User login
Managing maternal and infant mental health
An overwhelmed mother presents to your office with her 2-month-old son for his check-up. She seems distant and dysphoric, often shrugging her shoulders with an empty stare when asked about her son’s development. Her baby cries loudly in her arms and you can see that she is uncomfortable soothing him as she frantically rocks him back and forth. He appears to have gained little weight since the last appointment occurring 6 days post partum and his mother describes him as “difficult and fussy all the time.” The father was unable to attend the appointment due to work obligations and often leaves the baby alone with the mother for 10 hours per day. As you examine her son, you counsel the mother on how to care for her baby while also caring for herself. The mother immediately begins to sob into her hands and states: “I can’t do this anymore. I am not meant to be a mother.”
Major depressive disorder with peripartum onset – also known as postpartum depression – is a major public health concern that affects approximately 20% of women in industrial societies like the United States. It is among the most prevalent psychiatric disorders in the world and remains largely underdiagnosed because of lack of access to care, symptom underreporting secondary to stigma, and lack of education regarding illness.1 Adequate treatment of perinatal depression is of paramount importance, as this condition can have significant negative consequences for both mother and child.
Infants raised by depressed mothers show early disruptions in social and emotional development, including diminished security of attachment with their mothers and reduced ability to self-regulate.2 Later in development, the offspring of depressed mothers are at greater risk for psychopathology – most notably anxiety and depression as well as impaired social behavior. 3,4 Rates of depression in school-aged and adolescent children of depressed mothers have been reported to be between 20% and 41%.4 Not only are rates of depression higher, but depression in children of depressed parents, relative to depression in same-age children of nondepressed parents, has an earlier age of onset, longer duration, and is associated with greater functional impairment and risk of relapse.5
In addition, evidence shows that infants of depressed mothers show more negative affect and more self-directed regulatory behaviors, while toddlers show more dysregulated aggression and heightened mood lability.6 Given that these infants also already have an increased genetic risk for depression and anxiety, it is essential that mothers are identified and treated early to prevent these early disruptions to the parent-child relationship.
Pediatricians sit at the intersection of motherhood and infant development. This offers a unique opportunity to influence the trajectory of the child through bolstering supports for the mother. Understandably, time is limited during these brief touchpoints occurring over the first postpartum year, although a heartfelt “How are you?” can make all the difference. In asking this simple question in a disarming way, you may prevent multiple adverse childhood experiences for your tiniest patients.
Further, evidence has shown that toxic stress experienced during sensitive periods of brain development in infants and young children can negatively affect brain architecture. Brain pathways that are rarely used are pruned away, whereas pathways that are readily accessed grow stronger. If children are exposed to toxic stress, whether it be from abuse, mental illness of a caregiver such as severe maternal depression, witnessed domestic violence, or worse, they may begin to experience the world as dangerous and uncertain. This can strengthen connections in parts of the brain associated with fear, arousal, and emotional regulation at the cost of other parts of the brain associated with learning and safety.
Particularly focusing on infancy through preschool, children depend on sensitive, responsive caregivers to learn how to understand emotions and begin to self-soothe. Pediatricians have access to this critical period and can help lead the way toward secure attachment between mother and child. Through taking this dyadic, integrated approach, not only can downstream problems in the child be attenuated or even prevented (that is, disrupted social-emotional development and depression/anxiety), but a mother’s identity can form around her strengths in parenting rather than negative cognitive distortions. Here are some ways to quickly assess a mother for major depressive disorder with peripartum onset so that treatment can be secured, allowing children to develop and learn in a safe, supportive, loving environment:
- Add a standardized instrument to the check-in process during baby’s first year of life. The Edinburgh Postnatal Depression Scale (EPDS) is the most commonly used screening tool, consisting of 10 questions with a score of 10 or greater suggestive of maternal depression. Recently, it was found that the EPDS may be further abbreviated to a three-question version with a sensitivity of 95% and a negative predictive value of 98%.
- Dedicate 5 minutes during each appointment to ask the mother, in earnest, how she is doing and to create space to hear her concerns. This high-yield discussion can be the catalyst the mother needs to identify that something is not right.
- Obtain collateral information from the mother’s partner, if available, in a way that feels collaborative and supportive. You may ask the partner during the appointment if they have any concerns about how both parents are coping with their new parenting roles.
- If the mother has multiple risk factors for major depressive disorder with peripartum onset – past history of depression, family history of perinatal depression, lack of social supports, or past history of major depressive disorder with peripartum onset with an earlier child (elevating their risk to about 50%) – you may dedicate a bit more time to assess the patient and/or provide mental health resources directly upon wrapping up the appointment.
- Finally, you may add an educational blurb about major depressive disorder with peripartum onset in all after-visit summaries for new parents and infants with a list of mental health resources that includes reproductive psychiatrists, therapists, and a link to robust resources like Postpartum Support International.
By taking the extra step to leverage the relationship between mother and infant at this highly vulnerable time, you have the ability to positively affect the trajectory of a family. And, at the end of the day, this dyadic approach to patient care is the secret ingredient to improved outcomes all around.
References
1. Muzik M and Hamilton SE. Matern Child Health J. 2016;20(11):2268-79.
2. Granat A et al. Emotion. 2017;17(1):11-27.
3. Conroy S et al. J Am Acad Child Adolesc Psychiatry. 2012;51(1):51-61.
4. Goodman SH. Annu Rev Clin Psychol. 2007;3:107-35.
5. Keller MB et al. Arch Gen Psychiatry. 1986;43(10):930-7.
6. Tronick EZ and Gianino AF. New Dir Child Dev. 1986;34:5-11.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences, program director of the child and adolescent psychiatry fellowship, and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior in Los Angeles.
An overwhelmed mother presents to your office with her 2-month-old son for his check-up. She seems distant and dysphoric, often shrugging her shoulders with an empty stare when asked about her son’s development. Her baby cries loudly in her arms and you can see that she is uncomfortable soothing him as she frantically rocks him back and forth. He appears to have gained little weight since the last appointment occurring 6 days post partum and his mother describes him as “difficult and fussy all the time.” The father was unable to attend the appointment due to work obligations and often leaves the baby alone with the mother for 10 hours per day. As you examine her son, you counsel the mother on how to care for her baby while also caring for herself. The mother immediately begins to sob into her hands and states: “I can’t do this anymore. I am not meant to be a mother.”
Major depressive disorder with peripartum onset – also known as postpartum depression – is a major public health concern that affects approximately 20% of women in industrial societies like the United States. It is among the most prevalent psychiatric disorders in the world and remains largely underdiagnosed because of lack of access to care, symptom underreporting secondary to stigma, and lack of education regarding illness.1 Adequate treatment of perinatal depression is of paramount importance, as this condition can have significant negative consequences for both mother and child.
Infants raised by depressed mothers show early disruptions in social and emotional development, including diminished security of attachment with their mothers and reduced ability to self-regulate.2 Later in development, the offspring of depressed mothers are at greater risk for psychopathology – most notably anxiety and depression as well as impaired social behavior. 3,4 Rates of depression in school-aged and adolescent children of depressed mothers have been reported to be between 20% and 41%.4 Not only are rates of depression higher, but depression in children of depressed parents, relative to depression in same-age children of nondepressed parents, has an earlier age of onset, longer duration, and is associated with greater functional impairment and risk of relapse.5
In addition, evidence shows that infants of depressed mothers show more negative affect and more self-directed regulatory behaviors, while toddlers show more dysregulated aggression and heightened mood lability.6 Given that these infants also already have an increased genetic risk for depression and anxiety, it is essential that mothers are identified and treated early to prevent these early disruptions to the parent-child relationship.
Pediatricians sit at the intersection of motherhood and infant development. This offers a unique opportunity to influence the trajectory of the child through bolstering supports for the mother. Understandably, time is limited during these brief touchpoints occurring over the first postpartum year, although a heartfelt “How are you?” can make all the difference. In asking this simple question in a disarming way, you may prevent multiple adverse childhood experiences for your tiniest patients.
Further, evidence has shown that toxic stress experienced during sensitive periods of brain development in infants and young children can negatively affect brain architecture. Brain pathways that are rarely used are pruned away, whereas pathways that are readily accessed grow stronger. If children are exposed to toxic stress, whether it be from abuse, mental illness of a caregiver such as severe maternal depression, witnessed domestic violence, or worse, they may begin to experience the world as dangerous and uncertain. This can strengthen connections in parts of the brain associated with fear, arousal, and emotional regulation at the cost of other parts of the brain associated with learning and safety.
Particularly focusing on infancy through preschool, children depend on sensitive, responsive caregivers to learn how to understand emotions and begin to self-soothe. Pediatricians have access to this critical period and can help lead the way toward secure attachment between mother and child. Through taking this dyadic, integrated approach, not only can downstream problems in the child be attenuated or even prevented (that is, disrupted social-emotional development and depression/anxiety), but a mother’s identity can form around her strengths in parenting rather than negative cognitive distortions. Here are some ways to quickly assess a mother for major depressive disorder with peripartum onset so that treatment can be secured, allowing children to develop and learn in a safe, supportive, loving environment:
- Add a standardized instrument to the check-in process during baby’s first year of life. The Edinburgh Postnatal Depression Scale (EPDS) is the most commonly used screening tool, consisting of 10 questions with a score of 10 or greater suggestive of maternal depression. Recently, it was found that the EPDS may be further abbreviated to a three-question version with a sensitivity of 95% and a negative predictive value of 98%.
- Dedicate 5 minutes during each appointment to ask the mother, in earnest, how she is doing and to create space to hear her concerns. This high-yield discussion can be the catalyst the mother needs to identify that something is not right.
- Obtain collateral information from the mother’s partner, if available, in a way that feels collaborative and supportive. You may ask the partner during the appointment if they have any concerns about how both parents are coping with their new parenting roles.
- If the mother has multiple risk factors for major depressive disorder with peripartum onset – past history of depression, family history of perinatal depression, lack of social supports, or past history of major depressive disorder with peripartum onset with an earlier child (elevating their risk to about 50%) – you may dedicate a bit more time to assess the patient and/or provide mental health resources directly upon wrapping up the appointment.
- Finally, you may add an educational blurb about major depressive disorder with peripartum onset in all after-visit summaries for new parents and infants with a list of mental health resources that includes reproductive psychiatrists, therapists, and a link to robust resources like Postpartum Support International.
By taking the extra step to leverage the relationship between mother and infant at this highly vulnerable time, you have the ability to positively affect the trajectory of a family. And, at the end of the day, this dyadic approach to patient care is the secret ingredient to improved outcomes all around.
References
1. Muzik M and Hamilton SE. Matern Child Health J. 2016;20(11):2268-79.
2. Granat A et al. Emotion. 2017;17(1):11-27.
3. Conroy S et al. J Am Acad Child Adolesc Psychiatry. 2012;51(1):51-61.
4. Goodman SH. Annu Rev Clin Psychol. 2007;3:107-35.
5. Keller MB et al. Arch Gen Psychiatry. 1986;43(10):930-7.
6. Tronick EZ and Gianino AF. New Dir Child Dev. 1986;34:5-11.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences, program director of the child and adolescent psychiatry fellowship, and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior in Los Angeles.
An overwhelmed mother presents to your office with her 2-month-old son for his check-up. She seems distant and dysphoric, often shrugging her shoulders with an empty stare when asked about her son’s development. Her baby cries loudly in her arms and you can see that she is uncomfortable soothing him as she frantically rocks him back and forth. He appears to have gained little weight since the last appointment occurring 6 days post partum and his mother describes him as “difficult and fussy all the time.” The father was unable to attend the appointment due to work obligations and often leaves the baby alone with the mother for 10 hours per day. As you examine her son, you counsel the mother on how to care for her baby while also caring for herself. The mother immediately begins to sob into her hands and states: “I can’t do this anymore. I am not meant to be a mother.”
Major depressive disorder with peripartum onset – also known as postpartum depression – is a major public health concern that affects approximately 20% of women in industrial societies like the United States. It is among the most prevalent psychiatric disorders in the world and remains largely underdiagnosed because of lack of access to care, symptom underreporting secondary to stigma, and lack of education regarding illness.1 Adequate treatment of perinatal depression is of paramount importance, as this condition can have significant negative consequences for both mother and child.
Infants raised by depressed mothers show early disruptions in social and emotional development, including diminished security of attachment with their mothers and reduced ability to self-regulate.2 Later in development, the offspring of depressed mothers are at greater risk for psychopathology – most notably anxiety and depression as well as impaired social behavior. 3,4 Rates of depression in school-aged and adolescent children of depressed mothers have been reported to be between 20% and 41%.4 Not only are rates of depression higher, but depression in children of depressed parents, relative to depression in same-age children of nondepressed parents, has an earlier age of onset, longer duration, and is associated with greater functional impairment and risk of relapse.5
In addition, evidence shows that infants of depressed mothers show more negative affect and more self-directed regulatory behaviors, while toddlers show more dysregulated aggression and heightened mood lability.6 Given that these infants also already have an increased genetic risk for depression and anxiety, it is essential that mothers are identified and treated early to prevent these early disruptions to the parent-child relationship.
Pediatricians sit at the intersection of motherhood and infant development. This offers a unique opportunity to influence the trajectory of the child through bolstering supports for the mother. Understandably, time is limited during these brief touchpoints occurring over the first postpartum year, although a heartfelt “How are you?” can make all the difference. In asking this simple question in a disarming way, you may prevent multiple adverse childhood experiences for your tiniest patients.
Further, evidence has shown that toxic stress experienced during sensitive periods of brain development in infants and young children can negatively affect brain architecture. Brain pathways that are rarely used are pruned away, whereas pathways that are readily accessed grow stronger. If children are exposed to toxic stress, whether it be from abuse, mental illness of a caregiver such as severe maternal depression, witnessed domestic violence, or worse, they may begin to experience the world as dangerous and uncertain. This can strengthen connections in parts of the brain associated with fear, arousal, and emotional regulation at the cost of other parts of the brain associated with learning and safety.
Particularly focusing on infancy through preschool, children depend on sensitive, responsive caregivers to learn how to understand emotions and begin to self-soothe. Pediatricians have access to this critical period and can help lead the way toward secure attachment between mother and child. Through taking this dyadic, integrated approach, not only can downstream problems in the child be attenuated or even prevented (that is, disrupted social-emotional development and depression/anxiety), but a mother’s identity can form around her strengths in parenting rather than negative cognitive distortions. Here are some ways to quickly assess a mother for major depressive disorder with peripartum onset so that treatment can be secured, allowing children to develop and learn in a safe, supportive, loving environment:
- Add a standardized instrument to the check-in process during baby’s first year of life. The Edinburgh Postnatal Depression Scale (EPDS) is the most commonly used screening tool, consisting of 10 questions with a score of 10 or greater suggestive of maternal depression. Recently, it was found that the EPDS may be further abbreviated to a three-question version with a sensitivity of 95% and a negative predictive value of 98%.
- Dedicate 5 minutes during each appointment to ask the mother, in earnest, how she is doing and to create space to hear her concerns. This high-yield discussion can be the catalyst the mother needs to identify that something is not right.
- Obtain collateral information from the mother’s partner, if available, in a way that feels collaborative and supportive. You may ask the partner during the appointment if they have any concerns about how both parents are coping with their new parenting roles.
- If the mother has multiple risk factors for major depressive disorder with peripartum onset – past history of depression, family history of perinatal depression, lack of social supports, or past history of major depressive disorder with peripartum onset with an earlier child (elevating their risk to about 50%) – you may dedicate a bit more time to assess the patient and/or provide mental health resources directly upon wrapping up the appointment.
- Finally, you may add an educational blurb about major depressive disorder with peripartum onset in all after-visit summaries for new parents and infants with a list of mental health resources that includes reproductive psychiatrists, therapists, and a link to robust resources like Postpartum Support International.
By taking the extra step to leverage the relationship between mother and infant at this highly vulnerable time, you have the ability to positively affect the trajectory of a family. And, at the end of the day, this dyadic approach to patient care is the secret ingredient to improved outcomes all around.
References
1. Muzik M and Hamilton SE. Matern Child Health J. 2016;20(11):2268-79.
2. Granat A et al. Emotion. 2017;17(1):11-27.
3. Conroy S et al. J Am Acad Child Adolesc Psychiatry. 2012;51(1):51-61.
4. Goodman SH. Annu Rev Clin Psychol. 2007;3:107-35.
5. Keller MB et al. Arch Gen Psychiatry. 1986;43(10):930-7.
6. Tronick EZ and Gianino AF. New Dir Child Dev. 1986;34:5-11.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences, program director of the child and adolescent psychiatry fellowship, and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior in Los Angeles.
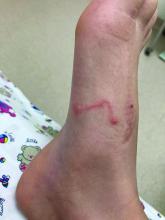
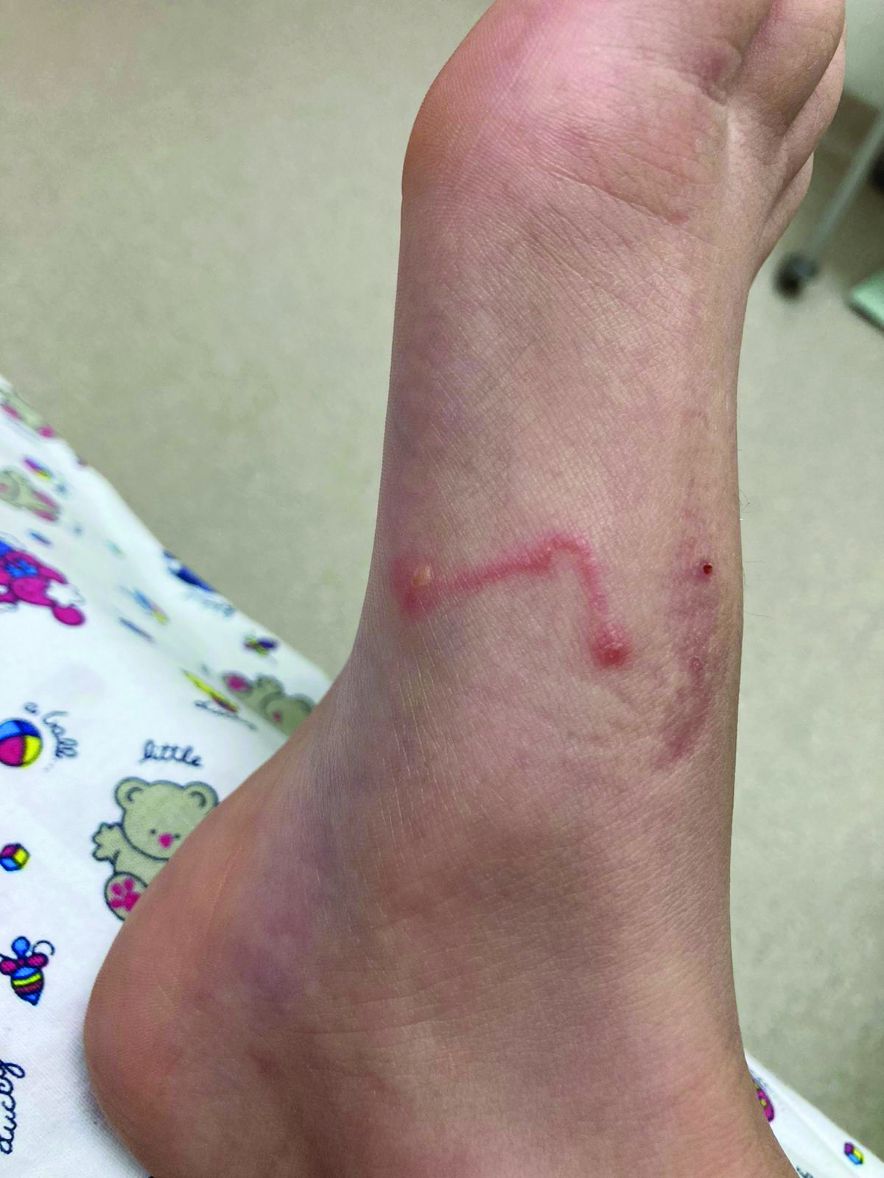
A 9-year-old girl was evaluated for a week-long history of rash on the feet
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
Her mother reported recent travel to a beachside city in Colombia. A review of systems was negative. She was not taking any other medications or vitamin supplements. There were no pets at home and no other affected family members. Physical exam was notable for an erythematous curvilinear plaque on the feet and a small vesicle.
Polio: The unwanted sequel
Summer, since 1975, is traditionally a time for the BIG blockbusters to hit theaters. Some are new, others are sequels in successful franchises. Some anticipated, some not as much.
And, in summer 2022, we have the least-wanted sequel in modern history – Polio II: The Return.
Of course, this sequel isn’t in the theaters (unless the concessions staff isn’t washing their hands), definitely isn’t funny, and could potentially cost a lot more money than the latest Marvel Cinematic Universe flick.
Personally and professionally, I’m in the middle generation on the disease. I’m young enough that I never had to worry about catching it or having afflicted classmates. But, as a doctor, I’m old enough to still see the consequences. Like most neurologists, I have a handful of patients who had childhood polio, and still deal with the chronic weakness (and consequent pain and orthopedic issues it brings). Signing off on braces and other mobility aids for them is still commonplace.
One of my attendings in residency was the renowned Parkinson’s disease expert Abraham Lieberman. On rounds it was impossible not to notice his marked limp, a consequence of childhood polio, and he’d tell us what it was like, being a 6-year-old boy and dealing with the disease. You learn as much from hearing firsthand experiences as you do from textbooks.
And now the virus is showing up again. A few victims, a lot of virions circulating in waste water, but it shouldn’t be there at all.
We aren’t in the era when schoolchildren died or were crippled by it. Elementary school kids today don’t see classmates catch polio and never return to school, or see their grieving parents.
To take 1 year: More than 3,000 American children died of polio in 1952, and more than 21,000 were left with lifelong paralysis – many of them still among us.
When you think of an iron lung, you think of polio.
Those were the casualties in a war to save future generations from this, along with smallpox and other horrors.
But today, that war is mostly forgotten. And now scientific evidence is drowned out by whatever’s on Facebook and the hard-earned miracle of vaccination is ignored in favor of a nonmedical “social influencer” on YouTube.
So The majority of the population likely has nothing to worry about. But there may be segments that are hit hard, and when they are they will never accept the obvious reasons why. It will be part of a cover-up, or a conspiracy, or whatever the guy on Parler told them it was.
As doctors, we’re in the middle. We have to give patients the best recommendations we can, based on learning, evidence, and experience, but at the same time have to recognize their autonomy. I’m not following someone around to make sure they get vaccinated, or take the medication I prescribed.
But we’re also the ones who can be held legally responsible for bad outcomes, regardless of the actual facts of the matter. On the flip side, you don’t hear about someone suing a Facebook “influencer” for doling out inaccurate, potentially fatal, medical advice.
So cracks appear in herd immunity, and leaks will happen.
A few generations of neurologists, including mine, have completed training without considering polio in a differential diagnosis. It would, of course, get bandied about in grand rounds or at the conference table, but none of us really took it seriously. To us residents it was more of historical note. “Gone with the Wind” and the “Wizard of Oz” both came out in 1939, and while we all knew of them, none of us were going to be watching them at the theaters.
Unlike them, though, polio is trying make it back to prime time. It’s a sequel nobody wanted.
But here it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Summer, since 1975, is traditionally a time for the BIG blockbusters to hit theaters. Some are new, others are sequels in successful franchises. Some anticipated, some not as much.
And, in summer 2022, we have the least-wanted sequel in modern history – Polio II: The Return.
Of course, this sequel isn’t in the theaters (unless the concessions staff isn’t washing their hands), definitely isn’t funny, and could potentially cost a lot more money than the latest Marvel Cinematic Universe flick.
Personally and professionally, I’m in the middle generation on the disease. I’m young enough that I never had to worry about catching it or having afflicted classmates. But, as a doctor, I’m old enough to still see the consequences. Like most neurologists, I have a handful of patients who had childhood polio, and still deal with the chronic weakness (and consequent pain and orthopedic issues it brings). Signing off on braces and other mobility aids for them is still commonplace.
One of my attendings in residency was the renowned Parkinson’s disease expert Abraham Lieberman. On rounds it was impossible not to notice his marked limp, a consequence of childhood polio, and he’d tell us what it was like, being a 6-year-old boy and dealing with the disease. You learn as much from hearing firsthand experiences as you do from textbooks.
And now the virus is showing up again. A few victims, a lot of virions circulating in waste water, but it shouldn’t be there at all.
We aren’t in the era when schoolchildren died or were crippled by it. Elementary school kids today don’t see classmates catch polio and never return to school, or see their grieving parents.
To take 1 year: More than 3,000 American children died of polio in 1952, and more than 21,000 were left with lifelong paralysis – many of them still among us.
When you think of an iron lung, you think of polio.
Those were the casualties in a war to save future generations from this, along with smallpox and other horrors.
But today, that war is mostly forgotten. And now scientific evidence is drowned out by whatever’s on Facebook and the hard-earned miracle of vaccination is ignored in favor of a nonmedical “social influencer” on YouTube.
So The majority of the population likely has nothing to worry about. But there may be segments that are hit hard, and when they are they will never accept the obvious reasons why. It will be part of a cover-up, or a conspiracy, or whatever the guy on Parler told them it was.
As doctors, we’re in the middle. We have to give patients the best recommendations we can, based on learning, evidence, and experience, but at the same time have to recognize their autonomy. I’m not following someone around to make sure they get vaccinated, or take the medication I prescribed.
But we’re also the ones who can be held legally responsible for bad outcomes, regardless of the actual facts of the matter. On the flip side, you don’t hear about someone suing a Facebook “influencer” for doling out inaccurate, potentially fatal, medical advice.
So cracks appear in herd immunity, and leaks will happen.
A few generations of neurologists, including mine, have completed training without considering polio in a differential diagnosis. It would, of course, get bandied about in grand rounds or at the conference table, but none of us really took it seriously. To us residents it was more of historical note. “Gone with the Wind” and the “Wizard of Oz” both came out in 1939, and while we all knew of them, none of us were going to be watching them at the theaters.
Unlike them, though, polio is trying make it back to prime time. It’s a sequel nobody wanted.
But here it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Summer, since 1975, is traditionally a time for the BIG blockbusters to hit theaters. Some are new, others are sequels in successful franchises. Some anticipated, some not as much.
And, in summer 2022, we have the least-wanted sequel in modern history – Polio II: The Return.
Of course, this sequel isn’t in the theaters (unless the concessions staff isn’t washing their hands), definitely isn’t funny, and could potentially cost a lot more money than the latest Marvel Cinematic Universe flick.
Personally and professionally, I’m in the middle generation on the disease. I’m young enough that I never had to worry about catching it or having afflicted classmates. But, as a doctor, I’m old enough to still see the consequences. Like most neurologists, I have a handful of patients who had childhood polio, and still deal with the chronic weakness (and consequent pain and orthopedic issues it brings). Signing off on braces and other mobility aids for them is still commonplace.
One of my attendings in residency was the renowned Parkinson’s disease expert Abraham Lieberman. On rounds it was impossible not to notice his marked limp, a consequence of childhood polio, and he’d tell us what it was like, being a 6-year-old boy and dealing with the disease. You learn as much from hearing firsthand experiences as you do from textbooks.
And now the virus is showing up again. A few victims, a lot of virions circulating in waste water, but it shouldn’t be there at all.
We aren’t in the era when schoolchildren died or were crippled by it. Elementary school kids today don’t see classmates catch polio and never return to school, or see their grieving parents.
To take 1 year: More than 3,000 American children died of polio in 1952, and more than 21,000 were left with lifelong paralysis – many of them still among us.
When you think of an iron lung, you think of polio.
Those were the casualties in a war to save future generations from this, along with smallpox and other horrors.
But today, that war is mostly forgotten. And now scientific evidence is drowned out by whatever’s on Facebook and the hard-earned miracle of vaccination is ignored in favor of a nonmedical “social influencer” on YouTube.
So The majority of the population likely has nothing to worry about. But there may be segments that are hit hard, and when they are they will never accept the obvious reasons why. It will be part of a cover-up, or a conspiracy, or whatever the guy on Parler told them it was.
As doctors, we’re in the middle. We have to give patients the best recommendations we can, based on learning, evidence, and experience, but at the same time have to recognize their autonomy. I’m not following someone around to make sure they get vaccinated, or take the medication I prescribed.
But we’re also the ones who can be held legally responsible for bad outcomes, regardless of the actual facts of the matter. On the flip side, you don’t hear about someone suing a Facebook “influencer” for doling out inaccurate, potentially fatal, medical advice.
So cracks appear in herd immunity, and leaks will happen.
A few generations of neurologists, including mine, have completed training without considering polio in a differential diagnosis. It would, of course, get bandied about in grand rounds or at the conference table, but none of us really took it seriously. To us residents it was more of historical note. “Gone with the Wind” and the “Wizard of Oz” both came out in 1939, and while we all knew of them, none of us were going to be watching them at the theaters.
Unlike them, though, polio is trying make it back to prime time. It’s a sequel nobody wanted.
But here it is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Acute otitis media pneumococcal disease burden in children due to serotypes not included in vaccines
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).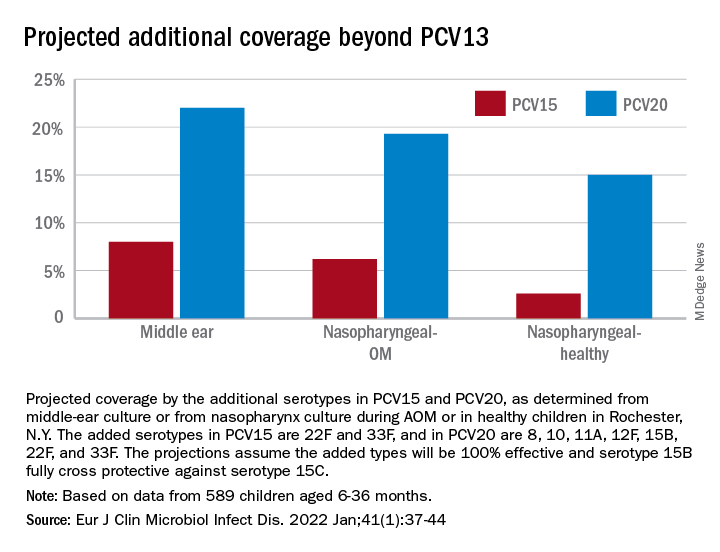
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
Growing pains? ... Rubbish
I’m pretty sure my ancestors came from Europe. And, as far as I know, I have no relatives in Australia. But, I must have some cosmic relationship with the Land Down Under because as I review articles for these columns I have an uncanny attraction to those coming out of Australia. Most of them are about sleep, one of my obsessions, and in general they address simple questions that no one has thought to ask.
My most recent Australia-based nugget appeared in the August edition of Pediatrics.
The researchers in Sidney were seeking to define “growing pains” by embarking on an extensive review of the medical literature. Beginning with thousands of articles, they winnowed these down to 145 studies. They found “there was extremely poor consensus between studies.” The most consistent components were the lower limb, bilaterality, evening onset, a normal physical assessment, and an episodic or recurrent course. However, all of these factors were mentioned in 50% or less of the articles they reviewed. The investigators wisely concluded that clinicians “should be wary of relying on the diagnosis to direct treatment decisions.”
This may seem like one small step for pediatrics. You may have reassured parents that none of your patients ever died of “growing pains” and the condition would eventually resolve. Hopefully, you were correct and that your case rate fatality is zero. But I suspect it wouldn’t take too long to unearth a wealth of malpractices cases in which another pediatrician’s patient died with an illness whose eventual discovery was tragically delayed by a period of false reassurance and diagnosis that the child merely had growing pains.
I can’t remember which of my sage instructors told me to never use “growing pains” as a diagnosis. It may have just been something I stumbled upon as my clinical experience grew. While holding firm to my commitment to never use it as a diagnosis, it became abundantly clear that I was seeing a large group of children (toddlers to early adolescents) who were experiencing lower leg pains in the early evening, often bad enough to wake them.
It took a bit longer to discover that most often these painful episodes occurred in children who were acutely or chronically sleep deprived. Occasionally, the pain would come on days in which the child had been unusually physically active. However, in most cases there was little correlation with lower limb activity.
I will admit that my observations were colored by my growing obsession that sleep deprivation is the root of many evils, including the phenomenon known as attention-deficit/hyperactivity disorder. I was even bold enough to include it in my one of the books I have written (Is My Child Overtired? Simon & Schuster, 2001). Nonetheless, I am still convinced that every investigation of a child with evening leg pains should include a thorough history of the child’s sleep history.
The bottom line is that these Australian researchers have done us a great favor with their research. However, I think they should have made a bolder statement in their conclusion. It is clear to me that “growing pains” should be removed as a diagnosis and no longer be reimbursed by third-party payers.
The void created by that action should spur some research into a better-defined diagnosis of the condition. If you want to use my tack and label it “nocturnal leg pains of childhood” and suggest better sleep hygiene, I will be flattered. But more importantly, take the time to take a good history, do a thorough exam, and then follow up, follow up, follow up, until the problem resolves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I’m pretty sure my ancestors came from Europe. And, as far as I know, I have no relatives in Australia. But, I must have some cosmic relationship with the Land Down Under because as I review articles for these columns I have an uncanny attraction to those coming out of Australia. Most of them are about sleep, one of my obsessions, and in general they address simple questions that no one has thought to ask.
My most recent Australia-based nugget appeared in the August edition of Pediatrics.
The researchers in Sidney were seeking to define “growing pains” by embarking on an extensive review of the medical literature. Beginning with thousands of articles, they winnowed these down to 145 studies. They found “there was extremely poor consensus between studies.” The most consistent components were the lower limb, bilaterality, evening onset, a normal physical assessment, and an episodic or recurrent course. However, all of these factors were mentioned in 50% or less of the articles they reviewed. The investigators wisely concluded that clinicians “should be wary of relying on the diagnosis to direct treatment decisions.”
This may seem like one small step for pediatrics. You may have reassured parents that none of your patients ever died of “growing pains” and the condition would eventually resolve. Hopefully, you were correct and that your case rate fatality is zero. But I suspect it wouldn’t take too long to unearth a wealth of malpractices cases in which another pediatrician’s patient died with an illness whose eventual discovery was tragically delayed by a period of false reassurance and diagnosis that the child merely had growing pains.
I can’t remember which of my sage instructors told me to never use “growing pains” as a diagnosis. It may have just been something I stumbled upon as my clinical experience grew. While holding firm to my commitment to never use it as a diagnosis, it became abundantly clear that I was seeing a large group of children (toddlers to early adolescents) who were experiencing lower leg pains in the early evening, often bad enough to wake them.
It took a bit longer to discover that most often these painful episodes occurred in children who were acutely or chronically sleep deprived. Occasionally, the pain would come on days in which the child had been unusually physically active. However, in most cases there was little correlation with lower limb activity.
I will admit that my observations were colored by my growing obsession that sleep deprivation is the root of many evils, including the phenomenon known as attention-deficit/hyperactivity disorder. I was even bold enough to include it in my one of the books I have written (Is My Child Overtired? Simon & Schuster, 2001). Nonetheless, I am still convinced that every investigation of a child with evening leg pains should include a thorough history of the child’s sleep history.
The bottom line is that these Australian researchers have done us a great favor with their research. However, I think they should have made a bolder statement in their conclusion. It is clear to me that “growing pains” should be removed as a diagnosis and no longer be reimbursed by third-party payers.
The void created by that action should spur some research into a better-defined diagnosis of the condition. If you want to use my tack and label it “nocturnal leg pains of childhood” and suggest better sleep hygiene, I will be flattered. But more importantly, take the time to take a good history, do a thorough exam, and then follow up, follow up, follow up, until the problem resolves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I’m pretty sure my ancestors came from Europe. And, as far as I know, I have no relatives in Australia. But, I must have some cosmic relationship with the Land Down Under because as I review articles for these columns I have an uncanny attraction to those coming out of Australia. Most of them are about sleep, one of my obsessions, and in general they address simple questions that no one has thought to ask.
My most recent Australia-based nugget appeared in the August edition of Pediatrics.
The researchers in Sidney were seeking to define “growing pains” by embarking on an extensive review of the medical literature. Beginning with thousands of articles, they winnowed these down to 145 studies. They found “there was extremely poor consensus between studies.” The most consistent components were the lower limb, bilaterality, evening onset, a normal physical assessment, and an episodic or recurrent course. However, all of these factors were mentioned in 50% or less of the articles they reviewed. The investigators wisely concluded that clinicians “should be wary of relying on the diagnosis to direct treatment decisions.”
This may seem like one small step for pediatrics. You may have reassured parents that none of your patients ever died of “growing pains” and the condition would eventually resolve. Hopefully, you were correct and that your case rate fatality is zero. But I suspect it wouldn’t take too long to unearth a wealth of malpractices cases in which another pediatrician’s patient died with an illness whose eventual discovery was tragically delayed by a period of false reassurance and diagnosis that the child merely had growing pains.
I can’t remember which of my sage instructors told me to never use “growing pains” as a diagnosis. It may have just been something I stumbled upon as my clinical experience grew. While holding firm to my commitment to never use it as a diagnosis, it became abundantly clear that I was seeing a large group of children (toddlers to early adolescents) who were experiencing lower leg pains in the early evening, often bad enough to wake them.
It took a bit longer to discover that most often these painful episodes occurred in children who were acutely or chronically sleep deprived. Occasionally, the pain would come on days in which the child had been unusually physically active. However, in most cases there was little correlation with lower limb activity.
I will admit that my observations were colored by my growing obsession that sleep deprivation is the root of many evils, including the phenomenon known as attention-deficit/hyperactivity disorder. I was even bold enough to include it in my one of the books I have written (Is My Child Overtired? Simon & Schuster, 2001). Nonetheless, I am still convinced that every investigation of a child with evening leg pains should include a thorough history of the child’s sleep history.
The bottom line is that these Australian researchers have done us a great favor with their research. However, I think they should have made a bolder statement in their conclusion. It is clear to me that “growing pains” should be removed as a diagnosis and no longer be reimbursed by third-party payers.
The void created by that action should spur some research into a better-defined diagnosis of the condition. If you want to use my tack and label it “nocturnal leg pains of childhood” and suggest better sleep hygiene, I will be flattered. But more importantly, take the time to take a good history, do a thorough exam, and then follow up, follow up, follow up, until the problem resolves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
First weeks back to school: An uneasy transition
Parents are relieved when school starts up again in the fall. Kids also are eager to see their friends and go on to the next level of learning.
Or are they?
This year brings a greater mix of feelings than usual for many families.
Many parents and children have new worries: Are children going to be safe at school from COVID, bullies, and shooters? Are they going to be ready to learn at this next level after the intermittent schooling of the past 2+ pandemic years of Zoom school, home school, or no school? Are they going to be able to separate after months of closeness/entanglement? Are they going to be able to catch up academically and fit in socially?
Children may have additional worries about how they have changed over the pandemic. Will my former friends still accept me now that I am heavier, showing puberty, experiencing acne, or feeling depressed or anxious?
While most of these worries occurred in some form after other summer breaks, they may be exacerbated by the length and degree of uncertainty we have all been through.
Often, health supervision visits are happy reunions with our patients when we hear about their growth and goals. We hope that is true this year, too, but we need to be vigilant and open to discussing the worries just mentioned.
What can we do to help ease this magnified transition?
First, we need to be open to their worries. Echoing back their concerns and noting how they are understandable and common can be reassuring when families have been isolated and missing interactions that might have made this clear. Second, we can remind them of the steps that assist in any transition. Now more than ever they need to collect information by visiting the new classroom, meeting teachers, and attending open house meet-and-greets. Older students may do better by looking over textbooks or a syllabus to see what will be covered. Making an effort to meet kids and families new to the school is a kind gesture but also helps the experienced child take some initiative and feel more confident.
Setting up an organizational system for homework from the start is valuable as work gets harder and is especially important for kids with ADHD. Single-subject folders, an assignment book tracking short-term and long-term projects, a plan for a specific homework time and place, a bookbag checklist by the door, or even a homework buddy and duplicate textbooks may be needed. Any kind of active steps toward organization can reduce anxiety.
Third, adjusting to the new schedule can take time. The most important adjustment is resetting the child’s sleep-wake cycle. You can recommend a move of 1 hour per day closer to the required wake up time and a corresponding bedtime that affords at least 8 hours (for tweens and teens; 9-12 hours for younger children), then maintaining the sleep schedule within 1 hour 7 days per week. Keep phones and tablets out of the bedroom. If children over 4 (including teens) have been napping over the summer, this needs to stop. Shifting mealtimes to fit the new schedule helps. Ensuring that lights are dimmed in the evening and bright in the morning has been shown to help the brain adjust.
A “new school year” is a good time for families to set new goals. Summer is often a time of fun, freedom, and new things. Parents may need your encouragement to exert leadership after months of cutting slack for their kids during COVID. Setting new goals such as greater responsibilities, music lessons, or household rules can be balanced by higher allowance and new earned privileges. Planning things to look forward to in the new year can be a family activity with a pleasant tone rather than just evoking protest. Suggest involving everyone in brainstorming crazy, out-of-the-box ideas (large and small) without censorship at first – for instance, go on a Mars mission; have pizza for breakfast; get yoga lessons; borrow binoculars to see Saturn; have a dog party! Everyone should be heard and their creativity celebrated. The list can then be narrowed down and marked on a calendar, starting soon.
Wait, you are hearing, how do we get our child off media to achieve this? Changing the rules about media use is never easy, and more now than ever. It is not just that kids are addicted to media, but it has been their main connection to peers during the pandemic. The “information” about/from peers, cliques, bullies, and world news may also be contributing to anxiety about returning to school. They may feel that they “need to know” even though it is upsetting. You can help kids verbalize the pros and cons of media use and possible addiction for themselves. How important media is to them needs to be acknowledged but ownership of the device and the final rules about this life-altering exposure must belong to the parents.
Sharing the AAP Family Media Plan to set proportions of time for school, homework, exercise, media (less than 2 hours for nonhomework), fun, and sleep can set an objective structure for the conversation. Parents may need to change their own media habits too!
While we pediatricians may normalize worries to reassure patients and parents, we also need to be alert to children and families in need of help. Many children have developed significant anxiety, depression, or substance use during the pandemic while out of our oversight but may not bring it up. Bereavement, which affected so many families during the pandemic, may not resolve smoothly. Families may have lost support, jobs, housing, or health insurance and need help connecting with assistance. Use of screening tools can ensure these are not missed, while remembering that functional impairment (social, academic, daily living, distress) is what differentiates normal from abnormal. We may be able to counsel them ourselves or refer them.
All this may be happening for you and your family, too. It can be difficult to assist others when we are struggling ourselves. We have been called on to cope when everything has been uncertain and our patients are sad, angry, or distrustful, with no end to the stress in sight. Sharing with colleagues, taking a break, or getting help for yourself may need to be a new goal for the school year, too.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Parents are relieved when school starts up again in the fall. Kids also are eager to see their friends and go on to the next level of learning.
Or are they?
This year brings a greater mix of feelings than usual for many families.
Many parents and children have new worries: Are children going to be safe at school from COVID, bullies, and shooters? Are they going to be ready to learn at this next level after the intermittent schooling of the past 2+ pandemic years of Zoom school, home school, or no school? Are they going to be able to separate after months of closeness/entanglement? Are they going to be able to catch up academically and fit in socially?
Children may have additional worries about how they have changed over the pandemic. Will my former friends still accept me now that I am heavier, showing puberty, experiencing acne, or feeling depressed or anxious?
While most of these worries occurred in some form after other summer breaks, they may be exacerbated by the length and degree of uncertainty we have all been through.
Often, health supervision visits are happy reunions with our patients when we hear about their growth and goals. We hope that is true this year, too, but we need to be vigilant and open to discussing the worries just mentioned.
What can we do to help ease this magnified transition?
First, we need to be open to their worries. Echoing back their concerns and noting how they are understandable and common can be reassuring when families have been isolated and missing interactions that might have made this clear. Second, we can remind them of the steps that assist in any transition. Now more than ever they need to collect information by visiting the new classroom, meeting teachers, and attending open house meet-and-greets. Older students may do better by looking over textbooks or a syllabus to see what will be covered. Making an effort to meet kids and families new to the school is a kind gesture but also helps the experienced child take some initiative and feel more confident.
Setting up an organizational system for homework from the start is valuable as work gets harder and is especially important for kids with ADHD. Single-subject folders, an assignment book tracking short-term and long-term projects, a plan for a specific homework time and place, a bookbag checklist by the door, or even a homework buddy and duplicate textbooks may be needed. Any kind of active steps toward organization can reduce anxiety.
Third, adjusting to the new schedule can take time. The most important adjustment is resetting the child’s sleep-wake cycle. You can recommend a move of 1 hour per day closer to the required wake up time and a corresponding bedtime that affords at least 8 hours (for tweens and teens; 9-12 hours for younger children), then maintaining the sleep schedule within 1 hour 7 days per week. Keep phones and tablets out of the bedroom. If children over 4 (including teens) have been napping over the summer, this needs to stop. Shifting mealtimes to fit the new schedule helps. Ensuring that lights are dimmed in the evening and bright in the morning has been shown to help the brain adjust.
A “new school year” is a good time for families to set new goals. Summer is often a time of fun, freedom, and new things. Parents may need your encouragement to exert leadership after months of cutting slack for their kids during COVID. Setting new goals such as greater responsibilities, music lessons, or household rules can be balanced by higher allowance and new earned privileges. Planning things to look forward to in the new year can be a family activity with a pleasant tone rather than just evoking protest. Suggest involving everyone in brainstorming crazy, out-of-the-box ideas (large and small) without censorship at first – for instance, go on a Mars mission; have pizza for breakfast; get yoga lessons; borrow binoculars to see Saturn; have a dog party! Everyone should be heard and their creativity celebrated. The list can then be narrowed down and marked on a calendar, starting soon.
Wait, you are hearing, how do we get our child off media to achieve this? Changing the rules about media use is never easy, and more now than ever. It is not just that kids are addicted to media, but it has been their main connection to peers during the pandemic. The “information” about/from peers, cliques, bullies, and world news may also be contributing to anxiety about returning to school. They may feel that they “need to know” even though it is upsetting. You can help kids verbalize the pros and cons of media use and possible addiction for themselves. How important media is to them needs to be acknowledged but ownership of the device and the final rules about this life-altering exposure must belong to the parents.
Sharing the AAP Family Media Plan to set proportions of time for school, homework, exercise, media (less than 2 hours for nonhomework), fun, and sleep can set an objective structure for the conversation. Parents may need to change their own media habits too!
While we pediatricians may normalize worries to reassure patients and parents, we also need to be alert to children and families in need of help. Many children have developed significant anxiety, depression, or substance use during the pandemic while out of our oversight but may not bring it up. Bereavement, which affected so many families during the pandemic, may not resolve smoothly. Families may have lost support, jobs, housing, or health insurance and need help connecting with assistance. Use of screening tools can ensure these are not missed, while remembering that functional impairment (social, academic, daily living, distress) is what differentiates normal from abnormal. We may be able to counsel them ourselves or refer them.
All this may be happening for you and your family, too. It can be difficult to assist others when we are struggling ourselves. We have been called on to cope when everything has been uncertain and our patients are sad, angry, or distrustful, with no end to the stress in sight. Sharing with colleagues, taking a break, or getting help for yourself may need to be a new goal for the school year, too.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Parents are relieved when school starts up again in the fall. Kids also are eager to see their friends and go on to the next level of learning.
Or are they?
This year brings a greater mix of feelings than usual for many families.
Many parents and children have new worries: Are children going to be safe at school from COVID, bullies, and shooters? Are they going to be ready to learn at this next level after the intermittent schooling of the past 2+ pandemic years of Zoom school, home school, or no school? Are they going to be able to separate after months of closeness/entanglement? Are they going to be able to catch up academically and fit in socially?
Children may have additional worries about how they have changed over the pandemic. Will my former friends still accept me now that I am heavier, showing puberty, experiencing acne, or feeling depressed or anxious?
While most of these worries occurred in some form after other summer breaks, they may be exacerbated by the length and degree of uncertainty we have all been through.
Often, health supervision visits are happy reunions with our patients when we hear about their growth and goals. We hope that is true this year, too, but we need to be vigilant and open to discussing the worries just mentioned.
What can we do to help ease this magnified transition?
First, we need to be open to their worries. Echoing back their concerns and noting how they are understandable and common can be reassuring when families have been isolated and missing interactions that might have made this clear. Second, we can remind them of the steps that assist in any transition. Now more than ever they need to collect information by visiting the new classroom, meeting teachers, and attending open house meet-and-greets. Older students may do better by looking over textbooks or a syllabus to see what will be covered. Making an effort to meet kids and families new to the school is a kind gesture but also helps the experienced child take some initiative and feel more confident.
Setting up an organizational system for homework from the start is valuable as work gets harder and is especially important for kids with ADHD. Single-subject folders, an assignment book tracking short-term and long-term projects, a plan for a specific homework time and place, a bookbag checklist by the door, or even a homework buddy and duplicate textbooks may be needed. Any kind of active steps toward organization can reduce anxiety.
Third, adjusting to the new schedule can take time. The most important adjustment is resetting the child’s sleep-wake cycle. You can recommend a move of 1 hour per day closer to the required wake up time and a corresponding bedtime that affords at least 8 hours (for tweens and teens; 9-12 hours for younger children), then maintaining the sleep schedule within 1 hour 7 days per week. Keep phones and tablets out of the bedroom. If children over 4 (including teens) have been napping over the summer, this needs to stop. Shifting mealtimes to fit the new schedule helps. Ensuring that lights are dimmed in the evening and bright in the morning has been shown to help the brain adjust.
A “new school year” is a good time for families to set new goals. Summer is often a time of fun, freedom, and new things. Parents may need your encouragement to exert leadership after months of cutting slack for their kids during COVID. Setting new goals such as greater responsibilities, music lessons, or household rules can be balanced by higher allowance and new earned privileges. Planning things to look forward to in the new year can be a family activity with a pleasant tone rather than just evoking protest. Suggest involving everyone in brainstorming crazy, out-of-the-box ideas (large and small) without censorship at first – for instance, go on a Mars mission; have pizza for breakfast; get yoga lessons; borrow binoculars to see Saturn; have a dog party! Everyone should be heard and their creativity celebrated. The list can then be narrowed down and marked on a calendar, starting soon.
Wait, you are hearing, how do we get our child off media to achieve this? Changing the rules about media use is never easy, and more now than ever. It is not just that kids are addicted to media, but it has been their main connection to peers during the pandemic. The “information” about/from peers, cliques, bullies, and world news may also be contributing to anxiety about returning to school. They may feel that they “need to know” even though it is upsetting. You can help kids verbalize the pros and cons of media use and possible addiction for themselves. How important media is to them needs to be acknowledged but ownership of the device and the final rules about this life-altering exposure must belong to the parents.
Sharing the AAP Family Media Plan to set proportions of time for school, homework, exercise, media (less than 2 hours for nonhomework), fun, and sleep can set an objective structure for the conversation. Parents may need to change their own media habits too!
While we pediatricians may normalize worries to reassure patients and parents, we also need to be alert to children and families in need of help. Many children have developed significant anxiety, depression, or substance use during the pandemic while out of our oversight but may not bring it up. Bereavement, which affected so many families during the pandemic, may not resolve smoothly. Families may have lost support, jobs, housing, or health insurance and need help connecting with assistance. Use of screening tools can ensure these are not missed, while remembering that functional impairment (social, academic, daily living, distress) is what differentiates normal from abnormal. We may be able to counsel them ourselves or refer them.
All this may be happening for you and your family, too. It can be difficult to assist others when we are struggling ourselves. We have been called on to cope when everything has been uncertain and our patients are sad, angry, or distrustful, with no end to the stress in sight. Sharing with colleagues, taking a break, or getting help for yourself may need to be a new goal for the school year, too.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Saddled with med school debt, yet left out of loan forgiveness plans
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.
The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
In a recently obtained plan by Politico, the Biden administration is zeroing in on a broad student loan forgiveness plan to be released imminently. The plan would broadly forgive $10,000 in federal student loans, including graduate and PLUS loans. However, there’s a rub: The plan restricts the forgiveness to those with incomes below $150,000.
This would unfairly exclude many in health care from receiving this forgiveness, an egregious oversight given how much health care providers have sacrificed during the pandemic.
What was proposed?
Previously, it was reported that the Biden administration was considering this same amount of forgiveness, but with plans to exclude borrowers by either career or income. Student loan payments have been on an extended CARES Act forbearance since March 2020, with payment resumption planned for Aug. 31. The administration has said that they would deliver a plan for further extensions before this date and have repeatedly teased including forgiveness.
Forgiveness for some ...
Forgiving $10,000 of federal student loans would relieve some 15 million borrowers of student debt, roughly one-third of the 45 million borrowers with debt.
This would provide a massive boost to these borrowers (who disproportionately are female, low-income, and non-White), many of whom were targeted by predatory institutions whose education didn’t offer any actual tangible benefit to their earnings. While this is a group that absolutely ought to have their loans forgiven, drawing an income line inappropriately restricts those in health care from receiving any forgiveness.
... But not for others
Someone making an annual gross income of $150,000 is in the 80th percentile of earners in the United States (for comparison, the top 1% took home more than $505,000 in 2021). What student loan borrowers make up the remaining 20%? Overwhelmingly, health care providers occupy that tier: physicians, dentists, veterinarians, and advanced-practice nurses.
These schools leave their graduates with some of the highest student loan burdens, with veterinarians, dentists, and physicians having the highest debt-to-income ratios of any professional careers.
Flat forgiveness is regressive
Forgiving any student debt is the right direction. Too may have fallen victim to an industry without quality control, appropriate regulation, or price control. Quite the opposite, the blank-check model of student loan financing has led to an arms race as it comes to capital improvements in university spending.

The price of medical schools has risen more than four times as fast as inflation over the past 30 years, with dental and veterinary schools and nursing education showing similarly exaggerated price increases. Trainees in these fields are more likely to have taken on six-figure debt, with average debt loads at graduation in the table below. While $10,000 will move the proverbial needle less for these borrowers, does that mean they should be excluded?
Health care workers’ income declines during the pandemic
Now, over 2½ years since the start of the COVID pandemic, multiple reports have demonstrated that health care workers have suffered a loss in income. This loss in income was never compensated for, as the Paycheck Protection Program and the individual economic stimuli typically excluded doctors and high earners.
COVID and the hazard tax
As a provider during the COVID-19 pandemic, I didn’t ask for hazard pay. I supported those who did but recognized their requests were more ceremonial than they were likely to be successful.
However, I flatly reject the idea that my fellow health care practitioners are not deserving of student loan forgiveness simply based on an arbitrary income threshold. Health care providers are saddled with high debt burden, have suffered lost income, and have given of themselves during a devastating pandemic, where more than 1 million perished in the United States.
Bottom line
Health care workers should not be excluded from student loan forgiveness. Sadly, the Biden administration has signaled that they are dropping career-based exclusions in favor of more broadly harmful income-based forgiveness restrictions. This will disproportionately harm physicians and other health care workers.
These practitioners have suffered financially as a result of working through the COVID pandemic; should they also be forced to shoulder another financial injury by being excluded from student loan forgiveness?
Dr. Palmer is the chief operating officer and cofounder of Panacea Financial. He is also a practicing pediatric hospitalist at Boston Children’s Hospital and is on faculty at Harvard Medical School, also in Boston.
A version of this article first appeared on Medscape.com.
The role of aspirin today
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the faculty of medicine at the University of Duisburg-Essen in Germany.
Usually in this video series, I report on interesting scientific studies in the field of neurology published in the last month. But I have to admit, June was a lousy month for new science in neurology. Therefore, this month I’d like to take a different approach and tell you about a very interesting, old drug.
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering aspirin’s antiplatelet activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study that showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the U.S. Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A history of efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin, compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective, compared with placebo, in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
Ladies and gentlemen, a drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany). A complete list of his financial disclosures is available at the link below.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the faculty of medicine at the University of Duisburg-Essen in Germany.
Usually in this video series, I report on interesting scientific studies in the field of neurology published in the last month. But I have to admit, June was a lousy month for new science in neurology. Therefore, this month I’d like to take a different approach and tell you about a very interesting, old drug.
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering aspirin’s antiplatelet activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study that showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the U.S. Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A history of efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin, compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective, compared with placebo, in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
Ladies and gentlemen, a drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany). A complete list of his financial disclosures is available at the link below.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the faculty of medicine at the University of Duisburg-Essen in Germany.
Usually in this video series, I report on interesting scientific studies in the field of neurology published in the last month. But I have to admit, June was a lousy month for new science in neurology. Therefore, this month I’d like to take a different approach and tell you about a very interesting, old drug.
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering aspirin’s antiplatelet activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study that showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the U.S. Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A history of efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin, compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective, compared with placebo, in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
Ladies and gentlemen, a drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany). A complete list of his financial disclosures is available at the link below.
A version of this article first appeared on Medscape.com.
No guarantees
Recently Sermo had an interesting case report. A young woman, a few hours after undergoing cupping and acupuncture to her upper back, developed dyspnea and presented to the emergency department. There she was found to have a pneumothorax requiring chest tube placement.
I’m certainly not an expert on pneumothoraces, cupping, or acupuncture. Maybe the occurrence is coincidental, though it certainly is temporally concerning.
If I were to cause a pneumothorax doing an electromyography and nerve conduction velocity of the chest wall or upper back, I’m sure I’d have a lot to answer for. Beyond just arranging care for the patient and explaining things to her and her family members, I’d probably have to deal with a board investigation and/or malpractice claim.
Yet, in my experience, people who provide such services rarely face legal accountability, whereas their counterparts in allopathic medicine regularly do so. How many late-night TV attorney ads have you seen that say “have you been injured by an acupuncturist?”
Me, neither.
I’m not going to go into the questions of whether acupuncture, or even cupping, do anything at all. But this case also raises the point that people tend to think of “alternative” medical treatments as things that, while of unclear benefit, are generally harmless.
The fact is that There probably never will be. No matter how well trained and intentioned the person doing it is, there is always the chance of something going wrong. Human error, mechanical failure, bad luck. As they say, dung happens.
In medicine we think about the risk-benefit ratio and proceed accordingly. But the risk, no matter how low, is never zero. People need to understand this applies to pretty much everything health-related. Even over-the-counter supplements, no matter how great their claims may sound (also unproven) have their issues.
Caveat emptor.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently Sermo had an interesting case report. A young woman, a few hours after undergoing cupping and acupuncture to her upper back, developed dyspnea and presented to the emergency department. There she was found to have a pneumothorax requiring chest tube placement.
I’m certainly not an expert on pneumothoraces, cupping, or acupuncture. Maybe the occurrence is coincidental, though it certainly is temporally concerning.
If I were to cause a pneumothorax doing an electromyography and nerve conduction velocity of the chest wall or upper back, I’m sure I’d have a lot to answer for. Beyond just arranging care for the patient and explaining things to her and her family members, I’d probably have to deal with a board investigation and/or malpractice claim.
Yet, in my experience, people who provide such services rarely face legal accountability, whereas their counterparts in allopathic medicine regularly do so. How many late-night TV attorney ads have you seen that say “have you been injured by an acupuncturist?”
Me, neither.
I’m not going to go into the questions of whether acupuncture, or even cupping, do anything at all. But this case also raises the point that people tend to think of “alternative” medical treatments as things that, while of unclear benefit, are generally harmless.
The fact is that There probably never will be. No matter how well trained and intentioned the person doing it is, there is always the chance of something going wrong. Human error, mechanical failure, bad luck. As they say, dung happens.
In medicine we think about the risk-benefit ratio and proceed accordingly. But the risk, no matter how low, is never zero. People need to understand this applies to pretty much everything health-related. Even over-the-counter supplements, no matter how great their claims may sound (also unproven) have their issues.
Caveat emptor.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently Sermo had an interesting case report. A young woman, a few hours after undergoing cupping and acupuncture to her upper back, developed dyspnea and presented to the emergency department. There she was found to have a pneumothorax requiring chest tube placement.
I’m certainly not an expert on pneumothoraces, cupping, or acupuncture. Maybe the occurrence is coincidental, though it certainly is temporally concerning.
If I were to cause a pneumothorax doing an electromyography and nerve conduction velocity of the chest wall or upper back, I’m sure I’d have a lot to answer for. Beyond just arranging care for the patient and explaining things to her and her family members, I’d probably have to deal with a board investigation and/or malpractice claim.
Yet, in my experience, people who provide such services rarely face legal accountability, whereas their counterparts in allopathic medicine regularly do so. How many late-night TV attorney ads have you seen that say “have you been injured by an acupuncturist?”
Me, neither.
I’m not going to go into the questions of whether acupuncture, or even cupping, do anything at all. But this case also raises the point that people tend to think of “alternative” medical treatments as things that, while of unclear benefit, are generally harmless.
The fact is that There probably never will be. No matter how well trained and intentioned the person doing it is, there is always the chance of something going wrong. Human error, mechanical failure, bad luck. As they say, dung happens.
In medicine we think about the risk-benefit ratio and proceed accordingly. But the risk, no matter how low, is never zero. People need to understand this applies to pretty much everything health-related. Even over-the-counter supplements, no matter how great their claims may sound (also unproven) have their issues.
Caveat emptor.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Endometriosis and infertility – Combining a chronic physical and emotional pain
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.