User login
How does gender-affirming hormone therapy affect QOL in transgender patients?
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.
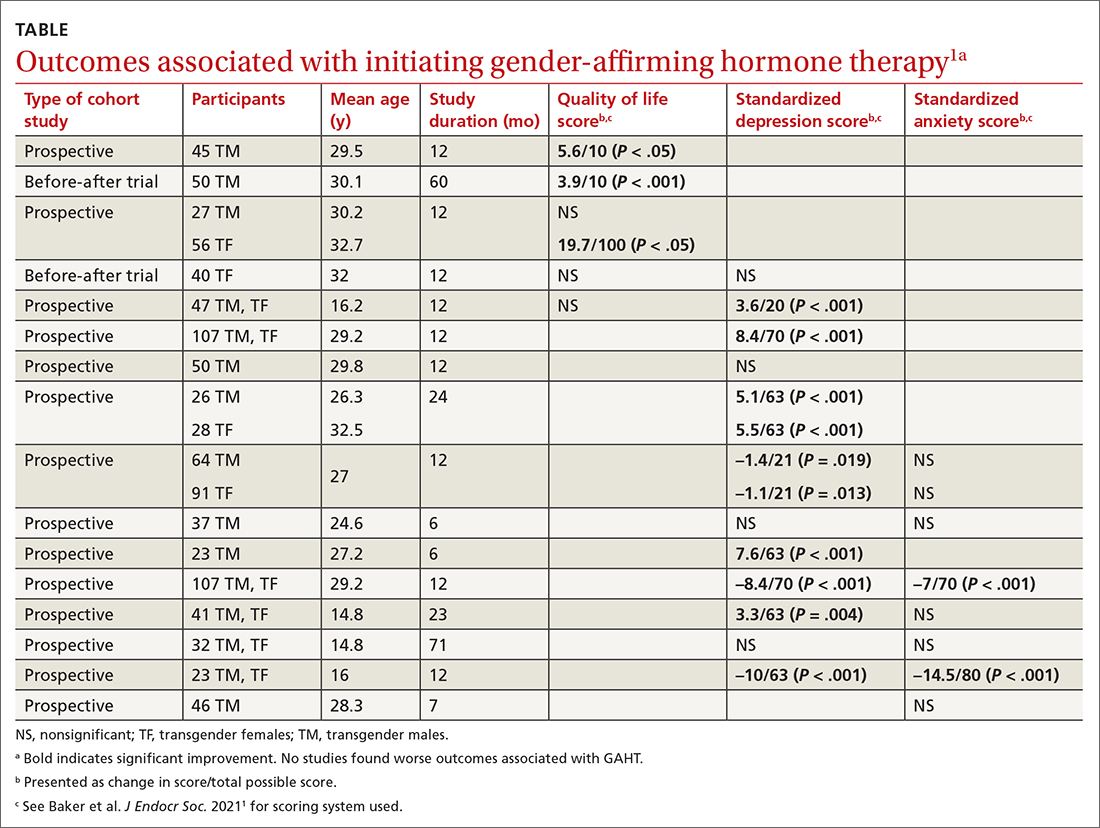
The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
EVIDENCE-BASED ANSWER:
There are modest effects on depression but not anxiety. Gender-affirming hormone therapy (GAHT) is associated with modest improvements in standardized scores for quality of life (QOL) and depression in adult male-to-female and female-to-male transgender people and modest improvements in depression scores in transgender adolescents, but the effect on anxiety is uncertain (strength of recommendation [SOR]: B, based on a preponderance of low-quality prospective cohort studies with inconsistent results).
GAHT is associated with reduced gender dysphoria and decreased suicidality (SOR: B, based on a prospective cohort study). However, there is insufficient evidence to determine any effect on suicide completion. No studies associated GAHT with worsened QOL, depression, or anxiety scores.
Mood stabilizers, particularly lithium, potential lifesavers in bipolar disorder
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.
Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
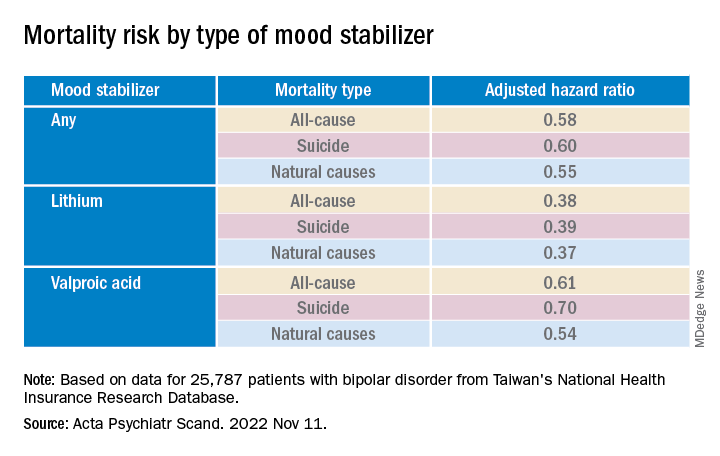
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.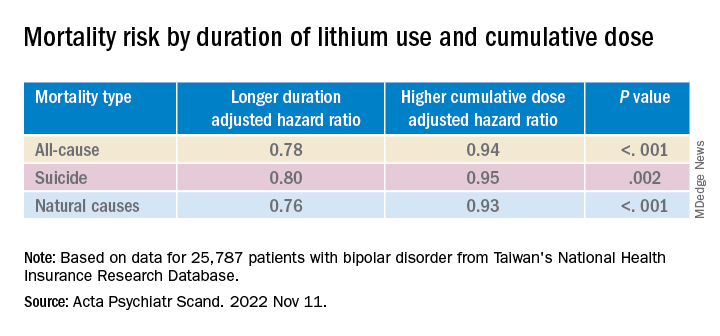
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.

Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.

Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM ACTA PSYCHIATRICA SCANDINAVICA
Digital treatment may help relieve PTSD, panic disorder
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.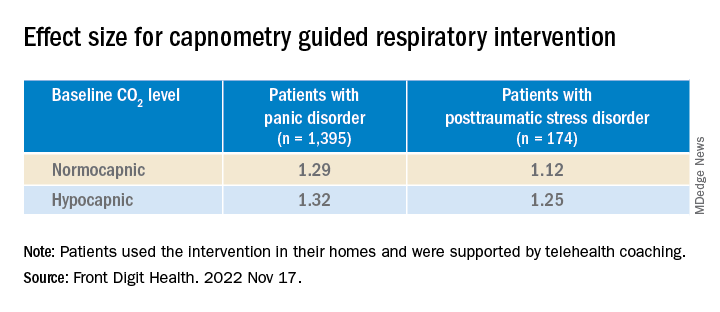
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN DIGITAL HEALTH
How your voice could reveal hidden disease
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
No, you can’t see a different doctor: We need zero tolerance of patient bias
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Poison centers fielding more calls about teen cannabis use
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Teens’ undisclosed dieting may precede anorexia nervosa diagnosis
Adolescents later diagnosed with anorexia nervosa (AN) likely embark on the trajectory to AN with undisclosed dieting for weight loss at about age 14, a study of teens and parents found.
In the interview-based study, both adolescents and their parents described a similar prediagnosis sequence of behavioral changes occurring over roughly 1 year to 18 months, but parents lagged some 6 months behind in noticing their children’s disordered eating.
The findings suggest that even teens of normal weight should be asked about their eating habits and monitored more closely for contact with those who endorse these potentially harmful eating behaviors, according to Lisa M. Ranzenhofer, PhD, assistant professor of clinical psychology in psychiatry at Columbia University Medical Center in New York, and colleagues. Their report is in the Journal of Adolescent Health.
