User login
FDA: MiniMed 670G now available for younger diabetes patients
The MiniMed 670G hybrid closed loop system has been approved to help manage basal insulin levels in patients aged 7-13 years who have type 1 diabetes, according to a Food and Drug Administration announcement.
The system, manufactured by Medtronic, automatically measures insulin levels every 5 minutes using an included sensor and then delivers insulin as needed through its insulin pump and attached infusion patch.
As part of this approval for children aged 7-13 years, the FDA is requiring the product developer to perform a postmarket study to evaluate how the device performs in this age group in real-world settings.
“Caregivers and families of young patients with diabetes face unique challenges in managing this disease, in particular the round-the-clock glucose monitoring that can be disruptive to people’s lives,” FDA Commissioner Scott Gottlieb, MD, said in a statement.
The device was approved in September 2017 for use in patients aged 14 years and older.
Read more about this approval in the full FDA announcement.
The MiniMed 670G hybrid closed loop system has been approved to help manage basal insulin levels in patients aged 7-13 years who have type 1 diabetes, according to a Food and Drug Administration announcement.
The system, manufactured by Medtronic, automatically measures insulin levels every 5 minutes using an included sensor and then delivers insulin as needed through its insulin pump and attached infusion patch.
As part of this approval for children aged 7-13 years, the FDA is requiring the product developer to perform a postmarket study to evaluate how the device performs in this age group in real-world settings.
“Caregivers and families of young patients with diabetes face unique challenges in managing this disease, in particular the round-the-clock glucose monitoring that can be disruptive to people’s lives,” FDA Commissioner Scott Gottlieb, MD, said in a statement.
The device was approved in September 2017 for use in patients aged 14 years and older.
Read more about this approval in the full FDA announcement.
The MiniMed 670G hybrid closed loop system has been approved to help manage basal insulin levels in patients aged 7-13 years who have type 1 diabetes, according to a Food and Drug Administration announcement.
The system, manufactured by Medtronic, automatically measures insulin levels every 5 minutes using an included sensor and then delivers insulin as needed through its insulin pump and attached infusion patch.
As part of this approval for children aged 7-13 years, the FDA is requiring the product developer to perform a postmarket study to evaluate how the device performs in this age group in real-world settings.
“Caregivers and families of young patients with diabetes face unique challenges in managing this disease, in particular the round-the-clock glucose monitoring that can be disruptive to people’s lives,” FDA Commissioner Scott Gottlieb, MD, said in a statement.
The device was approved in September 2017 for use in patients aged 14 years and older.
Read more about this approval in the full FDA announcement.
FDA okays fully implantable continuous glucose monitor/mobile app combo for diabetes
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
to transmit continuous information about blood glucose levels for people with diabetes.
The sensor-mobile app combo, called the Eversense Continuous Glucose Monitoring (CGM) system, is designed to supplant the need for frequent blood sampling to monitor blood glucose levels.
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” said FDA commissioner Scott Gottlieb, MD, in the agency’s press release announcing the approval. The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor.
The FDA’s approval was based on data from 125 patients with type 1 and type 2 diabetes who used the CGM system. The bulk of clinical data was acquired from PRECISE II, which enrolled 90 patients with type 1 and type 2 diabetes. When compared with levels returned from concurrently performed conventional home glucose monitoring, the CGM system achieved a mean absolute relative difference (MARD) of 8.8% (95% confidence interval, 8.1%-9.3%). This was less than the prespecified accuracy goal of 20% MARD (P less than .0001).
During the nonrandomized, blinded, prospective PRECISE II trial, 91% of the implanted sensors were functioning through the end of 90 days. A variation of the Eversense CGM, the Eversense CGM XL, has been approved for use up to 180 days in Europe.
The overall rate of serious adverse events among patients participating in the Eversense CGM trials was less than 1%. “The safety of this novel system will also be evaluated in a post-approval study,” wrote FDA officials in the press release.
In addition to adverse effects related to the outpatient procedure in which the glucose sensor is implanted subcutaneously, the FDA said that allergic reactions, ongoing pain, discomfort, scarring, and skin changes are possible with use of the CGM. Though the system sends frequent blood glucose measurements to the accompanying mobile app, missed alerts might still result in hypo- or hyperglycemia.
The Eversense CGM is marketed by Senseonics, which funded the studies underpinning approval.
Ethical violations scuttle NIH’s big alcohol study
A controversial study on abdominal aortic aneurysm screening; the importance of healthy lifestyle in diabetes; how NIH scientists corrupted a big alcohol study; and how cardiologists fare in starting salaries.
Listen to MDedge Cardiocast for all the details on the week’s top news.
A controversial study on abdominal aortic aneurysm screening; the importance of healthy lifestyle in diabetes; how NIH scientists corrupted a big alcohol study; and how cardiologists fare in starting salaries.
Listen to MDedge Cardiocast for all the details on the week’s top news.
A controversial study on abdominal aortic aneurysm screening; the importance of healthy lifestyle in diabetes; how NIH scientists corrupted a big alcohol study; and how cardiologists fare in starting salaries.
Listen to MDedge Cardiocast for all the details on the week’s top news.
Preview of ADA/EASD statement on hyperglycemia
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
Clinical trials to look for at ADA 2018
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
ADA punts photography ban to presenters
Last year’s American Diabetes Association annual meeting gobbled up a lot of social media attention, most of it criticizing the organization’s ban on photography in sessions. This year, it’s the presenters who’ll be in the position of deciding whether to allow photography.
During ADA 2017, attendees tweeting photos of presentation slides were surprised to get tweets from the association saying, “Thanks for joining us! Photography isn’t allowed at #2017ADA presentations; we’d appreciate if you could delete this tweet.”
