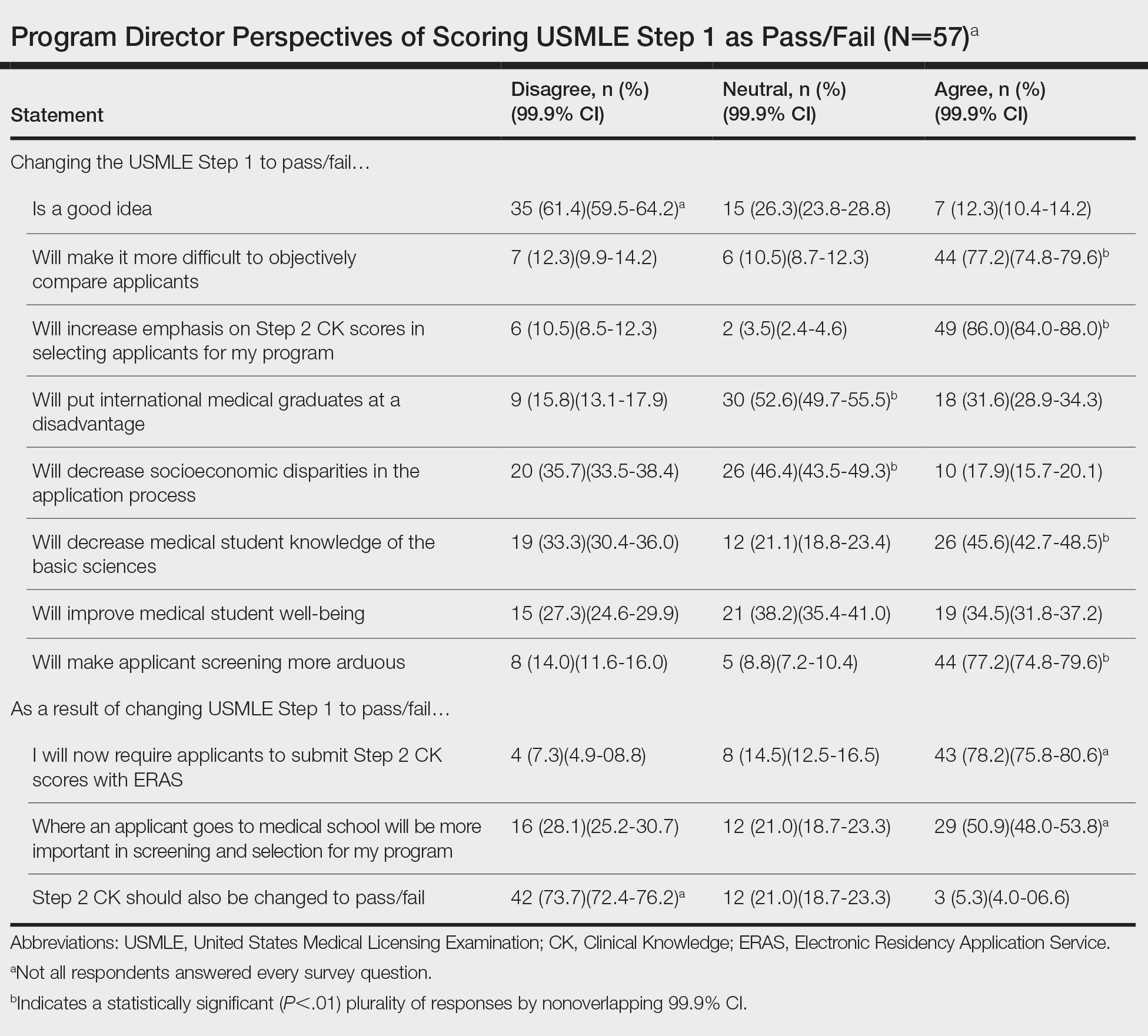
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Nivolumab-Induced Granuloma Annulare
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
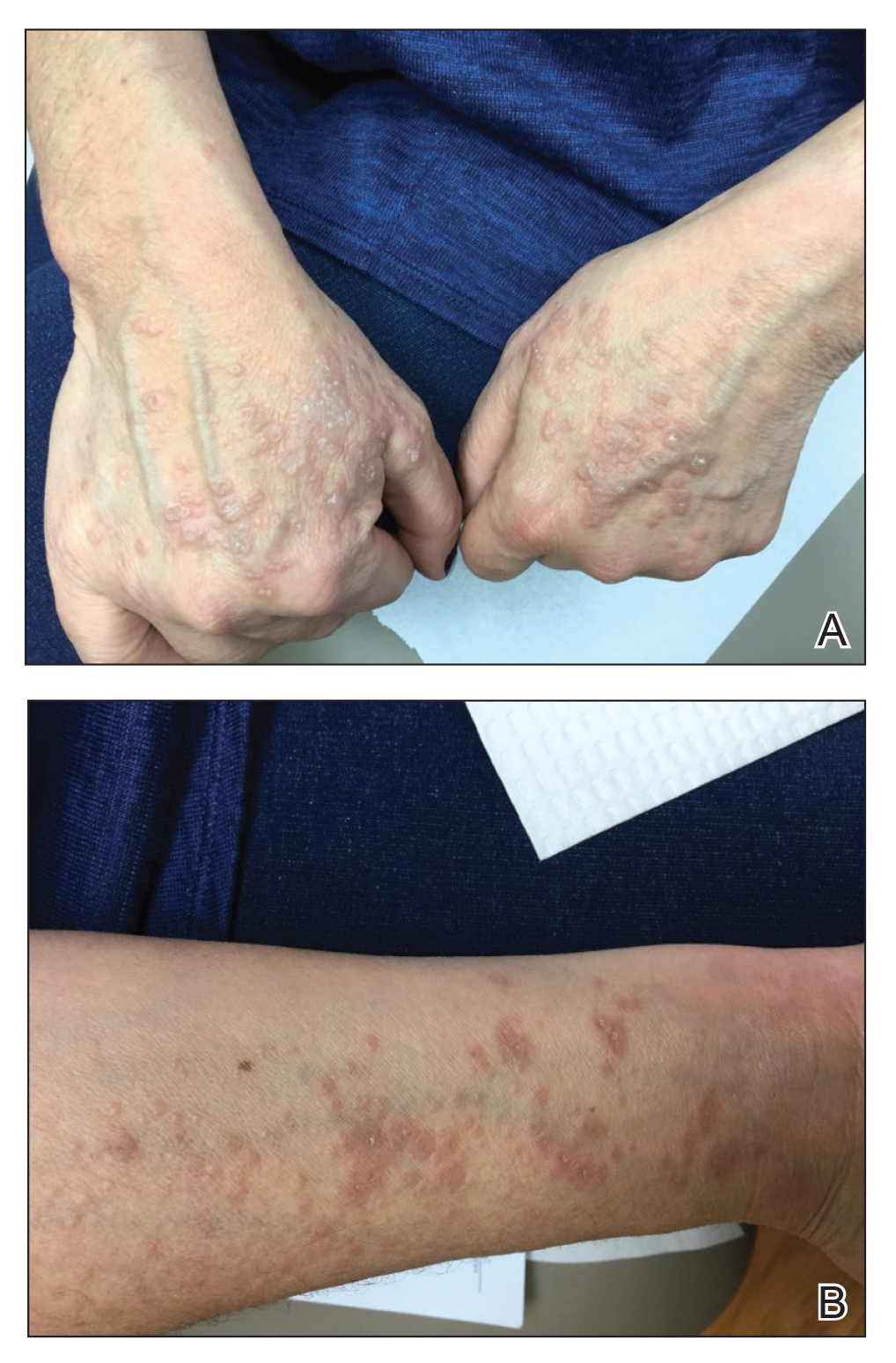
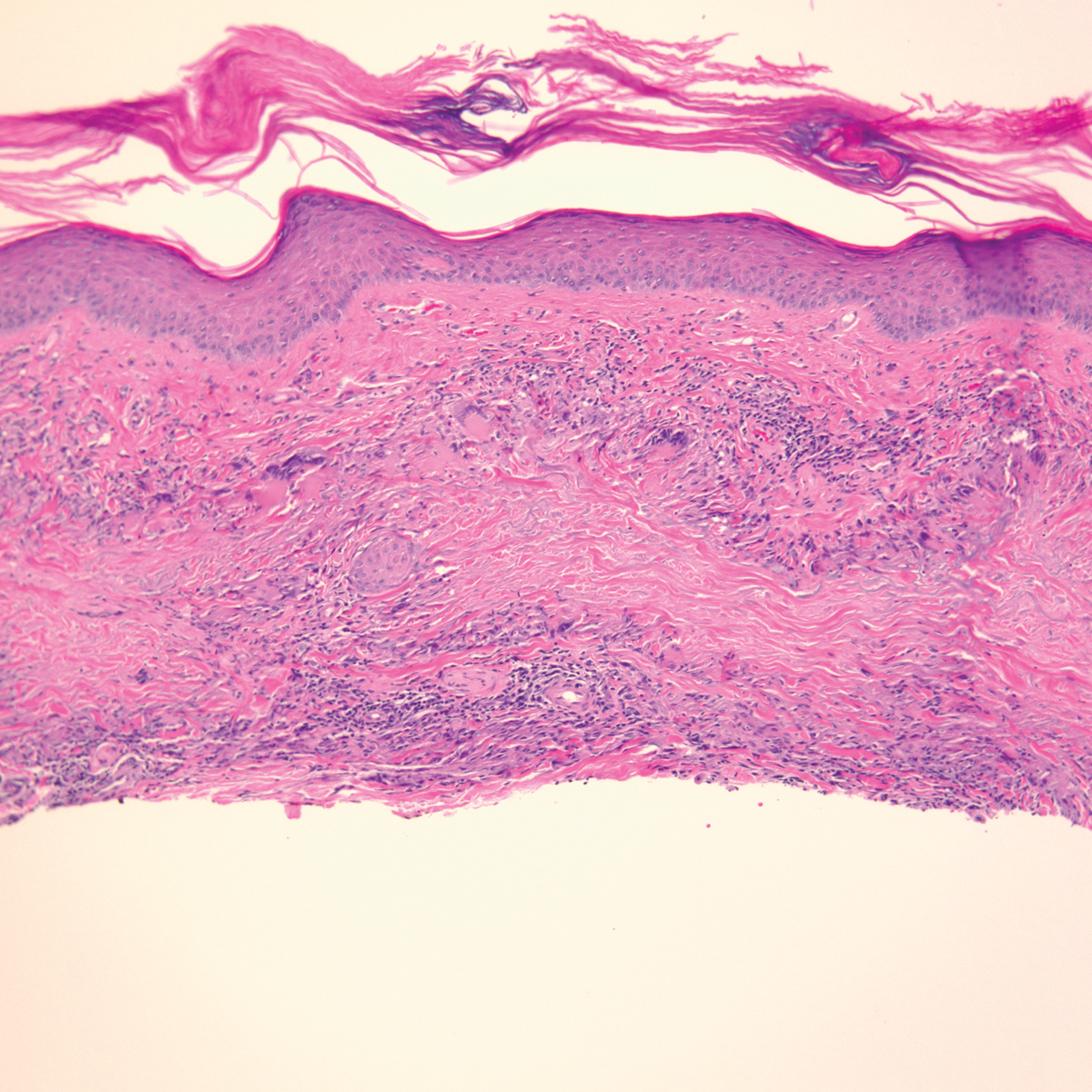
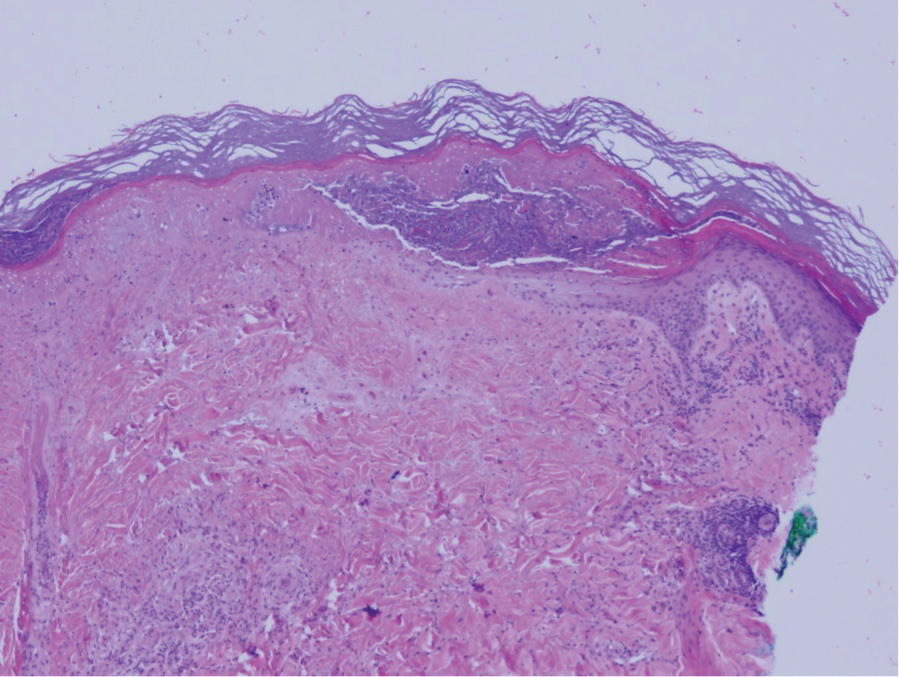
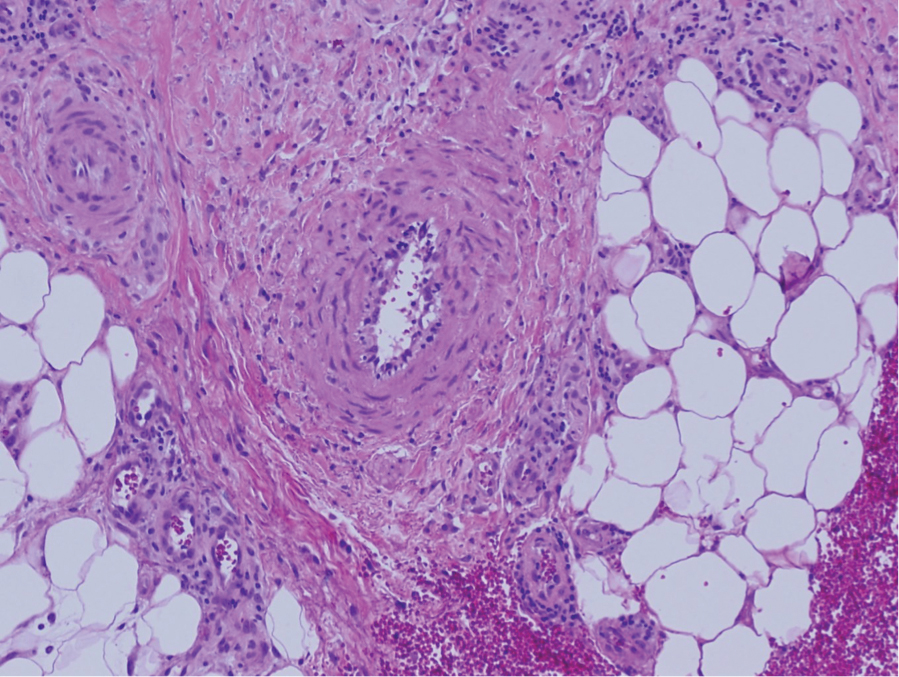
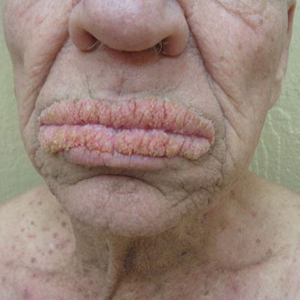
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.
A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.
A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.
A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Practice Points
- Immune-related adverse events (irAEs) frequently occur in patients on immunotherapy, with the skin representing the most common site of involvement.
- Although rare, granulomatous reactions such as granuloma annulare increasingly are recognized as potential irAEs.
- Clinicians should be aware of this novel association to accurately diagnose and effectively treat adverse reactions in patients receiving immunotherapy.
Atopic Dermatitis
The Comparison
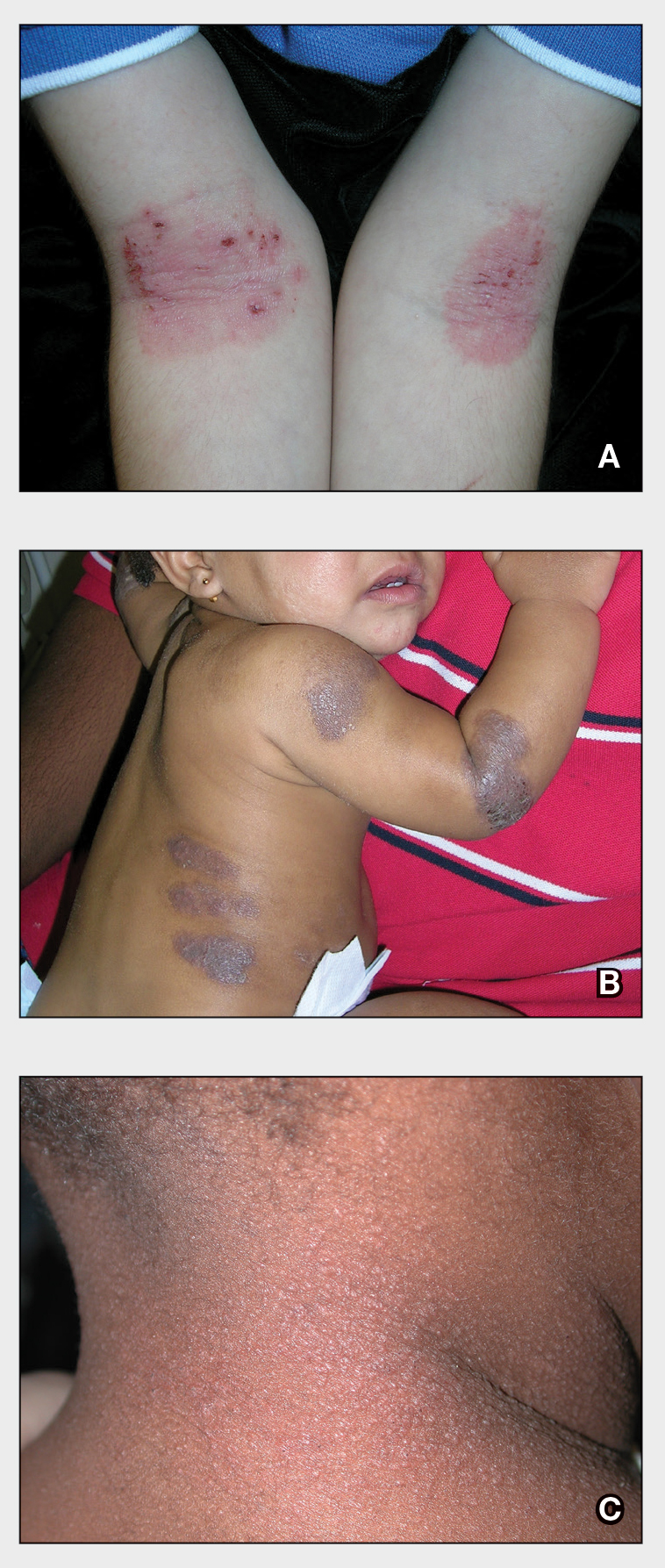
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
The Comparison
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-ofpocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016 /j.anai.2019.05.014
- Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016 /j.jid.2018.10.029
- Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
Atrophic Lesions in a Pregnant Woman
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
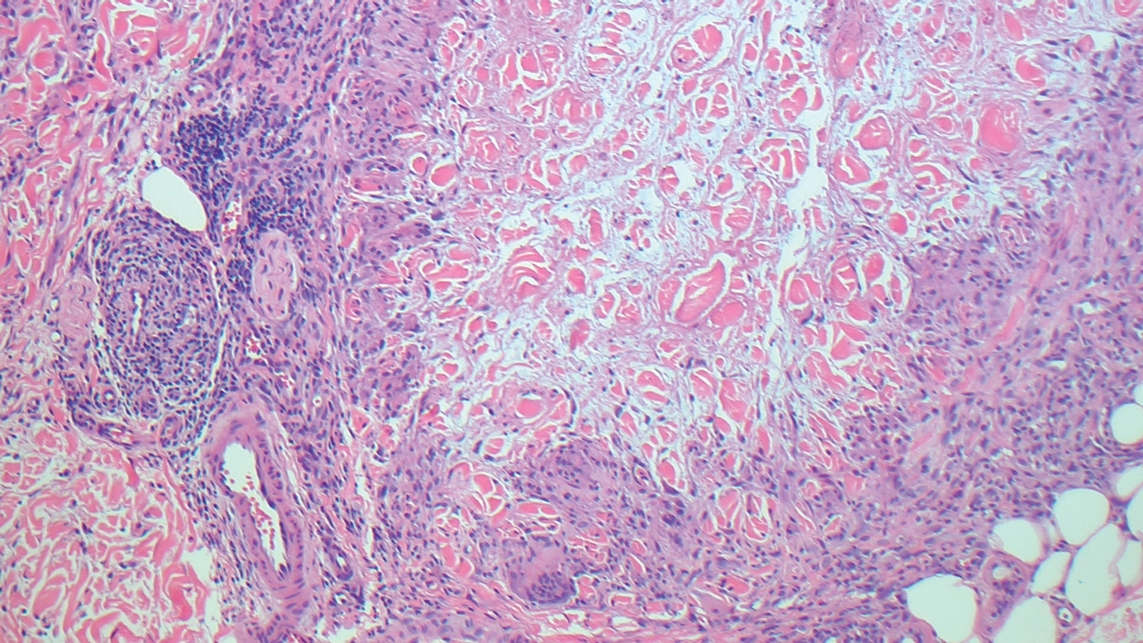
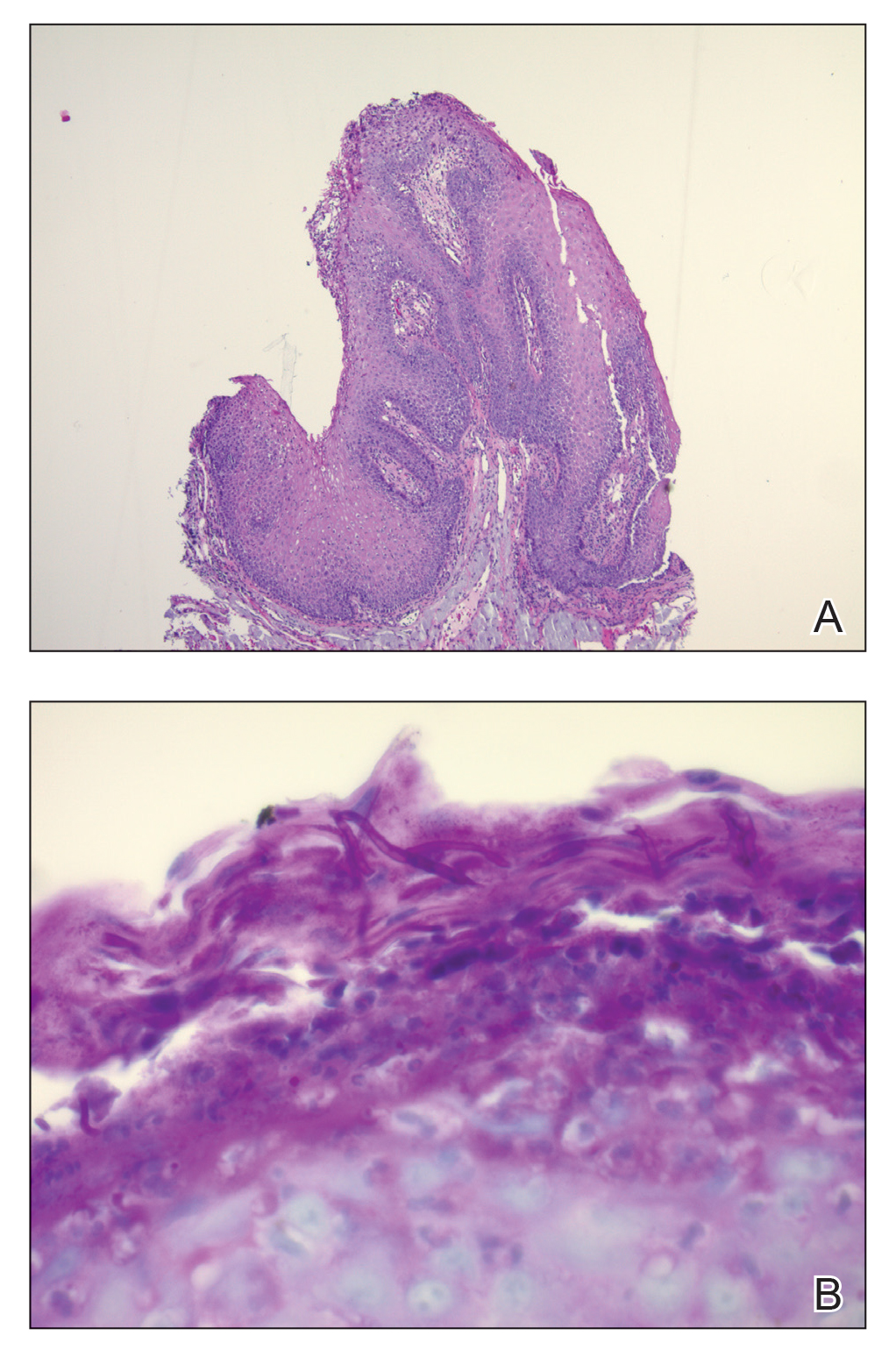
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5
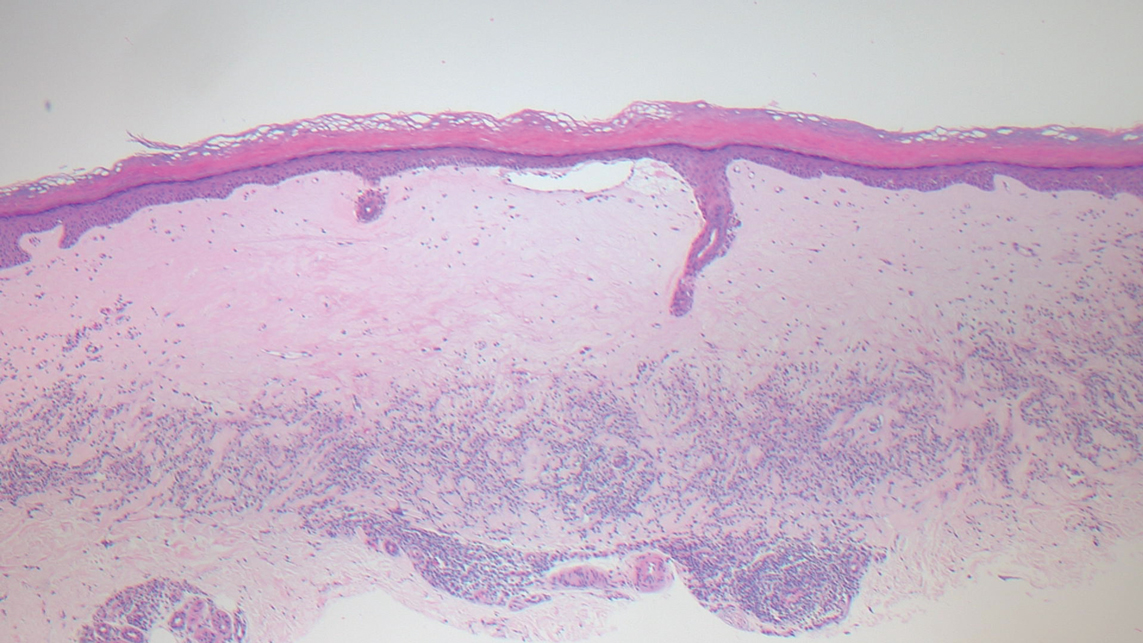
Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6
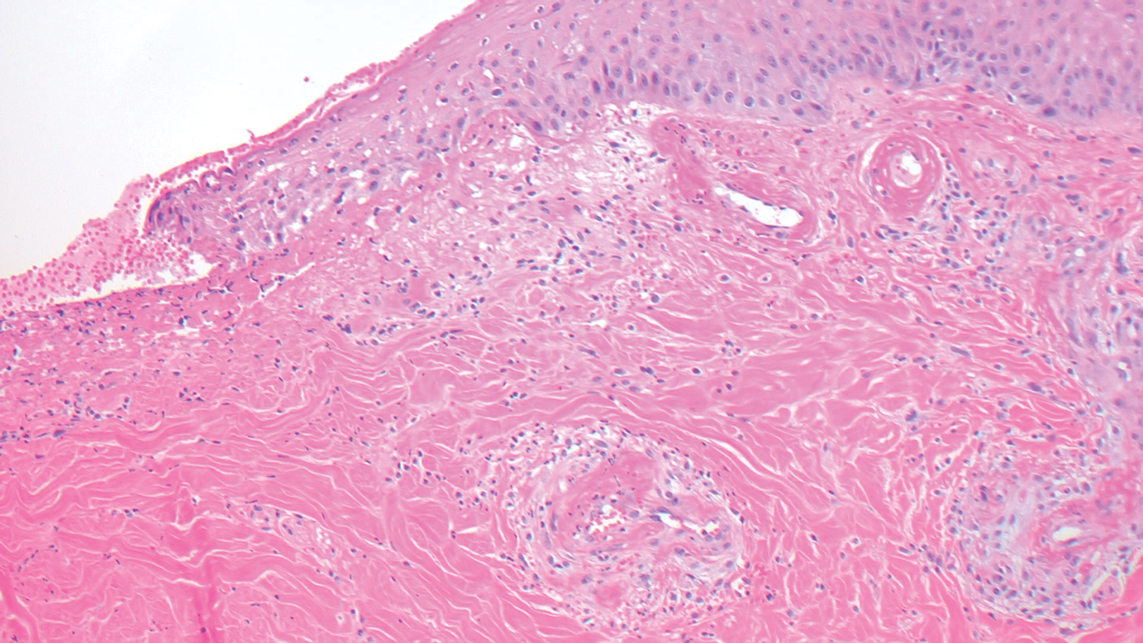
Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7
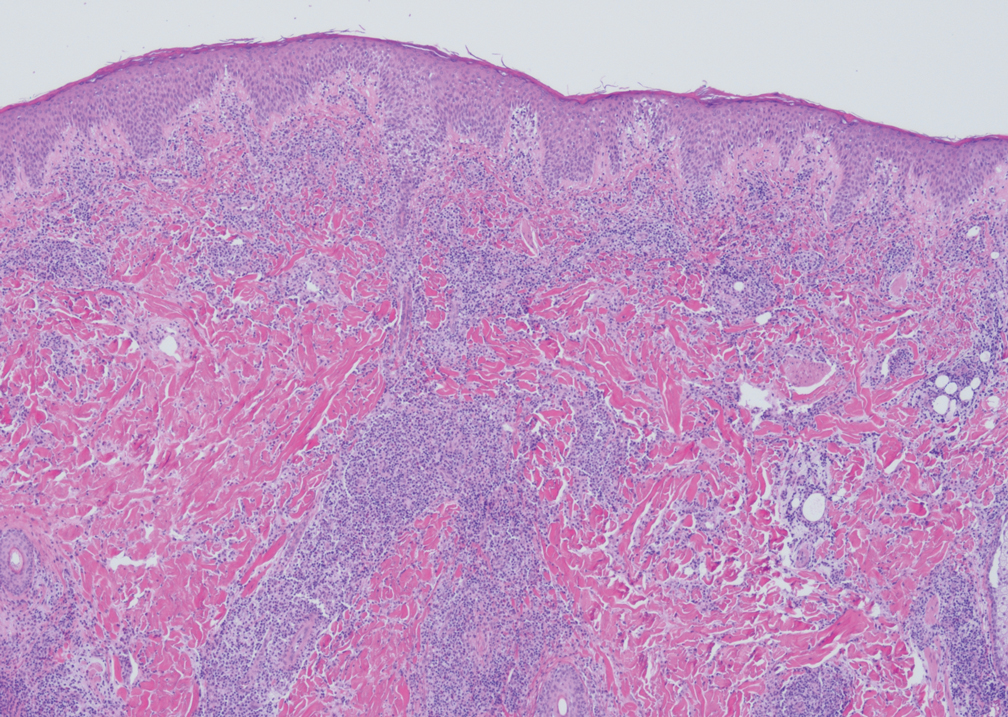
Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8
Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5
Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6
Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7
Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8
Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5
Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6
Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7
Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8
Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
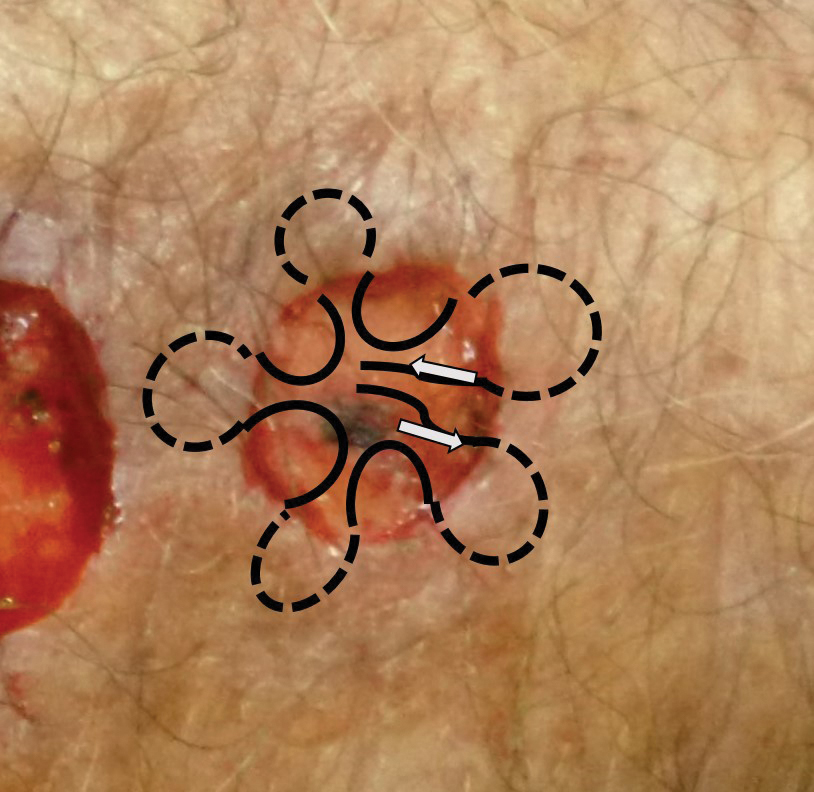
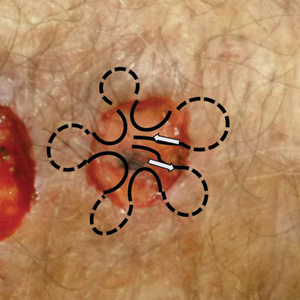
A 36-year-old pregnant woman presented with painful erythematous papules on the palms and fingers of 2 months’ duration. Similar lesions developed on the thighs and feet several weeks later. Two tender macules with central areas of porcelain white scarring rimmed by telangiectases on the right foot also were present. A punch biopsy of these lesions demonstrated a wedge-shaped area of ischemic necrosis associated with dermal mucin without associated necrobiosis. Fibrin thrombi were seen within several small dermal vessels and were associated with a perivascular lymphocytic infiltrate. Endotheliitis was observed within a deep dermal vessel. Laboratory workup including syphilis IgG, antinuclear antibodies, extractable nuclear antigen antibodies, anti–double-stranded DNA, antistreptolysin O antibodies, Russell viper venom time, cryoglobulin, hepatitis screening, perinuclear antineutrophil cytoplasmic antibodies (ANCA), and cytoplasmic ANCA was unremarkable. Hypercoagulable studies including prothrombin gene mutation, factor V Leiden, plasminogen, proteins C and S, antithrombin III, homocysteine, and antiphospholipid IgM and IgG antibodies were notable only for heterozygosity for factor V Leiden.
Reporting Biopsy Margin Status for Cutaneous Basal Cell Carcinoma: To Do or Not to Do
To the Editor:
In an interesting analysis, Brady and Hossler1 (Cutis. 2020;106:315-317) highlighted the limitations of histopathologic biopsy margin evaluation for cutaneous basal cell carcinoma (BCC). Taking into consideration the high prevalence of BCC and its medical and economic impact on the health care system, the issue raised by the authors is an important one. They proposed that pathologists may omit reporting margins or clarify the limitations in their reports. It is a valid suggestion; however, in practice, margin evaluation is not always a simple process and is influenced by a number of factors.
