User login
Advanced training in hepatology
Unlike previous hepatologists, who were trained through gastroenterology programs, most new practitioners seek advanced training in a fellowship year focused exclusively on hepatology.
Like practitioners in many medical subspecialties, transplant hepatologists have varied career goals and responsibilities. Hepatologists who continue to specifically practice transplant hepatology are affiliated with a liver transplant center, which is generally a hospital-based practice. However, most hepatologists also treat nontransplant hepatology patients and some who have completed advanced hepatology training focus exclusively on these patients or provide community-based care for transplant recipients from other centers. Caring for patients with end-stage liver disease and liver transplant recipients can be clinically demanding but also very rewarding. There are also many opportunities for academic pursuits within a hepatology career including areas in urgent need of clinical and basic investigation, clinical trials for novel agents to treat common diseases, education (including leadership in advanced hepatology training), and involvement in professional societies such as the American Gastroenterological Association (AGA) and American Association for the Study of Liver Disease (AASLD).
What are the opportunities for advanced hepatology training?
In 1999, the AASLD determined that the practice of transplant hepatology required its own specialized knowledge and that most practicing gastroenterologists did not consider themselves adequately prepared to care for patients with advanced liver disease.1,2 The following year, the AASLD applied to the American Board of Internal Medicine (ABIM) to develop formalized liver transplant training. After several years of debate and development, the first ABIM certification exam in transplant hepatology was held in 2006 and is now offered every 2 years.2
There are currently three pathways to achieve advanced training in hepatology. The traditional pathway is a 1-year Accreditation Council for Graduate Medical Education (ACGME) transplant fellowship that is separate from, and must follow completion of, a gastroenterology fellowship. There are currently 51 ACGME-accredited 1-year transplant hepatology fellowships in the United States. These fellowships are only at institutions with ACGME-accredited training in internal medicine and gastroenterology as well. The full and updated list of programs can be found on the ACGME website.3 The second pathway is the relatively new ABIM “pilot” program during which the transplant hepatology fellowship year is combined with the third year of gastroenterology fellowship (discussed in detail below). Finally, there remain many 1-year training programs that are not ACGME-accredited, may not be associated with a gastroenterology fellowship program, and do have not regulated requirements for entry. Trainees who complete non-ACGME programs are not candidates for ABIM board certification.
How does one apply for transplant hepatology fellowship?
Transplant hepatology fellowships do not participate in a match system. Therefore, the interviews and offers for training spots may occur at different times depending on the program and the region of the country. In general, fellows apply by the fall of their second year of gastroenterology fellowship in order to begin training after graduating from the third year of fellowship. Each program has its individual approach to the application process and most have this information available on a website as to how to apply. A complete list of ACGME-accredited programs along with the program directors and contact information is available on the ABIM website.3
What is the gastroenterology/transplant hepatology pilot training program?
The AASLD and ABIM have developed a combined gastroenterology and transplant hepatology pilot fellowship training program that allows eligible gastroenterology fellows to spend their third year training in transplant hepatology. This approach has the potential to shorten the total training from 4 years to 3. In addition, if all gastroenterology and transplant hepatology competencies are achieved by the end of the third year, fellows approved to be in this program are eligible to take both gastroenterology and transplant hepatology ABIM certification exams.
Any ACGME-accredited gastroenterology fellowship program that has an accredited hepatology counterpart is eligible to participate in this pilot. Eligible programs and fellows must apply to AASLD during the fellow’s second year. The fellow applicant must complete all clinical gastroenterology requirements before the end of the second year of fellowship and be on a trajectory to meet competency milestones, as the majority of the third year will focus on hepatology.
Since 2012, 59 fellows from 31 programs have participated in this pilot program.4 If you are interested in participating in this pilot program at your institution, it is important to confer with program directors as early as possible to meet all training requirements. In addition, applications are submitted to the Pilot Steering Taskforce during the fellow’s second year for review. This is not meant to be a competitive process and all fellows who meet the criteria are approved.
This track may not be ideal for all fellows interested in advanced and transplant hepatology. In particular, there may be a trade-off between achieving clinical competency in a shortened training period and pursuing scholarly activity. This pilot program is designed to be an intensive clinical track, so fellows who wish to focus on research should discuss with their program directors whether this is the best approach.
What has been your career path after advanced training in hepatology?
I first became interested in hepatology during my inpatient rotations as a medical student. This interest led me to become involved in research in this area very early in my career. The current structure of the fellowship as well as the board certification exam were both developed while I was in training and I adjusted my plans to complete 3 years of gastroenterology fellowship followed by an ACGME-accredited liver transplant fellowship year. Since completing training, I have worked as an attending at an academic medical center in a large liver transplant program and continue to care for patients with all forms of liver disease. In addition, I continued to pursue research as a large component of my job and now have NIH funding and direct the Transplant Clinical Research Center at Columbia University. Finally, I have always been devoted to education and am the program director for the transplant hepatology fellowship at our institution.
What is the future of advanced hepatology training?
The current transplant hepatology training system has evolved significantly since its inception, including development of curricula, ongoing modification of training requirements, and the development of the innovative pilot program. However, there are issues that continue to be debated by the community. For example, it is not certain when or if the combined gastroenterology and transplant hepatology pilot program will become a permanent pathway for training or how best to select fellows for this approach.
Hepatology continues to be a very dynamic area of medicine. With diseases such as nonalcoholic fatty liver disease and hepatocellular carcinoma on the rise, the urgent need for training in HCV treatment to combat the global epidemic of viral hepatitis, and the growing number of patients on the liver transplant waiting list, there has never been a more exciting time to choose hepatology as a career.
References
1. Luxon BA. So you want to be a hepatologist? Gastroenterology. 2013;145(6):1182-5.
2. Bacon BR, Grosso LJ, Freedman N, Althouse LA. Subspecialty certification in transplant hepatology. Liver Transpl. 2007;13(11):1479-81.
3. https://apps.acgme.org/ads/public/reports/report/1.
4. https://www.aasld.org/events-professional-development/educational-learning-faq.
Dr. Verna is assistant professor of medicine, program director, transplant hepatology fellowship, director of clinical research, Transplant Clinical Research Center, Columbia University Medical Center, New York.
Unlike previous hepatologists, who were trained through gastroenterology programs, most new practitioners seek advanced training in a fellowship year focused exclusively on hepatology.
Like practitioners in many medical subspecialties, transplant hepatologists have varied career goals and responsibilities. Hepatologists who continue to specifically practice transplant hepatology are affiliated with a liver transplant center, which is generally a hospital-based practice. However, most hepatologists also treat nontransplant hepatology patients and some who have completed advanced hepatology training focus exclusively on these patients or provide community-based care for transplant recipients from other centers. Caring for patients with end-stage liver disease and liver transplant recipients can be clinically demanding but also very rewarding. There are also many opportunities for academic pursuits within a hepatology career including areas in urgent need of clinical and basic investigation, clinical trials for novel agents to treat common diseases, education (including leadership in advanced hepatology training), and involvement in professional societies such as the American Gastroenterological Association (AGA) and American Association for the Study of Liver Disease (AASLD).
What are the opportunities for advanced hepatology training?
In 1999, the AASLD determined that the practice of transplant hepatology required its own specialized knowledge and that most practicing gastroenterologists did not consider themselves adequately prepared to care for patients with advanced liver disease.1,2 The following year, the AASLD applied to the American Board of Internal Medicine (ABIM) to develop formalized liver transplant training. After several years of debate and development, the first ABIM certification exam in transplant hepatology was held in 2006 and is now offered every 2 years.2
There are currently three pathways to achieve advanced training in hepatology. The traditional pathway is a 1-year Accreditation Council for Graduate Medical Education (ACGME) transplant fellowship that is separate from, and must follow completion of, a gastroenterology fellowship. There are currently 51 ACGME-accredited 1-year transplant hepatology fellowships in the United States. These fellowships are only at institutions with ACGME-accredited training in internal medicine and gastroenterology as well. The full and updated list of programs can be found on the ACGME website.3 The second pathway is the relatively new ABIM “pilot” program during which the transplant hepatology fellowship year is combined with the third year of gastroenterology fellowship (discussed in detail below). Finally, there remain many 1-year training programs that are not ACGME-accredited, may not be associated with a gastroenterology fellowship program, and do have not regulated requirements for entry. Trainees who complete non-ACGME programs are not candidates for ABIM board certification.
How does one apply for transplant hepatology fellowship?
Transplant hepatology fellowships do not participate in a match system. Therefore, the interviews and offers for training spots may occur at different times depending on the program and the region of the country. In general, fellows apply by the fall of their second year of gastroenterology fellowship in order to begin training after graduating from the third year of fellowship. Each program has its individual approach to the application process and most have this information available on a website as to how to apply. A complete list of ACGME-accredited programs along with the program directors and contact information is available on the ABIM website.3
What is the gastroenterology/transplant hepatology pilot training program?
The AASLD and ABIM have developed a combined gastroenterology and transplant hepatology pilot fellowship training program that allows eligible gastroenterology fellows to spend their third year training in transplant hepatology. This approach has the potential to shorten the total training from 4 years to 3. In addition, if all gastroenterology and transplant hepatology competencies are achieved by the end of the third year, fellows approved to be in this program are eligible to take both gastroenterology and transplant hepatology ABIM certification exams.
Any ACGME-accredited gastroenterology fellowship program that has an accredited hepatology counterpart is eligible to participate in this pilot. Eligible programs and fellows must apply to AASLD during the fellow’s second year. The fellow applicant must complete all clinical gastroenterology requirements before the end of the second year of fellowship and be on a trajectory to meet competency milestones, as the majority of the third year will focus on hepatology.
Since 2012, 59 fellows from 31 programs have participated in this pilot program.4 If you are interested in participating in this pilot program at your institution, it is important to confer with program directors as early as possible to meet all training requirements. In addition, applications are submitted to the Pilot Steering Taskforce during the fellow’s second year for review. This is not meant to be a competitive process and all fellows who meet the criteria are approved.
This track may not be ideal for all fellows interested in advanced and transplant hepatology. In particular, there may be a trade-off between achieving clinical competency in a shortened training period and pursuing scholarly activity. This pilot program is designed to be an intensive clinical track, so fellows who wish to focus on research should discuss with their program directors whether this is the best approach.
What has been your career path after advanced training in hepatology?
I first became interested in hepatology during my inpatient rotations as a medical student. This interest led me to become involved in research in this area very early in my career. The current structure of the fellowship as well as the board certification exam were both developed while I was in training and I adjusted my plans to complete 3 years of gastroenterology fellowship followed by an ACGME-accredited liver transplant fellowship year. Since completing training, I have worked as an attending at an academic medical center in a large liver transplant program and continue to care for patients with all forms of liver disease. In addition, I continued to pursue research as a large component of my job and now have NIH funding and direct the Transplant Clinical Research Center at Columbia University. Finally, I have always been devoted to education and am the program director for the transplant hepatology fellowship at our institution.
What is the future of advanced hepatology training?
The current transplant hepatology training system has evolved significantly since its inception, including development of curricula, ongoing modification of training requirements, and the development of the innovative pilot program. However, there are issues that continue to be debated by the community. For example, it is not certain when or if the combined gastroenterology and transplant hepatology pilot program will become a permanent pathway for training or how best to select fellows for this approach.
Hepatology continues to be a very dynamic area of medicine. With diseases such as nonalcoholic fatty liver disease and hepatocellular carcinoma on the rise, the urgent need for training in HCV treatment to combat the global epidemic of viral hepatitis, and the growing number of patients on the liver transplant waiting list, there has never been a more exciting time to choose hepatology as a career.
References
1. Luxon BA. So you want to be a hepatologist? Gastroenterology. 2013;145(6):1182-5.
2. Bacon BR, Grosso LJ, Freedman N, Althouse LA. Subspecialty certification in transplant hepatology. Liver Transpl. 2007;13(11):1479-81.
3. https://apps.acgme.org/ads/public/reports/report/1.
4. https://www.aasld.org/events-professional-development/educational-learning-faq.
Dr. Verna is assistant professor of medicine, program director, transplant hepatology fellowship, director of clinical research, Transplant Clinical Research Center, Columbia University Medical Center, New York.
Unlike previous hepatologists, who were trained through gastroenterology programs, most new practitioners seek advanced training in a fellowship year focused exclusively on hepatology.
Like practitioners in many medical subspecialties, transplant hepatologists have varied career goals and responsibilities. Hepatologists who continue to specifically practice transplant hepatology are affiliated with a liver transplant center, which is generally a hospital-based practice. However, most hepatologists also treat nontransplant hepatology patients and some who have completed advanced hepatology training focus exclusively on these patients or provide community-based care for transplant recipients from other centers. Caring for patients with end-stage liver disease and liver transplant recipients can be clinically demanding but also very rewarding. There are also many opportunities for academic pursuits within a hepatology career including areas in urgent need of clinical and basic investigation, clinical trials for novel agents to treat common diseases, education (including leadership in advanced hepatology training), and involvement in professional societies such as the American Gastroenterological Association (AGA) and American Association for the Study of Liver Disease (AASLD).
What are the opportunities for advanced hepatology training?
In 1999, the AASLD determined that the practice of transplant hepatology required its own specialized knowledge and that most practicing gastroenterologists did not consider themselves adequately prepared to care for patients with advanced liver disease.1,2 The following year, the AASLD applied to the American Board of Internal Medicine (ABIM) to develop formalized liver transplant training. After several years of debate and development, the first ABIM certification exam in transplant hepatology was held in 2006 and is now offered every 2 years.2
There are currently three pathways to achieve advanced training in hepatology. The traditional pathway is a 1-year Accreditation Council for Graduate Medical Education (ACGME) transplant fellowship that is separate from, and must follow completion of, a gastroenterology fellowship. There are currently 51 ACGME-accredited 1-year transplant hepatology fellowships in the United States. These fellowships are only at institutions with ACGME-accredited training in internal medicine and gastroenterology as well. The full and updated list of programs can be found on the ACGME website.3 The second pathway is the relatively new ABIM “pilot” program during which the transplant hepatology fellowship year is combined with the third year of gastroenterology fellowship (discussed in detail below). Finally, there remain many 1-year training programs that are not ACGME-accredited, may not be associated with a gastroenterology fellowship program, and do have not regulated requirements for entry. Trainees who complete non-ACGME programs are not candidates for ABIM board certification.
How does one apply for transplant hepatology fellowship?
Transplant hepatology fellowships do not participate in a match system. Therefore, the interviews and offers for training spots may occur at different times depending on the program and the region of the country. In general, fellows apply by the fall of their second year of gastroenterology fellowship in order to begin training after graduating from the third year of fellowship. Each program has its individual approach to the application process and most have this information available on a website as to how to apply. A complete list of ACGME-accredited programs along with the program directors and contact information is available on the ABIM website.3
What is the gastroenterology/transplant hepatology pilot training program?
The AASLD and ABIM have developed a combined gastroenterology and transplant hepatology pilot fellowship training program that allows eligible gastroenterology fellows to spend their third year training in transplant hepatology. This approach has the potential to shorten the total training from 4 years to 3. In addition, if all gastroenterology and transplant hepatology competencies are achieved by the end of the third year, fellows approved to be in this program are eligible to take both gastroenterology and transplant hepatology ABIM certification exams.
Any ACGME-accredited gastroenterology fellowship program that has an accredited hepatology counterpart is eligible to participate in this pilot. Eligible programs and fellows must apply to AASLD during the fellow’s second year. The fellow applicant must complete all clinical gastroenterology requirements before the end of the second year of fellowship and be on a trajectory to meet competency milestones, as the majority of the third year will focus on hepatology.
Since 2012, 59 fellows from 31 programs have participated in this pilot program.4 If you are interested in participating in this pilot program at your institution, it is important to confer with program directors as early as possible to meet all training requirements. In addition, applications are submitted to the Pilot Steering Taskforce during the fellow’s second year for review. This is not meant to be a competitive process and all fellows who meet the criteria are approved.
This track may not be ideal for all fellows interested in advanced and transplant hepatology. In particular, there may be a trade-off between achieving clinical competency in a shortened training period and pursuing scholarly activity. This pilot program is designed to be an intensive clinical track, so fellows who wish to focus on research should discuss with their program directors whether this is the best approach.
What has been your career path after advanced training in hepatology?
I first became interested in hepatology during my inpatient rotations as a medical student. This interest led me to become involved in research in this area very early in my career. The current structure of the fellowship as well as the board certification exam were both developed while I was in training and I adjusted my plans to complete 3 years of gastroenterology fellowship followed by an ACGME-accredited liver transplant fellowship year. Since completing training, I have worked as an attending at an academic medical center in a large liver transplant program and continue to care for patients with all forms of liver disease. In addition, I continued to pursue research as a large component of my job and now have NIH funding and direct the Transplant Clinical Research Center at Columbia University. Finally, I have always been devoted to education and am the program director for the transplant hepatology fellowship at our institution.
What is the future of advanced hepatology training?
The current transplant hepatology training system has evolved significantly since its inception, including development of curricula, ongoing modification of training requirements, and the development of the innovative pilot program. However, there are issues that continue to be debated by the community. For example, it is not certain when or if the combined gastroenterology and transplant hepatology pilot program will become a permanent pathway for training or how best to select fellows for this approach.
Hepatology continues to be a very dynamic area of medicine. With diseases such as nonalcoholic fatty liver disease and hepatocellular carcinoma on the rise, the urgent need for training in HCV treatment to combat the global epidemic of viral hepatitis, and the growing number of patients on the liver transplant waiting list, there has never been a more exciting time to choose hepatology as a career.
References
1. Luxon BA. So you want to be a hepatologist? Gastroenterology. 2013;145(6):1182-5.
2. Bacon BR, Grosso LJ, Freedman N, Althouse LA. Subspecialty certification in transplant hepatology. Liver Transpl. 2007;13(11):1479-81.
3. https://apps.acgme.org/ads/public/reports/report/1.
4. https://www.aasld.org/events-professional-development/educational-learning-faq.
Dr. Verna is assistant professor of medicine, program director, transplant hepatology fellowship, director of clinical research, Transplant Clinical Research Center, Columbia University Medical Center, New York.
Life and health are not even across the U.S.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.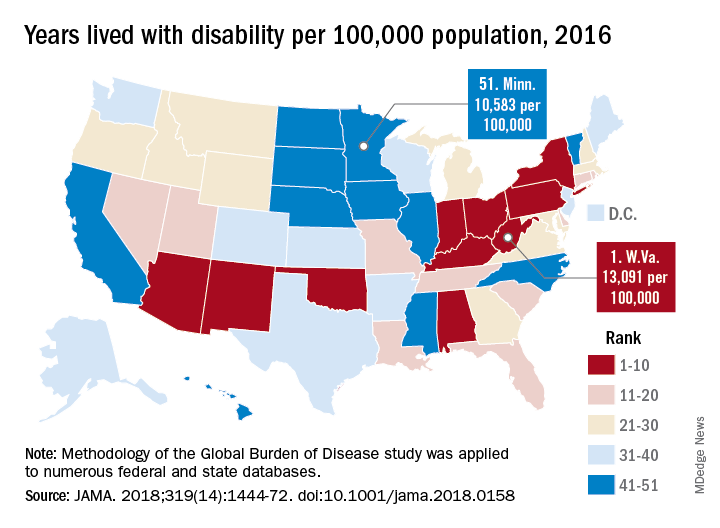
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
FROM JAMA
Key clinical point: While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors.
Major finding: Life expectancy ranged from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, and previously decreasing death rates for adults have reversed in 19 states.
Study details: A U.S. state-level analysis of results from the Global Burden of Disease (GBD) study illustrating trends in diseases, injuries, risk factors, and deaths from 1990 to 2016.
Disclosures: The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Study authors reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
Source: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-1472.
VIDEO: Biomarker accurately predicted primary nonfunction after liver transplant
, researchers reported in Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Glycomic alterations of immunoglobulin G “represent inflammatory disturbances in the liver that [mean it] will fail after transplantation,” wrote Xavier Verhelst, MD, of Ghent (Belgium) University Hospital, and his associates. The new glycomarker “could be a tool to safely select high-risk organs for liver transplantation that otherwise would be discarded from the donor pool based on a conventional clinical assessment,” and also could help prevent engraftment failures. “To our knowledge, not a single biomarker has demonstrated the same accuracy today,” they wrote in the April issue of Gastroenterology.
Chronic shortages of donor livers contribute to morbidity and death worldwide. However, relaxing donor criteria is controversial because of the increased risk of primary nonfunction, which affects some 2%-10% of liver transplantation patients, and early allograft dysfunction, which is even more common. Although no reliable scoring systems or biomarkers have been able to predict these outcomes prior to transplantation, clinical glycomics of serum has proven useful for diagnosing hepatic fibrosis, cirrhosis, and hepatocellular carcinoma, and for distinguishing hepatic steatosis from nonalcoholic steatohepatitis. “Perfusate biomarkers are an attractive alternative [to] liver biopsy or serum markers, because perfusate is believed to represent the condition of the entire liver parenchyma and is easy to collect in large volumes,” the researchers wrote.
Accordingly, they studied 66 patients who underwent liver transplantation at a single center in Belgium and a separate validation cohort of 56 transplantation recipients from two centers. The most common reason for liver transplantation was decompensated cirrhosis secondary to alcoholism, followed by chronic hepatitis C or B virus infection, acute liver failure, and polycystic liver disease. Donor grafts were transported using cold static storage (21° C), and hepatic veins were flushed to collect perfusate before transplantation. Protein-linked N-glycans was isolated from these perfusate samples and analyzed with a multicapillary electrophoresis-based ABI3130 sequencer.
The four patients in the primary study cohort who developed primary nonfunction resembled the others in terms of all clinical and demographic parameters except that they had a markedly increased concentration (P less than .0001) of a single-glycan, agalacto core-alpha-1,6-fucosylated biantennary glycan, dubbed NGA2F. The single patient in the validation cohort who developed primary nonfunction also had a significantly increased concentration of NGA2F (P = .037). There were no false positives in either cohort, and a 13% cutoff for perfusate NGA2F level identified primary nonfunction with 100% accuracy, the researchers said. In a multivariable model of donor risk index and perfusate markers, only NGA2F was prognostic for developing primary nonfunction (P less than .0001).
The researchers found no specific glycomic signature for early allograft dysfunction, perhaps because it is more complex and multifactorial, they wrote. Although electrophoresis testing took 48 hours, work is underway to shorten this to a “clinically acceptable time frame,” they added. They recommended multicenter studies to validate their findings.
Funders included the Research Fund – Flanders and Ghent University. The researchers reported having no conflicts of interest.
SOURCE: Verhelst X et al. Gastroenterology 2018 Jan 6. doi: 10.1053/j.gastro.2017.12.027.
, researchers reported in Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Glycomic alterations of immunoglobulin G “represent inflammatory disturbances in the liver that [mean it] will fail after transplantation,” wrote Xavier Verhelst, MD, of Ghent (Belgium) University Hospital, and his associates. The new glycomarker “could be a tool to safely select high-risk organs for liver transplantation that otherwise would be discarded from the donor pool based on a conventional clinical assessment,” and also could help prevent engraftment failures. “To our knowledge, not a single biomarker has demonstrated the same accuracy today,” they wrote in the April issue of Gastroenterology.
Chronic shortages of donor livers contribute to morbidity and death worldwide. However, relaxing donor criteria is controversial because of the increased risk of primary nonfunction, which affects some 2%-10% of liver transplantation patients, and early allograft dysfunction, which is even more common. Although no reliable scoring systems or biomarkers have been able to predict these outcomes prior to transplantation, clinical glycomics of serum has proven useful for diagnosing hepatic fibrosis, cirrhosis, and hepatocellular carcinoma, and for distinguishing hepatic steatosis from nonalcoholic steatohepatitis. “Perfusate biomarkers are an attractive alternative [to] liver biopsy or serum markers, because perfusate is believed to represent the condition of the entire liver parenchyma and is easy to collect in large volumes,” the researchers wrote.
