User login
New evaluation and management of CPT codes for telemedicine in 2025
. The AMA says the new codes are designed to bring the coding system up to date with the changing landscape of health care and reflect the realities of modern medical practice.
The 17 new CPT codes will encompass a variety of telemedicine services. While the official language of the codes has not been released yet, codes for telemedicine visits using a real-time audio-visual platform could be organized similarly to existing office/outpatient E/M visits (99202-99205, 99212-99215). As part of these revisions, the current telephone E/M codes (99441-99443) will be deleted and replaced with new codes for audio-only E/M.
Implementation of so many codes will require health care providers and systems to adapt their documentation and coding practices. Typically, the exact language and code numbers for new and revised codes are not released to the public until fall of the preceding year, leaving only a few months for practices to educate their physicians and coding staff and prepare their internal systems for implementation starting Jan. 1, 2025. However, given the significant education and systems changes that will be necessary to prepare for so many new codes, we will advocate that the AMA release this information in early 2024.
Additionally, the reimbursement for telemedicine services may not ultimately be the same as for in-person E/M office visits. The AMA/Specialty Society RVS Update Committee (RUC) provides recommendations to the Centers for Medicare & Medicaid Services (CMS) for consideration in developing Relative Value Units (RVUs) for new procedures, including the telemedicine codes. The RUC’s recommendations for the telemedicine codes are not yet publicly available. However, it is important to note that regardless of the RUC recommendations, CMS makes all final decisions about Medicare payment. CMS could decide to set the payments for the telemedicine codes at parity with in-person office E/M visits or less than, more than, or some combination at the individual code level.
If payments for telemedicine visits are set at parity with or higher than office E/M visits, practices can focus primarily on physician and staff education and system implementation of the new codes. However, if telemedicine visit payments are less than in-person E/M office visits, it would have significant implications for practices, providers, and patients. Providers might be discouraged from offering virtual care, leading to a disparity in the availability of telehealth services, with patients in some areas or with certain conditions having limited access. Additionally, not all patients have access to a smartphone or stable internet. Research has shown increased use of audio-only visits among marginalized groups including African Americans, non-English speakers, older patients, those with public insurance as opposed to private insurance and patients living in rural communities and communities with low broadband access. For these patients, audio-only is a lifeline that allows them to access needed care.
Hughes HK, Hasselfeld BW, Greene JA. Health Care Access on the Line - Audio-Only Visits and Digitally Inclusive Care. N Engl J Med. 2022 Nov 17;387(20):1823-1826. doi: 10.1056/NEJMp2118292. Epub 2022 Nov 12. PMID: 36373819.
Chen J, Li KY, Andino J, Hill CE, Ng S, Steppe E, Ellimoottil C. Predictors of Audio-Only Versus Video Telehealth Visits During the COVID-19 Pandemic. J Gen Intern Med. 2022 Apr;37(5):1138-1144. doi: 10.1007/s11606-021-07172-y. Epub 2021 Nov 17. PMID: 34791589; PMCID: PMC8597874.
If payment for audio-only is significantly less than in-person office E/M payments, practices may not offer this option furthering health care inequities.
Beyond the extensive preparation needed and the financial implications, there could be impacts to coverage policies. Currently, telemedicine coverage is triggered by reporting the appropriate office E/M level visit with telemedicine modifier 95. If the new telemedicine codes are no longer tied to the in-person codes, laws requiring payers to provide coverage and parity may need to be adjusted accordingly or they could become less effective. If coverage parity is not maintained, that may lead to changes in practice that could also worsen access and health disparities. Some insurers have already started rolling back coverages. Recently, Aetna decided to stop covering telemedicine visits as of Dec. 1, 2023.
Other insurers may follow suit.
As practices prepare for 2024, tracking insurance coverage policies for telemedicine, staying alert for information from the AMA about the new telemedicine CPT codes, and monitoring the proposed payments for telemedicine that CMS will release in late June to early July in the 2025 Medicare Physician Fee Schedule proposed rule will be important. Participation in advocacy efforts will be critical once the full details are released by the AMA and CMS about the new telemedicine codes and their proposed values. The AGA is monitoring this issue and will continue to fight to reduce burden to physicians and practices, which includes fighting for payment parity with in-person office E/M visits and maintaining coverage benefits for patients.
The authors have reported no conflicts of interest.
. The AMA says the new codes are designed to bring the coding system up to date with the changing landscape of health care and reflect the realities of modern medical practice.
The 17 new CPT codes will encompass a variety of telemedicine services. While the official language of the codes has not been released yet, codes for telemedicine visits using a real-time audio-visual platform could be organized similarly to existing office/outpatient E/M visits (99202-99205, 99212-99215). As part of these revisions, the current telephone E/M codes (99441-99443) will be deleted and replaced with new codes for audio-only E/M.
Implementation of so many codes will require health care providers and systems to adapt their documentation and coding practices. Typically, the exact language and code numbers for new and revised codes are not released to the public until fall of the preceding year, leaving only a few months for practices to educate their physicians and coding staff and prepare their internal systems for implementation starting Jan. 1, 2025. However, given the significant education and systems changes that will be necessary to prepare for so many new codes, we will advocate that the AMA release this information in early 2024.
Additionally, the reimbursement for telemedicine services may not ultimately be the same as for in-person E/M office visits. The AMA/Specialty Society RVS Update Committee (RUC) provides recommendations to the Centers for Medicare & Medicaid Services (CMS) for consideration in developing Relative Value Units (RVUs) for new procedures, including the telemedicine codes. The RUC’s recommendations for the telemedicine codes are not yet publicly available. However, it is important to note that regardless of the RUC recommendations, CMS makes all final decisions about Medicare payment. CMS could decide to set the payments for the telemedicine codes at parity with in-person office E/M visits or less than, more than, or some combination at the individual code level.
If payments for telemedicine visits are set at parity with or higher than office E/M visits, practices can focus primarily on physician and staff education and system implementation of the new codes. However, if telemedicine visit payments are less than in-person E/M office visits, it would have significant implications for practices, providers, and patients. Providers might be discouraged from offering virtual care, leading to a disparity in the availability of telehealth services, with patients in some areas or with certain conditions having limited access. Additionally, not all patients have access to a smartphone or stable internet. Research has shown increased use of audio-only visits among marginalized groups including African Americans, non-English speakers, older patients, those with public insurance as opposed to private insurance and patients living in rural communities and communities with low broadband access. For these patients, audio-only is a lifeline that allows them to access needed care.
Hughes HK, Hasselfeld BW, Greene JA. Health Care Access on the Line - Audio-Only Visits and Digitally Inclusive Care. N Engl J Med. 2022 Nov 17;387(20):1823-1826. doi: 10.1056/NEJMp2118292. Epub 2022 Nov 12. PMID: 36373819.
Chen J, Li KY, Andino J, Hill CE, Ng S, Steppe E, Ellimoottil C. Predictors of Audio-Only Versus Video Telehealth Visits During the COVID-19 Pandemic. J Gen Intern Med. 2022 Apr;37(5):1138-1144. doi: 10.1007/s11606-021-07172-y. Epub 2021 Nov 17. PMID: 34791589; PMCID: PMC8597874.
If payment for audio-only is significantly less than in-person office E/M payments, practices may not offer this option furthering health care inequities.
Beyond the extensive preparation needed and the financial implications, there could be impacts to coverage policies. Currently, telemedicine coverage is triggered by reporting the appropriate office E/M level visit with telemedicine modifier 95. If the new telemedicine codes are no longer tied to the in-person codes, laws requiring payers to provide coverage and parity may need to be adjusted accordingly or they could become less effective. If coverage parity is not maintained, that may lead to changes in practice that could also worsen access and health disparities. Some insurers have already started rolling back coverages. Recently, Aetna decided to stop covering telemedicine visits as of Dec. 1, 2023.
Other insurers may follow suit.
As practices prepare for 2024, tracking insurance coverage policies for telemedicine, staying alert for information from the AMA about the new telemedicine CPT codes, and monitoring the proposed payments for telemedicine that CMS will release in late June to early July in the 2025 Medicare Physician Fee Schedule proposed rule will be important. Participation in advocacy efforts will be critical once the full details are released by the AMA and CMS about the new telemedicine codes and their proposed values. The AGA is monitoring this issue and will continue to fight to reduce burden to physicians and practices, which includes fighting for payment parity with in-person office E/M visits and maintaining coverage benefits for patients.
The authors have reported no conflicts of interest.
. The AMA says the new codes are designed to bring the coding system up to date with the changing landscape of health care and reflect the realities of modern medical practice.
The 17 new CPT codes will encompass a variety of telemedicine services. While the official language of the codes has not been released yet, codes for telemedicine visits using a real-time audio-visual platform could be organized similarly to existing office/outpatient E/M visits (99202-99205, 99212-99215). As part of these revisions, the current telephone E/M codes (99441-99443) will be deleted and replaced with new codes for audio-only E/M.
Implementation of so many codes will require health care providers and systems to adapt their documentation and coding practices. Typically, the exact language and code numbers for new and revised codes are not released to the public until fall of the preceding year, leaving only a few months for practices to educate their physicians and coding staff and prepare their internal systems for implementation starting Jan. 1, 2025. However, given the significant education and systems changes that will be necessary to prepare for so many new codes, we will advocate that the AMA release this information in early 2024.
Additionally, the reimbursement for telemedicine services may not ultimately be the same as for in-person E/M office visits. The AMA/Specialty Society RVS Update Committee (RUC) provides recommendations to the Centers for Medicare & Medicaid Services (CMS) for consideration in developing Relative Value Units (RVUs) for new procedures, including the telemedicine codes. The RUC’s recommendations for the telemedicine codes are not yet publicly available. However, it is important to note that regardless of the RUC recommendations, CMS makes all final decisions about Medicare payment. CMS could decide to set the payments for the telemedicine codes at parity with in-person office E/M visits or less than, more than, or some combination at the individual code level.
If payments for telemedicine visits are set at parity with or higher than office E/M visits, practices can focus primarily on physician and staff education and system implementation of the new codes. However, if telemedicine visit payments are less than in-person E/M office visits, it would have significant implications for practices, providers, and patients. Providers might be discouraged from offering virtual care, leading to a disparity in the availability of telehealth services, with patients in some areas or with certain conditions having limited access. Additionally, not all patients have access to a smartphone or stable internet. Research has shown increased use of audio-only visits among marginalized groups including African Americans, non-English speakers, older patients, those with public insurance as opposed to private insurance and patients living in rural communities and communities with low broadband access. For these patients, audio-only is a lifeline that allows them to access needed care.
Hughes HK, Hasselfeld BW, Greene JA. Health Care Access on the Line - Audio-Only Visits and Digitally Inclusive Care. N Engl J Med. 2022 Nov 17;387(20):1823-1826. doi: 10.1056/NEJMp2118292. Epub 2022 Nov 12. PMID: 36373819.
Chen J, Li KY, Andino J, Hill CE, Ng S, Steppe E, Ellimoottil C. Predictors of Audio-Only Versus Video Telehealth Visits During the COVID-19 Pandemic. J Gen Intern Med. 2022 Apr;37(5):1138-1144. doi: 10.1007/s11606-021-07172-y. Epub 2021 Nov 17. PMID: 34791589; PMCID: PMC8597874.
If payment for audio-only is significantly less than in-person office E/M payments, practices may not offer this option furthering health care inequities.
Beyond the extensive preparation needed and the financial implications, there could be impacts to coverage policies. Currently, telemedicine coverage is triggered by reporting the appropriate office E/M level visit with telemedicine modifier 95. If the new telemedicine codes are no longer tied to the in-person codes, laws requiring payers to provide coverage and parity may need to be adjusted accordingly or they could become less effective. If coverage parity is not maintained, that may lead to changes in practice that could also worsen access and health disparities. Some insurers have already started rolling back coverages. Recently, Aetna decided to stop covering telemedicine visits as of Dec. 1, 2023.
Other insurers may follow suit.
As practices prepare for 2024, tracking insurance coverage policies for telemedicine, staying alert for information from the AMA about the new telemedicine CPT codes, and monitoring the proposed payments for telemedicine that CMS will release in late June to early July in the 2025 Medicare Physician Fee Schedule proposed rule will be important. Participation in advocacy efforts will be critical once the full details are released by the AMA and CMS about the new telemedicine codes and their proposed values. The AGA is monitoring this issue and will continue to fight to reduce burden to physicians and practices, which includes fighting for payment parity with in-person office E/M visits and maintaining coverage benefits for patients.
The authors have reported no conflicts of interest.
Hypertensive disorders of pregnancy and high stroke risk in Black women
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
Even one night in the ED raises risk for death
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
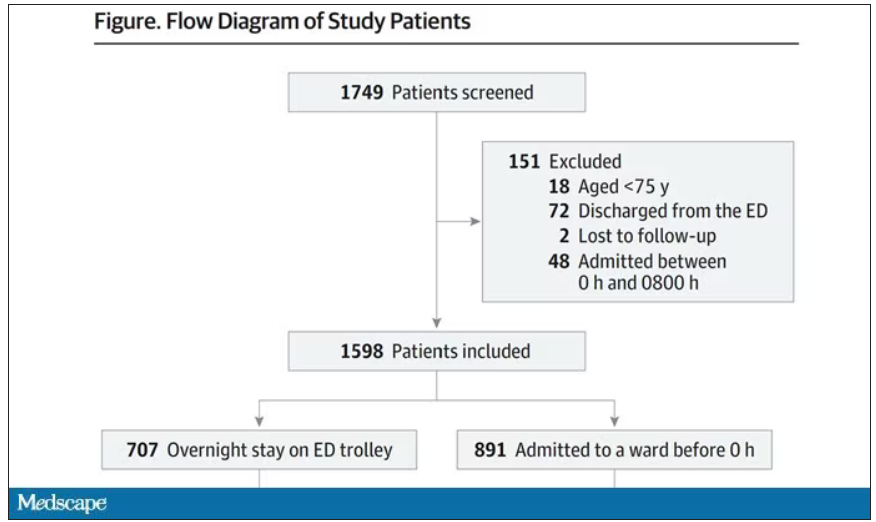
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
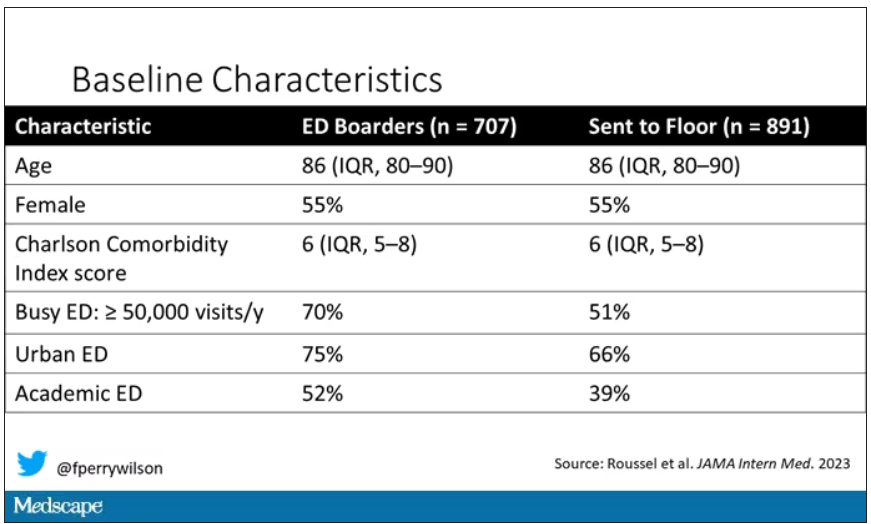
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
The placebo effect
As I noted in my last column, I recently had a generic cold.
One of the more irritating aspects is that I usually get a cough that lasts a few weeks afterwards, and, like most people, I try to do something about it. So I load up on various over-the-counter remedies.
I have no idea if they work, or if I’m shelling out for a placebo. I’m not alone in buying these, or they wouldn’t be on the market, or making money, at all.
But the placebo effect is pretty strong. Phenylephrine has been around since 1938. It’s sold on its own and is an ingredient in almost every anti-cough/cold combination medication out there (NyQuil, DayQuil, Robitussin Multi-Symptom, and their many generic store brands). Millions of people use it every year.
Yet, after sifting through piles of accumulated data, the Food and Drug Administration announced earlier this year that phenylephrine ... doesn’t do anything. Zip. Zero. Nada. When compared with a placebo in controlled trials, you couldn’t tell the difference between them. So now the use of it is being questioned. CVS has started pulling it off their shelves, and I suspect other pharmacies will follow.
But back to my cough. A time-honored tradition in American childhood is having to cram down Robitussin and gagging from its nasty taste (the cherry and orange flavoring don’t make a difference, it tastes terrible no matter what you do). So that gets ingrained into us, and to this day I, and most adults, reach for a bottle of dextromethorphan when they have a cough.
But the evidence for that is spotty, too. Several studies have shown equivocal, if any, evidence to suggest it helps with coughs, though others have shown some. Nothing really amazing though.
But we still buy it by the gallon when we’re sick, because we want something, anything, that will make us better. Even if we’re doing so more from hope than conviction.
There’s also the old standby of cough drops, which have been used for more than 3,000 years. Ingredients vary, but menthol is probably the most common one. I go through those, too. I keep a bag in my desk at work. In medical school, during cold season, it was in my backpack. I remember sitting in the Creighton library to study, quietly sucking on a lozenge to keep my cough from disturbing other students.
But even then, the evidence is iffy as to whether they do anything. In fact, one interesting (though small) study in 2018 suggested they may actually prolong coughs.
The fact is that we are all susceptible to the placebo effect, regardless of how much we know about illness and medication. Maybe these things work, maybe they don’t, but it’s a valid question. How often do we let wishful thinking beat objective data?
Probably more often than we want to admit.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As I noted in my last column, I recently had a generic cold.
One of the more irritating aspects is that I usually get a cough that lasts a few weeks afterwards, and, like most people, I try to do something about it. So I load up on various over-the-counter remedies.
I have no idea if they work, or if I’m shelling out for a placebo. I’m not alone in buying these, or they wouldn’t be on the market, or making money, at all.
But the placebo effect is pretty strong. Phenylephrine has been around since 1938. It’s sold on its own and is an ingredient in almost every anti-cough/cold combination medication out there (NyQuil, DayQuil, Robitussin Multi-Symptom, and their many generic store brands). Millions of people use it every year.
Yet, after sifting through piles of accumulated data, the Food and Drug Administration announced earlier this year that phenylephrine ... doesn’t do anything. Zip. Zero. Nada. When compared with a placebo in controlled trials, you couldn’t tell the difference between them. So now the use of it is being questioned. CVS has started pulling it off their shelves, and I suspect other pharmacies will follow.
But back to my cough. A time-honored tradition in American childhood is having to cram down Robitussin and gagging from its nasty taste (the cherry and orange flavoring don’t make a difference, it tastes terrible no matter what you do). So that gets ingrained into us, and to this day I, and most adults, reach for a bottle of dextromethorphan when they have a cough.
But the evidence for that is spotty, too. Several studies have shown equivocal, if any, evidence to suggest it helps with coughs, though others have shown some. Nothing really amazing though.
But we still buy it by the gallon when we’re sick, because we want something, anything, that will make us better. Even if we’re doing so more from hope than conviction.
There’s also the old standby of cough drops, which have been used for more than 3,000 years. Ingredients vary, but menthol is probably the most common one. I go through those, too. I keep a bag in my desk at work. In medical school, during cold season, it was in my backpack. I remember sitting in the Creighton library to study, quietly sucking on a lozenge to keep my cough from disturbing other students.
But even then, the evidence is iffy as to whether they do anything. In fact, one interesting (though small) study in 2018 suggested they may actually prolong coughs.
The fact is that we are all susceptible to the placebo effect, regardless of how much we know about illness and medication. Maybe these things work, maybe they don’t, but it’s a valid question. How often do we let wishful thinking beat objective data?
Probably more often than we want to admit.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As I noted in my last column, I recently had a generic cold.
One of the more irritating aspects is that I usually get a cough that lasts a few weeks afterwards, and, like most people, I try to do something about it. So I load up on various over-the-counter remedies.
I have no idea if they work, or if I’m shelling out for a placebo. I’m not alone in buying these, or they wouldn’t be on the market, or making money, at all.
But the placebo effect is pretty strong. Phenylephrine has been around since 1938. It’s sold on its own and is an ingredient in almost every anti-cough/cold combination medication out there (NyQuil, DayQuil, Robitussin Multi-Symptom, and their many generic store brands). Millions of people use it every year.
Yet, after sifting through piles of accumulated data, the Food and Drug Administration announced earlier this year that phenylephrine ... doesn’t do anything. Zip. Zero. Nada. When compared with a placebo in controlled trials, you couldn’t tell the difference between them. So now the use of it is being questioned. CVS has started pulling it off their shelves, and I suspect other pharmacies will follow.
But back to my cough. A time-honored tradition in American childhood is having to cram down Robitussin and gagging from its nasty taste (the cherry and orange flavoring don’t make a difference, it tastes terrible no matter what you do). So that gets ingrained into us, and to this day I, and most adults, reach for a bottle of dextromethorphan when they have a cough.
But the evidence for that is spotty, too. Several studies have shown equivocal, if any, evidence to suggest it helps with coughs, though others have shown some. Nothing really amazing though.
But we still buy it by the gallon when we’re sick, because we want something, anything, that will make us better. Even if we’re doing so more from hope than conviction.
There’s also the old standby of cough drops, which have been used for more than 3,000 years. Ingredients vary, but menthol is probably the most common one. I go through those, too. I keep a bag in my desk at work. In medical school, during cold season, it was in my backpack. I remember sitting in the Creighton library to study, quietly sucking on a lozenge to keep my cough from disturbing other students.
But even then, the evidence is iffy as to whether they do anything. In fact, one interesting (though small) study in 2018 suggested they may actually prolong coughs.
The fact is that we are all susceptible to the placebo effect, regardless of how much we know about illness and medication. Maybe these things work, maybe they don’t, but it’s a valid question. How often do we let wishful thinking beat objective data?
Probably more often than we want to admit.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Knowing when enough is enough
“On which side of the bed did you get up this morning?” Obviously, your inquisitor assumes that to avoid clumsily crawling over your sleeping partner you always get up on the side with the table stacked with unread books.
You know as well as I do that you have just received a totally undisguised comment on your recent behavior that has been several shades less than cheery. You may have already sensed your own grumpiness. Do you have an explanation? Did the commute leave you with a case of unresolved road rage? Did you wake up feeling unrested? How often does that happen? Do you think you are getting enough sleep?
A few weeks ago I wrote a Letters From Maine column in which I shared a study suggesting that the regularity of an individual’s sleep pattern may, in many cases, be more important than his or her total number of hours slept. In that same column I wrote that sleep scientists don’t as yet have a good definition of sleep irregularity, nor can they give us any more than a broad range for the total number of hours a person needs to maintain wellness.
How do you determine whether you are getting enough sleep? Do you keep a chart of how many times you were asked which side of the bed you got up on in a week? Or is it how you feel in the morning? Is it when you instantly doze off any time you sit down in a quiet place?
Although many adults are clueless (or in denial) that they are sleep deprived, generally if you ask them and take a brief history they will tell you. On the other hand, determining when a child, particularly one who is preverbal, is sleep deprived is a bit more difficult. Asking the patient isn’t going to give you the answer. You must rely on parental observations. And, to some extent, this can be difficult because parents are, by definition, learning on the job. They may not realize the symptoms and behaviors they are seeing in their child are the result of sleep deficiency.
Over the last half century of observing children, I have developed a very low threshold for diagnosing sleep deprivation. Basically, any child who is cranky and not obviously sick is overtired until proven otherwise. For example, colic does not appear on my frequently used, or in fact ever used, list of diagnoses. Colicky is an adjective that I may use to describe some episodic pain or behavior, but colic as a working diagnosis? Never.
When presented with a child who has already been diagnosed with “colic” by its aunt or the lady next door, this is when the astute pediatrician must be at his or her best. If a thorough history, including sleep pattern, yields no obvious evidence of illness, the next step should be some sleep coaching. However, this is where the “until proven otherwise” thing becomes important, because not providing close follow-up and continuing to keep an open mind for the less likely coexisting conditions can be dangerous and certainly not in the patient’s best interest.
For the older child crankiness, temper tantrums, mood disorders and signs and symptoms often (some might say too often) associated with attention-deficit disorder should trigger an immediate investigation of sleep habits and appropriate advice. Less well-known conditions associated with sleep deprivation are migraine and nocturnal leg pains, often mislabeled as growing pains.
The physicians planning on using sleep as a therapeutic modality is going to quickly run into several challenges. First is convincing the parents, the patient, and the family that the condition is to a greater or lesser degree the result of sleep deprivation. Because sleep is still underappreciated as a component of wellness, this is often not an easy sell.
Second, everyone must accept that altering sleep patterns regardless of age is often not easy and will not be achieved in 1 night or 2. Keeping up the drumbeat of encouragement with close follow-up is critical. Parents must be continually reminded that sleep is being used as a medicine and the dose is not measured in hours. The improvement in symptoms will tell us when enough is enough.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
“On which side of the bed did you get up this morning?” Obviously, your inquisitor assumes that to avoid clumsily crawling over your sleeping partner you always get up on the side with the table stacked with unread books.
You know as well as I do that you have just received a totally undisguised comment on your recent behavior that has been several shades less than cheery. You may have already sensed your own grumpiness. Do you have an explanation? Did the commute leave you with a case of unresolved road rage? Did you wake up feeling unrested? How often does that happen? Do you think you are getting enough sleep?
A few weeks ago I wrote a Letters From Maine column in which I shared a study suggesting that the regularity of an individual’s sleep pattern may, in many cases, be more important than his or her total number of hours slept. In that same column I wrote that sleep scientists don’t as yet have a good definition of sleep irregularity, nor can they give us any more than a broad range for the total number of hours a person needs to maintain wellness.
How do you determine whether you are getting enough sleep? Do you keep a chart of how many times you were asked which side of the bed you got up on in a week? Or is it how you feel in the morning? Is it when you instantly doze off any time you sit down in a quiet place?
Although many adults are clueless (or in denial) that they are sleep deprived, generally if you ask them and take a brief history they will tell you. On the other hand, determining when a child, particularly one who is preverbal, is sleep deprived is a bit more difficult. Asking the patient isn’t going to give you the answer. You must rely on parental observations. And, to some extent, this can be difficult because parents are, by definition, learning on the job. They may not realize the symptoms and behaviors they are seeing in their child are the result of sleep deficiency.
Over the last half century of observing children, I have developed a very low threshold for diagnosing sleep deprivation. Basically, any child who is cranky and not obviously sick is overtired until proven otherwise. For example, colic does not appear on my frequently used, or in fact ever used, list of diagnoses. Colicky is an adjective that I may use to describe some episodic pain or behavior, but colic as a working diagnosis? Never.
When presented with a child who has already been diagnosed with “colic” by its aunt or the lady next door, this is when the astute pediatrician must be at his or her best. If a thorough history, including sleep pattern, yields no obvious evidence of illness, the next step should be some sleep coaching. However, this is where the “until proven otherwise” thing becomes important, because not providing close follow-up and continuing to keep an open mind for the less likely coexisting conditions can be dangerous and certainly not in the patient’s best interest.
For the older child crankiness, temper tantrums, mood disorders and signs and symptoms often (some might say too often) associated with attention-deficit disorder should trigger an immediate investigation of sleep habits and appropriate advice. Less well-known conditions associated with sleep deprivation are migraine and nocturnal leg pains, often mislabeled as growing pains.
The physicians planning on using sleep as a therapeutic modality is going to quickly run into several challenges. First is convincing the parents, the patient, and the family that the condition is to a greater or lesser degree the result of sleep deprivation. Because sleep is still underappreciated as a component of wellness, this is often not an easy sell.
Second, everyone must accept that altering sleep patterns regardless of age is often not easy and will not be achieved in 1 night or 2. Keeping up the drumbeat of encouragement with close follow-up is critical. Parents must be continually reminded that sleep is being used as a medicine and the dose is not measured in hours. The improvement in symptoms will tell us when enough is enough.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
“On which side of the bed did you get up this morning?” Obviously, your inquisitor assumes that to avoid clumsily crawling over your sleeping partner you always get up on the side with the table stacked with unread books.
You know as well as I do that you have just received a totally undisguised comment on your recent behavior that has been several shades less than cheery. You may have already sensed your own grumpiness. Do you have an explanation? Did the commute leave you with a case of unresolved road rage? Did you wake up feeling unrested? How often does that happen? Do you think you are getting enough sleep?
A few weeks ago I wrote a Letters From Maine column in which I shared a study suggesting that the regularity of an individual’s sleep pattern may, in many cases, be more important than his or her total number of hours slept. In that same column I wrote that sleep scientists don’t as yet have a good definition of sleep irregularity, nor can they give us any more than a broad range for the total number of hours a person needs to maintain wellness.
How do you determine whether you are getting enough sleep? Do you keep a chart of how many times you were asked which side of the bed you got up on in a week? Or is it how you feel in the morning? Is it when you instantly doze off any time you sit down in a quiet place?
Although many adults are clueless (or in denial) that they are sleep deprived, generally if you ask them and take a brief history they will tell you. On the other hand, determining when a child, particularly one who is preverbal, is sleep deprived is a bit more difficult. Asking the patient isn’t going to give you the answer. You must rely on parental observations. And, to some extent, this can be difficult because parents are, by definition, learning on the job. They may not realize the symptoms and behaviors they are seeing in their child are the result of sleep deficiency.
Over the last half century of observing children, I have developed a very low threshold for diagnosing sleep deprivation. Basically, any child who is cranky and not obviously sick is overtired until proven otherwise. For example, colic does not appear on my frequently used, or in fact ever used, list of diagnoses. Colicky is an adjective that I may use to describe some episodic pain or behavior, but colic as a working diagnosis? Never.
When presented with a child who has already been diagnosed with “colic” by its aunt or the lady next door, this is when the astute pediatrician must be at his or her best. If a thorough history, including sleep pattern, yields no obvious evidence of illness, the next step should be some sleep coaching. However, this is where the “until proven otherwise” thing becomes important, because not providing close follow-up and continuing to keep an open mind for the less likely coexisting conditions can be dangerous and certainly not in the patient’s best interest.
For the older child crankiness, temper tantrums, mood disorders and signs and symptoms often (some might say too often) associated with attention-deficit disorder should trigger an immediate investigation of sleep habits and appropriate advice. Less well-known conditions associated with sleep deprivation are migraine and nocturnal leg pains, often mislabeled as growing pains.
The physicians planning on using sleep as a therapeutic modality is going to quickly run into several challenges. First is convincing the parents, the patient, and the family that the condition is to a greater or lesser degree the result of sleep deprivation. Because sleep is still underappreciated as a component of wellness, this is often not an easy sell.
Second, everyone must accept that altering sleep patterns regardless of age is often not easy and will not be achieved in 1 night or 2. Keeping up the drumbeat of encouragement with close follow-up is critical. Parents must be continually reminded that sleep is being used as a medicine and the dose is not measured in hours. The improvement in symptoms will tell us when enough is enough.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
525,600 minutes ... how does one measure a year as President?
For the first time since CHEST 2011, when I was the Scientific Program Committee Vice Chair, I was able to return to beautiful Hawaiʻi as the organization’s President, which was such a big coincidence that it felt almost like fate.
During my time on stage at the CHEST 2023 Opening Session, I reflected on the last (at the time) 9 months and shared how truly humbled I have been to lead such a group of leaders and doers. I’m continually amazed at the energy of our members and our staff. In my 25 years as a member, I thought I knew all that CHEST did, but there is so much more happening than any one person realizes. From creating and implementing patient care initiatives to drafting and endorsing statements advocating for better access to health care, there is a tremendous amount accomplished by this organization every year.
One notable accomplishment of this particular year is that not only was CHEST 2023 our largest meeting ever, but I’m proud to share that we also had more medical students, residents, and fellows than any other year, with over 2,000 attendees in-training.
This is a great reflection of the work we’ve been doing to expand the CHEST community – both to physicians earlier in their careers and also to the whole care team. We are putting a dedicated focus toward welcoming and creating a sense of belonging for every clinician. The first step toward this inclusion is the creation of the new CHEST interest groups – Respiratory Care, which is dedicated to the field, and Women in Chest Medicine, which is a more inclusive evolution of the previous Women & Pulmonary group.
This year, we also established CHEST organizational values. The result of a tremendous effort from an advisory committee, CHEST leaders, members, and staff, these values – Community, Inclusivity, Innovation, Advocacy, and Integrity – are reflective of the CHEST organization and will guide decisions for years to come.
They also serve to elevate the work we are doing in social responsibility and health equity, within both of which we’ve made great strides. CHEST philanthropy evolved from what was known as the CHEST Foundation, with a new strategic focus, and we continue working to create opportunities to expand diversity within health care, including the new CHEST mentor/mentee sponsorship fellowship in partnership with the Association of Pulmonary and Critical Care Medicine Program Directors.
Though I could go on for eternity describing all we did at CHEST this year, the reality is that at the end of the next month, as we ring in the new year, I will cede the presidency to the incredibly accomplished and capable Jack Buckley, MD, MPH, FCCP, who will take the reins of our great organization.
For now, in my parting words to you, I encourage everyone to stay in touch. I am always reachable by email and would love to hear your thoughts on CHEST – reflections on this past year, ideas about where we’re going, and suggestions for what we’re missing. The role of the President (and, to some extent, the Immediate Past President) is to be a steward of the needs of the CHEST members, and it’s been a true honor being your 2023 CHEST President.
For the first time since CHEST 2011, when I was the Scientific Program Committee Vice Chair, I was able to return to beautiful Hawaiʻi as the organization’s President, which was such a big coincidence that it felt almost like fate.
During my time on stage at the CHEST 2023 Opening Session, I reflected on the last (at the time) 9 months and shared how truly humbled I have been to lead such a group of leaders and doers. I’m continually amazed at the energy of our members and our staff. In my 25 years as a member, I thought I knew all that CHEST did, but there is so much more happening than any one person realizes. From creating and implementing patient care initiatives to drafting and endorsing statements advocating for better access to health care, there is a tremendous amount accomplished by this organization every year.
One notable accomplishment of this particular year is that not only was CHEST 2023 our largest meeting ever, but I’m proud to share that we also had more medical students, residents, and fellows than any other year, with over 2,000 attendees in-training.
This is a great reflection of the work we’ve been doing to expand the CHEST community – both to physicians earlier in their careers and also to the whole care team. We are putting a dedicated focus toward welcoming and creating a sense of belonging for every clinician. The first step toward this inclusion is the creation of the new CHEST interest groups – Respiratory Care, which is dedicated to the field, and Women in Chest Medicine, which is a more inclusive evolution of the previous Women & Pulmonary group.
This year, we also established CHEST organizational values. The result of a tremendous effort from an advisory committee, CHEST leaders, members, and staff, these values – Community, Inclusivity, Innovation, Advocacy, and Integrity – are reflective of the CHEST organization and will guide decisions for years to come.
They also serve to elevate the work we are doing in social responsibility and health equity, within both of which we’ve made great strides. CHEST philanthropy evolved from what was known as the CHEST Foundation, with a new strategic focus, and we continue working to create opportunities to expand diversity within health care, including the new CHEST mentor/mentee sponsorship fellowship in partnership with the Association of Pulmonary and Critical Care Medicine Program Directors.
Though I could go on for eternity describing all we did at CHEST this year, the reality is that at the end of the next month, as we ring in the new year, I will cede the presidency to the incredibly accomplished and capable Jack Buckley, MD, MPH, FCCP, who will take the reins of our great organization.
For now, in my parting words to you, I encourage everyone to stay in touch. I am always reachable by email and would love to hear your thoughts on CHEST – reflections on this past year, ideas about where we’re going, and suggestions for what we’re missing. The role of the President (and, to some extent, the Immediate Past President) is to be a steward of the needs of the CHEST members, and it’s been a true honor being your 2023 CHEST President.
For the first time since CHEST 2011, when I was the Scientific Program Committee Vice Chair, I was able to return to beautiful Hawaiʻi as the organization’s President, which was such a big coincidence that it felt almost like fate.
During my time on stage at the CHEST 2023 Opening Session, I reflected on the last (at the time) 9 months and shared how truly humbled I have been to lead such a group of leaders and doers. I’m continually amazed at the energy of our members and our staff. In my 25 years as a member, I thought I knew all that CHEST did, but there is so much more happening than any one person realizes. From creating and implementing patient care initiatives to drafting and endorsing statements advocating for better access to health care, there is a tremendous amount accomplished by this organization every year.
One notable accomplishment of this particular year is that not only was CHEST 2023 our largest meeting ever, but I’m proud to share that we also had more medical students, residents, and fellows than any other year, with over 2,000 attendees in-training.
This is a great reflection of the work we’ve been doing to expand the CHEST community – both to physicians earlier in their careers and also to the whole care team. We are putting a dedicated focus toward welcoming and creating a sense of belonging for every clinician. The first step toward this inclusion is the creation of the new CHEST interest groups – Respiratory Care, which is dedicated to the field, and Women in Chest Medicine, which is a more inclusive evolution of the previous Women & Pulmonary group.
This year, we also established CHEST organizational values. The result of a tremendous effort from an advisory committee, CHEST leaders, members, and staff, these values – Community, Inclusivity, Innovation, Advocacy, and Integrity – are reflective of the CHEST organization and will guide decisions for years to come.
They also serve to elevate the work we are doing in social responsibility and health equity, within both of which we’ve made great strides. CHEST philanthropy evolved from what was known as the CHEST Foundation, with a new strategic focus, and we continue working to create opportunities to expand diversity within health care, including the new CHEST mentor/mentee sponsorship fellowship in partnership with the Association of Pulmonary and Critical Care Medicine Program Directors.
Though I could go on for eternity describing all we did at CHEST this year, the reality is that at the end of the next month, as we ring in the new year, I will cede the presidency to the incredibly accomplished and capable Jack Buckley, MD, MPH, FCCP, who will take the reins of our great organization.
For now, in my parting words to you, I encourage everyone to stay in touch. I am always reachable by email and would love to hear your thoughts on CHEST – reflections on this past year, ideas about where we’re going, and suggestions for what we’re missing. The role of the President (and, to some extent, the Immediate Past President) is to be a steward of the needs of the CHEST members, and it’s been a true honor being your 2023 CHEST President.
New pharmacological interventions for residual excessive daytime sleepiness in OSA
Residual excessive daytime sleepiness (REDS) is defined as the urge to sleep during the day despite an intention to remain alert after optimal treatment of obstructive sleep apnea (OSA). This is a distressing outcome with an estimated prevalence of 9% to 22% among patients with OSA (Pépin JL, et al. Eur Respir J. 2009;33[5]:1062). The pathophysiology of the condition is complex, and experimental studies conducted on animal models have demonstrated that chronic sleep fragmentation and chronic intermittent hypoxia can result in detrimental effects on wake-promoting neurons. Additionally, there is evidence of heightened oxidative stress and alterations in melatonin secretion, with the severity and duration of the disease playing a significant role in the manifestation of these effects (Javaheri S, et al. Chest. 2020;158[2]:776). It is considered a diagnosis of exclusion, with the assessment being mostly subjective. Prior to diagnosing REDS, it is crucial to optimize positive airway pressure (PAP) therapy and nocturnal ventilation, ensure sufficient adherence to sleep hygiene practices, and exclude the presence of other sleep disorders. The Epworth Sleepiness Scale (ESS) score is widely utilized as a primary clinical tool in the assessment of sleepiness. To enhance the precision of this score, it is advantageous to take input from both family members and friends. Additional objective assessments that could be considered include the utilization of the Multiple Sleep Latency Test (MSLT) or the Maintenance of Wakefulness Test (MWT).
Off-label use of traditional central nervous system stimulants, like amphetamine or methylphenidate, in these patients is almost extinct. The potential for abuse and negative consequences outweighs the potential benefits. FDA-approved medications for treatment of REDS in OSA include modafinil, armodafinil, and solriamfetol in the United States.
Historically, modafinil and armodafinil are the first-line and most commonly used wake-promoting agents. Both agents bind to the dopamine transporter and inhibit dopamine reuptake. They have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA treated with CPAP. A meta-analysis of 10 randomized, placebo-controlled trials of modafinil and armodafinil found that they were better than placebo by 2.2 points on the ESS score and 3 minutes on the MWT (Maintenance of Wakefulness Test) (Chapman JL, et al. Eur Respir J. 2016;47[5]:1420). Both drugs have common adverse effects of headache, nausea, nervousness, insomnia, dizziness, rhinitis, and diarrhea. Drug interaction with CYP3A4/5 substrates and oral contraceptives is a concern with these medications. In 2010, the European Medicines Agency restricted the use of modafinil only to patients with narcolepsy, considering its cardiovascular and neuropsychiatric risks (European Medicines Agency website; press release, July 22, 2010).
Solriamfetol is the newest medication being utilized for EDS in OSA and is approved in both the United States and Europe for this indication. It is a dopamine and norepinephrine reuptake inhibitor with a simultaneous effect on both transporters. It has been effective in improving wakefulness and reducing sleepiness in patients with residual OSA. In the landmark trial TONES 3, dose-dependent (37.5, 75, 150, and 300 mg/day) effects were observed, with improvements in ESS scores of –1.9 to –4.7 points and sleep latency in MWT by 4.5 to 12.8 minutes (Schweitzer PK, et al. Am J Respir Crit Care Med. 2019;199[11]:1421). The current recommended dosing for REDS in OSA is to start with the lowest dose of 37.5 mg/day and increase to the maximum dose of 150 mg/day by titrating up every 3 days if needed. A recent meta-analysis showed an indirect treatment comparison between efficacy and safety among the medications solriamfetol, modafinil, and armodafinil (Ronnebaum S, et al. J Clin Sleep Med. 2021;17[12]:2543). Six parallel-arm, placebo-controlled, randomized, controlled trials were looked at. The ESS score, MWT20 sleep latency, and CGI-C (Clinical Global Impression of Change) all got better in comparison to the placebo. Relative to the comparators and placebo at 12 weeks, solriamfetol at 150 mg and 300 mg had the highest degree of improvement in all the outcomes studied. Common adverse effects of solriamfetol include headache, nausea, decreased appetite, insomnia, dry mouth, anxiety, and minimal increase in blood pressure and heart rate. The adverse effects in terms of blood pressure and heart rate change have a dose-dependent relationship, and serial vitals monitoring is recommended for patients every 6 months to a year. This medication is contraindicated in patients receiving concomitant monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of an MAOI because of the risk of hypertensive reactions. Solriamfetol is renally excreted, so dose adjustment is needed in patients with moderate to severe renal impairment. It is not recommended for use in end-stage renal disease (eGFR <15 mL/min/1.73 m2) (SUNOSI. Full prescribing information. Axsome; revised 06/2023. https://www.sunosihcp.com/assets/files/sunosi.en.uspi.pdf. Accessed: Sept 24, 2023). Solriamfetol demonstrates a comparatively shorter half-life when compared with traditional pharmaceuticals like modafinil and armodafinil, implying the possibility of a decreased duration of its effects. The effect in question may exhibit interpersonal diversity in its impact on quality of life when applied in a therapeutic setting.
Pitolisant is another potential medication to treat REDS in patients with OSA. While only approved for treating EDS and cataplexy in adult US patients with narcolepsy, it is currently approved for REDS in OSA in Europe (Ozawade. European Medicines Agency. Last updated 12/05/2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozawade#product-information-section. Accessed: Oct 2, 2023). It is a selective histamine H3 receptor antagonist and an inverse agonist of the presynaptic H3 receptor. The fact that this medication is not scheduled and has a negligible or nonexistent risk of abuse is one of its advantages. It is dosed once daily, and the most frequent adverse effects include headaches and insomnia. A prolonged QT interval was observed in a few patients; caution is needed with concomitant use of other medications with known similar effects. Dosage modification is recommended in patients with moderate hepatic impairment and moderate to severe renal impairment. Drug interactions are also observed with the concomitant use of CYP2D6 inhibitors and CYP3A4 inducers. Pitolisant may reduce the efficacy of hormonal contraception, including up to 21 days after its discontinuation (WAKIX. Full prescribing information. Harmony biosciences; revised 12/2022.https://wakixhcp.com/pdf/wakix-tablets-pi.pdf. Accessed: Sept 24, 2023).
Dr. Mechineni is Sleep Attending Physician, Ascension Illinois, Alexian Brothers Medical Center, Chicago. Dr. Sahni is Assistant Professor of Clinical Medicine, Associate Program Director, Sleep Medicine Fellowship; Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago.
Residual excessive daytime sleepiness (REDS) is defined as the urge to sleep during the day despite an intention to remain alert after optimal treatment of obstructive sleep apnea (OSA). This is a distressing outcome with an estimated prevalence of 9% to 22% among patients with OSA (Pépin JL, et al. Eur Respir J. 2009;33[5]:1062). The pathophysiology of the condition is complex, and experimental studies conducted on animal models have demonstrated that chronic sleep fragmentation and chronic intermittent hypoxia can result in detrimental effects on wake-promoting neurons. Additionally, there is evidence of heightened oxidative stress and alterations in melatonin secretion, with the severity and duration of the disease playing a significant role in the manifestation of these effects (Javaheri S, et al. Chest. 2020;158[2]:776). It is considered a diagnosis of exclusion, with the assessment being mostly subjective. Prior to diagnosing REDS, it is crucial to optimize positive airway pressure (PAP) therapy and nocturnal ventilation, ensure sufficient adherence to sleep hygiene practices, and exclude the presence of other sleep disorders. The Epworth Sleepiness Scale (ESS) score is widely utilized as a primary clinical tool in the assessment of sleepiness. To enhance the precision of this score, it is advantageous to take input from both family members and friends. Additional objective assessments that could be considered include the utilization of the Multiple Sleep Latency Test (MSLT) or the Maintenance of Wakefulness Test (MWT).
Off-label use of traditional central nervous system stimulants, like amphetamine or methylphenidate, in these patients is almost extinct. The potential for abuse and negative consequences outweighs the potential benefits. FDA-approved medications for treatment of REDS in OSA include modafinil, armodafinil, and solriamfetol in the United States.
Historically, modafinil and armodafinil are the first-line and most commonly used wake-promoting agents. Both agents bind to the dopamine transporter and inhibit dopamine reuptake. They have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA treated with CPAP. A meta-analysis of 10 randomized, placebo-controlled trials of modafinil and armodafinil found that they were better than placebo by 2.2 points on the ESS score and 3 minutes on the MWT (Maintenance of Wakefulness Test) (Chapman JL, et al. Eur Respir J. 2016;47[5]:1420). Both drugs have common adverse effects of headache, nausea, nervousness, insomnia, dizziness, rhinitis, and diarrhea. Drug interaction with CYP3A4/5 substrates and oral contraceptives is a concern with these medications. In 2010, the European Medicines Agency restricted the use of modafinil only to patients with narcolepsy, considering its cardiovascular and neuropsychiatric risks (European Medicines Agency website; press release, July 22, 2010).
Solriamfetol is the newest medication being utilized for EDS in OSA and is approved in both the United States and Europe for this indication. It is a dopamine and norepinephrine reuptake inhibitor with a simultaneous effect on both transporters. It has been effective in improving wakefulness and reducing sleepiness in patients with residual OSA. In the landmark trial TONES 3, dose-dependent (37.5, 75, 150, and 300 mg/day) effects were observed, with improvements in ESS scores of –1.9 to –4.7 points and sleep latency in MWT by 4.5 to 12.8 minutes (Schweitzer PK, et al. Am J Respir Crit Care Med. 2019;199[11]:1421). The current recommended dosing for REDS in OSA is to start with the lowest dose of 37.5 mg/day and increase to the maximum dose of 150 mg/day by titrating up every 3 days if needed. A recent meta-analysis showed an indirect treatment comparison between efficacy and safety among the medications solriamfetol, modafinil, and armodafinil (Ronnebaum S, et al. J Clin Sleep Med. 2021;17[12]:2543). Six parallel-arm, placebo-controlled, randomized, controlled trials were looked at. The ESS score, MWT20 sleep latency, and CGI-C (Clinical Global Impression of Change) all got better in comparison to the placebo. Relative to the comparators and placebo at 12 weeks, solriamfetol at 150 mg and 300 mg had the highest degree of improvement in all the outcomes studied. Common adverse effects of solriamfetol include headache, nausea, decreased appetite, insomnia, dry mouth, anxiety, and minimal increase in blood pressure and heart rate. The adverse effects in terms of blood pressure and heart rate change have a dose-dependent relationship, and serial vitals monitoring is recommended for patients every 6 months to a year. This medication is contraindicated in patients receiving concomitant monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of an MAOI because of the risk of hypertensive reactions. Solriamfetol is renally excreted, so dose adjustment is needed in patients with moderate to severe renal impairment. It is not recommended for use in end-stage renal disease (eGFR <15 mL/min/1.73 m2) (SUNOSI. Full prescribing information. Axsome; revised 06/2023. https://www.sunosihcp.com/assets/files/sunosi.en.uspi.pdf. Accessed: Sept 24, 2023). Solriamfetol demonstrates a comparatively shorter half-life when compared with traditional pharmaceuticals like modafinil and armodafinil, implying the possibility of a decreased duration of its effects. The effect in question may exhibit interpersonal diversity in its impact on quality of life when applied in a therapeutic setting.
Pitolisant is another potential medication to treat REDS in patients with OSA. While only approved for treating EDS and cataplexy in adult US patients with narcolepsy, it is currently approved for REDS in OSA in Europe (Ozawade. European Medicines Agency. Last updated 12/05/2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozawade#product-information-section. Accessed: Oct 2, 2023). It is a selective histamine H3 receptor antagonist and an inverse agonist of the presynaptic H3 receptor. The fact that this medication is not scheduled and has a negligible or nonexistent risk of abuse is one of its advantages. It is dosed once daily, and the most frequent adverse effects include headaches and insomnia. A prolonged QT interval was observed in a few patients; caution is needed with concomitant use of other medications with known similar effects. Dosage modification is recommended in patients with moderate hepatic impairment and moderate to severe renal impairment. Drug interactions are also observed with the concomitant use of CYP2D6 inhibitors and CYP3A4 inducers. Pitolisant may reduce the efficacy of hormonal contraception, including up to 21 days after its discontinuation (WAKIX. Full prescribing information. Harmony biosciences; revised 12/2022.https://wakixhcp.com/pdf/wakix-tablets-pi.pdf. Accessed: Sept 24, 2023).
Dr. Mechineni is Sleep Attending Physician, Ascension Illinois, Alexian Brothers Medical Center, Chicago. Dr. Sahni is Assistant Professor of Clinical Medicine, Associate Program Director, Sleep Medicine Fellowship; Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago.
Residual excessive daytime sleepiness (REDS) is defined as the urge to sleep during the day despite an intention to remain alert after optimal treatment of obstructive sleep apnea (OSA). This is a distressing outcome with an estimated prevalence of 9% to 22% among patients with OSA (Pépin JL, et al. Eur Respir J. 2009;33[5]:1062). The pathophysiology of the condition is complex, and experimental studies conducted on animal models have demonstrated that chronic sleep fragmentation and chronic intermittent hypoxia can result in detrimental effects on wake-promoting neurons. Additionally, there is evidence of heightened oxidative stress and alterations in melatonin secretion, with the severity and duration of the disease playing a significant role in the manifestation of these effects (Javaheri S, et al. Chest. 2020;158[2]:776). It is considered a diagnosis of exclusion, with the assessment being mostly subjective. Prior to diagnosing REDS, it is crucial to optimize positive airway pressure (PAP) therapy and nocturnal ventilation, ensure sufficient adherence to sleep hygiene practices, and exclude the presence of other sleep disorders. The Epworth Sleepiness Scale (ESS) score is widely utilized as a primary clinical tool in the assessment of sleepiness. To enhance the precision of this score, it is advantageous to take input from both family members and friends. Additional objective assessments that could be considered include the utilization of the Multiple Sleep Latency Test (MSLT) or the Maintenance of Wakefulness Test (MWT).
Off-label use of traditional central nervous system stimulants, like amphetamine or methylphenidate, in these patients is almost extinct. The potential for abuse and negative consequences outweighs the potential benefits. FDA-approved medications for treatment of REDS in OSA include modafinil, armodafinil, and solriamfetol in the United States.
Historically, modafinil and armodafinil are the first-line and most commonly used wake-promoting agents. Both agents bind to the dopamine transporter and inhibit dopamine reuptake. They have demonstrated efficacy in reducing EDS and improving wakefulness in patients with OSA treated with CPAP. A meta-analysis of 10 randomized, placebo-controlled trials of modafinil and armodafinil found that they were better than placebo by 2.2 points on the ESS score and 3 minutes on the MWT (Maintenance of Wakefulness Test) (Chapman JL, et al. Eur Respir J. 2016;47[5]:1420). Both drugs have common adverse effects of headache, nausea, nervousness, insomnia, dizziness, rhinitis, and diarrhea. Drug interaction with CYP3A4/5 substrates and oral contraceptives is a concern with these medications. In 2010, the European Medicines Agency restricted the use of modafinil only to patients with narcolepsy, considering its cardiovascular and neuropsychiatric risks (European Medicines Agency website; press release, July 22, 2010).
Solriamfetol is the newest medication being utilized for EDS in OSA and is approved in both the United States and Europe for this indication. It is a dopamine and norepinephrine reuptake inhibitor with a simultaneous effect on both transporters. It has been effective in improving wakefulness and reducing sleepiness in patients with residual OSA. In the landmark trial TONES 3, dose-dependent (37.5, 75, 150, and 300 mg/day) effects were observed, with improvements in ESS scores of –1.9 to –4.7 points and sleep latency in MWT by 4.5 to 12.8 minutes (Schweitzer PK, et al. Am J Respir Crit Care Med. 2019;199[11]:1421). The current recommended dosing for REDS in OSA is to start with the lowest dose of 37.5 mg/day and increase to the maximum dose of 150 mg/day by titrating up every 3 days if needed. A recent meta-analysis showed an indirect treatment comparison between efficacy and safety among the medications solriamfetol, modafinil, and armodafinil (Ronnebaum S, et al. J Clin Sleep Med. 2021;17[12]:2543). Six parallel-arm, placebo-controlled, randomized, controlled trials were looked at. The ESS score, MWT20 sleep latency, and CGI-C (Clinical Global Impression of Change) all got better in comparison to the placebo. Relative to the comparators and placebo at 12 weeks, solriamfetol at 150 mg and 300 mg had the highest degree of improvement in all the outcomes studied. Common adverse effects of solriamfetol include headache, nausea, decreased appetite, insomnia, dry mouth, anxiety, and minimal increase in blood pressure and heart rate. The adverse effects in terms of blood pressure and heart rate change have a dose-dependent relationship, and serial vitals monitoring is recommended for patients every 6 months to a year. This medication is contraindicated in patients receiving concomitant monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of an MAOI because of the risk of hypertensive reactions. Solriamfetol is renally excreted, so dose adjustment is needed in patients with moderate to severe renal impairment. It is not recommended for use in end-stage renal disease (eGFR <15 mL/min/1.73 m2) (SUNOSI. Full prescribing information. Axsome; revised 06/2023. https://www.sunosihcp.com/assets/files/sunosi.en.uspi.pdf. Accessed: Sept 24, 2023). Solriamfetol demonstrates a comparatively shorter half-life when compared with traditional pharmaceuticals like modafinil and armodafinil, implying the possibility of a decreased duration of its effects. The effect in question may exhibit interpersonal diversity in its impact on quality of life when applied in a therapeutic setting.
Pitolisant is another potential medication to treat REDS in patients with OSA. While only approved for treating EDS and cataplexy in adult US patients with narcolepsy, it is currently approved for REDS in OSA in Europe (Ozawade. European Medicines Agency. Last updated 12/05/2022. https://www.ema.europa.eu/en/medicines/human/EPAR/ozawade#product-information-section. Accessed: Oct 2, 2023). It is a selective histamine H3 receptor antagonist and an inverse agonist of the presynaptic H3 receptor. The fact that this medication is not scheduled and has a negligible or nonexistent risk of abuse is one of its advantages. It is dosed once daily, and the most frequent adverse effects include headaches and insomnia. A prolonged QT interval was observed in a few patients; caution is needed with concomitant use of other medications with known similar effects. Dosage modification is recommended in patients with moderate hepatic impairment and moderate to severe renal impairment. Drug interactions are also observed with the concomitant use of CYP2D6 inhibitors and CYP3A4 inducers. Pitolisant may reduce the efficacy of hormonal contraception, including up to 21 days after its discontinuation (WAKIX. Full prescribing information. Harmony biosciences; revised 12/2022.https://wakixhcp.com/pdf/wakix-tablets-pi.pdf. Accessed: Sept 24, 2023).
Dr. Mechineni is Sleep Attending Physician, Ascension Illinois, Alexian Brothers Medical Center, Chicago. Dr. Sahni is Assistant Professor of Clinical Medicine, Associate Program Director, Sleep Medicine Fellowship; Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago.
Sedative use in older adults after critical illness
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
One-year anniversary
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
It’s been a year since I have become editor-in-chief of The New Gastroenterologist and I am so grateful for our readers and contributors. Thank you for being a part of the TNG family. , including the increasing armamentarium of inflammatory bowel disease treatments, taking on leadership roles in diversity, equity, and inclusion, and tackling financial planning.
In this issue’s In Focus, Drs. Ariela K. Holmer, Shannon Chang, and Lisa Malter break down the factors that contribute to selecting therapies for moderate to severe IBD, such as disease activity and severity, extraintestinal manifestations, safety, prior anti–tumor necrosis factor exposure, perianal disease, and patient preference.
Drs. Michael G. Rubeiz, Kemmian D. Johnson, and Juan Reyes Genere continue our journey with IBD in the Short Clinical Review section, describing advances in endoscopic therapies in IBD. They review the resection of colitis dysplasia and management of luminal strictures with dilation and stricturotomy.
Early-career faculty are being requested to spearhead diversity, equity, and inclusion (DEI) efforts at their institutions or for their groups. Drs. Cassandra D.L. Fritz and Nicolette Juliana Rodriguez highlight important aspects that should be considered prior to taking on DEI roles.
In the Finance section, Dr. Animesh Jain answers five common questions for young gastroenterologists. He addresses student loans, disability insurance, life insurance, retirement, and buying a first house.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The first biologic therapy for IBD, infliximab, was only approved 25 years ago in 1998.
Yours truly,
Judy A Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Five personal finance questions for the young GI
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance