User login
New consensus recommendations on bleeding in acquired hemophilia
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
FROM HAEMOPHILIA
Firearm-related deaths show recent increase
After years of relative stability, firearm-related mortality in the United States rose sharply starting in 2015, according to analysis of a national mortality database.

U.S. firearm mortality was 10.4 per 100,000 person-years during 1999-2014, with the high in that period occurring in 2012 and dropping each of the next 2 years – compared with 11.8 per 100,000 during 2015-2017, an increase of 13.8%, Jason E. Goldstick, PhD, and associates wrote Oct. 8 in Health Affairs.
The majority of the 612,310 firearm deaths over the entire study period were suicides, with the proportion rising slightly from 58.6% in 1999-2014 to 60.0% in 2015-2017. Homicides made up 38.5% of deaths in 1999-2014 and 37.9% in 2015-2017, while the combined share of unintentional and undetermined deaths dropped from 2.9% to 2.1%, the investigators reported.
Dr. Goldstick of the University of Michigan, Ann Arbor, said in a separate written statement.
The geographic broadness can be seen when the change in mortality from 1999-2014 to 2015-2017 was calculated for each locale: 29 states had an increase of more than 20% and only 3 states (California, New York, and Rhode Island) and the District of Columbia had a decrease of at least 12.5%, they said. The data came from the Centers for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research tool.
The different trends among states and subpopulations make it difficult to offer policy-based interventions. “The epidemiology of firearm violence is complex and varies based on the mechanism of death, demographic group under study, and regionally specific culture, making a one-size-fits-all solution inappropriate,” Dr. Goldstick and associates wrote.
The study was funded mainly by a grant from the National Institute of Child Health and Human Development. The investigators did not provide any information on conflicts of interest.
SOURCE: Goldstick JE et al. Health Aff. 2019;38(10):1646-52.
After years of relative stability, firearm-related mortality in the United States rose sharply starting in 2015, according to analysis of a national mortality database.

U.S. firearm mortality was 10.4 per 100,000 person-years during 1999-2014, with the high in that period occurring in 2012 and dropping each of the next 2 years – compared with 11.8 per 100,000 during 2015-2017, an increase of 13.8%, Jason E. Goldstick, PhD, and associates wrote Oct. 8 in Health Affairs.
The majority of the 612,310 firearm deaths over the entire study period were suicides, with the proportion rising slightly from 58.6% in 1999-2014 to 60.0% in 2015-2017. Homicides made up 38.5% of deaths in 1999-2014 and 37.9% in 2015-2017, while the combined share of unintentional and undetermined deaths dropped from 2.9% to 2.1%, the investigators reported.
Dr. Goldstick of the University of Michigan, Ann Arbor, said in a separate written statement.
The geographic broadness can be seen when the change in mortality from 1999-2014 to 2015-2017 was calculated for each locale: 29 states had an increase of more than 20% and only 3 states (California, New York, and Rhode Island) and the District of Columbia had a decrease of at least 12.5%, they said. The data came from the Centers for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research tool.
The different trends among states and subpopulations make it difficult to offer policy-based interventions. “The epidemiology of firearm violence is complex and varies based on the mechanism of death, demographic group under study, and regionally specific culture, making a one-size-fits-all solution inappropriate,” Dr. Goldstick and associates wrote.
The study was funded mainly by a grant from the National Institute of Child Health and Human Development. The investigators did not provide any information on conflicts of interest.
SOURCE: Goldstick JE et al. Health Aff. 2019;38(10):1646-52.
After years of relative stability, firearm-related mortality in the United States rose sharply starting in 2015, according to analysis of a national mortality database.

U.S. firearm mortality was 10.4 per 100,000 person-years during 1999-2014, with the high in that period occurring in 2012 and dropping each of the next 2 years – compared with 11.8 per 100,000 during 2015-2017, an increase of 13.8%, Jason E. Goldstick, PhD, and associates wrote Oct. 8 in Health Affairs.
The majority of the 612,310 firearm deaths over the entire study period were suicides, with the proportion rising slightly from 58.6% in 1999-2014 to 60.0% in 2015-2017. Homicides made up 38.5% of deaths in 1999-2014 and 37.9% in 2015-2017, while the combined share of unintentional and undetermined deaths dropped from 2.9% to 2.1%, the investigators reported.
Dr. Goldstick of the University of Michigan, Ann Arbor, said in a separate written statement.
The geographic broadness can be seen when the change in mortality from 1999-2014 to 2015-2017 was calculated for each locale: 29 states had an increase of more than 20% and only 3 states (California, New York, and Rhode Island) and the District of Columbia had a decrease of at least 12.5%, they said. The data came from the Centers for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research tool.
The different trends among states and subpopulations make it difficult to offer policy-based interventions. “The epidemiology of firearm violence is complex and varies based on the mechanism of death, demographic group under study, and regionally specific culture, making a one-size-fits-all solution inappropriate,” Dr. Goldstick and associates wrote.
The study was funded mainly by a grant from the National Institute of Child Health and Human Development. The investigators did not provide any information on conflicts of interest.
SOURCE: Goldstick JE et al. Health Aff. 2019;38(10):1646-52.
FROM HEALTH AFFAIRS
Novel research aims to improve ED care in sickle cell disease
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
REPORTING FROM AN NIH WEBINAR
A complication of enoxaparin injection
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
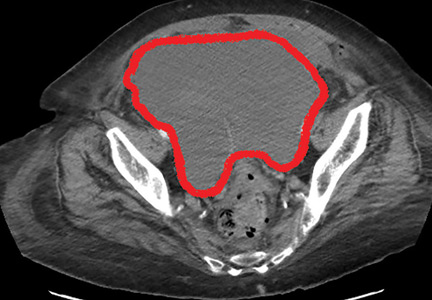
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 66-year-old man with abnormal thyroid function tests
A 66-year-old man presented to the emergency department with increasing shortness of breath and productive cough, which had begun 5 days earlier. Three years previously, he had been diagnosed with chronic obstructive pulmonary disease (COPD).
One week before the current presentation, he developed a sore throat, rhinorrhea, and nasal congestion, and the shortness of breath had started 2 days after that. Although he could speak in sentences, he was breathless even at rest. His dyspnea was associated with noisy breathing and cough productive of yellowish sputum; there was no hemoptysis. He reported fever, but he had no chills, night sweats, chest pain, or paroxysmal nocturnal dyspnea. The review of other systems was unremarkable.
His COPD was known to be mild, in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, group A. His postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was less than 0.70, and his FEV1 was 84% of predicted. Apart from mild intermittent cough with white sputum, his COPD had been under good control with inhaled ipratropium 4 times daily and inhaled albuterol as needed. He said he did not have shortness of breath except when hurrying on level ground or walking up a slight hill (Modified Medical Research Council dyspnea scale grade 1; COPD Assessment Test score < 10). In the last 3 years, he had 2 exacerbations of COPD, 1 year apart, both requiring oral prednisone and antibiotic therapy.
Other relevant history included hypertension and dyslipidemia of 15-year duration, for which he was taking candesartan 16 mg twice daily and atorvastatin 20 mg daily. He was compliant with his medications.
Though he usually received an influenza vaccine every year, he did not get it the previous year. Also, 3 years previously, he received the 23-valent pneumococcal polysaccharide vaccine (PPSV23), and the year before that he received the pneumococcal conjugate vaccine (PCV13). In addition, he was immunized against herpes zoster and tetanus.
The patient had smoked 1 pack per day for the past 38 years. His primary care physician had advised him many times to quit smoking. He had enrolled in a smoking cessation program 2 years previously, in which he received varenicline in addition to behavioral counseling in the form of motivational interviewing and a telephone quit-line. Nevertheless, he continued to smoke.
He was a retired engineer. He did not drink alcohol or use illicit drugs.
PHYSICAL EXAMINATION
On physical examination, the patient was sitting up in bed, leaning forward. He was alert and oriented but was breathing rapidly and looked sick. He had no cyanosis, clubbing, pallor, or jaundice. His blood pressure was 145/90 mm Hg, heart rate 110 beats per minute and regular, respiratory rate 29 breaths per minute, and oral temperature 38.1°C (100.6°F). His oxygen saturation was 88% while breathing room air. His body mass index was 27.1 kg/m2.
His throat was mildly congested. His neck veins were flat, and there were no carotid bruits. His thyroid examination was normal, without goiter, nodules, or tenderness.
Intercostal retractions were noted around the anterolateral costal margins. He had no chest wall deformities. Chest expansion was reduced bilaterally. There was hyperresonance bilaterally. Expiratory wheezes were heard over both lungs, without crackles.
His heart had no murmurs or added sounds. There was no lower-limb edema or swelling. The rest of his physical examination was unremarkable.
Results of initial laboratory testing are shown in Table 1.
Assessment: A 66-year-old man with GOLD grade 1, group A COPD, presenting with a severe exacerbation, most likely due to viral bronchitis.
INITIAL MANAGEMENT
The patient was given oxygen 28% by Venturi mask, and his oxygen saturation went up to 90%. He was started on nebulized albuterol 2.5 mg with ipratropium bromide 500 µg every 4 hours, prednisone 40 mg orally daily for 5 days, and ceftriaxone 1 g intravenously every 24 hours. The first dose of each medication was given in the emergency department.
The patient was then admitted to a progressive care unit, where he was placed on noninvasive positive pressure ventilation, continuous cardiac monitoring, and pulse oximetry. He was started on enoxaparin 40 mg subcutaneously daily to prevent venous thromboembolism, and the oral medications he had been taking at home were continued. Because he was receiving a glucocorticoid, his blood glucose was monitored in the fasting state, 2 hours after each meal, and as needed.
Two hours after he started noninvasive positive pressure ventilation, his arterial blood gases were remeasured and showed the following results:
- pH 7.35
- Partial pressure of carbon dioxide (Paco2) 52 mm Hg
- Bicarbonate 28 mmol/L
- Partial pressure of oxygen (Pao2) 60 mm Hg
- Oxygen saturation 90%.
HOSPITAL COURSE
On hospital day 3, his dyspnea had slightly improved. His respiratory rate was 26 to 28 breaths per minute. His oxygen saturation remained between 90% and 92%.
At 10:21 pm, his cardiac monitor showed an episode of focal atrial tachycardia at a rate of 129 beats per minute that lasted for 3 minutes and 21 seconds, terminating spontaneously. He denied any change in his clinical condition during the episode, with no chest pain, palpitation, or change in dyspnea. There was no change in his vital signs. He had another similar asymptomatic episode lasting 4 minutes and 9 seconds at 6:30 am of hospital day 4.
Because of these episodes, the attending physician ordered thyroid function tests.
THYROID FUNCTION TESTING
1. Which thyroid function test is most likely to be helpful in the assessment of this patient’s thyroid status?
- Serum thyroid-stimulating hormone (TSH) alone
- Serum TSH and total thyroxine (T4)
- Serum TSH and total triiodothyronine (T3)
- Serum TSH and free T4
- Serum TSH and free T3
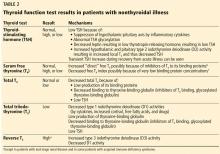
There are several tests to assess thyroid function: the serum TSH, total T4, free T4, total T3, and free T3 concentrations.1
In normal physiology, TSH from the pituitary stimulates the thyroid gland to produce and secrete T4 and T3, which in turn inhibit TSH secretion through negative feedback. A negative log-linear relation exists between serum free T4 and TSH levels.2 Thus, the serum free T4 level can remain within the normal reference range even if the TSH level is high or low.
TSH assays can have different detection limits. A third-generation TSH assay with a detection limit of 0.01 mU/L is recommended for use in clinical practice.3
TSH testing alone. Given its superior sensitivity and specificity, serum TSH measurement is considered the best single test for assessing thyroid function in most cases.4 Nevertheless, measurement of the serum TSH level alone could be misleading in several situations, eg, hypothalamic or pituitary disorders, recent treatment of thyrotoxicosis, impaired sensitivity to thyroid hormone, and acute nonthyroidal illness.4
Free vs total T4 and T3 levels
Serum total T4 includes a fraction that is bound, mainly to thyroxin-binding globulin, and a very small unbound (free) fraction. The same applies to T3. Only free thyroid hormones represent the “active” fraction available for interaction with their protein receptors in the nucleus.8 Patients with conditions that can affect the thyroid-binding protein concentrations usually have altered serum total T4 and T3 levels, whereas their free hormone concentrations remain normal. Accordingly, measurement of free hormone levels, especially free T4, is usually recommended.
Although equilibrium dialysis is the method most likely to provide an accurate serum free T4 measurement, it is not commonly used because of its limited availability and high cost. Thus, most commercial laboratories use “direct” free T4 measurement or, to a lesser degree, the free T4 index.9 However, none of the currently available free T4 tests actually measure free T4 directly; rather, they estimate it.10
Commercial laboratories can provide a direct free T3 estimate, but it may be less reliable than total T3. If serum T3 measurement is indicated, serum total T3 is usually measured. However, total T3 measurement is rarely indicated for patients with hypothyroidism because it usually remains within the normal reference range.11 Nevertheless, serum total T3 measurement could be useful in patients with T3 toxicosis and in those who are acutely ill.
Accordingly, in acutely ill hospitalized patients like ours, measuring serum TSH using a third-generation assay and free T4 is essential to assess thyroid function. Many clinicians also measure serum total T3.
CASE CONTINUED: LOW TSH, LOW-NORMAL FREE T4, LOW TOTAL T3
The attending physician ordered serum TSH, free T4, and total T3 measurements, which yielded the following:
- TSH 0.1 mU/L (0.5–5.0)
- Total T3 55 ng/dL (80–180)
- Free T4 0.9 ng/dL (0.9–2.4).
2. Which best explains this patient’s abnormal thyroid test results?
- His acute illness
- Central hypothyroidism due to pituitary infarction
- His albuterol therapy
- Subclinical thyrotoxicosis
- Hashimoto thyroiditis
Since euthyroid patients with an acute illness may have abnormal thyroid test results (Table 2),5–7 thyroid function testing is not recommended unless there is a strong indication for it, such as new-onset atrial fibrillation, atrial flutter, or focal atrial tachycardia.1 In such patients, it is important to know whether the test abnormalities represent true thyroid disorder or are the result of a nonthyroidal illness.
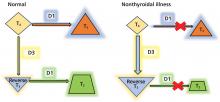
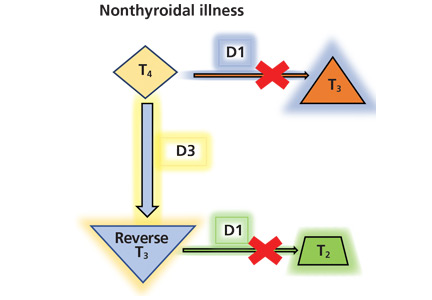
Thyroid function testing in patients with nonthyroidal illness usually shows low serum total T3, normal or low serum TSH, and normal, low, or high serum free T4. However, transient mild serum TSH elevation can be seen in some patients during the recovery period.16 These abnormalities with their mechanisms are shown in Table 2.5–7 In several commercial kits, serum direct free T4 can be falsely decreased or increased.8
THE DIFFERENTIAL DIAGNOSIS
Our patient had low serum TSH, low-normal serum direct free T4, and low serum total T3. This profile could be caused by a nonthyroidal illness, “true” central hypothyroidism, or his glucocorticoid treatment. The reason we use the term “true” in this setting is that some experts suggest that the thyroid function test abnormalities in patients with acute nonthyroidal illness represent a transient central hypothyroidism.17 The clinical presentation is key in differentiating true central hypothyroidism from nonthyroidal illness.
In addition, measuring serum cortisol may help to differentiate between the 2 states, as it would be elevated in patients with nonthyroidal illness as part of a stress response but low in patients with true central hypothyroidism, since it is usually part of combined pituitary hormone deficiency.18 Of note, some critically ill patients have low serum cortisol because of transient central adrenal insufficiency.19,20
The serum concentration of reverse T3 has been suggested as a way to differentiate between hypothyroidism (low) and nonthyroidal illness (high); however, further studies showed that it does not reliably differentiate between the conditions.21
GLUCOCORTICOIDS AND THYROID FUNCTION TESTS
By inhibiting D1, glucocorticoids can decrease peripheral conversion of T4 to T3 and thus decrease serum total T3. This effect depends on the type and dose of the glucocorticoid and the duration of therapy.
In one study,22 there was a significant reduction in serum total T3 concentration 24 hours after a single oral dose of dexamethasone 12 mg in normal participants. This effect lasted 48 hours, after which serum total T3 returned to its pretreatment level.
In another study,23 a daily oral dose of betamethasone 1.5 mg for 5 days did not significantly reduce the serum total T3 in healthy volunteers, but a daily dose of 3 mg did. This effect was more pronounced at a daily dose of 4.5 mg, whereas a dose of 6.0 mg had no further effect.
Long-term glucocorticoid therapy also decreases serum total T4 and total T3 by lowering serum thyroid-binding globulin.24
Finally, glucocorticoids can decrease TSH secretion by directly inhibiting thyrotropin-releasing hormone.25,26 However, chronic hypercortisolism, whether endogenous or exogenous, does not cause clinically central hypothyroidism, possibly because of the negative feedback mechanism of low thyroid hormones on the pituitary and the hypothalamus.27
Other drugs including dopamine, dopamine agonists, dobutamine, and somatostatin analogues can suppress serum TSH. As with glucocorticoids, these drugs do not cause clinically evident central hypothyroidism.28 Bexarotene, a retinoid X receptor ligand used in the treatment of cutaneous T-cell lymphoma, has been reported to cause clinically evident central hypothyroidism by suppressing TSH and increasing T4 clearance.29
BETA-BLOCKERS, BETA-AGONISTS AND THYROID FUNCTION
While there is general agreement that beta-adrenergic antagonists (beta-blockers) do not affect the serum TSH concentration, conflicting data have been reported concerning their effect on other thyroid function tests. This may be due to several factors, including dose, duration of therapy, the patient’s thyroid status, and differences in laboratory methodology.30
In studies of propranolol, serum total T4 concentrations did not change or were increased with daily doses of 160 mg or more in both euthyroid participants and hyperthyroid patients31–33; serum total T3 concentrations did not change or were decreased with 40 mg or more daily34; and serum reverse T3 concentrations were increased with daily doses of 80 mg or more.31 It is most likely that propranolol exerts these changes by inhibiting D1 activity in peripheral tissues.
Furthermore, a significant decrease in serum total T3 concentrations was observed in hyperthyroid patients treated with atenolol 100 mg daily, metoprolol 100 mg daily, and alprenolol 100 mg daily, but not with sotalol 80 mg daily or nadolol (up to 240 mg daily).35,36
On the other hand, beta-adrenergic agonists have not been reported to cause significant changes in thyroid function tests.37
SUBCLINICAL THYROTOXICOSIS OR HASHIMOTO THYROIDITIS?
Our patient’s thyroid function test results are more likely due to his nonthyroidal illness and glucocorticoid therapy, as there is no clinical evidence to point to a hypothalamic-pituitary disorder accounting for true central hypothyroidism.
The other options mentioned in question 2 are unlikely to explain our patient’s thyroid function test results.
Subclinical thyrotoxicosis is characterized by suppressed serum TSH, but both serum free T4 and total T3 remain within the normal reference ranges. In addition, the serum TSH level may help to differentiate between thyrotoxicosis and nonthyroidal illness. In the former, serum TSH is usually suppressed (< 0.01 mU/L), whereas in the latter it is usually low but detectable (0.05– 0.3 mU/L).38,39
Hashimoto thyroiditis is a chronic autoimmune thyroid disease characterized by diffuse lymphocytic infiltration of the thyroid gland. Almost all patients with Hashimoto thyroiditis have elevated levels of antibodies to thyroid peroxidase or thyroglobulin.40 Clinically, patients with Hashimoto thyroiditis can either be hypothyroid or have normal thyroid function, which is not the case in our patient.
CASE CONTINUED
An endocrinologist, consulted for a second opinion, agreed that the patient’s thyroid function test results were most likely due to his nonthyroidal illness and glucocorticoid therapy.
3. In view of the endocrinologist’s opinion, which should be the next step in the management of the patient’s thyroid condition?
- Start levothyroxine (T4) therapy
- Start liothyronine (T3) therapy
- Start N-acetylcysteine therapy
- Start thyrotropin-releasing hormone therapy
- Remeasure thyroid hormones after full recovery from his acute illness
It is not clear whether the changes in thyroid hormone levels during an acute illness are a pathologic alteration for which thyroid hormone therapy may be beneficial, or a physiologic adaptation for which such therapy would not be indicated.41
However, current data argue against thyroid hormone therapy using T4 or T3 for patients with nonthyroidal illness syndrome (also called euthyroid sick syndrome).42 Indeed, several randomized controlled trials showed that thyroid hormone therapy is not beneficial in such patients and may be detrimental.41,43
Therapies other than thyroid hormone have been investigated to ameliorate thyroid hormone abnormalities in patients with nonthyroidal illness. These include N-acetylcysteine, thyrotropin-releasing hormone therapy, and nutritional support.
