User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Higher endovascular thrombectomy volumes yield better stroke outcomes
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
REPORTING FROM ISC 2020
TNK dose in large-vessel stroke: 0.25 mg/kg is sufficient
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
As costs for neurologic drugs rise, adherence to therapy drops
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
FROM NEUROLOGY
ARCADIA: Predicting risk of atrial cardiopathy poststroke
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.
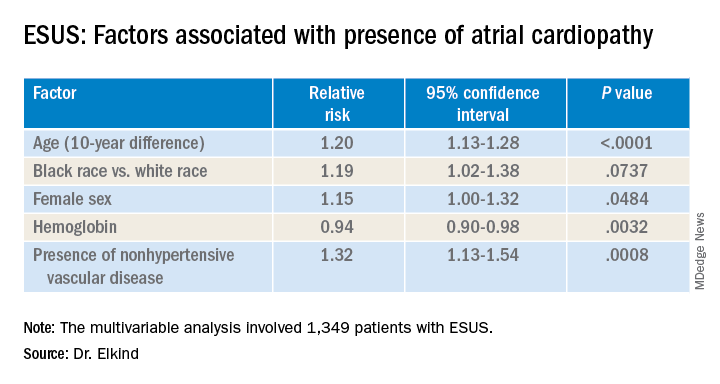
Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
REPORTING FROM ISC 2020
Shingles vaccine linked to lower stroke risk
LOS ANGELES – Prevention of shingles with the Zoster Vaccine Live may reduce the risk of subsequent stroke among older adults as well, the first study to examine this association suggests. Shingles vaccination was linked to a 20% decrease in stroke risk in people younger than 80 years of age in the large Medicare cohort study. Older participants showed a 10% reduced risk, according to data released in advance of formal presentation at this week’s International Stroke Conference, sponsored by the American Heart Association.
Reductions were seen for both ischemic and hemorrhagic events.
“Our findings might encourage people age 50 or older to get vaccinated against shingles and to prevent shingles-associated stroke risk,” Quanhe Yang, PhD, lead study author and senior scientist at the Centers for Disease Control and Prevention, said in an interview.
Dr. Yang and colleagues evaluated the only shingles vaccine available at the time of the study, Zoster Vaccine Live (Zostavax). However, the CDC now calls an adjuvanted, nonlive recombinant vaccine (Shingrix) the preferred shingles vaccine for healthy adults aged 50 years and older. Shingrix was approved in 2017. Zostavax, approved in 2006, can still be used in healthy adults aged 60 years and older, the agency states.
A reduction in inflammation from Zoster Vaccine Live may be the mechanism by which stroke risk is reduced, Dr. Yang said. The newer vaccine, which the CDC notes is more than 90% effective, might provide even greater protection against stroke, although more research is needed, he added.
Interestingly, prior research suggested that, once a person develops shingles, it may be too late. Dr. Yang and colleagues showed vaccination or antiviral treatment after a shingles episode was not effective at reducing stroke risk in research presented at the 2019 International Stroke Conference.
Shingles can present as a painful reactivation of chickenpox, also known as the varicella-zoster virus. Shingles is also common; Dr. Yang estimated one in three people who had chickenpox will develop the condition at some point in their lifetime. In addition, researchers have linked shingles to an elevated risk of stroke.
To assess the vaccine’s protective effect on stroke, Dr. Yang and colleagues reviewed health records for 1.38 million Medicare recipients. All participants were aged 66 years or older, had no history of stroke at baseline, and received the Zoster Vaccine Live during 2008-2016. The investigators compared the stroke rate in this vaccinated group with the rate in a matched control group of the same number of Medicare fee-for-service beneficiaries who did not receive the vaccination. They adjusted their analysis for age, sex, race, medications, and comorbidities.
The overall decrease of 16% in stroke risk associated with vaccination included a 12% drop in hemorrhagic stroke and 18% decrease in ischemic stroke over a median follow-up of 3.9 years follow-up (interquartile range, 2.7-5.4).