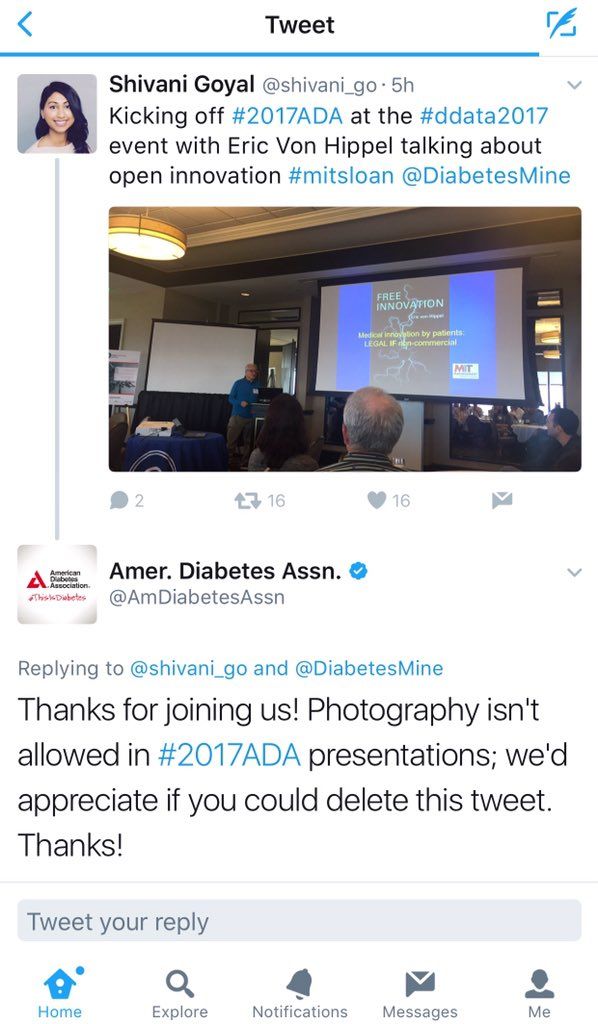
Social media responded with fury, news outlets wrote about it, and the uproar came to dominate public perception of the meeting. While the ADA claimed the policy was to protect intellectual property of unpublished data, critics countered that instant information is the norm and that medical innovation depends on it.
This year, it will be up to the presenters to decide whether their slides can be photographed and shared. The organizers state: “Each presenter/study author will announce, verbally and visually on a slide at the beginning of their presentation, whether or not he/she approves of photos being taken of their slides.”
So watch for the slides, and share your #2018ADA experiences and reactions with us at @ClinEndoNews.
Last year’s American Diabetes Association annual meeting gobbled up a lot of social media attention, most of it criticizing the organization’s ban on photography in sessions. This year, it’s the presenters who’ll be in the position of deciding whether to allow photography.
During ADA 2017, attendees tweeting photos of presentation slides were surprised to get tweets from the association saying, “Thanks for joining us! Photography isn’t allowed at #2017ADA presentations; we’d appreciate if you could delete this tweet.”

Social media responded with fury, news outlets wrote about it, and the uproar came to dominate public perception of the meeting. While the ADA claimed the policy was to protect intellectual property of unpublished data, critics countered that instant information is the norm and that medical innovation depends on it.
This year, it will be up to the presenters to decide whether their slides can be photographed and shared. The organizers state: “Each presenter/study author will announce, verbally and visually on a slide at the beginning of their presentation, whether or not he/she approves of photos being taken of their slides.”
So watch for the slides, and share your #2018ADA experiences and reactions with us at @ClinEndoNews.
Last year’s American Diabetes Association annual meeting gobbled up a lot of social media attention, most of it criticizing the organization’s ban on photography in sessions. This year, it’s the presenters who’ll be in the position of deciding whether to allow photography.
During ADA 2017, attendees tweeting photos of presentation slides were surprised to get tweets from the association saying, “Thanks for joining us! Photography isn’t allowed at #2017ADA presentations; we’d appreciate if you could delete this tweet.”

Social media responded with fury, news outlets wrote about it, and the uproar came to dominate public perception of the meeting. While the ADA claimed the policy was to protect intellectual property of unpublished data, critics countered that instant information is the norm and that medical innovation depends on it.
This year, it will be up to the presenters to decide whether their slides can be photographed and shared. The organizers state: “Each presenter/study author will announce, verbally and visually on a slide at the beginning of their presentation, whether or not he/she approves of photos being taken of their slides.”
So watch for the slides, and share your #2018ADA experiences and reactions with us at @ClinEndoNews.
Pancreatic cancer has a pancreatopathy distinct from type 2 diabetes
WASHINGTON –
“The endocrinopathies associated with type 2 diabetes mellitus and pancreatic cancer are distinct, the former represented by an increase in glucagon and the latter characterized by a reduction in islet size,” said Sajan Nagpal, MD, of the Mayo Clinic in Rochester, Minn., at Digestive Disease Week 2018®. “These data lend insight to a unique pathophysiology of diabetes that results from pancreatic cancer.”
Prior to presenting his results, Dr. Nagpal pointed out an interesting relationship between pancreatic cancer and diabetes.
“Pancreatic cancer and diabetes have a very unique, two-way relationship. While long-standing diabetes [LSDM] increases the risk of developing pancreatic cancer by 1.5-2 times over a person’s lifetime, pancreatic cancer itself causes a paraneoplastic form of diabetes. A person with a new diagnosis of diabetes mellitus has a five to eight times increased risk of being diagnosed with pancreatic cancer as compared to the general population within 3 years.“
This form of diabetes is referred to as pancreatic cancer–induced new onset diabetes (PC-NOD).
Dr. Nagpal and his team conducted a study that included 46 patients: 16 patients with pancreatic cancer (5 with LSDM, 5 NOD, and 6 without diabetes mellitus), 15 patients with T2DM (9 LSDM, 6 NOD), and 15 controls matched for age and body mass index. NOD was defined as a diabetes mellitus diagnosis less than 3 years from the date of autopsy or pancreatic cancer diagnosis. All pancreatic specimens were resected from pancreatic cancer patients or obtained as autopsy specimens. Researchers performed islet morphometric studies utilizing immunofluorescence analysis with specific insulin and glucagon antibodies.
The results of the study showed that patients with pancreatic cancer had islet sizes that were about a third smaller than those in the patients with T2DM and the healthy controls (P = .005). This held true for both LSDM and NOD pancreatic cancer patients, compared with the T2DM and healthy control groups. Researchers also found that insulin to glucagon (I:G) ratios were preserved in pancreatic cancer patients, whereas they were lower in patients with T2DM because of a higher percentage of glucagon in the islets (P = .08). Additionally, islet amyloid was much higher in T2DM patients (66.7%) versus patients with diabetes associated with pancreatic cancer (55.6%) and healthy controls (13.3%; P = .01).
There were several limitations to this study, including the small number of patients in each group and how pancreatic islet characteristics can vary based on their location. Finally, ductal obstruction can cause changes to islet morphology.
Along with the pathological changes found by Dr. Nagpal and his research team, PC-NOD and T2DM also have unique clinical profiles. As patients with pancreatic cancer approach their diagnosis, they begin to lose weight. This begins about 1 year before the patient’s diagnosis of pancreatic cancer. Conversely, these patients have worsening fasting glucose levels despite the fact that they are losing weight. The paradoxical relationship between weight loss and hyperglycemia are what distinguishes PC-NOD from T2DM, according to Dr. Nagpal. In patients with PC-NOD, worsening hyperglycemia happens over a periods of months, compared with the gradual increase over the course of years seen in T2DM.
The differences between these diseases may be caused by differences in their pancreatopathy.
When discussing the pancreatopathy associated with T2DM, Dr. Nagpal pointed out that there is a decrease in the I:G ratio, compared with those seen in patients without diabetes. The prevailing theory is that this decrease in the I:G ratio is caused by beta-cell apoptosis and transdifferentiation to alpha-cells. T2DM patients also have increased amyloid deposits, the result of increased islet amyloid polypeptide.
”Pancreatic cancer provides subtle metabolic clues, such as worsening glucose tolerance and weight loss, that can serve as potential targets for its early detection.”
According to Dr. Nagpal, more research is needed on pathogenesis of PC-NOD. Identification of biomarkers for screening may be possible in patients with new onset diabetes.
Dr. Nagpal had no financial conflicts of interest to report.
SOURCE: Nagpal S et al. DDW 2018, Abstract 392.
WASHINGTON –
“The endocrinopathies associated with type 2 diabetes mellitus and pancreatic cancer are distinct, the former represented by an increase in glucagon and the latter characterized by a reduction in islet size,” said Sajan Nagpal, MD, of the Mayo Clinic in Rochester, Minn., at Digestive Disease Week 2018®. “These data lend insight to a unique pathophysiology of diabetes that results from pancreatic cancer.”
Prior to presenting his results, Dr. Nagpal pointed out an interesting relationship between pancreatic cancer and diabetes.
“Pancreatic cancer and diabetes have a very unique, two-way relationship. While long-standing diabetes [LSDM] increases the risk of developing pancreatic cancer by 1.5-2 times over a person’s lifetime, pancreatic cancer itself causes a paraneoplastic form of diabetes. A person with a new diagnosis of diabetes mellitus has a five to eight times increased risk of being diagnosed with pancreatic cancer as compared to the general population within 3 years.“
This form of diabetes is referred to as pancreatic cancer–induced new onset diabetes (PC-NOD).
Dr. Nagpal and his team conducted a study that included 46 patients: 16 patients with pancreatic cancer (5 with LSDM, 5 NOD, and 6 without diabetes mellitus), 15 patients with T2DM (9 LSDM, 6 NOD), and 15 controls matched for age and body mass index. NOD was defined as a diabetes mellitus diagnosis less than 3 years from the date of autopsy or pancreatic cancer diagnosis. All pancreatic specimens were resected from pancreatic cancer patients or obtained as autopsy specimens. Researchers performed islet morphometric studies utilizing immunofluorescence analysis with specific insulin and glucagon antibodies.
The results of the study showed that patients with pancreatic cancer had islet sizes that were about a third smaller than those in the patients with T2DM and the healthy controls (P = .005). This held true for both LSDM and NOD pancreatic cancer patients, compared with the T2DM and healthy control groups. Researchers also found that insulin to glucagon (I:G) ratios were preserved in pancreatic cancer patients, whereas they were lower in patients with T2DM because of a higher percentage of glucagon in the islets (P = .08). Additionally, islet amyloid was much higher in T2DM patients (66.7%) versus patients with diabetes associated with pancreatic cancer (55.6%) and healthy controls (13.3%; P = .01).
There were several limitations to this study, including the small number of patients in each group and how pancreatic islet characteristics can vary based on their location. Finally, ductal obstruction can cause changes to islet morphology.
Along with the pathological changes found by Dr. Nagpal and his research team, PC-NOD and T2DM also have unique clinical profiles. As patients with pancreatic cancer approach their diagnosis, they begin to lose weight. This begins about 1 year before the patient’s diagnosis of pancreatic cancer. Conversely, these patients have worsening fasting glucose levels despite the fact that they are losing weight. The paradoxical relationship between weight loss and hyperglycemia are what distinguishes PC-NOD from T2DM, according to Dr. Nagpal. In patients with PC-NOD, worsening hyperglycemia happens over a periods of months, compared with the gradual increase over the course of years seen in T2DM.
The differences between these diseases may be caused by differences in their pancreatopathy.
When discussing the pancreatopathy associated with T2DM, Dr. Nagpal pointed out that there is a decrease in the I:G ratio, compared with those seen in patients without diabetes. The prevailing theory is that this decrease in the I:G ratio is caused by beta-cell apoptosis and transdifferentiation to alpha-cells. T2DM patients also have increased amyloid deposits, the result of increased islet amyloid polypeptide.
”Pancreatic cancer provides subtle metabolic clues, such as worsening glucose tolerance and weight loss, that can serve as potential targets for its early detection.”
According to Dr. Nagpal, more research is needed on pathogenesis of PC-NOD. Identification of biomarkers for screening may be possible in patients with new onset diabetes.
Dr. Nagpal had no financial conflicts of interest to report.
SOURCE: Nagpal S et al. DDW 2018, Abstract 392.
WASHINGTON –
“The endocrinopathies associated with type 2 diabetes mellitus and pancreatic cancer are distinct, the former represented by an increase in glucagon and the latter characterized by a reduction in islet size,” said Sajan Nagpal, MD, of the Mayo Clinic in Rochester, Minn., at Digestive Disease Week 2018®. “These data lend insight to a unique pathophysiology of diabetes that results from pancreatic cancer.”
Prior to presenting his results, Dr. Nagpal pointed out an interesting relationship between pancreatic cancer and diabetes.
“Pancreatic cancer and diabetes have a very unique, two-way relationship. While long-standing diabetes [LSDM] increases the risk of developing pancreatic cancer by 1.5-2 times over a person’s lifetime, pancreatic cancer itself causes a paraneoplastic form of diabetes. A person with a new diagnosis of diabetes mellitus has a five to eight times increased risk of being diagnosed with pancreatic cancer as compared to the general population within 3 years.“
This form of diabetes is referred to as pancreatic cancer–induced new onset diabetes (PC-NOD).
