User login
TRK inhibitor shows ‘striking’ activity, durability across diverse adult and pediatric cancers
CHICAGO – Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, finds an analysis of three trials reported at the annual meeting of the American Society of Clinical Oncology.
Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, lead author David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York explained in a press briefing.
Dr. Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase I and II trials. Results showed an overall response rate of 76%, and the large majority of responses were still ongoing at 12 months.
“I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
On the basis of these promising data, the drug’s manufacturer, Loxo Oncology, plans to submit a New Drug Application to the Food and Drug Administration later this year or early next year. Larotrectinib has already been granted both orphan drug designation (for drugs used to treat rare conditions) and breakthrough therapy designation (for drugs used to treat serious conditions showing greater efficacy than available therapies).
A randomized trial pitting larotrectinib against other therapies is unlikely given the low prevalence of TRK fusions, the lack of treatment options for the fairly heavily pretreated trial patients, and the drug’s impressive performance, according to Dr. Hyman.
“The efficacy is so striking that it really exceeds almost any existing standard of care for solid tumors,” he elaborated. “There is hardly any chemotherapy or targeted therapy that has a response rate or durability that looks like larotrectinib in these patients.”
Expert perspective
The data for larotrectinib “really bring us into a new era where treatment is truly based on mutation, not location,” said Sumanta Kumar Pal, MD, a medical oncologist at City of Hope, in Duarte, Calif. “When I was in training, which was not too long ago, it really would have been a pipe dream to think that we could have treated cancers independent of their site of origin. … With the data presented by Dr. Hyman for larotrectinib, we may now be poised to treat many cancers in a manner that is agnostic of their site of origin and that is instead based on molecular criteria.
TRK testing
Several next-generation sequencing–based tests already available clinically can pick up TRK fusions, Dr. Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering includes fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions.
“The list price for these tests is in the kind of low thousands of dollars, which equates essentially to a PET scan for the cancer patient,” he noted. In cancers where sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr. Hyman stressed. “My personal opinion is that this [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment … [so that they] don’t become too sick, as we see in our own experience as well, and don’t have an opportunity to be treated even when the test results come back positive. So I would generally advocate early testing.”
Study details
For the study, which was funded by Loxo Oncology, the investigators analyzed data from three trials in which patients with advanced TRK fusion–positive solid cancers received larotrectinib (LOXO-101): a phase I trial among 8 adult patients, a phase I/II trial among 12 pediatric patients (SCOUT), and a phase II “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“I want to emphasize that these patients were identified by local testing,” Dr. Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So this in a sense really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is actually just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of beginning therapy,” Dr. Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. Fully 79% of responses were still ongoing at 12 months. Median progression-free survival was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is unclear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr. Hyman speculated. Six patients developed acquired resistance to larotrectinib; five of them were found to have an identical resistance mutation, and two went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that appears to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
Dr. Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
CHICAGO – Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, finds an analysis of three trials reported at the annual meeting of the American Society of Clinical Oncology.
Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, lead author David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York explained in a press briefing.
Dr. Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase I and II trials. Results showed an overall response rate of 76%, and the large majority of responses were still ongoing at 12 months.
“I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
On the basis of these promising data, the drug’s manufacturer, Loxo Oncology, plans to submit a New Drug Application to the Food and Drug Administration later this year or early next year. Larotrectinib has already been granted both orphan drug designation (for drugs used to treat rare conditions) and breakthrough therapy designation (for drugs used to treat serious conditions showing greater efficacy than available therapies).
A randomized trial pitting larotrectinib against other therapies is unlikely given the low prevalence of TRK fusions, the lack of treatment options for the fairly heavily pretreated trial patients, and the drug’s impressive performance, according to Dr. Hyman.
“The efficacy is so striking that it really exceeds almost any existing standard of care for solid tumors,” he elaborated. “There is hardly any chemotherapy or targeted therapy that has a response rate or durability that looks like larotrectinib in these patients.”
Expert perspective
The data for larotrectinib “really bring us into a new era where treatment is truly based on mutation, not location,” said Sumanta Kumar Pal, MD, a medical oncologist at City of Hope, in Duarte, Calif. “When I was in training, which was not too long ago, it really would have been a pipe dream to think that we could have treated cancers independent of their site of origin. … With the data presented by Dr. Hyman for larotrectinib, we may now be poised to treat many cancers in a manner that is agnostic of their site of origin and that is instead based on molecular criteria.
TRK testing
Several next-generation sequencing–based tests already available clinically can pick up TRK fusions, Dr. Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering includes fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions.
“The list price for these tests is in the kind of low thousands of dollars, which equates essentially to a PET scan for the cancer patient,” he noted. In cancers where sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr. Hyman stressed. “My personal opinion is that this [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment … [so that they] don’t become too sick, as we see in our own experience as well, and don’t have an opportunity to be treated even when the test results come back positive. So I would generally advocate early testing.”
Study details
For the study, which was funded by Loxo Oncology, the investigators analyzed data from three trials in which patients with advanced TRK fusion–positive solid cancers received larotrectinib (LOXO-101): a phase I trial among 8 adult patients, a phase I/II trial among 12 pediatric patients (SCOUT), and a phase II “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“I want to emphasize that these patients were identified by local testing,” Dr. Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So this in a sense really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is actually just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of beginning therapy,” Dr. Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. Fully 79% of responses were still ongoing at 12 months. Median progression-free survival was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is unclear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr. Hyman speculated. Six patients developed acquired resistance to larotrectinib; five of them were found to have an identical resistance mutation, and two went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that appears to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
Dr. Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
CHICAGO – Larotrectinib, an oral inhibitor of tropomyosin receptor kinase (TRK), has durable efficacy across diverse adult and pediatric cancers that harbor a genetic aberration known as TRK fusion, finds an analysis of three trials reported at the annual meeting of the American Society of Clinical Oncology.
Fusion of a TRK gene with an unrelated gene leads to uncontrolled signaling in the TRK pathway, potentially causing tumor growth and addiction to this input, lead author David Hyman, MD, chief of early drug development at Memorial Sloan Kettering Cancer Center in New York explained in a press briefing.
Dr. Hyman and his colleagues analyzed data from 55 patients having 17 discrete types of advanced cancer harboring TRK fusions who were treated with larotrectinib in phase I and II trials. Results showed an overall response rate of 76%, and the large majority of responses were still ongoing at 12 months.
“I believe these data support larotrectinib as a potential new standard of care for these patients,” he said. “However, I want to emphasize that really recognizing this benefit in the community will require that we test patients more universally for the presence of TRK fusions or other tumor-agnostic biomarkers, such as microsatellite instability.”
On the basis of these promising data, the drug’s manufacturer, Loxo Oncology, plans to submit a New Drug Application to the Food and Drug Administration later this year or early next year. Larotrectinib has already been granted both orphan drug designation (for drugs used to treat rare conditions) and breakthrough therapy designation (for drugs used to treat serious conditions showing greater efficacy than available therapies).
A randomized trial pitting larotrectinib against other therapies is unlikely given the low prevalence of TRK fusions, the lack of treatment options for the fairly heavily pretreated trial patients, and the drug’s impressive performance, according to Dr. Hyman.
“The efficacy is so striking that it really exceeds almost any existing standard of care for solid tumors,” he elaborated. “There is hardly any chemotherapy or targeted therapy that has a response rate or durability that looks like larotrectinib in these patients.”
Expert perspective
The data for larotrectinib “really bring us into a new era where treatment is truly based on mutation, not location,” said Sumanta Kumar Pal, MD, a medical oncologist at City of Hope, in Duarte, Calif. “When I was in training, which was not too long ago, it really would have been a pipe dream to think that we could have treated cancers independent of their site of origin. … With the data presented by Dr. Hyman for larotrectinib, we may now be poised to treat many cancers in a manner that is agnostic of their site of origin and that is instead based on molecular criteria.
TRK testing
Several next-generation sequencing–based tests already available clinically can pick up TRK fusions, Dr. Hyman pointed out. “But it is important for the ordering physician to understand whether the tests they are ordering includes fusion detection and, if it’s an option, to select it. Otherwise, they will not find TRK fusions.
“The list price for these tests is in the kind of low thousands of dollars, which equates essentially to a PET scan for the cancer patient,” he noted. In cancers where sequential single-gene testing is already being done as standard of care, there is “minimal” incremental cost of instead using comprehensive testing that would detect TRK fusions.
Oncologists should be aware that obtaining test results can take weeks, Dr. Hyman stressed. “My personal opinion is that this [testing] should be more broadly adopted and should be adopted at a point in the patient’s treatment … [so that they] don’t become too sick, as we see in our own experience as well, and don’t have an opportunity to be treated even when the test results come back positive. So I would generally advocate early testing.”
Study details
For the study, which was funded by Loxo Oncology, the investigators analyzed data from three trials in which patients with advanced TRK fusion–positive solid cancers received larotrectinib (LOXO-101): a phase I trial among 8 adult patients, a phase I/II trial among 12 pediatric patients (SCOUT), and a phase II “basket” trial among 35 adult and adolescent patients (NAVIGATE).
“I want to emphasize that these patients were identified by local testing,” Dr. Hyman noted. “We did not perform central screening to find the TRK fusions, and in fact, 50 different laboratories identified the 55 patients. So this in a sense really represents the real-world identification of these patients.”
In an integrated analysis, the overall rate of confirmed response as assessed by investigators was 76%, with complete response in 12% of patients and partial response in 64%. Two patients had such deep tumor regression that they experienced downstaging enabling them to undergo potentially curative surgery. Efficacy was consistent regardless of tumor type, which TRK gene was affected, and the fusion partner gene.
Median time to response was 1.8 months. “This is actually just a reflection of when the first scan was obtained. But in the clinic, patients reported dramatic improvement of their symptoms within days of beginning therapy,” Dr. Hyman said.
With a median follow-up of 5.8 months, the median duration of response was not yet reached. Fully 79% of responses were still ongoing at 12 months. Median progression-free survival was likewise not reached; the 12-month rate was 63%.
