User login
Are Food Emulsifiers Associated With Increased Cancer Risk?
Food emulsifiers are among the most widespread food additives.
Ultraprocessed foods constitute a significant part of our diet, representing approximately 30% of energy intake in France.
Large epidemiologic studies have already linked diets rich in ultraprocessed products to an increased risk for cardiovascular diseases, diabetes, obesity, and mortality. Possible explanations for this association include the presence of additives, particularly emulsifiers. These additives are intended to improve the texture and shelf life of foods.
Recent experimental studies have shown that emulsifiers alter the gut microbiota and may lead to low-grade inflammation. Dysbiosis and chronic inflammation not only increase the risk for inflammatory bowel diseases but are also implicated in the etiology of several other chronic pathologies and certain extraintestinal cancers.
The NutriNet-Santé study provided extensive information on the dietary habits of > 100,000 French participants. A new analysis was conducted, examining the possible link between the presence of emulsifiers in the diet and cancer occurrence. Data from 92,000 participants (78.8% women) were utilized. They covered an average follow-up of 6.7 years, during which 2604 cancer cases were diagnosed, including 750 breast cancers, 322 prostate cancers, and 207 colorectal cancers.
In this cohort, the risk for cancer increased with a higher presence in the diet of products containing certain emulsifiers widely used in industrial food in Europe: Carrageenans (E407), mono- and diglycerides of fatty acids (E471), pectins (E440), and sodium carbonate (E500).
Notably, the highest consumption of mono- and diglycerides of fatty acids (E471) was associated with a 15% increase in the risk for all types of cancer, a 24% increase in breast cancer risk, and a 46% increase in prostate cancer risk. The highest consumption of carrageenans (E407) was associated with a 28% increase in breast cancer risk.
In an analysis by menopausal status, the risk for breast cancer before menopause was associated with high consumption of diphosphates (E450; 45% increase), pectins (E440; 55% increase), and sodium bicarbonate (E500; 48% increase). No link was found between emulsifier consumption and colorectal cancer risk. While some associations were observed for other emulsifiers, they did not persist in sensitivity analyses.
The European Food Safety Agency recently evaluated the risks of emulsifiers, however, and found no safety issues or need to limit daily consumption of several of them, notably E471.
It is certain that cancer is multifactorial, and a single factor (here, exposure to emulsifiers) will not significantly increase the risk. However, while not essential to human health, emulsifiers are widely prevalent in the global market. Therefore, if causality is established, the increased risk could translate into a significant number of preventable cancers at the population level. Confirmation of this causal link will need to be obtained through experimental and epidemiological studies.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Food emulsifiers are among the most widespread food additives.
Ultraprocessed foods constitute a significant part of our diet, representing approximately 30% of energy intake in France.
Large epidemiologic studies have already linked diets rich in ultraprocessed products to an increased risk for cardiovascular diseases, diabetes, obesity, and mortality. Possible explanations for this association include the presence of additives, particularly emulsifiers. These additives are intended to improve the texture and shelf life of foods.
Recent experimental studies have shown that emulsifiers alter the gut microbiota and may lead to low-grade inflammation. Dysbiosis and chronic inflammation not only increase the risk for inflammatory bowel diseases but are also implicated in the etiology of several other chronic pathologies and certain extraintestinal cancers.
The NutriNet-Santé study provided extensive information on the dietary habits of > 100,000 French participants. A new analysis was conducted, examining the possible link between the presence of emulsifiers in the diet and cancer occurrence. Data from 92,000 participants (78.8% women) were utilized. They covered an average follow-up of 6.7 years, during which 2604 cancer cases were diagnosed, including 750 breast cancers, 322 prostate cancers, and 207 colorectal cancers.
In this cohort, the risk for cancer increased with a higher presence in the diet of products containing certain emulsifiers widely used in industrial food in Europe: Carrageenans (E407), mono- and diglycerides of fatty acids (E471), pectins (E440), and sodium carbonate (E500).
Notably, the highest consumption of mono- and diglycerides of fatty acids (E471) was associated with a 15% increase in the risk for all types of cancer, a 24% increase in breast cancer risk, and a 46% increase in prostate cancer risk. The highest consumption of carrageenans (E407) was associated with a 28% increase in breast cancer risk.
In an analysis by menopausal status, the risk for breast cancer before menopause was associated with high consumption of diphosphates (E450; 45% increase), pectins (E440; 55% increase), and sodium bicarbonate (E500; 48% increase). No link was found between emulsifier consumption and colorectal cancer risk. While some associations were observed for other emulsifiers, they did not persist in sensitivity analyses.
The European Food Safety Agency recently evaluated the risks of emulsifiers, however, and found no safety issues or need to limit daily consumption of several of them, notably E471.
It is certain that cancer is multifactorial, and a single factor (here, exposure to emulsifiers) will not significantly increase the risk. However, while not essential to human health, emulsifiers are widely prevalent in the global market. Therefore, if causality is established, the increased risk could translate into a significant number of preventable cancers at the population level. Confirmation of this causal link will need to be obtained through experimental and epidemiological studies.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Food emulsifiers are among the most widespread food additives.
Ultraprocessed foods constitute a significant part of our diet, representing approximately 30% of energy intake in France.
Large epidemiologic studies have already linked diets rich in ultraprocessed products to an increased risk for cardiovascular diseases, diabetes, obesity, and mortality. Possible explanations for this association include the presence of additives, particularly emulsifiers. These additives are intended to improve the texture and shelf life of foods.
Recent experimental studies have shown that emulsifiers alter the gut microbiota and may lead to low-grade inflammation. Dysbiosis and chronic inflammation not only increase the risk for inflammatory bowel diseases but are also implicated in the etiology of several other chronic pathologies and certain extraintestinal cancers.
The NutriNet-Santé study provided extensive information on the dietary habits of > 100,000 French participants. A new analysis was conducted, examining the possible link between the presence of emulsifiers in the diet and cancer occurrence. Data from 92,000 participants (78.8% women) were utilized. They covered an average follow-up of 6.7 years, during which 2604 cancer cases were diagnosed, including 750 breast cancers, 322 prostate cancers, and 207 colorectal cancers.
In this cohort, the risk for cancer increased with a higher presence in the diet of products containing certain emulsifiers widely used in industrial food in Europe: Carrageenans (E407), mono- and diglycerides of fatty acids (E471), pectins (E440), and sodium carbonate (E500).
Notably, the highest consumption of mono- and diglycerides of fatty acids (E471) was associated with a 15% increase in the risk for all types of cancer, a 24% increase in breast cancer risk, and a 46% increase in prostate cancer risk. The highest consumption of carrageenans (E407) was associated with a 28% increase in breast cancer risk.
In an analysis by menopausal status, the risk for breast cancer before menopause was associated with high consumption of diphosphates (E450; 45% increase), pectins (E440; 55% increase), and sodium bicarbonate (E500; 48% increase). No link was found between emulsifier consumption and colorectal cancer risk. While some associations were observed for other emulsifiers, they did not persist in sensitivity analyses.
The European Food Safety Agency recently evaluated the risks of emulsifiers, however, and found no safety issues or need to limit daily consumption of several of them, notably E471.
It is certain that cancer is multifactorial, and a single factor (here, exposure to emulsifiers) will not significantly increase the risk. However, while not essential to human health, emulsifiers are widely prevalent in the global market. Therefore, if causality is established, the increased risk could translate into a significant number of preventable cancers at the population level. Confirmation of this causal link will need to be obtained through experimental and epidemiological studies.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Unleashing Our Immune Response to Quash Cancer
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
This article was originally published on February 10 in Eric Topol’s substack “Ground Truths.”
It’s astounding how devious cancer cells and tumor tissue can be. This week in Science we learned how certain lung cancer cells can function like “Catch Me If You Can” — changing their driver mutation and cell identity to escape targeted therapy. This histologic transformation, as seen in an experimental model, is just one of so many cancer tricks that we are learning about.
Recently, as shown by single-cell sequencing, cancer cells can steal the mitochondria from T cells, a double whammy that turbocharges cancer cells with the hijacked fuel supply and, at the same time, dismantles the immune response.
Last week, we saw how tumor cells can release a virus-like protein that unleashes a vicious autoimmune response.
And then there’s the finding that cancer cell spread predominantly is occurring while we sleep.
As I previously reviewed, the ability for cancer cells to hijack neurons and neural circuits is now well established, no less their ability to reprogram neurons to become adrenergic and stimulate tumor progression, and interfere with the immune response. Stay tuned on that for a new Ground Truths podcast with Prof Michelle Monje, a leader in cancer neuroscience, which will post soon.
Add advancing age’s immunosenescence as yet another challenge to the long and growing list of formidable ways that cancer cells, and the tumor microenvironment, evade our immune response.
An Ever-Expanding Armamentarium
Immune Checkpoint Inhibitors
The field of immunotherapies took off with the immune checkpoint inhibitors, first approved by the FDA in 2011, that take the brakes off of T cells, with the programmed death-1 (PD-1), PD-ligand1, and anti-CTLA-4 monoclonal antibodies.
But we’re clearly learning they are not enough to prevail over cancer with common recurrences, only short term success in most patients, with some notable exceptions. Adding other immune response strategies, such as a vaccine, or antibody-drug conjugates, or engineered T cells, are showing improved chances for success.
Therapeutic Cancer Vaccines
There are many therapeutic cancer vaccines in the works, as reviewed in depth here.
Here’s a list of ongoing clinical trials of cancer vaccines. You’ll note most of these are on top of a checkpoint inhibitor and use personalized neoantigens (cancer cell surface proteins) derived from sequencing (whole-exome or whole genome, RNA-sequencing and HLA-profiling) the patient’s tumor.
An example of positive findings is with the combination of an mRNA-nanoparticle vaccine with up to 34 personalized neoantigens and pembrolizumab (Keytruda) vs pembrolizumab alone in advanced melanoma after resection, with improved outcomes at 3-year follow-up, cutting death or relapse rate in half.
Antibody-Drug Conjugates (ADC)
There is considerable excitement about antibody-drug conjugates (ADC) whereby a linker is used to attach a chemotherapy agent to the checkpoint inhibitor antibody, specifically targeting the cancer cell and facilitating entry of the chemotherapy into the cell. Akin to these are bispecific antibodies (BiTEs, binding to a tumor antigen and T cell receptor simultaneously), both of these conjugates acting as “biologic” or “guided” missiles.
A very good example of the potency of an ADC was seen in a “HER2-low” breast cancer randomized trial. The absence or very low expression or amplification of the HER2 receptor is common in breast cancer and successful treatment has been elusive. A randomized trial of an ADC (trastuzumab deruxtecan) compared to physician’s choice therapy demonstrated a marked success for progression-free survival in HER2-low patients, which was characterized as “unheard-of success” by media coverage.
This strategy is being used to target some of the most difficult cancer driver mutations such as TP53 and KRAS.
Oncolytic Viruses
Modifying viruses to infect the tumor and make it more visible to the immune system, potentiating anti-tumor responses, known as oncolytic viruses, have been proposed as a way to rev up the immune response for a long time but without positive Phase 3 clinical trials.
After decades of failure, a recent trial in refractory bladder cancer showed marked success, along with others, summarized here, now providing very encouraging results. It looks like oncolytic viruses are on a comeback path.
Engineering T Cells (Chimeric Antigen Receptor [CAR-T])
As I recently reviewed, there are over 500 ongoing clinical trials to build on the success of the first CAR-T approval for leukemia 7 years ago. I won’t go through that all again here, but to reiterate most of the success to date has been in “liquid” blood (leukemia and lymphoma) cancer tumors. This week in Nature is the discovery of a T cell cancer mutation, a gene fusion CARD11-PIK3R3, from a T cell lymphoma that can potentially be used to augment CAR-T efficacy. It has pronounced and prolonged effects in the experimental model. Instead of 1 million cells needed for treatment, even 20,000 were enough to melt the tumor. This is a noteworthy discovery since CAR-T work to date has largely not exploited such naturally occurring mutations, while instead concentrating on those seen in the patient’s set of key tumor mutations.
As currently conceived, CAR-T, and what is being referred to more broadly as adoptive cell therapies, involves removing T cells from the patient’s body and engineering their activation, then reintroducing them back to the patient. This is laborious, technically difficult, and very expensive. Recently, the idea of achieving all of this via an injection of virus that specifically infects T cells and inserts the genes needed, was advanced by two biotech companies with preclinical results, one in non-human primates.
Gearing up to meet the challenge of solid tumor CAR-T intervention, there’s more work using CRISPR genome editing of T cell receptors. A.I. is increasingly being exploited to process the data from sequencing and identify optimal neoantigens.
Instead of just CAR-T, we’re seeing the emergence of CAR-macrophage and CAR-natural killer (NK) cells strategies, and rapidly expanding potential combinations of all the strategies I’ve mentioned. No less, there’s been maturation of on-off suicide switches programmed in, to limit cytokine release and promote safety of these interventions. Overall, major side effects of immunotherapies are not only cytokine release syndromes, but also include interstitial pneumonitis and neurotoxicity.
Summary
Given the multitude of ways cancer cells and tumor tissue can evade our immune response, durably successful treatment remains a daunting challenge. But the ingenuity of so many different approaches to unleash our immune response, and their combinations, provides considerable hope that we’ll increasingly meet the challenge in the years ahead. We have clearly learned that combining different immunotherapy strategies will be essential for many patients with the most resilient solid tumors.
Of concern, as noted by a recent editorial in The Lancet, entitled “Cancer Research Equity: Innovations For The Many, Not The Few,” is that these individualized, sophisticated strategies are not scalable; they will have limited reach and benefit. The movement towards “off the shelf” CAR-T and inexpensive, orally active checkpoint inhibitors may help mitigate this issue.
Notwithstanding this important concern, we’re seeing an array of diverse and potent immunotherapy strategies that are providing highly encouraging results, engendering more excitement than we’ve seen in this space for some time. These should propel substantial improvements in outcomes for patients in the years ahead. It can’t happen soon enough.
Thanks for reading this edition of Ground Truths. If you found it informative, please share it with your colleagues.
Dr. Topol has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for Dexcom; Illumina; Molecular Stethoscope; Quest Diagnostics; Blue Cross Blue Shield Association. Received research grant from National Institutes of Health.
A version of this article appeared on Medscape.com.
The Evolving Treatment Paradigm for Diffuse Large B-Cell Lymphoma
Non-Hodgkin lymphomas (NHLs) are cancers that arise in a type of white blood cell called the lymphocyte. NHLs are divided into B- and T-cell subtypes, as well as aggressive and indolent forms. Management varies widely depending on the disease type. We will focus on the most common type of NHL, diffuse large B-cell lymphoma (DLBCL), for which there have been significant treatment advances in recent years.
DLBCL is curable in about two-thirds of patients using chemoimmunotherapy. The longstanding frontline treatment for this disease has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In 2023, an antibody-drug conjugate against the B-cell surface protein CD79b, polatuzumab vedotin, was approved by the US Food and Drug Administration (FDA) in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for newly diagnosed DLBCL based on an improvement in progression-free survival at 2 years in patients with high-risk disease features enrolled in the POLARIX study.
For patients who do not respond to the initial treatment or in whom the disease recurs, the historical standard of care treatment strategy was high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Unfortunately, this approach is not feasible or not successful in a significant percentage of patients with relapsed or refractory DLBCL.
A newer strategy for DLBCL is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are collected from a patient and genetically modified to target a protein on the lymphoma cells called CD19. This type of treatment was initially approved in the third-line setting for DLBCL based on the ZUMA-1 (axi-cel), JULIET (tisa-cel), and TRANSCEND (liso-cel) clinical trials. More recently, in 2022, 2 of these agents received approval in the second-line setting in patients who relapse or are refractory to initial treatment within 1 year; axi-cel was approved based on the ZUMA-7 trial and liso-cel was approved based on the TRANSFORM trial.
Unfortunately, not all patients are eligible for ASCT and CAR T-cell therapy due to factors including age, comorbidities, and disease characteristics. Some patients prefer alternative therapies based on the potential side effects of CAR T-cell therapy and ASCT. Toxicities associated with CAR T-cell therapy include an inflammatory response called cytokine release syndrome and neurologic events.
For patients who are not eligible for or who relapse after ASCT or CAR T-cell therapy, several alternative treatment options are FDA approved. Novel strategies include polatuzumab vedotin with bendamustine and rituximab and tafasitamab plus lenalidomide. Tafasitamab is a monoclonal antibody against CD19 and lenalidomide is an oral anticancer agent originally approved for use in multiple myeloma. Lenalidomide is also effective and commonly used in other NHL subtypes.
In 2023, a new category of treatment called bispecific antibodies was approved in patients with DLBCL in whom the disease recurs after 2 lines of therapy. These drugs (epcoritamab and glofitamab) are a form of immunotherapy that connects B cells with T cells to enable a person’s own immune system to better fight the lymphoma. While these drugs can have similar toxicities as CAR T-cell therapy, the severity and incidence are much lower. In contrast to CAR T-cell therapy, which requires only 1 infusion, these drugs are given regularly in either subcutaneous or intravenous form for several months.
Two other FDA-approved treatment options for relapsed and refractory DLBCL are loncastuximab tesirine, an antibody-drug conjugate targeting CD19 with approval based on the results of the LOTIS-2 trial, and the oral selective inhibitor of nuclear export called selinexor, based on the results from the SADAL trial. Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Selinexor is approved for use in adults with relapsed or refractory DLBCL who have received at least 2 types of systemic therapy. Trials investigating these agents in combination with other novel treatments are ongoing.
The treatment landscape for DLBCL has changed markedly over the past several years. Therapies can be tailored for individual patients based on their disease status and characteristics, comorbidities, and treatment preferences. Research with novel strategies continues with the goal of a cure for all patients diagnosed with DLBCL.
Non-Hodgkin lymphomas (NHLs) are cancers that arise in a type of white blood cell called the lymphocyte. NHLs are divided into B- and T-cell subtypes, as well as aggressive and indolent forms. Management varies widely depending on the disease type. We will focus on the most common type of NHL, diffuse large B-cell lymphoma (DLBCL), for which there have been significant treatment advances in recent years.
DLBCL is curable in about two-thirds of patients using chemoimmunotherapy. The longstanding frontline treatment for this disease has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In 2023, an antibody-drug conjugate against the B-cell surface protein CD79b, polatuzumab vedotin, was approved by the US Food and Drug Administration (FDA) in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for newly diagnosed DLBCL based on an improvement in progression-free survival at 2 years in patients with high-risk disease features enrolled in the POLARIX study.
For patients who do not respond to the initial treatment or in whom the disease recurs, the historical standard of care treatment strategy was high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Unfortunately, this approach is not feasible or not successful in a significant percentage of patients with relapsed or refractory DLBCL.
A newer strategy for DLBCL is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are collected from a patient and genetically modified to target a protein on the lymphoma cells called CD19. This type of treatment was initially approved in the third-line setting for DLBCL based on the ZUMA-1 (axi-cel), JULIET (tisa-cel), and TRANSCEND (liso-cel) clinical trials. More recently, in 2022, 2 of these agents received approval in the second-line setting in patients who relapse or are refractory to initial treatment within 1 year; axi-cel was approved based on the ZUMA-7 trial and liso-cel was approved based on the TRANSFORM trial.
Unfortunately, not all patients are eligible for ASCT and CAR T-cell therapy due to factors including age, comorbidities, and disease characteristics. Some patients prefer alternative therapies based on the potential side effects of CAR T-cell therapy and ASCT. Toxicities associated with CAR T-cell therapy include an inflammatory response called cytokine release syndrome and neurologic events.
For patients who are not eligible for or who relapse after ASCT or CAR T-cell therapy, several alternative treatment options are FDA approved. Novel strategies include polatuzumab vedotin with bendamustine and rituximab and tafasitamab plus lenalidomide. Tafasitamab is a monoclonal antibody against CD19 and lenalidomide is an oral anticancer agent originally approved for use in multiple myeloma. Lenalidomide is also effective and commonly used in other NHL subtypes.
In 2023, a new category of treatment called bispecific antibodies was approved in patients with DLBCL in whom the disease recurs after 2 lines of therapy. These drugs (epcoritamab and glofitamab) are a form of immunotherapy that connects B cells with T cells to enable a person’s own immune system to better fight the lymphoma. While these drugs can have similar toxicities as CAR T-cell therapy, the severity and incidence are much lower. In contrast to CAR T-cell therapy, which requires only 1 infusion, these drugs are given regularly in either subcutaneous or intravenous form for several months.
Two other FDA-approved treatment options for relapsed and refractory DLBCL are loncastuximab tesirine, an antibody-drug conjugate targeting CD19 with approval based on the results of the LOTIS-2 trial, and the oral selective inhibitor of nuclear export called selinexor, based on the results from the SADAL trial. Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Selinexor is approved for use in adults with relapsed or refractory DLBCL who have received at least 2 types of systemic therapy. Trials investigating these agents in combination with other novel treatments are ongoing.
The treatment landscape for DLBCL has changed markedly over the past several years. Therapies can be tailored for individual patients based on their disease status and characteristics, comorbidities, and treatment preferences. Research with novel strategies continues with the goal of a cure for all patients diagnosed with DLBCL.
Non-Hodgkin lymphomas (NHLs) are cancers that arise in a type of white blood cell called the lymphocyte. NHLs are divided into B- and T-cell subtypes, as well as aggressive and indolent forms. Management varies widely depending on the disease type. We will focus on the most common type of NHL, diffuse large B-cell lymphoma (DLBCL), for which there have been significant treatment advances in recent years.
DLBCL is curable in about two-thirds of patients using chemoimmunotherapy. The longstanding frontline treatment for this disease has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In 2023, an antibody-drug conjugate against the B-cell surface protein CD79b, polatuzumab vedotin, was approved by the US Food and Drug Administration (FDA) in combination with R-CHP (rituximab, cyclophosphamide, doxorubicin, prednisone) for newly diagnosed DLBCL based on an improvement in progression-free survival at 2 years in patients with high-risk disease features enrolled in the POLARIX study.
For patients who do not respond to the initial treatment or in whom the disease recurs, the historical standard of care treatment strategy was high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Unfortunately, this approach is not feasible or not successful in a significant percentage of patients with relapsed or refractory DLBCL.
A newer strategy for DLBCL is chimeric antigen receptor (CAR) T-cell therapy. In this treatment, T cells are collected from a patient and genetically modified to target a protein on the lymphoma cells called CD19. This type of treatment was initially approved in the third-line setting for DLBCL based on the ZUMA-1 (axi-cel), JULIET (tisa-cel), and TRANSCEND (liso-cel) clinical trials. More recently, in 2022, 2 of these agents received approval in the second-line setting in patients who relapse or are refractory to initial treatment within 1 year; axi-cel was approved based on the ZUMA-7 trial and liso-cel was approved based on the TRANSFORM trial.
Unfortunately, not all patients are eligible for ASCT and CAR T-cell therapy due to factors including age, comorbidities, and disease characteristics. Some patients prefer alternative therapies based on the potential side effects of CAR T-cell therapy and ASCT. Toxicities associated with CAR T-cell therapy include an inflammatory response called cytokine release syndrome and neurologic events.
For patients who are not eligible for or who relapse after ASCT or CAR T-cell therapy, several alternative treatment options are FDA approved. Novel strategies include polatuzumab vedotin with bendamustine and rituximab and tafasitamab plus lenalidomide. Tafasitamab is a monoclonal antibody against CD19 and lenalidomide is an oral anticancer agent originally approved for use in multiple myeloma. Lenalidomide is also effective and commonly used in other NHL subtypes.
In 2023, a new category of treatment called bispecific antibodies was approved in patients with DLBCL in whom the disease recurs after 2 lines of therapy. These drugs (epcoritamab and glofitamab) are a form of immunotherapy that connects B cells with T cells to enable a person’s own immune system to better fight the lymphoma. While these drugs can have similar toxicities as CAR T-cell therapy, the severity and incidence are much lower. In contrast to CAR T-cell therapy, which requires only 1 infusion, these drugs are given regularly in either subcutaneous or intravenous form for several months.
Two other FDA-approved treatment options for relapsed and refractory DLBCL are loncastuximab tesirine, an antibody-drug conjugate targeting CD19 with approval based on the results of the LOTIS-2 trial, and the oral selective inhibitor of nuclear export called selinexor, based on the results from the SADAL trial. Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Selinexor is approved for use in adults with relapsed or refractory DLBCL who have received at least 2 types of systemic therapy. Trials investigating these agents in combination with other novel treatments are ongoing.
The treatment landscape for DLBCL has changed markedly over the past several years. Therapies can be tailored for individual patients based on their disease status and characteristics, comorbidities, and treatment preferences. Research with novel strategies continues with the goal of a cure for all patients diagnosed with DLBCL.
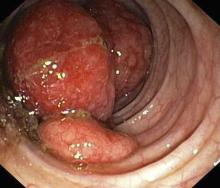
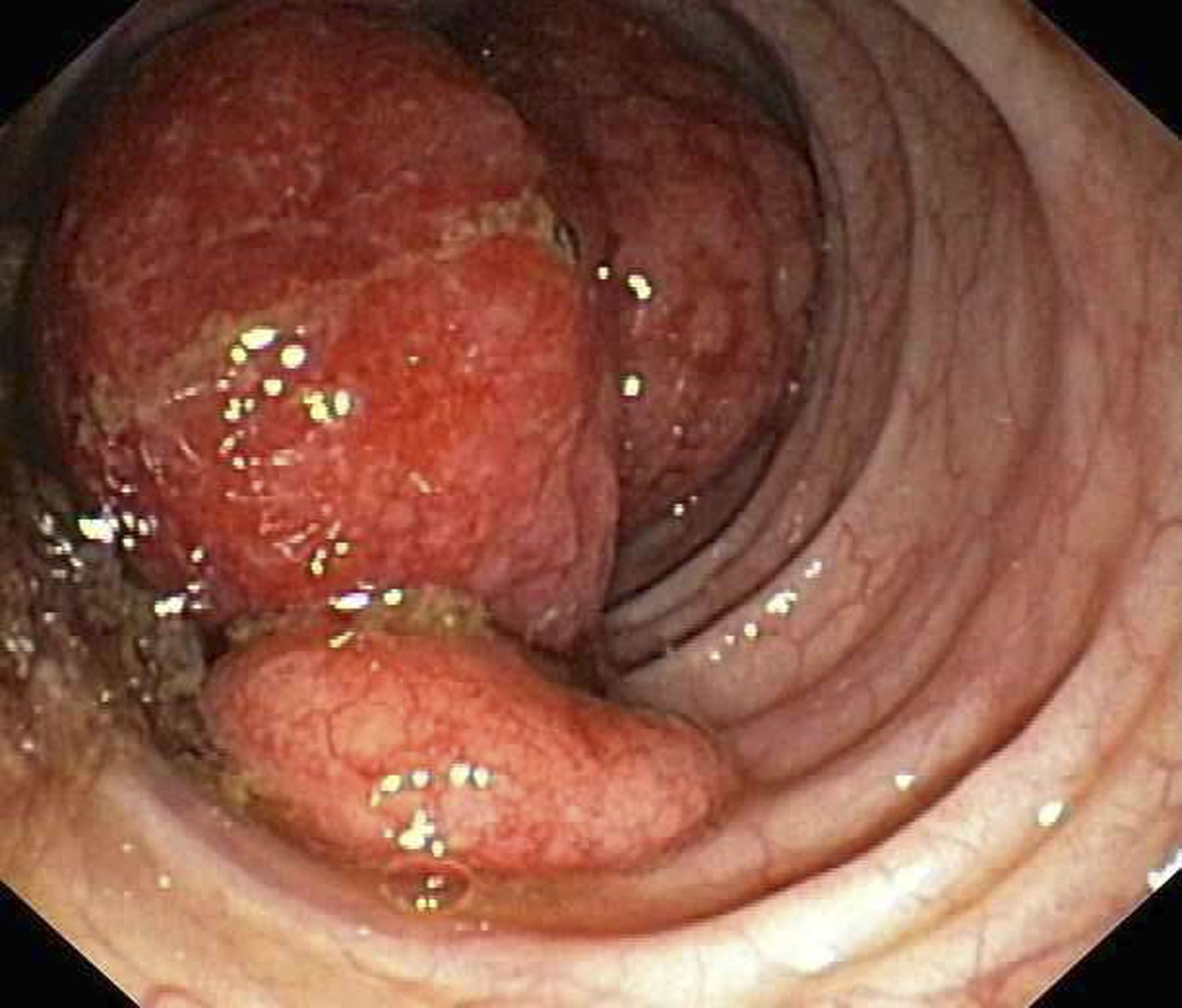
Abdominal distention and pain
Given the patient's symptomatology, laboratory studies, and the histopathology and immunophenotyping of the polypoid lesions in the transverse colon, this patient is diagnosed with advanced mantle cell lymphoma (MCL). The gastroenterologist shares the findings with the patient, and over the next several days, a multidisciplinary team forms to guide the patient through potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all non-Hodgkin lymphomas are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. One of the most frequent areas for extra-nodal MCL presentation is the gastrointestinal tract. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, which are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test, also help diagnose MCL. Gastrointestinal involvement of MCL typically presents as lymphoid polyposis on colonoscopy imaging and can appear in the colon, ileum, stomach, and duodenum.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options and associated adverse events, as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, when used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's symptomatology, laboratory studies, and the histopathology and immunophenotyping of the polypoid lesions in the transverse colon, this patient is diagnosed with advanced mantle cell lymphoma (MCL). The gastroenterologist shares the findings with the patient, and over the next several days, a multidisciplinary team forms to guide the patient through potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all non-Hodgkin lymphomas are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. One of the most frequent areas for extra-nodal MCL presentation is the gastrointestinal tract. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, which are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test, also help diagnose MCL. Gastrointestinal involvement of MCL typically presents as lymphoid polyposis on colonoscopy imaging and can appear in the colon, ileum, stomach, and duodenum.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options and associated adverse events, as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, when used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's symptomatology, laboratory studies, and the histopathology and immunophenotyping of the polypoid lesions in the transverse colon, this patient is diagnosed with advanced mantle cell lymphoma (MCL). The gastroenterologist shares the findings with the patient, and over the next several days, a multidisciplinary team forms to guide the patient through potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all non-Hodgkin lymphomas are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. One of the most frequent areas for extra-nodal MCL presentation is the gastrointestinal tract. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, which are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test, also help diagnose MCL. Gastrointestinal involvement of MCL typically presents as lymphoid polyposis on colonoscopy imaging and can appear in the colon, ileum, stomach, and duodenum.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options and associated adverse events, as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, when used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 60-year-old man presents to his primary care physician with weight loss, constipation, and abdominal distention and pain as well as fatigue and night sweats that have lasted for several months. The physician orders a complete blood count with differential and an ultrasound of the abdomen. Lab studies reveal anemia and cytopenias; ultrasound reveals hepatosplenomegaly and abdominal lymphadenopathy. The physician refers the patient to gastroenterology; he undergoes a colonoscopy. Multiple polypoid lesions are found throughout the transverse colon. Immunophenotyping shows CD5 and CD20 expression but a lack of CD23 and CD10 expression; cyclin D1 is overexpressed. Additional blood studies show lymphocytosis > 4000/μL, elevated lactate dehydrogenase levels, abnormal liver function tests, and a negative result on Coombs test.
Mantle Cell Lymphoma: Drug Combo Improves PFS
Still, “in the countries where ibrutinib is indicated, this combination should be a new standard therapy for relapsed/refractory mantle cell lymphoma,” Michael Wang, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in a media briefing at the annual meeting of the American Society of Hematology.
Its use would be off label, according to the authors of the industry-funded trial, because no nation has approved the combination therapy for MCL, a rare, aggressive form of non-Hodgkin lymphoma.
As Dr. Wang noted, ibrutinib (a Bruton tyrosine kinase inhibitor) is approved by the Food and Drug Administration to treat MCL, while venetoclax (a BCL-2 inhibitor) is approved for chronic lymphocytic leukemia and previously untreated acute myeloid leukemia. “The combination of these two agents leverages complementary modes of action and has demonstrated synergistic anti-tumor activity in preclinical models of mantle cell lymphoma,” he said. And “in patients with relapsed/refractory mantle cell lymphoma, promising clinical activity has also been observed in early-phase studies.”
For the multinational, randomized, phase 3, double-blind SYMPATICO study, researchers assigned 267 adults with relapsed/refractory MCL after 1-5 prior therapies 1:1 to receive oral ibrutinib 560 mg daily with oral venetoclax (standard 5-wk ramp-up to a target dose of 400 mg once daily) or placebo for 2 years. Then they continued with ibrutinib alone until progressive disease or unacceptable toxicity.
The study began in 2017. The median age of patients was 68, and the numbers of patients in each group were 134 (both drugs) and 133 (ibrutinib plus placebo).
At a median of 51.2 months, median PFS — the primary endpoint — was higher in the combination group vs. ibrutinib alone (31.9 vs. 22.1 months, hazard ratio [HR]=0.65, 95% CI, 0.47–0.88, P = .0052). While overall survival was higher in the combination group vs. ibrutinib alone, an interim analysis found that the difference was not statistically significant (44.9 months vs. 38.6 months, 95% CI, HR = 0.85, 0.62-1.19, P = .3465).
When questioned about this finding at the ASH news briefing, Dr. Wang said that 170 events are needed for a full overall survival analysis, and there are just 144 now. The study may reach that point in early 2025, he said.
Over a median treatment duration of 22.0 months for the combination treatment and 17.7 months for ibrutinib alone, grade ≥ 3 adverse events occurred in 84% and 76% of patients, respectively. At 60%, the level of serious adverse events was the same in both groups.
In an interview, Brian T. Hill, MD, PhD, of Cleveland Clinic, noted that in general, MCL “has a pretty relentless pattern of relapses and disease progression without an easy cure in the vast majority of patients.”
Ibrutinib has revolutionized treatment over the past decade with generally manageable side effects, and clinicians are now turning to other Bruton tyrosine kinase inhibitors, he said. Still, “there is a need for improving the durability and the response rates second-line treatment or beyond,” Dr. Hill said.
The new study is important since it’s the first randomized trial “that demonstrates that additional venetoclax significantly improves not only response rates, but also progression-free survival with a trend toward overall survival,” he said. “The toxicity profile doesn’t really seem to be significantly more worse than what we might expect with each agent given individually.”
However, Dr. Hill noted that “it’s a relatively small study and relatively short follow-up.”
It may be difficult to get an ibrutinib-venetoclax combination approved today since ibrutinib is no longer the preferred Bruton tyrosine kinase inhibitor for clinicians, he said.
Pharmacyclics, maker of ibrutinib, is the study sponsor and Janssen is a collaborator.
Dr. Wang reports research funding Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, and VelosBio. Other authors report multiple and various relationships with industry. Dr. Hill discloses research funding and consulting relationships with Pharmacyclics, AbbVie, BeiGene, and AstraZeneca.
Still, “in the countries where ibrutinib is indicated, this combination should be a new standard therapy for relapsed/refractory mantle cell lymphoma,” Michael Wang, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in a media briefing at the annual meeting of the American Society of Hematology.
Its use would be off label, according to the authors of the industry-funded trial, because no nation has approved the combination therapy for MCL, a rare, aggressive form of non-Hodgkin lymphoma.
As Dr. Wang noted, ibrutinib (a Bruton tyrosine kinase inhibitor) is approved by the Food and Drug Administration to treat MCL, while venetoclax (a BCL-2 inhibitor) is approved for chronic lymphocytic leukemia and previously untreated acute myeloid leukemia. “The combination of these two agents leverages complementary modes of action and has demonstrated synergistic anti-tumor activity in preclinical models of mantle cell lymphoma,” he said. And “in patients with relapsed/refractory mantle cell lymphoma, promising clinical activity has also been observed in early-phase studies.”
For the multinational, randomized, phase 3, double-blind SYMPATICO study, researchers assigned 267 adults with relapsed/refractory MCL after 1-5 prior therapies 1:1 to receive oral ibrutinib 560 mg daily with oral venetoclax (standard 5-wk ramp-up to a target dose of 400 mg once daily) or placebo for 2 years. Then they continued with ibrutinib alone until progressive disease or unacceptable toxicity.
The study began in 2017. The median age of patients was 68, and the numbers of patients in each group were 134 (both drugs) and 133 (ibrutinib plus placebo).
At a median of 51.2 months, median PFS — the primary endpoint — was higher in the combination group vs. ibrutinib alone (31.9 vs. 22.1 months, hazard ratio [HR]=0.65, 95% CI, 0.47–0.88, P = .0052). While overall survival was higher in the combination group vs. ibrutinib alone, an interim analysis found that the difference was not statistically significant (44.9 months vs. 38.6 months, 95% CI, HR = 0.85, 0.62-1.19, P = .3465).
When questioned about this finding at the ASH news briefing, Dr. Wang said that 170 events are needed for a full overall survival analysis, and there are just 144 now. The study may reach that point in early 2025, he said.
Over a median treatment duration of 22.0 months for the combination treatment and 17.7 months for ibrutinib alone, grade ≥ 3 adverse events occurred in 84% and 76% of patients, respectively. At 60%, the level of serious adverse events was the same in both groups.
In an interview, Brian T. Hill, MD, PhD, of Cleveland Clinic, noted that in general, MCL “has a pretty relentless pattern of relapses and disease progression without an easy cure in the vast majority of patients.”
Ibrutinib has revolutionized treatment over the past decade with generally manageable side effects, and clinicians are now turning to other Bruton tyrosine kinase inhibitors, he said. Still, “there is a need for improving the durability and the response rates second-line treatment or beyond,” Dr. Hill said.
The new study is important since it’s the first randomized trial “that demonstrates that additional venetoclax significantly improves not only response rates, but also progression-free survival with a trend toward overall survival,” he said. “The toxicity profile doesn’t really seem to be significantly more worse than what we might expect with each agent given individually.”
However, Dr. Hill noted that “it’s a relatively small study and relatively short follow-up.”
It may be difficult to get an ibrutinib-venetoclax combination approved today since ibrutinib is no longer the preferred Bruton tyrosine kinase inhibitor for clinicians, he said.
Pharmacyclics, maker of ibrutinib, is the study sponsor and Janssen is a collaborator.
Dr. Wang reports research funding Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, and VelosBio. Other authors report multiple and various relationships with industry. Dr. Hill discloses research funding and consulting relationships with Pharmacyclics, AbbVie, BeiGene, and AstraZeneca.
Still, “in the countries where ibrutinib is indicated, this combination should be a new standard therapy for relapsed/refractory mantle cell lymphoma,” Michael Wang, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in a media briefing at the annual meeting of the American Society of Hematology.
Its use would be off label, according to the authors of the industry-funded trial, because no nation has approved the combination therapy for MCL, a rare, aggressive form of non-Hodgkin lymphoma.
As Dr. Wang noted, ibrutinib (a Bruton tyrosine kinase inhibitor) is approved by the Food and Drug Administration to treat MCL, while venetoclax (a BCL-2 inhibitor) is approved for chronic lymphocytic leukemia and previously untreated acute myeloid leukemia. “The combination of these two agents leverages complementary modes of action and has demonstrated synergistic anti-tumor activity in preclinical models of mantle cell lymphoma,” he said. And “in patients with relapsed/refractory mantle cell lymphoma, promising clinical activity has also been observed in early-phase studies.”
For the multinational, randomized, phase 3, double-blind SYMPATICO study, researchers assigned 267 adults with relapsed/refractory MCL after 1-5 prior therapies 1:1 to receive oral ibrutinib 560 mg daily with oral venetoclax (standard 5-wk ramp-up to a target dose of 400 mg once daily) or placebo for 2 years. Then they continued with ibrutinib alone until progressive disease or unacceptable toxicity.
The study began in 2017. The median age of patients was 68, and the numbers of patients in each group were 134 (both drugs) and 133 (ibrutinib plus placebo).
At a median of 51.2 months, median PFS — the primary endpoint — was higher in the combination group vs. ibrutinib alone (31.9 vs. 22.1 months, hazard ratio [HR]=0.65, 95% CI, 0.47–0.88, P = .0052). While overall survival was higher in the combination group vs. ibrutinib alone, an interim analysis found that the difference was not statistically significant (44.9 months vs. 38.6 months, 95% CI, HR = 0.85, 0.62-1.19, P = .3465).
When questioned about this finding at the ASH news briefing, Dr. Wang said that 170 events are needed for a full overall survival analysis, and there are just 144 now. The study may reach that point in early 2025, he said.
Over a median treatment duration of 22.0 months for the combination treatment and 17.7 months for ibrutinib alone, grade ≥ 3 adverse events occurred in 84% and 76% of patients, respectively. At 60%, the level of serious adverse events was the same in both groups.
In an interview, Brian T. Hill, MD, PhD, of Cleveland Clinic, noted that in general, MCL “has a pretty relentless pattern of relapses and disease progression without an easy cure in the vast majority of patients.”
Ibrutinib has revolutionized treatment over the past decade with generally manageable side effects, and clinicians are now turning to other Bruton tyrosine kinase inhibitors, he said. Still, “there is a need for improving the durability and the response rates second-line treatment or beyond,” Dr. Hill said.
The new study is important since it’s the first randomized trial “that demonstrates that additional venetoclax significantly improves not only response rates, but also progression-free survival with a trend toward overall survival,” he said. “The toxicity profile doesn’t really seem to be significantly more worse than what we might expect with each agent given individually.”
However, Dr. Hill noted that “it’s a relatively small study and relatively short follow-up.”
It may be difficult to get an ibrutinib-venetoclax combination approved today since ibrutinib is no longer the preferred Bruton tyrosine kinase inhibitor for clinicians, he said.
Pharmacyclics, maker of ibrutinib, is the study sponsor and Janssen is a collaborator.
Dr. Wang reports research funding Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, and VelosBio. Other authors report multiple and various relationships with industry. Dr. Hill discloses research funding and consulting relationships with Pharmacyclics, AbbVie, BeiGene, and AstraZeneca.
FROM ASH 2023
FDA approves pirtobrutinib for previously treated CLL/SLL
The agent was initially approved in January 2023 for patients with mantle cell lymphoma who had previously received a BTK inhibitor.
Like the mantle cell approval, the CLL/SLL approval was based on findings from the open-label, single-arm, phase 1/2 BRUIN study that included adults with at least two prior lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor.
The trial included 108 patients with either CLL or SLL. Overall, patients demonstrated an overall response rate of 72%, all of which were partial responses, and median duration of response of 12.2 months.
Before starting pirtobrutinib, 77% of patients with CLL or SLL had discontinued their last BTK inhibitor for refractory or progressive disease.
