User login
Successful COVID-19 Surge Management With Monoclonal Antibody Infusion in Emergency Department Patients
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
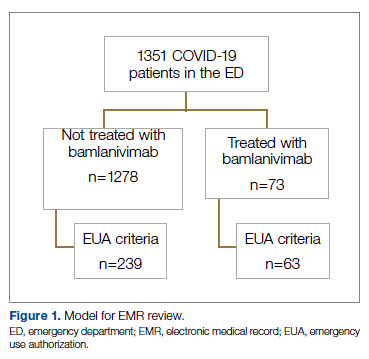
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
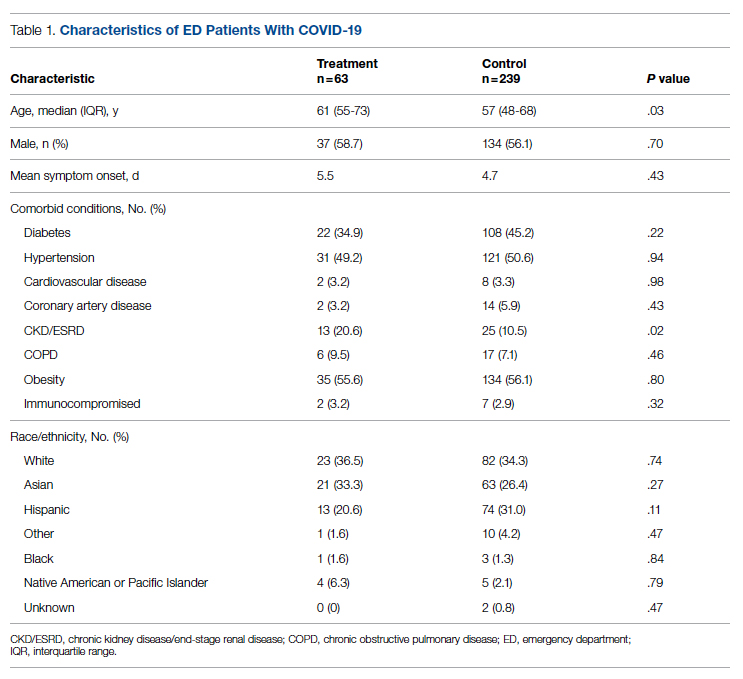
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
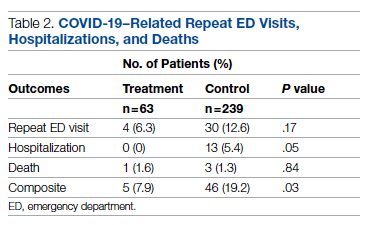
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
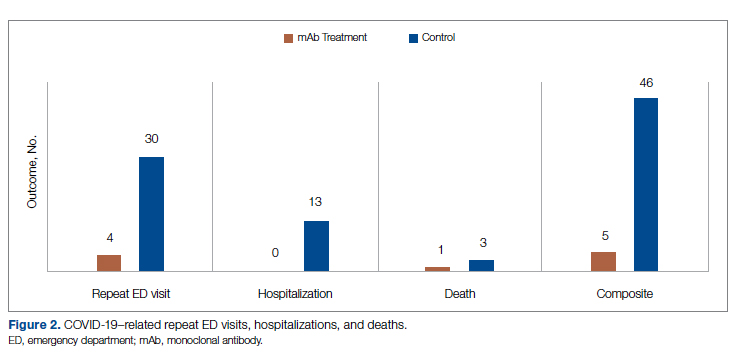
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
Antibiotics vs. placebo in acute uncomplicated diverticulitis
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Study shows wider gaps, broader inequities in U.S. sex education than 25 years ago
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
FROM THE JOURNAL OF ADOLESCENT HEALTH
HIV: Syringe services fill the gap when clinicians refuse to prescribe PrEP to people who inject drugs
Not that long ago, a man in his mid-20s whom Morgan Farrington calls Kiddo showed up at her house with a fever, chills, and nausea. He was increasingly out of it. These were all signs of an abscess from missing a vein and using someone else’s syringe, said Farrington, founder of Goodworks, in Huntsville, Alabama.
But it wasn’t just an abscess. It was four blood clots and sepsis. He’d been craving his next fix of heroin so hard, and the relief that comes from the act of shooting up itself, that he’d dug a 3-day-old blood shot – a used syringe with someone else’s blood in it – out of the garbage and had used it.
“He was almost dead,” said Farrington. “Another day, maybe two, he would have been dead, for sure, for sure.”
Farrington gets it. She has her own history of injection drug use. She knows the compulsion to, in her words, “shoot up sugar water just to get a hit.” And that’s fine, she said. “I just wish that he would do so with his own safety in mind.”
So when he got out of the hospital a few weeks later, Farrington talked to him about not just clean syringes but also HIV pre-exposure prophylaxis (PrEP), the daily HIV prevention pills that have been found to be up to 84% protective against HIV in people who inject drugs. Both approaches – syringe services and PrEP – play a key role in the president’s new National HIV/AIDS Strategy. The strategy calls for expanding access to both services in traditional and nontraditional settings but doesn’t include mechanisms for that to happen.
Of the 1.2 million people in the U.S. who could benefit from PrEP, according to the Centers for Disease Control and Prevention, 23% are using it. But according to data published in 2020, just 0% and 5% of people who inject drugs who could benefit are using it. And most, like the guy Farrington continues to talk to, don’t even know it exists.
People who inject drugs are willing, clinicians may not be
Clinicians have a role to play, but right now, many clinicians act as gatekeepers, picking and choosing whom they’ll offer PrEP to. In 2014, just 1% of PrEP prescribers said they had prescribed the prevention pill to people who inject drugs. And recent data published in AIDS and Behavior showed that clinicians who expressed negative attitudes about people who injected drugs were less likely to offer to prescribe PrEP to a theoretical man who injected drugs asking for PrEP. There was a paradox in there, however: Clinicians were also more likely to think men who inject drugs were at high risk for acquiring HIV. But they also believed those men would be less likely to adhere, less safety conscious, and less responsible than gay and bisexual men. So, the investigators found that despite need, clinicians were more likely to prescribe to men at risk via sex than men at risk via injection drug use.
