User login
Aquatic Antagonists: Dermatologic Injuries From Sea Urchins (Echinoidea)
Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
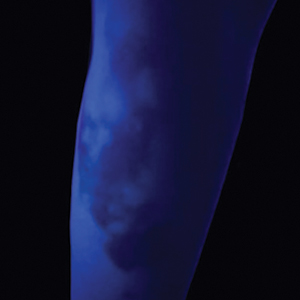
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
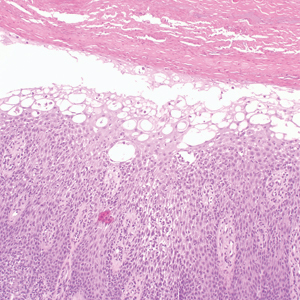
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
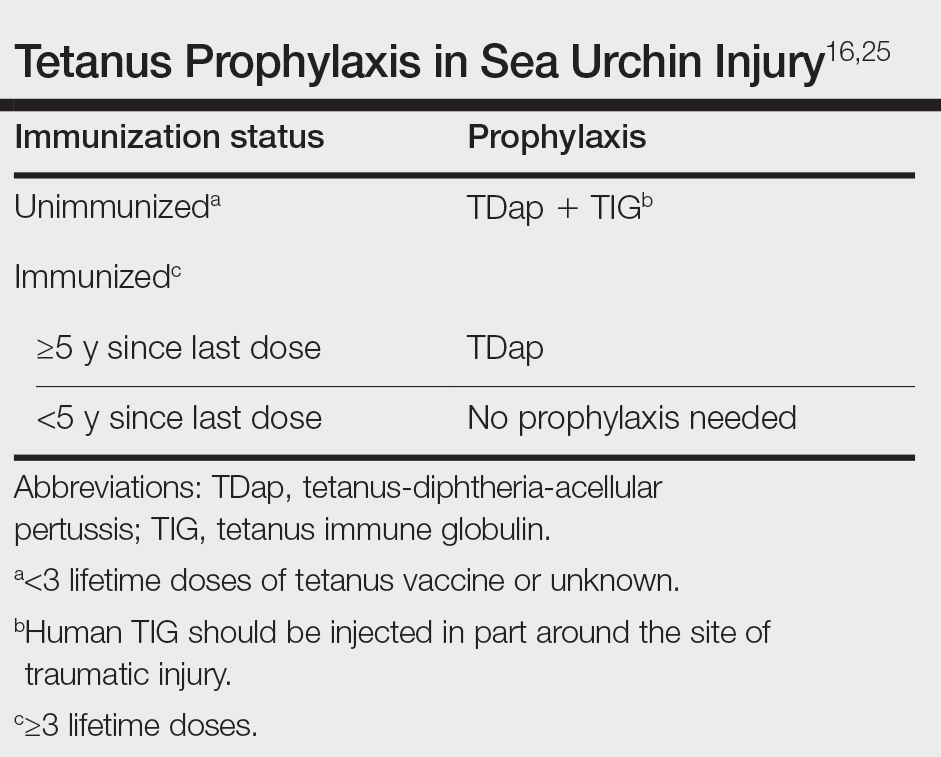
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.
- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.

Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.

- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
Practice Points
- Sea urchin spines easily become embedded in human skin upon contact and cause localized pain, edema, and black or purple pinpoint markings.
- Immediate treatment includes soaking in hot water (113 12°F [45 12°C]) for 30 to 90 minutes to inactivate proinflammatory compounds, followed by extraction of the spines.
- Successful methods of spine removal include the use of forceps and a hypodermic needle, as well as excision, liquid nitrogen, and punch biopsy.
- Prompt removal of the spines can reduce the incidence of delayed granulomatous reactions, synovitis, and sea urchin arthritis.
Central Centrifugal Cicatricial Alopecia in Males: Analysis of Time to Diagnosis and Disease Severity
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
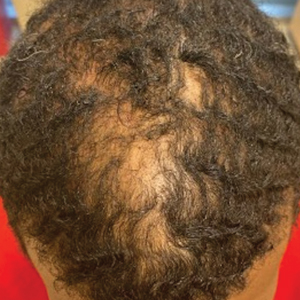
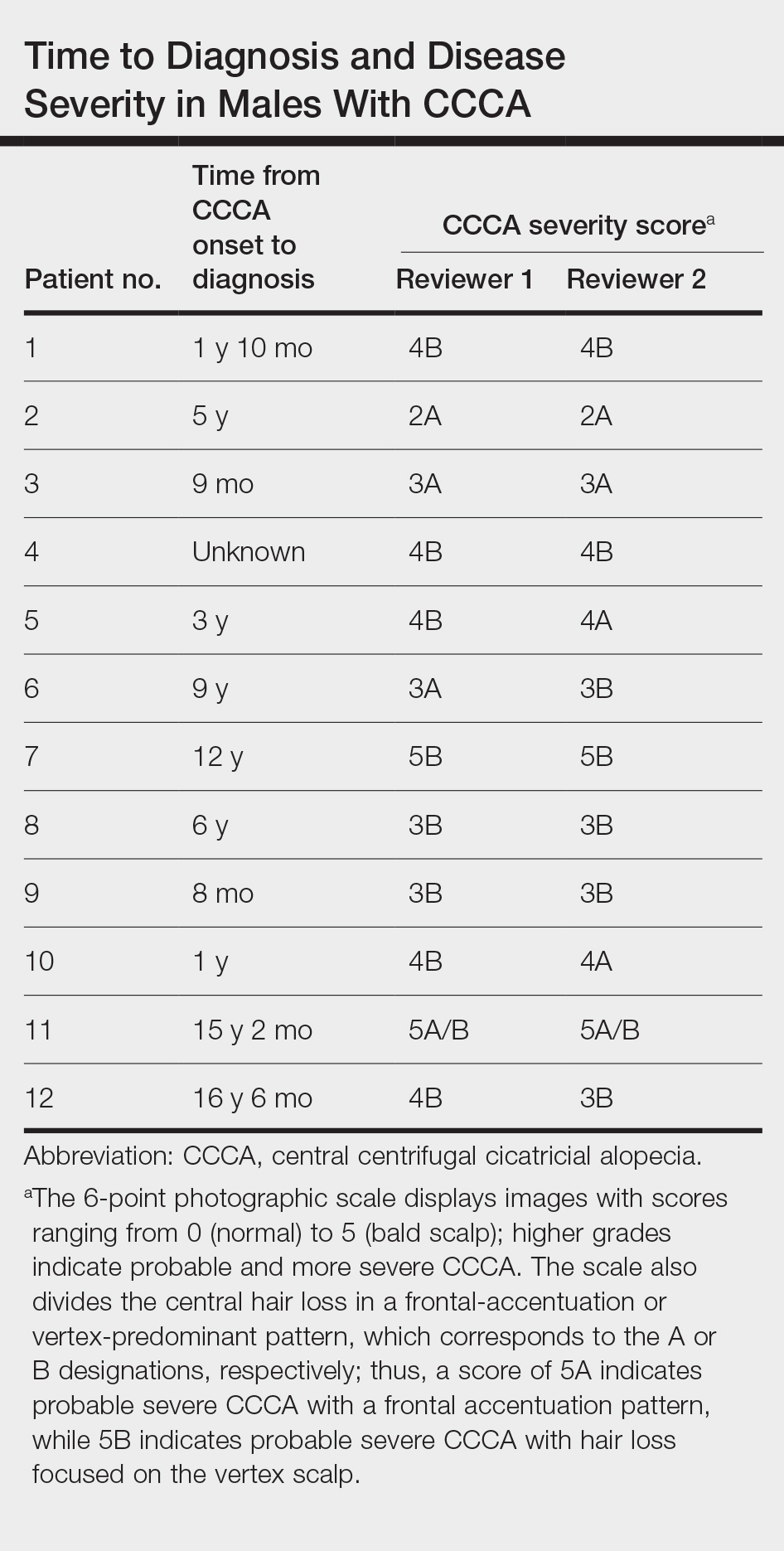
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.
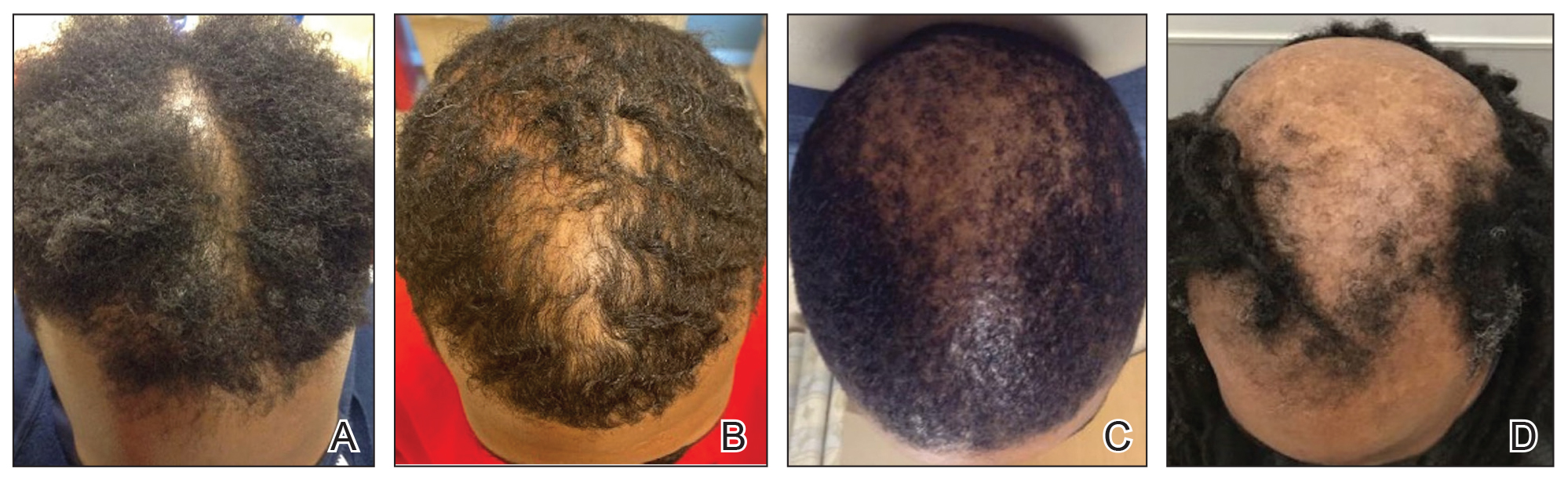
Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
Practice Points
- Most males with central centrifugal cicatricial alopecia (CCCA) experience considerable diagnostic delays and typically present to dermatology with late-stage disease.
- Dermatologists should consider CCCA in the differential diagnosis for adult Black males with alopecia.
- More research is needed to explore advanced CCCA in males, including factors limiting timely diagnosis and the impact on quality of life in this population.
The Impact of the Recent Supreme Court Ruling on the Dermatology Recruitment Pipeline
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
Practice Points
- The 2023 ruling by the Supreme Court of the United States on the use of race-based criteria in college admissions may have implications for the selection of individuals into the dermatology workforce.
- We highlight the impacts of these decisions at the college, medical school, and dermatology residency levels and provide context for future directions in the selection processes for practicing dermatologists.
Inflammatory Bowel Disease Highlights From Digestive Disease Week 2024
Highlights in ulcerative colitis (UC) and Crohn's disease (CD) from Digestive Disease Week® (DDW) 2024 are reported on by Dr. Andres Yarur from Cedars Sinai Medical Center in Los Angeles.
Dr. Yarur opens by discussing two phase 3 studies focused on risankizumab (RZB), which is currently approved for treatment of CD and has shown efficacy in UC. The first showed an induction period extended from 12 to 24 weeks resulted in clinical response in more than half of patients with UC.
The second study compared maintenance therapy with RZB to ustekinumab in patients with CD and found that RZB resulted in a higher rate of remission.
Dr. Yarur next looks at a study that explored use of darvadstrocel, an allogeneic stem cell therapy, in a subset of patients with CD and complex perianal fistulas. The disappointing results of the ADMIRE-CD II trial showed no benefit over placebo.
Patients hospitalized with UC, a population with few therapeutic options, were the focus of the next study. The TRIUMPH study explored use of the Janus kinase inhibitor tofacitinib for these patients and found that clinical response was achieved by 58.3% of them by day 7.
The final study addressed a clinical challenge: devising the optimal vaccination strategy for patients on immunosuppressive or anti–tumor necrosis factor therapies. Dr. Yarur reports that the study found an intensified pneumococcal vaccine regimen was more immunogenic and provided immunity for a longer duration than did the standard regimen.
--
Andres J. Yarur, MD, Associate Professor of Medicine, Cedars Sinai Medical Center, Los Angeles, California
Andres J. Yarur, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Takeda; Pfizer; Arena; AbbVie; Bristol Myers Squibb; Boehringer Ingelheim; Celltrion
Highlights in ulcerative colitis (UC) and Crohn's disease (CD) from Digestive Disease Week® (DDW) 2024 are reported on by Dr. Andres Yarur from Cedars Sinai Medical Center in Los Angeles.
Dr. Yarur opens by discussing two phase 3 studies focused on risankizumab (RZB), which is currently approved for treatment of CD and has shown efficacy in UC. The first showed an induction period extended from 12 to 24 weeks resulted in clinical response in more than half of patients with UC.
The second study compared maintenance therapy with RZB to ustekinumab in patients with CD and found that RZB resulted in a higher rate of remission.
Dr. Yarur next looks at a study that explored use of darvadstrocel, an allogeneic stem cell therapy, in a subset of patients with CD and complex perianal fistulas. The disappointing results of the ADMIRE-CD II trial showed no benefit over placebo.
Patients hospitalized with UC, a population with few therapeutic options, were the focus of the next study. The TRIUMPH study explored use of the Janus kinase inhibitor tofacitinib for these patients and found that clinical response was achieved by 58.3% of them by day 7.
The final study addressed a clinical challenge: devising the optimal vaccination strategy for patients on immunosuppressive or anti–tumor necrosis factor therapies. Dr. Yarur reports that the study found an intensified pneumococcal vaccine regimen was more immunogenic and provided immunity for a longer duration than did the standard regimen.
--
Andres J. Yarur, MD, Associate Professor of Medicine, Cedars Sinai Medical Center, Los Angeles, California
Andres J. Yarur, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Takeda; Pfizer; Arena; AbbVie; Bristol Myers Squibb; Boehringer Ingelheim; Celltrion
Highlights in ulcerative colitis (UC) and Crohn's disease (CD) from Digestive Disease Week® (DDW) 2024 are reported on by Dr. Andres Yarur from Cedars Sinai Medical Center in Los Angeles.
Dr. Yarur opens by discussing two phase 3 studies focused on risankizumab (RZB), which is currently approved for treatment of CD and has shown efficacy in UC. The first showed an induction period extended from 12 to 24 weeks resulted in clinical response in more than half of patients with UC.
The second study compared maintenance therapy with RZB to ustekinumab in patients with CD and found that RZB resulted in a higher rate of remission.
Dr. Yarur next looks at a study that explored use of darvadstrocel, an allogeneic stem cell therapy, in a subset of patients with CD and complex perianal fistulas. The disappointing results of the ADMIRE-CD II trial showed no benefit over placebo.
Patients hospitalized with UC, a population with few therapeutic options, were the focus of the next study. The TRIUMPH study explored use of the Janus kinase inhibitor tofacitinib for these patients and found that clinical response was achieved by 58.3% of them by day 7.
The final study addressed a clinical challenge: devising the optimal vaccination strategy for patients on immunosuppressive or anti–tumor necrosis factor therapies. Dr. Yarur reports that the study found an intensified pneumococcal vaccine regimen was more immunogenic and provided immunity for a longer duration than did the standard regimen.
--
Andres J. Yarur, MD, Associate Professor of Medicine, Cedars Sinai Medical Center, Los Angeles, California
Andres J. Yarur, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Takeda; Pfizer; Arena; AbbVie; Bristol Myers Squibb; Boehringer Ingelheim; Celltrion

COPD Highlights From ATS 2024
The latest research on treatment of patients with COPD presented at the American Thoracic Society (ATS) 2024 annual meeting is reported on by Diego J. Maselli, MD, FCCP, CHEST Physician Editorial Board Member, from UT Health San Antonio in Texas.
Dr. Maselli discusses the phase 2a COURSE study, which looked at patients with moderate to severe COPD to determine whether novel tezepelumab would help reduce exacerbations over 52 weeks. The study reached a nonsignificant numerical reduction in the annual rate vs placebo, but Dr. Maselli suggests that outcomes in patients. with high blood eosinophil counts merit further study.
Next, Dr. Maselli discusses the phase 3 NOTUS trial, looking at the efficacy and safety of the monoclonal antibody dupilumab in patients with moderate to severe COPD. The researchers found a 34% reduction in exacerbations in the dupilumab group vs placebo after 52 weeks.
He then details a 272-patient study looking at nebulized ensifentrine, a dual inhibitor of PDE3 and PDE4. The study demonstrated improved lung function as well as a reduction in exacerbation rate to patients with moderate to severe COPD treated with ensifentrined added to long-acting beta agonists-inhaled corticosteroid maintenance therapy.
Finally, Dr. Maselli highlights the MAZI study, a large retrospective analysis comparing the mortality rate in patients with COPD taking single-inhaler triple therapy (SITT) vs multiple-inhaler triple therapy (MITT). The researchers found that SITT was superior to MITT.
--
Diego J. Maselli, MD, FCCP, Professor, Chief, Division of Pulmonary Diseases & Critical Care, UT Health San Antonio, Texas
Diego J. Maselli, MD, FCCP has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: GSK; AstraZeneca; Sanofi/Regeneron; Amgen
Received research grant from: Gates Foundation; COPD Foundation; NIH
The latest research on treatment of patients with COPD presented at the American Thoracic Society (ATS) 2024 annual meeting is reported on by Diego J. Maselli, MD, FCCP, CHEST Physician Editorial Board Member, from UT Health San Antonio in Texas.
Dr. Maselli discusses the phase 2a COURSE study, which looked at patients with moderate to severe COPD to determine whether novel tezepelumab would help reduce exacerbations over 52 weeks. The study reached a nonsignificant numerical reduction in the annual rate vs placebo, but Dr. Maselli suggests that outcomes in patients. with high blood eosinophil counts merit further study.
Next, Dr. Maselli discusses the phase 3 NOTUS trial, looking at the efficacy and safety of the monoclonal antibody dupilumab in patients with moderate to severe COPD. The researchers found a 34% reduction in exacerbations in the dupilumab group vs placebo after 52 weeks.
He then details a 272-patient study looking at nebulized ensifentrine, a dual inhibitor of PDE3 and PDE4. The study demonstrated improved lung function as well as a reduction in exacerbation rate to patients with moderate to severe COPD treated with ensifentrined added to long-acting beta agonists-inhaled corticosteroid maintenance therapy.
Finally, Dr. Maselli highlights the MAZI study, a large retrospective analysis comparing the mortality rate in patients with COPD taking single-inhaler triple therapy (SITT) vs multiple-inhaler triple therapy (MITT). The researchers found that SITT was superior to MITT.
--
Diego J. Maselli, MD, FCCP, Professor, Chief, Division of Pulmonary Diseases & Critical Care, UT Health San Antonio, Texas
Diego J. Maselli, MD, FCCP has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: GSK; AstraZeneca; Sanofi/Regeneron; Amgen
Received research grant from: Gates Foundation; COPD Foundation; NIH
The latest research on treatment of patients with COPD presented at the American Thoracic Society (ATS) 2024 annual meeting is reported on by Diego J. Maselli, MD, FCCP, CHEST Physician Editorial Board Member, from UT Health San Antonio in Texas.
Dr. Maselli discusses the phase 2a COURSE study, which looked at patients with moderate to severe COPD to determine whether novel tezepelumab would help reduce exacerbations over 52 weeks. The study reached a nonsignificant numerical reduction in the annual rate vs placebo, but Dr. Maselli suggests that outcomes in patients. with high blood eosinophil counts merit further study.
Next, Dr. Maselli discusses the phase 3 NOTUS trial, looking at the efficacy and safety of the monoclonal antibody dupilumab in patients with moderate to severe COPD. The researchers found a 34% reduction in exacerbations in the dupilumab group vs placebo after 52 weeks.
He then details a 272-patient study looking at nebulized ensifentrine, a dual inhibitor of PDE3 and PDE4. The study demonstrated improved lung function as well as a reduction in exacerbation rate to patients with moderate to severe COPD treated with ensifentrined added to long-acting beta agonists-inhaled corticosteroid maintenance therapy.
Finally, Dr. Maselli highlights the MAZI study, a large retrospective analysis comparing the mortality rate in patients with COPD taking single-inhaler triple therapy (SITT) vs multiple-inhaler triple therapy (MITT). The researchers found that SITT was superior to MITT.
--
Diego J. Maselli, MD, FCCP, Professor, Chief, Division of Pulmonary Diseases & Critical Care, UT Health San Antonio, Texas
Diego J. Maselli, MD, FCCP has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: GSK; AstraZeneca; Sanofi/Regeneron; Amgen
Received research grant from: Gates Foundation; COPD Foundation; NIH

Erythematous Flaky Rash on the Toe
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13
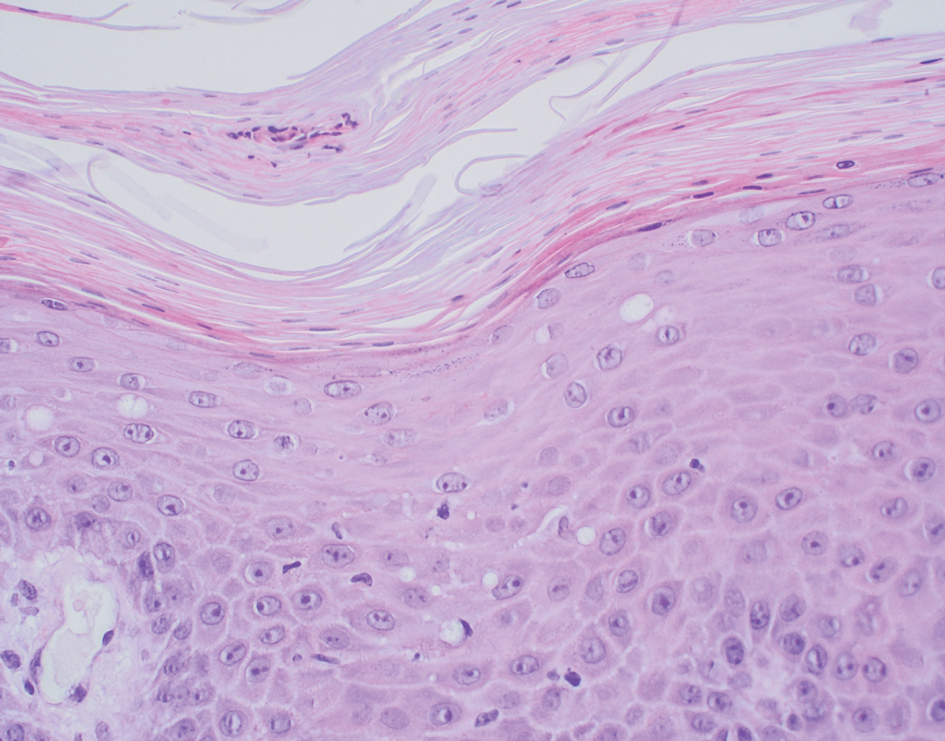
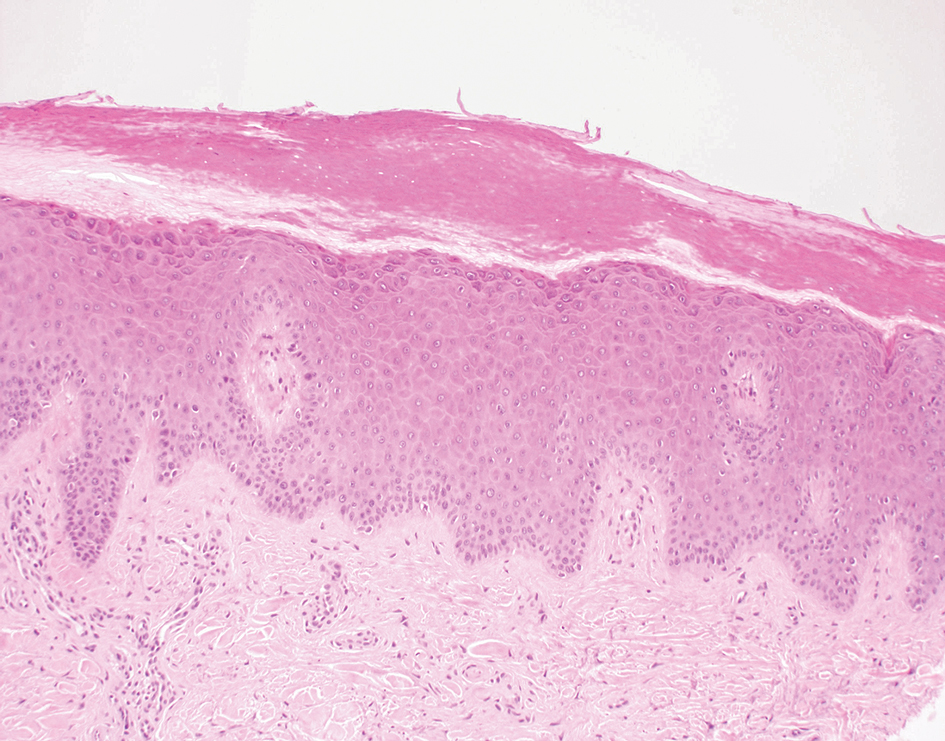
The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13
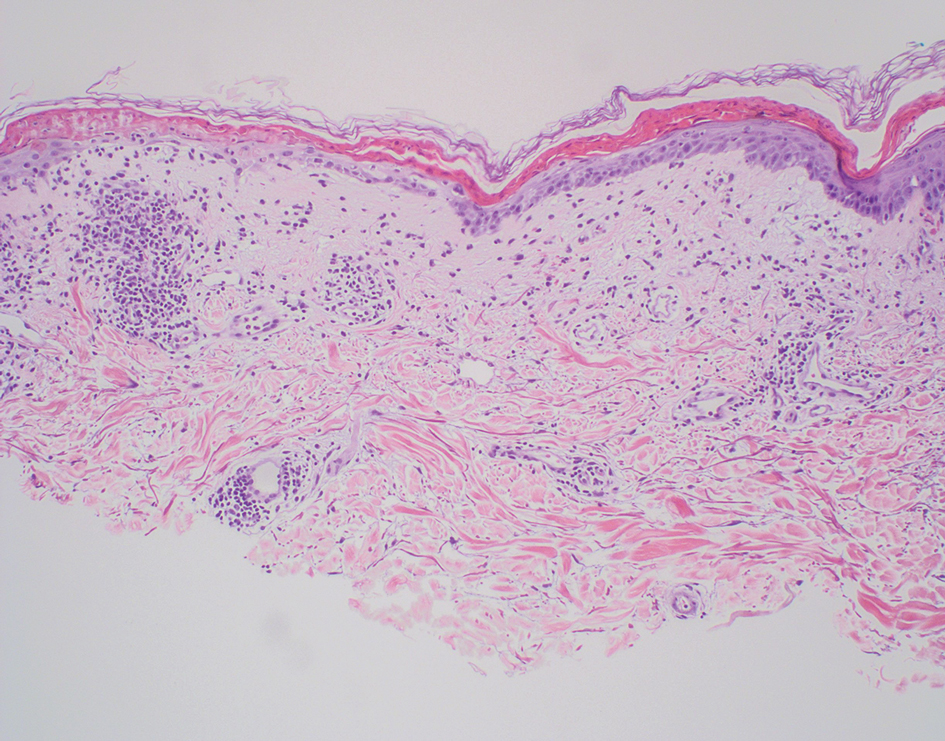
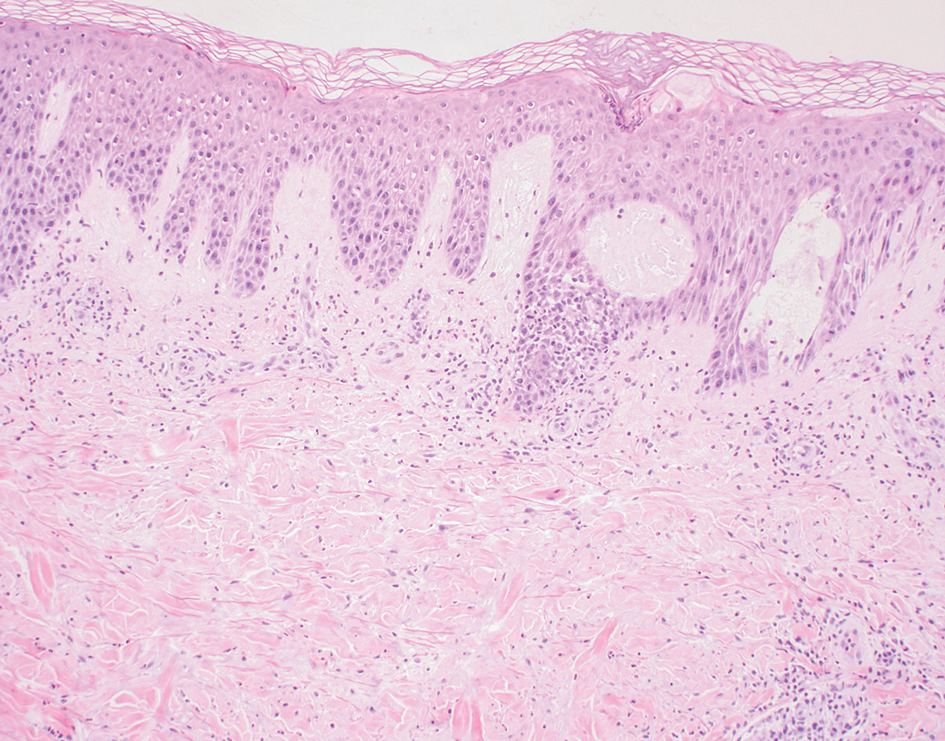
The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13


