User login
Author Q&A: Intravenous Immunoglobulin for Treatment of COVID-19 in Select Patients
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Improving Epistaxis Knowledge and Management Among Nursing Staff
From the University of Chicago Medical Center, Chicago, IL.
Abstract
Background: Epistaxis is a common chief complaint addressed by otolaryngologists. A review of the literature showed that there is a deficit in epistaxis education within the nursing community. Conversations with our nursing colleagues confirmed this unmet demand.
Objective: This quality improvement project aimed to increase general epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds among our nursing staff.
Methods: Data were collected through a survey administered before and after our intervention. The survey tested general epistaxis knowledge and assessed comfort and confidence in stopping epistaxis. Our intervention was an educational session covering pertinent epistaxis etiology and management. Quality improvement principles were used to optimize delivery of the intervention.
Results: A total of 51 nurses participated in the project. After participating in the in-service educational session, nurses answered significantly more epistaxis general knowledge questions correctly (mean [SD] difference, 2.07 [1.10] questions; 95% CI, 1.74-2.39; P < .001). There was no statistically significant difference in additional correct questions when stratified by clinical experience or clinical setting (P = .128 and P = 0.446, respectively). Nurses also reported feeling significantly more comfortable and significantly more confident in managing nosebleeds after the in-service (P = .007 and P < 0.001, respectively); 74.46% of nurses had an improvement in comfort level in managing epistaxis and 43.90% of nurses had an improvement in confidence in stopping epistaxis. After we moved the educational session from mid-shift to shift change, the nursing staff reported more satisfaction while maintaining similar improvements in knowledge and confidence.
Conclusion: We were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. Nurses of varying clinical experience and different clinical settings benefitted equally from our intervention.
Keywords: nosebleed; in-service; quality improvement.
Epistaxis, or nosebleed, is estimated to be the chief complaint in 1 in 200 emergency department visits in the United States.1 Additionally, it represents up to one-third of otolaryngology-related emergency room admissions.2 There is no existing literature, to our best knowledge, specifically investigating the incidence of epistaxis after a patient is admitted. Anecdotally, inpatients who develop epistaxis account for an appreciable number of consults to otolaryngology (ENT). Epistaxis is a cross-disciplinary issue, occurring in a range of clinical settings. For example, patients with epistaxis can present to the emergency department or to an outpatient primary care clinic before being referred to ENT. Additionally, inpatients on many different services can develop spontaneous epistaxis due to a variety of environmental and iatrogenic factors, such as dry air, use of nasal cannula, and initiation of anticoagulation. Based on the experience of our ENT providers and discussions with our nursing colleagues, we concluded that there was an interest in epistaxis management training among our nursing workforce.
The presence of unmet demand for epistaxis education among our nursing colleagues was supported by our literature review. A study performed in England surveyed emergency department nurses on first aid measures for management of epistaxis, including ideal head positioning, location of pressure application, and duration of pressure application.3 Overall, only 12% to 14% of the nursing staff answered all 3 questions correctly.3 Additionally, 73% to 78% of the nursing staff felt that their training in epistaxis management was inadequate, and 88% desired further training in epistaxis management.3 If generalized, this study confirms the demand for further epistaxis education among nurses.
In-services have previously been shown to be effective educational tools within the nursing community. A study in Ethiopia that evaluated pain management knowledge and attitudes before and after an in-service found a significant improvement in mean rank score of nurses’ knowledge and attitudes regarding pain management after they participated in the in-service.4 Scores on the knowledge survey improved from 41.4% before the intervention to 63.0% post intervention.4 A study in Connecticut evaluated nurses’ confidence in discussing suicidal ideation with patients and knowledge surrounding suicide precautions.5 After participating in an in-service, nurses were significantly more confident in discussing suicidal ideation with patients; application of appropriate suicide precautions also increased after the in-service.5
Our aim was for nurses to have an improvement in overall epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds after attending our in-service. Additionally, an overarching priority was to provide high-quality epistaxis education based on the literature and best practice guidelines.
Methods
Setting
This study was carried out at an 811-bed quaternary care center located in Chicago, Illinois. In fiscal year 2021, there were 91 643 emergency department visits and 33 805 hospital admissions. At our flagship hospital, 2658 patients were diagnosed with epistaxis during fiscal year 2021. The emergency department saw 533 patients with epistaxis, with 342 requiring admission and 191 being discharged. Separately, 566 inpatients received a diagnosis of epistaxis during their admission. The remainder of the patients with epistaxis were seen on an outpatient basis.
Data Collection
Data were collected from nurses on 5 different inpatient units. An email with information about the in-service was sent to the nurse managers of the inpatient units. These 5 units were included because the nurse managers responded to the email and facilitated delivery of the in-service. Data collection took place from August to December 2020.
Intervention
A quality improvement team composed of a resident physician champion, nurse educators, and nurse managers was formed. The physician champion was a senior otolaryngology resident who was responsible for designing and administering the pre-test, in-service, and post test. The nurse educators and nurse managers helped coordinate times for the in-service and promoted the in-service for their staff.
Our intervention was an educational in-service, a technique that is commonly used at our institution for nurse education. In-services typically involve delivering a lecture on a clinically relevant topic to a group of nurses on a unit. In developing the in-service, a top priority was to present high-quality evidence-based material. There is an abundance of information in the literature surrounding epistaxis management. The clinical practice guideline published by the American Academy of Otolaryngology lists nasal compression, application of vasoconstrictors, nasal packing, and nasal cautery as first-line treatments for the management of epistaxis.6 Nasal packing and nasal cautery tend to be perceived as interventions that require a certain level of expertise and specialized supplies. As such, these interventions are not often performed by floor nurses. In contrast, nasal compression and application of vasoconstrictors require only a few easily accessible supplies, and the risks are relatively minimal. When performing nasal compression, the clinical practice guidelines recommend firm, sustained compression to the lower third of the nose for 5 minutes or longer.6 Topical vasoconstrictors are generally underutilized in epistaxis management. In a study looking at a random sample of all US emergency department visits from 1992 to 2001, only 18% of visits used an epistaxis-related medication.2 Oxymetazoline hydrochloride is a topical vasoconstrictor that is commonly used as a nasal decongestant. However, its vasoconstrictor properties also make it a useful tool for controlling epistaxis. In a study looking at emergency department visits at the University of Texas Health Science Center, 65% of patients had resolution of nosebleed with application of oxymetazoline hydrochloride as the only intervention, with another 18% experiencing resolution of nosebleed with a combination of oxymetazoline hydrochloride and silver nitrate cautery.7 Based on review of the literature, nasal compression and application of vasoconstrictors seemed to be low-resource interventions with minimal morbidity. Therefore, management centered around nasal compression and use of topical vasoconstrictors seemed appropriate for our nursing staff.
The in-service included information about the etiology and management of epistaxis. Particular emphasis was placed on addressing and debunking common misconceptions about nosebleed management. With regards to management, our presentation focused on the use of topical vasoconstrictors and firm pressure to the lower third of the nose for at least 5 minutes. Nasal packing and nasal cautery were presented as procedures that ENT would perform. After the in-service, questions from the nurses were answered as time permitted.
Testing and Outcomes
A pre-test was administered before each in-service. The pre-test components comprised a knowledge survey and a descriptive survey. The general epistaxis knowledge questions on the pre-test included the location of blood vessels most commonly responsible for nosebleeds, the ideal positioning of a patient during a nosebleed, the appropriate location to hold pressure during a nosebleed, and the appropriate duration to hold pressure during a nosebleed. The descriptive survey portion asked nurses to rate whether they felt “very comfortable,” “comfortable,” “uncomfortable,” or “very uncomfortable” managing nosebleeds. It also asked whether nurses thought they would be able to “always,” “usually,” “rarely,” or “never” stop nosebleeds on the floor. We collected demographic information, including gender identity, years of clinical experience, and primary clinical environment.
The post test asked the same questions as the pre-test and was administered immediately after the in-service in order to assess its impact. We also established an ongoing dialogue with our nursing colleagues to obtain feedback on the sessions.
Primary outcomes of interest were the difference in general epistaxis knowledge questions answered correctly between the pre-test and the post test; the difference in comfort level in managing epistaxis before and after the in-service; and the difference in confidence to stop nosebleeds before and after the in-service. A secondary outcome was determining the audience for the in-service. Specifically, we wanted to determine whether there were different outcomes based on clinical setting or years of clinical experience. If nurses in a certain clinical environment or beyond a certain experience level did not show significant improvement from pre-test to post test, we would not target them for the in-service. Another secondary outcome was determining optimal timing for delivery of the in-service. We wanted to determine if there was a nursing preference for delivering the in-service at mid-shift vs shift change.
Analysis
Statistical calculations were performed using Stata 15 (StataCorp LLC). A P value < .05 was considered to be statistically significant. Where applicable, 95% confidence intervals (CI) were calculated. T-test was used to determine whether there was a statistically significant difference between pre-test and post-test epistaxis knowledge question scores. T-test was also used to determine whether there was a statistically significant difference in test scores between nurses receiving the in-service at mid-shift vs shift change. Pearson chi-squared tests were used to determine if there was a statistically significant difference between pre-test and post-test perceptions of epistaxis management, and to investigate outcomes between different subsets of nurses.
SQUIRE 2.0 guidelines were utilized to provide a framework for this project and to structure the manuscript.8 This study met criteria for exemption from institutional review board approval.
Results
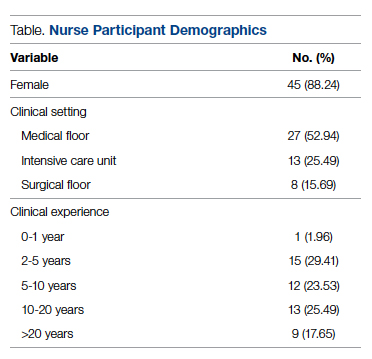
Fifty-one nurses took part in this project (Table). The majority of participants identified as female (88.24%), and just over half worked on medical floors (52.94%), with most of the remainder working in intensive care (25.49%) and surgical (15.69%) settings. There was a wide range of clinical experience, with 1.96% reporting 0 to 1 years of experience, 29.41% reporting 2 to 5 years, 23.53% reporting 5 to 10 years, 25.49% reporting 10 to 20 years, and 17.65% reporting more than 20 years.
There were unanswered questions on both the pre-test and post test. There was no consistently unanswered question. Omitted answers on the epistaxis knowledge questions were recorded as an “incorrect” answer. Omitted answers on the perception questions were considered null values and not considered in final analysis.
Primary Measures
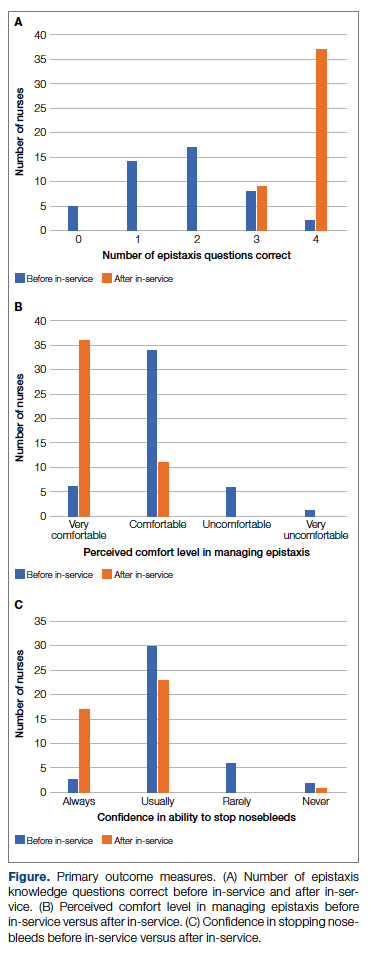
General epistaxis knowledge (Figure, part A) improved from the pre-test, where out of 4 questions, the mean (SD) score was 1.74 (1.02) correct questions, to the post-test, where out of 4 questions, the mean score was 3.80 (0.40) correct questions. After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (mean difference, 2.07 [1.10]; 95% CI, 1.74-2.39; P < .001), and 80.43% of them got a perfect score on the epistaxis knowledge questions.
The second primary measure was the difference in comfort level in managing nosebleed. After participating in the in-service, nurses felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), with 74.46% of nurses having an improved comfort level managing nosebleeds. Before the in-service, 12.76% of nurses felt “very comfortable” in managing nosebleeds vs more than three-quarters (76.59%) after the in-service. Of those who answered that they felt “comfortable” managing nosebleeds on the pre-test, 82.35% improved to feeling “very comfortable” in managing nosebleeds. Before the in-service, 14.89% of nurses felt “uncomfortable” or “very uncomfortable” in managing nosebleeds, and this decreased to 0 post intervention. After the in-service, 100.00% of nurses felt “comfortable” or “very comfortable” in managing nosebleeds.
After receiving the in-service, nurses felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001), with 43.90% of them having an improvement in confidence in stopping epistaxis. Before the in-service, 7.31% of nurses felt that they would “always” be able to stop a nose-bleed, and this increased to 41.46% after the in-service. Of those who answered that they felt that they would “usually” be able to stop a nosebleed on the pre-test, 36.67% changed their answer to state that they would “always” be able to stop a nosebleed on the post test. Before the in-service, 19.51% of nurses felt that they would “rarely” or “never” be able to stop a nosebleed, and this decreased to 2.44% after the in-service.
Secondary Measures
All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. However, to determine whether there was a population who would benefit most from the in-service, we stratified the data by years of clinical experience. There was no statistically significant difference in whether nurses with varying clinical experience learned something new (P = .148): 100% of nurses with 0-1 years of experience, 80.00% of nurses with 2-5 years of experience, 100% of nurses with 5-10 years of experience, 69.23% of nurses with 10-20 years of experience, and 100% of nurses with >20 years of experience “strongly agreed” that they learned something new from this in-service. There was no statistically significant difference on the post test compared to the pre-test in additional correct questions when stratified by clinical experience (P = .128). Second, when we stratified by clinical setting, we did not find a statistically significant difference in whether nurses in different clinical settings learned something new (P = .929): 88.89% of nurses in the medical setting, 87.50% of nurses in the surgical setting, and 84.62% of nurses in the intensive care setting “strongly agreed” that they learned something new from this presentation. On investigating additional questions correct on the post test compared to the pre-test, there was no statistically significant difference in additional correct questions when stratified by clinical setting (P = .446).
Optimal timing of the in-service was another important outcome. Initially, the in-service was administered at mid-shift, with 9 nurses participating at mid-shift, but our nursing colleagues gave unanimous feedback that this was a suboptimal time for delivery of an in-service. We changed the timing of the in-service to shift change; 42 nurses received the in-service at shift-change. There was no statistically significant difference in scores on the epistaxis knowledge questions between these two groups (P = .123). This indicated to us that changing the timing of the delivery resulted in similarly improved outcomes while having the added benefit of being preferred by our nursing colleagues.
Discussion
In undertaking this project, our primary aims were to improve epistaxis knowledge and perceived management in our nursing staff. Among our nursing staff, we were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. We also found that nurses of varying clinical experience and different clinical settings benefited equally from our intervention. Using quality improvement principles, we optimized our delivery. Our in-service focused on educating nurses to use epistaxis management techniques that were resource-efficient and low risk.
After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (Figure, part A; mean difference, 2.07 questions [1.10]; 95% CI, 1.74-2.39; P < .001), felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), and felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001). Based on these results, we successfully achieved our primary aims.
Our secondary aim was to determine the audience that would benefit the most from the in-service. All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. There was no statistically significant difference in whether nurses of varying clinical experience learned something new (P = .148) or in additional correct questions when stratified by clinical experience (P =.128). Also, there was no statistically significant difference in whether nurses in different clinical settings learned something new (P = .929) or in additional correct questions when stratified by clinical setting (P = .446). These results indicated to us that all participants learned something new and that there was no specific target audience, but rather that all participants benefitted from our session.
Our nursing colleagues gave us feedback that the timing of the in-service during mid-shift was not ideal. It was difficult to gather nurses mid-shift due to pressing patient-care duties. Nurses also found it difficult to give their full attention at this time. Nurses, nurse educators, and nurse managers suggested that we conduct the in-service at shift change in order to capture a larger population and take advantage of time relatively free of clinical duties. Giving the in-service at a time with relatively fewer clinical responsibilities allowed for a more robust question-and-answer session. It also allowed our nursing colleagues to pay full attention to the in-service. There was no statistically significant difference in epistaxis general knowledge questions answered correctly; this indicates that the quality of the education session did not vary greatly. However, our nursing colleagues strongly preferred the in-service at shift change. By making this modification to our intervention, we were able to optimize our intervention.
The previously mentioned study in England reported that only 12% to 14% of their nursing staff got a perfect score on epistaxis knowledge questions. Prior to our study, there was no literature investigating the impact of an in-service on epistaxis knowledge. After our intervention, 80.43% of our nurses got a perfect score on the epistaxis knowledge questions. We believe that this is a fair comparison because our post-test questions were identical to the survey questions used in the previously mentioned study in England, with the addition of one question.3 Further, the findings of our study are consistent with other studies regarding the positive effect of in-service education on knowledge and attitudes surrounding clinical topics. Similar to the study in Ethiopia investigating nurses’ knowledge surrounding pain management, our study noted a significant improvement in nurses’ knowledge after participating in the in-service.4 Also, when comparing our study to the study performed in Connecticut investigating nurses’ confidence surrounding suicide precautions, we found a similar significant improvement in confidence in management after participating in the in-service.5
Given our reliance on a survey as a tool to collect information, our study was subject to nonresponse bias. For each main outcome question, there was a handful of nonresponders. While this likely indicated either overlooking a question or deferring to answer due to clinical inexperience or nonapplicable clinical role, it is possible that this may have represented a respondent who did not benefit from the in-service. Another source of possible bias is sampling bias. Attempts were made to capture a wide range of nurses at the in-service. However, if a nurse was not interested in the topic material, whether due to abundant clinical experience or disinterest, it is possible that they may not have attended. Additionally, the cohort was selected purely based on responses from nursing managers to the initial email. It is possible that nonresponding units may have benefitted differently from this in-service.
There were several limitations within our analysis. We did not collect data assessing the long-term retention of epistaxis knowledge and management techniques. It is possible that epistaxis knowledge, comfort in managing nosebleeds, and perceived confidence in stopping nosebleeds decreased back to baseline several months after the in-service. Ideally, we would have been able to collect this data to assess retention of the in-service information. Unfortunately, a significant number of nurses who initially participated in the project became lost to follow-up, making such data collection impossible. Additionally, there was no assessment of actual ability to stop nosebleeds before vs after this in-service. Perceived management of epistaxis vs actual management of epistaxis are 2 vastly different things. However, this data would have been difficult to collect, and it likely would not have been in the best interest of patients, especially before the in-service was administered. As an improvement to this project, we could have assessed how many nosebleeds nurses had seen and successfully stopped after the in-service. As previously mentioned, this was not possible due to losing a significant number of nurses to follow-up. Finally, we did not collect objective data on preference for administration of in-service at mid-shift vs shift change. We relied on subjective data from conversations with our colleagues. By collecting objective data, we could have supported this change to our intervention with data.
The primary challenge to sustainability for this intervention is nursing turnover. With each wave of departing nurses and new nursing hires, the difficulty of ensuring a consistent knowledge base and management standards within our nursing workforce became clearer. After optimizing our intervention, our solution was to provide a hospital-wide in-service, which was recorded and uploaded to an institution-wide in-service library. In this way, a nurse with the desire to learn about epistaxis management could access the material at any point in time. Another solution would have been to appoint champions for epistaxis management within each major department to deliver the epistaxis in-service to new hires and new rotators within the department. However, given the turnover witnessed in our study cohort, this may not be sustainable long term.
Conclusion
Epistaxis is a chief complaint that can present in many different clinical settings and situations. Therefore, the ability to stop epistaxis in a timely and effective fashion is valuable. Our study demonstrated that in-services can improve epistaxis knowledge and improve perceived epistaxis management. Ideally, this intervention will lead to improved patient care. Given that epistaxis is a ubiquitous issue, this study may benefit other institutions who want to improve care for patients with epistaxis.
Next steps for this intervention include utilizing in-services for epistaxis education at other institutions and collecting long-term data within our own institution. Collecting long-term data would allow us to assess the retention of epistaxis knowledge from our in-service.
Acknowledgments: The author thanks the nurse managers, nurse educators, and staff nurses involved in this project, as well as Dr. Louis Portugal for providing mentorship throughout this process and Dr. Dara Adams for assisting with statistical analysis.
Corresponding author: Avery Nelson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC 1035, Chicago, IL 60637; avery.nelson@uchospitals.edu
Disclosures: None reported.
1. Pallin DJ, Chng Y-M, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81. doi:10.1016/j.annemergmed.2004.12.014
2. Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: An investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32(5):361-365. doi:10.1111/j.1749-4486.2007.01530.x
3. Hakim N, Mummadi SM, Jolly K, et al. Nurse-led epistaxis management within the emergency department. Br J Nurs. 2018;27(1):41-46. doi:10.12968/bjon.2018.27.1.41
4. Germossa GN, Sjetne IS, Hellesø R. The impact of an in-service educational program on nurses’ knowledge and attitudes regarding pain management in an Ethiopian University Hospital. Front Public Health. 2018;6:229. doi:10.3389/fpubh.2018.00229
5. Manister NN, Murray S, Burke JM, Finegan M, McKiernan ME. Effectiveness of nursing education to prevent inpatient suicide. J Contin Educ Nurs. 2017;48(9):413-419. doi:10.3928/00220124-20170816-07
6. Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: nosebleed (epistaxis) executive summary. Otolaryngol Head Neck Surg. 2020;162(1):S1-S38. doi:10.1177/0194599819890327
7. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104(9 Part 1):704-706. doi:10.1177/000348949510400906
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0—standards for quality improvement reporting excellence—revised publication guidelines from a detailed consensus process. J Am Coll Surg. 2016;222(3):317-323. doi:10.1016/j.jamcollsurg.2015.07.456
From the University of Chicago Medical Center, Chicago, IL.
Abstract
Background: Epistaxis is a common chief complaint addressed by otolaryngologists. A review of the literature showed that there is a deficit in epistaxis education within the nursing community. Conversations with our nursing colleagues confirmed this unmet demand.
Objective: This quality improvement project aimed to increase general epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds among our nursing staff.
Methods: Data were collected through a survey administered before and after our intervention. The survey tested general epistaxis knowledge and assessed comfort and confidence in stopping epistaxis. Our intervention was an educational session covering pertinent epistaxis etiology and management. Quality improvement principles were used to optimize delivery of the intervention.
Results: A total of 51 nurses participated in the project. After participating in the in-service educational session, nurses answered significantly more epistaxis general knowledge questions correctly (mean [SD] difference, 2.07 [1.10] questions; 95% CI, 1.74-2.39; P < .001). There was no statistically significant difference in additional correct questions when stratified by clinical experience or clinical setting (P = .128 and P = 0.446, respectively). Nurses also reported feeling significantly more comfortable and significantly more confident in managing nosebleeds after the in-service (P = .007 and P < 0.001, respectively); 74.46% of nurses had an improvement in comfort level in managing epistaxis and 43.90% of nurses had an improvement in confidence in stopping epistaxis. After we moved the educational session from mid-shift to shift change, the nursing staff reported more satisfaction while maintaining similar improvements in knowledge and confidence.
Conclusion: We were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. Nurses of varying clinical experience and different clinical settings benefitted equally from our intervention.
Keywords: nosebleed; in-service; quality improvement.
Epistaxis, or nosebleed, is estimated to be the chief complaint in 1 in 200 emergency department visits in the United States.1 Additionally, it represents up to one-third of otolaryngology-related emergency room admissions.2 There is no existing literature, to our best knowledge, specifically investigating the incidence of epistaxis after a patient is admitted. Anecdotally, inpatients who develop epistaxis account for an appreciable number of consults to otolaryngology (ENT). Epistaxis is a cross-disciplinary issue, occurring in a range of clinical settings. For example, patients with epistaxis can present to the emergency department or to an outpatient primary care clinic before being referred to ENT. Additionally, inpatients on many different services can develop spontaneous epistaxis due to a variety of environmental and iatrogenic factors, such as dry air, use of nasal cannula, and initiation of anticoagulation. Based on the experience of our ENT providers and discussions with our nursing colleagues, we concluded that there was an interest in epistaxis management training among our nursing workforce.
The presence of unmet demand for epistaxis education among our nursing colleagues was supported by our literature review. A study performed in England surveyed emergency department nurses on first aid measures for management of epistaxis, including ideal head positioning, location of pressure application, and duration of pressure application.3 Overall, only 12% to 14% of the nursing staff answered all 3 questions correctly.3 Additionally, 73% to 78% of the nursing staff felt that their training in epistaxis management was inadequate, and 88% desired further training in epistaxis management.3 If generalized, this study confirms the demand for further epistaxis education among nurses.
In-services have previously been shown to be effective educational tools within the nursing community. A study in Ethiopia that evaluated pain management knowledge and attitudes before and after an in-service found a significant improvement in mean rank score of nurses’ knowledge and attitudes regarding pain management after they participated in the in-service.4 Scores on the knowledge survey improved from 41.4% before the intervention to 63.0% post intervention.4 A study in Connecticut evaluated nurses’ confidence in discussing suicidal ideation with patients and knowledge surrounding suicide precautions.5 After participating in an in-service, nurses were significantly more confident in discussing suicidal ideation with patients; application of appropriate suicide precautions also increased after the in-service.5
Our aim was for nurses to have an improvement in overall epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds after attending our in-service. Additionally, an overarching priority was to provide high-quality epistaxis education based on the literature and best practice guidelines.
Methods
Setting
This study was carried out at an 811-bed quaternary care center located in Chicago, Illinois. In fiscal year 2021, there were 91 643 emergency department visits and 33 805 hospital admissions. At our flagship hospital, 2658 patients were diagnosed with epistaxis during fiscal year 2021. The emergency department saw 533 patients with epistaxis, with 342 requiring admission and 191 being discharged. Separately, 566 inpatients received a diagnosis of epistaxis during their admission. The remainder of the patients with epistaxis were seen on an outpatient basis.
Data Collection
Data were collected from nurses on 5 different inpatient units. An email with information about the in-service was sent to the nurse managers of the inpatient units. These 5 units were included because the nurse managers responded to the email and facilitated delivery of the in-service. Data collection took place from August to December 2020.
Intervention
A quality improvement team composed of a resident physician champion, nurse educators, and nurse managers was formed. The physician champion was a senior otolaryngology resident who was responsible for designing and administering the pre-test, in-service, and post test. The nurse educators and nurse managers helped coordinate times for the in-service and promoted the in-service for their staff.
Our intervention was an educational in-service, a technique that is commonly used at our institution for nurse education. In-services typically involve delivering a lecture on a clinically relevant topic to a group of nurses on a unit. In developing the in-service, a top priority was to present high-quality evidence-based material. There is an abundance of information in the literature surrounding epistaxis management. The clinical practice guideline published by the American Academy of Otolaryngology lists nasal compression, application of vasoconstrictors, nasal packing, and nasal cautery as first-line treatments for the management of epistaxis.6 Nasal packing and nasal cautery tend to be perceived as interventions that require a certain level of expertise and specialized supplies. As such, these interventions are not often performed by floor nurses. In contrast, nasal compression and application of vasoconstrictors require only a few easily accessible supplies, and the risks are relatively minimal. When performing nasal compression, the clinical practice guidelines recommend firm, sustained compression to the lower third of the nose for 5 minutes or longer.6 Topical vasoconstrictors are generally underutilized in epistaxis management. In a study looking at a random sample of all US emergency department visits from 1992 to 2001, only 18% of visits used an epistaxis-related medication.2 Oxymetazoline hydrochloride is a topical vasoconstrictor that is commonly used as a nasal decongestant. However, its vasoconstrictor properties also make it a useful tool for controlling epistaxis. In a study looking at emergency department visits at the University of Texas Health Science Center, 65% of patients had resolution of nosebleed with application of oxymetazoline hydrochloride as the only intervention, with another 18% experiencing resolution of nosebleed with a combination of oxymetazoline hydrochloride and silver nitrate cautery.7 Based on review of the literature, nasal compression and application of vasoconstrictors seemed to be low-resource interventions with minimal morbidity. Therefore, management centered around nasal compression and use of topical vasoconstrictors seemed appropriate for our nursing staff.
The in-service included information about the etiology and management of epistaxis. Particular emphasis was placed on addressing and debunking common misconceptions about nosebleed management. With regards to management, our presentation focused on the use of topical vasoconstrictors and firm pressure to the lower third of the nose for at least 5 minutes. Nasal packing and nasal cautery were presented as procedures that ENT would perform. After the in-service, questions from the nurses were answered as time permitted.
Testing and Outcomes
A pre-test was administered before each in-service. The pre-test components comprised a knowledge survey and a descriptive survey. The general epistaxis knowledge questions on the pre-test included the location of blood vessels most commonly responsible for nosebleeds, the ideal positioning of a patient during a nosebleed, the appropriate location to hold pressure during a nosebleed, and the appropriate duration to hold pressure during a nosebleed. The descriptive survey portion asked nurses to rate whether they felt “very comfortable,” “comfortable,” “uncomfortable,” or “very uncomfortable” managing nosebleeds. It also asked whether nurses thought they would be able to “always,” “usually,” “rarely,” or “never” stop nosebleeds on the floor. We collected demographic information, including gender identity, years of clinical experience, and primary clinical environment.
The post test asked the same questions as the pre-test and was administered immediately after the in-service in order to assess its impact. We also established an ongoing dialogue with our nursing colleagues to obtain feedback on the sessions.
Primary outcomes of interest were the difference in general epistaxis knowledge questions answered correctly between the pre-test and the post test; the difference in comfort level in managing epistaxis before and after the in-service; and the difference in confidence to stop nosebleeds before and after the in-service. A secondary outcome was determining the audience for the in-service. Specifically, we wanted to determine whether there were different outcomes based on clinical setting or years of clinical experience. If nurses in a certain clinical environment or beyond a certain experience level did not show significant improvement from pre-test to post test, we would not target them for the in-service. Another secondary outcome was determining optimal timing for delivery of the in-service. We wanted to determine if there was a nursing preference for delivering the in-service at mid-shift vs shift change.
Analysis
Statistical calculations were performed using Stata 15 (StataCorp LLC). A P value < .05 was considered to be statistically significant. Where applicable, 95% confidence intervals (CI) were calculated. T-test was used to determine whether there was a statistically significant difference between pre-test and post-test epistaxis knowledge question scores. T-test was also used to determine whether there was a statistically significant difference in test scores between nurses receiving the in-service at mid-shift vs shift change. Pearson chi-squared tests were used to determine if there was a statistically significant difference between pre-test and post-test perceptions of epistaxis management, and to investigate outcomes between different subsets of nurses.
SQUIRE 2.0 guidelines were utilized to provide a framework for this project and to structure the manuscript.8 This study met criteria for exemption from institutional review board approval.
Results
Fifty-one nurses took part in this project (Table). The majority of participants identified as female (88.24%), and just over half worked on medical floors (52.94%), with most of the remainder working in intensive care (25.49%) and surgical (15.69%) settings. There was a wide range of clinical experience, with 1.96% reporting 0 to 1 years of experience, 29.41% reporting 2 to 5 years, 23.53% reporting 5 to 10 years, 25.49% reporting 10 to 20 years, and 17.65% reporting more than 20 years.
There were unanswered questions on both the pre-test and post test. There was no consistently unanswered question. Omitted answers on the epistaxis knowledge questions were recorded as an “incorrect” answer. Omitted answers on the perception questions were considered null values and not considered in final analysis.
Primary Measures
General epistaxis knowledge (Figure, part A) improved from the pre-test, where out of 4 questions, the mean (SD) score was 1.74 (1.02) correct questions, to the post-test, where out of 4 questions, the mean score was 3.80 (0.40) correct questions. After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (mean difference, 2.07 [1.10]; 95% CI, 1.74-2.39; P < .001), and 80.43% of them got a perfect score on the epistaxis knowledge questions.
The second primary measure was the difference in comfort level in managing nosebleed. After participating in the in-service, nurses felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), with 74.46% of nurses having an improved comfort level managing nosebleeds. Before the in-service, 12.76% of nurses felt “very comfortable” in managing nosebleeds vs more than three-quarters (76.59%) after the in-service. Of those who answered that they felt “comfortable” managing nosebleeds on the pre-test, 82.35% improved to feeling “very comfortable” in managing nosebleeds. Before the in-service, 14.89% of nurses felt “uncomfortable” or “very uncomfortable” in managing nosebleeds, and this decreased to 0 post intervention. After the in-service, 100.00% of nurses felt “comfortable” or “very comfortable” in managing nosebleeds.
After receiving the in-service, nurses felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001), with 43.90% of them having an improvement in confidence in stopping epistaxis. Before the in-service, 7.31% of nurses felt that they would “always” be able to stop a nose-bleed, and this increased to 41.46% after the in-service. Of those who answered that they felt that they would “usually” be able to stop a nosebleed on the pre-test, 36.67% changed their answer to state that they would “always” be able to stop a nosebleed on the post test. Before the in-service, 19.51% of nurses felt that they would “rarely” or “never” be able to stop a nosebleed, and this decreased to 2.44% after the in-service.
Secondary Measures
All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. However, to determine whether there was a population who would benefit most from the in-service, we stratified the data by years of clinical experience. There was no statistically significant difference in whether nurses with varying clinical experience learned something new (P = .148): 100% of nurses with 0-1 years of experience, 80.00% of nurses with 2-5 years of experience, 100% of nurses with 5-10 years of experience, 69.23% of nurses with 10-20 years of experience, and 100% of nurses with >20 years of experience “strongly agreed” that they learned something new from this in-service. There was no statistically significant difference on the post test compared to the pre-test in additional correct questions when stratified by clinical experience (P = .128). Second, when we stratified by clinical setting, we did not find a statistically significant difference in whether nurses in different clinical settings learned something new (P = .929): 88.89% of nurses in the medical setting, 87.50% of nurses in the surgical setting, and 84.62% of nurses in the intensive care setting “strongly agreed” that they learned something new from this presentation. On investigating additional questions correct on the post test compared to the pre-test, there was no statistically significant difference in additional correct questions when stratified by clinical setting (P = .446).
Optimal timing of the in-service was another important outcome. Initially, the in-service was administered at mid-shift, with 9 nurses participating at mid-shift, but our nursing colleagues gave unanimous feedback that this was a suboptimal time for delivery of an in-service. We changed the timing of the in-service to shift change; 42 nurses received the in-service at shift-change. There was no statistically significant difference in scores on the epistaxis knowledge questions between these two groups (P = .123). This indicated to us that changing the timing of the delivery resulted in similarly improved outcomes while having the added benefit of being preferred by our nursing colleagues.
Discussion
In undertaking this project, our primary aims were to improve epistaxis knowledge and perceived management in our nursing staff. Among our nursing staff, we were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. We also found that nurses of varying clinical experience and different clinical settings benefited equally from our intervention. Using quality improvement principles, we optimized our delivery. Our in-service focused on educating nurses to use epistaxis management techniques that were resource-efficient and low risk.
After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (Figure, part A; mean difference, 2.07 questions [1.10]; 95% CI, 1.74-2.39; P < .001), felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), and felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001). Based on these results, we successfully achieved our primary aims.
Our secondary aim was to determine the audience that would benefit the most from the in-service. All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. There was no statistically significant difference in whether nurses of varying clinical experience learned something new (P = .148) or in additional correct questions when stratified by clinical experience (P =.128). Also, there was no statistically significant difference in whether nurses in different clinical settings learned something new (P = .929) or in additional correct questions when stratified by clinical setting (P = .446). These results indicated to us that all participants learned something new and that there was no specific target audience, but rather that all participants benefitted from our session.
Our nursing colleagues gave us feedback that the timing of the in-service during mid-shift was not ideal. It was difficult to gather nurses mid-shift due to pressing patient-care duties. Nurses also found it difficult to give their full attention at this time. Nurses, nurse educators, and nurse managers suggested that we conduct the in-service at shift change in order to capture a larger population and take advantage of time relatively free of clinical duties. Giving the in-service at a time with relatively fewer clinical responsibilities allowed for a more robust question-and-answer session. It also allowed our nursing colleagues to pay full attention to the in-service. There was no statistically significant difference in epistaxis general knowledge questions answered correctly; this indicates that the quality of the education session did not vary greatly. However, our nursing colleagues strongly preferred the in-service at shift change. By making this modification to our intervention, we were able to optimize our intervention.
The previously mentioned study in England reported that only 12% to 14% of their nursing staff got a perfect score on epistaxis knowledge questions. Prior to our study, there was no literature investigating the impact of an in-service on epistaxis knowledge. After our intervention, 80.43% of our nurses got a perfect score on the epistaxis knowledge questions. We believe that this is a fair comparison because our post-test questions were identical to the survey questions used in the previously mentioned study in England, with the addition of one question.3 Further, the findings of our study are consistent with other studies regarding the positive effect of in-service education on knowledge and attitudes surrounding clinical topics. Similar to the study in Ethiopia investigating nurses’ knowledge surrounding pain management, our study noted a significant improvement in nurses’ knowledge after participating in the in-service.4 Also, when comparing our study to the study performed in Connecticut investigating nurses’ confidence surrounding suicide precautions, we found a similar significant improvement in confidence in management after participating in the in-service.5
Given our reliance on a survey as a tool to collect information, our study was subject to nonresponse bias. For each main outcome question, there was a handful of nonresponders. While this likely indicated either overlooking a question or deferring to answer due to clinical inexperience or nonapplicable clinical role, it is possible that this may have represented a respondent who did not benefit from the in-service. Another source of possible bias is sampling bias. Attempts were made to capture a wide range of nurses at the in-service. However, if a nurse was not interested in the topic material, whether due to abundant clinical experience or disinterest, it is possible that they may not have attended. Additionally, the cohort was selected purely based on responses from nursing managers to the initial email. It is possible that nonresponding units may have benefitted differently from this in-service.
There were several limitations within our analysis. We did not collect data assessing the long-term retention of epistaxis knowledge and management techniques. It is possible that epistaxis knowledge, comfort in managing nosebleeds, and perceived confidence in stopping nosebleeds decreased back to baseline several months after the in-service. Ideally, we would have been able to collect this data to assess retention of the in-service information. Unfortunately, a significant number of nurses who initially participated in the project became lost to follow-up, making such data collection impossible. Additionally, there was no assessment of actual ability to stop nosebleeds before vs after this in-service. Perceived management of epistaxis vs actual management of epistaxis are 2 vastly different things. However, this data would have been difficult to collect, and it likely would not have been in the best interest of patients, especially before the in-service was administered. As an improvement to this project, we could have assessed how many nosebleeds nurses had seen and successfully stopped after the in-service. As previously mentioned, this was not possible due to losing a significant number of nurses to follow-up. Finally, we did not collect objective data on preference for administration of in-service at mid-shift vs shift change. We relied on subjective data from conversations with our colleagues. By collecting objective data, we could have supported this change to our intervention with data.
The primary challenge to sustainability for this intervention is nursing turnover. With each wave of departing nurses and new nursing hires, the difficulty of ensuring a consistent knowledge base and management standards within our nursing workforce became clearer. After optimizing our intervention, our solution was to provide a hospital-wide in-service, which was recorded and uploaded to an institution-wide in-service library. In this way, a nurse with the desire to learn about epistaxis management could access the material at any point in time. Another solution would have been to appoint champions for epistaxis management within each major department to deliver the epistaxis in-service to new hires and new rotators within the department. However, given the turnover witnessed in our study cohort, this may not be sustainable long term.
Conclusion
Epistaxis is a chief complaint that can present in many different clinical settings and situations. Therefore, the ability to stop epistaxis in a timely and effective fashion is valuable. Our study demonstrated that in-services can improve epistaxis knowledge and improve perceived epistaxis management. Ideally, this intervention will lead to improved patient care. Given that epistaxis is a ubiquitous issue, this study may benefit other institutions who want to improve care for patients with epistaxis.
Next steps for this intervention include utilizing in-services for epistaxis education at other institutions and collecting long-term data within our own institution. Collecting long-term data would allow us to assess the retention of epistaxis knowledge from our in-service.
Acknowledgments: The author thanks the nurse managers, nurse educators, and staff nurses involved in this project, as well as Dr. Louis Portugal for providing mentorship throughout this process and Dr. Dara Adams for assisting with statistical analysis.
Corresponding author: Avery Nelson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC 1035, Chicago, IL 60637; avery.nelson@uchospitals.edu
Disclosures: None reported.
From the University of Chicago Medical Center, Chicago, IL.
Abstract
Background: Epistaxis is a common chief complaint addressed by otolaryngologists. A review of the literature showed that there is a deficit in epistaxis education within the nursing community. Conversations with our nursing colleagues confirmed this unmet demand.
Objective: This quality improvement project aimed to increase general epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds among our nursing staff.
Methods: Data were collected through a survey administered before and after our intervention. The survey tested general epistaxis knowledge and assessed comfort and confidence in stopping epistaxis. Our intervention was an educational session covering pertinent epistaxis etiology and management. Quality improvement principles were used to optimize delivery of the intervention.
Results: A total of 51 nurses participated in the project. After participating in the in-service educational session, nurses answered significantly more epistaxis general knowledge questions correctly (mean [SD] difference, 2.07 [1.10] questions; 95% CI, 1.74-2.39; P < .001). There was no statistically significant difference in additional correct questions when stratified by clinical experience or clinical setting (P = .128 and P = 0.446, respectively). Nurses also reported feeling significantly more comfortable and significantly more confident in managing nosebleeds after the in-service (P = .007 and P < 0.001, respectively); 74.46% of nurses had an improvement in comfort level in managing epistaxis and 43.90% of nurses had an improvement in confidence in stopping epistaxis. After we moved the educational session from mid-shift to shift change, the nursing staff reported more satisfaction while maintaining similar improvements in knowledge and confidence.
Conclusion: We were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. Nurses of varying clinical experience and different clinical settings benefitted equally from our intervention.
Keywords: nosebleed; in-service; quality improvement.
Epistaxis, or nosebleed, is estimated to be the chief complaint in 1 in 200 emergency department visits in the United States.1 Additionally, it represents up to one-third of otolaryngology-related emergency room admissions.2 There is no existing literature, to our best knowledge, specifically investigating the incidence of epistaxis after a patient is admitted. Anecdotally, inpatients who develop epistaxis account for an appreciable number of consults to otolaryngology (ENT). Epistaxis is a cross-disciplinary issue, occurring in a range of clinical settings. For example, patients with epistaxis can present to the emergency department or to an outpatient primary care clinic before being referred to ENT. Additionally, inpatients on many different services can develop spontaneous epistaxis due to a variety of environmental and iatrogenic factors, such as dry air, use of nasal cannula, and initiation of anticoagulation. Based on the experience of our ENT providers and discussions with our nursing colleagues, we concluded that there was an interest in epistaxis management training among our nursing workforce.
The presence of unmet demand for epistaxis education among our nursing colleagues was supported by our literature review. A study performed in England surveyed emergency department nurses on first aid measures for management of epistaxis, including ideal head positioning, location of pressure application, and duration of pressure application.3 Overall, only 12% to 14% of the nursing staff answered all 3 questions correctly.3 Additionally, 73% to 78% of the nursing staff felt that their training in epistaxis management was inadequate, and 88% desired further training in epistaxis management.3 If generalized, this study confirms the demand for further epistaxis education among nurses.
In-services have previously been shown to be effective educational tools within the nursing community. A study in Ethiopia that evaluated pain management knowledge and attitudes before and after an in-service found a significant improvement in mean rank score of nurses’ knowledge and attitudes regarding pain management after they participated in the in-service.4 Scores on the knowledge survey improved from 41.4% before the intervention to 63.0% post intervention.4 A study in Connecticut evaluated nurses’ confidence in discussing suicidal ideation with patients and knowledge surrounding suicide precautions.5 After participating in an in-service, nurses were significantly more confident in discussing suicidal ideation with patients; application of appropriate suicide precautions also increased after the in-service.5
Our aim was for nurses to have an improvement in overall epistaxis knowledge, perceived comfort level managing nosebleeds, and perceived ability to stop nosebleeds after attending our in-service. Additionally, an overarching priority was to provide high-quality epistaxis education based on the literature and best practice guidelines.
Methods
Setting
This study was carried out at an 811-bed quaternary care center located in Chicago, Illinois. In fiscal year 2021, there were 91 643 emergency department visits and 33 805 hospital admissions. At our flagship hospital, 2658 patients were diagnosed with epistaxis during fiscal year 2021. The emergency department saw 533 patients with epistaxis, with 342 requiring admission and 191 being discharged. Separately, 566 inpatients received a diagnosis of epistaxis during their admission. The remainder of the patients with epistaxis were seen on an outpatient basis.
Data Collection
Data were collected from nurses on 5 different inpatient units. An email with information about the in-service was sent to the nurse managers of the inpatient units. These 5 units were included because the nurse managers responded to the email and facilitated delivery of the in-service. Data collection took place from August to December 2020.
Intervention
A quality improvement team composed of a resident physician champion, nurse educators, and nurse managers was formed. The physician champion was a senior otolaryngology resident who was responsible for designing and administering the pre-test, in-service, and post test. The nurse educators and nurse managers helped coordinate times for the in-service and promoted the in-service for their staff.
Our intervention was an educational in-service, a technique that is commonly used at our institution for nurse education. In-services typically involve delivering a lecture on a clinically relevant topic to a group of nurses on a unit. In developing the in-service, a top priority was to present high-quality evidence-based material. There is an abundance of information in the literature surrounding epistaxis management. The clinical practice guideline published by the American Academy of Otolaryngology lists nasal compression, application of vasoconstrictors, nasal packing, and nasal cautery as first-line treatments for the management of epistaxis.6 Nasal packing and nasal cautery tend to be perceived as interventions that require a certain level of expertise and specialized supplies. As such, these interventions are not often performed by floor nurses. In contrast, nasal compression and application of vasoconstrictors require only a few easily accessible supplies, and the risks are relatively minimal. When performing nasal compression, the clinical practice guidelines recommend firm, sustained compression to the lower third of the nose for 5 minutes or longer.6 Topical vasoconstrictors are generally underutilized in epistaxis management. In a study looking at a random sample of all US emergency department visits from 1992 to 2001, only 18% of visits used an epistaxis-related medication.2 Oxymetazoline hydrochloride is a topical vasoconstrictor that is commonly used as a nasal decongestant. However, its vasoconstrictor properties also make it a useful tool for controlling epistaxis. In a study looking at emergency department visits at the University of Texas Health Science Center, 65% of patients had resolution of nosebleed with application of oxymetazoline hydrochloride as the only intervention, with another 18% experiencing resolution of nosebleed with a combination of oxymetazoline hydrochloride and silver nitrate cautery.7 Based on review of the literature, nasal compression and application of vasoconstrictors seemed to be low-resource interventions with minimal morbidity. Therefore, management centered around nasal compression and use of topical vasoconstrictors seemed appropriate for our nursing staff.
The in-service included information about the etiology and management of epistaxis. Particular emphasis was placed on addressing and debunking common misconceptions about nosebleed management. With regards to management, our presentation focused on the use of topical vasoconstrictors and firm pressure to the lower third of the nose for at least 5 minutes. Nasal packing and nasal cautery were presented as procedures that ENT would perform. After the in-service, questions from the nurses were answered as time permitted.
Testing and Outcomes
A pre-test was administered before each in-service. The pre-test components comprised a knowledge survey and a descriptive survey. The general epistaxis knowledge questions on the pre-test included the location of blood vessels most commonly responsible for nosebleeds, the ideal positioning of a patient during a nosebleed, the appropriate location to hold pressure during a nosebleed, and the appropriate duration to hold pressure during a nosebleed. The descriptive survey portion asked nurses to rate whether they felt “very comfortable,” “comfortable,” “uncomfortable,” or “very uncomfortable” managing nosebleeds. It also asked whether nurses thought they would be able to “always,” “usually,” “rarely,” or “never” stop nosebleeds on the floor. We collected demographic information, including gender identity, years of clinical experience, and primary clinical environment.
The post test asked the same questions as the pre-test and was administered immediately after the in-service in order to assess its impact. We also established an ongoing dialogue with our nursing colleagues to obtain feedback on the sessions.
Primary outcomes of interest were the difference in general epistaxis knowledge questions answered correctly between the pre-test and the post test; the difference in comfort level in managing epistaxis before and after the in-service; and the difference in confidence to stop nosebleeds before and after the in-service. A secondary outcome was determining the audience for the in-service. Specifically, we wanted to determine whether there were different outcomes based on clinical setting or years of clinical experience. If nurses in a certain clinical environment or beyond a certain experience level did not show significant improvement from pre-test to post test, we would not target them for the in-service. Another secondary outcome was determining optimal timing for delivery of the in-service. We wanted to determine if there was a nursing preference for delivering the in-service at mid-shift vs shift change.
Analysis
Statistical calculations were performed using Stata 15 (StataCorp LLC). A P value < .05 was considered to be statistically significant. Where applicable, 95% confidence intervals (CI) were calculated. T-test was used to determine whether there was a statistically significant difference between pre-test and post-test epistaxis knowledge question scores. T-test was also used to determine whether there was a statistically significant difference in test scores between nurses receiving the in-service at mid-shift vs shift change. Pearson chi-squared tests were used to determine if there was a statistically significant difference between pre-test and post-test perceptions of epistaxis management, and to investigate outcomes between different subsets of nurses.
SQUIRE 2.0 guidelines were utilized to provide a framework for this project and to structure the manuscript.8 This study met criteria for exemption from institutional review board approval.
Results
Fifty-one nurses took part in this project (Table). The majority of participants identified as female (88.24%), and just over half worked on medical floors (52.94%), with most of the remainder working in intensive care (25.49%) and surgical (15.69%) settings. There was a wide range of clinical experience, with 1.96% reporting 0 to 1 years of experience, 29.41% reporting 2 to 5 years, 23.53% reporting 5 to 10 years, 25.49% reporting 10 to 20 years, and 17.65% reporting more than 20 years.
There were unanswered questions on both the pre-test and post test. There was no consistently unanswered question. Omitted answers on the epistaxis knowledge questions were recorded as an “incorrect” answer. Omitted answers on the perception questions were considered null values and not considered in final analysis.
Primary Measures
General epistaxis knowledge (Figure, part A) improved from the pre-test, where out of 4 questions, the mean (SD) score was 1.74 (1.02) correct questions, to the post-test, where out of 4 questions, the mean score was 3.80 (0.40) correct questions. After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (mean difference, 2.07 [1.10]; 95% CI, 1.74-2.39; P < .001), and 80.43% of them got a perfect score on the epistaxis knowledge questions.
The second primary measure was the difference in comfort level in managing nosebleed. After participating in the in-service, nurses felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), with 74.46% of nurses having an improved comfort level managing nosebleeds. Before the in-service, 12.76% of nurses felt “very comfortable” in managing nosebleeds vs more than three-quarters (76.59%) after the in-service. Of those who answered that they felt “comfortable” managing nosebleeds on the pre-test, 82.35% improved to feeling “very comfortable” in managing nosebleeds. Before the in-service, 14.89% of nurses felt “uncomfortable” or “very uncomfortable” in managing nosebleeds, and this decreased to 0 post intervention. After the in-service, 100.00% of nurses felt “comfortable” or “very comfortable” in managing nosebleeds.
After receiving the in-service, nurses felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001), with 43.90% of them having an improvement in confidence in stopping epistaxis. Before the in-service, 7.31% of nurses felt that they would “always” be able to stop a nose-bleed, and this increased to 41.46% after the in-service. Of those who answered that they felt that they would “usually” be able to stop a nosebleed on the pre-test, 36.67% changed their answer to state that they would “always” be able to stop a nosebleed on the post test. Before the in-service, 19.51% of nurses felt that they would “rarely” or “never” be able to stop a nosebleed, and this decreased to 2.44% after the in-service.
Secondary Measures
All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. However, to determine whether there was a population who would benefit most from the in-service, we stratified the data by years of clinical experience. There was no statistically significant difference in whether nurses with varying clinical experience learned something new (P = .148): 100% of nurses with 0-1 years of experience, 80.00% of nurses with 2-5 years of experience, 100% of nurses with 5-10 years of experience, 69.23% of nurses with 10-20 years of experience, and 100% of nurses with >20 years of experience “strongly agreed” that they learned something new from this in-service. There was no statistically significant difference on the post test compared to the pre-test in additional correct questions when stratified by clinical experience (P = .128). Second, when we stratified by clinical setting, we did not find a statistically significant difference in whether nurses in different clinical settings learned something new (P = .929): 88.89% of nurses in the medical setting, 87.50% of nurses in the surgical setting, and 84.62% of nurses in the intensive care setting “strongly agreed” that they learned something new from this presentation. On investigating additional questions correct on the post test compared to the pre-test, there was no statistically significant difference in additional correct questions when stratified by clinical setting (P = .446).
Optimal timing of the in-service was another important outcome. Initially, the in-service was administered at mid-shift, with 9 nurses participating at mid-shift, but our nursing colleagues gave unanimous feedback that this was a suboptimal time for delivery of an in-service. We changed the timing of the in-service to shift change; 42 nurses received the in-service at shift-change. There was no statistically significant difference in scores on the epistaxis knowledge questions between these two groups (P = .123). This indicated to us that changing the timing of the delivery resulted in similarly improved outcomes while having the added benefit of being preferred by our nursing colleagues.
Discussion
In undertaking this project, our primary aims were to improve epistaxis knowledge and perceived management in our nursing staff. Among our nursing staff, we were able to significantly increase epistaxis knowledge, improve comfort levels managing epistaxis, and improve confidence in successful epistaxis management. We also found that nurses of varying clinical experience and different clinical settings benefited equally from our intervention. Using quality improvement principles, we optimized our delivery. Our in-service focused on educating nurses to use epistaxis management techniques that were resource-efficient and low risk.
After participating in the in-service, nurses answered significantly more questions about epistaxis general knowledge correctly (Figure, part A; mean difference, 2.07 questions [1.10]; 95% CI, 1.74-2.39; P < .001), felt significantly more comfortable in managing nosebleeds (Figure, part B; P = .007), and felt significantly more confident in stopping nosebleeds (Figure, part C; P < .001). Based on these results, we successfully achieved our primary aims.
Our secondary aim was to determine the audience that would benefit the most from the in-service. All of the nurses who participated either “strongly agreed” or “agreed” that they learned something new from the in-service. There was no statistically significant difference in whether nurses of varying clinical experience learned something new (P = .148) or in additional correct questions when stratified by clinical experience (P =.128). Also, there was no statistically significant difference in whether nurses in different clinical settings learned something new (P = .929) or in additional correct questions when stratified by clinical setting (P = .446). These results indicated to us that all participants learned something new and that there was no specific target audience, but rather that all participants benefitted from our session.
Our nursing colleagues gave us feedback that the timing of the in-service during mid-shift was not ideal. It was difficult to gather nurses mid-shift due to pressing patient-care duties. Nurses also found it difficult to give their full attention at this time. Nurses, nurse educators, and nurse managers suggested that we conduct the in-service at shift change in order to capture a larger population and take advantage of time relatively free of clinical duties. Giving the in-service at a time with relatively fewer clinical responsibilities allowed for a more robust question-and-answer session. It also allowed our nursing colleagues to pay full attention to the in-service. There was no statistically significant difference in epistaxis general knowledge questions answered correctly; this indicates that the quality of the education session did not vary greatly. However, our nursing colleagues strongly preferred the in-service at shift change. By making this modification to our intervention, we were able to optimize our intervention.
The previously mentioned study in England reported that only 12% to 14% of their nursing staff got a perfect score on epistaxis knowledge questions. Prior to our study, there was no literature investigating the impact of an in-service on epistaxis knowledge. After our intervention, 80.43% of our nurses got a perfect score on the epistaxis knowledge questions. We believe that this is a fair comparison because our post-test questions were identical to the survey questions used in the previously mentioned study in England, with the addition of one question.3 Further, the findings of our study are consistent with other studies regarding the positive effect of in-service education on knowledge and attitudes surrounding clinical topics. Similar to the study in Ethiopia investigating nurses’ knowledge surrounding pain management, our study noted a significant improvement in nurses’ knowledge after participating in the in-service.4 Also, when comparing our study to the study performed in Connecticut investigating nurses’ confidence surrounding suicide precautions, we found a similar significant improvement in confidence in management after participating in the in-service.5
Given our reliance on a survey as a tool to collect information, our study was subject to nonresponse bias. For each main outcome question, there was a handful of nonresponders. While this likely indicated either overlooking a question or deferring to answer due to clinical inexperience or nonapplicable clinical role, it is possible that this may have represented a respondent who did not benefit from the in-service. Another source of possible bias is sampling bias. Attempts were made to capture a wide range of nurses at the in-service. However, if a nurse was not interested in the topic material, whether due to abundant clinical experience or disinterest, it is possible that they may not have attended. Additionally, the cohort was selected purely based on responses from nursing managers to the initial email. It is possible that nonresponding units may have benefitted differently from this in-service.
There were several limitations within our analysis. We did not collect data assessing the long-term retention of epistaxis knowledge and management techniques. It is possible that epistaxis knowledge, comfort in managing nosebleeds, and perceived confidence in stopping nosebleeds decreased back to baseline several months after the in-service. Ideally, we would have been able to collect this data to assess retention of the in-service information. Unfortunately, a significant number of nurses who initially participated in the project became lost to follow-up, making such data collection impossible. Additionally, there was no assessment of actual ability to stop nosebleeds before vs after this in-service. Perceived management of epistaxis vs actual management of epistaxis are 2 vastly different things. However, this data would have been difficult to collect, and it likely would not have been in the best interest of patients, especially before the in-service was administered. As an improvement to this project, we could have assessed how many nosebleeds nurses had seen and successfully stopped after the in-service. As previously mentioned, this was not possible due to losing a significant number of nurses to follow-up. Finally, we did not collect objective data on preference for administration of in-service at mid-shift vs shift change. We relied on subjective data from conversations with our colleagues. By collecting objective data, we could have supported this change to our intervention with data.
The primary challenge to sustainability for this intervention is nursing turnover. With each wave of departing nurses and new nursing hires, the difficulty of ensuring a consistent knowledge base and management standards within our nursing workforce became clearer. After optimizing our intervention, our solution was to provide a hospital-wide in-service, which was recorded and uploaded to an institution-wide in-service library. In this way, a nurse with the desire to learn about epistaxis management could access the material at any point in time. Another solution would have been to appoint champions for epistaxis management within each major department to deliver the epistaxis in-service to new hires and new rotators within the department. However, given the turnover witnessed in our study cohort, this may not be sustainable long term.
Conclusion
Epistaxis is a chief complaint that can present in many different clinical settings and situations. Therefore, the ability to stop epistaxis in a timely and effective fashion is valuable. Our study demonstrated that in-services can improve epistaxis knowledge and improve perceived epistaxis management. Ideally, this intervention will lead to improved patient care. Given that epistaxis is a ubiquitous issue, this study may benefit other institutions who want to improve care for patients with epistaxis.
Next steps for this intervention include utilizing in-services for epistaxis education at other institutions and collecting long-term data within our own institution. Collecting long-term data would allow us to assess the retention of epistaxis knowledge from our in-service.
Acknowledgments: The author thanks the nurse managers, nurse educators, and staff nurses involved in this project, as well as Dr. Louis Portugal for providing mentorship throughout this process and Dr. Dara Adams for assisting with statistical analysis.
Corresponding author: Avery Nelson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC 1035, Chicago, IL 60637; avery.nelson@uchospitals.edu
Disclosures: None reported.
1. Pallin DJ, Chng Y-M, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81. doi:10.1016/j.annemergmed.2004.12.014
2. Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: An investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32(5):361-365. doi:10.1111/j.1749-4486.2007.01530.x
3. Hakim N, Mummadi SM, Jolly K, et al. Nurse-led epistaxis management within the emergency department. Br J Nurs. 2018;27(1):41-46. doi:10.12968/bjon.2018.27.1.41
4. Germossa GN, Sjetne IS, Hellesø R. The impact of an in-service educational program on nurses’ knowledge and attitudes regarding pain management in an Ethiopian University Hospital. Front Public Health. 2018;6:229. doi:10.3389/fpubh.2018.00229
5. Manister NN, Murray S, Burke JM, Finegan M, McKiernan ME. Effectiveness of nursing education to prevent inpatient suicide. J Contin Educ Nurs. 2017;48(9):413-419. doi:10.3928/00220124-20170816-07
6. Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: nosebleed (epistaxis) executive summary. Otolaryngol Head Neck Surg. 2020;162(1):S1-S38. doi:10.1177/0194599819890327
7. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104(9 Part 1):704-706. doi:10.1177/000348949510400906
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0—standards for quality improvement reporting excellence—revised publication guidelines from a detailed consensus process. J Am Coll Surg. 2016;222(3):317-323. doi:10.1016/j.jamcollsurg.2015.07.456
1. Pallin DJ, Chng Y-M, McKay MP, et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81. doi:10.1016/j.annemergmed.2004.12.014
2. Walker TWM, Macfarlane TV, McGarry GW. The epidemiology and chronobiology of epistaxis: An investigation of Scottish hospital admissions 1995-2004. Clin Otolaryngol. 2007;32(5):361-365. doi:10.1111/j.1749-4486.2007.01530.x
3. Hakim N, Mummadi SM, Jolly K, et al. Nurse-led epistaxis management within the emergency department. Br J Nurs. 2018;27(1):41-46. doi:10.12968/bjon.2018.27.1.41
4. Germossa GN, Sjetne IS, Hellesø R. The impact of an in-service educational program on nurses’ knowledge and attitudes regarding pain management in an Ethiopian University Hospital. Front Public Health. 2018;6:229. doi:10.3389/fpubh.2018.00229
5. Manister NN, Murray S, Burke JM, Finegan M, McKiernan ME. Effectiveness of nursing education to prevent inpatient suicide. J Contin Educ Nurs. 2017;48(9):413-419. doi:10.3928/00220124-20170816-07
6. Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: nosebleed (epistaxis) executive summary. Otolaryngol Head Neck Surg. 2020;162(1):S1-S38. doi:10.1177/0194599819890327
7. Krempl GA, Noorily AD. Use of oxymetazoline in the management of epistaxis. Ann Otol Rhinol Laryngol. 1995;104(9 Part 1):704-706. doi:10.1177/000348949510400906
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0—standards for quality improvement reporting excellence—revised publication guidelines from a detailed consensus process. J Am Coll Surg. 2016;222(3):317-323. doi:10.1016/j.jamcollsurg.2015.07.456
Comorbidity Coding and Its Impact on Hospital Complexity: Reply
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).
We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
mxs2157@med.miami.edu
Disclosures: None reported.
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).
We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
mxs2157@med.miami.edu
Disclosures: None reported.
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).
We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
mxs2157@med.miami.edu
Disclosures: None reported.
Comorbidity Coding and Its Impact on Hospital Complexity
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
carlos.kerguelen@fsfb.org.co
Disclosures: None reported.
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
carlos.kerguelen@fsfb.org.co
Disclosures: None reported.
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
carlos.kerguelen@fsfb.org.co
Disclosures: None reported.
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
Supporting Patients on Complex Care Journeys: How Technology Can Bridge the Gaps
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; jamie@memorahealth.com
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; jamie@memorahealth.com
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; jamie@memorahealth.com
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
The Mission of Continuous Improvement in Health Care: A New Era for Clinical Outcomes Management
This issue of the Journal of Clinical Outcomes (JCOM) debuts a new cover design that brings forward the articles and features in each issue. Although the Journal’s cover has a new look, JCOM’s goals remain the same—improving care by disseminating evidence of quality improvement in health care and sharing access to the medical literature with our readers. We continue our mission to promote the best medical practice by providing clinicians with updates and communicating advances that lead to measurable improvement in health care delivery, quality, and outcomes.
As we continue the work of improving health care quality, knowledge gaps and unmet needs in the literature remain. These unmet needs are evident throughout all phases of health care delivery. Moreover, the Institutes of Medicine report that centered on efforts to build a safer health care environment by redesigning health care processes remains salient.1 The journey to continuous improvement in health care, where we achieve threshold change in the quality of each process and across the entire health care system, requires collective effort. Such efforts include establishing clear metrics and measurements for improvement goals throughout the patient’s journey through diagnosis, treatment, transitions of care, and disease management.2,3 To address evidence and knowledge gaps in the literature, JCOM publishes reports of original studies and quality improvement projects as well as reviews, providing its 30,000 readers with new evidence to implement in daily practice. We welcome submissions of original research reports, reports of quality improvement projects that follow the SQUIRE 2.0 standards,4 and perspectives on developments and innovations in health care delivery.
The next chapter in health care delivery improvement will encompass value-based care.5 This new era of clinical outcomes management will dictate the metrics and outcomes reporting6 and how to plan future investments. The value-based phase will increase innovation and shape policies that advance population health, transforming every step in the care delivery journey.7 The next phase in health care delivery will also create a viable financial structure while implementing effective performance measures for optimal outcomes through patient-centered care and optimization of cost and care strategies. In light of health care’s evolution toward a value-based model, JCOM welcomes submissions of manuscripts that explore themes central to this model, including patient-centered care, implementation of best practices, system design, safety, cost-effectiveness, and the balance between cost optimization and quality. For JCOM’s authors and readers, our editorial team remains commited to the highest standards in timely publishing to support our community through our collective expertise and dedication to quality improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000.
2. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-10. doi:10.1136/bmjqs-2014-003675
3. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(14 Suppl 9):S3-9. doi:10.2146/ajhp070190
4. Revised Standards for Quality Improvement Reporting Excellence. SQUIRE 2.0. Accessed July 25, 2022. http://squire-statement.org
5. Gray M. Value based healthcare. BMJ. 2017;356:j437. doi:10.1136/bmj.j437
6. What is value-based healthcare? NEJM Catalyst. January 1, 2017. Accessed July 25, 2022. catalyst.nejm.org/doi/full/10.1056/CAT.17.0558
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
This issue of the Journal of Clinical Outcomes (JCOM) debuts a new cover design that brings forward the articles and features in each issue. Although the Journal’s cover has a new look, JCOM’s goals remain the same—improving care by disseminating evidence of quality improvement in health care and sharing access to the medical literature with our readers. We continue our mission to promote the best medical practice by providing clinicians with updates and communicating advances that lead to measurable improvement in health care delivery, quality, and outcomes.
As we continue the work of improving health care quality, knowledge gaps and unmet needs in the literature remain. These unmet needs are evident throughout all phases of health care delivery. Moreover, the Institutes of Medicine report that centered on efforts to build a safer health care environment by redesigning health care processes remains salient.1 The journey to continuous improvement in health care, where we achieve threshold change in the quality of each process and across the entire health care system, requires collective effort. Such efforts include establishing clear metrics and measurements for improvement goals throughout the patient’s journey through diagnosis, treatment, transitions of care, and disease management.2,3 To address evidence and knowledge gaps in the literature, JCOM publishes reports of original studies and quality improvement projects as well as reviews, providing its 30,000 readers with new evidence to implement in daily practice. We welcome submissions of original research reports, reports of quality improvement projects that follow the SQUIRE 2.0 standards,4 and perspectives on developments and innovations in health care delivery.
The next chapter in health care delivery improvement will encompass value-based care.5 This new era of clinical outcomes management will dictate the metrics and outcomes reporting6 and how to plan future investments. The value-based phase will increase innovation and shape policies that advance population health, transforming every step in the care delivery journey.7 The next phase in health care delivery will also create a viable financial structure while implementing effective performance measures for optimal outcomes through patient-centered care and optimization of cost and care strategies. In light of health care’s evolution toward a value-based model, JCOM welcomes submissions of manuscripts that explore themes central to this model, including patient-centered care, implementation of best practices, system design, safety, cost-effectiveness, and the balance between cost optimization and quality. For JCOM’s authors and readers, our editorial team remains commited to the highest standards in timely publishing to support our community through our collective expertise and dedication to quality improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
This issue of the Journal of Clinical Outcomes (JCOM) debuts a new cover design that brings forward the articles and features in each issue. Although the Journal’s cover has a new look, JCOM’s goals remain the same—improving care by disseminating evidence of quality improvement in health care and sharing access to the medical literature with our readers. We continue our mission to promote the best medical practice by providing clinicians with updates and communicating advances that lead to measurable improvement in health care delivery, quality, and outcomes.
As we continue the work of improving health care quality, knowledge gaps and unmet needs in the literature remain. These unmet needs are evident throughout all phases of health care delivery. Moreover, the Institutes of Medicine report that centered on efforts to build a safer health care environment by redesigning health care processes remains salient.1 The journey to continuous improvement in health care, where we achieve threshold change in the quality of each process and across the entire health care system, requires collective effort. Such efforts include establishing clear metrics and measurements for improvement goals throughout the patient’s journey through diagnosis, treatment, transitions of care, and disease management.2,3 To address evidence and knowledge gaps in the literature, JCOM publishes reports of original studies and quality improvement projects as well as reviews, providing its 30,000 readers with new evidence to implement in daily practice. We welcome submissions of original research reports, reports of quality improvement projects that follow the SQUIRE 2.0 standards,4 and perspectives on developments and innovations in health care delivery.
The next chapter in health care delivery improvement will encompass value-based care.5 This new era of clinical outcomes management will dictate the metrics and outcomes reporting6 and how to plan future investments. The value-based phase will increase innovation and shape policies that advance population health, transforming every step in the care delivery journey.7 The next phase in health care delivery will also create a viable financial structure while implementing effective performance measures for optimal outcomes through patient-centered care and optimization of cost and care strategies. In light of health care’s evolution toward a value-based model, JCOM welcomes submissions of manuscripts that explore themes central to this model, including patient-centered care, implementation of best practices, system design, safety, cost-effectiveness, and the balance between cost optimization and quality. For JCOM’s authors and readers, our editorial team remains commited to the highest standards in timely publishing to support our community through our collective expertise and dedication to quality improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; ebarkoudah@bwh.harvard.edu
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000.
2. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-10. doi:10.1136/bmjqs-2014-003675
3. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(14 Suppl 9):S3-9. doi:10.2146/ajhp070190
4. Revised Standards for Quality Improvement Reporting Excellence. SQUIRE 2.0. Accessed July 25, 2022. http://squire-statement.org
5. Gray M. Value based healthcare. BMJ. 2017;356:j437. doi:10.1136/bmj.j437
6. What is value-based healthcare? NEJM Catalyst. January 1, 2017. Accessed July 25, 2022. catalyst.nejm.org/doi/full/10.1056/CAT.17.0558
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
1. Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US); 2000.
2. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-10. doi:10.1136/bmjqs-2014-003675
3. Bates DW. Preventing medication errors: a summary. Am J Health Syst Pharm. 2007;64(14 Suppl 9):S3-9. doi:10.2146/ajhp070190
4. Revised Standards for Quality Improvement Reporting Excellence. SQUIRE 2.0. Accessed July 25, 2022. http://squire-statement.org
5. Gray M. Value based healthcare. BMJ. 2017;356:j437. doi:10.1136/bmj.j437
6. What is value-based healthcare? NEJM Catalyst. January 1, 2017. Accessed July 25, 2022. catalyst.nejm.org/doi/full/10.1056/CAT.17.0558
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
Geriatric-Centered Interdisciplinary Care Pathway Reduces Delirium in Hospitalized Older Adults With Traumatic Injury
Study 1 Overview (Park et al)
Objective: To examine whether implementation of a geriatric trauma clinical pathway is associated with reduced rates of delirium in older adults with traumatic injury.
Design: Retrospective case-control study of electronic health records.
Setting and participants: Eligible patients were persons aged 65 years or older who were admitted to the trauma service and did not undergo an operation. A Geriatric Trauma Care Pathway was developed by a multidisciplinary Stanford Quality Pathways team and formally launched on November 1, 2018. The clinical pathway was designed to incorporate geriatric best practices, which included order sets (eg, age-appropriate nonpharmacological interventions and pharmacological dosages), guidelines (eg, Institute for Healthcare Improvement Age-Friendly Health systems 4M framework), automated consultations (comprehensive geriatric assessment), and escalation pathways executed by a multidisciplinary team (eg, pain, bowel, and sleep regulation). The clinical pathway began with admission to the emergency department (ED) (ie, automatic trigger of geriatric trauma care admission order set), daily multidisciplinary team meetings during acute hospitalization, and a transitional care team consultation for postdischarge follow-up or home visit.
Main outcome measures: The primary outcome was delirium as determined by a positive Confusion Assessment Method (CAM) score or a diagnosis of delirium by the clinical team. The secondary outcome was hospital length of stay (LOS). Process measures for pathway compliance (eg, achieving adequate pain control, early mobilization, advance care planning) were assessed. Outcome measures were compared between patients who underwent the Geriatric Trauma Care Pathway intervention (postimplementation group) vs patients who were treated prior to pathway implementation (baseline pre-implementation group).
Main results: Of the 859 eligible patients, 712 were included in the analysis (442 [62.1%] in the baseline pre-implementation group and 270 [37.9%] in the postimplementation group); mean (SD) age was 81.4 (9.1) years, and 394 (55.3%) were women. The injury mechanism was similar between groups, with falls being the most common cause of injury (247 [55.9%] in the baseline group vs 162 [60.0%] in the postimplementation group; P = .43). Injuries as measured by Injury Severity Score (ISS) were minor or moderate in both groups (261 [59.0%] in baseline group vs 168 [62.2%] in postimplementation group; P = .87). The adjusted odds ratio (OR) for delirium in the postimplementation group was lower compared to the baseline pre-implementation group (OR, 0.54; 95% CI, 0.37-0.80; P < .001). Measures of advance care planning in the postimplementation group improved, including more frequent goals-of-care documentation (53.7% in postimplementation group vs 16.7% in baseline group; P < .001) and a shortened time to first goals-of-care discussion upon presenting to the ED (36 hours in postimplementation group vs 50 hours in baseline group; P = .03).
Conclusion: Implementation of a multidisciplinary geriatric trauma clinical pathway for older adults with traumatic injury at a single level I trauma center was associated with reduced rates of delirium.
Study 2 Overview (Bryant et al)
Objective: To determine whether an interdisciplinary care pathway for frail trauma patients can improve in-hospital mortality, complications, and 30-day readmissions.
Design: Retrospective cohort study of frail patients.
Setting and participants: Eligible patients were persons aged 65 years or older who were admitted to the trauma service and survived more than 24 hours; admitted to and discharged from the trauma unit; and determined to be pre-frail or frail by a geriatrician’s assessment. A Frailty Identification and Care Pathway designed to reduce delirium and complications in frail older trauma patients was developed by a multidisciplinary team and implemented in 2016. The standardized evidence-based interdisciplinary care pathway included utilization of order sets and interventions for delirium prevention, early ambulation, bowel and pain regimens, nutrition and physical therapy consults, medication management, care-goal setting, and geriatric assessments.
Main outcome measures: The main outcomes were delirium as determined by a positive CAM score, major complications as defined by the Trauma Quality Improvement Project, in-hospital mortality, and 30-day hospital readmission. Outcome measures were compared between patients who underwent Frailty Identification and Care Pathway intervention (postintervention group) vs patients who were treated prior to pathway implementation (pre-intervention group).
Main results: A total of 269 frail patients were included in the analysis (125 in pre-intervention group vs 144 in postintervention group). Patient demographic and admission characteristics were similar between the 2 groups: mean age was 83.5 (7.1) years, 60.6% were women, and median ISS was 10 (interquartile range [IQR], 9-14). The injury mechanism was similar between groups, with falls accounting for 92.8% and 86.1% of injuries in the pre-intervention and postintervention groups, respectively (P = .07). In univariate analysis, the Frailty Identification and Care Pathway intervention was associated with a significant reduction in delirium (12.5% vs 21.6%, P = .04) and 30-day hospital readmission (2.7% vs 9.6%, P = .01) compared to patients in the pre-intervention group. However, rates of major complications (28.5% vs 28.0%, P = 0.93) and in-hospital mortality (4.2% vs 7.2%, P = .28) were similar between the pre-intervention and postintervention groups. In multivariate logistic regression models adjusted for patient characteristics (age, sex, race, ISS), patients in the postintervention group had lower delirium (OR, 0.44; 95% CI, 0.22-0.88; P = .02) and 30-day hospital readmission (OR, 0.25; 95% CI, 0.07-0.84; P = .02) rates compared to those in the pre-intervention group.
Conclusion: Implementation of an interdisciplinary care protocol for frail geriatric trauma patients significantly decreased their risks for in-hospital delirium and 30-day hospital readmission.
Commentary
Traumatic injuries in older adults are associated with higher morbidity and mortality compared to younger patients, with falls and motor vehicle accidents accounting for a majority of these injuries. Astoundingly, up to one-third of this vulnerable population presenting to hospitals with an ISS greater than 15 may die during hospitalization.1 As a result, a large number of studies and clinical trials have focused on interventions that are designed to reduce fall risks, and hence reduce adverse consequences of traumatic injuries that may arise after falls.2 However, this emphasis on falls prevention has overshadowed a need to develop effective geriatric-centered clinical interventions that aim to improve outcomes in older adults who present to hospitals with traumatic injuries. Furthermore, frailty—a geriatric syndrome indicative of an increased state of vulnerability and predictive of adverse outcomes such as delirium—is highly prevalent in older patients with traumatic injury.3 Thus, there is an urgent need to develop novel, hospital-based, traumatic injury–targeting strategies that incorporate a thoughtful redesign of the care framework that includes evidence-based interventions for geriatric syndromes such as delirium and frailty.
The study reported by Park et al (Study 1) represents the latest effort to evaluate inpatient management strategies designed to improve outcomes in hospitalized older adults who have sustained traumatic injury. Through the implementation of a novel multidisciplinary Geriatric Trauma Care Pathway that incorporates geriatric best practices, this intervention was found to be associated with a 46% lower risk of in-hospital delirium. Because of the inclusion of all age-eligible patients across all strata of traumatic injuries, rather than preselecting for those at the highest risk for poor clinical outcomes, the benefits of this intervention extend to those with minor or moderate injury severity. Furthermore, the improvement in delirium (ie, the primary outcome) is particularly meaningful given that delirium is one of the most common hospital-associated complications that increase hospital LOS, discharge to an institution, and mortality in older adults. Finally, the study’s observed reduced time to a first goals-of-care discussion and increased frequency of goals-of-care documentation after intervention should not be overlooked. The improvements in these 2 process measures are highly significant given that advanced care planning, an intervention that helps to align patients’ values, goals, and treatments, is completed at substantially lower rates in older adults in the acute hospital setting.4
Similarly, in an earlier published study, Bryant and colleagues (Study 2) also show that a geriatric-focused interdisciplinary trauma care pathway is associated with delirium risk reduction in hospitalized older trauma patients. Much like Study 1, the Frailty Identification and Care Pathway utilized in Study 2 is an evidence-based interdisciplinary care pathway that includes the use of geriatric assessments, order sets, and geriatric best practices. Moreover, its exclusive inclusion of pre-frail and frail older patients (ie, those at higher risk for poor outcomes) with moderate injury severity (median ISS of 10 [IQR, 9-14]) suggests that this type of care pathway benefits hospitalized older trauma patients, who are particularly vulnerable to adverse complications such as delirium. Moreover, the successful utilization of the FRAIL questionnaire, a validated frailty screening tool, by surgical residents in the ED to initiate this care pathway demonstrates the feasibility of its use in expediting frailty screening in older patients in trauma care.
Application for Clinical Practice and System Implementation
Findings from the 2 studies discussed in this review indicate that implementation of interdisciplinary clinical care pathways predicated on evidence-based geriatric principles and best practices is a promising approach to reduce delirium in hospitalized older trauma patients. These studies have helped to lay the groundwork in outlining the roadmaps (eg, processes and infrastructures) needed to create such clinical pathways. These key elements include: (1) integration of a multidisciplinary committee (eg, representation from trauma, emergency, and geriatric medicine, nursing, physical and occupational therapy, pharmacy, social work) in pathway design and implementation; (2) adaption of evidence-based geriatric best practices (eg, the Institute for Healthcare Improvement Age-Friendly Health System 4M framework [medication, mentation, mobility, what matters]) to prioritize interventions and to design a pathway that incorporates these features; (3) incorporation of comprehensive geriatric assessment by interdisciplinary providers; (4) utilization of validated clinical instruments to assess physical and cognitive functions, frailty, delirium, and social determinants of health; (5) modification of electronic health record systems to encompass order sets that incorporate evidence-based, nonpharmacological and pharmacological interventions to manage symptoms (eg, delirium, pain, bowel movement, sleep, immobility, polypharmacy) essential to quality geriatric care; and (6) integration of patient and caregiver preferences via goals-of-care discussions and corresponding documentation and communication of these goals.
Additionally, these 2 studies imparted some strategies that may facilitate the implementation of interdisciplinary clinical care pathways in trauma care. Examples of such facilitators include: (1) collaboration with champions within each specialty to reinforce education and buy-in; (2) creation of automatically triggered order sets upon patient presentation to the ED that unites distinct features of clinical pathways; (3) adaption and reorganization of existing hospital infrastructures and resources to meet the needs of clinical pathways implementation (eg, utilizing information technology resources to develop electronic health record order sets; using quality department to develop clinical pathway guidelines and electronic outcome dashboards); and (4) development of individualized patient and caregiver education materials based on care needs (eg, principles of delirium prevention and preservation of mobility during hospitalization) to prepare and engage these stakeholders in patient care and recovery.
Practice Points
- A geriatric interdisciplinary care model can be effectively applied to the management of acute trauma in older patients.
- Interdisciplinary clinical pathways should incorporate geriatric best practices and guidelines and age-appropriate order sets to prioritize and integrate care.
—Fred Ko, MD, MS
1. Hashmi A, Ibrahim-Zada I, Rhee P, et al. Predictors of mortality in geriatric trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2014;76(3):894-901. doi:10.1097/TA.0b013e3182ab0763
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:10.1002/14651858.CD012221.pub2
3. Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149(8):766-772. doi:10.1001/jamasurg.2014.296
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10(2):164-174. doi:10.1136/bmjspcare-2019-002093
Study 1 Overview (Park et al)
Objective: To examine whether implementation of a geriatric trauma clinical pathway is associated with reduced rates of delirium in older adults with traumatic injury.
Design: Retrospective case-control study of electronic health records.
Setting and participants: Eligible patients were persons aged 65 years or older who were admitted to the trauma service and did not undergo an operation. A Geriatric Trauma Care Pathway was developed by a multidisciplinary Stanford Quality Pathways team and formally launched on November 1, 2018. The clinical pathway was designed to incorporate geriatric best practices, which included order sets (eg, age-appropriate nonpharmacological interventions and pharmacological dosages), guidelines (eg, Institute for Healthcare Improvement Age-Friendly Health systems 4M framework), automated consultations (comprehensive geriatric assessment), and escalation pathways executed by a multidisciplinary team (eg, pain, bowel, and sleep regulation). The clinical pathway began with admission to the emergency department (ED) (ie, automatic trigger of geriatric trauma care admission order set), daily multidisciplinary team meetings during acute hospitalization, and a transitional care team consultation for postdischarge follow-up or home visit.
Main outcome measures: The primary outcome was delirium as determined by a positive Confusion Assessment Method (CAM) score or a diagnosis of delirium by the clinical team. The secondary outcome was hospital length of stay (LOS). Process measures for pathway compliance (eg, achieving adequate pain control, early mobilization, advance care planning) were assessed. Outcome measures were compared between patients who underwent the Geriatric Trauma Care Pathway intervention (postimplementation group) vs patients who were treated prior to pathway implementation (baseline pre-implementation group).
Main results: Of the 859 eligible patients, 712 were included in the analysis (442 [62.1%] in the baseline pre-implementation group and 270 [37.9%] in the postimplementation group); mean (SD) age was 81.4 (9.1) years, and 394 (55.3%) were women. The injury mechanism was similar between groups, with falls being the most common cause of injury (247 [55.9%] in the baseline group vs 162 [60.0%] in the postimplementation group; P = .43). Injuries as measured by Injury Severity Score (ISS) were minor or moderate in both groups (261 [59.0%] in baseline group vs 168 [62.2%] in postimplementation group; P = .87). The adjusted odds ratio (OR) for delirium in the postimplementation group was lower compared to the baseline pre-implementation group (OR, 0.54; 95% CI, 0.37-0.80; P < .001). Measures of advance care planning in the postimplementation group improved, including more frequent goals-of-care documentation (53.7% in postimplementation group vs 16.7% in baseline group; P < .001) and a shortened time to first goals-of-care discussion upon presenting to the ED (36 hours in postimplementation group vs 50 hours in baseline group; P = .03).
Conclusion: Implementation of a multidisciplinary geriatric trauma clinical pathway for older adults with traumatic injury at a single level I trauma center was associated with reduced rates of delirium.
Study 2 Overview (Bryant et al)
Objective: To determine whether an interdisciplinary care pathway for frail trauma patients can improve in-hospital mortality, complications, and 30-day readmissions.
Design: Retrospective cohort study of frail patients.
Setting and participants: Eligible patients were persons aged 65 years or older who were admitted to the trauma service and survived more than 24 hours; admitted to and discharged from the trauma unit; and determined to be pre-frail or frail by a geriatrician’s assessment. A Frailty Identification and Care Pathway designed to reduce delirium and complications in frail older trauma patients was developed by a multidisciplinary team and implemented in 2016. The standardized evidence-based interdisciplinary care pathway included utilization of order sets and interventions for delirium prevention, early ambulation, bowel and pain regimens, nutrition and physical therapy consults, medication management, care-goal setting, and geriatric assessments.
Main outcome measures: The main outcomes were delirium as determined by a positive CAM score, major complications as defined by the Trauma Quality Improvement Project, in-hospital mortality, and 30-day hospital readmission. Outcome measures were compared between patients who underwent Frailty Identification and Care Pathway intervention (postintervention group) vs patients who were treated prior to pathway implementation (pre-intervention group).
Main results: A total of 269 frail patients were included in the analysis (125 in pre-intervention group vs 144 in postintervention group). Patient demographic and admission characteristics were similar between the 2 groups: mean age was 83.5 (7.1) years, 60.6% were women, and median ISS was 10 (interquartile range [IQR], 9-14). The injury mechanism was similar between groups, with falls accounting for 92.8% and 86.1% of injuries in the pre-intervention and postintervention groups, respectively (P = .07). In univariate analysis, the Frailty Identification and Care Pathway intervention was associated with a significant reduction in delirium (12.5% vs 21.6%, P = .04) and 30-day hospital readmission (2.7% vs 9.6%, P = .01) compared to patients in the pre-intervention group. However, rates of major complications (28.5% vs 28.0%, P = 0.93) and in-hospital mortality (4.2% vs 7.2%, P = .28) were similar between the pre-intervention and postintervention groups. In multivariate logistic regression models adjusted for patient characteristics (age, sex, race, ISS), patients in the postintervention group had lower delirium (OR, 0.44; 95% CI, 0.22-0.88; P = .02) and 30-day hospital readmission (OR, 0.25; 95% CI, 0.07-0.84; P = .02) rates compared to those in the pre-intervention group.
Conclusion: Implementation of an interdisciplinary care protocol for frail geriatric trauma patients significantly decreased their risks for in-hospital delirium and 30-day hospital readmission.
Commentary
Traumatic injuries in older adults are associated with higher morbidity and mortality compared to younger patients, with falls and motor vehicle accidents accounting for a majority of these injuries. Astoundingly, up to one-third of this vulnerable population presenting to hospitals with an ISS greater than 15 may die during hospitalization.1 As a result, a large number of studies and clinical trials have focused on interventions that are designed to reduce fall risks, and hence reduce adverse consequences of traumatic injuries that may arise after falls.2 However, this emphasis on falls prevention has overshadowed a need to develop effective geriatric-centered clinical interventions that aim to improve outcomes in older adults who present to hospitals with traumatic injuries. Furthermore, frailty—a geriatric syndrome indicative of an increased state of vulnerability and predictive of adverse outcomes such as delirium—is highly prevalent in older patients with traumatic injury.3 Thus, there is an urgent need to develop novel, hospital-based, traumatic injury–targeting strategies that incorporate a thoughtful redesign of the care framework that includes evidence-based interventions for geriatric syndromes such as delirium and frailty.
The study reported by Park et al (Study 1) represents the latest effort to evaluate inpatient management strategies designed to improve outcomes in hospitalized older adults who have sustained traumatic injury. Through the implementation of a novel multidisciplinary Geriatric Trauma Care Pathway that incorporates geriatric best practices, this intervention was found to be associated with a 46% lower risk of in-hospital delirium. Because of the inclusion of all age-eligible patients across all strata of traumatic injuries, rather than preselecting for those at the highest risk for poor clinical outcomes, the benefits of this intervention extend to those with minor or moderate injury severity. Furthermore, the improvement in delirium (ie, the primary outcome) is particularly meaningful given that delirium is one of the most common hospital-associated complications that increase hospital LOS, discharge to an institution, and mortality in older adults. Finally, the study’s observed reduced time to a first goals-of-care discussion and increased frequency of goals-of-care documentation after intervention should not be overlooked. The improvements in these 2 process measures are highly significant given that advanced care planning, an intervention that helps to align patients’ values, goals, and treatments, is completed at substantially lower rates in older adults in the acute hospital setting.4
Similarly, in an earlier published study, Bryant and colleagues (Study 2) also show that a geriatric-focused interdisciplinary trauma care pathway is associated with delirium risk reduction in hospitalized older trauma patients. Much like Study 1, the Frailty Identification and Care Pathway utilized in Study 2 is an evidence-based interdisciplinary care pathway that includes the use of geriatric assessments, order sets, and geriatric best practices. Moreover, its exclusive inclusion of pre-frail and frail older patients (ie, those at higher risk for poor outcomes) with moderate injury severity (median ISS of 10 [IQR, 9-14]) suggests that this type of care pathway benefits hospitalized older trauma patients, who are particularly vulnerable to adverse complications such as delirium. Moreover, the successful utilization of the FRAIL questionnaire, a validated frailty screening tool, by surgical residents in the ED to initiate this care pathway demonstrates the feasibility of its use in expediting frailty screening in older patients in trauma care.
Application for Clinical Practice and System Implementation
Findings from the 2 studies discussed in this review indicate that implementation of interdisciplinary clinical care pathways predicated on evidence-based geriatric principles and best practices is a promising approach to reduce delirium in hospitalized older trauma patients. These studies have helped to lay the groundwork in outlining the roadmaps (eg, processes and infrastructures) needed to create such clinical pathways. These key elements include: (1) integration of a multidisciplinary committee (eg, representation from trauma, emergency, and geriatric medicine, nursing, physical and occupational therapy, pharmacy, social work) in pathway design and implementation; (2) adaption of evidence-based geriatric best practices (eg, the Institute for Healthcare Improvement Age-Friendly Health System 4M framework [medication, mentation, mobility, what matters]) to prioritize interventions and to design a pathway that incorporates these features; (3) incorporation of comprehensive geriatric assessment by interdisciplinary providers; (4) utilization of validated clinical instruments to assess physical and cognitive functions, frailty, delirium, and social determinants of health; (5) modification of electronic health record systems to encompass order sets that incorporate evidence-based, nonpharmacological and pharmacological interventions to manage symptoms (eg, delirium, pain, bowel movement, sleep, immobility, polypharmacy) essential to quality geriatric care; and (6) integration of patient and caregiver preferences via goals-of-care discussions and corresponding documentation and communication of these goals.
Additionally, these 2 studies imparted some strategies that may facilitate the implementation of interdisciplinary clinical care pathways in trauma care. Examples of such facilitators include: (1) collaboration with champions within each specialty to reinforce education and buy-in; (2) creation of automatically triggered order sets upon patient presentation to the ED that unites distinct features of clinical pathways; (3) adaption and reorganization of existing hospital infrastructures and resources to meet the needs of clinical pathways implementation (eg, utilizing information technology resources to develop electronic health record order sets; using quality department to develop clinical pathway guidelines and electronic outcome dashboards); and (4) development of individualized patient and caregiver education materials based on care needs (eg, principles of delirium prevention and preservation of mobility during hospitalization) to prepare and engage these stakeholders in patient care and recovery.
Practice Points
- A geriatric interdisciplinary care model can be effectively applied to the management of acute trauma in older patients.
- Interdisciplinary clinical pathways should incorporate geriatric best practices and guidelines and age-appropriate order sets to prioritize and integrate care.
—Fred Ko, MD, MS
Study 1 Overview (Park et al)
Objective: To examine whether implementation of a geriatric trauma clinical pathway is associated with reduced rates of delirium in older adults with traumatic injury.
Design: Retrospective case-control study of electronic health records.
Setting and participants: Eligible patients were persons aged 65 years or older who were admitted to the trauma service and did not undergo an operation. A Geriatric Trauma Care Pathway was developed by a multidisciplinary Stanford Quality Pathways team and formally launched on November 1, 2018. The clinical pathway was designed to incorporate geriatric best practices, which included order sets (eg, age-appropriate nonpharmacological interventions and pharmacological dosages), guidelines (eg, Institute for Healthcare Improvement Age-Friendly Health systems 4M framework), automated consultations (comprehensive geriatric assessment), and escalation pathways executed by a multidisciplinary team (eg, pain, bowel, and sleep regulation). The clinical pathway began with admission to the emergency department (ED) (ie, automatic trigger of geriatric trauma care admission order set), daily multidisciplinary team meetings during acute hospitalization, and a transitional care team consultation for postdischarge follow-up or home visit.
Main outcome measures: The primary outcome was delirium as determined by a positive Confusion Assessment Method (CAM) score or a diagnosis of delirium by the clinical team. The secondary outcome was hospital length of stay (LOS). Process measures for pathway compliance (eg, achieving adequate pain control, early mobilization, advance care planning) were assessed. Outcome measures were compared between patients who underwent the Geriatric Trauma Care Pathway intervention (postimplementation group) vs patients who were treated prior to pathway implementation (baseline pre-implementation group).
Main results: Of the 859 eligible patients, 712 were included in the analysis (442 [62.1%] in the baseline pre-implementation group and 270 [37.9%] in the postimplementation group); mean (SD) age was 81.4 (9.1) years, and 394 (55.3%) were women. The injury mechanism was similar between groups, with falls being the most common cause of injury (247 [55.9%] in the baseline group vs 162 [60.0%] in the postimplementation group; P = .43). Injuries as measured by Injury Severity Score (ISS) were minor or moderate in both groups (261 [59.0%] in baseline group vs 168 [62.2%] in postimplementation group; P = .87). The adjusted odds ratio (OR) for delirium in the postimplementation group was lower compared to the baseline pre-implementation group (OR, 0.54; 95% CI, 0.37-0.80; P < .001). Measures of advance care planning in the postimplementation group improved, including more frequent goals-of-care documentation (53.7% in postimplementation group vs 16.7% in baseline group; P < .001) and a shortened time to first goals-of-care discussion upon presenting to the ED (36 hours in postimplementation group vs 50 hours in baseline group; P = .03).
Conclusion: Implementation of a multidisciplinary geriatric trauma clinical pathway for older adults with traumatic injury at a single level I trauma center was associated with reduced rates of delirium.
Study 2 Overview (Bryant et al)
Objective: To determine whether an interdisciplinary care pathway for frail trauma patients can improve in-hospital mortality, complications, and 30-day readmissions.
Design: Retrospective cohort study of frail patients.
Setting and participants: Eligible patients were persons aged 65 years or older who were admitted to the trauma service and survived more than 24 hours; admitted to and discharged from the trauma unit; and determined to be pre-frail or frail by a geriatrician’s assessment. A Frailty Identification and Care Pathway designed to reduce delirium and complications in frail older trauma patients was developed by a multidisciplinary team and implemented in 2016. The standardized evidence-based interdisciplinary care pathway included utilization of order sets and interventions for delirium prevention, early ambulation, bowel and pain regimens, nutrition and physical therapy consults, medication management, care-goal setting, and geriatric assessments.
Main outcome measures: The main outcomes were delirium as determined by a positive CAM score, major complications as defined by the Trauma Quality Improvement Project, in-hospital mortality, and 30-day hospital readmission. Outcome measures were compared between patients who underwent Frailty Identification and Care Pathway intervention (postintervention group) vs patients who were treated prior to pathway implementation (pre-intervention group).
Main results: A total of 269 frail patients were included in the analysis (125 in pre-intervention group vs 144 in postintervention group). Patient demographic and admission characteristics were similar between the 2 groups: mean age was 83.5 (7.1) years, 60.6% were women, and median ISS was 10 (interquartile range [IQR], 9-14). The injury mechanism was similar between groups, with falls accounting for 92.8% and 86.1% of injuries in the pre-intervention and postintervention groups, respectively (P = .07). In univariate analysis, the Frailty Identification and Care Pathway intervention was associated with a significant reduction in delirium (12.5% vs 21.6%, P = .04) and 30-day hospital readmission (2.7% vs 9.6%, P = .01) compared to patients in the pre-intervention group. However, rates of major complications (28.5% vs 28.0%, P = 0.93) and in-hospital mortality (4.2% vs 7.2%, P = .28) were similar between the pre-intervention and postintervention groups. In multivariate logistic regression models adjusted for patient characteristics (age, sex, race, ISS), patients in the postintervention group had lower delirium (OR, 0.44; 95% CI, 0.22-0.88; P = .02) and 30-day hospital readmission (OR, 0.25; 95% CI, 0.07-0.84; P = .02) rates compared to those in the pre-intervention group.
Conclusion: Implementation of an interdisciplinary care protocol for frail geriatric trauma patients significantly decreased their risks for in-hospital delirium and 30-day hospital readmission.
Commentary
Traumatic injuries in older adults are associated with higher morbidity and mortality compared to younger patients, with falls and motor vehicle accidents accounting for a majority of these injuries. Astoundingly, up to one-third of this vulnerable population presenting to hospitals with an ISS greater than 15 may die during hospitalization.1 As a result, a large number of studies and clinical trials have focused on interventions that are designed to reduce fall risks, and hence reduce adverse consequences of traumatic injuries that may arise after falls.2 However, this emphasis on falls prevention has overshadowed a need to develop effective geriatric-centered clinical interventions that aim to improve outcomes in older adults who present to hospitals with traumatic injuries. Furthermore, frailty—a geriatric syndrome indicative of an increased state of vulnerability and predictive of adverse outcomes such as delirium—is highly prevalent in older patients with traumatic injury.3 Thus, there is an urgent need to develop novel, hospital-based, traumatic injury–targeting strategies that incorporate a thoughtful redesign of the care framework that includes evidence-based interventions for geriatric syndromes such as delirium and frailty.
The study reported by Park et al (Study 1) represents the latest effort to evaluate inpatient management strategies designed to improve outcomes in hospitalized older adults who have sustained traumatic injury. Through the implementation of a novel multidisciplinary Geriatric Trauma Care Pathway that incorporates geriatric best practices, this intervention was found to be associated with a 46% lower risk of in-hospital delirium. Because of the inclusion of all age-eligible patients across all strata of traumatic injuries, rather than preselecting for those at the highest risk for poor clinical outcomes, the benefits of this intervention extend to those with minor or moderate injury severity. Furthermore, the improvement in delirium (ie, the primary outcome) is particularly meaningful given that delirium is one of the most common hospital-associated complications that increase hospital LOS, discharge to an institution, and mortality in older adults. Finally, the study’s observed reduced time to a first goals-of-care discussion and increased frequency of goals-of-care documentation after intervention should not be overlooked. The improvements in these 2 process measures are highly significant given that advanced care planning, an intervention that helps to align patients’ values, goals, and treatments, is completed at substantially lower rates in older adults in the acute hospital setting.4
Similarly, in an earlier published study, Bryant and colleagues (Study 2) also show that a geriatric-focused interdisciplinary trauma care pathway is associated with delirium risk reduction in hospitalized older trauma patients. Much like Study 1, the Frailty Identification and Care Pathway utilized in Study 2 is an evidence-based interdisciplinary care pathway that includes the use of geriatric assessments, order sets, and geriatric best practices. Moreover, its exclusive inclusion of pre-frail and frail older patients (ie, those at higher risk for poor outcomes) with moderate injury severity (median ISS of 10 [IQR, 9-14]) suggests that this type of care pathway benefits hospitalized older trauma patients, who are particularly vulnerable to adverse complications such as delirium. Moreover, the successful utilization of the FRAIL questionnaire, a validated frailty screening tool, by surgical residents in the ED to initiate this care pathway demonstrates the feasibility of its use in expediting frailty screening in older patients in trauma care.
Application for Clinical Practice and System Implementation
Findings from the 2 studies discussed in this review indicate that implementation of interdisciplinary clinical care pathways predicated on evidence-based geriatric principles and best practices is a promising approach to reduce delirium in hospitalized older trauma patients. These studies have helped to lay the groundwork in outlining the roadmaps (eg, processes and infrastructures) needed to create such clinical pathways. These key elements include: (1) integration of a multidisciplinary committee (eg, representation from trauma, emergency, and geriatric medicine, nursing, physical and occupational therapy, pharmacy, social work) in pathway design and implementation; (2) adaption of evidence-based geriatric best practices (eg, the Institute for Healthcare Improvement Age-Friendly Health System 4M framework [medication, mentation, mobility, what matters]) to prioritize interventions and to design a pathway that incorporates these features; (3) incorporation of comprehensive geriatric assessment by interdisciplinary providers; (4) utilization of validated clinical instruments to assess physical and cognitive functions, frailty, delirium, and social determinants of health; (5) modification of electronic health record systems to encompass order sets that incorporate evidence-based, nonpharmacological and pharmacological interventions to manage symptoms (eg, delirium, pain, bowel movement, sleep, immobility, polypharmacy) essential to quality geriatric care; and (6) integration of patient and caregiver preferences via goals-of-care discussions and corresponding documentation and communication of these goals.
Additionally, these 2 studies imparted some strategies that may facilitate the implementation of interdisciplinary clinical care pathways in trauma care. Examples of such facilitators include: (1) collaboration with champions within each specialty to reinforce education and buy-in; (2) creation of automatically triggered order sets upon patient presentation to the ED that unites distinct features of clinical pathways; (3) adaption and reorganization of existing hospital infrastructures and resources to meet the needs of clinical pathways implementation (eg, utilizing information technology resources to develop electronic health record order sets; using quality department to develop clinical pathway guidelines and electronic outcome dashboards); and (4) development of individualized patient and caregiver education materials based on care needs (eg, principles of delirium prevention and preservation of mobility during hospitalization) to prepare and engage these stakeholders in patient care and recovery.
Practice Points
- A geriatric interdisciplinary care model can be effectively applied to the management of acute trauma in older patients.
- Interdisciplinary clinical pathways should incorporate geriatric best practices and guidelines and age-appropriate order sets to prioritize and integrate care.
—Fred Ko, MD, MS
1. Hashmi A, Ibrahim-Zada I, Rhee P, et al. Predictors of mortality in geriatric trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2014;76(3):894-901. doi:10.1097/TA.0b013e3182ab0763
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:10.1002/14651858.CD012221.pub2
3. Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149(8):766-772. doi:10.1001/jamasurg.2014.296
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10(2):164-174. doi:10.1136/bmjspcare-2019-002093
1. Hashmi A, Ibrahim-Zada I, Rhee P, et al. Predictors of mortality in geriatric trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2014;76(3):894-901. doi:10.1097/TA.0b013e3182ab0763
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:10.1002/14651858.CD012221.pub2
3. Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149(8):766-772. doi:10.1001/jamasurg.2014.296
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10(2):164-174. doi:10.1136/bmjspcare-2019-002093
Hospital-acquired pneumonia is killing patients, yet there is a simple way to stop it
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Effect of Pharmacist Interventions on Hospital Readmissions for Home-Based Primary Care Veterans
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
Registered Dietitian Nutritionists’ Role in Hospital in Home
Hospital in Home (HIH) is the delivery of acute care services in a patient’s home as an alternative to hospitalization.1 Compared with traditional inpatient care, HIH programs have been associated with reduced costs, as well as patient and caregiver satisfaction, diseasespecific outcomes, and mortality rates that were similar or improved compared with inpatient admissions.1-4
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) and other hospital systems are increasingly adopting HIH models.2-4 At the time of this writing, there were 12 HIH programs in VHA (personal communication, D. Cooper, 2/28/2022). In addition to physicians and nurses, the interdisciplinary HIH team may include a pharmacist, social worker, and registered dietitian nutritionist (RDN).2,5 HIH programs have been shown to improve nutritional status as measured by the Mini Nutritional Assessment Score, but overall, there is a paucity of published information regarding the provision of nutrition care in HIH.6 The role of the RDN has varied within VHA. Some sites, such as the Sacramento VA Medical Center in California, include a distinct RDN position on the HIH team, whereas others, such as the Spark M. Matsunaga VA Medical Center in Honolulu, Hawaii, and the James A. Haley Veterans’ Hospital in Tampa, Florida, consult clinic RDNs.
Since HIH programs typically treat conditions for which diet is an inherent part of the treatment (eg, congestive heart failure [CHF]), there is a need to precisely define the role of the RDN within the HIH model.2,3,7 Drawing from my experience as an HIH RDN, I will describe how the inclusion of an RDN position within the HIH team is optimal for health care delivery and how HIH practitioners can best utilize RDN services.
RDN Role in HIH Team
Delegating nutrition services to an RDN enhances patient care by empowering HIH team members to function at the highest level of their scope of practice. RDNs have been recognized by physicians as the most qualified health care professionals to help patients with diet-related conditions, such as obesity, and physicians also have reported a desire for additional training in nutrition.8 Although home-health nurses have frequently performed nutrition assessments and interventions, survey results have indicated that many nurses do not feel confident in teaching complex nutritional information.9 In my experience, many HIH patients are nutritionally complex, with more than one condition requiring nutrition intervention. For example, patients may be admitted to HIH for management of CHF, but they may also have diabetes mellitus (DM), obesity, and low socioeconomic status. The HIH RDN can address the nutrition aspects of these conditions, freeing time for physicians and nurses to focus on their respective areas of expertise.9,10 Moreover, the RDN can also provide dietary education to the HIH team to increase their knowledge of nutritional topics and promote consistent messaging to patients.
Including an RDN on the HIH team enables patients to have comprehensive, personalized nutrition care. Rather than merely offering generalized nutrition education, RDNs are trained to provide medical nutrition therapy (MNT), which has been shown to improve health outcomes and be cost-effective for conditions such as type 2 DM, chronic kidney disease, hypertension, and obesity.10,11 In MNT, RDNs use the standardized 4-stepnutrition care process (NCP).12 The Table shows examples of how the NCP can be applied in HIH settings. Furthermore, in my experience, MNT from an RDN also contributes to patient satisfaction. Subjective observations from my team have indicated that patients often express more confidence in managing their diets by the time of HIH discharge.
RDNs can guide physicians and pharmacists in ordering oral nutrition supplements (ONS). Within the VHA, a “food first” approach is preferred to increase caloric intake, and patients must meet specific criteria for prescription of an ONS.13 Furthermore, ONS designed for specific medical conditions (eg, chronic kidney disease) are considered nonformulary and require an RDN evaluation.13 Including an RDN on the HIH team allows this evaluation process to begin early in the patient’s admission to the program and ensures that provision of ONS is clinically appropriate and cost-effective.
Care Coordination
HIH is highly interdisciplinary. Team members perform their respective roles and communicate with the team throughout the day. RDNs can help monitor patients and alert physicians for changes in blood glucose, gastrointestinal concerns, and weight. This is especially helpful for patients who do not have a planned nursing visit on the day of an RDN evaluation. The HIH RDN can also collaborate with other team members to address patient needs. For example, for patients with limited financial resources, the HIH RDN can provide nutrition education regarding cooking on a budget, and the HIH social worker can arrange free or low-cost meal services.
Tips
When hiring an HIH RDN, seek candidates with experience in inpatient, outpatient, and home care settings. As a hybrid of these 3 areas, the HIH RDN position requires a unique combination of acute care skills and health coaching. Additionally, in my experience, the HIH RDN interacts more frequently with the HIH team than other RDN colleagues, so it is important that candidates can work independently and take initiative. This type of position would not be suitable for entry-level RDNs.
Stagger HIH team visits to prevent overwhelming the patient and caregivers. Early in our program, my team quickly learned that patients and caregivers can feel overwhelmed with too many home visits upon admission to HIH. After seeing multiple HIH team members the same day, they were often too tired to focus well on diet education during my visit. Staggering visits (eg, completing the initial nutrition assessment 1 day to 1 week after the initial medical and pharmacy visits) has been an effective strategy to address this problem. Furthermore, some patients prefer that the initial RDN appointment is conducted by telephone, with an inperson reassessment the following week. In my experience, HIH workflow is dynamic by nature, so it is crucial to remain flexible and accommodate individual patient needs as much as possible.
Dietary behavior change is a long-term process, and restrictive hospital diets can be challenging to replicate at home. In a hospital setting, clinicians can order a specialized diet (eg, low sodium with fluid restriction for CHF patients), whereas efforts to implement these restrictions in the home setting can be cumbersome and negatively impact quality of life.7,14 Nevertheless, the effectiveness of medical treatment is compromised when patients do not adhere to dietary recommendations. Meal delivery services that offer specialized diets can be a useful resource for patients and caregivers who are unable to cook, and the HIH RDN can assist patients in ordering these services.
HIH patients may vary in terms of readiness to make dietary changes, and in addition to nutrition education, nutrition counseling is usually needed to effect behavior change. My team has found that consideration of the transtheoretical/ stages of change model can be a helpful approach. 15 The HIH RDN can tailor nutrition interventions to the patient’s stage of change. For example, for patients in the precontemplation stage, the HIH RDN would focus on providing information and addressing emotional aspects of dietary change. In contrast, for patients in the action stage of change, the HIH RDN might emphasize behavioral skill training and social support.15 Particularly for patients in the early stages of change, it may be unrealistic to expect full adoption of the recommended diet within the 30 days of the HIH program. However, by acknowledging the reality of the patient’s stage of change, the HIH RDN and team can then collaborate to support the patient in moving toward the next stage. Patients who are not ready for dietary behavior change during the 30 days of HIH may benefit from longer-term support, and the HIH RDN can arrange followup care with an outpatient RDN.
Conclusions
As the HIH model continues to be adopted across the VHA and other health care systems, it is crucial to consider the value and expertise of an RDN for guiding nutrition care in the HIH setting. The HIH RDN contributes to optimal health care delivery by leading nutritional aspects of patient care, offering personalized MNT, and coordinating and collaborating with team members to meet individual patient needs. An RDN can serve as a valuable resource for nutrition information and enhance the team’s overall services, with the potential to impact clinical outcomes and patient satisfaction.
1. Levine DM, Ouchi K, Blanchfield B, et al. Hospitallevel care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172(2):77-85. doi:10.7326/M19-0600
2. Cai S, Grubbs A, Makineni R, Kinosian B, Phibbs CS, Intrator O. Evaluation of the Cincinnati Veterans Affairs medical center hospital-in-home program. J Am Geriatr Soc. 2018;66(7):1392-1398. doi:10.1111/jgs.15382
3. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
4. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. doi:10.1001/jamainternmed.2016.5974
5. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1144: Hospital in Home program, Appendix A, Hospital in Home program standards. January 19, 2021. Accessed May 5, 2022. https://www .va.gov/VHApublications/ViewPublication.asp?pub _ID=9157
6. Tibaldi V, Isaia G, Scarafiotti C, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Arch Intern Med. 2009;169(17):1569-1575. doi:10.1001/archinternmed.2009.267
7. Abshire M, Xu J, Baptiste D, et al. Nutritional interventions in heart failure: a systematic review of the literature. J Card Fail. 2015;21(12):989-999. doi:10.1016/j.cardfail.2015.10.004
8. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open. 2012;2(6):e001871. Published 2012 Dec 20. doi:10.1136/bmjopen-2012-001871
9. Sousa AM. Benefits of dietitian home visits. J Am Diet Assoc. 1994;94(10):1149-1151. doi:10.1016/0002-8223(94)91136-3
10. Casas-Agustench P, Megías-Rangil I, Babio N. Economic benefit of dietetic-nutritional treatment in the multidisciplinary primary care team. Beneficio económico del tratamiento dietético-nutricional en el equipo multidisciplinario de atención primaria. Nutr Hosp. 2020;37(4):863-874. doi:10.20960/nh.03025
11. Lee J, Briggs Early K, Kovesdy CP, Lancaster K, Brown N, Steiber AL. The impact of RDNs on non-communicable diseases: proceedings from The State of Food and Nutrition Series Forum. J Acad Nutr Diet. 2022;122(1):166-174. doi:10.1016/j.jand.2021.02.021
12. Academy of Nutrition and Dietetics. Evidence analysis library, nutrition care process. Accessed May 5, 2022. https://www.andeal.org/ncp
13. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1438, clinical nutrition management and therapy. Appendix A, nutrition support therapy. September 19, 2019. Accessed January 20, 2022. https://www.va.gov/VHAPUBLICATIONS/ViewPublication .asp?pub_ID=8512
14. Vogelzang JL. Fifteen ways to enhance client outcomes by using your registered dietitian. Home Healthc Nurse. 2002;20(4):227-229. doi:10.1097/00004045-200204000-00005
15. Kristal AR, Glanz K, Curry SJ, Patterson RE. How can stages of change be best used in dietary interventions?. J Am Diet Assoc. 1999;99(6):679-684. doi:10.1016/S0002-8223(99)00165-0
Hospital in Home (HIH) is the delivery of acute care services in a patient’s home as an alternative to hospitalization.1 Compared with traditional inpatient care, HIH programs have been associated with reduced costs, as well as patient and caregiver satisfaction, diseasespecific outcomes, and mortality rates that were similar or improved compared with inpatient admissions.1-4
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) and other hospital systems are increasingly adopting HIH models.2-4 At the time of this writing, there were 12 HIH programs in VHA (personal communication, D. Cooper, 2/28/2022). In addition to physicians and nurses, the interdisciplinary HIH team may include a pharmacist, social worker, and registered dietitian nutritionist (RDN).2,5 HIH programs have been shown to improve nutritional status as measured by the Mini Nutritional Assessment Score, but overall, there is a paucity of published information regarding the provision of nutrition care in HIH.6 The role of the RDN has varied within VHA. Some sites, such as the Sacramento VA Medical Center in California, include a distinct RDN position on the HIH team, whereas others, such as the Spark M. Matsunaga VA Medical Center in Honolulu, Hawaii, and the James A. Haley Veterans’ Hospital in Tampa, Florida, consult clinic RDNs.
Since HIH programs typically treat conditions for which diet is an inherent part of the treatment (eg, congestive heart failure [CHF]), there is a need to precisely define the role of the RDN within the HIH model.2,3,7 Drawing from my experience as an HIH RDN, I will describe how the inclusion of an RDN position within the HIH team is optimal for health care delivery and how HIH practitioners can best utilize RDN services.
RDN Role in HIH Team
Delegating nutrition services to an RDN enhances patient care by empowering HIH team members to function at the highest level of their scope of practice. RDNs have been recognized by physicians as the most qualified health care professionals to help patients with diet-related conditions, such as obesity, and physicians also have reported a desire for additional training in nutrition.8 Although home-health nurses have frequently performed nutrition assessments and interventions, survey results have indicated that many nurses do not feel confident in teaching complex nutritional information.9 In my experience, many HIH patients are nutritionally complex, with more than one condition requiring nutrition intervention. For example, patients may be admitted to HIH for management of CHF, but they may also have diabetes mellitus (DM), obesity, and low socioeconomic status. The HIH RDN can address the nutrition aspects of these conditions, freeing time for physicians and nurses to focus on their respective areas of expertise.9,10 Moreover, the RDN can also provide dietary education to the HIH team to increase their knowledge of nutritional topics and promote consistent messaging to patients.
Including an RDN on the HIH team enables patients to have comprehensive, personalized nutrition care. Rather than merely offering generalized nutrition education, RDNs are trained to provide medical nutrition therapy (MNT), which has been shown to improve health outcomes and be cost-effective for conditions such as type 2 DM, chronic kidney disease, hypertension, and obesity.10,11 In MNT, RDNs use the standardized 4-stepnutrition care process (NCP).12 The Table shows examples of how the NCP can be applied in HIH settings. Furthermore, in my experience, MNT from an RDN also contributes to patient satisfaction. Subjective observations from my team have indicated that patients often express more confidence in managing their diets by the time of HIH discharge.
RDNs can guide physicians and pharmacists in ordering oral nutrition supplements (ONS). Within the VHA, a “food first” approach is preferred to increase caloric intake, and patients must meet specific criteria for prescription of an ONS.13 Furthermore, ONS designed for specific medical conditions (eg, chronic kidney disease) are considered nonformulary and require an RDN evaluation.13 Including an RDN on the HIH team allows this evaluation process to begin early in the patient’s admission to the program and ensures that provision of ONS is clinically appropriate and cost-effective.
Care Coordination
HIH is highly interdisciplinary. Team members perform their respective roles and communicate with the team throughout the day. RDNs can help monitor patients and alert physicians for changes in blood glucose, gastrointestinal concerns, and weight. This is especially helpful for patients who do not have a planned nursing visit on the day of an RDN evaluation. The HIH RDN can also collaborate with other team members to address patient needs. For example, for patients with limited financial resources, the HIH RDN can provide nutrition education regarding cooking on a budget, and the HIH social worker can arrange free or low-cost meal services.
Tips
When hiring an HIH RDN, seek candidates with experience in inpatient, outpatient, and home care settings. As a hybrid of these 3 areas, the HIH RDN position requires a unique combination of acute care skills and health coaching. Additionally, in my experience, the HIH RDN interacts more frequently with the HIH team than other RDN colleagues, so it is important that candidates can work independently and take initiative. This type of position would not be suitable for entry-level RDNs.
Stagger HIH team visits to prevent overwhelming the patient and caregivers. Early in our program, my team quickly learned that patients and caregivers can feel overwhelmed with too many home visits upon admission to HIH. After seeing multiple HIH team members the same day, they were often too tired to focus well on diet education during my visit. Staggering visits (eg, completing the initial nutrition assessment 1 day to 1 week after the initial medical and pharmacy visits) has been an effective strategy to address this problem. Furthermore, some patients prefer that the initial RDN appointment is conducted by telephone, with an inperson reassessment the following week. In my experience, HIH workflow is dynamic by nature, so it is crucial to remain flexible and accommodate individual patient needs as much as possible.
Dietary behavior change is a long-term process, and restrictive hospital diets can be challenging to replicate at home. In a hospital setting, clinicians can order a specialized diet (eg, low sodium with fluid restriction for CHF patients), whereas efforts to implement these restrictions in the home setting can be cumbersome and negatively impact quality of life.7,14 Nevertheless, the effectiveness of medical treatment is compromised when patients do not adhere to dietary recommendations. Meal delivery services that offer specialized diets can be a useful resource for patients and caregivers who are unable to cook, and the HIH RDN can assist patients in ordering these services.
HIH patients may vary in terms of readiness to make dietary changes, and in addition to nutrition education, nutrition counseling is usually needed to effect behavior change. My team has found that consideration of the transtheoretical/ stages of change model can be a helpful approach. 15 The HIH RDN can tailor nutrition interventions to the patient’s stage of change. For example, for patients in the precontemplation stage, the HIH RDN would focus on providing information and addressing emotional aspects of dietary change. In contrast, for patients in the action stage of change, the HIH RDN might emphasize behavioral skill training and social support.15 Particularly for patients in the early stages of change, it may be unrealistic to expect full adoption of the recommended diet within the 30 days of the HIH program. However, by acknowledging the reality of the patient’s stage of change, the HIH RDN and team can then collaborate to support the patient in moving toward the next stage. Patients who are not ready for dietary behavior change during the 30 days of HIH may benefit from longer-term support, and the HIH RDN can arrange followup care with an outpatient RDN.
Conclusions
As the HIH model continues to be adopted across the VHA and other health care systems, it is crucial to consider the value and expertise of an RDN for guiding nutrition care in the HIH setting. The HIH RDN contributes to optimal health care delivery by leading nutritional aspects of patient care, offering personalized MNT, and coordinating and collaborating with team members to meet individual patient needs. An RDN can serve as a valuable resource for nutrition information and enhance the team’s overall services, with the potential to impact clinical outcomes and patient satisfaction.
Hospital in Home (HIH) is the delivery of acute care services in a patient’s home as an alternative to hospitalization.1 Compared with traditional inpatient care, HIH programs have been associated with reduced costs, as well as patient and caregiver satisfaction, diseasespecific outcomes, and mortality rates that were similar or improved compared with inpatient admissions.1-4
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) and other hospital systems are increasingly adopting HIH models.2-4 At the time of this writing, there were 12 HIH programs in VHA (personal communication, D. Cooper, 2/28/2022). In addition to physicians and nurses, the interdisciplinary HIH team may include a pharmacist, social worker, and registered dietitian nutritionist (RDN).2,5 HIH programs have been shown to improve nutritional status as measured by the Mini Nutritional Assessment Score, but overall, there is a paucity of published information regarding the provision of nutrition care in HIH.6 The role of the RDN has varied within VHA. Some sites, such as the Sacramento VA Medical Center in California, include a distinct RDN position on the HIH team, whereas others, such as the Spark M. Matsunaga VA Medical Center in Honolulu, Hawaii, and the James A. Haley Veterans’ Hospital in Tampa, Florida, consult clinic RDNs.
Since HIH programs typically treat conditions for which diet is an inherent part of the treatment (eg, congestive heart failure [CHF]), there is a need to precisely define the role of the RDN within the HIH model.2,3,7 Drawing from my experience as an HIH RDN, I will describe how the inclusion of an RDN position within the HIH team is optimal for health care delivery and how HIH practitioners can best utilize RDN services.
RDN Role in HIH Team
Delegating nutrition services to an RDN enhances patient care by empowering HIH team members to function at the highest level of their scope of practice. RDNs have been recognized by physicians as the most qualified health care professionals to help patients with diet-related conditions, such as obesity, and physicians also have reported a desire for additional training in nutrition.8 Although home-health nurses have frequently performed nutrition assessments and interventions, survey results have indicated that many nurses do not feel confident in teaching complex nutritional information.9 In my experience, many HIH patients are nutritionally complex, with more than one condition requiring nutrition intervention. For example, patients may be admitted to HIH for management of CHF, but they may also have diabetes mellitus (DM), obesity, and low socioeconomic status. The HIH RDN can address the nutrition aspects of these conditions, freeing time for physicians and nurses to focus on their respective areas of expertise.9,10 Moreover, the RDN can also provide dietary education to the HIH team to increase their knowledge of nutritional topics and promote consistent messaging to patients.
Including an RDN on the HIH team enables patients to have comprehensive, personalized nutrition care. Rather than merely offering generalized nutrition education, RDNs are trained to provide medical nutrition therapy (MNT), which has been shown to improve health outcomes and be cost-effective for conditions such as type 2 DM, chronic kidney disease, hypertension, and obesity.10,11 In MNT, RDNs use the standardized 4-stepnutrition care process (NCP).12 The Table shows examples of how the NCP can be applied in HIH settings. Furthermore, in my experience, MNT from an RDN also contributes to patient satisfaction. Subjective observations from my team have indicated that patients often express more confidence in managing their diets by the time of HIH discharge.
RDNs can guide physicians and pharmacists in ordering oral nutrition supplements (ONS). Within the VHA, a “food first” approach is preferred to increase caloric intake, and patients must meet specific criteria for prescription of an ONS.13 Furthermore, ONS designed for specific medical conditions (eg, chronic kidney disease) are considered nonformulary and require an RDN evaluation.13 Including an RDN on the HIH team allows this evaluation process to begin early in the patient’s admission to the program and ensures that provision of ONS is clinically appropriate and cost-effective.
Care Coordination
HIH is highly interdisciplinary. Team members perform their respective roles and communicate with the team throughout the day. RDNs can help monitor patients and alert physicians for changes in blood glucose, gastrointestinal concerns, and weight. This is especially helpful for patients who do not have a planned nursing visit on the day of an RDN evaluation. The HIH RDN can also collaborate with other team members to address patient needs. For example, for patients with limited financial resources, the HIH RDN can provide nutrition education regarding cooking on a budget, and the HIH social worker can arrange free or low-cost meal services.
Tips
When hiring an HIH RDN, seek candidates with experience in inpatient, outpatient, and home care settings. As a hybrid of these 3 areas, the HIH RDN position requires a unique combination of acute care skills and health coaching. Additionally, in my experience, the HIH RDN interacts more frequently with the HIH team than other RDN colleagues, so it is important that candidates can work independently and take initiative. This type of position would not be suitable for entry-level RDNs.
Stagger HIH team visits to prevent overwhelming the patient and caregivers. Early in our program, my team quickly learned that patients and caregivers can feel overwhelmed with too many home visits upon admission to HIH. After seeing multiple HIH team members the same day, they were often too tired to focus well on diet education during my visit. Staggering visits (eg, completing the initial nutrition assessment 1 day to 1 week after the initial medical and pharmacy visits) has been an effective strategy to address this problem. Furthermore, some patients prefer that the initial RDN appointment is conducted by telephone, with an inperson reassessment the following week. In my experience, HIH workflow is dynamic by nature, so it is crucial to remain flexible and accommodate individual patient needs as much as possible.
Dietary behavior change is a long-term process, and restrictive hospital diets can be challenging to replicate at home. In a hospital setting, clinicians can order a specialized diet (eg, low sodium with fluid restriction for CHF patients), whereas efforts to implement these restrictions in the home setting can be cumbersome and negatively impact quality of life.7,14 Nevertheless, the effectiveness of medical treatment is compromised when patients do not adhere to dietary recommendations. Meal delivery services that offer specialized diets can be a useful resource for patients and caregivers who are unable to cook, and the HIH RDN can assist patients in ordering these services.
HIH patients may vary in terms of readiness to make dietary changes, and in addition to nutrition education, nutrition counseling is usually needed to effect behavior change. My team has found that consideration of the transtheoretical/ stages of change model can be a helpful approach. 15 The HIH RDN can tailor nutrition interventions to the patient’s stage of change. For example, for patients in the precontemplation stage, the HIH RDN would focus on providing information and addressing emotional aspects of dietary change. In contrast, for patients in the action stage of change, the HIH RDN might emphasize behavioral skill training and social support.15 Particularly for patients in the early stages of change, it may be unrealistic to expect full adoption of the recommended diet within the 30 days of the HIH program. However, by acknowledging the reality of the patient’s stage of change, the HIH RDN and team can then collaborate to support the patient in moving toward the next stage. Patients who are not ready for dietary behavior change during the 30 days of HIH may benefit from longer-term support, and the HIH RDN can arrange followup care with an outpatient RDN.
Conclusions
As the HIH model continues to be adopted across the VHA and other health care systems, it is crucial to consider the value and expertise of an RDN for guiding nutrition care in the HIH setting. The HIH RDN contributes to optimal health care delivery by leading nutritional aspects of patient care, offering personalized MNT, and coordinating and collaborating with team members to meet individual patient needs. An RDN can serve as a valuable resource for nutrition information and enhance the team’s overall services, with the potential to impact clinical outcomes and patient satisfaction.
1. Levine DM, Ouchi K, Blanchfield B, et al. Hospitallevel care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172(2):77-85. doi:10.7326/M19-0600
2. Cai S, Grubbs A, Makineni R, Kinosian B, Phibbs CS, Intrator O. Evaluation of the Cincinnati Veterans Affairs medical center hospital-in-home program. J Am Geriatr Soc. 2018;66(7):1392-1398. doi:10.1111/jgs.15382
3. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
4. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. doi:10.1001/jamainternmed.2016.5974
5. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1144: Hospital in Home program, Appendix A, Hospital in Home program standards. January 19, 2021. Accessed May 5, 2022. https://www .va.gov/VHApublications/ViewPublication.asp?pub _ID=9157
6. Tibaldi V, Isaia G, Scarafiotti C, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Arch Intern Med. 2009;169(17):1569-1575. doi:10.1001/archinternmed.2009.267
7. Abshire M, Xu J, Baptiste D, et al. Nutritional interventions in heart failure: a systematic review of the literature. J Card Fail. 2015;21(12):989-999. doi:10.1016/j.cardfail.2015.10.004
8. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open. 2012;2(6):e001871. Published 2012 Dec 20. doi:10.1136/bmjopen-2012-001871
9. Sousa AM. Benefits of dietitian home visits. J Am Diet Assoc. 1994;94(10):1149-1151. doi:10.1016/0002-8223(94)91136-3
10. Casas-Agustench P, Megías-Rangil I, Babio N. Economic benefit of dietetic-nutritional treatment in the multidisciplinary primary care team. Beneficio económico del tratamiento dietético-nutricional en el equipo multidisciplinario de atención primaria. Nutr Hosp. 2020;37(4):863-874. doi:10.20960/nh.03025
11. Lee J, Briggs Early K, Kovesdy CP, Lancaster K, Brown N, Steiber AL. The impact of RDNs on non-communicable diseases: proceedings from The State of Food and Nutrition Series Forum. J Acad Nutr Diet. 2022;122(1):166-174. doi:10.1016/j.jand.2021.02.021
12. Academy of Nutrition and Dietetics. Evidence analysis library, nutrition care process. Accessed May 5, 2022. https://www.andeal.org/ncp
13. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1438, clinical nutrition management and therapy. Appendix A, nutrition support therapy. September 19, 2019. Accessed January 20, 2022. https://www.va.gov/VHAPUBLICATIONS/ViewPublication .asp?pub_ID=8512
14. Vogelzang JL. Fifteen ways to enhance client outcomes by using your registered dietitian. Home Healthc Nurse. 2002;20(4):227-229. doi:10.1097/00004045-200204000-00005
15. Kristal AR, Glanz K, Curry SJ, Patterson RE. How can stages of change be best used in dietary interventions?. J Am Diet Assoc. 1999;99(6):679-684. doi:10.1016/S0002-8223(99)00165-0
1. Levine DM, Ouchi K, Blanchfield B, et al. Hospitallevel care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172(2):77-85. doi:10.7326/M19-0600
2. Cai S, Grubbs A, Makineni R, Kinosian B, Phibbs CS, Intrator O. Evaluation of the Cincinnati Veterans Affairs medical center hospital-in-home program. J Am Geriatr Soc. 2018;66(7):1392-1398. doi:10.1111/jgs.15382
3. Cai S, Laurel PA, Makineni R, Marks ML. Evaluation of a hospital-in-home program implemented among veterans. Am J Manag Care. 2017;23(8):482-487.
4. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. doi:10.1001/jamainternmed.2016.5974
5. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1144: Hospital in Home program, Appendix A, Hospital in Home program standards. January 19, 2021. Accessed May 5, 2022. https://www .va.gov/VHApublications/ViewPublication.asp?pub _ID=9157
6. Tibaldi V, Isaia G, Scarafiotti C, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Arch Intern Med. 2009;169(17):1569-1575. doi:10.1001/archinternmed.2009.267
7. Abshire M, Xu J, Baptiste D, et al. Nutritional interventions in heart failure: a systematic review of the literature. J Card Fail. 2015;21(12):989-999. doi:10.1016/j.cardfail.2015.10.004
8. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open. 2012;2(6):e001871. Published 2012 Dec 20. doi:10.1136/bmjopen-2012-001871
9. Sousa AM. Benefits of dietitian home visits. J Am Diet Assoc. 1994;94(10):1149-1151. doi:10.1016/0002-8223(94)91136-3
10. Casas-Agustench P, Megías-Rangil I, Babio N. Economic benefit of dietetic-nutritional treatment in the multidisciplinary primary care team. Beneficio económico del tratamiento dietético-nutricional en el equipo multidisciplinario de atención primaria. Nutr Hosp. 2020;37(4):863-874. doi:10.20960/nh.03025
11. Lee J, Briggs Early K, Kovesdy CP, Lancaster K, Brown N, Steiber AL. The impact of RDNs on non-communicable diseases: proceedings from The State of Food and Nutrition Series Forum. J Acad Nutr Diet. 2022;122(1):166-174. doi:10.1016/j.jand.2021.02.021
12. Academy of Nutrition and Dietetics. Evidence analysis library, nutrition care process. Accessed May 5, 2022. https://www.andeal.org/ncp
13. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1438, clinical nutrition management and therapy. Appendix A, nutrition support therapy. September 19, 2019. Accessed January 20, 2022. https://www.va.gov/VHAPUBLICATIONS/ViewPublication .asp?pub_ID=8512
14. Vogelzang JL. Fifteen ways to enhance client outcomes by using your registered dietitian. Home Healthc Nurse. 2002;20(4):227-229. doi:10.1097/00004045-200204000-00005
15. Kristal AR, Glanz K, Curry SJ, Patterson RE. How can stages of change be best used in dietary interventions?. J Am Diet Assoc. 1999;99(6):679-684. doi:10.1016/S0002-8223(99)00165-0