User login
Abatacept appears safe for RA patients with COPD
Concerns about abatacept (Orencia)-related lung disease in patients with rheumatoid arthritis appear to be unfounded, a large U.S. database review has determined.
Safety signals seen in a small subset of patients in the 2006 ASSURE study likely arose from chance, Samy Suissa, PhD, and his colleagues wrote in Seminars in Arthritis & Rheumatism. Patients in that study with preexisting chronic obstructive pulmonary disease (COPD) were 84% more likely to develop an exacerbation or other lung disorder than were those taking a placebo comparator.
The new database study contradicted that finding.
“Our study suggests that these numerical differences in ASSURE are expected results of random variation and thus compatible with chance,” Dr. Suissa of Jewish General Hospital and McGill University, both in Montreal, and his coauthors wrote. “Moreover, our findings are consistent with two studies of the safety of abatacept in the context of interstitial lung disease, albeit a different respiratory disease than COPD.”
The year-long ASSURE trial reported on the safety of abatacept in 959 patients versus 482 assigned to placebo. The safety signal arose in a subgroup of 54 patients with COPD, 37 of whom were assigned to abatacept and 17 to placebo. Among these were four serious respiratory adverse events (COPD exacerbation, worsening of COPD, bronchitis, and pneumonia) in four patients taking abatacept and none in the placebo arm.
“It is useful to note that this difference of 11% versus 0% rate is compatible with chance [exact P = .31], while the exact 95% confidence interval for the odds ratio is wide and includes unity ... The trial also reported more mild-moderate respiratory events with abatacept than with placebo [43.2% vs. 23.5%], including cough, rhonchi, COPD exacerbation, COPD, dyspnea, and nasal congestion. This difference resulted in an odds ration of 1.84,” with a wide confidence interval that included unity (0.48-8.63).
Nevertheless, these findings led to the addition of a warning in the prescribing insert: “Adult COPD patients treated with Orencia developed adverse events more frequently than those treated with placebo, including COPD exacerbations, cough, rhonchi, and dyspnea. Use of Orencia in patients with RA and COPD should be undertaken with caution and such patients should be monitored for worsening of their respiratory status.”
Dr. Suissa’s team used the U.S. MarketScan prescribing database to asses the risk of respiratory adverse events associated with abatacept, compared with other biologic disease-modifying antirheumatic drugs (DMARDs) in a real-world setting.
The cohort comprised 1,807 patients with rheumatoid arthritis and COPD who started a new prescription for abatacept, matched in time to 3,547 who initiated another biologic DMARD. The primary endpoint was the combined risk of hospitalization for COPD exacerbation, bronchitis, and hospitalized pneumonia or influenza.
The most common comparator biologic DMARDs were etanercept, adalimumab, rituximab, and infliximab.
For patients with COPD and comparator patients, the incidence rates for COPD exacerbation were 1.2 per 100 person-years with abatacept and 2.1 per 100 person-years with a different biologic DMARD; for bronchitis, the respective rates were 4.2 and 5.3; for hospitalized pneumonia or influenza, 3.6 and 2.6; and for outpatient pneumonia or flu, 14.7 and 14.4. For the combined endpoint, the incidence rate was 8.7 per 100 person-years with abatacept and 9.9 per 100 person-years with other biologic DMARDs.
The adjusted hazard ratio of the combined endpoint with abatacept versus that with other biologic DMARDs was a nonsignificant risk of 0.87. The hazard ratio with abatacept was 0.60 for hospitalized COPD; 0.80 for bronchitis; 1.39 for hospitalized pneumonia and flu; and 1.05 for outpatient pneumonia and flu. None of these associations was statistically significant.
“One exception was the risk of hospitalization for pneumonia or influenza, which was higher with abatacept among patients with more severe COPD,” at 6.99, than it was with other biologic DMARDs, the authors noted.
Dr. Suissa disclosed relationships with AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Novartis.
SOURCE: Suissa S et al. Semin Arthritis Rheum. 2019 Mar 16. doi: 10.1016j.semarthrit.2019.03.007.
Concerns about abatacept (Orencia)-related lung disease in patients with rheumatoid arthritis appear to be unfounded, a large U.S. database review has determined.
Safety signals seen in a small subset of patients in the 2006 ASSURE study likely arose from chance, Samy Suissa, PhD, and his colleagues wrote in Seminars in Arthritis & Rheumatism. Patients in that study with preexisting chronic obstructive pulmonary disease (COPD) were 84% more likely to develop an exacerbation or other lung disorder than were those taking a placebo comparator.
The new database study contradicted that finding.
“Our study suggests that these numerical differences in ASSURE are expected results of random variation and thus compatible with chance,” Dr. Suissa of Jewish General Hospital and McGill University, both in Montreal, and his coauthors wrote. “Moreover, our findings are consistent with two studies of the safety of abatacept in the context of interstitial lung disease, albeit a different respiratory disease than COPD.”
The year-long ASSURE trial reported on the safety of abatacept in 959 patients versus 482 assigned to placebo. The safety signal arose in a subgroup of 54 patients with COPD, 37 of whom were assigned to abatacept and 17 to placebo. Among these were four serious respiratory adverse events (COPD exacerbation, worsening of COPD, bronchitis, and pneumonia) in four patients taking abatacept and none in the placebo arm.
“It is useful to note that this difference of 11% versus 0% rate is compatible with chance [exact P = .31], while the exact 95% confidence interval for the odds ratio is wide and includes unity ... The trial also reported more mild-moderate respiratory events with abatacept than with placebo [43.2% vs. 23.5%], including cough, rhonchi, COPD exacerbation, COPD, dyspnea, and nasal congestion. This difference resulted in an odds ration of 1.84,” with a wide confidence interval that included unity (0.48-8.63).
Nevertheless, these findings led to the addition of a warning in the prescribing insert: “Adult COPD patients treated with Orencia developed adverse events more frequently than those treated with placebo, including COPD exacerbations, cough, rhonchi, and dyspnea. Use of Orencia in patients with RA and COPD should be undertaken with caution and such patients should be monitored for worsening of their respiratory status.”
Dr. Suissa’s team used the U.S. MarketScan prescribing database to asses the risk of respiratory adverse events associated with abatacept, compared with other biologic disease-modifying antirheumatic drugs (DMARDs) in a real-world setting.
The cohort comprised 1,807 patients with rheumatoid arthritis and COPD who started a new prescription for abatacept, matched in time to 3,547 who initiated another biologic DMARD. The primary endpoint was the combined risk of hospitalization for COPD exacerbation, bronchitis, and hospitalized pneumonia or influenza.
The most common comparator biologic DMARDs were etanercept, adalimumab, rituximab, and infliximab.
For patients with COPD and comparator patients, the incidence rates for COPD exacerbation were 1.2 per 100 person-years with abatacept and 2.1 per 100 person-years with a different biologic DMARD; for bronchitis, the respective rates were 4.2 and 5.3; for hospitalized pneumonia or influenza, 3.6 and 2.6; and for outpatient pneumonia or flu, 14.7 and 14.4. For the combined endpoint, the incidence rate was 8.7 per 100 person-years with abatacept and 9.9 per 100 person-years with other biologic DMARDs.
The adjusted hazard ratio of the combined endpoint with abatacept versus that with other biologic DMARDs was a nonsignificant risk of 0.87. The hazard ratio with abatacept was 0.60 for hospitalized COPD; 0.80 for bronchitis; 1.39 for hospitalized pneumonia and flu; and 1.05 for outpatient pneumonia and flu. None of these associations was statistically significant.
“One exception was the risk of hospitalization for pneumonia or influenza, which was higher with abatacept among patients with more severe COPD,” at 6.99, than it was with other biologic DMARDs, the authors noted.
Dr. Suissa disclosed relationships with AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Novartis.
SOURCE: Suissa S et al. Semin Arthritis Rheum. 2019 Mar 16. doi: 10.1016j.semarthrit.2019.03.007.
Concerns about abatacept (Orencia)-related lung disease in patients with rheumatoid arthritis appear to be unfounded, a large U.S. database review has determined.
Safety signals seen in a small subset of patients in the 2006 ASSURE study likely arose from chance, Samy Suissa, PhD, and his colleagues wrote in Seminars in Arthritis & Rheumatism. Patients in that study with preexisting chronic obstructive pulmonary disease (COPD) were 84% more likely to develop an exacerbation or other lung disorder than were those taking a placebo comparator.
The new database study contradicted that finding.
“Our study suggests that these numerical differences in ASSURE are expected results of random variation and thus compatible with chance,” Dr. Suissa of Jewish General Hospital and McGill University, both in Montreal, and his coauthors wrote. “Moreover, our findings are consistent with two studies of the safety of abatacept in the context of interstitial lung disease, albeit a different respiratory disease than COPD.”
The year-long ASSURE trial reported on the safety of abatacept in 959 patients versus 482 assigned to placebo. The safety signal arose in a subgroup of 54 patients with COPD, 37 of whom were assigned to abatacept and 17 to placebo. Among these were four serious respiratory adverse events (COPD exacerbation, worsening of COPD, bronchitis, and pneumonia) in four patients taking abatacept and none in the placebo arm.
“It is useful to note that this difference of 11% versus 0% rate is compatible with chance [exact P = .31], while the exact 95% confidence interval for the odds ratio is wide and includes unity ... The trial also reported more mild-moderate respiratory events with abatacept than with placebo [43.2% vs. 23.5%], including cough, rhonchi, COPD exacerbation, COPD, dyspnea, and nasal congestion. This difference resulted in an odds ration of 1.84,” with a wide confidence interval that included unity (0.48-8.63).
Nevertheless, these findings led to the addition of a warning in the prescribing insert: “Adult COPD patients treated with Orencia developed adverse events more frequently than those treated with placebo, including COPD exacerbations, cough, rhonchi, and dyspnea. Use of Orencia in patients with RA and COPD should be undertaken with caution and such patients should be monitored for worsening of their respiratory status.”
Dr. Suissa’s team used the U.S. MarketScan prescribing database to asses the risk of respiratory adverse events associated with abatacept, compared with other biologic disease-modifying antirheumatic drugs (DMARDs) in a real-world setting.
The cohort comprised 1,807 patients with rheumatoid arthritis and COPD who started a new prescription for abatacept, matched in time to 3,547 who initiated another biologic DMARD. The primary endpoint was the combined risk of hospitalization for COPD exacerbation, bronchitis, and hospitalized pneumonia or influenza.
The most common comparator biologic DMARDs were etanercept, adalimumab, rituximab, and infliximab.
For patients with COPD and comparator patients, the incidence rates for COPD exacerbation were 1.2 per 100 person-years with abatacept and 2.1 per 100 person-years with a different biologic DMARD; for bronchitis, the respective rates were 4.2 and 5.3; for hospitalized pneumonia or influenza, 3.6 and 2.6; and for outpatient pneumonia or flu, 14.7 and 14.4. For the combined endpoint, the incidence rate was 8.7 per 100 person-years with abatacept and 9.9 per 100 person-years with other biologic DMARDs.
The adjusted hazard ratio of the combined endpoint with abatacept versus that with other biologic DMARDs was a nonsignificant risk of 0.87. The hazard ratio with abatacept was 0.60 for hospitalized COPD; 0.80 for bronchitis; 1.39 for hospitalized pneumonia and flu; and 1.05 for outpatient pneumonia and flu. None of these associations was statistically significant.
“One exception was the risk of hospitalization for pneumonia or influenza, which was higher with abatacept among patients with more severe COPD,” at 6.99, than it was with other biologic DMARDs, the authors noted.
Dr. Suissa disclosed relationships with AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Novartis.
SOURCE: Suissa S et al. Semin Arthritis Rheum. 2019 Mar 16. doi: 10.1016j.semarthrit.2019.03.007.
FROM SEMINARS IN ARTHRITIS & RHEUMATISM
Powerful breast-implant testimony constrained by limited evidence
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
Rituximab does not improve fatigue symptoms of ME/CFS
according to results from the phase 3 RituxME trial.
“The lack of clinical effect of B-cell depletion in this trial weakens the case for an important role of B lymphocytes in ME/CFS but does not exclude an immunologic basis,” Øystein Fluge, MD, PhD, of the department of oncology and medical physics at Haukeland University Hospital in Bergen, Norway, and his colleagues wrote April 1 in Annals of Internal Medicine.
The investigators noted that the basis for testing the effects of a B-cell–depleting intervention on clinical symptoms in patients with ME/CFS came from observations of its potential benefit in a subgroup of patients in previous studies. Dr. Fluge and his colleagues performed a three-patient case series in their own group that found benefit for patients who received rituximab for treatment of CFS (BMC Neurol. 2009 May 8;9:28. doi: 10.1186/1471-2377-9-28). A phase 2 trial of 30 patients with CFS also performed by his own group found improved fatigue scores in 66.7% of patients in the rituximab group, compared with placebo (PLOS One. 2011 Oct 19. doi: 10.1371/journal.pone.0026358).
In the double-blinded RituxME trial, 151 patients with ME/CFS from four university hospitals and one general hospital in Norway were recruited and randomized to receive infusions of rituximab (n = 77) or placebo (n = 74). The patients were aged 18-65 years old and had the disease ranging from 2 years to 15 years. Patients reported and rated their ME/CFS symptoms at baseline as well as completed forms for the SF-36, Hospital Anxiety and Depression Scale, Fatigue Severity Scale, and modified DePaul Symptom Questionnaire out to 24 months. The rituximab group received two infusions at 500 mg/m2 across body surface area at 2 weeks apart. They then received 500-mg maintenance infusions at 3 months, 6 months, 9 months, and 12 months where they also self-reported changes in ME/CFS symptoms.
There were no significant differences between groups regarding fatigue score at any follow-up period, with an average between-group difference of 0.02 at 24 months (95% confidence interval, –0.27 to 0.31). The overall response rate was 26% with rituximab and 35% with placebo. Dr. Fluge and his colleagues also noted no significant differences regarding SF-36 scores, function level, and fatigue severity between groups.
Adverse event rates were higher in the rituximab group (63 patients; 82%) than in the placebo group (48 patients; 65%), and these were more often attributed to treatment for those taking rituximab (26 patients [34%]) than for placebo (12 patients [16%]). Adverse events requiring hospitalization also occurred more often among those taking rituximab (31 events in 20 patients [26%]) than for those who took placebo (16 events in 14 patients [19%]).
Some of the weaknesses of the trial included its use of self-referral and self-reported symptom scores, which might have been subject to recall bias. In commenting on the difference in outcome between the phase 3 trial and others, Dr. Fluge and his associates said the discrepancy could potentially be high expectations in the placebo group, unknown factors surrounding symptom variation in ME/CFS patients, and unknown patient selection effects.
“[T]he negative outcome of RituxME should spur research to assess patient subgroups and further elucidate disease mechanisms, of which recently disclosed impairment of energy metabolism may be important,” Dr. Fluge and his coauthors wrote.
The trial was funded by grants to the researchers from the Norwegian Research Council, the Norwegian Regional Health Trusts, the MEandYou Foundation, the Norwegian ME Association, and the legacy of Torstein Hereid.
SOURCE: Fluge Ø et al. Ann Intern Med. 2019 Apr 1. doi: 10.7326/M18-1451
The RituxME trial’s results weaken the case for the use of rituximab to treat myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), but there are opportunities to test other treatments targeting immunologic abnormalities in ME/CFS, Peter C. Rowe, MD, of Johns Hopkins University, Baltimore, wrote in a related editorial.
“Persons with ME/CFS often meet criteria for several comorbid conditions, each of which could flare during a trial, possibly obscuring a true beneficial effect of an intervention,” Dr. Rowe wrote.
Trials with open treatment periods, in which ME/CFS patients all receive rituximab and then are grouped based on nontargeted conditions, could be warranted to “allow better control” of these conditions. Other trial designs could include randomizing patients to continue or discontinue therapy for responders, he added.
“The profound level of impaired function of affected individuals warrants a new commitment to hypothesis-driven clinical trials that incorporate and expand on the methodological sophistication of the rituximab trial,” Dr. Rowe wrote.
Dr. Rowe is with Johns Hopkins University, Baltimore. These comments summarize his editorial in response to Fluge et al. (Ann Intern Med. 2019 Apr 1. doi: 10.7326/M19-0643). Dr. Rowe reported receiving grants from the National Institutes of Health and is a scientific advisory board member for Solve ME/CFS, all outside the submitted work.
The RituxME trial’s results weaken the case for the use of rituximab to treat myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), but there are opportunities to test other treatments targeting immunologic abnormalities in ME/CFS, Peter C. Rowe, MD, of Johns Hopkins University, Baltimore, wrote in a related editorial.
“Persons with ME/CFS often meet criteria for several comorbid conditions, each of which could flare during a trial, possibly obscuring a true beneficial effect of an intervention,” Dr. Rowe wrote.
Trials with open treatment periods, in which ME/CFS patients all receive rituximab and then are grouped based on nontargeted conditions, could be warranted to “allow better control” of these conditions. Other trial designs could include randomizing patients to continue or discontinue therapy for responders, he added.
“The profound level of impaired function of affected individuals warrants a new commitment to hypothesis-driven clinical trials that incorporate and expand on the methodological sophistication of the rituximab trial,” Dr. Rowe wrote.
Dr. Rowe is with Johns Hopkins University, Baltimore. These comments summarize his editorial in response to Fluge et al. (Ann Intern Med. 2019 Apr 1. doi: 10.7326/M19-0643). Dr. Rowe reported receiving grants from the National Institutes of Health and is a scientific advisory board member for Solve ME/CFS, all outside the submitted work.
The RituxME trial’s results weaken the case for the use of rituximab to treat myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), but there are opportunities to test other treatments targeting immunologic abnormalities in ME/CFS, Peter C. Rowe, MD, of Johns Hopkins University, Baltimore, wrote in a related editorial.
“Persons with ME/CFS often meet criteria for several comorbid conditions, each of which could flare during a trial, possibly obscuring a true beneficial effect of an intervention,” Dr. Rowe wrote.
Trials with open treatment periods, in which ME/CFS patients all receive rituximab and then are grouped based on nontargeted conditions, could be warranted to “allow better control” of these conditions. Other trial designs could include randomizing patients to continue or discontinue therapy for responders, he added.
“The profound level of impaired function of affected individuals warrants a new commitment to hypothesis-driven clinical trials that incorporate and expand on the methodological sophistication of the rituximab trial,” Dr. Rowe wrote.
Dr. Rowe is with Johns Hopkins University, Baltimore. These comments summarize his editorial in response to Fluge et al. (Ann Intern Med. 2019 Apr 1. doi: 10.7326/M19-0643). Dr. Rowe reported receiving grants from the National Institutes of Health and is a scientific advisory board member for Solve ME/CFS, all outside the submitted work.
according to results from the phase 3 RituxME trial.
“The lack of clinical effect of B-cell depletion in this trial weakens the case for an important role of B lymphocytes in ME/CFS but does not exclude an immunologic basis,” Øystein Fluge, MD, PhD, of the department of oncology and medical physics at Haukeland University Hospital in Bergen, Norway, and his colleagues wrote April 1 in Annals of Internal Medicine.
The investigators noted that the basis for testing the effects of a B-cell–depleting intervention on clinical symptoms in patients with ME/CFS came from observations of its potential benefit in a subgroup of patients in previous studies. Dr. Fluge and his colleagues performed a three-patient case series in their own group that found benefit for patients who received rituximab for treatment of CFS (BMC Neurol. 2009 May 8;9:28. doi: 10.1186/1471-2377-9-28). A phase 2 trial of 30 patients with CFS also performed by his own group found improved fatigue scores in 66.7% of patients in the rituximab group, compared with placebo (PLOS One. 2011 Oct 19. doi: 10.1371/journal.pone.0026358).
In the double-blinded RituxME trial, 151 patients with ME/CFS from four university hospitals and one general hospital in Norway were recruited and randomized to receive infusions of rituximab (n = 77) or placebo (n = 74). The patients were aged 18-65 years old and had the disease ranging from 2 years to 15 years. Patients reported and rated their ME/CFS symptoms at baseline as well as completed forms for the SF-36, Hospital Anxiety and Depression Scale, Fatigue Severity Scale, and modified DePaul Symptom Questionnaire out to 24 months. The rituximab group received two infusions at 500 mg/m2 across body surface area at 2 weeks apart. They then received 500-mg maintenance infusions at 3 months, 6 months, 9 months, and 12 months where they also self-reported changes in ME/CFS symptoms.
There were no significant differences between groups regarding fatigue score at any follow-up period, with an average between-group difference of 0.02 at 24 months (95% confidence interval, –0.27 to 0.31). The overall response rate was 26% with rituximab and 35% with placebo. Dr. Fluge and his colleagues also noted no significant differences regarding SF-36 scores, function level, and fatigue severity between groups.
Adverse event rates were higher in the rituximab group (63 patients; 82%) than in the placebo group (48 patients; 65%), and these were more often attributed to treatment for those taking rituximab (26 patients [34%]) than for placebo (12 patients [16%]). Adverse events requiring hospitalization also occurred more often among those taking rituximab (31 events in 20 patients [26%]) than for those who took placebo (16 events in 14 patients [19%]).
Some of the weaknesses of the trial included its use of self-referral and self-reported symptom scores, which might have been subject to recall bias. In commenting on the difference in outcome between the phase 3 trial and others, Dr. Fluge and his associates said the discrepancy could potentially be high expectations in the placebo group, unknown factors surrounding symptom variation in ME/CFS patients, and unknown patient selection effects.
“[T]he negative outcome of RituxME should spur research to assess patient subgroups and further elucidate disease mechanisms, of which recently disclosed impairment of energy metabolism may be important,” Dr. Fluge and his coauthors wrote.
The trial was funded by grants to the researchers from the Norwegian Research Council, the Norwegian Regional Health Trusts, the MEandYou Foundation, the Norwegian ME Association, and the legacy of Torstein Hereid.
SOURCE: Fluge Ø et al. Ann Intern Med. 2019 Apr 1. doi: 10.7326/M18-1451
according to results from the phase 3 RituxME trial.
“The lack of clinical effect of B-cell depletion in this trial weakens the case for an important role of B lymphocytes in ME/CFS but does not exclude an immunologic basis,” Øystein Fluge, MD, PhD, of the department of oncology and medical physics at Haukeland University Hospital in Bergen, Norway, and his colleagues wrote April 1 in Annals of Internal Medicine.
The investigators noted that the basis for testing the effects of a B-cell–depleting intervention on clinical symptoms in patients with ME/CFS came from observations of its potential benefit in a subgroup of patients in previous studies. Dr. Fluge and his colleagues performed a three-patient case series in their own group that found benefit for patients who received rituximab for treatment of CFS (BMC Neurol. 2009 May 8;9:28. doi: 10.1186/1471-2377-9-28). A phase 2 trial of 30 patients with CFS also performed by his own group found improved fatigue scores in 66.7% of patients in the rituximab group, compared with placebo (PLOS One. 2011 Oct 19. doi: 10.1371/journal.pone.0026358).
In the double-blinded RituxME trial, 151 patients with ME/CFS from four university hospitals and one general hospital in Norway were recruited and randomized to receive infusions of rituximab (n = 77) or placebo (n = 74). The patients were aged 18-65 years old and had the disease ranging from 2 years to 15 years. Patients reported and rated their ME/CFS symptoms at baseline as well as completed forms for the SF-36, Hospital Anxiety and Depression Scale, Fatigue Severity Scale, and modified DePaul Symptom Questionnaire out to 24 months. The rituximab group received two infusions at 500 mg/m2 across body surface area at 2 weeks apart. They then received 500-mg maintenance infusions at 3 months, 6 months, 9 months, and 12 months where they also self-reported changes in ME/CFS symptoms.
There were no significant differences between groups regarding fatigue score at any follow-up period, with an average between-group difference of 0.02 at 24 months (95% confidence interval, –0.27 to 0.31). The overall response rate was 26% with rituximab and 35% with placebo. Dr. Fluge and his colleagues also noted no significant differences regarding SF-36 scores, function level, and fatigue severity between groups.
Adverse event rates were higher in the rituximab group (63 patients; 82%) than in the placebo group (48 patients; 65%), and these were more often attributed to treatment for those taking rituximab (26 patients [34%]) than for placebo (12 patients [16%]). Adverse events requiring hospitalization also occurred more often among those taking rituximab (31 events in 20 patients [26%]) than for those who took placebo (16 events in 14 patients [19%]).
Some of the weaknesses of the trial included its use of self-referral and self-reported symptom scores, which might have been subject to recall bias. In commenting on the difference in outcome between the phase 3 trial and others, Dr. Fluge and his associates said the discrepancy could potentially be high expectations in the placebo group, unknown factors surrounding symptom variation in ME/CFS patients, and unknown patient selection effects.
“[T]he negative outcome of RituxME should spur research to assess patient subgroups and further elucidate disease mechanisms, of which recently disclosed impairment of energy metabolism may be important,” Dr. Fluge and his coauthors wrote.
The trial was funded by grants to the researchers from the Norwegian Research Council, the Norwegian Regional Health Trusts, the MEandYou Foundation, the Norwegian ME Association, and the legacy of Torstein Hereid.
SOURCE: Fluge Ø et al. Ann Intern Med. 2019 Apr 1. doi: 10.7326/M18-1451
FROM ANNALS OF INTERNAL MEDICINE
Click for Credit: Suicide in Medicaid youth; persistent back pain; more
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
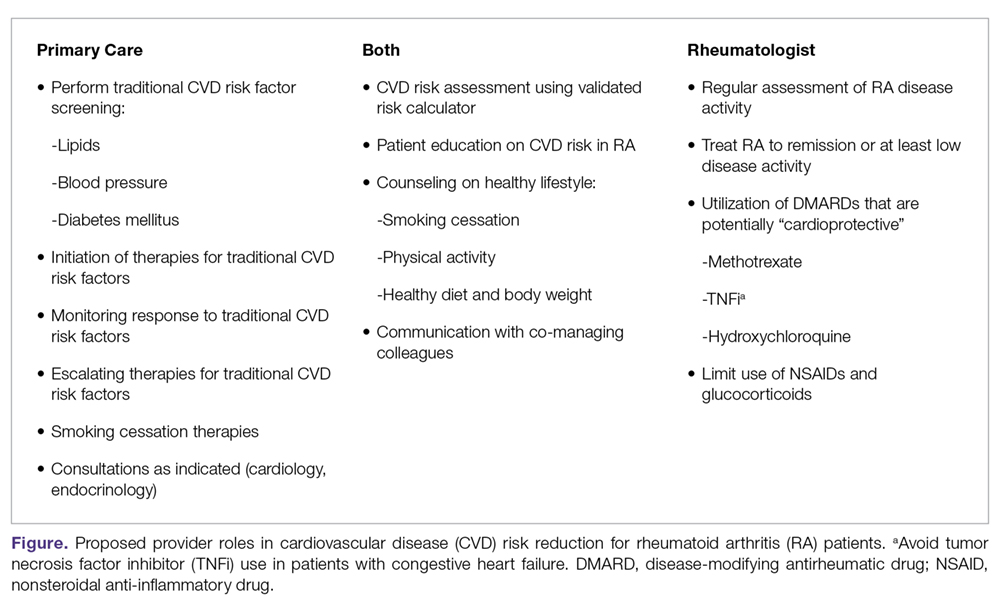
Management of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
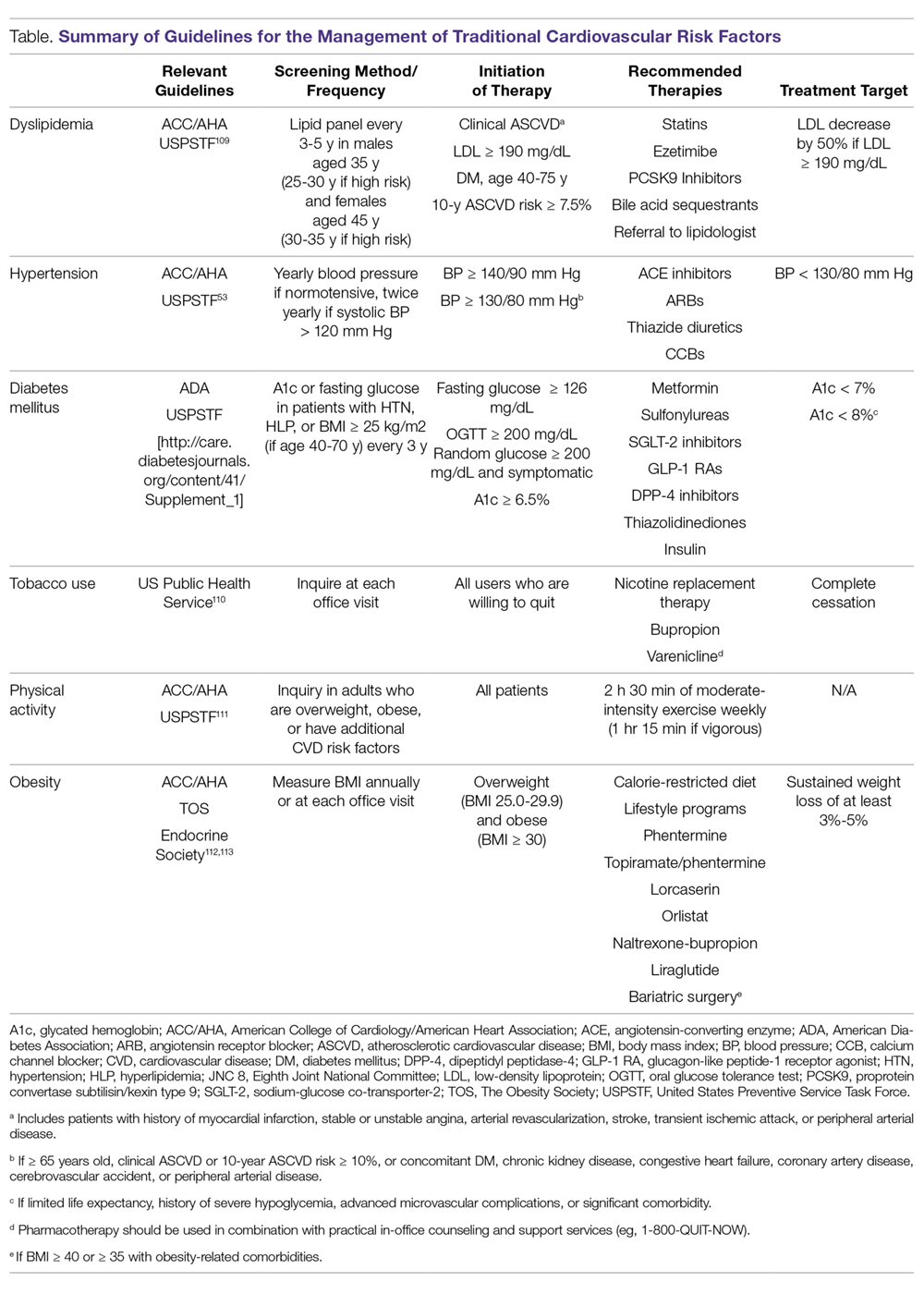
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
Flu shot can be given irrespective of the time of last methotrexate dose
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
Study details: A post hoc analysis of a randomized, controlled trial involving 316 patients with rheumatoid arthritis who continued or stopped methotrexate for 2 weeks following influenza vaccination.
Disclosures: The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
Source: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187
Cimzia becomes first FDA-approved treatment for nonradiographic axial spondyloarthritis
, with objective evidence of inflammation, making it the first treatment approved by the agency for the condition.
The FDA approved the tumor necrosis factor inhibitor based on results from a randomized clinical trial in 317 adult patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had elevated C-reactive protein levels and/or sacroiliitis (inflammation of the sacroiliac joints) on MRI.
The trial entailed 52 weeks of double-blind therapy with certolizumab at a starting dose of 400 mg on weeks 0, 2, and 4 followed by 200 mg every 2 weeks, or placebo. The Ankylosing Spondylitis Disease Activity Score Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866).
The overall safety profile observed in the Cimzia treatment group was consistent with the known safety profile of certolizumab.
Cimzia was first approved in 2008 and has FDA-approved indications for adult patients with Crohn’s disease, moderate to severe rheumatoid arthritis, active ankylosing spondylitis and moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
, with objective evidence of inflammation, making it the first treatment approved by the agency for the condition.
The FDA approved the tumor necrosis factor inhibitor based on results from a randomized clinical trial in 317 adult patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had elevated C-reactive protein levels and/or sacroiliitis (inflammation of the sacroiliac joints) on MRI.
The trial entailed 52 weeks of double-blind therapy with certolizumab at a starting dose of 400 mg on weeks 0, 2, and 4 followed by 200 mg every 2 weeks, or placebo. The Ankylosing Spondylitis Disease Activity Score Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866).
The overall safety profile observed in the Cimzia treatment group was consistent with the known safety profile of certolizumab.
Cimzia was first approved in 2008 and has FDA-approved indications for adult patients with Crohn’s disease, moderate to severe rheumatoid arthritis, active ankylosing spondylitis and moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
, with objective evidence of inflammation, making it the first treatment approved by the agency for the condition.
The FDA approved the tumor necrosis factor inhibitor based on results from a randomized clinical trial in 317 adult patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had elevated C-reactive protein levels and/or sacroiliitis (inflammation of the sacroiliac joints) on MRI.
The trial entailed 52 weeks of double-blind therapy with certolizumab at a starting dose of 400 mg on weeks 0, 2, and 4 followed by 200 mg every 2 weeks, or placebo. The Ankylosing Spondylitis Disease Activity Score Major Improvement rate, defined as at least a 2-point improvement from baseline, was 47% in the active treatment arm, compared with 7% on placebo. The Assessment in Ankylosing Spondylitis International Society 40% response rate, a more patient-reported outcome measure, was 57% in the certolizumab group and 16% in controls (Arthritis Rheumatol. 2019 March 8. doi: 10.1002/art.40866).
The overall safety profile observed in the Cimzia treatment group was consistent with the known safety profile of certolizumab.
Cimzia was first approved in 2008 and has FDA-approved indications for adult patients with Crohn’s disease, moderate to severe rheumatoid arthritis, active ankylosing spondylitis and moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
A panel of four biomarkers of cartilage metabolism, metabolic syndrome, and inflammation could help physicians to distinguish between osteoarthritis and psoriatic arthritis, new research suggests.
Such a test for distinguishing between the two conditions, which have “similarities in the distribution of joints involved,” could offer a way to make earlier diagnoses and avoid inappropriate treatment, according to Vinod Chandran, MD, PhD, of the department of medicine at the University of Toronto and Toronto Western Hospital and his colleagues. Dr. Chandran was first author on a study published online in Annals of the Rheumatic Diseases that analyzed serum samples from the University of Toronto Psoriatic Arthritis Program and University Health Network Arthritis Program for differences in certain biomarkers from 201 individuals with osteoarthritis, 77 with psoriatic arthritis, and 76 healthy controls.
The samples were tested for 15 biomarkers, including those related to cartilage metabolism (cartilage oligomeric matrix protein and hyaluronan), to metabolic syndrome (adiponectin, adipsin, resistin, hepatocyte growth factor, insulin, and leptin), and to inflammation (C-reactive protein, interleukin-1-beta, interleukin-6, interleukin-8, tumor necrosis factor alpha, monocyte chemoattractant protein–1, and nerve growth factor).
Researchers found that levels of 12 of these markers were different in patients with psoriatic arthritis, osteoarthritis, or controls, and 9 markers showed altered expression in psoriatic arthritis, compared with osteoarthritis.
Further analysis showed that levels of cartilage oligomeric matrix protein, resistin, monocyte chemoattractant protein–1, and nerve growth factor were significantly different between patients with psoriatic arthritis and those with osteoarthritis. The ROC curve for a model based on these four biomarkers that also incorporated age and sex had an area under the curve of 0.9984.
Researchers then validated the four biomarkers in an independent set of 75 patients with osteoarthritis and 73 with psoriatic arthritis and found these biomarkers were able to discriminate between the two conditions beyond what would be achieved based on age and sex alone.
The authors noted that previous research has observed high expression of monocyte chemoattractant protein–1 and resistin in patients with psoriatic arthritis when compared with those with osteoarthritis.
Nerve growth factor has been seen at elevated levels in the synovial fluid of individuals with osteoarthritis and is known to play a role in the chronic pain associated with that disease.
Similarly, higher cartilage oligomeric matrix protein levels are associated with a higher risk of knee osteoarthritis.
However, the authors noted that individuals with osteoarthritis in the study were all undergoing joint replacement surgery and therefore may not be typical of patients presenting to family practices or rheumatology clinics.
The University of Toronto Psoriatic Arthritis Program is supported by the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Chandran V et al. Ann Rheum Dis. 2019 Mar 25. doi: 10.1136/annrheumdis-2018-214737.
A panel of four biomarkers of cartilage metabolism, metabolic syndrome, and inflammation could help physicians to distinguish between osteoarthritis and psoriatic arthritis, new research suggests.
Such a test for distinguishing between the two conditions, which have “similarities in the distribution of joints involved,” could offer a way to make earlier diagnoses and avoid inappropriate treatment, according to Vinod Chandran, MD, PhD, of the department of medicine at the University of Toronto and Toronto Western Hospital and his colleagues. Dr. Chandran was first author on a study published online in Annals of the Rheumatic Diseases that analyzed serum samples from the University of Toronto Psoriatic Arthritis Program and University Health Network Arthritis Program for differences in certain biomarkers from 201 individuals with osteoarthritis, 77 with psoriatic arthritis, and 76 healthy controls.
The samples were tested for 15 biomarkers, including those related to cartilage metabolism (cartilage oligomeric matrix protein and hyaluronan), to metabolic syndrome (adiponectin, adipsin, resistin, hepatocyte growth factor, insulin, and leptin), and to inflammation (C-reactive protein, interleukin-1-beta, interleukin-6, interleukin-8, tumor necrosis factor alpha, monocyte chemoattractant protein–1, and nerve growth factor).
Researchers found that levels of 12 of these markers were different in patients with psoriatic arthritis, osteoarthritis, or controls, and 9 markers showed altered expression in psoriatic arthritis, compared with osteoarthritis.
Further analysis showed that levels of cartilage oligomeric matrix protein, resistin, monocyte chemoattractant protein–1, and nerve growth factor were significantly different between patients with psoriatic arthritis and those with osteoarthritis. The ROC curve for a model based on these four biomarkers that also incorporated age and sex had an area under the curve of 0.9984.
Researchers then validated the four biomarkers in an independent set of 75 patients with osteoarthritis and 73 with psoriatic arthritis and found these biomarkers were able to discriminate between the two conditions beyond what would be achieved based on age and sex alone.
The authors noted that previous research has observed high expression of monocyte chemoattractant protein–1 and resistin in patients with psoriatic arthritis when compared with those with osteoarthritis.
Nerve growth factor has been seen at elevated levels in the synovial fluid of individuals with osteoarthritis and is known to play a role in the chronic pain associated with that disease.
Similarly, higher cartilage oligomeric matrix protein levels are associated with a higher risk of knee osteoarthritis.
However, the authors noted that individuals with osteoarthritis in the study were all undergoing joint replacement surgery and therefore may not be typical of patients presenting to family practices or rheumatology clinics.
The University of Toronto Psoriatic Arthritis Program is supported by the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Chandran V et al. Ann Rheum Dis. 2019 Mar 25. doi: 10.1136/annrheumdis-2018-214737.
A panel of four biomarkers of cartilage metabolism, metabolic syndrome, and inflammation could help physicians to distinguish between osteoarthritis and psoriatic arthritis, new research suggests.
Such a test for distinguishing between the two conditions, which have “similarities in the distribution of joints involved,” could offer a way to make earlier diagnoses and avoid inappropriate treatment, according to Vinod Chandran, MD, PhD, of the department of medicine at the University of Toronto and Toronto Western Hospital and his colleagues. Dr. Chandran was first author on a study published online in Annals of the Rheumatic Diseases that analyzed serum samples from the University of Toronto Psoriatic Arthritis Program and University Health Network Arthritis Program for differences in certain biomarkers from 201 individuals with osteoarthritis, 77 with psoriatic arthritis, and 76 healthy controls.
The samples were tested for 15 biomarkers, including those related to cartilage metabolism (cartilage oligomeric matrix protein and hyaluronan), to metabolic syndrome (adiponectin, adipsin, resistin, hepatocyte growth factor, insulin, and leptin), and to inflammation (C-reactive protein, interleukin-1-beta, interleukin-6, interleukin-8, tumor necrosis factor alpha, monocyte chemoattractant protein–1, and nerve growth factor).
Researchers found that levels of 12 of these markers were different in patients with psoriatic arthritis, osteoarthritis, or controls, and 9 markers showed altered expression in psoriatic arthritis, compared with osteoarthritis.
Further analysis showed that levels of cartilage oligomeric matrix protein, resistin, monocyte chemoattractant protein–1, and nerve growth factor were significantly different between patients with psoriatic arthritis and those with osteoarthritis. The ROC curve for a model based on these four biomarkers that also incorporated age and sex had an area under the curve of 0.9984.
Researchers then validated the four biomarkers in an independent set of 75 patients with osteoarthritis and 73 with psoriatic arthritis and found these biomarkers were able to discriminate between the two conditions beyond what would be achieved based on age and sex alone.
The authors noted that previous research has observed high expression of monocyte chemoattractant protein–1 and resistin in patients with psoriatic arthritis when compared with those with osteoarthritis.
Nerve growth factor has been seen at elevated levels in the synovial fluid of individuals with osteoarthritis and is known to play a role in the chronic pain associated with that disease.
Similarly, higher cartilage oligomeric matrix protein levels are associated with a higher risk of knee osteoarthritis.
However, the authors noted that individuals with osteoarthritis in the study were all undergoing joint replacement surgery and therefore may not be typical of patients presenting to family practices or rheumatology clinics.
The University of Toronto Psoriatic Arthritis Program is supported by the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Chandran V et al. Ann Rheum Dis. 2019 Mar 25. doi: 10.1136/annrheumdis-2018-214737.
FROM ANNALS OF THE RHEUMATIC DISEASES
New postmenopausal osteoporosis guidelines emphasize patient priorities
NEW ORLEANS – In new guidelines for the pharmacologic management of osteoporosis, bisphosphonates have been identified as the first-line therapy with denosumab (Prolia) listed as an acceptable alternative that is particularly well suited for high-risk patients, according to a presentation at the annual meeting of the Endocrine Society.
“We hope our guideline will not only improve patient care but provide confidence in treatment,” reported guideline writing committee member Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough.
The new guidelines are evidence based, relying on randomized, controlled trials to evaluate the data quality of treatment options with GRADE methodology, but Dr. Rosen said that the guideline writing committee also considered patient preferences because of concerns about the abundant evidence that adherence to pharmacologic therapies for osteoporosis is poor.
“There is a considerable gap in the treatment of osteoporosis. Most women will not take anti-osteoporosis therapies despite their efficacy, and those who do often stop,” Dr. Rosen observed. He said it was the intention of the writing committee to provide acceptable recommendations with a clear outline of benefits and risks in order to enlist patients more successfully in understanding and participating in fracture prevention.
The Endocrine Society guidelines, which are available online and will soon appear in print (J Clin Endocrinol Metab. 2019;104:1-28), are focused on pharmacologic management and therefore differ from guidelines on diagnosis and treatment published previously by the American Association of Clinical Endocrinologists (AACE) (Endocr Pract. 2016;22[Suppl 4]:1-42).
The AACE guidelines, which devote considerable space to prevention, indicated that bisphosphonates should “be generally considered as initial options for most patients who are candidates for treatment.” The AACE guidelines identify denosumab as the “treatment of choice” in patients with renal insufficiency (although not in those on dialysis or with end-stage renal disease).
In outlining some of the key features of the new guidelines at ENDO 2019, Dr. Rosen drew attention to a call for reevaluation of the need for bisphosphonates after patients have been on this therapy for 3 or more years. For those found at this time to be at low or moderate risk of fracture, a drug holiday is recommended based on guideline-cited evidence that bisphosphonates offer a residual therapeutic effect after stopping.
However, stopping is not recommended in those who remain at high risk. In these patients, bone density should be monitored at regular intervals for the goal of switching or intensifying therapy if needed. This includes use of teriparatide (Forteo) or abaloparatide (Tymlos) for periods of up to 2 years in patients with a history of severe or multiple fractures. These and other choices are included in a detailed algorithm covering both low- and high-risk patients.
Although many postmenopausal women hope to avoid pharmacologic therapy with high dietary intake of calcium and vitamin D, Dr. Rosen stressed the limited benefit of these nutrients in preventing fracture for those with established osteoporosis. While acknowledging that calcium and vitamin D enhance mineralization and maintenance of bone mass, he characterized them as “supplements” once pharmacologic therapies are indicated.
Unsurprisingly, patients prefer oral therapies that are effective but with a low burden of adverse events, according to a review of evidence undertaken by the guideline committee. Cost was a less important consideration. Dr. Rosen indicated that recognizing patient goals and priorities while explaining relative risks might engage patients in selecting a therapy to which they are willing to adhere.
He reported having no relevant financial relationships.
NEW ORLEANS – In new guidelines for the pharmacologic management of osteoporosis, bisphosphonates have been identified as the first-line therapy with denosumab (Prolia) listed as an acceptable alternative that is particularly well suited for high-risk patients, according to a presentation at the annual meeting of the Endocrine Society.
“We hope our guideline will not only improve patient care but provide confidence in treatment,” reported guideline writing committee member Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough.
The new guidelines are evidence based, relying on randomized, controlled trials to evaluate the data quality of treatment options with GRADE methodology, but Dr. Rosen said that the guideline writing committee also considered patient preferences because of concerns about the abundant evidence that adherence to pharmacologic therapies for osteoporosis is poor.
“There is a considerable gap in the treatment of osteoporosis. Most women will not take anti-osteoporosis therapies despite their efficacy, and those who do often stop,” Dr. Rosen observed. He said it was the intention of the writing committee to provide acceptable recommendations with a clear outline of benefits and risks in order to enlist patients more successfully in understanding and participating in fracture prevention.
The Endocrine Society guidelines, which are available online and will soon appear in print (J Clin Endocrinol Metab. 2019;104:1-28), are focused on pharmacologic management and therefore differ from guidelines on diagnosis and treatment published previously by the American Association of Clinical Endocrinologists (AACE) (Endocr Pract. 2016;22[Suppl 4]:1-42).
The AACE guidelines, which devote considerable space to prevention, indicated that bisphosphonates should “be generally considered as initial options for most patients who are candidates for treatment.” The AACE guidelines identify denosumab as the “treatment of choice” in patients with renal insufficiency (although not in those on dialysis or with end-stage renal disease).
In outlining some of the key features of the new guidelines at ENDO 2019, Dr. Rosen drew attention to a call for reevaluation of the need for bisphosphonates after patients have been on this therapy for 3 or more years. For those found at this time to be at low or moderate risk of fracture, a drug holiday is recommended based on guideline-cited evidence that bisphosphonates offer a residual therapeutic effect after stopping.
However, stopping is not recommended in those who remain at high risk. In these patients, bone density should be monitored at regular intervals for the goal of switching or intensifying therapy if needed. This includes use of teriparatide (Forteo) or abaloparatide (Tymlos) for periods of up to 2 years in patients with a history of severe or multiple fractures. These and other choices are included in a detailed algorithm covering both low- and high-risk patients.
Although many postmenopausal women hope to avoid pharmacologic therapy with high dietary intake of calcium and vitamin D, Dr. Rosen stressed the limited benefit of these nutrients in preventing fracture for those with established osteoporosis. While acknowledging that calcium and vitamin D enhance mineralization and maintenance of bone mass, he characterized them as “supplements” once pharmacologic therapies are indicated.
Unsurprisingly, patients prefer oral therapies that are effective but with a low burden of adverse events, according to a review of evidence undertaken by the guideline committee. Cost was a less important consideration. Dr. Rosen indicated that recognizing patient goals and priorities while explaining relative risks might engage patients in selecting a therapy to which they are willing to adhere.
He reported having no relevant financial relationships.
NEW ORLEANS – In new guidelines for the pharmacologic management of osteoporosis, bisphosphonates have been identified as the first-line therapy with denosumab (Prolia) listed as an acceptable alternative that is particularly well suited for high-risk patients, according to a presentation at the annual meeting of the Endocrine Society.
“We hope our guideline will not only improve patient care but provide confidence in treatment,” reported guideline writing committee member Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough.
The new guidelines are evidence based, relying on randomized, controlled trials to evaluate the data quality of treatment options with GRADE methodology, but Dr. Rosen said that the guideline writing committee also considered patient preferences because of concerns about the abundant evidence that adherence to pharmacologic therapies for osteoporosis is poor.
“There is a considerable gap in the treatment of osteoporosis. Most women will not take anti-osteoporosis therapies despite their efficacy, and those who do often stop,” Dr. Rosen observed. He said it was the intention of the writing committee to provide acceptable recommendations with a clear outline of benefits and risks in order to enlist patients more successfully in understanding and participating in fracture prevention.
The Endocrine Society guidelines, which are available online and will soon appear in print (J Clin Endocrinol Metab. 2019;104:1-28), are focused on pharmacologic management and therefore differ from guidelines on diagnosis and treatment published previously by the American Association of Clinical Endocrinologists (AACE) (Endocr Pract. 2016;22[Suppl 4]:1-42).
The AACE guidelines, which devote considerable space to prevention, indicated that bisphosphonates should “be generally considered as initial options for most patients who are candidates for treatment.” The AACE guidelines identify denosumab as the “treatment of choice” in patients with renal insufficiency (although not in those on dialysis or with end-stage renal disease).
In outlining some of the key features of the new guidelines at ENDO 2019, Dr. Rosen drew attention to a call for reevaluation of the need for bisphosphonates after patients have been on this therapy for 3 or more years. For those found at this time to be at low or moderate risk of fracture, a drug holiday is recommended based on guideline-cited evidence that bisphosphonates offer a residual therapeutic effect after stopping.
However, stopping is not recommended in those who remain at high risk. In these patients, bone density should be monitored at regular intervals for the goal of switching or intensifying therapy if needed. This includes use of teriparatide (Forteo) or abaloparatide (Tymlos) for periods of up to 2 years in patients with a history of severe or multiple fractures. These and other choices are included in a detailed algorithm covering both low- and high-risk patients.
Although many postmenopausal women hope to avoid pharmacologic therapy with high dietary intake of calcium and vitamin D, Dr. Rosen stressed the limited benefit of these nutrients in preventing fracture for those with established osteoporosis. While acknowledging that calcium and vitamin D enhance mineralization and maintenance of bone mass, he characterized them as “supplements” once pharmacologic therapies are indicated.
Unsurprisingly, patients prefer oral therapies that are effective but with a low burden of adverse events, according to a review of evidence undertaken by the guideline committee. Cost was a less important consideration. Dr. Rosen indicated that recognizing patient goals and priorities while explaining relative risks might engage patients in selecting a therapy to which they are willing to adhere.
He reported having no relevant financial relationships.
REPORTING FROM ENDO 2019
Biologics boost work outcomes in axial spondyloarthritis
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019