impairment who are taking medications that interfere with potassium excretion, no significant differences were observed between the treatment and placebo groups over 6.5 years in the CANVAS program, she added, noting that “this was also supported by the lack of imbalance between the laboratory changes for serum potassium in the two groups.”
There also were no differences seen between the treatment and placebo groups in the rates of all serious AEs or in the rates of AEs leading to discontinuation, she said.
Canagliflozin has been generally well tolerated in both placebo-controlled trials and trials in which the SGLT2 inhibitor was compared with other active treatments. The non-CANVAS studies used for comparison in the current analysis included phase 3/4 canagliflozin clinical development program studies lasting up to 104 weeks and involving a general T2DM patient population, Dr. Hollander noted.
The CANVAS program, which was launched in 2009, included patients with T2DM and established cardiovascular disease or high cardiovascular disease risk who received a 2-week placebo run-in followed by placebo or either 100- or 300-mg doses of canagliflozin. CANVAS participants had hemoglobin A1c of 7%-10.5%; estimated glomerular filtration rate of 30 mL/min per 1.72m2 or greater; age of 30 years or greater plus a history of a prior cardiovascular event, or age of 50 years or greater with at least 2 cardiovascular risk factors, including diabetes for 10 years or more; systolic blood pressure greater than 140 mm Hg on at least one medication; current smoking status; micro- or macroalbuminuria; and an HDL cholesterol level less than 1 mmol/L.
The current analysis provides the longest-term safety data to date for the program, Dr. Hollander said.
The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
SOURCE: Hollander P et al. ADA 2018, Abstract 259-OR.
ORLANDO – according to the latest safety data from the CANVAS (Canagliflozin Cardiovascular Assessment Study) program.
CANVAS included two studies (CANVAS and CANVAS-R) involving a total of 10,142 patients, which established the superiority of canagliflozin (Invokana) over placebo for reducing the risk of a three-point major adverse cardiac event endpoint, including cardiovascular death, nonfatal MI, and nonfatal stroke. The sodium-glucose cotransporter-2 (SGLT2) inhibitor also improved other cardiovascular outcomes.
For the current analysis, outcomes in the CANVAS participants were compared with those from a general population of 8,114 patients with type 2 diabetes mellitus (T2DM) who participated in 12 non-CANVAS studies of canagliflozin. As previously reported, the risks for fracture or amputation were novel safety findings associated with canagliflozin in the CANVAS program and, in the current analysis, the incidence of fractures per 1,000 patient-years in CANVAS was 15.4 vs. 11.9 with treatment vs. placebo, whereas no significant difference was seen in the non-CANVAS studies (incidence rate of 11.8 vs. 10.8 per 1,000 patient-years for treatment vs. placebo), Priscilla Hollander, MD, reported at the annual meeting of the American Diabetes Association.
Of note, when the CANVAS and CANVAS-R studies were compared, the imbalance was seen only in CANVAS (incidence rates of 16.9 vs. 10.9 for treatment vs. placebo [hazard ratio, 1.55], compared with incidence rates of 11.3 and 13.2 , respectively, in CANVAS-R [HR, 0.86]), said Dr. Hollander, Baylor Scott & White Endocrine Center in Dallas.
“Ongoing analyses are trying to determine why there is a difference between the two studies,” she noted.
For the novel safety finding of increased amputation risk with canagliflozin, an excess of three events per 1,000 patient years was seen in both CANVAS (incidence of 6.3 vs. 3.4; HR, 1.97) and CANVAS-R (incidence of 5.9 vs. 2.8; HR, 2.12). No difference in risk was seen among the non-CANVAS population (incidence of 0.5 and 2.2 with treatment vs. placebo; HR, 0.23).
“Amputations were primarily at the level of the toe or the metatarsal. Patients with a history of amputation or peripheral vascular disease had the highest risk of amputation,” she said, adding that this was true in both treatment and placebo groups.
“Again, ongoing analyses are being done to look at the mechanism in this regard,” she said.
For safety outcomes known to be related to the mechanism of SGLT2 inhibition, including osmotic diuresis, volume depletion, and genital mycotic infection (GMI), similar differences between canagliflozin and placebo groups were seen in the CANVAS and non-CANVAS studies at 6.5 years, Dr. Hollander said.
Hazard ratios in the canagliflozin vs. placebo groups for the CANVAS and non-CANVAS studies, respectively, were 2.80 and 2.66 for osmotic diuresis, 1.44 and 1.35 for volume depletion, 4.37 and 4.32 for female GMI, and 3.76 and 6.26 for male GMI.
No imbalances were observed in other AEs of interest – including hypoglycemia, urinary tract infections, or hypersensitivity reactions – in either the CANVAS or the non-CANVAS studies.
“The point estimate for [diabetic ketoacidosis] was 2.3, but with very wide confidence intervals due to a very low number of events, so it really did not reach significance,” Dr. Hollander noted. “Again, due to the mechanism of action of canagliflozin, and the warning for acute kidney injury on the label, renal adverse events were also of interest, but there was no imbalance observed in the renal-related AEs between the CANVAS program and the non-CANVAS program.”
A closer look at renal-related adverse events (AEs) of interest in the CANVAS program only (not in comparison with the non-CANVAS findings) also showed no significant difference with canagliflozin vs. placebo in blood creatinine increase, blood urea increase, glomerular filtration rate decrease, acute kidney injury, renal impairment, renal failure, oliguria, acute prerenal failure, hypercreatininemia, nephritis, or prerenal failure, she said.
Furthermore, although hyperkalemia is noted as a risk with canagliflozin in patients with moderate renal impairment who are taking medications that interfere with potassium excretion, no significant differences were observed between the treatment and placebo groups over 6.5 years in the CANVAS program, she added, noting that “this was also supported by the lack of imbalance between the laboratory changes for serum potassium in the two groups.”
There also were no differences seen between the treatment and placebo groups in the rates of all serious AEs or in the rates of AEs leading to discontinuation, she said.
Canagliflozin has been generally well tolerated in both placebo-controlled trials and trials in which the SGLT2 inhibitor was compared with other active treatments. The non-CANVAS studies used for comparison in the current analysis included phase 3/4 canagliflozin clinical development program studies lasting up to 104 weeks and involving a general T2DM patient population, Dr. Hollander noted.
The CANVAS program, which was launched in 2009, included patients with T2DM and established cardiovascular disease or high cardiovascular disease risk who received a 2-week placebo run-in followed by placebo or either 100- or 300-mg doses of canagliflozin. CANVAS participants had hemoglobin A1c of 7%-10.5%; estimated glomerular filtration rate of 30 mL/min per 1.72m2 or greater; age of 30 years or greater plus a history of a prior cardiovascular event, or age of 50 years or greater with at least 2 cardiovascular risk factors, including diabetes for 10 years or more; systolic blood pressure greater than 140 mm Hg on at least one medication; current smoking status; micro- or macroalbuminuria; and an HDL cholesterol level less than 1 mmol/L.
The current analysis provides the longest-term safety data to date for the program, Dr. Hollander said.
The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
SOURCE: Hollander P et al. ADA 2018, Abstract 259-OR.
REPORTING FROM ADA 2018
Key clinical point: Canagliflozin is generally well tolerated for up to 6.5 years in patients with T2DM and high CV risk.
Major finding: Similar differences between canagliflozin and placebo groups for outcomes related to the mechanism of SGLT2 inhibition were seen in the CANVAS and non-CANVAS studies at 6.5 years.
Study details: A comparison of safety outcomes in 10,142 patients in CANVAS and 8,114 patients in non-CANVAS studies.
Disclosures: The CANVAS Program is sponsored by Janssen Research & Development. Dr. Hollander is an advisory panel member for Eli Lilly, Merck, and Novo Nordisk.
Source: Hollander P et al. ADA 2018 Abstract 259-OR.
Uric acid tied to pediatric diabetic kidney disease
ORLANDO – , according to a 7-year investigation of 539 children.
Every 1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02).
The finding adds to growing evidence that serum uric acid (SUA) isn’t just a marker of diabetic kidney disease, but a contributor to it. “There is definitely” cross-talk between gout and diabetes, said lead investigator Petter Bjornstad, MD, assistant professor of pediatric endocrinology at the University of Colorado, Aurora.
Elevated SUA is common in both conditions and a risk factor for kidney disease. Newer studies have linked higher levels to nephron number decline and other pathologies, perhaps through renal inflammation. Allopurinol, the traditional uric acid lowering agent in gout, is already under investigation to prevent kidney decline in adults with type 1 diabetes mellitus. There’s also evidence that the potent uric acid lowering agent, febuxostat (Uloric), attenuates hypofiltration in early diabetic kidney disease.
The 539 children, all part of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, were assessed annually over a mean of 5.7 years. At baseline, they were 13.9 years old and had T2DM for 7.9 months, on average. The mean body mass index was 34.6 kg/m2, mean hemoglobin A1c was 6%.
Almost 20% of the children were hypertensive at baseline (130/80 mm Hg or higher); 26% were hyperuricemic (6.8 mg/dL or higher); and 6.1% had elevated urine albumin excretion (urine albumin creatinine ratio of at least 30 mg/g), a marker of renal pathology. At the end of follow-up, 18% had elevated albumin excretion and 37.4% were hypertensive.
“Hyperuricemia was common in youth with type 2 diabetes,” just as it’s been shown in adults with the disease. “Higher baseline SUA independently increase[s] risk for onset of hypertension and elevated urine albumin excretion,” Dr. Bjornstad said.
However, the association between SUA and elevated albumin excretion was statistically significant only in boys – 36% of the study population – and non-Hispanic whites, 20% of the subjects, after adjustment for BMI, hemoglobin A1c, estimated glomerular filtration rate, and use of ACE inhibitors and angiotensin II receptor blockers.
The National Institutes of Health funded the work. Dr. Bjornstad is a consultant for Boehringer Ingelheim.
SOURCE: Bjornstad P et al. ADA 2018, abstract 339-OR.
ORLANDO – , according to a 7-year investigation of 539 children.
Every 1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02).
The finding adds to growing evidence that serum uric acid (SUA) isn’t just a marker of diabetic kidney disease, but a contributor to it. “There is definitely” cross-talk between gout and diabetes, said lead investigator Petter Bjornstad, MD, assistant professor of pediatric endocrinology at the University of Colorado, Aurora.
Elevated SUA is common in both conditions and a risk factor for kidney disease. Newer studies have linked higher levels to nephron number decline and other pathologies, perhaps through renal inflammation. Allopurinol, the traditional uric acid lowering agent in gout, is already under investigation to prevent kidney decline in adults with type 1 diabetes mellitus. There’s also evidence that the potent uric acid lowering agent, febuxostat (Uloric), attenuates hypofiltration in early diabetic kidney disease.
The 539 children, all part of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, were assessed annually over a mean of 5.7 years. At baseline, they were 13.9 years old and had T2DM for 7.9 months, on average. The mean body mass index was 34.6 kg/m2, mean hemoglobin A1c was 6%.
Almost 20% of the children were hypertensive at baseline (130/80 mm Hg or higher); 26% were hyperuricemic (6.8 mg/dL or higher); and 6.1% had elevated urine albumin excretion (urine albumin creatinine ratio of at least 30 mg/g), a marker of renal pathology. At the end of follow-up, 18% had elevated albumin excretion and 37.4% were hypertensive.
“Hyperuricemia was common in youth with type 2 diabetes,” just as it’s been shown in adults with the disease. “Higher baseline SUA independently increase[s] risk for onset of hypertension and elevated urine albumin excretion,” Dr. Bjornstad said.
However, the association between SUA and elevated albumin excretion was statistically significant only in boys – 36% of the study population – and non-Hispanic whites, 20% of the subjects, after adjustment for BMI, hemoglobin A1c, estimated glomerular filtration rate, and use of ACE inhibitors and angiotensin II receptor blockers.
The National Institutes of Health funded the work. Dr. Bjornstad is a consultant for Boehringer Ingelheim.
SOURCE: Bjornstad P et al. ADA 2018, abstract 339-OR.
ORLANDO – , according to a 7-year investigation of 539 children.
Every 1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02).
The finding adds to growing evidence that serum uric acid (SUA) isn’t just a marker of diabetic kidney disease, but a contributor to it. “There is definitely” cross-talk between gout and diabetes, said lead investigator Petter Bjornstad, MD, assistant professor of pediatric endocrinology at the University of Colorado, Aurora.
Elevated SUA is common in both conditions and a risk factor for kidney disease. Newer studies have linked higher levels to nephron number decline and other pathologies, perhaps through renal inflammation. Allopurinol, the traditional uric acid lowering agent in gout, is already under investigation to prevent kidney decline in adults with type 1 diabetes mellitus. There’s also evidence that the potent uric acid lowering agent, febuxostat (Uloric), attenuates hypofiltration in early diabetic kidney disease.
The 539 children, all part of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial, were assessed annually over a mean of 5.7 years. At baseline, they were 13.9 years old and had T2DM for 7.9 months, on average. The mean body mass index was 34.6 kg/m2, mean hemoglobin A1c was 6%.
Almost 20% of the children were hypertensive at baseline (130/80 mm Hg or higher); 26% were hyperuricemic (6.8 mg/dL or higher); and 6.1% had elevated urine albumin excretion (urine albumin creatinine ratio of at least 30 mg/g), a marker of renal pathology. At the end of follow-up, 18% had elevated albumin excretion and 37.4% were hypertensive.
“Hyperuricemia was common in youth with type 2 diabetes,” just as it’s been shown in adults with the disease. “Higher baseline SUA independently increase[s] risk for onset of hypertension and elevated urine albumin excretion,” Dr. Bjornstad said.
However, the association between SUA and elevated albumin excretion was statistically significant only in boys – 36% of the study population – and non-Hispanic whites, 20% of the subjects, after adjustment for BMI, hemoglobin A1c, estimated glomerular filtration rate, and use of ACE inhibitors and angiotensin II receptor blockers.
The National Institutes of Health funded the work. Dr. Bjornstad is a consultant for Boehringer Ingelheim.
SOURCE: Bjornstad P et al. ADA 2018, abstract 339-OR.
REPORTING FROM ADA 2018
Key clinical point: Serum uric acid lowering might help prevent kidney disease in children with T2DM.
Major finding: Every1-mg/dL climb in baseline serum uric acid increased the risk of subsequent elevated urine albumin excretion 1.23 fold, after adjustment for potential confounders (P = .02)
Study details: Seven-year investigation of 539 children with new-onset T2DM.
Disclosures: The National Institutes of Health funded the work. The study lead is a consultant for Boehringer Ingelheim.
Source: Bjornstad P et al. ADA 2018 Abstract 339-OR.
Study spotlights risk factors for albuminuria in youth with T2DM
TORONTO – When Brandy Wicklow, MD, began her pediatric endocrinology fellowship at McGill University in 2006, about 12 per 100,000 children in Manitoba, Canada, were diagnosed with type 2 diabetes mellitus each year. By 2016 that rate had more than doubled, to 26 per 100,000 children.
“If you look just at indigenous youth in our province, it’s probably one of the highest rates ever reported, with 95 per 100,000 Manitoba First Nation children diagnosed with type 2 diabetes,” said Dr. Wicklow, a pediatric endocrinologist at the University of Manitoba and the Children’s Hospital Research Institute of Manitoba.
Many indigenous populations also face an increased risk for primary renal disease. One study reviewed the charts 90 of Canadian First Nation children and adolescents with T2DM (Diabetes Care. 2009;32[5]:786-90). Of 10 who had renal biopsies performed, nine had immune complex disease/glomerulosclerosis, two had mild diabetes-related lesions, and seven had focal segmental glomerulosclerosis (FSGS); yet none had classic nephropathy. An analysis of Chinese youth that included 216 renal biopsies yielded similar findings (Intl Urol Nephrol. 2012;45[1]:173-9).
It’s also known that early-onset T2DM is associated with substantially increased incidence of end-stage renal disease (ESRD) and mortality in middle age. For example, one study of Pima Indians found that those who were diagnosed with T2DM earlier than 20 years of age had a one in five chance of developing ESRD, while those who were diagnosed at age 20 years or older had a one in two chance of ESRD (JAMA. 2006;296[4]:421-6). In a separate analysis, researchers estimated the remaining lifetime risks for ESRD among Aboriginal people in Australia with and without diabetes (Diabetes Res Clin Pract. 2014;103[3]:e24-6). The value for young adults with diabetes was high, about one in two at the age of 30 years, while it decreased with age to one in seven at 60 years.
“One of the first biomarkers we see in terms of renal disease in kids with T2DM is albuminuria,” Dr. Wicklow said at the Pediatric Academic Societies meeting. “The question is, why do kids with type 2 get more renal disease than kids with type 1 diabetes?” The SEARCH for Diabetes in Youth (SEARCH) study from 2006 found that hypertension, increased body mass index, increased weight circumference, and increased lipids were factors, while the SEARCH study from 2015 found that ethnicity, increased weight to height ratio, and mean arterial pressure were factors.
“Insulin resistance is significantly associated with albuminuria,” Dr. Wicklow continued. “It’s also been shown to be associated with hyperfiltration. Some of the markers of insulin resistance are important but they make up about 19% of the variance between type 1 and type 2, which means there are other variables that we’re not measuring.”
Enter ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study that Dr. Wicklow and her associates launched in 2014 at eight centers in Canada. It aims to examine the biopsychosocial risk factors for albuminuria in youth with T2DM and the mechanisms for renal injury. “Our theoretical framework was that biological exposures that we are aware of, such as glycemic control, hypertension, and lipids, would all be important in the development of albuminuria and renal disease in kids,” said Dr. Wicklow, who is the study’s coprimary investigator along with Allison Dart, MD. “But what we thought was novel was that psychological exposures either as socioeconomic status or as mental health factors would also directly impinge on renal health with respect to chronic inflammation in the body, inflammation in the kidneys, and long-term kidney damage.”
The first phase of ICARE involved a detailed phenotypic assessment of youth, including anthropometrics, biochemistry, 24-hour ambulatory blood pressure monitoring, overnight urine collections for albumin excretion, renal ultrasound, and iohexol-derived glomerular filtration rate (GFR). Phase 2 included an evaluation of psychological factors, including hair-derived cortisol; validated questionnaires for perceived stress, distress, and resiliency; and a detailed evaluation of systemic and urine inflammatory biomarkers. Annual follow-up is carried out to assess temporal associations between clinical risk factors and renal outcomes, including progression of albuminuria.
At the meeting, Dr. Wicklow reported on 187 youth enrolled to date. Of these, 96% were of indigenous ethnicity, 57 had albuminuria and 130 did not, and the mean ages of the groups were 16 years and 15 years, respectively. At baseline, a higher proportion of those in the albuminuria group were female (74% vs. 64% of those in the no albuminuria group, respectively), had a higher mean hemoglobin A1c (11% vs. 9%), and had hypertension (94% vs. 72%). She noted that upon presentation to the clinic, only 23% of participants had HbA1c levels less than 7%, only 26% had ranges between 7% and 9%, and about 40% did not have any hypertension. Of those who did, 27% had nighttime-only hypertension, and only 2% had daytime-only hypertension.
“The other risk factor these kids have for developing ESRD is that the majority were exposed to diabetes in pregnancy,” Dr. Wicklow said. “Murine models of maternal diabetes exposure have demonstrated that offspring have small kidneys, less ureteric bud branching, and a lower number of nephrons. Most of the human clinical cohort studies look at associations between development of diabetes and parental hypertension, maternal smoking, and maternal education. There is likely an impact at birth that sets these kids up for development of type 2 diabetes.”
In addition, results from clinical cohort studies have found that depression, mental stress, and distress are high in youth with T2DM. “Preliminary data suggest that if you have positive mental health, or coping strategies, or someone has worked through this with you and you are resilient, you might benefit in terms of overall glycemic control,” she said. For example, ICARE investigators have found that the higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by monocyte chemotactic protein-1 (MCP-1; P = .02). “Mental health seems to be something that can directly impact your health from a biological standpoint, and we might be able to find biomarkers of that risk,” Dr. Wicklow said. “Where does the stress come from? Most of my patients are indigenous, so it’s not surprising that the history in Canada of colonization of residential schools has left a lasting impression on these families and communities in terms of loss of language, loss of culture, and loss of land. There’s a community-based stress and a family-based stress that these children feel.”
Social factors also play a big role. She presented baseline findings from 196 youth with T2DM and 456 with T1DM, including measures such as the Socioeconomic Factor Index-Version 2 (SEFI-2), a way to assess socioeconomic characteristics based on Canadian Census data that reflects nonmedical social determinants of health. “It looks at factors like number of rooms in the house, single-parent households, maternal education attainment, and family income,” Dr. Wicklow explained. “The higher the SEFI-2 score, the lower your socioeconomic status is for the area you live in. Kids with T2DM generally live in areas of lower SES and lower socioeconomic index. They often live far away from health care providers. Many do not attend school and many are not with their biologic families, so we’ve had a lot of issues addressing child and family services, in particular in the phase of a chronic illness where our expectation is one thing and the family’s and community’s expectations of what’s realistic in terms of treatment and goals is another. We also have a lot of adolescent pregnancies.”
To date, about 80% of youth with T1D have seen a health care provider within the first year after transition from the pediatric diabetes clinic, compared with just over 50% of kids with T2D. “We transition youth with T1DM to internists, while our youth with T2DM go to itinerant physicians often back in their communities and/or rural family physicians,” she said. Between baseline and year 2, the rate of hospital admissions remained similar among T1DM at 11.6 and 11.8 admissions per 100 patient-years, respectively, but the number of hospital admissions for T2DM patients jumped from 20.1 to 25.5 admissions per 100 patient-years. “Kids with type 2 are showing up in the hospital a lot more than those with type 1 diabetes, but not for diabetes-related diagnoses,” Dr. Wicklow said. “We’re starting to look through the data now, and most of our kids are showing up with mental health complaints and issues. That’s why they’re getting hospitalized.”
Among ICARE study participants who have completed 3 years of follow-up, about 52% had albuminuria at their baseline visit and 48% sustained albuminuria throughout the study. About 26% progressed from normal levels of albuminuria to microalbuminuria, from microalbuminuria to macroalbuminuria, or from normal levels of albuminuria to macroalbuminuria. In addition, 16% persisted in the category that they were in, and 10% regressed. “The good news is, some of our kids get better over time,” Dr. Wicklow said. “The bad news is that the majority do not.”
Going forward, Dr. Wicklow and her associates work with an ICARE advisory group composed of children and families “who sit with us and talk about what mental health needs might be important, and how we should organize our study in a follow-up of the kids, to try and answer some of the questions that are important,” she said. “Working with the concept of the study’s theoretical framework, they acknowledged that the biological exposures are important, but they were also concerned about food security, finding strength/resilience within the community, and finding coping factors in terms of keeping themselves healthy with their diabetes. For some communities, they are concerned with basic needs. We’re working with them to help them progress, and to figure out how to best study children with type 2 diabetes.”
ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba (DREAM) theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
TORONTO – When Brandy Wicklow, MD, began her pediatric endocrinology fellowship at McGill University in 2006, about 12 per 100,000 children in Manitoba, Canada, were diagnosed with type 2 diabetes mellitus each year. By 2016 that rate had more than doubled, to 26 per 100,000 children.
“If you look just at indigenous youth in our province, it’s probably one of the highest rates ever reported, with 95 per 100,000 Manitoba First Nation children diagnosed with type 2 diabetes,” said Dr. Wicklow, a pediatric endocrinologist at the University of Manitoba and the Children’s Hospital Research Institute of Manitoba.
Many indigenous populations also face an increased risk for primary renal disease. One study reviewed the charts 90 of Canadian First Nation children and adolescents with T2DM (Diabetes Care. 2009;32[5]:786-90). Of 10 who had renal biopsies performed, nine had immune complex disease/glomerulosclerosis, two had mild diabetes-related lesions, and seven had focal segmental glomerulosclerosis (FSGS); yet none had classic nephropathy. An analysis of Chinese youth that included 216 renal biopsies yielded similar findings (Intl Urol Nephrol. 2012;45[1]:173-9).
It’s also known that early-onset T2DM is associated with substantially increased incidence of end-stage renal disease (ESRD) and mortality in middle age. For example, one study of Pima Indians found that those who were diagnosed with T2DM earlier than 20 years of age had a one in five chance of developing ESRD, while those who were diagnosed at age 20 years or older had a one in two chance of ESRD (JAMA. 2006;296[4]:421-6). In a separate analysis, researchers estimated the remaining lifetime risks for ESRD among Aboriginal people in Australia with and without diabetes (Diabetes Res Clin Pract. 2014;103[3]:e24-6). The value for young adults with diabetes was high, about one in two at the age of 30 years, while it decreased with age to one in seven at 60 years.
“One of the first biomarkers we see in terms of renal disease in kids with T2DM is albuminuria,” Dr. Wicklow said at the Pediatric Academic Societies meeting. “The question is, why do kids with type 2 get more renal disease than kids with type 1 diabetes?” The SEARCH for Diabetes in Youth (SEARCH) study from 2006 found that hypertension, increased body mass index, increased weight circumference, and increased lipids were factors, while the SEARCH study from 2015 found that ethnicity, increased weight to height ratio, and mean arterial pressure were factors.
“Insulin resistance is significantly associated with albuminuria,” Dr. Wicklow continued. “It’s also been shown to be associated with hyperfiltration. Some of the markers of insulin resistance are important but they make up about 19% of the variance between type 1 and type 2, which means there are other variables that we’re not measuring.”
Enter ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study that Dr. Wicklow and her associates launched in 2014 at eight centers in Canada. It aims to examine the biopsychosocial risk factors for albuminuria in youth with T2DM and the mechanisms for renal injury. “Our theoretical framework was that biological exposures that we are aware of, such as glycemic control, hypertension, and lipids, would all be important in the development of albuminuria and renal disease in kids,” said Dr. Wicklow, who is the study’s coprimary investigator along with Allison Dart, MD. “But what we thought was novel was that psychological exposures either as socioeconomic status or as mental health factors would also directly impinge on renal health with respect to chronic inflammation in the body, inflammation in the kidneys, and long-term kidney damage.”
The first phase of ICARE involved a detailed phenotypic assessment of youth, including anthropometrics, biochemistry, 24-hour ambulatory blood pressure monitoring, overnight urine collections for albumin excretion, renal ultrasound, and iohexol-derived glomerular filtration rate (GFR). Phase 2 included an evaluation of psychological factors, including hair-derived cortisol; validated questionnaires for perceived stress, distress, and resiliency; and a detailed evaluation of systemic and urine inflammatory biomarkers. Annual follow-up is carried out to assess temporal associations between clinical risk factors and renal outcomes, including progression of albuminuria.
At the meeting, Dr. Wicklow reported on 187 youth enrolled to date. Of these, 96% were of indigenous ethnicity, 57 had albuminuria and 130 did not, and the mean ages of the groups were 16 years and 15 years, respectively. At baseline, a higher proportion of those in the albuminuria group were female (74% vs. 64% of those in the no albuminuria group, respectively), had a higher mean hemoglobin A1c (11% vs. 9%), and had hypertension (94% vs. 72%). She noted that upon presentation to the clinic, only 23% of participants had HbA1c levels less than 7%, only 26% had ranges between 7% and 9%, and about 40% did not have any hypertension. Of those who did, 27% had nighttime-only hypertension, and only 2% had daytime-only hypertension.
“The other risk factor these kids have for developing ESRD is that the majority were exposed to diabetes in pregnancy,” Dr. Wicklow said. “Murine models of maternal diabetes exposure have demonstrated that offspring have small kidneys, less ureteric bud branching, and a lower number of nephrons. Most of the human clinical cohort studies look at associations between development of diabetes and parental hypertension, maternal smoking, and maternal education. There is likely an impact at birth that sets these kids up for development of type 2 diabetes.”
In addition, results from clinical cohort studies have found that depression, mental stress, and distress are high in youth with T2DM. “Preliminary data suggest that if you have positive mental health, or coping strategies, or someone has worked through this with you and you are resilient, you might benefit in terms of overall glycemic control,” she said. For example, ICARE investigators have found that the higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by monocyte chemotactic protein-1 (MCP-1; P = .02). “Mental health seems to be something that can directly impact your health from a biological standpoint, and we might be able to find biomarkers of that risk,” Dr. Wicklow said. “Where does the stress come from? Most of my patients are indigenous, so it’s not surprising that the history in Canada of colonization of residential schools has left a lasting impression on these families and communities in terms of loss of language, loss of culture, and loss of land. There’s a community-based stress and a family-based stress that these children feel.”
Social factors also play a big role. She presented baseline findings from 196 youth with T2DM and 456 with T1DM, including measures such as the Socioeconomic Factor Index-Version 2 (SEFI-2), a way to assess socioeconomic characteristics based on Canadian Census data that reflects nonmedical social determinants of health. “It looks at factors like number of rooms in the house, single-parent households, maternal education attainment, and family income,” Dr. Wicklow explained. “The higher the SEFI-2 score, the lower your socioeconomic status is for the area you live in. Kids with T2DM generally live in areas of lower SES and lower socioeconomic index. They often live far away from health care providers. Many do not attend school and many are not with their biologic families, so we’ve had a lot of issues addressing child and family services, in particular in the phase of a chronic illness where our expectation is one thing and the family’s and community’s expectations of what’s realistic in terms of treatment and goals is another. We also have a lot of adolescent pregnancies.”
To date, about 80% of youth with T1D have seen a health care provider within the first year after transition from the pediatric diabetes clinic, compared with just over 50% of kids with T2D. “We transition youth with T1DM to internists, while our youth with T2DM go to itinerant physicians often back in their communities and/or rural family physicians,” she said. Between baseline and year 2, the rate of hospital admissions remained similar among T1DM at 11.6 and 11.8 admissions per 100 patient-years, respectively, but the number of hospital admissions for T2DM patients jumped from 20.1 to 25.5 admissions per 100 patient-years. “Kids with type 2 are showing up in the hospital a lot more than those with type 1 diabetes, but not for diabetes-related diagnoses,” Dr. Wicklow said. “We’re starting to look through the data now, and most of our kids are showing up with mental health complaints and issues. That’s why they’re getting hospitalized.”
Among ICARE study participants who have completed 3 years of follow-up, about 52% had albuminuria at their baseline visit and 48% sustained albuminuria throughout the study. About 26% progressed from normal levels of albuminuria to microalbuminuria, from microalbuminuria to macroalbuminuria, or from normal levels of albuminuria to macroalbuminuria. In addition, 16% persisted in the category that they were in, and 10% regressed. “The good news is, some of our kids get better over time,” Dr. Wicklow said. “The bad news is that the majority do not.”
Going forward, Dr. Wicklow and her associates work with an ICARE advisory group composed of children and families “who sit with us and talk about what mental health needs might be important, and how we should organize our study in a follow-up of the kids, to try and answer some of the questions that are important,” she said. “Working with the concept of the study’s theoretical framework, they acknowledged that the biological exposures are important, but they were also concerned about food security, finding strength/resilience within the community, and finding coping factors in terms of keeping themselves healthy with their diabetes. For some communities, they are concerned with basic needs. We’re working with them to help them progress, and to figure out how to best study children with type 2 diabetes.”
ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba (DREAM) theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
TORONTO – When Brandy Wicklow, MD, began her pediatric endocrinology fellowship at McGill University in 2006, about 12 per 100,000 children in Manitoba, Canada, were diagnosed with type 2 diabetes mellitus each year. By 2016 that rate had more than doubled, to 26 per 100,000 children.
“If you look just at indigenous youth in our province, it’s probably one of the highest rates ever reported, with 95 per 100,000 Manitoba First Nation children diagnosed with type 2 diabetes,” said Dr. Wicklow, a pediatric endocrinologist at the University of Manitoba and the Children’s Hospital Research Institute of Manitoba.
Many indigenous populations also face an increased risk for primary renal disease. One study reviewed the charts 90 of Canadian First Nation children and adolescents with T2DM (Diabetes Care. 2009;32[5]:786-90). Of 10 who had renal biopsies performed, nine had immune complex disease/glomerulosclerosis, two had mild diabetes-related lesions, and seven had focal segmental glomerulosclerosis (FSGS); yet none had classic nephropathy. An analysis of Chinese youth that included 216 renal biopsies yielded similar findings (Intl Urol Nephrol. 2012;45[1]:173-9).
It’s also known that early-onset T2DM is associated with substantially increased incidence of end-stage renal disease (ESRD) and mortality in middle age. For example, one study of Pima Indians found that those who were diagnosed with T2DM earlier than 20 years of age had a one in five chance of developing ESRD, while those who were diagnosed at age 20 years or older had a one in two chance of ESRD (JAMA. 2006;296[4]:421-6). In a separate analysis, researchers estimated the remaining lifetime risks for ESRD among Aboriginal people in Australia with and without diabetes (Diabetes Res Clin Pract. 2014;103[3]:e24-6). The value for young adults with diabetes was high, about one in two at the age of 30 years, while it decreased with age to one in seven at 60 years.
“One of the first biomarkers we see in terms of renal disease in kids with T2DM is albuminuria,” Dr. Wicklow said at the Pediatric Academic Societies meeting. “The question is, why do kids with type 2 get more renal disease than kids with type 1 diabetes?” The SEARCH for Diabetes in Youth (SEARCH) study from 2006 found that hypertension, increased body mass index, increased weight circumference, and increased lipids were factors, while the SEARCH study from 2015 found that ethnicity, increased weight to height ratio, and mean arterial pressure were factors.
“Insulin resistance is significantly associated with albuminuria,” Dr. Wicklow continued. “It’s also been shown to be associated with hyperfiltration. Some of the markers of insulin resistance are important but they make up about 19% of the variance between type 1 and type 2, which means there are other variables that we’re not measuring.”
Enter ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study that Dr. Wicklow and her associates launched in 2014 at eight centers in Canada. It aims to examine the biopsychosocial risk factors for albuminuria in youth with T2DM and the mechanisms for renal injury. “Our theoretical framework was that biological exposures that we are aware of, such as glycemic control, hypertension, and lipids, would all be important in the development of albuminuria and renal disease in kids,” said Dr. Wicklow, who is the study’s coprimary investigator along with Allison Dart, MD. “But what we thought was novel was that psychological exposures either as socioeconomic status or as mental health factors would also directly impinge on renal health with respect to chronic inflammation in the body, inflammation in the kidneys, and long-term kidney damage.”
The first phase of ICARE involved a detailed phenotypic assessment of youth, including anthropometrics, biochemistry, 24-hour ambulatory blood pressure monitoring, overnight urine collections for albumin excretion, renal ultrasound, and iohexol-derived glomerular filtration rate (GFR). Phase 2 included an evaluation of psychological factors, including hair-derived cortisol; validated questionnaires for perceived stress, distress, and resiliency; and a detailed evaluation of systemic and urine inflammatory biomarkers. Annual follow-up is carried out to assess temporal associations between clinical risk factors and renal outcomes, including progression of albuminuria.
At the meeting, Dr. Wicklow reported on 187 youth enrolled to date. Of these, 96% were of indigenous ethnicity, 57 had albuminuria and 130 did not, and the mean ages of the groups were 16 years and 15 years, respectively. At baseline, a higher proportion of those in the albuminuria group were female (74% vs. 64% of those in the no albuminuria group, respectively), had a higher mean hemoglobin A1c (11% vs. 9%), and had hypertension (94% vs. 72%). She noted that upon presentation to the clinic, only 23% of participants had HbA1c levels less than 7%, only 26% had ranges between 7% and 9%, and about 40% did not have any hypertension. Of those who did, 27% had nighttime-only hypertension, and only 2% had daytime-only hypertension.
“The other risk factor these kids have for developing ESRD is that the majority were exposed to diabetes in pregnancy,” Dr. Wicklow said. “Murine models of maternal diabetes exposure have demonstrated that offspring have small kidneys, less ureteric bud branching, and a lower number of nephrons. Most of the human clinical cohort studies look at associations between development of diabetes and parental hypertension, maternal smoking, and maternal education. There is likely an impact at birth that sets these kids up for development of type 2 diabetes.”
In addition, results from clinical cohort studies have found that depression, mental stress, and distress are high in youth with T2DM. “Preliminary data suggest that if you have positive mental health, or coping strategies, or someone has worked through this with you and you are resilient, you might benefit in terms of overall glycemic control,” she said. For example, ICARE investigators have found that the higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by monocyte chemotactic protein-1 (MCP-1; P = .02). “Mental health seems to be something that can directly impact your health from a biological standpoint, and we might be able to find biomarkers of that risk,” Dr. Wicklow said. “Where does the stress come from? Most of my patients are indigenous, so it’s not surprising that the history in Canada of colonization of residential schools has left a lasting impression on these families and communities in terms of loss of language, loss of culture, and loss of land. There’s a community-based stress and a family-based stress that these children feel.”
Social factors also play a big role. She presented baseline findings from 196 youth with T2DM and 456 with T1DM, including measures such as the Socioeconomic Factor Index-Version 2 (SEFI-2), a way to assess socioeconomic characteristics based on Canadian Census data that reflects nonmedical social determinants of health. “It looks at factors like number of rooms in the house, single-parent households, maternal education attainment, and family income,” Dr. Wicklow explained. “The higher the SEFI-2 score, the lower your socioeconomic status is for the area you live in. Kids with T2DM generally live in areas of lower SES and lower socioeconomic index. They often live far away from health care providers. Many do not attend school and many are not with their biologic families, so we’ve had a lot of issues addressing child and family services, in particular in the phase of a chronic illness where our expectation is one thing and the family’s and community’s expectations of what’s realistic in terms of treatment and goals is another. We also have a lot of adolescent pregnancies.”
To date, about 80% of youth with T1D have seen a health care provider within the first year after transition from the pediatric diabetes clinic, compared with just over 50% of kids with T2D. “We transition youth with T1DM to internists, while our youth with T2DM go to itinerant physicians often back in their communities and/or rural family physicians,” she said. Between baseline and year 2, the rate of hospital admissions remained similar among T1DM at 11.6 and 11.8 admissions per 100 patient-years, respectively, but the number of hospital admissions for T2DM patients jumped from 20.1 to 25.5 admissions per 100 patient-years. “Kids with type 2 are showing up in the hospital a lot more than those with type 1 diabetes, but not for diabetes-related diagnoses,” Dr. Wicklow said. “We’re starting to look through the data now, and most of our kids are showing up with mental health complaints and issues. That’s why they’re getting hospitalized.”
Among ICARE study participants who have completed 3 years of follow-up, about 52% had albuminuria at their baseline visit and 48% sustained albuminuria throughout the study. About 26% progressed from normal levels of albuminuria to microalbuminuria, from microalbuminuria to macroalbuminuria, or from normal levels of albuminuria to macroalbuminuria. In addition, 16% persisted in the category that they were in, and 10% regressed. “The good news is, some of our kids get better over time,” Dr. Wicklow said. “The bad news is that the majority do not.”
Going forward, Dr. Wicklow and her associates work with an ICARE advisory group composed of children and families “who sit with us and talk about what mental health needs might be important, and how we should organize our study in a follow-up of the kids, to try and answer some of the questions that are important,” she said. “Working with the concept of the study’s theoretical framework, they acknowledged that the biological exposures are important, but they were also concerned about food security, finding strength/resilience within the community, and finding coping factors in terms of keeping themselves healthy with their diabetes. For some communities, they are concerned with basic needs. We’re working with them to help them progress, and to figure out how to best study children with type 2 diabetes.”
ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba (DREAM) theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
AT PAS 18
Key clinical point: Mental health may indirectly increase inflammation, which contributes to kidney health.
Major finding: The higher the score on the Kessler Psychological Distress Scale (K6), the greater the risk of renal inflammation as measured by MCP-1 (P = .02).
Study details: Preliminary results from ICARE (Improving Renal Complications in Adolescents with Type 2 Diabetes through Research), an ongoing prospective cohort study.
Disclosures: ICARE has received support from Diabetes Canada, Research Manitoba, the Canadian Institutes of Health Research, the Children’s Hospital Research Institute of Manitoba (specifically the Diabetes Research Envisioned and Accomplished in Manitoba [DREAM] theme), and the University of Manitoba. Dr. Wicklow reported having no financial disclosures.
Diabetes Mellitus Federal Health Data Trends (FULL)
Diabetes mellitus (DM) is the seventh leading cause of death in the U.S., but after decades of rapid growth, both the incidence and prevalence appear to have leveled off. The VA spends $1.5 billion annually to treat patients with DM. Veterans are 2.5 times more likely than nonveterans to have diabetes, and many have comorbid conditions, including obesity, hypoglycemia, hypertension, dyslipidemia, cardiovascular disease, stroke, blindness, kidney disease, and amputations. Obesity and DM remain closely related, and more than two-thirds of women veterans with DM also have obesity.
A number of factors seem to increase the risk of type 2 DM for veterans. For example, DM is associated with exposure to herbicides, such as Agent Orange; past physical strain with chronic pain, and degenerative joint damage. Certain factors also increase risk of obesity, such as advanced age and low income, as well as limited access to healthy and high-quality foods. High-risk ethnic groups for diabetes include African Americans, Hispanics, Native Americans, Asians, and Pacific Islanders. Veterans with prediabetes, hypertension, low highdensity lipoprotein cholesterol levels, high triglyceride levels, and insufficient physical activity also are at increased risk.
Click here to read the digital edition.
Diabetes mellitus (DM) is the seventh leading cause of death in the U.S., but after decades of rapid growth, both the incidence and prevalence appear to have leveled off. The VA spends $1.5 billion annually to treat patients with DM. Veterans are 2.5 times more likely than nonveterans to have diabetes, and many have comorbid conditions, including obesity, hypoglycemia, hypertension, dyslipidemia, cardiovascular disease, stroke, blindness, kidney disease, and amputations. Obesity and DM remain closely related, and more than two-thirds of women veterans with DM also have obesity.
A number of factors seem to increase the risk of type 2 DM for veterans. For example, DM is associated with exposure to herbicides, such as Agent Orange; past physical strain with chronic pain, and degenerative joint damage. Certain factors also increase risk of obesity, such as advanced age and low income, as well as limited access to healthy and high-quality foods. High-risk ethnic groups for diabetes include African Americans, Hispanics, Native Americans, Asians, and Pacific Islanders. Veterans with prediabetes, hypertension, low highdensity lipoprotein cholesterol levels, high triglyceride levels, and insufficient physical activity also are at increased risk.
Click here to read the digital edition.
Diabetes mellitus (DM) is the seventh leading cause of death in the U.S., but after decades of rapid growth, both the incidence and prevalence appear to have leveled off. The VA spends $1.5 billion annually to treat patients with DM. Veterans are 2.5 times more likely than nonveterans to have diabetes, and many have comorbid conditions, including obesity, hypoglycemia, hypertension, dyslipidemia, cardiovascular disease, stroke, blindness, kidney disease, and amputations. Obesity and DM remain closely related, and more than two-thirds of women veterans with DM also have obesity.
A number of factors seem to increase the risk of type 2 DM for veterans. For example, DM is associated with exposure to herbicides, such as Agent Orange; past physical strain with chronic pain, and degenerative joint damage. Certain factors also increase risk of obesity, such as advanced age and low income, as well as limited access to healthy and high-quality foods. High-risk ethnic groups for diabetes include African Americans, Hispanics, Native Americans, Asians, and Pacific Islanders. Veterans with prediabetes, hypertension, low highdensity lipoprotein cholesterol levels, high triglyceride levels, and insufficient physical activity also are at increased risk.
Click here to read the digital edition.
Chronic kidney disease is 40% more common in T2DM than T1DM
ORLANDO – A new analysis of more than 1.5 million U.S. subjects with diabetes found that chronic kidney disease (CKD) is much more common in type 2 diabetes mellitus (T2DM) than in type 1 diabetes mellitus (T1DM) – 44% vs. 32%, respectively. The research also provides more evidence that albumin testing can provide crucial warning signs of future kidney trouble.
“Our data suggest – but don’t really prove – that there’s a lot more eGFR testing than there is albumin testing,” said nephrologist and study coauthor Michael Cressman, DO, of Covance, the drug development business of LabCorp, in an interview at the annual scientific sessions of the American Diabetes Association. “It is very important to measure albumin in the urine in order to identify patients who are at highest risk of progressive renal disease. There you identify people for whom you really want to maximize all the available treatments.”
According to the study, previous research has estimated that 25% of U.S. adults with diabetes have CKD (eGFR less than 60 ml/min per 1.73m2 or an albumin to creatinine ratio equal to or greater than 30 mg/g), but the difference in rates between T1DM and T2DM has been unclear.
Researchers analyzed LabCorp laboratory data on blood from for 48,036 adults with T1DM and 1,461,915 with T2DM. The analysis included ACR and CKD-EPI calculator for eGFR measurements from 2014-2017.
The researchers tracked declines in eGFR in patients who had more than three eGFR readings over at least 1 year.
Researchers found that the rate of CKD was 40% higher in patients with T2DM than it was in those with T1DM (44% vs. 32%, respectively; P less than .001), as was the prevalence of subjects considered to be at high or very high risk (18% vs. 12%, respectively; P less than .001).
These findings didn’t surprise Dr. Cressman, who said the higher ages of subjects with T2DM could explain the gap since they were more likely to have been exposed to hypertension for longer amounts of time.
Researchers also reported that the median eGFR decline (ml/min per year) was especially high in those with macroalbuminuria: –3.80 in T1DM and –3.58 in T2DM.
“Although MA [macroalbuminuria] is uncommon and most frequently observed in patients with normal or only mildly reduced eGFR, it was a potent predictor of eGFR decline in both T1DM and T2DM,” the researchers wrote.
“While it’s been known for a while that it’s bad to have albumin, this is more of a strong reinforcing piece of data,” Dr. Cressman said. “When you read about these things and it’s an epidemiological study or a clinical trial, it kind of loses its flavor. These are actual patients. A doctor could look at this data and say, ‘I ought to be checking this [albumin].’ It’s sort of an obvious rationale for what the guidelines say.”
No study funding was reported. Dr. Cressman reported employment by Covance. Other study authors variously report no disclosures or employment by Covance and its parent company LabCorp and stock/shareholding in LabCorp.
SOURCE: Cressman M et al. ADA 2018, Abstract 544-P.
ORLANDO – A new analysis of more than 1.5 million U.S. subjects with diabetes found that chronic kidney disease (CKD) is much more common in type 2 diabetes mellitus (T2DM) than in type 1 diabetes mellitus (T1DM) – 44% vs. 32%, respectively. The research also provides more evidence that albumin testing can provide crucial warning signs of future kidney trouble.
“Our data suggest – but don’t really prove – that there’s a lot more eGFR testing than there is albumin testing,” said nephrologist and study coauthor Michael Cressman, DO, of Covance, the drug development business of LabCorp, in an interview at the annual scientific sessions of the American Diabetes Association. “It is very important to measure albumin in the urine in order to identify patients who are at highest risk of progressive renal disease. There you identify people for whom you really want to maximize all the available treatments.”
According to the study, previous research has estimated that 25% of U.S. adults with diabetes have CKD (eGFR less than 60 ml/min per 1.73m2 or an albumin to creatinine ratio equal to or greater than 30 mg/g), but the difference in rates between T1DM and T2DM has been unclear.
Researchers analyzed LabCorp laboratory data on blood from for 48,036 adults with T1DM and 1,461,915 with T2DM. The analysis included ACR and CKD-EPI calculator for eGFR measurements from 2014-2017.
The researchers tracked declines in eGFR in patients who had more than three eGFR readings over at least 1 year.
Researchers found that the rate of CKD was 40% higher in patients with T2DM than it was in those with T1DM (44% vs. 32%, respectively; P less than .001), as was the prevalence of subjects considered to be at high or very high risk (18% vs. 12%, respectively; P less than .001).
These findings didn’t surprise Dr. Cressman, who said the higher ages of subjects with T2DM could explain the gap since they were more likely to have been exposed to hypertension for longer amounts of time.
Researchers also reported that the median eGFR decline (ml/min per year) was especially high in those with macroalbuminuria: –3.80 in T1DM and –3.58 in T2DM.
“Although MA [macroalbuminuria] is uncommon and most frequently observed in patients with normal or only mildly reduced eGFR, it was a potent predictor of eGFR decline in both T1DM and T2DM,” the researchers wrote.
“While it’s been known for a while that it’s bad to have albumin, this is more of a strong reinforcing piece of data,” Dr. Cressman said. “When you read about these things and it’s an epidemiological study or a clinical trial, it kind of loses its flavor. These are actual patients. A doctor could look at this data and say, ‘I ought to be checking this [albumin].’ It’s sort of an obvious rationale for what the guidelines say.”
No study funding was reported. Dr. Cressman reported employment by Covance. Other study authors variously report no disclosures or employment by Covance and its parent company LabCorp and stock/shareholding in LabCorp.
SOURCE: Cressman M et al. ADA 2018, Abstract 544-P.
ORLANDO – A new analysis of more than 1.5 million U.S. subjects with diabetes found that chronic kidney disease (CKD) is much more common in type 2 diabetes mellitus (T2DM) than in type 1 diabetes mellitus (T1DM) – 44% vs. 32%, respectively. The research also provides more evidence that albumin testing can provide crucial warning signs of future kidney trouble.
“Our data suggest – but don’t really prove – that there’s a lot more eGFR testing than there is albumin testing,” said nephrologist and study coauthor Michael Cressman, DO, of Covance, the drug development business of LabCorp, in an interview at the annual scientific sessions of the American Diabetes Association. “It is very important to measure albumin in the urine in order to identify patients who are at highest risk of progressive renal disease. There you identify people for whom you really want to maximize all the available treatments.”
According to the study, previous research has estimated that 25% of U.S. adults with diabetes have CKD (eGFR less than 60 ml/min per 1.73m2 or an albumin to creatinine ratio equal to or greater than 30 mg/g), but the difference in rates between T1DM and T2DM has been unclear.
Researchers analyzed LabCorp laboratory data on blood from for 48,036 adults with T1DM and 1,461,915 with T2DM. The analysis included ACR and CKD-EPI calculator for eGFR measurements from 2014-2017.
The researchers tracked declines in eGFR in patients who had more than three eGFR readings over at least 1 year.
Researchers found that the rate of CKD was 40% higher in patients with T2DM than it was in those with T1DM (44% vs. 32%, respectively; P less than .001), as was the prevalence of subjects considered to be at high or very high risk (18% vs. 12%, respectively; P less than .001).
These findings didn’t surprise Dr. Cressman, who said the higher ages of subjects with T2DM could explain the gap since they were more likely to have been exposed to hypertension for longer amounts of time.
Researchers also reported that the median eGFR decline (ml/min per year) was especially high in those with macroalbuminuria: –3.80 in T1DM and –3.58 in T2DM.
“Although MA [macroalbuminuria] is uncommon and most frequently observed in patients with normal or only mildly reduced eGFR, it was a potent predictor of eGFR decline in both T1DM and T2DM,” the researchers wrote.
“While it’s been known for a while that it’s bad to have albumin, this is more of a strong reinforcing piece of data,” Dr. Cressman said. “When you read about these things and it’s an epidemiological study or a clinical trial, it kind of loses its flavor. These are actual patients. A doctor could look at this data and say, ‘I ought to be checking this [albumin].’ It’s sort of an obvious rationale for what the guidelines say.”
No study funding was reported. Dr. Cressman reported employment by Covance. Other study authors variously report no disclosures or employment by Covance and its parent company LabCorp and stock/shareholding in LabCorp.
SOURCE: Cressman M et al. ADA 2018, Abstract 544-P.
REPORTING FROM ADA 2018
Key clinical point: CKD is significantly more common in patients with T2DM than those with T1DM, and albumin testing provides crucial warning signs.
Major finding: Of subjects with T2DM, 44% had signs of CKD, compared with 32% of those with T1DM.
Study details: Analysis of LabCorp blood testing of more than 1.5 million U.S. adults with diabetes from 2014-2017.
Disclosures: No study funding was reported. Authors reported various disclosures, mostly employment for Covance or its parent company, LabCorp.
Source: Cressman M et al. ADA 2018, Abstract 544-P.
CANVAS program analysis: Canagliflozin benefits patients with T2DM and poor kidney function
ORLANDO – according to an analysis of data from the CANVAS program.
The findings suggest that it may be time to reconsider limitations on usage of the sodium-glucose transporter 2 inhibitor in patients with poor kidney function, Dick de Zeeuw, MD, PhD, said at the annual scientific sessions of the American Diabetes Association.
Despite the smaller glycemic effects in patients with reduced estimated glomerular filtration rate (eGFR) seen in CANVAS (CANagliflozin cardioVascular Assessment Study), the relative effects on the primary – and most other – cardiovascular outcomes in CANVAS were similar in patients with low (less than 60 mL/min per 1.72 m2 [2,029 patients]) versus high (greater than 60 mL/min per 1.72 m2 [8,101 patients]) eGFR, and across four eGFR subgroups (less than 45 mL/min per 1.72 m2 [554 patients], 45-60 mL/min per 1.72 m2 [1,485 patients], 60-90 mL/min per1.72 m2 [5,625 patients], and 90 or greater mL/min per 1.72 m2 [2,684 patients]), reported Dr. de Zeeuw of the University Medical Center Groningen, the Netherlands.
An exception was a possible finding of heterogeneity of treatment effect for the exploratory outcome of stroke, he noted.
As expected, the effects of canagliflozin versus placebo on the surrogate marker of HbA1c was significantly smaller with lower renal function, with reductions of 0.35%, 0.45%, 0.57%, and 0.76% across the four eGFR subgroups, respectively.
“But most interestingly, the three other cardiovascular risk markers show no difference in the effect of canagliflozin when we compare them for the four different eGFR groups,” he said, explaining that reductions in systolic blood pressure, body weight, and albuminuria were “very consistent” across the groups.
For the CANVAS 3-point major cardiac adverse event primary endpoint, which included a composite of cardiovascular death, nonfatal MI, or nonfatal stroke (and which was reduced by 14% [hazard ratio, 0.86] with canagliflozin treatment in the primary CANVAS analysis), no significant heterogeneity for treatment effect was noted between the high versus low eGFR groups or the four eGFR groups (P = .08 and .33, respectively), Dr. de Zeeuw said.
“If anything, there was more effect in the low eGFR group than in the high eGFR group [HRs, 0.92 and 0.70, respectively; 0.65, 0.71, 0.95, and 0.84 for the four eGFR groups, respectively]”, he added, noting that the same was true for the cardiovascular death and MI components of the primary endpoint.
For stroke, however, possible heterogeneity by baseline kidney function was noted. In the primary CANVAS analysis, canagliflozin was associated with a nonsignificant 13% reduction in stroke risk (HR, 0.87), and the hazard ratios for low versus high eGFR in the current analysis were 0.50 and 1.01, respectively, and in the four eGFR groups they were 0.32, 0.56, 0.89, and 1.42 , respectively (P = .01 for both comparisons).
For the secondary endpoints of hospitalizations for heart failure and renal function (a composite of eGFR, end-stage kidney disease, or renal death), which both were important outcomes in the CANVAS program with each showing highly significant reductions with canagliflozin treatment versus placebo in the primary analysis (HRs, 0.67 and 0.60), no heterogeneity by eGFR was seen in the current analysis.
As for safety outcomes, which included serious adverse events, adverse events leading to treatment discontinuation, amputation, fractures, and renal safety (serious renal-related adverse events, serious acute kidney injury, and serious hyperkalemia), no heterogeneity was seen in the outcomes across eGFR groups, he said.
“The CANVAS program ... is a composite of two cardiovascular safety trials that have been conducted over the past [8+] years,” Dr. de Zeeuw said, noting that participants included patients with type 2 diabetes mellitus and cardiovascular disease or high cardiovascular risk who were treated with either 300 mg or 100 mg of canagliflozin or with placebo after a 2-week placebo run-in.
The outcomes favored canagliflozin across all endpoints, and particularly for the secondary endpoints of hospitalization for heart failure and the composite of renal outcomes.
“The interest [in terms of the current analysis] is that this drug supposedly works better in those that have a functioning kidney and a filtering kidney, where the tubule is actually the target of this drug. It was anticipated that there would be less effect of this drug on low renal functions,” he said, adding that although the effect on HbA1c is progressively attenuated with declining renal function, as expected, the effects on other cardiovascular risk markers were, surprisingly, very comparable across the eGFR subgroups.
“And with that, more importantly, the effect on the hard outcomes – like the primary outcomes, most of the CV outcomes [maybe except stroke], the renal and safety outcomes – are very consistent across the different kidney function levels,” he said, adding that these beneficial findings in patients with 30-45 eGFR who are at high cardiovascular and renal risk suggest that reconsideration of the current limitation on the use of canagliflozin inhibitors to patients with eGFR above 45 is warranted.
The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe.
SOURCE: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
ORLANDO – according to an analysis of data from the CANVAS program.
The findings suggest that it may be time to reconsider limitations on usage of the sodium-glucose transporter 2 inhibitor in patients with poor kidney function, Dick de Zeeuw, MD, PhD, said at the annual scientific sessions of the American Diabetes Association.
Despite the smaller glycemic effects in patients with reduced estimated glomerular filtration rate (eGFR) seen in CANVAS (CANagliflozin cardioVascular Assessment Study), the relative effects on the primary – and most other – cardiovascular outcomes in CANVAS were similar in patients with low (less than 60 mL/min per 1.72 m2 [2,029 patients]) versus high (greater than 60 mL/min per 1.72 m2 [8,101 patients]) eGFR, and across four eGFR subgroups (less than 45 mL/min per 1.72 m2 [554 patients], 45-60 mL/min per 1.72 m2 [1,485 patients], 60-90 mL/min per1.72 m2 [5,625 patients], and 90 or greater mL/min per 1.72 m2 [2,684 patients]), reported Dr. de Zeeuw of the University Medical Center Groningen, the Netherlands.
An exception was a possible finding of heterogeneity of treatment effect for the exploratory outcome of stroke, he noted.
As expected, the effects of canagliflozin versus placebo on the surrogate marker of HbA1c was significantly smaller with lower renal function, with reductions of 0.35%, 0.45%, 0.57%, and 0.76% across the four eGFR subgroups, respectively.
“But most interestingly, the three other cardiovascular risk markers show no difference in the effect of canagliflozin when we compare them for the four different eGFR groups,” he said, explaining that reductions in systolic blood pressure, body weight, and albuminuria were “very consistent” across the groups.
For the CANVAS 3-point major cardiac adverse event primary endpoint, which included a composite of cardiovascular death, nonfatal MI, or nonfatal stroke (and which was reduced by 14% [hazard ratio, 0.86] with canagliflozin treatment in the primary CANVAS analysis), no significant heterogeneity for treatment effect was noted between the high versus low eGFR groups or the four eGFR groups (P = .08 and .33, respectively), Dr. de Zeeuw said.
“If anything, there was more effect in the low eGFR group than in the high eGFR group [HRs, 0.92 and 0.70, respectively; 0.65, 0.71, 0.95, and 0.84 for the four eGFR groups, respectively]”, he added, noting that the same was true for the cardiovascular death and MI components of the primary endpoint.
For stroke, however, possible heterogeneity by baseline kidney function was noted. In the primary CANVAS analysis, canagliflozin was associated with a nonsignificant 13% reduction in stroke risk (HR, 0.87), and the hazard ratios for low versus high eGFR in the current analysis were 0.50 and 1.01, respectively, and in the four eGFR groups they were 0.32, 0.56, 0.89, and 1.42 , respectively (P = .01 for both comparisons).
For the secondary endpoints of hospitalizations for heart failure and renal function (a composite of eGFR, end-stage kidney disease, or renal death), which both were important outcomes in the CANVAS program with each showing highly significant reductions with canagliflozin treatment versus placebo in the primary analysis (HRs, 0.67 and 0.60), no heterogeneity by eGFR was seen in the current analysis.
As for safety outcomes, which included serious adverse events, adverse events leading to treatment discontinuation, amputation, fractures, and renal safety (serious renal-related adverse events, serious acute kidney injury, and serious hyperkalemia), no heterogeneity was seen in the outcomes across eGFR groups, he said.
“The CANVAS program ... is a composite of two cardiovascular safety trials that have been conducted over the past [8+] years,” Dr. de Zeeuw said, noting that participants included patients with type 2 diabetes mellitus and cardiovascular disease or high cardiovascular risk who were treated with either 300 mg or 100 mg of canagliflozin or with placebo after a 2-week placebo run-in.
The outcomes favored canagliflozin across all endpoints, and particularly for the secondary endpoints of hospitalization for heart failure and the composite of renal outcomes.
“The interest [in terms of the current analysis] is that this drug supposedly works better in those that have a functioning kidney and a filtering kidney, where the tubule is actually the target of this drug. It was anticipated that there would be less effect of this drug on low renal functions,” he said, adding that although the effect on HbA1c is progressively attenuated with declining renal function, as expected, the effects on other cardiovascular risk markers were, surprisingly, very comparable across the eGFR subgroups.
“And with that, more importantly, the effect on the hard outcomes – like the primary outcomes, most of the CV outcomes [maybe except stroke], the renal and safety outcomes – are very consistent across the different kidney function levels,” he said, adding that these beneficial findings in patients with 30-45 eGFR who are at high cardiovascular and renal risk suggest that reconsideration of the current limitation on the use of canagliflozin inhibitors to patients with eGFR above 45 is warranted.
The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe.
SOURCE: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
ORLANDO – according to an analysis of data from the CANVAS program.
The findings suggest that it may be time to reconsider limitations on usage of the sodium-glucose transporter 2 inhibitor in patients with poor kidney function, Dick de Zeeuw, MD, PhD, said at the annual scientific sessions of the American Diabetes Association.
Despite the smaller glycemic effects in patients with reduced estimated glomerular filtration rate (eGFR) seen in CANVAS (CANagliflozin cardioVascular Assessment Study), the relative effects on the primary – and most other – cardiovascular outcomes in CANVAS were similar in patients with low (less than 60 mL/min per 1.72 m2 [2,029 patients]) versus high (greater than 60 mL/min per 1.72 m2 [8,101 patients]) eGFR, and across four eGFR subgroups (less than 45 mL/min per 1.72 m2 [554 patients], 45-60 mL/min per 1.72 m2 [1,485 patients], 60-90 mL/min per1.72 m2 [5,625 patients], and 90 or greater mL/min per 1.72 m2 [2,684 patients]), reported Dr. de Zeeuw of the University Medical Center Groningen, the Netherlands.
An exception was a possible finding of heterogeneity of treatment effect for the exploratory outcome of stroke, he noted.
As expected, the effects of canagliflozin versus placebo on the surrogate marker of HbA1c was significantly smaller with lower renal function, with reductions of 0.35%, 0.45%, 0.57%, and 0.76% across the four eGFR subgroups, respectively.
“But most interestingly, the three other cardiovascular risk markers show no difference in the effect of canagliflozin when we compare them for the four different eGFR groups,” he said, explaining that reductions in systolic blood pressure, body weight, and albuminuria were “very consistent” across the groups.
For the CANVAS 3-point major cardiac adverse event primary endpoint, which included a composite of cardiovascular death, nonfatal MI, or nonfatal stroke (and which was reduced by 14% [hazard ratio, 0.86] with canagliflozin treatment in the primary CANVAS analysis), no significant heterogeneity for treatment effect was noted between the high versus low eGFR groups or the four eGFR groups (P = .08 and .33, respectively), Dr. de Zeeuw said.
“If anything, there was more effect in the low eGFR group than in the high eGFR group [HRs, 0.92 and 0.70, respectively; 0.65, 0.71, 0.95, and 0.84 for the four eGFR groups, respectively]”, he added, noting that the same was true for the cardiovascular death and MI components of the primary endpoint.
For stroke, however, possible heterogeneity by baseline kidney function was noted. In the primary CANVAS analysis, canagliflozin was associated with a nonsignificant 13% reduction in stroke risk (HR, 0.87), and the hazard ratios for low versus high eGFR in the current analysis were 0.50 and 1.01, respectively, and in the four eGFR groups they were 0.32, 0.56, 0.89, and 1.42 , respectively (P = .01 for both comparisons).
For the secondary endpoints of hospitalizations for heart failure and renal function (a composite of eGFR, end-stage kidney disease, or renal death), which both were important outcomes in the CANVAS program with each showing highly significant reductions with canagliflozin treatment versus placebo in the primary analysis (HRs, 0.67 and 0.60), no heterogeneity by eGFR was seen in the current analysis.
As for safety outcomes, which included serious adverse events, adverse events leading to treatment discontinuation, amputation, fractures, and renal safety (serious renal-related adverse events, serious acute kidney injury, and serious hyperkalemia), no heterogeneity was seen in the outcomes across eGFR groups, he said.
“The CANVAS program ... is a composite of two cardiovascular safety trials that have been conducted over the past [8+] years,” Dr. de Zeeuw said, noting that participants included patients with type 2 diabetes mellitus and cardiovascular disease or high cardiovascular risk who were treated with either 300 mg or 100 mg of canagliflozin or with placebo after a 2-week placebo run-in.
The outcomes favored canagliflozin across all endpoints, and particularly for the secondary endpoints of hospitalization for heart failure and the composite of renal outcomes.
“The interest [in terms of the current analysis] is that this drug supposedly works better in those that have a functioning kidney and a filtering kidney, where the tubule is actually the target of this drug. It was anticipated that there would be less effect of this drug on low renal functions,” he said, adding that although the effect on HbA1c is progressively attenuated with declining renal function, as expected, the effects on other cardiovascular risk markers were, surprisingly, very comparable across the eGFR subgroups.
“And with that, more importantly, the effect on the hard outcomes – like the primary outcomes, most of the CV outcomes [maybe except stroke], the renal and safety outcomes – are very consistent across the different kidney function levels,” he said, adding that these beneficial findings in patients with 30-45 eGFR who are at high cardiovascular and renal risk suggest that reconsideration of the current limitation on the use of canagliflozin inhibitors to patients with eGFR above 45 is warranted.
The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe.
SOURCE: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
REPORTING FROM ADA 2018
Key clinical point: Cardioprotective benefits of canagliflozin in patients with type 2 diabetes mellitus were apparent across varying levels of kidney function.
Major finding: No heterogeneity for treatment effect for the primary major cardiac adverse event endpoint was noted between the high versus low estimated glomerular filtration rate groups (P = .08).
Study details: An analysis of data from the CANVAS program studies involving 10,142 patients.
Disclosures: The CANVAS program was sponsored by Janssen Research & Development. Dr. de Zeeuw is a consultant and/or speaker for AbbVie, Astellas, Bayer, Boehringer Ingelheim, Fresenius, Janssen, and Mitsubishi Tanabe Pharma.
Source: de Zeeuw D et al. ADA 2018, Abstract 258-OR.
Diabetes risk may rise with work hours
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.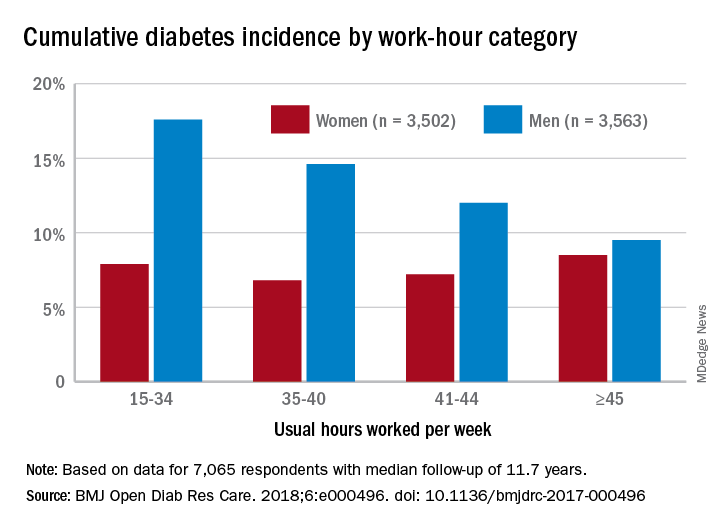
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
Men have a higher risk overall for developing diabetes, 12.2%, compared with 7.5% for women, but the risk for women increases as they work more hours per week, which is not the case for men, according to the results of a 12-year Canadian study that included over 7,000 workers.
Among the 3,502 women in the study, those who worked 45 or more hours per week had a cumulative diabetes incidence of 8.5% over the median 11.7 years of follow-up. Diabetes incidence was 7.2% for women who worked 41-44 hours a week, 6.8% for those who worked 35-40 hours, and 7.9% among women who worked 15-34 hours weekly, Mahée Gilbert-Ouimet, PhD, of the Institute for Work & Health, Toronto, and her associates reported in BMJ Open Diabetes Research & Care.
For the 3,563 men included in the study, diabetes incidence was 9.5% for those who worked at least 45 hours a week versus 12% for those who worked 41-44 hours, 14.6% for men working 35-40 hours weekly, and 17.6% among those who put in 15-34 hours, the investigators wrote.
Hazard ratios for working 45 or more hours, compared with 35-40 hours, were 1.63 for women and 0.81 for men after adjustment for age, level of education, working conditions, and other factors, although the effect was significant only for women, they noted.
“Considering the rapid and substantial increase of diabetes prevalence in Canada and worldwide, identifying modifiable risk factors, such as long work hours, is of major importance to improve prevention and orient policy making as it could prevent numerous cases of diabetes and diabetes-related chronic diseases,” Dr. Gilbert-Ouimet and her associates wrote.
The study was supported by the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. None of the investigators declared any conflicts of interest.
SOURCE: Gilbert-Ouimet M et al. BMJ Open Diab Res Care. 2018. doi: 10.1136/bmjdrc-2017-000496.
FROM BMJ OPEN DIABETES RESEARCH & CARE
High risk of low glucose? Hospital alerts promise a crucial heads-up
ORLANDO – Researchers have been able to sustain a dramatic reduction in hypoglycemia incidents at nine St. Louis–area hospitals, thanks to a computer algorithm that warns medical staff when patients appear to be on the road to dangerously low blood sugar levels.
The 6-year retrospective system-wide study, which was released at the annual scientific sessions of the American Diabetes Association, found that the use of the alert system lowered the annual occurrence of severe hypoglycemia events by 41% at the hospitals.
In at-risk patients – those with blood glucose levels under 90 mg/dL – the system considers several variables, such as their weight, creatinine clearance, insulin therapy, and basal insulin doses. If the algorithm considers that a patient is at high risk of a sub–40-mg/dL glucose level – dangerously low – it sends a single alert to medical staff during the patient’s stay.
The idea is that the real-time alerts will go to nurses or pharmacists who will review patient charts and then contact physicians. The doctors are expected to “make clinically appropriate changes,” Dr. Tobin said.
Earlier, Dr. Tobin and colleagues prospectively analyzed the alert system’s effectiveness at a single hospital for 5 months. The trial, a cohort intervention study, tracked 655 patients with a blood glucose level under 90 mg/dL.
In 2014, the researchers reported the results of that trial: The alert identified 390 of the patients as being at high risk for severe hypoglycemia (blood glucose under 40 mg/dL). The frequency of severe hypoglycemia events was just 3.1% in this population vs. 9.7% in unalerted patients who were also deemed to be at high risk (J Hosp Med. 2014[9]: 621-6).
For the new study, researchers extended the alert system to nine hospitals and tracked its use from 2011 to 2017.
During all visits, the number of severe hypoglycemic events fell from 2.9 to 1.7 per 1,000 at-risk patient days. (P less than .001)
At one hospital, Dr. Tobin said, the average monthly number of severe hypoglycemia incidents fell from 40 to 12.
Researchers found that the average blood glucose level post alert was 93 mg/dL vs. 74 mg/dL before alert. They also reported that the system-wide total of alerts per year ranged from 4,142 to 5,649.
“The current data reflected in our poster show that the alert process is sustainable over a wide range of clinical settings, including community hospitals of various size and complexity, as well as academic medical centers,” Dr. Tobin said.
The alert system had no effect on hyperglycemia, Dr. Tobin said.
In regard to expense, Dr. Tobin said it’s small because the alert system uses existing current staff and computer systems. Setup costs, he said, included programming, creating the alert infrastructure, and staff training
No study funding is reported. Dr. Tobin reports relationships with Novo Nordisk (advisory board, speaker’s bureau) and MannKind (speaker’s bureau). The other authors report no relevant disclosures.
SOURCE: Tobin G et al. ADA 2018. Abstract 397-P.
ORLANDO – Researchers have been able to sustain a dramatic reduction in hypoglycemia incidents at nine St. Louis–area hospitals, thanks to a computer algorithm that warns medical staff when patients appear to be on the road to dangerously low blood sugar levels.
The 6-year retrospective system-wide study, which was released at the annual scientific sessions of the American Diabetes Association, found that the use of the alert system lowered the annual occurrence of severe hypoglycemia events by 41% at the hospitals.
In at-risk patients – those with blood glucose levels under 90 mg/dL – the system considers several variables, such as their weight, creatinine clearance, insulin therapy, and basal insulin doses. If the algorithm considers that a patient is at high risk of a sub–40-mg/dL glucose level – dangerously low – it sends a single alert to medical staff during the patient’s stay.
The idea is that the real-time alerts will go to nurses or pharmacists who will review patient charts and then contact physicians. The doctors are expected to “make clinically appropriate changes,” Dr. Tobin said.
Earlier, Dr. Tobin and colleagues prospectively analyzed the alert system’s effectiveness at a single hospital for 5 months. The trial, a cohort intervention study, tracked 655 patients with a blood glucose level under 90 mg/dL.
In 2014, the researchers reported the results of that trial: The alert identified 390 of the patients as being at high risk for severe hypoglycemia (blood glucose under 40 mg/dL). The frequency of severe hypoglycemia events was just 3.1% in this population vs. 9.7% in unalerted patients who were also deemed to be at high risk (J Hosp Med. 2014[9]: 621-6).
For the new study, researchers extended the alert system to nine hospitals and tracked its use from 2011 to 2017.
During all visits, the number of severe hypoglycemic events fell from 2.9 to 1.7 per 1,000 at-risk patient days. (P less than .001)
At one hospital, Dr. Tobin said, the average monthly number of severe hypoglycemia incidents fell from 40 to 12.
Researchers found that the average blood glucose level post alert was 93 mg/dL vs. 74 mg/dL before alert. They also reported that the system-wide total of alerts per year ranged from 4,142 to 5,649.
“The current data reflected in our poster show that the alert process is sustainable over a wide range of clinical settings, including community hospitals of various size and complexity, as well as academic medical centers,” Dr. Tobin said.
The alert system had no effect on hyperglycemia, Dr. Tobin said.
In regard to expense, Dr. Tobin said it’s small because the alert system uses existing current staff and computer systems. Setup costs, he said, included programming, creating the alert infrastructure, and staff training
No study funding is reported. Dr. Tobin reports relationships with Novo Nordisk (advisory board, speaker’s bureau) and MannKind (speaker’s bureau). The other authors report no relevant disclosures.
SOURCE: Tobin G et al. ADA 2018. Abstract 397-P.
ORLANDO – Researchers have been able to sustain a dramatic reduction in hypoglycemia incidents at nine St. Louis–area hospitals, thanks to a computer algorithm that warns medical staff when patients appear to be on the road to dangerously low blood sugar levels.
The 6-year retrospective system-wide study, which was released at the annual scientific sessions of the American Diabetes Association, found that the use of the alert system lowered the annual occurrence of severe hypoglycemia events by 41% at the hospitals.
In at-risk patients – those with blood glucose levels under 90 mg/dL – the system considers several variables, such as their weight, creatinine clearance, insulin therapy, and basal insulin doses. If the algorithm considers that a patient is at high risk of a sub–40-mg/dL glucose level – dangerously low – it sends a single alert to medical staff during the patient’s stay.
The idea is that the real-time alerts will go to nurses or pharmacists who will review patient charts and then contact physicians. The doctors are expected to “make clinically appropriate changes,” Dr. Tobin said.
Earlier, Dr. Tobin and colleagues prospectively analyzed the alert system’s effectiveness at a single hospital for 5 months. The trial, a cohort intervention study, tracked 655 patients with a blood glucose level under 90 mg/dL.
In 2014, the researchers reported the results of that trial: The alert identified 390 of the patients as being at high risk for severe hypoglycemia (blood glucose under 40 mg/dL). The frequency of severe hypoglycemia events was just 3.1% in this population vs. 9.7% in unalerted patients who were also deemed to be at high risk (J Hosp Med. 2014[9]: 621-6).
For the new study, researchers extended the alert system to nine hospitals and tracked its use from 2011 to 2017.
During all visits, the number of severe hypoglycemic events fell from 2.9 to 1.7 per 1,000 at-risk patient days. (P less than .001)
At one hospital, Dr. Tobin said, the average monthly number of severe hypoglycemia incidents fell from 40 to 12.
Researchers found that the average blood glucose level post alert was 93 mg/dL vs. 74 mg/dL before alert. They also reported that the system-wide total of alerts per year ranged from 4,142 to 5,649.
“The current data reflected in our poster show that the alert process is sustainable over a wide range of clinical settings, including community hospitals of various size and complexity, as well as academic medical centers,” Dr. Tobin said.
The alert system had no effect on hyperglycemia, Dr. Tobin said.
In regard to expense, Dr. Tobin said it’s small because the alert system uses existing current staff and computer systems. Setup costs, he said, included programming, creating the alert infrastructure, and staff training
No study funding is reported. Dr. Tobin reports relationships with Novo Nordisk (advisory board, speaker’s bureau) and MannKind (speaker’s bureau). The other authors report no relevant disclosures.
SOURCE: Tobin G et al. ADA 2018. Abstract 397-P.
REPORTING FROM ADA 2018
Key clinical point: Hospitals were able to sustain lower numbers of severe hypoglycemia events over 6 years by using a prewarning alert system.
Major finding: The number of severe hypoglycemic events (below 40 mg/dL) fell from 2.9 per 1,000 at-risk patient-days to 1.7 per 1,000 at-risk patient-days.
Study details: Retrospective, system-wide study of nine hospitals with alert system in place from 2011 to 2017.
Disclosures: No funding is reported. One author reports relationships with Novo Nordisk and MannKind. The other authors report no relevant disclosures.
Source: Tobin G et al. ADA 2018. Abstract 397-P.