User login
For MD-IQ use only
Linear Vulvar Lesions
The Diagnosis: Vestibular Papillomatosis
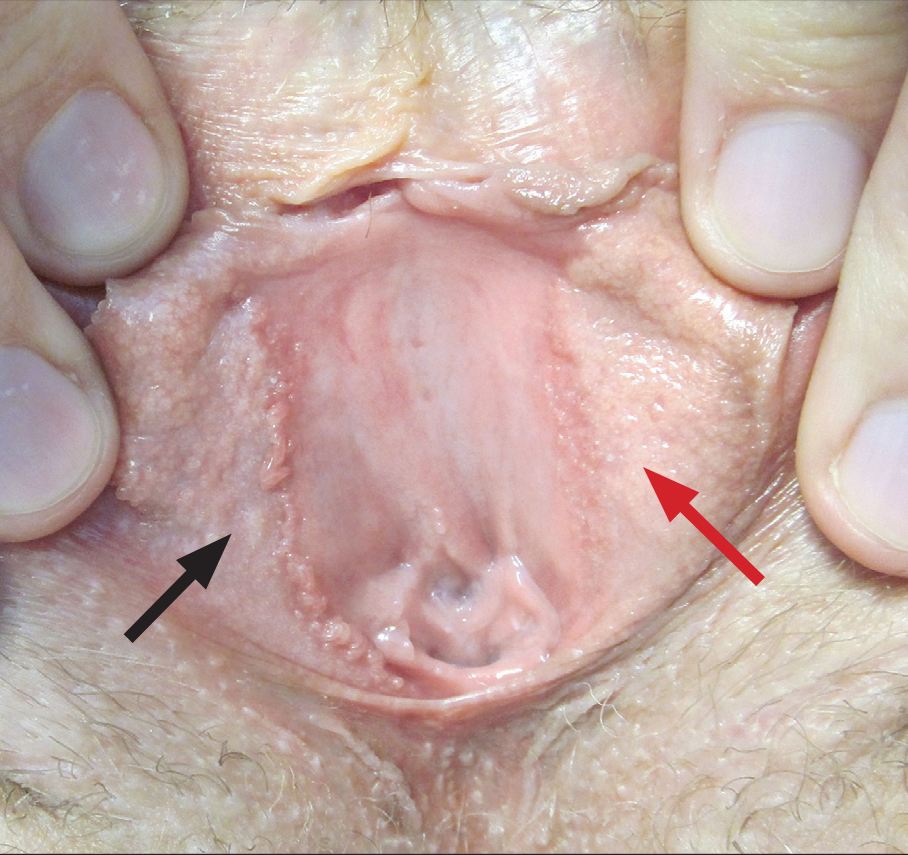
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
A 30-year-old woman with congenital absence of the uterus presented to dermatology for a second opinion of vulvar lesions that were first noted during adolescence. The patient reported that the lesions had not changed and were painful during sexual intercourse. The lesions were otherwise asymptomatic, and she had no additional relevant medical history or family history of similar lesions. She denied any history of sexually transmitted infections. Physical examination revealed multiple, soft, flesh-colored, 1- to 2-mm, discrete and coalescing, filiform papules distributed symmetrically in a linear array on the inner aspect of the bilateral medial labia minora. The rest of the mucocutaneous examination was normal.
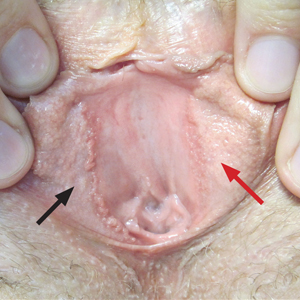
The lesions on the left medial labia minora were treated with low-voltage (3.0 V) electrodesiccation following local anesthesia with 1% lidocaine (red arrow), while the lesions on the right medial labia minora were left untreated (black arrow). The clinical image shows the left labia minora approximately 1 month after treatment; the papules on the right labia minora were unchanged from the prior examination.
Gone But Not Forgotten: How VA Remembers
Caring for veterans at the end of their lives is a great honor. The US Department of Veterans Affairs (VA) health care professionals (HCPs) find meaning and take pride in providing this care. We are there to support the patient and their family and loved ones around the time of death. When our patients die, we feel the loss and grieve as well. VA health care providers look to our teams to set up rituals that pay tribute to the veteran and to show respect and gratitude for our role in these moments. It is important to recognize the bonds we share and the grief we feel when a veteran dies. The relationships we form, the recognition of loss, and the honoring of the veterans help nourish and maintain us.
Although the number of VA inpatient deaths nationwide has been declining steadily for years, internal reporting by the Palliative and Hospice Care Program Office has shown that the percentage of VA inpatient deaths that occur in hospice settings has steadily grown. Since 2013, more veterans die in VA inpatient hospice beds than in any other hospital setting. Therefore, it is useful to take stock of the way hospice and palliative care providers and staff process and provide support so that they can continue to provide service to veterans.
In the same way that all loss and grief are unique, there are many diverse rituals across VA facilities. This article highlights some of the unique traditions that hospice and palliative care teams have adopted to embrace this remembrance. We hope that by sharing these practices others will be inspired to find ways to reflect on their work and honor the lives of veterans.
The authors reached out to VA palliative care colleagues across the country via the Veterans Health Administration National Hospice and Palliative Care listserve to ask: How does your team practice remembrance? Palliative care providers responded and shared the unique ways they and their teams reflect on these losses.
There are many moments for reflection from the time of death to the weeks and months after, to the entire year of cumulative loss. Some observances start around the time of death. Susan MacDonald, RN, GEC, from Erie VA Medical Center (VAMC) in Pennsylvania reported that following the death of a veteran in the hospice unit, there is a bedside remembrance that includes the chaplain, care team, family, and other loved ones. At the John D. Dingell VAMC in Detroit, Michigan, the clinical chaplain leads a memorial service after a community living center (CLC) resident dies.
Several VAMCs, such as Detroit and Erie, have an Honors Escort or Final Salute. In these ceremonies, family, employees, residents, and other veterans line the hallways to honor the veteran on their departure from the building.1 At the VA Maine Healthcare System, Kate MacFawn, nurse manager, Inpatient Hospice Unit, explained, “We debrief every death the day after it occurs. The doc[tor]s check in with the nursing staff on each shift, and the rest of the multidisciplinary team discusses [it] in our morning report.”
Palliative care providers consider the physical spaces where the veteran has spent those last moments and the void that is left. Karen Pickler, staff chaplain at Northport VAMC Hospice Unit recounts:
At the time of death, we decorate the tray table with the veteran’s picture, a flag, and an angel. In the CLC they will have a memorial service on Friday if a resident has died that week. This is for the unit and staff. In the past, other residents were not informed of the death. This way, the relationships between residents are honored as well as their natural families.
At VA Boston Healthcare System (VABHS) in Massachusetts in the Inpatient Hospice Unit-CLC, after a veteran dies, a flag, a strand of lights, and a rose in a vase are placed outside the veteran’s room to mark the absence. The VABHS remembrance practice has evolved over time based on input from the team. According to Noah Whiddon, LICSW, CLC complex case coordinator, at a weekly interdisciplinary team (IDT) meeting, the names of veterans who have died in the past week are read, and there is a commemorative ribbon cutting. “Any team member may write the last name of the deceased veteran on the ribbon and place it into a vase,” he said. “One of the nurse team members felt that a moment of silence would be appropriate, and we have added that.”
Every 6 months, VABHS holds a flag burning ceremony to appropriately dispose of worn out flags. Veterans and families are invited. The commemorative ribbons are packaged and burned at this ceremony with the following explanation of the ritual:
We’d like to take a moment to reflect on the lives of veterans we’ve lost in the last 6 months. Each week we remember the veterans for whom we have cared who have passed away. As part of this, we cut a ribbon and inscribe their name on it to commemorate their memory. We might have known these veterans for a few days or for a few years, but each of their lives had meaning for us. The courage that our veterans demonstrate at the end of their lives is an extension of the bravery they displayed in their service to our country. Today we will burn their commemorative ribbons with our country’s flag in tribute to and respect for their selflessness to our country. Please join us in a few moments of silence as their ribbons burn together with our flag.
In the VABHS acute care hospital, the palliative care IDT reserves 30 minutes, twice monthly for a chaplain-led remembrance. A large bowl-shaped shell is placed in the center of the table with smaller shells around it. Any team member can read the names of veterans who have died in prior weeks and share a memory of the patient or family, and then place a smaller shell into the larger bowl. This represents the transition from the smallest part of the universe back into the larger part. At the end, a moment of silence is observed or a poem is read. This tradition was brought to the team by the palliative care chaplain, Douglas Falls, MDiv.
