User login
For MD-IQ use only
A First Look at the VA MISSION Act Veteran Health Administration Medical School Scholarship and Loan Repayment Programs
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
As one of 4 statutory missions, the US Department of Veterans Affairs (VA) educates and trains health professionals to enhance the quality of and timely access to care provided to veterans within the Veterans Health Administration (VHA). To achieve its mission to
Despite its long-term success affiliating with medical schools, VA has continued to be challenged by physician staff shortages with wide variability in the number and specialty of available health care professionals across facilities.3,4 A 2020 VA Office of Inspector General report on VHA occupational staffing shortages concluded that numerous physician specialties were difficult to recruit due to a lack of qualified applicants, noncompetitive salary, and less desirable geographic locations.3
Federal health professions scholarship programs and loan repayment programs have long been used to address physician shortages.4 Focusing on physician shortages in underserved areas in the US, the Emergency Health Personnel Act of 1970 and its subsequent amendments paved the way for various federal medical school scholarship and loan repayment programs.5 Similarly, physician shortages in the armed forces were mitigated through the Uniformed Services Health Professions Revitalization Act of 1972 (USHPRA).6,7
In 2018, Congress passed the VA MISSION (Maintaining Internal Systems and Strengthening Integrated Outside Networks) Act, which included sections designed to alleviate physician shortages in the VHA.8 These sections authorized scholarships similar to those offered by the US Department of Defense (DoD) and loan repayment programs. Section 301 created the Health Professions Scholarship Program (HPSP), which offers scholarships for physicians and dentists. Section 302 increased the maximum debt reduction through the Education Debt Reduction Program (EDRP). Section 303 authorizes the Specialty Education Loan Repayment Program (SELRP), which provides for repayment of educational loans for physicians in specialties deemed necessary for VA. Finally, Section 304 created the Veterans Healing Veterans (VHV), a pilot scholarship specifically for veteran medical students.
Program Characteristics
Health Professions Scholarship
The VA HPSP is a program for physicians and dentists that extends from 2020 to 2033. The HPSP funds the costs of tuition, fees, and provides a stipend with a service obligation of 18 months for each year of support. The program is authorized for 10 years and must provide a minimum of 50 scholarships annually for physicians or dentists based on VHA needs. Applications are screened based on criteria that include a commitment to rural or underserved populations, veteran status, grade point average, essays, and letters of recommendation. Although the minimum required number of scholarships annually is 50, VA anticipates providing 1000 scholarships over 10 years with an aim to significantly increase the number physicians at VHA facilities (Table 1).
Implemented in 2020, the VHV was a 1-year pilot program. It offered scholarships to 2 veterans attending medical school at each of the 5 Teague-Cranston and the 4 Historically Black College and University (HBCU) medical schools (Table 2). The intent of the program was to determine the feasibility of increasing the pool of veteran physicians at VHA. Eligible applicants were notified of the scholarship opportunity through the American Medical College Application Service or through the medical school. Applicants must have separated from military service within the preceding 10 years of being admitted to medical school. In exchange for full tuition, fees, a monthly stipend, and rotation travel costs, the recipients accepted a 4-year clinical service obligation at VA facilities after completing their residency training.
Specialty Education Loan Repayment
The SELRP is a loan repayment program available to recently graduated physicians. Applicants must have graduated from an accredited medical or osteopathic school, matched to an accredited residency program and be ≥ 2 years from completion of residency. The specialties qualifying for SELRP are determined through an analysis of succession planning by the VA Office of Workforce Management and Consulting and change based on VA physician workforce needs. The SELRP provides loan repayment in the amount of $40,000 per year for up to 4 years, with a service obligation of 1 year for each $40,000 of support. In April 2021, VA began accepting applications from the eligible specialties of family medicine, internal medicine, gastroenterology, psychiatry, emergency medicine, and geriatrics.
Education Debt Reduction
The EDRP offers debt relief to clinicians in the most difficult to recruit professions, including physicians (generalists and specialists), registered nurses, licensed practical nurses, social workers, and psychologists. The list of difficult to recruit positions is developed annually by VA facilities. Annual reimbursements through the program may be used for tuition and expenses, such as fees, books, supplies, equipment, and other materials. In 2018, through the MISSION Act Section 302, the annual loan repayment was increased from $24,000 to $40,000, and the maximum level of support was increased from $120,000 to $200,000 over 5 years. Recipients receive reimbursement for loan repayment at the end of each year or service period and recipients are not required to remain in VA for 5 years.
Program Results
Health Professions Scholarship
For academic years 2020/2021 and 2021/2022, 126 HPSP applications from both allopathic and osteopathic schools were submitted and 51 scholarships were awarded (Table 3). Assuming an average residency length of 4 years, VHA estimates that these awards will yield 204 service-year equivalents by 2029.
Veterans Healing Veterans
In the VHV program, scholarship recipients came from 5 Teague-Cranston schools; 2 at University of South Carolina, 2 at East Tennessee State University, 2 at Wright State University, 1 at Texas A&M College of Medicine, 1 at Marshall University; and 3 HBCUs; 2 at Howard University, 1 at Morehouse School of Medicine and 1 at Meharry Medical College. The Charles R. Drew University of Medicine and Science did not nominate any students for the scholarship. Assuming all recipients complete postgraduate training, the VHV scholarship program will provide an additional 12 veteran physicians to serve at VA for at least 4 years each (48 service years).
Specialty Education Loan Repayment
Fourteen applicants have been approved, including 5 in psychiatry, 4 in family medicine, 3 in internal medicine, 1 in emergency medicine, and 1 in geriatrics. The mean loan repayment is anticipated to be $110,000 and equating to 38.5 VA service years or a mean of 2.3 years of service obligation per individual for the first cohort. The program has no termination date, and with continued funding, VA anticipates granting 100 loan repayments annually.
Education Debt Reduction
Since 2018, 1,546 VA physicians have received EDRP awards. Due to the increased reimbursement provided through the MISSION Act, average physician award amounts have increased from $96,090 in 2018 to $142,557 in 2019 and $148,302 in 2020.
Conclusions
The VA physician scholarship and loan repayment programs outlined in the MISSION Act build on the success of existing federal scholarship programs by providing opportunities for physician trainees to alleviate educational debt and explore a VA health professions career.
Looking ahead, VA must focus on measuring the success of the MISSION scholarship and loan repayment programs by tracking rates of acceptance and student graduation, residency and fellowship completion, and placement in VA medical facilities—both for the service obligation and future employment. Ultimately, the total impact on VA staffing, especially at rural and underresourced sites, will determine the success of the MISSION programs.
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
1. VA Policy Memorandum #2. Policy in Association of Veterans’ Hospitals with Medical Schools. US Department of Veterans Affairs. January 20, 1946. Accessed February 17, 2022. https://www.va.gov/oaa/Archive/PolicyMemo2.pdf 2. Gilman SC, Chang BK, Zeiss RA, Dougherty MB, Marks WJ, Ludke DA, Cox M. “The academic mission of the Department of Veterans Affairs.” In: Praeger Handbook of Veterans’ Health: History, Challenges, Issues, and Developments. Praeger; 2012:53-82.
3. Office of Inspector General, Veterans Health Administration OIG Determination of VHA Occupational Staffing Shortages FY2020. US Department of Veterans Affairs. Published September 23, 2020. Accessed February 17, 2022. https://www.va.gov/oig/pubs/VAOIG-20-01249-259.pdf
4. Hussey PS, Ringel J, et al. Resources and capabilities of the Department of Veterans Affairs to provide timely and accessible care to veterans. Rand Health Q. 2015;5(4). Accessed February 17, 2022. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1100/RR1165z2/RAND_RR1165z2.pdf
5. Lynch A, Best T, Gutierrez SC, Daily JA. What Should I Do With My Student Loans? A Proposed Strategy for Educational Debt Management. J Grad Med Educ. 2018;10(1):11-15. doi:10.4300/JGME-D-17-00279.1
6. The Uniformed Services Health Professions Revitalization Act of 1972, PL 92-426. US Government Publishing Office. Published 1972. Accessed February 17, 2022. https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg713.pdf
7. Armed Forces Health Professions Financial Assistance Programs, 10 USC § 105 (2006).
8. ‘‘VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018’’. H.R. 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 17, 2022. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
Telescoping Stents to Maintain a 3-Way Patency of the Airway
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
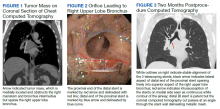
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
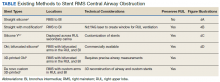
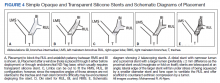
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
A Pioneer in Women’s Federal Practice
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
Is a progression-free survival benefit alone really worth $10,000 a month?
In the field of lung cancer, and more broadly in oncology, many of our biggest advances in 2021 have come as clinically meaningful improvements in surrogate endpoints – disease-free survival, progression-free survival, and sometimes even pathologic complete response rate.
I have historically been most compelled to consider new findings to be practice-changing when they improve overall survival or quality of life – the endpoints that translate to direct benefits for patients. However, I also feel it is appropriate to call surrogate endpoints practice-changing when they can predict improvements in overall survival or quality of life.
Take the PACIFIC trial, which assessed maintenance durvalumab after concurrent chemoradiation for unresectable stage III non–small cell lung cancer (NSCLC).
Back in 2017, I was initially unconvinced by the interim phase 3 data that were presented in a press release that highlighted the disease-free survival benefit. However, after examining additional data more closely, I saw the dramatic improvement in time to distant relapse or death was overwhelmingly likely to predict an improvement in overall survival – a benefit that the data subsequently bore out.
More recently, the disease-free survival results for adjuvant osimertinib in resected endothelial growth factor receptor mutation–positive NSCLC and adjuvant atezolizumab in resected programmed death-ligand 1–positive stage II-IIIA NSCLC have led to excitement about Food and Drug Administration approvals for these therapies. Although there is reason to be cautious about the likelihood of an overall survival benefit with either therapy – particularly for patients with low programmed death-ligand 1 who receive atezolizumab – I think that the results are promising enough to discuss these treatment options with appropriate patients.
Some argue, however, that overall survival is not necessarily a critical goal and that certain surrogate endpoints are inherently beneficial. Patients and oncologists may, for instance, view delaying disease progression as a win, even if overall survival remains the same.
I appreciate the view that favorable scan results are an achievement, even without a survival benefit. Patients appreciate the good news, and it is gratifying for us to deliver it. However, what remains unspoken is whether the benefit can be provided at a reasonable value given the financial costs associated with the new treatment.
In the United States, we consider the physician-patient relationship to be autonomous and even revered, but we conveniently ignore the fact that both are deciding on treatments that are funded by people who are not represented in the room. And in a health care system that fails to cover basic cancer care needs as well as other critical, high-value interventions for both the uninsured and underinsured, we should acknowledge that our decisions redirect limited resources from others.
Is it the best use of $10,000 per month for a new drug that improves disease-free survival but not overall survival?
At the same time, we also have to remain vigilant and reflect on whether we are echoing the marketing messages of the companies selling these treatments. Having recently watched the excellent Hulu series Dopesick, which realistically portrays the medical community’s egregious overuse of Oxycontin at the behest of Purdue Pharmaceuticals, it is striking to see how effectively the pharmaceutical industry can co-opt stakeholders. Very few physicians or patients have expertise in health care policy with broad societal perspective, yet subspecialists offer edicts as if society should dedicate unlimited resources first and foremost to our career focus or personal cause.
I certainly appreciate the appeal of surrogate endpoints in a world in which we hope to offer novel therapies to patients in a timely fashion. In the next few years, some of our most promising data in oncology will demand that we consider whether surrogate endpoints are practice-changing. We are facing a fundamental question: Are we using these surrogate endpoints to predict overall survival or quality of life or do these endpoints stand on their own as practice-changing metrics?
We need to acknowledge that our primary clinical focus is not the only one that deserves our attention, particularly when our treatment decisions are, in fact, spending other people’s money. We should be asking not whether we prefer to deliver good news after a scan, but whether that alone is enough to justify the high cost of a new treatment without an overall survival benefit.
Dr. West disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, and Merck; serving as a speaker or a member of a speakers bureau for Ariad/Takeda, AstraZeneca, and Genentech/Roche; and receiving income from Eli Lilly. A version of this article first appeared on Medscape.com.
In the field of lung cancer, and more broadly in oncology, many of our biggest advances in 2021 have come as clinically meaningful improvements in surrogate endpoints – disease-free survival, progression-free survival, and sometimes even pathologic complete response rate.
I have historically been most compelled to consider new findings to be practice-changing when they improve overall survival or quality of life – the endpoints that translate to direct benefits for patients. However, I also feel it is appropriate to call surrogate endpoints practice-changing when they can predict improvements in overall survival or quality of life.
Take the PACIFIC trial, which assessed maintenance durvalumab after concurrent chemoradiation for unresectable stage III non–small cell lung cancer (NSCLC).
Back in 2017, I was initially unconvinced by the interim phase 3 data that were presented in a press release that highlighted the disease-free survival benefit. However, after examining additional data more closely, I saw the dramatic improvement in time to distant relapse or death was overwhelmingly likely to predict an improvement in overall survival – a benefit that the data subsequently bore out.
More recently, the disease-free survival results for adjuvant osimertinib in resected endothelial growth factor receptor mutation–positive NSCLC and adjuvant atezolizumab in resected programmed death-ligand 1–positive stage II-IIIA NSCLC have led to excitement about Food and Drug Administration approvals for these therapies. Although there is reason to be cautious about the likelihood of an overall survival benefit with either therapy – particularly for patients with low programmed death-ligand 1 who receive atezolizumab – I think that the results are promising enough to discuss these treatment options with appropriate patients.
Some argue, however, that overall survival is not necessarily a critical goal and that certain surrogate endpoints are inherently beneficial. Patients and oncologists may, for instance, view delaying disease progression as a win, even if overall survival remains the same.
I appreciate the view that favorable scan results are an achievement, even without a survival benefit. Patients appreciate the good news, and it is gratifying for us to deliver it. However, what remains unspoken is whether the benefit can be provided at a reasonable value given the financial costs associated with the new treatment.
In the United States, we consider the physician-patient relationship to be autonomous and even revered, but we conveniently ignore the fact that both are deciding on treatments that are funded by people who are not represented in the room. And in a health care system that fails to cover basic cancer care needs as well as other critical, high-value interventions for both the uninsured and underinsured, we should acknowledge that our decisions redirect limited resources from others.
Is it the best use of $10,000 per month for a new drug that improves disease-free survival but not overall survival?
At the same time, we also have to remain vigilant and reflect on whether we are echoing the marketing messages of the companies selling these treatments. Having recently watched the excellent Hulu series Dopesick, which realistically portrays the medical community’s egregious overuse of Oxycontin at the behest of Purdue Pharmaceuticals, it is striking to see how effectively the pharmaceutical industry can co-opt stakeholders. Very few physicians or patients have expertise in health care policy with broad societal perspective, yet subspecialists offer edicts as if society should dedicate unlimited resources first and foremost to our career focus or personal cause.
I certainly appreciate the appeal of surrogate endpoints in a world in which we hope to offer novel therapies to patients in a timely fashion. In the next few years, some of our most promising data in oncology will demand that we consider whether surrogate endpoints are practice-changing. We are facing a fundamental question: Are we using these surrogate endpoints to predict overall survival or quality of life or do these endpoints stand on their own as practice-changing metrics?
We need to acknowledge that our primary clinical focus is not the only one that deserves our attention, particularly when our treatment decisions are, in fact, spending other people’s money. We should be asking not whether we prefer to deliver good news after a scan, but whether that alone is enough to justify the high cost of a new treatment without an overall survival benefit.
Dr. West disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, and Merck; serving as a speaker or a member of a speakers bureau for Ariad/Takeda, AstraZeneca, and Genentech/Roche; and receiving income from Eli Lilly. A version of this article first appeared on Medscape.com.
In the field of lung cancer, and more broadly in oncology, many of our biggest advances in 2021 have come as clinically meaningful improvements in surrogate endpoints – disease-free survival, progression-free survival, and sometimes even pathologic complete response rate.
I have historically been most compelled to consider new findings to be practice-changing when they improve overall survival or quality of life – the endpoints that translate to direct benefits for patients. However, I also feel it is appropriate to call surrogate endpoints practice-changing when they can predict improvements in overall survival or quality of life.
Take the PACIFIC trial, which assessed maintenance durvalumab after concurrent chemoradiation for unresectable stage III non–small cell lung cancer (NSCLC).
Back in 2017, I was initially unconvinced by the interim phase 3 data that were presented in a press release that highlighted the disease-free survival benefit. However, after examining additional data more closely, I saw the dramatic improvement in time to distant relapse or death was overwhelmingly likely to predict an improvement in overall survival – a benefit that the data subsequently bore out.
More recently, the disease-free survival results for adjuvant osimertinib in resected endothelial growth factor receptor mutation–positive NSCLC and adjuvant atezolizumab in resected programmed death-ligand 1–positive stage II-IIIA NSCLC have led to excitement about Food and Drug Administration approvals for these therapies. Although there is reason to be cautious about the likelihood of an overall survival benefit with either therapy – particularly for patients with low programmed death-ligand 1 who receive atezolizumab – I think that the results are promising enough to discuss these treatment options with appropriate patients.
Some argue, however, that overall survival is not necessarily a critical goal and that certain surrogate endpoints are inherently beneficial. Patients and oncologists may, for instance, view delaying disease progression as a win, even if overall survival remains the same.
I appreciate the view that favorable scan results are an achievement, even without a survival benefit. Patients appreciate the good news, and it is gratifying for us to deliver it. However, what remains unspoken is whether the benefit can be provided at a reasonable value given the financial costs associated with the new treatment.
In the United States, we consider the physician-patient relationship to be autonomous and even revered, but we conveniently ignore the fact that both are deciding on treatments that are funded by people who are not represented in the room. And in a health care system that fails to cover basic cancer care needs as well as other critical, high-value interventions for both the uninsured and underinsured, we should acknowledge that our decisions redirect limited resources from others.
Is it the best use of $10,000 per month for a new drug that improves disease-free survival but not overall survival?
At the same time, we also have to remain vigilant and reflect on whether we are echoing the marketing messages of the companies selling these treatments. Having recently watched the excellent Hulu series Dopesick, which realistically portrays the medical community’s egregious overuse of Oxycontin at the behest of Purdue Pharmaceuticals, it is striking to see how effectively the pharmaceutical industry can co-opt stakeholders. Very few physicians or patients have expertise in health care policy with broad societal perspective, yet subspecialists offer edicts as if society should dedicate unlimited resources first and foremost to our career focus or personal cause.
I certainly appreciate the appeal of surrogate endpoints in a world in which we hope to offer novel therapies to patients in a timely fashion. In the next few years, some of our most promising data in oncology will demand that we consider whether surrogate endpoints are practice-changing. We are facing a fundamental question: Are we using these surrogate endpoints to predict overall survival or quality of life or do these endpoints stand on their own as practice-changing metrics?
We need to acknowledge that our primary clinical focus is not the only one that deserves our attention, particularly when our treatment decisions are, in fact, spending other people’s money. We should be asking not whether we prefer to deliver good news after a scan, but whether that alone is enough to justify the high cost of a new treatment without an overall survival benefit.
Dr. West disclosed serving as a director, officer, partner, employee, adviser, consultant, or trustee for Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, and Merck; serving as a speaker or a member of a speakers bureau for Ariad/Takeda, AstraZeneca, and Genentech/Roche; and receiving income from Eli Lilly. A version of this article first appeared on Medscape.com.
Aaron Rodgers’s Panchakarma ‘cleanse’ is a dangerous play
Green Bay Packers quarterback Aaron Rodgers recently made headlines when he said he finished a 12-day detoxification process that is said to cleanse the body from within.
Registered dietitians who spoke to this news organizastion about the Panchakarma cleanse were quick to debunk it.
“There is no scientific evidence that supports a cleanse,” says Jessica DeGore, a registered dietitian and owner of Pittsburgh-based Dietitian Jess. “Our kidneys, GI systems, and liver all work to keep us healthy and rid us of toxins.”
The Panchakarma cleanse has roots in the ancient Indian alternative medicine approach known as ayurveda. Its 12-day approach includes such actions as self-induced vomiting; enemas; “nasya,” which means eliminating toxins through the nose; and even bloodletting in an effort to “detoxify” the blood.
All of it is misguided, says Alyssa Pike, a registered dietitian and senior manager of nutrition communications at the International Food Information Council.
“Certainly, there are medical procedures that require a fast of some kind for an extended period, and some choose to engage in a fast for religious or spiritual reasons,” she says, “but those are both very different from doing a voluntary ‘cleanse’ or ‘detox’ diet for purported health benefits.”
In fact, the idea that our bodies are full of “toxins” is simply incorrect.
“There isn’t a real medical definition of the word ‘toxins,’” says Ms. DeGore. “If you really had toxins in your body, you’d need emergency medical care, not a cleanse.”
Harmful advice
The entire notion of cleansing, whether Mr. Rodgers’s favored method or another, has gathered steam in the past few decades as celebrities like Gwyneth Paltrow peddle their favored methods for health.
“It’s easy for people to buy into these ideas when they see beautiful celebrities touting their methods for taking care of themselves,” says Ms. DeGore. “But behind the scenes, they receive support we can’t see or access to keep them well.”
Fans of Mr. Rodgers, Ms. Paltrow, and the like easily forget that these public figures have no medical credentials to support what they are pushing. And the celebrities often profit from their claims in the form of books and products related to them, leaving them anything but an unbiased resource.
In the case of Mr. Rodgers’s Panchakarma cleanse, there are real health risks in following its principles, says registered dietitian nutritionist Tiffany Godwin, director of nutrition and wellness at Connections Wellness Group.
“From a medical standpoint, engaging in activities such as induced vomiting, forced diarrhea, and enema use pose a high risk of extreme dehydration,” she says. “Dehydration can lead to fatigue, headaches, and dizziness at best. At worst, it can lead to seizures, kidney failure, coma, and death.”
Also, a cleanse that is designed to rid your body of toxins may introduce them to your body if you are using herbal medicines.
“Some of the products used in ayurvedic medicine contain herbs, metals, minerals, or other materials that may be harmful if used improperly,” Ms. Pike explains. “Ayurvedic medicines are regulated as dietary supplements rather than as drugs in the United States, so they are not required to meet the safety and efficacy standards for conventional medicines.”
When it comes to ayurveda, which is based on ancient writings that rely on a “natural” or holistic approach to physical and mental health, there is scant research or clinical trials in Western medical journals to support the approach. So people interested in following the practices should always consult with a doctor before trying them.
Mr. Rodgers’s approach includes a “nasal herbal remedy,” for instance.
“Tread very lightly with herbs and supplements,” advises Ms. DeGore. “We have the FDA to put drugs through a rigorous process before they approve them. These supplements are unregulated and don’t go through the same processes.”
Another danger is that when “cleansing,” you are starving your body of the nutrients it needs.
“When we vomit, or have diarrhea, we are not simply losing a mass amount of fluid from our bodies, but we are also losing essential electrolytes and minerals,” says Ms. Godwin.
Instead, say the registered dietitians, you can help your body by feeding it what it really needs.
“Eating plenty of fiber-rich foods such as fruits, veggies, beans, legumes, and whole grains, for example, keeps our GI tract moving and grooving, creating an ideal environment for our gut to use the useful things, and get rid of the not so useful,” says Ms. Godwin. “These systems can be compromised in different disease states, such as liver disease, kidney disease, and other GI disorders like Crohn’s or ulcerative colitis. With these disease states, however, cleanses can be even more harmful.”
Cleansing practices can also be a very slippery slope for people struggling with disordered eating.
“When celebrities promote these cleanses, they often bring in impressionable people,” says Ms. DeGore. “These approaches are stripping your body of nutritional needs and inducing disruptive behaviors. Ironically, they will slow down your metabolism, eventually leading to weight gain when you return to normal eating.”
With the Panchakarma cleanse, the 12-day length of cleansing is particularly alarming, says Ms. DeGore.
“Even after 5 days, you cannot think clearly and will have nasty side effects,” she says.
At the end of the day, whether it’s Mr. Rodgers, Ms. Paltrow, or another celebrity, all of the dietitians recommend steering clear of their advice when it comes to health and nutrition.
“Be wary of celebrities, influencers, or anyone who tries to persuade you to try an extreme cleanse or ‘too good to be true’ diet,” says Ms. Pike. “These can be dangerous for your health, physically and mentally.”
A version of this article first appeared on WebMD.com.
Green Bay Packers quarterback Aaron Rodgers recently made headlines when he said he finished a 12-day detoxification process that is said to cleanse the body from within.
Registered dietitians who spoke to this news organizastion about the Panchakarma cleanse were quick to debunk it.
“There is no scientific evidence that supports a cleanse,” says Jessica DeGore, a registered dietitian and owner of Pittsburgh-based Dietitian Jess. “Our kidneys, GI systems, and liver all work to keep us healthy and rid us of toxins.”
The Panchakarma cleanse has roots in the ancient Indian alternative medicine approach known as ayurveda. Its 12-day approach includes such actions as self-induced vomiting; enemas; “nasya,” which means eliminating toxins through the nose; and even bloodletting in an effort to “detoxify” the blood.
All of it is misguided, says Alyssa Pike, a registered dietitian and senior manager of nutrition communications at the International Food Information Council.
“Certainly, there are medical procedures that require a fast of some kind for an extended period, and some choose to engage in a fast for religious or spiritual reasons,” she says, “but those are both very different from doing a voluntary ‘cleanse’ or ‘detox’ diet for purported health benefits.”
In fact, the idea that our bodies are full of “toxins” is simply incorrect.
“There isn’t a real medical definition of the word ‘toxins,’” says Ms. DeGore. “If you really had toxins in your body, you’d need emergency medical care, not a cleanse.”
Harmful advice
The entire notion of cleansing, whether Mr. Rodgers’s favored method or another, has gathered steam in the past few decades as celebrities like Gwyneth Paltrow peddle their favored methods for health.
“It’s easy for people to buy into these ideas when they see beautiful celebrities touting their methods for taking care of themselves,” says Ms. DeGore. “But behind the scenes, they receive support we can’t see or access to keep them well.”
Fans of Mr. Rodgers, Ms. Paltrow, and the like easily forget that these public figures have no medical credentials to support what they are pushing. And the celebrities often profit from their claims in the form of books and products related to them, leaving them anything but an unbiased resource.
In the case of Mr. Rodgers’s Panchakarma cleanse, there are real health risks in following its principles, says registered dietitian nutritionist Tiffany Godwin, director of nutrition and wellness at Connections Wellness Group.
“From a medical standpoint, engaging in activities such as induced vomiting, forced diarrhea, and enema use pose a high risk of extreme dehydration,” she says. “Dehydration can lead to fatigue, headaches, and dizziness at best. At worst, it can lead to seizures, kidney failure, coma, and death.”
Also, a cleanse that is designed to rid your body of toxins may introduce them to your body if you are using herbal medicines.
“Some of the products used in ayurvedic medicine contain herbs, metals, minerals, or other materials that may be harmful if used improperly,” Ms. Pike explains. “Ayurvedic medicines are regulated as dietary supplements rather than as drugs in the United States, so they are not required to meet the safety and efficacy standards for conventional medicines.”
When it comes to ayurveda, which is based on ancient writings that rely on a “natural” or holistic approach to physical and mental health, there is scant research or clinical trials in Western medical journals to support the approach. So people interested in following the practices should always consult with a doctor before trying them.
Mr. Rodgers’s approach includes a “nasal herbal remedy,” for instance.
“Tread very lightly with herbs and supplements,” advises Ms. DeGore. “We have the FDA to put drugs through a rigorous process before they approve them. These supplements are unregulated and don’t go through the same processes.”
Another danger is that when “cleansing,” you are starving your body of the nutrients it needs.
“When we vomit, or have diarrhea, we are not simply losing a mass amount of fluid from our bodies, but we are also losing essential electrolytes and minerals,” says Ms. Godwin.
Instead, say the registered dietitians, you can help your body by feeding it what it really needs.
“Eating plenty of fiber-rich foods such as fruits, veggies, beans, legumes, and whole grains, for example, keeps our GI tract moving and grooving, creating an ideal environment for our gut to use the useful things, and get rid of the not so useful,” says Ms. Godwin. “These systems can be compromised in different disease states, such as liver disease, kidney disease, and other GI disorders like Crohn’s or ulcerative colitis. With these disease states, however, cleanses can be even more harmful.”
Cleansing practices can also be a very slippery slope for people struggling with disordered eating.
“When celebrities promote these cleanses, they often bring in impressionable people,” says Ms. DeGore. “These approaches are stripping your body of nutritional needs and inducing disruptive behaviors. Ironically, they will slow down your metabolism, eventually leading to weight gain when you return to normal eating.”
With the Panchakarma cleanse, the 12-day length of cleansing is particularly alarming, says Ms. DeGore.
“Even after 5 days, you cannot think clearly and will have nasty side effects,” she says.
At the end of the day, whether it’s Mr. Rodgers, Ms. Paltrow, or another celebrity, all of the dietitians recommend steering clear of their advice when it comes to health and nutrition.
“Be wary of celebrities, influencers, or anyone who tries to persuade you to try an extreme cleanse or ‘too good to be true’ diet,” says Ms. Pike. “These can be dangerous for your health, physically and mentally.”
A version of this article first appeared on WebMD.com.
Green Bay Packers quarterback Aaron Rodgers recently made headlines when he said he finished a 12-day detoxification process that is said to cleanse the body from within.
Registered dietitians who spoke to this news organizastion about the Panchakarma cleanse were quick to debunk it.
“There is no scientific evidence that supports a cleanse,” says Jessica DeGore, a registered dietitian and owner of Pittsburgh-based Dietitian Jess. “Our kidneys, GI systems, and liver all work to keep us healthy and rid us of toxins.”
The Panchakarma cleanse has roots in the ancient Indian alternative medicine approach known as ayurveda. Its 12-day approach includes such actions as self-induced vomiting; enemas; “nasya,” which means eliminating toxins through the nose; and even bloodletting in an effort to “detoxify” the blood.
All of it is misguided, says Alyssa Pike, a registered dietitian and senior manager of nutrition communications at the International Food Information Council.
“Certainly, there are medical procedures that require a fast of some kind for an extended period, and some choose to engage in a fast for religious or spiritual reasons,” she says, “but those are both very different from doing a voluntary ‘cleanse’ or ‘detox’ diet for purported health benefits.”
In fact, the idea that our bodies are full of “toxins” is simply incorrect.
“There isn’t a real medical definition of the word ‘toxins,’” says Ms. DeGore. “If you really had toxins in your body, you’d need emergency medical care, not a cleanse.”
Harmful advice
The entire notion of cleansing, whether Mr. Rodgers’s favored method or another, has gathered steam in the past few decades as celebrities like Gwyneth Paltrow peddle their favored methods for health.
“It’s easy for people to buy into these ideas when they see beautiful celebrities touting their methods for taking care of themselves,” says Ms. DeGore. “But behind the scenes, they receive support we can’t see or access to keep them well.”
Fans of Mr. Rodgers, Ms. Paltrow, and the like easily forget that these public figures have no medical credentials to support what they are pushing. And the celebrities often profit from their claims in the form of books and products related to them, leaving them anything but an unbiased resource.
In the case of Mr. Rodgers’s Panchakarma cleanse, there are real health risks in following its principles, says registered dietitian nutritionist Tiffany Godwin, director of nutrition and wellness at Connections Wellness Group.
“From a medical standpoint, engaging in activities such as induced vomiting, forced diarrhea, and enema use pose a high risk of extreme dehydration,” she says. “Dehydration can lead to fatigue, headaches, and dizziness at best. At worst, it can lead to seizures, kidney failure, coma, and death.”
Also, a cleanse that is designed to rid your body of toxins may introduce them to your body if you are using herbal medicines.
“Some of the products used in ayurvedic medicine contain herbs, metals, minerals, or other materials that may be harmful if used improperly,” Ms. Pike explains. “Ayurvedic medicines are regulated as dietary supplements rather than as drugs in the United States, so they are not required to meet the safety and efficacy standards for conventional medicines.”
When it comes to ayurveda, which is based on ancient writings that rely on a “natural” or holistic approach to physical and mental health, there is scant research or clinical trials in Western medical journals to support the approach. So people interested in following the practices should always consult with a doctor before trying them.
Mr. Rodgers’s approach includes a “nasal herbal remedy,” for instance.
“Tread very lightly with herbs and supplements,” advises Ms. DeGore. “We have the FDA to put drugs through a rigorous process before they approve them. These supplements are unregulated and don’t go through the same processes.”
Another danger is that when “cleansing,” you are starving your body of the nutrients it needs.
“When we vomit, or have diarrhea, we are not simply losing a mass amount of fluid from our bodies, but we are also losing essential electrolytes and minerals,” says Ms. Godwin.
Instead, say the registered dietitians, you can help your body by feeding it what it really needs.
“Eating plenty of fiber-rich foods such as fruits, veggies, beans, legumes, and whole grains, for example, keeps our GI tract moving and grooving, creating an ideal environment for our gut to use the useful things, and get rid of the not so useful,” says Ms. Godwin. “These systems can be compromised in different disease states, such as liver disease, kidney disease, and other GI disorders like Crohn’s or ulcerative colitis. With these disease states, however, cleanses can be even more harmful.”
Cleansing practices can also be a very slippery slope for people struggling with disordered eating.
“When celebrities promote these cleanses, they often bring in impressionable people,” says Ms. DeGore. “These approaches are stripping your body of nutritional needs and inducing disruptive behaviors. Ironically, they will slow down your metabolism, eventually leading to weight gain when you return to normal eating.”
With the Panchakarma cleanse, the 12-day length of cleansing is particularly alarming, says Ms. DeGore.
“Even after 5 days, you cannot think clearly and will have nasty side effects,” she says.
At the end of the day, whether it’s Mr. Rodgers, Ms. Paltrow, or another celebrity, all of the dietitians recommend steering clear of their advice when it comes to health and nutrition.
“Be wary of celebrities, influencers, or anyone who tries to persuade you to try an extreme cleanse or ‘too good to be true’ diet,” says Ms. Pike. “These can be dangerous for your health, physically and mentally.”
A version of this article first appeared on WebMD.com.
Can liquid biopsy predict oropharyngeal cancer recurrence?
PHOENIX – A liquid biopsy test may accurately predict recurrence of human papillomavirus (HPV)–driven oropharyngeal squamous cell carcinoma (OPSCC) earlier than standard clinical and imaging assessments, a new analysis indicates.
Of 80 patients who tested positive for circulating tumor tissue–modified viral (TTMV)-HPV DNA during surveillance, 74% (n = 59) had no other evidence of disease or had indeterminate disease status.
And of those patients, 93% (n = 55) “later had proven recurrent, metastatic disease on imaging and/or biopsy,” according to Glenn Hanna, MD, from the Dana-Farber Cancer Institute, Boston, who presented the results Feb. 24 at the 2022 Multidisciplinary Head and Neck Cancers Symposium.
“This is the first study to demonstrate broad clinical utility and validity of the biomarker in HPV-driven oropharyngeal cancer,” Dr. Hanna said in a press release.
Although patients with HPV-driven OPSCC generally have favorable outcomes, up to 25% will experience recurrence after treatment.
Post-treatment surveillance currently relies on physical examinations and imaging, but Dr. Hanna and colleagues wanted to determine whether a routine circulating cell-free TTMV-HPV DNA test could detect occult recurrence sooner.
Dr. Hanna and colleagues analyzed the records of 1,076 patients with HPV-driven OPSCC at 118 sites in the U.S. who had completed therapy more than 3 months previously and undergone an TTMV-HPV DNA test (NavDx, Naveris) between June 2020 and November 2021.
The results of the test, which used ultrasensitive digital droplet PCR to identify HPV subtypes 16, 18, 31, 33, and 35, were compared with subsequent clinical evidence of OPSCC via nasopharyngolaryngoscopy, radiologic evaluations, or tissue biopsy.
Approximately 7% of the patients tested positive (n = 80) for circulating TTMV-HPV DNA. Of those, 26.2% (n = 21) had known clinical recurrence, while 73.8% (n = 59) had no other evidence of disease or an intermediate disease status.
Among those with no clinical evidence of recurrence, 93.2% (n = 55) had their recurrence subsequently confirmed using imaging or biopsy. Of the 4 remaining patients, 2 had clinically suspicious lesions, and 2 had no other evidence of disease.
Overall, the data indicate that the biomarker test demonstrated a 95% positive predictive value (76 of 80 patients) for recurrence or persistence of HPV-driven OPSCC.
According to Dr. Hanna, a positive TTMV-HPV DNA test was the first indicator of recurrence for 72% of patients, and almost half of recurrences were detected more than 12 months after completing therapy.
“Incorporating a test for TTMV-HPV DNA into routine post-treatment follow-up can enable physicians to detect recurrent cancers earlier and allow us to start recommended interventions more quickly to improve outcomes,” Dr. Hanna said in the release.
The study was supported by Naveris, which developed the TTMV-HPV DNA test studied. Dr. Hanna declares relationships with Actuate Therapeutics, Altor BioScience, Bicara, BMS, GSK, Merck, Regeneron, Sanofi/Genzyme, and others.
A version of this article first appeared on Medscape.com.
PHOENIX – A liquid biopsy test may accurately predict recurrence of human papillomavirus (HPV)–driven oropharyngeal squamous cell carcinoma (OPSCC) earlier than standard clinical and imaging assessments, a new analysis indicates.
Of 80 patients who tested positive for circulating tumor tissue–modified viral (TTMV)-HPV DNA during surveillance, 74% (n = 59) had no other evidence of disease or had indeterminate disease status.
And of those patients, 93% (n = 55) “later had proven recurrent, metastatic disease on imaging and/or biopsy,” according to Glenn Hanna, MD, from the Dana-Farber Cancer Institute, Boston, who presented the results Feb. 24 at the 2022 Multidisciplinary Head and Neck Cancers Symposium.
“This is the first study to demonstrate broad clinical utility and validity of the biomarker in HPV-driven oropharyngeal cancer,” Dr. Hanna said in a press release.
Although patients with HPV-driven OPSCC generally have favorable outcomes, up to 25% will experience recurrence after treatment.
Post-treatment surveillance currently relies on physical examinations and imaging, but Dr. Hanna and colleagues wanted to determine whether a routine circulating cell-free TTMV-HPV DNA test could detect occult recurrence sooner.
Dr. Hanna and colleagues analyzed the records of 1,076 patients with HPV-driven OPSCC at 118 sites in the U.S. who had completed therapy more than 3 months previously and undergone an TTMV-HPV DNA test (NavDx, Naveris) between June 2020 and November 2021.
The results of the test, which used ultrasensitive digital droplet PCR to identify HPV subtypes 16, 18, 31, 33, and 35, were compared with subsequent clinical evidence of OPSCC via nasopharyngolaryngoscopy, radiologic evaluations, or tissue biopsy.
Approximately 7% of the patients tested positive (n = 80) for circulating TTMV-HPV DNA. Of those, 26.2% (n = 21) had known clinical recurrence, while 73.8% (n = 59) had no other evidence of disease or an intermediate disease status.
Among those with no clinical evidence of recurrence, 93.2% (n = 55) had their recurrence subsequently confirmed using imaging or biopsy. Of the 4 remaining patients, 2 had clinically suspicious lesions, and 2 had no other evidence of disease.
Overall, the data indicate that the biomarker test demonstrated a 95% positive predictive value (76 of 80 patients) for recurrence or persistence of HPV-driven OPSCC.
According to Dr. Hanna, a positive TTMV-HPV DNA test was the first indicator of recurrence for 72% of patients, and almost half of recurrences were detected more than 12 months after completing therapy.
“Incorporating a test for TTMV-HPV DNA into routine post-treatment follow-up can enable physicians to detect recurrent cancers earlier and allow us to start recommended interventions more quickly to improve outcomes,” Dr. Hanna said in the release.
The study was supported by Naveris, which developed the TTMV-HPV DNA test studied. Dr. Hanna declares relationships with Actuate Therapeutics, Altor BioScience, Bicara, BMS, GSK, Merck, Regeneron, Sanofi/Genzyme, and others.
A version of this article first appeared on Medscape.com.
PHOENIX – A liquid biopsy test may accurately predict recurrence of human papillomavirus (HPV)–driven oropharyngeal squamous cell carcinoma (OPSCC) earlier than standard clinical and imaging assessments, a new analysis indicates.
Of 80 patients who tested positive for circulating tumor tissue–modified viral (TTMV)-HPV DNA during surveillance, 74% (n = 59) had no other evidence of disease or had indeterminate disease status.
And of those patients, 93% (n = 55) “later had proven recurrent, metastatic disease on imaging and/or biopsy,” according to Glenn Hanna, MD, from the Dana-Farber Cancer Institute, Boston, who presented the results Feb. 24 at the 2022 Multidisciplinary Head and Neck Cancers Symposium.
“This is the first study to demonstrate broad clinical utility and validity of the biomarker in HPV-driven oropharyngeal cancer,” Dr. Hanna said in a press release.
Although patients with HPV-driven OPSCC generally have favorable outcomes, up to 25% will experience recurrence after treatment.
Post-treatment surveillance currently relies on physical examinations and imaging, but Dr. Hanna and colleagues wanted to determine whether a routine circulating cell-free TTMV-HPV DNA test could detect occult recurrence sooner.
Dr. Hanna and colleagues analyzed the records of 1,076 patients with HPV-driven OPSCC at 118 sites in the U.S. who had completed therapy more than 3 months previously and undergone an TTMV-HPV DNA test (NavDx, Naveris) between June 2020 and November 2021.
The results of the test, which used ultrasensitive digital droplet PCR to identify HPV subtypes 16, 18, 31, 33, and 35, were compared with subsequent clinical evidence of OPSCC via nasopharyngolaryngoscopy, radiologic evaluations, or tissue biopsy.
Approximately 7% of the patients tested positive (n = 80) for circulating TTMV-HPV DNA. Of those, 26.2% (n = 21) had known clinical recurrence, while 73.8% (n = 59) had no other evidence of disease or an intermediate disease status.
Among those with no clinical evidence of recurrence, 93.2% (n = 55) had their recurrence subsequently confirmed using imaging or biopsy. Of the 4 remaining patients, 2 had clinically suspicious lesions, and 2 had no other evidence of disease.
Overall, the data indicate that the biomarker test demonstrated a 95% positive predictive value (76 of 80 patients) for recurrence or persistence of HPV-driven OPSCC.
According to Dr. Hanna, a positive TTMV-HPV DNA test was the first indicator of recurrence for 72% of patients, and almost half of recurrences were detected more than 12 months after completing therapy.
“Incorporating a test for TTMV-HPV DNA into routine post-treatment follow-up can enable physicians to detect recurrent cancers earlier and allow us to start recommended interventions more quickly to improve outcomes,” Dr. Hanna said in the release.
The study was supported by Naveris, which developed the TTMV-HPV DNA test studied. Dr. Hanna declares relationships with Actuate Therapeutics, Altor BioScience, Bicara, BMS, GSK, Merck, Regeneron, Sanofi/Genzyme, and others.
A version of this article first appeared on Medscape.com.
Tastier chocolate may be healthier chocolate
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Practicing across state lines: A challenge for telemental health
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
Dressing in blue
On the first Friday in March, it has become an annual tradition to dress in blue to promote colorectal cancer awareness. Twitter feeds are filled with photos of members of our gastroenterology community (sometimes entire endoscopy units!) swathed in various shades of blue. This tradition was started in the mid-2000’s by a patient diagnosed with early-onset colorectal cancer who planned a fund raiser at her daughter’s elementary school where students were encouraged to wear a blue outfit and make a $1 donation to support awareness of this deadly but preventable cancer. What was once a local effort has now grown into a national phenomenon, and a powerful opportunity for the medical community to educate patients, friends, and family regarding risk factors for colorectal cancer and the importance of timely and effective screening. But while raising awareness is vital, it is only an initial step in the complex process of optimizing delivery of screening services and improving cancer outcomes through prevention and early detection.
In this month’s issue of GIHN, we report on a study from Cancer demonstrating the effectiveness of Spanish-speaking patient navigators in boosting colorectal cancer screening rates among Hispanic patients. We also highlight a quality improvement initiative at a large academic medical center demonstrating the impact of an electronic “primer” message delivered through the patient portal on screening completion rates in a mailed fecal immunochemical test outreach program. Finally, in this month’s Practice Management Toolbox column, Dr. Brill and Dr. Lieberman advise us on how to prepare for upcoming coverage changes impacting screening colonoscopy – a result of AGA’s tireless efforts to eliminate financial barriers impeding access to colorectal cancer screening.
As always, thank you for being a dedicated reader and please stay safe out there. Better days are ahead.
Megan A. Adams, MD, JD, MSc
Editor in Chief
On the first Friday in March, it has become an annual tradition to dress in blue to promote colorectal cancer awareness. Twitter feeds are filled with photos of members of our gastroenterology community (sometimes entire endoscopy units!) swathed in various shades of blue. This tradition was started in the mid-2000’s by a patient diagnosed with early-onset colorectal cancer who planned a fund raiser at her daughter’s elementary school where students were encouraged to wear a blue outfit and make a $1 donation to support awareness of this deadly but preventable cancer. What was once a local effort has now grown into a national phenomenon, and a powerful opportunity for the medical community to educate patients, friends, and family regarding risk factors for colorectal cancer and the importance of timely and effective screening. But while raising awareness is vital, it is only an initial step in the complex process of optimizing delivery of screening services and improving cancer outcomes through prevention and early detection.
In this month’s issue of GIHN, we report on a study from Cancer demonstrating the effectiveness of Spanish-speaking patient navigators in boosting colorectal cancer screening rates among Hispanic patients. We also highlight a quality improvement initiative at a large academic medical center demonstrating the impact of an electronic “primer” message delivered through the patient portal on screening completion rates in a mailed fecal immunochemical test outreach program. Finally, in this month’s Practice Management Toolbox column, Dr. Brill and Dr. Lieberman advise us on how to prepare for upcoming coverage changes impacting screening colonoscopy – a result of AGA’s tireless efforts to eliminate financial barriers impeding access to colorectal cancer screening.
As always, thank you for being a dedicated reader and please stay safe out there. Better days are ahead.
Megan A. Adams, MD, JD, MSc
Editor in Chief
On the first Friday in March, it has become an annual tradition to dress in blue to promote colorectal cancer awareness. Twitter feeds are filled with photos of members of our gastroenterology community (sometimes entire endoscopy units!) swathed in various shades of blue. This tradition was started in the mid-2000’s by a patient diagnosed with early-onset colorectal cancer who planned a fund raiser at her daughter’s elementary school where students were encouraged to wear a blue outfit and make a $1 donation to support awareness of this deadly but preventable cancer. What was once a local effort has now grown into a national phenomenon, and a powerful opportunity for the medical community to educate patients, friends, and family regarding risk factors for colorectal cancer and the importance of timely and effective screening. But while raising awareness is vital, it is only an initial step in the complex process of optimizing delivery of screening services and improving cancer outcomes through prevention and early detection.
In this month’s issue of GIHN, we report on a study from Cancer demonstrating the effectiveness of Spanish-speaking patient navigators in boosting colorectal cancer screening rates among Hispanic patients. We also highlight a quality improvement initiative at a large academic medical center demonstrating the impact of an electronic “primer” message delivered through the patient portal on screening completion rates in a mailed fecal immunochemical test outreach program. Finally, in this month’s Practice Management Toolbox column, Dr. Brill and Dr. Lieberman advise us on how to prepare for upcoming coverage changes impacting screening colonoscopy – a result of AGA’s tireless efforts to eliminate financial barriers impeding access to colorectal cancer screening.
As always, thank you for being a dedicated reader and please stay safe out there. Better days are ahead.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Autism spectrum disorder: Keys to early detection and accurate diagnosis
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334