User login
Telemedicine in primary care
How to effectively utilize this tool
By now it is well known that the COVID-19 pandemic has significantly disrupted primary care. Office visits and revenues have precipitously dropped as physicians and patients alike fear in-person visits may increase their risks of contracting the virus. However, telemedicine has emerged as a lifeline of sorts for many practices, enabling them to conduct visits and maintain contact with patients.
Telemedicine is likely to continue to serve as a tool for primary care providers to improve access to convenient, cost-effective, high-quality care after the pandemic. Another benefit of telemedicine is it can help maintain a portion of a practice’s revenue stream for physicians during uncertain times.
Indeed, the nation has seen recent progress toward telemedicine parity, which refers to the concept of reimbursing providers’ telehealth visits at the same rates as similar in-person visits.
A challenge to adopting telemedicine is that it calls for adjusting established workflows for in-person encounters. A practice cannot simply replicate in-person processes to work for telehealth. While both in-person and virtual visits require adherence to HIPAA, for example, how you actually protect patient privacy will call for different measures. Harking back to the early days of EMR implementation, one does not need to like the telemedicine platform or process, but come to terms with the fact that it is a tool that is here to stay to deliver patient care.
Treat your practice like a laboratory
Adoption may vary between practices depending on many factors, including clinicians’ comfort with technology, clinical tolerance and triage rules for nontouch encounters, state regulations, and more. Every provider group should begin experimenting with telemedicine in specific ways that make sense for them.
One physician may practice telemedicine full-time while the rest abstain, or perhaps the practice prefers to offer telemedicine services during specific hours on specific days. Don’t be afraid to start slowly when you’re trying something new – but do get started with telehealth. It will increasingly be a mainstream medium and more patients will come to expect it.
Train the entire team
Many primary care practices do not enjoy the resources of an information technology team, so all team members essentially need to learn the new skill of telemedicine usage, in addition to assisting patients. That can’t happen without staff buy-in, so it is essential that everyone from the office manager to medical assistants have the training they need to make the technology work. Juggling schedules for telehealth and in-office, activating an account through email, starting and joining a telehealth meeting, and preparing a patient for a visit are just a handful of basic tasks your staff should be trained to do to contribute to the successful integration of telehealth.
Educate and encourage patients to use telehealth
While unfamiliarity with technology may represent a roadblock for some patients, others resist telemedicine simply because no one has explained to them why it’s so important and the benefits it can hold for them. Education and communication are critical, including the sometimes painstaking work of slowly walking patients through the process of performing important functions on the telemedicine app. By providing them with some friendly coaching, patients won’t feel lost or abandoned during what for some may be an unfamiliar and frustrating process.
Manage more behavioral health
Different states and health plans incentivize primary practices for integrating behavioral health into their offerings. Rather than dismiss this addition to your own practice as too cumbersome to take on, I would recommend using telehealth to expand behavioral health care services.
If your practice is working toward a team-based, interdisciplinary approach to care delivery, behavioral health is a critical component. While other elements of this “whole person” health care may be better suited for an office visit, the vast majority of behavioral health services can be delivered virtually.
To decide if your patient may benefit from behavioral health care, the primary care provider (PCP) can conduct a screening via telehealth. Once the screening is complete, the PCP can discuss results and refer the patient to a mental health professional – all via telehealth. While patients may be reluctant to receive behavioral health treatment, perhaps because of stigma or inexperience, they may appreciate the telemedicine option as they can remain in the comfort and familiarity of their homes.
Collaborative Care is both an in-person and virtual model that allows PCP practices to offer behavioral health services in a cost effective way by utilizing a psychiatrist as a “consultant” to the practice as opposed to hiring a full-time psychiatrist. All services within the Collaborative Care Model can be offered via telehealth, and all major insurance providers reimburse primary care providers for delivering Collaborative Care.
When PCPs provide behavioral health treatment as an “extension” of the primary care service offerings, the stigma is reduced and more patients are willing to accept the care they need.
Many areas of the country suffer from a lack of access to behavioral health specialists. In rural counties, for example, the nearest therapist may be located over an hour away. By integrating behavioral telehealth services into your practice’s offerings, you can remove geographic and transportation obstacles to care for your patient population.
Doing this can lead to providing more culturally competent care. It’s important that you’re able to offer mental health services to your patients from a professional with a similar ethnic or racial background. Language barriers and cultural differences may limit a provider’s ability to treat a patient, particularly if the patient faces health disparities related to race or ethnicity. If your practice needs to look outside of your community to tap into a more diverse pool of providers to better meet your patients’ needs, telehealth makes it easier to do that.
Adopting telemedicine for consultative patient visits offers primary care a path toward restoring patient volume and hope for a postpandemic future.
Mark Stephan, MD, is chief medical officer at Equality Health, a whole-health delivery system. He practiced family medicine for 19 years, including hospital medicine and obstetrics in rural and urban settings. Dr. Stephan has no conflicts related to the content of this piece.
How to effectively utilize this tool
How to effectively utilize this tool
By now it is well known that the COVID-19 pandemic has significantly disrupted primary care. Office visits and revenues have precipitously dropped as physicians and patients alike fear in-person visits may increase their risks of contracting the virus. However, telemedicine has emerged as a lifeline of sorts for many practices, enabling them to conduct visits and maintain contact with patients.
Telemedicine is likely to continue to serve as a tool for primary care providers to improve access to convenient, cost-effective, high-quality care after the pandemic. Another benefit of telemedicine is it can help maintain a portion of a practice’s revenue stream for physicians during uncertain times.
Indeed, the nation has seen recent progress toward telemedicine parity, which refers to the concept of reimbursing providers’ telehealth visits at the same rates as similar in-person visits.
A challenge to adopting telemedicine is that it calls for adjusting established workflows for in-person encounters. A practice cannot simply replicate in-person processes to work for telehealth. While both in-person and virtual visits require adherence to HIPAA, for example, how you actually protect patient privacy will call for different measures. Harking back to the early days of EMR implementation, one does not need to like the telemedicine platform or process, but come to terms with the fact that it is a tool that is here to stay to deliver patient care.
Treat your practice like a laboratory
Adoption may vary between practices depending on many factors, including clinicians’ comfort with technology, clinical tolerance and triage rules for nontouch encounters, state regulations, and more. Every provider group should begin experimenting with telemedicine in specific ways that make sense for them.
One physician may practice telemedicine full-time while the rest abstain, or perhaps the practice prefers to offer telemedicine services during specific hours on specific days. Don’t be afraid to start slowly when you’re trying something new – but do get started with telehealth. It will increasingly be a mainstream medium and more patients will come to expect it.
Train the entire team
Many primary care practices do not enjoy the resources of an information technology team, so all team members essentially need to learn the new skill of telemedicine usage, in addition to assisting patients. That can’t happen without staff buy-in, so it is essential that everyone from the office manager to medical assistants have the training they need to make the technology work. Juggling schedules for telehealth and in-office, activating an account through email, starting and joining a telehealth meeting, and preparing a patient for a visit are just a handful of basic tasks your staff should be trained to do to contribute to the successful integration of telehealth.
Educate and encourage patients to use telehealth
While unfamiliarity with technology may represent a roadblock for some patients, others resist telemedicine simply because no one has explained to them why it’s so important and the benefits it can hold for them. Education and communication are critical, including the sometimes painstaking work of slowly walking patients through the process of performing important functions on the telemedicine app. By providing them with some friendly coaching, patients won’t feel lost or abandoned during what for some may be an unfamiliar and frustrating process.
Manage more behavioral health
Different states and health plans incentivize primary practices for integrating behavioral health into their offerings. Rather than dismiss this addition to your own practice as too cumbersome to take on, I would recommend using telehealth to expand behavioral health care services.
If your practice is working toward a team-based, interdisciplinary approach to care delivery, behavioral health is a critical component. While other elements of this “whole person” health care may be better suited for an office visit, the vast majority of behavioral health services can be delivered virtually.
To decide if your patient may benefit from behavioral health care, the primary care provider (PCP) can conduct a screening via telehealth. Once the screening is complete, the PCP can discuss results and refer the patient to a mental health professional – all via telehealth. While patients may be reluctant to receive behavioral health treatment, perhaps because of stigma or inexperience, they may appreciate the telemedicine option as they can remain in the comfort and familiarity of their homes.
Collaborative Care is both an in-person and virtual model that allows PCP practices to offer behavioral health services in a cost effective way by utilizing a psychiatrist as a “consultant” to the practice as opposed to hiring a full-time psychiatrist. All services within the Collaborative Care Model can be offered via telehealth, and all major insurance providers reimburse primary care providers for delivering Collaborative Care.
When PCPs provide behavioral health treatment as an “extension” of the primary care service offerings, the stigma is reduced and more patients are willing to accept the care they need.
Many areas of the country suffer from a lack of access to behavioral health specialists. In rural counties, for example, the nearest therapist may be located over an hour away. By integrating behavioral telehealth services into your practice’s offerings, you can remove geographic and transportation obstacles to care for your patient population.
Doing this can lead to providing more culturally competent care. It’s important that you’re able to offer mental health services to your patients from a professional with a similar ethnic or racial background. Language barriers and cultural differences may limit a provider’s ability to treat a patient, particularly if the patient faces health disparities related to race or ethnicity. If your practice needs to look outside of your community to tap into a more diverse pool of providers to better meet your patients’ needs, telehealth makes it easier to do that.
Adopting telemedicine for consultative patient visits offers primary care a path toward restoring patient volume and hope for a postpandemic future.
Mark Stephan, MD, is chief medical officer at Equality Health, a whole-health delivery system. He practiced family medicine for 19 years, including hospital medicine and obstetrics in rural and urban settings. Dr. Stephan has no conflicts related to the content of this piece.
By now it is well known that the COVID-19 pandemic has significantly disrupted primary care. Office visits and revenues have precipitously dropped as physicians and patients alike fear in-person visits may increase their risks of contracting the virus. However, telemedicine has emerged as a lifeline of sorts for many practices, enabling them to conduct visits and maintain contact with patients.
Telemedicine is likely to continue to serve as a tool for primary care providers to improve access to convenient, cost-effective, high-quality care after the pandemic. Another benefit of telemedicine is it can help maintain a portion of a practice’s revenue stream for physicians during uncertain times.
Indeed, the nation has seen recent progress toward telemedicine parity, which refers to the concept of reimbursing providers’ telehealth visits at the same rates as similar in-person visits.
A challenge to adopting telemedicine is that it calls for adjusting established workflows for in-person encounters. A practice cannot simply replicate in-person processes to work for telehealth. While both in-person and virtual visits require adherence to HIPAA, for example, how you actually protect patient privacy will call for different measures. Harking back to the early days of EMR implementation, one does not need to like the telemedicine platform or process, but come to terms with the fact that it is a tool that is here to stay to deliver patient care.
Treat your practice like a laboratory
Adoption may vary between practices depending on many factors, including clinicians’ comfort with technology, clinical tolerance and triage rules for nontouch encounters, state regulations, and more. Every provider group should begin experimenting with telemedicine in specific ways that make sense for them.
One physician may practice telemedicine full-time while the rest abstain, or perhaps the practice prefers to offer telemedicine services during specific hours on specific days. Don’t be afraid to start slowly when you’re trying something new – but do get started with telehealth. It will increasingly be a mainstream medium and more patients will come to expect it.
Train the entire team
Many primary care practices do not enjoy the resources of an information technology team, so all team members essentially need to learn the new skill of telemedicine usage, in addition to assisting patients. That can’t happen without staff buy-in, so it is essential that everyone from the office manager to medical assistants have the training they need to make the technology work. Juggling schedules for telehealth and in-office, activating an account through email, starting and joining a telehealth meeting, and preparing a patient for a visit are just a handful of basic tasks your staff should be trained to do to contribute to the successful integration of telehealth.
Educate and encourage patients to use telehealth
While unfamiliarity with technology may represent a roadblock for some patients, others resist telemedicine simply because no one has explained to them why it’s so important and the benefits it can hold for them. Education and communication are critical, including the sometimes painstaking work of slowly walking patients through the process of performing important functions on the telemedicine app. By providing them with some friendly coaching, patients won’t feel lost or abandoned during what for some may be an unfamiliar and frustrating process.
Manage more behavioral health
Different states and health plans incentivize primary practices for integrating behavioral health into their offerings. Rather than dismiss this addition to your own practice as too cumbersome to take on, I would recommend using telehealth to expand behavioral health care services.
If your practice is working toward a team-based, interdisciplinary approach to care delivery, behavioral health is a critical component. While other elements of this “whole person” health care may be better suited for an office visit, the vast majority of behavioral health services can be delivered virtually.
To decide if your patient may benefit from behavioral health care, the primary care provider (PCP) can conduct a screening via telehealth. Once the screening is complete, the PCP can discuss results and refer the patient to a mental health professional – all via telehealth. While patients may be reluctant to receive behavioral health treatment, perhaps because of stigma or inexperience, they may appreciate the telemedicine option as they can remain in the comfort and familiarity of their homes.
Collaborative Care is both an in-person and virtual model that allows PCP practices to offer behavioral health services in a cost effective way by utilizing a psychiatrist as a “consultant” to the practice as opposed to hiring a full-time psychiatrist. All services within the Collaborative Care Model can be offered via telehealth, and all major insurance providers reimburse primary care providers for delivering Collaborative Care.
When PCPs provide behavioral health treatment as an “extension” of the primary care service offerings, the stigma is reduced and more patients are willing to accept the care they need.
Many areas of the country suffer from a lack of access to behavioral health specialists. In rural counties, for example, the nearest therapist may be located over an hour away. By integrating behavioral telehealth services into your practice’s offerings, you can remove geographic and transportation obstacles to care for your patient population.
Doing this can lead to providing more culturally competent care. It’s important that you’re able to offer mental health services to your patients from a professional with a similar ethnic or racial background. Language barriers and cultural differences may limit a provider’s ability to treat a patient, particularly if the patient faces health disparities related to race or ethnicity. If your practice needs to look outside of your community to tap into a more diverse pool of providers to better meet your patients’ needs, telehealth makes it easier to do that.
Adopting telemedicine for consultative patient visits offers primary care a path toward restoring patient volume and hope for a postpandemic future.
Mark Stephan, MD, is chief medical officer at Equality Health, a whole-health delivery system. He practiced family medicine for 19 years, including hospital medicine and obstetrics in rural and urban settings. Dr. Stephan has no conflicts related to the content of this piece.
Chronicles of cancer: A history of mammography, part 1
Technological imperatives
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
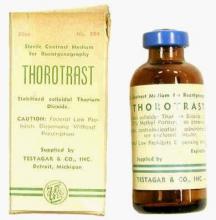
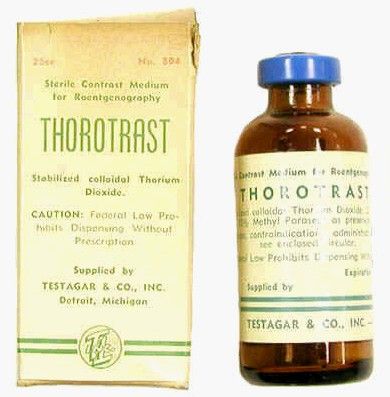
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Technological imperatives
Technological imperatives
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Welcome to HM20 Virtual
Welcome to the HM20 virtual conference! We’re glad to have you join us to virtually experience sessions from our most popular SHM annual conference tracks including Rapid Fire, Clinical Updates, and High-Value Care! We also have added some new timely topics given our current times that you won’t want to miss. We encourage you to engage with the larger community via social media at #HM20Virtual.
HM20 in San Diego, scheduled originally for April 2020, was trending to be the highest in-person attended SHM annual conference with a fantastic line-up of offerings. Unfortunately, then came our pandemic, or pandemics. In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 in San Diego because of the continued spread of COVID-19. Canceling the in-person conference during this unprecedented time was the right thing to do. I have valued the SHM leadership team and the larger SHM community for their support in being even more engaged on the front lines and with each other across our world during this time.
The COVID-19 pandemic has created a systemic challenge for health care systems across the nation. As hospitalists continue to be on the front lines of care and also innovations, organizations have leveraged telemedicine to support their patients, protect their clinicians, and conserve scarce resources. It is hospital medicine that has been on the front lines of change and adaptations and have led in this pandemic in many organizations across the nation and the world.
Unfortunately, known health disparities have also been amplified and there came an acute worsening of the chronic issues in this nation. On March 13, 2020, 26-year-old Breonna Taylor was shot after police forcibly entered her home. Armaud Arbery was shot and killed by armed neighbors while running through a neighborhood in Brunswick, Ga. Then on May 25, 5 miles from where I call home here in the Twin Cities in Minnesota, George Floyd, a 46-year-old father arrested for suspected use of a counterfeit $20 bill, died after police kneeled on his neck for over 8 minutes. This pandemic has also shaken up the status quo and laid bare a lot of our country’s long and deep-seated issues – from massive economic inequities to ongoing racial disparities to immigration concerns. It’s woken a lot of our valued hospitalists to the fact that the old ways of doing things just don’t work.
I’m grateful our society has taken steps to speak into these timely topics, and to share via publications, Twitter chats, advocacy items, and more! I want to encourage all of us to use the immense network of our hospitalist communities to comfort each other, learn, grow, and engage. We have not achieved big changes by ourselves. We’ve created valued offerings and innovative changes, and we’ve led on the front lines, in policies and procedures, by doing it together. Meaningful change requires allies in a common cause. We stand with our black and brown brothers and sisters who are particularly attuned to injustice, inequality, and struggle. We in hospital medicine stand up with many others who are struggling, our African American, Latin American, Native American, immigrant, LGBTQ+ communities. This intersection of the crisis of the COVID-19 pandemic and the racism pandemic have led us to a pivotal point in the arc of change and justice. I invite you to comfort each other, learn from each other, and act together in this community. To this end we have included timely resources in our HM20 virtual offering on these topics.
This year has been a big transition year. Not only did 2020 usher in a new decade, along with COVID-19 and our double pandemic, SHM has also had important transitions within its senior leadership. We say farewell to Larry Wellikson, MD, who has been at the helm of SHM since the beginning. On behalf of this annual conference, we want to celebrate and thank you, Larry, for your years of dedication and service to SHM. You have taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team may gather under a bigger tent for education, community, and for the betterment of care for our patients.
We extend a welcome to Eric Howell, MD, who succeeds Dr. Wellikson as SHM’s CEO. We also welcome Danielle Scheurer, MD, as the new SHM president, succeeding the great leadership offered this past year by Christopher Frost, MD. In addition, Jerome C. Siy, MD, was voted president-elect, Dr. Rachel Thompson, MD, was elected treasurer, Kris Rehm, MD, was voted secretary, and Darlene Tad-y, MD, was elected to the board of directors. We welcome these new officers.
HM20 Virtual will consist of prerecorded on-demand sessions that can be viewed at your convenience as well as live Q&A and attendee networking that will take place during specific dates/times. A few of the top-rated sessions from our historically popular tracks include: Update in Clinical Practice Guidelines, Antibiotics Made Ridiculously Simple, Getting to Know Oncology Emergencies, Inpatient Pain Management in the Era of the Opioid Epidemic, Updates in Heart Failure, and Hyponatremia: Don’t Drink the Water. Additionally, we have some of our perennial favorites including the Update in Hospital Medicine and Top Pediatric Articles of 2019. There will be COVID-19 specific content from expertise throughout the nation focusing on care pathways, clinical updates, telemedicine, point-of-care ultrasound, and more! To view the HM20 Virtual Opening Session and discover what you can expect in this educational experience, click here.
The Journal of Hospital Medicine has had a large presence in our meetings for many years. We are grateful for Samir Shah, MD, and his leadership during this double pandemic, for identifying areas where we can advance the field responsibly in the face of relatively limited evidence, and rapidly evolving news. As part of his commitment, all JHM articles related to COVID-19 and published during the pandemic are open access. A pre-COVID goal that has been realized during the pandemic was to bring more of the journal into our annual conference and the conference contents into the journal. We are proud to say this has been a great collaboration, particularly during this pandemic, and much thanks to Dr. Shah’s leadership for highlighting timely pieces. Kimberly Manning, MD, had an especially powerful piece on the topic of racism and our double pandemic, and she is a featured speaker during our HM20 Virtual offering, under the same title as her article: “When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics.” Additionally, Manpreet Malik, MD, and I will be copresenting on a timely topic about the “Immigrant Hospitalist during COVID-19.”
Aside from these sessions for HM20 Virtual, the real can’t miss(es) for the conference are the Research, Innovations, and Clinical Vignette (RIV) posters sessions. I am grateful for the leadership of Stephanie Mueller, MD, who served as chair for this year’s RIV. This unique year has led to the hosting of a virtual poster competition with judging and the opening of a virtual gallery. We are so pleased to be able to share and highlight the work of many of learners and staff hospitalists! I love that a hospitalist on one side of the country can help provide pearls on a case, an innovation, or a research idea that can help improve diagnosis for a patient at the other side of the country. Keep an eye on SHM’s social media and the presentation by Dr. Mueller for announcements of the winners.
A favorite reason many of us attend the annual conference is for the people and community. We wanted to keep this value as we shifted to a virtual offering. Networking will occur through a variety of offerings including Simulive sessions and Special Interest Forums. Simulive sessions will run for 3 weeks from August 11 to August 27. For those of you new to the term, Simulive may sound like a made-up word, but it is an actual amalgamation of a prerecorded webinar and a live interaction (simulated + live = Simulive). Simulive allows the faculty to sit in on their prerecorded session and interact with the audience via the chat feature during the live scheduled recording and spend time afterwards for a live Q&A from the audience.
There will also be over 20 Special Interest Forums hosted in the evenings after these Simulive sessions have concluded to give you a chance to connect with individuals, share experiences, and have meaningful discussions that can directly impact your practice. Samples of the forums include: Diversity and Inclusion, Rural Hospital Medicine, Pediatrics, NP/PA, Perioperative and Comanagement, Health Information Technology, and Point of Care Ultrasound! Take a look at the HM20 registration page for further information. You will receive direct information on how to attend. We encourage you to join!
HM20 also features a virtual 5K! Whether you run on a treadmill or jog in your neighborhood or local park, you can participate in HM20’s Virtual Fun Run or Walk. To participate, simply run your 5K during the weeks of HM20 Virtual and when you’re done, fill out our form to log your time. We encourage you to post a picture on social media as well with #HM20Virtual. You’ll also receive a certificate of completion at the close of HM20 Virtual.
All HM20 Virtual sessions will be available as on-demand after August 31. HM20 virtual offers more than 60 CME hours and over 35 MOC hours that you can claim at your convenience! That’s the most amount of CME and MOC ever offered at SHM for an event! This conference would not be possible without the tireless and relentless effort of SHM staff and leadership, our terrific speakers and faculty, and all the volunteer committee members of SHM. A huge thanks to the Annual Conference Committee who had the charge to develop the content for the Annual Conference, including topics, speakers, and learning objectives. I am grateful to have had the opportunity to serve on this committee for the past 7 years and to lead HM20 this year. Thanks to Brittany Evans, Hayleigh Lawrence, and Michelle Kann for their valued support this past year from an SHM staff perspective; to my assistant course director, Dan Steinberg, MD; and to the immediate past course director, Dustin Smith, MD, for their support.
Once again, we are excited to have you join, and we hope this conference elevates your education in hospital medicine, advances your career, stimulates innovative thinking, and provides you with enduring networking opportunities. We sincerely thank you for attending HM20 Virtual. Welcome!
Dr. Mathews is chief of hospital medicine at Regions Hospital, HealthPartners in St. Paul, Minn., an associate professor at the University of Minnesota, Minneapolis, and course director of HM20.
Welcome to the HM20 virtual conference! We’re glad to have you join us to virtually experience sessions from our most popular SHM annual conference tracks including Rapid Fire, Clinical Updates, and High-Value Care! We also have added some new timely topics given our current times that you won’t want to miss. We encourage you to engage with the larger community via social media at #HM20Virtual.
HM20 in San Diego, scheduled originally for April 2020, was trending to be the highest in-person attended SHM annual conference with a fantastic line-up of offerings. Unfortunately, then came our pandemic, or pandemics. In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 in San Diego because of the continued spread of COVID-19. Canceling the in-person conference during this unprecedented time was the right thing to do. I have valued the SHM leadership team and the larger SHM community for their support in being even more engaged on the front lines and with each other across our world during this time.
The COVID-19 pandemic has created a systemic challenge for health care systems across the nation. As hospitalists continue to be on the front lines of care and also innovations, organizations have leveraged telemedicine to support their patients, protect their clinicians, and conserve scarce resources. It is hospital medicine that has been on the front lines of change and adaptations and have led in this pandemic in many organizations across the nation and the world.
Unfortunately, known health disparities have also been amplified and there came an acute worsening of the chronic issues in this nation. On March 13, 2020, 26-year-old Breonna Taylor was shot after police forcibly entered her home. Armaud Arbery was shot and killed by armed neighbors while running through a neighborhood in Brunswick, Ga. Then on May 25, 5 miles from where I call home here in the Twin Cities in Minnesota, George Floyd, a 46-year-old father arrested for suspected use of a counterfeit $20 bill, died after police kneeled on his neck for over 8 minutes. This pandemic has also shaken up the status quo and laid bare a lot of our country’s long and deep-seated issues – from massive economic inequities to ongoing racial disparities to immigration concerns. It’s woken a lot of our valued hospitalists to the fact that the old ways of doing things just don’t work.
I’m grateful our society has taken steps to speak into these timely topics, and to share via publications, Twitter chats, advocacy items, and more! I want to encourage all of us to use the immense network of our hospitalist communities to comfort each other, learn, grow, and engage. We have not achieved big changes by ourselves. We’ve created valued offerings and innovative changes, and we’ve led on the front lines, in policies and procedures, by doing it together. Meaningful change requires allies in a common cause. We stand with our black and brown brothers and sisters who are particularly attuned to injustice, inequality, and struggle. We in hospital medicine stand up with many others who are struggling, our African American, Latin American, Native American, immigrant, LGBTQ+ communities. This intersection of the crisis of the COVID-19 pandemic and the racism pandemic have led us to a pivotal point in the arc of change and justice. I invite you to comfort each other, learn from each other, and act together in this community. To this end we have included timely resources in our HM20 virtual offering on these topics.
This year has been a big transition year. Not only did 2020 usher in a new decade, along with COVID-19 and our double pandemic, SHM has also had important transitions within its senior leadership. We say farewell to Larry Wellikson, MD, who has been at the helm of SHM since the beginning. On behalf of this annual conference, we want to celebrate and thank you, Larry, for your years of dedication and service to SHM. You have taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team may gather under a bigger tent for education, community, and for the betterment of care for our patients.
We extend a welcome to Eric Howell, MD, who succeeds Dr. Wellikson as SHM’s CEO. We also welcome Danielle Scheurer, MD, as the new SHM president, succeeding the great leadership offered this past year by Christopher Frost, MD. In addition, Jerome C. Siy, MD, was voted president-elect, Dr. Rachel Thompson, MD, was elected treasurer, Kris Rehm, MD, was voted secretary, and Darlene Tad-y, MD, was elected to the board of directors. We welcome these new officers.
HM20 Virtual will consist of prerecorded on-demand sessions that can be viewed at your convenience as well as live Q&A and attendee networking that will take place during specific dates/times. A few of the top-rated sessions from our historically popular tracks include: Update in Clinical Practice Guidelines, Antibiotics Made Ridiculously Simple, Getting to Know Oncology Emergencies, Inpatient Pain Management in the Era of the Opioid Epidemic, Updates in Heart Failure, and Hyponatremia: Don’t Drink the Water. Additionally, we have some of our perennial favorites including the Update in Hospital Medicine and Top Pediatric Articles of 2019. There will be COVID-19 specific content from expertise throughout the nation focusing on care pathways, clinical updates, telemedicine, point-of-care ultrasound, and more! To view the HM20 Virtual Opening Session and discover what you can expect in this educational experience, click here.
The Journal of Hospital Medicine has had a large presence in our meetings for many years. We are grateful for Samir Shah, MD, and his leadership during this double pandemic, for identifying areas where we can advance the field responsibly in the face of relatively limited evidence, and rapidly evolving news. As part of his commitment, all JHM articles related to COVID-19 and published during the pandemic are open access. A pre-COVID goal that has been realized during the pandemic was to bring more of the journal into our annual conference and the conference contents into the journal. We are proud to say this has been a great collaboration, particularly during this pandemic, and much thanks to Dr. Shah’s leadership for highlighting timely pieces. Kimberly Manning, MD, had an especially powerful piece on the topic of racism and our double pandemic, and she is a featured speaker during our HM20 Virtual offering, under the same title as her article: “When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics.” Additionally, Manpreet Malik, MD, and I will be copresenting on a timely topic about the “Immigrant Hospitalist during COVID-19.”
Aside from these sessions for HM20 Virtual, the real can’t miss(es) for the conference are the Research, Innovations, and Clinical Vignette (RIV) posters sessions. I am grateful for the leadership of Stephanie Mueller, MD, who served as chair for this year’s RIV. This unique year has led to the hosting of a virtual poster competition with judging and the opening of a virtual gallery. We are so pleased to be able to share and highlight the work of many of learners and staff hospitalists! I love that a hospitalist on one side of the country can help provide pearls on a case, an innovation, or a research idea that can help improve diagnosis for a patient at the other side of the country. Keep an eye on SHM’s social media and the presentation by Dr. Mueller for announcements of the winners.
A favorite reason many of us attend the annual conference is for the people and community. We wanted to keep this value as we shifted to a virtual offering. Networking will occur through a variety of offerings including Simulive sessions and Special Interest Forums. Simulive sessions will run for 3 weeks from August 11 to August 27. For those of you new to the term, Simulive may sound like a made-up word, but it is an actual amalgamation of a prerecorded webinar and a live interaction (simulated + live = Simulive). Simulive allows the faculty to sit in on their prerecorded session and interact with the audience via the chat feature during the live scheduled recording and spend time afterwards for a live Q&A from the audience.
There will also be over 20 Special Interest Forums hosted in the evenings after these Simulive sessions have concluded to give you a chance to connect with individuals, share experiences, and have meaningful discussions that can directly impact your practice. Samples of the forums include: Diversity and Inclusion, Rural Hospital Medicine, Pediatrics, NP/PA, Perioperative and Comanagement, Health Information Technology, and Point of Care Ultrasound! Take a look at the HM20 registration page for further information. You will receive direct information on how to attend. We encourage you to join!
HM20 also features a virtual 5K! Whether you run on a treadmill or jog in your neighborhood or local park, you can participate in HM20’s Virtual Fun Run or Walk. To participate, simply run your 5K during the weeks of HM20 Virtual and when you’re done, fill out our form to log your time. We encourage you to post a picture on social media as well with #HM20Virtual. You’ll also receive a certificate of completion at the close of HM20 Virtual.
All HM20 Virtual sessions will be available as on-demand after August 31. HM20 virtual offers more than 60 CME hours and over 35 MOC hours that you can claim at your convenience! That’s the most amount of CME and MOC ever offered at SHM for an event! This conference would not be possible without the tireless and relentless effort of SHM staff and leadership, our terrific speakers and faculty, and all the volunteer committee members of SHM. A huge thanks to the Annual Conference Committee who had the charge to develop the content for the Annual Conference, including topics, speakers, and learning objectives. I am grateful to have had the opportunity to serve on this committee for the past 7 years and to lead HM20 this year. Thanks to Brittany Evans, Hayleigh Lawrence, and Michelle Kann for their valued support this past year from an SHM staff perspective; to my assistant course director, Dan Steinberg, MD; and to the immediate past course director, Dustin Smith, MD, for their support.
Once again, we are excited to have you join, and we hope this conference elevates your education in hospital medicine, advances your career, stimulates innovative thinking, and provides you with enduring networking opportunities. We sincerely thank you for attending HM20 Virtual. Welcome!
Dr. Mathews is chief of hospital medicine at Regions Hospital, HealthPartners in St. Paul, Minn., an associate professor at the University of Minnesota, Minneapolis, and course director of HM20.
Welcome to the HM20 virtual conference! We’re glad to have you join us to virtually experience sessions from our most popular SHM annual conference tracks including Rapid Fire, Clinical Updates, and High-Value Care! We also have added some new timely topics given our current times that you won’t want to miss. We encourage you to engage with the larger community via social media at #HM20Virtual.
HM20 in San Diego, scheduled originally for April 2020, was trending to be the highest in-person attended SHM annual conference with a fantastic line-up of offerings. Unfortunately, then came our pandemic, or pandemics. In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 in San Diego because of the continued spread of COVID-19. Canceling the in-person conference during this unprecedented time was the right thing to do. I have valued the SHM leadership team and the larger SHM community for their support in being even more engaged on the front lines and with each other across our world during this time.
The COVID-19 pandemic has created a systemic challenge for health care systems across the nation. As hospitalists continue to be on the front lines of care and also innovations, organizations have leveraged telemedicine to support their patients, protect their clinicians, and conserve scarce resources. It is hospital medicine that has been on the front lines of change and adaptations and have led in this pandemic in many organizations across the nation and the world.
Unfortunately, known health disparities have also been amplified and there came an acute worsening of the chronic issues in this nation. On March 13, 2020, 26-year-old Breonna Taylor was shot after police forcibly entered her home. Armaud Arbery was shot and killed by armed neighbors while running through a neighborhood in Brunswick, Ga. Then on May 25, 5 miles from where I call home here in the Twin Cities in Minnesota, George Floyd, a 46-year-old father arrested for suspected use of a counterfeit $20 bill, died after police kneeled on his neck for over 8 minutes. This pandemic has also shaken up the status quo and laid bare a lot of our country’s long and deep-seated issues – from massive economic inequities to ongoing racial disparities to immigration concerns. It’s woken a lot of our valued hospitalists to the fact that the old ways of doing things just don’t work.
I’m grateful our society has taken steps to speak into these timely topics, and to share via publications, Twitter chats, advocacy items, and more! I want to encourage all of us to use the immense network of our hospitalist communities to comfort each other, learn, grow, and engage. We have not achieved big changes by ourselves. We’ve created valued offerings and innovative changes, and we’ve led on the front lines, in policies and procedures, by doing it together. Meaningful change requires allies in a common cause. We stand with our black and brown brothers and sisters who are particularly attuned to injustice, inequality, and struggle. We in hospital medicine stand up with many others who are struggling, our African American, Latin American, Native American, immigrant, LGBTQ+ communities. This intersection of the crisis of the COVID-19 pandemic and the racism pandemic have led us to a pivotal point in the arc of change and justice. I invite you to comfort each other, learn from each other, and act together in this community. To this end we have included timely resources in our HM20 virtual offering on these topics.
This year has been a big transition year. Not only did 2020 usher in a new decade, along with COVID-19 and our double pandemic, SHM has also had important transitions within its senior leadership. We say farewell to Larry Wellikson, MD, who has been at the helm of SHM since the beginning. On behalf of this annual conference, we want to celebrate and thank you, Larry, for your years of dedication and service to SHM. You have taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team may gather under a bigger tent for education, community, and for the betterment of care for our patients.
We extend a welcome to Eric Howell, MD, who succeeds Dr. Wellikson as SHM’s CEO. We also welcome Danielle Scheurer, MD, as the new SHM president, succeeding the great leadership offered this past year by Christopher Frost, MD. In addition, Jerome C. Siy, MD, was voted president-elect, Dr. Rachel Thompson, MD, was elected treasurer, Kris Rehm, MD, was voted secretary, and Darlene Tad-y, MD, was elected to the board of directors. We welcome these new officers.
HM20 Virtual will consist of prerecorded on-demand sessions that can be viewed at your convenience as well as live Q&A and attendee networking that will take place during specific dates/times. A few of the top-rated sessions from our historically popular tracks include: Update in Clinical Practice Guidelines, Antibiotics Made Ridiculously Simple, Getting to Know Oncology Emergencies, Inpatient Pain Management in the Era of the Opioid Epidemic, Updates in Heart Failure, and Hyponatremia: Don’t Drink the Water. Additionally, we have some of our perennial favorites including the Update in Hospital Medicine and Top Pediatric Articles of 2019. There will be COVID-19 specific content from expertise throughout the nation focusing on care pathways, clinical updates, telemedicine, point-of-care ultrasound, and more! To view the HM20 Virtual Opening Session and discover what you can expect in this educational experience, click here.
The Journal of Hospital Medicine has had a large presence in our meetings for many years. We are grateful for Samir Shah, MD, and his leadership during this double pandemic, for identifying areas where we can advance the field responsibly in the face of relatively limited evidence, and rapidly evolving news. As part of his commitment, all JHM articles related to COVID-19 and published during the pandemic are open access. A pre-COVID goal that has been realized during the pandemic was to bring more of the journal into our annual conference and the conference contents into the journal. We are proud to say this has been a great collaboration, particularly during this pandemic, and much thanks to Dr. Shah’s leadership for highlighting timely pieces. Kimberly Manning, MD, had an especially powerful piece on the topic of racism and our double pandemic, and she is a featured speaker during our HM20 Virtual offering, under the same title as her article: “When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics.” Additionally, Manpreet Malik, MD, and I will be copresenting on a timely topic about the “Immigrant Hospitalist during COVID-19.”
Aside from these sessions for HM20 Virtual, the real can’t miss(es) for the conference are the Research, Innovations, and Clinical Vignette (RIV) posters sessions. I am grateful for the leadership of Stephanie Mueller, MD, who served as chair for this year’s RIV. This unique year has led to the hosting of a virtual poster competition with judging and the opening of a virtual gallery. We are so pleased to be able to share and highlight the work of many of learners and staff hospitalists! I love that a hospitalist on one side of the country can help provide pearls on a case, an innovation, or a research idea that can help improve diagnosis for a patient at the other side of the country. Keep an eye on SHM’s social media and the presentation by Dr. Mueller for announcements of the winners.
A favorite reason many of us attend the annual conference is for the people and community. We wanted to keep this value as we shifted to a virtual offering. Networking will occur through a variety of offerings including Simulive sessions and Special Interest Forums. Simulive sessions will run for 3 weeks from August 11 to August 27. For those of you new to the term, Simulive may sound like a made-up word, but it is an actual amalgamation of a prerecorded webinar and a live interaction (simulated + live = Simulive). Simulive allows the faculty to sit in on their prerecorded session and interact with the audience via the chat feature during the live scheduled recording and spend time afterwards for a live Q&A from the audience.
There will also be over 20 Special Interest Forums hosted in the evenings after these Simulive sessions have concluded to give you a chance to connect with individuals, share experiences, and have meaningful discussions that can directly impact your practice. Samples of the forums include: Diversity and Inclusion, Rural Hospital Medicine, Pediatrics, NP/PA, Perioperative and Comanagement, Health Information Technology, and Point of Care Ultrasound! Take a look at the HM20 registration page for further information. You will receive direct information on how to attend. We encourage you to join!
HM20 also features a virtual 5K! Whether you run on a treadmill or jog in your neighborhood or local park, you can participate in HM20’s Virtual Fun Run or Walk. To participate, simply run your 5K during the weeks of HM20 Virtual and when you’re done, fill out our form to log your time. We encourage you to post a picture on social media as well with #HM20Virtual. You’ll also receive a certificate of completion at the close of HM20 Virtual.
All HM20 Virtual sessions will be available as on-demand after August 31. HM20 virtual offers more than 60 CME hours and over 35 MOC hours that you can claim at your convenience! That’s the most amount of CME and MOC ever offered at SHM for an event! This conference would not be possible without the tireless and relentless effort of SHM staff and leadership, our terrific speakers and faculty, and all the volunteer committee members of SHM. A huge thanks to the Annual Conference Committee who had the charge to develop the content for the Annual Conference, including topics, speakers, and learning objectives. I am grateful to have had the opportunity to serve on this committee for the past 7 years and to lead HM20 this year. Thanks to Brittany Evans, Hayleigh Lawrence, and Michelle Kann for their valued support this past year from an SHM staff perspective; to my assistant course director, Dan Steinberg, MD; and to the immediate past course director, Dustin Smith, MD, for their support.
Once again, we are excited to have you join, and we hope this conference elevates your education in hospital medicine, advances your career, stimulates innovative thinking, and provides you with enduring networking opportunities. We sincerely thank you for attending HM20 Virtual. Welcome!
Dr. Mathews is chief of hospital medicine at Regions Hospital, HealthPartners in St. Paul, Minn., an associate professor at the University of Minnesota, Minneapolis, and course director of HM20.
When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Exploring cannabis use by older adults
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
A doctor conquers his demons
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Web-based fellowship interviews in the era of COVID 19: Tips and tricks
Fellowship interviews are an essential step – arguably the most important step – in the process of matching candidates to training programs. Until recently, most programs relied exclusively on on-site face-to face interviews. Since the appearance of the COVID-19 pandemic, the medical field has utilized web-based platforms. Despite inherent limitations, virtual meetings appear to be effective in providing patient care and in conducting administrative meetings.1,2
Because of uncertainty related to the pandemic, including changing guidelines regarding social distancing and travel restrictions, fellowship programs are expected to comply with CDC,3 state, and federal recommendations to avoid nonessential travel. Therefore, conducting web-based interviews exclusively will likely become a necessity.
While there may be some disadvantages to web-based interviews, many candidates find the overall experience satisfactory, thereby allowing them to understand the programs, express themselves, and comfortably rank the programs, as two studies have shown.4,5 Programs and candidates are encouraged to adapt to this new reality in order to achieve a successful match. After all, there are many potential advantages of web-based interviews. In addition to eliminating the risk of COVID-19 acquisition, web-based interviews have been described as helping to improve scheduling flexibility, reduce the financial burden, and allow conducting more interviews for candidates and programs (Table 1).6-8
There are different styles to the web-based interview.9 Some programs choose to offer a single group interview (or the so-called panel interview) in which all the interviewing faculties invite each candidate at a time. Alternatively, programs might choose to conduct separate interviews by each faculty in which the candidates would alternate. For the latter option, the program could use a single invitation link or multiple invitation links for each session.
General tips for a successful interview:9
1. Be pleasant and professional: Your communication with the program should reflect excellent manners and a professional attitude with everyone (i.e., faculties, coordinators, and fellows).
2. Know yourself and what you want: Review your CV and personal statement and reflect on your achievements, strengths and weaknesses. Identify examples from your experience that would speak well of you as a person and as a physician.
3. Communication is key:
- Respond to the interview invitations promptly.
- Send a brief thank you email to the interviewers and the coordinator. Avoid being generic; mention specific points of discussion and show your interest in the program.
- Proofread your emails carefully. Well-written emails that are devoid of grammatical or spelling errors send a positive message about the candidate.
4. Do your homework:
- Read the information posted on the website carefully and take notes. This should provide you with useful information to use when you rank the programs and could lead to questions that you might want to ask your interviewers. Besides, asking questions that are answered on the website reflects poorly on the candidate.
- Pay attention to various clues that could reflect how organized and how academically oriented a program is. For example, a program that provides details about their didactic lectures sends a message that quality teaching is a priority. On the other hand, a program that has a website that hasn’t been updated for years could dissuade rather than recruit applicants.
- Read about the faculty, their areas of interests, and publications. Learn how their names are pronounced and use them during the interview.
- Read about the city where the program is. It shows interest in the area where you might be living and will help you to stand out among candidates.
5. Be prepared for the classics; be honest, genuine, and authentic. Think about these common prompts:
- Tell me about yourself.
- Why did you choose gastroenterology?
- Where do you see yourself in 5 years?
- Why would you like to come to the city where the program is?
- Are there any certain areas in gastroenterology that you’re interested in more (e.g., hepatology, motility, IBD, advanced endoscopy)?
6. It is likely your interviewer will ask if you have questions. Ask questions that further allow you to assess the program and your fit into the program.
- What aspects of the program are you most proud of?
- Where would you like to see this program in 5 years?
- What keeps you at this program?
Tips for a successful web-based interview9,10 (Table 2):
1. Pay attention to the time zone of the city of the program. Be ready at least 10 minutes before the interview.
2. Ensure a fast and stable Internet service for an uninterrupted interview experience. Consider using an ethernet cable. Have a back-up plan such as using a phone as a hotspot.
3. Use a quiet and private room, preferably at home. Be aware of the background. A simple decoration is acceptable.
4. Consider recording yourself using the same device you’ll use for the interview to make sure audio and video are functioning properly.
5. When scheduling more than one interview in 1 day, allow at least 2 hours between interviews to avoid scheduling conflicts caused by unanticipated delays related to technical issues.
6. Have immediate access to the invitation link(s) that you received. Add the interviews to your device’s calendar. Note that sometimes a new invitation link is generated last minute because of technical issues.
7. While the advice for physical interviews is to turn off your phone (and smart watch), you’ll have to keep your phone on but on silent for the virtual interviews. Sometimes, you’ll receive a phone call from the program to update you about any last-minute changes.
7. It is recommended that a laptop or a tablet with a camera with good resolution and a microphone be used rather than using a phone. A wide screen allows better communication. Disable notification on that device to avoid interruptions.
8. Sit comfortably with the device being at or just below eye level. Avoid distractions and maintain eye contact.
9. Familiarize yourself with the platform used and its functions. Double check the audio and video before each interview.
10. Put your device on a desk or table to improve stability; don’t hold it in your hand.
11. Find a place where the view is best and your face appears in the middle of the screen; not too far or too close. Use a well-lit room but don’t have a source of light behind you. Many platforms allow you to select a background or blur the background. A background that is monochromatic and not distracting is recommended.
12. Use a pen and a paper to take notes during the interview. You would use these notes to generate “thank you” or “interest in program” emails. Additionally, they will be a helpful reference when ranking programs.
13. Do not type. Typing is much louder to the interviewer and can be distracting.
14. Dress professionally, just as you dress for an on-site interview. You never know when you might have to stand up during the interview for unplanned reasons.
15. Do a practice interview. Have a colleague set up a virtual web session using any available platform. This will allow you to get feedback on your dress, background, acoustics, and general ability to answer questions.
References
1. Dig Dis Sci. 2019;64:1150-7.
2. BMJ Open. 2017;7:e016242.
3. Centers for Disease Control and Prevention. Coronavirus and Travel in the United States, 2020.
4. J Bone Joint Surg Am. 2017;99:e114.
5. Am J Gastroenterol. 2014;109:155-9.
6. West J Emerg Med. 2018;19:80-6.
7. Int J Med Educ. 2016;7:102-8.
8. Aparajit Naram M. How COVID-19 changed our fellowship interview process for the better. KevinMD.com. April 17, 2020.
9. Association of American Medical Colleges. Virtual interviews: Tips for medical school applicants, 2020. Updated May 14, 2020.
10. Top 5 video interviewing tips for residency and fellowship programs. Thalamus: Connecting the Docs. April 2, 2020, 2020.
Dr. Kiwan is chief fellow in gastroenterology, division of gastroenterology, at Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center, all in Detroit. Dr. Judd is an assistant professor and associate program director in the division of gastroenterology, Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center. Dr. Al Masalmeh is in the department of internal medicine, Wayne State University, Detroit Medical Center. Dr. Levine is professor and the vice chair for education, Wayne State University, Detroit Medical Center.
Fellowship interviews are an essential step – arguably the most important step – in the process of matching candidates to training programs. Until recently, most programs relied exclusively on on-site face-to face interviews. Since the appearance of the COVID-19 pandemic, the medical field has utilized web-based platforms. Despite inherent limitations, virtual meetings appear to be effective in providing patient care and in conducting administrative meetings.1,2
Because of uncertainty related to the pandemic, including changing guidelines regarding social distancing and travel restrictions, fellowship programs are expected to comply with CDC,3 state, and federal recommendations to avoid nonessential travel. Therefore, conducting web-based interviews exclusively will likely become a necessity.
While there may be some disadvantages to web-based interviews, many candidates find the overall experience satisfactory, thereby allowing them to understand the programs, express themselves, and comfortably rank the programs, as two studies have shown.4,5 Programs and candidates are encouraged to adapt to this new reality in order to achieve a successful match. After all, there are many potential advantages of web-based interviews. In addition to eliminating the risk of COVID-19 acquisition, web-based interviews have been described as helping to improve scheduling flexibility, reduce the financial burden, and allow conducting more interviews for candidates and programs (Table 1).6-8
There are different styles to the web-based interview.9 Some programs choose to offer a single group interview (or the so-called panel interview) in which all the interviewing faculties invite each candidate at a time. Alternatively, programs might choose to conduct separate interviews by each faculty in which the candidates would alternate. For the latter option, the program could use a single invitation link or multiple invitation links for each session.
General tips for a successful interview:9
1. Be pleasant and professional: Your communication with the program should reflect excellent manners and a professional attitude with everyone (i.e., faculties, coordinators, and fellows).
2. Know yourself and what you want: Review your CV and personal statement and reflect on your achievements, strengths and weaknesses. Identify examples from your experience that would speak well of you as a person and as a physician.
3. Communication is key:
- Respond to the interview invitations promptly.
- Send a brief thank you email to the interviewers and the coordinator. Avoid being generic; mention specific points of discussion and show your interest in the program.
- Proofread your emails carefully. Well-written emails that are devoid of grammatical or spelling errors send a positive message about the candidate.
4. Do your homework:
- Read the information posted on the website carefully and take notes. This should provide you with useful information to use when you rank the programs and could lead to questions that you might want to ask your interviewers. Besides, asking questions that are answered on the website reflects poorly on the candidate.
- Pay attention to various clues that could reflect how organized and how academically oriented a program is. For example, a program that provides details about their didactic lectures sends a message that quality teaching is a priority. On the other hand, a program that has a website that hasn’t been updated for years could dissuade rather than recruit applicants.
- Read about the faculty, their areas of interests, and publications. Learn how their names are pronounced and use them during the interview.
- Read about the city where the program is. It shows interest in the area where you might be living and will help you to stand out among candidates.
5. Be prepared for the classics; be honest, genuine, and authentic. Think about these common prompts:
- Tell me about yourself.
- Why did you choose gastroenterology?
- Where do you see yourself in 5 years?
- Why would you like to come to the city where the program is?
- Are there any certain areas in gastroenterology that you’re interested in more (e.g., hepatology, motility, IBD, advanced endoscopy)?
6. It is likely your interviewer will ask if you have questions. Ask questions that further allow you to assess the program and your fit into the program.
- What aspects of the program are you most proud of?
- Where would you like to see this program in 5 years?
- What keeps you at this program?
Tips for a successful web-based interview9,10 (Table 2):
1. Pay attention to the time zone of the city of the program. Be ready at least 10 minutes before the interview.
2. Ensure a fast and stable Internet service for an uninterrupted interview experience. Consider using an ethernet cable. Have a back-up plan such as using a phone as a hotspot.
3. Use a quiet and private room, preferably at home. Be aware of the background. A simple decoration is acceptable.
4. Consider recording yourself using the same device you’ll use for the interview to make sure audio and video are functioning properly.
5. When scheduling more than one interview in 1 day, allow at least 2 hours between interviews to avoid scheduling conflicts caused by unanticipated delays related to technical issues.
6. Have immediate access to the invitation link(s) that you received. Add the interviews to your device’s calendar. Note that sometimes a new invitation link is generated last minute because of technical issues.
7. While the advice for physical interviews is to turn off your phone (and smart watch), you’ll have to keep your phone on but on silent for the virtual interviews. Sometimes, you’ll receive a phone call from the program to update you about any last-minute changes.
7. It is recommended that a laptop or a tablet with a camera with good resolution and a microphone be used rather than using a phone. A wide screen allows better communication. Disable notification on that device to avoid interruptions.
8. Sit comfortably with the device being at or just below eye level. Avoid distractions and maintain eye contact.
9. Familiarize yourself with the platform used and its functions. Double check the audio and video before each interview.
10. Put your device on a desk or table to improve stability; don’t hold it in your hand.
11. Find a place where the view is best and your face appears in the middle of the screen; not too far or too close. Use a well-lit room but don’t have a source of light behind you. Many platforms allow you to select a background or blur the background. A background that is monochromatic and not distracting is recommended.
12. Use a pen and a paper to take notes during the interview. You would use these notes to generate “thank you” or “interest in program” emails. Additionally, they will be a helpful reference when ranking programs.
13. Do not type. Typing is much louder to the interviewer and can be distracting.
14. Dress professionally, just as you dress for an on-site interview. You never know when you might have to stand up during the interview for unplanned reasons.
15. Do a practice interview. Have a colleague set up a virtual web session using any available platform. This will allow you to get feedback on your dress, background, acoustics, and general ability to answer questions.
References
1. Dig Dis Sci. 2019;64:1150-7.
2. BMJ Open. 2017;7:e016242.
3. Centers for Disease Control and Prevention. Coronavirus and Travel in the United States, 2020.
4. J Bone Joint Surg Am. 2017;99:e114.
5. Am J Gastroenterol. 2014;109:155-9.
6. West J Emerg Med. 2018;19:80-6.
7. Int J Med Educ. 2016;7:102-8.
8. Aparajit Naram M. How COVID-19 changed our fellowship interview process for the better. KevinMD.com. April 17, 2020.
9. Association of American Medical Colleges. Virtual interviews: Tips for medical school applicants, 2020. Updated May 14, 2020.
10. Top 5 video interviewing tips for residency and fellowship programs. Thalamus: Connecting the Docs. April 2, 2020, 2020.
Dr. Kiwan is chief fellow in gastroenterology, division of gastroenterology, at Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center, all in Detroit. Dr. Judd is an assistant professor and associate program director in the division of gastroenterology, Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center. Dr. Al Masalmeh is in the department of internal medicine, Wayne State University, Detroit Medical Center. Dr. Levine is professor and the vice chair for education, Wayne State University, Detroit Medical Center.
Fellowship interviews are an essential step – arguably the most important step – in the process of matching candidates to training programs. Until recently, most programs relied exclusively on on-site face-to face interviews. Since the appearance of the COVID-19 pandemic, the medical field has utilized web-based platforms. Despite inherent limitations, virtual meetings appear to be effective in providing patient care and in conducting administrative meetings.1,2
Because of uncertainty related to the pandemic, including changing guidelines regarding social distancing and travel restrictions, fellowship programs are expected to comply with CDC,3 state, and federal recommendations to avoid nonessential travel. Therefore, conducting web-based interviews exclusively will likely become a necessity.
While there may be some disadvantages to web-based interviews, many candidates find the overall experience satisfactory, thereby allowing them to understand the programs, express themselves, and comfortably rank the programs, as two studies have shown.4,5 Programs and candidates are encouraged to adapt to this new reality in order to achieve a successful match. After all, there are many potential advantages of web-based interviews. In addition to eliminating the risk of COVID-19 acquisition, web-based interviews have been described as helping to improve scheduling flexibility, reduce the financial burden, and allow conducting more interviews for candidates and programs (Table 1).6-8
There are different styles to the web-based interview.9 Some programs choose to offer a single group interview (or the so-called panel interview) in which all the interviewing faculties invite each candidate at a time. Alternatively, programs might choose to conduct separate interviews by each faculty in which the candidates would alternate. For the latter option, the program could use a single invitation link or multiple invitation links for each session.
General tips for a successful interview:9
1. Be pleasant and professional: Your communication with the program should reflect excellent manners and a professional attitude with everyone (i.e., faculties, coordinators, and fellows).
2. Know yourself and what you want: Review your CV and personal statement and reflect on your achievements, strengths and weaknesses. Identify examples from your experience that would speak well of you as a person and as a physician.
3. Communication is key:
- Respond to the interview invitations promptly.
- Send a brief thank you email to the interviewers and the coordinator. Avoid being generic; mention specific points of discussion and show your interest in the program.
- Proofread your emails carefully. Well-written emails that are devoid of grammatical or spelling errors send a positive message about the candidate.
4. Do your homework:
- Read the information posted on the website carefully and take notes. This should provide you with useful information to use when you rank the programs and could lead to questions that you might want to ask your interviewers. Besides, asking questions that are answered on the website reflects poorly on the candidate.
- Pay attention to various clues that could reflect how organized and how academically oriented a program is. For example, a program that provides details about their didactic lectures sends a message that quality teaching is a priority. On the other hand, a program that has a website that hasn’t been updated for years could dissuade rather than recruit applicants.
- Read about the faculty, their areas of interests, and publications. Learn how their names are pronounced and use them during the interview.
- Read about the city where the program is. It shows interest in the area where you might be living and will help you to stand out among candidates.
5. Be prepared for the classics; be honest, genuine, and authentic. Think about these common prompts:
- Tell me about yourself.
- Why did you choose gastroenterology?
- Where do you see yourself in 5 years?
- Why would you like to come to the city where the program is?
- Are there any certain areas in gastroenterology that you’re interested in more (e.g., hepatology, motility, IBD, advanced endoscopy)?
6. It is likely your interviewer will ask if you have questions. Ask questions that further allow you to assess the program and your fit into the program.
- What aspects of the program are you most proud of?
- Where would you like to see this program in 5 years?
- What keeps you at this program?
Tips for a successful web-based interview9,10 (Table 2):
1. Pay attention to the time zone of the city of the program. Be ready at least 10 minutes before the interview.
2. Ensure a fast and stable Internet service for an uninterrupted interview experience. Consider using an ethernet cable. Have a back-up plan such as using a phone as a hotspot.
3. Use a quiet and private room, preferably at home. Be aware of the background. A simple decoration is acceptable.
4. Consider recording yourself using the same device you’ll use for the interview to make sure audio and video are functioning properly.
5. When scheduling more than one interview in 1 day, allow at least 2 hours between interviews to avoid scheduling conflicts caused by unanticipated delays related to technical issues.
6. Have immediate access to the invitation link(s) that you received. Add the interviews to your device’s calendar. Note that sometimes a new invitation link is generated last minute because of technical issues.
7. While the advice for physical interviews is to turn off your phone (and smart watch), you’ll have to keep your phone on but on silent for the virtual interviews. Sometimes, you’ll receive a phone call from the program to update you about any last-minute changes.
7. It is recommended that a laptop or a tablet with a camera with good resolution and a microphone be used rather than using a phone. A wide screen allows better communication. Disable notification on that device to avoid interruptions.
8. Sit comfortably with the device being at or just below eye level. Avoid distractions and maintain eye contact.
9. Familiarize yourself with the platform used and its functions. Double check the audio and video before each interview.
10. Put your device on a desk or table to improve stability; don’t hold it in your hand.
11. Find a place where the view is best and your face appears in the middle of the screen; not too far or too close. Use a well-lit room but don’t have a source of light behind you. Many platforms allow you to select a background or blur the background. A background that is monochromatic and not distracting is recommended.
12. Use a pen and a paper to take notes during the interview. You would use these notes to generate “thank you” or “interest in program” emails. Additionally, they will be a helpful reference when ranking programs.
13. Do not type. Typing is much louder to the interviewer and can be distracting.
14. Dress professionally, just as you dress for an on-site interview. You never know when you might have to stand up during the interview for unplanned reasons.
15. Do a practice interview. Have a colleague set up a virtual web session using any available platform. This will allow you to get feedback on your dress, background, acoustics, and general ability to answer questions.
References
1. Dig Dis Sci. 2019;64:1150-7.
2. BMJ Open. 2017;7:e016242.
3. Centers for Disease Control and Prevention. Coronavirus and Travel in the United States, 2020.
4. J Bone Joint Surg Am. 2017;99:e114.
5. Am J Gastroenterol. 2014;109:155-9.
6. West J Emerg Med. 2018;19:80-6.
7. Int J Med Educ. 2016;7:102-8.
8. Aparajit Naram M. How COVID-19 changed our fellowship interview process for the better. KevinMD.com. April 17, 2020.
9. Association of American Medical Colleges. Virtual interviews: Tips for medical school applicants, 2020. Updated May 14, 2020.
10. Top 5 video interviewing tips for residency and fellowship programs. Thalamus: Connecting the Docs. April 2, 2020, 2020.
Dr. Kiwan is chief fellow in gastroenterology, division of gastroenterology, at Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center, all in Detroit. Dr. Judd is an assistant professor and associate program director in the division of gastroenterology, Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center. Dr. Al Masalmeh is in the department of internal medicine, Wayne State University, Detroit Medical Center. Dr. Levine is professor and the vice chair for education, Wayne State University, Detroit Medical Center.
Reflections from PHM’s chief fellow
The education of a new generation of subspecialists
Editor’s note: The Hospitalist is excited to debut a quarterly Pediatric Hospital Medicine Fellows column with this article by pediatric hospitalist Dr. Adam Cohen.
In June 2019, I was offered the new role of chief fellow of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. After messaging colleagues and friends at PHM fellowships across the country, I discovered that I wasn’t only Baylor’s first chief fellow of PHM, but I was the only chief fellow of PHM in the nation.
At first, this seemed to be a daunting prospect that left me wondering what my experiences would be like. However, as any good academician knows, the only way to properly answer a question with such existential considerations is a literature review.
While the role of chief fellow exists in other pediatric subspecialty fellowships, the literature on this role is not yet developed. I focused my literature review on using the chief resident role as a surrogate. The chief resident position is filled with opportunities to work administratively and educationally and even has the potential to drive interinstitutional educational change.1 However, many chief residents feel their administrative roles outweigh their educational ones.2,3 This worried me, as the administrative side of program leadership was something that I had little experience in. Would I be weighed down with answering emails and fielding grievances from other fellows? While I did occasionally have that responsibility, my experiences as a chief fellow meant being intimately involved in one program’s response and growth during a national change to PHM as a field, while also coaching those from other programs on how to respond to these many changes.
The dawn of this new era of PHM saw the first board-certified hospitalists crowned and the first fellowships accredited by the Accreditation Council for Graduate Medical Education within the past academic year. I experienced this in a unique position as a chief fellow – an insider as part of the administration and an outsider as a prospective specialist. Prior to the recent accreditation and certification, PHM fellowship graduates were becoming successful academic physicians. A 2014 study of over 80% of all graduated PHM fellows showed nearly all had academic positions in which they taught students and residents. Many of these graduates also participated in research, with two-thirds being the first author on at least one peer-reviewed article.4
However, we also know that, prior to accreditation, fellowship training was varied, with clinical time ranging from 20% to 65%, in addition to wide variability in billing practices, scholarly practices, and the ability to pursue advanced nonclinical training, such as coursework or master’s degrees in quality improvement or education.5 With PHM fellowships becoming accredited and hospitalists becoming board certified, this is going to change, hopefully for the better.
National accrediting bodies like the ACGME create standards for programs to follow, but as a field we have to make sure we know what those standards mean for our future fellows and our educators. At my own program, these standards meant a significant reduction in clinical time, which was the main way fellows obtained content mastery in PHM. There were also concerns from practicing hospitalists about what it would mean if they did not or could not “grandfather in” to board certification. Would they be pushed out of their jobs or forced into less desirable ones? Would they be able to continue teaching and working with fellows?
As I reflect on experiencing this tumultuous time of change for our specialty, my main takeaway is that board certification of PHM faculty and accreditation of fellowships is an important step to creating the next generation of productive academic hospitalists. The greatest benefit for PHM fellows is that ACGME accreditation mandates that they be treated as learners, and not just junior attendings who are paid less. Many programs rely on fellow billing to fund fellowships, which can create a culture where the focus falls away from exploring a wide variety of educational opportunities and toward an exclusive or near-exclusive service-learning model.
This old model can come at the expense of opportunities such as conferences or secondary degrees. Under ACGME accreditation, fellowships will also be required to provide a regimented system of mentorship and support, more than just nonclinical time, to allow fellows to follow their interests and passions, whether that be in clinical hospital medicine, education, quality, advocacy or more. When these fellows graduate and become board certified, they will truly have recognition as specialists in the field, and be able to advance the field in any setting they choose to practice.
Like any change, this shift in our field also comes with our fair share of risks. Fellowship programs have to be careful about what they take away from an accreditation process that can be incredibly time-consuming and difficult. Leadership at these programs need to look critically at the changes they are required to make, and ensure they are integrated intelligently in a way that benefits the fellows.
At Baylor, while a decrease in clinical time was required, our leadership saw it as an opportunity to implement active learning and assessment techniques to improve clinical mastery with less clinical time. While many programs may need to make significant changes to align with ACGME standards, a key lesson in education is that these changes also need to reflect the goal of the program, to create expert academicians, clinicians, and leaders in PHM.
One of the largest challenges brought about by these changes is how we take into account pediatric hospitalists with clinical expertise who either are not academically oriented or are not eligible for board certification. Excluding them from participating in fellowship training or as productive members of our groups can create a hidden curriculum that board certification and academic practice are the only way forward in our field. We also risk excluding those with the ability to fill the largest need in our specialty, those who practice clinically in the community.6
We must ensure that our desire to have productive academic faculty does not result in the loss of those with clinical expertise, both for the care of our patients and the education of our learners. Whether that solution lies with alternative certification procedures or through thoughtful hiring and educational policies is yet to be seen.
Overall, as PHM’s chief fellow this past academic year, I found that we have a lot to be excited for as our field continues to grow. With this growth, we need be careful about how we move forward with the standardization of our training, education, and faculty practices to align with our core values of excellent care for children and advancement of our field to meet their needs and the needs of our medical system. I am grateful to the many PHM leaders and providers who have thoughtfully stimulated so much growth in the field and paved the way for current and future generations of fellows to benefit from that growth.
Dr. Cohen is an assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine and Texas Children’s Hospital. He graduated from PHM fellowship in June 2020 at Baylor, dedicating himself to developing expertise in medical education. He would like to thank Dr. Michelle Lopez for her assistance in revising this article.
References
1. Myers RE et al. Pediatric chief resident exchange program: A novel method to share educational ideas across training programs. Acad Pediatr. 2019. doi: S1876-2859(19)30386-9.
2. Norris T et al. Do program directors and their chief residents view the role of chief resident similarly? Family Medicine. 1996;28(5):343-5.
3. Dabrow SM et al. Two perspectives on the educational and administrative roles of the pediatric chief resident. J Grad Med Educ. 2011;3(1):17-20.
4. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
5. Shah NH et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-8.
6. Leyenaar JK et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-9.
The education of a new generation of subspecialists
The education of a new generation of subspecialists
Editor’s note: The Hospitalist is excited to debut a quarterly Pediatric Hospital Medicine Fellows column with this article by pediatric hospitalist Dr. Adam Cohen.
In June 2019, I was offered the new role of chief fellow of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. After messaging colleagues and friends at PHM fellowships across the country, I discovered that I wasn’t only Baylor’s first chief fellow of PHM, but I was the only chief fellow of PHM in the nation.
At first, this seemed to be a daunting prospect that left me wondering what my experiences would be like. However, as any good academician knows, the only way to properly answer a question with such existential considerations is a literature review.
While the role of chief fellow exists in other pediatric subspecialty fellowships, the literature on this role is not yet developed. I focused my literature review on using the chief resident role as a surrogate. The chief resident position is filled with opportunities to work administratively and educationally and even has the potential to drive interinstitutional educational change.1 However, many chief residents feel their administrative roles outweigh their educational ones.2,3 This worried me, as the administrative side of program leadership was something that I had little experience in. Would I be weighed down with answering emails and fielding grievances from other fellows? While I did occasionally have that responsibility, my experiences as a chief fellow meant being intimately involved in one program’s response and growth during a national change to PHM as a field, while also coaching those from other programs on how to respond to these many changes.
The dawn of this new era of PHM saw the first board-certified hospitalists crowned and the first fellowships accredited by the Accreditation Council for Graduate Medical Education within the past academic year. I experienced this in a unique position as a chief fellow – an insider as part of the administration and an outsider as a prospective specialist. Prior to the recent accreditation and certification, PHM fellowship graduates were becoming successful academic physicians. A 2014 study of over 80% of all graduated PHM fellows showed nearly all had academic positions in which they taught students and residents. Many of these graduates also participated in research, with two-thirds being the first author on at least one peer-reviewed article.4
However, we also know that, prior to accreditation, fellowship training was varied, with clinical time ranging from 20% to 65%, in addition to wide variability in billing practices, scholarly practices, and the ability to pursue advanced nonclinical training, such as coursework or master’s degrees in quality improvement or education.5 With PHM fellowships becoming accredited and hospitalists becoming board certified, this is going to change, hopefully for the better.
National accrediting bodies like the ACGME create standards for programs to follow, but as a field we have to make sure we know what those standards mean for our future fellows and our educators. At my own program, these standards meant a significant reduction in clinical time, which was the main way fellows obtained content mastery in PHM. There were also concerns from practicing hospitalists about what it would mean if they did not or could not “grandfather in” to board certification. Would they be pushed out of their jobs or forced into less desirable ones? Would they be able to continue teaching and working with fellows?
As I reflect on experiencing this tumultuous time of change for our specialty, my main takeaway is that board certification of PHM faculty and accreditation of fellowships is an important step to creating the next generation of productive academic hospitalists. The greatest benefit for PHM fellows is that ACGME accreditation mandates that they be treated as learners, and not just junior attendings who are paid less. Many programs rely on fellow billing to fund fellowships, which can create a culture where the focus falls away from exploring a wide variety of educational opportunities and toward an exclusive or near-exclusive service-learning model.
This old model can come at the expense of opportunities such as conferences or secondary degrees. Under ACGME accreditation, fellowships will also be required to provide a regimented system of mentorship and support, more than just nonclinical time, to allow fellows to follow their interests and passions, whether that be in clinical hospital medicine, education, quality, advocacy or more. When these fellows graduate and become board certified, they will truly have recognition as specialists in the field, and be able to advance the field in any setting they choose to practice.
Like any change, this shift in our field also comes with our fair share of risks. Fellowship programs have to be careful about what they take away from an accreditation process that can be incredibly time-consuming and difficult. Leadership at these programs need to look critically at the changes they are required to make, and ensure they are integrated intelligently in a way that benefits the fellows.
At Baylor, while a decrease in clinical time was required, our leadership saw it as an opportunity to implement active learning and assessment techniques to improve clinical mastery with less clinical time. While many programs may need to make significant changes to align with ACGME standards, a key lesson in education is that these changes also need to reflect the goal of the program, to create expert academicians, clinicians, and leaders in PHM.
One of the largest challenges brought about by these changes is how we take into account pediatric hospitalists with clinical expertise who either are not academically oriented or are not eligible for board certification. Excluding them from participating in fellowship training or as productive members of our groups can create a hidden curriculum that board certification and academic practice are the only way forward in our field. We also risk excluding those with the ability to fill the largest need in our specialty, those who practice clinically in the community.6
We must ensure that our desire to have productive academic faculty does not result in the loss of those with clinical expertise, both for the care of our patients and the education of our learners. Whether that solution lies with alternative certification procedures or through thoughtful hiring and educational policies is yet to be seen.
Overall, as PHM’s chief fellow this past academic year, I found that we have a lot to be excited for as our field continues to grow. With this growth, we need be careful about how we move forward with the standardization of our training, education, and faculty practices to align with our core values of excellent care for children and advancement of our field to meet their needs and the needs of our medical system. I am grateful to the many PHM leaders and providers who have thoughtfully stimulated so much growth in the field and paved the way for current and future generations of fellows to benefit from that growth.
Dr. Cohen is an assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine and Texas Children’s Hospital. He graduated from PHM fellowship in June 2020 at Baylor, dedicating himself to developing expertise in medical education. He would like to thank Dr. Michelle Lopez for her assistance in revising this article.
References
1. Myers RE et al. Pediatric chief resident exchange program: A novel method to share educational ideas across training programs. Acad Pediatr. 2019. doi: S1876-2859(19)30386-9.
2. Norris T et al. Do program directors and their chief residents view the role of chief resident similarly? Family Medicine. 1996;28(5):343-5.
3. Dabrow SM et al. Two perspectives on the educational and administrative roles of the pediatric chief resident. J Grad Med Educ. 2011;3(1):17-20.
4. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
5. Shah NH et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-8.
6. Leyenaar JK et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-9.
Editor’s note: The Hospitalist is excited to debut a quarterly Pediatric Hospital Medicine Fellows column with this article by pediatric hospitalist Dr. Adam Cohen.
In June 2019, I was offered the new role of chief fellow of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. After messaging colleagues and friends at PHM fellowships across the country, I discovered that I wasn’t only Baylor’s first chief fellow of PHM, but I was the only chief fellow of PHM in the nation.
At first, this seemed to be a daunting prospect that left me wondering what my experiences would be like. However, as any good academician knows, the only way to properly answer a question with such existential considerations is a literature review.
While the role of chief fellow exists in other pediatric subspecialty fellowships, the literature on this role is not yet developed. I focused my literature review on using the chief resident role as a surrogate. The chief resident position is filled with opportunities to work administratively and educationally and even has the potential to drive interinstitutional educational change.1 However, many chief residents feel their administrative roles outweigh their educational ones.2,3 This worried me, as the administrative side of program leadership was something that I had little experience in. Would I be weighed down with answering emails and fielding grievances from other fellows? While I did occasionally have that responsibility, my experiences as a chief fellow meant being intimately involved in one program’s response and growth during a national change to PHM as a field, while also coaching those from other programs on how to respond to these many changes.
The dawn of this new era of PHM saw the first board-certified hospitalists crowned and the first fellowships accredited by the Accreditation Council for Graduate Medical Education within the past academic year. I experienced this in a unique position as a chief fellow – an insider as part of the administration and an outsider as a prospective specialist. Prior to the recent accreditation and certification, PHM fellowship graduates were becoming successful academic physicians. A 2014 study of over 80% of all graduated PHM fellows showed nearly all had academic positions in which they taught students and residents. Many of these graduates also participated in research, with two-thirds being the first author on at least one peer-reviewed article.4
However, we also know that, prior to accreditation, fellowship training was varied, with clinical time ranging from 20% to 65%, in addition to wide variability in billing practices, scholarly practices, and the ability to pursue advanced nonclinical training, such as coursework or master’s degrees in quality improvement or education.5 With PHM fellowships becoming accredited and hospitalists becoming board certified, this is going to change, hopefully for the better.
National accrediting bodies like the ACGME create standards for programs to follow, but as a field we have to make sure we know what those standards mean for our future fellows and our educators. At my own program, these standards meant a significant reduction in clinical time, which was the main way fellows obtained content mastery in PHM. There were also concerns from practicing hospitalists about what it would mean if they did not or could not “grandfather in” to board certification. Would they be pushed out of their jobs or forced into less desirable ones? Would they be able to continue teaching and working with fellows?
As I reflect on experiencing this tumultuous time of change for our specialty, my main takeaway is that board certification of PHM faculty and accreditation of fellowships is an important step to creating the next generation of productive academic hospitalists. The greatest benefit for PHM fellows is that ACGME accreditation mandates that they be treated as learners, and not just junior attendings who are paid less. Many programs rely on fellow billing to fund fellowships, which can create a culture where the focus falls away from exploring a wide variety of educational opportunities and toward an exclusive or near-exclusive service-learning model.
This old model can come at the expense of opportunities such as conferences or secondary degrees. Under ACGME accreditation, fellowships will also be required to provide a regimented system of mentorship and support, more than just nonclinical time, to allow fellows to follow their interests and passions, whether that be in clinical hospital medicine, education, quality, advocacy or more. When these fellows graduate and become board certified, they will truly have recognition as specialists in the field, and be able to advance the field in any setting they choose to practice.
Like any change, this shift in our field also comes with our fair share of risks. Fellowship programs have to be careful about what they take away from an accreditation process that can be incredibly time-consuming and difficult. Leadership at these programs need to look critically at the changes they are required to make, and ensure they are integrated intelligently in a way that benefits the fellows.
At Baylor, while a decrease in clinical time was required, our leadership saw it as an opportunity to implement active learning and assessment techniques to improve clinical mastery with less clinical time. While many programs may need to make significant changes to align with ACGME standards, a key lesson in education is that these changes also need to reflect the goal of the program, to create expert academicians, clinicians, and leaders in PHM.
One of the largest challenges brought about by these changes is how we take into account pediatric hospitalists with clinical expertise who either are not academically oriented or are not eligible for board certification. Excluding them from participating in fellowship training or as productive members of our groups can create a hidden curriculum that board certification and academic practice are the only way forward in our field. We also risk excluding those with the ability to fill the largest need in our specialty, those who practice clinically in the community.6
We must ensure that our desire to have productive academic faculty does not result in the loss of those with clinical expertise, both for the care of our patients and the education of our learners. Whether that solution lies with alternative certification procedures or through thoughtful hiring and educational policies is yet to be seen.
Overall, as PHM’s chief fellow this past academic year, I found that we have a lot to be excited for as our field continues to grow. With this growth, we need be careful about how we move forward with the standardization of our training, education, and faculty practices to align with our core values of excellent care for children and advancement of our field to meet their needs and the needs of our medical system. I am grateful to the many PHM leaders and providers who have thoughtfully stimulated so much growth in the field and paved the way for current and future generations of fellows to benefit from that growth.
Dr. Cohen is an assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine and Texas Children’s Hospital. He graduated from PHM fellowship in June 2020 at Baylor, dedicating himself to developing expertise in medical education. He would like to thank Dr. Michelle Lopez for her assistance in revising this article.
References
1. Myers RE et al. Pediatric chief resident exchange program: A novel method to share educational ideas across training programs. Acad Pediatr. 2019. doi: S1876-2859(19)30386-9.
2. Norris T et al. Do program directors and their chief residents view the role of chief resident similarly? Family Medicine. 1996;28(5):343-5.
3. Dabrow SM et al. Two perspectives on the educational and administrative roles of the pediatric chief resident. J Grad Med Educ. 2011;3(1):17-20.
4. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
5. Shah NH et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-8.
6. Leyenaar JK et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-9.
Why are we still talking about hydroxychloroquine?
This is getting pretty ridiculous. The number of well-done, evidence-based trials of hydroxychloroquine in COVID-19 showing minimal-to-no benefit is increasing. There are still studies that show benefit in certain cases, but many of them are small-scale or even anecdotal.
How long is this going to go on? If the evidence supporting its use were to be put through the standard Food and Drug Administration approval panels it wouldn’t have a chance.
Yet, because it’s become a political football (like masks), science and rational research are tossed out the window. At the end of July we were all treated to videos of Dr. Stella Immanuel claiming the drug is a cure. Dr. Immanuel may have medical credentials, but she also supports beliefs that space aliens and the Illuminati are involved in running governments, and that multiple gynecologic disorders are caused by sexual relations with demons and witches during dreams.
Even so, her hydroxychloroquine statements were given heavy play during a news cycle, then endorsed by the president and his supporters, all with very little immediate background provided for other claims she’s made in the past.
Medicine is a science. Politics shouldn’t be. Continuing to give it to sick people, despite the growing evidence against it, violates the “do-no-harm” tenet of our field.
There was no shame in trying it and failing. This is the process through which all treatments are tested. If they work (such as with penicillin, for example) that’s wonderful. If they fail (such as with countless Alzheimer’s trials) we learn what doesn’t work and move on.
But to keep claiming success where there isn’t any moves beyond science and into things that whiff of a hoax, such as 1989’s cold fusion or recurrent claims of capturing Bigfoot.
With an implacable enemy such as COVID-19 at the door, money and effort need to be focused on finding what works, not on putting stale milk back in the refrigerator and hoping it comes out fresh.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This is getting pretty ridiculous. The number of well-done, evidence-based trials of hydroxychloroquine in COVID-19 showing minimal-to-no benefit is increasing. There are still studies that show benefit in certain cases, but many of them are small-scale or even anecdotal.
How long is this going to go on? If the evidence supporting its use were to be put through the standard Food and Drug Administration approval panels it wouldn’t have a chance.
Yet, because it’s become a political football (like masks), science and rational research are tossed out the window. At the end of July we were all treated to videos of Dr. Stella Immanuel claiming the drug is a cure. Dr. Immanuel may have medical credentials, but she also supports beliefs that space aliens and the Illuminati are involved in running governments, and that multiple gynecologic disorders are caused by sexual relations with demons and witches during dreams.
Even so, her hydroxychloroquine statements were given heavy play during a news cycle, then endorsed by the president and his supporters, all with very little immediate background provided for other claims she’s made in the past.
Medicine is a science. Politics shouldn’t be. Continuing to give it to sick people, despite the growing evidence against it, violates the “do-no-harm” tenet of our field.
There was no shame in trying it and failing. This is the process through which all treatments are tested. If they work (such as with penicillin, for example) that’s wonderful. If they fail (such as with countless Alzheimer’s trials) we learn what doesn’t work and move on.
But to keep claiming success where there isn’t any moves beyond science and into things that whiff of a hoax, such as 1989’s cold fusion or recurrent claims of capturing Bigfoot.
With an implacable enemy such as COVID-19 at the door, money and effort need to be focused on finding what works, not on putting stale milk back in the refrigerator and hoping it comes out fresh.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This is getting pretty ridiculous. The number of well-done, evidence-based trials of hydroxychloroquine in COVID-19 showing minimal-to-no benefit is increasing. There are still studies that show benefit in certain cases, but many of them are small-scale or even anecdotal.
How long is this going to go on? If the evidence supporting its use were to be put through the standard Food and Drug Administration approval panels it wouldn’t have a chance.
Yet, because it’s become a political football (like masks), science and rational research are tossed out the window. At the end of July we were all treated to videos of Dr. Stella Immanuel claiming the drug is a cure. Dr. Immanuel may have medical credentials, but she also supports beliefs that space aliens and the Illuminati are involved in running governments, and that multiple gynecologic disorders are caused by sexual relations with demons and witches during dreams.
Even so, her hydroxychloroquine statements were given heavy play during a news cycle, then endorsed by the president and his supporters, all with very little immediate background provided for other claims she’s made in the past.
Medicine is a science. Politics shouldn’t be. Continuing to give it to sick people, despite the growing evidence against it, violates the “do-no-harm” tenet of our field.
There was no shame in trying it and failing. This is the process through which all treatments are tested. If they work (such as with penicillin, for example) that’s wonderful. If they fail (such as with countless Alzheimer’s trials) we learn what doesn’t work and move on.
But to keep claiming success where there isn’t any moves beyond science and into things that whiff of a hoax, such as 1989’s cold fusion or recurrent claims of capturing Bigfoot.
With an implacable enemy such as COVID-19 at the door, money and effort need to be focused on finding what works, not on putting stale milk back in the refrigerator and hoping it comes out fresh.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Letter from the Editor: Stay safe and, please, wear a mask
“Beginning immediately, the University of Michigan will require all students, staff, faculty, and visitors to wear a face covering that covers the mouth and nose while anywhere on campus grounds.” (Mark S. Schlissel MD, PhD – President, University of Michigan – July 15).
Executive Order 2020-147 (Michigan’s Governor Whitmer) mandated appropriate facial covering for all indoor spaces and crowded outdoor spaces. Additionally, businesses will be held responsible if they allow entry to anyone not wearing a mask.
While enforcement is proving to be a nightmare, masking combined with social distancing, hand washing, and staying home are the only effective levers we have to slow the spread of COVID-19. As of today, 138,000 Americans have died, and we anticipate 240,000 deaths by November 1. By now, most of us (myself included) have had a friend or relative die of this virus. America is not winning this battle and we have yet to see an effective, coordinated national response. Four forces are killing our citizens: COVID-19, structural racism, economic/health inequities, and divisive politics. We should do better.
Although Michigan and Minnesota (my home states) have slowed the virus enough to maintain resource capacity, just last weekend a single house party in a suburb near Ann Arbor resulted in 40 new infections. Thirty-nine states have rising case numbers, hospitalizations, and deaths. We are still in the early innings of this game. Michigan Medicine is actively planning our response to a second surge, which will be combined with increases of influenza and RSV infections.
This month we continue to cover the rapidly emerging information about COVID-19 and digestive implications. There are other interesting articles, including guidance around BRCA risk for colorectal cancer, eosinophilic esophagitis, probiotics, and the emerging impact of AI on endoscopy. Enjoy – stay safe, wash hands, socially distance, and please, wear a mask.
“Respect science, respect nature, respect each other” (Thomas Friedman).
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Beginning immediately, the University of Michigan will require all students, staff, faculty, and visitors to wear a face covering that covers the mouth and nose while anywhere on campus grounds.” (Mark S. Schlissel MD, PhD – President, University of Michigan – July 15).
Executive Order 2020-147 (Michigan’s Governor Whitmer) mandated appropriate facial covering for all indoor spaces and crowded outdoor spaces. Additionally, businesses will be held responsible if they allow entry to anyone not wearing a mask.
While enforcement is proving to be a nightmare, masking combined with social distancing, hand washing, and staying home are the only effective levers we have to slow the spread of COVID-19. As of today, 138,000 Americans have died, and we anticipate 240,000 deaths by November 1. By now, most of us (myself included) have had a friend or relative die of this virus. America is not winning this battle and we have yet to see an effective, coordinated national response. Four forces are killing our citizens: COVID-19, structural racism, economic/health inequities, and divisive politics. We should do better.
Although Michigan and Minnesota (my home states) have slowed the virus enough to maintain resource capacity, just last weekend a single house party in a suburb near Ann Arbor resulted in 40 new infections. Thirty-nine states have rising case numbers, hospitalizations, and deaths. We are still in the early innings of this game. Michigan Medicine is actively planning our response to a second surge, which will be combined with increases of influenza and RSV infections.
This month we continue to cover the rapidly emerging information about COVID-19 and digestive implications. There are other interesting articles, including guidance around BRCA risk for colorectal cancer, eosinophilic esophagitis, probiotics, and the emerging impact of AI on endoscopy. Enjoy – stay safe, wash hands, socially distance, and please, wear a mask.
“Respect science, respect nature, respect each other” (Thomas Friedman).
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Beginning immediately, the University of Michigan will require all students, staff, faculty, and visitors to wear a face covering that covers the mouth and nose while anywhere on campus grounds.” (Mark S. Schlissel MD, PhD – President, University of Michigan – July 15).
Executive Order 2020-147 (Michigan’s Governor Whitmer) mandated appropriate facial covering for all indoor spaces and crowded outdoor spaces. Additionally, businesses will be held responsible if they allow entry to anyone not wearing a mask.
While enforcement is proving to be a nightmare, masking combined with social distancing, hand washing, and staying home are the only effective levers we have to slow the spread of COVID-19. As of today, 138,000 Americans have died, and we anticipate 240,000 deaths by November 1. By now, most of us (myself included) have had a friend or relative die of this virus. America is not winning this battle and we have yet to see an effective, coordinated national response. Four forces are killing our citizens: COVID-19, structural racism, economic/health inequities, and divisive politics. We should do better.
Although Michigan and Minnesota (my home states) have slowed the virus enough to maintain resource capacity, just last weekend a single house party in a suburb near Ann Arbor resulted in 40 new infections. Thirty-nine states have rising case numbers, hospitalizations, and deaths. We are still in the early innings of this game. Michigan Medicine is actively planning our response to a second surge, which will be combined with increases of influenza and RSV infections.
This month we continue to cover the rapidly emerging information about COVID-19 and digestive implications. There are other interesting articles, including guidance around BRCA risk for colorectal cancer, eosinophilic esophagitis, probiotics, and the emerging impact of AI on endoscopy. Enjoy – stay safe, wash hands, socially distance, and please, wear a mask.
“Respect science, respect nature, respect each other” (Thomas Friedman).
John I. Allen, MD, MBA, AGAF
Editor in Chief