User login
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
Cancer mortality continues to decline while cancer incidence rises in women
according to the Annual Report to the Nation on the Status of Cancer.
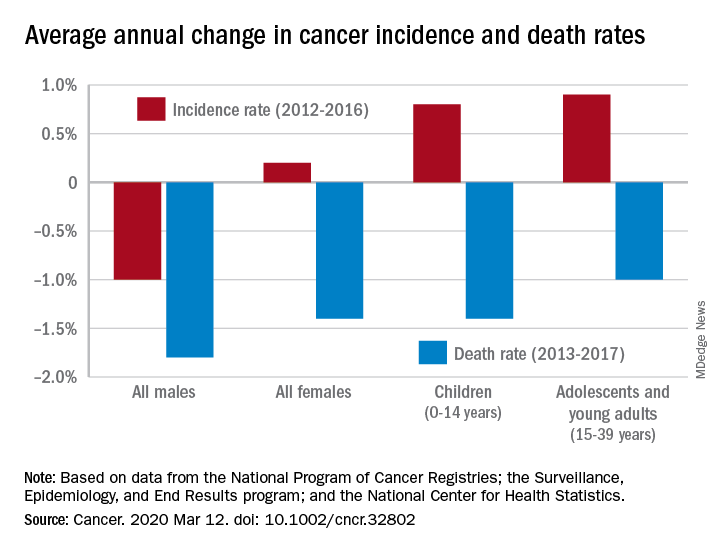
During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
according to the Annual Report to the Nation on the Status of Cancer.

During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
according to the Annual Report to the Nation on the Status of Cancer.

During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
FROM CANCER
Botox: A new option for endometriosis pain?
NATIONAL HARBOR, MD. – Botulinum toxin injection into the vagina appears to relieve pain associated with endometriosis by relaxing the pelvic floor muscles, new research suggests.
In a randomized study, women with surgically diagnosed endometriosis who had chronic pelvic pain despite optimal surgical and hormonal treatment had less pain after injection vs their counterparts who received placebo.
This result suggests pelvic floor spasm may be an important factor in endometriosis-associated pelvic pain, the investigators note.
“Botulinum toxin injection offers an alternative approach for women with pelvic pain,” lead author Barbara Illowsky Karp, MD, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., told Medscape Medical News.
“We focused on endometriosis, but there are reasons to think it may be effective for pelvic pain from other causes if there is spasm of the muscle,” said Karp, a neurologist who has used botulinum toxin therapeutically since it was first developed in the 1980s.
She noted that it is unknown whether the toxin will work in women who do not have actual spasm, “but the effect on spasm is not the sole effect of toxin,” as demonstrated by its use in other pain conditions.
“It seems to have a direct effect on the pain pathways in the nervous system as well,” Karp added.
The study findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Less pain
The investigators randomly assigned 29 women between the ages of 18 and 50 years to receive injections with 100 U onabotulinumtoxinA (n = 15) or saline placebo (n = 14).
All of the women had endometriosis with chronic pelvic pain lasting at least 3 months (mean time, 6 years) and confirmed pelvic floor spasm as a main pain generator.
One month after treatment, participants were asked if they had improvement in their pain.
“Our primary outcome was just simply asking the women if they felt better or not, because we were blinded as to what treatment they received. One month is typically when the toxin reaches its maximal effect,” Karp said.
At 1 month, 11 women in the placebo group reported that they had no benefit, compared with only 4 women in the botulinum toxin group (P = .027).
The botulinum toxin group reported a greater degree of benefit compared with the placebo group (P = .030) and greater percent of improvement (P = .034).
Neither group reported substantial changes in pain rating on the visual analog scale. However, a definite pain score is often difficult to measure in women with chronic pelvic pain, coinvestigator Pamela Stratton, MD, a gynecologist in Bethesda, said.
“Some women report their pain as a 2, some as an 8. Also, women may have a lot of pain one day and not have that much pain another day, and how do you measure that? This is why we have not focused solely on the pain score but instead wanted the women to tell us if they were improved or not,” Stratton told Medscape Medical News.
Disability worsened considerably in the placebo group, but remained consistent in the botulinum toxin group. Five patients in the botulinum toxin group were able to reduce pain medication compared with one patient in the placebo group.
“Compelling” findings but early days
Commenting on the findings for Medscape Medical News, Ann E. Hansen, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, noted that this “preliminary study” showed some benefit for a complex and challenging-to-treat syndrome.
“Injection of botulinum toxin prevents local muscle contraction, thus effectively relieving a variety of neuromuscular conditions such as torticollis; spasticity; pain syndromes such as headache and migraine; and some neurologic disorders, for instance, overactive bladder,” said Hansen, who was not involved with the research.
“Using botulinum toxin injection to target pelvic floor muscle spasm, a known pain generator in women suffering from chronic pelvic pain, makes sense. Future studies will be helpful in elucidating optimal treatment protocols for this debilitating condition,” she added.
Also commenting for Medscape Medical News, Kathryn T. Hall, PhD, MPH, Brigham & Women’s Hospital, Boston, Massachusetts, called the results “quite compelling” although, “it’s still early days.”
“It remains to be seen if the treatment effect will endure or if side effects will emerge. Hopefully all will go well,” Hall said.
The study was funded by an unrestricted grant from the National Institutes of Health. Allergan provided the botulinum toxin that was used in the study. Karp, Stratton, Hansen, and Hall have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Botulinum toxin injection into the vagina appears to relieve pain associated with endometriosis by relaxing the pelvic floor muscles, new research suggests.
In a randomized study, women with surgically diagnosed endometriosis who had chronic pelvic pain despite optimal surgical and hormonal treatment had less pain after injection vs their counterparts who received placebo.
This result suggests pelvic floor spasm may be an important factor in endometriosis-associated pelvic pain, the investigators note.
“Botulinum toxin injection offers an alternative approach for women with pelvic pain,” lead author Barbara Illowsky Karp, MD, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., told Medscape Medical News.
“We focused on endometriosis, but there are reasons to think it may be effective for pelvic pain from other causes if there is spasm of the muscle,” said Karp, a neurologist who has used botulinum toxin therapeutically since it was first developed in the 1980s.
She noted that it is unknown whether the toxin will work in women who do not have actual spasm, “but the effect on spasm is not the sole effect of toxin,” as demonstrated by its use in other pain conditions.
“It seems to have a direct effect on the pain pathways in the nervous system as well,” Karp added.
The study findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Less pain
The investigators randomly assigned 29 women between the ages of 18 and 50 years to receive injections with 100 U onabotulinumtoxinA (n = 15) or saline placebo (n = 14).
All of the women had endometriosis with chronic pelvic pain lasting at least 3 months (mean time, 6 years) and confirmed pelvic floor spasm as a main pain generator.
One month after treatment, participants were asked if they had improvement in their pain.
“Our primary outcome was just simply asking the women if they felt better or not, because we were blinded as to what treatment they received. One month is typically when the toxin reaches its maximal effect,” Karp said.
At 1 month, 11 women in the placebo group reported that they had no benefit, compared with only 4 women in the botulinum toxin group (P = .027).
The botulinum toxin group reported a greater degree of benefit compared with the placebo group (P = .030) and greater percent of improvement (P = .034).
Neither group reported substantial changes in pain rating on the visual analog scale. However, a definite pain score is often difficult to measure in women with chronic pelvic pain, coinvestigator Pamela Stratton, MD, a gynecologist in Bethesda, said.
“Some women report their pain as a 2, some as an 8. Also, women may have a lot of pain one day and not have that much pain another day, and how do you measure that? This is why we have not focused solely on the pain score but instead wanted the women to tell us if they were improved or not,” Stratton told Medscape Medical News.
Disability worsened considerably in the placebo group, but remained consistent in the botulinum toxin group. Five patients in the botulinum toxin group were able to reduce pain medication compared with one patient in the placebo group.
“Compelling” findings but early days
Commenting on the findings for Medscape Medical News, Ann E. Hansen, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, noted that this “preliminary study” showed some benefit for a complex and challenging-to-treat syndrome.
“Injection of botulinum toxin prevents local muscle contraction, thus effectively relieving a variety of neuromuscular conditions such as torticollis; spasticity; pain syndromes such as headache and migraine; and some neurologic disorders, for instance, overactive bladder,” said Hansen, who was not involved with the research.
“Using botulinum toxin injection to target pelvic floor muscle spasm, a known pain generator in women suffering from chronic pelvic pain, makes sense. Future studies will be helpful in elucidating optimal treatment protocols for this debilitating condition,” she added.
Also commenting for Medscape Medical News, Kathryn T. Hall, PhD, MPH, Brigham & Women’s Hospital, Boston, Massachusetts, called the results “quite compelling” although, “it’s still early days.”
“It remains to be seen if the treatment effect will endure or if side effects will emerge. Hopefully all will go well,” Hall said.
The study was funded by an unrestricted grant from the National Institutes of Health. Allergan provided the botulinum toxin that was used in the study. Karp, Stratton, Hansen, and Hall have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Botulinum toxin injection into the vagina appears to relieve pain associated with endometriosis by relaxing the pelvic floor muscles, new research suggests.
In a randomized study, women with surgically diagnosed endometriosis who had chronic pelvic pain despite optimal surgical and hormonal treatment had less pain after injection vs their counterparts who received placebo.
This result suggests pelvic floor spasm may be an important factor in endometriosis-associated pelvic pain, the investigators note.
“Botulinum toxin injection offers an alternative approach for women with pelvic pain,” lead author Barbara Illowsky Karp, MD, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., told Medscape Medical News.
“We focused on endometriosis, but there are reasons to think it may be effective for pelvic pain from other causes if there is spasm of the muscle,” said Karp, a neurologist who has used botulinum toxin therapeutically since it was first developed in the 1980s.
She noted that it is unknown whether the toxin will work in women who do not have actual spasm, “but the effect on spasm is not the sole effect of toxin,” as demonstrated by its use in other pain conditions.
“It seems to have a direct effect on the pain pathways in the nervous system as well,” Karp added.
The study findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Less pain
The investigators randomly assigned 29 women between the ages of 18 and 50 years to receive injections with 100 U onabotulinumtoxinA (n = 15) or saline placebo (n = 14).
All of the women had endometriosis with chronic pelvic pain lasting at least 3 months (mean time, 6 years) and confirmed pelvic floor spasm as a main pain generator.
One month after treatment, participants were asked if they had improvement in their pain.
“Our primary outcome was just simply asking the women if they felt better or not, because we were blinded as to what treatment they received. One month is typically when the toxin reaches its maximal effect,” Karp said.
At 1 month, 11 women in the placebo group reported that they had no benefit, compared with only 4 women in the botulinum toxin group (P = .027).
The botulinum toxin group reported a greater degree of benefit compared with the placebo group (P = .030) and greater percent of improvement (P = .034).
Neither group reported substantial changes in pain rating on the visual analog scale. However, a definite pain score is often difficult to measure in women with chronic pelvic pain, coinvestigator Pamela Stratton, MD, a gynecologist in Bethesda, said.
“Some women report their pain as a 2, some as an 8. Also, women may have a lot of pain one day and not have that much pain another day, and how do you measure that? This is why we have not focused solely on the pain score but instead wanted the women to tell us if they were improved or not,” Stratton told Medscape Medical News.
Disability worsened considerably in the placebo group, but remained consistent in the botulinum toxin group. Five patients in the botulinum toxin group were able to reduce pain medication compared with one patient in the placebo group.
“Compelling” findings but early days
Commenting on the findings for Medscape Medical News, Ann E. Hansen, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, noted that this “preliminary study” showed some benefit for a complex and challenging-to-treat syndrome.
“Injection of botulinum toxin prevents local muscle contraction, thus effectively relieving a variety of neuromuscular conditions such as torticollis; spasticity; pain syndromes such as headache and migraine; and some neurologic disorders, for instance, overactive bladder,” said Hansen, who was not involved with the research.
“Using botulinum toxin injection to target pelvic floor muscle spasm, a known pain generator in women suffering from chronic pelvic pain, makes sense. Future studies will be helpful in elucidating optimal treatment protocols for this debilitating condition,” she added.
Also commenting for Medscape Medical News, Kathryn T. Hall, PhD, MPH, Brigham & Women’s Hospital, Boston, Massachusetts, called the results “quite compelling” although, “it’s still early days.”
“It remains to be seen if the treatment effect will endure or if side effects will emerge. Hopefully all will go well,” Hall said.
The study was funded by an unrestricted grant from the National Institutes of Health. Allergan provided the botulinum toxin that was used in the study. Karp, Stratton, Hansen, and Hall have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Antifungal drug appears safe for pregnancy
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
FROM JAMA DERMATOLOGY
Fezolinetant safe, effective for menopausal vasomotor symptoms
The selective neurokinin 3 receptor antagonist Graeme L. Fraser, PhD, of Ogeda, a subsidiary of Astellas Pharma, and associates reported in Menopause.
The investigators conducted a randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study between July 19, 2017, and Sept. 19, 2018, in 287 women who completed the full 12-week trial. The women were aged between 41 and 65 years, were menopausal, and had moderate to severe vasomotor symptoms (VMS) with an incidence of at least 50 episodes per week. The majority of the women were white, 25% were black, 1% were Asian, and 1% were “other.”
The reduction in VMS episodes in patients who received fezolinetant ranged from 1.9 to 3.5 episodes per day at week 4 and from 1.8 to 2.6 per day at week 12. The mean difference from placebo in VMS severity score was –0.4 to –1 at week 4 and was –0.2 to –0.6 at week 12. At least a 50% reduction in VMS frequency at week 12 was achieved by 81%-95% of patients who received fezolinetant, compared with 59% of those who received placebo.
Treatment-emergent adverse events were generally mild to moderate, with the most common events including nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough. Of the five severe adverse events reported, only two were considered related to treatment – cholelithiasis and drug-induced liver injury. A total of 21 patients discontinued because of adverse events.
“Further evaluation of fezolinetant in larger and longer phase 3 trials of women with VMS associated with menopause is warranted to more fully characterize its efficacy and safety profile,” Dr. Fraser and colleagues concluded.
The study was funded by Astellas Pharma. The investigators reported numerous conflicts of interest with pharmaceutical companies.
SOURCE: Fraser GL et al. Menopause. 2020 Feb 24. doi: 10.1097/GME.0000000000001510.
The selective neurokinin 3 receptor antagonist Graeme L. Fraser, PhD, of Ogeda, a subsidiary of Astellas Pharma, and associates reported in Menopause.
The investigators conducted a randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study between July 19, 2017, and Sept. 19, 2018, in 287 women who completed the full 12-week trial. The women were aged between 41 and 65 years, were menopausal, and had moderate to severe vasomotor symptoms (VMS) with an incidence of at least 50 episodes per week. The majority of the women were white, 25% were black, 1% were Asian, and 1% were “other.”
The reduction in VMS episodes in patients who received fezolinetant ranged from 1.9 to 3.5 episodes per day at week 4 and from 1.8 to 2.6 per day at week 12. The mean difference from placebo in VMS severity score was –0.4 to –1 at week 4 and was –0.2 to –0.6 at week 12. At least a 50% reduction in VMS frequency at week 12 was achieved by 81%-95% of patients who received fezolinetant, compared with 59% of those who received placebo.
Treatment-emergent adverse events were generally mild to moderate, with the most common events including nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough. Of the five severe adverse events reported, only two were considered related to treatment – cholelithiasis and drug-induced liver injury. A total of 21 patients discontinued because of adverse events.
“Further evaluation of fezolinetant in larger and longer phase 3 trials of women with VMS associated with menopause is warranted to more fully characterize its efficacy and safety profile,” Dr. Fraser and colleagues concluded.
The study was funded by Astellas Pharma. The investigators reported numerous conflicts of interest with pharmaceutical companies.
SOURCE: Fraser GL et al. Menopause. 2020 Feb 24. doi: 10.1097/GME.0000000000001510.
The selective neurokinin 3 receptor antagonist Graeme L. Fraser, PhD, of Ogeda, a subsidiary of Astellas Pharma, and associates reported in Menopause.
The investigators conducted a randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study between July 19, 2017, and Sept. 19, 2018, in 287 women who completed the full 12-week trial. The women were aged between 41 and 65 years, were menopausal, and had moderate to severe vasomotor symptoms (VMS) with an incidence of at least 50 episodes per week. The majority of the women were white, 25% were black, 1% were Asian, and 1% were “other.”
The reduction in VMS episodes in patients who received fezolinetant ranged from 1.9 to 3.5 episodes per day at week 4 and from 1.8 to 2.6 per day at week 12. The mean difference from placebo in VMS severity score was –0.4 to –1 at week 4 and was –0.2 to –0.6 at week 12. At least a 50% reduction in VMS frequency at week 12 was achieved by 81%-95% of patients who received fezolinetant, compared with 59% of those who received placebo.
Treatment-emergent adverse events were generally mild to moderate, with the most common events including nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough. Of the five severe adverse events reported, only two were considered related to treatment – cholelithiasis and drug-induced liver injury. A total of 21 patients discontinued because of adverse events.
“Further evaluation of fezolinetant in larger and longer phase 3 trials of women with VMS associated with menopause is warranted to more fully characterize its efficacy and safety profile,” Dr. Fraser and colleagues concluded.
The study was funded by Astellas Pharma. The investigators reported numerous conflicts of interest with pharmaceutical companies.
SOURCE: Fraser GL et al. Menopause. 2020 Feb 24. doi: 10.1097/GME.0000000000001510.
FROM MENOPAUSE
High BMI does not complicate postpartum tubal ligation
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
REPORTING FROM THE PREGNANCY MEETING
Prenatal test market booms as patients grapple with results
When she was 4 months pregnant, Angela Crawley waited for 30 minutes in a private room to hear the results of her noninvasive prenatal testing. Her ultrasound had been flagged as high risk by the radiologist and she agreed to undergo further testing to gather information on the health of her unborn child.
As she waited for her genetic counseling appointment, she noticed somber expressions on the faces of her health team and picked up on hushed tones.
It had taken 2 years to become pregnant and the joy she felt attending prenatal care appointments was fading into a sense of dread as she sat in that small room and the minutes ticked by.
Crawley – a scientist in the chronic disease program at the Ottawa Hospital Research Institute, assistant professor at the University of Ottawa, and adjunct research professor at Carleton University in Ontario, Canada – is more qualified than most patients to absorb health information and make appropriate decisions.
And yet, “I was completely unprepared,” she told Medscape Medical News as she reflected on what she now refers to as some of the darkest days of her life. “It was a nightmare and it was such a confusing, scary time.”
Crawley is among the more than 6 million women from at least 90 countries who have undergone noninvasive prenatal testing. During pregnancy, a mother’s bloodstream contains a mix of cell-free DNA from her own cells and from placental cells, which is usually identical to the DNA of the fetus. Analysis of cell-free DNA can lead to the early detection of genetic disorders.
Testing is most often used to look for chromosomal disorders that are caused by the presence of an extra chromosome, like in trisomy 21 in the case of Down syndrome or extra or missing copies of the X and Y chromosomes in other disorders. The accuracy of the test tends to vary, depending on the condition being assessed.
Cell-free DNA testing has reduced the number of invasive prenatal diagnostic procedures, some of which can lead to miscarriage, and this noninvasive option made sense to Crawley and was covered by government health insurance.
With a market projected to surpass $13 billion by the year 2027, some experts speculate that prenatal genetic testing is the most rapidly adopted test in human history. Globally, noninvasive prenatal tests cost $500 to $3,000 for patients who pay out of pocket, and all those screening options are amassing valuable genetic data troves.
The pioneer of noninvasive prenatal testing, Dennis Lo, PhD, from the Chinese University of Hong Kong, told Medscape Medical News that the success of using cell-free DNA came after a long, winding road of rejected grant applications and scientific skepticism.
“Initially, people did not think this would be useful for assessing chromosomal abnormalities because the thinking at the time was that we would need to count them,” Lo said.
But he was enchanted by early glimpses of the capability of cell-free DNA, and felt driven to pursue unconventional research ideas even though there were significant hurdles to overcome in the lab.
“We were detecting fetal Y chromosomes in women. At first, it was just scientific curiosity,” said Lo. “At the time, people worried that fetal cells would persist from one pregnancy to the next, but we discovered that fetal DNA actually clears very quickly and does not progress into the next pregnancy,” he explained. “This is very important because it won’t alter the accuracy of the test.”
Gripped by the scientific mystery, the researcher put in long hours at the lab. “I’m fortunate I have a very understanding wife who is herself a scientist,” he said. After a particularly long stretch without quality time together, Lo and his spouse, Alice Wong, went to see a Harry Potter movie.
As Lo viewed the Harry Potter H through 3D glasses, he was suddenly reminded of the male human karyotype.
“I saw the vertical stripes of the H and it hit me,” he told Medscape Medical News. “There are two sets of chromosomes.” The average human karyotype contains 22 pairs of autosomal chromosomes and one pair of sex chromosomes.
“Our complex genetic conundrum was cracked in the middle of a Harry Potter movie in a moment when I felt completely relaxed,” he recalled. “My wife said: ‘You can’t even watch a movie properly.’ ”
Back at the lab, Lo shared his Harry Potter–inspired concept and the team got to work.
In December 2019, Lo received the Fudan-Zhongzhi Science Award in Shanghai from Nobel laureate physicist Samuel Chao Chung Ting, chair of the award committee. The prize honors fundamental and groundbreaking achievements in biomedicine, and the laureate receives ¥3 million (about U.S. $428,550), donated by Zhongzhi Enterprise Group.
This honor was 30 years in the making, Lo told Medscape Medical News. “I’m pleased to experience public recognition and this is a high honor in China,” he added.
“Noninvasive prenatal testing is better than anything we’ve ever had before,” said Ronald Wapner, MD, from the Columbia University Irving Medical Center in New York City, who taught a course on the transition of prenatal diagnostics from amniocentesis to whole-genome sequencing at the recent Society for Maternal–Fetal Medicine 2020 Annual Pregnancy Meeting.
“We now have the capability to improve healthcare decision-making in utero and at birth,” he told Medscape Medical News. “It’s remarkable.”
But, Wapner said, the market grew too fast. “The National Institutes of Health didn’t even play a role in these fast-paced developments. Traditional governing bodies and authorities were bypassed as cytogenetic labs marketed directly to physicians and patients,” he explained.
One of the major problems with the rapid uptake in testing is a lack of preparation for patients like Crawley.
The clinician who delivered her test results was not feeling well, so “she spoke through a surgical mask,” Crawley reported. “I was trying to understand what she was saying, but it was an uncomfortable exchange.”
Crawley had undergone prenatal genetic testing because her ultrasound had shown irregularities in fetal leg measurements. The genetic tests confirmed no anomalies in the chromosome count, but that was it.
“There was no prognosis, just vague numbers that no one seemed to know what to do with,” Crawley recalled.
With concern about growth measurements, the conversation moved quickly to options, including termination. Crawley said the dialogue felt jarring and moved too quickly for her to process all the information and possible courses of action.
She was told she could terminate and “try again to get pregnant.” But Crawley was 39 years old and had been trying to conceive for 2 years.
“It was devastating,” she said. “No one sat down with me before this appointment to learn about my values or preferences, and I left that conversation with more questions than I had before I arrived. I went home and had one the worst weekends of my life. My husband and I felt so overwhelmed, grieved, and alone.”
Pretest counseling can be as important as any subsequent genetic counseling, said Blair Stevens, a prenatal expert from the National Society of Genetic Counselors and a genetic counselor at the University of Texas Health Science Center in Houston.
“Information is valuable, but it can also be toxic, depending on what individuals intend to do with what they learn,” she explained. “We cannot unknow or unhear details, so it’s really important to work with patients in advance to make sure their preferences guide any planning.”
Uncertainty can be very unsettling, she acknowledged. “It’s important to help patients balance any ambiguity, so if there is a 20% risk, there is also an 80% chance of another, perhaps more favorable, outcome.”
Most clinicians don’t have the time to fully assess patient goals and align counseling approaches to individual needs, Stevens explained. And public interest in prenatal testing has outpaced clinical best practices as competing labs race to expand offerings and add options to screening tests to grab a piece of the global market, which is now about 130 million births per year.
“These are not scientifically sound additions and we need more evidence,” Stevens said. “There is a right way to handle this, and labs and clinicians need to collaborate on responsible methods to test and integrate expanding options.”
The blue and pink elephant in the room
“The reality is that most people don’t have a super high risk for chromosomal irregularities,” said Stevens. “Most people are more interested in learning the sex of their baby in early pregnancy than in any actual desire for genetic information.” Noninvasive prenatal testing can detect fetal sex as early as 9 weeks into a pregnancy, whereas ultrasound might not detect it until about 18 weeks.
“Honestly? I think the growing popularity of gender-reveal parties is what is actually driving the push for more prenatal testing,” she added. “The problem is that a couple eager to learn the sex of their baby may wind up with way more information than they expected and have trouble processing unanticipated risk.”
In February, five national medical organizations in the United States partnered with the Reproductive Genetics Technology Consortium to develop consensus recommendations and guidelines for prenatal genetic testing.
The National Society of Genetic Counselors and the Society for Maternal–Fetal Medicine are among the new members that will provide a forum through which commercial laboratories can communicate about new technologies and obtain input and guidance on emerging options.
Wapner, who is a member of the consortium, said he hopes thought leaders will be at the forefront to guide this next chapter of prenatal screening. “So much money is pouring into all this testing; let’s make sure we are making the right, most essential screening decisions,” he said.
“Science typically advances more rapidly than the ethical and legal framework to support decision-making, and it’s important for society to put protections in place,” Lo acknowledged.
The misuse of screening and unethical sex-selection efforts in Asia and elsewhere in the world, where males are highly valued and females are more likely to be aborted, is dismaying, he told Medscape Medical News. “These are exploitations of the science.”
In addition to scientific misuse like sex selection, data breaches are becoming a huge concern as companies amass large amounts of valuable genetic information.
Data for ransom
In Canada, where Crawley took her test, LifeLabs – the country’s largest laboratory testing company and a provider of genetic testing – paid a ransom after a major cyberattack led to the theft of lab results for 85,000 people in Ontario and the personal information of 15 million customers.
LifeLabs paid an undisclosed sum to retrieve the data, the company reported on December 17, and hired cybersecurity experts to assess the damage. The company is offering security protection services, including identity theft and fraud protection insurance, to customers.
“This has served as a reminder that we need to stay ahead of cybercrime, which has become a pervasive issue around the world in all sectors,” Charles Brown, president and chief executive officer of LifeLabs, wrote in a letter to customers. “You entrust us with important health information, and we take that responsibility very seriously.”
The United States has led the world in the commercial push for more prenatal testing. Other countries in Europe, for example, have proceeded with caution and have integrated the technologies with more controls. Hong Kong, where the inventor of the test is based, has been among the slowest to adopt the practice.
“I have been lobbying for 8 years for Hong Kong to offer testing,” said Lo. “I think Hong Kong has been too slow to integrate, but the United States probably moved too quickly. There is a balance that I think countries like the Netherlands have found; they take the aim of screening into account, along with justice and societal aspects.”
“Ideally, we will develop a great pretest model triage tool to help guide patients through this process,” Stevens said. “And we have to make sure the data they receive are clinically useful and backed up by evidence to safeguard the care of every patient.”
The practice of medicine is meticulously designed to assess and mitigate risk, “but this sensible objective can also be extremely negative in focus, with not-so-great delivery of information,” she acknowledged. Each individual’s tolerance for uncertainty and ability to cope in the face of adversity varies. “These are complex conversations that require time and empathy, and the details matter,” she added.
“In my home state of Texas, where there is a large religious base, there is not as much drive for advance prenatal genetic information,” Stevens explained. “We see a real advocacy movement emerging and a need for information from patients first because these can’t really be clinician-led decisions,” she pointed out. “Patients come to us undergoing not just the physical changes of pregnancy, but also emotional transformation as they transition to become parents. They may be nauseous or already sleep-deprived and they need our help,” she added.
Crawley could feel the fluttering of fetal movements in her womb and said she felt connected to her child, but she remembered her trip to Ireland when she and her husband drank too much and they likely conceived. Irrational thoughts crept in: “Maybe it was something we did. What about my swimming; could it have been harmful?”
Apprehensions lingered as she waited to meet her specialist. Would the child grow and be able to walk? Be held back by disabling joint pain? Crawley sat down with her doctor at the high-risk clinic to discuss the possibilities.
“I don’t see anything to be alarmed about. She’s probably going to be small,” said the obstetrician.
“She?!” Crawley had opted not to learn the sex of her baby, unlike so many other parents she knew, but her hope for her baby’s good health soared above the accidental disclosure.
“Everything changed in that moment,” Crawley said. “I knew that we were going to be okay no matter what happened next.”
Crawley’s pregnancy progressed to term and she gave birth to a healthy baby girl who is now 3 years old and dances ballet. Her beloved daughter is shorter than some of the other dancers in her class, but her mom says she hasn’t missed a beat. “The world is a better place because my daughter is in it,” Crawley said. “This, I know for sure.”
This article first appeared on Medscape.com.
When she was 4 months pregnant, Angela Crawley waited for 30 minutes in a private room to hear the results of her noninvasive prenatal testing. Her ultrasound had been flagged as high risk by the radiologist and she agreed to undergo further testing to gather information on the health of her unborn child.
As she waited for her genetic counseling appointment, she noticed somber expressions on the faces of her health team and picked up on hushed tones.
It had taken 2 years to become pregnant and the joy she felt attending prenatal care appointments was fading into a sense of dread as she sat in that small room and the minutes ticked by.
Crawley – a scientist in the chronic disease program at the Ottawa Hospital Research Institute, assistant professor at the University of Ottawa, and adjunct research professor at Carleton University in Ontario, Canada – is more qualified than most patients to absorb health information and make appropriate decisions.
And yet, “I was completely unprepared,” she told Medscape Medical News as she reflected on what she now refers to as some of the darkest days of her life. “It was a nightmare and it was such a confusing, scary time.”
Crawley is among the more than 6 million women from at least 90 countries who have undergone noninvasive prenatal testing. During pregnancy, a mother’s bloodstream contains a mix of cell-free DNA from her own cells and from placental cells, which is usually identical to the DNA of the fetus. Analysis of cell-free DNA can lead to the early detection of genetic disorders.
Testing is most often used to look for chromosomal disorders that are caused by the presence of an extra chromosome, like in trisomy 21 in the case of Down syndrome or extra or missing copies of the X and Y chromosomes in other disorders. The accuracy of the test tends to vary, depending on the condition being assessed.
Cell-free DNA testing has reduced the number of invasive prenatal diagnostic procedures, some of which can lead to miscarriage, and this noninvasive option made sense to Crawley and was covered by government health insurance.
With a market projected to surpass $13 billion by the year 2027, some experts speculate that prenatal genetic testing is the most rapidly adopted test in human history. Globally, noninvasive prenatal tests cost $500 to $3,000 for patients who pay out of pocket, and all those screening options are amassing valuable genetic data troves.
The pioneer of noninvasive prenatal testing, Dennis Lo, PhD, from the Chinese University of Hong Kong, told Medscape Medical News that the success of using cell-free DNA came after a long, winding road of rejected grant applications and scientific skepticism.
“Initially, people did not think this would be useful for assessing chromosomal abnormalities because the thinking at the time was that we would need to count them,” Lo said.
But he was enchanted by early glimpses of the capability of cell-free DNA, and felt driven to pursue unconventional research ideas even though there were significant hurdles to overcome in the lab.
“We were detecting fetal Y chromosomes in women. At first, it was just scientific curiosity,” said Lo. “At the time, people worried that fetal cells would persist from one pregnancy to the next, but we discovered that fetal DNA actually clears very quickly and does not progress into the next pregnancy,” he explained. “This is very important because it won’t alter the accuracy of the test.”
Gripped by the scientific mystery, the researcher put in long hours at the lab. “I’m fortunate I have a very understanding wife who is herself a scientist,” he said. After a particularly long stretch without quality time together, Lo and his spouse, Alice Wong, went to see a Harry Potter movie.
As Lo viewed the Harry Potter H through 3D glasses, he was suddenly reminded of the male human karyotype.
“I saw the vertical stripes of the H and it hit me,” he told Medscape Medical News. “There are two sets of chromosomes.” The average human karyotype contains 22 pairs of autosomal chromosomes and one pair of sex chromosomes.
“Our complex genetic conundrum was cracked in the middle of a Harry Potter movie in a moment when I felt completely relaxed,” he recalled. “My wife said: ‘You can’t even watch a movie properly.’ ”
Back at the lab, Lo shared his Harry Potter–inspired concept and the team got to work.
In December 2019, Lo received the Fudan-Zhongzhi Science Award in Shanghai from Nobel laureate physicist Samuel Chao Chung Ting, chair of the award committee. The prize honors fundamental and groundbreaking achievements in biomedicine, and the laureate receives ¥3 million (about U.S. $428,550), donated by Zhongzhi Enterprise Group.
This honor was 30 years in the making, Lo told Medscape Medical News. “I’m pleased to experience public recognition and this is a high honor in China,” he added.
“Noninvasive prenatal testing is better than anything we’ve ever had before,” said Ronald Wapner, MD, from the Columbia University Irving Medical Center in New York City, who taught a course on the transition of prenatal diagnostics from amniocentesis to whole-genome sequencing at the recent Society for Maternal–Fetal Medicine 2020 Annual Pregnancy Meeting.
“We now have the capability to improve healthcare decision-making in utero and at birth,” he told Medscape Medical News. “It’s remarkable.”
But, Wapner said, the market grew too fast. “The National Institutes of Health didn’t even play a role in these fast-paced developments. Traditional governing bodies and authorities were bypassed as cytogenetic labs marketed directly to physicians and patients,” he explained.
One of the major problems with the rapid uptake in testing is a lack of preparation for patients like Crawley.
The clinician who delivered her test results was not feeling well, so “she spoke through a surgical mask,” Crawley reported. “I was trying to understand what she was saying, but it was an uncomfortable exchange.”
Crawley had undergone prenatal genetic testing because her ultrasound had shown irregularities in fetal leg measurements. The genetic tests confirmed no anomalies in the chromosome count, but that was it.
“There was no prognosis, just vague numbers that no one seemed to know what to do with,” Crawley recalled.
With concern about growth measurements, the conversation moved quickly to options, including termination. Crawley said the dialogue felt jarring and moved too quickly for her to process all the information and possible courses of action.
She was told she could terminate and “try again to get pregnant.” But Crawley was 39 years old and had been trying to conceive for 2 years.
“It was devastating,” she said. “No one sat down with me before this appointment to learn about my values or preferences, and I left that conversation with more questions than I had before I arrived. I went home and had one the worst weekends of my life. My husband and I felt so overwhelmed, grieved, and alone.”
Pretest counseling can be as important as any subsequent genetic counseling, said Blair Stevens, a prenatal expert from the National Society of Genetic Counselors and a genetic counselor at the University of Texas Health Science Center in Houston.
“Information is valuable, but it can also be toxic, depending on what individuals intend to do with what they learn,” she explained. “We cannot unknow or unhear details, so it’s really important to work with patients in advance to make sure their preferences guide any planning.”
Uncertainty can be very unsettling, she acknowledged. “It’s important to help patients balance any ambiguity, so if there is a 20% risk, there is also an 80% chance of another, perhaps more favorable, outcome.”
Most clinicians don’t have the time to fully assess patient goals and align counseling approaches to individual needs, Stevens explained. And public interest in prenatal testing has outpaced clinical best practices as competing labs race to expand offerings and add options to screening tests to grab a piece of the global market, which is now about 130 million births per year.
“These are not scientifically sound additions and we need more evidence,” Stevens said. “There is a right way to handle this, and labs and clinicians need to collaborate on responsible methods to test and integrate expanding options.”
The blue and pink elephant in the room
“The reality is that most people don’t have a super high risk for chromosomal irregularities,” said Stevens. “Most people are more interested in learning the sex of their baby in early pregnancy than in any actual desire for genetic information.” Noninvasive prenatal testing can detect fetal sex as early as 9 weeks into a pregnancy, whereas ultrasound might not detect it until about 18 weeks.
“Honestly? I think the growing popularity of gender-reveal parties is what is actually driving the push for more prenatal testing,” she added. “The problem is that a couple eager to learn the sex of their baby may wind up with way more information than they expected and have trouble processing unanticipated risk.”
In February, five national medical organizations in the United States partnered with the Reproductive Genetics Technology Consortium to develop consensus recommendations and guidelines for prenatal genetic testing.
The National Society of Genetic Counselors and the Society for Maternal–Fetal Medicine are among the new members that will provide a forum through which commercial laboratories can communicate about new technologies and obtain input and guidance on emerging options.
Wapner, who is a member of the consortium, said he hopes thought leaders will be at the forefront to guide this next chapter of prenatal screening. “So much money is pouring into all this testing; let’s make sure we are making the right, most essential screening decisions,” he said.
“Science typically advances more rapidly than the ethical and legal framework to support decision-making, and it’s important for society to put protections in place,” Lo acknowledged.
The misuse of screening and unethical sex-selection efforts in Asia and elsewhere in the world, where males are highly valued and females are more likely to be aborted, is dismaying, he told Medscape Medical News. “These are exploitations of the science.”
In addition to scientific misuse like sex selection, data breaches are becoming a huge concern as companies amass large amounts of valuable genetic information.
Data for ransom
In Canada, where Crawley took her test, LifeLabs – the country’s largest laboratory testing company and a provider of genetic testing – paid a ransom after a major cyberattack led to the theft of lab results for 85,000 people in Ontario and the personal information of 15 million customers.
LifeLabs paid an undisclosed sum to retrieve the data, the company reported on December 17, and hired cybersecurity experts to assess the damage. The company is offering security protection services, including identity theft and fraud protection insurance, to customers.
“This has served as a reminder that we need to stay ahead of cybercrime, which has become a pervasive issue around the world in all sectors,” Charles Brown, president and chief executive officer of LifeLabs, wrote in a letter to customers. “You entrust us with important health information, and we take that responsibility very seriously.”
The United States has led the world in the commercial push for more prenatal testing. Other countries in Europe, for example, have proceeded with caution and have integrated the technologies with more controls. Hong Kong, where the inventor of the test is based, has been among the slowest to adopt the practice.
“I have been lobbying for 8 years for Hong Kong to offer testing,” said Lo. “I think Hong Kong has been too slow to integrate, but the United States probably moved too quickly. There is a balance that I think countries like the Netherlands have found; they take the aim of screening into account, along with justice and societal aspects.”
“Ideally, we will develop a great pretest model triage tool to help guide patients through this process,” Stevens said. “And we have to make sure the data they receive are clinically useful and backed up by evidence to safeguard the care of every patient.”
The practice of medicine is meticulously designed to assess and mitigate risk, “but this sensible objective can also be extremely negative in focus, with not-so-great delivery of information,” she acknowledged. Each individual’s tolerance for uncertainty and ability to cope in the face of adversity varies. “These are complex conversations that require time and empathy, and the details matter,” she added.
“In my home state of Texas, where there is a large religious base, there is not as much drive for advance prenatal genetic information,” Stevens explained. “We see a real advocacy movement emerging and a need for information from patients first because these can’t really be clinician-led decisions,” she pointed out. “Patients come to us undergoing not just the physical changes of pregnancy, but also emotional transformation as they transition to become parents. They may be nauseous or already sleep-deprived and they need our help,” she added.
Crawley could feel the fluttering of fetal movements in her womb and said she felt connected to her child, but she remembered her trip to Ireland when she and her husband drank too much and they likely conceived. Irrational thoughts crept in: “Maybe it was something we did. What about my swimming; could it have been harmful?”
Apprehensions lingered as she waited to meet her specialist. Would the child grow and be able to walk? Be held back by disabling joint pain? Crawley sat down with her doctor at the high-risk clinic to discuss the possibilities.
“I don’t see anything to be alarmed about. She’s probably going to be small,” said the obstetrician.
“She?!” Crawley had opted not to learn the sex of her baby, unlike so many other parents she knew, but her hope for her baby’s good health soared above the accidental disclosure.
“Everything changed in that moment,” Crawley said. “I knew that we were going to be okay no matter what happened next.”
Crawley’s pregnancy progressed to term and she gave birth to a healthy baby girl who is now 3 years old and dances ballet. Her beloved daughter is shorter than some of the other dancers in her class, but her mom says she hasn’t missed a beat. “The world is a better place because my daughter is in it,” Crawley said. “This, I know for sure.”
This article first appeared on Medscape.com.
When she was 4 months pregnant, Angela Crawley waited for 30 minutes in a private room to hear the results of her noninvasive prenatal testing. Her ultrasound had been flagged as high risk by the radiologist and she agreed to undergo further testing to gather information on the health of her unborn child.
As she waited for her genetic counseling appointment, she noticed somber expressions on the faces of her health team and picked up on hushed tones.
It had taken 2 years to become pregnant and the joy she felt attending prenatal care appointments was fading into a sense of dread as she sat in that small room and the minutes ticked by.
Crawley – a scientist in the chronic disease program at the Ottawa Hospital Research Institute, assistant professor at the University of Ottawa, and adjunct research professor at Carleton University in Ontario, Canada – is more qualified than most patients to absorb health information and make appropriate decisions.
And yet, “I was completely unprepared,” she told Medscape Medical News as she reflected on what she now refers to as some of the darkest days of her life. “It was a nightmare and it was such a confusing, scary time.”
Crawley is among the more than 6 million women from at least 90 countries who have undergone noninvasive prenatal testing. During pregnancy, a mother’s bloodstream contains a mix of cell-free DNA from her own cells and from placental cells, which is usually identical to the DNA of the fetus. Analysis of cell-free DNA can lead to the early detection of genetic disorders.
Testing is most often used to look for chromosomal disorders that are caused by the presence of an extra chromosome, like in trisomy 21 in the case of Down syndrome or extra or missing copies of the X and Y chromosomes in other disorders. The accuracy of the test tends to vary, depending on the condition being assessed.
Cell-free DNA testing has reduced the number of invasive prenatal diagnostic procedures, some of which can lead to miscarriage, and this noninvasive option made sense to Crawley and was covered by government health insurance.
With a market projected to surpass $13 billion by the year 2027, some experts speculate that prenatal genetic testing is the most rapidly adopted test in human history. Globally, noninvasive prenatal tests cost $500 to $3,000 for patients who pay out of pocket, and all those screening options are amassing valuable genetic data troves.
The pioneer of noninvasive prenatal testing, Dennis Lo, PhD, from the Chinese University of Hong Kong, told Medscape Medical News that the success of using cell-free DNA came after a long, winding road of rejected grant applications and scientific skepticism.
“Initially, people did not think this would be useful for assessing chromosomal abnormalities because the thinking at the time was that we would need to count them,” Lo said.
But he was enchanted by early glimpses of the capability of cell-free DNA, and felt driven to pursue unconventional research ideas even though there were significant hurdles to overcome in the lab.
“We were detecting fetal Y chromosomes in women. At first, it was just scientific curiosity,” said Lo. “At the time, people worried that fetal cells would persist from one pregnancy to the next, but we discovered that fetal DNA actually clears very quickly and does not progress into the next pregnancy,” he explained. “This is very important because it won’t alter the accuracy of the test.”
Gripped by the scientific mystery, the researcher put in long hours at the lab. “I’m fortunate I have a very understanding wife who is herself a scientist,” he said. After a particularly long stretch without quality time together, Lo and his spouse, Alice Wong, went to see a Harry Potter movie.
As Lo viewed the Harry Potter H through 3D glasses, he was suddenly reminded of the male human karyotype.
“I saw the vertical stripes of the H and it hit me,” he told Medscape Medical News. “There are two sets of chromosomes.” The average human karyotype contains 22 pairs of autosomal chromosomes and one pair of sex chromosomes.
“Our complex genetic conundrum was cracked in the middle of a Harry Potter movie in a moment when I felt completely relaxed,” he recalled. “My wife said: ‘You can’t even watch a movie properly.’ ”
Back at the lab, Lo shared his Harry Potter–inspired concept and the team got to work.
In December 2019, Lo received the Fudan-Zhongzhi Science Award in Shanghai from Nobel laureate physicist Samuel Chao Chung Ting, chair of the award committee. The prize honors fundamental and groundbreaking achievements in biomedicine, and the laureate receives ¥3 million (about U.S. $428,550), donated by Zhongzhi Enterprise Group.
This honor was 30 years in the making, Lo told Medscape Medical News. “I’m pleased to experience public recognition and this is a high honor in China,” he added.
“Noninvasive prenatal testing is better than anything we’ve ever had before,” said Ronald Wapner, MD, from the Columbia University Irving Medical Center in New York City, who taught a course on the transition of prenatal diagnostics from amniocentesis to whole-genome sequencing at the recent Society for Maternal–Fetal Medicine 2020 Annual Pregnancy Meeting.
“We now have the capability to improve healthcare decision-making in utero and at birth,” he told Medscape Medical News. “It’s remarkable.”
But, Wapner said, the market grew too fast. “The National Institutes of Health didn’t even play a role in these fast-paced developments. Traditional governing bodies and authorities were bypassed as cytogenetic labs marketed directly to physicians and patients,” he explained.
One of the major problems with the rapid uptake in testing is a lack of preparation for patients like Crawley.
The clinician who delivered her test results was not feeling well, so “she spoke through a surgical mask,” Crawley reported. “I was trying to understand what she was saying, but it was an uncomfortable exchange.”
Crawley had undergone prenatal genetic testing because her ultrasound had shown irregularities in fetal leg measurements. The genetic tests confirmed no anomalies in the chromosome count, but that was it.
“There was no prognosis, just vague numbers that no one seemed to know what to do with,” Crawley recalled.
With concern about growth measurements, the conversation moved quickly to options, including termination. Crawley said the dialogue felt jarring and moved too quickly for her to process all the information and possible courses of action.
She was told she could terminate and “try again to get pregnant.” But Crawley was 39 years old and had been trying to conceive for 2 years.
“It was devastating,” she said. “No one sat down with me before this appointment to learn about my values or preferences, and I left that conversation with more questions than I had before I arrived. I went home and had one the worst weekends of my life. My husband and I felt so overwhelmed, grieved, and alone.”
Pretest counseling can be as important as any subsequent genetic counseling, said Blair Stevens, a prenatal expert from the National Society of Genetic Counselors and a genetic counselor at the University of Texas Health Science Center in Houston.
“Information is valuable, but it can also be toxic, depending on what individuals intend to do with what they learn,” she explained. “We cannot unknow or unhear details, so it’s really important to work with patients in advance to make sure their preferences guide any planning.”
Uncertainty can be very unsettling, she acknowledged. “It’s important to help patients balance any ambiguity, so if there is a 20% risk, there is also an 80% chance of another, perhaps more favorable, outcome.”
Most clinicians don’t have the time to fully assess patient goals and align counseling approaches to individual needs, Stevens explained. And public interest in prenatal testing has outpaced clinical best practices as competing labs race to expand offerings and add options to screening tests to grab a piece of the global market, which is now about 130 million births per year.
“These are not scientifically sound additions and we need more evidence,” Stevens said. “There is a right way to handle this, and labs and clinicians need to collaborate on responsible methods to test and integrate expanding options.”
The blue and pink elephant in the room
“The reality is that most people don’t have a super high risk for chromosomal irregularities,” said Stevens. “Most people are more interested in learning the sex of their baby in early pregnancy than in any actual desire for genetic information.” Noninvasive prenatal testing can detect fetal sex as early as 9 weeks into a pregnancy, whereas ultrasound might not detect it until about 18 weeks.
“Honestly? I think the growing popularity of gender-reveal parties is what is actually driving the push for more prenatal testing,” she added. “The problem is that a couple eager to learn the sex of their baby may wind up with way more information than they expected and have trouble processing unanticipated risk.”
In February, five national medical organizations in the United States partnered with the Reproductive Genetics Technology Consortium to develop consensus recommendations and guidelines for prenatal genetic testing.
The National Society of Genetic Counselors and the Society for Maternal–Fetal Medicine are among the new members that will provide a forum through which commercial laboratories can communicate about new technologies and obtain input and guidance on emerging options.
Wapner, who is a member of the consortium, said he hopes thought leaders will be at the forefront to guide this next chapter of prenatal screening. “So much money is pouring into all this testing; let’s make sure we are making the right, most essential screening decisions,” he said.
“Science typically advances more rapidly than the ethical and legal framework to support decision-making, and it’s important for society to put protections in place,” Lo acknowledged.
The misuse of screening and unethical sex-selection efforts in Asia and elsewhere in the world, where males are highly valued and females are more likely to be aborted, is dismaying, he told Medscape Medical News. “These are exploitations of the science.”
In addition to scientific misuse like sex selection, data breaches are becoming a huge concern as companies amass large amounts of valuable genetic information.
Data for ransom
In Canada, where Crawley took her test, LifeLabs – the country’s largest laboratory testing company and a provider of genetic testing – paid a ransom after a major cyberattack led to the theft of lab results for 85,000 people in Ontario and the personal information of 15 million customers.
LifeLabs paid an undisclosed sum to retrieve the data, the company reported on December 17, and hired cybersecurity experts to assess the damage. The company is offering security protection services, including identity theft and fraud protection insurance, to customers.
“This has served as a reminder that we need to stay ahead of cybercrime, which has become a pervasive issue around the world in all sectors,” Charles Brown, president and chief executive officer of LifeLabs, wrote in a letter to customers. “You entrust us with important health information, and we take that responsibility very seriously.”
The United States has led the world in the commercial push for more prenatal testing. Other countries in Europe, for example, have proceeded with caution and have integrated the technologies with more controls. Hong Kong, where the inventor of the test is based, has been among the slowest to adopt the practice.
“I have been lobbying for 8 years for Hong Kong to offer testing,” said Lo. “I think Hong Kong has been too slow to integrate, but the United States probably moved too quickly. There is a balance that I think countries like the Netherlands have found; they take the aim of screening into account, along with justice and societal aspects.”
“Ideally, we will develop a great pretest model triage tool to help guide patients through this process,” Stevens said. “And we have to make sure the data they receive are clinically useful and backed up by evidence to safeguard the care of every patient.”
The practice of medicine is meticulously designed to assess and mitigate risk, “but this sensible objective can also be extremely negative in focus, with not-so-great delivery of information,” she acknowledged. Each individual’s tolerance for uncertainty and ability to cope in the face of adversity varies. “These are complex conversations that require time and empathy, and the details matter,” she added.
“In my home state of Texas, where there is a large religious base, there is not as much drive for advance prenatal genetic information,” Stevens explained. “We see a real advocacy movement emerging and a need for information from patients first because these can’t really be clinician-led decisions,” she pointed out. “Patients come to us undergoing not just the physical changes of pregnancy, but also emotional transformation as they transition to become parents. They may be nauseous or already sleep-deprived and they need our help,” she added.
Crawley could feel the fluttering of fetal movements in her womb and said she felt connected to her child, but she remembered her trip to Ireland when she and her husband drank too much and they likely conceived. Irrational thoughts crept in: “Maybe it was something we did. What about my swimming; could it have been harmful?”
Apprehensions lingered as she waited to meet her specialist. Would the child grow and be able to walk? Be held back by disabling joint pain? Crawley sat down with her doctor at the high-risk clinic to discuss the possibilities.
“I don’t see anything to be alarmed about. She’s probably going to be small,” said the obstetrician.
“She?!” Crawley had opted not to learn the sex of her baby, unlike so many other parents she knew, but her hope for her baby’s good health soared above the accidental disclosure.
“Everything changed in that moment,” Crawley said. “I knew that we were going to be okay no matter what happened next.”
Crawley’s pregnancy progressed to term and she gave birth to a healthy baby girl who is now 3 years old and dances ballet. Her beloved daughter is shorter than some of the other dancers in her class, but her mom says she hasn’t missed a beat. “The world is a better place because my daughter is in it,” Crawley said. “This, I know for sure.”
This article first appeared on Medscape.com.
Stillbirth linked to end-stage renal disease
and they were also at greater risk for chronic kidney disease (CKD), according to findings published in the American Journal of Obstetrics & Gynecology.
Peter M Barrett, MB, of the University College Cork (Ireland), and colleagues conducted a population-based cohort study using data from the Swedish Medical Birth Register, National Patient Register, and the Swedish Renal Register to identify women who had live births and stillbirths. They then used anonymized unique personal identification numbers to cross-reference the registries.
From a full cohort of nearly 2 million women who gave birth during 1973-2012, and during a median follow-up of 20.7 years, 13,032 women experienced stillbirth, which, until 2008, was defined as fetal death after 28 weeks’ gestation, and after 2008, as occurring after 22 weeks. Women were excluded if they had any diagnosis of renal disease before their first pregnancy, as well as for a history of cardiovascular disease, chronic hypertension, diabetes, and certain other conditions at baseline.
Overall, 18,017 women developed CKD, and 1,283 developed ESRD. The fully adjusted model showed adjusted hazard ratios of 1.26 for CKD (95% confidence interval, 1.09-1.45) and 2.25 for ESRD (95% CI, 1.55-3.25) in women who had experienced stillbirth, compared with those who had not experienced stillbirth.
The researchers reported that associations between stillbirth and renal disease existed independently of underlying medical and obstetric comorbidities, such as congenital malformations, being small for gestational age, and preeclampsia, and that when those comorbidities were excluded, “the associations between stillbirth and maternal renal disease were strengthened (CKD: aHR, 1.33; 95% CI, 1.13-1.57; and ESRD: aHR, 2.95; 95% CI, 1.86-4.68).”
In addition, they noted that there was no significant association between stillbirth and either CKD or ESRD in women who had prepregnancy medical comorbidities (CKD: aHR, 1.13; 95% CI 0.73-1.75; and ESRD: aHR 1.49; 95% CI, 0.78-2.85).
“Further research is required to better understand the underlying pathophysiology of this association and to determine whether affected women would benefit from closer surveillance and follow-up for future hypertension and renal disease,” the authors concluded.
The work was performed within the Irish Clinical Academic Training Programme and was funded by grants from several organizations. The authors reported no conflicts of interest.
SOURCE: Barret PM et al. Am J Obstet Gynecol. 2020 Feb 26. doi: 10.1016/j.ajog.2020.02.031.
and they were also at greater risk for chronic kidney disease (CKD), according to findings published in the American Journal of Obstetrics & Gynecology.
Peter M Barrett, MB, of the University College Cork (Ireland), and colleagues conducted a population-based cohort study using data from the Swedish Medical Birth Register, National Patient Register, and the Swedish Renal Register to identify women who had live births and stillbirths. They then used anonymized unique personal identification numbers to cross-reference the registries.
From a full cohort of nearly 2 million women who gave birth during 1973-2012, and during a median follow-up of 20.7 years, 13,032 women experienced stillbirth, which, until 2008, was defined as fetal death after 28 weeks’ gestation, and after 2008, as occurring after 22 weeks. Women were excluded if they had any diagnosis of renal disease before their first pregnancy, as well as for a history of cardiovascular disease, chronic hypertension, diabetes, and certain other conditions at baseline.
Overall, 18,017 women developed CKD, and 1,283 developed ESRD. The fully adjusted model showed adjusted hazard ratios of 1.26 for CKD (95% confidence interval, 1.09-1.45) and 2.25 for ESRD (95% CI, 1.55-3.25) in women who had experienced stillbirth, compared with those who had not experienced stillbirth.
The researchers reported that associations between stillbirth and renal disease existed independently of underlying medical and obstetric comorbidities, such as congenital malformations, being small for gestational age, and preeclampsia, and that when those comorbidities were excluded, “the associations between stillbirth and maternal renal disease were strengthened (CKD: aHR, 1.33; 95% CI, 1.13-1.57; and ESRD: aHR, 2.95; 95% CI, 1.86-4.68).”
In addition, they noted that there was no significant association between stillbirth and either CKD or ESRD in women who had prepregnancy medical comorbidities (CKD: aHR, 1.13; 95% CI 0.73-1.75; and ESRD: aHR 1.49; 95% CI, 0.78-2.85).
“Further research is required to better understand the underlying pathophysiology of this association and to determine whether affected women would benefit from closer surveillance and follow-up for future hypertension and renal disease,” the authors concluded.
The work was performed within the Irish Clinical Academic Training Programme and was funded by grants from several organizations. The authors reported no conflicts of interest.
SOURCE: Barret PM et al. Am J Obstet Gynecol. 2020 Feb 26. doi: 10.1016/j.ajog.2020.02.031.
and they were also at greater risk for chronic kidney disease (CKD), according to findings published in the American Journal of Obstetrics & Gynecology.
Peter M Barrett, MB, of the University College Cork (Ireland), and colleagues conducted a population-based cohort study using data from the Swedish Medical Birth Register, National Patient Register, and the Swedish Renal Register to identify women who had live births and stillbirths. They then used anonymized unique personal identification numbers to cross-reference the registries.
From a full cohort of nearly 2 million women who gave birth during 1973-2012, and during a median follow-up of 20.7 years, 13,032 women experienced stillbirth, which, until 2008, was defined as fetal death after 28 weeks’ gestation, and after 2008, as occurring after 22 weeks. Women were excluded if they had any diagnosis of renal disease before their first pregnancy, as well as for a history of cardiovascular disease, chronic hypertension, diabetes, and certain other conditions at baseline.
Overall, 18,017 women developed CKD, and 1,283 developed ESRD. The fully adjusted model showed adjusted hazard ratios of 1.26 for CKD (95% confidence interval, 1.09-1.45) and 2.25 for ESRD (95% CI, 1.55-3.25) in women who had experienced stillbirth, compared with those who had not experienced stillbirth.
The researchers reported that associations between stillbirth and renal disease existed independently of underlying medical and obstetric comorbidities, such as congenital malformations, being small for gestational age, and preeclampsia, and that when those comorbidities were excluded, “the associations between stillbirth and maternal renal disease were strengthened (CKD: aHR, 1.33; 95% CI, 1.13-1.57; and ESRD: aHR, 2.95; 95% CI, 1.86-4.68).”
In addition, they noted that there was no significant association between stillbirth and either CKD or ESRD in women who had prepregnancy medical comorbidities (CKD: aHR, 1.13; 95% CI 0.73-1.75; and ESRD: aHR 1.49; 95% CI, 0.78-2.85).
“Further research is required to better understand the underlying pathophysiology of this association and to determine whether affected women would benefit from closer surveillance and follow-up for future hypertension and renal disease,” the authors concluded.
The work was performed within the Irish Clinical Academic Training Programme and was funded by grants from several organizations. The authors reported no conflicts of interest.
SOURCE: Barret PM et al. Am J Obstet Gynecol. 2020 Feb 26. doi: 10.1016/j.ajog.2020.02.031.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
2020 Update on gynecologic cancer
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
Exercise needn’t be strenuous to reduce heart risk
PHOENIX – results from two studies presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting showed.
In one study, women who walked 2,100-4,500 steps each day reduced their risk of dying from cardiovascular disease by up to 38%, compared with those who walked fewer than 2,100 steps each day. In addition, women who walked more than 4,500 steps each day reduced their risk of cardiovascular disease (CVD) mortality risk by 48%.
The findings come from an ancillary analysis of the Women’s Health Study known as the Objective Physical Activity and Cardiovascular Health (OPACH) Study.
“Our work shows that both light-intensity and moderate-/vigorous-intensity steps are associated with reduced risk of cardiovascular disease death,” lead author Andrea Z. LaCroix, PhD, said in an interview. “And our previous studies show that all movement while standing, stepping, or just moving about at whatever intensity you choose, appears to have cardiovascular benefits, whereas long hours spent sedentary, especially prolonged sitting bouts are associated with increased risk of cardiovascular disease. These new findings on steps are best interpreted as showing that moving instead of sitting is good for your heart and blood vessels as we get older. Find the things you love to do and get moving.”
For OPACH, 6,379 women with an average age of 79 years wore ActiGraph GT3X+ triaxial accelerometers on their wrist for 7 days during 2012-2014, as a way to ascertain the number of steps they took. The researchers followed the study participants to March 1, 2019, and used Cox proportional hazard models to estimate CVD mortality across four quartiles of steps per day, adjusted for age, race/ethnicity, education, smoking, alcohol consumption, self-reported health, comorbidities, and physical function. The lowest quartile reference category was less than 2,108 steps per day. The second, third and fourth quartiles were: 2,108 to fewer than 3,136 steps, 3,136 to fewer than 4,499, and 4,500 and above.
Dr. LaCroix, distinguished professor and chief of epidemiology at the University of California, San Diego, reported that women who walked 2,100-4,500 steps daily reduced their risk of dying from CVD by up to up to 38%, compared with women who walked fewer than 2,100 daily steps. The women who walked more than 4,500 steps per day reduced their risk by 48%.
She noted that, for many years, common wisdom was that 10,000 steps per day should be used as a general fitness target, [but] that goal “was never evidence based, and so far, emerging evidence using accelerometers to measure steps shows benefit way below the level of 10,000 steps.” Dr. LaCroix added that, in this study, “we were able separate steps taken at a light intensity of energy expenditure versus a moderate or vigorous level of energy expenditure. This is like comparing slower versus faster steps. Both influenced the risk of CVD death and we found no evidence that faster steps were more beneficial for reducing risk of CVD death than slower steps. So, the main message I want my demographic [women aged over 60] to understand is that all movement appears to be good for your heart.”
Barry A. Franklin, PhD, director of preventive cardiology and cardiac rehabilitation at Beaumont Health in Royal Oak, Mich., characterized the study findings as “good news” but not entirely surprising. “It goes along with other research showing that the biggest bang from the buck is going from the least fit, least active cohort, which we call the bottom 20%, to the next lowest level,” he said in an interview. “So, by simply doing some steps, certainly less than 10,000, there were significant benefits for this older age group.”
Dr. LaCroix acknowledged certain limitations of the OPACH study, including the fact that it did not include men or women aged younger than 60 years. In addition, the accelerometer used in this and other studies may measure fewer steps than women are actually taking. “Devices vary in their accuracy,” she said. “If you are tracking steps, try to aim for 4,500 or a little more, but know that every step counts.”
In a separate study, researchers found that an increase of 30 minutes per day of low-intensity physical activity (LIPA) may lower the risk of death among older adults, regardless of the amount of moderate to vigorous physical activity (MVPA) participants are involved in or whether they have impaired physical function. In addition, an increase of 30 minutes of sedentary time per day may increase the risk of death regardless of the amount of MVPA or whether participants have impaired physical function.
Those are key findings from an analysis of 1,262 participants in the Framingham Offspring Study.
“Given that MVPA tends to decline with age, particularly during the mid- to late-life transition, promoting LIPA and reducing sedentary time may be a more practical alternative among older adults for reducing the risk of mortality,” lead author Joowon Lee, PhD, said in an interview at the meeting sponsored by the American Heart Association.
According to Dr. Lee, a postdoctoral fellow at Boston University, prior studies found that the inverse association between MVPA and cardiovascular and all-cause mortality among older adults. “However, we focused on sedentary and light-intensity physical activity, which is prevalent in older adult population,” he said. “Additionally, we looked at the association between physical activity and mortality after excluding participants with frailty as a sensitivity analysis.”
The researchers drew from accelerometry-derived physical activity data from 1,262 Framingham Offspring Study participants at their ninth examination (2011-2014). The mean age of the subjects was 69 years, 54% were women, and they had worn the accelerometers at least 10 hours per day for at least 4 days prior to the exam visit. The researchers used multivariable Cox proportional hazards regression models to relate physician activity and sedentary time with all-cause mortality adjusting for potential confounders.
During a median follow-up of 4.8 years, 67 study participants died. Dr. Lee and colleagues observed that higher total physical activity, LIPA, adherence to physical activity guidelines (at least 150 minutes of activity each week), and lower sedentary time were associated with a lower risk of all-cause mortality. Specifically, they were 67% less likely to die of any cause if they spent at least 150 minutes per week in moderate to vigorous physical activity, compared with those who did not. In addition, the researchers found that each 30-minute interval of LIPA, such as doing household chores or casual walking, was associated with a 20% lower risk of dying from any cause. On the other hand, every additional 30 minutes of being sedentary was related to a 32% higher risk of dying from any cause. The results remained statistically significant even after excluding those with frailty.
“In the present analysis, an increase of 10 minutes in MVPA was not associated with the risk of all-cause mortality although meeting physical activity guidelines [MVPA of at least 150 minutes per week] was the strongest factor associated with the risk of all-cause mortality,” Dr. Lee said.
He acknowledged certain limitations of the analysis, including the fact that the study participants were white individuals with European ancestry. “Additionally, a small number of mortality events were observed in the current investigation,” he said. “So, an additional study of larger multiethnic samples of older adults is warranted to confirm our findings.”
“We tell people: ‘You need 30 minutes of moderate intensity exercise most days of the week,’ ” Dr. Franklin said. “That’s true, but a classic study in Lancet showed that if you do 12 or 15 minutes of moderate exercise, not 30 minutes, you also get a 14% reduction in mortality. Some exercise is better than none, and for older adults, they don’t even have to do moderate intensity exercise to get benefits.”
Dr. LaCroix’s study was funded by the National Heart, Lung, and Blood Institute; Dr. LaCroix reported having no financial disclosures. Dr. Lee’s study was supported by the National Heart, Lung, and Blood Institute; Dr. Lee reported having no disclosures.
SOURCES: LaCroix A et al. Epi/Lifestyle 2020, Abstract 30; Lee J et al. Epi/Lifestyle 2020, Abstract 31.
PHOENIX – results from two studies presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting showed.
In one study, women who walked 2,100-4,500 steps each day reduced their risk of dying from cardiovascular disease by up to 38%, compared with those who walked fewer than 2,100 steps each day. In addition, women who walked more than 4,500 steps each day reduced their risk of cardiovascular disease (CVD) mortality risk by 48%.
The findings come from an ancillary analysis of the Women’s Health Study known as the Objective Physical Activity and Cardiovascular Health (OPACH) Study.
“Our work shows that both light-intensity and moderate-/vigorous-intensity steps are associated with reduced risk of cardiovascular disease death,” lead author Andrea Z. LaCroix, PhD, said in an interview. “And our previous studies show that all movement while standing, stepping, or just moving about at whatever intensity you choose, appears to have cardiovascular benefits, whereas long hours spent sedentary, especially prolonged sitting bouts are associated with increased risk of cardiovascular disease. These new findings on steps are best interpreted as showing that moving instead of sitting is good for your heart and blood vessels as we get older. Find the things you love to do and get moving.”
For OPACH, 6,379 women with an average age of 79 years wore ActiGraph GT3X+ triaxial accelerometers on their wrist for 7 days during 2012-2014, as a way to ascertain the number of steps they took. The researchers followed the study participants to March 1, 2019, and used Cox proportional hazard models to estimate CVD mortality across four quartiles of steps per day, adjusted for age, race/ethnicity, education, smoking, alcohol consumption, self-reported health, comorbidities, and physical function. The lowest quartile reference category was less than 2,108 steps per day. The second, third and fourth quartiles were: 2,108 to fewer than 3,136 steps, 3,136 to fewer than 4,499, and 4,500 and above.
Dr. LaCroix, distinguished professor and chief of epidemiology at the University of California, San Diego, reported that women who walked 2,100-4,500 steps daily reduced their risk of dying from CVD by up to up to 38%, compared with women who walked fewer than 2,100 daily steps. The women who walked more than 4,500 steps per day reduced their risk by 48%.
She noted that, for many years, common wisdom was that 10,000 steps per day should be used as a general fitness target, [but] that goal “was never evidence based, and so far, emerging evidence using accelerometers to measure steps shows benefit way below the level of 10,000 steps.” Dr. LaCroix added that, in this study, “we were able separate steps taken at a light intensity of energy expenditure versus a moderate or vigorous level of energy expenditure. This is like comparing slower versus faster steps. Both influenced the risk of CVD death and we found no evidence that faster steps were more beneficial for reducing risk of CVD death than slower steps. So, the main message I want my demographic [women aged over 60] to understand is that all movement appears to be good for your heart.”
Barry A. Franklin, PhD, director of preventive cardiology and cardiac rehabilitation at Beaumont Health in Royal Oak, Mich., characterized the study findings as “good news” but not entirely surprising. “It goes along with other research showing that the biggest bang from the buck is going from the least fit, least active cohort, which we call the bottom 20%, to the next lowest level,” he said in an interview. “So, by simply doing some steps, certainly less than 10,000, there were significant benefits for this older age group.”
Dr. LaCroix acknowledged certain limitations of the OPACH study, including the fact that it did not include men or women aged younger than 60 years. In addition, the accelerometer used in this and other studies may measure fewer steps than women are actually taking. “Devices vary in their accuracy,” she said. “If you are tracking steps, try to aim for 4,500 or a little more, but know that every step counts.”
In a separate study, researchers found that an increase of 30 minutes per day of low-intensity physical activity (LIPA) may lower the risk of death among older adults, regardless of the amount of moderate to vigorous physical activity (MVPA) participants are involved in or whether they have impaired physical function. In addition, an increase of 30 minutes of sedentary time per day may increase the risk of death regardless of the amount of MVPA or whether participants have impaired physical function.
Those are key findings from an analysis of 1,262 participants in the Framingham Offspring Study.
“Given that MVPA tends to decline with age, particularly during the mid- to late-life transition, promoting LIPA and reducing sedentary time may be a more practical alternative among older adults for reducing the risk of mortality,” lead author Joowon Lee, PhD, said in an interview at the meeting sponsored by the American Heart Association.
According to Dr. Lee, a postdoctoral fellow at Boston University, prior studies found that the inverse association between MVPA and cardiovascular and all-cause mortality among older adults. “However, we focused on sedentary and light-intensity physical activity, which is prevalent in older adult population,” he said. “Additionally, we looked at the association between physical activity and mortality after excluding participants with frailty as a sensitivity analysis.”
The researchers drew from accelerometry-derived physical activity data from 1,262 Framingham Offspring Study participants at their ninth examination (2011-2014). The mean age of the subjects was 69 years, 54% were women, and they had worn the accelerometers at least 10 hours per day for at least 4 days prior to the exam visit. The researchers used multivariable Cox proportional hazards regression models to relate physician activity and sedentary time with all-cause mortality adjusting for potential confounders.
During a median follow-up of 4.8 years, 67 study participants died. Dr. Lee and colleagues observed that higher total physical activity, LIPA, adherence to physical activity guidelines (at least 150 minutes of activity each week), and lower sedentary time were associated with a lower risk of all-cause mortality. Specifically, they were 67% less likely to die of any cause if they spent at least 150 minutes per week in moderate to vigorous physical activity, compared with those who did not. In addition, the researchers found that each 30-minute interval of LIPA, such as doing household chores or casual walking, was associated with a 20% lower risk of dying from any cause. On the other hand, every additional 30 minutes of being sedentary was related to a 32% higher risk of dying from any cause. The results remained statistically significant even after excluding those with frailty.
“In the present analysis, an increase of 10 minutes in MVPA was not associated with the risk of all-cause mortality although meeting physical activity guidelines [MVPA of at least 150 minutes per week] was the strongest factor associated with the risk of all-cause mortality,” Dr. Lee said.
He acknowledged certain limitations of the analysis, including the fact that the study participants were white individuals with European ancestry. “Additionally, a small number of mortality events were observed in the current investigation,” he said. “So, an additional study of larger multiethnic samples of older adults is warranted to confirm our findings.”
“We tell people: ‘You need 30 minutes of moderate intensity exercise most days of the week,’ ” Dr. Franklin said. “That’s true, but a classic study in Lancet showed that if you do 12 or 15 minutes of moderate exercise, not 30 minutes, you also get a 14% reduction in mortality. Some exercise is better than none, and for older adults, they don’t even have to do moderate intensity exercise to get benefits.”
Dr. LaCroix’s study was funded by the National Heart, Lung, and Blood Institute; Dr. LaCroix reported having no financial disclosures. Dr. Lee’s study was supported by the National Heart, Lung, and Blood Institute; Dr. Lee reported having no disclosures.
SOURCES: LaCroix A et al. Epi/Lifestyle 2020, Abstract 30; Lee J et al. Epi/Lifestyle 2020, Abstract 31.
PHOENIX – results from two studies presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting showed.
In one study, women who walked 2,100-4,500 steps each day reduced their risk of dying from cardiovascular disease by up to 38%, compared with those who walked fewer than 2,100 steps each day. In addition, women who walked more than 4,500 steps each day reduced their risk of cardiovascular disease (CVD) mortality risk by 48%.
The findings come from an ancillary analysis of the Women’s Health Study known as the Objective Physical Activity and Cardiovascular Health (OPACH) Study.
“Our work shows that both light-intensity and moderate-/vigorous-intensity steps are associated with reduced risk of cardiovascular disease death,” lead author Andrea Z. LaCroix, PhD, said in an interview. “And our previous studies show that all movement while standing, stepping, or just moving about at whatever intensity you choose, appears to have cardiovascular benefits, whereas long hours spent sedentary, especially prolonged sitting bouts are associated with increased risk of cardiovascular disease. These new findings on steps are best interpreted as showing that moving instead of sitting is good for your heart and blood vessels as we get older. Find the things you love to do and get moving.”
For OPACH, 6,379 women with an average age of 79 years wore ActiGraph GT3X+ triaxial accelerometers on their wrist for 7 days during 2012-2014, as a way to ascertain the number of steps they took. The researchers followed the study participants to March 1, 2019, and used Cox proportional hazard models to estimate CVD mortality across four quartiles of steps per day, adjusted for age, race/ethnicity, education, smoking, alcohol consumption, self-reported health, comorbidities, and physical function. The lowest quartile reference category was less than 2,108 steps per day. The second, third and fourth quartiles were: 2,108 to fewer than 3,136 steps, 3,136 to fewer than 4,499, and 4,500 and above.
Dr. LaCroix, distinguished professor and chief of epidemiology at the University of California, San Diego, reported that women who walked 2,100-4,500 steps daily reduced their risk of dying from CVD by up to up to 38%, compared with women who walked fewer than 2,100 daily steps. The women who walked more than 4,500 steps per day reduced their risk by 48%.
She noted that, for many years, common wisdom was that 10,000 steps per day should be used as a general fitness target, [but] that goal “was never evidence based, and so far, emerging evidence using accelerometers to measure steps shows benefit way below the level of 10,000 steps.” Dr. LaCroix added that, in this study, “we were able separate steps taken at a light intensity of energy expenditure versus a moderate or vigorous level of energy expenditure. This is like comparing slower versus faster steps. Both influenced the risk of CVD death and we found no evidence that faster steps were more beneficial for reducing risk of CVD death than slower steps. So, the main message I want my demographic [women aged over 60] to understand is that all movement appears to be good for your heart.”
Barry A. Franklin, PhD, director of preventive cardiology and cardiac rehabilitation at Beaumont Health in Royal Oak, Mich., characterized the study findings as “good news” but not entirely surprising. “It goes along with other research showing that the biggest bang from the buck is going from the least fit, least active cohort, which we call the bottom 20%, to the next lowest level,” he said in an interview. “So, by simply doing some steps, certainly less than 10,000, there were significant benefits for this older age group.”
Dr. LaCroix acknowledged certain limitations of the OPACH study, including the fact that it did not include men or women aged younger than 60 years. In addition, the accelerometer used in this and other studies may measure fewer steps than women are actually taking. “Devices vary in their accuracy,” she said. “If you are tracking steps, try to aim for 4,500 or a little more, but know that every step counts.”
In a separate study, researchers found that an increase of 30 minutes per day of low-intensity physical activity (LIPA) may lower the risk of death among older adults, regardless of the amount of moderate to vigorous physical activity (MVPA) participants are involved in or whether they have impaired physical function. In addition, an increase of 30 minutes of sedentary time per day may increase the risk of death regardless of the amount of MVPA or whether participants have impaired physical function.
Those are key findings from an analysis of 1,262 participants in the Framingham Offspring Study.
“Given that MVPA tends to decline with age, particularly during the mid- to late-life transition, promoting LIPA and reducing sedentary time may be a more practical alternative among older adults for reducing the risk of mortality,” lead author Joowon Lee, PhD, said in an interview at the meeting sponsored by the American Heart Association.
According to Dr. Lee, a postdoctoral fellow at Boston University, prior studies found that the inverse association between MVPA and cardiovascular and all-cause mortality among older adults. “However, we focused on sedentary and light-intensity physical activity, which is prevalent in older adult population,” he said. “Additionally, we looked at the association between physical activity and mortality after excluding participants with frailty as a sensitivity analysis.”
The researchers drew from accelerometry-derived physical activity data from 1,262 Framingham Offspring Study participants at their ninth examination (2011-2014). The mean age of the subjects was 69 years, 54% were women, and they had worn the accelerometers at least 10 hours per day for at least 4 days prior to the exam visit. The researchers used multivariable Cox proportional hazards regression models to relate physician activity and sedentary time with all-cause mortality adjusting for potential confounders.
During a median follow-up of 4.8 years, 67 study participants died. Dr. Lee and colleagues observed that higher total physical activity, LIPA, adherence to physical activity guidelines (at least 150 minutes of activity each week), and lower sedentary time were associated with a lower risk of all-cause mortality. Specifically, they were 67% less likely to die of any cause if they spent at least 150 minutes per week in moderate to vigorous physical activity, compared with those who did not. In addition, the researchers found that each 30-minute interval of LIPA, such as doing household chores or casual walking, was associated with a 20% lower risk of dying from any cause. On the other hand, every additional 30 minutes of being sedentary was related to a 32% higher risk of dying from any cause. The results remained statistically significant even after excluding those with frailty.
“In the present analysis, an increase of 10 minutes in MVPA was not associated with the risk of all-cause mortality although meeting physical activity guidelines [MVPA of at least 150 minutes per week] was the strongest factor associated with the risk of all-cause mortality,” Dr. Lee said.
He acknowledged certain limitations of the analysis, including the fact that the study participants were white individuals with European ancestry. “Additionally, a small number of mortality events were observed in the current investigation,” he said. “So, an additional study of larger multiethnic samples of older adults is warranted to confirm our findings.”
“We tell people: ‘You need 30 minutes of moderate intensity exercise most days of the week,’ ” Dr. Franklin said. “That’s true, but a classic study in Lancet showed that if you do 12 or 15 minutes of moderate exercise, not 30 minutes, you also get a 14% reduction in mortality. Some exercise is better than none, and for older adults, they don’t even have to do moderate intensity exercise to get benefits.”
Dr. LaCroix’s study was funded by the National Heart, Lung, and Blood Institute; Dr. LaCroix reported having no financial disclosures. Dr. Lee’s study was supported by the National Heart, Lung, and Blood Institute; Dr. Lee reported having no disclosures.
SOURCES: LaCroix A et al. Epi/Lifestyle 2020, Abstract 30; Lee J et al. Epi/Lifestyle 2020, Abstract 31.
REPORTING FROM EPI/LIFESTYLE 2020