Some studies showed that giving N-acetylcysteine, an antioxidant, increased serum T3 and decreased serum reverse T3 concentrations in patients with acute myocardial infarction.44 Nevertheless, the mortality rate and length of hospitalization were not affected. Further studies are needed to know whether N-acetylcysteine therapy is beneficial for such patients.
Similarly, a study using a thyrotropin-releasing hormone analogue along with growth hormone-releasing peptide 2 showed an increase in serum TSH, T4, and T3 levels in critically ill patients.45 The benefit of this therapy has yet to be determined. On the other hand, early nutritional support was reported to prevent thyroid hormonal changes in patients postoperatively.46
Measuring thyroid hormone levels after full recovery is the most appropriate next step in our patient, as the changes in thyroid hormone concentrations subside as the acute illness resolves.47
CASE CONTINUED
The patient continued to improve. On hospital day 6, he was feeling better but still had mild respiratory distress. There had been no further episodes of arrhythmia since day 4. His blood pressure was 136/86 mm Hg, heart rate 88 beats per minute and regular, respiratory rate 18 breaths per minute, and oral temperature 37.1°C. His oxygen saturation was 92% on room air.
Before discharge, he was encouraged to quit smoking. He was offered behavioral counseling and medication therapy, but he only said that he would think about it. He was discharged on oral cefixime for 4 more days and was instructed to switch to a long-acting bronchodilator along with his other home medications and to return in 1 week to have his thyroid hormones checked.
One week later, his laboratory results were:
- TSH 11.2 mU/L (reference range 0.5–5.0)
- Free T4 1.2 ng/dL (0.9–2.4)
- Total T3 92 ng/dL (80–180).
Clinically, the patient was euthyroid, and examination of his thyroid was unremarkable.
4. Based on these last test results, which statement is correct?
- Levothyroxine therapy should be started
- His serum TSH elevation is most likely transient
- Thyroid ultrasonography is strongly indicated
- A radioactive iodine uptake study should be performed
- Measurement of thyroid-stimulating immunoglobulins is indicated
During recovery from nonthyroidal illness, some patients may have elevated serum TSH levels that are usually transient and modest (< 20 mU/L).48 Normalization of the thyroid function tests including serum TSH may take several weeks49 or months.50 However, a systematic review found that the likelihood of permanent primary hypothyroidism is high in patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness.51
Ultrasonography is useful for evaluating patients with thyroid nodules or goiter but is of little benefit for patients like ours, in whom the thyroid is normal on examination.
Similarly, a radioactive iodine uptake study is not indicated, as it is principally used to help differentiate between types of thyrotoxicosis. (Radioactive iodine is also used to treat differentiated thyroid cancer.)
Thyroid-stimulating immunoglobins are TSH receptor-stimulating antibodies that cause Graves disease. Nevertheless, measuring them is not routinely indicated for its diagnosis. However, their measurement is of significant help in the diagnosis of Graves disease if a radioactive iodine uptake study cannot be performed (as in pregnancy) and in atypical presentations such as euthyroid Graves ophthalmopathy.52 Other indications for thyroid-stimulating immunoglobin measurement are beyond the scope of the article. Our patient’s test results are not consistent with hyperthyroidism, so measuring thyroid-stimulating immunoglobins is not indicated.
CASE CONCLUSION: BETTER, BUT STILL SMOKING
The patient missed his 1-month clinic follow-up, but he visited the clinic for follow-up 3 months later. He was feeling well with no complaints. Test results including serum TSH, free T4, and total T3 were within normal ranges. His COPD was under control, with an FEV1 88% of predicted.
He was again encouraged to quit smoking and was offered drug therapy and behavioral counseling, but he declined. In addition, he was instructed to adhere to his annual influenza vaccination.
KEY POINTS
- In patients with acute illness, it is recommended that thyroid function not be assessed unless there is a strong indication.
- If thyroid function assessment is indicated for critically ill patients, serum TSH and free T4 concentrations should be measured. Some clinicians also measure serum total T3 level.
- Thyroid function testing in critically ill patients usually shows low serum total T3, normal or low serum TSH, and normal or low serum free T4.
- Many drugs can alter thyroid hormone levels.
- Thyroid hormone therapy is not recommended for critically ill patients with low T3, low T4, or both.
- During recovery from nonthyroidal illness, some patients may have mild elevation in serum TSH levels (< 20 mU/L).
- Thyroid hormone levels may take several weeks or months to return to normal after the acute illness.
- Patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness are more likely to have permanent primary hypothyroidism.
- Lamb EJ, Martin J. Thyroid function tests: often justified in the acutely ill. Ann Clin Biochem 2000; 37(pt 2):158–164. doi:10.1258/0004563001899159
- Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990; 70(2):453–460. doi:10.1210/jcem-70-2-453
- Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989; 69(3):684–688. doi:10.1210/jcem-69-3-684
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27(6):745–762. doi:10.1016/j.beem.2013.10.003
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 2005; 15(8):883–897. doi:10.1089/thy.2005.15.883
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal ilnesses. Ann Intern Med 1983; 98(6):946–957. doi:10.7326/0003-4819-98-6-946
- Chopra IJ, Solomon DH, Hepner HW, Mortenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med 1979; 90(6):905–912. doi:10.7326/0003-4819-90-6-905
- Pontecorvi A, Robbins J. The plasma membrane and thyroid hormone entry into cells. Trends Endocrinol Metab 1989; 1(2):90–94. pmid:18411097
- Hennemann G, Krenning EP. Pitfalls in the interpretation of thyroid function tests in old age and non-thyroidal illness. Horm Res 1987; 26(1–4):100–104. doi:10.1159/000180688
- Baloch Z, Carayon P, Conte-Devolx B, et al; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13(1):3–126. doi:10.1089/105072503321086962
- Lum S, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984; 73(2):570–575. doi:10.1172/JCI111245
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol 2016; 6(3):1387–1428. doi:10.1002/cphy.c150027
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015; 225(3):R67–R81. doi:10.1530/JOE-15-0133
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Teco GN, Mean JF. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3, 5, 3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985; 60(4):666–672. doi:10.1210/jcem-60-4-666
- Peeters RP, Debaveye Y, Fliers E, Visser TJ. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22(1):41–55. doi:10.1016/j.ccc.2005.08.006
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33(8):1391–1396. pmid:3301067
- Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36(3):657–672. doi:10.1016/j.ecl.2007.04.007
- Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97(9):3068–3078. doi:10.1210/jc.2012-1616
- Kidess AI, Caplan RH, Reynertson RH, Wickus GG, Goodnough DE. Transient corticotropin deficiency in critical illness. Mayo Clin Proc 1993; 68(5):435–441. doi:10.1016/s0025-6196(12)60188-8
- Lamberts SW, Bruining HA, De Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337(18):1285–1292. doi:10.1056/NEJM199710303371807
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Duick DS, Warren DW, Nicoloff JT, Otis CL, Croxson MS. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab 1974; 39(6):1151–1154. doi:10.1210/jcem-39-6-1151
- Gamstedt A, Järnerot G, Kågedal B. Dose related effects of betamethasone on iodothyronines and thyroid hormone-binding proteins in serum. Acta Endocrinol (Copenh) 1981; 96(4):484–490. doi:10.1530/acta.0.0960484
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48(11):2096–2103. doi:10.1172/JCI106176
- Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49(10):1922–1929. doi:10.1172/JCI106411
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25):1688–1694. doi:10.1056/NEJM199512213332507
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 2009; 23(6):793–800. doi:10.1016/j.beem.2009.08.003
- Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor–selective ligands. N Engl J Med 1999; 340(14):1075–1079. doi:10.1056/NEJM199904083401404
- Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8(6):581–587. doi:10.1111/j.1365-2125.1979.tb01048.x
- Faber J, Friis T, Kirkegaard C, et al. Serum T4, T3 and reverse T3 during treatment with propranolol in hyperthyroidism, L-T4 treated myxedema and in normal man. Horm Metab Res 1979; 11(1):34–36. doi:10.1055/s-0028-1092678
- Kristensen BO, Weeke J. Propranolol-induced increments in total and free serum thyroxine in patients with essential hypertension. Clin Pharmacol Ther 1977; 22(6):864–867. doi:10.1002/cpt1977226864
- Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3(2):273–277. doi:10.1111/j.1365-2125.1976.tb00603.x
- Lotti G, Delitala G, Devilla L, Alagna S, Masala A. Reduction of plasma triiodothyronine (T3) induced by propranolol. Clin Endocrinol 1977; 6(6):405–410. doi:10.1111/j.1365-2265.1977.tb03322.x
- Perrild H, Hansen JM, Skovsted L, Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf) 1983; 18(2):139–142. pmid:6133659
- Reeves RA, From GL, Paul W, Leenen FH. Nadolol, propranolol, and thyroid hormones: evidence for a membrane-stabilizing action of propranolol. Clin Pharmacol Ther 1985; 37(2):157–161. doi:10.1038/clpt.1985.28
- Walker N, Jung RT, Jennings G, James WP. The effect of a beta-receptor agonist (salbutamol) on peripheral thyroid metabolism in euthyroid subjects. Horm Metab Res 1981; 13(10):590–591. doi:10.1055/s-2007-1019346
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54(2):300–306. doi:10.1210/jcem-54-2-300
- Docter R, Krenning E, De Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993; 39(5):499–518. pmid:8252737
- Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990; 71(3):661–669. doi:10.1210/jcem-71-3-661
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22(1):57–86. doi:10.1016/j.ccc.2005.10.001
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 2009; 94(10):3663–3675. doi:10.1210/jc.2009-0899
- Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99(12):4537–4545. doi:10.1210/jc.2014-2192
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999; 84(4):1311–1323. doi:10.1210/jcem.84.4.5636
- Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 2013; 98(3):1006–1013. doi:10.1210/jc.2012-2809
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011; 10(2):117–124. doi:10.14310/horm.2002.1301
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62(4):717–722. doi:10.1210/jcem-62-4-717
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160(4):503–515. doi:10.1530/EJE-08-0837
- Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc 1988; 63(12):1214–1222. doi:10.1016/s0025-6196(12)65408-1
- Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med 1999; 159(7):658–665. pmid:10218744
- Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013; 98(6):2247–2255. doi:10.1210/jc.2012-4309
A 66-year-old man presented to the emergency department with increasing shortness of breath and productive cough, which had begun 5 days earlier. Three years previously, he had been diagnosed with chronic obstructive pulmonary disease (COPD).
One week before the current presentation, he developed a sore throat, rhinorrhea, and nasal congestion, and the shortness of breath had started 2 days after that. Although he could speak in sentences, he was breathless even at rest. His dyspnea was associated with noisy breathing and cough productive of yellowish sputum; there was no hemoptysis. He reported fever, but he had no chills, night sweats, chest pain, or paroxysmal nocturnal dyspnea. The review of other systems was unremarkable.
His COPD was known to be mild, in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, group A. His postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was less than 0.70, and his FEV1 was 84% of predicted. Apart from mild intermittent cough with white sputum, his COPD had been under good control with inhaled ipratropium 4 times daily and inhaled albuterol as needed. He said he did not have shortness of breath except when hurrying on level ground or walking up a slight hill (Modified Medical Research Council dyspnea scale grade 1; COPD Assessment Test score < 10). In the last 3 years, he had 2 exacerbations of COPD, 1 year apart, both requiring oral prednisone and antibiotic therapy.
Other relevant history included hypertension and dyslipidemia of 15-year duration, for which he was taking candesartan 16 mg twice daily and atorvastatin 20 mg daily. He was compliant with his medications.
Though he usually received an influenza vaccine every year, he did not get it the previous year. Also, 3 years previously, he received the 23-valent pneumococcal polysaccharide vaccine (PPSV23), and the year before that he received the pneumococcal conjugate vaccine (PCV13). In addition, he was immunized against herpes zoster and tetanus.
The patient had smoked 1 pack per day for the past 38 years. His primary care physician had advised him many times to quit smoking. He had enrolled in a smoking cessation program 2 years previously, in which he received varenicline in addition to behavioral counseling in the form of motivational interviewing and a telephone quit-line. Nevertheless, he continued to smoke.
He was a retired engineer. He did not drink alcohol or use illicit drugs.
PHYSICAL EXAMINATION
On physical examination, the patient was sitting up in bed, leaning forward. He was alert and oriented but was breathing rapidly and looked sick. He had no cyanosis, clubbing, pallor, or jaundice. His blood pressure was 145/90 mm Hg, heart rate 110 beats per minute and regular, respiratory rate 29 breaths per minute, and oral temperature 38.1°C (100.6°F). His oxygen saturation was 88% while breathing room air. His body mass index was 27.1 kg/m2.
His throat was mildly congested. His neck veins were flat, and there were no carotid bruits. His thyroid examination was normal, without goiter, nodules, or tenderness.
Intercostal retractions were noted around the anterolateral costal margins. He had no chest wall deformities. Chest expansion was reduced bilaterally. There was hyperresonance bilaterally. Expiratory wheezes were heard over both lungs, without crackles.
His heart had no murmurs or added sounds. There was no lower-limb edema or swelling. The rest of his physical examination was unremarkable.
Results of initial laboratory testing are shown in Table 1.
Assessment: A 66-year-old man with GOLD grade 1, group A COPD, presenting with a severe exacerbation, most likely due to viral bronchitis.
INITIAL MANAGEMENT
The patient was given oxygen 28% by Venturi mask, and his oxygen saturation went up to 90%. He was started on nebulized albuterol 2.5 mg with ipratropium bromide 500 µg every 4 hours, prednisone 40 mg orally daily for 5 days, and ceftriaxone 1 g intravenously every 24 hours. The first dose of each medication was given in the emergency department.
The patient was then admitted to a progressive care unit, where he was placed on noninvasive positive pressure ventilation, continuous cardiac monitoring, and pulse oximetry. He was started on enoxaparin 40 mg subcutaneously daily to prevent venous thromboembolism, and the oral medications he had been taking at home were continued. Because he was receiving a glucocorticoid, his blood glucose was monitored in the fasting state, 2 hours after each meal, and as needed.
Two hours after he started noninvasive positive pressure ventilation, his arterial blood gases were remeasured and showed the following results:
- pH 7.35
- Partial pressure of carbon dioxide (Paco2) 52 mm Hg
- Bicarbonate 28 mmol/L
- Partial pressure of oxygen (Pao2) 60 mm Hg
- Oxygen saturation 90%.
HOSPITAL COURSE
On hospital day 3, his dyspnea had slightly improved. His respiratory rate was 26 to 28 breaths per minute. His oxygen saturation remained between 90% and 92%.
At 10:21 pm, his cardiac monitor showed an episode of focal atrial tachycardia at a rate of 129 beats per minute that lasted for 3 minutes and 21 seconds, terminating spontaneously. He denied any change in his clinical condition during the episode, with no chest pain, palpitation, or change in dyspnea. There was no change in his vital signs. He had another similar asymptomatic episode lasting 4 minutes and 9 seconds at 6:30 am of hospital day 4.
Because of these episodes, the attending physician ordered thyroid function tests.
THYROID FUNCTION TESTING
1. Which thyroid function test is most likely to be helpful in the assessment of this patient’s thyroid status?
- Serum thyroid-stimulating hormone (TSH) alone
- Serum TSH and total thyroxine (T4)
- Serum TSH and total triiodothyronine (T3)
- Serum TSH and free T4
- Serum TSH and free T3
There are several tests to assess thyroid function: the serum TSH, total T4, free T4, total T3, and free T3 concentrations.1
In normal physiology, TSH from the pituitary stimulates the thyroid gland to produce and secrete T4 and T3, which in turn inhibit TSH secretion through negative feedback. A negative log-linear relation exists between serum free T4 and TSH levels.2 Thus, the serum free T4 level can remain within the normal reference range even if the TSH level is high or low.
TSH assays can have different detection limits. A third-generation TSH assay with a detection limit of 0.01 mU/L is recommended for use in clinical practice.3
TSH testing alone. Given its superior sensitivity and specificity, serum TSH measurement is considered the best single test for assessing thyroid function in most cases.4 Nevertheless, measurement of the serum TSH level alone could be misleading in several situations, eg, hypothalamic or pituitary disorders, recent treatment of thyrotoxicosis, impaired sensitivity to thyroid hormone, and acute nonthyroidal illness.4
Free vs total T4 and T3 levels
Serum total T4 includes a fraction that is bound, mainly to thyroxin-binding globulin, and a very small unbound (free) fraction. The same applies to T3. Only free thyroid hormones represent the “active” fraction available for interaction with their protein receptors in the nucleus.8 Patients with conditions that can affect the thyroid-binding protein concentrations usually have altered serum total T4 and T3 levels, whereas their free hormone concentrations remain normal. Accordingly, measurement of free hormone levels, especially free T4, is usually recommended.
Although equilibrium dialysis is the method most likely to provide an accurate serum free T4 measurement, it is not commonly used because of its limited availability and high cost. Thus, most commercial laboratories use “direct” free T4 measurement or, to a lesser degree, the free T4 index.9 However, none of the currently available free T4 tests actually measure free T4 directly; rather, they estimate it.10
Commercial laboratories can provide a direct free T3 estimate, but it may be less reliable than total T3. If serum T3 measurement is indicated, serum total T3 is usually measured. However, total T3 measurement is rarely indicated for patients with hypothyroidism because it usually remains within the normal reference range.11 Nevertheless, serum total T3 measurement could be useful in patients with T3 toxicosis and in those who are acutely ill.
Accordingly, in acutely ill hospitalized patients like ours, measuring serum TSH using a third-generation assay and free T4 is essential to assess thyroid function. Many clinicians also measure serum total T3.
CASE CONTINUED: LOW TSH, LOW-NORMAL FREE T4, LOW TOTAL T3
The attending physician ordered serum TSH, free T4, and total T3 measurements, which yielded the following:
- TSH 0.1 mU/L (0.5–5.0)
- Total T3 55 ng/dL (80–180)
- Free T4 0.9 ng/dL (0.9–2.4).
2. Which best explains this patient’s abnormal thyroid test results?
- His acute illness
- Central hypothyroidism due to pituitary infarction
- His albuterol therapy
- Subclinical thyrotoxicosis
- Hashimoto thyroiditis
Since euthyroid patients with an acute illness may have abnormal thyroid test results (Table 2),5–7 thyroid function testing is not recommended unless there is a strong indication for it, such as new-onset atrial fibrillation, atrial flutter, or focal atrial tachycardia.1 In such patients, it is important to know whether the test abnormalities represent true thyroid disorder or are the result of a nonthyroidal illness.
Thyroid function testing in patients with nonthyroidal illness usually shows low serum total T3, normal or low serum TSH, and normal, low, or high serum free T4. However, transient mild serum TSH elevation can be seen in some patients during the recovery period.16 These abnormalities with their mechanisms are shown in Table 2.5–7 In several commercial kits, serum direct free T4 can be falsely decreased or increased.8
THE DIFFERENTIAL DIAGNOSIS
Our patient had low serum TSH, low-normal serum direct free T4, and low serum total T3. This profile could be caused by a nonthyroidal illness, “true” central hypothyroidism, or his glucocorticoid treatment. The reason we use the term “true” in this setting is that some experts suggest that the thyroid function test abnormalities in patients with acute nonthyroidal illness represent a transient central hypothyroidism.17 The clinical presentation is key in differentiating true central hypothyroidism from nonthyroidal illness.
In addition, measuring serum cortisol may help to differentiate between the 2 states, as it would be elevated in patients with nonthyroidal illness as part of a stress response but low in patients with true central hypothyroidism, since it is usually part of combined pituitary hormone deficiency.18 Of note, some critically ill patients have low serum cortisol because of transient central adrenal insufficiency.19,20
The serum concentration of reverse T3 has been suggested as a way to differentiate between hypothyroidism (low) and nonthyroidal illness (high); however, further studies showed that it does not reliably differentiate between the conditions.21
GLUCOCORTICOIDS AND THYROID FUNCTION TESTS
By inhibiting D1, glucocorticoids can decrease peripheral conversion of T4 to T3 and thus decrease serum total T3. This effect depends on the type and dose of the glucocorticoid and the duration of therapy.
In one study,22 there was a significant reduction in serum total T3 concentration 24 hours after a single oral dose of dexamethasone 12 mg in normal participants. This effect lasted 48 hours, after which serum total T3 returned to its pretreatment level.
In another study,23 a daily oral dose of betamethasone 1.5 mg for 5 days did not significantly reduce the serum total T3 in healthy volunteers, but a daily dose of 3 mg did. This effect was more pronounced at a daily dose of 4.5 mg, whereas a dose of 6.0 mg had no further effect.
Long-term glucocorticoid therapy also decreases serum total T4 and total T3 by lowering serum thyroid-binding globulin.24
Finally, glucocorticoids can decrease TSH secretion by directly inhibiting thyrotropin-releasing hormone.25,26 However, chronic hypercortisolism, whether endogenous or exogenous, does not cause clinically central hypothyroidism, possibly because of the negative feedback mechanism of low thyroid hormones on the pituitary and the hypothalamus.27
Other drugs including dopamine, dopamine agonists, dobutamine, and somatostatin analogues can suppress serum TSH. As with glucocorticoids, these drugs do not cause clinically evident central hypothyroidism.28 Bexarotene, a retinoid X receptor ligand used in the treatment of cutaneous T-cell lymphoma, has been reported to cause clinically evident central hypothyroidism by suppressing TSH and increasing T4 clearance.29
BETA-BLOCKERS, BETA-AGONISTS AND THYROID FUNCTION
While there is general agreement that beta-adrenergic antagonists (beta-blockers) do not affect the serum TSH concentration, conflicting data have been reported concerning their effect on other thyroid function tests. This may be due to several factors, including dose, duration of therapy, the patient’s thyroid status, and differences in laboratory methodology.30
In studies of propranolol, serum total T4 concentrations did not change or were increased with daily doses of 160 mg or more in both euthyroid participants and hyperthyroid patients31–33; serum total T3 concentrations did not change or were decreased with 40 mg or more daily34; and serum reverse T3 concentrations were increased with daily doses of 80 mg or more.31 It is most likely that propranolol exerts these changes by inhibiting D1 activity in peripheral tissues.
Furthermore, a significant decrease in serum total T3 concentrations was observed in hyperthyroid patients treated with atenolol 100 mg daily, metoprolol 100 mg daily, and alprenolol 100 mg daily, but not with sotalol 80 mg daily or nadolol (up to 240 mg daily).35,36
On the other hand, beta-adrenergic agonists have not been reported to cause significant changes in thyroid function tests.37
SUBCLINICAL THYROTOXICOSIS OR HASHIMOTO THYROIDITIS?
Our patient’s thyroid function test results are more likely due to his nonthyroidal illness and glucocorticoid therapy, as there is no clinical evidence to point to a hypothalamic-pituitary disorder accounting for true central hypothyroidism.
The other options mentioned in question 2 are unlikely to explain our patient’s thyroid function test results.
Subclinical thyrotoxicosis is characterized by suppressed serum TSH, but both serum free T4 and total T3 remain within the normal reference ranges. In addition, the serum TSH level may help to differentiate between thyrotoxicosis and nonthyroidal illness. In the former, serum TSH is usually suppressed (< 0.01 mU/L), whereas in the latter it is usually low but detectable (0.05– 0.3 mU/L).38,39
Hashimoto thyroiditis is a chronic autoimmune thyroid disease characterized by diffuse lymphocytic infiltration of the thyroid gland. Almost all patients with Hashimoto thyroiditis have elevated levels of antibodies to thyroid peroxidase or thyroglobulin.40 Clinically, patients with Hashimoto thyroiditis can either be hypothyroid or have normal thyroid function, which is not the case in our patient.
CASE CONTINUED
An endocrinologist, consulted for a second opinion, agreed that the patient’s thyroid function test results were most likely due to his nonthyroidal illness and glucocorticoid therapy.
3. In view of the endocrinologist’s opinion, which should be the next step in the management of the patient’s thyroid condition?
- Start levothyroxine (T4) therapy
- Start liothyronine (T3) therapy
- Start N-acetylcysteine therapy
- Start thyrotropin-releasing hormone therapy
- Remeasure thyroid hormones after full recovery from his acute illness
It is not clear whether the changes in thyroid hormone levels during an acute illness are a pathologic alteration for which thyroid hormone therapy may be beneficial, or a physiologic adaptation for which such therapy would not be indicated.41
However, current data argue against thyroid hormone therapy using T4 or T3 for patients with nonthyroidal illness syndrome (also called euthyroid sick syndrome).42 Indeed, several randomized controlled trials showed that thyroid hormone therapy is not beneficial in such patients and may be detrimental.41,43
Therapies other than thyroid hormone have been investigated to ameliorate thyroid hormone abnormalities in patients with nonthyroidal illness. These include N-acetylcysteine, thyrotropin-releasing hormone therapy, and nutritional support.
Some studies showed that giving N-acetylcysteine, an antioxidant, increased serum T3 and decreased serum reverse T3 concentrations in patients with acute myocardial infarction.44 Nevertheless, the mortality rate and length of hospitalization were not affected. Further studies are needed to know whether N-acetylcysteine therapy is beneficial for such patients.
Similarly, a study using a thyrotropin-releasing hormone analogue along with growth hormone-releasing peptide 2 showed an increase in serum TSH, T4, and T3 levels in critically ill patients.45 The benefit of this therapy has yet to be determined. On the other hand, early nutritional support was reported to prevent thyroid hormonal changes in patients postoperatively.46
Measuring thyroid hormone levels after full recovery is the most appropriate next step in our patient, as the changes in thyroid hormone concentrations subside as the acute illness resolves.47
CASE CONTINUED
The patient continued to improve. On hospital day 6, he was feeling better but still had mild respiratory distress. There had been no further episodes of arrhythmia since day 4. His blood pressure was 136/86 mm Hg, heart rate 88 beats per minute and regular, respiratory rate 18 breaths per minute, and oral temperature 37.1°C. His oxygen saturation was 92% on room air.
Before discharge, he was encouraged to quit smoking. He was offered behavioral counseling and medication therapy, but he only said that he would think about it. He was discharged on oral cefixime for 4 more days and was instructed to switch to a long-acting bronchodilator along with his other home medications and to return in 1 week to have his thyroid hormones checked.
One week later, his laboratory results were:
- TSH 11.2 mU/L (reference range 0.5–5.0)
- Free T4 1.2 ng/dL (0.9–2.4)
- Total T3 92 ng/dL (80–180).
Clinically, the patient was euthyroid, and examination of his thyroid was unremarkable.
4. Based on these last test results, which statement is correct?
- Levothyroxine therapy should be started
- His serum TSH elevation is most likely transient
- Thyroid ultrasonography is strongly indicated
- A radioactive iodine uptake study should be performed
- Measurement of thyroid-stimulating immunoglobulins is indicated
During recovery from nonthyroidal illness, some patients may have elevated serum TSH levels that are usually transient and modest (< 20 mU/L).48 Normalization of the thyroid function tests including serum TSH may take several weeks49 or months.50 However, a systematic review found that the likelihood of permanent primary hypothyroidism is high in patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness.51
Ultrasonography is useful for evaluating patients with thyroid nodules or goiter but is of little benefit for patients like ours, in whom the thyroid is normal on examination.
Similarly, a radioactive iodine uptake study is not indicated, as it is principally used to help differentiate between types of thyrotoxicosis. (Radioactive iodine is also used to treat differentiated thyroid cancer.)
Thyroid-stimulating immunoglobins are TSH receptor-stimulating antibodies that cause Graves disease. Nevertheless, measuring them is not routinely indicated for its diagnosis. However, their measurement is of significant help in the diagnosis of Graves disease if a radioactive iodine uptake study cannot be performed (as in pregnancy) and in atypical presentations such as euthyroid Graves ophthalmopathy.52 Other indications for thyroid-stimulating immunoglobin measurement are beyond the scope of the article. Our patient’s test results are not consistent with hyperthyroidism, so measuring thyroid-stimulating immunoglobins is not indicated.
CASE CONCLUSION: BETTER, BUT STILL SMOKING
The patient missed his 1-month clinic follow-up, but he visited the clinic for follow-up 3 months later. He was feeling well with no complaints. Test results including serum TSH, free T4, and total T3 were within normal ranges. His COPD was under control, with an FEV1 88% of predicted.
He was again encouraged to quit smoking and was offered drug therapy and behavioral counseling, but he declined. In addition, he was instructed to adhere to his annual influenza vaccination.
KEY POINTS
- In patients with acute illness, it is recommended that thyroid function not be assessed unless there is a strong indication.
- If thyroid function assessment is indicated for critically ill patients, serum TSH and free T4 concentrations should be measured. Some clinicians also measure serum total T3 level.
- Thyroid function testing in critically ill patients usually shows low serum total T3, normal or low serum TSH, and normal or low serum free T4.
- Many drugs can alter thyroid hormone levels.
- Thyroid hormone therapy is not recommended for critically ill patients with low T3, low T4, or both.
- During recovery from nonthyroidal illness, some patients may have mild elevation in serum TSH levels (< 20 mU/L).
- Thyroid hormone levels may take several weeks or months to return to normal after the acute illness.
- Patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness are more likely to have permanent primary hypothyroidism.
A 66-year-old man presented to the emergency department with increasing shortness of breath and productive cough, which had begun 5 days earlier. Three years previously, he had been diagnosed with chronic obstructive pulmonary disease (COPD).
One week before the current presentation, he developed a sore throat, rhinorrhea, and nasal congestion, and the shortness of breath had started 2 days after that. Although he could speak in sentences, he was breathless even at rest. His dyspnea was associated with noisy breathing and cough productive of yellowish sputum; there was no hemoptysis. He reported fever, but he had no chills, night sweats, chest pain, or paroxysmal nocturnal dyspnea. The review of other systems was unremarkable.
His COPD was known to be mild, in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade 1, group A. His postbronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) was less than 0.70, and his FEV1 was 84% of predicted. Apart from mild intermittent cough with white sputum, his COPD had been under good control with inhaled ipratropium 4 times daily and inhaled albuterol as needed. He said he did not have shortness of breath except when hurrying on level ground or walking up a slight hill (Modified Medical Research Council dyspnea scale grade 1; COPD Assessment Test score < 10). In the last 3 years, he had 2 exacerbations of COPD, 1 year apart, both requiring oral prednisone and antibiotic therapy.
Other relevant history included hypertension and dyslipidemia of 15-year duration, for which he was taking candesartan 16 mg twice daily and atorvastatin 20 mg daily. He was compliant with his medications.
Though he usually received an influenza vaccine every year, he did not get it the previous year. Also, 3 years previously, he received the 23-valent pneumococcal polysaccharide vaccine (PPSV23), and the year before that he received the pneumococcal conjugate vaccine (PCV13). In addition, he was immunized against herpes zoster and tetanus.
The patient had smoked 1 pack per day for the past 38 years. His primary care physician had advised him many times to quit smoking. He had enrolled in a smoking cessation program 2 years previously, in which he received varenicline in addition to behavioral counseling in the form of motivational interviewing and a telephone quit-line. Nevertheless, he continued to smoke.
He was a retired engineer. He did not drink alcohol or use illicit drugs.
PHYSICAL EXAMINATION
On physical examination, the patient was sitting up in bed, leaning forward. He was alert and oriented but was breathing rapidly and looked sick. He had no cyanosis, clubbing, pallor, or jaundice. His blood pressure was 145/90 mm Hg, heart rate 110 beats per minute and regular, respiratory rate 29 breaths per minute, and oral temperature 38.1°C (100.6°F). His oxygen saturation was 88% while breathing room air. His body mass index was 27.1 kg/m2.