The leading treatment-emergent adverse events were fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%). “This is an extremely well tolerated therapy with only 13% of patients requiring any form of dose modification and not a single patient discontinuing due to adverse events,” he said.
It is unclear why some patients had apparent primary resistance to larotrectinib, but their TRK fusion test results may have been incorrect, Dr. Hyman speculated. Six patients developed acquired resistance to larotrectinib; five of them were found to have an identical resistance mutation, and two went on to receive and have a response to LOXO-195, a next-generation TRK inhibitor that appears to retain activity in the presence of this mutation (Cancer Discov. 2017 June 3. doi: 10.1158/2159-8290.CD-17-0507).
Dr. Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology.
AT ASCO 2017
Key clinical point:
Major finding: The overall response rate was 76%, and 79% of responses were still ongoing at 12 months.
Data source: An integrated analysis of phase I and II trials among 55 children and adults having 17 discrete types of advanced cancer with TRK fusions.
Disclosures: Dr. Hyman disclosed that he has a consulting or advisory role with Atara Biotherapeutics, Chugai Pharma, and CytomX Therapeutics, and that he receives research funding from AstraZeneca and Puma Biotechnology. The study was funded by Loxo Oncology.
Immune-agonist combo has activity against several tumor types
CHICAGO – A combination of the programmed death 1 (PD-1) inhibitor nivolumab (Opdivo) with an experimental immune-enhancing monoclonal antibody induced clinical responses in patients with several different solid tumor types, including some patients who had disease progression on a PD-1 inhibitor, investigators reported.
The investigational agent, euphoniously named BMS-986156 (986156), is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR).
BMS-986156156 “induces potent antitumor immunity by several mechanisms. First, it increases T-effector cell survival and function. Second, it promotes T-regulatory cell depletion and reduction through its conversion to other immune cells. As well, it reduces T-reg-mediated suppression of T-effector cells,” said Lillian L Siu, MD, from the Princess Margaret Hospital in Toronto.
In preclinical studies, the combination of an anti-GITR and an anti-PD-1 agent showed synergistic activity against murine tumor models.
Dr. Siu and colleagues conducted a phase I/IIa study of BMS-986156 with or without nivolumab in 66 patients with advanced solid tumors.
The 29 patients assigned to BMS-986156 monotherapy were started at 10 mg every 2 weeks, which was gradually titrated upward to find the maximum tolerated dose of 240 mg Q2 weeks.
The 37 patients assigned to the combination were started on a dose of 30-mg nivolumab and 240-mg BMS-986156. The nivolumab dose but not the BMS-986156 dose was then titrated upward to a maximum tolerated dose of 240 mg for each agent. This dose was based on pharmacodynamic and pharmacokinetic studies.
Tumor types included melanoma, cervical, colon, breast, renal, pancreatic, and ovarian cancers and cholangiocarcinoma.
Approximately one-third of patients in the monotherapy arm and nearly half of those in the combination arm had undergone three or more prior therapies for cancer. Seven patients in the monotherapy group and five in the combination group had previously received a PD-1 or PD-L1 inhibitor.
The median duration of treatment ranged from 7 to 15.5 weeks for 156 monotherapy and 8 to 18 weeks for the combination.
Safe and well tolerated
There were no dose-limiting toxicities or treatment-related deaths in either study arm, and patients tolerated both BMS-986156 monotherapy and the combination well. There were no grade 3 or 4 adverse events in the monotherapy arm.
“In the combination arm, the toxicity is very consistent with that observed with nivolumab monotherapy alone,” Dr. Siu said.
The only grade 4 event in this group was an increase in blood creatine phosphokinase. In this group, there were six grade 3 adverse events, including one each of colitis, dehydration, fatigue and increases in hepatic enzymes, lipase increase, and lung infection.
In pharmacokinetic studies, the action of the combinations was linear, with dose-related increases in exposure, and the combination had low immunogenicity, with no patients developing persistent antidrug antibodies.
The combination was also associated with increases in natural killer and CD8 cells in peripheral blood. Immunophenotyping of patients treated with the 240/240-mg dose of the combination showed increased proliferation and activation of CD8 effector cells, central memory cells, and CD4 cells.
Early promise
Dr. Siu reviewed interim efficacy results for the five patients treated with the combination who had responses.
For example, a 44-year-old woman with metastatic cervical cancer – a tumor type known to have high levels of GITR expression – had received more than three prior lines of therapy, including chemotherapy with a vascular endothelial growth factor inhibitor. She had a partial response with the combination, with an approximately 62% reduction in tumor burden. She had an ongoing response to the combination at the time of data cutoff in March 2017.
Two other patients had partial responses after progression on an anti-PD-1 agents, including one with nasopharyngeal cancer who had received three prior lines of therapy, including chemotherapy and a PD-1 inhibitor. This patient had an approximately 43% reduction in tumor burden, with a 17-week duration of response and ongoing response at data cutoff.
The other patient was a 59-year-old with malignant melanoma that had advanced on pembrolizumab (Keytruda). This patient too had received three prior lines of therapy, including a BRAF inhibitor, anti-PD-1, and BRAF/MEK inhibitor combination.
This patient had a response of 24-week duration at the time of data cutoff. It is ongoing, Dr. Liu said.
“This combination of immune agonists was safe with a low incidence of severe toxicity, and there was no maximum tolerated dose; however, the maximum administered dose may not be the most effective dose to move forward,” commented Siwen Hu-Lieskovan MD, PhD, from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, the invited discussant.
She noted that activity of the combination has been seen in a wide range of tumor histologies but added that further biomarker studies will be critical for identifying patients who are likely to respond.
The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
CHICAGO – A combination of the programmed death 1 (PD-1) inhibitor nivolumab (Opdivo) with an experimental immune-enhancing monoclonal antibody induced clinical responses in patients with several different solid tumor types, including some patients who had disease progression on a PD-1 inhibitor, investigators reported.
The investigational agent, euphoniously named BMS-986156 (986156), is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR).
BMS-986156156 “induces potent antitumor immunity by several mechanisms. First, it increases T-effector cell survival and function. Second, it promotes T-regulatory cell depletion and reduction through its conversion to other immune cells. As well, it reduces T-reg-mediated suppression of T-effector cells,” said Lillian L Siu, MD, from the Princess Margaret Hospital in Toronto.
In preclinical studies, the combination of an anti-GITR and an anti-PD-1 agent showed synergistic activity against murine tumor models.
Dr. Siu and colleagues conducted a phase I/IIa study of BMS-986156 with or without nivolumab in 66 patients with advanced solid tumors.
The 29 patients assigned to BMS-986156 monotherapy were started at 10 mg every 2 weeks, which was gradually titrated upward to find the maximum tolerated dose of 240 mg Q2 weeks.
The 37 patients assigned to the combination were started on a dose of 30-mg nivolumab and 240-mg BMS-986156. The nivolumab dose but not the BMS-986156 dose was then titrated upward to a maximum tolerated dose of 240 mg for each agent. This dose was based on pharmacodynamic and pharmacokinetic studies.
Tumor types included melanoma, cervical, colon, breast, renal, pancreatic, and ovarian cancers and cholangiocarcinoma.
Approximately one-third of patients in the monotherapy arm and nearly half of those in the combination arm had undergone three or more prior therapies for cancer. Seven patients in the monotherapy group and five in the combination group had previously received a PD-1 or PD-L1 inhibitor.
The median duration of treatment ranged from 7 to 15.5 weeks for 156 monotherapy and 8 to 18 weeks for the combination.
Safe and well tolerated
There were no dose-limiting toxicities or treatment-related deaths in either study arm, and patients tolerated both BMS-986156 monotherapy and the combination well. There were no grade 3 or 4 adverse events in the monotherapy arm.
“In the combination arm, the toxicity is very consistent with that observed with nivolumab monotherapy alone,” Dr. Siu said.
The only grade 4 event in this group was an increase in blood creatine phosphokinase. In this group, there were six grade 3 adverse events, including one each of colitis, dehydration, fatigue and increases in hepatic enzymes, lipase increase, and lung infection.
In pharmacokinetic studies, the action of the combinations was linear, with dose-related increases in exposure, and the combination had low immunogenicity, with no patients developing persistent antidrug antibodies.
The combination was also associated with increases in natural killer and CD8 cells in peripheral blood. Immunophenotyping of patients treated with the 240/240-mg dose of the combination showed increased proliferation and activation of CD8 effector cells, central memory cells, and CD4 cells.
Early promise
Dr. Siu reviewed interim efficacy results for the five patients treated with the combination who had responses.
For example, a 44-year-old woman with metastatic cervical cancer – a tumor type known to have high levels of GITR expression – had received more than three prior lines of therapy, including chemotherapy with a vascular endothelial growth factor inhibitor. She had a partial response with the combination, with an approximately 62% reduction in tumor burden. She had an ongoing response to the combination at the time of data cutoff in March 2017.
Two other patients had partial responses after progression on an anti-PD-1 agents, including one with nasopharyngeal cancer who had received three prior lines of therapy, including chemotherapy and a PD-1 inhibitor. This patient had an approximately 43% reduction in tumor burden, with a 17-week duration of response and ongoing response at data cutoff.
The other patient was a 59-year-old with malignant melanoma that had advanced on pembrolizumab (Keytruda). This patient too had received three prior lines of therapy, including a BRAF inhibitor, anti-PD-1, and BRAF/MEK inhibitor combination.
This patient had a response of 24-week duration at the time of data cutoff. It is ongoing, Dr. Liu said.
“This combination of immune agonists was safe with a low incidence of severe toxicity, and there was no maximum tolerated dose; however, the maximum administered dose may not be the most effective dose to move forward,” commented Siwen Hu-Lieskovan MD, PhD, from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, the invited discussant.
She noted that activity of the combination has been seen in a wide range of tumor histologies but added that further biomarker studies will be critical for identifying patients who are likely to respond.
The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
CHICAGO – A combination of the programmed death 1 (PD-1) inhibitor nivolumab (Opdivo) with an experimental immune-enhancing monoclonal antibody induced clinical responses in patients with several different solid tumor types, including some patients who had disease progression on a PD-1 inhibitor, investigators reported.
The investigational agent, euphoniously named BMS-986156 (986156), is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR).
BMS-986156156 “induces potent antitumor immunity by several mechanisms. First, it increases T-effector cell survival and function. Second, it promotes T-regulatory cell depletion and reduction through its conversion to other immune cells. As well, it reduces T-reg-mediated suppression of T-effector cells,” said Lillian L Siu, MD, from the Princess Margaret Hospital in Toronto.
In preclinical studies, the combination of an anti-GITR and an anti-PD-1 agent showed synergistic activity against murine tumor models.
Dr. Siu and colleagues conducted a phase I/IIa study of BMS-986156 with or without nivolumab in 66 patients with advanced solid tumors.
The 29 patients assigned to BMS-986156 monotherapy were started at 10 mg every 2 weeks, which was gradually titrated upward to find the maximum tolerated dose of 240 mg Q2 weeks.
The 37 patients assigned to the combination were started on a dose of 30-mg nivolumab and 240-mg BMS-986156. The nivolumab dose but not the BMS-986156 dose was then titrated upward to a maximum tolerated dose of 240 mg for each agent. This dose was based on pharmacodynamic and pharmacokinetic studies.
Tumor types included melanoma, cervical, colon, breast, renal, pancreatic, and ovarian cancers and cholangiocarcinoma.
Approximately one-third of patients in the monotherapy arm and nearly half of those in the combination arm had undergone three or more prior therapies for cancer. Seven patients in the monotherapy group and five in the combination group had previously received a PD-1 or PD-L1 inhibitor.
The median duration of treatment ranged from 7 to 15.5 weeks for 156 monotherapy and 8 to 18 weeks for the combination.
Safe and well tolerated
There were no dose-limiting toxicities or treatment-related deaths in either study arm, and patients tolerated both BMS-986156 monotherapy and the combination well. There were no grade 3 or 4 adverse events in the monotherapy arm.
“In the combination arm, the toxicity is very consistent with that observed with nivolumab monotherapy alone,” Dr. Siu said.
The only grade 4 event in this group was an increase in blood creatine phosphokinase. In this group, there were six grade 3 adverse events, including one each of colitis, dehydration, fatigue and increases in hepatic enzymes, lipase increase, and lung infection.
In pharmacokinetic studies, the action of the combinations was linear, with dose-related increases in exposure, and the combination had low immunogenicity, with no patients developing persistent antidrug antibodies.
The combination was also associated with increases in natural killer and CD8 cells in peripheral blood. Immunophenotyping of patients treated with the 240/240-mg dose of the combination showed increased proliferation and activation of CD8 effector cells, central memory cells, and CD4 cells.
Early promise
Dr. Siu reviewed interim efficacy results for the five patients treated with the combination who had responses.
For example, a 44-year-old woman with metastatic cervical cancer – a tumor type known to have high levels of GITR expression – had received more than three prior lines of therapy, including chemotherapy with a vascular endothelial growth factor inhibitor. She had a partial response with the combination, with an approximately 62% reduction in tumor burden. She had an ongoing response to the combination at the time of data cutoff in March 2017.
Two other patients had partial responses after progression on an anti-PD-1 agents, including one with nasopharyngeal cancer who had received three prior lines of therapy, including chemotherapy and a PD-1 inhibitor. This patient had an approximately 43% reduction in tumor burden, with a 17-week duration of response and ongoing response at data cutoff.
The other patient was a 59-year-old with malignant melanoma that had advanced on pembrolizumab (Keytruda). This patient too had received three prior lines of therapy, including a BRAF inhibitor, anti-PD-1, and BRAF/MEK inhibitor combination.
This patient had a response of 24-week duration at the time of data cutoff. It is ongoing, Dr. Liu said.
“This combination of immune agonists was safe with a low incidence of severe toxicity, and there was no maximum tolerated dose; however, the maximum administered dose may not be the most effective dose to move forward,” commented Siwen Hu-Lieskovan MD, PhD, from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, the invited discussant.
She noted that activity of the combination has been seen in a wide range of tumor histologies but added that further biomarker studies will be critical for identifying patients who are likely to respond.
The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
AT ASCO 2017
Key clinical point: A combination of a GITR-agonist and anti-PD-1 agent was safe and produced partial responses in patients with heavily pretreated advanced cancers.
Major finding: Two patients with cancers that had progression on a PD-1 inhibitor had durable partial responses.
Data source: A phase I/IIa dose-finding and safety study of BMS986156 alone or in combination with nivolumab (Opdivo).
Disclosures: The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising for several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
Segmental Vitiligo–like Hypopigmentation Associated With Metastatic Melanoma
To the Editor:
Melanoma-associated hypopigmentation frequently has been reported during the disease course and can include different characteristics such as regression of the primary melanoma and/or its metastases as well as common vitiligolike hypopigmentation at sites distant from the melanoma.1,2 Among patients who present with hypopigmentation, the most common clinical presentation is hypopigmented patches in a bilateral symmetric distribution that is similar to vitiligo.1 We report a case of segmental vitiligo–like hypopigmentation associated with melanoma.
RELATED ARTICLE: Novel Melanoma Therapies and Their Side Effects
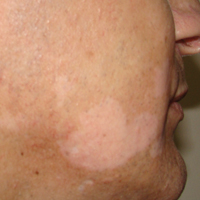
A 37-year-old man presented with achromic patches on the right side of the neck and lower face of 2 months’ duration. He had a history of melanoma (Breslow thickness, 1.37 mm; mitotic rate, 4/mm2) on the right retroauricular region that was treated by wide local excision 12 years prior; after 10 years, he began to have headaches. At that time, imaging studies including computed tomography, magnetic resonance imaging, and positron emission tomography–computed tomography revealed multiple nodules on the brain, lungs, pancreas, left scapula, and left suprarenal gland. A lung biopsy confirmed metastatic melanoma. Intr
On physical examination using a Wood lamp at the current presentation 2 months later, the achromic patches were linearly distributed on the inferior portion of the right cheek (Figure). A 2×3-cm atrophic scar was present on the right retroauricular region. No regional or distant lymph nodes were enlarged or hard on examination. Although vitiligo is diagnosed using clinical findings,3 a biopsy was performed and showed absence of melanocytes at the dermoepidermal junction (hematoxylin and eosin stain) and complete absence of melanin pigment (Fontana-Masson stain). The patient was treated with topical tacrolimus with poor improvement after 2 months.
The relationship between melanoma and vitiligolike hypopigmentation is a fascinating and controversial topic. Its association is considered to be a consequence of the immune-mediated response against antigens shared by normal melanocytes and melanoma cells.4 Vitiligolike hypopigmentation occurs in 2.8%2 of melanoma patients and is reported in metastatic disease1 as well as those undergoing immunotherapy with or without chemotherapy.5 Its development in patients with stage III or IV melanoma seems to represent an independent positive prognostic factor2 and correlates with a better therapeutic outcome in patients undergoing treatment with biotherapy.5
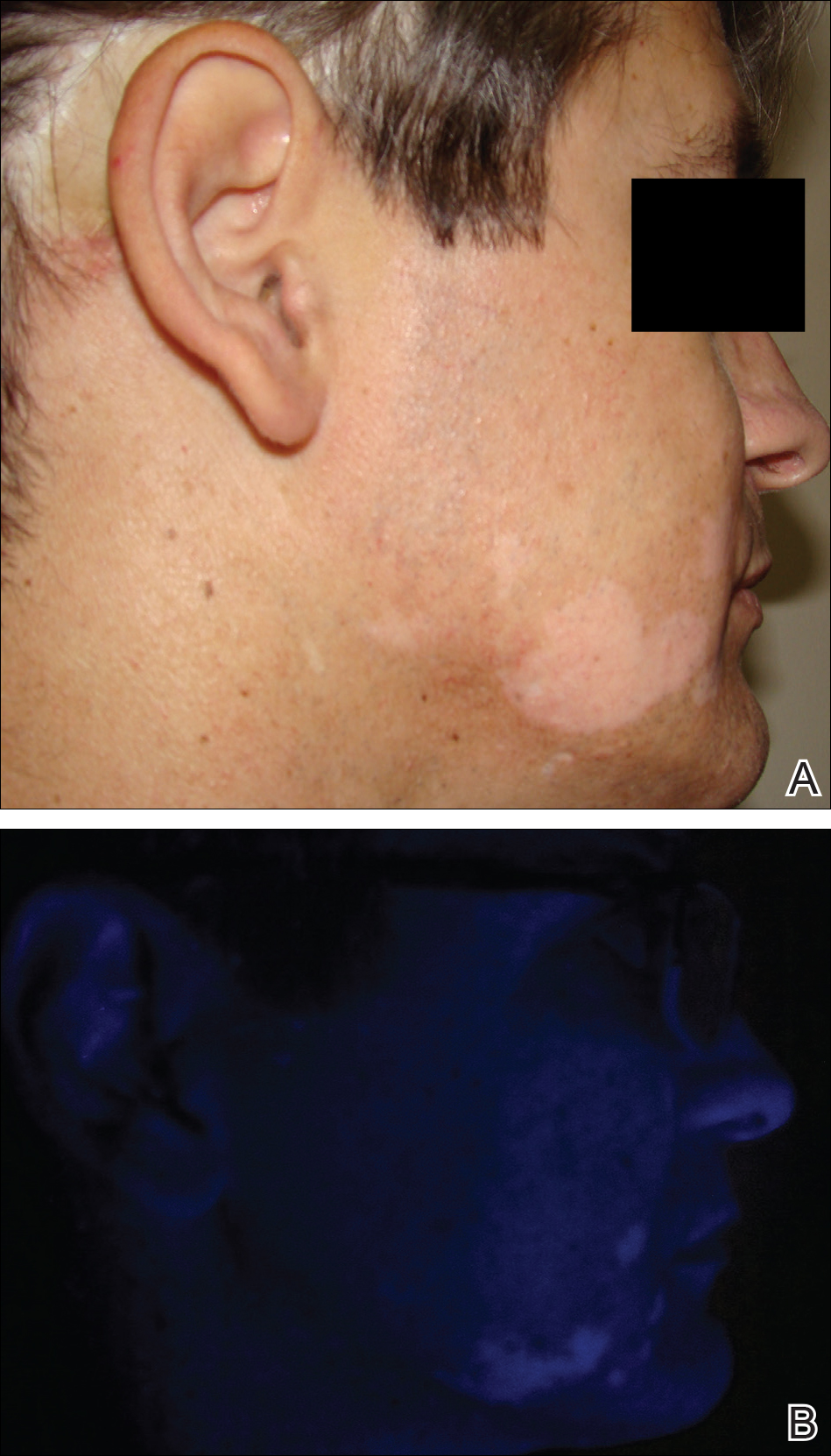
In most cases, the onset of achromic lesions follows the diagnosis of melanoma. Hypopigmentation appears on average 4.8 years after the initial diagnosis and approximately 1 to 2 years after lymph node or distant metastasis.1 In our case, it occurred 12 years after the initial diagnosis and 2 years after metastatic disease was diagnosed.
Despite having widespread metastatic melanoma, our patient only developed achromic patches on the area near the prior melanoma. However, most affected patients present with hypopigmented patches in a bilateral symmetric distribution pattern similar to common vitiligo. No correlation has been found between the hypopigmentation distribution and the location of the primary tumor.1
Because fotemustine is not likely to induce hypopigmentation, we believe that the vitiligolike hypopigmentation in our patient was related to an immune-mediated response associated with melanoma. To help explain our findings, one hypothesis considered was that cutaneous mosaicism may be involved in segmental vitiligo.6 The tumor may have triggered an immune response that affected a close susceptible area of mosaic vitiligo, leading to these clinical findings.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053-1059.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409-414.
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27-35.
- Becker JC, Guldberg P, Zeuthen J, et al. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033-1038.
- Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658-2663.
- Van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56-64.
To the Editor:
Melanoma-associated hypopigmentation frequently has been reported during the disease course and can include different characteristics such as regression of the primary melanoma and/or its metastases as well as common vitiligolike hypopigmentation at sites distant from the melanoma.1,2 Among patients who present with hypopigmentation, the most common clinical presentation is hypopigmented patches in a bilateral symmetric distribution that is similar to vitiligo.1 We report a case of segmental vitiligo–like hypopigmentation associated with melanoma.
RELATED ARTICLE: Novel Melanoma Therapies and Their Side Effects
A 37-year-old man presented with achromic patches on the right side of the neck and lower face of 2 months’ duration. He had a history of melanoma (Breslow thickness, 1.37 mm; mitotic rate, 4/mm2) on the right retroauricular region that was treated by wide local excision 12 years prior; after 10 years, he began to have headaches. At that time, imaging studies including computed tomography, magnetic resonance imaging, and positron emission tomography–computed tomography revealed multiple nodules on the brain, lungs, pancreas, left scapula, and left suprarenal gland. A lung biopsy confirmed metastatic melanoma. Intr
On physical examination using a Wood lamp at the current presentation 2 months later, the achromic patches were linearly distributed on the inferior portion of the right cheek (Figure). A 2×3-cm atrophic scar was present on the right retroauricular region. No regional or distant lymph nodes were enlarged or hard on examination. Although vitiligo is diagnosed using clinical findings,3 a biopsy was performed and showed absence of melanocytes at the dermoepidermal junction (hematoxylin and eosin stain) and complete absence of melanin pigment (Fontana-Masson stain). The patient was treated with topical tacrolimus with poor improvement after 2 months.
The relationship between melanoma and vitiligolike hypopigmentation is a fascinating and controversial topic. Its association is considered to be a consequence of the immune-mediated response against antigens shared by normal melanocytes and melanoma cells.4 Vitiligolike hypopigmentation occurs in 2.8%2 of melanoma patients and is reported in metastatic disease1 as well as those undergoing immunotherapy with or without chemotherapy.5 Its development in patients with stage III or IV melanoma seems to represent an independent positive prognostic factor2 and correlates with a better therapeutic outcome in patients undergoing treatment with biotherapy.5

In most cases, the onset of achromic lesions follows the diagnosis of melanoma. Hypopigmentation appears on average 4.8 years after the initial diagnosis and approximately 1 to 2 years after lymph node or distant metastasis.1 In our case, it occurred 12 years after the initial diagnosis and 2 years after metastatic disease was diagnosed.
Despite having widespread metastatic melanoma, our patient only developed achromic patches on the area near the prior melanoma. However, most affected patients present with hypopigmented patches in a bilateral symmetric distribution pattern similar to common vitiligo. No correlation has been found between the hypopigmentation distribution and the location of the primary tumor.1
Because fotemustine is not likely to induce hypopigmentation, we believe that the vitiligolike hypopigmentation in our patient was related to an immune-mediated response associated with melanoma. To help explain our findings, one hypothesis considered was that cutaneous mosaicism may be involved in segmental vitiligo.6 The tumor may have triggered an immune response that affected a close susceptible area of mosaic vitiligo, leading to these clinical findings.
To the Editor:
Melanoma-associated hypopigmentation frequently has been reported during the disease course and can include different characteristics such as regression of the primary melanoma and/or its metastases as well as common vitiligolike hypopigmentation at sites distant from the melanoma.1,2 Among patients who present with hypopigmentation, the most common clinical presentation is hypopigmented patches in a bilateral symmetric distribution that is similar to vitiligo.1 We report a case of segmental vitiligo–like hypopigmentation associated with melanoma.
RELATED ARTICLE: Novel Melanoma Therapies and Their Side Effects
A 37-year-old man presented with achromic patches on the right side of the neck and lower face of 2 months’ duration. He had a history of melanoma (Breslow thickness, 1.37 mm; mitotic rate, 4/mm2) on the right retroauricular region that was treated by wide local excision 12 years prior; after 10 years, he began to have headaches. At that time, imaging studies including computed tomography, magnetic resonance imaging, and positron emission tomography–computed tomography revealed multiple nodules on the brain, lungs, pancreas, left scapula, and left suprarenal gland. A lung biopsy confirmed metastatic melanoma. Intr
On physical examination using a Wood lamp at the current presentation 2 months later, the achromic patches were linearly distributed on the inferior portion of the right cheek (Figure). A 2×3-cm atrophic scar was present on the right retroauricular region. No regional or distant lymph nodes were enlarged or hard on examination. Although vitiligo is diagnosed using clinical findings,3 a biopsy was performed and showed absence of melanocytes at the dermoepidermal junction (hematoxylin and eosin stain) and complete absence of melanin pigment (Fontana-Masson stain). The patient was treated with topical tacrolimus with poor improvement after 2 months.
The relationship between melanoma and vitiligolike hypopigmentation is a fascinating and controversial topic. Its association is considered to be a consequence of the immune-mediated response against antigens shared by normal melanocytes and melanoma cells.4 Vitiligolike hypopigmentation occurs in 2.8%2 of melanoma patients and is reported in metastatic disease1 as well as those undergoing immunotherapy with or without chemotherapy.5 Its development in patients with stage III or IV melanoma seems to represent an independent positive prognostic factor2 and correlates with a better therapeutic outcome in patients undergoing treatment with biotherapy.5

In most cases, the onset of achromic lesions follows the diagnosis of melanoma. Hypopigmentation appears on average 4.8 years after the initial diagnosis and approximately 1 to 2 years after lymph node or distant metastasis.1 In our case, it occurred 12 years after the initial diagnosis and 2 years after metastatic disease was diagnosed.
Despite having widespread metastatic melanoma, our patient only developed achromic patches on the area near the prior melanoma. However, most affected patients present with hypopigmented patches in a bilateral symmetric distribution pattern similar to common vitiligo. No correlation has been found between the hypopigmentation distribution and the location of the primary tumor.1
Because fotemustine is not likely to induce hypopigmentation, we believe that the vitiligolike hypopigmentation in our patient was related to an immune-mediated response associated with melanoma. To help explain our findings, one hypothesis considered was that cutaneous mosaicism may be involved in segmental vitiligo.6 The tumor may have triggered an immune response that affected a close susceptible area of mosaic vitiligo, leading to these clinical findings.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053-1059.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409-414.
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27-35.
- Becker JC, Guldberg P, Zeuthen J, et al. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033-1038.
- Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658-2663.
- Van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56-64.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053-1059.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409-414.
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27-35.
- Becker JC, Guldberg P, Zeuthen J, et al. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033-1038.
- Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658-2663.
- Van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56-64.
Prac
- Melanoma-associated hypopigmentation usually manifests as common vitiligo; however, little is known about the pathophysiology of segmental vitiligo–like hypopigmentation associated with melanoma.
- This case of segmental vitiligo–like hypopigmentation associated with melanoma sheds light on possible autoimmune and mosaic disease etiology.
In Vivo Reflectance Confocal Microscopy
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
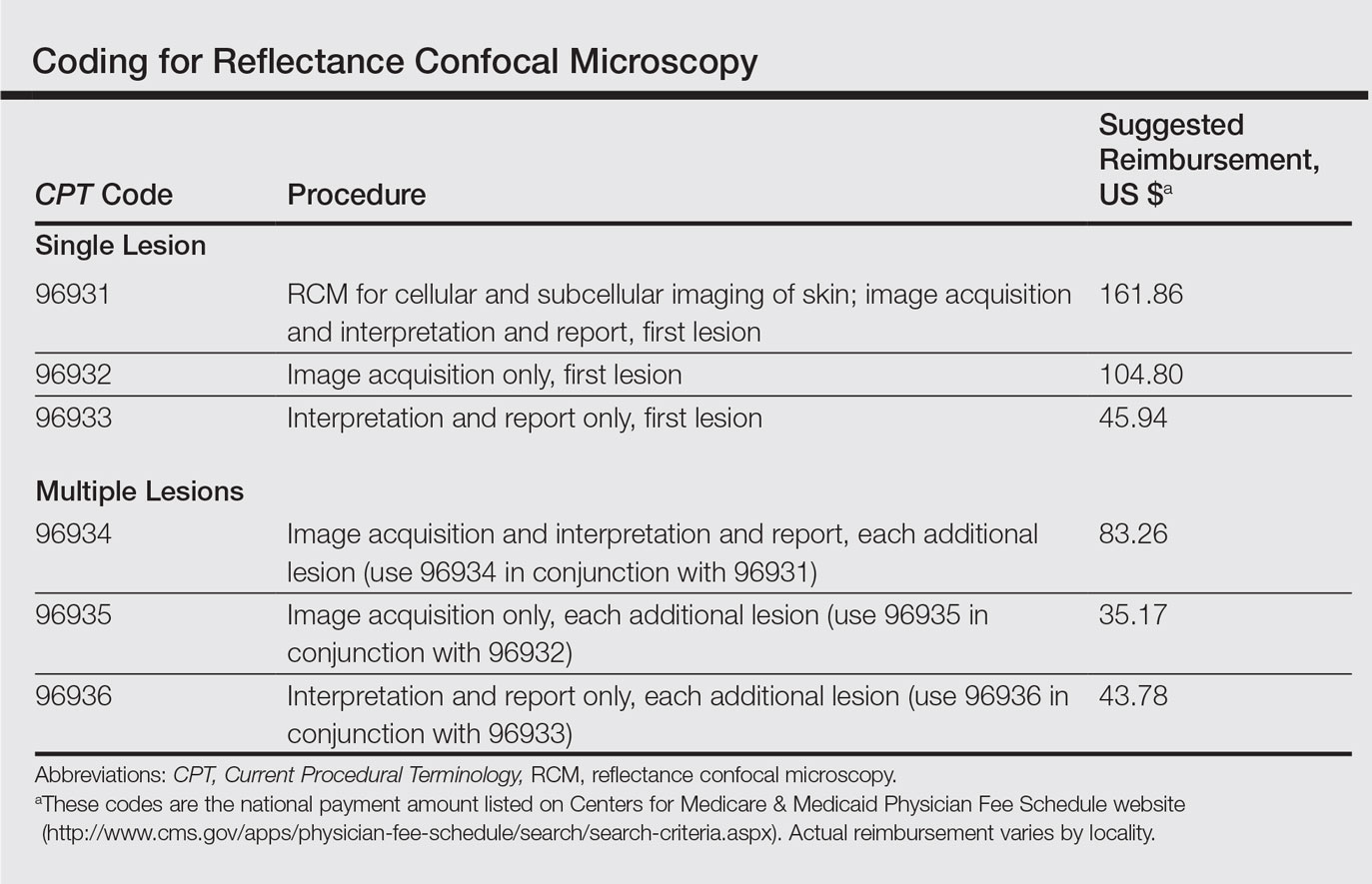
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
Practice Points
- Reflectance confocal microscopy (RCM) recently received Category I Current Procedural Terminology codes for reimbursement comparable to a skin biopsy.
- When used in combination with dermoscopy, RCM has been shown to increase diagnostic accuracy of skin cancer.
- Reflectance confocal microscopy also is useful in surgical treatment planning and monitoring nonsurgical treatments over time.
- Limitations of RCM imaging include low imaging depth, difficulty in imaging certain areas of the skin, learning curve for interpreting these images, and the cost of equipment.
How to best evaluate children’s melanocytic lesions for melanoma
Children often present for evaluation of a melanocytic lesion that is new, evolving, or worrisome to parents and caregivers.
“In the pediatric health care system, malignant melanoma is considered an especially heinous crime. In San Diego, dedicated pediatric providers who identify this disease are members of an elite squad known as the mole patrol unit.” Dr. Sheila Fallon Friedlander opened her presentation on pediatric moles with this tongue-in-cheek statement, adapted from the popular TV show “Law and Order SVU,” at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego.
Only 104 cases were diagnosed in children aged less than 10 years, and the melanoma incidence in this age group was relatively unchanging from 1973 to 2009. Dr. Friedlander further emphasized, “Pediatric melanoma is extremely uncommon in patients less than 10 years of age, but more likely to be atypical.”
She continued by describing a group of surgical oncologists at MD Anderson Cancer Center in Houston, who conducted a retrospective review of children with cutaneous melanoma between 1988 and 2007 included in the SEER database, to determine the influence of age on disease presentation. Preadolescents younger than age 10 years were more ethnically diverse (nonwhite), more frequently presented with nontruncal primary melanocytic lesions, and increasingly were diagnosed with advanced disease, compared with their adolescent counterparts (J Pediatr Surg. 2013 Nov;48[11]:2207-13).
Congenital melanocytic nevi (CMN) may have increased risk for malignant potential, and can be challenging for pediatric providers to manage. Among all CMN, the increase in melanoma risk is estimated as less than 1%. The risk for malignant melanoma is further increased in individuals with large or giant CMN (greater than 20 cm diameter adult size), with an absolute risk of approximately 2%-5%. The number of satellite nevi also is considered in risk stratification. The presence of greater than 20 satellite nevi is associated with a greater than fivefold risk of neurocutaneous melanosis. There is no documented association between an increased quantity of satellite nevi and malignant melanoma.
“One particularly challenging pigmented lesion identified among pediatric patients is a Spitz nevus,” according to Dr. Friedlander. This lesion presents with greater cytologic atypia than other benign congenital and acquired nevi, and often clinically mimics malignant melanoma if identified in adults. There also exists a subset of atypical Spitz nevi, consisting of lesions with greater cytologic atypia than benign Spitz nevi. A retrospective review at Massachusetts General Hospital, Boston, of 157 cases of Spitz-type melanocytic lesions identified between 1987 and 2002 revealed increased melanoma risk, minimal mortality, and moderate risk of regional lymph node metastasis (Arch Dermatol. 2011;147[10]:1173-9).
“Classic pediatric Spitz nevi with typical clinical features and history may be managed conservatively with clinical monitoring alone, but those with concerning features such as bleeding, asymmetry, or ulceration should be excised with clear margins,” Dr. Friedlander emphasized. She discouraged sentinel lymph node biopsy, however, given the positive outcomes of 24 patients at Boston Children’s Hospital with atypical Spitz nevi treated with excision alone, published by Cerrato et al. (Pediatr Dermatol. 2011 Dec 30;29[4]:448-53).
“In light of the rising incidence of pediatric melanoma, we need to identify high-risk patients, educate about mole surveillance, and encourage sun protection,” Dr. Friedlander stressed. Children with phenotype of Fitzpatrick I (fair skin, blonde or red hair, and blue eye color) are at highest risk, as are those with a high density of freckles who burn easily and tan poorly. Further risk factors highlighted include excessive sun exposure, indoor tanning, use of phototoxic medications, immunosuppression, and genetics. The first and best line of defense against harmful ultraviolet radiation is covering up (clothing with a tight weave, wet suits, and hats).
The American Academy of Pediatrics encourages staying in the shade when possible, and limiting sun exposure during the peak sun intensity hours, between 10 a.m. and 4 p.m. When physical protection is not possible, the American Academy of Dermatology endorses the application of water resistant, broad spectrum SPF of greater than 30 at least every 2 hours.
Children often present for evaluation of a melanocytic lesion that is new, evolving, or worrisome to parents and caregivers.
“In the pediatric health care system, malignant melanoma is considered an especially heinous crime. In San Diego, dedicated pediatric providers who identify this disease are members of an elite squad known as the mole patrol unit.” Dr. Sheila Fallon Friedlander opened her presentation on pediatric moles with this tongue-in-cheek statement, adapted from the popular TV show “Law and Order SVU,” at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego.
Only 104 cases were diagnosed in children aged less than 10 years, and the melanoma incidence in this age group was relatively unchanging from 1973 to 2009. Dr. Friedlander further emphasized, “Pediatric melanoma is extremely uncommon in patients less than 10 years of age, but more likely to be atypical.”
She continued by describing a group of surgical oncologists at MD Anderson Cancer Center in Houston, who conducted a retrospective review of children with cutaneous melanoma between 1988 and 2007 included in the SEER database, to determine the influence of age on disease presentation. Preadolescents younger than age 10 years were more ethnically diverse (nonwhite), more frequently presented with nontruncal primary melanocytic lesions, and increasingly were diagnosed with advanced disease, compared with their adolescent counterparts (J Pediatr Surg. 2013 Nov;48[11]:2207-13).
Congenital melanocytic nevi (CMN) may have increased risk for malignant potential, and can be challenging for pediatric providers to manage. Among all CMN, the increase in melanoma risk is estimated as less than 1%. The risk for malignant melanoma is further increased in individuals with large or giant CMN (greater than 20 cm diameter adult size), with an absolute risk of approximately 2%-5%. The number of satellite nevi also is considered in risk stratification. The presence of greater than 20 satellite nevi is associated with a greater than fivefold risk of neurocutaneous melanosis. There is no documented association between an increased quantity of satellite nevi and malignant melanoma.
“One particularly challenging pigmented lesion identified among pediatric patients is a Spitz nevus,” according to Dr. Friedlander. This lesion presents with greater cytologic atypia than other benign congenital and acquired nevi, and often clinically mimics malignant melanoma if identified in adults. There also exists a subset of atypical Spitz nevi, consisting of lesions with greater cytologic atypia than benign Spitz nevi. A retrospective review at Massachusetts General Hospital, Boston, of 157 cases of Spitz-type melanocytic lesions identified between 1987 and 2002 revealed increased melanoma risk, minimal mortality, and moderate risk of regional lymph node metastasis (Arch Dermatol. 2011;147[10]:1173-9).
“Classic pediatric Spitz nevi with typical clinical features and history may be managed conservatively with clinical monitoring alone, but those with concerning features such as bleeding, asymmetry, or ulceration should be excised with clear margins,” Dr. Friedlander emphasized. She discouraged sentinel lymph node biopsy, however, given the positive outcomes of 24 patients at Boston Children’s Hospital with atypical Spitz nevi treated with excision alone, published by Cerrato et al. (Pediatr Dermatol. 2011 Dec 30;29[4]:448-53).
“In light of the rising incidence of pediatric melanoma, we need to identify high-risk patients, educate about mole surveillance, and encourage sun protection,” Dr. Friedlander stressed. Children with phenotype of Fitzpatrick I (fair skin, blonde or red hair, and blue eye color) are at highest risk, as are those with a high density of freckles who burn easily and tan poorly. Further risk factors highlighted include excessive sun exposure, indoor tanning, use of phototoxic medications, immunosuppression, and genetics. The first and best line of defense against harmful ultraviolet radiation is covering up (clothing with a tight weave, wet suits, and hats).
The American Academy of Pediatrics encourages staying in the shade when possible, and limiting sun exposure during the peak sun intensity hours, between 10 a.m. and 4 p.m. When physical protection is not possible, the American Academy of Dermatology endorses the application of water resistant, broad spectrum SPF of greater than 30 at least every 2 hours.
Children often present for evaluation of a melanocytic lesion that is new, evolving, or worrisome to parents and caregivers.
“In the pediatric health care system, malignant melanoma is considered an especially heinous crime. In San Diego, dedicated pediatric providers who identify this disease are members of an elite squad known as the mole patrol unit.” Dr. Sheila Fallon Friedlander opened her presentation on pediatric moles with this tongue-in-cheek statement, adapted from the popular TV show “Law and Order SVU,” at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego.
Only 104 cases were diagnosed in children aged less than 10 years, and the melanoma incidence in this age group was relatively unchanging from 1973 to 2009. Dr. Friedlander further emphasized, “Pediatric melanoma is extremely uncommon in patients less than 10 years of age, but more likely to be atypical.”
She continued by describing a group of surgical oncologists at MD Anderson Cancer Center in Houston, who conducted a retrospective review of children with cutaneous melanoma between 1988 and 2007 included in the SEER database, to determine the influence of age on disease presentation. Preadolescents younger than age 10 years were more ethnically diverse (nonwhite), more frequently presented with nontruncal primary melanocytic lesions, and increasingly were diagnosed with advanced disease, compared with their adolescent counterparts (J Pediatr Surg. 2013 Nov;48[11]:2207-13).
Congenital melanocytic nevi (CMN) may have increased risk for malignant potential, and can be challenging for pediatric providers to manage. Among all CMN, the increase in melanoma risk is estimated as less than 1%. The risk for malignant melanoma is further increased in individuals with large or giant CMN (greater than 20 cm diameter adult size), with an absolute risk of approximately 2%-5%. The number of satellite nevi also is considered in risk stratification. The presence of greater than 20 satellite nevi is associated with a greater than fivefold risk of neurocutaneous melanosis. There is no documented association between an increased quantity of satellite nevi and malignant melanoma.
“One particularly challenging pigmented lesion identified among pediatric patients is a Spitz nevus,” according to Dr. Friedlander. This lesion presents with greater cytologic atypia than other benign congenital and acquired nevi, and often clinically mimics malignant melanoma if identified in adults. There also exists a subset of atypical Spitz nevi, consisting of lesions with greater cytologic atypia than benign Spitz nevi. A retrospective review at Massachusetts General Hospital, Boston, of 157 cases of Spitz-type melanocytic lesions identified between 1987 and 2002 revealed increased melanoma risk, minimal mortality, and moderate risk of regional lymph node metastasis (Arch Dermatol. 2011;147[10]:1173-9).
“Classic pediatric Spitz nevi with typical clinical features and history may be managed conservatively with clinical monitoring alone, but those with concerning features such as bleeding, asymmetry, or ulceration should be excised with clear margins,” Dr. Friedlander emphasized. She discouraged sentinel lymph node biopsy, however, given the positive outcomes of 24 patients at Boston Children’s Hospital with atypical Spitz nevi treated with excision alone, published by Cerrato et al. (Pediatr Dermatol. 2011 Dec 30;29[4]:448-53).
“In light of the rising incidence of pediatric melanoma, we need to identify high-risk patients, educate about mole surveillance, and encourage sun protection,” Dr. Friedlander stressed. Children with phenotype of Fitzpatrick I (fair skin, blonde or red hair, and blue eye color) are at highest risk, as are those with a high density of freckles who burn easily and tan poorly. Further risk factors highlighted include excessive sun exposure, indoor tanning, use of phototoxic medications, immunosuppression, and genetics. The first and best line of defense against harmful ultraviolet radiation is covering up (clothing with a tight weave, wet suits, and hats).
The American Academy of Pediatrics encourages staying in the shade when possible, and limiting sun exposure during the peak sun intensity hours, between 10 a.m. and 4 p.m. When physical protection is not possible, the American Academy of Dermatology endorses the application of water resistant, broad spectrum SPF of greater than 30 at least every 2 hours.
Mole count predicted melanoma death, especially among men
PORTLAND, ORE. – Among white men, the presence of at least one cutaneous nevus measuring 3 mm or more significantly predicted death from melanoma, in an adjusted analysis of a large prospective cohort study.
Mole count also predicted melanoma death among white women, but the association reached statistical significance only when women had at least three cutaneous nevi measuring 3 mm or more, Eunyoung Cho, ScD, said at the annual meeting of the Society for Investigative Dermatology. The reasons why these associations varied by sex is unclear, although previous studies have documented higher rates of melanoma death among men than among women, and even male physicians tend to seek health care less frequently than their female counterparts, Dr. Cho said in her poster presentation.
In the Nurses’ Health Study, white women with at least three moles measuring at least 3 mm in diameter were at significantly increased risk of dying of melanoma, compared with those with no moles that size (hazard ratio, 2.5; 95% confidence interval, 1.5-4.1), even after the investigators controlled for many other potential confounders, including sunburn history, skin reaction to sun during childhood, tanning ability, family history of melanoma, personal history of nonmelanoma skin cancer, age, activity level, smoking, body mass index, alcohol intake, and hair color. Women with one or two moles also showed a trend toward increased risk of melanoma death (HR, 1.4), but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.9-2.3).
The investigators estimated that among white women, each additional mole measuring 3 mm or more conferred about a 12% increase in the melanoma death rate, even after confounders were controlled for.
In the Health Professionals Follow-Up Study, men with one or two moles of at least 3 mm had about twice the melanoma death rate as men without moles of this size (HR, 2.0; 95% CI, 1.3-3.3), even after investigators controlled for potential confounders. The risk of melanoma death was even greater among men with at least three moles (HR, 4.0; 95% CI, 2.5-6.2), and the difference in rates was statistically significant (P less than .0001). After confounders were accounted for, each additional mole measuring at least 3 mm conferred a 20% increase in the rate of melanoma death.
A different picture emerged after narrowing the adjusted analyses to include only people diagnosed with melanoma: In this group, mole count did not predict melanoma death among women, but continued to do so among men with melanoma who had at least three moles at baseline (HR, 1.8; 95% CI, 1.1-3.0), Dr. Cho reported. Among men, higher mole count also predicted melanoma of at least 1-mm Breslow thickness, an important prognostic factor, she added. Hazard ratios for these “thicker melanomas” were 1.9 (95% CI, 1.1-3.3) among men with one or two moles, and 2.5 (95% CI, 1.5-4.4) among men with three or more moles. Among women with melanoma, mole count did not predict Breslow thickness.
The extent to which sex affected trends in this analysis highlights the need for more studies of sex and other phenotypic risk factors for melanoma death, Dr. Cho concluded. She presented on behalf of lead author Wen-Qing Li, PhD, also of Brown University.
The National Institutes of Health and the Dermatology Foundation provided funding. Dr. Cho and Dr. Li had no relevant financial disclosures.
PORTLAND, ORE. – Among white men, the presence of at least one cutaneous nevus measuring 3 mm or more significantly predicted death from melanoma, in an adjusted analysis of a large prospective cohort study.
Mole count also predicted melanoma death among white women, but the association reached statistical significance only when women had at least three cutaneous nevi measuring 3 mm or more, Eunyoung Cho, ScD, said at the annual meeting of the Society for Investigative Dermatology. The reasons why these associations varied by sex is unclear, although previous studies have documented higher rates of melanoma death among men than among women, and even male physicians tend to seek health care less frequently than their female counterparts, Dr. Cho said in her poster presentation.
In the Nurses’ Health Study, white women with at least three moles measuring at least 3 mm in diameter were at significantly increased risk of dying of melanoma, compared with those with no moles that size (hazard ratio, 2.5; 95% confidence interval, 1.5-4.1), even after the investigators controlled for many other potential confounders, including sunburn history, skin reaction to sun during childhood, tanning ability, family history of melanoma, personal history of nonmelanoma skin cancer, age, activity level, smoking, body mass index, alcohol intake, and hair color. Women with one or two moles also showed a trend toward increased risk of melanoma death (HR, 1.4), but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.9-2.3).
The investigators estimated that among white women, each additional mole measuring 3 mm or more conferred about a 12% increase in the melanoma death rate, even after confounders were controlled for.
In the Health Professionals Follow-Up Study, men with one or two moles of at least 3 mm had about twice the melanoma death rate as men without moles of this size (HR, 2.0; 95% CI, 1.3-3.3), even after investigators controlled for potential confounders. The risk of melanoma death was even greater among men with at least three moles (HR, 4.0; 95% CI, 2.5-6.2), and the difference in rates was statistically significant (P less than .0001). After confounders were accounted for, each additional mole measuring at least 3 mm conferred a 20% increase in the rate of melanoma death.
A different picture emerged after narrowing the adjusted analyses to include only people diagnosed with melanoma: In this group, mole count did not predict melanoma death among women, but continued to do so among men with melanoma who had at least three moles at baseline (HR, 1.8; 95% CI, 1.1-3.0), Dr. Cho reported. Among men, higher mole count also predicted melanoma of at least 1-mm Breslow thickness, an important prognostic factor, she added. Hazard ratios for these “thicker melanomas” were 1.9 (95% CI, 1.1-3.3) among men with one or two moles, and 2.5 (95% CI, 1.5-4.4) among men with three or more moles. Among women with melanoma, mole count did not predict Breslow thickness.
The extent to which sex affected trends in this analysis highlights the need for more studies of sex and other phenotypic risk factors for melanoma death, Dr. Cho concluded. She presented on behalf of lead author Wen-Qing Li, PhD, also of Brown University.
The National Institutes of Health and the Dermatology Foundation provided funding. Dr. Cho and Dr. Li had no relevant financial disclosures.
PORTLAND, ORE. – Among white men, the presence of at least one cutaneous nevus measuring 3 mm or more significantly predicted death from melanoma, in an adjusted analysis of a large prospective cohort study.
Mole count also predicted melanoma death among white women, but the association reached statistical significance only when women had at least three cutaneous nevi measuring 3 mm or more, Eunyoung Cho, ScD, said at the annual meeting of the Society for Investigative Dermatology. The reasons why these associations varied by sex is unclear, although previous studies have documented higher rates of melanoma death among men than among women, and even male physicians tend to seek health care less frequently than their female counterparts, Dr. Cho said in her poster presentation.
In the Nurses’ Health Study, white women with at least three moles measuring at least 3 mm in diameter were at significantly increased risk of dying of melanoma, compared with those with no moles that size (hazard ratio, 2.5; 95% confidence interval, 1.5-4.1), even after the investigators controlled for many other potential confounders, including sunburn history, skin reaction to sun during childhood, tanning ability, family history of melanoma, personal history of nonmelanoma skin cancer, age, activity level, smoking, body mass index, alcohol intake, and hair color. Women with one or two moles also showed a trend toward increased risk of melanoma death (HR, 1.4), but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.9-2.3).
The investigators estimated that among white women, each additional mole measuring 3 mm or more conferred about a 12% increase in the melanoma death rate, even after confounders were controlled for.
In the Health Professionals Follow-Up Study, men with one or two moles of at least 3 mm had about twice the melanoma death rate as men without moles of this size (HR, 2.0; 95% CI, 1.3-3.3), even after investigators controlled for potential confounders. The risk of melanoma death was even greater among men with at least three moles (HR, 4.0; 95% CI, 2.5-6.2), and the difference in rates was statistically significant (P less than .0001). After confounders were accounted for, each additional mole measuring at least 3 mm conferred a 20% increase in the rate of melanoma death.
A different picture emerged after narrowing the adjusted analyses to include only people diagnosed with melanoma: In this group, mole count did not predict melanoma death among women, but continued to do so among men with melanoma who had at least three moles at baseline (HR, 1.8; 95% CI, 1.1-3.0), Dr. Cho reported. Among men, higher mole count also predicted melanoma of at least 1-mm Breslow thickness, an important prognostic factor, she added. Hazard ratios for these “thicker melanomas” were 1.9 (95% CI, 1.1-3.3) among men with one or two moles, and 2.5 (95% CI, 1.5-4.4) among men with three or more moles. Among women with melanoma, mole count did not predict Breslow thickness.
The extent to which sex affected trends in this analysis highlights the need for more studies of sex and other phenotypic risk factors for melanoma death, Dr. Cho concluded. She presented on behalf of lead author Wen-Qing Li, PhD, also of Brown University.
The National Institutes of Health and the Dermatology Foundation provided funding. Dr. Cho and Dr. Li had no relevant financial disclosures.
AT SID 2017
Key clinical point: Mole count was an independent risk factor for melanoma death among men and, to a lesser extent, among women.
Major finding: Adjusted hazard ratios were 2.0 among white men with one or two moles at least 3 mm in diameter and 4.0 among those with at least three moles, but among white women, the association was not significant unless they had at least three moles (HR, 2.5).
Data source: Adjusted analyses of 77,288 white women from the Nurses’ Health Study and 32,455 white men from the Health Professionals Follow-Up Study for 1986 through 2012.
Disclosures: The National Institutes of Health and the Dermatology Foundation provided funding for the study. Dr. Cho and Dr. Li had no relevant financial disclosures.
Modern estrogen ‘microdoses’ in contraceptives did not increase risk of melanoma
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
PORTLAND, ORE. – Long-term exposure to commonly used estrogen-based contraceptives was not associated with malignant melanoma in a single-center retrospective study of more than 77,000 women.
This null result belied decades-old studies performed when contraceptives contained much higher doses of estrogen, Kelly A. Mueller of Northwestern University, Chicago, said in a poster presented at the annual meeting of the Society for Investigative Dermatology. Current microdosing of ethinyl estradiol (EE) “is not associated with subsequent diagnosis of melanoma in our population, and is not inconsistent with the full prescribing information for commonly prescribed contraceptives containing EE,” she said. The study also shows how large retrospective analyses of long-term follow-up data can inform pharmacovigilance for rare, serious medical events.
To help clarify whether current microdosing (10-40 mcg/day) of EE can increase melanoma risk, the researchers compared 2,425 women prescribed oral, vaginal ring, or skin patch EE contraceptives for at least 12 months with 74,868 unexposed women. For both groups, initial clinical encounters occurred between 2001 and 2011, women were followed for at least 5 years, and none had a baseline history of melanoma or exogenous estrogen exposure. The data source was the Northwestern Medicine Enterprise Data Warehouse, which integrates electronic medical records from more than 4 million patients in the urban Midwest.
When first seen, patients tended to be in their late 20s and ranged in age between 18 and 40 years. Excluding cutaneous malignant melanomas diagnosed within 12 months of initial contraceptive prescription left three cases in the exposed group and 194 cases in the unexposed group, which translated to statistically similar rates of melanoma (0.1% and 0.3%, respectively; P = 0.3). The three cases in the exposed group were diagnosed between 37 and 92 months after initial prescription of EE contraceptives, but “the limited sample size for the outcome of interest did not allow for further analyses,” she reported. Nevertheless, the findings suggest no link between long-term microdosing of EE exposure and cutaneous melanoma, Ms. Mueller added.
The National Institutes of Health helps support the Northwestern Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
AT SID 2017
Key clinical point: Long-term exposure to modern “microdoses” of ethinyl estradiol in contraceptives was not associated with malignant melanoma.
Major finding: Rates were 0.1% in the exposed group and 0.3% in the unexposed group (P = .3).
Data source: A retrospective cohort study of 77,293 women.
Disclosures: The National Institutes of Health helps support the Northwestern Medicine Enterprise Data Warehouse. Ms. Mueller and her associates had no relevant financial conflicts of interest.
Chemoprevention: Thinking outside the box
WAILEA, HAWAII – Nicotinamide is one of the rare proposed agents for skin cancer chemoprevention distinguished by dirt cheap cost combined with a highly reassuring safety profile plus evidence of efficacy – which, together, make it a reasonable option in high risk patients, according to Daniel M. Siegel, MD.
Other agents that fit into that category include the tropical rainforest fern Polypodium leucotomos and milk thistle, added Dr. Siegel, a dermatologist at the State University of New York, Brooklyn.
“That’s a really interesting one. I don’t know if, 5 years from now, we’ll all be taking low-dose rapamycin as an antiaging drug, but we might, especially if someone figures out the ideal dose,” he said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
Nicotinamide
In the case of nicotinamide, the efficacy is actually supported by published level 1 evidence in the form of a highly positive 1-year, double-blind, randomized, placebo-controlled phase III clinical trial.
“You can Google ‘nicotinamide’ and find it at places like Costco and Trader Joe’s for less than 6 cents per day. That makes for a really good risk/benefit ratio. A nickel a day: That’s a cheap one. That’s one where I’d say, ‘Why not?’ It seems to be safe,” Dr. Siegel said.
In the phase III ONTRAC trial, Australian investigators randomized 386 patients who averaged roughly eight nonmelanoma skin cancers in the past 5 years to either 500 mg of oral nicotinamide twice daily or matched placebo for 12 months. During the study period, the nicotinamide group had a statistically significant and clinically meaningful 23% reduction in new nonmelanoma skin cancers, compared with the control group. They also had 13% fewer actinic keratoses at 12 months than controls. And the side effect profile mirrored that of placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26).
“Nicotinamide is vitamin B3. It’s not niacin. It doesn’t cause flushing and other vasodilatory effects. It’s actually pretty innocuous,” Dr. Siegel said.
In laboratory studies, nicotinamide has been shown to enhance DNA repair following UV exposure, as well as curb UV-induced immunosuppression.
Polypodium leucotomos Samambaia
This plant, commonly known as calaguala in the Spanish-speaking tropics and samambaia in Brazil, has a centuries-long tradition of safe medicinal use. It is commercially available over-the-counter (OTC) as a standardized product called Heliocare, designed to avoid the guesswork involved in topical sunscreen application. Each capsule contains 240 mg of an extract of P. leucotomos. Dr. Siegel said he takes it daily when he’s in a sunny locale, such as Hawaii.
Milk thistle
This plant, known as Silybum marianum, has silymarin as its bioactive compound. Dermatologist Haines Ely, MD, of the University of California, Davis, has reported therapeutic success using it in porphyria cutanea tarda and other conditions. It has been shown to inhibit photocarcinogenesis in animal studies.
Dr. Siegel said that, while Dr. Ely has told him his preferred preparation is a German OTC product, milk thistle seeds can be found in health food stores, ground to a powder using a coffee bean grinder, and used as a food supplement. Like Polypodium leucotomos and nicotinamide, milk thistle is nontoxic.
Rapamycin
This macrolide compound is produced by the bacterium Streptomyces hygroscopicus. Rapamycin is an immunosuppressant used to coat coronary stents and prevent rejection of transplanted organs. It is an mechanistic target of rapamycin signaling pathway inhibitor being studied as a cancer prevention and antiaging agent.
Science magazine called the discovery that rapamycin increased the lifespan of mice one of the top scientific breakthroughs of 2009. Subsequent animal studies have established that the extended lifespan wasn’t solely the result of rapamycin’s antineoplastic effects but of across-the-board delayed onset of all the major age-related diseases. Thus, rapamycin could turn out to be a true antiaging agent, in Dr. Siegel’s view.
Studies in humans are underway. Researchers at Novartis have reported that a rapamycin-related compound curbed the typical decline in immune function that accompanies aging as reflected in a 20% enhancement in the response to influenza vaccine in elderly volunteers (Sci Transl Med. 2014 Dec 24;6[268]:268ra179).
Dr. Siegel reported serving as a consultant to Ferndale, which markets Heliocare. The SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Nicotinamide is one of the rare proposed agents for skin cancer chemoprevention distinguished by dirt cheap cost combined with a highly reassuring safety profile plus evidence of efficacy – which, together, make it a reasonable option in high risk patients, according to Daniel M. Siegel, MD.
Other agents that fit into that category include the tropical rainforest fern Polypodium leucotomos and milk thistle, added Dr. Siegel, a dermatologist at the State University of New York, Brooklyn.
“That’s a really interesting one. I don’t know if, 5 years from now, we’ll all be taking low-dose rapamycin as an antiaging drug, but we might, especially if someone figures out the ideal dose,” he said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
Nicotinamide
In the case of nicotinamide, the efficacy is actually supported by published level 1 evidence in the form of a highly positive 1-year, double-blind, randomized, placebo-controlled phase III clinical trial.
“You can Google ‘nicotinamide’ and find it at places like Costco and Trader Joe’s for less than 6 cents per day. That makes for a really good risk/benefit ratio. A nickel a day: That’s a cheap one. That’s one where I’d say, ‘Why not?’ It seems to be safe,” Dr. Siegel said.
In the phase III ONTRAC trial, Australian investigators randomized 386 patients who averaged roughly eight nonmelanoma skin cancers in the past 5 years to either 500 mg of oral nicotinamide twice daily or matched placebo for 12 months. During the study period, the nicotinamide group had a statistically significant and clinically meaningful 23% reduction in new nonmelanoma skin cancers, compared with the control group. They also had 13% fewer actinic keratoses at 12 months than controls. And the side effect profile mirrored that of placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26).
“Nicotinamide is vitamin B3. It’s not niacin. It doesn’t cause flushing and other vasodilatory effects. It’s actually pretty innocuous,” Dr. Siegel said.
In laboratory studies, nicotinamide has been shown to enhance DNA repair following UV exposure, as well as curb UV-induced immunosuppression.
Polypodium leucotomos Samambaia
This plant, commonly known as calaguala in the Spanish-speaking tropics and samambaia in Brazil, has a centuries-long tradition of safe medicinal use. It is commercially available over-the-counter (OTC) as a standardized product called Heliocare, designed to avoid the guesswork involved in topical sunscreen application. Each capsule contains 240 mg of an extract of P. leucotomos. Dr. Siegel said he takes it daily when he’s in a sunny locale, such as Hawaii.
Milk thistle
This plant, known as Silybum marianum, has silymarin as its bioactive compound. Dermatologist Haines Ely, MD, of the University of California, Davis, has reported therapeutic success using it in porphyria cutanea tarda and other conditions. It has been shown to inhibit photocarcinogenesis in animal studies.
Dr. Siegel said that, while Dr. Ely has told him his preferred preparation is a German OTC product, milk thistle seeds can be found in health food stores, ground to a powder using a coffee bean grinder, and used as a food supplement. Like Polypodium leucotomos and nicotinamide, milk thistle is nontoxic.
Rapamycin
This macrolide compound is produced by the bacterium Streptomyces hygroscopicus. Rapamycin is an immunosuppressant used to coat coronary stents and prevent rejection of transplanted organs. It is an mechanistic target of rapamycin signaling pathway inhibitor being studied as a cancer prevention and antiaging agent.
Science magazine called the discovery that rapamycin increased the lifespan of mice one of the top scientific breakthroughs of 2009. Subsequent animal studies have established that the extended lifespan wasn’t solely the result of rapamycin’s antineoplastic effects but of across-the-board delayed onset of all the major age-related diseases. Thus, rapamycin could turn out to be a true antiaging agent, in Dr. Siegel’s view.
Studies in humans are underway. Researchers at Novartis have reported that a rapamycin-related compound curbed the typical decline in immune function that accompanies aging as reflected in a 20% enhancement in the response to influenza vaccine in elderly volunteers (Sci Transl Med. 2014 Dec 24;6[268]:268ra179).
Dr. Siegel reported serving as a consultant to Ferndale, which markets Heliocare. The SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Nicotinamide is one of the rare proposed agents for skin cancer chemoprevention distinguished by dirt cheap cost combined with a highly reassuring safety profile plus evidence of efficacy – which, together, make it a reasonable option in high risk patients, according to Daniel M. Siegel, MD.
Other agents that fit into that category include the tropical rainforest fern Polypodium leucotomos and milk thistle, added Dr. Siegel, a dermatologist at the State University of New York, Brooklyn.
“That’s a really interesting one. I don’t know if, 5 years from now, we’ll all be taking low-dose rapamycin as an antiaging drug, but we might, especially if someone figures out the ideal dose,” he said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
Nicotinamide
In the case of nicotinamide, the efficacy is actually supported by published level 1 evidence in the form of a highly positive 1-year, double-blind, randomized, placebo-controlled phase III clinical trial.
“You can Google ‘nicotinamide’ and find it at places like Costco and Trader Joe’s for less than 6 cents per day. That makes for a really good risk/benefit ratio. A nickel a day: That’s a cheap one. That’s one where I’d say, ‘Why not?’ It seems to be safe,” Dr. Siegel said.
In the phase III ONTRAC trial, Australian investigators randomized 386 patients who averaged roughly eight nonmelanoma skin cancers in the past 5 years to either 500 mg of oral nicotinamide twice daily or matched placebo for 12 months. During the study period, the nicotinamide group had a statistically significant and clinically meaningful 23% reduction in new nonmelanoma skin cancers, compared with the control group. They also had 13% fewer actinic keratoses at 12 months than controls. And the side effect profile mirrored that of placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26).
“Nicotinamide is vitamin B3. It’s not niacin. It doesn’t cause flushing and other vasodilatory effects. It’s actually pretty innocuous,” Dr. Siegel said.
In laboratory studies, nicotinamide has been shown to enhance DNA repair following UV exposure, as well as curb UV-induced immunosuppression.
Polypodium leucotomos Samambaia
This plant, commonly known as calaguala in the Spanish-speaking tropics and samambaia in Brazil, has a centuries-long tradition of safe medicinal use. It is commercially available over-the-counter (OTC) as a standardized product called Heliocare, designed to avoid the guesswork involved in topical sunscreen application. Each capsule contains 240 mg of an extract of P. leucotomos. Dr. Siegel said he takes it daily when he’s in a sunny locale, such as Hawaii.
Milk thistle
This plant, known as Silybum marianum, has silymarin as its bioactive compound. Dermatologist Haines Ely, MD, of the University of California, Davis, has reported therapeutic success using it in porphyria cutanea tarda and other conditions. It has been shown to inhibit photocarcinogenesis in animal studies.
Dr. Siegel said that, while Dr. Ely has told him his preferred preparation is a German OTC product, milk thistle seeds can be found in health food stores, ground to a powder using a coffee bean grinder, and used as a food supplement. Like Polypodium leucotomos and nicotinamide, milk thistle is nontoxic.
Rapamycin
This macrolide compound is produced by the bacterium Streptomyces hygroscopicus. Rapamycin is an immunosuppressant used to coat coronary stents and prevent rejection of transplanted organs. It is an mechanistic target of rapamycin signaling pathway inhibitor being studied as a cancer prevention and antiaging agent.
Science magazine called the discovery that rapamycin increased the lifespan of mice one of the top scientific breakthroughs of 2009. Subsequent animal studies have established that the extended lifespan wasn’t solely the result of rapamycin’s antineoplastic effects but of across-the-board delayed onset of all the major age-related diseases. Thus, rapamycin could turn out to be a true antiaging agent, in Dr. Siegel’s view.
Studies in humans are underway. Researchers at Novartis have reported that a rapamycin-related compound curbed the typical decline in immune function that accompanies aging as reflected in a 20% enhancement in the response to influenza vaccine in elderly volunteers (Sci Transl Med. 2014 Dec 24;6[268]:268ra179).
Dr. Siegel reported serving as a consultant to Ferndale, which markets Heliocare. The SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
SLE linked to subsequent risk of malignant melanoma
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
AT SID 2017
Key clinical point: Compared with controls, patients with systemic lupus erythematosus (SLE) were at significantly increased risk of later being diagnosed with malignant melanoma.
Major finding: Ten patients with SLE (0.4%) were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), a statistically significant difference (P = .03).
Data source: Electronic medical record reviews of 2,351 patients with SLE and 1,676 patients with systemic sclerosis (controls) between 2000 and 2016.
Disclosures: The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
Total-Body Photography in Skin Cancer Screening: The Clinical Utility of Standardized Imaging
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Practice Points
- Advances in technology have the potential to provide affordable standardized total-body photography platforms.
- Total-body photography augments the clinical examination and plays a role in clinical decision-making.
- Total-body photography has the potential to become a part of the total-body skin examination and increase access to dermatologic care.