User login
Sperm Appear to Have a Nonreproductive Function
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brazilian researchers have identified a previously unrecognized function of sperm that is unrelated to reproduction. A study of 13 patients admitted to the Hospital das Clínicas da Universidade de São Paulo with moderate to severe COVID-19 showed that male gametes released extracellular traps (in a process called ETosis) in response to the infection. This immune response, which is common to macrophages and neutrophils, had never been observed in mammalian reproductive cells.
“It opens up a new line of research,” said Jorge Hallak, a professor at the University of São Paulo School of Medicine, São Paulo, Brazil, and first author of the article published in Andrology. “This may be an innovative mechanism, or it may have always existed, and no one knew.”
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in cells more than 3 months after infection in 11 participants, although polymerase chain reaction tests were negative. These findings suggest the potential for drafting a protocol or guidance on when to attempt a pregnancy. “My concern is with assisted reproduction, in which, in general, only one basic spermogram is done, without diagnostic investigation or serology for coronavirus,” said Hallak.
Symptomatic infections hinder the reproductive process because symptoms such as high fever impair cell function by triggering increased DNA fragmentation, reduced mitochondrial activity, decreased acrosome reaction, and cell death, thus affecting sperm count and gamete mobility.
The new findings indicate that the impact of SARS-CoV-2 infection can continue for as long as 90 days after symptoms and signs disappear and affect sperm count and gamete quality for even longer. “With the sperm selection technique, you are at risk of taking a cell with viruses and injecting it into the egg. It is not known what changes this may cause to the embryo,” said Hallak.
The expert emphasized that the finding contributes to the understanding of reproductive difficulties that previously had no plausible explanation. It serves as a warning against negligence in the evaluation of men in assisted reproductive treatments.
Daniel Zylberstein, urologist and member of the Brazilian Association of Assisted Reproduction, who did not participate in the research, noted that the result comes from a small study that should be expanded to try to develop guidance for doctors.
“There is still no protocol for these cases. The ideal approach would be to wait for complete spermatogenesis, which takes about 3 months, before putting patients on treatment. This often does not happen, and treatment begins shortly after clinical recovery. In the case of moderate to severe COVID-19, this period should be longer than 90 days,” he said.
The study suggests establishing a quarantine period for reproduction until the sperm are free of the virus, said Zylberstein. “With infected sperm, it makes no sense to start reproductive treatment. This sperm is spending energy to fight the pathogen. Assisted reproduction is expensive and exhaustive and may not have the expected outcome because of SARS-CoV-2 infectivity.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM ANDROLOGY
Maternal Immunization to Prevent Serious Respiratory Illness
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.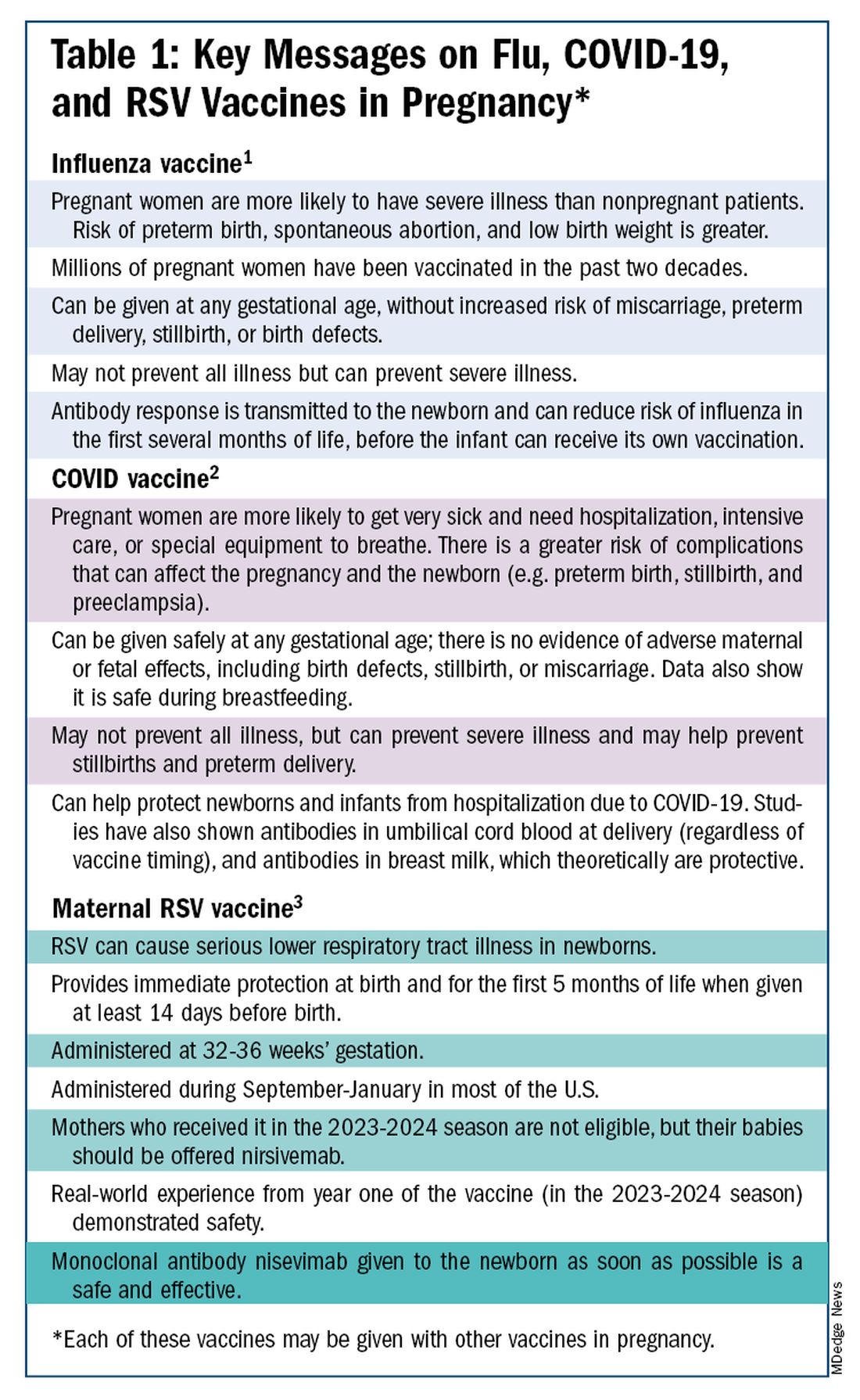
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)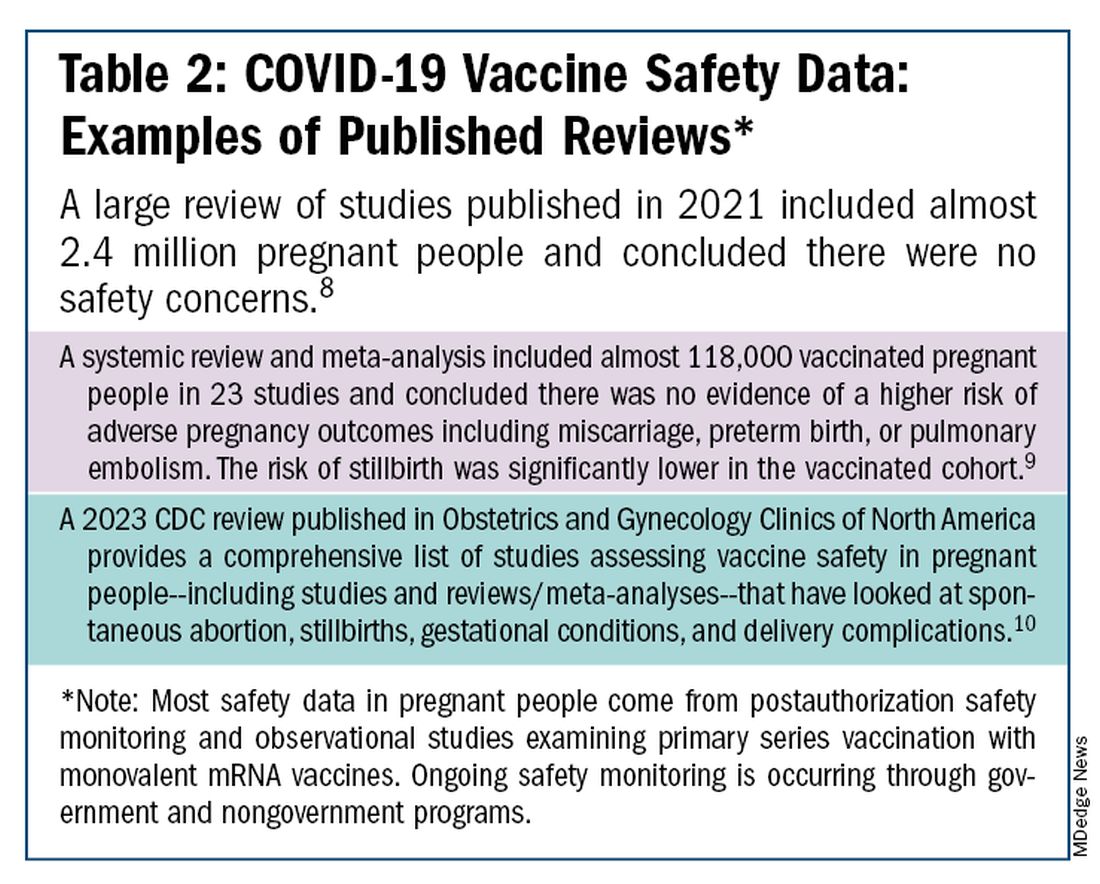
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Vancomycin AUC-Dosing Initiative at a Regional Antibiotic Stewardship Collaborative
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.
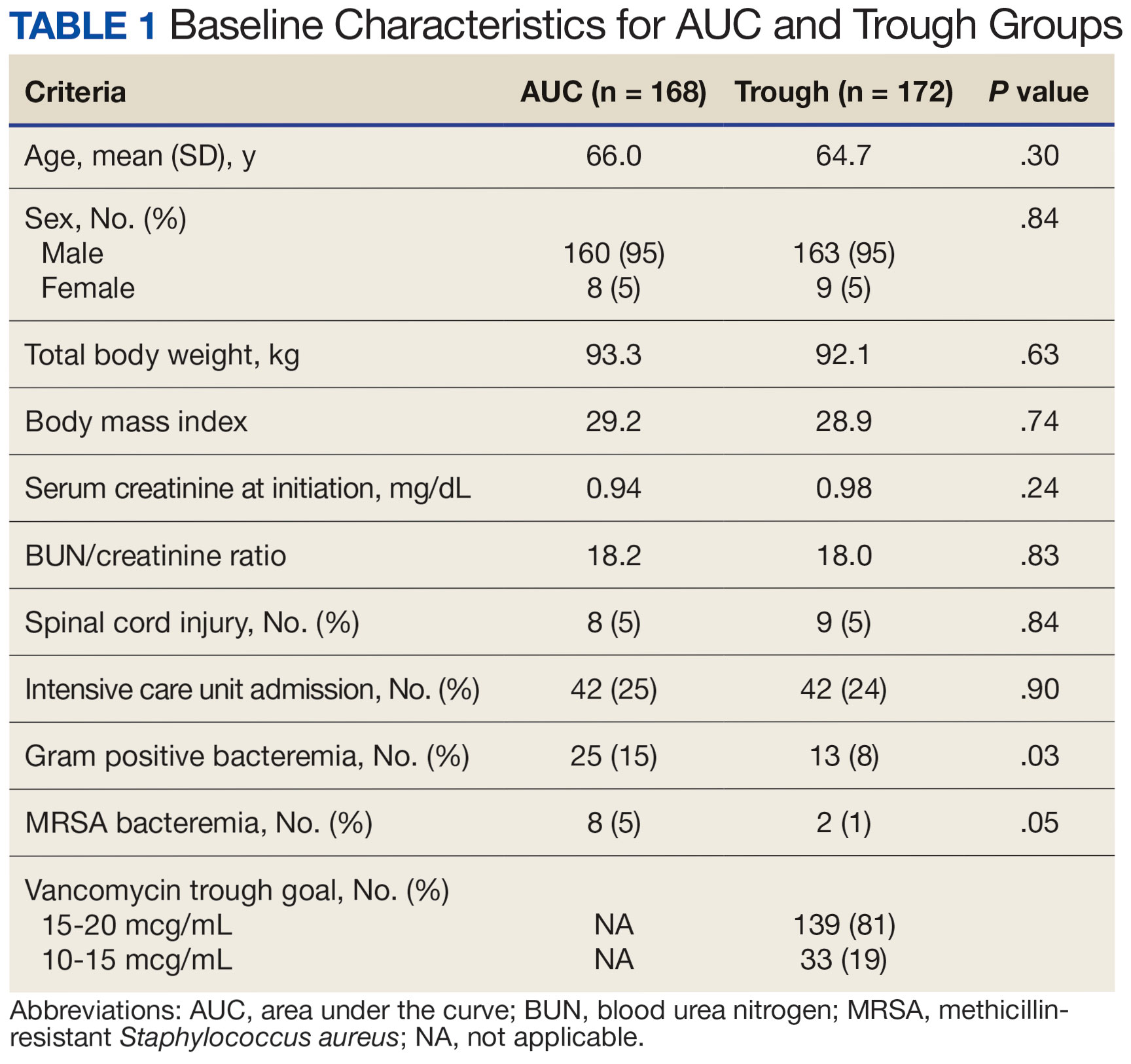

We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.


We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.


We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
Reduced Vaccination Rates Contribute to Rising Pertussis Numbers
New data from the Centers for Disease Control and Prevention (CDC) show significant spikes in pertussis cases compared with last year, especially in several urban areas including New York, Illinois, Florida, and Colorado.
Notably, the current pertussis case count in Illinois as of September 21, 2024, was five times higher than the total cases in 2023 (1058 vs 50). New York City alone had reported 624 cases as of September 21, compared with 38 cases in 2023.
Additional data from the CDC on vaccination coverage and exemptions of school-aged children showed an increase from 3.0% last year to 3.3% in 2024 of children who were exempted from recommended vaccination requirements. Although nearly 93% of kindergarteners in the United States received recommended vaccines (including Tdap), similar to last year, this number shows a steady decline from 94% in the 2021-2021 school year and 93% in the 2021-2022 school year, according to previous CDC reports.
What’s Happening in the Clinic
Clinical experience and the most recent CDC data point to under vaccination as a driver of the increased pertussis cases this year, David J. Cennimo, MD, associate professor of medicine and pediatrics in the division of infectious disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
Although the pertussis vaccination rates in infancy are still very good, clinicians are seeing a drop-off in school-aged children and adults, and the lingering anti-vaccine efforts from the COVID-19 pandemic period are undoubtedly playing a part, said Dr. Cennimo. “Unfortunately, pertussis is contagious, and the vaccine effectiveness wears off. Having decreased numbers of people protected results in more rapid spread,” he said.
Dr. Cennimo agreed that the number of cases in the United States is underreported, and even higher than the data suggest. “I’m sure of it; the initial clinical presentation may be mistaken for a viral upper respiratory tract infection (common cold),” he told this news organization.
Many older children and adults with pertussis do not manifest the classic “whooping cough” seen in infants and young children, so making a clinical diagnosis can be difficult, he said. “One classical component of the illness is a prolonged cough. I have wondered if some people now reporting a lingering cough had pertussis that was missed,” Dr. Cennimo noted.
“Clinicians should stress the value of boosters in a vaccine-preventable illness where we know immunity wanes overtime,” Dr. Cennimo said. “We have a great remedy in the Tdap vaccine, which we should all be getting very 10 years,” he said.
He also emphasized that clinicians remind pregnant women of the current recommendations to receive the Tdap vaccine for every pregnancy. “Vaccination during pregnancy is the best way to protect both the pregnant person and the newborn.
Even for the vaccine hesitant, this vaccine has a long track record of safety so should not be a significant concern,” he said.
The ultimate take-home message is not a new one, and applies to all illnesses, Dr. Cennimo told this news organization. Simply put, “Stay home if you are sick. Social distancing is not just for COVID-19,” he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
New data from the Centers for Disease Control and Prevention (CDC) show significant spikes in pertussis cases compared with last year, especially in several urban areas including New York, Illinois, Florida, and Colorado.
Notably, the current pertussis case count in Illinois as of September 21, 2024, was five times higher than the total cases in 2023 (1058 vs 50). New York City alone had reported 624 cases as of September 21, compared with 38 cases in 2023.
Additional data from the CDC on vaccination coverage and exemptions of school-aged children showed an increase from 3.0% last year to 3.3% in 2024 of children who were exempted from recommended vaccination requirements. Although nearly 93% of kindergarteners in the United States received recommended vaccines (including Tdap), similar to last year, this number shows a steady decline from 94% in the 2021-2021 school year and 93% in the 2021-2022 school year, according to previous CDC reports.
What’s Happening in the Clinic
Clinical experience and the most recent CDC data point to under vaccination as a driver of the increased pertussis cases this year, David J. Cennimo, MD, associate professor of medicine and pediatrics in the division of infectious disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
Although the pertussis vaccination rates in infancy are still very good, clinicians are seeing a drop-off in school-aged children and adults, and the lingering anti-vaccine efforts from the COVID-19 pandemic period are undoubtedly playing a part, said Dr. Cennimo. “Unfortunately, pertussis is contagious, and the vaccine effectiveness wears off. Having decreased numbers of people protected results in more rapid spread,” he said.
Dr. Cennimo agreed that the number of cases in the United States is underreported, and even higher than the data suggest. “I’m sure of it; the initial clinical presentation may be mistaken for a viral upper respiratory tract infection (common cold),” he told this news organization.
Many older children and adults with pertussis do not manifest the classic “whooping cough” seen in infants and young children, so making a clinical diagnosis can be difficult, he said. “One classical component of the illness is a prolonged cough. I have wondered if some people now reporting a lingering cough had pertussis that was missed,” Dr. Cennimo noted.
“Clinicians should stress the value of boosters in a vaccine-preventable illness where we know immunity wanes overtime,” Dr. Cennimo said. “We have a great remedy in the Tdap vaccine, which we should all be getting very 10 years,” he said.
He also emphasized that clinicians remind pregnant women of the current recommendations to receive the Tdap vaccine for every pregnancy. “Vaccination during pregnancy is the best way to protect both the pregnant person and the newborn.
Even for the vaccine hesitant, this vaccine has a long track record of safety so should not be a significant concern,” he said.
The ultimate take-home message is not a new one, and applies to all illnesses, Dr. Cennimo told this news organization. Simply put, “Stay home if you are sick. Social distancing is not just for COVID-19,” he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
New data from the Centers for Disease Control and Prevention (CDC) show significant spikes in pertussis cases compared with last year, especially in several urban areas including New York, Illinois, Florida, and Colorado.
Notably, the current pertussis case count in Illinois as of September 21, 2024, was five times higher than the total cases in 2023 (1058 vs 50). New York City alone had reported 624 cases as of September 21, compared with 38 cases in 2023.
Additional data from the CDC on vaccination coverage and exemptions of school-aged children showed an increase from 3.0% last year to 3.3% in 2024 of children who were exempted from recommended vaccination requirements. Although nearly 93% of kindergarteners in the United States received recommended vaccines (including Tdap), similar to last year, this number shows a steady decline from 94% in the 2021-2021 school year and 93% in the 2021-2022 school year, according to previous CDC reports.
What’s Happening in the Clinic
Clinical experience and the most recent CDC data point to under vaccination as a driver of the increased pertussis cases this year, David J. Cennimo, MD, associate professor of medicine and pediatrics in the division of infectious disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
Although the pertussis vaccination rates in infancy are still very good, clinicians are seeing a drop-off in school-aged children and adults, and the lingering anti-vaccine efforts from the COVID-19 pandemic period are undoubtedly playing a part, said Dr. Cennimo. “Unfortunately, pertussis is contagious, and the vaccine effectiveness wears off. Having decreased numbers of people protected results in more rapid spread,” he said.
Dr. Cennimo agreed that the number of cases in the United States is underreported, and even higher than the data suggest. “I’m sure of it; the initial clinical presentation may be mistaken for a viral upper respiratory tract infection (common cold),” he told this news organization.
Many older children and adults with pertussis do not manifest the classic “whooping cough” seen in infants and young children, so making a clinical diagnosis can be difficult, he said. “One classical component of the illness is a prolonged cough. I have wondered if some people now reporting a lingering cough had pertussis that was missed,” Dr. Cennimo noted.
“Clinicians should stress the value of boosters in a vaccine-preventable illness where we know immunity wanes overtime,” Dr. Cennimo said. “We have a great remedy in the Tdap vaccine, which we should all be getting very 10 years,” he said.
He also emphasized that clinicians remind pregnant women of the current recommendations to receive the Tdap vaccine for every pregnancy. “Vaccination during pregnancy is the best way to protect both the pregnant person and the newborn.
Even for the vaccine hesitant, this vaccine has a long track record of safety so should not be a significant concern,” he said.
The ultimate take-home message is not a new one, and applies to all illnesses, Dr. Cennimo told this news organization. Simply put, “Stay home if you are sick. Social distancing is not just for COVID-19,” he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Disseminated Gonococcal Infection of Pharyngeal Origin: Test All Anatomic Sites
To the Editor:
Gonococcal infections, which are caused by the sexually transmitted, gram-negative diplococcus Neisseria gonorrhoeae, are a current and increasing threat to public health. Between 2012 and 2021, the rate of gonococcal infection in the United States increased 137.8% in men and 64.9% in women,1 with an estimated 1.5 million new gonococcal infections occurring each year in the United States as of 2021.2Neisseria gonorrhoeae is the second most common bacterial sexually transmitted infection (STI), and patients with gonococcal infection frequently are coinfected with Chlamydia trachomatis, which is the most common bacterial STI. Uncomplicated gonococcal infection (also known as gonorrhea) most commonly causes asymptomatic cervicovaginal infection in women and symptomatic urethral infection in men.2 Other uncomplicated manifestations include rectal infection, which can be asymptomatic or manifest with anal pruritus, anal discharge, or tenesmus, and oropharyngeal infection, which can be asymptomatic or manifest with throat pain. If uncomplicated gonococcal infections are left untreated or are incompletely treated, serious complications including septic arthritis, myositis, osteomyelitis, myocarditis, endocarditis, and meningitis might occur.2-5 Ascending, locally invasive infections can cause epididymitis or pelvic inflammatory disease, which is an important cause of infertility in women.2,3 Gonococcal conjunctivitis also can occur, particularly when neonates are exposed to bacteria during vaginal delivery. Although rare, gonococcal bacteria can disseminate widely, with an estimated 0.5% to 3% of uncomplicated gonococcal infections progressing to disseminated gonococcal infection (DGI).3-6 Because DGI can mimic other systemic conditions, including a variety of bacterial and viral infections as well as inflammatory conditions, it can be difficult to diagnose without a high index of clinical suspicion. We present a case of DGI diagnosed based on dermatologic expertise and pharyngeal molecular testing.
A 30-year-old man presented to the emergency department with a rash on the extremeities as well as emesis, fever, sore throat, and severe arthralgia in the wrists, hands, knees, and feet of 2 days’ duration. The patient also had experienced several months of dysuria. He reported daily use of the recreational drug ketamine, multiple new male sexual partners, and unprotected oral and receptive anal sex in recent months. He denied any history of STIs. Physical examination demonstrated tender edematous wrists and fingers, papulovesicles on erythematous bases on the palms, and purpuric macules scattered on the legs (Figure 1). The patient also had tonsillar edema with notable white tonsillar exudate.
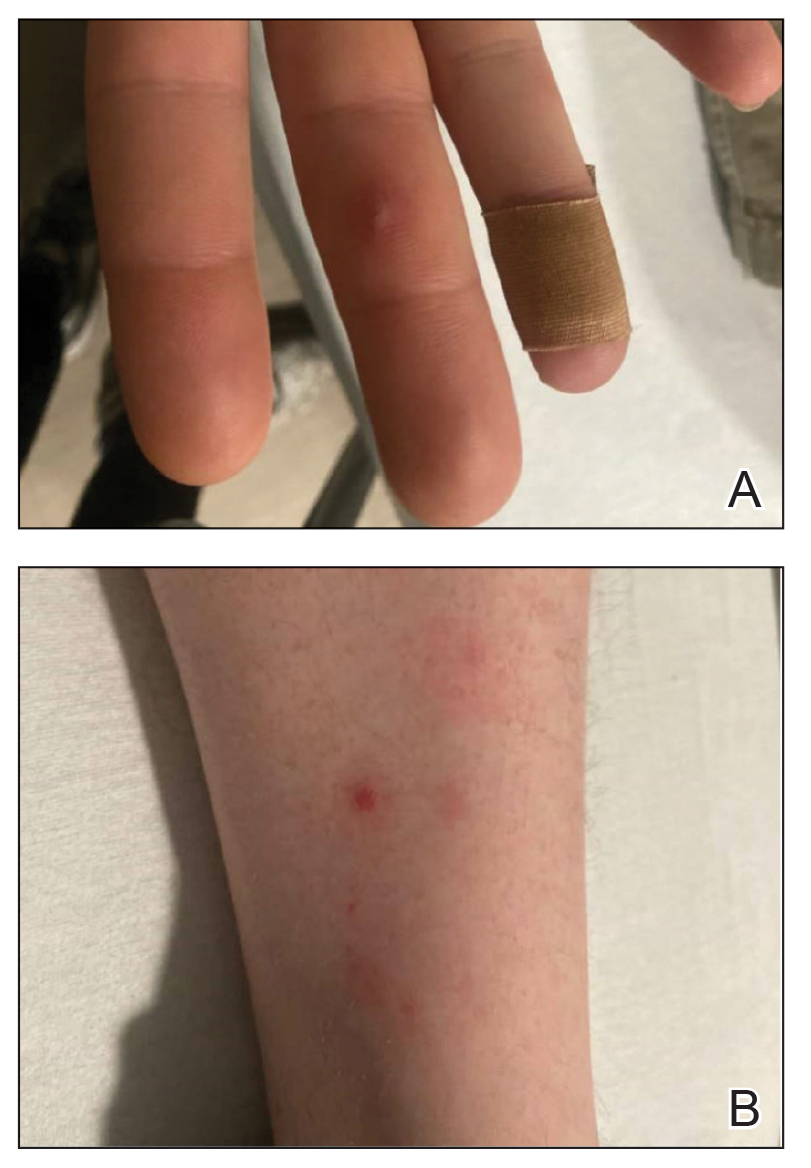
A shave biopsy performed on a papulovesicular lesion on the right thigh showed an intact epidermis with minimal spongiosis and no viral cytopathic changes. There was dermal edema with a moderate superficial and deep neutrophilic infiltrate, mild karyorrhexis, and focal dermal necrosis (Figure 2). Rare acute vasculitis with intravascular fibrin was seen. Periodic acid-Schiff stain for fungi, Gram stain for bacteria, and immunostains for human herpesviruses 1 and 2 were negative.