“We know that adolescents often have eating disorder behaviors long before they’re diagnosed, so we developed this interview as a tool to figure out how long a maladaptive behavior has been present,” Dr. Ranzenhofer said in an interview. “Most studies that report illness duration do so based on diagnosis, so this interview provides a more fine-grained assessment of the duration of problematic behavior, which may help improve understanding of the impact of duration on outcome, and hopefully facilitate better methods for early detection.” Since healthy adolescents are often seen once per year at an annual pediatrician visit, she added, teens engaging in significant dieting might benefit from more frequent monitoring since this behavior can evolve into an eating disorder over a relatively short time frame.
AN is associated with significant medical and psychiatric comorbidity and has a mortality rate among the highest of any psychiatric illness, the authors noted.
The study
The study cohort consisted of 71 girls ages 12-18 years participating in research from 2017 to 2021 at the Eating Disorders Research Unit of New York (N.Y.) State Psychiatric Institute. Patients had either the restricting or binge-eating/purging subtype of AN as diagnosed by the Eating Disorder Assessment–5 questionnaire. A semistructured 15-minute interview with the girls and their parents explored food restriction, dieting, loss of control/binge eating, purging, excessive/compulsive exercise, weight history, and amenorrhea.
Both parents and children were asked whether and when the children had been underweight or overweight, and whether and when primary amenorrhea (no menarche) or secondary amenorrhea (periods missed for 3 months) became evident. Dieting was defined as “deliberately changing eating patterns in any way to influence your shape or weight,” and restriction as “deliberately cutting down on the amount of food that you are eating, in order to change your shape or weight.” Loss-of-control eating was defined as “feeling unable to stop eating or control what or how much you are eating.”
In other characterizations, purging was defined as making yourself vomit on purpose, taking diuretics, or feeling driven to engage in these behaviors. Questions on exercise explored whether children might feel anxious when they do not exercise or inclined to exercise even if sick or injured, with excessive exercise defined as “Feeling like you must exercise, might continue exercising, sometimes in secret, if parents or doctors have told you to stop.”
Other questions focused on use of diuretics or laxatives and other strategies to compensate for calories consumed.
Responses revealed that restriction, underweight, dieting, and excessive exercise were present in most of the sample, while purging, loss-of-control eating, and overweight were reported by fewer than a third. With dieting typically emerging first around age 14, the other behaviors tended to manifest from age 14 to 14 and a half. The average age of formal diagnosis was just over 15 years. Parent-child dyads showed good agreement on the presence and timing of all behaviors except for dieting, for which children reported onset about 6 months earlier or longer duration compared with parents.
Although older age at the time of interview was associated with a lower body mass index percentile and higher eating disorder score, neither age of onset nor duration of disordered eating was associated with severity when researchers controlled for current age.
Telltale signs for parents
“For teens starting at a healthy weight, significant and intentional weight loss of more than 5-10 pounds can be a cause for concern,” Dr. Ranzenhofer said. Missed periods, refusing meals, skipping meals, fighting or arguing about eating, and withdrawal from normal activities and relationships are other signs of disordered eating. For overweight or obese teens, rapid weight loss and weight loss above and beyond that recommended are also concerning.
As for compulsive exercise, she said, “Altered exercise behavior might look like exercise that interferes with other activities, for example, being late to school or not doing homework in order to exercise.” Other red flags would be physical activity that varies considerably from that of peers, for instance, going running after a 2-hour sports practice and an inflexible routine that precludes being able to skip a day.
“All adolescents, male and female, should be screened regardless of weight trends – underweight, overweight, obese, or normal weight – regarding their body image and thoughts of dieting,” said Margaret E. Thew, DNP, FNP-BC, of the Medical College of Wisconsin, and medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, commenting on the study but not involved in it. “Most adolescents make decisions to lose weight after trying to ‘eat healthy’ but may take an aggressive approach when they don’t see the weight loss they hope to see.”
According to Ms. Thew, the study findings support the benefit of giving medical caregivers and parents training on the red flags regarding eating disorders to foster early detection. “These include starting a new fad diet, eliminating foods, ‘healthy eating,’ over-exercising, skipping meals, or no longer eating foods they previously loved.”
