User login
ED visits for kids with suicidal thoughts increasing: Study
A new study sheds light on the escalating youth suicide crisis, revealing that emergency room visits for suicidal thoughts among kids and teens steeply increased even before the start of the COVID-19 pandemic.
Emergency room visits for “suicidal ideation” (or suicidal thoughts) among 5- to 19-year-olds increased 59% from 2016 to 2021, and hospitalizations rose 57% from fall 2019 to the fall of 2020, according to the study published in Pediatrics.
“A lot of people have talked about mental health problems in youth during the pandemic, but it was happening before the pandemic,” said author Audrey Brewer, MD, MPH, in a news release from the Ann and Robert H. Lurie Children’s Hospital of Chicago. “This has been an issue for so long, and it’s getting worse.”
Researchers looked at data for 81,105 emergency room visits across 205 Illinois hospitals from 2016 to 2021 for kids between the ages of 5 and 19.
The researchers found “there was a very sharp spike in fall 2019, followed by a similar spike during the pandemic fall of 2020, with the highest number of monthly visits during October 2020,” the authors said. “Youth aged 14-17 years had the highest frequency of [suicidal ideation emergency room] monthly visits, with visits in this group greater than the other age groups combined.”
Last year, the Centers for Disease Control and Prevention announced that suicide is the second leading cause of death among 10- to 19-year-olds.
The new research is being called a benchmark because it evaluates emergency room data for suicidal thoughts – a critical point of care for serving youths’ mental health needs. The data showed that providers were increasingly likely to list suicidal thoughts as the main diagnosis.
“Suicidal ideation can be thought about as two types: actively thinking about suicide or having thoughts, but not having a plan,” Dr. Brewer said in the news release. “That could be the difference in why someone might get admitted to the hospital.”
The researchers hypothesize that care in 2019 (when the initial spike occurred) was delayed in the early days of the pandemic, and that delay possibly contributed to the increase in providers identifying suicidal ideation as the main diagnosis.
“The early pandemic period coincided with constrained access to pediatric mental health services through schools, pediatric primary care homes, and mental health clinics for many children and their families,” the authors wrote. “The proportion of child mental health visits increased relative to other types as patients avoided ED visits during the early wave of the COVID-19 pandemic. Thus, the increase in hospitalizations during fall 2020 may reflect patients’ deferring care until symptoms became even more severe.”
Other health care scholars agreed the study spurred questions about whether the pandemic was truly the source of the crisis.
“Was it the pandemic that exacerbated the increase or is this a growing trend?” wrote Lisa M. Horowitz, PhD, MPH, and Jeffrey A. Bridge, PhD, in a commentary published along with the study. “These rising rates underscore the worsening mental health crisis for youth, as noted by the 2022 Surgeon General report and several youth mental health organizations.”
A version of this article first appeared on WebMD.com.
A new study sheds light on the escalating youth suicide crisis, revealing that emergency room visits for suicidal thoughts among kids and teens steeply increased even before the start of the COVID-19 pandemic.
Emergency room visits for “suicidal ideation” (or suicidal thoughts) among 5- to 19-year-olds increased 59% from 2016 to 2021, and hospitalizations rose 57% from fall 2019 to the fall of 2020, according to the study published in Pediatrics.
“A lot of people have talked about mental health problems in youth during the pandemic, but it was happening before the pandemic,” said author Audrey Brewer, MD, MPH, in a news release from the Ann and Robert H. Lurie Children’s Hospital of Chicago. “This has been an issue for so long, and it’s getting worse.”
Researchers looked at data for 81,105 emergency room visits across 205 Illinois hospitals from 2016 to 2021 for kids between the ages of 5 and 19.
The researchers found “there was a very sharp spike in fall 2019, followed by a similar spike during the pandemic fall of 2020, with the highest number of monthly visits during October 2020,” the authors said. “Youth aged 14-17 years had the highest frequency of [suicidal ideation emergency room] monthly visits, with visits in this group greater than the other age groups combined.”
Last year, the Centers for Disease Control and Prevention announced that suicide is the second leading cause of death among 10- to 19-year-olds.
The new research is being called a benchmark because it evaluates emergency room data for suicidal thoughts – a critical point of care for serving youths’ mental health needs. The data showed that providers were increasingly likely to list suicidal thoughts as the main diagnosis.
“Suicidal ideation can be thought about as two types: actively thinking about suicide or having thoughts, but not having a plan,” Dr. Brewer said in the news release. “That could be the difference in why someone might get admitted to the hospital.”
The researchers hypothesize that care in 2019 (when the initial spike occurred) was delayed in the early days of the pandemic, and that delay possibly contributed to the increase in providers identifying suicidal ideation as the main diagnosis.
“The early pandemic period coincided with constrained access to pediatric mental health services through schools, pediatric primary care homes, and mental health clinics for many children and their families,” the authors wrote. “The proportion of child mental health visits increased relative to other types as patients avoided ED visits during the early wave of the COVID-19 pandemic. Thus, the increase in hospitalizations during fall 2020 may reflect patients’ deferring care until symptoms became even more severe.”
Other health care scholars agreed the study spurred questions about whether the pandemic was truly the source of the crisis.
“Was it the pandemic that exacerbated the increase or is this a growing trend?” wrote Lisa M. Horowitz, PhD, MPH, and Jeffrey A. Bridge, PhD, in a commentary published along with the study. “These rising rates underscore the worsening mental health crisis for youth, as noted by the 2022 Surgeon General report and several youth mental health organizations.”
A version of this article first appeared on WebMD.com.
A new study sheds light on the escalating youth suicide crisis, revealing that emergency room visits for suicidal thoughts among kids and teens steeply increased even before the start of the COVID-19 pandemic.
Emergency room visits for “suicidal ideation” (or suicidal thoughts) among 5- to 19-year-olds increased 59% from 2016 to 2021, and hospitalizations rose 57% from fall 2019 to the fall of 2020, according to the study published in Pediatrics.
“A lot of people have talked about mental health problems in youth during the pandemic, but it was happening before the pandemic,” said author Audrey Brewer, MD, MPH, in a news release from the Ann and Robert H. Lurie Children’s Hospital of Chicago. “This has been an issue for so long, and it’s getting worse.”
Researchers looked at data for 81,105 emergency room visits across 205 Illinois hospitals from 2016 to 2021 for kids between the ages of 5 and 19.
The researchers found “there was a very sharp spike in fall 2019, followed by a similar spike during the pandemic fall of 2020, with the highest number of monthly visits during October 2020,” the authors said. “Youth aged 14-17 years had the highest frequency of [suicidal ideation emergency room] monthly visits, with visits in this group greater than the other age groups combined.”
Last year, the Centers for Disease Control and Prevention announced that suicide is the second leading cause of death among 10- to 19-year-olds.
The new research is being called a benchmark because it evaluates emergency room data for suicidal thoughts – a critical point of care for serving youths’ mental health needs. The data showed that providers were increasingly likely to list suicidal thoughts as the main diagnosis.
“Suicidal ideation can be thought about as two types: actively thinking about suicide or having thoughts, but not having a plan,” Dr. Brewer said in the news release. “That could be the difference in why someone might get admitted to the hospital.”
The researchers hypothesize that care in 2019 (when the initial spike occurred) was delayed in the early days of the pandemic, and that delay possibly contributed to the increase in providers identifying suicidal ideation as the main diagnosis.
“The early pandemic period coincided with constrained access to pediatric mental health services through schools, pediatric primary care homes, and mental health clinics for many children and their families,” the authors wrote. “The proportion of child mental health visits increased relative to other types as patients avoided ED visits during the early wave of the COVID-19 pandemic. Thus, the increase in hospitalizations during fall 2020 may reflect patients’ deferring care until symptoms became even more severe.”
Other health care scholars agreed the study spurred questions about whether the pandemic was truly the source of the crisis.
“Was it the pandemic that exacerbated the increase or is this a growing trend?” wrote Lisa M. Horowitz, PhD, MPH, and Jeffrey A. Bridge, PhD, in a commentary published along with the study. “These rising rates underscore the worsening mental health crisis for youth, as noted by the 2022 Surgeon General report and several youth mental health organizations.”
A version of this article first appeared on WebMD.com.
FROM PEDIATRICS
Watching violent TV in preschool linked with emotional, behavioral issues at age 12
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
FROM THE JOURNAL OF DEVELOPMENTAL & BEHAVIORAL PEDIATRICS
Study finds high rate of psychiatric burden in cosmetic dermatology patients
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Medicaid Expansion and Veterans’ Reliance on the VA for Depression Care
The US Department of Veterans Affairs (VA) is the largest integrated health care system in the United States, providing care for more than 9 million veterans.1 With veterans experiencing mental health conditions like posttraumatic stress disorder (PTSD), substance use disorders, and other serious mental illnesses (SMI) at higher rates compared with the general population, the VA plays an important role in the provision of mental health services.2-5 Since the implementation of its Mental Health Strategic Plan in 2004, the VA has overseen the development of a wide array of mental health programs geared toward the complex needs of veterans. Research has demonstrated VA care outperforming Medicaid-reimbursed services in terms of the percentage of veterans filling antidepressants for at least 12 weeks after initiation of treatment for major depressive disorder (MDD), as well as posthospitalization follow-up.6
Eligible veterans enrolled in the VA often also seek non-VA care. Medicaid covers nearly 10% of all nonelderly veterans, and of these veterans, 39% rely solely on Medicaid for health care access.7 Today, Medicaid is the largest payer for mental health services in the US, providing coverage for approximately 27% of Americans who have SMI and helping fulfill unmet mental health needs.8,9 Understanding which of these systems veterans choose to use, and under which circumstances, is essential in guiding the allocation of limited health care resources.10
Beyond Medicaid, alternatives to VA care may include TRICARE, Medicare, Indian Health Services, and employer-based or self-purchased private insurance. While these options potentially increase convenience, choice, and access to health care practitioners (HCPs) and services not available at local VA systems, cross-system utilization with poor integration may cause care coordination and continuity problems, such as medication mismanagement and opioid overdose, unnecessary duplicate utilization, and possible increased mortality.11-15 As recent national legislative changes, such as the Patient Protection and Affordable Care Act (ACA), Veterans Access, Choice and Accountability Act, and the VA MISSION Act, continue to shift the health care landscape for veterans, questions surrounding how veterans are changing their health care use become significant.16,17
Here, we approach the impacts of Medicaid expansion on veterans’ reliance on the VA for mental health services with a unique lens. We leverage a difference-in-difference design to study 2 historical Medicaid expansions in Arizona (AZ) and New York (NY), which extended eligibility to childless adults in 2001. Prior Medicaid dual-eligible mental health research investigated reliance shifts during the immediate postenrollment year in a subset of veterans newly enrolled in Medicaid.18 However, this study took place in a period of relative policy stability. In contrast, we investigate the potential effects of a broad policy shift by analyzing state-level changes in veterans’ reliance over 6 years after a statewide Medicaid expansion. We match expansion states with demographically similar nonexpansion states to account for unobserved trends and confounding effects. Prior studies have used this method to evaluate post-Medicaid expansion mortality changes and changes in veteran dual enrollment and hospitalizations.10,19 While a study of ACA Medicaid expansion states would be ideal, Medicaid data from most states were only available through 2014 at the time of this analysis. Our study offers a quasi-experimental framework leveraging longitudinal data that can be applied as more post-ACA data become available.
Given the rising incidence of suicide among veterans, understanding care-seeking behaviors for depression among veterans is important as it is the most common psychiatric condition found in those who died by suicide.20,21 Furthermore, depression may be useful as a clinical proxy for mental health policy impacts, given that the Patient Health Questionnaire-9 (PHQ-9) screening tool is well validated and increasingly research accessible, and it is a chronic condition responsive to both well-managed pharmacologic treatment and psychotherapeutic interventions.22,23
In this study, we quantify the change in care-seeking behavior for depression among veterans after Medicaid expansion, using a quasi-experimental design. We hypothesize that new access to Medicaid would be associated with a shift away from using VA services for depression. Given the income-dependent eligibility requirements of Medicaid, we also hypothesize that veterans who qualified for VA coverage due to low income, determined by a regional means test (Priority group 5, “income-eligible”), would be more likely to shift care compared with those whose serviced-connected conditions related to their military service (Priority groups 1-4, “service-connected”) provide VA access.
Methods
To investigate the relative changes in veterans’ reliance on the VA for depression care after the 2001 NY and AZ Medicaid expansions We used a retrospective, difference-in-difference analysis. Our comparison pairings, based on prior demographic analyses were as follows: NY with Pennsylvania(PA); AZ with New Mexico and Nevada (NM/NV).19 The time frame of our analysis was 1999 to 2006, with pre- and postexpansion periods defined as 1999 to 2000 and 2001 to 2006, respectively.
Data
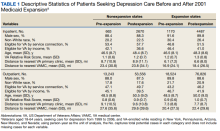
We included veterans aged 18 to 64 years, seeking care for depression from 1999 to 2006, who were also VA-enrolled and residing in our states of interest. We counted veterans as enrolled in Medicaid if they were enrolled at least 1 month in a given year.
Using similar methods like those used in prior studies, we selected patients with encounters documenting depression as the primary outpatient or inpatient diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 296.2x for a single episode of major depressive disorder, 296.3x for a recurrent episode of MDD, 300.4 for dysthymia, and 311.0 for depression not otherwise specified.18,24 We used data from the Medicaid Analytic eXtract files (MAX) for Medicaid data and the VA Corporate Data Warehouse (CDW) for VA data. We chose 1999 as the first study year because it was the earliest year MAX data were available.
Our final sample included 1833 person-years pre-expansion and 7157 postexpansion in our inpatient analysis, as well as 31,767 person-years pre-expansion and 130,382 postexpansion in our outpatient analysis.
Outcomes and Variables
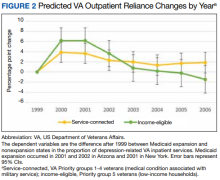
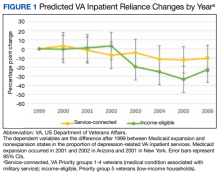
Our primary outcomes were comparative shifts in VA reliance between expansion and nonexpansion states after Medicaid expansion for both inpatient and outpatient depression care. For each year of study, we calculated a veteran’s VA reliance by aggregating the number of days with depression-related encounters at the VA and dividing by the total number of days with a VA or Medicaid depression-related encounters for the year. To provide context to these shifts in VA reliance, we further analyzed the changes in the proportion of annual VA-Medicaid dual users and annual per capita utilization of depression care across the VA and Medicaid.
We conducted subanalyses by income-eligible and service-connected veterans and adjusted our models for age, non-White race, sex, distances to the nearest inpatient and outpatient VA facilities, and VA Relative Risk Score, which is a measure of disease burden and clinical complexity validated specifically for veterans.25
Statistical Analysis
We used fractional logistic regression to model the adjusted effect of Medicaid expansion on VA reliance for depression care. In parallel, we leveraged ordered logit regression and negative binomial regression models to examine the proportion of VA-Medicaid dual users and the per capita utilization of Medicaid and VA depression care, respectively. To estimate the difference-in-difference effects, we used the interaction term of 2 categorical variables—expansion vs nonexpansion states and pre- vs postexpansion status—as the independent variable. We then calculated the average marginal effects with 95% CIs to estimate the differences in outcomes between expansion and nonexpansion states from pre- to postexpansion periods, as well as year-by-year shifts as a robustness check. We conducted these analyses using Stata MP, version 15.
Results
Baseline and postexpansion characteristics
VA Reliance
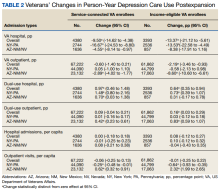
Overall, we observed postexpansion decreases in VA reliance for depression care
At the state level, reliance on the VA for inpatient depression care in NY decreased by 13.53 pp (95% CI, -22.58 to -4.49) for income-eligible veterans and 16.67 pp (95% CI, -24.53 to -8.80) for service-connected veterans. No relative differences were observed in the outpatient comparisons for both income-eligible (-0.58 pp; 95% CI, -2.13 to 0.98) and service-connected (0.05 pp; 95% CI, -1.00 to 1.10) veterans. In AZ, Medicaid expansion was associated with decreased VA reliance for outpatient depression care among income-eligible veterans (-8.60 pp; 95% CI, -10.60 to -6.61), greater than that for service-connected veterans (-2.89 pp; 95% CI, -4.02 to -1.77). This decrease in VA reliance was significant in the inpatient context only for service-connected veterans (-4.55 pp; 95% CI, -8.14 to -0.97), not income-eligible veterans (-8.38 pp; 95% CI, -17.91 to 1.16).
By applying the aggregate pp changes toward the postexpansion number of visits across both expansion and nonexpansion states, we found that expansion of Medicaid across all our study states would have resulted in 996 fewer hospitalizations and 10,109 fewer outpatient visits for depression at VA in the postexpansion period vs if no states had chosen to expand Medicaid.
Dual Use/Per Capita Utilization
Overall, Medicaid expansion was associated with greater dual use for inpatient depression care—a 0.97-pp (95% CI, 0.46 to 1.48) increase among service-connected veterans and a 0.64-pp (95% CI, 0.35 to 0.94) increase among income-eligible veterans.
At the state level, NY similarly showed increases in dual use among both service-connected (1.48 pp; 95% CI, 0.80 to 2.16) and income-eligible veterans (0.73 pp; 95% CI, 0.39 to 1.07) after Medicaid expansion. However, dual use in AZ increased significantly only among service-connected veterans (0.70 pp; 95% CI, 0.03 to 1.38), not income-eligible veterans (0.31 pp; 95% CI, -0.17 to 0.78).
Among outpatient visits, Medicaid expansion was associated with increased dual use only for income-eligible veterans (0.16 pp; 95% CI, 0.03-0.29), and not service-connected veterans (0.09 pp; 95% CI, -0.04 to 0.21). State-level analyses showed that Medicaid expansion in NY was not associated with changes in dual use for either service-connected (0.01 pp; 95% CI, -0.16 to 0.17) or income-eligible veterans (0.03 pp; 95% CI, -0.12 to 0.18), while expansion in AZ was associated with increases in dual use among both service-connected (0.42 pp; 95% CI, 0.23 to 0.61) and income-eligible veterans (0.83 pp; 95% CI, 0.59 to 1.07).
Concerning per capita utilization of depression care after Medicaid expansion, analyses showed no detectable changes for either inpatient or outpatient services, among both service-connected and income-eligible veterans. However, while this pattern held at the state level among hospitalizations, outpatient visit results showed divergent trends between AZ and NY. In NY, Medicaid expansion was associated with decreased per capita utilization of outpatient depression care among both service-connected (-0.25 visits annually; 95% CI, -0.48 to -0.01) and income-eligible veterans (-0.64 visits annually; 95% CI, -0.93 to -0.35). In AZ, Medicaid expansion was associated with increased per capita utilization of outpatient depression care among both service-connected (0.62 visits annually; 95% CI, 0.32-0.91) and income-eligible veterans (2.32 visits annually; 95% CI, 1.99-2.65).
Discussion
Our study quantified changes in depression-related health care utilization after Medicaid expansions in NY and AZ in 2001. Overall, the balance of evidence indicated that Medicaid expansion was associated with decreased reliance on the VA for depression-related services. There was an exception: income-eligible veterans in AZ did not shift their hospital care away from the VA in a statistically discernible way, although the point estimate was lower. More broadly, these findings concerning veterans’ reliance varied not only in inpatient vs outpatient services and income- vs service-connected eligibility, but also in the state-level contexts of veteran dual users and per capita utilization.
Given that the overall per capita utilization of depression care was unchanged from pre- to postexpansion periods, one might interpret the decreases in VA reliance and increases in Medicaid-VA dual users as a substitution effect from VA care to non-VA care. This could be plausible for hospitalizations where state-level analyses showed similarly stable levels of per capita utilization. However, state-level trends in our outpatient utilization analysis, especially with a substantial 2.32 pp increase in annual per capita visits among income-eligible veterans in AZ, leave open the possibility that in some cases veterans may be complementing VA care with Medicaid-reimbursed services.
The causes underlying these differences in reliance shifts between NY and AZ are likely also influenced by the policy contexts of their respective Medicaid expansions. For example, in 1999, NY passed Kendra’s Law, which established a procedure for obtaining court orders for assisted outpatient mental health treatment for individuals deemed unlikely to survive safely in the community.26 A reasonable inference is that there was less unfulfilled outpatient mental health need in NY under the existing accessibility provisioned by Kendra’s Law. In addition, while both states extended coverage to childless adults under 100% of the Federal Poverty level (FPL), the AZ Medicaid expansion was via a voters’ initiative and extended family coverage to 200% FPL vs 150% FPL for families in NY. Given that the AZ Medicaid expansion enjoyed both broader public participation and generosity in terms of eligibility, its uptake and therefore effect size may have been larger than in NY for nonacute outpatient care.
Our findings contribute to the growing body of literature surrounding the changes in health care utilization after Medicaid expansion, specifically for a newly dual-eligible population of veterans seeking mental health services for depression. While prior research concerning Medicare dual-enrolled veterans has shown high reliance on the VA for both mental health diagnoses and services, scholars have established the association of Medicaid enrollment with decreased VA reliance.27-29 Our analysis is the first to investigate state-level effects of Medicaid expansion on VA reliance for a single mental health condition using a natural experimental framework. We focus on a population that includes a large portion of veterans who are newly Medicaid-eligible due to a sweeping policy change and use demographically matched nonexpansion states to draw comparisons in VA reliance for depression care. Our findings of Medicaid expansion–associated decreases in VA reliance for depression care complement prior literature that describe Medicaid enrollment–associated decreases in VA reliance for overall mental health care.
Implications
From a systems-level perspective, the implications of shifting services away from the VA are complex and incompletely understood. The VA lacks interoperability with the electronic health records (EHRs) used by Medicaid clinicians. Consequently, significant issues of service duplication and incomplete clinical data exist for veterans seeking treatment outside of the VA system, posing health care quality and safety concerns.30 On one hand, Medicaid access is associated with increased health care utilization attributed to filling unmet needs for Medicare dual enrollees, as well as increased prescription filling for psychiatric medications.31,32 Furthermore, the only randomized control trial of Medicaid expansion to date was associated with a 9-pp decrease in positive screening rates for depression among those who received access at around 2 years postexpansion.33 On the other hand, the VA has developed a mental health system tailored to the particular needs of veterans, and health care practitioners at the VA have significantly greater rates of military cultural competency compared to those in nonmilitary settings (70% vs 24% in the TRICARE network and 8% among those with no military or TRICARE affiliation).34 Compared to individuals seeking mental health services with private insurance plans, veterans were about twice as likely to receive appropriate treatment for schizophrenia and depression at the VA.35 These documented strengths of VA mental health care may together help explain the small absolute number of visits that were associated with shifts away from VA overall after Medicaid expansion.
Finally, it is worth considering extrinsic factors that influence utilization among newly dual-eligible veterans. For example, hospitalizations are less likely to be planned than outpatient services, translating to a greater importance of proximity to a nearby medical facility than a veteran’s preference of where to seek care. In the same vein, major VA medical centers are fewer and more distant on average than VA outpatient clinics, therefore reducing the advantage of a Medicaid-reimbursed outpatient clinic in terms of distance.36 These realities may partially explain the proportionally larger shifts away from the VA for hospitalizations compared to outpatient care for depression.
Limitations and Future Directions
Our results should be interpreted within methodological and data limitations. With only 2 states in our sample, NY demonstrably skewed overall results, contributing 1.7 to 3 times more observations than AZ across subanalyses—a challenge also cited by Sommers and colleagues.19 Our veteran groupings were also unable to distinguish those veterans classified as service-connected who may also have qualified by income-eligible criteria (which would tend to understate the size of results) and those veterans who gained and then lost Medicaid coverage in a given year. Our study also faces limitations in generalizability and establishing causality. First, we included only 2 historical state Medicaid expansions, compared with the 38 states and Washington, DC, that have now expanded Medicaid to date under the ACA. Just in the 2 states from our study, we noted significant heterogeneity in the shifts associated with Medicaid expansion, which makes extrapolating specific trends difficult. Differences in underlying health care resources, legislation, and other external factors may limit the applicability of Medicaid expansion in the era of the ACA, as well as the Veterans Choice and MISSION acts. Second, while we leveraged a difference-in-difference analysis using demographically matched, neighboring comparison states, our findings are nevertheless drawn from observational data obviating causality. VA data for other sources of coverage such as private insurance are limited and not included in our study, and MAX datasets vary by quality across states, translating to potential gaps in our study cohort.28
Moving forward, our study demonstrates the potential for applying a natural experimental approach to studying dual-eligible veterans at the interface of Medicaid expansion. We focused on changes in VA reliance for the specific condition of depression and, in doing so, invite further inquiry into the impact of state mental health policy on outcomes more proximate to veterans’ outcomes. Clinical indicators, such as rates of antidepressant filling, utilization and duration of psychotherapy, and PHQ-9 scores, can similarly be investigated by natural experimental design. While current limits of administrative data and the siloing of EHRs may pose barriers to some of these avenues of research, multidisciplinary methodologies and data querying innovations such as natural language processing algorithms for clinical notes hold exciting opportunities to bridge the gap between policy and clinical efficacy.
Conclusions
This study applied a difference-in-difference analysis and found that Medicaid expansion is associated with decreases in VA reliance for both inpatient and outpatient services for depression. As additional data are generated from the Medicaid expansions of the ACA, similarly robust methods should be applied to further explore the impacts associated with such policy shifts and open the door to a better understanding of implications at the clinical level.
Acknowledgments
We acknowledge the efforts of Janine Wong, who proofread and formatted the manuscript.
1. US Department of Veterans Affairs, Veterans Health Administration. About VA. 2019. Updated September 27, 2022. Accessed September 29, 2022. https://www.va.gov/health/
2. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19. doi:10.3109/00048670903393597
3. Lan CW, Fiellin DA, Barry DT, et al. The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict. 2016;25(1):7-24. doi:10.1111/ajad.12319
4. Grant BF, Saha TD, June Ruan W, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiat. 2016;73(1):39-47. doi:10.1001/jamapsychiatry.015.2132
5. Pemberton MR, Forman-Hoffman VL, Lipari RN, Ashley OS, Heller DC, Williams MR. Prevalence of past year substance use and mental illness by veteran status in a nationally representative sample. CBHSQ Data Review. Published November 9, 2016. Accessed October 6, 2022. https://www.samhsa.gov/data/report/prevalence-past-year-substance-use-and-mental-illness-veteran-status-nationally
6. Watkins KE, Pincus HA, Smith B, et al. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. 2011. Accessed September 29, 2022. https://www.rand.org/pubs/technical_reports/TR956.html
7. Henry J. Kaiser Family Foundation. Medicaid’s role in covering veterans. June 29, 2017. Accessed September 29, 2022. https://www.kff.org/infographic/medicaids-role-in-covering-veterans
8. Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: detailed tables. September 7, 2017. Accessed September 29, 2022. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
9. Wen H, Druss BG, Cummings JR. Effect of Medicaid expansions on health insurance coverage and access to care among low-income adults with behavioral health conditions. Health Serv Res. 2015;50:1787-1809. doi:10.1111/1475-6773.12411
10. O’Mahen PN, Petersen LA. Effects of state-level Medicaid expansion on Veterans Health Administration dual enrollment and utilization: potential implications for future coverage expansions. Med Care. 2020;58(6):526-533. doi:10.1097/MLR.0000000000001327
11. Ono SS, Dziak KM, Wittrock SM, et al. Treating dual-use patients across two health care systems: a qualitative study. Fed Pract. 2015;32(8):32-37.
12. Weeks WB, Mahar PJ, Wright SM. Utilization of VA and Medicare services by Medicare-eligible veterans: the impact of additional access points in a rural setting. J Healthc Manag. 2005;50(2):95-106.
13. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare part d drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248-255. doi:10.2105/AJPH.2017.304174
14. Coughlin SS, Young L. A review of dual health care system use by veterans with cardiometabolic disease. J Hosp Manag Health Policy. 2018;2:39. doi:10.21037/jhmhp.2018.07.05
15. Radomski TR, Zhao X, Thorpe CT, et al. The impact of medication-based risk adjustment on the association between veteran health outcomes and dual health system use. J Gen Intern Med. 2017;32(9):967-973. doi:10.1007/s11606-017-4064-4
16. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2020;35(5):1567-1570. doi:10.1007/s11606-019-05404-w
17. VA MISSION Act of 2018, 38 USC §101 (2018). https://www.govinfo.gov/app/details/USCODE-2018-title38/USCODE-2018-title38-partI-chap1-sec101
18. Vanneman ME, Phibbs CS, Dally SK, Trivedi AN, Yoon J. The impact of Medicaid enrollment on Veterans Health Administration enrollees’ behavioral health services use. Health Serv Res. 2018;53(suppl 3):5238-5259. doi:10.1111/1475-6773.13062
19. Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025-1034. doi:10.1056/NEJMsa1202099
20. US Department of Veterans Affairs Office of Mental Health. 2019 national veteran suicide prevention annual report. 2019. Accessed September 29, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
21. Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1-3):17-28. doi:10.1016/j.jad.2013.01.004
22. Adekkanattu P, Sholle ET, DeFerio J, Pathak J, Johnson SB, Campion TR Jr. Ascertaining depression severity by extracting Patient Health Questionnaire-9 (PHQ-9) scores from clinical notes. AMIA Annu Symp Proc. 2018;2018:147-156.
23. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788-796. doi:10.1038/nrn2345
24. Cully JA, Zimmer M, Khan MM, Petersen LA. Quality of depression care and its impact on health service use and mortality among veterans. Psychiatr Serv. 2008;59(12):1399-1405. doi:10.1176/ps.2008.59.12.1399
25. Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768-773. doi:10.1097/01.mlr.0000218786.44722.14
26. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2016;67(4):391-396. doi:10.1176/appi.ps.201400537
27. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45(3):762-791. doi:10.1111/j.1475-6773.2010.01107.x
28. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Use of Veterans Affairs and Medicaid services for dually enrolled veterans. Health Serv Res. 2018;53(3):1539-1561. doi:10.1111/1475-6773.12727
29. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Veterans’ reliance on VA care by type of service and distance to VA for nonelderly VA-Medicaid dual enrollees. Med Care. 2019;57(3):225-229. doi:10.1097/MLR.0000000000001066
30. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. doi:10.7205/MILMED-D-13-00342
31. Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006;6(1):88. doi:10.1186/1471-2458-6-88
32. Henry J. Kaiser Family Foundation. Facilitating access to mental health services: a look at Medicaid, private insurance, and the uninsured. November 27, 2017. Accessed September 29, 2022. https://www.kff.org/medicaid/fact-sheet/facilitating-access-to-mental-health-services-a-look-at-medicaid-private-insurance-and-the-uninsured
33. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment - effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713-1722. doi:10.1056/NEJMsa1212321
34. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. 2014. Accessed September 29, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf
35. Kizer KW, Dudley RA. Extreme makeover: transformation of the Veterans Health Care System. Annu Rev Public Health. 2009;30(1):313-339. doi:10.1146/annurev.publhealth.29.020907.090940
36. Brennan KJ. Kendra’s Law: final report on the status of assisted outpatient treatment, appendix 2. 2002. Accessed September 29, 2022. https://omh.ny.gov/omhweb/kendra_web/finalreport/appendix2.htm
The US Department of Veterans Affairs (VA) is the largest integrated health care system in the United States, providing care for more than 9 million veterans.1 With veterans experiencing mental health conditions like posttraumatic stress disorder (PTSD), substance use disorders, and other serious mental illnesses (SMI) at higher rates compared with the general population, the VA plays an important role in the provision of mental health services.2-5 Since the implementation of its Mental Health Strategic Plan in 2004, the VA has overseen the development of a wide array of mental health programs geared toward the complex needs of veterans. Research has demonstrated VA care outperforming Medicaid-reimbursed services in terms of the percentage of veterans filling antidepressants for at least 12 weeks after initiation of treatment for major depressive disorder (MDD), as well as posthospitalization follow-up.6
Eligible veterans enrolled in the VA often also seek non-VA care. Medicaid covers nearly 10% of all nonelderly veterans, and of these veterans, 39% rely solely on Medicaid for health care access.7 Today, Medicaid is the largest payer for mental health services in the US, providing coverage for approximately 27% of Americans who have SMI and helping fulfill unmet mental health needs.8,9 Understanding which of these systems veterans choose to use, and under which circumstances, is essential in guiding the allocation of limited health care resources.10
Beyond Medicaid, alternatives to VA care may include TRICARE, Medicare, Indian Health Services, and employer-based or self-purchased private insurance. While these options potentially increase convenience, choice, and access to health care practitioners (HCPs) and services not available at local VA systems, cross-system utilization with poor integration may cause care coordination and continuity problems, such as medication mismanagement and opioid overdose, unnecessary duplicate utilization, and possible increased mortality.11-15 As recent national legislative changes, such as the Patient Protection and Affordable Care Act (ACA), Veterans Access, Choice and Accountability Act, and the VA MISSION Act, continue to shift the health care landscape for veterans, questions surrounding how veterans are changing their health care use become significant.16,17
Here, we approach the impacts of Medicaid expansion on veterans’ reliance on the VA for mental health services with a unique lens. We leverage a difference-in-difference design to study 2 historical Medicaid expansions in Arizona (AZ) and New York (NY), which extended eligibility to childless adults in 2001. Prior Medicaid dual-eligible mental health research investigated reliance shifts during the immediate postenrollment year in a subset of veterans newly enrolled in Medicaid.18 However, this study took place in a period of relative policy stability. In contrast, we investigate the potential effects of a broad policy shift by analyzing state-level changes in veterans’ reliance over 6 years after a statewide Medicaid expansion. We match expansion states with demographically similar nonexpansion states to account for unobserved trends and confounding effects. Prior studies have used this method to evaluate post-Medicaid expansion mortality changes and changes in veteran dual enrollment and hospitalizations.10,19 While a study of ACA Medicaid expansion states would be ideal, Medicaid data from most states were only available through 2014 at the time of this analysis. Our study offers a quasi-experimental framework leveraging longitudinal data that can be applied as more post-ACA data become available.
Given the rising incidence of suicide among veterans, understanding care-seeking behaviors for depression among veterans is important as it is the most common psychiatric condition found in those who died by suicide.20,21 Furthermore, depression may be useful as a clinical proxy for mental health policy impacts, given that the Patient Health Questionnaire-9 (PHQ-9) screening tool is well validated and increasingly research accessible, and it is a chronic condition responsive to both well-managed pharmacologic treatment and psychotherapeutic interventions.22,23
In this study, we quantify the change in care-seeking behavior for depression among veterans after Medicaid expansion, using a quasi-experimental design. We hypothesize that new access to Medicaid would be associated with a shift away from using VA services for depression. Given the income-dependent eligibility requirements of Medicaid, we also hypothesize that veterans who qualified for VA coverage due to low income, determined by a regional means test (Priority group 5, “income-eligible”), would be more likely to shift care compared with those whose serviced-connected conditions related to their military service (Priority groups 1-4, “service-connected”) provide VA access.
Methods
To investigate the relative changes in veterans’ reliance on the VA for depression care after the 2001 NY and AZ Medicaid expansions We used a retrospective, difference-in-difference analysis. Our comparison pairings, based on prior demographic analyses were as follows: NY with Pennsylvania(PA); AZ with New Mexico and Nevada (NM/NV).19 The time frame of our analysis was 1999 to 2006, with pre- and postexpansion periods defined as 1999 to 2000 and 2001 to 2006, respectively.
Data
We included veterans aged 18 to 64 years, seeking care for depression from 1999 to 2006, who were also VA-enrolled and residing in our states of interest. We counted veterans as enrolled in Medicaid if they were enrolled at least 1 month in a given year.
Using similar methods like those used in prior studies, we selected patients with encounters documenting depression as the primary outpatient or inpatient diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 296.2x for a single episode of major depressive disorder, 296.3x for a recurrent episode of MDD, 300.4 for dysthymia, and 311.0 for depression not otherwise specified.18,24 We used data from the Medicaid Analytic eXtract files (MAX) for Medicaid data and the VA Corporate Data Warehouse (CDW) for VA data. We chose 1999 as the first study year because it was the earliest year MAX data were available.
Our final sample included 1833 person-years pre-expansion and 7157 postexpansion in our inpatient analysis, as well as 31,767 person-years pre-expansion and 130,382 postexpansion in our outpatient analysis.
Outcomes and Variables
Our primary outcomes were comparative shifts in VA reliance between expansion and nonexpansion states after Medicaid expansion for both inpatient and outpatient depression care. For each year of study, we calculated a veteran’s VA reliance by aggregating the number of days with depression-related encounters at the VA and dividing by the total number of days with a VA or Medicaid depression-related encounters for the year. To provide context to these shifts in VA reliance, we further analyzed the changes in the proportion of annual VA-Medicaid dual users and annual per capita utilization of depression care across the VA and Medicaid.
We conducted subanalyses by income-eligible and service-connected veterans and adjusted our models for age, non-White race, sex, distances to the nearest inpatient and outpatient VA facilities, and VA Relative Risk Score, which is a measure of disease burden and clinical complexity validated specifically for veterans.25
Statistical Analysis
We used fractional logistic regression to model the adjusted effect of Medicaid expansion on VA reliance for depression care. In parallel, we leveraged ordered logit regression and negative binomial regression models to examine the proportion of VA-Medicaid dual users and the per capita utilization of Medicaid and VA depression care, respectively. To estimate the difference-in-difference effects, we used the interaction term of 2 categorical variables—expansion vs nonexpansion states and pre- vs postexpansion status—as the independent variable. We then calculated the average marginal effects with 95% CIs to estimate the differences in outcomes between expansion and nonexpansion states from pre- to postexpansion periods, as well as year-by-year shifts as a robustness check. We conducted these analyses using Stata MP, version 15.
Results
Baseline and postexpansion characteristics
VA Reliance
Overall, we observed postexpansion decreases in VA reliance for depression care
At the state level, reliance on the VA for inpatient depression care in NY decreased by 13.53 pp (95% CI, -22.58 to -4.49) for income-eligible veterans and 16.67 pp (95% CI, -24.53 to -8.80) for service-connected veterans. No relative differences were observed in the outpatient comparisons for both income-eligible (-0.58 pp; 95% CI, -2.13 to 0.98) and service-connected (0.05 pp; 95% CI, -1.00 to 1.10) veterans. In AZ, Medicaid expansion was associated with decreased VA reliance for outpatient depression care among income-eligible veterans (-8.60 pp; 95% CI, -10.60 to -6.61), greater than that for service-connected veterans (-2.89 pp; 95% CI, -4.02 to -1.77). This decrease in VA reliance was significant in the inpatient context only for service-connected veterans (-4.55 pp; 95% CI, -8.14 to -0.97), not income-eligible veterans (-8.38 pp; 95% CI, -17.91 to 1.16).
By applying the aggregate pp changes toward the postexpansion number of visits across both expansion and nonexpansion states, we found that expansion of Medicaid across all our study states would have resulted in 996 fewer hospitalizations and 10,109 fewer outpatient visits for depression at VA in the postexpansion period vs if no states had chosen to expand Medicaid.
Dual Use/Per Capita Utilization
Overall, Medicaid expansion was associated with greater dual use for inpatient depression care—a 0.97-pp (95% CI, 0.46 to 1.48) increase among service-connected veterans and a 0.64-pp (95% CI, 0.35 to 0.94) increase among income-eligible veterans.
At the state level, NY similarly showed increases in dual use among both service-connected (1.48 pp; 95% CI, 0.80 to 2.16) and income-eligible veterans (0.73 pp; 95% CI, 0.39 to 1.07) after Medicaid expansion. However, dual use in AZ increased significantly only among service-connected veterans (0.70 pp; 95% CI, 0.03 to 1.38), not income-eligible veterans (0.31 pp; 95% CI, -0.17 to 0.78).
Among outpatient visits, Medicaid expansion was associated with increased dual use only for income-eligible veterans (0.16 pp; 95% CI, 0.03-0.29), and not service-connected veterans (0.09 pp; 95% CI, -0.04 to 0.21). State-level analyses showed that Medicaid expansion in NY was not associated with changes in dual use for either service-connected (0.01 pp; 95% CI, -0.16 to 0.17) or income-eligible veterans (0.03 pp; 95% CI, -0.12 to 0.18), while expansion in AZ was associated with increases in dual use among both service-connected (0.42 pp; 95% CI, 0.23 to 0.61) and income-eligible veterans (0.83 pp; 95% CI, 0.59 to 1.07).
Concerning per capita utilization of depression care after Medicaid expansion, analyses showed no detectable changes for either inpatient or outpatient services, among both service-connected and income-eligible veterans. However, while this pattern held at the state level among hospitalizations, outpatient visit results showed divergent trends between AZ and NY. In NY, Medicaid expansion was associated with decreased per capita utilization of outpatient depression care among both service-connected (-0.25 visits annually; 95% CI, -0.48 to -0.01) and income-eligible veterans (-0.64 visits annually; 95% CI, -0.93 to -0.35). In AZ, Medicaid expansion was associated with increased per capita utilization of outpatient depression care among both service-connected (0.62 visits annually; 95% CI, 0.32-0.91) and income-eligible veterans (2.32 visits annually; 95% CI, 1.99-2.65).
Discussion
Our study quantified changes in depression-related health care utilization after Medicaid expansions in NY and AZ in 2001. Overall, the balance of evidence indicated that Medicaid expansion was associated with decreased reliance on the VA for depression-related services. There was an exception: income-eligible veterans in AZ did not shift their hospital care away from the VA in a statistically discernible way, although the point estimate was lower. More broadly, these findings concerning veterans’ reliance varied not only in inpatient vs outpatient services and income- vs service-connected eligibility, but also in the state-level contexts of veteran dual users and per capita utilization.
Given that the overall per capita utilization of depression care was unchanged from pre- to postexpansion periods, one might interpret the decreases in VA reliance and increases in Medicaid-VA dual users as a substitution effect from VA care to non-VA care. This could be plausible for hospitalizations where state-level analyses showed similarly stable levels of per capita utilization. However, state-level trends in our outpatient utilization analysis, especially with a substantial 2.32 pp increase in annual per capita visits among income-eligible veterans in AZ, leave open the possibility that in some cases veterans may be complementing VA care with Medicaid-reimbursed services.
The causes underlying these differences in reliance shifts between NY and AZ are likely also influenced by the policy contexts of their respective Medicaid expansions. For example, in 1999, NY passed Kendra’s Law, which established a procedure for obtaining court orders for assisted outpatient mental health treatment for individuals deemed unlikely to survive safely in the community.26 A reasonable inference is that there was less unfulfilled outpatient mental health need in NY under the existing accessibility provisioned by Kendra’s Law. In addition, while both states extended coverage to childless adults under 100% of the Federal Poverty level (FPL), the AZ Medicaid expansion was via a voters’ initiative and extended family coverage to 200% FPL vs 150% FPL for families in NY. Given that the AZ Medicaid expansion enjoyed both broader public participation and generosity in terms of eligibility, its uptake and therefore effect size may have been larger than in NY for nonacute outpatient care.
Our findings contribute to the growing body of literature surrounding the changes in health care utilization after Medicaid expansion, specifically for a newly dual-eligible population of veterans seeking mental health services for depression. While prior research concerning Medicare dual-enrolled veterans has shown high reliance on the VA for both mental health diagnoses and services, scholars have established the association of Medicaid enrollment with decreased VA reliance.27-29 Our analysis is the first to investigate state-level effects of Medicaid expansion on VA reliance for a single mental health condition using a natural experimental framework. We focus on a population that includes a large portion of veterans who are newly Medicaid-eligible due to a sweeping policy change and use demographically matched nonexpansion states to draw comparisons in VA reliance for depression care. Our findings of Medicaid expansion–associated decreases in VA reliance for depression care complement prior literature that describe Medicaid enrollment–associated decreases in VA reliance for overall mental health care.
Implications
From a systems-level perspective, the implications of shifting services away from the VA are complex and incompletely understood. The VA lacks interoperability with the electronic health records (EHRs) used by Medicaid clinicians. Consequently, significant issues of service duplication and incomplete clinical data exist for veterans seeking treatment outside of the VA system, posing health care quality and safety concerns.30 On one hand, Medicaid access is associated with increased health care utilization attributed to filling unmet needs for Medicare dual enrollees, as well as increased prescription filling for psychiatric medications.31,32 Furthermore, the only randomized control trial of Medicaid expansion to date was associated with a 9-pp decrease in positive screening rates for depression among those who received access at around 2 years postexpansion.33 On the other hand, the VA has developed a mental health system tailored to the particular needs of veterans, and health care practitioners at the VA have significantly greater rates of military cultural competency compared to those in nonmilitary settings (70% vs 24% in the TRICARE network and 8% among those with no military or TRICARE affiliation).34 Compared to individuals seeking mental health services with private insurance plans, veterans were about twice as likely to receive appropriate treatment for schizophrenia and depression at the VA.35 These documented strengths of VA mental health care may together help explain the small absolute number of visits that were associated with shifts away from VA overall after Medicaid expansion.
Finally, it is worth considering extrinsic factors that influence utilization among newly dual-eligible veterans. For example, hospitalizations are less likely to be planned than outpatient services, translating to a greater importance of proximity to a nearby medical facility than a veteran’s preference of where to seek care. In the same vein, major VA medical centers are fewer and more distant on average than VA outpatient clinics, therefore reducing the advantage of a Medicaid-reimbursed outpatient clinic in terms of distance.36 These realities may partially explain the proportionally larger shifts away from the VA for hospitalizations compared to outpatient care for depression.
Limitations and Future Directions
Our results should be interpreted within methodological and data limitations. With only 2 states in our sample, NY demonstrably skewed overall results, contributing 1.7 to 3 times more observations than AZ across subanalyses—a challenge also cited by Sommers and colleagues.19 Our veteran groupings were also unable to distinguish those veterans classified as service-connected who may also have qualified by income-eligible criteria (which would tend to understate the size of results) and those veterans who gained and then lost Medicaid coverage in a given year. Our study also faces limitations in generalizability and establishing causality. First, we included only 2 historical state Medicaid expansions, compared with the 38 states and Washington, DC, that have now expanded Medicaid to date under the ACA. Just in the 2 states from our study, we noted significant heterogeneity in the shifts associated with Medicaid expansion, which makes extrapolating specific trends difficult. Differences in underlying health care resources, legislation, and other external factors may limit the applicability of Medicaid expansion in the era of the ACA, as well as the Veterans Choice and MISSION acts. Second, while we leveraged a difference-in-difference analysis using demographically matched, neighboring comparison states, our findings are nevertheless drawn from observational data obviating causality. VA data for other sources of coverage such as private insurance are limited and not included in our study, and MAX datasets vary by quality across states, translating to potential gaps in our study cohort.28
Moving forward, our study demonstrates the potential for applying a natural experimental approach to studying dual-eligible veterans at the interface of Medicaid expansion. We focused on changes in VA reliance for the specific condition of depression and, in doing so, invite further inquiry into the impact of state mental health policy on outcomes more proximate to veterans’ outcomes. Clinical indicators, such as rates of antidepressant filling, utilization and duration of psychotherapy, and PHQ-9 scores, can similarly be investigated by natural experimental design. While current limits of administrative data and the siloing of EHRs may pose barriers to some of these avenues of research, multidisciplinary methodologies and data querying innovations such as natural language processing algorithms for clinical notes hold exciting opportunities to bridge the gap between policy and clinical efficacy.
Conclusions
This study applied a difference-in-difference analysis and found that Medicaid expansion is associated with decreases in VA reliance for both inpatient and outpatient services for depression. As additional data are generated from the Medicaid expansions of the ACA, similarly robust methods should be applied to further explore the impacts associated with such policy shifts and open the door to a better understanding of implications at the clinical level.
Acknowledgments
We acknowledge the efforts of Janine Wong, who proofread and formatted the manuscript.
The US Department of Veterans Affairs (VA) is the largest integrated health care system in the United States, providing care for more than 9 million veterans.1 With veterans experiencing mental health conditions like posttraumatic stress disorder (PTSD), substance use disorders, and other serious mental illnesses (SMI) at higher rates compared with the general population, the VA plays an important role in the provision of mental health services.2-5 Since the implementation of its Mental Health Strategic Plan in 2004, the VA has overseen the development of a wide array of mental health programs geared toward the complex needs of veterans. Research has demonstrated VA care outperforming Medicaid-reimbursed services in terms of the percentage of veterans filling antidepressants for at least 12 weeks after initiation of treatment for major depressive disorder (MDD), as well as posthospitalization follow-up.6
Eligible veterans enrolled in the VA often also seek non-VA care. Medicaid covers nearly 10% of all nonelderly veterans, and of these veterans, 39% rely solely on Medicaid for health care access.7 Today, Medicaid is the largest payer for mental health services in the US, providing coverage for approximately 27% of Americans who have SMI and helping fulfill unmet mental health needs.8,9 Understanding which of these systems veterans choose to use, and under which circumstances, is essential in guiding the allocation of limited health care resources.10
Beyond Medicaid, alternatives to VA care may include TRICARE, Medicare, Indian Health Services, and employer-based or self-purchased private insurance. While these options potentially increase convenience, choice, and access to health care practitioners (HCPs) and services not available at local VA systems, cross-system utilization with poor integration may cause care coordination and continuity problems, such as medication mismanagement and opioid overdose, unnecessary duplicate utilization, and possible increased mortality.11-15 As recent national legislative changes, such as the Patient Protection and Affordable Care Act (ACA), Veterans Access, Choice and Accountability Act, and the VA MISSION Act, continue to shift the health care landscape for veterans, questions surrounding how veterans are changing their health care use become significant.16,17
Here, we approach the impacts of Medicaid expansion on veterans’ reliance on the VA for mental health services with a unique lens. We leverage a difference-in-difference design to study 2 historical Medicaid expansions in Arizona (AZ) and New York (NY), which extended eligibility to childless adults in 2001. Prior Medicaid dual-eligible mental health research investigated reliance shifts during the immediate postenrollment year in a subset of veterans newly enrolled in Medicaid.18 However, this study took place in a period of relative policy stability. In contrast, we investigate the potential effects of a broad policy shift by analyzing state-level changes in veterans’ reliance over 6 years after a statewide Medicaid expansion. We match expansion states with demographically similar nonexpansion states to account for unobserved trends and confounding effects. Prior studies have used this method to evaluate post-Medicaid expansion mortality changes and changes in veteran dual enrollment and hospitalizations.10,19 While a study of ACA Medicaid expansion states would be ideal, Medicaid data from most states were only available through 2014 at the time of this analysis. Our study offers a quasi-experimental framework leveraging longitudinal data that can be applied as more post-ACA data become available.
Given the rising incidence of suicide among veterans, understanding care-seeking behaviors for depression among veterans is important as it is the most common psychiatric condition found in those who died by suicide.20,21 Furthermore, depression may be useful as a clinical proxy for mental health policy impacts, given that the Patient Health Questionnaire-9 (PHQ-9) screening tool is well validated and increasingly research accessible, and it is a chronic condition responsive to both well-managed pharmacologic treatment and psychotherapeutic interventions.22,23
In this study, we quantify the change in care-seeking behavior for depression among veterans after Medicaid expansion, using a quasi-experimental design. We hypothesize that new access to Medicaid would be associated with a shift away from using VA services for depression. Given the income-dependent eligibility requirements of Medicaid, we also hypothesize that veterans who qualified for VA coverage due to low income, determined by a regional means test (Priority group 5, “income-eligible”), would be more likely to shift care compared with those whose serviced-connected conditions related to their military service (Priority groups 1-4, “service-connected”) provide VA access.
Methods
To investigate the relative changes in veterans’ reliance on the VA for depression care after the 2001 NY and AZ Medicaid expansions We used a retrospective, difference-in-difference analysis. Our comparison pairings, based on prior demographic analyses were as follows: NY with Pennsylvania(PA); AZ with New Mexico and Nevada (NM/NV).19 The time frame of our analysis was 1999 to 2006, with pre- and postexpansion periods defined as 1999 to 2000 and 2001 to 2006, respectively.
Data
We included veterans aged 18 to 64 years, seeking care for depression from 1999 to 2006, who were also VA-enrolled and residing in our states of interest. We counted veterans as enrolled in Medicaid if they were enrolled at least 1 month in a given year.
Using similar methods like those used in prior studies, we selected patients with encounters documenting depression as the primary outpatient or inpatient diagnosis using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 296.2x for a single episode of major depressive disorder, 296.3x for a recurrent episode of MDD, 300.4 for dysthymia, and 311.0 for depression not otherwise specified.18,24 We used data from the Medicaid Analytic eXtract files (MAX) for Medicaid data and the VA Corporate Data Warehouse (CDW) for VA data. We chose 1999 as the first study year because it was the earliest year MAX data were available.
Our final sample included 1833 person-years pre-expansion and 7157 postexpansion in our inpatient analysis, as well as 31,767 person-years pre-expansion and 130,382 postexpansion in our outpatient analysis.
Outcomes and Variables
Our primary outcomes were comparative shifts in VA reliance between expansion and nonexpansion states after Medicaid expansion for both inpatient and outpatient depression care. For each year of study, we calculated a veteran’s VA reliance by aggregating the number of days with depression-related encounters at the VA and dividing by the total number of days with a VA or Medicaid depression-related encounters for the year. To provide context to these shifts in VA reliance, we further analyzed the changes in the proportion of annual VA-Medicaid dual users and annual per capita utilization of depression care across the VA and Medicaid.
We conducted subanalyses by income-eligible and service-connected veterans and adjusted our models for age, non-White race, sex, distances to the nearest inpatient and outpatient VA facilities, and VA Relative Risk Score, which is a measure of disease burden and clinical complexity validated specifically for veterans.25
Statistical Analysis
We used fractional logistic regression to model the adjusted effect of Medicaid expansion on VA reliance for depression care. In parallel, we leveraged ordered logit regression and negative binomial regression models to examine the proportion of VA-Medicaid dual users and the per capita utilization of Medicaid and VA depression care, respectively. To estimate the difference-in-difference effects, we used the interaction term of 2 categorical variables—expansion vs nonexpansion states and pre- vs postexpansion status—as the independent variable. We then calculated the average marginal effects with 95% CIs to estimate the differences in outcomes between expansion and nonexpansion states from pre- to postexpansion periods, as well as year-by-year shifts as a robustness check. We conducted these analyses using Stata MP, version 15.
Results
Baseline and postexpansion characteristics
VA Reliance
Overall, we observed postexpansion decreases in VA reliance for depression care
At the state level, reliance on the VA for inpatient depression care in NY decreased by 13.53 pp (95% CI, -22.58 to -4.49) for income-eligible veterans and 16.67 pp (95% CI, -24.53 to -8.80) for service-connected veterans. No relative differences were observed in the outpatient comparisons for both income-eligible (-0.58 pp; 95% CI, -2.13 to 0.98) and service-connected (0.05 pp; 95% CI, -1.00 to 1.10) veterans. In AZ, Medicaid expansion was associated with decreased VA reliance for outpatient depression care among income-eligible veterans (-8.60 pp; 95% CI, -10.60 to -6.61), greater than that for service-connected veterans (-2.89 pp; 95% CI, -4.02 to -1.77). This decrease in VA reliance was significant in the inpatient context only for service-connected veterans (-4.55 pp; 95% CI, -8.14 to -0.97), not income-eligible veterans (-8.38 pp; 95% CI, -17.91 to 1.16).
By applying the aggregate pp changes toward the postexpansion number of visits across both expansion and nonexpansion states, we found that expansion of Medicaid across all our study states would have resulted in 996 fewer hospitalizations and 10,109 fewer outpatient visits for depression at VA in the postexpansion period vs if no states had chosen to expand Medicaid.
Dual Use/Per Capita Utilization
Overall, Medicaid expansion was associated with greater dual use for inpatient depression care—a 0.97-pp (95% CI, 0.46 to 1.48) increase among service-connected veterans and a 0.64-pp (95% CI, 0.35 to 0.94) increase among income-eligible veterans.
At the state level, NY similarly showed increases in dual use among both service-connected (1.48 pp; 95% CI, 0.80 to 2.16) and income-eligible veterans (0.73 pp; 95% CI, 0.39 to 1.07) after Medicaid expansion. However, dual use in AZ increased significantly only among service-connected veterans (0.70 pp; 95% CI, 0.03 to 1.38), not income-eligible veterans (0.31 pp; 95% CI, -0.17 to 0.78).
Among outpatient visits, Medicaid expansion was associated with increased dual use only for income-eligible veterans (0.16 pp; 95% CI, 0.03-0.29), and not service-connected veterans (0.09 pp; 95% CI, -0.04 to 0.21). State-level analyses showed that Medicaid expansion in NY was not associated with changes in dual use for either service-connected (0.01 pp; 95% CI, -0.16 to 0.17) or income-eligible veterans (0.03 pp; 95% CI, -0.12 to 0.18), while expansion in AZ was associated with increases in dual use among both service-connected (0.42 pp; 95% CI, 0.23 to 0.61) and income-eligible veterans (0.83 pp; 95% CI, 0.59 to 1.07).
Concerning per capita utilization of depression care after Medicaid expansion, analyses showed no detectable changes for either inpatient or outpatient services, among both service-connected and income-eligible veterans. However, while this pattern held at the state level among hospitalizations, outpatient visit results showed divergent trends between AZ and NY. In NY, Medicaid expansion was associated with decreased per capita utilization of outpatient depression care among both service-connected (-0.25 visits annually; 95% CI, -0.48 to -0.01) and income-eligible veterans (-0.64 visits annually; 95% CI, -0.93 to -0.35). In AZ, Medicaid expansion was associated with increased per capita utilization of outpatient depression care among both service-connected (0.62 visits annually; 95% CI, 0.32-0.91) and income-eligible veterans (2.32 visits annually; 95% CI, 1.99-2.65).
Discussion
Our study quantified changes in depression-related health care utilization after Medicaid expansions in NY and AZ in 2001. Overall, the balance of evidence indicated that Medicaid expansion was associated with decreased reliance on the VA for depression-related services. There was an exception: income-eligible veterans in AZ did not shift their hospital care away from the VA in a statistically discernible way, although the point estimate was lower. More broadly, these findings concerning veterans’ reliance varied not only in inpatient vs outpatient services and income- vs service-connected eligibility, but also in the state-level contexts of veteran dual users and per capita utilization.
Given that the overall per capita utilization of depression care was unchanged from pre- to postexpansion periods, one might interpret the decreases in VA reliance and increases in Medicaid-VA dual users as a substitution effect from VA care to non-VA care. This could be plausible for hospitalizations where state-level analyses showed similarly stable levels of per capita utilization. However, state-level trends in our outpatient utilization analysis, especially with a substantial 2.32 pp increase in annual per capita visits among income-eligible veterans in AZ, leave open the possibility that in some cases veterans may be complementing VA care with Medicaid-reimbursed services.
The causes underlying these differences in reliance shifts between NY and AZ are likely also influenced by the policy contexts of their respective Medicaid expansions. For example, in 1999, NY passed Kendra’s Law, which established a procedure for obtaining court orders for assisted outpatient mental health treatment for individuals deemed unlikely to survive safely in the community.26 A reasonable inference is that there was less unfulfilled outpatient mental health need in NY under the existing accessibility provisioned by Kendra’s Law. In addition, while both states extended coverage to childless adults under 100% of the Federal Poverty level (FPL), the AZ Medicaid expansion was via a voters’ initiative and extended family coverage to 200% FPL vs 150% FPL for families in NY. Given that the AZ Medicaid expansion enjoyed both broader public participation and generosity in terms of eligibility, its uptake and therefore effect size may have been larger than in NY for nonacute outpatient care.
Our findings contribute to the growing body of literature surrounding the changes in health care utilization after Medicaid expansion, specifically for a newly dual-eligible population of veterans seeking mental health services for depression. While prior research concerning Medicare dual-enrolled veterans has shown high reliance on the VA for both mental health diagnoses and services, scholars have established the association of Medicaid enrollment with decreased VA reliance.27-29 Our analysis is the first to investigate state-level effects of Medicaid expansion on VA reliance for a single mental health condition using a natural experimental framework. We focus on a population that includes a large portion of veterans who are newly Medicaid-eligible due to a sweeping policy change and use demographically matched nonexpansion states to draw comparisons in VA reliance for depression care. Our findings of Medicaid expansion–associated decreases in VA reliance for depression care complement prior literature that describe Medicaid enrollment–associated decreases in VA reliance for overall mental health care.
Implications
From a systems-level perspective, the implications of shifting services away from the VA are complex and incompletely understood. The VA lacks interoperability with the electronic health records (EHRs) used by Medicaid clinicians. Consequently, significant issues of service duplication and incomplete clinical data exist for veterans seeking treatment outside of the VA system, posing health care quality and safety concerns.30 On one hand, Medicaid access is associated with increased health care utilization attributed to filling unmet needs for Medicare dual enrollees, as well as increased prescription filling for psychiatric medications.31,32 Furthermore, the only randomized control trial of Medicaid expansion to date was associated with a 9-pp decrease in positive screening rates for depression among those who received access at around 2 years postexpansion.33 On the other hand, the VA has developed a mental health system tailored to the particular needs of veterans, and health care practitioners at the VA have significantly greater rates of military cultural competency compared to those in nonmilitary settings (70% vs 24% in the TRICARE network and 8% among those with no military or TRICARE affiliation).34 Compared to individuals seeking mental health services with private insurance plans, veterans were about twice as likely to receive appropriate treatment for schizophrenia and depression at the VA.35 These documented strengths of VA mental health care may together help explain the small absolute number of visits that were associated with shifts away from VA overall after Medicaid expansion.
Finally, it is worth considering extrinsic factors that influence utilization among newly dual-eligible veterans. For example, hospitalizations are less likely to be planned than outpatient services, translating to a greater importance of proximity to a nearby medical facility than a veteran’s preference of where to seek care. In the same vein, major VA medical centers are fewer and more distant on average than VA outpatient clinics, therefore reducing the advantage of a Medicaid-reimbursed outpatient clinic in terms of distance.36 These realities may partially explain the proportionally larger shifts away from the VA for hospitalizations compared to outpatient care for depression.
Limitations and Future Directions
Our results should be interpreted within methodological and data limitations. With only 2 states in our sample, NY demonstrably skewed overall results, contributing 1.7 to 3 times more observations than AZ across subanalyses—a challenge also cited by Sommers and colleagues.19 Our veteran groupings were also unable to distinguish those veterans classified as service-connected who may also have qualified by income-eligible criteria (which would tend to understate the size of results) and those veterans who gained and then lost Medicaid coverage in a given year. Our study also faces limitations in generalizability and establishing causality. First, we included only 2 historical state Medicaid expansions, compared with the 38 states and Washington, DC, that have now expanded Medicaid to date under the ACA. Just in the 2 states from our study, we noted significant heterogeneity in the shifts associated with Medicaid expansion, which makes extrapolating specific trends difficult. Differences in underlying health care resources, legislation, and other external factors may limit the applicability of Medicaid expansion in the era of the ACA, as well as the Veterans Choice and MISSION acts. Second, while we leveraged a difference-in-difference analysis using demographically matched, neighboring comparison states, our findings are nevertheless drawn from observational data obviating causality. VA data for other sources of coverage such as private insurance are limited and not included in our study, and MAX datasets vary by quality across states, translating to potential gaps in our study cohort.28
Moving forward, our study demonstrates the potential for applying a natural experimental approach to studying dual-eligible veterans at the interface of Medicaid expansion. We focused on changes in VA reliance for the specific condition of depression and, in doing so, invite further inquiry into the impact of state mental health policy on outcomes more proximate to veterans’ outcomes. Clinical indicators, such as rates of antidepressant filling, utilization and duration of psychotherapy, and PHQ-9 scores, can similarly be investigated by natural experimental design. While current limits of administrative data and the siloing of EHRs may pose barriers to some of these avenues of research, multidisciplinary methodologies and data querying innovations such as natural language processing algorithms for clinical notes hold exciting opportunities to bridge the gap between policy and clinical efficacy.
Conclusions
This study applied a difference-in-difference analysis and found that Medicaid expansion is associated with decreases in VA reliance for both inpatient and outpatient services for depression. As additional data are generated from the Medicaid expansions of the ACA, similarly robust methods should be applied to further explore the impacts associated with such policy shifts and open the door to a better understanding of implications at the clinical level.
Acknowledgments
We acknowledge the efforts of Janine Wong, who proofread and formatted the manuscript.
1. US Department of Veterans Affairs, Veterans Health Administration. About VA. 2019. Updated September 27, 2022. Accessed September 29, 2022. https://www.va.gov/health/
2. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19. doi:10.3109/00048670903393597
3. Lan CW, Fiellin DA, Barry DT, et al. The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict. 2016;25(1):7-24. doi:10.1111/ajad.12319
4. Grant BF, Saha TD, June Ruan W, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiat. 2016;73(1):39-47. doi:10.1001/jamapsychiatry.015.2132
5. Pemberton MR, Forman-Hoffman VL, Lipari RN, Ashley OS, Heller DC, Williams MR. Prevalence of past year substance use and mental illness by veteran status in a nationally representative sample. CBHSQ Data Review. Published November 9, 2016. Accessed October 6, 2022. https://www.samhsa.gov/data/report/prevalence-past-year-substance-use-and-mental-illness-veteran-status-nationally
6. Watkins KE, Pincus HA, Smith B, et al. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. 2011. Accessed September 29, 2022. https://www.rand.org/pubs/technical_reports/TR956.html
7. Henry J. Kaiser Family Foundation. Medicaid’s role in covering veterans. June 29, 2017. Accessed September 29, 2022. https://www.kff.org/infographic/medicaids-role-in-covering-veterans
8. Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: detailed tables. September 7, 2017. Accessed September 29, 2022. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
9. Wen H, Druss BG, Cummings JR. Effect of Medicaid expansions on health insurance coverage and access to care among low-income adults with behavioral health conditions. Health Serv Res. 2015;50:1787-1809. doi:10.1111/1475-6773.12411
10. O’Mahen PN, Petersen LA. Effects of state-level Medicaid expansion on Veterans Health Administration dual enrollment and utilization: potential implications for future coverage expansions. Med Care. 2020;58(6):526-533. doi:10.1097/MLR.0000000000001327
11. Ono SS, Dziak KM, Wittrock SM, et al. Treating dual-use patients across two health care systems: a qualitative study. Fed Pract. 2015;32(8):32-37.
12. Weeks WB, Mahar PJ, Wright SM. Utilization of VA and Medicare services by Medicare-eligible veterans: the impact of additional access points in a rural setting. J Healthc Manag. 2005;50(2):95-106.
13. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare part d drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248-255. doi:10.2105/AJPH.2017.304174
14. Coughlin SS, Young L. A review of dual health care system use by veterans with cardiometabolic disease. J Hosp Manag Health Policy. 2018;2:39. doi:10.21037/jhmhp.2018.07.05
15. Radomski TR, Zhao X, Thorpe CT, et al. The impact of medication-based risk adjustment on the association between veteran health outcomes and dual health system use. J Gen Intern Med. 2017;32(9):967-973. doi:10.1007/s11606-017-4064-4
16. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2020;35(5):1567-1570. doi:10.1007/s11606-019-05404-w
17. VA MISSION Act of 2018, 38 USC §101 (2018). https://www.govinfo.gov/app/details/USCODE-2018-title38/USCODE-2018-title38-partI-chap1-sec101
18. Vanneman ME, Phibbs CS, Dally SK, Trivedi AN, Yoon J. The impact of Medicaid enrollment on Veterans Health Administration enrollees’ behavioral health services use. Health Serv Res. 2018;53(suppl 3):5238-5259. doi:10.1111/1475-6773.13062
19. Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025-1034. doi:10.1056/NEJMsa1202099
20. US Department of Veterans Affairs Office of Mental Health. 2019 national veteran suicide prevention annual report. 2019. Accessed September 29, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
21. Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1-3):17-28. doi:10.1016/j.jad.2013.01.004
22. Adekkanattu P, Sholle ET, DeFerio J, Pathak J, Johnson SB, Campion TR Jr. Ascertaining depression severity by extracting Patient Health Questionnaire-9 (PHQ-9) scores from clinical notes. AMIA Annu Symp Proc. 2018;2018:147-156.
23. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788-796. doi:10.1038/nrn2345
24. Cully JA, Zimmer M, Khan MM, Petersen LA. Quality of depression care and its impact on health service use and mortality among veterans. Psychiatr Serv. 2008;59(12):1399-1405. doi:10.1176/ps.2008.59.12.1399
25. Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768-773. doi:10.1097/01.mlr.0000218786.44722.14
26. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2016;67(4):391-396. doi:10.1176/appi.ps.201400537
27. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45(3):762-791. doi:10.1111/j.1475-6773.2010.01107.x
28. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Use of Veterans Affairs and Medicaid services for dually enrolled veterans. Health Serv Res. 2018;53(3):1539-1561. doi:10.1111/1475-6773.12727
29. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Veterans’ reliance on VA care by type of service and distance to VA for nonelderly VA-Medicaid dual enrollees. Med Care. 2019;57(3):225-229. doi:10.1097/MLR.0000000000001066
30. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. doi:10.7205/MILMED-D-13-00342
31. Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006;6(1):88. doi:10.1186/1471-2458-6-88
32. Henry J. Kaiser Family Foundation. Facilitating access to mental health services: a look at Medicaid, private insurance, and the uninsured. November 27, 2017. Accessed September 29, 2022. https://www.kff.org/medicaid/fact-sheet/facilitating-access-to-mental-health-services-a-look-at-medicaid-private-insurance-and-the-uninsured
33. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment - effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713-1722. doi:10.1056/NEJMsa1212321
34. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. 2014. Accessed September 29, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf
35. Kizer KW, Dudley RA. Extreme makeover: transformation of the Veterans Health Care System. Annu Rev Public Health. 2009;30(1):313-339. doi:10.1146/annurev.publhealth.29.020907.090940
36. Brennan KJ. Kendra’s Law: final report on the status of assisted outpatient treatment, appendix 2. 2002. Accessed September 29, 2022. https://omh.ny.gov/omhweb/kendra_web/finalreport/appendix2.htm
1. US Department of Veterans Affairs, Veterans Health Administration. About VA. 2019. Updated September 27, 2022. Accessed September 29, 2022. https://www.va.gov/health/
2. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19. doi:10.3109/00048670903393597
3. Lan CW, Fiellin DA, Barry DT, et al. The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict. 2016;25(1):7-24. doi:10.1111/ajad.12319
4. Grant BF, Saha TD, June Ruan W, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiat. 2016;73(1):39-47. doi:10.1001/jamapsychiatry.015.2132
5. Pemberton MR, Forman-Hoffman VL, Lipari RN, Ashley OS, Heller DC, Williams MR. Prevalence of past year substance use and mental illness by veteran status in a nationally representative sample. CBHSQ Data Review. Published November 9, 2016. Accessed October 6, 2022. https://www.samhsa.gov/data/report/prevalence-past-year-substance-use-and-mental-illness-veteran-status-nationally
6. Watkins KE, Pincus HA, Smith B, et al. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. 2011. Accessed September 29, 2022. https://www.rand.org/pubs/technical_reports/TR956.html
7. Henry J. Kaiser Family Foundation. Medicaid’s role in covering veterans. June 29, 2017. Accessed September 29, 2022. https://www.kff.org/infographic/medicaids-role-in-covering-veterans
8. Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: detailed tables. September 7, 2017. Accessed September 29, 2022. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf
9. Wen H, Druss BG, Cummings JR. Effect of Medicaid expansions on health insurance coverage and access to care among low-income adults with behavioral health conditions. Health Serv Res. 2015;50:1787-1809. doi:10.1111/1475-6773.12411
10. O’Mahen PN, Petersen LA. Effects of state-level Medicaid expansion on Veterans Health Administration dual enrollment and utilization: potential implications for future coverage expansions. Med Care. 2020;58(6):526-533. doi:10.1097/MLR.0000000000001327
11. Ono SS, Dziak KM, Wittrock SM, et al. Treating dual-use patients across two health care systems: a qualitative study. Fed Pract. 2015;32(8):32-37.
12. Weeks WB, Mahar PJ, Wright SM. Utilization of VA and Medicare services by Medicare-eligible veterans: the impact of additional access points in a rural setting. J Healthc Manag. 2005;50(2):95-106.
13. Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of Department of Veterans Affairs and Medicare part d drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248-255. doi:10.2105/AJPH.2017.304174
14. Coughlin SS, Young L. A review of dual health care system use by veterans with cardiometabolic disease. J Hosp Manag Health Policy. 2018;2:39. doi:10.21037/jhmhp.2018.07.05
15. Radomski TR, Zhao X, Thorpe CT, et al. The impact of medication-based risk adjustment on the association between veteran health outcomes and dual health system use. J Gen Intern Med. 2017;32(9):967-973. doi:10.1007/s11606-017-4064-4
16. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2020;35(5):1567-1570. doi:10.1007/s11606-019-05404-w
17. VA MISSION Act of 2018, 38 USC §101 (2018). https://www.govinfo.gov/app/details/USCODE-2018-title38/USCODE-2018-title38-partI-chap1-sec101
18. Vanneman ME, Phibbs CS, Dally SK, Trivedi AN, Yoon J. The impact of Medicaid enrollment on Veterans Health Administration enrollees’ behavioral health services use. Health Serv Res. 2018;53(suppl 3):5238-5259. doi:10.1111/1475-6773.13062
19. Sommers BD, Baicker K, Epstein AM. Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367(11):1025-1034. doi:10.1056/NEJMsa1202099
20. US Department of Veterans Affairs Office of Mental Health. 2019 national veteran suicide prevention annual report. 2019. Accessed September 29, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
21. Hawton K, Casañas I Comabella C, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147(1-3):17-28. doi:10.1016/j.jad.2013.01.004
22. Adekkanattu P, Sholle ET, DeFerio J, Pathak J, Johnson SB, Campion TR Jr. Ascertaining depression severity by extracting Patient Health Questionnaire-9 (PHQ-9) scores from clinical notes. AMIA Annu Symp Proc. 2018;2018:147-156.
23. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788-796. doi:10.1038/nrn2345
24. Cully JA, Zimmer M, Khan MM, Petersen LA. Quality of depression care and its impact on health service use and mortality among veterans. Psychiatr Serv. 2008;59(12):1399-1405. doi:10.1176/ps.2008.59.12.1399
25. Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care. 2006;44(8):768-773. doi:10.1097/01.mlr.0000218786.44722.14
26. Watkins KE, Smith B, Akincigil A, et al. The quality of medication treatment for mental disorders in the Department of Veterans Affairs and in private-sector plans. Psychiatr Serv. 2016;67(4):391-396. doi:10.1176/appi.ps.201400537
27. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled veterans. Health Serv Res. 2010;45(3):762-791. doi:10.1111/j.1475-6773.2010.01107.x
28. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Use of Veterans Affairs and Medicaid services for dually enrolled veterans. Health Serv Res. 2018;53(3):1539-1561. doi:10.1111/1475-6773.12727
29. Yoon J, Vanneman ME, Dally SK, Trivedi AN, Phibbs Ciaran S. Veterans’ reliance on VA care by type of service and distance to VA for nonelderly VA-Medicaid dual enrollees. Med Care. 2019;57(3):225-229. doi:10.1097/MLR.0000000000001066
30. Gaglioti A, Cozad A, Wittrock S, et al. Non-VA primary care providers’ perspectives on comanagement for rural veterans. Mil Med. 2014;179(11):1236-1243. doi:10.7205/MILMED-D-13-00342
31. Moon S, Shin J. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health. 2006;6(1):88. doi:10.1186/1471-2458-6-88
32. Henry J. Kaiser Family Foundation. Facilitating access to mental health services: a look at Medicaid, private insurance, and the uninsured. November 27, 2017. Accessed September 29, 2022. https://www.kff.org/medicaid/fact-sheet/facilitating-access-to-mental-health-services-a-look-at-medicaid-private-insurance-and-the-uninsured
33. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment - effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713-1722. doi:10.1056/NEJMsa1212321
34. Tanielian T, Farris C, Batka C, et al. Ready to serve: community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families. 2014. Accessed September 29, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR806/RAND_RR806.pdf
35. Kizer KW, Dudley RA. Extreme makeover: transformation of the Veterans Health Care System. Annu Rev Public Health. 2009;30(1):313-339. doi:10.1146/annurev.publhealth.29.020907.090940
36. Brennan KJ. Kendra’s Law: final report on the status of assisted outpatient treatment, appendix 2. 2002. Accessed September 29, 2022. https://omh.ny.gov/omhweb/kendra_web/finalreport/appendix2.htm
Challenges and Considerations in Treating Negative and Cognitive Symptoms of Schizophrenia Spectrum Disorders
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
The danger when doctors don’t get mental health help
As medical professionals, you’re continually exposed to overwork, burnout, stressful situations, and challenging ethical decisions. Yet seeking help for mental health care may be last on your to-do list – or completely off your radar.
That’s sad and dangerous, since the American College of Emergency Physicians said 300-400 physicians die by suicide each year, and the stigma keeps 69% of female physicians from seeking mental health care, according to a prepandemic study.
In the 2022 Medscape Physician Suicide Report, 11% of female doctors and 9% of male doctors said they have had thoughts of suicide, and 64% experienced colloquial depression (feeling down, sad, or blue).
What’s more, physicians are typically seen as strong and capable and are often put on a pedestal by loved ones, patients, and the public and thought of as superhuman. No wonder it isn’t easy when you need to take time away to decompress and treat your mental well-being.
“There is a real fear for physicians when it comes to getting mental health care,” said Emil Tsai, MD, PhD, MAS, professor at the department of psychiatry and behavioral sciences at the University of California, Los Angeles, and an internationally reputed scientist in neurosciences and brain disorders.
The fear, said Dr. Tsai, comes from the stigma of mental health issues, potential repercussions to employment, and conceivable medical board suspension or revocation of your medical license.
Dr. Tsai said in an interview that to combat anxiety about “punishment” that many physicians fear when seeking care for their mental health, we must allow physicians to take time away from their day-to-day patient care for respite and treatment without reprisal.
Since the medical profession is high stress and has a high depression and suicide rate, finding solutions is imperative. And physicians must feel supported enough to seek treatment when needed. So how can we normalize seeking mental health care among physicians?
Get honest about stress and burnout
The only way to normalize any behavior is to be open and candid, Dr. Tsai said in an interview. The mental health conversation must occur across the board, not just within the medical profession.
“The greatest thing we can do to try and lift the burden that we place on physicians is to be willing to talk and be honest about the stress that physicians deal with and the importance of everyone feeling free to seek treatment and rest to strengthen their mental health,” said Dr. Tsai.
The more we talk about mental health and its treatment, the more we lessen the stigma, said Dr. Tsai. That could be more employer-employee check-ins, counseling as part of physician wellness, and programs structured so as not to construe a penal system.
“Mental health in the medical profession is a big issue and one that has to be met with the same compassion and care as it should be for any patient. We have annual physical checkups. Why don’t we offer annual mental health checkups for all, physicians included?” asked Dr. Tsai.
Evaluate the workload
Elizabeth Lombardo, PhD, psychologist, coach, and global keynote speaker, thinks that health care employers should reexamine their physicians’ workloads to see if they’re contributing to mental health issues.
The conversation on mental health in the workplace shouldn’t be about whether a certain person can handle stressors that are “normal” for health care settings. Instead, workplace managers in health care institutions should redefine workloads to ensure that physicians aren’t too heavily burdened with responsibilities that can cause overwork, burnout, and mental health problems,” she said.
Lessen the stigma
Even when physicians want to seek help for their psychological struggles, they may be weary of how their colleagues would react if they knew.
Raffaello Antonino, MD, clinical director at Therapy Central in London, said several underlying fears may exist at a physician’s core that prevent them from seeking care – being seen as weak, being judged as unfit to practice medicine, and the notion that “something is wrong with them.”
Dr. Antonino said we need to understand that physicians face challenges of bereavement and trauma derived from losing patients and the inability to save someone’s life. “These issues can easily develop into an accumulation of difficult, unprocessed emotions, later arising in symptoms and signs of PTSD, anxiety, and depression.”
Education is the best way to end this stigma, just like with any form of prejudice and stereotypes. For instance, we know that health care professionals are at risk of developing burnout. So, educating physicians on the symptoms and management of burnout and its consequences and prevention strategies is a must.
“Imagine what could happen if there were regular opportunities to work through the day’s events before signing out from a shift. The idea that individual weekly therapy is the only way to relieve mental distress is false,” said Lori McIsaac Bewsher, MSW, RSW, a trauma therapist and owner of a trauma-focused mental health clinic in New Brunswick.
“There are ways of integrating individual care into our doctor’s offices and hospitals that can be brief, effective, and confidential. The best way to introduce these interventions is early and collectively; no one is immune to the potential impact of exposure to trauma. The earlier these interventions can be accessed, the better the outcomes for everyone,” she said.
Dr. Antonino suggests, perhaps in the future, organizations can have “burnout checks” or mental health wellness checks for physicians akin to how we also have quick examinations for various physical ailments. What if physicians regularly answered a 10-question mental health survey as part of a burnout or trauma prevention strategy?
“Theirs is a profession and an identity which is often linked with a sense of strength, leadership and [benevolent] power: adjectives, which on the surface one might see as incompatible with what instead, unfortunately, and wrongly, may be associated with mental health issues,” said Dr. Antonino.
Keep it private
When it comes to removing the stigma from mental health care and treatment for physicians, privacy is top of mind. There needs to be some form of privacy protection for physicians who seek professional help for mental health reasons. Dr. Lombardo said physicians need to have the choice to keep their mental health journeys private. “Ideally, normalization should mean openly conversing about mental health, but for physicians, it can be a matter of life or death for their career, so the choice to remain private is something that should be afforded to them.”
Along those lines, the American Medical Association is pushing for system changes in legislative and regulatory arenas to support the mental health of practicing physicians, residents, and medical students. The organization is also urging health systems and state medical licensing bodies to remove questions on their applications that ask about prior treatment for mental health conditions.
Among many programs across the country, the Foundation of the Pennsylvania Medical Society has also created a Physicians’ Health Program, which provides confidential assessment, counseling, and referral services for physicians with mental health concerns.
“All of these initiatives are important in helping to destigmatize mental health issues among physicians,” said Harold Hong, MD, a board-certified psychiatrist in Raleigh, N.C.
Hail the benefits of treatment
Dr. Hong said to continue to destigmatize mental health among physicians and normalize its treatment, we not only have to emphasize how attending to mental health has individual benefits but also how it helps us help our patients.
“One key aspect that perhaps underpins this issue is the still present separation between mental and physical health, between mind and body, Dr. Hong said in an interview. “Feeling sad or angry or anxious should become a fact of life, a characteristic of being human, just like catching a cold or breaking a leg.”
It’s a normalization that, perhaps more than anything else, can lead the way for improving physicians’ mental health outcomes while also improving them for the rest of society. When society can finally see the health and well-being of someone in both their psychological and physical status, some of the stigmas may dissipate, and perhaps more physicians’ lives can be saved.
A version of this article first appeared on Medscape.com.
As medical professionals, you’re continually exposed to overwork, burnout, stressful situations, and challenging ethical decisions. Yet seeking help for mental health care may be last on your to-do list – or completely off your radar.
That’s sad and dangerous, since the American College of Emergency Physicians said 300-400 physicians die by suicide each year, and the stigma keeps 69% of female physicians from seeking mental health care, according to a prepandemic study.
In the 2022 Medscape Physician Suicide Report, 11% of female doctors and 9% of male doctors said they have had thoughts of suicide, and 64% experienced colloquial depression (feeling down, sad, or blue).
What’s more, physicians are typically seen as strong and capable and are often put on a pedestal by loved ones, patients, and the public and thought of as superhuman. No wonder it isn’t easy when you need to take time away to decompress and treat your mental well-being.
“There is a real fear for physicians when it comes to getting mental health care,” said Emil Tsai, MD, PhD, MAS, professor at the department of psychiatry and behavioral sciences at the University of California, Los Angeles, and an internationally reputed scientist in neurosciences and brain disorders.
The fear, said Dr. Tsai, comes from the stigma of mental health issues, potential repercussions to employment, and conceivable medical board suspension or revocation of your medical license.
Dr. Tsai said in an interview that to combat anxiety about “punishment” that many physicians fear when seeking care for their mental health, we must allow physicians to take time away from their day-to-day patient care for respite and treatment without reprisal.
Since the medical profession is high stress and has a high depression and suicide rate, finding solutions is imperative. And physicians must feel supported enough to seek treatment when needed. So how can we normalize seeking mental health care among physicians?
Get honest about stress and burnout
The only way to normalize any behavior is to be open and candid, Dr. Tsai said in an interview. The mental health conversation must occur across the board, not just within the medical profession.
“The greatest thing we can do to try and lift the burden that we place on physicians is to be willing to talk and be honest about the stress that physicians deal with and the importance of everyone feeling free to seek treatment and rest to strengthen their mental health,” said Dr. Tsai.
The more we talk about mental health and its treatment, the more we lessen the stigma, said Dr. Tsai. That could be more employer-employee check-ins, counseling as part of physician wellness, and programs structured so as not to construe a penal system.
“Mental health in the medical profession is a big issue and one that has to be met with the same compassion and care as it should be for any patient. We have annual physical checkups. Why don’t we offer annual mental health checkups for all, physicians included?” asked Dr. Tsai.
Evaluate the workload
Elizabeth Lombardo, PhD, psychologist, coach, and global keynote speaker, thinks that health care employers should reexamine their physicians’ workloads to see if they’re contributing to mental health issues.
The conversation on mental health in the workplace shouldn’t be about whether a certain person can handle stressors that are “normal” for health care settings. Instead, workplace managers in health care institutions should redefine workloads to ensure that physicians aren’t too heavily burdened with responsibilities that can cause overwork, burnout, and mental health problems,” she said.
Lessen the stigma
Even when physicians want to seek help for their psychological struggles, they may be weary of how their colleagues would react if they knew.
Raffaello Antonino, MD, clinical director at Therapy Central in London, said several underlying fears may exist at a physician’s core that prevent them from seeking care – being seen as weak, being judged as unfit to practice medicine, and the notion that “something is wrong with them.”
Dr. Antonino said we need to understand that physicians face challenges of bereavement and trauma derived from losing patients and the inability to save someone’s life. “These issues can easily develop into an accumulation of difficult, unprocessed emotions, later arising in symptoms and signs of PTSD, anxiety, and depression.”
Education is the best way to end this stigma, just like with any form of prejudice and stereotypes. For instance, we know that health care professionals are at risk of developing burnout. So, educating physicians on the symptoms and management of burnout and its consequences and prevention strategies is a must.
“Imagine what could happen if there were regular opportunities to work through the day’s events before signing out from a shift. The idea that individual weekly therapy is the only way to relieve mental distress is false,” said Lori McIsaac Bewsher, MSW, RSW, a trauma therapist and owner of a trauma-focused mental health clinic in New Brunswick.
“There are ways of integrating individual care into our doctor’s offices and hospitals that can be brief, effective, and confidential. The best way to introduce these interventions is early and collectively; no one is immune to the potential impact of exposure to trauma. The earlier these interventions can be accessed, the better the outcomes for everyone,” she said.
Dr. Antonino suggests, perhaps in the future, organizations can have “burnout checks” or mental health wellness checks for physicians akin to how we also have quick examinations for various physical ailments. What if physicians regularly answered a 10-question mental health survey as part of a burnout or trauma prevention strategy?
“Theirs is a profession and an identity which is often linked with a sense of strength, leadership and [benevolent] power: adjectives, which on the surface one might see as incompatible with what instead, unfortunately, and wrongly, may be associated with mental health issues,” said Dr. Antonino.
Keep it private
When it comes to removing the stigma from mental health care and treatment for physicians, privacy is top of mind. There needs to be some form of privacy protection for physicians who seek professional help for mental health reasons. Dr. Lombardo said physicians need to have the choice to keep their mental health journeys private. “Ideally, normalization should mean openly conversing about mental health, but for physicians, it can be a matter of life or death for their career, so the choice to remain private is something that should be afforded to them.”
Along those lines, the American Medical Association is pushing for system changes in legislative and regulatory arenas to support the mental health of practicing physicians, residents, and medical students. The organization is also urging health systems and state medical licensing bodies to remove questions on their applications that ask about prior treatment for mental health conditions.
Among many programs across the country, the Foundation of the Pennsylvania Medical Society has also created a Physicians’ Health Program, which provides confidential assessment, counseling, and referral services for physicians with mental health concerns.
“All of these initiatives are important in helping to destigmatize mental health issues among physicians,” said Harold Hong, MD, a board-certified psychiatrist in Raleigh, N.C.
Hail the benefits of treatment
Dr. Hong said to continue to destigmatize mental health among physicians and normalize its treatment, we not only have to emphasize how attending to mental health has individual benefits but also how it helps us help our patients.
“One key aspect that perhaps underpins this issue is the still present separation between mental and physical health, between mind and body, Dr. Hong said in an interview. “Feeling sad or angry or anxious should become a fact of life, a characteristic of being human, just like catching a cold or breaking a leg.”
It’s a normalization that, perhaps more than anything else, can lead the way for improving physicians’ mental health outcomes while also improving them for the rest of society. When society can finally see the health and well-being of someone in both their psychological and physical status, some of the stigmas may dissipate, and perhaps more physicians’ lives can be saved.
A version of this article first appeared on Medscape.com.
As medical professionals, you’re continually exposed to overwork, burnout, stressful situations, and challenging ethical decisions. Yet seeking help for mental health care may be last on your to-do list – or completely off your radar.
That’s sad and dangerous, since the American College of Emergency Physicians said 300-400 physicians die by suicide each year, and the stigma keeps 69% of female physicians from seeking mental health care, according to a prepandemic study.
In the 2022 Medscape Physician Suicide Report, 11% of female doctors and 9% of male doctors said they have had thoughts of suicide, and 64% experienced colloquial depression (feeling down, sad, or blue).
What’s more, physicians are typically seen as strong and capable and are often put on a pedestal by loved ones, patients, and the public and thought of as superhuman. No wonder it isn’t easy when you need to take time away to decompress and treat your mental well-being.
“There is a real fear for physicians when it comes to getting mental health care,” said Emil Tsai, MD, PhD, MAS, professor at the department of psychiatry and behavioral sciences at the University of California, Los Angeles, and an internationally reputed scientist in neurosciences and brain disorders.
The fear, said Dr. Tsai, comes from the stigma of mental health issues, potential repercussions to employment, and conceivable medical board suspension or revocation of your medical license.
Dr. Tsai said in an interview that to combat anxiety about “punishment” that many physicians fear when seeking care for their mental health, we must allow physicians to take time away from their day-to-day patient care for respite and treatment without reprisal.
Since the medical profession is high stress and has a high depression and suicide rate, finding solutions is imperative. And physicians must feel supported enough to seek treatment when needed. So how can we normalize seeking mental health care among physicians?
Get honest about stress and burnout
The only way to normalize any behavior is to be open and candid, Dr. Tsai said in an interview. The mental health conversation must occur across the board, not just within the medical profession.
“The greatest thing we can do to try and lift the burden that we place on physicians is to be willing to talk and be honest about the stress that physicians deal with and the importance of everyone feeling free to seek treatment and rest to strengthen their mental health,” said Dr. Tsai.
The more we talk about mental health and its treatment, the more we lessen the stigma, said Dr. Tsai. That could be more employer-employee check-ins, counseling as part of physician wellness, and programs structured so as not to construe a penal system.
“Mental health in the medical profession is a big issue and one that has to be met with the same compassion and care as it should be for any patient. We have annual physical checkups. Why don’t we offer annual mental health checkups for all, physicians included?” asked Dr. Tsai.
Evaluate the workload
Elizabeth Lombardo, PhD, psychologist, coach, and global keynote speaker, thinks that health care employers should reexamine their physicians’ workloads to see if they’re contributing to mental health issues.
The conversation on mental health in the workplace shouldn’t be about whether a certain person can handle stressors that are “normal” for health care settings. Instead, workplace managers in health care institutions should redefine workloads to ensure that physicians aren’t too heavily burdened with responsibilities that can cause overwork, burnout, and mental health problems,” she said.
Lessen the stigma
Even when physicians want to seek help for their psychological struggles, they may be weary of how their colleagues would react if they knew.
Raffaello Antonino, MD, clinical director at Therapy Central in London, said several underlying fears may exist at a physician’s core that prevent them from seeking care – being seen as weak, being judged as unfit to practice medicine, and the notion that “something is wrong with them.”
Dr. Antonino said we need to understand that physicians face challenges of bereavement and trauma derived from losing patients and the inability to save someone’s life. “These issues can easily develop into an accumulation of difficult, unprocessed emotions, later arising in symptoms and signs of PTSD, anxiety, and depression.”
Education is the best way to end this stigma, just like with any form of prejudice and stereotypes. For instance, we know that health care professionals are at risk of developing burnout. So, educating physicians on the symptoms and management of burnout and its consequences and prevention strategies is a must.
“Imagine what could happen if there were regular opportunities to work through the day’s events before signing out from a shift. The idea that individual weekly therapy is the only way to relieve mental distress is false,” said Lori McIsaac Bewsher, MSW, RSW, a trauma therapist and owner of a trauma-focused mental health clinic in New Brunswick.
“There are ways of integrating individual care into our doctor’s offices and hospitals that can be brief, effective, and confidential. The best way to introduce these interventions is early and collectively; no one is immune to the potential impact of exposure to trauma. The earlier these interventions can be accessed, the better the outcomes for everyone,” she said.
Dr. Antonino suggests, perhaps in the future, organizations can have “burnout checks” or mental health wellness checks for physicians akin to how we also have quick examinations for various physical ailments. What if physicians regularly answered a 10-question mental health survey as part of a burnout or trauma prevention strategy?
“Theirs is a profession and an identity which is often linked with a sense of strength, leadership and [benevolent] power: adjectives, which on the surface one might see as incompatible with what instead, unfortunately, and wrongly, may be associated with mental health issues,” said Dr. Antonino.
Keep it private
When it comes to removing the stigma from mental health care and treatment for physicians, privacy is top of mind. There needs to be some form of privacy protection for physicians who seek professional help for mental health reasons. Dr. Lombardo said physicians need to have the choice to keep their mental health journeys private. “Ideally, normalization should mean openly conversing about mental health, but for physicians, it can be a matter of life or death for their career, so the choice to remain private is something that should be afforded to them.”
Along those lines, the American Medical Association is pushing for system changes in legislative and regulatory arenas to support the mental health of practicing physicians, residents, and medical students. The organization is also urging health systems and state medical licensing bodies to remove questions on their applications that ask about prior treatment for mental health conditions.
Among many programs across the country, the Foundation of the Pennsylvania Medical Society has also created a Physicians’ Health Program, which provides confidential assessment, counseling, and referral services for physicians with mental health concerns.
“All of these initiatives are important in helping to destigmatize mental health issues among physicians,” said Harold Hong, MD, a board-certified psychiatrist in Raleigh, N.C.
Hail the benefits of treatment
Dr. Hong said to continue to destigmatize mental health among physicians and normalize its treatment, we not only have to emphasize how attending to mental health has individual benefits but also how it helps us help our patients.
“One key aspect that perhaps underpins this issue is the still present separation between mental and physical health, between mind and body, Dr. Hong said in an interview. “Feeling sad or angry or anxious should become a fact of life, a characteristic of being human, just like catching a cold or breaking a leg.”
It’s a normalization that, perhaps more than anything else, can lead the way for improving physicians’ mental health outcomes while also improving them for the rest of society. When society can finally see the health and well-being of someone in both their psychological and physical status, some of the stigmas may dissipate, and perhaps more physicians’ lives can be saved.
A version of this article first appeared on Medscape.com.
Clozapine underutilized in treatment-resistant schizophrenia
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
With a little help from your friends
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Do scare tactics work?
I suspect that you have heard about or maybe read the recent Associated Press story reporting that four daycare workers in Hamilton, Miss., have been charged with felony child abuse for intentionally scaring the children “who didn’t clean up or act good” by wearing a Halloween mask and yelling in their faces. I can have some sympathy for those among us who choose to spend their days tending a flock of sometimes unruly and mischievous toddlers and preschoolers. But, I think one would be hard pressed to find very many adults who would condone the strategy of these misguided daycare providers. Not surprisingly, the parents of some of these children describe their children as traumatized and having disordered sleep.
The news report of this incident in Mississippi doesn’t tell us if these daycare providers had used this tactic in the past. One wonders whether they had found less dramatic verbal threats just weren’t as effective as they had hoped and so decided to go all out.
How effective is fear in changing behavior? Certainly, we have all experienced situations in which a frightening experience has caused us to avoid places, people, and activities. But, is a fear-focused strategy one that health care providers should include in their quiver as we try to mold patient behavior? As luck would have it, 2 weeks before this news story broke I encountered a global study from 84 countries that sought to answer this question (Affect Sci. 2022 Sep. doi: 10.1007/s42761-022-00128-3).
Using the WHO four-point advice about COVID prevention (stay home/avoid shops/use face covering/isolate if exposed) as a model the researchers around the world reviewed the responses of 16,000 individuals. They found that there was no difference in the effectiveness of the message whether it was framed as a negative (“you have so much to lose”) or a positive (“you have so much to gain”). However, investigators observed that the negatively framed presentations generated significantly more anxiety in the respondents. The authors of the paper conclude that if there is no significant difference in the effectiveness, why would we chose a negatively framed presentation that is likely to generate anxiety that we know is associated with increased morbidity and mortality. From a purely public health perspective, it doesn’t make sense and is counterproductive.
I guess if we look back to the old carrot and stick metaphor we shouldn’t be surprised by the findings in this paper. If one’s only goal is to get a group of young preschoolers to behave by scaring the b’geezes out of them with a mask or a threat of bodily punishment, then go for it. Scare tactics will probably work just as well as offering a well-chosen reward system. However, the devil is in the side effects. It’s the same argument that I give to parents who argue that spanking works. Of course it does, but it has a narrow margin for safety and can set up ripples of negative side effects that can destroy healthy parent-child relationships.
The bottom line of this story is the sad truth that somewhere along the line someone failed to effectively train these four daycare workers. But, do we as health care providers need to rethink our training? Have we forgotten our commitment to “First do no harm?” As we craft our messaging have we thought enough about the potential side effects of our attempts at scaring the public into following our suggestions?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect that you have heard about or maybe read the recent Associated Press story reporting that four daycare workers in Hamilton, Miss., have been charged with felony child abuse for intentionally scaring the children “who didn’t clean up or act good” by wearing a Halloween mask and yelling in their faces. I can have some sympathy for those among us who choose to spend their days tending a flock of sometimes unruly and mischievous toddlers and preschoolers. But, I think one would be hard pressed to find very many adults who would condone the strategy of these misguided daycare providers. Not surprisingly, the parents of some of these children describe their children as traumatized and having disordered sleep.
The news report of this incident in Mississippi doesn’t tell us if these daycare providers had used this tactic in the past. One wonders whether they had found less dramatic verbal threats just weren’t as effective as they had hoped and so decided to go all out.
How effective is fear in changing behavior? Certainly, we have all experienced situations in which a frightening experience has caused us to avoid places, people, and activities. But, is a fear-focused strategy one that health care providers should include in their quiver as we try to mold patient behavior? As luck would have it, 2 weeks before this news story broke I encountered a global study from 84 countries that sought to answer this question (Affect Sci. 2022 Sep. doi: 10.1007/s42761-022-00128-3).
Using the WHO four-point advice about COVID prevention (stay home/avoid shops/use face covering/isolate if exposed) as a model the researchers around the world reviewed the responses of 16,000 individuals. They found that there was no difference in the effectiveness of the message whether it was framed as a negative (“you have so much to lose”) or a positive (“you have so much to gain”). However, investigators observed that the negatively framed presentations generated significantly more anxiety in the respondents. The authors of the paper conclude that if there is no significant difference in the effectiveness, why would we chose a negatively framed presentation that is likely to generate anxiety that we know is associated with increased morbidity and mortality. From a purely public health perspective, it doesn’t make sense and is counterproductive.
I guess if we look back to the old carrot and stick metaphor we shouldn’t be surprised by the findings in this paper. If one’s only goal is to get a group of young preschoolers to behave by scaring the b’geezes out of them with a mask or a threat of bodily punishment, then go for it. Scare tactics will probably work just as well as offering a well-chosen reward system. However, the devil is in the side effects. It’s the same argument that I give to parents who argue that spanking works. Of course it does, but it has a narrow margin for safety and can set up ripples of negative side effects that can destroy healthy parent-child relationships.
The bottom line of this story is the sad truth that somewhere along the line someone failed to effectively train these four daycare workers. But, do we as health care providers need to rethink our training? Have we forgotten our commitment to “First do no harm?” As we craft our messaging have we thought enough about the potential side effects of our attempts at scaring the public into following our suggestions?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect that you have heard about or maybe read the recent Associated Press story reporting that four daycare workers in Hamilton, Miss., have been charged with felony child abuse for intentionally scaring the children “who didn’t clean up or act good” by wearing a Halloween mask and yelling in their faces. I can have some sympathy for those among us who choose to spend their days tending a flock of sometimes unruly and mischievous toddlers and preschoolers. But, I think one would be hard pressed to find very many adults who would condone the strategy of these misguided daycare providers. Not surprisingly, the parents of some of these children describe their children as traumatized and having disordered sleep.
The news report of this incident in Mississippi doesn’t tell us if these daycare providers had used this tactic in the past. One wonders whether they had found less dramatic verbal threats just weren’t as effective as they had hoped and so decided to go all out.
How effective is fear in changing behavior? Certainly, we have all experienced situations in which a frightening experience has caused us to avoid places, people, and activities. But, is a fear-focused strategy one that health care providers should include in their quiver as we try to mold patient behavior? As luck would have it, 2 weeks before this news story broke I encountered a global study from 84 countries that sought to answer this question (Affect Sci. 2022 Sep. doi: 10.1007/s42761-022-00128-3).
Using the WHO four-point advice about COVID prevention (stay home/avoid shops/use face covering/isolate if exposed) as a model the researchers around the world reviewed the responses of 16,000 individuals. They found that there was no difference in the effectiveness of the message whether it was framed as a negative (“you have so much to lose”) or a positive (“you have so much to gain”). However, investigators observed that the negatively framed presentations generated significantly more anxiety in the respondents. The authors of the paper conclude that if there is no significant difference in the effectiveness, why would we chose a negatively framed presentation that is likely to generate anxiety that we know is associated with increased morbidity and mortality. From a purely public health perspective, it doesn’t make sense and is counterproductive.
I guess if we look back to the old carrot and stick metaphor we shouldn’t be surprised by the findings in this paper. If one’s only goal is to get a group of young preschoolers to behave by scaring the b’geezes out of them with a mask or a threat of bodily punishment, then go for it. Scare tactics will probably work just as well as offering a well-chosen reward system. However, the devil is in the side effects. It’s the same argument that I give to parents who argue that spanking works. Of course it does, but it has a narrow margin for safety and can set up ripples of negative side effects that can destroy healthy parent-child relationships.
The bottom line of this story is the sad truth that somewhere along the line someone failed to effectively train these four daycare workers. But, do we as health care providers need to rethink our training? Have we forgotten our commitment to “First do no harm?” As we craft our messaging have we thought enough about the potential side effects of our attempts at scaring the public into following our suggestions?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Single dose of psilocybin for major depression tied to short-term remission
, new research shows.
In the largest study of psilocybin for TRD to date, results of the phase 2b randomized, double-blind trial show participants in the 25-mg dose group experienced a significant reduction in depressive symptoms for at least 3 weeks vs. patients in the 10-mg or 1-mg group, which served as the control group.
Investigators found that 29% of participants who received the 25-mg dose were in remission 3 weeks after the treatment and 37% had at least a 50% drop in depression scores. However, at the 3-month mark, only 20% of those on the 25-mg dose experienced significant improvement.
The change from baseline to week 3 in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score was significantly better with a 25-mg dose than with a 1-mg dose; there was no significant difference between the 10-mg dose and the 1-mg dose, the investigators reported.
The response rate was high for those receiving the 25-mg dose, lead investigator Guy Goodwin, MD, DPhil, told reporters attending a press briefing.
“It’s important to understand that response rates in these patients are usually somewhere between 10% and 20%, and we are seeing remission rates at three weeks of 30%,” he said.
Dr. Goodwin is chief medical officer of COMPASS Pathways, the company that funded the trial and created COMP360, the synthetic formulation of psilocybin used in the trial, and professor emeritus of psychiatry at the University of Oxford, England.
Based on the results of the trial it was announced that a phase 3 trial will launch in December.
The study was published online in the New England Journal of Medicine.
Further research planned
Psilocybin has been under investigation for TRD for some time, including one study that compared it with the antidepressant escitalopram (Lexapro) with promising results.
In the current study the researchers sought to find an acceptable, efficacious dose and the safety of a synthetic formulation of the drug administered in combination with psychological support.
The multicenter study was conducted at 22 sites in 10 countries and included 233 participants with TRD and evaluated the safety and efficacy of one of three doses. The study’s primary endpoint was change from baseline to 3 weeks in MADRS scores in patients with TRD. The scale runs from 0 to 60 with higher scores indicating more severe depression.
Participants were randomly assigned to receive 25 mg of psilocybin (n = 79), 10 mg (n = 75) or 1 mg (n = 79). Those taking medications discontinued them at least 2 weeks before the baseline visit. The mean MADRS score was 32 or 33 in each study group.
There was a 3- to 6-week run-up period to the study in which each participant met with a study therapist about three times to build trust and prepare for the psychedelic experience.
On the day of psilocybin administration, each participant listened to a tailored music playlist and wore eye shades while reclining in a comfortable chair to direct attention inwardly.
The psychotherapy sessions lasted 6-8 hours, and two therapists were always present. The following day, participants returned for an “integration” session with the therapists that was designed to help the participants explore insights from their session.
MADRS scores were measured at baseline, the day following psilocybin administration, and at weeks 1, 3, 6, 9, and 12.
Participants were asked to stay off standard antidepressant treatment during the first 3 weeks of the trial but could be restarted at any time if deemed necessary by a trial investigator.
Mean changes from baseline to week 3 in MADRS scores were −12.0 for 25-mg, −7.9 for 10-mg, and −5.4 for 1-mg groups. The difference between the 25-mg group and 1-mg group was −6.6 (95% confidence interval [CI], −10.2 to −2.9; P < .001 and between the 10-mg group and 1-mg group was −2.5 (95% CI, −6.2 to 1.2; P = .18).
The investigators reported that in the 25-mg group, the incidences of response and remission at 3 weeks, but not sustained response at 12 weeks, were generally supportive of the primary results.
Up to 84% of those who received the 25-mg dosage reported adverse events, with the occurrence dropping slightly with each dosage group. The most frequent adverse events included headache, nausea, dizziness, and fatigue, and occurred only on administration day.
Among those who received the 25-mg dose of psilocybin, two participants reported suicidal thoughts during the 3 weeks following treatment, and 3 months post treatment, three patients exhibited suicidal behavior.
Dr. Goodwin noted that these participants had a prior history of suicidal behavior. Two participants in the 10-mg group also had suicidal thoughts. However, the investigators also noted that suicidal ideation, behavior, or self-injury occurred in all dose groups.
The researchers noted that longer and larger trials, including comparisons with existing depression treatments, are needed to determine the safety and efficacy of psilocybin for TRD.
Intriguing, sobering
In an accompanying editorial, Bertha Madras, PhD, McLean Hospital, Belmont, Mass., and Harvard Medical School, Boston, noted “the findings are both intriguing and sobering. The highest dose (25 mg), but not the intermediate dose (10 mg), resulted in significantly lower levels of depressive symptoms after 3 weeks than the lowest dose (1 mg, which served as a control), but the 37% incidence of response with the 25-mg dose was numerically lower than that in large trials of conventional antidepressants and less robust than in a trial showing similar efficacies of psilocybin and a selective serotonin reuptake inhibitor.”
Also sobering, she noted, were the high percentages of adverse events in the 25-mg group and suicidal ideation and behavior. Dr. Madras also wondered if “legalization and commercialization [of psychedelics] are allied with the medical movement, psychedelic shops and ‘clinics’ could proliferate even for vulnerable populations, and rigorously designed medical protocols will be compromised.
“Nevertheless,” she concluded, “it is provocative that these agents show some short-term benefit for depression in selected populations.”
Dr. Goodwin is CMO of Compass Pathways, which funded the study. He and several coauthors disclosed relationships with industry. Dr. Madras reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In the largest study of psilocybin for TRD to date, results of the phase 2b randomized, double-blind trial show participants in the 25-mg dose group experienced a significant reduction in depressive symptoms for at least 3 weeks vs. patients in the 10-mg or 1-mg group, which served as the control group.
Investigators found that 29% of participants who received the 25-mg dose were in remission 3 weeks after the treatment and 37% had at least a 50% drop in depression scores. However, at the 3-month mark, only 20% of those on the 25-mg dose experienced significant improvement.
The change from baseline to week 3 in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score was significantly better with a 25-mg dose than with a 1-mg dose; there was no significant difference between the 10-mg dose and the 1-mg dose, the investigators reported.
The response rate was high for those receiving the 25-mg dose, lead investigator Guy Goodwin, MD, DPhil, told reporters attending a press briefing.
“It’s important to understand that response rates in these patients are usually somewhere between 10% and 20%, and we are seeing remission rates at three weeks of 30%,” he said.
Dr. Goodwin is chief medical officer of COMPASS Pathways, the company that funded the trial and created COMP360, the synthetic formulation of psilocybin used in the trial, and professor emeritus of psychiatry at the University of Oxford, England.
Based on the results of the trial it was announced that a phase 3 trial will launch in December.
The study was published online in the New England Journal of Medicine.
Further research planned
Psilocybin has been under investigation for TRD for some time, including one study that compared it with the antidepressant escitalopram (Lexapro) with promising results.
In the current study the researchers sought to find an acceptable, efficacious dose and the safety of a synthetic formulation of the drug administered in combination with psychological support.
The multicenter study was conducted at 22 sites in 10 countries and included 233 participants with TRD and evaluated the safety and efficacy of one of three doses. The study’s primary endpoint was change from baseline to 3 weeks in MADRS scores in patients with TRD. The scale runs from 0 to 60 with higher scores indicating more severe depression.
Participants were randomly assigned to receive 25 mg of psilocybin (n = 79), 10 mg (n = 75) or 1 mg (n = 79). Those taking medications discontinued them at least 2 weeks before the baseline visit. The mean MADRS score was 32 or 33 in each study group.
There was a 3- to 6-week run-up period to the study in which each participant met with a study therapist about three times to build trust and prepare for the psychedelic experience.
On the day of psilocybin administration, each participant listened to a tailored music playlist and wore eye shades while reclining in a comfortable chair to direct attention inwardly.
The psychotherapy sessions lasted 6-8 hours, and two therapists were always present. The following day, participants returned for an “integration” session with the therapists that was designed to help the participants explore insights from their session.
MADRS scores were measured at baseline, the day following psilocybin administration, and at weeks 1, 3, 6, 9, and 12.
Participants were asked to stay off standard antidepressant treatment during the first 3 weeks of the trial but could be restarted at any time if deemed necessary by a trial investigator.
Mean changes from baseline to week 3 in MADRS scores were −12.0 for 25-mg, −7.9 for 10-mg, and −5.4 for 1-mg groups. The difference between the 25-mg group and 1-mg group was −6.6 (95% confidence interval [CI], −10.2 to −2.9; P < .001 and between the 10-mg group and 1-mg group was −2.5 (95% CI, −6.2 to 1.2; P = .18).
The investigators reported that in the 25-mg group, the incidences of response and remission at 3 weeks, but not sustained response at 12 weeks, were generally supportive of the primary results.
Up to 84% of those who received the 25-mg dosage reported adverse events, with the occurrence dropping slightly with each dosage group. The most frequent adverse events included headache, nausea, dizziness, and fatigue, and occurred only on administration day.
Among those who received the 25-mg dose of psilocybin, two participants reported suicidal thoughts during the 3 weeks following treatment, and 3 months post treatment, three patients exhibited suicidal behavior.
Dr. Goodwin noted that these participants had a prior history of suicidal behavior. Two participants in the 10-mg group also had suicidal thoughts. However, the investigators also noted that suicidal ideation, behavior, or self-injury occurred in all dose groups.
The researchers noted that longer and larger trials, including comparisons with existing depression treatments, are needed to determine the safety and efficacy of psilocybin for TRD.
Intriguing, sobering
In an accompanying editorial, Bertha Madras, PhD, McLean Hospital, Belmont, Mass., and Harvard Medical School, Boston, noted “the findings are both intriguing and sobering. The highest dose (25 mg), but not the intermediate dose (10 mg), resulted in significantly lower levels of depressive symptoms after 3 weeks than the lowest dose (1 mg, which served as a control), but the 37% incidence of response with the 25-mg dose was numerically lower than that in large trials of conventional antidepressants and less robust than in a trial showing similar efficacies of psilocybin and a selective serotonin reuptake inhibitor.”
Also sobering, she noted, were the high percentages of adverse events in the 25-mg group and suicidal ideation and behavior. Dr. Madras also wondered if “legalization and commercialization [of psychedelics] are allied with the medical movement, psychedelic shops and ‘clinics’ could proliferate even for vulnerable populations, and rigorously designed medical protocols will be compromised.
“Nevertheless,” she concluded, “it is provocative that these agents show some short-term benefit for depression in selected populations.”
Dr. Goodwin is CMO of Compass Pathways, which funded the study. He and several coauthors disclosed relationships with industry. Dr. Madras reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In the largest study of psilocybin for TRD to date, results of the phase 2b randomized, double-blind trial show participants in the 25-mg dose group experienced a significant reduction in depressive symptoms for at least 3 weeks vs. patients in the 10-mg or 1-mg group, which served as the control group.
Investigators found that 29% of participants who received the 25-mg dose were in remission 3 weeks after the treatment and 37% had at least a 50% drop in depression scores. However, at the 3-month mark, only 20% of those on the 25-mg dose experienced significant improvement.
The change from baseline to week 3 in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score was significantly better with a 25-mg dose than with a 1-mg dose; there was no significant difference between the 10-mg dose and the 1-mg dose, the investigators reported.
The response rate was high for those receiving the 25-mg dose, lead investigator Guy Goodwin, MD, DPhil, told reporters attending a press briefing.
“It’s important to understand that response rates in these patients are usually somewhere between 10% and 20%, and we are seeing remission rates at three weeks of 30%,” he said.
Dr. Goodwin is chief medical officer of COMPASS Pathways, the company that funded the trial and created COMP360, the synthetic formulation of psilocybin used in the trial, and professor emeritus of psychiatry at the University of Oxford, England.
Based on the results of the trial it was announced that a phase 3 trial will launch in December.
The study was published online in the New England Journal of Medicine.
Further research planned
Psilocybin has been under investigation for TRD for some time, including one study that compared it with the antidepressant escitalopram (Lexapro) with promising results.
In the current study the researchers sought to find an acceptable, efficacious dose and the safety of a synthetic formulation of the drug administered in combination with psychological support.
The multicenter study was conducted at 22 sites in 10 countries and included 233 participants with TRD and evaluated the safety and efficacy of one of three doses. The study’s primary endpoint was change from baseline to 3 weeks in MADRS scores in patients with TRD. The scale runs from 0 to 60 with higher scores indicating more severe depression.
Participants were randomly assigned to receive 25 mg of psilocybin (n = 79), 10 mg (n = 75) or 1 mg (n = 79). Those taking medications discontinued them at least 2 weeks before the baseline visit. The mean MADRS score was 32 or 33 in each study group.
There was a 3- to 6-week run-up period to the study in which each participant met with a study therapist about three times to build trust and prepare for the psychedelic experience.
On the day of psilocybin administration, each participant listened to a tailored music playlist and wore eye shades while reclining in a comfortable chair to direct attention inwardly.
The psychotherapy sessions lasted 6-8 hours, and two therapists were always present. The following day, participants returned for an “integration” session with the therapists that was designed to help the participants explore insights from their session.
MADRS scores were measured at baseline, the day following psilocybin administration, and at weeks 1, 3, 6, 9, and 12.
Participants were asked to stay off standard antidepressant treatment during the first 3 weeks of the trial but could be restarted at any time if deemed necessary by a trial investigator.
Mean changes from baseline to week 3 in MADRS scores were −12.0 for 25-mg, −7.9 for 10-mg, and −5.4 for 1-mg groups. The difference between the 25-mg group and 1-mg group was −6.6 (95% confidence interval [CI], −10.2 to −2.9; P < .001 and between the 10-mg group and 1-mg group was −2.5 (95% CI, −6.2 to 1.2; P = .18).
The investigators reported that in the 25-mg group, the incidences of response and remission at 3 weeks, but not sustained response at 12 weeks, were generally supportive of the primary results.
Up to 84% of those who received the 25-mg dosage reported adverse events, with the occurrence dropping slightly with each dosage group. The most frequent adverse events included headache, nausea, dizziness, and fatigue, and occurred only on administration day.
Among those who received the 25-mg dose of psilocybin, two participants reported suicidal thoughts during the 3 weeks following treatment, and 3 months post treatment, three patients exhibited suicidal behavior.
Dr. Goodwin noted that these participants had a prior history of suicidal behavior. Two participants in the 10-mg group also had suicidal thoughts. However, the investigators also noted that suicidal ideation, behavior, or self-injury occurred in all dose groups.
The researchers noted that longer and larger trials, including comparisons with existing depression treatments, are needed to determine the safety and efficacy of psilocybin for TRD.
Intriguing, sobering
In an accompanying editorial, Bertha Madras, PhD, McLean Hospital, Belmont, Mass., and Harvard Medical School, Boston, noted “the findings are both intriguing and sobering. The highest dose (25 mg), but not the intermediate dose (10 mg), resulted in significantly lower levels of depressive symptoms after 3 weeks than the lowest dose (1 mg, which served as a control), but the 37% incidence of response with the 25-mg dose was numerically lower than that in large trials of conventional antidepressants and less robust than in a trial showing similar efficacies of psilocybin and a selective serotonin reuptake inhibitor.”
Also sobering, she noted, were the high percentages of adverse events in the 25-mg group and suicidal ideation and behavior. Dr. Madras also wondered if “legalization and commercialization [of psychedelics] are allied with the medical movement, psychedelic shops and ‘clinics’ could proliferate even for vulnerable populations, and rigorously designed medical protocols will be compromised.
“Nevertheless,” she concluded, “it is provocative that these agents show some short-term benefit for depression in selected populations.”
Dr. Goodwin is CMO of Compass Pathways, which funded the study. He and several coauthors disclosed relationships with industry. Dr. Madras reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE