User login
Telemedicine meets menopause in customized patient care service
Women facing issues related to perimenopause and menopause can consult their primary care physicians or ob.gyns. through telemedicine visits, but
The Cusp doesn’t claim to replace routine gynecologic care. Rather, it focuses on perimenopause and menopause symptoms specifically, and states that its physicians, some of whom are certified by the North American Menopause Society, provide expertise in menopause beyond what patients might receive as part of a typical ob.gyn. visit.
The Cusp is a for-profit organization, a group of physicians, nurse practitioners, and technologists who focus on integrated care for women in perimenopause and beyond. The aim is to leverage technology as a way to connect women to the care platform to book physician and nurse practitioner visits virtually and to have all of the information about their care centralized in one place.
According to the website, most patients who sign up for a care plan check in with their providers at least once a month to monitor their symptoms and tweak treatment strategies. Patients who sign up are prompted to download an app, which then becomes the main tool for scheduling future visits, tracking symptoms, and communicating with providers.
The Cusp launched in early 2019, before the advent of the COVID-19 pandemic, but the pandemic has accelerated the acceptance across medical specialties, suggesting that telemedicine is here to stay, according to Mindy Goldman, MD, professor of gynecology and gynecologic surgery at the University of California, San Francisco, and director of the Gynecology Center for Cancer Survivors and At-Risk Women at UCSF, who also serves as a medical adviser to the Cusp.
Partnering with technology companies allows opportunities to provide care in areas where there are gaps, such as menopause management, she said. Many clinicians in primary care and ob.gyn. care don’t have the time or training to discuss menopause management in depth with patients, and patient interviews conducted by the Cusp before launching the site showed that this was an area of need.
“One thing that is really unique about the Cusp is that we brought together experts to provide care in both in an evidence-based and holistic fashion,” Dr. Goldman emphasized.
The Cusp’s medical team includes physician and nurse practitioner menopause experts with backgrounds including not only ob.gyn. but also psychiatry, integrative medicine, and naturopathic medicine, with plans to add endocrinology and dermatology as well. This holistic approach allows the Cusp to tailor care based on what each woman is looking for, with evidence-based expertise to support treatment decisions, said Dr. Goldman, whose advisory role includes helping to develop patient treatment protocols and services.
If a woman wants to begin treating symptoms with a naturopathic approach, the team will provide protocols that take current guidelines into account. Regular visits, approximately once a month or as needed, allow for collaboration with the Cusp’s specialists to provide consistent care that is very comprehensive, she said.
One of the benefits of the Cusp is the opportunity for “frequent touchpoints” in which providers reach out to patients via text, email, or video. Although a traditional medical visit may include some initial discussion of menopause and treatment plans, the Cusp offers “a more seamless way to address needs on an ongoing basis,” to provide more complete patient care, Dr. Goldman said.
“We are constantly asking women what they are looking for in menopause care,” and a recurring question was about hormone testing, she said. Nontraditional practitioners may offer hormone testing as a way of individualizing care that also involves compounded formulations, and other treatments that are not standard of care. “In all of our protocols we follow what is recommended by standard organizations such as ACOG [American College of Obstetricians and Gynecologists] and NAMS.”
The Cusp’s newest service is an at-home hormone test currently for women in New York and California, but the company plans to expand this service. The hormone test, while not essential, is another tool to guide menopause management, and having a sense of when menopause will occur “gives us a chance to talk to people about behavioral changes and time to personalize a treatment protocol,” Dr. Goldman said.
The test is based in part on the anti-Müllerian hormone, which recent studies have shown is useful in predicting time to menopause. This, in combination with other hormone tests and other clinical information, will allow the Cusp’s menopause specialists to help women in perimenopause gain perspective on their symptoms and design a treatment plan that can evolve as their needs change, she explained.
“The more information you know about when menopause is going to be happening, you can tailor your treatment plan,” Dr. Goldman said. For example, a woman who may be 2 years away from menopause might consider a naturopathic approach at first, and switch to a different therapy as menopause occurs. “We know that the risks of cardiovascular disease and bone loss increase after menopause, and knowing the time to menopause gives us more guidance when educating patients about healthy lifestyle habits such as exercise and dietary changes that can help reduce these risks.”
The Cusp allows patients to use money in flexible spending accounts or health savings accounts to pay for the program. If doctors require lab tests or other procedures, these are covered through the patients’ regular health insurance as they would be if requested by a primary care physician or other health care professional.
Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn., commented that part of the value in a telehealth site such as the Cusp is to serve as “a resource for reproductively aging women to understand what is happening to them.”
Any way to improve education on the topic of menopause is empowering to women, said Dr. Pal, professor of obstetrics, gynecology, and reproductive sciences at Yale. “This is an opportunity for patients to have access to a directed evaluation” of menopause-related symptoms. Then, when women visit their regular health care provider in person, they are well-equipped with knowledge to ask more informed questions and discuss a wide range of treatment options.
Dr. Pal noted that the hormone test is less valuable than the interaction between physicians and patients, whether online or in person.
“Menopause is a Monday morning quarterback diagnosis,” she said, emphasizing that, not only is a year without menses part of the diagnosis of menopause, many women in perimenopause can have wide fluctuations in hormone levels, so a test is more of a snapshot than a diagnostic tool, and that the results might cause unnecessary angst and concerns for patients.
However, part of the value of a telehealth site that focuses on menopause is that it gives women a place to learn more about their biology and to clarify their questions about symptoms and become aware of a range of treatment options. Telehealth consultations also can help women recognize how other factors such as lifestyle modifications can play a role in menopause symptoms, and how modifying these factors may provide some relief, she said.
Dr. Pal said she would be cautious about the idea of prescribing without seeing the patient in person, but noted that telehealth sites such as the Cusp can be a win-win to enhance women’s health when used in combination with regular in-person visits to an ob.gyn. The added value in patients’ being able to discuss their concerns and to learn more about their symptoms means that they will be better informed to develop a menopause management strategy in partnership with their providers, said Dr. Pal, who is not associated with the Cusp.
Dr. Goldman disclosed receiving compensation from the Cusp for her advisory work. She also holds stock options in the company. Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board, had no financial conflicts to disclose.
Women facing issues related to perimenopause and menopause can consult their primary care physicians or ob.gyns. through telemedicine visits, but
The Cusp doesn’t claim to replace routine gynecologic care. Rather, it focuses on perimenopause and menopause symptoms specifically, and states that its physicians, some of whom are certified by the North American Menopause Society, provide expertise in menopause beyond what patients might receive as part of a typical ob.gyn. visit.
The Cusp is a for-profit organization, a group of physicians, nurse practitioners, and technologists who focus on integrated care for women in perimenopause and beyond. The aim is to leverage technology as a way to connect women to the care platform to book physician and nurse practitioner visits virtually and to have all of the information about their care centralized in one place.
According to the website, most patients who sign up for a care plan check in with their providers at least once a month to monitor their symptoms and tweak treatment strategies. Patients who sign up are prompted to download an app, which then becomes the main tool for scheduling future visits, tracking symptoms, and communicating with providers.
The Cusp launched in early 2019, before the advent of the COVID-19 pandemic, but the pandemic has accelerated the acceptance across medical specialties, suggesting that telemedicine is here to stay, according to Mindy Goldman, MD, professor of gynecology and gynecologic surgery at the University of California, San Francisco, and director of the Gynecology Center for Cancer Survivors and At-Risk Women at UCSF, who also serves as a medical adviser to the Cusp.
Partnering with technology companies allows opportunities to provide care in areas where there are gaps, such as menopause management, she said. Many clinicians in primary care and ob.gyn. care don’t have the time or training to discuss menopause management in depth with patients, and patient interviews conducted by the Cusp before launching the site showed that this was an area of need.
“One thing that is really unique about the Cusp is that we brought together experts to provide care in both in an evidence-based and holistic fashion,” Dr. Goldman emphasized.
The Cusp’s medical team includes physician and nurse practitioner menopause experts with backgrounds including not only ob.gyn. but also psychiatry, integrative medicine, and naturopathic medicine, with plans to add endocrinology and dermatology as well. This holistic approach allows the Cusp to tailor care based on what each woman is looking for, with evidence-based expertise to support treatment decisions, said Dr. Goldman, whose advisory role includes helping to develop patient treatment protocols and services.
If a woman wants to begin treating symptoms with a naturopathic approach, the team will provide protocols that take current guidelines into account. Regular visits, approximately once a month or as needed, allow for collaboration with the Cusp’s specialists to provide consistent care that is very comprehensive, she said.
One of the benefits of the Cusp is the opportunity for “frequent touchpoints” in which providers reach out to patients via text, email, or video. Although a traditional medical visit may include some initial discussion of menopause and treatment plans, the Cusp offers “a more seamless way to address needs on an ongoing basis,” to provide more complete patient care, Dr. Goldman said.
“We are constantly asking women what they are looking for in menopause care,” and a recurring question was about hormone testing, she said. Nontraditional practitioners may offer hormone testing as a way of individualizing care that also involves compounded formulations, and other treatments that are not standard of care. “In all of our protocols we follow what is recommended by standard organizations such as ACOG [American College of Obstetricians and Gynecologists] and NAMS.”
The Cusp’s newest service is an at-home hormone test currently for women in New York and California, but the company plans to expand this service. The hormone test, while not essential, is another tool to guide menopause management, and having a sense of when menopause will occur “gives us a chance to talk to people about behavioral changes and time to personalize a treatment protocol,” Dr. Goldman said.
The test is based in part on the anti-Müllerian hormone, which recent studies have shown is useful in predicting time to menopause. This, in combination with other hormone tests and other clinical information, will allow the Cusp’s menopause specialists to help women in perimenopause gain perspective on their symptoms and design a treatment plan that can evolve as their needs change, she explained.
“The more information you know about when menopause is going to be happening, you can tailor your treatment plan,” Dr. Goldman said. For example, a woman who may be 2 years away from menopause might consider a naturopathic approach at first, and switch to a different therapy as menopause occurs. “We know that the risks of cardiovascular disease and bone loss increase after menopause, and knowing the time to menopause gives us more guidance when educating patients about healthy lifestyle habits such as exercise and dietary changes that can help reduce these risks.”
The Cusp allows patients to use money in flexible spending accounts or health savings accounts to pay for the program. If doctors require lab tests or other procedures, these are covered through the patients’ regular health insurance as they would be if requested by a primary care physician or other health care professional.
Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn., commented that part of the value in a telehealth site such as the Cusp is to serve as “a resource for reproductively aging women to understand what is happening to them.”
Any way to improve education on the topic of menopause is empowering to women, said Dr. Pal, professor of obstetrics, gynecology, and reproductive sciences at Yale. “This is an opportunity for patients to have access to a directed evaluation” of menopause-related symptoms. Then, when women visit their regular health care provider in person, they are well-equipped with knowledge to ask more informed questions and discuss a wide range of treatment options.
Dr. Pal noted that the hormone test is less valuable than the interaction between physicians and patients, whether online or in person.
“Menopause is a Monday morning quarterback diagnosis,” she said, emphasizing that, not only is a year without menses part of the diagnosis of menopause, many women in perimenopause can have wide fluctuations in hormone levels, so a test is more of a snapshot than a diagnostic tool, and that the results might cause unnecessary angst and concerns for patients.
However, part of the value of a telehealth site that focuses on menopause is that it gives women a place to learn more about their biology and to clarify their questions about symptoms and become aware of a range of treatment options. Telehealth consultations also can help women recognize how other factors such as lifestyle modifications can play a role in menopause symptoms, and how modifying these factors may provide some relief, she said.
Dr. Pal said she would be cautious about the idea of prescribing without seeing the patient in person, but noted that telehealth sites such as the Cusp can be a win-win to enhance women’s health when used in combination with regular in-person visits to an ob.gyn. The added value in patients’ being able to discuss their concerns and to learn more about their symptoms means that they will be better informed to develop a menopause management strategy in partnership with their providers, said Dr. Pal, who is not associated with the Cusp.
Dr. Goldman disclosed receiving compensation from the Cusp for her advisory work. She also holds stock options in the company. Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board, had no financial conflicts to disclose.
Women facing issues related to perimenopause and menopause can consult their primary care physicians or ob.gyns. through telemedicine visits, but
The Cusp doesn’t claim to replace routine gynecologic care. Rather, it focuses on perimenopause and menopause symptoms specifically, and states that its physicians, some of whom are certified by the North American Menopause Society, provide expertise in menopause beyond what patients might receive as part of a typical ob.gyn. visit.
The Cusp is a for-profit organization, a group of physicians, nurse practitioners, and technologists who focus on integrated care for women in perimenopause and beyond. The aim is to leverage technology as a way to connect women to the care platform to book physician and nurse practitioner visits virtually and to have all of the information about their care centralized in one place.
According to the website, most patients who sign up for a care plan check in with their providers at least once a month to monitor their symptoms and tweak treatment strategies. Patients who sign up are prompted to download an app, which then becomes the main tool for scheduling future visits, tracking symptoms, and communicating with providers.
The Cusp launched in early 2019, before the advent of the COVID-19 pandemic, but the pandemic has accelerated the acceptance across medical specialties, suggesting that telemedicine is here to stay, according to Mindy Goldman, MD, professor of gynecology and gynecologic surgery at the University of California, San Francisco, and director of the Gynecology Center for Cancer Survivors and At-Risk Women at UCSF, who also serves as a medical adviser to the Cusp.
Partnering with technology companies allows opportunities to provide care in areas where there are gaps, such as menopause management, she said. Many clinicians in primary care and ob.gyn. care don’t have the time or training to discuss menopause management in depth with patients, and patient interviews conducted by the Cusp before launching the site showed that this was an area of need.
“One thing that is really unique about the Cusp is that we brought together experts to provide care in both in an evidence-based and holistic fashion,” Dr. Goldman emphasized.
The Cusp’s medical team includes physician and nurse practitioner menopause experts with backgrounds including not only ob.gyn. but also psychiatry, integrative medicine, and naturopathic medicine, with plans to add endocrinology and dermatology as well. This holistic approach allows the Cusp to tailor care based on what each woman is looking for, with evidence-based expertise to support treatment decisions, said Dr. Goldman, whose advisory role includes helping to develop patient treatment protocols and services.
If a woman wants to begin treating symptoms with a naturopathic approach, the team will provide protocols that take current guidelines into account. Regular visits, approximately once a month or as needed, allow for collaboration with the Cusp’s specialists to provide consistent care that is very comprehensive, she said.
One of the benefits of the Cusp is the opportunity for “frequent touchpoints” in which providers reach out to patients via text, email, or video. Although a traditional medical visit may include some initial discussion of menopause and treatment plans, the Cusp offers “a more seamless way to address needs on an ongoing basis,” to provide more complete patient care, Dr. Goldman said.
“We are constantly asking women what they are looking for in menopause care,” and a recurring question was about hormone testing, she said. Nontraditional practitioners may offer hormone testing as a way of individualizing care that also involves compounded formulations, and other treatments that are not standard of care. “In all of our protocols we follow what is recommended by standard organizations such as ACOG [American College of Obstetricians and Gynecologists] and NAMS.”
The Cusp’s newest service is an at-home hormone test currently for women in New York and California, but the company plans to expand this service. The hormone test, while not essential, is another tool to guide menopause management, and having a sense of when menopause will occur “gives us a chance to talk to people about behavioral changes and time to personalize a treatment protocol,” Dr. Goldman said.
The test is based in part on the anti-Müllerian hormone, which recent studies have shown is useful in predicting time to menopause. This, in combination with other hormone tests and other clinical information, will allow the Cusp’s menopause specialists to help women in perimenopause gain perspective on their symptoms and design a treatment plan that can evolve as their needs change, she explained.
“The more information you know about when menopause is going to be happening, you can tailor your treatment plan,” Dr. Goldman said. For example, a woman who may be 2 years away from menopause might consider a naturopathic approach at first, and switch to a different therapy as menopause occurs. “We know that the risks of cardiovascular disease and bone loss increase after menopause, and knowing the time to menopause gives us more guidance when educating patients about healthy lifestyle habits such as exercise and dietary changes that can help reduce these risks.”
The Cusp allows patients to use money in flexible spending accounts or health savings accounts to pay for the program. If doctors require lab tests or other procedures, these are covered through the patients’ regular health insurance as they would be if requested by a primary care physician or other health care professional.
Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn., commented that part of the value in a telehealth site such as the Cusp is to serve as “a resource for reproductively aging women to understand what is happening to them.”
Any way to improve education on the topic of menopause is empowering to women, said Dr. Pal, professor of obstetrics, gynecology, and reproductive sciences at Yale. “This is an opportunity for patients to have access to a directed evaluation” of menopause-related symptoms. Then, when women visit their regular health care provider in person, they are well-equipped with knowledge to ask more informed questions and discuss a wide range of treatment options.
Dr. Pal noted that the hormone test is less valuable than the interaction between physicians and patients, whether online or in person.
“Menopause is a Monday morning quarterback diagnosis,” she said, emphasizing that, not only is a year without menses part of the diagnosis of menopause, many women in perimenopause can have wide fluctuations in hormone levels, so a test is more of a snapshot than a diagnostic tool, and that the results might cause unnecessary angst and concerns for patients.
However, part of the value of a telehealth site that focuses on menopause is that it gives women a place to learn more about their biology and to clarify their questions about symptoms and become aware of a range of treatment options. Telehealth consultations also can help women recognize how other factors such as lifestyle modifications can play a role in menopause symptoms, and how modifying these factors may provide some relief, she said.
Dr. Pal said she would be cautious about the idea of prescribing without seeing the patient in person, but noted that telehealth sites such as the Cusp can be a win-win to enhance women’s health when used in combination with regular in-person visits to an ob.gyn. The added value in patients’ being able to discuss their concerns and to learn more about their symptoms means that they will be better informed to develop a menopause management strategy in partnership with their providers, said Dr. Pal, who is not associated with the Cusp.
Dr. Goldman disclosed receiving compensation from the Cusp for her advisory work. She also holds stock options in the company. Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board, had no financial conflicts to disclose.
Appendix may be common site of endometriosis
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
FROM SGS 2020
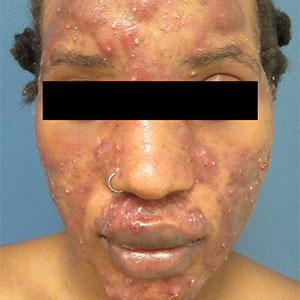
Large, painful facial cysts
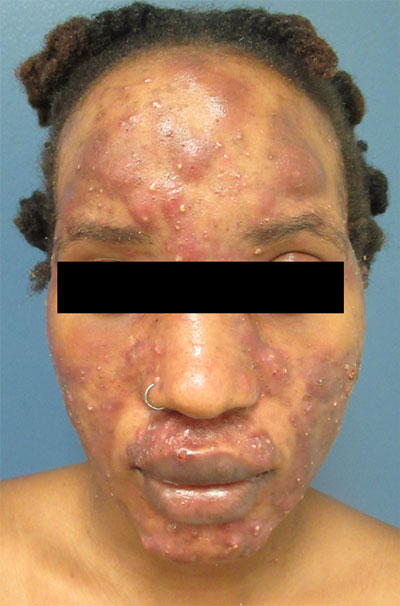
The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
Cohort study finds a twofold greater psoriasis risk linked to a PCOS diagnosis
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
HPV test is preferred method for cervical cancer screening: ACS
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening. The key recommendation is that primary human papillomavirus (HPV) testing is the preferred screening method, starting at the age of 25 and repeated every 5 years.
In the past, guidelines for cervical cancer screening recommended cytology (the Pap test) starting at 21 years of age and repeated every 3 years. In more recent years, cotesting (with both Pap and HPV tests) has been recommended.
Since the last ACS guidelines on cervical cancer screening were published in 2012, two HPV tests have been approved by the Food and Drug Administration (FDA) for use in primary HPV screening.
The new “streamlined recommendations can improve compliance and reduce potential harms,” commented Debbie Saslow, PhD, managing director, HPV/GYN Cancers, American Cancer Society.
The updated guidelines were published online July 30 in CA: A Cancer Journal for Clinicians.
“We now have stronger evidence to support starting cervical cancer screening at a later age and to recommend screening with the HPV test as the preferred test,” Saslow told Medscape Medical News. This also reflects the phasing out of cytology and cotesting, she added.
“This update is based on decades of studies comparing the effectiveness of HPV testing to cytology and is bolstered by evidence of the impact of HPV vaccination, including a dramatic decline in cervical precancers and, more recently, cervical cancers among young women,” she said.
The American Society for Colposcopy and Cervical Pathology (ASCCP) said that it was preparing a response to these new guidelines, as is the American College of Obstetricians and Gynecologists (ACOG).
Cotesting or cytology alone
The updated guidelines recommend primary HPV testing as the preferred screening method for all women with a cervix. If primary HPV testing is not available, women should be screened with cotesting, which should also be performed every 5 years.
If only cytology is available, then women should be screened every 3 years.
The ACS authors point out that cotesting or cytology testing alone is still an acceptable option for cervical cancer screening, insofar as primary HPV testing using FDA-approved tests may not be available in some settings.
As more laboratories in the United States transition to FDA-approved tests for primary HPV testing, it is expected that the use of cotesting or cytology alone will be phased out.
The new guidelines also emphasize that women may discontinue screening at the age of 65 if they have not had cervical intraepitheal neoplasia of grade 2 or higher within the past 25 years and if they have tested negative over the past 10 years on all past screens.
The authors caution that past screens should only be considered negative if the patient has had two consecutive negative HPV tests or two consecutive negative cotests or three consecutive negative cytology tests within the past 10 years.
“These criteria do not apply to individuals who are currently under surveillance for abnormal screening results,” the authors state.
Women older than 65 for whom adequate documentation of prior screening is not available should continue to be screened until criteria for screening discontinuation are met, they add.
Screening may be discontinued among women with a limited life expectancy.
HPV vaccination
The authors note that HPV vaccination is expected to substantially change cervical cancer screening strategies.
In 2018, the National Immunization Survey–Teen, involving adolescents aged 13 to 17 years, showed that 68.1% of female patients were up to date on HPV vaccine recommendations, as were 51.1% of male patients.
“Cytology-based screening is much less efficient in vaccinated populations, as abnormal cytology disproportionately identifies minor abnormalities resulting from HPV types that are associated with lower cancer risk,” the reports’ authors point out.
As the prevalence of high-grade cervical abnormalities and the incidence of cervical cancer continue to decline, “the proportion of false-positive findings [on cytology alone] is expected to increase significantly,” they caution.
As a result, the ACS suggests that physicians will likely have to consider a patient’s vaccination status in tandem with cervical cancer screening results to arrive at an accurate assessment.
Raising starting age to 25 years
Saslow also noted that there were several reasons why it is now recommended that screening begin at the age of 25 instead of the age of 21, as in earlier guidelines.
“Firstly, less than 1% of cervical cancers are diagnosed before the age of 25 – so this is about 130 cases per year,” she explained.
Thanks to HPV vaccination, this percentage is further declining, “so screening is just not beneficial at this age,” Saslow emphasized.
Furthermore, the rate of false positives is much higher in younger patients, and a false-positive result can have a negative impact on pregnancy outcomes, she added.
Saslow also dismissed an article in favor of cotesting instead of HPV testing alone. That study, carried out by researchers at Quest Diagnostics and the University of Pittsburgh Medical Center, recommended cotesting, claiming that primary HPV testing is significantly less likely to detect cervical precancers or cervical cancer than cotesting.
“These data come from parties with a vested interest in preserving cytology as a screening test,” Saslow told Medscape Medical News. She noted that “these findings are not at all credible as judged by the scientific community.”
On the basis of their own modeling, ACS researchers estimate that “starting with primary HPV testing at age 25 will prevent 13% more cervical cancers and 7% more cervical cancer deaths” in comparison with cytology (Pap testing alone) beginning at the age of 21, then cotesting at the age of 30, Saslow said in a statement.
“Our model showed we could do that with a 9% increase in follow-up procedures but with 45% fewer tests required overall,” she added.
The new recommendations are not expected to create any change in the type or amount of care required by providers, and patients will not notice any difference, inasmuch as cotesting and primary HPV testing are performed the same way in the examination room, she added.
“Resistance [to the changes] is expected – and is already occurring – from laboratories and manufacturers of tests that will no longer be used once we transition from cotesting and, less commonly, Pap testing to primary HPV testing,” Saslow said.
However, providers need to be aware that HPV infection, as with any sexually transmitted disease, is associated with a certain stigma, and they need to take care in discussing potential HPV infection with their patients.
Good method
Medscape Medical News approached Mark Einstein, MD, president of the ASCCP and professor and chair of obstetrics, gynecology, and reproductive health at Rutgers Biomedical and Health Sciences, Newark, New Jersey, to comment on the new guidelines.
“First and foremost,” he said, “everything we want to do when it comes to screening is to maximize the identification of picking up a cancer and minimize the risk or potential harm of not only screening itself but of missing cancers, so any strategy that improves on the sensitivity of picking up a cancer is a good method.”
Nevertheless, inasmuch as the ASCCP is one of the foremost organizations involved in cervical cancer screening and management, its members need more time to take a closer look at the updated ACS guidelines before they, together with sister organizations, such as the ACOG, release an official statement as to whether or not they fully endorse the new guidelines.
The United States Preventive Services Task Force recently endorsed primary HPV testing (starting at age 30), but it also said that an alternative strategy is cotesting for women between 30 and 65 years of age, Einstein observed.
Asked to comment on the article from Quest Diagnostics and the University of Pittsburgh that recommended cotesting instead of primary HPV testing, Einstein said that suggestion should not be dismissed out of hand.
The ASCCP has asked the authors of that study for their data in order conduct an independent assessment of it, largely because the study was retrospective in nature. Because of that, “there may have been a few pieces of information that were missing in true real-time fashion,” he said. “Not having [both the primary HPV testing and the cytology results] in front of me might change the next thing I might recommend to the patient,” Einstein explained.
The bottom line is that, when comparing primary HPV testing alone, cytology alone, and cotesting and rates of cervical cancer at 5 years, “the biggest driver for true performance of positive predictive value is HPV,” Einstein said.
Nevertheless, cotesting does bring more information into the equation compared with primary HPV testing alone, although it also increases the potential for harm, including the harm of overtesting and conducting needless colposcopies, he added.
That said, starting primary HPV testing at the age of 25 rather than the age of 30, as was previously recommended, is very likely to lead to detection of spurious HPV infections because HPV infections are very common among women in their 20s, Einstein pointed out.
“This, too, could potentially lead to more colposcopies, which may cause harm from the procedure itself but also create a certain amount of anxiety and concern, so there is some harm in testing for HPV at an earlier age as well,” Einstein said.
Saslow and Einstein have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening. The key recommendation is that primary human papillomavirus (HPV) testing is the preferred screening method, starting at the age of 25 and repeated every 5 years.
In the past, guidelines for cervical cancer screening recommended cytology (the Pap test) starting at 21 years of age and repeated every 3 years. In more recent years, cotesting (with both Pap and HPV tests) has been recommended.
Since the last ACS guidelines on cervical cancer screening were published in 2012, two HPV tests have been approved by the Food and Drug Administration (FDA) for use in primary HPV screening.
The new “streamlined recommendations can improve compliance and reduce potential harms,” commented Debbie Saslow, PhD, managing director, HPV/GYN Cancers, American Cancer Society.
The updated guidelines were published online July 30 in CA: A Cancer Journal for Clinicians.
“We now have stronger evidence to support starting cervical cancer screening at a later age and to recommend screening with the HPV test as the preferred test,” Saslow told Medscape Medical News. This also reflects the phasing out of cytology and cotesting, she added.
“This update is based on decades of studies comparing the effectiveness of HPV testing to cytology and is bolstered by evidence of the impact of HPV vaccination, including a dramatic decline in cervical precancers and, more recently, cervical cancers among young women,” she said.
The American Society for Colposcopy and Cervical Pathology (ASCCP) said that it was preparing a response to these new guidelines, as is the American College of Obstetricians and Gynecologists (ACOG).
Cotesting or cytology alone
The updated guidelines recommend primary HPV testing as the preferred screening method for all women with a cervix. If primary HPV testing is not available, women should be screened with cotesting, which should also be performed every 5 years.
If only cytology is available, then women should be screened every 3 years.
The ACS authors point out that cotesting or cytology testing alone is still an acceptable option for cervical cancer screening, insofar as primary HPV testing using FDA-approved tests may not be available in some settings.
As more laboratories in the United States transition to FDA-approved tests for primary HPV testing, it is expected that the use of cotesting or cytology alone will be phased out.
The new guidelines also emphasize that women may discontinue screening at the age of 65 if they have not had cervical intraepitheal neoplasia of grade 2 or higher within the past 25 years and if they have tested negative over the past 10 years on all past screens.
The authors caution that past screens should only be considered negative if the patient has had two consecutive negative HPV tests or two consecutive negative cotests or three consecutive negative cytology tests within the past 10 years.
“These criteria do not apply to individuals who are currently under surveillance for abnormal screening results,” the authors state.
Women older than 65 for whom adequate documentation of prior screening is not available should continue to be screened until criteria for screening discontinuation are met, they add.
Screening may be discontinued among women with a limited life expectancy.
HPV vaccination
The authors note that HPV vaccination is expected to substantially change cervical cancer screening strategies.
In 2018, the National Immunization Survey–Teen, involving adolescents aged 13 to 17 years, showed that 68.1% of female patients were up to date on HPV vaccine recommendations, as were 51.1% of male patients.
“Cytology-based screening is much less efficient in vaccinated populations, as abnormal cytology disproportionately identifies minor abnormalities resulting from HPV types that are associated with lower cancer risk,” the reports’ authors point out.
As the prevalence of high-grade cervical abnormalities and the incidence of cervical cancer continue to decline, “the proportion of false-positive findings [on cytology alone] is expected to increase significantly,” they caution.
As a result, the ACS suggests that physicians will likely have to consider a patient’s vaccination status in tandem with cervical cancer screening results to arrive at an accurate assessment.
Raising starting age to 25 years
Saslow also noted that there were several reasons why it is now recommended that screening begin at the age of 25 instead of the age of 21, as in earlier guidelines.
“Firstly, less than 1% of cervical cancers are diagnosed before the age of 25 – so this is about 130 cases per year,” she explained.
Thanks to HPV vaccination, this percentage is further declining, “so screening is just not beneficial at this age,” Saslow emphasized.
Furthermore, the rate of false positives is much higher in younger patients, and a false-positive result can have a negative impact on pregnancy outcomes, she added.
Saslow also dismissed an article in favor of cotesting instead of HPV testing alone. That study, carried out by researchers at Quest Diagnostics and the University of Pittsburgh Medical Center, recommended cotesting, claiming that primary HPV testing is significantly less likely to detect cervical precancers or cervical cancer than cotesting.
“These data come from parties with a vested interest in preserving cytology as a screening test,” Saslow told Medscape Medical News. She noted that “these findings are not at all credible as judged by the scientific community.”
On the basis of their own modeling, ACS researchers estimate that “starting with primary HPV testing at age 25 will prevent 13% more cervical cancers and 7% more cervical cancer deaths” in comparison with cytology (Pap testing alone) beginning at the age of 21, then cotesting at the age of 30, Saslow said in a statement.
“Our model showed we could do that with a 9% increase in follow-up procedures but with 45% fewer tests required overall,” she added.
The new recommendations are not expected to create any change in the type or amount of care required by providers, and patients will not notice any difference, inasmuch as cotesting and primary HPV testing are performed the same way in the examination room, she added.
“Resistance [to the changes] is expected – and is already occurring – from laboratories and manufacturers of tests that will no longer be used once we transition from cotesting and, less commonly, Pap testing to primary HPV testing,” Saslow said.
However, providers need to be aware that HPV infection, as with any sexually transmitted disease, is associated with a certain stigma, and they need to take care in discussing potential HPV infection with their patients.
Good method
Medscape Medical News approached Mark Einstein, MD, president of the ASCCP and professor and chair of obstetrics, gynecology, and reproductive health at Rutgers Biomedical and Health Sciences, Newark, New Jersey, to comment on the new guidelines.
“First and foremost,” he said, “everything we want to do when it comes to screening is to maximize the identification of picking up a cancer and minimize the risk or potential harm of not only screening itself but of missing cancers, so any strategy that improves on the sensitivity of picking up a cancer is a good method.”
Nevertheless, inasmuch as the ASCCP is one of the foremost organizations involved in cervical cancer screening and management, its members need more time to take a closer look at the updated ACS guidelines before they, together with sister organizations, such as the ACOG, release an official statement as to whether or not they fully endorse the new guidelines.
The United States Preventive Services Task Force recently endorsed primary HPV testing (starting at age 30), but it also said that an alternative strategy is cotesting for women between 30 and 65 years of age, Einstein observed.
Asked to comment on the article from Quest Diagnostics and the University of Pittsburgh that recommended cotesting instead of primary HPV testing, Einstein said that suggestion should not be dismissed out of hand.
The ASCCP has asked the authors of that study for their data in order conduct an independent assessment of it, largely because the study was retrospective in nature. Because of that, “there may have been a few pieces of information that were missing in true real-time fashion,” he said. “Not having [both the primary HPV testing and the cytology results] in front of me might change the next thing I might recommend to the patient,” Einstein explained.
The bottom line is that, when comparing primary HPV testing alone, cytology alone, and cotesting and rates of cervical cancer at 5 years, “the biggest driver for true performance of positive predictive value is HPV,” Einstein said.
Nevertheless, cotesting does bring more information into the equation compared with primary HPV testing alone, although it also increases the potential for harm, including the harm of overtesting and conducting needless colposcopies, he added.
That said, starting primary HPV testing at the age of 25 rather than the age of 30, as was previously recommended, is very likely to lead to detection of spurious HPV infections because HPV infections are very common among women in their 20s, Einstein pointed out.
“This, too, could potentially lead to more colposcopies, which may cause harm from the procedure itself but also create a certain amount of anxiety and concern, so there is some harm in testing for HPV at an earlier age as well,” Einstein said.
Saslow and Einstein have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening. The key recommendation is that primary human papillomavirus (HPV) testing is the preferred screening method, starting at the age of 25 and repeated every 5 years.
In the past, guidelines for cervical cancer screening recommended cytology (the Pap test) starting at 21 years of age and repeated every 3 years. In more recent years, cotesting (with both Pap and HPV tests) has been recommended.
Since the last ACS guidelines on cervical cancer screening were published in 2012, two HPV tests have been approved by the Food and Drug Administration (FDA) for use in primary HPV screening.
The new “streamlined recommendations can improve compliance and reduce potential harms,” commented Debbie Saslow, PhD, managing director, HPV/GYN Cancers, American Cancer Society.
The updated guidelines were published online July 30 in CA: A Cancer Journal for Clinicians.
“We now have stronger evidence to support starting cervical cancer screening at a later age and to recommend screening with the HPV test as the preferred test,” Saslow told Medscape Medical News. This also reflects the phasing out of cytology and cotesting, she added.
“This update is based on decades of studies comparing the effectiveness of HPV testing to cytology and is bolstered by evidence of the impact of HPV vaccination, including a dramatic decline in cervical precancers and, more recently, cervical cancers among young women,” she said.
The American Society for Colposcopy and Cervical Pathology (ASCCP) said that it was preparing a response to these new guidelines, as is the American College of Obstetricians and Gynecologists (ACOG).
Cotesting or cytology alone
The updated guidelines recommend primary HPV testing as the preferred screening method for all women with a cervix. If primary HPV testing is not available, women should be screened with cotesting, which should also be performed every 5 years.
If only cytology is available, then women should be screened every 3 years.
The ACS authors point out that cotesting or cytology testing alone is still an acceptable option for cervical cancer screening, insofar as primary HPV testing using FDA-approved tests may not be available in some settings.
As more laboratories in the United States transition to FDA-approved tests for primary HPV testing, it is expected that the use of cotesting or cytology alone will be phased out.
The new guidelines also emphasize that women may discontinue screening at the age of 65 if they have not had cervical intraepitheal neoplasia of grade 2 or higher within the past 25 years and if they have tested negative over the past 10 years on all past screens.
The authors caution that past screens should only be considered negative if the patient has had two consecutive negative HPV tests or two consecutive negative cotests or three consecutive negative cytology tests within the past 10 years.
“These criteria do not apply to individuals who are currently under surveillance for abnormal screening results,” the authors state.
Women older than 65 for whom adequate documentation of prior screening is not available should continue to be screened until criteria for screening discontinuation are met, they add.
Screening may be discontinued among women with a limited life expectancy.
HPV vaccination
The authors note that HPV vaccination is expected to substantially change cervical cancer screening strategies.
In 2018, the National Immunization Survey–Teen, involving adolescents aged 13 to 17 years, showed that 68.1% of female patients were up to date on HPV vaccine recommendations, as were 51.1% of male patients.
“Cytology-based screening is much less efficient in vaccinated populations, as abnormal cytology disproportionately identifies minor abnormalities resulting from HPV types that are associated with lower cancer risk,” the reports’ authors point out.
As the prevalence of high-grade cervical abnormalities and the incidence of cervical cancer continue to decline, “the proportion of false-positive findings [on cytology alone] is expected to increase significantly,” they caution.
As a result, the ACS suggests that physicians will likely have to consider a patient’s vaccination status in tandem with cervical cancer screening results to arrive at an accurate assessment.
Raising starting age to 25 years
Saslow also noted that there were several reasons why it is now recommended that screening begin at the age of 25 instead of the age of 21, as in earlier guidelines.
“Firstly, less than 1% of cervical cancers are diagnosed before the age of 25 – so this is about 130 cases per year,” she explained.
Thanks to HPV vaccination, this percentage is further declining, “so screening is just not beneficial at this age,” Saslow emphasized.
Furthermore, the rate of false positives is much higher in younger patients, and a false-positive result can have a negative impact on pregnancy outcomes, she added.
Saslow also dismissed an article in favor of cotesting instead of HPV testing alone. That study, carried out by researchers at Quest Diagnostics and the University of Pittsburgh Medical Center, recommended cotesting, claiming that primary HPV testing is significantly less likely to detect cervical precancers or cervical cancer than cotesting.
“These data come from parties with a vested interest in preserving cytology as a screening test,” Saslow told Medscape Medical News. She noted that “these findings are not at all credible as judged by the scientific community.”
On the basis of their own modeling, ACS researchers estimate that “starting with primary HPV testing at age 25 will prevent 13% more cervical cancers and 7% more cervical cancer deaths” in comparison with cytology (Pap testing alone) beginning at the age of 21, then cotesting at the age of 30, Saslow said in a statement.
“Our model showed we could do that with a 9% increase in follow-up procedures but with 45% fewer tests required overall,” she added.
The new recommendations are not expected to create any change in the type or amount of care required by providers, and patients will not notice any difference, inasmuch as cotesting and primary HPV testing are performed the same way in the examination room, she added.
“Resistance [to the changes] is expected – and is already occurring – from laboratories and manufacturers of tests that will no longer be used once we transition from cotesting and, less commonly, Pap testing to primary HPV testing,” Saslow said.
However, providers need to be aware that HPV infection, as with any sexually transmitted disease, is associated with a certain stigma, and they need to take care in discussing potential HPV infection with their patients.
Good method
Medscape Medical News approached Mark Einstein, MD, president of the ASCCP and professor and chair of obstetrics, gynecology, and reproductive health at Rutgers Biomedical and Health Sciences, Newark, New Jersey, to comment on the new guidelines.
“First and foremost,” he said, “everything we want to do when it comes to screening is to maximize the identification of picking up a cancer and minimize the risk or potential harm of not only screening itself but of missing cancers, so any strategy that improves on the sensitivity of picking up a cancer is a good method.”
Nevertheless, inasmuch as the ASCCP is one of the foremost organizations involved in cervical cancer screening and management, its members need more time to take a closer look at the updated ACS guidelines before they, together with sister organizations, such as the ACOG, release an official statement as to whether or not they fully endorse the new guidelines.
The United States Preventive Services Task Force recently endorsed primary HPV testing (starting at age 30), but it also said that an alternative strategy is cotesting for women between 30 and 65 years of age, Einstein observed.
Asked to comment on the article from Quest Diagnostics and the University of Pittsburgh that recommended cotesting instead of primary HPV testing, Einstein said that suggestion should not be dismissed out of hand.
The ASCCP has asked the authors of that study for their data in order conduct an independent assessment of it, largely because the study was retrospective in nature. Because of that, “there may have been a few pieces of information that were missing in true real-time fashion,” he said. “Not having [both the primary HPV testing and the cytology results] in front of me might change the next thing I might recommend to the patient,” Einstein explained.
The bottom line is that, when comparing primary HPV testing alone, cytology alone, and cotesting and rates of cervical cancer at 5 years, “the biggest driver for true performance of positive predictive value is HPV,” Einstein said.
Nevertheless, cotesting does bring more information into the equation compared with primary HPV testing alone, although it also increases the potential for harm, including the harm of overtesting and conducting needless colposcopies, he added.
That said, starting primary HPV testing at the age of 25 rather than the age of 30, as was previously recommended, is very likely to lead to detection of spurious HPV infections because HPV infections are very common among women in their 20s, Einstein pointed out.
“This, too, could potentially lead to more colposcopies, which may cause harm from the procedure itself but also create a certain amount of anxiety and concern, so there is some harm in testing for HPV at an earlier age as well,” Einstein said.
Saslow and Einstein have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fracture risk prediction: No benefit to repeat BMD testing in postmenopausal women
On the basis of the findings, published online in JAMA Internal Medicine, the authors recommend against routine repeat testing in postmenopausal women. Other experts, however, caution that the results may not be so broadly generalizable.
For the investigation, Carolyn J. Crandall, MD, of the division of general internal medicine and health services research at the University of California, Los Angeles, and colleagues analyzed data from 7,419 women enrolled in the prospective Women’s Health Initiative study and who underwent baseline and repeat dual-energy x-ray absorptiometry (DXA) between 1993 and 2010. The researchers excluded patients who reported using bisphosphonates, calcitonin, or selective estrogen-receptor modulators, those with a history of major osteoporotic fracture, or those who lacked follow-up visits. The mean body mass index (BMI) of the study population was 28.7 kg/m2, and the mean age was 66.1 years.
The mean follow-up after the repeat BMD test was 9.0 years, during which period 732 (9.9%) of the women experienced a major osteoporotic fracture, and 139 (1.9%) experienced hip fractures.
To determine whether repeat testing improved fracture risk discrimination, the researchers calculated area under the receiver operating characteristic curve (AUROC) for baseline BMD, absolute change in BMD, and the combination of baseline BMD and change in BMD.
With respect to any major osteoporotic fracture risk, the AUROC values for total hip BMD at baseline, change in total hip BMD at 3 years, and the combination of the two, respectively, were 0.61 (95% confidence interval, 0.59-0.63), 0.53 (95% CI, 0.51-0.55), and 0.61 (95% CI, 0.59-0.63). For hip fracture risk, the respective AUROC values were 0.71 (95% CI, 0.67-0.75), 0.61 (95% CI, 0.56-0.65), and 0.73 (95% CI, 0.69-0.77), the authors reported.
Similar results were observed for femoral neck and lumbar spine BMD measurements. The associations between BMD changes and fracture risk were consistent across age, race, ethnicity, BMI, and baseline BMD T-score subgroups.
Although baseline BMD and change in BMD were independently associated with incident fracture, the association was stronger for lower baseline BMD than the 3-year absolute change in BMD, the authors stated.
The findings, which are consistent with those of previous investigations that involved older adults, are notable because of the age range of the population, according to the authors. “To our knowledge, this is the first prospective study that addressed this issue in a study cohort that included younger postmenopausal U.S. women,” they wrote. “Forty-four percent of our study population was younger than 65 years.”
The authors wrote that, given the lack of benefit associated with repeat BMD testing, such tests should no longer be routinely performed. “Our findings further suggest that resources should be devoted to increasing the underuse of baseline BMD testing among women aged [between] 65 and 85 years, one-quarter of whom do not receive an initial BMD test.”
However, some experts are not comfortable with the broad recommendation to skip repeat testing in the general population. “This is a great study, and it gives important information. However, we know, even in the real world, that patients can lose BMD in this time frame and not really fracture. This does not mean that they will not fracture further down the road,” said Pauline Camacho, MD, director of Loyola University Medical Center’s Osteoporosis and Metabolic Bone Disease Center in Chicago,. “The value of doing BMD goes beyond predicting fracture risk. It also helps assess patient compliance and detect the presence of uncorrected secondary causes of osteoporosis that are limiting the response to therapy, including failure to absorb oral bisphosphonates, vitamin D deficiency, or hyperparathyroidism.”
In addition, patients for whom treatment is initiated would want to know whether it’s working. “Seeing the BMD response to therapy is helpful to both clinicians and patients,” Dr. Camacho said in an interview.
Another concern is the study population. “The study was designed to assess the clinical utility of repeating a screening BMD test in a population of low-risk women -- older postmenopausal women with remarkably good BMD on initial testing,” according to E. Michael Lewiecki, MD, vice president of the National Osteoporosis Foundation and director of the New Mexico Clinical Research and Osteoporosis Center in Albuquerque. “Not surprisingly, with what we know about the expected age-related rate of bone loss, there was only a modest decrease in BMD and little clinical utility in repeating DXA in 3 years. However, repeat testing is an important component in the care of many patients seen in clinical practice.”
There are numerous situations in clinical practice in which repeat BMD testing can enhance patient care and potentially improve outcomes, Dr. Lewiecki said in an interview. “Repeating BMD 1-2 years after starting osteoporosis therapy is a useful way to assess response and determine whether the patient is on a pathway to achieving an acceptable level of fracture risk with a strategy called treat to target.”
Additionally, patients starting high-dose glucocorticoids who are at high risk for rapid bone loss may benefit from undergoing baseline BMD testing and having a follow-up test 1 year later or even sooner, he said. Further, for early postmenopausal women, the rate of bone loss may be accelerated and may be faster than age-related bone loss later in life. For this reason, “close monitoring of BMD may be used to determine when a treatment threshold has been crossed and pharmacological therapy is indicated.”
The most important message from this study for clinicians and healthcare policymakers is not the relative value of the repeat BMD testing, Dr. Lewiecki stated. Rather, it is the call to action regarding the underuse of BMD testing. “There is a global crisis in the care of osteoporosis that is characterized by underdiagnosis and undertreatment of patients at risk for fracture. Many patients who could benefit from treatment to reduce fracture risk are not receiving it, resulting in disability and deaths from fractures that might have been prevented. We need more bone density testing in appropriately selected patients to identify high-risk patients and intervene to reduce fracture risk,” he said. “DXA is an inexpensive and highly versatile clinical tool with many applications in clinical practice. When used wisely, it can be extraordinarily useful to identify and monitor high-risk patients, with the goal of reducing the burden of osteoporotic fractures.”
The barriers to performing baseline BMD measurement in this population are poorly understood and not well researched, Dr. Crandall said in an interview. “I expect that they relate to the multiple competing demands on primary care physicians, who are, for example, trying to juggle hypertension, a sprained ankle, diabetes, and complex social situations simultaneously with identifying appropriate candidates for osteoporosis screening and considering numerous other screening guidelines.”
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The study authors reported relationships with multiple companies, including Amgen, Pfizer, Bayer, Mithra, Norton Rose Fulbright, TherapeuticsMD, AbbVie, Radius, and Allergan. Dr. Camacho reported relationships with Amgen and Shire. Dr. Lewiecki reported relationships with Amgen, Radius Health, Alexion, Samsung Bioepis, Sandoz, Mereo, and Bindex.
A version of this article originally appeared on Medscape.com.
On the basis of the findings, published online in JAMA Internal Medicine, the authors recommend against routine repeat testing in postmenopausal women. Other experts, however, caution that the results may not be so broadly generalizable.
For the investigation, Carolyn J. Crandall, MD, of the division of general internal medicine and health services research at the University of California, Los Angeles, and colleagues analyzed data from 7,419 women enrolled in the prospective Women’s Health Initiative study and who underwent baseline and repeat dual-energy x-ray absorptiometry (DXA) between 1993 and 2010. The researchers excluded patients who reported using bisphosphonates, calcitonin, or selective estrogen-receptor modulators, those with a history of major osteoporotic fracture, or those who lacked follow-up visits. The mean body mass index (BMI) of the study population was 28.7 kg/m2, and the mean age was 66.1 years.
The mean follow-up after the repeat BMD test was 9.0 years, during which period 732 (9.9%) of the women experienced a major osteoporotic fracture, and 139 (1.9%) experienced hip fractures.
To determine whether repeat testing improved fracture risk discrimination, the researchers calculated area under the receiver operating characteristic curve (AUROC) for baseline BMD, absolute change in BMD, and the combination of baseline BMD and change in BMD.
With respect to any major osteoporotic fracture risk, the AUROC values for total hip BMD at baseline, change in total hip BMD at 3 years, and the combination of the two, respectively, were 0.61 (95% confidence interval, 0.59-0.63), 0.53 (95% CI, 0.51-0.55), and 0.61 (95% CI, 0.59-0.63). For hip fracture risk, the respective AUROC values were 0.71 (95% CI, 0.67-0.75), 0.61 (95% CI, 0.56-0.65), and 0.73 (95% CI, 0.69-0.77), the authors reported.
Similar results were observed for femoral neck and lumbar spine BMD measurements. The associations between BMD changes and fracture risk were consistent across age, race, ethnicity, BMI, and baseline BMD T-score subgroups.
Although baseline BMD and change in BMD were independently associated with incident fracture, the association was stronger for lower baseline BMD than the 3-year absolute change in BMD, the authors stated.
The findings, which are consistent with those of previous investigations that involved older adults, are notable because of the age range of the population, according to the authors. “To our knowledge, this is the first prospective study that addressed this issue in a study cohort that included younger postmenopausal U.S. women,” they wrote. “Forty-four percent of our study population was younger than 65 years.”
The authors wrote that, given the lack of benefit associated with repeat BMD testing, such tests should no longer be routinely performed. “Our findings further suggest that resources should be devoted to increasing the underuse of baseline BMD testing among women aged [between] 65 and 85 years, one-quarter of whom do not receive an initial BMD test.”
However, some experts are not comfortable with the broad recommendation to skip repeat testing in the general population. “This is a great study, and it gives important information. However, we know, even in the real world, that patients can lose BMD in this time frame and not really fracture. This does not mean that they will not fracture further down the road,” said Pauline Camacho, MD, director of Loyola University Medical Center’s Osteoporosis and Metabolic Bone Disease Center in Chicago,. “The value of doing BMD goes beyond predicting fracture risk. It also helps assess patient compliance and detect the presence of uncorrected secondary causes of osteoporosis that are limiting the response to therapy, including failure to absorb oral bisphosphonates, vitamin D deficiency, or hyperparathyroidism.”
In addition, patients for whom treatment is initiated would want to know whether it’s working. “Seeing the BMD response to therapy is helpful to both clinicians and patients,” Dr. Camacho said in an interview.
Another concern is the study population. “The study was designed to assess the clinical utility of repeating a screening BMD test in a population of low-risk women -- older postmenopausal women with remarkably good BMD on initial testing,” according to E. Michael Lewiecki, MD, vice president of the National Osteoporosis Foundation and director of the New Mexico Clinical Research and Osteoporosis Center in Albuquerque. “Not surprisingly, with what we know about the expected age-related rate of bone loss, there was only a modest decrease in BMD and little clinical utility in repeating DXA in 3 years. However, repeat testing is an important component in the care of many patients seen in clinical practice.”
There are numerous situations in clinical practice in which repeat BMD testing can enhance patient care and potentially improve outcomes, Dr. Lewiecki said in an interview. “Repeating BMD 1-2 years after starting osteoporosis therapy is a useful way to assess response and determine whether the patient is on a pathway to achieving an acceptable level of fracture risk with a strategy called treat to target.”
Additionally, patients starting high-dose glucocorticoids who are at high risk for rapid bone loss may benefit from undergoing baseline BMD testing and having a follow-up test 1 year later or even sooner, he said. Further, for early postmenopausal women, the rate of bone loss may be accelerated and may be faster than age-related bone loss later in life. For this reason, “close monitoring of BMD may be used to determine when a treatment threshold has been crossed and pharmacological therapy is indicated.”
The most important message from this study for clinicians and healthcare policymakers is not the relative value of the repeat BMD testing, Dr. Lewiecki stated. Rather, it is the call to action regarding the underuse of BMD testing. “There is a global crisis in the care of osteoporosis that is characterized by underdiagnosis and undertreatment of patients at risk for fracture. Many patients who could benefit from treatment to reduce fracture risk are not receiving it, resulting in disability and deaths from fractures that might have been prevented. We need more bone density testing in appropriately selected patients to identify high-risk patients and intervene to reduce fracture risk,” he said. “DXA is an inexpensive and highly versatile clinical tool with many applications in clinical practice. When used wisely, it can be extraordinarily useful to identify and monitor high-risk patients, with the goal of reducing the burden of osteoporotic fractures.”
The barriers to performing baseline BMD measurement in this population are poorly understood and not well researched, Dr. Crandall said in an interview. “I expect that they relate to the multiple competing demands on primary care physicians, who are, for example, trying to juggle hypertension, a sprained ankle, diabetes, and complex social situations simultaneously with identifying appropriate candidates for osteoporosis screening and considering numerous other screening guidelines.”
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The study authors reported relationships with multiple companies, including Amgen, Pfizer, Bayer, Mithra, Norton Rose Fulbright, TherapeuticsMD, AbbVie, Radius, and Allergan. Dr. Camacho reported relationships with Amgen and Shire. Dr. Lewiecki reported relationships with Amgen, Radius Health, Alexion, Samsung Bioepis, Sandoz, Mereo, and Bindex.
A version of this article originally appeared on Medscape.com.
On the basis of the findings, published online in JAMA Internal Medicine, the authors recommend against routine repeat testing in postmenopausal women. Other experts, however, caution that the results may not be so broadly generalizable.
For the investigation, Carolyn J. Crandall, MD, of the division of general internal medicine and health services research at the University of California, Los Angeles, and colleagues analyzed data from 7,419 women enrolled in the prospective Women’s Health Initiative study and who underwent baseline and repeat dual-energy x-ray absorptiometry (DXA) between 1993 and 2010. The researchers excluded patients who reported using bisphosphonates, calcitonin, or selective estrogen-receptor modulators, those with a history of major osteoporotic fracture, or those who lacked follow-up visits. The mean body mass index (BMI) of the study population was 28.7 kg/m2, and the mean age was 66.1 years.
The mean follow-up after the repeat BMD test was 9.0 years, during which period 732 (9.9%) of the women experienced a major osteoporotic fracture, and 139 (1.9%) experienced hip fractures.
To determine whether repeat testing improved fracture risk discrimination, the researchers calculated area under the receiver operating characteristic curve (AUROC) for baseline BMD, absolute change in BMD, and the combination of baseline BMD and change in BMD.
With respect to any major osteoporotic fracture risk, the AUROC values for total hip BMD at baseline, change in total hip BMD at 3 years, and the combination of the two, respectively, were 0.61 (95% confidence interval, 0.59-0.63), 0.53 (95% CI, 0.51-0.55), and 0.61 (95% CI, 0.59-0.63). For hip fracture risk, the respective AUROC values were 0.71 (95% CI, 0.67-0.75), 0.61 (95% CI, 0.56-0.65), and 0.73 (95% CI, 0.69-0.77), the authors reported.
Similar results were observed for femoral neck and lumbar spine BMD measurements. The associations between BMD changes and fracture risk were consistent across age, race, ethnicity, BMI, and baseline BMD T-score subgroups.
Although baseline BMD and change in BMD were independently associated with incident fracture, the association was stronger for lower baseline BMD than the 3-year absolute change in BMD, the authors stated.
The findings, which are consistent with those of previous investigations that involved older adults, are notable because of the age range of the population, according to the authors. “To our knowledge, this is the first prospective study that addressed this issue in a study cohort that included younger postmenopausal U.S. women,” they wrote. “Forty-four percent of our study population was younger than 65 years.”
The authors wrote that, given the lack of benefit associated with repeat BMD testing, such tests should no longer be routinely performed. “Our findings further suggest that resources should be devoted to increasing the underuse of baseline BMD testing among women aged [between] 65 and 85 years, one-quarter of whom do not receive an initial BMD test.”
However, some experts are not comfortable with the broad recommendation to skip repeat testing in the general population. “This is a great study, and it gives important information. However, we know, even in the real world, that patients can lose BMD in this time frame and not really fracture. This does not mean that they will not fracture further down the road,” said Pauline Camacho, MD, director of Loyola University Medical Center’s Osteoporosis and Metabolic Bone Disease Center in Chicago,. “The value of doing BMD goes beyond predicting fracture risk. It also helps assess patient compliance and detect the presence of uncorrected secondary causes of osteoporosis that are limiting the response to therapy, including failure to absorb oral bisphosphonates, vitamin D deficiency, or hyperparathyroidism.”
In addition, patients for whom treatment is initiated would want to know whether it’s working. “Seeing the BMD response to therapy is helpful to both clinicians and patients,” Dr. Camacho said in an interview.
Another concern is the study population. “The study was designed to assess the clinical utility of repeating a screening BMD test in a population of low-risk women -- older postmenopausal women with remarkably good BMD on initial testing,” according to E. Michael Lewiecki, MD, vice president of the National Osteoporosis Foundation and director of the New Mexico Clinical Research and Osteoporosis Center in Albuquerque. “Not surprisingly, with what we know about the expected age-related rate of bone loss, there was only a modest decrease in BMD and little clinical utility in repeating DXA in 3 years. However, repeat testing is an important component in the care of many patients seen in clinical practice.”
There are numerous situations in clinical practice in which repeat BMD testing can enhance patient care and potentially improve outcomes, Dr. Lewiecki said in an interview. “Repeating BMD 1-2 years after starting osteoporosis therapy is a useful way to assess response and determine whether the patient is on a pathway to achieving an acceptable level of fracture risk with a strategy called treat to target.”
Additionally, patients starting high-dose glucocorticoids who are at high risk for rapid bone loss may benefit from undergoing baseline BMD testing and having a follow-up test 1 year later or even sooner, he said. Further, for early postmenopausal women, the rate of bone loss may be accelerated and may be faster than age-related bone loss later in life. For this reason, “close monitoring of BMD may be used to determine when a treatment threshold has been crossed and pharmacological therapy is indicated.”
The most important message from this study for clinicians and healthcare policymakers is not the relative value of the repeat BMD testing, Dr. Lewiecki stated. Rather, it is the call to action regarding the underuse of BMD testing. “There is a global crisis in the care of osteoporosis that is characterized by underdiagnosis and undertreatment of patients at risk for fracture. Many patients who could benefit from treatment to reduce fracture risk are not receiving it, resulting in disability and deaths from fractures that might have been prevented. We need more bone density testing in appropriately selected patients to identify high-risk patients and intervene to reduce fracture risk,” he said. “DXA is an inexpensive and highly versatile clinical tool with many applications in clinical practice. When used wisely, it can be extraordinarily useful to identify and monitor high-risk patients, with the goal of reducing the burden of osteoporotic fractures.”
The barriers to performing baseline BMD measurement in this population are poorly understood and not well researched, Dr. Crandall said in an interview. “I expect that they relate to the multiple competing demands on primary care physicians, who are, for example, trying to juggle hypertension, a sprained ankle, diabetes, and complex social situations simultaneously with identifying appropriate candidates for osteoporosis screening and considering numerous other screening guidelines.”
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The study authors reported relationships with multiple companies, including Amgen, Pfizer, Bayer, Mithra, Norton Rose Fulbright, TherapeuticsMD, AbbVie, Radius, and Allergan. Dr. Camacho reported relationships with Amgen and Shire. Dr. Lewiecki reported relationships with Amgen, Radius Health, Alexion, Samsung Bioepis, Sandoz, Mereo, and Bindex.
A version of this article originally appeared on Medscape.com.
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY
AAP report aims to educate providers on female genital mutilation/cutting
Although female genital mutilation or cutting (FGM/C) is outlawed in much of the world, it still occurs for cultural reasons despite having no medical benefit, according to a clinical report from the American Academy of Pediatrics.
FGM/C is mainly performed on children and adolescents, but most of the research and teaching to date has addressed the impact of FGM/C on women of childbearing age and management during pregnancy and post partum, wrote Janine Young, MD, of the University of Colorado Denver in Aurora and colleagues. They are members of the AAP section on global health, committee on medical liability and risk management, or the committee on bioethics.
Published in Pediatrics, the report provides “the first comprehensive summary of FGM/C in children and includes education regarding a standard-of-care approach for examination of external female genitalia at all health supervision examinations, diagnosis, complications, management, treatment, culturally sensitive discussion and counseling approaches, and legal and ethical considerations,” they wrote.
The World Health Organization categorizes FGM/C into four subtypes. “Type I includes cutting of the glans or part of the body of the clitoris and/or prepuce; type II includes excision of the clitoris and labia minora, with or without excision of the labia majora; type III, infibulation, includes cutting and apposing the labia minora and/or majora over the urethral meatus and vaginal opening to significantly narrow it and may include clitoral excision; and type IV includes piercing, scraping, nicking, stretching, or otherwise injuring the external female genitalia without removing any genital tissue and includes practices that do not fall into the other three categories,” the authors wrote. Of these, type III is associated with the greatest long-term morbidity.
Data suggest that the prevalence and type of FGM/C varies by region, with the highest prevalence of type III in East Africa, where 82%-99% of girls reported FGM/C and 34%-79% of these cases involved type III, the authors reported.
Generally, pediatric health care providers in the United States have limited knowledge of FGM/C in the absence of any required courses on diagnosis or treatment for most primary care specialties. However, clinicians should be aware of possible risk factors, including a mother or sibling with a history of FGM/C, or patients with a country of origin, birth country, or travel history to a country where FGM/C is practiced, Dr. Young and associates noted.
They recommend that but acknowledged the challenges in raising the topic and addressing it in a culturally sensitive way. “Experts suggest that health care providers ask the patient or parent the term they use to name female genital cutting” and avoid the term mutilation, which may be offensive or misunderstood.
Many girls who have undergone FGM/C were too young to remember, the authors note. “Instead, it is advisable that the FGM/C clinical history taking include both the girl and parent or guardian once rapport has been established.”
Review potential medical complications if FGM/C is identified, and plans should be made for follow-up visits to monitor development of complications, the authors said. In addition, engage in a culturally sensitive discussion with teenagers, who may or may not have known about their FGM/C. In some cases, parents and caregivers may not have known about the FGM/C, which may be a community practice in some cultures with decisions made by other family members or authority figures.
“It is important for health care providers to assess each patient individually and make no assumptions about her and her parents’ beliefs regarding FGM/C,” Dr. Young and associates emphasized. “Mothers and fathers may or may not hold discordant views about FGM/C, and some clinical experts suggest that mothers who have themselves undergone FGM/C may nonetheless oppose subjecting their daughters to this practice. Instead, treating patients and caregivers with respect, sensitivity, and professionalism will encourage them to return and supports health-seeking behavior.”
The report presents 11 specific recommendations, including that health care providers should not perform any type of FGM/C and actively counsel families against such practices. In addition, children should have external genitalia checked at all health supervision examinations (with the consent of the guardian and/or child), and an assessment for FGM/C should be documented in the health records of patients with risk factors.
Notably, “[i]f genital examination findings are equivocal for the presence of FGM/C and risk factors for FGM/ C are present, a specialist trained in identification of FGM/C should be consulted,” Dr. Young and associates recommended. They also recommended defibulation for all girls and teenagers with type III FGM/C, especially for those with complications, and the procedure should be performed by an experienced pediatric gynecologist, gynecologist, urologist, or urogynecologist.
Finally, “[i]f FGM/C is suspected to have occurred in the United States, or as vacation cutting after immigration to the United States, the child should be evaluated for potential abuse. ... Expressed intention to engage in FGM/C, either in the United States or abroad, should also prompt a report to CPS [child protective services] if the child’s parent or caregiver cannot be dissuaded,” the authors wrote.
The report also includes case examples and expert analyses from legal and medical ethics experts to provide additional guidance for clinicians.
“This work seeks to educate pediatric health care providers on the occurrence of FGM/C, and the broader applications to the patients/population it impacts as well as the intersecting issues of diagnosis, complications, treatment, counseling needs, and the ethical and legal implications,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
However, challenges in implementing the recommendations “relate to the complexity of the issue and also the need for greater education of primary providers,” Dr. Jay said. “The overall message for providers, I believe, is a greater understanding of the practice [of FGM/C] as most providers have limited knowledge of this practice in the United States.”
“I believe the case-based presentations allow for a better understanding of how best to approach patients and families,” she added.
Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said, “I think one of largest barriers to implementing the strategies [from] this report is the limited knowledge of FGM/C by most clinicians.”
“In general, many pediatricians are uncomfortable with genital examinations,” she said in an interview. “I suspect most feel uncomfortable with identifying FGM/C versus other genital pathology and may not have ready access to FGM/C experts. Additionally, having these difficult conversations with families about this sensitive topic may be challenging,” said Dr. Curran. “Fortunately, this report is incredibly comprehensive, providing extensive background into FGM/C, effectively using diagrams and pictures, and explaining the legal and ethical issues that arise in the care of these patients.”
“Ultimately, I think there will need to be more education within medical training and further research into FGM/C,” Dr. Curran added. “Clinicians should be knowledgeable about FGM/C, including prevalence, identification, health complications, and treatment, as well as legal and ethical implications.” However, “in addition to knowledge, clinicians must be able to navigate counseling patients and their families around this culturally sensitive topic.”
The report is thorough and well written, yet “there still remains significant gaps in knowledge about FGM/C in children and adolescents,” she said. “I think future research into prevalence, along with the health effects of FGM/C, including its impact on mental and sexual health, in the pediatric population will be essential.”
The study received no outside funding. Coauthor Christa Johnson-Agbakwu, MD, disclosed a grant relationship with Arizona State University from the 2018 copyright of “Female Genital Mutilation/Cutting (FGM/C): A Visual Reference and Learning Tool for Health Care Professionals.” The other researchers had no financial conflicts to disclose. Dr. Jay and Dr. Curran had no relevant financial conflicts to disclose. They are members of the Pediatric News editorial advisory board.
SOURCE: Young J et al. Pediatrics. 2020 Jul 27. doi: 10.1542/peds.2020-1012.
Although female genital mutilation or cutting (FGM/C) is outlawed in much of the world, it still occurs for cultural reasons despite having no medical benefit, according to a clinical report from the American Academy of Pediatrics.
FGM/C is mainly performed on children and adolescents, but most of the research and teaching to date has addressed the impact of FGM/C on women of childbearing age and management during pregnancy and post partum, wrote Janine Young, MD, of the University of Colorado Denver in Aurora and colleagues. They are members of the AAP section on global health, committee on medical liability and risk management, or the committee on bioethics.
Published in Pediatrics, the report provides “the first comprehensive summary of FGM/C in children and includes education regarding a standard-of-care approach for examination of external female genitalia at all health supervision examinations, diagnosis, complications, management, treatment, culturally sensitive discussion and counseling approaches, and legal and ethical considerations,” they wrote.
The World Health Organization categorizes FGM/C into four subtypes. “Type I includes cutting of the glans or part of the body of the clitoris and/or prepuce; type II includes excision of the clitoris and labia minora, with or without excision of the labia majora; type III, infibulation, includes cutting and apposing the labia minora and/or majora over the urethral meatus and vaginal opening to significantly narrow it and may include clitoral excision; and type IV includes piercing, scraping, nicking, stretching, or otherwise injuring the external female genitalia without removing any genital tissue and includes practices that do not fall into the other three categories,” the authors wrote. Of these, type III is associated with the greatest long-term morbidity.
Data suggest that the prevalence and type of FGM/C varies by region, with the highest prevalence of type III in East Africa, where 82%-99% of girls reported FGM/C and 34%-79% of these cases involved type III, the authors reported.
Generally, pediatric health care providers in the United States have limited knowledge of FGM/C in the absence of any required courses on diagnosis or treatment for most primary care specialties. However, clinicians should be aware of possible risk factors, including a mother or sibling with a history of FGM/C, or patients with a country of origin, birth country, or travel history to a country where FGM/C is practiced, Dr. Young and associates noted.
They recommend that but acknowledged the challenges in raising the topic and addressing it in a culturally sensitive way. “Experts suggest that health care providers ask the patient or parent the term they use to name female genital cutting” and avoid the term mutilation, which may be offensive or misunderstood.
Many girls who have undergone FGM/C were too young to remember, the authors note. “Instead, it is advisable that the FGM/C clinical history taking include both the girl and parent or guardian once rapport has been established.”
Review potential medical complications if FGM/C is identified, and plans should be made for follow-up visits to monitor development of complications, the authors said. In addition, engage in a culturally sensitive discussion with teenagers, who may or may not have known about their FGM/C. In some cases, parents and caregivers may not have known about the FGM/C, which may be a community practice in some cultures with decisions made by other family members or authority figures.
“It is important for health care providers to assess each patient individually and make no assumptions about her and her parents’ beliefs regarding FGM/C,” Dr. Young and associates emphasized. “Mothers and fathers may or may not hold discordant views about FGM/C, and some clinical experts suggest that mothers who have themselves undergone FGM/C may nonetheless oppose subjecting their daughters to this practice. Instead, treating patients and caregivers with respect, sensitivity, and professionalism will encourage them to return and supports health-seeking behavior.”
The report presents 11 specific recommendations, including that health care providers should not perform any type of FGM/C and actively counsel families against such practices. In addition, children should have external genitalia checked at all health supervision examinations (with the consent of the guardian and/or child), and an assessment for FGM/C should be documented in the health records of patients with risk factors.
Notably, “[i]f genital examination findings are equivocal for the presence of FGM/C and risk factors for FGM/ C are present, a specialist trained in identification of FGM/C should be consulted,” Dr. Young and associates recommended. They also recommended defibulation for all girls and teenagers with type III FGM/C, especially for those with complications, and the procedure should be performed by an experienced pediatric gynecologist, gynecologist, urologist, or urogynecologist.
Finally, “[i]f FGM/C is suspected to have occurred in the United States, or as vacation cutting after immigration to the United States, the child should be evaluated for potential abuse. ... Expressed intention to engage in FGM/C, either in the United States or abroad, should also prompt a report to CPS [child protective services] if the child’s parent or caregiver cannot be dissuaded,” the authors wrote.
The report also includes case examples and expert analyses from legal and medical ethics experts to provide additional guidance for clinicians.
“This work seeks to educate pediatric health care providers on the occurrence of FGM/C, and the broader applications to the patients/population it impacts as well as the intersecting issues of diagnosis, complications, treatment, counseling needs, and the ethical and legal implications,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
However, challenges in implementing the recommendations “relate to the complexity of the issue and also the need for greater education of primary providers,” Dr. Jay said. “The overall message for providers, I believe, is a greater understanding of the practice [of FGM/C] as most providers have limited knowledge of this practice in the United States.”
“I believe the case-based presentations allow for a better understanding of how best to approach patients and families,” she added.
Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said, “I think one of largest barriers to implementing the strategies [from] this report is the limited knowledge of FGM/C by most clinicians.”
“In general, many pediatricians are uncomfortable with genital examinations,” she said in an interview. “I suspect most feel uncomfortable with identifying FGM/C versus other genital pathology and may not have ready access to FGM/C experts. Additionally, having these difficult conversations with families about this sensitive topic may be challenging,” said Dr. Curran. “Fortunately, this report is incredibly comprehensive, providing extensive background into FGM/C, effectively using diagrams and pictures, and explaining the legal and ethical issues that arise in the care of these patients.”
“Ultimately, I think there will need to be more education within medical training and further research into FGM/C,” Dr. Curran added. “Clinicians should be knowledgeable about FGM/C, including prevalence, identification, health complications, and treatment, as well as legal and ethical implications.” However, “in addition to knowledge, clinicians must be able to navigate counseling patients and their families around this culturally sensitive topic.”
The report is thorough and well written, yet “there still remains significant gaps in knowledge about FGM/C in children and adolescents,” she said. “I think future research into prevalence, along with the health effects of FGM/C, including its impact on mental and sexual health, in the pediatric population will be essential.”
The study received no outside funding. Coauthor Christa Johnson-Agbakwu, MD, disclosed a grant relationship with Arizona State University from the 2018 copyright of “Female Genital Mutilation/Cutting (FGM/C): A Visual Reference and Learning Tool for Health Care Professionals.” The other researchers had no financial conflicts to disclose. Dr. Jay and Dr. Curran had no relevant financial conflicts to disclose. They are members of the Pediatric News editorial advisory board.
SOURCE: Young J et al. Pediatrics. 2020 Jul 27. doi: 10.1542/peds.2020-1012.
Although female genital mutilation or cutting (FGM/C) is outlawed in much of the world, it still occurs for cultural reasons despite having no medical benefit, according to a clinical report from the American Academy of Pediatrics.
FGM/C is mainly performed on children and adolescents, but most of the research and teaching to date has addressed the impact of FGM/C on women of childbearing age and management during pregnancy and post partum, wrote Janine Young, MD, of the University of Colorado Denver in Aurora and colleagues. They are members of the AAP section on global health, committee on medical liability and risk management, or the committee on bioethics.
Published in Pediatrics, the report provides “the first comprehensive summary of FGM/C in children and includes education regarding a standard-of-care approach for examination of external female genitalia at all health supervision examinations, diagnosis, complications, management, treatment, culturally sensitive discussion and counseling approaches, and legal and ethical considerations,” they wrote.
The World Health Organization categorizes FGM/C into four subtypes. “Type I includes cutting of the glans or part of the body of the clitoris and/or prepuce; type II includes excision of the clitoris and labia minora, with or without excision of the labia majora; type III, infibulation, includes cutting and apposing the labia minora and/or majora over the urethral meatus and vaginal opening to significantly narrow it and may include clitoral excision; and type IV includes piercing, scraping, nicking, stretching, or otherwise injuring the external female genitalia without removing any genital tissue and includes practices that do not fall into the other three categories,” the authors wrote. Of these, type III is associated with the greatest long-term morbidity.
Data suggest that the prevalence and type of FGM/C varies by region, with the highest prevalence of type III in East Africa, where 82%-99% of girls reported FGM/C and 34%-79% of these cases involved type III, the authors reported.
Generally, pediatric health care providers in the United States have limited knowledge of FGM/C in the absence of any required courses on diagnosis or treatment for most primary care specialties. However, clinicians should be aware of possible risk factors, including a mother or sibling with a history of FGM/C, or patients with a country of origin, birth country, or travel history to a country where FGM/C is practiced, Dr. Young and associates noted.
They recommend that but acknowledged the challenges in raising the topic and addressing it in a culturally sensitive way. “Experts suggest that health care providers ask the patient or parent the term they use to name female genital cutting” and avoid the term mutilation, which may be offensive or misunderstood.
Many girls who have undergone FGM/C were too young to remember, the authors note. “Instead, it is advisable that the FGM/C clinical history taking include both the girl and parent or guardian once rapport has been established.”
Review potential medical complications if FGM/C is identified, and plans should be made for follow-up visits to monitor development of complications, the authors said. In addition, engage in a culturally sensitive discussion with teenagers, who may or may not have known about their FGM/C. In some cases, parents and caregivers may not have known about the FGM/C, which may be a community practice in some cultures with decisions made by other family members or authority figures.
“It is important for health care providers to assess each patient individually and make no assumptions about her and her parents’ beliefs regarding FGM/C,” Dr. Young and associates emphasized. “Mothers and fathers may or may not hold discordant views about FGM/C, and some clinical experts suggest that mothers who have themselves undergone FGM/C may nonetheless oppose subjecting their daughters to this practice. Instead, treating patients and caregivers with respect, sensitivity, and professionalism will encourage them to return and supports health-seeking behavior.”
The report presents 11 specific recommendations, including that health care providers should not perform any type of FGM/C and actively counsel families against such practices. In addition, children should have external genitalia checked at all health supervision examinations (with the consent of the guardian and/or child), and an assessment for FGM/C should be documented in the health records of patients with risk factors.
Notably, “[i]f genital examination findings are equivocal for the presence of FGM/C and risk factors for FGM/ C are present, a specialist trained in identification of FGM/C should be consulted,” Dr. Young and associates recommended. They also recommended defibulation for all girls and teenagers with type III FGM/C, especially for those with complications, and the procedure should be performed by an experienced pediatric gynecologist, gynecologist, urologist, or urogynecologist.
Finally, “[i]f FGM/C is suspected to have occurred in the United States, or as vacation cutting after immigration to the United States, the child should be evaluated for potential abuse. ... Expressed intention to engage in FGM/C, either in the United States or abroad, should also prompt a report to CPS [child protective services] if the child’s parent or caregiver cannot be dissuaded,” the authors wrote.
The report also includes case examples and expert analyses from legal and medical ethics experts to provide additional guidance for clinicians.
“This work seeks to educate pediatric health care providers on the occurrence of FGM/C, and the broader applications to the patients/population it impacts as well as the intersecting issues of diagnosis, complications, treatment, counseling needs, and the ethical and legal implications,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview.
However, challenges in implementing the recommendations “relate to the complexity of the issue and also the need for greater education of primary providers,” Dr. Jay said. “The overall message for providers, I believe, is a greater understanding of the practice [of FGM/C] as most providers have limited knowledge of this practice in the United States.”
“I believe the case-based presentations allow for a better understanding of how best to approach patients and families,” she added.
Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said, “I think one of largest barriers to implementing the strategies [from] this report is the limited knowledge of FGM/C by most clinicians.”
“In general, many pediatricians are uncomfortable with genital examinations,” she said in an interview. “I suspect most feel uncomfortable with identifying FGM/C versus other genital pathology and may not have ready access to FGM/C experts. Additionally, having these difficult conversations with families about this sensitive topic may be challenging,” said Dr. Curran. “Fortunately, this report is incredibly comprehensive, providing extensive background into FGM/C, effectively using diagrams and pictures, and explaining the legal and ethical issues that arise in the care of these patients.”
“Ultimately, I think there will need to be more education within medical training and further research into FGM/C,” Dr. Curran added. “Clinicians should be knowledgeable about FGM/C, including prevalence, identification, health complications, and treatment, as well as legal and ethical implications.” However, “in addition to knowledge, clinicians must be able to navigate counseling patients and their families around this culturally sensitive topic.”
The report is thorough and well written, yet “there still remains significant gaps in knowledge about FGM/C in children and adolescents,” she said. “I think future research into prevalence, along with the health effects of FGM/C, including its impact on mental and sexual health, in the pediatric population will be essential.”
The study received no outside funding. Coauthor Christa Johnson-Agbakwu, MD, disclosed a grant relationship with Arizona State University from the 2018 copyright of “Female Genital Mutilation/Cutting (FGM/C): A Visual Reference and Learning Tool for Health Care Professionals.” The other researchers had no financial conflicts to disclose. Dr. Jay and Dr. Curran had no relevant financial conflicts to disclose. They are members of the Pediatric News editorial advisory board.
SOURCE: Young J et al. Pediatrics. 2020 Jul 27. doi: 10.1542/peds.2020-1012.
FROM PEDIATRICS
Postmenopausal use of estrogen alone lowers breast cancer cases, deaths
A new follow-up study of menopausal hormone therapy found that prior use of conjugated equine estrogen (CEE) decreased both breast cancer incidence and mortality, while prior use of CEE plus medroxyprogesterone acetate (MPA) was associated with an increase in incidence.
“Prior use of CEE alone is, to our knowledge, the first pharmacologic intervention demonstrated to be associated with a statistically significantly reduction in deaths from breast cancer,” wrote Rowan T. Chlebowski, MD, PhD, of the Lundquist Institute for Biomedical Innovation in Torrance, Calif., and his coauthors. The study was published July 28 in JAMA.
To further investigate the outcomes of the Women’s Health Initiative in regard to hormone therapy and breast cancer risk, the researchers analyzed the long-term follow-up of two randomized trials that included 27,347 postmenopausal women with no prior breast cancer and negative mammograms at baseline. Their mean (SD) age was 63.4 (7.2) years. Enrollment took place from 1993 to 1998; participants were contacted for follow-up every 6 months through 2005 and annually from then on. Mortality data were gathered from follow-up and the National Death Index.
The first trial included 16,608 women with a uterus. Among these women, 8,506 received 0.625 mg/day of CEE plus 2.5 mg/day of MPA, and 8,102 received placebo. The second trial included 10,739 women who’d gotten a hysterectomy, 5,310 of whom received 0.625 mg/day of CEE alone and 5,429 of whom received placebo. The first trial ended in 2002 after a median intervention period of 5.6 years, and the second trial ended in 2004 after a period of 7.2 years.
An analysis in 2015 found that CEE alone was associated with lower risk of breast cancer and CEE plus MPA was associated with increased risk.
The current analysis confirmed that, after a median of 20.3 years of follow-up, and with mortality data now available for more than 98% of participants, CEE alone was associated with fewer cases of breast cancer (238 cases, annualized rate 0.30%), compared with placebo (296 cases, annualized rate 0.37%; hazard ratio 0.78; 95% confidence interval, 0.65-0.93; P = .005).
Furthermore, CEE alone was also associated with lower mortality (30 deaths, annualized mortality rate 0.031%), compared with placebo (46 deaths, annualized mortality rate 0.046%; HR 0.60; 95% CI, 0.37-0.97; P = .04).
By comparison, CEE plus MPA was linked with more cases of breast cancer (584 cases, annualized rate 0.45%) than placebo (447 cases, annualized rate 0.36%; HR 1.28; 95% CI, 1.13-1.45; P < .001). In regard to mortality, there was no statistically significant difference between CEE plus MPA (71 deaths, annualized mortality rate 0.045%) and placebo (53 deaths, annualized mortality rate 0.035%; HR 1.35; 95% CI, 0.94-1.95; P = .11).
“The big thing to think about is estrogen alone reducing breast cancer mortality by 40%,” said Dr. Chlebowski in an interview. “None of the other interventions, including tamoxifen, had any change on mortality. This should change the way we look at breast cancer prevention, though we might have to be a little creative about it. I think you have to be a little away from menopause for it to reduce breast cancer. But we wanted to start that debate.
“On the other hand,” he said, “a woman takes estrogen plus progestin and when you look at that curve, it’s staying about 25% increased. You take it for 5.6 years and the increase continues through 20 years, so you’re maybe buying a lifetime of increase in breast cancer by taking estrogen plus progestin for 5 years.”
He also highlighted the comprehensiveness of the mortality data, noting that “when you hook up to the National Death Index, they find 98% of all deaths in the United States. That’s really remarkable; you retain the whole power of the randomization. It means our data, between the death index and our follow-up of participants, is essentially complete.”
Use of hormone therapy, and decoding the outcomes, remains ‘complex’
Decades after the data were gathered from the Women’s Health Initiative clinical trials, they continue to assist researchers and patients alike, wrote Christina A. Minami, MD, of Brigham and Women’s Hospital in Boston and Rachel A. Freedman, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
That said, in regard to the findings of this latest analysis, “many questions still remain on whether (and how) a hormone therapy intervention that occurred many years earlier may continue to affect breast cancer risk and mortality at 20 years,” they wrote. They noted that it’s “impossible” to isolate how exposure to certain therapies can impact long-term outcomes, and that a high percentage of patients who discontinued the drugs during each trial muddy the waters even further.
“Decisions to initiate these medications remain complex,” they added, emphasizing that breast cancer risk is just one of many factors that physicians must consider when considering hormone therapy for their patients.
Dr. Chlebowski and his coauthors acknowledged their study’s limitations, including the use of very specifically administered and formulated dosages making their findings “not necessarily generalizable to other preparations.” In addition, they noted the significant percentage of patients – 54% with CEE alone and 42% with CEE plus MPA – who discontinued drug usage during their respective trials.
The Women’s Health Initiative is supported by the National Institutes of Health and the Department of Health and Human Services. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various government organizations, foundations, and pharmaceutical companies. The editorial’s authors reported no conflicts of interest.
SOURCE: Chlebowski RT et al. JAMA. 2020 Jul 28. doi: 10.1001/jama.2020.9482.
A new follow-up study of menopausal hormone therapy found that prior use of conjugated equine estrogen (CEE) decreased both breast cancer incidence and mortality, while prior use of CEE plus medroxyprogesterone acetate (MPA) was associated with an increase in incidence.
“Prior use of CEE alone is, to our knowledge, the first pharmacologic intervention demonstrated to be associated with a statistically significantly reduction in deaths from breast cancer,” wrote Rowan T. Chlebowski, MD, PhD, of the Lundquist Institute for Biomedical Innovation in Torrance, Calif., and his coauthors. The study was published July 28 in JAMA.
To further investigate the outcomes of the Women’s Health Initiative in regard to hormone therapy and breast cancer risk, the researchers analyzed the long-term follow-up of two randomized trials that included 27,347 postmenopausal women with no prior breast cancer and negative mammograms at baseline. Their mean (SD) age was 63.4 (7.2) years. Enrollment took place from 1993 to 1998; participants were contacted for follow-up every 6 months through 2005 and annually from then on. Mortality data were gathered from follow-up and the National Death Index.
The first trial included 16,608 women with a uterus. Among these women, 8,506 received 0.625 mg/day of CEE plus 2.5 mg/day of MPA, and 8,102 received placebo. The second trial included 10,739 women who’d gotten a hysterectomy, 5,310 of whom received 0.625 mg/day of CEE alone and 5,429 of whom received placebo. The first trial ended in 2002 after a median intervention period of 5.6 years, and the second trial ended in 2004 after a period of 7.2 years.
An analysis in 2015 found that CEE alone was associated with lower risk of breast cancer and CEE plus MPA was associated with increased risk.
The current analysis confirmed that, after a median of 20.3 years of follow-up, and with mortality data now available for more than 98% of participants, CEE alone was associated with fewer cases of breast cancer (238 cases, annualized rate 0.30%), compared with placebo (296 cases, annualized rate 0.37%; hazard ratio 0.78; 95% confidence interval, 0.65-0.93; P = .005).
Furthermore, CEE alone was also associated with lower mortality (30 deaths, annualized mortality rate 0.031%), compared with placebo (46 deaths, annualized mortality rate 0.046%; HR 0.60; 95% CI, 0.37-0.97; P = .04).
By comparison, CEE plus MPA was linked with more cases of breast cancer (584 cases, annualized rate 0.45%) than placebo (447 cases, annualized rate 0.36%; HR 1.28; 95% CI, 1.13-1.45; P < .001). In regard to mortality, there was no statistically significant difference between CEE plus MPA (71 deaths, annualized mortality rate 0.045%) and placebo (53 deaths, annualized mortality rate 0.035%; HR 1.35; 95% CI, 0.94-1.95; P = .11).
“The big thing to think about is estrogen alone reducing breast cancer mortality by 40%,” said Dr. Chlebowski in an interview. “None of the other interventions, including tamoxifen, had any change on mortality. This should change the way we look at breast cancer prevention, though we might have to be a little creative about it. I think you have to be a little away from menopause for it to reduce breast cancer. But we wanted to start that debate.
“On the other hand,” he said, “a woman takes estrogen plus progestin and when you look at that curve, it’s staying about 25% increased. You take it for 5.6 years and the increase continues through 20 years, so you’re maybe buying a lifetime of increase in breast cancer by taking estrogen plus progestin for 5 years.”
He also highlighted the comprehensiveness of the mortality data, noting that “when you hook up to the National Death Index, they find 98% of all deaths in the United States. That’s really remarkable; you retain the whole power of the randomization. It means our data, between the death index and our follow-up of participants, is essentially complete.”
Use of hormone therapy, and decoding the outcomes, remains ‘complex’
Decades after the data were gathered from the Women’s Health Initiative clinical trials, they continue to assist researchers and patients alike, wrote Christina A. Minami, MD, of Brigham and Women’s Hospital in Boston and Rachel A. Freedman, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
That said, in regard to the findings of this latest analysis, “many questions still remain on whether (and how) a hormone therapy intervention that occurred many years earlier may continue to affect breast cancer risk and mortality at 20 years,” they wrote. They noted that it’s “impossible” to isolate how exposure to certain therapies can impact long-term outcomes, and that a high percentage of patients who discontinued the drugs during each trial muddy the waters even further.
“Decisions to initiate these medications remain complex,” they added, emphasizing that breast cancer risk is just one of many factors that physicians must consider when considering hormone therapy for their patients.
Dr. Chlebowski and his coauthors acknowledged their study’s limitations, including the use of very specifically administered and formulated dosages making their findings “not necessarily generalizable to other preparations.” In addition, they noted the significant percentage of patients – 54% with CEE alone and 42% with CEE plus MPA – who discontinued drug usage during their respective trials.
The Women’s Health Initiative is supported by the National Institutes of Health and the Department of Health and Human Services. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various government organizations, foundations, and pharmaceutical companies. The editorial’s authors reported no conflicts of interest.
SOURCE: Chlebowski RT et al. JAMA. 2020 Jul 28. doi: 10.1001/jama.2020.9482.
A new follow-up study of menopausal hormone therapy found that prior use of conjugated equine estrogen (CEE) decreased both breast cancer incidence and mortality, while prior use of CEE plus medroxyprogesterone acetate (MPA) was associated with an increase in incidence.
“Prior use of CEE alone is, to our knowledge, the first pharmacologic intervention demonstrated to be associated with a statistically significantly reduction in deaths from breast cancer,” wrote Rowan T. Chlebowski, MD, PhD, of the Lundquist Institute for Biomedical Innovation in Torrance, Calif., and his coauthors. The study was published July 28 in JAMA.
To further investigate the outcomes of the Women’s Health Initiative in regard to hormone therapy and breast cancer risk, the researchers analyzed the long-term follow-up of two randomized trials that included 27,347 postmenopausal women with no prior breast cancer and negative mammograms at baseline. Their mean (SD) age was 63.4 (7.2) years. Enrollment took place from 1993 to 1998; participants were contacted for follow-up every 6 months through 2005 and annually from then on. Mortality data were gathered from follow-up and the National Death Index.
The first trial included 16,608 women with a uterus. Among these women, 8,506 received 0.625 mg/day of CEE plus 2.5 mg/day of MPA, and 8,102 received placebo. The second trial included 10,739 women who’d gotten a hysterectomy, 5,310 of whom received 0.625 mg/day of CEE alone and 5,429 of whom received placebo. The first trial ended in 2002 after a median intervention period of 5.6 years, and the second trial ended in 2004 after a period of 7.2 years.
An analysis in 2015 found that CEE alone was associated with lower risk of breast cancer and CEE plus MPA was associated with increased risk.
The current analysis confirmed that, after a median of 20.3 years of follow-up, and with mortality data now available for more than 98% of participants, CEE alone was associated with fewer cases of breast cancer (238 cases, annualized rate 0.30%), compared with placebo (296 cases, annualized rate 0.37%; hazard ratio 0.78; 95% confidence interval, 0.65-0.93; P = .005).
Furthermore, CEE alone was also associated with lower mortality (30 deaths, annualized mortality rate 0.031%), compared with placebo (46 deaths, annualized mortality rate 0.046%; HR 0.60; 95% CI, 0.37-0.97; P = .04).
By comparison, CEE plus MPA was linked with more cases of breast cancer (584 cases, annualized rate 0.45%) than placebo (447 cases, annualized rate 0.36%; HR 1.28; 95% CI, 1.13-1.45; P < .001). In regard to mortality, there was no statistically significant difference between CEE plus MPA (71 deaths, annualized mortality rate 0.045%) and placebo (53 deaths, annualized mortality rate 0.035%; HR 1.35; 95% CI, 0.94-1.95; P = .11).
“The big thing to think about is estrogen alone reducing breast cancer mortality by 40%,” said Dr. Chlebowski in an interview. “None of the other interventions, including tamoxifen, had any change on mortality. This should change the way we look at breast cancer prevention, though we might have to be a little creative about it. I think you have to be a little away from menopause for it to reduce breast cancer. But we wanted to start that debate.
“On the other hand,” he said, “a woman takes estrogen plus progestin and when you look at that curve, it’s staying about 25% increased. You take it for 5.6 years and the increase continues through 20 years, so you’re maybe buying a lifetime of increase in breast cancer by taking estrogen plus progestin for 5 years.”
He also highlighted the comprehensiveness of the mortality data, noting that “when you hook up to the National Death Index, they find 98% of all deaths in the United States. That’s really remarkable; you retain the whole power of the randomization. It means our data, between the death index and our follow-up of participants, is essentially complete.”
Use of hormone therapy, and decoding the outcomes, remains ‘complex’
Decades after the data were gathered from the Women’s Health Initiative clinical trials, they continue to assist researchers and patients alike, wrote Christina A. Minami, MD, of Brigham and Women’s Hospital in Boston and Rachel A. Freedman, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
That said, in regard to the findings of this latest analysis, “many questions still remain on whether (and how) a hormone therapy intervention that occurred many years earlier may continue to affect breast cancer risk and mortality at 20 years,” they wrote. They noted that it’s “impossible” to isolate how exposure to certain therapies can impact long-term outcomes, and that a high percentage of patients who discontinued the drugs during each trial muddy the waters even further.
“Decisions to initiate these medications remain complex,” they added, emphasizing that breast cancer risk is just one of many factors that physicians must consider when considering hormone therapy for their patients.
Dr. Chlebowski and his coauthors acknowledged their study’s limitations, including the use of very specifically administered and formulated dosages making their findings “not necessarily generalizable to other preparations.” In addition, they noted the significant percentage of patients – 54% with CEE alone and 42% with CEE plus MPA – who discontinued drug usage during their respective trials.
The Women’s Health Initiative is supported by the National Institutes of Health and the Department of Health and Human Services. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various government organizations, foundations, and pharmaceutical companies. The editorial’s authors reported no conflicts of interest.
SOURCE: Chlebowski RT et al. JAMA. 2020 Jul 28. doi: 10.1001/jama.2020.9482.
FROM JAMA
The fix is in: AIM bundles to combat maternal morbidity and mortality
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.