User login
Fourth patient cleared of HIV after stem cell transplant for blood cancer
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
AT AIDS 2022
Children and COVID: Many parents see vaccine as the greater risk
New COVID-19 cases rose for the second week in a row as cumulative cases among U.S. children passed the 14-million mark, but a recent survey shows that more than half of parents believe that the vaccine is a greater risk to children under age 5 years than the virus.
In a Kaiser Family Foundation survey conducted July 7-17, 53% of parents with children aged 6 months to 5 years said that the vaccine is “a bigger risk to their child’s health than getting infected with COVID-19, compared to 44% who say getting infected is the bigger risk,” KFF reported July 26.
More than 4 out of 10 of respondents (43%) said that they will “definitely not” get their eligible children vaccinated, while only 7% said that their children had already received it and 10% said their children would get it as soon as possible, according to the KFF survey, which had an overall sample size of 1,847 adults, including an oversample of 471 parents of children under age 5.
Vaccine initiation has been slow in the first month since it was approved for the youngest children. Just 2.8% of all eligible children under age 5 had received an initial dose as of July 19, compared with first-month uptake figures of more than 18% for the 5- to 11-year-olds and 27% for those aged 12-15, based on data from the Centers for Disease Control and Prevention.
The current rates for vaccination in those aged 5 and older look like this: 70.2% of 12- to 17-year-olds have received at least one dose, versus 37.1% of those aged 5-11. Just over 60% of the older children were fully vaccinated as of July 19, as were 30.2% of the 5- to 11-year-olds, the CDC reported on its COVID Data Tracker.
Number of new cases hits 2-month high
Despite the vaccine, SARS-CoV-2 and its various mutations have continued with their summer travels. With 92,000 newly infected children added for the week of July 15-21, there have now been a total of 14,003,497 pediatric cases reported since the start of the pandemic, which works out to 18.6% of cases in all ages, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
The 92,000 new cases represent an increase of almost 22% over the previous week and mark the highest 1-week count since May, when the total passed 100,000 for 2 consecutive weeks. More recently the trend had seemed more stable as weekly cases dropped twice and rose twice as the total hovered around 70,000, based on the data collected by the AAP and CHA from state and territorial health departments.
A different scenario has played out for emergency department visits and hospital admissions, which have risen steadily since the beginning of April. The admission rate for children aged 0-17, which was just 0.13 new patients per 100,000 population on April 11, was up to 0.44 per 100,000 on July 21. By comparison, the highest rate reached last year during the Delta surge was 0.47 per 100,000, based on CDC data.
The 7-day average of emergency dept. visits among the youngest age group, 0-11 years, shows the same general increase as hospital admissions, but the older children have diverged form that path (see graph). For those aged 12-15 and 16-17, hospitalizations started dropping in late May and into mid-June before climbing again, although more slowly than for the youngest group, the CDC data show.
The ED visit rate with diagnosed COVID among those aged 0-11, measured at 6.1% of all visits on July 19, is, in fact, considerably higher than at any time during the Delta surge last year, when it never passed 4.0%, although much lower than peak Omicron (14.1%). That 6.1% was also higher than any other age group on that day, adults included, the CDC said.
New COVID-19 cases rose for the second week in a row as cumulative cases among U.S. children passed the 14-million mark, but a recent survey shows that more than half of parents believe that the vaccine is a greater risk to children under age 5 years than the virus.
In a Kaiser Family Foundation survey conducted July 7-17, 53% of parents with children aged 6 months to 5 years said that the vaccine is “a bigger risk to their child’s health than getting infected with COVID-19, compared to 44% who say getting infected is the bigger risk,” KFF reported July 26.
More than 4 out of 10 of respondents (43%) said that they will “definitely not” get their eligible children vaccinated, while only 7% said that their children had already received it and 10% said their children would get it as soon as possible, according to the KFF survey, which had an overall sample size of 1,847 adults, including an oversample of 471 parents of children under age 5.
Vaccine initiation has been slow in the first month since it was approved for the youngest children. Just 2.8% of all eligible children under age 5 had received an initial dose as of July 19, compared with first-month uptake figures of more than 18% for the 5- to 11-year-olds and 27% for those aged 12-15, based on data from the Centers for Disease Control and Prevention.
The current rates for vaccination in those aged 5 and older look like this: 70.2% of 12- to 17-year-olds have received at least one dose, versus 37.1% of those aged 5-11. Just over 60% of the older children were fully vaccinated as of July 19, as were 30.2% of the 5- to 11-year-olds, the CDC reported on its COVID Data Tracker.
Number of new cases hits 2-month high
Despite the vaccine, SARS-CoV-2 and its various mutations have continued with their summer travels. With 92,000 newly infected children added for the week of July 15-21, there have now been a total of 14,003,497 pediatric cases reported since the start of the pandemic, which works out to 18.6% of cases in all ages, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
The 92,000 new cases represent an increase of almost 22% over the previous week and mark the highest 1-week count since May, when the total passed 100,000 for 2 consecutive weeks. More recently the trend had seemed more stable as weekly cases dropped twice and rose twice as the total hovered around 70,000, based on the data collected by the AAP and CHA from state and territorial health departments.
A different scenario has played out for emergency department visits and hospital admissions, which have risen steadily since the beginning of April. The admission rate for children aged 0-17, which was just 0.13 new patients per 100,000 population on April 11, was up to 0.44 per 100,000 on July 21. By comparison, the highest rate reached last year during the Delta surge was 0.47 per 100,000, based on CDC data.
The 7-day average of emergency dept. visits among the youngest age group, 0-11 years, shows the same general increase as hospital admissions, but the older children have diverged form that path (see graph). For those aged 12-15 and 16-17, hospitalizations started dropping in late May and into mid-June before climbing again, although more slowly than for the youngest group, the CDC data show.
The ED visit rate with diagnosed COVID among those aged 0-11, measured at 6.1% of all visits on July 19, is, in fact, considerably higher than at any time during the Delta surge last year, when it never passed 4.0%, although much lower than peak Omicron (14.1%). That 6.1% was also higher than any other age group on that day, adults included, the CDC said.
New COVID-19 cases rose for the second week in a row as cumulative cases among U.S. children passed the 14-million mark, but a recent survey shows that more than half of parents believe that the vaccine is a greater risk to children under age 5 years than the virus.
In a Kaiser Family Foundation survey conducted July 7-17, 53% of parents with children aged 6 months to 5 years said that the vaccine is “a bigger risk to their child’s health than getting infected with COVID-19, compared to 44% who say getting infected is the bigger risk,” KFF reported July 26.
More than 4 out of 10 of respondents (43%) said that they will “definitely not” get their eligible children vaccinated, while only 7% said that their children had already received it and 10% said their children would get it as soon as possible, according to the KFF survey, which had an overall sample size of 1,847 adults, including an oversample of 471 parents of children under age 5.
Vaccine initiation has been slow in the first month since it was approved for the youngest children. Just 2.8% of all eligible children under age 5 had received an initial dose as of July 19, compared with first-month uptake figures of more than 18% for the 5- to 11-year-olds and 27% for those aged 12-15, based on data from the Centers for Disease Control and Prevention.
