User login
Landmark ALLIANCE results offer tenofovir guidance in HIV/HBV coinfection
MONTREAL – Interim results of ALLIANCE, the first head-to-head trial comparing two different tenofovir-containing antiretroviral regimens for the treatment of HIV and hepatitis B (HBV) coinfection, demonstrate the superiority of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) over dolutegravir plus tenofovir disoproxil fumarate (DTG + F/TDF), researchers reported at a meeting of the International AIDS Society.
, with more HBV DNA suppression and significantly more seroconversion, reported lead investigator Anchalee Avihingsanon, MD, PhD, at a press conference during the meeting. Dr. Avihingsanon heads the medical department of the HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT) at the Thai Red Cross AIDS Research Centre, Bangkok.
The ongoing phase 3, multicountry study has 48-week results for 243 participants, who were HIV/HBV coinfected and treatment naive. All subjects received three pills of ART per day, with blinded randomization to (active B/F/TAF + placebo DTG + placebo TDF/FTC or placebo B/F/TAF + active DTG + active TDF/FTC). The primary endpoints at 48 weeks were proportion of participants with HIV-1 RNA less than 50 copies/mL and plasma HBV DNA less than 29 IU/mL.
For the HIV endpoint, results showed both the B/F/TAF and DTG + F/TDF arms had high rates of suppression (95% and 91%, respectively, P = .21), but the B/F/TAF group had significantly higher rates of HBV DNA suppression (63% vs 43.4%, P = .0023) and HBeAg seroconversion (23.3% vs. 11.3%), with numerically higher, but not statistically significant differences in HBsAg loss/seroconversion (12.6% vs. 5.8% and 8.4% vs. 3.3%), HBeAg loss (25.6% vs 14.4%), and ALT normalization (73.3% vs 55.3%).
No participant developed treatment-emergent HIV-1 drug resistance while on B/F/TAF, and there were few study-drug–related AEs or discontinuations, she reported.
“There is hardly any good reason to give the two-pill DTG regimen over single-tablet BTG/TAF/FTC in HBV-coinfected people living with HIV [PLWH],” commented Babafemi Taiwo, MD, chief of infectious diseases and professor of medicine at Northwestern University in Evanston, Ill., who was not involved in the research. “This gives me confidence to prescribe bictegravir/TAF/FTC, which has the added advantage of being a single-tablet formulation, to HBV coinfected PLWH,” he said in an interview. However, he added, the results “call for some head-scratching since TAF is not known to be better than TDF for HBV treatment in persons without HIV.”
“The lower response rate of the TDF group is still poorly understood,” agreed Dr. Avihingsanon, emphasizing that “HBV and HIV/HBV are not the same, and TDF and TAF are also different. TAF has slightly more drug-drug interactions than TDF. I guess its end product in the liver might be higher. What is exciting to me is that there was such a high rate of HBsAg loss and HBs seroconversion in HIV/HBV coinfection, which is totally different from HBV monoinfection [< 1% at 48 weeks]. For me as an investigator, this important finding has additional benefit to further explore the immunologic outcome for possible HBV cure strategy.” She said the study remains blinded until week 96, at which time further data may shed light on this question.
“Perhaps a larger study would help clarify impact of TAF versus TDF on measures that did not achieve statistical significance in this study. Long-term follow up to better understand the clinical implications of these results could be helpful as well,” Dr. Taiwo added.
The study was funded by Gilead. Dr. Avihingsanon reported no relevant disclosures. Dr. Taiwo disclosed that he has served as consultant to ViiV/GlaxoSmithKline, Johnson & Johnson, and Merck, and consulted for Gilead on COVID.
A version of this article first appeared on Medscape.com.
MONTREAL – Interim results of ALLIANCE, the first head-to-head trial comparing two different tenofovir-containing antiretroviral regimens for the treatment of HIV and hepatitis B (HBV) coinfection, demonstrate the superiority of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) over dolutegravir plus tenofovir disoproxil fumarate (DTG + F/TDF), researchers reported at a meeting of the International AIDS Society.
, with more HBV DNA suppression and significantly more seroconversion, reported lead investigator Anchalee Avihingsanon, MD, PhD, at a press conference during the meeting. Dr. Avihingsanon heads the medical department of the HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT) at the Thai Red Cross AIDS Research Centre, Bangkok.
The ongoing phase 3, multicountry study has 48-week results for 243 participants, who were HIV/HBV coinfected and treatment naive. All subjects received three pills of ART per day, with blinded randomization to (active B/F/TAF + placebo DTG + placebo TDF/FTC or placebo B/F/TAF + active DTG + active TDF/FTC). The primary endpoints at 48 weeks were proportion of participants with HIV-1 RNA less than 50 copies/mL and plasma HBV DNA less than 29 IU/mL.
For the HIV endpoint, results showed both the B/F/TAF and DTG + F/TDF arms had high rates of suppression (95% and 91%, respectively, P = .21), but the B/F/TAF group had significantly higher rates of HBV DNA suppression (63% vs 43.4%, P = .0023) and HBeAg seroconversion (23.3% vs. 11.3%), with numerically higher, but not statistically significant differences in HBsAg loss/seroconversion (12.6% vs. 5.8% and 8.4% vs. 3.3%), HBeAg loss (25.6% vs 14.4%), and ALT normalization (73.3% vs 55.3%).
No participant developed treatment-emergent HIV-1 drug resistance while on B/F/TAF, and there were few study-drug–related AEs or discontinuations, she reported.
“There is hardly any good reason to give the two-pill DTG regimen over single-tablet BTG/TAF/FTC in HBV-coinfected people living with HIV [PLWH],” commented Babafemi Taiwo, MD, chief of infectious diseases and professor of medicine at Northwestern University in Evanston, Ill., who was not involved in the research. “This gives me confidence to prescribe bictegravir/TAF/FTC, which has the added advantage of being a single-tablet formulation, to HBV coinfected PLWH,” he said in an interview. However, he added, the results “call for some head-scratching since TAF is not known to be better than TDF for HBV treatment in persons without HIV.”
“The lower response rate of the TDF group is still poorly understood,” agreed Dr. Avihingsanon, emphasizing that “HBV and HIV/HBV are not the same, and TDF and TAF are also different. TAF has slightly more drug-drug interactions than TDF. I guess its end product in the liver might be higher. What is exciting to me is that there was such a high rate of HBsAg loss and HBs seroconversion in HIV/HBV coinfection, which is totally different from HBV monoinfection [< 1% at 48 weeks]. For me as an investigator, this important finding has additional benefit to further explore the immunologic outcome for possible HBV cure strategy.” She said the study remains blinded until week 96, at which time further data may shed light on this question.
“Perhaps a larger study would help clarify impact of TAF versus TDF on measures that did not achieve statistical significance in this study. Long-term follow up to better understand the clinical implications of these results could be helpful as well,” Dr. Taiwo added.
The study was funded by Gilead. Dr. Avihingsanon reported no relevant disclosures. Dr. Taiwo disclosed that he has served as consultant to ViiV/GlaxoSmithKline, Johnson & Johnson, and Merck, and consulted for Gilead on COVID.
A version of this article first appeared on Medscape.com.
MONTREAL – Interim results of ALLIANCE, the first head-to-head trial comparing two different tenofovir-containing antiretroviral regimens for the treatment of HIV and hepatitis B (HBV) coinfection, demonstrate the superiority of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) over dolutegravir plus tenofovir disoproxil fumarate (DTG + F/TDF), researchers reported at a meeting of the International AIDS Society.
, with more HBV DNA suppression and significantly more seroconversion, reported lead investigator Anchalee Avihingsanon, MD, PhD, at a press conference during the meeting. Dr. Avihingsanon heads the medical department of the HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT) at the Thai Red Cross AIDS Research Centre, Bangkok.
The ongoing phase 3, multicountry study has 48-week results for 243 participants, who were HIV/HBV coinfected and treatment naive. All subjects received three pills of ART per day, with blinded randomization to (active B/F/TAF + placebo DTG + placebo TDF/FTC or placebo B/F/TAF + active DTG + active TDF/FTC). The primary endpoints at 48 weeks were proportion of participants with HIV-1 RNA less than 50 copies/mL and plasma HBV DNA less than 29 IU/mL.
For the HIV endpoint, results showed both the B/F/TAF and DTG + F/TDF arms had high rates of suppression (95% and 91%, respectively, P = .21), but the B/F/TAF group had significantly higher rates of HBV DNA suppression (63% vs 43.4%, P = .0023) and HBeAg seroconversion (23.3% vs. 11.3%), with numerically higher, but not statistically significant differences in HBsAg loss/seroconversion (12.6% vs. 5.8% and 8.4% vs. 3.3%), HBeAg loss (25.6% vs 14.4%), and ALT normalization (73.3% vs 55.3%).
No participant developed treatment-emergent HIV-1 drug resistance while on B/F/TAF, and there were few study-drug–related AEs or discontinuations, she reported.
“There is hardly any good reason to give the two-pill DTG regimen over single-tablet BTG/TAF/FTC in HBV-coinfected people living with HIV [PLWH],” commented Babafemi Taiwo, MD, chief of infectious diseases and professor of medicine at Northwestern University in Evanston, Ill., who was not involved in the research. “This gives me confidence to prescribe bictegravir/TAF/FTC, which has the added advantage of being a single-tablet formulation, to HBV coinfected PLWH,” he said in an interview. However, he added, the results “call for some head-scratching since TAF is not known to be better than TDF for HBV treatment in persons without HIV.”
“The lower response rate of the TDF group is still poorly understood,” agreed Dr. Avihingsanon, emphasizing that “HBV and HIV/HBV are not the same, and TDF and TAF are also different. TAF has slightly more drug-drug interactions than TDF. I guess its end product in the liver might be higher. What is exciting to me is that there was such a high rate of HBsAg loss and HBs seroconversion in HIV/HBV coinfection, which is totally different from HBV monoinfection [< 1% at 48 weeks]. For me as an investigator, this important finding has additional benefit to further explore the immunologic outcome for possible HBV cure strategy.” She said the study remains blinded until week 96, at which time further data may shed light on this question.
“Perhaps a larger study would help clarify impact of TAF versus TDF on measures that did not achieve statistical significance in this study. Long-term follow up to better understand the clinical implications of these results could be helpful as well,” Dr. Taiwo added.
The study was funded by Gilead. Dr. Avihingsanon reported no relevant disclosures. Dr. Taiwo disclosed that he has served as consultant to ViiV/GlaxoSmithKline, Johnson & Johnson, and Merck, and consulted for Gilead on COVID.
A version of this article first appeared on Medscape.com.
AT AIDS 2022
Author Q&A: Intravenous Immunoglobulin for Treatment of COVID-19 in Select Patients
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Doxycycline cuts STI risk in men and trans women having sex with men
MONTREAL – (PrEP). The results of the open-label DoxyPEP trial were reported at a press conference at a meeting of the International AIDS Society.
“It is time to take action on the data that we have and really think about incorporating it into guidelines and rolling this out in a safe and thoughtful way,” said co-principal investigator Annie Luetkemeyer, MD, of Zuckerberg San Francisco General Hospital, and professor of medicine at the University of California, San Francisco (UCSF).
The open-label trial, conducted in Seattle and San Francisco, randomized MSM/TGW living with HIV or on PrEP, and with a history of N. gonorrhoeae (GC), C. trachomatis (CT), or early syphilis in the past year, to either doxycycline or none within 72 hours of having condomless sex. It was stopped early in May when a planned interim analysis showed those randomized to take doxycycline had substantially fewer STIs than participants assigned to the control group.
The intent-to-treat analysis included 501 patients with at least one quarter of follow-up: 327 taking PrEP and 174 living with HIV. Among those taking PrEP, new STIs (GC, CT or syphilis) occurred in 31.9% of control participants vs. 10.7% of those taking doxycycline – a reduction of 66% per quarter (P < .001). Among participants living with HIV, new STIs occurred in 30.5% of controls vs. 11.8% taking doxycycline, for a 62% reduction in STIs per quarter (P < .0001).
“Participants reported taking doxycycline 87% of the time after having condomless sex, about half of participants took fewer than 10 doses per month, 30% took 10-20 doses per month, and 16% took more than 20 doses of doxycycline per month,” said Dr. Luetkemeyer, adding that there were no serious – grade 2 or greater – adverse events, and “the majority of participants reported that taking doxy was acceptable or very acceptable.”
Asked how broadly doxycycline prophylaxis could be used in other populations, Dr. Luetkemeyer was cautious. “Our study participants had a very high rate of new STIs – a 30% incidence per quarter and using doxyPEP was well tolerated and very effective to reduce new STIs. However, this is a fairly limited population,” she said. “Whether doxyPEP should be considered for other groups, such as women on PrEP or with an elevated risk for STIs, will need more data which will be forthcoming from ongoing studies.”
Dr. Luetkemeyer said her group is looking at three possible risks of antibiotic resistance with the doxyPEP regimen: the risk to bystander bacteria such as Staphylococcus aureus or commensal neisseria; the impact on the gut; and the risk of resistance to antibiotic treatments for STI.
For the latter, “we don’t really think this is going to be an issue in chlamydia and syphilis, and we’re looking carefully at gonorrhea,” she said, adding that it will be challenging to get definitive data from this particular study because of its short follow-up.
“Available culture data from those who had gonorrhea infections during the study demonstrated a relatively low rate of tetracycline resistance, which is a proxy for doxycycline resistance, at 20%. ... However, larger studies and population-based surveillance of those taking doxycycline as PEP are needed to understand if doxycycline use could drive the element of tetracycline resistance in gonorrhea,” she said, emphasizing that doxycycline is not used to treat active gonorrhea infections.
Calling the doxyPEP regimen a “game-changing strategy,” Sharon Lewin, AO, PhD, president-elect of the International AIDS Society, said many physicians are already prescribing it off label based on the IPERGAY study (N Engl J Med. 2015; 373:2237-46) “but there’s a clear need for more evidence to guide the use of this intervention.”
“This study has huge implications for clinical care,” said Monica Gandhi, MD, MPH, an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at UCSF. “Although the data on drug resistance is very important to evaluate, we should certainly consider at this point using doxycycline PEP within 72 hours of condomless sex for our patients for STI prevention,” she said in an interview.
“In our practice, we are very excited about the possibility of a simple one-pill postexposure prophylactic agent (doxycycline 200 mg) to reduce the risk of a number of STIs. We have used PEP for HIV infection for a number of years and are very familiar with the concept of preventing infections after an exposure,” said Dr. Gandhi, director of the UCSF Center for AIDS Research and medical director of the HIV Clinic (“Ward 86”) at San Francisco General Hospital. “We are planning to institute doxycycline as PEP at my clinic after the release of these findings and will follow the remainder of the study findings closely.”
The trial was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, through grant R01AI143439. It was conducted at the HIV clinic at Zuckerberg San Francisco General Hospital and the San Francisco City Clinic, both part of the San Francisco Department of Public Health, and the Madison Clinic and the Sexual Health Clinic at Harborview Medical Center, both at the University of Washington. Medications were provided by Mayne Pharmaceuticals, and lab support by Hologic & Cepheid.
Dr. Lewin has the following disclosures: investigator-initiated, industry-funded research for Gilead, Viiv, Merck; scientific advisory board (honoraria paid to her personally) for Gilead, Merck, Viiv, Esfam, Immunocore, Vaxxinity; collaborative research (nonfunded) for AbbVie, Genentech, BMS. Dr. Luetkemeyer and Dr. Gandhi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – (PrEP). The results of the open-label DoxyPEP trial were reported at a press conference at a meeting of the International AIDS Society.
“It is time to take action on the data that we have and really think about incorporating it into guidelines and rolling this out in a safe and thoughtful way,” said co-principal investigator Annie Luetkemeyer, MD, of Zuckerberg San Francisco General Hospital, and professor of medicine at the University of California, San Francisco (UCSF).
The open-label trial, conducted in Seattle and San Francisco, randomized MSM/TGW living with HIV or on PrEP, and with a history of N. gonorrhoeae (GC), C. trachomatis (CT), or early syphilis in the past year, to either doxycycline or none within 72 hours of having condomless sex. It was stopped early in May when a planned interim analysis showed those randomized to take doxycycline had substantially fewer STIs than participants assigned to the control group.
The intent-to-treat analysis included 501 patients with at least one quarter of follow-up: 327 taking PrEP and 174 living with HIV. Among those taking PrEP, new STIs (GC, CT or syphilis) occurred in 31.9% of control participants vs. 10.7% of those taking doxycycline – a reduction of 66% per quarter (P < .001). Among participants living with HIV, new STIs occurred in 30.5% of controls vs. 11.8% taking doxycycline, for a 62% reduction in STIs per quarter (P < .0001).
“Participants reported taking doxycycline 87% of the time after having condomless sex, about half of participants took fewer than 10 doses per month, 30% took 10-20 doses per month, and 16% took more than 20 doses of doxycycline per month,” said Dr. Luetkemeyer, adding that there were no serious – grade 2 or greater – adverse events, and “the majority of participants reported that taking doxy was acceptable or very acceptable.”
Asked how broadly doxycycline prophylaxis could be used in other populations, Dr. Luetkemeyer was cautious. “Our study participants had a very high rate of new STIs – a 30% incidence per quarter and using doxyPEP was well tolerated and very effective to reduce new STIs. However, this is a fairly limited population,” she said. “Whether doxyPEP should be considered for other groups, such as women on PrEP or with an elevated risk for STIs, will need more data which will be forthcoming from ongoing studies.”
Dr. Luetkemeyer said her group is looking at three possible risks of antibiotic resistance with the doxyPEP regimen: the risk to bystander bacteria such as Staphylococcus aureus or commensal neisseria; the impact on the gut; and the risk of resistance to antibiotic treatments for STI.
For the latter, “we don’t really think this is going to be an issue in chlamydia and syphilis, and we’re looking carefully at gonorrhea,” she said, adding that it will be challenging to get definitive data from this particular study because of its short follow-up.
“Available culture data from those who had gonorrhea infections during the study demonstrated a relatively low rate of tetracycline resistance, which is a proxy for doxycycline resistance, at 20%. ... However, larger studies and population-based surveillance of those taking doxycycline as PEP are needed to understand if doxycycline use could drive the element of tetracycline resistance in gonorrhea,” she said, emphasizing that doxycycline is not used to treat active gonorrhea infections.
Calling the doxyPEP regimen a “game-changing strategy,” Sharon Lewin, AO, PhD, president-elect of the International AIDS Society, said many physicians are already prescribing it off label based on the IPERGAY study (N Engl J Med. 2015; 373:2237-46) “but there’s a clear need for more evidence to guide the use of this intervention.”
“This study has huge implications for clinical care,” said Monica Gandhi, MD, MPH, an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at UCSF. “Although the data on drug resistance is very important to evaluate, we should certainly consider at this point using doxycycline PEP within 72 hours of condomless sex for our patients for STI prevention,” she said in an interview.
“In our practice, we are very excited about the possibility of a simple one-pill postexposure prophylactic agent (doxycycline 200 mg) to reduce the risk of a number of STIs. We have used PEP for HIV infection for a number of years and are very familiar with the concept of preventing infections after an exposure,” said Dr. Gandhi, director of the UCSF Center for AIDS Research and medical director of the HIV Clinic (“Ward 86”) at San Francisco General Hospital. “We are planning to institute doxycycline as PEP at my clinic after the release of these findings and will follow the remainder of the study findings closely.”
The trial was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, through grant R01AI143439. It was conducted at the HIV clinic at Zuckerberg San Francisco General Hospital and the San Francisco City Clinic, both part of the San Francisco Department of Public Health, and the Madison Clinic and the Sexual Health Clinic at Harborview Medical Center, both at the University of Washington. Medications were provided by Mayne Pharmaceuticals, and lab support by Hologic & Cepheid.
Dr. Lewin has the following disclosures: investigator-initiated, industry-funded research for Gilead, Viiv, Merck; scientific advisory board (honoraria paid to her personally) for Gilead, Merck, Viiv, Esfam, Immunocore, Vaxxinity; collaborative research (nonfunded) for AbbVie, Genentech, BMS. Dr. Luetkemeyer and Dr. Gandhi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – (PrEP). The results of the open-label DoxyPEP trial were reported at a press conference at a meeting of the International AIDS Society.
“It is time to take action on the data that we have and really think about incorporating it into guidelines and rolling this out in a safe and thoughtful way,” said co-principal investigator Annie Luetkemeyer, MD, of Zuckerberg San Francisco General Hospital, and professor of medicine at the University of California, San Francisco (UCSF).
The open-label trial, conducted in Seattle and San Francisco, randomized MSM/TGW living with HIV or on PrEP, and with a history of N. gonorrhoeae (GC), C. trachomatis (CT), or early syphilis in the past year, to either doxycycline or none within 72 hours of having condomless sex. It was stopped early in May when a planned interim analysis showed those randomized to take doxycycline had substantially fewer STIs than participants assigned to the control group.
The intent-to-treat analysis included 501 patients with at least one quarter of follow-up: 327 taking PrEP and 174 living with HIV. Among those taking PrEP, new STIs (GC, CT or syphilis) occurred in 31.9% of control participants vs. 10.7% of those taking doxycycline – a reduction of 66% per quarter (P < .001). Among participants living with HIV, new STIs occurred in 30.5% of controls vs. 11.8% taking doxycycline, for a 62% reduction in STIs per quarter (P < .0001).
“Participants reported taking doxycycline 87% of the time after having condomless sex, about half of participants took fewer than 10 doses per month, 30% took 10-20 doses per month, and 16% took more than 20 doses of doxycycline per month,” said Dr. Luetkemeyer, adding that there were no serious – grade 2 or greater – adverse events, and “the majority of participants reported that taking doxy was acceptable or very acceptable.”
Asked how broadly doxycycline prophylaxis could be used in other populations, Dr. Luetkemeyer was cautious. “Our study participants had a very high rate of new STIs – a 30% incidence per quarter and using doxyPEP was well tolerated and very effective to reduce new STIs. However, this is a fairly limited population,” she said. “Whether doxyPEP should be considered for other groups, such as women on PrEP or with an elevated risk for STIs, will need more data which will be forthcoming from ongoing studies.”
Dr. Luetkemeyer said her group is looking at three possible risks of antibiotic resistance with the doxyPEP regimen: the risk to bystander bacteria such as Staphylococcus aureus or commensal neisseria; the impact on the gut; and the risk of resistance to antibiotic treatments for STI.
For the latter, “we don’t really think this is going to be an issue in chlamydia and syphilis, and we’re looking carefully at gonorrhea,” she said, adding that it will be challenging to get definitive data from this particular study because of its short follow-up.
“Available culture data from those who had gonorrhea infections during the study demonstrated a relatively low rate of tetracycline resistance, which is a proxy for doxycycline resistance, at 20%. ... However, larger studies and population-based surveillance of those taking doxycycline as PEP are needed to understand if doxycycline use could drive the element of tetracycline resistance in gonorrhea,” she said, emphasizing that doxycycline is not used to treat active gonorrhea infections.
Calling the doxyPEP regimen a “game-changing strategy,” Sharon Lewin, AO, PhD, president-elect of the International AIDS Society, said many physicians are already prescribing it off label based on the IPERGAY study (N Engl J Med. 2015; 373:2237-46) “but there’s a clear need for more evidence to guide the use of this intervention.”
“This study has huge implications for clinical care,” said Monica Gandhi, MD, MPH, an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at UCSF. “Although the data on drug resistance is very important to evaluate, we should certainly consider at this point using doxycycline PEP within 72 hours of condomless sex for our patients for STI prevention,” she said in an interview.
“In our practice, we are very excited about the possibility of a simple one-pill postexposure prophylactic agent (doxycycline 200 mg) to reduce the risk of a number of STIs. We have used PEP for HIV infection for a number of years and are very familiar with the concept of preventing infections after an exposure,” said Dr. Gandhi, director of the UCSF Center for AIDS Research and medical director of the HIV Clinic (“Ward 86”) at San Francisco General Hospital. “We are planning to institute doxycycline as PEP at my clinic after the release of these findings and will follow the remainder of the study findings closely.”
The trial was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, through grant R01AI143439. It was conducted at the HIV clinic at Zuckerberg San Francisco General Hospital and the San Francisco City Clinic, both part of the San Francisco Department of Public Health, and the Madison Clinic and the Sexual Health Clinic at Harborview Medical Center, both at the University of Washington. Medications were provided by Mayne Pharmaceuticals, and lab support by Hologic & Cepheid.
Dr. Lewin has the following disclosures: investigator-initiated, industry-funded research for Gilead, Viiv, Merck; scientific advisory board (honoraria paid to her personally) for Gilead, Merck, Viiv, Esfam, Immunocore, Vaxxinity; collaborative research (nonfunded) for AbbVie, Genentech, BMS. Dr. Luetkemeyer and Dr. Gandhi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AIDS 2022
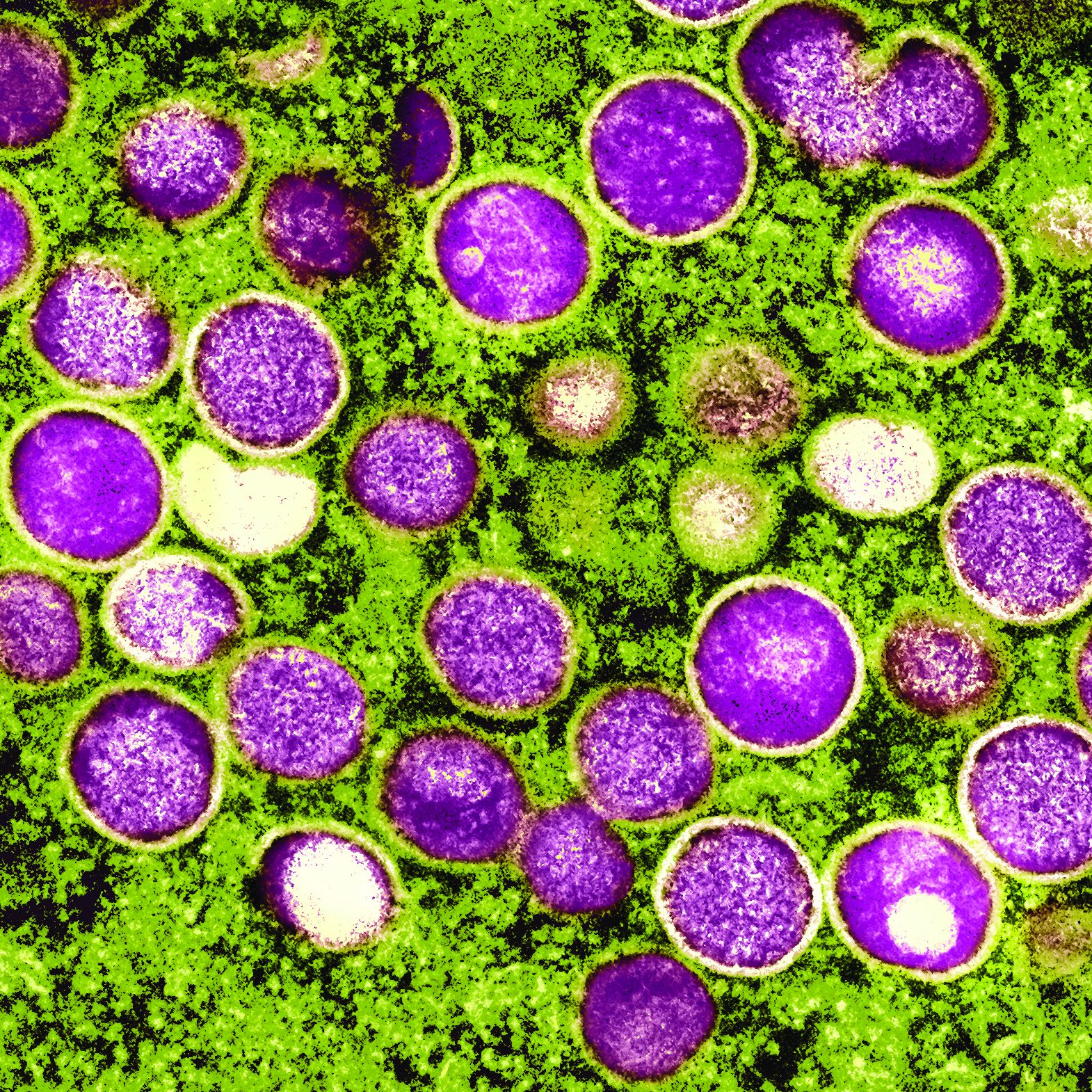
U.S. clears 786,000 monkeypox vaccine doses for distribution
More than 780,000 doses of the JYNNEOS monkeypox vaccine will be available in the United States beginning July 29, the Department of Health & Human Services announced on July 28 in a press call.
HHS Secretary Xavier Becerra urged local and state public health departments to use these doses for preventive vaccination efforts to stay ahead of the virus and end the outbreak, noting that the HHS and Centers for Disease Control and Prevention do not control how vaccines are distributed at state and local levels. “We don’t have the authority to tell them what to do,” he said during the call. “We need them to work with us.”
As of July 28, there were 4,907 reported cases of monkeypox in the United States and officials expect cases will continue to rise in the coming weeks.
The vaccine is manufactured by the small Danish company Bavarian Nordic. These additional 786,000 doses were previously stored at a plant in Denmark, awaiting the completion of an inspection and authorization of the vaccine plant by the Food and Drug Administration. The agency announced on July 27 that both the vaccine doses and the manufacturing plant met standards.
With the announcement of these additional doses, the vaccine allocation plan is also being updated to take into account two important factors: the number of people at high risk in a jurisdiction and the number of new cases reported since the last vaccine allocation.
“This update gives greater weight to prioritizing vaccines to areas with the greatest number of people at risk, which includes men who have sex with men who have HIV or who are eligible for HIV pre-exposure prophylaxis, while still considering where we are seeing cases increase,” said Capt. Jennifer McQuiston, DVM, deputy director of the division of high consequence pathogens and pathology at the CDC.
Capt.McQuiston also provided additional demographic information on the U.S. outbreak. The median age of people with confirmed cases is 35 years old, with a range from 17 to 76. (This does not include the two cases in children reported on July 22.) Of the cases where sex at birth was provided, 99% were individuals assigned male sex at birth. In cases with reported ethnicity and race, 37% were non-Hispanic White people, 31% were Hispanic/Latino, 27% were Black or African American, and 4% were of Asian descent. The most common symptoms were rash – present in 99% of cases – malaise, fever, and swollen lymph nodes.
HHS and CDC did not have data on how many people have received at least one dose of the monkeypox vaccine. When asked how many people need to be fully vaccinated against monkeypox to contain the outbreak, Mr. Becerra did not provide an estimate but implied that preventive vaccination could help limit the number of vaccines needed and expressed optimism about quelling the outbreak in the United States. “We believe that we have done everything we can at the federal level to work with our state and local partners and communities affected to make sure we can stay ahead of this and end this outbreak,” he said, “but everybody’s got to do their part.”
A version of this article first appeared on Medscape.com.
More than 780,000 doses of the JYNNEOS monkeypox vaccine will be available in the United States beginning July 29, the Department of Health & Human Services announced on July 28 in a press call.
HHS Secretary Xavier Becerra urged local and state public health departments to use these doses for preventive vaccination efforts to stay ahead of the virus and end the outbreak, noting that the HHS and Centers for Disease Control and Prevention do not control how vaccines are distributed at state and local levels. “We don’t have the authority to tell them what to do,” he said during the call. “We need them to work with us.”
As of July 28, there were 4,907 reported cases of monkeypox in the United States and officials expect cases will continue to rise in the coming weeks.
The vaccine is manufactured by the small Danish company Bavarian Nordic. These additional 786,000 doses were previously stored at a plant in Denmark, awaiting the completion of an inspection and authorization of the vaccine plant by the Food and Drug Administration. The agency announced on July 27 that both the vaccine doses and the manufacturing plant met standards.
With the announcement of these additional doses, the vaccine allocation plan is also being updated to take into account two important factors: the number of people at high risk in a jurisdiction and the number of new cases reported since the last vaccine allocation.
“This update gives greater weight to prioritizing vaccines to areas with the greatest number of people at risk, which includes men who have sex with men who have HIV or who are eligible for HIV pre-exposure prophylaxis, while still considering where we are seeing cases increase,” said Capt. Jennifer McQuiston, DVM, deputy director of the division of high consequence pathogens and pathology at the CDC.
Capt.McQuiston also provided additional demographic information on the U.S. outbreak. The median age of people with confirmed cases is 35 years old, with a range from 17 to 76. (This does not include the two cases in children reported on July 22.) Of the cases where sex at birth was provided, 99% were individuals assigned male sex at birth. In cases with reported ethnicity and race, 37% were non-Hispanic White people, 31% were Hispanic/Latino, 27% were Black or African American, and 4% were of Asian descent. The most common symptoms were rash – present in 99% of cases – malaise, fever, and swollen lymph nodes.
HHS and CDC did not have data on how many people have received at least one dose of the monkeypox vaccine. When asked how many people need to be fully vaccinated against monkeypox to contain the outbreak, Mr. Becerra did not provide an estimate but implied that preventive vaccination could help limit the number of vaccines needed and expressed optimism about quelling the outbreak in the United States. “We believe that we have done everything we can at the federal level to work with our state and local partners and communities affected to make sure we can stay ahead of this and end this outbreak,” he said, “but everybody’s got to do their part.”
A version of this article first appeared on Medscape.com.
More than 780,000 doses of the JYNNEOS monkeypox vaccine will be available in the United States beginning July 29, the Department of Health & Human Services announced on July 28 in a press call.
HHS Secretary Xavier Becerra urged local and state public health departments to use these doses for preventive vaccination efforts to stay ahead of the virus and end the outbreak, noting that the HHS and Centers for Disease Control and Prevention do not control how vaccines are distributed at state and local levels. “We don’t have the authority to tell them what to do,” he said during the call. “We need them to work with us.”
As of July 28, there were 4,907 reported cases of monkeypox in the United States and officials expect cases will continue to rise in the coming weeks.
The vaccine is manufactured by the small Danish company Bavarian Nordic. These additional 786,000 doses were previously stored at a plant in Denmark, awaiting the completion of an inspection and authorization of the vaccine plant by the Food and Drug Administration. The agency announced on July 27 that both the vaccine doses and the manufacturing plant met standards.
With the announcement of these additional doses, the vaccine allocation plan is also being updated to take into account two important factors: the number of people at high risk in a jurisdiction and the number of new cases reported since the last vaccine allocation.
“This update gives greater weight to prioritizing vaccines to areas with the greatest number of people at risk, which includes men who have sex with men who have HIV or who are eligible for HIV pre-exposure prophylaxis, while still considering where we are seeing cases increase,” said Capt. Jennifer McQuiston, DVM, deputy director of the division of high consequence pathogens and pathology at the CDC.
Capt.McQuiston also provided additional demographic information on the U.S. outbreak. The median age of people with confirmed cases is 35 years old, with a range from 17 to 76. (This does not include the two cases in children reported on July 22.) Of the cases where sex at birth was provided, 99% were individuals assigned male sex at birth. In cases with reported ethnicity and race, 37% were non-Hispanic White people, 31% were Hispanic/Latino, 27% were Black or African American, and 4% were of Asian descent. The most common symptoms were rash – present in 99% of cases – malaise, fever, and swollen lymph nodes.
HHS and CDC did not have data on how many people have received at least one dose of the monkeypox vaccine. When asked how many people need to be fully vaccinated against monkeypox to contain the outbreak, Mr. Becerra did not provide an estimate but implied that preventive vaccination could help limit the number of vaccines needed and expressed optimism about quelling the outbreak in the United States. “We believe that we have done everything we can at the federal level to work with our state and local partners and communities affected to make sure we can stay ahead of this and end this outbreak,” he said, “but everybody’s got to do their part.”
A version of this article first appeared on Medscape.com.
Prolonged remission in patient with HIV may open new avenues to functional cure
MONTREAL – The case of a patient in an HIV study whose viral load dropped to undetectable levels and whose immune cells soared has captured the attention of organizers at a meeting of the International AIDS Society.
Although the 59-year-old woman is one of many who are known as posttreatment controllers (PTCs) – having been in remission for more than 15 years after stopping antiretroviral therapy (ART) –
“This case opens new avenues in the HIV functional-cure field,” lead investigator Núria Climent, PhD, of the HIV unit at Hospital Clinic-IDIBAPS/University of Barcelona, told this news organization.
“As far as we know, this is the first time that the gamma-delta T cells have been identified in a PTC, and concerning the memory-like NK cells, there are very few published data and only sparse information presented in several congresses,” she said, explaining that these cells “have a high capacity to inhibit the replication of the virus in vitro. For that reason, we think that this PTC has cells able to dramatically reduce the virus amount. We think that the potential capacity to increase these cells in this PTC woman could be not only mediated by especial genetic factors ... but also mediated by early ART treatment and might be by the immunomediated treatment.”
The findings suggest the potential for “increasing the amount of those memory-like NK cells and gamma-delta T cells in order to translate this potent antiviral activity in new therapies to achieve an HIV functional cure,” she said, adding: “As far as we know, aiming to increase these specific cells has never been done before in people living with HIV.”
In a press conference during the meeting, Dr. Climent explained that the patient was enrolled in a study in which she received a combination of ART and immunomodulatory therapy. This involved a combination of cyclosporine A, low-dose interleukin 2, granulocyte macrophage colony-stimulating factor, and pegylated interferon alfa-2b.
“None of the other 19 patients included in the trial controlled viral replication,” senior investigator Jose Miro, MD, PhD, also from the HIV unit at Hospital Clinic-IDIBAPS/University of Barcelona, told this news organization.
Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the conference, said in an interview that although the significance of the case is unclear, the IAS selected it as a highlight for the meeting. “It is important for clinicians to understand the complexities in interpreting these case reports. Their patients are probably likely to ask them about the report, and it’s important [that] they can explain it to them.”
Dr. Lewin, who is professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity in Melbourne, added that it is impossible to determine the mechanism of action from a single case report. “We don’t know if the intervention played a role or if this person is a ‘posttreatment controller,’ which has been previously described many times,” she said in an interview. “In this patient, the virus is at very low, but controlled, levels, and virus could be grown out. While it’s still exciting and important, this is really what we would consider a remission. The intense study of a single case such as this is certainly worthwhile and important but can only provide new ideas for research. So, I don’t think we can draw any conclusion on the role of NK cells, et cetera. We need much larger case series or controlled trials to reach any conclusion on the reasons for her remission.”
Dr. Climent disclosed no relevant financial conflicts of interest. Dr. Lewin has disclosed investigator-initiated industry-funded research (Gilead, ViiV, Merck), scientific advisory board honoraria paid to her personally (Gilead, Merck, ViiV, Esfam, Immunocore, Vaxxinity), and nonfunded collaborative research (AbbVie, Genentech, Bristol-Myers Squibb).
A version of this article first appeared on Medscape.com.
MONTREAL – The case of a patient in an HIV study whose viral load dropped to undetectable levels and whose immune cells soared has captured the attention of organizers at a meeting of the International AIDS Society.
Although the 59-year-old woman is one of many who are known as posttreatment controllers (PTCs) – having been in remission for more than 15 years after stopping antiretroviral therapy (ART) –
“This case opens new avenues in the HIV functional-cure field,” lead investigator Núria Climent, PhD, of the HIV unit at Hospital Clinic-IDIBAPS/University of Barcelona, told this news organization.
“As far as we know, this is the first time that the gamma-delta T cells have been identified in a PTC, and concerning the memory-like NK cells, there are very few published data and only sparse information presented in several congresses,” she said, explaining that these cells “have a high capacity to inhibit the replication of the virus in vitro. For that reason, we think that this PTC has cells able to dramatically reduce the virus amount. We think that the potential capacity to increase these cells in this PTC woman could be not only mediated by especial genetic factors ... but also mediated by early ART treatment and might be by the immunomediated treatment.”
The findings suggest the potential for “increasing the amount of those memory-like NK cells and gamma-delta T cells in order to translate this potent antiviral activity in new therapies to achieve an HIV functional cure,” she said, adding: “As far as we know, aiming to increase these specific cells has never been done before in people living with HIV.”
In a press conference during the meeting, Dr. Climent explained that the patient was enrolled in a study in which she received a combination of ART and immunomodulatory therapy. This involved a combination of cyclosporine A, low-dose interleukin 2, granulocyte macrophage colony-stimulating factor, and pegylated interferon alfa-2b.
“None of the other 19 patients included in the trial controlled viral replication,” senior investigator Jose Miro, MD, PhD, also from the HIV unit at Hospital Clinic-IDIBAPS/University of Barcelona, told this news organization.
Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the conference, said in an interview that although the significance of the case is unclear, the IAS selected it as a highlight for the meeting. “It is important for clinicians to understand the complexities in interpreting these case reports. Their patients are probably likely to ask them about the report, and it’s important [that] they can explain it to them.”
Dr. Lewin, who is professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity in Melbourne, added that it is impossible to determine the mechanism of action from a single case report. “We don’t know if the intervention played a role or if this person is a ‘posttreatment controller,’ which has been previously described many times,” she said in an interview. “In this patient, the virus is at very low, but controlled, levels, and virus could be grown out. While it’s still exciting and important, this is really what we would consider a remission. The intense study of a single case such as this is certainly worthwhile and important but can only provide new ideas for research. So, I don’t think we can draw any conclusion on the role of NK cells, et cetera. We need much larger case series or controlled trials to reach any conclusion on the reasons for her remission.”
Dr. Climent disclosed no relevant financial conflicts of interest. Dr. Lewin has disclosed investigator-initiated industry-funded research (Gilead, ViiV, Merck), scientific advisory board honoraria paid to her personally (Gilead, Merck, ViiV, Esfam, Immunocore, Vaxxinity), and nonfunded collaborative research (AbbVie, Genentech, Bristol-Myers Squibb).
A version of this article first appeared on Medscape.com.
MONTREAL – The case of a patient in an HIV study whose viral load dropped to undetectable levels and whose immune cells soared has captured the attention of organizers at a meeting of the International AIDS Society.
Although the 59-year-old woman is one of many who are known as posttreatment controllers (PTCs) – having been in remission for more than 15 years after stopping antiretroviral therapy (ART) –
“This case opens new avenues in the HIV functional-cure field,” lead investigator Núria Climent, PhD, of the HIV unit at Hospital Clinic-IDIBAPS/University of Barcelona, told this news organization.
“As far as we know, this is the first time that the gamma-delta T cells have been identified in a PTC, and concerning the memory-like NK cells, there are very few published data and only sparse information presented in several congresses,” she said, explaining that these cells “have a high capacity to inhibit the replication of the virus in vitro. For that reason, we think that this PTC has cells able to dramatically reduce the virus amount. We think that the potential capacity to increase these cells in this PTC woman could be not only mediated by especial genetic factors ... but also mediated by early ART treatment and might be by the immunomediated treatment.”
The findings suggest the potential for “increasing the amount of those memory-like NK cells and gamma-delta T cells in order to translate this potent antiviral activity in new therapies to achieve an HIV functional cure,” she said, adding: “As far as we know, aiming to increase these specific cells has never been done before in people living with HIV.”
In a press conference during the meeting, Dr. Climent explained that the patient was enrolled in a study in which she received a combination of ART and immunomodulatory therapy. This involved a combination of cyclosporine A, low-dose interleukin 2, granulocyte macrophage colony-stimulating factor, and pegylated interferon alfa-2b.
“None of the other 19 patients included in the trial controlled viral replication,” senior investigator Jose Miro, MD, PhD, also from the HIV unit at Hospital Clinic-IDIBAPS/University of Barcelona, told this news organization.
Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the conference, said in an interview that although the significance of the case is unclear, the IAS selected it as a highlight for the meeting. “It is important for clinicians to understand the complexities in interpreting these case reports. Their patients are probably likely to ask them about the report, and it’s important [that] they can explain it to them.”
Dr. Lewin, who is professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity in Melbourne, added that it is impossible to determine the mechanism of action from a single case report. “We don’t know if the intervention played a role or if this person is a ‘posttreatment controller,’ which has been previously described many times,” she said in an interview. “In this patient, the virus is at very low, but controlled, levels, and virus could be grown out. While it’s still exciting and important, this is really what we would consider a remission. The intense study of a single case such as this is certainly worthwhile and important but can only provide new ideas for research. So, I don’t think we can draw any conclusion on the role of NK cells, et cetera. We need much larger case series or controlled trials to reach any conclusion on the reasons for her remission.”
Dr. Climent disclosed no relevant financial conflicts of interest. Dr. Lewin has disclosed investigator-initiated industry-funded research (Gilead, ViiV, Merck), scientific advisory board honoraria paid to her personally (Gilead, Merck, ViiV, Esfam, Immunocore, Vaxxinity), and nonfunded collaborative research (AbbVie, Genentech, Bristol-Myers Squibb).
A version of this article first appeared on Medscape.com.
AT AIDS 2022
Potentially deadly bacteria detected in U.S. soil
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
Pandemic tied to misdiagnosis of rare pneumonia
Psittacosis, a rare disease, has been underdiagnosed or misdiagnosed during the COVID-19 pandemic, likely because the symptoms of the disease are similar to COVID-19 symptoms, researchers suggest on the basis of data from 32 individuals.
Diagnosis of and screening for COVID-19 continues to increase; however, cases of atypical pneumonia caused by uncommon pathogens, which presents with similar symptoms, may be missed, wrote Qiaoqiao Yin, MS, of Zhejiang Provincial People’s Hospital, China, and colleagues.
“The clinical manifestations of human psittacosis can present as rapidly progressing severe pneumonia, acute respiratory distress syndrome, sepsis, and multiple organ failure,” but human cases have not been well studied, they say.
In a study published in the International Journal of Infectious Diseases, the researchers reviewed data from 32 adults diagnosed with Chlamydia psittaci pneumonia during the COVID-19 pandemic between April 2020 and June 2021 in China. The median age of the patients was 63 years, 20 were men, and 20 had underlying diseases.
A total of 17 patients presented with fever, cough, and expectoration of yellow-white sputum. At the time of hospital admission, three patients had myalgia, two had headache, and two had hypertension. The patients were originally suspected of having COVID-19.
all of which could be observed in COVID-19 patients as well, the researchers wrote.
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing were used to rule out COVID-19. The researchers then used metagenomic next-generation sequencing (mNGS) to identify the disease-causing pathogens. They collected 18 bronchoalveolar lavage fluid (BALF) samples, 9 peripheral blood samples, and 5 sputum samples. The mNGS identified C. psittaci as the suspected pathogen within 48 hours. Suspected C. psittaci infections were confirmed by endpoint PCR for the BALF and sputum samples and six of nine blood samples, “indicating a lower sensitivity of PCR compared to mNGS for blood samples,” the researchers say. No other potential pathogens were identified.
Psittacosis is common in birds but is rare in humans. C. psittaci is responsible for 1%-8% of cases involving community-acquired pneumonia in China, the researchers note. Although poultry is a source of infection, 25 of the patients in the study did not report a history of exposure to poultry or pigeons at the time of their initial hospital admission. Many patients may be unaware of exposures to poultry, which further complicates the C. psittaci diagnosis, they note.
All patients were treated with doxycycline-based regimens and showed improvement.
The findings were limited by several factors, including the lack of a definitive diagnostic tool for C. psittaci and the lack of convalescent serum samples to confirm cases, the researchers note. In addition, molecular detections for PCR are unavailable in most hospitals in China, they say. The results represent the largest known collection of suspected C. psittaci pneumonia cases and highlight the need for clinician vigilance and awareness of this rare condition, especially in light of the potential for misdiagnosis during the ongoing COVID-19 pandemic, they conclude.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psittacosis, a rare disease, has been underdiagnosed or misdiagnosed during the COVID-19 pandemic, likely because the symptoms of the disease are similar to COVID-19 symptoms, researchers suggest on the basis of data from 32 individuals.
Diagnosis of and screening for COVID-19 continues to increase; however, cases of atypical pneumonia caused by uncommon pathogens, which presents with similar symptoms, may be missed, wrote Qiaoqiao Yin, MS, of Zhejiang Provincial People’s Hospital, China, and colleagues.
“The clinical manifestations of human psittacosis can present as rapidly progressing severe pneumonia, acute respiratory distress syndrome, sepsis, and multiple organ failure,” but human cases have not been well studied, they say.
In a study published in the International Journal of Infectious Diseases, the researchers reviewed data from 32 adults diagnosed with Chlamydia psittaci pneumonia during the COVID-19 pandemic between April 2020 and June 2021 in China. The median age of the patients was 63 years, 20 were men, and 20 had underlying diseases.
A total of 17 patients presented with fever, cough, and expectoration of yellow-white sputum. At the time of hospital admission, three patients had myalgia, two had headache, and two had hypertension. The patients were originally suspected of having COVID-19.
all of which could be observed in COVID-19 patients as well, the researchers wrote.
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing were used to rule out COVID-19. The researchers then used metagenomic next-generation sequencing (mNGS) to identify the disease-causing pathogens. They collected 18 bronchoalveolar lavage fluid (BALF) samples, 9 peripheral blood samples, and 5 sputum samples. The mNGS identified C. psittaci as the suspected pathogen within 48 hours. Suspected C. psittaci infections were confirmed by endpoint PCR for the BALF and sputum samples and six of nine blood samples, “indicating a lower sensitivity of PCR compared to mNGS for blood samples,” the researchers say. No other potential pathogens were identified.
Psittacosis is common in birds but is rare in humans. C. psittaci is responsible for 1%-8% of cases involving community-acquired pneumonia in China, the researchers note. Although poultry is a source of infection, 25 of the patients in the study did not report a history of exposure to poultry or pigeons at the time of their initial hospital admission. Many patients may be unaware of exposures to poultry, which further complicates the C. psittaci diagnosis, they note.
All patients were treated with doxycycline-based regimens and showed improvement.
The findings were limited by several factors, including the lack of a definitive diagnostic tool for C. psittaci and the lack of convalescent serum samples to confirm cases, the researchers note. In addition, molecular detections for PCR are unavailable in most hospitals in China, they say. The results represent the largest known collection of suspected C. psittaci pneumonia cases and highlight the need for clinician vigilance and awareness of this rare condition, especially in light of the potential for misdiagnosis during the ongoing COVID-19 pandemic, they conclude.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psittacosis, a rare disease, has been underdiagnosed or misdiagnosed during the COVID-19 pandemic, likely because the symptoms of the disease are similar to COVID-19 symptoms, researchers suggest on the basis of data from 32 individuals.
Diagnosis of and screening for COVID-19 continues to increase; however, cases of atypical pneumonia caused by uncommon pathogens, which presents with similar symptoms, may be missed, wrote Qiaoqiao Yin, MS, of Zhejiang Provincial People’s Hospital, China, and colleagues.
“The clinical manifestations of human psittacosis can present as rapidly progressing severe pneumonia, acute respiratory distress syndrome, sepsis, and multiple organ failure,” but human cases have not been well studied, they say.
In a study published in the International Journal of Infectious Diseases, the researchers reviewed data from 32 adults diagnosed with Chlamydia psittaci pneumonia during the COVID-19 pandemic between April 2020 and June 2021 in China. The median age of the patients was 63 years, 20 were men, and 20 had underlying diseases.
A total of 17 patients presented with fever, cough, and expectoration of yellow-white sputum. At the time of hospital admission, three patients had myalgia, two had headache, and two had hypertension. The patients were originally suspected of having COVID-19.
all of which could be observed in COVID-19 patients as well, the researchers wrote.
Reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) testing were used to rule out COVID-19. The researchers then used metagenomic next-generation sequencing (mNGS) to identify the disease-causing pathogens. They collected 18 bronchoalveolar lavage fluid (BALF) samples, 9 peripheral blood samples, and 5 sputum samples. The mNGS identified C. psittaci as the suspected pathogen within 48 hours. Suspected C. psittaci infections were confirmed by endpoint PCR for the BALF and sputum samples and six of nine blood samples, “indicating a lower sensitivity of PCR compared to mNGS for blood samples,” the researchers say. No other potential pathogens were identified.
Psittacosis is common in birds but is rare in humans. C. psittaci is responsible for 1%-8% of cases involving community-acquired pneumonia in China, the researchers note. Although poultry is a source of infection, 25 of the patients in the study did not report a history of exposure to poultry or pigeons at the time of their initial hospital admission. Many patients may be unaware of exposures to poultry, which further complicates the C. psittaci diagnosis, they note.
All patients were treated with doxycycline-based regimens and showed improvement.
The findings were limited by several factors, including the lack of a definitive diagnostic tool for C. psittaci and the lack of convalescent serum samples to confirm cases, the researchers note. In addition, molecular detections for PCR are unavailable in most hospitals in China, they say. The results represent the largest known collection of suspected C. psittaci pneumonia cases and highlight the need for clinician vigilance and awareness of this rare condition, especially in light of the potential for misdiagnosis during the ongoing COVID-19 pandemic, they conclude.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Linezolid succeeds against gram-positive bacterial infections in ICU
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Approximately 80% of patients in intensive care showed clinical improvement in gram-positive bacterial infections after treatment with linezolid, based on data from more than 300 individuals.
Bacterial infections remain a challenge in the management of critically ill patients, as many gram-positive pathogens have become resistant to multiple drug options, Aijia Ma, MD, of West China Hospital of Sichuan University, and colleagues wrote.
Linezolid has demonstrated effectiveness against MRSA and skin and soft-tissue infections (SSTIs), but its use in critically ill patients with gram-positive infections in the ICU has not been characterized, they said.
In a multicenter, real-world study published in the Journal of Intensive Medicine, the researchers reviewed data from 52 hospitals between June 2018 and December 2019. The study population included 366 patients admitted to the ICU with a clinical or laboratory diagnosis of a gram-positive bacterial infection. Patients were treated with linezolid injections (200 mg/100 mL) and followed up once a day until 48 hours after discontinuing therapy, transferring out of the ICU, or death. Most of the patients (243) were older than 65 years; 90 were aged 18-65 years, and 30 were younger than 18 years. Approximately two-thirds (67%) were men. The primary outcome of clinical efficacy was success (cured or improved).
Linezolid was used as second-line and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (31% MRSA; 12.6% methicillin-susceptible S. aureus [MSSA]) followed by Enterococci (6.7% vancomycin resistant, 9.2% vancomycin susceptible) and Streptococcus pneumoniae (3.4% multidrug resistant, 1.7% non–multidrug resistant).
Overall, 82.2% of patients met the criteria for clinical success; 34 (9.3%) were cured and 267 (73%) improved. Clinical success rates for first-line and second-line linezolid therapy were 79.9% and 83.6%, respectively. Failure rates for linezolid were higher for second-line versus first-line treatment (9.5% vs. 5.2%).
The clinical success rate was highest against MSSA (93.3%), followed by MRSA (83.8%). The average daily linezolid dose was 1,109 mg, and the mean treatment time was 5.1 days.
A total of eight patients (2.2%) reported linezolid-related adverse events, and four patients discontinued the medication because of them; none reported treatment-related serious adverse events. The low incidence of thrombocytopenia in the current study (two patients), compared with previous studies may have been related to avoidance of linezolid for at-risk patients as determined by clinicians, and the relatively short duration of linezolid use, the researchers wrote.
The study findings were limited by several factors, including the observational design and inability to compare the efficacy of different drugs; the small sample size; and the lack of data on drugs used prior to ICU admission, the researchers noted. Other limitations included the low detection rate of gram-positive bacteria and potential underreporting of adverse events.
However, the although clinicians will need to pay close attention to possible side effects and evaluate patient conditions on an individual basis before using linezolid in the clinic, they concluded.
The study was supported by grants from West China Hospital of Sichuan University. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INTENSIVE MEDICINE
Single dose of HPV vaccine is ‘game changer,’ says WHO
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
To gauge monkeypox spread, researchers eye cases in women
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.