User login
Cross-contamination of Pathology Specimens: A Cautionary Tale
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.
The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
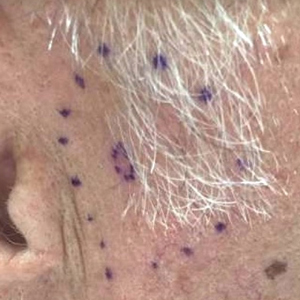
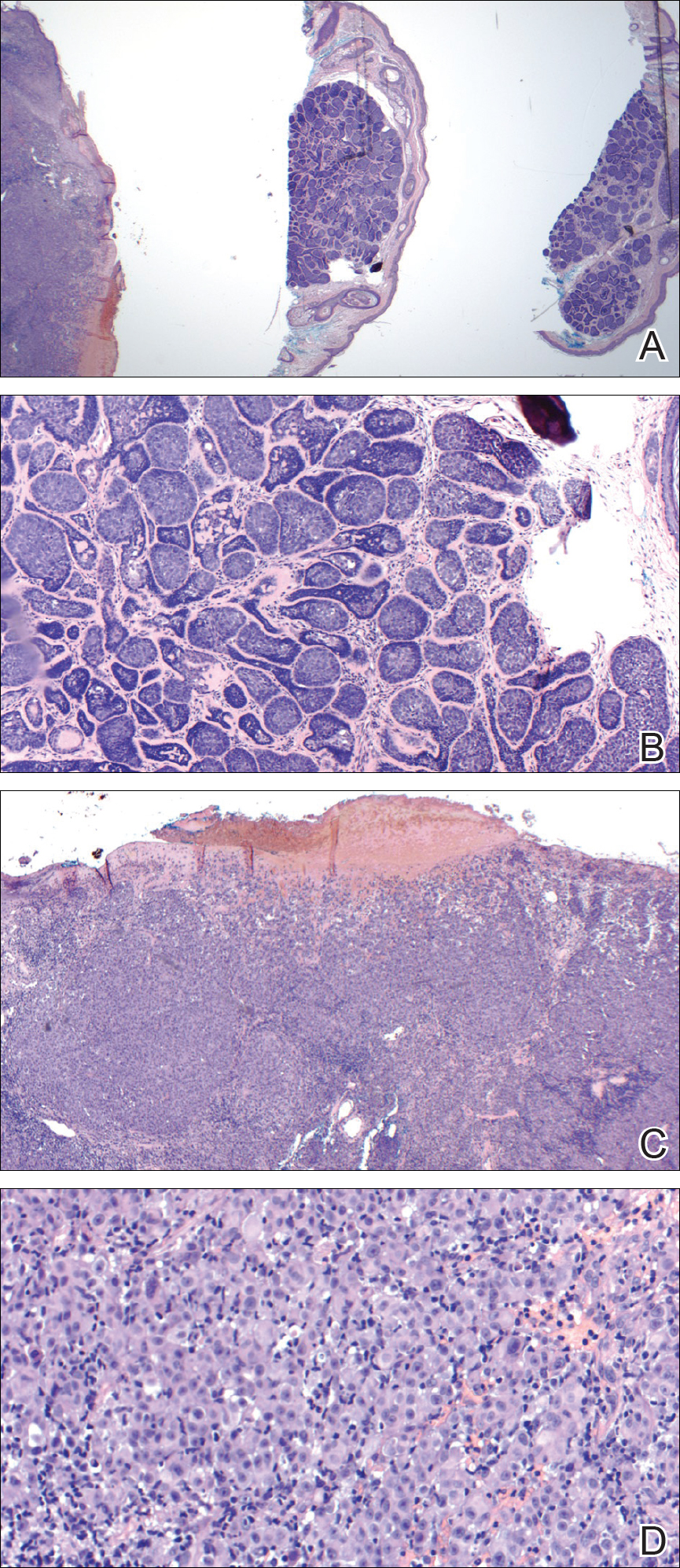
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.
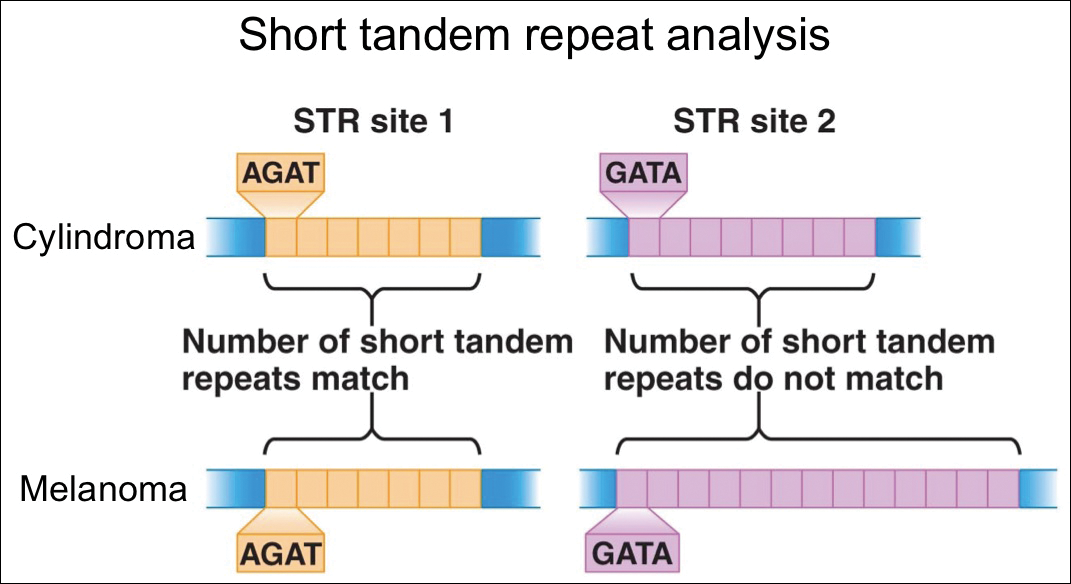
For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.
Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.

The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.

For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.

Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
Cross-contamination of pathology specimens is a rare but nonnegligible source of potential morbidity in clinical practice. Contaminant tissue fragments, colloquially referred to as floaters, typically are readily identifiable based on obvious cytomorphologic differences, especially if the tissues arise from different organs; however, one cannot rely on such distinctions in a pathology laboratory dedicated to a single organ system (eg, dermatopathology). The inability to identify quickly and confidently the presence of a contaminant puts the patient at risk for misdiagnosis, which can lead to unnecessary morbidity or even mortality in the case of cancer misdiagnosis. Studies that have been conducted to estimate the incidence of this type of error have suggested an overall incidence rate between approximately 1% and 3%.1,2 Awareness of this phenomenon and careful scrutiny when the histopathologic evidence diverges considerably from the clinical impression is critical for minimizing the negative outcomes that could result from the presence of contaminant tissue. We present a case in which cross-contamination of a pathology specimen led to an initial erroneous diagnosis of an aggressive cutaneous melanoma in a patient with a benign adnexal neoplasm.
Case Report
A 72-year-old man was referred to the Pigmented Lesion and Melanoma Program at Stanford University Medical Center and Cancer Institute (Palo Alto, California) for evaluation and treatment of a presumed stage IIB melanoma on the right preauricular cheek based on a shave biopsy that had been performed (<1 month prior) by his local dermatology provider and subsequently read by an affiliated out-of-state dermatopathology laboratory. Per the clinical history that was gathered at the current presentation, neither the patient nor his wife had noticed the lesion prior to his dermatology provider pointing it out on the day of the biopsy. Additionally, he denied associated pain, bleeding, or ulceration. According to outside medical records, the referring dermatology provider described the lesion as a 4-mm pink pearly papule with telangiectasia favoring a diagnosis of basal cell carcinoma, and a diagnostic shave biopsy was performed. On presentation to our clinic, physical examination of the right preauricular cheek revealed a 4×3-mm depressed erythematous scar with no evidence of residual pigmentation or nodularity (Figure 1). There was no clinically appreciable regional lymphadenopathy.

The original dermatopathology report indicated an invasive melanoma with the following pathologic characteristics: superficial spreading type, Breslow depth of at least 2.16 mm, ulceration, and a mitotic index of 8 mitotic figures/mm2 with transection of the invasive component at the peripheral and deep margins. There was no evidence of regression, perineural invasion, lymphovascular invasion, or microsatellites. Interestingly, the report indicated that there also was a basaloid proliferation with features of cylindroma in the same pathology slide adjacent to the aggressive invasive melanoma that was described. Given the complexity of cases referred to our academic center, the standard of care includes internal dermatopathology review of all outside pathology specimens. This review proved critical to this patient’s care in light of the considerable divergence of the initial pathologic diagnosis and the reported clinical features of the lesion.
Internal review of the single pathology slide received from the referring provider showed a total of 4 sections, 3 of which are shown here (Figure 2A). Three sections, including the one not shown, were all consistent with a diagnosis of cylindroma and showed no evidence of a melanocytic proliferation (Figure 2B). However, the fourth section demonstrated marked morphologic dissimilarity compared to the other 3 sections. This outlier section showed a thick cutaneous melanoma with a Breslow depth of at least 2.1 mm, ulceration, a mitotic rate of 12 mitotic figures/mm2, and broad transection of the invasive component at the peripheral and deep margins (Figures 2C and 2D). Correlation with the gross description of tissue processing on the original pathology report indicating that the specimen had been trisected raised suspicion that the fourth and very dissimilar section could be a contaminant from another source that was incorporated into our patient’s histologic sections during processing. Taken together, these discrepancies made the diagnosis of cylindroma alone far more likely than cutaneous melanoma, but we needed conclusive evidence given the dramatic difference in prognosis and management between a cylindroma and an aggressive cutaneous melanoma.

For further diagnostic clarification, we performed polymorphic short tandem repeat (STR) analysis, a well-described forensic pathology technique, to determine if the melanoma and cylindroma specimens derived from different patients, as we hypothesized. This analysis revealed differences in all but one DNA locus tested between the cylindroma specimen and the melanoma specimen, confirming our hypothesis (Figure 3). Subsequent discussion of the case with staff from the dermatopathology laboratory that processed this specimen provided further support for our suspicion that the invasive melanoma specimen was part of a case processed prior to our patient’s benign lesion. Therefore, the wide local excision for treatment of the suspected melanoma fortunately was canceled, and the patient did not require further treatment of the benign cylindroma. The patient expressed relief and gratitude for this critical clarification and change in management.

Comment
Shah et al3 reported a similar case in which a benign granuloma of the lung masqueraded as a squamous cell carcinoma due to histopathologic contamination. Although few similar cases have been described in the literature, the risk posed by such contamination is remarkable, regardless of whether it occurs during specimen grossing, embedding, sectioning, or staining.1,4,5 This risk is amplified in facilities that process specimens originating predominantly from a single organ system or tissue type, as is often the case in dedicated dermatopathology laboratories. In this scenario, it is unlikely that one could use the presence of tissues from 2 different organ systems on a single slide as a way of easily recognizing the presence of a contaminant and rectifying the error. Additionally, the presence of malignant cells in the contaminant further complicates the problem and requires an investigation that can conclusively distinguish the contaminant from the patient’s actual tissue.
In our case, our dermatology and dermatopathology teams partnered with our molecular pathology team to find a solution. Polymorphic STR analysis via polymerase chain reaction amplification is a sensitive method employed commonly in forensic DNA laboratories for determining whether a sample submitted as evidence belongs to a given suspect.6 Although much more commonly used in forensics, STR analysis does have known roles in clinical medicine, such as chimerism testing after bone marrow or allogeneic stem cell transplantation.7 Given the relatively short period of time it takes along with the convenience of commercially available kits, a high discriminative ability, and well-validated interpretation procedures, STR analysis is an excellent method for determining if a given tissue sample came from a given patient, which is what was needed in our case.
The combined clinical, histopathologic, and molecular data in our case allowed for confident clarification of our patient’s diagnosis, sparing him the morbidity of wide local excision on the face, sentinel lymph node biopsy, and emotional distress associated with a diagnosis of aggressive cutaneous melanoma. Our case highlights the critical importance of internal review of pathology specimens in ensuring proper diagnosis and management and reminds us that, though rare, accidental contamination during processing of pathology specimens is a potential adverse event that must be considered, especially when a pathologic finding diverges considerably from what is anticipated based on the patient’s history and physical examination.
Acknowledgment
The authors express gratitude to the patient described herein who graciously provided permission for us to publish his case and clinical photography.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
- Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009-1014.
- Alam M, Shah AD, Ali S, et al. Floaters in Mohs micrographic surgery [published online June 27, 2013]. Dermatol Surg. 2013;39:1317-1322.
- Shah PA, Prat MP, Hostler DC. Benign granuloma masquerading as squamous cell carcinoma due to a “floater.” Hawaii J Med Public Health. 2017;76(11, suppl 2):19-21.
- Platt E, Sommer P, McDonald L, et al. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973-978.
- Layfield LJ, Witt BL, Metzger KG, et al. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767-772.
- Butler JM. Forensic DNA testing. Cold Spring Harb Protoc. 2011;2011:1438-1450.
- Manasatienkij C, Ra-ngabpai C. Clinical application of forensic DNA analysis: a literature review. J Med Assoc Thai. 2012;95:1357-1363.
Resident Pearl
- Although cross-contamination of pathology specimens is rare, it does occur and can impact diagnosis and management if detected early.
Which Patients Have the Best Chance With Checkpoint Inhibitors?
Checkpoint inhibitors are so new that not enough patients have received them to allow clinicians to predict who will benefit most. But researchers from the National Cancer Institute, Center for Cancer Institute; Harvard University in Cambridge, Massachusetts; University of Pennsylvania in Philadelphia; and University of Maryland in College Park may have found a clue: A gene expression predictor.
They began by looking at neuroblastoma cases where the immune system seemed to mount “an unprompted, successful immune response” to cancer, causing spontaneous tumor regression. The researchers were able to define gene expression features that separated regressing from nonregressing disease.
The researchers then computed Immuno-PREdictive Scores (IMPRES) for each patient sample. The higher the score, the more likely was spontaneous regression. Analyzing 297 samples from several studies, they found the predictor identified nearly all patients who responded to the inhibitors and more than half of those who did not. “Importantly,” the researchers say, their predictor was accurate across many different melanoma patient datasets.
Checkpoint inhibitors are so new that not enough patients have received them to allow clinicians to predict who will benefit most. But researchers from the National Cancer Institute, Center for Cancer Institute; Harvard University in Cambridge, Massachusetts; University of Pennsylvania in Philadelphia; and University of Maryland in College Park may have found a clue: A gene expression predictor.
They began by looking at neuroblastoma cases where the immune system seemed to mount “an unprompted, successful immune response” to cancer, causing spontaneous tumor regression. The researchers were able to define gene expression features that separated regressing from nonregressing disease.
The researchers then computed Immuno-PREdictive Scores (IMPRES) for each patient sample. The higher the score, the more likely was spontaneous regression. Analyzing 297 samples from several studies, they found the predictor identified nearly all patients who responded to the inhibitors and more than half of those who did not. “Importantly,” the researchers say, their predictor was accurate across many different melanoma patient datasets.
Checkpoint inhibitors are so new that not enough patients have received them to allow clinicians to predict who will benefit most. But researchers from the National Cancer Institute, Center for Cancer Institute; Harvard University in Cambridge, Massachusetts; University of Pennsylvania in Philadelphia; and University of Maryland in College Park may have found a clue: A gene expression predictor.
They began by looking at neuroblastoma cases where the immune system seemed to mount “an unprompted, successful immune response” to cancer, causing spontaneous tumor regression. The researchers were able to define gene expression features that separated regressing from nonregressing disease.
The researchers then computed Immuno-PREdictive Scores (IMPRES) for each patient sample. The higher the score, the more likely was spontaneous regression. Analyzing 297 samples from several studies, they found the predictor identified nearly all patients who responded to the inhibitors and more than half of those who did not. “Importantly,” the researchers say, their predictor was accurate across many different melanoma patient datasets.
Epacadostat plus pembrolizumab shows promise in advanced solid tumors
Epacadostat, a highly selective oral inhibitor of the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme, was well tolerated when combined with pembrolizumab and demonstrated encouraging antitumor activity in multiple types of advanced solid tumors, according to the results of a phase l/ll trial.
Tumors may evade immunosurveillance through upregulation of the IDO1 enzyme, and thus there is a great interest in developing combination therapies that can target various immune evasion pathways to improve therapeutic response and outcomes. In this study, the authors evaluated the investigational agent epacadostat combined with pembrolizumab in 62 patients with advanced solid tumors.
In the dose escalation phase, patents received increasing doses of oral epacadostat (25, 50, 100, or 300 mg) twice per day plus intravenous pembrolizumab 2 mg/kg or 200 mg every 3 weeks. During the safety expansion, epacadostat at 50, 100, or 300 mg was given twice per day, plus pembrolizumab 200 mg every 3 weeks. The maximum tolerated dose of epacadostat in combination with pembrolizumab was not reached.
Objective responses (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) occurred in 12 (55%) of 22 patients with melanoma and in patients with non–small-cell lung cancer, renal cell carcinoma, endometrial adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck, reported Tara C. Mitchell, MD, of the Abramson Cancer Center, University of Pennsylvania, Philadelphia, and her colleagues. The report is in the Journal of Clinical Oncology.
The authors observed that there was antitumor activity at all epacadostat doses and in several tumor types. A complete response was achieved by 8 patients (treatment naive melanoma [5 patients] and previously treated for advanced/ metastatic melanoma, endometrial adenocarcinoma [EA], or urothelial carcinoma [UC] [1 patient each]), while 17 patients achieved a partial response (treatment-naive melanoma [6 patients], non–small cell lung cancer [NSCLC] [5 patients], renal cell carcinoma [RCC] and UC [2 patients each], and EA and squamous cell carcinoma of the head and neck [1 patient each]).
Most patients (n = 52, 84%) experienced treatment-related adverse events (TRAEs), the most frequently observed being fatigue (36%), rash (36%), arthralgia (24%), pruritus (23%), and nausea (21%). Grade 3/4 TRAEs occurred in 24% of patients, and 7 patients (11%) discontinued their treatment because of TRAEs. There were no deaths associated with TRAEs.
“The safety profile observed with epacadostat plus pembrolizumab compares favorably with studies of other combination immunotherapies,” wrote Dr. Mitchell and her colleagues. “Although not powered to evaluate efficacy, the phase I portion of this study showed that epacadostat plus pembrolizumab had encouraging and durable antitumor activity,” they said.
SOURCE: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
Epacadostat, a highly selective oral inhibitor of the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme, was well tolerated when combined with pembrolizumab and demonstrated encouraging antitumor activity in multiple types of advanced solid tumors, according to the results of a phase l/ll trial.
Tumors may evade immunosurveillance through upregulation of the IDO1 enzyme, and thus there is a great interest in developing combination therapies that can target various immune evasion pathways to improve therapeutic response and outcomes. In this study, the authors evaluated the investigational agent epacadostat combined with pembrolizumab in 62 patients with advanced solid tumors.
In the dose escalation phase, patents received increasing doses of oral epacadostat (25, 50, 100, or 300 mg) twice per day plus intravenous pembrolizumab 2 mg/kg or 200 mg every 3 weeks. During the safety expansion, epacadostat at 50, 100, or 300 mg was given twice per day, plus pembrolizumab 200 mg every 3 weeks. The maximum tolerated dose of epacadostat in combination with pembrolizumab was not reached.
Objective responses (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) occurred in 12 (55%) of 22 patients with melanoma and in patients with non–small-cell lung cancer, renal cell carcinoma, endometrial adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck, reported Tara C. Mitchell, MD, of the Abramson Cancer Center, University of Pennsylvania, Philadelphia, and her colleagues. The report is in the Journal of Clinical Oncology.
The authors observed that there was antitumor activity at all epacadostat doses and in several tumor types. A complete response was achieved by 8 patients (treatment naive melanoma [5 patients] and previously treated for advanced/ metastatic melanoma, endometrial adenocarcinoma [EA], or urothelial carcinoma [UC] [1 patient each]), while 17 patients achieved a partial response (treatment-naive melanoma [6 patients], non–small cell lung cancer [NSCLC] [5 patients], renal cell carcinoma [RCC] and UC [2 patients each], and EA and squamous cell carcinoma of the head and neck [1 patient each]).
Most patients (n = 52, 84%) experienced treatment-related adverse events (TRAEs), the most frequently observed being fatigue (36%), rash (36%), arthralgia (24%), pruritus (23%), and nausea (21%). Grade 3/4 TRAEs occurred in 24% of patients, and 7 patients (11%) discontinued their treatment because of TRAEs. There were no deaths associated with TRAEs.
“The safety profile observed with epacadostat plus pembrolizumab compares favorably with studies of other combination immunotherapies,” wrote Dr. Mitchell and her colleagues. “Although not powered to evaluate efficacy, the phase I portion of this study showed that epacadostat plus pembrolizumab had encouraging and durable antitumor activity,” they said.
SOURCE: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
Epacadostat, a highly selective oral inhibitor of the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme, was well tolerated when combined with pembrolizumab and demonstrated encouraging antitumor activity in multiple types of advanced solid tumors, according to the results of a phase l/ll trial.
Tumors may evade immunosurveillance through upregulation of the IDO1 enzyme, and thus there is a great interest in developing combination therapies that can target various immune evasion pathways to improve therapeutic response and outcomes. In this study, the authors evaluated the investigational agent epacadostat combined with pembrolizumab in 62 patients with advanced solid tumors.
In the dose escalation phase, patents received increasing doses of oral epacadostat (25, 50, 100, or 300 mg) twice per day plus intravenous pembrolizumab 2 mg/kg or 200 mg every 3 weeks. During the safety expansion, epacadostat at 50, 100, or 300 mg was given twice per day, plus pembrolizumab 200 mg every 3 weeks. The maximum tolerated dose of epacadostat in combination with pembrolizumab was not reached.
Objective responses (per Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) occurred in 12 (55%) of 22 patients with melanoma and in patients with non–small-cell lung cancer, renal cell carcinoma, endometrial adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma of the head and neck, reported Tara C. Mitchell, MD, of the Abramson Cancer Center, University of Pennsylvania, Philadelphia, and her colleagues. The report is in the Journal of Clinical Oncology.
The authors observed that there was antitumor activity at all epacadostat doses and in several tumor types. A complete response was achieved by 8 patients (treatment naive melanoma [5 patients] and previously treated for advanced/ metastatic melanoma, endometrial adenocarcinoma [EA], or urothelial carcinoma [UC] [1 patient each]), while 17 patients achieved a partial response (treatment-naive melanoma [6 patients], non–small cell lung cancer [NSCLC] [5 patients], renal cell carcinoma [RCC] and UC [2 patients each], and EA and squamous cell carcinoma of the head and neck [1 patient each]).
Most patients (n = 52, 84%) experienced treatment-related adverse events (TRAEs), the most frequently observed being fatigue (36%), rash (36%), arthralgia (24%), pruritus (23%), and nausea (21%). Grade 3/4 TRAEs occurred in 24% of patients, and 7 patients (11%) discontinued their treatment because of TRAEs. There were no deaths associated with TRAEs.
“The safety profile observed with epacadostat plus pembrolizumab compares favorably with studies of other combination immunotherapies,” wrote Dr. Mitchell and her colleagues. “Although not powered to evaluate efficacy, the phase I portion of this study showed that epacadostat plus pembrolizumab had encouraging and durable antitumor activity,” they said.
SOURCE: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Epacadostat plus pembrolizumab showed antitumor activity and tolerability in patients with advanced solid tumors.
Major finding: Among 62 patients, 25 achieved an objective response.
Study details: Phase l/ll clinical trial of 62 patients with advanced solid tumors.
Disclosures: Incyte and Merck funded the study. All of the authors have disclosed relationships with industry, including the study sponsor.
Source: Mitchell TC et al. J Clin Oncol. 2018 Sep 28. doi: 10.1200/JCO.2018.78.9602.
Xanthogranulomatous Reaction to Trametinib for Metastatic Malignant Melanoma
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
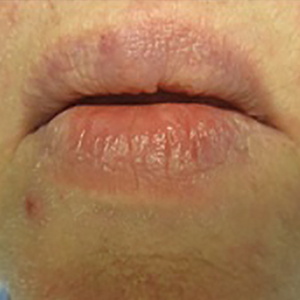
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
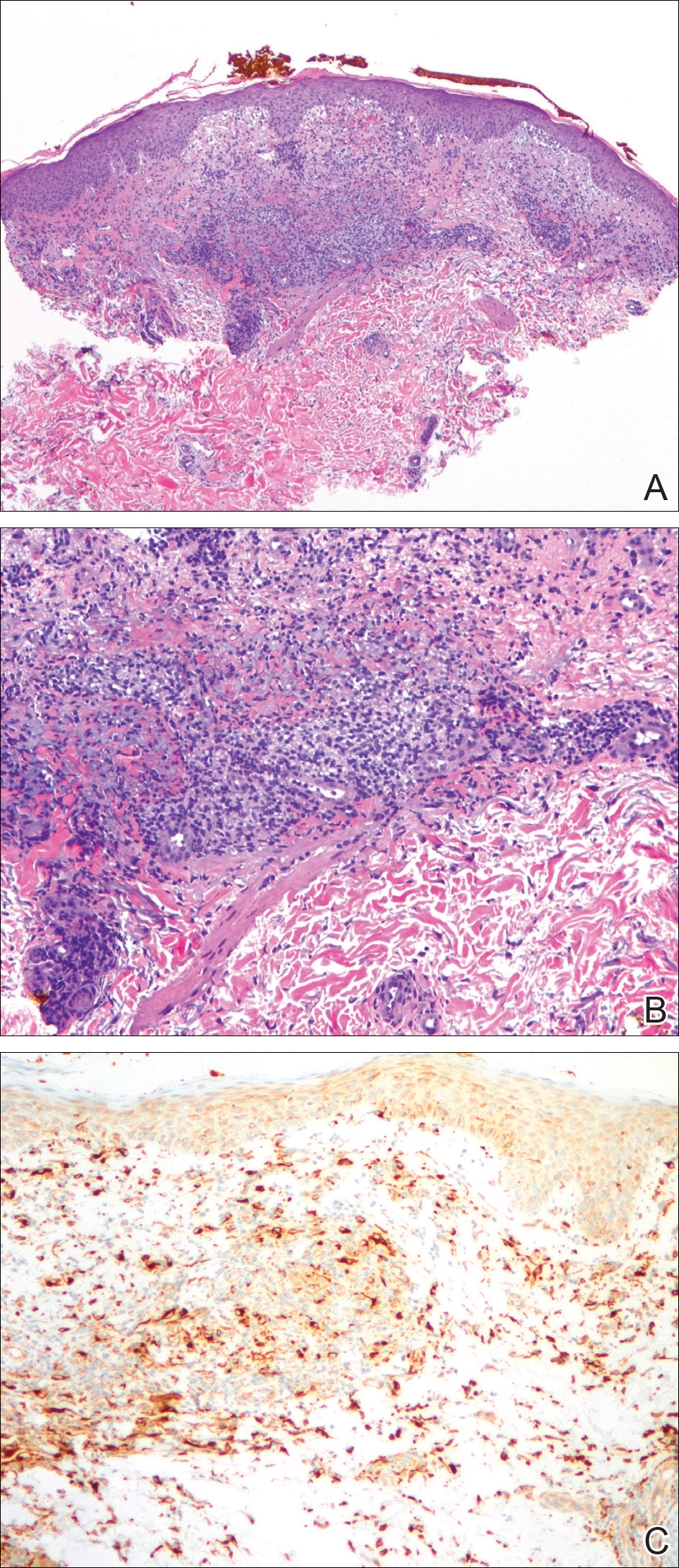
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.
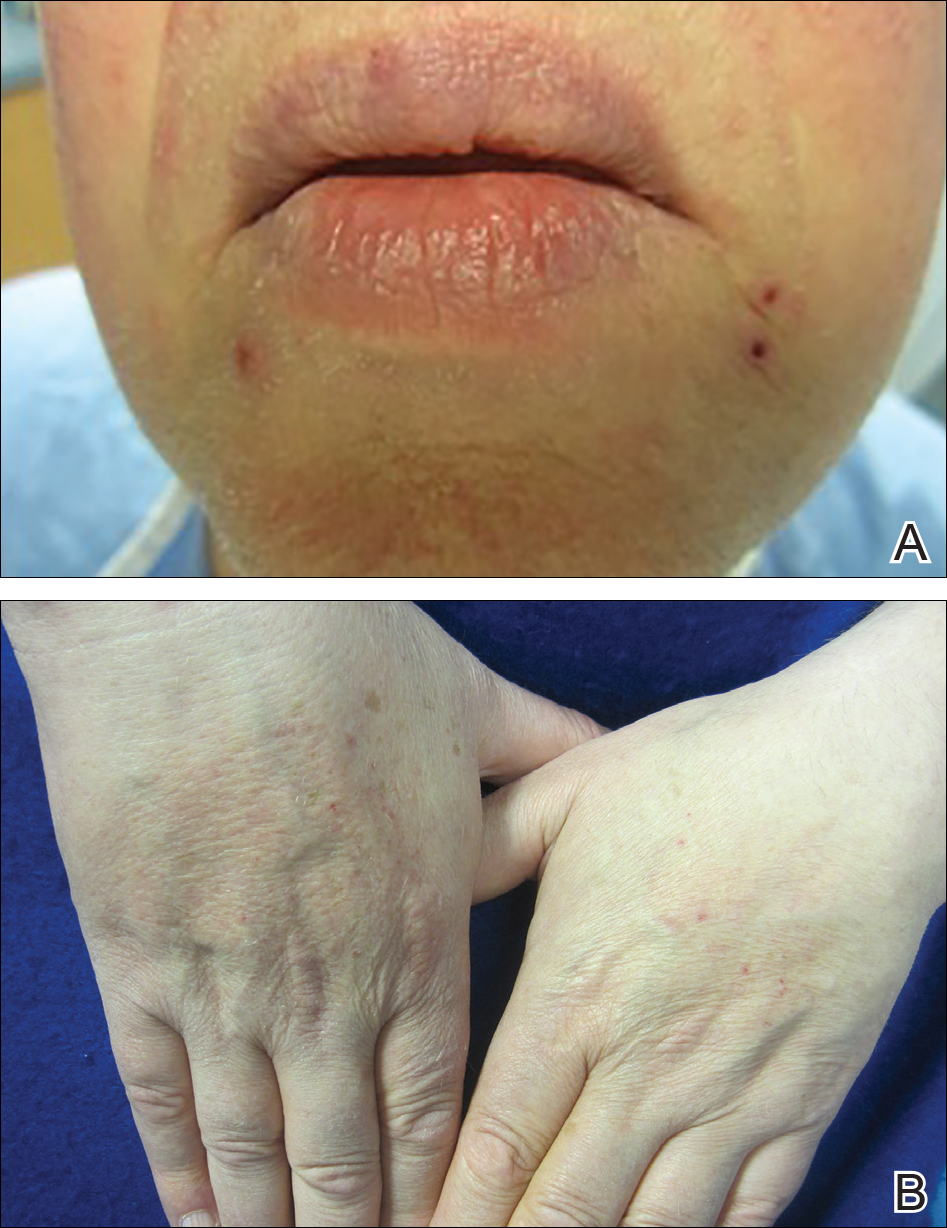
Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.
Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
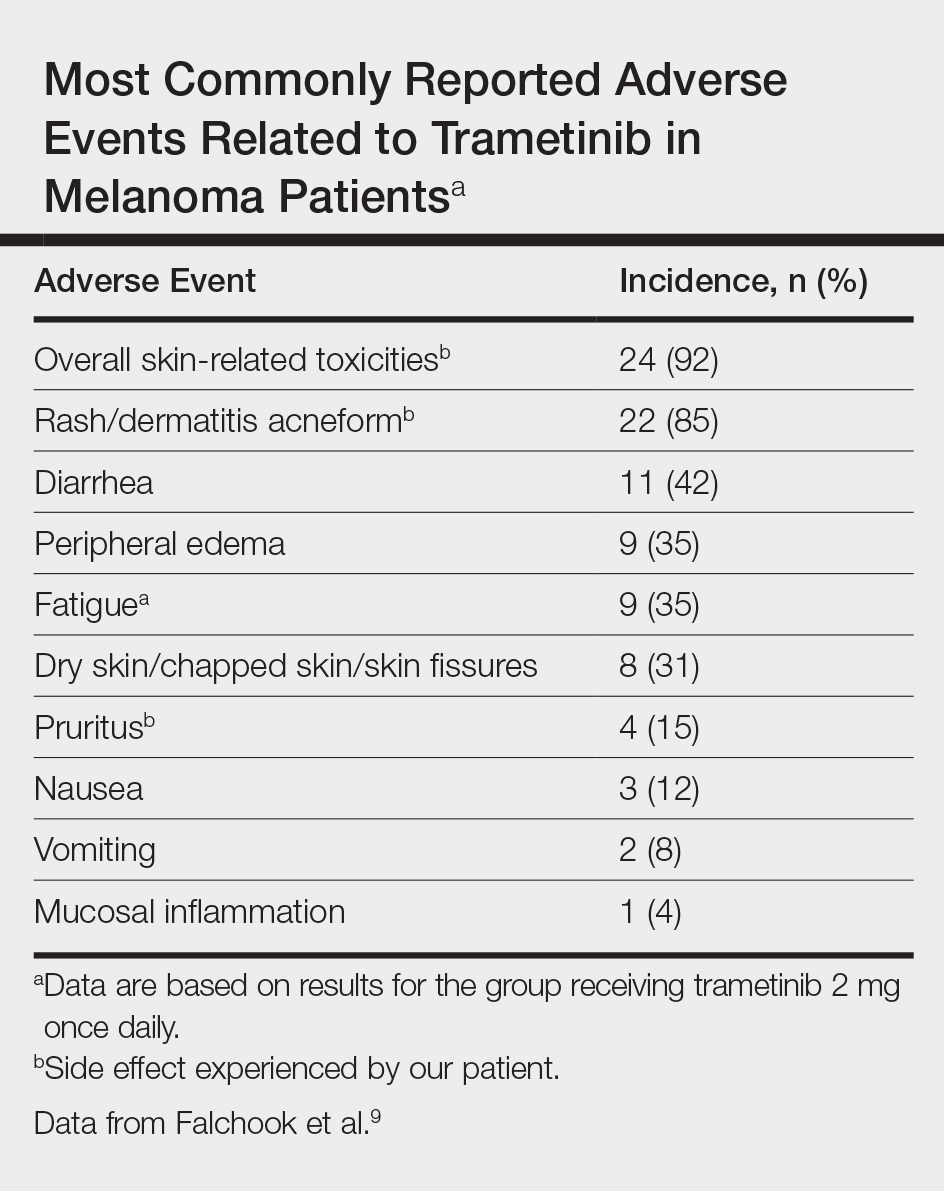
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
Practice Points
- With the discovery of molecular targeting in melanoma, BRAF and MEK inhibitors have been increasingly utilized as therapies in metastatic melanoma management.
- Trametinib, a MEK inhibitor, is commonly associated with cutaneous adverse reactions, particularly acneform eruptions.
- We report a patient on trametinib who developed an eruption with an unusual xanthogranulomatous reaction pattern noted on histology.
Mobile App Rankings in Dermatology
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
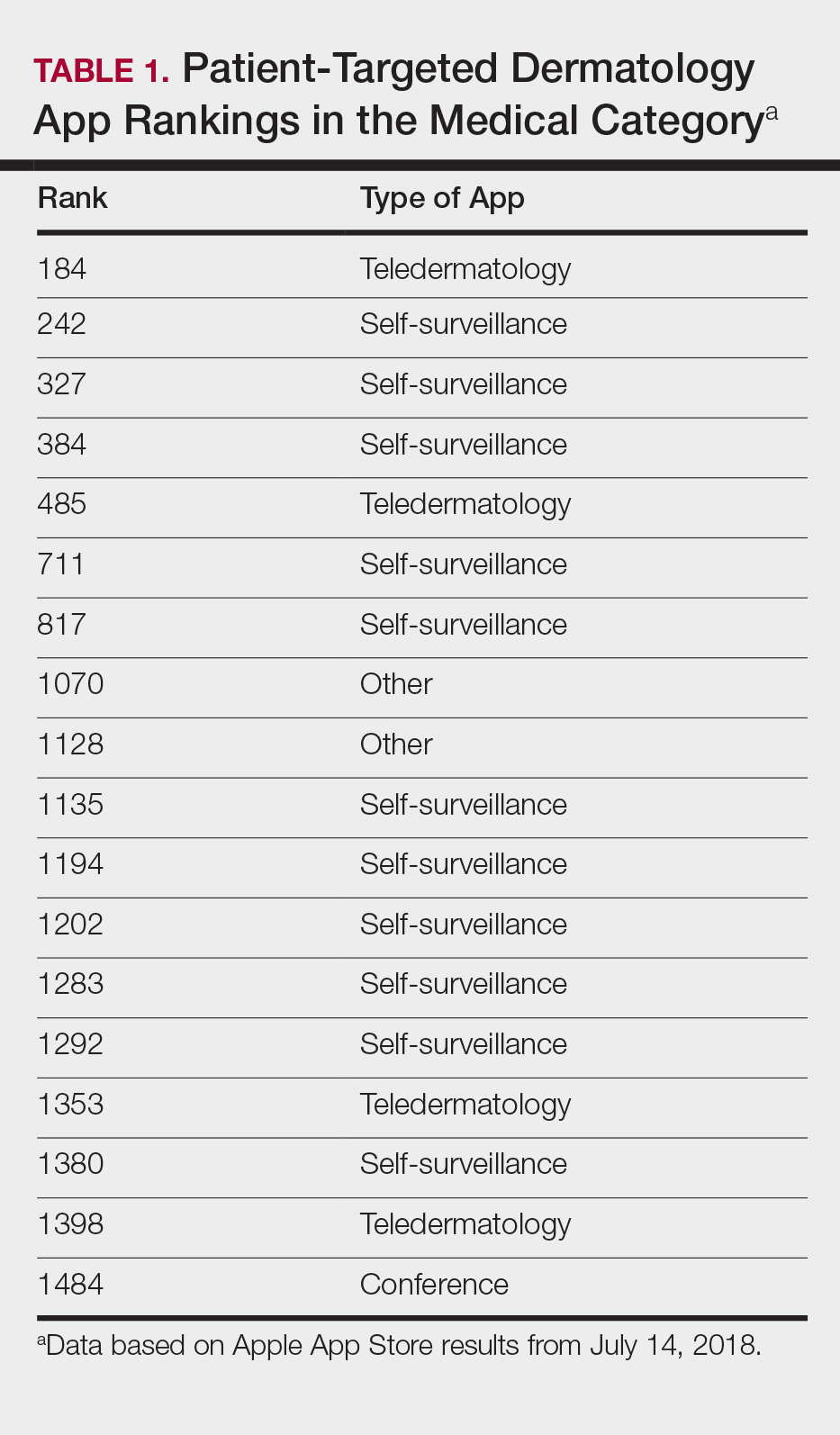
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).
Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).
Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).
Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
Practice Points
- As mobile application (app) usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in dermatology education. As such, it will become more critical to develop formal scientific standards.
- The most desired dermatology apps for patients were apps that allowed them to be proactive with their health.
- There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps.
Checkpoint inhibitor linked to antiphospholipid syndrome in melanoma patient
A patient with melanoma experienced antiphospholipid syndrome following multiple infusions of the PD-1 inhibitor pembrolizumab, according to authors of a recent case report.
Presence of Raynaud phenomenon and high levels of antiphospholipid antibodies led to the diagnosis of antiphospholipid syndrome in the patient, who had stage IIIB unresectable melanoma.
This report provides additional evidence that this syndrome is an immune-related adverse event associated with checkpoint inhibitor therapy, said Alexandra Picard, MD, of Hôpital Archet, Nice, France, and coauthors.
“Due to the increased use of anti PD-1 therapies, clinicians should be aware of this new potential immune-mediated toxic effect that manifests as antiphospholipid syndrome,” the researchers wrote. The report is in JAMA Dermatology.
“Great caution” should be exercised when considering use of immune checkpoint inhibitors in patients with a history of antiphospholipid syndrome, the authors added.
The woman in this report was over 60 years of age and had first presented with superficial melanoma on her right calf, followed by recurrent lymph node metastases over the next few years, all of which were surgically treated.
Following a PET-CT scan showing a new metastatic lymph node, the woman started pembrolizumab 2 mg/kg every 3 weeks and had a partial response within 3 months, the investigators reported.
After the tenth infusion, however, the patient developed bilateral secondary Raynaud phenomenon that followed a typical discoloration sequence and resulted in a necrotic lesion at the tip of one finger.
The patient had no personal or family history of Raynaud phenomenon.
While beta2-glycoprotein 1 antibodies were not elevated, laboratory tests did show anticardiolipin antibodies and lupus anticoagulants at elevated levels, the investigators said, noting that repeat testing at 12 weeks confirmed positivity of antiphospholipid antibodies.
The Raynaud phenomenon disappeared and the necrotic lesion healed after pembrolizumab was stopped and prednisolone treatment was started, they added.
No recurrence of either was noted at the last follow-up.
Previous reports have described antiphospholipid syndrome in advanced melanoma patients treated with alfa-2b interferon alone or in combination with anti-interleukin 2, the authors said in their discussion of the case.
In addition, there has been another recent report of antiphospholipid syndrome associated with the CTLA4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab, they said. In that case, testing for antiphospholipid antibodies revealed elevated beta2-glycoprotein 1 antibody levels.
“We hypothesize that [antiphospholipid syndrome] is a kind of autoimmunity induced by anti–PD-1 due to the expansive expression of the immune system against tumor cells,” the researchers wrote.
Although a case of cancer-associated antiphospholipid syndrome could not be ruled out in the present report, the rapid and complete resolution of symptoms after treatment discontinuation suggested that pembrolizumab, a “known immunostimulant,” was the cause, they said.
While antibodies against PD-1 have improved melanoma prognosis, they are associated with a wide range of immune-related adverse effects in the skin, gastrointestinal tract, liver, and endocrine system, Dr. Picard and coauthors noted.
They reported having no conflicts of interest.
SOURCE: Sanchez A, et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
A patient with melanoma experienced antiphospholipid syndrome following multiple infusions of the PD-1 inhibitor pembrolizumab, according to authors of a recent case report.
Presence of Raynaud phenomenon and high levels of antiphospholipid antibodies led to the diagnosis of antiphospholipid syndrome in the patient, who had stage IIIB unresectable melanoma.
This report provides additional evidence that this syndrome is an immune-related adverse event associated with checkpoint inhibitor therapy, said Alexandra Picard, MD, of Hôpital Archet, Nice, France, and coauthors.
“Due to the increased use of anti PD-1 therapies, clinicians should be aware of this new potential immune-mediated toxic effect that manifests as antiphospholipid syndrome,” the researchers wrote. The report is in JAMA Dermatology.
“Great caution” should be exercised when considering use of immune checkpoint inhibitors in patients with a history of antiphospholipid syndrome, the authors added.
The woman in this report was over 60 years of age and had first presented with superficial melanoma on her right calf, followed by recurrent lymph node metastases over the next few years, all of which were surgically treated.
Following a PET-CT scan showing a new metastatic lymph node, the woman started pembrolizumab 2 mg/kg every 3 weeks and had a partial response within 3 months, the investigators reported.
After the tenth infusion, however, the patient developed bilateral secondary Raynaud phenomenon that followed a typical discoloration sequence and resulted in a necrotic lesion at the tip of one finger.
The patient had no personal or family history of Raynaud phenomenon.
While beta2-glycoprotein 1 antibodies were not elevated, laboratory tests did show anticardiolipin antibodies and lupus anticoagulants at elevated levels, the investigators said, noting that repeat testing at 12 weeks confirmed positivity of antiphospholipid antibodies.
The Raynaud phenomenon disappeared and the necrotic lesion healed after pembrolizumab was stopped and prednisolone treatment was started, they added.
No recurrence of either was noted at the last follow-up.
Previous reports have described antiphospholipid syndrome in advanced melanoma patients treated with alfa-2b interferon alone or in combination with anti-interleukin 2, the authors said in their discussion of the case.
In addition, there has been another recent report of antiphospholipid syndrome associated with the CTLA4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab, they said. In that case, testing for antiphospholipid antibodies revealed elevated beta2-glycoprotein 1 antibody levels.
“We hypothesize that [antiphospholipid syndrome] is a kind of autoimmunity induced by anti–PD-1 due to the expansive expression of the immune system against tumor cells,” the researchers wrote.
Although a case of cancer-associated antiphospholipid syndrome could not be ruled out in the present report, the rapid and complete resolution of symptoms after treatment discontinuation suggested that pembrolizumab, a “known immunostimulant,” was the cause, they said.
While antibodies against PD-1 have improved melanoma prognosis, they are associated with a wide range of immune-related adverse effects in the skin, gastrointestinal tract, liver, and endocrine system, Dr. Picard and coauthors noted.
They reported having no conflicts of interest.
SOURCE: Sanchez A, et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
A patient with melanoma experienced antiphospholipid syndrome following multiple infusions of the PD-1 inhibitor pembrolizumab, according to authors of a recent case report.
Presence of Raynaud phenomenon and high levels of antiphospholipid antibodies led to the diagnosis of antiphospholipid syndrome in the patient, who had stage IIIB unresectable melanoma.
This report provides additional evidence that this syndrome is an immune-related adverse event associated with checkpoint inhibitor therapy, said Alexandra Picard, MD, of Hôpital Archet, Nice, France, and coauthors.
“Due to the increased use of anti PD-1 therapies, clinicians should be aware of this new potential immune-mediated toxic effect that manifests as antiphospholipid syndrome,” the researchers wrote. The report is in JAMA Dermatology.
“Great caution” should be exercised when considering use of immune checkpoint inhibitors in patients with a history of antiphospholipid syndrome, the authors added.
The woman in this report was over 60 years of age and had first presented with superficial melanoma on her right calf, followed by recurrent lymph node metastases over the next few years, all of which were surgically treated.
Following a PET-CT scan showing a new metastatic lymph node, the woman started pembrolizumab 2 mg/kg every 3 weeks and had a partial response within 3 months, the investigators reported.
After the tenth infusion, however, the patient developed bilateral secondary Raynaud phenomenon that followed a typical discoloration sequence and resulted in a necrotic lesion at the tip of one finger.
The patient had no personal or family history of Raynaud phenomenon.
While beta2-glycoprotein 1 antibodies were not elevated, laboratory tests did show anticardiolipin antibodies and lupus anticoagulants at elevated levels, the investigators said, noting that repeat testing at 12 weeks confirmed positivity of antiphospholipid antibodies.
The Raynaud phenomenon disappeared and the necrotic lesion healed after pembrolizumab was stopped and prednisolone treatment was started, they added.
No recurrence of either was noted at the last follow-up.
Previous reports have described antiphospholipid syndrome in advanced melanoma patients treated with alfa-2b interferon alone or in combination with anti-interleukin 2, the authors said in their discussion of the case.
In addition, there has been another recent report of antiphospholipid syndrome associated with the CTLA4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab, they said. In that case, testing for antiphospholipid antibodies revealed elevated beta2-glycoprotein 1 antibody levels.
“We hypothesize that [antiphospholipid syndrome] is a kind of autoimmunity induced by anti–PD-1 due to the expansive expression of the immune system against tumor cells,” the researchers wrote.
Although a case of cancer-associated antiphospholipid syndrome could not be ruled out in the present report, the rapid and complete resolution of symptoms after treatment discontinuation suggested that pembrolizumab, a “known immunostimulant,” was the cause, they said.
While antibodies against PD-1 have improved melanoma prognosis, they are associated with a wide range of immune-related adverse effects in the skin, gastrointestinal tract, liver, and endocrine system, Dr. Picard and coauthors noted.
They reported having no conflicts of interest.
SOURCE: Sanchez A, et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
FROM JAMA DERMATOLOGY
Key clinical point: Antiphospholipid syndrome appears to be an immune-related adverse event associated with anti-PD-1 therapy.
Major finding: A melanoma patient receiving pembrolizumab was diagnosed with antiphospholipid syndrome that resolved following discontinuation of that treatment.
Study details: Case report of a woman in her 60s with stage IIIB unresectable melanoma who was treated with pembrolizumab 2 mg/kg every 3 weeks.
Disclosures: The authors reported no conflicts of interest.
Source: Sanchez A et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
Re-excision unnecessary in moderately dysplastic nevi with positive margins
ORLANDO – Re-excisions are not needed when clinically excised moderately dysplastic nevi have positive histologic margins, based on results of a retrospective study of 438 patients who were treated at nine academic medical centers in the United States.
Not a single patient in the study developed melanoma at the excision site after an average follow-up of 6.9 years, and at least 3 years in all cases, said Elizabeth G. Berry, MD, of Emory University, Atlanta, and Atlanta Veterans Administration Medical Center, one of the study investigators.
The finding “really has the potential to change how we manage these lesions. You don’t need to cut [these patients] again. You can watch them. Close observation with routine skin surveillance is reasonable,” Dr. Berry said at the International Investigative Dermatology meeting.
Routine skin exams are essential for patients with a history of dysplastic nevi as these patients are at risk for developing melanoma. Indeed, in this study, 100 patients (22.8%) subsequently developed melanomas at a site other than the location of their biopsy.
The study included 438 patients who had 467 biopsies that indicated incomplete excision of a moderately dysplastic nevus from 1990 to 2014. Patients were at least 18 years old and were an average of 47 years old. About half had a history of dysplastic nevi, and a third had a history of melanoma.
All of their biopsies for moderately dysplastic nevi had positive margins, but patients had no clinically apparent residual pigment at their excision sites. Lesions were equally as likely to be removed by shave and punch biopsies, and the majority of the nevi were located on the trunk. Complete excision was the intent in all cases.
To control for interobserver variability, the centers submitted a total of 40 slides for central dermatopathology review, which found agreement in 35 cases (87.8%). Two of the remaining five cases were downgraded to mild dysplasia, two were upgraded to severe, and one patient was upgraded to melanoma in situ, but hasn’t had a recurrence after 5 years of follow-up.
Controlling for age, sex, and family history, a patient history of dysplastic nevus prior to the biopsy doubled the risk of a subsequent melanoma (P = .017), and a history of melanoma increased it almost eightfold (P less than .001).
Knowing these risk factors, patients with a history of dysplastic nevi “need to have more frequent total body skin exams. What that frequency is, we don’t know,” Dr. Berry said.
The investigators reported they had no relevant disclosures.
SOURCE: Kim CC et al. IID 2018, Abstract 571.
ORLANDO – Re-excisions are not needed when clinically excised moderately dysplastic nevi have positive histologic margins, based on results of a retrospective study of 438 patients who were treated at nine academic medical centers in the United States.
Not a single patient in the study developed melanoma at the excision site after an average follow-up of 6.9 years, and at least 3 years in all cases, said Elizabeth G. Berry, MD, of Emory University, Atlanta, and Atlanta Veterans Administration Medical Center, one of the study investigators.
The finding “really has the potential to change how we manage these lesions. You don’t need to cut [these patients] again. You can watch them. Close observation with routine skin surveillance is reasonable,” Dr. Berry said at the International Investigative Dermatology meeting.
Routine skin exams are essential for patients with a history of dysplastic nevi as these patients are at risk for developing melanoma. Indeed, in this study, 100 patients (22.8%) subsequently developed melanomas at a site other than the location of their biopsy.
The study included 438 patients who had 467 biopsies that indicated incomplete excision of a moderately dysplastic nevus from 1990 to 2014. Patients were at least 18 years old and were an average of 47 years old. About half had a history of dysplastic nevi, and a third had a history of melanoma.
All of their biopsies for moderately dysplastic nevi had positive margins, but patients had no clinically apparent residual pigment at their excision sites. Lesions were equally as likely to be removed by shave and punch biopsies, and the majority of the nevi were located on the trunk. Complete excision was the intent in all cases.
To control for interobserver variability, the centers submitted a total of 40 slides for central dermatopathology review, which found agreement in 35 cases (87.8%). Two of the remaining five cases were downgraded to mild dysplasia, two were upgraded to severe, and one patient was upgraded to melanoma in situ, but hasn’t had a recurrence after 5 years of follow-up.
Controlling for age, sex, and family history, a patient history of dysplastic nevus prior to the biopsy doubled the risk of a subsequent melanoma (P = .017), and a history of melanoma increased it almost eightfold (P less than .001).
Knowing these risk factors, patients with a history of dysplastic nevi “need to have more frequent total body skin exams. What that frequency is, we don’t know,” Dr. Berry said.
The investigators reported they had no relevant disclosures.
SOURCE: Kim CC et al. IID 2018, Abstract 571.
ORLANDO – Re-excisions are not needed when clinically excised moderately dysplastic nevi have positive histologic margins, based on results of a retrospective study of 438 patients who were treated at nine academic medical centers in the United States.
Not a single patient in the study developed melanoma at the excision site after an average follow-up of 6.9 years, and at least 3 years in all cases, said Elizabeth G. Berry, MD, of Emory University, Atlanta, and Atlanta Veterans Administration Medical Center, one of the study investigators.
The finding “really has the potential to change how we manage these lesions. You don’t need to cut [these patients] again. You can watch them. Close observation with routine skin surveillance is reasonable,” Dr. Berry said at the International Investigative Dermatology meeting.
Routine skin exams are essential for patients with a history of dysplastic nevi as these patients are at risk for developing melanoma. Indeed, in this study, 100 patients (22.8%) subsequently developed melanomas at a site other than the location of their biopsy.
The study included 438 patients who had 467 biopsies that indicated incomplete excision of a moderately dysplastic nevus from 1990 to 2014. Patients were at least 18 years old and were an average of 47 years old. About half had a history of dysplastic nevi, and a third had a history of melanoma.
All of their biopsies for moderately dysplastic nevi had positive margins, but patients had no clinically apparent residual pigment at their excision sites. Lesions were equally as likely to be removed by shave and punch biopsies, and the majority of the nevi were located on the trunk. Complete excision was the intent in all cases.
To control for interobserver variability, the centers submitted a total of 40 slides for central dermatopathology review, which found agreement in 35 cases (87.8%). Two of the remaining five cases were downgraded to mild dysplasia, two were upgraded to severe, and one patient was upgraded to melanoma in situ, but hasn’t had a recurrence after 5 years of follow-up.
Controlling for age, sex, and family history, a patient history of dysplastic nevus prior to the biopsy doubled the risk of a subsequent melanoma (P = .017), and a history of melanoma increased it almost eightfold (P less than .001).
Knowing these risk factors, patients with a history of dysplastic nevi “need to have more frequent total body skin exams. What that frequency is, we don’t know,” Dr. Berry said.
The investigators reported they had no relevant disclosures.
SOURCE: Kim CC et al. IID 2018, Abstract 571.
REPORTING FROM IID 2018
Vemurafenib-Induced Plantar Hyperkeratosis
To the Editor:
Vemurafenib, a selective BRAF inhibitor, is a chemotherapeutic agent used in the treatment of metastatic melanoma with BRAF mutations. It has been associated with various cutaneous side effects. We report a case of metastatic melanoma with acquired plantar hyperkeratosis secondary to vemurafenib therapy.
A 49-year-old man presented for evaluation of a pigmented plaque on the left pretibial region that had been enlarging over the last 2 months. The lesion had been diagnosed as folliculitis by his primary care physician 1 month prior to the current presentation and was being treated with oral antibiotics. The patient reported occasional bleeding from the lesion but denied other symptoms. Physical examination revealed a 1.4-cm pigmented plaque distributed over the left shin. Excisional biopsy was performed to rule out melanoma. Histopathology revealed well-circumscribed and symmetric proliferation of nested and single atypical melanocytes throughout all layers to the deep reticular dermis, confirming a clinical diagnosis of malignant melanoma. The lesion demonstrated angiolymphatic invasion, mitotic activity, and a Breslow depth of 2.5 mm. The patient underwent wide local excision with 3-cm margins and left inguinal sentinel lymph node biopsy; 2 of 14 lymph nodes were positive for melanoma. Positron emission tomography–computed tomography was negative for further metastatic disease. The patient underwent isolated limb perfusion with ipilimumab, but treatment was discontinued due to regional progression of multiple cutaneous metastases that were positive for the BRAF V600E mutation.
The patient was then started on vemurafenib therapy. Within 2 weeks, the patient reported various cutaneous symptoms, including morbilliform drug eruption covering approximately 70% of the body surface area that resolved with topical steroids and oral antihistamines, as well as the appearance of melanocytic nevi on the posterior neck, back, and abdomen. After 5 months of vemurafenib therapy, the patient began to develop hyperkeratosis of the bilateral soles of the feet (Figure). A diagnosis of acquired plantar hyperkeratosis secondary to vemurafenib therapy was made. Treatment with keratolytics was initiated and vemurafenib was not discontinued. The patient died approximately 1 year after therapy was started.
Metastatic melanoma is challenging to treat and continues to have a high mortality rate; however, newer chemotherapeutic agents targeting specific mutations found in melanoma, including the BRAF V600E mutation, are promising.
The US Food and Drug Administration first approved vemurafenib, a selective BRAF inhibitor, in 2011 for treatment of metastatic melanoma. Activating BRAF mutations have been detected in up to 60% of cutaneous melanomas.1 In the majority of these mutations, valine (V) is inserted at codon 600 instead of glutamic acid (E); therefore, the mutation is named V600E.2 In a phase 3 trial of 675 metastatic melanoma patients with positive V600E who were randomized to receive either vemurafenib or dacarbazine, the overall survival rate in the vemurafenib group improved by 84% versus 64% in the dacarbazine group at 6 months.3
Vemurafenib and other BRAF inhibitors have been associated with multiple cutaneous side effects, including rash, alopecia, squamous cell carcinoma, photosensitivity, evolution of existing nevi, and less commonly palmoplantar hyperkeratosis.2-5 Constitutional symptoms including arthralgia, nausea, and fatigue also have been commonly reported.2-5 In several large studies comprising 1138 patients, cutaneous side effects were seen in 92% to 95% of patients.3,5 Adverse effects caused interruption or modification of therapy in 38% of patients.3
Palmoplantar keratoderma is a known side effect of vemurafenib therapy, but it is less commonly reported than other cutaneous adverse effects. It is believed that vemurafenib has the ability to paradoxically activate the mitogen-activated protein kinase pathway, leading to keratinocyte proliferation in cells without BRAF mutations.6-8 In the phase 3 trial, approximately 23% to 30% of patients developed some form of hyperkeratosis.5 Comparatively, 64% of patients developed a rash and 23% developed cutaneous squamous cell carcinoma. Incidence of palmoplantar hyperkeratosis was similar in the vemurafenib and dabrafenib groups (6% vs 8%).3,9 Development of keratoderma also has been associated with other multikinase inhibitors (eg, sorafenib, sunitinib).10,11
In our case, the patient displayed multiple side effects while undergoing vemurafenib therapy. Within the first 2 weeks of therapy, he experienced a drug eruption that affected approximately 70% of the body surface area. The eruption resolved with topical steroids and oral antihistamines. The patient also noted the appearance of several new melanocytic nevi on the posterior neck as well as several evolving nevi on the back and abdomen. Five months into the treatment cycle, the patient began to develop hyperkeratosis on the bilateral plantar feet. Treatment consisted of keratolytics. Vemurafenib therapy was not discontinued secondary to any adverse effects.
Vemurafenib and other BRAF inhibitors are efficacious in the treatment of metastatic melanoma with V600E mutations. The use of these therapies is likely to continue and increase in the future. BRAF inhibitors have been associated with a variety of side effects, including palmoplantar hyperkeratosis. Awareness of and appropriate response to adverse reactions is essential to proper patient care and continuation of potentially life-extending therapies.
- Davies H, Bignell GR, Cox C, et al. Mutations in the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Cohen PR, Bedikian AY, Kim KB. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Rinderknecht JD, Goldinger SM, Rozati S, et al. RASopathic skin eruptions during vemurafenib therapy [published online March 13, 2014]. PLoS One. 2013;8:e58721.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18:314-322.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Su F, Bradley WD, Wang Q, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969-978.
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
- Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886-892.
- Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges. 2010;8:652-661.
To the Editor:
Vemurafenib, a selective BRAF inhibitor, is a chemotherapeutic agent used in the treatment of metastatic melanoma with BRAF mutations. It has been associated with various cutaneous side effects. We report a case of metastatic melanoma with acquired plantar hyperkeratosis secondary to vemurafenib therapy.
A 49-year-old man presented for evaluation of a pigmented plaque on the left pretibial region that had been enlarging over the last 2 months. The lesion had been diagnosed as folliculitis by his primary care physician 1 month prior to the current presentation and was being treated with oral antibiotics. The patient reported occasional bleeding from the lesion but denied other symptoms. Physical examination revealed a 1.4-cm pigmented plaque distributed over the left shin. Excisional biopsy was performed to rule out melanoma. Histopathology revealed well-circumscribed and symmetric proliferation of nested and single atypical melanocytes throughout all layers to the deep reticular dermis, confirming a clinical diagnosis of malignant melanoma. The lesion demonstrated angiolymphatic invasion, mitotic activity, and a Breslow depth of 2.5 mm. The patient underwent wide local excision with 3-cm margins and left inguinal sentinel lymph node biopsy; 2 of 14 lymph nodes were positive for melanoma. Positron emission tomography–computed tomography was negative for further metastatic disease. The patient underwent isolated limb perfusion with ipilimumab, but treatment was discontinued due to regional progression of multiple cutaneous metastases that were positive for the BRAF V600E mutation.
The patient was then started on vemurafenib therapy. Within 2 weeks, the patient reported various cutaneous symptoms, including morbilliform drug eruption covering approximately 70% of the body surface area that resolved with topical steroids and oral antihistamines, as well as the appearance of melanocytic nevi on the posterior neck, back, and abdomen. After 5 months of vemurafenib therapy, the patient began to develop hyperkeratosis of the bilateral soles of the feet (Figure). A diagnosis of acquired plantar hyperkeratosis secondary to vemurafenib therapy was made. Treatment with keratolytics was initiated and vemurafenib was not discontinued. The patient died approximately 1 year after therapy was started.
Metastatic melanoma is challenging to treat and continues to have a high mortality rate; however, newer chemotherapeutic agents targeting specific mutations found in melanoma, including the BRAF V600E mutation, are promising.

The US Food and Drug Administration first approved vemurafenib, a selective BRAF inhibitor, in 2011 for treatment of metastatic melanoma. Activating BRAF mutations have been detected in up to 60% of cutaneous melanomas.1 In the majority of these mutations, valine (V) is inserted at codon 600 instead of glutamic acid (E); therefore, the mutation is named V600E.2 In a phase 3 trial of 675 metastatic melanoma patients with positive V600E who were randomized to receive either vemurafenib or dacarbazine, the overall survival rate in the vemurafenib group improved by 84% versus 64% in the dacarbazine group at 6 months.3
Vemurafenib and other BRAF inhibitors have been associated with multiple cutaneous side effects, including rash, alopecia, squamous cell carcinoma, photosensitivity, evolution of existing nevi, and less commonly palmoplantar hyperkeratosis.2-5 Constitutional symptoms including arthralgia, nausea, and fatigue also have been commonly reported.2-5 In several large studies comprising 1138 patients, cutaneous side effects were seen in 92% to 95% of patients.3,5 Adverse effects caused interruption or modification of therapy in 38% of patients.3
Palmoplantar keratoderma is a known side effect of vemurafenib therapy, but it is less commonly reported than other cutaneous adverse effects. It is believed that vemurafenib has the ability to paradoxically activate the mitogen-activated protein kinase pathway, leading to keratinocyte proliferation in cells without BRAF mutations.6-8 In the phase 3 trial, approximately 23% to 30% of patients developed some form of hyperkeratosis.5 Comparatively, 64% of patients developed a rash and 23% developed cutaneous squamous cell carcinoma. Incidence of palmoplantar hyperkeratosis was similar in the vemurafenib and dabrafenib groups (6% vs 8%).3,9 Development of keratoderma also has been associated with other multikinase inhibitors (eg, sorafenib, sunitinib).10,11
In our case, the patient displayed multiple side effects while undergoing vemurafenib therapy. Within the first 2 weeks of therapy, he experienced a drug eruption that affected approximately 70% of the body surface area. The eruption resolved with topical steroids and oral antihistamines. The patient also noted the appearance of several new melanocytic nevi on the posterior neck as well as several evolving nevi on the back and abdomen. Five months into the treatment cycle, the patient began to develop hyperkeratosis on the bilateral plantar feet. Treatment consisted of keratolytics. Vemurafenib therapy was not discontinued secondary to any adverse effects.
