User login
EADV: Investigational biologic rocks psoriasis world
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
COPENHAGEN – An investigational biologic agent that selectively inhibits the p19 subunit of interleukin-23 was the talk of the 2015 EADV congress based upon its striking outperformance of ustekinumab in a head-to-head phase II study in psoriasis patients.
Not only did the investigational agent, known for now as BI 655066, achieve substantially higher rates of PASI 90 – clear or almost clear skin – and PASI 100, but it did so much faster and maintained those stellar results far longer off treatment than with ustekinumab (Stelara), Dr. Kim A. Papp reported at the annual congress of the European Academy of Dermatology and Venereology.
“These data make an irrefutable case for IL-23 as instrumental in the expression of psoriasis,” declared Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“What these data show is that selective blockade of IL-23 p19 clearly provides a remarkable response, whether we measure it as PASI 75, PASI 90, or PASI 100, compared with ustekinumab, which provides a very robust response itself. And when we look at the safety profile – admittedly in a small number of patients and for a short period of time – we see there are no real differences in safety signals. Obviously, we’re very encouraged to move forward with long-term phase III investigations,” Dr. Papp added.
The multicenter, double-blind phase II study included 166 patients with moderate to severe plaque psoriasis who were randomized to one of four treatment arms: BI 655066 at 90 or 180 mg given subcutaneously at weeks 0, 4, and 16; weight-based ustekinumab at 45 or 90 mg given on the same schedule; or a single 18-mg dose of BI 655066. Dr. Papp presented the results at 36 weeks, fully 20 weeks after the final dose was given.
Significantly more patients taking the investigational agent achieved PASI targets than did those taking ustekinumab, a highly effective medication. Here are the eye-popping 36-week results:
• PASI 90: 81% with BI 655066 at 180 mg, 69% with the lower dose, 30% with ustekinumab, and 7% with a single 18-mg dose of BI 655066.
• PASI 100: 54% and 43% with high- and low-dose BI 655066, respectively, 15% with ustekinumab, and 0% with single-dose BI 655066.
• PASI 75: 93% and 88% with high- and low-dose BI 655066; 55% with ustekinumab; and 19% after single-dose BI 655066.
One of the most intriguing study findings, according to Dr. Papp, is that the slope of the PASI 100 response curve in BI 655066–treated patients was still rising at week 20, a month after the final dose.
“It suggests that if you were to continue treating these patients, you might anticipate an even higher response,” the dermatologist said.
Another notable finding was the accelerated speed of response to the investigational agent: 57 days after the first dose of BI 655066, 50% of patients had a PASI 90 response.
“That means from the time you initiate therapy, you can expect half of your patients to get to PASI 90 in less than 2 months time,” Dr. Papp observed.
In contrast, it took 117 days – basically twice as long – for half of the ustekinumab group to achieve PASI 90.
Turning to the durability of response, Dr. Papp noted that it took 169 days for half of patients in the ustekinumab group to lose their PASI 90 response after treatment stopped, compared with 225 days in the BI 655066 90-mg group. That endpoint was never reached in the BI 655066 180-mg group before the study’s end.
Audience reaction to the study results was “wow.”
“Obviously, the data are terrific. Your mother couldn’t have invented better data,” declared Dr. Alice B. Gottlieb, professor of dermatology and dermatologist in chief at Tufts Medical Center, Boston.
She posed a question: Why are the BI 655066 response rates so much higher and longer lasting than with other biologics, including ustekinumab, which inhibits both IL-23 and IL-12?
“I think one key factor in the durability of the response is the pathway,” Dr. Papp replied. “Exactly why blockade of IL-23 p19 leads to this durable clinical response is unclear, but we can posit that we’re depopulating the Th-17 cells. IL-23 is necessary for Th-17 cell survival. So if you block the water, you ain’t going to grow the grass. I’m speculating here, but I think we’re removing that clone of cells, and it’s only once we start to allow them to repopulate that we then see recurrence of the disease.”
BI 655066 is being developed by Boehringer Ingelheim. Dr. Papp serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: An investigational inhibitor of the p19 subunit of interleukin-23 is generating efficacy results never before seen in psoriasis.
Major finding: The PASI 90 response rate was 81% fully 20 weeks after the third and final dose of BI 655066 at 180 mg, compared with 30% for ustekinumab.
Data source: This prospective, multicenter, double-blind study included 166 patients with moderate to severe plaque psoriasis who were randomized to various doses of ustekinumab or BI 655066 and followed for 36 weeks.
Disclosures: The study was sponsored by Boehringer Ingelheim. The presenter serves as an investigator for and consultant to Boehringer Ingelheim and numerous other pharmaceutical companies.
Infections from endemic fungi, mycobacteria rare in patients on TNFIs
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The incidence of select mycobacterial and fungal infections in patients taking TNFIs is low.
Major finding: Only 0.51% of patients taking TNFIs developed the mycobacterial and fungal infections targeted in this study.
Data source: A case-control study of 30,772 patients taking TNFIs.
Disclosures: The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
Topical calcipotriene-betamethasone combination approved for plaque psoriasis
A combination of calcipotriene and betamethasone dipropionate in a foam formulation has been approved by the Food and Drug Administration for the topical treatment of plaque psoriasis in adults, according to the manufacturer.
The product will be marketed as Enstilar (calcipotriene and betamethasone dipropionate) foam, 0.005%/0.064%, by Leo Pharma.
Each gram contains 52.2 mcg calcipotriene hydrate (equivalent to 50 mcg of calcipotriene) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone); it is applied to affected areas once a day for up to 4 weeks, according to the prescribing information, which states that patient should not use more than 60 g every 4 days.
The vitamin D analog-corticosteroid combination product was compared with treatment with the two individual components and/or a vehicle only, in more than 700 patients with plaque psoriasis in two studies. At baseline, about two-thirds of the patients had moderate disease, 14%-15% had mild disease, and 10% had moderately severe disease, based on a 5-point Investigator’s Global Assessment scale. The proportion of patients who were considered clear or almost clear at 4 weeks, based on IGA scale results, was the efficacy endpoint defining treatment success. Among patients with mild disease at baseline, treatment was considered successful if they were considered clear at 4 weeks.
In one study of 302 patients, 45% treated with the combination product had achieved treatment success at week 4 vs. 30.7% of those treated with betamethasone and 14.9% treated with calcipotriene, according to the prescribing information. In the second study, 323 patients were treated with the combination product and 103 received the vehicle. At 4 weeks, 53.3% of those treated with the foam met the efficacy endpoint vs. 4.8% of those in the vehicle group.
Application site irritation, application site pruritus, folliculitis, skin hypopigmentation, hypercalcemia, urticaria, and psoriasis exacerbations were among the adverse reactions reported in fewer than 1% of those treated with the combination product. The warnings and precautions section of the prescribing information includes a statement noting that the product contains propellants that are flammable, and that patients should be advised to “avoid fire, flame, or smoking during and immediately after using this product.”
This approval is for an adult population. As a postmarketing commitment, the FDA is requiring that the company conduct a study in pediatric patients with plaque psoriasis, according to the agency’s approval letter, dated Oct. 16. That study is described as an open-label study to assess the effect of the product on calcium metabolism in pediatric patients aged 12-16 years, with plaque psoriasis of the scalp and body. In a subgroup of these children, pharmacokinetics and hypothalamic-pituitary axis suppression also will be assessed, the letter says.
Serious adverse events associated with this product should be reported to the FDA’s MedWatch program at 800-332-1088, or http://www.fda.gov/Safety/MedWatch/default.htm.
A combination of calcipotriene and betamethasone dipropionate in a foam formulation has been approved by the Food and Drug Administration for the topical treatment of plaque psoriasis in adults, according to the manufacturer.
The product will be marketed as Enstilar (calcipotriene and betamethasone dipropionate) foam, 0.005%/0.064%, by Leo Pharma.
Each gram contains 52.2 mcg calcipotriene hydrate (equivalent to 50 mcg of calcipotriene) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone); it is applied to affected areas once a day for up to 4 weeks, according to the prescribing information, which states that patient should not use more than 60 g every 4 days.
The vitamin D analog-corticosteroid combination product was compared with treatment with the two individual components and/or a vehicle only, in more than 700 patients with plaque psoriasis in two studies. At baseline, about two-thirds of the patients had moderate disease, 14%-15% had mild disease, and 10% had moderately severe disease, based on a 5-point Investigator’s Global Assessment scale. The proportion of patients who were considered clear or almost clear at 4 weeks, based on IGA scale results, was the efficacy endpoint defining treatment success. Among patients with mild disease at baseline, treatment was considered successful if they were considered clear at 4 weeks.
In one study of 302 patients, 45% treated with the combination product had achieved treatment success at week 4 vs. 30.7% of those treated with betamethasone and 14.9% treated with calcipotriene, according to the prescribing information. In the second study, 323 patients were treated with the combination product and 103 received the vehicle. At 4 weeks, 53.3% of those treated with the foam met the efficacy endpoint vs. 4.8% of those in the vehicle group.
Application site irritation, application site pruritus, folliculitis, skin hypopigmentation, hypercalcemia, urticaria, and psoriasis exacerbations were among the adverse reactions reported in fewer than 1% of those treated with the combination product. The warnings and precautions section of the prescribing information includes a statement noting that the product contains propellants that are flammable, and that patients should be advised to “avoid fire, flame, or smoking during and immediately after using this product.”
This approval is for an adult population. As a postmarketing commitment, the FDA is requiring that the company conduct a study in pediatric patients with plaque psoriasis, according to the agency’s approval letter, dated Oct. 16. That study is described as an open-label study to assess the effect of the product on calcium metabolism in pediatric patients aged 12-16 years, with plaque psoriasis of the scalp and body. In a subgroup of these children, pharmacokinetics and hypothalamic-pituitary axis suppression also will be assessed, the letter says.
Serious adverse events associated with this product should be reported to the FDA’s MedWatch program at 800-332-1088, or http://www.fda.gov/Safety/MedWatch/default.htm.
A combination of calcipotriene and betamethasone dipropionate in a foam formulation has been approved by the Food and Drug Administration for the topical treatment of plaque psoriasis in adults, according to the manufacturer.
The product will be marketed as Enstilar (calcipotriene and betamethasone dipropionate) foam, 0.005%/0.064%, by Leo Pharma.
Each gram contains 52.2 mcg calcipotriene hydrate (equivalent to 50 mcg of calcipotriene) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone); it is applied to affected areas once a day for up to 4 weeks, according to the prescribing information, which states that patient should not use more than 60 g every 4 days.
The vitamin D analog-corticosteroid combination product was compared with treatment with the two individual components and/or a vehicle only, in more than 700 patients with plaque psoriasis in two studies. At baseline, about two-thirds of the patients had moderate disease, 14%-15% had mild disease, and 10% had moderately severe disease, based on a 5-point Investigator’s Global Assessment scale. The proportion of patients who were considered clear or almost clear at 4 weeks, based on IGA scale results, was the efficacy endpoint defining treatment success. Among patients with mild disease at baseline, treatment was considered successful if they were considered clear at 4 weeks.
In one study of 302 patients, 45% treated with the combination product had achieved treatment success at week 4 vs. 30.7% of those treated with betamethasone and 14.9% treated with calcipotriene, according to the prescribing information. In the second study, 323 patients were treated with the combination product and 103 received the vehicle. At 4 weeks, 53.3% of those treated with the foam met the efficacy endpoint vs. 4.8% of those in the vehicle group.
Application site irritation, application site pruritus, folliculitis, skin hypopigmentation, hypercalcemia, urticaria, and psoriasis exacerbations were among the adverse reactions reported in fewer than 1% of those treated with the combination product. The warnings and precautions section of the prescribing information includes a statement noting that the product contains propellants that are flammable, and that patients should be advised to “avoid fire, flame, or smoking during and immediately after using this product.”
This approval is for an adult population. As a postmarketing commitment, the FDA is requiring that the company conduct a study in pediatric patients with plaque psoriasis, according to the agency’s approval letter, dated Oct. 16. That study is described as an open-label study to assess the effect of the product on calcium metabolism in pediatric patients aged 12-16 years, with plaque psoriasis of the scalp and body. In a subgroup of these children, pharmacokinetics and hypothalamic-pituitary axis suppression also will be assessed, the letter says.
Serious adverse events associated with this product should be reported to the FDA’s MedWatch program at 800-332-1088, or http://www.fda.gov/Safety/MedWatch/default.htm.
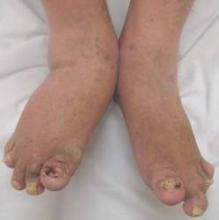
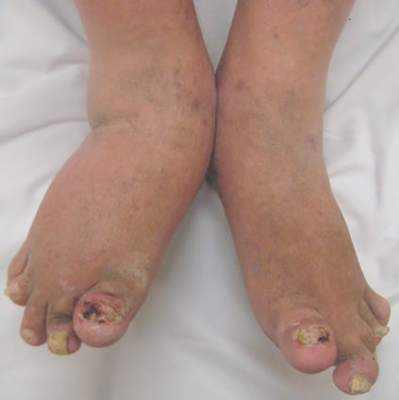
Dactylitis warns of future damage in psoriatic arthritis
Baseline radiographic damage and the presence of dactylitis at 5 years are independent predictors of further joint destruction in people with psoriatic arthritis, according to follow-up data from a psoriatic arthritis registry.
The analysis of hand and foot radiographic findings from 72 patients (29 men, 43 women) participating in the Swedish Early Psoriatic Arthritis Registry (SwePsA) registry found that radiographic progression in early PsA was generally slow but was substantial in men. At entry to the registry, the patients had a mean age of about 48 years and had symptoms for a median of 12 months. Most had polyarticular disease as a result of the registry’s protocol, and none had arthritis mutilans or axial disease only. Three men and none of the women had combined axial and peripheral disease, reported Dr. Mats Geijer of the Center for Medical Imaging and Physiology at Skåne University Hospital, Lund, Sweden, and his colleagues (J Rheumatol. 2015 Oct 15. doi: 10.3899/jrheum.150165).
Only 4 (14%) of the men did not have radiographic changes at 5 years, compared with 17 (40%) women, making male sex a risk factor for structural abnormality (odds ratio, 4.09; 95% confidence interval, 1.2-13.8; P = .024). Most of the radiographic changes in men occurred in the feet. Baseline radiographic changes also were a significant predictor of additional radiographic damage at 5 years (OR, 3.4; 95% CI, 2.2-5.2; P less than .001).
In a multivariate linear regression analysis, only baseline radiographic score and the presence of dactylitis at 5 years were independent predictors of a high radiographic score at 5-year follow-up.
The findings suggested that men improve clinically but develop radiographic changes, whereas women show less clinical improvement but do not develop joint damage to the same extent as men. This gender-divergent behavior could be taken into consideration when planning treatment and care for patients, the authors said.
For example, women may require more interventions against functional deterioration and help coping with pain and men may need follow-up radiography, especially in the presence of dactylitis, high disease activity, and a known propensity for radiographic destruction, they suggested.
“Radiography and scoring the hands and feet at baseline are important and cannot be substituted with clinical signs, especially in men,” they concluded.
The study was supported by independent grants from the Swedish Psoriasis Foundation, Skåne University Hospital (SUS Fonder) and Pfizer.
Baseline radiographic damage and the presence of dactylitis at 5 years are independent predictors of further joint destruction in people with psoriatic arthritis, according to follow-up data from a psoriatic arthritis registry.
The analysis of hand and foot radiographic findings from 72 patients (29 men, 43 women) participating in the Swedish Early Psoriatic Arthritis Registry (SwePsA) registry found that radiographic progression in early PsA was generally slow but was substantial in men. At entry to the registry, the patients had a mean age of about 48 years and had symptoms for a median of 12 months. Most had polyarticular disease as a result of the registry’s protocol, and none had arthritis mutilans or axial disease only. Three men and none of the women had combined axial and peripheral disease, reported Dr. Mats Geijer of the Center for Medical Imaging and Physiology at Skåne University Hospital, Lund, Sweden, and his colleagues (J Rheumatol. 2015 Oct 15. doi: 10.3899/jrheum.150165).
Only 4 (14%) of the men did not have radiographic changes at 5 years, compared with 17 (40%) women, making male sex a risk factor for structural abnormality (odds ratio, 4.09; 95% confidence interval, 1.2-13.8; P = .024). Most of the radiographic changes in men occurred in the feet. Baseline radiographic changes also were a significant predictor of additional radiographic damage at 5 years (OR, 3.4; 95% CI, 2.2-5.2; P less than .001).
In a multivariate linear regression analysis, only baseline radiographic score and the presence of dactylitis at 5 years were independent predictors of a high radiographic score at 5-year follow-up.
The findings suggested that men improve clinically but develop radiographic changes, whereas women show less clinical improvement but do not develop joint damage to the same extent as men. This gender-divergent behavior could be taken into consideration when planning treatment and care for patients, the authors said.
For example, women may require more interventions against functional deterioration and help coping with pain and men may need follow-up radiography, especially in the presence of dactylitis, high disease activity, and a known propensity for radiographic destruction, they suggested.
“Radiography and scoring the hands and feet at baseline are important and cannot be substituted with clinical signs, especially in men,” they concluded.
The study was supported by independent grants from the Swedish Psoriasis Foundation, Skåne University Hospital (SUS Fonder) and Pfizer.
Baseline radiographic damage and the presence of dactylitis at 5 years are independent predictors of further joint destruction in people with psoriatic arthritis, according to follow-up data from a psoriatic arthritis registry.
The analysis of hand and foot radiographic findings from 72 patients (29 men, 43 women) participating in the Swedish Early Psoriatic Arthritis Registry (SwePsA) registry found that radiographic progression in early PsA was generally slow but was substantial in men. At entry to the registry, the patients had a mean age of about 48 years and had symptoms for a median of 12 months. Most had polyarticular disease as a result of the registry’s protocol, and none had arthritis mutilans or axial disease only. Three men and none of the women had combined axial and peripheral disease, reported Dr. Mats Geijer of the Center for Medical Imaging and Physiology at Skåne University Hospital, Lund, Sweden, and his colleagues (J Rheumatol. 2015 Oct 15. doi: 10.3899/jrheum.150165).
Only 4 (14%) of the men did not have radiographic changes at 5 years, compared with 17 (40%) women, making male sex a risk factor for structural abnormality (odds ratio, 4.09; 95% confidence interval, 1.2-13.8; P = .024). Most of the radiographic changes in men occurred in the feet. Baseline radiographic changes also were a significant predictor of additional radiographic damage at 5 years (OR, 3.4; 95% CI, 2.2-5.2; P less than .001).
In a multivariate linear regression analysis, only baseline radiographic score and the presence of dactylitis at 5 years were independent predictors of a high radiographic score at 5-year follow-up.
The findings suggested that men improve clinically but develop radiographic changes, whereas women show less clinical improvement but do not develop joint damage to the same extent as men. This gender-divergent behavior could be taken into consideration when planning treatment and care for patients, the authors said.
For example, women may require more interventions against functional deterioration and help coping with pain and men may need follow-up radiography, especially in the presence of dactylitis, high disease activity, and a known propensity for radiographic destruction, they suggested.
“Radiography and scoring the hands and feet at baseline are important and cannot be substituted with clinical signs, especially in men,” they concluded.
The study was supported by independent grants from the Swedish Psoriasis Foundation, Skåne University Hospital (SUS Fonder) and Pfizer.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Men with early psoriatic arthritis tend to improve clinically but develop radiographic changes over a 5-year period, whereas women show less clinical improvement but do not develop joint damage to the same extent as men.
Major finding: Only 4 (14%) of the men did not have radiographic changes at 5 years, compared with 17 (40%) women, making male sex a risk factor for structural abnormality (OR, 4.09; 95% CI, 1.2-13.8; P = .024).
Data source: A longitudinal cohort study of 72 patients with psoriatic arthritis from the Swedish Early Psoriatic Arthritis Registry.
Disclosures: The study was supported by independent grants from the Swedish Psoriasis Foundation, Skåne University Hospital (SUS Fonder) and Pfizer.
EADV: Latest gruesome twosome: Psoriasis spawns renal disease
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
AT THE EADV CONGRESS
Key clinical point: Patients with severe but not mild psoriasis are at increased risk of new-onset chronic kidney disease and end-stage renal disease.
Major finding: Severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD. Comorbid psoriatic arthritis further boosted those risks.
Data source: This retrospective cohort study included 4,633 consecutive patients diagnosed with psoriasis in Taiwan.
Disclosures: Dr. Chi reported having no financial conflicts regarding this government-funded study.
EADV: Long-term weight loss curbs psoriasis severity
COPENHAGEN – Long-term weight loss achieved by obese patients with psoriasis provides long-lasting reductions in psoriasis severity, Dr. Peter Jensen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a prospective 64-week observational study that was a follow-up to his earlier 16-week randomized, controlled clinical trial. The earlier study (JAMA Dermatol. 2013 Jul;149[7]:795-801) generated enormous interest because it provided the first level I evidence that weight loss by obese psoriasis patients brings clinically meaningful improvement in their skin disease as well as achieving well-established metabolic and cardiovascular risk reduction benefits.
In the randomized trial, 60 consecutive obese patients with psoriasis and a mean baseline Psoriasis Area and Severity Index (PASI) of 5.4 and a body mass index of 34.4 kg/m2 were assigned to a dietary intervention or given general advice to eat healthy foods. The intervention entailed 8 weeks of a low-energy liquid diet featuring a daily total nutrition intake of 800-1,000 kcal in the form of fortified drinks and soups. This was followed by 8 weeks in which regular foods were reintroduced at 1,200 kcal/day. At week 16, the intervention group had lost an average of 15.4 kg more body weight than controls. Their mean PASI was 2.0 points lower as well, according to Dr. Jensen, a dermatologist at the University of Copenhagen.
In the observational follow-up study, the original control group was offered the opportunity to participate in the 16-week weight loss intervention. All patients were then prospectively followed for 48 weeks after completing the dietary intervention. Psoriasis medications weren’t changed during the study period. The questions Dr. Jensen and coinvestigators sought to answer through the follow-up study were, first, can patients keep the weight off long term without a structured maintenance program and, if so, do their PASI scores stay low or do they creep back up?
Only 32 of the original 60 patients completed the full 64-week study. Among the completers, as is typical in weight loss studies, there was a gradual weight regain. Nonetheless, at 64 weeks, patients still maintained a mean 10-kg weight loss, compared with baseline, or two-thirds of the weight loss achieved during the 16-week dietary intervention.
Most importantly from a dermatologic perspective, the positive effect upon disease severity was maintained despite the regain of one-third of the initial weight loss, with patients showing a clinically important mean 3-point reduction in PASI, compared with baseline, Dr. Jensen noted.
Audience members were enthusiastic about the study findings but asked if the results are widely applicable. In other words, was this an unusually highly motivated group of patients?
“Our experience is that people really would like to try this. It was no problem to get participation,” according to Dr. Jensen.
Study limitations included the small sample size and the fact that this was a patient cohort with relatively mild psoriasis. In the future, it would be informative to study the effects of weight loss in obese patients with higher baseline PASI scores, the dermatologist said.
The study was funded by the Danish Academy of Dermatology and various research foundations. Dr. Jensen reported having no financial conflicts.
COPENHAGEN – Long-term weight loss achieved by obese patients with psoriasis provides long-lasting reductions in psoriasis severity, Dr. Peter Jensen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a prospective 64-week observational study that was a follow-up to his earlier 16-week randomized, controlled clinical trial. The earlier study (JAMA Dermatol. 2013 Jul;149[7]:795-801) generated enormous interest because it provided the first level I evidence that weight loss by obese psoriasis patients brings clinically meaningful improvement in their skin disease as well as achieving well-established metabolic and cardiovascular risk reduction benefits.
In the randomized trial, 60 consecutive obese patients with psoriasis and a mean baseline Psoriasis Area and Severity Index (PASI) of 5.4 and a body mass index of 34.4 kg/m2 were assigned to a dietary intervention or given general advice to eat healthy foods. The intervention entailed 8 weeks of a low-energy liquid diet featuring a daily total nutrition intake of 800-1,000 kcal in the form of fortified drinks and soups. This was followed by 8 weeks in which regular foods were reintroduced at 1,200 kcal/day. At week 16, the intervention group had lost an average of 15.4 kg more body weight than controls. Their mean PASI was 2.0 points lower as well, according to Dr. Jensen, a dermatologist at the University of Copenhagen.
In the observational follow-up study, the original control group was offered the opportunity to participate in the 16-week weight loss intervention. All patients were then prospectively followed for 48 weeks after completing the dietary intervention. Psoriasis medications weren’t changed during the study period. The questions Dr. Jensen and coinvestigators sought to answer through the follow-up study were, first, can patients keep the weight off long term without a structured maintenance program and, if so, do their PASI scores stay low or do they creep back up?
Only 32 of the original 60 patients completed the full 64-week study. Among the completers, as is typical in weight loss studies, there was a gradual weight regain. Nonetheless, at 64 weeks, patients still maintained a mean 10-kg weight loss, compared with baseline, or two-thirds of the weight loss achieved during the 16-week dietary intervention.
Most importantly from a dermatologic perspective, the positive effect upon disease severity was maintained despite the regain of one-third of the initial weight loss, with patients showing a clinically important mean 3-point reduction in PASI, compared with baseline, Dr. Jensen noted.
Audience members were enthusiastic about the study findings but asked if the results are widely applicable. In other words, was this an unusually highly motivated group of patients?
“Our experience is that people really would like to try this. It was no problem to get participation,” according to Dr. Jensen.
Study limitations included the small sample size and the fact that this was a patient cohort with relatively mild psoriasis. In the future, it would be informative to study the effects of weight loss in obese patients with higher baseline PASI scores, the dermatologist said.
The study was funded by the Danish Academy of Dermatology and various research foundations. Dr. Jensen reported having no financial conflicts.
COPENHAGEN – Long-term weight loss achieved by obese patients with psoriasis provides long-lasting reductions in psoriasis severity, Dr. Peter Jensen reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a prospective 64-week observational study that was a follow-up to his earlier 16-week randomized, controlled clinical trial. The earlier study (JAMA Dermatol. 2013 Jul;149[7]:795-801) generated enormous interest because it provided the first level I evidence that weight loss by obese psoriasis patients brings clinically meaningful improvement in their skin disease as well as achieving well-established metabolic and cardiovascular risk reduction benefits.
In the randomized trial, 60 consecutive obese patients with psoriasis and a mean baseline Psoriasis Area and Severity Index (PASI) of 5.4 and a body mass index of 34.4 kg/m2 were assigned to a dietary intervention or given general advice to eat healthy foods. The intervention entailed 8 weeks of a low-energy liquid diet featuring a daily total nutrition intake of 800-1,000 kcal in the form of fortified drinks and soups. This was followed by 8 weeks in which regular foods were reintroduced at 1,200 kcal/day. At week 16, the intervention group had lost an average of 15.4 kg more body weight than controls. Their mean PASI was 2.0 points lower as well, according to Dr. Jensen, a dermatologist at the University of Copenhagen.
In the observational follow-up study, the original control group was offered the opportunity to participate in the 16-week weight loss intervention. All patients were then prospectively followed for 48 weeks after completing the dietary intervention. Psoriasis medications weren’t changed during the study period. The questions Dr. Jensen and coinvestigators sought to answer through the follow-up study were, first, can patients keep the weight off long term without a structured maintenance program and, if so, do their PASI scores stay low or do they creep back up?
Only 32 of the original 60 patients completed the full 64-week study. Among the completers, as is typical in weight loss studies, there was a gradual weight regain. Nonetheless, at 64 weeks, patients still maintained a mean 10-kg weight loss, compared with baseline, or two-thirds of the weight loss achieved during the 16-week dietary intervention.
Most importantly from a dermatologic perspective, the positive effect upon disease severity was maintained despite the regain of one-third of the initial weight loss, with patients showing a clinically important mean 3-point reduction in PASI, compared with baseline, Dr. Jensen noted.
Audience members were enthusiastic about the study findings but asked if the results are widely applicable. In other words, was this an unusually highly motivated group of patients?
“Our experience is that people really would like to try this. It was no problem to get participation,” according to Dr. Jensen.
Study limitations included the small sample size and the fact that this was a patient cohort with relatively mild psoriasis. In the future, it would be informative to study the effects of weight loss in obese patients with higher baseline PASI scores, the dermatologist said.
The study was funded by the Danish Academy of Dermatology and various research foundations. Dr. Jensen reported having no financial conflicts.
AT THE EADV CONGRESS
Key clinical point: Long-term weight loss by obese patients with psoriasi pays dividends in terms of sustained clinically meaningful reduction in PASI scores.
Major finding: At 64 weeks of follow-up, obese patients were able to maintain two-thirds of the mean 15.4-kg weight loss achieved through a 16-week dietary intervention, and their mean PASI scores were 3 points lower than the mean score of 5.4 points at baseline.
Data source: This was a prospective observational study of 32 obese psoriasis patients who completed 48 weeks of additional follow-up after a 16-week low-energy dietary intervention.
Disclosures: The study was funded by the Danish Academy of Dermatology and various research foundations. The presenter reported having no financial conflicts.
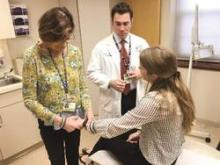
Combined dermatology-rheumatology clinics improve care, spark new research
At the Rheumatic Skin Disease Clinic in Manhasset, N.Y., dermatologist Dr. Amit Garg regularly discusses patient cases and develops care plans alongside rheumatologists after each specialist separately visits the same patient. The dual dermatology-rheumatology clinic has led to countless benefits, including improved quality of care for patients and multi-discipline training for new and veteran physicians, said Dr. Garg, clinic codirector and chair of the dermatology department at North Shore–Long Island Jewish Health System in New York.
Perhaps the greatest advantage of the combined clinic, however, is the research inspiration generated by the collaboration. “Working closely with colleagues in another discipline makes the environment really ripe for new ideas based on observations we may see in the patients we’re treating,” Dr. Garg said in an interview. “It’s amazing how when you’re thinking through a patient’s care together, how quickly you appreciate where the gaps are in our understanding of disease and evaluation and management of disease. From there, you take it to the next step of planning research projects or protocols to essentially fill those gaps in knowledge.”
Dr. Garg’s clinic is one of a growing number at U.S. academic medical centers that combine dermatologic and rheumatologic care for the management of psoriatic arthritis (PsA) and other interrelated skin and musculoskeletal diseases. Clinic operations vary from virtual consults, to simultaneous assessments, to sequential patient visits, but all focus on collaborative care and disease management. The institutions using such models recently united to form the Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network, a national organization that develops research initiatives and assists other centers in creating similar programs. At least 10 institutions are now participating in the network, including NYU Langone Medical Center, New York; Harvard University’s Brigham and Women’s Hospital, Boston; and the National Psoriasis Foundation, Portland, Ore.
Combining dermatology and rheumatology care through a dual clinic follows an ongoing trend by academic medical centers to integrate specialties that treat overlapping conditions, said Dr. Alexis R. Ogdie-Beatty, a rheumatologist at the University of Pennsylvania Health System in Philadelphia and director of the Penn Psoriatic Arthritis Program.
“It was kind of a natural thing for rheumatic disease because our diseases are multisystem in nature in most cases,” Dr. Ogdie-Beatty said in an interview. “I think the increasing knowledge of the need for communication [between specialists] drove this.”
Dr. Ogdie-Beatty leads a virtual rheumatology-dermatology clinic at the University of Pennsylvania where she meets weekly with two dermatologists to discuss research studies, shared patients, and case management. The physicians primarily see patients separately, but occasionally will visit patients at the same time, she said. In addition, dermatologists on the team immediately contact Dr. Ogdie-Beatty if they are seeing a patient who might benefit from an assessment by the rheumatologist and vice versa, she said.
“One of our favorite parts is learning from each other,” Dr. Ogdie-Beatty said in an interview. “I’ve learned a lot about skin disease and psoriasis, and they’ve learned about inflammatory arthritis and management. The ultimate impact for the patient is to have more well rounded care so that both the skin disease and the arthritis are being taken care of in a comprehensive way.”
Dermatology and rheumatology medical students and residents exposed to such clinics have the unique opportunity to learn about disease overlap and strengthen their understanding of the other’s specialty, added Dr. Joseph F. Merola, codirector of the Center for Skin and Related Musculoskeletal Diseases, a combined clinic at Brigham and Women’s Hospital in Boston. Dr. Merola’s center also has created a fellowship program in which either a dermatologist or rheumatologist can spend 1 year training at the clinic.
Research opportunities abound at the combined clinics, physicians said. At the Center for Skin and Related Musculoskeletal Diseases for example, physicians have developed new outcome metrics for measuring psoriasis and screening for PsA, Dr. Merola said. Physicians also commonly refer clinic patients for clinical trials, he said.
“It’s a great place for patients who have been through many other therapies,” said Dr. Merola, who practices dermatology and rheumatology. “If they are at the end of the line, we have a number of clinical trials that we can offer them. Because it tends to be a referral center, these are patients who have more complex disease, so being able to offer them clinical trials and multiple opinions has been really key.”
Dr. Ogdie-Beatty’s clinic is participating in a variety of research studies, including an analysis of vascular inflammation in psoriasis patients. The study is examining markers of early joint inflammation among psoriasis patients who don’t have symptoms of joint pain. Another initiative focuses on how to implement psoriasis and PsA disease activity measures into the medical record, Dr. Ogdie-Beatty said.
At a combined dermatology-rheumatology clinic within the University Health Network, Toronto, doctors are studying the incidence of arthritis and comorbidities in patients with psoriasis, said Dr. Vinod Chandran, a rheumatologist at the University of Toronto. Another ongoing study focuses on identifying risk factors for PsA in patients with psoriasis. The Toronto clinic is participating in the studies as part of the International Psoriasis and Arthritis Research Team.
“There are a number of research opportunities in the combined clinic since a detailed clinical evaluation is extremely important in biomarker and translational studies,” said Dr. Chandran, who codirects the psoriatic arthritis program at the Centre for Prognosis Studies in the Rheumatic Diseases, Toronto. “The clinic also provides an opportunity to participate in phase II and III clinical trials.”
Managing the combined derm-rheum clinics does come with challenges, doctors said. The obstacles are primarily logistical, and include identifying the space, time, and scheduling for the clinic, Dr. Garg said. Other challenges revolve around reimbursement, such as figuring out the best payment structure and ensuring that insurers will cover the simultaneous care, Dr. Merola said. Generally, it also takes longer to see the patient, and physicians may not be able to see as many patients together per day as they could alone, he added. However, physicians stress that the benefits of the combined clinics far outweigh the negatives.
“We’ve always felt the value has been there because we feel the patients really appreciate the service,” he said. “And we think the quality of care around what we do is better.”
On Twitter@legal_med
At the Rheumatic Skin Disease Clinic in Manhasset, N.Y., dermatologist Dr. Amit Garg regularly discusses patient cases and develops care plans alongside rheumatologists after each specialist separately visits the same patient. The dual dermatology-rheumatology clinic has led to countless benefits, including improved quality of care for patients and multi-discipline training for new and veteran physicians, said Dr. Garg, clinic codirector and chair of the dermatology department at North Shore–Long Island Jewish Health System in New York.
Perhaps the greatest advantage of the combined clinic, however, is the research inspiration generated by the collaboration. “Working closely with colleagues in another discipline makes the environment really ripe for new ideas based on observations we may see in the patients we’re treating,” Dr. Garg said in an interview. “It’s amazing how when you’re thinking through a patient’s care together, how quickly you appreciate where the gaps are in our understanding of disease and evaluation and management of disease. From there, you take it to the next step of planning research projects or protocols to essentially fill those gaps in knowledge.”
Dr. Garg’s clinic is one of a growing number at U.S. academic medical centers that combine dermatologic and rheumatologic care for the management of psoriatic arthritis (PsA) and other interrelated skin and musculoskeletal diseases. Clinic operations vary from virtual consults, to simultaneous assessments, to sequential patient visits, but all focus on collaborative care and disease management. The institutions using such models recently united to form the Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network, a national organization that develops research initiatives and assists other centers in creating similar programs. At least 10 institutions are now participating in the network, including NYU Langone Medical Center, New York; Harvard University’s Brigham and Women’s Hospital, Boston; and the National Psoriasis Foundation, Portland, Ore.
Combining dermatology and rheumatology care through a dual clinic follows an ongoing trend by academic medical centers to integrate specialties that treat overlapping conditions, said Dr. Alexis R. Ogdie-Beatty, a rheumatologist at the University of Pennsylvania Health System in Philadelphia and director of the Penn Psoriatic Arthritis Program.
“It was kind of a natural thing for rheumatic disease because our diseases are multisystem in nature in most cases,” Dr. Ogdie-Beatty said in an interview. “I think the increasing knowledge of the need for communication [between specialists] drove this.”
Dr. Ogdie-Beatty leads a virtual rheumatology-dermatology clinic at the University of Pennsylvania where she meets weekly with two dermatologists to discuss research studies, shared patients, and case management. The physicians primarily see patients separately, but occasionally will visit patients at the same time, she said. In addition, dermatologists on the team immediately contact Dr. Ogdie-Beatty if they are seeing a patient who might benefit from an assessment by the rheumatologist and vice versa, she said.
“One of our favorite parts is learning from each other,” Dr. Ogdie-Beatty said in an interview. “I’ve learned a lot about skin disease and psoriasis, and they’ve learned about inflammatory arthritis and management. The ultimate impact for the patient is to have more well rounded care so that both the skin disease and the arthritis are being taken care of in a comprehensive way.”
Dermatology and rheumatology medical students and residents exposed to such clinics have the unique opportunity to learn about disease overlap and strengthen their understanding of the other’s specialty, added Dr. Joseph F. Merola, codirector of the Center for Skin and Related Musculoskeletal Diseases, a combined clinic at Brigham and Women’s Hospital in Boston. Dr. Merola’s center also has created a fellowship program in which either a dermatologist or rheumatologist can spend 1 year training at the clinic.
Research opportunities abound at the combined clinics, physicians said. At the Center for Skin and Related Musculoskeletal Diseases for example, physicians have developed new outcome metrics for measuring psoriasis and screening for PsA, Dr. Merola said. Physicians also commonly refer clinic patients for clinical trials, he said.
“It’s a great place for patients who have been through many other therapies,” said Dr. Merola, who practices dermatology and rheumatology. “If they are at the end of the line, we have a number of clinical trials that we can offer them. Because it tends to be a referral center, these are patients who have more complex disease, so being able to offer them clinical trials and multiple opinions has been really key.”
Dr. Ogdie-Beatty’s clinic is participating in a variety of research studies, including an analysis of vascular inflammation in psoriasis patients. The study is examining markers of early joint inflammation among psoriasis patients who don’t have symptoms of joint pain. Another initiative focuses on how to implement psoriasis and PsA disease activity measures into the medical record, Dr. Ogdie-Beatty said.
At a combined dermatology-rheumatology clinic within the University Health Network, Toronto, doctors are studying the incidence of arthritis and comorbidities in patients with psoriasis, said Dr. Vinod Chandran, a rheumatologist at the University of Toronto. Another ongoing study focuses on identifying risk factors for PsA in patients with psoriasis. The Toronto clinic is participating in the studies as part of the International Psoriasis and Arthritis Research Team.
“There are a number of research opportunities in the combined clinic since a detailed clinical evaluation is extremely important in biomarker and translational studies,” said Dr. Chandran, who codirects the psoriatic arthritis program at the Centre for Prognosis Studies in the Rheumatic Diseases, Toronto. “The clinic also provides an opportunity to participate in phase II and III clinical trials.”
Managing the combined derm-rheum clinics does come with challenges, doctors said. The obstacles are primarily logistical, and include identifying the space, time, and scheduling for the clinic, Dr. Garg said. Other challenges revolve around reimbursement, such as figuring out the best payment structure and ensuring that insurers will cover the simultaneous care, Dr. Merola said. Generally, it also takes longer to see the patient, and physicians may not be able to see as many patients together per day as they could alone, he added. However, physicians stress that the benefits of the combined clinics far outweigh the negatives.
“We’ve always felt the value has been there because we feel the patients really appreciate the service,” he said. “And we think the quality of care around what we do is better.”
On Twitter@legal_med
At the Rheumatic Skin Disease Clinic in Manhasset, N.Y., dermatologist Dr. Amit Garg regularly discusses patient cases and develops care plans alongside rheumatologists after each specialist separately visits the same patient. The dual dermatology-rheumatology clinic has led to countless benefits, including improved quality of care for patients and multi-discipline training for new and veteran physicians, said Dr. Garg, clinic codirector and chair of the dermatology department at North Shore–Long Island Jewish Health System in New York.
Perhaps the greatest advantage of the combined clinic, however, is the research inspiration generated by the collaboration. “Working closely with colleagues in another discipline makes the environment really ripe for new ideas based on observations we may see in the patients we’re treating,” Dr. Garg said in an interview. “It’s amazing how when you’re thinking through a patient’s care together, how quickly you appreciate where the gaps are in our understanding of disease and evaluation and management of disease. From there, you take it to the next step of planning research projects or protocols to essentially fill those gaps in knowledge.”
Dr. Garg’s clinic is one of a growing number at U.S. academic medical centers that combine dermatologic and rheumatologic care for the management of psoriatic arthritis (PsA) and other interrelated skin and musculoskeletal diseases. Clinic operations vary from virtual consults, to simultaneous assessments, to sequential patient visits, but all focus on collaborative care and disease management. The institutions using such models recently united to form the Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network, a national organization that develops research initiatives and assists other centers in creating similar programs. At least 10 institutions are now participating in the network, including NYU Langone Medical Center, New York; Harvard University’s Brigham and Women’s Hospital, Boston; and the National Psoriasis Foundation, Portland, Ore.
Combining dermatology and rheumatology care through a dual clinic follows an ongoing trend by academic medical centers to integrate specialties that treat overlapping conditions, said Dr. Alexis R. Ogdie-Beatty, a rheumatologist at the University of Pennsylvania Health System in Philadelphia and director of the Penn Psoriatic Arthritis Program.
“It was kind of a natural thing for rheumatic disease because our diseases are multisystem in nature in most cases,” Dr. Ogdie-Beatty said in an interview. “I think the increasing knowledge of the need for communication [between specialists] drove this.”
Dr. Ogdie-Beatty leads a virtual rheumatology-dermatology clinic at the University of Pennsylvania where she meets weekly with two dermatologists to discuss research studies, shared patients, and case management. The physicians primarily see patients separately, but occasionally will visit patients at the same time, she said. In addition, dermatologists on the team immediately contact Dr. Ogdie-Beatty if they are seeing a patient who might benefit from an assessment by the rheumatologist and vice versa, she said.
“One of our favorite parts is learning from each other,” Dr. Ogdie-Beatty said in an interview. “I’ve learned a lot about skin disease and psoriasis, and they’ve learned about inflammatory arthritis and management. The ultimate impact for the patient is to have more well rounded care so that both the skin disease and the arthritis are being taken care of in a comprehensive way.”
Dermatology and rheumatology medical students and residents exposed to such clinics have the unique opportunity to learn about disease overlap and strengthen their understanding of the other’s specialty, added Dr. Joseph F. Merola, codirector of the Center for Skin and Related Musculoskeletal Diseases, a combined clinic at Brigham and Women’s Hospital in Boston. Dr. Merola’s center also has created a fellowship program in which either a dermatologist or rheumatologist can spend 1 year training at the clinic.
Research opportunities abound at the combined clinics, physicians said. At the Center for Skin and Related Musculoskeletal Diseases for example, physicians have developed new outcome metrics for measuring psoriasis and screening for PsA, Dr. Merola said. Physicians also commonly refer clinic patients for clinical trials, he said.
“It’s a great place for patients who have been through many other therapies,” said Dr. Merola, who practices dermatology and rheumatology. “If they are at the end of the line, we have a number of clinical trials that we can offer them. Because it tends to be a referral center, these are patients who have more complex disease, so being able to offer them clinical trials and multiple opinions has been really key.”
Dr. Ogdie-Beatty’s clinic is participating in a variety of research studies, including an analysis of vascular inflammation in psoriasis patients. The study is examining markers of early joint inflammation among psoriasis patients who don’t have symptoms of joint pain. Another initiative focuses on how to implement psoriasis and PsA disease activity measures into the medical record, Dr. Ogdie-Beatty said.
At a combined dermatology-rheumatology clinic within the University Health Network, Toronto, doctors are studying the incidence of arthritis and comorbidities in patients with psoriasis, said Dr. Vinod Chandran, a rheumatologist at the University of Toronto. Another ongoing study focuses on identifying risk factors for PsA in patients with psoriasis. The Toronto clinic is participating in the studies as part of the International Psoriasis and Arthritis Research Team.
“There are a number of research opportunities in the combined clinic since a detailed clinical evaluation is extremely important in biomarker and translational studies,” said Dr. Chandran, who codirects the psoriatic arthritis program at the Centre for Prognosis Studies in the Rheumatic Diseases, Toronto. “The clinic also provides an opportunity to participate in phase II and III clinical trials.”
Managing the combined derm-rheum clinics does come with challenges, doctors said. The obstacles are primarily logistical, and include identifying the space, time, and scheduling for the clinic, Dr. Garg said. Other challenges revolve around reimbursement, such as figuring out the best payment structure and ensuring that insurers will cover the simultaneous care, Dr. Merola said. Generally, it also takes longer to see the patient, and physicians may not be able to see as many patients together per day as they could alone, he added. However, physicians stress that the benefits of the combined clinics far outweigh the negatives.
“We’ve always felt the value has been there because we feel the patients really appreciate the service,” he said. “And we think the quality of care around what we do is better.”
On Twitter@legal_med
Psoriasis severity correlates with aortic vascular inflammation
The severity of psoriasis appears to correlate with the degree of aortic vascular inflammation in patients who have psoriasis, according to a prospective observational study comparing patients with psoriasis with age- and sex-matched controls.
To better characterize the known relationship between psoriatic skin disease and atherosclerosis, they assessed vascular inflammation using 18F-fluorodeoxyglucose–positron emission tomography/computed tomography (FDG-PET/CT) of the aorta in 60 adults with moderate to severe psoriasis and 22 healthy control subjects (mean age, 44 years) during a 2-year period. FDG uptake in the blood vessel wall denotes activated endothelial cells and cellular infiltration in noncalcified atherosclerotic plaques and is a direct, reliable marker of vascular inflammation, said Dr. Haley B. Naik of the cardiovascular and pulmonary branch, National Heart, Lung, and Blood Institute in Bethesda Md., and her associates. The study was published online on Oct. 8 (Arterioscler Thromb Vasc Biol. 2015 Oct 8. doi: 10.1161/ATVBAHA.115.306460).
As the severity of psoriasis increased, as measured by scores on the Psoriasis Area Severity Index (PASI), the degree of vascular inflammation also increased. This association was stronger than would be expected, given the psoriasis patients’ already increased cardiovascular risk based on traditional risk factors (their higher incidence of obesity, dyslipidemia, hypertension, diabetes, and smoking) and their elevated Framingham risk scores and higher levels of C-reactive protein. This suggests that “psoriasis plaques are biologically active in promoting inflammation at remote sites,” the investigators concluded.
In addition, they found that the absolute neutrophil count in the circulation was significantly elevated in psoriasis patients, compared with control subjects (mean, 3.7 vs. 2.9). Immunophenotyping by flow cytometry identified a significant increase in neutrophil frequencies in whole blood (mean, 65.2 vs. 56.3). Protein markers of neutrophil activation also were significantly elevated. Levels of these markers correlated with both psoriasis severity and vascular inflammation, suggesting that the two disorders may share the same immune-mediated mechanism.
“Our observation that the psoriatic atherosclerotic milieu is upregulated in concert with markers of neutrophil activation underscores the concept that atherosclerosis may involve complex neutrophil biology, which future studies should focus on elucidating, especially after intervention,” Dr. Naik and her associates added.
The severity of psoriasis appears to correlate with the degree of aortic vascular inflammation in patients who have psoriasis, according to a prospective observational study comparing patients with psoriasis with age- and sex-matched controls.
To better characterize the known relationship between psoriatic skin disease and atherosclerosis, they assessed vascular inflammation using 18F-fluorodeoxyglucose–positron emission tomography/computed tomography (FDG-PET/CT) of the aorta in 60 adults with moderate to severe psoriasis and 22 healthy control subjects (mean age, 44 years) during a 2-year period. FDG uptake in the blood vessel wall denotes activated endothelial cells and cellular infiltration in noncalcified atherosclerotic plaques and is a direct, reliable marker of vascular inflammation, said Dr. Haley B. Naik of the cardiovascular and pulmonary branch, National Heart, Lung, and Blood Institute in Bethesda Md., and her associates. The study was published online on Oct. 8 (Arterioscler Thromb Vasc Biol. 2015 Oct 8. doi: 10.1161/ATVBAHA.115.306460).
As the severity of psoriasis increased, as measured by scores on the Psoriasis Area Severity Index (PASI), the degree of vascular inflammation also increased. This association was stronger than would be expected, given the psoriasis patients’ already increased cardiovascular risk based on traditional risk factors (their higher incidence of obesity, dyslipidemia, hypertension, diabetes, and smoking) and their elevated Framingham risk scores and higher levels of C-reactive protein. This suggests that “psoriasis plaques are biologically active in promoting inflammation at remote sites,” the investigators concluded.
In addition, they found that the absolute neutrophil count in the circulation was significantly elevated in psoriasis patients, compared with control subjects (mean, 3.7 vs. 2.9). Immunophenotyping by flow cytometry identified a significant increase in neutrophil frequencies in whole blood (mean, 65.2 vs. 56.3). Protein markers of neutrophil activation also were significantly elevated. Levels of these markers correlated with both psoriasis severity and vascular inflammation, suggesting that the two disorders may share the same immune-mediated mechanism.
“Our observation that the psoriatic atherosclerotic milieu is upregulated in concert with markers of neutrophil activation underscores the concept that atherosclerosis may involve complex neutrophil biology, which future studies should focus on elucidating, especially after intervention,” Dr. Naik and her associates added.
The severity of psoriasis appears to correlate with the degree of aortic vascular inflammation in patients who have psoriasis, according to a prospective observational study comparing patients with psoriasis with age- and sex-matched controls.
To better characterize the known relationship between psoriatic skin disease and atherosclerosis, they assessed vascular inflammation using 18F-fluorodeoxyglucose–positron emission tomography/computed tomography (FDG-PET/CT) of the aorta in 60 adults with moderate to severe psoriasis and 22 healthy control subjects (mean age, 44 years) during a 2-year period. FDG uptake in the blood vessel wall denotes activated endothelial cells and cellular infiltration in noncalcified atherosclerotic plaques and is a direct, reliable marker of vascular inflammation, said Dr. Haley B. Naik of the cardiovascular and pulmonary branch, National Heart, Lung, and Blood Institute in Bethesda Md., and her associates. The study was published online on Oct. 8 (Arterioscler Thromb Vasc Biol. 2015 Oct 8. doi: 10.1161/ATVBAHA.115.306460).
As the severity of psoriasis increased, as measured by scores on the Psoriasis Area Severity Index (PASI), the degree of vascular inflammation also increased. This association was stronger than would be expected, given the psoriasis patients’ already increased cardiovascular risk based on traditional risk factors (their higher incidence of obesity, dyslipidemia, hypertension, diabetes, and smoking) and their elevated Framingham risk scores and higher levels of C-reactive protein. This suggests that “psoriasis plaques are biologically active in promoting inflammation at remote sites,” the investigators concluded.
In addition, they found that the absolute neutrophil count in the circulation was significantly elevated in psoriasis patients, compared with control subjects (mean, 3.7 vs. 2.9). Immunophenotyping by flow cytometry identified a significant increase in neutrophil frequencies in whole blood (mean, 65.2 vs. 56.3). Protein markers of neutrophil activation also were significantly elevated. Levels of these markers correlated with both psoriasis severity and vascular inflammation, suggesting that the two disorders may share the same immune-mediated mechanism.
“Our observation that the psoriatic atherosclerotic milieu is upregulated in concert with markers of neutrophil activation underscores the concept that atherosclerosis may involve complex neutrophil biology, which future studies should focus on elucidating, especially after intervention,” Dr. Naik and her associates added.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: The severity of psoriasis correlates with the degree of aortic vascular inflammation.
Major finding: The absolute neutrophil count in the circulation was significantly elevated in psoriasis patients compared with control subjects (mean, 3.7 vs. 2.9), and immunophenotyping by flow cytometry confirmed a significant increase in neutrophil frequencies in whole blood (mean, 65.2 vs. 56.3).
Data source: A prospective cross-sectional observational study compared vascular inflammation in 60 adults with psoriasis and 20 sex- and age-matched control subjects.
Disclosures: This study was supported by the National Heart, Lung, and Blood Institute and the National Psoriasis Foundation. Dr. Naik and her coauthors reported having no relevant financial disclosures.
Tumor Necrosis Factor Inhibitors and Multiple Sclerosis: A Closer Look
With the advent of tumor necrosis factor (TNF) inhibitors, one of the major topics of discussion is the relationship of the drug class with new or worsening multiple sclerosis (MS). Exacerbation of previously quiescent MS and the onset of other demyelinating diseases such as optic neuritis have been reported in patients taking TNF inhibitors. Symptoms included paraesthesia, visual disturbance, and confusion.
Therefore, controversy surrounds the use of TNF-α inhibitors in patients who are more predisposed to developing MS, specifically first-degree relatives of MS patients. The American Academy of Dermatology guidelines for TNF inhibitors state the following: “Because there is an association between anti-TNF therapy and demyelinating diseases (ie, MS), TNF inhibitors should not be used in patients with MS or other demyelinating diseases; first-degree relatives of patients with MS have an increased risk of developing MS, with a sibling relative risk of between 18 and 36, evidence strongly suggests that TNF inhibitors should not be used in first-degree relatives of patients with MS.”
Mansouri et al (J Drugs Dermatol. 2015;14:876-878) presented data suggesting that the number needed to treat is at least an order of magnitude smaller than the number needed to harm across all comparisons of anti–TNF-α agents and first-degree relative relationships. Based on these data, the authors suggest that physicians could weigh the treatment options available and work closely with neurological colleagues when prescribing anti–TNF-α therapy in this patient population, which could be an alternative approach to practicing absolute prohibition of anti–TNF-α agents in patients who have a first-degree relative with MS.
What’s the issue?
This article presents interesting data concerning the risk-benefit of using TNF inhibitors in a potential at-risk population. We are fortunate to have many treatment options available, but in many cases, a TNF inhibitor may be preferable. How will this information affect your use of TNF inhibitors in patients with a first-degree relative with MS?
With the advent of tumor necrosis factor (TNF) inhibitors, one of the major topics of discussion is the relationship of the drug class with new or worsening multiple sclerosis (MS). Exacerbation of previously quiescent MS and the onset of other demyelinating diseases such as optic neuritis have been reported in patients taking TNF inhibitors. Symptoms included paraesthesia, visual disturbance, and confusion.
Therefore, controversy surrounds the use of TNF-α inhibitors in patients who are more predisposed to developing MS, specifically first-degree relatives of MS patients. The American Academy of Dermatology guidelines for TNF inhibitors state the following: “Because there is an association between anti-TNF therapy and demyelinating diseases (ie, MS), TNF inhibitors should not be used in patients with MS or other demyelinating diseases; first-degree relatives of patients with MS have an increased risk of developing MS, with a sibling relative risk of between 18 and 36, evidence strongly suggests that TNF inhibitors should not be used in first-degree relatives of patients with MS.”
Mansouri et al (J Drugs Dermatol. 2015;14:876-878) presented data suggesting that the number needed to treat is at least an order of magnitude smaller than the number needed to harm across all comparisons of anti–TNF-α agents and first-degree relative relationships. Based on these data, the authors suggest that physicians could weigh the treatment options available and work closely with neurological colleagues when prescribing anti–TNF-α therapy in this patient population, which could be an alternative approach to practicing absolute prohibition of anti–TNF-α agents in patients who have a first-degree relative with MS.
What’s the issue?
This article presents interesting data concerning the risk-benefit of using TNF inhibitors in a potential at-risk population. We are fortunate to have many treatment options available, but in many cases, a TNF inhibitor may be preferable. How will this information affect your use of TNF inhibitors in patients with a first-degree relative with MS?
With the advent of tumor necrosis factor (TNF) inhibitors, one of the major topics of discussion is the relationship of the drug class with new or worsening multiple sclerosis (MS). Exacerbation of previously quiescent MS and the onset of other demyelinating diseases such as optic neuritis have been reported in patients taking TNF inhibitors. Symptoms included paraesthesia, visual disturbance, and confusion.
Therefore, controversy surrounds the use of TNF-α inhibitors in patients who are more predisposed to developing MS, specifically first-degree relatives of MS patients. The American Academy of Dermatology guidelines for TNF inhibitors state the following: “Because there is an association between anti-TNF therapy and demyelinating diseases (ie, MS), TNF inhibitors should not be used in patients with MS or other demyelinating diseases; first-degree relatives of patients with MS have an increased risk of developing MS, with a sibling relative risk of between 18 and 36, evidence strongly suggests that TNF inhibitors should not be used in first-degree relatives of patients with MS.”
Mansouri et al (J Drugs Dermatol. 2015;14:876-878) presented data suggesting that the number needed to treat is at least an order of magnitude smaller than the number needed to harm across all comparisons of anti–TNF-α agents and first-degree relative relationships. Based on these data, the authors suggest that physicians could weigh the treatment options available and work closely with neurological colleagues when prescribing anti–TNF-α therapy in this patient population, which could be an alternative approach to practicing absolute prohibition of anti–TNF-α agents in patients who have a first-degree relative with MS.
What’s the issue?
This article presents interesting data concerning the risk-benefit of using TNF inhibitors in a potential at-risk population. We are fortunate to have many treatment options available, but in many cases, a TNF inhibitor may be preferable. How will this information affect your use of TNF inhibitors in patients with a first-degree relative with MS?
PsA confers higher risk of cardiovascular disease, events
Cardiovascular disease and major adverse cardiovascular events are significantly more common in patients with psoriatic arthritis (PsA) than in those without, according to two case-control studies involving individuals in the longitudinal, population-based United Kingdom Clinical Practice Research Datalink.
Dr. Lin Li and associates determined that the incidence of cardiovascular disease (CVD) in patients with PsA was 12.8 per 1,000 person-years, compared with 9.6 per 1,000 person-years in the non-PsA group, giving an incidence rate ratio (IRR) of 1.33. The investigators defined CVD as arrhythmias, ischemic heart disease, angina, myocardial infarction, stroke, pericardial disease, pulmonary hypertension, and sudden death, and only counted cases that had one of these diagnoses during the 1988-2012 study period.
In a separate cohort from the U.K. database, the researchers selected individuals who had a major adverse cardiovascular event (MACE) – myocardial infarctions, strokes, and sudden deaths – since entering the cohort. The MACE incidence in the PsA group was 4.6 per 1,000 person-years and 3.5 per 1,000 person-years in the non-PsA group, yielding an IRR of 1.30.
Rates of CVD and MACE within the PsA group were higher in those who had received a prescription for systemic therapy. Patients who had taken corticosteroids had the highest rates of CVD and MACE, compared with those who had taken disease-modifying antirheumatic drugs, biologics, or immunosuppressants.
“The higher risk in treated patients with PsA compared to non-treated patients may be explained by the severity of the PsA disease: Patients who receive treatments are likely to have more severe disease than those who do not,” the investigators noted.
Find the full study in the Journal of Clinical Rheumatology (2015. doi: 10.1097/RHU.0000000000000306).
Cardiovascular disease and major adverse cardiovascular events are significantly more common in patients with psoriatic arthritis (PsA) than in those without, according to two case-control studies involving individuals in the longitudinal, population-based United Kingdom Clinical Practice Research Datalink.
Dr. Lin Li and associates determined that the incidence of cardiovascular disease (CVD) in patients with PsA was 12.8 per 1,000 person-years, compared with 9.6 per 1,000 person-years in the non-PsA group, giving an incidence rate ratio (IRR) of 1.33. The investigators defined CVD as arrhythmias, ischemic heart disease, angina, myocardial infarction, stroke, pericardial disease, pulmonary hypertension, and sudden death, and only counted cases that had one of these diagnoses during the 1988-2012 study period.
In a separate cohort from the U.K. database, the researchers selected individuals who had a major adverse cardiovascular event (MACE) – myocardial infarctions, strokes, and sudden deaths – since entering the cohort. The MACE incidence in the PsA group was 4.6 per 1,000 person-years and 3.5 per 1,000 person-years in the non-PsA group, yielding an IRR of 1.30.
Rates of CVD and MACE within the PsA group were higher in those who had received a prescription for systemic therapy. Patients who had taken corticosteroids had the highest rates of CVD and MACE, compared with those who had taken disease-modifying antirheumatic drugs, biologics, or immunosuppressants.
“The higher risk in treated patients with PsA compared to non-treated patients may be explained by the severity of the PsA disease: Patients who receive treatments are likely to have more severe disease than those who do not,” the investigators noted.
Find the full study in the Journal of Clinical Rheumatology (2015. doi: 10.1097/RHU.0000000000000306).
Cardiovascular disease and major adverse cardiovascular events are significantly more common in patients with psoriatic arthritis (PsA) than in those without, according to two case-control studies involving individuals in the longitudinal, population-based United Kingdom Clinical Practice Research Datalink.
Dr. Lin Li and associates determined that the incidence of cardiovascular disease (CVD) in patients with PsA was 12.8 per 1,000 person-years, compared with 9.6 per 1,000 person-years in the non-PsA group, giving an incidence rate ratio (IRR) of 1.33. The investigators defined CVD as arrhythmias, ischemic heart disease, angina, myocardial infarction, stroke, pericardial disease, pulmonary hypertension, and sudden death, and only counted cases that had one of these diagnoses during the 1988-2012 study period.
In a separate cohort from the U.K. database, the researchers selected individuals who had a major adverse cardiovascular event (MACE) – myocardial infarctions, strokes, and sudden deaths – since entering the cohort. The MACE incidence in the PsA group was 4.6 per 1,000 person-years and 3.5 per 1,000 person-years in the non-PsA group, yielding an IRR of 1.30.
Rates of CVD and MACE within the PsA group were higher in those who had received a prescription for systemic therapy. Patients who had taken corticosteroids had the highest rates of CVD and MACE, compared with those who had taken disease-modifying antirheumatic drugs, biologics, or immunosuppressants.
“The higher risk in treated patients with PsA compared to non-treated patients may be explained by the severity of the PsA disease: Patients who receive treatments are likely to have more severe disease than those who do not,” the investigators noted.
Find the full study in the Journal of Clinical Rheumatology (2015. doi: 10.1097/RHU.0000000000000306).