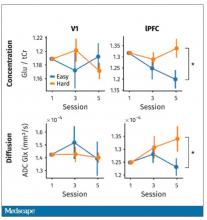
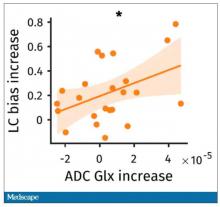
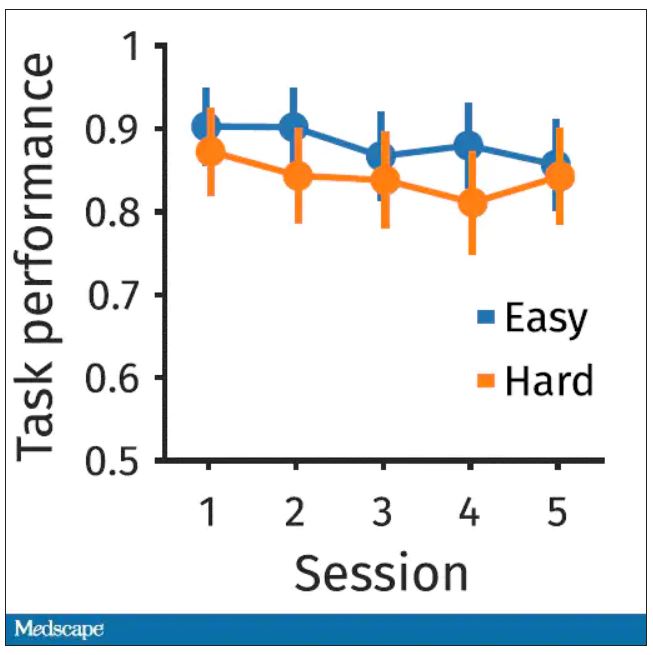
User login
Sexual function in transfeminine patients following gender-affirming vaginoplasty
For many patients, sexual function is an important component of a healthy quality of life.1 However, to many transgender individuals, their sexual organs are often a source of gender dysphoria, which can significantly inhibit sexual activity with their partners. Patients who seek gender-affirming surgery not only hope to have these feelings of dysphoria alleviated but also desire improvement in sexual function after surgery. While the medical and psychiatric criteria for patients seeking vaginoplasty procedures are well established by the World Professional Association for Transgender Health,2 there is little guidance surrounding the discourse surgeons should have regarding sexual function pre- and postsurgery.
Setting realistic expectations is one of the major challenges surgeons and patients alike face in preoperative and postoperative encounters. Patients not only are tasked with recovering from a major surgical procedure, but must also now learn their new anatomy, which includes learning how to urinate, maintain proper neovaginal hygiene, and experience sexual pleasure.
Given the permanence of these procedures and the possibility of loss of sexual function, the surgeon must ensure that patients truly comprehend the nature of the procedure and its complications. During the preoperative consultation, the surgeon must inquire about any desire for future fertility, discuss any history of pelvic radiation, epispadias, hypospadias, current erectile dysfunction, libido, comorbid medical conditions (such as diabetes or smoking), current sexual practices, and overall patient goals regarding their surgical outcome.
The vast majority of patients state they will experience a significant decrease in gender dysphoria with the removal of their current natal male genitalia.1 However, some patients have very specific preferences regarding the cosmetic appearance of vulvar structures. Others have more functional concerns about neovaginal depth and the ability to have receptive penetrative intercourse. It is important to note that not all transgender women have male partners. Furthermore, whether patients have male or female partners, some patients do not desire the ability to have penetrative intercourse and/or do not want to undergo the potential complications of a full-depth vaginoplasty. In these patients, offering a “shallow depth” vaginoplasty may be acceptable.
It is useful in the consultation to discuss a patient’s sexual partners and sexual practices in order to best determine the type of procedure that may be appropriate for a patient. In my practice, I emphasize that full-depth vaginoplasties require a lifelong commitment of dilation to maintain patency. Unlike cisgender women, patients must also douche to ensure appropriate vaginal hygiene. Regarding cosmetic preferences patients may have, it is essential to educate patients on the significant variation in the appearance of vulvar structures among both cisgender and transgender women.
During the surgical consultation, I review which structures from their natal genitalia are removed and which structures are utilized to create the neo–vulvar-vaginal anatomy. The testicles and spermatic cord are excised. The dorsal neurovascular bundle of the penile shaft and portion of the dorsal aspect of the glans penis are used to create the neoclitoris. A combination of penile shaft skin and scrotal skin is used to line the neovaginal canal. The erectile tissue of the penile shaft is also resected and the natal urethra is shortened and spatulated to create the urethral plate and urethral meatus. I also remind patients that the prostate remains intact during vaginoplasty procedures. Unless patients undergo the colonic interposition vaginoplasty and in some cases the peritoneal vaginoplasty, the neovaginal canal is not self-lubricating, nor will patients experience ejaculation after surgery. In the presurgical period, I often remind patients that the location of erogenous sensation after surgery will be altered and the method by which they self-stimulate will also be different. It is also essential to document whether patients can achieve satisfactory orgasms presurgically in order to determine adequate sexual function in the postoperative period.
It cannot be emphasized enough that the best predictor of unsatisfactory sexual function after genital gender-affirming surgery is poor sexual function prior to surgery.1,3
Retention of sexual function after gender-affirming genital surgery is common, with studies citing a range of 70%-90% of patients reporting their ability to regularly achieve an orgasm after surgery.1,4 In some cases, patients will report issues with sexual function after surgery despite having no prior history of sexual dysfunction. If patients present with complaints of postsurgical anorgasmia, the provider should rule out insufficient time for wound healing and resolution of surgery-site pain, and determine if there was an intraoperative injury to the neurovascular bundle or significant clitoral necrosis. A thorough genital exam should include a sensory examination of the neoclitoris and the introitus and neovaginal canal for signs of scarring, stenosis, loss of vaginal depth, or high-tone pelvic-floor dysfunction.
Unfortunately, if the neurovascular bundle is injured or if a patient experienced clitoral necrosis, the likelihood of a patient regaining sensation is decreased, although there are currently no studies examining the exact rates. It is also important to reassure patients that wound healing after surgery and relearning sexual function is not linear. I encourage patients to initially self-stimulate without a partner as they learn their new anatomy in order to remove any potential performance anxiety a partner could cause immediately after surgery. Similar to the approach to sexual dysfunction in cisgender patients, referral to a specialist in sexual health and/or pelvic floor physical therapy are useful adjuncts, depending on the findings from the physical exam and patient symptoms.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Garcia MM. Clin Plastic Surg. 2018;45:437-46.
2. Eli Coleman WB et al. “Standards of care for the health of transsexual, transgender, and gender non-conforming people” 7th version. World Professional Association for Transgender Health: 2012.
3. Garcia MM et al. Transl Androl Urol. 2014;3:156.
4. Ferrando CA, Bowers ML. “Genital gender confirmation surgery for patients assigned male at birth” In: Ferrando CA, ed. “Comprehensive care for the transgender patient” Philadelphia: Elsevier, 2020:82-92.
For many patients, sexual function is an important component of a healthy quality of life.1 However, to many transgender individuals, their sexual organs are often a source of gender dysphoria, which can significantly inhibit sexual activity with their partners. Patients who seek gender-affirming surgery not only hope to have these feelings of dysphoria alleviated but also desire improvement in sexual function after surgery. While the medical and psychiatric criteria for patients seeking vaginoplasty procedures are well established by the World Professional Association for Transgender Health,2 there is little guidance surrounding the discourse surgeons should have regarding sexual function pre- and postsurgery.
Setting realistic expectations is one of the major challenges surgeons and patients alike face in preoperative and postoperative encounters. Patients not only are tasked with recovering from a major surgical procedure, but must also now learn their new anatomy, which includes learning how to urinate, maintain proper neovaginal hygiene, and experience sexual pleasure.
Given the permanence of these procedures and the possibility of loss of sexual function, the surgeon must ensure that patients truly comprehend the nature of the procedure and its complications. During the preoperative consultation, the surgeon must inquire about any desire for future fertility, discuss any history of pelvic radiation, epispadias, hypospadias, current erectile dysfunction, libido, comorbid medical conditions (such as diabetes or smoking), current sexual practices, and overall patient goals regarding their surgical outcome.
The vast majority of patients state they will experience a significant decrease in gender dysphoria with the removal of their current natal male genitalia.1 However, some patients have very specific preferences regarding the cosmetic appearance of vulvar structures. Others have more functional concerns about neovaginal depth and the ability to have receptive penetrative intercourse. It is important to note that not all transgender women have male partners. Furthermore, whether patients have male or female partners, some patients do not desire the ability to have penetrative intercourse and/or do not want to undergo the potential complications of a full-depth vaginoplasty. In these patients, offering a “shallow depth” vaginoplasty may be acceptable.
It is useful in the consultation to discuss a patient’s sexual partners and sexual practices in order to best determine the type of procedure that may be appropriate for a patient. In my practice, I emphasize that full-depth vaginoplasties require a lifelong commitment of dilation to maintain patency. Unlike cisgender women, patients must also douche to ensure appropriate vaginal hygiene. Regarding cosmetic preferences patients may have, it is essential to educate patients on the significant variation in the appearance of vulvar structures among both cisgender and transgender women.
During the surgical consultation, I review which structures from their natal genitalia are removed and which structures are utilized to create the neo–vulvar-vaginal anatomy. The testicles and spermatic cord are excised. The dorsal neurovascular bundle of the penile shaft and portion of the dorsal aspect of the glans penis are used to create the neoclitoris. A combination of penile shaft skin and scrotal skin is used to line the neovaginal canal. The erectile tissue of the penile shaft is also resected and the natal urethra is shortened and spatulated to create the urethral plate and urethral meatus. I also remind patients that the prostate remains intact during vaginoplasty procedures. Unless patients undergo the colonic interposition vaginoplasty and in some cases the peritoneal vaginoplasty, the neovaginal canal is not self-lubricating, nor will patients experience ejaculation after surgery. In the presurgical period, I often remind patients that the location of erogenous sensation after surgery will be altered and the method by which they self-stimulate will also be different. It is also essential to document whether patients can achieve satisfactory orgasms presurgically in order to determine adequate sexual function in the postoperative period.
It cannot be emphasized enough that the best predictor of unsatisfactory sexual function after genital gender-affirming surgery is poor sexual function prior to surgery.1,3
Retention of sexual function after gender-affirming genital surgery is common, with studies citing a range of 70%-90% of patients reporting their ability to regularly achieve an orgasm after surgery.1,4 In some cases, patients will report issues with sexual function after surgery despite having no prior history of sexual dysfunction. If patients present with complaints of postsurgical anorgasmia, the provider should rule out insufficient time for wound healing and resolution of surgery-site pain, and determine if there was an intraoperative injury to the neurovascular bundle or significant clitoral necrosis. A thorough genital exam should include a sensory examination of the neoclitoris and the introitus and neovaginal canal for signs of scarring, stenosis, loss of vaginal depth, or high-tone pelvic-floor dysfunction.
Unfortunately, if the neurovascular bundle is injured or if a patient experienced clitoral necrosis, the likelihood of a patient regaining sensation is decreased, although there are currently no studies examining the exact rates. It is also important to reassure patients that wound healing after surgery and relearning sexual function is not linear. I encourage patients to initially self-stimulate without a partner as they learn their new anatomy in order to remove any potential performance anxiety a partner could cause immediately after surgery. Similar to the approach to sexual dysfunction in cisgender patients, referral to a specialist in sexual health and/or pelvic floor physical therapy are useful adjuncts, depending on the findings from the physical exam and patient symptoms.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Garcia MM. Clin Plastic Surg. 2018;45:437-46.
2. Eli Coleman WB et al. “Standards of care for the health of transsexual, transgender, and gender non-conforming people” 7th version. World Professional Association for Transgender Health: 2012.
3. Garcia MM et al. Transl Androl Urol. 2014;3:156.
4. Ferrando CA, Bowers ML. “Genital gender confirmation surgery for patients assigned male at birth” In: Ferrando CA, ed. “Comprehensive care for the transgender patient” Philadelphia: Elsevier, 2020:82-92.
For many patients, sexual function is an important component of a healthy quality of life.1 However, to many transgender individuals, their sexual organs are often a source of gender dysphoria, which can significantly inhibit sexual activity with their partners. Patients who seek gender-affirming surgery not only hope to have these feelings of dysphoria alleviated but also desire improvement in sexual function after surgery. While the medical and psychiatric criteria for patients seeking vaginoplasty procedures are well established by the World Professional Association for Transgender Health,2 there is little guidance surrounding the discourse surgeons should have regarding sexual function pre- and postsurgery.
Setting realistic expectations is one of the major challenges surgeons and patients alike face in preoperative and postoperative encounters. Patients not only are tasked with recovering from a major surgical procedure, but must also now learn their new anatomy, which includes learning how to urinate, maintain proper neovaginal hygiene, and experience sexual pleasure.
Given the permanence of these procedures and the possibility of loss of sexual function, the surgeon must ensure that patients truly comprehend the nature of the procedure and its complications. During the preoperative consultation, the surgeon must inquire about any desire for future fertility, discuss any history of pelvic radiation, epispadias, hypospadias, current erectile dysfunction, libido, comorbid medical conditions (such as diabetes or smoking), current sexual practices, and overall patient goals regarding their surgical outcome.
The vast majority of patients state they will experience a significant decrease in gender dysphoria with the removal of their current natal male genitalia.1 However, some patients have very specific preferences regarding the cosmetic appearance of vulvar structures. Others have more functional concerns about neovaginal depth and the ability to have receptive penetrative intercourse. It is important to note that not all transgender women have male partners. Furthermore, whether patients have male or female partners, some patients do not desire the ability to have penetrative intercourse and/or do not want to undergo the potential complications of a full-depth vaginoplasty. In these patients, offering a “shallow depth” vaginoplasty may be acceptable.
It is useful in the consultation to discuss a patient’s sexual partners and sexual practices in order to best determine the type of procedure that may be appropriate for a patient. In my practice, I emphasize that full-depth vaginoplasties require a lifelong commitment of dilation to maintain patency. Unlike cisgender women, patients must also douche to ensure appropriate vaginal hygiene. Regarding cosmetic preferences patients may have, it is essential to educate patients on the significant variation in the appearance of vulvar structures among both cisgender and transgender women.
During the surgical consultation, I review which structures from their natal genitalia are removed and which structures are utilized to create the neo–vulvar-vaginal anatomy. The testicles and spermatic cord are excised. The dorsal neurovascular bundle of the penile shaft and portion of the dorsal aspect of the glans penis are used to create the neoclitoris. A combination of penile shaft skin and scrotal skin is used to line the neovaginal canal. The erectile tissue of the penile shaft is also resected and the natal urethra is shortened and spatulated to create the urethral plate and urethral meatus. I also remind patients that the prostate remains intact during vaginoplasty procedures. Unless patients undergo the colonic interposition vaginoplasty and in some cases the peritoneal vaginoplasty, the neovaginal canal is not self-lubricating, nor will patients experience ejaculation after surgery. In the presurgical period, I often remind patients that the location of erogenous sensation after surgery will be altered and the method by which they self-stimulate will also be different. It is also essential to document whether patients can achieve satisfactory orgasms presurgically in order to determine adequate sexual function in the postoperative period.
It cannot be emphasized enough that the best predictor of unsatisfactory sexual function after genital gender-affirming surgery is poor sexual function prior to surgery.1,3
Retention of sexual function after gender-affirming genital surgery is common, with studies citing a range of 70%-90% of patients reporting their ability to regularly achieve an orgasm after surgery.1,4 In some cases, patients will report issues with sexual function after surgery despite having no prior history of sexual dysfunction. If patients present with complaints of postsurgical anorgasmia, the provider should rule out insufficient time for wound healing and resolution of surgery-site pain, and determine if there was an intraoperative injury to the neurovascular bundle or significant clitoral necrosis. A thorough genital exam should include a sensory examination of the neoclitoris and the introitus and neovaginal canal for signs of scarring, stenosis, loss of vaginal depth, or high-tone pelvic-floor dysfunction.
Unfortunately, if the neurovascular bundle is injured or if a patient experienced clitoral necrosis, the likelihood of a patient regaining sensation is decreased, although there are currently no studies examining the exact rates. It is also important to reassure patients that wound healing after surgery and relearning sexual function is not linear. I encourage patients to initially self-stimulate without a partner as they learn their new anatomy in order to remove any potential performance anxiety a partner could cause immediately after surgery. Similar to the approach to sexual dysfunction in cisgender patients, referral to a specialist in sexual health and/or pelvic floor physical therapy are useful adjuncts, depending on the findings from the physical exam and patient symptoms.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Garcia MM. Clin Plastic Surg. 2018;45:437-46.