According to the CDC, to qualify for PrEP, the only requirements for people who inject drugs are testing negative for HIV and not sharing injection equipment – whether their injecting partners have confirmed HIV or whether their HIV status is unknown.
“As long as PrEP is a prescription, medication providers are really going to determine who accesses PrEP and who does not,” said study lead author Sarah Calabrese, PhD, assistant professor of psychiatry at George Washington University. “Even if you do anticipate that a patient might have adherence struggles. The solution is not withholding something that could be beneficial to them. The solution is supporting them to take that beneficial medication.”
And it appears that providers and regular people like Farrington have stepped into the access vacuum, with a decidedly harm-reduction approach: syringe services programs. While there’s no national data on how many people receive PrEP through needle exchange programs, those programs are the natural place to offer other health care services, said Hansel Tookes, MD, assistant professor of medicine at the University of Miami and founder of the Infectious Disease Elimination Act (IDEA) Syringe Exchange program and clinic. At the clinic, 80% of the people living with HIV have undetectable viral loads – a sign of good adherence to medication and general health. Previous research suggests that when people who inject drugs find out about PrEP, 57% are game for trying it. But early work suggests that people who inject drugs might need to access PrEP in a different way from other people who use PrEP.
Dr. Tookes is currently conducting a study looking at whether referring people who inject drugs out from needle exchanges to PrEP prescribers is as effective as offering it on site at the exchanges.
“My experience in the past 5 years of being faculty at the university and being a cofounder of a program like IDEA is that we really, if we’re going to be successful with engaging people who inject drugs in things like PrEP, we have to, like all things harm reduction, meet them where they’re at, both physically and mentally, and on their own terms,” said Dr. Tookes. “What better place than a syringe services program?”
Where people are: the exchanges
That’s where community health worker Farrington and others come in. More than 400 syringe access programs that exist in North America have PrEP programs, according to the North American Syringe Exchange Network (NASEN), and 86 of them report offering access to PrEP, either directly or through referrals. It’s an HIV prevention one-two punch: PrEP protects a person once they are exposed to HIV, and needle exchanges themselves reduce HIV transmission rates by reducing the odds that people will engage in behavior that exposes them to the virus in the first place.
So far, PrEP access for people who inject drugs looks different everywhere. At Las Vegas’ Huntridge Family Clinic, people can come to the lobby and pick up clean supplies from a syringe exchange vending machine, and while they’re there, talk to nurse practitioner Rob Phoenix, MSN, APRN, about HIV prevention.
In Cincinnati, where Adam Reilly, CDCA, runs a Ryan White–funded PrEP program out of the nonprofit Caracole, PrEP navigators go out with the syringe services vans run by the county health department and can connect them with providers willing to prescribe it. In Alabama, where needle exchanges are illegal, Farrington works as a community health worker through the North Alabama Area Health Education Center to go in to Huntsville’s legal tent cities to offer HIV and hepatitis C testing and tell them about PrEP. In Philadelphia, Drexel University, the city Department of Health, and Prevention Point Philadelphia co-offer PrEP through Prevention Point, which increased the number of people who inject drugs taking PrEP from just two to three a year to 584 times in 2021, according to Andres Freire, director of harm reduction health services at Prevention Point Philadelphia.
“Co-locating a PrEP clinic with our syringe-services program is the most effective means of delivering care to people who use drugs,” he said. “It is a friendly, nonstigmatizing place, as well as a place where individuals are already coming for services.”
At Dr. Tookes’ IDEA clinic and its PrEP study, people who inject drugs can not only get clean supplies, they can get a PrEP prescription on site and store their medications at the exchange so they don’t get stolen or used by others. And that idea didn’t come from him.
“It was one of my patients,” he said. “That person gave me an idea that impacted the health of hundreds of people in Miami.”
Indeed, Boston Health Care for the Homeless Program does the same. New data showed that what really worked for people who injected drugs in the group was not just medication storage on site but also PrEP prescriptions that lasted just a week at a time, or even same-day prescribing, as well as the program’s PrEP nurses showing up in person to their communities. That program managed to get PrEP referrals to 239 people, 152 of whom started taking PrEP. Six months later, 22 people were still using it.
But Dr. Tookes’s is a rare study on PrEP among people who inject drugs. The only data so far on the efficacy of PrEP for this group come from a 2013 study out of Thailand. Angela Bazzi, PhD, an associate professor of family medicine and public health at the University of California, San Diego, who studied the Boston program, said the dearth of research into effective ways of getting PrEP to people who inject drugs is fueling a negative feedback loop, where people who inject drugs and their providers largely don’t know about the HIV prevention pills, don’t see research on it, and therefore think it won’t work in people who inject drugs.
“There’s been a systematic exclusion of people who inject drugs from HIV prevention drug trials,” Dr. Bazzi told this news organization. Together with colleagues she wrote a viewpoint on the issue that was published in the International Journal of Drug Policy. “It really extends into effectiveness research, public health research, and clinical practice. We argued that the stigma surrounding addiction is the key driver of this.”
This is especially important, she said, because the U.S. Food and Drug Administration had been expected to make an approval decision on an injectable form of PrEP by Jan. 2021. That drug, cabotegravir, has been found to work for a month at a time. Injection drug users were excluded from the primary clinical trial of that drug, though a ViiV Healthcare spokesperson said the company is planning an after-market study in people who inject drugs some time in the future.
An incomplete solution
But syringe services aren’t enough, said Mr. Reilly. For one thing, public funding of PrEP programs can limit things like where navigators can send people. For instance, in Ohio, Mr. Reilly’s team can cover the costs of PrEP for people who inject drugs – but only with certain providers. State law prohibits them from contracting with Planned Parenthood.
Also, syringe services aren’t available everywhere. In Pennsylvania, where syringe services are legal only in the counties containing Philadelphia and Pittsburgh, the state’s two large cities, funding for basic syringe services precludes expanding services to offer PrEP.
“A lot of our focus has to stay with making sure our folks have access to the harm-reduction supplies they need, because the number of people we are seeing has grown exponentially during the pandemic,” said Katie Houston, a coordinator for Prevention Point Pittsburgh, which tries to address its clients’s PrEP needs by holding syringe-services distribution at a local clinic that provides PrEP. “Getting funding for our core supplies like syringes, crack pipes, and the works is extremely difficult because many grants/foundations don’t want to fund these supplies. And with the growing number of SSPs, the funding that has been available is being spread thin.”
And that means that traditional clinicians still have an important role to play, said Mr. Reilly.
“Syringe services programs are supposed to now provide treatment for hepatitis C and make sure people get on PrEP?” he said. “That seems like medical providers’s job.”
As for Farrington, operating as a solo health worker without the benefit of exchanges to help people like the young man who came to her house that night, she’ll keep going in to tent city and inviting sick people who inject drugs into her home to offer them what she can. She can’t legally offer syringe services. But she can keep checking in on people and offering them the help that’s available.
Recently, she saw that young man again. He was in a better place. He had found a place to live for the winter, so he wouldn’t have to stay in the hammock in someone’s yard when the temperatures dipped. And that was going a long way to stabilize everything else in his life. He’s still shooting up, she said, but having housing is making it easier for him to moderate his use. As for PrEP, he hasn’t started on that, either. But Farrington hasn’t given up hope.
“Not yet,” she said.
Dr. Tookes reports receiving research funding from Gilead Sciences. Dr. Calabrese reports receiving conference travel funding from Gilead Sciences. Farrington, Dr. Bazzi, Ms. Houston, Mr. Freire, and Mr. Reilly reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Not that long ago, a man in his mid-20s whom Morgan Farrington calls Kiddo showed up at her house with a fever, chills, and nausea. He was increasingly out of it. These were all signs of an abscess from missing a vein and using someone else’s syringe, said Farrington, founder of Goodworks, in Huntsville, Alabama.
But it wasn’t just an abscess. It was four blood clots and sepsis. He’d been craving his next fix of heroin so hard, and the relief that comes from the act of shooting up itself, that he’d dug a 3-day-old blood shot – a used syringe with someone else’s blood in it – out of the garbage and had used it.
“He was almost dead,” said Farrington. “Another day, maybe two, he would have been dead, for sure, for sure.”
Farrington gets it. She has her own history of injection drug use. She knows the compulsion to, in her words, “shoot up sugar water just to get a hit.” And that’s fine, she said. “I just wish that he would do so with his own safety in mind.”
So when he got out of the hospital a few weeks later, Farrington talked to him about not just clean syringes but also HIV pre-exposure prophylaxis (PrEP), the daily HIV prevention pills that have been found to be up to 84% protective against HIV in people who inject drugs. Both approaches – syringe services and PrEP – play a key role in the president’s new National HIV/AIDS Strategy. The strategy calls for expanding access to both services in traditional and nontraditional settings but doesn’t include mechanisms for that to happen.
Of the 1.2 million people in the U.S. who could benefit from PrEP, according to the Centers for Disease Control and Prevention, 23% are using it. But according to data published in 2020, just 0% and 5% of people who inject drugs who could benefit are using it. And most, like the guy Farrington continues to talk to, don’t even know it exists.
People who inject drugs are willing, clinicians may not be
Clinicians have a role to play, but right now, many clinicians act as gatekeepers, picking and choosing whom they’ll offer PrEP to. In 2014, just 1% of PrEP prescribers said they had prescribed the prevention pill to people who inject drugs. And recent data published in AIDS and Behavior showed that clinicians who expressed negative attitudes about people who injected drugs were less likely to offer to prescribe PrEP to a theoretical man who injected drugs asking for PrEP. There was a paradox in there, however: Clinicians were also more likely to think men who inject drugs were at high risk for acquiring HIV. But they also believed those men would be less likely to adhere, less safety conscious, and less responsible than gay and bisexual men. So, the investigators found that despite need, clinicians were more likely to prescribe to men at risk via sex than men at risk via injection drug use.
According to the CDC, to qualify for PrEP, the only requirements for people who inject drugs are testing negative for HIV and not sharing injection equipment – whether their injecting partners have confirmed HIV or whether their HIV status is unknown.
“As long as PrEP is a prescription, medication providers are really going to determine who accesses PrEP and who does not,” said study lead author Sarah Calabrese, PhD, assistant professor of psychiatry at George Washington University. “Even if you do anticipate that a patient might have adherence struggles. The solution is not withholding something that could be beneficial to them. The solution is supporting them to take that beneficial medication.”
And it appears that providers and regular people like Farrington have stepped into the access vacuum, with a decidedly harm-reduction approach: syringe services programs. While there’s no national data on how many people receive PrEP through needle exchange programs, those programs are the natural place to offer other health care services, said Hansel Tookes, MD, assistant professor of medicine at the University of Miami and founder of the Infectious Disease Elimination Act (IDEA) Syringe Exchange program and clinic. At the clinic, 80% of the people living with HIV have undetectable viral loads – a sign of good adherence to medication and general health. Previous research suggests that when people who inject drugs find out about PrEP, 57% are game for trying it. But early work suggests that people who inject drugs might need to access PrEP in a different way from other people who use PrEP.
Dr. Tookes is currently conducting a study looking at whether referring people who inject drugs out from needle exchanges to PrEP prescribers is as effective as offering it on site at the exchanges.
“My experience in the past 5 years of being faculty at the university and being a cofounder of a program like IDEA is that we really, if we’re going to be successful with engaging people who inject drugs in things like PrEP, we have to, like all things harm reduction, meet them where they’re at, both physically and mentally, and on their own terms,” said Dr. Tookes. “What better place than a syringe services program?”
Where people are: the exchanges
That’s where community health worker Farrington and others come in. More than 400 syringe access programs that exist in North America have PrEP programs, according to the North American Syringe Exchange Network (NASEN), and 86 of them report offering access to PrEP, either directly or through referrals. It’s an HIV prevention one-two punch: PrEP protects a person once they are exposed to HIV, and needle exchanges themselves reduce HIV transmission rates by reducing the odds that people will engage in behavior that exposes them to the virus in the first place.
So far, PrEP access for people who inject drugs looks different everywhere. At Las Vegas’ Huntridge Family Clinic, people can come to the lobby and pick up clean supplies from a syringe exchange vending machine, and while they’re there, talk to nurse practitioner Rob Phoenix, MSN, APRN, about HIV prevention.
In Cincinnati, where Adam Reilly, CDCA, runs a Ryan White–funded PrEP program out of the nonprofit Caracole, PrEP navigators go out with the syringe services vans run by the county health department and can connect them with providers willing to prescribe it. In Alabama, where needle exchanges are illegal, Farrington works as a community health worker through the North Alabama Area Health Education Center to go in to Huntsville’s legal tent cities to offer HIV and hepatitis C testing and tell them about PrEP. In Philadelphia, Drexel University, the city Department of Health, and Prevention Point Philadelphia co-offer PrEP through Prevention Point, which increased the number of people who inject drugs taking PrEP from just two to three a year to 584 times in 2021, according to Andres Freire, director of harm reduction health services at Prevention Point Philadelphia.
“Co-locating a PrEP clinic with our syringe-services program is the most effective means of delivering care to people who use drugs,” he said. “It is a friendly, nonstigmatizing place, as well as a place where individuals are already coming for services.”
At Dr. Tookes’ IDEA clinic and its PrEP study, people who inject drugs can not only get clean supplies, they can get a PrEP prescription on site and store their medications at the exchange so they don’t get stolen or used by others. And that idea didn’t come from him.
“It was one of my patients,” he said. “That person gave me an idea that impacted the health of hundreds of people in Miami.”
Indeed, Boston Health Care for the Homeless Program does the same. New data showed that what really worked for people who injected drugs in the group was not just medication storage on site but also PrEP prescriptions that lasted just a week at a time, or even same-day prescribing, as well as the program’s PrEP nurses showing up in person to their communities. That program managed to get PrEP referrals to 239 people, 152 of whom started taking PrEP. Six months later, 22 people were still using it.
But Dr. Tookes’s is a rare study on PrEP among people who inject drugs. The only data so far on the efficacy of PrEP for this group come from a 2013 study out of Thailand. Angela Bazzi, PhD, an associate professor of family medicine and public health at the University of California, San Diego, who studied the Boston program, said the dearth of research into effective ways of getting PrEP to people who inject drugs is fueling a negative feedback loop, where people who inject drugs and their providers largely don’t know about the HIV prevention pills, don’t see research on it, and therefore think it won’t work in people who inject drugs.
“There’s been a systematic exclusion of people who inject drugs from HIV prevention drug trials,” Dr. Bazzi told this news organization. Together with colleagues she wrote a viewpoint on the issue that was published in the International Journal of Drug Policy. “It really extends into effectiveness research, public health research, and clinical practice. We argued that the stigma surrounding addiction is the key driver of this.”
This is especially important, she said, because the U.S. Food and Drug Administration had been expected to make an approval decision on an injectable form of PrEP by Jan. 2021. That drug, cabotegravir, has been found to work for a month at a time. Injection drug users were excluded from the primary clinical trial of that drug, though a ViiV Healthcare spokesperson said the company is planning an after-market study in people who inject drugs some time in the future.
An incomplete solution
But syringe services aren’t enough, said Mr. Reilly. For one thing, public funding of PrEP programs can limit things like where navigators can send people. For instance, in Ohio, Mr. Reilly’s team can cover the costs of PrEP for people who inject drugs – but only with certain providers. State law prohibits them from contracting with Planned Parenthood.
Also, syringe services aren’t available everywhere. In Pennsylvania, where syringe services are legal only in the counties containing Philadelphia and Pittsburgh, the state’s two large cities, funding for basic syringe services precludes expanding services to offer PrEP.
“A lot of our focus has to stay with making sure our folks have access to the harm-reduction supplies they need, because the number of people we are seeing has grown exponentially during the pandemic,” said Katie Houston, a coordinator for Prevention Point Pittsburgh, which tries to address its clients’s PrEP needs by holding syringe-services distribution at a local clinic that provides PrEP. “Getting funding for our core supplies like syringes, crack pipes, and the works is extremely difficult because many grants/foundations don’t want to fund these supplies. And with the growing number of SSPs, the funding that has been available is being spread thin.”
And that means that traditional clinicians still have an important role to play, said Mr. Reilly.
“Syringe services programs are supposed to now provide treatment for hepatitis C and make sure people get on PrEP?” he said. “That seems like medical providers’s job.”
As for Farrington, operating as a solo health worker without the benefit of exchanges to help people like the young man who came to her house that night, she’ll keep going in to tent city and inviting sick people who inject drugs into her home to offer them what she can. She can’t legally offer syringe services. But she can keep checking in on people and offering them the help that’s available.
Recently, she saw that young man again. He was in a better place. He had found a place to live for the winter, so he wouldn’t have to stay in the hammock in someone’s yard when the temperatures dipped. And that was going a long way to stabilize everything else in his life. He’s still shooting up, she said, but having housing is making it easier for him to moderate his use. As for PrEP, he hasn’t started on that, either. But Farrington hasn’t given up hope.
“Not yet,” she said.
Dr. Tookes reports receiving research funding from Gilead Sciences. Dr. Calabrese reports receiving conference travel funding from Gilead Sciences. Farrington, Dr. Bazzi, Ms. Houston, Mr. Freire, and Mr. Reilly reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Not that long ago, a man in his mid-20s whom Morgan Farrington calls Kiddo showed up at her house with a fever, chills, and nausea. He was increasingly out of it. These were all signs of an abscess from missing a vein and using someone else’s syringe, said Farrington, founder of Goodworks, in Huntsville, Alabama.
But it wasn’t just an abscess. It was four blood clots and sepsis. He’d been craving his next fix of heroin so hard, and the relief that comes from the act of shooting up itself, that he’d dug a 3-day-old blood shot – a used syringe with someone else’s blood in it – out of the garbage and had used it.
“He was almost dead,” said Farrington. “Another day, maybe two, he would have been dead, for sure, for sure.”
Farrington gets it. She has her own history of injection drug use. She knows the compulsion to, in her words, “shoot up sugar water just to get a hit.” And that’s fine, she said. “I just wish that he would do so with his own safety in mind.”
So when he got out of the hospital a few weeks later, Farrington talked to him about not just clean syringes but also HIV pre-exposure prophylaxis (PrEP), the daily HIV prevention pills that have been found to be up to 84% protective against HIV in people who inject drugs. Both approaches – syringe services and PrEP – play a key role in the president’s new National HIV/AIDS Strategy. The strategy calls for expanding access to both services in traditional and nontraditional settings but doesn’t include mechanisms for that to happen.
Of the 1.2 million people in the U.S. who could benefit from PrEP, according to the Centers for Disease Control and Prevention, 23% are using it. But according to data published in 2020, just 0% and 5% of people who inject drugs who could benefit are using it. And most, like the guy Farrington continues to talk to, don’t even know it exists.
People who inject drugs are willing, clinicians may not be
Clinicians have a role to play, but right now, many clinicians act as gatekeepers, picking and choosing whom they’ll offer PrEP to. In 2014, just 1% of PrEP prescribers said they had prescribed the prevention pill to people who inject drugs. And recent data published in AIDS and Behavior showed that clinicians who expressed negative attitudes about people who injected drugs were less likely to offer to prescribe PrEP to a theoretical man who injected drugs asking for PrEP. There was a paradox in there, however: Clinicians were also more likely to think men who inject drugs were at high risk for acquiring HIV. But they also believed those men would be less likely to adhere, less safety conscious, and less responsible than gay and bisexual men. So, the investigators found that despite need, clinicians were more likely to prescribe to men at risk via sex than men at risk via injection drug use.
According to the CDC, to qualify for PrEP, the only requirements for people who inject drugs are testing negative for HIV and not sharing injection equipment – whether their injecting partners have confirmed HIV or whether their HIV status is unknown.
“As long as PrEP is a prescription, medication providers are really going to determine who accesses PrEP and who does not,” said study lead author Sarah Calabrese, PhD, assistant professor of psychiatry at George Washington University. “Even if you do anticipate that a patient might have adherence struggles. The solution is not withholding something that could be beneficial to them. The solution is supporting them to take that beneficial medication.”
And it appears that providers and regular people like Farrington have stepped into the access vacuum, with a decidedly harm-reduction approach: syringe services programs. While there’s no national data on how many people receive PrEP through needle exchange programs, those programs are the natural place to offer other health care services, said Hansel Tookes, MD, assistant professor of medicine at the University of Miami and founder of the Infectious Disease Elimination Act (IDEA) Syringe Exchange program and clinic. At the clinic, 80% of the people living with HIV have undetectable viral loads – a sign of good adherence to medication and general health. Previous research suggests that when people who inject drugs find out about PrEP, 57% are game for trying it. But early work suggests that people who inject drugs might need to access PrEP in a different way from other people who use PrEP.
Dr. Tookes is currently conducting a study looking at whether referring people who inject drugs out from needle exchanges to PrEP prescribers is as effective as offering it on site at the exchanges.
“My experience in the past 5 years of being faculty at the university and being a cofounder of a program like IDEA is that we really, if we’re going to be successful with engaging people who inject drugs in things like PrEP, we have to, like all things harm reduction, meet them where they’re at, both physically and mentally, and on their own terms,” said Dr. Tookes. “What better place than a syringe services program?”
Where people are: the exchanges
That’s where community health worker Farrington and others come in. More than 400 syringe access programs that exist in North America have PrEP programs, according to the North American Syringe Exchange Network (NASEN), and 86 of them report offering access to PrEP, either directly or through referrals. It’s an HIV prevention one-two punch: PrEP protects a person once they are exposed to HIV, and needle exchanges themselves reduce HIV transmission rates by reducing the odds that people will engage in behavior that exposes them to the virus in the first place.
So far, PrEP access for people who inject drugs looks different everywhere. At Las Vegas’ Huntridge Family Clinic, people can come to the lobby and pick up clean supplies from a syringe exchange vending machine, and while they’re there, talk to nurse practitioner Rob Phoenix, MSN, APRN, about HIV prevention.
In Cincinnati, where Adam Reilly, CDCA, runs a Ryan White–funded PrEP program out of the nonprofit Caracole, PrEP navigators go out with the syringe services vans run by the county health department and can connect them with providers willing to prescribe it. In Alabama, where needle exchanges are illegal, Farrington works as a community health worker through the North Alabama Area Health Education Center to go in to Huntsville’s legal tent cities to offer HIV and hepatitis C testing and tell them about PrEP. In Philadelphia, Drexel University, the city Department of Health, and Prevention Point Philadelphia co-offer PrEP through Prevention Point, which increased the number of people who inject drugs taking PrEP from just two to three a year to 584 times in 2021, according to Andres Freire, director of harm reduction health services at Prevention Point Philadelphia.
“Co-locating a PrEP clinic with our syringe-services program is the most effective means of delivering care to people who use drugs,” he said. “It is a friendly, nonstigmatizing place, as well as a place where individuals are already coming for services.”
At Dr. Tookes’ IDEA clinic and its PrEP study, people who inject drugs can not only get clean supplies, they can get a PrEP prescription on site and store their medications at the exchange so they don’t get stolen or used by others. And that idea didn’t come from him.
“It was one of my patients,” he said. “That person gave me an idea that impacted the health of hundreds of people in Miami.”
Indeed, Boston Health Care for the Homeless Program does the same. New data showed that what really worked for people who injected drugs in the group was not just medication storage on site but also PrEP prescriptions that lasted just a week at a time, or even same-day prescribing, as well as the program’s PrEP nurses showing up in person to their communities. That program managed to get PrEP referrals to 239 people, 152 of whom started taking PrEP. Six months later, 22 people were still using it.
But Dr. Tookes’s is a rare study on PrEP among people who inject drugs. The only data so far on the efficacy of PrEP for this group come from a 2013 study out of Thailand. Angela Bazzi, PhD, an associate professor of family medicine and public health at the University of California, San Diego, who studied the Boston program, said the dearth of research into effective ways of getting PrEP to people who inject drugs is fueling a negative feedback loop, where people who inject drugs and their providers largely don’t know about the HIV prevention pills, don’t see research on it, and therefore think it won’t work in people who inject drugs.
“There’s been a systematic exclusion of people who inject drugs from HIV prevention drug trials,” Dr. Bazzi told this news organization. Together with colleagues she wrote a viewpoint on the issue that was published in the International Journal of Drug Policy. “It really extends into effectiveness research, public health research, and clinical practice. We argued that the stigma surrounding addiction is the key driver of this.”
This is especially important, she said, because the U.S. Food and Drug Administration had been expected to make an approval decision on an injectable form of PrEP by Jan. 2021. That drug, cabotegravir, has been found to work for a month at a time. Injection drug users were excluded from the primary clinical trial of that drug, though a ViiV Healthcare spokesperson said the company is planning an after-market study in people who inject drugs some time in the future.
An incomplete solution
But syringe services aren’t enough, said Mr. Reilly. For one thing, public funding of PrEP programs can limit things like where navigators can send people. For instance, in Ohio, Mr. Reilly’s team can cover the costs of PrEP for people who inject drugs – but only with certain providers. State law prohibits them from contracting with Planned Parenthood.
Also, syringe services aren’t available everywhere. In Pennsylvania, where syringe services are legal only in the counties containing Philadelphia and Pittsburgh, the state’s two large cities, funding for basic syringe services precludes expanding services to offer PrEP.
“A lot of our focus has to stay with making sure our folks have access to the harm-reduction supplies they need, because the number of people we are seeing has grown exponentially during the pandemic,” said Katie Houston, a coordinator for Prevention Point Pittsburgh, which tries to address its clients’s PrEP needs by holding syringe-services distribution at a local clinic that provides PrEP. “Getting funding for our core supplies like syringes, crack pipes, and the works is extremely difficult because many grants/foundations don’t want to fund these supplies. And with the growing number of SSPs, the funding that has been available is being spread thin.”
And that means that traditional clinicians still have an important role to play, said Mr. Reilly.
“Syringe services programs are supposed to now provide treatment for hepatitis C and make sure people get on PrEP?” he said. “That seems like medical providers’s job.”
As for Farrington, operating as a solo health worker without the benefit of exchanges to help people like the young man who came to her house that night, she’ll keep going in to tent city and inviting sick people who inject drugs into her home to offer them what she can. She can’t legally offer syringe services. But she can keep checking in on people and offering them the help that’s available.
Recently, she saw that young man again. He was in a better place. He had found a place to live for the winter, so he wouldn’t have to stay in the hammock in someone’s yard when the temperatures dipped. And that was going a long way to stabilize everything else in his life. He’s still shooting up, she said, but having housing is making it easier for him to moderate his use. As for PrEP, he hasn’t started on that, either. But Farrington hasn’t given up hope.
“Not yet,” she said.
Dr. Tookes reports receiving research funding from Gilead Sciences. Dr. Calabrese reports receiving conference travel funding from Gilead Sciences. Farrington, Dr. Bazzi, Ms. Houston, Mr. Freire, and Mr. Reilly reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Single-dose HPV vaccination highly effective
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
A single dose of human papillomavirus (HPV) vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens, according to results from the KEN SHE trial, based in Kenya.
The findings, published on the preprint server Research Square and presented Nov. 17 at the 34th International Papillomavirus Conference in Toronto, bring “renewed energy to the push to make cervical cancer the first cancer to be wiped out globally,” according to co–principal investigator Ruanne V. Barnabas, PhD, a professor of global health at the University of Washington, Seattle.
Decision-makers will consider these findings, which have not yet been peer-reviewed, along with other evidence to determine if dosing-schedule changes are warranted, she told this news organization.
In a press release, Samuel Kariuki, PhD, acting director general, Kenya Medical Research Institute, who was not involved in the research, called the findings a “game changer” that could “substantially reduce the incidence of HPV-attributable cervical cancer.”
Between 2018 and 2019, Dr. Barnabas and her colleagues enrolled 2,275 sexually active, HPV-vaccine–naive women in Kenya in their study. The women, 15-20 years of age, were randomly assigned to receive a bivalent vaccine (HPV 16/18), a nonavalent vaccine (HPV 16/18/31/33/45/52/58/6/11), or a vaccine against meningococcal meningitis.
Most participants (57%) were between 15 and 17 years of age, and 61% reported one lifetime sexual partner. The women underwent genital and cervical swabs at enrollment to test for HPV DNA and had blood drawn to test for antibodies. During 18 months of follow-up, they had cervical swabs every 6 months and a vaginal swab at 3 months to test for HPV DNA.
