User login
Presence of autoantibodies most predictive of long COVID in study
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
FROM CELL
Dapivirine vaginal ring for HIV prevention no longer under consideration by the FDA
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: United States passes 10 million total cases
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.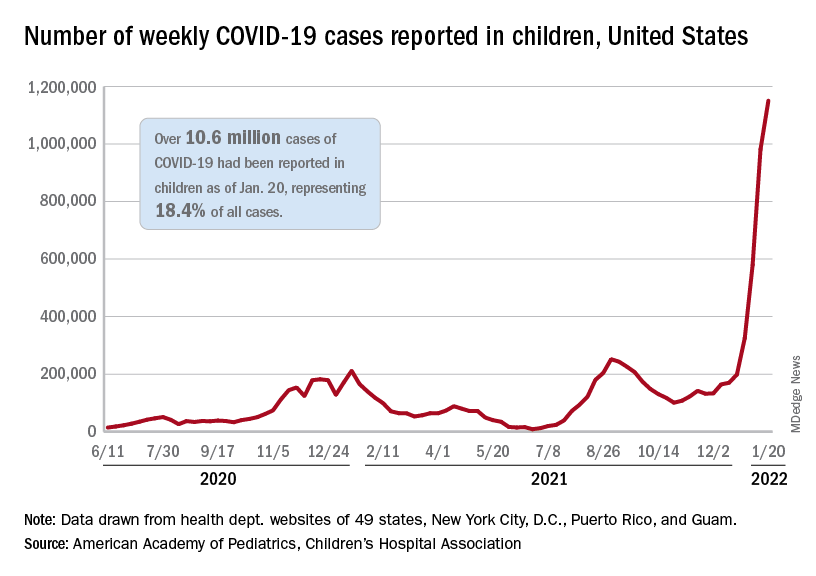
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.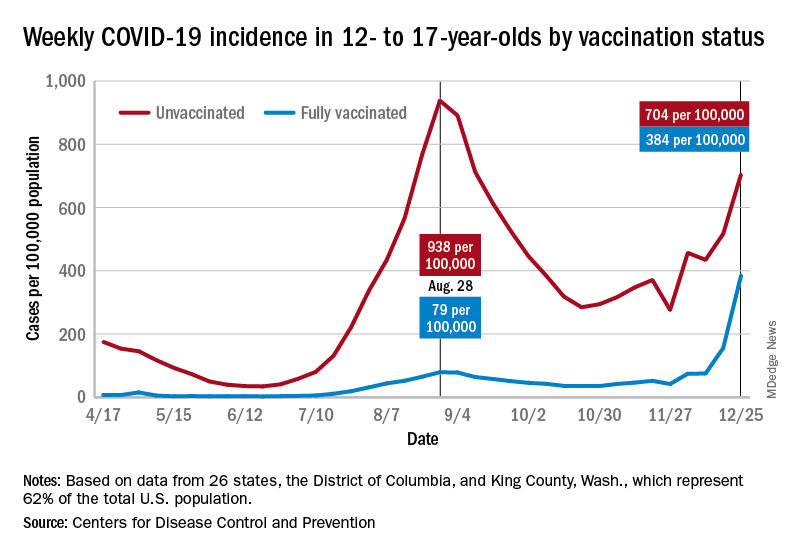
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Ways to make sure 2022 doesn’t stink for docs
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Alternative birthing practices tied to neonatal infection risk
Increasingly popular alternative peripartum practices such as water immersion and nonseverance of the umbilical cord may increase the risk of infections in newborns, a new clinical report from the American Academy of Pediatrics found.
Another perinatal measure potentially raising infection risk was placentophagy, according to a review led by Dawn Nolt, MD, MPH, a professor of pediatric infectious diseases at Oregon Health & Science University, Portland.
“Awareness of emerging alternative peripartum and neonatal practices helps pediatricians provide counseling to families before birth and to appropriately evaluate and treat neonates who have been exposed to these practices,” Dr. Nolt and colleagues wrote online in Pediatrics.
Amid growing inquiries made from women seeking a positive and meaningful birth experience through alternative approaches as well as reports of possibly related illness in newborns, Dr. Nolt’s group reviewed observational studies, case series, and medical society guidance on the risks associated with seven alternative birthing practices.
Based on their summation, it was not possible to quantify the actual risk associated with any one practice. “But of the seven we reviewed, as an infectious disease pediatrician I would say the most discernible immediate risk is likely attached to nonseverance of the cord,” Dr. Nolt said in an interview. “Left attached, the tissue can potentially necrote and transfer bacteria directly to the child.”
The authors made the following recommendations:
- Water immersion for labor and delivery. While this can increase the comfort of the mother in the first stages of labor, the water can become contaminated and increase the infant’s exposure to water-borne pathogens such as Legionella and Pseudomonas. It is not recommended after the second stage of labor and if offered, requires rigorous prophylactic and infection-control measures. This practice has also been linked to aspiration, drowning, hyponatremia, cord rupture, and death.
- Vaginal seeding. The skin, noses, and mouths of infants born by cesarean section are inoculated with swabs of vaginal fluid in order to expose them to vaginal bacteria that positively influence the infant’s microbiome. Of no known benefit, this measure can expose newborns to microbes such as group B Streptococcus and herpes simplex virus. Infants born by C-section receiving vaginal seeding should be evaluated the same way as those delivered vaginally.
- Umbilical cord nonseverance. Colloquially known as lotus birth, this is another practice with no evidence of advantage but with the potential to raise the risk of neonatal sepsis owing to the presence of necrotic umbilical or placental tissue. Some parents may view the placenta as a spiritual entity and fail to recognize it may be contaminated with harmful pathogens. Any placenta and umbilical cord attached to a febrile or ill-seeming neonate should be immediately removed.
- Placentophagy. Proponents believe placental consumption has antidepressive, analgesic, galactogogic, and nutritional properties. But eating raw, cooked, or dehydrated afterbirth tissue – viewed by some as a spiritual event – can expose a neonate to flora from the mother’s genitourinary tract and other sources encountered during preparation. Placentophagy has been associated with a case of recurrent late-onset group B streptococcal sepsis in a newborn. Strict food-handling practices at the level for raw meat should be maintained.
- HBV vaccine deferral. Viewed as “a critical safety net in preventing HBV infection,” the birth dose of the hepatitis B virus vaccine should not be postponed except for medical reasons. An estimated 1,000 new perinatally acquired HBV cases occurred annually in the United States from 2000 to 2009.
- Deferral of ocular prophylaxis. While ocular prophylaxis with topical erythromycin protects against gonococcal ophthalmia neonatorum, particularly in infants of high-risk mothers, it is not effective against other common pathogens. Parents and health care providers have recently questioned the need for its routine application, with concerns including its limited range of effectiveness as well as antibiotic resistance and shortages. With adequate prenatal testing, the risk of this neonatal conjunctivitis is significantly reduced, and deferral of prophylaxis may be considered in low-risk situations although it may be mandated by state legislation.
- Delayed bathing. The practice of delaying the infant’s first bath until several hours after birth may have several benefits. These include the initiation and exclusivity of breastfeeding, decreased mother/child separation time and risk of hypothermia, and protection of the neonatal skin microbiome. It should be discouraged, however, in neonates exposed to active herpes simplex virus lesions or whose mothers have a known history of HIV infection.
When women inquire about alternative practices, physicians need to strike a diplomatic balance between respecting women’s wishes and the benefits they hope to gain and at the same time informing them of potential risks, Dr. Nolt said. “The conversation we want to have with them should show compassion and sympathy but also tell them what the medical literature shows.” Patient and doctor should engage in shared decision-making about the safety of various alternative approaches.
“Over the last decade information on a variety of birth practices have become more widely available through social media and other Internet forums, which certainly has increased the variety of questions to health professionals, Amy C. Hermesch, MD, PhD, director of obstetric services at OHSC, said in an interview.
“We counsel about rare but serious risk, as noted in Dr. Nolt’s article,” said Dr. Hermesch, who was not involved in the AAP report. Most important is a discussion about appropriate pregnancy risk stratification. “For example, persons considering water immersion birth, probably the most common one I get inquiries about, should have an otherwise uncomplicated pregnancy with good mobility to get in and out of tub in the event of an emergency.”
While adverse events can happen during any birth, she sees these more often in mothers who underestimate the risk level of their situation or pregnancy when declining provider-recommended interventions. “I encourage pregnant persons to find a health care professional they trust who is knowledgeable about the benefits and the risk of all birth environments and interventions.”
Dr. Hermesch added that most alternative practices have little data to guide decisions, so she offers professional society recommendations, evidence review, and her own professional experiences. “The patient must weight the risk and benefits in the context of their value system and sometimes this means not following my advice or recommendations. My medical recommendation with the best of intentions does not remove patient autonomy.”
This report had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Hermesch had no competing interests to declare.
Increasingly popular alternative peripartum practices such as water immersion and nonseverance of the umbilical cord may increase the risk of infections in newborns, a new clinical report from the American Academy of Pediatrics found.
Another perinatal measure potentially raising infection risk was placentophagy, according to a review led by Dawn Nolt, MD, MPH, a professor of pediatric infectious diseases at Oregon Health & Science University, Portland.
“Awareness of emerging alternative peripartum and neonatal practices helps pediatricians provide counseling to families before birth and to appropriately evaluate and treat neonates who have been exposed to these practices,” Dr. Nolt and colleagues wrote online in Pediatrics.
Amid growing inquiries made from women seeking a positive and meaningful birth experience through alternative approaches as well as reports of possibly related illness in newborns, Dr. Nolt’s group reviewed observational studies, case series, and medical society guidance on the risks associated with seven alternative birthing practices.
Based on their summation, it was not possible to quantify the actual risk associated with any one practice. “But of the seven we reviewed, as an infectious disease pediatrician I would say the most discernible immediate risk is likely attached to nonseverance of the cord,” Dr. Nolt said in an interview. “Left attached, the tissue can potentially necrote and transfer bacteria directly to the child.”
The authors made the following recommendations:
- Water immersion for labor and delivery. While this can increase the comfort of the mother in the first stages of labor, the water can become contaminated and increase the infant’s exposure to water-borne pathogens such as Legionella and Pseudomonas. It is not recommended after the second stage of labor and if offered, requires rigorous prophylactic and infection-control measures. This practice has also been linked to aspiration, drowning, hyponatremia, cord rupture, and death.
- Vaginal seeding. The skin, noses, and mouths of infants born by cesarean section are inoculated with swabs of vaginal fluid in order to expose them to vaginal bacteria that positively influence the infant’s microbiome. Of no known benefit, this measure can expose newborns to microbes such as group B Streptococcus and herpes simplex virus. Infants born by C-section receiving vaginal seeding should be evaluated the same way as those delivered vaginally.
- Umbilical cord nonseverance. Colloquially known as lotus birth, this is another practice with no evidence of advantage but with the potential to raise the risk of neonatal sepsis owing to the presence of necrotic umbilical or placental tissue. Some parents may view the placenta as a spiritual entity and fail to recognize it may be contaminated with harmful pathogens. Any placenta and umbilical cord attached to a febrile or ill-seeming neonate should be immediately removed.
- Placentophagy. Proponents believe placental consumption has antidepressive, analgesic, galactogogic, and nutritional properties. But eating raw, cooked, or dehydrated afterbirth tissue – viewed by some as a spiritual event – can expose a neonate to flora from the mother’s genitourinary tract and other sources encountered during preparation. Placentophagy has been associated with a case of recurrent late-onset group B streptococcal sepsis in a newborn. Strict food-handling practices at the level for raw meat should be maintained.
- HBV vaccine deferral. Viewed as “a critical safety net in preventing HBV infection,” the birth dose of the hepatitis B virus vaccine should not be postponed except for medical reasons. An estimated 1,000 new perinatally acquired HBV cases occurred annually in the United States from 2000 to 2009.
- Deferral of ocular prophylaxis. While ocular prophylaxis with topical erythromycin protects against gonococcal ophthalmia neonatorum, particularly in infants of high-risk mothers, it is not effective against other common pathogens. Parents and health care providers have recently questioned the need for its routine application, with concerns including its limited range of effectiveness as well as antibiotic resistance and shortages. With adequate prenatal testing, the risk of this neonatal conjunctivitis is significantly reduced, and deferral of prophylaxis may be considered in low-risk situations although it may be mandated by state legislation.
- Delayed bathing. The practice of delaying the infant’s first bath until several hours after birth may have several benefits. These include the initiation and exclusivity of breastfeeding, decreased mother/child separation time and risk of hypothermia, and protection of the neonatal skin microbiome. It should be discouraged, however, in neonates exposed to active herpes simplex virus lesions or whose mothers have a known history of HIV infection.
When women inquire about alternative practices, physicians need to strike a diplomatic balance between respecting women’s wishes and the benefits they hope to gain and at the same time informing them of potential risks, Dr. Nolt said. “The conversation we want to have with them should show compassion and sympathy but also tell them what the medical literature shows.” Patient and doctor should engage in shared decision-making about the safety of various alternative approaches.
“Over the last decade information on a variety of birth practices have become more widely available through social media and other Internet forums, which certainly has increased the variety of questions to health professionals, Amy C. Hermesch, MD, PhD, director of obstetric services at OHSC, said in an interview.
“We counsel about rare but serious risk, as noted in Dr. Nolt’s article,” said Dr. Hermesch, who was not involved in the AAP report. Most important is a discussion about appropriate pregnancy risk stratification. “For example, persons considering water immersion birth, probably the most common one I get inquiries about, should have an otherwise uncomplicated pregnancy with good mobility to get in and out of tub in the event of an emergency.”
While adverse events can happen during any birth, she sees these more often in mothers who underestimate the risk level of their situation or pregnancy when declining provider-recommended interventions. “I encourage pregnant persons to find a health care professional they trust who is knowledgeable about the benefits and the risk of all birth environments and interventions.”
Dr. Hermesch added that most alternative practices have little data to guide decisions, so she offers professional society recommendations, evidence review, and her own professional experiences. “The patient must weight the risk and benefits in the context of their value system and sometimes this means not following my advice or recommendations. My medical recommendation with the best of intentions does not remove patient autonomy.”
This report had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Hermesch had no competing interests to declare.
Increasingly popular alternative peripartum practices such as water immersion and nonseverance of the umbilical cord may increase the risk of infections in newborns, a new clinical report from the American Academy of Pediatrics found.
Another perinatal measure potentially raising infection risk was placentophagy, according to a review led by Dawn Nolt, MD, MPH, a professor of pediatric infectious diseases at Oregon Health & Science University, Portland.
“Awareness of emerging alternative peripartum and neonatal practices helps pediatricians provide counseling to families before birth and to appropriately evaluate and treat neonates who have been exposed to these practices,” Dr. Nolt and colleagues wrote online in Pediatrics.
Amid growing inquiries made from women seeking a positive and meaningful birth experience through alternative approaches as well as reports of possibly related illness in newborns, Dr. Nolt’s group reviewed observational studies, case series, and medical society guidance on the risks associated with seven alternative birthing practices.
Based on their summation, it was not possible to quantify the actual risk associated with any one practice. “But of the seven we reviewed, as an infectious disease pediatrician I would say the most discernible immediate risk is likely attached to nonseverance of the cord,” Dr. Nolt said in an interview. “Left attached, the tissue can potentially necrote and transfer bacteria directly to the child.”
The authors made the following recommendations:
- Water immersion for labor and delivery. While this can increase the comfort of the mother in the first stages of labor, the water can become contaminated and increase the infant’s exposure to water-borne pathogens such as Legionella and Pseudomonas. It is not recommended after the second stage of labor and if offered, requires rigorous prophylactic and infection-control measures. This practice has also been linked to aspiration, drowning, hyponatremia, cord rupture, and death.
- Vaginal seeding. The skin, noses, and mouths of infants born by cesarean section are inoculated with swabs of vaginal fluid in order to expose them to vaginal bacteria that positively influence the infant’s microbiome. Of no known benefit, this measure can expose newborns to microbes such as group B Streptococcus and herpes simplex virus. Infants born by C-section receiving vaginal seeding should be evaluated the same way as those delivered vaginally.