She added that times of transition are key junctures to watch: The transition from grade school to middle school, middle to high school, and high school to college. “These tend to provoke eating disorder onset or relapse of eating disorder thoughts and behaviors after diagnosis,” Ms. Thew said. “It would benefit the patient to screen for concerns about disordered eating and provide resources, including consultation with a dietitian, as appropriate.”
This study was supported by grants from the National Institute of Mental Health and the Hilda and Preston Davis Foundation. Coauthor Joanna E. Steinglass, MD, disclosed receiving royalties from UpToDate. Ms. Thew disclosed no competing interests with regard to her comments.
Adolescents later diagnosed with anorexia nervosa (AN) likely embark on the trajectory to AN with undisclosed dieting for weight loss at about age 14, a study of teens and parents found.
In the interview-based study, both adolescents and their parents described a similar prediagnosis sequence of behavioral changes occurring over roughly 1 year to 18 months, but parents lagged some 6 months behind in noticing their children’s disordered eating.
The findings suggest that even teens of normal weight should be asked about their eating habits and monitored more closely for contact with those who endorse these potentially harmful eating behaviors, according to Lisa M. Ranzenhofer, PhD, assistant professor of clinical psychology in psychiatry at Columbia University Medical Center in New York, and colleagues. Their report is in the Journal of Adolescent Health.
“We know that adolescents often have eating disorder behaviors long before they’re diagnosed, so we developed this interview as a tool to figure out how long a maladaptive behavior has been present,” Dr. Ranzenhofer said in an interview. “Most studies that report illness duration do so based on diagnosis, so this interview provides a more fine-grained assessment of the duration of problematic behavior, which may help improve understanding of the impact of duration on outcome, and hopefully facilitate better methods for early detection.” Since healthy adolescents are often seen once per year at an annual pediatrician visit, she added, teens engaging in significant dieting might benefit from more frequent monitoring since this behavior can evolve into an eating disorder over a relatively short time frame.
AN is associated with significant medical and psychiatric comorbidity and has a mortality rate among the highest of any psychiatric illness, the authors noted.
The study
The study cohort consisted of 71 girls ages 12-18 years participating in research from 2017 to 2021 at the Eating Disorders Research Unit of New York (N.Y.) State Psychiatric Institute. Patients had either the restricting or binge-eating/purging subtype of AN as diagnosed by the Eating Disorder Assessment–5 questionnaire. A semistructured 15-minute interview with the girls and their parents explored food restriction, dieting, loss of control/binge eating, purging, excessive/compulsive exercise, weight history, and amenorrhea.
Both parents and children were asked whether and when the children had been underweight or overweight, and whether and when primary amenorrhea (no menarche) or secondary amenorrhea (periods missed for 3 months) became evident. Dieting was defined as “deliberately changing eating patterns in any way to influence your shape or weight,” and restriction as “deliberately cutting down on the amount of food that you are eating, in order to change your shape or weight.” Loss-of-control eating was defined as “feeling unable to stop eating or control what or how much you are eating.”
In other characterizations, purging was defined as making yourself vomit on purpose, taking diuretics, or feeling driven to engage in these behaviors. Questions on exercise explored whether children might feel anxious when they do not exercise or inclined to exercise even if sick or injured, with excessive exercise defined as “Feeling like you must exercise, might continue exercising, sometimes in secret, if parents or doctors have told you to stop.”
Other questions focused on use of diuretics or laxatives and other strategies to compensate for calories consumed.
Responses revealed that restriction, underweight, dieting, and excessive exercise were present in most of the sample, while purging, loss-of-control eating, and overweight were reported by fewer than a third. With dieting typically emerging first around age 14, the other behaviors tended to manifest from age 14 to 14 and a half. The average age of formal diagnosis was just over 15 years. Parent-child dyads showed good agreement on the presence and timing of all behaviors except for dieting, for which children reported onset about 6 months earlier or longer duration compared with parents.
Although older age at the time of interview was associated with a lower body mass index percentile and higher eating disorder score, neither age of onset nor duration of disordered eating was associated with severity when researchers controlled for current age.
Telltale signs for parents
“For teens starting at a healthy weight, significant and intentional weight loss of more than 5-10 pounds can be a cause for concern,” Dr. Ranzenhofer said. Missed periods, refusing meals, skipping meals, fighting or arguing about eating, and withdrawal from normal activities and relationships are other signs of disordered eating. For overweight or obese teens, rapid weight loss and weight loss above and beyond that recommended are also concerning.
As for compulsive exercise, she said, “Altered exercise behavior might look like exercise that interferes with other activities, for example, being late to school or not doing homework in order to exercise.” Other red flags would be physical activity that varies considerably from that of peers, for instance, going running after a 2-hour sports practice and an inflexible routine that precludes being able to skip a day.
“All adolescents, male and female, should be screened regardless of weight trends – underweight, overweight, obese, or normal weight – regarding their body image and thoughts of dieting,” said Margaret E. Thew, DNP, FNP-BC, of the Medical College of Wisconsin, and medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, commenting on the study but not involved in it. “Most adolescents make decisions to lose weight after trying to ‘eat healthy’ but may take an aggressive approach when they don’t see the weight loss they hope to see.”
According to Ms. Thew, the study findings support the benefit of giving medical caregivers and parents training on the red flags regarding eating disorders to foster early detection. “These include starting a new fad diet, eliminating foods, ‘healthy eating,’ over-exercising, skipping meals, or no longer eating foods they previously loved.”
She added that times of transition are key junctures to watch: The transition from grade school to middle school, middle to high school, and high school to college. “These tend to provoke eating disorder onset or relapse of eating disorder thoughts and behaviors after diagnosis,” Ms. Thew said. “It would benefit the patient to screen for concerns about disordered eating and provide resources, including consultation with a dietitian, as appropriate.”
This study was supported by grants from the National Institute of Mental Health and the Hilda and Preston Davis Foundation. Coauthor Joanna E. Steinglass, MD, disclosed receiving royalties from UpToDate. Ms. Thew disclosed no competing interests with regard to her comments.
Adolescents later diagnosed with anorexia nervosa (AN) likely embark on the trajectory to AN with undisclosed dieting for weight loss at about age 14, a study of teens and parents found.
In the interview-based study, both adolescents and their parents described a similar prediagnosis sequence of behavioral changes occurring over roughly 1 year to 18 months, but parents lagged some 6 months behind in noticing their children’s disordered eating.
The findings suggest that even teens of normal weight should be asked about their eating habits and monitored more closely for contact with those who endorse these potentially harmful eating behaviors, according to Lisa M. Ranzenhofer, PhD, assistant professor of clinical psychology in psychiatry at Columbia University Medical Center in New York, and colleagues. Their report is in the Journal of Adolescent Health.
“We know that adolescents often have eating disorder behaviors long before they’re diagnosed, so we developed this interview as a tool to figure out how long a maladaptive behavior has been present,” Dr. Ranzenhofer said in an interview. “Most studies that report illness duration do so based on diagnosis, so this interview provides a more fine-grained assessment of the duration of problematic behavior, which may help improve understanding of the impact of duration on outcome, and hopefully facilitate better methods for early detection.” Since healthy adolescents are often seen once per year at an annual pediatrician visit, she added, teens engaging in significant dieting might benefit from more frequent monitoring since this behavior can evolve into an eating disorder over a relatively short time frame.
AN is associated with significant medical and psychiatric comorbidity and has a mortality rate among the highest of any psychiatric illness, the authors noted.
The study
The study cohort consisted of 71 girls ages 12-18 years participating in research from 2017 to 2021 at the Eating Disorders Research Unit of New York (N.Y.) State Psychiatric Institute. Patients had either the restricting or binge-eating/purging subtype of AN as diagnosed by the Eating Disorder Assessment–5 questionnaire. A semistructured 15-minute interview with the girls and their parents explored food restriction, dieting, loss of control/binge eating, purging, excessive/compulsive exercise, weight history, and amenorrhea.
