User login
Arthritis joint pain, inactivity vary greatly across U.S.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
FROM MMWR
Interosseous tendon inflammation is common prior to RA
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
REPORTING FROM RWCS 2019
Click for Credit: Migraine & stroke risk; Aspirin for CV events; more
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Positive psoriatic arthritis screens occur often in psoriasis patients
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Benlysta approved for children with SLE
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
FDA approves new etanercept biosimilar, Eticovo
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
Twitter chat recap: Take-homes from LUPUS 2019
Despite negative trial findings, belimumab (Benlysta) remains a valid option for black lupus patients, so long as they have high disease activity and positive serology.
That was just one of the many useful messages from a robust question-and-answer session on Twitter April 23, about important findings from the recent LUPUS 2019 Congress in San Francisco. The Twitter chat was hosted by MDedge Rheumatology and led by Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both associate professors of rheumatology at the University of California, San Francisco (UCSF). The chat included scores of posts from over a dozen participants, most of them rheumatologists, and it’s worth a recap.
The EMBRACE trial
The belimumab EMBRACE trial was the first topic up to bat. The Food and Drug Administration ordered GlaxoSmithKline to conduct the trial as a condition of approval for lupus in 2011; phase 3 trials found no benefit among a small number of black subjects and even a suggestion of harm.
Although lupus is highly prevalent among black people, and outcomes are generally worse, EMBRACE was the first lupus trial to enroll an entirely black population; 345 patients were treated for a year at 10 mg/kg IV every 4 weeks. Inclusion criteria included disease activity scores of at least 8.
Overall, 49% of belimumab patients, and 42% on placebo, had a positive response, which meant a drop of 4 or more disease activity points, among other things. The difference was not statistically significant (P = .11).
However, GSK’s data showed a statistically significant benefit in favor of belimumab among people who entered with a disease activity score of at least 10 (53% vs. 41% for placebo), as well as for those with low complement levels (47% vs. 25%) and both anti–double stranded DNA antibodies and low complement (45% vs. 24%). Response rates were also significantly higher among the 57% of subjects who lived outside of the United States and Canada.
So what to make of the results?

“The EMBRACE efficacy data seems to reinforce what we already know: belimumab doesn’t work in a lot of patients, and it [has] the best chance of working in patients with higher baseline disease activity,” tweeted Dr. Yazdany.
As for prescribing, Sarah Patterson, MD, a postdoctoral fellow in the UCSF Division of Rheumatology, tweeted that the results “support the use of belimumab for black lupus patients with high disease activity” and positive serology.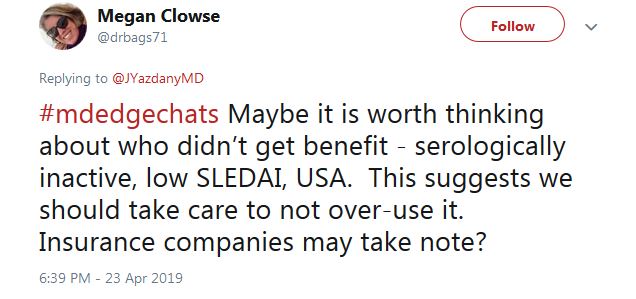
For those who don’t fit the treatment profile, “we should take care to not over-use it,” said Megan Clowse, MD, an associate professor of rheumatology at Duke University, Durham, N.C., in a tweet.
The HCQ adherence fail
Poor hydroxychloroquine (HCQ) adherence came up next on Twitter. The chat participants agreed it’s a huge problem, but no one knows why. Perhaps it’s because patients don’t feel a therapeutic effect or perhaps because GI problems and other side effects are worse than doctors think. Maybe there’s simply not enough social support to encourage people to stay on the drug, even though it’s the single most important medication in lupus.
A nine-study meta-analysis presented at LUPUS 2019 suggested a solution: blood levels. The odds of nonadherence were three times higher in patients below threshold HCQ levels, and although not statistically significant, the mean lupus disease activity index score was more than 3 points higher.
A rheumatologist on the chat said that he’s already checking them.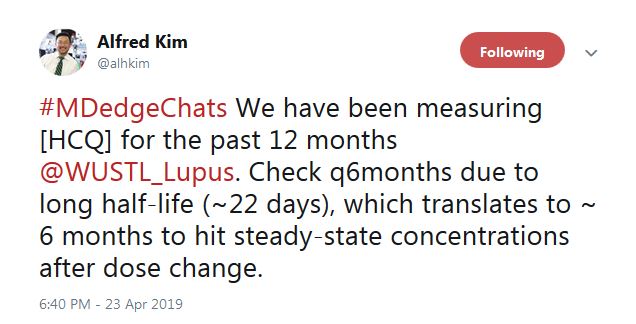
“We have been measuring HCQ for the past 12 months” at Washington University, St. Louis, according to Alfred Kim, MD, PhD, an assistant professor of rheumatology at the school. The data are just now coming in, he said, but it seems to be catching people.
That raised another question on the chat, however: What do you do with people who aren’t down with the program? They’ll be out the door and gone with the wrong words.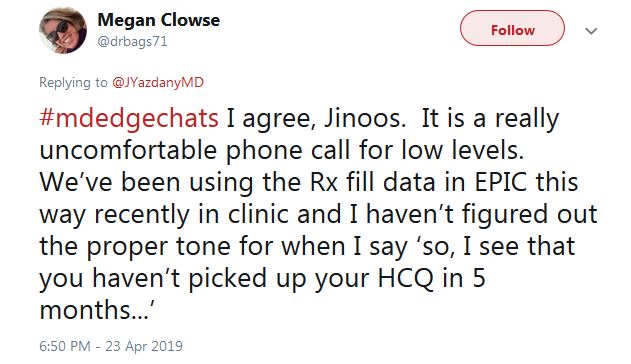
“I haven’t figured out the proper tone for when I say ‘So, I see that you haven’t picked up your HCQ in 5 months,’ ” tweeted Dr. Clowse. “It is a really uncomfortable phone call.”
Dr. Kim said that “I ‘gotcha’ them all the time. Guess what typically goes up after they get that 1st HCQ test? Their HCQ levels ... But this rise isn’t durable in our short experience. It goes back down later.”
He tweeted that overall “this is an opportunity to educate patients on the benefits of HCQ ... Most actually do not [know] why they are taking this medication, in our experience.” In another tweet, Dr. Kim said “I tell them I care,” and that checking HCQ levels “is one way of demonstrating how I want to improve their outcomes.”
Tiffany from #LupusChat thought that it’s time for doctors to sit down with patient advocates and hash it out. She tweeted that “I truly feel this would be a positive 1st step ... a two-way conversation that’s non-judgmental and focused on improving” outcomes and quality of life.
Baricitinib for lupus?
The final topic was baricitinib (Olumiant).
There were modest indications of benefit at 4 mg/day oral after 6 months in a phase 2 trial with 314 people. There were also serious infections in 6% versus 2% on 2 mg/day and 1% on placebo. One patient in the 4-mg/day arm (1%) developed deep vein thrombosis (DVT), but they were positive for antiphospholipid antibodies, which raise the clot risk.
Baricitinib is FDA approved as second line at 2 mg/day for adult rheumatoid arthritis; there’s a black box warning of malignancies, thrombosis, and serious infections.
“I’m not sure” the risk-benefit is in the right direction for baricitinib in lupus. “There were a significant number of serious infections ... for not a lot of effect on” disease activity, tweeted the Twitter chat coleader, Dr. Schmajuk. In a separate tweet, she said that, even in nonlupus patients, “we are avoiding [Janus kinase inhibitors] in patients with DVT risk factors. If I were a patient, [I’m] not sure I would want to take the risk.”
“Future studies with larger sample size and longer follow up ... are needed to address some of the concerns related to DVT,” said Zahi Touma, MD, an assistant professor of rheumatology at the University of Toronto.
Despite negative trial findings, belimumab (Benlysta) remains a valid option for black lupus patients, so long as they have high disease activity and positive serology.
That was just one of the many useful messages from a robust question-and-answer session on Twitter April 23, about important findings from the recent LUPUS 2019 Congress in San Francisco. The Twitter chat was hosted by MDedge Rheumatology and led by Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both associate professors of rheumatology at the University of California, San Francisco (UCSF). The chat included scores of posts from over a dozen participants, most of them rheumatologists, and it’s worth a recap.
The EMBRACE trial
The belimumab EMBRACE trial was the first topic up to bat. The Food and Drug Administration ordered GlaxoSmithKline to conduct the trial as a condition of approval for lupus in 2011; phase 3 trials found no benefit among a small number of black subjects and even a suggestion of harm.
Although lupus is highly prevalent among black people, and outcomes are generally worse, EMBRACE was the first lupus trial to enroll an entirely black population; 345 patients were treated for a year at 10 mg/kg IV every 4 weeks. Inclusion criteria included disease activity scores of at least 8.
Overall, 49% of belimumab patients, and 42% on placebo, had a positive response, which meant a drop of 4 or more disease activity points, among other things. The difference was not statistically significant (P = .11).
However, GSK’s data showed a statistically significant benefit in favor of belimumab among people who entered with a disease activity score of at least 10 (53% vs. 41% for placebo), as well as for those with low complement levels (47% vs. 25%) and both anti–double stranded DNA antibodies and low complement (45% vs. 24%). Response rates were also significantly higher among the 57% of subjects who lived outside of the United States and Canada.
So what to make of the results?

“The EMBRACE efficacy data seems to reinforce what we already know: belimumab doesn’t work in a lot of patients, and it [has] the best chance of working in patients with higher baseline disease activity,” tweeted Dr. Yazdany.
As for prescribing, Sarah Patterson, MD, a postdoctoral fellow in the UCSF Division of Rheumatology, tweeted that the results “support the use of belimumab for black lupus patients with high disease activity” and positive serology.
For those who don’t fit the treatment profile, “we should take care to not over-use it,” said Megan Clowse, MD, an associate professor of rheumatology at Duke University, Durham, N.C., in a tweet.
The HCQ adherence fail
Poor hydroxychloroquine (HCQ) adherence came up next on Twitter. The chat participants agreed it’s a huge problem, but no one knows why. Perhaps it’s because patients don’t feel a therapeutic effect or perhaps because GI problems and other side effects are worse than doctors think. Maybe there’s simply not enough social support to encourage people to stay on the drug, even though it’s the single most important medication in lupus.
A nine-study meta-analysis presented at LUPUS 2019 suggested a solution: blood levels. The odds of nonadherence were three times higher in patients below threshold HCQ levels, and although not statistically significant, the mean lupus disease activity index score was more than 3 points higher.
A rheumatologist on the chat said that he’s already checking them.
“We have been measuring HCQ for the past 12 months” at Washington University, St. Louis, according to Alfred Kim, MD, PhD, an assistant professor of rheumatology at the school. The data are just now coming in, he said, but it seems to be catching people.
That raised another question on the chat, however: What do you do with people who aren’t down with the program? They’ll be out the door and gone with the wrong words.
“I haven’t figured out the proper tone for when I say ‘So, I see that you haven’t picked up your HCQ in 5 months,’ ” tweeted Dr. Clowse. “It is a really uncomfortable phone call.”
Dr. Kim said that “I ‘gotcha’ them all the time. Guess what typically goes up after they get that 1st HCQ test? Their HCQ levels ... But this rise isn’t durable in our short experience. It goes back down later.”
He tweeted that overall “this is an opportunity to educate patients on the benefits of HCQ ... Most actually do not [know] why they are taking this medication, in our experience.” In another tweet, Dr. Kim said “I tell them I care,” and that checking HCQ levels “is one way of demonstrating how I want to improve their outcomes.”
Tiffany from #LupusChat thought that it’s time for doctors to sit down with patient advocates and hash it out. She tweeted that “I truly feel this would be a positive 1st step ... a two-way conversation that’s non-judgmental and focused on improving” outcomes and quality of life.
Baricitinib for lupus?
The final topic was baricitinib (Olumiant).
There were modest indications of benefit at 4 mg/day oral after 6 months in a phase 2 trial with 314 people. There were also serious infections in 6% versus 2% on 2 mg/day and 1% on placebo. One patient in the 4-mg/day arm (1%) developed deep vein thrombosis (DVT), but they were positive for antiphospholipid antibodies, which raise the clot risk.
Baricitinib is FDA approved as second line at 2 mg/day for adult rheumatoid arthritis; there’s a black box warning of malignancies, thrombosis, and serious infections.
“I’m not sure” the risk-benefit is in the right direction for baricitinib in lupus. “There were a significant number of serious infections ... for not a lot of effect on” disease activity, tweeted the Twitter chat coleader, Dr. Schmajuk. In a separate tweet, she said that, even in nonlupus patients, “we are avoiding [Janus kinase inhibitors] in patients with DVT risk factors. If I were a patient, [I’m] not sure I would want to take the risk.”
“Future studies with larger sample size and longer follow up ... are needed to address some of the concerns related to DVT,” said Zahi Touma, MD, an assistant professor of rheumatology at the University of Toronto.
Despite negative trial findings, belimumab (Benlysta) remains a valid option for black lupus patients, so long as they have high disease activity and positive serology.
That was just one of the many useful messages from a robust question-and-answer session on Twitter April 23, about important findings from the recent LUPUS 2019 Congress in San Francisco. The Twitter chat was hosted by MDedge Rheumatology and led by Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both associate professors of rheumatology at the University of California, San Francisco (UCSF). The chat included scores of posts from over a dozen participants, most of them rheumatologists, and it’s worth a recap.
The EMBRACE trial
The belimumab EMBRACE trial was the first topic up to bat. The Food and Drug Administration ordered GlaxoSmithKline to conduct the trial as a condition of approval for lupus in 2011; phase 3 trials found no benefit among a small number of black subjects and even a suggestion of harm.
Although lupus is highly prevalent among black people, and outcomes are generally worse, EMBRACE was the first lupus trial to enroll an entirely black population; 345 patients were treated for a year at 10 mg/kg IV every 4 weeks. Inclusion criteria included disease activity scores of at least 8.
Overall, 49% of belimumab patients, and 42% on placebo, had a positive response, which meant a drop of 4 or more disease activity points, among other things. The difference was not statistically significant (P = .11).
However, GSK’s data showed a statistically significant benefit in favor of belimumab among people who entered with a disease activity score of at least 10 (53% vs. 41% for placebo), as well as for those with low complement levels (47% vs. 25%) and both anti–double stranded DNA antibodies and low complement (45% vs. 24%). Response rates were also significantly higher among the 57% of subjects who lived outside of the United States and Canada.
So what to make of the results?

“The EMBRACE efficacy data seems to reinforce what we already know: belimumab doesn’t work in a lot of patients, and it [has] the best chance of working in patients with higher baseline disease activity,” tweeted Dr. Yazdany.
As for prescribing, Sarah Patterson, MD, a postdoctoral fellow in the UCSF Division of Rheumatology, tweeted that the results “support the use of belimumab for black lupus patients with high disease activity” and positive serology.
For those who don’t fit the treatment profile, “we should take care to not over-use it,” said Megan Clowse, MD, an associate professor of rheumatology at Duke University, Durham, N.C., in a tweet.
The HCQ adherence fail
Poor hydroxychloroquine (HCQ) adherence came up next on Twitter. The chat participants agreed it’s a huge problem, but no one knows why. Perhaps it’s because patients don’t feel a therapeutic effect or perhaps because GI problems and other side effects are worse than doctors think. Maybe there’s simply not enough social support to encourage people to stay on the drug, even though it’s the single most important medication in lupus.
A nine-study meta-analysis presented at LUPUS 2019 suggested a solution: blood levels. The odds of nonadherence were three times higher in patients below threshold HCQ levels, and although not statistically significant, the mean lupus disease activity index score was more than 3 points higher.
A rheumatologist on the chat said that he’s already checking them.
“We have been measuring HCQ for the past 12 months” at Washington University, St. Louis, according to Alfred Kim, MD, PhD, an assistant professor of rheumatology at the school. The data are just now coming in, he said, but it seems to be catching people.
That raised another question on the chat, however: What do you do with people who aren’t down with the program? They’ll be out the door and gone with the wrong words.
“I haven’t figured out the proper tone for when I say ‘So, I see that you haven’t picked up your HCQ in 5 months,’ ” tweeted Dr. Clowse. “It is a really uncomfortable phone call.”
Dr. Kim said that “I ‘gotcha’ them all the time. Guess what typically goes up after they get that 1st HCQ test? Their HCQ levels ... But this rise isn’t durable in our short experience. It goes back down later.”
He tweeted that overall “this is an opportunity to educate patients on the benefits of HCQ ... Most actually do not [know] why they are taking this medication, in our experience.” In another tweet, Dr. Kim said “I tell them I care,” and that checking HCQ levels “is one way of demonstrating how I want to improve their outcomes.”
Tiffany from #LupusChat thought that it’s time for doctors to sit down with patient advocates and hash it out. She tweeted that “I truly feel this would be a positive 1st step ... a two-way conversation that’s non-judgmental and focused on improving” outcomes and quality of life.
Baricitinib for lupus?
The final topic was baricitinib (Olumiant).
There were modest indications of benefit at 4 mg/day oral after 6 months in a phase 2 trial with 314 people. There were also serious infections in 6% versus 2% on 2 mg/day and 1% on placebo. One patient in the 4-mg/day arm (1%) developed deep vein thrombosis (DVT), but they were positive for antiphospholipid antibodies, which raise the clot risk.
Baricitinib is FDA approved as second line at 2 mg/day for adult rheumatoid arthritis; there’s a black box warning of malignancies, thrombosis, and serious infections.
“I’m not sure” the risk-benefit is in the right direction for baricitinib in lupus. “There were a significant number of serious infections ... for not a lot of effect on” disease activity, tweeted the Twitter chat coleader, Dr. Schmajuk. In a separate tweet, she said that, even in nonlupus patients, “we are avoiding [Janus kinase inhibitors] in patients with DVT risk factors. If I were a patient, [I’m] not sure I would want to take the risk.”
“Future studies with larger sample size and longer follow up ... are needed to address some of the concerns related to DVT,” said Zahi Touma, MD, an assistant professor of rheumatology at the University of Toronto.
FROM LUPUS 2019
Measuring hydroxychloroquine blood levels could inform safe, optimal dosing
SAN FRANCISCO – , according to an investigation of the Hopkins Lupus Cohort, an ongoing longitudinal study of lupus patients in the Baltimore area.
As innocuous as the assertions seem, they are anything but. They directly contradict a 2014 investigation from Kaiser Permanente that put the retinopathy risk after 20 years at almost 40%; that finding led directly to an American Academy of Ophthalmology recommendation to reduce the maximum hydroxychloroquine dose from 6.5 mg/kg per day ideal weight to 5 mg/kg real weight, where it remains to this day.
Meanwhile, very few rheumatologists have access to hydroxychloroquine (HCQ) blood levels because most commercial labs don’t offer them. Plasma testing is widely available, but it’s nowhere near as good, according to Michelle Petri, MD, a rheumatology professor at Johns Hopkins University, Baltimore; director of the Hopkins Lupus Cohort; and a respected authority on lupus management.
“The Kaiser Permanente study was very worrisome,” she said. “I remember that I thought it didn’t fit my practice at all; I don’t see 40%. It made me even more concerned when the ophthalmologists” reduced the dose, “because hydroxychloroquine is the most important medicine I have for my lupus patients; it is the only one that improves survival. We don’t want to scare our patients into thinking that 40% of them are going to have retinopathy.”
Dueling studies
Dr. Petri’s concerns led her and her team to launch their own investigation; they followed 537 Baltimore cohort members on HCQ as they went through eye exams by Hopkins retinopathy specialists, often with optical coherence tomography (OCT). With a sensitivity of 93% and specificity of 84%, OCT is the best screening method available.
“We found that the risk of retinopathy is not nearly as high as Kaiser Permanente found,” just 11.46% (11/96) with 16-20 years of use, and 8% (6/75) with 21 or more years. On average, “the risk is probably about 10% after 16 or more years, not 40%,” Dr. Petri said at an international congress on systemic lupus erythematosus.
Patients with “possible” retinopathy were not included in the analysis.
When asked for comment, Ronald Melles, MD, a Kaiser ophthalmologist in Redwood City, Calif.; one of the two authors on the Kaiser study; and an author on the subsequent AAO recommendations, stood by his work.
“A rate of 12% retinopathy after 16 years of use ... seems right in line with what we found.” However, “the fact that the rate went down to 8%” after 20 years does not make sense; “the longer you are on the medicine, the more likely you would be to develop the toxicity,” he said.
Maybe the fluctuation had to do with the fact that there were only 75 patients in the Hopkins study on HCQ past 20 years, whereas “we looked at 2,361 patients, and 238 were on the medication for” 20 years or more. Patients over 5.0 mg/kg per day had a 5.67-fold higher risk”of retinopathy, he said (univariate analysis, P less than .001).
Dr. Petri wasn’t buying it. The across the board recommendation was made “without any recognition that if you reduce the dose, you reduce the benefit,” she said.
A new referee: blood levels
Dr. Petri and her team also found that HCQ blood levels correlated with retinopathy, and it was a direct relationship. Patients in the highest maximum tertile (1,753-6,281 ng/mL) had a retinopathy rate of 6.7%, a good deal higher than patients in lower tertiles. It was the same story with the highest mean tertile (1,117-3,513 ng/mL). Retinopathy in that group occurred in 7.9% versus 3.7% or less in lower tertiles. The findings were statistically significant.
Patients in the third tertile “are at the greatest risk, so I reduce their dose,” but “I do not want to reduce the dose across the board” to 5 mg/kg per day; that’s overreach. The tertile approach, if it pans out, might be a better way, she said.
The problem with plasma levels is that HCQ binds to red blood cells, so plasma levels are artificially low and do not indicate the true HCQ load. For now, just one company in the United States offers HCQ blood levels: Exagen.
“We have to get the big companies to start offering” this, and “I want rheumatologists to adopt it. I am lucky at Hopkins [because] we have our own homegrown blood level assay,” she said.
Dr. Melles agreed that tracking blood level makes sense, “but the literature I am aware of has not been able to closely correlate either lupus disease activity or retinal toxicity with blood levels. Also, we have seen some patients at lower doses develop toxicity and other patients on higher doses without any detectable changes.”
Still, “we would like to see [this] studied more, perhaps with newer analytic methods,” said his coauthor on the Kaiser study, and also the lead author on the AAO guidelines, Michael Marmor, MD, professor emeritus of ophthalmology at Stanford (Calif.) University.
In the end, on the same team
Dr. Petri said there is interest among some of her fellow members of the American College of Rheumatology to work with AAO to revise the guidelines. “Until then,” she said, “I want the ophthalmologists to withdraw” them.
She’s worried about undertreatment and believes that the previous AAO guideline, up to 6.5 mg/kg per day ideal weight, was fine, “with some understanding that there are high-risk groups, such as the elderly and people with renal impairment, where the dose should be reduced.”
“No matter how obese a patient is, I cap it at 400 mg/day,” she said, and, with the luxury of HCQ blood level testing, “no matter the weight, if the person is in the upper tertile, I reduce the dose.”
Dr. Marmor agreed that “if rheumatologists prescribe 5 mg/kg real weight and do not stress compliance, some patients may indeed be underdosed.”
“However, that is a fault of the doctor and patient relationship,” he said, “not the guideline; we do not feel it ethical to prescribe higher doses which could increase toxicity in reliable patients ... just because some patients might be unreliable.”
Overall, “I have not heard complaints from rheumatologists in our area, who try hard to follow the current recommendations. ... any doctor can use the dose he or she feels is necessary for a patient. Several recent reports [also] suggest the incidence of toxicity is falling now with usage of AAO guidelines, [and] I am not aware of any data” showing that management has become less effective, he said.
In the meantime, “I assure you that AAO wants ... to serve both specialties, and will change the guidelines when there is new, defensible data,” he added.
The Hopkins team found that the risk of HCQ retinopathy was highest in men and white patients, as well as older people. Body mass index and hypertension also predicted retina issues.
“As screening tests are frequently abnormal due to causes other than HCQ ... stopping [it] based on an abnormal test without confirmation from a retinopathy expert could needlessly deprive an SLE patient of an important medication,” they said.
The Hopkins Lupus Cohort is funded by the National Institutes of Health. The physicians didn’t have any relevant disclosures.
SOURCES: Petri M et al. Lupus Sci Med. 2019;6(suppl 1). Abstracts 15 and 16.
SAN FRANCISCO – , according to an investigation of the Hopkins Lupus Cohort, an ongoing longitudinal study of lupus patients in the Baltimore area.
As innocuous as the assertions seem, they are anything but. They directly contradict a 2014 investigation from Kaiser Permanente that put the retinopathy risk after 20 years at almost 40%; that finding led directly to an American Academy of Ophthalmology recommendation to reduce the maximum hydroxychloroquine dose from 6.5 mg/kg per day ideal weight to 5 mg/kg real weight, where it remains to this day.
Meanwhile, very few rheumatologists have access to hydroxychloroquine (HCQ) blood levels because most commercial labs don’t offer them. Plasma testing is widely available, but it’s nowhere near as good, according to Michelle Petri, MD, a rheumatology professor at Johns Hopkins University, Baltimore; director of the Hopkins Lupus Cohort; and a respected authority on lupus management.
“The Kaiser Permanente study was very worrisome,” she said. “I remember that I thought it didn’t fit my practice at all; I don’t see 40%. It made me even more concerned when the ophthalmologists” reduced the dose, “because hydroxychloroquine is the most important medicine I have for my lupus patients; it is the only one that improves survival. We don’t want to scare our patients into thinking that 40% of them are going to have retinopathy.”
Dueling studies
Dr. Petri’s concerns led her and her team to launch their own investigation; they followed 537 Baltimore cohort members on HCQ as they went through eye exams by Hopkins retinopathy specialists, often with optical coherence tomography (OCT). With a sensitivity of 93% and specificity of 84%, OCT is the best screening method available.
“We found that the risk of retinopathy is not nearly as high as Kaiser Permanente found,” just 11.46% (11/96) with 16-20 years of use, and 8% (6/75) with 21 or more years. On average, “the risk is probably about 10% after 16 or more years, not 40%,” Dr. Petri said at an international congress on systemic lupus erythematosus.
Patients with “possible” retinopathy were not included in the analysis.
When asked for comment, Ronald Melles, MD, a Kaiser ophthalmologist in Redwood City, Calif.; one of the two authors on the Kaiser study; and an author on the subsequent AAO recommendations, stood by his work.
“A rate of 12% retinopathy after 16 years of use ... seems right in line with what we found.” However, “the fact that the rate went down to 8%” after 20 years does not make sense; “the longer you are on the medicine, the more likely you would be to develop the toxicity,” he said.
Maybe the fluctuation had to do with the fact that there were only 75 patients in the Hopkins study on HCQ past 20 years, whereas “we looked at 2,361 patients, and 238 were on the medication for” 20 years or more. Patients over 5.0 mg/kg per day had a 5.67-fold higher risk”of retinopathy, he said (univariate analysis, P less than .001).
Dr. Petri wasn’t buying it. The across the board recommendation was made “without any recognition that if you reduce the dose, you reduce the benefit,” she said.
A new referee: blood levels
Dr. Petri and her team also found that HCQ blood levels correlated with retinopathy, and it was a direct relationship. Patients in the highest maximum tertile (1,753-6,281 ng/mL) had a retinopathy rate of 6.7%, a good deal higher than patients in lower tertiles. It was the same story with the highest mean tertile (1,117-3,513 ng/mL). Retinopathy in that group occurred in 7.9% versus 3.7% or less in lower tertiles. The findings were statistically significant.
Patients in the third tertile “are at the greatest risk, so I reduce their dose,” but “I do not want to reduce the dose across the board” to 5 mg/kg per day; that’s overreach. The tertile approach, if it pans out, might be a better way, she said.
The problem with plasma levels is that HCQ binds to red blood cells, so plasma levels are artificially low and do not indicate the true HCQ load. For now, just one company in the United States offers HCQ blood levels: Exagen.
“We have to get the big companies to start offering” this, and “I want rheumatologists to adopt it. I am lucky at Hopkins [because] we have our own homegrown blood level assay,” she said.
Dr. Melles agreed that tracking blood level makes sense, “but the literature I am aware of has not been able to closely correlate either lupus disease activity or retinal toxicity with blood levels. Also, we have seen some patients at lower doses develop toxicity and other patients on higher doses without any detectable changes.”
Still, “we would like to see [this] studied more, perhaps with newer analytic methods,” said his coauthor on the Kaiser study, and also the lead author on the AAO guidelines, Michael Marmor, MD, professor emeritus of ophthalmology at Stanford (Calif.) University.
In the end, on the same team
Dr. Petri said there is interest among some of her fellow members of the American College of Rheumatology to work with AAO to revise the guidelines. “Until then,” she said, “I want the ophthalmologists to withdraw” them.
She’s worried about undertreatment and believes that the previous AAO guideline, up to 6.5 mg/kg per day ideal weight, was fine, “with some understanding that there are high-risk groups, such as the elderly and people with renal impairment, where the dose should be reduced.”
“No matter how obese a patient is, I cap it at 400 mg/day,” she said, and, with the luxury of HCQ blood level testing, “no matter the weight, if the person is in the upper tertile, I reduce the dose.”
Dr. Marmor agreed that “if rheumatologists prescribe 5 mg/kg real weight and do not stress compliance, some patients may indeed be underdosed.”
“However, that is a fault of the doctor and patient relationship,” he said, “not the guideline; we do not feel it ethical to prescribe higher doses which could increase toxicity in reliable patients ... just because some patients might be unreliable.”
Overall, “I have not heard complaints from rheumatologists in our area, who try hard to follow the current recommendations. ... any doctor can use the dose he or she feels is necessary for a patient. Several recent reports [also] suggest the incidence of toxicity is falling now with usage of AAO guidelines, [and] I am not aware of any data” showing that management has become less effective, he said.
In the meantime, “I assure you that AAO wants ... to serve both specialties, and will change the guidelines when there is new, defensible data,” he added.
The Hopkins team found that the risk of HCQ retinopathy was highest in men and white patients, as well as older people. Body mass index and hypertension also predicted retina issues.
“As screening tests are frequently abnormal due to causes other than HCQ ... stopping [it] based on an abnormal test without confirmation from a retinopathy expert could needlessly deprive an SLE patient of an important medication,” they said.
The Hopkins Lupus Cohort is funded by the National Institutes of Health. The physicians didn’t have any relevant disclosures.
SOURCES: Petri M et al. Lupus Sci Med. 2019;6(suppl 1). Abstracts 15 and 16.
SAN FRANCISCO – , according to an investigation of the Hopkins Lupus Cohort, an ongoing longitudinal study of lupus patients in the Baltimore area.
As innocuous as the assertions seem, they are anything but. They directly contradict a 2014 investigation from Kaiser Permanente that put the retinopathy risk after 20 years at almost 40%; that finding led directly to an American Academy of Ophthalmology recommendation to reduce the maximum hydroxychloroquine dose from 6.5 mg/kg per day ideal weight to 5 mg/kg real weight, where it remains to this day.
Meanwhile, very few rheumatologists have access to hydroxychloroquine (HCQ) blood levels because most commercial labs don’t offer them. Plasma testing is widely available, but it’s nowhere near as good, according to Michelle Petri, MD, a rheumatology professor at Johns Hopkins University, Baltimore; director of the Hopkins Lupus Cohort; and a respected authority on lupus management.
“The Kaiser Permanente study was very worrisome,” she said. “I remember that I thought it didn’t fit my practice at all; I don’t see 40%. It made me even more concerned when the ophthalmologists” reduced the dose, “because hydroxychloroquine is the most important medicine I have for my lupus patients; it is the only one that improves survival. We don’t want to scare our patients into thinking that 40% of them are going to have retinopathy.”
Dueling studies
Dr. Petri’s concerns led her and her team to launch their own investigation; they followed 537 Baltimore cohort members on HCQ as they went through eye exams by Hopkins retinopathy specialists, often with optical coherence tomography (OCT). With a sensitivity of 93% and specificity of 84%, OCT is the best screening method available.
“We found that the risk of retinopathy is not nearly as high as Kaiser Permanente found,” just 11.46% (11/96) with 16-20 years of use, and 8% (6/75) with 21 or more years. On average, “the risk is probably about 10% after 16 or more years, not 40%,” Dr. Petri said at an international congress on systemic lupus erythematosus.
Patients with “possible” retinopathy were not included in the analysis.
When asked for comment, Ronald Melles, MD, a Kaiser ophthalmologist in Redwood City, Calif.; one of the two authors on the Kaiser study; and an author on the subsequent AAO recommendations, stood by his work.
“A rate of 12% retinopathy after 16 years of use ... seems right in line with what we found.” However, “the fact that the rate went down to 8%” after 20 years does not make sense; “the longer you are on the medicine, the more likely you would be to develop the toxicity,” he said.
Maybe the fluctuation had to do with the fact that there were only 75 patients in the Hopkins study on HCQ past 20 years, whereas “we looked at 2,361 patients, and 238 were on the medication for” 20 years or more. Patients over 5.0 mg/kg per day had a 5.67-fold higher risk”of retinopathy, he said (univariate analysis, P less than .001).
Dr. Petri wasn’t buying it. The across the board recommendation was made “without any recognition that if you reduce the dose, you reduce the benefit,” she said.
A new referee: blood levels
Dr. Petri and her team also found that HCQ blood levels correlated with retinopathy, and it was a direct relationship. Patients in the highest maximum tertile (1,753-6,281 ng/mL) had a retinopathy rate of 6.7%, a good deal higher than patients in lower tertiles. It was the same story with the highest mean tertile (1,117-3,513 ng/mL). Retinopathy in that group occurred in 7.9% versus 3.7% or less in lower tertiles. The findings were statistically significant.
Patients in the third tertile “are at the greatest risk, so I reduce their dose,” but “I do not want to reduce the dose across the board” to 5 mg/kg per day; that’s overreach. The tertile approach, if it pans out, might be a better way, she said.
The problem with plasma levels is that HCQ binds to red blood cells, so plasma levels are artificially low and do not indicate the true HCQ load. For now, just one company in the United States offers HCQ blood levels: Exagen.
“We have to get the big companies to start offering” this, and “I want rheumatologists to adopt it. I am lucky at Hopkins [because] we have our own homegrown blood level assay,” she said.
Dr. Melles agreed that tracking blood level makes sense, “but the literature I am aware of has not been able to closely correlate either lupus disease activity or retinal toxicity with blood levels. Also, we have seen some patients at lower doses develop toxicity and other patients on higher doses without any detectable changes.”
Still, “we would like to see [this] studied more, perhaps with newer analytic methods,” said his coauthor on the Kaiser study, and also the lead author on the AAO guidelines, Michael Marmor, MD, professor emeritus of ophthalmology at Stanford (Calif.) University.
In the end, on the same team
Dr. Petri said there is interest among some of her fellow members of the American College of Rheumatology to work with AAO to revise the guidelines. “Until then,” she said, “I want the ophthalmologists to withdraw” them.
She’s worried about undertreatment and believes that the previous AAO guideline, up to 6.5 mg/kg per day ideal weight, was fine, “with some understanding that there are high-risk groups, such as the elderly and people with renal impairment, where the dose should be reduced.”
“No matter how obese a patient is, I cap it at 400 mg/day,” she said, and, with the luxury of HCQ blood level testing, “no matter the weight, if the person is in the upper tertile, I reduce the dose.”
Dr. Marmor agreed that “if rheumatologists prescribe 5 mg/kg real weight and do not stress compliance, some patients may indeed be underdosed.”
“However, that is a fault of the doctor and patient relationship,” he said, “not the guideline; we do not feel it ethical to prescribe higher doses which could increase toxicity in reliable patients ... just because some patients might be unreliable.”
Overall, “I have not heard complaints from rheumatologists in our area, who try hard to follow the current recommendations. ... any doctor can use the dose he or she feels is necessary for a patient. Several recent reports [also] suggest the incidence of toxicity is falling now with usage of AAO guidelines, [and] I am not aware of any data” showing that management has become less effective, he said.
In the meantime, “I assure you that AAO wants ... to serve both specialties, and will change the guidelines when there is new, defensible data,” he added.
The Hopkins team found that the risk of HCQ retinopathy was highest in men and white patients, as well as older people. Body mass index and hypertension also predicted retina issues.
“As screening tests are frequently abnormal due to causes other than HCQ ... stopping [it] based on an abnormal test without confirmation from a retinopathy expert could needlessly deprive an SLE patient of an important medication,” they said.
The Hopkins Lupus Cohort is funded by the National Institutes of Health. The physicians didn’t have any relevant disclosures.
SOURCES: Petri M et al. Lupus Sci Med. 2019;6(suppl 1). Abstracts 15 and 16.
REPORTING FROM LUPUS 2019
CD40 ligand–binding protein safely lowered RA disease activity
A nonantibody scaffold protein that targets the CD40 ligand appears to dampen down autoimmune responses without the thromboembolic complications seen in trials of monoclonal antibodies against the CD40 ligand.
In a paper published in Science Translational Medicine, researchers presented the results of a phase 1a study in 59 healthy volunteers and phase 1b proof-of-concept study in 57 individuals with rheumatoid arthritis. Participants received either varying dosages of CD40 ligand (CD40L)–binding protein VIB4920 – a single dose in the healthy volunteers and seven doses in the phase 1b study – or placebo.
Jodi L. Karnell, PhD, of Viela Bio in Gaithersburg, Md., and coauthors, wrote that the CD40/CD40L pathway is known to play a key role in humoral immune responses and in the pathogenesis of several autoimmune diseases.
However, previous clinical trials of compounds targeting CD40L were stopped early because of an increased risk of adverse thromboembolic events related to platelet aggregation, despite showing potential benefits in lupus and immune thrombocytopenic purpura.
Preclinical studies of VIB4920 found that it blocked the expansion of CD40L-dependent human B cells without showing any signs of platelet aggregation. The authors said the platelet aggregation had been linked to a particular region of anti-CD40L monoclonal antibodies, but VIB4920 was engineered using a protein scaffold that did not contain that region.
In healthy volunteers, researchers saw a dose-dependent suppression of antibody production and reductions in B-cell proliferation, in recall response to a T cell–dependent antigen.
In individuals with rheumatoid arthritis, more than half of those treated with the two highest dosages of VIB4920 achieved low disease activity state or clinical remission by 12 weeks. Overall, there was also a significant decrease in disease activity, and dose-dependent decreases in rheumatoid factor autoantibodies and Vectra DA biomarker score, which is a composite of twelve rheumatoid arthritis–related biomarkers.
“The consistency of improvement across a variety of clinical and laboratory outcome measures further supports the potential clinical efficacy of VIB4920,” the authors wrote.
Researchers saw a similar rate of adverse events in the placebo and treatment arms of the study.
“VIB4920 represents an alternative to monoclonal antibody–based targeting of CD40L, which does not induce platelet aggregation in vitro and demonstrates a favorable safety profile in early clinical evaluation.”
The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or of Viela Bio.
SOURCE: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584
A nonantibody scaffold protein that targets the CD40 ligand appears to dampen down autoimmune responses without the thromboembolic complications seen in trials of monoclonal antibodies against the CD40 ligand.
In a paper published in Science Translational Medicine, researchers presented the results of a phase 1a study in 59 healthy volunteers and phase 1b proof-of-concept study in 57 individuals with rheumatoid arthritis. Participants received either varying dosages of CD40 ligand (CD40L)–binding protein VIB4920 – a single dose in the healthy volunteers and seven doses in the phase 1b study – or placebo.
Jodi L. Karnell, PhD, of Viela Bio in Gaithersburg, Md., and coauthors, wrote that the CD40/CD40L pathway is known to play a key role in humoral immune responses and in the pathogenesis of several autoimmune diseases.
However, previous clinical trials of compounds targeting CD40L were stopped early because of an increased risk of adverse thromboembolic events related to platelet aggregation, despite showing potential benefits in lupus and immune thrombocytopenic purpura.
Preclinical studies of VIB4920 found that it blocked the expansion of CD40L-dependent human B cells without showing any signs of platelet aggregation. The authors said the platelet aggregation had been linked to a particular region of anti-CD40L monoclonal antibodies, but VIB4920 was engineered using a protein scaffold that did not contain that region.
In healthy volunteers, researchers saw a dose-dependent suppression of antibody production and reductions in B-cell proliferation, in recall response to a T cell–dependent antigen.
In individuals with rheumatoid arthritis, more than half of those treated with the two highest dosages of VIB4920 achieved low disease activity state or clinical remission by 12 weeks. Overall, there was also a significant decrease in disease activity, and dose-dependent decreases in rheumatoid factor autoantibodies and Vectra DA biomarker score, which is a composite of twelve rheumatoid arthritis–related biomarkers.
“The consistency of improvement across a variety of clinical and laboratory outcome measures further supports the potential clinical efficacy of VIB4920,” the authors wrote.
Researchers saw a similar rate of adverse events in the placebo and treatment arms of the study.
“VIB4920 represents an alternative to monoclonal antibody–based targeting of CD40L, which does not induce platelet aggregation in vitro and demonstrates a favorable safety profile in early clinical evaluation.”
The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or of Viela Bio.
SOURCE: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584
A nonantibody scaffold protein that targets the CD40 ligand appears to dampen down autoimmune responses without the thromboembolic complications seen in trials of monoclonal antibodies against the CD40 ligand.
In a paper published in Science Translational Medicine, researchers presented the results of a phase 1a study in 59 healthy volunteers and phase 1b proof-of-concept study in 57 individuals with rheumatoid arthritis. Participants received either varying dosages of CD40 ligand (CD40L)–binding protein VIB4920 – a single dose in the healthy volunteers and seven doses in the phase 1b study – or placebo.
Jodi L. Karnell, PhD, of Viela Bio in Gaithersburg, Md., and coauthors, wrote that the CD40/CD40L pathway is known to play a key role in humoral immune responses and in the pathogenesis of several autoimmune diseases.
However, previous clinical trials of compounds targeting CD40L were stopped early because of an increased risk of adverse thromboembolic events related to platelet aggregation, despite showing potential benefits in lupus and immune thrombocytopenic purpura.
Preclinical studies of VIB4920 found that it blocked the expansion of CD40L-dependent human B cells without showing any signs of platelet aggregation. The authors said the platelet aggregation had been linked to a particular region of anti-CD40L monoclonal antibodies, but VIB4920 was engineered using a protein scaffold that did not contain that region.
In healthy volunteers, researchers saw a dose-dependent suppression of antibody production and reductions in B-cell proliferation, in recall response to a T cell–dependent antigen.
In individuals with rheumatoid arthritis, more than half of those treated with the two highest dosages of VIB4920 achieved low disease activity state or clinical remission by 12 weeks. Overall, there was also a significant decrease in disease activity, and dose-dependent decreases in rheumatoid factor autoantibodies and Vectra DA biomarker score, which is a composite of twelve rheumatoid arthritis–related biomarkers.
“The consistency of improvement across a variety of clinical and laboratory outcome measures further supports the potential clinical efficacy of VIB4920,” the authors wrote.
Researchers saw a similar rate of adverse events in the placebo and treatment arms of the study.
“VIB4920 represents an alternative to monoclonal antibody–based targeting of CD40L, which does not induce platelet aggregation in vitro and demonstrates a favorable safety profile in early clinical evaluation.”
The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or of Viela Bio.
SOURCE: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: Treatment with higher doses of VIB4920 is associated with low disease activity or remission in 50% of rheumatoid arthritis patients.
Study details: Phase 1a and 1b study in 59 healthy individuals and 57 people with rheumatoid arthritis.
Disclosures: The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or Viela Bio.
Source: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584.
FDA approves IL-23 inhibitor risankizumab for treating plaque psoriasis
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.