- Umbilical cord nonseverance. Colloquially known as lotus birth, this is another practice with no evidence of advantage but with the potential to raise the risk of neonatal sepsis owing to the presence of necrotic umbilical or placental tissue. Some parents may view the placenta as a spiritual entity and fail to recognize it may be contaminated with harmful pathogens. Any placenta and umbilical cord attached to a febrile or ill-seeming neonate should be immediately removed.
- Placentophagy. Proponents believe placental consumption has antidepressive, analgesic, galactogogic, and nutritional properties. But eating raw, cooked, or dehydrated afterbirth tissue – viewed by some as a spiritual event – can expose a neonate to flora from the mother’s genitourinary tract and other sources encountered during preparation. Placentophagy has been associated with a case of recurrent late-onset group B streptococcal sepsis in a newborn. Strict food-handling practices at the level for raw meat should be maintained.
- HBV vaccine deferral. Viewed as “a critical safety net in preventing HBV infection,” the birth dose of the hepatitis B virus vaccine should not be postponed except for medical reasons. An estimated 1,000 new perinatally acquired HBV cases occurred annually in the United States from 2000 to 2009.
- Deferral of ocular prophylaxis. While ocular prophylaxis with topical erythromycin protects against gonococcal ophthalmia neonatorum, particularly in infants of high-risk mothers, it is not effective against other common pathogens. Parents and health care providers have recently questioned the need for its routine application, with concerns including its limited range of effectiveness as well as antibiotic resistance and shortages. With adequate prenatal testing, the risk of this neonatal conjunctivitis is significantly reduced, and deferral of prophylaxis may be considered in low-risk situations although it may be mandated by state legislation.
- Delayed bathing. The practice of delaying the infant’s first bath until several hours after birth may have several benefits. These include the initiation and exclusivity of breastfeeding, decreased mother/child separation time and risk of hypothermia, and protection of the neonatal skin microbiome. It should be discouraged, however, in neonates exposed to active herpes simplex virus lesions or whose mothers have a known history of HIV infection.
When women inquire about alternative practices, physicians need to strike a diplomatic balance between respecting women’s wishes and the benefits they hope to gain and at the same time informing them of potential risks, Dr. Nolt said. “The conversation we want to have with them should show compassion and sympathy but also tell them what the medical literature shows.” Patient and doctor should engage in shared decision-making about the safety of various alternative approaches.
“Over the last decade information on a variety of birth practices have become more widely available through social media and other Internet forums, which certainly has increased the variety of questions to health professionals, Amy C. Hermesch, MD, PhD, director of obstetric services at OHSC, said in an interview.
“We counsel about rare but serious risk, as noted in Dr. Nolt’s article,” said Dr. Hermesch, who was not involved in the AAP report. Most important is a discussion about appropriate pregnancy risk stratification. “For example, persons considering water immersion birth, probably the most common one I get inquiries about, should have an otherwise uncomplicated pregnancy with good mobility to get in and out of tub in the event of an emergency.”
While adverse events can happen during any birth, she sees these more often in mothers who underestimate the risk level of their situation or pregnancy when declining provider-recommended interventions. “I encourage pregnant persons to find a health care professional they trust who is knowledgeable about the benefits and the risk of all birth environments and interventions.”
Dr. Hermesch added that most alternative practices have little data to guide decisions, so she offers professional society recommendations, evidence review, and her own professional experiences. “The patient must weight the risk and benefits in the context of their value system and sometimes this means not following my advice or recommendations. My medical recommendation with the best of intentions does not remove patient autonomy.”
This report had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Hermesch had no competing interests to declare.
FROM PEDIATRICS
Antimicrobial resistance linked to 1.2 million global deaths in 2019
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
Antibiotic choices for inpatients with SSTIs vary by race
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
FROM JAMA NETWORK OPEN
Severe outcomes increased in youth hospitalized after positive COVID-19 test
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Pediatric community-acquired pneumonia: 5 days of antibiotics better than 10 days
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
Appendectomy or antibiotics? Large trial helps decision-making
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY









