User login
Work-life balance dwarfs pay in female doctors’ top concerns
who responded to a new Medscape survey, far outpacing concerns about pay.
A psychiatrist who responded to the survey commented, “I’ve been trying to use all my vacation to spend time with my spouse. I’m always apologizing for being late, not being able to go to an event due to my work schedule, and missing out on life with my husband.”
Nearly two thirds (64%) said the balance was their top concern whereas 43% put pay at the top.
Medscape surveyed more than 3,000 women physicians about how they deal with parenthood, work pressures, and relationships in Women Physicians 2020: The Issues They Care About.
Almost all are making personal trade-offs
An overwhelming percentage (94%) said they have had to make personal trade-offs for work obligations.
“Women are more likely to make work compromises to benefit their families,” a cardiologist responded. “I won’t/can’t take a position that would disrupt my husband’s community ties, my children’s schooling, and relationships with family.”
More than one-third of women (36%) said that being a woman had a negative or very negative impact on their compensation. Only 4% said their gender had a positive or very positive impact on pay and 59% said gender had no effect.
The Medscape Physician Compensation Report 2020 showed male specialists made 31% more than their female counterparts and male primary care physicians earned 25% more.
Some factors may help explain some of the difference, but others remain unclear.
Poor negotiating skills have long been cited as a reason women get paid less; in this survey 39% said they were unskilled or very unskilled in salary negotiations, compared with 28% who said they were skilled or very skilled in those talks.
Katie Donovan, founder of Equal Pay Negotiations, reports that only 30% of women negotiate pay at all, compared with 46% of men.
Additionally, women tend to gravitate in specialties that don’t pay as well.
They are poorly represented in some of the highest-paying specialties: orthopedics (9%), urology (12%), and cardiology (14%).
“Society’s view of women as caretaker is powerful,” a radiologist commented. “Women feel like they need to choose specialties where they can work part-time or flexible time in order to be the primary caretaker at home.”
Confidence high in leadership abilities
The survey asked women about their confidence in taking a leadership role, and 90% answered that they were confident about taking such a role. However, only half said they had a leadership or supervisory role.
According to the American Medical Association, women make up 3% of healthcare chief medical officers, 6% of department chairs, and 9% of division leaders.
Asked whether women have experienced gender inequity in the workplace, respondents were almost evenly split, but hospital-based physicians at 61% were more likely to report inequity than were 42% of office-based physicians.
A family physician responded, “I have experienced gender inequality more from administrators than from my male colleagues. I think it’s coming from corporate more than from medical professionals.”
In this survey, 3% said their male colleagues were unsupportive of gender equality in the workplace.
The survey responses indicate most women physicians who have children are also conflicted as parents regarding their careers. Almost two-thirds (64%) said they were always or often conflicted with these dueling priorities; only 8% said they sometimes or rarely are.
Those conflicts start even before having children. More than half in this survey (52%) said their career influenced the number of children they have.
A family physician said, “I delayed starting a family because of my career. That affected my fertility and made it hard to complete [in-vitro fertilization].”
Family responsibilities meet stigma
Half of the respondents said women physicians are stigmatized for taking a full maternity leave (6 weeks or longer). An even higher percentage (65%) said women are stigmatized for taking more flexible or fewer hours to accommodate family responsibilities.
A 2019 survey of 844 physician mothers found that physicians who took maternity leave received lower peer evaluation scores, lost potential income, and reported experiencing discrimination. One-quarter of the participants (25.8%) reported experiencing discrimination related to breastfeeding or breast milk pumping upon their return to work.
Burnout at work puts stress on primary relationships, 63% of respondents said, although 24% said it did not strain those relationships. Thirteen percent of women gave the response “not applicable.”
“I try to be present when I’m home, but to be honest, I don’t deal with it very well,” a family physician commented.
A version of this article originally appeared on Medscape.com.
who responded to a new Medscape survey, far outpacing concerns about pay.
A psychiatrist who responded to the survey commented, “I’ve been trying to use all my vacation to spend time with my spouse. I’m always apologizing for being late, not being able to go to an event due to my work schedule, and missing out on life with my husband.”
Nearly two thirds (64%) said the balance was their top concern whereas 43% put pay at the top.
Medscape surveyed more than 3,000 women physicians about how they deal with parenthood, work pressures, and relationships in Women Physicians 2020: The Issues They Care About.
Almost all are making personal trade-offs
An overwhelming percentage (94%) said they have had to make personal trade-offs for work obligations.
“Women are more likely to make work compromises to benefit their families,” a cardiologist responded. “I won’t/can’t take a position that would disrupt my husband’s community ties, my children’s schooling, and relationships with family.”
More than one-third of women (36%) said that being a woman had a negative or very negative impact on their compensation. Only 4% said their gender had a positive or very positive impact on pay and 59% said gender had no effect.
The Medscape Physician Compensation Report 2020 showed male specialists made 31% more than their female counterparts and male primary care physicians earned 25% more.
Some factors may help explain some of the difference, but others remain unclear.
Poor negotiating skills have long been cited as a reason women get paid less; in this survey 39% said they were unskilled or very unskilled in salary negotiations, compared with 28% who said they were skilled or very skilled in those talks.
Katie Donovan, founder of Equal Pay Negotiations, reports that only 30% of women negotiate pay at all, compared with 46% of men.
Additionally, women tend to gravitate in specialties that don’t pay as well.
They are poorly represented in some of the highest-paying specialties: orthopedics (9%), urology (12%), and cardiology (14%).
“Society’s view of women as caretaker is powerful,” a radiologist commented. “Women feel like they need to choose specialties where they can work part-time or flexible time in order to be the primary caretaker at home.”
Confidence high in leadership abilities
The survey asked women about their confidence in taking a leadership role, and 90% answered that they were confident about taking such a role. However, only half said they had a leadership or supervisory role.
According to the American Medical Association, women make up 3% of healthcare chief medical officers, 6% of department chairs, and 9% of division leaders.
Asked whether women have experienced gender inequity in the workplace, respondents were almost evenly split, but hospital-based physicians at 61% were more likely to report inequity than were 42% of office-based physicians.
A family physician responded, “I have experienced gender inequality more from administrators than from my male colleagues. I think it’s coming from corporate more than from medical professionals.”
In this survey, 3% said their male colleagues were unsupportive of gender equality in the workplace.
The survey responses indicate most women physicians who have children are also conflicted as parents regarding their careers. Almost two-thirds (64%) said they were always or often conflicted with these dueling priorities; only 8% said they sometimes or rarely are.
Those conflicts start even before having children. More than half in this survey (52%) said their career influenced the number of children they have.
A family physician said, “I delayed starting a family because of my career. That affected my fertility and made it hard to complete [in-vitro fertilization].”
Family responsibilities meet stigma
Half of the respondents said women physicians are stigmatized for taking a full maternity leave (6 weeks or longer). An even higher percentage (65%) said women are stigmatized for taking more flexible or fewer hours to accommodate family responsibilities.
A 2019 survey of 844 physician mothers found that physicians who took maternity leave received lower peer evaluation scores, lost potential income, and reported experiencing discrimination. One-quarter of the participants (25.8%) reported experiencing discrimination related to breastfeeding or breast milk pumping upon their return to work.
Burnout at work puts stress on primary relationships, 63% of respondents said, although 24% said it did not strain those relationships. Thirteen percent of women gave the response “not applicable.”
“I try to be present when I’m home, but to be honest, I don’t deal with it very well,” a family physician commented.
A version of this article originally appeared on Medscape.com.
who responded to a new Medscape survey, far outpacing concerns about pay.
A psychiatrist who responded to the survey commented, “I’ve been trying to use all my vacation to spend time with my spouse. I’m always apologizing for being late, not being able to go to an event due to my work schedule, and missing out on life with my husband.”
Nearly two thirds (64%) said the balance was their top concern whereas 43% put pay at the top.
Medscape surveyed more than 3,000 women physicians about how they deal with parenthood, work pressures, and relationships in Women Physicians 2020: The Issues They Care About.
Almost all are making personal trade-offs
An overwhelming percentage (94%) said they have had to make personal trade-offs for work obligations.
“Women are more likely to make work compromises to benefit their families,” a cardiologist responded. “I won’t/can’t take a position that would disrupt my husband’s community ties, my children’s schooling, and relationships with family.”
More than one-third of women (36%) said that being a woman had a negative or very negative impact on their compensation. Only 4% said their gender had a positive or very positive impact on pay and 59% said gender had no effect.
The Medscape Physician Compensation Report 2020 showed male specialists made 31% more than their female counterparts and male primary care physicians earned 25% more.
Some factors may help explain some of the difference, but others remain unclear.
Poor negotiating skills have long been cited as a reason women get paid less; in this survey 39% said they were unskilled or very unskilled in salary negotiations, compared with 28% who said they were skilled or very skilled in those talks.
Katie Donovan, founder of Equal Pay Negotiations, reports that only 30% of women negotiate pay at all, compared with 46% of men.
Additionally, women tend to gravitate in specialties that don’t pay as well.
They are poorly represented in some of the highest-paying specialties: orthopedics (9%), urology (12%), and cardiology (14%).
“Society’s view of women as caretaker is powerful,” a radiologist commented. “Women feel like they need to choose specialties where they can work part-time or flexible time in order to be the primary caretaker at home.”
Confidence high in leadership abilities
The survey asked women about their confidence in taking a leadership role, and 90% answered that they were confident about taking such a role. However, only half said they had a leadership or supervisory role.
According to the American Medical Association, women make up 3% of healthcare chief medical officers, 6% of department chairs, and 9% of division leaders.
Asked whether women have experienced gender inequity in the workplace, respondents were almost evenly split, but hospital-based physicians at 61% were more likely to report inequity than were 42% of office-based physicians.
A family physician responded, “I have experienced gender inequality more from administrators than from my male colleagues. I think it’s coming from corporate more than from medical professionals.”
In this survey, 3% said their male colleagues were unsupportive of gender equality in the workplace.
The survey responses indicate most women physicians who have children are also conflicted as parents regarding their careers. Almost two-thirds (64%) said they were always or often conflicted with these dueling priorities; only 8% said they sometimes or rarely are.
Those conflicts start even before having children. More than half in this survey (52%) said their career influenced the number of children they have.
A family physician said, “I delayed starting a family because of my career. That affected my fertility and made it hard to complete [in-vitro fertilization].”
Family responsibilities meet stigma
Half of the respondents said women physicians are stigmatized for taking a full maternity leave (6 weeks or longer). An even higher percentage (65%) said women are stigmatized for taking more flexible or fewer hours to accommodate family responsibilities.
A 2019 survey of 844 physician mothers found that physicians who took maternity leave received lower peer evaluation scores, lost potential income, and reported experiencing discrimination. One-quarter of the participants (25.8%) reported experiencing discrimination related to breastfeeding or breast milk pumping upon their return to work.
Burnout at work puts stress on primary relationships, 63% of respondents said, although 24% said it did not strain those relationships. Thirteen percent of women gave the response “not applicable.”
“I try to be present when I’m home, but to be honest, I don’t deal with it very well,” a family physician commented.
A version of this article originally appeared on Medscape.com.
Ex-nursing assistant pleads guilty in West Virginia insulin deaths
A former nursing assistant and Army veteran pleaded guilty to federal murder charges this week in connection with the 2017-2018 deaths of seven patients in a West Virginia veteran’s hospital, according to news reports.
Prosecutors said in court documents filed on July 13 that Reta Mays, 46, injected lethal doses of insulin into seven veterans at the Louis A. Johnson VA Medical Center (VAMC) in rural Clarksburg, W.Va.
Their blood glucose levels plummeted, and each died shortly after their injections, according to the Tennessean.
An eighth patient, a 92-year-old man whom Mays is accused of assaulting with an insulin injection, initially survived after staff were able to stabilize him but died 2 weeks later at a nursing home, NPR reports.
According to NPR, US Attorney Jarod Douglas told the court Tuesday that the medical investigator could not determine whether the insulin contributed to the man’s death but that it was Mays’ intention to kill him.
“No one watched while she injected them with lethal doses of insulin during an 11-month killing rampage,” the Washington Post reported.
No motive offered
The Post article said no motive has been established, but after a 2-year investigation into a pattern of suspicious deaths that took the hospital almost a year to detect, Mays, who had denied any wrongdoing in multiple interviews with investigators, told a federal judge she preyed on some of the country›s most vulnerable service members.
An attorney for Mays, Brian Kornbrath, contacted by Medscape Medical News, said: “The defense team decided that we would have no public comment at this time.”
According to court documents from the Northern District of West Virginia, Mays was charged with seven counts of second-degree murder and one count of assault with intent to commit murder in connection with the patient who died later.
Mays was hired at the VAMC in Clarksburg in June 2015. She worked from 7:30 PM to 8:00 AM in the medical surgical unit, court documents say.
According to the documents, “VAMC Clarksburg did not require a nursing assistant to have a certification or licensure for initial appointment or as a condition of continuing employment.”
The documents indicate that in June 2018, a hospitalist employed by VAMC Clarksburg reported concern about several deaths from unexplained hypoglycemic events in the same ward and noted that many of the affected patients did not have diabetes.
By that time, according to the Tennessean, “at least eight patients had died under suspicious circumstances. Several had been embalmed and buried, destroying potential evidence. One veteran had been cremated.”
An internal investigation began, followed by a criminal investigation, and in July 2018, Mays was removed from patient care.
Mays fired in 2019 because of lies on resume; claims suffers from PTSD
The Post reports that Mays was fired from the hospital in 2019, 7 months after she was banned from patient care, «after it was discovered she had lied about her qualifications on her resume.»
Court documents indicate that her duties included acting as a sitter for patients, checking vital signs, intake and output, and testing blood glucose levels, but she was not qualified to administer medications, including insulin.
Similarities in the deaths were evident, the Post reported. Citing sources familiar with the case, the report said, “elderly patients in private rooms were injected in their abdomen and limbs with insulin the hospital had not ordered.”
The Post reported that Mays sobbed by the end of the hearing on Tuesday.
The article notes that Mays has three sons and served in the Army National Guard from November 2000 to April 2001 and again from February 2003 to May 2004, when she was deployed to Iraq and Kuwait. She told the judge she was taking medication for posttraumatic stress disorder.
By pleading guilty, she waived her right to have the case presented to a grand jury. A sentencing hearing has not been scheduled, the Post reports.
NPR notes that prosecutors have requested that Mays serve seven consecutive life sentences and an additional 20 years in prison.
“Our hearts go out to those affected by these tragic deaths”
A spokesman for VAMC Clarksburg said in a statement to Medscape Medical News: “Our hearts go out to those affected by these tragic deaths. Clarksburg VA Medical Center discovered these allegations and reported them to VA›s independent inspector general more than 2 years ago. Clarksburg VA Medical Center also fired the individual at the center of the allegations.
“We’re glad the Department of Justice stepped in to push this investigation across the finish line and hopeful our court system will deliver the justice Clarksburg-area Veterans and families deserve.”
According to the Tennessean, Michael Missal, inspector general for the Department of Veteran Affairs, said the agency is investigating the hospital’s practices, “including medication management and communications among staffers.”
This article first appeared on Medscape.com.
A former nursing assistant and Army veteran pleaded guilty to federal murder charges this week in connection with the 2017-2018 deaths of seven patients in a West Virginia veteran’s hospital, according to news reports.
Prosecutors said in court documents filed on July 13 that Reta Mays, 46, injected lethal doses of insulin into seven veterans at the Louis A. Johnson VA Medical Center (VAMC) in rural Clarksburg, W.Va.
Their blood glucose levels plummeted, and each died shortly after their injections, according to the Tennessean.
An eighth patient, a 92-year-old man whom Mays is accused of assaulting with an insulin injection, initially survived after staff were able to stabilize him but died 2 weeks later at a nursing home, NPR reports.
According to NPR, US Attorney Jarod Douglas told the court Tuesday that the medical investigator could not determine whether the insulin contributed to the man’s death but that it was Mays’ intention to kill him.
“No one watched while she injected them with lethal doses of insulin during an 11-month killing rampage,” the Washington Post reported.
No motive offered
The Post article said no motive has been established, but after a 2-year investigation into a pattern of suspicious deaths that took the hospital almost a year to detect, Mays, who had denied any wrongdoing in multiple interviews with investigators, told a federal judge she preyed on some of the country›s most vulnerable service members.
An attorney for Mays, Brian Kornbrath, contacted by Medscape Medical News, said: “The defense team decided that we would have no public comment at this time.”
According to court documents from the Northern District of West Virginia, Mays was charged with seven counts of second-degree murder and one count of assault with intent to commit murder in connection with the patient who died later.
Mays was hired at the VAMC in Clarksburg in June 2015. She worked from 7:30 PM to 8:00 AM in the medical surgical unit, court documents say.
According to the documents, “VAMC Clarksburg did not require a nursing assistant to have a certification or licensure for initial appointment or as a condition of continuing employment.”
The documents indicate that in June 2018, a hospitalist employed by VAMC Clarksburg reported concern about several deaths from unexplained hypoglycemic events in the same ward and noted that many of the affected patients did not have diabetes.
By that time, according to the Tennessean, “at least eight patients had died under suspicious circumstances. Several had been embalmed and buried, destroying potential evidence. One veteran had been cremated.”
An internal investigation began, followed by a criminal investigation, and in July 2018, Mays was removed from patient care.
Mays fired in 2019 because of lies on resume; claims suffers from PTSD
The Post reports that Mays was fired from the hospital in 2019, 7 months after she was banned from patient care, «after it was discovered she had lied about her qualifications on her resume.»
Court documents indicate that her duties included acting as a sitter for patients, checking vital signs, intake and output, and testing blood glucose levels, but she was not qualified to administer medications, including insulin.
Similarities in the deaths were evident, the Post reported. Citing sources familiar with the case, the report said, “elderly patients in private rooms were injected in their abdomen and limbs with insulin the hospital had not ordered.”
The Post reported that Mays sobbed by the end of the hearing on Tuesday.
The article notes that Mays has three sons and served in the Army National Guard from November 2000 to April 2001 and again from February 2003 to May 2004, when she was deployed to Iraq and Kuwait. She told the judge she was taking medication for posttraumatic stress disorder.
By pleading guilty, she waived her right to have the case presented to a grand jury. A sentencing hearing has not been scheduled, the Post reports.
NPR notes that prosecutors have requested that Mays serve seven consecutive life sentences and an additional 20 years in prison.
“Our hearts go out to those affected by these tragic deaths”
A spokesman for VAMC Clarksburg said in a statement to Medscape Medical News: “Our hearts go out to those affected by these tragic deaths. Clarksburg VA Medical Center discovered these allegations and reported them to VA›s independent inspector general more than 2 years ago. Clarksburg VA Medical Center also fired the individual at the center of the allegations.
“We’re glad the Department of Justice stepped in to push this investigation across the finish line and hopeful our court system will deliver the justice Clarksburg-area Veterans and families deserve.”
According to the Tennessean, Michael Missal, inspector general for the Department of Veteran Affairs, said the agency is investigating the hospital’s practices, “including medication management and communications among staffers.”
This article first appeared on Medscape.com.
A former nursing assistant and Army veteran pleaded guilty to federal murder charges this week in connection with the 2017-2018 deaths of seven patients in a West Virginia veteran’s hospital, according to news reports.
Prosecutors said in court documents filed on July 13 that Reta Mays, 46, injected lethal doses of insulin into seven veterans at the Louis A. Johnson VA Medical Center (VAMC) in rural Clarksburg, W.Va.
Their blood glucose levels plummeted, and each died shortly after their injections, according to the Tennessean.
An eighth patient, a 92-year-old man whom Mays is accused of assaulting with an insulin injection, initially survived after staff were able to stabilize him but died 2 weeks later at a nursing home, NPR reports.
According to NPR, US Attorney Jarod Douglas told the court Tuesday that the medical investigator could not determine whether the insulin contributed to the man’s death but that it was Mays’ intention to kill him.
“No one watched while she injected them with lethal doses of insulin during an 11-month killing rampage,” the Washington Post reported.
No motive offered
The Post article said no motive has been established, but after a 2-year investigation into a pattern of suspicious deaths that took the hospital almost a year to detect, Mays, who had denied any wrongdoing in multiple interviews with investigators, told a federal judge she preyed on some of the country›s most vulnerable service members.
An attorney for Mays, Brian Kornbrath, contacted by Medscape Medical News, said: “The defense team decided that we would have no public comment at this time.”
According to court documents from the Northern District of West Virginia, Mays was charged with seven counts of second-degree murder and one count of assault with intent to commit murder in connection with the patient who died later.
Mays was hired at the VAMC in Clarksburg in June 2015. She worked from 7:30 PM to 8:00 AM in the medical surgical unit, court documents say.
According to the documents, “VAMC Clarksburg did not require a nursing assistant to have a certification or licensure for initial appointment or as a condition of continuing employment.”
The documents indicate that in June 2018, a hospitalist employed by VAMC Clarksburg reported concern about several deaths from unexplained hypoglycemic events in the same ward and noted that many of the affected patients did not have diabetes.
By that time, according to the Tennessean, “at least eight patients had died under suspicious circumstances. Several had been embalmed and buried, destroying potential evidence. One veteran had been cremated.”
An internal investigation began, followed by a criminal investigation, and in July 2018, Mays was removed from patient care.
Mays fired in 2019 because of lies on resume; claims suffers from PTSD
The Post reports that Mays was fired from the hospital in 2019, 7 months after she was banned from patient care, «after it was discovered she had lied about her qualifications on her resume.»
Court documents indicate that her duties included acting as a sitter for patients, checking vital signs, intake and output, and testing blood glucose levels, but she was not qualified to administer medications, including insulin.
Similarities in the deaths were evident, the Post reported. Citing sources familiar with the case, the report said, “elderly patients in private rooms were injected in their abdomen and limbs with insulin the hospital had not ordered.”
The Post reported that Mays sobbed by the end of the hearing on Tuesday.
The article notes that Mays has three sons and served in the Army National Guard from November 2000 to April 2001 and again from February 2003 to May 2004, when she was deployed to Iraq and Kuwait. She told the judge she was taking medication for posttraumatic stress disorder.
By pleading guilty, she waived her right to have the case presented to a grand jury. A sentencing hearing has not been scheduled, the Post reports.
NPR notes that prosecutors have requested that Mays serve seven consecutive life sentences and an additional 20 years in prison.
“Our hearts go out to those affected by these tragic deaths”
A spokesman for VAMC Clarksburg said in a statement to Medscape Medical News: “Our hearts go out to those affected by these tragic deaths. Clarksburg VA Medical Center discovered these allegations and reported them to VA›s independent inspector general more than 2 years ago. Clarksburg VA Medical Center also fired the individual at the center of the allegations.
“We’re glad the Department of Justice stepped in to push this investigation across the finish line and hopeful our court system will deliver the justice Clarksburg-area Veterans and families deserve.”
According to the Tennessean, Michael Missal, inspector general for the Department of Veteran Affairs, said the agency is investigating the hospital’s practices, “including medication management and communications among staffers.”
This article first appeared on Medscape.com.
Residents, fellows will get minimum 6 weeks leave for caregiving
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
Intubation boxes may do more harm than good in COVID-19 risk
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Primary care practices may lose about $68k per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
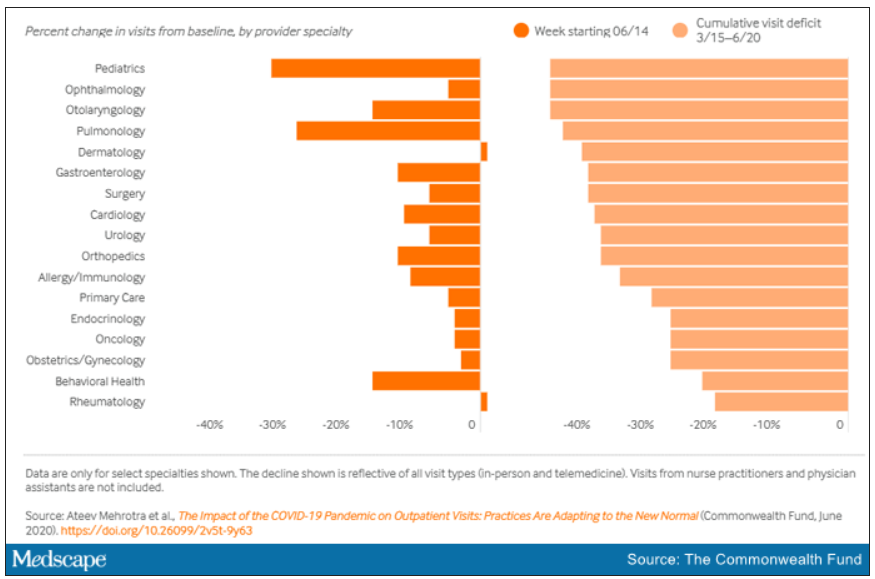
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Physician shortage grows in latest projections
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Many physicians live within their means and save, survey shows
Although about two of five physicians report a net worth of between $1 million and $5 million, half are under the million dollars and about half believe in living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.

Along with that somewhat prudent lifestyle comes savings, with
Those habits may help some navigate the financial upheaval in medicine brought about by COVID-19.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic.
The authors of the report note that by some estimates, primary care offices have seen a 55% drop in revenue because of the pandemic, and specialists have been hard hit with the suspension of most elective procedures.
Primary care offices are seeing fewer patients and are limiting hours, and some offices have been forced to close. Others have stemmed the losses by introducing telemedicine options.
Before COVID-19, average incomes had continued to rise – this year to $243,000 (a 2.5% boost from last year’s $237,000) for primary care physicians and $346,000 for specialists (a 1.5% rise from last year’s $341,000).
About half of physicians (42%) reported a net worth of $1 million to $5 million, and 8% reported a net worth of more than $5 million. Fifty percent of physicians had a net worth of less than $1 million.
Those figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%).
Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000.
Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
43% live below their means
Asked about habits regarding saving, 43% of physicians reported they live below their means. Half said they live at their means, and 7% said they live above their means.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., recommends in the report trying to save 20% of annual gross salary.
More than a third of physicians who responded (39%) said they put more than $2,000/month into tax-deferred retirement or college savings, but Dr. Greenwald acknowledged that this may become more challenging.
“Many have seen the employer match in their retirement plans reduced or eliminated through the end of 2020, with what comes in 2021 as yet undefined,” he said.
A smaller percentage (26%) answered that they put more than $2,000 a month into a taxable retirement or college savings account each month.
Home size by specialty
Mortgages on a primary residence were the top reasons for debt (63%), followed by car loans (37%), personal education loans (26%), and credit card balances (25%).
Half of specialists and 61% of primary care physicians live in homes with up to 3,000 square feet. Only 7% of PCPs and 12% of specialists live in homes with 5000 square feet or more.
At 22%, plastic surgeons and orthopedists were the most likely groups to have houses with the largest square footage, according to the survey.
About one in four physicians in five specialties (urology, cardiology, plastic surgery, otolaryngology, and critical care) reported that they had mortgages of more than $500,000.
Standard financial advice, the report authors note, is that a mortgage should take up no more than 28% of monthly gross income.
Another large source of debt came from student loans. Close to 80% of graduating medical students have educational debt. The average balance for graduating students in 2018 was $196,520, the report authors state.
Those in physical medicine/rehabilitation and family medicine were most likely to still be paying off student debt (34% said they were). Conversely, half as many nephrologists and rheumatologists (15%) and gastroenterologists (14%) reported that they were paying off educational debt.
Only 11% of physicians said they were currently free of any debt.
Most physicians in the survey (72%) reported that they had not experienced a significant financial loss in the past year.
For those who did experience such a loss, the top reason given was related to a bad investment or the stock market (9%).
Cost-cutting strategies
Revenue reduction will likely lead to spending less this year as the pandemic challenges continue.
Survey respondents offered their most effective cost-cutting strategies.
A hospitalist said, “Half of every bonus goes into the investment account, no matter how much.”
“We add an extra amount to the principal of our monthly mortgage payment,” an internist said.
A pediatrician offered, “I bring my lunch to work every day and don’t eat in restaurants often.”
This article first appeared on Medscape.com.
Although about two of five physicians report a net worth of between $1 million and $5 million, half are under the million dollars and about half believe in living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.

Along with that somewhat prudent lifestyle comes savings, with
Those habits may help some navigate the financial upheaval in medicine brought about by COVID-19.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic.
The authors of the report note that by some estimates, primary care offices have seen a 55% drop in revenue because of the pandemic, and specialists have been hard hit with the suspension of most elective procedures.
Primary care offices are seeing fewer patients and are limiting hours, and some offices have been forced to close. Others have stemmed the losses by introducing telemedicine options.
Before COVID-19, average incomes had continued to rise – this year to $243,000 (a 2.5% boost from last year’s $237,000) for primary care physicians and $346,000 for specialists (a 1.5% rise from last year’s $341,000).
About half of physicians (42%) reported a net worth of $1 million to $5 million, and 8% reported a net worth of more than $5 million. Fifty percent of physicians had a net worth of less than $1 million.
Those figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%).
Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000.
Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
43% live below their means
Asked about habits regarding saving, 43% of physicians reported they live below their means. Half said they live at their means, and 7% said they live above their means.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., recommends in the report trying to save 20% of annual gross salary.
More than a third of physicians who responded (39%) said they put more than $2,000/month into tax-deferred retirement or college savings, but Dr. Greenwald acknowledged that this may become more challenging.
“Many have seen the employer match in their retirement plans reduced or eliminated through the end of 2020, with what comes in 2021 as yet undefined,” he said.
A smaller percentage (26%) answered that they put more than $2,000 a month into a taxable retirement or college savings account each month.
Home size by specialty
Mortgages on a primary residence were the top reasons for debt (63%), followed by car loans (37%), personal education loans (26%), and credit card balances (25%).
Half of specialists and 61% of primary care physicians live in homes with up to 3,000 square feet. Only 7% of PCPs and 12% of specialists live in homes with 5000 square feet or more.
At 22%, plastic surgeons and orthopedists were the most likely groups to have houses with the largest square footage, according to the survey.
About one in four physicians in five specialties (urology, cardiology, plastic surgery, otolaryngology, and critical care) reported that they had mortgages of more than $500,000.
Standard financial advice, the report authors note, is that a mortgage should take up no more than 28% of monthly gross income.
Another large source of debt came from student loans. Close to 80% of graduating medical students have educational debt. The average balance for graduating students in 2018 was $196,520, the report authors state.
Those in physical medicine/rehabilitation and family medicine were most likely to still be paying off student debt (34% said they were). Conversely, half as many nephrologists and rheumatologists (15%) and gastroenterologists (14%) reported that they were paying off educational debt.
Only 11% of physicians said they were currently free of any debt.
Most physicians in the survey (72%) reported that they had not experienced a significant financial loss in the past year.
For those who did experience such a loss, the top reason given was related to a bad investment or the stock market (9%).
Cost-cutting strategies
Revenue reduction will likely lead to spending less this year as the pandemic challenges continue.
Survey respondents offered their most effective cost-cutting strategies.
A hospitalist said, “Half of every bonus goes into the investment account, no matter how much.”
“We add an extra amount to the principal of our monthly mortgage payment,” an internist said.
A pediatrician offered, “I bring my lunch to work every day and don’t eat in restaurants often.”
This article first appeared on Medscape.com.
Although about two of five physicians report a net worth of between $1 million and $5 million, half are under the million dollars and about half believe in living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.

Along with that somewhat prudent lifestyle comes savings, with
Those habits may help some navigate the financial upheaval in medicine brought about by COVID-19.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic.
The authors of the report note that by some estimates, primary care offices have seen a 55% drop in revenue because of the pandemic, and specialists have been hard hit with the suspension of most elective procedures.
Primary care offices are seeing fewer patients and are limiting hours, and some offices have been forced to close. Others have stemmed the losses by introducing telemedicine options.
Before COVID-19, average incomes had continued to rise – this year to $243,000 (a 2.5% boost from last year’s $237,000) for primary care physicians and $346,000 for specialists (a 1.5% rise from last year’s $341,000).
About half of physicians (42%) reported a net worth of $1 million to $5 million, and 8% reported a net worth of more than $5 million. Fifty percent of physicians had a net worth of less than $1 million.
Those figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%).
Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000.
Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
43% live below their means
Asked about habits regarding saving, 43% of physicians reported they live below their means. Half said they live at their means, and 7% said they live above their means.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., recommends in the report trying to save 20% of annual gross salary.
More than a third of physicians who responded (39%) said they put more than $2,000/month into tax-deferred retirement or college savings, but Dr. Greenwald acknowledged that this may become more challenging.
“Many have seen the employer match in their retirement plans reduced or eliminated through the end of 2020, with what comes in 2021 as yet undefined,” he said.
A smaller percentage (26%) answered that they put more than $2,000 a month into a taxable retirement or college savings account each month.
Home size by specialty
Mortgages on a primary residence were the top reasons for debt (63%), followed by car loans (37%), personal education loans (26%), and credit card balances (25%).
Half of specialists and 61% of primary care physicians live in homes with up to 3,000 square feet. Only 7% of PCPs and 12% of specialists live in homes with 5000 square feet or more.
At 22%, plastic surgeons and orthopedists were the most likely groups to have houses with the largest square footage, according to the survey.
About one in four physicians in five specialties (urology, cardiology, plastic surgery, otolaryngology, and critical care) reported that they had mortgages of more than $500,000.
Standard financial advice, the report authors note, is that a mortgage should take up no more than 28% of monthly gross income.
Another large source of debt came from student loans. Close to 80% of graduating medical students have educational debt. The average balance for graduating students in 2018 was $196,520, the report authors state.
Those in physical medicine/rehabilitation and family medicine were most likely to still be paying off student debt (34% said they were). Conversely, half as many nephrologists and rheumatologists (15%) and gastroenterologists (14%) reported that they were paying off educational debt.
Only 11% of physicians said they were currently free of any debt.
Most physicians in the survey (72%) reported that they had not experienced a significant financial loss in the past year.
For those who did experience such a loss, the top reason given was related to a bad investment or the stock market (9%).
Cost-cutting strategies
Revenue reduction will likely lead to spending less this year as the pandemic challenges continue.
Survey respondents offered their most effective cost-cutting strategies.
A hospitalist said, “Half of every bonus goes into the investment account, no matter how much.”
“We add an extra amount to the principal of our monthly mortgage payment,” an internist said.
A pediatrician offered, “I bring my lunch to work every day and don’t eat in restaurants often.”
This article first appeared on Medscape.com.
Lancet, NEJM retract studies on hydroxychloroquine for COVID-19
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
Scientific doubt tempers COVID-19 vaccine optimism
US government and industry projections that a COVID-19 vaccine will be ready by this fall or even January would take compressing what usually takes at least a decade into months, with little room for error or safety surprises.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said last week.
But Fauci said a more realistic timeline is still 12 to 18 months, and experts interviewed by Medscape Medical News agree. They say that although recent developments are encouraging, history and scientific reason say the day when a COVID-19 vaccine is widely available will not come this year and may not come by the end of 2021.
The encouraging signals come primarily from two recent announcements: the $1.2 billion United States backing last week of one vaccine platform and the announcement on May 18 that the first human trials of another have produced some positive phase 1 results.
Recent developments
On May 21, the US Department of Health and Human Services (HHS) under “Operation Warp Speed” announced that the US will give AstraZeneca $1.2 billion “to make available at least 300 million doses of a coronavirus vaccine called AZD1222, with the first doses delivered as early as October 2020.”
On May 18, the Massachusetts-based biotechnology company Moderna announced that phase 1 clinical results showed that its vaccine candidate, which uses a new messenger RNA (mRNA) technology, appeared safe. Eight participants in the human trials were able to produce neutralizing antibodies that researchers believe are important in developing protection from the virus.
Moderna Chief Medical Officer Tal Zaks, MD, PhD told CNN that if the vaccine candidate does well in phase 2, “it could be ready by January 2021.”
The two candidates are among 10 in clinical trials for the SARS-CoV-2 virus, according to the World Health Organization (WHO). The AstraZeneca/ AZD1222 candidate (also called ChAdOx1 nCoV-19, in collaboration with the University of Oxford) has entered phase 2/3.
Moderna’s candidate and another being developed in Beijing, China, are in phase 2, WHO reports. As of yesterday, 115 other candidates are in preclinical evaluation.
Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas, told Medscape Medical News it’s important to realize that, in the case of the $1.2 billion US investment, “what they’re talking about is manufacturing.”
The idea, she said, is to pay AstraZeneca up front so that manufacturing can start before it is known whether the vaccine candidate is safe or effective, the reverse of how the clinical trial process usually works.
That way, if the candidate is deemed safe and effective, time is not lost by then deciding how to make it and distribute it.
By the end of this year, she said, “Maybe we will have many vaccines made and stored in a refrigerator somewhere. But between now and December, there’s absolutely no way you can show efficacy of the vaccine at the same time you confirm that it’s safe.”
“Take these things with a grain of salt”
Animal testing for the AstraZeneca candidate, made in partnership with the University of Oxford in the United Kingdom, has yielded lackluster results, according to results on the preprint server BioRxiv, which have not been peer-reviewed.
“The results were not bad, but they were not gangbusters,” Bottazzi said. The results show the vaccine offered only partial protection.
“Partial protection is better than no protection,” she noted. “You have to take these things with a grain of salt. We don’t know what’s going to happen in humans.”
As for the Moderna candidate, Bottazzi said, “the good news is they found an appropriate safety profile. But from an eight-person group to make the extrapolation that they have efficacy — it’s unrealistic.”
Nicole Lurie, MD, MSPH, is senior adviser to the CEO for the Coalition for Epidemic Preparedness Innovation (CEPI), a nongovernmental organization funded by the Wellcome Trust, the Bill and Melinda Gates Foundation, the European Commission, and eight countries (Australia, Belgium, Canada, Ethiopia, Germany, Japan, Norway, and the United Kingdom) charged with supporting development of vaccines for pathogens on WHO’s priority list.
She and her colleagues write in a paper published online in the New England Journal of Medicine on March 30 that “it typically takes multiple candidates and many years to produce a licensed vaccine.”
The fastest time for developing a vaccine to date is 4 years, for the mumps vaccine, licensed in 1967.
As to whether she would expect a rollout of any vaccine by the end of the year, Lurie told Medscape Medical News, “If everything goes according to plan in every way, shape or form, well then maybe you can get there. But I wouldn’t hold my breath.”
Lurie and her colleagues write that “it’s far from certain that these new platforms will be scalable or that existing capacity can provide sufficient quantities of vaccine fast enough.”
On a call with reporters today, leaders of some of the words largest pharmaceutical companies said that one of the key bottlenecks is the sheer number of vials needed in order to distribute billions of doses of a successful vaccine.
Pfizer CEO Albert Bourla, DVM, PhD, said, “Typically we are producing vaccines in single-dose vials. We are exploring with governments right now if it would be more convenient if there were 5-dose vials or 10-dose vials. I think we can resolve a significant part of the bottleneck.”
Despite the challenges, experts interviewed for this article agree that it will be possible to make a vaccine for COVID-19. They don’t expect attempts to meet the same complications that HIV researchers have seen over decades as the virus continues to confound with mutations.
Fred Ledley, MD, director of the Center for Integration of Science and Industry at Bentley University in Waltham, Massachusetts, told Medscape Medical News, “There doesn’t appear to be anything terribly diabolical about this virus. The mutation rate doesn’t appear to be anything like HIV. It appears to have some big, ugly proteins on the surface, which is good for vaccines — proteins with a lot of physical features look distinguishable from healthy cells. Signs all point to that it should be possible to make a vaccine.”
History raises safety concerns
However, Ledley said, “The idea of doing it in 6 months is largely unrealistic.”
He says 18 months is more realistic, primarily because of the sheer number of people that would have to be enrolled in a phase 3 study to truly test whether the endpoints are being met.
Vaccines are given to healthy volunteers. If safety signals arise, they may not be apparent until massive numbers of people are tested in phase 3.
“You’re never going to see the rates cut to 0%, but to see the difference between 10 people getting sick and seven people getting sick, takes very, very large numbers,” Ledley said. “There’s no way that can be done in 6 months. You’re talking about tens of thousands of people enrolled.”
He notes at this point it’s unclear what the endpoints will be and what the safety thresholds will be after consideration of risks and benefit.
Another big question for Ledley: “We don’t know what type of immunity we need to protect us against the virus. Do you just need the antibodies in your blood or do you need cells that are primed to attack the virus? Is it more of a chemical clearance or do the cells need to physically go in and digest the virus?”
History also points to the need for rigorous safety precautions that scientists fear could be compromised as trial phases overlap and processes are run in parallel instead of one step at a time.
An early batch of the Salk vaccine for polio in 1955, for example, turned out to be contaminated and caused paralysis in some children and 10 deaths, he points out.
CEPI’s Lurie adds that early candidates for another coronavirus, severe acute respiratory syndrome (SARS), “caused a reaction in the lungs that was very dangerous” before development was halted.
She also pointed to previous findings that a vaccine for dengue fever could worsen the disease in some people through a phenomenon called antibody-dependent enhancement.
Lurie and colleagues write in their paper that “it’s critical that vaccines also be developed using the tried-and-true methods, even if they may take longer to enter clinical trials or to result in large numbers of doses.”
Live attenuated vaccine
Raul Andino, PhD, a virologist at the University of California San Francisco, is among the scientists working with a tried-and-true method — a live attenuated vaccine — and he told Medscape Medical News he’s predicting it will take 2 years to develop.
He said it is cheaper to produce because scientists just have to learn how to grow the virus. Because the technology is already proven, a live attenuated vaccine could be rapidly produced on a worldwide scale.
The hope is also that a live attenuated vaccine would be given once in a lifetime and therefore be more affordable, especially in poorer countries.
“While a Moderna vaccine might be good for Europe and the United States,” he said, “It’s not going to be good for Africa, India, Brazil.”
Andino said, “I would bet money” that the front-runner vaccines so far will not be one-time vaccines.
He points out that most of the vaccine candidates are trying to protect people from disease. While there’s nothing wrong with that, he said, “In my opinion that is the lower-hanging fruit.”
“In my mind we need something that interrupts the chain of transmission and induces protection,” Andino said, important for developing herd immunity.
The reason this type of approach takes longer is because you are introducing a weakened form of the virus to the body and you have to make sure it doesn’t cause disease, not just in a small test population, but in populations who may be more susceptible to the disease, Andino said.
A call for unified strategies
Universities, countries, international consortiums, and public-private partnerships are all racing to find several safe and effective vaccines as no one entity will likely be able to provide the global solution.
Some of the efforts involve overlap of entities but with different focuses.
Along with “Operation Warp Speed” and CEPI, other collaborations include Gavi the Vaccine Alliance, whose core partners include WHO, UNICEF, the World Bank, and the Gates Foundation; and “Accelerating Therapeutic Interventions and Vaccines (ACTIV) partnership,” led by the National Institutes of Health.
Industry partners in ACTIV (18 biopharmaceutical companies), according to a May 18 article published online in the Journal of the American Medical Association, have said they will contribute their respective clinical trial capacities, regardless of which agent is studied.
Some, however, have called for more streamlining of efforts.
“Ideally we’d be working together,” Lurie told Medscape Medical News.
“I’m hopeful we will find ways to collaborate scientifically,” she said. “The US government’s responsibility is to make doses for the US. CEPI’s responsibility is to make doses for the world. A big focus of CEPI is to make sure we have manufacturing capacity outside of the US so those doses can be available to the world and they don’t get seized by wealthy countries.”
Bottazzi, Ledley, Lurie, and Andino report no relevant financial relationships.
This article first appeared on Medscape.com.
US government and industry projections that a COVID-19 vaccine will be ready by this fall or even January would take compressing what usually takes at least a decade into months, with little room for error or safety surprises.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said last week.
But Fauci said a more realistic timeline is still 12 to 18 months, and experts interviewed by Medscape Medical News agree. They say that although recent developments are encouraging, history and scientific reason say the day when a COVID-19 vaccine is widely available will not come this year and may not come by the end of 2021.
The encouraging signals come primarily from two recent announcements: the $1.2 billion United States backing last week of one vaccine platform and the announcement on May 18 that the first human trials of another have produced some positive phase 1 results.
Recent developments
On May 21, the US Department of Health and Human Services (HHS) under “Operation Warp Speed” announced that the US will give AstraZeneca $1.2 billion “to make available at least 300 million doses of a coronavirus vaccine called AZD1222, with the first doses delivered as early as October 2020.”
On May 18, the Massachusetts-based biotechnology company Moderna announced that phase 1 clinical results showed that its vaccine candidate, which uses a new messenger RNA (mRNA) technology, appeared safe. Eight participants in the human trials were able to produce neutralizing antibodies that researchers believe are important in developing protection from the virus.
Moderna Chief Medical Officer Tal Zaks, MD, PhD told CNN that if the vaccine candidate does well in phase 2, “it could be ready by January 2021.”
The two candidates are among 10 in clinical trials for the SARS-CoV-2 virus, according to the World Health Organization (WHO). The AstraZeneca/ AZD1222 candidate (also called ChAdOx1 nCoV-19, in collaboration with the University of Oxford) has entered phase 2/3.
Moderna’s candidate and another being developed in Beijing, China, are in phase 2, WHO reports. As of yesterday, 115 other candidates are in preclinical evaluation.
Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas, told Medscape Medical News it’s important to realize that, in the case of the $1.2 billion US investment, “what they’re talking about is manufacturing.”
The idea, she said, is to pay AstraZeneca up front so that manufacturing can start before it is known whether the vaccine candidate is safe or effective, the reverse of how the clinical trial process usually works.
That way, if the candidate is deemed safe and effective, time is not lost by then deciding how to make it and distribute it.
By the end of this year, she said, “Maybe we will have many vaccines made and stored in a refrigerator somewhere. But between now and December, there’s absolutely no way you can show efficacy of the vaccine at the same time you confirm that it’s safe.”
“Take these things with a grain of salt”
Animal testing for the AstraZeneca candidate, made in partnership with the University of Oxford in the United Kingdom, has yielded lackluster results, according to results on the preprint server BioRxiv, which have not been peer-reviewed.
“The results were not bad, but they were not gangbusters,” Bottazzi said. The results show the vaccine offered only partial protection.
“Partial protection is better than no protection,” she noted. “You have to take these things with a grain of salt. We don’t know what’s going to happen in humans.”
As for the Moderna candidate, Bottazzi said, “the good news is they found an appropriate safety profile. But from an eight-person group to make the extrapolation that they have efficacy — it’s unrealistic.”
Nicole Lurie, MD, MSPH, is senior adviser to the CEO for the Coalition for Epidemic Preparedness Innovation (CEPI), a nongovernmental organization funded by the Wellcome Trust, the Bill and Melinda Gates Foundation, the European Commission, and eight countries (Australia, Belgium, Canada, Ethiopia, Germany, Japan, Norway, and the United Kingdom) charged with supporting development of vaccines for pathogens on WHO’s priority list.
She and her colleagues write in a paper published online in the New England Journal of Medicine on March 30 that “it typically takes multiple candidates and many years to produce a licensed vaccine.”
The fastest time for developing a vaccine to date is 4 years, for the mumps vaccine, licensed in 1967.
As to whether she would expect a rollout of any vaccine by the end of the year, Lurie told Medscape Medical News, “If everything goes according to plan in every way, shape or form, well then maybe you can get there. But I wouldn’t hold my breath.”
Lurie and her colleagues write that “it’s far from certain that these new platforms will be scalable or that existing capacity can provide sufficient quantities of vaccine fast enough.”
On a call with reporters today, leaders of some of the words largest pharmaceutical companies said that one of the key bottlenecks is the sheer number of vials needed in order to distribute billions of doses of a successful vaccine.
Pfizer CEO Albert Bourla, DVM, PhD, said, “Typically we are producing vaccines in single-dose vials. We are exploring with governments right now if it would be more convenient if there were 5-dose vials or 10-dose vials. I think we can resolve a significant part of the bottleneck.”
Despite the challenges, experts interviewed for this article agree that it will be possible to make a vaccine for COVID-19. They don’t expect attempts to meet the same complications that HIV researchers have seen over decades as the virus continues to confound with mutations.
Fred Ledley, MD, director of the Center for Integration of Science and Industry at Bentley University in Waltham, Massachusetts, told Medscape Medical News, “There doesn’t appear to be anything terribly diabolical about this virus. The mutation rate doesn’t appear to be anything like HIV. It appears to have some big, ugly proteins on the surface, which is good for vaccines — proteins with a lot of physical features look distinguishable from healthy cells. Signs all point to that it should be possible to make a vaccine.”
History raises safety concerns
However, Ledley said, “The idea of doing it in 6 months is largely unrealistic.”
He says 18 months is more realistic, primarily because of the sheer number of people that would have to be enrolled in a phase 3 study to truly test whether the endpoints are being met.
Vaccines are given to healthy volunteers. If safety signals arise, they may not be apparent until massive numbers of people are tested in phase 3.
“You’re never going to see the rates cut to 0%, but to see the difference between 10 people getting sick and seven people getting sick, takes very, very large numbers,” Ledley said. “There’s no way that can be done in 6 months. You’re talking about tens of thousands of people enrolled.”
He notes at this point it’s unclear what the endpoints will be and what the safety thresholds will be after consideration of risks and benefit.
Another big question for Ledley: “We don’t know what type of immunity we need to protect us against the virus. Do you just need the antibodies in your blood or do you need cells that are primed to attack the virus? Is it more of a chemical clearance or do the cells need to physically go in and digest the virus?”
History also points to the need for rigorous safety precautions that scientists fear could be compromised as trial phases overlap and processes are run in parallel instead of one step at a time.
An early batch of the Salk vaccine for polio in 1955, for example, turned out to be contaminated and caused paralysis in some children and 10 deaths, he points out.
CEPI’s Lurie adds that early candidates for another coronavirus, severe acute respiratory syndrome (SARS), “caused a reaction in the lungs that was very dangerous” before development was halted.
She also pointed to previous findings that a vaccine for dengue fever could worsen the disease in some people through a phenomenon called antibody-dependent enhancement.
Lurie and colleagues write in their paper that “it’s critical that vaccines also be developed using the tried-and-true methods, even if they may take longer to enter clinical trials or to result in large numbers of doses.”
Live attenuated vaccine
Raul Andino, PhD, a virologist at the University of California San Francisco, is among the scientists working with a tried-and-true method — a live attenuated vaccine — and he told Medscape Medical News he’s predicting it will take 2 years to develop.
He said it is cheaper to produce because scientists just have to learn how to grow the virus. Because the technology is already proven, a live attenuated vaccine could be rapidly produced on a worldwide scale.
The hope is also that a live attenuated vaccine would be given once in a lifetime and therefore be more affordable, especially in poorer countries.
“While a Moderna vaccine might be good for Europe and the United States,” he said, “It’s not going to be good for Africa, India, Brazil.”
Andino said, “I would bet money” that the front-runner vaccines so far will not be one-time vaccines.
He points out that most of the vaccine candidates are trying to protect people from disease. While there’s nothing wrong with that, he said, “In my opinion that is the lower-hanging fruit.”
“In my mind we need something that interrupts the chain of transmission and induces protection,” Andino said, important for developing herd immunity.
The reason this type of approach takes longer is because you are introducing a weakened form of the virus to the body and you have to make sure it doesn’t cause disease, not just in a small test population, but in populations who may be more susceptible to the disease, Andino said.
A call for unified strategies
Universities, countries, international consortiums, and public-private partnerships are all racing to find several safe and effective vaccines as no one entity will likely be able to provide the global solution.
Some of the efforts involve overlap of entities but with different focuses.
Along with “Operation Warp Speed” and CEPI, other collaborations include Gavi the Vaccine Alliance, whose core partners include WHO, UNICEF, the World Bank, and the Gates Foundation; and “Accelerating Therapeutic Interventions and Vaccines (ACTIV) partnership,” led by the National Institutes of Health.
Industry partners in ACTIV (18 biopharmaceutical companies), according to a May 18 article published online in the Journal of the American Medical Association, have said they will contribute their respective clinical trial capacities, regardless of which agent is studied.
Some, however, have called for more streamlining of efforts.
“Ideally we’d be working together,” Lurie told Medscape Medical News.
“I’m hopeful we will find ways to collaborate scientifically,” she said. “The US government’s responsibility is to make doses for the US. CEPI’s responsibility is to make doses for the world. A big focus of CEPI is to make sure we have manufacturing capacity outside of the US so those doses can be available to the world and they don’t get seized by wealthy countries.”
Bottazzi, Ledley, Lurie, and Andino report no relevant financial relationships.
This article first appeared on Medscape.com.
US government and industry projections that a COVID-19 vaccine will be ready by this fall or even January would take compressing what usually takes at least a decade into months, with little room for error or safety surprises.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said last week.
But Fauci said a more realistic timeline is still 12 to 18 months, and experts interviewed by Medscape Medical News agree. They say that although recent developments are encouraging, history and scientific reason say the day when a COVID-19 vaccine is widely available will not come this year and may not come by the end of 2021.
The encouraging signals come primarily from two recent announcements: the $1.2 billion United States backing last week of one vaccine platform and the announcement on May 18 that the first human trials of another have produced some positive phase 1 results.
Recent developments
On May 21, the US Department of Health and Human Services (HHS) under “Operation Warp Speed” announced that the US will give AstraZeneca $1.2 billion “to make available at least 300 million doses of a coronavirus vaccine called AZD1222, with the first doses delivered as early as October 2020.”
On May 18, the Massachusetts-based biotechnology company Moderna announced that phase 1 clinical results showed that its vaccine candidate, which uses a new messenger RNA (mRNA) technology, appeared safe. Eight participants in the human trials were able to produce neutralizing antibodies that researchers believe are important in developing protection from the virus.
Moderna Chief Medical Officer Tal Zaks, MD, PhD told CNN that if the vaccine candidate does well in phase 2, “it could be ready by January 2021.”
The two candidates are among 10 in clinical trials for the SARS-CoV-2 virus, according to the World Health Organization (WHO). The AstraZeneca/ AZD1222 candidate (also called ChAdOx1 nCoV-19, in collaboration with the University of Oxford) has entered phase 2/3.
Moderna’s candidate and another being developed in Beijing, China, are in phase 2, WHO reports. As of yesterday, 115 other candidates are in preclinical evaluation.
Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas, told Medscape Medical News it’s important to realize that, in the case of the $1.2 billion US investment, “what they’re talking about is manufacturing.”
The idea, she said, is to pay AstraZeneca up front so that manufacturing can start before it is known whether the vaccine candidate is safe or effective, the reverse of how the clinical trial process usually works.
That way, if the candidate is deemed safe and effective, time is not lost by then deciding how to make it and distribute it.
By the end of this year, she said, “Maybe we will have many vaccines made and stored in a refrigerator somewhere. But between now and December, there’s absolutely no way you can show efficacy of the vaccine at the same time you confirm that it’s safe.”
“Take these things with a grain of salt”
Animal testing for the AstraZeneca candidate, made in partnership with the University of Oxford in the United Kingdom, has yielded lackluster results, according to results on the preprint server BioRxiv, which have not been peer-reviewed.
“The results were not bad, but they were not gangbusters,” Bottazzi said. The results show the vaccine offered only partial protection.
“Partial protection is better than no protection,” she noted. “You have to take these things with a grain of salt. We don’t know what’s going to happen in humans.”
As for the Moderna candidate, Bottazzi said, “the good news is they found an appropriate safety profile. But from an eight-person group to make the extrapolation that they have efficacy — it’s unrealistic.”
Nicole Lurie, MD, MSPH, is senior adviser to the CEO for the Coalition for Epidemic Preparedness Innovation (CEPI), a nongovernmental organization funded by the Wellcome Trust, the Bill and Melinda Gates Foundation, the European Commission, and eight countries (Australia, Belgium, Canada, Ethiopia, Germany, Japan, Norway, and the United Kingdom) charged with supporting development of vaccines for pathogens on WHO’s priority list.
She and her colleagues write in a paper published online in the New England Journal of Medicine on March 30 that “it typically takes multiple candidates and many years to produce a licensed vaccine.”
The fastest time for developing a vaccine to date is 4 years, for the mumps vaccine, licensed in 1967.
As to whether she would expect a rollout of any vaccine by the end of the year, Lurie told Medscape Medical News, “If everything goes according to plan in every way, shape or form, well then maybe you can get there. But I wouldn’t hold my breath.”
Lurie and her colleagues write that “it’s far from certain that these new platforms will be scalable or that existing capacity can provide sufficient quantities of vaccine fast enough.”
On a call with reporters today, leaders of some of the words largest pharmaceutical companies said that one of the key bottlenecks is the sheer number of vials needed in order to distribute billions of doses of a successful vaccine.
Pfizer CEO Albert Bourla, DVM, PhD, said, “Typically we are producing vaccines in single-dose vials. We are exploring with governments right now if it would be more convenient if there were 5-dose vials or 10-dose vials. I think we can resolve a significant part of the bottleneck.”
Despite the challenges, experts interviewed for this article agree that it will be possible to make a vaccine for COVID-19. They don’t expect attempts to meet the same complications that HIV researchers have seen over decades as the virus continues to confound with mutations.
Fred Ledley, MD, director of the Center for Integration of Science and Industry at Bentley University in Waltham, Massachusetts, told Medscape Medical News, “There doesn’t appear to be anything terribly diabolical about this virus. The mutation rate doesn’t appear to be anything like HIV. It appears to have some big, ugly proteins on the surface, which is good for vaccines — proteins with a lot of physical features look distinguishable from healthy cells. Signs all point to that it should be possible to make a vaccine.”
History raises safety concerns
However, Ledley said, “The idea of doing it in 6 months is largely unrealistic.”
He says 18 months is more realistic, primarily because of the sheer number of people that would have to be enrolled in a phase 3 study to truly test whether the endpoints are being met.
Vaccines are given to healthy volunteers. If safety signals arise, they may not be apparent until massive numbers of people are tested in phase 3.
“You’re never going to see the rates cut to 0%, but to see the difference between 10 people getting sick and seven people getting sick, takes very, very large numbers,” Ledley said. “There’s no way that can be done in 6 months. You’re talking about tens of thousands of people enrolled.”
He notes at this point it’s unclear what the endpoints will be and what the safety thresholds will be after consideration of risks and benefit.
Another big question for Ledley: “We don’t know what type of immunity we need to protect us against the virus. Do you just need the antibodies in your blood or do you need cells that are primed to attack the virus? Is it more of a chemical clearance or do the cells need to physically go in and digest the virus?”
History also points to the need for rigorous safety precautions that scientists fear could be compromised as trial phases overlap and processes are run in parallel instead of one step at a time.
An early batch of the Salk vaccine for polio in 1955, for example, turned out to be contaminated and caused paralysis in some children and 10 deaths, he points out.
CEPI’s Lurie adds that early candidates for another coronavirus, severe acute respiratory syndrome (SARS), “caused a reaction in the lungs that was very dangerous” before development was halted.
She also pointed to previous findings that a vaccine for dengue fever could worsen the disease in some people through a phenomenon called antibody-dependent enhancement.
Lurie and colleagues write in their paper that “it’s critical that vaccines also be developed using the tried-and-true methods, even if they may take longer to enter clinical trials or to result in large numbers of doses.”
Live attenuated vaccine
Raul Andino, PhD, a virologist at the University of California San Francisco, is among the scientists working with a tried-and-true method — a live attenuated vaccine — and he told Medscape Medical News he’s predicting it will take 2 years to develop.
He said it is cheaper to produce because scientists just have to learn how to grow the virus. Because the technology is already proven, a live attenuated vaccine could be rapidly produced on a worldwide scale.
The hope is also that a live attenuated vaccine would be given once in a lifetime and therefore be more affordable, especially in poorer countries.
“While a Moderna vaccine might be good for Europe and the United States,” he said, “It’s not going to be good for Africa, India, Brazil.”
Andino said, “I would bet money” that the front-runner vaccines so far will not be one-time vaccines.
He points out that most of the vaccine candidates are trying to protect people from disease. While there’s nothing wrong with that, he said, “In my opinion that is the lower-hanging fruit.”
“In my mind we need something that interrupts the chain of transmission and induces protection,” Andino said, important for developing herd immunity.
The reason this type of approach takes longer is because you are introducing a weakened form of the virus to the body and you have to make sure it doesn’t cause disease, not just in a small test population, but in populations who may be more susceptible to the disease, Andino said.
A call for unified strategies
Universities, countries, international consortiums, and public-private partnerships are all racing to find several safe and effective vaccines as no one entity will likely be able to provide the global solution.
Some of the efforts involve overlap of entities but with different focuses.
Along with “Operation Warp Speed” and CEPI, other collaborations include Gavi the Vaccine Alliance, whose core partners include WHO, UNICEF, the World Bank, and the Gates Foundation; and “Accelerating Therapeutic Interventions and Vaccines (ACTIV) partnership,” led by the National Institutes of Health.
Industry partners in ACTIV (18 biopharmaceutical companies), according to a May 18 article published online in the Journal of the American Medical Association, have said they will contribute their respective clinical trial capacities, regardless of which agent is studied.
Some, however, have called for more streamlining of efforts.
“Ideally we’d be working together,” Lurie told Medscape Medical News.
“I’m hopeful we will find ways to collaborate scientifically,” she said. “The US government’s responsibility is to make doses for the US. CEPI’s responsibility is to make doses for the world. A big focus of CEPI is to make sure we have manufacturing capacity outside of the US so those doses can be available to the world and they don’t get seized by wealthy countries.”
Bottazzi, Ledley, Lurie, and Andino report no relevant financial relationships.
This article first appeared on Medscape.com.
Family physicians have lowest incentive bonuses, survey finds
according to the Medscape Family Medicine Physician Compensation Report 2020.
This year’s survey was the first to ask about bonuses, and it showed strong contrasts between specialties. Family physicians’ bonuses averaged $24,000, whereas orthopedists’ were four times higher, at $96,000.
Two-thirds of family physicians (67%), similar to physicians overall, reported that bonuses had no influence on the number of hours worked.
More than half of all physicians in the survey (56%) said they got such bonuses.
Family physicians’ pay was up $3,000 from last year, to $234,000, but still ranked near the bottom in comparison with other specialties. Only physicians in public health/preventive medicine and pediatrics made less, both at $232,000.
The top four specialties in pay were the same this year as they were last year and ranked in the same order: Orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. In a study from the Medical Group Management Association, researchers estimated that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
Male family physicians continue to make more than their female colleagues, with this year’s difference at 26% ($257,000 vs. $205,000). Male specialists overall in the survey made 31% more than their female counterparts.
Few claims denied
A bright spot in compensation was that family physicians have among the lowest rates (14%) of claims that are denied or that need to be resubmitted. Plastic surgeons have twice that rate (28%) of rejected claims.
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but health care professionals spend about $118 per claim on appeals.”
Family physicians were in the middle of the pack as far as how much time was spent on paperwork. On average, they spent 15.9 hours a week on the tasks. Intensivists spent the most, at 19.1 hours each week, and ophthalmologists spent the least, at 9.8 hours per week.
Although 73% of physicians overall said they had no plans to stop accepting new and current Medicare and Medicaid patients, only 65% of family physicians answered that way. Seventeen percent said they would stop taking new Medicare patients, and 9% said they wouldn’t take new Medicaid patients; 15% had not made those decisions yet.
Rules and regulations are the biggest challenges
Asked about their biggest challenges, 29% of family physicians put “having so many rules and regulations” at the top. Next came working with an electronic health records system, followed by dealing with difficult patients.
The biggest reward, they said again this year, was “gratitude/relationships with patients” (34% ranked it at the top), followed by “knowing I’m making the world a better place” (25%), “being very good at what I do/finding answers, diagnoses” (18%), and “making good money at a job that I like” (10%).
Most family practices employ advanced practice providers (62% employed NPs, and 43% employed PAs). Fewer than one-third employed neither.
Of the family medicine physicians who did work with advanced practice providers in their offices, half (50%) said they improved profitability, 45% said they had no effect, and 5% said they decreased profitability.
A version of this article originally appeared on Medscape.com.
according to the Medscape Family Medicine Physician Compensation Report 2020.
This year’s survey was the first to ask about bonuses, and it showed strong contrasts between specialties. Family physicians’ bonuses averaged $24,000, whereas orthopedists’ were four times higher, at $96,000.
Two-thirds of family physicians (67%), similar to physicians overall, reported that bonuses had no influence on the number of hours worked.
More than half of all physicians in the survey (56%) said they got such bonuses.
Family physicians’ pay was up $3,000 from last year, to $234,000, but still ranked near the bottom in comparison with other specialties. Only physicians in public health/preventive medicine and pediatrics made less, both at $232,000.
The top four specialties in pay were the same this year as they were last year and ranked in the same order: Orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. In a study from the Medical Group Management Association, researchers estimated that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
Male family physicians continue to make more than their female colleagues, with this year’s difference at 26% ($257,000 vs. $205,000). Male specialists overall in the survey made 31% more than their female counterparts.
Few claims denied
A bright spot in compensation was that family physicians have among the lowest rates (14%) of claims that are denied or that need to be resubmitted. Plastic surgeons have twice that rate (28%) of rejected claims.
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but health care professionals spend about $118 per claim on appeals.”
Family physicians were in the middle of the pack as far as how much time was spent on paperwork. On average, they spent 15.9 hours a week on the tasks. Intensivists spent the most, at 19.1 hours each week, and ophthalmologists spent the least, at 9.8 hours per week.
Although 73% of physicians overall said they had no plans to stop accepting new and current Medicare and Medicaid patients, only 65% of family physicians answered that way. Seventeen percent said they would stop taking new Medicare patients, and 9% said they wouldn’t take new Medicaid patients; 15% had not made those decisions yet.
Rules and regulations are the biggest challenges
Asked about their biggest challenges, 29% of family physicians put “having so many rules and regulations” at the top. Next came working with an electronic health records system, followed by dealing with difficult patients.
The biggest reward, they said again this year, was “gratitude/relationships with patients” (34% ranked it at the top), followed by “knowing I’m making the world a better place” (25%), “being very good at what I do/finding answers, diagnoses” (18%), and “making good money at a job that I like” (10%).
Most family practices employ advanced practice providers (62% employed NPs, and 43% employed PAs). Fewer than one-third employed neither.
Of the family medicine physicians who did work with advanced practice providers in their offices, half (50%) said they improved profitability, 45% said they had no effect, and 5% said they decreased profitability.
A version of this article originally appeared on Medscape.com.
according to the Medscape Family Medicine Physician Compensation Report 2020.
This year’s survey was the first to ask about bonuses, and it showed strong contrasts between specialties. Family physicians’ bonuses averaged $24,000, whereas orthopedists’ were four times higher, at $96,000.
Two-thirds of family physicians (67%), similar to physicians overall, reported that bonuses had no influence on the number of hours worked.
More than half of all physicians in the survey (56%) said they got such bonuses.
Family physicians’ pay was up $3,000 from last year, to $234,000, but still ranked near the bottom in comparison with other specialties. Only physicians in public health/preventive medicine and pediatrics made less, both at $232,000.
The top four specialties in pay were the same this year as they were last year and ranked in the same order: Orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. In a study from the Medical Group Management Association, researchers estimated that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
Male family physicians continue to make more than their female colleagues, with this year’s difference at 26% ($257,000 vs. $205,000). Male specialists overall in the survey made 31% more than their female counterparts.
Few claims denied
A bright spot in compensation was that family physicians have among the lowest rates (14%) of claims that are denied or that need to be resubmitted. Plastic surgeons have twice that rate (28%) of rejected claims.
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but health care professionals spend about $118 per claim on appeals.”
Family physicians were in the middle of the pack as far as how much time was spent on paperwork. On average, they spent 15.9 hours a week on the tasks. Intensivists spent the most, at 19.1 hours each week, and ophthalmologists spent the least, at 9.8 hours per week.
Although 73% of physicians overall said they had no plans to stop accepting new and current Medicare and Medicaid patients, only 65% of family physicians answered that way. Seventeen percent said they would stop taking new Medicare patients, and 9% said they wouldn’t take new Medicaid patients; 15% had not made those decisions yet.
Rules and regulations are the biggest challenges
Asked about their biggest challenges, 29% of family physicians put “having so many rules and regulations” at the top. Next came working with an electronic health records system, followed by dealing with difficult patients.
The biggest reward, they said again this year, was “gratitude/relationships with patients” (34% ranked it at the top), followed by “knowing I’m making the world a better place” (25%), “being very good at what I do/finding answers, diagnoses” (18%), and “making good money at a job that I like” (10%).
Most family practices employ advanced practice providers (62% employed NPs, and 43% employed PAs). Fewer than one-third employed neither.
Of the family medicine physicians who did work with advanced practice providers in their offices, half (50%) said they improved profitability, 45% said they had no effect, and 5% said they decreased profitability.
A version of this article originally appeared on Medscape.com.