The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13


The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13


The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13


The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
A 62-year-old man presented with an erythematous flaky rash associated with burning pain on the right medial second toe that persisted for several months. Prior treatment with econazole, ciclopirox, and oral amoxicillin had failed. A shave biopsy was performed.
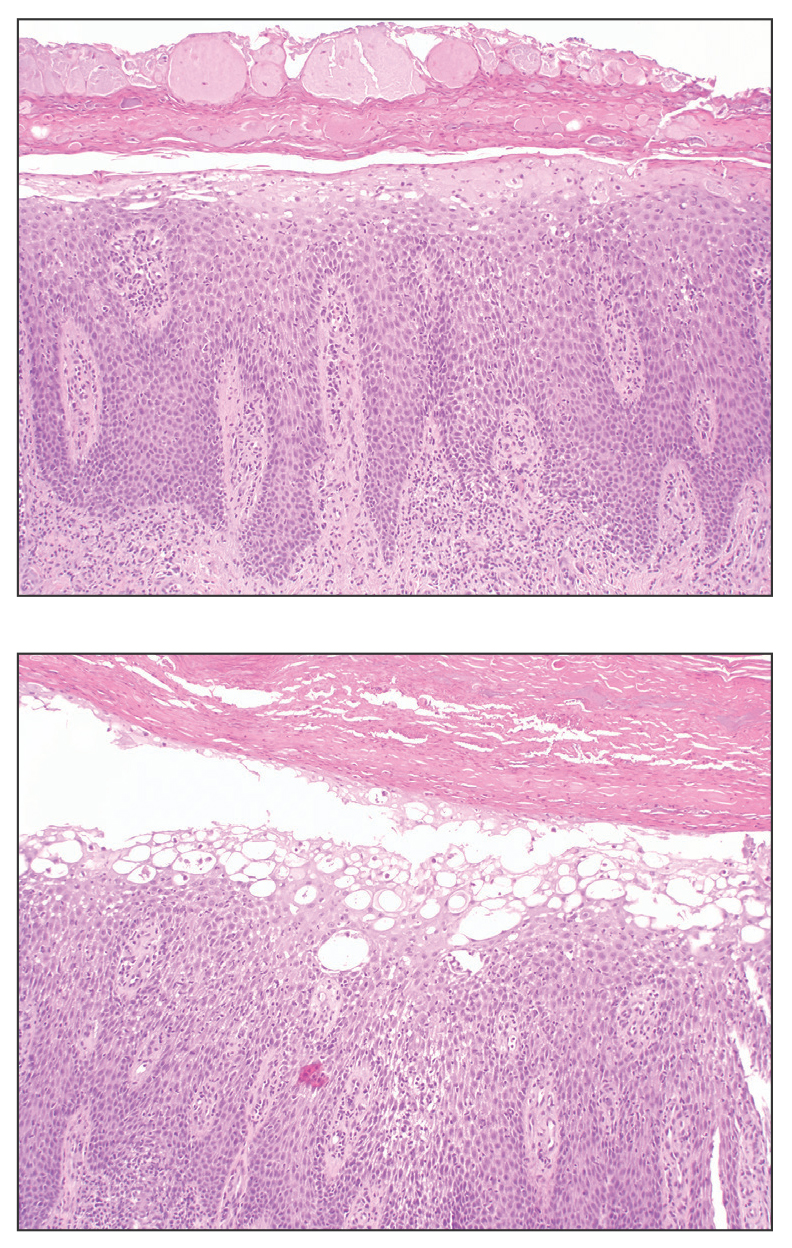
Latest Breakthroughs in Molluscum Contagiosum Therapy
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Overuse of Hematocrit Testing After Elective General Surgery at a Veterans Affairs Medical Center
It is common practice to routinely measure postoperative hematocrit levels at US Department of Veterans Affairs (VA) hospitals for a wide range of elective general surgeries. While hematocrit measurement is a low-cost test, the high frequency with which these tests are performed may drastically increase overall costs.
Numerous studies have suggested that physicians overuse laboratory testing.1-10 Kohli and colleagues recommended that the routine practice of obtaining postoperative hematocrit tests following elective gynecologic surgery be abandoned.1 A similar recommendation was made by Olus and colleagues after studying uneventful, unplanned cesarean sections and by Wu and colleagues after investigating routine laboratory tests post total hip arthroplasty.2,3
To our knowledge, a study assessing routine postoperative hematocrit testing in elective general surgery has not yet been conducted. Many laboratory tests ordered in the perioperative period are not indicated, including complete blood count (CBC), electrolytes, and coagulation studies.4 Based on the results of these studies, we expected that the routine measurement of postoperative hematocrit levels after elective general surgeries at VA medical centers would not be cost effective. A PubMed search for articles published from 1990 to 2023 using the search terms “hematocrit,” “hemoglobin,” “general,” “surgery,” “routine,” and “cost” or “cost-effectiveness,” suggests that the clinical usefulness of postoperative hematocrit testing has not been well studied in the general surgery setting. The purpose of this study was to determine the clinical utility and associated cost of measuring routine postoperative hematocrit levels in order to generate a guide as to when the practice is warranted following common elective general surgery.
Although gynecologic textbooks may describe recommendations of routine hematocrit checking after elective gynecologic operations, one has difficulty finding the same recommendations in general surgery textbooks.1 However, it is common practice for surgical residents and attending surgeons to routinely order hematocrit on postoperative day-1 to ensure that the operation did not result in unsuspected anemia that then would need treatment (either with fluids or a blood transfusion). Many other surgeons rely on clinical factors such as tachycardia, oliguria, or hypotension to trigger a hematocrit (and other laboratory) tests. Our hypothesis is that the latter group has chosen the most cost-effective and prudent practice. One problem with checking the hematocrit routinely, as with any other screening test, is what to do with an abnormal result, assuming an asymptomatic patient? If the postoperative hematocrit is lower than expected given the estimated blood loss (EBL), what is one to do?
Methods
This retrospective case-control study conducted at the New Mexico VA Health Care System (NMVAHCS) in Albuquerque compared data for patients who received transfusion within 72 hours of elective surgeries vs patients who did not. Patients who underwent elective general surgery from January 2011 through December 2014 were included. An elective general surgery was defined as surgery performed following an outpatient preoperative anesthesia evaluation ≥ 30 days prior to operation. Patients who underwent emergency operations, and those with baseline anemia (preoperative hematocrit < 30%), and those transfused > 72 hours after their operation were excluded. The NMVAHCSInstitutional Review Board approved this study (No. 15-H184).
A detailed record review was conducted to collect data on demographics and other preoperative risk factors, including age, sex, body mass index (BMI), race and ethnicity, cardiac and pulmonary comorbidities, tobacco use, alcohol intake, diabetes, American Society of Anesthesiologists Physical Status Classification, metabolic equivalent of task, hematologic conditions, and renal disease.
For each procedure, we recorded the type of elective general surgery performed, the diagnosis/indication, pre- and postoperative hemoglobin/hematocrit, intraoperative EBL, length of operation, surgical wound class, length of hospital stay (LOS), intensive care unit (ICU) status, number of hematocrit tests, cardiovascular risk of operation (defined by anesthesia assessment), presence or absence of malignancy, preoperative platelet count, albumin level, preoperative prothrombin time/activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin A1c, and incidence of transfusion. Signs and symptoms of anemia were recorded as present if the postoperative vital signs suggested low intravascular volume (pulse > 120 beats/minute, systolic blood pressure < 90 mm Hg, or vasoactive medication requirement [per anesthesia postoperative note]) or if the patient reported or exhibited symptoms of dizziness or fatigue or evidence of clinically apparent bleeding (ie, hematoma formation). Laboratory charges for hematocrit tests and CBC at the NMAVAHCS were used to assess cost.11
To stratify the transfusion risk, patients were distributed among 3 groups based on the following criteria: discharged home the same day as surgery; admitted but did not have postoperative hematocrit testing; and admitted and had postoperative hematocrit testing. We also stratified operations into low or high risk based on the risk for postoperative transfusion (Figure). Recognizing that the American College of Chest Physicians guidelines for perioperative management of antithrombotic therapy places bowel resection in a high-risk category, we designated a surgery as high risk when ≥ 2 patients in the transfusion group had that type of surgery over the 4 years of the study.12 Otherwise, the operations were deemed low risk.
Statistical Analysis
Numeric analysis used t tests and Binary and categorical variables used Fisher exact tests. P value ≤ .05 was considered statistically significant. SAS software was used for all statistical analyses.
Results
From 2011 through 2014, 1531 patients had elective general surgery at NMVAHCS. Twenty-two patients with preoperative anemia (hematocrit < 30%) and 1 patient who received a transfusion > 72 hours after the operation were excluded. Most elective operations (70%, n = 1075) were performed on an outpatient basis; none involved transfusion. Inguinal hernia repair was most common with 479 operations; 17 patients were treated inpatient of which 2 patients had routine postoperative hematocrit checks; (neither received transfusion). One patient with inguinal hernia surgery received transfusion without routine postoperative hematocrit monitoring.
Of 112 partial colon resections, 1 patient had a postoperative transfusion; and all but 3 received postoperative hematocrit monitoring. Nineteen patients undergoing partial colon resection had a clinical indication for postoperative hematocrit monitoring. None of the 5 patients with partial gastrectomy received a postoperative transfusion. Of 121 elective cholecystectomies, no patients had postoperative transfusion, whereas 34 had postoperative hematocrit monitoring; only 2 patients had a clinical reason for the hematocrit monitoring.
Of 430 elective inpatient operations, 12 received transfusions and 288 patients had ≥ 1 postoperative hematocrit test (67%). All hematocrit tests were requested by the attending surgeon, resident surgeon, or the surgical ICU team. Of the group that had postoperative hematocrit monitoring, there was an average of 4.4 postoperative hematocrit tests per patient (range, 1-44).
There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 Five of the 12 patients received intraoperative transfusions while 7 were transfused within 72 hours postoperation. All but 1 patient receiving transfusion had EBL > 199 mL (range, 5-3000; mean, 950 mL; median, 500 mL) and/or signs or symptoms of anemia or other indications for measurement of the postoperative hematocrit. There were no statistically significant differences in patients’ age, sex, BMI, or race and ethnicity between groups receiving and not receiving transfusion (Table 1).
When comparing the transfusion vs the nontransfusion groups (after excluding those with clinical preoperative anemia) the risk factors for transfusion included: relatively low mean preoperative hematocrit (mean, 36.9% vs 42.7%, respectively; P = .003), low postoperative hematocrit (mean, 30.2% vs 37.1%, respectively; P < .001), high EBL (mean, 844 mL vs 109 mL, respectively; P = .005), large infusion of intraoperative fluids (mean, 4625 mL vs 2505 mL, respectively; P = .005), longer duration of operation (mean, 397 min vs 183 min, respectively; P < .001), and longer LOS (mean, 14.5 d vs 4.9 d, respectively; P < .001) (Table 2). Similarly, we found an increased risk for transfusion with high/intermediate cardiovascular risk (vs low), any wound not classified as clean, ICU stay, and postoperative symptoms of anemia.
We found no increased risk for transfusion with ethanol, tobacco, warfarin, or clopidogrel use; polycythemia; thrombocytopenia; preoperative INR; preoperative aPTT; preoperative albumin; Hemoglobin A1c; or diabetes mellitus; or for operations performed for malignancy. Ten patients in the ICU received transfusion (5.8%) compared with 2 patients (0.8%) not admitted to the ICU.
Operations were deemed high risk when ≥ 2 of patients having that operation received transfusions within 72 hours of their operation. There were 15 abdominoperineal resections; 3 of these received transfusions (20%). There were 7 total abdominal colectomies; 3 of these received transfusions (43%). We therefore had 22 high-risk operations, 6 of which were transfused (27%).
Discussion
Routine measurement of postoperative hematocrit levels after elective general surgery at NMVAHCS was not necessary. There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 We found that routine postoperative hematocrit measurements to assess anemia had little or no effect on clinical decision-making or clinical outcomes.
According to our results, 88% of initial hematocrit tests after elective partial colectomies could have been eliminated; only 32 of 146 patients demonstrated a clinical reason for postoperative hematocrit testing. Similarly, 36 of 40 postcholecystectomy hematocrit tests (90%) could have been eliminated had the surgeons relied on clinical signs indicating possible postoperative anemia (none were transfused). Excluding patients with major intraoperative blood loss (> 300 mL), only 29 of 288 (10%) patients who had postoperative hematocrit tests had a clinical indication for a postoperative hematocrit test (ie, symptoms of anemia and/or active bleeding). One patient with inguinal hernia surgery who received transfusion was taking an anticoagulant and had a clinically indicated hematocrit test for a large hematoma that eventually required reoperation.
Our study found that routine hematocrit checks may actually increase the risk that a patient would receive an unnecessary transfusion. For instance, one elderly patient, after a right colectomy, had 6 hematocrit levels while on a heparin drip and received transfusion despite being asymptomatic. His lowest hematocrit level prior to transfusion was 23.7%. This patient had a total of 18 hematocrit tests. His EBL was 350 mL and his first postoperative HCT level was 33.1%. In another instance, a patient undergoing abdominoperineal resection had a transfusion on postoperative day 1, despite being hypertensive, with a hematocrit that ranged from 26% before transfusion to 31% after the transfusion. These 2 cases illustrate what has been shown in a recent study: A substantial number of patients with colorectal cancer receive unnecessary transfusions.14 On the other hand, one ileostomy closure patient had 33 hematocrit tests, yet his initial postoperative hematocrit was 37%, and he never received a transfusion. With low-risk surgeries, clinical judgment should dictate when a postoperative hematocrit level is needed. This strategy would have eliminated 206 unnecessary initial postoperative hematocrit tests (72%), could have decreased the number of unnecessary transfusions, and would have saved NMVAHCS about $1600 annually.
Abdominoperineal resections and total abdominal colectomies accounted for a high proportion of transfusions in our study. Inpatient elective operations can be risk stratified and have routine hematocrit tests ordered for patients at high risk. The probability of transfusion was greater in high-risk vs low-risk surgeries; 27% (6 of 22 patients) vs 2% (6 of 408 patients), respectively (P < .001). Since 14 of the 22 patients undergoing high-risk operation already had clinical reasons for a postoperative hematocrit test, we only need to add the remaining 8 patients with high-risk operations to the 74 who had a clinical reason for a hematocrit test and conclude that 82 of 430 patients (19%) had a clinical reason for a hematocrit test, either from signs or symptoms of blood loss or because they were in a high-risk group.
While our elective general surgery cases may not represent many general surgery programs in the US and VA health care systems, we can extrapolate cost savings using the same cost analyses outlined by Kohli and colleagues.1 Assuming 1.9 million elective inpatient general surgeries per year in the United States with an average cost of $21 per CBC, the annual cost of universal postoperative hematocrit testing would be $40 million.11,15 If postoperative hematocrit testing were 70% consistent with our findings, the annual cost for hematocrit tests on 51% of the inpatient general surgeries would be approximately $20.4 million. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our finding that 19% were deemed necessary) results in an annual savings of $30 million. This conservative estimate could be even higher since there were 4.4 hematocrit tests per patient; therefore, we have about $132 million in savings.
Assuming 181,384 elective VA inpatient general surgeries each year, costing $7.14 per CBC (the NMVAHCS cost), the VA could save $1.3 million annually. If postoperative HCT testing were 70% consistent with our findings, the annual cost for hematocrit tests on 50.4% of inpatient general surgery operations would be about $653,000. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our 19%) results in annual VA savings of $330,000. This conservative estimate could be even higher since there were on average 4.4 hematocrit levels per patient; therefore, we estimate that annual savings for the VA of about $1.45 million.
Limitations
The retrospective chart review nature of this study may have led to selection bias. Only a small number of patients received a transfusion, which may have skewed the data. This study population comes from a single VA medical center; this patient population may not be reflective of other VA medical centers or the US population as a whole. Given that NMVAHCS does not perform hepatic, esophageal, pancreas, or transplant operations, the potential savings to both the US and the VA may be overestimated, but this could be studied in the future by VA medical centers that perform more complex operations.
Conclusions
This study found that over a 4-year period routine postoperative hematocrit tests for patients undergoing elective general surgery at a VA medical center were not necessary. General surgeons routinely order various pre- and postoperative laboratory tests despite their limited utility. Reduction in unneeded routine tests could result in notable savings to the VA without compromising quality of care.
Only general surgery patients undergoing operations that carry a high risk for needing a blood transfusion should have a routine postoperative hematocrit testing. In our study population, the chance of an elective colectomy, cholecystectomy, or hernia patient needing a transfusion was rare. This strategy could eliminate a considerable number of unnecessary blood tests and would potentially yield significant savings.
1. Kohli N, Mallipeddi PK, Neff JM, Sze EH, Roat TW. Routine hematocrit after elective gynecologic surgery. Obstet Gynecol. 2000;95(6 Pt 1):847-850. doi:10.1016/s0029-7844(00)00796-1
2. Olus A, Orhan, U, Murat A, et al. Do asymptomatic patients require routine hemoglobin testing following uneventful, unplanned cesarean sections? Arch Gynecol Obstet. 2010;281(2):195-199. doi:10.1007/s00404-009-1093-1
3. Wu XD, Zhu ZL, Xiao P, Liu JC, Wang JW, Huang W. Are routine postoperative laboratory tests necessary after primary total hip arthroplasty? J Arthroplasty. 2020;35(10):2892-2898. doi:10.1016/j.arth.2020.04.097
4. Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anesthesiol Clin Pharmacol. 2011;27(2):174-179. doi:10.4103/0970-9185.81824
5. Aghajanian A, Grimes DA. Routine prothrombin time determination before elective gynecologic operations. Obstet Gynecol. 1991;78(5 Pt 1):837-839.
6. Ransom SB, McNeeley SG, Malone JM Jr. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol. 1996;175(5):1201-1203. doi:10.1016/s0002-9378(96)70028-5
7. Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol. 1995;86(3):346-348. doi:10.1016/0029-7844(95)00187-V
8. Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. doi:10.1097/ALN.0b013e31823c1067
9. Weil IA, Seicean S, Neuhauser D, Schiltz NK, Seicean A. Use and utility of hemostatic screening in adults undergoing elective, non-cardiac surgery. PLoS One. 2015;10(12):e0139139. doi:10.1371/journal.pone.0139139
10. Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing non-cardiac surgery. JAMA. 2007;297(22):2481-2488. doi:10.1001/jama.297.22.2481
11. Healthcare Bluebook. Complete blood count (CBC) with differential. Accessed March 28, 2024. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?id=214&dataset=lab
12. Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243. doi:10.1016/j.chest.2022.07.025
13. Randall JA, Wagner KT, Brody F. Perioperative transfusions in veterans following noncardiac procedures. J Laparoendosc Adv Surg Tech A. 2023;33(10):923-931. doi:10.1089/lap. 2023.0307
14. Tartter PI, Barron DM. Unnecessary blood transfusions in elective colorectal cancer surgery. Transfusion. 1985;25(2):113-115. doi:10.1046/j.1537-2995.1985.25285169199.x
15. Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Healthcare Cost and Utilization Project statistical brief #223. May 2017. Revised July 2020. Agency for Healthcare Research and Quality. Accessed February 26, 2024. https://hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
16. US Department of Veterans Affairs, National Surgery Office. Quarterly report: Q3 of fiscal year 2017. VISN operative complexity summary [Source not verified].
It is common practice to routinely measure postoperative hematocrit levels at US Department of Veterans Affairs (VA) hospitals for a wide range of elective general surgeries. While hematocrit measurement is a low-cost test, the high frequency with which these tests are performed may drastically increase overall costs.
Numerous studies have suggested that physicians overuse laboratory testing.1-10 Kohli and colleagues recommended that the routine practice of obtaining postoperative hematocrit tests following elective gynecologic surgery be abandoned.1 A similar recommendation was made by Olus and colleagues after studying uneventful, unplanned cesarean sections and by Wu and colleagues after investigating routine laboratory tests post total hip arthroplasty.2,3
To our knowledge, a study assessing routine postoperative hematocrit testing in elective general surgery has not yet been conducted. Many laboratory tests ordered in the perioperative period are not indicated, including complete blood count (CBC), electrolytes, and coagulation studies.4 Based on the results of these studies, we expected that the routine measurement of postoperative hematocrit levels after elective general surgeries at VA medical centers would not be cost effective. A PubMed search for articles published from 1990 to 2023 using the search terms “hematocrit,” “hemoglobin,” “general,” “surgery,” “routine,” and “cost” or “cost-effectiveness,” suggests that the clinical usefulness of postoperative hematocrit testing has not been well studied in the general surgery setting. The purpose of this study was to determine the clinical utility and associated cost of measuring routine postoperative hematocrit levels in order to generate a guide as to when the practice is warranted following common elective general surgery.
Although gynecologic textbooks may describe recommendations of routine hematocrit checking after elective gynecologic operations, one has difficulty finding the same recommendations in general surgery textbooks.1 However, it is common practice for surgical residents and attending surgeons to routinely order hematocrit on postoperative day-1 to ensure that the operation did not result in unsuspected anemia that then would need treatment (either with fluids or a blood transfusion). Many other surgeons rely on clinical factors such as tachycardia, oliguria, or hypotension to trigger a hematocrit (and other laboratory) tests. Our hypothesis is that the latter group has chosen the most cost-effective and prudent practice. One problem with checking the hematocrit routinely, as with any other screening test, is what to do with an abnormal result, assuming an asymptomatic patient? If the postoperative hematocrit is lower than expected given the estimated blood loss (EBL), what is one to do?
Methods
This retrospective case-control study conducted at the New Mexico VA Health Care System (NMVAHCS) in Albuquerque compared data for patients who received transfusion within 72 hours of elective surgeries vs patients who did not. Patients who underwent elective general surgery from January 2011 through December 2014 were included. An elective general surgery was defined as surgery performed following an outpatient preoperative anesthesia evaluation ≥ 30 days prior to operation. Patients who underwent emergency operations, and those with baseline anemia (preoperative hematocrit < 30%), and those transfused > 72 hours after their operation were excluded. The NMVAHCSInstitutional Review Board approved this study (No. 15-H184).
A detailed record review was conducted to collect data on demographics and other preoperative risk factors, including age, sex, body mass index (BMI), race and ethnicity, cardiac and pulmonary comorbidities, tobacco use, alcohol intake, diabetes, American Society of Anesthesiologists Physical Status Classification, metabolic equivalent of task, hematologic conditions, and renal disease.
For each procedure, we recorded the type of elective general surgery performed, the diagnosis/indication, pre- and postoperative hemoglobin/hematocrit, intraoperative EBL, length of operation, surgical wound class, length of hospital stay (LOS), intensive care unit (ICU) status, number of hematocrit tests, cardiovascular risk of operation (defined by anesthesia assessment), presence or absence of malignancy, preoperative platelet count, albumin level, preoperative prothrombin time/activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin A1c, and incidence of transfusion. Signs and symptoms of anemia were recorded as present if the postoperative vital signs suggested low intravascular volume (pulse > 120 beats/minute, systolic blood pressure < 90 mm Hg, or vasoactive medication requirement [per anesthesia postoperative note]) or if the patient reported or exhibited symptoms of dizziness or fatigue or evidence of clinically apparent bleeding (ie, hematoma formation). Laboratory charges for hematocrit tests and CBC at the NMAVAHCS were used to assess cost.11
To stratify the transfusion risk, patients were distributed among 3 groups based on the following criteria: discharged home the same day as surgery; admitted but did not have postoperative hematocrit testing; and admitted and had postoperative hematocrit testing. We also stratified operations into low or high risk based on the risk for postoperative transfusion (Figure). Recognizing that the American College of Chest Physicians guidelines for perioperative management of antithrombotic therapy places bowel resection in a high-risk category, we designated a surgery as high risk when ≥ 2 patients in the transfusion group had that type of surgery over the 4 years of the study.12 Otherwise, the operations were deemed low risk.
Statistical Analysis
Numeric analysis used t tests and Binary and categorical variables used Fisher exact tests. P value ≤ .05 was considered statistically significant. SAS software was used for all statistical analyses.
Results
From 2011 through 2014, 1531 patients had elective general surgery at NMVAHCS. Twenty-two patients with preoperative anemia (hematocrit < 30%) and 1 patient who received a transfusion > 72 hours after the operation were excluded. Most elective operations (70%, n = 1075) were performed on an outpatient basis; none involved transfusion. Inguinal hernia repair was most common with 479 operations; 17 patients were treated inpatient of which 2 patients had routine postoperative hematocrit checks; (neither received transfusion). One patient with inguinal hernia surgery received transfusion without routine postoperative hematocrit monitoring.
Of 112 partial colon resections, 1 patient had a postoperative transfusion; and all but 3 received postoperative hematocrit monitoring. Nineteen patients undergoing partial colon resection had a clinical indication for postoperative hematocrit monitoring. None of the 5 patients with partial gastrectomy received a postoperative transfusion. Of 121 elective cholecystectomies, no patients had postoperative transfusion, whereas 34 had postoperative hematocrit monitoring; only 2 patients had a clinical reason for the hematocrit monitoring.
Of 430 elective inpatient operations, 12 received transfusions and 288 patients had ≥ 1 postoperative hematocrit test (67%). All hematocrit tests were requested by the attending surgeon, resident surgeon, or the surgical ICU team. Of the group that had postoperative hematocrit monitoring, there was an average of 4.4 postoperative hematocrit tests per patient (range, 1-44).
There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 Five of the 12 patients received intraoperative transfusions while 7 were transfused within 72 hours postoperation. All but 1 patient receiving transfusion had EBL > 199 mL (range, 5-3000; mean, 950 mL; median, 500 mL) and/or signs or symptoms of anemia or other indications for measurement of the postoperative hematocrit. There were no statistically significant differences in patients’ age, sex, BMI, or race and ethnicity between groups receiving and not receiving transfusion (Table 1).
When comparing the transfusion vs the nontransfusion groups (after excluding those with clinical preoperative anemia) the risk factors for transfusion included: relatively low mean preoperative hematocrit (mean, 36.9% vs 42.7%, respectively; P = .003), low postoperative hematocrit (mean, 30.2% vs 37.1%, respectively; P < .001), high EBL (mean, 844 mL vs 109 mL, respectively; P = .005), large infusion of intraoperative fluids (mean, 4625 mL vs 2505 mL, respectively; P = .005), longer duration of operation (mean, 397 min vs 183 min, respectively; P < .001), and longer LOS (mean, 14.5 d vs 4.9 d, respectively; P < .001) (Table 2). Similarly, we found an increased risk for transfusion with high/intermediate cardiovascular risk (vs low), any wound not classified as clean, ICU stay, and postoperative symptoms of anemia.
We found no increased risk for transfusion with ethanol, tobacco, warfarin, or clopidogrel use; polycythemia; thrombocytopenia; preoperative INR; preoperative aPTT; preoperative albumin; Hemoglobin A1c; or diabetes mellitus; or for operations performed for malignancy. Ten patients in the ICU received transfusion (5.8%) compared with 2 patients (0.8%) not admitted to the ICU.
Operations were deemed high risk when ≥ 2 of patients having that operation received transfusions within 72 hours of their operation. There were 15 abdominoperineal resections; 3 of these received transfusions (20%). There were 7 total abdominal colectomies; 3 of these received transfusions (43%). We therefore had 22 high-risk operations, 6 of which were transfused (27%).
Discussion
Routine measurement of postoperative hematocrit levels after elective general surgery at NMVAHCS was not necessary. There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 We found that routine postoperative hematocrit measurements to assess anemia had little or no effect on clinical decision-making or clinical outcomes.
According to our results, 88% of initial hematocrit tests after elective partial colectomies could have been eliminated; only 32 of 146 patients demonstrated a clinical reason for postoperative hematocrit testing. Similarly, 36 of 40 postcholecystectomy hematocrit tests (90%) could have been eliminated had the surgeons relied on clinical signs indicating possible postoperative anemia (none were transfused). Excluding patients with major intraoperative blood loss (> 300 mL), only 29 of 288 (10%) patients who had postoperative hematocrit tests had a clinical indication for a postoperative hematocrit test (ie, symptoms of anemia and/or active bleeding). One patient with inguinal hernia surgery who received transfusion was taking an anticoagulant and had a clinically indicated hematocrit test for a large hematoma that eventually required reoperation.
Our study found that routine hematocrit checks may actually increase the risk that a patient would receive an unnecessary transfusion. For instance, one elderly patient, after a right colectomy, had 6 hematocrit levels while on a heparin drip and received transfusion despite being asymptomatic. His lowest hematocrit level prior to transfusion was 23.7%. This patient had a total of 18 hematocrit tests. His EBL was 350 mL and his first postoperative HCT level was 33.1%. In another instance, a patient undergoing abdominoperineal resection had a transfusion on postoperative day 1, despite being hypertensive, with a hematocrit that ranged from 26% before transfusion to 31% after the transfusion. These 2 cases illustrate what has been shown in a recent study: A substantial number of patients with colorectal cancer receive unnecessary transfusions.14 On the other hand, one ileostomy closure patient had 33 hematocrit tests, yet his initial postoperative hematocrit was 37%, and he never received a transfusion. With low-risk surgeries, clinical judgment should dictate when a postoperative hematocrit level is needed. This strategy would have eliminated 206 unnecessary initial postoperative hematocrit tests (72%), could have decreased the number of unnecessary transfusions, and would have saved NMVAHCS about $1600 annually.
Abdominoperineal resections and total abdominal colectomies accounted for a high proportion of transfusions in our study. Inpatient elective operations can be risk stratified and have routine hematocrit tests ordered for patients at high risk. The probability of transfusion was greater in high-risk vs low-risk surgeries; 27% (6 of 22 patients) vs 2% (6 of 408 patients), respectively (P < .001). Since 14 of the 22 patients undergoing high-risk operation already had clinical reasons for a postoperative hematocrit test, we only need to add the remaining 8 patients with high-risk operations to the 74 who had a clinical reason for a hematocrit test and conclude that 82 of 430 patients (19%) had a clinical reason for a hematocrit test, either from signs or symptoms of blood loss or because they were in a high-risk group.
While our elective general surgery cases may not represent many general surgery programs in the US and VA health care systems, we can extrapolate cost savings using the same cost analyses outlined by Kohli and colleagues.1 Assuming 1.9 million elective inpatient general surgeries per year in the United States with an average cost of $21 per CBC, the annual cost of universal postoperative hematocrit testing would be $40 million.11,15 If postoperative hematocrit testing were 70% consistent with our findings, the annual cost for hematocrit tests on 51% of the inpatient general surgeries would be approximately $20.4 million. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our finding that 19% were deemed necessary) results in an annual savings of $30 million. This conservative estimate could be even higher since there were 4.4 hematocrit tests per patient; therefore, we have about $132 million in savings.
Assuming 181,384 elective VA inpatient general surgeries each year, costing $7.14 per CBC (the NMVAHCS cost), the VA could save $1.3 million annually. If postoperative HCT testing were 70% consistent with our findings, the annual cost for hematocrit tests on 50.4% of inpatient general surgery operations would be about $653,000. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our 19%) results in annual VA savings of $330,000. This conservative estimate could be even higher since there were on average 4.4 hematocrit levels per patient; therefore, we estimate that annual savings for the VA of about $1.45 million.
Limitations
The retrospective chart review nature of this study may have led to selection bias. Only a small number of patients received a transfusion, which may have skewed the data. This study population comes from a single VA medical center; this patient population may not be reflective of other VA medical centers or the US population as a whole. Given that NMVAHCS does not perform hepatic, esophageal, pancreas, or transplant operations, the potential savings to both the US and the VA may be overestimated, but this could be studied in the future by VA medical centers that perform more complex operations.
Conclusions
This study found that over a 4-year period routine postoperative hematocrit tests for patients undergoing elective general surgery at a VA medical center were not necessary. General surgeons routinely order various pre- and postoperative laboratory tests despite their limited utility. Reduction in unneeded routine tests could result in notable savings to the VA without compromising quality of care.
Only general surgery patients undergoing operations that carry a high risk for needing a blood transfusion should have a routine postoperative hematocrit testing. In our study population, the chance of an elective colectomy, cholecystectomy, or hernia patient needing a transfusion was rare. This strategy could eliminate a considerable number of unnecessary blood tests and would potentially yield significant savings.
It is common practice to routinely measure postoperative hematocrit levels at US Department of Veterans Affairs (VA) hospitals for a wide range of elective general surgeries. While hematocrit measurement is a low-cost test, the high frequency with which these tests are performed may drastically increase overall costs.
Numerous studies have suggested that physicians overuse laboratory testing.1-10 Kohli and colleagues recommended that the routine practice of obtaining postoperative hematocrit tests following elective gynecologic surgery be abandoned.1 A similar recommendation was made by Olus and colleagues after studying uneventful, unplanned cesarean sections and by Wu and colleagues after investigating routine laboratory tests post total hip arthroplasty.2,3
To our knowledge, a study assessing routine postoperative hematocrit testing in elective general surgery has not yet been conducted. Many laboratory tests ordered in the perioperative period are not indicated, including complete blood count (CBC), electrolytes, and coagulation studies.4 Based on the results of these studies, we expected that the routine measurement of postoperative hematocrit levels after elective general surgeries at VA medical centers would not be cost effective. A PubMed search for articles published from 1990 to 2023 using the search terms “hematocrit,” “hemoglobin,” “general,” “surgery,” “routine,” and “cost” or “cost-effectiveness,” suggests that the clinical usefulness of postoperative hematocrit testing has not been well studied in the general surgery setting. The purpose of this study was to determine the clinical utility and associated cost of measuring routine postoperative hematocrit levels in order to generate a guide as to when the practice is warranted following common elective general surgery.
Although gynecologic textbooks may describe recommendations of routine hematocrit checking after elective gynecologic operations, one has difficulty finding the same recommendations in general surgery textbooks.1 However, it is common practice for surgical residents and attending surgeons to routinely order hematocrit on postoperative day-1 to ensure that the operation did not result in unsuspected anemia that then would need treatment (either with fluids or a blood transfusion). Many other surgeons rely on clinical factors such as tachycardia, oliguria, or hypotension to trigger a hematocrit (and other laboratory) tests. Our hypothesis is that the latter group has chosen the most cost-effective and prudent practice. One problem with checking the hematocrit routinely, as with any other screening test, is what to do with an abnormal result, assuming an asymptomatic patient? If the postoperative hematocrit is lower than expected given the estimated blood loss (EBL), what is one to do?
Methods
This retrospective case-control study conducted at the New Mexico VA Health Care System (NMVAHCS) in Albuquerque compared data for patients who received transfusion within 72 hours of elective surgeries vs patients who did not. Patients who underwent elective general surgery from January 2011 through December 2014 were included. An elective general surgery was defined as surgery performed following an outpatient preoperative anesthesia evaluation ≥ 30 days prior to operation. Patients who underwent emergency operations, and those with baseline anemia (preoperative hematocrit < 30%), and those transfused > 72 hours after their operation were excluded. The NMVAHCSInstitutional Review Board approved this study (No. 15-H184).
A detailed record review was conducted to collect data on demographics and other preoperative risk factors, including age, sex, body mass index (BMI), race and ethnicity, cardiac and pulmonary comorbidities, tobacco use, alcohol intake, diabetes, American Society of Anesthesiologists Physical Status Classification, metabolic equivalent of task, hematologic conditions, and renal disease.
For each procedure, we recorded the type of elective general surgery performed, the diagnosis/indication, pre- and postoperative hemoglobin/hematocrit, intraoperative EBL, length of operation, surgical wound class, length of hospital stay (LOS), intensive care unit (ICU) status, number of hematocrit tests, cardiovascular risk of operation (defined by anesthesia assessment), presence or absence of malignancy, preoperative platelet count, albumin level, preoperative prothrombin time/activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin A1c, and incidence of transfusion. Signs and symptoms of anemia were recorded as present if the postoperative vital signs suggested low intravascular volume (pulse > 120 beats/minute, systolic blood pressure < 90 mm Hg, or vasoactive medication requirement [per anesthesia postoperative note]) or if the patient reported or exhibited symptoms of dizziness or fatigue or evidence of clinically apparent bleeding (ie, hematoma formation). Laboratory charges for hematocrit tests and CBC at the NMAVAHCS were used to assess cost.11
To stratify the transfusion risk, patients were distributed among 3 groups based on the following criteria: discharged home the same day as surgery; admitted but did not have postoperative hematocrit testing; and admitted and had postoperative hematocrit testing. We also stratified operations into low or high risk based on the risk for postoperative transfusion (Figure). Recognizing that the American College of Chest Physicians guidelines for perioperative management of antithrombotic therapy places bowel resection in a high-risk category, we designated a surgery as high risk when ≥ 2 patients in the transfusion group had that type of surgery over the 4 years of the study.12 Otherwise, the operations were deemed low risk.
Statistical Analysis
Numeric analysis used t tests and Binary and categorical variables used Fisher exact tests. P value ≤ .05 was considered statistically significant. SAS software was used for all statistical analyses.
Results
From 2011 through 2014, 1531 patients had elective general surgery at NMVAHCS. Twenty-two patients with preoperative anemia (hematocrit < 30%) and 1 patient who received a transfusion > 72 hours after the operation were excluded. Most elective operations (70%, n = 1075) were performed on an outpatient basis; none involved transfusion. Inguinal hernia repair was most common with 479 operations; 17 patients were treated inpatient of which 2 patients had routine postoperative hematocrit checks; (neither received transfusion). One patient with inguinal hernia surgery received transfusion without routine postoperative hematocrit monitoring.
Of 112 partial colon resections, 1 patient had a postoperative transfusion; and all but 3 received postoperative hematocrit monitoring. Nineteen patients undergoing partial colon resection had a clinical indication for postoperative hematocrit monitoring. None of the 5 patients with partial gastrectomy received a postoperative transfusion. Of 121 elective cholecystectomies, no patients had postoperative transfusion, whereas 34 had postoperative hematocrit monitoring; only 2 patients had a clinical reason for the hematocrit monitoring.
Of 430 elective inpatient operations, 12 received transfusions and 288 patients had ≥ 1 postoperative hematocrit test (67%). All hematocrit tests were requested by the attending surgeon, resident surgeon, or the surgical ICU team. Of the group that had postoperative hematocrit monitoring, there was an average of 4.4 postoperative hematocrit tests per patient (range, 1-44).
There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 Five of the 12 patients received intraoperative transfusions while 7 were transfused within 72 hours postoperation. All but 1 patient receiving transfusion had EBL > 199 mL (range, 5-3000; mean, 950 mL; median, 500 mL) and/or signs or symptoms of anemia or other indications for measurement of the postoperative hematocrit. There were no statistically significant differences in patients’ age, sex, BMI, or race and ethnicity between groups receiving and not receiving transfusion (Table 1).
When comparing the transfusion vs the nontransfusion groups (after excluding those with clinical preoperative anemia) the risk factors for transfusion included: relatively low mean preoperative hematocrit (mean, 36.9% vs 42.7%, respectively; P = .003), low postoperative hematocrit (mean, 30.2% vs 37.1%, respectively; P < .001), high EBL (mean, 844 mL vs 109 mL, respectively; P = .005), large infusion of intraoperative fluids (mean, 4625 mL vs 2505 mL, respectively; P = .005), longer duration of operation (mean, 397 min vs 183 min, respectively; P < .001), and longer LOS (mean, 14.5 d vs 4.9 d, respectively; P < .001) (Table 2). Similarly, we found an increased risk for transfusion with high/intermediate cardiovascular risk (vs low), any wound not classified as clean, ICU stay, and postoperative symptoms of anemia.
We found no increased risk for transfusion with ethanol, tobacco, warfarin, or clopidogrel use; polycythemia; thrombocytopenia; preoperative INR; preoperative aPTT; preoperative albumin; Hemoglobin A1c; or diabetes mellitus; or for operations performed for malignancy. Ten patients in the ICU received transfusion (5.8%) compared with 2 patients (0.8%) not admitted to the ICU.
Operations were deemed high risk when ≥ 2 of patients having that operation received transfusions within 72 hours of their operation. There were 15 abdominoperineal resections; 3 of these received transfusions (20%). There were 7 total abdominal colectomies; 3 of these received transfusions (43%). We therefore had 22 high-risk operations, 6 of which were transfused (27%).
Discussion
Routine measurement of postoperative hematocrit levels after elective general surgery at NMVAHCS was not necessary. There were 12 transfusions for inpatients (2.8%), which is similar to the findings of a recent study of VA general surgery (2.3%).13 We found that routine postoperative hematocrit measurements to assess anemia had little or no effect on clinical decision-making or clinical outcomes.
According to our results, 88% of initial hematocrit tests after elective partial colectomies could have been eliminated; only 32 of 146 patients demonstrated a clinical reason for postoperative hematocrit testing. Similarly, 36 of 40 postcholecystectomy hematocrit tests (90%) could have been eliminated had the surgeons relied on clinical signs indicating possible postoperative anemia (none were transfused). Excluding patients with major intraoperative blood loss (> 300 mL), only 29 of 288 (10%) patients who had postoperative hematocrit tests had a clinical indication for a postoperative hematocrit test (ie, symptoms of anemia and/or active bleeding). One patient with inguinal hernia surgery who received transfusion was taking an anticoagulant and had a clinically indicated hematocrit test for a large hematoma that eventually required reoperation.
Our study found that routine hematocrit checks may actually increase the risk that a patient would receive an unnecessary transfusion. For instance, one elderly patient, after a right colectomy, had 6 hematocrit levels while on a heparin drip and received transfusion despite being asymptomatic. His lowest hematocrit level prior to transfusion was 23.7%. This patient had a total of 18 hematocrit tests. His EBL was 350 mL and his first postoperative HCT level was 33.1%. In another instance, a patient undergoing abdominoperineal resection had a transfusion on postoperative day 1, despite being hypertensive, with a hematocrit that ranged from 26% before transfusion to 31% after the transfusion. These 2 cases illustrate what has been shown in a recent study: A substantial number of patients with colorectal cancer receive unnecessary transfusions.14 On the other hand, one ileostomy closure patient had 33 hematocrit tests, yet his initial postoperative hematocrit was 37%, and he never received a transfusion. With low-risk surgeries, clinical judgment should dictate when a postoperative hematocrit level is needed. This strategy would have eliminated 206 unnecessary initial postoperative hematocrit tests (72%), could have decreased the number of unnecessary transfusions, and would have saved NMVAHCS about $1600 annually.
Abdominoperineal resections and total abdominal colectomies accounted for a high proportion of transfusions in our study. Inpatient elective operations can be risk stratified and have routine hematocrit tests ordered for patients at high risk. The probability of transfusion was greater in high-risk vs low-risk surgeries; 27% (6 of 22 patients) vs 2% (6 of 408 patients), respectively (P < .001). Since 14 of the 22 patients undergoing high-risk operation already had clinical reasons for a postoperative hematocrit test, we only need to add the remaining 8 patients with high-risk operations to the 74 who had a clinical reason for a hematocrit test and conclude that 82 of 430 patients (19%) had a clinical reason for a hematocrit test, either from signs or symptoms of blood loss or because they were in a high-risk group.
While our elective general surgery cases may not represent many general surgery programs in the US and VA health care systems, we can extrapolate cost savings using the same cost analyses outlined by Kohli and colleagues.1 Assuming 1.9 million elective inpatient general surgeries per year in the United States with an average cost of $21 per CBC, the annual cost of universal postoperative hematocrit testing would be $40 million.11,15 If postoperative hematocrit testing were 70% consistent with our findings, the annual cost for hematocrit tests on 51% of the inpatient general surgeries would be approximately $20.4 million. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our finding that 19% were deemed necessary) results in an annual savings of $30 million. This conservative estimate could be even higher since there were 4.4 hematocrit tests per patient; therefore, we have about $132 million in savings.
Assuming 181,384 elective VA inpatient general surgeries each year, costing $7.14 per CBC (the NMVAHCS cost), the VA could save $1.3 million annually. If postoperative HCT testing were 70% consistent with our findings, the annual cost for hematocrit tests on 50.4% of inpatient general surgery operations would be about $653,000. A reduction in routine hematocrit testing to 25% of all inpatient general surgeries (vs our 19%) results in annual VA savings of $330,000. This conservative estimate could be even higher since there were on average 4.4 hematocrit levels per patient; therefore, we estimate that annual savings for the VA of about $1.45 million.
Limitations
The retrospective chart review nature of this study may have led to selection bias. Only a small number of patients received a transfusion, which may have skewed the data. This study population comes from a single VA medical center; this patient population may not be reflective of other VA medical centers or the US population as a whole. Given that NMVAHCS does not perform hepatic, esophageal, pancreas, or transplant operations, the potential savings to both the US and the VA may be overestimated, but this could be studied in the future by VA medical centers that perform more complex operations.
Conclusions
This study found that over a 4-year period routine postoperative hematocrit tests for patients undergoing elective general surgery at a VA medical center were not necessary. General surgeons routinely order various pre- and postoperative laboratory tests despite their limited utility. Reduction in unneeded routine tests could result in notable savings to the VA without compromising quality of care.
Only general surgery patients undergoing operations that carry a high risk for needing a blood transfusion should have a routine postoperative hematocrit testing. In our study population, the chance of an elective colectomy, cholecystectomy, or hernia patient needing a transfusion was rare. This strategy could eliminate a considerable number of unnecessary blood tests and would potentially yield significant savings.
1. Kohli N, Mallipeddi PK, Neff JM, Sze EH, Roat TW. Routine hematocrit after elective gynecologic surgery. Obstet Gynecol. 2000;95(6 Pt 1):847-850. doi:10.1016/s0029-7844(00)00796-1
2. Olus A, Orhan, U, Murat A, et al. Do asymptomatic patients require routine hemoglobin testing following uneventful, unplanned cesarean sections? Arch Gynecol Obstet. 2010;281(2):195-199. doi:10.1007/s00404-009-1093-1
3. Wu XD, Zhu ZL, Xiao P, Liu JC, Wang JW, Huang W. Are routine postoperative laboratory tests necessary after primary total hip arthroplasty? J Arthroplasty. 2020;35(10):2892-2898. doi:10.1016/j.arth.2020.04.097
4. Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anesthesiol Clin Pharmacol. 2011;27(2):174-179. doi:10.4103/0970-9185.81824
5. Aghajanian A, Grimes DA. Routine prothrombin time determination before elective gynecologic operations. Obstet Gynecol. 1991;78(5 Pt 1):837-839.
6. Ransom SB, McNeeley SG, Malone JM Jr. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol. 1996;175(5):1201-1203. doi:10.1016/s0002-9378(96)70028-5
7. Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol. 1995;86(3):346-348. doi:10.1016/0029-7844(95)00187-V
8. Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. doi:10.1097/ALN.0b013e31823c1067
9. Weil IA, Seicean S, Neuhauser D, Schiltz NK, Seicean A. Use and utility of hemostatic screening in adults undergoing elective, non-cardiac surgery. PLoS One. 2015;10(12):e0139139. doi:10.1371/journal.pone.0139139
10. Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing non-cardiac surgery. JAMA. 2007;297(22):2481-2488. doi:10.1001/jama.297.22.2481
11. Healthcare Bluebook. Complete blood count (CBC) with differential. Accessed March 28, 2024. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?id=214&dataset=lab
12. Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243. doi:10.1016/j.chest.2022.07.025
13. Randall JA, Wagner KT, Brody F. Perioperative transfusions in veterans following noncardiac procedures. J Laparoendosc Adv Surg Tech A. 2023;33(10):923-931. doi:10.1089/lap. 2023.0307
14. Tartter PI, Barron DM. Unnecessary blood transfusions in elective colorectal cancer surgery. Transfusion. 1985;25(2):113-115. doi:10.1046/j.1537-2995.1985.25285169199.x
15. Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Healthcare Cost and Utilization Project statistical brief #223. May 2017. Revised July 2020. Agency for Healthcare Research and Quality. Accessed February 26, 2024. https://hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
16. US Department of Veterans Affairs, National Surgery Office. Quarterly report: Q3 of fiscal year 2017. VISN operative complexity summary [Source not verified].
1. Kohli N, Mallipeddi PK, Neff JM, Sze EH, Roat TW. Routine hematocrit after elective gynecologic surgery. Obstet Gynecol. 2000;95(6 Pt 1):847-850. doi:10.1016/s0029-7844(00)00796-1
2. Olus A, Orhan, U, Murat A, et al. Do asymptomatic patients require routine hemoglobin testing following uneventful, unplanned cesarean sections? Arch Gynecol Obstet. 2010;281(2):195-199. doi:10.1007/s00404-009-1093-1
3. Wu XD, Zhu ZL, Xiao P, Liu JC, Wang JW, Huang W. Are routine postoperative laboratory tests necessary after primary total hip arthroplasty? J Arthroplasty. 2020;35(10):2892-2898. doi:10.1016/j.arth.2020.04.097
4. Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anesthesiol Clin Pharmacol. 2011;27(2):174-179. doi:10.4103/0970-9185.81824
5. Aghajanian A, Grimes DA. Routine prothrombin time determination before elective gynecologic operations. Obstet Gynecol. 1991;78(5 Pt 1):837-839.
6. Ransom SB, McNeeley SG, Malone JM Jr. A cost-effectiveness evaluation of preoperative type-and-screen testing for vaginal hysterectomy. Am J Obstet Gynecol. 1996;175(5):1201-1203. doi:10.1016/s0002-9378(96)70028-5
7. Ransom SB, McNeeley SG, Hosseini RB. Cost-effectiveness of routine blood type and screen testing before elective laparoscopy. Obstet Gynecol. 1995;86(3):346-348. doi:10.1016/0029-7844(95)00187-V
8. Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. doi:10.1097/ALN.0b013e31823c1067
9. Weil IA, Seicean S, Neuhauser D, Schiltz NK, Seicean A. Use and utility of hemostatic screening in adults undergoing elective, non-cardiac surgery. PLoS One. 2015;10(12):e0139139. doi:10.1371/journal.pone.0139139
10. Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing non-cardiac surgery. JAMA. 2007;297(22):2481-2488. doi:10.1001/jama.297.22.2481
11. Healthcare Bluebook. Complete blood count (CBC) with differential. Accessed March 28, 2024. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?id=214&dataset=lab
12. Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243. doi:10.1016/j.chest.2022.07.025
13. Randall JA, Wagner KT, Brody F. Perioperative transfusions in veterans following noncardiac procedures. J Laparoendosc Adv Surg Tech A. 2023;33(10):923-931. doi:10.1089/lap. 2023.0307
14. Tartter PI, Barron DM. Unnecessary blood transfusions in elective colorectal cancer surgery. Transfusion. 1985;25(2):113-115. doi:10.1046/j.1537-2995.1985.25285169199.x
15. Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Healthcare Cost and Utilization Project statistical brief #223. May 2017. Revised July 2020. Agency for Healthcare Research and Quality. Accessed February 26, 2024. https://hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
16. US Department of Veterans Affairs, National Surgery Office. Quarterly report: Q3 of fiscal year 2017. VISN operative complexity summary [Source not verified].
Oxidative Stress in Patients With Melasma: An Evaluation of the Correlation of the Thiol/Disulfide Homeostasis Parameters and Modified MASI Score
Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4
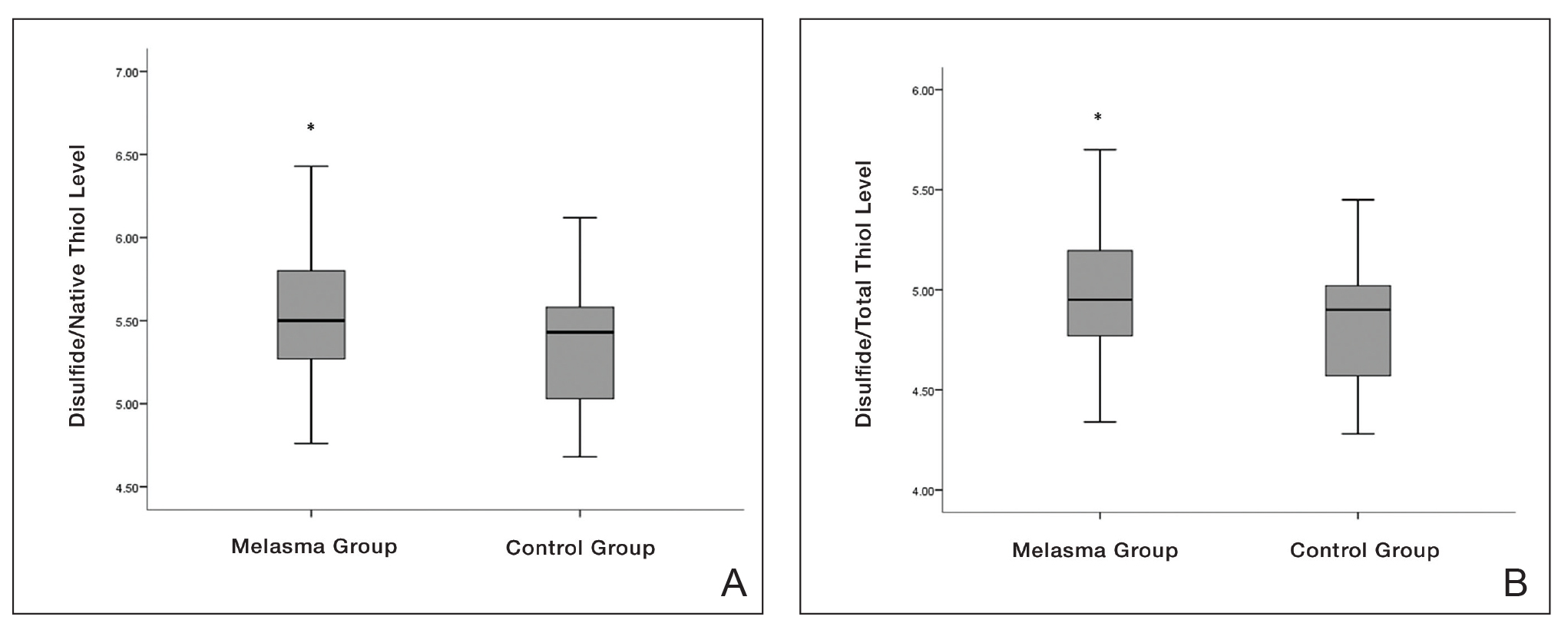

Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reportsin the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.

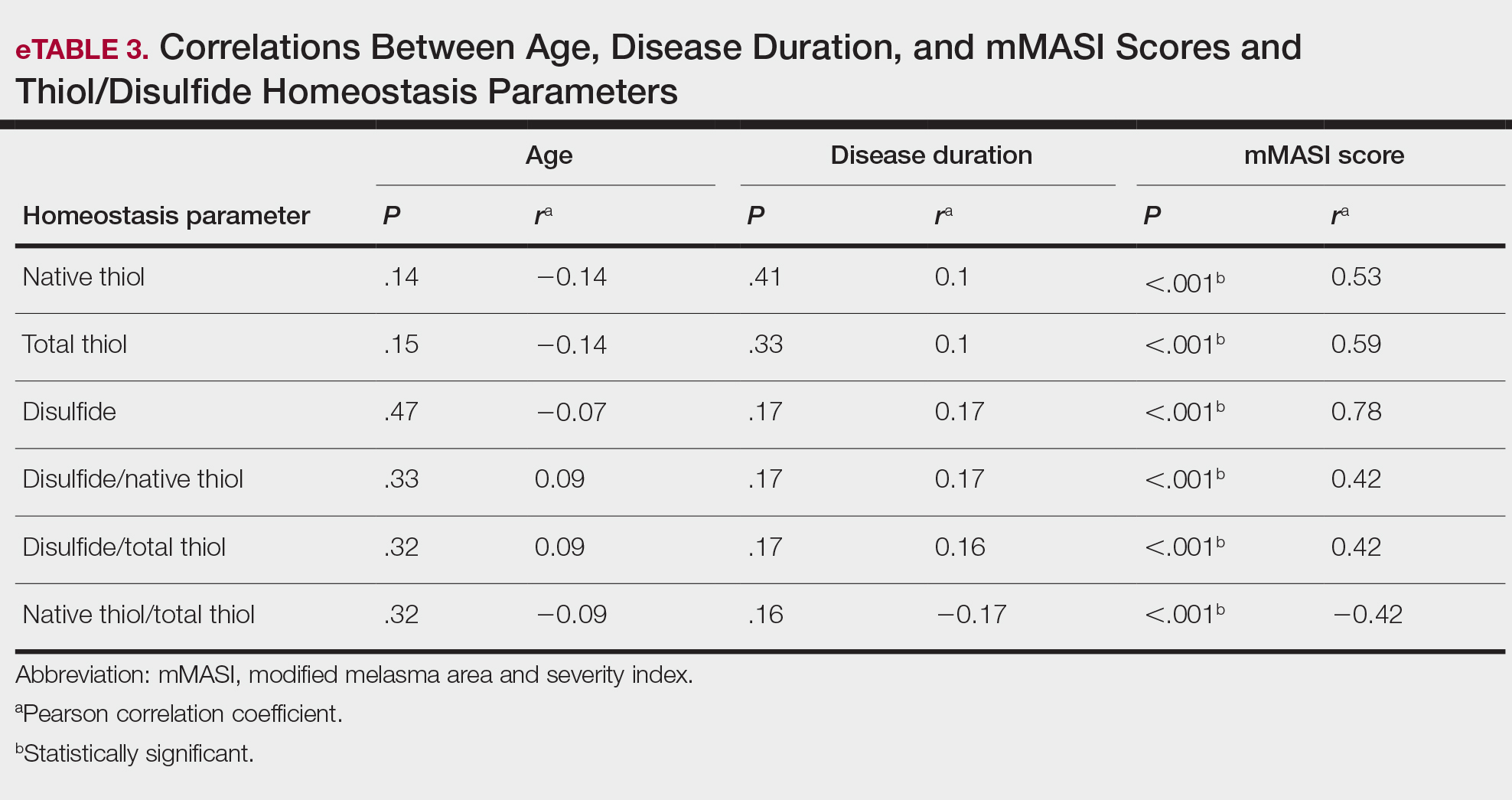
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4


Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reportsin the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.



Melasma is an acquired hyperpigmentation disorder characterized by irregular brown macules and patches that usually appear on sun-exposed areas of the skin. The term melasma originates from the Greek word melas meaning black.1 Facial melasma is divided into 2 groups according to its clinical distribution: centrofacial lesions are located in the center of the face (eg, the glabellar, frontal, nasal, zygomatic, upper lip, chin areas), and peripheral lesions manifest on the frontotemporal, preauricular, and mandibular regions.1,2 There is debate on the categorization of zygomatic (or malar) melasma; some researchers argue it should be categorized independent of other areas, while others include malar melasma in the centrofacial group because of its frequent association with the centrofacial type, especially with glabellar lesions.2 Mandibular melasma is rare and occurs mostly in postmenopausal women after intense sun exposure.1,2 Although the etiopathogenesis of the disease is not clearly known, increased melanogenesis, extracellular matrix alterations, inflammation, and angiogenesis are assumed to play a role.3 Various risk factors such as genetic predisposition, UV radiation (UVR) exposure, pregnancy, thyroid dysfunction, and exogenous hormones (eg, oral contraceptives, hormone replacement therapy) have been identified; phototoxic drugs, anticonvulsants, and some cosmetics also have been implicated.4,5 Exposure to UVR is thought to be the main triggering environmental factor by inducing both melanin production and oxidative stress.5 However, it also has been shown that visible light can induce hyperpigmentation in darker skin types.6
The presence of oxidative stress in melasma recently has become an intriguing topic of interest. First, the presence of oxidative stress in the etiopathogenesis of melasma was thought to be based on the effectiveness of antioxidants in treatment. A few studies also have confirmed the presence of oxidative stress in melasma.7-10 Classically, oxidative stress can be described as a disturbance in the balance between oxidants and antioxidants. Reactive oxygen species (ROS) are highly reactive molecules due to the unpaired electrons in their structure. Although ROS are present at low levels in physiologic conditions and are involved in critical physiologic events, they damage cellular components such as fat, protein, and nucleic acid at high concentrations.5
Dynamic thiol/disulfide homeostasis is one of the most important markers of oxidative stress in biological systems. Thiols are organic compounds containing a sulfhydryl (-SH) group. Thiols are considered highly potent antioxidants because they reduce unstable free radicals by donating electrons. They are the first antioxidants to be depleted in an oxidative environment.11,12 In case of oxidative stress, they transform into reversible forms called disulfide bridges between 2 thiol groups. Disulfide bridges can be reduced back to thiol groups, which is how dynamic thiol/disulfide homeostasis is maintained. Dynamic thiol/disulfide homeostasis is responsible for cellular events such as antioxidant defense, signal transduction, regulation of enzyme function, and apoptosis.11,12
The aim of this study was to evaluate the presence of oxidative stress in melasma by comparing dynamic thiol/disulfide homeostasis in patients with melasma compared with age- and sex-matched healthy controls.
Materials and Methods
Participants and Eligibility Criteria—We conducted a prospective study in a tertiary-care hospital (Ankara Bilkent City Hospital [Ankara, Turkey]) of patients with melasma who were followed from October 2021 to October 2022 compared with age- and sex-matched healthy volunteers. Ethics committee approval was obtained from Ankara Bilkent City Hospital before the study (E2-21-881)(13.10.2021). Written informed consent was obtained from all participants, and all were older than 18 years. Patients were excluded if there was the presence of any systemic disease or dermatologic disease other than melasma; smoking or alcohol use; any use of vitamins, food supplements, or any medication in the last 3 months; or pregnancy.
Melasma Severity—The modified melasma area and severity index (mMASI) score was used to determine the severity of melasma. The score is calculated from assessments of the darkness of the pigmentation and the percentage of affected area on the face. The mMASI score is the sum of the darkness score (D); area score (A); and separate fixed coefficients for the forehead, as well as the right malar, left malar, and chin regions.13 The mMASI score, with a range of 0 to 24, is a reliable and objective marker in the calculation of melasma severity.4
Biochemical Analysis of Samples—The 6-cc peripheral fasting venous blood samples obtained from the study participants were centrifuged at 1500 g for 10 minutes, and the separated sera were stored in a freezer at −80 °C until the time of analysis. When the study was completed, the disulfide and thiol values were analyzed. Serum native and total thiol concentrations indicating thiol/disulfide homeostasis were calculated by a new fully automatic colorimetric method developed by Erel and Neselioglu.14 Using this method, short disulfide bonds are first reduced with sodium borohydride solution to form free-functional thiol groups, and then the unused sodium borohydride is removed using formaldehyde. Finally, all thiol groups are reacted with 5,5’-dithiobis-(2-nitrobenzoic) acid (Ellman reagent), and all thiol groups are detected after reaction with 5,5’-dithiobis-(2-nitrobenzoic) acid. When a disulfide bond (−S−S−) is reduced, 2 thiol groups are formed. For this reason, half of the difference between total thiol (-SH + the amount of thiol formed by the reduction of disulfides) and native thiol (-SH) corresponds to the dynamic disulfide amount (total thiol − native thiol/2).14
Statistical Analysis—Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were presented as numbers and percentages for categorical variables, and numerical variables were presented as mean, SD, median, minimum, maximum, 25th quartile, and 75th quartile. The conformity of the variables to normal distribution was examined using visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). In pairwise group comparisons for numerical variables, a Mann-Whitney U test was used when normal distribution was not met, and a t test was used when normal distribution was met. The statistical significance level was accepted as P<.05.
Results
Our study included 67 patients with melasma and 41 healthy age- and sex-matched controls. Of the participants with melasma, 60 (89.5%) were female and 7 (10.5%) were male. The control group was similar to the melasma group in terms of sex (87.8% female vs 12.2% male [P=.59]). The mean age (SD) was 33.1 (6.7) years in the melasma group and 31.9 (6.7) years in the control group. Age was similar across both groups (P=.41). All participants were of Asian race, and Fitzpatrick skin types (types II–IV) were similar across both groups.
Fifty-four (80.6%) participants had centrofacial melasma and 13 (19.4%) had mixed-type melasma. The mMASI score ranged from 3 to 20; the mean (SD) mMASI score was 11.28 (3.2). Disease duration ranged from 2 to 72 months; the mean (SD) disease duration was 12.26 (6.3) months. The demographics and clinical characteristics of the study group are shown in eTable 1.
eTable 2 provides a summary of disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios in the study population. Disulfide/native thiol and disulfide/total thiol ratios were higher in melasma patients (Figure 1), whereas the native thiol/total thiol ratio was higher in the control group (P=.025, P=.025, and P=.026, respectively).
All correlations between age, disease duration, and mMASI scores and disulfide, native thiol, and total thiol levels, as well as disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios, are summarized in eTable 3. No significant correlation was observed between age and disease duration and disulfide, native thiol, and total thiol levels or disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratios.
We independently assessed whether Fitzpatrick skin types II, III, and IV exhibited distinct levels of oxidative stress in clinical melasma. There were no significant correlations with Fitzpatrick skin type (disulfide/native thiol, P=.25; disulfide/total thiol, P=.19). We further evaluated if the thiol/disulfide parameters were correlated with duration of melasma by dividing the melasma patients into 3 groups (<6 months [n=12], 6–18 months [n=32], >18 months [n=23]), but there was not any significant correlation (disulfide/native thiol, P=.15; disulfide/total thiol, P=.15). We also divided our patients into 3 groups according to age (<27 years [n=14], 27–36 years [n=33], >36 years [n=20]). There was no correlation of the parameters with age (disulfide/native thiol, P=.15; disulfide/total thiol, P=.14).
There was a positive correlation between mMASI score and disulfide, native thiol, and total thiol levels and disulfide/native thiol and disulfide/total thiol ratios, as well as a negative correlation between mMASI score and native thiol/total thiol ratio. The correlations between mMASI scores and disulfide/native thiol and disulfide/total thiol ratios are shown in Figure 2 and eTable 3.
Comment
Melasma is a common condition that may cause psychosocial problems in affected patients and negatively affect quality of life.1 It occurs in all races but is more common in individuals with darker skin types (eg, Fitzpatrick skin types III and IV). Although melasma is more common in women during reproductive years (50%–70%), it also has been observed in 10% to 30% of men.5
Treatment options include topical bleaching agents, chemical peels, and laser therapy, as well as discontinuation of medications that may potentially trigger melasma; use of broad-spectrum sunscreens also is recommended.4 Vitamins A, C, and E, as well as niacinamide, are used in the treatment of melasma, especially for their antioxidant properties. The key role of antioxidants in the treatment of melasma supports the importance of oxidative stress in the pathogenesis.7 Melasma often is challenging to treat, particularly the mixed or dermal types, due to their stubborn nature. This condition poses a considerable therapeutic challenge for dermatologists.4


Oxidative stress and oxidant-antioxidant imbalance previously have been studied in various diseases, but research investigating the presence of oxidative stress in melasma are limited.7-10 Exposure of the skin to polluted air and intense UVR, as well as some food by-products, cosmetics, and drugs (eg, oral contraceptives), can directly or indirectly cause ROS production in the skin. Reactive oxygen species are thought to be involved in the pathophysiology of melasma by affecting apoptotic pathways and causing cell proliferation. The intermediate heme pathway has pro-oxidant effects and produces ROS and metabolites such as redox-active quinines. Exposure to UVR leads to the generation of ROS, highlighting the role of oxidative stress in the onset of melasma. 5
In any cutaneous disease in which oxidative stress plays a role, oxidant and antioxidant levels may be expected to vary both locally and systemically; however, measurement of oxidative stress markers in serum instead of skin is technically and economically more advantageous.8 Firstly, serum collection is less invasive and technically simpler than skin biopsies. Drawing blood is a routine procedure that requires minimal specialized equipment and training compared to the extraction and processing of skin samples. Secondly, analyzing serum samples generally is less expensive than processing skin tissue.8
In our study, we evaluated dynamic thiol/disulfide homeostasis in serum to investigate the presence of oxidative stress in the setting of melasma. Functional sulfhydryl (-SH) groups in thiols act as substrates for antioxidant enzymes and as free-radical scavengers. They constitute one of the most powerful defense systems against the unwanted effects of ROS. Thiols, which become the main target of ROS under oxidative stress, oxidize with oxidant molecules and form disulfide bridges.15
Thiol/disulfide homeostasis has been studied many times in dermatologic diseases,16-19 and the results obtained from these studies are heterogenous depending on the extent of oxidative damage. It has been shown that thiol/disulfide homeostasis plays a role in oxidative stress in conditions such as psoriasis,17 seborrheic dermatitis,11 atopic dermatitits,18 and rosacea.19 In our study, disulfide/native thiol and disulfide/total thiol levels were significantly higher (both P=.025) in the melasma group compared with the control group, which indicates that the thiol/disulfide balance in patients with melasma is shifted to disulfide formation and thiols are oxidized to disulfide bonds in the presence of oxidative stress.
Seçkin et al7 evaluated the role of oxidative stress in the pathogenesis of melasma and found that the serum levels of the antioxidants superoxide dismutase and glutathione peroxidase were significantly higher in the patient group compared with the control group (both P<.001). They also found that the levels of nitric oxide (another antioxidant) were increased in the patient group and the levels of protein carbonyl (an oxidative metabolite) were significantly lower (both P<.001). These findings indicated that free-radical damage may be involved in the pathogenesis of melasma
In a study of 75 patients with melasma, serum levels of the antioxidants melatonin and catalase were significantly (P<.001 and P=.001, respectively) lower in the melasma group compared with the control group, while serum levels of the oxidants protein carbonyl and nitric oxide were significantly higher (P=.002 and P=.001, respectively). No significant correlation was found between oxidative stress parameters and melasma severity.8
Choubey et al9 found that serum malondialdehyde (an end product of lipid peroxidation), superoxide dismutase, and glutathione peroxidase levels were significantly higher in the melasma group (n=50) compared with the control group (n=50)(all P<.001). In addition, a significant positive correlation (correlation coefficient, +0.307; P<.05) was found between serum malondialdehyde levels and melasma severity. The mean age (SD) of the patients was 32.22 (6.377) years, and the female (n=41) to male (n=9) ratio was 4.55:1. The most common melasma pattern was centrofacial, followed by malar.9
In a study with 50 melasma patients and 50 controls, Rahimi et al10 examined bilirubin and uric acid levels, which are major extracellular antioxidants. The mean age (SD) at disease onset was 32.6 (6.7) years, and the mean MASI score (SD) was 18.1 (9). Serum bilirubin levels were found to be higher in the melasma group than in the control group and were correlated with disease severity. No significant difference in uric acid levels was found between the groups, and no correlation was found between MASI score and bilirubin and uric acid levels.10
In our study, the melasma group was similar to those in other reportsin the literature regarding gender distribution, mean age, and melasma pattern.7-10 Additionally, the correlation of mMASI score with disulfide/native thiol and disulfide/total thiol values in the melasma group suggested that oxidative stress also is correlated with melasma severity.
Thiol-based treatments such as n-acetyl cysteine, which contains a thiol compound, may be helpful in melasma.20 In a double-blind, placebo-controlled study, topical n-acetyl cysteine combined with hydroquinone 2% was used in 10 female patients with melasma. Mild to strong bleaching of the skin was observed in 90% (9/10) of the patients.21 Systemic use of n-acetyl cysteine in melasma also may be a potential research topic.
Major limitations of our study were the small sample size and lack of measurement of oxidative stress parameters in the skin concurrently with serum.
Conclusion
In our study, the presence of oxidative stress in melasma was demonstrated by evaluating thiol/disulfide homeostasis—one of the strongest markers of oxidative stress. Oxidative stress also correlated with melasma disease severity in our analysis. The data obtained in this study may contribute to understanding the etiopathogenesis of melasma and may open new horizons in treatment; however, more comprehensive studies should be conducted to support our findings.



- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Tamega Ade A, Miot LD, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151-156.
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Abou-Taleb DA, Ibrahim AK, Youssef EM, et al. Reliability, validity, and sensitivity to change overtime of the modified melasma area and severity index score. Dermatol Surg. 2017;43:210-217.
- Katiyar S, Yadav D. Correlation of oxidative stress with melasma: an overview. Curr Pharm Des. 2022;28:225-231.
- Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092-2097.
- Seçkin HY, Kalkan G, Bas¸ Y, et al. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33:212-217.
- Sarkar R, Devadasan S, Choubey V, et al. Melatonin and oxidative stress in melasma—an unexplored territory; a prospective study. Int J Dermatol. 2020;59:572-575.
- Choubey V, Sarkar R, Garg V, et al. Role of oxidative stress in melasma: a prospective study on serum and blood markers of oxidative stress in melasma patients. Int J Dermatol. 2017;56:939-943.
- Rahimi H, Mirnezami M, Yazdabadi A. Bilirubin as a new antioxidant in melasma. J Cosmet Dermatol. 2022;21:5800-5803.
- Emre S, Kalkan G, Erdog˘an S, et al. Dynamic thiol/disulfide balance in patients with seborrheic dermatitis: a case-control study. Saudi J Med Med Sci. 2020;8:12-16.
- Erel Ö, Erdog˘an S. Thiol-disulfide homeostasis: an integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728-1738.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.E1-E2.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332.
- Guzelcicek A, Cakirca G, Erel O, et al. Assessment of thiol/disulfide balance as an oxidative stress marker in children with β-thalassemia major. Pak J Med Sci. 2019;35:161-165.
- Georgescu SR, Mitran CI, Mitran MI, et al. Thiol-Disulfide homeostasis in skin diseases. J Clin Med. 2022;11:1507.
- Üstüner P, Balevi A, Özdemir M, et al. The role of thiol/disulfide homeostasis in psoriasis: can it be a new marker for inflammation? Turk Arch Dermatol Venereol. 2018;52:120-125.
- Karacan G, Ercan N, Bostanci I, et al. A novel oxidative stress marker of atopic dermatitis in infants: Thiol–disulfide balance. Arch Dermatol Res. 2020;312:697-703.
- Demir Pektas S, Cinar N, Pektas G, et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol. 2021;11:14477.
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659.
- Njoo MD, Menke HE, Pavel W, et al. N-acetylcysteine as a bleaching agent in the treatment of melasma. J Eur Acad Dermatol Venereol. 1997;9:86-87.
Practice Points
- Melasma is a common pigmentation disorder that causes brown or grayish patches on the skin.
- Disulfide/native thiol and disulfide/total thiol ratios were higher in patients with melasma compared with controls, which indicated the presence of oxidative stress in melasma.
- The evaluation of modified melasma area and severity index score with disulfide/native thiol and disulfide/total thiol values suggests that oxidative stress is correlated with melasma disease severity.
Need a Wood Lamp Alternative? Grab Your Smartphone
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).
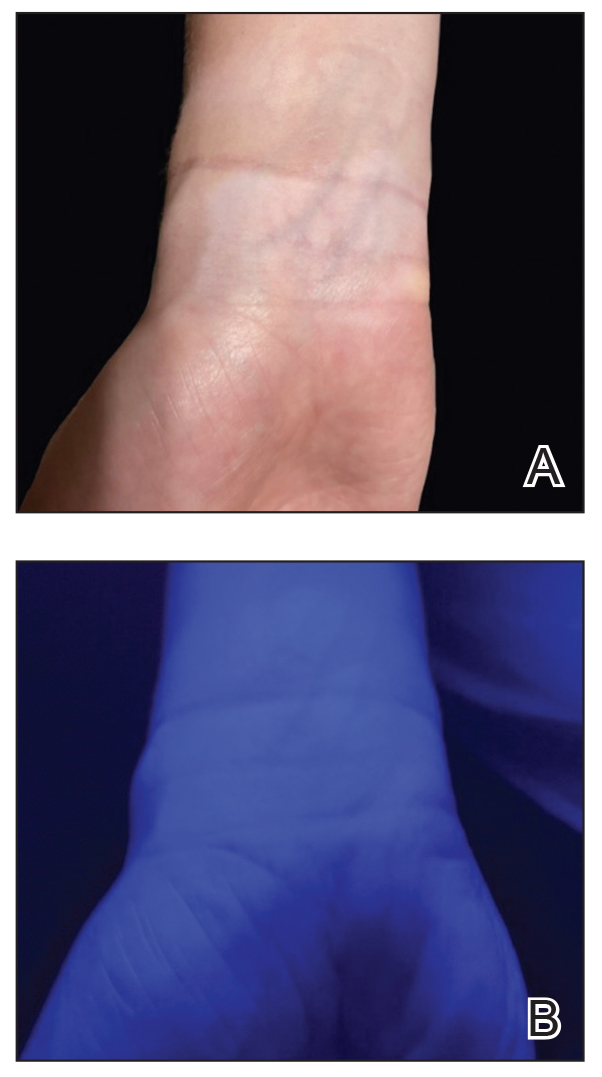
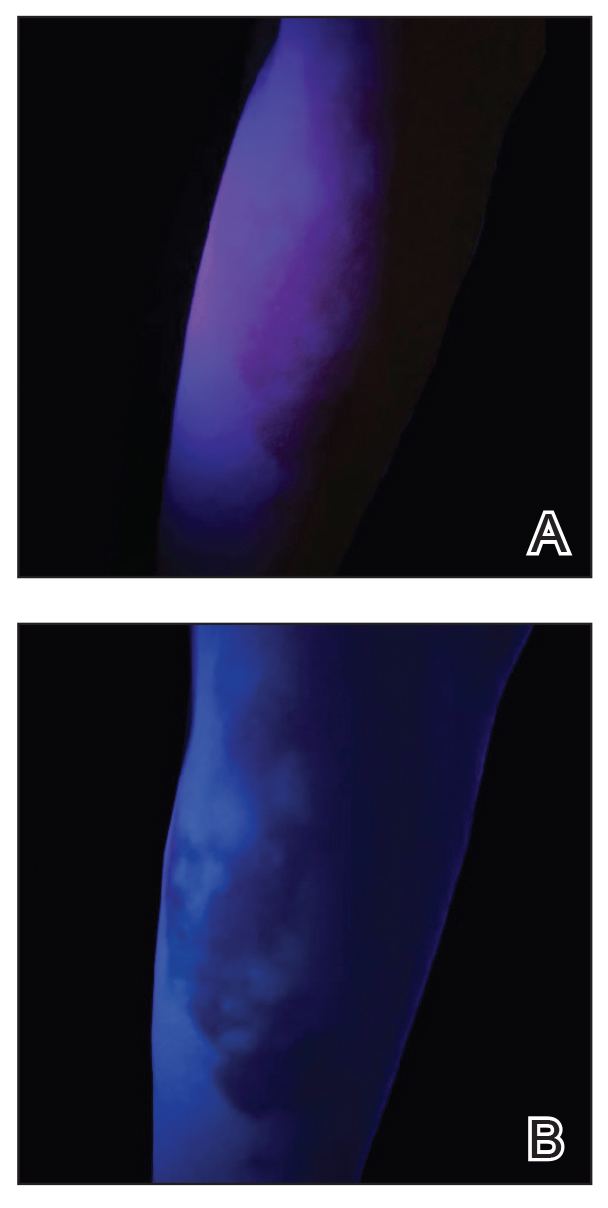
Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
Practice Gap
The Wood lamp commonly is used as a diagnostic tool for pigmentary skin conditions (eg, vitiligo) or skin conditions that exhibit fluorescence (eg, erythrasma).1 Recently, its diagnostic efficacy has extended to scabies, in which it unveils a distinctive wavy, bluish-white, linear fluorescence upon illumination.2
Functionally, the Wood lamp operates by subjecting phosphors to UV light within the wavelength range of 320 to 400 nm, inducing fluorescence in substances such as collagen and elastin. In the context of vitiligo, this process manifests as a preferential chalk white fluorescence in areas lacking melanin.1
Despite its demonstrated effectiveness, the Wood lamp is not without limitations. It comes with a notable financial investment ranging from $70 to $500, requires periodic maintenance such as light bulb replacements, and can be unwieldy.3 Furthermore, its reliance on a power source poses a challenge in settings where immediate access to convenient power outlets is limited, such as inpatient and rural dermatology clinics. These limitations underscore the need for alternative solutions and innovations to address challenges and ensure accessibility in diverse health care environments.
The Tools
Free smartphone applications (apps), such as Ultraviolet Light-UV Lamp by AppBrain or Blacklight UV Light Simulator by That Smile, can simulate UV light and functionally serve as a Wood lamp.
The Technique
UV light apps use LED or organic LED screen pixels to emit a blue light equivalent at 467 nm.4 Although these apps are not designed specifically for dermatologic uses, they are mostly free, widely available for Android and iPhone users, and portable. Importantly, they can demonstrate good performance in visualizing vitiligo, as shown in Figure 1—albeit perhaps not reaching the same level as the Wood lamp (Figure 2).


Because these UV light apps are not regulated and their efficacy for medical use has not been firmly established, the Wood lamp remains the gold standard. Therefore, we propose the use of UV light apps in situations when a Wood lamp is not available or convenient, such as in rural, inpatient, or international health care settings.
Practice Implications
Exploring and adopting these free alternatives can contribute to improved accessibility and diagnostic capabilities in diverse health care environments, particularly for communities facing financial constraints. Continued research and validation of these apps in clinical settings will be essential to establish their reliability and effectiveness in enhancing diagnostic practices.
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9
- Dyer JM, Foy VM. Revealing the unseen: a review of Wood’s lamp in dermatology. J Clin Aesthet Dermatol. 2022;15:25-30.
- Scanni G. Facilitations in the clinical diagnosis of human scabies through the use of ultraviolet light (UV-scab scanning): a case-series study. Trop Med Infect Dis. 2022;7:422. doi:10.3390/tropicalmed7120422
- USA Medical and Surgical Supplies. Top 9 medical diagnostic applications for a Woods lamp. February 26, 2019. Accessed May 20, 2024.
- Huang Y, Hsiang E-L, Deng M-Y, et al. Mini-led, micro-led and OLED displays: present status and future perspectives. Light Sci Appl. 2020;9:105. doi:10.1038/s41377-020-0341-9