The adjusted hazard ratios comparing the vaccinated with control groups were 0.84 (95% confidence interval, 0.83-0.85) for all stroke; 0.82 (95% CI, 0.81-0.83) for acute ischemic stroke; and 0.88 (95% CI, 0.84-0.91) for hemorrhagic stroke.
The vaccinated group experienced 42,267 stroke events during that time. This rate included 33,510 acute ischemic strokes and 4,318 hemorrhagic strokes. At the same time, 48,139 strokes occurred in the control group. The breakdown included 39,334 ischemic and 4,713 hemorrhagic events.
“Approximately 1 million people in the United States get shingles each year, yet there is a vaccine to help prevent it,” Dr. Yang stated in a news release. “Our study results may encourage people ages 50 and older to follow the recommendation and get vaccinated against shingles. You are reducing the risk of shingles, and at the same time, you may be reducing your risk of stroke.”
“Further studies are needed to confirm our findings of association between Zostavax vaccine and risk of stroke,” Dr. Yang said.
Because the CDC Advisory Committee on Immunization Practices recommended Shingrix vaccine only for healthy adults 50 years and older in 2017, there were insufficient data in Medicare to study the association between that vaccine and risk of stroke at the time of the current study.
“However, two doses of Shingrix are more than 90% effective at preventing shingles and postherpetic neuralgia, and higher than that of Zostavax,” Dr. Yang said.
‘Very intriguing’ research
“This is a very interesting study,” Ralph L. Sacco, MD, past president of the American Heart Association, said in a video commentary released in advance of the conference. It was a very large sample, he noted, and those older than age 60 years who had the vaccine were protected with a lower risk for both ischemic and hemorrhagic stroke.
“So it is very intriguing,” added Dr. Sacco, chairman of the department of neurology at the University of Miami. “We know things like shingles can increase inflammation and increase the risk of stroke,” Dr. Sacco said, “but this is the first time in a very large Medicare database that it was shown that those who had the vaccine had a lower risk of stroke.”
The CDC funded this study. Dr. Yang and Dr. Sacco have disclosed no relevant financial relationships.
SOURCE: Yang Q et al. ISC 2020, Abstract TP493.
This article first appeared on Medscape.com.
LOS ANGELES – Prevention of shingles with the Zoster Vaccine Live may reduce the risk of subsequent stroke among older adults as well, the first study to examine this association suggests. Shingles vaccination was linked to a 20% decrease in stroke risk in people younger than 80 years of age in the large Medicare cohort study. Older participants showed a 10% reduced risk, according to data released in advance of formal presentation at this week’s International Stroke Conference, sponsored by the American Heart Association.
Reductions were seen for both ischemic and hemorrhagic events.
“Our findings might encourage people age 50 or older to get vaccinated against shingles and to prevent shingles-associated stroke risk,” Quanhe Yang, PhD, lead study author and senior scientist at the Centers for Disease Control and Prevention, said in an interview.
Dr. Yang and colleagues evaluated the only shingles vaccine available at the time of the study, Zoster Vaccine Live (Zostavax). However, the CDC now calls an adjuvanted, nonlive recombinant vaccine (Shingrix) the preferred shingles vaccine for healthy adults aged 50 years and older. Shingrix was approved in 2017. Zostavax, approved in 2006, can still be used in healthy adults aged 60 years and older, the agency states.
A reduction in inflammation from Zoster Vaccine Live may be the mechanism by which stroke risk is reduced, Dr. Yang said. The newer vaccine, which the CDC notes is more than 90% effective, might provide even greater protection against stroke, although more research is needed, he added.
Interestingly, prior research suggested that, once a person develops shingles, it may be too late. Dr. Yang and colleagues showed vaccination or antiviral treatment after a shingles episode was not effective at reducing stroke risk in research presented at the 2019 International Stroke Conference.
Shingles can present as a painful reactivation of chickenpox, also known as the varicella-zoster virus. Shingles is also common; Dr. Yang estimated one in three people who had chickenpox will develop the condition at some point in their lifetime. In addition, researchers have linked shingles to an elevated risk of stroke.