The subject of optimum margins for BCC has been debated over decades now; however, ambiguity and lack of definitive guidelines on certain aspects still remain, leading to a lack of standardization and variability in reporting, which opens potential for error. In anatomical pathology, the biopsies for malignancies are interpreted to confirm diagnosis and perform risk assessment, with evaluation of margins generally reserved for subsequent definitive resections. Typically, margins are not required by clinicians or reported by pathologists in common endoscopic (eg, stomach, colon) or needle core (eg, prostate, breast) biopsies. Skin holds a rather unique position in which margin evaluation is not just limited to excisions. With the exception of samples generated from electrodesiccation and curettage, it is common practice by some laboratories to report margins on most specimens of cutaneous malignancies.
In simple terms, when margins are labeled negative there should be no residual disease, and when they are deemed positive there should be disease still persisting in the patient. Margin evaluation for BCC on biopsies falls short on both fronts. In one analysis, 24% (34/143) of shave biopsies reported with negative margins displayed residual BCC in ensuing re-excisions (negative predictive value: 76%).2 Standard bread-loafing, en-face margins and inking for orientation utilized to provide a thorough margin evaluation of excisions cannot be optimally achieved on small skin biopsies. Microscopic sections for analysis are 2-dimensional representations of 3-dimensional structures. Slides prepared can miss deeply embedded outermost margins, positioned parallel to the plane of sectioning, thereby creating blind spots where margins cannot be precisely assessed and generating an inherent limitation in evaluation. Exhaustive deeper levels done routinely can address this issue to a certain degree; however, it can be an impractical solution with cost implications and delay in turnaround time.
Conversely, it also is common to encounter absence of residual BCC in re-excisions in which the original biopsy margins were labeled positive. In one analysis, 49% of BCC patients (n=100) with positive biopsy margins did not display residual neoplasm on following re-excisions.3 Localized biopsy site immune response as a cause of postbiopsy regression of residual tumor has been hypothesized to produce this phenomenon. Moreover, initial biopsies may eliminate the majority of the tumor with only minimal disease persisting. Re-excisions submitted in toto allow for a systematic examination; however, areas in between sections still remain where minute residual tumor may hide. Searching for such occult foci generally is not aggressively pursued via deeper levels unless the margins of re-excision are in question.
Superficial-type BCC (or superficial multifocal BCC) is a major factor in precluding precise biopsy margin evaluation. In a study where initial biopsies reported with negative margins displayed residual BCC in subsequent re-excisions, 91% (31/34) of residual BCCs were of superficial variety.2 Clinically, superficial BCC frequently has indistinct borders with subtle subclinical peripheral progression. It has a tendency to expand radially, with the clinical appearance deceptively smaller than its true extent. In a plane of histopathologic section, superficial BCC may exhibit skip zones within the epidermis. Even though the margin may seem uninvolved on the slide, a noncontiguous focus may still emerge beyond the “negative” margin. Because superficial pattern is not unusual as one of the components of mixed histology (composite) BCC, this issue is not just limited to tumors specifically designated as superficial type.4
The intent of a procedure is important to recognize. If a biopsy is done with the intention of diagnosis only, the pathologic assessment can be limited to tumor identification and core data elements, with margin evaluation reserved for excisions done with therapeutic intent. However, the intent is not always clear, which adds to ambiguity on when to report margins. It is not uncommon to find saucerization shaves or large punch biopsies for BCC carried out with a therapeutic intent. The status of margin is desired in such samples; however, the intent is not always clearly communicated on requisitions. To avoid any gaps in communication, some pathologists may err on the side of caution and start routinely reporting margins on biopsies.
Taking into account the inaccuracy of margin assessment in biopsies, an argument for omitting margin reporting is plausible. Although dermatologists are the major contributors of skin samples, pathology laboratories cater to a broader clientele. Other physicians from different surgical and medical specialities also perform skin biopsies, and catering to a variety of specialities adds another layer of complexity. A dermatologist may appreciate the debate regarding reliability of margins; however, a physician from another speciality who is not as familiar with the diseases of the integument may lack proper understanding. Omitting margin reporting may lead to misinterpretations or false assumptions, such as, “The margins must be uninvolved, otherwise the pathologist would have said something.” This also can generate additional phone or email inquiries and second review requests. Rather than completely omitting them, another strategy can be to report margins in more quantitative terms. One reporting approach is to have 3 categories of involved, uninvolved, and uninvolved but close for margins less than 1 mm. The cases in the third category may require greater scrutiny by deeper levels or an added caveat in the comment addressing the limitation. If the status of margins is not reported due to a certain reason, a short comment can be added to explain the reason.
In sum, clinicians should recognize that “margin negative” on skin biopsy does not always equate to “completely excised.” Margin status on biopsies is a data item that essentially provides a probability of margin clearance. Completely omitting the margin status on all biopsies may not be the most prudent approach; however, improved guidelines and modifications to enhance the reporting are definitely required.
References
- Brady MC, Hossler EW. Reliability of biopsy margin status for basal cell carcinoma: a retrospective study. Cutis. 2020;106:315-317.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52-56.
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg. 2006;32:542-551.
Continue to: Author's Response...
Authors’ Response
We appreciate the thorough and thoughtful comments in the Letter to the Editor. We agree with the author’s assertion that negative margins on skin specimens does not equate to “completely excised” and that the intent of the clinician is not always clear, even when the pathologist has ready access to the clinician’s notes, as was the case for the majority of specimens included in our study.
There is already variability in how pathologists report margins, including the specific verbiage used, at least for melanocytic lesions.1 The choice of whether or not to report margins and the meaning of those margins is complex due to the uncertainty inherent in margin assessment. Quantifying this uncertainty was the main reason for our study. Ultimately, the pathologist’s decision on whether and how to report margins should be focused on improving patient outcomes. There are benefits and drawbacks to all approaches, and our goal is to provide more information for clinicians and pathologists so that they may better care for their patients. Understanding the limitations of margins on submitted skin specimens—whether margins are reported or not—can only serve to guide improve clinical decision-making.
We also agree that the breadth of specialties of submitting clinicians make reporting of margins difficult, and there is likely similar breadth in their understanding of the nuances of margin assessment and reports. The solution to this problem is adequate education regarding the limitations of a pathology report, and specifically what is meant when margins are (or are not) reported on skin specimens. How to best educate the myriad clinicians who submit biopsies is, of course, the ultimate challenge.
We hope that our study adds information to this ongoing debate regarding margin status reporting, and we appreciate the discussion points raised by the author.
Eric Hossler, MD; Mary Brady, MD
From the Department of Dermatology, Geisinger Health System, Danville, Pennsylvania.
The authors report no conflict of interest.
Reference
- Sellheyer K, Bergfeld WF, Stewart E, et al. Evaluation of surgical margins in melanocytic lesions: a survey among 152 dermatopathologists.J Cutan Pathol. 2005;32:293-299.
To the Editor:
In an interesting analysis, Brady and Hossler1 (Cutis. 2020;106:315-317) highlighted the limitations of histopathologic biopsy margin evaluation for cutaneous basal cell carcinoma (BCC). Taking into consideration the high prevalence of BCC and its medical and economic impact on the health care system, the issue raised by the authors is an important one. They proposed that pathologists may omit reporting margins or clarify the limitations in their reports. It is a valid suggestion; however, in practice, margin evaluation is not always a simple process and is influenced by a number of factors.
The subject of optimum margins for BCC has been debated over decades now; however, ambiguity and lack of definitive guidelines on certain aspects still remain, leading to a lack of standardization and variability in reporting, which opens potential for error. In anatomical pathology, the biopsies for malignancies are interpreted to confirm diagnosis and perform risk assessment, with evaluation of margins generally reserved for subsequent definitive resections. Typically, margins are not required by clinicians or reported by pathologists in common endoscopic (eg, stomach, colon) or needle core (eg, prostate, breast) biopsies. Skin holds a rather unique position in which margin evaluation is not just limited to excisions. With the exception of samples generated from electrodesiccation and curettage, it is common practice by some laboratories to report margins on most specimens of cutaneous malignancies.
In simple terms, when margins are labeled negative there should be no residual disease, and when they are deemed positive there should be disease still persisting in the patient. Margin evaluation for BCC on biopsies falls short on both fronts. In one analysis, 24% (34/143) of shave biopsies reported with negative margins displayed residual BCC in ensuing re-excisions (negative predictive value: 76%).2 Standard bread-loafing, en-face margins and inking for orientation utilized to provide a thorough margin evaluation of excisions cannot be optimally achieved on small skin biopsies. Microscopic sections for analysis are 2-dimensional representations of 3-dimensional structures. Slides prepared can miss deeply embedded outermost margins, positioned parallel to the plane of sectioning, thereby creating blind spots where margins cannot be precisely assessed and generating an inherent limitation in evaluation. Exhaustive deeper levels done routinely can address this issue to a certain degree; however, it can be an impractical solution with cost implications and delay in turnaround time.
Conversely, it also is common to encounter absence of residual BCC in re-excisions in which the original biopsy margins were labeled positive. In one analysis, 49% of BCC patients (n=100) with positive biopsy margins did not display residual neoplasm on following re-excisions.3 Localized biopsy site immune response as a cause of postbiopsy regression of residual tumor has been hypothesized to produce this phenomenon. Moreover, initial biopsies may eliminate the majority of the tumor with only minimal disease persisting. Re-excisions submitted in toto allow for a systematic examination; however, areas in between sections still remain where minute residual tumor may hide. Searching for such occult foci generally is not aggressively pursued via deeper levels unless the margins of re-excision are in question.
Superficial-type BCC (or superficial multifocal BCC) is a major factor in precluding precise biopsy margin evaluation. In a study where initial biopsies reported with negative margins displayed residual BCC in subsequent re-excisions, 91% (31/34) of residual BCCs were of superficial variety.2 Clinically, superficial BCC frequently has indistinct borders with subtle subclinical peripheral progression. It has a tendency to expand radially, with the clinical appearance deceptively smaller than its true extent. In a plane of histopathologic section, superficial BCC may exhibit skip zones within the epidermis. Even though the margin may seem uninvolved on the slide, a noncontiguous focus may still emerge beyond the “negative” margin. Because superficial pattern is not unusual as one of the components of mixed histology (composite) BCC, this issue is not just limited to tumors specifically designated as superficial type.4
The intent of a procedure is important to recognize. If a biopsy is done with the intention of diagnosis only, the pathologic assessment can be limited to tumor identification and core data elements, with margin evaluation reserved for excisions done with therapeutic intent. However, the intent is not always clear, which adds to ambiguity on when to report margins. It is not uncommon to find saucerization shaves or large punch biopsies for BCC carried out with a therapeutic intent. The status of margin is desired in such samples; however, the intent is not always clearly communicated on requisitions. To avoid any gaps in communication, some pathologists may err on the side of caution and start routinely reporting margins on biopsies.
Taking into account the inaccuracy of margin assessment in biopsies, an argument for omitting margin reporting is plausible. Although dermatologists are the major contributors of skin samples, pathology laboratories cater to a broader clientele. Other physicians from different surgical and medical specialities also perform skin biopsies, and catering to a variety of specialities adds another layer of complexity. A dermatologist may appreciate the debate regarding reliability of margins; however, a physician from another speciality who is not as familiar with the diseases of the integument may lack proper understanding. Omitting margin reporting may lead to misinterpretations or false assumptions, such as, “The margins must be uninvolved, otherwise the pathologist would have said something.” This also can generate additional phone or email inquiries and second review requests. Rather than completely omitting them, another strategy can be to report margins in more quantitative terms. One reporting approach is to have 3 categories of involved, uninvolved, and uninvolved but close for margins less than 1 mm. The cases in the third category may require greater scrutiny by deeper levels or an added caveat in the comment addressing the limitation. If the status of margins is not reported due to a certain reason, a short comment can be added to explain the reason.
In sum, clinicians should recognize that “margin negative” on skin biopsy does not always equate to “completely excised.” Margin status on biopsies is a data item that essentially provides a probability of margin clearance. Completely omitting the margin status on all biopsies may not be the most prudent approach; however, improved guidelines and modifications to enhance the reporting are definitely required.
References
- Brady MC, Hossler EW. Reliability of biopsy margin status for basal cell carcinoma: a retrospective study. Cutis. 2020;106:315-317.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52-56.
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg. 2006;32:542-551.
Continue to: Author's Response...
Authors’ Response
We appreciate the thorough and thoughtful comments in the Letter to the Editor. We agree with the author’s assertion that negative margins on skin specimens does not equate to “completely excised” and that the intent of the clinician is not always clear, even when the pathologist has ready access to the clinician’s notes, as was the case for the majority of specimens included in our study.
There is already variability in how pathologists report margins, including the specific verbiage used, at least for melanocytic lesions.1 The choice of whether or not to report margins and the meaning of those margins is complex due to the uncertainty inherent in margin assessment. Quantifying this uncertainty was the main reason for our study. Ultimately, the pathologist’s decision on whether and how to report margins should be focused on improving patient outcomes. There are benefits and drawbacks to all approaches, and our goal is to provide more information for clinicians and pathologists so that they may better care for their patients. Understanding the limitations of margins on submitted skin specimens—whether margins are reported or not—can only serve to guide improve clinical decision-making.
We also agree that the breadth of specialties of submitting clinicians make reporting of margins difficult, and there is likely similar breadth in their understanding of the nuances of margin assessment and reports. The solution to this problem is adequate education regarding the limitations of a pathology report, and specifically what is meant when margins are (or are not) reported on skin specimens. How to best educate the myriad clinicians who submit biopsies is, of course, the ultimate challenge.
We hope that our study adds information to this ongoing debate regarding margin status reporting, and we appreciate the discussion points raised by the author.
Eric Hossler, MD; Mary Brady, MD
From the Department of Dermatology, Geisinger Health System, Danville, Pennsylvania.
The authors report no conflict of interest.
Reference
- Sellheyer K, Bergfeld WF, Stewart E, et al. Evaluation of surgical margins in melanocytic lesions: a survey among 152 dermatopathologists.J Cutan Pathol. 2005;32:293-299.
To the Editor:
In an interesting analysis, Brady and Hossler1 (Cutis. 2020;106:315-317) highlighted the limitations of histopathologic biopsy margin evaluation for cutaneous basal cell carcinoma (BCC). Taking into consideration the high prevalence of BCC and its medical and economic impact on the health care system, the issue raised by the authors is an important one. They proposed that pathologists may omit reporting margins or clarify the limitations in their reports. It is a valid suggestion; however, in practice, margin evaluation is not always a simple process and is influenced by a number of factors.
The subject of optimum margins for BCC has been debated over decades now; however, ambiguity and lack of definitive guidelines on certain aspects still remain, leading to a lack of standardization and variability in reporting, which opens potential for error. In anatomical pathology, the biopsies for malignancies are interpreted to confirm diagnosis and perform risk assessment, with evaluation of margins generally reserved for subsequent definitive resections. Typically, margins are not required by clinicians or reported by pathologists in common endoscopic (eg, stomach, colon) or needle core (eg, prostate, breast) biopsies. Skin holds a rather unique position in which margin evaluation is not just limited to excisions. With the exception of samples generated from electrodesiccation and curettage, it is common practice by some laboratories to report margins on most specimens of cutaneous malignancies.
In simple terms, when margins are labeled negative there should be no residual disease, and when they are deemed positive there should be disease still persisting in the patient. Margin evaluation for BCC on biopsies falls short on both fronts. In one analysis, 24% (34/143) of shave biopsies reported with negative margins displayed residual BCC in ensuing re-excisions (negative predictive value: 76%).2 Standard bread-loafing, en-face margins and inking for orientation utilized to provide a thorough margin evaluation of excisions cannot be optimally achieved on small skin biopsies. Microscopic sections for analysis are 2-dimensional representations of 3-dimensional structures. Slides prepared can miss deeply embedded outermost margins, positioned parallel to the plane of sectioning, thereby creating blind spots where margins cannot be precisely assessed and generating an inherent limitation in evaluation. Exhaustive deeper levels done routinely can address this issue to a certain degree; however, it can be an impractical solution with cost implications and delay in turnaround time.
Conversely, it also is common to encounter absence of residual BCC in re-excisions in which the original biopsy margins were labeled positive. In one analysis, 49% of BCC patients (n=100) with positive biopsy margins did not display residual neoplasm on following re-excisions.3 Localized biopsy site immune response as a cause of postbiopsy regression of residual tumor has been hypothesized to produce this phenomenon. Moreover, initial biopsies may eliminate the majority of the tumor with only minimal disease persisting. Re-excisions submitted in toto allow for a systematic examination; however, areas in between sections still remain where minute residual tumor may hide. Searching for such occult foci generally is not aggressively pursued via deeper levels unless the margins of re-excision are in question.
Superficial-type BCC (or superficial multifocal BCC) is a major factor in precluding precise biopsy margin evaluation. In a study where initial biopsies reported with negative margins displayed residual BCC in subsequent re-excisions, 91% (31/34) of residual BCCs were of superficial variety.2 Clinically, superficial BCC frequently has indistinct borders with subtle subclinical peripheral progression. It has a tendency to expand radially, with the clinical appearance deceptively smaller than its true extent. In a plane of histopathologic section, superficial BCC may exhibit skip zones within the epidermis. Even though the margin may seem uninvolved on the slide, a noncontiguous focus may still emerge beyond the “negative” margin. Because superficial pattern is not unusual as one of the components of mixed histology (composite) BCC, this issue is not just limited to tumors specifically designated as superficial type.4
The intent of a procedure is important to recognize. If a biopsy is done with the intention of diagnosis only, the pathologic assessment can be limited to tumor identification and core data elements, with margin evaluation reserved for excisions done with therapeutic intent. However, the intent is not always clear, which adds to ambiguity on when to report margins. It is not uncommon to find saucerization shaves or large punch biopsies for BCC carried out with a therapeutic intent. The status of margin is desired in such samples; however, the intent is not always clearly communicated on requisitions. To avoid any gaps in communication, some pathologists may err on the side of caution and start routinely reporting margins on biopsies.
Taking into account the inaccuracy of margin assessment in biopsies, an argument for omitting margin reporting is plausible. Although dermatologists are the major contributors of skin samples, pathology laboratories cater to a broader clientele. Other physicians from different surgical and medical specialities also perform skin biopsies, and catering to a variety of specialities adds another layer of complexity. A dermatologist may appreciate the debate regarding reliability of margins; however, a physician from another speciality who is not as familiar with the diseases of the integument may lack proper understanding. Omitting margin reporting may lead to misinterpretations or false assumptions, such as, “The margins must be uninvolved, otherwise the pathologist would have said something.” This also can generate additional phone or email inquiries and second review requests. Rather than completely omitting them, another strategy can be to report margins in more quantitative terms. One reporting approach is to have 3 categories of involved, uninvolved, and uninvolved but close for margins less than 1 mm. The cases in the third category may require greater scrutiny by deeper levels or an added caveat in the comment addressing the limitation. If the status of margins is not reported due to a certain reason, a short comment can be added to explain the reason.
In sum, clinicians should recognize that “margin negative” on skin biopsy does not always equate to “completely excised.” Margin status on biopsies is a data item that essentially provides a probability of margin clearance. Completely omitting the margin status on all biopsies may not be the most prudent approach; however, improved guidelines and modifications to enhance the reporting are definitely required.
References
- Brady MC, Hossler EW. Reliability of biopsy margin status for basal cell carcinoma: a retrospective study. Cutis. 2020;106:315-317.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Yuan Y, Duff ML, Sammons DL, et al. Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases. 2014;2:52-56.
- Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg. 2006;32:542-551.
Continue to: Author's Response...
Authors’ Response
We appreciate the thorough and thoughtful comments in the Letter to the Editor. We agree with the author’s assertion that negative margins on skin specimens does not equate to “completely excised” and that the intent of the clinician is not always clear, even when the pathologist has ready access to the clinician’s notes, as was the case for the majority of specimens included in our study.
There is already variability in how pathologists report margins, including the specific verbiage used, at least for melanocytic lesions.1 The choice of whether or not to report margins and the meaning of those margins is complex due to the uncertainty inherent in margin assessment. Quantifying this uncertainty was the main reason for our study. Ultimately, the pathologist’s decision on whether and how to report margins should be focused on improving patient outcomes. There are benefits and drawbacks to all approaches, and our goal is to provide more information for clinicians and pathologists so that they may better care for their patients. Understanding the limitations of margins on submitted skin specimens—whether margins are reported or not—can only serve to guide improve clinical decision-making.
We also agree that the breadth of specialties of submitting clinicians make reporting of margins difficult, and there is likely similar breadth in their understanding of the nuances of margin assessment and reports. The solution to this problem is adequate education regarding the limitations of a pathology report, and specifically what is meant when margins are (or are not) reported on skin specimens. How to best educate the myriad clinicians who submit biopsies is, of course, the ultimate challenge.
We hope that our study adds information to this ongoing debate regarding margin status reporting, and we appreciate the discussion points raised by the author.
Eric Hossler, MD; Mary Brady, MD
From the Department of Dermatology, Geisinger Health System, Danville, Pennsylvania.
The authors report no conflict of interest.
Reference
- Sellheyer K, Bergfeld WF, Stewart E, et al. Evaluation of surgical margins in melanocytic lesions: a survey among 152 dermatopathologists.J Cutan Pathol. 2005;32:293-299.
USMLE Step 1 Changes: Dermatology Program Director Perspectives and Implications
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
Practice Points
- The changes to US Medical Licensing Examination (USMLE) Step 1 were met with mixed reactions from dermatology program directors.
- These changes likely will increase the emphasis on USMLE Step 2 and other objective measures.
Wound Healing on the Dorsal Hands: An Intrapatient Comparison of Primary Closure, Purse-String Closure, and Secondary Intention
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).
Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).
Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
Practice Gap
Many cutaneous surgery wounds can be closed primarily; however, in certain cases, other repair options might be appropriate and should be evaluated on a case-by-case basis with input from the patient. Defects on the dorsal aspect of the hands—where nonmelanoma skin cancer is common and reserve tissue is limited—often heal by secondary intention with good cosmetic and functional results. Patients often express a desire to reduce the time spent in the surgical suite and restrictions on postoperative activity, making secondary intention healing more appealing. An additional advantage is obviation of the need to remove additional tissue in the form of Burow triangles, which would lead to a longer wound. The major disadvantage of secondary intention healing is longer time to wound maturity; we often minimize this disadvantage with purse-string closure to decrease the size of the wound defect, which can be done quickly and without removing additional tissue.
The Technique
An elderly man had 3 nonmelanoma skin cancers—all on the dorsal aspect of the left hand—that were treated on the same day, leaving 3 similar wound defects after Mohs micrographic surgery. The wound defects (distal to proximal) measured 12 mm, 12 mm, and 10 mm in diameter (Figure 1) and were repaired by primary closure, secondary intention, and purse-string circumferential closure, respectively. Purse-string closure1 was performed with a 4-0 polyglactin 901 suture and left to heal without external sutures (Figure 2). Figure 3 shows the 3 types of repairs immediately following closure. All wounds healed with excellent and essentially equivalent cosmetic results, with excellent patient satisfaction at 6-month follow-up (Figure 4).
Practical Implications
Our case illustrates different modalities of wound repair during precisely the same time frame and essentially on the same location. Skin of the dorsal hand often is tight; depending on the size of the defect, large primary closure can be tedious to perform, can lead to increased wound tension and risk of dehiscence, and can be uncomfortable for the patient during healing. However, primary closure typically will lead to faster healing.
Secondary intention healing and purse-string closure require less surgery and therefore cost less; these modalities yield similar cosmesis and satisfaction. In the appropriate context, secondary intention has been highlighted as a suitable alternative to primary closure2-4; in our experience (and that of others5), patient satisfaction is not diminished with healing by secondary intention. Purse-string closure also can minimize wound size and healing time.
For small shallow wounds on the dorsal hand, dermatologic surgeons should have confidence that secondary intention healing, with or without wound reduction using purse-string repair, likely will lead to acceptable cosmetic and functional results. Of course, repair should be tailored to the circumstances and wishes of the individual patient.
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
- Peled IJ, Zagher U, Wexler MR. Purse-string suture for reduction and closure of skin defects. Ann Plast Surg. 1985;14:465-469. doi:10.1097/00000637-198505000-00012
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106. doi:10.1016/0738-081x(84)90031-2
- Fazio MJ, Zitelli JA. Principles of reconstruction following excision of nonmelanoma skin cancer. Clin Dermatol. 1995;13:601-616. doi:10.1016/0738-081x(95)00099-2
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38:647-653. doi:10.1111/j.1524-4725.2011.02258.x
- Stebbins WG, Gusev J, Higgins HW 2nd, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol. 2015;73:865-867.e1. doi:10.1016/j.jaad.2015.07.019
Oral Verrucous Plaques in a Patient With Urothelial Cancer
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.
Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.
Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
The Diagnosis: Paraneoplastic Acanthosis Nigricans
Histopathologic examination demonstrated verrucous epidermal hyperplasia (Figure, A). Fungal organisms were identified with an Alcian blue and periodic acid-Schiff stain (Figure, B). The organisms demonstrated a vertical orientation in relation to the mucosal surface, which was consistent with candidal organisms.
Given the rapid eruption of these plaques, the distribution on the oral and palmar surfaces (tripe palms), and the minimal improvement with both systemic steroids and antifungal treatment, a diagnosis of paraneoplastic acanthosis nigricans with secondary candidal infection was made. Drug-induced cheilitis was considered; however, improvement with discontinuation of the suspected offending drug would have been expected. Although chronic mucocutaneous candidiasis was possible, more prompt improvement upon initiation of systemic antifungal therapy would have been observed. Oral Crohn disease should be included in the differential, but it was unlikely given the lack of granulomas on pathology and absence of history of gastrointestinal tract symptoms. Melkersson-Rosenthal syndrome also was unlikely given the lack of facial nerve palsy as well as the lack of granulomas on pathology. Furthermore, none of these options would be associated with tripe palms, as seen in our patient.
Acanthosis nigricans is a localized skin disorder characterized by hyperpigmented velvety plaques arising in flexural and intertriginous regions. Although most cases (80%) are associated with idiopathic or benign conditions, the link between acanthosis nigricans and an underlying malignancy has been well documented.1-3 Most commonly associated with an underlying intra-abdominal malignancy (often gastric carcinoma), the lesions of paraneoplastic acanthosis nigricans are indistinguishable from their benign counterparts.1,4 When the condition presents abruptly and extensively in a nonobese patient, prompt workup for malignancy should be initiated. Rapid onset and atypical distribution (ie, palmar, perioral, or mucosal) more commonly is associated with a paraneoplastic etiology.5,6
Histopathology for acanthosis nigricans shows hyperkeratosis and epidermal papillomatosis. Horn pseudocyst formation is possible, but usually no hyperpigmentation is observed. The findings typically are indistinguishable from seborrheic keratoses, epidermal nevi, or lesions of confluent and reticulated papillomatosis of Gougerot and Carteaud.2
The underlying pathogenesis of acanthosis nigricans is poorly understood. In the benign subtype, insulin resistance commonly has been described. In the paraneoplastic subtype, it is proposed that the tumor produces a transforming growth factor that mimics epidermal growth factor and leads to keratinocyte proliferation.7,8 Paraneoplastic acanthosis nigricans has the potential to arise at any point of tumor development, further contributing to the diagnostic challenge. Treatment of the skin lesions involves management of the underlying malignancy. Unfortunately, many such malignancies often are at an advanced stage, and subsequent prognosis is poor.2
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
- Shah A, Jack A, Liu H, et al. Neoplastic/paraneoplastic dermatitis, fasciitis, and panniculitis. Rheum Dis Clin North Am. 2011;37:573-592.
- Chairatchaneeboon M, Kim EJ. Cutaneous paraneoplastic syndromes. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick's Dermatology. 9th ed. McGraw-Hill Education; 2019:2441-2464.
- Lee HC, Ker KJ, Chong WS. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. 2017;15:208.
- Mohrenschlager M, Vocks E, Wessner DB, et al. Tripe palms and malignant acanthosis nigricans: cutaneous signs of imminent metastasis in bladder cancer? J Urol. 2001;165:1629-1630.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101.
A 75-year-old nonobese man with metastatic urothelial carcinoma presented for evaluation and treatment of swollen lips. The patient stated that his lips began to swell and crack shortly after beginning pembrolizumab approximately 5 months prior. The swelling had progressively worsened, prompting discontinuation of the pembrolizumab by oncology about 2 months prior to presentation to our dermatology clinic. He reported slight improvement after the discontinuation of pembrolizumab, and he had since been started on carboplatin and gemcitabine. He previously was treated with oral corticosteroids without improvement. His oncologist started him on oral fluconazole for treatment of oral thrush on the day of presentation to our clinic. Physical examination revealed diffuse papillomatous and verrucous plaques of the upper and lower lips with involvement of the buccal mucosa. He also had deep fissures and white plaques on the tongue. Velvety hyperpigmented plaques were noted in the axillae, and he had confluent thickening of the palms. A 3-mm punch biopsy from the lower lip was performed. The patient subsequently was evaluated 2 weeks after the initial appointment, and minor improvement in the oral verrucous hyperplasia was noted following antifungal therapy, with resolution of the candidiasis.
Reexamining the Role of Diet in Dermatology
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Practice Points
- Patients are increasingly interested in dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.
- Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
- There remains a lack of randomized controlled trials assessing the efficacy of various dietary interventions in the dermatologic setting.
E/M Coding in 2021: The Times (and More) Are A-Changin’
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Practice Points
- The outpatient evaluation and management (E/M) codes have undergone substantial changes that took effect January 1, 2021.
- Outpatient E/M visits are now coded based on time or level of medical decision-making (MDM).
- Time now includes all preservice, intraservice, and postservice time the physician spends with the patient on the date of the encounter.
- Many of the key definitions used in order to determine level of MDM have been streamlined and updated.