User login
Beginning estrogen soon after menopause slows atherosclerosis progression
PHOENIX – Oral estrogen therapy taken within 6 years after the onset of menopause significantly reduced progression of lipid deposition in the carotid arterial wall, compared with placebo. However, starting oral estrogen 10 years after menopause did not confer a similar benefit.
“The clinical practice of estradiol therapy has been nothing short of a roller coaster ride,” lead study author Roksana Karim, PhD, MBBS, said in an interview at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association. “Clinicians have been sort of conservative in terms of prescribing estradiol therapy. But over the last 2 decades things have changed, and eventually the timing hypothesis evolved based on the final analysis of the Women’s Health Initiative results as well.”
The findings come from a secondary analysis of the Early Versus Late Intervention Trial With Estradiol (ELITE), which examined the effects of oral 17-beta-estradiol (estrogen) on the progression of early atherosclerosis and cognitive decline in healthy postmenopausal women.
In the original trial, 643 healthy postmenopausal women were randomized to receive 1 mg/day of estradiol or a placebo pill either within 6 years after the onset of menopause or more than a decade after menopause (N Engl J Med 2016;374[13]:1221-31). All study participants took estradiol or placebo daily for an average of 5 years. The study’s initial findings showed that the mean carotid intima-media thickness progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo, but only in women who initiated hormone therapy within 6 years of menopause onset.
For the current analysis, researchers led by Dr. Karim looked further into estradiol’s impact on heart health by using echogenicity to analyze lipids in the arterial wall among the ELITE participants. The main outcome of interest was gray-scale median (GSM, unitless), a qualitative measure of atherosclerosis based on echogenicity obtained by high-resolution ultrasonography of the common carotid arterial wall. Whereas higher GSM values result with plaques rich in calcium and fibrous tissue, lower GSM values indicate more lipid deposition.
Dr. Karim, an associate professor of clinical preventive medicine at the University of Southern California, Los Angeles, and colleagues assessed GSM and serum concentrations of estradiol every 6 months over a median 5-year trial period, and used linear mixed effects regression models to compare the rate of GSM progression between the randomized groups within time-since-menopause strata.
The researchers found that effect of estradiol on the annual rate of GSM progression significantly differed between women in the early and late postmenopause groups (P for interaction = .006). Specifically, the annual GSM progression rate among women in early postmenopause fell by 0.30 per year in women taking estradiol, compared with 1.41 per year in those in the placebo group (P less than .0001), indicating significantly more atherosclerosis in the placebo group. On the other hand, the annual GSM progression rate was not significantly different between the estradiol and placebo groups among the late postmenopausal women (P = .37).
“I think this should comfort clinicians in terms of prescribing estradiol therapy to women who don’t have any contraindications and who are within 6 years of menopause,” Dr. Karim said. “Accumulation of lipids is the key event for atherosclerosis progression.” She and her colleagues also observed that the positive association between mean on-trial serum estradiol levels and GSM progression rate was stronger and significant among early postmenopausal women (P = .008), compared with women in the late postmenopausal group (P = .003). However, this differential association between estradiol level and GSM progression rate was not statistically significant (P for interaction = .33).
“This study is important and raises a critical question: Is there a time period where getting hormone therapy would be most beneficial for the heart?” Nieca Goldberg, MD, medical director of the New York University women’s heart program and senior advisor for women’s health strategy at NYU Langone Health, said in an interview. “I think more studies and more analyses are needed, but we haven’t changed the indications for estradiol. We’re not giving estradiol to prevent progression of heart disease. We use estradiol hormone therapy as indicated for women who are having menopausal symptoms.”
Dr. Karim and colleagues plan to conduct a follow-up analysis from the same cohort of ELITE study participants to validate the findings by assessing lipid particles and markers of inflammation.
She reported having no financial disclosures. The study was funded by the National Institute on Aging.
SOURCE: Karim R et al. Epi/Lifestyle 2020, Abstract MP09.
PHOENIX – Oral estrogen therapy taken within 6 years after the onset of menopause significantly reduced progression of lipid deposition in the carotid arterial wall, compared with placebo. However, starting oral estrogen 10 years after menopause did not confer a similar benefit.
“The clinical practice of estradiol therapy has been nothing short of a roller coaster ride,” lead study author Roksana Karim, PhD, MBBS, said in an interview at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association. “Clinicians have been sort of conservative in terms of prescribing estradiol therapy. But over the last 2 decades things have changed, and eventually the timing hypothesis evolved based on the final analysis of the Women’s Health Initiative results as well.”
The findings come from a secondary analysis of the Early Versus Late Intervention Trial With Estradiol (ELITE), which examined the effects of oral 17-beta-estradiol (estrogen) on the progression of early atherosclerosis and cognitive decline in healthy postmenopausal women.
In the original trial, 643 healthy postmenopausal women were randomized to receive 1 mg/day of estradiol or a placebo pill either within 6 years after the onset of menopause or more than a decade after menopause (N Engl J Med 2016;374[13]:1221-31). All study participants took estradiol or placebo daily for an average of 5 years. The study’s initial findings showed that the mean carotid intima-media thickness progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo, but only in women who initiated hormone therapy within 6 years of menopause onset.
For the current analysis, researchers led by Dr. Karim looked further into estradiol’s impact on heart health by using echogenicity to analyze lipids in the arterial wall among the ELITE participants. The main outcome of interest was gray-scale median (GSM, unitless), a qualitative measure of atherosclerosis based on echogenicity obtained by high-resolution ultrasonography of the common carotid arterial wall. Whereas higher GSM values result with plaques rich in calcium and fibrous tissue, lower GSM values indicate more lipid deposition.
Dr. Karim, an associate professor of clinical preventive medicine at the University of Southern California, Los Angeles, and colleagues assessed GSM and serum concentrations of estradiol every 6 months over a median 5-year trial period, and used linear mixed effects regression models to compare the rate of GSM progression between the randomized groups within time-since-menopause strata.
The researchers found that effect of estradiol on the annual rate of GSM progression significantly differed between women in the early and late postmenopause groups (P for interaction = .006). Specifically, the annual GSM progression rate among women in early postmenopause fell by 0.30 per year in women taking estradiol, compared with 1.41 per year in those in the placebo group (P less than .0001), indicating significantly more atherosclerosis in the placebo group. On the other hand, the annual GSM progression rate was not significantly different between the estradiol and placebo groups among the late postmenopausal women (P = .37).
“I think this should comfort clinicians in terms of prescribing estradiol therapy to women who don’t have any contraindications and who are within 6 years of menopause,” Dr. Karim said. “Accumulation of lipids is the key event for atherosclerosis progression.” She and her colleagues also observed that the positive association between mean on-trial serum estradiol levels and GSM progression rate was stronger and significant among early postmenopausal women (P = .008), compared with women in the late postmenopausal group (P = .003). However, this differential association between estradiol level and GSM progression rate was not statistically significant (P for interaction = .33).
“This study is important and raises a critical question: Is there a time period where getting hormone therapy would be most beneficial for the heart?” Nieca Goldberg, MD, medical director of the New York University women’s heart program and senior advisor for women’s health strategy at NYU Langone Health, said in an interview. “I think more studies and more analyses are needed, but we haven’t changed the indications for estradiol. We’re not giving estradiol to prevent progression of heart disease. We use estradiol hormone therapy as indicated for women who are having menopausal symptoms.”
Dr. Karim and colleagues plan to conduct a follow-up analysis from the same cohort of ELITE study participants to validate the findings by assessing lipid particles and markers of inflammation.
She reported having no financial disclosures. The study was funded by the National Institute on Aging.
SOURCE: Karim R et al. Epi/Lifestyle 2020, Abstract MP09.
PHOENIX – Oral estrogen therapy taken within 6 years after the onset of menopause significantly reduced progression of lipid deposition in the carotid arterial wall, compared with placebo. However, starting oral estrogen 10 years after menopause did not confer a similar benefit.
“The clinical practice of estradiol therapy has been nothing short of a roller coaster ride,” lead study author Roksana Karim, PhD, MBBS, said in an interview at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association. “Clinicians have been sort of conservative in terms of prescribing estradiol therapy. But over the last 2 decades things have changed, and eventually the timing hypothesis evolved based on the final analysis of the Women’s Health Initiative results as well.”
The findings come from a secondary analysis of the Early Versus Late Intervention Trial With Estradiol (ELITE), which examined the effects of oral 17-beta-estradiol (estrogen) on the progression of early atherosclerosis and cognitive decline in healthy postmenopausal women.
In the original trial, 643 healthy postmenopausal women were randomized to receive 1 mg/day of estradiol or a placebo pill either within 6 years after the onset of menopause or more than a decade after menopause (N Engl J Med 2016;374[13]:1221-31). All study participants took estradiol or placebo daily for an average of 5 years. The study’s initial findings showed that the mean carotid intima-media thickness progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo, but only in women who initiated hormone therapy within 6 years of menopause onset.
For the current analysis, researchers led by Dr. Karim looked further into estradiol’s impact on heart health by using echogenicity to analyze lipids in the arterial wall among the ELITE participants. The main outcome of interest was gray-scale median (GSM, unitless), a qualitative measure of atherosclerosis based on echogenicity obtained by high-resolution ultrasonography of the common carotid arterial wall. Whereas higher GSM values result with plaques rich in calcium and fibrous tissue, lower GSM values indicate more lipid deposition.
Dr. Karim, an associate professor of clinical preventive medicine at the University of Southern California, Los Angeles, and colleagues assessed GSM and serum concentrations of estradiol every 6 months over a median 5-year trial period, and used linear mixed effects regression models to compare the rate of GSM progression between the randomized groups within time-since-menopause strata.
The researchers found that effect of estradiol on the annual rate of GSM progression significantly differed between women in the early and late postmenopause groups (P for interaction = .006). Specifically, the annual GSM progression rate among women in early postmenopause fell by 0.30 per year in women taking estradiol, compared with 1.41 per year in those in the placebo group (P less than .0001), indicating significantly more atherosclerosis in the placebo group. On the other hand, the annual GSM progression rate was not significantly different between the estradiol and placebo groups among the late postmenopausal women (P = .37).
“I think this should comfort clinicians in terms of prescribing estradiol therapy to women who don’t have any contraindications and who are within 6 years of menopause,” Dr. Karim said. “Accumulation of lipids is the key event for atherosclerosis progression.” She and her colleagues also observed that the positive association between mean on-trial serum estradiol levels and GSM progression rate was stronger and significant among early postmenopausal women (P = .008), compared with women in the late postmenopausal group (P = .003). However, this differential association between estradiol level and GSM progression rate was not statistically significant (P for interaction = .33).
“This study is important and raises a critical question: Is there a time period where getting hormone therapy would be most beneficial for the heart?” Nieca Goldberg, MD, medical director of the New York University women’s heart program and senior advisor for women’s health strategy at NYU Langone Health, said in an interview. “I think more studies and more analyses are needed, but we haven’t changed the indications for estradiol. We’re not giving estradiol to prevent progression of heart disease. We use estradiol hormone therapy as indicated for women who are having menopausal symptoms.”
Dr. Karim and colleagues plan to conduct a follow-up analysis from the same cohort of ELITE study participants to validate the findings by assessing lipid particles and markers of inflammation.
She reported having no financial disclosures. The study was funded by the National Institute on Aging.
SOURCE: Karim R et al. Epi/Lifestyle 2020, Abstract MP09.
REPORTING FROM EPI/LIFESTYLE 2020
Family history of MI may increase CVD mortality after bilateral salpingo-oophorectomy
Family history of premature MI (FHPMI) in women with bilateral salpingo-oophorectomy (BSO) modifies the increased mortality associated with heart disease and cardiovascular disease in those women, according to an analysis published in Menopause.
Duke Appiah, PhD, of the department of public health at Texas Tech University Health Sciences Center, Lubbock, and colleagues drew data for 4,066 postmenopausal women aged 40 years and older from the National Health and Nutrition Examination Survey III (1988-1994). Women were excluded if they had partial or unilateral oophorectomy; unknown or missing age at menopause; or prevalent MI, stroke, or heart failure, which left a sample of 2,763 women for the analysis.
Women with BSO were considered postmenopausal if they had not experienced a menstrual period within the previous 12 months. Women were asked whether any blood relatives, and especially any first-degree relatives, had a heart attack before age 50 years, which was considered premature MI. The average age at baseline was 62 years. Of those 2,763 women, 610 women had BSO, 338 had FHPMI, and 95 had both, which yields weighted proportions of 24%, 15%, and 5%, respectively.
When compared with having neither factor, presence of any FHPMI was modestly associated with increased risk of mortality from heart disease (HD), cardiovascular disease (CVD), and all causes in the multivariable adjusted analysis, and having undergone BSO was not significantly associated with any of those on its own. However, the combination of those two factors yielded much higher multivariable adjusted hazard ratios – HD mortality, 2.88; CVD mortality, 2.05; and all-cause mortality, 1.58.
These multivariable adjusted HRs were even more dramatic with first-degree FHPMI and BSO: 3.51 for HD mortality, 2.55 for CVD mortality, and 1.63 for all-cause mortality.
In the women who had the combination of FHPMI and BSO, the elevated risks of HD, CVD, and all-cause mortality “were stronger in women who underwent BSO before the age of 45 years than among those who had this procedure at or after the age of 45 years,” reported Dr. Appiah and colleagues. A significantly elevated risk of HD, CVD, or all-cause mortality was not evident in women with BSO alone “regardless of age at surgery.”
“This study provides additional evidence that removal of the ovaries before the natural age of menopause is associated with multiple adverse long-term health outcomes, including cardiovascular disease and early mortality and should be strongly discouraged in women who are not at increased genetic risk for ovarian cancer,” Stephanie Faubion, MD, medical director of North American of Menopause Science, commented in a press release. She was not involved in the study.
Limitations of the study include how FHPMI was self-reported; however, the investigators suggested that, given findings of other research regarding reporting family history (Genet Epidemiol. 1999;17:141-50), the true rate may actually have been underreported. The investigators cited the large, population-based sample size as one of the study’s strengths, suggesting it helps make the findings generalizable.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Appiah D et al. Menopause. 2020 Feb. doi: 10.1097/GME.0000000000001522.
Family history of premature MI (FHPMI) in women with bilateral salpingo-oophorectomy (BSO) modifies the increased mortality associated with heart disease and cardiovascular disease in those women, according to an analysis published in Menopause.
Duke Appiah, PhD, of the department of public health at Texas Tech University Health Sciences Center, Lubbock, and colleagues drew data for 4,066 postmenopausal women aged 40 years and older from the National Health and Nutrition Examination Survey III (1988-1994). Women were excluded if they had partial or unilateral oophorectomy; unknown or missing age at menopause; or prevalent MI, stroke, or heart failure, which left a sample of 2,763 women for the analysis.
Women with BSO were considered postmenopausal if they had not experienced a menstrual period within the previous 12 months. Women were asked whether any blood relatives, and especially any first-degree relatives, had a heart attack before age 50 years, which was considered premature MI. The average age at baseline was 62 years. Of those 2,763 women, 610 women had BSO, 338 had FHPMI, and 95 had both, which yields weighted proportions of 24%, 15%, and 5%, respectively.
When compared with having neither factor, presence of any FHPMI was modestly associated with increased risk of mortality from heart disease (HD), cardiovascular disease (CVD), and all causes in the multivariable adjusted analysis, and having undergone BSO was not significantly associated with any of those on its own. However, the combination of those two factors yielded much higher multivariable adjusted hazard ratios – HD mortality, 2.88; CVD mortality, 2.05; and all-cause mortality, 1.58.
These multivariable adjusted HRs were even more dramatic with first-degree FHPMI and BSO: 3.51 for HD mortality, 2.55 for CVD mortality, and 1.63 for all-cause mortality.
In the women who had the combination of FHPMI and BSO, the elevated risks of HD, CVD, and all-cause mortality “were stronger in women who underwent BSO before the age of 45 years than among those who had this procedure at or after the age of 45 years,” reported Dr. Appiah and colleagues. A significantly elevated risk of HD, CVD, or all-cause mortality was not evident in women with BSO alone “regardless of age at surgery.”
“This study provides additional evidence that removal of the ovaries before the natural age of menopause is associated with multiple adverse long-term health outcomes, including cardiovascular disease and early mortality and should be strongly discouraged in women who are not at increased genetic risk for ovarian cancer,” Stephanie Faubion, MD, medical director of North American of Menopause Science, commented in a press release. She was not involved in the study.
Limitations of the study include how FHPMI was self-reported; however, the investigators suggested that, given findings of other research regarding reporting family history (Genet Epidemiol. 1999;17:141-50), the true rate may actually have been underreported. The investigators cited the large, population-based sample size as one of the study’s strengths, suggesting it helps make the findings generalizable.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Appiah D et al. Menopause. 2020 Feb. doi: 10.1097/GME.0000000000001522.
Family history of premature MI (FHPMI) in women with bilateral salpingo-oophorectomy (BSO) modifies the increased mortality associated with heart disease and cardiovascular disease in those women, according to an analysis published in Menopause.
Duke Appiah, PhD, of the department of public health at Texas Tech University Health Sciences Center, Lubbock, and colleagues drew data for 4,066 postmenopausal women aged 40 years and older from the National Health and Nutrition Examination Survey III (1988-1994). Women were excluded if they had partial or unilateral oophorectomy; unknown or missing age at menopause; or prevalent MI, stroke, or heart failure, which left a sample of 2,763 women for the analysis.
Women with BSO were considered postmenopausal if they had not experienced a menstrual period within the previous 12 months. Women were asked whether any blood relatives, and especially any first-degree relatives, had a heart attack before age 50 years, which was considered premature MI. The average age at baseline was 62 years. Of those 2,763 women, 610 women had BSO, 338 had FHPMI, and 95 had both, which yields weighted proportions of 24%, 15%, and 5%, respectively.
When compared with having neither factor, presence of any FHPMI was modestly associated with increased risk of mortality from heart disease (HD), cardiovascular disease (CVD), and all causes in the multivariable adjusted analysis, and having undergone BSO was not significantly associated with any of those on its own. However, the combination of those two factors yielded much higher multivariable adjusted hazard ratios – HD mortality, 2.88; CVD mortality, 2.05; and all-cause mortality, 1.58.
These multivariable adjusted HRs were even more dramatic with first-degree FHPMI and BSO: 3.51 for HD mortality, 2.55 for CVD mortality, and 1.63 for all-cause mortality.
In the women who had the combination of FHPMI and BSO, the elevated risks of HD, CVD, and all-cause mortality “were stronger in women who underwent BSO before the age of 45 years than among those who had this procedure at or after the age of 45 years,” reported Dr. Appiah and colleagues. A significantly elevated risk of HD, CVD, or all-cause mortality was not evident in women with BSO alone “regardless of age at surgery.”
“This study provides additional evidence that removal of the ovaries before the natural age of menopause is associated with multiple adverse long-term health outcomes, including cardiovascular disease and early mortality and should be strongly discouraged in women who are not at increased genetic risk for ovarian cancer,” Stephanie Faubion, MD, medical director of North American of Menopause Science, commented in a press release. She was not involved in the study.
Limitations of the study include how FHPMI was self-reported; however, the investigators suggested that, given findings of other research regarding reporting family history (Genet Epidemiol. 1999;17:141-50), the true rate may actually have been underreported. The investigators cited the large, population-based sample size as one of the study’s strengths, suggesting it helps make the findings generalizable.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Appiah D et al. Menopause. 2020 Feb. doi: 10.1097/GME.0000000000001522.
FROM MENOPAUSE
In a public health crisis, obstetric collaboration is mission-critical
With the novel coronavirus (COVID-19) monopolizing the news cycle, fear and misinformation are at an all-time high. Public health officials and physicians are accelerating education outreach to the public to address misinformation, and identify and care for patients who may have been exposed to the virus.
In times of public health crises, pregnant women have unique and pressing concerns about their personal health and the health of their unborn children. While not often mentioned in major news coverage, obstetricians play a critical role during health crises because of their uniquely personal role with patients during all stages of pregnancy, providing this vulnerable population with the most up-to-date information and following the latest guidelines for recommended care.
Unfortunately, COVID-19 is breaking unfamiliar new ground. We know that pregnant women are at higher risk for viral infection – annually, influenza is a grim reminder that pregnant women are more immunocompromised than the general public – but we do not yet have data to confirm or refute that pregnant women have a higher susceptibility to COVID-19 than the rest of the adult population. We also do not know enough about COVID-19 transmission, including whether the virus can cross the transplacental barrier to affect a fetus, or whether it can be transmitted through breast milk.
As private practice community obstetricians work to protect their patients during this public health crisis, Ob hospitalists can play an important role in supporting them in the provision of patient care.
First, Ob hospitalists are highly-trained specialists who can help ensure that pregnant patients who seek care at the hospital – either with viral symptoms or with separate pregnancy-related concerns – are protected during triage until the treating community obstetrician can take the reins.
When a pregnant woman presents at a hospital, in most cases she will bypass the ED and instead be sent directly to the labor and delivery (L&D) unit. During a viral outbreak, there are two major concerns with this approach. For one thing, it means an immunocompromised woman is being sent through the hospital to get to L&D, and along the path, is exposed to every airborne pathogen in the facility (and, if she is already infected, exposes others along the way). In addition, in hospitals without an Ob hospitalist on site, the patient generally is not immediately triaged by a physician, physician’s assistant, or nurse practitioner upon arrival because those clinicians are not consistently on site in L&D.
In times of viral pandemics, new approaches are warranted. For hospitals with contracted L&D management with hospitalists, hospitalists work closely with department heads to implement protocols loosely based on the Emergency Severity Index (ESI) model established by the Agency for Healthcare Research and Quality. Just as the ESI algorithm guides clinical stratification of patients, in times of reported viral outbreaks, L&D should consider triage of all pregnant women at higher levels of acuity, regardless of presentation status. In particular, if they show clinical symptoms, they should be masked, accompanied to the L&D unit by protected personnel, separated from other patients in areas of forced proximity such as hallways and elevators, and triaged in a secure single-patient room with a closed door (ideally at negative pressure relative to the surrounding areas).
If the patient has traveled to an area of outbreak, reports exposure to travelers who have visited high-risk areas, has had contact with individuals who tested positive for COVID-19, or exhibits any clinical symptoms of COVID-19 (fever, dry cough, fatigue, etc.), her care management should adhere to standing hospital emergency protocols. Following consultation with the assigned community obstetrician, the Ob hospitalist and hospital staff should contact their local/state health departments immediately for all cases of patients who show symptoms to determine if the patient meets requirements for a person under investigation (PUI) for COVID-19. The state/local health department will work with clinicians to collect, store, and ship clinical specimens appropriately. Very ill patients may need to be treated in an intensive care setting where respiratory status can be closely monitored.
At Ob Hospitalist Group, our body of evidence from our large national footprint has informed the development of standard sets of protocols for delivery complications such as preeclampsia and postpartum hemorrhage, as well as a cesarean section reduction toolkit to combat medically unnecessary cesarean sections. OB hospitalists therefore can assist with refining COVID-19 protocols specifically for the L&D setting, using evidence-based data to tailor protocols to address public health emergencies as they evolve.
The second way that Ob hospitalists can support their colleagues is by covering L&D 24/7 so that community obstetricians can focus on other pressing medical needs. From our experience with other outbreaks such as severe acute respiratory syndrome (SARS) and influenza, we anticipate that obstetricians in private practice likely will have their hands full juggling a regular patient load, fielding calls from concerned patients, and caring for infected or ill patients who are being treated in an outpatient setting. Adding to that plate the need to rush to the hospital to clinically assess a patient for COVID-19 or for a delivery only compounds stress and exhaustion. At Ob Hospitalist Group, our hospitalist programs provide coverage and support to community obstetricians until they can arrive at the hospital or when the woman has no assigned obstetrician, reducing the pressure on community obstetricians to rush through their schedules.
Diagnostic and pharmaceutical companies are collaborating with public health officials to expedite diagnostic testing staff, hospital treatment capacity, vaccines, and even early therapies that may help to minimize severity. But right now, as clinicians work to protect their vulnerable patients, a close collaboration between community obstetricians and Ob hospitalists will help to keep patients and health care personnel safe and healthy – a goal that should apply not only to public health crises, but to the provision of maternal care every day.
Dr. Simon is chief medical officer at Ob Hospitalist Group (OBHG), is a board-certified ob.gyn., and former head of the department of obstetrics and gynecology for a U.S. hospital. He has no relevant conflicts of interest or financial disclosures. Email him at obnews@mdedge.com.
With the novel coronavirus (COVID-19) monopolizing the news cycle, fear and misinformation are at an all-time high. Public health officials and physicians are accelerating education outreach to the public to address misinformation, and identify and care for patients who may have been exposed to the virus.
In times of public health crises, pregnant women have unique and pressing concerns about their personal health and the health of their unborn children. While not often mentioned in major news coverage, obstetricians play a critical role during health crises because of their uniquely personal role with patients during all stages of pregnancy, providing this vulnerable population with the most up-to-date information and following the latest guidelines for recommended care.
Unfortunately, COVID-19 is breaking unfamiliar new ground. We know that pregnant women are at higher risk for viral infection – annually, influenza is a grim reminder that pregnant women are more immunocompromised than the general public – but we do not yet have data to confirm or refute that pregnant women have a higher susceptibility to COVID-19 than the rest of the adult population. We also do not know enough about COVID-19 transmission, including whether the virus can cross the transplacental barrier to affect a fetus, or whether it can be transmitted through breast milk.
As private practice community obstetricians work to protect their patients during this public health crisis, Ob hospitalists can play an important role in supporting them in the provision of patient care.
First, Ob hospitalists are highly-trained specialists who can help ensure that pregnant patients who seek care at the hospital – either with viral symptoms or with separate pregnancy-related concerns – are protected during triage until the treating community obstetrician can take the reins.
When a pregnant woman presents at a hospital, in most cases she will bypass the ED and instead be sent directly to the labor and delivery (L&D) unit. During a viral outbreak, there are two major concerns with this approach. For one thing, it means an immunocompromised woman is being sent through the hospital to get to L&D, and along the path, is exposed to every airborne pathogen in the facility (and, if she is already infected, exposes others along the way). In addition, in hospitals without an Ob hospitalist on site, the patient generally is not immediately triaged by a physician, physician’s assistant, or nurse practitioner upon arrival because those clinicians are not consistently on site in L&D.
In times of viral pandemics, new approaches are warranted. For hospitals with contracted L&D management with hospitalists, hospitalists work closely with department heads to implement protocols loosely based on the Emergency Severity Index (ESI) model established by the Agency for Healthcare Research and Quality. Just as the ESI algorithm guides clinical stratification of patients, in times of reported viral outbreaks, L&D should consider triage of all pregnant women at higher levels of acuity, regardless of presentation status. In particular, if they show clinical symptoms, they should be masked, accompanied to the L&D unit by protected personnel, separated from other patients in areas of forced proximity such as hallways and elevators, and triaged in a secure single-patient room with a closed door (ideally at negative pressure relative to the surrounding areas).
If the patient has traveled to an area of outbreak, reports exposure to travelers who have visited high-risk areas, has had contact with individuals who tested positive for COVID-19, or exhibits any clinical symptoms of COVID-19 (fever, dry cough, fatigue, etc.), her care management should adhere to standing hospital emergency protocols. Following consultation with the assigned community obstetrician, the Ob hospitalist and hospital staff should contact their local/state health departments immediately for all cases of patients who show symptoms to determine if the patient meets requirements for a person under investigation (PUI) for COVID-19. The state/local health department will work with clinicians to collect, store, and ship clinical specimens appropriately. Very ill patients may need to be treated in an intensive care setting where respiratory status can be closely monitored.
At Ob Hospitalist Group, our body of evidence from our large national footprint has informed the development of standard sets of protocols for delivery complications such as preeclampsia and postpartum hemorrhage, as well as a cesarean section reduction toolkit to combat medically unnecessary cesarean sections. OB hospitalists therefore can assist with refining COVID-19 protocols specifically for the L&D setting, using evidence-based data to tailor protocols to address public health emergencies as they evolve.
The second way that Ob hospitalists can support their colleagues is by covering L&D 24/7 so that community obstetricians can focus on other pressing medical needs. From our experience with other outbreaks such as severe acute respiratory syndrome (SARS) and influenza, we anticipate that obstetricians in private practice likely will have their hands full juggling a regular patient load, fielding calls from concerned patients, and caring for infected or ill patients who are being treated in an outpatient setting. Adding to that plate the need to rush to the hospital to clinically assess a patient for COVID-19 or for a delivery only compounds stress and exhaustion. At Ob Hospitalist Group, our hospitalist programs provide coverage and support to community obstetricians until they can arrive at the hospital or when the woman has no assigned obstetrician, reducing the pressure on community obstetricians to rush through their schedules.
Diagnostic and pharmaceutical companies are collaborating with public health officials to expedite diagnostic testing staff, hospital treatment capacity, vaccines, and even early therapies that may help to minimize severity. But right now, as clinicians work to protect their vulnerable patients, a close collaboration between community obstetricians and Ob hospitalists will help to keep patients and health care personnel safe and healthy – a goal that should apply not only to public health crises, but to the provision of maternal care every day.
Dr. Simon is chief medical officer at Ob Hospitalist Group (OBHG), is a board-certified ob.gyn., and former head of the department of obstetrics and gynecology for a U.S. hospital. He has no relevant conflicts of interest or financial disclosures. Email him at obnews@mdedge.com.
With the novel coronavirus (COVID-19) monopolizing the news cycle, fear and misinformation are at an all-time high. Public health officials and physicians are accelerating education outreach to the public to address misinformation, and identify and care for patients who may have been exposed to the virus.
In times of public health crises, pregnant women have unique and pressing concerns about their personal health and the health of their unborn children. While not often mentioned in major news coverage, obstetricians play a critical role during health crises because of their uniquely personal role with patients during all stages of pregnancy, providing this vulnerable population with the most up-to-date information and following the latest guidelines for recommended care.
Unfortunately, COVID-19 is breaking unfamiliar new ground. We know that pregnant women are at higher risk for viral infection – annually, influenza is a grim reminder that pregnant women are more immunocompromised than the general public – but we do not yet have data to confirm or refute that pregnant women have a higher susceptibility to COVID-19 than the rest of the adult population. We also do not know enough about COVID-19 transmission, including whether the virus can cross the transplacental barrier to affect a fetus, or whether it can be transmitted through breast milk.
As private practice community obstetricians work to protect their patients during this public health crisis, Ob hospitalists can play an important role in supporting them in the provision of patient care.
First, Ob hospitalists are highly-trained specialists who can help ensure that pregnant patients who seek care at the hospital – either with viral symptoms or with separate pregnancy-related concerns – are protected during triage until the treating community obstetrician can take the reins.
When a pregnant woman presents at a hospital, in most cases she will bypass the ED and instead be sent directly to the labor and delivery (L&D) unit. During a viral outbreak, there are two major concerns with this approach. For one thing, it means an immunocompromised woman is being sent through the hospital to get to L&D, and along the path, is exposed to every airborne pathogen in the facility (and, if she is already infected, exposes others along the way). In addition, in hospitals without an Ob hospitalist on site, the patient generally is not immediately triaged by a physician, physician’s assistant, or nurse practitioner upon arrival because those clinicians are not consistently on site in L&D.
In times of viral pandemics, new approaches are warranted. For hospitals with contracted L&D management with hospitalists, hospitalists work closely with department heads to implement protocols loosely based on the Emergency Severity Index (ESI) model established by the Agency for Healthcare Research and Quality. Just as the ESI algorithm guides clinical stratification of patients, in times of reported viral outbreaks, L&D should consider triage of all pregnant women at higher levels of acuity, regardless of presentation status. In particular, if they show clinical symptoms, they should be masked, accompanied to the L&D unit by protected personnel, separated from other patients in areas of forced proximity such as hallways and elevators, and triaged in a secure single-patient room with a closed door (ideally at negative pressure relative to the surrounding areas).
If the patient has traveled to an area of outbreak, reports exposure to travelers who have visited high-risk areas, has had contact with individuals who tested positive for COVID-19, or exhibits any clinical symptoms of COVID-19 (fever, dry cough, fatigue, etc.), her care management should adhere to standing hospital emergency protocols. Following consultation with the assigned community obstetrician, the Ob hospitalist and hospital staff should contact their local/state health departments immediately for all cases of patients who show symptoms to determine if the patient meets requirements for a person under investigation (PUI) for COVID-19. The state/local health department will work with clinicians to collect, store, and ship clinical specimens appropriately. Very ill patients may need to be treated in an intensive care setting where respiratory status can be closely monitored.
At Ob Hospitalist Group, our body of evidence from our large national footprint has informed the development of standard sets of protocols for delivery complications such as preeclampsia and postpartum hemorrhage, as well as a cesarean section reduction toolkit to combat medically unnecessary cesarean sections. OB hospitalists therefore can assist with refining COVID-19 protocols specifically for the L&D setting, using evidence-based data to tailor protocols to address public health emergencies as they evolve.
The second way that Ob hospitalists can support their colleagues is by covering L&D 24/7 so that community obstetricians can focus on other pressing medical needs. From our experience with other outbreaks such as severe acute respiratory syndrome (SARS) and influenza, we anticipate that obstetricians in private practice likely will have their hands full juggling a regular patient load, fielding calls from concerned patients, and caring for infected or ill patients who are being treated in an outpatient setting. Adding to that plate the need to rush to the hospital to clinically assess a patient for COVID-19 or for a delivery only compounds stress and exhaustion. At Ob Hospitalist Group, our hospitalist programs provide coverage and support to community obstetricians until they can arrive at the hospital or when the woman has no assigned obstetrician, reducing the pressure on community obstetricians to rush through their schedules.
Diagnostic and pharmaceutical companies are collaborating with public health officials to expedite diagnostic testing staff, hospital treatment capacity, vaccines, and even early therapies that may help to minimize severity. But right now, as clinicians work to protect their vulnerable patients, a close collaboration between community obstetricians and Ob hospitalists will help to keep patients and health care personnel safe and healthy – a goal that should apply not only to public health crises, but to the provision of maternal care every day.
Dr. Simon is chief medical officer at Ob Hospitalist Group (OBHG), is a board-certified ob.gyn., and former head of the department of obstetrics and gynecology for a U.S. hospital. He has no relevant conflicts of interest or financial disclosures. Email him at obnews@mdedge.com.
Mammography does not reduce breast cancer deaths in women 75 and older
While more than half of women aged 75 years and older receive annual mammograms, they do not see a reduced risk of death from breast cancer, compared with women who have stopped regular screening, according to a study published in Annals of Internal Medicine.
The lack of benefit is not because older women’s cancer risk is low; a third of breast cancer deaths occur in women diagnosed at or after age 70 years, according to study author Xabier García-Albéniz, MD, PhD, of Harvard University in Boston, and colleagues.
The lack of benefit is not because mammography is less effective in women older than 75 years; indeed, it becomes a better diagnostic tool as women age, said Otis Brawley, MD, of Johns Hopkins University, Baltimore, the author of an editorial related to the study. Rather, the lack of benefit is because breast cancer treatment in older women is less successful, he clarified.
Study details
Dr. García-Albéniz and colleagues looked at data from 1,058,013 women enrolled in Medicare across the United States during 2000-2008. All subjects were aged 70-84 years and had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer.
There are little randomized trial data available on mammography and breast cancer deaths for women in their early 70s and none for women older than 75 years. To compensate for this, the researchers aimed to emulate a prospective trial by looking at deaths over an 8-year period for women aged 70 and older who either continued annual screening or stopped it. The investigators conducted separate analyses for women aged 70-74 years and those 75-84 years of age.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but this did not translate to serious reductions in death.
In the continued-screening group, the estimated 8-year risk for breast cancer was 5.5% in women aged 70-74 and 5.8% in women aged 75-84 years. Among women who stopped screening, the estimated 8-year risk for breast cancer was 3.9% in both age groups.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was slightly reduced with continued screening: 2.7 deaths per 1,000 women, compared with 3.7 deaths per 1,000 women for those who stopped screening. The risk difference was –1.0 deaths per 1,000 women, and the hazard ratio was 0.78.
Among women aged 75-84 years, there was no difference in estimated 8-year risk for breast cancer death. Women treated under a continued screening protocol had 3.8 deaths per 1,000, while the stop-screening group had 3.7 deaths per 1,000. The risk difference was 0.07 deaths per 1,000 women, and the hazard ratio was 1.00.
Interpreting the results
In the editorial accompanying this study, Dr. Brawley praised its design as “especially useful in breast cancer screening,” as “prospective randomized studies of mammography are not feasible and are perhaps no longer ethical in older women … because mammography is so widely accepted.”
In an interview, Dr. Brawley stressed that the findings do not argue for denying women aged 75 years and older mammography screening. Decisions about screening require a value judgment tailored to each individual patient’s perceived risks and benefits, he said.
In the absence of randomized trial evidence, “the jury will always be out” on the benefits of regular mammography for women 75 and older, Dr. Brawley said. “A clinical trial or a modeling study always tells you about an average person who doesn’t exist,” he added. “I predict that, in the future, we will have more parameters to tell us, ‘this is a person who’s 80 years old who is likely to benefit from screening; this is a person who is 75 years old who is unlikely to benefit.’ ”
And focusing too much on screening, he said, can divert attention from a key driver of breast cancer mortality in older women: inadequate treatment.
In the United States, Dr. Brawley said, “There’s a lot of emphasis on screening but fewer people writing about the fact that nearly 40% of American women get less than optimal treatment once they’re diagnosed.”
Dr. Brawley cited a 2013 modeling study showing that improvements in delivering current treatments would save more women even if screening rates remained unaltered (Cancer. 2013 Jul 15;119[14]:2541-8).
Among women in their 70s and 80s, Dr. Brawley said, some of the barriers to effective breast cancer care aren’t related to treatment efficacy but to travel and other logistical issues that can become more pronounced with age. “Unfortunately, there’s very little research on why, for women in their 70s and 80s, the treatments don’t work as well as they work in women 20 years younger,” he said.
Dr. García-Albéniz and colleagues’ study was funded by the National Institutes of Health. One coauthor reported financial ties to industry. Dr. Brawley discloses no conflicts of interest related to his editorial.
SOURCE: García-Albéniz X et al. Ann Intern Med 2020. doi: 10.7326/M18-1199.
While more than half of women aged 75 years and older receive annual mammograms, they do not see a reduced risk of death from breast cancer, compared with women who have stopped regular screening, according to a study published in Annals of Internal Medicine.
The lack of benefit is not because older women’s cancer risk is low; a third of breast cancer deaths occur in women diagnosed at or after age 70 years, according to study author Xabier García-Albéniz, MD, PhD, of Harvard University in Boston, and colleagues.
The lack of benefit is not because mammography is less effective in women older than 75 years; indeed, it becomes a better diagnostic tool as women age, said Otis Brawley, MD, of Johns Hopkins University, Baltimore, the author of an editorial related to the study. Rather, the lack of benefit is because breast cancer treatment in older women is less successful, he clarified.
Study details
Dr. García-Albéniz and colleagues looked at data from 1,058,013 women enrolled in Medicare across the United States during 2000-2008. All subjects were aged 70-84 years and had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer.
There are little randomized trial data available on mammography and breast cancer deaths for women in their early 70s and none for women older than 75 years. To compensate for this, the researchers aimed to emulate a prospective trial by looking at deaths over an 8-year period for women aged 70 and older who either continued annual screening or stopped it. The investigators conducted separate analyses for women aged 70-74 years and those 75-84 years of age.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but this did not translate to serious reductions in death.
In the continued-screening group, the estimated 8-year risk for breast cancer was 5.5% in women aged 70-74 and 5.8% in women aged 75-84 years. Among women who stopped screening, the estimated 8-year risk for breast cancer was 3.9% in both age groups.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was slightly reduced with continued screening: 2.7 deaths per 1,000 women, compared with 3.7 deaths per 1,000 women for those who stopped screening. The risk difference was –1.0 deaths per 1,000 women, and the hazard ratio was 0.78.
Among women aged 75-84 years, there was no difference in estimated 8-year risk for breast cancer death. Women treated under a continued screening protocol had 3.8 deaths per 1,000, while the stop-screening group had 3.7 deaths per 1,000. The risk difference was 0.07 deaths per 1,000 women, and the hazard ratio was 1.00.
Interpreting the results
In the editorial accompanying this study, Dr. Brawley praised its design as “especially useful in breast cancer screening,” as “prospective randomized studies of mammography are not feasible and are perhaps no longer ethical in older women … because mammography is so widely accepted.”
In an interview, Dr. Brawley stressed that the findings do not argue for denying women aged 75 years and older mammography screening. Decisions about screening require a value judgment tailored to each individual patient’s perceived risks and benefits, he said.
In the absence of randomized trial evidence, “the jury will always be out” on the benefits of regular mammography for women 75 and older, Dr. Brawley said. “A clinical trial or a modeling study always tells you about an average person who doesn’t exist,” he added. “I predict that, in the future, we will have more parameters to tell us, ‘this is a person who’s 80 years old who is likely to benefit from screening; this is a person who is 75 years old who is unlikely to benefit.’ ”
And focusing too much on screening, he said, can divert attention from a key driver of breast cancer mortality in older women: inadequate treatment.
In the United States, Dr. Brawley said, “There’s a lot of emphasis on screening but fewer people writing about the fact that nearly 40% of American women get less than optimal treatment once they’re diagnosed.”
Dr. Brawley cited a 2013 modeling study showing that improvements in delivering current treatments would save more women even if screening rates remained unaltered (Cancer. 2013 Jul 15;119[14]:2541-8).
Among women in their 70s and 80s, Dr. Brawley said, some of the barriers to effective breast cancer care aren’t related to treatment efficacy but to travel and other logistical issues that can become more pronounced with age. “Unfortunately, there’s very little research on why, for women in their 70s and 80s, the treatments don’t work as well as they work in women 20 years younger,” he said.
Dr. García-Albéniz and colleagues’ study was funded by the National Institutes of Health. One coauthor reported financial ties to industry. Dr. Brawley discloses no conflicts of interest related to his editorial.
SOURCE: García-Albéniz X et al. Ann Intern Med 2020. doi: 10.7326/M18-1199.
While more than half of women aged 75 years and older receive annual mammograms, they do not see a reduced risk of death from breast cancer, compared with women who have stopped regular screening, according to a study published in Annals of Internal Medicine.
The lack of benefit is not because older women’s cancer risk is low; a third of breast cancer deaths occur in women diagnosed at or after age 70 years, according to study author Xabier García-Albéniz, MD, PhD, of Harvard University in Boston, and colleagues.
The lack of benefit is not because mammography is less effective in women older than 75 years; indeed, it becomes a better diagnostic tool as women age, said Otis Brawley, MD, of Johns Hopkins University, Baltimore, the author of an editorial related to the study. Rather, the lack of benefit is because breast cancer treatment in older women is less successful, he clarified.
Study details
Dr. García-Albéniz and colleagues looked at data from 1,058,013 women enrolled in Medicare across the United States during 2000-2008. All subjects were aged 70-84 years and had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer.
There are little randomized trial data available on mammography and breast cancer deaths for women in their early 70s and none for women older than 75 years. To compensate for this, the researchers aimed to emulate a prospective trial by looking at deaths over an 8-year period for women aged 70 and older who either continued annual screening or stopped it. The investigators conducted separate analyses for women aged 70-74 years and those 75-84 years of age.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but this did not translate to serious reductions in death.
In the continued-screening group, the estimated 8-year risk for breast cancer was 5.5% in women aged 70-74 and 5.8% in women aged 75-84 years. Among women who stopped screening, the estimated 8-year risk for breast cancer was 3.9% in both age groups.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was slightly reduced with continued screening: 2.7 deaths per 1,000 women, compared with 3.7 deaths per 1,000 women for those who stopped screening. The risk difference was –1.0 deaths per 1,000 women, and the hazard ratio was 0.78.
Among women aged 75-84 years, there was no difference in estimated 8-year risk for breast cancer death. Women treated under a continued screening protocol had 3.8 deaths per 1,000, while the stop-screening group had 3.7 deaths per 1,000. The risk difference was 0.07 deaths per 1,000 women, and the hazard ratio was 1.00.
Interpreting the results
In the editorial accompanying this study, Dr. Brawley praised its design as “especially useful in breast cancer screening,” as “prospective randomized studies of mammography are not feasible and are perhaps no longer ethical in older women … because mammography is so widely accepted.”
In an interview, Dr. Brawley stressed that the findings do not argue for denying women aged 75 years and older mammography screening. Decisions about screening require a value judgment tailored to each individual patient’s perceived risks and benefits, he said.
In the absence of randomized trial evidence, “the jury will always be out” on the benefits of regular mammography for women 75 and older, Dr. Brawley said. “A clinical trial or a modeling study always tells you about an average person who doesn’t exist,” he added. “I predict that, in the future, we will have more parameters to tell us, ‘this is a person who’s 80 years old who is likely to benefit from screening; this is a person who is 75 years old who is unlikely to benefit.’ ”
And focusing too much on screening, he said, can divert attention from a key driver of breast cancer mortality in older women: inadequate treatment.
In the United States, Dr. Brawley said, “There’s a lot of emphasis on screening but fewer people writing about the fact that nearly 40% of American women get less than optimal treatment once they’re diagnosed.”
Dr. Brawley cited a 2013 modeling study showing that improvements in delivering current treatments would save more women even if screening rates remained unaltered (Cancer. 2013 Jul 15;119[14]:2541-8).
Among women in their 70s and 80s, Dr. Brawley said, some of the barriers to effective breast cancer care aren’t related to treatment efficacy but to travel and other logistical issues that can become more pronounced with age. “Unfortunately, there’s very little research on why, for women in their 70s and 80s, the treatments don’t work as well as they work in women 20 years younger,” he said.
Dr. García-Albéniz and colleagues’ study was funded by the National Institutes of Health. One coauthor reported financial ties to industry. Dr. Brawley discloses no conflicts of interest related to his editorial.
SOURCE: García-Albéniz X et al. Ann Intern Med 2020. doi: 10.7326/M18-1199.
FROM ANNALS OF INTERNAL MEDICINE
ERAS protocol for cesarean delivery reduces opioid usage
GRAPEVINE, TEX. – An enhanced recovery after surgery (ERAS) pathway for cesarean delivery decreased postoperative opioid usage by 62% in one health care organization, researchers reported at the Pregnancy Meeting. The protocol incorporates a stepwise approach to pain control with no scheduled postoperative opioids.
Abington Jefferson Health, which includes two hospitals in Pennsylvania, implemented an ERAS pathway for all cesarean deliveries in October 2018. Kathryn Ruymann, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Ruymann is an obstetrics and gynecology resident at Abington Jefferson Health.
Prior to the ERAS protocol, 99%-100% of patients took an opioid during the postoperative period. “With ERAS, 26% of patients never took an opioid during the postop period,” Dr. Ruymann and her associates reported. “Pain scores decreased with ERAS for postoperative days 1-3 and remained unchanged on day 4.”
One in 300 opioid-naive patients who receives opioids after cesarean delivery becomes a persistent user, one study has shown (Am J Obstet Gynecol. 2016 Sep; 215(3):353.e1-18). “ERAS pathways integrate evidence-based interventions before, during, and after surgery to optimize outcomes, specifically to decrease postoperative opioid use,” the researchers said.
While other surgical fields have adopted ERAS pathways, more research is needed in obstetrics, said Dr. Ruymann. More than 4,500 women deliver at Abington Jefferson Health each year, and about a third undergo cesarean deliveries.
The organization’s ERAS pathway incorporates preoperative education, fasting guidelines, and intraoperative analgesia, nausea prophylaxis, and antimicrobial therapy. Under the new protocol, postoperative analgesia includes scheduled administration of nonopioid medications, including celecoxib and acetaminophen. In addition, patients may take 5-10 mg of oxycodone orally every 4 hours as needed, and hydromorphone 0.4 mg IV as needed may be used for refractory pain. In addition, patients should resume eating as soon as tolerated and be out of bed within 4 hours after surgery, according to the protocol. Postoperative management of pruritus and instructions on how to wean off opioids at home are among the other elements of the enhanced recovery plan.
To examine postoperative opioid usage before and after implementation of the ERAS pathway, the investigators conducted a retrospective cohort study of 316 women who underwent cesarean delivery 3 months before the start of the ERAS pathway and 267 who underwent cesarean delivery 3 months after. The researchers used an application developed in Qlik Sense, a data analytics platform, to calculate opioid usage.
Mean postoperative opioid use decreased by 62%. The reduction in opioid use remained 8 months after starting the ERAS pathway.
“An ERAS pathway for [cesarean delivery] decreases postoperative opioid usage by integrating a multimodal stepwise approach to pain control and recovery,” the researchers said. “Standardized order sets and departmentwide education were crucial in the success of ERAS. Additional research is needed to evaluate the impact of unique components of ERAS in order to optimize this pathway.”
The researchers had no disclosures.
SOURCE: Ruymann K et al. Am J Obstet Gynecol. 2020 Jan;222(1):S212, Abstract 315.
GRAPEVINE, TEX. – An enhanced recovery after surgery (ERAS) pathway for cesarean delivery decreased postoperative opioid usage by 62% in one health care organization, researchers reported at the Pregnancy Meeting. The protocol incorporates a stepwise approach to pain control with no scheduled postoperative opioids.
Abington Jefferson Health, which includes two hospitals in Pennsylvania, implemented an ERAS pathway for all cesarean deliveries in October 2018. Kathryn Ruymann, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Ruymann is an obstetrics and gynecology resident at Abington Jefferson Health.
Prior to the ERAS protocol, 99%-100% of patients took an opioid during the postoperative period. “With ERAS, 26% of patients never took an opioid during the postop period,” Dr. Ruymann and her associates reported. “Pain scores decreased with ERAS for postoperative days 1-3 and remained unchanged on day 4.”
One in 300 opioid-naive patients who receives opioids after cesarean delivery becomes a persistent user, one study has shown (Am J Obstet Gynecol. 2016 Sep; 215(3):353.e1-18). “ERAS pathways integrate evidence-based interventions before, during, and after surgery to optimize outcomes, specifically to decrease postoperative opioid use,” the researchers said.
While other surgical fields have adopted ERAS pathways, more research is needed in obstetrics, said Dr. Ruymann. More than 4,500 women deliver at Abington Jefferson Health each year, and about a third undergo cesarean deliveries.
The organization’s ERAS pathway incorporates preoperative education, fasting guidelines, and intraoperative analgesia, nausea prophylaxis, and antimicrobial therapy. Under the new protocol, postoperative analgesia includes scheduled administration of nonopioid medications, including celecoxib and acetaminophen. In addition, patients may take 5-10 mg of oxycodone orally every 4 hours as needed, and hydromorphone 0.4 mg IV as needed may be used for refractory pain. In addition, patients should resume eating as soon as tolerated and be out of bed within 4 hours after surgery, according to the protocol. Postoperative management of pruritus and instructions on how to wean off opioids at home are among the other elements of the enhanced recovery plan.
To examine postoperative opioid usage before and after implementation of the ERAS pathway, the investigators conducted a retrospective cohort study of 316 women who underwent cesarean delivery 3 months before the start of the ERAS pathway and 267 who underwent cesarean delivery 3 months after. The researchers used an application developed in Qlik Sense, a data analytics platform, to calculate opioid usage.
Mean postoperative opioid use decreased by 62%. The reduction in opioid use remained 8 months after starting the ERAS pathway.
“An ERAS pathway for [cesarean delivery] decreases postoperative opioid usage by integrating a multimodal stepwise approach to pain control and recovery,” the researchers said. “Standardized order sets and departmentwide education were crucial in the success of ERAS. Additional research is needed to evaluate the impact of unique components of ERAS in order to optimize this pathway.”
The researchers had no disclosures.
SOURCE: Ruymann K et al. Am J Obstet Gynecol. 2020 Jan;222(1):S212, Abstract 315.
GRAPEVINE, TEX. – An enhanced recovery after surgery (ERAS) pathway for cesarean delivery decreased postoperative opioid usage by 62% in one health care organization, researchers reported at the Pregnancy Meeting. The protocol incorporates a stepwise approach to pain control with no scheduled postoperative opioids.
Abington Jefferson Health, which includes two hospitals in Pennsylvania, implemented an ERAS pathway for all cesarean deliveries in October 2018. Kathryn Ruymann, MD, said at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Ruymann is an obstetrics and gynecology resident at Abington Jefferson Health.
Prior to the ERAS protocol, 99%-100% of patients took an opioid during the postoperative period. “With ERAS, 26% of patients never took an opioid during the postop period,” Dr. Ruymann and her associates reported. “Pain scores decreased with ERAS for postoperative days 1-3 and remained unchanged on day 4.”
One in 300 opioid-naive patients who receives opioids after cesarean delivery becomes a persistent user, one study has shown (Am J Obstet Gynecol. 2016 Sep; 215(3):353.e1-18). “ERAS pathways integrate evidence-based interventions before, during, and after surgery to optimize outcomes, specifically to decrease postoperative opioid use,” the researchers said.
While other surgical fields have adopted ERAS pathways, more research is needed in obstetrics, said Dr. Ruymann. More than 4,500 women deliver at Abington Jefferson Health each year, and about a third undergo cesarean deliveries.
The organization’s ERAS pathway incorporates preoperative education, fasting guidelines, and intraoperative analgesia, nausea prophylaxis, and antimicrobial therapy. Under the new protocol, postoperative analgesia includes scheduled administration of nonopioid medications, including celecoxib and acetaminophen. In addition, patients may take 5-10 mg of oxycodone orally every 4 hours as needed, and hydromorphone 0.4 mg IV as needed may be used for refractory pain. In addition, patients should resume eating as soon as tolerated and be out of bed within 4 hours after surgery, according to the protocol. Postoperative management of pruritus and instructions on how to wean off opioids at home are among the other elements of the enhanced recovery plan.
To examine postoperative opioid usage before and after implementation of the ERAS pathway, the investigators conducted a retrospective cohort study of 316 women who underwent cesarean delivery 3 months before the start of the ERAS pathway and 267 who underwent cesarean delivery 3 months after. The researchers used an application developed in Qlik Sense, a data analytics platform, to calculate opioid usage.
Mean postoperative opioid use decreased by 62%. The reduction in opioid use remained 8 months after starting the ERAS pathway.
“An ERAS pathway for [cesarean delivery] decreases postoperative opioid usage by integrating a multimodal stepwise approach to pain control and recovery,” the researchers said. “Standardized order sets and departmentwide education were crucial in the success of ERAS. Additional research is needed to evaluate the impact of unique components of ERAS in order to optimize this pathway.”
The researchers had no disclosures.
SOURCE: Ruymann K et al. Am J Obstet Gynecol. 2020 Jan;222(1):S212, Abstract 315.
REPORTING FROM THE PREGNANCY MEETING
Refining your approach to hypothyroidism treatment
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
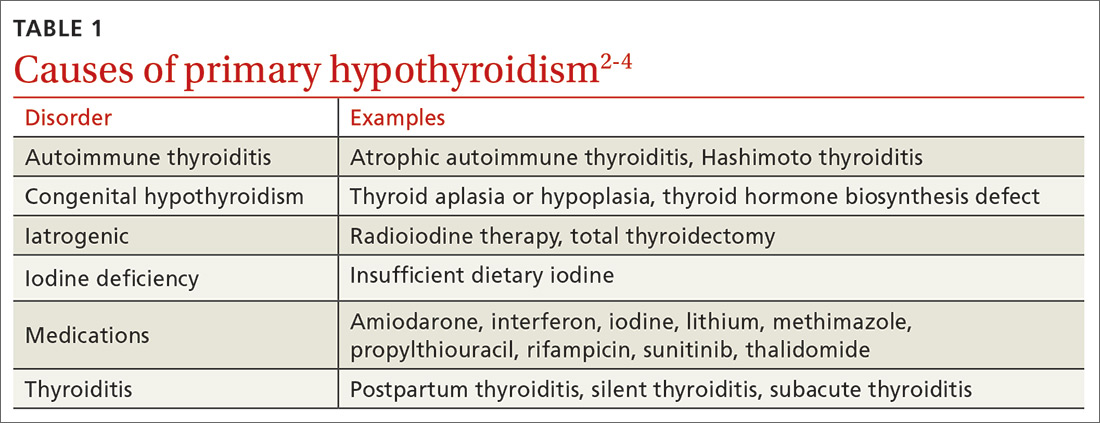
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
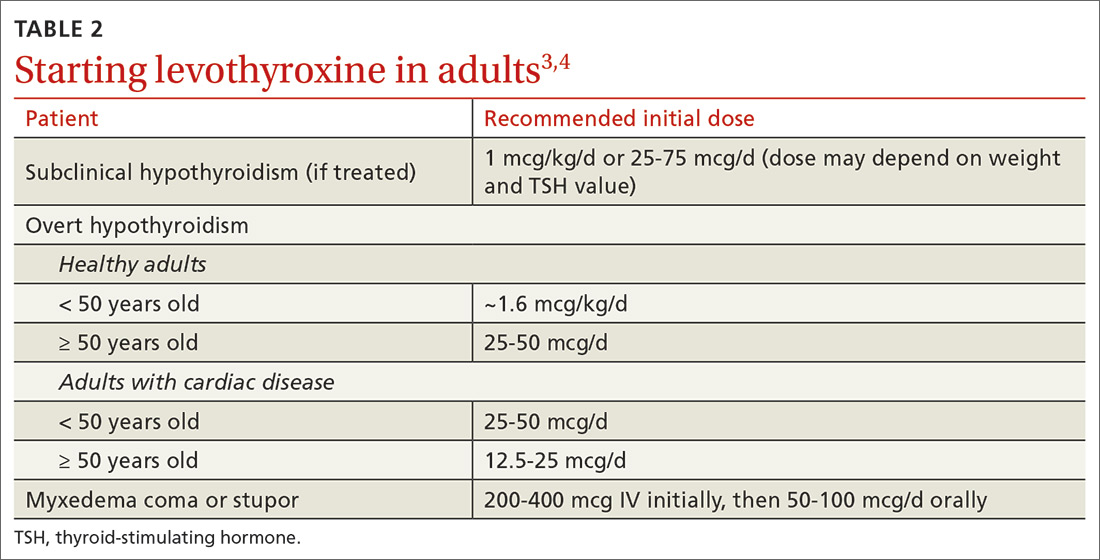
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
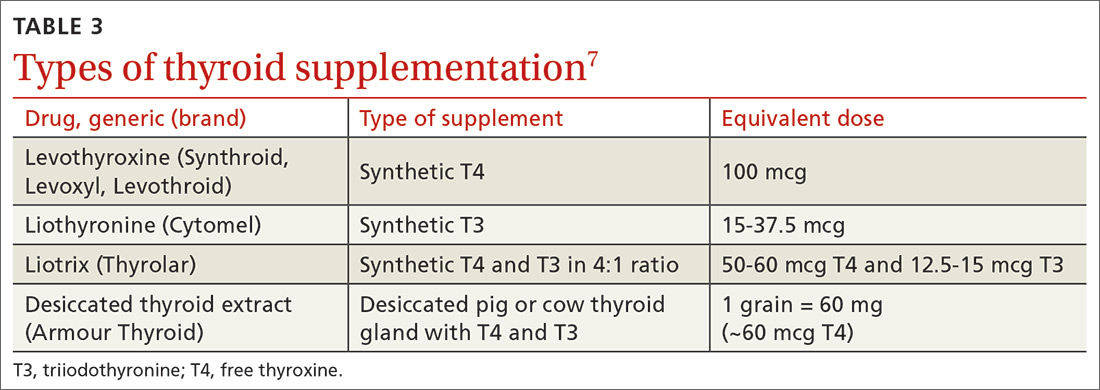
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine 1.6 mcg/kg/d for healthy adult patients < 50 years of age with overt hypothyroidism. B
› Consider lower initial doses of levothyroxine in patients with cardiac disease (12.5-50 mcg/d) or subclinical hypothyroidism (25-75 mcg/d). B
› Titrate levothyroxine by 12.5 to 25 mcg/d at 6- to 8-week intervals based on thyroid-stimulating hormone measurements, comorbidities, and symptoms. C
› Closely monitor and provide thyroid supplementation to female patients who are pregnant or of reproductive age with concomitant hypothyroidism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fever, abdominal pain, and adnexal mass
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
New guideline offers recommendations for reproductive health in patients with rheumatic diseases
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
FROM ARTHRITIS & RHEUMATOLOGY
In gestational diabetes, early postpartum glucose testing is a winner
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
REPORTING FROM THE PREGNANCY MEETING
Increased risk of infection seen in patients with MS
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
womensWEST PALM BEACH, FLA. – Patients with multiple sclerosis (MS) are at increased risk for most types of infection, with the highest risk associated with renal tract infections, according to an analysis of Department of Defense data.
Susan Jick, DSc, director of the Boston Collaborative Drug Surveillance Program and professor of epidemiology and biostatistics at Boston University, and colleagues sought to understand the rates at which infections occur because they are known to be a common cause of comorbidity and death in patients with MS.
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Dr. Jick and associates presented rates of infection in patients with MS after MS diagnosis, compared with a matched population of patients without MS. The MS cohort included patients who had MS diagnosed and treated between January 2004 and August 2017. Patients had medical history available for at least 1 year before MS diagnosis and at least one prescription for an MS disease-modifying treatment.
Patients without MS were matched to patients with MS 10:1 based on age, sex, geographic region, and cohort entry date. For each patient, the researchers identified the first diagnosed infection of each type after cohort entry. They followed patients until loss of eligibility, death, or end of data collection.
In all, the study included 8,695 patients with MS and 86,934 matched patients without MS. The median age at cohort entry was 41 years, and 71% were female. Median duration of follow-up after study entry was about 6 years. Patients with MS were more likely to have an infection in the year before cohort entry, compared with non-MS patients (43.9% vs. 36.3%).
After cohort entry, the incidence rate of any infection was higher among patients with MS, compared with non-MS patients (4,805 vs. 2,731 per 10,000 person-years; IR ratio, 1.76). In addition, the IR of hospitalized infection was higher among MS patients (125 vs. 51.3 per 10,000 person-years; IRR, 2.43). The IR also was increased for several other types of infections, including renal, skin, fungal, pneumonia or influenza, and other infections (such as rickettsial and spirochetal diseases, helminthiases, and nonsyphilitic and nongonococcal venereal diseases). Eye or ear, respiratory or throat, and viral IRRs “were marginally elevated,” the investigators wrote.
In both cohorts, females had a higher risk of infection than males did. The rate of renal tract infection was more than fourfold higher among females, compared with males, in both cohorts. Relative to non-MS patients, however, men with MS had a higher IRR for renal tract infection than women with MS did (2.47 vs. 1.90).
“The risk for any opportunistic infection was slightly increased among MS patients,” the researchers wrote (520 vs. 338 per 10,000 person-years; IRR, 1.54). This was particularly true for candidiasis (252 vs. 166 per 10,000 person-years; IRR, 1.52) and herpes virus infection (221 vs. 150 per 10,000 person-years; IRR, 1.47). “There were few cases of tuberculosis, hepatitis B infection, or hepatitis C infection,” they noted.
The study was funded by a grant from Celgene, a subsidiary of Bristol-Myers Squibb. Four authors are employees of Bristol-Myers Squibb, and one author works for a company that does business with Celgene.
SOURCE: Jick S et al. ACTRIMS Forum 2020, Abstract P086.
REPORTING FROM ACTRIMS FORUM 2020