Both parents and children were asked whether and when the children had been underweight or overweight, and whether and when primary amenorrhea (no menarche) or secondary amenorrhea (periods missed for 3 months) became evident. Dieting was defined as “deliberately changing eating patterns in any way to influence your shape or weight,” and restriction as “deliberately cutting down on the amount of food that you are eating, in order to change your shape or weight.” Loss-of-control eating was defined as “feeling unable to stop eating or control what or how much you are eating.”
In other characterizations, purging was defined as making yourself vomit on purpose, taking diuretics, or feeling driven to engage in these behaviors. Questions on exercise explored whether children might feel anxious when they do not exercise or inclined to exercise even if sick or injured, with excessive exercise defined as “Feeling like you must exercise, might continue exercising, sometimes in secret, if parents or doctors have told you to stop.”
Other questions focused on use of diuretics or laxatives and other strategies to compensate for calories consumed.
Responses revealed that restriction, underweight, dieting, and excessive exercise were present in most of the sample, while purging, loss-of-control eating, and overweight were reported by fewer than a third. With dieting typically emerging first around age 14, the other behaviors tended to manifest from age 14 to 14 and a half. The average age of formal diagnosis was just over 15 years. Parent-child dyads showed good agreement on the presence and timing of all behaviors except for dieting, for which children reported onset about 6 months earlier or longer duration compared with parents.
Although older age at the time of interview was associated with a lower body mass index percentile and higher eating disorder score, neither age of onset nor duration of disordered eating was associated with severity when researchers controlled for current age.
Telltale signs for parents
“For teens starting at a healthy weight, significant and intentional weight loss of more than 5-10 pounds can be a cause for concern,” Dr. Ranzenhofer said. Missed periods, refusing meals, skipping meals, fighting or arguing about eating, and withdrawal from normal activities and relationships are other signs of disordered eating. For overweight or obese teens, rapid weight loss and weight loss above and beyond that recommended are also concerning.
As for compulsive exercise, she said, “Altered exercise behavior might look like exercise that interferes with other activities, for example, being late to school or not doing homework in order to exercise.” Other red flags would be physical activity that varies considerably from that of peers, for instance, going running after a 2-hour sports practice and an inflexible routine that precludes being able to skip a day.
“All adolescents, male and female, should be screened regardless of weight trends – underweight, overweight, obese, or normal weight – regarding their body image and thoughts of dieting,” said Margaret E. Thew, DNP, FNP-BC, of the Medical College of Wisconsin, and medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, commenting on the study but not involved in it. “Most adolescents make decisions to lose weight after trying to ‘eat healthy’ but may take an aggressive approach when they don’t see the weight loss they hope to see.”
According to Ms. Thew, the study findings support the benefit of giving medical caregivers and parents training on the red flags regarding eating disorders to foster early detection. “These include starting a new fad diet, eliminating foods, ‘healthy eating,’ over-exercising, skipping meals, or no longer eating foods they previously loved.”
She added that times of transition are key junctures to watch: The transition from grade school to middle school, middle to high school, and high school to college. “These tend to provoke eating disorder onset or relapse of eating disorder thoughts and behaviors after diagnosis,” Ms. Thew said. “It would benefit the patient to screen for concerns about disordered eating and provide resources, including consultation with a dietitian, as appropriate.”
This study was supported by grants from the National Institute of Mental Health and the Hilda and Preston Davis Foundation. Coauthor Joanna E. Steinglass, MD, disclosed receiving royalties from UpToDate. Ms. Thew disclosed no competing interests with regard to her comments.
FROM JOURNAL OF ADOLESCENT HEALTH
Does dopamine dysregulation cause schizophrenia?
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Mind the geriatrician gap
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
Ultraprocessed foods tied to faster rate of cognitive decline
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY









