User login
ACIP simplifies adult vaccinations for HepB and pneumonia
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
To boost HIV screening, ED nurses need institutional support
, according to a national survey of ED nurses. Nearly 43% of respondents said they had received “little” or “very little” HIV education as part of their professional development and practice.
This lack of continuing HIV education “often translated into attitudes that did not support the policy” of routine HIV screening in EDs, lead author Candace Elam, DNP, a family nurse practitioner at the Institute of Family Health in the Bronx, New York City, told this news organization. “But more than individual attitudes, what came out most clearly in the research was that organizational support for HIV screening in EDs was the one factor that could make or break whether an emergency nurse performs HIV screening,” she said. This includes working routine HIV screening into ED workflows and providing resources to streamline screening and testing efforts.
In 2006, the Centers for Disease Control and Prevention released guidance recommending routine HIV screening in all healthcare settings, including urgent care and EDs. Elam, who conducted the research as a student at Rutgers University School of Nursing in New Brunswick, N.J., noticed during her time as an ED nurse that, while her department had a policy supporting routine HIV screening, the practice was not consistent across all nursing staff. To find out how HIV screening varied nationally, Elam ran a national survey from Oct. through Dec. 2020, recruiting participants both by email outreach and Facebook.
In the 30- to 45-minute survey, respondents reported:
- Demographic information
- Knowledge of the CDC HIV screening recommendations
- Workplace HIV screening policy
- Self-reported performance of HIV screening
- Beliefs and attitudes pertaining to HIV screening
Overall, 371 individuals from 43 states filled out at least some part of the survey, and 171 individuals completed it. Of the 251 individuals who answered whether their EDs routinely conducted HIV screening, 76.9% responded affirmatively. Overall, 28.5% of respondents thought HIV screening was “not important” or “not at all important.” Nearly half – 47.6% – reported never offering HIV testing to all eligible patients regardless of risk factors, and only 14.3% reported offering testing all of the time. Only 25% of participants said they received “adequate” or “a lot” of HIV-related nursing education, and 42.9% reported “little” or “very little” education.
“For the most part, those of us working in hospitals, all the education that we get about HIV took place in school,” Elam said. “So, if you went to school in the early 2000s or in the 1990s, you don’t know much else.” Elam noted that she keeps informed on HIV research issues because it is an area of interest, but the hospital she had worked at did not contribute much to her knowledge.
Elam also found that in practice there were several barriers to performing screening, such as lack of availability of a dedicated HIV educator, tester, or counselors; not knowing where to refer patients who had a positive HIV test result; and insufficient time to address positive HIV test results in ED practice.
“A lot of these things are outside an individual nurse’s control,” said Elam, and can result in missing patients who would benefit from care. Lisa Leimer, RN, a nurse at Primary Health Care in Des Moines, works with patients after they have been diagnosed with HIV, but noted that many of her patients could have been identified earlier. “Once we get someone, you look back at medical records and you see that they have been in and out of the hospital,” she said. “There’s been multiple encounters,” she said.
Prioritizing HIV screening in all healthcare settings and including HIV education for all medical professionals – not just nurses – could help in the continuing battle against HIV. “So much has changed in the world of HIV,” she said. “We’re trying to end the epidemic, and it could happen if we identified, diagnosed, and treated the people that are living with it.”
Elam and Leimer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a national survey of ED nurses. Nearly 43% of respondents said they had received “little” or “very little” HIV education as part of their professional development and practice.
This lack of continuing HIV education “often translated into attitudes that did not support the policy” of routine HIV screening in EDs, lead author Candace Elam, DNP, a family nurse practitioner at the Institute of Family Health in the Bronx, New York City, told this news organization. “But more than individual attitudes, what came out most clearly in the research was that organizational support for HIV screening in EDs was the one factor that could make or break whether an emergency nurse performs HIV screening,” she said. This includes working routine HIV screening into ED workflows and providing resources to streamline screening and testing efforts.
In 2006, the Centers for Disease Control and Prevention released guidance recommending routine HIV screening in all healthcare settings, including urgent care and EDs. Elam, who conducted the research as a student at Rutgers University School of Nursing in New Brunswick, N.J., noticed during her time as an ED nurse that, while her department had a policy supporting routine HIV screening, the practice was not consistent across all nursing staff. To find out how HIV screening varied nationally, Elam ran a national survey from Oct. through Dec. 2020, recruiting participants both by email outreach and Facebook.
In the 30- to 45-minute survey, respondents reported:
- Demographic information
- Knowledge of the CDC HIV screening recommendations
- Workplace HIV screening policy
- Self-reported performance of HIV screening
- Beliefs and attitudes pertaining to HIV screening
Overall, 371 individuals from 43 states filled out at least some part of the survey, and 171 individuals completed it. Of the 251 individuals who answered whether their EDs routinely conducted HIV screening, 76.9% responded affirmatively. Overall, 28.5% of respondents thought HIV screening was “not important” or “not at all important.” Nearly half – 47.6% – reported never offering HIV testing to all eligible patients regardless of risk factors, and only 14.3% reported offering testing all of the time. Only 25% of participants said they received “adequate” or “a lot” of HIV-related nursing education, and 42.9% reported “little” or “very little” education.
“For the most part, those of us working in hospitals, all the education that we get about HIV took place in school,” Elam said. “So, if you went to school in the early 2000s or in the 1990s, you don’t know much else.” Elam noted that she keeps informed on HIV research issues because it is an area of interest, but the hospital she had worked at did not contribute much to her knowledge.
Elam also found that in practice there were several barriers to performing screening, such as lack of availability of a dedicated HIV educator, tester, or counselors; not knowing where to refer patients who had a positive HIV test result; and insufficient time to address positive HIV test results in ED practice.
“A lot of these things are outside an individual nurse’s control,” said Elam, and can result in missing patients who would benefit from care. Lisa Leimer, RN, a nurse at Primary Health Care in Des Moines, works with patients after they have been diagnosed with HIV, but noted that many of her patients could have been identified earlier. “Once we get someone, you look back at medical records and you see that they have been in and out of the hospital,” she said. “There’s been multiple encounters,” she said.
Prioritizing HIV screening in all healthcare settings and including HIV education for all medical professionals – not just nurses – could help in the continuing battle against HIV. “So much has changed in the world of HIV,” she said. “We’re trying to end the epidemic, and it could happen if we identified, diagnosed, and treated the people that are living with it.”
Elam and Leimer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a national survey of ED nurses. Nearly 43% of respondents said they had received “little” or “very little” HIV education as part of their professional development and practice.
This lack of continuing HIV education “often translated into attitudes that did not support the policy” of routine HIV screening in EDs, lead author Candace Elam, DNP, a family nurse practitioner at the Institute of Family Health in the Bronx, New York City, told this news organization. “But more than individual attitudes, what came out most clearly in the research was that organizational support for HIV screening in EDs was the one factor that could make or break whether an emergency nurse performs HIV screening,” she said. This includes working routine HIV screening into ED workflows and providing resources to streamline screening and testing efforts.
In 2006, the Centers for Disease Control and Prevention released guidance recommending routine HIV screening in all healthcare settings, including urgent care and EDs. Elam, who conducted the research as a student at Rutgers University School of Nursing in New Brunswick, N.J., noticed during her time as an ED nurse that, while her department had a policy supporting routine HIV screening, the practice was not consistent across all nursing staff. To find out how HIV screening varied nationally, Elam ran a national survey from Oct. through Dec. 2020, recruiting participants both by email outreach and Facebook.
In the 30- to 45-minute survey, respondents reported:
- Demographic information
- Knowledge of the CDC HIV screening recommendations
- Workplace HIV screening policy
- Self-reported performance of HIV screening
- Beliefs and attitudes pertaining to HIV screening
Overall, 371 individuals from 43 states filled out at least some part of the survey, and 171 individuals completed it. Of the 251 individuals who answered whether their EDs routinely conducted HIV screening, 76.9% responded affirmatively. Overall, 28.5% of respondents thought HIV screening was “not important” or “not at all important.” Nearly half – 47.6% – reported never offering HIV testing to all eligible patients regardless of risk factors, and only 14.3% reported offering testing all of the time. Only 25% of participants said they received “adequate” or “a lot” of HIV-related nursing education, and 42.9% reported “little” or “very little” education.