Vemurafenib and other BRAF inhibitors are efficacious in the treatment of metastatic melanoma with V600E mutations. The use of these therapies is likely to continue and increase in the future. BRAF inhibitors have been associated with a variety of side effects, including palmoplantar hyperkeratosis. Awareness of and appropriate response to adverse reactions is essential to proper patient care and continuation of potentially life-extending therapies.
To the Editor:
Vemurafenib, a selective BRAF inhibitor, is a chemotherapeutic agent used in the treatment of metastatic melanoma with BRAF mutations. It has been associated with various cutaneous side effects. We report a case of metastatic melanoma with acquired plantar hyperkeratosis secondary to vemurafenib therapy.
A 49-year-old man presented for evaluation of a pigmented plaque on the left pretibial region that had been enlarging over the last 2 months. The lesion had been diagnosed as folliculitis by his primary care physician 1 month prior to the current presentation and was being treated with oral antibiotics. The patient reported occasional bleeding from the lesion but denied other symptoms. Physical examination revealed a 1.4-cm pigmented plaque distributed over the left shin. Excisional biopsy was performed to rule out melanoma. Histopathology revealed well-circumscribed and symmetric proliferation of nested and single atypical melanocytes throughout all layers to the deep reticular dermis, confirming a clinical diagnosis of malignant melanoma. The lesion demonstrated angiolymphatic invasion, mitotic activity, and a Breslow depth of 2.5 mm. The patient underwent wide local excision with 3-cm margins and left inguinal sentinel lymph node biopsy; 2 of 14 lymph nodes were positive for melanoma. Positron emission tomography–computed tomography was negative for further metastatic disease. The patient underwent isolated limb perfusion with ipilimumab, but treatment was discontinued due to regional progression of multiple cutaneous metastases that were positive for the BRAF V600E mutation.
The patient was then started on vemurafenib therapy. Within 2 weeks, the patient reported various cutaneous symptoms, including morbilliform drug eruption covering approximately 70% of the body surface area that resolved with topical steroids and oral antihistamines, as well as the appearance of melanocytic nevi on the posterior neck, back, and abdomen. After 5 months of vemurafenib therapy, the patient began to develop hyperkeratosis of the bilateral soles of the feet (Figure). A diagnosis of acquired plantar hyperkeratosis secondary to vemurafenib therapy was made. Treatment with keratolytics was initiated and vemurafenib was not discontinued. The patient died approximately 1 year after therapy was started.
Metastatic melanoma is challenging to treat and continues to have a high mortality rate; however, newer chemotherapeutic agents targeting specific mutations found in melanoma, including the BRAF V600E mutation, are promising.

The US Food and Drug Administration first approved vemurafenib, a selective BRAF inhibitor, in 2011 for treatment of metastatic melanoma. Activating BRAF mutations have been detected in up to 60% of cutaneous melanomas.1 In the majority of these mutations, valine (V) is inserted at codon 600 instead of glutamic acid (E); therefore, the mutation is named V600E.2 In a phase 3 trial of 675 metastatic melanoma patients with positive V600E who were randomized to receive either vemurafenib or dacarbazine, the overall survival rate in the vemurafenib group improved by 84% versus 64% in the dacarbazine group at 6 months.3
Vemurafenib and other BRAF inhibitors have been associated with multiple cutaneous side effects, including rash, alopecia, squamous cell carcinoma, photosensitivity, evolution of existing nevi, and less commonly palmoplantar hyperkeratosis.2-5 Constitutional symptoms including arthralgia, nausea, and fatigue also have been commonly reported.2-5 In several large studies comprising 1138 patients, cutaneous side effects were seen in 92% to 95% of patients.3,5 Adverse effects caused interruption or modification of therapy in 38% of patients.3
Palmoplantar keratoderma is a known side effect of vemurafenib therapy, but it is less commonly reported than other cutaneous adverse effects. It is believed that vemurafenib has the ability to paradoxically activate the mitogen-activated protein kinase pathway, leading to keratinocyte proliferation in cells without BRAF mutations.6-8 In the phase 3 trial, approximately 23% to 30% of patients developed some form of hyperkeratosis.5 Comparatively, 64% of patients developed a rash and 23% developed cutaneous squamous cell carcinoma. Incidence of palmoplantar hyperkeratosis was similar in the vemurafenib and dabrafenib groups (6% vs 8%).3,9 Development of keratoderma also has been associated with other multikinase inhibitors (eg, sorafenib, sunitinib).10,11
In our case, the patient displayed multiple side effects while undergoing vemurafenib therapy. Within the first 2 weeks of therapy, he experienced a drug eruption that affected approximately 70% of the body surface area. The eruption resolved with topical steroids and oral antihistamines. The patient also noted the appearance of several new melanocytic nevi on the posterior neck as well as several evolving nevi on the back and abdomen. Five months into the treatment cycle, the patient began to develop hyperkeratosis on the bilateral plantar feet. Treatment consisted of keratolytics. Vemurafenib therapy was not discontinued secondary to any adverse effects.
Vemurafenib and other BRAF inhibitors are efficacious in the treatment of metastatic melanoma with V600E mutations. The use of these therapies is likely to continue and increase in the future. BRAF inhibitors have been associated with a variety of side effects, including palmoplantar hyperkeratosis. Awareness of and appropriate response to adverse reactions is essential to proper patient care and continuation of potentially life-extending therapies.
- Davies H, Bignell GR, Cox C, et al. Mutations in the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Cohen PR, Bedikian AY, Kim KB. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Rinderknecht JD, Goldinger SM, Rozati S, et al. RASopathic skin eruptions during vemurafenib therapy [published online March 13, 2014]. PLoS One. 2013;8:e58721.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18:314-322.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Su F, Bradley WD, Wang Q, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969-978.
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
- Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886-892.
- Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges. 2010;8:652-661.
- Davies H, Bignell GR, Cox C, et al. Mutations in the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Cohen PR, Bedikian AY, Kim KB. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Rinderknecht JD, Goldinger SM, Rozati S, et al. RASopathic skin eruptions during vemurafenib therapy [published online March 13, 2014]. PLoS One. 2013;8:e58721.
- Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18:314-322.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Su F, Bradley WD, Wang Q, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969-978.
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
- Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886-892.
- Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges. 2010;8:652-661.
Practice Points
- BRAF inhibitors such as vemurafenib are associated with a high incidence of cutaneous side effects, including rash, hyperkeratosis, and cutaneous squamous cell carcinoma.
- Practitioners should be aware of these side effects and their management to avoid discontinuation or interruption of therapy.
Uveal melanoma: NCCN provides first pathway-based guidelines
ORLANDO – Uveal melanoma has little in common with it’s cutaneous namesake, and its distinct characteristics necessitated the development of specific guidelines for diagnosis and management, which were released earlier this year by the National Comprehensive Cancer Network (NCCN).
Unlike cutaneous melanoma, uveal melanoma is usually treated with radiotherapy rather than surgery, and primary treatment is based on tumor size, according to Christopher A. Barker, MD, a radiation oncologist and director of clinical investigations in the department of radiation at Memorial Sloan Kettering Cancer Center, New York.
Further, molecular testing aids in prognosis in uveal melanoma but not in predicting treatment response as it can in cutaneous disease, and recurrences of uveal melanoma are typically distant – usually occurring in the liver – rather than in the skin or lymph nodes as with cutaneous melanoma.
These and other diagnosis- and treatment-related issues are outlined in the new guidelines, which are the first developed by the NCCN for uveal melanoma.
Guidelines exist in several countries, including Australia, the United Kingdom, Canada, and the United States (published by The American Brachytherapy Society), but until now none have provided pathway-based strategies for the management of all stages of this rare disease that affects about 1 in 200,000 Americans, typically Caucasians in their 50s, 60s, or 70s, Dr. Barker, a member of the NCCN Melanoma guidelines panel and the uveal melanoma subcommittee, said at the NCCN’s annual meeting where he presented the guidelines.
The median age of diagnosis is 60 years, he noted.
The NCCN guidelines specifically address melanoma arising in the choroid and ciliary body of the uvea. The choroid is the predominant site of uveal melanoma origin, and tumors arising there may involve the ciliary body as well, although the latter is a rare site of melanoma origin. The iris is also a rare site of origin, and tumors arising there are typically indolent in nature and thus are not part of the new guidelines, he explained.
Risk factors include choroidal nevi, ocular melanocytosis, and familial uveal melanoma associated with germline BAP1 mutation, neurofibromatosis, or dysplastic nevus syndrome; cutaneous melanoma is not a risk factor, he said.
The guidelines address clinical presentation, diagnostic work-up, and staging; primary treatments; and metastatic risks and follow-up imaging.
Presentation, diagnosis, and staging
About two-thirds of patients with uveal melanoma present because of changes in their vision, and about a third present with no new symptoms and are diagnosed during routine evaluation, Dr. Barker said.
“History and physical exam, and specifically attention to any prior malignancies, is important,” he said. “A comprehensive eye examination is absolutely vital to the evaluation and staging of patients with uveal melanoma.”
Numerous additional testing options, including autofluorescence of the ocular fundus, retinal fluorescein angiography of the ocular fundus, and transillumination, among others, are listed in the guidelines, which note that MRI is sometimes needed to confirm diagnosis.
Biopsies, however, are generally only performed to confirm diagnosis if needed or for prognostic analysis for risk stratification.
Staging is determined mainly by tumor size, which is known to be associated with outcomes in patients with uveal melanoma, and is based on criteria from both the Collaborative Ocular Melanoma Study (COMS) staging system and the American Joint Committee on Cancer (AJCC) staging manual, Dr. Barker said.
The COMS system was developed based on separate studies of small, medium, and large tumors and helped define primary tumor management and establish existing standards of care. The AJCC system was developed subsequently and focuses more on tumor features that may improve clinical predictions.
Primary treatments
Options for primary treatment for small tumors (largest diameter 5-16 mm and thickness less than 2.5 mm) include plaque brachytherapy, partical beam radiation, and laser ablation in highly select patients. For medium tumors (18mm or less at largest diameter and thickness of 2.5-10 mm), they include plaque brachytherapy, particle beam radiation, and enucleation, according to the guidelines.
Large tumors can be treated with radiotherapy (preferably particle beam radiation, Dr. Barker said) or enucleation. Large tumors are those greater than 18mm with any thickness, those with thickness greater than 10mm with any diameter, and those with thickness greater than 8mm with optic nerve involvement.
In patients for whom surgical removal is selected, “ there are a few unusual situations where additional local adjuvant therapy might be considered,” Dr. Barker said, explaining, for example, that the presence of microscopically positive or close margins after enucleation without evidence of gross residual disease in the orbit may be observed or may warrant map biopsy and/or particle beam or photon beam radiotherapy to the orbit.
For visible extraocular tumors or suspicion of gross disease in the orbit, biopsy of the extraocular tissue is recommended when possible, along with either intraoperative cryotherapy, orbital exenteration, or particle beam or photon beam radiotherapy to the orbit, he added.
Metastatic risks and follow-up imaging
Recent studies, including Cancer Genome Atlas Research Network studies, have elucidated the genomics of both uveal and cutaneous melanomas, and they show “a completely different mutation spectrum” for uveal melanoma, Dr. Barker said.
These genomic studies demonstrate that cutaneous melanoma involves mutations that predict response to certain treatments, while those found in uveal melanoma do not, but they are, however, associated with the probability of metastasis, he said.
For example, various studies have shown that BRAF mutation in cutaneous melanoma predicts response to RAF or MEK inhibition and that the overall number of mutations in cutaneous melanoma may predict response to immunotherapy.
In uveal melanoma, alterations in ElFAX1, SF3B1, or BAP1 have been shown to indicate low, intermediate, and high risk of metastasis, respectively.
Other factors associated with the risk of metastasis after primary tumor treatment in uveal melanoma include clinical factors (tumor size, ciliary body involvement, and extraocular extension); histopathologic factors (spindle or epithelioid cell type); and cytogenetics (status of chromosomes 3, 6, and 8), he said.
An AJCC Ophthalmic Oncology Task Force study published in 2015, for example, showed that the 5-year metastasis-free survival was 97% for AJCC stage T1 disease, 85% for stage T2, 77% for stage T3, and 61% for stage T4. T-stage modifiers, which are based on particular tumor characteristics, such as ciliary body involvement (CBI) and extraocular extension (EXE), were also associated with the risk of distant metastasis: 5-year metastasis-free survival was 90%, 72%, 54% and 33% for AJCC T_a (no CBI or EXE), T_b (CBI only), T_c (EXE only), and T_d tumors (CBI and EXE), respectively.
Follow-up imaging after primary tumor treatment should be based on the most likely site of metastatic recurrence, which is the liver in 90% of uveal melanoma metastases.
“For this reason, surveillance of high-risk patients with uveal melanoma should include specific imaging of the liver,” Dr. Barker said, who noted that the NCCN risk stratification–based surveillance guidelines categorize patients as either low, medium, or high risk for metastasis based on the various characteristics that can affect risk.
“These risk groups help clinicians identify how often imaging should be performed in their surveillance strategy,” he said, adding that those who are high risk based on BAP1 mutation, PRAME mutation, CBI, or EXE, for example, should undergo imaging to evaluate signs or symptoms every 3-6 months for 5 years, every 6-12 months for 10 years, and then as clinically indicated thereafter.
The guideline calls for less stringent imaging for low- and medium-risk patients.
“Now, what happens when distant metastases are identified? Unfortunately there is no single systemic therapy that has proven to be most effective for uveal melanoma,” he said. “For this reason, the NCCN guideline encourages clinical trial participation whenever possible for patients who develop distant metastasis.”
This is because drugs effective for cutaneous melanoma are far less effective for uveal melanoma, but do elicit response in some patients and can be considered, he explained.
“Moreover, because the liver is the site of metastasis most often, and is often the exclusive site of metastasis, liver metastasis–directed therapy is considered part of the management of patients with uveal melanoma,” he said, adding that this can involve resection, ablation, chemo/radio embolization, or regional perfusion.”
The NCCN Uveal Melanoma Guidelines were developed by a panel of experts from various institutions based on the available evidence and on consensus; they are category 2A (based on lower-level evidence with uniform NCCN consensus that the intervention is appropriate).
Dr. Barker reported receiving clinical research support from Amgen, Bristol-Myers Squibb, Elekta, Merck, University of California San Francisco, and University of Florida Health Cancer Center Orlando, and serving as an advisor, consultant, or expert witness for Pfizer.
ORLANDO – Uveal melanoma has little in common with it’s cutaneous namesake, and its distinct characteristics necessitated the development of specific guidelines for diagnosis and management, which were released earlier this year by the National Comprehensive Cancer Network (NCCN).
Unlike cutaneous melanoma, uveal melanoma is usually treated with radiotherapy rather than surgery, and primary treatment is based on tumor size, according to Christopher A. Barker, MD, a radiation oncologist and director of clinical investigations in the department of radiation at Memorial Sloan Kettering Cancer Center, New York.
Further, molecular testing aids in prognosis in uveal melanoma but not in predicting treatment response as it can in cutaneous disease, and recurrences of uveal melanoma are typically distant – usually occurring in the liver – rather than in the skin or lymph nodes as with cutaneous melanoma.
These and other diagnosis- and treatment-related issues are outlined in the new guidelines, which are the first developed by the NCCN for uveal melanoma.
Guidelines exist in several countries, including Australia, the United Kingdom, Canada, and the United States (published by The American Brachytherapy Society), but until now none have provided pathway-based strategies for the management of all stages of this rare disease that affects about 1 in 200,000 Americans, typically Caucasians in their 50s, 60s, or 70s, Dr. Barker, a member of the NCCN Melanoma guidelines panel and the uveal melanoma subcommittee, said at the NCCN’s annual meeting where he presented the guidelines.
The median age of diagnosis is 60 years, he noted.
The NCCN guidelines specifically address melanoma arising in the choroid and ciliary body of the uvea. The choroid is the predominant site of uveal melanoma origin, and tumors arising there may involve the ciliary body as well, although the latter is a rare site of melanoma origin. The iris is also a rare site of origin, and tumors arising there are typically indolent in nature and thus are not part of the new guidelines, he explained.
Risk factors include choroidal nevi, ocular melanocytosis, and familial uveal melanoma associated with germline BAP1 mutation, neurofibromatosis, or dysplastic nevus syndrome; cutaneous melanoma is not a risk factor, he said.
The guidelines address clinical presentation, diagnostic work-up, and staging; primary treatments; and metastatic risks and follow-up imaging.
Presentation, diagnosis, and staging
About two-thirds of patients with uveal melanoma present because of changes in their vision, and about a third present with no new symptoms and are diagnosed during routine evaluation, Dr. Barker said.
“History and physical exam, and specifically attention to any prior malignancies, is important,” he said. “A comprehensive eye examination is absolutely vital to the evaluation and staging of patients with uveal melanoma.”
Numerous additional testing options, including autofluorescence of the ocular fundus, retinal fluorescein angiography of the ocular fundus, and transillumination, among others, are listed in the guidelines, which note that MRI is sometimes needed to confirm diagnosis.
Biopsies, however, are generally only performed to confirm diagnosis if needed or for prognostic analysis for risk stratification.
Staging is determined mainly by tumor size, which is known to be associated with outcomes in patients with uveal melanoma, and is based on criteria from both the Collaborative Ocular Melanoma Study (COMS) staging system and the American Joint Committee on Cancer (AJCC) staging manual, Dr. Barker said.
The COMS system was developed based on separate studies of small, medium, and large tumors and helped define primary tumor management and establish existing standards of care. The AJCC system was developed subsequently and focuses more on tumor features that may improve clinical predictions.
Primary treatments
Options for primary treatment for small tumors (largest diameter 5-16 mm and thickness less than 2.5 mm) include plaque brachytherapy, partical beam radiation, and laser ablation in highly select patients. For medium tumors (18mm or less at largest diameter and thickness of 2.5-10 mm), they include plaque brachytherapy, particle beam radiation, and enucleation, according to the guidelines.
Large tumors can be treated with radiotherapy (preferably particle beam radiation, Dr. Barker said) or enucleation. Large tumors are those greater than 18mm with any thickness, those with thickness greater than 10mm with any diameter, and those with thickness greater than 8mm with optic nerve involvement.
In patients for whom surgical removal is selected, “ there are a few unusual situations where additional local adjuvant therapy might be considered,” Dr. Barker said, explaining, for example, that the presence of microscopically positive or close margins after enucleation without evidence of gross residual disease in the orbit may be observed or may warrant map biopsy and/or particle beam or photon beam radiotherapy to the orbit.
For visible extraocular tumors or suspicion of gross disease in the orbit, biopsy of the extraocular tissue is recommended when possible, along with either intraoperative cryotherapy, orbital exenteration, or particle beam or photon beam radiotherapy to the orbit, he added.
Metastatic risks and follow-up imaging
Recent studies, including Cancer Genome Atlas Research Network studies, have elucidated the genomics of both uveal and cutaneous melanomas, and they show “a completely different mutation spectrum” for uveal melanoma, Dr. Barker said.
These genomic studies demonstrate that cutaneous melanoma involves mutations that predict response to certain treatments, while those found in uveal melanoma do not, but they are, however, associated with the probability of metastasis, he said.
For example, various studies have shown that BRAF mutation in cutaneous melanoma predicts response to RAF or MEK inhibition and that the overall number of mutations in cutaneous melanoma may predict response to immunotherapy.
In uveal melanoma, alterations in ElFAX1, SF3B1, or BAP1 have been shown to indicate low, intermediate, and high risk of metastasis, respectively.
Other factors associated with the risk of metastasis after primary tumor treatment in uveal melanoma include clinical factors (tumor size, ciliary body involvement, and extraocular extension); histopathologic factors (spindle or epithelioid cell type); and cytogenetics (status of chromosomes 3, 6, and 8), he said.
An AJCC Ophthalmic Oncology Task Force study published in 2015, for example, showed that the 5-year metastasis-free survival was 97% for AJCC stage T1 disease, 85% for stage T2, 77% for stage T3, and 61% for stage T4. T-stage modifiers, which are based on particular tumor characteristics, such as ciliary body involvement (CBI) and extraocular extension (EXE), were also associated with the risk of distant metastasis: 5-year metastasis-free survival was 90%, 72%, 54% and 33% for AJCC T_a (no CBI or EXE), T_b (CBI only), T_c (EXE only), and T_d tumors (CBI and EXE), respectively.
Follow-up imaging after primary tumor treatment should be based on the most likely site of metastatic recurrence, which is the liver in 90% of uveal melanoma metastases.
“For this reason, surveillance of high-risk patients with uveal melanoma should include specific imaging of the liver,” Dr. Barker said, who noted that the NCCN risk stratification–based surveillance guidelines categorize patients as either low, medium, or high risk for metastasis based on the various characteristics that can affect risk.
“These risk groups help clinicians identify how often imaging should be performed in their surveillance strategy,” he said, adding that those who are high risk based on BAP1 mutation, PRAME mutation, CBI, or EXE, for example, should undergo imaging to evaluate signs or symptoms every 3-6 months for 5 years, every 6-12 months for 10 years, and then as clinically indicated thereafter.
The guideline calls for less stringent imaging for low- and medium-risk patients.
“Now, what happens when distant metastases are identified? Unfortunately there is no single systemic therapy that has proven to be most effective for uveal melanoma,” he said. “For this reason, the NCCN guideline encourages clinical trial participation whenever possible for patients who develop distant metastasis.”
This is because drugs effective for cutaneous melanoma are far less effective for uveal melanoma, but do elicit response in some patients and can be considered, he explained.
“Moreover, because the liver is the site of metastasis most often, and is often the exclusive site of metastasis, liver metastasis–directed therapy is considered part of the management of patients with uveal melanoma,” he said, adding that this can involve resection, ablation, chemo/radio embolization, or regional perfusion.”
The NCCN Uveal Melanoma Guidelines were developed by a panel of experts from various institutions based on the available evidence and on consensus; they are category 2A (based on lower-level evidence with uniform NCCN consensus that the intervention is appropriate).
Dr. Barker reported receiving clinical research support from Amgen, Bristol-Myers Squibb, Elekta, Merck, University of California San Francisco, and University of Florida Health Cancer Center Orlando, and serving as an advisor, consultant, or expert witness for Pfizer.
ORLANDO – Uveal melanoma has little in common with it’s cutaneous namesake, and its distinct characteristics necessitated the development of specific guidelines for diagnosis and management, which were released earlier this year by the National Comprehensive Cancer Network (NCCN).
Unlike cutaneous melanoma, uveal melanoma is usually treated with radiotherapy rather than surgery, and primary treatment is based on tumor size, according to Christopher A. Barker, MD, a radiation oncologist and director of clinical investigations in the department of radiation at Memorial Sloan Kettering Cancer Center, New York.
Further, molecular testing aids in prognosis in uveal melanoma but not in predicting treatment response as it can in cutaneous disease, and recurrences of uveal melanoma are typically distant – usually occurring in the liver – rather than in the skin or lymph nodes as with cutaneous melanoma.
These and other diagnosis- and treatment-related issues are outlined in the new guidelines, which are the first developed by the NCCN for uveal melanoma.
Guidelines exist in several countries, including Australia, the United Kingdom, Canada, and the United States (published by The American Brachytherapy Society), but until now none have provided pathway-based strategies for the management of all stages of this rare disease that affects about 1 in 200,000 Americans, typically Caucasians in their 50s, 60s, or 70s, Dr. Barker, a member of the NCCN Melanoma guidelines panel and the uveal melanoma subcommittee, said at the NCCN’s annual meeting where he presented the guidelines.
The median age of diagnosis is 60 years, he noted.
The NCCN guidelines specifically address melanoma arising in the choroid and ciliary body of the uvea. The choroid is the predominant site of uveal melanoma origin, and tumors arising there may involve the ciliary body as well, although the latter is a rare site of melanoma origin. The iris is also a rare site of origin, and tumors arising there are typically indolent in nature and thus are not part of the new guidelines, he explained.
Risk factors include choroidal nevi, ocular melanocytosis, and familial uveal melanoma associated with germline BAP1 mutation, neurofibromatosis, or dysplastic nevus syndrome; cutaneous melanoma is not a risk factor, he said.
The guidelines address clinical presentation, diagnostic work-up, and staging; primary treatments; and metastatic risks and follow-up imaging.
Presentation, diagnosis, and staging
About two-thirds of patients with uveal melanoma present because of changes in their vision, and about a third present with no new symptoms and are diagnosed during routine evaluation, Dr. Barker said.
“History and physical exam, and specifically attention to any prior malignancies, is important,” he said. “A comprehensive eye examination is absolutely vital to the evaluation and staging of patients with uveal melanoma.”
Numerous additional testing options, including autofluorescence of the ocular fundus, retinal fluorescein angiography of the ocular fundus, and transillumination, among others, are listed in the guidelines, which note that MRI is sometimes needed to confirm diagnosis.
Biopsies, however, are generally only performed to confirm diagnosis if needed or for prognostic analysis for risk stratification.
Staging is determined mainly by tumor size, which is known to be associated with outcomes in patients with uveal melanoma, and is based on criteria from both the Collaborative Ocular Melanoma Study (COMS) staging system and the American Joint Committee on Cancer (AJCC) staging manual, Dr. Barker said.
The COMS system was developed based on separate studies of small, medium, and large tumors and helped define primary tumor management and establish existing standards of care. The AJCC system was developed subsequently and focuses more on tumor features that may improve clinical predictions.
Primary treatments
Options for primary treatment for small tumors (largest diameter 5-16 mm and thickness less than 2.5 mm) include plaque brachytherapy, partical beam radiation, and laser ablation in highly select patients. For medium tumors (18mm or less at largest diameter and thickness of 2.5-10 mm), they include plaque brachytherapy, particle beam radiation, and enucleation, according to the guidelines.
Large tumors can be treated with radiotherapy (preferably particle beam radiation, Dr. Barker said) or enucleation. Large tumors are those greater than 18mm with any thickness, those with thickness greater than 10mm with any diameter, and those with thickness greater than 8mm with optic nerve involvement.
In patients for whom surgical removal is selected, “ there are a few unusual situations where additional local adjuvant therapy might be considered,” Dr. Barker said, explaining, for example, that the presence of microscopically positive or close margins after enucleation without evidence of gross residual disease in the orbit may be observed or may warrant map biopsy and/or particle beam or photon beam radiotherapy to the orbit.
For visible extraocular tumors or suspicion of gross disease in the orbit, biopsy of the extraocular tissue is recommended when possible, along with either intraoperative cryotherapy, orbital exenteration, or particle beam or photon beam radiotherapy to the orbit, he added.
Metastatic risks and follow-up imaging
Recent studies, including Cancer Genome Atlas Research Network studies, have elucidated the genomics of both uveal and cutaneous melanomas, and they show “a completely different mutation spectrum” for uveal melanoma, Dr. Barker said.
These genomic studies demonstrate that cutaneous melanoma involves mutations that predict response to certain treatments, while those found in uveal melanoma do not, but they are, however, associated with the probability of metastasis, he said.
For example, various studies have shown that BRAF mutation in cutaneous melanoma predicts response to RAF or MEK inhibition and that the overall number of mutations in cutaneous melanoma may predict response to immunotherapy.
In uveal melanoma, alterations in ElFAX1, SF3B1, or BAP1 have been shown to indicate low, intermediate, and high risk of metastasis, respectively.
Other factors associated with the risk of metastasis after primary tumor treatment in uveal melanoma include clinical factors (tumor size, ciliary body involvement, and extraocular extension); histopathologic factors (spindle or epithelioid cell type); and cytogenetics (status of chromosomes 3, 6, and 8), he said.
An AJCC Ophthalmic Oncology Task Force study published in 2015, for example, showed that the 5-year metastasis-free survival was 97% for AJCC stage T1 disease, 85% for stage T2, 77% for stage T3, and 61% for stage T4. T-stage modifiers, which are based on particular tumor characteristics, such as ciliary body involvement (CBI) and extraocular extension (EXE), were also associated with the risk of distant metastasis: 5-year metastasis-free survival was 90%, 72%, 54% and 33% for AJCC T_a (no CBI or EXE), T_b (CBI only), T_c (EXE only), and T_d tumors (CBI and EXE), respectively.
Follow-up imaging after primary tumor treatment should be based on the most likely site of metastatic recurrence, which is the liver in 90% of uveal melanoma metastases.
“For this reason, surveillance of high-risk patients with uveal melanoma should include specific imaging of the liver,” Dr. Barker said, who noted that the NCCN risk stratification–based surveillance guidelines categorize patients as either low, medium, or high risk for metastasis based on the various characteristics that can affect risk.
“These risk groups help clinicians identify how often imaging should be performed in their surveillance strategy,” he said, adding that those who are high risk based on BAP1 mutation, PRAME mutation, CBI, or EXE, for example, should undergo imaging to evaluate signs or symptoms every 3-6 months for 5 years, every 6-12 months for 10 years, and then as clinically indicated thereafter.
The guideline calls for less stringent imaging for low- and medium-risk patients.
“Now, what happens when distant metastases are identified? Unfortunately there is no single systemic therapy that has proven to be most effective for uveal melanoma,” he said. “For this reason, the NCCN guideline encourages clinical trial participation whenever possible for patients who develop distant metastasis.”
This is because drugs effective for cutaneous melanoma are far less effective for uveal melanoma, but do elicit response in some patients and can be considered, he explained.
“Moreover, because the liver is the site of metastasis most often, and is often the exclusive site of metastasis, liver metastasis–directed therapy is considered part of the management of patients with uveal melanoma,” he said, adding that this can involve resection, ablation, chemo/radio embolization, or regional perfusion.”
The NCCN Uveal Melanoma Guidelines were developed by a panel of experts from various institutions based on the available evidence and on consensus; they are category 2A (based on lower-level evidence with uniform NCCN consensus that the intervention is appropriate).
Dr. Barker reported receiving clinical research support from Amgen, Bristol-Myers Squibb, Elekta, Merck, University of California San Francisco, and University of Florida Health Cancer Center Orlando, and serving as an advisor, consultant, or expert witness for Pfizer.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
First combo trial of mTOR/BRAF inhibition shows potential
A combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus and the BRAF inhibitor vemurafenib appears safe for patients with advanced, BRAF-mutated, solid tumors that had progressed on BRAF and/or MEK therapy, investigators reported.
The combination provided partial responses in a variety of tumor types, and half of the patients achieved stable disease, reported Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center in Houston and his coauthors.
“Activation of alternative parallel signaling pathways such as the PI3K–mTOR pathway have been hypothesized to contribute to primary and acquired resistance to BRAF-targeted therapy,” the authors wrote in JCO Precision Oncology. Preclinical studies have supported this hypothesis, which led to the present study; it is the first to evaluate a combination of a BRAF inhibitor and an mTOR inhibitor.
The open-label, phase 1 trial included 20 patients with BRAF-mutated, advanced cancer that had progressed on BRAF and/or MEK therapy. Solid tumor types included melanoma (n = 7), glioma (n = 5), thyroid cancer (n = 4), appendiceal carcinoma (n = 1), colorectal cancer (n = 1), non–small cell lung cancer (NSCLC; n =1), and cancer of unknown primary (n = 1). More than half of the patients had already been treated with surgery, clinical trial therapy, radiation therapy, or chemotherapy. The median adult age was 64 years; two pediatric patients were aged 10 and 13 years.
Dose-escalation revealed a maximum-tolerated dose of everolimus 5 mg orally once a day and vemurafenib 720 mg orally twice a day. Across doses, the most common grade 3 adverse events were fatigue (20%) and rash (15%), followed distantly by anemia, thrombocytopenia, hyperglycemia, or hypertriglyceridemia, which occurred in one patient each.
Responses were evaluated in 18 patients. Of these, 22% had partial responses and 50% achieved stable disease. Partial responses occurred in patients with pleomorphic xanthoastrocytoma, optic nerve glioma, melanoma, and NSCLC.
“Our trial demonstrates that the combination of vemurafenib and everolimus can be tolerated in patients with advanced malignancies,” the authors concluded. “Our trial also demonstrates that the addition of an mTOR inhibitor to everolimus treatment is able to overcome resistance to BRAF and/or MEK inhibition in a subset of patients with BRAF-mutant advanced cancers.”
The authors reported affiliations with Baxter, Bayer, Novartis, Roche, Trovagene, and others.
SOURCE: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
A combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus and the BRAF inhibitor vemurafenib appears safe for patients with advanced, BRAF-mutated, solid tumors that had progressed on BRAF and/or MEK therapy, investigators reported.
The combination provided partial responses in a variety of tumor types, and half of the patients achieved stable disease, reported Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center in Houston and his coauthors.
“Activation of alternative parallel signaling pathways such as the PI3K–mTOR pathway have been hypothesized to contribute to primary and acquired resistance to BRAF-targeted therapy,” the authors wrote in JCO Precision Oncology. Preclinical studies have supported this hypothesis, which led to the present study; it is the first to evaluate a combination of a BRAF inhibitor and an mTOR inhibitor.
The open-label, phase 1 trial included 20 patients with BRAF-mutated, advanced cancer that had progressed on BRAF and/or MEK therapy. Solid tumor types included melanoma (n = 7), glioma (n = 5), thyroid cancer (n = 4), appendiceal carcinoma (n = 1), colorectal cancer (n = 1), non–small cell lung cancer (NSCLC; n =1), and cancer of unknown primary (n = 1). More than half of the patients had already been treated with surgery, clinical trial therapy, radiation therapy, or chemotherapy. The median adult age was 64 years; two pediatric patients were aged 10 and 13 years.
Dose-escalation revealed a maximum-tolerated dose of everolimus 5 mg orally once a day and vemurafenib 720 mg orally twice a day. Across doses, the most common grade 3 adverse events were fatigue (20%) and rash (15%), followed distantly by anemia, thrombocytopenia, hyperglycemia, or hypertriglyceridemia, which occurred in one patient each.
Responses were evaluated in 18 patients. Of these, 22% had partial responses and 50% achieved stable disease. Partial responses occurred in patients with pleomorphic xanthoastrocytoma, optic nerve glioma, melanoma, and NSCLC.
“Our trial demonstrates that the combination of vemurafenib and everolimus can be tolerated in patients with advanced malignancies,” the authors concluded. “Our trial also demonstrates that the addition of an mTOR inhibitor to everolimus treatment is able to overcome resistance to BRAF and/or MEK inhibition in a subset of patients with BRAF-mutant advanced cancers.”
The authors reported affiliations with Baxter, Bayer, Novartis, Roche, Trovagene, and others.
SOURCE: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
A combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus and the BRAF inhibitor vemurafenib appears safe for patients with advanced, BRAF-mutated, solid tumors that had progressed on BRAF and/or MEK therapy, investigators reported.
The combination provided partial responses in a variety of tumor types, and half of the patients achieved stable disease, reported Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center in Houston and his coauthors.
“Activation of alternative parallel signaling pathways such as the PI3K–mTOR pathway have been hypothesized to contribute to primary and acquired resistance to BRAF-targeted therapy,” the authors wrote in JCO Precision Oncology. Preclinical studies have supported this hypothesis, which led to the present study; it is the first to evaluate a combination of a BRAF inhibitor and an mTOR inhibitor.
The open-label, phase 1 trial included 20 patients with BRAF-mutated, advanced cancer that had progressed on BRAF and/or MEK therapy. Solid tumor types included melanoma (n = 7), glioma (n = 5), thyroid cancer (n = 4), appendiceal carcinoma (n = 1), colorectal cancer (n = 1), non–small cell lung cancer (NSCLC; n =1), and cancer of unknown primary (n = 1). More than half of the patients had already been treated with surgery, clinical trial therapy, radiation therapy, or chemotherapy. The median adult age was 64 years; two pediatric patients were aged 10 and 13 years.
Dose-escalation revealed a maximum-tolerated dose of everolimus 5 mg orally once a day and vemurafenib 720 mg orally twice a day. Across doses, the most common grade 3 adverse events were fatigue (20%) and rash (15%), followed distantly by anemia, thrombocytopenia, hyperglycemia, or hypertriglyceridemia, which occurred in one patient each.
Responses were evaluated in 18 patients. Of these, 22% had partial responses and 50% achieved stable disease. Partial responses occurred in patients with pleomorphic xanthoastrocytoma, optic nerve glioma, melanoma, and NSCLC.
“Our trial demonstrates that the combination of vemurafenib and everolimus can be tolerated in patients with advanced malignancies,” the authors concluded. “Our trial also demonstrates that the addition of an mTOR inhibitor to everolimus treatment is able to overcome resistance to BRAF and/or MEK inhibition in a subset of patients with BRAF-mutant advanced cancers.”
The authors reported affiliations with Baxter, Bayer, Novartis, Roche, Trovagene, and others.
SOURCE: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.
FROM JCO PRECISION ONCOLOGY
Key clinical point: A combination of the mTOR inhibitor everolimus and the BRAF inhibitor vemurafenib is safe and effective against some treatment-refractory, BRAF-mutated solid tumors.
Major finding: Twenty-two percent (22%) of patients had a partial response to therapy.
Study details: A phase I, dose-escalation trial involving 20 patients with advanced, BRAF-mutated cancer that had progressed on MEK and/or BRAF inhibitor therapy.
Disclosures: The authors reported affiliations with Bayer, Baxter, Novartis, Roche, Trovagene, and others.
Source: Subbiah V et al. JCO Prec Oncol. 2018 Sep 13. doi: 10.1200/PO.18.00189.