The current rates for vaccination in those aged 5 and older look like this: 70.2% of 12- to 17-year-olds have received at least one dose, versus 37.1% of those aged 5-11. Just over 60% of the older children were fully vaccinated as of July 19, as were 30.2% of the 5- to 11-year-olds, the CDC reported on its COVID Data Tracker.
Number of new cases hits 2-month high
Despite the vaccine, SARS-CoV-2 and its various mutations have continued with their summer travels. With 92,000 newly infected children added for the week of July 15-21, there have now been a total of 14,003,497 pediatric cases reported since the start of the pandemic, which works out to 18.6% of cases in all ages, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
The 92,000 new cases represent an increase of almost 22% over the previous week and mark the highest 1-week count since May, when the total passed 100,000 for 2 consecutive weeks. More recently the trend had seemed more stable as weekly cases dropped twice and rose twice as the total hovered around 70,000, based on the data collected by the AAP and CHA from state and territorial health departments.
A different scenario has played out for emergency department visits and hospital admissions, which have risen steadily since the beginning of April. The admission rate for children aged 0-17, which was just 0.13 new patients per 100,000 population on April 11, was up to 0.44 per 100,000 on July 21. By comparison, the highest rate reached last year during the Delta surge was 0.47 per 100,000, based on CDC data.
The 7-day average of emergency dept. visits among the youngest age group, 0-11 years, shows the same general increase as hospital admissions, but the older children have diverged form that path (see graph). For those aged 12-15 and 16-17, hospitalizations started dropping in late May and into mid-June before climbing again, although more slowly than for the youngest group, the CDC data show.
The ED visit rate with diagnosed COVID among those aged 0-11, measured at 6.1% of all visits on July 19, is, in fact, considerably higher than at any time during the Delta surge last year, when it never passed 4.0%, although much lower than peak Omicron (14.1%). That 6.1% was also higher than any other age group on that day, adults included, the CDC said.
Two distinct phenotypes of COVID-related myocarditis emerge
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Monkeypox: Large study highlights new symptoms
Single ulcers, anal lesions, and mouth sores are all unique symptoms of the current monkeypox outbreak, according to the largest international monkeypox case series to date. These findings underscore the need to broaden case definitions for the disease, researchers say.
“While we expected various skin problems and rashes, we also found that 1 in 10 people had only a single skin lesion in the genital area, and 15% had anal and/or rectal pain,” John Thornhill, MD, PhD, the lead author of the research, said in a press release. Dr. Thornhill is a consultant physician in sexual health and HIV and a clinical senior lecturer at Barts NHS Health Trust and Queen Mary University of London. “These different presentations highlight that monkeypox infections could be missed or easily confused with common sexually transmitted infections such as syphilis or herpes,” he said.
Since April 2022, more than 15,000 cases of monkeypox have been reported in 66 countries where the virus was previously not known to be present. The virus, a less severe cousin of smallpox, is endemic to areas of central and west Africa.
In a study published in the New England Journal of Medicine, researchers reported clinical details and outcomes of 528 monkeypox infections across 16 countries. All the cases were diagnosed between April 27 and June 24, 2022. Ninety-five percent of the cases were suspected to have been transmitted through sexual activity, 98% of patients identified as gay or bisexual men, and 75% of the patients were White. The median age of patients in this case series was 38 years, and 90% of infections occurred in Europe. Forty-one percent of patients were HIV-positive, and 96% of these individuals were receiving antiretroviral therapy. Among patients whose HIV status was negative or unknown, 57% reported using preexposure prophylaxis against HIV. About 3 in 10 (29%) individuals tested positive for concurrent sexually transmitted infections.
Nearly three out of four patients (73%) had anogenital lesions, and 41% had mucosal lesions. Fifty-four patients had one genital lesion, and 64% had fewer than 10 lesions in total. Fever (62%), swollen lymph nodes (56%), lethargy (41%), and myalgia (31%) were commonly reported symptoms prior to the development of the rash. Seventy patients (13%) required hospitalization, most commonly for severe anorectal pain and soft-tissue superinfection. Just 5% of patients received monkeypox-specific treatment: intravenous or topical cidofovir (2%), tecovirimat (2%), and vaccinia immune globulin (<1%).
The study “importantly reinforces our current understanding that the overwhelming majority of cases have been sexually associated, predominantly in men who have sex with men,” Jeffrey Klausner, MD, PhD, an infectious disease specialist at the University of Southern California, Los Angeles, said in an interview with this news organization. He was not involved with the research. “Anyone can get monkeypox, but it is most effectively spread through what we call dense networks – where there is a frequent, close personal contact,” he said. “It just happens that gay men and other men who have sex with men have some of those networks.”
The fact that most lesions are present in the genital and anal region – which is unique to this outbreak – points to transmission of the infection during intimate contact, he noted. Still, there is not enough evidence to suggest that monkeypox is spread through sexual transmission. While most semen samples in the study tested positive for monkeypox viral DNA, it is not known whether there is enough virus present to cause transmission, Dr. Thornhill said. He noted that more research is needed.
Dr. Klausner also emphasized the importance of developing new tests to diagnose monkeypox earlier to prevent spread. The lab test for monkeypox requires a swab from a lesion, but this study showed that most patients had notable symptoms prior to developing the standard rash or lesions, he said. Reliable tests using saliva or throat swabs could help detect infections faster, he noted. Patients are thought to be most contagious when they develop lesions, Dr. Klausner said, so diagnosing patients before this stage would allow them to be isolated sooner.
The California-based lab company Flow Health announced a saliva-based PCR test for monkeypox on July 9, although the Food and Drug Administration cautioned that test results from other sample types beside lesion swabs may be inaccurate. “The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing,” the agency said in a statement on July 15. “Testing samples not taken from a lesion may lead to false test results.”
Dr. Klausner reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Single ulcers, anal lesions, and mouth sores are all unique symptoms of the current monkeypox outbreak, according to the largest international monkeypox case series to date. These findings underscore the need to broaden case definitions for the disease, researchers say.
“While we expected various skin problems and rashes, we also found that 1 in 10 people had only a single skin lesion in the genital area, and 15% had anal and/or rectal pain,” John Thornhill, MD, PhD, the lead author of the research, said in a press release. Dr. Thornhill is a consultant physician in sexual health and HIV and a clinical senior lecturer at Barts NHS Health Trust and Queen Mary University of London. “These different presentations highlight that monkeypox infections could be missed or easily confused with common sexually transmitted infections such as syphilis or herpes,” he said.
Since April 2022, more than 15,000 cases of monkeypox have been reported in 66 countries where the virus was previously not known to be present. The virus, a less severe cousin of smallpox, is endemic to areas of central and west Africa.
In a study published in the New England Journal of Medicine, researchers reported clinical details and outcomes of 528 monkeypox infections across 16 countries. All the cases were diagnosed between April 27 and June 24, 2022. Ninety-five percent of the cases were suspected to have been transmitted through sexual activity, 98% of patients identified as gay or bisexual men, and 75% of the patients were White. The median age of patients in this case series was 38 years, and 90% of infections occurred in Europe. Forty-one percent of patients were HIV-positive, and 96% of these individuals were receiving antiretroviral therapy. Among patients whose HIV status was negative or unknown, 57% reported using preexposure prophylaxis against HIV. About 3 in 10 (29%) individuals tested positive for concurrent sexually transmitted infections.
Nearly three out of four patients (73%) had anogenital lesions, and 41% had mucosal lesions. Fifty-four patients had one genital lesion, and 64% had fewer than 10 lesions in total. Fever (62%), swollen lymph nodes (56%), lethargy (41%), and myalgia (31%) were commonly reported symptoms prior to the development of the rash. Seventy patients (13%) required hospitalization, most commonly for severe anorectal pain and soft-tissue superinfection. Just 5% of patients received monkeypox-specific treatment: intravenous or topical cidofovir (2%), tecovirimat (2%), and vaccinia immune globulin (<1%).
The study “importantly reinforces our current understanding that the overwhelming majority of cases have been sexually associated, predominantly in men who have sex with men,” Jeffrey Klausner, MD, PhD, an infectious disease specialist at the University of Southern California, Los Angeles, said in an interview with this news organization. He was not involved with the research. “Anyone can get monkeypox, but it is most effectively spread through what we call dense networks – where there is a frequent, close personal contact,” he said. “It just happens that gay men and other men who have sex with men have some of those networks.”
The fact that most lesions are present in the genital and anal region – which is unique to this outbreak – points to transmission of the infection during intimate contact, he noted. Still, there is not enough evidence to suggest that monkeypox is spread through sexual transmission. While most semen samples in the study tested positive for monkeypox viral DNA, it is not known whether there is enough virus present to cause transmission, Dr. Thornhill said. He noted that more research is needed.
Dr. Klausner also emphasized the importance of developing new tests to diagnose monkeypox earlier to prevent spread. The lab test for monkeypox requires a swab from a lesion, but this study showed that most patients had notable symptoms prior to developing the standard rash or lesions, he said. Reliable tests using saliva or throat swabs could help detect infections faster, he noted. Patients are thought to be most contagious when they develop lesions, Dr. Klausner said, so diagnosing patients before this stage would allow them to be isolated sooner.
The California-based lab company Flow Health announced a saliva-based PCR test for monkeypox on July 9, although the Food and Drug Administration cautioned that test results from other sample types beside lesion swabs may be inaccurate. “The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing,” the agency said in a statement on July 15. “Testing samples not taken from a lesion may lead to false test results.”
Dr. Klausner reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Single ulcers, anal lesions, and mouth sores are all unique symptoms of the current monkeypox outbreak, according to the largest international monkeypox case series to date. These findings underscore the need to broaden case definitions for the disease, researchers say.
“While we expected various skin problems and rashes, we also found that 1 in 10 people had only a single skin lesion in the genital area, and 15% had anal and/or rectal pain,” John Thornhill, MD, PhD, the lead author of the research, said in a press release. Dr. Thornhill is a consultant physician in sexual health and HIV and a clinical senior lecturer at Barts NHS Health Trust and Queen Mary University of London. “These different presentations highlight that monkeypox infections could be missed or easily confused with common sexually transmitted infections such as syphilis or herpes,” he said.
Since April 2022, more than 15,000 cases of monkeypox have been reported in 66 countries where the virus was previously not known to be present. The virus, a less severe cousin of smallpox, is endemic to areas of central and west Africa.
In a study published in the New England Journal of Medicine, researchers reported clinical details and outcomes of 528 monkeypox infections across 16 countries. All the cases were diagnosed between April 27 and June 24, 2022. Ninety-five percent of the cases were suspected to have been transmitted through sexual activity, 98% of patients identified as gay or bisexual men, and 75% of the patients were White. The median age of patients in this case series was 38 years, and 90% of infections occurred in Europe. Forty-one percent of patients were HIV-positive, and 96% of these individuals were receiving antiretroviral therapy. Among patients whose HIV status was negative or unknown, 57% reported using preexposure prophylaxis against HIV. About 3 in 10 (29%) individuals tested positive for concurrent sexually transmitted infections.
Nearly three out of four patients (73%) had anogenital lesions, and 41% had mucosal lesions. Fifty-four patients had one genital lesion, and 64% had fewer than 10 lesions in total. Fever (62%), swollen lymph nodes (56%), lethargy (41%), and myalgia (31%) were commonly reported symptoms prior to the development of the rash. Seventy patients (13%) required hospitalization, most commonly for severe anorectal pain and soft-tissue superinfection. Just 5% of patients received monkeypox-specific treatment: intravenous or topical cidofovir (2%), tecovirimat (2%), and vaccinia immune globulin (<1%).
The study “importantly reinforces our current understanding that the overwhelming majority of cases have been sexually associated, predominantly in men who have sex with men,” Jeffrey Klausner, MD, PhD, an infectious disease specialist at the University of Southern California, Los Angeles, said in an interview with this news organization. He was not involved with the research. “Anyone can get monkeypox, but it is most effectively spread through what we call dense networks – where there is a frequent, close personal contact,” he said. “It just happens that gay men and other men who have sex with men have some of those networks.”
The fact that most lesions are present in the genital and anal region – which is unique to this outbreak – points to transmission of the infection during intimate contact, he noted. Still, there is not enough evidence to suggest that monkeypox is spread through sexual transmission. While most semen samples in the study tested positive for monkeypox viral DNA, it is not known whether there is enough virus present to cause transmission, Dr. Thornhill said. He noted that more research is needed.
Dr. Klausner also emphasized the importance of developing new tests to diagnose monkeypox earlier to prevent spread. The lab test for monkeypox requires a swab from a lesion, but this study showed that most patients had notable symptoms prior to developing the standard rash or lesions, he said. Reliable tests using saliva or throat swabs could help detect infections faster, he noted. Patients are thought to be most contagious when they develop lesions, Dr. Klausner said, so diagnosing patients before this stage would allow them to be isolated sooner.
The California-based lab company Flow Health announced a saliva-based PCR test for monkeypox on July 9, although the Food and Drug Administration cautioned that test results from other sample types beside lesion swabs may be inaccurate. “The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing,” the agency said in a statement on July 15. “Testing samples not taken from a lesion may lead to false test results.”
Dr. Klausner reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Science lags behind for kids with long COVID
Emma Sherman, a 13-year-old girl in Ascot, England, woke up to a dizzying aura of blind spots and flashing lights in her field of vision. It was May 2020, and she also had crippling nausea and headaches. By August, her dizziness was so overwhelming, she couldn’t hold her head up, lying in her mother’s lap for hours, too fatigued to attend school.
The former competitive gymnast, who had hoped to try out for the cheerleading squad, now used a wheelchair and was a shadow of her former self. She had been diagnosed with COVID-induced postural orthostatic tachycardia syndrome, a condition often caused by an infection that results in a higher heart rate, extreme nausea, dizziness, and fatigue.
“I was so into sports before I got long COVID, and afterwards I could barely walk,” Emma said.
Even minor movements sent her heart rate sky-high. Her long chestnut hair turned gray and fell out in clumps. In the hospital, she was pricked and prodded, her blood tested for numerous conditions.
“They ran every scan known to man and took an MRI of her brain,” said Emma’s mother, Marie Sherman. “All was clear.”
Emma’s pediatrician determined that the teen had long COVID after having had a mild case of the virus in March, about 2 months before her puzzling symptoms began. But beyond a positive antibody test, doctors have found little evidence of what was causing Emma’s symptoms.