“For the most part, those of us working in hospitals, all the education that we get about HIV took place in school,” Elam said. “So, if you went to school in the early 2000s or in the 1990s, you don’t know much else.” Elam noted that she keeps informed on HIV research issues because it is an area of interest, but the hospital she had worked at did not contribute much to her knowledge.
Elam also found that in practice there were several barriers to performing screening, such as lack of availability of a dedicated HIV educator, tester, or counselors; not knowing where to refer patients who had a positive HIV test result; and insufficient time to address positive HIV test results in ED practice.
“A lot of these things are outside an individual nurse’s control,” said Elam, and can result in missing patients who would benefit from care. Lisa Leimer, RN, a nurse at Primary Health Care in Des Moines, works with patients after they have been diagnosed with HIV, but noted that many of her patients could have been identified earlier. “Once we get someone, you look back at medical records and you see that they have been in and out of the hospital,” she said. “There’s been multiple encounters,” she said.
Prioritizing HIV screening in all healthcare settings and including HIV education for all medical professionals – not just nurses – could help in the continuing battle against HIV. “So much has changed in the world of HIV,” she said. “We’re trying to end the epidemic, and it could happen if we identified, diagnosed, and treated the people that are living with it.”
Elam and Leimer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases up again after dropping for 8 weeks
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.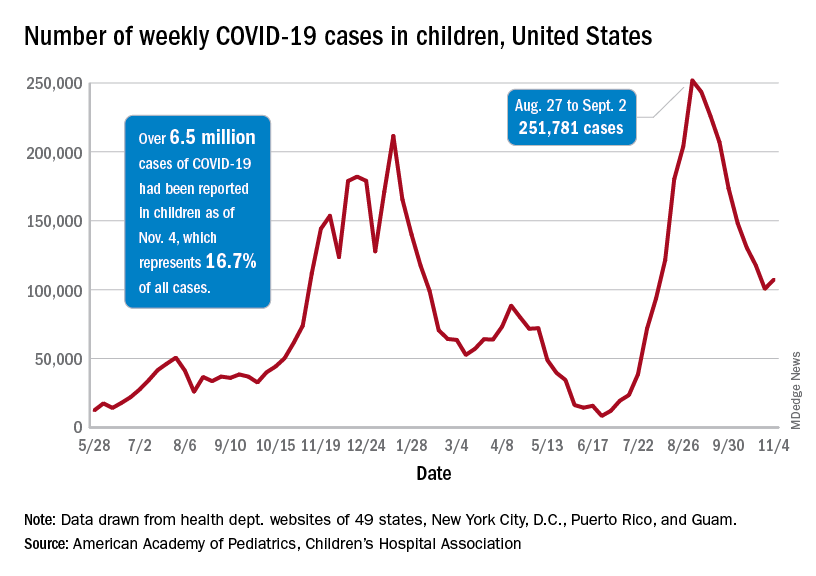
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
72-year-old man • fever • new-onset urinary frequency • altered mental state • Dx?
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; tenzin.tsewang@phhs.org
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; tenzin.tsewang@phhs.org
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; tenzin.tsewang@phhs.org
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
More than half of people living with HIV have coronary plaque
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Expected spike in acute flaccid myelitis did not occur in 2020
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
FROM MMWR
Does zinc really help treat colds?
A new study published in BMJ Open adds to the evidence that zinc is effective against viral respiratory infections, such as colds.
Jennifer Hunter, PhD, BMed, of Western Sydney University’s NICM Health Research Institute, New South Wales, Australia, and colleagues conducted a meta-analysis of 28 randomized controlled trials (RCTs). They searched 17 English and Chinese databases to identify the trials and then used the Cochrane rapid review technique for the analysis.
The trials included 5,446 adults who had received zinc in a variety of formulations and routes — oral, sublingual, and nasal spray. The researchers separately analyzed whether zinc prevented or treated respiratory tract infections (RTIs)
Oral or intranasal zinc prevented five RTIs per 100 person-months (95% CI, 1 – 8; numbers needed to treat, 20). There was a 32% lower relative risk (RR) of developing mild to moderate symptoms consistent with a viral RTI.
Use of zinc was also associated with an 87% lower risk of developing moderately severe symptoms (incidence rate ratio, 0.13; 95% CI, 0.04 – 0.38) and a 28% lower risk of developing milder symptoms. The largest reductions in RR were for moderately severe symptoms consistent with an influenza-like illness.
Symptoms resolved 2 days earlier with sublingual or intranasal zinc compared with placebo (95% CI, 0.61 – 3.50; very low-certainty quality of evidence). There were clinically significant reductions in day 3 symptom severity scores (mean difference, -1.20 points; 95% CI, -0.66 to -1.74; low-certainty quality of evidence) but not in overall symptom severity. Participants who used sublingual or topical nasal zinc early in the course of illness were 1.8 times more likely to recover before those who used a placebo.
However, the investigators found no benefit of zinc when patients were inoculated with rhinovirus; there was no reduction in the risk of developing a cold. Asked about this disparity, Dr. Hunter said, “It might well be that when inoculating people to make sure they get infected, you give them a really high dose of the virus. [This] doesn’t really mimic what happens in the real world.”
On the downside of supplemental zinc, there were more side effects among those who used zinc, including nausea or gastrointestinal discomfort, mouth irritation, or soreness from sublingual lozenges (RR, 1.41; 95% CI, 1.17 – 1.69; number needed to harm, 7; moderate-certainty quality of evidence). The risk for a serious adverse event, such as loss of smell or copper deficiency, was low. Although not found in these studies, postmarketing studies have found that there is a risk for severe and in some cases permanent loss of smell associated with the use of nasal gels or sprays containing zinc. Three such products were recalled from the market.
The trial could not provide answers about the comparative efficacy of different types of zinc formulations, nor could the investigators recommend specific doses. The trial was not designed to assess zinc for the prevention or treatment of COVID-19.
Asked for independent comment, pediatrician Aamer Imdad, MBBS, assistant professor at the State University of New York Upstate Medical University, Syracuse, told this news organization, “It’s a very comprehensive review for zinc-related studies in adults” but was challenging because of the “significant clinical heterogeneity in the population.”
Dr. Imdad explained that zinc has “absolutely” been shown to be effective for children with diarrhea. The World Health Organization has recommended it since 2004. “The way it works in diarrhea is that it helps with the regeneration of the epithelium.... It also improves the immunity itself, especially the cell-mediated immunity.” He raised the question of whether it might work similarly in the respiratory tract. Dr. Imdad has a long-standing interest in the use of zinc for pediatric infections. Regarding this study, he concluded, “I think we still need to know the nuts and bolts of this intervention before we can recommend it more specifically.”
Dr. Hunter said, “We don’t have any high-quality studies that have evaluated zinc orally as treatment once you’re actually infected and have symptoms of the cold or influenza, or COVID.”
Asked about zinc’s possible role, Dr. Hunter said, “So I do think it gives us a viable alternative. More people are going, ‘What can I do?’ And you know as well as I do people come to you, and [they say], ‘Well, just give me something. Even if it’s a day or a little bit of symptom relief, anything to make me feel better that isn’t going to hurt me and doesn’t have any major risks.’ So I think in the short term, clinicians and consumers can consider trying it.”
Dr. Hunter was not keen on giving zinc to family members after they develop an RTI: “Consider it. But I don’t think we have enough evidence to say definitely yes.” But she does see a potential role for “people who are at risk of suboptimal zinc absorption, like people who are taking a variety of pharmaceuticals [notably proton pump inhibitors] that block or reduce the absorption of zinc, people with a whole lot of the chronic diseases that we know are associated with an increased risk of worse outcomes from respiratory viral infections, and older adults. Yes, I think [for] those high-risk groups, you could consider using zinc, either in a moderate dose longer term or in a higher dose for very short bursts of, like, 1 to 2 weeks.”
Dr. Hunter concluded, “Up until now, we all commonly thought that zinc’s role was only for people who were zinc deficient, and now we’ve got some signals pointing towards its potential role as an anti-infective and anti-inflammatory agent in people who don’t have zinc deficiency.”
But both Dr. Hunter and Dr. Imdad emphasized that zinc is not a game changer. There is a hint that it produces a small benefit in prevention and may slightly shorten the duration of RTIs. More research is needed.
Dr. Hunter has received payment for providing expert advice about traditional, complementary, and integrative medicine, including nutraceuticals, to industry, government bodies, and nongovernmental organizations and has spoken at workshops, seminars, and conferences for which registration, travel, and/or accommodation has been paid for by the organizers. Dr. Imdad has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study published in BMJ Open adds to the evidence that zinc is effective against viral respiratory infections, such as colds.
Jennifer Hunter, PhD, BMed, of Western Sydney University’s NICM Health Research Institute, New South Wales, Australia, and colleagues conducted a meta-analysis of 28 randomized controlled trials (RCTs). They searched 17 English and Chinese databases to identify the trials and then used the Cochrane rapid review technique for the analysis.
The trials included 5,446 adults who had received zinc in a variety of formulations and routes — oral, sublingual, and nasal spray. The researchers separately analyzed whether zinc prevented or treated respiratory tract infections (RTIs)
Oral or intranasal zinc prevented five RTIs per 100 person-months (95% CI, 1 – 8; numbers needed to treat, 20). There was a 32% lower relative risk (RR) of developing mild to moderate symptoms consistent with a viral RTI.
Use of zinc was also associated with an 87% lower risk of developing moderately severe symptoms (incidence rate ratio, 0.13; 95% CI, 0.04 – 0.38) and a 28% lower risk of developing milder symptoms. The largest reductions in RR were for moderately severe symptoms consistent with an influenza-like illness.
Symptoms resolved 2 days earlier with sublingual or intranasal zinc compared with placebo (95% CI, 0.61 – 3.50; very low-certainty quality of evidence). There were clinically significant reductions in day 3 symptom severity scores (mean difference, -1.20 points; 95% CI, -0.66 to -1.74; low-certainty quality of evidence) but not in overall symptom severity. Participants who used sublingual or topical nasal zinc early in the course of illness were 1.8 times more likely to recover before those who used a placebo.
However, the investigators found no benefit of zinc when patients were inoculated with rhinovirus; there was no reduction in the risk of developing a cold. Asked about this disparity, Dr. Hunter said, “It might well be that when inoculating people to make sure they get infected, you give them a really high dose of the virus. [This] doesn’t really mimic what happens in the real world.”
On the downside of supplemental zinc, there were more side effects among those who used zinc, including nausea or gastrointestinal discomfort, mouth irritation, or soreness from sublingual lozenges (RR, 1.41; 95% CI, 1.17 – 1.69; number needed to harm, 7; moderate-certainty quality of evidence). The risk for a serious adverse event, such as loss of smell or copper deficiency, was low. Although not found in these studies, postmarketing studies have found that there is a risk for severe and in some cases permanent loss of smell associated with the use of nasal gels or sprays containing zinc. Three such products were recalled from the market.
The trial could not provide answers about the comparative efficacy of different types of zinc formulations, nor could the investigators recommend specific doses. The trial was not designed to assess zinc for the prevention or treatment of COVID-19.
Asked for independent comment, pediatrician Aamer Imdad, MBBS, assistant professor at the State University of New York Upstate Medical University, Syracuse, told this news organization, “It’s a very comprehensive review for zinc-related studies in adults” but was challenging because of the “significant clinical heterogeneity in the population.”
Dr. Imdad explained that zinc has “absolutely” been shown to be effective for children with diarrhea. The World Health Organization has recommended it since 2004. “The way it works in diarrhea is that it helps with the regeneration of the epithelium.... It also improves the immunity itself, especially the cell-mediated immunity.” He raised the question of whether it might work similarly in the respiratory tract. Dr. Imdad has a long-standing interest in the use of zinc for pediatric infections. Regarding this study, he concluded, “I think we still need to know the nuts and bolts of this intervention before we can recommend it more specifically.”
Dr. Hunter said, “We don’t have any high-quality studies that have evaluated zinc orally as treatment once you’re actually infected and have symptoms of the cold or influenza, or COVID.”
Asked about zinc’s possible role, Dr. Hunter said, “So I do think it gives us a viable alternative. More people are going, ‘What can I do?’ And you know as well as I do people come to you, and [they say], ‘Well, just give me something. Even if it’s a day or a little bit of symptom relief, anything to make me feel better that isn’t going to hurt me and doesn’t have any major risks.’ So I think in the short term, clinicians and consumers can consider trying it.”
Dr. Hunter was not keen on giving zinc to family members after they develop an RTI: “Consider it. But I don’t think we have enough evidence to say definitely yes.” But she does see a potential role for “people who are at risk of suboptimal zinc absorption, like people who are taking a variety of pharmaceuticals [notably proton pump inhibitors] that block or reduce the absorption of zinc, people with a whole lot of the chronic diseases that we know are associated with an increased risk of worse outcomes from respiratory viral infections, and older adults. Yes, I think [for] those high-risk groups, you could consider using zinc, either in a moderate dose longer term or in a higher dose for very short bursts of, like, 1 to 2 weeks.”
Dr. Hunter concluded, “Up until now, we all commonly thought that zinc’s role was only for people who were zinc deficient, and now we’ve got some signals pointing towards its potential role as an anti-infective and anti-inflammatory agent in people who don’t have zinc deficiency.”
But both Dr. Hunter and Dr. Imdad emphasized that zinc is not a game changer. There is a hint that it produces a small benefit in prevention and may slightly shorten the duration of RTIs. More research is needed.
Dr. Hunter has received payment for providing expert advice about traditional, complementary, and integrative medicine, including nutraceuticals, to industry, government bodies, and nongovernmental organizations and has spoken at workshops, seminars, and conferences for which registration, travel, and/or accommodation has been paid for by the organizers. Dr. Imdad has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study published in BMJ Open adds to the evidence that zinc is effective against viral respiratory infections, such as colds.
Jennifer Hunter, PhD, BMed, of Western Sydney University’s NICM Health Research Institute, New South Wales, Australia, and colleagues conducted a meta-analysis of 28 randomized controlled trials (RCTs). They searched 17 English and Chinese databases to identify the trials and then used the Cochrane rapid review technique for the analysis.
The trials included 5,446 adults who had received zinc in a variety of formulations and routes — oral, sublingual, and nasal spray. The researchers separately analyzed whether zinc prevented or treated respiratory tract infections (RTIs)
Oral or intranasal zinc prevented five RTIs per 100 person-months (95% CI, 1 – 8; numbers needed to treat, 20). There was a 32% lower relative risk (RR) of developing mild to moderate symptoms consistent with a viral RTI.
Use of zinc was also associated with an 87% lower risk of developing moderately severe symptoms (incidence rate ratio, 0.13; 95% CI, 0.04 – 0.38) and a 28% lower risk of developing milder symptoms. The largest reductions in RR were for moderately severe symptoms consistent with an influenza-like illness.
Symptoms resolved 2 days earlier with sublingual or intranasal zinc compared with placebo (95% CI, 0.61 – 3.50; very low-certainty quality of evidence). There were clinically significant reductions in day 3 symptom severity scores (mean difference, -1.20 points; 95% CI, -0.66 to -1.74; low-certainty quality of evidence) but not in overall symptom severity. Participants who used sublingual or topical nasal zinc early in the course of illness were 1.8 times more likely to recover before those who used a placebo.
