User login
Cervical Pannus Without Rheumatoid Arthritis or Trauma
Although usually seen in patients with rheumatoid arthritis, cervical pannus also can develop in patients who have had spine surgery.
Cervical pannus is a disease that could easily develop in an active-duty soldier or veteran. The disease has been associated with trauma and rheumatoid arthritis, or can be idiopathic. For years, cervical pannus has been closely tied to rheumatoid arthritis; however, a study published in 2019 showed that only 28% of patients with cervical pannus had an associated diagnosis of rheumatoid arthritis.1 In the same study, 18% of patients had undergone some type of prior cervical spine surgery as the next most common cause. The condition also can occur years after an injury.
Background
In the US, 42,000 veterans are living with spinal cord disease, and thousands of these veterans have surgery every year.2 Service men and women and veterans are at risk for cervical pannus as they age especially if they have a history of rheumatoid arthritis, cervical spine surgery, trauma, and numerous other causes. It is critical for health care providers who treat this population to understand cervical pannus, how to recognize it, and how to identify patients at risk. A cervical pannus can be life threatening if not detected and treated properly.
There is no clear definition for cervical pannus. Some researchers think of it as the chronically inflamed synovial membrane in patients with rheumatoid arthritis (RA); others consider it as a specialized synovial membrane derived from vascular soft tissue structures at or near the bone synovial membrane.3 The pathogenesis for developing a pannus is not well understood, and little is known when a pannus begins or its initial location. A pannus formation can occur in any synovial joint in the body, such as wrists, metacarpophalangeal joint, proximal interphalangeal joint, and cervical joints.
A cervical pannus can cause serious complications. It can lead to a cervical subluxation in up to 4% of patients with RA, or it also can occur spontaneously in some patients without RA especially those with trauma or cancer.4
There are 2 suggested mechanisms by which the synovial membrane proliferates. It was originally believed that T cells from the chronic inflamed joint lead to the pannus formation by initiating an autoimmune reaction through the production of different cytokines against arthritogenic agents.3-5 These cytokines increase inflammation by recruiting neutrophils and activating various kinds of macrophages that might lead to increased osteoclast activity.6 Osteoclastic activity can damage bone and allow the synovium to penetrate the bone, forming the pannus.
Another proposed mechanism is that the synovial cells hyperpolarize and hypertrophy automatically without T-cell help by expressing oncogenes and their proteins.3 In either case, angiogenesis follows this proliferation and increases the influx of inflammatory cells into the joints, which can lead to more destruction.7 This increase in blood supply to the synovial membrane is important in the growth of the pannus and can have a damaging effect to cartilage, bone, and joints.4,7
Cervical pannus can progress in patients with prolonged use of corticosteroids.8 Because a pannus can put pressure on any segment of the cervical spine and the cranio-cervical junction leading to cervical instability, patients with this condition may present with a variety of clinical symptoms.9 The most frequently reported clinical features include neck pain, easy fatigability, difficulty walking, abnormal gait, increased clumsiness, and numbness and tingling in the arms. Patients also may complain of neck stiffness and decreased neck motion.10Cervical pannus is most frequently seen in patients with RA. However, patients without a RA diagnosis and incidental atlantoaxial pannus on cervical spine magnetic resonance imaging (MRI) are unlikely to have previously undiagnosed RA.11
Case Presentation
A 70-year-old white woman presented to the neurology clinic at Gretna Medical Center in Virginia in December 2016 with constant headache and imbalance that started in September 2016. She characterized the pain as predominately pressure (6 on a 10-point pain scale) with occasional shooting pains. The pain started at the left occipital lobe and radiated toward the left temporal lobe and left eye. The patient also stated that it was very difficult to lay her head down on a pillow to sleep and that she had to use a recliner in order to sleep over the past 3 months. She reported that the headache felt slightly worse if she had a lot of repetitive head and neck movements during the day. There was no photophobia, phonophobia, nausea, vomiting, facial paresthesias, lacrimation, nasal congestion, confusion, or impaired speech.
The patient’s lack of balance, which resulted in an unsteady gait, had started 1 month before and had increased significantly in the past 2 to 3 weeks. She stated that the unsteady gait was associated with numbness in her right upper and lower extremities, although more intense in the right lower extremity. Aside from the headaches, paresthesia, and unsteady gait, the patient reported no other major symptoms. She did not smoke tobacco or drink alcohol. Her family history revealed that her brothers had heart disease.
The patient’s vital signs at physical examination included heart rate, 83 beats per minute; blood pressure, 159/75 mm hg; temporal temperature, 97.9 °F; and respiratory rate, 20 breaths per minute. The patient’s gait was unsteady, needing stabilization by holding on to her husband’s arm, slightly favoring right lower extremity. Finger-to-nose test, rapid alternating movements, heel-knee-shin testing were all normal. The Romberg sign was positive. The patient could rise on toes and heels with slight balance disturbance. Deep tendon reflexes and reflexes in the upper and lower extremities was symmetric 2+ bilaterally. Musculoskeletal examination revealed strength and tone in all major muscle groups and demonstrated symmetrical movements with no fasciculation noted. A rheumatologic evaluation showed no abnormalities, including inspection of hands, feet, major joints, and other range of motion, besides her neck. The rest of the physical, cognitive, and neurologic examination findings were otherwise unremarkable. A routine rheumatologic laboratory evaluation was negative.
A head computed tomography ordered before coming to the clinic showed normal results. An MRI of the head was obtained to evaluate for ischemic cause or structural abnormality (Figures 1 and 2). Given the patient’s presentation and the pattern seen on the MRI results, it was determined that large pannus posterior to the dens, severely narrowing the spinal canal, was most likely the diagnosis. A second opinion confirmed the diagnosis, and a second MRI revealed stabilization with no signs of enhancement.
The patient was advised to meet with a neurosurgeon to remove the pannus. The patient agreed on occiput to C2 posterior instrument arthrodesis as well as decompression. A plain film radiograph showed C2-occipital repair after surgery (Figure 3). The patient recovered in the neurosurgical intensive care unit, and the rest of the recovery was uncomplicated. She showed some improvement in her headaches and unsteady gait. A postoperative pathologic evaluation of tissue was not available. She was referred to a rheumatologist to rule out an autoimmune disease as the cause for this pannus, but no autoimmune disease was found.
Discussion
Cervical pannus is relatively uncommon in those without RA. However, there are multiple reasons that a patient could develop a cervical pannus. Cervical pannus in RA and cervical pannus without RA may mimic each other clinically, but medical management is distinctly different. Consequently, a rheumatology consult is necessary to ensure that there is no undiagnosed autoimmune disorder. Our patient did not have RA, and a neurosurgery intervention was needed to manage her headaches and unsteady gait. Although we could not isolate a cause of this patient’s cervical pannus development, we believed that nonintervention would adversely affect this patient.
The course of pannus progression can be fatal especially if left untreated.12 MRI can detect a pannus and may be helpful for planning surgery.13 Surgical resection has been the treatment of choice for patients with neurologic symptoms.14 However, some cases have reported resolution of pannus associated with RA and other forms of chronic atlantoaxial instability only after posterior stabilization.14In order to manage pannus, cervical spine examination for the diagnosis of cervical involvement is encouraged to prevent morbidity and mortality.13 There are new data that demonstrated the potential of using retinoid X receptor agonists, such as bexarotene, as a treatment against the development and progression of pannus.14
Conclusions
We present a patient with cervical pannus disease without RA whose diagnosis was based on the pathognomonic pattern seen on MRI. She showed a clinically significant recovery with an occiput to C2 posterior instrument arthrodesis as well as decompression. She showed marked improvements in her headaches and unsteady gait. This case report highlights the importance of realizing cervical pannus as a disease found in patients without RA. It serves as an alert to clinicians for timely detection, diagnosis, and initiation of treatment to prevent mortality and long-term neurologic sequelae of cervical pannus.
Although further studies of early diagnosis and treatment for cervical pannus are warranted, we propose that including pannus in a differential diagnosis for patients with no RA could be lifesaving.
1. Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37(6):783-789.
2. Henderson DR. Vertical atlanto-axial subluxation in rheumatoid arthritis. Rheumatol Rehabil. 1975;14(1):31-38.
3. Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(suppl 2):S4-S14.
4. Wang R, Zhang L, Zhang X, et al. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur J Immunol. 2002;32(4):1090-1098.
5. Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62(suppl 2):ii60-ii67.
6. Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine (Phila Pa 1976). 1998;23(24):2755-2766.
7. Alaya Z, Lataoui S, Amri D, Zaghouani H, Bouajina E. Atlantoaxial instability: an exceptional complication of ankylosing spondylitis. Egypt Rheumatol. 2018;40(2):141-143.
8. Walter KD, Tassone JC. Atlantoaxial instability. In: Micheli LJ, ed. Encyclopedia of Sports Medicine. Thousand Oaks, CA: SAGE Reference; 2011:122-124.
9. Joyce AA, Williams JN, Shi J, Mandell JC, Isaac Z, Ermann J. Atlanto-axial pannus in patients with and without rheumatoid arthritis. J Rheumatol. 2019;46(11):1431-1437.
10. Neva MH, Myllykangas-Luosujärvi R, Kautiainen H, Kauppi M. Mortality associated with cervical spine disorders: a population-based study of 1666 patients with rheumatoid arthritis who died in Finland in 1989. Rheumatology (Oxford). 2001;40(2):123-127.
11. Mallory GW, Halasz SR, Clarke MJ. Advances in the treatment of cervical rheumatoid: less surgery and less morbidity. World J Orthop. 2014;5(3):292-303.
12. Lagares A, Arrese I, Pascual B, Gòmez PA, Ramos A, Lobato RD. Pannus resolution after occipitocervical fusion in a non-rheumatoid atlanto-axial instability. Eur Spine J. 2006;15(3):366-369.
13. Chung J, Bak KH, Yi H-J, Chun HJ, Ryu JI, Han M-H. Upper cervical subluxation and cervicomedullary junction compression in patients with rheumatoid arthritis. J Korean Neurosurg Soc. 2019;62(6):661-670.
14. Li Y, Xing Q, Wei Y, et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast‑like synoviocytes. Int J Mol Med. 2019;44(5):1963-1970.
Although usually seen in patients with rheumatoid arthritis, cervical pannus also can develop in patients who have had spine surgery.
Although usually seen in patients with rheumatoid arthritis, cervical pannus also can develop in patients who have had spine surgery.
Cervical pannus is a disease that could easily develop in an active-duty soldier or veteran. The disease has been associated with trauma and rheumatoid arthritis, or can be idiopathic. For years, cervical pannus has been closely tied to rheumatoid arthritis; however, a study published in 2019 showed that only 28% of patients with cervical pannus had an associated diagnosis of rheumatoid arthritis.1 In the same study, 18% of patients had undergone some type of prior cervical spine surgery as the next most common cause. The condition also can occur years after an injury.
Background
In the US, 42,000 veterans are living with spinal cord disease, and thousands of these veterans have surgery every year.2 Service men and women and veterans are at risk for cervical pannus as they age especially if they have a history of rheumatoid arthritis, cervical spine surgery, trauma, and numerous other causes. It is critical for health care providers who treat this population to understand cervical pannus, how to recognize it, and how to identify patients at risk. A cervical pannus can be life threatening if not detected and treated properly.
There is no clear definition for cervical pannus. Some researchers think of it as the chronically inflamed synovial membrane in patients with rheumatoid arthritis (RA); others consider it as a specialized synovial membrane derived from vascular soft tissue structures at or near the bone synovial membrane.3 The pathogenesis for developing a pannus is not well understood, and little is known when a pannus begins or its initial location. A pannus formation can occur in any synovial joint in the body, such as wrists, metacarpophalangeal joint, proximal interphalangeal joint, and cervical joints.
A cervical pannus can cause serious complications. It can lead to a cervical subluxation in up to 4% of patients with RA, or it also can occur spontaneously in some patients without RA especially those with trauma or cancer.4
There are 2 suggested mechanisms by which the synovial membrane proliferates. It was originally believed that T cells from the chronic inflamed joint lead to the pannus formation by initiating an autoimmune reaction through the production of different cytokines against arthritogenic agents.3-5 These cytokines increase inflammation by recruiting neutrophils and activating various kinds of macrophages that might lead to increased osteoclast activity.6 Osteoclastic activity can damage bone and allow the synovium to penetrate the bone, forming the pannus.
Another proposed mechanism is that the synovial cells hyperpolarize and hypertrophy automatically without T-cell help by expressing oncogenes and their proteins.3 In either case, angiogenesis follows this proliferation and increases the influx of inflammatory cells into the joints, which can lead to more destruction.7 This increase in blood supply to the synovial membrane is important in the growth of the pannus and can have a damaging effect to cartilage, bone, and joints.4,7
Cervical pannus can progress in patients with prolonged use of corticosteroids.8 Because a pannus can put pressure on any segment of the cervical spine and the cranio-cervical junction leading to cervical instability, patients with this condition may present with a variety of clinical symptoms.9 The most frequently reported clinical features include neck pain, easy fatigability, difficulty walking, abnormal gait, increased clumsiness, and numbness and tingling in the arms. Patients also may complain of neck stiffness and decreased neck motion.10Cervical pannus is most frequently seen in patients with RA. However, patients without a RA diagnosis and incidental atlantoaxial pannus on cervical spine magnetic resonance imaging (MRI) are unlikely to have previously undiagnosed RA.11
Case Presentation
A 70-year-old white woman presented to the neurology clinic at Gretna Medical Center in Virginia in December 2016 with constant headache and imbalance that started in September 2016. She characterized the pain as predominately pressure (6 on a 10-point pain scale) with occasional shooting pains. The pain started at the left occipital lobe and radiated toward the left temporal lobe and left eye. The patient also stated that it was very difficult to lay her head down on a pillow to sleep and that she had to use a recliner in order to sleep over the past 3 months. She reported that the headache felt slightly worse if she had a lot of repetitive head and neck movements during the day. There was no photophobia, phonophobia, nausea, vomiting, facial paresthesias, lacrimation, nasal congestion, confusion, or impaired speech.
The patient’s lack of balance, which resulted in an unsteady gait, had started 1 month before and had increased significantly in the past 2 to 3 weeks. She stated that the unsteady gait was associated with numbness in her right upper and lower extremities, although more intense in the right lower extremity. Aside from the headaches, paresthesia, and unsteady gait, the patient reported no other major symptoms. She did not smoke tobacco or drink alcohol. Her family history revealed that her brothers had heart disease.
The patient’s vital signs at physical examination included heart rate, 83 beats per minute; blood pressure, 159/75 mm hg; temporal temperature, 97.9 °F; and respiratory rate, 20 breaths per minute. The patient’s gait was unsteady, needing stabilization by holding on to her husband’s arm, slightly favoring right lower extremity. Finger-to-nose test, rapid alternating movements, heel-knee-shin testing were all normal. The Romberg sign was positive. The patient could rise on toes and heels with slight balance disturbance. Deep tendon reflexes and reflexes in the upper and lower extremities was symmetric 2+ bilaterally. Musculoskeletal examination revealed strength and tone in all major muscle groups and demonstrated symmetrical movements with no fasciculation noted. A rheumatologic evaluation showed no abnormalities, including inspection of hands, feet, major joints, and other range of motion, besides her neck. The rest of the physical, cognitive, and neurologic examination findings were otherwise unremarkable. A routine rheumatologic laboratory evaluation was negative.
A head computed tomography ordered before coming to the clinic showed normal results. An MRI of the head was obtained to evaluate for ischemic cause or structural abnormality (Figures 1 and 2). Given the patient’s presentation and the pattern seen on the MRI results, it was determined that large pannus posterior to the dens, severely narrowing the spinal canal, was most likely the diagnosis. A second opinion confirmed the diagnosis, and a second MRI revealed stabilization with no signs of enhancement.
The patient was advised to meet with a neurosurgeon to remove the pannus. The patient agreed on occiput to C2 posterior instrument arthrodesis as well as decompression. A plain film radiograph showed C2-occipital repair after surgery (Figure 3). The patient recovered in the neurosurgical intensive care unit, and the rest of the recovery was uncomplicated. She showed some improvement in her headaches and unsteady gait. A postoperative pathologic evaluation of tissue was not available. She was referred to a rheumatologist to rule out an autoimmune disease as the cause for this pannus, but no autoimmune disease was found.
Discussion
Cervical pannus is relatively uncommon in those without RA. However, there are multiple reasons that a patient could develop a cervical pannus. Cervical pannus in RA and cervical pannus without RA may mimic each other clinically, but medical management is distinctly different. Consequently, a rheumatology consult is necessary to ensure that there is no undiagnosed autoimmune disorder. Our patient did not have RA, and a neurosurgery intervention was needed to manage her headaches and unsteady gait. Although we could not isolate a cause of this patient’s cervical pannus development, we believed that nonintervention would adversely affect this patient.
The course of pannus progression can be fatal especially if left untreated.12 MRI can detect a pannus and may be helpful for planning surgery.13 Surgical resection has been the treatment of choice for patients with neurologic symptoms.14 However, some cases have reported resolution of pannus associated with RA and other forms of chronic atlantoaxial instability only after posterior stabilization.14In order to manage pannus, cervical spine examination for the diagnosis of cervical involvement is encouraged to prevent morbidity and mortality.13 There are new data that demonstrated the potential of using retinoid X receptor agonists, such as bexarotene, as a treatment against the development and progression of pannus.14
Conclusions
We present a patient with cervical pannus disease without RA whose diagnosis was based on the pathognomonic pattern seen on MRI. She showed a clinically significant recovery with an occiput to C2 posterior instrument arthrodesis as well as decompression. She showed marked improvements in her headaches and unsteady gait. This case report highlights the importance of realizing cervical pannus as a disease found in patients without RA. It serves as an alert to clinicians for timely detection, diagnosis, and initiation of treatment to prevent mortality and long-term neurologic sequelae of cervical pannus.
Although further studies of early diagnosis and treatment for cervical pannus are warranted, we propose that including pannus in a differential diagnosis for patients with no RA could be lifesaving.
Cervical pannus is a disease that could easily develop in an active-duty soldier or veteran. The disease has been associated with trauma and rheumatoid arthritis, or can be idiopathic. For years, cervical pannus has been closely tied to rheumatoid arthritis; however, a study published in 2019 showed that only 28% of patients with cervical pannus had an associated diagnosis of rheumatoid arthritis.1 In the same study, 18% of patients had undergone some type of prior cervical spine surgery as the next most common cause. The condition also can occur years after an injury.
Background
In the US, 42,000 veterans are living with spinal cord disease, and thousands of these veterans have surgery every year.2 Service men and women and veterans are at risk for cervical pannus as they age especially if they have a history of rheumatoid arthritis, cervical spine surgery, trauma, and numerous other causes. It is critical for health care providers who treat this population to understand cervical pannus, how to recognize it, and how to identify patients at risk. A cervical pannus can be life threatening if not detected and treated properly.
There is no clear definition for cervical pannus. Some researchers think of it as the chronically inflamed synovial membrane in patients with rheumatoid arthritis (RA); others consider it as a specialized synovial membrane derived from vascular soft tissue structures at or near the bone synovial membrane.3 The pathogenesis for developing a pannus is not well understood, and little is known when a pannus begins or its initial location. A pannus formation can occur in any synovial joint in the body, such as wrists, metacarpophalangeal joint, proximal interphalangeal joint, and cervical joints.
A cervical pannus can cause serious complications. It can lead to a cervical subluxation in up to 4% of patients with RA, or it also can occur spontaneously in some patients without RA especially those with trauma or cancer.4
There are 2 suggested mechanisms by which the synovial membrane proliferates. It was originally believed that T cells from the chronic inflamed joint lead to the pannus formation by initiating an autoimmune reaction through the production of different cytokines against arthritogenic agents.3-5 These cytokines increase inflammation by recruiting neutrophils and activating various kinds of macrophages that might lead to increased osteoclast activity.6 Osteoclastic activity can damage bone and allow the synovium to penetrate the bone, forming the pannus.
Another proposed mechanism is that the synovial cells hyperpolarize and hypertrophy automatically without T-cell help by expressing oncogenes and their proteins.3 In either case, angiogenesis follows this proliferation and increases the influx of inflammatory cells into the joints, which can lead to more destruction.7 This increase in blood supply to the synovial membrane is important in the growth of the pannus and can have a damaging effect to cartilage, bone, and joints.4,7
Cervical pannus can progress in patients with prolonged use of corticosteroids.8 Because a pannus can put pressure on any segment of the cervical spine and the cranio-cervical junction leading to cervical instability, patients with this condition may present with a variety of clinical symptoms.9 The most frequently reported clinical features include neck pain, easy fatigability, difficulty walking, abnormal gait, increased clumsiness, and numbness and tingling in the arms. Patients also may complain of neck stiffness and decreased neck motion.10Cervical pannus is most frequently seen in patients with RA. However, patients without a RA diagnosis and incidental atlantoaxial pannus on cervical spine magnetic resonance imaging (MRI) are unlikely to have previously undiagnosed RA.11
Case Presentation
A 70-year-old white woman presented to the neurology clinic at Gretna Medical Center in Virginia in December 2016 with constant headache and imbalance that started in September 2016. She characterized the pain as predominately pressure (6 on a 10-point pain scale) with occasional shooting pains. The pain started at the left occipital lobe and radiated toward the left temporal lobe and left eye. The patient also stated that it was very difficult to lay her head down on a pillow to sleep and that she had to use a recliner in order to sleep over the past 3 months. She reported that the headache felt slightly worse if she had a lot of repetitive head and neck movements during the day. There was no photophobia, phonophobia, nausea, vomiting, facial paresthesias, lacrimation, nasal congestion, confusion, or impaired speech.
The patient’s lack of balance, which resulted in an unsteady gait, had started 1 month before and had increased significantly in the past 2 to 3 weeks. She stated that the unsteady gait was associated with numbness in her right upper and lower extremities, although more intense in the right lower extremity. Aside from the headaches, paresthesia, and unsteady gait, the patient reported no other major symptoms. She did not smoke tobacco or drink alcohol. Her family history revealed that her brothers had heart disease.
The patient’s vital signs at physical examination included heart rate, 83 beats per minute; blood pressure, 159/75 mm hg; temporal temperature, 97.9 °F; and respiratory rate, 20 breaths per minute. The patient’s gait was unsteady, needing stabilization by holding on to her husband’s arm, slightly favoring right lower extremity. Finger-to-nose test, rapid alternating movements, heel-knee-shin testing were all normal. The Romberg sign was positive. The patient could rise on toes and heels with slight balance disturbance. Deep tendon reflexes and reflexes in the upper and lower extremities was symmetric 2+ bilaterally. Musculoskeletal examination revealed strength and tone in all major muscle groups and demonstrated symmetrical movements with no fasciculation noted. A rheumatologic evaluation showed no abnormalities, including inspection of hands, feet, major joints, and other range of motion, besides her neck. The rest of the physical, cognitive, and neurologic examination findings were otherwise unremarkable. A routine rheumatologic laboratory evaluation was negative.
A head computed tomography ordered before coming to the clinic showed normal results. An MRI of the head was obtained to evaluate for ischemic cause or structural abnormality (Figures 1 and 2). Given the patient’s presentation and the pattern seen on the MRI results, it was determined that large pannus posterior to the dens, severely narrowing the spinal canal, was most likely the diagnosis. A second opinion confirmed the diagnosis, and a second MRI revealed stabilization with no signs of enhancement.
The patient was advised to meet with a neurosurgeon to remove the pannus. The patient agreed on occiput to C2 posterior instrument arthrodesis as well as decompression. A plain film radiograph showed C2-occipital repair after surgery (Figure 3). The patient recovered in the neurosurgical intensive care unit, and the rest of the recovery was uncomplicated. She showed some improvement in her headaches and unsteady gait. A postoperative pathologic evaluation of tissue was not available. She was referred to a rheumatologist to rule out an autoimmune disease as the cause for this pannus, but no autoimmune disease was found.
Discussion
Cervical pannus is relatively uncommon in those without RA. However, there are multiple reasons that a patient could develop a cervical pannus. Cervical pannus in RA and cervical pannus without RA may mimic each other clinically, but medical management is distinctly different. Consequently, a rheumatology consult is necessary to ensure that there is no undiagnosed autoimmune disorder. Our patient did not have RA, and a neurosurgery intervention was needed to manage her headaches and unsteady gait. Although we could not isolate a cause of this patient’s cervical pannus development, we believed that nonintervention would adversely affect this patient.
The course of pannus progression can be fatal especially if left untreated.12 MRI can detect a pannus and may be helpful for planning surgery.13 Surgical resection has been the treatment of choice for patients with neurologic symptoms.14 However, some cases have reported resolution of pannus associated with RA and other forms of chronic atlantoaxial instability only after posterior stabilization.14In order to manage pannus, cervical spine examination for the diagnosis of cervical involvement is encouraged to prevent morbidity and mortality.13 There are new data that demonstrated the potential of using retinoid X receptor agonists, such as bexarotene, as a treatment against the development and progression of pannus.14
Conclusions
We present a patient with cervical pannus disease without RA whose diagnosis was based on the pathognomonic pattern seen on MRI. She showed a clinically significant recovery with an occiput to C2 posterior instrument arthrodesis as well as decompression. She showed marked improvements in her headaches and unsteady gait. This case report highlights the importance of realizing cervical pannus as a disease found in patients without RA. It serves as an alert to clinicians for timely detection, diagnosis, and initiation of treatment to prevent mortality and long-term neurologic sequelae of cervical pannus.
Although further studies of early diagnosis and treatment for cervical pannus are warranted, we propose that including pannus in a differential diagnosis for patients with no RA could be lifesaving.
1. Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37(6):783-789.
2. Henderson DR. Vertical atlanto-axial subluxation in rheumatoid arthritis. Rheumatol Rehabil. 1975;14(1):31-38.
3. Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(suppl 2):S4-S14.
4. Wang R, Zhang L, Zhang X, et al. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur J Immunol. 2002;32(4):1090-1098.
5. Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62(suppl 2):ii60-ii67.
6. Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine (Phila Pa 1976). 1998;23(24):2755-2766.
7. Alaya Z, Lataoui S, Amri D, Zaghouani H, Bouajina E. Atlantoaxial instability: an exceptional complication of ankylosing spondylitis. Egypt Rheumatol. 2018;40(2):141-143.
8. Walter KD, Tassone JC. Atlantoaxial instability. In: Micheli LJ, ed. Encyclopedia of Sports Medicine. Thousand Oaks, CA: SAGE Reference; 2011:122-124.
9. Joyce AA, Williams JN, Shi J, Mandell JC, Isaac Z, Ermann J. Atlanto-axial pannus in patients with and without rheumatoid arthritis. J Rheumatol. 2019;46(11):1431-1437.
10. Neva MH, Myllykangas-Luosujärvi R, Kautiainen H, Kauppi M. Mortality associated with cervical spine disorders: a population-based study of 1666 patients with rheumatoid arthritis who died in Finland in 1989. Rheumatology (Oxford). 2001;40(2):123-127.
11. Mallory GW, Halasz SR, Clarke MJ. Advances in the treatment of cervical rheumatoid: less surgery and less morbidity. World J Orthop. 2014;5(3):292-303.
12. Lagares A, Arrese I, Pascual B, Gòmez PA, Ramos A, Lobato RD. Pannus resolution after occipitocervical fusion in a non-rheumatoid atlanto-axial instability. Eur Spine J. 2006;15(3):366-369.
13. Chung J, Bak KH, Yi H-J, Chun HJ, Ryu JI, Han M-H. Upper cervical subluxation and cervicomedullary junction compression in patients with rheumatoid arthritis. J Korean Neurosurg Soc. 2019;62(6):661-670.
14. Li Y, Xing Q, Wei Y, et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast‑like synoviocytes. Int J Mol Med. 2019;44(5):1963-1970.
1. Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37(6):783-789.
2. Henderson DR. Vertical atlanto-axial subluxation in rheumatoid arthritis. Rheumatol Rehabil. 1975;14(1):31-38.
3. Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(suppl 2):S4-S14.
4. Wang R, Zhang L, Zhang X, et al. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur J Immunol. 2002;32(4):1090-1098.
5. Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62(suppl 2):ii60-ii67.
6. Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine (Phila Pa 1976). 1998;23(24):2755-2766.
7. Alaya Z, Lataoui S, Amri D, Zaghouani H, Bouajina E. Atlantoaxial instability: an exceptional complication of ankylosing spondylitis. Egypt Rheumatol. 2018;40(2):141-143.
8. Walter KD, Tassone JC. Atlantoaxial instability. In: Micheli LJ, ed. Encyclopedia of Sports Medicine. Thousand Oaks, CA: SAGE Reference; 2011:122-124.
9. Joyce AA, Williams JN, Shi J, Mandell JC, Isaac Z, Ermann J. Atlanto-axial pannus in patients with and without rheumatoid arthritis. J Rheumatol. 2019;46(11):1431-1437.
10. Neva MH, Myllykangas-Luosujärvi R, Kautiainen H, Kauppi M. Mortality associated with cervical spine disorders: a population-based study of 1666 patients with rheumatoid arthritis who died in Finland in 1989. Rheumatology (Oxford). 2001;40(2):123-127.
11. Mallory GW, Halasz SR, Clarke MJ. Advances in the treatment of cervical rheumatoid: less surgery and less morbidity. World J Orthop. 2014;5(3):292-303.
12. Lagares A, Arrese I, Pascual B, Gòmez PA, Ramos A, Lobato RD. Pannus resolution after occipitocervical fusion in a non-rheumatoid atlanto-axial instability. Eur Spine J. 2006;15(3):366-369.
13. Chung J, Bak KH, Yi H-J, Chun HJ, Ryu JI, Han M-H. Upper cervical subluxation and cervicomedullary junction compression in patients with rheumatoid arthritis. J Korean Neurosurg Soc. 2019;62(6):661-670.
14. Li Y, Xing Q, Wei Y, et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast‑like synoviocytes. Int J Mol Med. 2019;44(5):1963-1970.
Water-only fasting may reduce chemo modifications, hospital admissions
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
FROM SGO 2020
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
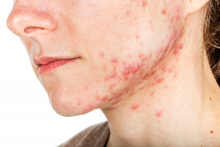
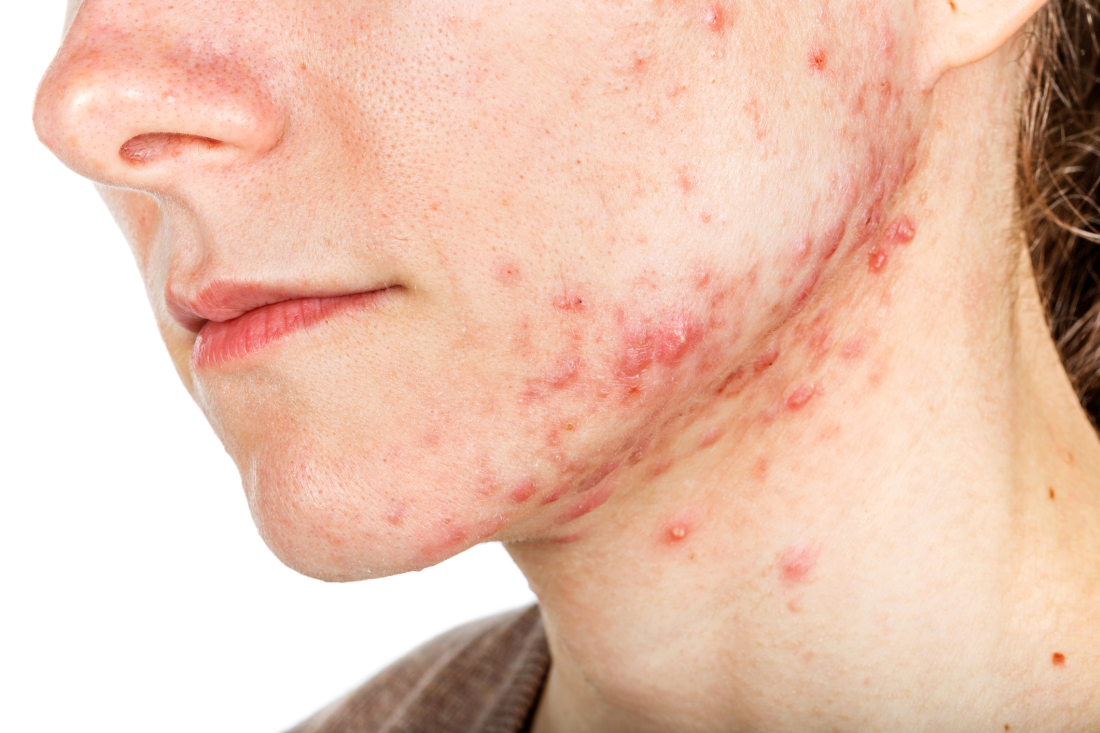
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
FDA removes pregnancy category C warning from certain MS medications
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
PFAS exposure in pregnancy tied to obesity risk in granddaughters
Exposure during pregnancy to a specific per- and polyfluoroalkyl substance (PFAS), combined with a low cholesterol level, is linked to a heightened risk of abdominal and whole-body obesity in granddaughters, according to a new analysis of the Child Health and Development Studies, which have been ongoing since the 1960s.
Researchers directly measured levels of N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA) in blood samples from the grandmothers, which had been taken shortly after delivery, and then analyzed measures of obesity and other metabolic factors in their daughters at ages 30 years and 50 years, and their granddaughters at age 20.
PFASs are synthetic compounds commonly used as oil and water repellents; coatings for cookware, carpets, and textiles; and as firefighting foams. The compounds do not break down in the environment or the human body and accumulate over time. They are known to disrupt the endocrine system.
EtFOSAA is a metabolite of a raw material used in the manufacturing of packaging and paper products, and itself gets converted to perfluorooctane sulfonic acid (PFOS), which is extremely stable in the environment and within organisms, leading to bioaccumulation that has the potential to span generations, Barbara A. Cohn, PhD, director of child health and development studies at the Public Health Institute in Berkeley, Calif., said during a virtual press conference held by The Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Abdominal obesity was defined as a waist circumference of more than 34.6 inches (88 cm), and whole-body obesity was defined as a body mass index of more than 30 kg/m2. Findings from a previous study drawn from the same cohort showed that exposure to EtFOSAA, combined with high maternal cholesterol levels, was linked to increased risk of breast cancer in daughters.
“I want to emphasize that we don’t understand the mechanism, but we do know that this finding [from the current study], if it is confirmed, has implications for the current epidemic of obesity. Exposure to these compounds is very widespread, [having] started in the 1940s and 50s, and is consistent with the timing of the obesity epidemic,” said Dr. Cohn, during the virtual press conference.
Robert Sargis, MD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, said the mechanistic connection could be complex. “It’s a combination of the possibility that the chemicals themselves are passed down either through breast milk or across the placenta, or that the biological impact is somehow coded epigenetically, and then that epigenetic code is somehow passed on to subsequent generations,” he said in an interview. He was not associated with the research.
Dr. Cohn said her team is investigating both of those possibilities through analysis of the existing blood samples. “There are implications for PFAS clean-up if [these findings are] confirmed, and there’s an opportunity for setting up precautions for pregnant women on how they can try to avoid this contamination to [offset] a rekindling of this generational effect 60 years down the road,” Dr. Cohn added.
Daughters of the original participants (now grandmothers) were measured at an average age of 50, and the granddaughters, at an average of 20 (219 dyads, 657 women in total). Daughters also reported their weight at age 30, which was close to the mean age at which they had given birth. This allowed the researchers to control for obesity present during gestation of the granddaughters.
The researchers analyzed EtFOSAA, PFOS, and cholesterol levels from archived blood samples taken from grandmothers within 3 days of delivery. There was an association between EtFOSAA and self-reported obesity at age 30 in daughters, as well as measured abdominal, whole-body obesity, and blood pressure at age 20 in granddaughters, and all were modified by low cholesterol levels (25% interquartile) in grandmothers (P < .05).
In granddaughters, the combined risk of abdominal and whole-body obesity was 2.3-fold higher in those whose grandmothers were in the top 25% of EtFOSAA exposure, compared with those whose grandmothers were in the lowest 25% (95% confidence interval, 1.1-4.8). Those associations remained after adjustments for race, being overweight in early pregnancy (BMI, >25 kg/m2), and serum PFOS levels.
Although the weight of daughters did not affect the association between the granddaughters’ risk for obesity risk and EtFOSAA levels in grandmothers, it did predict high metabolic risk in granddaughters. That suggests that the burden may be building over generations. “Independently, their mothers themselves are heavier and fatter, and that heaviness of the mother is also a source of increasing body size for the granddaughter. We have a multiplying, very ugly situation that may be helping us to understand this really quick rise of obesity,” said Dr. Cohn.
She also emphasized that PFAS may not be the only culprit in fueling obesity. “Most of us believe that there is sufficient data in the animal studies and, now, growing data in human studies, to suggest that these obesogens exist and are contributing to the health problems that are going to be following the obesity epidemic in young people now.”
Dr. Cohn noted that the study is limited by its lack of a control group.
The California Breast Research Foundation, the National Institutes of Health, and the State of California funded the study. Dr. Cohn and Dr. Sargis reported no relevant financial disclosures.
The study abstract will be published in the Journal of the Endocrine Society. In addition to a series of news conferences held on March 30-31, the society will host ENDO Online 2020 during June 8-22 with programming for clinicians and researchers.
SOURCE: Cohn B et al. ENDO 2020, Abstract LB132.
Exposure during pregnancy to a specific per- and polyfluoroalkyl substance (PFAS), combined with a low cholesterol level, is linked to a heightened risk of abdominal and whole-body obesity in granddaughters, according to a new analysis of the Child Health and Development Studies, which have been ongoing since the 1960s.
Researchers directly measured levels of N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA) in blood samples from the grandmothers, which had been taken shortly after delivery, and then analyzed measures of obesity and other metabolic factors in their daughters at ages 30 years and 50 years, and their granddaughters at age 20.
PFASs are synthetic compounds commonly used as oil and water repellents; coatings for cookware, carpets, and textiles; and as firefighting foams. The compounds do not break down in the environment or the human body and accumulate over time. They are known to disrupt the endocrine system.
EtFOSAA is a metabolite of a raw material used in the manufacturing of packaging and paper products, and itself gets converted to perfluorooctane sulfonic acid (PFOS), which is extremely stable in the environment and within organisms, leading to bioaccumulation that has the potential to span generations, Barbara A. Cohn, PhD, director of child health and development studies at the Public Health Institute in Berkeley, Calif., said during a virtual press conference held by The Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Abdominal obesity was defined as a waist circumference of more than 34.6 inches (88 cm), and whole-body obesity was defined as a body mass index of more than 30 kg/m2. Findings from a previous study drawn from the same cohort showed that exposure to EtFOSAA, combined with high maternal cholesterol levels, was linked to increased risk of breast cancer in daughters.
“I want to emphasize that we don’t understand the mechanism, but we do know that this finding [from the current study], if it is confirmed, has implications for the current epidemic of obesity. Exposure to these compounds is very widespread, [having] started in the 1940s and 50s, and is consistent with the timing of the obesity epidemic,” said Dr. Cohn, during the virtual press conference.
Robert Sargis, MD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, said the mechanistic connection could be complex. “It’s a combination of the possibility that the chemicals themselves are passed down either through breast milk or across the placenta, or that the biological impact is somehow coded epigenetically, and then that epigenetic code is somehow passed on to subsequent generations,” he said in an interview. He was not associated with the research.
Dr. Cohn said her team is investigating both of those possibilities through analysis of the existing blood samples. “There are implications for PFAS clean-up if [these findings are] confirmed, and there’s an opportunity for setting up precautions for pregnant women on how they can try to avoid this contamination to [offset] a rekindling of this generational effect 60 years down the road,” Dr. Cohn added.
Daughters of the original participants (now grandmothers) were measured at an average age of 50, and the granddaughters, at an average of 20 (219 dyads, 657 women in total). Daughters also reported their weight at age 30, which was close to the mean age at which they had given birth. This allowed the researchers to control for obesity present during gestation of the granddaughters.
The researchers analyzed EtFOSAA, PFOS, and cholesterol levels from archived blood samples taken from grandmothers within 3 days of delivery. There was an association between EtFOSAA and self-reported obesity at age 30 in daughters, as well as measured abdominal, whole-body obesity, and blood pressure at age 20 in granddaughters, and all were modified by low cholesterol levels (25% interquartile) in grandmothers (P < .05).
In granddaughters, the combined risk of abdominal and whole-body obesity was 2.3-fold higher in those whose grandmothers were in the top 25% of EtFOSAA exposure, compared with those whose grandmothers were in the lowest 25% (95% confidence interval, 1.1-4.8). Those associations remained after adjustments for race, being overweight in early pregnancy (BMI, >25 kg/m2), and serum PFOS levels.
Although the weight of daughters did not affect the association between the granddaughters’ risk for obesity risk and EtFOSAA levels in grandmothers, it did predict high metabolic risk in granddaughters. That suggests that the burden may be building over generations. “Independently, their mothers themselves are heavier and fatter, and that heaviness of the mother is also a source of increasing body size for the granddaughter. We have a multiplying, very ugly situation that may be helping us to understand this really quick rise of obesity,” said Dr. Cohn.
She also emphasized that PFAS may not be the only culprit in fueling obesity. “Most of us believe that there is sufficient data in the animal studies and, now, growing data in human studies, to suggest that these obesogens exist and are contributing to the health problems that are going to be following the obesity epidemic in young people now.”
Dr. Cohn noted that the study is limited by its lack of a control group.
The California Breast Research Foundation, the National Institutes of Health, and the State of California funded the study. Dr. Cohn and Dr. Sargis reported no relevant financial disclosures.
The study abstract will be published in the Journal of the Endocrine Society. In addition to a series of news conferences held on March 30-31, the society will host ENDO Online 2020 during June 8-22 with programming for clinicians and researchers.
SOURCE: Cohn B et al. ENDO 2020, Abstract LB132.
Exposure during pregnancy to a specific per- and polyfluoroalkyl substance (PFAS), combined with a low cholesterol level, is linked to a heightened risk of abdominal and whole-body obesity in granddaughters, according to a new analysis of the Child Health and Development Studies, which have been ongoing since the 1960s.
Researchers directly measured levels of N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA) in blood samples from the grandmothers, which had been taken shortly after delivery, and then analyzed measures of obesity and other metabolic factors in their daughters at ages 30 years and 50 years, and their granddaughters at age 20.
PFASs are synthetic compounds commonly used as oil and water repellents; coatings for cookware, carpets, and textiles; and as firefighting foams. The compounds do not break down in the environment or the human body and accumulate over time. They are known to disrupt the endocrine system.
EtFOSAA is a metabolite of a raw material used in the manufacturing of packaging and paper products, and itself gets converted to perfluorooctane sulfonic acid (PFOS), which is extremely stable in the environment and within organisms, leading to bioaccumulation that has the potential to span generations, Barbara A. Cohn, PhD, director of child health and development studies at the Public Health Institute in Berkeley, Calif., said during a virtual press conference held by The Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Abdominal obesity was defined as a waist circumference of more than 34.6 inches (88 cm), and whole-body obesity was defined as a body mass index of more than 30 kg/m2. Findings from a previous study drawn from the same cohort showed that exposure to EtFOSAA, combined with high maternal cholesterol levels, was linked to increased risk of breast cancer in daughters.
“I want to emphasize that we don’t understand the mechanism, but we do know that this finding [from the current study], if it is confirmed, has implications for the current epidemic of obesity. Exposure to these compounds is very widespread, [having] started in the 1940s and 50s, and is consistent with the timing of the obesity epidemic,” said Dr. Cohn, during the virtual press conference.
Robert Sargis, MD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, said the mechanistic connection could be complex. “It’s a combination of the possibility that the chemicals themselves are passed down either through breast milk or across the placenta, or that the biological impact is somehow coded epigenetically, and then that epigenetic code is somehow passed on to subsequent generations,” he said in an interview. He was not associated with the research.
Dr. Cohn said her team is investigating both of those possibilities through analysis of the existing blood samples. “There are implications for PFAS clean-up if [these findings are] confirmed, and there’s an opportunity for setting up precautions for pregnant women on how they can try to avoid this contamination to [offset] a rekindling of this generational effect 60 years down the road,” Dr. Cohn added.
Daughters of the original participants (now grandmothers) were measured at an average age of 50, and the granddaughters, at an average of 20 (219 dyads, 657 women in total). Daughters also reported their weight at age 30, which was close to the mean age at which they had given birth. This allowed the researchers to control for obesity present during gestation of the granddaughters.
The researchers analyzed EtFOSAA, PFOS, and cholesterol levels from archived blood samples taken from grandmothers within 3 days of delivery. There was an association between EtFOSAA and self-reported obesity at age 30 in daughters, as well as measured abdominal, whole-body obesity, and blood pressure at age 20 in granddaughters, and all were modified by low cholesterol levels (25% interquartile) in grandmothers (P < .05).
In granddaughters, the combined risk of abdominal and whole-body obesity was 2.3-fold higher in those whose grandmothers were in the top 25% of EtFOSAA exposure, compared with those whose grandmothers were in the lowest 25% (95% confidence interval, 1.1-4.8). Those associations remained after adjustments for race, being overweight in early pregnancy (BMI, >25 kg/m2), and serum PFOS levels.
Although the weight of daughters did not affect the association between the granddaughters’ risk for obesity risk and EtFOSAA levels in grandmothers, it did predict high metabolic risk in granddaughters. That suggests that the burden may be building over generations. “Independently, their mothers themselves are heavier and fatter, and that heaviness of the mother is also a source of increasing body size for the granddaughter. We have a multiplying, very ugly situation that may be helping us to understand this really quick rise of obesity,” said Dr. Cohn.
She also emphasized that PFAS may not be the only culprit in fueling obesity. “Most of us believe that there is sufficient data in the animal studies and, now, growing data in human studies, to suggest that these obesogens exist and are contributing to the health problems that are going to be following the obesity epidemic in young people now.”
Dr. Cohn noted that the study is limited by its lack of a control group.
The California Breast Research Foundation, the National Institutes of Health, and the State of California funded the study. Dr. Cohn and Dr. Sargis reported no relevant financial disclosures.
The study abstract will be published in the Journal of the Endocrine Society. In addition to a series of news conferences held on March 30-31, the society will host ENDO Online 2020 during June 8-22 with programming for clinicians and researchers.
SOURCE: Cohn B et al. ENDO 2020, Abstract LB132.
FROM ENDO 2020
Barriers to clinical trial participation revealed by gynecologic cancer patients
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
FROM SGO 2020
Pembrolizumab Plus Neoadjuvant Chemotherapy Improves Pathologic Complete Response Rates in Triple-Negative Breast Cancer
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
Novel drugs approved in 2019
In 2019, the Food and Drug Administration approved 42 drugs, 6 of which will not be discussed here because of space limitations: recarbrio, a three-drug combination, containing imipenem, cilastatin, and relebactam; polatuzumab (Polivy) combined with bendamustine and a rituximab product; pretomanid combined with bedaquiline and linezolid; romosozumab (Evenity) for postmenopausal women; and alpelisib (Piqray) for postmenopausal women. In addition, darolutamide (Nubeqa) will not be included because it is indicated for the treatment of patients with prostate cancer. The remaining 36 agents are listed alphabetically below with the trade names in parentheses.
The molecular weights (if available), rounded to the nearest whole number, are shown in parentheses.
Air polymer-type a intrauterine foam (ExEm Foam), an ultrasound contrast agent, is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility. Animal studies have not been conducted, and the agent is contraindicated in pregnancy.
Afamelanotide implant (Scenesse) (1,647) is a melanocortin 1 receptor agonist that is indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria. The drug caused no embryofetal toxicity in two species of rats. The molecular weight suggests that it will not cross the placenta, at least early in pregnancy.
Alpelisib (Piqray) (441) is a kinase inhibitor that is combined with fulvestrant for the treatment of advanced breast cancer in women and men. The molecular weight suggests that it can cross the human placenta. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Bremelanotide (Vyleesi) (1,025) is indicated for the treatment of premenopausal women with hypoactive sexual disorder. The drug caused fetal harm in dogs and mice. If a pregnant woman is exposed to the drug, health care providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 877-411-2510.
Brolucizumab (Beovu) (26,000) is a human vascular endothelial growth factor that is indicated for the treatment of neovascular age-related macular degeneration. In animals, it caused malformations, embryofetal resorption, and decreased fetal weight. Other adverse effects were follicular development, corpus luteum function, and fertility.
Caplacizumab (Cablivi) (28,000) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenia purpura, in combination with plasma exchange and immunosuppressive therapy. If used in pregnancy, there is a risk of hemorrhage in the mother and fetus. In guinea pigs given intramuscular doses of the drug, there was no evidence of adverse developmental outcomes.
Cefiderocol (Fetroja) (3,044) is an IV cephalosporin antibiotic indicated for the treatment of urinary tract infections, including pyelonephritis. The manufacturer states that it should be used in patients 18 years of age or older who have limited or no alternative treatment options. Consistent with other cephalosporins, no developmental adverse effects were observed in rats and mice.
Cenobamate (Xcopri) (268) is indicated for the treatment of partial-onset seizures in adults. In pregnant animals given the drug, there was increased embryo-fetal mortality, decreased fetal and offspring body weight, and neurobehavioral and reproductive impairment in offspring. If a pregnant woman receives this drug, encourage her to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling the toll-free number 1-888-233-2334.
Crizanlizumab (Adakveo) (146,000) is indicated to reduce the frequency of vaso-occlusive crises in patients with sickle cell disease. In monkeys given doses slightly higher than those given to humans, there was increased fetal loss (abortions/stillbirths).
Entrectinib (Rozlytrek) (561) is a kinase inhibitor indicated for the treatment of cancer. The drug was teratogenic in rats. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Erdafitinib (Balversa) (447), a kinase inhibitor, is indicated for the treatment of locally advanced or metastatic urothelial carcinoma. In rats given doses during organogenesis with maternal exposures less than human exposures, the drug was teratogenic and caused embryofetal death. The manufacturer states that women of reproductive potential should use effective contraception during treatment and for 1 month after the last dose. The same advice was provided for male patients with female partners of reproductive potential. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fedratinib (Inrebic) (616), a kinase inhibitor, is indicated for patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The drug was teratogenic in rats when doses that were about 0.1 times the human exposure based on AUC (area under the curve) at the recommended daily dose during organogenesis. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fluorodopa f18 (214) is a radioactive diagnostic agent. It is indicated for use in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for evaluation of adult patients with suspected parkinsonian syndromes. The potential for adverse pregnant outcomes is based on the radiation dose and the gestational timing of exposure.
Givosiran sodium (Givlaari) (17,2460) is an aminolevulinate synthase 1-directed small interfering RNA given subcutaneously. It is indicated for the treatment of adults with acute hepatic porphyria. Doses less than 10 times the human dose in rats and rabbits produced maternal toxicity. In rats there was increased postimplantation loss, and in rats there was skeletal variation (incomplete ossification of pubes).
Golodirsen (Vyondys 53) (8,647) is indicated for the treatment of Duchenne muscular dystrophy given intravenously. There are no human or animal data available to assess the use of this drug during pregnancy.
Istradefylline (Nourianz) (384) is an adenosine receptor antagonist given orally. It is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. In pregnant rats and rabbits, the drug was related to teratogenicity, embryo-fetal and offspring mortality, and growth deficits at clinically relevant exposures.
Lasmiditan (Reyvow) (436), a serotonin receptor agonist, is indicated for acute treatment of migraine with or without aura. In animals, the drug caused increased incidences of fetal defects, increased embryo-fetal and offspring mortality, and decreased fetal body weight at maternal exposures less than (rabbits) or greater than (rat) those observed clinically.
Lefamulin (Xenleta) (568) is an antibacterial agent available for oral and IV administration. They are indicated for the treatment of community-acquired bacterial pneumonia. The drug was teratogenic in rats at systemic exposures lower than those in humans, an increased incidence of post-implantation fetal loss and stillbirths, and decreased fetal body weights and ossification. There was also an apparent delay in sexual maturation in rats.
Luspatercept (Reblozyl) (76,000) is given subcutaneously for the treatment of anemia in patients with beta thalassemia who require regular red blood cell transfusions. In rats and rabbits, the drug cause increased embryo-fetal mortality, alteration to growth, and structural defects at exposures (based on AUC) that were about 13 times (rats) and 18 times (rabbits) the maximum recommended human dose.
Pexidartinib (Turalio) (454) is an oral kinase inhibitor that is indicated for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not amenable with surgery. In rats and rabbits, the drug caused malformations, increased post-implantation loss, and abortion at exposures nearly equal to the human exposure. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Pitolisant HCl (Wakix) (296) is an histamine-3 receptor antagonist/inverse agonist indicated for the treatment of excessive daytime sleepiness in patients with narcolepsy. The drug has caused maternal and embryofetal toxicity in rats and rabbits at doses greater than and equal to 13 times and greater than 4 times the maximum human dose, respectively. The manufacturer has a pregnancy exposure registry that patients can contact at 1-800-833-7460.
Prabotulinum toxin A (Jeuveau) (900,000) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity. The drug caused no adverse embryo-fetal in rats with doses up to 12 times the human dose.
Risankizumab-rzaa (Skyrizi) (molecular weight unknown), an interleukin-23 antagonist, is used for the treatment of moderate-to-severe plaque psoriasis. In pregnant monkeys, doses that were 20 times the maximum human dose increased fetal/infant loss.
Selinexor (Xpovio) (443) is an oral nuclear export inhibitor given in combination with dexamethasone for the treatment of relapsed or refractory myeloma. At doses lower than those used clinically, the drug caused structural abnormalities and alterations to growth in fetal rats.
Siponimod (Mayzent) (1,149) is an oral sphingosine 1-phosphate receptor modulator. It is indicated for the treatment of relapsing forms of multiple sclerosis. At low doses, the drug caused embryotoxicity and fetotoxicity in rats and rabbits including embryofetal deaths and abortions. The drug was teratogenic in both species.
Solriamfetol (Sunosi) (231) is an oral dopamine and norepinephrine reuptake inhibitor that is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. The drug caused maternal and fetal toxicities in rats and rabbits and was teratogenic. The manufacturer has a pregnancy exposure registry to monitor pregnancy outcomes. Health care providers or patients can enroll in the program by calling 1-877-283-6220 or contacting the company.
Tafamidis meglumine (Vyndaqel) (503) and tafamidis (Vyndamax) (308) are indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis to reduce cardiovascular mortality and cardiovascular-related hospitalization. In rabbits and rats, use of the drugs during pregnancy caused birth defects, embryo-fetal mortality, and fetal body weight reduction. Limited available data with Vyndaqel use in human pregnancy at a dose of 20 mg/day have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see package insert).
Tenapanor (Ibsrela) (1,218) is indicated for the treatment of irritable bowel syndrome with constipation. The drug is minimally absorbed systemically, with plasma concentrations below the limit of quantification. No adverse maternal or fetal outcomes in rats or rabbits were observed. As reported by the manufacturer, in a small number of pregnant women, no drug-induced adverse maternal or fetal outcomes were identified.
Triclabendazole (Egaten) (360), an oral anthelmintic, is indicated for the treatment of fascioliasis. The drug was not teratogenic in mice and rabbits.
Trifarotene (Aklief) (460) cream is a retinoid that is indicated for the topical treatment of acne vulgaris. Animal data was related to oral retinoids and it not applicable to this agent. The manufacturer reported that available data from the use of the cream in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Upadacitinib (Rinvoq) (389) is an oral Janus inhibitor. It is indicated for the treatment of moderate to severe active rheumatoid arthritis in patients who have had an inadequate response or intolerance to methotrexate. The drug caused increases in fetal malformations when given to rats and rabbits during organogenesis.
Voxelotor (Oxbryta) (337) is an oral hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease. In rats and rabbits, there was no evidence of adverse developmental outcomes.
Zanubrutinib (Brukinsa) (472), an oral kinase inhibitor, is indicated for the treatment of mantle cell lymphoma. The drug caused embryofetal toxicity in pregnant rats, including malformations. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Breastfeeding
Brexanolone (Zulresso) (319) is indicated for the treatment of postpartum depression. It is given as a continuous IV infusion over 60 hours. The drug, at exposures close to those seen in humans, did not cause structural defects in rabbits and rats, but did cause fetal toxicity. Because patients are at risk of excessive sedation or sudden loss of consciousness when receiving the drug, it is only available through a restricted program called the ZULRESSO REMS. Health care providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 844-405-6185. To obtain a list of health care facilities enrolled in the program call 844-472-4379.
Nearly all of the above drugs will cross into a woman’s colostrum during the first 48 hours post partum. These amounts should be very small, but not breastfeeding is the best choice.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
In 2019, the Food and Drug Administration approved 42 drugs, 6 of which will not be discussed here because of space limitations: recarbrio, a three-drug combination, containing imipenem, cilastatin, and relebactam; polatuzumab (Polivy) combined with bendamustine and a rituximab product; pretomanid combined with bedaquiline and linezolid; romosozumab (Evenity) for postmenopausal women; and alpelisib (Piqray) for postmenopausal women. In addition, darolutamide (Nubeqa) will not be included because it is indicated for the treatment of patients with prostate cancer. The remaining 36 agents are listed alphabetically below with the trade names in parentheses.
The molecular weights (if available), rounded to the nearest whole number, are shown in parentheses.
Air polymer-type a intrauterine foam (ExEm Foam), an ultrasound contrast agent, is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility. Animal studies have not been conducted, and the agent is contraindicated in pregnancy.
Afamelanotide implant (Scenesse) (1,647) is a melanocortin 1 receptor agonist that is indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria. The drug caused no embryofetal toxicity in two species of rats. The molecular weight suggests that it will not cross the placenta, at least early in pregnancy.
Alpelisib (Piqray) (441) is a kinase inhibitor that is combined with fulvestrant for the treatment of advanced breast cancer in women and men. The molecular weight suggests that it can cross the human placenta. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Bremelanotide (Vyleesi) (1,025) is indicated for the treatment of premenopausal women with hypoactive sexual disorder. The drug caused fetal harm in dogs and mice. If a pregnant woman is exposed to the drug, health care providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 877-411-2510.
Brolucizumab (Beovu) (26,000) is a human vascular endothelial growth factor that is indicated for the treatment of neovascular age-related macular degeneration. In animals, it caused malformations, embryofetal resorption, and decreased fetal weight. Other adverse effects were follicular development, corpus luteum function, and fertility.
Caplacizumab (Cablivi) (28,000) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenia purpura, in combination with plasma exchange and immunosuppressive therapy. If used in pregnancy, there is a risk of hemorrhage in the mother and fetus. In guinea pigs given intramuscular doses of the drug, there was no evidence of adverse developmental outcomes.
Cefiderocol (Fetroja) (3,044) is an IV cephalosporin antibiotic indicated for the treatment of urinary tract infections, including pyelonephritis. The manufacturer states that it should be used in patients 18 years of age or older who have limited or no alternative treatment options. Consistent with other cephalosporins, no developmental adverse effects were observed in rats and mice.
Cenobamate (Xcopri) (268) is indicated for the treatment of partial-onset seizures in adults. In pregnant animals given the drug, there was increased embryo-fetal mortality, decreased fetal and offspring body weight, and neurobehavioral and reproductive impairment in offspring. If a pregnant woman receives this drug, encourage her to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling the toll-free number 1-888-233-2334.
Crizanlizumab (Adakveo) (146,000) is indicated to reduce the frequency of vaso-occlusive crises in patients with sickle cell disease. In monkeys given doses slightly higher than those given to humans, there was increased fetal loss (abortions/stillbirths).
Entrectinib (Rozlytrek) (561) is a kinase inhibitor indicated for the treatment of cancer. The drug was teratogenic in rats. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Erdafitinib (Balversa) (447), a kinase inhibitor, is indicated for the treatment of locally advanced or metastatic urothelial carcinoma. In rats given doses during organogenesis with maternal exposures less than human exposures, the drug was teratogenic and caused embryofetal death. The manufacturer states that women of reproductive potential should use effective contraception during treatment and for 1 month after the last dose. The same advice was provided for male patients with female partners of reproductive potential. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fedratinib (Inrebic) (616), a kinase inhibitor, is indicated for patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The drug was teratogenic in rats when doses that were about 0.1 times the human exposure based on AUC (area under the curve) at the recommended daily dose during organogenesis. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fluorodopa f18 (214) is a radioactive diagnostic agent. It is indicated for use in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for evaluation of adult patients with suspected parkinsonian syndromes. The potential for adverse pregnant outcomes is based on the radiation dose and the gestational timing of exposure.
Givosiran sodium (Givlaari) (17,2460) is an aminolevulinate synthase 1-directed small interfering RNA given subcutaneously. It is indicated for the treatment of adults with acute hepatic porphyria. Doses less than 10 times the human dose in rats and rabbits produced maternal toxicity. In rats there was increased postimplantation loss, and in rats there was skeletal variation (incomplete ossification of pubes).
Golodirsen (Vyondys 53) (8,647) is indicated for the treatment of Duchenne muscular dystrophy given intravenously. There are no human or animal data available to assess the use of this drug during pregnancy.
Istradefylline (Nourianz) (384) is an adenosine receptor antagonist given orally. It is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. In pregnant rats and rabbits, the drug was related to teratogenicity, embryo-fetal and offspring mortality, and growth deficits at clinically relevant exposures.
Lasmiditan (Reyvow) (436), a serotonin receptor agonist, is indicated for acute treatment of migraine with or without aura. In animals, the drug caused increased incidences of fetal defects, increased embryo-fetal and offspring mortality, and decreased fetal body weight at maternal exposures less than (rabbits) or greater than (rat) those observed clinically.
Lefamulin (Xenleta) (568) is an antibacterial agent available for oral and IV administration. They are indicated for the treatment of community-acquired bacterial pneumonia. The drug was teratogenic in rats at systemic exposures lower than those in humans, an increased incidence of post-implantation fetal loss and stillbirths, and decreased fetal body weights and ossification. There was also an apparent delay in sexual maturation in rats.
Luspatercept (Reblozyl) (76,000) is given subcutaneously for the treatment of anemia in patients with beta thalassemia who require regular red blood cell transfusions. In rats and rabbits, the drug cause increased embryo-fetal mortality, alteration to growth, and structural defects at exposures (based on AUC) that were about 13 times (rats) and 18 times (rabbits) the maximum recommended human dose.
Pexidartinib (Turalio) (454) is an oral kinase inhibitor that is indicated for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not amenable with surgery. In rats and rabbits, the drug caused malformations, increased post-implantation loss, and abortion at exposures nearly equal to the human exposure. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Pitolisant HCl (Wakix) (296) is an histamine-3 receptor antagonist/inverse agonist indicated for the treatment of excessive daytime sleepiness in patients with narcolepsy. The drug has caused maternal and embryofetal toxicity in rats and rabbits at doses greater than and equal to 13 times and greater than 4 times the maximum human dose, respectively. The manufacturer has a pregnancy exposure registry that patients can contact at 1-800-833-7460.
Prabotulinum toxin A (Jeuveau) (900,000) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity. The drug caused no adverse embryo-fetal in rats with doses up to 12 times the human dose.
Risankizumab-rzaa (Skyrizi) (molecular weight unknown), an interleukin-23 antagonist, is used for the treatment of moderate-to-severe plaque psoriasis. In pregnant monkeys, doses that were 20 times the maximum human dose increased fetal/infant loss.
Selinexor (Xpovio) (443) is an oral nuclear export inhibitor given in combination with dexamethasone for the treatment of relapsed or refractory myeloma. At doses lower than those used clinically, the drug caused structural abnormalities and alterations to growth in fetal rats.
Siponimod (Mayzent) (1,149) is an oral sphingosine 1-phosphate receptor modulator. It is indicated for the treatment of relapsing forms of multiple sclerosis. At low doses, the drug caused embryotoxicity and fetotoxicity in rats and rabbits including embryofetal deaths and abortions. The drug was teratogenic in both species.
Solriamfetol (Sunosi) (231) is an oral dopamine and norepinephrine reuptake inhibitor that is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. The drug caused maternal and fetal toxicities in rats and rabbits and was teratogenic. The manufacturer has a pregnancy exposure registry to monitor pregnancy outcomes. Health care providers or patients can enroll in the program by calling 1-877-283-6220 or contacting the company.
Tafamidis meglumine (Vyndaqel) (503) and tafamidis (Vyndamax) (308) are indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis to reduce cardiovascular mortality and cardiovascular-related hospitalization. In rabbits and rats, use of the drugs during pregnancy caused birth defects, embryo-fetal mortality, and fetal body weight reduction. Limited available data with Vyndaqel use in human pregnancy at a dose of 20 mg/day have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see package insert).
Tenapanor (Ibsrela) (1,218) is indicated for the treatment of irritable bowel syndrome with constipation. The drug is minimally absorbed systemically, with plasma concentrations below the limit of quantification. No adverse maternal or fetal outcomes in rats or rabbits were observed. As reported by the manufacturer, in a small number of pregnant women, no drug-induced adverse maternal or fetal outcomes were identified.
Triclabendazole (Egaten) (360), an oral anthelmintic, is indicated for the treatment of fascioliasis. The drug was not teratogenic in mice and rabbits.
Trifarotene (Aklief) (460) cream is a retinoid that is indicated for the topical treatment of acne vulgaris. Animal data was related to oral retinoids and it not applicable to this agent. The manufacturer reported that available data from the use of the cream in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Upadacitinib (Rinvoq) (389) is an oral Janus inhibitor. It is indicated for the treatment of moderate to severe active rheumatoid arthritis in patients who have had an inadequate response or intolerance to methotrexate. The drug caused increases in fetal malformations when given to rats and rabbits during organogenesis.
Voxelotor (Oxbryta) (337) is an oral hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease. In rats and rabbits, there was no evidence of adverse developmental outcomes.
Zanubrutinib (Brukinsa) (472), an oral kinase inhibitor, is indicated for the treatment of mantle cell lymphoma. The drug caused embryofetal toxicity in pregnant rats, including malformations. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Breastfeeding
Brexanolone (Zulresso) (319) is indicated for the treatment of postpartum depression. It is given as a continuous IV infusion over 60 hours. The drug, at exposures close to those seen in humans, did not cause structural defects in rabbits and rats, but did cause fetal toxicity. Because patients are at risk of excessive sedation or sudden loss of consciousness when receiving the drug, it is only available through a restricted program called the ZULRESSO REMS. Health care providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 844-405-6185. To obtain a list of health care facilities enrolled in the program call 844-472-4379.
Nearly all of the above drugs will cross into a woman’s colostrum during the first 48 hours post partum. These amounts should be very small, but not breastfeeding is the best choice.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
In 2019, the Food and Drug Administration approved 42 drugs, 6 of which will not be discussed here because of space limitations: recarbrio, a three-drug combination, containing imipenem, cilastatin, and relebactam; polatuzumab (Polivy) combined with bendamustine and a rituximab product; pretomanid combined with bedaquiline and linezolid; romosozumab (Evenity) for postmenopausal women; and alpelisib (Piqray) for postmenopausal women. In addition, darolutamide (Nubeqa) will not be included because it is indicated for the treatment of patients with prostate cancer. The remaining 36 agents are listed alphabetically below with the trade names in parentheses.
The molecular weights (if available), rounded to the nearest whole number, are shown in parentheses.
Air polymer-type a intrauterine foam (ExEm Foam), an ultrasound contrast agent, is indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility. Animal studies have not been conducted, and the agent is contraindicated in pregnancy.
Afamelanotide implant (Scenesse) (1,647) is a melanocortin 1 receptor agonist that is indicated to increase pain-free light exposure in adult patients with a history of phototoxic reactions from erythropoietic protoporphyria. The drug caused no embryofetal toxicity in two species of rats. The molecular weight suggests that it will not cross the placenta, at least early in pregnancy.
Alpelisib (Piqray) (441) is a kinase inhibitor that is combined with fulvestrant for the treatment of advanced breast cancer in women and men. The molecular weight suggests that it can cross the human placenta. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Bremelanotide (Vyleesi) (1,025) is indicated for the treatment of premenopausal women with hypoactive sexual disorder. The drug caused fetal harm in dogs and mice. If a pregnant woman is exposed to the drug, health care providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 877-411-2510.
Brolucizumab (Beovu) (26,000) is a human vascular endothelial growth factor that is indicated for the treatment of neovascular age-related macular degeneration. In animals, it caused malformations, embryofetal resorption, and decreased fetal weight. Other adverse effects were follicular development, corpus luteum function, and fertility.
Caplacizumab (Cablivi) (28,000) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenia purpura, in combination with plasma exchange and immunosuppressive therapy. If used in pregnancy, there is a risk of hemorrhage in the mother and fetus. In guinea pigs given intramuscular doses of the drug, there was no evidence of adverse developmental outcomes.
Cefiderocol (Fetroja) (3,044) is an IV cephalosporin antibiotic indicated for the treatment of urinary tract infections, including pyelonephritis. The manufacturer states that it should be used in patients 18 years of age or older who have limited or no alternative treatment options. Consistent with other cephalosporins, no developmental adverse effects were observed in rats and mice.
Cenobamate (Xcopri) (268) is indicated for the treatment of partial-onset seizures in adults. In pregnant animals given the drug, there was increased embryo-fetal mortality, decreased fetal and offspring body weight, and neurobehavioral and reproductive impairment in offspring. If a pregnant woman receives this drug, encourage her to enroll in the North American Antiepileptic Drug Pregnancy Registry by calling the toll-free number 1-888-233-2334.
Crizanlizumab (Adakveo) (146,000) is indicated to reduce the frequency of vaso-occlusive crises in patients with sickle cell disease. In monkeys given doses slightly higher than those given to humans, there was increased fetal loss (abortions/stillbirths).
Entrectinib (Rozlytrek) (561) is a kinase inhibitor indicated for the treatment of cancer. The drug was teratogenic in rats. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Erdafitinib (Balversa) (447), a kinase inhibitor, is indicated for the treatment of locally advanced or metastatic urothelial carcinoma. In rats given doses during organogenesis with maternal exposures less than human exposures, the drug was teratogenic and caused embryofetal death. The manufacturer states that women of reproductive potential should use effective contraception during treatment and for 1 month after the last dose. The same advice was provided for male patients with female partners of reproductive potential. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fedratinib (Inrebic) (616), a kinase inhibitor, is indicated for patients with intermediate-2 or high-risk primary or secondary myelofibrosis. The drug was teratogenic in rats when doses that were about 0.1 times the human exposure based on AUC (area under the curve) at the recommended daily dose during organogenesis. It is contraindicated in pregnancy because it can cause embryofetal toxicity.
Fluorodopa f18 (214) is a radioactive diagnostic agent. It is indicated for use in positron emission tomography to visualize dopaminergic nerve terminals in the striatum for evaluation of adult patients with suspected parkinsonian syndromes. The potential for adverse pregnant outcomes is based on the radiation dose and the gestational timing of exposure.
Givosiran sodium (Givlaari) (17,2460) is an aminolevulinate synthase 1-directed small interfering RNA given subcutaneously. It is indicated for the treatment of adults with acute hepatic porphyria. Doses less than 10 times the human dose in rats and rabbits produced maternal toxicity. In rats there was increased postimplantation loss, and in rats there was skeletal variation (incomplete ossification of pubes).
Golodirsen (Vyondys 53) (8,647) is indicated for the treatment of Duchenne muscular dystrophy given intravenously. There are no human or animal data available to assess the use of this drug during pregnancy.
Istradefylline (Nourianz) (384) is an adenosine receptor antagonist given orally. It is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. In pregnant rats and rabbits, the drug was related to teratogenicity, embryo-fetal and offspring mortality, and growth deficits at clinically relevant exposures.
Lasmiditan (Reyvow) (436), a serotonin receptor agonist, is indicated for acute treatment of migraine with or without aura. In animals, the drug caused increased incidences of fetal defects, increased embryo-fetal and offspring mortality, and decreased fetal body weight at maternal exposures less than (rabbits) or greater than (rat) those observed clinically.
Lefamulin (Xenleta) (568) is an antibacterial agent available for oral and IV administration. They are indicated for the treatment of community-acquired bacterial pneumonia. The drug was teratogenic in rats at systemic exposures lower than those in humans, an increased incidence of post-implantation fetal loss and stillbirths, and decreased fetal body weights and ossification. There was also an apparent delay in sexual maturation in rats.
Luspatercept (Reblozyl) (76,000) is given subcutaneously for the treatment of anemia in patients with beta thalassemia who require regular red blood cell transfusions. In rats and rabbits, the drug cause increased embryo-fetal mortality, alteration to growth, and structural defects at exposures (based on AUC) that were about 13 times (rats) and 18 times (rabbits) the maximum recommended human dose.
Pexidartinib (Turalio) (454) is an oral kinase inhibitor that is indicated for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not amenable with surgery. In rats and rabbits, the drug caused malformations, increased post-implantation loss, and abortion at exposures nearly equal to the human exposure. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Pitolisant HCl (Wakix) (296) is an histamine-3 receptor antagonist/inverse agonist indicated for the treatment of excessive daytime sleepiness in patients with narcolepsy. The drug has caused maternal and embryofetal toxicity in rats and rabbits at doses greater than and equal to 13 times and greater than 4 times the maximum human dose, respectively. The manufacturer has a pregnancy exposure registry that patients can contact at 1-800-833-7460.
Prabotulinum toxin A (Jeuveau) (900,000) is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity. The drug caused no adverse embryo-fetal in rats with doses up to 12 times the human dose.
Risankizumab-rzaa (Skyrizi) (molecular weight unknown), an interleukin-23 antagonist, is used for the treatment of moderate-to-severe plaque psoriasis. In pregnant monkeys, doses that were 20 times the maximum human dose increased fetal/infant loss.
Selinexor (Xpovio) (443) is an oral nuclear export inhibitor given in combination with dexamethasone for the treatment of relapsed or refractory myeloma. At doses lower than those used clinically, the drug caused structural abnormalities and alterations to growth in fetal rats.
Siponimod (Mayzent) (1,149) is an oral sphingosine 1-phosphate receptor modulator. It is indicated for the treatment of relapsing forms of multiple sclerosis. At low doses, the drug caused embryotoxicity and fetotoxicity in rats and rabbits including embryofetal deaths and abortions. The drug was teratogenic in both species.
Solriamfetol (Sunosi) (231) is an oral dopamine and norepinephrine reuptake inhibitor that is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. The drug caused maternal and fetal toxicities in rats and rabbits and was teratogenic. The manufacturer has a pregnancy exposure registry to monitor pregnancy outcomes. Health care providers or patients can enroll in the program by calling 1-877-283-6220 or contacting the company.
Tafamidis meglumine (Vyndaqel) (503) and tafamidis (Vyndamax) (308) are indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis to reduce cardiovascular mortality and cardiovascular-related hospitalization. In rabbits and rats, use of the drugs during pregnancy caused birth defects, embryo-fetal mortality, and fetal body weight reduction. Limited available data with Vyndaqel use in human pregnancy at a dose of 20 mg/day have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see package insert).
Tenapanor (Ibsrela) (1,218) is indicated for the treatment of irritable bowel syndrome with constipation. The drug is minimally absorbed systemically, with plasma concentrations below the limit of quantification. No adverse maternal or fetal outcomes in rats or rabbits were observed. As reported by the manufacturer, in a small number of pregnant women, no drug-induced adverse maternal or fetal outcomes were identified.
Triclabendazole (Egaten) (360), an oral anthelmintic, is indicated for the treatment of fascioliasis. The drug was not teratogenic in mice and rabbits.
Trifarotene (Aklief) (460) cream is a retinoid that is indicated for the topical treatment of acne vulgaris. Animal data was related to oral retinoids and it not applicable to this agent. The manufacturer reported that available data from the use of the cream in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Upadacitinib (Rinvoq) (389) is an oral Janus inhibitor. It is indicated for the treatment of moderate to severe active rheumatoid arthritis in patients who have had an inadequate response or intolerance to methotrexate. The drug caused increases in fetal malformations when given to rats and rabbits during organogenesis.
Voxelotor (Oxbryta) (337) is an oral hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease. In rats and rabbits, there was no evidence of adverse developmental outcomes.
Zanubrutinib (Brukinsa) (472), an oral kinase inhibitor, is indicated for the treatment of mantle cell lymphoma. The drug caused embryofetal toxicity in pregnant rats, including malformations. It is contraindicated in pregnancy because it can cause embryo-fetal toxicity.
Breastfeeding
Brexanolone (Zulresso) (319) is indicated for the treatment of postpartum depression. It is given as a continuous IV infusion over 60 hours. The drug, at exposures close to those seen in humans, did not cause structural defects in rabbits and rats, but did cause fetal toxicity. Because patients are at risk of excessive sedation or sudden loss of consciousness when receiving the drug, it is only available through a restricted program called the ZULRESSO REMS. Health care providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 844-405-6185. To obtain a list of health care facilities enrolled in the program call 844-472-4379.
Nearly all of the above drugs will cross into a woman’s colostrum during the first 48 hours post partum. These amounts should be very small, but not breastfeeding is the best choice.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
Study challenges role of birth canal exposure in newborn microbiome establishment
During parturient transmission of gut bacteria from mothers to infants, the dominant maternal source of bacteria is rectal, according to investigators.
This challenges the hypothesis that exposure to the birth canal explains major differences in gut bacteria between infants born vaginally and those born via C-section, reported Moran Yassour, PhD, of Hebrew University in Jerusalem.
“It’s not how and if you entered the birth canal, but rather how you exited it,” Dr. Yassour said during a presentation at the annual Gut Microbiota for Health World Summit.
According to Dr. Yassour, a number of investigators have evaluated vertical transmission of gut bacteria from mothers to newborns, but most began collecting data a week or more after birth, potentially missing critical information.
“We wanted to generate large-scale, paired, longitudinal data, which means that we had [samples from] both mothers and children, and we wanted to start at birth,” Dr. Yassour said at the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
Dr. Yassour explained that newborns delivered vaginally often exhibit Bacteroides in their gut, whereas babies born via C-section do not exhibit these bacteria until 6-18 months of age; however, the vaginal microbiome typically lacks Bacteroides, making the birth canal an unlikely source. This disconnect served as the impetus for the present investigation, Dr. Yassour said.
The study, which is available as a preprint, involved 73 mothers and their infants. To determine the impact of birth canal exposure, the investigators compared gut bacteria of infants born vaginally with those born via pre-labor C-section (no exposure to the birth canal), and those born via post-labor C-section (exposure to the birth canal).
Initial results were surprising, Dr. Yassour said, as 54% of babies delivered via C-section had Bacteroides in their stool during the first week. But in the second week, 94% of the C-section group lacked Bacteroides, which aligns with characteristic findings and suggests failure of colonization, rather than complete lack of exposure.
Out of the 24 infants with persistent Bacteroides colonization, 22 (92%) were born vaginally, compared with 2 (8%) born via pre-labor C-section, and none born via post-labor C-section. This pattern was maintained in a multivariate analysis that accounted for antibiotic use and exposure to formula, both of which are more common among mothers that give birth via C-section.
The investigators also conducted a strain-level analysis of mothers and infants using metagenomic sequencing. Across all time points, 90% of matched maternal-infant strains were detected in babies delivered vaginally.
“[W]e found evidence for mother-to-child transmission of rectal rather than vaginal strains,” the investigators wrote. “These results challenge birth canal exposure as the dominant factor in infant gut microbiome establishment and implicate colonization efficiency rather than exposure as a dictating factor of the newborn gut microbiome composition.”
Dr. Yassour said that these findings may have an immediate effect on clinical practice.
“People have reported the practice of smearing babies that were born by C-section with vaginal fluids in the sense of trying to recapitulate the microbial signature that we find in kids born vaginally,” Dr. Yassour said. “But it’s probably not the vaginal fluid that we need to smear; it’s probably the proximity to the rectum and the bowel movements that happen during delivery ... and that is what’s causing this initial seeding from mother to child.”
Dr. Yassour disclosed no conflicts of interest.
SOURCE: Yassour M et al. GMFH 2020.
During parturient transmission of gut bacteria from mothers to infants, the dominant maternal source of bacteria is rectal, according to investigators.
This challenges the hypothesis that exposure to the birth canal explains major differences in gut bacteria between infants born vaginally and those born via C-section, reported Moran Yassour, PhD, of Hebrew University in Jerusalem.
“It’s not how and if you entered the birth canal, but rather how you exited it,” Dr. Yassour said during a presentation at the annual Gut Microbiota for Health World Summit.
According to Dr. Yassour, a number of investigators have evaluated vertical transmission of gut bacteria from mothers to newborns, but most began collecting data a week or more after birth, potentially missing critical information.
“We wanted to generate large-scale, paired, longitudinal data, which means that we had [samples from] both mothers and children, and we wanted to start at birth,” Dr. Yassour said at the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
Dr. Yassour explained that newborns delivered vaginally often exhibit Bacteroides in their gut, whereas babies born via C-section do not exhibit these bacteria until 6-18 months of age; however, the vaginal microbiome typically lacks Bacteroides, making the birth canal an unlikely source. This disconnect served as the impetus for the present investigation, Dr. Yassour said.
The study, which is available as a preprint, involved 73 mothers and their infants. To determine the impact of birth canal exposure, the investigators compared gut bacteria of infants born vaginally with those born via pre-labor C-section (no exposure to the birth canal), and those born via post-labor C-section (exposure to the birth canal).
Initial results were surprising, Dr. Yassour said, as 54% of babies delivered via C-section had Bacteroides in their stool during the first week. But in the second week, 94% of the C-section group lacked Bacteroides, which aligns with characteristic findings and suggests failure of colonization, rather than complete lack of exposure.
Out of the 24 infants with persistent Bacteroides colonization, 22 (92%) were born vaginally, compared with 2 (8%) born via pre-labor C-section, and none born via post-labor C-section. This pattern was maintained in a multivariate analysis that accounted for antibiotic use and exposure to formula, both of which are more common among mothers that give birth via C-section.
The investigators also conducted a strain-level analysis of mothers and infants using metagenomic sequencing. Across all time points, 90% of matched maternal-infant strains were detected in babies delivered vaginally.
“[W]e found evidence for mother-to-child transmission of rectal rather than vaginal strains,” the investigators wrote. “These results challenge birth canal exposure as the dominant factor in infant gut microbiome establishment and implicate colonization efficiency rather than exposure as a dictating factor of the newborn gut microbiome composition.”
Dr. Yassour said that these findings may have an immediate effect on clinical practice.
“People have reported the practice of smearing babies that were born by C-section with vaginal fluids in the sense of trying to recapitulate the microbial signature that we find in kids born vaginally,” Dr. Yassour said. “But it’s probably not the vaginal fluid that we need to smear; it’s probably the proximity to the rectum and the bowel movements that happen during delivery ... and that is what’s causing this initial seeding from mother to child.”
Dr. Yassour disclosed no conflicts of interest.
SOURCE: Yassour M et al. GMFH 2020.
During parturient transmission of gut bacteria from mothers to infants, the dominant maternal source of bacteria is rectal, according to investigators.
This challenges the hypothesis that exposure to the birth canal explains major differences in gut bacteria between infants born vaginally and those born via C-section, reported Moran Yassour, PhD, of Hebrew University in Jerusalem.
“It’s not how and if you entered the birth canal, but rather how you exited it,” Dr. Yassour said during a presentation at the annual Gut Microbiota for Health World Summit.
According to Dr. Yassour, a number of investigators have evaluated vertical transmission of gut bacteria from mothers to newborns, but most began collecting data a week or more after birth, potentially missing critical information.
“We wanted to generate large-scale, paired, longitudinal data, which means that we had [samples from] both mothers and children, and we wanted to start at birth,” Dr. Yassour said at the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
Dr. Yassour explained that newborns delivered vaginally often exhibit Bacteroides in their gut, whereas babies born via C-section do not exhibit these bacteria until 6-18 months of age; however, the vaginal microbiome typically lacks Bacteroides, making the birth canal an unlikely source. This disconnect served as the impetus for the present investigation, Dr. Yassour said.
The study, which is available as a preprint, involved 73 mothers and their infants. To determine the impact of birth canal exposure, the investigators compared gut bacteria of infants born vaginally with those born via pre-labor C-section (no exposure to the birth canal), and those born via post-labor C-section (exposure to the birth canal).
Initial results were surprising, Dr. Yassour said, as 54% of babies delivered via C-section had Bacteroides in their stool during the first week. But in the second week, 94% of the C-section group lacked Bacteroides, which aligns with characteristic findings and suggests failure of colonization, rather than complete lack of exposure.
Out of the 24 infants with persistent Bacteroides colonization, 22 (92%) were born vaginally, compared with 2 (8%) born via pre-labor C-section, and none born via post-labor C-section. This pattern was maintained in a multivariate analysis that accounted for antibiotic use and exposure to formula, both of which are more common among mothers that give birth via C-section.
The investigators also conducted a strain-level analysis of mothers and infants using metagenomic sequencing. Across all time points, 90% of matched maternal-infant strains were detected in babies delivered vaginally.
“[W]e found evidence for mother-to-child transmission of rectal rather than vaginal strains,” the investigators wrote. “These results challenge birth canal exposure as the dominant factor in infant gut microbiome establishment and implicate colonization efficiency rather than exposure as a dictating factor of the newborn gut microbiome composition.”
Dr. Yassour said that these findings may have an immediate effect on clinical practice.
“People have reported the practice of smearing babies that were born by C-section with vaginal fluids in the sense of trying to recapitulate the microbial signature that we find in kids born vaginally,” Dr. Yassour said. “But it’s probably not the vaginal fluid that we need to smear; it’s probably the proximity to the rectum and the bowel movements that happen during delivery ... and that is what’s causing this initial seeding from mother to child.”
Dr. Yassour disclosed no conflicts of interest.
SOURCE: Yassour M et al. GMFH 2020.
FROM GMFH 2020
HCV screening risk factors in pregnant women need updating
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
FROM OBSTETRICS & GYNECOLOGY