To assess the vaccine’s protective effect on stroke, Dr. Yang and colleagues reviewed health records for 1.38 million Medicare recipients. All participants were aged 66 years or older, had no history of stroke at baseline, and received the Zoster Vaccine Live during 2008-2016. The investigators compared the stroke rate in this vaccinated group with the rate in a matched control group of the same number of Medicare fee-for-service beneficiaries who did not receive the vaccination. They adjusted their analysis for age, sex, race, medications, and comorbidities.
The overall decrease of 16% in stroke risk associated with vaccination included a 12% drop in hemorrhagic stroke and 18% decrease in ischemic stroke over a median follow-up of 3.9 years follow-up (interquartile range, 2.7-5.4).
The adjusted hazard ratios comparing the vaccinated with control groups were 0.84 (95% confidence interval, 0.83-0.85) for all stroke; 0.82 (95% CI, 0.81-0.83) for acute ischemic stroke; and 0.88 (95% CI, 0.84-0.91) for hemorrhagic stroke.
The vaccinated group experienced 42,267 stroke events during that time. This rate included 33,510 acute ischemic strokes and 4,318 hemorrhagic strokes. At the same time, 48,139 strokes occurred in the control group. The breakdown included 39,334 ischemic and 4,713 hemorrhagic events.
“Approximately 1 million people in the United States get shingles each year, yet there is a vaccine to help prevent it,” Dr. Yang stated in a news release. “Our study results may encourage people ages 50 and older to follow the recommendation and get vaccinated against shingles. You are reducing the risk of shingles, and at the same time, you may be reducing your risk of stroke.”
“Further studies are needed to confirm our findings of association between Zostavax vaccine and risk of stroke,” Dr. Yang said.
Because the CDC Advisory Committee on Immunization Practices recommended Shingrix vaccine only for healthy adults 50 years and older in 2017, there were insufficient data in Medicare to study the association between that vaccine and risk of stroke at the time of the current study.
“However, two doses of Shingrix are more than 90% effective at preventing shingles and postherpetic neuralgia, and higher than that of Zostavax,” Dr. Yang said.
‘Very intriguing’ research
“This is a very interesting study,” Ralph L. Sacco, MD, past president of the American Heart Association, said in a video commentary released in advance of the conference. It was a very large sample, he noted, and those older than age 60 years who had the vaccine were protected with a lower risk for both ischemic and hemorrhagic stroke.
“So it is very intriguing,” added Dr. Sacco, chairman of the department of neurology at the University of Miami. “We know things like shingles can increase inflammation and increase the risk of stroke,” Dr. Sacco said, “but this is the first time in a very large Medicare database that it was shown that those who had the vaccine had a lower risk of stroke.”
The CDC funded this study. Dr. Yang and Dr. Sacco have disclosed no relevant financial relationships.
SOURCE: Yang Q et al. ISC 2020, Abstract TP493.
This article first appeared on Medscape.com.
LOS ANGELES – Prevention of shingles with the Zoster Vaccine Live may reduce the risk of subsequent stroke among older adults as well, the first study to examine this association suggests. Shingles vaccination was linked to a 20% decrease in stroke risk in people younger than 80 years of age in the large Medicare cohort study. Older participants showed a 10% reduced risk, according to data released in advance of formal presentation at this week’s International Stroke Conference, sponsored by the American Heart Association.
Reductions were seen for both ischemic and hemorrhagic events.
“Our findings might encourage people age 50 or older to get vaccinated against shingles and to prevent shingles-associated stroke risk,” Quanhe Yang, PhD, lead study author and senior scientist at the Centers for Disease Control and Prevention, said in an interview.
Dr. Yang and colleagues evaluated the only shingles vaccine available at the time of the study, Zoster Vaccine Live (Zostavax). However, the CDC now calls an adjuvanted, nonlive recombinant vaccine (Shingrix) the preferred shingles vaccine for healthy adults aged 50 years and older. Shingrix was approved in 2017. Zostavax, approved in 2006, can still be used in healthy adults aged 60 years and older, the agency states.
A reduction in inflammation from Zoster Vaccine Live may be the mechanism by which stroke risk is reduced, Dr. Yang said. The newer vaccine, which the CDC notes is more than 90% effective, might provide even greater protection against stroke, although more research is needed, he added.
Interestingly, prior research suggested that, once a person develops shingles, it may be too late. Dr. Yang and colleagues showed vaccination or antiviral treatment after a shingles episode was not effective at reducing stroke risk in research presented at the 2019 International Stroke Conference.
Shingles can present as a painful reactivation of chickenpox, also known as the varicella-zoster virus. Shingles is also common; Dr. Yang estimated one in three people who had chickenpox will develop the condition at some point in their lifetime. In addition, researchers have linked shingles to an elevated risk of stroke.
To assess the vaccine’s protective effect on stroke, Dr. Yang and colleagues reviewed health records for 1.38 million Medicare recipients. All participants were aged 66 years or older, had no history of stroke at baseline, and received the Zoster Vaccine Live during 2008-2016. The investigators compared the stroke rate in this vaccinated group with the rate in a matched control group of the same number of Medicare fee-for-service beneficiaries who did not receive the vaccination. They adjusted their analysis for age, sex, race, medications, and comorbidities.
The overall decrease of 16% in stroke risk associated with vaccination included a 12% drop in hemorrhagic stroke and 18% decrease in ischemic stroke over a median follow-up of 3.9 years follow-up (interquartile range, 2.7-5.4).
The adjusted hazard ratios comparing the vaccinated with control groups were 0.84 (95% confidence interval, 0.83-0.85) for all stroke; 0.82 (95% CI, 0.81-0.83) for acute ischemic stroke; and 0.88 (95% CI, 0.84-0.91) for hemorrhagic stroke.
The vaccinated group experienced 42,267 stroke events during that time. This rate included 33,510 acute ischemic strokes and 4,318 hemorrhagic strokes. At the same time, 48,139 strokes occurred in the control group. The breakdown included 39,334 ischemic and 4,713 hemorrhagic events.
“Approximately 1 million people in the United States get shingles each year, yet there is a vaccine to help prevent it,” Dr. Yang stated in a news release. “Our study results may encourage people ages 50 and older to follow the recommendation and get vaccinated against shingles. You are reducing the risk of shingles, and at the same time, you may be reducing your risk of stroke.”
“Further studies are needed to confirm our findings of association between Zostavax vaccine and risk of stroke,” Dr. Yang said.
Because the CDC Advisory Committee on Immunization Practices recommended Shingrix vaccine only for healthy adults 50 years and older in 2017, there were insufficient data in Medicare to study the association between that vaccine and risk of stroke at the time of the current study.
“However, two doses of Shingrix are more than 90% effective at preventing shingles and postherpetic neuralgia, and higher than that of Zostavax,” Dr. Yang said.
‘Very intriguing’ research
“This is a very interesting study,” Ralph L. Sacco, MD, past president of the American Heart Association, said in a video commentary released in advance of the conference. It was a very large sample, he noted, and those older than age 60 years who had the vaccine were protected with a lower risk for both ischemic and hemorrhagic stroke.
“So it is very intriguing,” added Dr. Sacco, chairman of the department of neurology at the University of Miami. “We know things like shingles can increase inflammation and increase the risk of stroke,” Dr. Sacco said, “but this is the first time in a very large Medicare database that it was shown that those who had the vaccine had a lower risk of stroke.”
The CDC funded this study. Dr. Yang and Dr. Sacco have disclosed no relevant financial relationships.
SOURCE: Yang Q et al. ISC 2020, Abstract TP493.
This article first appeared on Medscape.com.
REPORTING FROM ISC 2020
Stroke risk tied to diabetic retinopathy may not be modifiable
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
REPORTING FROM ISC 2020
Carotid endarterectomy surpasses stenting in elderly, asymptomatic patients
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
REPORTING FROM ISC 2020
‘Momentous’ USMLE change: New pass/fail format stuns medicine
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
‘A glimmer of hope’ for stroke/mortality benefit with AFib catheter ablation
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
Zilucoplan improved efficacy outcomes in myasthenia gravis
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
FROM JAMA NEUROLOGY