However, the investigators found no benefit of zinc when patients were inoculated with rhinovirus; there was no reduction in the risk of developing a cold. Asked about this disparity, Dr. Hunter said, “It might well be that when inoculating people to make sure they get infected, you give them a really high dose of the virus. [This] doesn’t really mimic what happens in the real world.”
On the downside of supplemental zinc, there were more side effects among those who used zinc, including nausea or gastrointestinal discomfort, mouth irritation, or soreness from sublingual lozenges (RR, 1.41; 95% CI, 1.17 – 1.69; number needed to harm, 7; moderate-certainty quality of evidence). The risk for a serious adverse event, such as loss of smell or copper deficiency, was low. Although not found in these studies, postmarketing studies have found that there is a risk for severe and in some cases permanent loss of smell associated with the use of nasal gels or sprays containing zinc. Three such products were recalled from the market.
The trial could not provide answers about the comparative efficacy of different types of zinc formulations, nor could the investigators recommend specific doses. The trial was not designed to assess zinc for the prevention or treatment of COVID-19.
Asked for independent comment, pediatrician Aamer Imdad, MBBS, assistant professor at the State University of New York Upstate Medical University, Syracuse, told this news organization, “It’s a very comprehensive review for zinc-related studies in adults” but was challenging because of the “significant clinical heterogeneity in the population.”
Dr. Imdad explained that zinc has “absolutely” been shown to be effective for children with diarrhea. The World Health Organization has recommended it since 2004. “The way it works in diarrhea is that it helps with the regeneration of the epithelium.... It also improves the immunity itself, especially the cell-mediated immunity.” He raised the question of whether it might work similarly in the respiratory tract. Dr. Imdad has a long-standing interest in the use of zinc for pediatric infections. Regarding this study, he concluded, “I think we still need to know the nuts and bolts of this intervention before we can recommend it more specifically.”
Dr. Hunter said, “We don’t have any high-quality studies that have evaluated zinc orally as treatment once you’re actually infected and have symptoms of the cold or influenza, or COVID.”
Asked about zinc’s possible role, Dr. Hunter said, “So I do think it gives us a viable alternative. More people are going, ‘What can I do?’ And you know as well as I do people come to you, and [they say], ‘Well, just give me something. Even if it’s a day or a little bit of symptom relief, anything to make me feel better that isn’t going to hurt me and doesn’t have any major risks.’ So I think in the short term, clinicians and consumers can consider trying it.”
Dr. Hunter was not keen on giving zinc to family members after they develop an RTI: “Consider it. But I don’t think we have enough evidence to say definitely yes.” But she does see a potential role for “people who are at risk of suboptimal zinc absorption, like people who are taking a variety of pharmaceuticals [notably proton pump inhibitors] that block or reduce the absorption of zinc, people with a whole lot of the chronic diseases that we know are associated with an increased risk of worse outcomes from respiratory viral infections, and older adults. Yes, I think [for] those high-risk groups, you could consider using zinc, either in a moderate dose longer term or in a higher dose for very short bursts of, like, 1 to 2 weeks.”
Dr. Hunter concluded, “Up until now, we all commonly thought that zinc’s role was only for people who were zinc deficient, and now we’ve got some signals pointing towards its potential role as an anti-infective and anti-inflammatory agent in people who don’t have zinc deficiency.”
But both Dr. Hunter and Dr. Imdad emphasized that zinc is not a game changer. There is a hint that it produces a small benefit in prevention and may slightly shorten the duration of RTIs. More research is needed.
Dr. Hunter has received payment for providing expert advice about traditional, complementary, and integrative medicine, including nutraceuticals, to industry, government bodies, and nongovernmental organizations and has spoken at workshops, seminars, and conferences for which registration, travel, and/or accommodation has been paid for by the organizers. Dr. Imdad has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Maraviroc, metformin fail to control NAFLD in people with HIV
The MAVMET study, the first randomized controlled trial of – and in some cases, prolonged use actually increased liver fat.
And that means clinicians like Yvonne Gilleece, MB BCh, who was not involved in the study but does run a liver clinic in England for people living with HIV, are returning to the one intervention proven to work. “As yet, the only thing that is proven to have a very positive effect that is published is weight loss,” said Dr. Gilleece, who runs the clinic at Brighton and Sussex University Hospital. “You don’t put someone on these particular drugs, particularly this combination, easily. MAVMET has really demonstrated that, actually, it’s not effective, and it’s not particularly beneficial to patients.”
The MAVMET trial data was presented at the 18th European AIDS Conference,
There was good reason to think maraviroc might work. A 2018 study in the journal Hepatology found that one of maraviroc’s molecular cousins, cenicriviroc, significantly reduced fibrosis in people with NAFLD. Dr. Gilleece is co-investigator of another study of maraviroc in NAFLD, the HEPMARC trial, which is wrapping up now. In addition to those studies, there are other potential treatments in ongoing trials, including semaglutide, which is being studied in the United States under the study name SLIM LIVER.
MAVMET enrolled 90 people living with HIV from six clinical sites in London who were 35 or older and who had at least one marker for NAFLD, such as abnormal liver lab results. But 70% qualified via imaging- and/or biopsy-confirmed NAFLD. Almost all participants (93%) were men and 81% were White. The trial excluded people who were pregnant or breastfeeding. The median age was 52, and the participants met the criteria for overweight but not obesity, with a median BMI of 28.
In other words, participants generally had fatty livers without the inflammation that characterizes the more aggressive nonalcoholic steatohepatitis (NASH). Clinicians can’t yet differentiate between those who will continue to have asymptomatic fatty liver and those who will progress to NASH and potentially need a liver transplant.
All people living with HIV in the trial had undetectable viral loads and were on HIV treatment. Nearly 1 in 5 (19%) were using a treatment regimen containing tenofovir alafenamide (TAF), which has been associated with weight gain. Nearly half were on integrase strand inhibitors.
Investigators divided the participants up into four groups: 24 people stayed on their HIV treatment and added nothing else; 23 people took maraviroc only; 21 took metformin only; and the final group took both maraviroc and metformin. Across groups, liver fat at baseline was 8.9%, and 78% had mild hepatic steatosis.
After taking the medications for 48 weeks, participants returned to clinic to be scanned via MRI proton density fat fraction (MRI-PDFF), which has been found to successfully measure liver fat. However, because of the COVID-19 pandemic, 20 of the 83 people who returned to the clinic came later than 48 weeks after the trial began.
When investigators looked at the results, they didn’t see what they hypothesized, said Sarah Pett, professor of infectious diseases at University College, London: The scatter plot graph of change in weight looked, well, scattershot: People who didn’t take any additional treatment sometimes lost more liver fat than those on treatment. In fact, the mean liver fat percentage rose by 2.2% in the maraviroc group, 1.3% in the metformin group, and 0.8% in the combination group. The control group saw an increase of 1.4% – meaning that there was no difference between the change in fat between those on treatment and those not.
What’s more, those who had delayed scans – and stayed on their treatment for a median of an additional 16 weeks – saw their liver fat increase even more.
In an interview, Dr. Pett called the results “disappointing.” “The numbers are quite small, but we still didn’t expect this,” she said. “It’s not explained by lockdown weight gain, although we still have to look in detail at how alcohol consumption could have contributed.”
There were also some limits to what the design of this particular trial could tell the researchers. For instance, nearly half of the participants in the maraviroc group, a third of the people in the metformin group, and 36% of those in the combination group had hepatic steatosis grades of 0, meaning that their livers were healthy. And MRI-PDFF becomes less reliable at that level.
“One of the regrets is that perhaps we should have done FibroScan [ultrasound], as well,” Dr. Pett said. The consequence is that the study may have undercounted the fat level by using MRI-PFDD.
“This suggests that the surrogate markers of NAFLD used in MAVMET were not very sensitive to those with a higher percentage of fat,” Dr. Pett said during her presentation. “We were really trying to be pragmatic and not require an MRI at screening.”
Whatever the case, she said that the failure of this particular treatment just highlights the growing need to look more seriously, and more collaboratively, at fat and liver health in people living with HIV.
“We need to really focus on setting up large cohorts of people living with HIV to look in a rigorous way at weight gain, changes in waist circumference, ectopic fat, capture fatty liver disease index scores, and cardiovascular risk, to acquire some longitudinal data,” she said. “And [we need to] join with our fellow researchers in overweight and obesity medicine and hepatology to make sure that people living with HIV are included in new treatments for NASH, as several large RCTs have excluded [people living with HIV].”
From Dr. Gilleece’s perspective, it also just speaks to how far the field has to go in identifying those with asymptomatic fatty livers from those who will progress to fibrosis and potentially need liver transplants.
“MAVMET shows the difficulty in managing NAFLD,” she said. “It seems quite an innocuous disease, because for the majority of people it’s not going to cause a problem in their lifetime. But the reality is, for some it will, and we don’t really know how to treat it.”
Dr. Gilleece has disclosed no relevant financial relationships. Dr. Pett reported receiving funding for trials from Gilead Sciences and Janssen-Cilag. ViiV Healthcare funded the MAVMET trial.
A version of this article first appeared on Medscape.com.
The MAVMET study, the first randomized controlled trial of – and in some cases, prolonged use actually increased liver fat.
And that means clinicians like Yvonne Gilleece, MB BCh, who was not involved in the study but does run a liver clinic in England for people living with HIV, are returning to the one intervention proven to work. “As yet, the only thing that is proven to have a very positive effect that is published is weight loss,” said Dr. Gilleece, who runs the clinic at Brighton and Sussex University Hospital. “You don’t put someone on these particular drugs, particularly this combination, easily. MAVMET has really demonstrated that, actually, it’s not effective, and it’s not particularly beneficial to patients.”
The MAVMET trial data was presented at the 18th European AIDS Conference,
There was good reason to think maraviroc might work. A 2018 study in the journal Hepatology found that one of maraviroc’s molecular cousins, cenicriviroc, significantly reduced fibrosis in people with NAFLD. Dr. Gilleece is co-investigator of another study of maraviroc in NAFLD, the HEPMARC trial, which is wrapping up now. In addition to those studies, there are other potential treatments in ongoing trials, including semaglutide, which is being studied in the United States under the study name SLIM LIVER.
MAVMET enrolled 90 people living with HIV from six clinical sites in London who were 35 or older and who had at least one marker for NAFLD, such as abnormal liver lab results. But 70% qualified via imaging- and/or biopsy-confirmed NAFLD. Almost all participants (93%) were men and 81% were White. The trial excluded people who were pregnant or breastfeeding. The median age was 52, and the participants met the criteria for overweight but not obesity, with a median BMI of 28.
In other words, participants generally had fatty livers without the inflammation that characterizes the more aggressive nonalcoholic steatohepatitis (NASH). Clinicians can’t yet differentiate between those who will continue to have asymptomatic fatty liver and those who will progress to NASH and potentially need a liver transplant.
All people living with HIV in the trial had undetectable viral loads and were on HIV treatment. Nearly 1 in 5 (19%) were using a treatment regimen containing tenofovir alafenamide (TAF), which has been associated with weight gain. Nearly half were on integrase strand inhibitors.
Investigators divided the participants up into four groups: 24 people stayed on their HIV treatment and added nothing else; 23 people took maraviroc only; 21 took metformin only; and the final group took both maraviroc and metformin. Across groups, liver fat at baseline was 8.9%, and 78% had mild hepatic steatosis.
After taking the medications for 48 weeks, participants returned to clinic to be scanned via MRI proton density fat fraction (MRI-PDFF), which has been found to successfully measure liver fat. However, because of the COVID-19 pandemic, 20 of the 83 people who returned to the clinic came later than 48 weeks after the trial began.
When investigators looked at the results, they didn’t see what they hypothesized, said Sarah Pett, professor of infectious diseases at University College, London: The scatter plot graph of change in weight looked, well, scattershot: People who didn’t take any additional treatment sometimes lost more liver fat than those on treatment. In fact, the mean liver fat percentage rose by 2.2% in the maraviroc group, 1.3% in the metformin group, and 0.8% in the combination group. The control group saw an increase of 1.4% – meaning that there was no difference between the change in fat between those on treatment and those not.
What’s more, those who had delayed scans – and stayed on their treatment for a median of an additional 16 weeks – saw their liver fat increase even more.
In an interview, Dr. Pett called the results “disappointing.” “The numbers are quite small, but we still didn’t expect this,” she said. “It’s not explained by lockdown weight gain, although we still have to look in detail at how alcohol consumption could have contributed.”
There were also some limits to what the design of this particular trial could tell the researchers. For instance, nearly half of the participants in the maraviroc group, a third of the people in the metformin group, and 36% of those in the combination group had hepatic steatosis grades of 0, meaning that their livers were healthy. And MRI-PDFF becomes less reliable at that level.
“One of the regrets is that perhaps we should have done FibroScan [ultrasound], as well,” Dr. Pett said. The consequence is that the study may have undercounted the fat level by using MRI-PFDD.
“This suggests that the surrogate markers of NAFLD used in MAVMET were not very sensitive to those with a higher percentage of fat,” Dr. Pett said during her presentation. “We were really trying to be pragmatic and not require an MRI at screening.”
Whatever the case, she said that the failure of this particular treatment just highlights the growing need to look more seriously, and more collaboratively, at fat and liver health in people living with HIV.
“We need to really focus on setting up large cohorts of people living with HIV to look in a rigorous way at weight gain, changes in waist circumference, ectopic fat, capture fatty liver disease index scores, and cardiovascular risk, to acquire some longitudinal data,” she said. “And [we need to] join with our fellow researchers in overweight and obesity medicine and hepatology to make sure that people living with HIV are included in new treatments for NASH, as several large RCTs have excluded [people living with HIV].”
From Dr. Gilleece’s perspective, it also just speaks to how far the field has to go in identifying those with asymptomatic fatty livers from those who will progress to fibrosis and potentially need liver transplants.
“MAVMET shows the difficulty in managing NAFLD,” she said. “It seems quite an innocuous disease, because for the majority of people it’s not going to cause a problem in their lifetime. But the reality is, for some it will, and we don’t really know how to treat it.”
Dr. Gilleece has disclosed no relevant financial relationships. Dr. Pett reported receiving funding for trials from Gilead Sciences and Janssen-Cilag. ViiV Healthcare funded the MAVMET trial.
A version of this article first appeared on Medscape.com.
The MAVMET study, the first randomized controlled trial of – and in some cases, prolonged use actually increased liver fat.
And that means clinicians like Yvonne Gilleece, MB BCh, who was not involved in the study but does run a liver clinic in England for people living with HIV, are returning to the one intervention proven to work. “As yet, the only thing that is proven to have a very positive effect that is published is weight loss,” said Dr. Gilleece, who runs the clinic at Brighton and Sussex University Hospital. “You don’t put someone on these particular drugs, particularly this combination, easily. MAVMET has really demonstrated that, actually, it’s not effective, and it’s not particularly beneficial to patients.”
The MAVMET trial data was presented at the 18th European AIDS Conference,
There was good reason to think maraviroc might work. A 2018 study in the journal Hepatology found that one of maraviroc’s molecular cousins, cenicriviroc, significantly reduced fibrosis in people with NAFLD. Dr. Gilleece is co-investigator of another study of maraviroc in NAFLD, the HEPMARC trial, which is wrapping up now. In addition to those studies, there are other potential treatments in ongoing trials, including semaglutide, which is being studied in the United States under the study name SLIM LIVER.
MAVMET enrolled 90 people living with HIV from six clinical sites in London who were 35 or older and who had at least one marker for NAFLD, such as abnormal liver lab results. But 70% qualified via imaging- and/or biopsy-confirmed NAFLD. Almost all participants (93%) were men and 81% were White. The trial excluded people who were pregnant or breastfeeding. The median age was 52, and the participants met the criteria for overweight but not obesity, with a median BMI of 28.
In other words, participants generally had fatty livers without the inflammation that characterizes the more aggressive nonalcoholic steatohepatitis (NASH). Clinicians can’t yet differentiate between those who will continue to have asymptomatic fatty liver and those who will progress to NASH and potentially need a liver transplant.
All people living with HIV in the trial had undetectable viral loads and were on HIV treatment. Nearly 1 in 5 (19%) were using a treatment regimen containing tenofovir alafenamide (TAF), which has been associated with weight gain. Nearly half were on integrase strand inhibitors.
Investigators divided the participants up into four groups: 24 people stayed on their HIV treatment and added nothing else; 23 people took maraviroc only; 21 took metformin only; and the final group took both maraviroc and metformin. Across groups, liver fat at baseline was 8.9%, and 78% had mild hepatic steatosis.
After taking the medications for 48 weeks, participants returned to clinic to be scanned via MRI proton density fat fraction (MRI-PDFF), which has been found to successfully measure liver fat. However, because of the COVID-19 pandemic, 20 of the 83 people who returned to the clinic came later than 48 weeks after the trial began.
When investigators looked at the results, they didn’t see what they hypothesized, said Sarah Pett, professor of infectious diseases at University College, London: The scatter plot graph of change in weight looked, well, scattershot: People who didn’t take any additional treatment sometimes lost more liver fat than those on treatment. In fact, the mean liver fat percentage rose by 2.2% in the maraviroc group, 1.3% in the metformin group, and 0.8% in the combination group. The control group saw an increase of 1.4% – meaning that there was no difference between the change in fat between those on treatment and those not.
What’s more, those who had delayed scans – and stayed on their treatment for a median of an additional 16 weeks – saw their liver fat increase even more.
In an interview, Dr. Pett called the results “disappointing.” “The numbers are quite small, but we still didn’t expect this,” she said. “It’s not explained by lockdown weight gain, although we still have to look in detail at how alcohol consumption could have contributed.”
There were also some limits to what the design of this particular trial could tell the researchers. For instance, nearly half of the participants in the maraviroc group, a third of the people in the metformin group, and 36% of those in the combination group had hepatic steatosis grades of 0, meaning that their livers were healthy. And MRI-PDFF becomes less reliable at that level.
“One of the regrets is that perhaps we should have done FibroScan [ultrasound], as well,” Dr. Pett said. The consequence is that the study may have undercounted the fat level by using MRI-PFDD.
“This suggests that the surrogate markers of NAFLD used in MAVMET were not very sensitive to those with a higher percentage of fat,” Dr. Pett said during her presentation. “We were really trying to be pragmatic and not require an MRI at screening.”
Whatever the case, she said that the failure of this particular treatment just highlights the growing need to look more seriously, and more collaboratively, at fat and liver health in people living with HIV.
“We need to really focus on setting up large cohorts of people living with HIV to look in a rigorous way at weight gain, changes in waist circumference, ectopic fat, capture fatty liver disease index scores, and cardiovascular risk, to acquire some longitudinal data,” she said. “And [we need to] join with our fellow researchers in overweight and obesity medicine and hepatology to make sure that people living with HIV are included in new treatments for NASH, as several large RCTs have excluded [people living with HIV].”
From Dr. Gilleece’s perspective, it also just speaks to how far the field has to go in identifying those with asymptomatic fatty livers from those who will progress to fibrosis and potentially need liver transplants.
“MAVMET shows the difficulty in managing NAFLD,” she said. “It seems quite an innocuous disease, because for the majority of people it’s not going to cause a problem in their lifetime. But the reality is, for some it will, and we don’t really know how to treat it.”
Dr. Gilleece has disclosed no relevant financial relationships. Dr. Pett reported receiving funding for trials from Gilead Sciences and Janssen-Cilag. ViiV Healthcare funded the MAVMET trial.
A version of this article first appeared on Medscape.com.
ACIP recommends universal HBV vaccination for adults under 60, expands recommendations for vaccines against orthopoxviruses and Ebola
The group also voted to expand recommendations for vaccinating people at risk for occupational exposure to Ebola and to recommend Jynneos, a smallpox and monkeypox vaccine, for at-risk populations.
The recommendations were approved Nov. 3.
Previously, ACIP recommended HBV vaccination for unvaccinated adults at increased risk for infection because of sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, HIV infection, and travel to areas with high to intermediate levels of HBV infection. But experts agreed a new strategy was needed, as previously falling rates of HBV have plateaued. “The past decade has illustrated that risk-based screening has got us as far as it can take us,” Mark Weng, MD, a lieutenant commander in the U.S. Public Health Service and lead of the ACIP Hepatitis Vaccine Working Group, said during the meeting.
There are 1.9 million people living with chronic HBV in the United States, with over 20,000 new acute infections every year. Rates are highest among those in their 40s and 50s, Dr. Weng noted.
The group debated whether to apply the universal recommendation to all ages, but in a close vote (eight yes, seven no), ACIP included an age cutoff of 59. The majority argued that adults 60 and older are at lower risk for infection and vaccination efforts targeting younger adults would be more effective. Those 60 and older would continue to follow the risk-based guidelines, but anyone, regardless of age, can receive the vaccine if they wish to be protected, the group added.
The CDC director as well as several professional societies need to approve the recommendation before it becomes public policy.
ACIP also voted to recommend the following:
- Adding updated recommendations to the 2022 immunization schedules for children, adolescents, and adults, including dengue vaccination for children aged 9-16 years in endemic areas and in adults over 65 and those aged 19-64 with certain chronic conditions.
- The use of Jynneos, a smallpox and monkeypox vaccine, as an alternative to ACAM2000 for those at risk for occupational exposure.
- Pre-exposure vaccination of health care personnel involved in the transport and treatment of suspected Ebola patients at special treatment centers, or lab and support staff working with or handling specimens that may contain the Ebola virus.
A version of this article first appeared on Medscape.com.
The group also voted to expand recommendations for vaccinating people at risk for occupational exposure to Ebola and to recommend Jynneos, a smallpox and monkeypox vaccine, for at-risk populations.
The recommendations were approved Nov. 3.
Previously, ACIP recommended HBV vaccination for unvaccinated adults at increased risk for infection because of sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, HIV infection, and travel to areas with high to intermediate levels of HBV infection. But experts agreed a new strategy was needed, as previously falling rates of HBV have plateaued. “The past decade has illustrated that risk-based screening has got us as far as it can take us,” Mark Weng, MD, a lieutenant commander in the U.S. Public Health Service and lead of the ACIP Hepatitis Vaccine Working Group, said during the meeting.
There are 1.9 million people living with chronic HBV in the United States, with over 20,000 new acute infections every year. Rates are highest among those in their 40s and 50s, Dr. Weng noted.
The group debated whether to apply the universal recommendation to all ages, but in a close vote (eight yes, seven no), ACIP included an age cutoff of 59. The majority argued that adults 60 and older are at lower risk for infection and vaccination efforts targeting younger adults would be more effective. Those 60 and older would continue to follow the risk-based guidelines, but anyone, regardless of age, can receive the vaccine if they wish to be protected, the group added.
The CDC director as well as several professional societies need to approve the recommendation before it becomes public policy.
ACIP also voted to recommend the following:
- Adding updated recommendations to the 2022 immunization schedules for children, adolescents, and adults, including dengue vaccination for children aged 9-16 years in endemic areas and in adults over 65 and those aged 19-64 with certain chronic conditions.
- The use of Jynneos, a smallpox and monkeypox vaccine, as an alternative to ACAM2000 for those at risk for occupational exposure.
- Pre-exposure vaccination of health care personnel involved in the transport and treatment of suspected Ebola patients at special treatment centers, or lab and support staff working with or handling specimens that may contain the Ebola virus.
A version of this article first appeared on Medscape.com.
The group also voted to expand recommendations for vaccinating people at risk for occupational exposure to Ebola and to recommend Jynneos, a smallpox and monkeypox vaccine, for at-risk populations.
The recommendations were approved Nov. 3.
Previously, ACIP recommended HBV vaccination for unvaccinated adults at increased risk for infection because of sexual exposure, percutaneous or mucosal exposure to blood, hepatitis C infection, chronic liver disease, end-stage renal disease, HIV infection, and travel to areas with high to intermediate levels of HBV infection. But experts agreed a new strategy was needed, as previously falling rates of HBV have plateaued. “The past decade has illustrated that risk-based screening has got us as far as it can take us,” Mark Weng, MD, a lieutenant commander in the U.S. Public Health Service and lead of the ACIP Hepatitis Vaccine Working Group, said during the meeting.
There are 1.9 million people living with chronic HBV in the United States, with over 20,000 new acute infections every year. Rates are highest among those in their 40s and 50s, Dr. Weng noted.
The group debated whether to apply the universal recommendation to all ages, but in a close vote (eight yes, seven no), ACIP included an age cutoff of 59. The majority argued that adults 60 and older are at lower risk for infection and vaccination efforts targeting younger adults would be more effective. Those 60 and older would continue to follow the risk-based guidelines, but anyone, regardless of age, can receive the vaccine if they wish to be protected, the group added.
The CDC director as well as several professional societies need to approve the recommendation before it becomes public policy.
ACIP also voted to recommend the following:
- Adding updated recommendations to the 2022 immunization schedules for children, adolescents, and adults, including dengue vaccination for children aged 9-16 years in endemic areas and in adults over 65 and those aged 19-64 with certain chronic conditions.
- The use of Jynneos, a smallpox and monkeypox vaccine, as an alternative to ACAM2000 for those at risk for occupational exposure.
- Pre-exposure vaccination of health care personnel involved in the transport and treatment of suspected Ebola patients at special treatment centers, or lab and support staff working with or handling specimens that may contain the Ebola virus.
A version of this article first appeared on Medscape.com.
HIV prescription mandate controversy reaches the Supreme Court
A firestorm of controversy over access to HIV medications and protection against discriminatory insurance practices has been making its way through U.S. district courts for the past 3 years, pitting HIV patients against pharmacy benefits managers and, ostensibly, the healthcare industry itself.
.
At odds are whether or not mandatory mail-order requirements for specialty medications violate specific provisions of the Patient Protection and Affordable Care Act (ACA) and the Rehabilitation Act of 1973, both of which prohibit discrimination by programs that receive federal funds.
An amicus brief submitted on October 29 by the Center for Health Law and Policy Innovation (CHLPI) of Harvard Law School on behalf of five John Does and a number of medical practitioners and practitioner organizations underscores the degree to which advances in HIV treatment, viral suppression, and care linkage — not to mention the national mandate to end the AIDS epidemic by 2030 — might ultimately be affected.
“We decided to file the brief at the Supreme Court level because we wanted to make sure that the perspectives of people living with HIV, their providers, and advocates were in the record,” Maryanne Tomazic, a clinical instructor at CHLPI, toldthis news organization.
“It’s important for the court to consider why robust access to prescription drug coverage and pharmacy services are so important for people living with HIV, and why it’s not appropriate to compromise access to antiretroviral therapy,” she explained.
A bitter pill, regardless of who swallows it
CVS Pharmacy Inc. v. Doe focuses on a legal concept known as “disparate impact discrimination,” which refers to a policy that appears neutral but unintentionally discriminates against a protected class of people (eg, on the basis of sex, age, or ethnicity).
The Supreme Court’s decision in the case will address a central question: did CVS Pharmacy, Caremark, and Caremark Specialty Pharmacy (“CVS”) discriminate against the respondents by requiring that they obtain specialty medications (including those for HIV) by mail order or drop shipment for pickup, or, alternatively, pay out-of-network prices for these medications at non-CVS pharmacies?
The decision will also address whether the ACA’s inclusion of clause 504 of the Rehabilitation Act, which prohibits protected class discrimination, allows patients to challenge terms and conditions of their healthcare plans, a decision that has broad and far-reaching implications for insurers’ abilities to set plan restrictions and pricing.
A spokesperson for CVS declined to comment when contacted by this news organization but provided a link to an April 9, 2021 SCOTUS blog post about the filing. In its court filing, CVS contended that the program applies to all specialty medications (not just HIV) and simply reflects the cost/complexity of specialty medications.
Not everyone agrees that cost is the most important issue at play. Indeed, a critical take-away for practitioners is how mandated mail-order pharmacy programs can disrupt coordination of care.
“In the traditional model, the physician is talking to the pharmacists [or] talking with the patient, and you have kind of triangular communication model that helps not only the patient stay engaged in care but [also] allows the healthcare provider team to adjust the medication quickly without delay,” said Ms. Tomazic.
The John Doe statements in the original case highlight these concerns. They focus on how mandatory mail orders restrict highly personable relationships with local specialty pharmacists who are familiar with their patients’ medical histories as well as their medication dosing and adjustments and who regularly communicate with the complete care team on the patients’ behalf.
“JOHN DOE THREE and others depend on these types of long standing relationships with local pharmacists to maximize the benefits of HIV/AIDS medications and treat the complex and ever-changing needs of the HIV/AIDS patients,” wrote attorneys in the 2018 class action filing.
Other issues raised by the suit involve the following: privacy with respect to medication pickup; specialty care customer representatives’ lack of understanding and knowledge of HIV medications; incomplete prescription fills; late medication deliveries; exposure of medications to the elements; work and employment interruptions; and restrictions on early fills and reorders, which increase the risk for missed doses and potentially serious health problems, including interruptions in viral suppression and resistance.
Discrimination issues also raised
CHLPI’s amicus joins several others in support of the unique needs of persons with HIV, especially in Black and Hispanic/Latino communities, which are disproportionately affected by HIV.
A press release distributed by the National Association for the Advancement of Colored People Legal Defense and Educational Fund (LDF) reinforces the idea that not only are Black people more likely to have a disability other groups, owing to the country’s legacy of racial inequality, but also that they are likely to encounter unique forms of discrimination and specific barriers to full participation in society, further underscoring the need for disparate impact liability to address unfair policies and practices.
“Inequity in access to resources, including healthcare, further amplifies the instance and persistence of disabilities among Black people,” LDF attorneys wrote in the brief.
“We saw with COVID-19 that [mail-order prescription] programs can serve in a supportive role in access to care,” said Ms. Tomazic. “But we don’t want those programs to be mandated, and we don’t want to forget about communities where these kinds of programs are simply not a viable option,” she said.
Oral arguments in the case begin on December 7. A decision is expected some months later.
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
A firestorm of controversy over access to HIV medications and protection against discriminatory insurance practices has been making its way through U.S. district courts for the past 3 years, pitting HIV patients against pharmacy benefits managers and, ostensibly, the healthcare industry itself.
.
At odds are whether or not mandatory mail-order requirements for specialty medications violate specific provisions of the Patient Protection and Affordable Care Act (ACA) and the Rehabilitation Act of 1973, both of which prohibit discrimination by programs that receive federal funds.
An amicus brief submitted on October 29 by the Center for Health Law and Policy Innovation (CHLPI) of Harvard Law School on behalf of five John Does and a number of medical practitioners and practitioner organizations underscores the degree to which advances in HIV treatment, viral suppression, and care linkage — not to mention the national mandate to end the AIDS epidemic by 2030 — might ultimately be affected.
“We decided to file the brief at the Supreme Court level because we wanted to make sure that the perspectives of people living with HIV, their providers, and advocates were in the record,” Maryanne Tomazic, a clinical instructor at CHLPI, toldthis news organization.
“It’s important for the court to consider why robust access to prescription drug coverage and pharmacy services are so important for people living with HIV, and why it’s not appropriate to compromise access to antiretroviral therapy,” she explained.
A bitter pill, regardless of who swallows it
CVS Pharmacy Inc. v. Doe focuses on a legal concept known as “disparate impact discrimination,” which refers to a policy that appears neutral but unintentionally discriminates against a protected class of people (eg, on the basis of sex, age, or ethnicity).
The Supreme Court’s decision in the case will address a central question: did CVS Pharmacy, Caremark, and Caremark Specialty Pharmacy (“CVS”) discriminate against the respondents by requiring that they obtain specialty medications (including those for HIV) by mail order or drop shipment for pickup, or, alternatively, pay out-of-network prices for these medications at non-CVS pharmacies?
The decision will also address whether the ACA’s inclusion of clause 504 of the Rehabilitation Act, which prohibits protected class discrimination, allows patients to challenge terms and conditions of their healthcare plans, a decision that has broad and far-reaching implications for insurers’ abilities to set plan restrictions and pricing.
A spokesperson for CVS declined to comment when contacted by this news organization but provided a link to an April 9, 2021 SCOTUS blog post about the filing. In its court filing, CVS contended that the program applies to all specialty medications (not just HIV) and simply reflects the cost/complexity of specialty medications.
Not everyone agrees that cost is the most important issue at play. Indeed, a critical take-away for practitioners is how mandated mail-order pharmacy programs can disrupt coordination of care.
“In the traditional model, the physician is talking to the pharmacists [or] talking with the patient, and you have kind of triangular communication model that helps not only the patient stay engaged in care but [also] allows the healthcare provider team to adjust the medication quickly without delay,” said Ms. Tomazic.
The John Doe statements in the original case highlight these concerns. They focus on how mandatory mail orders restrict highly personable relationships with local specialty pharmacists who are familiar with their patients’ medical histories as well as their medication dosing and adjustments and who regularly communicate with the complete care team on the patients’ behalf.
“JOHN DOE THREE and others depend on these types of long standing relationships with local pharmacists to maximize the benefits of HIV/AIDS medications and treat the complex and ever-changing needs of the HIV/AIDS patients,” wrote attorneys in the 2018 class action filing.
Other issues raised by the suit involve the following: privacy with respect to medication pickup; specialty care customer representatives’ lack of understanding and knowledge of HIV medications; incomplete prescription fills; late medication deliveries; exposure of medications to the elements; work and employment interruptions; and restrictions on early fills and reorders, which increase the risk for missed doses and potentially serious health problems, including interruptions in viral suppression and resistance.
Discrimination issues also raised
CHLPI’s amicus joins several others in support of the unique needs of persons with HIV, especially in Black and Hispanic/Latino communities, which are disproportionately affected by HIV.
A press release distributed by the National Association for the Advancement of Colored People Legal Defense and Educational Fund (LDF) reinforces the idea that not only are Black people more likely to have a disability other groups, owing to the country’s legacy of racial inequality, but also that they are likely to encounter unique forms of discrimination and specific barriers to full participation in society, further underscoring the need for disparate impact liability to address unfair policies and practices.
“Inequity in access to resources, including healthcare, further amplifies the instance and persistence of disabilities among Black people,” LDF attorneys wrote in the brief.
“We saw with COVID-19 that [mail-order prescription] programs can serve in a supportive role in access to care,” said Ms. Tomazic. “But we don’t want those programs to be mandated, and we don’t want to forget about communities where these kinds of programs are simply not a viable option,” she said.
Oral arguments in the case begin on December 7. A decision is expected some months later.
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
A firestorm of controversy over access to HIV medications and protection against discriminatory insurance practices has been making its way through U.S. district courts for the past 3 years, pitting HIV patients against pharmacy benefits managers and, ostensibly, the healthcare industry itself.
.
At odds are whether or not mandatory mail-order requirements for specialty medications violate specific provisions of the Patient Protection and Affordable Care Act (ACA) and the Rehabilitation Act of 1973, both of which prohibit discrimination by programs that receive federal funds.
An amicus brief submitted on October 29 by the Center for Health Law and Policy Innovation (CHLPI) of Harvard Law School on behalf of five John Does and a number of medical practitioners and practitioner organizations underscores the degree to which advances in HIV treatment, viral suppression, and care linkage — not to mention the national mandate to end the AIDS epidemic by 2030 — might ultimately be affected.
“We decided to file the brief at the Supreme Court level because we wanted to make sure that the perspectives of people living with HIV, their providers, and advocates were in the record,” Maryanne Tomazic, a clinical instructor at CHLPI, toldthis news organization.
“It’s important for the court to consider why robust access to prescription drug coverage and pharmacy services are so important for people living with HIV, and why it’s not appropriate to compromise access to antiretroviral therapy,” she explained.
A bitter pill, regardless of who swallows it
CVS Pharmacy Inc. v. Doe focuses on a legal concept known as “disparate impact discrimination,” which refers to a policy that appears neutral but unintentionally discriminates against a protected class of people (eg, on the basis of sex, age, or ethnicity).
The Supreme Court’s decision in the case will address a central question: did CVS Pharmacy, Caremark, and Caremark Specialty Pharmacy (“CVS”) discriminate against the respondents by requiring that they obtain specialty medications (including those for HIV) by mail order or drop shipment for pickup, or, alternatively, pay out-of-network prices for these medications at non-CVS pharmacies?
The decision will also address whether the ACA’s inclusion of clause 504 of the Rehabilitation Act, which prohibits protected class discrimination, allows patients to challenge terms and conditions of their healthcare plans, a decision that has broad and far-reaching implications for insurers’ abilities to set plan restrictions and pricing.
A spokesperson for CVS declined to comment when contacted by this news organization but provided a link to an April 9, 2021 SCOTUS blog post about the filing. In its court filing, CVS contended that the program applies to all specialty medications (not just HIV) and simply reflects the cost/complexity of specialty medications.
Not everyone agrees that cost is the most important issue at play. Indeed, a critical take-away for practitioners is how mandated mail-order pharmacy programs can disrupt coordination of care.
“In the traditional model, the physician is talking to the pharmacists [or] talking with the patient, and you have kind of triangular communication model that helps not only the patient stay engaged in care but [also] allows the healthcare provider team to adjust the medication quickly without delay,” said Ms. Tomazic.
The John Doe statements in the original case highlight these concerns. They focus on how mandatory mail orders restrict highly personable relationships with local specialty pharmacists who are familiar with their patients’ medical histories as well as their medication dosing and adjustments and who regularly communicate with the complete care team on the patients’ behalf.
“JOHN DOE THREE and others depend on these types of long standing relationships with local pharmacists to maximize the benefits of HIV/AIDS medications and treat the complex and ever-changing needs of the HIV/AIDS patients,” wrote attorneys in the 2018 class action filing.
Other issues raised by the suit involve the following: privacy with respect to medication pickup; specialty care customer representatives’ lack of understanding and knowledge of HIV medications; incomplete prescription fills; late medication deliveries; exposure of medications to the elements; work and employment interruptions; and restrictions on early fills and reorders, which increase the risk for missed doses and potentially serious health problems, including interruptions in viral suppression and resistance.
Discrimination issues also raised
CHLPI’s amicus joins several others in support of the unique needs of persons with HIV, especially in Black and Hispanic/Latino communities, which are disproportionately affected by HIV.
A press release distributed by the National Association for the Advancement of Colored People Legal Defense and Educational Fund (LDF) reinforces the idea that not only are Black people more likely to have a disability other groups, owing to the country’s legacy of racial inequality, but also that they are likely to encounter unique forms of discrimination and specific barriers to full participation in society, further underscoring the need for disparate impact liability to address unfair policies and practices.
“Inequity in access to resources, including healthcare, further amplifies the instance and persistence of disabilities among Black people,” LDF attorneys wrote in the brief.
“We saw with COVID-19 that [mail-order prescription] programs can serve in a supportive role in access to care,” said Ms. Tomazic. “But we don’t want those programs to be mandated, and we don’t want to forget about communities where these kinds of programs are simply not a viable option,” she said.
Oral arguments in the case begin on December 7. A decision is expected some months later.
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.

