User login
Two doses HPV vaccine are as good as three against genital warts
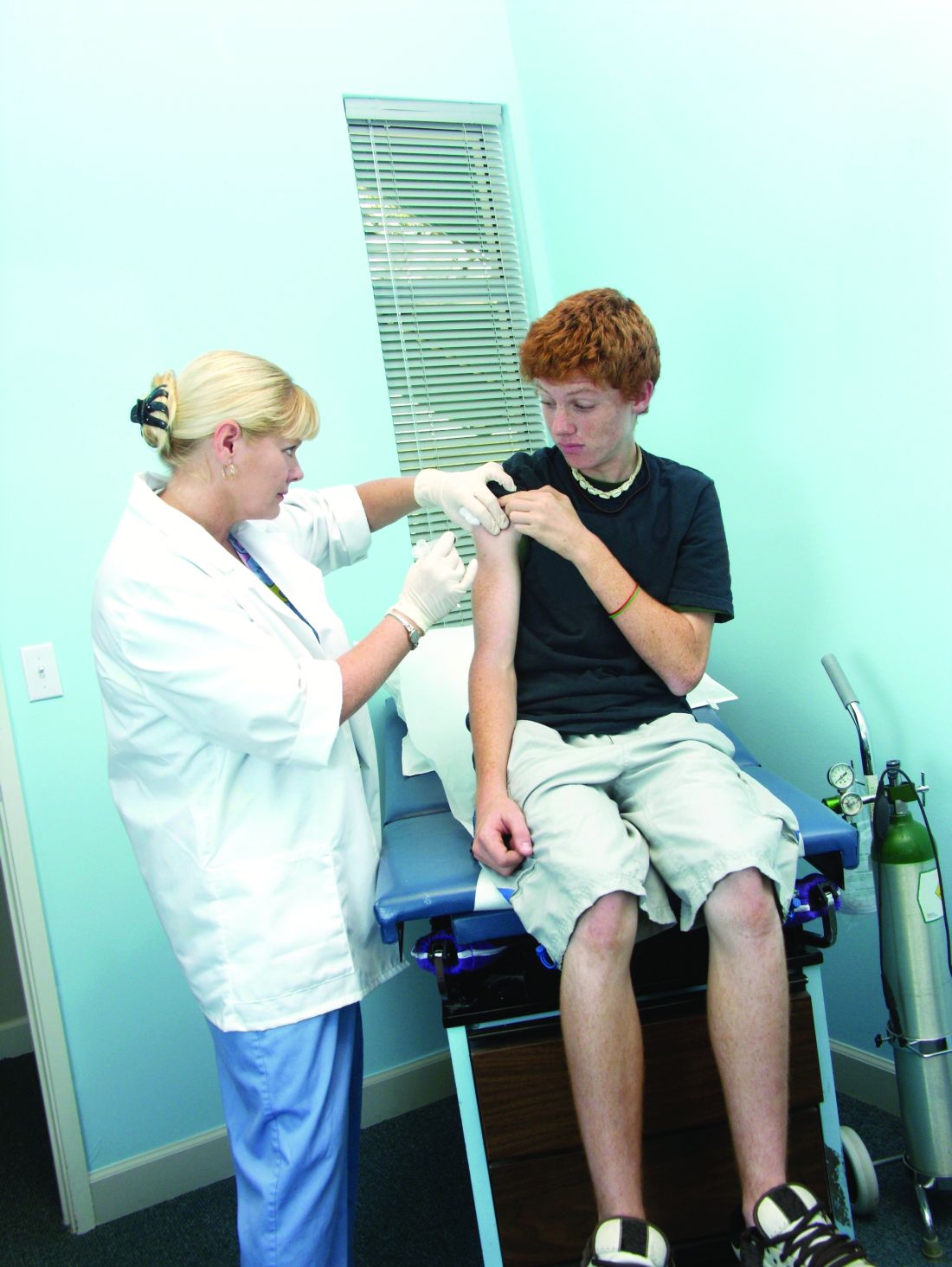
Receiving two doses of human papillomavirus (HPV) vaccine at 5-month intervals or longer appears to provide similar protection against genital warts as three doses among girls initiating the series before age 15 years, reported Rebecca B. Perkins, MD, of Boston University, and her associates.
Of 387,906 adolescent females, 8% received 1 dose of HPV vaccine, 9% received 2 doses, 31% received 3 doses, and 52% remained unvaccinated. The mean age of the girls in the study was 15 years, and average length of follow-up was 6 years. The girls were aged 9-18 years on Jan.1, 2007, and the exposure period began at that time for unvaccinated girls or on the date of the last HPV vaccine injection for those receiving the vaccine. Among girls receiving more than 1 dose, 60% received their second dose within 3 months of their first dose (on time), and 47% received their third dose within 5 months of their second dose.
“Although reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes,” Dr. Perkins and her associates said. “Human papillomavirus vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount,” they noted.
The study used data from the Truven Health Analytics MarketScan Commercial Claims Database, covering enrollees and dependents from about half of provider-sponsored U.S. health insurance plans.
Read more at (Sex Transm Dis. 2017 Jun. doi: 10.1097/OLQ.0000000000000615).
Receiving two doses of human papillomavirus (HPV) vaccine at 5-month intervals or longer appears to provide similar protection against genital warts as three doses among girls initiating the series before age 15 years, reported Rebecca B. Perkins, MD, of Boston University, and her associates.
Of 387,906 adolescent females, 8% received 1 dose of HPV vaccine, 9% received 2 doses, 31% received 3 doses, and 52% remained unvaccinated. The mean age of the girls in the study was 15 years, and average length of follow-up was 6 years. The girls were aged 9-18 years on Jan.1, 2007, and the exposure period began at that time for unvaccinated girls or on the date of the last HPV vaccine injection for those receiving the vaccine. Among girls receiving more than 1 dose, 60% received their second dose within 3 months of their first dose (on time), and 47% received their third dose within 5 months of their second dose.
“Although reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes,” Dr. Perkins and her associates said. “Human papillomavirus vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount,” they noted.
The study used data from the Truven Health Analytics MarketScan Commercial Claims Database, covering enrollees and dependents from about half of provider-sponsored U.S. health insurance plans.
Read more at (Sex Transm Dis. 2017 Jun. doi: 10.1097/OLQ.0000000000000615).
Receiving two doses of human papillomavirus (HPV) vaccine at 5-month intervals or longer appears to provide similar protection against genital warts as three doses among girls initiating the series before age 15 years, reported Rebecca B. Perkins, MD, of Boston University, and her associates.
Of 387,906 adolescent females, 8% received 1 dose of HPV vaccine, 9% received 2 doses, 31% received 3 doses, and 52% remained unvaccinated. The mean age of the girls in the study was 15 years, and average length of follow-up was 6 years. The girls were aged 9-18 years on Jan.1, 2007, and the exposure period began at that time for unvaccinated girls or on the date of the last HPV vaccine injection for those receiving the vaccine. Among girls receiving more than 1 dose, 60% received their second dose within 3 months of their first dose (on time), and 47% received their third dose within 5 months of their second dose.
“Although reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes,” Dr. Perkins and her associates said. “Human papillomavirus vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount,” they noted.
The study used data from the Truven Health Analytics MarketScan Commercial Claims Database, covering enrollees and dependents from about half of provider-sponsored U.S. health insurance plans.
Read more at (Sex Transm Dis. 2017 Jun. doi: 10.1097/OLQ.0000000000000615).
FROM SEXUALLY TRANSMITTED DISEASES
Preventing herpes zoster through vaccination: New developments
Herpes zoster (HZ), or shingles, represents a reactivation of the varicella-zoster virus (VZV). Following primary infection, usually in childhood, the virus typically lies dormant in the dorsal root and sensory nerve ganglia for decades. The precise mechanism of reactivation is not well understood, but it is associated with a decline in cell-mediated immunity that occurs with advancing age, immune-compromising conditions such as HIV infection and cancer, or immunosuppressive therapies, including corticosteroids.1 HZ is usually a self-limited disease characterized by unilateral dermatomal rash and pain, but can cause disseminated infection in immunocompromised individuals.2
Treatment with antiviral medications within 72 hours of rash onset can reduce acute HZ symptoms.1 However, antiviral agents are only minimally effective in preventing postherpetic neuralgia, the most common complication of HZ.3 Therefore, efforts to reduce the burden of HZ morbidity have focused on prevention through vaccination.
Currently, the only shingles vaccine approved by the US Food and Drug Administration (FDA) is Zostavax (Merck), which contains the live-attenuated Oka strain of VZV at a concentration 14 times greater than that of the varicella vaccine (Varivax, Merck). The live-attenuated vaccine boosts VZV-specific cell-mediated immunity, preventing reactivation of the latent virus.
In this article, we describe the burden of disease and review recent developments in the literature on HZ vaccine, including duration of efficacy, uptake and barriers to vaccination, cost-effectiveness, and the outlook for future vaccines.
INCIDENCE INCREASES WITH AGE
The incidence of herpes zoster in the general population is between 3 and 5 per 1,000 person-years4 and increases with age, especially after age 60 when the incidence can approach 13 to 15 per 1,000 person-years.5,6 An estimated 1 million new cases occur each year in the United States, and about 6% of patients experience a second episode of HZ within 8 years.7,8 In immunocompromised patients, the incidence of HZ is 2 to 10 times higher than in the general population.9
The incidence of HZ has been increasing for reasons that are unclear. After varicella vaccine was introduced into the routine childhood immunization schedule in 1995, it was hypothesized that the resultant decrease in primary varicella infections would remove a natural source of immune boosting and cause an increase in HZ incidence for up to 20 years.10 However, recent studies demonstrate that the observed increase in HZ incidence actually predates the introduction of varicella vaccine,11–13 and the widespread use of varicella vaccine has not resulted in an increase in the incidence of HZ.14
Other potential explanations for the rise in reported incidence include increasing awareness among patients, who might previously not have sought care and among physicians, who may be more likely to make the diagnosis. Advertisement of new treatments for HZ, including gabapentin and capsaicin, probably began to increase awareness in the 1990s, as did promotion of the HZ vaccine after its licensure in 2006.
HZ can occur in people who have been vaccinated against varicella due to reactivation of the vaccine-strain virus, but the risk is lower than after infection with wild-type varicella.15 Given that the varicella vaccine has been routinely used in children for only 20 years, the long-term effect of varicella vaccination on the incidence of HZ in elderly people is unknown.
Serious complications
HZ can cause rare but serious complications including encephalitis, herpes ophthalmicus, herpes oticus, myelitis, and retinitis.1 These can lead to long-term disability including unilateral blindness and deafness.
The most common and debilitating complication is postherpetic neuralgia, a persistent pain lasting at least 3 months, with a mean duration of 3.3 years and sometimes as long as 10 years.16 Postherpetic neuralgia occurs in 8% to 32% of patients after acute HZ,4 and the incidence increases with age, being most common after age 70. The chronic pain of postherpetic neuralgia has a significant adverse impact on patients’ quality of life, including physical disability and emotional distress.17 Some pain is intense, and anecdotal reports of patients committing suicide were included in the Advisory Committee on Immunization Practices (ACIP) recommendations regarding herpes zoster vaccine.18
HZ and its complications also impose a substantial economic burden on society.19 In a population-based study, the mean direct medical costs of HZ ranged from $620 to $1,160 (2015 dollars) depending on age,20 and the mean costs of postherpetic neuralgia were 2 to 5 times higher than that.20–22 Immunocompromised patients had costs 2 to 3 times higher than those of immunocompetent adults.23 In addition, for employed patients, HZ resulted in an average loss of 32 hours of work due to absenteeism and 84 hours due to presenteeism (ie, working while sick and therefore suboptimally).24
Assuming there are 1 million cases of HZ each year, if 8% to 32% of patients go on to develop postherpetic neuralgia, that would translate into approximately $1 to $2 billion in direct medical costs. With 60% of adult patients working,25 at an average wage of $23.23 per hour,26 HZ illness could be responsible for another $1.6 billion in lost productivity.
EFFICACY AND SAFETY OF HZ VACCINE
In 2006, the FDA approved the live-attenuated Oka strain VZV vaccine for prevention of HZ and postherpetic neuralgia in adults age 60 and older based on findings from the Shingles Prevention Study (SPS).27
The Shingles Prevention Study
This multicenter randomized placebo-controlled trial27 enrolled 38,546 immunocompetent persons age 60 and older. Subjects in the intervention group received a single dose of live-attenuated vaccine, and all participants were followed for up to 4.9 years after vaccination.
HZ occurred in 315 (1.636%) of the 19,254 participants in the vaccine group and in 642 (3.336%) of the 19,247 participants in the placebo group, an absolute risk reduction of 1.7%, number needed to treat 59, relative risk reduction 51%, P < .001. Similarly, postherpetic neuralgia occurred in 27 (0.140%) of the 19,254 vaccine recipients and in 80 (0.416%) of the placebo recipients (an absolute risk reduction of 0.276%, number needed to treat 362, relative risk reduction 66%, P < .001). The investigators calculated that vaccination reduced the overall burden of illness by 61% (Table 1).
The efficacy against HZ incidence decreased with age,28 but the efficacy against postherpetic neuralgia did not. In addition, vaccine recipients who developed HZ generally had less severe manifestations.
The safety of the vaccine was assessed for all participants in the SPS. In addition, one-sixth of SPS participants were enrolled in a safety substudy. These participants completed a detailed report card regarding all medically important events within the first 42 days. Forty-eight percent of the vaccine group and 17% of the placebo group (P < .05) experienced adverse events, primarily at the injection site. Less than 1% of all local reactions were severe.29 Serious adverse events were rare (< 2%), but occurred significantly more often in the vaccinated group.
Short-Term Persistence Substudy
Short-term efficacy of the live-attenuated vaccine (up to 7 years) was assessed in the Short-Term Persistence Substudy (STPS), which involved 14,270 of the initial participants and reported yearly and overall vaccine efficacy.30 After 5 years, the yearly efficacy against postherpetic neuralgia incidence declined to 32% and was no longer statistically significant. Efficacy against HZ incidence and burden of illness displayed the same pattern. After the end of the STPS, all subjects in the placebo group received vaccination.
Long-Term Persistence Substudy
Those in the intervention group were followed for an additional 4 years in the Long-Term Persistence Substudy (LTPS).31 Due to the lack of concurrent controls in the LTPS, the authors used regression models based on historical controls to estimate contemporary population incidence of HZ and postherpetic neuralgia for comparison.
Efficacy continued to decline over time, and by 10 years after vaccination there was no difference between vaccinated patients and historical controls in the rate of any end point (ie, efficacy declined to zero).
A trial of booster vaccination
Because many patients are vaccinated at age 60, waning immunity could leave them vulnerable to HZ and postherpetic neuralgia by age 70. A potential solution would be to give a booster dose after 10 years.
A recent phase 3 clinical trial of adults age 70 years and older found that a booster dose of live-attenuated vaccine was as safe and immunogenic as an initial dose.32 While antibody responses were similar in the boosted group and the newly vaccinated group, cell-mediated immunity was higher in the boosted group.
Because prevention of HZ is generally via cell-mediated immunity, the booster might be more effective than the initial vaccination, but clinical trials measuring actual cases prevented will be required to prove it. A booster dose is not currently recommended.
A trial of vaccination in adults 50 to 59
In 2011, the FDA extended its approval of HZ vaccine for use in adults ages 50 to 59.33
In a randomized, double-blind, placebo-controlled trial in this age group,33 the vaccine reduced HZ incidence by almost 70% (absolute risk reduction 0.614%, number needed to treat 156; Table 1), but the severity of HZ cases was not affected. There were too few cases of postherpetic neuralgia to assess the efficacy for this end point. The study followed patients for only 1.5 years after vaccination, so the duration of efficacy is unknown.
As in the older recipients, the vaccine was well tolerated; injection-site reactions and headache were the major adverse effects reported among vaccine recipients.33
INDICATIONS AND CONTRAINDICATIONS
Although HZ vaccine is licensed for use in adults age 50 and older, the ACIP recommends it only for immunocompetent adults age 60 and older. At this time, the ACIP does not recommend HZ vaccine in those younger than 60 because of the low risk of HZ in this age group.34
Any person age 60 or older should receive a single dose of the live-attenuated HZ vaccine subcutaneously, regardless of past history of HZ.
The vaccine is contraindicated in patients who have a history of allergic reaction to any vaccine component, immunosuppression or immunodeficiency conditions, and pregnancy. Specifically, people who will receive immunosuppressive therapies should have the vaccine at least 14 days before beginning treatment. Antiviral medications such as acyclovir, famciclovir, and valacyclovir should be discontinued at least 24 hours before vaccination and not resumed until 14 days later. Patients taking high-dose corticosteroids for more than 2 weeks should not be vaccinated until at least 1 month after therapy is completed.
In contrast, HZ vaccine is not contraindicated for leukemia patients who are in remission and who have not received chemotherapy or radiation for at least 3 months, or for patients receiving short-term, low-to-moderate dose, topical, intra-articular, bursal, or tendon injections of corticosteroids. Patients on low-dose methotrexate, azathioprine, or 6-mercaptopurine can also receive the vaccine.18
VACCINATION RATES ARE LOW
Although the vaccine has been recommended since 2008, uptake has been slow. Figure 1 shows the rate of HZ vaccination in adults age 60 and older surveyed in the National Health Interview Survey from 2007 to 2013.35 Eight years after the vaccine was licensed, only 28% of eligible patients had been vaccinated. Assuming the current rate of increase remains constant, it will take 7 more years to reach a 60% coverage rate—the same as for pneumococcal vaccine36—and 18 years to reach universal coverage.
Barriers to vaccination
Several barriers to HZ vaccination might account for the slow uptake.
For the first few years the vaccine was available, the requirement to store it frozen presented an obstacle for some physicians.37 Physicians may also have been discouraged by the cumbersome Medicare reimbursement process because while the administration fee is covered through Medicare Part B, the live-attenuated vaccine is reimbursed only through Medicare Part D, a benefit that varies by plans. Other barriers to physicians are supply shortages, high up-front costs, and uncertainties regarding the duration of vaccine protection, its safety, and side effects.38–40
Patient barriers include lack of physician recommendation, lack of familiarity with the vaccine, high out-of-pocket costs, the perception that they are at low risk for HZ, underestimation of the pain associated with HZ and postherpetic neuralgia, and fear of vaccine adverse effects.39,41,42
Interventions to increase vaccination rates
Certain interventions have been shown to increase vaccination adherence in general and HZ vaccination in particular. In randomized trials involving other vaccines, electronic medical record reminders supporting panel management or nurse-initiated protocols have been proven to increase vaccination rates, but these methods have not been tested for HZ vaccine specifically.43,44
In an observational study, Chaudhry et al found that the number of HZ vaccinations administered at the Mayo Clinic increased 43% in one practice and 54% in another after the implementation of an electronic alert.45 A randomized controlled trial showed that an informational package discussing HZ and the vaccine sent to patients via either their electronic personal health record or traditional mail increased HZ vaccination by almost 3 times.46
Pharmacists can also influence vaccination rates. States that provide full immunization privileges to pharmacists have vaccination rates significantly higher than states with restricted or no authorization.47
COST-EFFECTIVENESS CONSIDERATIONS
Unlike the Centers for Medicare and Medicaid Services, the ACIP does consider cost-effectiveness in their vaccine recommendations. Because of the morbidity associated with HZ and postherpetic neuralgia as well as the economic impact, vaccination is generally considered cost-effective for adults age 60 and older.48,49
Analyses have demonstrated that cost-effectiveness hinges on 4 factors: initial vaccine efficacy, the duration of efficacy, the age-specific incidence of HZ, and the cost of the vaccine.
For patients ages 50 to 59, the incidence of HZ is low, and because the duration of vaccine efficacy is short even though initial vaccine efficacy is high, vaccination in this age group offers poor value.50 At older ages, the incidence of HZ and postherpetic neuralgia rises, making vaccination more cost-effective. After age 60, the vaccine is cost-effective at all ages, although age 70 appears to offer the optimal trade-off between increasing incidence and declining vaccine efficacy.48,49
For patients who plan to be vaccinated only once, waiting until age 70 would appear to offer the best value.51 For those who are willing to receive a booster dose, the optimal age for vaccination is unknown, but will likely depend on the effectiveness, cost, and duration of the booster.
A NEW HZ VACCINE
In 2015, GlaxoSmithKline tested a new HZ vaccine containing a single VZV glycoprotein in an AS01B adjuvant system (HZ/su vaccine).52 In a phase 3 randomized trial involving 15,411 immunocompetent persons age 50 and older, a 2-dose schedule of HZ/su vaccine was 97% effective in preventing HZ (Table 1).53 Importantly, the vaccine was equally effective in older patients.
This vaccine also had a high rate of adverse reactions, with 17% of vaccine recipients vs 3% of placebo recipients reporting events that prevented normal everyday activities for at least 1 day. However, the rate of serious adverse reactions was the same in both groups (9%). The company announced that they intended to submit a regulatory application for HZ/su vaccine in the second half of 2016.54
Because of its high efficacy, HZ/su vaccine has the potential to change practice, but several issues must be resolved before it can supplant the current vaccine.
First, the AS01B adjuvant is not currently licensed in the United States, so it is unclear if the HZ/su vaccine can get FDA approval.52,55
Second, there are several questions about the efficacy of the vaccine, including long-term efficacy, efficacy in the elderly, and efficacy in the case of a patient receiving only 1 of the 2 required doses.
Third, the impact of HZ/su vaccine on complications such as postherpetic neuralgia has not been established. The clinical trial (NCT01165229) examining vaccine efficacy against postherpetic neuralgia incidence and other complications in adults age 70 and older has recently been completed and data should be available soon. Given the extremely high efficacy against HZ, it is likely that it will be close to 100% effective against this complication.
Fourth, there is uncertainty as to how the HZ/su vaccine should be used in patients who have already received the live-attenuated vaccine, if it is determined that a booster is necessary.
Finally, the vaccine is not yet priced. Given its superior effectiveness, particularly in older individuals, competitive pricing could dramatically affect the market. How Medicare or other insurers cover the new vaccine will likely influence its acceptance.
HZ VACCINATION OF IMMUNOCOMPROMISED PATIENTS
Immunocompromised patients are at highest risk for developing HZ. Unfortunately, there are currently no HZ vaccines approved for use in this population. The current live-attenuated vaccine has been demonstrated to be safe, well tolerated, and immunogenic in patients age 60 and older who are receiving chronic or maintenance low to moderate doses of corticosteroids.56
A clinical trial is being conducted to assess the immunogenicity, clinical effectiveness, and safety of the vaccine in rheumatoid arthritis patients receiving antitumor necrosis factor therapy (NCT01967316). Other trials are examining vaccine efficacy and safety in patients with solid organ tumors prior to chemotherapy (NCT02444936) and in patients who will be undergoing living donor kidney transplantation (NCT00940940). Researchers are also investigating the possibility of vaccinating allogeneic stem cell donors before donation in order to protect transplant recipients against HZ (NCT01573182).
ZVHT and HZ/su vaccination in immunocompromised patients
Heat-treated varicella-zoster vaccine (ZVHT) is a potential alternative for immunocompromised patients. A 4-dose regimen has been proven to reduce the risk of HZ in patients receiving autologous hematopoietic-cell transplants for non-Hodgkin or Hodgkin lymphoma.57
In another trial, the 4-dose ZVHT was safe and elicited significant VZV-specific T-cell response through 28 days in immunosuppressed patients with solid tumor malignancy, hematologic malignancy, human immunodeficiency virus infection with CD4 counts of 200 cells/mm3 or less, and autologous hematopoietic-cell transplants. The T-cell response was poor in allogeneic hematopoietic-cell transplant recipients, however.58
Because the HZ/su vaccine does not contain live virus, it seems particularly promising for immunocompromised patients. In phase 1 and 2 studies, a 3-dose regimen has been shown to be safe and immunogenic in hematopoietic-cell transplant recipients and HIV-infected adults with CD4 count higher than 200 cells/mm3.59,60 A phase 3 trial assessing the efficacy of HZ/su vaccine in autologous hematopoietic-cell transplant recipients is under way (NCT01610414). Changes in recommendations for HZ vaccine in these most vulnerable populations await the results of these studies.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl 1):S1–S26.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(suppl):21–26.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833.
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420.
- Yawn BP, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–1349.
- Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42:325–334.
- Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211–219.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–745.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–340.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–1005.
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002–2007.
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989; 159:1000–1001.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356–363.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–262.
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–794.
- Dworkin RH, White R, O’Connor AB, Hawkins K. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008; 9:348–353.
- White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–792.
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155–169.
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639–645.
- US Bureau of Labor Statistics. Labor force statistics from the current population survey. www.bls.gov/web/empsit/cpseea13.htm. Accessed April 6, 2017.
- US Bureau of Labor Statistic. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm. Accessed April 6, 2017.
- Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- Food and Drug Administration (FDA). FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax. www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198b2_1.pdf. Accessed April 6, 2017.
- Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545–554.
- Schmader KE, Oxman MN, Levin MJ, et al; Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–1328.
- Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–909.
- Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22.
- Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–928.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC). Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–731.
- Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(1):1–36. Accessed April 12, 2017.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010; 51:197–213.
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555–560.
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–887.
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008; 197(suppl 2):S216–S223.
- Joon Lee T, Hayes S, Cummings DM, et al. Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med 2013; 26:45–51.
- Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine 2009; 27:192–196.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011; 171:1552–1558.
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med 1999; 14:351–356.
- Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19:263–266.
- Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med 2013; 126:832.e1–832.e6.
- Taitel MS, Fensterheim LE, Cannon AE, Cohen ES. Improving pneumococcal and herpes zoster vaccination uptake: expanding pharmacist privileges. Am J Manag Care 2013; 19:e309–e313.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–136.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–497.
- Le P, Rothberg MB. Determining the optimal age to vaccinate against herpes zoster: a cost-effectiveness analysis. Society for Medical Decision Making 37th Annual North American Meeting. St. Louis, MO; October 18-21, 2015.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–263.
- Lal H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096.
- GlaxoSmithKline plc. GSK’s candidate shingles vaccine demonstrates 90% efficacy against shingles in people 70 years of age and over. www.gsk.com/en-gb/media/press-releases/gsk-s-candidate-shingles-vaccine-demonstrates-90-efficacy-against-shingles-in-people-70-years-of-age-and-over/. Accessed April 6, 2017.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–1608.
- Russell AF, Parrino J, Fisher CL Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015; 33:3129–3134.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013; 208:1375–1385.
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–1287.
Herpes zoster (HZ), or shingles, represents a reactivation of the varicella-zoster virus (VZV). Following primary infection, usually in childhood, the virus typically lies dormant in the dorsal root and sensory nerve ganglia for decades. The precise mechanism of reactivation is not well understood, but it is associated with a decline in cell-mediated immunity that occurs with advancing age, immune-compromising conditions such as HIV infection and cancer, or immunosuppressive therapies, including corticosteroids.1 HZ is usually a self-limited disease characterized by unilateral dermatomal rash and pain, but can cause disseminated infection in immunocompromised individuals.2
Treatment with antiviral medications within 72 hours of rash onset can reduce acute HZ symptoms.1 However, antiviral agents are only minimally effective in preventing postherpetic neuralgia, the most common complication of HZ.3 Therefore, efforts to reduce the burden of HZ morbidity have focused on prevention through vaccination.
Currently, the only shingles vaccine approved by the US Food and Drug Administration (FDA) is Zostavax (Merck), which contains the live-attenuated Oka strain of VZV at a concentration 14 times greater than that of the varicella vaccine (Varivax, Merck). The live-attenuated vaccine boosts VZV-specific cell-mediated immunity, preventing reactivation of the latent virus.
In this article, we describe the burden of disease and review recent developments in the literature on HZ vaccine, including duration of efficacy, uptake and barriers to vaccination, cost-effectiveness, and the outlook for future vaccines.
INCIDENCE INCREASES WITH AGE
The incidence of herpes zoster in the general population is between 3 and 5 per 1,000 person-years4 and increases with age, especially after age 60 when the incidence can approach 13 to 15 per 1,000 person-years.5,6 An estimated 1 million new cases occur each year in the United States, and about 6% of patients experience a second episode of HZ within 8 years.7,8 In immunocompromised patients, the incidence of HZ is 2 to 10 times higher than in the general population.9
The incidence of HZ has been increasing for reasons that are unclear. After varicella vaccine was introduced into the routine childhood immunization schedule in 1995, it was hypothesized that the resultant decrease in primary varicella infections would remove a natural source of immune boosting and cause an increase in HZ incidence for up to 20 years.10 However, recent studies demonstrate that the observed increase in HZ incidence actually predates the introduction of varicella vaccine,11–13 and the widespread use of varicella vaccine has not resulted in an increase in the incidence of HZ.14
Other potential explanations for the rise in reported incidence include increasing awareness among patients, who might previously not have sought care and among physicians, who may be more likely to make the diagnosis. Advertisement of new treatments for HZ, including gabapentin and capsaicin, probably began to increase awareness in the 1990s, as did promotion of the HZ vaccine after its licensure in 2006.