Bimonthly bereavement meetings are held at the James A. Haley Veterans’ Hospital-Pasco County branch, and each veteran who has died is remembered. Whoever wants to share is welcome. “We conclude with a poem, usually shared by the physician, but it can be any team member,” explained Linda Falzetta-Gross, ARNP-BC. “This process is led by the team social worker. In the past, we used to ring a bell prior to each name.”
Bells also are used at the Greater West Los Angeles VAMC in California. At the weekly clinic, veterans who have died are remembered, and each team member has an opportunity to share memories. “We ring a Tibetan bell for a moment without words,” explains Geoffrey Tyrell, palliative care chaplain. “It is introduced with a few words to allow new staff members in our clinic to participate, as a moment of mindfulness to let go of our words and to go inside, to see what we might need for our own wellness.” Afterward the chaplain says a few words and wishes for peace for the veterans and their families. The team also has responded well to more participatory group activities, such as placing rocks in a bowl of water, to symbolize letting go of something that has been difficult.
Additionally, there are practices of a larger scope. Many VAMCs have established facility-wide memorial services annually, biannually, or quarterly. At this time, families and staff come together to remember and honor veterans who have died within the VAMC. These memorials might involve a variety of service lines, such as chaplaincy, voluntary services, nursing, and social work and may consist of an honor guard, music, and readings. In accordance with the Health Insurance Portability and Accountability Act (HIPAA) and privacy regulations, only family members of deceased veterans may speak the names at the ceremony unless written consent is given. At the Tennessee Valley Healthcare System in Nashville, family members may stand and give the name of the person they are honoring. Balloons are released, stories are told, and a poem or appropriate passage is read. Families are given a book pinned with a flag, according to Jennifer C. Crenshaw, clinical staff chaplain. Family members are moved knowing that the VA remembers their loved ones even months after they are gone.
Due to the overwhelming positive feedback from veterans’ families who participated in these ceremonies, on January 24, 2018, Carolyn Clancy, MD, VHA Executive-in-Charge, Office of the Under Secretary for Health issued a memorandum requesting that all VAMCs immediately adopt this best practice: to host periodic ceremonies to publicly recognize and honor deceased veterans in the presence of their families, VA care providers, veterans service organizations and community members. Clancy recommended calling the ceremonies “The Last Roll Call Ceremony of Remembrance.”2
These rituals are a small sample of the rich diversity of practice in VAs across the country. What unifies VA palliative care providers is our mission to serve the veterans, honor their deaths, show respect and gratitude, and recognize that we, too, feel the pain of loss. We mark these moments with solemnity and beauty—a bell, a poem, a prayer—to honor our shared experience caring for veterans.
1. Saint S. A VA hospital you may not know: The Final Salute, and how much we doctors care. https://theconversation.com/a-va-hospital-you-may-not-know-the-final-salute-and-how-much-we-doctors-care-94217. Published March 30, 2018. Accessed May 8, 2019.
2. Clancy CM. VAIQ Memorandum 7866347: Implementation of the last roll call ceremony of remembrance at all Veterans Affairs medical centers. Published 2018.
Caring for veterans at the end of their lives is a great honor. The US Department of Veterans Affairs (VA) health care professionals (HCPs) find meaning and take pride in providing this care. We are there to support the patient and their family and loved ones around the time of death. When our patients die, we feel the loss and grieve as well. VA health care providers look to our teams to set up rituals that pay tribute to the veteran and to show respect and gratitude for our role in these moments. It is important to recognize the bonds we share and the grief we feel when a veteran dies. The relationships we form, the recognition of loss, and the honoring of the veterans help nourish and maintain us.
Although the number of VA inpatient deaths nationwide has been declining steadily for years, internal reporting by the Palliative and Hospice Care Program Office has shown that the percentage of VA inpatient deaths that occur in hospice settings has steadily grown. Since 2013, more veterans die in VA inpatient hospice beds than in any other hospital setting. Therefore, it is useful to take stock of the way hospice and palliative care providers and staff process and provide support so that they can continue to provide service to veterans.
In the same way that all loss and grief are unique, there are many diverse rituals across VA facilities. This article highlights some of the unique traditions that hospice and palliative care teams have adopted to embrace this remembrance. We hope that by sharing these practices others will be inspired to find ways to reflect on their work and honor the lives of veterans.
The authors reached out to VA palliative care colleagues across the country via the Veterans Health Administration National Hospice and Palliative Care listserve to ask: How does your team practice remembrance? Palliative care providers responded and shared the unique ways they and their teams reflect on these losses.
There are many moments for reflection from the time of death to the weeks and months after, to the entire year of cumulative loss. Some observances start around the time of death. Susan MacDonald, RN, GEC, from Erie VA Medical Center (VAMC) in Pennsylvania reported that following the death of a veteran in the hospice unit, there is a bedside remembrance that includes the chaplain, care team, family, and other loved ones. At the John D. Dingell VAMC in Detroit, Michigan, the clinical chaplain leads a memorial service after a community living center (CLC) resident dies.
Several VAMCs, such as Detroit and Erie, have an Honors Escort or Final Salute. In these ceremonies, family, employees, residents, and other veterans line the hallways to honor the veteran on their departure from the building.1 At the VA Maine Healthcare System, Kate MacFawn, nurse manager, Inpatient Hospice Unit, explained, “We debrief every death the day after it occurs. The doc[tor]s check in with the nursing staff on each shift, and the rest of the multidisciplinary team discusses [it] in our morning report.”
Palliative care providers consider the physical spaces where the veteran has spent those last moments and the void that is left. Karen Pickler, staff chaplain at Northport VAMC Hospice Unit recounts:
At the time of death, we decorate the tray table with the veteran’s picture, a flag, and an angel. In the CLC they will have a memorial service on Friday if a resident has died that week. This is for the unit and staff. In the past, other residents were not informed of the death. This way, the relationships between residents are honored as well as their natural families.
At VA Boston Healthcare System (VABHS) in Massachusetts in the Inpatient Hospice Unit-CLC, after a veteran dies, a flag, a strand of lights, and a rose in a vase are placed outside the veteran’s room to mark the absence. The VABHS remembrance practice has evolved over time based on input from the team. According to Noah Whiddon, LICSW, CLC complex case coordinator, at a weekly interdisciplinary team (IDT) meeting, the names of veterans who have died in the past week are read, and there is a commemorative ribbon cutting. “Any team member may write the last name of the deceased veteran on the ribbon and place it into a vase,” he said. “One of the nurse team members felt that a moment of silence would be appropriate, and we have added that.”
Every 6 months, VABHS holds a flag burning ceremony to appropriately dispose of worn out flags. Veterans and families are invited. The commemorative ribbons are packaged and burned at this ceremony with the following explanation of the ritual:
We’d like to take a moment to reflect on the lives of veterans we’ve lost in the last 6 months. Each week we remember the veterans for whom we have cared who have passed away. As part of this, we cut a ribbon and inscribe their name on it to commemorate their memory. We might have known these veterans for a few days or for a few years, but each of their lives had meaning for us. The courage that our veterans demonstrate at the end of their lives is an extension of the bravery they displayed in their service to our country. Today we will burn their commemorative ribbons with our country’s flag in tribute to and respect for their selflessness to our country. Please join us in a few moments of silence as their ribbons burn together with our flag.
In the VABHS acute care hospital, the palliative care IDT reserves 30 minutes, twice monthly for a chaplain-led remembrance. A large bowl-shaped shell is placed in the center of the table with smaller shells around it. Any team member can read the names of veterans who have died in prior weeks and share a memory of the patient or family, and then place a smaller shell into the larger bowl. This represents the transition from the smallest part of the universe back into the larger part. At the end, a moment of silence is observed or a poem is read. This tradition was brought to the team by the palliative care chaplain, Douglas Falls, MDiv.
Bimonthly bereavement meetings are held at the James A. Haley Veterans’ Hospital-Pasco County branch, and each veteran who has died is remembered. Whoever wants to share is welcome. “We conclude with a poem, usually shared by the physician, but it can be any team member,” explained Linda Falzetta-Gross, ARNP-BC. “This process is led by the team social worker. In the past, we used to ring a bell prior to each name.”
Bells also are used at the Greater West Los Angeles VAMC in California. At the weekly clinic, veterans who have died are remembered, and each team member has an opportunity to share memories. “We ring a Tibetan bell for a moment without words,” explains Geoffrey Tyrell, palliative care chaplain. “It is introduced with a few words to allow new staff members in our clinic to participate, as a moment of mindfulness to let go of our words and to go inside, to see what we might need for our own wellness.” Afterward the chaplain says a few words and wishes for peace for the veterans and their families. The team also has responded well to more participatory group activities, such as placing rocks in a bowl of water, to symbolize letting go of something that has been difficult.
Additionally, there are practices of a larger scope. Many VAMCs have established facility-wide memorial services annually, biannually, or quarterly. At this time, families and staff come together to remember and honor veterans who have died within the VAMC. These memorials might involve a variety of service lines, such as chaplaincy, voluntary services, nursing, and social work and may consist of an honor guard, music, and readings. In accordance with the Health Insurance Portability and Accountability Act (HIPAA) and privacy regulations, only family members of deceased veterans may speak the names at the ceremony unless written consent is given. At the Tennessee Valley Healthcare System in Nashville, family members may stand and give the name of the person they are honoring. Balloons are released, stories are told, and a poem or appropriate passage is read. Families are given a book pinned with a flag, according to Jennifer C. Crenshaw, clinical staff chaplain. Family members are moved knowing that the VA remembers their loved ones even months after they are gone.
Due to the overwhelming positive feedback from veterans’ families who participated in these ceremonies, on January 24, 2018, Carolyn Clancy, MD, VHA Executive-in-Charge, Office of the Under Secretary for Health issued a memorandum requesting that all VAMCs immediately adopt this best practice: to host periodic ceremonies to publicly recognize and honor deceased veterans in the presence of their families, VA care providers, veterans service organizations and community members. Clancy recommended calling the ceremonies “The Last Roll Call Ceremony of Remembrance.”2
These rituals are a small sample of the rich diversity of practice in VAs across the country. What unifies VA palliative care providers is our mission to serve the veterans, honor their deaths, show respect and gratitude, and recognize that we, too, feel the pain of loss. We mark these moments with solemnity and beauty—a bell, a poem, a prayer—to honor our shared experience caring for veterans.
Caring for veterans at the end of their lives is a great honor. The US Department of Veterans Affairs (VA) health care professionals (HCPs) find meaning and take pride in providing this care. We are there to support the patient and their family and loved ones around the time of death. When our patients die, we feel the loss and grieve as well. VA health care providers look to our teams to set up rituals that pay tribute to the veteran and to show respect and gratitude for our role in these moments. It is important to recognize the bonds we share and the grief we feel when a veteran dies. The relationships we form, the recognition of loss, and the honoring of the veterans help nourish and maintain us.
Although the number of VA inpatient deaths nationwide has been declining steadily for years, internal reporting by the Palliative and Hospice Care Program Office has shown that the percentage of VA inpatient deaths that occur in hospice settings has steadily grown. Since 2013, more veterans die in VA inpatient hospice beds than in any other hospital setting. Therefore, it is useful to take stock of the way hospice and palliative care providers and staff process and provide support so that they can continue to provide service to veterans.
In the same way that all loss and grief are unique, there are many diverse rituals across VA facilities. This article highlights some of the unique traditions that hospice and palliative care teams have adopted to embrace this remembrance. We hope that by sharing these practices others will be inspired to find ways to reflect on their work and honor the lives of veterans.
The authors reached out to VA palliative care colleagues across the country via the Veterans Health Administration National Hospice and Palliative Care listserve to ask: How does your team practice remembrance? Palliative care providers responded and shared the unique ways they and their teams reflect on these losses.
There are many moments for reflection from the time of death to the weeks and months after, to the entire year of cumulative loss. Some observances start around the time of death. Susan MacDonald, RN, GEC, from Erie VA Medical Center (VAMC) in Pennsylvania reported that following the death of a veteran in the hospice unit, there is a bedside remembrance that includes the chaplain, care team, family, and other loved ones. At the John D. Dingell VAMC in Detroit, Michigan, the clinical chaplain leads a memorial service after a community living center (CLC) resident dies.
Several VAMCs, such as Detroit and Erie, have an Honors Escort or Final Salute. In these ceremonies, family, employees, residents, and other veterans line the hallways to honor the veteran on their departure from the building.1 At the VA Maine Healthcare System, Kate MacFawn, nurse manager, Inpatient Hospice Unit, explained, “We debrief every death the day after it occurs. The doc[tor]s check in with the nursing staff on each shift, and the rest of the multidisciplinary team discusses [it] in our morning report.”
Palliative care providers consider the physical spaces where the veteran has spent those last moments and the void that is left. Karen Pickler, staff chaplain at Northport VAMC Hospice Unit recounts:
At the time of death, we decorate the tray table with the veteran’s picture, a flag, and an angel. In the CLC they will have a memorial service on Friday if a resident has died that week. This is for the unit and staff. In the past, other residents were not informed of the death. This way, the relationships between residents are honored as well as their natural families.
At VA Boston Healthcare System (VABHS) in Massachusetts in the Inpatient Hospice Unit-CLC, after a veteran dies, a flag, a strand of lights, and a rose in a vase are placed outside the veteran’s room to mark the absence. The VABHS remembrance practice has evolved over time based on input from the team. According to Noah Whiddon, LICSW, CLC complex case coordinator, at a weekly interdisciplinary team (IDT) meeting, the names of veterans who have died in the past week are read, and there is a commemorative ribbon cutting. “Any team member may write the last name of the deceased veteran on the ribbon and place it into a vase,” he said. “One of the nurse team members felt that a moment of silence would be appropriate, and we have added that.”
Every 6 months, VABHS holds a flag burning ceremony to appropriately dispose of worn out flags. Veterans and families are invited. The commemorative ribbons are packaged and burned at this ceremony with the following explanation of the ritual:
We’d like to take a moment to reflect on the lives of veterans we’ve lost in the last 6 months. Each week we remember the veterans for whom we have cared who have passed away. As part of this, we cut a ribbon and inscribe their name on it to commemorate their memory. We might have known these veterans for a few days or for a few years, but each of their lives had meaning for us. The courage that our veterans demonstrate at the end of their lives is an extension of the bravery they displayed in their service to our country. Today we will burn their commemorative ribbons with our country’s flag in tribute to and respect for their selflessness to our country. Please join us in a few moments of silence as their ribbons burn together with our flag.
In the VABHS acute care hospital, the palliative care IDT reserves 30 minutes, twice monthly for a chaplain-led remembrance. A large bowl-shaped shell is placed in the center of the table with smaller shells around it. Any team member can read the names of veterans who have died in prior weeks and share a memory of the patient or family, and then place a smaller shell into the larger bowl. This represents the transition from the smallest part of the universe back into the larger part. At the end, a moment of silence is observed or a poem is read. This tradition was brought to the team by the palliative care chaplain, Douglas Falls, MDiv.
Bimonthly bereavement meetings are held at the James A. Haley Veterans’ Hospital-Pasco County branch, and each veteran who has died is remembered. Whoever wants to share is welcome. “We conclude with a poem, usually shared by the physician, but it can be any team member,” explained Linda Falzetta-Gross, ARNP-BC. “This process is led by the team social worker. In the past, we used to ring a bell prior to each name.”
Bells also are used at the Greater West Los Angeles VAMC in California. At the weekly clinic, veterans who have died are remembered, and each team member has an opportunity to share memories. “We ring a Tibetan bell for a moment without words,” explains Geoffrey Tyrell, palliative care chaplain. “It is introduced with a few words to allow new staff members in our clinic to participate, as a moment of mindfulness to let go of our words and to go inside, to see what we might need for our own wellness.” Afterward the chaplain says a few words and wishes for peace for the veterans and their families. The team also has responded well to more participatory group activities, such as placing rocks in a bowl of water, to symbolize letting go of something that has been difficult.
Additionally, there are practices of a larger scope. Many VAMCs have established facility-wide memorial services annually, biannually, or quarterly. At this time, families and staff come together to remember and honor veterans who have died within the VAMC. These memorials might involve a variety of service lines, such as chaplaincy, voluntary services, nursing, and social work and may consist of an honor guard, music, and readings. In accordance with the Health Insurance Portability and Accountability Act (HIPAA) and privacy regulations, only family members of deceased veterans may speak the names at the ceremony unless written consent is given. At the Tennessee Valley Healthcare System in Nashville, family members may stand and give the name of the person they are honoring. Balloons are released, stories are told, and a poem or appropriate passage is read. Families are given a book pinned with a flag, according to Jennifer C. Crenshaw, clinical staff chaplain. Family members are moved knowing that the VA remembers their loved ones even months after they are gone.
Due to the overwhelming positive feedback from veterans’ families who participated in these ceremonies, on January 24, 2018, Carolyn Clancy, MD, VHA Executive-in-Charge, Office of the Under Secretary for Health issued a memorandum requesting that all VAMCs immediately adopt this best practice: to host periodic ceremonies to publicly recognize and honor deceased veterans in the presence of their families, VA care providers, veterans service organizations and community members. Clancy recommended calling the ceremonies “The Last Roll Call Ceremony of Remembrance.”2
These rituals are a small sample of the rich diversity of practice in VAs across the country. What unifies VA palliative care providers is our mission to serve the veterans, honor their deaths, show respect and gratitude, and recognize that we, too, feel the pain of loss. We mark these moments with solemnity and beauty—a bell, a poem, a prayer—to honor our shared experience caring for veterans.
1. Saint S. A VA hospital you may not know: The Final Salute, and how much we doctors care. https://theconversation.com/a-va-hospital-you-may-not-know-the-final-salute-and-how-much-we-doctors-care-94217. Published March 30, 2018. Accessed May 8, 2019.
2. Clancy CM. VAIQ Memorandum 7866347: Implementation of the last roll call ceremony of remembrance at all Veterans Affairs medical centers. Published 2018.
1. Saint S. A VA hospital you may not know: The Final Salute, and how much we doctors care. https://theconversation.com/a-va-hospital-you-may-not-know-the-final-salute-and-how-much-we-doctors-care-94217. Published March 30, 2018. Accessed May 8, 2019.
2. Clancy CM. VAIQ Memorandum 7866347: Implementation of the last roll call ceremony of remembrance at all Veterans Affairs medical centers. Published 2018.
GI practice consolidation continues
Digestive Disease Week® (DDW) 2019 is now history. This was the 50th anniversary of DDW and again, it lived up to its reputation as the world’s foremost meeting dedicated to digestive diseases. GI & Hepatology News will publish multiple articles highlighting the best of DDW in the coming months.
The AGA Presidential Plenary session is an annual DDW highlight. This year’s session did not disappoint and was attended by a large crowd. David Lieberman, MD, AGAF (outgoing AGA president) and Hashem B. El-Serag MD, MPH, AGAF (incoming AGA president) moderated the session. Outstanding presentations about management of obesity, new findings in IBD, the use of virtual reality in the treatment of functional abdominal pain, and findings from a long-term colorectal cancer screening trial were some of the key presentations.
Recent behind-the-scenes work by the AGA is paying off for its members and the larger GI community. The AGA was again awarded an NIH-funded grant to advance its education and training of under-represented minorities. This is the second NIH grant given to the AGA, who now has become a leader in diversity and inclusive education. The AGA has strengthened its close bond with the Crohn's and Colitis Foundation, adding to its portfolio of scientific and clinical offerings focused on IBD. The AGA Center for Gut Microbiome Research and Education has emerged as one of the best sources of education and research about the microbiome’s impact on digestive health.
On the business front, there are tectonic changes occurring. In 2018, three large GI practices were sold to private equity companies and each has completed multiple arbitrage plays (acquisition of smaller practices), growing to over 200 physicians. This year we will see 6-10 additional private equity acquisitions and will likely see one or more GI practices of 500-1000 providers. This consolidation will have profound implications for the practice of gastroenterology and will provide some interesting opportunities to conduct population-based research for physicians who can capture that potential through academic-community partnerships.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Digestive Disease Week® (DDW) 2019 is now history. This was the 50th anniversary of DDW and again, it lived up to its reputation as the world’s foremost meeting dedicated to digestive diseases. GI & Hepatology News will publish multiple articles highlighting the best of DDW in the coming months.
The AGA Presidential Plenary session is an annual DDW highlight. This year’s session did not disappoint and was attended by a large crowd. David Lieberman, MD, AGAF (outgoing AGA president) and Hashem B. El-Serag MD, MPH, AGAF (incoming AGA president) moderated the session. Outstanding presentations about management of obesity, new findings in IBD, the use of virtual reality in the treatment of functional abdominal pain, and findings from a long-term colorectal cancer screening trial were some of the key presentations.
Recent behind-the-scenes work by the AGA is paying off for its members and the larger GI community. The AGA was again awarded an NIH-funded grant to advance its education and training of under-represented minorities. This is the second NIH grant given to the AGA, who now has become a leader in diversity and inclusive education. The AGA has strengthened its close bond with the Crohn's and Colitis Foundation, adding to its portfolio of scientific and clinical offerings focused on IBD. The AGA Center for Gut Microbiome Research and Education has emerged as one of the best sources of education and research about the microbiome’s impact on digestive health.
On the business front, there are tectonic changes occurring. In 2018, three large GI practices were sold to private equity companies and each has completed multiple arbitrage plays (acquisition of smaller practices), growing to over 200 physicians. This year we will see 6-10 additional private equity acquisitions and will likely see one or more GI practices of 500-1000 providers. This consolidation will have profound implications for the practice of gastroenterology and will provide some interesting opportunities to conduct population-based research for physicians who can capture that potential through academic-community partnerships.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Digestive Disease Week® (DDW) 2019 is now history. This was the 50th anniversary of DDW and again, it lived up to its reputation as the world’s foremost meeting dedicated to digestive diseases. GI & Hepatology News will publish multiple articles highlighting the best of DDW in the coming months.
The AGA Presidential Plenary session is an annual DDW highlight. This year’s session did not disappoint and was attended by a large crowd. David Lieberman, MD, AGAF (outgoing AGA president) and Hashem B. El-Serag MD, MPH, AGAF (incoming AGA president) moderated the session. Outstanding presentations about management of obesity, new findings in IBD, the use of virtual reality in the treatment of functional abdominal pain, and findings from a long-term colorectal cancer screening trial were some of the key presentations.
Recent behind-the-scenes work by the AGA is paying off for its members and the larger GI community. The AGA was again awarded an NIH-funded grant to advance its education and training of under-represented minorities. This is the second NIH grant given to the AGA, who now has become a leader in diversity and inclusive education. The AGA has strengthened its close bond with the Crohn's and Colitis Foundation, adding to its portfolio of scientific and clinical offerings focused on IBD. The AGA Center for Gut Microbiome Research and Education has emerged as one of the best sources of education and research about the microbiome’s impact on digestive health.
On the business front, there are tectonic changes occurring. In 2018, three large GI practices were sold to private equity companies and each has completed multiple arbitrage plays (acquisition of smaller practices), growing to over 200 physicians. This year we will see 6-10 additional private equity acquisitions and will likely see one or more GI practices of 500-1000 providers. This consolidation will have profound implications for the practice of gastroenterology and will provide some interesting opportunities to conduct population-based research for physicians who can capture that potential through academic-community partnerships.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Steroids May Not Benefit Patients With Mild Asthma
The gold-standard treatment is no more effective than placebo for patients with mild persistent asthma, say researchers from the Steroids in Eosinophil Negative Asthma (SIENA) study, funded by the National Heart, Lung, and Blood Institute.
The researchers divided 295 participants into groups based on low- or high-sputum eosinophil levels and assigned them randomly to each of 3 treatment groups for 12-week periods: inhaled steroids (mometasone), a long-acting muscarinic antagonist (LAMA) (tiotropium), or placebo.
Surprisingly, 221 participants—nearly 73%—were classified as having low-sputum eosinophils (< 2%), a much higher frequency than the researchers expected. And of those, the number who responded better to steroids was no different from the number responding to placebo. Of the Eos-low group, 60% had better symptom control with LAMA; 40% had better symptom control with placebo.
By contrast, patients classified as “Eos-high” were nearly 3 times as likely to respond to inhaled steroids compared with placebo.
Other research has indicated that about half the population with mild persistent asthma have < 2% sputum eosinophils and are not likely to respond well to steroids. But laboratory tests to measure sputum eosinophils are not routinely used in most clinics, the researchers say.
The difference between the groups is not large enough to conclude that patients are more likely to do better on LAMA drugs, the researchers say, but their study highlights the need to look for alternatives to inhaled steroids for patients with mild asthma.
The research underscores the value of customizing treatments to help people with asthma, said James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “This study adds to a growing body of evidence that different patients with mild asthma should be treated differently, perhaps using biomarkers like sputum eosinophils to select which drugs should be used—a precision medicine approach.”
The gold-standard treatment is no more effective than placebo for patients with mild persistent asthma, say researchers from the Steroids in Eosinophil Negative Asthma (SIENA) study, funded by the National Heart, Lung, and Blood Institute.
The researchers divided 295 participants into groups based on low- or high-sputum eosinophil levels and assigned them randomly to each of 3 treatment groups for 12-week periods: inhaled steroids (mometasone), a long-acting muscarinic antagonist (LAMA) (tiotropium), or placebo.
Surprisingly, 221 participants—nearly 73%—were classified as having low-sputum eosinophils (< 2%), a much higher frequency than the researchers expected. And of those, the number who responded better to steroids was no different from the number responding to placebo. Of the Eos-low group, 60% had better symptom control with LAMA; 40% had better symptom control with placebo.
By contrast, patients classified as “Eos-high” were nearly 3 times as likely to respond to inhaled steroids compared with placebo.
Other research has indicated that about half the population with mild persistent asthma have < 2% sputum eosinophils and are not likely to respond well to steroids. But laboratory tests to measure sputum eosinophils are not routinely used in most clinics, the researchers say.
The difference between the groups is not large enough to conclude that patients are more likely to do better on LAMA drugs, the researchers say, but their study highlights the need to look for alternatives to inhaled steroids for patients with mild asthma.
The research underscores the value of customizing treatments to help people with asthma, said James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “This study adds to a growing body of evidence that different patients with mild asthma should be treated differently, perhaps using biomarkers like sputum eosinophils to select which drugs should be used—a precision medicine approach.”
The gold-standard treatment is no more effective than placebo for patients with mild persistent asthma, say researchers from the Steroids in Eosinophil Negative Asthma (SIENA) study, funded by the National Heart, Lung, and Blood Institute.
The researchers divided 295 participants into groups based on low- or high-sputum eosinophil levels and assigned them randomly to each of 3 treatment groups for 12-week periods: inhaled steroids (mometasone), a long-acting muscarinic antagonist (LAMA) (tiotropium), or placebo.
Surprisingly, 221 participants—nearly 73%—were classified as having low-sputum eosinophils (< 2%), a much higher frequency than the researchers expected. And of those, the number who responded better to steroids was no different from the number responding to placebo. Of the Eos-low group, 60% had better symptom control with LAMA; 40% had better symptom control with placebo.
By contrast, patients classified as “Eos-high” were nearly 3 times as likely to respond to inhaled steroids compared with placebo.
Other research has indicated that about half the population with mild persistent asthma have < 2% sputum eosinophils and are not likely to respond well to steroids. But laboratory tests to measure sputum eosinophils are not routinely used in most clinics, the researchers say.
The difference between the groups is not large enough to conclude that patients are more likely to do better on LAMA drugs, the researchers say, but their study highlights the need to look for alternatives to inhaled steroids for patients with mild asthma.
The research underscores the value of customizing treatments to help people with asthma, said James Kiley, PhD, director of the Division of Lung Diseases at NHLBI. “This study adds to a growing body of evidence that different patients with mild asthma should be treated differently, perhaps using biomarkers like sputum eosinophils to select which drugs should be used—a precision medicine approach.”
SPIRITT: What does ‘spirituality’ mean?
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
When your patient is a physician: Overcoming the challenges
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
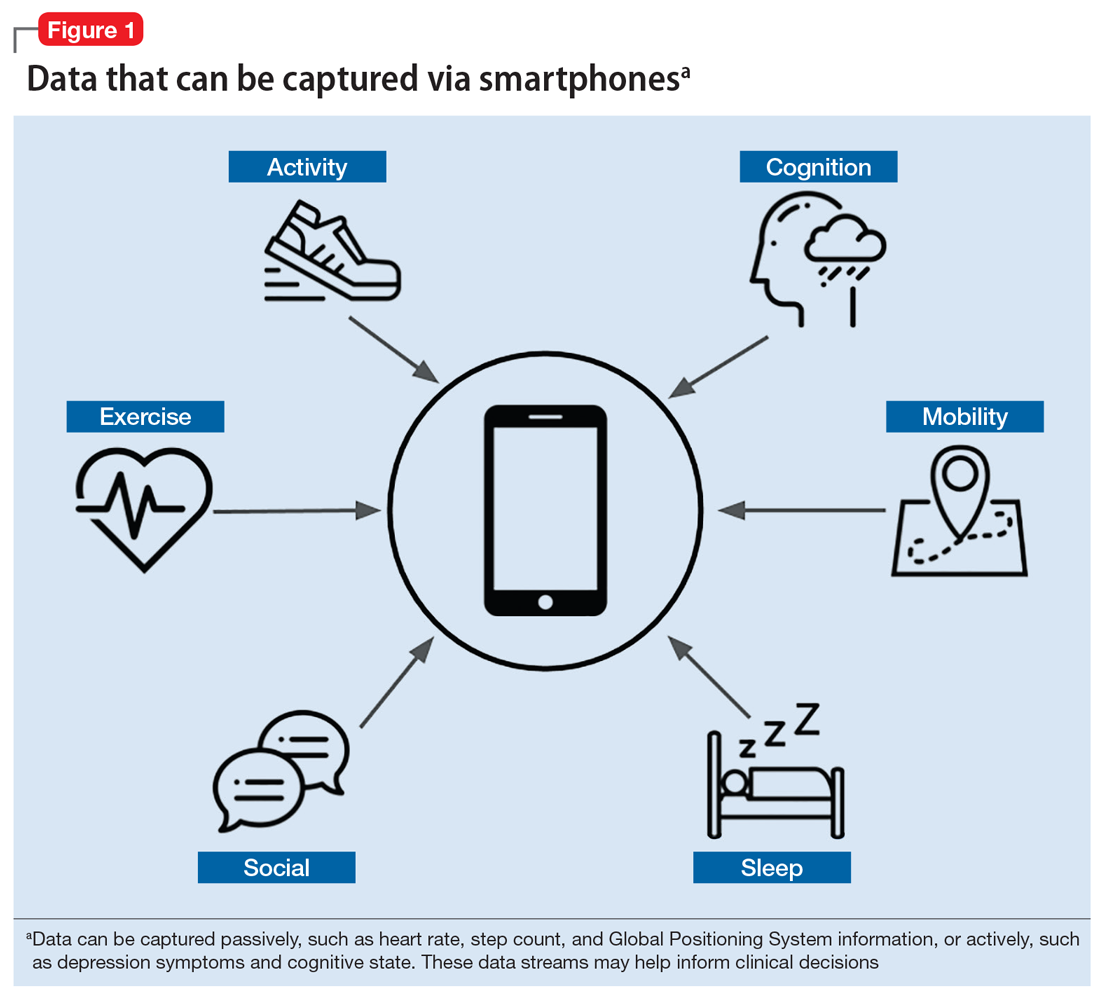
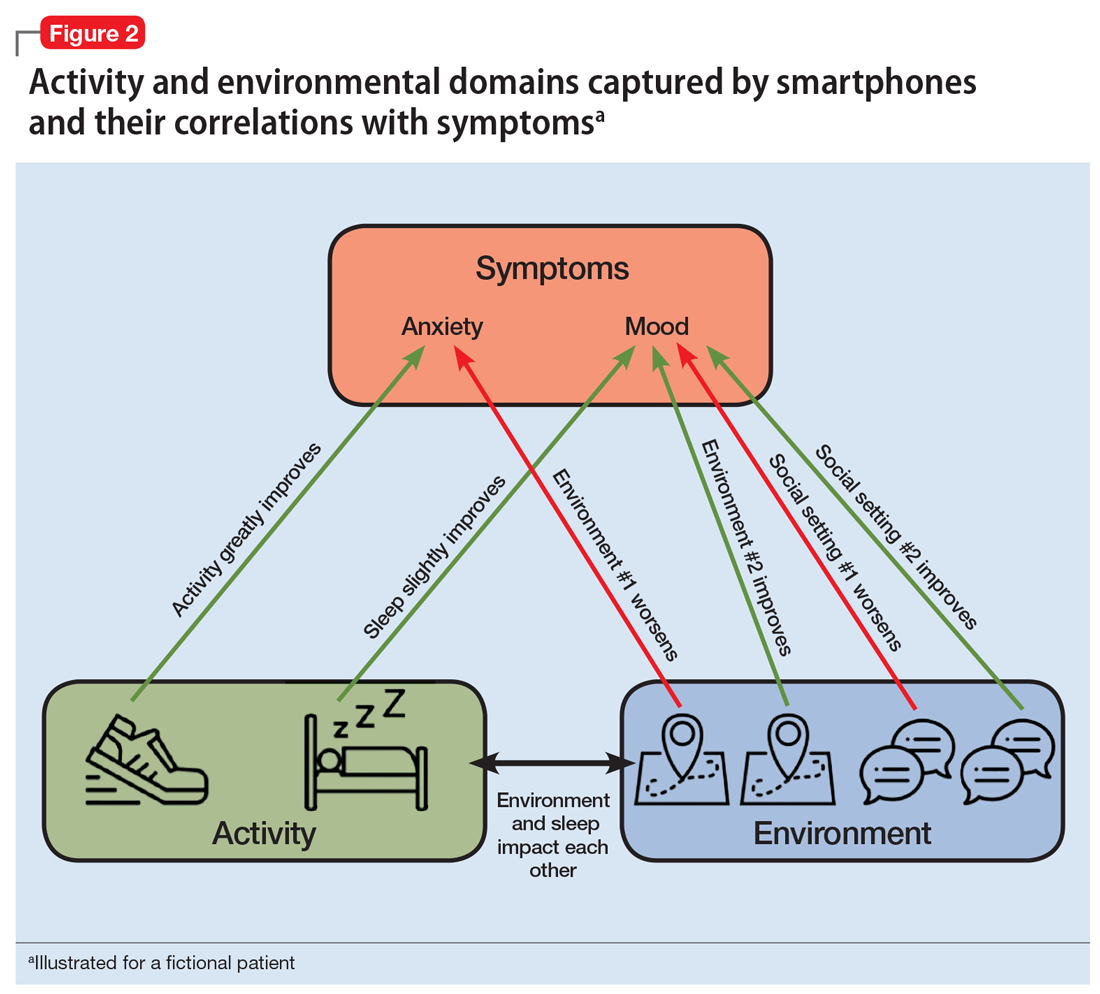
Mobile apps and mental health: Using technology to quantify real-time clinical risk
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
In today’s global society, smartphones are ubiquitous, used by >2.5 billion people.1 They provide limitless availability of on-demand services and resources, unparalleled computing power by size, and the ability to connect with anyone in the world.
Digital applications and new mobile technologies can be used to change the nature of the psychiatrist–patient relationship. The future of clinical practice is changing with the help of smartphones and apps. Diagnosis, follow-up, and treatment will never look the same as we come to better understand and apply emerging technologies.2
Both Android and iOS—the 2 largest mobile operating systems by market share3—provide outlets for the dissemination of mobile applications. There are currently >10,000 mental health–related apps available for download.4 One particular use case of mental health–related apps is digital phenotyping.
In this article, we aim to:
- define digital phenotyping
- explore the potential advances in patient care afforded by emerging technology
- discuss the ethical dilemmas and future of mental health apps.
The possibilities of digital phenotyping
Digital phenotyping is capturing a patient’s real-time clinical state using digital technology to better understand the patient’s state outside of the clinic. While digital phenotyping may seem new, the concepts behind it are grounded in good clinical care.
For example, it is important to assess sleep and physical activity for nearly all patients, regardless of diagnosis. However, the patient’s retrospective recollection of sleep, mood, and other clinically relevant metrics is often unreliable, especially when visits are months apart. With smartphones, it is possible to automatically collect metrics for sleep, activity, mood, and much more in real time from the convenience of our patients’ personal devices (Figure 1).
Smartphones can capture a seemingly endless number of data streams, from patient-interfacing active data, such as journal entries, messaging, and games, to data that is captured passively, such as screen time, Global Positioning System information, and step count. Clinicians can work with patients to customize which digital phenotyping data they would like to capture. In one study, researchers worked with 17 patients with schizophrenia by capturing self-reported surveys, anonymized phone call logs, and location data to see if they could predict relapse by observing variations in how patients interact with their smartphones.5 They observed that the rate of behavioral anomalies was 71% higher in the 2 weeks prior to relapse than during other periods. The data captured by the smartphone will depend on the patient and the clinical needs. Some clinicians may only want to collect data on step count and screen time to learn if a patient is overusing his or her smartphone, which might be related to becoming less physically active.
Continue to: One novel data stream...
One novel data stream offered by smartphone digital phenotyping is cognition. While we know that impaired cognition is a core symptom of schizophrenia, and that cognition is affected by depression and anxiety, cognitive symptoms are clinically challenging to quantify. Thus, the cognitive burden of mental illness and the cognitive effects of treatment are often overlooked. However, smartphones are beginning to offer a novel means of capturing a patient’s cognitive state through the use of common clinical tests. For example, the Trail Making Test measures visual attention and executive function by having participants connect dots that differ in number, color, or shape in an ascending pattern.6 By having patients perform this test on a smartphone, clinicians can utilize the touchscreen to capture the user’s discrete actions, such as time to completion and misclicks. These data can be used to build novel measures of cognitive performance that can account for learning bias and other confounding variables.7 While these digital cognitive biomarkers are still in active research, it is likely that they will quickly be developed for broad clinical use.
In addition to the novel data offered by digital phenotyping, another benefit is the low cost and ease of use. Unlike wearable devices such as smartwatches, which can also offer data on steps and sleep, smartphone-based digital phenotyping does not require patients to purchase or use additional devices. Running on patients’ smartphones, digital phenotyping offers the ability to capture rich and continuous health data without added effort or cost. Given that the average person interacts with their phone more than 2,600 times per day,8 smartphones are well suited for capturing large amounts of information that may provide insights into patients’ mental health.
For illnesses such as depression and anxiety, the clinical relevance of digital phenotyping is in the ability to capture symptoms as they occur in context. Figure 2 provides a simplified example of how we can learn that for this fictitious patient, exercise greatly improves anxiety, whereas being in a certain environment worsens it. Other insights about sleep and social settings could also provide further information about the context of the patient’s symptoms. While these correlations alone will not lead to better clinical outcomes, it is easy to imagine how such data could help a patient and clinician start a conversation about making impactful changes.
Continue to: Case report...
Case report: Digital phenotyping
To illustrate how digital phenotyping could be put to clinical use, we created the following case report of a fictional patient who agrees to be monitored via her smartphone.
Consider a hypothetical patient we will call Ms. T who is in her mid-20s and has been diagnosed with schizophrenia. On a follow-up visit, she says she has insomnia. She also reports having a recent loss of appetite and higher levels of anxiety. After reviewing her smartphone data (Figure 3), the clinician sees an inversely proportional relationship between her sleep quality and symptoms of anxiety, psychosis, and depression, which suggests that these symptoms might be due to poor sleep. Her step count has been fairly stable, indicating that there is no significant correlation between physical activity and her other symptoms.
Continue to: The clinician shows...
The clinician shows Ms. T the data to help her understand why a trial of cognitive-behavioral therapy for insomnia, or at least improving sleep hygiene, may offer several benefits. The clinician advises her to continue to use the app to help assess her response to these interventions and monitor her progress in real time.
Dilemma: The ethics of continuous observation
The rich data captured by digital phenotyping afford many clinical opportunities, but also raise concerns. Among these are 3 significant ethical implications.
Firstly, the same data that may help a clinician learn about what environments are associated with less anxiety for the patient may also reveal personal details about where that patient has been or with whom they have interacted. In the wrong hands, such personal data could cause harm. And even in the hands of a trusted clinician, a breach in the patient’s privacy begs the question: “Should such information be anyone’s business at all?”
Secondly, many apps that offer digital phenotyping could also store patient data—something that currently pervades social media and causes reasonable discomfort for many people. You might have personally encountered this with social media platforms such as Facebook. When it comes to mobile mental health apps, clinicians should carefully understand the data usage agreement of any digital phenotyping app they wish to use and then share this information with their patients.
Finally, while it is possible to collect the types of data outlined in this article, less is known about how to use it directly in clinical care. Understanding for each patient which data streams are most meaningful and which data streams are noise that should be ignored is an area of ongoing research. A good first step may be to begin with data streams that are known to be clinically relevant and valuable, such as sleep and physical activity.9-11
Continue to: Discussion...
Discussion: Genomic sequencing and digital phenotyping
Although smartphones can gather a wide range of active and passive data, other data streams hold potential for predicting relapse and performing other clinically relevant actions. One data stream that could be of clinical use is genomic sequencing.12 The genotyping of patients provides a wealth of information about the underlying biology, and genomic sequencing has never been cheaper.13
Combining the data gathered via digital phenotyping with that of genotyping could help elucidate the mechanisms by which specific diseases and symptoms occur. This could be very promising to better understand and treat our patients. However, as is the case with genomics, digital phenotyping has important ethical implications. If used in the proper way to benefit our patients, the future for this new method is bright.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
1. Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions). https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/. Accessed April 29, 2019.
2. Thibaut F. Digital applications: the future in psychiatry? Dialogues Clin Neurosci. 2016;18(2):123.
3. Statista. Global market share held by the leading smartphone operating systems in sales to end users from 1st quarter 2009 to 2nd quarter 2018. https://www.statista.com/statistics/266136/global-market-share-held-by-smartphone-operating-systems/. Accessed April 19, 2019.
4. Torous J, Roberts L. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
5. Barnett I, Torous J, Staples P, et al. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology. 2018;43(8):1660-1666.
6. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220-221.
7. Brouillette RM, Foil H, Fontenot S, et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PloS One. 2013;8(6):e65925. doi: 10.1371/journal.pone.0065925.
8. Winnick W. Putting a finger on our phone obsession. dscout. https://blog.dscout.com/mobile-touches. Published June 16, 2016. Accessed April 29, 2019.
9. Waite F, Myers E, Harvey AG, et al. Treating sleep problems in patients with schizophrenia. Behav Cogn Psychother. 2016;44(3):273-287.
10. Mcgurk SR, Mueser KT, Xie H, et al. (2015). Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852-861.
11. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546-556.
12. Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258-267.
13. National Human Genome Research Institute. The cost of sequencing a human genome. https://www.genome.gov/27565109/the-cost-of-sequencing-a-human-genome/. Updated July 6, 2016. Accessed April 29, 2019.
It’s time to implement measurement-based care in psychiatric practice
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at aborayascip@gmail.com or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at aborayascip@gmail.com or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
In an editorial published in Current Psychiatry 10 years ago, I cited a stunning fact based on a readers’ survey: 98% of psychiatrists did not use any of the 4 clinical rating scales that are routinely used in the clinical trials required for FDA approval of medications for psychotic, mood, and anxiety disorders.1
As a follow-up, Ahmed Aboraya, MD, DrPH, and I would like to report on the state of measurement-based care (MBC), a term coined by Trivedi in 2006 and defined by Fortney as “the systematic administration of symptom rating scales and use of the results to drive clinical decision making at the level of the individual patient.”2
We will start with the creator of modern rating scales, Father Thomas Verner Moore (1877-1969), who is considered one of the most underrecognized legends in the history of modern psychiatry. Moore was a psychologist and psychiatrist who can lay claim to 3 major achievements in psychiatry: the creation of rating scales in psychiatry, the use of factor analysis to deconstruct psychosis, and the formulation of specific definitions for symptoms and signs of psychopathology. Moore’s 1933 book described the rating scales used in his research.3
Since that time, researchers have continued to invent clinician-rated scales, self-report scales, and other measures in psychiatry. The Handbook of Psychiatric Measures, which was published in 2000 by the American Psychiatric Association Task Force chaired by AJ Rush Jr., includes >240 measures covering adult and child psychiatric disorders.4
Recent research has shown the superiority of MBC compared with usual standard care (USC) in improving patient outcomes.2,5-7 A recent well-designed, blind-rater, randomized trial by Guo et al8 showed that MBC is more effective than USC both in achieving response and remission, and reducing the time to response and remission. Given the evidence of the benefits of MBC in improving patient outcomes, and the plethora of reliable and validated rating scales, an important question arises: Why has MBC not yet been established as the standard of care in psychiatric clinical practice? There are many barriers to implementing MBC,9 including:
- time constraints (most commonly cited reason by psychiatrists)
- mismatch between clinical needs and the content of the measure (ie, rating scales are designed for research and not for clinicians’ use)
- measurements produced by rating scales may not always be clinically relevant
- administering rating scales may interfere with establishing rapport with patients
- some measures, such as standardized diagnostic interviews, can be cumbersome, unwieldy, and complicated
- the lack of formal training for most clinicians (among the top barriers for residents and faculty)
- lack of availability of training manuals and protocols.
Clinician researchers have started to adapt and invent instruments that can be used in clinical settings. For more than 20 years, Mark Zimmerman, MD, has been the principal investigator of the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project, aimed at integrating the assessment methods of researchers into routine clinical practice.10 Zimmerman has developed self-report scales and outcome measures such as the Psychiatric Diagnostic Screening Questionnaire (PDSQ), the Clinically Useful Depression Outcome Scale (CUDOS), the Standardized Clinical Outcome Rating for Depression (SCOR-D), the Clinically Useful Anxiety Outcome Scale (CUXOS), the Remission from Depression Questionnaire (RDQ), and the Clinically Useful Patient Satisfaction Scale (CUPSS).11-18
We have been critical of the utility of the existing diagnostic interviews and rating scales. I (AA) developed the Standard for Clinicians’ Interview in Psychiatry (SCIP) as a MBC tool that addresses the most common barriers that clinicians face.9,19-23 The SCIP includes 18 clinician-rated scales for the following symptom domains: generalized anxiety, obsessions, compulsions, posttraumatic stress, depression, mania, delusions, hallucinations, disorganized thoughts, aggression, negative symptoms, alcohol use, drug use, attention deficit, hyperactivity, anorexia, binge-eating, and bulimia. The SCIP rating scales meet the criteria for MBC because they are efficient, reliable, and valid. They reflect how clinicians assess psychiatric disorders, and are relevant to decision-making. Both self-report and clinician-rated scales are important MBC tools and complementary to each other. The choice to use self-report scales, clinician-rated scales, or both depends on several factors, including the clinical setting (inpatient or outpatient), psychiatric diagnoses, and patient characteristics. No measure or scale will ever replace a seasoned and experienced clinician who has been evaluating and treating real-world patients for years. Just as thermometers, stethoscopes, and laboratories help other types of physicians to reach accurate diagnoses and provide appropriate management, the use of MBC by psychiatrists will enhance the accuracy of diagnoses and improve the outcomes of care.
Continue to: On a positive note...
On a positive note, I (AA) have completed a MBC curriculum for training psychiatry residents that includes 11 videotaped interviews with actual patients covering the major adult psychiatric disorders: generalized anxiety, panic, depressive, posttraumatic stress, bipolar, psychotic, eating, and attention-deficit/hyperactivity. The interviews show and teach how to rate psychopathology items, how to score the dimensions, and how to evaluate the severity of the disorder(s). All of the SCIP’s 18 scales have been uploaded into the Epic electronic health record (EHR) system at West Virginia University hospitals. A pilot project for implementing MBC in the treatment of adult psychiatric disorders at the West Virginia University residency program and other programs is underway. If we instruct residents in MBC during their psychiatric training, they will likely practice it for the rest of their clinical careers. Except for a minority of clinicians who are involved in clinical trials and who use rating scales in practice, most practicing clinicians were never trained to use scales. For more information about the MBC curriculum and videotapes, contact Dr. Aboraya at aborayascip@gmail.com or visit www.scip-psychiatry.com.
Today, some of the barriers that impede the implementation of MBC in psychiatric practice have been resolved, but much more work remains. Now is the time to implement MBC and provide an answer to AJ Rush, who asked, “Isn’t it about time to employ measurement-based care in practice?”24 The 3 main ingredients for MBC implementation—useful measures, integration of EHR, and health information technologies—exist today. We strongly encourage psychiatrists, nurse practitioners, and other mental health professionals to adopt MBC in their daily practice.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
1. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
2. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2016;68(2):179-188.
3. Moore TV. The essential psychoses and their fundamental syndromes. Baltimore, MD: Williams & Wilkins; 1933.
4. Rush AJ. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000.
5. Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cogn Behav Pract. 2015;22(1):49-59.
6. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61-S71.
7. Harding KJ, Rush AJ, Arbuckle M, et al. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136-1143.
8. Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004-1013.
9. Aboraya A, Nasrallah HA, Elswick D, et al. Measurement-based care in psychiatry: past, present and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
10. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
11. Zimmerman M, Mattia JI. The reliability and validity of a screening questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. J Clin Psychiatry. 1999;60(10):677-683.
12. Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42(3):175-189.
13. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140.
14. Zimmerman M, Posternak MA, Chelminski I, et al. Standardized clinical outcome rating scale for depression for use in clinical practice. Depress Anxiety. 2005;22(1):36-40.
15. Zimmerman M, Chelminski I, Young D, et al. A clinically useful anxiety outcome scale. J Clin Psychiatry. 2010;71(5):534-542.
16. Zimmerman M, Galione JN, Attiullah N, et al. Depressed patients’ perspectives of 2 measures of outcome: the Quick Inventory of Depressive Symptomatology (QIDS) and the Remission from Depression Questionnaire (RDQ). Ann Clin Psychiatry. 2011;23(3):208-212.
17. Zimmerman M, Martinez JH, Attiullah N, et al. The remission from depression questionnaire as an outcome measure in the treatment of depression. Depress Anxiety. 2014;31(6):533-538.
18. Zimmerman M, Gazarian D, Multach M, et al. A clinically useful self-report measure of psychiatric patients’ satisfaction with the initial evaluation. Psychiatry Res. 2017;252:38-44.
19. Aboraya A. The validity results of the Standard for Clinicians’ Interview in Psychiatry (SCIP). Schizophrenia Bulletin. 2015;41(Suppl 1):S103-S104.
20. Aboraya A. Instruction manual for the Standard for Clinicians’ Interview in Psychiatry (SCIP). http://innovationscns.com/wp-content/uploads/SCIP_Instruction_Manual.pdf. Accessed April 29, 2019.
21. Aboraya A, El-Missiry A, Barlowe J, et al. The reliability of the Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional and numeric output. Schizophr Res. 2014;156(2-3):174-183.
22. Aboraya A, Nasrallah HA, Muvvala S, et al. The Standard for Clinicians’ Interview in Psychiatry (SCIP): a clinician-administered tool with categorical, dimensional, and numeric output-conceptual development, design, and description of the SCIP. Innov Clin Neurosci. 2016;13(5-6):31-77.
23. Aboraya A, Nasrallah HA. Perspectives on the Positive and Negative Syndrome Scale (PANSS): Use, misuse, drawbacks, and a new alternative for schizophrenia research. Ann Clin Psychiatry. 2016;28(2):125-131.
24. Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934-936.
Caring for patients on probation or parole
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Burnout Among Vascular Surgeons – A Report From the SVS Wellness Committee
Physician burnout has been linked to medical errors, decreased patient satisfaction, and reduced career longevity. In light of the increasing prevalence of cardiovascular disease, vascular surgeon burnout presents a legitimate public health concern because of the impact on the adequacy of the vascular surgery workforce. Dawn Coleman, MD, and her colleagues, performed a study on behalf of the Society for Vascular Surgery (SVS) Wellness Task Force to define the prevalence of burnout among practicing vascular surgeons, and to identify the risk factors for burnout. Such information will be used to facilitate future SVS initiatives to avert this crisis.
In Thursday’s von Liebig Forum, Dr. Coleman, of the University of Michigan, Ann Arbor, will present the results of their 2018 anonymous survey of active SVS members. The survey used a validated burnout assessment, Maslach Burnout Index (MBI), embedded in a questionnaire that also captured demographic and practice-related characteristics.
The survey was personalized for the specialty and did allow for free text. according to Dr. Coleman, and “we specifically analyzed emotional exhaustion, one dimension of burnout.”
The accepted threshold of a score of 27 or greater on the MBI Emotional Exhaustion module was used to identify surgeons suffering from burnout. Risk factors for such were identified using bivariate analyses (Chi-square, Kruskal-Wallis), and multivariate logistic regression models were developed to identify independent risk factors for burnout, she added.
Dr. Coleman will present the results from the 960 out of 2,905 active SVS members who responded to the survey (33%). After excluding retired surgeons and incomplete submissions, responses from 872 practicing vascular surgeons were finally analyzed. The mean respondent age was 49.7 years; and the majority of respondents (81%) were men. The primary practice settings were academic (40%), community practice (41%), Veterans Administration hospital (3.3%), active military practice (1.5%), or “other.” Mean years in practice was 15.7.
Overall, 30% of the respondents met criteria for burnout, 37% screened positive for symptoms of depression in the past month and 8% supported thoughts of taking their own life during the last 12 months.
By unadjusted analysis, factors significantly associated with burnout included clinical work hours, on-call frequency, electronic medical record/documentation requirements, perceived conflict between work and personal responsibilities, and physical pain. Multivariate analysis revealed age, work-related physical pain, and conflict between work and personal responsibilities as independent risk factors for burnout, said Dr. Coleman.
“Approximately one-third of practicing vascular surgeons self-report burnout and depression, according to our survey. Advancing age, physical pain, and work-life conflict are each independent predictors for burnout among vascular surgeons. These findings will facilitate SVS efforts to improve vascular surgeon well-being, in an effort to mitigate the personal, economic, and social impact of vascular surgeon burnout,” Dr. Coleman concluded.
See more on the work of the SVS Wellness Task Force in the June issue of Vascular Specialist.
Physician burnout has been linked to medical errors, decreased patient satisfaction, and reduced career longevity. In light of the increasing prevalence of cardiovascular disease, vascular surgeon burnout presents a legitimate public health concern because of the impact on the adequacy of the vascular surgery workforce. Dawn Coleman, MD, and her colleagues, performed a study on behalf of the Society for Vascular Surgery (SVS) Wellness Task Force to define the prevalence of burnout among practicing vascular surgeons, and to identify the risk factors for burnout. Such information will be used to facilitate future SVS initiatives to avert this crisis.
In Thursday’s von Liebig Forum, Dr. Coleman, of the University of Michigan, Ann Arbor, will present the results of their 2018 anonymous survey of active SVS members. The survey used a validated burnout assessment, Maslach Burnout Index (MBI), embedded in a questionnaire that also captured demographic and practice-related characteristics.
The survey was personalized for the specialty and did allow for free text. according to Dr. Coleman, and “we specifically analyzed emotional exhaustion, one dimension of burnout.”
The accepted threshold of a score of 27 or greater on the MBI Emotional Exhaustion module was used to identify surgeons suffering from burnout. Risk factors for such were identified using bivariate analyses (Chi-square, Kruskal-Wallis), and multivariate logistic regression models were developed to identify independent risk factors for burnout, she added.
Dr. Coleman will present the results from the 960 out of 2,905 active SVS members who responded to the survey (33%). After excluding retired surgeons and incomplete submissions, responses from 872 practicing vascular surgeons were finally analyzed. The mean respondent age was 49.7 years; and the majority of respondents (81%) were men. The primary practice settings were academic (40%), community practice (41%), Veterans Administration hospital (3.3%), active military practice (1.5%), or “other.” Mean years in practice was 15.7.
Overall, 30% of the respondents met criteria for burnout, 37% screened positive for symptoms of depression in the past month and 8% supported thoughts of taking their own life during the last 12 months.
By unadjusted analysis, factors significantly associated with burnout included clinical work hours, on-call frequency, electronic medical record/documentation requirements, perceived conflict between work and personal responsibilities, and physical pain. Multivariate analysis revealed age, work-related physical pain, and conflict between work and personal responsibilities as independent risk factors for burnout, said Dr. Coleman.
“Approximately one-third of practicing vascular surgeons self-report burnout and depression, according to our survey. Advancing age, physical pain, and work-life conflict are each independent predictors for burnout among vascular surgeons. These findings will facilitate SVS efforts to improve vascular surgeon well-being, in an effort to mitigate the personal, economic, and social impact of vascular surgeon burnout,” Dr. Coleman concluded.
See more on the work of the SVS Wellness Task Force in the June issue of Vascular Specialist.
Physician burnout has been linked to medical errors, decreased patient satisfaction, and reduced career longevity. In light of the increasing prevalence of cardiovascular disease, vascular surgeon burnout presents a legitimate public health concern because of the impact on the adequacy of the vascular surgery workforce. Dawn Coleman, MD, and her colleagues, performed a study on behalf of the Society for Vascular Surgery (SVS) Wellness Task Force to define the prevalence of burnout among practicing vascular surgeons, and to identify the risk factors for burnout. Such information will be used to facilitate future SVS initiatives to avert this crisis.
In Thursday’s von Liebig Forum, Dr. Coleman, of the University of Michigan, Ann Arbor, will present the results of their 2018 anonymous survey of active SVS members. The survey used a validated burnout assessment, Maslach Burnout Index (MBI), embedded in a questionnaire that also captured demographic and practice-related characteristics.
The survey was personalized for the specialty and did allow for free text. according to Dr. Coleman, and “we specifically analyzed emotional exhaustion, one dimension of burnout.”
The accepted threshold of a score of 27 or greater on the MBI Emotional Exhaustion module was used to identify surgeons suffering from burnout. Risk factors for such were identified using bivariate analyses (Chi-square, Kruskal-Wallis), and multivariate logistic regression models were developed to identify independent risk factors for burnout, she added.
Dr. Coleman will present the results from the 960 out of 2,905 active SVS members who responded to the survey (33%). After excluding retired surgeons and incomplete submissions, responses from 872 practicing vascular surgeons were finally analyzed. The mean respondent age was 49.7 years; and the majority of respondents (81%) were men. The primary practice settings were academic (40%), community practice (41%), Veterans Administration hospital (3.3%), active military practice (1.5%), or “other.” Mean years in practice was 15.7.
Overall, 30% of the respondents met criteria for burnout, 37% screened positive for symptoms of depression in the past month and 8% supported thoughts of taking their own life during the last 12 months.
By unadjusted analysis, factors significantly associated with burnout included clinical work hours, on-call frequency, electronic medical record/documentation requirements, perceived conflict between work and personal responsibilities, and physical pain. Multivariate analysis revealed age, work-related physical pain, and conflict between work and personal responsibilities as independent risk factors for burnout, said Dr. Coleman.
“Approximately one-third of practicing vascular surgeons self-report burnout and depression, according to our survey. Advancing age, physical pain, and work-life conflict are each independent predictors for burnout among vascular surgeons. These findings will facilitate SVS efforts to improve vascular surgeon well-being, in an effort to mitigate the personal, economic, and social impact of vascular surgeon burnout,” Dr. Coleman concluded.
See more on the work of the SVS Wellness Task Force in the June issue of Vascular Specialist.