User login
Pulmonary arterial hypertension: Promising results for investigational agents and catheter-based denervation
PHILADELPHIA — Promise that the unmet need for more effective pulmonary artery hypertension treatments may soon be met was in strong evidence in research into three strategies presented at this year’s recent American Heart Association scientific sessions; one was based on an ancient Chinese herb epimedium (yin yang huo or horny goat weed) commonly used for treating sexual dysfunction and directly related to the phosphodiesterase inhibitors sildenafil, vardenafil, and tadalafil (sold as Viagra, Levitra, and Cialis). A second studied sotatercept, an investigational, potential first-in-class activin signaling inhibitor biologic, and a third evaluated physically ablating the baroreceptor nerves that stimulate vasoconstriction of the pulmonary artery via catheter-based techniques.
Until as recently as the late 1970s, a pulmonary arterial hypertension diagnosis was a uniformly fatal one.1 While associated with pulmonary and right ventricle remodeling, and leads toward heart failure and death. The complex underlying pathogenesis was divided into six groups by the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, and includes as its most common features pulmonary artery endothelial cell dysfunction, pulmonary artery smooth muscle cell proliferation and migration, and dysregulated fibroblast activity leading to dysregulated vasoconstriction, micro and in-situ vascular thrombosis, vascular fibrosis and pathogenic remodeling of pulmonary vessels.1 The threshold mean arterial pressure (mPAP) for pulmonary arterial hypertension was defined by the 6th [WSPH] at mPAP ≥ 20 mm Hg, twice the upper limit of a normal mPAP of 14.0 ± 3.3 mm Hg as reported by Kovacs et al. in 2018.2
Pathways for current therapies
Current drugs for pulmonary arterial hypertension focus on three signaling pathways, including the endothelin receptor, prostacyclin and nitric oxide pathways, stated Zhi-Cheng Jing, MD, professor of medicine, head of the cardiology department at Peking Union Medical College Hospital, Peking, China. While the phosphodiesterase 5 inhibitors sildenafil and tadalafil, which target the nitric oxide pathway, came into wide use after Food and Drug Administration approval, the need for higher PDE5-selectivity remains, Dr. Jing said. Structurally modified from the active ingredient in epimedium, TPN171H is an investigational PDE5 inhibitor which has shown several favorable features: a greater PDE5 selectivity than both sildenafil and tadalafil in vitro, an ability to decrease right ventricular systolic pressure and alleviate arterial remodeling in animal studies, and safety and tolerability in healthy human subjects.
The current randomized, double-blind, placebo-and active-controlled phase IIa study assessed the hemodynamic impact of a single oral dose of TPN171H in 60 pulmonary arterial hypertension patients (mean age ~34 years, 83.3% female), all with negative vasodilation test results and in WHO class 2 or 3. Only patients aged 18-75 years with group 1 pulmonary arterial hypertension of idiopathic, connective tissue disorder, or repaired congenital heart defects etiology were included. Patients were divided into six groups: placebo, TPN171H at 2.5, 5, and 10 milligrams, and tadalafil at 20 and 40 milligrams.
For the primary endpoint of maximum decrease in pulmonary vascular resistance (PVR), significant reductions vs. placebo were found only for the TPN171H 5-mg group (–41.2% vs. –24.4%; P = .008) and for the 20-mg (–39.8%) and 40-mg (–37.6%) tadalafil groups (both P < .05). What was not seen in the tadalafil groups, but was evident in the TPN171H 5-mg group, was a significant reduction in the secondary endpoint of PVR/SVR (systolic vascular resistance) at 2, 3, and 5 hours (all P < .05). “As we know,” Dr. Jing said in an interview, “the PDE5 inhibitor functions as a vasodilator, having an impact on both pulmonary circulation and systemic circulation. So, to evaluate the selectivity for pulmonary circulation is crucial when exploring a novel drug for pulmonary arterial hypertension. The change of PVR/SVR ratio from baseline is an indicator for selectivity for pulmonary circulation and implies that TPN171H has good PDE5 selectivity in the pulmonary vasculature,” Dr. Jing said.
TPN171H was well tolerated with no serious adverse effects (vomiting 10% and headache 10% were most common with no discontinuations).
TGF-signaling pathway
A meta-analysis of randomized controlled trials of sotatercept, an investigational fusion protein under priority FDA review that modulates the TGF-beta superfamily signaling pathway, looked at PVR, pulmonary arterial pressure (PAP), right arterial pressure (RAP) and N-terminal pro-brain natriuretic peptide (NT-proBNP). A literature search by corresponding author Vamsikalyan Borra, MD, Knapp Medical Center, Weslaco, Texas, and colleagues identified two trials (STELLAR and PULSAR) comprising 429 patients with pulmonary arterial hypertension. The experimental arms (sotatercept) had 237 patients (mean age ~49 years, ~82% female) and the placebo arm had 192 patients (mean age ~47 years, ~80% female).
A pooled analysis showed significant reductions with sotatercept in PVR (standardization mean difference [SMD] = –1.00, 95% confidence interval [CI] = –1.2, –.79, P < .001), PAP (SMD = –1.34, 95% CI = 1.6, –1.08, P < .001), RAP (SMD = –0.66, 95% CI = –0.93, –0.39, P < .001), and the levels of NT-proBNP (SMD = –0.64, 95% CI = –1.01, –0.27, P < .001) at 24 weeks from baseline. The sotatercept safety profile was favorable, with lower overall incidence of adverse events (84.8% vs. 87.5%) and fewer adverse events leading to death (0.4% vs. 3.1%) compared with placebo. Further investigation is needed, however, according to Dr. Borra, into the higher frequency of reported thrombocytopenia (71.7% vs. 20.8%) with sotatercept. “Our findings,” Dr. Borra said in a poster session, “suggest that sotatercept is an effective treatment option for pulmonary arterial hypertension, with the potential to improve both pulmonary and cardiac function.”
Denervation technique
Catheter-based ablation techniques, most commonly using thermal energy, target the afferent and efferent fibers of the baroreceptor reflex in the main pulmonary artery trunk and bifurcation involved in elevated pulmonary artery pressure. Mounica Vorla, MD, Carle Foundation Hospital, Urbana, Illinois, and colleagues conducted an updated systematic review and meta-analysis of the safety and efficacy of pulmonary artery denervation (PADN) for pulmonary arterial hypertension in seven clinical trials with 506 patients with moderate-severe pulmonary arterial hypertension conducted from 2013 to 2022.
Compared with placebo, PADN treatment was associated with a significant reduction in mean pulmonary artery pressure (weighted mean difference [WMD] = –6.9 mm Hg; 95% CI = –9.7, –4.1; P < .01; I2 = 61) and pulmonary vascular resistance (WMD = –3.2; 95% CI = –5.4, –0.9; P = .005). PADN improvements in cardiac output were also statistically significant (WMD = 0.3; 95% CI = 0.07, 0.6; P = .012), with numerical improvement in 6-minute walking distance (WMD = 67.7; 95% CI = –3.73, 139.2; P = .06) in the PADN group. Side effects were less common in the PADN group as compared with the placebo group, Dr. Vorla reported. She concluded, “This updated meta-analysis supports PADN as a safe and efficacious therapy for severe pulmonary arterial hypertension.” The authors noted limitations imposed by the small sample size, large data heterogeneity, and medium-quality literature. Larger randomized, controlled trials with clinical endpoints comparing PADN with optimal medical therapy are needed, they stated.
References
1. Shah AJ et al. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int J Mol Sci. 2023 Mar 19;24(6):5850. doi: 10.3390/ijms24065850. PMID: 36982922; PMCID: PMC10058689.
2. Kovacs G et al. Pulmonary Vascular Involvement in Chronic Obstructive Pulmonary Disease. Is There a Pulmonary Vascular Phenotype? Am J Respir Crit Care Med. 2018 Oct 15;198(8):1000-11. doi: 10.1164/rccm.201801-0095PP. PMID: 29746142.
PHILADELPHIA — Promise that the unmet need for more effective pulmonary artery hypertension treatments may soon be met was in strong evidence in research into three strategies presented at this year’s recent American Heart Association scientific sessions; one was based on an ancient Chinese herb epimedium (yin yang huo or horny goat weed) commonly used for treating sexual dysfunction and directly related to the phosphodiesterase inhibitors sildenafil, vardenafil, and tadalafil (sold as Viagra, Levitra, and Cialis). A second studied sotatercept, an investigational, potential first-in-class activin signaling inhibitor biologic, and a third evaluated physically ablating the baroreceptor nerves that stimulate vasoconstriction of the pulmonary artery via catheter-based techniques.
Until as recently as the late 1970s, a pulmonary arterial hypertension diagnosis was a uniformly fatal one.1 While associated with pulmonary and right ventricle remodeling, and leads toward heart failure and death. The complex underlying pathogenesis was divided into six groups by the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, and includes as its most common features pulmonary artery endothelial cell dysfunction, pulmonary artery smooth muscle cell proliferation and migration, and dysregulated fibroblast activity leading to dysregulated vasoconstriction, micro and in-situ vascular thrombosis, vascular fibrosis and pathogenic remodeling of pulmonary vessels.1 The threshold mean arterial pressure (mPAP) for pulmonary arterial hypertension was defined by the 6th [WSPH] at mPAP ≥ 20 mm Hg, twice the upper limit of a normal mPAP of 14.0 ± 3.3 mm Hg as reported by Kovacs et al. in 2018.2
Pathways for current therapies
Current drugs for pulmonary arterial hypertension focus on three signaling pathways, including the endothelin receptor, prostacyclin and nitric oxide pathways, stated Zhi-Cheng Jing, MD, professor of medicine, head of the cardiology department at Peking Union Medical College Hospital, Peking, China. While the phosphodiesterase 5 inhibitors sildenafil and tadalafil, which target the nitric oxide pathway, came into wide use after Food and Drug Administration approval, the need for higher PDE5-selectivity remains, Dr. Jing said. Structurally modified from the active ingredient in epimedium, TPN171H is an investigational PDE5 inhibitor which has shown several favorable features: a greater PDE5 selectivity than both sildenafil and tadalafil in vitro, an ability to decrease right ventricular systolic pressure and alleviate arterial remodeling in animal studies, and safety and tolerability in healthy human subjects.
The current randomized, double-blind, placebo-and active-controlled phase IIa study assessed the hemodynamic impact of a single oral dose of TPN171H in 60 pulmonary arterial hypertension patients (mean age ~34 years, 83.3% female), all with negative vasodilation test results and in WHO class 2 or 3. Only patients aged 18-75 years with group 1 pulmonary arterial hypertension of idiopathic, connective tissue disorder, or repaired congenital heart defects etiology were included. Patients were divided into six groups: placebo, TPN171H at 2.5, 5, and 10 milligrams, and tadalafil at 20 and 40 milligrams.
For the primary endpoint of maximum decrease in pulmonary vascular resistance (PVR), significant reductions vs. placebo were found only for the TPN171H 5-mg group (–41.2% vs. –24.4%; P = .008) and for the 20-mg (–39.8%) and 40-mg (–37.6%) tadalafil groups (both P < .05). What was not seen in the tadalafil groups, but was evident in the TPN171H 5-mg group, was a significant reduction in the secondary endpoint of PVR/SVR (systolic vascular resistance) at 2, 3, and 5 hours (all P < .05). “As we know,” Dr. Jing said in an interview, “the PDE5 inhibitor functions as a vasodilator, having an impact on both pulmonary circulation and systemic circulation. So, to evaluate the selectivity for pulmonary circulation is crucial when exploring a novel drug for pulmonary arterial hypertension. The change of PVR/SVR ratio from baseline is an indicator for selectivity for pulmonary circulation and implies that TPN171H has good PDE5 selectivity in the pulmonary vasculature,” Dr. Jing said.
TPN171H was well tolerated with no serious adverse effects (vomiting 10% and headache 10% were most common with no discontinuations).
TGF-signaling pathway
A meta-analysis of randomized controlled trials of sotatercept, an investigational fusion protein under priority FDA review that modulates the TGF-beta superfamily signaling pathway, looked at PVR, pulmonary arterial pressure (PAP), right arterial pressure (RAP) and N-terminal pro-brain natriuretic peptide (NT-proBNP). A literature search by corresponding author Vamsikalyan Borra, MD, Knapp Medical Center, Weslaco, Texas, and colleagues identified two trials (STELLAR and PULSAR) comprising 429 patients with pulmonary arterial hypertension. The experimental arms (sotatercept) had 237 patients (mean age ~49 years, ~82% female) and the placebo arm had 192 patients (mean age ~47 years, ~80% female).
A pooled analysis showed significant reductions with sotatercept in PVR (standardization mean difference [SMD] = –1.00, 95% confidence interval [CI] = –1.2, –.79, P < .001), PAP (SMD = –1.34, 95% CI = 1.6, –1.08, P < .001), RAP (SMD = –0.66, 95% CI = –0.93, –0.39, P < .001), and the levels of NT-proBNP (SMD = –0.64, 95% CI = –1.01, –0.27, P < .001) at 24 weeks from baseline. The sotatercept safety profile was favorable, with lower overall incidence of adverse events (84.8% vs. 87.5%) and fewer adverse events leading to death (0.4% vs. 3.1%) compared with placebo. Further investigation is needed, however, according to Dr. Borra, into the higher frequency of reported thrombocytopenia (71.7% vs. 20.8%) with sotatercept. “Our findings,” Dr. Borra said in a poster session, “suggest that sotatercept is an effective treatment option for pulmonary arterial hypertension, with the potential to improve both pulmonary and cardiac function.”
Denervation technique
Catheter-based ablation techniques, most commonly using thermal energy, target the afferent and efferent fibers of the baroreceptor reflex in the main pulmonary artery trunk and bifurcation involved in elevated pulmonary artery pressure. Mounica Vorla, MD, Carle Foundation Hospital, Urbana, Illinois, and colleagues conducted an updated systematic review and meta-analysis of the safety and efficacy of pulmonary artery denervation (PADN) for pulmonary arterial hypertension in seven clinical trials with 506 patients with moderate-severe pulmonary arterial hypertension conducted from 2013 to 2022.
Compared with placebo, PADN treatment was associated with a significant reduction in mean pulmonary artery pressure (weighted mean difference [WMD] = –6.9 mm Hg; 95% CI = –9.7, –4.1; P < .01; I2 = 61) and pulmonary vascular resistance (WMD = –3.2; 95% CI = –5.4, –0.9; P = .005). PADN improvements in cardiac output were also statistically significant (WMD = 0.3; 95% CI = 0.07, 0.6; P = .012), with numerical improvement in 6-minute walking distance (WMD = 67.7; 95% CI = –3.73, 139.2; P = .06) in the PADN group. Side effects were less common in the PADN group as compared with the placebo group, Dr. Vorla reported. She concluded, “This updated meta-analysis supports PADN as a safe and efficacious therapy for severe pulmonary arterial hypertension.” The authors noted limitations imposed by the small sample size, large data heterogeneity, and medium-quality literature. Larger randomized, controlled trials with clinical endpoints comparing PADN with optimal medical therapy are needed, they stated.
References
1. Shah AJ et al. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int J Mol Sci. 2023 Mar 19;24(6):5850. doi: 10.3390/ijms24065850. PMID: 36982922; PMCID: PMC10058689.
2. Kovacs G et al. Pulmonary Vascular Involvement in Chronic Obstructive Pulmonary Disease. Is There a Pulmonary Vascular Phenotype? Am J Respir Crit Care Med. 2018 Oct 15;198(8):1000-11. doi: 10.1164/rccm.201801-0095PP. PMID: 29746142.
PHILADELPHIA — Promise that the unmet need for more effective pulmonary artery hypertension treatments may soon be met was in strong evidence in research into three strategies presented at this year’s recent American Heart Association scientific sessions; one was based on an ancient Chinese herb epimedium (yin yang huo or horny goat weed) commonly used for treating sexual dysfunction and directly related to the phosphodiesterase inhibitors sildenafil, vardenafil, and tadalafil (sold as Viagra, Levitra, and Cialis). A second studied sotatercept, an investigational, potential first-in-class activin signaling inhibitor biologic, and a third evaluated physically ablating the baroreceptor nerves that stimulate vasoconstriction of the pulmonary artery via catheter-based techniques.
Until as recently as the late 1970s, a pulmonary arterial hypertension diagnosis was a uniformly fatal one.1 While associated with pulmonary and right ventricle remodeling, and leads toward heart failure and death. The complex underlying pathogenesis was divided into six groups by the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, and includes as its most common features pulmonary artery endothelial cell dysfunction, pulmonary artery smooth muscle cell proliferation and migration, and dysregulated fibroblast activity leading to dysregulated vasoconstriction, micro and in-situ vascular thrombosis, vascular fibrosis and pathogenic remodeling of pulmonary vessels.1 The threshold mean arterial pressure (mPAP) for pulmonary arterial hypertension was defined by the 6th [WSPH] at mPAP ≥ 20 mm Hg, twice the upper limit of a normal mPAP of 14.0 ± 3.3 mm Hg as reported by Kovacs et al. in 2018.2
Pathways for current therapies
Current drugs for pulmonary arterial hypertension focus on three signaling pathways, including the endothelin receptor, prostacyclin and nitric oxide pathways, stated Zhi-Cheng Jing, MD, professor of medicine, head of the cardiology department at Peking Union Medical College Hospital, Peking, China. While the phosphodiesterase 5 inhibitors sildenafil and tadalafil, which target the nitric oxide pathway, came into wide use after Food and Drug Administration approval, the need for higher PDE5-selectivity remains, Dr. Jing said. Structurally modified from the active ingredient in epimedium, TPN171H is an investigational PDE5 inhibitor which has shown several favorable features: a greater PDE5 selectivity than both sildenafil and tadalafil in vitro, an ability to decrease right ventricular systolic pressure and alleviate arterial remodeling in animal studies, and safety and tolerability in healthy human subjects.
The current randomized, double-blind, placebo-and active-controlled phase IIa study assessed the hemodynamic impact of a single oral dose of TPN171H in 60 pulmonary arterial hypertension patients (mean age ~34 years, 83.3% female), all with negative vasodilation test results and in WHO class 2 or 3. Only patients aged 18-75 years with group 1 pulmonary arterial hypertension of idiopathic, connective tissue disorder, or repaired congenital heart defects etiology were included. Patients were divided into six groups: placebo, TPN171H at 2.5, 5, and 10 milligrams, and tadalafil at 20 and 40 milligrams.
For the primary endpoint of maximum decrease in pulmonary vascular resistance (PVR), significant reductions vs. placebo were found only for the TPN171H 5-mg group (–41.2% vs. –24.4%; P = .008) and for the 20-mg (–39.8%) and 40-mg (–37.6%) tadalafil groups (both P < .05). What was not seen in the tadalafil groups, but was evident in the TPN171H 5-mg group, was a significant reduction in the secondary endpoint of PVR/SVR (systolic vascular resistance) at 2, 3, and 5 hours (all P < .05). “As we know,” Dr. Jing said in an interview, “the PDE5 inhibitor functions as a vasodilator, having an impact on both pulmonary circulation and systemic circulation. So, to evaluate the selectivity for pulmonary circulation is crucial when exploring a novel drug for pulmonary arterial hypertension. The change of PVR/SVR ratio from baseline is an indicator for selectivity for pulmonary circulation and implies that TPN171H has good PDE5 selectivity in the pulmonary vasculature,” Dr. Jing said.
TPN171H was well tolerated with no serious adverse effects (vomiting 10% and headache 10% were most common with no discontinuations).
TGF-signaling pathway
A meta-analysis of randomized controlled trials of sotatercept, an investigational fusion protein under priority FDA review that modulates the TGF-beta superfamily signaling pathway, looked at PVR, pulmonary arterial pressure (PAP), right arterial pressure (RAP) and N-terminal pro-brain natriuretic peptide (NT-proBNP). A literature search by corresponding author Vamsikalyan Borra, MD, Knapp Medical Center, Weslaco, Texas, and colleagues identified two trials (STELLAR and PULSAR) comprising 429 patients with pulmonary arterial hypertension. The experimental arms (sotatercept) had 237 patients (mean age ~49 years, ~82% female) and the placebo arm had 192 patients (mean age ~47 years, ~80% female).
A pooled analysis showed significant reductions with sotatercept in PVR (standardization mean difference [SMD] = –1.00, 95% confidence interval [CI] = –1.2, –.79, P < .001), PAP (SMD = –1.34, 95% CI = 1.6, –1.08, P < .001), RAP (SMD = –0.66, 95% CI = –0.93, –0.39, P < .001), and the levels of NT-proBNP (SMD = –0.64, 95% CI = –1.01, –0.27, P < .001) at 24 weeks from baseline. The sotatercept safety profile was favorable, with lower overall incidence of adverse events (84.8% vs. 87.5%) and fewer adverse events leading to death (0.4% vs. 3.1%) compared with placebo. Further investigation is needed, however, according to Dr. Borra, into the higher frequency of reported thrombocytopenia (71.7% vs. 20.8%) with sotatercept. “Our findings,” Dr. Borra said in a poster session, “suggest that sotatercept is an effective treatment option for pulmonary arterial hypertension, with the potential to improve both pulmonary and cardiac function.”
Denervation technique
Catheter-based ablation techniques, most commonly using thermal energy, target the afferent and efferent fibers of the baroreceptor reflex in the main pulmonary artery trunk and bifurcation involved in elevated pulmonary artery pressure. Mounica Vorla, MD, Carle Foundation Hospital, Urbana, Illinois, and colleagues conducted an updated systematic review and meta-analysis of the safety and efficacy of pulmonary artery denervation (PADN) for pulmonary arterial hypertension in seven clinical trials with 506 patients with moderate-severe pulmonary arterial hypertension conducted from 2013 to 2022.
Compared with placebo, PADN treatment was associated with a significant reduction in mean pulmonary artery pressure (weighted mean difference [WMD] = –6.9 mm Hg; 95% CI = –9.7, –4.1; P < .01; I2 = 61) and pulmonary vascular resistance (WMD = –3.2; 95% CI = –5.4, –0.9; P = .005). PADN improvements in cardiac output were also statistically significant (WMD = 0.3; 95% CI = 0.07, 0.6; P = .012), with numerical improvement in 6-minute walking distance (WMD = 67.7; 95% CI = –3.73, 139.2; P = .06) in the PADN group. Side effects were less common in the PADN group as compared with the placebo group, Dr. Vorla reported. She concluded, “This updated meta-analysis supports PADN as a safe and efficacious therapy for severe pulmonary arterial hypertension.” The authors noted limitations imposed by the small sample size, large data heterogeneity, and medium-quality literature. Larger randomized, controlled trials with clinical endpoints comparing PADN with optimal medical therapy are needed, they stated.
References
1. Shah AJ et al. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int J Mol Sci. 2023 Mar 19;24(6):5850. doi: 10.3390/ijms24065850. PMID: 36982922; PMCID: PMC10058689.
2. Kovacs G et al. Pulmonary Vascular Involvement in Chronic Obstructive Pulmonary Disease. Is There a Pulmonary Vascular Phenotype? Am J Respir Crit Care Med. 2018 Oct 15;198(8):1000-11. doi: 10.1164/rccm.201801-0095PP. PMID: 29746142.
FROM AHA 2023
Smoking alters salivary microbiota in potential path to disease risk
TOPLINE:
Salivary microbiota changes caused by cigarette smoking may affect metabolic pathways and increase disease risk.
METHODOLOGY:
The researchers analyzed health information and data on the composition of salivary microbiota from 1601 adult participants in the Cooperative Health Research in South Tyrol (CHRIS) microbiome study (CHRISMB); CHRIS is an ongoing study in Italy.
The average age of the study population was 45 years; 53% were female, and 45% were current or former smokers.
The researchers hypothesized that changes in salivary microbial composition would be associated with smoking, with more nitrate-reducing bacteria present, and that nitrate reduction pathways would be reduced in smokers.
TAKEAWAY:
The researchers identified 44 genera that differed in the salivary microbiota of current smokers and nonsmokers. In smokers, seven genera in the phylum Proteobacteria were decreased and six in the phylum Actinobacteria were increased compared with nonsmokers; these phyla contain primarily aerobic and anaerobic taxa, respectively.
Some microbiota changes were significantly associated with daily smoking intensity; genera from the classes Betaproteobacteria (Lautropia or Neisseria), Gammaproteobacteria (Cardiobacterium), and Flavobacteriia (Capnocytophaga) decreased significantly with increased grams of tobacco smoked per day, measured in 5-g increments.
Smoking was associated with changes in the salivary microbiota; the nitrate reduction pathway was significantly lower in smokers compared with nonsmokers, and these decreases were consistent with previous studies of decreased cardiovascular events in former smokers.
However, the salivary microbiota of smokers who had quit for at least 5 years resembled that of individuals who had never smoked.
IN PRACTICE:
“Decreased microbial nitrate reduction pathway abundance in smokers may provide an additional explanation for the effect of smoking on cardiovascular and periodontal diseases risk, a hypothesis which should be tested in future studies,” the researchers wrote.
SOURCE:
The lead author of the study was Giacomo Antonello, MD, of Eurac Research, Affiliated Institute of the University of Lübeck, Bolzano, Italy. The study was published online in Scientific Reports (a Nature journal) on November 2, 2023.
LIMITATIONS:
The cross-sectional design and lack of professional assessment of tooth and gum health were limiting factors, as were potential confounding factors including medication use, diet, and alcohol intake.
DISCLOSURES:
The study was supported by the Department of Innovation, Research and University of the Autonomous Province of Bolzano-South Tyrol and by the European Regional Development Fund. The CHRISMB microbiota data generation was funded by the National Institute of Dental and Craniofacial Research. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
Salivary microbiota changes caused by cigarette smoking may affect metabolic pathways and increase disease risk.
METHODOLOGY:
The researchers analyzed health information and data on the composition of salivary microbiota from 1601 adult participants in the Cooperative Health Research in South Tyrol (CHRIS) microbiome study (CHRISMB); CHRIS is an ongoing study in Italy.
The average age of the study population was 45 years; 53% were female, and 45% were current or former smokers.
The researchers hypothesized that changes in salivary microbial composition would be associated with smoking, with more nitrate-reducing bacteria present, and that nitrate reduction pathways would be reduced in smokers.
TAKEAWAY:
The researchers identified 44 genera that differed in the salivary microbiota of current smokers and nonsmokers. In smokers, seven genera in the phylum Proteobacteria were decreased and six in the phylum Actinobacteria were increased compared with nonsmokers; these phyla contain primarily aerobic and anaerobic taxa, respectively.
Some microbiota changes were significantly associated with daily smoking intensity; genera from the classes Betaproteobacteria (Lautropia or Neisseria), Gammaproteobacteria (Cardiobacterium), and Flavobacteriia (Capnocytophaga) decreased significantly with increased grams of tobacco smoked per day, measured in 5-g increments.
Smoking was associated with changes in the salivary microbiota; the nitrate reduction pathway was significantly lower in smokers compared with nonsmokers, and these decreases were consistent with previous studies of decreased cardiovascular events in former smokers.
However, the salivary microbiota of smokers who had quit for at least 5 years resembled that of individuals who had never smoked.
IN PRACTICE:
“Decreased microbial nitrate reduction pathway abundance in smokers may provide an additional explanation for the effect of smoking on cardiovascular and periodontal diseases risk, a hypothesis which should be tested in future studies,” the researchers wrote.
SOURCE:
The lead author of the study was Giacomo Antonello, MD, of Eurac Research, Affiliated Institute of the University of Lübeck, Bolzano, Italy. The study was published online in Scientific Reports (a Nature journal) on November 2, 2023.
LIMITATIONS:
The cross-sectional design and lack of professional assessment of tooth and gum health were limiting factors, as were potential confounding factors including medication use, diet, and alcohol intake.
DISCLOSURES:
The study was supported by the Department of Innovation, Research and University of the Autonomous Province of Bolzano-South Tyrol and by the European Regional Development Fund. The CHRISMB microbiota data generation was funded by the National Institute of Dental and Craniofacial Research. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
Salivary microbiota changes caused by cigarette smoking may affect metabolic pathways and increase disease risk.
METHODOLOGY:
The researchers analyzed health information and data on the composition of salivary microbiota from 1601 adult participants in the Cooperative Health Research in South Tyrol (CHRIS) microbiome study (CHRISMB); CHRIS is an ongoing study in Italy.
The average age of the study population was 45 years; 53% were female, and 45% were current or former smokers.
The researchers hypothesized that changes in salivary microbial composition would be associated with smoking, with more nitrate-reducing bacteria present, and that nitrate reduction pathways would be reduced in smokers.
TAKEAWAY:
The researchers identified 44 genera that differed in the salivary microbiota of current smokers and nonsmokers. In smokers, seven genera in the phylum Proteobacteria were decreased and six in the phylum Actinobacteria were increased compared with nonsmokers; these phyla contain primarily aerobic and anaerobic taxa, respectively.
Some microbiota changes were significantly associated with daily smoking intensity; genera from the classes Betaproteobacteria (Lautropia or Neisseria), Gammaproteobacteria (Cardiobacterium), and Flavobacteriia (Capnocytophaga) decreased significantly with increased grams of tobacco smoked per day, measured in 5-g increments.
Smoking was associated with changes in the salivary microbiota; the nitrate reduction pathway was significantly lower in smokers compared with nonsmokers, and these decreases were consistent with previous studies of decreased cardiovascular events in former smokers.
However, the salivary microbiota of smokers who had quit for at least 5 years resembled that of individuals who had never smoked.
IN PRACTICE:
“Decreased microbial nitrate reduction pathway abundance in smokers may provide an additional explanation for the effect of smoking on cardiovascular and periodontal diseases risk, a hypothesis which should be tested in future studies,” the researchers wrote.
SOURCE:
The lead author of the study was Giacomo Antonello, MD, of Eurac Research, Affiliated Institute of the University of Lübeck, Bolzano, Italy. The study was published online in Scientific Reports (a Nature journal) on November 2, 2023.
LIMITATIONS:
The cross-sectional design and lack of professional assessment of tooth and gum health were limiting factors, as were potential confounding factors including medication use, diet, and alcohol intake.
DISCLOSURES:
The study was supported by the Department of Innovation, Research and University of the Autonomous Province of Bolzano-South Tyrol and by the European Regional Development Fund. The CHRISMB microbiota data generation was funded by the National Institute of Dental and Craniofacial Research. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Discontinuation Schedule of Inhaled Corticosteroids in Patients With Chronic Obstructive Pulmonary Disease
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
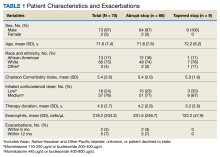
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
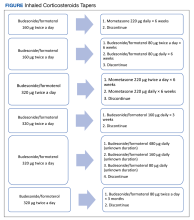
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
Secondhand smoke exposure linked to migraine, severe headache
TOPLINE:
, with effects of exposure varying depending on body mass index (BMI) and level of physical activity, new research shows.
METHODOLOGY:
Investigators analyzed data on 4,560 participants (median age, 43 years; 60% female; 71.5% White) from the 1999-2004 National Health and Nutrition Examination Survey.
Participants were aged 20 years or older and had never smoked.
Migraine headache status was determined by asking whether participants experienced severe headaches or migraines during the previous 3 months.
SHS exposure was categorized as unexposed (serum cotinine levels <0.05 ng/mL and no smoker in the home), low (0.05 ng/mL ≤ serum cotinine level <1 ng/mL), or heavy (1 ng/mL ≤ serum cotinine level ≤ 10 ng/mL).
TAKEAWAY:
In all, 919 (20%) participants had severe headaches or migraines.
After adjustment for demographic and lifestyle factors (including medication use), heavy SHS exposure was positively associated with severe headache or migraine (adjusted odds ratio [aOR], 2.02; 95% CI, 1.19-3.43).
No significant association was found between low SHS exposure and severe headaches or migraine (aOR, 1.15; 95% CI, 0.91-1.47).
In participants who were sedentary (P=.016) and those with a BMI <25 (P=.001), significant associations between SHS and severe headache or migraine were observed.
IN PRACTICE:
Noting a linear dose-response relationship between cotinine and severe headaches or migraine, the investigators write, “These findings underscore the need for stronger regulation of tobacco exposure, particularly in homes and public places.”
SOURCE:
Junpeng Wu, MMc, and Haitang Wang, MD, of Southern Medical University in Guangzhou, China, and their colleagues conducted the study. It was published online in Headache.
LIMITATIONS:
The study could not establish causal relationships between SHS and migraine or severe headache. In addition, the half-life of serum cotinine is 15-40 hours and thus this measure can reflect only recent SHS exposure.
DISCLOSURES:
The study was not funded. The investigators reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
, with effects of exposure varying depending on body mass index (BMI) and level of physical activity, new research shows.
METHODOLOGY:
Investigators analyzed data on 4,560 participants (median age, 43 years; 60% female; 71.5% White) from the 1999-2004 National Health and Nutrition Examination Survey.
Participants were aged 20 years or older and had never smoked.
Migraine headache status was determined by asking whether participants experienced severe headaches or migraines during the previous 3 months.
SHS exposure was categorized as unexposed (serum cotinine levels <0.05 ng/mL and no smoker in the home), low (0.05 ng/mL ≤ serum cotinine level <1 ng/mL), or heavy (1 ng/mL ≤ serum cotinine level ≤ 10 ng/mL).
TAKEAWAY:
In all, 919 (20%) participants had severe headaches or migraines.
After adjustment for demographic and lifestyle factors (including medication use), heavy SHS exposure was positively associated with severe headache or migraine (adjusted odds ratio [aOR], 2.02; 95% CI, 1.19-3.43).
No significant association was found between low SHS exposure and severe headaches or migraine (aOR, 1.15; 95% CI, 0.91-1.47).
In participants who were sedentary (P=.016) and those with a BMI <25 (P=.001), significant associations between SHS and severe headache or migraine were observed.
IN PRACTICE:
Noting a linear dose-response relationship between cotinine and severe headaches or migraine, the investigators write, “These findings underscore the need for stronger regulation of tobacco exposure, particularly in homes and public places.”
SOURCE:
Junpeng Wu, MMc, and Haitang Wang, MD, of Southern Medical University in Guangzhou, China, and their colleagues conducted the study. It was published online in Headache.
LIMITATIONS:
The study could not establish causal relationships between SHS and migraine or severe headache. In addition, the half-life of serum cotinine is 15-40 hours and thus this measure can reflect only recent SHS exposure.
DISCLOSURES:
The study was not funded. The investigators reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
, with effects of exposure varying depending on body mass index (BMI) and level of physical activity, new research shows.
METHODOLOGY:
Investigators analyzed data on 4,560 participants (median age, 43 years; 60% female; 71.5% White) from the 1999-2004 National Health and Nutrition Examination Survey.
Participants were aged 20 years or older and had never smoked.
Migraine headache status was determined by asking whether participants experienced severe headaches or migraines during the previous 3 months.
SHS exposure was categorized as unexposed (serum cotinine levels <0.05 ng/mL and no smoker in the home), low (0.05 ng/mL ≤ serum cotinine level <1 ng/mL), or heavy (1 ng/mL ≤ serum cotinine level ≤ 10 ng/mL).
TAKEAWAY:
In all, 919 (20%) participants had severe headaches or migraines.
After adjustment for demographic and lifestyle factors (including medication use), heavy SHS exposure was positively associated with severe headache or migraine (adjusted odds ratio [aOR], 2.02; 95% CI, 1.19-3.43).
No significant association was found between low SHS exposure and severe headaches or migraine (aOR, 1.15; 95% CI, 0.91-1.47).
In participants who were sedentary (P=.016) and those with a BMI <25 (P=.001), significant associations between SHS and severe headache or migraine were observed.
IN PRACTICE:
Noting a linear dose-response relationship between cotinine and severe headaches or migraine, the investigators write, “These findings underscore the need for stronger regulation of tobacco exposure, particularly in homes and public places.”
SOURCE:
Junpeng Wu, MMc, and Haitang Wang, MD, of Southern Medical University in Guangzhou, China, and their colleagues conducted the study. It was published online in Headache.
LIMITATIONS:
The study could not establish causal relationships between SHS and migraine or severe headache. In addition, the half-life of serum cotinine is 15-40 hours and thus this measure can reflect only recent SHS exposure.
DISCLOSURES:
The study was not funded. The investigators reported no disclosures.
A version of this article appeared on Medscape.com.
All-oral regimen succeeds for rifampin-resistant tuberculosis
A combination oral-only therapy of bedaquiline, pretomanid, and linezolid was significantly more effective than standard care in preventing unfavorable outcomes in patients with treatment-resistant tuberculosis, based on data from more than 500 individuals.
, and data on the use of new and repurposed drug combinations are lacking, wrote Bern-Thomas Nyang’wa, MBBS, of Médecins Sans Frontières, Amsterdam, and colleagues.
In a study known as the TB-PRACTECAL trial, the researchers enrolled 552 pulmonary rifampin-resistant tuberculosis patients aged 15 years and older to examine several new and repurposed drug combinations. The participants were randomized in a 1:1:1:1 ratio to treatment with 36-80 weeks of standard care; 24-week oral bedaquiline, pretomanid, and linezolid (BPaL); BPaL plus clofazimine (BPaLC); or BPaL plus moxifloxacin (BPaLM) . This was followed by stage two of the trial, in which participants were randomized 1:1 to receive standard care or BPaLM. The current study, published in The Lancet Respiratory Medicine, reported the stage two findings; the primary outcome was a composite of unfavorable outcomes at 72 weeks including death, treatment failure, treatment discontinuation, recurrence of tuberculosis, or loss to follow-up.
The modified intent-to-treat population included 138 patients in the BPaLM group and 137 patients in the standard care group. In this population, 56 (41%) of 137 participants in the standard care group and 16 (12%) of 137 participants in the BPaLM group met criteria for the unfavorable outcome at 72 weeks; noninferiority and superiority were significantly greater in the BPaLM group (P < .0001).
Early discontinuation was the main reason patients met the unfavorable outcome criteria (89% of standard care patients and 69% of BPaLM patients); adverse events accounted for 23% of discontinuations in the standard care group and 64% of discontinuations in the BPaLM group.
However, fewer patients in the BPaLM group experienced grade 3 or higher adverse events compared with the standard care group (23% vs. 48%). The most common adverse events included hepatic disorders, cardiac disorders, and anemia.
In addition, all subgroup analyses favored BPaLM over standard care at 72 weeks including subgroups based on sex, age, disease severity, re-treatment status, and smoking status.
The findings were limited by several factors including the changes to standard of care over the course of the study, potential bias because the study was stopped for efficacy, and inclusion of loss to follow-up as part of the composite unfavorable outcome, the researchers noted.
Remaining research questions include the optimal dose of linezolid, whether use of alternative fluoroquinolones would yield similar results, and whether the results would generalize to populations including children, pregnant women, and patients with extrapulmonary tuberculosis, they added.
However, the results support BPaLM as the preferred treatment for adults and adolescents with pulmonary rifampin-resistant TB, the researchers concluded.
BPaLM poised to improve TB care
Before 2020, treatment for rifampin-resistant tuberculosis was 9-20 months in duration, toxic, and inadequately effective, and new treatment regimens are urgently needed, Mary Jo Farmer, MD, a pulmonary and critical care specialist at the University of Massachusetts Baystate Health Regional Campus, Springfield, said in an interview.
“The BPaL-based regimens perform better than the 9- to 20-month standard of care, are shorter in duration, have a lower pill burden, improve quality of life, and are cost-effective,” she said. “The BPaL regimens have the potential to improve outcomes for thousands of patients with rifampin-resistant tuberculosis.”
“The 24-week oral regimen consisting of bedaquiline, pretomanid, linezolid and moxifloxacin is noninferior to standard of care for treatment of patients with pulmonary rifampin-resistant tuberculosis, and this BPaLM regimen was added to the WHO guidance for treatment of this condition in 2022,” said Dr. Farmer, who was not involved in the study. “It remains to be seen if BPaLM will become the preferred regimen for adolescents and adults with pulmonary rifampin-resistant tuberculosis,” she said.
Dr. Farmer agreed with the study authors that the optimal dose of linezolid, optimal duration of treatment, and the role of dose reduction remain unknown, and pharmacokinetic studies are needed to identify these parameters.
The study was supported by Médecins Sans Frontières. TB Alliance donated pretomanid to the study prior to its commercialization. The researchers had no financial conflicts to disclose. Dr. Farmer had no financial conflicts to disclose, but serves on the editorial advisory board of CHEST Physician.
A combination oral-only therapy of bedaquiline, pretomanid, and linezolid was significantly more effective than standard care in preventing unfavorable outcomes in patients with treatment-resistant tuberculosis, based on data from more than 500 individuals.
, and data on the use of new and repurposed drug combinations are lacking, wrote Bern-Thomas Nyang’wa, MBBS, of Médecins Sans Frontières, Amsterdam, and colleagues.
In a study known as the TB-PRACTECAL trial, the researchers enrolled 552 pulmonary rifampin-resistant tuberculosis patients aged 15 years and older to examine several new and repurposed drug combinations. The participants were randomized in a 1:1:1:1 ratio to treatment with 36-80 weeks of standard care; 24-week oral bedaquiline, pretomanid, and linezolid (BPaL); BPaL plus clofazimine (BPaLC); or BPaL plus moxifloxacin (BPaLM) . This was followed by stage two of the trial, in which participants were randomized 1:1 to receive standard care or BPaLM. The current study, published in The Lancet Respiratory Medicine, reported the stage two findings; the primary outcome was a composite of unfavorable outcomes at 72 weeks including death, treatment failure, treatment discontinuation, recurrence of tuberculosis, or loss to follow-up.
The modified intent-to-treat population included 138 patients in the BPaLM group and 137 patients in the standard care group. In this population, 56 (41%) of 137 participants in the standard care group and 16 (12%) of 137 participants in the BPaLM group met criteria for the unfavorable outcome at 72 weeks; noninferiority and superiority were significantly greater in the BPaLM group (P < .0001).
Early discontinuation was the main reason patients met the unfavorable outcome criteria (89% of standard care patients and 69% of BPaLM patients); adverse events accounted for 23% of discontinuations in the standard care group and 64% of discontinuations in the BPaLM group.
However, fewer patients in the BPaLM group experienced grade 3 or higher adverse events compared with the standard care group (23% vs. 48%). The most common adverse events included hepatic disorders, cardiac disorders, and anemia.
In addition, all subgroup analyses favored BPaLM over standard care at 72 weeks including subgroups based on sex, age, disease severity, re-treatment status, and smoking status.
The findings were limited by several factors including the changes to standard of care over the course of the study, potential bias because the study was stopped for efficacy, and inclusion of loss to follow-up as part of the composite unfavorable outcome, the researchers noted.
Remaining research questions include the optimal dose of linezolid, whether use of alternative fluoroquinolones would yield similar results, and whether the results would generalize to populations including children, pregnant women, and patients with extrapulmonary tuberculosis, they added.
However, the results support BPaLM as the preferred treatment for adults and adolescents with pulmonary rifampin-resistant TB, the researchers concluded.
BPaLM poised to improve TB care
Before 2020, treatment for rifampin-resistant tuberculosis was 9-20 months in duration, toxic, and inadequately effective, and new treatment regimens are urgently needed, Mary Jo Farmer, MD, a pulmonary and critical care specialist at the University of Massachusetts Baystate Health Regional Campus, Springfield, said in an interview.
“The BPaL-based regimens perform better than the 9- to 20-month standard of care, are shorter in duration, have a lower pill burden, improve quality of life, and are cost-effective,” she said. “The BPaL regimens have the potential to improve outcomes for thousands of patients with rifampin-resistant tuberculosis.”
“The 24-week oral regimen consisting of bedaquiline, pretomanid, linezolid and moxifloxacin is noninferior to standard of care for treatment of patients with pulmonary rifampin-resistant tuberculosis, and this BPaLM regimen was added to the WHO guidance for treatment of this condition in 2022,” said Dr. Farmer, who was not involved in the study. “It remains to be seen if BPaLM will become the preferred regimen for adolescents and adults with pulmonary rifampin-resistant tuberculosis,” she said.
Dr. Farmer agreed with the study authors that the optimal dose of linezolid, optimal duration of treatment, and the role of dose reduction remain unknown, and pharmacokinetic studies are needed to identify these parameters.
The study was supported by Médecins Sans Frontières. TB Alliance donated pretomanid to the study prior to its commercialization. The researchers had no financial conflicts to disclose. Dr. Farmer had no financial conflicts to disclose, but serves on the editorial advisory board of CHEST Physician.
A combination oral-only therapy of bedaquiline, pretomanid, and linezolid was significantly more effective than standard care in preventing unfavorable outcomes in patients with treatment-resistant tuberculosis, based on data from more than 500 individuals.
, and data on the use of new and repurposed drug combinations are lacking, wrote Bern-Thomas Nyang’wa, MBBS, of Médecins Sans Frontières, Amsterdam, and colleagues.
In a study known as the TB-PRACTECAL trial, the researchers enrolled 552 pulmonary rifampin-resistant tuberculosis patients aged 15 years and older to examine several new and repurposed drug combinations. The participants were randomized in a 1:1:1:1 ratio to treatment with 36-80 weeks of standard care; 24-week oral bedaquiline, pretomanid, and linezolid (BPaL); BPaL plus clofazimine (BPaLC); or BPaL plus moxifloxacin (BPaLM) . This was followed by stage two of the trial, in which participants were randomized 1:1 to receive standard care or BPaLM. The current study, published in The Lancet Respiratory Medicine, reported the stage two findings; the primary outcome was a composite of unfavorable outcomes at 72 weeks including death, treatment failure, treatment discontinuation, recurrence of tuberculosis, or loss to follow-up.
The modified intent-to-treat population included 138 patients in the BPaLM group and 137 patients in the standard care group. In this population, 56 (41%) of 137 participants in the standard care group and 16 (12%) of 137 participants in the BPaLM group met criteria for the unfavorable outcome at 72 weeks; noninferiority and superiority were significantly greater in the BPaLM group (P < .0001).
Early discontinuation was the main reason patients met the unfavorable outcome criteria (89% of standard care patients and 69% of BPaLM patients); adverse events accounted for 23% of discontinuations in the standard care group and 64% of discontinuations in the BPaLM group.
However, fewer patients in the BPaLM group experienced grade 3 or higher adverse events compared with the standard care group (23% vs. 48%). The most common adverse events included hepatic disorders, cardiac disorders, and anemia.
In addition, all subgroup analyses favored BPaLM over standard care at 72 weeks including subgroups based on sex, age, disease severity, re-treatment status, and smoking status.
The findings were limited by several factors including the changes to standard of care over the course of the study, potential bias because the study was stopped for efficacy, and inclusion of loss to follow-up as part of the composite unfavorable outcome, the researchers noted.
Remaining research questions include the optimal dose of linezolid, whether use of alternative fluoroquinolones would yield similar results, and whether the results would generalize to populations including children, pregnant women, and patients with extrapulmonary tuberculosis, they added.
However, the results support BPaLM as the preferred treatment for adults and adolescents with pulmonary rifampin-resistant TB, the researchers concluded.
BPaLM poised to improve TB care
Before 2020, treatment for rifampin-resistant tuberculosis was 9-20 months in duration, toxic, and inadequately effective, and new treatment regimens are urgently needed, Mary Jo Farmer, MD, a pulmonary and critical care specialist at the University of Massachusetts Baystate Health Regional Campus, Springfield, said in an interview.
“The BPaL-based regimens perform better than the 9- to 20-month standard of care, are shorter in duration, have a lower pill burden, improve quality of life, and are cost-effective,” she said. “The BPaL regimens have the potential to improve outcomes for thousands of patients with rifampin-resistant tuberculosis.”
“The 24-week oral regimen consisting of bedaquiline, pretomanid, linezolid and moxifloxacin is noninferior to standard of care for treatment of patients with pulmonary rifampin-resistant tuberculosis, and this BPaLM regimen was added to the WHO guidance for treatment of this condition in 2022,” said Dr. Farmer, who was not involved in the study. “It remains to be seen if BPaLM will become the preferred regimen for adolescents and adults with pulmonary rifampin-resistant tuberculosis,” she said.
Dr. Farmer agreed with the study authors that the optimal dose of linezolid, optimal duration of treatment, and the role of dose reduction remain unknown, and pharmacokinetic studies are needed to identify these parameters.
The study was supported by Médecins Sans Frontières. TB Alliance donated pretomanid to the study prior to its commercialization. The researchers had no financial conflicts to disclose. Dr. Farmer had no financial conflicts to disclose, but serves on the editorial advisory board of CHEST Physician.
FROM LANCET RESPIRATORY MEDICINE
Is air filtration the best public health intervention against respiratory viruses?
This transcript has been edited for clarity.
When it comes to the public health fight against respiratory viruses – COVID, flu, RSV, and so on – it has always struck me as strange how staunchly basically any intervention is opposed. Masking was, of course, the prototypical entrenched warfare of opposing ideologies, with advocates pointing to studies suggesting the efficacy of masking to prevent transmission and advocating for broad masking recommendations, and detractors citing studies that suggested masks were ineffective and characterizing masking policies as fascist overreach. I’ll admit that I was always perplexed by this a bit, as that particular intervention seemed so benign – a bit annoying, I guess, but not crazy.
I have come to appreciate what I call status quo bias, which is the tendency to reject any policy, advice, or intervention that would force you, as an individual, to change your usual behavior. We just don’t like to do that. It has made me think that the most successful public health interventions might be the ones that take the individual out of the loop. And air quality control seems an ideal fit here. Here is a potential intervention where you, the individual, have to do precisely nothing. The status quo is preserved. We just, you know, have cleaner indoor air.
But even the suggestion of air treatment systems as a bulwark against respiratory virus transmission has been met with not just skepticism but cynicism, and perhaps even defeatism. It seems that there are those out there who think there really is nothing we can do. Sickness is interpreted in a Calvinistic framework: You become ill because it is your pre-destiny. But maybe air treatment could actually work. It seems like it might, if a new paper from PLOS One is to be believed.
What we’re talking about is a study titled “Bipolar Ionization Rapidly Inactivates Real-World, Airborne Concentrations of Infective Respiratory Viruses” – a highly controlled, laboratory-based analysis of a bipolar ionization system which seems to rapidly reduce viral counts in the air.
The proposed mechanism of action is pretty simple. The ionization system – which, don’t worry, has been shown not to produce ozone – spits out positively and negatively charged particles, which float around the test chamber, designed to look like a pretty standard room that you might find in an office or a school.
Virus is then injected into the chamber through an aerosolization machine, to achieve concentrations on the order of what you might get standing within 6 feet or so of someone actively infected with COVID while they are breathing and talking.
The idea is that those ions stick to the virus particles, similar to how a balloon sticks to the wall after you rub it on your hair, and that tends to cause them to clump together and settle on surfaces more rapidly, and thus get farther away from their ports of entry to the human system: nose, mouth, and eyes. But the ions may also interfere with viruses’ ability to bind to cellular receptors, even in the air.
To quantify viral infectivity, the researchers used a biological system. Basically, you take air samples and expose a petri dish of cells to them and see how many cells die. Fewer cells dying, less infective. Under control conditions, you can see that virus infectivity does decrease over time. Time zero here is the end of a SARS-CoV-2 aerosolization.
This may simply reflect the fact that virus particles settle out of the air. But As you can see, within about an hour, you have almost no infective virus detectable. That’s fairly impressive.
Now, I’m not saying that this is a panacea, but it is certainly worth considering the use of technologies like these if we are going to revamp the infrastructure of our offices and schools. And, of course, it would be nice to see this tested in a rigorous clinical trial with actual infected people, not cells, as the outcome. But I continue to be encouraged by interventions like this which, to be honest, ask very little of us as individuals. Maybe it’s time we accept the things, or people, that we cannot change.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the public health fight against respiratory viruses – COVID, flu, RSV, and so on – it has always struck me as strange how staunchly basically any intervention is opposed. Masking was, of course, the prototypical entrenched warfare of opposing ideologies, with advocates pointing to studies suggesting the efficacy of masking to prevent transmission and advocating for broad masking recommendations, and detractors citing studies that suggested masks were ineffective and characterizing masking policies as fascist overreach. I’ll admit that I was always perplexed by this a bit, as that particular intervention seemed so benign – a bit annoying, I guess, but not crazy.
I have come to appreciate what I call status quo bias, which is the tendency to reject any policy, advice, or intervention that would force you, as an individual, to change your usual behavior. We just don’t like to do that. It has made me think that the most successful public health interventions might be the ones that take the individual out of the loop. And air quality control seems an ideal fit here. Here is a potential intervention where you, the individual, have to do precisely nothing. The status quo is preserved. We just, you know, have cleaner indoor air.
But even the suggestion of air treatment systems as a bulwark against respiratory virus transmission has been met with not just skepticism but cynicism, and perhaps even defeatism. It seems that there are those out there who think there really is nothing we can do. Sickness is interpreted in a Calvinistic framework: You become ill because it is your pre-destiny. But maybe air treatment could actually work. It seems like it might, if a new paper from PLOS One is to be believed.
What we’re talking about is a study titled “Bipolar Ionization Rapidly Inactivates Real-World, Airborne Concentrations of Infective Respiratory Viruses” – a highly controlled, laboratory-based analysis of a bipolar ionization system which seems to rapidly reduce viral counts in the air.
The proposed mechanism of action is pretty simple. The ionization system – which, don’t worry, has been shown not to produce ozone – spits out positively and negatively charged particles, which float around the test chamber, designed to look like a pretty standard room that you might find in an office or a school.
Virus is then injected into the chamber through an aerosolization machine, to achieve concentrations on the order of what you might get standing within 6 feet or so of someone actively infected with COVID while they are breathing and talking.
The idea is that those ions stick to the virus particles, similar to how a balloon sticks to the wall after you rub it on your hair, and that tends to cause them to clump together and settle on surfaces more rapidly, and thus get farther away from their ports of entry to the human system: nose, mouth, and eyes. But the ions may also interfere with viruses’ ability to bind to cellular receptors, even in the air.
To quantify viral infectivity, the researchers used a biological system. Basically, you take air samples and expose a petri dish of cells to them and see how many cells die. Fewer cells dying, less infective. Under control conditions, you can see that virus infectivity does decrease over time. Time zero here is the end of a SARS-CoV-2 aerosolization.
This may simply reflect the fact that virus particles settle out of the air. But As you can see, within about an hour, you have almost no infective virus detectable. That’s fairly impressive.
Now, I’m not saying that this is a panacea, but it is certainly worth considering the use of technologies like these if we are going to revamp the infrastructure of our offices and schools. And, of course, it would be nice to see this tested in a rigorous clinical trial with actual infected people, not cells, as the outcome. But I continue to be encouraged by interventions like this which, to be honest, ask very little of us as individuals. Maybe it’s time we accept the things, or people, that we cannot change.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the public health fight against respiratory viruses – COVID, flu, RSV, and so on – it has always struck me as strange how staunchly basically any intervention is opposed. Masking was, of course, the prototypical entrenched warfare of opposing ideologies, with advocates pointing to studies suggesting the efficacy of masking to prevent transmission and advocating for broad masking recommendations, and detractors citing studies that suggested masks were ineffective and characterizing masking policies as fascist overreach. I’ll admit that I was always perplexed by this a bit, as that particular intervention seemed so benign – a bit annoying, I guess, but not crazy.
I have come to appreciate what I call status quo bias, which is the tendency to reject any policy, advice, or intervention that would force you, as an individual, to change your usual behavior. We just don’t like to do that. It has made me think that the most successful public health interventions might be the ones that take the individual out of the loop. And air quality control seems an ideal fit here. Here is a potential intervention where you, the individual, have to do precisely nothing. The status quo is preserved. We just, you know, have cleaner indoor air.
But even the suggestion of air treatment systems as a bulwark against respiratory virus transmission has been met with not just skepticism but cynicism, and perhaps even defeatism. It seems that there are those out there who think there really is nothing we can do. Sickness is interpreted in a Calvinistic framework: You become ill because it is your pre-destiny. But maybe air treatment could actually work. It seems like it might, if a new paper from PLOS One is to be believed.
What we’re talking about is a study titled “Bipolar Ionization Rapidly Inactivates Real-World, Airborne Concentrations of Infective Respiratory Viruses” – a highly controlled, laboratory-based analysis of a bipolar ionization system which seems to rapidly reduce viral counts in the air.
The proposed mechanism of action is pretty simple. The ionization system – which, don’t worry, has been shown not to produce ozone – spits out positively and negatively charged particles, which float around the test chamber, designed to look like a pretty standard room that you might find in an office or a school.
Virus is then injected into the chamber through an aerosolization machine, to achieve concentrations on the order of what you might get standing within 6 feet or so of someone actively infected with COVID while they are breathing and talking.
The idea is that those ions stick to the virus particles, similar to how a balloon sticks to the wall after you rub it on your hair, and that tends to cause them to clump together and settle on surfaces more rapidly, and thus get farther away from their ports of entry to the human system: nose, mouth, and eyes. But the ions may also interfere with viruses’ ability to bind to cellular receptors, even in the air.
To quantify viral infectivity, the researchers used a biological system. Basically, you take air samples and expose a petri dish of cells to them and see how many cells die. Fewer cells dying, less infective. Under control conditions, you can see that virus infectivity does decrease over time. Time zero here is the end of a SARS-CoV-2 aerosolization.
This may simply reflect the fact that virus particles settle out of the air. But As you can see, within about an hour, you have almost no infective virus detectable. That’s fairly impressive.
Now, I’m not saying that this is a panacea, but it is certainly worth considering the use of technologies like these if we are going to revamp the infrastructure of our offices and schools. And, of course, it would be nice to see this tested in a rigorous clinical trial with actual infected people, not cells, as the outcome. But I continue to be encouraged by interventions like this which, to be honest, ask very little of us as individuals. Maybe it’s time we accept the things, or people, that we cannot change.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Quitting tobacco can improve lung health in COPD
Reducing exposure to tobacco smoke may reduce the burden of chronic obstructive pulmonary disease, and public health measures are needed, according to a new Tobacco Knowledge Summary from the World Health Organization.
“Smoking is a major risk factor for COPD and leads to airway inflammation and remodeling associated with lung destruction,” and contributes to approximately 70% of COPD cases worldwide, according to the statement.
Types of tobacco exposure include not only traditional smoked tobacco products (cigarettes, cigars, pipes, water pipes, kreteks, and bidis), but also smokeless tobacco, heated tobacco products, and electronic nicotine delivery systems; the addition of chemicals and flavors can increase the appeal of tobacco products and promote addiction, the authors wrote. Hookahs and water pipes “are at least as detrimental to lung health as smoking cigarettes and should not be considered as a safe alternative,” they added.
The risk of COPD extends to new e-cigarette products, the authors noted. A study in the American Journal of Preventive Medicine showed that current users of e-cigarettes had a 75% increased risk of developing COPD compared with individuals who have never used e-cigarettes.
Individuals with COPD also face an increased risk of cardiovascular disease and type 2 diabetes, and smokers with COPD who quit not only improve their COPD but also reduce their risk of developing these conditions, the authors said.
Mechanism of action explored
The authors noted how tobacco smoking may cause COPD when inhaled particles are deposited through the airway.
Growing evidence suggests that extracellular vesicles may play a role in the development of lung disorders such as COPD, and cigarette smoke can have an impact through this channel. A study published in the American Journal of Respiratory and Critical Care Medicine offered evidence of a potential link between exposure to cigarette smoke and the generation of a unique extracellular vesicle population that could promote the development of lung damage. In the study, Matthew C. Madison, MD, of the University of Alabama, Birmingham, and colleagues examined activity in extracellular vesicles from the bronchoalveolar lavage (BAL) fluid of smoke-exposed mice and human smokers who were otherwise healthy.
The researchers found that airway extracellular vesicles in mice or humans exposed to cigarette smoke had the ability to cause rapid lung damage when transferred into naive recipient mice. The results provide a new model that can inform preclinical COPD research, they wrote.
Public health action needed
“In recognition of COPD and Lung Cancer Awareness Month, the World Health Organization (WHO) emphasizes the impact of various forms of tobacco use on COPD,” Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
“This article focuses on the different types of tobacco exposure, the health care burden associated with COPD, and the risk of developing lung cancer. It also addresses the high-risk groups, especially youth, underscoring the importance of public education and the implementation of restrictions on tobacco use to combat these growing concerns,” she said.
“Education, awareness, and targeted interventions are essential for smoking cessation and COPD management,” said Dr. Narendra. “These elements are key to informing the public about smoking risks, encouraging behavioral change, and ultimately reducing the incidence of smoking-related diseases,” she emphasized.
The WHO statement called for population-level interventions including brief advice to tobacco users, toll-free quit lines, pharmacological interventions, use of messaging and chatbots to provide quit support, and the WHO quit tobacco mobile app.
“It is imperative that all tobacco users, particularly those living in low- to middle-income countries, have access to comprehensive cessation support aligned with WHO recommendations,” the authors wrote.
Finally, the authors emphasized the need to protect children and teens from the dangers of tobacco use through product regulation and to expose the tobacco industry’s marketing tactics.
“The article offers a comprehensive look at different types of tobacco exposure and their contribution to the development of COPD,” Dr. Narendra told this news organization. “Notably, it presents groundbreaking evidence of a strong association between the use of electronic nicotine delivery systems (ENDS) and heated tobacco products to development of COPD; additionally, it provides valuable guidance on smoking cessation resources for physicians to help patients quit smoking,” she said.
Looking ahead, more research is needed on “developing and sustaining state-specific or population-specific interventions for effective smoking cessation programs, and reducing the burden of COPD,” Dr. Narendra said.
The study by Madison and colleagues was supported by the National Heart, Lung, and Blood Institute, the National Institute of General Medical Science, the U.S. Veterans Affairs Administration, the Cystic Fibrosis Foundation Research Development Program, and the Veterans Affairs Merit grant.
Additional financial support came from Imperial College London, a Wellcome Trust Senior Research Fellowship, and Rosetrees Trust/The Stoneygate Trust.
Dr. Narendra had no financial conflicts to disclose but serves as a member of the editorial board of CHEST Physician.
Reducing exposure to tobacco smoke may reduce the burden of chronic obstructive pulmonary disease, and public health measures are needed, according to a new Tobacco Knowledge Summary from the World Health Organization.
“Smoking is a major risk factor for COPD and leads to airway inflammation and remodeling associated with lung destruction,” and contributes to approximately 70% of COPD cases worldwide, according to the statement.
Types of tobacco exposure include not only traditional smoked tobacco products (cigarettes, cigars, pipes, water pipes, kreteks, and bidis), but also smokeless tobacco, heated tobacco products, and electronic nicotine delivery systems; the addition of chemicals and flavors can increase the appeal of tobacco products and promote addiction, the authors wrote. Hookahs and water pipes “are at least as detrimental to lung health as smoking cigarettes and should not be considered as a safe alternative,” they added.
The risk of COPD extends to new e-cigarette products, the authors noted. A study in the American Journal of Preventive Medicine showed that current users of e-cigarettes had a 75% increased risk of developing COPD compared with individuals who have never used e-cigarettes.
Individuals with COPD also face an increased risk of cardiovascular disease and type 2 diabetes, and smokers with COPD who quit not only improve their COPD but also reduce their risk of developing these conditions, the authors said.
Mechanism of action explored
The authors noted how tobacco smoking may cause COPD when inhaled particles are deposited through the airway.
Growing evidence suggests that extracellular vesicles may play a role in the development of lung disorders such as COPD, and cigarette smoke can have an impact through this channel. A study published in the American Journal of Respiratory and Critical Care Medicine offered evidence of a potential link between exposure to cigarette smoke and the generation of a unique extracellular vesicle population that could promote the development of lung damage. In the study, Matthew C. Madison, MD, of the University of Alabama, Birmingham, and colleagues examined activity in extracellular vesicles from the bronchoalveolar lavage (BAL) fluid of smoke-exposed mice and human smokers who were otherwise healthy.
The researchers found that airway extracellular vesicles in mice or humans exposed to cigarette smoke had the ability to cause rapid lung damage when transferred into naive recipient mice. The results provide a new model that can inform preclinical COPD research, they wrote.
Public health action needed
“In recognition of COPD and Lung Cancer Awareness Month, the World Health Organization (WHO) emphasizes the impact of various forms of tobacco use on COPD,” Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
“This article focuses on the different types of tobacco exposure, the health care burden associated with COPD, and the risk of developing lung cancer. It also addresses the high-risk groups, especially youth, underscoring the importance of public education and the implementation of restrictions on tobacco use to combat these growing concerns,” she said.
“Education, awareness, and targeted interventions are essential for smoking cessation and COPD management,” said Dr. Narendra. “These elements are key to informing the public about smoking risks, encouraging behavioral change, and ultimately reducing the incidence of smoking-related diseases,” she emphasized.
The WHO statement called for population-level interventions including brief advice to tobacco users, toll-free quit lines, pharmacological interventions, use of messaging and chatbots to provide quit support, and the WHO quit tobacco mobile app.
“It is imperative that all tobacco users, particularly those living in low- to middle-income countries, have access to comprehensive cessation support aligned with WHO recommendations,” the authors wrote.
Finally, the authors emphasized the need to protect children and teens from the dangers of tobacco use through product regulation and to expose the tobacco industry’s marketing tactics.
“The article offers a comprehensive look at different types of tobacco exposure and their contribution to the development of COPD,” Dr. Narendra told this news organization. “Notably, it presents groundbreaking evidence of a strong association between the use of electronic nicotine delivery systems (ENDS) and heated tobacco products to development of COPD; additionally, it provides valuable guidance on smoking cessation resources for physicians to help patients quit smoking,” she said.
Looking ahead, more research is needed on “developing and sustaining state-specific or population-specific interventions for effective smoking cessation programs, and reducing the burden of COPD,” Dr. Narendra said.
The study by Madison and colleagues was supported by the National Heart, Lung, and Blood Institute, the National Institute of General Medical Science, the U.S. Veterans Affairs Administration, the Cystic Fibrosis Foundation Research Development Program, and the Veterans Affairs Merit grant.
Additional financial support came from Imperial College London, a Wellcome Trust Senior Research Fellowship, and Rosetrees Trust/The Stoneygate Trust.
Dr. Narendra had no financial conflicts to disclose but serves as a member of the editorial board of CHEST Physician.
Reducing exposure to tobacco smoke may reduce the burden of chronic obstructive pulmonary disease, and public health measures are needed, according to a new Tobacco Knowledge Summary from the World Health Organization.
“Smoking is a major risk factor for COPD and leads to airway inflammation and remodeling associated with lung destruction,” and contributes to approximately 70% of COPD cases worldwide, according to the statement.
Types of tobacco exposure include not only traditional smoked tobacco products (cigarettes, cigars, pipes, water pipes, kreteks, and bidis), but also smokeless tobacco, heated tobacco products, and electronic nicotine delivery systems; the addition of chemicals and flavors can increase the appeal of tobacco products and promote addiction, the authors wrote. Hookahs and water pipes “are at least as detrimental to lung health as smoking cigarettes and should not be considered as a safe alternative,” they added.
The risk of COPD extends to new e-cigarette products, the authors noted. A study in the American Journal of Preventive Medicine showed that current users of e-cigarettes had a 75% increased risk of developing COPD compared with individuals who have never used e-cigarettes.
Individuals with COPD also face an increased risk of cardiovascular disease and type 2 diabetes, and smokers with COPD who quit not only improve their COPD but also reduce their risk of developing these conditions, the authors said.
Mechanism of action explored
The authors noted how tobacco smoking may cause COPD when inhaled particles are deposited through the airway.
Growing evidence suggests that extracellular vesicles may play a role in the development of lung disorders such as COPD, and cigarette smoke can have an impact through this channel. A study published in the American Journal of Respiratory and Critical Care Medicine offered evidence of a potential link between exposure to cigarette smoke and the generation of a unique extracellular vesicle population that could promote the development of lung damage. In the study, Matthew C. Madison, MD, of the University of Alabama, Birmingham, and colleagues examined activity in extracellular vesicles from the bronchoalveolar lavage (BAL) fluid of smoke-exposed mice and human smokers who were otherwise healthy.
The researchers found that airway extracellular vesicles in mice or humans exposed to cigarette smoke had the ability to cause rapid lung damage when transferred into naive recipient mice. The results provide a new model that can inform preclinical COPD research, they wrote.
Public health action needed
“In recognition of COPD and Lung Cancer Awareness Month, the World Health Organization (WHO) emphasizes the impact of various forms of tobacco use on COPD,” Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
“This article focuses on the different types of tobacco exposure, the health care burden associated with COPD, and the risk of developing lung cancer. It also addresses the high-risk groups, especially youth, underscoring the importance of public education and the implementation of restrictions on tobacco use to combat these growing concerns,” she said.
“Education, awareness, and targeted interventions are essential for smoking cessation and COPD management,” said Dr. Narendra. “These elements are key to informing the public about smoking risks, encouraging behavioral change, and ultimately reducing the incidence of smoking-related diseases,” she emphasized.
The WHO statement called for population-level interventions including brief advice to tobacco users, toll-free quit lines, pharmacological interventions, use of messaging and chatbots to provide quit support, and the WHO quit tobacco mobile app.
“It is imperative that all tobacco users, particularly those living in low- to middle-income countries, have access to comprehensive cessation support aligned with WHO recommendations,” the authors wrote.
Finally, the authors emphasized the need to protect children and teens from the dangers of tobacco use through product regulation and to expose the tobacco industry’s marketing tactics.
“The article offers a comprehensive look at different types of tobacco exposure and their contribution to the development of COPD,” Dr. Narendra told this news organization. “Notably, it presents groundbreaking evidence of a strong association between the use of electronic nicotine delivery systems (ENDS) and heated tobacco products to development of COPD; additionally, it provides valuable guidance on smoking cessation resources for physicians to help patients quit smoking,” she said.
Looking ahead, more research is needed on “developing and sustaining state-specific or population-specific interventions for effective smoking cessation programs, and reducing the burden of COPD,” Dr. Narendra said.
The study by Madison and colleagues was supported by the National Heart, Lung, and Blood Institute, the National Institute of General Medical Science, the U.S. Veterans Affairs Administration, the Cystic Fibrosis Foundation Research Development Program, and the Veterans Affairs Merit grant.
Additional financial support came from Imperial College London, a Wellcome Trust Senior Research Fellowship, and Rosetrees Trust/The Stoneygate Trust.
Dr. Narendra had no financial conflicts to disclose but serves as a member of the editorial board of CHEST Physician.
Conditional recommendations rule in new SARD-associated interstitial lung disease guidelines
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
AT ACR 2023
Chest pain with long COVID common but undertreated
And chronic chest discomfort may persist in some individuals for years after COVID, warranting future studies of reliable treatments and pain management in this population, a new study shows.
“Recent studies have shown that chest pain occurs in as many as 89% of patients who qualify as having long COVID,” said Ansley Poole, an undergraduate student at the University of South Florida, Tampa, who conducted the research under the supervision of Christine Hunt, DO, and her colleagues at Mayo Clinic, Jacksonville, Fla.
The findings, though preliminary, shed light on the prevalence, current treatments, and ongoing challenges in managing symptoms of long COVID, said Ms. Poole, who presented the research at the annual Pain Medicine Meeting sponsored by the American Society of Regional Anesthesia and Pain Medicine.
Long COVID, which affects an estimated 18 million Americans, manifests approximately 12 weeks after the initial infection and can persist for 2 months or more. Ms. Poole and her team set out to identify risk factors, treatment options, and outcomes for patients dealing with post-COVID chest discomfort.
The study involved a retrospective chart review of 520 patients from the Mayo Clinic network, narrowed down to a final sample of 104. To be included, patients had to report chest discomfort 3-6 months post COVID that continued for 3-6 months after presentation, with no history of chronic chest pain before the infection.
The researchers identified no standardized method for the treatment or management of chest pain linked to long COVID. “Patients were prescribed multiple different treatments, including opioids, post-COVID treatment programs, anticoagulants, steroids, and even psychological programs,” Ms. Poole said.
The median age of the patients was around 50 years; more than 65% were female and over 90% identified as White. More than half (55%) had received one or more vaccine doses at the time of infection. The majority were classified as overweight or obese at the time of their SARS-CoV-2 infection.
Of the 104 patients analyzed, 30 were referred to one or more subspecialties within the pain medicine department, 23 were hospitalized, and 9 were admitted to the intensive care unit or critical care.
“Fifty-three of our patients visited the ER one or more times after COVID because of chest discomfort; however, only six were admitted for over 24 hours, indicating possible overuse of emergency services,” Ms. Poole noted.
Overall, chest pain was described as intermittent instead of constant, which may have been a barrier to providing adequate and timely treatment. The inconsistent presence of pain contributed to the prolonged suffering some patients experienced, Ms. Poole noted.
The study identified several comorbidities, potentially complicating the treatment and etiology of chest pain. These comorbidities – when combined with COVID-related chest pain – contributed to the wide array of prescribed treatments, including steroids, anticoagulants, beta blockers, and physical therapy. Chest pain also seldom stood alone; it was often accompanied by other long COVID–related symptoms, such as shortness of breath.
“Our current analysis indicates that chest pain continues on for years in many individuals, suggesting that COVID-related chest pain may be resistant to treatment,” Ms. Poole reported.
The observed heterogeneity in treatments and outcomes in patients experiencing long-term chest discomfort after COVID infection underscores the need for future studies to establish reliable treatment and management protocols for this population, said Dalia Elmofty, MD, an associate professor of anesthesia and critical care at the University of Chicago, who was not involved in the study. “There are things about COVID that we don’t fully understand. As we’re seeing its consequences and trying to understand its etiology, we recognize the need for further research,” Dr. Elmofty said.
“So many different disease pathologies came out of COVID, whether it’s organ pathology, myofascial pathology, or autoimmune pathology, and all of that is obviously linked to pain,” Dr. Elmofty told this news organization. “It’s an area of research that we are going to have to devote a lot of time to in order to understand, but I think we’re still in the very early phases, trying to fit the pieces of the puzzle together.”
Ms. Poole and Dr. Elmofty report no relevant financial relationships.
A version of this article appeared on Medscape.com.
And chronic chest discomfort may persist in some individuals for years after COVID, warranting future studies of reliable treatments and pain management in this population, a new study shows.
“Recent studies have shown that chest pain occurs in as many as 89% of patients who qualify as having long COVID,” said Ansley Poole, an undergraduate student at the University of South Florida, Tampa, who conducted the research under the supervision of Christine Hunt, DO, and her colleagues at Mayo Clinic, Jacksonville, Fla.
The findings, though preliminary, shed light on the prevalence, current treatments, and ongoing challenges in managing symptoms of long COVID, said Ms. Poole, who presented the research at the annual Pain Medicine Meeting sponsored by the American Society of Regional Anesthesia and Pain Medicine.
Long COVID, which affects an estimated 18 million Americans, manifests approximately 12 weeks after the initial infection and can persist for 2 months or more. Ms. Poole and her team set out to identify risk factors, treatment options, and outcomes for patients dealing with post-COVID chest discomfort.
The study involved a retrospective chart review of 520 patients from the Mayo Clinic network, narrowed down to a final sample of 104. To be included, patients had to report chest discomfort 3-6 months post COVID that continued for 3-6 months after presentation, with no history of chronic chest pain before the infection.
The researchers identified no standardized method for the treatment or management of chest pain linked to long COVID. “Patients were prescribed multiple different treatments, including opioids, post-COVID treatment programs, anticoagulants, steroids, and even psychological programs,” Ms. Poole said.
The median age of the patients was around 50 years; more than 65% were female and over 90% identified as White. More than half (55%) had received one or more vaccine doses at the time of infection. The majority were classified as overweight or obese at the time of their SARS-CoV-2 infection.
Of the 104 patients analyzed, 30 were referred to one or more subspecialties within the pain medicine department, 23 were hospitalized, and 9 were admitted to the intensive care unit or critical care.
“Fifty-three of our patients visited the ER one or more times after COVID because of chest discomfort; however, only six were admitted for over 24 hours, indicating possible overuse of emergency services,” Ms. Poole noted.
Overall, chest pain was described as intermittent instead of constant, which may have been a barrier to providing adequate and timely treatment. The inconsistent presence of pain contributed to the prolonged suffering some patients experienced, Ms. Poole noted.
The study identified several comorbidities, potentially complicating the treatment and etiology of chest pain. These comorbidities – when combined with COVID-related chest pain – contributed to the wide array of prescribed treatments, including steroids, anticoagulants, beta blockers, and physical therapy. Chest pain also seldom stood alone; it was often accompanied by other long COVID–related symptoms, such as shortness of breath.
“Our current analysis indicates that chest pain continues on for years in many individuals, suggesting that COVID-related chest pain may be resistant to treatment,” Ms. Poole reported.
The observed heterogeneity in treatments and outcomes in patients experiencing long-term chest discomfort after COVID infection underscores the need for future studies to establish reliable treatment and management protocols for this population, said Dalia Elmofty, MD, an associate professor of anesthesia and critical care at the University of Chicago, who was not involved in the study. “There are things about COVID that we don’t fully understand. As we’re seeing its consequences and trying to understand its etiology, we recognize the need for further research,” Dr. Elmofty said.
“So many different disease pathologies came out of COVID, whether it’s organ pathology, myofascial pathology, or autoimmune pathology, and all of that is obviously linked to pain,” Dr. Elmofty told this news organization. “It’s an area of research that we are going to have to devote a lot of time to in order to understand, but I think we’re still in the very early phases, trying to fit the pieces of the puzzle together.”
Ms. Poole and Dr. Elmofty report no relevant financial relationships.
A version of this article appeared on Medscape.com.
And chronic chest discomfort may persist in some individuals for years after COVID, warranting future studies of reliable treatments and pain management in this population, a new study shows.
“Recent studies have shown that chest pain occurs in as many as 89% of patients who qualify as having long COVID,” said Ansley Poole, an undergraduate student at the University of South Florida, Tampa, who conducted the research under the supervision of Christine Hunt, DO, and her colleagues at Mayo Clinic, Jacksonville, Fla.
The findings, though preliminary, shed light on the prevalence, current treatments, and ongoing challenges in managing symptoms of long COVID, said Ms. Poole, who presented the research at the annual Pain Medicine Meeting sponsored by the American Society of Regional Anesthesia and Pain Medicine.
Long COVID, which affects an estimated 18 million Americans, manifests approximately 12 weeks after the initial infection and can persist for 2 months or more. Ms. Poole and her team set out to identify risk factors, treatment options, and outcomes for patients dealing with post-COVID chest discomfort.
The study involved a retrospective chart review of 520 patients from the Mayo Clinic network, narrowed down to a final sample of 104. To be included, patients had to report chest discomfort 3-6 months post COVID that continued for 3-6 months after presentation, with no history of chronic chest pain before the infection.
The researchers identified no standardized method for the treatment or management of chest pain linked to long COVID. “Patients were prescribed multiple different treatments, including opioids, post-COVID treatment programs, anticoagulants, steroids, and even psychological programs,” Ms. Poole said.
The median age of the patients was around 50 years; more than 65% were female and over 90% identified as White. More than half (55%) had received one or more vaccine doses at the time of infection. The majority were classified as overweight or obese at the time of their SARS-CoV-2 infection.
Of the 104 patients analyzed, 30 were referred to one or more subspecialties within the pain medicine department, 23 were hospitalized, and 9 were admitted to the intensive care unit or critical care.
“Fifty-three of our patients visited the ER one or more times after COVID because of chest discomfort; however, only six were admitted for over 24 hours, indicating possible overuse of emergency services,” Ms. Poole noted.
Overall, chest pain was described as intermittent instead of constant, which may have been a barrier to providing adequate and timely treatment. The inconsistent presence of pain contributed to the prolonged suffering some patients experienced, Ms. Poole noted.
The study identified several comorbidities, potentially complicating the treatment and etiology of chest pain. These comorbidities – when combined with COVID-related chest pain – contributed to the wide array of prescribed treatments, including steroids, anticoagulants, beta blockers, and physical therapy. Chest pain also seldom stood alone; it was often accompanied by other long COVID–related symptoms, such as shortness of breath.
“Our current analysis indicates that chest pain continues on for years in many individuals, suggesting that COVID-related chest pain may be resistant to treatment,” Ms. Poole reported.
The observed heterogeneity in treatments and outcomes in patients experiencing long-term chest discomfort after COVID infection underscores the need for future studies to establish reliable treatment and management protocols for this population, said Dalia Elmofty, MD, an associate professor of anesthesia and critical care at the University of Chicago, who was not involved in the study. “There are things about COVID that we don’t fully understand. As we’re seeing its consequences and trying to understand its etiology, we recognize the need for further research,” Dr. Elmofty said.
“So many different disease pathologies came out of COVID, whether it’s organ pathology, myofascial pathology, or autoimmune pathology, and all of that is obviously linked to pain,” Dr. Elmofty told this news organization. “It’s an area of research that we are going to have to devote a lot of time to in order to understand, but I think we’re still in the very early phases, trying to fit the pieces of the puzzle together.”
Ms. Poole and Dr. Elmofty report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Intense exercise may lead to colds. A new study tells us why
Can too much of a healthy habit become bad?
Lots of evidence shows that regular exercise wards off respiratory infections such as colds, flu, and COVID-19. However, according to a new study.
The findings come as we enter another possible tripledemic this winter, with an increase in COVID, flu, and respiratory syncytial virus (RSV). Public health officials are on alert for a potentially severe flu season, following high flu activity this year in Australia (which can help predict how bad the U.S. flu season will be).
Studies show that the risk for acute respiratory infections is lower in people who exercise regularly. Physically active people are also less likely to suffer severe outcomes from COVID.
But while inactivity has emerged as a potential risk factor for respiratory infections, scientists have long proposed that too much activity, particularly of a prolonged and highly intense nature, may also increase susceptibility.
“The theory suggests that a short-term suppression of the immune system following intense exercise leads to an increase in susceptibility to infection, especially upper respiratory illness,” said Choukri Ben Mamoun, PhD, professor of medicine (infectious diseases) and microbial pathogenesis at the Yale Institute for Global Health, New Haven, Conn. Researchers have documented a greater incidence of upper respiratory illness “among both highly trained and healthy untrained individuals following increased activity during competition or heaving training blocks.”
That’s important if you treat athletes or patients with physically demanding jobs that push them to their physical limits, such as firefighters, police officers, or military personnel.
The new study was small but sheds light on a possible mechanism. Researchers tested blood, saliva, and urine samples from 11 firefighters before and 10 minutes after intense exercise designed to mimic wildfire fighting. The firefighters hiked over hilly terrain for 45 minutes in humid weather wearing up to 44 pounds of wildland gear.
After the workout, subjects had fewer proinflammatory cytokines and ceramides, and more antimicrobial peptides, changes that indicate a greater susceptibility to infection, researchers said. A systematic review adds weight to their findings, revealing a handful of studies in marathon runners, firefighters, soldiers, and soccer players that found an increase in respiratory symptoms after strenuous workouts.
“The relationship between exercise and the immune system is complex and varies from person to person,” said Dr. Mamoun, who was not part of the study. “Physicians can use this study’s findings to provide individualized exercise recommendations.”
An adaptive mechanism gone awry
During intense exercise, the body may reduce airway inflammation to help you breathe, say the authors. The boost in antimicrobial peptides found in the saliva samples could be the body’s way of compensating for the diminished immune function.
Antimicrobial peptides are part of the immune response but they’re “usually not very effective for viral infections,” said lead author Ernesto Nakayasu, PhD, senior research scientist at the Pacific Northwest National Laboratory, a U.S. Department of Energy lab in Richland, Washington. “That’s why we think it may make you more exposed to respiratory infections.”
The drop in proinflammatory molecules had an inverse relationship with opiorphin, a peripheral tissue vasodilator thought to increase blood flow and improve oxygen delivery to the muscles during exercise. This may be an adaptive mechanism to improve gas exchange in response to greater oxygen demand.
But as with many adaptive mechanisms, this one may have an unintended consequence. Fewer proinflammatory molecules on patrol may leave you more vulnerable to infection. Plus, during intense exercise, people tend to breathe through their mouths, bypassing the nasal barriers and allowing more microbes – including viruses – to penetrate and deposit in the distal airways of the lungs.
Advice for patients
More research is needed to know exactly how long and how strenuously one needs to exercise to trigger these immune changes, Dr. Nakayasu said.
As shown by their lactate accumulation (an indicator of anaerobic metabolism), the firefighters in the study outpaced the average person’s aerobic respiratory capacity, meaning the average person doing moderate exercise likely wouldn’t trigger these changes.
“Regular moderate exercise is generally associated with better health outcomes [and] improved immune function,” said Dr. Mamoun. For those who exercise to the extreme, proper rest and recovery are “essential for maintaining a robust immune system,” Dr. Mamoun said.
And of course, you can encourage patients to get vaccinated. Young, healthy patients may assume they don’t need COVID-19 or flu shots, as indicated by a recent survey that found one-third of Americans feel they don’t need these vaccinations if they’re not high risk.
A version of this article first appeared on Medscape.com.
Can too much of a healthy habit become bad?
Lots of evidence shows that regular exercise wards off respiratory infections such as colds, flu, and COVID-19. However, according to a new study.
The findings come as we enter another possible tripledemic this winter, with an increase in COVID, flu, and respiratory syncytial virus (RSV). Public health officials are on alert for a potentially severe flu season, following high flu activity this year in Australia (which can help predict how bad the U.S. flu season will be).
Studies show that the risk for acute respiratory infections is lower in people who exercise regularly. Physically active people are also less likely to suffer severe outcomes from COVID.
But while inactivity has emerged as a potential risk factor for respiratory infections, scientists have long proposed that too much activity, particularly of a prolonged and highly intense nature, may also increase susceptibility.
“The theory suggests that a short-term suppression of the immune system following intense exercise leads to an increase in susceptibility to infection, especially upper respiratory illness,” said Choukri Ben Mamoun, PhD, professor of medicine (infectious diseases) and microbial pathogenesis at the Yale Institute for Global Health, New Haven, Conn. Researchers have documented a greater incidence of upper respiratory illness “among both highly trained and healthy untrained individuals following increased activity during competition or heaving training blocks.”
That’s important if you treat athletes or patients with physically demanding jobs that push them to their physical limits, such as firefighters, police officers, or military personnel.
The new study was small but sheds light on a possible mechanism. Researchers tested blood, saliva, and urine samples from 11 firefighters before and 10 minutes after intense exercise designed to mimic wildfire fighting. The firefighters hiked over hilly terrain for 45 minutes in humid weather wearing up to 44 pounds of wildland gear.
After the workout, subjects had fewer proinflammatory cytokines and ceramides, and more antimicrobial peptides, changes that indicate a greater susceptibility to infection, researchers said. A systematic review adds weight to their findings, revealing a handful of studies in marathon runners, firefighters, soldiers, and soccer players that found an increase in respiratory symptoms after strenuous workouts.
“The relationship between exercise and the immune system is complex and varies from person to person,” said Dr. Mamoun, who was not part of the study. “Physicians can use this study’s findings to provide individualized exercise recommendations.”
An adaptive mechanism gone awry
During intense exercise, the body may reduce airway inflammation to help you breathe, say the authors. The boost in antimicrobial peptides found in the saliva samples could be the body’s way of compensating for the diminished immune function.
Antimicrobial peptides are part of the immune response but they’re “usually not very effective for viral infections,” said lead author Ernesto Nakayasu, PhD, senior research scientist at the Pacific Northwest National Laboratory, a U.S. Department of Energy lab in Richland, Washington. “That’s why we think it may make you more exposed to respiratory infections.”
The drop in proinflammatory molecules had an inverse relationship with opiorphin, a peripheral tissue vasodilator thought to increase blood flow and improve oxygen delivery to the muscles during exercise. This may be an adaptive mechanism to improve gas exchange in response to greater oxygen demand.
But as with many adaptive mechanisms, this one may have an unintended consequence. Fewer proinflammatory molecules on patrol may leave you more vulnerable to infection. Plus, during intense exercise, people tend to breathe through their mouths, bypassing the nasal barriers and allowing more microbes – including viruses – to penetrate and deposit in the distal airways of the lungs.
Advice for patients
More research is needed to know exactly how long and how strenuously one needs to exercise to trigger these immune changes, Dr. Nakayasu said.
As shown by their lactate accumulation (an indicator of anaerobic metabolism), the firefighters in the study outpaced the average person’s aerobic respiratory capacity, meaning the average person doing moderate exercise likely wouldn’t trigger these changes.
“Regular moderate exercise is generally associated with better health outcomes [and] improved immune function,” said Dr. Mamoun. For those who exercise to the extreme, proper rest and recovery are “essential for maintaining a robust immune system,” Dr. Mamoun said.
And of course, you can encourage patients to get vaccinated. Young, healthy patients may assume they don’t need COVID-19 or flu shots, as indicated by a recent survey that found one-third of Americans feel they don’t need these vaccinations if they’re not high risk.
A version of this article first appeared on Medscape.com.
Can too much of a healthy habit become bad?
Lots of evidence shows that regular exercise wards off respiratory infections such as colds, flu, and COVID-19. However, according to a new study.
The findings come as we enter another possible tripledemic this winter, with an increase in COVID, flu, and respiratory syncytial virus (RSV). Public health officials are on alert for a potentially severe flu season, following high flu activity this year in Australia (which can help predict how bad the U.S. flu season will be).
Studies show that the risk for acute respiratory infections is lower in people who exercise regularly. Physically active people are also less likely to suffer severe outcomes from COVID.
But while inactivity has emerged as a potential risk factor for respiratory infections, scientists have long proposed that too much activity, particularly of a prolonged and highly intense nature, may also increase susceptibility.
“The theory suggests that a short-term suppression of the immune system following intense exercise leads to an increase in susceptibility to infection, especially upper respiratory illness,” said Choukri Ben Mamoun, PhD, professor of medicine (infectious diseases) and microbial pathogenesis at the Yale Institute for Global Health, New Haven, Conn. Researchers have documented a greater incidence of upper respiratory illness “among both highly trained and healthy untrained individuals following increased activity during competition or heaving training blocks.”
That’s important if you treat athletes or patients with physically demanding jobs that push them to their physical limits, such as firefighters, police officers, or military personnel.
The new study was small but sheds light on a possible mechanism. Researchers tested blood, saliva, and urine samples from 11 firefighters before and 10 minutes after intense exercise designed to mimic wildfire fighting. The firefighters hiked over hilly terrain for 45 minutes in humid weather wearing up to 44 pounds of wildland gear.
After the workout, subjects had fewer proinflammatory cytokines and ceramides, and more antimicrobial peptides, changes that indicate a greater susceptibility to infection, researchers said. A systematic review adds weight to their findings, revealing a handful of studies in marathon runners, firefighters, soldiers, and soccer players that found an increase in respiratory symptoms after strenuous workouts.
“The relationship between exercise and the immune system is complex and varies from person to person,” said Dr. Mamoun, who was not part of the study. “Physicians can use this study’s findings to provide individualized exercise recommendations.”
An adaptive mechanism gone awry
During intense exercise, the body may reduce airway inflammation to help you breathe, say the authors. The boost in antimicrobial peptides found in the saliva samples could be the body’s way of compensating for the diminished immune function.
Antimicrobial peptides are part of the immune response but they’re “usually not very effective for viral infections,” said lead author Ernesto Nakayasu, PhD, senior research scientist at the Pacific Northwest National Laboratory, a U.S. Department of Energy lab in Richland, Washington. “That’s why we think it may make you more exposed to respiratory infections.”
The drop in proinflammatory molecules had an inverse relationship with opiorphin, a peripheral tissue vasodilator thought to increase blood flow and improve oxygen delivery to the muscles during exercise. This may be an adaptive mechanism to improve gas exchange in response to greater oxygen demand.
But as with many adaptive mechanisms, this one may have an unintended consequence. Fewer proinflammatory molecules on patrol may leave you more vulnerable to infection. Plus, during intense exercise, people tend to breathe through their mouths, bypassing the nasal barriers and allowing more microbes – including viruses – to penetrate and deposit in the distal airways of the lungs.
Advice for patients
More research is needed to know exactly how long and how strenuously one needs to exercise to trigger these immune changes, Dr. Nakayasu said.
As shown by their lactate accumulation (an indicator of anaerobic metabolism), the firefighters in the study outpaced the average person’s aerobic respiratory capacity, meaning the average person doing moderate exercise likely wouldn’t trigger these changes.
“Regular moderate exercise is generally associated with better health outcomes [and] improved immune function,” said Dr. Mamoun. For those who exercise to the extreme, proper rest and recovery are “essential for maintaining a robust immune system,” Dr. Mamoun said.
And of course, you can encourage patients to get vaccinated. Young, healthy patients may assume they don’t need COVID-19 or flu shots, as indicated by a recent survey that found one-third of Americans feel they don’t need these vaccinations if they’re not high risk.
A version of this article first appeared on Medscape.com.
FROM MILITARY MEDICAL RESEARCH