For Emma and others with long COVID, there are no medications shown to directly target the condition. Instead, caregivers target their symptoms, which include nausea, dizziness, fatigue, headaches, and a racing heart, said Laura Malone, MD, codirector of the Johns Hopkins Kennedy Krieger Pediatric Post–COVID-19 Rehabilitation Clinic in Baltimore.
“Right now, it’s a rehabilitation-based approach focused on improving symptoms and functioning so that kids can go back to their usual activities as much as possible,” she says.
Depression and anxiety are common, although doctors are struggling to figure out whether COVID is changing the brain or whether mental health symptoms result from all the life disruptions. There’s little research to show how may kids have depression because of long COVID. Dr. Malone said about half of her patients at the Kennedy Krieger Institute›s long COVID clinic are also dealing with mental health issues.
Patients with headaches, dizziness, and nausea are given pain and nausea medications and recommendations for a healthy diet with added fruits and vegetables, monounsaturated fats, lower sodium, unprocessed foods, and whole grains. Kids with irregular or racing heart rates are referred to cardiologists and potentially prescribed beta-blockers to treat their heart arrhythmias, while children with breathing problems may be referred to pulmonologists and those with depression to a psychiatrist.
Still, many patients like Emma go to their doctors with phantom symptoms that don’t show up on scans or blood tests.
“We’re not seeing any evidence of structural damage to the brain, for example,” said Dr. Malone. “When we do MRIs, they often come out normal.”
It’s possible that the virus lingers in some patients, said Rajeev Fernando, MD, an infectious disease specialist and a fellow at Harvard Medical School, Boston. Kids’ strong immune systems often fend off problems that can be noticed. But on the inside, dead fragments of the virus persist, floating in hidden parts of the body and activating the immune system long after the threat has passed.
The virus can be in the gut and in the brain, which may help explain why symptoms like brain fog and nausea can linger in children.
“The immune system doesn’t recognize whether fragments of the virus are dead or alive. It continues to think it’s fighting active COVID,” said Dr. Fernando.
There is little data on how long symptoms last, Dr. Fernando said, as well as how many kids get them and why some are more vulnerable than others. Some research has found that about 5%-15% of children with COVID may get long COVID, but the statistics vary globally.
“Children with long COVID have largely been ignored. And while we’re talking about it now, we’ve got some work to do,” said Dr. Fernando.
As for Emma, she recovered in January of 2021, heading back to school and her friends, although her cardiologist advised her to skip gym classes.
“For the first time in months, I was feeling like myself again,” she said.
But the coronavirus found its way to Emma again. Although she was fully vaccinated in the fall of 2021, when the Omicron variant swept the world late that year, she was infected again.
“When the wave of Omicron descended, Emma was like a sitting duck,” her mother said.
She was bedridden with a high fever and cough. The cold-like symptoms eventually went away, but the issues in her gut stuck around. Since then, Emma has had extreme nausea, losing most of the weight she had gained back.
For her part, Ms. Sherman has found solace in a group called Long COVID Kids, a nonprofit in Europe and the United States. The group is raising awareness about the condition in kids to increase funding, boost understanding, and improve treatment and outcomes.
“There’s nothing worse than watching your child suffer and not being able to do anything about it,” she said. “I tell Emma all the time: If I could just crawl in your body and take it, I would do it in a second.”
Emma is hoping for a fresh start with her family’s move in the coming weeks to Sotogrande in southern Spain.
“I miss the simplest things like going for a run, going to the fair with my friends, and just feeling well,” she said. “I have a long list of things I’ll do once this is all done.”
A version of this article first appeared on WebMD.com.
Emma Sherman, a 13-year-old girl in Ascot, England, woke up to a dizzying aura of blind spots and flashing lights in her field of vision. It was May 2020, and she also had crippling nausea and headaches. By August, her dizziness was so overwhelming, she couldn’t hold her head up, lying in her mother’s lap for hours, too fatigued to attend school.
The former competitive gymnast, who had hoped to try out for the cheerleading squad, now used a wheelchair and was a shadow of her former self. She had been diagnosed with COVID-induced postural orthostatic tachycardia syndrome, a condition often caused by an infection that results in a higher heart rate, extreme nausea, dizziness, and fatigue.
“I was so into sports before I got long COVID, and afterwards I could barely walk,” Emma said.
Even minor movements sent her heart rate sky-high. Her long chestnut hair turned gray and fell out in clumps. In the hospital, she was pricked and prodded, her blood tested for numerous conditions.
“They ran every scan known to man and took an MRI of her brain,” said Emma’s mother, Marie Sherman. “All was clear.”
Emma’s pediatrician determined that the teen had long COVID after having had a mild case of the virus in March, about 2 months before her puzzling symptoms began. But beyond a positive antibody test, doctors have found little evidence of what was causing Emma’s symptoms.
For Emma and others with long COVID, there are no medications shown to directly target the condition. Instead, caregivers target their symptoms, which include nausea, dizziness, fatigue, headaches, and a racing heart, said Laura Malone, MD, codirector of the Johns Hopkins Kennedy Krieger Pediatric Post–COVID-19 Rehabilitation Clinic in Baltimore.
“Right now, it’s a rehabilitation-based approach focused on improving symptoms and functioning so that kids can go back to their usual activities as much as possible,” she says.
Depression and anxiety are common, although doctors are struggling to figure out whether COVID is changing the brain or whether mental health symptoms result from all the life disruptions. There’s little research to show how may kids have depression because of long COVID. Dr. Malone said about half of her patients at the Kennedy Krieger Institute›s long COVID clinic are also dealing with mental health issues.
Patients with headaches, dizziness, and nausea are given pain and nausea medications and recommendations for a healthy diet with added fruits and vegetables, monounsaturated fats, lower sodium, unprocessed foods, and whole grains. Kids with irregular or racing heart rates are referred to cardiologists and potentially prescribed beta-blockers to treat their heart arrhythmias, while children with breathing problems may be referred to pulmonologists and those with depression to a psychiatrist.
Still, many patients like Emma go to their doctors with phantom symptoms that don’t show up on scans or blood tests.
“We’re not seeing any evidence of structural damage to the brain, for example,” said Dr. Malone. “When we do MRIs, they often come out normal.”
It’s possible that the virus lingers in some patients, said Rajeev Fernando, MD, an infectious disease specialist and a fellow at Harvard Medical School, Boston. Kids’ strong immune systems often fend off problems that can be noticed. But on the inside, dead fragments of the virus persist, floating in hidden parts of the body and activating the immune system long after the threat has passed.
The virus can be in the gut and in the brain, which may help explain why symptoms like brain fog and nausea can linger in children.
“The immune system doesn’t recognize whether fragments of the virus are dead or alive. It continues to think it’s fighting active COVID,” said Dr. Fernando.
There is little data on how long symptoms last, Dr. Fernando said, as well as how many kids get them and why some are more vulnerable than others. Some research has found that about 5%-15% of children with COVID may get long COVID, but the statistics vary globally.
“Children with long COVID have largely been ignored. And while we’re talking about it now, we’ve got some work to do,” said Dr. Fernando.
As for Emma, she recovered in January of 2021, heading back to school and her friends, although her cardiologist advised her to skip gym classes.
“For the first time in months, I was feeling like myself again,” she said.
But the coronavirus found its way to Emma again. Although she was fully vaccinated in the fall of 2021, when the Omicron variant swept the world late that year, she was infected again.
“When the wave of Omicron descended, Emma was like a sitting duck,” her mother said.
She was bedridden with a high fever and cough. The cold-like symptoms eventually went away, but the issues in her gut stuck around. Since then, Emma has had extreme nausea, losing most of the weight she had gained back.
For her part, Ms. Sherman has found solace in a group called Long COVID Kids, a nonprofit in Europe and the United States. The group is raising awareness about the condition in kids to increase funding, boost understanding, and improve treatment and outcomes.
“There’s nothing worse than watching your child suffer and not being able to do anything about it,” she said. “I tell Emma all the time: If I could just crawl in your body and take it, I would do it in a second.”
Emma is hoping for a fresh start with her family’s move in the coming weeks to Sotogrande in southern Spain.
“I miss the simplest things like going for a run, going to the fair with my friends, and just feeling well,” she said. “I have a long list of things I’ll do once this is all done.”
A version of this article first appeared on WebMD.com.
Emma Sherman, a 13-year-old girl in Ascot, England, woke up to a dizzying aura of blind spots and flashing lights in her field of vision. It was May 2020, and she also had crippling nausea and headaches. By August, her dizziness was so overwhelming, she couldn’t hold her head up, lying in her mother’s lap for hours, too fatigued to attend school.
The former competitive gymnast, who had hoped to try out for the cheerleading squad, now used a wheelchair and was a shadow of her former self. She had been diagnosed with COVID-induced postural orthostatic tachycardia syndrome, a condition often caused by an infection that results in a higher heart rate, extreme nausea, dizziness, and fatigue.
“I was so into sports before I got long COVID, and afterwards I could barely walk,” Emma said.
Even minor movements sent her heart rate sky-high. Her long chestnut hair turned gray and fell out in clumps. In the hospital, she was pricked and prodded, her blood tested for numerous conditions.
“They ran every scan known to man and took an MRI of her brain,” said Emma’s mother, Marie Sherman. “All was clear.”
Emma’s pediatrician determined that the teen had long COVID after having had a mild case of the virus in March, about 2 months before her puzzling symptoms began. But beyond a positive antibody test, doctors have found little evidence of what was causing Emma’s symptoms.
For Emma and others with long COVID, there are no medications shown to directly target the condition. Instead, caregivers target their symptoms, which include nausea, dizziness, fatigue, headaches, and a racing heart, said Laura Malone, MD, codirector of the Johns Hopkins Kennedy Krieger Pediatric Post–COVID-19 Rehabilitation Clinic in Baltimore.
“Right now, it’s a rehabilitation-based approach focused on improving symptoms and functioning so that kids can go back to their usual activities as much as possible,” she says.
Depression and anxiety are common, although doctors are struggling to figure out whether COVID is changing the brain or whether mental health symptoms result from all the life disruptions. There’s little research to show how may kids have depression because of long COVID. Dr. Malone said about half of her patients at the Kennedy Krieger Institute›s long COVID clinic are also dealing with mental health issues.
Patients with headaches, dizziness, and nausea are given pain and nausea medications and recommendations for a healthy diet with added fruits and vegetables, monounsaturated fats, lower sodium, unprocessed foods, and whole grains. Kids with irregular or racing heart rates are referred to cardiologists and potentially prescribed beta-blockers to treat their heart arrhythmias, while children with breathing problems may be referred to pulmonologists and those with depression to a psychiatrist.
Still, many patients like Emma go to their doctors with phantom symptoms that don’t show up on scans or blood tests.
“We’re not seeing any evidence of structural damage to the brain, for example,” said Dr. Malone. “When we do MRIs, they often come out normal.”
It’s possible that the virus lingers in some patients, said Rajeev Fernando, MD, an infectious disease specialist and a fellow at Harvard Medical School, Boston. Kids’ strong immune systems often fend off problems that can be noticed. But on the inside, dead fragments of the virus persist, floating in hidden parts of the body and activating the immune system long after the threat has passed.
The virus can be in the gut and in the brain, which may help explain why symptoms like brain fog and nausea can linger in children.
“The immune system doesn’t recognize whether fragments of the virus are dead or alive. It continues to think it’s fighting active COVID,” said Dr. Fernando.
There is little data on how long symptoms last, Dr. Fernando said, as well as how many kids get them and why some are more vulnerable than others. Some research has found that about 5%-15% of children with COVID may get long COVID, but the statistics vary globally.
“Children with long COVID have largely been ignored. And while we’re talking about it now, we’ve got some work to do,” said Dr. Fernando.
As for Emma, she recovered in January of 2021, heading back to school and her friends, although her cardiologist advised her to skip gym classes.
“For the first time in months, I was feeling like myself again,” she said.
But the coronavirus found its way to Emma again. Although she was fully vaccinated in the fall of 2021, when the Omicron variant swept the world late that year, she was infected again.
“When the wave of Omicron descended, Emma was like a sitting duck,” her mother said.
She was bedridden with a high fever and cough. The cold-like symptoms eventually went away, but the issues in her gut stuck around. Since then, Emma has had extreme nausea, losing most of the weight she had gained back.
For her part, Ms. Sherman has found solace in a group called Long COVID Kids, a nonprofit in Europe and the United States. The group is raising awareness about the condition in kids to increase funding, boost understanding, and improve treatment and outcomes.
“There’s nothing worse than watching your child suffer and not being able to do anything about it,” she said. “I tell Emma all the time: If I could just crawl in your body and take it, I would do it in a second.”
Emma is hoping for a fresh start with her family’s move in the coming weeks to Sotogrande in southern Spain.
“I miss the simplest things like going for a run, going to the fair with my friends, and just feeling well,” she said. “I have a long list of things I’ll do once this is all done.”
A version of this article first appeared on WebMD.com.
Should monkeypox be considered an STD? Experts debate
As the number of monkeypox cases keeps growing, a discussion has opened on whether it should be considered a sexually transmitted disease like herpes, gonorrhea, or HIV.
Monkeypox is almost always spread through skin-to-skin contact and, in the West, many of the cases have occurred among men who have sex with men.
But health experts say that doesn’t make it an STD – at least not in “the classic sense.”
“Monkeypox is not a sexually transmitted disease in the classic sense (by which it’s spread in the semen or vaginal fluids), but it is spread by close physical contact with lesions,” infectious diseases expert Robert L. Murphy, MD, of Northwestern Medicine, Chicago, said in a news release.
He said the current monkeypox outbreak was more like a meningitis outbreak among gay men a few years ago.
Rowland Kao, PhD, a professor of veterinary epidemiology and data science at the University of Edinburgh, said that an “STD is one where intimate, sexual contact is critical to the transmission – where sexual acts are central to the transmission,” Newsweek reported.
“Some infections are transmitted by any type of close contact, of which sexual activity is one. Monkeypox is one of those –
But calling monkeypox an STD could deter measures to limit its spread, another expert told Newsweek.
“My uneasiness about labeling it as an STD is that for most STDs, wearing a condom or avoiding penetration or direct oral-anal/oral-genital contact is a good way of preventing transmission,” said Paul Hunter, MD, a professor of health protection at the University of East Anglia, Norwich, England.
“But for monkeypox, even just naked cuddling is a big risk. So labeling it an STD could actually work against control if people felt they just had to wear a condom.”
Denise Dewald, MD, a pediatric specialist at University Hospitals Cleveland Medical Center, said monkeypox is not an STD – but it could become an entrenched virus.
“Monkeypox will become established in the pediatric and general population and will transmit through daycares and schools,” she tweeted. “It is not an STD. It is like MRSA. This isn’t rocket science.”
One thing is certain: More and more people are getting monkeypox. It’s been endemic in Western and Central Africa for years, and cases in Europe and North America were identified in May.
Globally, more than 14,000 cases have been identified, World Health Organization Director-General Tedros Adhanom Ghebreyesus said on July 20, according to the Center for Infectious Disease Research and Policy. Five people in Africa have died. In the United Kingdom, more than 2,100 cases have been identified.
In the United States, more than 2,500 confirmed monkeypox cases have been detected, with cases reported from every state except Alaska, Maine, Montana, Mississippi, Vermont, and Wyoming, the CDC said on July 21.
A version of this article first appeared on WebMD.com.
As the number of monkeypox cases keeps growing, a discussion has opened on whether it should be considered a sexually transmitted disease like herpes, gonorrhea, or HIV.
Monkeypox is almost always spread through skin-to-skin contact and, in the West, many of the cases have occurred among men who have sex with men.
But health experts say that doesn’t make it an STD – at least not in “the classic sense.”
“Monkeypox is not a sexually transmitted disease in the classic sense (by which it’s spread in the semen or vaginal fluids), but it is spread by close physical contact with lesions,” infectious diseases expert Robert L. Murphy, MD, of Northwestern Medicine, Chicago, said in a news release.
He said the current monkeypox outbreak was more like a meningitis outbreak among gay men a few years ago.
Rowland Kao, PhD, a professor of veterinary epidemiology and data science at the University of Edinburgh, said that an “STD is one where intimate, sexual contact is critical to the transmission – where sexual acts are central to the transmission,” Newsweek reported.
“Some infections are transmitted by any type of close contact, of which sexual activity is one. Monkeypox is one of those –
But calling monkeypox an STD could deter measures to limit its spread, another expert told Newsweek.
“My uneasiness about labeling it as an STD is that for most STDs, wearing a condom or avoiding penetration or direct oral-anal/oral-genital contact is a good way of preventing transmission,” said Paul Hunter, MD, a professor of health protection at the University of East Anglia, Norwich, England.
“But for monkeypox, even just naked cuddling is a big risk. So labeling it an STD could actually work against control if people felt they just had to wear a condom.”
Denise Dewald, MD, a pediatric specialist at University Hospitals Cleveland Medical Center, said monkeypox is not an STD – but it could become an entrenched virus.
“Monkeypox will become established in the pediatric and general population and will transmit through daycares and schools,” she tweeted. “It is not an STD. It is like MRSA. This isn’t rocket science.”
One thing is certain: More and more people are getting monkeypox. It’s been endemic in Western and Central Africa for years, and cases in Europe and North America were identified in May.
Globally, more than 14,000 cases have been identified, World Health Organization Director-General Tedros Adhanom Ghebreyesus said on July 20, according to the Center for Infectious Disease Research and Policy. Five people in Africa have died. In the United Kingdom, more than 2,100 cases have been identified.
In the United States, more than 2,500 confirmed monkeypox cases have been detected, with cases reported from every state except Alaska, Maine, Montana, Mississippi, Vermont, and Wyoming, the CDC said on July 21.
A version of this article first appeared on WebMD.com.
As the number of monkeypox cases keeps growing, a discussion has opened on whether it should be considered a sexually transmitted disease like herpes, gonorrhea, or HIV.
Monkeypox is almost always spread through skin-to-skin contact and, in the West, many of the cases have occurred among men who have sex with men.
But health experts say that doesn’t make it an STD – at least not in “the classic sense.”
“Monkeypox is not a sexually transmitted disease in the classic sense (by which it’s spread in the semen or vaginal fluids), but it is spread by close physical contact with lesions,” infectious diseases expert Robert L. Murphy, MD, of Northwestern Medicine, Chicago, said in a news release.
He said the current monkeypox outbreak was more like a meningitis outbreak among gay men a few years ago.
Rowland Kao, PhD, a professor of veterinary epidemiology and data science at the University of Edinburgh, said that an “STD is one where intimate, sexual contact is critical to the transmission – where sexual acts are central to the transmission,” Newsweek reported.
“Some infections are transmitted by any type of close contact, of which sexual activity is one. Monkeypox is one of those –
But calling monkeypox an STD could deter measures to limit its spread, another expert told Newsweek.
“My uneasiness about labeling it as an STD is that for most STDs, wearing a condom or avoiding penetration or direct oral-anal/oral-genital contact is a good way of preventing transmission,” said Paul Hunter, MD, a professor of health protection at the University of East Anglia, Norwich, England.
“But for monkeypox, even just naked cuddling is a big risk. So labeling it an STD could actually work against control if people felt they just had to wear a condom.”
Denise Dewald, MD, a pediatric specialist at University Hospitals Cleveland Medical Center, said monkeypox is not an STD – but it could become an entrenched virus.
“Monkeypox will become established in the pediatric and general population and will transmit through daycares and schools,” she tweeted. “It is not an STD. It is like MRSA. This isn’t rocket science.”
One thing is certain: More and more people are getting monkeypox. It’s been endemic in Western and Central Africa for years, and cases in Europe and North America were identified in May.
Globally, more than 14,000 cases have been identified, World Health Organization Director-General Tedros Adhanom Ghebreyesus said on July 20, according to the Center for Infectious Disease Research and Policy. Five people in Africa have died. In the United Kingdom, more than 2,100 cases have been identified.
In the United States, more than 2,500 confirmed monkeypox cases have been detected, with cases reported from every state except Alaska, Maine, Montana, Mississippi, Vermont, and Wyoming, the CDC said on July 21.
A version of this article first appeared on WebMD.com.
Clinical characteristics of recurrent RIME elucidated in chart review
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – , in a single-center retrospective study. In addition, 71% of patients with recurrent disease experienced 1-2 recurrences – episodes that were generally milder and occurred at variable intervals.
Those are among key findings from the study of 50 patients with RIME, presented by Catherina X. Pan at the annual meeting of the Society for Pediatric Dermatology.
Reactive infectious mucocutaneous eruption (RIME) is a novel term encompassing an array of rare, parainfectious mucositis diseases, noted Ms. Pan, a fourth-year medical student at Harvard Medical School, Boston. Previously known as Mycoplasma pneumoniae-induced rash and mucositis (MIRM), common clinical characteristics of RIME include less than 10% body surface area involvement of polymorphic skin lesions (vesiculobullous or targetoid macules/papules); erosive oral, genital, and/or ocular mucositis involving more than two sites, and evidence of prior infection including but not limited to upper respiratory infection, fever, and cough.
In addition to M. pneumoniae, other pathogens have been implicated, she said. “While the underlying etiology of the disease is not entirely clear, it’s become increasingly known that RIME tends to recur in a subset of patients.”
A cohort study of 13 patients with RIME found that Black race, male sex, and older age were predominant among the five patients who developed recurrent disease.
The estimated recurrence rate is between 8% and 38%, but the clinical characteristics of patients who develop recurrent RIME tend to be poorly understood, Ms. Pan said.
Along with her mentor, Sadaf Hussain, MD, of the department of dermatology at Boston Children’s Hospital, Ms. Pan conducted a retrospective chart review to characterize the clinical history and course of disease in patients diagnosed with recurrent RIME. They extracted data between January of 2000 and March of 2022 using ICD-10 codes used by board-certified dermatologists at Boston Children’s Hospital, as well as a text search for RIME or MIRM in the dermatology notes. Patients were included if they had a RIME/MIRM diagnosis by a board-certified dermatologist and/or infection on PCR/serology and mucositis involvement with limited skin involvement.
The study population included 50 patients: 24 with recurrent RIME and 26 with isolated RIME. The majority (66%) were male, and the mean age of RIME onset was between 11 and 12 years old, which is up to two years younger than previously reported in the case series of 13 patients. Most of the study participants (79%) were White, but there were no significant differences in patients who had recurrent RIME and those who had isolated RIME in terms of age, sex, or race.
Isolated vs. recurrent RIME
However, compared with patients who had isolated RIME, a greater proportion of those with recurrent RIME had a history of atopic disease (46% vs. 23%, respectively; P = .136), as well as a history of tonsillectomy and adenoidectomy (25% vs. 4%; P = .045). “This has not been previously observed, but it may generate a hypothesis that patients with a history of frequent infection as well as amplified immune responses may be associated with disease recurrence,” Ms. Pan said.
The average number of episodes among patients with recurrent RIME was 3.5 and the interval between episodes was variable, at a mean of 10.2 months. Ms. Pan reported that 71% of recurrent RIME patients experienced 1-2 episodes, although one patient experienced 9 episodes.
Clinically, episodes among all patients with RIME were characterized by infectious prodromal symptoms (69%), oral lesions (95%), ocular lesions (60%), genital lesions (41%) and cutaneous lesions (40%). However, RIME recurrences were less severe and more atypical, with 49% involving only one mucosal surface and 29% involving two mucosal surfaces. Also, except for oral lesions, rates of infectious prodromal symptoms and other lesions significantly decreased among recurrences compared with initial RIME.
“Notably, we found that M. pneumoniae was the most common known cause of RIME, particularly among the initial episodes,” Ms. Pan said. “However, 61% of recurrent RIME episodes did not have a known cause in terms of infectious etiology. And, concordant with prior studies, we also found decreased severity [of RIME recurrences] as indicated by decreased rates of emergency department presentation, hospitalization, and duration of hospitalization.”
In other findings, psychiatric complications such as anxiety and depression followed the onset of RIME in 33% of those with recurrent disease and 22% of those with isolated disease. In addition, the three most common treatments among all 50 patients were systemic steroids, topical steroids, and M. pneumoniae-specific antibiotics.
“While RIME is considered as typically milder than Stevens-Johnson syndrome and toxic epidermal necrolysis with low mortality rates, it can lead to severe complications including conjunctival shrinkage, corneal ulceration and scarring, blindness, and oral, ocular, urogenital synechiae,” Ms. Pan noted. “Increased use of corticosteroids and steroid-sparing agents such as IVIG have also been observed. Multidisciplinary care with ophthalmology, urology, and mental health services is critical.”
She acknowledged certain limitations of the study, including its retrospective, single-center design, and the possibility that milder cases may have been excluded due to a lack of accurate diagnosis or referral.
Carrie C. Coughlin, MD, who was asked to comment on the study results, pointed out that nearly half (24) of patients in the cohort experienced recurrent RIME. “This is a high proportion, suggesting counseling about the possibility of recurrence is more important than previously thought,” said Dr. Coughlin, director of the section of pediatric dermatology Washington University/St. Louis Children’s Hospital.
“Fortunately, recurrent cases tended to be less severe. However, many patients had more than one recurrence, making this challenging for affected patients.”
The researchers reported having no financial disclosures. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Milium cysts on hands; hypertrichosis on face
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
A 55-YEAR-OLD MAN with hypertension and untreated hepatitis C virus (HCV) was referred to the Dermatology Clinic after reporting a 2-year history of photosensitivity and intermittent episodes of blistering and scars on the dorsal side of his hands and feet. No alcohol consumption or drug use was reported.
Physical examination revealed small and shallow erosions on the dorsal aspect of the hands and feet (but no visible blisters) and milium cysts (FIGURE 1A). Additionally, hypertrichosis and hyperpigmentation were observed in the zygomatic areas (FIGURE 1B). Complete blood count and kidney function test results were within normal ranges. Liver function tests showed slightly elevated levels of alanine aminotransferase (79 U/L; normal range, 0-41 U/L), aspartate aminotransferase (62 U/L; normal range, 0-40 U/L), and ferritin (121 ng/mL; normal range, 30-100 ng/mL). Serologies for syphilis, HIV, and hepatitis B virus were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
The porphyrias are a group of metabolic diseases that affect the heme biosynthesis. They can be classified into 1 of 3 groups, according to clinical features:
- acute hepatic porphyrias, with neurovisceral symptoms (eg, acute intermittent porphyria),
- nonblistering cutaneous porphyrias, with severe photosensitivity but without bullae formation (eg, erythropoietic protoporphyria), or
- blistering cutaneous porphyrias (eg, PCT, hepatoerythropoietic porphyria, and variegate porphyria).
PCT is the most common type of porphyria, with a global prevalence of 1 per 10,000 people.1,2 It affects adults after the third or fourth decade of life.
PCT involves dysfunction of the uroporphyrinogen decarboxylase enzyme (UROD), the fifth enzyme in heme biosynthesis, which catalyzes the conversion of uroporphyrinogen to coproporphyrinogen. This dysfunction causes the accumulation of porphyrinogens that are auto-oxidized to photosensitizing porphyrins.1-4 PCT can be classified as “sporadic” or “familial” based on the absence or presence of UROD mutation. Approximately 80% of cases of PCT are sporadic.2
In sporadic PCT, triggers for UROD dysfunction include alcohol use, use of estrogens, hemochromatosis or iron overload, chronic HCV infection, and HIV infection.1-4 HCV (which this patient had) is the most common infection associated with sporadic PCT, with a prevalence of about 50% among these patients.5
Continue to: Dermatologic manifestations of PCT
Dermatologic manifestations of PCT include photosensitivity, skin fragility, vesicles, bullae, erosions, and crusts observed in sun-exposed areas. A nonvirilizing type of hypertrichosis may appear prominently on the temples and the cheeks.2-4 After blisters rupture, atrophy and scarring occur. Milia cysts can form on the dorsal side of the hands and fingers. Less common manifestations include pruritus, scarring alopecia, sclerodermatous changes, and periorbital purple-red suffusion.
Hepatic involvement is demonstrated with elevated serum transaminases and gamma-glutamyl transpeptidase. Hepatomegaly is common, and cirrhosis manifests in 30% to 40% of patients.2-5 On liver biopsy, some degree of siderosis is found in 80% of patients with PCT, and most of them have increased levels of serum iron. The incidence of hepatocellular carcinoma in patients with PCT is greater than in patients with other liver diseases.2
A Wood lamp can be a useful diagnostic first step
Plasma or urine porphyrin lab tests are the gold standard for PCT diagnosis. These tests can be followed by more specific tests (eg, porphyrin fractionation) to exclude other forms of porphyria. However, if plasma or urine porphyrin testing is not readily available, a good first step is a Wood lamp exam, which can be performed on urine or stool. (Plasma or urine porphyrin testing may ultimately be necessary if there is doubt about the diagnosis following the Wood lamp screening.) Histopathologic examination does not confirm the diagnosis of PCT4; however, it can be helpful in differential diagnosis.
Wood lamp is a source of long-wave UV light (320 to 400 nm), visualized as a purple or violet light. When porphyrins are present in a urine sample, a red-pink fluorescence may be seen.3,4,6 The Wood lamp examination should be performed in a completely dark room after the lamp has been warmed up for about 1 minute; time should be allowed for the clinician’s vision to adapt to the dark.6 There are no data regarding the sensitivity or specificity of the Wood lamp test in the diagnosis of PCT.
These conditions also cause skin fragility and photosensitivity
The differential diagnosis for PCT includes diseases that also cause skin fragility, blistering, or photosensitivity, such as pseudoporphyria, bullous systemic lupus erythematosus (SLE), and epidermolysis bullosa acquisita (EBA).3
Continue to: In pseudoporphyria
In pseudoporphyria, the clinical findings may be indistinguishable from PCT. Thus, the patient’s history will be especially important; suspect pseudoporphyria if the patient has a history of chronic renal failure or use of a photosensitizing drug.1,3
Bullous SLE usually manifests with systemic involvement and widespread, tense bullae. Serologic investigation will demonstrate the presence of antinuclear antibodies in high titers (> 1:80), as well as other circulating autoantibodies.
Skin lesions of EBA usually manifest with skin fragility and noninflammatory tense bullae in traumatized skin, such as the extensor surfaces of the hands, feet, and fingers.
None of the above-mentioned diagnoses manifest with hypertrichosis or red-pink fluorescent urine on Wood lamp, and results of porphyrin studies would be normal.3
Address triggers, provide treatment
Once the diagnosis is confirmed, steps must be taken to avoid triggering factors, such as any alcohol consumption, use of estrogen, sun exposure (until plasma porphyrin levels are normal), and potential sources of excessive iron intake.
Two therapeutic options are available for treating PCT—whether it’s sporadic or familial. Phlebotomy sessions reduce iron overload and iron depletion and may prevent the formation of a porphomethene inhibitor of UROD. The other treatment option is antimalarial agents—usually hydroxychloroquine— and is indicated for patients with lower serum ferritin levels.1-4 In patients with HCV-associated PCT, effective treatment of the infection has resulted in resolution of the PCT, in some cases.3
Treatment involving phlebotomy or an antimalarial agent can be stopped when plasma porphyrins reach normal levels.
Our patient was initially managed with 2 sessions of phlebotomy. He subsequently received treatment for the HCV infection at another hospital.
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
1. Handler NS, Handler MZ, Stephany MP, et. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:e106-e117.doi: 10.1111/ijd.13580
2. Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin Liver Dis. 2007;27:99-108.doi: 10.1055/s-2006-960173
3. Callen JP. Hepatitis C viral infection and porphyria cutanea tarda. Am J Med Sci. 2017;354:5-6. doi: 10.1016/j.amjms.2017.06.009
4. Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi: 10.1016/j.bpg.2010.07.002
5. Gisbert JP, García-Buey L, Pajares JM, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627.doi: 10.1016/s0168-8278(03)00346-5
6. Asawanonda P, Taylor CR. Wood’s light in dermatology. Int J Dermatol. 1999;38:801-807. doi: 10.1046/j.1365-4362.1999.00794.x
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.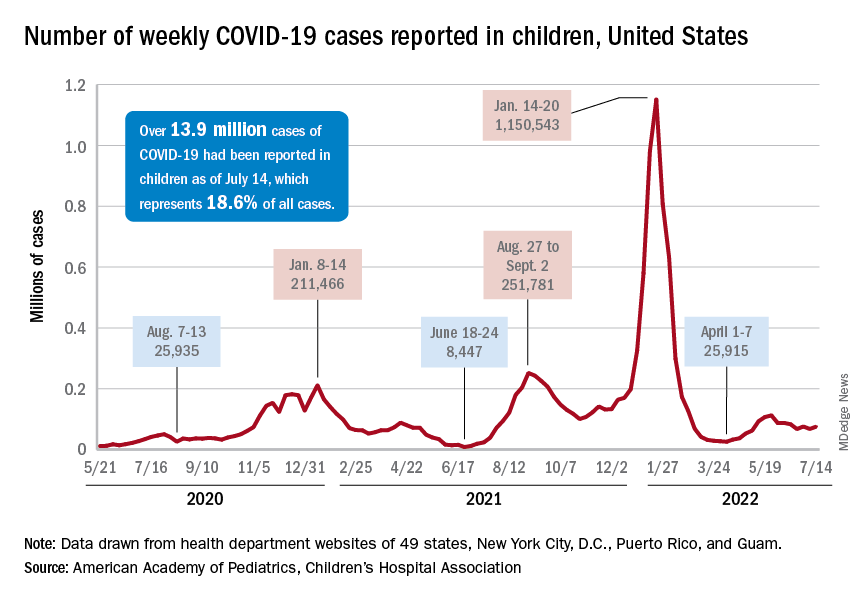
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.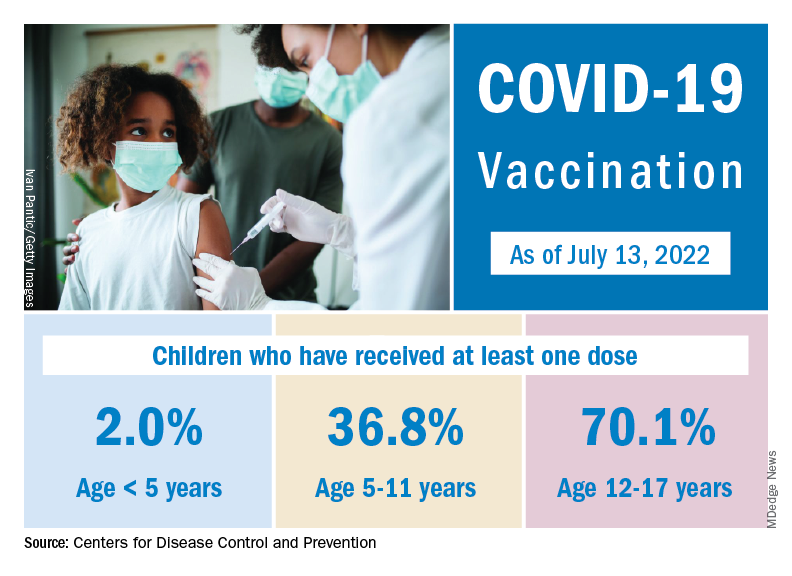
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
CDC warns about potentially deadly virus in infants
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.