2. Eli Coleman WB et al. “Standards of care for the health of transsexual, transgender, and gender non-conforming people” 7th version. World Professional Association for Transgender Health: 2012.
3. Garcia MM et al. Transl Androl Urol. 2014;3:156.
4. Ferrando CA, Bowers ML. “Genital gender confirmation surgery for patients assigned male at birth” In: Ferrando CA, ed. “Comprehensive care for the transgender patient” Philadelphia: Elsevier, 2020:82-92.
Exercise limitations in COPD – not everyone needs more inhalers
Chronic obstructive pulmonary disease (COPD) is defined by airway obstruction and alveolar damage caused by exposure to noxious air particles. The physiologic results include varying degrees of gas-exchange abnormality and mechanical respiratory limitation, often in the form of dynamic hyperinflation. There’s a third major contributor, though – skeletal muscle deconditioning. Only one of these abnormalities responds to inhalers.
When your patients with COPD report dyspnea or exercise intolerance, what do you do? Do you attempt to determine its character to pinpoint its origin? Do you quiz them about their baseline activity levels to quantify their conditioning? I bet you get right to the point and order a cardiopulmonary exercise test (CPET). That way you’ll be able to tease out all the contributors. Nah. Most likely you add an inhaler before continuing to rush through your COPD quality metrics: Vaccines? Check. Lung cancer screening? Check. Smoking cessation? Check.
The physiology of dyspnea and exercise limitation in COPD has been extensively studied. Work-of-breathing, dynamic hyperinflation, and gas-exchange inefficiencies interact with each other in complex ways to produce symptoms. The presence of deconditioning simply magnifies the existing abnormalities within the respiratory system by creating more strain at lower work rates. Acute exacerbations (AECOPD) and oral corticosteroids further aggravate skeletal muscle dysfunction.
The Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) Report directs clinicians to use inhalers to manage dyspnea. If they’re already on one inhaler, they get another. This continues until they’re stabilized on a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS). The GOLD report also advises pulmonary rehabilitation for any patient with grade B through D disease. Unfortunately, the pulmonary rehabilitation recommendation is buried in the text and doesn’t appear within the popularized pharmacologic algorithms in the report’s figures.
The data for adding inhalers on top of each other to reduce AECOPD and improve overall quality of life (QOL) are good. However, although GOLD tells us to keep adding inhalers for the dyspneic patient with COPD, the authors acknowledge that this hasn’t been systematically tested. The difference? A statement doesn’t require the same formal, rigorous scientific analysis known as the GRADE approach. Using this kind of analysis, a recent clinical practice guideline by the American Thoracic Society found no benefit in dyspnea or respiratory QOL with step-up from inhaler monotherapy.
Inhalers won’t do anything for gas-exchange inefficiencies and deconditioning, at least not directly. A recent CPET study from the CanCOLD network found ventilatory inefficiency in 23% of GOLD 1 and 26% of GOLD 2-4 COPD patients. The numbers were higher for those who reported dyspnea. Skeletal muscle dysfunction rates are equally high.
Thus, dyspnea and exercise intolerance are major determinants of QOL in COPD, but inhalers will only get you so far. At a minimum, make sure you get an activity/exercise history from your patients with COPD. For those who are sedentary, provide an exercise prescription (really, it’s not that hard to do). If dyspnea persists despite LABA or LAMA monotherapy, clarify the complaint before doubling down. Finally, try to get the patient into a good pulmonary rehabilitation program. They’ll thank you afterwards.
Dr. Holley is Associate Professor, department of medicine, Uniformed Services University of the Health Sciences and Program Director, Pulmonary and Critical Care Medical Fellowship, department of medicine, Walter Reed National Military Medical Center, both in Bethesda, Md. He reported receiving research grants from Fisher-Paykel and receiving income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease (COPD) is defined by airway obstruction and alveolar damage caused by exposure to noxious air particles. The physiologic results include varying degrees of gas-exchange abnormality and mechanical respiratory limitation, often in the form of dynamic hyperinflation. There’s a third major contributor, though – skeletal muscle deconditioning. Only one of these abnormalities responds to inhalers.
When your patients with COPD report dyspnea or exercise intolerance, what do you do? Do you attempt to determine its character to pinpoint its origin? Do you quiz them about their baseline activity levels to quantify their conditioning? I bet you get right to the point and order a cardiopulmonary exercise test (CPET). That way you’ll be able to tease out all the contributors. Nah. Most likely you add an inhaler before continuing to rush through your COPD quality metrics: Vaccines? Check. Lung cancer screening? Check. Smoking cessation? Check.
The physiology of dyspnea and exercise limitation in COPD has been extensively studied. Work-of-breathing, dynamic hyperinflation, and gas-exchange inefficiencies interact with each other in complex ways to produce symptoms. The presence of deconditioning simply magnifies the existing abnormalities within the respiratory system by creating more strain at lower work rates. Acute exacerbations (AECOPD) and oral corticosteroids further aggravate skeletal muscle dysfunction.
The Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) Report directs clinicians to use inhalers to manage dyspnea. If they’re already on one inhaler, they get another. This continues until they’re stabilized on a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS). The GOLD report also advises pulmonary rehabilitation for any patient with grade B through D disease. Unfortunately, the pulmonary rehabilitation recommendation is buried in the text and doesn’t appear within the popularized pharmacologic algorithms in the report’s figures.
The data for adding inhalers on top of each other to reduce AECOPD and improve overall quality of life (QOL) are good. However, although GOLD tells us to keep adding inhalers for the dyspneic patient with COPD, the authors acknowledge that this hasn’t been systematically tested. The difference? A statement doesn’t require the same formal, rigorous scientific analysis known as the GRADE approach. Using this kind of analysis, a recent clinical practice guideline by the American Thoracic Society found no benefit in dyspnea or respiratory QOL with step-up from inhaler monotherapy.
Inhalers won’t do anything for gas-exchange inefficiencies and deconditioning, at least not directly. A recent CPET study from the CanCOLD network found ventilatory inefficiency in 23% of GOLD 1 and 26% of GOLD 2-4 COPD patients. The numbers were higher for those who reported dyspnea. Skeletal muscle dysfunction rates are equally high.
Thus, dyspnea and exercise intolerance are major determinants of QOL in COPD, but inhalers will only get you so far. At a minimum, make sure you get an activity/exercise history from your patients with COPD. For those who are sedentary, provide an exercise prescription (really, it’s not that hard to do). If dyspnea persists despite LABA or LAMA monotherapy, clarify the complaint before doubling down. Finally, try to get the patient into a good pulmonary rehabilitation program. They’ll thank you afterwards.
Dr. Holley is Associate Professor, department of medicine, Uniformed Services University of the Health Sciences and Program Director, Pulmonary and Critical Care Medical Fellowship, department of medicine, Walter Reed National Military Medical Center, both in Bethesda, Md. He reported receiving research grants from Fisher-Paykel and receiving income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease (COPD) is defined by airway obstruction and alveolar damage caused by exposure to noxious air particles. The physiologic results include varying degrees of gas-exchange abnormality and mechanical respiratory limitation, often in the form of dynamic hyperinflation. There’s a third major contributor, though – skeletal muscle deconditioning. Only one of these abnormalities responds to inhalers.
When your patients with COPD report dyspnea or exercise intolerance, what do you do? Do you attempt to determine its character to pinpoint its origin? Do you quiz them about their baseline activity levels to quantify their conditioning? I bet you get right to the point and order a cardiopulmonary exercise test (CPET). That way you’ll be able to tease out all the contributors. Nah. Most likely you add an inhaler before continuing to rush through your COPD quality metrics: Vaccines? Check. Lung cancer screening? Check. Smoking cessation? Check.
The physiology of dyspnea and exercise limitation in COPD has been extensively studied. Work-of-breathing, dynamic hyperinflation, and gas-exchange inefficiencies interact with each other in complex ways to produce symptoms. The presence of deconditioning simply magnifies the existing abnormalities within the respiratory system by creating more strain at lower work rates. Acute exacerbations (AECOPD) and oral corticosteroids further aggravate skeletal muscle dysfunction.
The Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) Report directs clinicians to use inhalers to manage dyspnea. If they’re already on one inhaler, they get another. This continues until they’re stabilized on a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS). The GOLD report also advises pulmonary rehabilitation for any patient with grade B through D disease. Unfortunately, the pulmonary rehabilitation recommendation is buried in the text and doesn’t appear within the popularized pharmacologic algorithms in the report’s figures.
The data for adding inhalers on top of each other to reduce AECOPD and improve overall quality of life (QOL) are good. However, although GOLD tells us to keep adding inhalers for the dyspneic patient with COPD, the authors acknowledge that this hasn’t been systematically tested. The difference? A statement doesn’t require the same formal, rigorous scientific analysis known as the GRADE approach. Using this kind of analysis, a recent clinical practice guideline by the American Thoracic Society found no benefit in dyspnea or respiratory QOL with step-up from inhaler monotherapy.
Inhalers won’t do anything for gas-exchange inefficiencies and deconditioning, at least not directly. A recent CPET study from the CanCOLD network found ventilatory inefficiency in 23% of GOLD 1 and 26% of GOLD 2-4 COPD patients. The numbers were higher for those who reported dyspnea. Skeletal muscle dysfunction rates are equally high.
Thus, dyspnea and exercise intolerance are major determinants of QOL in COPD, but inhalers will only get you so far. At a minimum, make sure you get an activity/exercise history from your patients with COPD. For those who are sedentary, provide an exercise prescription (really, it’s not that hard to do). If dyspnea persists despite LABA or LAMA monotherapy, clarify the complaint before doubling down. Finally, try to get the patient into a good pulmonary rehabilitation program. They’ll thank you afterwards.
Dr. Holley is Associate Professor, department of medicine, Uniformed Services University of the Health Sciences and Program Director, Pulmonary and Critical Care Medical Fellowship, department of medicine, Walter Reed National Military Medical Center, both in Bethesda, Md. He reported receiving research grants from Fisher-Paykel and receiving income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
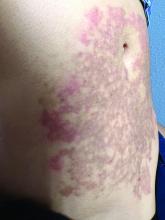
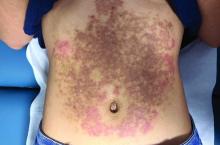
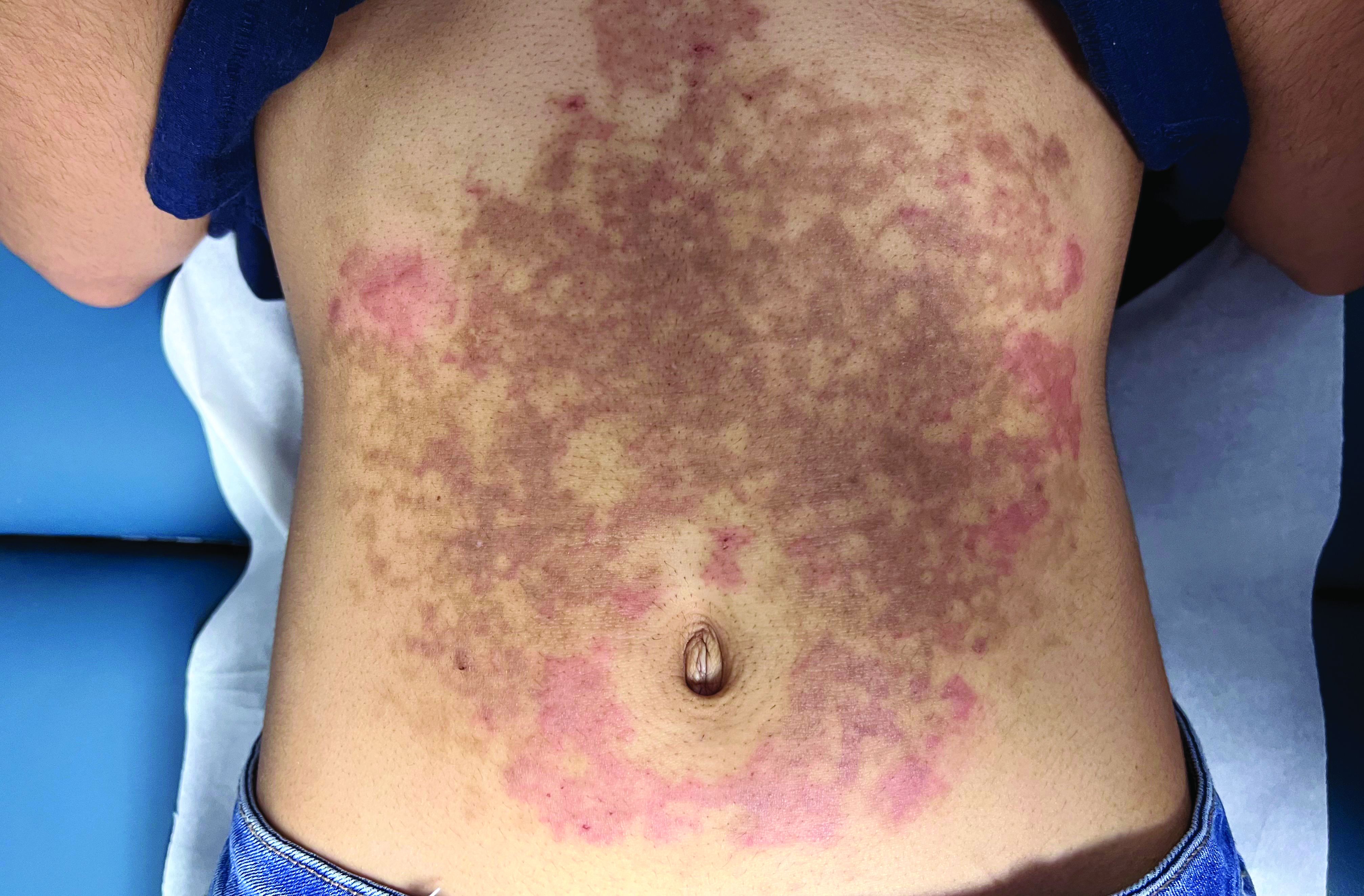
A White female presented with pruritic, reticulated, erythematous plaques on the abdomen
It is characterized by pruritic, erythematous papules, papulovesicles, and vesicles that appear in a reticular pattern, most commonly on the trunk. The lesions are typically followed by postinflammatory hyperpigmentation (PIH).
Although PP has been described in people of all races, ages, and sexes, it is predominantly observed in Japan, often in female young adults. Triggers may include a ketogenic diet, diabetes mellitus, and pregnancy. Friction and contact allergic reactions to chrome or nickel have been proposed as exogenous trigger factors. Individual cases of Sjögren’s syndrome, Helicobacter pylori infections, and adult Still syndrome have also been associated with recurrent eruptions.
The diagnosis of PP is made both clinically and by biopsy. The histological features vary according to the stage of the disease. In early-stage disease, superficial and perivascular infiltration of neutrophils are prominent. Later stages are characterized by spongiosis and necrotic keratinocytes.
The first-line therapy for prurigo pigmentosa is oral minocycline. However, for some patients, doxycycline, macrolide antibiotics, or dapsone may be indicated. Adding carbohydrates to a keto diet may be helpful. In this patient, a punch biopsy was performed, which revealed an interface dermatitis with eosinophils and neutrophils, consistent with prurigo pigmentosa. The cause of her PP remains idiopathic. She was treated with 100 mg doxycycline twice a day, which resulted in a resolution of active lesions. The patient did have postinflammatory hyperpigmentation.
This case and photo were submitted by Brooke Resh Sateesh, MD, of San Diego Family Dermatology, San Diego, California, and Mina Zulal, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany. Dr. Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Beutler et al. Am J Clin Dermatol. 2015 Dec;16(6):533-43.
2. Kim et al. J Dermatol. 2012 Nov;39(11):891-7.
3. Mufti et al. JAAD Int. 2021 Apr 10;3:79-87.
It is characterized by pruritic, erythematous papules, papulovesicles, and vesicles that appear in a reticular pattern, most commonly on the trunk. The lesions are typically followed by postinflammatory hyperpigmentation (PIH).
Although PP has been described in people of all races, ages, and sexes, it is predominantly observed in Japan, often in female young adults. Triggers may include a ketogenic diet, diabetes mellitus, and pregnancy. Friction and contact allergic reactions to chrome or nickel have been proposed as exogenous trigger factors. Individual cases of Sjögren’s syndrome, Helicobacter pylori infections, and adult Still syndrome have also been associated with recurrent eruptions.
The diagnosis of PP is made both clinically and by biopsy. The histological features vary according to the stage of the disease. In early-stage disease, superficial and perivascular infiltration of neutrophils are prominent. Later stages are characterized by spongiosis and necrotic keratinocytes.
The first-line therapy for prurigo pigmentosa is oral minocycline. However, for some patients, doxycycline, macrolide antibiotics, or dapsone may be indicated. Adding carbohydrates to a keto diet may be helpful. In this patient, a punch biopsy was performed, which revealed an interface dermatitis with eosinophils and neutrophils, consistent with prurigo pigmentosa. The cause of her PP remains idiopathic. She was treated with 100 mg doxycycline twice a day, which resulted in a resolution of active lesions. The patient did have postinflammatory hyperpigmentation.
This case and photo were submitted by Brooke Resh Sateesh, MD, of San Diego Family Dermatology, San Diego, California, and Mina Zulal, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany. Dr. Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Beutler et al. Am J Clin Dermatol. 2015 Dec;16(6):533-43.
2. Kim et al. J Dermatol. 2012 Nov;39(11):891-7.
3. Mufti et al. JAAD Int. 2021 Apr 10;3:79-87.
It is characterized by pruritic, erythematous papules, papulovesicles, and vesicles that appear in a reticular pattern, most commonly on the trunk. The lesions are typically followed by postinflammatory hyperpigmentation (PIH).
Although PP has been described in people of all races, ages, and sexes, it is predominantly observed in Japan, often in female young adults. Triggers may include a ketogenic diet, diabetes mellitus, and pregnancy. Friction and contact allergic reactions to chrome or nickel have been proposed as exogenous trigger factors. Individual cases of Sjögren’s syndrome, Helicobacter pylori infections, and adult Still syndrome have also been associated with recurrent eruptions.
The diagnosis of PP is made both clinically and by biopsy. The histological features vary according to the stage of the disease. In early-stage disease, superficial and perivascular infiltration of neutrophils are prominent. Later stages are characterized by spongiosis and necrotic keratinocytes.
The first-line therapy for prurigo pigmentosa is oral minocycline. However, for some patients, doxycycline, macrolide antibiotics, or dapsone may be indicated. Adding carbohydrates to a keto diet may be helpful. In this patient, a punch biopsy was performed, which revealed an interface dermatitis with eosinophils and neutrophils, consistent with prurigo pigmentosa. The cause of her PP remains idiopathic. She was treated with 100 mg doxycycline twice a day, which resulted in a resolution of active lesions. The patient did have postinflammatory hyperpigmentation.
This case and photo were submitted by Brooke Resh Sateesh, MD, of San Diego Family Dermatology, San Diego, California, and Mina Zulal, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany. Dr. Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Beutler et al. Am J Clin Dermatol. 2015 Dec;16(6):533-43.
2. Kim et al. J Dermatol. 2012 Nov;39(11):891-7.
3. Mufti et al. JAAD Int. 2021 Apr 10;3:79-87.
‘Stop pretending’ there’s a magic formula to weight loss
Is there a diet or weight-loss program out there that doesn’t work for those who stick with it during its first 12 weeks?
Truly, the world’s most backwards, upside-down, anti-science, nonsensical diets work over the short haul, fueled by the fact that short-term suffering for weight loss is a skill set that humanity has assiduously cultivated for at least the past 100 years. We’re really good at it!
It’s the keeping the weight off, though, that’s the hitch. Which leads me to the question, why are medical journals, even preeminent nonpredatory ones, publishing 12-week weight-loss program studies as if they have value? And does anyone truly imagine that after over 100 years of trying, there’ll be a short-term diet or program that’ll have the durable, reproducible results that no other short-term diet or program ever has?
Take this study published by Obesity: “Pragmatic implementation of a fully automated online obesity treatment in primary care.” It details a 12-week online, automated, weight-loss program that led completers to lose the roughly 5% of weight that many diets and programs see lost over their first 12 weeks. By its description, aside from its automated provision, the program sounds like pretty much the same boilerplate weight management advice and recommendations that haven’t been shown to lead large numbers of people to sustain long-term weight loss.
Participants were provided with weekly lessons which no doubt in some manner told them that high-calorie foods had high numbers of calories and should be minimized, along with other weight-loss secrets. Users were to upload weekly self-monitored weight, energy intake, and exercise minutes and were told to use a food diary. Their goal was losing 10% of their body weight by consuming 1,200-1,500 calories per day if they weighed less than 250 pounds (113 kg) and 1,500-1,800 calories if they weighed more than 250 pounds, while also telling them to aim for 200 minutes per week of moderate- to vigorous-intensity physical activity.
What was found was wholly unsurprising. Perhaps speaking to the tremendous and wide-ranging degrees of privilege that are required to prioritize intentional behavior change in the name of health, 79% of those who were given a prescription for the program either didn’t start it or stopped it before the end of the first week.
Of those who actually started the program and completed more than 1 week, despite having been selected as appropriate and interested participants by their physicians, only 20% watched all of the automated programs’ video lessons while only 32% actually bothered to submit all 12 weeks of weight data. Of course, the authors found that those who watched the greatest number of videos and submitted the most self-reported weights lost more weight and ascribed that loss to the program. What the authors did not entertain was the possibility that those who weren’t losing weight, or who were gaining, might simply be less inclined to continue with a program that wasn’t leading them to their desired outcomes or to want to submit their lack of loss or gains.
Short-term weight-loss studies help no one and when, as in this case, the outcomes aren’t even mediocre, and the completion and engagement rates are terrible, the study is still presented as significant and important. This bolsters the harmful stereotype that weight management is achievable by way of simple messages and generic goals. It suggests that it’s individuals who fail programs by not trying hard enough and that those who do, or who want it the most, will succeed. It may also lead patients and clinicians to second-guess the use of antiobesity medications, the current generation of which lead to far greater weight loss and reproducibility than any behavioral program or diet ever has.
The good news here at least is that the small percentage of participants who made it through this program’s 12 weeks are being randomly assigned to differing 9-month maintenance programs which at least will then lead to a 1-year analysis on the completers.
Why this study was published now, rather than pushed until the 1-year data were available, speaks to the pervasiveness of the toxic weight-biased notion that simple education will overcome the physiology forged over millions of years of extreme dietary insecurity.
Our food environment is a veritable floodplain of hyperpalatable foods, and social determinants of health make intentional behavior change in the name of health an unattainable luxury for a huge swath of the population.
Dr. Freedhoff is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health and receiving research grants from Novo Nordisk. A version of this article first appeared on Medscape.com.
Is there a diet or weight-loss program out there that doesn’t work for those who stick with it during its first 12 weeks?
Truly, the world’s most backwards, upside-down, anti-science, nonsensical diets work over the short haul, fueled by the fact that short-term suffering for weight loss is a skill set that humanity has assiduously cultivated for at least the past 100 years. We’re really good at it!
It’s the keeping the weight off, though, that’s the hitch. Which leads me to the question, why are medical journals, even preeminent nonpredatory ones, publishing 12-week weight-loss program studies as if they have value? And does anyone truly imagine that after over 100 years of trying, there’ll be a short-term diet or program that’ll have the durable, reproducible results that no other short-term diet or program ever has?
Take this study published by Obesity: “Pragmatic implementation of a fully automated online obesity treatment in primary care.” It details a 12-week online, automated, weight-loss program that led completers to lose the roughly 5% of weight that many diets and programs see lost over their first 12 weeks. By its description, aside from its automated provision, the program sounds like pretty much the same boilerplate weight management advice and recommendations that haven’t been shown to lead large numbers of people to sustain long-term weight loss.
Participants were provided with weekly lessons which no doubt in some manner told them that high-calorie foods had high numbers of calories and should be minimized, along with other weight-loss secrets. Users were to upload weekly self-monitored weight, energy intake, and exercise minutes and were told to use a food diary. Their goal was losing 10% of their body weight by consuming 1,200-1,500 calories per day if they weighed less than 250 pounds (113 kg) and 1,500-1,800 calories if they weighed more than 250 pounds, while also telling them to aim for 200 minutes per week of moderate- to vigorous-intensity physical activity.
What was found was wholly unsurprising. Perhaps speaking to the tremendous and wide-ranging degrees of privilege that are required to prioritize intentional behavior change in the name of health, 79% of those who were given a prescription for the program either didn’t start it or stopped it before the end of the first week.
Of those who actually started the program and completed more than 1 week, despite having been selected as appropriate and interested participants by their physicians, only 20% watched all of the automated programs’ video lessons while only 32% actually bothered to submit all 12 weeks of weight data. Of course, the authors found that those who watched the greatest number of videos and submitted the most self-reported weights lost more weight and ascribed that loss to the program. What the authors did not entertain was the possibility that those who weren’t losing weight, or who were gaining, might simply be less inclined to continue with a program that wasn’t leading them to their desired outcomes or to want to submit their lack of loss or gains.
Short-term weight-loss studies help no one and when, as in this case, the outcomes aren’t even mediocre, and the completion and engagement rates are terrible, the study is still presented as significant and important. This bolsters the harmful stereotype that weight management is achievable by way of simple messages and generic goals. It suggests that it’s individuals who fail programs by not trying hard enough and that those who do, or who want it the most, will succeed. It may also lead patients and clinicians to second-guess the use of antiobesity medications, the current generation of which lead to far greater weight loss and reproducibility than any behavioral program or diet ever has.
The good news here at least is that the small percentage of participants who made it through this program’s 12 weeks are being randomly assigned to differing 9-month maintenance programs which at least will then lead to a 1-year analysis on the completers.
Why this study was published now, rather than pushed until the 1-year data were available, speaks to the pervasiveness of the toxic weight-biased notion that simple education will overcome the physiology forged over millions of years of extreme dietary insecurity.
Our food environment is a veritable floodplain of hyperpalatable foods, and social determinants of health make intentional behavior change in the name of health an unattainable luxury for a huge swath of the population.
Dr. Freedhoff is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health and receiving research grants from Novo Nordisk. A version of this article first appeared on Medscape.com.
Is there a diet or weight-loss program out there that doesn’t work for those who stick with it during its first 12 weeks?
Truly, the world’s most backwards, upside-down, anti-science, nonsensical diets work over the short haul, fueled by the fact that short-term suffering for weight loss is a skill set that humanity has assiduously cultivated for at least the past 100 years. We’re really good at it!
It’s the keeping the weight off, though, that’s the hitch. Which leads me to the question, why are medical journals, even preeminent nonpredatory ones, publishing 12-week weight-loss program studies as if they have value? And does anyone truly imagine that after over 100 years of trying, there’ll be a short-term diet or program that’ll have the durable, reproducible results that no other short-term diet or program ever has?
Take this study published by Obesity: “Pragmatic implementation of a fully automated online obesity treatment in primary care.” It details a 12-week online, automated, weight-loss program that led completers to lose the roughly 5% of weight that many diets and programs see lost over their first 12 weeks. By its description, aside from its automated provision, the program sounds like pretty much the same boilerplate weight management advice and recommendations that haven’t been shown to lead large numbers of people to sustain long-term weight loss.
Participants were provided with weekly lessons which no doubt in some manner told them that high-calorie foods had high numbers of calories and should be minimized, along with other weight-loss secrets. Users were to upload weekly self-monitored weight, energy intake, and exercise minutes and were told to use a food diary. Their goal was losing 10% of their body weight by consuming 1,200-1,500 calories per day if they weighed less than 250 pounds (113 kg) and 1,500-1,800 calories if they weighed more than 250 pounds, while also telling them to aim for 200 minutes per week of moderate- to vigorous-intensity physical activity.
What was found was wholly unsurprising. Perhaps speaking to the tremendous and wide-ranging degrees of privilege that are required to prioritize intentional behavior change in the name of health, 79% of those who were given a prescription for the program either didn’t start it or stopped it before the end of the first week.
Of those who actually started the program and completed more than 1 week, despite having been selected as appropriate and interested participants by their physicians, only 20% watched all of the automated programs’ video lessons while only 32% actually bothered to submit all 12 weeks of weight data. Of course, the authors found that those who watched the greatest number of videos and submitted the most self-reported weights lost more weight and ascribed that loss to the program. What the authors did not entertain was the possibility that those who weren’t losing weight, or who were gaining, might simply be less inclined to continue with a program that wasn’t leading them to their desired outcomes or to want to submit their lack of loss or gains.
Short-term weight-loss studies help no one and when, as in this case, the outcomes aren’t even mediocre, and the completion and engagement rates are terrible, the study is still presented as significant and important. This bolsters the harmful stereotype that weight management is achievable by way of simple messages and generic goals. It suggests that it’s individuals who fail programs by not trying hard enough and that those who do, or who want it the most, will succeed. It may also lead patients and clinicians to second-guess the use of antiobesity medications, the current generation of which lead to far greater weight loss and reproducibility than any behavioral program or diet ever has.
The good news here at least is that the small percentage of participants who made it through this program’s 12 weeks are being randomly assigned to differing 9-month maintenance programs which at least will then lead to a 1-year analysis on the completers.
Why this study was published now, rather than pushed until the 1-year data were available, speaks to the pervasiveness of the toxic weight-biased notion that simple education will overcome the physiology forged over millions of years of extreme dietary insecurity.
Our food environment is a veritable floodplain of hyperpalatable foods, and social determinants of health make intentional behavior change in the name of health an unattainable luxury for a huge swath of the population.
Dr. Freedhoff is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health and receiving research grants from Novo Nordisk. A version of this article first appeared on Medscape.com.
Understanding the relationship between life satisfaction and cognitive decline
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Reliably solving complex problems
The James Webb Space Telescope (JWST) is an engineering marvel. Costing over $10 billion, it should be. The project cost overrun was 900%. The launch was delayed by more than a decade. The Human Genome Project from 1990 to 2003 was completed slightly ahead of schedule and for less than the $4-$5 billion original estimates. This HGP success story is partly because of private entrepreneurial involvement. The Superconducting Super Collider in Texas spent $2 billion but never got off the ground. Successfully shepherding huge public projects like these involves the art of politics and management as well as science.
Whatever the earlier missteps, the JWST project is now performing above expectations. It has launched, taken up residence a million miles from Earth, deployed its mirrors (a process that had more than 300 possible single points of failure, any one of which would reduce the thing to scrap metal), and been calibrated. The JWST has even been dented by a micrometeoroid – sort of like a parking lot ding on the door of your brand new car. The first images are visually amazing and producing new scientific insights. This is a pinnacle of scientific achievement.
What characteristics enable such an achievement? How do we foster those same characteristics in the practice of medicine and medical research? Will the success of the JWST increase and restore the public’s trust in science and scientists?
After all the bickering over vaccines and masks for the past 2+ years, medical science could use a boost. The gravitas of scientists, and indeed all experts, has diminished over the 5 decades since humans walked on the moon. It has been harmed by mercenary scientists who sought to sow doubt about whether smoking caused cancer and whether fossil fuels created climate change. No proof was needed, just doubt.
The trust in science has also been harmed by the vast amount of published medical research that is wrong. An effort was made 20 years ago to rid research of the bias of taking money from drug companies. To my observation, that change produced only a small benefit that has been overwhelmed by the unintended harms. The large, well-funded academic labs of full-time researchers have been replaced with unfunded, undertrained, and inadequately supported part-time junior faculty trying to publish enough articles to be promoted. In my opinion, this change is worse than funding from Big Pharma. (Disclosure – I worked in industry prior to graduate school.)
The pressure to publish reduces skepticism, so more incorrect data are published. The small size of these amateur studies produces unconvincing conclusions that feed an industry of meta-analysis that tries to overcome the deficiencies of the individual studies. This fragmented, biased approach is not how you build, launch, deploy, and operate the JWST, which requires very high reliability.
This approach is not working well for pediatrics either. I look at the history of the recommended workup of the febrile young infant from the 1980s until today. I see constant changes to the guidelines but no real progress toward a validated, evidence-based approach. A similar history is behind treatment of neonatal hyperbilirubinemia. In the 1994 publication, there was a movement toward being less aggressive. The 2004 and 2009 editions increased the frequency of screening and phototherapy. Now, the 2022 guidelines have moved in the direction we were headed in the 1990s. The workup of infants and children with possible urinary tract infections has undergone a similar trajectory. So has the screening for neonatal herpes infections. The practice changes are more like Brownian motion than real progress. This inconsistency has led me to be skeptical of the process the American Academy of Pediatrics uses to create guidelines.
Part of solving complex problems is allowing all stakeholders’ voices to be heard. On Jan. 28, 1986, seconds after liftoff, the space shuttle Challenger exploded. In the aftermath, it was determined that some engineers had expressed concern about the very cold weather that morning. The rubber in the O-ring would not be as flexible as designed. Their objection was not listened to. The O-ring failed, the fuel tank exploded, and the ship and crew were lost. It is a lesson many engineers of my generation took to heart. Do not suppress voices.
For example, 1 year ago (September 2021), the Royal Australian and New Zealand College of Psychiatrists published a position statement, “Recognising and addressing the mental health needs of people experiencing gender dysphoria/gender incongruence.” The statement expressed concern about the marked increase in incidence of rapid-onset gender dysphoria and therefore urged more thorough assessment by psychiatry before embarking on puberty-blocking therapies. The RANZCP position is at variance with recent trends in the United States. The topic was censored at the 2021 AAP national conference. Lately, I have heard the words disinformation and homophobic used to describe my RANZCP colleagues. I have been comparing AAP, Britain’s National Institute for Health and Care Excellence, and Royal Children’s Hospital Melbourne guidelines for 20 years. The variation is enlightening. I do not know the correct answer to treating gender dysphoria, but I know suppressing viewpoints and debate leads to exploding spaceships.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
The James Webb Space Telescope (JWST) is an engineering marvel. Costing over $10 billion, it should be. The project cost overrun was 900%. The launch was delayed by more than a decade. The Human Genome Project from 1990 to 2003 was completed slightly ahead of schedule and for less than the $4-$5 billion original estimates. This HGP success story is partly because of private entrepreneurial involvement. The Superconducting Super Collider in Texas spent $2 billion but never got off the ground. Successfully shepherding huge public projects like these involves the art of politics and management as well as science.
Whatever the earlier missteps, the JWST project is now performing above expectations. It has launched, taken up residence a million miles from Earth, deployed its mirrors (a process that had more than 300 possible single points of failure, any one of which would reduce the thing to scrap metal), and been calibrated. The JWST has even been dented by a micrometeoroid – sort of like a parking lot ding on the door of your brand new car. The first images are visually amazing and producing new scientific insights. This is a pinnacle of scientific achievement.
What characteristics enable such an achievement? How do we foster those same characteristics in the practice of medicine and medical research? Will the success of the JWST increase and restore the public’s trust in science and scientists?
After all the bickering over vaccines and masks for the past 2+ years, medical science could use a boost. The gravitas of scientists, and indeed all experts, has diminished over the 5 decades since humans walked on the moon. It has been harmed by mercenary scientists who sought to sow doubt about whether smoking caused cancer and whether fossil fuels created climate change. No proof was needed, just doubt.
The trust in science has also been harmed by the vast amount of published medical research that is wrong. An effort was made 20 years ago to rid research of the bias of taking money from drug companies. To my observation, that change produced only a small benefit that has been overwhelmed by the unintended harms. The large, well-funded academic labs of full-time researchers have been replaced with unfunded, undertrained, and inadequately supported part-time junior faculty trying to publish enough articles to be promoted. In my opinion, this change is worse than funding from Big Pharma. (Disclosure – I worked in industry prior to graduate school.)
The pressure to publish reduces skepticism, so more incorrect data are published. The small size of these amateur studies produces unconvincing conclusions that feed an industry of meta-analysis that tries to overcome the deficiencies of the individual studies. This fragmented, biased approach is not how you build, launch, deploy, and operate the JWST, which requires very high reliability.
This approach is not working well for pediatrics either. I look at the history of the recommended workup of the febrile young infant from the 1980s until today. I see constant changes to the guidelines but no real progress toward a validated, evidence-based approach. A similar history is behind treatment of neonatal hyperbilirubinemia. In the 1994 publication, there was a movement toward being less aggressive. The 2004 and 2009 editions increased the frequency of screening and phototherapy. Now, the 2022 guidelines have moved in the direction we were headed in the 1990s. The workup of infants and children with possible urinary tract infections has undergone a similar trajectory. So has the screening for neonatal herpes infections. The practice changes are more like Brownian motion than real progress. This inconsistency has led me to be skeptical of the process the American Academy of Pediatrics uses to create guidelines.
Part of solving complex problems is allowing all stakeholders’ voices to be heard. On Jan. 28, 1986, seconds after liftoff, the space shuttle Challenger exploded. In the aftermath, it was determined that some engineers had expressed concern about the very cold weather that morning. The rubber in the O-ring would not be as flexible as designed. Their objection was not listened to. The O-ring failed, the fuel tank exploded, and the ship and crew were lost. It is a lesson many engineers of my generation took to heart. Do not suppress voices.
For example, 1 year ago (September 2021), the Royal Australian and New Zealand College of Psychiatrists published a position statement, “Recognising and addressing the mental health needs of people experiencing gender dysphoria/gender incongruence.” The statement expressed concern about the marked increase in incidence of rapid-onset gender dysphoria and therefore urged more thorough assessment by psychiatry before embarking on puberty-blocking therapies. The RANZCP position is at variance with recent trends in the United States. The topic was censored at the 2021 AAP national conference. Lately, I have heard the words disinformation and homophobic used to describe my RANZCP colleagues. I have been comparing AAP, Britain’s National Institute for Health and Care Excellence, and Royal Children’s Hospital Melbourne guidelines for 20 years. The variation is enlightening. I do not know the correct answer to treating gender dysphoria, but I know suppressing viewpoints and debate leads to exploding spaceships.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
The James Webb Space Telescope (JWST) is an engineering marvel. Costing over $10 billion, it should be. The project cost overrun was 900%. The launch was delayed by more than a decade. The Human Genome Project from 1990 to 2003 was completed slightly ahead of schedule and for less than the $4-$5 billion original estimates. This HGP success story is partly because of private entrepreneurial involvement. The Superconducting Super Collider in Texas spent $2 billion but never got off the ground. Successfully shepherding huge public projects like these involves the art of politics and management as well as science.
Whatever the earlier missteps, the JWST project is now performing above expectations. It has launched, taken up residence a million miles from Earth, deployed its mirrors (a process that had more than 300 possible single points of failure, any one of which would reduce the thing to scrap metal), and been calibrated. The JWST has even been dented by a micrometeoroid – sort of like a parking lot ding on the door of your brand new car. The first images are visually amazing and producing new scientific insights. This is a pinnacle of scientific achievement.
What characteristics enable such an achievement? How do we foster those same characteristics in the practice of medicine and medical research? Will the success of the JWST increase and restore the public’s trust in science and scientists?
After all the bickering over vaccines and masks for the past 2+ years, medical science could use a boost. The gravitas of scientists, and indeed all experts, has diminished over the 5 decades since humans walked on the moon. It has been harmed by mercenary scientists who sought to sow doubt about whether smoking caused cancer and whether fossil fuels created climate change. No proof was needed, just doubt.
The trust in science has also been harmed by the vast amount of published medical research that is wrong. An effort was made 20 years ago to rid research of the bias of taking money from drug companies. To my observation, that change produced only a small benefit that has been overwhelmed by the unintended harms. The large, well-funded academic labs of full-time researchers have been replaced with unfunded, undertrained, and inadequately supported part-time junior faculty trying to publish enough articles to be promoted. In my opinion, this change is worse than funding from Big Pharma. (Disclosure – I worked in industry prior to graduate school.)
The pressure to publish reduces skepticism, so more incorrect data are published. The small size of these amateur studies produces unconvincing conclusions that feed an industry of meta-analysis that tries to overcome the deficiencies of the individual studies. This fragmented, biased approach is not how you build, launch, deploy, and operate the JWST, which requires very high reliability.
This approach is not working well for pediatrics either. I look at the history of the recommended workup of the febrile young infant from the 1980s until today. I see constant changes to the guidelines but no real progress toward a validated, evidence-based approach. A similar history is behind treatment of neonatal hyperbilirubinemia. In the 1994 publication, there was a movement toward being less aggressive. The 2004 and 2009 editions increased the frequency of screening and phototherapy. Now, the 2022 guidelines have moved in the direction we were headed in the 1990s. The workup of infants and children with possible urinary tract infections has undergone a similar trajectory. So has the screening for neonatal herpes infections. The practice changes are more like Brownian motion than real progress. This inconsistency has led me to be skeptical of the process the American Academy of Pediatrics uses to create guidelines.
Part of solving complex problems is allowing all stakeholders’ voices to be heard. On Jan. 28, 1986, seconds after liftoff, the space shuttle Challenger exploded. In the aftermath, it was determined that some engineers had expressed concern about the very cold weather that morning. The rubber in the O-ring would not be as flexible as designed. Their objection was not listened to. The O-ring failed, the fuel tank exploded, and the ship and crew were lost. It is a lesson many engineers of my generation took to heart. Do not suppress voices.
For example, 1 year ago (September 2021), the Royal Australian and New Zealand College of Psychiatrists published a position statement, “Recognising and addressing the mental health needs of people experiencing gender dysphoria/gender incongruence.” The statement expressed concern about the marked increase in incidence of rapid-onset gender dysphoria and therefore urged more thorough assessment by psychiatry before embarking on puberty-blocking therapies. The RANZCP position is at variance with recent trends in the United States. The topic was censored at the 2021 AAP national conference. Lately, I have heard the words disinformation and homophobic used to describe my RANZCP colleagues. I have been comparing AAP, Britain’s National Institute for Health and Care Excellence, and Royal Children’s Hospital Melbourne guidelines for 20 years. The variation is enlightening. I do not know the correct answer to treating gender dysphoria, but I know suppressing viewpoints and debate leads to exploding spaceships.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
Estrogen replacement therapy in endometrial cancer survivors
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
Why our brains wear out at the end of the day
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Dig like an archaeologist
You can observe a lot by watching. – Yogi Berra
He was a fit man in his 40s. Thick legs. Maybe he was a long-distance walker? The bones of his right arm were more developed than his left – a right-handed thrower. His lower left fibula was fractured from a severely rolled ankle. He carried a walking stick that was glossy in the middle from where he gripped it with his left hand, dragging his bad left foot along. Dental cavities tell the story of his diet: honey, carobs, dates. Carbon 14 dating confirms that he lived during the Chalcolithic period, approximately 6,000 years ago. He was likely a shepherd in the Judean Desert.
Isn’t it amazing how much we can know about another human even across such an enormous chasm of time? If you’d asked me when I was 11 what I wanted to be, I’d have said archaeologist. .
A 64-year-old woman with a 4-cm red, brown shiny plaque on her right calf. She burned it on her boyfriend’s Harley Davidson nearly 40 years ago. She wonders where he is now.
A 58-year-old man with a 3-inch scar on his right wrist. He fell off his 6-year-old’s skimboard. ORIF.
A 40-year-old woman with bilateral mastectomy scars.
A 66-year-old with a lichenified nodule on his left forearm. When I shaved it off, a quarter inch spicule of glass came out. It was from a car accident in his first car, a Chevy Impala. He saved the piece of glass as a souvenir.
A fit 50-year-old with extensive scars on his feet and ankles. “Yeah, I went ‘whistling-in’ on a training jump,” he said. He was a retired Navy Seal and raconteur with quite a tale about the day his parachute malfunctioned. Some well placed live oak trees is why he’s around for his skin screening.
A classic, rope-like open-heart scar on the chest of a thin, young, healthy, flaxen-haired woman. Dissected aorta.
A 30-something woman dressed in a pants suit with razor-thin parallel scars on her volar forearms and proximal thighs. She asks if any laser could remove them.
A rotund, hard-living, bearded man with chest and upper-arm tattoos of flames and nudie girls now mixed with the striking face of an old woman and three little kids: His mom and grandkids. He shows me where the fourth grandkid will go and gives me a bear hug to thank me for the care when he leaves.
Attending to these details shifts us from autopilot to present. It keeps us involved, holding our attention even if it’s the 20th skin screening or diabetic foot exam of the day. And what a gift to share in the intimate details of another’s life.
Like examining the minute details of an ancient bone, dig for the history with curiosity, pity, humility. The perfect moment for asking might be when you stand with your #15 blade ready to introduce a new scar and become part of this human’s story forever.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
You can observe a lot by watching. – Yogi Berra
He was a fit man in his 40s. Thick legs. Maybe he was a long-distance walker? The bones of his right arm were more developed than his left – a right-handed thrower. His lower left fibula was fractured from a severely rolled ankle. He carried a walking stick that was glossy in the middle from where he gripped it with his left hand, dragging his bad left foot along. Dental cavities tell the story of his diet: honey, carobs, dates. Carbon 14 dating confirms that he lived during the Chalcolithic period, approximately 6,000 years ago. He was likely a shepherd in the Judean Desert.
Isn’t it amazing how much we can know about another human even across such an enormous chasm of time? If you’d asked me when I was 11 what I wanted to be, I’d have said archaeologist. .
A 64-year-old woman with a 4-cm red, brown shiny plaque on her right calf. She burned it on her boyfriend’s Harley Davidson nearly 40 years ago. She wonders where he is now.
A 58-year-old man with a 3-inch scar on his right wrist. He fell off his 6-year-old’s skimboard. ORIF.
A 40-year-old woman with bilateral mastectomy scars.
A 66-year-old with a lichenified nodule on his left forearm. When I shaved it off, a quarter inch spicule of glass came out. It was from a car accident in his first car, a Chevy Impala. He saved the piece of glass as a souvenir.
A fit 50-year-old with extensive scars on his feet and ankles. “Yeah, I went ‘whistling-in’ on a training jump,” he said. He was a retired Navy Seal and raconteur with quite a tale about the day his parachute malfunctioned. Some well placed live oak trees is why he’s around for his skin screening.
A classic, rope-like open-heart scar on the chest of a thin, young, healthy, flaxen-haired woman. Dissected aorta.
A 30-something woman dressed in a pants suit with razor-thin parallel scars on her volar forearms and proximal thighs. She asks if any laser could remove them.
A rotund, hard-living, bearded man with chest and upper-arm tattoos of flames and nudie girls now mixed with the striking face of an old woman and three little kids: His mom and grandkids. He shows me where the fourth grandkid will go and gives me a bear hug to thank me for the care when he leaves.
Attending to these details shifts us from autopilot to present. It keeps us involved, holding our attention even if it’s the 20th skin screening or diabetic foot exam of the day. And what a gift to share in the intimate details of another’s life.
Like examining the minute details of an ancient bone, dig for the history with curiosity, pity, humility. The perfect moment for asking might be when you stand with your #15 blade ready to introduce a new scar and become part of this human’s story forever.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
You can observe a lot by watching. – Yogi Berra
He was a fit man in his 40s. Thick legs. Maybe he was a long-distance walker? The bones of his right arm were more developed than his left – a right-handed thrower. His lower left fibula was fractured from a severely rolled ankle. He carried a walking stick that was glossy in the middle from where he gripped it with his left hand, dragging his bad left foot along. Dental cavities tell the story of his diet: honey, carobs, dates. Carbon 14 dating confirms that he lived during the Chalcolithic period, approximately 6,000 years ago. He was likely a shepherd in the Judean Desert.
Isn’t it amazing how much we can know about another human even across such an enormous chasm of time? If you’d asked me when I was 11 what I wanted to be, I’d have said archaeologist. .
A 64-year-old woman with a 4-cm red, brown shiny plaque on her right calf. She burned it on her boyfriend’s Harley Davidson nearly 40 years ago. She wonders where he is now.
A 58-year-old man with a 3-inch scar on his right wrist. He fell off his 6-year-old’s skimboard. ORIF.
A 40-year-old woman with bilateral mastectomy scars.
A 66-year-old with a lichenified nodule on his left forearm. When I shaved it off, a quarter inch spicule of glass came out. It was from a car accident in his first car, a Chevy Impala. He saved the piece of glass as a souvenir.
A fit 50-year-old with extensive scars on his feet and ankles. “Yeah, I went ‘whistling-in’ on a training jump,” he said. He was a retired Navy Seal and raconteur with quite a tale about the day his parachute malfunctioned. Some well placed live oak trees is why he’s around for his skin screening.
A classic, rope-like open-heart scar on the chest of a thin, young, healthy, flaxen-haired woman. Dissected aorta.
A 30-something woman dressed in a pants suit with razor-thin parallel scars on her volar forearms and proximal thighs. She asks if any laser could remove them.
A rotund, hard-living, bearded man with chest and upper-arm tattoos of flames and nudie girls now mixed with the striking face of an old woman and three little kids: His mom and grandkids. He shows me where the fourth grandkid will go and gives me a bear hug to thank me for the care when he leaves.
Attending to these details shifts us from autopilot to present. It keeps us involved, holding our attention even if it’s the 20th skin screening or diabetic foot exam of the day. And what a gift to share in the intimate details of another’s life.
Like examining the minute details of an ancient bone, dig for the history with curiosity, pity, humility. The perfect moment for asking might be when you stand with your #15 blade ready to introduce a new scar and become part of this human’s story forever.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Postpartum psychosis: Does longitudinal course inform treatment?
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.