Accordingly, they studied 66 patients who underwent liver transplantation at a single center in Belgium and a separate validation cohort of 56 transplantation recipients from two centers. The most common reason for liver transplantation was decompensated cirrhosis secondary to alcoholism, followed by chronic hepatitis C or B virus infection, acute liver failure, and polycystic liver disease. Donor grafts were transported using cold static storage (21° C), and hepatic veins were flushed to collect perfusate before transplantation. Protein-linked N-glycans was isolated from these perfusate samples and analyzed with a multicapillary electrophoresis-based ABI3130 sequencer.
The four patients in the primary study cohort who developed primary nonfunction resembled the others in terms of all clinical and demographic parameters except that they had a markedly increased concentration (P less than .0001) of a single-glycan, agalacto core-alpha-1,6-fucosylated biantennary glycan, dubbed NGA2F. The single patient in the validation cohort who developed primary nonfunction also had a significantly increased concentration of NGA2F (P = .037). There were no false positives in either cohort, and a 13% cutoff for perfusate NGA2F level identified primary nonfunction with 100% accuracy, the researchers said. In a multivariable model of donor risk index and perfusate markers, only NGA2F was prognostic for developing primary nonfunction (P less than .0001).
The researchers found no specific glycomic signature for early allograft dysfunction, perhaps because it is more complex and multifactorial, they wrote. Although electrophoresis testing took 48 hours, work is underway to shorten this to a “clinically acceptable time frame,” they added. They recommended multicenter studies to validate their findings.
Funders included the Research Fund – Flanders and Ghent University. The researchers reported having no conflicts of interest.
SOURCE: Verhelst X et al. Gastroenterology 2018 Jan 6. doi: 10.1053/j.gastro.2017.12.027.
, researchers reported in Gastroenterology.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Glycomic alterations of immunoglobulin G “represent inflammatory disturbances in the liver that [mean it] will fail after transplantation,” wrote Xavier Verhelst, MD, of Ghent (Belgium) University Hospital, and his associates. The new glycomarker “could be a tool to safely select high-risk organs for liver transplantation that otherwise would be discarded from the donor pool based on a conventional clinical assessment,” and also could help prevent engraftment failures. “To our knowledge, not a single biomarker has demonstrated the same accuracy today,” they wrote in the April issue of Gastroenterology.
Chronic shortages of donor livers contribute to morbidity and death worldwide. However, relaxing donor criteria is controversial because of the increased risk of primary nonfunction, which affects some 2%-10% of liver transplantation patients, and early allograft dysfunction, which is even more common. Although no reliable scoring systems or biomarkers have been able to predict these outcomes prior to transplantation, clinical glycomics of serum has proven useful for diagnosing hepatic fibrosis, cirrhosis, and hepatocellular carcinoma, and for distinguishing hepatic steatosis from nonalcoholic steatohepatitis. “Perfusate biomarkers are an attractive alternative [to] liver biopsy or serum markers, because perfusate is believed to represent the condition of the entire liver parenchyma and is easy to collect in large volumes,” the researchers wrote.
Accordingly, they studied 66 patients who underwent liver transplantation at a single center in Belgium and a separate validation cohort of 56 transplantation recipients from two centers. The most common reason for liver transplantation was decompensated cirrhosis secondary to alcoholism, followed by chronic hepatitis C or B virus infection, acute liver failure, and polycystic liver disease. Donor grafts were transported using cold static storage (21° C), and hepatic veins were flushed to collect perfusate before transplantation. Protein-linked N-glycans was isolated from these perfusate samples and analyzed with a multicapillary electrophoresis-based ABI3130 sequencer.
The four patients in the primary study cohort who developed primary nonfunction resembled the others in terms of all clinical and demographic parameters except that they had a markedly increased concentration (P less than .0001) of a single-glycan, agalacto core-alpha-1,6-fucosylated biantennary glycan, dubbed NGA2F. The single patient in the validation cohort who developed primary nonfunction also had a significantly increased concentration of NGA2F (P = .037). There were no false positives in either cohort, and a 13% cutoff for perfusate NGA2F level identified primary nonfunction with 100% accuracy, the researchers said. In a multivariable model of donor risk index and perfusate markers, only NGA2F was prognostic for developing primary nonfunction (P less than .0001).
The researchers found no specific glycomic signature for early allograft dysfunction, perhaps because it is more complex and multifactorial, they wrote. Although electrophoresis testing took 48 hours, work is underway to shorten this to a “clinically acceptable time frame,” they added. They recommended multicenter studies to validate their findings.
Funders included the Research Fund – Flanders and Ghent University. The researchers reported having no conflicts of interest.
SOURCE: Verhelst X et al. Gastroenterology 2018 Jan 6. doi: 10.1053/j.gastro.2017.12.027.
FROM GASTROENTEROLOGY
Key clinical point: A glycomarker in donor liver perfusate was 100% accurate at predicting primary nonfunction after liver transplantation.
Major finding: In a multivariable model of donor risk index and perfusate markers, only the single-glycan, NGA2F was a significant predictor of primary nonfunction (P less than .0001).
Data source: A dual-center, prospective study of 66 liver transplant patients and a 55-member validation cohort.
Disclosures: Funders included the Research Fund – Flanders and Ghent University. The researchers reported having no conflicts of interest.
Source: Verhelst X et al. Gastroenterology 2018 Jan 6. doi: 10.1053/j.gastro.2017.12.027.
Survival worse with alcohol-related HCC, compared with other types
Hepatocellular carcinoma (HCC) related to alcohol use tends to be diagnosed at a later stage than HCC from other causes, which contributes to reduced overall survival among patients with alcoholic HCC, investigators in a prospective French study said.
Among 894 patients diagnosed with HCC, the adjusted median overall survival was 5.7 months for those with alcoholic HCC, compared with 9.7 months for those with nonalcoholic HCC (P = .0002), reported Charlotte E. Costentin, MD, of the Hopital Henri Mondor in Creteil, France, and colleagues.
“Various assumptions can be made to explain why patients with alcohol-related HCC have reduced survival in comparison with patients with non–alcohol-related HCC: a diagnosis at a later stage due to lower rates of HCC screening, worse liver function and/or ongoing alcohol consumption preventing curative options, and discrimination against alcoholic patients leading to less aggressive treatment options,” they wrote in a study published online in Cancer.
The investigators looked at data on clinical features and treatment allocation of patients in the CHANGH cohort (cohorte de Carcinomes Hepatocelulaires de l’Association des hepato-Gastroenterologues des Hopitaux Generaux), a prospective, observational cohort study.
Of 1,207 patients with complete data, 582 had isolated alcohol-related HCC, and 312 had non–alcohol-related HCC, which was caused by either nonalcoholic fatty liver, hepatitis C infections, hepatitis B infections, hemochromatosis, or other etiologies.
As noted before, the median overall survival adjusted for lead-time bias (the length of time between the detection of a disease and its usual diagnosis) was significantly shorter for patients with alcohol-related HCC.
In univariate analysis, alcohol-related HCC, compared with non–alcohol-related HCC, was an independent risk factor for worse overall survival (hazard ratio, 1.39; P = .0002).
Among patients in the alcohol-related HCC group, median overall survival adjusted for lead-time was 5.8 months for patients who had been abstinent for a median of 1 year, compared with 5.0 months for the nonabstinent patients, a difference that was not statistically significant.
In multivariate analysis, factors significantly associated with worse overall survival included advanced HCC at diagnosis (diffuse or metastatic HCC and/or macrovascular invasion), alkaline phosphatase score, alpha-fetoprotein levels, creatinine, performance status, Child-Pugh score, age plus alcohol-related disease, and male sex plus alcohol-related disease. However, alcohol-related versus non–alcohol-related HCC was no longer statistically significant in multivariate analysis.
They noted that for 199 patients who were diagnosed with HCC as part of a cirrhosis follow-up program, the median overall survival adjusted for lead-time was 11.7 months, compared with 5.4 months for patients whose HCC was detected incidentally (P less than .0001).
The investigators noted that other studies have shown that screening rates for HCC are lower in alcohol abusers and that the most common reason for a lack of screening was failure of clinician to order surveillance in patients with known cirrhosis. In addition, alcoholic patients are less likely to be compliant with screening.
“Importantly, Bucci et al (Aliment Pharmacol Ther. 2016 Feb;43[3]:385-99) observed similar survival between alcoholic patients and patients with hepatitis C virus among patients undergoing HCC surveillance according to guidelines. The poorer prognosis of alcohol-related HCC is, therefore, very likely to be related to an advanced stage at diagnosis due to screening failure instead of greater cancer aggressiveness,” they wrote.
“To improve prognosis of liver cancer in the alcoholic population, efforts should be made to implement effective screening programs for both cirrhosis and liver cancer and to improve access to alcoholism treatment services,” Dr. Costentin said in press release. “A smaller tumor burden and a better liver function at diagnosis should translate into higher rates of patients with alcohol-related liver cancer amenable to curative treatment such as tumor resection or ablation and liver transplantation.”
Dr. Costentin did not report conflicts of interest. Several of her coauthors reported personal fees from various companies outside the submitted work.
SOURCE: Costentin CE at al. Cancer. doi: 10.1002/cncr.31215.
Hepatocellular carcinoma (HCC) related to alcohol use tends to be diagnosed at a later stage than HCC from other causes, which contributes to reduced overall survival among patients with alcoholic HCC, investigators in a prospective French study said.
Among 894 patients diagnosed with HCC, the adjusted median overall survival was 5.7 months for those with alcoholic HCC, compared with 9.7 months for those with nonalcoholic HCC (P = .0002), reported Charlotte E. Costentin, MD, of the Hopital Henri Mondor in Creteil, France, and colleagues.
“Various assumptions can be made to explain why patients with alcohol-related HCC have reduced survival in comparison with patients with non–alcohol-related HCC: a diagnosis at a later stage due to lower rates of HCC screening, worse liver function and/or ongoing alcohol consumption preventing curative options, and discrimination against alcoholic patients leading to less aggressive treatment options,” they wrote in a study published online in Cancer.
The investigators looked at data on clinical features and treatment allocation of patients in the CHANGH cohort (cohorte de Carcinomes Hepatocelulaires de l’Association des hepato-Gastroenterologues des Hopitaux Generaux), a prospective, observational cohort study.
Of 1,207 patients with complete data, 582 had isolated alcohol-related HCC, and 312 had non–alcohol-related HCC, which was caused by either nonalcoholic fatty liver, hepatitis C infections, hepatitis B infections, hemochromatosis, or other etiologies.
As noted before, the median overall survival adjusted for lead-time bias (the length of time between the detection of a disease and its usual diagnosis) was significantly shorter for patients with alcohol-related HCC.
In univariate analysis, alcohol-related HCC, compared with non–alcohol-related HCC, was an independent risk factor for worse overall survival (hazard ratio, 1.39; P = .0002).
Among patients in the alcohol-related HCC group, median overall survival adjusted for lead-time was 5.8 months for patients who had been abstinent for a median of 1 year, compared with 5.0 months for the nonabstinent patients, a difference that was not statistically significant.
In multivariate analysis, factors significantly associated with worse overall survival included advanced HCC at diagnosis (diffuse or metastatic HCC and/or macrovascular invasion), alkaline phosphatase score, alpha-fetoprotein levels, creatinine, performance status, Child-Pugh score, age plus alcohol-related disease, and male sex plus alcohol-related disease. However, alcohol-related versus non–alcohol-related HCC was no longer statistically significant in multivariate analysis.
They noted that for 199 patients who were diagnosed with HCC as part of a cirrhosis follow-up program, the median overall survival adjusted for lead-time was 11.7 months, compared with 5.4 months for patients whose HCC was detected incidentally (P less than .0001).
The investigators noted that other studies have shown that screening rates for HCC are lower in alcohol abusers and that the most common reason for a lack of screening was failure of clinician to order surveillance in patients with known cirrhosis. In addition, alcoholic patients are less likely to be compliant with screening.
“Importantly, Bucci et al (Aliment Pharmacol Ther. 2016 Feb;43[3]:385-99) observed similar survival between alcoholic patients and patients with hepatitis C virus among patients undergoing HCC surveillance according to guidelines. The poorer prognosis of alcohol-related HCC is, therefore, very likely to be related to an advanced stage at diagnosis due to screening failure instead of greater cancer aggressiveness,” they wrote.
“To improve prognosis of liver cancer in the alcoholic population, efforts should be made to implement effective screening programs for both cirrhosis and liver cancer and to improve access to alcoholism treatment services,” Dr. Costentin said in press release. “A smaller tumor burden and a better liver function at diagnosis should translate into higher rates of patients with alcohol-related liver cancer amenable to curative treatment such as tumor resection or ablation and liver transplantation.”
Dr. Costentin did not report conflicts of interest. Several of her coauthors reported personal fees from various companies outside the submitted work.
SOURCE: Costentin CE at al. Cancer. doi: 10.1002/cncr.31215.
Hepatocellular carcinoma (HCC) related to alcohol use tends to be diagnosed at a later stage than HCC from other causes, which contributes to reduced overall survival among patients with alcoholic HCC, investigators in a prospective French study said.
Among 894 patients diagnosed with HCC, the adjusted median overall survival was 5.7 months for those with alcoholic HCC, compared with 9.7 months for those with nonalcoholic HCC (P = .0002), reported Charlotte E. Costentin, MD, of the Hopital Henri Mondor in Creteil, France, and colleagues.
“Various assumptions can be made to explain why patients with alcohol-related HCC have reduced survival in comparison with patients with non–alcohol-related HCC: a diagnosis at a later stage due to lower rates of HCC screening, worse liver function and/or ongoing alcohol consumption preventing curative options, and discrimination against alcoholic patients leading to less aggressive treatment options,” they wrote in a study published online in Cancer.
The investigators looked at data on clinical features and treatment allocation of patients in the CHANGH cohort (cohorte de Carcinomes Hepatocelulaires de l’Association des hepato-Gastroenterologues des Hopitaux Generaux), a prospective, observational cohort study.
Of 1,207 patients with complete data, 582 had isolated alcohol-related HCC, and 312 had non–alcohol-related HCC, which was caused by either nonalcoholic fatty liver, hepatitis C infections, hepatitis B infections, hemochromatosis, or other etiologies.
As noted before, the median overall survival adjusted for lead-time bias (the length of time between the detection of a disease and its usual diagnosis) was significantly shorter for patients with alcohol-related HCC.
In univariate analysis, alcohol-related HCC, compared with non–alcohol-related HCC, was an independent risk factor for worse overall survival (hazard ratio, 1.39; P = .0002).
Among patients in the alcohol-related HCC group, median overall survival adjusted for lead-time was 5.8 months for patients who had been abstinent for a median of 1 year, compared with 5.0 months for the nonabstinent patients, a difference that was not statistically significant.
In multivariate analysis, factors significantly associated with worse overall survival included advanced HCC at diagnosis (diffuse or metastatic HCC and/or macrovascular invasion), alkaline phosphatase score, alpha-fetoprotein levels, creatinine, performance status, Child-Pugh score, age plus alcohol-related disease, and male sex plus alcohol-related disease. However, alcohol-related versus non–alcohol-related HCC was no longer statistically significant in multivariate analysis.
They noted that for 199 patients who were diagnosed with HCC as part of a cirrhosis follow-up program, the median overall survival adjusted for lead-time was 11.7 months, compared with 5.4 months for patients whose HCC was detected incidentally (P less than .0001).
The investigators noted that other studies have shown that screening rates for HCC are lower in alcohol abusers and that the most common reason for a lack of screening was failure of clinician to order surveillance in patients with known cirrhosis. In addition, alcoholic patients are less likely to be compliant with screening.
“Importantly, Bucci et al (Aliment Pharmacol Ther. 2016 Feb;43[3]:385-99) observed similar survival between alcoholic patients and patients with hepatitis C virus among patients undergoing HCC surveillance according to guidelines. The poorer prognosis of alcohol-related HCC is, therefore, very likely to be related to an advanced stage at diagnosis due to screening failure instead of greater cancer aggressiveness,” they wrote.
“To improve prognosis of liver cancer in the alcoholic population, efforts should be made to implement effective screening programs for both cirrhosis and liver cancer and to improve access to alcoholism treatment services,” Dr. Costentin said in press release. “A smaller tumor burden and a better liver function at diagnosis should translate into higher rates of patients with alcohol-related liver cancer amenable to curative treatment such as tumor resection or ablation and liver transplantation.”
Dr. Costentin did not report conflicts of interest. Several of her coauthors reported personal fees from various companies outside the submitted work.
SOURCE: Costentin CE at al. Cancer. doi: 10.1002/cncr.31215.
FROM CANCER
Key clinical point: Patients with HCC from alcohol or other causes had better survival if they were under surveillance for cirrhosis.
Major finding: Adjusted median overall survival was 5.7 months with alcohol-related HCC versus 9.7 months for nonalcoholic HCC (P = .0002).
Study details: Analysis of data from a prospective observational cohort of 1,207 patients with HCC in France.
Disclosures: The study was supported by the Association Nationale des Hepato-Gastroenterologues des Hopitaux Généraux group and Roche Pharmaceuticals. Dr. Constentin did not report conflicts of interest. Several of her coauthors reported personal fees from various companies outside the submitted work.
Source: Costentin CE at al. Cancer. doi: 10.1002/cncr.31215.
Oral SGLT-2 inhibitor reduced liver fat in diabetics with NAFLD
CHICAGO – and improved ALT in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus, according to a study presented at the annual meeting of the Endocrine Society.
As insulin resistance is the mechanism for NAFLD development, this new addition to the list of drugs on offer to patients with diabetes could help decrease the chance of developing metabolic syndrome and cardiovascular disease.
“SGLT-2 inhibitors are newer antidiabetic agents that reduce blood glucose by promoting urinary glucose excretion,” said presenter Mohammad Shafi Kuchay, MD, DM, an endocrinologist at Medanta The Medicity, Gurugram, India. “NAFLD, which also increases the risk of type 2 diabetes, often responds to strategies that improve hyperglycemia.”
Dr. Kuchay and fellow investigators conducted a small, 20-week randomized controlled trial of 42 patients with type 2 diabetes and NAFLD.
Patients in the test group were mostly male and on average 50 years old, with baseline AST, ALT, and gamma-glutamyltransferase scores of 44.6 U/L, 64.3 U/L, and 65.8 U/L, respectively. Those randomized to the control group had similar characteristics.
After adding 10 mg of empagliflozin to their diabetes regimen, liver fat density in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001). The drop stands in sharp contrast to the control group, which decreased from 16.4% to 15.5% (P = .054). Measurement of liver fat density was made by MRI-derived proton density fat fraction (MRI-PDFF). This method has higher sensitivity for detecting changes in liver fat, compared with histology, explained Dr. Kuchay.
When broken down by individual liver fat, 25% of patients in the control group increased in liver fat, 50% had no significant change, and 25% decreased in liver fat, according to Dr. Kuchay.
In comparison, 77% of patients in the empagliflozin group had a decrease in liver fat, 23% had no change, and no patients saw an increase in liver fat.
When comparing levels of hemoglobin A1c between the two groups, both had a similarly significant reduction of around 2%, which Dr. Kuchay attributes to deliberate intervention by investigators.
Further studies will need to be conducted regarding the long-term effects of this treatment; however, using SGLT-2 to reduce liver fat could be a boon to preventing more serious liver diseases, concluded Dr. Kuchay.
“There are studies in which liver fat reduction led to improvement in inflammation and fibrosis,” said Dr. Kuchay in response to a question from the audience. “Because liver fat accumulation is the first inhibitor in the pathogenesis of more severe forms of liver disease, reducing liver fat should help improve patient outcomes.”
Dr. Kuchay reported no relevant financial disclosures.
Source: M. Kuchay et al. ENDO 2018, Abstract OR27-2.
CHICAGO – and improved ALT in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus, according to a study presented at the annual meeting of the Endocrine Society.
As insulin resistance is the mechanism for NAFLD development, this new addition to the list of drugs on offer to patients with diabetes could help decrease the chance of developing metabolic syndrome and cardiovascular disease.
“SGLT-2 inhibitors are newer antidiabetic agents that reduce blood glucose by promoting urinary glucose excretion,” said presenter Mohammad Shafi Kuchay, MD, DM, an endocrinologist at Medanta The Medicity, Gurugram, India. “NAFLD, which also increases the risk of type 2 diabetes, often responds to strategies that improve hyperglycemia.”
Dr. Kuchay and fellow investigators conducted a small, 20-week randomized controlled trial of 42 patients with type 2 diabetes and NAFLD.
Patients in the test group were mostly male and on average 50 years old, with baseline AST, ALT, and gamma-glutamyltransferase scores of 44.6 U/L, 64.3 U/L, and 65.8 U/L, respectively. Those randomized to the control group had similar characteristics.
After adding 10 mg of empagliflozin to their diabetes regimen, liver fat density in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001). The drop stands in sharp contrast to the control group, which decreased from 16.4% to 15.5% (P = .054). Measurement of liver fat density was made by MRI-derived proton density fat fraction (MRI-PDFF). This method has higher sensitivity for detecting changes in liver fat, compared with histology, explained Dr. Kuchay.
When broken down by individual liver fat, 25% of patients in the control group increased in liver fat, 50% had no significant change, and 25% decreased in liver fat, according to Dr. Kuchay.
In comparison, 77% of patients in the empagliflozin group had a decrease in liver fat, 23% had no change, and no patients saw an increase in liver fat.
When comparing levels of hemoglobin A1c between the two groups, both had a similarly significant reduction of around 2%, which Dr. Kuchay attributes to deliberate intervention by investigators.
Further studies will need to be conducted regarding the long-term effects of this treatment; however, using SGLT-2 to reduce liver fat could be a boon to preventing more serious liver diseases, concluded Dr. Kuchay.
“There are studies in which liver fat reduction led to improvement in inflammation and fibrosis,” said Dr. Kuchay in response to a question from the audience. “Because liver fat accumulation is the first inhibitor in the pathogenesis of more severe forms of liver disease, reducing liver fat should help improve patient outcomes.”
Dr. Kuchay reported no relevant financial disclosures.
Source: M. Kuchay et al. ENDO 2018, Abstract OR27-2.
CHICAGO – and improved ALT in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus, according to a study presented at the annual meeting of the Endocrine Society.
As insulin resistance is the mechanism for NAFLD development, this new addition to the list of drugs on offer to patients with diabetes could help decrease the chance of developing metabolic syndrome and cardiovascular disease.
“SGLT-2 inhibitors are newer antidiabetic agents that reduce blood glucose by promoting urinary glucose excretion,” said presenter Mohammad Shafi Kuchay, MD, DM, an endocrinologist at Medanta The Medicity, Gurugram, India. “NAFLD, which also increases the risk of type 2 diabetes, often responds to strategies that improve hyperglycemia.”
Dr. Kuchay and fellow investigators conducted a small, 20-week randomized controlled trial of 42 patients with type 2 diabetes and NAFLD.
Patients in the test group were mostly male and on average 50 years old, with baseline AST, ALT, and gamma-glutamyltransferase scores of 44.6 U/L, 64.3 U/L, and 65.8 U/L, respectively. Those randomized to the control group had similar characteristics.
After adding 10 mg of empagliflozin to their diabetes regimen, liver fat density in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001). The drop stands in sharp contrast to the control group, which decreased from 16.4% to 15.5% (P = .054). Measurement of liver fat density was made by MRI-derived proton density fat fraction (MRI-PDFF). This method has higher sensitivity for detecting changes in liver fat, compared with histology, explained Dr. Kuchay.
When broken down by individual liver fat, 25% of patients in the control group increased in liver fat, 50% had no significant change, and 25% decreased in liver fat, according to Dr. Kuchay.
In comparison, 77% of patients in the empagliflozin group had a decrease in liver fat, 23% had no change, and no patients saw an increase in liver fat.
When comparing levels of hemoglobin A1c between the two groups, both had a similarly significant reduction of around 2%, which Dr. Kuchay attributes to deliberate intervention by investigators.
Further studies will need to be conducted regarding the long-term effects of this treatment; however, using SGLT-2 to reduce liver fat could be a boon to preventing more serious liver diseases, concluded Dr. Kuchay.
“There are studies in which liver fat reduction led to improvement in inflammation and fibrosis,” said Dr. Kuchay in response to a question from the audience. “Because liver fat accumulation is the first inhibitor in the pathogenesis of more severe forms of liver disease, reducing liver fat should help improve patient outcomes.”
Dr. Kuchay reported no relevant financial disclosures.
Source: M. Kuchay et al. ENDO 2018, Abstract OR27-2.
REPORTING FROM ENDO 2018
Key clinical point: Empagliflozin reduced liver fat in patients with NAFLD and type 2 diabetes.
Major finding: MRI-PDFF in test patients decreased from 16.2% to 11.3% (P less than or equal to .0001), compared with control patients, who saw a decrease from 19.4% to 15.5% (P = .057)
Data source: Prospective, randomized, controlled trial of 60 patients with type 2 diabetes and NAFLD.
Disclosures: Dr. Kuchay reported no relevant financial disclosures.
Source: Kuchay M et al. ENDO 2018, Abstract OR27-2.
Switching to tenofovir alafenamide may benefit HBV patients
PHILADELPHIA – Tenofovir alafenamide, the newest kid on the block for treatment of chronic hepatitis B, not only has less bone and renal effects than tenofovir disoproxil, but now also appears to improve those parameters in patients switched over from the older tenofovir formulation, according to Paul Kwo, MD.
“Renal function, as well as hip and spine bone mineral density measurements, all improve after you flip,” said Dr. Kwo, director of hepatology at Stanford (Calif.) University.
Dr. Kwo described some of the latest data on the newer tenofovir formulation in a hepatitis B update he gave at the conference, jointly provided by Rutgers and Global Academy for Medical Education.
Tenofovir alafenamide, a nucleoside analogue reverse transcriptase inhibitor, was approved in November 2016 for treatment of adults with chronic hepatitis B virus (HBV) infection and compensated liver disease.
It has similar efficacy to tenofovir disoproxil, with fewer bone and renal effects, according to results of two large international phase 3 trials.
Some of the latest data, presented in October 2017 at The Liver Meeting in Washington, show that switching patients from tenofovir disoproxil to tenofovir alafenamide improved creatinine clearance and increased rates of alanine aminotransferase normalization, with sustained rates of virologic control, over 48 weeks of treatment.
Similar results were seen for bone mineral density. “It goes up over time, and you approach bone mineral density levels that are similar to [levels in] those who are on tenofovir alafenamide long term,” Dr. Kwo said, commenting on results of the study.
Compared with tenofovir disoproxil, tenofovir alafenamide is a slightly different prodrug of tenofovir, according to Dr. Kwo.
The approved dose of tenofovir alafenamide is 25 mg, compared with 300 mg for tenofovir disoproxil. “It’s more stable in the serum, so you don’t need higher levels, and you have fewer off-target effects,” Dr. Kwo said.
The two agents are “Coke and Pepsi” in terms of efficacy, he added, noting that comparative studies showed similar efficacy on endpoints of percentage HBV DNA less than 29 IU/mL and log10 HBV DNA change.
Very low rates of resistance are seen with first-line therapies for chronic hepatitis B, including entecavir and tenofovir disoproxil. “We wouldn’t expect (tenofovir alafenamide) to be any different, but nonetheless the surveillance has to happen,” Dr. Kwo said.
Tenofovir alafenamide is not yet listed in the official recommendations of the American Association for the Study of Liver Diseases, but it is in current guidelines from the European Association for the Study of the Liver.
The published EASL guidelines provide guidance on how tenofovir alafenamide fits into the treatment armamentarium for HBV.
Going by the EASL recommendations, age greater than 60 years, bone disease, and renal alterations are all good reasons to use tenofovir alafenamide as first-line therapy for hepatitis B, according to Dr. Kwo.
Dr. Kwo reported disclosures related to AbbVie, Allergan, Bristol-Myers Squibb, Conatus Pharmaceuticals, Dova Pharmaceuticals, DURECT, Gilead Sciences, Merck, and Shionogi.
Global Academy and this news organization are owned by the same company.
PHILADELPHIA – Tenofovir alafenamide, the newest kid on the block for treatment of chronic hepatitis B, not only has less bone and renal effects than tenofovir disoproxil, but now also appears to improve those parameters in patients switched over from the older tenofovir formulation, according to Paul Kwo, MD.
“Renal function, as well as hip and spine bone mineral density measurements, all improve after you flip,” said Dr. Kwo, director of hepatology at Stanford (Calif.) University.
Dr. Kwo described some of the latest data on the newer tenofovir formulation in a hepatitis B update he gave at the conference, jointly provided by Rutgers and Global Academy for Medical Education.
Tenofovir alafenamide, a nucleoside analogue reverse transcriptase inhibitor, was approved in November 2016 for treatment of adults with chronic hepatitis B virus (HBV) infection and compensated liver disease.
It has similar efficacy to tenofovir disoproxil, with fewer bone and renal effects, according to results of two large international phase 3 trials.
Some of the latest data, presented in October 2017 at The Liver Meeting in Washington, show that switching patients from tenofovir disoproxil to tenofovir alafenamide improved creatinine clearance and increased rates of alanine aminotransferase normalization, with sustained rates of virologic control, over 48 weeks of treatment.
Similar results were seen for bone mineral density. “It goes up over time, and you approach bone mineral density levels that are similar to [levels in] those who are on tenofovir alafenamide long term,” Dr. Kwo said, commenting on results of the study.
Compared with tenofovir disoproxil, tenofovir alafenamide is a slightly different prodrug of tenofovir, according to Dr. Kwo.
The approved dose of tenofovir alafenamide is 25 mg, compared with 300 mg for tenofovir disoproxil. “It’s more stable in the serum, so you don’t need higher levels, and you have fewer off-target effects,” Dr. Kwo said.
The two agents are “Coke and Pepsi” in terms of efficacy, he added, noting that comparative studies showed similar efficacy on endpoints of percentage HBV DNA less than 29 IU/mL and log10 HBV DNA change.
Very low rates of resistance are seen with first-line therapies for chronic hepatitis B, including entecavir and tenofovir disoproxil. “We wouldn’t expect (tenofovir alafenamide) to be any different, but nonetheless the surveillance has to happen,” Dr. Kwo said.
Tenofovir alafenamide is not yet listed in the official recommendations of the American Association for the Study of Liver Diseases, but it is in current guidelines from the European Association for the Study of the Liver.
The published EASL guidelines provide guidance on how tenofovir alafenamide fits into the treatment armamentarium for HBV.
Going by the EASL recommendations, age greater than 60 years, bone disease, and renal alterations are all good reasons to use tenofovir alafenamide as first-line therapy for hepatitis B, according to Dr. Kwo.
Dr. Kwo reported disclosures related to AbbVie, Allergan, Bristol-Myers Squibb, Conatus Pharmaceuticals, Dova Pharmaceuticals, DURECT, Gilead Sciences, Merck, and Shionogi.
Global Academy and this news organization are owned by the same company.
PHILADELPHIA – Tenofovir alafenamide, the newest kid on the block for treatment of chronic hepatitis B, not only has less bone and renal effects than tenofovir disoproxil, but now also appears to improve those parameters in patients switched over from the older tenofovir formulation, according to Paul Kwo, MD.
“Renal function, as well as hip and spine bone mineral density measurements, all improve after you flip,” said Dr. Kwo, director of hepatology at Stanford (Calif.) University.
Dr. Kwo described some of the latest data on the newer tenofovir formulation in a hepatitis B update he gave at the conference, jointly provided by Rutgers and Global Academy for Medical Education.
Tenofovir alafenamide, a nucleoside analogue reverse transcriptase inhibitor, was approved in November 2016 for treatment of adults with chronic hepatitis B virus (HBV) infection and compensated liver disease.
It has similar efficacy to tenofovir disoproxil, with fewer bone and renal effects, according to results of two large international phase 3 trials.
Some of the latest data, presented in October 2017 at The Liver Meeting in Washington, show that switching patients from tenofovir disoproxil to tenofovir alafenamide improved creatinine clearance and increased rates of alanine aminotransferase normalization, with sustained rates of virologic control, over 48 weeks of treatment.
Similar results were seen for bone mineral density. “It goes up over time, and you approach bone mineral density levels that are similar to [levels in] those who are on tenofovir alafenamide long term,” Dr. Kwo said, commenting on results of the study.
Compared with tenofovir disoproxil, tenofovir alafenamide is a slightly different prodrug of tenofovir, according to Dr. Kwo.
The approved dose of tenofovir alafenamide is 25 mg, compared with 300 mg for tenofovir disoproxil. “It’s more stable in the serum, so you don’t need higher levels, and you have fewer off-target effects,” Dr. Kwo said.
The two agents are “Coke and Pepsi” in terms of efficacy, he added, noting that comparative studies showed similar efficacy on endpoints of percentage HBV DNA less than 29 IU/mL and log10 HBV DNA change.
Very low rates of resistance are seen with first-line therapies for chronic hepatitis B, including entecavir and tenofovir disoproxil. “We wouldn’t expect (tenofovir alafenamide) to be any different, but nonetheless the surveillance has to happen,” Dr. Kwo said.
Tenofovir alafenamide is not yet listed in the official recommendations of the American Association for the Study of Liver Diseases, but it is in current guidelines from the European Association for the Study of the Liver.
The published EASL guidelines provide guidance on how tenofovir alafenamide fits into the treatment armamentarium for HBV.
Going by the EASL recommendations, age greater than 60 years, bone disease, and renal alterations are all good reasons to use tenofovir alafenamide as first-line therapy for hepatitis B, according to Dr. Kwo.
Dr. Kwo reported disclosures related to AbbVie, Allergan, Bristol-Myers Squibb, Conatus Pharmaceuticals, Dova Pharmaceuticals, DURECT, Gilead Sciences, Merck, and Shionogi.
Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Red meat intake linked to NAFLD risk
Higher dietary intake of red meat and processed meats such as salami may increase the risk of nonalcoholic fatty liver disease and insulin resistance, new research suggests.
In a cross-sectional study, published in the March 20 edition of the Journal of Hepatology, researchers used food-frequency questionnaires to examine red and processed meat consumption in 789 adults aged 40-70 years, including information on cooking methods.
They found that those who reported a total meat intake above the median had a significant 49% higher odds of nonalcoholic fatty liver disease (NAFLD) (95% confidence interval, 1.05-2.13; P = .028) and 63% greater odds of insulin resistance (95% CI, 1.12-2.37, P = .011), even after adjustment for potential confounders such as body mass index, physical activity, smoking, alcohol, and saturated fat and cholesterol intake.
Those whose intake of red and/or processed meat was above the median had a 47% greater odds of NAFLD (P = .031), and a 55% greater odds of insulin resistance (P = .020).
Even when the analysis was limited to nondiabetic participants, the study still showed a significant relationship between higher intake of red and processed meat, and insulin resistance (J Hepatol. 2018 Mar 20. doi: 10.1016/j.jhep.2018.01.015).
“It can be claimed that the harmful association with meat may, at least partially, be related to a generally less healthy diet or lifestyle characterizing people who eat more red or processed meat, rather than a causal effect of meat,” wrote Shira Zelber-Sagi, PhD, of the department of gastroenterology at Tel Aviv Medical Center, and coauthors. “However, in the current study we meticulously adjusted the association with meat for other nutritional and lifestyle parameters to minimize confounding as much as possible.”
There was also a significant association between unhealthy cooking methods such as frying, broiling, and grilling – which are known to increase the quantity of heterocyclic amines (HCA) in the meat – and insulin resistance.
Individuals who ate one or more portions of meat cooked by these methods showed a higher incidence of insulin resistance compared with those who ate fewer than one portion per week (36.00% vs. 22.20%, P = .004). Researchers also used the food-frequency questionnaires to calculate the quantity of participants’ HCA intake, and found a significantly higher odds of insulin resistance in individuals whose HCA intake was above the median.
Even among the 305 individuals with NAFLD, higher total meat intake, and higher red and processed meat intake, the prevalence of insulin resistance was higher.
In this group, high HCA intake and high consumption of meat cooked by the unhealthy methods were associated with a fourfold higher odds of insulin resistance.
“Potential mechanisms for NAFLD may be related to the formation of reactive species during HCA metabolism, which can cause oxidation of lipids, proteins, and nucleic acids, resulting in oxidative stress, cell damage, and loss of biological function,” the authors wrote. “HCAs were also demonstrated to be bioactive in adipocytes in vitro, leading to increased expression of genes related to inflammation, diabetes and cancer risk.”
The authors noted that their findings supported the recommendations in dietary guidelines for cardiometabolic health, which suggest no more than one to two 100-g servings per week of red meat, and no more than one 50-g serving per week of processed meats.
“Although the specific effect of different types of meat and their quantities in NAFLD requires further research, these recommendations may be helpful in the treatment of patients with NAFLD at least in terms of CVD and diabetes prevention, and maybe for NAFLD prevention by reducing insulin resistance.”
The Israeli Ministry of Health supported the study. No conflicts of interest were declared.
SOURCE: Zelber-Sagi S et al. J Hepatol. 2018 Mar 20. doi: 10.1016/j.jhep.2018.01.015.
Higher dietary intake of red meat and processed meats such as salami may increase the risk of nonalcoholic fatty liver disease and insulin resistance, new research suggests.
In a cross-sectional study, published in the March 20 edition of the Journal of Hepatology, researchers used food-frequency questionnaires to examine red and processed meat consumption in 789 adults aged 40-70 years, including information on cooking methods.
They found that those who reported a total meat intake above the median had a significant 49% higher odds of nonalcoholic fatty liver disease (NAFLD) (95% confidence interval, 1.05-2.13; P = .028) and 63% greater odds of insulin resistance (95% CI, 1.12-2.37, P = .011), even after adjustment for potential confounders such as body mass index, physical activity, smoking, alcohol, and saturated fat and cholesterol intake.
Those whose intake of red and/or processed meat was above the median had a 47% greater odds of NAFLD (P = .031), and a 55% greater odds of insulin resistance (P = .020).
Even when the analysis was limited to nondiabetic participants, the study still showed a significant relationship between higher intake of red and processed meat, and insulin resistance (J Hepatol. 2018 Mar 20. doi: 10.1016/j.jhep.2018.01.015).
“It can be claimed that the harmful association with meat may, at least partially, be related to a generally less healthy diet or lifestyle characterizing people who eat more red or processed meat, rather than a causal effect of meat,” wrote Shira Zelber-Sagi, PhD, of the department of gastroenterology at Tel Aviv Medical Center, and coauthors. “However, in the current study we meticulously adjusted the association with meat for other nutritional and lifestyle parameters to minimize confounding as much as possible.”
There was also a significant association between unhealthy cooking methods such as frying, broiling, and grilling – which are known to increase the quantity of heterocyclic amines (HCA) in the meat – and insulin resistance.
Individuals who ate one or more portions of meat cooked by these methods showed a higher incidence of insulin resistance compared with those who ate fewer than one portion per week (36.00% vs. 22.20%, P = .004). Researchers also used the food-frequency questionnaires to calculate the quantity of participants’ HCA intake, and found a significantly higher odds of insulin resistance in individuals whose HCA intake was above the median.
Even among the 305 individuals with NAFLD, higher total meat intake, and higher red and processed meat intake, the prevalence of insulin resistance was higher.
In this group, high HCA intake and high consumption of meat cooked by the unhealthy methods were associated with a fourfold higher odds of insulin resistance.
“Potential mechanisms for NAFLD may be related to the formation of reactive species during HCA metabolism, which can cause oxidation of lipids, proteins, and nucleic acids, resulting in oxidative stress, cell damage, and loss of biological function,” the authors wrote. “HCAs were also demonstrated to be bioactive in adipocytes in vitro, leading to increased expression of genes related to inflammation, diabetes and cancer risk.”
The authors noted that their findings supported the recommendations in dietary guidelines for cardiometabolic health, which suggest no more than one to two 100-g servings per week of red meat, and no more than one 50-g serving per week of processed meats.
“Although the specific effect of different types of meat and their quantities in NAFLD requires further research, these recommendations may be helpful in the treatment of patients with NAFLD at least in terms of CVD and diabetes prevention, and maybe for NAFLD prevention by reducing insulin resistance.”
The Israeli Ministry of Health supported the study. No conflicts of interest were declared.
SOURCE: Zelber-Sagi S et al. J Hepatol. 2018 Mar 20. doi: 10.1016/j.jhep.2018.01.015.
Higher dietary intake of red meat and processed meats such as salami may increase the risk of nonalcoholic fatty liver disease and insulin resistance, new research suggests.
In a cross-sectional study, published in the March 20 edition of the Journal of Hepatology, researchers used food-frequency questionnaires to examine red and processed meat consumption in 789 adults aged 40-70 years, including information on cooking methods.
They found that those who reported a total meat intake above the median had a significant 49% higher odds of nonalcoholic fatty liver disease (NAFLD) (95% confidence interval, 1.05-2.13; P = .028) and 63% greater odds of insulin resistance (95% CI, 1.12-2.37, P = .011), even after adjustment for potential confounders such as body mass index, physical activity, smoking, alcohol, and saturated fat and cholesterol intake.
Those whose intake of red and/or processed meat was above the median had a 47% greater odds of NAFLD (P = .031), and a 55% greater odds of insulin resistance (P = .020).
Even when the analysis was limited to nondiabetic participants, the study still showed a significant relationship between higher intake of red and processed meat, and insulin resistance (J Hepatol. 2018 Mar 20. doi: 10.1016/j.jhep.2018.01.015).
“It can be claimed that the harmful association with meat may, at least partially, be related to a generally less healthy diet or lifestyle characterizing people who eat more red or processed meat, rather than a causal effect of meat,” wrote Shira Zelber-Sagi, PhD, of the department of gastroenterology at Tel Aviv Medical Center, and coauthors. “However, in the current study we meticulously adjusted the association with meat for other nutritional and lifestyle parameters to minimize confounding as much as possible.”
There was also a significant association between unhealthy cooking methods such as frying, broiling, and grilling – which are known to increase the quantity of heterocyclic amines (HCA) in the meat – and insulin resistance.
Individuals who ate one or more portions of meat cooked by these methods showed a higher incidence of insulin resistance compared with those who ate fewer than one portion per week (36.00% vs. 22.20%, P = .004). Researchers also used the food-frequency questionnaires to calculate the quantity of participants’ HCA intake, and found a significantly higher odds of insulin resistance in individuals whose HCA intake was above the median.
Even among the 305 individuals with NAFLD, higher total meat intake, and higher red and processed meat intake, the prevalence of insulin resistance was higher.
In this group, high HCA intake and high consumption of meat cooked by the unhealthy methods were associated with a fourfold higher odds of insulin resistance.
“Potential mechanisms for NAFLD may be related to the formation of reactive species during HCA metabolism, which can cause oxidation of lipids, proteins, and nucleic acids, resulting in oxidative stress, cell damage, and loss of biological function,” the authors wrote. “HCAs were also demonstrated to be bioactive in adipocytes in vitro, leading to increased expression of genes related to inflammation, diabetes and cancer risk.”
The authors noted that their findings supported the recommendations in dietary guidelines for cardiometabolic health, which suggest no more than one to two 100-g servings per week of red meat, and no more than one 50-g serving per week of processed meats.
“Although the specific effect of different types of meat and their quantities in NAFLD requires further research, these recommendations may be helpful in the treatment of patients with NAFLD at least in terms of CVD and diabetes prevention, and maybe for NAFLD prevention by reducing insulin resistance.”
The Israeli Ministry of Health supported the study. No conflicts of interest were declared.
SOURCE: Zelber-Sagi S et al. J Hepatol. 2018 Mar 20. doi: 10.1016/j.jhep.2018.01.015.
FROM JOURNAL OF HEPATOLOGY
Key clinical point: High red meat intake increases the risk of nonalcoholic fatty liver disease.
Major finding: Higher meat intake was associated with a 49% greater odds of NAFLD.
Study details: A cross-sectional study of 789 adults.
Disclosures: The Israeli Ministry of Health supported the study. No conflicts of interest were declared.
Source: Zelber-Sagi S et al. J. Hepatol. 2018. doi: 10.1016/j.jhep.2018.01.015.
Pre-screening could help identify NAFLD biopsy candidates
PHILADELPHIA – When evaluating patients with established non-alcoholic fatty liver disease (NAFLD), using pre-screening criteria may help identify the subset of patients who should be subjected to liver biopsy, according to Vinod K. Rustgi, MD.
The recently described pre-screening criteria include a set of patient and disease characteristics that identify who might be at highest risk for non-alcoholic steatohepatitis (NASH) and fibrosis, said Dr. Rustgi, chief of hepatology at Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
“Eighty-five percent of the patients who fall into this category will actually have fibrosis on liver biopsy. So it’s a good way to screen out who you want to actually biopsy,” Dr. Rustgi said at the meeting, jointly provided by Rutgers and Global Academy for Medical Education.
Liver biopsy needs to be considered in patients with NAFLD who are at increased risk of having advanced fibrosis, according to the latest practice guidance on diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57).
While it’s the most accurate means to diagnose and stage severity of NASH, biopsy is invasive, costly, and associated with potential complications, according to a recent Hepatology review article (2017 Dec 9. doi: 10.1002/hep.29721).
Criteria to pinpoint patients at highest risk for NASH and fibrosis could be useful for streamlining clinical trial enrollment and limiting screening failures, according to authors who recently described the pre-screening criteria in the Journal of Hepatology (2018 Feb. doi: 10.1016/j.jhep.2017.10.015).
, or who have a vibration controlled transient elastography (VCTE, FibroScan) score of kPa greater than 8.5, or an AST/ALT ratio greater than 1, among several other criteria described in the article.
“The patients who have a low likelihood of NASH and fibrosis are those who are under the age of 40, who may not be diabetic, who have a elastography score of kPa less than 7, or an AST less than 20,” Dr. Rustgi said of the pre-screening criteria.
In his presentation, Dr. Rustgi provided other notes on when to biopsy as described in the January 2018 practice guidance from Hepatology.
In particular, the guidance states that presence of metabolic syndrome, NAFLD fibrosis score or Fibrosis 4 Score, or liver stiffness measured by VCTE or magnetic resonance elastography might be used to help identify patients at risk for steatohepatitis or advanced fibrosis.
Liver biopsy also should be considered in NAFLD when competing etiologies cannot be excluded except by a liver biopsy, according to the recent guidance.
Dr. Rustgi reported disclosures related to AbbVie, Genfit, and Gilead Sciences.
Global Academy for Medical Education and this news organization are owned by the same company.
PHILADELPHIA – When evaluating patients with established non-alcoholic fatty liver disease (NAFLD), using pre-screening criteria may help identify the subset of patients who should be subjected to liver biopsy, according to Vinod K. Rustgi, MD.
The recently described pre-screening criteria include a set of patient and disease characteristics that identify who might be at highest risk for non-alcoholic steatohepatitis (NASH) and fibrosis, said Dr. Rustgi, chief of hepatology at Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
“Eighty-five percent of the patients who fall into this category will actually have fibrosis on liver biopsy. So it’s a good way to screen out who you want to actually biopsy,” Dr. Rustgi said at the meeting, jointly provided by Rutgers and Global Academy for Medical Education.
Liver biopsy needs to be considered in patients with NAFLD who are at increased risk of having advanced fibrosis, according to the latest practice guidance on diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57).
While it’s the most accurate means to diagnose and stage severity of NASH, biopsy is invasive, costly, and associated with potential complications, according to a recent Hepatology review article (2017 Dec 9. doi: 10.1002/hep.29721).
Criteria to pinpoint patients at highest risk for NASH and fibrosis could be useful for streamlining clinical trial enrollment and limiting screening failures, according to authors who recently described the pre-screening criteria in the Journal of Hepatology (2018 Feb. doi: 10.1016/j.jhep.2017.10.015).
, or who have a vibration controlled transient elastography (VCTE, FibroScan) score of kPa greater than 8.5, or an AST/ALT ratio greater than 1, among several other criteria described in the article.
“The patients who have a low likelihood of NASH and fibrosis are those who are under the age of 40, who may not be diabetic, who have a elastography score of kPa less than 7, or an AST less than 20,” Dr. Rustgi said of the pre-screening criteria.
In his presentation, Dr. Rustgi provided other notes on when to biopsy as described in the January 2018 practice guidance from Hepatology.
In particular, the guidance states that presence of metabolic syndrome, NAFLD fibrosis score or Fibrosis 4 Score, or liver stiffness measured by VCTE or magnetic resonance elastography might be used to help identify patients at risk for steatohepatitis or advanced fibrosis.
Liver biopsy also should be considered in NAFLD when competing etiologies cannot be excluded except by a liver biopsy, according to the recent guidance.
Dr. Rustgi reported disclosures related to AbbVie, Genfit, and Gilead Sciences.
Global Academy for Medical Education and this news organization are owned by the same company.
PHILADELPHIA – When evaluating patients with established non-alcoholic fatty liver disease (NAFLD), using pre-screening criteria may help identify the subset of patients who should be subjected to liver biopsy, according to Vinod K. Rustgi, MD.
The recently described pre-screening criteria include a set of patient and disease characteristics that identify who might be at highest risk for non-alcoholic steatohepatitis (NASH) and fibrosis, said Dr. Rustgi, chief of hepatology at Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
“Eighty-five percent of the patients who fall into this category will actually have fibrosis on liver biopsy. So it’s a good way to screen out who you want to actually biopsy,” Dr. Rustgi said at the meeting, jointly provided by Rutgers and Global Academy for Medical Education.
Liver biopsy needs to be considered in patients with NAFLD who are at increased risk of having advanced fibrosis, according to the latest practice guidance on diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57).
While it’s the most accurate means to diagnose and stage severity of NASH, biopsy is invasive, costly, and associated with potential complications, according to a recent Hepatology review article (2017 Dec 9. doi: 10.1002/hep.29721).
Criteria to pinpoint patients at highest risk for NASH and fibrosis could be useful for streamlining clinical trial enrollment and limiting screening failures, according to authors who recently described the pre-screening criteria in the Journal of Hepatology (2018 Feb. doi: 10.1016/j.jhep.2017.10.015).
, or who have a vibration controlled transient elastography (VCTE, FibroScan) score of kPa greater than 8.5, or an AST/ALT ratio greater than 1, among several other criteria described in the article.
“The patients who have a low likelihood of NASH and fibrosis are those who are under the age of 40, who may not be diabetic, who have a elastography score of kPa less than 7, or an AST less than 20,” Dr. Rustgi said of the pre-screening criteria.
In his presentation, Dr. Rustgi provided other notes on when to biopsy as described in the January 2018 practice guidance from Hepatology.
In particular, the guidance states that presence of metabolic syndrome, NAFLD fibrosis score or Fibrosis 4 Score, or liver stiffness measured by VCTE or magnetic resonance elastography might be used to help identify patients at risk for steatohepatitis or advanced fibrosis.
Liver biopsy also should be considered in NAFLD when competing etiologies cannot be excluded except by a liver biopsy, according to the recent guidance.
Dr. Rustgi reported disclosures related to AbbVie, Genfit, and Gilead Sciences.
Global Academy for Medical Education and this news organization are owned by the same company.
REPORTING FROM DIGESTIVE DISEASES: NEW ADVANCES
Bioengineered liver models screen drugs and study liver injury
resulting in stabilized liver functions for several weeks in vitro. Studies have focused on using these models to investigate cell responses to drugs and other stimuli (for example, viruses and cell differentiation cues) to predict clinical outcomes. Gregory H. Underhill, PhD, from the department of bioengineering at the University of Illinois at Urbana-Champaign and Salman R. Khetani, PhD, from the department of bioengineering at the University of Illinois in Chicago presented a comprehensive review of the these advances in bioengineered liver models in Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2017.11.012).
Drug-induced liver injury (DILI) is a leading cause of drug attrition in the United States, with some marketed drugs causing cell necrosis, hepatitis, cholestasis, fibrosis, or a mixture of injury types. Although the Food and Drug Administration requires preclinical drug testing in animal models, differences in species-specific drug metabolism pathways and human genetics may result in inadequate identification of potential for human DILI. Some bioengineered liver models for in vitro studies are based on tissue engineering using high-throughput microarrays, protein micropatterning, microfluidics, specialized plates, biomaterial scaffolds, and bioprinting.
High-throughput cell microarrays enable systematic analysis of a large number of drugs or compounds at a relatively low cost. Several culture platforms have been developed using multiple sources of liver cells, including cancerous and immortalized cell lines. These platforms show enhanced capabilities to evaluate combinatorial effects of multiple signals with independent control of biochemical and biomechanical cues. For instance, a microchip platform for transducing 3-D liver cell cultures with genes for drug metabolism enzymes featuring 532 reaction vessels (micropillars and corresponding microwells) was able to provide information about certain enzyme combinations that led to drug toxicity in cells. The high-throughput cell microarrays are, however, primarily dependent on imaging-based readouts and have a limited ability to investigate cell responses to gradients of microenvironmental signals.
Liver development, physiology, and pathophysiology are dependent on homotypic and heterotypic interactions between parenchymal and nonparenchymal cells (NPCs). Cocultures with both liver- and nonliver-derived NPC types, in vitro, can induce liver functions transiently and have proven useful for investigating host responses to sepsis, mutagenesis, xenobiotic metabolism and toxicity, response to oxidative stress, lipid metabolism, and induction of the acute-phase response. Micropatterned cocultures (MPCCs) are designed to allow the use of different NPC types without significantly altering hepatocyte homotypic interactions. Cell-cell interactions can be precisely controlled to allow for stable functions for up to 4-6 weeks, whereas more randomly distributed cocultures have limited stability. Unlike randomly distributed cocultures, MPCCs can be infected with HBV, HCV, and malaria. Potential limitations of MPCCs include the requirement for specialized equipment and devices for patterning collagen for hepatocyte attachment.
Randomly distributed spheroids or organoids enable 3-D establishment of homotypic cell-cell interactions surrounded by an extracellular matrix. The spheroids can be further cocultured with NPCs that facilitate heterotypic cell-cell interactions and allow the evaluation of outcomes resulting from drugs and other stimuli. Hepatic spheroids maintain major liver functions for several weeks and have proven to be compatible with multiple applications within the drug development pipeline.
These spheroids showed greater sensitivity in identifying known hepatotoxic drugs than did short-term primary human hepatocyte (PHH) monolayers. PHHs secreted liver proteins, such as albumin, transferrin, and fibrinogen, and showed cytochrome-P450 activities for 77-90 days when cultured on a nylon scaffold containing a mixture of liver NPCs and PHHs.
Nanopillar plates can be used to create induced pluripotent stem cell–derived human hepatocyte-like cell (iHep) spheroids; although these spheroids showed some potential for initial drug toxicity screening, they had lower overall sensitivity than conventional PHH monolayers, which suggests that further maturation of iHeps is likely required.
Potential limitations of randomly distributed spheroids include necrosis of cells in the center of larger spheroids and the requirement for expensive confocal microscopy for high-content imaging of entire spheroid cultures. To overcome the limitation of disorganized cell type interactions over time within the randomly distributed spheroids/organoids, bioprinted human liver organoids are designed to allow precise control of cell placement.
Yet another bioengineered liver model is based on perfusion systems or bioreactors that enable dynamic fluid flow for nutrient and waste exchange. These so called liver-on-a-chip devices contain hepatocyte aggregates adhered to collagen-coated microchannel walls; these are then perfused at optimal flow rates both to meet the oxygen demands of the hepatocytes and deliver low shear stress to the cells that’s similar to what would be the case in vivo. Layered architectures can be created with single-chamber or multichamber, microfluidic device designs that can sustain cell functionality for 2-4 weeks.
Some of the limitations of perfusion systems include the potential binding of drugs to tubing and materials used, large dead volume requiring higher quantities of novel compounds for the treatment of cell cultures, low throughput, and washing away of built-up beneficial molecules with perfusion.
The ongoing development of more sophisticated engineering tools for manipulating cells in culture will lead to continued advances in bioengineered livers that will show improving sensitivity for the prediction of clinically relevant drug and disease outcomes.
This work was funded by National Institutes of Health grants. The author Dr. Khetani disclosed a conflict of interest with Ascendance Biotechnology, which has licensed the micropatterned coculture and related systems from Massachusetts Institute of Technology, Cambridge, and Colorado State University, Fort Collins, for commercial distribution. Dr. Underhill disclosed no conflicts.
SOURCE: Underhill GH and Khetani SR. Cell Molec Gastro Hepatol. 2017. doi: org/10.1016/j.jcmgh.2017.11.012.
Thirty to 50 new drugs are approved in the United States annually, which costs approximately $2.5 billion/drug in drug development costs. Nine out of 10 drugs never make it to market, and of those that do, adverse events affect their longevity. Hepatotoxicity is the most frequent adverse drug reaction, and drug-induced liver injury, which can lead to acute liver failure, occurs in a subset of affected patients. Understanding a drug’s risk of hepatotoxicity before patients start using it can not only save lives but also conceivably reduce the costs incurred by pharmaceutical companies, which are passed on to consumers.
However, just as we have seen with the limitations of the in vitro systems, bioartificial livers are unlikely to be successful unless they integrate the liver’s complex functions of protein synthesis, immune surveillance, energy homeostasis, and nutrient sensing. The future is bright, though, as biomedical scientists and bioengineers continue to push the envelope by advancing both in vitro and bioartificial technologies.
Rotonya Carr, MD, is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia. She receives research support from Intercept Pharmaceuticals.
Thirty to 50 new drugs are approved in the United States annually, which costs approximately $2.5 billion/drug in drug development costs. Nine out of 10 drugs never make it to market, and of those that do, adverse events affect their longevity. Hepatotoxicity is the most frequent adverse drug reaction, and drug-induced liver injury, which can lead to acute liver failure, occurs in a subset of affected patients. Understanding a drug’s risk of hepatotoxicity before patients start using it can not only save lives but also conceivably reduce the costs incurred by pharmaceutical companies, which are passed on to consumers.
However, just as we have seen with the limitations of the in vitro systems, bioartificial livers are unlikely to be successful unless they integrate the liver’s complex functions of protein synthesis, immune surveillance, energy homeostasis, and nutrient sensing. The future is bright, though, as biomedical scientists and bioengineers continue to push the envelope by advancing both in vitro and bioartificial technologies.
Rotonya Carr, MD, is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia. She receives research support from Intercept Pharmaceuticals.
