User login
AAN publishes ethical guidance on patient care during the pandemic
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
FROM NEUROLOGY
Blood pressure lowering lessens risk of dementia, cognitive decline
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
FROM JAMA
Yoga is a good adjunct to migraine therapy
in Neurology.
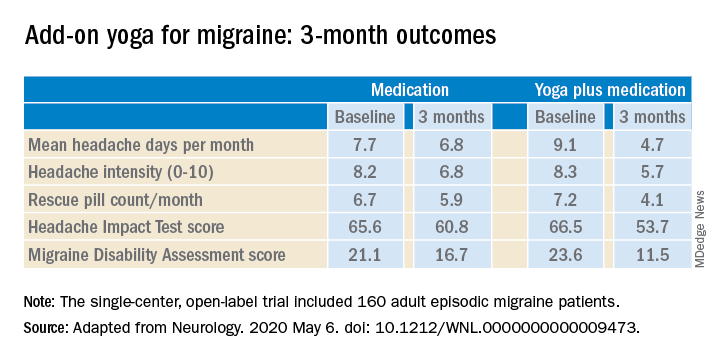
The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
FROM NEUROLOGY
Satralizumab monotherapy reduces NMOSD relapse rate
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
FROM THE LANCET NEUROLOGY
Video coaching may relieve anxiety and distress for long-distance cancer caregivers
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
FROM ASCO 2020
Mammography cuts risk for fatal breast cancers: New data
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Updated AAN advisory outlines when PFO closure may be option for patients with stroke
Patients with an embolic-appearing infarct who are younger than 60 years, have undergone a thorough evaluation to rule out other stroke mechanisms, and have discussed with doctors the potential risks and benefits may be candidates for the procedure.
“For patients with cryptogenic stroke and PFO, percutaneous PFO closure probably reduces the risk of stroke recurrence with [a hazard ratio] of 0.41 and an absolute risk reduction of 3.4% at 5 years; probably is associated with a periprocedural complication rate of 3.9%; and probably is associated with the development of serious nonperiprocedural atrial fibrillation, with a relative risk of 2.72,” according to the advisory authors’ meta-analysis.
Most procedural complications and instances of atrial fibrillation were “self-limited and are of uncertain long-term clinical consequence, given the lower rate of stroke in patients whose PFOs were closed,” the authors said. “Subgroup analysis suggests that the overall benefit seen across trials may not extend to those patients with small shunts and small, deep infarcts.” The authors estimated that the number of patients who need to be treated to prevent one stroke at 5 years is 29.
The advisory updates 2016 guidance that said clinicians should not routinely offer PFO closure outside of a research setting. Since then, three trials published in 2017 the New England Journal of Medicine (RESPECT, CLOSE, and REDUCE) and one trial published in 2018 in the Journal of the American College of Cardiology (DEFENSE-PFO) found that PFO closure reduces the risk of recurrent stroke in patients with a PFO who have had a cryptogenic stroke, compared with medical therapy alone. In addition, the Food and Drug Administration approved the Amplatzer PFO Occluder and Gore Cardioform Septal Occluder. These developments necessitated the practice advisory, the authors said. The advisory was published online April 29 in Neurology. It is endorsed by the American Heart Association/American Stroke Association, the Society for Cardiovascular Angiography and Interventions, and the European Academy of Neurology.
Systematic review
For the update, Steven R. Messé, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and a panel of neurologists, internists, and cardiologists with expertise in stroke and PFO systematically reviewed relevant randomized studies published through August 2019 and conducted meta-analyses to make their recommendations. The literature search identified eight articles that met inclusion criteria, including one article that provided follow-up from a trial that had been included in the previous practice advisory.
“The risk of a second stroke in people with PFO and no other possible causes of stroke is very low, approximately 1% per year while being treated with just medication alone,” Dr. Messé said in a news release. “Also, it is difficult to determine with absolute certainty that the PFO is the cause of a person’s stroke. So it is important that people with PFO are educated about the benefits and risks of PFO closure.” For patients who opt to take medication only, doctors may consider prescribing antiplatelet or anticoagulant drugs, according to the advisory. “All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed,” Dr. Messé and colleagues wrote. “However, specific antithrombotic management for patients with stroke thought to be caused by PFO remains uncertain.”
Calls for thorough work-up
“If an alternative plausible higher-risk mechanism of stroke is identified, it is likely that the PFO was an innocent bystander,” the authors said. “Secondary stroke prevention is optimized by targeting the most likely etiology of the preceding event. ... The randomized PFO closure trials all mandated thorough evaluations for participants before enrollment ... to rule out other stroke mechanisms; moreover, all studies required TEE [transesophageal echocardiography] to characterize the PFO and ensure that it was the most likely etiology for the initial event.”
In patients being considered for PFO closure, clinicians should obtain brain imaging to confirm stroke size and distribution (level B); obtain vascular imaging of the cervical and intracranial vessels to look for dissection, vasculopathy, and atherosclerosis (level B); and perform hypercoagulable studies (level B), according to the advisory. Clinicians must perform a baseline ECG to look for atrial fibrillation (level A), and patients thought to be at risk of atrial fibrillation should receive prolonged cardiac monitoring for at least 28 days (level B).
Before PFO closure, a clinician with expertise in stroke should assess the patient to ensure that the PFO is the most plausible mechanism of stroke (level B). “If a higher-risk alternative mechanism of stroke is identified, clinicians should not routinely recommend PFO closure (level B),” the authors said. Patients also should be assessed by a clinician with expertise in assessing the anatomic features of a PFO and performing PFO closure (level B).
The randomized trials focused on patients whose PFOs were closed within 6 months of a stroke, and registry studies are needed to assess long-term outcomes, noted Dr. Messé and colleagues. “It remains unclear whether closure provides a similar benefit in these patients who otherwise still fit the studies’ inclusion criteria,” the authors said. “Long-term and large-scale safety registries for patients who have received PFO closure are needed to assess the risk of device erosion, fracture, embolization, and thrombotic and endocarditis risks, and the effect of residual shunts and incidence of atrial fibrillation.”
About 25% of the general adult population has a PFO. “It’s important to note that having a PFO is common, and that most people with PFO will never know they have it because it usually does not cause any problems,” Dr. Messé said. “However, while there is generally a very low risk of stroke in patients with PFO, in younger people who have had a stroke without any other possible causes identified, closing the PFO may reduce the risk of having another stroke better than medication alone.”
The practice advisory was developed with financial support from the AAN. Dr. Messé and most of the authors had no relevant conflicts of interest. Several authors disclosed ties to medical device and pharmaceutical companies.
SOURCE: Messé SR et al. Neurology. 2020 Apr 29. doi: 10.1212/WNL.0000000000009443.
Patients with an embolic-appearing infarct who are younger than 60 years, have undergone a thorough evaluation to rule out other stroke mechanisms, and have discussed with doctors the potential risks and benefits may be candidates for the procedure.
“For patients with cryptogenic stroke and PFO, percutaneous PFO closure probably reduces the risk of stroke recurrence with [a hazard ratio] of 0.41 and an absolute risk reduction of 3.4% at 5 years; probably is associated with a periprocedural complication rate of 3.9%; and probably is associated with the development of serious nonperiprocedural atrial fibrillation, with a relative risk of 2.72,” according to the advisory authors’ meta-analysis.
Most procedural complications and instances of atrial fibrillation were “self-limited and are of uncertain long-term clinical consequence, given the lower rate of stroke in patients whose PFOs were closed,” the authors said. “Subgroup analysis suggests that the overall benefit seen across trials may not extend to those patients with small shunts and small, deep infarcts.” The authors estimated that the number of patients who need to be treated to prevent one stroke at 5 years is 29.
The advisory updates 2016 guidance that said clinicians should not routinely offer PFO closure outside of a research setting. Since then, three trials published in 2017 the New England Journal of Medicine (RESPECT, CLOSE, and REDUCE) and one trial published in 2018 in the Journal of the American College of Cardiology (DEFENSE-PFO) found that PFO closure reduces the risk of recurrent stroke in patients with a PFO who have had a cryptogenic stroke, compared with medical therapy alone. In addition, the Food and Drug Administration approved the Amplatzer PFO Occluder and Gore Cardioform Septal Occluder. These developments necessitated the practice advisory, the authors said. The advisory was published online April 29 in Neurology. It is endorsed by the American Heart Association/American Stroke Association, the Society for Cardiovascular Angiography and Interventions, and the European Academy of Neurology.
Systematic review
For the update, Steven R. Messé, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and a panel of neurologists, internists, and cardiologists with expertise in stroke and PFO systematically reviewed relevant randomized studies published through August 2019 and conducted meta-analyses to make their recommendations. The literature search identified eight articles that met inclusion criteria, including one article that provided follow-up from a trial that had been included in the previous practice advisory.
“The risk of a second stroke in people with PFO and no other possible causes of stroke is very low, approximately 1% per year while being treated with just medication alone,” Dr. Messé said in a news release. “Also, it is difficult to determine with absolute certainty that the PFO is the cause of a person’s stroke. So it is important that people with PFO are educated about the benefits and risks of PFO closure.” For patients who opt to take medication only, doctors may consider prescribing antiplatelet or anticoagulant drugs, according to the advisory. “All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed,” Dr. Messé and colleagues wrote. “However, specific antithrombotic management for patients with stroke thought to be caused by PFO remains uncertain.”
Calls for thorough work-up
“If an alternative plausible higher-risk mechanism of stroke is identified, it is likely that the PFO was an innocent bystander,” the authors said. “Secondary stroke prevention is optimized by targeting the most likely etiology of the preceding event. ... The randomized PFO closure trials all mandated thorough evaluations for participants before enrollment ... to rule out other stroke mechanisms; moreover, all studies required TEE [transesophageal echocardiography] to characterize the PFO and ensure that it was the most likely etiology for the initial event.”
In patients being considered for PFO closure, clinicians should obtain brain imaging to confirm stroke size and distribution (level B); obtain vascular imaging of the cervical and intracranial vessels to look for dissection, vasculopathy, and atherosclerosis (level B); and perform hypercoagulable studies (level B), according to the advisory. Clinicians must perform a baseline ECG to look for atrial fibrillation (level A), and patients thought to be at risk of atrial fibrillation should receive prolonged cardiac monitoring for at least 28 days (level B).
Before PFO closure, a clinician with expertise in stroke should assess the patient to ensure that the PFO is the most plausible mechanism of stroke (level B). “If a higher-risk alternative mechanism of stroke is identified, clinicians should not routinely recommend PFO closure (level B),” the authors said. Patients also should be assessed by a clinician with expertise in assessing the anatomic features of a PFO and performing PFO closure (level B).
The randomized trials focused on patients whose PFOs were closed within 6 months of a stroke, and registry studies are needed to assess long-term outcomes, noted Dr. Messé and colleagues. “It remains unclear whether closure provides a similar benefit in these patients who otherwise still fit the studies’ inclusion criteria,” the authors said. “Long-term and large-scale safety registries for patients who have received PFO closure are needed to assess the risk of device erosion, fracture, embolization, and thrombotic and endocarditis risks, and the effect of residual shunts and incidence of atrial fibrillation.”
About 25% of the general adult population has a PFO. “It’s important to note that having a PFO is common, and that most people with PFO will never know they have it because it usually does not cause any problems,” Dr. Messé said. “However, while there is generally a very low risk of stroke in patients with PFO, in younger people who have had a stroke without any other possible causes identified, closing the PFO may reduce the risk of having another stroke better than medication alone.”
The practice advisory was developed with financial support from the AAN. Dr. Messé and most of the authors had no relevant conflicts of interest. Several authors disclosed ties to medical device and pharmaceutical companies.
SOURCE: Messé SR et al. Neurology. 2020 Apr 29. doi: 10.1212/WNL.0000000000009443.
Patients with an embolic-appearing infarct who are younger than 60 years, have undergone a thorough evaluation to rule out other stroke mechanisms, and have discussed with doctors the potential risks and benefits may be candidates for the procedure.
“For patients with cryptogenic stroke and PFO, percutaneous PFO closure probably reduces the risk of stroke recurrence with [a hazard ratio] of 0.41 and an absolute risk reduction of 3.4% at 5 years; probably is associated with a periprocedural complication rate of 3.9%; and probably is associated with the development of serious nonperiprocedural atrial fibrillation, with a relative risk of 2.72,” according to the advisory authors’ meta-analysis.
Most procedural complications and instances of atrial fibrillation were “self-limited and are of uncertain long-term clinical consequence, given the lower rate of stroke in patients whose PFOs were closed,” the authors said. “Subgroup analysis suggests that the overall benefit seen across trials may not extend to those patients with small shunts and small, deep infarcts.” The authors estimated that the number of patients who need to be treated to prevent one stroke at 5 years is 29.
The advisory updates 2016 guidance that said clinicians should not routinely offer PFO closure outside of a research setting. Since then, three trials published in 2017 the New England Journal of Medicine (RESPECT, CLOSE, and REDUCE) and one trial published in 2018 in the Journal of the American College of Cardiology (DEFENSE-PFO) found that PFO closure reduces the risk of recurrent stroke in patients with a PFO who have had a cryptogenic stroke, compared with medical therapy alone. In addition, the Food and Drug Administration approved the Amplatzer PFO Occluder and Gore Cardioform Septal Occluder. These developments necessitated the practice advisory, the authors said. The advisory was published online April 29 in Neurology. It is endorsed by the American Heart Association/American Stroke Association, the Society for Cardiovascular Angiography and Interventions, and the European Academy of Neurology.
Systematic review
For the update, Steven R. Messé, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and a panel of neurologists, internists, and cardiologists with expertise in stroke and PFO systematically reviewed relevant randomized studies published through August 2019 and conducted meta-analyses to make their recommendations. The literature search identified eight articles that met inclusion criteria, including one article that provided follow-up from a trial that had been included in the previous practice advisory.
“The risk of a second stroke in people with PFO and no other possible causes of stroke is very low, approximately 1% per year while being treated with just medication alone,” Dr. Messé said in a news release. “Also, it is difficult to determine with absolute certainty that the PFO is the cause of a person’s stroke. So it is important that people with PFO are educated about the benefits and risks of PFO closure.” For patients who opt to take medication only, doctors may consider prescribing antiplatelet or anticoagulant drugs, according to the advisory. “All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed,” Dr. Messé and colleagues wrote. “However, specific antithrombotic management for patients with stroke thought to be caused by PFO remains uncertain.”
Calls for thorough work-up
“If an alternative plausible higher-risk mechanism of stroke is identified, it is likely that the PFO was an innocent bystander,” the authors said. “Secondary stroke prevention is optimized by targeting the most likely etiology of the preceding event. ... The randomized PFO closure trials all mandated thorough evaluations for participants before enrollment ... to rule out other stroke mechanisms; moreover, all studies required TEE [transesophageal echocardiography] to characterize the PFO and ensure that it was the most likely etiology for the initial event.”
In patients being considered for PFO closure, clinicians should obtain brain imaging to confirm stroke size and distribution (level B); obtain vascular imaging of the cervical and intracranial vessels to look for dissection, vasculopathy, and atherosclerosis (level B); and perform hypercoagulable studies (level B), according to the advisory. Clinicians must perform a baseline ECG to look for atrial fibrillation (level A), and patients thought to be at risk of atrial fibrillation should receive prolonged cardiac monitoring for at least 28 days (level B).
Before PFO closure, a clinician with expertise in stroke should assess the patient to ensure that the PFO is the most plausible mechanism of stroke (level B). “If a higher-risk alternative mechanism of stroke is identified, clinicians should not routinely recommend PFO closure (level B),” the authors said. Patients also should be assessed by a clinician with expertise in assessing the anatomic features of a PFO and performing PFO closure (level B).
The randomized trials focused on patients whose PFOs were closed within 6 months of a stroke, and registry studies are needed to assess long-term outcomes, noted Dr. Messé and colleagues. “It remains unclear whether closure provides a similar benefit in these patients who otherwise still fit the studies’ inclusion criteria,” the authors said. “Long-term and large-scale safety registries for patients who have received PFO closure are needed to assess the risk of device erosion, fracture, embolization, and thrombotic and endocarditis risks, and the effect of residual shunts and incidence of atrial fibrillation.”
About 25% of the general adult population has a PFO. “It’s important to note that having a PFO is common, and that most people with PFO will never know they have it because it usually does not cause any problems,” Dr. Messé said. “However, while there is generally a very low risk of stroke in patients with PFO, in younger people who have had a stroke without any other possible causes identified, closing the PFO may reduce the risk of having another stroke better than medication alone.”
The practice advisory was developed with financial support from the AAN. Dr. Messé and most of the authors had no relevant conflicts of interest. Several authors disclosed ties to medical device and pharmaceutical companies.
SOURCE: Messé SR et al. Neurology. 2020 Apr 29. doi: 10.1212/WNL.0000000000009443.
FROM NEUROLOGY
ASCO goes ahead online, as conference center is used as hospital
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Researchers identify a cause of L-DOPA–induced dyskinesia in Parkinson’s disease
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
FROM Science Advances
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY



