User login
Mask-wearing cuts new COVID-19 cases by 53%, study says
Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective tool against the coronavirus.
“Personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of COVID-19,” the study authors wrote.
The research team, which included public health and infectious disease specialists in Australia, China, and the U.K., evaluated 72 studies of COVID-19 precautions during the pandemic. They later looked at eight studies that focused on handwashing, mask wearing, and physical distancing.
Among six studies that looked at mask wearing, the researchers found a 53% reduction in COVID-19 cases. In the broader analysis with additional studies, wearing a mask reduced coronavirus transmission, cases, and deaths.
In one study across 200 countries, mandatory mask wearing resulted in nearly 46% fewer negative outcomes from COVID-19. In another study in the U.S., coronavirus transmission was reduced 29% in states where masks were mandatory.
But the research team couldn’t analyze the impact of the type of face mask used, the frequency of mask wearing, or the overall compliance with wearing face masks.
Among five studies that looked at physical distancing, the researchers found a 25% reduction in the rate of COVID-19. A study in the U.S. showed a 12% decrease in coronavirus transmission, while another study in Iran reported a reduction in COVID-19 mortality.
Handwashing interventions also suggested a substantial reduction of COVID-19 cases up to 53%, the researchers wrote. But in adjusted models, the results weren’t statistically significant due to the small number of studies included.
Other studies found significant decreases related to other public health measures, such as quarantines, broad lockdowns, border closures, school closures, business closures, and travel restrictions. Still, the research team couldn’t analyze the overall effectiveness of these measures due to the different ways the studies were conducted.
The study lines up with other research conducted so far during the pandemic, the research team wrote, which indicates that wearing masks and physical distancing can reduce transmission, cases, and deaths.
That said, more studies are needed, particularly now that vaccinations are available and contagious coronavirus variants have become prevalent.
“Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved,” they wrote.
“It is likely that further control of the COVID-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures,” they concluded.
A version of this article first appeared on WebMD.com.
Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective tool against the coronavirus.
“Personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of COVID-19,” the study authors wrote.
The research team, which included public health and infectious disease specialists in Australia, China, and the U.K., evaluated 72 studies of COVID-19 precautions during the pandemic. They later looked at eight studies that focused on handwashing, mask wearing, and physical distancing.
Among six studies that looked at mask wearing, the researchers found a 53% reduction in COVID-19 cases. In the broader analysis with additional studies, wearing a mask reduced coronavirus transmission, cases, and deaths.
In one study across 200 countries, mandatory mask wearing resulted in nearly 46% fewer negative outcomes from COVID-19. In another study in the U.S., coronavirus transmission was reduced 29% in states where masks were mandatory.
But the research team couldn’t analyze the impact of the type of face mask used, the frequency of mask wearing, or the overall compliance with wearing face masks.
Among five studies that looked at physical distancing, the researchers found a 25% reduction in the rate of COVID-19. A study in the U.S. showed a 12% decrease in coronavirus transmission, while another study in Iran reported a reduction in COVID-19 mortality.
Handwashing interventions also suggested a substantial reduction of COVID-19 cases up to 53%, the researchers wrote. But in adjusted models, the results weren’t statistically significant due to the small number of studies included.
Other studies found significant decreases related to other public health measures, such as quarantines, broad lockdowns, border closures, school closures, business closures, and travel restrictions. Still, the research team couldn’t analyze the overall effectiveness of these measures due to the different ways the studies were conducted.
The study lines up with other research conducted so far during the pandemic, the research team wrote, which indicates that wearing masks and physical distancing can reduce transmission, cases, and deaths.
That said, more studies are needed, particularly now that vaccinations are available and contagious coronavirus variants have become prevalent.
“Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved,” they wrote.
“It is likely that further control of the COVID-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures,” they concluded.
A version of this article first appeared on WebMD.com.
Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective tool against the coronavirus.
“Personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of COVID-19,” the study authors wrote.
The research team, which included public health and infectious disease specialists in Australia, China, and the U.K., evaluated 72 studies of COVID-19 precautions during the pandemic. They later looked at eight studies that focused on handwashing, mask wearing, and physical distancing.
Among six studies that looked at mask wearing, the researchers found a 53% reduction in COVID-19 cases. In the broader analysis with additional studies, wearing a mask reduced coronavirus transmission, cases, and deaths.
In one study across 200 countries, mandatory mask wearing resulted in nearly 46% fewer negative outcomes from COVID-19. In another study in the U.S., coronavirus transmission was reduced 29% in states where masks were mandatory.
But the research team couldn’t analyze the impact of the type of face mask used, the frequency of mask wearing, or the overall compliance with wearing face masks.
Among five studies that looked at physical distancing, the researchers found a 25% reduction in the rate of COVID-19. A study in the U.S. showed a 12% decrease in coronavirus transmission, while another study in Iran reported a reduction in COVID-19 mortality.
Handwashing interventions also suggested a substantial reduction of COVID-19 cases up to 53%, the researchers wrote. But in adjusted models, the results weren’t statistically significant due to the small number of studies included.
Other studies found significant decreases related to other public health measures, such as quarantines, broad lockdowns, border closures, school closures, business closures, and travel restrictions. Still, the research team couldn’t analyze the overall effectiveness of these measures due to the different ways the studies were conducted.
The study lines up with other research conducted so far during the pandemic, the research team wrote, which indicates that wearing masks and physical distancing can reduce transmission, cases, and deaths.
That said, more studies are needed, particularly now that vaccinations are available and contagious coronavirus variants have become prevalent.
“Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved,” they wrote.
“It is likely that further control of the COVID-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures,” they concluded.
A version of this article first appeared on WebMD.com.
FROM THE BMJ
Growing evidence supports repurposing antidepressants to treat COVID-19
Mounting evidence suggests selective serotonin reuptake inhibitors (SSRI) are associated with lower COVID-19 severity.
A large analysis of health records shows patients with COVID-19 taking an SSRI were significantly less likely to die of COVID-19 than a matched control group.
“We can’t tell if the drugs are causing these effects, but the statistical analysis is showing significant association. There’s power in the numbers,” Marina Sirota, PhD, University of California, San Francisco (UCSF), said in a statement.
The study was published online Nov. 15 in JAMA Network Open.
Data-driven approach
, including 3,401 patients who were prescribed SSRIs.
When compared with matched patients with COVID-19 taking SSRIs, patients taking fluoxetine were 28% less likely to die (relative risk, 0.72; 95% CI, 0.54-0.97; adjusted P = .03) and those taking either fluoxetine or fluvoxamine were 26% less likely to die (RR, 0.74; 95% CI, 0.55-0.99; adjusted P = .04) versus those not on these medications.
Patients with COVID-19 taking any kind of SSRI were 8% less likely to die than the matched controls (RR, 0.92; 95% CI, 0.85-0.99; adjusted P = .03).
“We observed a statistically significant reduction in mortality of COVID-19 patients who were already taking SSRIs. This is a demonstration of a data-driven approach for identifying new uses for existing drugs,” Dr. Sirota said in an interview.
“Our study simply shows an association between SSRIs and COVID-19 outcomes and doesn’t investigate the mechanism of action of why the drugs might work. Additional clinical trials need to be carried out before these drugs can be used in patients going forward,” she cautioned.
“There is currently an open-label trial investigating fluoxetine to reduce intubation and death after COVID-19. To our knowledge, there are no phase 3 randomized controlled trials taking place or planned,” study investigator Tomiko Oskotsky, MD, with UCSF, told this news organization.
Urgent need
The current results “confirm and expand on prior findings from observational, preclinical, and clinical studies suggesting that certain SSRI antidepressants, including fluoxetine or fluvoxamine, could be beneficial against COVID-19,” Nicolas Hoertel, MD, PhD, MPH, with Paris University and Corentin-Celton Hospital, France, writes in a linked editorial.
Dr. Hoertel notes that the anti-inflammatory properties of SSRIs may underlie their potential action against COVID-19, and other potential mechanisms may include reduction in platelet aggregation, decreased mast cell degranulation, increased melatonin levels, interference with endolysosomal viral trafficking, and antioxidant activities.
“Because most of the world’s population is currently unvaccinated and the COVID-19 pandemic is still active, effective treatments of COVID-19 – especially those that are easy to use, show good tolerability, can be administered orally, and have widespread availability at low cost to allow their use in resource-poor countries – are urgently needed to reduce COVID-19-related mortality and morbidity,” Dr. Hoertel points out.
“In this context, short-term use of fluoxetine or fluvoxamine, if proven effective, should be considered as a potential means of reaching this goal,” he adds.
The study was supported by the Christopher Hess Research Fund and, in part, by UCSF and the National Institutes of Health. Dr. Sirota has reported serving as a scientific advisor at Aria Pharmaceuticals. Dr. Hoertel has reported being listed as an inventor on a patent application related to methods of treating COVID-19, filed by Assistance Publique-Hopitaux de Paris, and receiving consulting fees and nonfinancial support from Lundbeck.
A version of this article first appeared on Medscape.com.
Mounting evidence suggests selective serotonin reuptake inhibitors (SSRI) are associated with lower COVID-19 severity.
A large analysis of health records shows patients with COVID-19 taking an SSRI were significantly less likely to die of COVID-19 than a matched control group.
“We can’t tell if the drugs are causing these effects, but the statistical analysis is showing significant association. There’s power in the numbers,” Marina Sirota, PhD, University of California, San Francisco (UCSF), said in a statement.
The study was published online Nov. 15 in JAMA Network Open.
Data-driven approach
, including 3,401 patients who were prescribed SSRIs.
When compared with matched patients with COVID-19 taking SSRIs, patients taking fluoxetine were 28% less likely to die (relative risk, 0.72; 95% CI, 0.54-0.97; adjusted P = .03) and those taking either fluoxetine or fluvoxamine were 26% less likely to die (RR, 0.74; 95% CI, 0.55-0.99; adjusted P = .04) versus those not on these medications.
Patients with COVID-19 taking any kind of SSRI were 8% less likely to die than the matched controls (RR, 0.92; 95% CI, 0.85-0.99; adjusted P = .03).
“We observed a statistically significant reduction in mortality of COVID-19 patients who were already taking SSRIs. This is a demonstration of a data-driven approach for identifying new uses for existing drugs,” Dr. Sirota said in an interview.
“Our study simply shows an association between SSRIs and COVID-19 outcomes and doesn’t investigate the mechanism of action of why the drugs might work. Additional clinical trials need to be carried out before these drugs can be used in patients going forward,” she cautioned.
“There is currently an open-label trial investigating fluoxetine to reduce intubation and death after COVID-19. To our knowledge, there are no phase 3 randomized controlled trials taking place or planned,” study investigator Tomiko Oskotsky, MD, with UCSF, told this news organization.
Urgent need
The current results “confirm and expand on prior findings from observational, preclinical, and clinical studies suggesting that certain SSRI antidepressants, including fluoxetine or fluvoxamine, could be beneficial against COVID-19,” Nicolas Hoertel, MD, PhD, MPH, with Paris University and Corentin-Celton Hospital, France, writes in a linked editorial.
Dr. Hoertel notes that the anti-inflammatory properties of SSRIs may underlie their potential action against COVID-19, and other potential mechanisms may include reduction in platelet aggregation, decreased mast cell degranulation, increased melatonin levels, interference with endolysosomal viral trafficking, and antioxidant activities.
“Because most of the world’s population is currently unvaccinated and the COVID-19 pandemic is still active, effective treatments of COVID-19 – especially those that are easy to use, show good tolerability, can be administered orally, and have widespread availability at low cost to allow their use in resource-poor countries – are urgently needed to reduce COVID-19-related mortality and morbidity,” Dr. Hoertel points out.
“In this context, short-term use of fluoxetine or fluvoxamine, if proven effective, should be considered as a potential means of reaching this goal,” he adds.
The study was supported by the Christopher Hess Research Fund and, in part, by UCSF and the National Institutes of Health. Dr. Sirota has reported serving as a scientific advisor at Aria Pharmaceuticals. Dr. Hoertel has reported being listed as an inventor on a patent application related to methods of treating COVID-19, filed by Assistance Publique-Hopitaux de Paris, and receiving consulting fees and nonfinancial support from Lundbeck.
A version of this article first appeared on Medscape.com.
Mounting evidence suggests selective serotonin reuptake inhibitors (SSRI) are associated with lower COVID-19 severity.
A large analysis of health records shows patients with COVID-19 taking an SSRI were significantly less likely to die of COVID-19 than a matched control group.
“We can’t tell if the drugs are causing these effects, but the statistical analysis is showing significant association. There’s power in the numbers,” Marina Sirota, PhD, University of California, San Francisco (UCSF), said in a statement.
The study was published online Nov. 15 in JAMA Network Open.
Data-driven approach
, including 3,401 patients who were prescribed SSRIs.
When compared with matched patients with COVID-19 taking SSRIs, patients taking fluoxetine were 28% less likely to die (relative risk, 0.72; 95% CI, 0.54-0.97; adjusted P = .03) and those taking either fluoxetine or fluvoxamine were 26% less likely to die (RR, 0.74; 95% CI, 0.55-0.99; adjusted P = .04) versus those not on these medications.
Patients with COVID-19 taking any kind of SSRI were 8% less likely to die than the matched controls (RR, 0.92; 95% CI, 0.85-0.99; adjusted P = .03).
“We observed a statistically significant reduction in mortality of COVID-19 patients who were already taking SSRIs. This is a demonstration of a data-driven approach for identifying new uses for existing drugs,” Dr. Sirota said in an interview.
“Our study simply shows an association between SSRIs and COVID-19 outcomes and doesn’t investigate the mechanism of action of why the drugs might work. Additional clinical trials need to be carried out before these drugs can be used in patients going forward,” she cautioned.
“There is currently an open-label trial investigating fluoxetine to reduce intubation and death after COVID-19. To our knowledge, there are no phase 3 randomized controlled trials taking place or planned,” study investigator Tomiko Oskotsky, MD, with UCSF, told this news organization.
Urgent need
The current results “confirm and expand on prior findings from observational, preclinical, and clinical studies suggesting that certain SSRI antidepressants, including fluoxetine or fluvoxamine, could be beneficial against COVID-19,” Nicolas Hoertel, MD, PhD, MPH, with Paris University and Corentin-Celton Hospital, France, writes in a linked editorial.
Dr. Hoertel notes that the anti-inflammatory properties of SSRIs may underlie their potential action against COVID-19, and other potential mechanisms may include reduction in platelet aggregation, decreased mast cell degranulation, increased melatonin levels, interference with endolysosomal viral trafficking, and antioxidant activities.
“Because most of the world’s population is currently unvaccinated and the COVID-19 pandemic is still active, effective treatments of COVID-19 – especially those that are easy to use, show good tolerability, can be administered orally, and have widespread availability at low cost to allow their use in resource-poor countries – are urgently needed to reduce COVID-19-related mortality and morbidity,” Dr. Hoertel points out.
“In this context, short-term use of fluoxetine or fluvoxamine, if proven effective, should be considered as a potential means of reaching this goal,” he adds.
The study was supported by the Christopher Hess Research Fund and, in part, by UCSF and the National Institutes of Health. Dr. Sirota has reported serving as a scientific advisor at Aria Pharmaceuticals. Dr. Hoertel has reported being listed as an inventor on a patent application related to methods of treating COVID-19, filed by Assistance Publique-Hopitaux de Paris, and receiving consulting fees and nonfinancial support from Lundbeck.
A version of this article first appeared on Medscape.com.
Faster testing possible for secondary ICU infections
The SARS-CoV-2 pandemic has given added impetus for metagenomic testing using nanopore sequencing to progress from a research tool to routine clinical application. A study led by researchers from Guy’s and St. Thomas’ NHS Foundation Trust has shown the potential for clinical metagenomics to become a same-day test for identifying secondary infection in ventilated ICU patients. Getting results in hours rather than days would help to ensure rapid treatment with the correct antibiotic, minimize unnecessary prescriptions, and thus reduce the growing menace of antimicrobial resistance.
‘SARS-CoV-2 has put considerable strain on ICUs’
The researchers point out that the setting of an intensive care unit involves frequent staff-patient contact that imparts a risk of secondary or nosocomial infection. In addition, invasive ventilation may introduce organisms into the lungs and lead to ventilator-acquired pneumonia. This carries a high mortality and is responsible for up to 70% of antimicrobial prescribing, with current guidelines requiring empiric antibiotics pending culture results, which typically takes 2-4 days.
Many of these infection problems worsened during SARS-CoV-2. Expanded critical care capacity raised the risk of nosocomial infections, with attendant increased antimicrobial prescriptions and the threat of antimicrobial resistance. In addition, treatment of COVID-19 patients with steroid therapy potentially exacerbates bacterial or fungal infections.
The researchers, from the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London, in collaboration with the Quadram Institute in Norwich, Oxford Nanopore Technologies, and Viapath, the U.K.’s largest independent pathology service provider, noted that the pandemic thus reinforced “a need for rapid comprehensive diagnostics to improve antimicrobial stewardship and help prevent emergence and transmission of multi-drug-resistant organisms.”
“As soon as the pandemic started, our scientists realized there would be a benefit to sequencing genomes of all bacteria and fungi causing infection in COVID-19 patients while on ICU,” said Professor Jonathan Edgeworth, who led the research team.
“Within a few weeks we showed it can diagnose secondary infection, target antibiotic treatment, and detect outbreaks much earlier than current technologies – all from a single sample.”
Proof-of-concept study
The team performed a proof-of-concept study of nanopore metagenomics sequencing – a type of DNA sequencing that allows direct rapid unbiased detection of all organisms present in a clinical sample – on 43 surplus respiratory samples from 34 intubated COVID-19 patients with suspected secondary bacterial or fungal pneumonia. Patients were drawn from seven ICUs at St. Thomas’ Hospital, London over a 9-week period between April 11 and June 15 2020, during the first wave of COVID-19.
Their median age was 52, 70% were male, 47% White, and 44% Black or minority ethnicities. Median length of stay was 32 days and mortality 24%. Samples sent for metagenomic analysis and culture included 10 bronchoalveolar lavages, 6 tracheal aspirates, and 27 non-direct bronchoalveolar lavages.
The study, published in Genome Medicine, showed that an 8-hour metagenomics workflow was 92% sensitive (95% CI, 75% to 99%) and 82% specific (95% CI, 57% to 96%) for bacterial identification, based on culture-positive and culture-negative samples, respectively.
The main Gram-negative bacteria identified were Klebsiella spp. (53%), Citrobacter spp. (15%), and E coli (9%). The main Gram-positive bacteria were S aureus (9%), C striatum (24%) and Enterococcus spp. (12%). In addition, C albicans, other Candida spp. and Aspergillus spp. were cultured from 38%, 15%, and 9% of patients, respectively.
In every case, the initial antibiotics prescribed according to prevailing guideline recommendations would have been modified by metagenomic sequencing demonstrating the presence or absence of β-lactam-resistant genes carried by Enterobacterales.
Next day results of sequencing also detected Aspergillus fumigatus in four samples, with results 100% concordant with quantitative PCR for both the four positive and 39 negative samples. It identified two multi-drug–resistant outbreaks, one involving K pneumoniae ST307 affecting four patients and one a C striatum outbreak involving 14 patients across three ICUs.
Thus, a single sample can provide enough genetic sequence data to compare pathogen genomes with a database and accurately identify patients carrying the same strain, enabling early detection of outbreaks. This is the first time this combined benefit of a single test has been demonstrated, the team say.
Gordon Sanghera, CEO of Oxford Nanopore commented that “rapidly characterizing co-infections for precision prescribing is a vital next step for both COVID-19 patients and respiratory disease in general.”
Dr. Andrew Page of the Quadram Institute said: “We have been working on metagenomics technology for the last 7 years. It is great to see it applied to patient care during the COVID-19 pandemic.”
He said in an interview: “The pandemic has accelerated the transition from using sequencing purely in research labs to using it in the clinic to rapidly provide clinicians with information they can use to improve outcomes for patients.”
Potential to inform antimicrobial prescribing and infection control
“Clinical metagenomic testing provides accurate pathogen detection and antibiotic resistance prediction in a same-day laboratory workflow, with assembled genomes available the next day for genomic surveillance,” the researchers say.
The technology “could fundamentally change the multi-disciplinary team approach to managing ICU infections.” It has the potential to improve initial targeted antimicrobial treatment and infection control decisions, as well as help rapidly detect unsuspected outbreaks of multi-drug–resistant pathogens.
Professor Edgeworth told this news organization that since the study, “secondary bacterial and fungal infections have increased, perhaps due to immunomodulatory treatments or just the length of time patients spend on ICU recovering from COVID-19. This makes rapid diagnosis even more important to ensure patients get more targeted antibiotics earlier, rather than relying on generic guidelines.”
The team “are planning to move respiratory metagenomics into pilot service under our Trust’s quality improvement framework,” he revealed. This will enable them to gather data on patient benefits.
“We also need to see how clinicians use these tests to improve antibiotic treatment, to stop antibiotics when not needed or to identify outbreaks earlier, and then how that translates into tangible benefits for individual patients and the wider NHS.”
He predicts that the technique will revolutionize the approach to prevention and treatment of serious infection in ICUs, and it is now planned to offer it as a clinical service for COVID-19 and influenza patients during the coming winter.
In addition, he said: “It can be equally applied to other samples such as tissue fluids and biopsies, including those removed at operation. It therefore has potential to impact on diagnostics for many clinical services, particularly if the progress is maintained at the current pace.”
This article first appeared on Medscape UK/Univadis.
The SARS-CoV-2 pandemic has given added impetus for metagenomic testing using nanopore sequencing to progress from a research tool to routine clinical application. A study led by researchers from Guy’s and St. Thomas’ NHS Foundation Trust has shown the potential for clinical metagenomics to become a same-day test for identifying secondary infection in ventilated ICU patients. Getting results in hours rather than days would help to ensure rapid treatment with the correct antibiotic, minimize unnecessary prescriptions, and thus reduce the growing menace of antimicrobial resistance.
‘SARS-CoV-2 has put considerable strain on ICUs’
The researchers point out that the setting of an intensive care unit involves frequent staff-patient contact that imparts a risk of secondary or nosocomial infection. In addition, invasive ventilation may introduce organisms into the lungs and lead to ventilator-acquired pneumonia. This carries a high mortality and is responsible for up to 70% of antimicrobial prescribing, with current guidelines requiring empiric antibiotics pending culture results, which typically takes 2-4 days.
Many of these infection problems worsened during SARS-CoV-2. Expanded critical care capacity raised the risk of nosocomial infections, with attendant increased antimicrobial prescriptions and the threat of antimicrobial resistance. In addition, treatment of COVID-19 patients with steroid therapy potentially exacerbates bacterial or fungal infections.
The researchers, from the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London, in collaboration with the Quadram Institute in Norwich, Oxford Nanopore Technologies, and Viapath, the U.K.’s largest independent pathology service provider, noted that the pandemic thus reinforced “a need for rapid comprehensive diagnostics to improve antimicrobial stewardship and help prevent emergence and transmission of multi-drug-resistant organisms.”
“As soon as the pandemic started, our scientists realized there would be a benefit to sequencing genomes of all bacteria and fungi causing infection in COVID-19 patients while on ICU,” said Professor Jonathan Edgeworth, who led the research team.
“Within a few weeks we showed it can diagnose secondary infection, target antibiotic treatment, and detect outbreaks much earlier than current technologies – all from a single sample.”
Proof-of-concept study
The team performed a proof-of-concept study of nanopore metagenomics sequencing – a type of DNA sequencing that allows direct rapid unbiased detection of all organisms present in a clinical sample – on 43 surplus respiratory samples from 34 intubated COVID-19 patients with suspected secondary bacterial or fungal pneumonia. Patients were drawn from seven ICUs at St. Thomas’ Hospital, London over a 9-week period between April 11 and June 15 2020, during the first wave of COVID-19.
Their median age was 52, 70% were male, 47% White, and 44% Black or minority ethnicities. Median length of stay was 32 days and mortality 24%. Samples sent for metagenomic analysis and culture included 10 bronchoalveolar lavages, 6 tracheal aspirates, and 27 non-direct bronchoalveolar lavages.
The study, published in Genome Medicine, showed that an 8-hour metagenomics workflow was 92% sensitive (95% CI, 75% to 99%) and 82% specific (95% CI, 57% to 96%) for bacterial identification, based on culture-positive and culture-negative samples, respectively.
The main Gram-negative bacteria identified were Klebsiella spp. (53%), Citrobacter spp. (15%), and E coli (9%). The main Gram-positive bacteria were S aureus (9%), C striatum (24%) and Enterococcus spp. (12%). In addition, C albicans, other Candida spp. and Aspergillus spp. were cultured from 38%, 15%, and 9% of patients, respectively.
In every case, the initial antibiotics prescribed according to prevailing guideline recommendations would have been modified by metagenomic sequencing demonstrating the presence or absence of β-lactam-resistant genes carried by Enterobacterales.
Next day results of sequencing also detected Aspergillus fumigatus in four samples, with results 100% concordant with quantitative PCR for both the four positive and 39 negative samples. It identified two multi-drug–resistant outbreaks, one involving K pneumoniae ST307 affecting four patients and one a C striatum outbreak involving 14 patients across three ICUs.
Thus, a single sample can provide enough genetic sequence data to compare pathogen genomes with a database and accurately identify patients carrying the same strain, enabling early detection of outbreaks. This is the first time this combined benefit of a single test has been demonstrated, the team say.
Gordon Sanghera, CEO of Oxford Nanopore commented that “rapidly characterizing co-infections for precision prescribing is a vital next step for both COVID-19 patients and respiratory disease in general.”
Dr. Andrew Page of the Quadram Institute said: “We have been working on metagenomics technology for the last 7 years. It is great to see it applied to patient care during the COVID-19 pandemic.”
He said in an interview: “The pandemic has accelerated the transition from using sequencing purely in research labs to using it in the clinic to rapidly provide clinicians with information they can use to improve outcomes for patients.”
Potential to inform antimicrobial prescribing and infection control
“Clinical metagenomic testing provides accurate pathogen detection and antibiotic resistance prediction in a same-day laboratory workflow, with assembled genomes available the next day for genomic surveillance,” the researchers say.
The technology “could fundamentally change the multi-disciplinary team approach to managing ICU infections.” It has the potential to improve initial targeted antimicrobial treatment and infection control decisions, as well as help rapidly detect unsuspected outbreaks of multi-drug–resistant pathogens.
Professor Edgeworth told this news organization that since the study, “secondary bacterial and fungal infections have increased, perhaps due to immunomodulatory treatments or just the length of time patients spend on ICU recovering from COVID-19. This makes rapid diagnosis even more important to ensure patients get more targeted antibiotics earlier, rather than relying on generic guidelines.”
The team “are planning to move respiratory metagenomics into pilot service under our Trust’s quality improvement framework,” he revealed. This will enable them to gather data on patient benefits.
“We also need to see how clinicians use these tests to improve antibiotic treatment, to stop antibiotics when not needed or to identify outbreaks earlier, and then how that translates into tangible benefits for individual patients and the wider NHS.”
He predicts that the technique will revolutionize the approach to prevention and treatment of serious infection in ICUs, and it is now planned to offer it as a clinical service for COVID-19 and influenza patients during the coming winter.
In addition, he said: “It can be equally applied to other samples such as tissue fluids and biopsies, including those removed at operation. It therefore has potential to impact on diagnostics for many clinical services, particularly if the progress is maintained at the current pace.”
This article first appeared on Medscape UK/Univadis.
The SARS-CoV-2 pandemic has given added impetus for metagenomic testing using nanopore sequencing to progress from a research tool to routine clinical application. A study led by researchers from Guy’s and St. Thomas’ NHS Foundation Trust has shown the potential for clinical metagenomics to become a same-day test for identifying secondary infection in ventilated ICU patients. Getting results in hours rather than days would help to ensure rapid treatment with the correct antibiotic, minimize unnecessary prescriptions, and thus reduce the growing menace of antimicrobial resistance.
‘SARS-CoV-2 has put considerable strain on ICUs’
The researchers point out that the setting of an intensive care unit involves frequent staff-patient contact that imparts a risk of secondary or nosocomial infection. In addition, invasive ventilation may introduce organisms into the lungs and lead to ventilator-acquired pneumonia. This carries a high mortality and is responsible for up to 70% of antimicrobial prescribing, with current guidelines requiring empiric antibiotics pending culture results, which typically takes 2-4 days.
Many of these infection problems worsened during SARS-CoV-2. Expanded critical care capacity raised the risk of nosocomial infections, with attendant increased antimicrobial prescriptions and the threat of antimicrobial resistance. In addition, treatment of COVID-19 patients with steroid therapy potentially exacerbates bacterial or fungal infections.
The researchers, from the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London, in collaboration with the Quadram Institute in Norwich, Oxford Nanopore Technologies, and Viapath, the U.K.’s largest independent pathology service provider, noted that the pandemic thus reinforced “a need for rapid comprehensive diagnostics to improve antimicrobial stewardship and help prevent emergence and transmission of multi-drug-resistant organisms.”
“As soon as the pandemic started, our scientists realized there would be a benefit to sequencing genomes of all bacteria and fungi causing infection in COVID-19 patients while on ICU,” said Professor Jonathan Edgeworth, who led the research team.
“Within a few weeks we showed it can diagnose secondary infection, target antibiotic treatment, and detect outbreaks much earlier than current technologies – all from a single sample.”
Proof-of-concept study
The team performed a proof-of-concept study of nanopore metagenomics sequencing – a type of DNA sequencing that allows direct rapid unbiased detection of all organisms present in a clinical sample – on 43 surplus respiratory samples from 34 intubated COVID-19 patients with suspected secondary bacterial or fungal pneumonia. Patients were drawn from seven ICUs at St. Thomas’ Hospital, London over a 9-week period between April 11 and June 15 2020, during the first wave of COVID-19.
Their median age was 52, 70% were male, 47% White, and 44% Black or minority ethnicities. Median length of stay was 32 days and mortality 24%. Samples sent for metagenomic analysis and culture included 10 bronchoalveolar lavages, 6 tracheal aspirates, and 27 non-direct bronchoalveolar lavages.
The study, published in Genome Medicine, showed that an 8-hour metagenomics workflow was 92% sensitive (95% CI, 75% to 99%) and 82% specific (95% CI, 57% to 96%) for bacterial identification, based on culture-positive and culture-negative samples, respectively.
The main Gram-negative bacteria identified were Klebsiella spp. (53%), Citrobacter spp. (15%), and E coli (9%). The main Gram-positive bacteria were S aureus (9%), C striatum (24%) and Enterococcus spp. (12%). In addition, C albicans, other Candida spp. and Aspergillus spp. were cultured from 38%, 15%, and 9% of patients, respectively.
In every case, the initial antibiotics prescribed according to prevailing guideline recommendations would have been modified by metagenomic sequencing demonstrating the presence or absence of β-lactam-resistant genes carried by Enterobacterales.
Next day results of sequencing also detected Aspergillus fumigatus in four samples, with results 100% concordant with quantitative PCR for both the four positive and 39 negative samples. It identified two multi-drug–resistant outbreaks, one involving K pneumoniae ST307 affecting four patients and one a C striatum outbreak involving 14 patients across three ICUs.
Thus, a single sample can provide enough genetic sequence data to compare pathogen genomes with a database and accurately identify patients carrying the same strain, enabling early detection of outbreaks. This is the first time this combined benefit of a single test has been demonstrated, the team say.
Gordon Sanghera, CEO of Oxford Nanopore commented that “rapidly characterizing co-infections for precision prescribing is a vital next step for both COVID-19 patients and respiratory disease in general.”
Dr. Andrew Page of the Quadram Institute said: “We have been working on metagenomics technology for the last 7 years. It is great to see it applied to patient care during the COVID-19 pandemic.”
He said in an interview: “The pandemic has accelerated the transition from using sequencing purely in research labs to using it in the clinic to rapidly provide clinicians with information they can use to improve outcomes for patients.”
Potential to inform antimicrobial prescribing and infection control
“Clinical metagenomic testing provides accurate pathogen detection and antibiotic resistance prediction in a same-day laboratory workflow, with assembled genomes available the next day for genomic surveillance,” the researchers say.
The technology “could fundamentally change the multi-disciplinary team approach to managing ICU infections.” It has the potential to improve initial targeted antimicrobial treatment and infection control decisions, as well as help rapidly detect unsuspected outbreaks of multi-drug–resistant pathogens.
Professor Edgeworth told this news organization that since the study, “secondary bacterial and fungal infections have increased, perhaps due to immunomodulatory treatments or just the length of time patients spend on ICU recovering from COVID-19. This makes rapid diagnosis even more important to ensure patients get more targeted antibiotics earlier, rather than relying on generic guidelines.”
The team “are planning to move respiratory metagenomics into pilot service under our Trust’s quality improvement framework,” he revealed. This will enable them to gather data on patient benefits.
“We also need to see how clinicians use these tests to improve antibiotic treatment, to stop antibiotics when not needed or to identify outbreaks earlier, and then how that translates into tangible benefits for individual patients and the wider NHS.”
He predicts that the technique will revolutionize the approach to prevention and treatment of serious infection in ICUs, and it is now planned to offer it as a clinical service for COVID-19 and influenza patients during the coming winter.
In addition, he said: “It can be equally applied to other samples such as tissue fluids and biopsies, including those removed at operation. It therefore has potential to impact on diagnostics for many clinical services, particularly if the progress is maintained at the current pace.”
This article first appeared on Medscape UK/Univadis.
Should you worry about picking up COVID or other infections from public bathrooms?
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
but some experts disagree with the study’s conclusions. The study was published in Science of the Total Environment.
Sotiris Vardoulakis, PhD, of the Australian National University, Canberra, and colleagues reviewed studies of infections associated with public washrooms.
The researchers used keywords to identify potential articles. After screening study abstracts to ensure that only publicly available washrooms with toilets, sinks, and hand dryers were included, 65 studies remained. The investigators excluded washrooms on public transportation (ships, planes, trains, and buses).
“What most of the studies concluded was that what’s really important is to have good hand hygiene and proper maintenance and ventilation of washrooms,” Dr. Vardoulakis said in an interview. “So if the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
There has been ongoing debate on whether electric hand dryers or paper towels are better. Some studies focused on hygiene. Others focused on the environmental cost of paper towels. One concern is that air dryers might spread germs further.
One study focused on the idea that the air recirculation from electric dryers may spread infective aerosols. Another study determined that the Airblade filters in some electric dryers clean more than 99% of the bacteria. The first study, published in Mayo Clinic Proceedings by Cunrui Huang, MMed, MSPH, and colleagues, concluded that “drying hands thoroughly with single-use, disposable paper towels is the preferred method of hand drying in terms of hand hygiene.” Many people prefer to use paper towels because they can be used as a barrier when opening the washroom door.
Dr. Vardoulakis dismissed the air-versus-paper debate, saying, “If the hand washing and drying is effective in the first place, it’s unlikely that the bathroom air or surfaces will pose an infectious disease transmission risk.”
Although Dr. Vardoulakis’ review did not find that public washrooms pose a risk for infection, other researchers have shown that some settings do pose problems. For example, toilet plumes are thought to have contributed to the 2003 outbreak of severe acute respiratory syndrome at the Amoy Gardens housing complex in Hong Kong and nearby buildings by aerosolization of fecal waste. Also, norovirus has long been shown to be transmitted by aerosolized particles in vomitus or stool.
Rodney E. Rohde, PhD, professor and chair, clinical lab science program, Texas State University, San Marcos, expressed concern about this systematic review in an interview with this news organization. “I believe one of the major limitations is that studies which involved restrooms on planes, hotels, camping (those camp kids are nasty), and other similar public-access restrooms MUST be included in this type of review. I also believe they excluded restrooms from low-income/rural areas. WHAT? Their ultimate conclusions seem to be in line with the most current understanding about hand hygiene (including drying without devices that create strong air currents, which may create widespread emission of microbes).”
In an interview, Emanuel Goldman, PhD, professor of microbiology, biochemistry, and molecular genetics, New Jersey Medical School, Newark, focused on the COVID-specific aspects of the review. “The chances are less than 1 in 10,000 of getting COVID from a fomite, and that’s very conservative,” he said. “I think it’s a lot lower than that. The virus is fragile. It dies very quickly outside of a human host.” He emphasized, “virtually no infectious virus has been found on fomites over the last 2 years. ... A big mistake in a lot of papers is they confuse viral RNA with the virus. It’s not the same. Viral RNA is the genetic material of the virus, but it also is the ghost of the virus after the virus is dead, and that’s what people are finding. They’re finding the ghost of the virus.”
Because “studies show that the transfer from a surface to fingers is in the neighborhood of 10% efficiency” and one’s fingers also kill the virus, “transmission through your fingers is not easy,” Dr. Goldman said. “You’ve got to really work at it to deliberately infect yourself” with COVID from a fomite.
Dr. Rohde’s conclusion about Dr. Vardoulakis’s review? “So, the question may be, have there been enough studies, in general, of these other areas to include in a review? Otherwise, can we really generalize from this study? I don’t think so.”
Dr. Goldman is not worried about COVID transmission in public bathrooms. His summation: “I think indoor dining is more risky than anything else right now.”
The study was funded by Dyson Technology. Dr. Vardoulakis is a member of the Dyson scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM SCIENCE OF THE TOTAL ENVIRONMENT
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
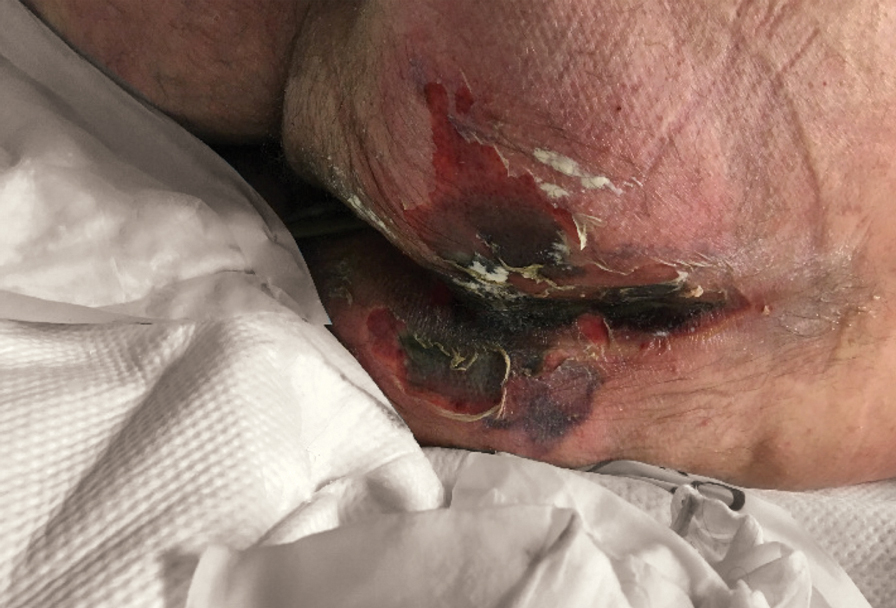
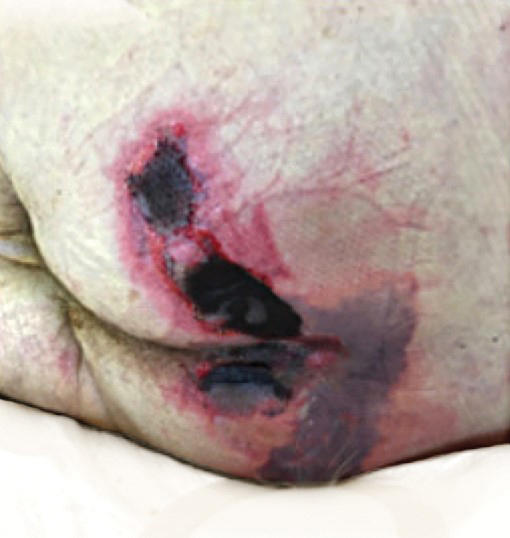
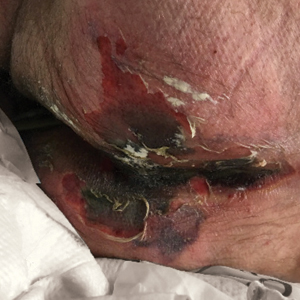
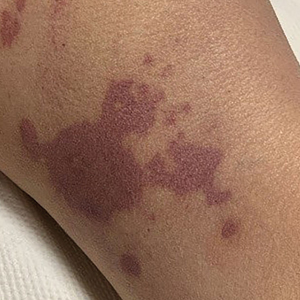
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
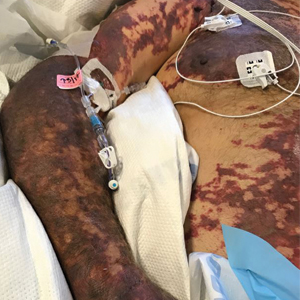
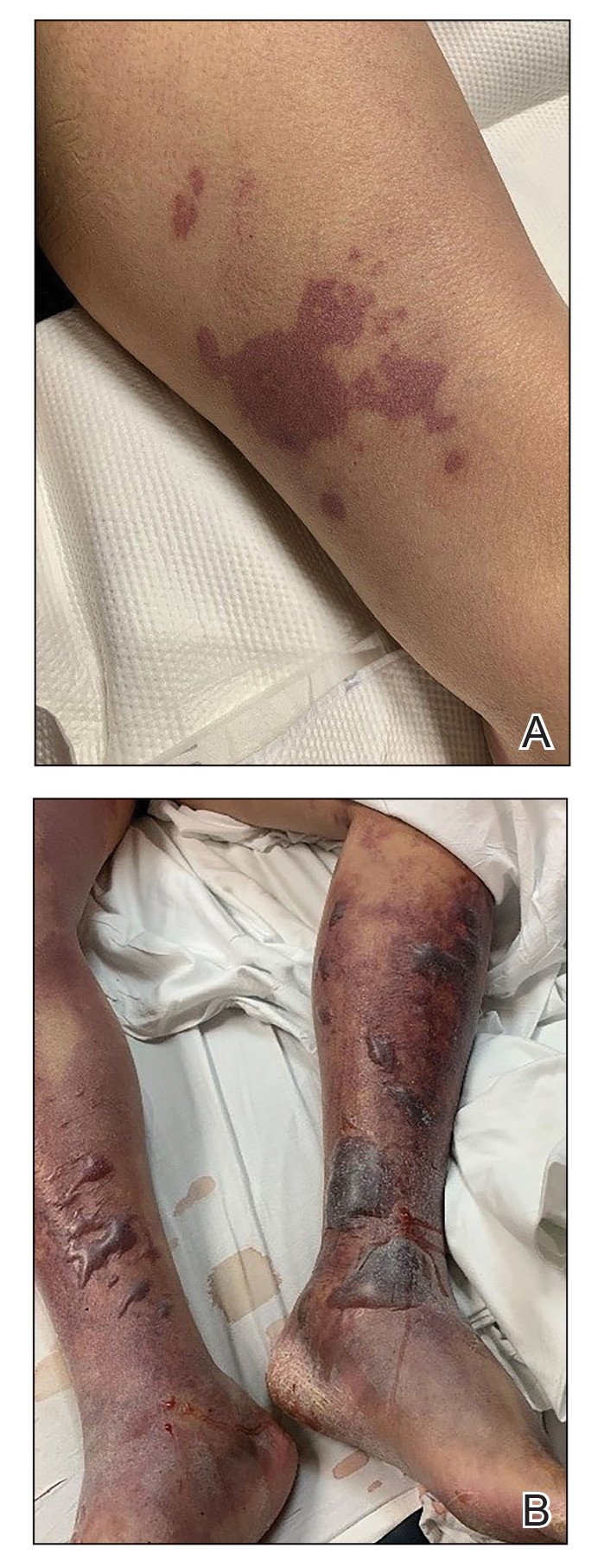
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
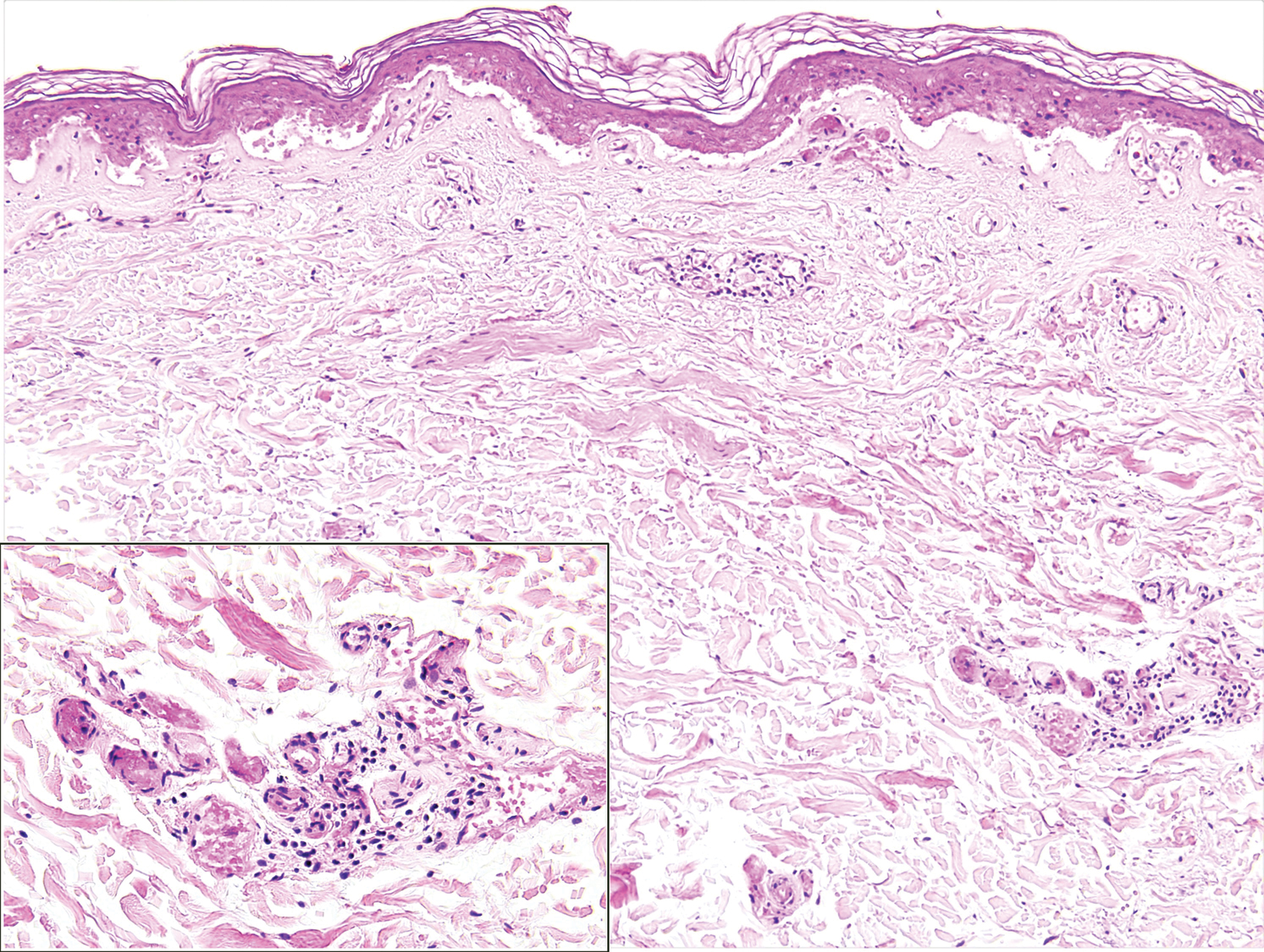
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.
Purpura Fulminans in an Asplenic Intravenous Drug User
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
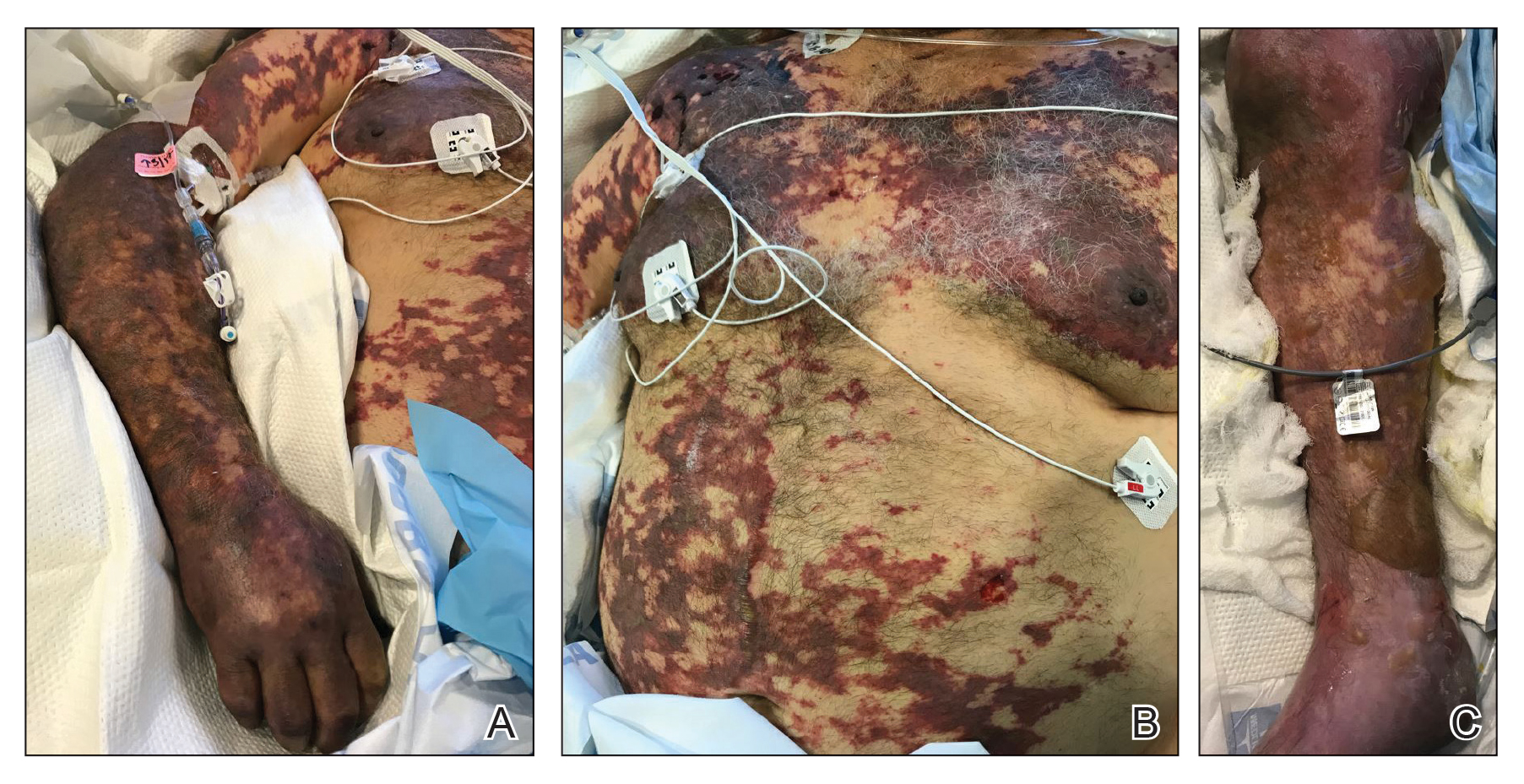
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
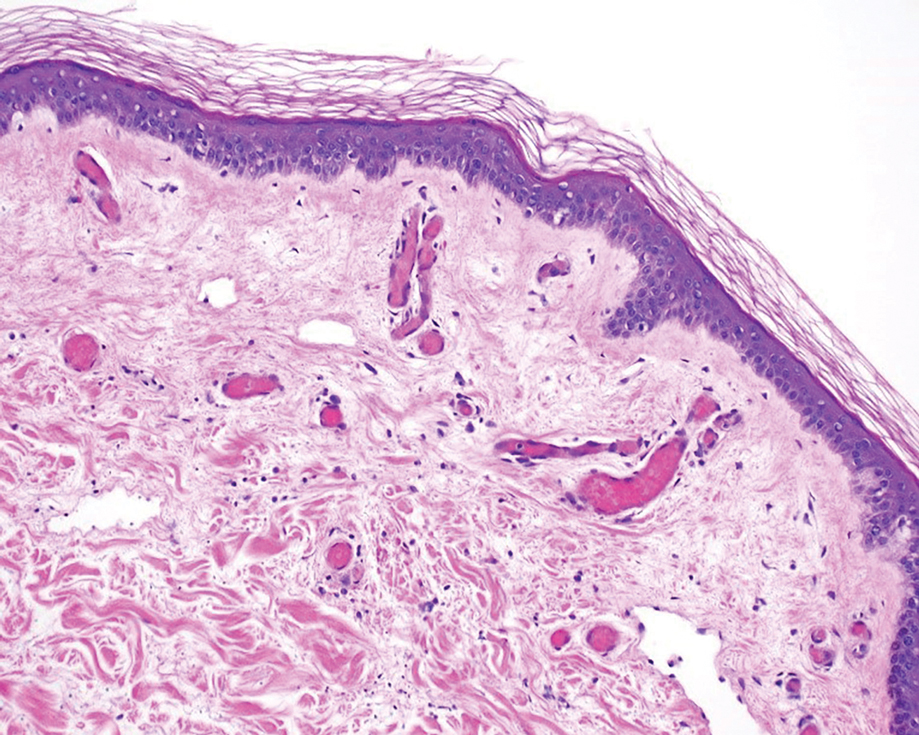
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
Practice Points
- Capnocytophaga species are fastidious, slow-growing microorganisms. It is important, therefore, to maintain a high degree of suspicion and alertthe microbiology laboratory to increase the likelihood of isolation.
- Patients should be cautioned regarding the need for prophylactic antibiotics in the event of an animal bite; asplenic patients are at particular risk for infection.
- In patients with severe purpura fulminans and a gangrenous limb, it is important to allow adequate time for demarcation of gangrene and not rush to amputation.
Purpura Fulminans Induced by Vibrio vulnificus
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.
Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3
Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.
Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3
Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
To the Editor:
Purpura fulminans (PF) is an acute, life-threatening condition characterized by intravascular thrombosis and hemorrhagic necrosis of the skin. It classically presents as retiform purpura with branched or angular purpuric lesions. Purpura fulminans often occurs in the setting of disseminated intravascular coagulation, secondary to sepsis, trauma, malignancy, autoimmune disease, and congenital or acquired protein C or S deficiency, among other abnormalities.1 Rapid identification and treatment of the underlying cause are mainstays of management. We report a case of PF secondary to Vibrio vulnificus infection and highlight the importance of timely consideration of this etiologic agent due to the high mortality rate and specific treatment required.
A 58-year-old man with liver cirrhosis and hepatitis B virus presented with pain, swelling, and localized erythema affecting both legs as well as a fever. He reported vomiting blood and an episode of bloody diarrhea over the preceding 24 hours. He denied exposure to sick contacts or a history of autoimmune disease. At initial presentation to the emergency department, physical examination revealed few scattered, sharply demarcated, erythematous to violaceous patches that rapidly progressed overnight to hemorrhagic bullae and widespread retiform purpuric patches on both legs (Figure 1). As the patient’s skin condition worsened, he had a blood pressure of 80/50 mm Hg and a pulse rate of 110/min. Serum analysis was notable for mild leukocytosis (10.74×109/L [reference range, 4.8–10.8×109/L), thrombocytopenia (39×109/L [reference range, 150–450×109/L]), and decreased C3 (25 mg/dL [reference range, 81–157 mg/dL]) and C4 (8 mg/dL [reference range, 13–39 mg/dL]). Laboratory findings also were remarkable for prothrombin time (23.3 seconds [reference range, 8.8–12.3 seconds]), partial thromboplastin time (52.5 seconds [reference range, 23.6–35.8 seconds]), and international normalized ratio (2.01 [reference range, 0.8–1.13]). Aspartate transaminase (237 U/L [reference range, 11–39 U/L]) and alanine transaminase (80 U/L [reference range, 11–35 U/L]) were elevated, while antineutrophil cytoplasmic antibodies, serum immunoglobulin, and cryoglobulins were unremarkable. Punch biopsies of the left thigh were performed, and histopathology revealed small vessel thrombosis and ischemic changes consistent with PF (Figure 2). Vancomycin, clindamycin, cefepime injection, and piperacillin-tazobactam were administered intravenously for empiric broad-spectrum sepsis coverage. Within hours, the patient experienced refractory septic shock with disseminated intravascular coagulation and died from pulmonary embolism and subsequent cardiac arrest. Tissue and blood cultures grew V vulnificus.
Vibrio vulnificus is a gram-negative bacillus and a rare cause of primary septicemia following consumption of shellfish, especially oysters. Wounds exposed to saltwater or brackish water contaminated with the microorganism can produce soft-tissue infections. Individuals with chronic liver disease are at greater risk for V vulnificus infection.2 The clinical presentation of V vulnificus includes early cellulitislike patches, late purpura with hemorrhagic bullae, and rapidly progressing shock.3
Mortality rates from V vulnificus infection are high.4 Therefore, it is recommended to presumptively diagnose V vulnificus septicemia in any individual at risk for infection who presents with the characteristic history in the setting of hypotension, fever, or septic shock. It is crucial for providers to be aware that broad-spectrum antibiotics commonly used for sepsis are inadequate for the treatment of V vulnificus. Immediate treatment with tetracycline (minocycline or doxycycline) and a third-generation cephalosporin (cefotaxime or ceftriaxone injection) or in combination with ciprofloxacin has been proven effective.4,5
Vibrio vulnificus rarely is described in the literature as inducing PF. In one previously reported case, the patient was otherwise healthy and managed to recover following antibiotic therapy and wound debridement,6 whereas in another case the patient had undiagnosed liver cirrhosis and died from the infection.6,7 In the latter case, the patient presented to the emergency department in a coma. Our patient did not have the clinical signs of sepsis upon initial presentation to the emergency department. It is possible the infection rapidly progressed because of his underlying liver disease. Genotyping analysis of V vulnificus has shown that strains with low pathogenicity can cause primary septicemia in humans.7
Our case reinforces the need to quickly recognize V vulnificus as a rare underlying cause of PF and administer the appropriate treatment.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586-592.
- Tacket CO, Brenner F, Blake PA. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558-561.
- Blake PA, Merson MH, Weaver RE et al. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N Engl J Med. 1979;300:1-5.
- Liu JW, Lee IK, Tang HJ, et al. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123.
- Chen SC, Lee YT, Tsai SJ, et al. Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period.J Antimicrob Chemother. 2012;67:488-493.
- Choi HJ, Lee DK, Lee MW et al. Vibrio vulnificus septicemia presenting as purpura fulminans. J Dermatol. 2005;32:48-51.
- Hori M, Nakayama A, Kitagawa D et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096.
Practice Points
- Purpura fulminans (PF) is a life-threatening condition characterized by intravascular coagulation and skin necrosis.
- Patients with underlying liver disease are at greater risk for PF secondary to Vibrio vulnificus infection.
- Given the high mortality rate, rapid identification of the etiologic agent and timely antibiotic treatment are necessary.
Second woman spontaneously clears HIV: ‘We think more are out there’
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It sounds like a fairy tale steeped in HIV stigma: A woman wakes up one morning and, poof, the HIV she’s been living with for 8 years is gone. But for a 30-year-old Argentinian woman from the aptly named village of Esperanza, that’s close to the truth, according to an article published in Annals of Internal Medicine.
The woman, the so-called Esperanza Patient, appears to be the second person whose immune system cleared the virus without the use of stem cell transplantation. The first was Loreen Willenberg, a California woman who, after living with HIV for 27 years, no longer had replicating HIV in her system. That case was reported last year.
“That’s the beauty of this name, right? Esperanza,” said Xu Yu, MD, principal investigator of the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University, Boston, referring to the Spanish word for “hope.” “This makes us hopeful that a natural cure of HIV is actually possible.”
Two other people appear to have cleared HIV, but only after full replacement of the immune system via stem cell transplantation – the Berlin Patient, Timothy Ray Brown, and the London Patient. Another man, from Brazil, appeared to have an undetectable viral load after receiving intensified antiretroviral treatment plus supplemental vitamin B3.
The rarest of the rare
The Esperanza Patient is among a rare group of people living with HIV called elite controllers. These people’s immune systems can control HIV without antiretrovirals. Most elite controllers’ immune systems, however, can’t mount the immune attack necessary to eliminate all replicating HIV from their systems. Instead, their immune systems control the virus without affecting the reservoirs where HIV continues to make copies of itself and can spread.
The Esperanza Patient and Ms. Willenberg, however, appear to be the rarest of the rare. Their own immune systems seem not only to have stopped HIV replication outside of reservoirs but also to have stormed those reservoirs and killed all virus that might have continued to replicate.
The two women are connected in another way: At an HIV conference in 2019, Dr. Yu was presenting data on Ms. Willenberg’s case. At that conference, she met Natalia Laufer, MD, PhD, associate researcher at the Instituto de Investigaciones Biomédicas en Retrovirs y SIDA at the University of Buenos Aires. Dr. Laufer had been studying the Esperanza Patient at the time and asked Dr. Yu whether she and her team at the Ragon Institute could help her sequence the patient’s HIV genome to see whether, indeed, the virus had been spontaneously cleared from the patient’s system.
So that’s what the pair did, in collaboration with several other researchers into cures for HIV. The Esperanza Patient first acquired HIV in 2013, but in the 8 years that followed, results of 10 conventional viral load tests indicated the virus was undetectable (that is, below the level of quantification for standard technology). During that time, the woman’s boyfriend, from whom she had acquired HIV, died of AIDS-defining illnesses. She subsequently married and had a baby. Both her partner and baby are HIV negative. She only received HIV treatment for 6 months while she was pregnant.
A fossil record of HIV
Yet, there was still HIV in the woman’s system. Dr. Laufer and Dr. Yu wanted to know whether that HIV was transmissible or whether it was a relic from when HIV was still replicating and was now defective and incapable of replicating. They performed extensive genome sequencing on nearly 1.2 billion cells that Dr. Laufer had taken from the patient’s blood in 2017, 2018, 2019, and 2020, an additional 503 million cells that were from the placenta of the baby she gave birth to in 2020, and 150 million resting CD4 T cells. Proviral sequencing was undertaken of the full DNA of the HIV to detect whether the virus was still intact. The DNA was then analyzed by use of an algorithm and was tested for mutations. The investigators tested the patient’s CD4 cells to determine whether the cells still harbored any latent HIV.
In this way, they conducted a full viral workup using tests that are far more sensitive than the viral load tests the woman had undergone in the clinic. The investigators then assessed the patient’s immune system to see what the various cells of the immune system could tell them about how well her natural immune system could identify and kill HIV. They isolated the Esperanza Patient’s immune cells and subjected those cells to HIV in the lab to see whether the cells could detect and eliminate the virus.
And just to be safe, they checked to make sure there were no antiretroviral drugs in the patient’s system.
What they found was that without treatment, her CD4 count hovered around 1,000 cells – a sign of a functioning immune system. DNA sequences revealed large chunks of missing DNA, and one sequence had an immune-induced hypermutation. In total, seven proviruses were found, but none were capable of replicating. The CD4 cells they evaluated showed no evidence of latent HIV.
In other words, they had uncovered a fossil record.
“These HIV-1 DNA products clearly indicate that this person was infected with HIV-1 in the past and that active cycles of viral replication had occurred at one point,” Dr. Yu and colleagues write in their recent article.
What may be more useful to researchers looking to turn this spontaneous cure into treatment for millions of people living with active HIV was the evidence that the woman’s immune system had trained itself to attack HIV through a number of genetic mutations. What they found, the researchers write, was evidence of “an incomplete seroconversion” – that is, when the patient was acquiring HIV, the infection was stopped in its tracks.
Yet, Dr. Yu and colleagues say that they can’t prove that the woman is fully cured of HIV.
“Although this might sound unsatisfying, it reflects an intrinsic limitation of scientific research,” they write. “Scientific concepts can never be proved through empirical data collection; they can only be disproved.”
There are more out there
Are these women the only ones to have spontaneously cleared HIV? That’s the question, said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. Just like they can’t disprove that the women cured themselves, they can’t prove that she and Ms. Willenberg are the only two people to have experienced this cure.
“We’re all struggling with this,” Dr. Dieffenbach told this news organization. “The goal is to get enough of these people so maybe there’s a road map to how to induce, trigger, change immunity. But this could well be a unique event at the time of initiation of infection. We just don’t know.”
What is needed, Dr. Yu said, is for clinicians to reach out to them regarding cases that could mimic the cases of Ms. Willenberg and the Esperanza Patient. Elaborate testing could then be conducted to see whether these cases are similar to those of Ms. Willenberg and the Esperanza Patient.
“We do think there are more out there,” Dr. Yu said in an interview.
Asked whether we’re still far away from applying these one-off cures to the millions of people taking HIV treatment daily, Dr. Yu responded, “We might be close. That’s the beauty of scientific discovery. We don’t know, but that’s why we need more engagement of the community and care providers to help us.”
The research was funded by the Bill and Melinda Gates Foundation and the National Institutes of Health. Dr. Yu and Dr. Dieffenbach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Text-based COVID monitoring system could reduce deaths, relieve ED in winter surge
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
COVID Watch, a text message–based remote monitoring program developed by the University of Pennsylvania Health System, was associated with a 68% reduction in the risk of death, compared with those who received usual care. This was the main finding of a paper published in the Annals of Internal Medicine.
The investigators also determined that patients who enrolled in the program were more likely to seek care in the ED and when they did, they came in on average 2 days sooner than those who received usual care.
“When our clinical team designed COVID Watch the goal was to facilitate hospital care for patients who require it, while supporting access to care for patients who can safely remain at home,” study author M. Kit Delgado, MD, MS, an assistant professor of emergency medicine and epidemiology at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
Researchers had initially hoped COVID Watch would relieve pressure on EDs, Dr. Delgado said.
Significantly lower mortality seen among COVID Watch group
For the study, Dr. Delgado and colleagues enrolled 3,488 patients in COVID Watch and 4,377 in the usual care group to compare outcomes at 30 and 60 days.
“We didn’t include patients who were diagnosed with COVID in the ER or hospital, so this is a lower-risk cohort of patients who test positive in outpatient settings,” Dr. Delgado noted. “Outpatients who received usual care and COVID Watch both had relatively low mortality, but it was significantly lower in those who were in COVID Watch.”
The researchers found that 3 patients in the COVID Watch group died within 30 days of their enrollment, compared with 12 in the control group. At 60 days after enrollment, 5 people within COVID Watch died, compared with 16 not using the system. More than one-third of the deaths in the usual care group occurred outside the hospital, compared with zero deaths among those in COVID Watch.
More than half of program participants were Black or Latino
The messaging system also reduced mortality rates among “all major racial and ethnic subgroups,” the researchers said, with more than 50% of the patients enrolled in COVID Watch having been Black or Latino.
“This is important because Black and Hispanic communities have experienced higher exposure and infection rates, decreased access to care, and have had higher mortality rates,” Dr. Delgado said. “Therefore, the results imply that this type of program could play a role in decreasing disparities in COVID outcomes if scaled more broadly.”
Outside expert: COVID Watch bring new approach to digital health monitoring
The study not only highlights the efficacy and sustainment of the COVID Watch program, but it sheds light on the possibility of using text message monitoring systems on other chronic disease conditions, said Jamie Faro, PhD, who was not involved in the study.
“It brings a new approach to health monitoring using digital means, which may lessen the burden on health care providers and be more cost effective than usual care approaches,” said Dr. Faro, who is assistant professor at the department of population and quantitative health sciences at the University of Massachusetts, Worcester. “Text messaging, which is used by over 80% of Americans, can allow us to reach a large percentage of the population for remote health care monitoring.”
Researchers of the current study said the findings “reveal a model for outpatient health system management of patients with COVID-19 and possibly other conditions where the early detection of clinical declines is critical.” Dr. Faro said that COVID Watch can have a measurable impact on an outcome that is truly life or death. However, it would be critical to understand how to reach those who either “were not offered or refused to take part in the program.”
The authors of the paper and Dr. Faro had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Children and COVID: Youngest vaccinees off to a slower start

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).

Specific figures for children aged 5-11 years are not yet available, but CDC data show that 1.55 million children under the age of 12 years had received at least one dose of COVID-19 vaccine as of Nov. 15, of whom almost 204,000 already had been vaccinated before Nov. 2. For children aged 12-15, the first 2 weeks after approval on May 12 produced almost 2.1 million vaccine initiations, according to the CDC’s COVID Data Tracker.
That dataset reveals several other noteworthy differences between the two age groups in the 10 days after approval:
- There were over 7,000 vaccine initiations on the first day in the 12-15 group; the younger group had 32.
- The older children reached 100,000 per day in 3 days; the younger children took 8 days.
- The older group topped 200,000 vaccinations per day on six different days; the younger group didn’t get above 175,000.
Children under 12 made up 27.5% of vaccine initiations in all age groups during the 2 weeks from Nov. 2 to Nov. 15, versus 3.4% for 12- to 15-year-olds and 1.2% for 16- and 17-year-olds, the CDC said, while also reporting that 3.6% of children under age 12 had received at least one dose of the COVID vaccine, compared with 57.8% of those aged 12-15 and 64.4% of 16- to 17-year-olds.
Meanwhile, the first full week of November marked the second consecutive increase in the number of weekly child COVID cases, with 122,000 reported for Nov. 5-11. The number of new cases has now surpassed 100,000 for 14 consecutive weeks, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report. That report, which covers state health departments, has not included current information from Alabama, Nebraska, and Texas since the summer.
Regionally, the increases over the past 2 weeks were spread out among the East, the Midwest, and the West, while the decline that had been going on for several weeks in the South has largely come to a halt. The states with the highest percent increases over those 2 weeks are all in New England: Maine, New Hampshire, and Vermont, the AAP and CHA noted. In a separate report, the AAP said that Vermont has the second-highest child vaccination rate (81%) in the country, just behind Massachusetts (82%).