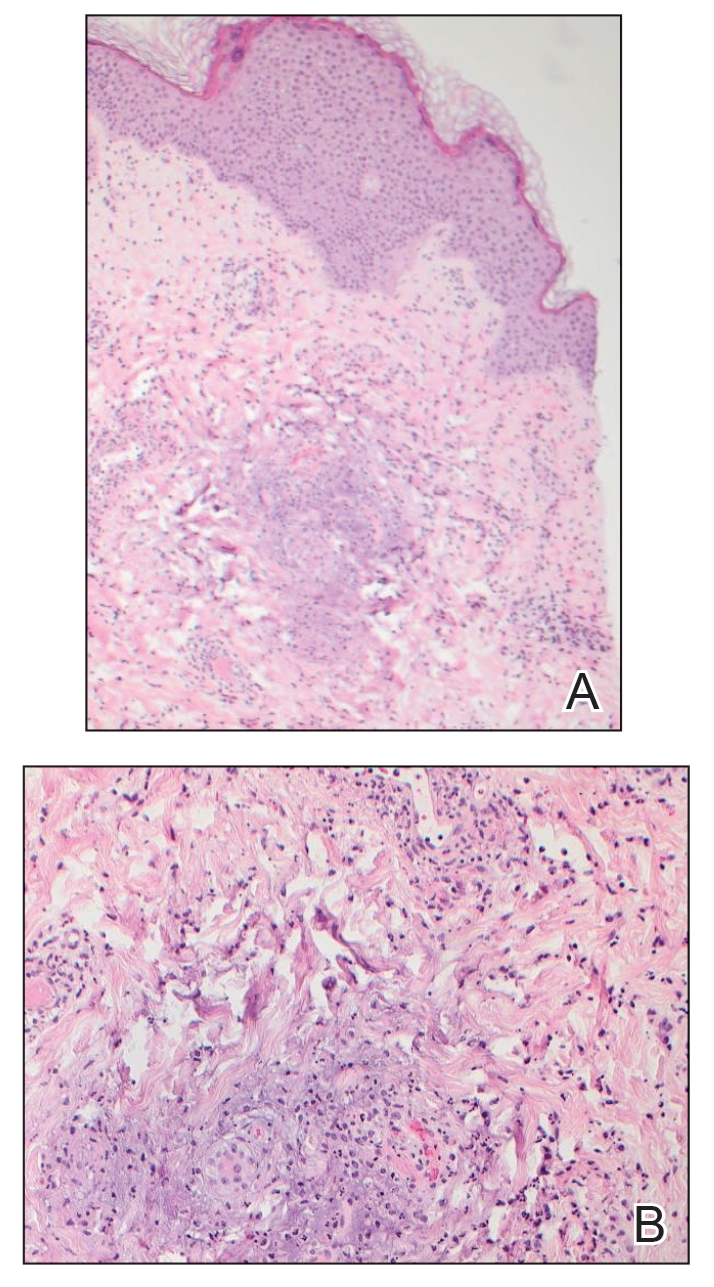
Laboratory studies revealed neutrophil-predominant leukocytosis (white blood cell count, 13.89×109/L [reference range, 4.5–11.0×109/L] with 78.2% neutrophils [reference range, 40.0%–70.0%]) as well as an elevated C-reactive protein level and erythrocyte sedimentation rate (19.98 mg/dL [reference range, <0.05 mg/dL] and 38 mm/h [reference range, 0–15 mm/h], respectively). His liver enzymes, kidney function, prothrombin time, and international normalized ratio were all normal. Urinalysis showed trace amounts of blood and protein, and urine culture was negative for pathogenic bacteria. A rapid plasma reagin test and a fifth-generation HIV antibody test were nonreactive, and bacterial blood cultures were negative for other infectious diseases. Nucleic acid amplification testing (NAAT) performed on a swab from a papulovesicular lesion was negative for human herpesviruses 1 and 2, varicella-zoster virus, orthopoxvirus, and mpox (monkeypox) virus. Based on recommendations from dermatology, NAATs for C trachomatis and N gonorrhoeae were performed on urine and on swabs from the patient’s rectum and pharynx; N gonorrhoeae was detected at the pharynx, but the other sites were negative for both bacteria. A diagnosis of DGI was made based on these results as well as the patient’s clinical presentation of fever, arthralgia, and papulovesicular skin lesions. The patient was treated with 1 g of intravenous ceftriaxone while in the hospital, but unfortunately, he was lost to follow-up and did not complete the full 1-week treatment course.
Disseminated gonococcal infection (also known as arthritis-dermatitis syndrome) is characterized by the abrupt onset of fever, skin lesions, and arthralgia in a symmetric and migratory distribution. Tenosynovitis involving the extensor tendons of the wrists, fingers, knees, and ankles (particularly the Achilles tendon) is characteristic. Skin manifestations usually include hemorrhagic vesicles and papulovesicles limited to the extremities, often with an acral distribution,2-5 though other cutaneous lesions have been described in DGI, including macules, purpura, periurethral abscesses, multifocal cellulitis, and necrotizing fasciitis.7 It is important to consider DGI in a patient who presents with acute systemic symptoms and any of these cutaneous manifestations, even in the absence of joint pain.
Diagnosis of DGI can be difficult, and surveillance is limited in the United States; therefore, the risk factors are somewhat unclear and might be changing. Traditional risk factors for DGI have included immunosuppression due to terminal complement deficiency, female sex, recent menstruation, and pregnancy, but recent data have shown that male sex, HIV infection, use of methamphetamines and other drugs, and use of the monoclonal antibody eculizumab for treatment of complement disorders have been associated with DGI.2,6-8 In the past decade, uncomplicated gonococcal infections have disproportionately affected Black patients, men who have sex with men, adults aged 20 to 25 years, and individuals living in the southern United States.1 It is unclear if the changing demographics of patients with DGI represent true risk factors for dissemination or simply reflect the changing demographics of patients at risk for uncomplicated gonococcal infection.6
Dermatologic expertise in the recognition of cutaneous manifestations of DGI is particularly important due to the limitations of diagnostic tools. The organism is fastidious and difficult to grow in vitro, thus cultures for N gonorrhoeae are not sensitive and require specialized media (eg, Thayer-Martin, modified New York City, or chocolate agar medium with additional antimicrobial agents).3 Molecular assays such as NAATs are more sensitive and specific than culture but are not 100% accurate.2,3,5 Finally, sterile sites such as joints, blood, or cerebrospinal fluid can be difficult to access, and specimens are not always available for specific microbial diagnosis; therefore, even when a gonococcal infection is identified at a mucosal source, physicians must use their clinical judgment to determine whether the mucosal infection is the cause of DGI or if the patient has a separate additional illness.
Once a diagnosis of gonococcal infection is made, any isolated gonococcal bacteria should be tested for antimicrobial susceptibility due to rising rates of drug resistance. Since at least the 1980s, N gonorrhoeae has steadily evolved to have some degree of resistance to most antimicrobials, and epidemiologic evidence indicates that this evolution is continuing.2 Current Centers for Disease Control and Prevention (CDC) recommendations are to treat uncomplicated gonococcal infections with 1 dose of ceftriaxone 500 mg intramuscularly in individuals weighing less than 150 kg (increase to 1 g in those ≥150 kg). Disseminated gonococcal infection requires more aggressive treatment with ceftriaxone 1 g intravenously or intramuscularly every 24 hours for at least 7 days and at a higher dose and for longer duration for patients with endocarditis or meningitis.2 If there is notable clinical improvement after 24 to 48 hours and antimicrobial susceptibility testing confirms an oral agent is appropriate, the patient can be switched to that oral agent to complete treatment. Also, if chlamydia has not been excluded in patients with any type of gonococcal infection, they also should be treated for chlamydia with doxycycline 100 mg twice daily, per CDC guidelines.2 Dermatologists should advocate for patients to be treated for DGI even if the diagnosis is clinical because of the potential for untreated or undertreated patients to progress, to develop additional antimicrobial resistant bacteria, and/or to transmit the infection to others.
This case highlights 2 important points about gonococcal infections and DGI. First, it is important to test and screen patients for gonococcal infection at genitourinary, rectal, and pharyngeal sites. Despite our patient’s report of dysuria, gonococcal infection was only detected via NAAT at the pharynx. As of 2021, CDC guidelines recommend not only testing for gonococcal infection in symptomatic patients at all mucosal sites but also screening all mucosal sites in asymptomatic individuals at high risk.2 Second, dermatologists’ specialized knowledge of cutaneous manifestations provides a valuable tool in the clinical diagnosis of DGI. In this patient, it was the dermatology team’s high index of concern for DGI that led to NAAT testing at all mucosal sites and resulted in an accurate diagnosis. Ultimately, dermatologists play an important role in the diagnosis and management of DGI.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2021. Accessed September 9, 2024. https://www.cdc.gov/std/statistics/2022/2021-STD-Surveillance-Report-PDF_ARCHIVED-2-16-24.pdf
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Skerlev M, Čulav-Košćak I. Gonorrhea: new challenges. Clin Dermatol. 2014;32:275-281. doi:10.1016/j.clindermatol.2013.08.010
- Mehrany K, Kist JM, O’Connor WJ, et al. Disseminated gonococcemia. Int J Dermatol. 2003;42:208-209. doi:10.1046/j.1365-4362.2003.01720.x
- Sciaudone M, Cope A, Mobley V, et al. Ten years of disseminated gonococcal infections in North Carolina: a review of cases from a large tertiary care hospital. Sex Transm Dis. 2023;50:410-414. doi:10.1097/OLQ.0000000000001794
- Weston EJ, Heidenga BL, Farley MM, et al. Surveillance for disseminated gonococcal infections, Active Bacterial Core surveillance (ABCs)—United States, 2015-2019. Clin Infect Dis. 2022;75:953-958. doi:10.1093/cid/ciac052
- Beatrous SV, Grisoli SB, Riahi RR, et al. Cutaneous manifestations of disseminated gonococcemia. Dermatol Online J. 2017;23:13030/qt33b24006
- Nettleton WD, Kent JB, Macomber K, et al. Notes from the field: ongoing cluster of highly related disseminated gonococcal infections—southwest Michigan, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:353-354. doi:10.15585/mmwr.mm6912az
To the Editor:
Gonococcal infections, which are caused by the sexually transmitted, gram-negative diplococcus Neisseria gonorrhoeae, are a current and increasing threat to public health. Between 2012 and 2021, the rate of gonococcal infection in the United States increased 137.8% in men and 64.9% in women,1 with an estimated 1.5 million new gonococcal infections occurring each year in the United States as of 2021.2Neisseria gonorrhoeae is the second most common bacterial sexually transmitted infection (STI), and patients with gonococcal infection frequently are coinfected with Chlamydia trachomatis, which is the most common bacterial STI. Uncomplicated gonococcal infection (also known as gonorrhea) most commonly causes asymptomatic cervicovaginal infection in women and symptomatic urethral infection in men.2 Other uncomplicated manifestations include rectal infection, which can be asymptomatic or manifest with anal pruritus, anal discharge, or tenesmus, and oropharyngeal infection, which can be asymptomatic or manifest with throat pain. If uncomplicated gonococcal infections are left untreated or are incompletely treated, serious complications including septic arthritis, myositis, osteomyelitis, myocarditis, endocarditis, and meningitis might occur.2-5 Ascending, locally invasive infections can cause epididymitis or pelvic inflammatory disease, which is an important cause of infertility in women.2,3 Gonococcal conjunctivitis also can occur, particularly when neonates are exposed to bacteria during vaginal delivery. Although rare, gonococcal bacteria can disseminate widely, with an estimated 0.5% to 3% of uncomplicated gonococcal infections progressing to disseminated gonococcal infection (DGI).3-6 Because DGI can mimic other systemic conditions, including a variety of bacterial and viral infections as well as inflammatory conditions, it can be difficult to diagnose without a high index of clinical suspicion. We present a case of DGI diagnosed based on dermatologic expertise and pharyngeal molecular testing.
A 30-year-old man presented to the emergency department with a rash on the extremeities as well as emesis, fever, sore throat, and severe arthralgia in the wrists, hands, knees, and feet of 2 days’ duration. The patient also had experienced several months of dysuria. He reported daily use of the recreational drug ketamine, multiple new male sexual partners, and unprotected oral and receptive anal sex in recent months. He denied any history of STIs. Physical examination demonstrated tender edematous wrists and fingers, papulovesicles on erythematous bases on the palms, and purpuric macules scattered on the legs (Figure 1). The patient also had tonsillar edema with notable white tonsillar exudate.

A shave biopsy performed on a papulovesicular lesion on the right thigh showed an intact epidermis with minimal spongiosis and no viral cytopathic changes. There was dermal edema with a moderate superficial and deep neutrophilic infiltrate, mild karyorrhexis, and focal dermal necrosis (Figure 2). Rare acute vasculitis with intravascular fibrin was seen. Periodic acid-Schiff stain for fungi, Gram stain for bacteria, and immunostains for human herpesviruses 1 and 2 were negative.

Laboratory studies revealed neutrophil-predominant leukocytosis (white blood cell count, 13.89×109/L [reference range, 4.5–11.0×109/L] with 78.2% neutrophils [reference range, 40.0%–70.0%]) as well as an elevated C-reactive protein level and erythrocyte sedimentation rate (19.98 mg/dL [reference range, <0.05 mg/dL] and 38 mm/h [reference range, 0–15 mm/h], respectively). His liver enzymes, kidney function, prothrombin time, and international normalized ratio were all normal. Urinalysis showed trace amounts of blood and protein, and urine culture was negative for pathogenic bacteria. A rapid plasma reagin test and a fifth-generation HIV antibody test were nonreactive, and bacterial blood cultures were negative for other infectious diseases. Nucleic acid amplification testing (NAAT) performed on a swab from a papulovesicular lesion was negative for human herpesviruses 1 and 2, varicella-zoster virus, orthopoxvirus, and mpox (monkeypox) virus. Based on recommendations from dermatology, NAATs for C trachomatis and N gonorrhoeae were performed on urine and on swabs from the patient’s rectum and pharynx; N gonorrhoeae was detected at the pharynx, but the other sites were negative for both bacteria. A diagnosis of DGI was made based on these results as well as the patient’s clinical presentation of fever, arthralgia, and papulovesicular skin lesions. The patient was treated with 1 g of intravenous ceftriaxone while in the hospital, but unfortunately, he was lost to follow-up and did not complete the full 1-week treatment course.
Disseminated gonococcal infection (also known as arthritis-dermatitis syndrome) is characterized by the abrupt onset of fever, skin lesions, and arthralgia in a symmetric and migratory distribution. Tenosynovitis involving the extensor tendons of the wrists, fingers, knees, and ankles (particularly the Achilles tendon) is characteristic. Skin manifestations usually include hemorrhagic vesicles and papulovesicles limited to the extremities, often with an acral distribution,2-5 though other cutaneous lesions have been described in DGI, including macules, purpura, periurethral abscesses, multifocal cellulitis, and necrotizing fasciitis.7 It is important to consider DGI in a patient who presents with acute systemic symptoms and any of these cutaneous manifestations, even in the absence of joint pain.
Diagnosis of DGI can be difficult, and surveillance is limited in the United States; therefore, the risk factors are somewhat unclear and might be changing. Traditional risk factors for DGI have included immunosuppression due to terminal complement deficiency, female sex, recent menstruation, and pregnancy, but recent data have shown that male sex, HIV infection, use of methamphetamines and other drugs, and use of the monoclonal antibody eculizumab for treatment of complement disorders have been associated with DGI.2,6-8 In the past decade, uncomplicated gonococcal infections have disproportionately affected Black patients, men who have sex with men, adults aged 20 to 25 years, and individuals living in the southern United States.1 It is unclear if the changing demographics of patients with DGI represent true risk factors for dissemination or simply reflect the changing demographics of patients at risk for uncomplicated gonococcal infection.6
Dermatologic expertise in the recognition of cutaneous manifestations of DGI is particularly important due to the limitations of diagnostic tools. The organism is fastidious and difficult to grow in vitro, thus cultures for N gonorrhoeae are not sensitive and require specialized media (eg, Thayer-Martin, modified New York City, or chocolate agar medium with additional antimicrobial agents).3 Molecular assays such as NAATs are more sensitive and specific than culture but are not 100% accurate.2,3,5 Finally, sterile sites such as joints, blood, or cerebrospinal fluid can be difficult to access, and specimens are not always available for specific microbial diagnosis; therefore, even when a gonococcal infection is identified at a mucosal source, physicians must use their clinical judgment to determine whether the mucosal infection is the cause of DGI or if the patient has a separate additional illness.
Once a diagnosis of gonococcal infection is made, any isolated gonococcal bacteria should be tested for antimicrobial susceptibility due to rising rates of drug resistance. Since at least the 1980s, N gonorrhoeae has steadily evolved to have some degree of resistance to most antimicrobials, and epidemiologic evidence indicates that this evolution is continuing.2 Current Centers for Disease Control and Prevention (CDC) recommendations are to treat uncomplicated gonococcal infections with 1 dose of ceftriaxone 500 mg intramuscularly in individuals weighing less than 150 kg (increase to 1 g in those ≥150 kg). Disseminated gonococcal infection requires more aggressive treatment with ceftriaxone 1 g intravenously or intramuscularly every 24 hours for at least 7 days and at a higher dose and for longer duration for patients with endocarditis or meningitis.2 If there is notable clinical improvement after 24 to 48 hours and antimicrobial susceptibility testing confirms an oral agent is appropriate, the patient can be switched to that oral agent to complete treatment. Also, if chlamydia has not been excluded in patients with any type of gonococcal infection, they also should be treated for chlamydia with doxycycline 100 mg twice daily, per CDC guidelines.2 Dermatologists should advocate for patients to be treated for DGI even if the diagnosis is clinical because of the potential for untreated or undertreated patients to progress, to develop additional antimicrobial resistant bacteria, and/or to transmit the infection to others.
This case highlights 2 important points about gonococcal infections and DGI. First, it is important to test and screen patients for gonococcal infection at genitourinary, rectal, and pharyngeal sites. Despite our patient’s report of dysuria, gonococcal infection was only detected via NAAT at the pharynx. As of 2021, CDC guidelines recommend not only testing for gonococcal infection in symptomatic patients at all mucosal sites but also screening all mucosal sites in asymptomatic individuals at high risk.2 Second, dermatologists’ specialized knowledge of cutaneous manifestations provides a valuable tool in the clinical diagnosis of DGI. In this patient, it was the dermatology team’s high index of concern for DGI that led to NAAT testing at all mucosal sites and resulted in an accurate diagnosis. Ultimately, dermatologists play an important role in the diagnosis and management of DGI.
To the Editor:
Gonococcal infections, which are caused by the sexually transmitted, gram-negative diplococcus Neisseria gonorrhoeae, are a current and increasing threat to public health. Between 2012 and 2021, the rate of gonococcal infection in the United States increased 137.8% in men and 64.9% in women,1 with an estimated 1.5 million new gonococcal infections occurring each year in the United States as of 2021.2Neisseria gonorrhoeae is the second most common bacterial sexually transmitted infection (STI), and patients with gonococcal infection frequently are coinfected with Chlamydia trachomatis, which is the most common bacterial STI. Uncomplicated gonococcal infection (also known as gonorrhea) most commonly causes asymptomatic cervicovaginal infection in women and symptomatic urethral infection in men.2 Other uncomplicated manifestations include rectal infection, which can be asymptomatic or manifest with anal pruritus, anal discharge, or tenesmus, and oropharyngeal infection, which can be asymptomatic or manifest with throat pain. If uncomplicated gonococcal infections are left untreated or are incompletely treated, serious complications including septic arthritis, myositis, osteomyelitis, myocarditis, endocarditis, and meningitis might occur.2-5 Ascending, locally invasive infections can cause epididymitis or pelvic inflammatory disease, which is an important cause of infertility in women.2,3 Gonococcal conjunctivitis also can occur, particularly when neonates are exposed to bacteria during vaginal delivery. Although rare, gonococcal bacteria can disseminate widely, with an estimated 0.5% to 3% of uncomplicated gonococcal infections progressing to disseminated gonococcal infection (DGI).3-6 Because DGI can mimic other systemic conditions, including a variety of bacterial and viral infections as well as inflammatory conditions, it can be difficult to diagnose without a high index of clinical suspicion. We present a case of DGI diagnosed based on dermatologic expertise and pharyngeal molecular testing.
A 30-year-old man presented to the emergency department with a rash on the extremeities as well as emesis, fever, sore throat, and severe arthralgia in the wrists, hands, knees, and feet of 2 days’ duration. The patient also had experienced several months of dysuria. He reported daily use of the recreational drug ketamine, multiple new male sexual partners, and unprotected oral and receptive anal sex in recent months. He denied any history of STIs. Physical examination demonstrated tender edematous wrists and fingers, papulovesicles on erythematous bases on the palms, and purpuric macules scattered on the legs (Figure 1). The patient also had tonsillar edema with notable white tonsillar exudate.

A shave biopsy performed on a papulovesicular lesion on the right thigh showed an intact epidermis with minimal spongiosis and no viral cytopathic changes. There was dermal edema with a moderate superficial and deep neutrophilic infiltrate, mild karyorrhexis, and focal dermal necrosis (Figure 2). Rare acute vasculitis with intravascular fibrin was seen. Periodic acid-Schiff stain for fungi, Gram stain for bacteria, and immunostains for human herpesviruses 1 and 2 were negative.

Laboratory studies revealed neutrophil-predominant leukocytosis (white blood cell count, 13.89×109/L [reference range, 4.5–11.0×109/L] with 78.2% neutrophils [reference range, 40.0%–70.0%]) as well as an elevated C-reactive protein level and erythrocyte sedimentation rate (19.98 mg/dL [reference range, <0.05 mg/dL] and 38 mm/h [reference range, 0–15 mm/h], respectively). His liver enzymes, kidney function, prothrombin time, and international normalized ratio were all normal. Urinalysis showed trace amounts of blood and protein, and urine culture was negative for pathogenic bacteria. A rapid plasma reagin test and a fifth-generation HIV antibody test were nonreactive, and bacterial blood cultures were negative for other infectious diseases. Nucleic acid amplification testing (NAAT) performed on a swab from a papulovesicular lesion was negative for human herpesviruses 1 and 2, varicella-zoster virus, orthopoxvirus, and mpox (monkeypox) virus. Based on recommendations from dermatology, NAATs for C trachomatis and N gonorrhoeae were performed on urine and on swabs from the patient’s rectum and pharynx; N gonorrhoeae was detected at the pharynx, but the other sites were negative for both bacteria. A diagnosis of DGI was made based on these results as well as the patient’s clinical presentation of fever, arthralgia, and papulovesicular skin lesions. The patient was treated with 1 g of intravenous ceftriaxone while in the hospital, but unfortunately, he was lost to follow-up and did not complete the full 1-week treatment course.
Disseminated gonococcal infection (also known as arthritis-dermatitis syndrome) is characterized by the abrupt onset of fever, skin lesions, and arthralgia in a symmetric and migratory distribution. Tenosynovitis involving the extensor tendons of the wrists, fingers, knees, and ankles (particularly the Achilles tendon) is characteristic. Skin manifestations usually include hemorrhagic vesicles and papulovesicles limited to the extremities, often with an acral distribution,2-5 though other cutaneous lesions have been described in DGI, including macules, purpura, periurethral abscesses, multifocal cellulitis, and necrotizing fasciitis.7 It is important to consider DGI in a patient who presents with acute systemic symptoms and any of these cutaneous manifestations, even in the absence of joint pain.
Diagnosis of DGI can be difficult, and surveillance is limited in the United States; therefore, the risk factors are somewhat unclear and might be changing. Traditional risk factors for DGI have included immunosuppression due to terminal complement deficiency, female sex, recent menstruation, and pregnancy, but recent data have shown that male sex, HIV infection, use of methamphetamines and other drugs, and use of the monoclonal antibody eculizumab for treatment of complement disorders have been associated with DGI.2,6-8 In the past decade, uncomplicated gonococcal infections have disproportionately affected Black patients, men who have sex with men, adults aged 20 to 25 years, and individuals living in the southern United States.1 It is unclear if the changing demographics of patients with DGI represent true risk factors for dissemination or simply reflect the changing demographics of patients at risk for uncomplicated gonococcal infection.6
Dermatologic expertise in the recognition of cutaneous manifestations of DGI is particularly important due to the limitations of diagnostic tools. The organism is fastidious and difficult to grow in vitro, thus cultures for N gonorrhoeae are not sensitive and require specialized media (eg, Thayer-Martin, modified New York City, or chocolate agar medium with additional antimicrobial agents).3 Molecular assays such as NAATs are more sensitive and specific than culture but are not 100% accurate.2,3,5 Finally, sterile sites such as joints, blood, or cerebrospinal fluid can be difficult to access, and specimens are not always available for specific microbial diagnosis; therefore, even when a gonococcal infection is identified at a mucosal source, physicians must use their clinical judgment to determine whether the mucosal infection is the cause of DGI or if the patient has a separate additional illness.
Once a diagnosis of gonococcal infection is made, any isolated gonococcal bacteria should be tested for antimicrobial susceptibility due to rising rates of drug resistance. Since at least the 1980s, N gonorrhoeae has steadily evolved to have some degree of resistance to most antimicrobials, and epidemiologic evidence indicates that this evolution is continuing.2 Current Centers for Disease Control and Prevention (CDC) recommendations are to treat uncomplicated gonococcal infections with 1 dose of ceftriaxone 500 mg intramuscularly in individuals weighing less than 150 kg (increase to 1 g in those ≥150 kg). Disseminated gonococcal infection requires more aggressive treatment with ceftriaxone 1 g intravenously or intramuscularly every 24 hours for at least 7 days and at a higher dose and for longer duration for patients with endocarditis or meningitis.2 If there is notable clinical improvement after 24 to 48 hours and antimicrobial susceptibility testing confirms an oral agent is appropriate, the patient can be switched to that oral agent to complete treatment. Also, if chlamydia has not been excluded in patients with any type of gonococcal infection, they also should be treated for chlamydia with doxycycline 100 mg twice daily, per CDC guidelines.2 Dermatologists should advocate for patients to be treated for DGI even if the diagnosis is clinical because of the potential for untreated or undertreated patients to progress, to develop additional antimicrobial resistant bacteria, and/or to transmit the infection to others.
This case highlights 2 important points about gonococcal infections and DGI. First, it is important to test and screen patients for gonococcal infection at genitourinary, rectal, and pharyngeal sites. Despite our patient’s report of dysuria, gonococcal infection was only detected via NAAT at the pharynx. As of 2021, CDC guidelines recommend not only testing for gonococcal infection in symptomatic patients at all mucosal sites but also screening all mucosal sites in asymptomatic individuals at high risk.2 Second, dermatologists’ specialized knowledge of cutaneous manifestations provides a valuable tool in the clinical diagnosis of DGI. In this patient, it was the dermatology team’s high index of concern for DGI that led to NAAT testing at all mucosal sites and resulted in an accurate diagnosis. Ultimately, dermatologists play an important role in the diagnosis and management of DGI.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2021. Accessed September 9, 2024. https://www.cdc.gov/std/statistics/2022/2021-STD-Surveillance-Report-PDF_ARCHIVED-2-16-24.pdf
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Skerlev M, Čulav-Košćak I. Gonorrhea: new challenges. Clin Dermatol. 2014;32:275-281. doi:10.1016/j.clindermatol.2013.08.010
- Mehrany K, Kist JM, O’Connor WJ, et al. Disseminated gonococcemia. Int J Dermatol. 2003;42:208-209. doi:10.1046/j.1365-4362.2003.01720.x
- Sciaudone M, Cope A, Mobley V, et al. Ten years of disseminated gonococcal infections in North Carolina: a review of cases from a large tertiary care hospital. Sex Transm Dis. 2023;50:410-414. doi:10.1097/OLQ.0000000000001794
- Weston EJ, Heidenga BL, Farley MM, et al. Surveillance for disseminated gonococcal infections, Active Bacterial Core surveillance (ABCs)—United States, 2015-2019. Clin Infect Dis. 2022;75:953-958. doi:10.1093/cid/ciac052
- Beatrous SV, Grisoli SB, Riahi RR, et al. Cutaneous manifestations of disseminated gonococcemia. Dermatol Online J. 2017;23:13030/qt33b24006
- Nettleton WD, Kent JB, Macomber K, et al. Notes from the field: ongoing cluster of highly related disseminated gonococcal infections—southwest Michigan, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:353-354. doi:10.15585/mmwr.mm6912az
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2021. Accessed September 9, 2024. https://www.cdc.gov/std/statistics/2022/2021-STD-Surveillance-Report-PDF_ARCHIVED-2-16-24.pdf
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Skerlev M, Čulav-Košćak I. Gonorrhea: new challenges. Clin Dermatol. 2014;32:275-281. doi:10.1016/j.clindermatol.2013.08.010
- Mehrany K, Kist JM, O’Connor WJ, et al. Disseminated gonococcemia. Int J Dermatol. 2003;42:208-209. doi:10.1046/j.1365-4362.2003.01720.x
- Sciaudone M, Cope A, Mobley V, et al. Ten years of disseminated gonococcal infections in North Carolina: a review of cases from a large tertiary care hospital. Sex Transm Dis. 2023;50:410-414. doi:10.1097/OLQ.0000000000001794
- Weston EJ, Heidenga BL, Farley MM, et al. Surveillance for disseminated gonococcal infections, Active Bacterial Core surveillance (ABCs)—United States, 2015-2019. Clin Infect Dis. 2022;75:953-958. doi:10.1093/cid/ciac052
- Beatrous SV, Grisoli SB, Riahi RR, et al. Cutaneous manifestations of disseminated gonococcemia. Dermatol Online J. 2017;23:13030/qt33b24006
- Nettleton WD, Kent JB, Macomber K, et al. Notes from the field: ongoing cluster of highly related disseminated gonococcal infections—southwest Michigan, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:353-354. doi:10.15585/mmwr.mm6912az
Practice Points
- Neisseria gonorrhoeae infections of the genitourinary system, rectum, and pharynx can disseminate and cause fever, joint pain, and hemorrhagic papulovesicles that can mimic other serious conditions and require dermatologic expertise to confirm.
- Patients with suspected disseminated gonococcal infection (DGI) as well as patients who are asymptomatic and at increased risk should have all possible anatomic sites of infection—the genitourinary system, rectum, and pharynx—tested with the appropriate molecular assays and culture when appropriate.
- Appropriate recognition and treatment of DGI is vital, as undertreatment can result in serious complications and contribute to the increasing global public health threat of antimicrobial-resistant gonococcal infections.
Public Health, Not Politics, Should Drive Mask Policies, Says Ethicist
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Six Tips on Coronavirus Testing for Doctors and Patients
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
COVID Levels Start to Dip, New Variant Emerges
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
How Experts Predicts This COVID and Flu Season Will Unfold
What’s the outlook for COVID-19 and flu this fall and winter? It’ll probably be a lot like last year, experts say.
“We currently expect this flu season to be comparable to last year’s season,” said Adrienne Keen, PhD, of the Centers for Disease Control and Prevention’s (CDC) Center for Forecasting and Outbreak Analytics. “We expect this year’s COVID-19 season peak to be similar to last year’s or lower.” The CDC is still analyzing COVID surveillance data from the summer and will update the forecast as more is learned.
For COVID, that means it won’t be as bad as the pandemic years, and for the flu, it’s a typical pre-pandemic season. But status quo does not mean great.
Between October 2023 and April 2024, as many as 75 million people got the flu in the United States, according to CDC estimates, resulting in up to 900,000 hospitalizations and between 17,000 and 100,000 deaths. In 2023, about 900,000 Americans were hospitalized with COVID and 75,000 died.
Getting vaccinated remains crucial, public health officials stressed.
Predicting COVID
Two key predictors of how bad an upcoming COVID season will be are the cycling of new variants and the population’s immunity (protection from an infectious disease that happens when a population is immune through vaccination or previous infection).
When new variants go up and immunity goes down, “we tend to see the increase in cases,” said Michael T. Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy and a professor of public health at the University of Minnesota, Minneapolis. But if the number of variants goes down and immunity levels go up, the outlook is more favorable.
The new COVID variant called XEC has been found in at least 25 states. On September 27, the CDC added the variant to the COVID tracker. It now accounts for 6% of US cases. This was expected, as the variant has been circulating in Europe, said Amesh Adalja, MD, a senior scholar and infectious disease expert at the Center for Health Security at Johns Hopkins University, Baltimore, Maryland.
“There will always be a new variant appearing, and one falling,” he said. “So the fact that this is happening is not surprising.”
Meanwhile, the summer COVID surge has provided postinfection immunity for some people. “What’s likely is, we are going to see substantial protection of the population for several months based on previous infection and in some cases vaccination,” Dr. Osterholm said. That means protection from serious illness, hospitalizations, and deaths (but not necessarily infection). That protection could last through the year or into early 2025.
The timing of 2024’s winter surge will likely be a bit later than 2023’s, said Andrew Pekosz, PhD, a professor and vice chair of molecular microbiology and immunology at Johns Hopkins University, Baltimore, “peaking just after the Christmas/New Year holiday.”
During the 2023-2024 season, weekly COVID hospitalizations peaked the week of Dec. 30, said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill and a member of the COVID-19 Scenario Modeling Hub.
But variants are unpredictable. “There’s a chance that the XEC variant may take off and spread, or might not,” said Dr. Adalja. As of September 28, the Omicron variant KP.3.1.1 was leading, accounting for 58.7% of US cases, according to the CDC.
While Dr. Adalja agreed that 2024’s COVID season will probably be like 2023’s, “we have to be prepared for cases and hospitalizations going up,” he said, “but not to the point of a crisis.” A return to lockdowns and social distancing is unlikely.
Still, older adults and others at higher risk of getting very sick from COVID should consider masking during travel, said Rajendram Rajnarayanan, PhD, MSc, an associate professor at the New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Jonesboro.
Flu Forecasts
Predicting flu season this early is hard, said Jeffrey Shaman, PhD, a professor of environmental health sciences and professor of climate at Colombia University, New York.
“You can look at the CDC forecast and use it as a very loose guide right now,” said Dr. Shaman, who won the CDC’s first “Predict the Influenza Season Challenge” in 2014. “Until there is actually flu, it’s like trying to predict the landfall of a hurricane.” Flu activity remained low as of September 14 (the most current data available), according to the CDC.
When flu activity picks up, typically in mid-October or November, experts look at the dominant strain, exposure to similar strains in previous years, and how well-matched the current flu vaccine is to that dominant strain, Dr. Shaman said. Vaccine makers must make an educated guess months in advance regarding which strain to target, to allow time for production.
The vaccination rate plays a role, too, but that tends to remain constant, Dr. Shaman said. According to the CDC, less than half of adults age 18 and up got a flu vaccination last year.
Experts also consider flu patterns in the Southern Hemisphere, where 2024 flu activity has mostly involved two subtypes of influenza A — H1N1 and H3N2 — and some influenza B, the CDC found.
How Well Do This Year’s Vaccines and Viruses Match Up?
The FDA has authorized three updated COVID vaccines for this fall. Novavax targets the JN.1 strain of SARS-CoV-2, the virus that causes COVID-19. Both mRNA vaccines, Moderna and Pfizer, target KP.2, a descendant of JN.1. All three target current predominant variants, and any one of them is recommended by the CDC.
The vaccines are a good “though not perfect match to virtually all the circulating variants of SARS-CoV-2,” said Dr. Pekosz.
Experts said that the shots will protect against the XEC variant.
“XEC and its sublineages are expected to be the dominant fall/winter variant group,” said Dr. Rajnarayanan.
This year’s flu vaccines, all trivalent (protecting against three viruses), will target the three strains expected to circulate — H1N1, H3N2, and influenza B (Victoria), according to the CDC.
People should still get vaccinated, Dr. Adalja said, and use home tests for flu and COVID and take antivirals promptly when needed. The goal should not be status quo but rather fewer COVID and flu hospitalizations and deaths.
A version of this article first appeared on WebMD.com.
What’s the outlook for COVID-19 and flu this fall and winter? It’ll probably be a lot like last year, experts say.
“We currently expect this flu season to be comparable to last year’s season,” said Adrienne Keen, PhD, of the Centers for Disease Control and Prevention’s (CDC) Center for Forecasting and Outbreak Analytics. “We expect this year’s COVID-19 season peak to be similar to last year’s or lower.” The CDC is still analyzing COVID surveillance data from the summer and will update the forecast as more is learned.
For COVID, that means it won’t be as bad as the pandemic years, and for the flu, it’s a typical pre-pandemic season. But status quo does not mean great.
Between October 2023 and April 2024, as many as 75 million people got the flu in the United States, according to CDC estimates, resulting in up to 900,000 hospitalizations and between 17,000 and 100,000 deaths. In 2023, about 900,000 Americans were hospitalized with COVID and 75,000 died.
Getting vaccinated remains crucial, public health officials stressed.
Predicting COVID
Two key predictors of how bad an upcoming COVID season will be are the cycling of new variants and the population’s immunity (protection from an infectious disease that happens when a population is immune through vaccination or previous infection).
When new variants go up and immunity goes down, “we tend to see the increase in cases,” said Michael T. Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy and a professor of public health at the University of Minnesota, Minneapolis. But if the number of variants goes down and immunity levels go up, the outlook is more favorable.
The new COVID variant called XEC has been found in at least 25 states. On September 27, the CDC added the variant to the COVID tracker. It now accounts for 6% of US cases. This was expected, as the variant has been circulating in Europe, said Amesh Adalja, MD, a senior scholar and infectious disease expert at the Center for Health Security at Johns Hopkins University, Baltimore, Maryland.
“There will always be a new variant appearing, and one falling,” he said. “So the fact that this is happening is not surprising.”
Meanwhile, the summer COVID surge has provided postinfection immunity for some people. “What’s likely is, we are going to see substantial protection of the population for several months based on previous infection and in some cases vaccination,” Dr. Osterholm said. That means protection from serious illness, hospitalizations, and deaths (but not necessarily infection). That protection could last through the year or into early 2025.
The timing of 2024’s winter surge will likely be a bit later than 2023’s, said Andrew Pekosz, PhD, a professor and vice chair of molecular microbiology and immunology at Johns Hopkins University, Baltimore, “peaking just after the Christmas/New Year holiday.”
During the 2023-2024 season, weekly COVID hospitalizations peaked the week of Dec. 30, said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill and a member of the COVID-19 Scenario Modeling Hub.
But variants are unpredictable. “There’s a chance that the XEC variant may take off and spread, or might not,” said Dr. Adalja. As of September 28, the Omicron variant KP.3.1.1 was leading, accounting for 58.7% of US cases, according to the CDC.
While Dr. Adalja agreed that 2024’s COVID season will probably be like 2023’s, “we have to be prepared for cases and hospitalizations going up,” he said, “but not to the point of a crisis.” A return to lockdowns and social distancing is unlikely.
Still, older adults and others at higher risk of getting very sick from COVID should consider masking during travel, said Rajendram Rajnarayanan, PhD, MSc, an associate professor at the New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Jonesboro.
Flu Forecasts
Predicting flu season this early is hard, said Jeffrey Shaman, PhD, a professor of environmental health sciences and professor of climate at Colombia University, New York.
“You can look at the CDC forecast and use it as a very loose guide right now,” said Dr. Shaman, who won the CDC’s first “Predict the Influenza Season Challenge” in 2014. “Until there is actually flu, it’s like trying to predict the landfall of a hurricane.” Flu activity remained low as of September 14 (the most current data available), according to the CDC.
When flu activity picks up, typically in mid-October or November, experts look at the dominant strain, exposure to similar strains in previous years, and how well-matched the current flu vaccine is to that dominant strain, Dr. Shaman said. Vaccine makers must make an educated guess months in advance regarding which strain to target, to allow time for production.
The vaccination rate plays a role, too, but that tends to remain constant, Dr. Shaman said. According to the CDC, less than half of adults age 18 and up got a flu vaccination last year.
Experts also consider flu patterns in the Southern Hemisphere, where 2024 flu activity has mostly involved two subtypes of influenza A — H1N1 and H3N2 — and some influenza B, the CDC found.
How Well Do This Year’s Vaccines and Viruses Match Up?
The FDA has authorized three updated COVID vaccines for this fall. Novavax targets the JN.1 strain of SARS-CoV-2, the virus that causes COVID-19. Both mRNA vaccines, Moderna and Pfizer, target KP.2, a descendant of JN.1. All three target current predominant variants, and any one of them is recommended by the CDC.
The vaccines are a good “though not perfect match to virtually all the circulating variants of SARS-CoV-2,” said Dr. Pekosz.
Experts said that the shots will protect against the XEC variant.
“XEC and its sublineages are expected to be the dominant fall/winter variant group,” said Dr. Rajnarayanan.
This year’s flu vaccines, all trivalent (protecting against three viruses), will target the three strains expected to circulate — H1N1, H3N2, and influenza B (Victoria), according to the CDC.
People should still get vaccinated, Dr. Adalja said, and use home tests for flu and COVID and take antivirals promptly when needed. The goal should not be status quo but rather fewer COVID and flu hospitalizations and deaths.
A version of this article first appeared on WebMD.com.
What’s the outlook for COVID-19 and flu this fall and winter? It’ll probably be a lot like last year, experts say.
“We currently expect this flu season to be comparable to last year’s season,” said Adrienne Keen, PhD, of the Centers for Disease Control and Prevention’s (CDC) Center for Forecasting and Outbreak Analytics. “We expect this year’s COVID-19 season peak to be similar to last year’s or lower.” The CDC is still analyzing COVID surveillance data from the summer and will update the forecast as more is learned.
For COVID, that means it won’t be as bad as the pandemic years, and for the flu, it’s a typical pre-pandemic season. But status quo does not mean great.
Between October 2023 and April 2024, as many as 75 million people got the flu in the United States, according to CDC estimates, resulting in up to 900,000 hospitalizations and between 17,000 and 100,000 deaths. In 2023, about 900,000 Americans were hospitalized with COVID and 75,000 died.
Getting vaccinated remains crucial, public health officials stressed.
Predicting COVID
Two key predictors of how bad an upcoming COVID season will be are the cycling of new variants and the population’s immunity (protection from an infectious disease that happens when a population is immune through vaccination or previous infection).
When new variants go up and immunity goes down, “we tend to see the increase in cases,” said Michael T. Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy and a professor of public health at the University of Minnesota, Minneapolis. But if the number of variants goes down and immunity levels go up, the outlook is more favorable.
The new COVID variant called XEC has been found in at least 25 states. On September 27, the CDC added the variant to the COVID tracker. It now accounts for 6% of US cases. This was expected, as the variant has been circulating in Europe, said Amesh Adalja, MD, a senior scholar and infectious disease expert at the Center for Health Security at Johns Hopkins University, Baltimore, Maryland.
“There will always be a new variant appearing, and one falling,” he said. “So the fact that this is happening is not surprising.”
Meanwhile, the summer COVID surge has provided postinfection immunity for some people. “What’s likely is, we are going to see substantial protection of the population for several months based on previous infection and in some cases vaccination,” Dr. Osterholm said. That means protection from serious illness, hospitalizations, and deaths (but not necessarily infection). That protection could last through the year or into early 2025.
The timing of 2024’s winter surge will likely be a bit later than 2023’s, said Andrew Pekosz, PhD, a professor and vice chair of molecular microbiology and immunology at Johns Hopkins University, Baltimore, “peaking just after the Christmas/New Year holiday.”
During the 2023-2024 season, weekly COVID hospitalizations peaked the week of Dec. 30, said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill and a member of the COVID-19 Scenario Modeling Hub.
But variants are unpredictable. “There’s a chance that the XEC variant may take off and spread, or might not,” said Dr. Adalja. As of September 28, the Omicron variant KP.3.1.1 was leading, accounting for 58.7% of US cases, according to the CDC.
While Dr. Adalja agreed that 2024’s COVID season will probably be like 2023’s, “we have to be prepared for cases and hospitalizations going up,” he said, “but not to the point of a crisis.” A return to lockdowns and social distancing is unlikely.
Still, older adults and others at higher risk of getting very sick from COVID should consider masking during travel, said Rajendram Rajnarayanan, PhD, MSc, an associate professor at the New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Jonesboro.
Flu Forecasts
Predicting flu season this early is hard, said Jeffrey Shaman, PhD, a professor of environmental health sciences and professor of climate at Colombia University, New York.
“You can look at the CDC forecast and use it as a very loose guide right now,” said Dr. Shaman, who won the CDC’s first “Predict the Influenza Season Challenge” in 2014. “Until there is actually flu, it’s like trying to predict the landfall of a hurricane.” Flu activity remained low as of September 14 (the most current data available), according to the CDC.
When flu activity picks up, typically in mid-October or November, experts look at the dominant strain, exposure to similar strains in previous years, and how well-matched the current flu vaccine is to that dominant strain, Dr. Shaman said. Vaccine makers must make an educated guess months in advance regarding which strain to target, to allow time for production.
The vaccination rate plays a role, too, but that tends to remain constant, Dr. Shaman said. According to the CDC, less than half of adults age 18 and up got a flu vaccination last year.
Experts also consider flu patterns in the Southern Hemisphere, where 2024 flu activity has mostly involved two subtypes of influenza A — H1N1 and H3N2 — and some influenza B, the CDC found.
How Well Do This Year’s Vaccines and Viruses Match Up?
The FDA has authorized three updated COVID vaccines for this fall. Novavax targets the JN.1 strain of SARS-CoV-2, the virus that causes COVID-19. Both mRNA vaccines, Moderna and Pfizer, target KP.2, a descendant of JN.1. All three target current predominant variants, and any one of them is recommended by the CDC.
The vaccines are a good “though not perfect match to virtually all the circulating variants of SARS-CoV-2,” said Dr. Pekosz.
Experts said that the shots will protect against the XEC variant.
“XEC and its sublineages are expected to be the dominant fall/winter variant group,” said Dr. Rajnarayanan.
This year’s flu vaccines, all trivalent (protecting against three viruses), will target the three strains expected to circulate — H1N1, H3N2, and influenza B (Victoria), according to the CDC.
People should still get vaccinated, Dr. Adalja said, and use home tests for flu and COVID and take antivirals promptly when needed. The goal should not be status quo but rather fewer COVID and flu hospitalizations and deaths.
A version of this article first appeared on WebMD.com.
Guidance for Practicing Primary Care: World Health Organization’s Updated Influenza Guidelines for 2024
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.