His throat was mildly congested. His neck veins were flat, and there were no carotid bruits. His thyroid examination was normal, without goiter, nodules, or tenderness.
Intercostal retractions were noted around the anterolateral costal margins. He had no chest wall deformities. Chest expansion was reduced bilaterally. There was hyperresonance bilaterally. Expiratory wheezes were heard over both lungs, without crackles.
His heart had no murmurs or added sounds. There was no lower-limb edema or swelling. The rest of his physical examination was unremarkable.
Results of initial laboratory testing are shown in Table 1.
Assessment: A 66-year-old man with GOLD grade 1, group A COPD, presenting with a severe exacerbation, most likely due to viral bronchitis.
INITIAL MANAGEMENT
The patient was given oxygen 28% by Venturi mask, and his oxygen saturation went up to 90%. He was started on nebulized albuterol 2.5 mg with ipratropium bromide 500 µg every 4 hours, prednisone 40 mg orally daily for 5 days, and ceftriaxone 1 g intravenously every 24 hours. The first dose of each medication was given in the emergency department.
The patient was then admitted to a progressive care unit, where he was placed on noninvasive positive pressure ventilation, continuous cardiac monitoring, and pulse oximetry. He was started on enoxaparin 40 mg subcutaneously daily to prevent venous thromboembolism, and the oral medications he had been taking at home were continued. Because he was receiving a glucocorticoid, his blood glucose was monitored in the fasting state, 2 hours after each meal, and as needed.
Two hours after he started noninvasive positive pressure ventilation, his arterial blood gases were remeasured and showed the following results:
- pH 7.35
- Partial pressure of carbon dioxide (Paco2) 52 mm Hg
- Bicarbonate 28 mmol/L
- Partial pressure of oxygen (Pao2) 60 mm Hg
- Oxygen saturation 90%.
HOSPITAL COURSE
On hospital day 3, his dyspnea had slightly improved. His respiratory rate was 26 to 28 breaths per minute. His oxygen saturation remained between 90% and 92%.
At 10:21 pm, his cardiac monitor showed an episode of focal atrial tachycardia at a rate of 129 beats per minute that lasted for 3 minutes and 21 seconds, terminating spontaneously. He denied any change in his clinical condition during the episode, with no chest pain, palpitation, or change in dyspnea. There was no change in his vital signs. He had another similar asymptomatic episode lasting 4 minutes and 9 seconds at 6:30 am of hospital day 4.
Because of these episodes, the attending physician ordered thyroid function tests.
THYROID FUNCTION TESTING
1. Which thyroid function test is most likely to be helpful in the assessment of this patient’s thyroid status?
- Serum thyroid-stimulating hormone (TSH) alone
- Serum TSH and total thyroxine (T4)
- Serum TSH and total triiodothyronine (T3)
- Serum TSH and free T4
- Serum TSH and free T3
There are several tests to assess thyroid function: the serum TSH, total T4, free T4, total T3, and free T3 concentrations.1
In normal physiology, TSH from the pituitary stimulates the thyroid gland to produce and secrete T4 and T3, which in turn inhibit TSH secretion through negative feedback. A negative log-linear relation exists between serum free T4 and TSH levels.2 Thus, the serum free T4 level can remain within the normal reference range even if the TSH level is high or low.
TSH assays can have different detection limits. A third-generation TSH assay with a detection limit of 0.01 mU/L is recommended for use in clinical practice.3
TSH testing alone. Given its superior sensitivity and specificity, serum TSH measurement is considered the best single test for assessing thyroid function in most cases.4 Nevertheless, measurement of the serum TSH level alone could be misleading in several situations, eg, hypothalamic or pituitary disorders, recent treatment of thyrotoxicosis, impaired sensitivity to thyroid hormone, and acute nonthyroidal illness.4
Free vs total T4 and T3 levels
Serum total T4 includes a fraction that is bound, mainly to thyroxin-binding globulin, and a very small unbound (free) fraction. The same applies to T3. Only free thyroid hormones represent the “active” fraction available for interaction with their protein receptors in the nucleus.8 Patients with conditions that can affect the thyroid-binding protein concentrations usually have altered serum total T4 and T3 levels, whereas their free hormone concentrations remain normal. Accordingly, measurement of free hormone levels, especially free T4, is usually recommended.
Although equilibrium dialysis is the method most likely to provide an accurate serum free T4 measurement, it is not commonly used because of its limited availability and high cost. Thus, most commercial laboratories use “direct” free T4 measurement or, to a lesser degree, the free T4 index.9 However, none of the currently available free T4 tests actually measure free T4 directly; rather, they estimate it.10
Commercial laboratories can provide a direct free T3 estimate, but it may be less reliable than total T3. If serum T3 measurement is indicated, serum total T3 is usually measured. However, total T3 measurement is rarely indicated for patients with hypothyroidism because it usually remains within the normal reference range.11 Nevertheless, serum total T3 measurement could be useful in patients with T3 toxicosis and in those who are acutely ill.
Accordingly, in acutely ill hospitalized patients like ours, measuring serum TSH using a third-generation assay and free T4 is essential to assess thyroid function. Many clinicians also measure serum total T3.
CASE CONTINUED: LOW TSH, LOW-NORMAL FREE T4, LOW TOTAL T3
The attending physician ordered serum TSH, free T4, and total T3 measurements, which yielded the following:
- TSH 0.1 mU/L (0.5–5.0)
- Total T3 55 ng/dL (80–180)
- Free T4 0.9 ng/dL (0.9–2.4).
2. Which best explains this patient’s abnormal thyroid test results?
- His acute illness
- Central hypothyroidism due to pituitary infarction
- His albuterol therapy
- Subclinical thyrotoxicosis
- Hashimoto thyroiditis
Since euthyroid patients with an acute illness may have abnormal thyroid test results (Table 2),5–7 thyroid function testing is not recommended unless there is a strong indication for it, such as new-onset atrial fibrillation, atrial flutter, or focal atrial tachycardia.1 In such patients, it is important to know whether the test abnormalities represent true thyroid disorder or are the result of a nonthyroidal illness.
Thyroid function testing in patients with nonthyroidal illness usually shows low serum total T3, normal or low serum TSH, and normal, low, or high serum free T4. However, transient mild serum TSH elevation can be seen in some patients during the recovery period.16 These abnormalities with their mechanisms are shown in Table 2.5–7 In several commercial kits, serum direct free T4 can be falsely decreased or increased.8
THE DIFFERENTIAL DIAGNOSIS
Our patient had low serum TSH, low-normal serum direct free T4, and low serum total T3. This profile could be caused by a nonthyroidal illness, “true” central hypothyroidism, or his glucocorticoid treatment. The reason we use the term “true” in this setting is that some experts suggest that the thyroid function test abnormalities in patients with acute nonthyroidal illness represent a transient central hypothyroidism.17 The clinical presentation is key in differentiating true central hypothyroidism from nonthyroidal illness.
In addition, measuring serum cortisol may help to differentiate between the 2 states, as it would be elevated in patients with nonthyroidal illness as part of a stress response but low in patients with true central hypothyroidism, since it is usually part of combined pituitary hormone deficiency.18 Of note, some critically ill patients have low serum cortisol because of transient central adrenal insufficiency.19,20
The serum concentration of reverse T3 has been suggested as a way to differentiate between hypothyroidism (low) and nonthyroidal illness (high); however, further studies showed that it does not reliably differentiate between the conditions.21
GLUCOCORTICOIDS AND THYROID FUNCTION TESTS
By inhibiting D1, glucocorticoids can decrease peripheral conversion of T4 to T3 and thus decrease serum total T3. This effect depends on the type and dose of the glucocorticoid and the duration of therapy.
In one study,22 there was a significant reduction in serum total T3 concentration 24 hours after a single oral dose of dexamethasone 12 mg in normal participants. This effect lasted 48 hours, after which serum total T3 returned to its pretreatment level.
In another study,23 a daily oral dose of betamethasone 1.5 mg for 5 days did not significantly reduce the serum total T3 in healthy volunteers, but a daily dose of 3 mg did. This effect was more pronounced at a daily dose of 4.5 mg, whereas a dose of 6.0 mg had no further effect.
Long-term glucocorticoid therapy also decreases serum total T4 and total T3 by lowering serum thyroid-binding globulin.24
Finally, glucocorticoids can decrease TSH secretion by directly inhibiting thyrotropin-releasing hormone.25,26 However, chronic hypercortisolism, whether endogenous or exogenous, does not cause clinically central hypothyroidism, possibly because of the negative feedback mechanism of low thyroid hormones on the pituitary and the hypothalamus.27
Other drugs including dopamine, dopamine agonists, dobutamine, and somatostatin analogues can suppress serum TSH. As with glucocorticoids, these drugs do not cause clinically evident central hypothyroidism.28 Bexarotene, a retinoid X receptor ligand used in the treatment of cutaneous T-cell lymphoma, has been reported to cause clinically evident central hypothyroidism by suppressing TSH and increasing T4 clearance.29
BETA-BLOCKERS, BETA-AGONISTS AND THYROID FUNCTION
While there is general agreement that beta-adrenergic antagonists (beta-blockers) do not affect the serum TSH concentration, conflicting data have been reported concerning their effect on other thyroid function tests. This may be due to several factors, including dose, duration of therapy, the patient’s thyroid status, and differences in laboratory methodology.30
In studies of propranolol, serum total T4 concentrations did not change or were increased with daily doses of 160 mg or more in both euthyroid participants and hyperthyroid patients31–33; serum total T3 concentrations did not change or were decreased with 40 mg or more daily34; and serum reverse T3 concentrations were increased with daily doses of 80 mg or more.31 It is most likely that propranolol exerts these changes by inhibiting D1 activity in peripheral tissues.
Furthermore, a significant decrease in serum total T3 concentrations was observed in hyperthyroid patients treated with atenolol 100 mg daily, metoprolol 100 mg daily, and alprenolol 100 mg daily, but not with sotalol 80 mg daily or nadolol (up to 240 mg daily).35,36
On the other hand, beta-adrenergic agonists have not been reported to cause significant changes in thyroid function tests.37
SUBCLINICAL THYROTOXICOSIS OR HASHIMOTO THYROIDITIS?
Our patient’s thyroid function test results are more likely due to his nonthyroidal illness and glucocorticoid therapy, as there is no clinical evidence to point to a hypothalamic-pituitary disorder accounting for true central hypothyroidism.
The other options mentioned in question 2 are unlikely to explain our patient’s thyroid function test results.
