User login
Skin Cancer Mortality in Patients With Skin of Color
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
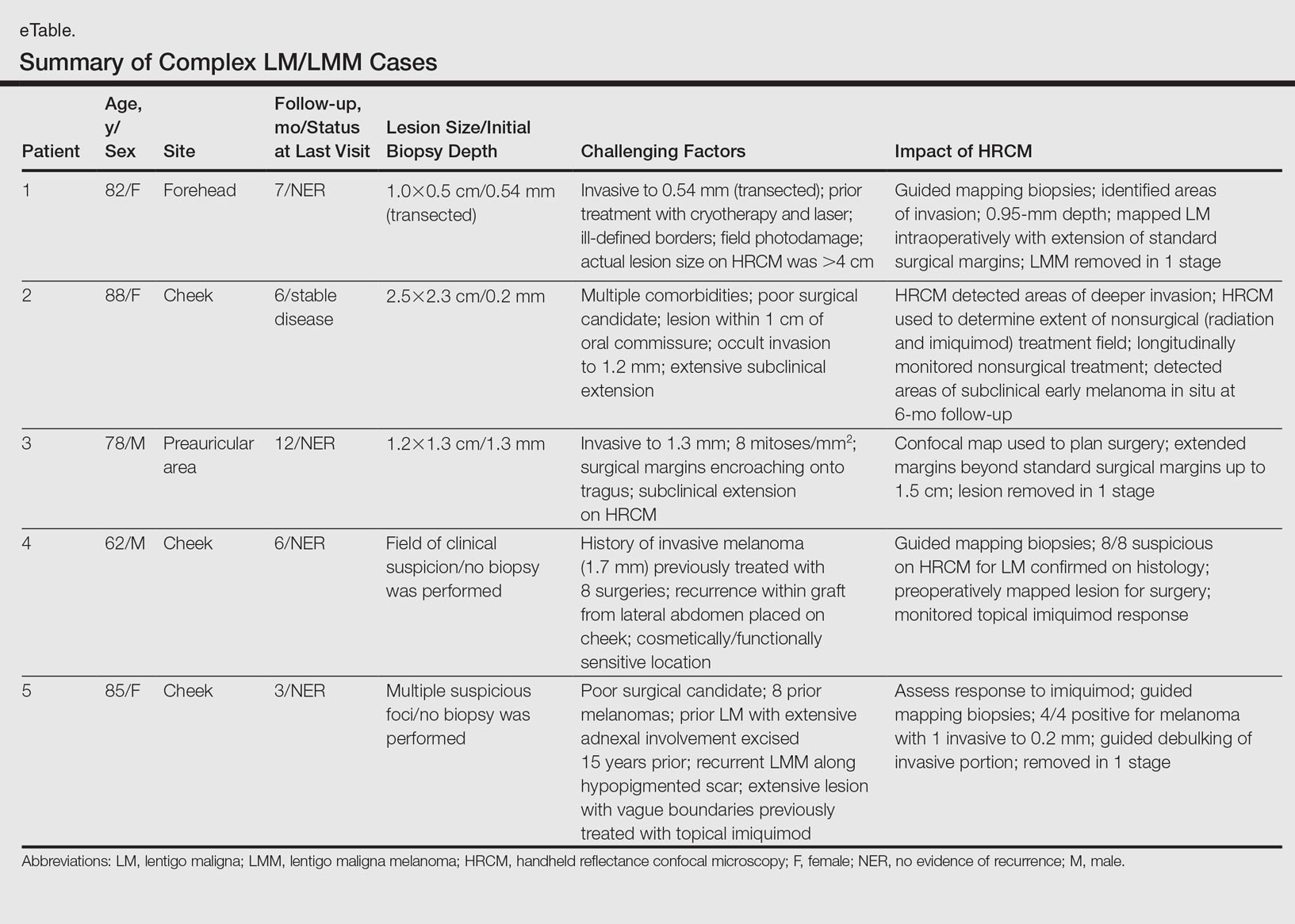
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
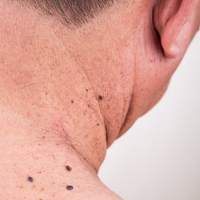
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
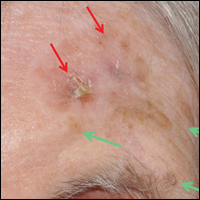
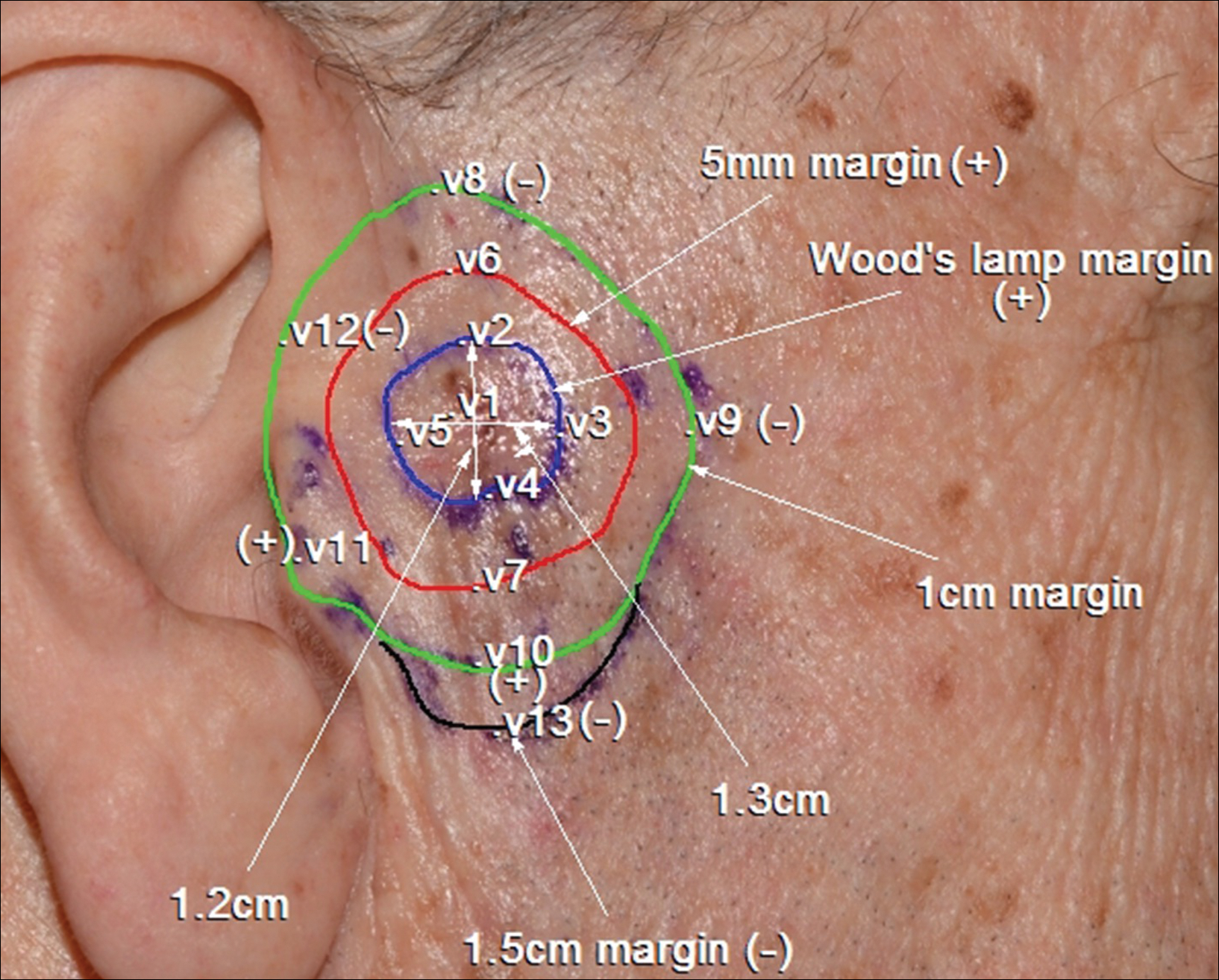
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.
Patient 4
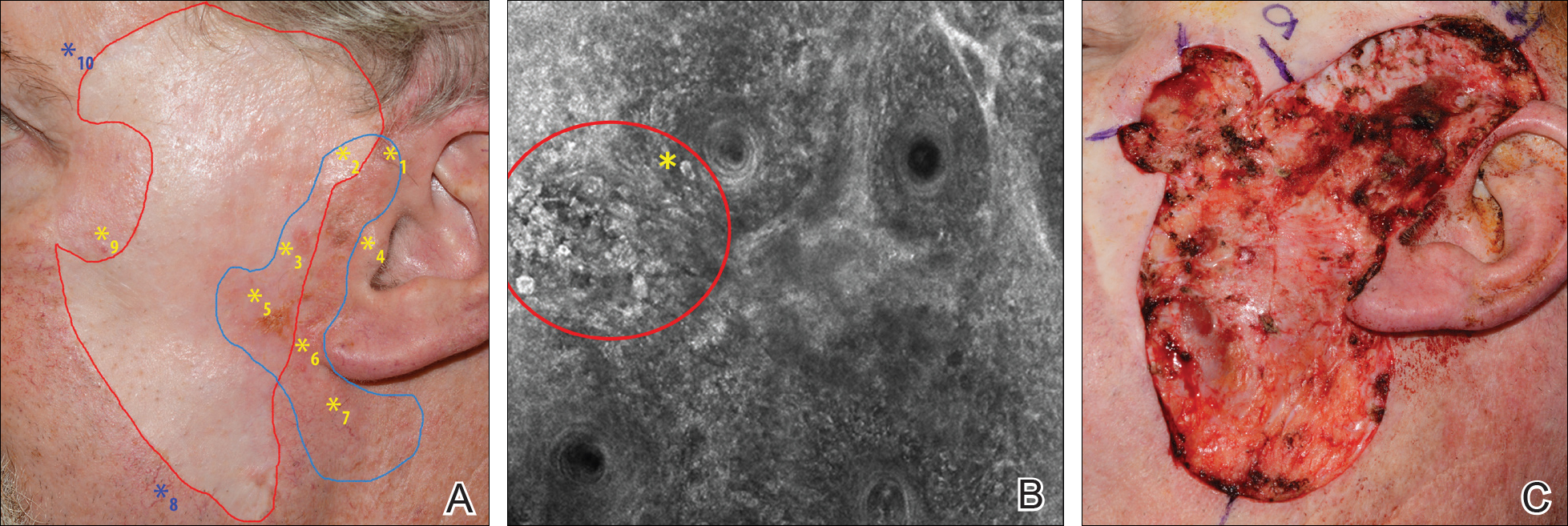
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.
Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Diagnosis and management of lentigo maligna (LM) and LM melanoma (LMM) is challenging due to their ill-defined margins and location mainly on the head and neck.
- Handheld reflectance confocal microscopy (RCM) has high diagnostic accuracy for LM/LMM and can be used in curved locations to assess large lesions.
- Handheld RCM can be a versatile tool in pretreatment decision-making, intraoperative surgical mapping, and posttreatment monitoring of both surgical and nonsurgical therapies for complex facial LM/LMM.
Study finds psoriasis link to melanoma and hematologic cancers
Patients with psoriasis may be at a greater risk of melanoma and hematologic cancers, compared with the general population, but treatments do not appear to increase risk, according to Shivani P. Reddy of the University of Illinois at Chicago, and associates.
In a retrospective cohort study, they identified 815,765 patients at Kaiser Permanente Southern California who had at least one medical encounter from January 2004 through December 2013. Of these patients, 8,161 (1%) met the diagnostic and inclusion criteria for psoriasis, and there were 7,167 (0.89%) cases of melanoma and 5,399 (0.66%) cases of lymphoma or leukemia.
Among the patients with psoriasis, there were 62 (0.87%) melanoma cases and 47 (0.87%) cases of lymphoma or leukemia.
Of the 109 patients with psoriasis who went on to develop melanoma or lymphoma, the time to diagnosis of melanoma or hematologic cancers was significantly less for patients with psoriasis than for patients without psoriasis (P = .01). The patients with psoriasis had a 1.53 times higher risk of developing a malignancy compared with patients without psoriasis (P less than .01) in the multivariable Cox proportional hazards model.
“There were no differences between patients with melanoma and hematologic cancer by treatment type,” which were phototherapy, tumor necrosis factor inhibitor therapy, and topical medications, they wrote.
“Our study demonstrates that the risk of hematologic cancer and melanoma is increased in patients with psoriasis over time, although this risk is not impacted by psoriasis therapies,” the researchers concluded. “Defining the risk of malignancy in these patients is important for proper workup and management.”
Read the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2016.09.047).
Patients with psoriasis may be at a greater risk of melanoma and hematologic cancers, compared with the general population, but treatments do not appear to increase risk, according to Shivani P. Reddy of the University of Illinois at Chicago, and associates.
In a retrospective cohort study, they identified 815,765 patients at Kaiser Permanente Southern California who had at least one medical encounter from January 2004 through December 2013. Of these patients, 8,161 (1%) met the diagnostic and inclusion criteria for psoriasis, and there were 7,167 (0.89%) cases of melanoma and 5,399 (0.66%) cases of lymphoma or leukemia.
Among the patients with psoriasis, there were 62 (0.87%) melanoma cases and 47 (0.87%) cases of lymphoma or leukemia.
Of the 109 patients with psoriasis who went on to develop melanoma or lymphoma, the time to diagnosis of melanoma or hematologic cancers was significantly less for patients with psoriasis than for patients without psoriasis (P = .01). The patients with psoriasis had a 1.53 times higher risk of developing a malignancy compared with patients without psoriasis (P less than .01) in the multivariable Cox proportional hazards model.
“There were no differences between patients with melanoma and hematologic cancer by treatment type,” which were phototherapy, tumor necrosis factor inhibitor therapy, and topical medications, they wrote.
“Our study demonstrates that the risk of hematologic cancer and melanoma is increased in patients with psoriasis over time, although this risk is not impacted by psoriasis therapies,” the researchers concluded. “Defining the risk of malignancy in these patients is important for proper workup and management.”
Read the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2016.09.047).
Patients with psoriasis may be at a greater risk of melanoma and hematologic cancers, compared with the general population, but treatments do not appear to increase risk, according to Shivani P. Reddy of the University of Illinois at Chicago, and associates.
In a retrospective cohort study, they identified 815,765 patients at Kaiser Permanente Southern California who had at least one medical encounter from January 2004 through December 2013. Of these patients, 8,161 (1%) met the diagnostic and inclusion criteria for psoriasis, and there were 7,167 (0.89%) cases of melanoma and 5,399 (0.66%) cases of lymphoma or leukemia.
Among the patients with psoriasis, there were 62 (0.87%) melanoma cases and 47 (0.87%) cases of lymphoma or leukemia.
Of the 109 patients with psoriasis who went on to develop melanoma or lymphoma, the time to diagnosis of melanoma or hematologic cancers was significantly less for patients with psoriasis than for patients without psoriasis (P = .01). The patients with psoriasis had a 1.53 times higher risk of developing a malignancy compared with patients without psoriasis (P less than .01) in the multivariable Cox proportional hazards model.
“There were no differences between patients with melanoma and hematologic cancer by treatment type,” which were phototherapy, tumor necrosis factor inhibitor therapy, and topical medications, they wrote.
“Our study demonstrates that the risk of hematologic cancer and melanoma is increased in patients with psoriasis over time, although this risk is not impacted by psoriasis therapies,” the researchers concluded. “Defining the risk of malignancy in these patients is important for proper workup and management.”
Read the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2016.09.047).
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Congenital melanocytic nevi in the very young tend to be benign
Histopathology findings of cytologic atypia, architectural disorder, and pagetoid spread are common in congenital melanocytic nevi (CMN) of all sizes in children aged 0-35 months, and tend to have benign outcomes, according to a retrospective study.
Emily A. Simons, MPH, and her associates at Boston Children’s Hospital studied 197 nevi in 179 patients with an average age of 14 months (range, 4 days to 35 months); 51% were female. Of those, 80% had skin types I-II, and 90% were white. The majority of the lesions involved the head or trunk and were predominantly medium in size, and 58% had a projected adult size of 1.5-10 cm. The study was retrospective; cases had been diagnosed between 1993 and 2013.
Cytologic atypia, architectural disorder, and pagetoid spread were the most frequent features – they were present in 73% of nevi and were closely associated. Combined histologic patterns of a blue nevus, spindle and Spitz nevus, or a deep penetrating nevus were identified in 40% of CMN. Proliferative nodules occurred in 5% of nevi.
Clinical outcomes were available for 130 patients, including 26 with large CMN and 8 with proliferative nodules. The children were alive and had not been diagnosed with melanoma at a mean follow-up of a mean of 8.4 years (range, 7 months to 21.3 years), even though margins of the last excision were positive in 41% of all CMN and in 77% of large CMN.
Malignant transformation of CMN certainly should be recognized, but the morbidity of overdiagnosis also needs to be considered. “Excision of larger CMN might require serial excisions under general anesthesia, the use of tissue expanders, and grafts,” Dr. Simons and her associates said.
Among the limitations of this study were that the majority of patients were white, so the results may not translate to children with darker skin types, they noted.