Thirty to 50 new drugs are approved in the United States annually, which costs approximately $2.5 billion/drug in drug development costs. Nine out of 10 drugs never make it to market, and of those that do, adverse events affect their longevity. Hepatotoxicity is the most frequent adverse drug reaction, and drug-induced liver injury, which can lead to acute liver failure, occurs in a subset of affected patients. Understanding a drug’s risk of hepatotoxicity before patients start using it can not only save lives but also conceivably reduce the costs incurred by pharmaceutical companies, which are passed on to consumers.
However, just as we have seen with the limitations of the in vitro systems, bioartificial livers are unlikely to be successful unless they integrate the liver’s complex functions of protein synthesis, immune surveillance, energy homeostasis, and nutrient sensing. The future is bright, though, as biomedical scientists and bioengineers continue to push the envelope by advancing both in vitro and bioartificial technologies.
Rotonya Carr, MD, is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia. She receives research support from Intercept Pharmaceuticals.
resulting in stabilized liver functions for several weeks in vitro. Studies have focused on using these models to investigate cell responses to drugs and other stimuli (for example, viruses and cell differentiation cues) to predict clinical outcomes. Gregory H. Underhill, PhD, from the department of bioengineering at the University of Illinois at Urbana-Champaign and Salman R. Khetani, PhD, from the department of bioengineering at the University of Illinois in Chicago presented a comprehensive review of the these advances in bioengineered liver models in Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2017.11.012).
Drug-induced liver injury (DILI) is a leading cause of drug attrition in the United States, with some marketed drugs causing cell necrosis, hepatitis, cholestasis, fibrosis, or a mixture of injury types. Although the Food and Drug Administration requires preclinical drug testing in animal models, differences in species-specific drug metabolism pathways and human genetics may result in inadequate identification of potential for human DILI. Some bioengineered liver models for in vitro studies are based on tissue engineering using high-throughput microarrays, protein micropatterning, microfluidics, specialized plates, biomaterial scaffolds, and bioprinting.
High-throughput cell microarrays enable systematic analysis of a large number of drugs or compounds at a relatively low cost. Several culture platforms have been developed using multiple sources of liver cells, including cancerous and immortalized cell lines. These platforms show enhanced capabilities to evaluate combinatorial effects of multiple signals with independent control of biochemical and biomechanical cues. For instance, a microchip platform for transducing 3-D liver cell cultures with genes for drug metabolism enzymes featuring 532 reaction vessels (micropillars and corresponding microwells) was able to provide information about certain enzyme combinations that led to drug toxicity in cells. The high-throughput cell microarrays are, however, primarily dependent on imaging-based readouts and have a limited ability to investigate cell responses to gradients of microenvironmental signals.
Liver development, physiology, and pathophysiology are dependent on homotypic and heterotypic interactions between parenchymal and nonparenchymal cells (NPCs). Cocultures with both liver- and nonliver-derived NPC types, in vitro, can induce liver functions transiently and have proven useful for investigating host responses to sepsis, mutagenesis, xenobiotic metabolism and toxicity, response to oxidative stress, lipid metabolism, and induction of the acute-phase response. Micropatterned cocultures (MPCCs) are designed to allow the use of different NPC types without significantly altering hepatocyte homotypic interactions. Cell-cell interactions can be precisely controlled to allow for stable functions for up to 4-6 weeks, whereas more randomly distributed cocultures have limited stability. Unlike randomly distributed cocultures, MPCCs can be infected with HBV, HCV, and malaria. Potential limitations of MPCCs include the requirement for specialized equipment and devices for patterning collagen for hepatocyte attachment.
Randomly distributed spheroids or organoids enable 3-D establishment of homotypic cell-cell interactions surrounded by an extracellular matrix. The spheroids can be further cocultured with NPCs that facilitate heterotypic cell-cell interactions and allow the evaluation of outcomes resulting from drugs and other stimuli. Hepatic spheroids maintain major liver functions for several weeks and have proven to be compatible with multiple applications within the drug development pipeline.
These spheroids showed greater sensitivity in identifying known hepatotoxic drugs than did short-term primary human hepatocyte (PHH) monolayers. PHHs secreted liver proteins, such as albumin, transferrin, and fibrinogen, and showed cytochrome-P450 activities for 77-90 days when cultured on a nylon scaffold containing a mixture of liver NPCs and PHHs.
Nanopillar plates can be used to create induced pluripotent stem cell–derived human hepatocyte-like cell (iHep) spheroids; although these spheroids showed some potential for initial drug toxicity screening, they had lower overall sensitivity than conventional PHH monolayers, which suggests that further maturation of iHeps is likely required.
Potential limitations of randomly distributed spheroids include necrosis of cells in the center of larger spheroids and the requirement for expensive confocal microscopy for high-content imaging of entire spheroid cultures. To overcome the limitation of disorganized cell type interactions over time within the randomly distributed spheroids/organoids, bioprinted human liver organoids are designed to allow precise control of cell placement.
Yet another bioengineered liver model is based on perfusion systems or bioreactors that enable dynamic fluid flow for nutrient and waste exchange. These so called liver-on-a-chip devices contain hepatocyte aggregates adhered to collagen-coated microchannel walls; these are then perfused at optimal flow rates both to meet the oxygen demands of the hepatocytes and deliver low shear stress to the cells that’s similar to what would be the case in vivo. Layered architectures can be created with single-chamber or multichamber, microfluidic device designs that can sustain cell functionality for 2-4 weeks.
Some of the limitations of perfusion systems include the potential binding of drugs to tubing and materials used, large dead volume requiring higher quantities of novel compounds for the treatment of cell cultures, low throughput, and washing away of built-up beneficial molecules with perfusion.
The ongoing development of more sophisticated engineering tools for manipulating cells in culture will lead to continued advances in bioengineered livers that will show improving sensitivity for the prediction of clinically relevant drug and disease outcomes.
This work was funded by National Institutes of Health grants. The author Dr. Khetani disclosed a conflict of interest with Ascendance Biotechnology, which has licensed the micropatterned coculture and related systems from Massachusetts Institute of Technology, Cambridge, and Colorado State University, Fort Collins, for commercial distribution. Dr. Underhill disclosed no conflicts.
SOURCE: Underhill GH and Khetani SR. Cell Molec Gastro Hepatol. 2017. doi: org/10.1016/j.jcmgh.2017.11.012.
resulting in stabilized liver functions for several weeks in vitro. Studies have focused on using these models to investigate cell responses to drugs and other stimuli (for example, viruses and cell differentiation cues) to predict clinical outcomes. Gregory H. Underhill, PhD, from the department of bioengineering at the University of Illinois at Urbana-Champaign and Salman R. Khetani, PhD, from the department of bioengineering at the University of Illinois in Chicago presented a comprehensive review of the these advances in bioengineered liver models in Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2017.11.012).
Drug-induced liver injury (DILI) is a leading cause of drug attrition in the United States, with some marketed drugs causing cell necrosis, hepatitis, cholestasis, fibrosis, or a mixture of injury types. Although the Food and Drug Administration requires preclinical drug testing in animal models, differences in species-specific drug metabolism pathways and human genetics may result in inadequate identification of potential for human DILI. Some bioengineered liver models for in vitro studies are based on tissue engineering using high-throughput microarrays, protein micropatterning, microfluidics, specialized plates, biomaterial scaffolds, and bioprinting.
High-throughput cell microarrays enable systematic analysis of a large number of drugs or compounds at a relatively low cost. Several culture platforms have been developed using multiple sources of liver cells, including cancerous and immortalized cell lines. These platforms show enhanced capabilities to evaluate combinatorial effects of multiple signals with independent control of biochemical and biomechanical cues. For instance, a microchip platform for transducing 3-D liver cell cultures with genes for drug metabolism enzymes featuring 532 reaction vessels (micropillars and corresponding microwells) was able to provide information about certain enzyme combinations that led to drug toxicity in cells. The high-throughput cell microarrays are, however, primarily dependent on imaging-based readouts and have a limited ability to investigate cell responses to gradients of microenvironmental signals.
Liver development, physiology, and pathophysiology are dependent on homotypic and heterotypic interactions between parenchymal and nonparenchymal cells (NPCs). Cocultures with both liver- and nonliver-derived NPC types, in vitro, can induce liver functions transiently and have proven useful for investigating host responses to sepsis, mutagenesis, xenobiotic metabolism and toxicity, response to oxidative stress, lipid metabolism, and induction of the acute-phase response. Micropatterned cocultures (MPCCs) are designed to allow the use of different NPC types without significantly altering hepatocyte homotypic interactions. Cell-cell interactions can be precisely controlled to allow for stable functions for up to 4-6 weeks, whereas more randomly distributed cocultures have limited stability. Unlike randomly distributed cocultures, MPCCs can be infected with HBV, HCV, and malaria. Potential limitations of MPCCs include the requirement for specialized equipment and devices for patterning collagen for hepatocyte attachment.
Randomly distributed spheroids or organoids enable 3-D establishment of homotypic cell-cell interactions surrounded by an extracellular matrix. The spheroids can be further cocultured with NPCs that facilitate heterotypic cell-cell interactions and allow the evaluation of outcomes resulting from drugs and other stimuli. Hepatic spheroids maintain major liver functions for several weeks and have proven to be compatible with multiple applications within the drug development pipeline.
These spheroids showed greater sensitivity in identifying known hepatotoxic drugs than did short-term primary human hepatocyte (PHH) monolayers. PHHs secreted liver proteins, such as albumin, transferrin, and fibrinogen, and showed cytochrome-P450 activities for 77-90 days when cultured on a nylon scaffold containing a mixture of liver NPCs and PHHs.
Nanopillar plates can be used to create induced pluripotent stem cell–derived human hepatocyte-like cell (iHep) spheroids; although these spheroids showed some potential for initial drug toxicity screening, they had lower overall sensitivity than conventional PHH monolayers, which suggests that further maturation of iHeps is likely required.
Potential limitations of randomly distributed spheroids include necrosis of cells in the center of larger spheroids and the requirement for expensive confocal microscopy for high-content imaging of entire spheroid cultures. To overcome the limitation of disorganized cell type interactions over time within the randomly distributed spheroids/organoids, bioprinted human liver organoids are designed to allow precise control of cell placement.
Yet another bioengineered liver model is based on perfusion systems or bioreactors that enable dynamic fluid flow for nutrient and waste exchange. These so called liver-on-a-chip devices contain hepatocyte aggregates adhered to collagen-coated microchannel walls; these are then perfused at optimal flow rates both to meet the oxygen demands of the hepatocytes and deliver low shear stress to the cells that’s similar to what would be the case in vivo. Layered architectures can be created with single-chamber or multichamber, microfluidic device designs that can sustain cell functionality for 2-4 weeks.
Some of the limitations of perfusion systems include the potential binding of drugs to tubing and materials used, large dead volume requiring higher quantities of novel compounds for the treatment of cell cultures, low throughput, and washing away of built-up beneficial molecules with perfusion.
The ongoing development of more sophisticated engineering tools for manipulating cells in culture will lead to continued advances in bioengineered livers that will show improving sensitivity for the prediction of clinically relevant drug and disease outcomes.
This work was funded by National Institutes of Health grants. The author Dr. Khetani disclosed a conflict of interest with Ascendance Biotechnology, which has licensed the micropatterned coculture and related systems from Massachusetts Institute of Technology, Cambridge, and Colorado State University, Fort Collins, for commercial distribution. Dr. Underhill disclosed no conflicts.
SOURCE: Underhill GH and Khetani SR. Cell Molec Gastro Hepatol. 2017. doi: org/10.1016/j.jcmgh.2017.11.012.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Tenofovir didn’t prevent hepatitis B transmission to newborns
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tenofovir was no different than placebo in preventing HBV transmission to newborns.
Major finding: The transmission rate was 0% in the tenofovir group and 2% in the placebo group (P = .12).
Study details: The study randomized 331 pregnant women to tenofovir or placebo from gestational week 28 to 2 months postpartum.
Disclosures: Dr. Jourdain had no financial disclosures; the National Institute of Child Health and Development sponsored the study.
Source: Jourdain G et al. N Engl J Med. 2018;378:911-23.