Subclinical thyrotoxicosis is characterized by suppressed serum TSH, but both serum free T4 and total T3 remain within the normal reference ranges. In addition, the serum TSH level may help to differentiate between thyrotoxicosis and nonthyroidal illness. In the former, serum TSH is usually suppressed (< 0.01 mU/L), whereas in the latter it is usually low but detectable (0.05– 0.3 mU/L).38,39
Hashimoto thyroiditis is a chronic autoimmune thyroid disease characterized by diffuse lymphocytic infiltration of the thyroid gland. Almost all patients with Hashimoto thyroiditis have elevated levels of antibodies to thyroid peroxidase or thyroglobulin.40 Clinically, patients with Hashimoto thyroiditis can either be hypothyroid or have normal thyroid function, which is not the case in our patient.
CASE CONTINUED
An endocrinologist, consulted for a second opinion, agreed that the patient’s thyroid function test results were most likely due to his nonthyroidal illness and glucocorticoid therapy.
3. In view of the endocrinologist’s opinion, which should be the next step in the management of the patient’s thyroid condition?
- Start levothyroxine (T4) therapy
- Start liothyronine (T3) therapy
- Start N-acetylcysteine therapy
- Start thyrotropin-releasing hormone therapy
- Remeasure thyroid hormones after full recovery from his acute illness
It is not clear whether the changes in thyroid hormone levels during an acute illness are a pathologic alteration for which thyroid hormone therapy may be beneficial, or a physiologic adaptation for which such therapy would not be indicated.41
However, current data argue against thyroid hormone therapy using T4 or T3 for patients with nonthyroidal illness syndrome (also called euthyroid sick syndrome).42 Indeed, several randomized controlled trials showed that thyroid hormone therapy is not beneficial in such patients and may be detrimental.41,43
Therapies other than thyroid hormone have been investigated to ameliorate thyroid hormone abnormalities in patients with nonthyroidal illness. These include N-acetylcysteine, thyrotropin-releasing hormone therapy, and nutritional support.
Some studies showed that giving N-acetylcysteine, an antioxidant, increased serum T3 and decreased serum reverse T3 concentrations in patients with acute myocardial infarction.44 Nevertheless, the mortality rate and length of hospitalization were not affected. Further studies are needed to know whether N-acetylcysteine therapy is beneficial for such patients.
Similarly, a study using a thyrotropin-releasing hormone analogue along with growth hormone-releasing peptide 2 showed an increase in serum TSH, T4, and T3 levels in critically ill patients.45 The benefit of this therapy has yet to be determined. On the other hand, early nutritional support was reported to prevent thyroid hormonal changes in patients postoperatively.46
Measuring thyroid hormone levels after full recovery is the most appropriate next step in our patient, as the changes in thyroid hormone concentrations subside as the acute illness resolves.47
CASE CONTINUED
The patient continued to improve. On hospital day 6, he was feeling better but still had mild respiratory distress. There had been no further episodes of arrhythmia since day 4. His blood pressure was 136/86 mm Hg, heart rate 88 beats per minute and regular, respiratory rate 18 breaths per minute, and oral temperature 37.1°C. His oxygen saturation was 92% on room air.
Before discharge, he was encouraged to quit smoking. He was offered behavioral counseling and medication therapy, but he only said that he would think about it. He was discharged on oral cefixime for 4 more days and was instructed to switch to a long-acting bronchodilator along with his other home medications and to return in 1 week to have his thyroid hormones checked.
One week later, his laboratory results were:
- TSH 11.2 mU/L (reference range 0.5–5.0)
- Free T4 1.2 ng/dL (0.9–2.4)
- Total T3 92 ng/dL (80–180).
Clinically, the patient was euthyroid, and examination of his thyroid was unremarkable.
4. Based on these last test results, which statement is correct?
- Levothyroxine therapy should be started
- His serum TSH elevation is most likely transient
- Thyroid ultrasonography is strongly indicated
- A radioactive iodine uptake study should be performed
- Measurement of thyroid-stimulating immunoglobulins is indicated
During recovery from nonthyroidal illness, some patients may have elevated serum TSH levels that are usually transient and modest (< 20 mU/L).48 Normalization of the thyroid function tests including serum TSH may take several weeks49 or months.50 However, a systematic review found that the likelihood of permanent primary hypothyroidism is high in patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness.51
Ultrasonography is useful for evaluating patients with thyroid nodules or goiter but is of little benefit for patients like ours, in whom the thyroid is normal on examination.
Similarly, a radioactive iodine uptake study is not indicated, as it is principally used to help differentiate between types of thyrotoxicosis. (Radioactive iodine is also used to treat differentiated thyroid cancer.)
Thyroid-stimulating immunoglobins are TSH receptor-stimulating antibodies that cause Graves disease. Nevertheless, measuring them is not routinely indicated for its diagnosis. However, their measurement is of significant help in the diagnosis of Graves disease if a radioactive iodine uptake study cannot be performed (as in pregnancy) and in atypical presentations such as euthyroid Graves ophthalmopathy.52 Other indications for thyroid-stimulating immunoglobin measurement are beyond the scope of the article. Our patient’s test results are not consistent with hyperthyroidism, so measuring thyroid-stimulating immunoglobins is not indicated.
CASE CONCLUSION: BETTER, BUT STILL SMOKING
The patient missed his 1-month clinic follow-up, but he visited the clinic for follow-up 3 months later. He was feeling well with no complaints. Test results including serum TSH, free T4, and total T3 were within normal ranges. His COPD was under control, with an FEV1 88% of predicted.
He was again encouraged to quit smoking and was offered drug therapy and behavioral counseling, but he declined. In addition, he was instructed to adhere to his annual influenza vaccination.
KEY POINTS
- In patients with acute illness, it is recommended that thyroid function not be assessed unless there is a strong indication.
- If thyroid function assessment is indicated for critically ill patients, serum TSH and free T4 concentrations should be measured. Some clinicians also measure serum total T3 level.
- Thyroid function testing in critically ill patients usually shows low serum total T3, normal or low serum TSH, and normal or low serum free T4.
- Many drugs can alter thyroid hormone levels.
- Thyroid hormone therapy is not recommended for critically ill patients with low T3, low T4, or both.
- During recovery from nonthyroidal illness, some patients may have mild elevation in serum TSH levels (< 20 mU/L).
- Thyroid hormone levels may take several weeks or months to return to normal after the acute illness.
- Patients with serum TSH levels higher than 20 mU/L during the recovery phase of their nonthyroidal illness are more likely to have permanent primary hypothyroidism.
- Lamb EJ, Martin J. Thyroid function tests: often justified in the acutely ill. Ann Clin Biochem 2000; 37(pt 2):158–164. doi:10.1258/0004563001899159
- Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990; 70(2):453–460. doi:10.1210/jcem-70-2-453
- Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989; 69(3):684–688. doi:10.1210/jcem-69-3-684
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27(6):745–762. doi:10.1016/j.beem.2013.10.003
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 2005; 15(8):883–897. doi:10.1089/thy.2005.15.883
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal ilnesses. Ann Intern Med 1983; 98(6):946–957. doi:10.7326/0003-4819-98-6-946
- Chopra IJ, Solomon DH, Hepner HW, Mortenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med 1979; 90(6):905–912. doi:10.7326/0003-4819-90-6-905
- Pontecorvi A, Robbins J. The plasma membrane and thyroid hormone entry into cells. Trends Endocrinol Metab 1989; 1(2):90–94. pmid:18411097
- Hennemann G, Krenning EP. Pitfalls in the interpretation of thyroid function tests in old age and non-thyroidal illness. Horm Res 1987; 26(1–4):100–104. doi:10.1159/000180688
- Baloch Z, Carayon P, Conte-Devolx B, et al; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13(1):3–126. doi:10.1089/105072503321086962
- Lum S, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984; 73(2):570–575. doi:10.1172/JCI111245
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol 2016; 6(3):1387–1428. doi:10.1002/cphy.c150027
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015; 225(3):R67–R81. doi:10.1530/JOE-15-0133
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Teco GN, Mean JF. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3, 5, 3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985; 60(4):666–672. doi:10.1210/jcem-60-4-666
- Peeters RP, Debaveye Y, Fliers E, Visser TJ. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22(1):41–55. doi:10.1016/j.ccc.2005.08.006
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33(8):1391–1396. pmid:3301067
- Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36(3):657–672. doi:10.1016/j.ecl.2007.04.007
- Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97(9):3068–3078. doi:10.1210/jc.2012-1616
- Kidess AI, Caplan RH, Reynertson RH, Wickus GG, Goodnough DE. Transient corticotropin deficiency in critical illness. Mayo Clin Proc 1993; 68(5):435–441. doi:10.1016/s0025-6196(12)60188-8
- Lamberts SW, Bruining HA, De Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337(18):1285–1292. doi:10.1056/NEJM199710303371807
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Duick DS, Warren DW, Nicoloff JT, Otis CL, Croxson MS. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab 1974; 39(6):1151–1154. doi:10.1210/jcem-39-6-1151
- Gamstedt A, Järnerot G, Kågedal B. Dose related effects of betamethasone on iodothyronines and thyroid hormone-binding proteins in serum. Acta Endocrinol (Copenh) 1981; 96(4):484–490. doi:10.1530/acta.0.0960484
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48(11):2096–2103. doi:10.1172/JCI106176
- Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49(10):1922–1929. doi:10.1172/JCI106411
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25):1688–1694. doi:10.1056/NEJM199512213332507
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 2009; 23(6):793–800. doi:10.1016/j.beem.2009.08.003
- Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor–selective ligands. N Engl J Med 1999; 340(14):1075–1079. doi:10.1056/NEJM199904083401404
- Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8(6):581–587. doi:10.1111/j.1365-2125.1979.tb01048.x
- Faber J, Friis T, Kirkegaard C, et al. Serum T4, T3 and reverse T3 during treatment with propranolol in hyperthyroidism, L-T4 treated myxedema and in normal man. Horm Metab Res 1979; 11(1):34–36. doi:10.1055/s-0028-1092678
- Kristensen BO, Weeke J. Propranolol-induced increments in total and free serum thyroxine in patients with essential hypertension. Clin Pharmacol Ther 1977; 22(6):864–867. doi:10.1002/cpt1977226864
- Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3(2):273–277. doi:10.1111/j.1365-2125.1976.tb00603.x
- Lotti G, Delitala G, Devilla L, Alagna S, Masala A. Reduction of plasma triiodothyronine (T3) induced by propranolol. Clin Endocrinol 1977; 6(6):405–410. doi:10.1111/j.1365-2265.1977.tb03322.x
- Perrild H, Hansen JM, Skovsted L, Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf) 1983; 18(2):139–142. pmid:6133659
- Reeves RA, From GL, Paul W, Leenen FH. Nadolol, propranolol, and thyroid hormones: evidence for a membrane-stabilizing action of propranolol. Clin Pharmacol Ther 1985; 37(2):157–161. doi:10.1038/clpt.1985.28
- Walker N, Jung RT, Jennings G, James WP. The effect of a beta-receptor agonist (salbutamol) on peripheral thyroid metabolism in euthyroid subjects. Horm Metab Res 1981; 13(10):590–591. doi:10.1055/s-2007-1019346
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54(2):300–306. doi:10.1210/jcem-54-2-300
- Docter R, Krenning E, De Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993; 39(5):499–518. pmid:8252737
- Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990; 71(3):661–669. doi:10.1210/jcem-71-3-661
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22(1):57–86. doi:10.1016/j.ccc.2005.10.001
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 2009; 94(10):3663–3675. doi:10.1210/jc.2009-0899
- Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99(12):4537–4545. doi:10.1210/jc.2014-2192
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999; 84(4):1311–1323. doi:10.1210/jcem.84.4.5636
- Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 2013; 98(3):1006–1013. doi:10.1210/jc.2012-2809
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011; 10(2):117–124. doi:10.14310/horm.2002.1301
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62(4):717–722. doi:10.1210/jcem-62-4-717
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160(4):503–515. doi:10.1530/EJE-08-0837
- Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc 1988; 63(12):1214–1222. doi:10.1016/s0025-6196(12)65408-1
- Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med 1999; 159(7):658–665. pmid:10218744
- Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013; 98(6):2247–2255. doi:10.1210/jc.2012-4309
- Lamb EJ, Martin J. Thyroid function tests: often justified in the acutely ill. Ann Clin Biochem 2000; 37(pt 2):158–164. doi:10.1258/0004563001899159
- Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990; 70(2):453–460. doi:10.1210/jcem-70-2-453
- Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989; 69(3):684–688. doi:10.1210/jcem-69-3-684
- Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013; 27(6):745–762. doi:10.1016/j.beem.2013.10.003
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 2005; 15(8):883–897. doi:10.1089/thy.2005.15.883
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal ilnesses. Ann Intern Med 1983; 98(6):946–957. doi:10.7326/0003-4819-98-6-946
- Chopra IJ, Solomon DH, Hepner HW, Mortenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med 1979; 90(6):905–912. doi:10.7326/0003-4819-90-6-905
- Pontecorvi A, Robbins J. The plasma membrane and thyroid hormone entry into cells. Trends Endocrinol Metab 1989; 1(2):90–94. pmid:18411097
- Hennemann G, Krenning EP. Pitfalls in the interpretation of thyroid function tests in old age and non-thyroidal illness. Horm Res 1987; 26(1–4):100–104. doi:10.1159/000180688
- Baloch Z, Carayon P, Conte-Devolx B, et al; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13(1):3–126. doi:10.1089/105072503321086962
- Lum S, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984; 73(2):570–575. doi:10.1172/JCI111245
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol 2016; 6(3):1387–1428. doi:10.1002/cphy.c150027
- de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015; 225(3):R67–R81. doi:10.1530/JOE-15-0133
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Teco GN, Mean JF. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3, 5, 3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985; 60(4):666–672. doi:10.1210/jcem-60-4-666
- Peeters RP, Debaveye Y, Fliers E, Visser TJ. Changes within the thyroid axis during critical illness. Crit Care Clin 2006; 22(1):41–55. doi:10.1016/j.ccc.2005.08.006
- Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem 1987; 33(8):1391–1396. pmid:3301067
- Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36(3):657–672. doi:10.1016/j.ecl.2007.04.007
- Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab 2012; 97(9):3068–3078. doi:10.1210/jc.2012-1616
- Kidess AI, Caplan RH, Reynertson RH, Wickus GG, Goodnough DE. Transient corticotropin deficiency in critical illness. Mayo Clin Proc 1993; 68(5):435–441. doi:10.1016/s0025-6196(12)60188-8
- Lamberts SW, Bruining HA, De Jong FH. Corticosteroid therapy in severe illness. N Engl J Med 1997; 337(18):1285–1292. doi:10.1056/NEJM199710303371807
- Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 1995; 5(6):435–441. doi:10.1089/thy.1995.5.435
- Duick DS, Warren DW, Nicoloff JT, Otis CL, Croxson MS. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab 1974; 39(6):1151–1154. doi:10.1210/jcem-39-6-1151
- Gamstedt A, Järnerot G, Kågedal B. Dose related effects of betamethasone on iodothyronines and thyroid hormone-binding proteins in serum. Acta Endocrinol (Copenh) 1981; 96(4):484–490. doi:10.1530/acta.0.0960484
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 1982; 3(2):164–217. doi:10.1210/edrv-3-2-164
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest 1969; 48(11):2096–2103. doi:10.1172/JCI106176
- Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970; 49(10):1922–1929. doi:10.1172/JCI106411
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25):1688–1694. doi:10.1056/NEJM199512213332507
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 2009; 23(6):793–800. doi:10.1016/j.beem.2009.08.003
- Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor–selective ligands. N Engl J Med 1999; 340(14):1075–1079. doi:10.1056/NEJM199904083401404
- Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8(6):581–587. doi:10.1111/j.1365-2125.1979.tb01048.x
- Faber J, Friis T, Kirkegaard C, et al. Serum T4, T3 and reverse T3 during treatment with propranolol in hyperthyroidism, L-T4 treated myxedema and in normal man. Horm Metab Res 1979; 11(1):34–36. doi:10.1055/s-0028-1092678
- Kristensen BO, Weeke J. Propranolol-induced increments in total and free serum thyroxine in patients with essential hypertension. Clin Pharmacol Ther 1977; 22(6):864–867. doi:10.1002/cpt1977226864
- Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3(2):273–277. doi:10.1111/j.1365-2125.1976.tb00603.x
- Lotti G, Delitala G, Devilla L, Alagna S, Masala A. Reduction of plasma triiodothyronine (T3) induced by propranolol. Clin Endocrinol 1977; 6(6):405–410. doi:10.1111/j.1365-2265.1977.tb03322.x
- Perrild H, Hansen JM, Skovsted L, Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clin Endocrinol (Oxf) 1983; 18(2):139–142. pmid:6133659
- Reeves RA, From GL, Paul W, Leenen FH. Nadolol, propranolol, and thyroid hormones: evidence for a membrane-stabilizing action of propranolol. Clin Pharmacol Ther 1985; 37(2):157–161. doi:10.1038/clpt.1985.28
- Walker N, Jung RT, Jennings G, James WP. The effect of a beta-receptor agonist (salbutamol) on peripheral thyroid metabolism in euthyroid subjects. Horm Metab Res 1981; 13(10):590–591. doi:10.1055/s-2007-1019346
- Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab 1982; 54(2):300–306. doi:10.1210/jcem-54-2-300
- Docter R, Krenning E, De Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993; 39(5):499–518. pmid:8252737
- Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990; 71(3):661–669. doi:10.1210/jcem-71-3-661
- De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22(1):57–86. doi:10.1016/j.ccc.2005.10.001
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 2009; 94(10):3663–3675. doi:10.1210/jc.2009-0899
- Vidart J, Wajner SM, Leite RS, et al. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: a randomized clinical trial. J Clin Endocrinol Metab 2014; 99(12):4537–4545. doi:10.1210/jc.2014-2192
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999; 84(4):1311–1323. doi:10.1210/jcem.84.4.5636
- Langouche L, Vander Perre S, Marques M, et al. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 2013; 98(3):1006–1013. doi:10.1210/jc.2012-2809
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011; 10(2):117–124. doi:10.14310/horm.2002.1301
- Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62(4):717–722. doi:10.1210/jcem-62-4-717
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160(4):503–515. doi:10.1530/EJE-08-0837
- Spencer CA. Clinical utility and cost-effectiveness of sensitive thyrotropin assays in ambulatory and hospitalized patients. Mayo Clin Proc 1988; 63(12):1214–1222. doi:10.1016/s0025-6196(12)65408-1
- Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med 1999; 159(7):658–665. pmid:10218744
- Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013; 98(6):2247–2255. doi:10.1210/jc.2012-4309
Cross at the Green, Not in Between
ANSWER
Aside from the usual artifacts from placement on a backboard and from the presence of monitoring devices and wires, the radiograph shows a displaced fracture of the right clavicle. No other significant abnormalities are present.
The patient was ultimately diagnosed with multiple orthopedic injuries, and orthopedics was called to evaluate and manage accordingly.

ANSWER
Aside from the usual artifacts from placement on a backboard and from the presence of monitoring devices and wires, the radiograph shows a displaced fracture of the right clavicle. No other significant abnormalities are present.
The patient was ultimately diagnosed with multiple orthopedic injuries, and orthopedics was called to evaluate and manage accordingly.

ANSWER
Aside from the usual artifacts from placement on a backboard and from the presence of monitoring devices and wires, the radiograph shows a displaced fracture of the right clavicle. No other significant abnormalities are present.
The patient was ultimately diagnosed with multiple orthopedic injuries, and orthopedics was called to evaluate and manage accordingly.
A 25-year-old man is brought to your facility by EMS transport following an accident while riding a bicycle. Witnesses say he was hit by a car when he suddenly tried to cross a busy intersection. They also report that he was not wearing a helmet and that he was thrown off the bike, landing several feet away.
Initial survey reveals a male who is arousable but nonverbal, just moaning and groaning. There are obvious deformities in both lower extremities and the right upper extremity. The patient’s blood pressure is 100/50 mm Hg and his heart rate, 130 beats/min. Pulse oximetry shows his O2 saturation to be 95% with 100% oxygen via nonrebreather mask. His pupils are equal and reactive bilaterally. Heart and lungs sound clear. Abdomen is soft.
While you continue examining the patient and begin your secondary survey, a portable chest radiograph is obtained (shown). What is your impression?
Early infusion of mononuclear cells may benefit stroke patients
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
FROM STEM CELLS
Peanut allergy pill gets thumbs-up from FDA advisory panel
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
FROM THE FDA ALLERGENIC PRODUCTS ADVISORY COMMITTEE MEETING
FDA issues warning for CDK 4/6 inhibitors
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
Older IBD patients are most at risk of postdischarge VTE
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Readmission for VTE in patients with inflammatory bowel diseases most often occurs within 60 days of discharge.
Major finding: The highest readmission risk was in patients between the ages of 66 and 80 (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01).
Study details: A retrospective cohort study of 1,160 IBD patients who had VTE readmissions via 2010-2014 data from the Nationwide Readmissions Database.
Disclosures: The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
Source: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.