“The diagnosis of malignant melanoma should be made with great caution in this population,” they concluded, pointing out that the histopathologic features alone (cytologic atypia, architectural disorder, and pagetoid spread) “should not be interpreted as evidence for potential malignant behavior or serve as grounds for further excision.”
Read more in the Journal of the American Academy of Dermatology (2017 May;76[5]941-7).
The authors had no relevant financial disorders.
Histopathology findings of cytologic atypia, architectural disorder, and pagetoid spread are common in congenital melanocytic nevi (CMN) of all sizes in children aged 0-35 months, and tend to have benign outcomes, according to a retrospective study.
Emily A. Simons, MPH, and her associates at Boston Children’s Hospital studied 197 nevi in 179 patients with an average age of 14 months (range, 4 days to 35 months); 51% were female. Of those, 80% had skin types I-II, and 90% were white. The majority of the lesions involved the head or trunk and were predominantly medium in size, and 58% had a projected adult size of 1.5-10 cm. The study was retrospective; cases had been diagnosed between 1993 and 2013.
Cytologic atypia, architectural disorder, and pagetoid spread were the most frequent features – they were present in 73% of nevi and were closely associated. Combined histologic patterns of a blue nevus, spindle and Spitz nevus, or a deep penetrating nevus were identified in 40% of CMN. Proliferative nodules occurred in 5% of nevi.
Clinical outcomes were available for 130 patients, including 26 with large CMN and 8 with proliferative nodules. The children were alive and had not been diagnosed with melanoma at a mean follow-up of a mean of 8.4 years (range, 7 months to 21.3 years), even though margins of the last excision were positive in 41% of all CMN and in 77% of large CMN.
Malignant transformation of CMN certainly should be recognized, but the morbidity of overdiagnosis also needs to be considered. “Excision of larger CMN might require serial excisions under general anesthesia, the use of tissue expanders, and grafts,” Dr. Simons and her associates said.
Among the limitations of this study were that the majority of patients were white, so the results may not translate to children with darker skin types, they noted.
“The diagnosis of malignant melanoma should be made with great caution in this population,” they concluded, pointing out that the histopathologic features alone (cytologic atypia, architectural disorder, and pagetoid spread) “should not be interpreted as evidence for potential malignant behavior or serve as grounds for further excision.”
Read more in the Journal of the American Academy of Dermatology (2017 May;76[5]941-7).
The authors had no relevant financial disorders.
Histopathology findings of cytologic atypia, architectural disorder, and pagetoid spread are common in congenital melanocytic nevi (CMN) of all sizes in children aged 0-35 months, and tend to have benign outcomes, according to a retrospective study.
Emily A. Simons, MPH, and her associates at Boston Children’s Hospital studied 197 nevi in 179 patients with an average age of 14 months (range, 4 days to 35 months); 51% were female. Of those, 80% had skin types I-II, and 90% were white. The majority of the lesions involved the head or trunk and were predominantly medium in size, and 58% had a projected adult size of 1.5-10 cm. The study was retrospective; cases had been diagnosed between 1993 and 2013.
Cytologic atypia, architectural disorder, and pagetoid spread were the most frequent features – they were present in 73% of nevi and were closely associated. Combined histologic patterns of a blue nevus, spindle and Spitz nevus, or a deep penetrating nevus were identified in 40% of CMN. Proliferative nodules occurred in 5% of nevi.
Clinical outcomes were available for 130 patients, including 26 with large CMN and 8 with proliferative nodules. The children were alive and had not been diagnosed with melanoma at a mean follow-up of a mean of 8.4 years (range, 7 months to 21.3 years), even though margins of the last excision were positive in 41% of all CMN and in 77% of large CMN.
Malignant transformation of CMN certainly should be recognized, but the morbidity of overdiagnosis also needs to be considered. “Excision of larger CMN might require serial excisions under general anesthesia, the use of tissue expanders, and grafts,” Dr. Simons and her associates said.
Among the limitations of this study were that the majority of patients were white, so the results may not translate to children with darker skin types, they noted.
“The diagnosis of malignant melanoma should be made with great caution in this population,” they concluded, pointing out that the histopathologic features alone (cytologic atypia, architectural disorder, and pagetoid spread) “should not be interpreted as evidence for potential malignant behavior or serve as grounds for further excision.”
Read more in the Journal of the American Academy of Dermatology (2017 May;76[5]941-7).
The authors had no relevant financial disorders.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
LGBT Access to Health Care: A Dermatologist’s Role in Building a Therapeutic Relationship
The last decade has been a period of advancement for the lesbian, gay, bisexual, and transgender (LGBT) community for legal protections and visibility. Although the journey to acceptance and equality is far from over, this progress has appropriately extended to medical academia as physicians search for ways to become more inclusive and effective care providers for their LGBT patients.1 In a recent cross-sectional study, Ginsberg et al2 examined the role for dermatologists in the care of transgender patients. The investigators concluded that dermatologists should play a larger role in a transgender patient’s physical transformation.2 It is our opinion that dermatologists need to be comfortable building rapport with LGBT patients and to become attuned to their specific needs to provide effective care.
When forging a relationship with an LGBT patient, assumptions can damage rapport. Two assumptions that should be avoided include presuming heterosexuality or, on the other hand, assuming risk for disease based on known LGBT status. A dermatologist who takes a cursory sexual history, or none at all, assuming his/her patient is heterosexual creates an environment in which a nonheterosexual patient feels uncomfortable being honest and open. Although there is enough literature to support the claim that some sexual minority groups have increased risk for sexually transmitted infections (STIs),3 it is dangerous to assume a patient’s risk based solely on sexual orientation. An abstinent patient or a patient in a long-term, monogamous, same-sex relationship, for instance, may feel stereotyped by a dermatologist who wants to screen him/her for an STI. The best step in building a therapeutic relationship is to cast out these assumptions and allow LGBT patients to be open about themselves and their sexual practices. Sexual histories should be asked in nonjudgmental ways that are related to the health of the patient, leading to relevant and useful information for their care. For example, ask patients, “Do you have sex with men, women, or both?” This question should be delivered in a matter-of-fact tone, which conveys to the patient that the provider merely wants an answer to guide patient care.
Dermatologists can tailor their encounters to the specific needs of sexual minority patients. The medical literature is rich with examples of conditions that occur at greater frequency in specific sexual minority groups. Sexually transmitted infections, particularly human immunodeficiency virus, are important causes of morbidity and mortality among sexual minorities, especially men who have sex with men (MSM).3,4 Anal and penile human papillomavirus (HPV) infection and HPV-associated anal carcinoma risk are increased in MSM.5,6 The literature has remained inconclusive on the use of anal Papanicolaou tests for diagnosis; however, dermatologists have a duty to at least examine the perianal and genital area of any patient at risk for HPV-related disease or STIs.7,8 For younger patients, the HPV vaccine can help prevent certain types of HPV infection and likely reduce a patient’s risk for condyloma acuminatum and other sequelae of the virus. Guidelines have been expanded to include men aged 13 to 21 years and up to 26 years.9 More research is needed to determine if detection and prevention of these types of HPV infection using the vaccine in MSM actually leads to a decreased incidence of anal carcinoma.
Certain LGBT groups may benefit from a dermatologist’s care outside the realm of infectious diseases. One study found that increased indoor tanning use in MSM correlated with increased risk for nonmelanoma skin cancer.10 Lesbians have been found to be less likely to pursue preventative health examinations in general, including skin checks.11 Finally, transgender patients can utilize dermatologists for help with transformative procedures and side effects of hormonal treatment such as androgenic acne.1,4
Cutaneous and beyond, the future of LGBT health care in the United States is affected by the institutions that train future physicians. There is a trend toward incorporating formal LGBT curricula into medical schools and academic centers.12 The Penn Medicine Program for LGBT Health (Philadelphia, Pennsylvania) is a pilot program geared toward both educating future clinicians and providing equal and unbiased care to LGBT patients.12 Programs such as this one give rise to a new generation of physicians who feel comfortable and aware of the needs of their LGBT patients.
In a time when LGBT patients are becoming more comfortable claiming their sexual and gender identities openly, there is a need for dermatologists to provide individualized unbiased care, which can best be achieved by building rapport through assumption-free history taking, performing thorough physical examinations that include the genital and perianal area, and passing these good practices on to trainees.
- Snyder JE. Trend analysis of medical publications about LGBT persons: 1950-2007. J Homosex. 2011;58:164-188.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community. J Am Acad Dermatol. 2016;74:303-308.
- Gee R. Primary care health issues among men who have sex with men. J Am Acad Nurse Pract. 2006;18:144-153.
- Katz KA, Furnish TJ. Dermatology-related epidemiologic and clinical concerns of men who have sex with men, women who have sex with women, and transgender individuals. Arch Dermatol. 2005;141:1303-1310.
- Fenkl EA, Jones SG, Schochet E, et al. HPV and anal cancer knowledge among HIV-infected and non-infected men who have sex with men [published online December 11, 2015]. LGBT Health. 2016;3:42-48. doi:10.1089/lgbt.2015.0086.
- Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE Study. J Natl Cancer Inst. 2005;97:896-905.
- Schofield AM, Sadler L, Nelson L, et al. A prospective study of anal cancer screening in HIV-positive and negative MSM. AIDS. 2016;30:1375-1383.
- Katz MH, Katz KA, Bernestein KT, et al. We need data on anal screening effectiveness before focusing on increasing it [published online September 23, 2010]. Am J Public Health. 2010;100:2016.
- Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-Valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300-304.
- Mansh M, Katz KA, Linos E, et al. Association of skin cancer and indoor tanning in sexual minority men and women. JAMA Dermatol. 2015;151:1308-1316.
- Conron KJ, Mimiaga MJ, Landers SJ. A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health. 2010;100:1953-1960.
- Yehia BR, Calder D, Flesch JD, et al. Advancing LGBT health at an academic medical center: a case study. LGBT Health. 2015;2:362-366.
The last decade has been a period of advancement for the lesbian, gay, bisexual, and transgender (LGBT) community for legal protections and visibility. Although the journey to acceptance and equality is far from over, this progress has appropriately extended to medical academia as physicians search for ways to become more inclusive and effective care providers for their LGBT patients.1 In a recent cross-sectional study, Ginsberg et al2 examined the role for dermatologists in the care of transgender patients. The investigators concluded that dermatologists should play a larger role in a transgender patient’s physical transformation.2 It is our opinion that dermatologists need to be comfortable building rapport with LGBT patients and to become attuned to their specific needs to provide effective care.
When forging a relationship with an LGBT patient, assumptions can damage rapport. Two assumptions that should be avoided include presuming heterosexuality or, on the other hand, assuming risk for disease based on known LGBT status. A dermatologist who takes a cursory sexual history, or none at all, assuming his/her patient is heterosexual creates an environment in which a nonheterosexual patient feels uncomfortable being honest and open. Although there is enough literature to support the claim that some sexual minority groups have increased risk for sexually transmitted infections (STIs),3 it is dangerous to assume a patient’s risk based solely on sexual orientation. An abstinent patient or a patient in a long-term, monogamous, same-sex relationship, for instance, may feel stereotyped by a dermatologist who wants to screen him/her for an STI. The best step in building a therapeutic relationship is to cast out these assumptions and allow LGBT patients to be open about themselves and their sexual practices. Sexual histories should be asked in nonjudgmental ways that are related to the health of the patient, leading to relevant and useful information for their care. For example, ask patients, “Do you have sex with men, women, or both?” This question should be delivered in a matter-of-fact tone, which conveys to the patient that the provider merely wants an answer to guide patient care.
Dermatologists can tailor their encounters to the specific needs of sexual minority patients. The medical literature is rich with examples of conditions that occur at greater frequency in specific sexual minority groups. Sexually transmitted infections, particularly human immunodeficiency virus, are important causes of morbidity and mortality among sexual minorities, especially men who have sex with men (MSM).3,4 Anal and penile human papillomavirus (HPV) infection and HPV-associated anal carcinoma risk are increased in MSM.5,6 The literature has remained inconclusive on the use of anal Papanicolaou tests for diagnosis; however, dermatologists have a duty to at least examine the perianal and genital area of any patient at risk for HPV-related disease or STIs.7,8 For younger patients, the HPV vaccine can help prevent certain types of HPV infection and likely reduce a patient’s risk for condyloma acuminatum and other sequelae of the virus. Guidelines have been expanded to include men aged 13 to 21 years and up to 26 years.9 More research is needed to determine if detection and prevention of these types of HPV infection using the vaccine in MSM actually leads to a decreased incidence of anal carcinoma.
Certain LGBT groups may benefit from a dermatologist’s care outside the realm of infectious diseases. One study found that increased indoor tanning use in MSM correlated with increased risk for nonmelanoma skin cancer.10 Lesbians have been found to be less likely to pursue preventative health examinations in general, including skin checks.11 Finally, transgender patients can utilize dermatologists for help with transformative procedures and side effects of hormonal treatment such as androgenic acne.1,4
Cutaneous and beyond, the future of LGBT health care in the United States is affected by the institutions that train future physicians. There is a trend toward incorporating formal LGBT curricula into medical schools and academic centers.12 The Penn Medicine Program for LGBT Health (Philadelphia, Pennsylvania) is a pilot program geared toward both educating future clinicians and providing equal and unbiased care to LGBT patients.12 Programs such as this one give rise to a new generation of physicians who feel comfortable and aware of the needs of their LGBT patients.
In a time when LGBT patients are becoming more comfortable claiming their sexual and gender identities openly, there is a need for dermatologists to provide individualized unbiased care, which can best be achieved by building rapport through assumption-free history taking, performing thorough physical examinations that include the genital and perianal area, and passing these good practices on to trainees.
The last decade has been a period of advancement for the lesbian, gay, bisexual, and transgender (LGBT) community for legal protections and visibility. Although the journey to acceptance and equality is far from over, this progress has appropriately extended to medical academia as physicians search for ways to become more inclusive and effective care providers for their LGBT patients.1 In a recent cross-sectional study, Ginsberg et al2 examined the role for dermatologists in the care of transgender patients. The investigators concluded that dermatologists should play a larger role in a transgender patient’s physical transformation.2 It is our opinion that dermatologists need to be comfortable building rapport with LGBT patients and to become attuned to their specific needs to provide effective care.
When forging a relationship with an LGBT patient, assumptions can damage rapport. Two assumptions that should be avoided include presuming heterosexuality or, on the other hand, assuming risk for disease based on known LGBT status. A dermatologist who takes a cursory sexual history, or none at all, assuming his/her patient is heterosexual creates an environment in which a nonheterosexual patient feels uncomfortable being honest and open. Although there is enough literature to support the claim that some sexual minority groups have increased risk for sexually transmitted infections (STIs),3 it is dangerous to assume a patient’s risk based solely on sexual orientation. An abstinent patient or a patient in a long-term, monogamous, same-sex relationship, for instance, may feel stereotyped by a dermatologist who wants to screen him/her for an STI. The best step in building a therapeutic relationship is to cast out these assumptions and allow LGBT patients to be open about themselves and their sexual practices. Sexual histories should be asked in nonjudgmental ways that are related to the health of the patient, leading to relevant and useful information for their care. For example, ask patients, “Do you have sex with men, women, or both?” This question should be delivered in a matter-of-fact tone, which conveys to the patient that the provider merely wants an answer to guide patient care.
Dermatologists can tailor their encounters to the specific needs of sexual minority patients. The medical literature is rich with examples of conditions that occur at greater frequency in specific sexual minority groups. Sexually transmitted infections, particularly human immunodeficiency virus, are important causes of morbidity and mortality among sexual minorities, especially men who have sex with men (MSM).3,4 Anal and penile human papillomavirus (HPV) infection and HPV-associated anal carcinoma risk are increased in MSM.5,6 The literature has remained inconclusive on the use of anal Papanicolaou tests for diagnosis; however, dermatologists have a duty to at least examine the perianal and genital area of any patient at risk for HPV-related disease or STIs.7,8 For younger patients, the HPV vaccine can help prevent certain types of HPV infection and likely reduce a patient’s risk for condyloma acuminatum and other sequelae of the virus. Guidelines have been expanded to include men aged 13 to 21 years and up to 26 years.9 More research is needed to determine if detection and prevention of these types of HPV infection using the vaccine in MSM actually leads to a decreased incidence of anal carcinoma.
Certain LGBT groups may benefit from a dermatologist’s care outside the realm of infectious diseases. One study found that increased indoor tanning use in MSM correlated with increased risk for nonmelanoma skin cancer.10 Lesbians have been found to be less likely to pursue preventative health examinations in general, including skin checks.11 Finally, transgender patients can utilize dermatologists for help with transformative procedures and side effects of hormonal treatment such as androgenic acne.1,4
Cutaneous and beyond, the future of LGBT health care in the United States is affected by the institutions that train future physicians. There is a trend toward incorporating formal LGBT curricula into medical schools and academic centers.12 The Penn Medicine Program for LGBT Health (Philadelphia, Pennsylvania) is a pilot program geared toward both educating future clinicians and providing equal and unbiased care to LGBT patients.12 Programs such as this one give rise to a new generation of physicians who feel comfortable and aware of the needs of their LGBT patients.
In a time when LGBT patients are becoming more comfortable claiming their sexual and gender identities openly, there is a need for dermatologists to provide individualized unbiased care, which can best be achieved by building rapport through assumption-free history taking, performing thorough physical examinations that include the genital and perianal area, and passing these good practices on to trainees.
- Snyder JE. Trend analysis of medical publications about LGBT persons: 1950-2007. J Homosex. 2011;58:164-188.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community. J Am Acad Dermatol. 2016;74:303-308.
- Gee R. Primary care health issues among men who have sex with men. J Am Acad Nurse Pract. 2006;18:144-153.
- Katz KA, Furnish TJ. Dermatology-related epidemiologic and clinical concerns of men who have sex with men, women who have sex with women, and transgender individuals. Arch Dermatol. 2005;141:1303-1310.
- Fenkl EA, Jones SG, Schochet E, et al. HPV and anal cancer knowledge among HIV-infected and non-infected men who have sex with men [published online December 11, 2015]. LGBT Health. 2016;3:42-48. doi:10.1089/lgbt.2015.0086.
- Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE Study. J Natl Cancer Inst. 2005;97:896-905.
- Schofield AM, Sadler L, Nelson L, et al. A prospective study of anal cancer screening in HIV-positive and negative MSM. AIDS. 2016;30:1375-1383.
- Katz MH, Katz KA, Bernestein KT, et al. We need data on anal screening effectiveness before focusing on increasing it [published online September 23, 2010]. Am J Public Health. 2010;100:2016.
- Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-Valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300-304.
- Mansh M, Katz KA, Linos E, et al. Association of skin cancer and indoor tanning in sexual minority men and women. JAMA Dermatol. 2015;151:1308-1316.
- Conron KJ, Mimiaga MJ, Landers SJ. A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health. 2010;100:1953-1960.
- Yehia BR, Calder D, Flesch JD, et al. Advancing LGBT health at an academic medical center: a case study. LGBT Health. 2015;2:362-366.
- Snyder JE. Trend analysis of medical publications about LGBT persons: 1950-2007. J Homosex. 2011;58:164-188.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community. J Am Acad Dermatol. 2016;74:303-308.
- Gee R. Primary care health issues among men who have sex with men. J Am Acad Nurse Pract. 2006;18:144-153.
- Katz KA, Furnish TJ. Dermatology-related epidemiologic and clinical concerns of men who have sex with men, women who have sex with women, and transgender individuals. Arch Dermatol. 2005;141:1303-1310.
- Fenkl EA, Jones SG, Schochet E, et al. HPV and anal cancer knowledge among HIV-infected and non-infected men who have sex with men [published online December 11, 2015]. LGBT Health. 2016;3:42-48. doi:10.1089/lgbt.2015.0086.
- Chin-Hong PV, Vittinghoff E, Cranston RD, et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE Study. J Natl Cancer Inst. 2005;97:896-905.
- Schofield AM, Sadler L, Nelson L, et al. A prospective study of anal cancer screening in HIV-positive and negative MSM. AIDS. 2016;30:1375-1383.
- Katz MH, Katz KA, Bernestein KT, et al. We need data on anal screening effectiveness before focusing on increasing it [published online September 23, 2010]. Am J Public Health. 2010;100:2016.
- Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-Valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300-304.
- Mansh M, Katz KA, Linos E, et al. Association of skin cancer and indoor tanning in sexual minority men and women. JAMA Dermatol. 2015;151:1308-1316.
- Conron KJ, Mimiaga MJ, Landers SJ. A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health. 2010;100:1953-1960.
- Yehia BR, Calder D, Flesch JD, et al. Advancing LGBT health at an academic medical center: a case study. LGBT Health. 2015;2:362-366.
Get ready for cancer immunotherapy-induced rheumatic diseases
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Systemic Therapy in Metastatic Melanoma
Melanoma is the most aggressive form of skin cancer, contributing to about 76,000 new cases and more than 9,000 deaths in 2014.1 Depending on the stage of the disease, 5-year melanoma survival can range from 15% to 97%. Patients with local and distant metastases have a 5-year survival of about 60% and 15%, respectively.2
The incidence of melanoma is rising, partly because of the increasing number of skin biopsies being performed.3 If melanoma is diagnosed early, surgical excision is the treatment of choice. In patients with oligometastatic disease (cancer that has spread, but only to 1 or a small number of sites), complete surgical excision of the metastases may provide prolonged overall survival (OS) and delay the need to use systemic therapy.4
Recently, many new drug therapies have shown promising results in clinical trials, which may improve the prognosis of metastatic disease. This article reviews currently available systemic treatment options for the management of metastatic melanoma, the role of cytotoxic chemotherapy and interleukin-2 (IL-2), and the latest therapies available, including immune checkpoint inhibitors.
Cytotoxic Chemotherapy and Interleukin-2
Cytotoxic chemotherapy does not have an established role in the initial treatment of metastatic melanoma. Currently, cytotoxic chemotherapy is used in patients who have not responded to immunotherapy or molecular targeted therapy. The most commonly used drugs include dacarbazine and its prodrug, temozolomide. Several studies have failed to demonstrate a survival benefit using a single-agent chemotherapy with either dacarbazine or temozolomide.5,6
Other agents used in metastatic melanoma include nitrosoureas (fotemustine), platinum compounds (cisplatin, carboplatin), vinca alkaloids (vincristine),
and taxanes (paclitaxel). None of these agents provide a survival benefit, but an objective response may be seen in a minority of cases. Combination chemotherapy regimens have not shown an advantage over singleagent dacarbazine or temozolomide.7,8
High-dose IL-2 has been used in cases of metastatic melanoma with good performance status (PS) and organ function. Studies have shown a complete response rate of 3% to 7% and a prolonged disease-free survival in a minority of patients.9-11 The use of highdose IL-2, however, is limited by the high incidence of adverse effects (AEs), which include bacterial sepsis, pulmonary edema, arrhythmias, fever, and on some occasions, death due to complications.10 The use of IL-2 requires admission of the patient to a specialized unit for AE monitoring and management. Because of its ability to “cure” a minority of patients, a role still exists for IL-2 therapy in the treatment of younger, healthy patients with no evidence of organ dysfunction at baseline.
Immune Checkpoint Inhibitors
Checkpoint inhibitors are a class of drugs that unmask the immune system to fight against cancer cells. This class of drugs has shown significant activity and survival advantage in recent phase 2 and 3 trials. The class includes the anticytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab and monoclonal antibodies targeting the programmed death 1 protein (PD-1) or its ligand (PD-L1).
Anti-CTLA-4 Antibodies: Ipilimumab
Cytotoxic T-lymphocyte antigen 4 is the antigen responsible for inhibition of cytotoxic T-cell-mediated immunity against foreign antigens presented by the antigen presenting cells (APCs). The APCs cause activation of the T cells when peptide fragments of intracellular proteins are presented in combination with mixed histocompatibility complex molecules. This step requires interaction of a costimulatory molecule (B7) on the APCs with a cluster of differentiation 28 protein (CD28) receptor located on T cells. CTLA-4 competes with CD28 to bind with the B7 molecule, thereby inhibiting the activation of the cytotoxic T cells (Figure 1). This pathway is thought to help with development of tolerance to host tissue antigens. Ipilimumab is a human monoclonal antibody that inhibits this CTLA-4 molecule and facilitates T-cell mediated antitumor activity.12 By blocking the CTLA-4 molecule, ipilimumab also mediates its autoimmune AEs on the host tissues.
Hodi and colleagues conducted a phase 3 trial of ipilimumab, including 676 patients who progressed after prior treatment for stage III or IV melanoma, and found that median OS was significantly better in the ipilimumab groups: 10 months in the ipilimumab plus gp100 peptide vaccine group vs 6.4 months in the gp100 vaccine alone group; 10.1 months in the ipilimumab alone group vs 6.4 months in the gp100 vaccine alone group.13 In another phase 3 trial comparing ipilimumab plus dacarbazine to dacarbazine alone, the ipilimumab group had a significantly improved OS (11.2 months vs 9.1 months).1 Survival rates with ipilimumab were prolonged for up to 3 years compared with the dacarbazine plus placebo group. However, the combination was associated with increased incidence of hepatotoxicity, thereby limiting its use.
A long-term survival analysis of 10 prospective and 2 retrospective studies of ipilimumab showed a median OS of 11.4 months and a long-term survival that began at 3 years with a plateau at 10 years of 21%, which was independent of prior therapy or ipilimumab dose.14 The immune-related AEs of ipilimumab are secondary to its activity against the host antigens and include dermatitis, enterocolitis, hepatitis, and endocrinopathies.15
A recent phase 2 trial studied the combination of ipilimumab with granulocyte-macrophage colonystimulating factor in 245 patients with stage III and IV melanoma. Median OS after 13 months was significantly higher with the combination compared with ipilimumab alone. The 1-year survival rate was 69% with
the combination and 53% with ipilimumab alone. There was no difference in the overall response rate (ORR) or progression-free survival (PFS) between the 2 groups. However, the AEs were significantly reduced with the combination (45% vs 58%).16 The dose of ipilimumab used in the trial was higher than the approved dose, making it difficult to apply the results in practice without further studies on the combination.
Anti-PD-1 Antibodies
Programmed death 1 ligands (PD-L1 and PD-L2) are expressed by tumor or stromal cells to inhibit the T-cell mediated antitumor activity. These ligands bind to the PD-1 protein on the surface of activated T cells to mediate their immunosuppressive effects. Interruption of this interaction by either anti-PD-1 antibodies or anti-PD-L1 antibodies facilitates tumor cell killing by activated T cells.17
Pembrozilumab and nivolumab are the 2 anti-PD-1 monoclonal antibodies that have been approved for treatment of metastatic melanoma. In a phase 1 trial
of pembrolizumab, 411 patients with advanced melanoma (consisting of both ipilimumab-naïve [IPI-N] and ipilimumab-treated [IPI-T] patients), ORR was 40% in IPI-N and 28% in IPI-T patients with a 1-year OS of 71% in all patients. Median PFS was 24 weeks in IPI-N and 23 weeks in IPI-T pts.18 There was no difference in outcomes and safety profiles across the various dosing regimens.18,19 Of note, pembrolizumab had antitumor activity irrespective of the PS, lactate dehydrogenase levels, BRAF (B-Raf proto-oncogene, serine/threonine kinase) gene mutation, metastatic stage, and number and type of prior therapy. In a subgroup analysis, 173 patients who had progression after treatment with ipilimumab were randomly assigned to pembrolizumab 2 mg/kg every 3 weeks (q3w) or 10 mg/kg q3w dosing regimens. Both groups had no significant difference in the ORR (26% in both) and safety profiles.20
In the 2012 KEYNOTE-002 clinical trial, a randomized phase 2 trial involving 540 patients with ipilimumab-refractory advanced melanoma, patients were randomized 1:1:1 to pembrolizumab 2 mg/kg or 10 mg/kg q3w or investigator-choice chemotherapy (control arm consisting of carboplatin plus paclitaxel, carboplatin, paclitaxel, dacarbazine, or temozolomide). The 6-month PFS was significantly improved with pembrolizumab (34% and 38% for pembrolizumab 2 mg/kg and 10 mg/kg, respectively) compared with 16% with chemotherapy. The ORR was significantly better with pembrolizumab (21% at 2 mg/kg, 25% at 10 mg/kg) compared with the control arm (4%).21 These findings led to the approval of pembrolizumab by the FDA for treatment of patients with advanced melanoma who have progressed on ipilimumab. Pembrolizumab is generally well tolerated. The most common AEs include fatigue, pruritus, and rash.
Nivolumab was studied in a recent phase 1 trial in which 107 patients with previously treated advanced melanoma were treated with escalated doses every
2 weeks.22 The 2-year and 3-year OS rates were 48% and 41%, respectively. Objective responses were seen in 32% of the patients. The median response duration was 23 months.23
The first phase 3 trial was conducted in 418 patients with previously untreated metastatic melanoma BRAF mutation. Patients were randomized to receive either nivolumab or dacarbazine. The PFS and OS were significantly better with nivolumab compared with dacarbazine (PFS 5.1 months vs 2.2 months; OS 73% vs 42% at 1 year).24 The AE profile of nivolumab is similar to pembrolizumab and includes lung, skin, endocrine, renal, and gastrointestinal tract toxicities.
Preliminary results of another phase 3 trial were presented at the European Society of Medical Oncology 2014 meeting. Patients with previously treated metastatic melanoma (ipilimumab or BRAF inhibitor) were randomized in a 2:1 ratio to receive either nivolumab or investigators’ choice chemotherapy (dacarbazine or carboplatin plus paclitaxel). The ORR was significantly better with nivolumab (32% vs 11%), and 95% of patients were still responding after 6 months. The nivolumab group showed a complete remission in 3% of the patients with 34% of the responses lasting ≥ 6 months.25 This led to the recent approval of nivolumab for patients with metastatic melanoma with a BRAF mutation who have advanced on ipilimumab. In the phase 3 NCT01844505 trial patients are being randomized to receive ipilimumab, nivolumab, or both.
A newer PD-1 inhibitor, pidilizumab, was studied in a phase 2 trial that included 103 patients with metastatic melanoma, 51% of whom had received therapy with ipilimumab. The ORR in the study group was relatively lower (6%), but the OS at 1 year was 64.5%.26 Further studies are underway to evaluate the role of this drug in metastatic melanoma.
The response with both nivolumab and pembrolizumab is durable as well as sustained, even after discontinuation of therapy. None of the deaths in the aforementioned studies were atributed to drug-related toxicities. As evidenced by current data, these 2 drugs hold a great promise for the management of patients who progress after therapy with anti-CTLA-4 antibodies.
Anti-PD-L1 Antibodies
The anti-PD-L1 monoclonal antibodies work in a similar way to the PD-1 inhibitors and block the interaction between the PD-1 and its ligand, PD-L1. This causes sustained activation of cytotoxic T cells and facilitates their antitumor activity. Two of PD-L1 inhibitors have shown clinical activity against metastatic melanoma.
BMS-936559, the first PD-L1 antibody, is being studied in a phase 1 trial that includes 55 patients with advanced melanoma along with 152 patients with other solid malignancies. Three patients achieved a complete response, and 5 patients had an objective response lasting 1 year. The ORR for melanoma was 17%, with disease stabilization of ≥ 24 weeks in 27% of the patients.27 Common AEs included infusion reactions, diarrhea, fatigue, rash, hypothyroidism, and hepatitis.
The second PD-L1 antibody, MPDL3280A, was studied in a phase 1 trial of 45 patients with metastatic melanoma. An ORR of 29% was observed, along with a 24-week PFS of 43%.28 Commonly noted AEs included hyperglycemia and elevated liver aminotransferases.
A newer PD-L1 inhibitor, MEDI4736, is being studied for advanced malignancies in 8 patients with melanoma. In preliminary analysis, MEDI4736 demonstrated a partial response in 1 out of 8 melanoma patients with a disease control rate of 46%.29 Although the PD-L1 inhibitors seem promising, more information will help discern their role in the management of metastatic melanoma.
Combined Anti-CTLA-4 Plus Anti-PD-1 Antibody
The combination of ipilimumab and the PD-1 inhibitor nivolumab was tested in a phase 1 trial in which both drugs were used concurrently as well as sequentially in metastatic melanoma.30 The 1- and 2-year OS in patients who were treated concurrently was 82% and 75%, respectively. Complete remission was seen in 17% of the patients, and the responses were seen irrespective of the BRAF mutation status. The responses were durable, and about 64% of the objective responses remain in remission at last follow-up.31 Grade 3 to grade 4 AEs were noted in 53% of the patients, with 11 patients requiring discontinuation of the medications. More studies are required to ascertain the optimum dosage of the combination prior to its approval for use in metastatic melanoma.
Molecular Targeted Therapy
The RAS-RAF–mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway is activated in almost 90% of patients
with melanoma.32 This pathway is normally required for the growth and survival of nonmalignant cells. In malignant transformation, mutations and/or overexpression is seen at various levels including KIT, NRAS, BRAF, and the MEK protein. This leads to activation of serine and threonine protein kinases, which lead to uncontrolled cell proliferation and survival.33
Novel therapeutic approaches have tried inhibiting one or more of these pathways for melanoma treatment. The most important mediator of tumorigenesis is BRAF, which is a downstream receptor of NRAS, and is mutated in almost 50% of melanoma cases.34 NRAS mutations are seen in 15% to 20% of cutaneous melanomas.35,36 After its activation, the RAF enzyme—coded by the BRAF gene—causes phosphorylation of the MEK protein, which activates ERK. This ERK activation leads to growth signaling and is the final pathway in several malignancies (Figure 2).37,38
BRAF Inhibitors
BRAF is the first mediator whose inhibition led to clinically significant outcomes in patients with melanoma. The most common BRAF mutation consists of the
substitution of glutamic acid for valine at amino acid 600 (V600E mutation) with majority of the remainder consisting of an alternate substitution (V600V or V600K).34 Vemurafenib and dabrafenib are the 2 BRAF inhibitors that have been shown to improve tumor regression, PFS, and OS considerably, especially in combination with a MEK protein inhibitor. In the phase 3 BRIM-3 trial, the vemurafenib group had a significantly prolonged PFS and OS compared with dacarbazine (13.6 months vs 9.7 months; 6.9 months vs 1.6 months, respectively). It was the first study to show improved survival with vemurafenib in both the V600E and V600K BRAF mutant melanomas.39
Another BRAF inhibitor, dabrafenib, was approved by the FDA for treatment of advanced melanoma with BRAF V600E mutation. It was tested in a phase 3 trial in which it was compared with dacarbazine in patients with advanced melanoma. Median OS in the dabrafenib arm was > 18 months and in dacarbazine arm > 15 months.40 Fifty-seven percent of the patients in dacarbazine arm were crossed over to the dabrafenib arm, thereby confounding the survival data for the former group. Another multicenter, phase 2 trial showed dabrafenib to have activity in melanoma patients with brain metastases, irrespective of previous therapy for the brain metastases.41 The long-term analysis of the BREAK-2 trial, which included 92 patients with metastatic melanoma treated with dabrafenib, showed a median OS of 12.9 months in BRAF V600K group and 13.1 months in BRAF V600E group.42
Adverse effects associated with BRAF inhibition include fatigue, rash, arthralgia, and photosensitivity reactions.43 Dermatologic complications may also include squamous cell carcinoma (SCC) (19%-26%), with keratoacanthoma being the most common subtype.44 These are believed to be likely secondary to the paradoxical activation of the MAPK signaling, since most of these lesions are found to have mutations in the RAS molecule.45 Other specific AEs of dabrafenib include hyperkeratosis (33%) and pyrexia (29%).42
Most patients treated with a BRAF inhibitor eventually have disease progression, likely secondary to reactivation of the MAPK pathway.46,47 This result has led to a heightened interest in combination therapies in an effort to improve outcomes. Combination therapy with ipilimumab and vemurafenib was studied and resulted in a higher incidence of hepatotoxicity (50%).48 However, no hepatotoxicity was seen in a phase 1 trial of combined dabrafenib and ipilimumab.49
Some studies have also suggested that extended BRAF inhibition after progression on a BRAF inhibitor may prolong survival.50,51 The phase 2 trial NCT01983124 is being conducted to evaluate the survival benefit with a combination of vemurafenib and a nitrosourea alkylating agent, fotemustine, in patients who have progressed on vemurafenib alone.
MEK Inhibitors
The inhibition of MEK can halt cell proliferation and induce apoptosis. The phase 3 METRIC trial, which compared the oral MEK inhibitor (trametinib) with chemotherapy, was conducted in 322 patients who had metastatic melanoma with a V600E or V600K BRAF mutation. The PFS and 6-month OS were significantly better with trametinib (4.8 months vs 1.5 months, 81% vs 66%) despite the crossover between the 2 groups.52 The AEs associated with trametinib included rash, diarrhea, and peripheral edema. Another phase 2 trial of trametinib including patients pretreated with a BRAF inhibitor showed no confirmed objective responses, 28% patients with stable disease, and minimal improvement in PFS (2 months). Among patients treated with prior chemotherapy and/or immunotherapy, trametinib showed significant improvement in complete responses, partial responses, stable disease, and the median PFS (2%, 23%, 51%, 4 months, respectively).53
The second MEK inhibitor, binimetinib, was studied in a phase 2 trial of advanced melanoma cases harboring a BRAF V600E or NRAS. Bimetinib demonstrated a PR in 20% cases of both the BRAF and NRAS mutant melanomas. Durable disease control was seen in 43% of the NRAS group and 32% of the BRAF group.54 The AE profile was similar to that seen with trametinib. Bimetinib is being studied in phase 1 and 2 trials with the CDK4/6 inhibitor as well as in the phase 3 trial NCT01763164 compared with dacarbazine in NRAS mutation positive melanomas.55
Selumetinib is a MEK inhibitor that has been compared with dacarbazine and temozolomide with no significant OS advantage. A novel highly specific inhibitor of MEK, cobimetinib, is currently being studied in combination with BRAF inhibitors.
Combined BRAF and MEK Inhibition
A randomized, double-blind, phase 3 study comparing the combination of dabrafenib and trametinib with dabrafenib and placebo in patients with advanced melanoma with a BRAF V600E mutation was presented at the 2014 American Society of Clinical Oncology meeting. Researchers found that after a median follow-up period of 9 months, there was a significant improvement with the combination in the PFS (9.3 months vs 8.8 months) and the ORR (67% vs 51%), with a similar incidence of AEs.56 The combination therapy group had fewer incidences of SCC of the skin but more incidence of pyrexia.
The combination of dabrafenib and trametinib was compared with vemurafenib monotherapy in a recent randomized phase 3 trial among 704 metastatic melanoma patients with a BRAF V600 mutation. Median PFS and ORR were significantly better with combination therapy compared with vemurafenib alone (11.4 months vs 7.3 months, 64% vs 51%, respectively). Overall survival rate at 1 year was significantly improved in the combination group as well (72% vs 65%).57 The incidence of SCC and keratoacanthoma was less in the combination (1%) compared with vemurafenib alone (18%). Another study investigating the coadministration and sequential administration of vemurafenib and trametinib is underway.58
The vemurafenib and cobimetinib combination was studied in a phase 3 trial of previously untreated unresectable locally advanced or metastatic BRAF V600
mutation-positive melanoma. The median PFS was 9.9 months in the combination group and 6.2 months in the control group. The interim analysis showed a 9-month survival rate of 81% in the combination group and 73% in the control group, with no significantly higher incidence of AEs in either arm.59 A longer follow-up will be needed to assess the OS benefit with the combination.
Encorafenib, a selective BRAF inhibitor, has been studied in a phase 1 trial in combination with binimetinib.60 This trial has paved the way to the initiation of a currently ongoing phase 3 trial (NCT01909453) comparing the combination with vemurafenib or encorafenib alone.
C-KIT Inhibitors
Mutations of c-KIT are seen more commonly in chronic sun damage-induced cutaneous melanomas, along with acral and mucosal melanomas.61,62 Earlier trials involving patients without selection for c-KIT mutation positivity failed to show benefit with imatinib. A single-arm, phase 2 trial of imatinib mesylate in patients with metastatic melanoma harboring the c-KIT mutation, an ORR of 23% was achieved, with a median PFS of 3.5 months.63 Imatinib showed an ORR of 29% in a phase 2 trial of mucosal, acral, and in chronic sun damage-induced melanoma patients with c-KIT amplifications and/or mutations. It was demonstrated that c-KIT amplification alone is not as responsive to imatinib compared with c-KIT mutation, suggesting that all patients with these specific melanomas should be tested for KIT mutation status.64
A second-generation c-KIT inhibitor, nilotinib, has shown some promising results with a favorable AE profile in small phase 2 trials.65,66 However, more clinical research will be needed before definite recommendations on its use in cutaneous melanomas can be made. Currently, its role seems to be limited to the management of acral, mucosal, and chronic sun damage-related melanomas with c-KIT mutations.
Future Directions
Angiogenesis promoters, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor, and interleukin-8, are overexpressed in melanoma. Bevacizumab, an anti-VEGF antibody, has been shown to have some benefit in combination with carboplatin and paclitaxel as a triple therapy.67 However, grade 3 AEs were seen in a portion of patients.
The phosphatidylinositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has also been studied as a target for melanoma therapy. Everolimus, an mTOR inhibitor, was studied in a phase 2 trial in combination with bevacizumab for treatment of metastatic melanoma. The combination showed improved median PFS and OS with the combination (4 months and 8.6 months, respectively), with 43% of patients alive after 12 months of follow-up.68 This study points to the direction of possible benefits with the combination of anti-VEGF and immunotherapy. A recent study failed to show survival advantage with combination of bevacizumab and temozolomide.69
Buparlisib (BKM120), a PI3K inhibitor, has been shown to have activity in vivo and in vitro against melanoma brain metastases.70 More studies need to be done to assess the possible combination with other established therapies.
Oblimersen is an antisense oligonucleotide that suppresses B-cell lymphoma-2, thereby suppressing its anti-apoptotic effect. The triple combination of oblimersen with temozolomide and albumin-bound paclitaxel has shown to be safe and efficacious in a phase 1 trial, thereby creating a need for further clinical trials.71
Treatment Approach
Systemic therapy for metastatic melanoma depends on several factors, including BRAF mutation status, functional status of the patient, disease burden, and severity of symptoms. Assessing the BRAF mutation status has become an important component in the management of patients with metastatic melanoma. It can help recognize patients who will benefit from molecular targeted therapy. In case of a BRAF-positive melanoma, treatment can be initiated with either immunotherapy or BRAF inhibitors. There are no randomized studies comparing immunotherapy to molecular targeted therapy.
Patients who have good PS and lymph node metastases can be treated initially with IL-2, which has the advantage of inducing cure in a minority of patients but should only be considered in patients with well-preserved organ function who can be monitored in an intensive care setting. On the other hand, patients who have bulky, symptomatic disease and poor PS should be treated initially with BRAF inhibitors. Combination of BRAF and MEK inhibitors can also be used and has an improved PFS and OS with potential to cause early tumor regression. There are studies to suggest suboptimal outcomes in patients who are treated with ipilimumab after progression on a BRAF inhibitor compared with initial treatment with ipilimumab followed by a BRAF inhibitor.72-74 However, all these studies are retrospective and there is no prospective data to suggest the above. BRAF mutation-positive patients who progress on a BRAF inhibitor
can be treated with PD-1 inhibitors.
Patients who do not have a BRAF mutation are unlikely to benefit from a BRAF inhibitor and primarily receive immunotherapy with ipilimumab or IL-2. Whenever possible, such patients should be enrolled in a clinical trial, as they have a poor prognosis. Patients who progress on ipilimumab can be treated with one of the PD-1 inhibitors (pembrolizumab, nivolumab). These PD-L1 inhibitors are still being investigated for use in such situations.
The role of chemotherapy in the management of metastatic melanoma has been limited by numerous studies showing significantly better survival with immunotherapy and molecular targeted therapy. Dacarbazine is the only FDA-approved drug for the treatment of melanoma. Its use is reserved mainly for patients who are not candidates for any of the other therapies available, including enrollment in a clinical trial.
Conclusion
Therapies for metastatic melanoma are in a state of flux. In the past decade, several new therapeutic agents have been introduced for the management of this potentially lethal disease. The treatment of metastatic melanoma has gradually shifted from cytotoxic chemotherapy toward a more individualized treatment that has a definite survival advantage over traditional counterparts. The advent of novel therapies has led to initiation of further studies to determine their role in the treatment of advanced melanoma, singly or in combination with other agents. In addition to evaluating new agents, more studies are needed to compare existing treatment modalities so that definitive treatment protocols can be formulated.
Acknowledgement
The authors would like to thank Felicia Ratnaraj, MD, for her assistance in creating the figures.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin.2014;64(1):9-29.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-6206.
3. Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma:
population based ecological study. BMJ. 2005;331(7515):481.
4. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011;117(20):4740-4746.
5. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention, & Therapy of Melanoma. New York, NY: Marcel Dekker;1997:219-225.
6. Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer. 2011;47(10):1476-1483.
7. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745-2751.
8. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with
or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31(3):373-379.
9. Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271(12):907-913.
10. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105-2116.
11. Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(suppl 1):S11-S14.
12. Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37(5):533-546.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
14. Schadendorf D, Hodi FS, Robert C, et. al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma [published online ahead of print February 9, 2015]. J Clin Oncol. pii:JCO.2014.56.2736.
15. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
16. Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312(17):1744-1753.
17. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
18. Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in 411 patients (pts) with melanoma (MEL) (Abstract LBA9000). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
19. Hamid O, Robert C, Ribas A, et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL) (abstract 3000). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
20. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014; 384(9948):1109-1117.
21. Dummer R, Daud A, Puzanov I, et. al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J Transl Med. 2015;13(suppl 1):O5.
22. Topalian SL, Sznol M, McDermott DF, et. al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030.
23. Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naive patients with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial (abstract 9002). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
24. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330.
25. Weber J, D’Angelo S, Gutzmer R, et al. A phase 3 randomized, open-label study of nivolumab versus investigator’s choice of chemotherapy in patients with advanced melanoma after prior anti-CTLA4 therapy (abstract LBA3). Paper presented at: European Society of Medical Oncology 2014 meeting; September 2014; Madrid, Spain.
26. Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma (abstract 9001). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
27. Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465.
28. Hamid O, Sosman JA, Lawrence DP, et. al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(15)(suppl): Abstract 9010.
29. Lutzky J, Antonia SJ, Blake-Haskins A, et. al. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol. 2014;32(15)(suppl): Abstract 3001.
30. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced
melanoma. N Engl J Med. 2013;369(2):122-133.
31. Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) (abstract LBA9003). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
32. Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9(17):6483-6488.
33. Wellbrock C, Hurlstone A. BRAF as therapeutic target in melanoma. Biochem Pharmacol. 2010;80(5):561-567.
34. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239-1246.
35. Ball NJ, Yohn JJ, Morelli JG, et al. Ras mutations in human melanoma: a marker of malignant progression. J Invest Dermatol. 1994;102(3):285-290.
36. Platz A, Ringborg U, Brahme EM, Lagerlöf B. Melanoma metastases from patients with hereditary cutaneous malignant melanoma contain a high frequency of N-ras activating mutations. Melanoma Res. 1994;4(3):169-177.
37. Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23(27):6771-6790.
38. Terai K, Matsuda M. The amino-terminal B-Raf-specific region mediates calcium-dependent homo- and hetero-dimerization of Raf. EMBO J. 2006;25(15):3556-3564.
39. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323-332.
40. Hauschild A, Grob JJ, Demidov LV, et al. An update on BREAK-3, a phase III, randomized trial: dabrafenib versus dacarbazine in patients with BRAF V600E-positive mutation metastatic melanoma (Abstract 9013). Paper presented at: American Society of Clinical Oncology 2013 meeting; May-June 2013; Chicago, IL.
41. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
42. Ascierto PA, Minor DR, Ribas A, et. al., Long-term safety and overall survival update for BREAK-2, a phase 2, single-arm, open-label study of dabrafenib in previously treated metastatic melanoma (NCT01153763). J Clin Oncol. 2014;32(15)(suppl): Abstract 9034.
43. Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with
BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety
study. Lancet Oncol. 2014;15(4):436-444.
44. Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18(3):314-322.
45. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207-215.
46. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
47. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358-365.
48. Ribas A, Hodi FS, Callahan M, et. al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2014;368(14):1365-1366.
49. Linette GP, Puzanov I, Callahan MK, et al. Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation–positive unresectable or metastatic melanoma (MM). J Clin Oncol. 2014;32(15)(suppl): Abstract 2511.
50. Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120(20):3142-3153.
51. Carlino MS, Gowrishankar K, Saunders CAB, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther. 2013;12(7):1332-1342.
52. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107-114.
53. Kim KB, Kefford R, Pavlick AC, et. al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31(1):482-489.
54. Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14(3):249-256.
55. Sosman JA, Kittaneh M, Lolkema MP, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity (abstract 9009). Paper presented at: 2014 American Society of Clinical Oncology meeting ; May-June 2014; Chicago, IL.
56. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877-1888.
57. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30-39.
58. Gogas H, Schadendorf D, Dummer R. Vemurafenib treatment in patients with BRAF-mutated melanoma failing MEK inhibition with trametinib. J Clin Oncol. 2014;32(15)(suppl): Abstract 9061.
59. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867-1876.
60. Kefford R, Miller WH, Tan DS, et al. Preliminary results from a phase Ib/II, openlabel, dose-escalation study of the oral BRAF inhibitor LGX818 in combination with the oral MEK1/2 inhibitor MEK162 in BRAF V600-dependent advanced solid tumors (abstract 9019). Paper presented at: 2013 American Society of Clinical Oncology meeting; May-June 2014; Chicago, IL.
61. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct
subtypes of melanoma. J Clin Oncol. 2006;24(26):4340-4346.
62. Jin SA, Chun SM, Choi YD, et al. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J Invest Dermatol. 2013;133(2):579-582.
63. Guo J, Si L, Kong Y et. al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904-2909.
64. Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182-3190.
65. Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2012;30(5): 2008-2014.
66. Lebbe C, Chevret S, Jouary T, et. al. Phase II multicentric uncontrolled national trial assessing the efficacy of nilotinib in the treatment of advanced melanomas with c-KIT mutation or amplification. J Clin Oncol. 2014;32(15)(suppl): Abstract 9032.
67. Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115(1):119-127.
68. Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma. Cancer. 2010;116(17): 4122-4129.
69. Dronca RS, Allred JB, Perez DG, et. al. Phase II study of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: a North Central Cancer Treatment Group study, N0675. Am J Clin Oncol. 2014;37(4):369-376.
70. Meier FE, Niessner H, Schmitz J, et al. The PI3K inhibitor BKM120 has potent antitumor activity in melanoma brain metastases in vitro and in vivo. J Clin Oncol. 2013;31(15)(suppl): Abstract e20050.
71. Ott PA, Chang J, Madden K, et al. Oblimersen in combination with temozolomide and albumin-bound paclitaxel in patients with advanced melanoma: a phase I trial. Cancer Chemother Pharmacol. 2013;71(1);183-191.
72. Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic
melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695-1701.
73. Ascierto PA, Margolin K. Ipilimumab before BRAF inhibitor treatment may be
more beneficial than vice versa for the majority of patients with advanced melanoma.
Cancer. 2014;120(11):1617-1619.
74. Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 2014;32(4):144-149.
Melanoma is the most aggressive form of skin cancer, contributing to about 76,000 new cases and more than 9,000 deaths in 2014.1 Depending on the stage of the disease, 5-year melanoma survival can range from 15% to 97%. Patients with local and distant metastases have a 5-year survival of about 60% and 15%, respectively.2
The incidence of melanoma is rising, partly because of the increasing number of skin biopsies being performed.3 If melanoma is diagnosed early, surgical excision is the treatment of choice. In patients with oligometastatic disease (cancer that has spread, but only to 1 or a small number of sites), complete surgical excision of the metastases may provide prolonged overall survival (OS) and delay the need to use systemic therapy.4
Recently, many new drug therapies have shown promising results in clinical trials, which may improve the prognosis of metastatic disease. This article reviews currently available systemic treatment options for the management of metastatic melanoma, the role of cytotoxic chemotherapy and interleukin-2 (IL-2), and the latest therapies available, including immune checkpoint inhibitors.
Cytotoxic Chemotherapy and Interleukin-2
Cytotoxic chemotherapy does not have an established role in the initial treatment of metastatic melanoma. Currently, cytotoxic chemotherapy is used in patients who have not responded to immunotherapy or molecular targeted therapy. The most commonly used drugs include dacarbazine and its prodrug, temozolomide. Several studies have failed to demonstrate a survival benefit using a single-agent chemotherapy with either dacarbazine or temozolomide.5,6
Other agents used in metastatic melanoma include nitrosoureas (fotemustine), platinum compounds (cisplatin, carboplatin), vinca alkaloids (vincristine),
and taxanes (paclitaxel). None of these agents provide a survival benefit, but an objective response may be seen in a minority of cases. Combination chemotherapy regimens have not shown an advantage over singleagent dacarbazine or temozolomide.7,8
High-dose IL-2 has been used in cases of metastatic melanoma with good performance status (PS) and organ function. Studies have shown a complete response rate of 3% to 7% and a prolonged disease-free survival in a minority of patients.9-11 The use of highdose IL-2, however, is limited by the high incidence of adverse effects (AEs), which include bacterial sepsis, pulmonary edema, arrhythmias, fever, and on some occasions, death due to complications.10 The use of IL-2 requires admission of the patient to a specialized unit for AE monitoring and management. Because of its ability to “cure” a minority of patients, a role still exists for IL-2 therapy in the treatment of younger, healthy patients with no evidence of organ dysfunction at baseline.
Immune Checkpoint Inhibitors
Checkpoint inhibitors are a class of drugs that unmask the immune system to fight against cancer cells. This class of drugs has shown significant activity and survival advantage in recent phase 2 and 3 trials. The class includes the anticytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab and monoclonal antibodies targeting the programmed death 1 protein (PD-1) or its ligand (PD-L1).
Anti-CTLA-4 Antibodies: Ipilimumab
Cytotoxic T-lymphocyte antigen 4 is the antigen responsible for inhibition of cytotoxic T-cell-mediated immunity against foreign antigens presented by the antigen presenting cells (APCs). The APCs cause activation of the T cells when peptide fragments of intracellular proteins are presented in combination with mixed histocompatibility complex molecules. This step requires interaction of a costimulatory molecule (B7) on the APCs with a cluster of differentiation 28 protein (CD28) receptor located on T cells. CTLA-4 competes with CD28 to bind with the B7 molecule, thereby inhibiting the activation of the cytotoxic T cells (Figure 1). This pathway is thought to help with development of tolerance to host tissue antigens. Ipilimumab is a human monoclonal antibody that inhibits this CTLA-4 molecule and facilitates T-cell mediated antitumor activity.12 By blocking the CTLA-4 molecule, ipilimumab also mediates its autoimmune AEs on the host tissues.
Hodi and colleagues conducted a phase 3 trial of ipilimumab, including 676 patients who progressed after prior treatment for stage III or IV melanoma, and found that median OS was significantly better in the ipilimumab groups: 10 months in the ipilimumab plus gp100 peptide vaccine group vs 6.4 months in the gp100 vaccine alone group; 10.1 months in the ipilimumab alone group vs 6.4 months in the gp100 vaccine alone group.13 In another phase 3 trial comparing ipilimumab plus dacarbazine to dacarbazine alone, the ipilimumab group had a significantly improved OS (11.2 months vs 9.1 months).1 Survival rates with ipilimumab were prolonged for up to 3 years compared with the dacarbazine plus placebo group. However, the combination was associated with increased incidence of hepatotoxicity, thereby limiting its use.
A long-term survival analysis of 10 prospective and 2 retrospective studies of ipilimumab showed a median OS of 11.4 months and a long-term survival that began at 3 years with a plateau at 10 years of 21%, which was independent of prior therapy or ipilimumab dose.14 The immune-related AEs of ipilimumab are secondary to its activity against the host antigens and include dermatitis, enterocolitis, hepatitis, and endocrinopathies.15
A recent phase 2 trial studied the combination of ipilimumab with granulocyte-macrophage colonystimulating factor in 245 patients with stage III and IV melanoma. Median OS after 13 months was significantly higher with the combination compared with ipilimumab alone. The 1-year survival rate was 69% with
the combination and 53% with ipilimumab alone. There was no difference in the overall response rate (ORR) or progression-free survival (PFS) between the 2 groups. However, the AEs were significantly reduced with the combination (45% vs 58%).16 The dose of ipilimumab used in the trial was higher than the approved dose, making it difficult to apply the results in practice without further studies on the combination.
Anti-PD-1 Antibodies
Programmed death 1 ligands (PD-L1 and PD-L2) are expressed by tumor or stromal cells to inhibit the T-cell mediated antitumor activity. These ligands bind to the PD-1 protein on the surface of activated T cells to mediate their immunosuppressive effects. Interruption of this interaction by either anti-PD-1 antibodies or anti-PD-L1 antibodies facilitates tumor cell killing by activated T cells.17
Pembrozilumab and nivolumab are the 2 anti-PD-1 monoclonal antibodies that have been approved for treatment of metastatic melanoma. In a phase 1 trial
of pembrolizumab, 411 patients with advanced melanoma (consisting of both ipilimumab-naïve [IPI-N] and ipilimumab-treated [IPI-T] patients), ORR was 40% in IPI-N and 28% in IPI-T patients with a 1-year OS of 71% in all patients. Median PFS was 24 weeks in IPI-N and 23 weeks in IPI-T pts.18 There was no difference in outcomes and safety profiles across the various dosing regimens.18,19 Of note, pembrolizumab had antitumor activity irrespective of the PS, lactate dehydrogenase levels, BRAF (B-Raf proto-oncogene, serine/threonine kinase) gene mutation, metastatic stage, and number and type of prior therapy. In a subgroup analysis, 173 patients who had progression after treatment with ipilimumab were randomly assigned to pembrolizumab 2 mg/kg every 3 weeks (q3w) or 10 mg/kg q3w dosing regimens. Both groups had no significant difference in the ORR (26% in both) and safety profiles.20
In the 2012 KEYNOTE-002 clinical trial, a randomized phase 2 trial involving 540 patients with ipilimumab-refractory advanced melanoma, patients were randomized 1:1:1 to pembrolizumab 2 mg/kg or 10 mg/kg q3w or investigator-choice chemotherapy (control arm consisting of carboplatin plus paclitaxel, carboplatin, paclitaxel, dacarbazine, or temozolomide). The 6-month PFS was significantly improved with pembrolizumab (34% and 38% for pembrolizumab 2 mg/kg and 10 mg/kg, respectively) compared with 16% with chemotherapy. The ORR was significantly better with pembrolizumab (21% at 2 mg/kg, 25% at 10 mg/kg) compared with the control arm (4%).21 These findings led to the approval of pembrolizumab by the FDA for treatment of patients with advanced melanoma who have progressed on ipilimumab. Pembrolizumab is generally well tolerated. The most common AEs include fatigue, pruritus, and rash.
Nivolumab was studied in a recent phase 1 trial in which 107 patients with previously treated advanced melanoma were treated with escalated doses every
2 weeks.22 The 2-year and 3-year OS rates were 48% and 41%, respectively. Objective responses were seen in 32% of the patients. The median response duration was 23 months.23
The first phase 3 trial was conducted in 418 patients with previously untreated metastatic melanoma BRAF mutation. Patients were randomized to receive either nivolumab or dacarbazine. The PFS and OS were significantly better with nivolumab compared with dacarbazine (PFS 5.1 months vs 2.2 months; OS 73% vs 42% at 1 year).24 The AE profile of nivolumab is similar to pembrolizumab and includes lung, skin, endocrine, renal, and gastrointestinal tract toxicities.
Preliminary results of another phase 3 trial were presented at the European Society of Medical Oncology 2014 meeting. Patients with previously treated metastatic melanoma (ipilimumab or BRAF inhibitor) were randomized in a 2:1 ratio to receive either nivolumab or investigators’ choice chemotherapy (dacarbazine or carboplatin plus paclitaxel). The ORR was significantly better with nivolumab (32% vs 11%), and 95% of patients were still responding after 6 months. The nivolumab group showed a complete remission in 3% of the patients with 34% of the responses lasting ≥ 6 months.25 This led to the recent approval of nivolumab for patients with metastatic melanoma with a BRAF mutation who have advanced on ipilimumab. In the phase 3 NCT01844505 trial patients are being randomized to receive ipilimumab, nivolumab, or both.
A newer PD-1 inhibitor, pidilizumab, was studied in a phase 2 trial that included 103 patients with metastatic melanoma, 51% of whom had received therapy with ipilimumab. The ORR in the study group was relatively lower (6%), but the OS at 1 year was 64.5%.26 Further studies are underway to evaluate the role of this drug in metastatic melanoma.
The response with both nivolumab and pembrolizumab is durable as well as sustained, even after discontinuation of therapy. None of the deaths in the aforementioned studies were atributed to drug-related toxicities. As evidenced by current data, these 2 drugs hold a great promise for the management of patients who progress after therapy with anti-CTLA-4 antibodies.
Anti-PD-L1 Antibodies
The anti-PD-L1 monoclonal antibodies work in a similar way to the PD-1 inhibitors and block the interaction between the PD-1 and its ligand, PD-L1. This causes sustained activation of cytotoxic T cells and facilitates their antitumor activity. Two of PD-L1 inhibitors have shown clinical activity against metastatic melanoma.
BMS-936559, the first PD-L1 antibody, is being studied in a phase 1 trial that includes 55 patients with advanced melanoma along with 152 patients with other solid malignancies. Three patients achieved a complete response, and 5 patients had an objective response lasting 1 year. The ORR for melanoma was 17%, with disease stabilization of ≥ 24 weeks in 27% of the patients.27 Common AEs included infusion reactions, diarrhea, fatigue, rash, hypothyroidism, and hepatitis.
The second PD-L1 antibody, MPDL3280A, was studied in a phase 1 trial of 45 patients with metastatic melanoma. An ORR of 29% was observed, along with a 24-week PFS of 43%.28 Commonly noted AEs included hyperglycemia and elevated liver aminotransferases.
A newer PD-L1 inhibitor, MEDI4736, is being studied for advanced malignancies in 8 patients with melanoma. In preliminary analysis, MEDI4736 demonstrated a partial response in 1 out of 8 melanoma patients with a disease control rate of 46%.29 Although the PD-L1 inhibitors seem promising, more information will help discern their role in the management of metastatic melanoma.
Combined Anti-CTLA-4 Plus Anti-PD-1 Antibody
The combination of ipilimumab and the PD-1 inhibitor nivolumab was tested in a phase 1 trial in which both drugs were used concurrently as well as sequentially in metastatic melanoma.30 The 1- and 2-year OS in patients who were treated concurrently was 82% and 75%, respectively. Complete remission was seen in 17% of the patients, and the responses were seen irrespective of the BRAF mutation status. The responses were durable, and about 64% of the objective responses remain in remission at last follow-up.31 Grade 3 to grade 4 AEs were noted in 53% of the patients, with 11 patients requiring discontinuation of the medications. More studies are required to ascertain the optimum dosage of the combination prior to its approval for use in metastatic melanoma.
Molecular Targeted Therapy
The RAS-RAF–mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway is activated in almost 90% of patients
with melanoma.32 This pathway is normally required for the growth and survival of nonmalignant cells. In malignant transformation, mutations and/or overexpression is seen at various levels including KIT, NRAS, BRAF, and the MEK protein. This leads to activation of serine and threonine protein kinases, which lead to uncontrolled cell proliferation and survival.33
Novel therapeutic approaches have tried inhibiting one or more of these pathways for melanoma treatment. The most important mediator of tumorigenesis is BRAF, which is a downstream receptor of NRAS, and is mutated in almost 50% of melanoma cases.34 NRAS mutations are seen in 15% to 20% of cutaneous melanomas.35,36 After its activation, the RAF enzyme—coded by the BRAF gene—causes phosphorylation of the MEK protein, which activates ERK. This ERK activation leads to growth signaling and is the final pathway in several malignancies (Figure 2).37,38
BRAF Inhibitors
BRAF is the first mediator whose inhibition led to clinically significant outcomes in patients with melanoma. The most common BRAF mutation consists of the
substitution of glutamic acid for valine at amino acid 600 (V600E mutation) with majority of the remainder consisting of an alternate substitution (V600V or V600K).34 Vemurafenib and dabrafenib are the 2 BRAF inhibitors that have been shown to improve tumor regression, PFS, and OS considerably, especially in combination with a MEK protein inhibitor. In the phase 3 BRIM-3 trial, the vemurafenib group had a significantly prolonged PFS and OS compared with dacarbazine (13.6 months vs 9.7 months; 6.9 months vs 1.6 months, respectively). It was the first study to show improved survival with vemurafenib in both the V600E and V600K BRAF mutant melanomas.39
Another BRAF inhibitor, dabrafenib, was approved by the FDA for treatment of advanced melanoma with BRAF V600E mutation. It was tested in a phase 3 trial in which it was compared with dacarbazine in patients with advanced melanoma. Median OS in the dabrafenib arm was > 18 months and in dacarbazine arm > 15 months.40 Fifty-seven percent of the patients in dacarbazine arm were crossed over to the dabrafenib arm, thereby confounding the survival data for the former group. Another multicenter, phase 2 trial showed dabrafenib to have activity in melanoma patients with brain metastases, irrespective of previous therapy for the brain metastases.41 The long-term analysis of the BREAK-2 trial, which included 92 patients with metastatic melanoma treated with dabrafenib, showed a median OS of 12.9 months in BRAF V600K group and 13.1 months in BRAF V600E group.42
Adverse effects associated with BRAF inhibition include fatigue, rash, arthralgia, and photosensitivity reactions.43 Dermatologic complications may also include squamous cell carcinoma (SCC) (19%-26%), with keratoacanthoma being the most common subtype.44 These are believed to be likely secondary to the paradoxical activation of the MAPK signaling, since most of these lesions are found to have mutations in the RAS molecule.45 Other specific AEs of dabrafenib include hyperkeratosis (33%) and pyrexia (29%).42
Most patients treated with a BRAF inhibitor eventually have disease progression, likely secondary to reactivation of the MAPK pathway.46,47 This result has led to a heightened interest in combination therapies in an effort to improve outcomes. Combination therapy with ipilimumab and vemurafenib was studied and resulted in a higher incidence of hepatotoxicity (50%).48 However, no hepatotoxicity was seen in a phase 1 trial of combined dabrafenib and ipilimumab.49
Some studies have also suggested that extended BRAF inhibition after progression on a BRAF inhibitor may prolong survival.50,51 The phase 2 trial NCT01983124 is being conducted to evaluate the survival benefit with a combination of vemurafenib and a nitrosourea alkylating agent, fotemustine, in patients who have progressed on vemurafenib alone.
MEK Inhibitors
The inhibition of MEK can halt cell proliferation and induce apoptosis. The phase 3 METRIC trial, which compared the oral MEK inhibitor (trametinib) with chemotherapy, was conducted in 322 patients who had metastatic melanoma with a V600E or V600K BRAF mutation. The PFS and 6-month OS were significantly better with trametinib (4.8 months vs 1.5 months, 81% vs 66%) despite the crossover between the 2 groups.52 The AEs associated with trametinib included rash, diarrhea, and peripheral edema. Another phase 2 trial of trametinib including patients pretreated with a BRAF inhibitor showed no confirmed objective responses, 28% patients with stable disease, and minimal improvement in PFS (2 months). Among patients treated with prior chemotherapy and/or immunotherapy, trametinib showed significant improvement in complete responses, partial responses, stable disease, and the median PFS (2%, 23%, 51%, 4 months, respectively).53
The second MEK inhibitor, binimetinib, was studied in a phase 2 trial of advanced melanoma cases harboring a BRAF V600E or NRAS. Bimetinib demonstrated a PR in 20% cases of both the BRAF and NRAS mutant melanomas. Durable disease control was seen in 43% of the NRAS group and 32% of the BRAF group.54 The AE profile was similar to that seen with trametinib. Bimetinib is being studied in phase 1 and 2 trials with the CDK4/6 inhibitor as well as in the phase 3 trial NCT01763164 compared with dacarbazine in NRAS mutation positive melanomas.55
Selumetinib is a MEK inhibitor that has been compared with dacarbazine and temozolomide with no significant OS advantage. A novel highly specific inhibitor of MEK, cobimetinib, is currently being studied in combination with BRAF inhibitors.
Combined BRAF and MEK Inhibition
A randomized, double-blind, phase 3 study comparing the combination of dabrafenib and trametinib with dabrafenib and placebo in patients with advanced melanoma with a BRAF V600E mutation was presented at the 2014 American Society of Clinical Oncology meeting. Researchers found that after a median follow-up period of 9 months, there was a significant improvement with the combination in the PFS (9.3 months vs 8.8 months) and the ORR (67% vs 51%), with a similar incidence of AEs.56 The combination therapy group had fewer incidences of SCC of the skin but more incidence of pyrexia.
The combination of dabrafenib and trametinib was compared with vemurafenib monotherapy in a recent randomized phase 3 trial among 704 metastatic melanoma patients with a BRAF V600 mutation. Median PFS and ORR were significantly better with combination therapy compared with vemurafenib alone (11.4 months vs 7.3 months, 64% vs 51%, respectively). Overall survival rate at 1 year was significantly improved in the combination group as well (72% vs 65%).57 The incidence of SCC and keratoacanthoma was less in the combination (1%) compared with vemurafenib alone (18%). Another study investigating the coadministration and sequential administration of vemurafenib and trametinib is underway.58
The vemurafenib and cobimetinib combination was studied in a phase 3 trial of previously untreated unresectable locally advanced or metastatic BRAF V600
mutation-positive melanoma. The median PFS was 9.9 months in the combination group and 6.2 months in the control group. The interim analysis showed a 9-month survival rate of 81% in the combination group and 73% in the control group, with no significantly higher incidence of AEs in either arm.59 A longer follow-up will be needed to assess the OS benefit with the combination.
Encorafenib, a selective BRAF inhibitor, has been studied in a phase 1 trial in combination with binimetinib.60 This trial has paved the way to the initiation of a currently ongoing phase 3 trial (NCT01909453) comparing the combination with vemurafenib or encorafenib alone.
C-KIT Inhibitors
Mutations of c-KIT are seen more commonly in chronic sun damage-induced cutaneous melanomas, along with acral and mucosal melanomas.61,62 Earlier trials involving patients without selection for c-KIT mutation positivity failed to show benefit with imatinib. A single-arm, phase 2 trial of imatinib mesylate in patients with metastatic melanoma harboring the c-KIT mutation, an ORR of 23% was achieved, with a median PFS of 3.5 months.63 Imatinib showed an ORR of 29% in a phase 2 trial of mucosal, acral, and in chronic sun damage-induced melanoma patients with c-KIT amplifications and/or mutations. It was demonstrated that c-KIT amplification alone is not as responsive to imatinib compared with c-KIT mutation, suggesting that all patients with these specific melanomas should be tested for KIT mutation status.64
A second-generation c-KIT inhibitor, nilotinib, has shown some promising results with a favorable AE profile in small phase 2 trials.65,66 However, more clinical research will be needed before definite recommendations on its use in cutaneous melanomas can be made. Currently, its role seems to be limited to the management of acral, mucosal, and chronic sun damage-related melanomas with c-KIT mutations.
Future Directions
Angiogenesis promoters, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor, and interleukin-8, are overexpressed in melanoma. Bevacizumab, an anti-VEGF antibody, has been shown to have some benefit in combination with carboplatin and paclitaxel as a triple therapy.67 However, grade 3 AEs were seen in a portion of patients.
The phosphatidylinositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has also been studied as a target for melanoma therapy. Everolimus, an mTOR inhibitor, was studied in a phase 2 trial in combination with bevacizumab for treatment of metastatic melanoma. The combination showed improved median PFS and OS with the combination (4 months and 8.6 months, respectively), with 43% of patients alive after 12 months of follow-up.68 This study points to the direction of possible benefits with the combination of anti-VEGF and immunotherapy. A recent study failed to show survival advantage with combination of bevacizumab and temozolomide.69
Buparlisib (BKM120), a PI3K inhibitor, has been shown to have activity in vivo and in vitro against melanoma brain metastases.70 More studies need to be done to assess the possible combination with other established therapies.
Oblimersen is an antisense oligonucleotide that suppresses B-cell lymphoma-2, thereby suppressing its anti-apoptotic effect. The triple combination of oblimersen with temozolomide and albumin-bound paclitaxel has shown to be safe and efficacious in a phase 1 trial, thereby creating a need for further clinical trials.71
Treatment Approach
Systemic therapy for metastatic melanoma depends on several factors, including BRAF mutation status, functional status of the patient, disease burden, and severity of symptoms. Assessing the BRAF mutation status has become an important component in the management of patients with metastatic melanoma. It can help recognize patients who will benefit from molecular targeted therapy. In case of a BRAF-positive melanoma, treatment can be initiated with either immunotherapy or BRAF inhibitors. There are no randomized studies comparing immunotherapy to molecular targeted therapy.
Patients who have good PS and lymph node metastases can be treated initially with IL-2, which has the advantage of inducing cure in a minority of patients but should only be considered in patients with well-preserved organ function who can be monitored in an intensive care setting. On the other hand, patients who have bulky, symptomatic disease and poor PS should be treated initially with BRAF inhibitors. Combination of BRAF and MEK inhibitors can also be used and has an improved PFS and OS with potential to cause early tumor regression. There are studies to suggest suboptimal outcomes in patients who are treated with ipilimumab after progression on a BRAF inhibitor compared with initial treatment with ipilimumab followed by a BRAF inhibitor.72-74 However, all these studies are retrospective and there is no prospective data to suggest the above. BRAF mutation-positive patients who progress on a BRAF inhibitor
can be treated with PD-1 inhibitors.
Patients who do not have a BRAF mutation are unlikely to benefit from a BRAF inhibitor and primarily receive immunotherapy with ipilimumab or IL-2. Whenever possible, such patients should be enrolled in a clinical trial, as they have a poor prognosis. Patients who progress on ipilimumab can be treated with one of the PD-1 inhibitors (pembrolizumab, nivolumab). These PD-L1 inhibitors are still being investigated for use in such situations.
The role of chemotherapy in the management of metastatic melanoma has been limited by numerous studies showing significantly better survival with immunotherapy and molecular targeted therapy. Dacarbazine is the only FDA-approved drug for the treatment of melanoma. Its use is reserved mainly for patients who are not candidates for any of the other therapies available, including enrollment in a clinical trial.
Conclusion
Therapies for metastatic melanoma are in a state of flux. In the past decade, several new therapeutic agents have been introduced for the management of this potentially lethal disease. The treatment of metastatic melanoma has gradually shifted from cytotoxic chemotherapy toward a more individualized treatment that has a definite survival advantage over traditional counterparts. The advent of novel therapies has led to initiation of further studies to determine their role in the treatment of advanced melanoma, singly or in combination with other agents. In addition to evaluating new agents, more studies are needed to compare existing treatment modalities so that definitive treatment protocols can be formulated.
Acknowledgement
The authors would like to thank Felicia Ratnaraj, MD, for her assistance in creating the figures.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Melanoma is the most aggressive form of skin cancer, contributing to about 76,000 new cases and more than 9,000 deaths in 2014.1 Depending on the stage of the disease, 5-year melanoma survival can range from 15% to 97%. Patients with local and distant metastases have a 5-year survival of about 60% and 15%, respectively.2
The incidence of melanoma is rising, partly because of the increasing number of skin biopsies being performed.3 If melanoma is diagnosed early, surgical excision is the treatment of choice. In patients with oligometastatic disease (cancer that has spread, but only to 1 or a small number of sites), complete surgical excision of the metastases may provide prolonged overall survival (OS) and delay the need to use systemic therapy.4
Recently, many new drug therapies have shown promising results in clinical trials, which may improve the prognosis of metastatic disease. This article reviews currently available systemic treatment options for the management of metastatic melanoma, the role of cytotoxic chemotherapy and interleukin-2 (IL-2), and the latest therapies available, including immune checkpoint inhibitors.
Cytotoxic Chemotherapy and Interleukin-2
Cytotoxic chemotherapy does not have an established role in the initial treatment of metastatic melanoma. Currently, cytotoxic chemotherapy is used in patients who have not responded to immunotherapy or molecular targeted therapy. The most commonly used drugs include dacarbazine and its prodrug, temozolomide. Several studies have failed to demonstrate a survival benefit using a single-agent chemotherapy with either dacarbazine or temozolomide.5,6
Other agents used in metastatic melanoma include nitrosoureas (fotemustine), platinum compounds (cisplatin, carboplatin), vinca alkaloids (vincristine),
and taxanes (paclitaxel). None of these agents provide a survival benefit, but an objective response may be seen in a minority of cases. Combination chemotherapy regimens have not shown an advantage over singleagent dacarbazine or temozolomide.7,8
High-dose IL-2 has been used in cases of metastatic melanoma with good performance status (PS) and organ function. Studies have shown a complete response rate of 3% to 7% and a prolonged disease-free survival in a minority of patients.9-11 The use of highdose IL-2, however, is limited by the high incidence of adverse effects (AEs), which include bacterial sepsis, pulmonary edema, arrhythmias, fever, and on some occasions, death due to complications.10 The use of IL-2 requires admission of the patient to a specialized unit for AE monitoring and management. Because of its ability to “cure” a minority of patients, a role still exists for IL-2 therapy in the treatment of younger, healthy patients with no evidence of organ dysfunction at baseline.
Immune Checkpoint Inhibitors
Checkpoint inhibitors are a class of drugs that unmask the immune system to fight against cancer cells. This class of drugs has shown significant activity and survival advantage in recent phase 2 and 3 trials. The class includes the anticytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab and monoclonal antibodies targeting the programmed death 1 protein (PD-1) or its ligand (PD-L1).
Anti-CTLA-4 Antibodies: Ipilimumab
Cytotoxic T-lymphocyte antigen 4 is the antigen responsible for inhibition of cytotoxic T-cell-mediated immunity against foreign antigens presented by the antigen presenting cells (APCs). The APCs cause activation of the T cells when peptide fragments of intracellular proteins are presented in combination with mixed histocompatibility complex molecules. This step requires interaction of a costimulatory molecule (B7) on the APCs with a cluster of differentiation 28 protein (CD28) receptor located on T cells. CTLA-4 competes with CD28 to bind with the B7 molecule, thereby inhibiting the activation of the cytotoxic T cells (Figure 1). This pathway is thought to help with development of tolerance to host tissue antigens. Ipilimumab is a human monoclonal antibody that inhibits this CTLA-4 molecule and facilitates T-cell mediated antitumor activity.12 By blocking the CTLA-4 molecule, ipilimumab also mediates its autoimmune AEs on the host tissues.
Hodi and colleagues conducted a phase 3 trial of ipilimumab, including 676 patients who progressed after prior treatment for stage III or IV melanoma, and found that median OS was significantly better in the ipilimumab groups: 10 months in the ipilimumab plus gp100 peptide vaccine group vs 6.4 months in the gp100 vaccine alone group; 10.1 months in the ipilimumab alone group vs 6.4 months in the gp100 vaccine alone group.13 In another phase 3 trial comparing ipilimumab plus dacarbazine to dacarbazine alone, the ipilimumab group had a significantly improved OS (11.2 months vs 9.1 months).1 Survival rates with ipilimumab were prolonged for up to 3 years compared with the dacarbazine plus placebo group. However, the combination was associated with increased incidence of hepatotoxicity, thereby limiting its use.
A long-term survival analysis of 10 prospective and 2 retrospective studies of ipilimumab showed a median OS of 11.4 months and a long-term survival that began at 3 years with a plateau at 10 years of 21%, which was independent of prior therapy or ipilimumab dose.14 The immune-related AEs of ipilimumab are secondary to its activity against the host antigens and include dermatitis, enterocolitis, hepatitis, and endocrinopathies.15
A recent phase 2 trial studied the combination of ipilimumab with granulocyte-macrophage colonystimulating factor in 245 patients with stage III and IV melanoma. Median OS after 13 months was significantly higher with the combination compared with ipilimumab alone. The 1-year survival rate was 69% with
the combination and 53% with ipilimumab alone. There was no difference in the overall response rate (ORR) or progression-free survival (PFS) between the 2 groups. However, the AEs were significantly reduced with the combination (45% vs 58%).16 The dose of ipilimumab used in the trial was higher than the approved dose, making it difficult to apply the results in practice without further studies on the combination.
Anti-PD-1 Antibodies
Programmed death 1 ligands (PD-L1 and PD-L2) are expressed by tumor or stromal cells to inhibit the T-cell mediated antitumor activity. These ligands bind to the PD-1 protein on the surface of activated T cells to mediate their immunosuppressive effects. Interruption of this interaction by either anti-PD-1 antibodies or anti-PD-L1 antibodies facilitates tumor cell killing by activated T cells.17
Pembrozilumab and nivolumab are the 2 anti-PD-1 monoclonal antibodies that have been approved for treatment of metastatic melanoma. In a phase 1 trial
of pembrolizumab, 411 patients with advanced melanoma (consisting of both ipilimumab-naïve [IPI-N] and ipilimumab-treated [IPI-T] patients), ORR was 40% in IPI-N and 28% in IPI-T patients with a 1-year OS of 71% in all patients. Median PFS was 24 weeks in IPI-N and 23 weeks in IPI-T pts.18 There was no difference in outcomes and safety profiles across the various dosing regimens.18,19 Of note, pembrolizumab had antitumor activity irrespective of the PS, lactate dehydrogenase levels, BRAF (B-Raf proto-oncogene, serine/threonine kinase) gene mutation, metastatic stage, and number and type of prior therapy. In a subgroup analysis, 173 patients who had progression after treatment with ipilimumab were randomly assigned to pembrolizumab 2 mg/kg every 3 weeks (q3w) or 10 mg/kg q3w dosing regimens. Both groups had no significant difference in the ORR (26% in both) and safety profiles.20
In the 2012 KEYNOTE-002 clinical trial, a randomized phase 2 trial involving 540 patients with ipilimumab-refractory advanced melanoma, patients were randomized 1:1:1 to pembrolizumab 2 mg/kg or 10 mg/kg q3w or investigator-choice chemotherapy (control arm consisting of carboplatin plus paclitaxel, carboplatin, paclitaxel, dacarbazine, or temozolomide). The 6-month PFS was significantly improved with pembrolizumab (34% and 38% for pembrolizumab 2 mg/kg and 10 mg/kg, respectively) compared with 16% with chemotherapy. The ORR was significantly better with pembrolizumab (21% at 2 mg/kg, 25% at 10 mg/kg) compared with the control arm (4%).21 These findings led to the approval of pembrolizumab by the FDA for treatment of patients with advanced melanoma who have progressed on ipilimumab. Pembrolizumab is generally well tolerated. The most common AEs include fatigue, pruritus, and rash.
Nivolumab was studied in a recent phase 1 trial in which 107 patients with previously treated advanced melanoma were treated with escalated doses every
2 weeks.22 The 2-year and 3-year OS rates were 48% and 41%, respectively. Objective responses were seen in 32% of the patients. The median response duration was 23 months.23
The first phase 3 trial was conducted in 418 patients with previously untreated metastatic melanoma BRAF mutation. Patients were randomized to receive either nivolumab or dacarbazine. The PFS and OS were significantly better with nivolumab compared with dacarbazine (PFS 5.1 months vs 2.2 months; OS 73% vs 42% at 1 year).24 The AE profile of nivolumab is similar to pembrolizumab and includes lung, skin, endocrine, renal, and gastrointestinal tract toxicities.
Preliminary results of another phase 3 trial were presented at the European Society of Medical Oncology 2014 meeting. Patients with previously treated metastatic melanoma (ipilimumab or BRAF inhibitor) were randomized in a 2:1 ratio to receive either nivolumab or investigators’ choice chemotherapy (dacarbazine or carboplatin plus paclitaxel). The ORR was significantly better with nivolumab (32% vs 11%), and 95% of patients were still responding after 6 months. The nivolumab group showed a complete remission in 3% of the patients with 34% of the responses lasting ≥ 6 months.25 This led to the recent approval of nivolumab for patients with metastatic melanoma with a BRAF mutation who have advanced on ipilimumab. In the phase 3 NCT01844505 trial patients are being randomized to receive ipilimumab, nivolumab, or both.
A newer PD-1 inhibitor, pidilizumab, was studied in a phase 2 trial that included 103 patients with metastatic melanoma, 51% of whom had received therapy with ipilimumab. The ORR in the study group was relatively lower (6%), but the OS at 1 year was 64.5%.26 Further studies are underway to evaluate the role of this drug in metastatic melanoma.
The response with both nivolumab and pembrolizumab is durable as well as sustained, even after discontinuation of therapy. None of the deaths in the aforementioned studies were atributed to drug-related toxicities. As evidenced by current data, these 2 drugs hold a great promise for the management of patients who progress after therapy with anti-CTLA-4 antibodies.
Anti-PD-L1 Antibodies
The anti-PD-L1 monoclonal antibodies work in a similar way to the PD-1 inhibitors and block the interaction between the PD-1 and its ligand, PD-L1. This causes sustained activation of cytotoxic T cells and facilitates their antitumor activity. Two of PD-L1 inhibitors have shown clinical activity against metastatic melanoma.
BMS-936559, the first PD-L1 antibody, is being studied in a phase 1 trial that includes 55 patients with advanced melanoma along with 152 patients with other solid malignancies. Three patients achieved a complete response, and 5 patients had an objective response lasting 1 year. The ORR for melanoma was 17%, with disease stabilization of ≥ 24 weeks in 27% of the patients.27 Common AEs included infusion reactions, diarrhea, fatigue, rash, hypothyroidism, and hepatitis.
The second PD-L1 antibody, MPDL3280A, was studied in a phase 1 trial of 45 patients with metastatic melanoma. An ORR of 29% was observed, along with a 24-week PFS of 43%.28 Commonly noted AEs included hyperglycemia and elevated liver aminotransferases.
A newer PD-L1 inhibitor, MEDI4736, is being studied for advanced malignancies in 8 patients with melanoma. In preliminary analysis, MEDI4736 demonstrated a partial response in 1 out of 8 melanoma patients with a disease control rate of 46%.29 Although the PD-L1 inhibitors seem promising, more information will help discern their role in the management of metastatic melanoma.
Combined Anti-CTLA-4 Plus Anti-PD-1 Antibody
The combination of ipilimumab and the PD-1 inhibitor nivolumab was tested in a phase 1 trial in which both drugs were used concurrently as well as sequentially in metastatic melanoma.30 The 1- and 2-year OS in patients who were treated concurrently was 82% and 75%, respectively. Complete remission was seen in 17% of the patients, and the responses were seen irrespective of the BRAF mutation status. The responses were durable, and about 64% of the objective responses remain in remission at last follow-up.31 Grade 3 to grade 4 AEs were noted in 53% of the patients, with 11 patients requiring discontinuation of the medications. More studies are required to ascertain the optimum dosage of the combination prior to its approval for use in metastatic melanoma.
Molecular Targeted Therapy
The RAS-RAF–mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway is activated in almost 90% of patients
with melanoma.32 This pathway is normally required for the growth and survival of nonmalignant cells. In malignant transformation, mutations and/or overexpression is seen at various levels including KIT, NRAS, BRAF, and the MEK protein. This leads to activation of serine and threonine protein kinases, which lead to uncontrolled cell proliferation and survival.33
Novel therapeutic approaches have tried inhibiting one or more of these pathways for melanoma treatment. The most important mediator of tumorigenesis is BRAF, which is a downstream receptor of NRAS, and is mutated in almost 50% of melanoma cases.34 NRAS mutations are seen in 15% to 20% of cutaneous melanomas.35,36 After its activation, the RAF enzyme—coded by the BRAF gene—causes phosphorylation of the MEK protein, which activates ERK. This ERK activation leads to growth signaling and is the final pathway in several malignancies (Figure 2).37,38
BRAF Inhibitors
BRAF is the first mediator whose inhibition led to clinically significant outcomes in patients with melanoma. The most common BRAF mutation consists of the
substitution of glutamic acid for valine at amino acid 600 (V600E mutation) with majority of the remainder consisting of an alternate substitution (V600V or V600K).34 Vemurafenib and dabrafenib are the 2 BRAF inhibitors that have been shown to improve tumor regression, PFS, and OS considerably, especially in combination with a MEK protein inhibitor. In the phase 3 BRIM-3 trial, the vemurafenib group had a significantly prolonged PFS and OS compared with dacarbazine (13.6 months vs 9.7 months; 6.9 months vs 1.6 months, respectively). It was the first study to show improved survival with vemurafenib in both the V600E and V600K BRAF mutant melanomas.39
Another BRAF inhibitor, dabrafenib, was approved by the FDA for treatment of advanced melanoma with BRAF V600E mutation. It was tested in a phase 3 trial in which it was compared with dacarbazine in patients with advanced melanoma. Median OS in the dabrafenib arm was > 18 months and in dacarbazine arm > 15 months.40 Fifty-seven percent of the patients in dacarbazine arm were crossed over to the dabrafenib arm, thereby confounding the survival data for the former group. Another multicenter, phase 2 trial showed dabrafenib to have activity in melanoma patients with brain metastases, irrespective of previous therapy for the brain metastases.41 The long-term analysis of the BREAK-2 trial, which included 92 patients with metastatic melanoma treated with dabrafenib, showed a median OS of 12.9 months in BRAF V600K group and 13.1 months in BRAF V600E group.42
Adverse effects associated with BRAF inhibition include fatigue, rash, arthralgia, and photosensitivity reactions.43 Dermatologic complications may also include squamous cell carcinoma (SCC) (19%-26%), with keratoacanthoma being the most common subtype.44 These are believed to be likely secondary to the paradoxical activation of the MAPK signaling, since most of these lesions are found to have mutations in the RAS molecule.45 Other specific AEs of dabrafenib include hyperkeratosis (33%) and pyrexia (29%).42
Most patients treated with a BRAF inhibitor eventually have disease progression, likely secondary to reactivation of the MAPK pathway.46,47 This result has led to a heightened interest in combination therapies in an effort to improve outcomes. Combination therapy with ipilimumab and vemurafenib was studied and resulted in a higher incidence of hepatotoxicity (50%).48 However, no hepatotoxicity was seen in a phase 1 trial of combined dabrafenib and ipilimumab.49
Some studies have also suggested that extended BRAF inhibition after progression on a BRAF inhibitor may prolong survival.50,51 The phase 2 trial NCT01983124 is being conducted to evaluate the survival benefit with a combination of vemurafenib and a nitrosourea alkylating agent, fotemustine, in patients who have progressed on vemurafenib alone.
MEK Inhibitors
The inhibition of MEK can halt cell proliferation and induce apoptosis. The phase 3 METRIC trial, which compared the oral MEK inhibitor (trametinib) with chemotherapy, was conducted in 322 patients who had metastatic melanoma with a V600E or V600K BRAF mutation. The PFS and 6-month OS were significantly better with trametinib (4.8 months vs 1.5 months, 81% vs 66%) despite the crossover between the 2 groups.52 The AEs associated with trametinib included rash, diarrhea, and peripheral edema. Another phase 2 trial of trametinib including patients pretreated with a BRAF inhibitor showed no confirmed objective responses, 28% patients with stable disease, and minimal improvement in PFS (2 months). Among patients treated with prior chemotherapy and/or immunotherapy, trametinib showed significant improvement in complete responses, partial responses, stable disease, and the median PFS (2%, 23%, 51%, 4 months, respectively).53
The second MEK inhibitor, binimetinib, was studied in a phase 2 trial of advanced melanoma cases harboring a BRAF V600E or NRAS. Bimetinib demonstrated a PR in 20% cases of both the BRAF and NRAS mutant melanomas. Durable disease control was seen in 43% of the NRAS group and 32% of the BRAF group.54 The AE profile was similar to that seen with trametinib. Bimetinib is being studied in phase 1 and 2 trials with the CDK4/6 inhibitor as well as in the phase 3 trial NCT01763164 compared with dacarbazine in NRAS mutation positive melanomas.55
Selumetinib is a MEK inhibitor that has been compared with dacarbazine and temozolomide with no significant OS advantage. A novel highly specific inhibitor of MEK, cobimetinib, is currently being studied in combination with BRAF inhibitors.
Combined BRAF and MEK Inhibition
A randomized, double-blind, phase 3 study comparing the combination of dabrafenib and trametinib with dabrafenib and placebo in patients with advanced melanoma with a BRAF V600E mutation was presented at the 2014 American Society of Clinical Oncology meeting. Researchers found that after a median follow-up period of 9 months, there was a significant improvement with the combination in the PFS (9.3 months vs 8.8 months) and the ORR (67% vs 51%), with a similar incidence of AEs.56 The combination therapy group had fewer incidences of SCC of the skin but more incidence of pyrexia.
The combination of dabrafenib and trametinib was compared with vemurafenib monotherapy in a recent randomized phase 3 trial among 704 metastatic melanoma patients with a BRAF V600 mutation. Median PFS and ORR were significantly better with combination therapy compared with vemurafenib alone (11.4 months vs 7.3 months, 64% vs 51%, respectively). Overall survival rate at 1 year was significantly improved in the combination group as well (72% vs 65%).57 The incidence of SCC and keratoacanthoma was less in the combination (1%) compared with vemurafenib alone (18%). Another study investigating the coadministration and sequential administration of vemurafenib and trametinib is underway.58
The vemurafenib and cobimetinib combination was studied in a phase 3 trial of previously untreated unresectable locally advanced or metastatic BRAF V600
mutation-positive melanoma. The median PFS was 9.9 months in the combination group and 6.2 months in the control group. The interim analysis showed a 9-month survival rate of 81% in the combination group and 73% in the control group, with no significantly higher incidence of AEs in either arm.59 A longer follow-up will be needed to assess the OS benefit with the combination.
Encorafenib, a selective BRAF inhibitor, has been studied in a phase 1 trial in combination with binimetinib.60 This trial has paved the way to the initiation of a currently ongoing phase 3 trial (NCT01909453) comparing the combination with vemurafenib or encorafenib alone.
C-KIT Inhibitors
Mutations of c-KIT are seen more commonly in chronic sun damage-induced cutaneous melanomas, along with acral and mucosal melanomas.61,62 Earlier trials involving patients without selection for c-KIT mutation positivity failed to show benefit with imatinib. A single-arm, phase 2 trial of imatinib mesylate in patients with metastatic melanoma harboring the c-KIT mutation, an ORR of 23% was achieved, with a median PFS of 3.5 months.63 Imatinib showed an ORR of 29% in a phase 2 trial of mucosal, acral, and in chronic sun damage-induced melanoma patients with c-KIT amplifications and/or mutations. It was demonstrated that c-KIT amplification alone is not as responsive to imatinib compared with c-KIT mutation, suggesting that all patients with these specific melanomas should be tested for KIT mutation status.64
A second-generation c-KIT inhibitor, nilotinib, has shown some promising results with a favorable AE profile in small phase 2 trials.65,66 However, more clinical research will be needed before definite recommendations on its use in cutaneous melanomas can be made. Currently, its role seems to be limited to the management of acral, mucosal, and chronic sun damage-related melanomas with c-KIT mutations.
Future Directions
Angiogenesis promoters, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor, and interleukin-8, are overexpressed in melanoma. Bevacizumab, an anti-VEGF antibody, has been shown to have some benefit in combination with carboplatin and paclitaxel as a triple therapy.67 However, grade 3 AEs were seen in a portion of patients.
The phosphatidylinositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has also been studied as a target for melanoma therapy. Everolimus, an mTOR inhibitor, was studied in a phase 2 trial in combination with bevacizumab for treatment of metastatic melanoma. The combination showed improved median PFS and OS with the combination (4 months and 8.6 months, respectively), with 43% of patients alive after 12 months of follow-up.68 This study points to the direction of possible benefits with the combination of anti-VEGF and immunotherapy. A recent study failed to show survival advantage with combination of bevacizumab and temozolomide.69
Buparlisib (BKM120), a PI3K inhibitor, has been shown to have activity in vivo and in vitro against melanoma brain metastases.70 More studies need to be done to assess the possible combination with other established therapies.
Oblimersen is an antisense oligonucleotide that suppresses B-cell lymphoma-2, thereby suppressing its anti-apoptotic effect. The triple combination of oblimersen with temozolomide and albumin-bound paclitaxel has shown to be safe and efficacious in a phase 1 trial, thereby creating a need for further clinical trials.71
Treatment Approach
Systemic therapy for metastatic melanoma depends on several factors, including BRAF mutation status, functional status of the patient, disease burden, and severity of symptoms. Assessing the BRAF mutation status has become an important component in the management of patients with metastatic melanoma. It can help recognize patients who will benefit from molecular targeted therapy. In case of a BRAF-positive melanoma, treatment can be initiated with either immunotherapy or BRAF inhibitors. There are no randomized studies comparing immunotherapy to molecular targeted therapy.
Patients who have good PS and lymph node metastases can be treated initially with IL-2, which has the advantage of inducing cure in a minority of patients but should only be considered in patients with well-preserved organ function who can be monitored in an intensive care setting. On the other hand, patients who have bulky, symptomatic disease and poor PS should be treated initially with BRAF inhibitors. Combination of BRAF and MEK inhibitors can also be used and has an improved PFS and OS with potential to cause early tumor regression. There are studies to suggest suboptimal outcomes in patients who are treated with ipilimumab after progression on a BRAF inhibitor compared with initial treatment with ipilimumab followed by a BRAF inhibitor.72-74 However, all these studies are retrospective and there is no prospective data to suggest the above. BRAF mutation-positive patients who progress on a BRAF inhibitor
can be treated with PD-1 inhibitors.
Patients who do not have a BRAF mutation are unlikely to benefit from a BRAF inhibitor and primarily receive immunotherapy with ipilimumab or IL-2. Whenever possible, such patients should be enrolled in a clinical trial, as they have a poor prognosis. Patients who progress on ipilimumab can be treated with one of the PD-1 inhibitors (pembrolizumab, nivolumab). These PD-L1 inhibitors are still being investigated for use in such situations.
The role of chemotherapy in the management of metastatic melanoma has been limited by numerous studies showing significantly better survival with immunotherapy and molecular targeted therapy. Dacarbazine is the only FDA-approved drug for the treatment of melanoma. Its use is reserved mainly for patients who are not candidates for any of the other therapies available, including enrollment in a clinical trial.
Conclusion
Therapies for metastatic melanoma are in a state of flux. In the past decade, several new therapeutic agents have been introduced for the management of this potentially lethal disease. The treatment of metastatic melanoma has gradually shifted from cytotoxic chemotherapy toward a more individualized treatment that has a definite survival advantage over traditional counterparts. The advent of novel therapies has led to initiation of further studies to determine their role in the treatment of advanced melanoma, singly or in combination with other agents. In addition to evaluating new agents, more studies are needed to compare existing treatment modalities so that definitive treatment protocols can be formulated.
Acknowledgement
The authors would like to thank Felicia Ratnaraj, MD, for her assistance in creating the figures.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin.2014;64(1):9-29.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-6206.
3. Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma:
population based ecological study. BMJ. 2005;331(7515):481.
4. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011;117(20):4740-4746.
5. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention, & Therapy of Melanoma. New York, NY: Marcel Dekker;1997:219-225.
6. Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer. 2011;47(10):1476-1483.
7. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745-2751.
8. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with
or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31(3):373-379.
9. Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271(12):907-913.
10. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105-2116.
11. Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(suppl 1):S11-S14.
12. Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37(5):533-546.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
14. Schadendorf D, Hodi FS, Robert C, et. al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma [published online ahead of print February 9, 2015]. J Clin Oncol. pii:JCO.2014.56.2736.
15. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
16. Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312(17):1744-1753.
17. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
18. Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in 411 patients (pts) with melanoma (MEL) (Abstract LBA9000). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
19. Hamid O, Robert C, Ribas A, et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL) (abstract 3000). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
20. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014; 384(9948):1109-1117.
21. Dummer R, Daud A, Puzanov I, et. al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J Transl Med. 2015;13(suppl 1):O5.
22. Topalian SL, Sznol M, McDermott DF, et. al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030.
23. Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naive patients with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial (abstract 9002). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
24. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330.
25. Weber J, D’Angelo S, Gutzmer R, et al. A phase 3 randomized, open-label study of nivolumab versus investigator’s choice of chemotherapy in patients with advanced melanoma after prior anti-CTLA4 therapy (abstract LBA3). Paper presented at: European Society of Medical Oncology 2014 meeting; September 2014; Madrid, Spain.
26. Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma (abstract 9001). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
27. Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465.
28. Hamid O, Sosman JA, Lawrence DP, et. al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(15)(suppl): Abstract 9010.
29. Lutzky J, Antonia SJ, Blake-Haskins A, et. al. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol. 2014;32(15)(suppl): Abstract 3001.
30. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced
melanoma. N Engl J Med. 2013;369(2):122-133.
31. Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) (abstract LBA9003). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
32. Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9(17):6483-6488.
33. Wellbrock C, Hurlstone A. BRAF as therapeutic target in melanoma. Biochem Pharmacol. 2010;80(5):561-567.
34. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239-1246.
35. Ball NJ, Yohn JJ, Morelli JG, et al. Ras mutations in human melanoma: a marker of malignant progression. J Invest Dermatol. 1994;102(3):285-290.
36. Platz A, Ringborg U, Brahme EM, Lagerlöf B. Melanoma metastases from patients with hereditary cutaneous malignant melanoma contain a high frequency of N-ras activating mutations. Melanoma Res. 1994;4(3):169-177.
37. Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23(27):6771-6790.
38. Terai K, Matsuda M. The amino-terminal B-Raf-specific region mediates calcium-dependent homo- and hetero-dimerization of Raf. EMBO J. 2006;25(15):3556-3564.
39. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323-332.
40. Hauschild A, Grob JJ, Demidov LV, et al. An update on BREAK-3, a phase III, randomized trial: dabrafenib versus dacarbazine in patients with BRAF V600E-positive mutation metastatic melanoma (Abstract 9013). Paper presented at: American Society of Clinical Oncology 2013 meeting; May-June 2013; Chicago, IL.
41. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
42. Ascierto PA, Minor DR, Ribas A, et. al., Long-term safety and overall survival update for BREAK-2, a phase 2, single-arm, open-label study of dabrafenib in previously treated metastatic melanoma (NCT01153763). J Clin Oncol. 2014;32(15)(suppl): Abstract 9034.
43. Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with
BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety
study. Lancet Oncol. 2014;15(4):436-444.
44. Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18(3):314-322.
45. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207-215.
46. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
47. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358-365.
48. Ribas A, Hodi FS, Callahan M, et. al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2014;368(14):1365-1366.
49. Linette GP, Puzanov I, Callahan MK, et al. Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation–positive unresectable or metastatic melanoma (MM). J Clin Oncol. 2014;32(15)(suppl): Abstract 2511.
50. Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120(20):3142-3153.
51. Carlino MS, Gowrishankar K, Saunders CAB, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther. 2013;12(7):1332-1342.
52. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107-114.
53. Kim KB, Kefford R, Pavlick AC, et. al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31(1):482-489.
54. Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14(3):249-256.
55. Sosman JA, Kittaneh M, Lolkema MP, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity (abstract 9009). Paper presented at: 2014 American Society of Clinical Oncology meeting ; May-June 2014; Chicago, IL.
56. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877-1888.
57. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30-39.
58. Gogas H, Schadendorf D, Dummer R. Vemurafenib treatment in patients with BRAF-mutated melanoma failing MEK inhibition with trametinib. J Clin Oncol. 2014;32(15)(suppl): Abstract 9061.
59. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867-1876.
60. Kefford R, Miller WH, Tan DS, et al. Preliminary results from a phase Ib/II, openlabel, dose-escalation study of the oral BRAF inhibitor LGX818 in combination with the oral MEK1/2 inhibitor MEK162 in BRAF V600-dependent advanced solid tumors (abstract 9019). Paper presented at: 2013 American Society of Clinical Oncology meeting; May-June 2014; Chicago, IL.
61. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct
subtypes of melanoma. J Clin Oncol. 2006;24(26):4340-4346.
62. Jin SA, Chun SM, Choi YD, et al. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J Invest Dermatol. 2013;133(2):579-582.
63. Guo J, Si L, Kong Y et. al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904-2909.
64. Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182-3190.
65. Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2012;30(5): 2008-2014.
66. Lebbe C, Chevret S, Jouary T, et. al. Phase II multicentric uncontrolled national trial assessing the efficacy of nilotinib in the treatment of advanced melanomas with c-KIT mutation or amplification. J Clin Oncol. 2014;32(15)(suppl): Abstract 9032.
67. Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115(1):119-127.
68. Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma. Cancer. 2010;116(17): 4122-4129.
69. Dronca RS, Allred JB, Perez DG, et. al. Phase II study of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: a North Central Cancer Treatment Group study, N0675. Am J Clin Oncol. 2014;37(4):369-376.
70. Meier FE, Niessner H, Schmitz J, et al. The PI3K inhibitor BKM120 has potent antitumor activity in melanoma brain metastases in vitro and in vivo. J Clin Oncol. 2013;31(15)(suppl): Abstract e20050.
71. Ott PA, Chang J, Madden K, et al. Oblimersen in combination with temozolomide and albumin-bound paclitaxel in patients with advanced melanoma: a phase I trial. Cancer Chemother Pharmacol. 2013;71(1);183-191.
72. Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic
melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695-1701.
73. Ascierto PA, Margolin K. Ipilimumab before BRAF inhibitor treatment may be
more beneficial than vice versa for the majority of patients with advanced melanoma.
Cancer. 2014;120(11):1617-1619.
74. Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 2014;32(4):144-149.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin.2014;64(1):9-29.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-6206.
3. Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma:
population based ecological study. BMJ. 2005;331(7515):481.
4. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011;117(20):4740-4746.
5. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention, & Therapy of Melanoma. New York, NY: Marcel Dekker;1997:219-225.
6. Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer. 2011;47(10):1476-1483.
7. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745-2751.
8. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with
or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31(3):373-379.
9. Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271(12):907-913.
10. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105-2116.
11. Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(suppl 1):S11-S14.
12. Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37(5):533-546.
13. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
14. Schadendorf D, Hodi FS, Robert C, et. al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma [published online ahead of print February 9, 2015]. J Clin Oncol. pii:JCO.2014.56.2736.
15. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
16. Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312(17):1744-1753.
17. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
18. Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in 411 patients (pts) with melanoma (MEL) (Abstract LBA9000). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
19. Hamid O, Robert C, Ribas A, et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL) (abstract 3000). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
20. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014; 384(9948):1109-1117.
21. Dummer R, Daud A, Puzanov I, et. al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J Transl Med. 2015;13(suppl 1):O5.
22. Topalian SL, Sznol M, McDermott DF, et. al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030.
23. Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naive patients with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial (abstract 9002). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
24. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330.
25. Weber J, D’Angelo S, Gutzmer R, et al. A phase 3 randomized, open-label study of nivolumab versus investigator’s choice of chemotherapy in patients with advanced melanoma after prior anti-CTLA4 therapy (abstract LBA3). Paper presented at: European Society of Medical Oncology 2014 meeting; September 2014; Madrid, Spain.
26. Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma (abstract 9001). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
27. Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465.
28. Hamid O, Sosman JA, Lawrence DP, et. al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(15)(suppl): Abstract 9010.
29. Lutzky J, Antonia SJ, Blake-Haskins A, et. al. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol. 2014;32(15)(suppl): Abstract 3001.
30. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced
melanoma. N Engl J Med. 2013;369(2):122-133.
31. Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) (abstract LBA9003). Paper presented at: 2014 American Society of Clinical Oncology (ASCO) meeting; May-June 2014; Chicago, IL.
32. Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9(17):6483-6488.
33. Wellbrock C, Hurlstone A. BRAF as therapeutic target in melanoma. Biochem Pharmacol. 2010;80(5):561-567.
34. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239-1246.
35. Ball NJ, Yohn JJ, Morelli JG, et al. Ras mutations in human melanoma: a marker of malignant progression. J Invest Dermatol. 1994;102(3):285-290.
36. Platz A, Ringborg U, Brahme EM, Lagerlöf B. Melanoma metastases from patients with hereditary cutaneous malignant melanoma contain a high frequency of N-ras activating mutations. Melanoma Res. 1994;4(3):169-177.
37. Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23(27):6771-6790.
38. Terai K, Matsuda M. The amino-terminal B-Raf-specific region mediates calcium-dependent homo- and hetero-dimerization of Raf. EMBO J. 2006;25(15):3556-3564.
39. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323-332.
40. Hauschild A, Grob JJ, Demidov LV, et al. An update on BREAK-3, a phase III, randomized trial: dabrafenib versus dacarbazine in patients with BRAF V600E-positive mutation metastatic melanoma (Abstract 9013). Paper presented at: American Society of Clinical Oncology 2013 meeting; May-June 2013; Chicago, IL.
41. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
42. Ascierto PA, Minor DR, Ribas A, et. al., Long-term safety and overall survival update for BREAK-2, a phase 2, single-arm, open-label study of dabrafenib in previously treated metastatic melanoma (NCT01153763). J Clin Oncol. 2014;32(15)(suppl): Abstract 9034.
43. Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with
BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety
study. Lancet Oncol. 2014;15(4):436-444.
44. Lacouture ME, Duvic M, Hauschild A, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist. 2013;18(3):314-322.
45. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207-215.
46. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
47. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358-365.
48. Ribas A, Hodi FS, Callahan M, et. al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2014;368(14):1365-1366.
49. Linette GP, Puzanov I, Callahan MK, et al. Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation–positive unresectable or metastatic melanoma (MM). J Clin Oncol. 2014;32(15)(suppl): Abstract 2511.
50. Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120(20):3142-3153.
51. Carlino MS, Gowrishankar K, Saunders CAB, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther. 2013;12(7):1332-1342.
52. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107-114.
53. Kim KB, Kefford R, Pavlick AC, et. al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31(1):482-489.
54. Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14(3):249-256.
55. Sosman JA, Kittaneh M, Lolkema MP, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity (abstract 9009). Paper presented at: 2014 American Society of Clinical Oncology meeting ; May-June 2014; Chicago, IL.
56. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877-1888.
57. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30-39.
58. Gogas H, Schadendorf D, Dummer R. Vemurafenib treatment in patients with BRAF-mutated melanoma failing MEK inhibition with trametinib. J Clin Oncol. 2014;32(15)(suppl): Abstract 9061.
59. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867-1876.
60. Kefford R, Miller WH, Tan DS, et al. Preliminary results from a phase Ib/II, openlabel, dose-escalation study of the oral BRAF inhibitor LGX818 in combination with the oral MEK1/2 inhibitor MEK162 in BRAF V600-dependent advanced solid tumors (abstract 9019). Paper presented at: 2013 American Society of Clinical Oncology meeting; May-June 2014; Chicago, IL.
61. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct
subtypes of melanoma. J Clin Oncol. 2006;24(26):4340-4346.
62. Jin SA, Chun SM, Choi YD, et al. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J Invest Dermatol. 2013;133(2):579-582.
63. Guo J, Si L, Kong Y et. al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904-2909.
64. Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182-3190.
65. Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2012;30(5): 2008-2014.
66. Lebbe C, Chevret S, Jouary T, et. al. Phase II multicentric uncontrolled national trial assessing the efficacy of nilotinib in the treatment of advanced melanomas with c-KIT mutation or amplification. J Clin Oncol. 2014;32(15)(suppl): Abstract 9032.
67. Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115(1):119-127.
68. Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma. Cancer. 2010;116(17): 4122-4129.
69. Dronca RS, Allred JB, Perez DG, et. al. Phase II study of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: a North Central Cancer Treatment Group study, N0675. Am J Clin Oncol. 2014;37(4):369-376.
70. Meier FE, Niessner H, Schmitz J, et al. The PI3K inhibitor BKM120 has potent antitumor activity in melanoma brain metastases in vitro and in vivo. J Clin Oncol. 2013;31(15)(suppl): Abstract e20050.
71. Ott PA, Chang J, Madden K, et al. Oblimersen in combination with temozolomide and albumin-bound paclitaxel in patients with advanced melanoma: a phase I trial. Cancer Chemother Pharmacol. 2013;71(1);183-191.
72. Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic
melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695-1701.
73. Ascierto PA, Margolin K. Ipilimumab before BRAF inhibitor treatment may be
more beneficial than vice versa for the majority of patients with advanced melanoma.
Cancer. 2014;120(11):1617-1619.
74. Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest. 2014;32(4):144-149.
For Latinos, misperceptions and lack of medical care make preventing melanoma risky business
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 17
Machine learning melanoma
What if an app could diagnose melanoma from a photo? That was my idea. In December 2009, Google introduced Google Goggles, an application that recognized images. At the time, I thought, “Wouldn’t it be neat if we could use this with telederm?” I even pitched it to a friend at the search giant. “Great idea!” he wrote back, placating me. For those uninitiated in innovation, “Great idea!” is a euphemism for “Yeah, we thought of that.”
Yes, it isn’t only mine; no doubt, many of you had this same idea: Let’s use amazing image interpretation capabilities from companies like Google or Apple to help us make diagnoses. Sounds simple. It isn’t. This is why most melanoma-finding apps are for entertainment purposes only – they don’t work.
So can melanoma be diagnosed from an app? A Stanford University team believes so. They trained a machine learning system to make dermatologic diagnoses from photos of skin lesions. To overcome previous barriers, they used open-sourced software from Google and awesome processors. For a start, they pretrained the program on over 1.28 million images. Then they fed it 128,450 images of known diagnoses.
Then, just as when Google’s AlphaGo algorithm challenged Lee Sedol, the world Go champion, the Stanford research team challenged 21 dermatologists. They had to choose if they would biopsy/treat or reassure patients based on photos of benign lesions, keratinocyte carcinomas, clinical melanomas, and dermoscopic melanomas. Guess who won?
In a stunning victory (or defeat, if you’re rooting for our team), the trained algorithm matched or outperformed all the dermatologists when scored on sensitivity-specificity curves. While we dermatologists, of course, use more than just a photo to diagnose skin cancer, many around the globe don’t have access to us. Based on these findings, they might need access only to a smartphone to get potentially life-saving advice.
But, what does this mean? Will we someday be outsourced to AI? Will a future POTUS promise to “bring back the doctor industry?” Not if we adapt. The future is bright – if we learn to apply machine learning in ways that can have an impact. (Brain + Computer > Brain.) Consider the following: An optimized ophthalmologist who reads retinal scans prediagnosed by a computer. A teledermatologist who uses AI to perform perfectly in diagnosing melanoma.
Patients have always wanted high quality and high touch care. In the history of medicine, we’ve never been better at both than we are today. Until tomorrow, when we’ll be better still.
Jeff Benabio, MD, MBA, is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com. He has no disclosures related to this column.
What if an app could diagnose melanoma from a photo? That was my idea. In December 2009, Google introduced Google Goggles, an application that recognized images. At the time, I thought, “Wouldn’t it be neat if we could use this with telederm?” I even pitched it to a friend at the search giant. “Great idea!” he wrote back, placating me. For those uninitiated in innovation, “Great idea!” is a euphemism for “Yeah, we thought of that.”
Yes, it isn’t only mine; no doubt, many of you had this same idea: Let’s use amazing image interpretation capabilities from companies like Google or Apple to help us make diagnoses. Sounds simple. It isn’t. This is why most melanoma-finding apps are for entertainment purposes only – they don’t work.
So can melanoma be diagnosed from an app? A Stanford University team believes so. They trained a machine learning system to make dermatologic diagnoses from photos of skin lesions. To overcome previous barriers, they used open-sourced software from Google and awesome processors. For a start, they pretrained the program on over 1.28 million images. Then they fed it 128,450 images of known diagnoses.
Then, just as when Google’s AlphaGo algorithm challenged Lee Sedol, the world Go champion, the Stanford research team challenged 21 dermatologists. They had to choose if they would biopsy/treat or reassure patients based on photos of benign lesions, keratinocyte carcinomas, clinical melanomas, and dermoscopic melanomas. Guess who won?
In a stunning victory (or defeat, if you’re rooting for our team), the trained algorithm matched or outperformed all the dermatologists when scored on sensitivity-specificity curves. While we dermatologists, of course, use more than just a photo to diagnose skin cancer, many around the globe don’t have access to us. Based on these findings, they might need access only to a smartphone to get potentially life-saving advice.
But, what does this mean? Will we someday be outsourced to AI? Will a future POTUS promise to “bring back the doctor industry?” Not if we adapt. The future is bright – if we learn to apply machine learning in ways that can have an impact. (Brain + Computer > Brain.) Consider the following: An optimized ophthalmologist who reads retinal scans prediagnosed by a computer. A teledermatologist who uses AI to perform perfectly in diagnosing melanoma.
Patients have always wanted high quality and high touch care. In the history of medicine, we’ve never been better at both than we are today. Until tomorrow, when we’ll be better still.
Jeff Benabio, MD, MBA, is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com. He has no disclosures related to this column.
What if an app could diagnose melanoma from a photo? That was my idea. In December 2009, Google introduced Google Goggles, an application that recognized images. At the time, I thought, “Wouldn’t it be neat if we could use this with telederm?” I even pitched it to a friend at the search giant. “Great idea!” he wrote back, placating me. For those uninitiated in innovation, “Great idea!” is a euphemism for “Yeah, we thought of that.”
Yes, it isn’t only mine; no doubt, many of you had this same idea: Let’s use amazing image interpretation capabilities from companies like Google or Apple to help us make diagnoses. Sounds simple. It isn’t. This is why most melanoma-finding apps are for entertainment purposes only – they don’t work.
So can melanoma be diagnosed from an app? A Stanford University team believes so. They trained a machine learning system to make dermatologic diagnoses from photos of skin lesions. To overcome previous barriers, they used open-sourced software from Google and awesome processors. For a start, they pretrained the program on over 1.28 million images. Then they fed it 128,450 images of known diagnoses.
Then, just as when Google’s AlphaGo algorithm challenged Lee Sedol, the world Go champion, the Stanford research team challenged 21 dermatologists. They had to choose if they would biopsy/treat or reassure patients based on photos of benign lesions, keratinocyte carcinomas, clinical melanomas, and dermoscopic melanomas. Guess who won?
In a stunning victory (or defeat, if you’re rooting for our team), the trained algorithm matched or outperformed all the dermatologists when scored on sensitivity-specificity curves. While we dermatologists, of course, use more than just a photo to diagnose skin cancer, many around the globe don’t have access to us. Based on these findings, they might need access only to a smartphone to get potentially life-saving advice.
But, what does this mean? Will we someday be outsourced to AI? Will a future POTUS promise to “bring back the doctor industry?” Not if we adapt. The future is bright – if we learn to apply machine learning in ways that can have an impact. (Brain + Computer > Brain.) Consider the following: An optimized ophthalmologist who reads retinal scans prediagnosed by a computer. A teledermatologist who uses AI to perform perfectly in diagnosing melanoma.
Patients have always wanted high quality and high touch care. In the history of medicine, we’ve never been better at both than we are today. Until tomorrow, when we’ll be better still.
Jeff Benabio, MD, MBA, is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com. He has no disclosures related to this column.