Dr. Nagpal and his team conducted a study that included 46 patients: 16 patients with pancreatic cancer (5 with LSDM, 5 NOD, and 6 without diabetes mellitus), 15 patients with T2DM (9 LSDM, 6 NOD), and 15 controls matched for age and body mass index. NOD was defined as a diabetes mellitus diagnosis less than 3 years from the date of autopsy or pancreatic cancer diagnosis. All pancreatic specimens were resected from pancreatic cancer patients or obtained as autopsy specimens. Researchers performed islet morphometric studies utilizing immunofluorescence analysis with specific insulin and glucagon antibodies.
The results of the study showed that patients with pancreatic cancer had islet sizes that were about a third smaller than those in the patients with T2DM and the healthy controls (P = .005). This held true for both LSDM and NOD pancreatic cancer patients, compared with the T2DM and healthy control groups. Researchers also found that insulin to glucagon (I:G) ratios were preserved in pancreatic cancer patients, whereas they were lower in patients with T2DM because of a higher percentage of glucagon in the islets (P = .08). Additionally, islet amyloid was much higher in T2DM patients (66.7%) versus patients with diabetes associated with pancreatic cancer (55.6%) and healthy controls (13.3%; P = .01).
There were several limitations to this study, including the small number of patients in each group and how pancreatic islet characteristics can vary based on their location. Finally, ductal obstruction can cause changes to islet morphology.
Along with the pathological changes found by Dr. Nagpal and his research team, PC-NOD and T2DM also have unique clinical profiles. As patients with pancreatic cancer approach their diagnosis, they begin to lose weight. This begins about 1 year before the patient’s diagnosis of pancreatic cancer. Conversely, these patients have worsening fasting glucose levels despite the fact that they are losing weight. The paradoxical relationship between weight loss and hyperglycemia are what distinguishes PC-NOD from T2DM, according to Dr. Nagpal. In patients with PC-NOD, worsening hyperglycemia happens over a periods of months, compared with the gradual increase over the course of years seen in T2DM.
The differences between these diseases may be caused by differences in their pancreatopathy.
When discussing the pancreatopathy associated with T2DM, Dr. Nagpal pointed out that there is a decrease in the I:G ratio, compared with those seen in patients without diabetes. The prevailing theory is that this decrease in the I:G ratio is caused by beta-cell apoptosis and transdifferentiation to alpha-cells. T2DM patients also have increased amyloid deposits, the result of increased islet amyloid polypeptide.
”Pancreatic cancer provides subtle metabolic clues, such as worsening glucose tolerance and weight loss, that can serve as potential targets for its early detection.”
According to Dr. Nagpal, more research is needed on pathogenesis of PC-NOD. Identification of biomarkers for screening may be possible in patients with new onset diabetes.
Dr. Nagpal had no financial conflicts of interest to report.
SOURCE: Nagpal S et al. DDW 2018, Abstract 392.
REPORTING FROM DDW 2018
Key clinical point: Pancreatic cancer has a distinct endocrine pancreatopathy.
Major finding: Patients with pancreatic cancer had islet sizes that were about a third smaller than those in patients with type 2 diabetes and healthy controls (P = .005).
Study details: Small study analyzing the pathology of pancreatic cancer resections and autopsy specimens.
Disclosures: The authors of this study had no financial conflicts to disclose.
Source: Nagpal S et al. DDW 2018, Abstract 392.
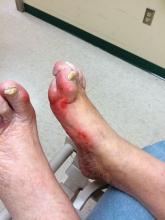
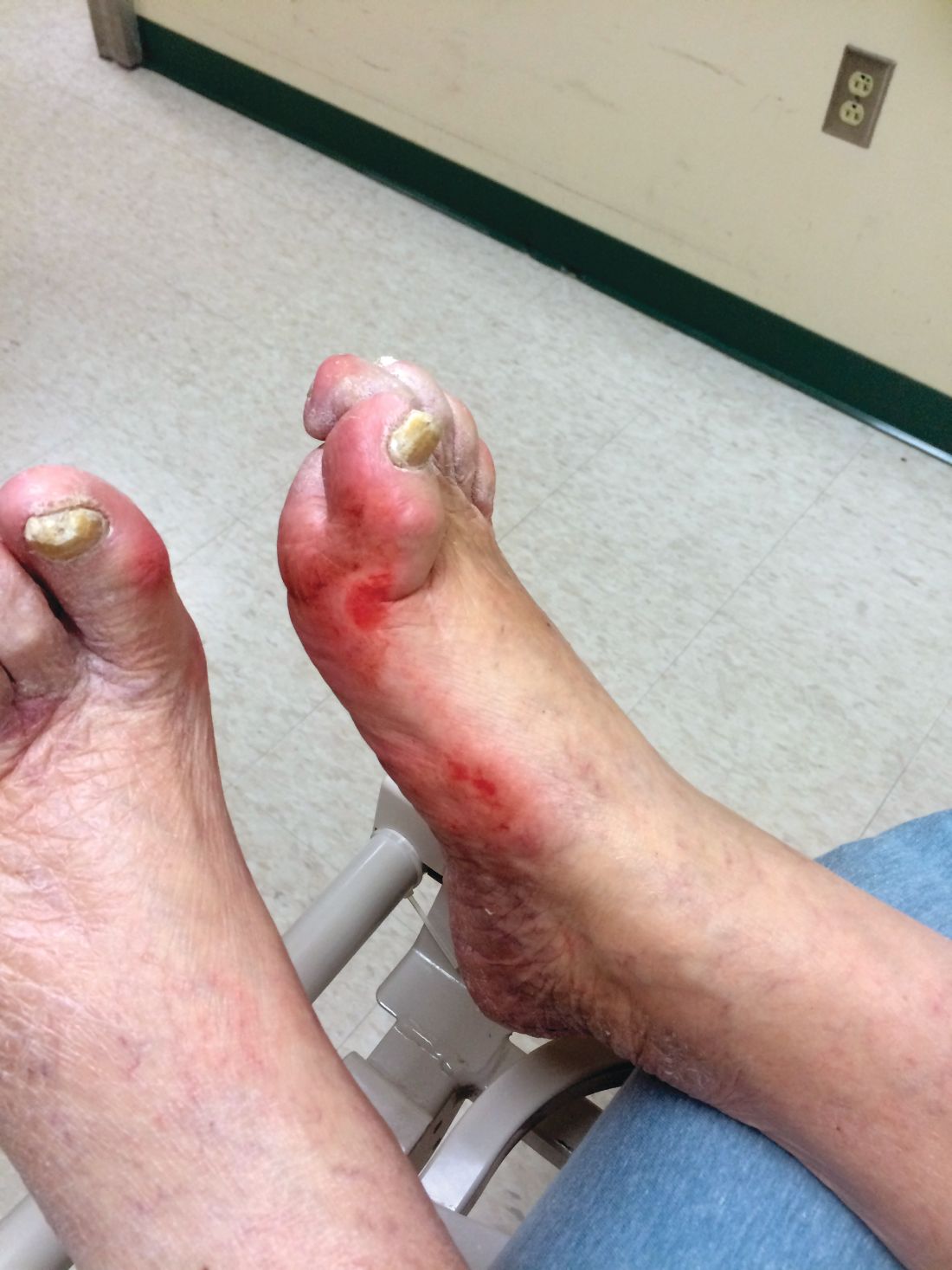
Diabetic foot ulcer healing is predictable by WIfI stage scores
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: The Wound, Ischemia, and foot Infection (WIfI) classification of diabetic foot ulcers provides a predictable primary outcome for wound healing at 1 year.
Major finding: Wound healing probability at 1 year was 94.1% for WIfI stage 1 wounds and 67.4% for stage 4 wounds.
Study details: A single-location, multidisciplinary-setting, retrospective study of 709 WIfI stage 1-4 wounds presented by 310 diabetic foot ulcer patients.
Disclosures: The authors reported no conflicts of interest.
Source: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
In T2DM, healthy lifestyle lowers CVD risk, mortality
, according to the results of a pooled analysis of two large observational cohort studies.
Relevant criteria included following a high-quality diet, not smoking, exercising moderately to vigorously for at least 2.5 hours per week, and limiting alcohol intake to 5-15 g of alcohol per day for women or 5-30 g/day for men. After the researchers controlled for possible confounders, individuals who met at least three of these criteria had about a 52% lower risk of new-onset CVD (adjusted hazard ratio, 0.48; 95% confidence interval, 0.40-0.59) and a 68% lower risk of CVD-related mortality (HR, 0.32; 95% CI, 0.22-0.47), said Gang Liu, PhD, of Harvard T.H. Chan School of Public Health, Boston, and his associates. “Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle,” they wrote. The report was published online June 18 in the Journal of the American College of Cardiology.
Cardiovascular disease is common in type 2 diabetes (T2DM), but few studies have examined the possible mitigating effects of healthy lifestyle. For this study, the researchers analyzed questionnaire data for 11,527 participants with T2DM diagnosed after enrollment in either the Nurses’ Health Study or the Health Professionals Follow-Up Study. Over an average follow-up time of 13.3 years, there were 2,311 incident cases of CVD, including 498 cases of stroke, and 858 deaths from CVD. The reduced risk of cardiovascular events remained significant even after the researchers controlled for factors such as body mass index, hypertension, hypercholesterolemia, use of antihypertensive agents, cholesterol lowering drugs, diabetes medication, and hemoglobin A1c.
Healthy lifestyle also was associated with significant reductions in the individual risk of coronary heart disease (HR, 0.53) and stroke (HR, 0.33), the investigators said. In this population, 40% of the risk of CVD mortality could be attributed to poor adherence to a healthy lifestyle, they added. Importantly, individuals who improved their lifestyle after a T2DM diagnosis had a significantly lower risk of CVD and CVD mortality than those who did not. The findings, they concluded, “support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2DM.”
The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
SOURCE: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76. doi: 10.1016/j.jacc.2018.04.027.
The findings send “a clear message” that health care promotion, advocacy, and research should keep focusing on healthy lifestyle factors, not only to improve glycemic control, but also to cut overall cardiovascular risk, experts wrote in an accompanying editorial.
The study supported a healthy lifestyle across the board, from overall CVD risk reduction to reduced risk of coronary heart disease or stroke, even after the researchers controlled for important potential confounders, wrote Kim Connelly, MBBS, PhD, Sumeet Gandhi, MD, and Edward Horton, MD. Their comments were published in Journal of the American College of Cardiology.
“Encouragingly, patients who increased the number of low-risk lifestyle factors from the time of initial diagnosis were also shown to have a lower incidence of cardiovascular disease,” they added.
But many questions persist, they noted. These include which diets are best, how much alcohol really is safe, whether there are minimum or maximum exercises thresholds, which type of exercise (if any) is best, how to monitor compliance, which health care professional should prescribe diet and exercise, and whether the findings are generalizable to groups of other ethnicities or socioeconomic levels.
Dr. Connelly and Dr. Gandhi are with University of Toronto. Dr. Horton is with Harvard University, Boston. Dr. Connelly disclosed ties to Servier, Boehringer Ingelheim, Janssen, Merck, AstraZeneca, and Novartis. Dr. Gandhi and Dr. Horton reported having no conflicts. These comments summarize their editorial (J Am Coll Cardiol. 2018;71:2877-79).
The findings send “a clear message” that health care promotion, advocacy, and research should keep focusing on healthy lifestyle factors, not only to improve glycemic control, but also to cut overall cardiovascular risk, experts wrote in an accompanying editorial.
The study supported a healthy lifestyle across the board, from overall CVD risk reduction to reduced risk of coronary heart disease or stroke, even after the researchers controlled for important potential confounders, wrote Kim Connelly, MBBS, PhD, Sumeet Gandhi, MD, and Edward Horton, MD. Their comments were published in Journal of the American College of Cardiology.
“Encouragingly, patients who increased the number of low-risk lifestyle factors from the time of initial diagnosis were also shown to have a lower incidence of cardiovascular disease,” they added.
But many questions persist, they noted. These include which diets are best, how much alcohol really is safe, whether there are minimum or maximum exercises thresholds, which type of exercise (if any) is best, how to monitor compliance, which health care professional should prescribe diet and exercise, and whether the findings are generalizable to groups of other ethnicities or socioeconomic levels.
Dr. Connelly and Dr. Gandhi are with University of Toronto. Dr. Horton is with Harvard University, Boston. Dr. Connelly disclosed ties to Servier, Boehringer Ingelheim, Janssen, Merck, AstraZeneca, and Novartis. Dr. Gandhi and Dr. Horton reported having no conflicts. These comments summarize their editorial (J Am Coll Cardiol. 2018;71:2877-79).
The findings send “a clear message” that health care promotion, advocacy, and research should keep focusing on healthy lifestyle factors, not only to improve glycemic control, but also to cut overall cardiovascular risk, experts wrote in an accompanying editorial.
The study supported a healthy lifestyle across the board, from overall CVD risk reduction to reduced risk of coronary heart disease or stroke, even after the researchers controlled for important potential confounders, wrote Kim Connelly, MBBS, PhD, Sumeet Gandhi, MD, and Edward Horton, MD. Their comments were published in Journal of the American College of Cardiology.
“Encouragingly, patients who increased the number of low-risk lifestyle factors from the time of initial diagnosis were also shown to have a lower incidence of cardiovascular disease,” they added.
But many questions persist, they noted. These include which diets are best, how much alcohol really is safe, whether there are minimum or maximum exercises thresholds, which type of exercise (if any) is best, how to monitor compliance, which health care professional should prescribe diet and exercise, and whether the findings are generalizable to groups of other ethnicities or socioeconomic levels.
Dr. Connelly and Dr. Gandhi are with University of Toronto. Dr. Horton is with Harvard University, Boston. Dr. Connelly disclosed ties to Servier, Boehringer Ingelheim, Janssen, Merck, AstraZeneca, and Novartis. Dr. Gandhi and Dr. Horton reported having no conflicts. These comments summarize their editorial (J Am Coll Cardiol. 2018;71:2877-79).
, according to the results of a pooled analysis of two large observational cohort studies.
Relevant criteria included following a high-quality diet, not smoking, exercising moderately to vigorously for at least 2.5 hours per week, and limiting alcohol intake to 5-15 g of alcohol per day for women or 5-30 g/day for men. After the researchers controlled for possible confounders, individuals who met at least three of these criteria had about a 52% lower risk of new-onset CVD (adjusted hazard ratio, 0.48; 95% confidence interval, 0.40-0.59) and a 68% lower risk of CVD-related mortality (HR, 0.32; 95% CI, 0.22-0.47), said Gang Liu, PhD, of Harvard T.H. Chan School of Public Health, Boston, and his associates. “Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle,” they wrote. The report was published online June 18 in the Journal of the American College of Cardiology.
Cardiovascular disease is common in type 2 diabetes (T2DM), but few studies have examined the possible mitigating effects of healthy lifestyle. For this study, the researchers analyzed questionnaire data for 11,527 participants with T2DM diagnosed after enrollment in either the Nurses’ Health Study or the Health Professionals Follow-Up Study. Over an average follow-up time of 13.3 years, there were 2,311 incident cases of CVD, including 498 cases of stroke, and 858 deaths from CVD. The reduced risk of cardiovascular events remained significant even after the researchers controlled for factors such as body mass index, hypertension, hypercholesterolemia, use of antihypertensive agents, cholesterol lowering drugs, diabetes medication, and hemoglobin A1c.
Healthy lifestyle also was associated with significant reductions in the individual risk of coronary heart disease (HR, 0.53) and stroke (HR, 0.33), the investigators said. In this population, 40% of the risk of CVD mortality could be attributed to poor adherence to a healthy lifestyle, they added. Importantly, individuals who improved their lifestyle after a T2DM diagnosis had a significantly lower risk of CVD and CVD mortality than those who did not. The findings, they concluded, “support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2DM.”
The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
SOURCE: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76. doi: 10.1016/j.jacc.2018.04.027.
, according to the results of a pooled analysis of two large observational cohort studies.
Relevant criteria included following a high-quality diet, not smoking, exercising moderately to vigorously for at least 2.5 hours per week, and limiting alcohol intake to 5-15 g of alcohol per day for women or 5-30 g/day for men. After the researchers controlled for possible confounders, individuals who met at least three of these criteria had about a 52% lower risk of new-onset CVD (adjusted hazard ratio, 0.48; 95% confidence interval, 0.40-0.59) and a 68% lower risk of CVD-related mortality (HR, 0.32; 95% CI, 0.22-0.47), said Gang Liu, PhD, of Harvard T.H. Chan School of Public Health, Boston, and his associates. “Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle,” they wrote. The report was published online June 18 in the Journal of the American College of Cardiology.
Cardiovascular disease is common in type 2 diabetes (T2DM), but few studies have examined the possible mitigating effects of healthy lifestyle. For this study, the researchers analyzed questionnaire data for 11,527 participants with T2DM diagnosed after enrollment in either the Nurses’ Health Study or the Health Professionals Follow-Up Study. Over an average follow-up time of 13.3 years, there were 2,311 incident cases of CVD, including 498 cases of stroke, and 858 deaths from CVD. The reduced risk of cardiovascular events remained significant even after the researchers controlled for factors such as body mass index, hypertension, hypercholesterolemia, use of antihypertensive agents, cholesterol lowering drugs, diabetes medication, and hemoglobin A1c.
Healthy lifestyle also was associated with significant reductions in the individual risk of coronary heart disease (HR, 0.53) and stroke (HR, 0.33), the investigators said. In this population, 40% of the risk of CVD mortality could be attributed to poor adherence to a healthy lifestyle, they added. Importantly, individuals who improved their lifestyle after a T2DM diagnosis had a significantly lower risk of CVD and CVD mortality than those who did not. The findings, they concluded, “support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2DM.”
The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
SOURCE: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76. doi: 10.1016/j.jacc.2018.04.027.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Eating a high-quality diet, not smoking, exercising for at least 150 minutes weekly, and drinking only moderate amounts of alcohol led to a statistically significant decrease in risk of cardiovascular disease among persons with type 2 diabetes mellitus.
Major finding: Over an average of 13.3 years of follow-up, the adjusted risk of CVD was 52% lower in participants with at least three of these healthy lifestyle factors compared with those with none (multivariate-adjusted HR, 0.48; 95% CI, 0.40-0.59).
Study details: Pooled analysis of data from 11,527 patients from the Nurses’ Health Study and the Health Professionals Follow-Up Study.
Disclosures: The National Institutes of Health provided funding. The investigators reported having no relevant conflicts of interest.
Source: Liu G et al. J Am Coll Cardiol. 2018;71:2867-76.
NIH cans study that relied on millions in funding from alcohol companies
NIH Francis Collins, MD, said the ethical violations resulted in a fundamentally flawed study that could not proceed.
“NIH has strong policies that detail the standards of conduct for NIH employees, including prohibiting the solicitation of gifts and promoting fairness in grant competitions. We take very seriously any violations of these standards,” Dr. Collins said in a statement, which added that the agency will take appropriate personnel actions.
While testifying before the Senate Appropriations Committee in mid-May on NIH’s budget request for 2019, Dr. Collins vowed not only to appropriately close the Moderate Alcohol and Cardiovascular Health (MACH) study, but to investigate whether other potential conflicts exist in other NIH-funded studies.
The story broke in mid-March, when The New York Times reported that scientists and officials from the National Institute on Alcohol Abuse and Alcoholism who were working on the MACH trial met at informational sessions with five liquor and beer companies in 2013 and 2014. The officials suggested that “the research might reflect favorably on moderate drinking, while institute officials pressed the groups for support,” according to documents obtained by the Times.
In all, the Times reported, the alcohol companies agreed to foot $67 million of the trial’s total $100 million bill. Such action violates NIH policy. An NIH report named those companies as Anheuser-Busch InBev, Carlsberg Breweries A/S, Diageo plc, Heineken, and Pernod Ricard USA LLC.
The MACH study was a multicenter, randomized clinical trial to determine the effects of one serving of alcohol (approximately 15 grams) daily, compared to no alcohol intake, on the rate of new cases of cardiovascular disease and the rate of new cases of diabetes among participants free of diabetes at baseline.
“The study was launched because some epidemiological studies have shown that moderate alcohol consumption has health benefits by reducing risk for coronary artery disease, type 2 diabetes, and rheumatoid arthritis,” according to the NIH statement. “The study aimed to enroll 7,800 participants. After a planning phase, it began enrollment on Feb. 5, 2018, and was suspended on May 10, 2018, at which time there were 105 participants enrolled.”
The trial was being led by researchers at Beth Israel Deaconess Medical Center, Boston.
In response to the public disclosure of the study’s funding, NIH convened a working group to ascertain:
- the circumstances that led to securing private funding for MACH trial
- the scientific premise of and planning for the MACH trial
- the process used to decide to support the MACH trial
- program development and oversight once funding was secured by the secured by the Foundation for NIH (FNIH)
- a review of the NIAAA portfolio prior to and during the leadership of the current NIAAA Director to assess what programmatic shifts, if any, could be discerned.
While noting that public-private partnerships are key to advancing science, the committee found that soliciting funds from alcoholic beverage companies for a study that could prove such beverages are beneficial, crossed the “firewall” between public funds and private resources. The committee recommended terminating the study.
The committee also recommended an expanded investigation into measures that would prevent NIH staff from soliciting external funds to support research programs.
The committee uncovered an email trail strongly suggesting that the solicitation of funds was planned and intended to be secretive.
According to the working group report, there was “frequent email correspondence among members of NIAAA senior staff, select extramural investigators (including the eventual PI of the MACH trial), and industry representatives occurred prior to involvement of the FNIH and the development of the NIH funding opportunity announcement for a multi-site clinical trial on moderate drinking and cardiovascular health. These communications appear to be an attempt to persuade industry to provide funding for the MACH trial. Moreover, these senior members of NIAAA staff appear to have purposefully kept other key members of NIAAA staff and the FNIH ignorant of these efforts. For example, correspondence between NIAAA staff draws attention to a February 2014 wine industry blog that reports that FNIH is initiating a search for industry funding to support a major clinical study on the health effects of moderate alcohol consumption. One senior staff member at NIAAA is unaware of any such potential planning, asking another senior staff member about the article. ‘... Anything seem broken here?’ even though such a trial to test moderate drinking effects on cardiovascular health should very likely involve the programmatic division to which this senior staff member belongs. In response to receiving the forwarded discussion, NIAAA senior leadership communicates among one other, ‘Best not to respond right now but we can’t keep him totally in the dark.’ "
The trial was also funded in part by NIAAA, which expected to commit $20 million to the overall project over 10 years, of which $4 million has been spent.
“The integrity of the NIH grants administrative process, peer review, and the quality of NIH-supported research must always be above reproach,” Dr. Collins said in the statement. “When any problems are uncovered, however, efforts to correct them must be swift and comprehensive.”
NIH Francis Collins, MD, said the ethical violations resulted in a fundamentally flawed study that could not proceed.
“NIH has strong policies that detail the standards of conduct for NIH employees, including prohibiting the solicitation of gifts and promoting fairness in grant competitions. We take very seriously any violations of these standards,” Dr. Collins said in a statement, which added that the agency will take appropriate personnel actions.
While testifying before the Senate Appropriations Committee in mid-May on NIH’s budget request for 2019, Dr. Collins vowed not only to appropriately close the Moderate Alcohol and Cardiovascular Health (MACH) study, but to investigate whether other potential conflicts exist in other NIH-funded studies.
The story broke in mid-March, when The New York Times reported that scientists and officials from the National Institute on Alcohol Abuse and Alcoholism who were working on the MACH trial met at informational sessions with five liquor and beer companies in 2013 and 2014. The officials suggested that “the research might reflect favorably on moderate drinking, while institute officials pressed the groups for support,” according to documents obtained by the Times.
In all, the Times reported, the alcohol companies agreed to foot $67 million of the trial’s total $100 million bill. Such action violates NIH policy. An NIH report named those companies as Anheuser-Busch InBev, Carlsberg Breweries A/S, Diageo plc, Heineken, and Pernod Ricard USA LLC.
The MACH study was a multicenter, randomized clinical trial to determine the effects of one serving of alcohol (approximately 15 grams) daily, compared to no alcohol intake, on the rate of new cases of cardiovascular disease and the rate of new cases of diabetes among participants free of diabetes at baseline.
“The study was launched because some epidemiological studies have shown that moderate alcohol consumption has health benefits by reducing risk for coronary artery disease, type 2 diabetes, and rheumatoid arthritis,” according to the NIH statement. “The study aimed to enroll 7,800 participants. After a planning phase, it began enrollment on Feb. 5, 2018, and was suspended on May 10, 2018, at which time there were 105 participants enrolled.”
The trial was being led by researchers at Beth Israel Deaconess Medical Center, Boston.
In response to the public disclosure of the study’s funding, NIH convened a working group to ascertain:
- the circumstances that led to securing private funding for MACH trial
- the scientific premise of and planning for the MACH trial
- the process used to decide to support the MACH trial
- program development and oversight once funding was secured by the secured by the Foundation for NIH (FNIH)
- a review of the NIAAA portfolio prior to and during the leadership of the current NIAAA Director to assess what programmatic shifts, if any, could be discerned.
While noting that public-private partnerships are key to advancing science, the committee found that soliciting funds from alcoholic beverage companies for a study that could prove such beverages are beneficial, crossed the “firewall” between public funds and private resources. The committee recommended terminating the study.
The committee also recommended an expanded investigation into measures that would prevent NIH staff from soliciting external funds to support research programs.
The committee uncovered an email trail strongly suggesting that the solicitation of funds was planned and intended to be secretive.
According to the working group report, there was “frequent email correspondence among members of NIAAA senior staff, select extramural investigators (including the eventual PI of the MACH trial), and industry representatives occurred prior to involvement of the FNIH and the development of the NIH funding opportunity announcement for a multi-site clinical trial on moderate drinking and cardiovascular health. These communications appear to be an attempt to persuade industry to provide funding for the MACH trial. Moreover, these senior members of NIAAA staff appear to have purposefully kept other key members of NIAAA staff and the FNIH ignorant of these efforts. For example, correspondence between NIAAA staff draws attention to a February 2014 wine industry blog that reports that FNIH is initiating a search for industry funding to support a major clinical study on the health effects of moderate alcohol consumption. One senior staff member at NIAAA is unaware of any such potential planning, asking another senior staff member about the article. ‘... Anything seem broken here?’ even though such a trial to test moderate drinking effects on cardiovascular health should very likely involve the programmatic division to which this senior staff member belongs. In response to receiving the forwarded discussion, NIAAA senior leadership communicates among one other, ‘Best not to respond right now but we can’t keep him totally in the dark.’ "
The trial was also funded in part by NIAAA, which expected to commit $20 million to the overall project over 10 years, of which $4 million has been spent.
“The integrity of the NIH grants administrative process, peer review, and the quality of NIH-supported research must always be above reproach,” Dr. Collins said in the statement. “When any problems are uncovered, however, efforts to correct them must be swift and comprehensive.”
NIH Francis Collins, MD, said the ethical violations resulted in a fundamentally flawed study that could not proceed.
“NIH has strong policies that detail the standards of conduct for NIH employees, including prohibiting the solicitation of gifts and promoting fairness in grant competitions. We take very seriously any violations of these standards,” Dr. Collins said in a statement, which added that the agency will take appropriate personnel actions.
While testifying before the Senate Appropriations Committee in mid-May on NIH’s budget request for 2019, Dr. Collins vowed not only to appropriately close the Moderate Alcohol and Cardiovascular Health (MACH) study, but to investigate whether other potential conflicts exist in other NIH-funded studies.
The story broke in mid-March, when The New York Times reported that scientists and officials from the National Institute on Alcohol Abuse and Alcoholism who were working on the MACH trial met at informational sessions with five liquor and beer companies in 2013 and 2014. The officials suggested that “the research might reflect favorably on moderate drinking, while institute officials pressed the groups for support,” according to documents obtained by the Times.
In all, the Times reported, the alcohol companies agreed to foot $67 million of the trial’s total $100 million bill. Such action violates NIH policy. An NIH report named those companies as Anheuser-Busch InBev, Carlsberg Breweries A/S, Diageo plc, Heineken, and Pernod Ricard USA LLC.
The MACH study was a multicenter, randomized clinical trial to determine the effects of one serving of alcohol (approximately 15 grams) daily, compared to no alcohol intake, on the rate of new cases of cardiovascular disease and the rate of new cases of diabetes among participants free of diabetes at baseline.
“The study was launched because some epidemiological studies have shown that moderate alcohol consumption has health benefits by reducing risk for coronary artery disease, type 2 diabetes, and rheumatoid arthritis,” according to the NIH statement. “The study aimed to enroll 7,800 participants. After a planning phase, it began enrollment on Feb. 5, 2018, and was suspended on May 10, 2018, at which time there were 105 participants enrolled.”
The trial was being led by researchers at Beth Israel Deaconess Medical Center, Boston.
In response to the public disclosure of the study’s funding, NIH convened a working group to ascertain:
- the circumstances that led to securing private funding for MACH trial
- the scientific premise of and planning for the MACH trial
- the process used to decide to support the MACH trial
- program development and oversight once funding was secured by the secured by the Foundation for NIH (FNIH)
- a review of the NIAAA portfolio prior to and during the leadership of the current NIAAA Director to assess what programmatic shifts, if any, could be discerned.
While noting that public-private partnerships are key to advancing science, the committee found that soliciting funds from alcoholic beverage companies for a study that could prove such beverages are beneficial, crossed the “firewall” between public funds and private resources. The committee recommended terminating the study.
The committee also recommended an expanded investigation into measures that would prevent NIH staff from soliciting external funds to support research programs.
The committee uncovered an email trail strongly suggesting that the solicitation of funds was planned and intended to be secretive.
According to the working group report, there was “frequent email correspondence among members of NIAAA senior staff, select extramural investigators (including the eventual PI of the MACH trial), and industry representatives occurred prior to involvement of the FNIH and the development of the NIH funding opportunity announcement for a multi-site clinical trial on moderate drinking and cardiovascular health. These communications appear to be an attempt to persuade industry to provide funding for the MACH trial. Moreover, these senior members of NIAAA staff appear to have purposefully kept other key members of NIAAA staff and the FNIH ignorant of these efforts. For example, correspondence between NIAAA staff draws attention to a February 2014 wine industry blog that reports that FNIH is initiating a search for industry funding to support a major clinical study on the health effects of moderate alcohol consumption. One senior staff member at NIAAA is unaware of any such potential planning, asking another senior staff member about the article. ‘... Anything seem broken here?’ even though such a trial to test moderate drinking effects on cardiovascular health should very likely involve the programmatic division to which this senior staff member belongs. In response to receiving the forwarded discussion, NIAAA senior leadership communicates among one other, ‘Best not to respond right now but we can’t keep him totally in the dark.’ "
The trial was also funded in part by NIAAA, which expected to commit $20 million to the overall project over 10 years, of which $4 million has been spent.
“The integrity of the NIH grants administrative process, peer review, and the quality of NIH-supported research must always be above reproach,” Dr. Collins said in the statement. “When any problems are uncovered, however, efforts to correct them must be swift and comprehensive.”