HZ can occur in people who have been vaccinated against varicella due to reactivation of the vaccine-strain virus, but the risk is lower than after infection with wild-type varicella.15 Given that the varicella vaccine has been routinely used in children for only 20 years, the long-term effect of varicella vaccination on the incidence of HZ in elderly people is unknown.
Serious complications
HZ can cause rare but serious complications including encephalitis, herpes ophthalmicus, herpes oticus, myelitis, and retinitis.1 These can lead to long-term disability including unilateral blindness and deafness.
The most common and debilitating complication is postherpetic neuralgia, a persistent pain lasting at least 3 months, with a mean duration of 3.3 years and sometimes as long as 10 years.16 Postherpetic neuralgia occurs in 8% to 32% of patients after acute HZ,4 and the incidence increases with age, being most common after age 70. The chronic pain of postherpetic neuralgia has a significant adverse impact on patients’ quality of life, including physical disability and emotional distress.17 Some pain is intense, and anecdotal reports of patients committing suicide were included in the Advisory Committee on Immunization Practices (ACIP) recommendations regarding herpes zoster vaccine.18
HZ and its complications also impose a substantial economic burden on society.19 In a population-based study, the mean direct medical costs of HZ ranged from $620 to $1,160 (2015 dollars) depending on age,20 and the mean costs of postherpetic neuralgia were 2 to 5 times higher than that.20–22 Immunocompromised patients had costs 2 to 3 times higher than those of immunocompetent adults.23 In addition, for employed patients, HZ resulted in an average loss of 32 hours of work due to absenteeism and 84 hours due to presenteeism (ie, working while sick and therefore suboptimally).24
Assuming there are 1 million cases of HZ each year, if 8% to 32% of patients go on to develop postherpetic neuralgia, that would translate into approximately $1 to $2 billion in direct medical costs. With 60% of adult patients working,25 at an average wage of $23.23 per hour,26 HZ illness could be responsible for another $1.6 billion in lost productivity.
EFFICACY AND SAFETY OF HZ VACCINE
In 2006, the FDA approved the live-attenuated Oka strain VZV vaccine for prevention of HZ and postherpetic neuralgia in adults age 60 and older based on findings from the Shingles Prevention Study (SPS).27
The Shingles Prevention Study
This multicenter randomized placebo-controlled trial27 enrolled 38,546 immunocompetent persons age 60 and older. Subjects in the intervention group received a single dose of live-attenuated vaccine, and all participants were followed for up to 4.9 years after vaccination.
HZ occurred in 315 (1.636%) of the 19,254 participants in the vaccine group and in 642 (3.336%) of the 19,247 participants in the placebo group, an absolute risk reduction of 1.7%, number needed to treat 59, relative risk reduction 51%, P < .001. Similarly, postherpetic neuralgia occurred in 27 (0.140%) of the 19,254 vaccine recipients and in 80 (0.416%) of the placebo recipients (an absolute risk reduction of 0.276%, number needed to treat 362, relative risk reduction 66%, P < .001). The investigators calculated that vaccination reduced the overall burden of illness by 61% (Table 1).
The efficacy against HZ incidence decreased with age,28 but the efficacy against postherpetic neuralgia did not. In addition, vaccine recipients who developed HZ generally had less severe manifestations.
The safety of the vaccine was assessed for all participants in the SPS. In addition, one-sixth of SPS participants were enrolled in a safety substudy. These participants completed a detailed report card regarding all medically important events within the first 42 days. Forty-eight percent of the vaccine group and 17% of the placebo group (P < .05) experienced adverse events, primarily at the injection site. Less than 1% of all local reactions were severe.29 Serious adverse events were rare (< 2%), but occurred significantly more often in the vaccinated group.
Short-Term Persistence Substudy
Short-term efficacy of the live-attenuated vaccine (up to 7 years) was assessed in the Short-Term Persistence Substudy (STPS), which involved 14,270 of the initial participants and reported yearly and overall vaccine efficacy.30 After 5 years, the yearly efficacy against postherpetic neuralgia incidence declined to 32% and was no longer statistically significant. Efficacy against HZ incidence and burden of illness displayed the same pattern. After the end of the STPS, all subjects in the placebo group received vaccination.
Long-Term Persistence Substudy
Those in the intervention group were followed for an additional 4 years in the Long-Term Persistence Substudy (LTPS).31 Due to the lack of concurrent controls in the LTPS, the authors used regression models based on historical controls to estimate contemporary population incidence of HZ and postherpetic neuralgia for comparison.
Efficacy continued to decline over time, and by 10 years after vaccination there was no difference between vaccinated patients and historical controls in the rate of any end point (ie, efficacy declined to zero).
A trial of booster vaccination
Because many patients are vaccinated at age 60, waning immunity could leave them vulnerable to HZ and postherpetic neuralgia by age 70. A potential solution would be to give a booster dose after 10 years.
A recent phase 3 clinical trial of adults age 70 years and older found that a booster dose of live-attenuated vaccine was as safe and immunogenic as an initial dose.32 While antibody responses were similar in the boosted group and the newly vaccinated group, cell-mediated immunity was higher in the boosted group.
Because prevention of HZ is generally via cell-mediated immunity, the booster might be more effective than the initial vaccination, but clinical trials measuring actual cases prevented will be required to prove it. A booster dose is not currently recommended.
A trial of vaccination in adults 50 to 59
In 2011, the FDA extended its approval of HZ vaccine for use in adults ages 50 to 59.33
In a randomized, double-blind, placebo-controlled trial in this age group,33 the vaccine reduced HZ incidence by almost 70% (absolute risk reduction 0.614%, number needed to treat 156; Table 1), but the severity of HZ cases was not affected. There were too few cases of postherpetic neuralgia to assess the efficacy for this end point. The study followed patients for only 1.5 years after vaccination, so the duration of efficacy is unknown.
As in the older recipients, the vaccine was well tolerated; injection-site reactions and headache were the major adverse effects reported among vaccine recipients.33
INDICATIONS AND CONTRAINDICATIONS
Although HZ vaccine is licensed for use in adults age 50 and older, the ACIP recommends it only for immunocompetent adults age 60 and older. At this time, the ACIP does not recommend HZ vaccine in those younger than 60 because of the low risk of HZ in this age group.34
Any person age 60 or older should receive a single dose of the live-attenuated HZ vaccine subcutaneously, regardless of past history of HZ.
The vaccine is contraindicated in patients who have a history of allergic reaction to any vaccine component, immunosuppression or immunodeficiency conditions, and pregnancy. Specifically, people who will receive immunosuppressive therapies should have the vaccine at least 14 days before beginning treatment. Antiviral medications such as acyclovir, famciclovir, and valacyclovir should be discontinued at least 24 hours before vaccination and not resumed until 14 days later. Patients taking high-dose corticosteroids for more than 2 weeks should not be vaccinated until at least 1 month after therapy is completed.
In contrast, HZ vaccine is not contraindicated for leukemia patients who are in remission and who have not received chemotherapy or radiation for at least 3 months, or for patients receiving short-term, low-to-moderate dose, topical, intra-articular, bursal, or tendon injections of corticosteroids. Patients on low-dose methotrexate, azathioprine, or 6-mercaptopurine can also receive the vaccine.18
VACCINATION RATES ARE LOW
Although the vaccine has been recommended since 2008, uptake has been slow. Figure 1 shows the rate of HZ vaccination in adults age 60 and older surveyed in the National Health Interview Survey from 2007 to 2013.35 Eight years after the vaccine was licensed, only 28% of eligible patients had been vaccinated. Assuming the current rate of increase remains constant, it will take 7 more years to reach a 60% coverage rate—the same as for pneumococcal vaccine36—and 18 years to reach universal coverage.
Barriers to vaccination
Several barriers to HZ vaccination might account for the slow uptake.
For the first few years the vaccine was available, the requirement to store it frozen presented an obstacle for some physicians.37 Physicians may also have been discouraged by the cumbersome Medicare reimbursement process because while the administration fee is covered through Medicare Part B, the live-attenuated vaccine is reimbursed only through Medicare Part D, a benefit that varies by plans. Other barriers to physicians are supply shortages, high up-front costs, and uncertainties regarding the duration of vaccine protection, its safety, and side effects.38–40
Patient barriers include lack of physician recommendation, lack of familiarity with the vaccine, high out-of-pocket costs, the perception that they are at low risk for HZ, underestimation of the pain associated with HZ and postherpetic neuralgia, and fear of vaccine adverse effects.39,41,42
Interventions to increase vaccination rates
Certain interventions have been shown to increase vaccination adherence in general and HZ vaccination in particular. In randomized trials involving other vaccines, electronic medical record reminders supporting panel management or nurse-initiated protocols have been proven to increase vaccination rates, but these methods have not been tested for HZ vaccine specifically.43,44
In an observational study, Chaudhry et al found that the number of HZ vaccinations administered at the Mayo Clinic increased 43% in one practice and 54% in another after the implementation of an electronic alert.45 A randomized controlled trial showed that an informational package discussing HZ and the vaccine sent to patients via either their electronic personal health record or traditional mail increased HZ vaccination by almost 3 times.46
Pharmacists can also influence vaccination rates. States that provide full immunization privileges to pharmacists have vaccination rates significantly higher than states with restricted or no authorization.47
COST-EFFECTIVENESS CONSIDERATIONS
Unlike the Centers for Medicare and Medicaid Services, the ACIP does consider cost-effectiveness in their vaccine recommendations. Because of the morbidity associated with HZ and postherpetic neuralgia as well as the economic impact, vaccination is generally considered cost-effective for adults age 60 and older.48,49
Analyses have demonstrated that cost-effectiveness hinges on 4 factors: initial vaccine efficacy, the duration of efficacy, the age-specific incidence of HZ, and the cost of the vaccine.
For patients ages 50 to 59, the incidence of HZ is low, and because the duration of vaccine efficacy is short even though initial vaccine efficacy is high, vaccination in this age group offers poor value.50 At older ages, the incidence of HZ and postherpetic neuralgia rises, making vaccination more cost-effective. After age 60, the vaccine is cost-effective at all ages, although age 70 appears to offer the optimal trade-off between increasing incidence and declining vaccine efficacy.48,49
For patients who plan to be vaccinated only once, waiting until age 70 would appear to offer the best value.51 For those who are willing to receive a booster dose, the optimal age for vaccination is unknown, but will likely depend on the effectiveness, cost, and duration of the booster.
A NEW HZ VACCINE
In 2015, GlaxoSmithKline tested a new HZ vaccine containing a single VZV glycoprotein in an AS01B adjuvant system (HZ/su vaccine).52 In a phase 3 randomized trial involving 15,411 immunocompetent persons age 50 and older, a 2-dose schedule of HZ/su vaccine was 97% effective in preventing HZ (Table 1).53 Importantly, the vaccine was equally effective in older patients.
This vaccine also had a high rate of adverse reactions, with 17% of vaccine recipients vs 3% of placebo recipients reporting events that prevented normal everyday activities for at least 1 day. However, the rate of serious adverse reactions was the same in both groups (9%). The company announced that they intended to submit a regulatory application for HZ/su vaccine in the second half of 2016.54
Because of its high efficacy, HZ/su vaccine has the potential to change practice, but several issues must be resolved before it can supplant the current vaccine.
First, the AS01B adjuvant is not currently licensed in the United States, so it is unclear if the HZ/su vaccine can get FDA approval.52,55
Second, there are several questions about the efficacy of the vaccine, including long-term efficacy, efficacy in the elderly, and efficacy in the case of a patient receiving only 1 of the 2 required doses.
Third, the impact of HZ/su vaccine on complications such as postherpetic neuralgia has not been established. The clinical trial (NCT01165229) examining vaccine efficacy against postherpetic neuralgia incidence and other complications in adults age 70 and older has recently been completed and data should be available soon. Given the extremely high efficacy against HZ, it is likely that it will be close to 100% effective against this complication.
Fourth, there is uncertainty as to how the HZ/su vaccine should be used in patients who have already received the live-attenuated vaccine, if it is determined that a booster is necessary.
Finally, the vaccine is not yet priced. Given its superior effectiveness, particularly in older individuals, competitive pricing could dramatically affect the market. How Medicare or other insurers cover the new vaccine will likely influence its acceptance.
HZ VACCINATION OF IMMUNOCOMPROMISED PATIENTS
Immunocompromised patients are at highest risk for developing HZ. Unfortunately, there are currently no HZ vaccines approved for use in this population. The current live-attenuated vaccine has been demonstrated to be safe, well tolerated, and immunogenic in patients age 60 and older who are receiving chronic or maintenance low to moderate doses of corticosteroids.56
A clinical trial is being conducted to assess the immunogenicity, clinical effectiveness, and safety of the vaccine in rheumatoid arthritis patients receiving antitumor necrosis factor therapy (NCT01967316). Other trials are examining vaccine efficacy and safety in patients with solid organ tumors prior to chemotherapy (NCT02444936) and in patients who will be undergoing living donor kidney transplantation (NCT00940940). Researchers are also investigating the possibility of vaccinating allogeneic stem cell donors before donation in order to protect transplant recipients against HZ (NCT01573182).
ZVHT and HZ/su vaccination in immunocompromised patients
Heat-treated varicella-zoster vaccine (ZVHT) is a potential alternative for immunocompromised patients. A 4-dose regimen has been proven to reduce the risk of HZ in patients receiving autologous hematopoietic-cell transplants for non-Hodgkin or Hodgkin lymphoma.57
In another trial, the 4-dose ZVHT was safe and elicited significant VZV-specific T-cell response through 28 days in immunosuppressed patients with solid tumor malignancy, hematologic malignancy, human immunodeficiency virus infection with CD4 counts of 200 cells/mm3 or less, and autologous hematopoietic-cell transplants. The T-cell response was poor in allogeneic hematopoietic-cell transplant recipients, however.58
Because the HZ/su vaccine does not contain live virus, it seems particularly promising for immunocompromised patients. In phase 1 and 2 studies, a 3-dose regimen has been shown to be safe and immunogenic in hematopoietic-cell transplant recipients and HIV-infected adults with CD4 count higher than 200 cells/mm3.59,60 A phase 3 trial assessing the efficacy of HZ/su vaccine in autologous hematopoietic-cell transplant recipients is under way (NCT01610414). Changes in recommendations for HZ vaccine in these most vulnerable populations await the results of these studies.
Herpes zoster (HZ), or shingles, represents a reactivation of the varicella-zoster virus (VZV). Following primary infection, usually in childhood, the virus typically lies dormant in the dorsal root and sensory nerve ganglia for decades. The precise mechanism of reactivation is not well understood, but it is associated with a decline in cell-mediated immunity that occurs with advancing age, immune-compromising conditions such as HIV infection and cancer, or immunosuppressive therapies, including corticosteroids.1 HZ is usually a self-limited disease characterized by unilateral dermatomal rash and pain, but can cause disseminated infection in immunocompromised individuals.2
Treatment with antiviral medications within 72 hours of rash onset can reduce acute HZ symptoms.1 However, antiviral agents are only minimally effective in preventing postherpetic neuralgia, the most common complication of HZ.3 Therefore, efforts to reduce the burden of HZ morbidity have focused on prevention through vaccination.
Currently, the only shingles vaccine approved by the US Food and Drug Administration (FDA) is Zostavax (Merck), which contains the live-attenuated Oka strain of VZV at a concentration 14 times greater than that of the varicella vaccine (Varivax, Merck). The live-attenuated vaccine boosts VZV-specific cell-mediated immunity, preventing reactivation of the latent virus.
In this article, we describe the burden of disease and review recent developments in the literature on HZ vaccine, including duration of efficacy, uptake and barriers to vaccination, cost-effectiveness, and the outlook for future vaccines.
INCIDENCE INCREASES WITH AGE
The incidence of herpes zoster in the general population is between 3 and 5 per 1,000 person-years4 and increases with age, especially after age 60 when the incidence can approach 13 to 15 per 1,000 person-years.5,6 An estimated 1 million new cases occur each year in the United States, and about 6% of patients experience a second episode of HZ within 8 years.7,8 In immunocompromised patients, the incidence of HZ is 2 to 10 times higher than in the general population.9
The incidence of HZ has been increasing for reasons that are unclear. After varicella vaccine was introduced into the routine childhood immunization schedule in 1995, it was hypothesized that the resultant decrease in primary varicella infections would remove a natural source of immune boosting and cause an increase in HZ incidence for up to 20 years.10 However, recent studies demonstrate that the observed increase in HZ incidence actually predates the introduction of varicella vaccine,11–13 and the widespread use of varicella vaccine has not resulted in an increase in the incidence of HZ.14
Other potential explanations for the rise in reported incidence include increasing awareness among patients, who might previously not have sought care and among physicians, who may be more likely to make the diagnosis. Advertisement of new treatments for HZ, including gabapentin and capsaicin, probably began to increase awareness in the 1990s, as did promotion of the HZ vaccine after its licensure in 2006.
HZ can occur in people who have been vaccinated against varicella due to reactivation of the vaccine-strain virus, but the risk is lower than after infection with wild-type varicella.15 Given that the varicella vaccine has been routinely used in children for only 20 years, the long-term effect of varicella vaccination on the incidence of HZ in elderly people is unknown.
Serious complications
HZ can cause rare but serious complications including encephalitis, herpes ophthalmicus, herpes oticus, myelitis, and retinitis.1 These can lead to long-term disability including unilateral blindness and deafness.
The most common and debilitating complication is postherpetic neuralgia, a persistent pain lasting at least 3 months, with a mean duration of 3.3 years and sometimes as long as 10 years.16 Postherpetic neuralgia occurs in 8% to 32% of patients after acute HZ,4 and the incidence increases with age, being most common after age 70. The chronic pain of postherpetic neuralgia has a significant adverse impact on patients’ quality of life, including physical disability and emotional distress.17 Some pain is intense, and anecdotal reports of patients committing suicide were included in the Advisory Committee on Immunization Practices (ACIP) recommendations regarding herpes zoster vaccine.18
HZ and its complications also impose a substantial economic burden on society.19 In a population-based study, the mean direct medical costs of HZ ranged from $620 to $1,160 (2015 dollars) depending on age,20 and the mean costs of postherpetic neuralgia were 2 to 5 times higher than that.20–22 Immunocompromised patients had costs 2 to 3 times higher than those of immunocompetent adults.23 In addition, for employed patients, HZ resulted in an average loss of 32 hours of work due to absenteeism and 84 hours due to presenteeism (ie, working while sick and therefore suboptimally).24
Assuming there are 1 million cases of HZ each year, if 8% to 32% of patients go on to develop postherpetic neuralgia, that would translate into approximately $1 to $2 billion in direct medical costs. With 60% of adult patients working,25 at an average wage of $23.23 per hour,26 HZ illness could be responsible for another $1.6 billion in lost productivity.
EFFICACY AND SAFETY OF HZ VACCINE
In 2006, the FDA approved the live-attenuated Oka strain VZV vaccine for prevention of HZ and postherpetic neuralgia in adults age 60 and older based on findings from the Shingles Prevention Study (SPS).27
The Shingles Prevention Study
This multicenter randomized placebo-controlled trial27 enrolled 38,546 immunocompetent persons age 60 and older. Subjects in the intervention group received a single dose of live-attenuated vaccine, and all participants were followed for up to 4.9 years after vaccination.
HZ occurred in 315 (1.636%) of the 19,254 participants in the vaccine group and in 642 (3.336%) of the 19,247 participants in the placebo group, an absolute risk reduction of 1.7%, number needed to treat 59, relative risk reduction 51%, P < .001. Similarly, postherpetic neuralgia occurred in 27 (0.140%) of the 19,254 vaccine recipients and in 80 (0.416%) of the placebo recipients (an absolute risk reduction of 0.276%, number needed to treat 362, relative risk reduction 66%, P < .001). The investigators calculated that vaccination reduced the overall burden of illness by 61% (Table 1).
The efficacy against HZ incidence decreased with age,28 but the efficacy against postherpetic neuralgia did not. In addition, vaccine recipients who developed HZ generally had less severe manifestations.
The safety of the vaccine was assessed for all participants in the SPS. In addition, one-sixth of SPS participants were enrolled in a safety substudy. These participants completed a detailed report card regarding all medically important events within the first 42 days. Forty-eight percent of the vaccine group and 17% of the placebo group (P < .05) experienced adverse events, primarily at the injection site. Less than 1% of all local reactions were severe.29 Serious adverse events were rare (< 2%), but occurred significantly more often in the vaccinated group.
Short-Term Persistence Substudy
Short-term efficacy of the live-attenuated vaccine (up to 7 years) was assessed in the Short-Term Persistence Substudy (STPS), which involved 14,270 of the initial participants and reported yearly and overall vaccine efficacy.30 After 5 years, the yearly efficacy against postherpetic neuralgia incidence declined to 32% and was no longer statistically significant. Efficacy against HZ incidence and burden of illness displayed the same pattern. After the end of the STPS, all subjects in the placebo group received vaccination.
Long-Term Persistence Substudy
Those in the intervention group were followed for an additional 4 years in the Long-Term Persistence Substudy (LTPS).31 Due to the lack of concurrent controls in the LTPS, the authors used regression models based on historical controls to estimate contemporary population incidence of HZ and postherpetic neuralgia for comparison.
Efficacy continued to decline over time, and by 10 years after vaccination there was no difference between vaccinated patients and historical controls in the rate of any end point (ie, efficacy declined to zero).
A trial of booster vaccination
Because many patients are vaccinated at age 60, waning immunity could leave them vulnerable to HZ and postherpetic neuralgia by age 70. A potential solution would be to give a booster dose after 10 years.
A recent phase 3 clinical trial of adults age 70 years and older found that a booster dose of live-attenuated vaccine was as safe and immunogenic as an initial dose.32 While antibody responses were similar in the boosted group and the newly vaccinated group, cell-mediated immunity was higher in the boosted group.
Because prevention of HZ is generally via cell-mediated immunity, the booster might be more effective than the initial vaccination, but clinical trials measuring actual cases prevented will be required to prove it. A booster dose is not currently recommended.
A trial of vaccination in adults 50 to 59
In 2011, the FDA extended its approval of HZ vaccine for use in adults ages 50 to 59.33
In a randomized, double-blind, placebo-controlled trial in this age group,33 the vaccine reduced HZ incidence by almost 70% (absolute risk reduction 0.614%, number needed to treat 156; Table 1), but the severity of HZ cases was not affected. There were too few cases of postherpetic neuralgia to assess the efficacy for this end point. The study followed patients for only 1.5 years after vaccination, so the duration of efficacy is unknown.
As in the older recipients, the vaccine was well tolerated; injection-site reactions and headache were the major adverse effects reported among vaccine recipients.33
INDICATIONS AND CONTRAINDICATIONS
Although HZ vaccine is licensed for use in adults age 50 and older, the ACIP recommends it only for immunocompetent adults age 60 and older. At this time, the ACIP does not recommend HZ vaccine in those younger than 60 because of the low risk of HZ in this age group.34
Any person age 60 or older should receive a single dose of the live-attenuated HZ vaccine subcutaneously, regardless of past history of HZ.
The vaccine is contraindicated in patients who have a history of allergic reaction to any vaccine component, immunosuppression or immunodeficiency conditions, and pregnancy. Specifically, people who will receive immunosuppressive therapies should have the vaccine at least 14 days before beginning treatment. Antiviral medications such as acyclovir, famciclovir, and valacyclovir should be discontinued at least 24 hours before vaccination and not resumed until 14 days later. Patients taking high-dose corticosteroids for more than 2 weeks should not be vaccinated until at least 1 month after therapy is completed.
In contrast, HZ vaccine is not contraindicated for leukemia patients who are in remission and who have not received chemotherapy or radiation for at least 3 months, or for patients receiving short-term, low-to-moderate dose, topical, intra-articular, bursal, or tendon injections of corticosteroids. Patients on low-dose methotrexate, azathioprine, or 6-mercaptopurine can also receive the vaccine.18
VACCINATION RATES ARE LOW
Although the vaccine has been recommended since 2008, uptake has been slow. Figure 1 shows the rate of HZ vaccination in adults age 60 and older surveyed in the National Health Interview Survey from 2007 to 2013.35 Eight years after the vaccine was licensed, only 28% of eligible patients had been vaccinated. Assuming the current rate of increase remains constant, it will take 7 more years to reach a 60% coverage rate—the same as for pneumococcal vaccine36—and 18 years to reach universal coverage.
Barriers to vaccination
Several barriers to HZ vaccination might account for the slow uptake.
For the first few years the vaccine was available, the requirement to store it frozen presented an obstacle for some physicians.37 Physicians may also have been discouraged by the cumbersome Medicare reimbursement process because while the administration fee is covered through Medicare Part B, the live-attenuated vaccine is reimbursed only through Medicare Part D, a benefit that varies by plans. Other barriers to physicians are supply shortages, high up-front costs, and uncertainties regarding the duration of vaccine protection, its safety, and side effects.38–40
Patient barriers include lack of physician recommendation, lack of familiarity with the vaccine, high out-of-pocket costs, the perception that they are at low risk for HZ, underestimation of the pain associated with HZ and postherpetic neuralgia, and fear of vaccine adverse effects.39,41,42
Interventions to increase vaccination rates
Certain interventions have been shown to increase vaccination adherence in general and HZ vaccination in particular. In randomized trials involving other vaccines, electronic medical record reminders supporting panel management or nurse-initiated protocols have been proven to increase vaccination rates, but these methods have not been tested for HZ vaccine specifically.43,44
In an observational study, Chaudhry et al found that the number of HZ vaccinations administered at the Mayo Clinic increased 43% in one practice and 54% in another after the implementation of an electronic alert.45 A randomized controlled trial showed that an informational package discussing HZ and the vaccine sent to patients via either their electronic personal health record or traditional mail increased HZ vaccination by almost 3 times.46
Pharmacists can also influence vaccination rates. States that provide full immunization privileges to pharmacists have vaccination rates significantly higher than states with restricted or no authorization.47
COST-EFFECTIVENESS CONSIDERATIONS
Unlike the Centers for Medicare and Medicaid Services, the ACIP does consider cost-effectiveness in their vaccine recommendations. Because of the morbidity associated with HZ and postherpetic neuralgia as well as the economic impact, vaccination is generally considered cost-effective for adults age 60 and older.48,49
Analyses have demonstrated that cost-effectiveness hinges on 4 factors: initial vaccine efficacy, the duration of efficacy, the age-specific incidence of HZ, and the cost of the vaccine.
For patients ages 50 to 59, the incidence of HZ is low, and because the duration of vaccine efficacy is short even though initial vaccine efficacy is high, vaccination in this age group offers poor value.50 At older ages, the incidence of HZ and postherpetic neuralgia rises, making vaccination more cost-effective. After age 60, the vaccine is cost-effective at all ages, although age 70 appears to offer the optimal trade-off between increasing incidence and declining vaccine efficacy.48,49
For patients who plan to be vaccinated only once, waiting until age 70 would appear to offer the best value.51 For those who are willing to receive a booster dose, the optimal age for vaccination is unknown, but will likely depend on the effectiveness, cost, and duration of the booster.
A NEW HZ VACCINE
In 2015, GlaxoSmithKline tested a new HZ vaccine containing a single VZV glycoprotein in an AS01B adjuvant system (HZ/su vaccine).52 In a phase 3 randomized trial involving 15,411 immunocompetent persons age 50 and older, a 2-dose schedule of HZ/su vaccine was 97% effective in preventing HZ (Table 1).53 Importantly, the vaccine was equally effective in older patients.
This vaccine also had a high rate of adverse reactions, with 17% of vaccine recipients vs 3% of placebo recipients reporting events that prevented normal everyday activities for at least 1 day. However, the rate of serious adverse reactions was the same in both groups (9%). The company announced that they intended to submit a regulatory application for HZ/su vaccine in the second half of 2016.54
Because of its high efficacy, HZ/su vaccine has the potential to change practice, but several issues must be resolved before it can supplant the current vaccine.
First, the AS01B adjuvant is not currently licensed in the United States, so it is unclear if the HZ/su vaccine can get FDA approval.52,55
Second, there are several questions about the efficacy of the vaccine, including long-term efficacy, efficacy in the elderly, and efficacy in the case of a patient receiving only 1 of the 2 required doses.
Third, the impact of HZ/su vaccine on complications such as postherpetic neuralgia has not been established. The clinical trial (NCT01165229) examining vaccine efficacy against postherpetic neuralgia incidence and other complications in adults age 70 and older has recently been completed and data should be available soon. Given the extremely high efficacy against HZ, it is likely that it will be close to 100% effective against this complication.
Fourth, there is uncertainty as to how the HZ/su vaccine should be used in patients who have already received the live-attenuated vaccine, if it is determined that a booster is necessary.
Finally, the vaccine is not yet priced. Given its superior effectiveness, particularly in older individuals, competitive pricing could dramatically affect the market. How Medicare or other insurers cover the new vaccine will likely influence its acceptance.
HZ VACCINATION OF IMMUNOCOMPROMISED PATIENTS
Immunocompromised patients are at highest risk for developing HZ. Unfortunately, there are currently no HZ vaccines approved for use in this population. The current live-attenuated vaccine has been demonstrated to be safe, well tolerated, and immunogenic in patients age 60 and older who are receiving chronic or maintenance low to moderate doses of corticosteroids.56
A clinical trial is being conducted to assess the immunogenicity, clinical effectiveness, and safety of the vaccine in rheumatoid arthritis patients receiving antitumor necrosis factor therapy (NCT01967316). Other trials are examining vaccine efficacy and safety in patients with solid organ tumors prior to chemotherapy (NCT02444936) and in patients who will be undergoing living donor kidney transplantation (NCT00940940). Researchers are also investigating the possibility of vaccinating allogeneic stem cell donors before donation in order to protect transplant recipients against HZ (NCT01573182).
ZVHT and HZ/su vaccination in immunocompromised patients
Heat-treated varicella-zoster vaccine (ZVHT) is a potential alternative for immunocompromised patients. A 4-dose regimen has been proven to reduce the risk of HZ in patients receiving autologous hematopoietic-cell transplants for non-Hodgkin or Hodgkin lymphoma.57
In another trial, the 4-dose ZVHT was safe and elicited significant VZV-specific T-cell response through 28 days in immunosuppressed patients with solid tumor malignancy, hematologic malignancy, human immunodeficiency virus infection with CD4 counts of 200 cells/mm3 or less, and autologous hematopoietic-cell transplants. The T-cell response was poor in allogeneic hematopoietic-cell transplant recipients, however.58
Because the HZ/su vaccine does not contain live virus, it seems particularly promising for immunocompromised patients. In phase 1 and 2 studies, a 3-dose regimen has been shown to be safe and immunogenic in hematopoietic-cell transplant recipients and HIV-infected adults with CD4 count higher than 200 cells/mm3.59,60 A phase 3 trial assessing the efficacy of HZ/su vaccine in autologous hematopoietic-cell transplant recipients is under way (NCT01610414). Changes in recommendations for HZ vaccine in these most vulnerable populations await the results of these studies.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl 1):S1–S26.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(suppl):21–26.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833.
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420.
- Yawn BP, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–1349.
- Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42:325–334.
- Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211–219.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–745.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–340.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–1005.
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002–2007.
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989; 159:1000–1001.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356–363.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–262.
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–794.
- Dworkin RH, White R, O’Connor AB, Hawkins K. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008; 9:348–353.
- White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–792.
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155–169.
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639–645.
- US Bureau of Labor Statistics. Labor force statistics from the current population survey. www.bls.gov/web/empsit/cpseea13.htm. Accessed April 6, 2017.
- US Bureau of Labor Statistic. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm. Accessed April 6, 2017.
- Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- Food and Drug Administration (FDA). FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax. www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198b2_1.pdf. Accessed April 6, 2017.
- Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545–554.
- Schmader KE, Oxman MN, Levin MJ, et al; Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–1328.
- Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–909.
- Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22.
- Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–928.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC). Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–731.
- Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(1):1–36. Accessed April 12, 2017.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010; 51:197–213.
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555–560.
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–887.
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008; 197(suppl 2):S216–S223.
- Joon Lee T, Hayes S, Cummings DM, et al. Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med 2013; 26:45–51.
- Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine 2009; 27:192–196.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011; 171:1552–1558.
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med 1999; 14:351–356.
- Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19:263–266.
- Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med 2013; 126:832.e1–832.e6.
- Taitel MS, Fensterheim LE, Cannon AE, Cohen ES. Improving pneumococcal and herpes zoster vaccination uptake: expanding pharmacist privileges. Am J Manag Care 2013; 19:e309–e313.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–136.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–497.
- Le P, Rothberg MB. Determining the optimal age to vaccinate against herpes zoster: a cost-effectiveness analysis. Society for Medical Decision Making 37th Annual North American Meeting. St. Louis, MO; October 18-21, 2015.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–263.
- Lal H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096.
- GlaxoSmithKline plc. GSK’s candidate shingles vaccine demonstrates 90% efficacy against shingles in people 70 years of age and over. www.gsk.com/en-gb/media/press-releases/gsk-s-candidate-shingles-vaccine-demonstrates-90-efficacy-against-shingles-in-people-70-years-of-age-and-over/. Accessed April 6, 2017.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–1608.
- Russell AF, Parrino J, Fisher CL Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015; 33:3129–3134.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013; 208:1375–1385.
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–1287.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(suppl 1):S1–S26.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9(suppl):21–26.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2014; 2:CD006866.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833.
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166.
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420.
- Yawn BP, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–1349.
- Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42:325–334.
- Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002; 44:211–219.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159:739–745.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332–340.
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis 2010; 50:1000–1005.
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002–2007.
- Plotkin SA, Starr SE, Connor K, Morton D. Zoster in normal children after varicella vaccine. J Infect Dis 1989; 159:1000–1001.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356–363.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37.
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of herpes zoster (HZ) infection. Hum Vaccin Immunother 2015; 11:245–262.
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–794.
- Dworkin RH, White R, O’Connor AB, Hawkins K. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008; 9:348–353.
- White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–792.
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155–169.
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639–645.
- US Bureau of Labor Statistics. Labor force statistics from the current population survey. www.bls.gov/web/empsit/cpseea13.htm. Accessed April 6, 2017.
- US Bureau of Labor Statistic. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm. Accessed April 6, 2017.
- Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–2284.
- Food and Drug Administration (FDA). FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax. www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198b2_1.pdf. Accessed April 6, 2017.
- Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545–554.
- Schmader KE, Oxman MN, Levin MJ, et al; Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320–1328.
- Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–909.
- Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22.
- Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–928.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC). Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–731.
- Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65(1):1–36. Accessed April 12, 2017.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:95–102.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis 2010; 51:197–213.
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555–560.
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–887.
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008; 197(suppl 2):S216–S223.
- Joon Lee T, Hayes S, Cummings DM, et al. Herpes zoster knowledge, prevalence, and vaccination rate by race. J Am Board Fam Med 2013; 26:45–51.
- Opstelten W, van Essen GA, Hak E. Determinants of non-compliance with herpes zoster vaccination in the community-dwelling elderly. Vaccine 2009; 27:192–196.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011; 171:1552–1558.
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gen Intern Med 1999; 14:351–356.
- Chaudhry R, Schietel SM, North F, Dejesus R, Kesman RL, Stroebel RJ. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19:263–266.
- Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med 2013; 126:832.e1–832.e6.
- Taitel MS, Fensterheim LE, Cannon AE, Cohen ES. Improving pneumococcal and herpes zoster vaccination uptake: expanding pharmacist privileges. Am J Manag Care 2013; 19:e309–e313.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–1653.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–136.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–497.
- Le P, Rothberg MB. Determining the optimal age to vaccinate against herpes zoster: a cost-effectiveness analysis. Society for Medical Decision Making 37th Annual North American Meeting. St. Louis, MO; October 18-21, 2015.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–263.
- Lal H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096.
- GlaxoSmithKline plc. GSK’s candidate shingles vaccine demonstrates 90% efficacy against shingles in people 70 years of age and over. www.gsk.com/en-gb/media/press-releases/gsk-s-candidate-shingles-vaccine-demonstrates-90-efficacy-against-shingles-in-people-70-years-of-age-and-over/. Accessed April 6, 2017.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–1608.
- Russell AF, Parrino J, Fisher CL Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015; 33:3129–3134.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013; 208:1375–1385.
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–1287.
KEY POINTS
- HZ continues to be an important public health problem, with substantial morbidity and economic impact. Because of the lack of effective treatment, vaccination provides the best strategy for disease mitigation.
- Physicians can reduce the impact of HZ by educating patients about its complications and recommending immunization for all patients age 60 and older. Patients can protect themselves by seeking vaccination.
- Vaccine protection wanes completely after 10 years, and physicians should be prepared to offer a booster dose should the Advisory Committee on Immunization Practices issue such recommendations.
- Newer vaccines offer promise for greater efficacy, especially for the elderly. For immunocompromised patients, a safe and effective vaccine may be available in the near future.
Thoughtful vaccination
Few in mainstream medicine doubt the efficacy of vaccination and the net positive value of a thoughtful vaccination policy. We have witnessed within a professional lifetime the virtual eradication, at least regionally, of a number of previously devastating infectious diseases including polio, smallpox, pertussis, and measles. With growing understanding of disease mechanisms, the potential value of vaccination has expanded to include preventing sequelae of certain infections and malignancy—a holy grail in oncology, the true prevention of cancer. Sporadic local resistance to uniform vaccination against measles has resulted in geographic reappearance of the disease, providing further support for a uniform approach to vaccination against communicable diseases, with some potential to opt out, perhaps with associated societal repercussions for those who do so.
For most clinicians, certainly those of us dealing with chronically ill or immunosuppressed patients, the decision to recommend annual influenza vaccination and pneumococcal vaccinations per guidelines is an easy one. Vaccination against certain infections provides some protection for the individual patient and for the population, contributing to “herd immunity” and helping to protect against the occurrence of pandemics. But this is not the case for all vaccines. For some vaccines the issues of immunity and vaccination are more individual and complicated and warrant more education, reflection, and conversation.
The vaccine to protect against human papillomavirus is effective at reducing the incidence of cervical and anal cancers triggered by infection with certain strains of human papillomavirus. The viral infection itself is not immediately life-threatening. Thus, patients (and their parents) are asked to consider vaccination against a sexually transmitted virus (before sexual transmission of infection) to prevent a possible malignancy later in life. This decision can create social angst. Perhaps less socially challenging, but medically more complicated, is vaccination against the hepatitis B virus (HBV). HBV is also sexually transmissible, but the source in many patients infected with this virus is unclear. The HBV vaccine helps protect against clinically meaningful hepatitis and chronic liver disease from HBV and also will reduce the occurrence of HBV-associated hepatocellular carcinoma and progression of cirrhosis in patients coinfected with hepatitis C virus.
More complex yet is vaccination against the varicella-zoster virus (VZV) to reduce the likelihood of shingles and its possible consequence, postherpetic neuralgia. Le, Sabella, and Rothberg, in this issue of the Journal, review clinical and public health challenges affecting the use of this vaccine.
We are almost all exposed to this virus as children, through natural infection (chickenpox) or vaccination; many infections are seemingly subclinical. My older son had the distinct misfortune of getting chickenpox in a quite memorable way, developing a concentrated collection of the pruritic vesicles underneath a newly placed arm cast. Whether we remember the initial infection or not, VZV sets up housekeeping and lies dormant for decades within sensory neurons. Decades later, it may erupt as shingles along the distribution of the infected nerves with characteristic painful vesicles, sometimes with persistent, extremely painful, and debilitating residua within the same dermatomal distribution: postherpetic neuralgia. The triggers for this fairly common scenario are only generically understood and include waning cellular immunity attributed to aging, malignancy, immunosuppressive medications, human immunodeficiency virus, and perhaps stress and depression.
The zoster vaccine is unique in that it is given to bolster already present cellular immunity to prevent clinical recurrence of the earlier infection, decades after the virus has been dormant—not, as for the other vaccines noted above, to prevent primary infection. It doesn’t matter if the patient has already experienced an episode of shingles. The currently available vaccine is a live-attenuated strain (Oka) of VZV. Another vaccine in clinical testing uses isolated viral components with adjuvant and thus eliminates current concerns of giving the live-attenuated vaccine to immunosuppressed and elderly patients, those who may benefit the most from it.
Fortunately, it seems that even significantly immunosuppressed and elderly patients tolerate the current vaccine, but at present it is suggested that these groups not receive the vaccine, and vaccination rates in these patients is low. Hopefully, additional data will accumulate regarding the safety of the vaccine and will permit its more widespread use within these patient groups, or a replacement “dead” vaccine will become available.
As Le et al nicely discuss, the current vaccine efficacy wanes over about 10 years, and then a booster vaccine should be considered, but there are few data to provide safety and efficacy outcome measures from patients who received a booster vaccination. The potential need to receive a booster injection after about a decade, the demonstrated greater efficacy against zoster when the initial vaccination is provided to patients ages 60 through 69 than in those over 70, and the lower absolute impact when given to patients ages 50 through 59 (baseline zoster incidence increases with age) should enter into the conversation with patients as to when they should receive the vaccine.
Additionally, there are the extremely provocative and as yet unanswered questions surrounding the implications of vaccination if VZV infection is a trigger of inflammatory vascular diseases such as giant cell arteritis in the elderly, as was proposed by the late Dr. Don Gilden.1
And yes, I did get the vaccine. I was 64 at the time.
- Gilden D, Nagel MA. Varicella zoster virus triggers the immunopathology of giant cell arteritis. Curr Opin Rheumatol 2016; 28:376–382.
Few in mainstream medicine doubt the efficacy of vaccination and the net positive value of a thoughtful vaccination policy. We have witnessed within a professional lifetime the virtual eradication, at least regionally, of a number of previously devastating infectious diseases including polio, smallpox, pertussis, and measles. With growing understanding of disease mechanisms, the potential value of vaccination has expanded to include preventing sequelae of certain infections and malignancy—a holy grail in oncology, the true prevention of cancer. Sporadic local resistance to uniform vaccination against measles has resulted in geographic reappearance of the disease, providing further support for a uniform approach to vaccination against communicable diseases, with some potential to opt out, perhaps with associated societal repercussions for those who do so.
For most clinicians, certainly those of us dealing with chronically ill or immunosuppressed patients, the decision to recommend annual influenza vaccination and pneumococcal vaccinations per guidelines is an easy one. Vaccination against certain infections provides some protection for the individual patient and for the population, contributing to “herd immunity” and helping to protect against the occurrence of pandemics. But this is not the case for all vaccines. For some vaccines the issues of immunity and vaccination are more individual and complicated and warrant more education, reflection, and conversation.
The vaccine to protect against human papillomavirus is effective at reducing the incidence of cervical and anal cancers triggered by infection with certain strains of human papillomavirus. The viral infection itself is not immediately life-threatening. Thus, patients (and their parents) are asked to consider vaccination against a sexually transmitted virus (before sexual transmission of infection) to prevent a possible malignancy later in life. This decision can create social angst. Perhaps less socially challenging, but medically more complicated, is vaccination against the hepatitis B virus (HBV). HBV is also sexually transmissible, but the source in many patients infected with this virus is unclear. The HBV vaccine helps protect against clinically meaningful hepatitis and chronic liver disease from HBV and also will reduce the occurrence of HBV-associated hepatocellular carcinoma and progression of cirrhosis in patients coinfected with hepatitis C virus.
More complex yet is vaccination against the varicella-zoster virus (VZV) to reduce the likelihood of shingles and its possible consequence, postherpetic neuralgia. Le, Sabella, and Rothberg, in this issue of the Journal, review clinical and public health challenges affecting the use of this vaccine.
We are almost all exposed to this virus as children, through natural infection (chickenpox) or vaccination; many infections are seemingly subclinical. My older son had the distinct misfortune of getting chickenpox in a quite memorable way, developing a concentrated collection of the pruritic vesicles underneath a newly placed arm cast. Whether we remember the initial infection or not, VZV sets up housekeeping and lies dormant for decades within sensory neurons. Decades later, it may erupt as shingles along the distribution of the infected nerves with characteristic painful vesicles, sometimes with persistent, extremely painful, and debilitating residua within the same dermatomal distribution: postherpetic neuralgia. The triggers for this fairly common scenario are only generically understood and include waning cellular immunity attributed to aging, malignancy, immunosuppressive medications, human immunodeficiency virus, and perhaps stress and depression.
The zoster vaccine is unique in that it is given to bolster already present cellular immunity to prevent clinical recurrence of the earlier infection, decades after the virus has been dormant—not, as for the other vaccines noted above, to prevent primary infection. It doesn’t matter if the patient has already experienced an episode of shingles. The currently available vaccine is a live-attenuated strain (Oka) of VZV. Another vaccine in clinical testing uses isolated viral components with adjuvant and thus eliminates current concerns of giving the live-attenuated vaccine to immunosuppressed and elderly patients, those who may benefit the most from it.
Fortunately, it seems that even significantly immunosuppressed and elderly patients tolerate the current vaccine, but at present it is suggested that these groups not receive the vaccine, and vaccination rates in these patients is low. Hopefully, additional data will accumulate regarding the safety of the vaccine and will permit its more widespread use within these patient groups, or a replacement “dead” vaccine will become available.
As Le et al nicely discuss, the current vaccine efficacy wanes over about 10 years, and then a booster vaccine should be considered, but there are few data to provide safety and efficacy outcome measures from patients who received a booster vaccination. The potential need to receive a booster injection after about a decade, the demonstrated greater efficacy against zoster when the initial vaccination is provided to patients ages 60 through 69 than in those over 70, and the lower absolute impact when given to patients ages 50 through 59 (baseline zoster incidence increases with age) should enter into the conversation with patients as to when they should receive the vaccine.
Additionally, there are the extremely provocative and as yet unanswered questions surrounding the implications of vaccination if VZV infection is a trigger of inflammatory vascular diseases such as giant cell arteritis in the elderly, as was proposed by the late Dr. Don Gilden.1
And yes, I did get the vaccine. I was 64 at the time.
Few in mainstream medicine doubt the efficacy of vaccination and the net positive value of a thoughtful vaccination policy. We have witnessed within a professional lifetime the virtual eradication, at least regionally, of a number of previously devastating infectious diseases including polio, smallpox, pertussis, and measles. With growing understanding of disease mechanisms, the potential value of vaccination has expanded to include preventing sequelae of certain infections and malignancy—a holy grail in oncology, the true prevention of cancer. Sporadic local resistance to uniform vaccination against measles has resulted in geographic reappearance of the disease, providing further support for a uniform approach to vaccination against communicable diseases, with some potential to opt out, perhaps with associated societal repercussions for those who do so.
For most clinicians, certainly those of us dealing with chronically ill or immunosuppressed patients, the decision to recommend annual influenza vaccination and pneumococcal vaccinations per guidelines is an easy one. Vaccination against certain infections provides some protection for the individual patient and for the population, contributing to “herd immunity” and helping to protect against the occurrence of pandemics. But this is not the case for all vaccines. For some vaccines the issues of immunity and vaccination are more individual and complicated and warrant more education, reflection, and conversation.
The vaccine to protect against human papillomavirus is effective at reducing the incidence of cervical and anal cancers triggered by infection with certain strains of human papillomavirus. The viral infection itself is not immediately life-threatening. Thus, patients (and their parents) are asked to consider vaccination against a sexually transmitted virus (before sexual transmission of infection) to prevent a possible malignancy later in life. This decision can create social angst. Perhaps less socially challenging, but medically more complicated, is vaccination against the hepatitis B virus (HBV). HBV is also sexually transmissible, but the source in many patients infected with this virus is unclear. The HBV vaccine helps protect against clinically meaningful hepatitis and chronic liver disease from HBV and also will reduce the occurrence of HBV-associated hepatocellular carcinoma and progression of cirrhosis in patients coinfected with hepatitis C virus.
More complex yet is vaccination against the varicella-zoster virus (VZV) to reduce the likelihood of shingles and its possible consequence, postherpetic neuralgia. Le, Sabella, and Rothberg, in this issue of the Journal, review clinical and public health challenges affecting the use of this vaccine.
We are almost all exposed to this virus as children, through natural infection (chickenpox) or vaccination; many infections are seemingly subclinical. My older son had the distinct misfortune of getting chickenpox in a quite memorable way, developing a concentrated collection of the pruritic vesicles underneath a newly placed arm cast. Whether we remember the initial infection or not, VZV sets up housekeeping and lies dormant for decades within sensory neurons. Decades later, it may erupt as shingles along the distribution of the infected nerves with characteristic painful vesicles, sometimes with persistent, extremely painful, and debilitating residua within the same dermatomal distribution: postherpetic neuralgia. The triggers for this fairly common scenario are only generically understood and include waning cellular immunity attributed to aging, malignancy, immunosuppressive medications, human immunodeficiency virus, and perhaps stress and depression.
The zoster vaccine is unique in that it is given to bolster already present cellular immunity to prevent clinical recurrence of the earlier infection, decades after the virus has been dormant—not, as for the other vaccines noted above, to prevent primary infection. It doesn’t matter if the patient has already experienced an episode of shingles. The currently available vaccine is a live-attenuated strain (Oka) of VZV. Another vaccine in clinical testing uses isolated viral components with adjuvant and thus eliminates current concerns of giving the live-attenuated vaccine to immunosuppressed and elderly patients, those who may benefit the most from it.
Fortunately, it seems that even significantly immunosuppressed and elderly patients tolerate the current vaccine, but at present it is suggested that these groups not receive the vaccine, and vaccination rates in these patients is low. Hopefully, additional data will accumulate regarding the safety of the vaccine and will permit its more widespread use within these patient groups, or a replacement “dead” vaccine will become available.
As Le et al nicely discuss, the current vaccine efficacy wanes over about 10 years, and then a booster vaccine should be considered, but there are few data to provide safety and efficacy outcome measures from patients who received a booster vaccination. The potential need to receive a booster injection after about a decade, the demonstrated greater efficacy against zoster when the initial vaccination is provided to patients ages 60 through 69 than in those over 70, and the lower absolute impact when given to patients ages 50 through 59 (baseline zoster incidence increases with age) should enter into the conversation with patients as to when they should receive the vaccine.
Additionally, there are the extremely provocative and as yet unanswered questions surrounding the implications of vaccination if VZV infection is a trigger of inflammatory vascular diseases such as giant cell arteritis in the elderly, as was proposed by the late Dr. Don Gilden.1
And yes, I did get the vaccine. I was 64 at the time.
- Gilden D, Nagel MA. Varicella zoster virus triggers the immunopathology of giant cell arteritis. Curr Opin Rheumatol 2016; 28:376–382.
- Gilden D, Nagel MA. Varicella zoster virus triggers the immunopathology of giant cell arteritis. Curr Opin Rheumatol 2016; 28:376–382.
U.S. yellow fever vaccine stocks could be depleted within months
Supply of the only U.S.-licensed yellow fever vaccine will be depleted by mid-2017 because of manufacturing issues, according to the Centers for Disease Control and Prevention.
Sanofi Pasteur, the manufacturer of the YF-VAX vaccine, notified the CDC and the Food and Drug Administration in 2016 there could be a shortage this year after the manufacturing complications during a factory switch over led to the loss of a large amount of vaccine supply, according to an article published online in the Morbidity and Mortality Weekly Report.
The CDC, Sanofi Pasteur, and the FDA are working to supplement the shortage. The manufacturer submitted an expanded investigational new drug (eIND) application to the FDA in September 2016 for marketing permission for Stamaril, an alternative vaccine manufactured by Sanofi Pasteur France and used in around 70 countries.
The application included planning for strategic distribution sites, which the CDC is determining using a tiered system based on volume of doses ordered in 2016.
As of April 2017, 250 civilian sites have been invited to participate in the eIND program, significantly less than the 4,000 currently distributing YF-VAX.
The CDC will “monitor for critical gaps in vaccine access and collaborate to address any issues, including considering the possibility of recruiting additional clinics to participate as necessary,” according to a statement.
Updates on the shortage will be available on the CDC travel health website.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Supply of the only U.S.-licensed yellow fever vaccine will be depleted by mid-2017 because of manufacturing issues, according to the Centers for Disease Control and Prevention.
Sanofi Pasteur, the manufacturer of the YF-VAX vaccine, notified the CDC and the Food and Drug Administration in 2016 there could be a shortage this year after the manufacturing complications during a factory switch over led to the loss of a large amount of vaccine supply, according to an article published online in the Morbidity and Mortality Weekly Report.
The CDC, Sanofi Pasteur, and the FDA are working to supplement the shortage. The manufacturer submitted an expanded investigational new drug (eIND) application to the FDA in September 2016 for marketing permission for Stamaril, an alternative vaccine manufactured by Sanofi Pasteur France and used in around 70 countries.
The application included planning for strategic distribution sites, which the CDC is determining using a tiered system based on volume of doses ordered in 2016.
As of April 2017, 250 civilian sites have been invited to participate in the eIND program, significantly less than the 4,000 currently distributing YF-VAX.
The CDC will “monitor for critical gaps in vaccine access and collaborate to address any issues, including considering the possibility of recruiting additional clinics to participate as necessary,” according to a statement.
Updates on the shortage will be available on the CDC travel health website.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Supply of the only U.S.-licensed yellow fever vaccine will be depleted by mid-2017 because of manufacturing issues, according to the Centers for Disease Control and Prevention.
Sanofi Pasteur, the manufacturer of the YF-VAX vaccine, notified the CDC and the Food and Drug Administration in 2016 there could be a shortage this year after the manufacturing complications during a factory switch over led to the loss of a large amount of vaccine supply, according to an article published online in the Morbidity and Mortality Weekly Report.
The CDC, Sanofi Pasteur, and the FDA are working to supplement the shortage. The manufacturer submitted an expanded investigational new drug (eIND) application to the FDA in September 2016 for marketing permission for Stamaril, an alternative vaccine manufactured by Sanofi Pasteur France and used in around 70 countries.
The application included planning for strategic distribution sites, which the CDC is determining using a tiered system based on volume of doses ordered in 2016.
As of April 2017, 250 civilian sites have been invited to participate in the eIND program, significantly less than the 4,000 currently distributing YF-VAX.
The CDC will “monitor for critical gaps in vaccine access and collaborate to address any issues, including considering the possibility of recruiting additional clinics to participate as necessary,” according to a statement.
Updates on the shortage will be available on the CDC travel health website.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM MMWR
Rotavirus vaccination in last decade cuts AGE hospitalization
There have been important reductions in hospitalization for acute gastroenteritis (AGE) and mortality since licensure of rotavirus vaccines 10 years ago, even in low-income countries with high child mortality, according to a new analysis of data from 27 countries.
In a search of articles published between Jan. 1, 2006, and Dec. 6, 2016, in the PubMed database, Eleanor Burnett of the division of viral diseases at the National Center for Immunization and Respiratory Diseases, Atlanta, and her associates identified 57 articles reporting on 59 data sources from 27 countries.
Among children younger than 5 years, the median percent reduction in AGE hospitalizations and/or ED visits was 38% overall, and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
The median reduction in AGE mortality among children younger than 1 year was 31% overall. In countries with medium and high child mortality, it was 45% and 30%, respectively.
The median reduction in AGE mortality in children younger than 5 years was 42% overall. In countries with medium and high child mortality, it was 50% and 36%, respectively. No estimates have been published from countries with low child mortality (J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix186).
“In several, but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection,” Ms. Burnett and her associates said. This population was not directly assessed in the studies.
The authors had no funding or conflicts of interest to disclose.
Decisions to introduce a new vaccine into a number of national immunization programs are influenced by many factors, including local disease burden, vaccine efficacy and safety, and the cost-effectiveness of the vaccines.
Introduction of rotavirus vaccination never started in earnest in Asia, a region with large birth cohort countries carrying substantial disease, but that lack has now turned an important corner, with India, Pakistan, and Bangladesh implementing or planning to implement rotavirus vaccination.
Also, two large African countries with high rotavirus mortality, Nigeria and Democratic Republic of Congo, are approved for Gavi (the Vaccine Alliance) funding to introduce the vaccines in 2018. The dramatic impact of rotavirus vaccines on rotavirus-associated hospitalizations and deaths described by Burnett et al. support the decisions by these large countries with high rotavirus mortality to introduce rotavirus vaccines and will lead to greater global health impacts.
Countries considering rotavirus vaccine introduction now will increasingly have additional data on vaccine safety and effectiveness. Burnett and her colleagues’ review will likely provide important information to these national immunization technical advisory groups and other decision-making bodies. This review will also be important for the earlier-adopter countries by way of validating their earlier introduction decisions.
Finally, the information will be of value to Gavi, UNICEF, and other international bodies tasked with providing resources and support for rotavirus vaccine introduction in low-income and lower-middle income countries.
Anthony Nelson, MD , is in the department of paediatrics at the Chinese University of Hong Kong, and Duncan Steele, PhD, is at the Bill & Melinda Gates Foundation, Seattle. These comments were excerpted from an editorial accompanying the article by Burnett et al. ( J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix187 ). The authors had no conflicts of interest or funding to disclose.
Decisions to introduce a new vaccine into a number of national immunization programs are influenced by many factors, including local disease burden, vaccine efficacy and safety, and the cost-effectiveness of the vaccines.
Introduction of rotavirus vaccination never started in earnest in Asia, a region with large birth cohort countries carrying substantial disease, but that lack has now turned an important corner, with India, Pakistan, and Bangladesh implementing or planning to implement rotavirus vaccination.
Also, two large African countries with high rotavirus mortality, Nigeria and Democratic Republic of Congo, are approved for Gavi (the Vaccine Alliance) funding to introduce the vaccines in 2018. The dramatic impact of rotavirus vaccines on rotavirus-associated hospitalizations and deaths described by Burnett et al. support the decisions by these large countries with high rotavirus mortality to introduce rotavirus vaccines and will lead to greater global health impacts.
Countries considering rotavirus vaccine introduction now will increasingly have additional data on vaccine safety and effectiveness. Burnett and her colleagues’ review will likely provide important information to these national immunization technical advisory groups and other decision-making bodies. This review will also be important for the earlier-adopter countries by way of validating their earlier introduction decisions.
Finally, the information will be of value to Gavi, UNICEF, and other international bodies tasked with providing resources and support for rotavirus vaccine introduction in low-income and lower-middle income countries.
Anthony Nelson, MD , is in the department of paediatrics at the Chinese University of Hong Kong, and Duncan Steele, PhD, is at the Bill & Melinda Gates Foundation, Seattle. These comments were excerpted from an editorial accompanying the article by Burnett et al. ( J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix187 ). The authors had no conflicts of interest or funding to disclose.
Decisions to introduce a new vaccine into a number of national immunization programs are influenced by many factors, including local disease burden, vaccine efficacy and safety, and the cost-effectiveness of the vaccines.
Introduction of rotavirus vaccination never started in earnest in Asia, a region with large birth cohort countries carrying substantial disease, but that lack has now turned an important corner, with India, Pakistan, and Bangladesh implementing or planning to implement rotavirus vaccination.
Also, two large African countries with high rotavirus mortality, Nigeria and Democratic Republic of Congo, are approved for Gavi (the Vaccine Alliance) funding to introduce the vaccines in 2018. The dramatic impact of rotavirus vaccines on rotavirus-associated hospitalizations and deaths described by Burnett et al. support the decisions by these large countries with high rotavirus mortality to introduce rotavirus vaccines and will lead to greater global health impacts.
Countries considering rotavirus vaccine introduction now will increasingly have additional data on vaccine safety and effectiveness. Burnett and her colleagues’ review will likely provide important information to these national immunization technical advisory groups and other decision-making bodies. This review will also be important for the earlier-adopter countries by way of validating their earlier introduction decisions.
Finally, the information will be of value to Gavi, UNICEF, and other international bodies tasked with providing resources and support for rotavirus vaccine introduction in low-income and lower-middle income countries.
Anthony Nelson, MD , is in the department of paediatrics at the Chinese University of Hong Kong, and Duncan Steele, PhD, is at the Bill & Melinda Gates Foundation, Seattle. These comments were excerpted from an editorial accompanying the article by Burnett et al. ( J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix187 ). The authors had no conflicts of interest or funding to disclose.
There have been important reductions in hospitalization for acute gastroenteritis (AGE) and mortality since licensure of rotavirus vaccines 10 years ago, even in low-income countries with high child mortality, according to a new analysis of data from 27 countries.
In a search of articles published between Jan. 1, 2006, and Dec. 6, 2016, in the PubMed database, Eleanor Burnett of the division of viral diseases at the National Center for Immunization and Respiratory Diseases, Atlanta, and her associates identified 57 articles reporting on 59 data sources from 27 countries.
Among children younger than 5 years, the median percent reduction in AGE hospitalizations and/or ED visits was 38% overall, and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
The median reduction in AGE mortality among children younger than 1 year was 31% overall. In countries with medium and high child mortality, it was 45% and 30%, respectively.
The median reduction in AGE mortality in children younger than 5 years was 42% overall. In countries with medium and high child mortality, it was 50% and 36%, respectively. No estimates have been published from countries with low child mortality (J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix186).
“In several, but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection,” Ms. Burnett and her associates said. This population was not directly assessed in the studies.
The authors had no funding or conflicts of interest to disclose.
There have been important reductions in hospitalization for acute gastroenteritis (AGE) and mortality since licensure of rotavirus vaccines 10 years ago, even in low-income countries with high child mortality, according to a new analysis of data from 27 countries.
In a search of articles published between Jan. 1, 2006, and Dec. 6, 2016, in the PubMed database, Eleanor Burnett of the division of viral diseases at the National Center for Immunization and Respiratory Diseases, Atlanta, and her associates identified 57 articles reporting on 59 data sources from 27 countries.
Among children younger than 5 years, the median percent reduction in AGE hospitalizations and/or ED visits was 38% overall, and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
The median reduction in AGE mortality among children younger than 1 year was 31% overall. In countries with medium and high child mortality, it was 45% and 30%, respectively.
The median reduction in AGE mortality in children younger than 5 years was 42% overall. In countries with medium and high child mortality, it was 50% and 36%, respectively. No estimates have been published from countries with low child mortality (J Infect Dis. 2017 Apr 18. doi: 10.1093/infdis/jix186).
“In several, but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection,” Ms. Burnett and her associates said. This population was not directly assessed in the studies.
The authors had no funding or conflicts of interest to disclose.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point:
Major finding: In children younger than 5 years, the median percent reduction in AGE hospitalizations and/or emergency department visits was 38% overall and 41%, 30%, and 46% in countries with low, medium, and high child mortality, respectively.
Data source: Meta-analysis of 57 articles reporting on 59 data sources from 27 countries between Jan. 1, 2006, and Dec. 6, 2016.
Disclosures: The authors had no funding or conflicts of interest to disclose.
International travel vaccination updates
There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.
There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.
There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.
WHO’s malaria pilot vaccine: No silver bullet, but a potential strike at malaria’s heart
EXPERT ANALYSIS FROM ECCMID 2017
VIENNA – The first malaria vaccine to enter a national pilot project is not a silver bullet against the disease that kills half a million every year, but it still might be powerful enough to significantly reduce global disease burden, and even impact transmission, according to infectious disease specialist Nick Beeching, MD.
The vaccine, RTS,S (Mosquirix; GlaxoSmithKline), will be tested in three African countries beginning next year, the World Health Organization announced on April 25. The pilot programs will target 720,000 children aged 5-17 months in high-risk areas of the three countries.
Even though it’s the first malaria vaccine to pass its pivotal phase III trial, RTS,S isn’t terribly effective by any standards, said Dr. Beeching of the Royal Liverpool (England) University.
April 25, 2017, is World Malaria Day, and Anthony S. Fauci, MD, and B. Fenton Hall, MD, PhD, of the U.S. National Institute of Allergy and Infectious Diseases, said in a statement, “Safe and effective vaccines are critical tools for future efforts to control, eliminate, and, ultimately, eradicate malaria. NIAID is supporting the development of numerous malaria vaccine candidates, 10 of which are in clinical trials. In 2015, an estimated 212 million new malaria cases and 429,000 deaths occurred. Nearly 90% of these cases were among children under the age of 5 years in Africa, where malaria claims the life of a child every 2 minutes.”
GSK has been working on this vaccine since 1985, according to the company’s RTS,S literature. It is a recombinant protein that targets the circumsporozoite protein of the Plasmodium falciparum parasite at an early stage, before it enters the liver and begins to embed in erythrocytes. The aim, Dr. Beeching said, was to develop an antigen that would mobilize the immune system from the moment a mosquito injected the sporozoites through a bite, “well before they have a chance to hide in the liver.”
The 2- and 3-year follow-up results of the phase III trial, conducted in 15,500 children, were published in the Lancet in 2015. RTS,S was administered as a three-dose series, plus a booster dose, beginning at 5 months of age. The primary immunizations were given with a minimum 4-week interval between doses, with the booster administered 18 months after the last dose.
The primary series reduced clinical cases by 26%. With the booster dose, cases were reduced by 39% overall. The vaccine averted 1,774 episodes of clinical malaria per 1,000 vaccinated children, and 983 cases per 1,000 vaccinated infants. But vaccine efficacy waned over time, disappearing completely in children who got only the three-dose series. The booster dose improved response stability somewhat; during the 12 months after the fourth dose, vaccine efficacy was about 25%.
Based on these results, GSK received approval from the European Medicines Agency in 2015, and the WHO recommended a large-scale implementation of the vaccine be carried out last year. GSK will provide the vaccine at no cost, and each country’s government will decide which regions to include in the pilot study.
This real-world use will put RTS,S to the ultimate test, Dr. Beeching said: “There is always the practical problem of how do you get four doses of vaccine into people. It’s easy to do in a clinical trial, but the operations and the logistics of getting it right on the ground are what really matter. We don’t know how good less than four doses would be, and we still don’t know how long the protective effect of the full series plus booster will last. I think there’s concern that it might wane with time.”
Still, he said, even a 39% reduction in disease burden is worth aggressively pursuing, not only because of the thousands of children’s lives that could be saved, but because unvaccinated children and adults could potentially be protected as well: “We could see a knock-on effect. By reducing the burden of malaria in children, it may also reduce transmission to other people who haven’t been vaccinated.”
The vaccine certainly won’t eradicate malaria, Dr. Beeching said. It needs to be viewed as an addition to WHO’s core vector control strategy, which includes insecticide-impregnated bed nets and mosquito eradication programs.
Cost is an unresolved issue. According to the Malaria Vaccine Initiative, which is partnering with GSK to launch RTS,S, the company won’t charge for the vaccine in the pilot project, and is committed to making sure the children who need it get it.
“In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms,” the company said in a press release. “The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access.
“GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around 5%, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.”
Finally, Dr. Beeching said, there’s no way to know to know how long any malaria vaccine would retain its effectiveness.
“Making a malaria vaccine has been a dream for years, and a tough one. The antigens change according to the stage of the parasite, and there is always continuous genetic variation. So there is a possibility of escape from vaccine coverage. These are very clever parasites,” he said.
Dr. Beeching has no financial interest in the vaccine.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
EXPERT ANALYSIS FROM ECCMID 2017
VIENNA – The first malaria vaccine to enter a national pilot project is not a silver bullet against the disease that kills half a million every year, but it still might be powerful enough to significantly reduce global disease burden, and even impact transmission, according to infectious disease specialist Nick Beeching, MD.
The vaccine, RTS,S (Mosquirix; GlaxoSmithKline), will be tested in three African countries beginning next year, the World Health Organization announced on April 25. The pilot programs will target 720,000 children aged 5-17 months in high-risk areas of the three countries.
Even though it’s the first malaria vaccine to pass its pivotal phase III trial, RTS,S isn’t terribly effective by any standards, said Dr. Beeching of the Royal Liverpool (England) University.
April 25, 2017, is World Malaria Day, and Anthony S. Fauci, MD, and B. Fenton Hall, MD, PhD, of the U.S. National Institute of Allergy and Infectious Diseases, said in a statement, “Safe and effective vaccines are critical tools for future efforts to control, eliminate, and, ultimately, eradicate malaria. NIAID is supporting the development of numerous malaria vaccine candidates, 10 of which are in clinical trials. In 2015, an estimated 212 million new malaria cases and 429,000 deaths occurred. Nearly 90% of these cases were among children under the age of 5 years in Africa, where malaria claims the life of a child every 2 minutes.”
GSK has been working on this vaccine since 1985, according to the company’s RTS,S literature. It is a recombinant protein that targets the circumsporozoite protein of the Plasmodium falciparum parasite at an early stage, before it enters the liver and begins to embed in erythrocytes. The aim, Dr. Beeching said, was to develop an antigen that would mobilize the immune system from the moment a mosquito injected the sporozoites through a bite, “well before they have a chance to hide in the liver.”
The 2- and 3-year follow-up results of the phase III trial, conducted in 15,500 children, were published in the Lancet in 2015. RTS,S was administered as a three-dose series, plus a booster dose, beginning at 5 months of age. The primary immunizations were given with a minimum 4-week interval between doses, with the booster administered 18 months after the last dose.
The primary series reduced clinical cases by 26%. With the booster dose, cases were reduced by 39% overall. The vaccine averted 1,774 episodes of clinical malaria per 1,000 vaccinated children, and 983 cases per 1,000 vaccinated infants. But vaccine efficacy waned over time, disappearing completely in children who got only the three-dose series. The booster dose improved response stability somewhat; during the 12 months after the fourth dose, vaccine efficacy was about 25%.
Based on these results, GSK received approval from the European Medicines Agency in 2015, and the WHO recommended a large-scale implementation of the vaccine be carried out last year. GSK will provide the vaccine at no cost, and each country’s government will decide which regions to include in the pilot study.
This real-world use will put RTS,S to the ultimate test, Dr. Beeching said: “There is always the practical problem of how do you get four doses of vaccine into people. It’s easy to do in a clinical trial, but the operations and the logistics of getting it right on the ground are what really matter. We don’t know how good less than four doses would be, and we still don’t know how long the protective effect of the full series plus booster will last. I think there’s concern that it might wane with time.”
Still, he said, even a 39% reduction in disease burden is worth aggressively pursuing, not only because of the thousands of children’s lives that could be saved, but because unvaccinated children and adults could potentially be protected as well: “We could see a knock-on effect. By reducing the burden of malaria in children, it may also reduce transmission to other people who haven’t been vaccinated.”
The vaccine certainly won’t eradicate malaria, Dr. Beeching said. It needs to be viewed as an addition to WHO’s core vector control strategy, which includes insecticide-impregnated bed nets and mosquito eradication programs.
Cost is an unresolved issue. According to the Malaria Vaccine Initiative, which is partnering with GSK to launch RTS,S, the company won’t charge for the vaccine in the pilot project, and is committed to making sure the children who need it get it.
“In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms,” the company said in a press release. “The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access.
“GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around 5%, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.”
Finally, Dr. Beeching said, there’s no way to know to know how long any malaria vaccine would retain its effectiveness.
“Making a malaria vaccine has been a dream for years, and a tough one. The antigens change according to the stage of the parasite, and there is always continuous genetic variation. So there is a possibility of escape from vaccine coverage. These are very clever parasites,” he said.
Dr. Beeching has no financial interest in the vaccine.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
EXPERT ANALYSIS FROM ECCMID 2017
VIENNA – The first malaria vaccine to enter a national pilot project is not a silver bullet against the disease that kills half a million every year, but it still might be powerful enough to significantly reduce global disease burden, and even impact transmission, according to infectious disease specialist Nick Beeching, MD.
The vaccine, RTS,S (Mosquirix; GlaxoSmithKline), will be tested in three African countries beginning next year, the World Health Organization announced on April 25. The pilot programs will target 720,000 children aged 5-17 months in high-risk areas of the three countries.
Even though it’s the first malaria vaccine to pass its pivotal phase III trial, RTS,S isn’t terribly effective by any standards, said Dr. Beeching of the Royal Liverpool (England) University.
April 25, 2017, is World Malaria Day, and Anthony S. Fauci, MD, and B. Fenton Hall, MD, PhD, of the U.S. National Institute of Allergy and Infectious Diseases, said in a statement, “Safe and effective vaccines are critical tools for future efforts to control, eliminate, and, ultimately, eradicate malaria. NIAID is supporting the development of numerous malaria vaccine candidates, 10 of which are in clinical trials. In 2015, an estimated 212 million new malaria cases and 429,000 deaths occurred. Nearly 90% of these cases were among children under the age of 5 years in Africa, where malaria claims the life of a child every 2 minutes.”
GSK has been working on this vaccine since 1985, according to the company’s RTS,S literature. It is a recombinant protein that targets the circumsporozoite protein of the Plasmodium falciparum parasite at an early stage, before it enters the liver and begins to embed in erythrocytes. The aim, Dr. Beeching said, was to develop an antigen that would mobilize the immune system from the moment a mosquito injected the sporozoites through a bite, “well before they have a chance to hide in the liver.”
The 2- and 3-year follow-up results of the phase III trial, conducted in 15,500 children, were published in the Lancet in 2015. RTS,S was administered as a three-dose series, plus a booster dose, beginning at 5 months of age. The primary immunizations were given with a minimum 4-week interval between doses, with the booster administered 18 months after the last dose.
The primary series reduced clinical cases by 26%. With the booster dose, cases were reduced by 39% overall. The vaccine averted 1,774 episodes of clinical malaria per 1,000 vaccinated children, and 983 cases per 1,000 vaccinated infants. But vaccine efficacy waned over time, disappearing completely in children who got only the three-dose series. The booster dose improved response stability somewhat; during the 12 months after the fourth dose, vaccine efficacy was about 25%.
Based on these results, GSK received approval from the European Medicines Agency in 2015, and the WHO recommended a large-scale implementation of the vaccine be carried out last year. GSK will provide the vaccine at no cost, and each country’s government will decide which regions to include in the pilot study.
This real-world use will put RTS,S to the ultimate test, Dr. Beeching said: “There is always the practical problem of how do you get four doses of vaccine into people. It’s easy to do in a clinical trial, but the operations and the logistics of getting it right on the ground are what really matter. We don’t know how good less than four doses would be, and we still don’t know how long the protective effect of the full series plus booster will last. I think there’s concern that it might wane with time.”
Still, he said, even a 39% reduction in disease burden is worth aggressively pursuing, not only because of the thousands of children’s lives that could be saved, but because unvaccinated children and adults could potentially be protected as well: “We could see a knock-on effect. By reducing the burden of malaria in children, it may also reduce transmission to other people who haven’t been vaccinated.”
The vaccine certainly won’t eradicate malaria, Dr. Beeching said. It needs to be viewed as an addition to WHO’s core vector control strategy, which includes insecticide-impregnated bed nets and mosquito eradication programs.
Cost is an unresolved issue. According to the Malaria Vaccine Initiative, which is partnering with GSK to launch RTS,S, the company won’t charge for the vaccine in the pilot project, and is committed to making sure the children who need it get it.
“In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms,” the company said in a press release. “The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access.
“GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around 5%, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.”
Finally, Dr. Beeching said, there’s no way to know to know how long any malaria vaccine would retain its effectiveness.
“Making a malaria vaccine has been a dream for years, and a tough one. The antigens change according to the stage of the parasite, and there is always continuous genetic variation. So there is a possibility of escape from vaccine coverage. These are very clever parasites,” he said.
Dr. Beeching has no financial interest in the vaccine.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Vaccine for Respiratory Syncytial Virus Enters Phase 1 Testing
A vaccine against respiratory syncytial virus (RSV) is entering a phase 1 safety and tolerability trial. The vaccine developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) is badly needed, according to Anthony Fauci, MD, director of NIAID. Although common and causing usually mild symptoms RSV infection also can lead to severe lower respiratory tract diseases, such as pneumonia and bronchiolitis in infants, children, the elderly, and immune-compromised patients. Globally, RSV infections cause upwards of 250,000 deaths each year. “RSV is underappreciated as a major cause of illness and death,” Fauci said.
The vaccine (DS-Cav1) will fill a void. Currently no vaccine is available to prevent RSV infection, and no drug is available to treat it. The monoclonal antibody palivizumab is approved for preventing lower respiratory tract disease caused by RSV in high-risk children, but is not approved for use in the general population.
The study, VRC 317, will enroll healthy adults aged 18 to 50. Participants will be assigned randomly to receive 2 injections 12 weeks apart with the investigational vaccine or the investigational vaccine with alum, a compound commonly added to vaccines to enhance the immune response.
Participants also will be randomly assigned to receive 1 of 3 doses (50, 150, or 500 μg) at both time points. To start, 5 people will receive the 50-µg dose. If they experience no serious adverse reactions attributable to the vaccine the other participants will be vaccinated with the higher doses.
The participants will return for 12 clinic visits over 44 weeks when researchers will conduct physical exams, collect blood samples, and test mucous samples to measure the immune response.
DS-Cav1 is the result of “years of research” at the Vaccine Research Center, the NIH says. Traditionally a vaccine is derived from a weakened or inactivated whole virus. By contrast, DS-Cav1 is a single, structurally engineered protein from the surface of RSV. Co-lead investigator Barney Graham, MD, PhD, deputy VRC director, says, “This work represents how new biological insights from basic research can lead to candidate vaccines for diseases of public health importance.”
A vaccine against respiratory syncytial virus (RSV) is entering a phase 1 safety and tolerability trial. The vaccine developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) is badly needed, according to Anthony Fauci, MD, director of NIAID. Although common and causing usually mild symptoms RSV infection also can lead to severe lower respiratory tract diseases, such as pneumonia and bronchiolitis in infants, children, the elderly, and immune-compromised patients. Globally, RSV infections cause upwards of 250,000 deaths each year. “RSV is underappreciated as a major cause of illness and death,” Fauci said.
The vaccine (DS-Cav1) will fill a void. Currently no vaccine is available to prevent RSV infection, and no drug is available to treat it. The monoclonal antibody palivizumab is approved for preventing lower respiratory tract disease caused by RSV in high-risk children, but is not approved for use in the general population.
The study, VRC 317, will enroll healthy adults aged 18 to 50. Participants will be assigned randomly to receive 2 injections 12 weeks apart with the investigational vaccine or the investigational vaccine with alum, a compound commonly added to vaccines to enhance the immune response.
Participants also will be randomly assigned to receive 1 of 3 doses (50, 150, or 500 μg) at both time points. To start, 5 people will receive the 50-µg dose. If they experience no serious adverse reactions attributable to the vaccine the other participants will be vaccinated with the higher doses.
The participants will return for 12 clinic visits over 44 weeks when researchers will conduct physical exams, collect blood samples, and test mucous samples to measure the immune response.
DS-Cav1 is the result of “years of research” at the Vaccine Research Center, the NIH says. Traditionally a vaccine is derived from a weakened or inactivated whole virus. By contrast, DS-Cav1 is a single, structurally engineered protein from the surface of RSV. Co-lead investigator Barney Graham, MD, PhD, deputy VRC director, says, “This work represents how new biological insights from basic research can lead to candidate vaccines for diseases of public health importance.”
A vaccine against respiratory syncytial virus (RSV) is entering a phase 1 safety and tolerability trial. The vaccine developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) is badly needed, according to Anthony Fauci, MD, director of NIAID. Although common and causing usually mild symptoms RSV infection also can lead to severe lower respiratory tract diseases, such as pneumonia and bronchiolitis in infants, children, the elderly, and immune-compromised patients. Globally, RSV infections cause upwards of 250,000 deaths each year. “RSV is underappreciated as a major cause of illness and death,” Fauci said.
The vaccine (DS-Cav1) will fill a void. Currently no vaccine is available to prevent RSV infection, and no drug is available to treat it. The monoclonal antibody palivizumab is approved for preventing lower respiratory tract disease caused by RSV in high-risk children, but is not approved for use in the general population.
The study, VRC 317, will enroll healthy adults aged 18 to 50. Participants will be assigned randomly to receive 2 injections 12 weeks apart with the investigational vaccine or the investigational vaccine with alum, a compound commonly added to vaccines to enhance the immune response.
Participants also will be randomly assigned to receive 1 of 3 doses (50, 150, or 500 μg) at both time points. To start, 5 people will receive the 50-µg dose. If they experience no serious adverse reactions attributable to the vaccine the other participants will be vaccinated with the higher doses.
The participants will return for 12 clinic visits over 44 weeks when researchers will conduct physical exams, collect blood samples, and test mucous samples to measure the immune response.
DS-Cav1 is the result of “years of research” at the Vaccine Research Center, the NIH says. Traditionally a vaccine is derived from a weakened or inactivated whole virus. By contrast, DS-Cav1 is a single, structurally engineered protein from the surface of RSV. Co-lead investigator Barney Graham, MD, PhD, deputy VRC director, says, “This work represents how new biological insights from basic research can lead to candidate vaccines for diseases of public health importance.”
Preterm infants face increased pertussis risk
Pertussis is more likely in infants who are born prematurely, compared with infants carried to term, according to Dr. Øystein Rolandsen Riise of the Norwegian Institute of Public Health, Oslo, and associates.
Using data from the Medical Birth Registry of Norway, 713,166 children were monitored until the age of 2 years from 1998 to 2010, during which time 968 cases of pertussis were laboratory confirmed. The incidence rate in term infants was 67.9 cases per 100,000 person-years, and was 115.2 cases per 100,000 person-years for preterm infants. The overall incidence rate ratio (IRR) of pertussis for preterm infants was 1.65, compared with term infants.
Hospitalization due to pertussis also was significantly more likely in preterm infants, with an overall IRR of 1.99, and infants born at 23-27 weeks again faced a greatly increased risk, with an IRR of 5.28.
Three-dose vaccine effectiveness against reported pertussis was 88.8% in term infants and 93% in preterm infants.
“Early and timely pediatric vaccinations as well as other strategies to prevent transmission to preterm infants are of utmost importance,” the investigators wrote.
Find the full study in the Pediatric Infectious Disease Journal (2017 May. doi: 10.1097/INF.0000000000001545).
Pertussis is more likely in infants who are born prematurely, compared with infants carried to term, according to Dr. Øystein Rolandsen Riise of the Norwegian Institute of Public Health, Oslo, and associates.
Using data from the Medical Birth Registry of Norway, 713,166 children were monitored until the age of 2 years from 1998 to 2010, during which time 968 cases of pertussis were laboratory confirmed. The incidence rate in term infants was 67.9 cases per 100,000 person-years, and was 115.2 cases per 100,000 person-years for preterm infants. The overall incidence rate ratio (IRR) of pertussis for preterm infants was 1.65, compared with term infants.
Hospitalization due to pertussis also was significantly more likely in preterm infants, with an overall IRR of 1.99, and infants born at 23-27 weeks again faced a greatly increased risk, with an IRR of 5.28.
Three-dose vaccine effectiveness against reported pertussis was 88.8% in term infants and 93% in preterm infants.
“Early and timely pediatric vaccinations as well as other strategies to prevent transmission to preterm infants are of utmost importance,” the investigators wrote.
Find the full study in the Pediatric Infectious Disease Journal (2017 May. doi: 10.1097/INF.0000000000001545).
Pertussis is more likely in infants who are born prematurely, compared with infants carried to term, according to Dr. Øystein Rolandsen Riise of the Norwegian Institute of Public Health, Oslo, and associates.
Using data from the Medical Birth Registry of Norway, 713,166 children were monitored until the age of 2 years from 1998 to 2010, during which time 968 cases of pertussis were laboratory confirmed. The incidence rate in term infants was 67.9 cases per 100,000 person-years, and was 115.2 cases per 100,000 person-years for preterm infants. The overall incidence rate ratio (IRR) of pertussis for preterm infants was 1.65, compared with term infants.
Hospitalization due to pertussis also was significantly more likely in preterm infants, with an overall IRR of 1.99, and infants born at 23-27 weeks again faced a greatly increased risk, with an IRR of 5.28.
Three-dose vaccine effectiveness against reported pertussis was 88.8% in term infants and 93% in preterm infants.
“Early and timely pediatric vaccinations as well as other strategies to prevent transmission to preterm infants are of utmost importance,” the investigators wrote.
Find the full study in the Pediatric Infectious Disease Journal (2017 May. doi: 10.1097/INF.0000000000001545).
Revaccinate HIV-infected teens with hepatitis B vaccine
Consider revaccinating all perinatally HIV-infected adolescents who did not initiate highly active antiretroviral therapy (HAART) at the time of initial infant hepatitis B virus (HBV) vaccination, with a three-dose HBV schedule, recommended researchers at Mahidol University, Bangkok, in a recent study.
In a prospective study from March 2012 to March 2014 ultimately involving 162 perinatally HIV-infected adolescents with immune reconstitution, only 3.6% were receiving HAART at the time of initial infant HBV vaccination and 96.3% had undetectable antihepatitis B surface antibodies (anti-HBs) at baseline. Adolescents with breakthrough HBV infection had been excluded from the cohort.
In a multivariate analysis, there was no independent factor that was associated with the presence of immune memory defined as anti-HBs greater than or equal to 100 mIU/mL, wrote lead researcher Keswadee Lapphra, MD, and her associates.
In a previous study, 71% of three-dose revaccinated persons retained protective antibodies against HBV at 3-year follow-up; this was a similar rate to that reported in healthy HIV-infected children after their infant primary HBV series (Vaccine. 2011 May 23;29[23]:3977-81), they said.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/inf.0000000000001613).
Consider revaccinating all perinatally HIV-infected adolescents who did not initiate highly active antiretroviral therapy (HAART) at the time of initial infant hepatitis B virus (HBV) vaccination, with a three-dose HBV schedule, recommended researchers at Mahidol University, Bangkok, in a recent study.
In a prospective study from March 2012 to March 2014 ultimately involving 162 perinatally HIV-infected adolescents with immune reconstitution, only 3.6% were receiving HAART at the time of initial infant HBV vaccination and 96.3% had undetectable antihepatitis B surface antibodies (anti-HBs) at baseline. Adolescents with breakthrough HBV infection had been excluded from the cohort.
In a multivariate analysis, there was no independent factor that was associated with the presence of immune memory defined as anti-HBs greater than or equal to 100 mIU/mL, wrote lead researcher Keswadee Lapphra, MD, and her associates.
In a previous study, 71% of three-dose revaccinated persons retained protective antibodies against HBV at 3-year follow-up; this was a similar rate to that reported in healthy HIV-infected children after their infant primary HBV series (Vaccine. 2011 May 23;29[23]:3977-81), they said.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/inf.0000000000001613).
Consider revaccinating all perinatally HIV-infected adolescents who did not initiate highly active antiretroviral therapy (HAART) at the time of initial infant hepatitis B virus (HBV) vaccination, with a three-dose HBV schedule, recommended researchers at Mahidol University, Bangkok, in a recent study.
In a prospective study from March 2012 to March 2014 ultimately involving 162 perinatally HIV-infected adolescents with immune reconstitution, only 3.6% were receiving HAART at the time of initial infant HBV vaccination and 96.3% had undetectable antihepatitis B surface antibodies (anti-HBs) at baseline. Adolescents with breakthrough HBV infection had been excluded from the cohort.
In a multivariate analysis, there was no independent factor that was associated with the presence of immune memory defined as anti-HBs greater than or equal to 100 mIU/mL, wrote lead researcher Keswadee Lapphra, MD, and her associates.
In a previous study, 71% of three-dose revaccinated persons retained protective antibodies against HBV at 3-year follow-up; this was a similar rate to that reported in healthy HIV-infected children after their infant primary HBV series (Vaccine. 2011 May 23;29[23]:3977-81), they said.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/inf.0000000000001613).
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL