User login
OA risk-reduction program targets injured knees
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
FROM CARC 2021
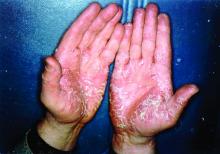
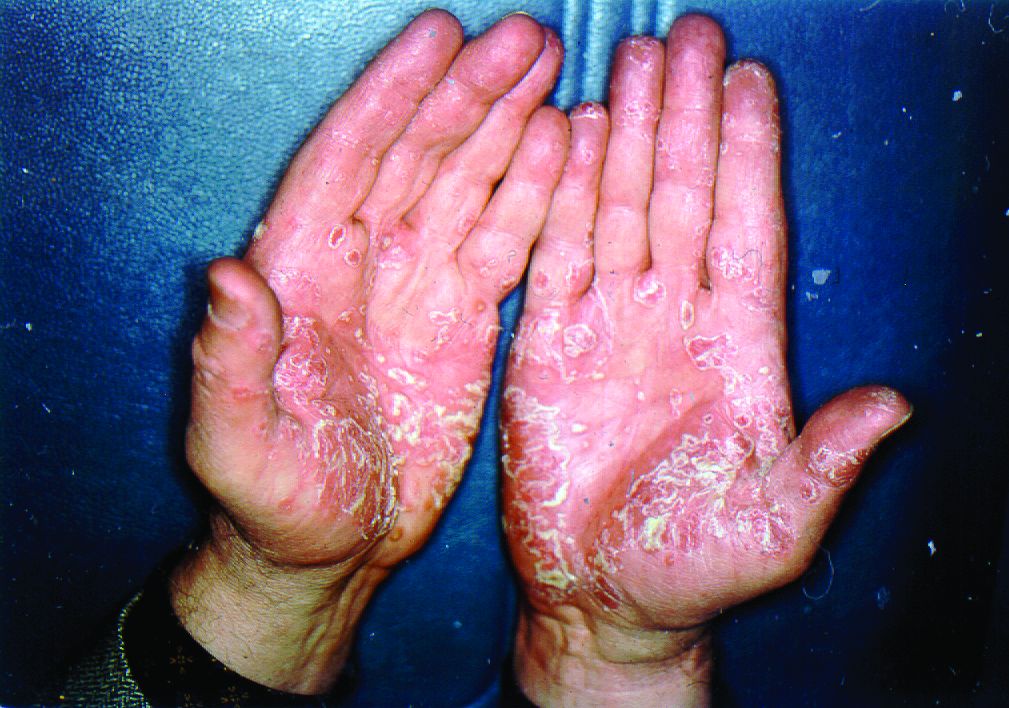
Pandemic puts patients with psoriatic disease off seeking medical help
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
FROM CARC 2021
Rheumatologic disease activity an important influencer of COVID-19 death risk
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
FROM ANNALS OF THE RHEUMATIC DISEASES
Entinostat doesn’t overcome endocrine resistance in advanced breast cancer
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
FROM SABCS 2020
Study: Doctors underreport side effects of breast irradiation
There is a substantial mismatch between patient and physician reports of toxicity during radiotherapy for breast cancer, according to an analysis of nearly 10,000 U.S. patients.
Researchers assessed physician underrecognition of four key symptoms – pain, pruritus, edema, and fatigue – during radiotherapy. Physicians underrecognized one of these four symptoms at least once in 53.2% of patients who reported having at least one substantial symptom.
“Understanding whether physicians detect when their patients are experiencing substantial toxicity is important, not only because recognition of symptoms is necessary for appropriate supportive care, but also because it influences what techniques and treatments we adopt,” said Reshma Jagsi, MD, DPhil, of the University of Michigan, Ann Arbor.
Dr. Jagsi presented the study results at the 2020 San Antonio Breast Cancer Symposium.
The underrecognition of symptoms during radiotherapy may reflect differences in physician or patient behaviors, according to Dr. Jagsi. “It’s absolutely something we need to understand better.”
Ian E. Krop, MD, PhD, of the Dana-Farber Cancer Institute in Boston, moderated the session where this research was presented and called the work “striking,” noting that it “clearly identifies an important area that needs improvement.”
“We need to do a better job. We as physicians need to listen more to our patients,” said Virginia Kaklamani, MD, of the University of Texas Health Science Center, San Antonio, and codirector of the SABCS 2020 meeting.
Comparing patient and physician reports
Dr. Jagsi and colleagues analyzed data on patients who had received radiotherapy after lumpectomy for breast cancer and had completed patient-reported outcome measures (PROs) as part of the Michigan Radiation Oncology Quality Consortium (MROQC). The MROQC registry collects data on patients receiving radiation for breast, lung, and prostate cancers, as well as for bone metastases.
Results of the PROs were compared with physician reports of toxicity as assessed using the Common Terminology Criteria for Adverse Events (CTCAE) system.
The researchers evaluated underrecognition of toxicity in 9,868 patients by comparing 37,593 independent paired observations from patients and their doctors. Patient and physician reports were made on the same day (n = 35,797) or within 3 days of each other (n = 1,796).
The comparison showed underrecognition of all four symptoms assessed – pain, pruritus, edema, and fatigue.
Underrecognition of pain was defined as patients reporting moderate pain while physicians graded pain as absent or as patients reporting severe pain while physicians rated pain as grade 1 or lower. Underrecognition of fatigue, bother from pruritus, or bother from edema were defined as physicians grading these symptoms as absent when patients reported fatigue or bother from pruritus/edema often or all of the time.
The percentage of observations with underrecognized symptoms was 30.9% for moderate to severe pain, 36.7% for frequent bother from pruritus, 51.4% for frequent bother from edema, and 18.8% for severe fatigue.
Factors independently associated with symptom underrecognition were younger age (odds ratio, 1.4 for <50 years and 1.2 for 50-59 years), Black or other non-White race (OR, 1.9 and 1.8, respectively), conventional fractionation (OR, 1.2), not having a supraclavicular field (OR, 1.3), and being treated at an academic center (OR, 1.1).
Underreporting worse in the time of COVID?
Data collection for this study ended before the start of the COVID-19 pandemic, but Dr. Jagsi expressed concern that the pandemic could lead to underrecognition of toxicity as well.
“We are doing more virtual visits, and I think the relationships between physicians and patients are a bit more strained,” Dr. Jagsi said. While virtual visits mean that patients can be seen safely, they are “not the same as being in the same room as one another.”
On the other hand, in-person visits during the pandemic may pose challenges as well. The need to wear masks during in-person consultations could lead to a lot of nonverbal communication being missed.
“I wouldn’t be surprised at all if underrecognition were worse in this context,” Dr. Jagsi said.
Encourage patients to speak up, use PROs
“I think we need to encourage patients that when we’ve told them that certain side effects are expected, it doesn’t mean that they shouldn’t tell us if they’re bothered by those side effects,” Dr. Jagsi said. “They’re not bothering us. They’re not troubling us to bring those symptoms to our attention, because there actually are things that we can do to help support them through the experience.”
Dr. Jagsi also said PROs should be included in clinical trials. Trials tend to rely on physician assessment of possible toxicity using the CTCAE system, but this can miss important symptoms that patients experience during radiotherapy.
The current study and MROQC were sponsored by Blue Cross Blue Shield of Michigan and the Blue Care Network as part of the BCBSM Value Partnership program. Dr. Jagsi disclosed financial relationships with Amgen, Equity Quotient, Genentech, Vizient, law firms, various foundations, the National Institutes of Health, and BCBSM for the MROQC. Dr. Kaklamani and Dr. Krop disclosed relationships with many pharmaceutical companies.
SOURCE: Jagsi R J et al. SABCS 2020, Abstract GS3-07.
There is a substantial mismatch between patient and physician reports of toxicity during radiotherapy for breast cancer, according to an analysis of nearly 10,000 U.S. patients.
Researchers assessed physician underrecognition of four key symptoms – pain, pruritus, edema, and fatigue – during radiotherapy. Physicians underrecognized one of these four symptoms at least once in 53.2% of patients who reported having at least one substantial symptom.
“Understanding whether physicians detect when their patients are experiencing substantial toxicity is important, not only because recognition of symptoms is necessary for appropriate supportive care, but also because it influences what techniques and treatments we adopt,” said Reshma Jagsi, MD, DPhil, of the University of Michigan, Ann Arbor.
Dr. Jagsi presented the study results at the 2020 San Antonio Breast Cancer Symposium.
The underrecognition of symptoms during radiotherapy may reflect differences in physician or patient behaviors, according to Dr. Jagsi. “It’s absolutely something we need to understand better.”
Ian E. Krop, MD, PhD, of the Dana-Farber Cancer Institute in Boston, moderated the session where this research was presented and called the work “striking,” noting that it “clearly identifies an important area that needs improvement.”
“We need to do a better job. We as physicians need to listen more to our patients,” said Virginia Kaklamani, MD, of the University of Texas Health Science Center, San Antonio, and codirector of the SABCS 2020 meeting.
Comparing patient and physician reports
Dr. Jagsi and colleagues analyzed data on patients who had received radiotherapy after lumpectomy for breast cancer and had completed patient-reported outcome measures (PROs) as part of the Michigan Radiation Oncology Quality Consortium (MROQC). The MROQC registry collects data on patients receiving radiation for breast, lung, and prostate cancers, as well as for bone metastases.
Results of the PROs were compared with physician reports of toxicity as assessed using the Common Terminology Criteria for Adverse Events (CTCAE) system.
The researchers evaluated underrecognition of toxicity in 9,868 patients by comparing 37,593 independent paired observations from patients and their doctors. Patient and physician reports were made on the same day (n = 35,797) or within 3 days of each other (n = 1,796).
The comparison showed underrecognition of all four symptoms assessed – pain, pruritus, edema, and fatigue.
Underrecognition of pain was defined as patients reporting moderate pain while physicians graded pain as absent or as patients reporting severe pain while physicians rated pain as grade 1 or lower. Underrecognition of fatigue, bother from pruritus, or bother from edema were defined as physicians grading these symptoms as absent when patients reported fatigue or bother from pruritus/edema often or all of the time.
The percentage of observations with underrecognized symptoms was 30.9% for moderate to severe pain, 36.7% for frequent bother from pruritus, 51.4% for frequent bother from edema, and 18.8% for severe fatigue.
Factors independently associated with symptom underrecognition were younger age (odds ratio, 1.4 for <50 years and 1.2 for 50-59 years), Black or other non-White race (OR, 1.9 and 1.8, respectively), conventional fractionation (OR, 1.2), not having a supraclavicular field (OR, 1.3), and being treated at an academic center (OR, 1.1).
Underreporting worse in the time of COVID?
Data collection for this study ended before the start of the COVID-19 pandemic, but Dr. Jagsi expressed concern that the pandemic could lead to underrecognition of toxicity as well.
“We are doing more virtual visits, and I think the relationships between physicians and patients are a bit more strained,” Dr. Jagsi said. While virtual visits mean that patients can be seen safely, they are “not the same as being in the same room as one another.”
On the other hand, in-person visits during the pandemic may pose challenges as well. The need to wear masks during in-person consultations could lead to a lot of nonverbal communication being missed.
“I wouldn’t be surprised at all if underrecognition were worse in this context,” Dr. Jagsi said.
Encourage patients to speak up, use PROs
“I think we need to encourage patients that when we’ve told them that certain side effects are expected, it doesn’t mean that they shouldn’t tell us if they’re bothered by those side effects,” Dr. Jagsi said. “They’re not bothering us. They’re not troubling us to bring those symptoms to our attention, because there actually are things that we can do to help support them through the experience.”
Dr. Jagsi also said PROs should be included in clinical trials. Trials tend to rely on physician assessment of possible toxicity using the CTCAE system, but this can miss important symptoms that patients experience during radiotherapy.
The current study and MROQC were sponsored by Blue Cross Blue Shield of Michigan and the Blue Care Network as part of the BCBSM Value Partnership program. Dr. Jagsi disclosed financial relationships with Amgen, Equity Quotient, Genentech, Vizient, law firms, various foundations, the National Institutes of Health, and BCBSM for the MROQC. Dr. Kaklamani and Dr. Krop disclosed relationships with many pharmaceutical companies.
SOURCE: Jagsi R J et al. SABCS 2020, Abstract GS3-07.
There is a substantial mismatch between patient and physician reports of toxicity during radiotherapy for breast cancer, according to an analysis of nearly 10,000 U.S. patients.
Researchers assessed physician underrecognition of four key symptoms – pain, pruritus, edema, and fatigue – during radiotherapy. Physicians underrecognized one of these four symptoms at least once in 53.2% of patients who reported having at least one substantial symptom.
“Understanding whether physicians detect when their patients are experiencing substantial toxicity is important, not only because recognition of symptoms is necessary for appropriate supportive care, but also because it influences what techniques and treatments we adopt,” said Reshma Jagsi, MD, DPhil, of the University of Michigan, Ann Arbor.
Dr. Jagsi presented the study results at the 2020 San Antonio Breast Cancer Symposium.
The underrecognition of symptoms during radiotherapy may reflect differences in physician or patient behaviors, according to Dr. Jagsi. “It’s absolutely something we need to understand better.”
Ian E. Krop, MD, PhD, of the Dana-Farber Cancer Institute in Boston, moderated the session where this research was presented and called the work “striking,” noting that it “clearly identifies an important area that needs improvement.”
“We need to do a better job. We as physicians need to listen more to our patients,” said Virginia Kaklamani, MD, of the University of Texas Health Science Center, San Antonio, and codirector of the SABCS 2020 meeting.
Comparing patient and physician reports
Dr. Jagsi and colleagues analyzed data on patients who had received radiotherapy after lumpectomy for breast cancer and had completed patient-reported outcome measures (PROs) as part of the Michigan Radiation Oncology Quality Consortium (MROQC). The MROQC registry collects data on patients receiving radiation for breast, lung, and prostate cancers, as well as for bone metastases.
Results of the PROs were compared with physician reports of toxicity as assessed using the Common Terminology Criteria for Adverse Events (CTCAE) system.
The researchers evaluated underrecognition of toxicity in 9,868 patients by comparing 37,593 independent paired observations from patients and their doctors. Patient and physician reports were made on the same day (n = 35,797) or within 3 days of each other (n = 1,796).
The comparison showed underrecognition of all four symptoms assessed – pain, pruritus, edema, and fatigue.
Underrecognition of pain was defined as patients reporting moderate pain while physicians graded pain as absent or as patients reporting severe pain while physicians rated pain as grade 1 or lower. Underrecognition of fatigue, bother from pruritus, or bother from edema were defined as physicians grading these symptoms as absent when patients reported fatigue or bother from pruritus/edema often or all of the time.
The percentage of observations with underrecognized symptoms was 30.9% for moderate to severe pain, 36.7% for frequent bother from pruritus, 51.4% for frequent bother from edema, and 18.8% for severe fatigue.
Factors independently associated with symptom underrecognition were younger age (odds ratio, 1.4 for <50 years and 1.2 for 50-59 years), Black or other non-White race (OR, 1.9 and 1.8, respectively), conventional fractionation (OR, 1.2), not having a supraclavicular field (OR, 1.3), and being treated at an academic center (OR, 1.1).
Underreporting worse in the time of COVID?
Data collection for this study ended before the start of the COVID-19 pandemic, but Dr. Jagsi expressed concern that the pandemic could lead to underrecognition of toxicity as well.
“We are doing more virtual visits, and I think the relationships between physicians and patients are a bit more strained,” Dr. Jagsi said. While virtual visits mean that patients can be seen safely, they are “not the same as being in the same room as one another.”
On the other hand, in-person visits during the pandemic may pose challenges as well. The need to wear masks during in-person consultations could lead to a lot of nonverbal communication being missed.
“I wouldn’t be surprised at all if underrecognition were worse in this context,” Dr. Jagsi said.
Encourage patients to speak up, use PROs
“I think we need to encourage patients that when we’ve told them that certain side effects are expected, it doesn’t mean that they shouldn’t tell us if they’re bothered by those side effects,” Dr. Jagsi said. “They’re not bothering us. They’re not troubling us to bring those symptoms to our attention, because there actually are things that we can do to help support them through the experience.”
Dr. Jagsi also said PROs should be included in clinical trials. Trials tend to rely on physician assessment of possible toxicity using the CTCAE system, but this can miss important symptoms that patients experience during radiotherapy.
The current study and MROQC were sponsored by Blue Cross Blue Shield of Michigan and the Blue Care Network as part of the BCBSM Value Partnership program. Dr. Jagsi disclosed financial relationships with Amgen, Equity Quotient, Genentech, Vizient, law firms, various foundations, the National Institutes of Health, and BCBSM for the MROQC. Dr. Kaklamani and Dr. Krop disclosed relationships with many pharmaceutical companies.
SOURCE: Jagsi R J et al. SABCS 2020, Abstract GS3-07.
FROM SABCS 2020
No edge for anastrozole over tamoxifen in DCIS
“Our analysis shows that there was really no significant difference in recurrences,” said investigator Ivana Sestak, PhD, of Queen Mary University of London.
Similarly, there were no significant differences in overall deaths or breast cancer deaths. On the other hand, there were “clear differences” in adverse events with the two treatments, Dr. Sestak said.
“[W]e observed an excess of endometrial cancer and ovarian cancers in women who were randomized to tamoxifen and an excess of fractures, strokes, and transient ischemic attacks for women who were randomized to anastrozole,” Dr. Sestak said.
She presented these results at the 2020 San Antonio Breast Cancer Symposium.
Comparing IBIS-II DCIS with prior results
“The long-term results of the IBIS-II [DCIS] trial are consistent with previous results,” said Halle Moore, MD, of the Cleveland Clinic, who was not involved in this study.
However, the results do contrast with the findings of the NSABP-B35 study, “which demonstrated a very modest advantage to anastrozole that was mostly limited to younger postmenopausal women,” she said.
Indeed, a significant 27% reduction in recurrence was seen with anastrozole, compared with tamoxifen at a median of 9 years of follow-up in the NSABP-B35 study. But, as Dr. Sestak pointed out, that reduction “was mainly observed during the posttreatment follow-up period and not during the active treatment period.”
In the IBIS-II DCIS study, there was a nonsignificant 11% reduction in breast cancer recurrence with anastrozole.
The investigators did examine the potential effect of age on the rate of breast cancer recurrence, but no significant differences were found. They also looked at the active and post–endocrine therapy treatment periods, all showing no benefit of one drug over the other.
“So we clearly cannot replicate the findings of the NSABP-B35 study,” Dr. Sestak acknowledged.
IBIS-II DCIS details and results
Aromatase inhibitors such as anastrozole have been shown to be more effective than tamoxifen for preventing recurrence in postmenopausal HR+ women with invasive breast cancer, but it wasn’t previously known if this included women with HR-positive DCIS.
The IBIS-II DCIS study was therefore designed to determine if there was any advantage of anastrazole over tamoxifen. The phase 3 trial enrolled 2,980 postmenopausal women with HR-positive DCIS. They were randomized to 5 years of anastrozole or 5 years of tamoxifen.
“Women were followed up during this active period of treatment by clinic visits,” explained Dr. Sestak. “Thereafter, we collected data via questionnaire, registry data sets, and clinic visits.”
Baseline characteristics were similar between the treatment arms. The median age was about 60 years in both arms. Similar proportions of patients had received hormone replacement therapy (44.2% in the tamoxifen arm and 46.8% in the anastrozole arm) or undergone radiotherapy (71.5% and 70.9%, respectively).
Results showed little difference in breast cancer recurrence rates. At a median follow-up of 11.6 years, the rate of recurrence was 9.7% with tamoxifen and 8.5% with anastrozole (hazard ratio, 0.89; P = .401).
Trends were seen favoring anastrozole in estrogen receptor–positive and HER2-negative women, but this was only while women were being actively treated.
Death rates were similar – 4.2% with anastrozole and 4.5% with tamoxifen (odds ratio, 0.93). There were three breast cancer deaths in each treatment arm.
Adverse events could be the deciding factor
“The main take-away from this presentation is that choice of adjuvant endocrine therapy for DCIS should be individualized based primarily on the different side effect profiles of the two medications,” Dr. Moore said.
During the trial, “a significant 34% increase in fractures was observed in women who received anastrozole, compared to tamoxifen [HR, 1.34; P = .013],” Dr. Sestak said.
“We also observed a threefold increase in strokes and transient ischemic attacks with anastrozole, compared to tamoxifen [HR, 3.10; P = .021 for both strokes and transient ischemic attacks],” Dr. Sestak added.
She acknowledged that this finding is inconsistent with what is known about aromatase inhibitors in general. It could be that, rather than anastrozole raising the risk of strokes and transient ischemic attacks, tamoxifen was having a beneficial effect. This could be a result of tamoxifen improving endothelial function by increasing vasodilation, “but it is really not clear what the mechanism is,” Dr. Sestak said.
Also contrary to what is known about tamoxifen was an excess of deaths because of endometrial cancer or ovarian cancers. Wherever possible, the pathology reports had been requested to confirm the cause of death, “so we are pretty sure that they are true ovarian cancers and not some other abdominal tumors,” Dr. Sestak said.
“We did observe very clear differences in terms of adverse events,” she said, adding that “improved understanding of adverse event profiles will help patients with HR-positive DCIS to make an informed decision regarding their treatment.”
The IBIS-II DCIS trial was funded by Cancer Research UK, the National Health and Medical Research Council Australia, Breast Cancer Research Foundation, AstraZeneca, and Sanofi Aventis. Two investigators disclosed relationships with AstraZeneca, and one disclosed a relationship with Cancer Research UK. Dr. Sestak and Dr. Moore had no relevant disclosures.
SOURCE: Sestak I et al. SABCS 2020, Abstract GS2-02.
“Our analysis shows that there was really no significant difference in recurrences,” said investigator Ivana Sestak, PhD, of Queen Mary University of London.
Similarly, there were no significant differences in overall deaths or breast cancer deaths. On the other hand, there were “clear differences” in adverse events with the two treatments, Dr. Sestak said.
“[W]e observed an excess of endometrial cancer and ovarian cancers in women who were randomized to tamoxifen and an excess of fractures, strokes, and transient ischemic attacks for women who were randomized to anastrozole,” Dr. Sestak said.
She presented these results at the 2020 San Antonio Breast Cancer Symposium.
Comparing IBIS-II DCIS with prior results
“The long-term results of the IBIS-II [DCIS] trial are consistent with previous results,” said Halle Moore, MD, of the Cleveland Clinic, who was not involved in this study.
However, the results do contrast with the findings of the NSABP-B35 study, “which demonstrated a very modest advantage to anastrozole that was mostly limited to younger postmenopausal women,” she said.
Indeed, a significant 27% reduction in recurrence was seen with anastrozole, compared with tamoxifen at a median of 9 years of follow-up in the NSABP-B35 study. But, as Dr. Sestak pointed out, that reduction “was mainly observed during the posttreatment follow-up period and not during the active treatment period.”
In the IBIS-II DCIS study, there was a nonsignificant 11% reduction in breast cancer recurrence with anastrozole.
The investigators did examine the potential effect of age on the rate of breast cancer recurrence, but no significant differences were found. They also looked at the active and post–endocrine therapy treatment periods, all showing no benefit of one drug over the other.
“So we clearly cannot replicate the findings of the NSABP-B35 study,” Dr. Sestak acknowledged.
IBIS-II DCIS details and results
Aromatase inhibitors such as anastrozole have been shown to be more effective than tamoxifen for preventing recurrence in postmenopausal HR+ women with invasive breast cancer, but it wasn’t previously known if this included women with HR-positive DCIS.
The IBIS-II DCIS study was therefore designed to determine if there was any advantage of anastrazole over tamoxifen. The phase 3 trial enrolled 2,980 postmenopausal women with HR-positive DCIS. They were randomized to 5 years of anastrozole or 5 years of tamoxifen.
“Women were followed up during this active period of treatment by clinic visits,” explained Dr. Sestak. “Thereafter, we collected data via questionnaire, registry data sets, and clinic visits.”
Baseline characteristics were similar between the treatment arms. The median age was about 60 years in both arms. Similar proportions of patients had received hormone replacement therapy (44.2% in the tamoxifen arm and 46.8% in the anastrozole arm) or undergone radiotherapy (71.5% and 70.9%, respectively).
Results showed little difference in breast cancer recurrence rates. At a median follow-up of 11.6 years, the rate of recurrence was 9.7% with tamoxifen and 8.5% with anastrozole (hazard ratio, 0.89; P = .401).
Trends were seen favoring anastrozole in estrogen receptor–positive and HER2-negative women, but this was only while women were being actively treated.
Death rates were similar – 4.2% with anastrozole and 4.5% with tamoxifen (odds ratio, 0.93). There were three breast cancer deaths in each treatment arm.
Adverse events could be the deciding factor
“The main take-away from this presentation is that choice of adjuvant endocrine therapy for DCIS should be individualized based primarily on the different side effect profiles of the two medications,” Dr. Moore said.
During the trial, “a significant 34% increase in fractures was observed in women who received anastrozole, compared to tamoxifen [HR, 1.34; P = .013],” Dr. Sestak said.
“We also observed a threefold increase in strokes and transient ischemic attacks with anastrozole, compared to tamoxifen [HR, 3.10; P = .021 for both strokes and transient ischemic attacks],” Dr. Sestak added.
She acknowledged that this finding is inconsistent with what is known about aromatase inhibitors in general. It could be that, rather than anastrozole raising the risk of strokes and transient ischemic attacks, tamoxifen was having a beneficial effect. This could be a result of tamoxifen improving endothelial function by increasing vasodilation, “but it is really not clear what the mechanism is,” Dr. Sestak said.
Also contrary to what is known about tamoxifen was an excess of deaths because of endometrial cancer or ovarian cancers. Wherever possible, the pathology reports had been requested to confirm the cause of death, “so we are pretty sure that they are true ovarian cancers and not some other abdominal tumors,” Dr. Sestak said.
“We did observe very clear differences in terms of adverse events,” she said, adding that “improved understanding of adverse event profiles will help patients with HR-positive DCIS to make an informed decision regarding their treatment.”
The IBIS-II DCIS trial was funded by Cancer Research UK, the National Health and Medical Research Council Australia, Breast Cancer Research Foundation, AstraZeneca, and Sanofi Aventis. Two investigators disclosed relationships with AstraZeneca, and one disclosed a relationship with Cancer Research UK. Dr. Sestak and Dr. Moore had no relevant disclosures.
SOURCE: Sestak I et al. SABCS 2020, Abstract GS2-02.
“Our analysis shows that there was really no significant difference in recurrences,” said investigator Ivana Sestak, PhD, of Queen Mary University of London.
Similarly, there were no significant differences in overall deaths or breast cancer deaths. On the other hand, there were “clear differences” in adverse events with the two treatments, Dr. Sestak said.
“[W]e observed an excess of endometrial cancer and ovarian cancers in women who were randomized to tamoxifen and an excess of fractures, strokes, and transient ischemic attacks for women who were randomized to anastrozole,” Dr. Sestak said.
She presented these results at the 2020 San Antonio Breast Cancer Symposium.
Comparing IBIS-II DCIS with prior results
“The long-term results of the IBIS-II [DCIS] trial are consistent with previous results,” said Halle Moore, MD, of the Cleveland Clinic, who was not involved in this study.
However, the results do contrast with the findings of the NSABP-B35 study, “which demonstrated a very modest advantage to anastrozole that was mostly limited to younger postmenopausal women,” she said.
Indeed, a significant 27% reduction in recurrence was seen with anastrozole, compared with tamoxifen at a median of 9 years of follow-up in the NSABP-B35 study. But, as Dr. Sestak pointed out, that reduction “was mainly observed during the posttreatment follow-up period and not during the active treatment period.”
In the IBIS-II DCIS study, there was a nonsignificant 11% reduction in breast cancer recurrence with anastrozole.
The investigators did examine the potential effect of age on the rate of breast cancer recurrence, but no significant differences were found. They also looked at the active and post–endocrine therapy treatment periods, all showing no benefit of one drug over the other.
“So we clearly cannot replicate the findings of the NSABP-B35 study,” Dr. Sestak acknowledged.
IBIS-II DCIS details and results
Aromatase inhibitors such as anastrozole have been shown to be more effective than tamoxifen for preventing recurrence in postmenopausal HR+ women with invasive breast cancer, but it wasn’t previously known if this included women with HR-positive DCIS.
The IBIS-II DCIS study was therefore designed to determine if there was any advantage of anastrazole over tamoxifen. The phase 3 trial enrolled 2,980 postmenopausal women with HR-positive DCIS. They were randomized to 5 years of anastrozole or 5 years of tamoxifen.
“Women were followed up during this active period of treatment by clinic visits,” explained Dr. Sestak. “Thereafter, we collected data via questionnaire, registry data sets, and clinic visits.”
Baseline characteristics were similar between the treatment arms. The median age was about 60 years in both arms. Similar proportions of patients had received hormone replacement therapy (44.2% in the tamoxifen arm and 46.8% in the anastrozole arm) or undergone radiotherapy (71.5% and 70.9%, respectively).
Results showed little difference in breast cancer recurrence rates. At a median follow-up of 11.6 years, the rate of recurrence was 9.7% with tamoxifen and 8.5% with anastrozole (hazard ratio, 0.89; P = .401).
Trends were seen favoring anastrozole in estrogen receptor–positive and HER2-negative women, but this was only while women were being actively treated.
Death rates were similar – 4.2% with anastrozole and 4.5% with tamoxifen (odds ratio, 0.93). There were three breast cancer deaths in each treatment arm.
Adverse events could be the deciding factor
“The main take-away from this presentation is that choice of adjuvant endocrine therapy for DCIS should be individualized based primarily on the different side effect profiles of the two medications,” Dr. Moore said.
During the trial, “a significant 34% increase in fractures was observed in women who received anastrozole, compared to tamoxifen [HR, 1.34; P = .013],” Dr. Sestak said.
“We also observed a threefold increase in strokes and transient ischemic attacks with anastrozole, compared to tamoxifen [HR, 3.10; P = .021 for both strokes and transient ischemic attacks],” Dr. Sestak added.
She acknowledged that this finding is inconsistent with what is known about aromatase inhibitors in general. It could be that, rather than anastrozole raising the risk of strokes and transient ischemic attacks, tamoxifen was having a beneficial effect. This could be a result of tamoxifen improving endothelial function by increasing vasodilation, “but it is really not clear what the mechanism is,” Dr. Sestak said.
Also contrary to what is known about tamoxifen was an excess of deaths because of endometrial cancer or ovarian cancers. Wherever possible, the pathology reports had been requested to confirm the cause of death, “so we are pretty sure that they are true ovarian cancers and not some other abdominal tumors,” Dr. Sestak said.
“We did observe very clear differences in terms of adverse events,” she said, adding that “improved understanding of adverse event profiles will help patients with HR-positive DCIS to make an informed decision regarding their treatment.”
The IBIS-II DCIS trial was funded by Cancer Research UK, the National Health and Medical Research Council Australia, Breast Cancer Research Foundation, AstraZeneca, and Sanofi Aventis. Two investigators disclosed relationships with AstraZeneca, and one disclosed a relationship with Cancer Research UK. Dr. Sestak and Dr. Moore had no relevant disclosures.
SOURCE: Sestak I et al. SABCS 2020, Abstract GS2-02.
FROM SABCS 2020
Omitting postop radiotherapy doesn’t affect survival in older breast cancer patients
Skipping whole-breast adjuvant radiotherapy does not appear to affect long-term survival in women age 65 and older who have had surgery for early-stage, hormone receptor–positive (HR+) breast cancer, according to 10-year follow-up of the phase 3 PRIME-2 study.
Although the risk for local recurrence was higher among patients who did not receive radiotherapy, the absolute risk for recurrence was still low, said study investigator Ian Kunkler, MRCPUK, FRCR, of Western General Hospital, University of Edinburgh in Scotland.
Dr. Kunkler presented results from PRIME-2 at the 2020 San Antonio Breast Cancer Symposium.
“In older patients, we have to carefully balance benefits [of radiotherapy] in terms of local control and survival against toxicities,” Dr. Kunkler said in an interview.
Omitting radiotherapy could help women avoid complications such as fatigue, changes to lung function, and an increased risk of cardiovascular damage.
“We think that these results should provide some reassurance that the omission of radiotherapy could be an option,” Dr. Kunkler said.
PRIME-2 results
The PRIME-2 study was a randomized trial that recruited 1,326 women with histologically confirmed, unilateral invasive breast cancer who were all 65 years or older.
For inclusion, the women had to have a tumor measuring 3 cm or less, have no nodal involvement, and be about to undergo breast-conserving surgery. Women also needed to be HR+ and be treated with adjuvant endocrine therapy.
The women were randomized 1:1 to receive adjuvant whole-breast irradiation at a dosing schedule of 40-50 Gy in 15-25 fractions or no radiotherapy in addition to adjuvant endocrine therapy.
The primary endpoint was the recurrence of breast cancer in the same breast at 10 years. There was a significantly lower rate of ipsilateral recurrence with radiotherapy than without it, at 0.9% and 9.8%, respectively (P = .00008).
Similarly, the 10-year rate of regional recurrence was significantly lower in the radiotherapy arm than in the no-radiotherapy arm (0.5% vs. 2.3%, P = .014).
However, there was no significant difference in the radiotherapy and nonradiotherapy arms when it came to distant recurrence (3.6% vs. 1.9%, P = .07), contralateral recurrence (2.2% vs. 1.2%, P = .20), or new, non–breast cancer (8.7% vs. 10.2%, P = .41).
The overall survival estimate at 10 years was 80.4% in women who did not receive radiotherapy and 81.0% in those who did (P = .68). Rates of metastasis-free survival were also similar (98.1% vs. 96.4%, P = .28).
“Most of these women are dying from non–breast cancer causes, reflecting the impact of competitive causes of non–breast cancer mortality,” Dr. Kunkler said.
Implications for practice
The current findings build on prior findings from the PRIME-2 study 5 years ago, which showed a small benefit of postoperative radiotherapy over no radiotherapy in reducing the rate of local recurrence. This led to the recommendation that postoperative radiotherapy might be safely omitted in some older women and influenced U.K. practice.
Indeed, Dr. Kunkler observed that U.K. guidelines have pretty much adopted the entry criteria for the PRIME-2 study (HR+, axillary node-negative [N0], T1–T2 up to 3 cm at the longest dimension, and clear margins) for the omission of radiotherapy.
“It’s had much less impact in the United States, where the usage of radiotherapy after breast-conserving surgery still remains very high,” Dr. Kunkler said.
He acknowledged that the current U.S. guidelines include the omission of radiotherapy in older women, but only those with much smaller (T1, N0) tumors, based on the findings of the Cancer and Leukemia Group B (CALGB) 9343 study.
“The findings from PRIME-2 so far seem consistent with long-term findings from CALGB 9343,” Matthew Katz, MD, of Lowell (Mass.) General Hospital Cancer Center, said in an interview.
However, “the median follow-up of the study was only 7 years, so it’s a little early to analyze 10-year data,” he added.
As to why leaving out radiotherapy in older women may be less common in the United States than in the U.K., Dr. Katz said it was probably due to a “tendency on the part of U.S. oncologists and cancer patients to lean more toward treatment to lower the risk of recurrence.
“When I discuss omitting radiation to women 70 or older with an early-stage, low risk breast cancer, the majority of people I see choose treatment,” he said. “The key is that a cancer patient can make informed choices about treatment based upon her or his values, looking at both the risks of cancer recurrence and the side effects of cancer treatments.”
“The decision as to whether radiotherapy is omitted or not has become a bit more nuanced,” since the PRIME-2 study started in 2003, Dr. Kunkler acknowledged.
He said there’s now evidence to suggest that shorter radiotherapy regimens may be beneficial. For example, the FAST-Forward trial showed that a regimen of 26 Gy in five fractions over 1 week was noninferior to a regimen of 40 Gy in 15 fractions over 3 weeks.
“There are really only two studies – the PRIME-2 study and the CALGB 9343 study – which are specific to an older age group,” Dr. Kunkler noted. “Most of the previous studies of breast-conserving surgery with or without radiotherapy receiving endocrine therapy have been predominantly in women under the age of 70. And indeed, 70 was often considered an exclusion criterion for randomized trials.”
PRIME-2 was funded by the Chief Scientist Office (Scottish Government) and the Breast Cancer Institute at the Western General Hospital in Edinburgh, Scotland. Neither Dr. Kunkler nor Dr. Katz had relevant disclosures.
SOURCE: Kunkler IH J et al. SABCS 2020, Abstract GS2-03.
Skipping whole-breast adjuvant radiotherapy does not appear to affect long-term survival in women age 65 and older who have had surgery for early-stage, hormone receptor–positive (HR+) breast cancer, according to 10-year follow-up of the phase 3 PRIME-2 study.
Although the risk for local recurrence was higher among patients who did not receive radiotherapy, the absolute risk for recurrence was still low, said study investigator Ian Kunkler, MRCPUK, FRCR, of Western General Hospital, University of Edinburgh in Scotland.
Dr. Kunkler presented results from PRIME-2 at the 2020 San Antonio Breast Cancer Symposium.
“In older patients, we have to carefully balance benefits [of radiotherapy] in terms of local control and survival against toxicities,” Dr. Kunkler said in an interview.
Omitting radiotherapy could help women avoid complications such as fatigue, changes to lung function, and an increased risk of cardiovascular damage.
“We think that these results should provide some reassurance that the omission of radiotherapy could be an option,” Dr. Kunkler said.
PRIME-2 results
The PRIME-2 study was a randomized trial that recruited 1,326 women with histologically confirmed, unilateral invasive breast cancer who were all 65 years or older.
For inclusion, the women had to have a tumor measuring 3 cm or less, have no nodal involvement, and be about to undergo breast-conserving surgery. Women also needed to be HR+ and be treated with adjuvant endocrine therapy.
The women were randomized 1:1 to receive adjuvant whole-breast irradiation at a dosing schedule of 40-50 Gy in 15-25 fractions or no radiotherapy in addition to adjuvant endocrine therapy.
The primary endpoint was the recurrence of breast cancer in the same breast at 10 years. There was a significantly lower rate of ipsilateral recurrence with radiotherapy than without it, at 0.9% and 9.8%, respectively (P = .00008).
Similarly, the 10-year rate of regional recurrence was significantly lower in the radiotherapy arm than in the no-radiotherapy arm (0.5% vs. 2.3%, P = .014).
However, there was no significant difference in the radiotherapy and nonradiotherapy arms when it came to distant recurrence (3.6% vs. 1.9%, P = .07), contralateral recurrence (2.2% vs. 1.2%, P = .20), or new, non–breast cancer (8.7% vs. 10.2%, P = .41).
The overall survival estimate at 10 years was 80.4% in women who did not receive radiotherapy and 81.0% in those who did (P = .68). Rates of metastasis-free survival were also similar (98.1% vs. 96.4%, P = .28).
“Most of these women are dying from non–breast cancer causes, reflecting the impact of competitive causes of non–breast cancer mortality,” Dr. Kunkler said.
Implications for practice
The current findings build on prior findings from the PRIME-2 study 5 years ago, which showed a small benefit of postoperative radiotherapy over no radiotherapy in reducing the rate of local recurrence. This led to the recommendation that postoperative radiotherapy might be safely omitted in some older women and influenced U.K. practice.
Indeed, Dr. Kunkler observed that U.K. guidelines have pretty much adopted the entry criteria for the PRIME-2 study (HR+, axillary node-negative [N0], T1–T2 up to 3 cm at the longest dimension, and clear margins) for the omission of radiotherapy.
“It’s had much less impact in the United States, where the usage of radiotherapy after breast-conserving surgery still remains very high,” Dr. Kunkler said.
He acknowledged that the current U.S. guidelines include the omission of radiotherapy in older women, but only those with much smaller (T1, N0) tumors, based on the findings of the Cancer and Leukemia Group B (CALGB) 9343 study.
“The findings from PRIME-2 so far seem consistent with long-term findings from CALGB 9343,” Matthew Katz, MD, of Lowell (Mass.) General Hospital Cancer Center, said in an interview.
However, “the median follow-up of the study was only 7 years, so it’s a little early to analyze 10-year data,” he added.
As to why leaving out radiotherapy in older women may be less common in the United States than in the U.K., Dr. Katz said it was probably due to a “tendency on the part of U.S. oncologists and cancer patients to lean more toward treatment to lower the risk of recurrence.
“When I discuss omitting radiation to women 70 or older with an early-stage, low risk breast cancer, the majority of people I see choose treatment,” he said. “The key is that a cancer patient can make informed choices about treatment based upon her or his values, looking at both the risks of cancer recurrence and the side effects of cancer treatments.”
“The decision as to whether radiotherapy is omitted or not has become a bit more nuanced,” since the PRIME-2 study started in 2003, Dr. Kunkler acknowledged.
He said there’s now evidence to suggest that shorter radiotherapy regimens may be beneficial. For example, the FAST-Forward trial showed that a regimen of 26 Gy in five fractions over 1 week was noninferior to a regimen of 40 Gy in 15 fractions over 3 weeks.
“There are really only two studies – the PRIME-2 study and the CALGB 9343 study – which are specific to an older age group,” Dr. Kunkler noted. “Most of the previous studies of breast-conserving surgery with or without radiotherapy receiving endocrine therapy have been predominantly in women under the age of 70. And indeed, 70 was often considered an exclusion criterion for randomized trials.”
PRIME-2 was funded by the Chief Scientist Office (Scottish Government) and the Breast Cancer Institute at the Western General Hospital in Edinburgh, Scotland. Neither Dr. Kunkler nor Dr. Katz had relevant disclosures.
SOURCE: Kunkler IH J et al. SABCS 2020, Abstract GS2-03.
Skipping whole-breast adjuvant radiotherapy does not appear to affect long-term survival in women age 65 and older who have had surgery for early-stage, hormone receptor–positive (HR+) breast cancer, according to 10-year follow-up of the phase 3 PRIME-2 study.
Although the risk for local recurrence was higher among patients who did not receive radiotherapy, the absolute risk for recurrence was still low, said study investigator Ian Kunkler, MRCPUK, FRCR, of Western General Hospital, University of Edinburgh in Scotland.
Dr. Kunkler presented results from PRIME-2 at the 2020 San Antonio Breast Cancer Symposium.
“In older patients, we have to carefully balance benefits [of radiotherapy] in terms of local control and survival against toxicities,” Dr. Kunkler said in an interview.
Omitting radiotherapy could help women avoid complications such as fatigue, changes to lung function, and an increased risk of cardiovascular damage.
“We think that these results should provide some reassurance that the omission of radiotherapy could be an option,” Dr. Kunkler said.
PRIME-2 results
The PRIME-2 study was a randomized trial that recruited 1,326 women with histologically confirmed, unilateral invasive breast cancer who were all 65 years or older.
For inclusion, the women had to have a tumor measuring 3 cm or less, have no nodal involvement, and be about to undergo breast-conserving surgery. Women also needed to be HR+ and be treated with adjuvant endocrine therapy.
The women were randomized 1:1 to receive adjuvant whole-breast irradiation at a dosing schedule of 40-50 Gy in 15-25 fractions or no radiotherapy in addition to adjuvant endocrine therapy.
The primary endpoint was the recurrence of breast cancer in the same breast at 10 years. There was a significantly lower rate of ipsilateral recurrence with radiotherapy than without it, at 0.9% and 9.8%, respectively (P = .00008).
Similarly, the 10-year rate of regional recurrence was significantly lower in the radiotherapy arm than in the no-radiotherapy arm (0.5% vs. 2.3%, P = .014).
However, there was no significant difference in the radiotherapy and nonradiotherapy arms when it came to distant recurrence (3.6% vs. 1.9%, P = .07), contralateral recurrence (2.2% vs. 1.2%, P = .20), or new, non–breast cancer (8.7% vs. 10.2%, P = .41).
The overall survival estimate at 10 years was 80.4% in women who did not receive radiotherapy and 81.0% in those who did (P = .68). Rates of metastasis-free survival were also similar (98.1% vs. 96.4%, P = .28).
“Most of these women are dying from non–breast cancer causes, reflecting the impact of competitive causes of non–breast cancer mortality,” Dr. Kunkler said.
Implications for practice
The current findings build on prior findings from the PRIME-2 study 5 years ago, which showed a small benefit of postoperative radiotherapy over no radiotherapy in reducing the rate of local recurrence. This led to the recommendation that postoperative radiotherapy might be safely omitted in some older women and influenced U.K. practice.
Indeed, Dr. Kunkler observed that U.K. guidelines have pretty much adopted the entry criteria for the PRIME-2 study (HR+, axillary node-negative [N0], T1–T2 up to 3 cm at the longest dimension, and clear margins) for the omission of radiotherapy.
“It’s had much less impact in the United States, where the usage of radiotherapy after breast-conserving surgery still remains very high,” Dr. Kunkler said.
He acknowledged that the current U.S. guidelines include the omission of radiotherapy in older women, but only those with much smaller (T1, N0) tumors, based on the findings of the Cancer and Leukemia Group B (CALGB) 9343 study.
“The findings from PRIME-2 so far seem consistent with long-term findings from CALGB 9343,” Matthew Katz, MD, of Lowell (Mass.) General Hospital Cancer Center, said in an interview.
However, “the median follow-up of the study was only 7 years, so it’s a little early to analyze 10-year data,” he added.
As to why leaving out radiotherapy in older women may be less common in the United States than in the U.K., Dr. Katz said it was probably due to a “tendency on the part of U.S. oncologists and cancer patients to lean more toward treatment to lower the risk of recurrence.
“When I discuss omitting radiation to women 70 or older with an early-stage, low risk breast cancer, the majority of people I see choose treatment,” he said. “The key is that a cancer patient can make informed choices about treatment based upon her or his values, looking at both the risks of cancer recurrence and the side effects of cancer treatments.”
“The decision as to whether radiotherapy is omitted or not has become a bit more nuanced,” since the PRIME-2 study started in 2003, Dr. Kunkler acknowledged.
He said there’s now evidence to suggest that shorter radiotherapy regimens may be beneficial. For example, the FAST-Forward trial showed that a regimen of 26 Gy in five fractions over 1 week was noninferior to a regimen of 40 Gy in 15 fractions over 3 weeks.
“There are really only two studies – the PRIME-2 study and the CALGB 9343 study – which are specific to an older age group,” Dr. Kunkler noted. “Most of the previous studies of breast-conserving surgery with or without radiotherapy receiving endocrine therapy have been predominantly in women under the age of 70. And indeed, 70 was often considered an exclusion criterion for randomized trials.”
PRIME-2 was funded by the Chief Scientist Office (Scottish Government) and the Breast Cancer Institute at the Western General Hospital in Edinburgh, Scotland. Neither Dr. Kunkler nor Dr. Katz had relevant disclosures.
SOURCE: Kunkler IH J et al. SABCS 2020, Abstract GS2-03.
FROM SABCS 2020
An all-oral option for advanced HR+, HER2– breast cancer?
These results suggest tesetaxel plus capecitabine is “a potential new treatment option” for this patient population, said study investigator Joyce O’Shaughnessy, MD, of Baylor University Medical Center and Texas Oncology, both in Dallas.
“This should launch an oral taxane into the clinical space, which will be a nice addition to the toolbox for treating advanced breast cancer, with real upsides for patients,” said Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the trial but commented on the results in an interview.
Another commenter was more critical of CONTESSA’s results, which were presented at the 2020 San Antonio Breast Cancer Symposium.
“Three months’ difference in PFS in this setting is meaningless without overall survival [OS] results,” Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, Portugal, said in a question submitted through the virtual meeting’s chat system.
At this point, the OS data are immature, and mature data won’t be available for another couple of years at least, according to the study’s protocol.
Dr. O’Shaughnessy defended the PFS result as being significant, however, saying it was comparable with outcomes seen previously with docetaxel-capecitabine and paclitaxel-gemcitabine combinations.
Other meeting attendees questioned why the waters had been muddied by testing the effects of tesetaxel in combination with capecitabine, albeit at a reduced dose, versus the approved full dose of capecitabine as monotherapy, particularly as a phase 2 trial had shown that tesetaxel demonstrated “significant activity” as monotherapy.
“The reason for the combination versus a monotherapy is because it was designed as a registration trial,” Dr. O’Shaughnessy explained. The trial was designed to be very similar to early taxane studies where docetaxel was assessed with or without capecitabine, or paclitaxel with or without gemcitabine.
“Probably we’re going to be using a doublet for patients who have virulent disease who really need a response,” Dr. O’Shaughnessy explained. She noted that the objective response rate was much higher with the tesetaxel-capecitabine combination than with capecitabine alone, and that result alone is “probably enough that we would utilize a doublet.”
The key thing is that it now gives patients an all-oral option, Dr. O’Shaughnessy said.
“The data are exciting because it would be terrific to have an orally available taxane chemotherapy,” agreed Dr. Burstein. “It is far more convenient for patients and opens access globally in places that do not have adequate resources for administration of IV therapeutics. Also, the data suggest that tesetaxel has a different side effect profile than IV taxane, with less neuropathy and less alopecia.”
Trial design
CONTESSA is an ongoing randomized, controlled trial that started in 2017 and is projected to end in early 2023. It is investigating the use of tesetaxel plus a reduced dose of capecitabine versus the approved dose of capecitabine alone in 685 women with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer who had previously been treated with a taxane.
Being intrinsically orally bioavailable and more soluble than the other taxanes means that tesetaxel has a much longer half-life that allows for a “more convenient treatment experience for patients,” Dr. O’Shaughnessy observed.
Indeed, because tesetaxel only needs to be dosed once every 3 weeks, patients in the trial received tesetaxel at 27 mg/m2 only on the first day of a 21-day treatment cycle. This was combined with a reduced, 825-mg/m2 dose of capecitabine, given orally twice-daily on days 2-14 but once daily on the evening of day 1 and on the morning of day 15.
The combination regimen was compared with the recommended full dose of capecitabine alone, 1,250 mg/m2 given orally twice daily on days 2-14 but once daily on the evening of day 1 and on the morning of day 15.
Efficacy and safety
PFS was 9.8 months with tesetaxel plus capecitabine and 6.9 months with capecitabine alone, representing a 2.9-month improvement with the combination (hazard ratio, 0.716; P = .003).
A similar PFS benefit was seen regardless of multiple predefined subgroups, such as age, baseline performance status, duration of disease-free interval before study entry, and the use of CDK4/6 inhibitors.
The objective response rate was 57% with tesetaxel plus capecitabine and 41% with capecitabine alone (P = .0002). The 24-week disease control rate was 67% and 50%, respectively (P < .0001).
The most frequent treatment-emergent adverse event seen with the tesetaxel-capecitabine combination was neutropenia, occurring in 76.9% of patients, compared with 22.6% of patients in the monotherapy arm. Rates of grade 3-4 neutropenia were much higher in the combination arm (32.6% and 38.3%, respectively) than in the monotherapy arm (7.4% and 0.9%, respectively).
The neutropenia seen was “generally manageable,” Dr. O’Shaughnessy said, primarily with dose reductions and granulocyte colony–stimulating factor as needed.
She pointed out that rates of grade 3 or higher neuropathy and grade 2 alopecia were low, a respective 5.9% and 8%, with the combination.
The dose of capecitabine used in the control arm was noted to be higher than that used in usual practice.
“This was because of the global nature of the study and the regulatory requirements globally,” Dr. O’Shaughnessy said.
“The dose-modification scheme was that patients could have a dose reduction at the first sign of grade 2 toxicity,” she added, giving investigators the flexibility to reduce the dose as soon as possible.
This study was sponsored by Odonate Therapeutics. Dr. O’Shaughnessy disclosed consulting fees from AbbVie, Agendia, AstraZeneca, Bristol-Myers Squibb, Celgene, Eisai, Genentech/Roche, Genomic Health, GRAIL, Heron, Immunomedics, Ipsen, Jounce, Lilly, Novartis, Odonate, Pfizer, Puma, and Seagen. Dr. Burstein and Dr. Cardoso had no relevant disclosures.
SOURCE: O’Shaughnessy J et al. SABCS 2020, Abstract GS4-01.
These results suggest tesetaxel plus capecitabine is “a potential new treatment option” for this patient population, said study investigator Joyce O’Shaughnessy, MD, of Baylor University Medical Center and Texas Oncology, both in Dallas.
“This should launch an oral taxane into the clinical space, which will be a nice addition to the toolbox for treating advanced breast cancer, with real upsides for patients,” said Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the trial but commented on the results in an interview.
Another commenter was more critical of CONTESSA’s results, which were presented at the 2020 San Antonio Breast Cancer Symposium.
“Three months’ difference in PFS in this setting is meaningless without overall survival [OS] results,” Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, Portugal, said in a question submitted through the virtual meeting’s chat system.
At this point, the OS data are immature, and mature data won’t be available for another couple of years at least, according to the study’s protocol.
Dr. O’Shaughnessy defended the PFS result as being significant, however, saying it was comparable with outcomes seen previously with docetaxel-capecitabine and paclitaxel-gemcitabine combinations.
Other meeting attendees questioned why the waters had been muddied by testing the effects of tesetaxel in combination with capecitabine, albeit at a reduced dose, versus the approved full dose of capecitabine as monotherapy, particularly as a phase 2 trial had shown that tesetaxel demonstrated “significant activity” as monotherapy.
“The reason for the combination versus a monotherapy is because it was designed as a registration trial,” Dr. O’Shaughnessy explained. The trial was designed to be very similar to early taxane studies where docetaxel was assessed with or without capecitabine, or paclitaxel with or without gemcitabine.
“Probably we’re going to be using a doublet for patients who have virulent disease who really need a response,” Dr. O’Shaughnessy explained. She noted that the objective response rate was much higher with the tesetaxel-capecitabine combination than with capecitabine alone, and that result alone is “probably enough that we would utilize a doublet.”
The key thing is that it now gives patients an all-oral option, Dr. O’Shaughnessy said.
“The data are exciting because it would be terrific to have an orally available taxane chemotherapy,” agreed Dr. Burstein. “It is far more convenient for patients and opens access globally in places that do not have adequate resources for administration of IV therapeutics. Also, the data suggest that tesetaxel has a different side effect profile than IV taxane, with less neuropathy and less alopecia.”
Trial design
CONTESSA is an ongoing randomized, controlled trial that started in 2017 and is projected to end in early 2023. It is investigating the use of tesetaxel plus a reduced dose of capecitabine versus the approved dose of capecitabine alone in 685 women with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer who had previously been treated with a taxane.
Being intrinsically orally bioavailable and more soluble than the other taxanes means that tesetaxel has a much longer half-life that allows for a “more convenient treatment experience for patients,” Dr. O’Shaughnessy observed.
Indeed, because tesetaxel only needs to be dosed once every 3 weeks, patients in the trial received tesetaxel at 27 mg/m2 only on the first day of a 21-day treatment cycle. This was combined with a reduced, 825-mg/m2 dose of capecitabine, given orally twice-daily on days 2-14 but once daily on the evening of day 1 and on the morning of day 15.
The combination regimen was compared with the recommended full dose of capecitabine alone, 1,250 mg/m2 given orally twice daily on days 2-14 but once daily on the evening of day 1 and on the morning of day 15.
Efficacy and safety
PFS was 9.8 months with tesetaxel plus capecitabine and 6.9 months with capecitabine alone, representing a 2.9-month improvement with the combination (hazard ratio, 0.716; P = .003).
A similar PFS benefit was seen regardless of multiple predefined subgroups, such as age, baseline performance status, duration of disease-free interval before study entry, and the use of CDK4/6 inhibitors.
The objective response rate was 57% with tesetaxel plus capecitabine and 41% with capecitabine alone (P = .0002). The 24-week disease control rate was 67% and 50%, respectively (P < .0001).
The most frequent treatment-emergent adverse event seen with the tesetaxel-capecitabine combination was neutropenia, occurring in 76.9% of patients, compared with 22.6% of patients in the monotherapy arm. Rates of grade 3-4 neutropenia were much higher in the combination arm (32.6% and 38.3%, respectively) than in the monotherapy arm (7.4% and 0.9%, respectively).
The neutropenia seen was “generally manageable,” Dr. O’Shaughnessy said, primarily with dose reductions and granulocyte colony–stimulating factor as needed.
She pointed out that rates of grade 3 or higher neuropathy and grade 2 alopecia were low, a respective 5.9% and 8%, with the combination.
The dose of capecitabine used in the control arm was noted to be higher than that used in usual practice.
“This was because of the global nature of the study and the regulatory requirements globally,” Dr. O’Shaughnessy said.
“The dose-modification scheme was that patients could have a dose reduction at the first sign of grade 2 toxicity,” she added, giving investigators the flexibility to reduce the dose as soon as possible.
This study was sponsored by Odonate Therapeutics. Dr. O’Shaughnessy disclosed consulting fees from AbbVie, Agendia, AstraZeneca, Bristol-Myers Squibb, Celgene, Eisai, Genentech/Roche, Genomic Health, GRAIL, Heron, Immunomedics, Ipsen, Jounce, Lilly, Novartis, Odonate, Pfizer, Puma, and Seagen. Dr. Burstein and Dr. Cardoso had no relevant disclosures.
SOURCE: O’Shaughnessy J et al. SABCS 2020, Abstract GS4-01.
These results suggest tesetaxel plus capecitabine is “a potential new treatment option” for this patient population, said study investigator Joyce O’Shaughnessy, MD, of Baylor University Medical Center and Texas Oncology, both in Dallas.
“This should launch an oral taxane into the clinical space, which will be a nice addition to the toolbox for treating advanced breast cancer, with real upsides for patients,” said Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the trial but commented on the results in an interview.
Another commenter was more critical of CONTESSA’s results, which were presented at the 2020 San Antonio Breast Cancer Symposium.
“Three months’ difference in PFS in this setting is meaningless without overall survival [OS] results,” Fatima Cardoso, MD, of Champalimaud Clinical Center in Lisbon, Portugal, said in a question submitted through the virtual meeting’s chat system.
At this point, the OS data are immature, and mature data won’t be available for another couple of years at least, according to the study’s protocol.
Dr. O’Shaughnessy defended the PFS result as being significant, however, saying it was comparable with outcomes seen previously with docetaxel-capecitabine and paclitaxel-gemcitabine combinations.
Other meeting attendees questioned why the waters had been muddied by testing the effects of tesetaxel in combination with capecitabine, albeit at a reduced dose, versus the approved full dose of capecitabine as monotherapy, particularly as a phase 2 trial had shown that tesetaxel demonstrated “significant activity” as monotherapy.
“The reason for the combination versus a monotherapy is because it was designed as a registration trial,” Dr. O’Shaughnessy explained. The trial was designed to be very similar to early taxane studies where docetaxel was assessed with or without capecitabine, or paclitaxel with or without gemcitabine.
“Probably we’re going to be using a doublet for patients who have virulent disease who really need a response,” Dr. O’Shaughnessy explained. She noted that the objective response rate was much higher with the tesetaxel-capecitabine combination than with capecitabine alone, and that result alone is “probably enough that we would utilize a doublet.”
The key thing is that it now gives patients an all-oral option, Dr. O’Shaughnessy said.
“The data are exciting because it would be terrific to have an orally available taxane chemotherapy,” agreed Dr. Burstein. “It is far more convenient for patients and opens access globally in places that do not have adequate resources for administration of IV therapeutics. Also, the data suggest that tesetaxel has a different side effect profile than IV taxane, with less neuropathy and less alopecia.”
Trial design
CONTESSA is an ongoing randomized, controlled trial that started in 2017 and is projected to end in early 2023. It is investigating the use of tesetaxel plus a reduced dose of capecitabine versus the approved dose of capecitabine alone in 685 women with hormone receptor–positive, HER2-negative locally advanced or metastatic breast cancer who had previously been treated with a taxane.
Being intrinsically orally bioavailable and more soluble than the other taxanes means that tesetaxel has a much longer half-life that allows for a “more convenient treatment experience for patients,” Dr. O’Shaughnessy observed.
Indeed, because tesetaxel only needs to be dosed once every 3 weeks, patients in the trial received tesetaxel at 27 mg/m2 only on the first day of a 21-day treatment cycle. This was combined with a reduced, 825-mg/m2 dose of capecitabine, given orally twice-daily on days 2-14 but once daily on the evening of day 1 and on the morning of day 15.
The combination regimen was compared with the recommended full dose of capecitabine alone, 1,250 mg/m2 given orally twice daily on days 2-14 but once daily on the evening of day 1 and on the morning of day 15.
Efficacy and safety
PFS was 9.8 months with tesetaxel plus capecitabine and 6.9 months with capecitabine alone, representing a 2.9-month improvement with the combination (hazard ratio, 0.716; P = .003).
A similar PFS benefit was seen regardless of multiple predefined subgroups, such as age, baseline performance status, duration of disease-free interval before study entry, and the use of CDK4/6 inhibitors.
The objective response rate was 57% with tesetaxel plus capecitabine and 41% with capecitabine alone (P = .0002). The 24-week disease control rate was 67% and 50%, respectively (P < .0001).
The most frequent treatment-emergent adverse event seen with the tesetaxel-capecitabine combination was neutropenia, occurring in 76.9% of patients, compared with 22.6% of patients in the monotherapy arm. Rates of grade 3-4 neutropenia were much higher in the combination arm (32.6% and 38.3%, respectively) than in the monotherapy arm (7.4% and 0.9%, respectively).
The neutropenia seen was “generally manageable,” Dr. O’Shaughnessy said, primarily with dose reductions and granulocyte colony–stimulating factor as needed.
She pointed out that rates of grade 3 or higher neuropathy and grade 2 alopecia were low, a respective 5.9% and 8%, with the combination.
The dose of capecitabine used in the control arm was noted to be higher than that used in usual practice.
“This was because of the global nature of the study and the regulatory requirements globally,” Dr. O’Shaughnessy said.
“The dose-modification scheme was that patients could have a dose reduction at the first sign of grade 2 toxicity,” she added, giving investigators the flexibility to reduce the dose as soon as possible.
This study was sponsored by Odonate Therapeutics. Dr. O’Shaughnessy disclosed consulting fees from AbbVie, Agendia, AstraZeneca, Bristol-Myers Squibb, Celgene, Eisai, Genentech/Roche, Genomic Health, GRAIL, Heron, Immunomedics, Ipsen, Jounce, Lilly, Novartis, Odonate, Pfizer, Puma, and Seagen. Dr. Burstein and Dr. Cardoso had no relevant disclosures.
SOURCE: O’Shaughnessy J et al. SABCS 2020, Abstract GS4-01.
FROM SABCS 2020
PENELOPE-B: Palbociclib disappoints in HR+, HER2– breast cancer
The CDK4/6 inhibitor palbociclib provides no significant benefit in patients with hormone receptor–positive (HR+), HER2-negative primary breast cancer, according to first results from the PENELOPE-B trial.
In this phase 3 trial, adding palbociclib to standard endocrine therapy did not improve invasive disease-free survival (iDFS) in patients who were at high risk of relapse following neoadjuvant chemotherapy (NACT).
There was no significant difference in iDFS rates with palbociclib or placebo at 2 years (88.3% vs. 84%), 3 years (81.2% vs. 77.7%), or even 4 years (73% vs. 72.4%), with a stratified hazard ratio of 0.93 (P = .525).
“This, unfortunately, was a negative trial,” said Ruth M. O’Regan, MD, of the University of Wisconsin–Madison, who independently commented after the trial’s virtual presentation at the 2020 San Antonio Breast Cancer Symposium.
Discussing the iDFS curves for palbociclib and placebo over time, Dr. O’Regan observed that there was a slight benefit for palbociclib in the range of 4.3%, compared with placebo at 2 years and 3.5% at 3 years. The benefit was small but “still meaningful,” she added. However, “at 4 years, you can see the curves have completely come together.”
“This begs the question of whether this trial was just treating patients with occult metastatic disease because I think these curves kind of mirror what we might see in the first-line metastatic setting,” Dr. O’Regan suggested.
Study details
The PENELOPE-B trial was designed to see if palbociclib could improve iDFS in women with HR+, HER2-negative primary breast cancer who were at high risk of relapse following NACT.
The clinical-pathologic stage plus estrogen receptor and grade (CPS-EG) staging system was used to identify patients at high risk for relapse. A CPS-EG score of 3 or higher or 2 or higher with nodal involvement was used as an entry criterion. This has been associated with a 3-year disease-free survival rate of 77%, noted Sibylle Loibl, MD, PhD, head of the German Breast Group in Neu-Isenburg, who presented PENELOPE-B data at the meeting.
The trial enrolled 1,250 women who did not have a pathological complete response after NACT. After surgery, with or without radiotherapy, patients were randomized to receive 13 cycles of either palbociclib or placebo (125 mg once daily; 21 days on and 7 days off treatment). All patients received concomitant endocrine therapy according to local standards.
“Although the compliance was lower in the palbociclib arm than the placebo arm, it was still satisfactory, and the relative dose intensity was over 80%,” Dr. Loibl noted.
The primary endpoint was iDFS rate. As noted previously, there were no significant between-arm differences in 2-, 3-, or 4-year iDFS rates.
Likewise, there were no significant differences between palbociclib and placebo in terms of the secondary endpoints, which included the type of iDFS event (distant recurrences, invasive locoregional recurrences, contralateral breast cancer, second primary invasive nonbreast cancer, and death without previous event).
Subgroup iDFS analyses showed no differences between the study arms. “No group could be identified with a higher benefit from palbociclib,” Dr. Loibl reported.
An interim overall survival (OS) analysis showed no significant differences between palbociclib and placebo. The 2-year OS rate was 96.3% with palbociclib and 94.5% with placebo. The 3-year OS rates were 93.6% and 90.5%, respectively. The 4-year OS rates were 90.4% and 87.3%, respectively.
“Today, the results of the PENELOPE-B study do not support the addition of 1 year of palbociclib to endocrine therapy. Long-term follow up from all neoadjuvant CDK4/6 inhibitor studies should continue and must be awaited,” Dr. Loibl concluded.
Findings in context
PENELOPE-B is the second phase 3 trial to show no benefit for palbociclib in the treatment of early high-risk breast cancer. The first was the PALLAS trial, which was reported only a few months ago at the European Society for Medical Oncology Virtual Congress 2020.
In PALLAS, palbociclib plus endocrine therapy was compared with endocrine therapy alone in the treatment of women with HR+, HER2-negative early breast cancer, but there was no additional benefit seen.
The results of both PENELOPE-B and PALLAS contrast those recently seen with another CDK4/6 inhibitor, abemaciclib, in HR+/HER2-negative early breast cancer.
Results of the phase 3 monarchE trial showed that, when abemaciclib was added to endocrine treatment, there was a significant (P = .0096) 25% relative risk reduction (HR, 0.75; 95% confidence interval, 0.60-0.93) in iDFS, compared with endocrine therapy alone. These results were also presented at the ESMO Virtual Congress 2020 and published in the Journal of Clinical Oncology.
While it’s not clear why one CDK4/6 inhibitor may be of benefit in HR+/HER2-negative early breast cancer while the other is not, “PENELOPE-B is clearly quite a different trial to the other two adjuvant CDK inhibitor trials,” Dr. O’Regan observed.
For one thing, PENELOPE-B participants were only eligible for the trial if they did not have a pathological complete response after NACT. In addition, PENELOPE-B is a smaller trial, enrolling well under a third of the number of patients who participated in the PALLAS and monarchE trials. Furthermore, the CPS-EG score, rather than anatomic staging, was used to determine eligibility.
“Abemaciclib could be a more effective CDK inhibitor; that’s most certainly a possibility,” Dr. O’Regan suggested. “However, it’s not supported by the metastatic first-line trials, which have remarkably similar hazard ratios and favor CDK inhibitors.”
Perhaps the shorter duration of palbociclib treatment in the PENELOPE-B trial (12 months vs. 24 months) had an effect.
“Clearly, we need to await the results of NATALEE that uses 3 years of ribociclib,” Dr. O’Regan said. It also remains to be seen if the results of the monarchE trial hold up with longer follow-up.
The PENELOPE-B trial was sponsored by the German Breast Group in collaboration with Pfizer, the AGO Study Group, NSABP Foundation, and the Breast International Group. Dr. Loibl disclosed grant and other support from Pfizer during the conduct of the study and relationships with other companies outside the submitted work. She also disclosed intellectual property rights and being a pending patent holder (EP14153692.0) for a method to predict response to anti-HER2–containing therapy and/or chemotherapy in patients with breast cancer, and she disclosed a relationship with Medscape, which is owned by the same company as MDedge. Dr. O’Regan disclosed relationships with Novartis, Eli Lilly, Genentech, PUMA, Macrogenics, Immunomedics, Biotheranostics, and Eisai.
SOURCE: Loibl S et al. SABCS 2020, Abstract GS1-02.
The CDK4/6 inhibitor palbociclib provides no significant benefit in patients with hormone receptor–positive (HR+), HER2-negative primary breast cancer, according to first results from the PENELOPE-B trial.
In this phase 3 trial, adding palbociclib to standard endocrine therapy did not improve invasive disease-free survival (iDFS) in patients who were at high risk of relapse following neoadjuvant chemotherapy (NACT).
There was no significant difference in iDFS rates with palbociclib or placebo at 2 years (88.3% vs. 84%), 3 years (81.2% vs. 77.7%), or even 4 years (73% vs. 72.4%), with a stratified hazard ratio of 0.93 (P = .525).
“This, unfortunately, was a negative trial,” said Ruth M. O’Regan, MD, of the University of Wisconsin–Madison, who independently commented after the trial’s virtual presentation at the 2020 San Antonio Breast Cancer Symposium.
Discussing the iDFS curves for palbociclib and placebo over time, Dr. O’Regan observed that there was a slight benefit for palbociclib in the range of 4.3%, compared with placebo at 2 years and 3.5% at 3 years. The benefit was small but “still meaningful,” she added. However, “at 4 years, you can see the curves have completely come together.”
“This begs the question of whether this trial was just treating patients with occult metastatic disease because I think these curves kind of mirror what we might see in the first-line metastatic setting,” Dr. O’Regan suggested.
Study details
The PENELOPE-B trial was designed to see if palbociclib could improve iDFS in women with HR+, HER2-negative primary breast cancer who were at high risk of relapse following NACT.
The clinical-pathologic stage plus estrogen receptor and grade (CPS-EG) staging system was used to identify patients at high risk for relapse. A CPS-EG score of 3 or higher or 2 or higher with nodal involvement was used as an entry criterion. This has been associated with a 3-year disease-free survival rate of 77%, noted Sibylle Loibl, MD, PhD, head of the German Breast Group in Neu-Isenburg, who presented PENELOPE-B data at the meeting.
The trial enrolled 1,250 women who did not have a pathological complete response after NACT. After surgery, with or without radiotherapy, patients were randomized to receive 13 cycles of either palbociclib or placebo (125 mg once daily; 21 days on and 7 days off treatment). All patients received concomitant endocrine therapy according to local standards.
“Although the compliance was lower in the palbociclib arm than the placebo arm, it was still satisfactory, and the relative dose intensity was over 80%,” Dr. Loibl noted.
The primary endpoint was iDFS rate. As noted previously, there were no significant between-arm differences in 2-, 3-, or 4-year iDFS rates.
Likewise, there were no significant differences between palbociclib and placebo in terms of the secondary endpoints, which included the type of iDFS event (distant recurrences, invasive locoregional recurrences, contralateral breast cancer, second primary invasive nonbreast cancer, and death without previous event).
Subgroup iDFS analyses showed no differences between the study arms. “No group could be identified with a higher benefit from palbociclib,” Dr. Loibl reported.
An interim overall survival (OS) analysis showed no significant differences between palbociclib and placebo. The 2-year OS rate was 96.3% with palbociclib and 94.5% with placebo. The 3-year OS rates were 93.6% and 90.5%, respectively. The 4-year OS rates were 90.4% and 87.3%, respectively.
“Today, the results of the PENELOPE-B study do not support the addition of 1 year of palbociclib to endocrine therapy. Long-term follow up from all neoadjuvant CDK4/6 inhibitor studies should continue and must be awaited,” Dr. Loibl concluded.
Findings in context
PENELOPE-B is the second phase 3 trial to show no benefit for palbociclib in the treatment of early high-risk breast cancer. The first was the PALLAS trial, which was reported only a few months ago at the European Society for Medical Oncology Virtual Congress 2020.
In PALLAS, palbociclib plus endocrine therapy was compared with endocrine therapy alone in the treatment of women with HR+, HER2-negative early breast cancer, but there was no additional benefit seen.
The results of both PENELOPE-B and PALLAS contrast those recently seen with another CDK4/6 inhibitor, abemaciclib, in HR+/HER2-negative early breast cancer.
Results of the phase 3 monarchE trial showed that, when abemaciclib was added to endocrine treatment, there was a significant (P = .0096) 25% relative risk reduction (HR, 0.75; 95% confidence interval, 0.60-0.93) in iDFS, compared with endocrine therapy alone. These results were also presented at the ESMO Virtual Congress 2020 and published in the Journal of Clinical Oncology.
While it’s not clear why one CDK4/6 inhibitor may be of benefit in HR+/HER2-negative early breast cancer while the other is not, “PENELOPE-B is clearly quite a different trial to the other two adjuvant CDK inhibitor trials,” Dr. O’Regan observed.
For one thing, PENELOPE-B participants were only eligible for the trial if they did not have a pathological complete response after NACT. In addition, PENELOPE-B is a smaller trial, enrolling well under a third of the number of patients who participated in the PALLAS and monarchE trials. Furthermore, the CPS-EG score, rather than anatomic staging, was used to determine eligibility.
“Abemaciclib could be a more effective CDK inhibitor; that’s most certainly a possibility,” Dr. O’Regan suggested. “However, it’s not supported by the metastatic first-line trials, which have remarkably similar hazard ratios and favor CDK inhibitors.”
Perhaps the shorter duration of palbociclib treatment in the PENELOPE-B trial (12 months vs. 24 months) had an effect.
“Clearly, we need to await the results of NATALEE that uses 3 years of ribociclib,” Dr. O’Regan said. It also remains to be seen if the results of the monarchE trial hold up with longer follow-up.
The PENELOPE-B trial was sponsored by the German Breast Group in collaboration with Pfizer, the AGO Study Group, NSABP Foundation, and the Breast International Group. Dr. Loibl disclosed grant and other support from Pfizer during the conduct of the study and relationships with other companies outside the submitted work. She also disclosed intellectual property rights and being a pending patent holder (EP14153692.0) for a method to predict response to anti-HER2–containing therapy and/or chemotherapy in patients with breast cancer, and she disclosed a relationship with Medscape, which is owned by the same company as MDedge. Dr. O’Regan disclosed relationships with Novartis, Eli Lilly, Genentech, PUMA, Macrogenics, Immunomedics, Biotheranostics, and Eisai.
SOURCE: Loibl S et al. SABCS 2020, Abstract GS1-02.
The CDK4/6 inhibitor palbociclib provides no significant benefit in patients with hormone receptor–positive (HR+), HER2-negative primary breast cancer, according to first results from the PENELOPE-B trial.
In this phase 3 trial, adding palbociclib to standard endocrine therapy did not improve invasive disease-free survival (iDFS) in patients who were at high risk of relapse following neoadjuvant chemotherapy (NACT).
There was no significant difference in iDFS rates with palbociclib or placebo at 2 years (88.3% vs. 84%), 3 years (81.2% vs. 77.7%), or even 4 years (73% vs. 72.4%), with a stratified hazard ratio of 0.93 (P = .525).
“This, unfortunately, was a negative trial,” said Ruth M. O’Regan, MD, of the University of Wisconsin–Madison, who independently commented after the trial’s virtual presentation at the 2020 San Antonio Breast Cancer Symposium.
Discussing the iDFS curves for palbociclib and placebo over time, Dr. O’Regan observed that there was a slight benefit for palbociclib in the range of 4.3%, compared with placebo at 2 years and 3.5% at 3 years. The benefit was small but “still meaningful,” she added. However, “at 4 years, you can see the curves have completely come together.”
“This begs the question of whether this trial was just treating patients with occult metastatic disease because I think these curves kind of mirror what we might see in the first-line metastatic setting,” Dr. O’Regan suggested.
Study details
The PENELOPE-B trial was designed to see if palbociclib could improve iDFS in women with HR+, HER2-negative primary breast cancer who were at high risk of relapse following NACT.
The clinical-pathologic stage plus estrogen receptor and grade (CPS-EG) staging system was used to identify patients at high risk for relapse. A CPS-EG score of 3 or higher or 2 or higher with nodal involvement was used as an entry criterion. This has been associated with a 3-year disease-free survival rate of 77%, noted Sibylle Loibl, MD, PhD, head of the German Breast Group in Neu-Isenburg, who presented PENELOPE-B data at the meeting.
The trial enrolled 1,250 women who did not have a pathological complete response after NACT. After surgery, with or without radiotherapy, patients were randomized to receive 13 cycles of either palbociclib or placebo (125 mg once daily; 21 days on and 7 days off treatment). All patients received concomitant endocrine therapy according to local standards.
“Although the compliance was lower in the palbociclib arm than the placebo arm, it was still satisfactory, and the relative dose intensity was over 80%,” Dr. Loibl noted.
The primary endpoint was iDFS rate. As noted previously, there were no significant between-arm differences in 2-, 3-, or 4-year iDFS rates.
Likewise, there were no significant differences between palbociclib and placebo in terms of the secondary endpoints, which included the type of iDFS event (distant recurrences, invasive locoregional recurrences, contralateral breast cancer, second primary invasive nonbreast cancer, and death without previous event).
Subgroup iDFS analyses showed no differences between the study arms. “No group could be identified with a higher benefit from palbociclib,” Dr. Loibl reported.
An interim overall survival (OS) analysis showed no significant differences between palbociclib and placebo. The 2-year OS rate was 96.3% with palbociclib and 94.5% with placebo. The 3-year OS rates were 93.6% and 90.5%, respectively. The 4-year OS rates were 90.4% and 87.3%, respectively.
“Today, the results of the PENELOPE-B study do not support the addition of 1 year of palbociclib to endocrine therapy. Long-term follow up from all neoadjuvant CDK4/6 inhibitor studies should continue and must be awaited,” Dr. Loibl concluded.
Findings in context
PENELOPE-B is the second phase 3 trial to show no benefit for palbociclib in the treatment of early high-risk breast cancer. The first was the PALLAS trial, which was reported only a few months ago at the European Society for Medical Oncology Virtual Congress 2020.
In PALLAS, palbociclib plus endocrine therapy was compared with endocrine therapy alone in the treatment of women with HR+, HER2-negative early breast cancer, but there was no additional benefit seen.
The results of both PENELOPE-B and PALLAS contrast those recently seen with another CDK4/6 inhibitor, abemaciclib, in HR+/HER2-negative early breast cancer.
Results of the phase 3 monarchE trial showed that, when abemaciclib was added to endocrine treatment, there was a significant (P = .0096) 25% relative risk reduction (HR, 0.75; 95% confidence interval, 0.60-0.93) in iDFS, compared with endocrine therapy alone. These results were also presented at the ESMO Virtual Congress 2020 and published in the Journal of Clinical Oncology.
While it’s not clear why one CDK4/6 inhibitor may be of benefit in HR+/HER2-negative early breast cancer while the other is not, “PENELOPE-B is clearly quite a different trial to the other two adjuvant CDK inhibitor trials,” Dr. O’Regan observed.
For one thing, PENELOPE-B participants were only eligible for the trial if they did not have a pathological complete response after NACT. In addition, PENELOPE-B is a smaller trial, enrolling well under a third of the number of patients who participated in the PALLAS and monarchE trials. Furthermore, the CPS-EG score, rather than anatomic staging, was used to determine eligibility.
“Abemaciclib could be a more effective CDK inhibitor; that’s most certainly a possibility,” Dr. O’Regan suggested. “However, it’s not supported by the metastatic first-line trials, which have remarkably similar hazard ratios and favor CDK inhibitors.”
Perhaps the shorter duration of palbociclib treatment in the PENELOPE-B trial (12 months vs. 24 months) had an effect.
“Clearly, we need to await the results of NATALEE that uses 3 years of ribociclib,” Dr. O’Regan said. It also remains to be seen if the results of the monarchE trial hold up with longer follow-up.
The PENELOPE-B trial was sponsored by the German Breast Group in collaboration with Pfizer, the AGO Study Group, NSABP Foundation, and the Breast International Group. Dr. Loibl disclosed grant and other support from Pfizer during the conduct of the study and relationships with other companies outside the submitted work. She also disclosed intellectual property rights and being a pending patent holder (EP14153692.0) for a method to predict response to anti-HER2–containing therapy and/or chemotherapy in patients with breast cancer, and she disclosed a relationship with Medscape, which is owned by the same company as MDedge. Dr. O’Regan disclosed relationships with Novartis, Eli Lilly, Genentech, PUMA, Macrogenics, Immunomedics, Biotheranostics, and Eisai.
SOURCE: Loibl S et al. SABCS 2020, Abstract GS1-02.
FROM SABCS 2020
JIA arthritis and uveitis flares ‘often run parallel’
Children with juvenile idiopathic arthritis–associated uveitis (JIA-U) are significantly more likely to experience a flare in their eye disease if their arthritis is also worsening, a team of U.S.-based researchers has found.
In a longitudinal cohort study, children with active arthritis at the time of a routine rheumatology assessment had an almost 2.5-fold increased risk of also having active uveitis 45 days before or after the assessment than did children whose arthritis was not flaring at the rheumatology assessment.
“We demonstrate that the two diseases often run parallel courses,” corresponding author Emily J. Liebling, MD, of the Children’s Hospital of Philadelphia and associates state in Arthritis Care & Research, noting that the magnitude of the association is striking.
“Although there are known risk factors associated with uveitis development in children with JIA, less data are available about factors associated with uveitis flare or activity,” said Sheila T. Angeles-Han, MD, MSc, of the departments of pediatrics and ophthalmology at Cincinnati Children’s Hospital Medical Center who commented on the study in an interview.
“If proven, this knowledge has the potential to impact practice patterns and current guidelines wherein a pediatric rheumatologist who evaluates a child with JIA-associated uveitis and finds active arthritis would request an expedited ophthalmic examination,” Dr. Angeles-Han suggested.
Dr. Angeles-Han led the development of the first American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of JIA-Associated Uveitis, which recommends regular screening for uveitis in all children with JIA. Children found to have uveitis should then be screened at least every 3 months, and more frequently if they are taking glucocorticoids and treatment is being tapered.
JIA-associated uveitis accounts for around 20%-40% of all cases of noninfectious childhood eye inflammation, and it can run an insidious and chronic course.
“Children with acute anterior uveitis are symptomatic and tend to have a painful red eye, thus prompting an ophthalmic evaluation,” Dr. Angeles-Han explained. “This is different from children with chronic anterior uveitis who tend not to have any symptoms, thus a screening examination is critical to detect ocular inflammation.”
While the ACR/AF guideline distinguishes between acute and chronic uveitis, Dr. Liebling and colleagues explain that they did not because their experience shows that “even patients with chronic anterior uveitis, typically thought to have silent disease, may exhibit symptoms of eye pain, redness, vision changes, and photophobia.”
Conversely, they say “the JIA subtypes usually associated with acute anterior uveitis may instead manifest as asymptomatic eye disease.”
For their study, Dr. Liebling and coinvestigators examined the records of children seen at the Children’s Hospital of Philadelphia over a 6.5-year period. For inclusion, children had to have a physician diagnosis of JIA of any subtype and a history of uveitis.
A total of 98 children were included in the retrospective evaluation; the median age at diagnosis of JIA was 3.3 years, and the median age at first uveitis diagnosis was 5.1 years. The majority (82%) were female, 69% were antinuclear antibody (ANA) positive, and 60% had oligoarthritis – all of which have been associated with having a higher risk for developing uveitis.
However, independent of these and several other factors, the probability of having active uveitis within 45 days of a rheumatology assessment was 65% in those with active arthritis versus 42% for those with no active joints.
Their data are based on 1,229 rheumatology visits that occurred between 2013 and 2019, with a median of 13 visits per patient. Overall, arthritis was defined as being active in 17% of visits, and active uveitis was observed in 18% of rheumatology visits.
Concordance between arthritis and uveitis activity was observed 73% of the time, the researchers reported. A sensitivity analysis that excluded children with the enthesitis-related arthritis subtype of JIA, who may not undergo frequent eye exams, did not change their findings.
Decreased odds of active uveitis at any time point were seen with the use of combination biologic and nonbiologic disease-modifying antirheumatic drugs. Years from uveitis diagnosis was also associated with lower odds of active uveitis over time.
Other factors associated with lower odds of uveitis were female sex, HLA-B27 positivity, and having any subtype of JIA other than the oligoarticular subtype.
Dr. Liebling and coinvestigators concluded that, contrary to the historical dogma, arthritis and uveitis do not run distinct and unrelated courses: “In patients with JIA-U, there is a significant temporal association between arthritis and uveitis disease activity.”
The study was sponsored by the Children’s Hospital of Philadelphia Rheumatology Research Fund. The investigators for the study had no financial support from commercial sources or any other potential conflicts of interest. Dr. Angeles-Han had no conflicts of interest to disclose.
SOURCE: Liebling EJ et al. Arthritis Care Res. 2020 Oct 12. doi: 10.1002/acr.24483.
Children with juvenile idiopathic arthritis–associated uveitis (JIA-U) are significantly more likely to experience a flare in their eye disease if their arthritis is also worsening, a team of U.S.-based researchers has found.
In a longitudinal cohort study, children with active arthritis at the time of a routine rheumatology assessment had an almost 2.5-fold increased risk of also having active uveitis 45 days before or after the assessment than did children whose arthritis was not flaring at the rheumatology assessment.
“We demonstrate that the two diseases often run parallel courses,” corresponding author Emily J. Liebling, MD, of the Children’s Hospital of Philadelphia and associates state in Arthritis Care & Research, noting that the magnitude of the association is striking.
“Although there are known risk factors associated with uveitis development in children with JIA, less data are available about factors associated with uveitis flare or activity,” said Sheila T. Angeles-Han, MD, MSc, of the departments of pediatrics and ophthalmology at Cincinnati Children’s Hospital Medical Center who commented on the study in an interview.
“If proven, this knowledge has the potential to impact practice patterns and current guidelines wherein a pediatric rheumatologist who evaluates a child with JIA-associated uveitis and finds active arthritis would request an expedited ophthalmic examination,” Dr. Angeles-Han suggested.
Dr. Angeles-Han led the development of the first American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of JIA-Associated Uveitis, which recommends regular screening for uveitis in all children with JIA. Children found to have uveitis should then be screened at least every 3 months, and more frequently if they are taking glucocorticoids and treatment is being tapered.
JIA-associated uveitis accounts for around 20%-40% of all cases of noninfectious childhood eye inflammation, and it can run an insidious and chronic course.
“Children with acute anterior uveitis are symptomatic and tend to have a painful red eye, thus prompting an ophthalmic evaluation,” Dr. Angeles-Han explained. “This is different from children with chronic anterior uveitis who tend not to have any symptoms, thus a screening examination is critical to detect ocular inflammation.”
While the ACR/AF guideline distinguishes between acute and chronic uveitis, Dr. Liebling and colleagues explain that they did not because their experience shows that “even patients with chronic anterior uveitis, typically thought to have silent disease, may exhibit symptoms of eye pain, redness, vision changes, and photophobia.”
Conversely, they say “the JIA subtypes usually associated with acute anterior uveitis may instead manifest as asymptomatic eye disease.”
For their study, Dr. Liebling and coinvestigators examined the records of children seen at the Children’s Hospital of Philadelphia over a 6.5-year period. For inclusion, children had to have a physician diagnosis of JIA of any subtype and a history of uveitis.
A total of 98 children were included in the retrospective evaluation; the median age at diagnosis of JIA was 3.3 years, and the median age at first uveitis diagnosis was 5.1 years. The majority (82%) were female, 69% were antinuclear antibody (ANA) positive, and 60% had oligoarthritis – all of which have been associated with having a higher risk for developing uveitis.
However, independent of these and several other factors, the probability of having active uveitis within 45 days of a rheumatology assessment was 65% in those with active arthritis versus 42% for those with no active joints.
Their data are based on 1,229 rheumatology visits that occurred between 2013 and 2019, with a median of 13 visits per patient. Overall, arthritis was defined as being active in 17% of visits, and active uveitis was observed in 18% of rheumatology visits.
Concordance between arthritis and uveitis activity was observed 73% of the time, the researchers reported. A sensitivity analysis that excluded children with the enthesitis-related arthritis subtype of JIA, who may not undergo frequent eye exams, did not change their findings.
Decreased odds of active uveitis at any time point were seen with the use of combination biologic and nonbiologic disease-modifying antirheumatic drugs. Years from uveitis diagnosis was also associated with lower odds of active uveitis over time.
Other factors associated with lower odds of uveitis were female sex, HLA-B27 positivity, and having any subtype of JIA other than the oligoarticular subtype.
Dr. Liebling and coinvestigators concluded that, contrary to the historical dogma, arthritis and uveitis do not run distinct and unrelated courses: “In patients with JIA-U, there is a significant temporal association between arthritis and uveitis disease activity.”
The study was sponsored by the Children’s Hospital of Philadelphia Rheumatology Research Fund. The investigators for the study had no financial support from commercial sources or any other potential conflicts of interest. Dr. Angeles-Han had no conflicts of interest to disclose.
SOURCE: Liebling EJ et al. Arthritis Care Res. 2020 Oct 12. doi: 10.1002/acr.24483.
Children with juvenile idiopathic arthritis–associated uveitis (JIA-U) are significantly more likely to experience a flare in their eye disease if their arthritis is also worsening, a team of U.S.-based researchers has found.
In a longitudinal cohort study, children with active arthritis at the time of a routine rheumatology assessment had an almost 2.5-fold increased risk of also having active uveitis 45 days before or after the assessment than did children whose arthritis was not flaring at the rheumatology assessment.
“We demonstrate that the two diseases often run parallel courses,” corresponding author Emily J. Liebling, MD, of the Children’s Hospital of Philadelphia and associates state in Arthritis Care & Research, noting that the magnitude of the association is striking.
“Although there are known risk factors associated with uveitis development in children with JIA, less data are available about factors associated with uveitis flare or activity,” said Sheila T. Angeles-Han, MD, MSc, of the departments of pediatrics and ophthalmology at Cincinnati Children’s Hospital Medical Center who commented on the study in an interview.
“If proven, this knowledge has the potential to impact practice patterns and current guidelines wherein a pediatric rheumatologist who evaluates a child with JIA-associated uveitis and finds active arthritis would request an expedited ophthalmic examination,” Dr. Angeles-Han suggested.
Dr. Angeles-Han led the development of the first American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of JIA-Associated Uveitis, which recommends regular screening for uveitis in all children with JIA. Children found to have uveitis should then be screened at least every 3 months, and more frequently if they are taking glucocorticoids and treatment is being tapered.
JIA-associated uveitis accounts for around 20%-40% of all cases of noninfectious childhood eye inflammation, and it can run an insidious and chronic course.
“Children with acute anterior uveitis are symptomatic and tend to have a painful red eye, thus prompting an ophthalmic evaluation,” Dr. Angeles-Han explained. “This is different from children with chronic anterior uveitis who tend not to have any symptoms, thus a screening examination is critical to detect ocular inflammation.”
While the ACR/AF guideline distinguishes between acute and chronic uveitis, Dr. Liebling and colleagues explain that they did not because their experience shows that “even patients with chronic anterior uveitis, typically thought to have silent disease, may exhibit symptoms of eye pain, redness, vision changes, and photophobia.”
Conversely, they say “the JIA subtypes usually associated with acute anterior uveitis may instead manifest as asymptomatic eye disease.”
For their study, Dr. Liebling and coinvestigators examined the records of children seen at the Children’s Hospital of Philadelphia over a 6.5-year period. For inclusion, children had to have a physician diagnosis of JIA of any subtype and a history of uveitis.
A total of 98 children were included in the retrospective evaluation; the median age at diagnosis of JIA was 3.3 years, and the median age at first uveitis diagnosis was 5.1 years. The majority (82%) were female, 69% were antinuclear antibody (ANA) positive, and 60% had oligoarthritis – all of which have been associated with having a higher risk for developing uveitis.
However, independent of these and several other factors, the probability of having active uveitis within 45 days of a rheumatology assessment was 65% in those with active arthritis versus 42% for those with no active joints.
Their data are based on 1,229 rheumatology visits that occurred between 2013 and 2019, with a median of 13 visits per patient. Overall, arthritis was defined as being active in 17% of visits, and active uveitis was observed in 18% of rheumatology visits.
Concordance between arthritis and uveitis activity was observed 73% of the time, the researchers reported. A sensitivity analysis that excluded children with the enthesitis-related arthritis subtype of JIA, who may not undergo frequent eye exams, did not change their findings.
Decreased odds of active uveitis at any time point were seen with the use of combination biologic and nonbiologic disease-modifying antirheumatic drugs. Years from uveitis diagnosis was also associated with lower odds of active uveitis over time.
Other factors associated with lower odds of uveitis were female sex, HLA-B27 positivity, and having any subtype of JIA other than the oligoarticular subtype.
Dr. Liebling and coinvestigators concluded that, contrary to the historical dogma, arthritis and uveitis do not run distinct and unrelated courses: “In patients with JIA-U, there is a significant temporal association between arthritis and uveitis disease activity.”
The study was sponsored by the Children’s Hospital of Philadelphia Rheumatology Research Fund. The investigators for the study had no financial support from commercial sources or any other potential conflicts of interest. Dr. Angeles-Han had no conflicts of interest to disclose.
SOURCE: Liebling EJ et al. Arthritis Care Res. 2020 Oct 12. doi: 10.1002/acr.24483.
FROM ARTHRITIS CARE & RESEARCH