User login
Tick-borne disease has become a national issue
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.
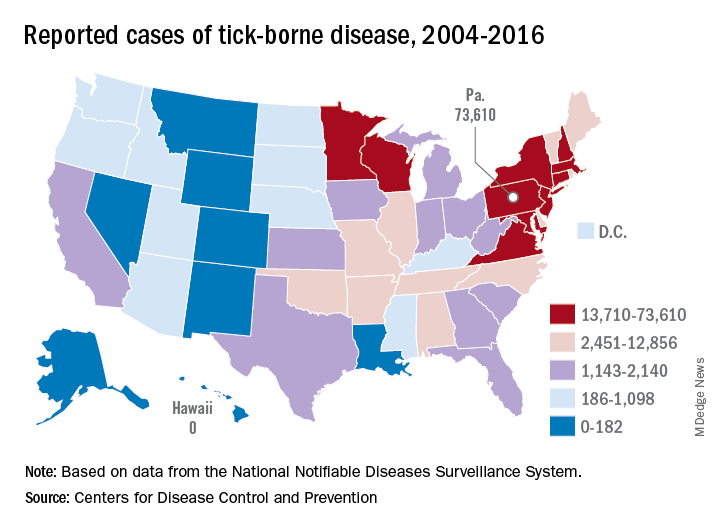
There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Does Vitamin D Supplementation Improve Lower Extremity Power and Function in Community-Dwelling Older Adults?
Study Overview
Objective. To test the effect of 12 months of vitamin D supplementation on lower-extremity power and function in older community-dwelling adults screened for low serum 25-hydroxyvitamin D (25(OH)D).
Design. A single-center, double-blind, randomized placebo-controlled study in which participants were assigned to 800 IU of vitamin D3 supplementation or placebo daily and were followed over a total period of 12 months.
Setting and participants. A total of 100 community-dwelling men and women aged ≥ 60 years with serum 25(OH)D ≤ 20 ng/mL at screening participated. Participants were prescreened by phone, and were excluded if they met any of the following exclusion criteria: vitamin D supplement use > 600 IU/day (for age 60-70 years) or > 800 IU/day (for age ≥ 71 years); vitamin D injection within the previous 3 months; > 2 falls or 1 fall with injury in past year; use of cane, walker, or other indoor walking aid; history of kidney stones within past 3 years; hypercalcemia (serum calcium > 10.8 mg/dL); renal dysfunction (glomerular filtration rate, < 30 mL/min); history of liver disease, sarcoidosis, lymphoma, dysphagia, or other gastrointestinal disorder; neuromuscular disorder affecting lower-extremity function; hip replacement within the past year; cancer treatment in the past 3 years; treatment with thiazide diuretics > 37.5 mg, teriparatide, denosumab, or bisphosphonates within the past 2 years; oral steroids (for > 3 weeks in the past 6 months); and use of fat malabsorption products or anticonvulsive therapy.
Main outcome measures. The primary outcome was leg extensor power assessed using a computer-interfaced bilateral Keiser pneumatic leg press. Secondary outcomes to measure physical function included: (1) backward tandem walk test (which is an indicator of balance and postural control during movement1); (2) Short Physical Performance Battery (SPPB) testing, which includes a balance assessment (ability to stand with feet positioned normally, semi-tandem, and tandem for 10s), a timed 4-m walk, and a chair stand test (time to complete 5 repeated chair stands); (3) stair climbing (ie, time to climb 10 steps, as a measure of knee extensor strength and functional capacity); and (4) handgrip strength (using a dynamometer). Lean tissue mass was assessed by dual X-ray absorptiometry (DEXA scan). Finally, other measures included serum total 25(OH)D levels measured at baseline, 4, 8, and 12 months, as well as 24-hour urine collection for urea-nitrogen and creatinine measurements.
Main results. Of the 2289 individuals screened for the study, 100 met eligibility criteria and underwent randomization to receive either 800 IU vitamin D supplementation daily (n = 49) or placebo (n = 51). Three patients (2 in vitamin D group and 1 in placebo group) were lost to follow up. The mean age of all participants was 69.6 ± 6.9 years. In the vitamin D group versus the control group, respectively, the percent male: female ratio was 66:34 versus 63:37, and percent Caucasian was 75% versus 82%. Mean body mass index was 28.2 ± 7.0 and mean serum 25(OH)D was 20.2 ± 6.7 ng/mL. At the end of the study (12 months), 70% of participants given vitamin D supplementation had 25(OH)D levels ≥ 30 ng/mL and all participants had levels ≥ 20 ng/mL. In the placebo group, the serum 25(OH)D level was ≥ 20 ng/mL in 54% and ≥ 30 ng/mL in 6%. The mean serum 25(OH)D level increased to 32.5 ± 5.1 ng/mL in the vitamin D–supplemented group, but no significant change was found in the placebo group (treatment × time, P < 0.001). Overall, the serum 1,25 (OH)2D3 levels did not differ between the 2 groups over the intervention period (time, P = 0.49; treatment × time, P = 0.27). Dietary intake of vitamin D, calcium, nitrogen, and protein did not differ or change over time between the 2 groups. The change in leg press power, function, and strength did not differ between the groups over 12 months (all treatment × time, P values ≥ 0.60). A total of 27 falls were reported (14 in vitamin D versus 9 in control group), of which 9 were associated with injuries. There was no significant change in lean body mass at the end of the study period in either group (treatment × time, P = 0.98).
Conclusion. In community-dwelling older adults with vitamin D deficiency (≤ 20 ng/mL), 12-month daily supplementation with 800 IU of vitamin D3 resulted in sufficient increases in serum 25(OH)D levels, but did not improve lower-extremity power, strength, or lean mass.
Commentary
Vitamin D deficiency is common in older adults (prevalence of about 41% in US adults ≥ 65 years old, according to Forrest et al2) and is likely due to dietary deficiency, reduced sun exposure (lifestyle), and decreased intestinal calcium absorption. As such, vitamin D deficiency has historically been a topic of debate and of interest in geriatric medicine, as it relates to muscle weakness, which in turn leads to increased susceptibility to falls.3 Interestingly, vitamin D receptors are expressed in human skeletal muscle,4 and in one study, 3-month supplementation of vitamin D led to an increase in type II skeletal muscle fibers in older women.5 Similarly, results from a meta-analysis of 5 randomized controlled trials (RCTs)6 showed that vitamin D supplementation may reduce fall risk in older adults by 22% (corrected odds ratio, 0.78; 95% confidence interval, 0.64-0.92). Thus, in keeping with this general theme of vitamin D supplementation yielding beneficial effects in clinical outcomes, clinicians have long accepted and practiced routine vitamin D supplementation in caring for older adults.
In more recent years, the role of vitamin D supplementation in primary care has become controversial,7 as observed in a recent paradigm shift of moving away from routine supplementation for fall and fracture prevention in clinical practice.8 In a recent meta-analysis of 33 RCTs in older community-dwelling adults, supplementation with vitamin D with or without calcium did not result in a reduction of hip fracture or total number of fractures.9 Moreover, the United States Preventive Services Task Force (USPSTF) recently published updated recommendations on the use of vitamin D supplementation for primary prevention of fractures10 and prevention of falls11 in community-dwelling adults. In these updated recommendations, the USPSTF indicated that insufficient evidence exists to re
Vitamin D supplementation is no longer routinely recommended for fall and fracture prevention. However, if we believe that poor lower extremity muscle strength is a risk factor for falls,12 then the question of whether vitamin D has a beneficial role in improving lower extremity strength in older adults needs to be addressed. Results regarding the effect of vitamin D supplementation on muscle function have so far been mixed. For example, in a randomized, double-blinded, placebo-controlled trial of 160 postmenopausal women with low vitamin D level (< 20 ng/mL), vitamin D3 supplementation at 1000 IU/day for 9 months showed a significant increase in lower extremity muscle strength.13 However, in another randomized double-blinded, placebo-controlled trial of 130 men aged 65 to 90 years with low vitamin D level (< 30 ng/mL) and an SPPB score of ≤ 9 (mild-moderate limitation in mobility), daily supplementation with 4000 IU of vitamin D3 for 9 months did not result in improved SPPB score or gait speed.14 In the study reported by Shea et al, the authors showed that 800 IU of daily vitamin D supplementation (consistent with the Institute of Medicine [IOM] recommendations for older adults15) in community-dwelling older adults with vitamin D deficiency (< 20 ng/mL) did not improve lower extremity muscle strength. This finding is significant in that it adds further evidence to support the rationale against using vitamin D supplementation for the sole purpose of improving lower extremity muscle function in older adults with vitamin D deficiency.
Valuable strengths of this study include its randomized, double-blinded, placebo-controlled trial design testing the IOM recommended dose of daily vitamin D supplementation for older adults. In addition, compared to some of the prior studies mentioned above, the study population included both males and females, although the final study population resulted in some gender bias (with male predominance). Moreover, participants were followed for a sufficient amount of time (1 year), with an excellent adherence rate (only 3 were lost to follow-up) and with corresponding improvement in vitamin D levels. Finally, the use of SPPB as a readout for primary outcome should also be commended, as this assessment is a well-validated method for measuring lower extremity function with scaled scores that predict poor outcomes.16 However, some limitations include the aforementioned predominance of male participants and Caucasian race in both intervention and control groups, as well as discrepancies between the measurement methods for serum vitamin D levels (ie, finger-stick cards versus clinical lab measurement) that may have underestimated the actual serum 25(OH)D levels.
Applications for Clinical Practice
While the null findings from the Shea and colleagues study are applicable to healthier community-dwelling older adults, they may not be generalizable to the care of more frail older patients due to their increased risks for falls and high vulnerability to adverse outcomes. Thus, further studies that account for baseline sarcopenia, frailty, and other fall-risk factors (eg, polypharmacy) are needed to better evaluate the value of vitamin D supplementation in this most vulnerable population.
— Caroline Park, MD, PhD, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai, New York, NY
1. Husu P, Suni J, Pasanen M, Miilunpalo S. Health-related fitness tests as predictors of difficulties in long-distance walking among high-functioning older adults. Aging Clin Exp Res. 2007;19:444-450.
2. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
3. Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:1253.
4. Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin-D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882-8891.
5. Sorensen OH, Lund BI, Saltin B, et al. Myopathy in bone loss ofaging - improvement by treatment with 1alpha-hydroxycholecalciferol and calcium. Clinical Science. 1979;56:157-161.
6. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls - A meta-analysis. JAMA. 2004;291:1999-2006.
7. Lewis JR SM, Daly RM. The vitamin D and calcium controversy: an update. Curr Opin Rheumatol. 2019;31:91-97.
8. Schwenk T. No value for routine vitamin D supplementation. NEJM Journal Watch. December 26, 2018.
9. Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482.
10. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
11. Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1696-1704.
12. Tinetti ME, Speechley M, Ginter SF. Risk-factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701-1707.
13. Cangussu LM, Nahas-Neto J, Orsatti CL, et al. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int. 2015;26:2413-2421.
14. Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc. 2017;65:323-331.
15. Ross CA TC, Yaktine AL, Del Valle HB. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. National Academies Press. 2011.
16. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556-561
Study Overview
Objective. To test the effect of 12 months of vitamin D supplementation on lower-extremity power and function in older community-dwelling adults screened for low serum 25-hydroxyvitamin D (25(OH)D).
Design. A single-center, double-blind, randomized placebo-controlled study in which participants were assigned to 800 IU of vitamin D3 supplementation or placebo daily and were followed over a total period of 12 months.
Setting and participants. A total of 100 community-dwelling men and women aged ≥ 60 years with serum 25(OH)D ≤ 20 ng/mL at screening participated. Participants were prescreened by phone, and were excluded if they met any of the following exclusion criteria: vitamin D supplement use > 600 IU/day (for age 60-70 years) or > 800 IU/day (for age ≥ 71 years); vitamin D injection within the previous 3 months; > 2 falls or 1 fall with injury in past year; use of cane, walker, or other indoor walking aid; history of kidney stones within past 3 years; hypercalcemia (serum calcium > 10.8 mg/dL); renal dysfunction (glomerular filtration rate, < 30 mL/min); history of liver disease, sarcoidosis, lymphoma, dysphagia, or other gastrointestinal disorder; neuromuscular disorder affecting lower-extremity function; hip replacement within the past year; cancer treatment in the past 3 years; treatment with thiazide diuretics > 37.5 mg, teriparatide, denosumab, or bisphosphonates within the past 2 years; oral steroids (for > 3 weeks in the past 6 months); and use of fat malabsorption products or anticonvulsive therapy.
Main outcome measures. The primary outcome was leg extensor power assessed using a computer-interfaced bilateral Keiser pneumatic leg press. Secondary outcomes to measure physical function included: (1) backward tandem walk test (which is an indicator of balance and postural control during movement1); (2) Short Physical Performance Battery (SPPB) testing, which includes a balance assessment (ability to stand with feet positioned normally, semi-tandem, and tandem for 10s), a timed 4-m walk, and a chair stand test (time to complete 5 repeated chair stands); (3) stair climbing (ie, time to climb 10 steps, as a measure of knee extensor strength and functional capacity); and (4) handgrip strength (using a dynamometer). Lean tissue mass was assessed by dual X-ray absorptiometry (DEXA scan). Finally, other measures included serum total 25(OH)D levels measured at baseline, 4, 8, and 12 months, as well as 24-hour urine collection for urea-nitrogen and creatinine measurements.
Main results. Of the 2289 individuals screened for the study, 100 met eligibility criteria and underwent randomization to receive either 800 IU vitamin D supplementation daily (n = 49) or placebo (n = 51). Three patients (2 in vitamin D group and 1 in placebo group) were lost to follow up. The mean age of all participants was 69.6 ± 6.9 years. In the vitamin D group versus the control group, respectively, the percent male: female ratio was 66:34 versus 63:37, and percent Caucasian was 75% versus 82%. Mean body mass index was 28.2 ± 7.0 and mean serum 25(OH)D was 20.2 ± 6.7 ng/mL. At the end of the study (12 months), 70% of participants given vitamin D supplementation had 25(OH)D levels ≥ 30 ng/mL and all participants had levels ≥ 20 ng/mL. In the placebo group, the serum 25(OH)D level was ≥ 20 ng/mL in 54% and ≥ 30 ng/mL in 6%. The mean serum 25(OH)D level increased to 32.5 ± 5.1 ng/mL in the vitamin D–supplemented group, but no significant change was found in the placebo group (treatment × time, P < 0.001). Overall, the serum 1,25 (OH)2D3 levels did not differ between the 2 groups over the intervention period (time, P = 0.49; treatment × time, P = 0.27). Dietary intake of vitamin D, calcium, nitrogen, and protein did not differ or change over time between the 2 groups. The change in leg press power, function, and strength did not differ between the groups over 12 months (all treatment × time, P values ≥ 0.60). A total of 27 falls were reported (14 in vitamin D versus 9 in control group), of which 9 were associated with injuries. There was no significant change in lean body mass at the end of the study period in either group (treatment × time, P = 0.98).
Conclusion. In community-dwelling older adults with vitamin D deficiency (≤ 20 ng/mL), 12-month daily supplementation with 800 IU of vitamin D3 resulted in sufficient increases in serum 25(OH)D levels, but did not improve lower-extremity power, strength, or lean mass.
Commentary
Vitamin D deficiency is common in older adults (prevalence of about 41% in US adults ≥ 65 years old, according to Forrest et al2) and is likely due to dietary deficiency, reduced sun exposure (lifestyle), and decreased intestinal calcium absorption. As such, vitamin D deficiency has historically been a topic of debate and of interest in geriatric medicine, as it relates to muscle weakness, which in turn leads to increased susceptibility to falls.3 Interestingly, vitamin D receptors are expressed in human skeletal muscle,4 and in one study, 3-month supplementation of vitamin D led to an increase in type II skeletal muscle fibers in older women.5 Similarly, results from a meta-analysis of 5 randomized controlled trials (RCTs)6 showed that vitamin D supplementation may reduce fall risk in older adults by 22% (corrected odds ratio, 0.78; 95% confidence interval, 0.64-0.92). Thus, in keeping with this general theme of vitamin D supplementation yielding beneficial effects in clinical outcomes, clinicians have long accepted and practiced routine vitamin D supplementation in caring for older adults.
In more recent years, the role of vitamin D supplementation in primary care has become controversial,7 as observed in a recent paradigm shift of moving away from routine supplementation for fall and fracture prevention in clinical practice.8 In a recent meta-analysis of 33 RCTs in older community-dwelling adults, supplementation with vitamin D with or without calcium did not result in a reduction of hip fracture or total number of fractures.9 Moreover, the United States Preventive Services Task Force (USPSTF) recently published updated recommendations on the use of vitamin D supplementation for primary prevention of fractures10 and prevention of falls11 in community-dwelling adults. In these updated recommendations, the USPSTF indicated that insufficient evidence exists to re
Vitamin D supplementation is no longer routinely recommended for fall and fracture prevention. However, if we believe that poor lower extremity muscle strength is a risk factor for falls,12 then the question of whether vitamin D has a beneficial role in improving lower extremity strength in older adults needs to be addressed. Results regarding the effect of vitamin D supplementation on muscle function have so far been mixed. For example, in a randomized, double-blinded, placebo-controlled trial of 160 postmenopausal women with low vitamin D level (< 20 ng/mL), vitamin D3 supplementation at 1000 IU/day for 9 months showed a significant increase in lower extremity muscle strength.13 However, in another randomized double-blinded, placebo-controlled trial of 130 men aged 65 to 90 years with low vitamin D level (< 30 ng/mL) and an SPPB score of ≤ 9 (mild-moderate limitation in mobility), daily supplementation with 4000 IU of vitamin D3 for 9 months did not result in improved SPPB score or gait speed.14 In the study reported by Shea et al, the authors showed that 800 IU of daily vitamin D supplementation (consistent with the Institute of Medicine [IOM] recommendations for older adults15) in community-dwelling older adults with vitamin D deficiency (< 20 ng/mL) did not improve lower extremity muscle strength. This finding is significant in that it adds further evidence to support the rationale against using vitamin D supplementation for the sole purpose of improving lower extremity muscle function in older adults with vitamin D deficiency.
Valuable strengths of this study include its randomized, double-blinded, placebo-controlled trial design testing the IOM recommended dose of daily vitamin D supplementation for older adults. In addition, compared to some of the prior studies mentioned above, the study population included both males and females, although the final study population resulted in some gender bias (with male predominance). Moreover, participants were followed for a sufficient amount of time (1 year), with an excellent adherence rate (only 3 were lost to follow-up) and with corresponding improvement in vitamin D levels. Finally, the use of SPPB as a readout for primary outcome should also be commended, as this assessment is a well-validated method for measuring lower extremity function with scaled scores that predict poor outcomes.16 However, some limitations include the aforementioned predominance of male participants and Caucasian race in both intervention and control groups, as well as discrepancies between the measurement methods for serum vitamin D levels (ie, finger-stick cards versus clinical lab measurement) that may have underestimated the actual serum 25(OH)D levels.
Applications for Clinical Practice
While the null findings from the Shea and colleagues study are applicable to healthier community-dwelling older adults, they may not be generalizable to the care of more frail older patients due to their increased risks for falls and high vulnerability to adverse outcomes. Thus, further studies that account for baseline sarcopenia, frailty, and other fall-risk factors (eg, polypharmacy) are needed to better evaluate the value of vitamin D supplementation in this most vulnerable population.
— Caroline Park, MD, PhD, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai, New York, NY
Study Overview
Objective. To test the effect of 12 months of vitamin D supplementation on lower-extremity power and function in older community-dwelling adults screened for low serum 25-hydroxyvitamin D (25(OH)D).
Design. A single-center, double-blind, randomized placebo-controlled study in which participants were assigned to 800 IU of vitamin D3 supplementation or placebo daily and were followed over a total period of 12 months.
Setting and participants. A total of 100 community-dwelling men and women aged ≥ 60 years with serum 25(OH)D ≤ 20 ng/mL at screening participated. Participants were prescreened by phone, and were excluded if they met any of the following exclusion criteria: vitamin D supplement use > 600 IU/day (for age 60-70 years) or > 800 IU/day (for age ≥ 71 years); vitamin D injection within the previous 3 months; > 2 falls or 1 fall with injury in past year; use of cane, walker, or other indoor walking aid; history of kidney stones within past 3 years; hypercalcemia (serum calcium > 10.8 mg/dL); renal dysfunction (glomerular filtration rate, < 30 mL/min); history of liver disease, sarcoidosis, lymphoma, dysphagia, or other gastrointestinal disorder; neuromuscular disorder affecting lower-extremity function; hip replacement within the past year; cancer treatment in the past 3 years; treatment with thiazide diuretics > 37.5 mg, teriparatide, denosumab, or bisphosphonates within the past 2 years; oral steroids (for > 3 weeks in the past 6 months); and use of fat malabsorption products or anticonvulsive therapy.
Main outcome measures. The primary outcome was leg extensor power assessed using a computer-interfaced bilateral Keiser pneumatic leg press. Secondary outcomes to measure physical function included: (1) backward tandem walk test (which is an indicator of balance and postural control during movement1); (2) Short Physical Performance Battery (SPPB) testing, which includes a balance assessment (ability to stand with feet positioned normally, semi-tandem, and tandem for 10s), a timed 4-m walk, and a chair stand test (time to complete 5 repeated chair stands); (3) stair climbing (ie, time to climb 10 steps, as a measure of knee extensor strength and functional capacity); and (4) handgrip strength (using a dynamometer). Lean tissue mass was assessed by dual X-ray absorptiometry (DEXA scan). Finally, other measures included serum total 25(OH)D levels measured at baseline, 4, 8, and 12 months, as well as 24-hour urine collection for urea-nitrogen and creatinine measurements.
Main results. Of the 2289 individuals screened for the study, 100 met eligibility criteria and underwent randomization to receive either 800 IU vitamin D supplementation daily (n = 49) or placebo (n = 51). Three patients (2 in vitamin D group and 1 in placebo group) were lost to follow up. The mean age of all participants was 69.6 ± 6.9 years. In the vitamin D group versus the control group, respectively, the percent male: female ratio was 66:34 versus 63:37, and percent Caucasian was 75% versus 82%. Mean body mass index was 28.2 ± 7.0 and mean serum 25(OH)D was 20.2 ± 6.7 ng/mL. At the end of the study (12 months), 70% of participants given vitamin D supplementation had 25(OH)D levels ≥ 30 ng/mL and all participants had levels ≥ 20 ng/mL. In the placebo group, the serum 25(OH)D level was ≥ 20 ng/mL in 54% and ≥ 30 ng/mL in 6%. The mean serum 25(OH)D level increased to 32.5 ± 5.1 ng/mL in the vitamin D–supplemented group, but no significant change was found in the placebo group (treatment × time, P < 0.001). Overall, the serum 1,25 (OH)2D3 levels did not differ between the 2 groups over the intervention period (time, P = 0.49; treatment × time, P = 0.27). Dietary intake of vitamin D, calcium, nitrogen, and protein did not differ or change over time between the 2 groups. The change in leg press power, function, and strength did not differ between the groups over 12 months (all treatment × time, P values ≥ 0.60). A total of 27 falls were reported (14 in vitamin D versus 9 in control group), of which 9 were associated with injuries. There was no significant change in lean body mass at the end of the study period in either group (treatment × time, P = 0.98).
Conclusion. In community-dwelling older adults with vitamin D deficiency (≤ 20 ng/mL), 12-month daily supplementation with 800 IU of vitamin D3 resulted in sufficient increases in serum 25(OH)D levels, but did not improve lower-extremity power, strength, or lean mass.
Commentary
Vitamin D deficiency is common in older adults (prevalence of about 41% in US adults ≥ 65 years old, according to Forrest et al2) and is likely due to dietary deficiency, reduced sun exposure (lifestyle), and decreased intestinal calcium absorption. As such, vitamin D deficiency has historically been a topic of debate and of interest in geriatric medicine, as it relates to muscle weakness, which in turn leads to increased susceptibility to falls.3 Interestingly, vitamin D receptors are expressed in human skeletal muscle,4 and in one study, 3-month supplementation of vitamin D led to an increase in type II skeletal muscle fibers in older women.5 Similarly, results from a meta-analysis of 5 randomized controlled trials (RCTs)6 showed that vitamin D supplementation may reduce fall risk in older adults by 22% (corrected odds ratio, 0.78; 95% confidence interval, 0.64-0.92). Thus, in keeping with this general theme of vitamin D supplementation yielding beneficial effects in clinical outcomes, clinicians have long accepted and practiced routine vitamin D supplementation in caring for older adults.
In more recent years, the role of vitamin D supplementation in primary care has become controversial,7 as observed in a recent paradigm shift of moving away from routine supplementation for fall and fracture prevention in clinical practice.8 In a recent meta-analysis of 33 RCTs in older community-dwelling adults, supplementation with vitamin D with or without calcium did not result in a reduction of hip fracture or total number of fractures.9 Moreover, the United States Preventive Services Task Force (USPSTF) recently published updated recommendations on the use of vitamin D supplementation for primary prevention of fractures10 and prevention of falls11 in community-dwelling adults. In these updated recommendations, the USPSTF indicated that insufficient evidence exists to re
Vitamin D supplementation is no longer routinely recommended for fall and fracture prevention. However, if we believe that poor lower extremity muscle strength is a risk factor for falls,12 then the question of whether vitamin D has a beneficial role in improving lower extremity strength in older adults needs to be addressed. Results regarding the effect of vitamin D supplementation on muscle function have so far been mixed. For example, in a randomized, double-blinded, placebo-controlled trial of 160 postmenopausal women with low vitamin D level (< 20 ng/mL), vitamin D3 supplementation at 1000 IU/day for 9 months showed a significant increase in lower extremity muscle strength.13 However, in another randomized double-blinded, placebo-controlled trial of 130 men aged 65 to 90 years with low vitamin D level (< 30 ng/mL) and an SPPB score of ≤ 9 (mild-moderate limitation in mobility), daily supplementation with 4000 IU of vitamin D3 for 9 months did not result in improved SPPB score or gait speed.14 In the study reported by Shea et al, the authors showed that 800 IU of daily vitamin D supplementation (consistent with the Institute of Medicine [IOM] recommendations for older adults15) in community-dwelling older adults with vitamin D deficiency (< 20 ng/mL) did not improve lower extremity muscle strength. This finding is significant in that it adds further evidence to support the rationale against using vitamin D supplementation for the sole purpose of improving lower extremity muscle function in older adults with vitamin D deficiency.
Valuable strengths of this study include its randomized, double-blinded, placebo-controlled trial design testing the IOM recommended dose of daily vitamin D supplementation for older adults. In addition, compared to some of the prior studies mentioned above, the study population included both males and females, although the final study population resulted in some gender bias (with male predominance). Moreover, participants were followed for a sufficient amount of time (1 year), with an excellent adherence rate (only 3 were lost to follow-up) and with corresponding improvement in vitamin D levels. Finally, the use of SPPB as a readout for primary outcome should also be commended, as this assessment is a well-validated method for measuring lower extremity function with scaled scores that predict poor outcomes.16 However, some limitations include the aforementioned predominance of male participants and Caucasian race in both intervention and control groups, as well as discrepancies between the measurement methods for serum vitamin D levels (ie, finger-stick cards versus clinical lab measurement) that may have underestimated the actual serum 25(OH)D levels.
Applications for Clinical Practice
While the null findings from the Shea and colleagues study are applicable to healthier community-dwelling older adults, they may not be generalizable to the care of more frail older patients due to their increased risks for falls and high vulnerability to adverse outcomes. Thus, further studies that account for baseline sarcopenia, frailty, and other fall-risk factors (eg, polypharmacy) are needed to better evaluate the value of vitamin D supplementation in this most vulnerable population.
— Caroline Park, MD, PhD, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai, New York, NY
1. Husu P, Suni J, Pasanen M, Miilunpalo S. Health-related fitness tests as predictors of difficulties in long-distance walking among high-functioning older adults. Aging Clin Exp Res. 2007;19:444-450.
2. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
3. Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:1253.
4. Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin-D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882-8891.
5. Sorensen OH, Lund BI, Saltin B, et al. Myopathy in bone loss ofaging - improvement by treatment with 1alpha-hydroxycholecalciferol and calcium. Clinical Science. 1979;56:157-161.
6. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls - A meta-analysis. JAMA. 2004;291:1999-2006.
7. Lewis JR SM, Daly RM. The vitamin D and calcium controversy: an update. Curr Opin Rheumatol. 2019;31:91-97.
8. Schwenk T. No value for routine vitamin D supplementation. NEJM Journal Watch. December 26, 2018.
9. Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482.
10. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
11. Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1696-1704.
12. Tinetti ME, Speechley M, Ginter SF. Risk-factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701-1707.
13. Cangussu LM, Nahas-Neto J, Orsatti CL, et al. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int. 2015;26:2413-2421.
14. Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc. 2017;65:323-331.
15. Ross CA TC, Yaktine AL, Del Valle HB. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. National Academies Press. 2011.
16. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556-561
1. Husu P, Suni J, Pasanen M, Miilunpalo S. Health-related fitness tests as predictors of difficulties in long-distance walking among high-functioning older adults. Aging Clin Exp Res. 2007;19:444-450.
2. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
3. Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:1253.
4. Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin-D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882-8891.
5. Sorensen OH, Lund BI, Saltin B, et al. Myopathy in bone loss ofaging - improvement by treatment with 1alpha-hydroxycholecalciferol and calcium. Clinical Science. 1979;56:157-161.
6. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls - A meta-analysis. JAMA. 2004;291:1999-2006.
7. Lewis JR SM, Daly RM. The vitamin D and calcium controversy: an update. Curr Opin Rheumatol. 2019;31:91-97.
8. Schwenk T. No value for routine vitamin D supplementation. NEJM Journal Watch. December 26, 2018.
9. Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482.
10. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
11. Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1696-1704.
12. Tinetti ME, Speechley M, Ginter SF. Risk-factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701-1707.
13. Cangussu LM, Nahas-Neto J, Orsatti CL, et al. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int. 2015;26:2413-2421.
14. Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc. 2017;65:323-331.
15. Ross CA TC, Yaktine AL, Del Valle HB. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. National Academies Press. 2011.
16. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556-561
Methotrexate significantly reduced knee OA pain
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.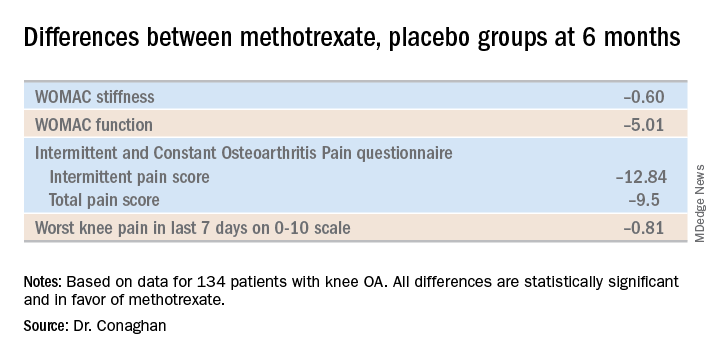
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
REPORTING FROM OARSI 2019
Click for Credit: Biomarkers for VTE risk; Exercise & concussion recovery; more
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Check for complementopathies in lupus pregnancy
SAN FRANCISCO – It’s important to check for complementopathies in pregnant women with lupus, according to Michelle Petri, MD, a professor of rheumatology at Johns Hopkins University, Baltimore.
A new diagnosis being developed at Hopkins and elsewhere, complementopathies involve an inappropriate activation of the alternative pathway of complement (APC), either from a mutation in a complement control protein, or, in the case of lupus, an autoantibody against one. They’ve been implicated as a major cause of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, a condition to which women with lupus are particularly prone.
Hopkins has developed a serum test to diagnose inappropriate APC activation in a few hours, the modified Ham test. When HELLP develops in a woman with a complementopathy, the complement inhibitor eculizumab (Soliris) is proving to be a safe alternative to pregnancy termination.
“I urge you to think about using the modified Ham test, because if it is positive, you can treat HELLP without having to stop the pregnancy,” said Dr. Petri, also codirector of the Hopkins Lupus Pregnancy Center.
Lupus management has come a long way from the days when women were counseled to avoid or terminate pregnancy. Risks remain, “but many pregnancies are successful. I think that for every woman with lupus, we do want to offer the possibility of successful pregnancy,” she said.
Disease control is key. Preterm birth, the most common adverse outcome in lupus, correlates closely with disease activity, and disease activity can be controlled with hydroxychloroquine, and, when needed, azathioprine and tacrolimus for renal flairs.
“But these kinds of basic lessons – we need hydroxychloroquine in pregnancy; we must control disease activity – are not heard out in the real world. Claims data have shown that pregnant women with lupus actually take fewer prescribed medications, and they have fewer rheumatology visits.” It’s a problem that needs to be addressed, Dr. Petri said.
Vitamin D is also important. Hopkins has shown that supplementation to hit a level of 40 ng/mL reduces both global and renal disease activity without toxicity; studies in the general population have shown reduced preeclampsia, preterm birth, and low birth weight, all concerns in lupus.
“I haven’t convinced the world of lupus how important vitamin D is,” but “I actually love it just as much as I love hydroxychloroquine,” Dr. Petri said.
Cosupplementation with calcium complicates matters. Together, they seem to reduce the risk of preeclampsia, but increase the risk of preterm birth. More needs to be known, so “for all of us with pregnancy cohorts, it’s time to start to record vitamin D and calcium levels so we can look at this,” she said.
Dr. Petri has worked with numerous companies.
SAN FRANCISCO – It’s important to check for complementopathies in pregnant women with lupus, according to Michelle Petri, MD, a professor of rheumatology at Johns Hopkins University, Baltimore.
A new diagnosis being developed at Hopkins and elsewhere, complementopathies involve an inappropriate activation of the alternative pathway of complement (APC), either from a mutation in a complement control protein, or, in the case of lupus, an autoantibody against one. They’ve been implicated as a major cause of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, a condition to which women with lupus are particularly prone.
Hopkins has developed a serum test to diagnose inappropriate APC activation in a few hours, the modified Ham test. When HELLP develops in a woman with a complementopathy, the complement inhibitor eculizumab (Soliris) is proving to be a safe alternative to pregnancy termination.
“I urge you to think about using the modified Ham test, because if it is positive, you can treat HELLP without having to stop the pregnancy,” said Dr. Petri, also codirector of the Hopkins Lupus Pregnancy Center.
Lupus management has come a long way from the days when women were counseled to avoid or terminate pregnancy. Risks remain, “but many pregnancies are successful. I think that for every woman with lupus, we do want to offer the possibility of successful pregnancy,” she said.
Disease control is key. Preterm birth, the most common adverse outcome in lupus, correlates closely with disease activity, and disease activity can be controlled with hydroxychloroquine, and, when needed, azathioprine and tacrolimus for renal flairs.
“But these kinds of basic lessons – we need hydroxychloroquine in pregnancy; we must control disease activity – are not heard out in the real world. Claims data have shown that pregnant women with lupus actually take fewer prescribed medications, and they have fewer rheumatology visits.” It’s a problem that needs to be addressed, Dr. Petri said.
Vitamin D is also important. Hopkins has shown that supplementation to hit a level of 40 ng/mL reduces both global and renal disease activity without toxicity; studies in the general population have shown reduced preeclampsia, preterm birth, and low birth weight, all concerns in lupus.
“I haven’t convinced the world of lupus how important vitamin D is,” but “I actually love it just as much as I love hydroxychloroquine,” Dr. Petri said.
Cosupplementation with calcium complicates matters. Together, they seem to reduce the risk of preeclampsia, but increase the risk of preterm birth. More needs to be known, so “for all of us with pregnancy cohorts, it’s time to start to record vitamin D and calcium levels so we can look at this,” she said.
Dr. Petri has worked with numerous companies.
SAN FRANCISCO – It’s important to check for complementopathies in pregnant women with lupus, according to Michelle Petri, MD, a professor of rheumatology at Johns Hopkins University, Baltimore.
A new diagnosis being developed at Hopkins and elsewhere, complementopathies involve an inappropriate activation of the alternative pathway of complement (APC), either from a mutation in a complement control protein, or, in the case of lupus, an autoantibody against one. They’ve been implicated as a major cause of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, a condition to which women with lupus are particularly prone.
Hopkins has developed a serum test to diagnose inappropriate APC activation in a few hours, the modified Ham test. When HELLP develops in a woman with a complementopathy, the complement inhibitor eculizumab (Soliris) is proving to be a safe alternative to pregnancy termination.
“I urge you to think about using the modified Ham test, because if it is positive, you can treat HELLP without having to stop the pregnancy,” said Dr. Petri, also codirector of the Hopkins Lupus Pregnancy Center.
Lupus management has come a long way from the days when women were counseled to avoid or terminate pregnancy. Risks remain, “but many pregnancies are successful. I think that for every woman with lupus, we do want to offer the possibility of successful pregnancy,” she said.
Disease control is key. Preterm birth, the most common adverse outcome in lupus, correlates closely with disease activity, and disease activity can be controlled with hydroxychloroquine, and, when needed, azathioprine and tacrolimus for renal flairs.
“But these kinds of basic lessons – we need hydroxychloroquine in pregnancy; we must control disease activity – are not heard out in the real world. Claims data have shown that pregnant women with lupus actually take fewer prescribed medications, and they have fewer rheumatology visits.” It’s a problem that needs to be addressed, Dr. Petri said.
Vitamin D is also important. Hopkins has shown that supplementation to hit a level of 40 ng/mL reduces both global and renal disease activity without toxicity; studies in the general population have shown reduced preeclampsia, preterm birth, and low birth weight, all concerns in lupus.
“I haven’t convinced the world of lupus how important vitamin D is,” but “I actually love it just as much as I love hydroxychloroquine,” Dr. Petri said.
Cosupplementation with calcium complicates matters. Together, they seem to reduce the risk of preeclampsia, but increase the risk of preterm birth. More needs to be known, so “for all of us with pregnancy cohorts, it’s time to start to record vitamin D and calcium levels so we can look at this,” she said.
Dr. Petri has worked with numerous companies.
EXPERT ANALYSIS FROM LUPUS 2019
Proinflammatory diets up rheumatoid arthritis risk
BIRMINGHAM, ENGLAND – Proinflammatory diets are associated with increased C-reactive protein (CRP) and subsequent rheumatoid arthritis (RA), according to combined data from the European Prospective Investigation of Cancer and Nutrition (EPIC) and Norfolk Arthritis Register (NOAR).
“There has always been a debate around this topic,” Max Yates, MBBS, PhD, said at the annual conference of the British Society for Rheumatology. “A quick online search will reveal a plethora of texts claiming to give definitive or the best advice for arthritis” and diet, he said, often from “questionable experts.”
“I think we’re all interested in diet,” observed Dr. Yates, of the University of East Anglia in Norwich (England), and “although the association between diet and arthritis is open to debate, previous studies have shown an association with those who have a lower intake of vitamin C and fiber.” The problem is one of credibility, he noted, so this was something that the NOAR investigators decided to look into with data from the Dietary Inflammatory Index (DII) collected from the EPIC cohort.
The DII is a literature-based, population-derived tool that has been used to determine the inflammatory potential of diet, Dr. Yates explained. Data show that inflammatory diets are is associated with increased levels of inflammatory markers including C-reactive protein (CRP) and interleukin (IL)-6. These diets include items such as trans and saturated fats, and fats from animal protein versus more anti-inflammatory items such as black tea, thyme, turmeric, and saffron.
“We are fortunate that NOAR is in the same geographic location as EPIC,” Dr. Yates said, and the two cohorts have been running alongside each other since the early 1990s. While NOAR has been collecting data on incident inflammatory polyarthritis since 1989, EPIC has been “intensively” collecting information on dietary and lifestyle factors and blood samples from its participants since 1993.
EPIC investigators have been “trailblazers” in recording of dietary data, Dr. Yates said. First using paper-based questionnaires and now smartphone apps that allow people to upload photos of what they are eating. Data are linked to both primary care practice and hospital records.
For the present study, data on 159 patients who participated in both NOAR and EPIC were used. Participants had RA according to the 2010 American College of Rheumatology criteria and had an average disease onset of 7 years after enrollment into NOAR and EPIC.
“Quite pleasingly, the dietary inflammatory index scores were associated with high-sensitivity CRP taken at baseline enrollment in 1993 to 1997, further validating the index again within another population,” Dr. Yates said.
Results showed that there was a significant association between the baseline DII score and subsequent development of RA, with an odds ratio of 1.90 comparing individuals with the highest and lowest DII scores (P less than .01).
When cases were matched by age, sex, and body mass index, however, there was only a trend for an association between inflammatory diets and RA onset. “We hope to identify more patients within EPIC to strengthen this association,” Dr. Yates said.
The results are consistent with data from the Nurses’ Health Study, Dr. Yates noted, adding that future research is needed to address whether dietary modification can demonstrate causality.
“Diet is one of the modifiable risk factors that we can use to tackle RA, and I think it’s about time we as a community take over this area and gave definitive advice.”
Dr. Yates presented the work on behalf of PhD student Ellie Sayer. Neither Dr. Yates nor Ms. Sayer had any conflicts of interests to disclose.
SOURCE: Sayer E et al. Rheumatology. 2019;58(suppl 3), Abstract 014.
BIRMINGHAM, ENGLAND – Proinflammatory diets are associated with increased C-reactive protein (CRP) and subsequent rheumatoid arthritis (RA), according to combined data from the European Prospective Investigation of Cancer and Nutrition (EPIC) and Norfolk Arthritis Register (NOAR).
“There has always been a debate around this topic,” Max Yates, MBBS, PhD, said at the annual conference of the British Society for Rheumatology. “A quick online search will reveal a plethora of texts claiming to give definitive or the best advice for arthritis” and diet, he said, often from “questionable experts.”
“I think we’re all interested in diet,” observed Dr. Yates, of the University of East Anglia in Norwich (England), and “although the association between diet and arthritis is open to debate, previous studies have shown an association with those who have a lower intake of vitamin C and fiber.” The problem is one of credibility, he noted, so this was something that the NOAR investigators decided to look into with data from the Dietary Inflammatory Index (DII) collected from the EPIC cohort.
The DII is a literature-based, population-derived tool that has been used to determine the inflammatory potential of diet, Dr. Yates explained. Data show that inflammatory diets are is associated with increased levels of inflammatory markers including C-reactive protein (CRP) and interleukin (IL)-6. These diets include items such as trans and saturated fats, and fats from animal protein versus more anti-inflammatory items such as black tea, thyme, turmeric, and saffron.
“We are fortunate that NOAR is in the same geographic location as EPIC,” Dr. Yates said, and the two cohorts have been running alongside each other since the early 1990s. While NOAR has been collecting data on incident inflammatory polyarthritis since 1989, EPIC has been “intensively” collecting information on dietary and lifestyle factors and blood samples from its participants since 1993.
EPIC investigators have been “trailblazers” in recording of dietary data, Dr. Yates said. First using paper-based questionnaires and now smartphone apps that allow people to upload photos of what they are eating. Data are linked to both primary care practice and hospital records.
For the present study, data on 159 patients who participated in both NOAR and EPIC were used. Participants had RA according to the 2010 American College of Rheumatology criteria and had an average disease onset of 7 years after enrollment into NOAR and EPIC.
“Quite pleasingly, the dietary inflammatory index scores were associated with high-sensitivity CRP taken at baseline enrollment in 1993 to 1997, further validating the index again within another population,” Dr. Yates said.
Results showed that there was a significant association between the baseline DII score and subsequent development of RA, with an odds ratio of 1.90 comparing individuals with the highest and lowest DII scores (P less than .01).
When cases were matched by age, sex, and body mass index, however, there was only a trend for an association between inflammatory diets and RA onset. “We hope to identify more patients within EPIC to strengthen this association,” Dr. Yates said.
The results are consistent with data from the Nurses’ Health Study, Dr. Yates noted, adding that future research is needed to address whether dietary modification can demonstrate causality.
“Diet is one of the modifiable risk factors that we can use to tackle RA, and I think it’s about time we as a community take over this area and gave definitive advice.”
Dr. Yates presented the work on behalf of PhD student Ellie Sayer. Neither Dr. Yates nor Ms. Sayer had any conflicts of interests to disclose.
SOURCE: Sayer E et al. Rheumatology. 2019;58(suppl 3), Abstract 014.
BIRMINGHAM, ENGLAND – Proinflammatory diets are associated with increased C-reactive protein (CRP) and subsequent rheumatoid arthritis (RA), according to combined data from the European Prospective Investigation of Cancer and Nutrition (EPIC) and Norfolk Arthritis Register (NOAR).
“There has always been a debate around this topic,” Max Yates, MBBS, PhD, said at the annual conference of the British Society for Rheumatology. “A quick online search will reveal a plethora of texts claiming to give definitive or the best advice for arthritis” and diet, he said, often from “questionable experts.”
“I think we’re all interested in diet,” observed Dr. Yates, of the University of East Anglia in Norwich (England), and “although the association between diet and arthritis is open to debate, previous studies have shown an association with those who have a lower intake of vitamin C and fiber.” The problem is one of credibility, he noted, so this was something that the NOAR investigators decided to look into with data from the Dietary Inflammatory Index (DII) collected from the EPIC cohort.
The DII is a literature-based, population-derived tool that has been used to determine the inflammatory potential of diet, Dr. Yates explained. Data show that inflammatory diets are is associated with increased levels of inflammatory markers including C-reactive protein (CRP) and interleukin (IL)-6. These diets include items such as trans and saturated fats, and fats from animal protein versus more anti-inflammatory items such as black tea, thyme, turmeric, and saffron.
“We are fortunate that NOAR is in the same geographic location as EPIC,” Dr. Yates said, and the two cohorts have been running alongside each other since the early 1990s. While NOAR has been collecting data on incident inflammatory polyarthritis since 1989, EPIC has been “intensively” collecting information on dietary and lifestyle factors and blood samples from its participants since 1993.
EPIC investigators have been “trailblazers” in recording of dietary data, Dr. Yates said. First using paper-based questionnaires and now smartphone apps that allow people to upload photos of what they are eating. Data are linked to both primary care practice and hospital records.
For the present study, data on 159 patients who participated in both NOAR and EPIC were used. Participants had RA according to the 2010 American College of Rheumatology criteria and had an average disease onset of 7 years after enrollment into NOAR and EPIC.
“Quite pleasingly, the dietary inflammatory index scores were associated with high-sensitivity CRP taken at baseline enrollment in 1993 to 1997, further validating the index again within another population,” Dr. Yates said.
Results showed that there was a significant association between the baseline DII score and subsequent development of RA, with an odds ratio of 1.90 comparing individuals with the highest and lowest DII scores (P less than .01).
When cases were matched by age, sex, and body mass index, however, there was only a trend for an association between inflammatory diets and RA onset. “We hope to identify more patients within EPIC to strengthen this association,” Dr. Yates said.
The results are consistent with data from the Nurses’ Health Study, Dr. Yates noted, adding that future research is needed to address whether dietary modification can demonstrate causality.
“Diet is one of the modifiable risk factors that we can use to tackle RA, and I think it’s about time we as a community take over this area and gave definitive advice.”
Dr. Yates presented the work on behalf of PhD student Ellie Sayer. Neither Dr. Yates nor Ms. Sayer had any conflicts of interests to disclose.
SOURCE: Sayer E et al. Rheumatology. 2019;58(suppl 3), Abstract 014.
REPORTING FROM BSR 2019
Scandinavian studies shed light on OA inheritance
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
REPORTING FROM OARSI 2019
High multimorbidity in axial spondyloarthritis
BIRMINGHAM, ENGLAND – Almost two-thirds of patients with axial spondyloarthritis (axSpA) have more than one medical condition, and these may cluster together, the results of a single-center, cross-sectional study have shown.
Of 419 patients, 61% had multiple conditions, with a median of at least one other condition in addition to axSpA, but some patients had as many as five. The most common other conditions in patients with axSpA were hypertension (19% of patients), depression (16%), and dyspepsia (11%).
In all, 15 clusters of conditions were found, each dominated by one or two conditions in addition to axSpA. The three largest clusters were hypertension and cardiovascular disease (n = 88), anxiety and depression (n = 50), and dyspepsia (n = 31). In the cohort, 69% of the patients were male; the mean age was 45 years.
“Multimorbidity and comorbidity both essentially mean the same thing but from different perspectives,” Sizheng Steven Zhao, MD, said at the annual conference of the British Society for Rheumatology.
“Many of us will be familiar with comorbidity and this is a concept that has one index disease at the center and we consider all other conditions to be coexisting. This is a model you typically see in hospital settings, so you go to see your rheumatologist for your axSpA and your cardiologist for your cardiovascular disease,” he explained.
“From a patient’s perspective, that’s probably a little less helpful to go from specialist to specialist, each only interested in one condition so, quite rightly, there’s been a push towards providing more holistic care with the patient at the center, and this is really encapsulated by the concept of multimorbidity, which is just defined as the coexistence of any two conditions.” The multimorbidity model is particularly suited to a primary care setting rather than a hospital setting, he added.
“Whatever you want to call it, it’s becoming more of a problem,” Dr. Zhao said. There is increasing multimorbidity as people age, and it’s a common problem in those with rheumatic diseases because of shared risk factors, the consequences of systemic inflammation, and how patients are treated.
Prior studies of multimorbidity in axSpA have either been of individual comorbid diseases or counts. Dr. Zhao, a clinical research fellow at Aintree University Hospital in Liverpool, England, and his associates looked at how coexisting conditions might cluster together to provide insight into how these might be better managed collectively in patients with axSpA.
The investigators used an adapted form of the Radner index for determining multimorbidity in patients consecutively seen at the Aintree University Hospital’s rheumatology clinic between 2010 and 2017. The Radner index was adapted to consider 40 chronic conditions, including fibromyalgia and osteoporosis but excluding rheumatic diseases and their extra-articular manifestations.
Dr. Zhao and his colleagues used regression models adjusted for age, gender, symptom duration, smoking status, body mass index, social deprivation, and NSAID use to see what, if any, effect having one of these clusters of multimorbid conditions might have on quality of life, general health, disease activity, and functional impairment measures. Patients with certain clusters of conditions had worse scores, particularly those with coexisting depression and/or anxiety and those with fibromyalgia, irritable bowel syndrome, or both in addition to axSpA.
“These are important conditions to be aware of in the management of axSpA patients,” Dr. Zhao said.
These data are consistent with what has already been published, with between 50% and 60% of patients having other conditions, particularly depression, said consultant rheumatologist Helena Marzo-Ortega, MD, of Leeds Teaching Hospitals NHS Trust, who chaired the session. “These are really important numbers for us to remember in the clinic,” she said.
None of the investigators reported having relevant disclosures.
SOURCE: Zhao S et al., Rheumatology 2019;58(suppl 3), Abstract 035.
BIRMINGHAM, ENGLAND – Almost two-thirds of patients with axial spondyloarthritis (axSpA) have more than one medical condition, and these may cluster together, the results of a single-center, cross-sectional study have shown.
Of 419 patients, 61% had multiple conditions, with a median of at least one other condition in addition to axSpA, but some patients had as many as five. The most common other conditions in patients with axSpA were hypertension (19% of patients), depression (16%), and dyspepsia (11%).
In all, 15 clusters of conditions were found, each dominated by one or two conditions in addition to axSpA. The three largest clusters were hypertension and cardiovascular disease (n = 88), anxiety and depression (n = 50), and dyspepsia (n = 31). In the cohort, 69% of the patients were male; the mean age was 45 years.
“Multimorbidity and comorbidity both essentially mean the same thing but from different perspectives,” Sizheng Steven Zhao, MD, said at the annual conference of the British Society for Rheumatology.
“Many of us will be familiar with comorbidity and this is a concept that has one index disease at the center and we consider all other conditions to be coexisting. This is a model you typically see in hospital settings, so you go to see your rheumatologist for your axSpA and your cardiologist for your cardiovascular disease,” he explained.
“From a patient’s perspective, that’s probably a little less helpful to go from specialist to specialist, each only interested in one condition so, quite rightly, there’s been a push towards providing more holistic care with the patient at the center, and this is really encapsulated by the concept of multimorbidity, which is just defined as the coexistence of any two conditions.” The multimorbidity model is particularly suited to a primary care setting rather than a hospital setting, he added.
“Whatever you want to call it, it’s becoming more of a problem,” Dr. Zhao said. There is increasing multimorbidity as people age, and it’s a common problem in those with rheumatic diseases because of shared risk factors, the consequences of systemic inflammation, and how patients are treated.
Prior studies of multimorbidity in axSpA have either been of individual comorbid diseases or counts. Dr. Zhao, a clinical research fellow at Aintree University Hospital in Liverpool, England, and his associates looked at how coexisting conditions might cluster together to provide insight into how these might be better managed collectively in patients with axSpA.
The investigators used an adapted form of the Radner index for determining multimorbidity in patients consecutively seen at the Aintree University Hospital’s rheumatology clinic between 2010 and 2017. The Radner index was adapted to consider 40 chronic conditions, including fibromyalgia and osteoporosis but excluding rheumatic diseases and their extra-articular manifestations.
Dr. Zhao and his colleagues used regression models adjusted for age, gender, symptom duration, smoking status, body mass index, social deprivation, and NSAID use to see what, if any, effect having one of these clusters of multimorbid conditions might have on quality of life, general health, disease activity, and functional impairment measures. Patients with certain clusters of conditions had worse scores, particularly those with coexisting depression and/or anxiety and those with fibromyalgia, irritable bowel syndrome, or both in addition to axSpA.
“These are important conditions to be aware of in the management of axSpA patients,” Dr. Zhao said.
These data are consistent with what has already been published, with between 50% and 60% of patients having other conditions, particularly depression, said consultant rheumatologist Helena Marzo-Ortega, MD, of Leeds Teaching Hospitals NHS Trust, who chaired the session. “These are really important numbers for us to remember in the clinic,” she said.
None of the investigators reported having relevant disclosures.
SOURCE: Zhao S et al., Rheumatology 2019;58(suppl 3), Abstract 035.
BIRMINGHAM, ENGLAND – Almost two-thirds of patients with axial spondyloarthritis (axSpA) have more than one medical condition, and these may cluster together, the results of a single-center, cross-sectional study have shown.
Of 419 patients, 61% had multiple conditions, with a median of at least one other condition in addition to axSpA, but some patients had as many as five. The most common other conditions in patients with axSpA were hypertension (19% of patients), depression (16%), and dyspepsia (11%).
In all, 15 clusters of conditions were found, each dominated by one or two conditions in addition to axSpA. The three largest clusters were hypertension and cardiovascular disease (n = 88), anxiety and depression (n = 50), and dyspepsia (n = 31). In the cohort, 69% of the patients were male; the mean age was 45 years.
“Multimorbidity and comorbidity both essentially mean the same thing but from different perspectives,” Sizheng Steven Zhao, MD, said at the annual conference of the British Society for Rheumatology.
“Many of us will be familiar with comorbidity and this is a concept that has one index disease at the center and we consider all other conditions to be coexisting. This is a model you typically see in hospital settings, so you go to see your rheumatologist for your axSpA and your cardiologist for your cardiovascular disease,” he explained.
“From a patient’s perspective, that’s probably a little less helpful to go from specialist to specialist, each only interested in one condition so, quite rightly, there’s been a push towards providing more holistic care with the patient at the center, and this is really encapsulated by the concept of multimorbidity, which is just defined as the coexistence of any two conditions.” The multimorbidity model is particularly suited to a primary care setting rather than a hospital setting, he added.
“Whatever you want to call it, it’s becoming more of a problem,” Dr. Zhao said. There is increasing multimorbidity as people age, and it’s a common problem in those with rheumatic diseases because of shared risk factors, the consequences of systemic inflammation, and how patients are treated.
Prior studies of multimorbidity in axSpA have either been of individual comorbid diseases or counts. Dr. Zhao, a clinical research fellow at Aintree University Hospital in Liverpool, England, and his associates looked at how coexisting conditions might cluster together to provide insight into how these might be better managed collectively in patients with axSpA.
The investigators used an adapted form of the Radner index for determining multimorbidity in patients consecutively seen at the Aintree University Hospital’s rheumatology clinic between 2010 and 2017. The Radner index was adapted to consider 40 chronic conditions, including fibromyalgia and osteoporosis but excluding rheumatic diseases and their extra-articular manifestations.
Dr. Zhao and his colleagues used regression models adjusted for age, gender, symptom duration, smoking status, body mass index, social deprivation, and NSAID use to see what, if any, effect having one of these clusters of multimorbid conditions might have on quality of life, general health, disease activity, and functional impairment measures. Patients with certain clusters of conditions had worse scores, particularly those with coexisting depression and/or anxiety and those with fibromyalgia, irritable bowel syndrome, or both in addition to axSpA.
“These are important conditions to be aware of in the management of axSpA patients,” Dr. Zhao said.
These data are consistent with what has already been published, with between 50% and 60% of patients having other conditions, particularly depression, said consultant rheumatologist Helena Marzo-Ortega, MD, of Leeds Teaching Hospitals NHS Trust, who chaired the session. “These are really important numbers for us to remember in the clinic,” she said.
None of the investigators reported having relevant disclosures.
SOURCE: Zhao S et al., Rheumatology 2019;58(suppl 3), Abstract 035.
REPORTING FROM BSR 2019
Tanezumab acts fast for OA pain relief
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
REPORTING FROM OARSI 2019
Key clinical point: The nerve growth factor inhibitor tanezumab brings rapid improvement in pain.
Major finding: Tanezumab-treated patients experienced significant pain reduction within 3-5 days after their first dose.
Study details: This was a phase 3, prospective, multicenter, double-blind, placebo-controlled trial in 696 patients with refractory pain attributable to knee or hip OA.
Disclosures: The presenter reported serving as a consultant to Pfizer and Eli Lilly, which cosponsored the phase 3 trial.
Source: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
Warfarin boosts OA risk in Rotterdam Study
TORONTO – The use of warfarin or related vitamin K antagonists was associated with a more than 100% increased risk of incident or progressive knee or hip osteoarthritis in the Rotterdam Study, Cindy G. Boer reported at the OARSI 2019 World Congress.
A biologically plausible mechanism exists for this relationship, added Ms. Boer, a PhD student with a special interest in the molecular genetics of OA at Erasmus University, Rotterdam, the Netherlands.
In a previous genetic study, she and her coinvestigators identified two genetic variants that result in loss of function of matrix Gla protein (MGP), a key inhibitor of cartilage calcification. They showed that the presence of these alleles was associated with a significantly increased risk of hand OA, which makes sense because increased calcification within a vulnerable joint promotes OA.
“The interesting thing here is that, in order for MGP to inhibit calcification, it needs to be gamma-carboxylated by vitamin K. If it’s not gamma-carboxylated it cannot inhibit calcification,” Ms. Boer said at the meeting sponsored by the Osteoarthritis Research Society International.
This observation led her to hypothesize that patients on warfarin or other vitamin K antagonists might have an increased risk of developing new-onset OA or, if they already had OA, of experiencing disease progression, since their medication inhibits MGP. To test this hypothesis, she and her coinvestigators turned to the landmark Rotterdam Study, a prospective, population-based cohort study of 15,000 participants, ongoing since 1990. Two large cohorts within the study had data available on the incidence and progression of radiographic knee and hip OA, one group over the course of 5 years of follow-up, the other with 10 years.
Serial x-rays of 8,845 knee joints were available, including 657 of warfarin users. Their rate of incident or progressive knee OA was 13%, compared with 5.9% in the nonusers in an analysis adjusted for age, sex, BMI, baseline OA status, and time between study visits.
In a similar vein, the rate of incident or progressive hip OA was 12% in the warfarin users, compared with 3.1% in nonusers.
About 80% of the OA endpoints in this analysis involved incident OA, defined radiographically as Kellgren-Lawrence grade 2. Progressive OA was defined as going from grade 2 at baseline to grade 3-4 during follow-up.
There was a signal of a treatment duration-related effect, with OA rates trending highest in individuals on warfarin for longer than 365 days, followed by those on the oral anticoagulant for more than 100 days, who in turn had higher rates than those on warfarin for less time.
Ms. Boer said an important next step in this research is to replicate the warfarin/OA association in an independent cohort. Also, she and her coworkers are now gathering OA incidence and progression data in patients on direct oral anticoagulants rather than warfarin to test the hypothesis that they will not have an increased rate of OA, compared with nonusers, since these newer agents don’t affect vitamin K. Of course, if they do turn out to have an elevated risk, it would point to one or more of the conditions for which oral anticoagulants are commonly prescribed as the explanation.
She reported having no financial conflicts regarding her study, sponsored by Erasmus University and the Dutch government.
TORONTO – The use of warfarin or related vitamin K antagonists was associated with a more than 100% increased risk of incident or progressive knee or hip osteoarthritis in the Rotterdam Study, Cindy G. Boer reported at the OARSI 2019 World Congress.
A biologically plausible mechanism exists for this relationship, added Ms. Boer, a PhD student with a special interest in the molecular genetics of OA at Erasmus University, Rotterdam, the Netherlands.
In a previous genetic study, she and her coinvestigators identified two genetic variants that result in loss of function of matrix Gla protein (MGP), a key inhibitor of cartilage calcification. They showed that the presence of these alleles was associated with a significantly increased risk of hand OA, which makes sense because increased calcification within a vulnerable joint promotes OA.
“The interesting thing here is that, in order for MGP to inhibit calcification, it needs to be gamma-carboxylated by vitamin K. If it’s not gamma-carboxylated it cannot inhibit calcification,” Ms. Boer said at the meeting sponsored by the Osteoarthritis Research Society International.
This observation led her to hypothesize that patients on warfarin or other vitamin K antagonists might have an increased risk of developing new-onset OA or, if they already had OA, of experiencing disease progression, since their medication inhibits MGP. To test this hypothesis, she and her coinvestigators turned to the landmark Rotterdam Study, a prospective, population-based cohort study of 15,000 participants, ongoing since 1990. Two large cohorts within the study had data available on the incidence and progression of radiographic knee and hip OA, one group over the course of 5 years of follow-up, the other with 10 years.
Serial x-rays of 8,845 knee joints were available, including 657 of warfarin users. Their rate of incident or progressive knee OA was 13%, compared with 5.9% in the nonusers in an analysis adjusted for age, sex, BMI, baseline OA status, and time between study visits.
In a similar vein, the rate of incident or progressive hip OA was 12% in the warfarin users, compared with 3.1% in nonusers.
About 80% of the OA endpoints in this analysis involved incident OA, defined radiographically as Kellgren-Lawrence grade 2. Progressive OA was defined as going from grade 2 at baseline to grade 3-4 during follow-up.
There was a signal of a treatment duration-related effect, with OA rates trending highest in individuals on warfarin for longer than 365 days, followed by those on the oral anticoagulant for more than 100 days, who in turn had higher rates than those on warfarin for less time.
Ms. Boer said an important next step in this research is to replicate the warfarin/OA association in an independent cohort. Also, she and her coworkers are now gathering OA incidence and progression data in patients on direct oral anticoagulants rather than warfarin to test the hypothesis that they will not have an increased rate of OA, compared with nonusers, since these newer agents don’t affect vitamin K. Of course, if they do turn out to have an elevated risk, it would point to one or more of the conditions for which oral anticoagulants are commonly prescribed as the explanation.
She reported having no financial conflicts regarding her study, sponsored by Erasmus University and the Dutch government.
TORONTO – The use of warfarin or related vitamin K antagonists was associated with a more than 100% increased risk of incident or progressive knee or hip osteoarthritis in the Rotterdam Study, Cindy G. Boer reported at the OARSI 2019 World Congress.
A biologically plausible mechanism exists for this relationship, added Ms. Boer, a PhD student with a special interest in the molecular genetics of OA at Erasmus University, Rotterdam, the Netherlands.
In a previous genetic study, she and her coinvestigators identified two genetic variants that result in loss of function of matrix Gla protein (MGP), a key inhibitor of cartilage calcification. They showed that the presence of these alleles was associated with a significantly increased risk of hand OA, which makes sense because increased calcification within a vulnerable joint promotes OA.
“The interesting thing here is that, in order for MGP to inhibit calcification, it needs to be gamma-carboxylated by vitamin K. If it’s not gamma-carboxylated it cannot inhibit calcification,” Ms. Boer said at the meeting sponsored by the Osteoarthritis Research Society International.
This observation led her to hypothesize that patients on warfarin or other vitamin K antagonists might have an increased risk of developing new-onset OA or, if they already had OA, of experiencing disease progression, since their medication inhibits MGP. To test this hypothesis, she and her coinvestigators turned to the landmark Rotterdam Study, a prospective, population-based cohort study of 15,000 participants, ongoing since 1990. Two large cohorts within the study had data available on the incidence and progression of radiographic knee and hip OA, one group over the course of 5 years of follow-up, the other with 10 years.
Serial x-rays of 8,845 knee joints were available, including 657 of warfarin users. Their rate of incident or progressive knee OA was 13%, compared with 5.9% in the nonusers in an analysis adjusted for age, sex, BMI, baseline OA status, and time between study visits.
In a similar vein, the rate of incident or progressive hip OA was 12% in the warfarin users, compared with 3.1% in nonusers.
About 80% of the OA endpoints in this analysis involved incident OA, defined radiographically as Kellgren-Lawrence grade 2. Progressive OA was defined as going from grade 2 at baseline to grade 3-4 during follow-up.
There was a signal of a treatment duration-related effect, with OA rates trending highest in individuals on warfarin for longer than 365 days, followed by those on the oral anticoagulant for more than 100 days, who in turn had higher rates than those on warfarin for less time.
Ms. Boer said an important next step in this research is to replicate the warfarin/OA association in an independent cohort. Also, she and her coworkers are now gathering OA incidence and progression data in patients on direct oral anticoagulants rather than warfarin to test the hypothesis that they will not have an increased rate of OA, compared with nonusers, since these newer agents don’t affect vitamin K. Of course, if they do turn out to have an elevated risk, it would point to one or more of the conditions for which oral anticoagulants are commonly prescribed as the explanation.
She reported having no financial conflicts regarding her study, sponsored by Erasmus University and the Dutch government.
REPORTING FROM OARSI 2019












