User login
Studies begin to pinpoint ways to diagnose SLE earlier
SAN FRANCISCO – A host of novel clinical and serologic findings that physicians can put to good use right now in helping to distinguish early SLE from its many mimickers have been identified in a large study conducted on four continents, Marta Mosca, MD, PhD, observed at an international congress on systemic lupus erythematosus.
These useful findings aren’t incorporated into the current American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) lupus classification criteria, which have come under criticism for limited sensitivity in identifying early SLE. Some of the novel findings provide support for increased suspicion of early SLE, while others suggest a need to veer in another direction and assess a patient for a disease other than lupus. The study has served to provide input for the proposed new ACR/EULAR weighted SLE classification criteria, although that scheme is meant to be used only for research and not in clinical practice, explained Dr. Mosca of the University of Pisa (Italy).
She was lead author of the four-continent study, which included 616 patients referred to experienced academic lupus centers for possible SLE with a symptom duration of less than 1 year. During up to 3 years of follow-up, 389 patients were diagnosed as having SLE by experienced rheumatologists based upon their clinical judgment, without any requirement to meet the full ACR or SLICC classification criteria. The other 227 patients were determined to be SLE mimickers with conditions including lymphoma, Sjögren’s syndrome, systemic sclerosis, interstitial lung disease, fibromyalgia, antinuclear antibody–positive thyroiditis, and undifferentiated connective tissue disease.
Dr. Mosca also highlighted key recent work by other investigators aimed at speeding the diagnosis of SLE and shortening the duration of what she called “the gray zone” of diagnostic uncertainty, when autoantibodies and insidious symptoms are present but not yet sufficient to make the diagnosis of SLE by conventional criteria. It’s well established that 60%-70% of patients with mild undifferentiated connective tissue disease will remain stable without evolving into SLE during long years of follow-up.
The ultimate objective of all this work is to try to change the natural history of the disease through targeted early aggressive therapy aimed at minimizing the extent of active disease and preventing severe organ involvement.
Among the key takeaways from the four-continent study led by Dr. Mosca: Fever not related to infection was far more prevalent in early SLE than in mimicking conditions, by a margin of 34.5% versus 13.7%. On the other hand, Raynaud’s phenomenon was more than twice as prevalent among patients with mimicking conditions: 22.1% in early SLE, compared with 48.5% in SLE mimickers. Sicca symptoms were present in just 4.4% of early SLE patients versus 34.4% of SLE mimickers. Only 0.3% of early SLE patients complained of dysphagia; the rate was 20-fold higher in the SLE mimickers. Rashes atypical for lupus were twice as frequent in the SLE mimicking conditions (Arthritis Rheumatol. 2019 Jan;71[1]:91-8).
Turning to key differentiating serologic findings, Dr. Mosca noted that anti-double stranded DNA (anti-dsDNA) and anti-Sm antibodies were present in 71.7% and 30.2% of early SLE patients, respectively, compared with 6.9% and 2.6% of SLE mimickers. Anticardiolipin IgM, a positive Coombs test, anti-beta2 glycoprotein-I antibodies, leukopenia, autoimmune hemolytic anemia, and hypocomplementemia were all significantly more common in the early SLE cohort.
In contrast, antibodies to Ro (SS-A) and La (SS-B) were of no value in separating early SLE from its mimickers, according to Dr. Mosca.
Other tipoffs to early SLE
Two separate teams of British researchers have advanced the field in a highly practical way. One group showed in a study of 1,739 newly diagnosed SLE patients and 6,956 controls that in the 5 years prior to diagnosis, the SLE group averaged 9.2 primary care visits per year, compared with 3.8 for controls. The visits clustered around nonspecific complaints of arthritis and arthralgias, alopecia, and rash (Arthritis Care Res. 2017 Jun;69[6]:833-41).
“An accumulating number of primary care office visits and referrals over time should raise suspicion,” Dr. Mosca said.
Other investigators, working with 1,426 cases of newly diagnosed SLE in the U.K. Clinical Practice Research Database, observed that the proportion of patients with disease manifestations in three or more British Isles Lupus Activity Group (BILAG) symptom domains rose from 18.7% at 3 years prior to diagnosis to 39.7% in the year before diagnosis (Lupus Sci Med. 2017 Feb 10;4[1]:e000172. doi: 10.1136/lupus-2016-000172).
“These patients accrue clinical manifestations. It’s not just one symptom, it’s more of a state of being unwell. This is a suspicious factor for the development of lupus,” she continued.
And just as patients who will eventually be diagnosed with SLE accrue a growing number of signs and symptoms during the run up to diagnosis, they also accrue multiple autoantibodies. Moreover, as demonstrated by a multicenter group of U.S. investigators, patients also develop elevated levels of multiple soluble inflammatory markers more than 3.5 years prior to diagnosis of SLE. These include interleukins-5 and -6 and interferon-gamma. And less than 10 months prior to being classified as having SLE, patients develop significantly higher levels of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand known as APRIL. The investigators developed a predictive model incorporating IL-5, -6, and interferon-gamma levels with antinuclear antibody status that identified future SLE patients with 84% accuracy more than 3.5 years before diagnosis. This could prove useful in selecting high-risk patients for clinical prevention trials (J Autoimmun. 2016 Nov;74:182-93).
Researchers at the University of Leeds (England) have also zeroed in on interferon activity as playing a key role in progression from asymptomatic antinuclear antigen positivity, which is present in up to 25% of the general population, to symptomatic autoimmune connective tissue disease, which affects less than 1%. A multivariate logistic regression analysis identified two independent predictors of development of autoimmune connective tissue disease within the next 12 months: a family history of autoimmune rheumatic disease, which was associated with an 8.2-fold increased risk; and positivity for a pattern of interferon-stimulated gene activity they call IFN-Score-B (Ann Rheum Dis. 2018 Oct;77[10]:1432-9).
All of this work has led up to what Dr. Mosca called “a glance into the future”: the National Institutes of Health–supported Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE), which is now recruiting patients. This randomized, double-blind, placebo-controlled multicenter U.S. trial involves patients who are antinuclear antibody positive at a titer of 1:80 or more plus one or two additional criteria from the SLICC classification scheme. Participants are being randomized to 96 weeks of hydroxychloroquine or placebo. The goal is to learn whether hydroxychloroquine can slow disease progression, with the primary endpoint being the number of SLICC criteria met at the study’s end. The trial will also scrutinize potential biomarkers that could be used to guide treatment decisions (Trials. 2018 Dec 20;19[1]:694. doi: 10.1186/s13063-018-3076-7).
Dr. Mosca reported serving as an adviser to UCB and Lilly.
SAN FRANCISCO – A host of novel clinical and serologic findings that physicians can put to good use right now in helping to distinguish early SLE from its many mimickers have been identified in a large study conducted on four continents, Marta Mosca, MD, PhD, observed at an international congress on systemic lupus erythematosus.
These useful findings aren’t incorporated into the current American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) lupus classification criteria, which have come under criticism for limited sensitivity in identifying early SLE. Some of the novel findings provide support for increased suspicion of early SLE, while others suggest a need to veer in another direction and assess a patient for a disease other than lupus. The study has served to provide input for the proposed new ACR/EULAR weighted SLE classification criteria, although that scheme is meant to be used only for research and not in clinical practice, explained Dr. Mosca of the University of Pisa (Italy).
She was lead author of the four-continent study, which included 616 patients referred to experienced academic lupus centers for possible SLE with a symptom duration of less than 1 year. During up to 3 years of follow-up, 389 patients were diagnosed as having SLE by experienced rheumatologists based upon their clinical judgment, without any requirement to meet the full ACR or SLICC classification criteria. The other 227 patients were determined to be SLE mimickers with conditions including lymphoma, Sjögren’s syndrome, systemic sclerosis, interstitial lung disease, fibromyalgia, antinuclear antibody–positive thyroiditis, and undifferentiated connective tissue disease.
Dr. Mosca also highlighted key recent work by other investigators aimed at speeding the diagnosis of SLE and shortening the duration of what she called “the gray zone” of diagnostic uncertainty, when autoantibodies and insidious symptoms are present but not yet sufficient to make the diagnosis of SLE by conventional criteria. It’s well established that 60%-70% of patients with mild undifferentiated connective tissue disease will remain stable without evolving into SLE during long years of follow-up.
The ultimate objective of all this work is to try to change the natural history of the disease through targeted early aggressive therapy aimed at minimizing the extent of active disease and preventing severe organ involvement.
Among the key takeaways from the four-continent study led by Dr. Mosca: Fever not related to infection was far more prevalent in early SLE than in mimicking conditions, by a margin of 34.5% versus 13.7%. On the other hand, Raynaud’s phenomenon was more than twice as prevalent among patients with mimicking conditions: 22.1% in early SLE, compared with 48.5% in SLE mimickers. Sicca symptoms were present in just 4.4% of early SLE patients versus 34.4% of SLE mimickers. Only 0.3% of early SLE patients complained of dysphagia; the rate was 20-fold higher in the SLE mimickers. Rashes atypical for lupus were twice as frequent in the SLE mimicking conditions (Arthritis Rheumatol. 2019 Jan;71[1]:91-8).
Turning to key differentiating serologic findings, Dr. Mosca noted that anti-double stranded DNA (anti-dsDNA) and anti-Sm antibodies were present in 71.7% and 30.2% of early SLE patients, respectively, compared with 6.9% and 2.6% of SLE mimickers. Anticardiolipin IgM, a positive Coombs test, anti-beta2 glycoprotein-I antibodies, leukopenia, autoimmune hemolytic anemia, and hypocomplementemia were all significantly more common in the early SLE cohort.
In contrast, antibodies to Ro (SS-A) and La (SS-B) were of no value in separating early SLE from its mimickers, according to Dr. Mosca.
Other tipoffs to early SLE
Two separate teams of British researchers have advanced the field in a highly practical way. One group showed in a study of 1,739 newly diagnosed SLE patients and 6,956 controls that in the 5 years prior to diagnosis, the SLE group averaged 9.2 primary care visits per year, compared with 3.8 for controls. The visits clustered around nonspecific complaints of arthritis and arthralgias, alopecia, and rash (Arthritis Care Res. 2017 Jun;69[6]:833-41).
“An accumulating number of primary care office visits and referrals over time should raise suspicion,” Dr. Mosca said.
Other investigators, working with 1,426 cases of newly diagnosed SLE in the U.K. Clinical Practice Research Database, observed that the proportion of patients with disease manifestations in three or more British Isles Lupus Activity Group (BILAG) symptom domains rose from 18.7% at 3 years prior to diagnosis to 39.7% in the year before diagnosis (Lupus Sci Med. 2017 Feb 10;4[1]:e000172. doi: 10.1136/lupus-2016-000172).
“These patients accrue clinical manifestations. It’s not just one symptom, it’s more of a state of being unwell. This is a suspicious factor for the development of lupus,” she continued.
And just as patients who will eventually be diagnosed with SLE accrue a growing number of signs and symptoms during the run up to diagnosis, they also accrue multiple autoantibodies. Moreover, as demonstrated by a multicenter group of U.S. investigators, patients also develop elevated levels of multiple soluble inflammatory markers more than 3.5 years prior to diagnosis of SLE. These include interleukins-5 and -6 and interferon-gamma. And less than 10 months prior to being classified as having SLE, patients develop significantly higher levels of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand known as APRIL. The investigators developed a predictive model incorporating IL-5, -6, and interferon-gamma levels with antinuclear antibody status that identified future SLE patients with 84% accuracy more than 3.5 years before diagnosis. This could prove useful in selecting high-risk patients for clinical prevention trials (J Autoimmun. 2016 Nov;74:182-93).
Researchers at the University of Leeds (England) have also zeroed in on interferon activity as playing a key role in progression from asymptomatic antinuclear antigen positivity, which is present in up to 25% of the general population, to symptomatic autoimmune connective tissue disease, which affects less than 1%. A multivariate logistic regression analysis identified two independent predictors of development of autoimmune connective tissue disease within the next 12 months: a family history of autoimmune rheumatic disease, which was associated with an 8.2-fold increased risk; and positivity for a pattern of interferon-stimulated gene activity they call IFN-Score-B (Ann Rheum Dis. 2018 Oct;77[10]:1432-9).
All of this work has led up to what Dr. Mosca called “a glance into the future”: the National Institutes of Health–supported Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE), which is now recruiting patients. This randomized, double-blind, placebo-controlled multicenter U.S. trial involves patients who are antinuclear antibody positive at a titer of 1:80 or more plus one or two additional criteria from the SLICC classification scheme. Participants are being randomized to 96 weeks of hydroxychloroquine or placebo. The goal is to learn whether hydroxychloroquine can slow disease progression, with the primary endpoint being the number of SLICC criteria met at the study’s end. The trial will also scrutinize potential biomarkers that could be used to guide treatment decisions (Trials. 2018 Dec 20;19[1]:694. doi: 10.1186/s13063-018-3076-7).
Dr. Mosca reported serving as an adviser to UCB and Lilly.
SAN FRANCISCO – A host of novel clinical and serologic findings that physicians can put to good use right now in helping to distinguish early SLE from its many mimickers have been identified in a large study conducted on four continents, Marta Mosca, MD, PhD, observed at an international congress on systemic lupus erythematosus.
These useful findings aren’t incorporated into the current American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) lupus classification criteria, which have come under criticism for limited sensitivity in identifying early SLE. Some of the novel findings provide support for increased suspicion of early SLE, while others suggest a need to veer in another direction and assess a patient for a disease other than lupus. The study has served to provide input for the proposed new ACR/EULAR weighted SLE classification criteria, although that scheme is meant to be used only for research and not in clinical practice, explained Dr. Mosca of the University of Pisa (Italy).
She was lead author of the four-continent study, which included 616 patients referred to experienced academic lupus centers for possible SLE with a symptom duration of less than 1 year. During up to 3 years of follow-up, 389 patients were diagnosed as having SLE by experienced rheumatologists based upon their clinical judgment, without any requirement to meet the full ACR or SLICC classification criteria. The other 227 patients were determined to be SLE mimickers with conditions including lymphoma, Sjögren’s syndrome, systemic sclerosis, interstitial lung disease, fibromyalgia, antinuclear antibody–positive thyroiditis, and undifferentiated connective tissue disease.
Dr. Mosca also highlighted key recent work by other investigators aimed at speeding the diagnosis of SLE and shortening the duration of what she called “the gray zone” of diagnostic uncertainty, when autoantibodies and insidious symptoms are present but not yet sufficient to make the diagnosis of SLE by conventional criteria. It’s well established that 60%-70% of patients with mild undifferentiated connective tissue disease will remain stable without evolving into SLE during long years of follow-up.
The ultimate objective of all this work is to try to change the natural history of the disease through targeted early aggressive therapy aimed at minimizing the extent of active disease and preventing severe organ involvement.
Among the key takeaways from the four-continent study led by Dr. Mosca: Fever not related to infection was far more prevalent in early SLE than in mimicking conditions, by a margin of 34.5% versus 13.7%. On the other hand, Raynaud’s phenomenon was more than twice as prevalent among patients with mimicking conditions: 22.1% in early SLE, compared with 48.5% in SLE mimickers. Sicca symptoms were present in just 4.4% of early SLE patients versus 34.4% of SLE mimickers. Only 0.3% of early SLE patients complained of dysphagia; the rate was 20-fold higher in the SLE mimickers. Rashes atypical for lupus were twice as frequent in the SLE mimicking conditions (Arthritis Rheumatol. 2019 Jan;71[1]:91-8).
Turning to key differentiating serologic findings, Dr. Mosca noted that anti-double stranded DNA (anti-dsDNA) and anti-Sm antibodies were present in 71.7% and 30.2% of early SLE patients, respectively, compared with 6.9% and 2.6% of SLE mimickers. Anticardiolipin IgM, a positive Coombs test, anti-beta2 glycoprotein-I antibodies, leukopenia, autoimmune hemolytic anemia, and hypocomplementemia were all significantly more common in the early SLE cohort.
In contrast, antibodies to Ro (SS-A) and La (SS-B) were of no value in separating early SLE from its mimickers, according to Dr. Mosca.
Other tipoffs to early SLE
Two separate teams of British researchers have advanced the field in a highly practical way. One group showed in a study of 1,739 newly diagnosed SLE patients and 6,956 controls that in the 5 years prior to diagnosis, the SLE group averaged 9.2 primary care visits per year, compared with 3.8 for controls. The visits clustered around nonspecific complaints of arthritis and arthralgias, alopecia, and rash (Arthritis Care Res. 2017 Jun;69[6]:833-41).
“An accumulating number of primary care office visits and referrals over time should raise suspicion,” Dr. Mosca said.
Other investigators, working with 1,426 cases of newly diagnosed SLE in the U.K. Clinical Practice Research Database, observed that the proportion of patients with disease manifestations in three or more British Isles Lupus Activity Group (BILAG) symptom domains rose from 18.7% at 3 years prior to diagnosis to 39.7% in the year before diagnosis (Lupus Sci Med. 2017 Feb 10;4[1]:e000172. doi: 10.1136/lupus-2016-000172).
“These patients accrue clinical manifestations. It’s not just one symptom, it’s more of a state of being unwell. This is a suspicious factor for the development of lupus,” she continued.
And just as patients who will eventually be diagnosed with SLE accrue a growing number of signs and symptoms during the run up to diagnosis, they also accrue multiple autoantibodies. Moreover, as demonstrated by a multicenter group of U.S. investigators, patients also develop elevated levels of multiple soluble inflammatory markers more than 3.5 years prior to diagnosis of SLE. These include interleukins-5 and -6 and interferon-gamma. And less than 10 months prior to being classified as having SLE, patients develop significantly higher levels of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand known as APRIL. The investigators developed a predictive model incorporating IL-5, -6, and interferon-gamma levels with antinuclear antibody status that identified future SLE patients with 84% accuracy more than 3.5 years before diagnosis. This could prove useful in selecting high-risk patients for clinical prevention trials (J Autoimmun. 2016 Nov;74:182-93).
Researchers at the University of Leeds (England) have also zeroed in on interferon activity as playing a key role in progression from asymptomatic antinuclear antigen positivity, which is present in up to 25% of the general population, to symptomatic autoimmune connective tissue disease, which affects less than 1%. A multivariate logistic regression analysis identified two independent predictors of development of autoimmune connective tissue disease within the next 12 months: a family history of autoimmune rheumatic disease, which was associated with an 8.2-fold increased risk; and positivity for a pattern of interferon-stimulated gene activity they call IFN-Score-B (Ann Rheum Dis. 2018 Oct;77[10]:1432-9).
All of this work has led up to what Dr. Mosca called “a glance into the future”: the National Institutes of Health–supported Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE), which is now recruiting patients. This randomized, double-blind, placebo-controlled multicenter U.S. trial involves patients who are antinuclear antibody positive at a titer of 1:80 or more plus one or two additional criteria from the SLICC classification scheme. Participants are being randomized to 96 weeks of hydroxychloroquine or placebo. The goal is to learn whether hydroxychloroquine can slow disease progression, with the primary endpoint being the number of SLICC criteria met at the study’s end. The trial will also scrutinize potential biomarkers that could be used to guide treatment decisions (Trials. 2018 Dec 20;19[1]:694. doi: 10.1186/s13063-018-3076-7).
Dr. Mosca reported serving as an adviser to UCB and Lilly.
REPORTING FROM LUPUS 2019
Treatment of Gout
Hip T scores can guide duration of osteoporosis therapy
according to Serge Ferrari, MD, and his colleagues.
Using 10 years of follow-up data from 1,343 women who took denosumab in the FREEDOM trial, Dr. Ferrari and his colleagues determined that a T score of at least –2.5 would be an appropriate target for this decision.
“A T-score unit increase of 1.0 was associated with a significant reduction in fracture risk for T scores up to, but no greater than, –2.0, suggesting that a T-score threshold of at least –2.0 would be an appropriate target for therapy to maximize treatment,” said Dr. Ferrari of the University of Geneva and his colleagues. “Further improvements in bone mineral density were not associated with major additional changes in 1-year nonvertebral fracture incidence.”
The findings “highlight the importance of the relationship between hip T score and fracture risk, which is maintained during long-term therapy with denosumab. Regular monitoring of bone mineral density during therapy may be useful to determine when fracture risk has reached a minimal threshold; treatment could therefore be suspended and/or consolidated, as in the case of a reversible therapy such as denosumab.”
SOURCE: Ferrari S et al. J Bone Miner Res. 2019 Mar 28. doi: 10.1002/jbmr.3722.
according to Serge Ferrari, MD, and his colleagues.
Using 10 years of follow-up data from 1,343 women who took denosumab in the FREEDOM trial, Dr. Ferrari and his colleagues determined that a T score of at least –2.5 would be an appropriate target for this decision.
“A T-score unit increase of 1.0 was associated with a significant reduction in fracture risk for T scores up to, but no greater than, –2.0, suggesting that a T-score threshold of at least –2.0 would be an appropriate target for therapy to maximize treatment,” said Dr. Ferrari of the University of Geneva and his colleagues. “Further improvements in bone mineral density were not associated with major additional changes in 1-year nonvertebral fracture incidence.”
The findings “highlight the importance of the relationship between hip T score and fracture risk, which is maintained during long-term therapy with denosumab. Regular monitoring of bone mineral density during therapy may be useful to determine when fracture risk has reached a minimal threshold; treatment could therefore be suspended and/or consolidated, as in the case of a reversible therapy such as denosumab.”
SOURCE: Ferrari S et al. J Bone Miner Res. 2019 Mar 28. doi: 10.1002/jbmr.3722.
according to Serge Ferrari, MD, and his colleagues.
Using 10 years of follow-up data from 1,343 women who took denosumab in the FREEDOM trial, Dr. Ferrari and his colleagues determined that a T score of at least –2.5 would be an appropriate target for this decision.
“A T-score unit increase of 1.0 was associated with a significant reduction in fracture risk for T scores up to, but no greater than, –2.0, suggesting that a T-score threshold of at least –2.0 would be an appropriate target for therapy to maximize treatment,” said Dr. Ferrari of the University of Geneva and his colleagues. “Further improvements in bone mineral density were not associated with major additional changes in 1-year nonvertebral fracture incidence.”
The findings “highlight the importance of the relationship between hip T score and fracture risk, which is maintained during long-term therapy with denosumab. Regular monitoring of bone mineral density during therapy may be useful to determine when fracture risk has reached a minimal threshold; treatment could therefore be suspended and/or consolidated, as in the case of a reversible therapy such as denosumab.”
SOURCE: Ferrari S et al. J Bone Miner Res. 2019 Mar 28. doi: 10.1002/jbmr.3722.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
Gut bacterium R. gnavus linked to lupus flares
SAN FRANCISCO –
Not only that, but those patients also had highly elevated antibodies to an endotoxin-like antigen released by one particular R. gnavus strain.
That antigen is “very proinflammatory, very immunogenic. We are wondering if this is actually [what drives] the immune activation that results in immune complexes in the glomeruli” of patients with lupus nephritis, said investigator Gregg Silverman, MD, a professor of medicine and pathology and head of the laboratory of B-cell immunobiology at New York University.
R. gnavus is an obligate anaerobe found in the guts of most people, but in lupus, it might be a problem.
“We are finding a very specific relationship with lupus patients and this bacteria – and this particular antibody,” Dr. Silverman explained in an interview at an international congress on systemic lupus erythematosus. “There’s an expansion of this particular bug, but also a contraction of others” as disease activity progresses.
“It speaks to an imbalance,” he added, and it suggests a role for probiotics or even fecal transplants to restore order.
“What if instead of killing the immune system” in lupus treatment, “we should be reducing or removing a single bacterium or a single molecule?” he asked.
Dr. Silverman is one of many researchers working to unravel the role of the human microbiome in both disease and health. His findings are preliminary, and, as he cautioned, correlation is not causation. But the implications are remarkable, Dr. Silverman noted.
SAN FRANCISCO –
Not only that, but those patients also had highly elevated antibodies to an endotoxin-like antigen released by one particular R. gnavus strain.
That antigen is “very proinflammatory, very immunogenic. We are wondering if this is actually [what drives] the immune activation that results in immune complexes in the glomeruli” of patients with lupus nephritis, said investigator Gregg Silverman, MD, a professor of medicine and pathology and head of the laboratory of B-cell immunobiology at New York University.
R. gnavus is an obligate anaerobe found in the guts of most people, but in lupus, it might be a problem.
“We are finding a very specific relationship with lupus patients and this bacteria – and this particular antibody,” Dr. Silverman explained in an interview at an international congress on systemic lupus erythematosus. “There’s an expansion of this particular bug, but also a contraction of others” as disease activity progresses.
“It speaks to an imbalance,” he added, and it suggests a role for probiotics or even fecal transplants to restore order.
“What if instead of killing the immune system” in lupus treatment, “we should be reducing or removing a single bacterium or a single molecule?” he asked.
Dr. Silverman is one of many researchers working to unravel the role of the human microbiome in both disease and health. His findings are preliminary, and, as he cautioned, correlation is not causation. But the implications are remarkable, Dr. Silverman noted.
SAN FRANCISCO –
Not only that, but those patients also had highly elevated antibodies to an endotoxin-like antigen released by one particular R. gnavus strain.
That antigen is “very proinflammatory, very immunogenic. We are wondering if this is actually [what drives] the immune activation that results in immune complexes in the glomeruli” of patients with lupus nephritis, said investigator Gregg Silverman, MD, a professor of medicine and pathology and head of the laboratory of B-cell immunobiology at New York University.
R. gnavus is an obligate anaerobe found in the guts of most people, but in lupus, it might be a problem.
“We are finding a very specific relationship with lupus patients and this bacteria – and this particular antibody,” Dr. Silverman explained in an interview at an international congress on systemic lupus erythematosus. “There’s an expansion of this particular bug, but also a contraction of others” as disease activity progresses.
“It speaks to an imbalance,” he added, and it suggests a role for probiotics or even fecal transplants to restore order.
“What if instead of killing the immune system” in lupus treatment, “we should be reducing or removing a single bacterium or a single molecule?” he asked.
Dr. Silverman is one of many researchers working to unravel the role of the human microbiome in both disease and health. His findings are preliminary, and, as he cautioned, correlation is not causation. But the implications are remarkable, Dr. Silverman noted.
REPORTING FROM LUPUS 2019
Combo B-cell depletion advances in SLE
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
REPORTING FROM LUPUS 2019
Hydroxychloroquine adherence in SLE: worse than you thought
SAN FRANCISCO – Routine office measurement of hydroxychloroquine blood levels in systemic lupus erythematosus (SLE) patients accomplishes two major objectives, Nathalie Costedoat-Chalumeau, MD, asserted at an international congress on systemic lupus erythematosus.
First, measuring hydroxychloroquine levels identifies the surprisingly large number of individuals who are severely nonadherent to this cornerstone of lupus therapy despite its excellent benefit/risk ratio. Also, serial measurements coupled with brief counseling have been shown to boost poor adherence rates, noted Dr. Costedoat-Chalumeau, professor of rheumatology at Paris Descartes University.
Numerous studies have documented startlingly low adherence to hydroxychloroquine among SLE patients. Some of the same studies show prescribing physicians are often clueless as to their patients’ adherence or lack thereof.
Just how bad is the adherence problem? A recent study of 10,406 Medicaid patients with SLE who started on hydroxychloroquine showed that 85% of them were nonadherent as defined by pharmacy refill data, indicating insufficient medication on hand to cover a minimum of 80% of days during at least 1 year of follow-up.
In a novel finding, the investigators also broke down the Medicaid data month by month and identified four broad patterns of adherence/nonadherence. A total of 17% of patients were persistently adherent throughout the first year after the drug was dispensed. Another 36% were persistent nonadherers right from the get-go. A further 24% remained partially adherent, dropping down to a plateau of 30%-40% monthly adherence after the first couple of months and staying there. And 23% dropped steadily from roughly 50% adherence at month 3 to nearly total nonadherence from month 9 onward. Overall, adherence in the Medicaid cohort declined over the course of the first year (Semin Arthritis Rheum. 2018 Oct;48[2]:205-13).
Dr. Costedoat-Chalumeau was the lead investigator in a large French multicenter clinical trial known as the PLUS Study, which established that increasing the hydroxychloroquine daily dose to raise blood levels to a target of at least 1,000 ng/mL didn’t reduce the risk of flares (Ann Rheum Dis. 2013;72[11]:1786-92).
“So there is no reason to use blood drug measurements to adjust hydroxychloroquine daily dose or blood levels to prevent SLE flares. But drug levels teach us something regarding adherence,” she said.
Why routinely measuring hydroxychloroquine levels matters
Dr. Costedoat-Chalumeau and other investigators have shown that whole blood drug levels below 200 ng/mL indicate a patient is severely nonadherent. In various studies, that’s 7%-29% of SLE patients who are supposedly on hydroxychloroquine.
Also, investigators at Johns Hopkins University, Baltimore, have analyzed prospective data from the Hopkins Lupus Cohort and determined that at the first clinic visit after going on a maximum of 400 mg/day of hydroxychloroquine, only 44% of participants had a blood drug level above the 500-ng/mL threshold indicative of adherence.
Importantly, however, the Hopkins researchers also demonstrated that with repeated brief counseling of nonadherent patients as to why hydroxychloroquine is the most important medication they take for their SLE, adherence climbed in stepwise fashion with each visit in which the drug blood level was assessed: With no prior measurement, adherence was 56%; with one prior measurement, it jumped to 69%; with two, 77% of patients were adherent to hydroxychloroquine; and with three or more prior blood level checks, adherence rose to 80% (J Rheumatol. 2015 Nov;42[11]:2092-7).
It is well established that hydroxychloroquine prevents SLE flares, protects against thrombotic events, diabetes, dyslipidemia, and lupus-induced organ damage, and improves survival. Dr. Costedoat-Chalumeau’s final words on hydroxychloroquine adherence: ”Drugs don’t work in people who don’t take them.”
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – Routine office measurement of hydroxychloroquine blood levels in systemic lupus erythematosus (SLE) patients accomplishes two major objectives, Nathalie Costedoat-Chalumeau, MD, asserted at an international congress on systemic lupus erythematosus.
First, measuring hydroxychloroquine levels identifies the surprisingly large number of individuals who are severely nonadherent to this cornerstone of lupus therapy despite its excellent benefit/risk ratio. Also, serial measurements coupled with brief counseling have been shown to boost poor adherence rates, noted Dr. Costedoat-Chalumeau, professor of rheumatology at Paris Descartes University.
Numerous studies have documented startlingly low adherence to hydroxychloroquine among SLE patients. Some of the same studies show prescribing physicians are often clueless as to their patients’ adherence or lack thereof.
Just how bad is the adherence problem? A recent study of 10,406 Medicaid patients with SLE who started on hydroxychloroquine showed that 85% of them were nonadherent as defined by pharmacy refill data, indicating insufficient medication on hand to cover a minimum of 80% of days during at least 1 year of follow-up.
In a novel finding, the investigators also broke down the Medicaid data month by month and identified four broad patterns of adherence/nonadherence. A total of 17% of patients were persistently adherent throughout the first year after the drug was dispensed. Another 36% were persistent nonadherers right from the get-go. A further 24% remained partially adherent, dropping down to a plateau of 30%-40% monthly adherence after the first couple of months and staying there. And 23% dropped steadily from roughly 50% adherence at month 3 to nearly total nonadherence from month 9 onward. Overall, adherence in the Medicaid cohort declined over the course of the first year (Semin Arthritis Rheum. 2018 Oct;48[2]:205-13).
Dr. Costedoat-Chalumeau was the lead investigator in a large French multicenter clinical trial known as the PLUS Study, which established that increasing the hydroxychloroquine daily dose to raise blood levels to a target of at least 1,000 ng/mL didn’t reduce the risk of flares (Ann Rheum Dis. 2013;72[11]:1786-92).
“So there is no reason to use blood drug measurements to adjust hydroxychloroquine daily dose or blood levels to prevent SLE flares. But drug levels teach us something regarding adherence,” she said.
Why routinely measuring hydroxychloroquine levels matters
Dr. Costedoat-Chalumeau and other investigators have shown that whole blood drug levels below 200 ng/mL indicate a patient is severely nonadherent. In various studies, that’s 7%-29% of SLE patients who are supposedly on hydroxychloroquine.
Also, investigators at Johns Hopkins University, Baltimore, have analyzed prospective data from the Hopkins Lupus Cohort and determined that at the first clinic visit after going on a maximum of 400 mg/day of hydroxychloroquine, only 44% of participants had a blood drug level above the 500-ng/mL threshold indicative of adherence.
Importantly, however, the Hopkins researchers also demonstrated that with repeated brief counseling of nonadherent patients as to why hydroxychloroquine is the most important medication they take for their SLE, adherence climbed in stepwise fashion with each visit in which the drug blood level was assessed: With no prior measurement, adherence was 56%; with one prior measurement, it jumped to 69%; with two, 77% of patients were adherent to hydroxychloroquine; and with three or more prior blood level checks, adherence rose to 80% (J Rheumatol. 2015 Nov;42[11]:2092-7).
It is well established that hydroxychloroquine prevents SLE flares, protects against thrombotic events, diabetes, dyslipidemia, and lupus-induced organ damage, and improves survival. Dr. Costedoat-Chalumeau’s final words on hydroxychloroquine adherence: ”Drugs don’t work in people who don’t take them.”
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – Routine office measurement of hydroxychloroquine blood levels in systemic lupus erythematosus (SLE) patients accomplishes two major objectives, Nathalie Costedoat-Chalumeau, MD, asserted at an international congress on systemic lupus erythematosus.
First, measuring hydroxychloroquine levels identifies the surprisingly large number of individuals who are severely nonadherent to this cornerstone of lupus therapy despite its excellent benefit/risk ratio. Also, serial measurements coupled with brief counseling have been shown to boost poor adherence rates, noted Dr. Costedoat-Chalumeau, professor of rheumatology at Paris Descartes University.
Numerous studies have documented startlingly low adherence to hydroxychloroquine among SLE patients. Some of the same studies show prescribing physicians are often clueless as to their patients’ adherence or lack thereof.
Just how bad is the adherence problem? A recent study of 10,406 Medicaid patients with SLE who started on hydroxychloroquine showed that 85% of them were nonadherent as defined by pharmacy refill data, indicating insufficient medication on hand to cover a minimum of 80% of days during at least 1 year of follow-up.
In a novel finding, the investigators also broke down the Medicaid data month by month and identified four broad patterns of adherence/nonadherence. A total of 17% of patients were persistently adherent throughout the first year after the drug was dispensed. Another 36% were persistent nonadherers right from the get-go. A further 24% remained partially adherent, dropping down to a plateau of 30%-40% monthly adherence after the first couple of months and staying there. And 23% dropped steadily from roughly 50% adherence at month 3 to nearly total nonadherence from month 9 onward. Overall, adherence in the Medicaid cohort declined over the course of the first year (Semin Arthritis Rheum. 2018 Oct;48[2]:205-13).
Dr. Costedoat-Chalumeau was the lead investigator in a large French multicenter clinical trial known as the PLUS Study, which established that increasing the hydroxychloroquine daily dose to raise blood levels to a target of at least 1,000 ng/mL didn’t reduce the risk of flares (Ann Rheum Dis. 2013;72[11]:1786-92).
“So there is no reason to use blood drug measurements to adjust hydroxychloroquine daily dose or blood levels to prevent SLE flares. But drug levels teach us something regarding adherence,” she said.
Why routinely measuring hydroxychloroquine levels matters
Dr. Costedoat-Chalumeau and other investigators have shown that whole blood drug levels below 200 ng/mL indicate a patient is severely nonadherent. In various studies, that’s 7%-29% of SLE patients who are supposedly on hydroxychloroquine.
Also, investigators at Johns Hopkins University, Baltimore, have analyzed prospective data from the Hopkins Lupus Cohort and determined that at the first clinic visit after going on a maximum of 400 mg/day of hydroxychloroquine, only 44% of participants had a blood drug level above the 500-ng/mL threshold indicative of adherence.
Importantly, however, the Hopkins researchers also demonstrated that with repeated brief counseling of nonadherent patients as to why hydroxychloroquine is the most important medication they take for their SLE, adherence climbed in stepwise fashion with each visit in which the drug blood level was assessed: With no prior measurement, adherence was 56%; with one prior measurement, it jumped to 69%; with two, 77% of patients were adherent to hydroxychloroquine; and with three or more prior blood level checks, adherence rose to 80% (J Rheumatol. 2015 Nov;42[11]:2092-7).
It is well established that hydroxychloroquine prevents SLE flares, protects against thrombotic events, diabetes, dyslipidemia, and lupus-induced organ damage, and improves survival. Dr. Costedoat-Chalumeau’s final words on hydroxychloroquine adherence: ”Drugs don’t work in people who don’t take them.”
She reported having no financial conflicts regarding her presentation.
REPORTING FROM LUPUS 2019
‘Type II’ SLE assessment catches what matters to patients
SAN FRANCISCO – For almost a year, lupus patients at Duke University in Durham, N.C., have been getting two physician global assessments, the usual one for classic “type I” disease, and a new one for nonspecific “type II” symptoms: fatigue, widespread pain, depression, sleep disturbance, and cognitive dysfunction.
It’s often the type II problems that affect patients the most, and what they are most concerned about; formally assessing them with the type II physician global assessment (PGA) – a 0- to 3-point visual analog scale – ensures they aren’t overlooked, said Jennifer Rogers, MD, assistant professor of rheumatology at Duke.
It “forces us to address these symptoms,” she said, and the approach seems to be working, according to a study Dr. Rogers presented at an international congress on systemic lupus erythematosus.
In the 5 months leading up to implementation of the PGA II in late spring 2018, type II problems had treatment recommendations in patients’ charts just 53% of the time; the number rose to 89% of the time during the PGA II’s first 5 months (P = .03). Type II PGA scores correlated strongly with patient-reported fibromyalgia and depression symptoms, but did not correlate with PGA scores for type I symptoms, such as nephritis and arthritis.
Type II problems are common in lupus. Patients’ joints might be fine, and their kidney disease in remission, but they can still feel miserable, and will often blame it on a lupus flare. Physicians who disagree end up at odds with their patients, Dr. Rogers explained.
“We decided to rethink how we address these patients, and came up with this new type I, type II categorization.” Now, when paints complain of brain fog, for example, “I say ‘yes, this is your lupus. I believe you,’ but we don’t need to give you more steroids or very expensive immunosuppressives for this. What you need to do is take your Cymbalta, work on your exercise, and maybe see your therapist,” she said.
It validates what people are going through, and builds trust. “Patients like it; they feel heard, and I walk out of the room, and I feel better,” she said.
During its first 5 months, 197 patients had PGAs for type II symptoms, along with type I PGAs. The average age of the patients was 46 years, and 92% were women.
Patients with predominately type II symptoms were more likely than were those with predominately type I disease to be depressed (84% versus 39%), and they reported higher lupus activity, greater symptom severity, and more severe fibromyalgia. The differences were statistically significant.
Type II treatments included medications in 60% of cases, exercise or physical therapy in almost 60% of cases, sleep studies or help with sleep hygiene in about 35%, and psychiatric or psychological referral in almost 20%. Less than 5% of patients were referred to a pain clinic.
There was no external funding for the study, and Dr. Rogers didn’t have any disclosures.
SOURCE: Rogers J et al. Lupus Sci Med. 2019;6[suppl 1]: Abstract 102. doi: 10.1136/lupus-2019-lsm.102
SAN FRANCISCO – For almost a year, lupus patients at Duke University in Durham, N.C., have been getting two physician global assessments, the usual one for classic “type I” disease, and a new one for nonspecific “type II” symptoms: fatigue, widespread pain, depression, sleep disturbance, and cognitive dysfunction.
It’s often the type II problems that affect patients the most, and what they are most concerned about; formally assessing them with the type II physician global assessment (PGA) – a 0- to 3-point visual analog scale – ensures they aren’t overlooked, said Jennifer Rogers, MD, assistant professor of rheumatology at Duke.
It “forces us to address these symptoms,” she said, and the approach seems to be working, according to a study Dr. Rogers presented at an international congress on systemic lupus erythematosus.
In the 5 months leading up to implementation of the PGA II in late spring 2018, type II problems had treatment recommendations in patients’ charts just 53% of the time; the number rose to 89% of the time during the PGA II’s first 5 months (P = .03). Type II PGA scores correlated strongly with patient-reported fibromyalgia and depression symptoms, but did not correlate with PGA scores for type I symptoms, such as nephritis and arthritis.
Type II problems are common in lupus. Patients’ joints might be fine, and their kidney disease in remission, but they can still feel miserable, and will often blame it on a lupus flare. Physicians who disagree end up at odds with their patients, Dr. Rogers explained.
“We decided to rethink how we address these patients, and came up with this new type I, type II categorization.” Now, when paints complain of brain fog, for example, “I say ‘yes, this is your lupus. I believe you,’ but we don’t need to give you more steroids or very expensive immunosuppressives for this. What you need to do is take your Cymbalta, work on your exercise, and maybe see your therapist,” she said.
It validates what people are going through, and builds trust. “Patients like it; they feel heard, and I walk out of the room, and I feel better,” she said.
During its first 5 months, 197 patients had PGAs for type II symptoms, along with type I PGAs. The average age of the patients was 46 years, and 92% were women.
Patients with predominately type II symptoms were more likely than were those with predominately type I disease to be depressed (84% versus 39%), and they reported higher lupus activity, greater symptom severity, and more severe fibromyalgia. The differences were statistically significant.
Type II treatments included medications in 60% of cases, exercise or physical therapy in almost 60% of cases, sleep studies or help with sleep hygiene in about 35%, and psychiatric or psychological referral in almost 20%. Less than 5% of patients were referred to a pain clinic.
There was no external funding for the study, and Dr. Rogers didn’t have any disclosures.
SOURCE: Rogers J et al. Lupus Sci Med. 2019;6[suppl 1]: Abstract 102. doi: 10.1136/lupus-2019-lsm.102
SAN FRANCISCO – For almost a year, lupus patients at Duke University in Durham, N.C., have been getting two physician global assessments, the usual one for classic “type I” disease, and a new one for nonspecific “type II” symptoms: fatigue, widespread pain, depression, sleep disturbance, and cognitive dysfunction.
It’s often the type II problems that affect patients the most, and what they are most concerned about; formally assessing them with the type II physician global assessment (PGA) – a 0- to 3-point visual analog scale – ensures they aren’t overlooked, said Jennifer Rogers, MD, assistant professor of rheumatology at Duke.
It “forces us to address these symptoms,” she said, and the approach seems to be working, according to a study Dr. Rogers presented at an international congress on systemic lupus erythematosus.
In the 5 months leading up to implementation of the PGA II in late spring 2018, type II problems had treatment recommendations in patients’ charts just 53% of the time; the number rose to 89% of the time during the PGA II’s first 5 months (P = .03). Type II PGA scores correlated strongly with patient-reported fibromyalgia and depression symptoms, but did not correlate with PGA scores for type I symptoms, such as nephritis and arthritis.
Type II problems are common in lupus. Patients’ joints might be fine, and their kidney disease in remission, but they can still feel miserable, and will often blame it on a lupus flare. Physicians who disagree end up at odds with their patients, Dr. Rogers explained.
“We decided to rethink how we address these patients, and came up with this new type I, type II categorization.” Now, when paints complain of brain fog, for example, “I say ‘yes, this is your lupus. I believe you,’ but we don’t need to give you more steroids or very expensive immunosuppressives for this. What you need to do is take your Cymbalta, work on your exercise, and maybe see your therapist,” she said.
It validates what people are going through, and builds trust. “Patients like it; they feel heard, and I walk out of the room, and I feel better,” she said.
During its first 5 months, 197 patients had PGAs for type II symptoms, along with type I PGAs. The average age of the patients was 46 years, and 92% were women.
Patients with predominately type II symptoms were more likely than were those with predominately type I disease to be depressed (84% versus 39%), and they reported higher lupus activity, greater symptom severity, and more severe fibromyalgia. The differences were statistically significant.
Type II treatments included medications in 60% of cases, exercise or physical therapy in almost 60% of cases, sleep studies or help with sleep hygiene in about 35%, and psychiatric or psychological referral in almost 20%. Less than 5% of patients were referred to a pain clinic.
There was no external funding for the study, and Dr. Rogers didn’t have any disclosures.
SOURCE: Rogers J et al. Lupus Sci Med. 2019;6[suppl 1]: Abstract 102. doi: 10.1136/lupus-2019-lsm.102
AT LUPUS 2019
Referral system aims to slash axial spondyloarthritis diagnostic delay
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.
But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.
But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.
But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low-dose IL-2 found effective in SLE
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.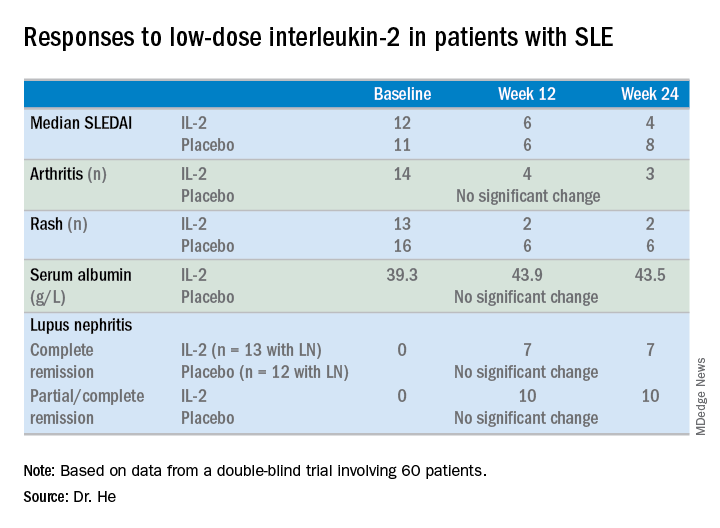
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
REPORTING FROM LUPUS 2019
Belimumab a bust for black SLE patients
SAN FRANCISCO – ordered by the Food and Drug Administration.
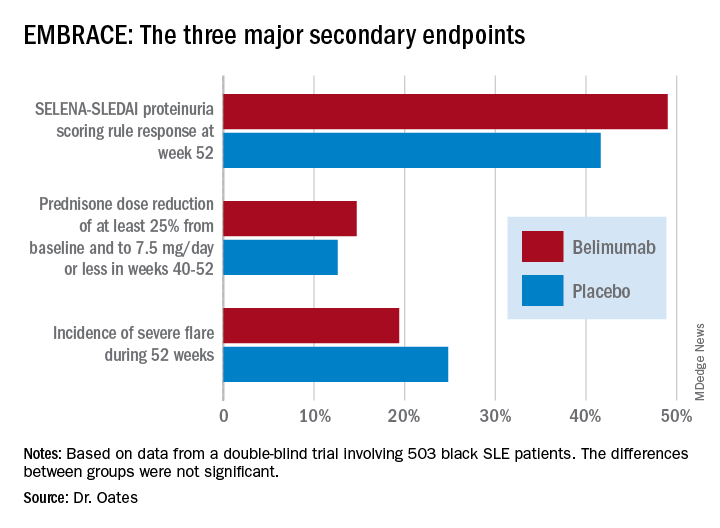
Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
SAN FRANCISCO – ordered by the Food and Drug Administration.

Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
SAN FRANCISCO – ordered by the Food and Drug Administration.

Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
REPORTING FROM LUPUS 2019