“Once patients with CLL or SLL have progressed on covalent BTK inhibitor and BCL-2 inhibitor therapies, treatments are limited and outcomes can be poor, making the approval of Jaypirca a meaningful advance and much-needed new treatment option for these patients,” William G. Wierda, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, said in an Eli Lilly press release.
Treatment during the study included the recommended dose of 200 mg given orally once daily until disease progression or unacceptable toxicity. Common adverse reactions that occurred in at least 20% of patients included fatigue, bruising, cough, musculoskeletal pain, COVID-19, diarrhea, pneumonia, abdominal pain, dyspnea, hemorrhage, edema, nausea, pyrexia, and headache. Grade 3 or 4 laboratory abnormalities occurring in more than 10% of patients included decreased neutrophil counts, anemia, and decreased platelet counts.
Serious infections occurred in 32% of patients, including fatal infections in 10% of patients. The prescribing information for pirtobrutinib includes warnings about infections, hemorrhage, cytopenias, cardiac arrhythmias, and secondary primary malignancies.
A version of this article first appeared on Medscape.com.
The agent was initially approved in January 2023 for patients with mantle cell lymphoma who had previously received a BTK inhibitor.
Like the mantle cell approval, the CLL/SLL approval was based on findings from the open-label, single-arm, phase 1/2 BRUIN study that included adults with at least two prior lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor.
The trial included 108 patients with either CLL or SLL. Overall, patients demonstrated an overall response rate of 72%, all of which were partial responses, and median duration of response of 12.2 months.
Before starting pirtobrutinib, 77% of patients with CLL or SLL had discontinued their last BTK inhibitor for refractory or progressive disease.
“Once patients with CLL or SLL have progressed on covalent BTK inhibitor and BCL-2 inhibitor therapies, treatments are limited and outcomes can be poor, making the approval of Jaypirca a meaningful advance and much-needed new treatment option for these patients,” William G. Wierda, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, said in an Eli Lilly press release.
Treatment during the study included the recommended dose of 200 mg given orally once daily until disease progression or unacceptable toxicity. Common adverse reactions that occurred in at least 20% of patients included fatigue, bruising, cough, musculoskeletal pain, COVID-19, diarrhea, pneumonia, abdominal pain, dyspnea, hemorrhage, edema, nausea, pyrexia, and headache. Grade 3 or 4 laboratory abnormalities occurring in more than 10% of patients included decreased neutrophil counts, anemia, and decreased platelet counts.
Serious infections occurred in 32% of patients, including fatal infections in 10% of patients. The prescribing information for pirtobrutinib includes warnings about infections, hemorrhage, cytopenias, cardiac arrhythmias, and secondary primary malignancies.
A version of this article first appeared on Medscape.com.
The agent was initially approved in January 2023 for patients with mantle cell lymphoma who had previously received a BTK inhibitor.
Like the mantle cell approval, the CLL/SLL approval was based on findings from the open-label, single-arm, phase 1/2 BRUIN study that included adults with at least two prior lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor.
The trial included 108 patients with either CLL or SLL. Overall, patients demonstrated an overall response rate of 72%, all of which were partial responses, and median duration of response of 12.2 months.
Before starting pirtobrutinib, 77% of patients with CLL or SLL had discontinued their last BTK inhibitor for refractory or progressive disease.
“Once patients with CLL or SLL have progressed on covalent BTK inhibitor and BCL-2 inhibitor therapies, treatments are limited and outcomes can be poor, making the approval of Jaypirca a meaningful advance and much-needed new treatment option for these patients,” William G. Wierda, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, said in an Eli Lilly press release.
Treatment during the study included the recommended dose of 200 mg given orally once daily until disease progression or unacceptable toxicity. Common adverse reactions that occurred in at least 20% of patients included fatigue, bruising, cough, musculoskeletal pain, COVID-19, diarrhea, pneumonia, abdominal pain, dyspnea, hemorrhage, edema, nausea, pyrexia, and headache. Grade 3 or 4 laboratory abnormalities occurring in more than 10% of patients included decreased neutrophil counts, anemia, and decreased platelet counts.
Serious infections occurred in 32% of patients, including fatal infections in 10% of patients. The prescribing information for pirtobrutinib includes warnings about infections, hemorrhage, cytopenias, cardiac arrhythmias, and secondary primary malignancies.
A version of this article first appeared on Medscape.com.
FDA OKs new agent to block chemotherapy-induced neutropenia
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
Efbemalenograstim joins other agents already on the U.S. market, including pegfilgrastim (Neulasta), that aim to reduce the incidence of chemotherapy-induced febrile neutropenia.
The approval of efbemalenograstim was based on two randomized trials. The first included 122 women with either metastatic or nonmetastatic breast cancer who were receiving doxorubicin and docetaxel. These patients were randomly assigned to receive either one subcutaneous injection of efbemalenograstim or placebo on the second day of their first chemotherapy cycle. All patients received efbemalenograstim on the second day of cycles two through four.
The mean duration of grade 4 neutropenia in the first cycle was 1.4 days with efbemalenograstim versus 4.3 days with placebo. Only 4.8% of patients who received efbemalenograstim experienced chemotherapy-induced febrile neutropenia, compared with 25.6% who received the placebo.
The new agent went up against pegfilgrastim in the second trial, which included 393 women who received docetaxel and cyclophosphamide as treatment for nonmetastatic breast cancer. These patients were randomly assigned to receive either a single subcutaneous injection of efbemalenograstim or pegfilgrastim on the second day of each cycle.
During the first cycle, patients in both arms of the trial experienced a mean of 0.2 days of grade 4 neutropenia.
The most common side effects associated with efbemalenograstim were nausea, anemia, and thrombocytopenia. Similar to pegfilgrastim’s label, efbemalenograstim’s label warns of possible splenic rupture, respiratory distress syndrome, sickle cell crisis, and other serious adverse events.
The FDA recommends a dose of 20 mg subcutaneous once per chemotherapy cycle.
A version of this article first appeared on Medscape.com.
FDA panel voices concerns over 2 lymphoma accelerated approvals
At a Nov. 16 meeting, the Oncologic Drugs Advisory Committee of the Food and Drug Administration reviewed the reasons for delays in confirmatory trials for pralatrexate (Folotyn) and belinostat (Beleodaq), both now owned by East Windsor, N.J.–based Acrotech. The FDA granted accelerated approval for pralatrexate in 2009 and belinostat in 2014.
“The consensus of the advisory committee is that we have significant concerns about the very prolonged delay and getting these confirmatory studies underway,” said Andy Chen, MD, PhD, of Oregon Health & Science University, Portland, who served as acting ODAC chair for the meeting.
Corporate ownership changes were among the reasons Acrotech cited for the long delays in producing the confirmatory research on pralatrexate and belinostat. Allos Therapeutics won the FDA approval of pralatrexate in 2009. In 2012, Spectrum Pharmaceuticals acquired Acrotech. Spectrum won approval of belinostat in 2014. Acrotech acquired Spectrum in 2019.
The FDA didn’t ask ODAC to take votes on any questions at the meeting. Instead, the FDA sought its expert feedback about how to address the prolonged delays with pralatrexate and belinostat research and, in general, how to promote more timely completion of confirmatory trials for drugs cleared by accelerated approval.
Pralatrexate and belinostat are both used to treat relapsed or refractory peripheral T-cell lymphoma, a rare and aggressive disease affecting about 10,000-15,000 people annually in the United States.
Through the accelerated approval process, the FDA seeks to speed medicines to people with fatal and serious conditions based on promising signs in clinical testing.
The initial pralatrexate and belinostat were based on phase 2, single-arm, monotherapy studies, with about 109 evaluable patients in the key pralatrexate study and 120 evaluable patients in the belinostat study. As is common, these phase 2 tests used measurements of cancer progression, known as the overall response rate.
The FDA then expects companies to show through more extensive testing that medicines cleared with accelerated approvals can deliver significant benefits, such as extending lives. When there are delays in confirmatory trials, patients can be exposed to medicines, often with significant side effects, that are unlikely to benefit them.
For example, the FDA granted an accelerated approval in 2011 for romidepsin for this use for peripheral T-cell lymphoma, the same condition for which pralatrexate and belinostat are used. But in 2021, Bristol-Myers Squibb withdrew the approval for that use of romidepsin when a confirmatory trial failed to meet the primary efficacy endpoint of progression free survival.
At the meeting, Richard Pazdur, MD, who leads oncology medicine at the FDA, urged Acrotech to shorten the time needed to determine whether its medicines deliver significant benefits to patients and thus merit full approval, or whether they too may fall short.
“We’re really in a situation where patients are caught in the middle here,” Dr. Pazdur said. “I feel very bad for that situation and very bad for the patients that they don’t have this information.”
‘Dangerous precedent’
The FDA in recent years has stepped up its efforts to get companies to complete their required studies on drugs cleared by accelerated approvals. The FDA has granted a total of 187 accelerated approvals for cancer drugs. Many of these cover new uses of established drugs and others serve to allow the introduction of new medicines.
For more than half of these cases, 96 of 187, the FDA already has learned that it made the right call in allowing early access to medicines. Companies have presented study results that confirmed the benefit of drugs and thus been able to convert accelerated approvals to traditional approvals.
But 27 of the 187 oncology accelerated approvals have been withdrawn. In these cases, subsequent research failed to establish the expected benefits of these cancer drugs.
And in 95 cases, the FDA and companies are still waiting for the results of studies to confirm the expected benefit of drugs granted accelerated approvals. The FDA classifies these as ongoing accelerated approvals. About 85% of these ongoing approvals were granted in the past 5 years, in contrast to 14 years for pralatrexate and 9 for belinostat.
“It sets a dangerous precedent for the other sponsors and drug companies to have such outliers from the same company,” said ODAC member Toni K. Choueiri, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston.
The current agreement between the FDA and Acrotech focuses on a phase 3 trial, SPI-BEL-301 as the confirmatory study. Acrotech’s plan is to start with dose optimization studies in part 1 of the trial, with part 2 meant to see if its medicines provide a significant benefit as measured by progression-free survival.
The plan is to compare treatments. One group of patients would get belinostat plus a common cancer regimen known as CHOP, another group would get pralatrexate plus the COP cancer regimen, which is CHOP without doxorubicin, and a third group would get CHOP.
Acrotech’s current time line is for part 1, which began in October, to finish by December 2025. Then the part 2 timeline would run from 2026 to 2030, with interim progression-free survival possible by 2028.
ODAC member Ashley Rosko, MD, a hematologist from Ohio State University, Columbus, asked Acrotech what steps it will take to try to speed recruitment for the study.
“We are going to implement many strategies,” including what’s called digital amplification, replied Ashish Anvekar, president of Acrotech. This will help identify patients and channel them toward participating clinical sites.
Alexander A. Vinks, PhD, PharmD, who served as a temporary member of ODAC for the Nov. 16 meeting, said many clinicians will not be excited about enrolling patients in this kind of large, traditionally designed study.
Dr. Vinks, who is professor emeritus at Cincinnati Children’s Hospital Medical Center and University of Cincinnati, now works with consultant group NDA, a firm that advises companies on developing drugs.
Dr. Vinks advised Acrotech should try “to pin down what is most likely a smaller study that could be simpler, but still give robust, informative data.”
At a Nov. 16 meeting, the Oncologic Drugs Advisory Committee of the Food and Drug Administration reviewed the reasons for delays in confirmatory trials for pralatrexate (Folotyn) and belinostat (Beleodaq), both now owned by East Windsor, N.J.–based Acrotech. The FDA granted accelerated approval for pralatrexate in 2009 and belinostat in 2014.
“The consensus of the advisory committee is that we have significant concerns about the very prolonged delay and getting these confirmatory studies underway,” said Andy Chen, MD, PhD, of Oregon Health & Science University, Portland, who served as acting ODAC chair for the meeting.
Corporate ownership changes were among the reasons Acrotech cited for the long delays in producing the confirmatory research on pralatrexate and belinostat. Allos Therapeutics won the FDA approval of pralatrexate in 2009. In 2012, Spectrum Pharmaceuticals acquired Acrotech. Spectrum won approval of belinostat in 2014. Acrotech acquired Spectrum in 2019.
The FDA didn’t ask ODAC to take votes on any questions at the meeting. Instead, the FDA sought its expert feedback about how to address the prolonged delays with pralatrexate and belinostat research and, in general, how to promote more timely completion of confirmatory trials for drugs cleared by accelerated approval.
Pralatrexate and belinostat are both used to treat relapsed or refractory peripheral T-cell lymphoma, a rare and aggressive disease affecting about 10,000-15,000 people annually in the United States.
Through the accelerated approval process, the FDA seeks to speed medicines to people with fatal and serious conditions based on promising signs in clinical testing.
The initial pralatrexate and belinostat were based on phase 2, single-arm, monotherapy studies, with about 109 evaluable patients in the key pralatrexate study and 120 evaluable patients in the belinostat study. As is common, these phase 2 tests used measurements of cancer progression, known as the overall response rate.
The FDA then expects companies to show through more extensive testing that medicines cleared with accelerated approvals can deliver significant benefits, such as extending lives. When there are delays in confirmatory trials, patients can be exposed to medicines, often with significant side effects, that are unlikely to benefit them.
For example, the FDA granted an accelerated approval in 2011 for romidepsin for this use for peripheral T-cell lymphoma, the same condition for which pralatrexate and belinostat are used. But in 2021, Bristol-Myers Squibb withdrew the approval for that use of romidepsin when a confirmatory trial failed to meet the primary efficacy endpoint of progression free survival.
At the meeting, Richard Pazdur, MD, who leads oncology medicine at the FDA, urged Acrotech to shorten the time needed to determine whether its medicines deliver significant benefits to patients and thus merit full approval, or whether they too may fall short.
“We’re really in a situation where patients are caught in the middle here,” Dr. Pazdur said. “I feel very bad for that situation and very bad for the patients that they don’t have this information.”
‘Dangerous precedent’
The FDA in recent years has stepped up its efforts to get companies to complete their required studies on drugs cleared by accelerated approvals. The FDA has granted a total of 187 accelerated approvals for cancer drugs. Many of these cover new uses of established drugs and others serve to allow the introduction of new medicines.
For more than half of these cases, 96 of 187, the FDA already has learned that it made the right call in allowing early access to medicines. Companies have presented study results that confirmed the benefit of drugs and thus been able to convert accelerated approvals to traditional approvals.
But 27 of the 187 oncology accelerated approvals have been withdrawn. In these cases, subsequent research failed to establish the expected benefits of these cancer drugs.
And in 95 cases, the FDA and companies are still waiting for the results of studies to confirm the expected benefit of drugs granted accelerated approvals. The FDA classifies these as ongoing accelerated approvals. About 85% of these ongoing approvals were granted in the past 5 years, in contrast to 14 years for pralatrexate and 9 for belinostat.
“It sets a dangerous precedent for the other sponsors and drug companies to have such outliers from the same company,” said ODAC member Toni K. Choueiri, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston.
The current agreement between the FDA and Acrotech focuses on a phase 3 trial, SPI-BEL-301 as the confirmatory study. Acrotech’s plan is to start with dose optimization studies in part 1 of the trial, with part 2 meant to see if its medicines provide a significant benefit as measured by progression-free survival.
The plan is to compare treatments. One group of patients would get belinostat plus a common cancer regimen known as CHOP, another group would get pralatrexate plus the COP cancer regimen, which is CHOP without doxorubicin, and a third group would get CHOP.
Acrotech’s current time line is for part 1, which began in October, to finish by December 2025. Then the part 2 timeline would run from 2026 to 2030, with interim progression-free survival possible by 2028.
ODAC member Ashley Rosko, MD, a hematologist from Ohio State University, Columbus, asked Acrotech what steps it will take to try to speed recruitment for the study.
“We are going to implement many strategies,” including what’s called digital amplification, replied Ashish Anvekar, president of Acrotech. This will help identify patients and channel them toward participating clinical sites.
Alexander A. Vinks, PhD, PharmD, who served as a temporary member of ODAC for the Nov. 16 meeting, said many clinicians will not be excited about enrolling patients in this kind of large, traditionally designed study.
Dr. Vinks, who is professor emeritus at Cincinnati Children’s Hospital Medical Center and University of Cincinnati, now works with consultant group NDA, a firm that advises companies on developing drugs.
Dr. Vinks advised Acrotech should try “to pin down what is most likely a smaller study that could be simpler, but still give robust, informative data.”
At a Nov. 16 meeting, the Oncologic Drugs Advisory Committee of the Food and Drug Administration reviewed the reasons for delays in confirmatory trials for pralatrexate (Folotyn) and belinostat (Beleodaq), both now owned by East Windsor, N.J.–based Acrotech. The FDA granted accelerated approval for pralatrexate in 2009 and belinostat in 2014.
“The consensus of the advisory committee is that we have significant concerns about the very prolonged delay and getting these confirmatory studies underway,” said Andy Chen, MD, PhD, of Oregon Health & Science University, Portland, who served as acting ODAC chair for the meeting.
Corporate ownership changes were among the reasons Acrotech cited for the long delays in producing the confirmatory research on pralatrexate and belinostat. Allos Therapeutics won the FDA approval of pralatrexate in 2009. In 2012, Spectrum Pharmaceuticals acquired Acrotech. Spectrum won approval of belinostat in 2014. Acrotech acquired Spectrum in 2019.
The FDA didn’t ask ODAC to take votes on any questions at the meeting. Instead, the FDA sought its expert feedback about how to address the prolonged delays with pralatrexate and belinostat research and, in general, how to promote more timely completion of confirmatory trials for drugs cleared by accelerated approval.
Pralatrexate and belinostat are both used to treat relapsed or refractory peripheral T-cell lymphoma, a rare and aggressive disease affecting about 10,000-15,000 people annually in the United States.
Through the accelerated approval process, the FDA seeks to speed medicines to people with fatal and serious conditions based on promising signs in clinical testing.
The initial pralatrexate and belinostat were based on phase 2, single-arm, monotherapy studies, with about 109 evaluable patients in the key pralatrexate study and 120 evaluable patients in the belinostat study. As is common, these phase 2 tests used measurements of cancer progression, known as the overall response rate.
The FDA then expects companies to show through more extensive testing that medicines cleared with accelerated approvals can deliver significant benefits, such as extending lives. When there are delays in confirmatory trials, patients can be exposed to medicines, often with significant side effects, that are unlikely to benefit them.
For example, the FDA granted an accelerated approval in 2011 for romidepsin for this use for peripheral T-cell lymphoma, the same condition for which pralatrexate and belinostat are used. But in 2021, Bristol-Myers Squibb withdrew the approval for that use of romidepsin when a confirmatory trial failed to meet the primary efficacy endpoint of progression free survival.
At the meeting, Richard Pazdur, MD, who leads oncology medicine at the FDA, urged Acrotech to shorten the time needed to determine whether its medicines deliver significant benefits to patients and thus merit full approval, or whether they too may fall short.
“We’re really in a situation where patients are caught in the middle here,” Dr. Pazdur said. “I feel very bad for that situation and very bad for the patients that they don’t have this information.”
‘Dangerous precedent’
The FDA in recent years has stepped up its efforts to get companies to complete their required studies on drugs cleared by accelerated approvals. The FDA has granted a total of 187 accelerated approvals for cancer drugs. Many of these cover new uses of established drugs and others serve to allow the introduction of new medicines.
For more than half of these cases, 96 of 187, the FDA already has learned that it made the right call in allowing early access to medicines. Companies have presented study results that confirmed the benefit of drugs and thus been able to convert accelerated approvals to traditional approvals.
But 27 of the 187 oncology accelerated approvals have been withdrawn. In these cases, subsequent research failed to establish the expected benefits of these cancer drugs.
And in 95 cases, the FDA and companies are still waiting for the results of studies to confirm the expected benefit of drugs granted accelerated approvals. The FDA classifies these as ongoing accelerated approvals. About 85% of these ongoing approvals were granted in the past 5 years, in contrast to 14 years for pralatrexate and 9 for belinostat.
“It sets a dangerous precedent for the other sponsors and drug companies to have such outliers from the same company,” said ODAC member Toni K. Choueiri, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston.
The current agreement between the FDA and Acrotech focuses on a phase 3 trial, SPI-BEL-301 as the confirmatory study. Acrotech’s plan is to start with dose optimization studies in part 1 of the trial, with part 2 meant to see if its medicines provide a significant benefit as measured by progression-free survival.
The plan is to compare treatments. One group of patients would get belinostat plus a common cancer regimen known as CHOP, another group would get pralatrexate plus the COP cancer regimen, which is CHOP without doxorubicin, and a third group would get CHOP.
Acrotech’s current time line is for part 1, which began in October, to finish by December 2025. Then the part 2 timeline would run from 2026 to 2030, with interim progression-free survival possible by 2028.
ODAC member Ashley Rosko, MD, a hematologist from Ohio State University, Columbus, asked Acrotech what steps it will take to try to speed recruitment for the study.
“We are going to implement many strategies,” including what’s called digital amplification, replied Ashish Anvekar, president of Acrotech. This will help identify patients and channel them toward participating clinical sites.
Alexander A. Vinks, PhD, PharmD, who served as a temporary member of ODAC for the Nov. 16 meeting, said many clinicians will not be excited about enrolling patients in this kind of large, traditionally designed study.
Dr. Vinks, who is professor emeritus at Cincinnati Children’s Hospital Medical Center and University of Cincinnati, now works with consultant group NDA, a firm that advises companies on developing drugs.
Dr. Vinks advised Acrotech should try “to pin down what is most likely a smaller study that could be simpler, but still give robust, informative data.”
Fatigue and night sweats
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's presentation of generalized lymphadenopathy, B symptoms, fatigue (probably from anemia), hepatosplenomegaly, immunophenotyping results of flow cell cytometry, and central nervous system (CNS) involvement, blastoid mantle cell lymphoma (MCL) is the most likely diagnosis. Although small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) and diffuse large B-cell lymphoma (DLBCL) most often occur in men 60-70 years old with similar clinical findings, an initial presentation with a stage IV involvement is rare; moreover, SLL/CLL and DLBCL are typically CD23 positive. Pleomorphic MCL displays larger and more pleomorphic cells with irregular nuclei, prominent nucleoli, and pale cytoplasm, resembling DLBCL.
MCL is a rare type of mature B-cell lymphoma that was first described in 1992 and was recognized by World Health Organization in 2001. MCL represents 3%-10% of all non-Hodgkin lymphoma cases, with an incidence between 0.50 and 1.0 per 100,000 population. Men are more likely than women to present with MCL by a ratio of 3:1, with a median age at presentation of 67 years. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. MCL usually affects the lymph nodes, with the spleen and bone marrow being significant sites of the disease. Stage IV disease is present in 70% of patients; the gastrointestinal tract, lung, pleura, and CNS are also frequently affected.
Besides classic MCL, several variants have been described that exhibit specific morphologic features, including small cell variant mimicking SLL marginal zone-like MCL (resembling marginal zone lymphoma), in situ mantle cell neoplasia (associated with indolent course), and two aggressive variants, including blastoid and pleomorphic MCL. These blastoid and pleomorphic variants are defined by cytomorphologic features; the criteria are somewhat subjective, but both are characterized by highly aggressive features and a dismal clinical course. In clinical cohorts, the frequency of these subsets varies widely but probably represents ∼10% of all cases.
Diagnosing MCL requires a multipronged approach. Lymph node biopsy and aspiration with immunophenotyping in MCL reveal monoclonal B cells expressing surface immunoglobulin, immunoglobulin M, or immunoglobulin D that are characteristically CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate and biopsy are used more for staging than diagnosis. Blood studies commonly reveal anemia and cytopenias secondary to bone marrow infiltration (with 20%-40% of cases showing lymphocytosis > 4000 cells/μL), abnormal liver function tests, and elevated lactate dehydrogenase when tumor burden is high. The term "blastoid mantle cell lymphoma" describes a morphologic subgroup of lymphomas with blastic features that morphologically resemble the lymphoblasts found in lymphoblastic lymphoma/leukemia (roundish nuclei, a narrow rim of cytoplasm, and finely dispersed chromatin).
MCL is associated with a poor prognosis; patients generally experience disease progression after chemotherapy, even with initial treatment response rates ranging from 50% to 70%. The 5-year survival rate is about 50% in the overall population, 75% in persons younger than 50 years, and 36% in those aged 75 years or older. A poorer prognosis is also associated with the presence of the blastoid variant, commonly associated with TP53 mutations. Median survival can vary by as much as 5 years, depending on the expression of cyclin D1 and other proliferation signature genes.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 65-year-old man presents to the oncology clinic with a 6-week history of fatigue, night sweats, and unintentional weight loss of 15 lb. He reports occasional fevers and generalized discomfort in his abdomen and has recently been experiencing painful headaches that are not relieved with nonsteroidal anti-inflammatory drugs. His medical history is otherwise unremarkable except for mild hypertension, for which he takes medication. His family history is unremarkable.
Physical examination reveals palpable lymph nodes in the neck, axilla, and inguinal regions; the spleen is palpable 3 cm below the left costal margin. A complete blood count shows anemia (hemoglobin level, 9.1g/dL) thrombocytopenia (platelet count, 90,000 cells/μL), and lymphocytosis (total leukocyte count, 5000 cells/μL); peripheral blood smear shows small, monomorphic lymphoid cells with oval-shaped nuclei and high nuclear-to-cytoplasmic ratio. Flow cytometry of lymph node biopsy is CD5-positive and pan B-cell antigen positive (eg, CD19, CD20, and CD22) but lacks expression of CD10 and CD23. A T2-weighted MRI is ordered.