The researchers detected 38 persistent HPV 16/18 infections in women who had tested negative for HPV 16/18 antibodies at enrollment and for HPV 16/18 DNA at enrollment and month 3 – one in each of the HPV-vaccine groups and 36 in the meningococcal group. This infection rate corresponded to a vaccine efficacy of 97.5% (P < .001) against HPV 16/18 for both the bivalent and nonavalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers write.
Among women negative for HPV 16/18/31/33/45/52/58 at the beginning of the trial, 33 had persistent infections: four in the nonavalent vaccine group and 29 in the meningococcal group, demonstrating an efficacy of 89% (P < .001) against all seven oncogenic strains contained in the vaccine.
Even if women tested positive for one strain of HPV, the vaccine protected them from other strains of the virus, the investigators noted.
Serious adverse events occurred in 4.5%-5.2% of participants across the study arms.
The KEN SHE trial comes 15 years after the U.S. Food and Drug Administration approved the first HPV vaccine – Merck’s Gardasil. Two others, Cervarix and Gardasil-9, have since been approved, but cost and supply issues have inhibited coverage, particularly in areas where the cervical cancer burden is high, the researchers noted.
Recent data indicate that just 15% of girls globally are vaccinated against HPV, but a single-dose vaccine would “simplify logistics and decrease costs,” thereby improving the chances of reaching the World Health Organization goal of vaccinating 90% of 15-year-old girls against HPV by 2030, Dr. Barnabas said in a press release about the trial.
Co–principal investigator Nelly Mugo, MBChB, MPH, senior principal clinical research scientist with the Center for Clinical Research at the Kenya Medical Research Institute in Nairobi, further emphasized the importance of the findings, noting in the press release that the “trial brings new energy to the elimination of cervical cancer. It brings great hope to the women living in countries like Kenya, who have a high burden of the disease.”
Dr. Mugo is also an associate research professor of global health at the University of Washington, Seattle.
Dr. Barnabas said women have been given multiple doses of the HPV vaccine because of “gaps in evidence for the effectiveness of a single-dose vaccine and concerns about clinically meaningful differences in efficacy.
“Observational data suggested that the single-dose HPV vaccine could have good efficacy, but because the data were not from randomized trials, that could have been from chance,” she explained, noting, however, that “sufficient evidence supported the decrease in doses from three to two doses for girls 15 years of age and younger.”
Going forward, the researchers will conduct immunobridging studies to other populations and will continue follow-up to assess the durability of single-dose efficacy, Dr. Barnabas said.
“The results from the KEN SHE trial support the use of single-dose HPV vaccination to increase access and coverage,” she concluded.
The KEN SHE trial was funded by the Bill & Melinda Gates Foundation (BMGF). Dr. Barnabas reports grants from BMGF and grants from King K. Holmes Professorship in STDs and AIDS during the conduct of the study, and grants from BMGF, National Institutes of Health, and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
HPV vaccines reduce cervical cancer rates in young females
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
Two different studies have found that, provided young females are immunized with the human papilloma virus (HPV) vaccine at a young enough age, both the incidence of and mortality from cervical cancer can be dramatically curtailed, data from the United Kingdom and to a lesser extent, the United States indicate.
In the U.K. study, published online in The Lancet, researchers showed that the national vaccination program against HPV, initiated in England in 2008, has all but eradicated cervical cancer and cervical intraepithelial neoplasia (CIN3) in young girls who received the vaccine at the age of 12 and 13 years (school year 8) prior to their sexual debut.
In this age group, cervical cancer rates were 87% lower than rates among previously nonvaccinated generations, while CIN3 rates were reduced by 97%, as researchers report. “It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding,” he added.
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, UK Health Security Agency, London, said in the same statement.
“This represents an important step forward in cervical cancer prevention, and we hope that these new results encourage uptake as the success of the vaccination programme relies not only on the efficacy of the vaccine but also the proportion of the population vaccinated,” she added.
Vanessa Saliba, MD, a consultant epidemiologist for the UK Health Security Agency, agreed, adding that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.”
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she reemphasized.
British HPV program
When initiated in 2008, the national HPV vaccination program used the bivalent, Cervarix vaccine against HPV 16 and 18. As researchers noted, these two HPV types are responsible for 70%-80% of all cervical cancers in England.
However, in 2012, the program switched to the quadrivalent HPV vaccine (Gardasil) which is also effective against two additional HPV types, 6 and 11, both of which cause genital warts. The program also originally recommended the three-dose regimen for both HPV vaccines.
Now, only two doses of the vaccine are given to girls under the age of 15 even though it has been shown that a single dose of the HPV vaccine provides good protection against persistent infection, with efficacy rates that are similar to that of three doses, as the authors point out.
Among the cohort eligible for vaccination at 12 or 13 years of age, 89% received at least one dose of the HPV vaccine while 85% of the same age group received all three shots.
Cancer registry
Data from a population-based cancer registry was used to estimate the early effect of the bivalent HPV program on the incidence of cervical cancer and CIN3 in England between January 2006 and June 2019. During the study interval, there were 27,946 diagnoses of cervical cancer and 318,058 diagnoses of CIN3, lead author Milena Falcaro, MD, King’s College London, and colleagues report. Participants were then analyzed separately according to their age at the time of vaccination and the incidence rates calculated for both cervical cancer and CIN3 in the three separate groups.
For slightly older girls who received the vaccine between 14 and 16 years of age (school year 10-11), cervical cancer was reduced by 62% while CIN3 rates were reduced by 75%. For those who received the vaccine between 16 and 18 years of age (school year 12-13), cervical cancer rates were reduced by 34% while CIN3 rates were reduced by 39%, study authors add.
Indeed, the authors estimate that by June 2019 there were approximately 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would otherwise have been expected in the vaccinated population in England.
The authors acknowledge that cervical cancer is rare in young women and vaccinated populations are still young. For example, the youngest recipients would have been immunized at the age of 12 in 2008 and would still be only 23 years old in 2019 when the study ended.
Thus, the authors emphasize that, because the vaccinated populations are still young, it’s too early to assess the full effect of HPV vaccination on cervical cancer rates.
Asked to comment on the study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, pointed out that results from the British study are very similar to those from a Swedish study assessing the effect of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. As an oncologist who has been treating cervical cancer for 40 years – particularly advanced cervical cancer – “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful,” he stressed.
Editorial commentary
Commenting on the findings, editorialists Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania, point out that published reports evaluating the effect of HPV vaccination on cervical cancer rates have been scarce until now.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by the WHO [World Health Organization],” the editorialists add.
Dr. Cruickshank and Dr. Grigore also suggest that the effect HPV vaccination is having on cervical cancer rates as shown in this study should also stimulate vaccination programs in low- and middle-income countries where cervical cancer is a far greater public health issue than it is in countries with established systems of vaccination and screening.
HPV vaccination in the United States
The HPV vaccination program is similarly reducing the incidence of and mortality from cervical cancer among younger women in the United States who are most likely to have received the vaccine. As reported by lead author, Justin Barnes, MD, Washington University, St. Louis, the incidence of cervical cancer dropped by 37.7% from 2001 through 2005 to 2010 through 2017 in girls and young women between 15 and 24 years of age.
The U.S. study was published online in JAMA Pediatrics.
“HPV vaccine coverage in the U.S. has improved over the last few years although it was quite poor for many years,” senior author of the U.K. study, Peter Sasieni, MD, King’s College London, said in an interview. “Thus, one would anticipate a lower impact on the population in the U.S., because vaccine uptake, particularly in those aged 11-14 years was so much lower than it was in the U.K.,” he noted.
SEER databases
National age-adjusted cervical cancer incidence and mortality data from January 2001 through December 2017 for women and girls between 15 and 39 years of age were obtained from the combined Surveillance, Epidemiology, and End Results as well as the National Program of Cancer Registries databases. Mortality data was obtained from the National Center for Health Statistics.
Investigators then compared percentage changes in the incidence of and mortality from cervical cancer from January 2001 through December 2005 during the prevaccination years to that observed between January 2010 through December 2017 during the postvaccination years. They also compared incidence and mortality rates in three different cohorts: females between 15 and 24 years of age, those between 25 and 29 years of age, and those between 30 and 39 years of age.
“The older two groups were included as comparison, given their low vaccination rates,” the authors explained. Results showed that, during the same study interval from 2001 through 2005 to 2010 through 2017, the incidence of cervical cancer dropped by only 16.1% in women between 25 and 29 years of age and by only 8% for women between 30 and 39 years of age, the investigators report.
Reductions in mortality from cervical cancer were only strikingly so in the youngest age group of females between 15 and 24 years of age, among whom there was a 43.3% reduction in mortality from 2001-2005 to 2010-2017, as Dr. Barnes and colleagues note.
This pattern changed substantially in women between the ages of 25 and 29, among whom there was a 4.3% increase in mortality from cervical cancer during the same study interval and a small, 4.7% reduction among women between 30 and 39 years of age, investigators add. In actual numbers, mortality rates from cervical cancer were very low at only 0.6 per 100,000 in females between 15 and 24 years of age.
This compared to a mortality rate of 0.57 per 100,000 in women between 25 and 29 years of age and 1.89 per 100,000 in the oldest age group. “These nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes notes.
“Thus, the current study adds to knowledge by quantitatively comparing changes in cervical cancer incidence by age-based vaccine eligibility and providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators add.
However, as the authors also point out, while the reduction in mortality from cervical cancer associated with HPV vaccination may translate to older age groups as HPV-vaccinated cohorts age, “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” they caution, “and efforts to further improve vaccination uptake remain important.”
None of the authors or the editorialists had any conflicts of interest to declare.
For older adults, smelling the roses may be more difficult
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Children and COVID: New cases, vaccinations both decline
States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.
FDA approves first drug for treatment of resistant cytomegalovirus infection
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the first treatment for posttransplant cytomegalovirus (CMV) that is resistant to other drugs.
There are an estimated 200,000 adult transplants every year globally. CMV, a type of herpes virus, is one of the most common infections in transplant patients, occurring in 16%-56% of solid organ transplant recipients and 30%-70% of hematopoietic stem cell transplant recipients, according to Takeda Pharmaceutical Company Limited, the company that manufactures Livtencity. For immunosuppressed transplant patients, CMV infection can lead to complications that include loss of the transplanted or organ or even death.
“Cytomegalovirus infections that are resistant or do not respond to available drugs are of even greater concern,” John Farley, MD, MPH, the director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement. “Today’s approval helps meet a significant unmet medical need by providing a treatment option for this patient population.”
Livtencity, which is taken orally, works by preventing the activity of the enzyme responsible for virus replication. The approval, announced Nov. 23, was based on a phase 3 clinical trial that compared Livtencity with conventional antiviral treatments in the achievement of CMV DNA concentration levels below what is measurable in transplant patients with CMV infection that is refractory or treatment-resistant. After 8 weeks, of the 235 patients who received Livtencity, 56% achieved this primary endpoint, compared with 24% of the 117 patients who received conventional antiviral treatments, the press release says.
The most reported adverse reactions of Livtencity were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
“We are grateful for the contributions of the patients and clinicians who participated in our clinical trials, as well as the dedication of our scientists and researchers,” Ramona Sequeira, president of the Takeda’s U.S. Business Unit and Global Portfolio Commercialization, said in a statement. “People undergoing transplants have a lengthy and complex health care journey; with the approval of this treatment, we’re proud to offer these individuals a new oral antiviral to fight CMV infection and disease.”
A version of this article first appeared on Medscape.com.
Big drop in U.S. cervical cancer rates, mortality in younger women
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS