User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
E/M Coding in 2021: The Times (and More) Are A-Changin’
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
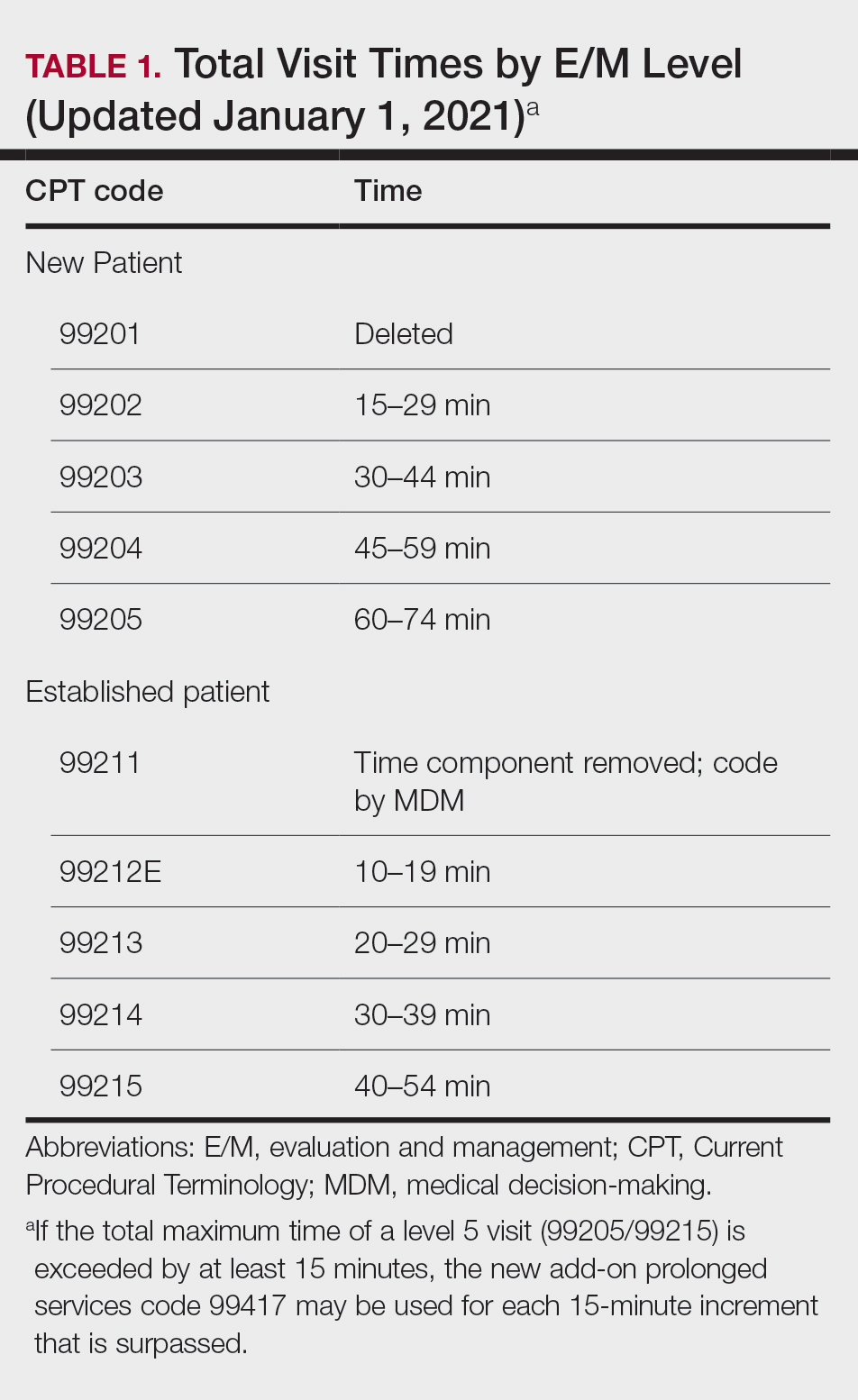
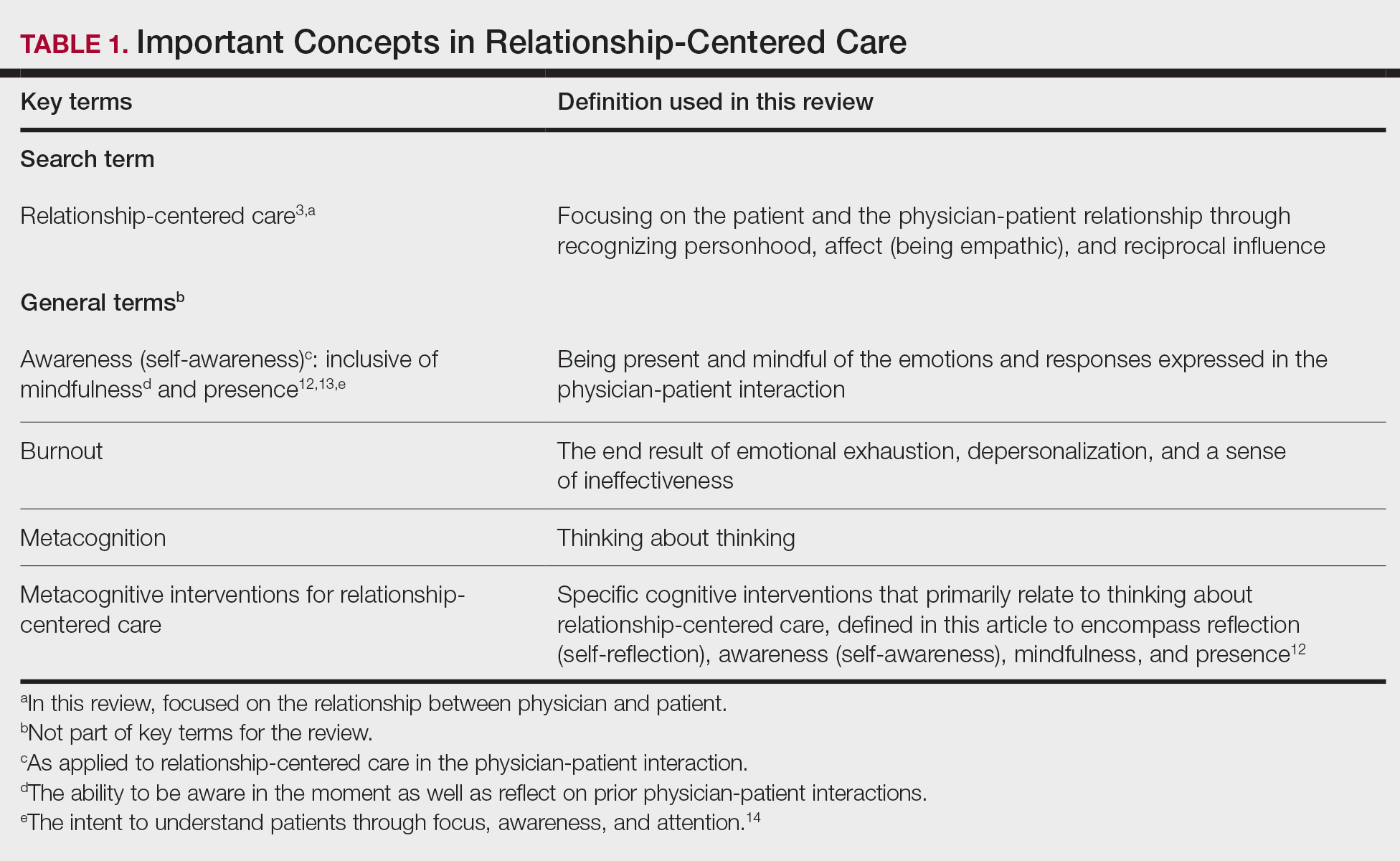
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
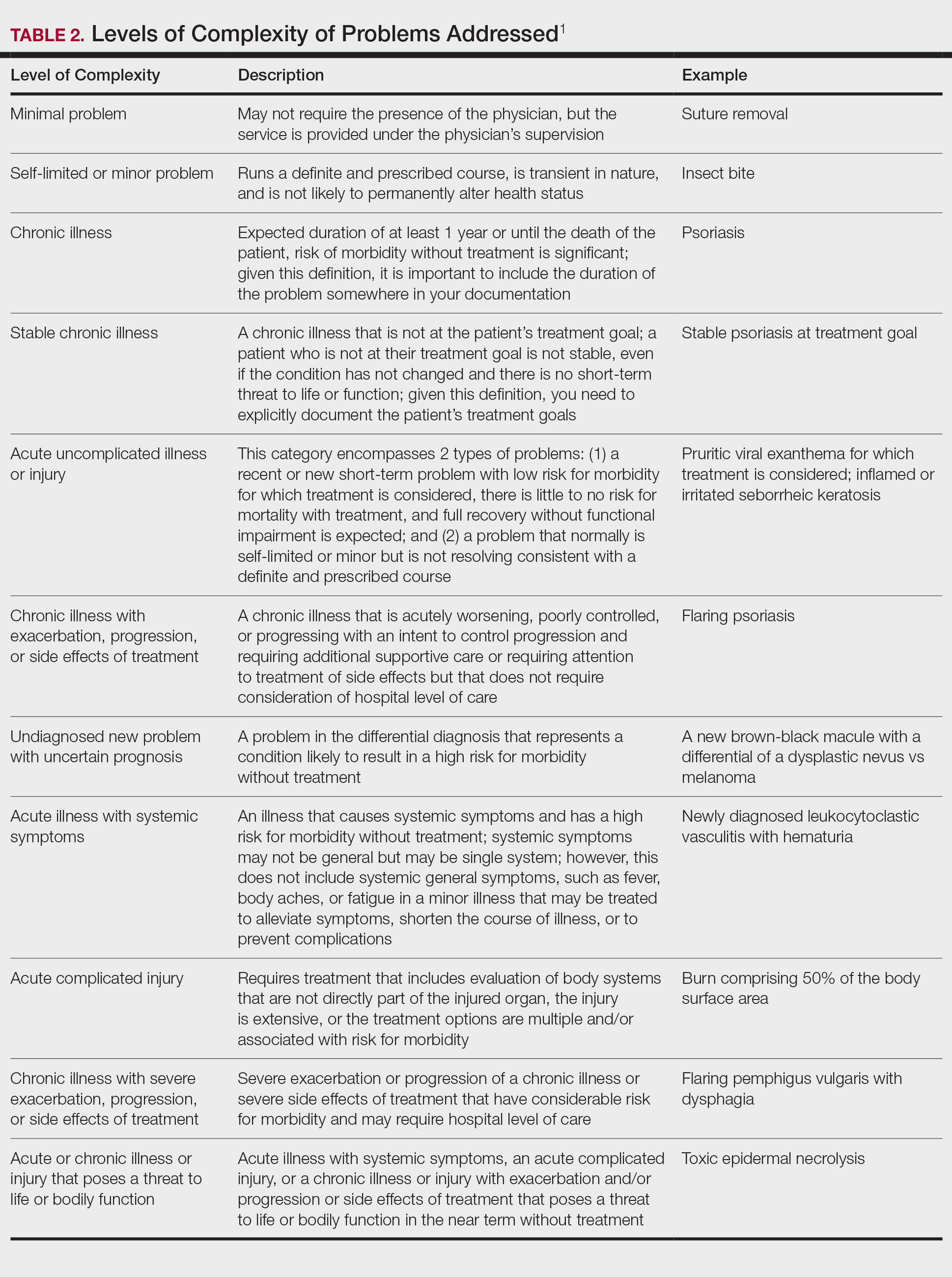
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
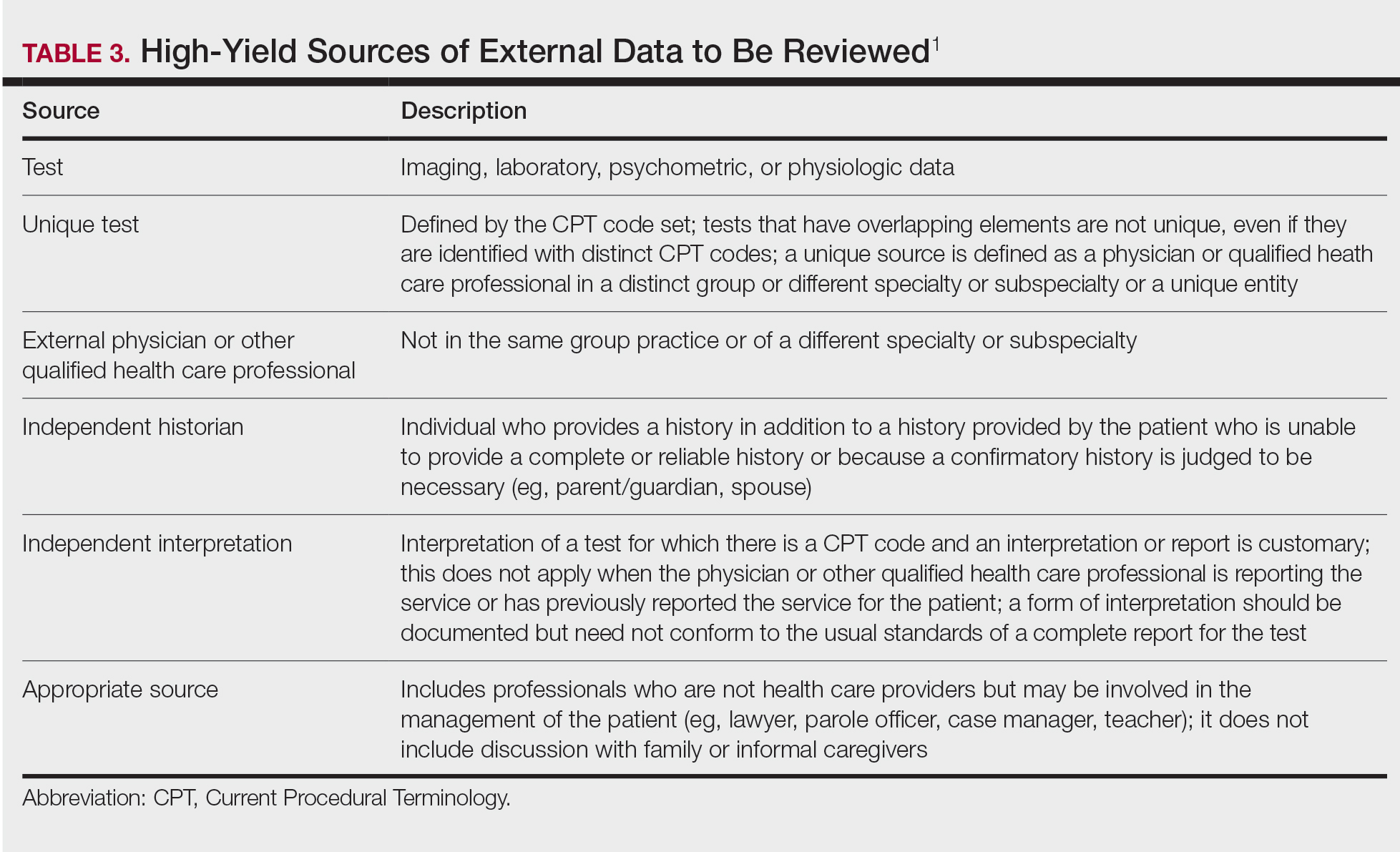
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
Effective on January 1, 2021, the outpatient evaluation and management (E/M) codes underwent substantial changes, which were the culmination of multiple years of revision and surveying via the American Medical Association (AMA) Relative Value Scale Update Committee and Current Procedural Terminology (RUC-CPT) process to streamline definitions and promote consistency as well as to decrease the administrative burden for all specialties within the house of medicine.1 These updates represent a notable change from the previous documentation requirements for this oft used family of codes. Herein, we break down some of the highlights of the changes and how they may be applied for some commonly used dermatologic diagnoses.
Time Is Time Is Time
Prior to the 2021 revisions, a physician generally could only code for an E/M level by time for a face-to-face encounter dominated by counseling and/or care coordination. With the new updates, any encounter can be coded by total time spent by the physician with the patient1; however, clinical staff time is not included. There also are now clear guidelines of the time ranges corresponding to the level of E/M,1 as noted in Table 1.
Importantly, time now includes not just face-to-face time with the patient but also any time on the date of the encounter that the physician is involved in the care of the patient when not reported with a separate code. This can include reviewing notes or data before or after the examination, care coordination, ordering laboratory tests, and providing any documentation related to the encounter. Importantly, this applies only when these activities are done on the date of the encounter.
If you work with a nurse practitioner or physician assistant (PA) who assists you and you are the one reporting the service, you cannot double-dip. For example, if your PA spends 10 minutes alone with a patient, you are in the room together for 5 minutes, the PA spends another 10 minutes alone with the patient afterward, and you do chart work for 10 minutes at the end of the day, the total time spent is 35 minutes, not 40 minutes, as you cannot count the time you and the PA spent together twice.
Decisions, Decisions
Evaluation and management coding also can be determined via the level of medical decision-making (MDM). Per the 2021 guidelines, MDM is comprised of 3 categories: (1) number and complexity of problems addressed at the encounter, (2) amount and/or complexity of data to be reviewed or analyzed, and (3) risk of complications and/or morbidity or mortality of patient management.1 To reach a certain overall E/M level, 2 of 3 categories must be met or exceeded. Let’s dive into each of these in a little more detail.
Number and Complexity of Problems Addressed at the Encounter
First, it is important to understand the definition of a problem addressed. Per AMA guidelines, this includes a disease, condition, illness, injury, symptom, sign, finding, complaint, or other matter addressed at the encounter that is evaluated or treated at the encounter by the physician. If the problem is referred to another provider without evaluation or consideration of treatment, it is not considered to be a problem addressed and cannot count toward this first category. An example could be a patient with a lump on the abdomen that you refer to plastic or general surgery for evaluation and treatment.
Once you have determined that you are addressing a problem, you will need to determine the level of complexity of the problem, as outlined in Table 2. Keep in mind that some entities and disease states in dermatology may fit the requirements of more than 1 level of complexity depending on the clinical situation, while there are many entities in dermatology that may not be perfectly captured by any of the levels described. In these situations, clinical judgement is required to determine where the problem would best fit. Importantly, whatever you decide, your documentation should support that decision.
Amount and/or Complexity of Data to Be Reviewed and Analyzed
This category encompasses any external notes reviewed, unique laboratory tests or imaging ordered or reviewed, the need for an independent historian or discussion with external health care providers or appropriate sources, or independent interpretation of tests. Some high-yield definitions in this category are outlined in Table 3.
Risk of Complications and/or Morbidity or Mortality of Patient Management
In this category, risk relates to both the patient’s diagnosis and treatment(s). Importantly, for treatment and diagnostic options, these include both the options selected and those considered but not selected. Risk is defined as the probability and/or consequences of an event and is based on the usual behavior and thought processes of a physician in the same specialty. In other words, think of the risk as compared to risk in the setting of other dermatologists diagnosing and/or treating the same condition.
Social determinants of health also play a part in this category and are defined as economic and social conditions that influence the health of individuals and communities. Social determinants of health can be indicated by the specific corresponding International Statistical Classification of Diseases, Tenth Revision code and may need to be included in your billing according to specific institutional or carrier guidelines if they are a factor in your level of MDM.
For the purposes of MDM, risk is stratified into minimal, low, moderate, and high. Some examples for each level are outlined in Table 4.
Putting It All Together
Once you have determined each of the above 3 categories, you can put them together into the MDM chart to ascertain the overall level of MDM. (The official AMA medical decision-making grid is available online [https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf]). Keep in mind that 2 of 3 columns in the table must be obtained in that level to reach an overall E/M level; for example, a visit that addresses 2 self-limited or minor problems (level 3) in which no data is reviewed (level 2) and involves prescribing a new medication (level 4), would be an overall level 3 visit.
Final Thoughts
The outpatient E/M guidelines have undergone substantial revisions; therefore, it is crucial to understand the updated definitions to ensure proper billing and documentation. History and physical examination documentation must be medically appropriate but are no longer used to determine overall E/M level; time and MDM are the sole options that can be used. Importantly, try to code as accurately as possible, documenting which problems were both noted and addressed. If you are unsure of a definition within the updated changes and MDM table, referencing the appropriate sources for guidance is recommended.
Although representing a considerable shift, the revaluation of this family of codes and the intended decrease in documentation burden has the ability to be a positive gain for dermatologists. Expect other code families to mirror these changes in the next few years.
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- American Medical Association. CPT® Evaluation and management (E/M) office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Accessed May 14, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
Practice Points
- The outpatient evaluation and management (E/M) codes have undergone substantial changes that took effect January 1, 2021.
- Outpatient E/M visits are now coded based on time or level of medical decision-making (MDM).
- Time now includes all preservice, intraservice, and postservice time the physician spends with the patient on the date of the encounter.
- Many of the key definitions used in order to determine level of MDM have been streamlined and updated.
COVID-19 Vaccine Reactions in Dermatology: “Filling” in the Gaps
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
How to Save a Limb: Identification of Pyoderma Gangrenosum
Case Report
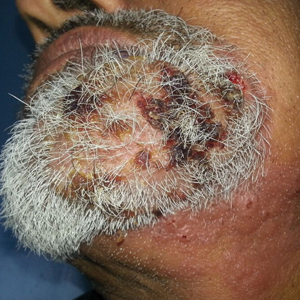
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
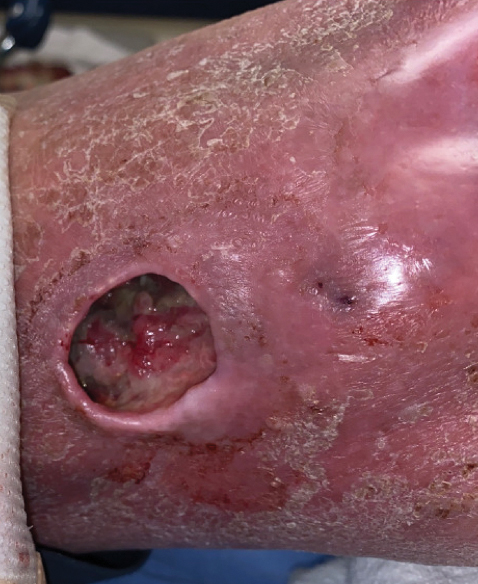
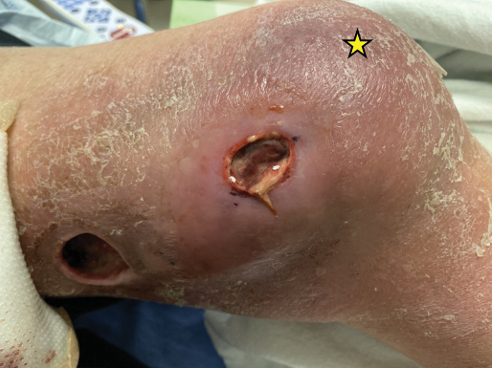
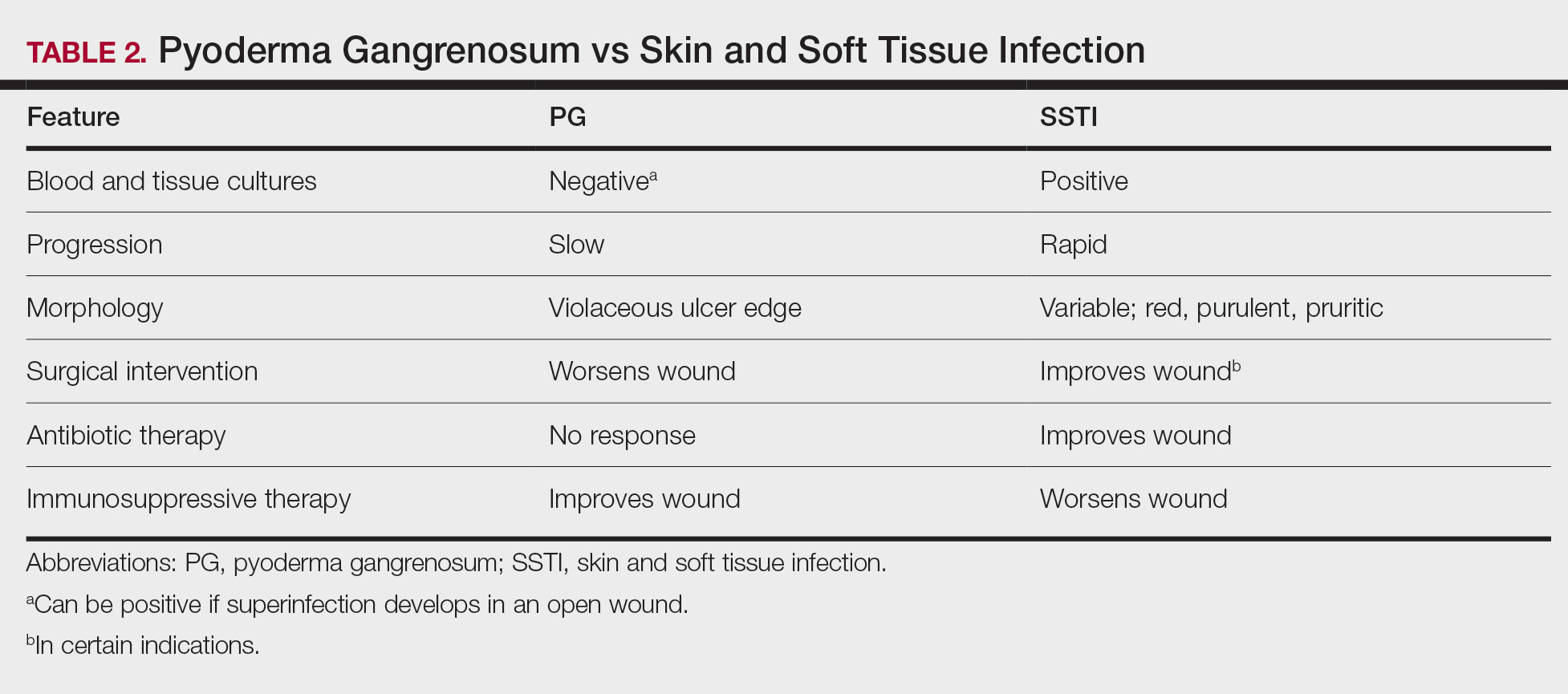
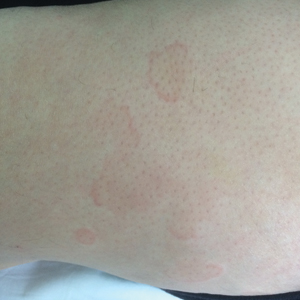
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.
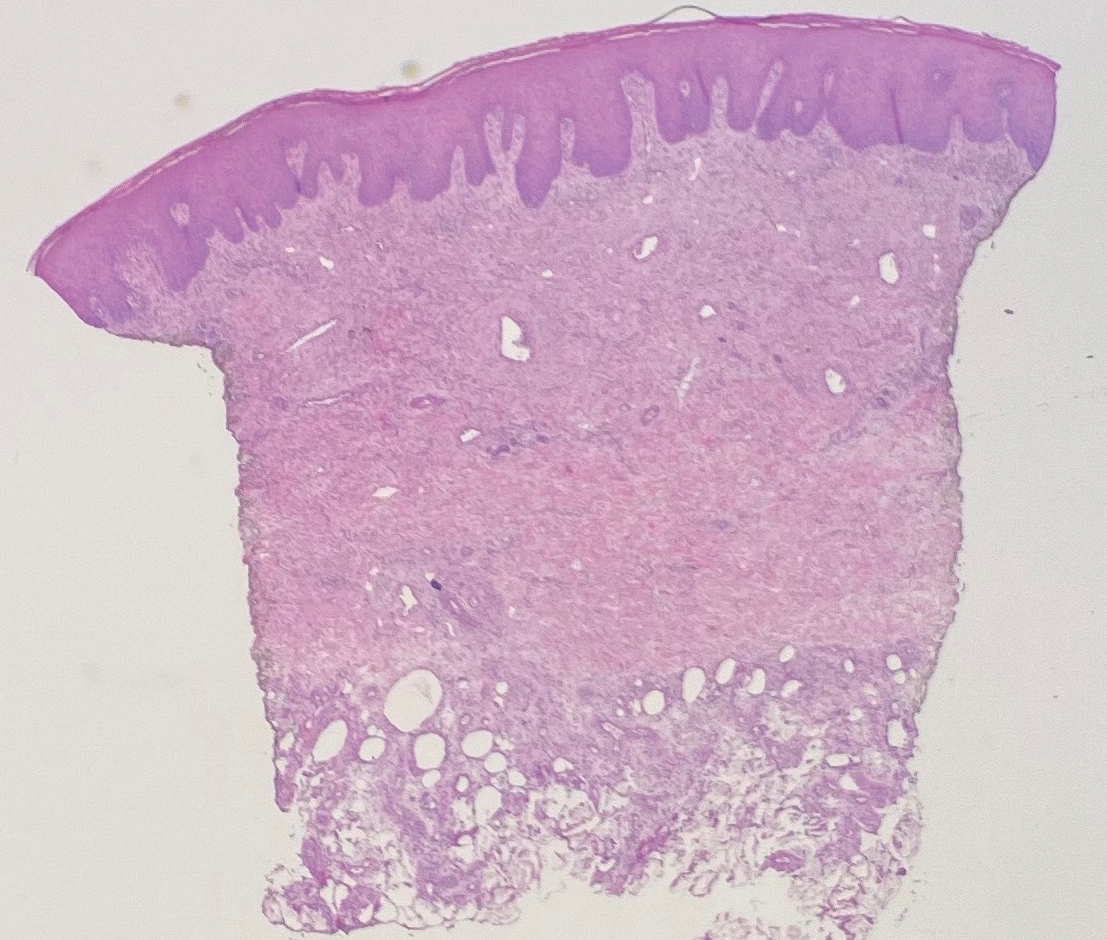
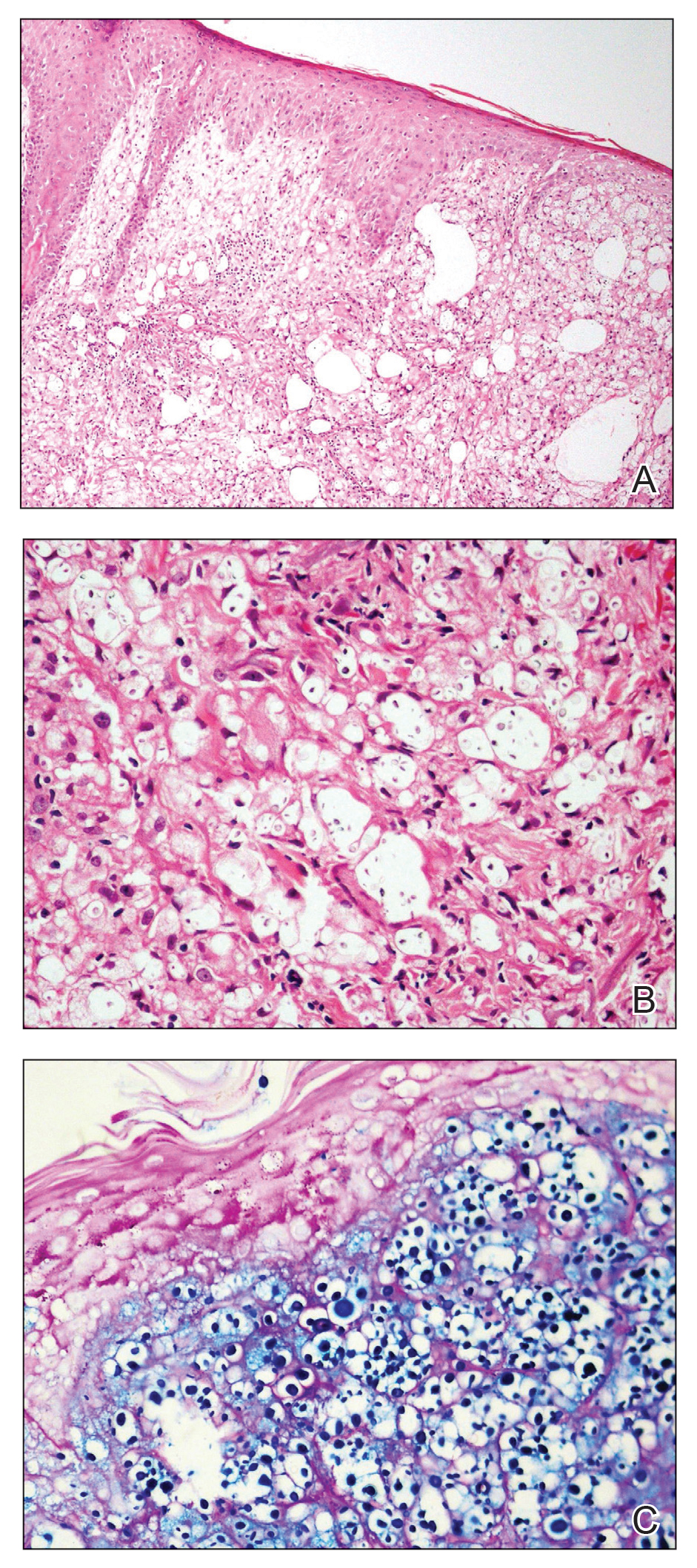
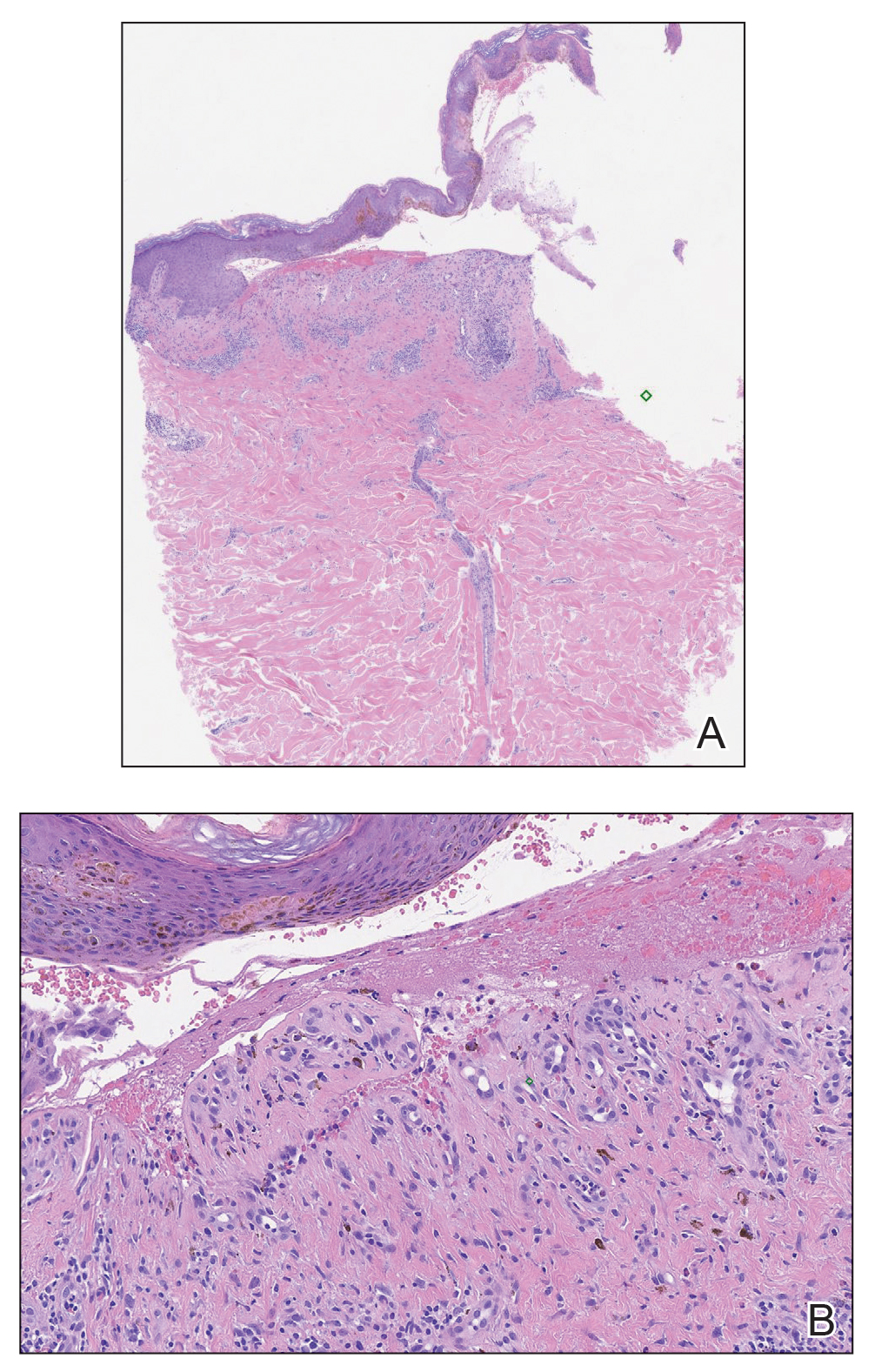
Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.
Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
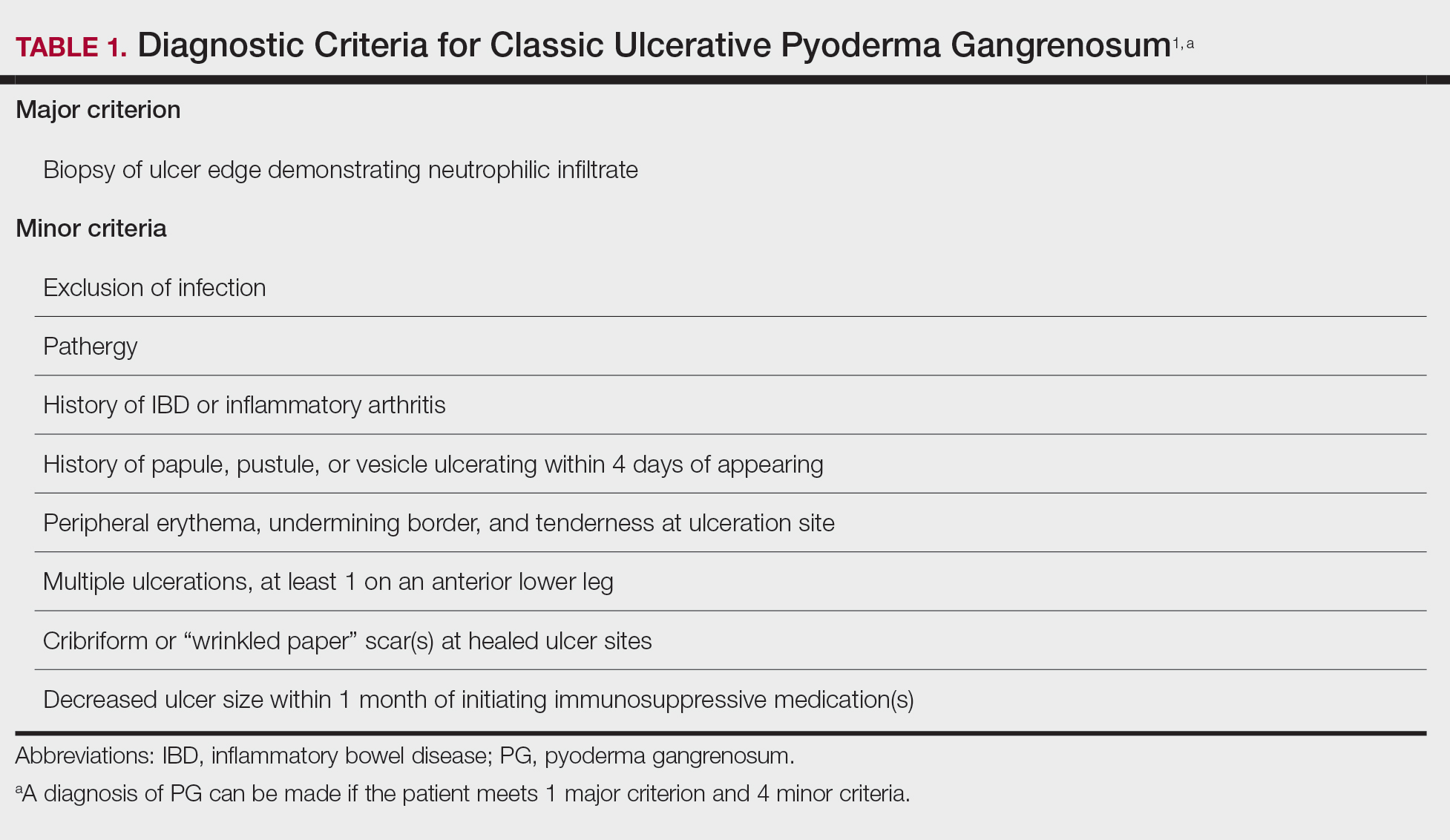
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6
Differential Diagnosis of PG
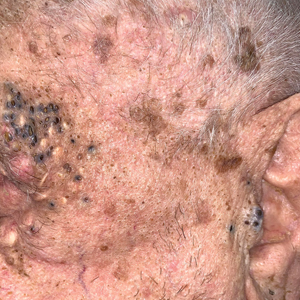
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.
Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.
Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.
Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6
Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.
Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
Case Report
A 67-year-old woman presented with a painful expanding ulcer on the left leg and a new nearby ulcer of 2 months’ duration. She initially was seen 2 months prior for a wound on the left knee due to a fall as well as cellulitis, which was treated with intravenous vancomycin and ceftriaxone. Wound cultures were negative for bacteria, and she was discharged without antibiotics. She presented to the emergency department 1 month later for malodorous discharge of the first ulcer with zero systemic inflammatory response syndrome criteria; no fever; and no abnormal heart rate, respiratory rate, or leukocyte count. She was discharged with wound care. After 3 weeks, she returned with a second ulcer and worsening drainage but zero systemic inflammatory response syndrome criteria. She had a medical history of Crohn disease with 9-year remission, atrial fibrillation, pacemaker, mitral valve replacement, chronic obstructive pulmonary disease, and a 51 pack-year smoking history.
Physical examination of the left leg revealed a 3×3-cm deep lesion (ulcer A) on the distal left thigh located superomedial to the knee (Figure 1) as well as a 2×1-cm deep lesion (ulcer B) on the anteromedial knee with undermining and tunneling (Figure 2). A large amount of malodorous tan bloody discharge was present on both ulcers. There were no signs of induration or crepitus.Due to concerns of skin and soft tissue infection (SSTI) or osteomyelitis, a bone scan and wound and blood cultures were ordered. The patient was started on vancomycin and piperacillin-tazobactam in the emergency department, which later was augmented with cefepime. Trauma surgery scheduled debridement for the following morning with suspicion of necrotizing fasciitis. Additional consultations were requested, including infectious disease, wound care, and dermatology. Dermatology evaluated the wound, performed a punch biopsy, and canceled debridement due to unclear diagnosis. The clinical differential at that time included pyoderma gangrenosum (PG), atypical vasculitis, or infection. Additional workup revealed positive antineutrophil cytoplasmic antibodies but negative proteinase 3 and myeloperoxidase, disfavoring vasculitis. Wound cultures grew Staphylococcus aureus and Pseudomonas aeruginosa.
Histologic evaluation revealed deep dermal necrosis with a mixed inflammatory infiltrate (Figure 3) and no organisms or vasculitis. Antibiotics were discontinued, and she was discharged on a 14-day course of prednisone 60 mg daily for empirical treatment of PG with dermatology follow-up. Medical management included a 6-month course of dapsone that was extended to 7 months because of an intensive care unit stay for a cerebrovascular accident. Daily dosing was as follows: 100 mg for 5 months, 50 mg for 1 month, and 25 mg for 1 month, then stopped. She was followed with serial complete blood cell count every 1 to 2 months and home-health wound care. One month after dapsone initiation, the ulcers decreased in size. Ulcer B was fully healed after 4 months, and ulcer A was nearly closed at 6 months without any new flares.
Comment
Pyoderma gangrenosum is a rare inflammatory skin condition that classically presents as tender papules or pustules evolving into painful ulcers, most commonly on the lower extremities. Pyoderma gangrenosum has a propensity to exhibit pathergy, the hyperreactivity of the skin in response to minor trauma. This phenomenon in PG manifests as the rapid evolution from pustule to ulceration with violaceous undermining borders.
Diagnosis of PG
Pyoderma gangrenosum has been described as a diagnosis of exclusion, as its findings frequently mimic SSTIs. Important findings to obtain are histology, history, ulcer morphology, and response to treatment.
In 2018, Maverakis et al1 proposed diagnostic criteria for classic ulcerative PG (Table 1). A diagnosis of PG can be made if the patient meets 1 major criterion and 4 minor criteria. Our case met 0 major criteria and 5 minor criteria: history of inflammatory bowel disease (IBD); history of pustule ulcerating within 4 days of appearing; peripheral erythema, undermining border, and tenderness at ulceration site; multiple ulcerations, with at least 1 on an anterior lower leg; and decreased ulcer size within 1 month of initiating immunosuppressive medication(s). Although our patient’s biopsy demonstrated a mixed infiltrate, PG was not excluded due to spontaneous resolution at the time of biopsy, emphasizing the need to biopsy subsequent new lesions if neutrophils are not initially seen.1 Pyoderma gangrenosum frequently is associated with IBD, most often Crohn disease, as seen in our patient.2-4 Although IBD classically is associated with smoking, studies have yet to conclude if smoking is a predictive factor of PG.5 Our patient presented with an initial ulcer that evolved into 2 ulcers, similar to a case of bilateral ulcers.6
Differential Diagnosis of PG
Other possible diagnoses to consider are SSTI and vasculitis, the latter being disfavored by no evidence of vasculitis on biopsy and negative titers for proteinase 3 and myeloperoxidase antibodies. However, the presence of either, similar to a mixed infiltrate, does not exclude a diagnosis of PG, as they can occur simultaneously. Consequently, superinfection of a chronically open wound can occur due to underlying PG.7 The differences between PG and SSTI are listed in Table 2.
Although we know PG involves neutrophilic dysfunction, the pathophysiology remains poorly understood, contributing to the lack of clinical guidelines.8 Therefore, the diagnosis of PG often is delayed and is associated with severe consequences such as necrotizing fasciitis, osteomyelitis, cosmetic morbidity, and limb amputation.9,10 Dermatologic consultation can aid in early diagnosis and avoid amputation.7,10 Amputation has been used as a last resort to preserve optimal outcomes in patients with severe PG.11
Management of PG
A gold standard of treatment of PG does not exist, but the goal is to promote wound healing. Patients with limited disease typically can be managed with wound care and topical steroids or calcineurin inhibitors, though data on efficacy are limited. However, our patient had more extensive disease and needed to be treated with systemic therapy. First-line therapy for extensive disease includes oral prednisone or cyclosporine for patients who cannot tolerate systemic corticosteroids.12 Second-line and adjunctive therapy options include dapsone, minocycline, methotrexate, and infliximab. Our patient was prescribed a 7-month course of dapsone with outpatient dermatology and demonstrated resolution of both ulcers. Dapsone was tapered from a daily dose of 100 mg to 50 mg to 25 mg to none over the course of 2 to 3 months. Close monitoring with wound care is recommended, and petroleum jelly can be used for dry skin around the lesion for comfort.
Conclusion
The diagnosis of PG is challenging because it relies heavily on clinical signs and often mimics SSTI. Gathering a detailed medical history is critical to make the diagnosis of PG. In a patient with associated features of PG, dermatologic consultation and biopsy of skin lesions should be considered. Physicians should evaluate for suspected PG prior to proceeding with surgical intervention to avoid unnecessary amputation. The diagnostic criteria for classic ulcerative PG are gaining wider acceptance and are a useful tool for clinicians.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- Bisarya K, Azzopardi S, Lye G, et al. Necrotizing fasciitis versus pyoderma gangrenosum: securing the correct diagnosis! a case report and literature review. Eplasty. 2011;11:E24.
- Perricone G, Vangeli M. Pyoderma gangrenosum in ulcerative colitis. N Engl J Med. 2018;379:E7.
- Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Ampuero J, Rojas-Feria M, Castro-Fernández M, et al. Predictive factors for erythema nodosum and pyoderma gangrenosum in inflammatory bowel disease. J Gastroenterol Hepatol. 2014;29:291-295.
- Ebner DW, Hu M, Poterucha TH. 29-year-old woman with fever and bilateral lower extremity lesions. Mayo Clin Proc. 2018;93:1659-1663.
- Marzak H, Von Hunolstein JJ, Lipsker D, et al. Management of a superinfected pyoderma gangrenosum after pacemaker implant. HeartRhythm Case Rep. 2018;5:63-65.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;2018:8907542.
- Haag CK, Nutan F, Cyrus JW, et al. Pyoderma gangrenosum misdiagnosis resulting in amputation: a review. J Trauma Acute Care Surg. 2019;86:307-313.
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol. 2019;155:79-84.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18:355-372.
Practice Points
- Pyoderma gangrenosum (PG) frequently is misdiagnosed due to its similar presentation to other skin and soft tissue infections (SSTIs). Patients with known risk factors for PG should be evaluated with a high index of suspicion to ensure early diagnosis and avoid serious complications. Common associations include inflammatory bowel disease (IBD), hematologic malignancies, and rheumatologic disorders.
- Response to treatment may be used to guide management when the diagnosis of SSTIs vs PG cannot be distinguished with clinical and histologic findings alone. In a worsening ulcer that has failed antibiotic therapy, clinicians should consider the diagnosis of PG and the risk of pathergy prior to surgical intervention such as debridement.
- Although typically a diagnosis of exclusion, clinicians can consider the use of diagnostic criteria for PG in patients of high clinical suspicion. A trial of immunosuppressants can be considered after infection has been ruled out.
Periorbital and Tragal Cutaneous Lesions
The Diagnosis: Favre-Racouchot Syndrome
Favre-Racouchot syndrome, also known as nodular elastosis with cysts and comedones, is seen in approximately 6% of adults aged 40 to 60 years and predominantly is observed in White males.1 Typically, patients have a history of prolonged recreational or occupational UV exposure and tobacco use. The diagnosis can be made clinically; no biopsy is necessary. If a biopsy is performed, histologic findings typically consist of notable actinic elastosis, epidermal atrophy, and comedones. The differential diagnosis includes acne comedones, colloid milium, milia, chloracne, and trichoepithelioma.2 Associated conditions that have been found concurrently include cutis rhomboidalis nuchae, actinic keratosis, erosive pustular dermatosis, actinic granuloma, and basal and squamous cell carcinomas.2
The pathogenesis, while not fully understood, seems to involve a combination of chronic UV radiation exposure and heavy cigarette smoking that eventually leads to cutaneous atrophy and keratinization of the pilosebaceous follicles as well as the formation of comedones.2 Radiation therapy also has been implicated as a possible causative agent of Favre-Racouchot syndrome.1 Clinically, symmetric distribution of large black comedones over the temporal and periorbital areas is seen surrounded by distinct signs of UV-damaged skin, including wrinkles and atrophic skin.3 Although there seems to be a synergistic effect between cigarette smoking and chronic UV exposure, evidence favors smoking as the major cause of this condition,4,5 which causes striking visual changes but is a benign process. UV protection and smoking cessation are the most important factors for prevention and limiting progression.
Treatment consists of typical comedonal therapies such as tretinoin or comedone extraction. Procedural options in conjunction with medical therapy include dermabrasion or laser therapy. Newer studies have shown promising results for both CO2 laser treatment and plasma exeresis.6 Plasma exeresis is a noninvasive technique that causes ionization of atmospheric gas between the device and tissue, ultimately causing sublimation of the target tissue.7 It is important to carefully evaluate and follow up with these patients due to their history of extensive UV exposure. Both short-term and long-term follow-up is recommended due to high rates of reoccurrence within 10 to 12 months and the dangers of chronic UV exposure– related malignancies.6
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part i. increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1-21; quiz 22-24. doi:10.1016/j.jaad.2004.03.013
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol. 2004;43:167-169. doi:10.1111/j.1365-4632.2004.01546.x
- Sonthalia S, Arora R, Chhabra N, et al. Favre-Racouchot syndrome. Indian Dermatol Online J. 2014;5(suppl 2):S128-S129. doi:10.4103/2229-5178.146192
- Keough GC, Laws RA, Elston DM. Favre-Racouchot syndrome: a case for smokers’ comedones. Arch Dermatol. 1997;133:796-797. doi:10.1001/archderm.133.6.796
- Muto H, Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health. 1989;44:171-174. doi:10.1080/00039896.1989.9935882
- Paganelli A, Mandel VD, Kaleci S, et al. Favre-Racouchot disease: systematic review and possible therapeutic strategies. J Eur Acad Dermatol Venereol. 2018;33:32-41. doi:10.1111/jdv.15184
- Rossi E, Paganelli A, Mandel VD, et al. Plasma exeresis treatment for epidermoid cysts: a minimal scarring technique. Dermatol Surg. 2018;44:1509-1515. doi:10.1097/dss.0000000000001604
The Diagnosis: Favre-Racouchot Syndrome
Favre-Racouchot syndrome, also known as nodular elastosis with cysts and comedones, is seen in approximately 6% of adults aged 40 to 60 years and predominantly is observed in White males.1 Typically, patients have a history of prolonged recreational or occupational UV exposure and tobacco use. The diagnosis can be made clinically; no biopsy is necessary. If a biopsy is performed, histologic findings typically consist of notable actinic elastosis, epidermal atrophy, and comedones. The differential diagnosis includes acne comedones, colloid milium, milia, chloracne, and trichoepithelioma.2 Associated conditions that have been found concurrently include cutis rhomboidalis nuchae, actinic keratosis, erosive pustular dermatosis, actinic granuloma, and basal and squamous cell carcinomas.2
The pathogenesis, while not fully understood, seems to involve a combination of chronic UV radiation exposure and heavy cigarette smoking that eventually leads to cutaneous atrophy and keratinization of the pilosebaceous follicles as well as the formation of comedones.2 Radiation therapy also has been implicated as a possible causative agent of Favre-Racouchot syndrome.1 Clinically, symmetric distribution of large black comedones over the temporal and periorbital areas is seen surrounded by distinct signs of UV-damaged skin, including wrinkles and atrophic skin.3 Although there seems to be a synergistic effect between cigarette smoking and chronic UV exposure, evidence favors smoking as the major cause of this condition,4,5 which causes striking visual changes but is a benign process. UV protection and smoking cessation are the most important factors for prevention and limiting progression.
Treatment consists of typical comedonal therapies such as tretinoin or comedone extraction. Procedural options in conjunction with medical therapy include dermabrasion or laser therapy. Newer studies have shown promising results for both CO2 laser treatment and plasma exeresis.6 Plasma exeresis is a noninvasive technique that causes ionization of atmospheric gas between the device and tissue, ultimately causing sublimation of the target tissue.7 It is important to carefully evaluate and follow up with these patients due to their history of extensive UV exposure. Both short-term and long-term follow-up is recommended due to high rates of reoccurrence within 10 to 12 months and the dangers of chronic UV exposure– related malignancies.6
The Diagnosis: Favre-Racouchot Syndrome
Favre-Racouchot syndrome, also known as nodular elastosis with cysts and comedones, is seen in approximately 6% of adults aged 40 to 60 years and predominantly is observed in White males.1 Typically, patients have a history of prolonged recreational or occupational UV exposure and tobacco use. The diagnosis can be made clinically; no biopsy is necessary. If a biopsy is performed, histologic findings typically consist of notable actinic elastosis, epidermal atrophy, and comedones. The differential diagnosis includes acne comedones, colloid milium, milia, chloracne, and trichoepithelioma.2 Associated conditions that have been found concurrently include cutis rhomboidalis nuchae, actinic keratosis, erosive pustular dermatosis, actinic granuloma, and basal and squamous cell carcinomas.2
The pathogenesis, while not fully understood, seems to involve a combination of chronic UV radiation exposure and heavy cigarette smoking that eventually leads to cutaneous atrophy and keratinization of the pilosebaceous follicles as well as the formation of comedones.2 Radiation therapy also has been implicated as a possible causative agent of Favre-Racouchot syndrome.1 Clinically, symmetric distribution of large black comedones over the temporal and periorbital areas is seen surrounded by distinct signs of UV-damaged skin, including wrinkles and atrophic skin.3 Although there seems to be a synergistic effect between cigarette smoking and chronic UV exposure, evidence favors smoking as the major cause of this condition,4,5 which causes striking visual changes but is a benign process. UV protection and smoking cessation are the most important factors for prevention and limiting progression.
Treatment consists of typical comedonal therapies such as tretinoin or comedone extraction. Procedural options in conjunction with medical therapy include dermabrasion or laser therapy. Newer studies have shown promising results for both CO2 laser treatment and plasma exeresis.6 Plasma exeresis is a noninvasive technique that causes ionization of atmospheric gas between the device and tissue, ultimately causing sublimation of the target tissue.7 It is important to carefully evaluate and follow up with these patients due to their history of extensive UV exposure. Both short-term and long-term follow-up is recommended due to high rates of reoccurrence within 10 to 12 months and the dangers of chronic UV exposure– related malignancies.6
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part i. increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1-21; quiz 22-24. doi:10.1016/j.jaad.2004.03.013
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol. 2004;43:167-169. doi:10.1111/j.1365-4632.2004.01546.x
- Sonthalia S, Arora R, Chhabra N, et al. Favre-Racouchot syndrome. Indian Dermatol Online J. 2014;5(suppl 2):S128-S129. doi:10.4103/2229-5178.146192
- Keough GC, Laws RA, Elston DM. Favre-Racouchot syndrome: a case for smokers’ comedones. Arch Dermatol. 1997;133:796-797. doi:10.1001/archderm.133.6.796
- Muto H, Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health. 1989;44:171-174. doi:10.1080/00039896.1989.9935882
- Paganelli A, Mandel VD, Kaleci S, et al. Favre-Racouchot disease: systematic review and possible therapeutic strategies. J Eur Acad Dermatol Venereol. 2018;33:32-41. doi:10.1111/jdv.15184
- Rossi E, Paganelli A, Mandel VD, et al. Plasma exeresis treatment for epidermoid cysts: a minimal scarring technique. Dermatol Surg. 2018;44:1509-1515. doi:10.1097/dss.0000000000001604
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part i. increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1-21; quiz 22-24. doi:10.1016/j.jaad.2004.03.013
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol. 2004;43:167-169. doi:10.1111/j.1365-4632.2004.01546.x
- Sonthalia S, Arora R, Chhabra N, et al. Favre-Racouchot syndrome. Indian Dermatol Online J. 2014;5(suppl 2):S128-S129. doi:10.4103/2229-5178.146192
- Keough GC, Laws RA, Elston DM. Favre-Racouchot syndrome: a case for smokers’ comedones. Arch Dermatol. 1997;133:796-797. doi:10.1001/archderm.133.6.796
- Muto H, Takizawa Y. Dioxins in cigarette smoke. Arch Environ Health. 1989;44:171-174. doi:10.1080/00039896.1989.9935882
- Paganelli A, Mandel VD, Kaleci S, et al. Favre-Racouchot disease: systematic review and possible therapeutic strategies. J Eur Acad Dermatol Venereol. 2018;33:32-41. doi:10.1111/jdv.15184
- Rossi E, Paganelli A, Mandel VD, et al. Plasma exeresis treatment for epidermoid cysts: a minimal scarring technique. Dermatol Surg. 2018;44:1509-1515. doi:10.1097/dss.0000000000001604
A 91-year-old White man with no personal or family history of skin cancer presented to the dermatology clinic for a total-body skin examination. A 6×5-cm grouped cluster of open comedones in the periorbital region and on the left tragus as well as surrounding actinic damaged skin with coarse rhytides, dyschromia, and lentigines were seen. He had a history of excessive UV exposure and noted that the lesions had been present for approximately 10 years. They were asymptomatic and remained unchanged since their onset.
Relationship-Centered Care in the Physician-Patient Interaction: Improving Your Understanding of Metacognitive Interventions
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
Communication and relationships cannot be taken for granted, particularly in the physician-patient relationship, where life-altering diagnoses may be given. With one diagnosis, someone’s life may be changed, and both physicians and patients need to be cognizant of the importance of a strong relationship and clear communication.
In the current US health care system, both physicians and patients often are not getting their needs met, and studies that include factors of race, ethnicity, and socioeconomic status suggest that physician-patient relationship barriers contribute to racial disparities in health care.1,2 Although patient-centered care is a widely recognized and upheld model, relationship-centered care between physician and patient involves focusing on the patient and the physician-patient relationship through recognizing personhood, affect (being empathic), and reciprocal influence.3,4 Although it is not necessarily intuitive because it can appear to be yet another task for busy physicians, relationship-centered care improves health care delivery for both physicians and patients through decreased physician burnout, reduced medical errors, and better patient outcomes and satisfaction.5,6
Every physician, patient, and physician-patient relationship is different; unlike the standard questions directed at a routine patient history focused on gathering data, there is no one-size-fits-all relationship-centered conversation.7-10 As with any successful interaction between 2 people, there is a certain amount of necessary self-awareness (Table 1)11 that allows for improvisation and appropriate responsiveness to what is seen, heard, and felt. Rather than attending solely to disease states, the focus of relationship-centered care is on patients, interpersonal interaction, and promoting health and well-being.15
This review summarizes the existing literature on relationship-centered care, introduces the use of metacognition (Table 1), and suggests creating simple habits to promote such care. The following databases were searched from inception through November 23, 2020, using the term relationship-centered care: MEDLINE (Ovid), EMBASE (Ovid), APA PsycInfo (Ovid), Scopus, Web of Science Core Collection, CINAHL Complete (EBSCO), Academic Search Premier (EBSCOhost), and ERIC (ProQuest). A total of 1772 records were retrieved through searches, and after deduplication of 1116 studies, 350 records were screened through a 2-part process. Articles were first screened by title and abstract for relevance to the relationship between physician and patient, with 185 studies deemed irrelevant (eg, pertaining to the relationship of veterinarian to animal). The remaining 165 studies were assessed for eligibility, with 69 further studies excluded for various reasons. The screening process resulted in 96 articles considered in this review.
Definitions/key terms, as used in this article, are listed in Table 1.
Background of Relationship-Centered Care
Given time constraints, the diagnosis and treatment of medical problems often are the focus of physicians. Although proper medical diagnosis and treatment are important, and their delivery is made possible by the physician having the appropriate knowledge, a physician-patient relationship that focuses solely on disease without acknowledging the patient creates a system that ultimately neglects both patients and physicians.15 This prevailing physician-patient relationship paradigm is suboptimal, and a proposed remedy is relationship-centered care, which focuses on relationships among the human beings in health care interactions.3 Relationship-centered care has 4 principles: (1) the personhood of each party must be recognized, (2) emotion is part of relationships, (3) relationships are reciprocal and not just one way, and (4) creating these types of relationships is morally valuable3 and beneficial to patient care.16
Assessment of the Need for Relationship-Centered Care
Relationship-centered care has been studied in physician-patient interactions in various health care settings.17-23 For at least 2 decades, relationship-centered care has been set forth as a model,4,24,25 but there are challenges. Physicians tend to overrate or underrate their communication skills in patient interactions.26,27 A given physician’s preferences often still seem to supersede those of the patient.3,28,29 The impetus to develop relationship-centered care skills generally needs to be internally driven,4,30 as, ultimately, physicians and patients have varying needs.4,31 However, providing physicians with a potential structure is helpful.32
A Solution: Metacognition in the Physician-Patient Interaction
Metacognition is important to integrating basic science knowledge into medical learning and practice,33,34 and it is no less important in translating interpersonal knowledge to the physician-patient interaction. Decreased metacognitive effort35 may underpin the decline in empathy seen with increasing medical training.36,37 Understanding how metacognitive practices foster relationship-centered care is important for teaching, developing, and maintaining that care.
Metacognition is already embedded in the fabric of the physician-patient interaction.33,34 The complex interplay of the physician-patient interview, patient examination, and integration of physical as well as ancillary data requires higher-order thinking and the ability to parse out that thinking successfully. As a concrete example, coming to a diagnosis requires thinking about what has been presented during the physician-patient interaction and considering what supports and suggests the disease while a list of potential differential diagnosis alternatives is being generated. Physicians are trained to apply this clinical reasoning approach to their patient care.
Conversely, although communication skills are a key component of doctoring,38 both between physician and patient as well as among other colleagues and staff, many physicians have never received formal training in communication skills,26,32,39 though it is now an integral part of medical school curricula.40 When such training is mandatory, less than 1% of physicians continue to believe that there was no benefit, even from a single 8-hour communications skills training session.41 Communication cannot be taught comprehensively in 8 hours; thus, the benefit of such training may be the end result of metacognition and increased self-awareness (Table 1).42,43
Building Relationship-Centered Care Through Metacognitive Attention
Metacognition as manifested by such self-awareness can build relationship-centered care.4 Self-awareness can be taught through mentorship or role models.44 Journaling,40 meditation, and appreciation of beauty and the arts45 can contribute, as well as more formal training programs,32,38,42 as offered by the Academy of Communication in Healthcare. Creating opportunities for patient empowerment also supports relationship-centered care, as does applying knowledge of implicit bias.46
Even without formal training, relationship-centered care can be built through attention to cues9—visual (eg, sitting down, other body language),47,48 auditory (eg, knocking, language, tone, conversational flow),48,49 and emotional (eg, clinical empathy, emotional intelligence)(Table 2). Such attention is familiar to everyone, not just physicians or patients, through interactions outside of health care; inattention may be due to the hidden curriculum or culture of medicine40 as well as real-time changes, such as the introduction of the electronic health record.51 Inattention to these cues also may be a result of context-specific knowledge, in which a physician’s real-life communication skills are not applied to the unique context of patient care.
Although the theoretical foundation of relationship-centered care is relatively complex,9 a simple formula that has improved patient experience is “The Big 3,” which entails (1) simply knocking before entering the examination room, (2) sitting, and (3) asking, “What is your main concern?”30 Another relatively simple technique would be to involve the patient with the electronic health record by sharing the screen with them.52 Learning about narrative medicine and developing skills to appreciate each patient’s story is another method to increase relationship-centered care,40,53 as is emotional intelligence.54 These interventions are simple to implement, and good relationship-centered care will save time, help manage patient visits more effectively, and aid in avoiding the urgent new concern that the patient adds at the end of the visit.55 The positive effect of these different interventions highlights that small changes (Table 2) can shift the prevailing culture of medicine to become more relationship centered.56
Metacognitive Attention Can Generate Habit
Taking metacognition a step further, these small interventions can become habit11,14,39 through self-awareness, deliberate practice, and feedback.43 Habit is generated by linking a given intervention to another defined cue. For example, placing a hand on a doorknob to enter an examination room can be the cue to generate a habit of entering with presence.14 Alternatively, before entering an examination room, taking 3 deep breaths can be the cue to trigger presence.14 Habits can be created in just 3 weeks,57 and other proposed cues to generate habits toward relationship-centered care are listed in Table 2. By creating habit through metacognitive attention, relationship-centered care will become something that happens subconsciously without further burdening physicians with another task. Asking patients for permission to record video of an interaction also can create opportunities for self-awareness and self-evaluation through rewatching the video.58
Final Thoughts
Physicians already have the tools to create relationship-centered care in physician-patient interactions. A critical mental shift is to develop habits and apply thinking patterns toward understanding and responding appropriately to patients of all ethnicities and their emotions in the physician-patient interaction. This shift is aided by metacognitive awareness (Table 1) and the development of useful habits (Table 2).
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
- Sanders L, Fortin AH VI, Schiff GD. Connecting with patients—the missing links. JAMA. 2020;323:33-34.
- Peck BM, Denney M. Disparities in the conduct of the medical encounter: the effects of physician and patient race and gender. SAGE Open. 2012;2:1-14.
- Beach MC, Inui T. Relationship-centered care. a constructive reframing. J Gen Intern Med. 2006;21(suppl 1):S3-S8.
- Tresolini CP, Pew-Fetzer Task Force. Health Professions Education and Relationship-Centered Care. Pew Health Professions Commission; 1994.
- Hojat M. Empathy in Health Professions Education and Patient Care. Springer; 2016.
- Wilkinson H, Whittington R, Perry L, et al. Examining the relationship between burnout and empathy in healthcare professionals: a systematic review. Burn Res. 2017;6:18-29.
- Frankel RM, Quill T. Integrating biopsychosocial and relationship-centered care into mainstream medical practice: a challenge that continues to produce positive results. Fam Syst Health. 2005;23:413-421.
- Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004;19:1163-1165.
- Ventres WB, Frankel RM. Shared presence in physician-patient communication: a graphic representation. Fam Syst Health. 2015;33:270-279.
- Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(suppl 1):S21-S27.
- Epstein RM. Mindful practice. JAMA. 1999;282:833-839.
- Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422-427.
- Rabow MW. Meaning and relationship-centered care: recommendations for clinicians attending to the spiritual distress of patients at the end of life. Ethics Med Public Health. 2019;9:57-62.
- Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323:70-81.
- Rakel DP, Guerrera MP, Bayles BP, et al. CAM education: promoting a salutogenic focus in health care. J Altern Complement Med. 2008;14:87-93.
- Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med. 2020;18:422-429.
- Berg GM, Ekengren F, Lee FA, et al. Patient satisfaction with surgeons in a trauma population: testing a structural equation model using perceptions of interpersonal and technical care. J Trauma Acute Care Surg. 2012;72:1316-1322.
- Nassar A, Weimer-Elder B, Kline M, et al. Developing an inpatient relationship-centered communication curriculum for surgical teams: pilot study. J Am Coll Surg. 2019;229(4 suppl 2):E48.
- Caldicott CV, Dunn KA, Frankel RM. Can patients tell when they are unwanted? “turfing” in residency training. Patient Educ Couns. 2005;56:104-111.
- Tucker Edmonds B, Mogul M, Shea JA. Understanding low-income African American women’s expectations, preferences, and priorities in prenatal care. Fam Community Health. 2015;38:149-157.
- Sundstrom B, Szabo C, Dempsey A. “My body. my choice:” a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice. J Health Commun. 2018;23:162-169.
- Block S, Billings JA. Nurturing humanism through teaching palliative care. Acad Med. 1998;73:763-765.
- Hebert RS, Schulz R, Copeland VC, et al. Preparing family caregivers for death and bereavement. insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37:3-12.
- Nundy S, Oswald J. Relationship-centered care: a new paradigm for population health management. Healthc (Amst). 2014;2:216-219.
- Sprague S. Relationship centered care. J S C Med Assoc. 2009;105:135-136.
- Roter DL, Frankel RM, Hall JA, et al. The expression of emotion through nonverbal behavior in medical visits. mechanisms and outcomes. J Gen Intern Med. 2006;21(suppl 1):S28-S34.
- Kenny DA, Veldhuijzen W, van der Weijden T, et al. Interpersonal perception in the context of doctor-patient relationships: a dyadic analysis of doctor-patient communication. Soc Sci Med. 2010;70:763-768.
- Tarzian AJ, Neal MT, O’Neil JA. Attitudes, experiences, and beliefs affecting end-of-life decision-making among homeless individuals. J Palliat Med. 2005;8:36-48.
- Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5-15.
- Sharieff GQ. MD to MD coaching: improving physician-patient experience scores: what works, what doesn’t. J Patient Exp. 2017;4:210-212.
- Duggan AP, Bradshaw YS, Swergold N, et al. When rapport building extends beyond affiliation: communication overaccommodation toward patients with disabilities. Perm J. 2011;15:23-30.
- Hirschmann K, Rosler G, Fortin AH VI. “For me, this has been transforming”: a qualitative analysis of interprofessional relationship-centered communication skills training. J Patient Exp. 2020;7:1007-1014.
- Hennrikus EF, Skolka MP, Hennrikus N. Applying metacognition through patient encounters and illness scripts to create a conceptual framework for basic science integration, storage, and retrieval. J Med Educ Curric Dev. 2018;5:2382120518777770.
- Eichbaum QG. Thinking about thinking and emotion: the metacognitive approach to the medical humanities that integrates the humanities with the basic and clinical sciences. Perm J. 2014;18:64-75.
- Stansfield RB, Schwartz A, O’Brien CL, et al. Development of a metacognitive effort construct of empathy during clinical training: a longitudinal study of the factor structure of the Jefferson Scale of Empathy. Adv Health Sci Educ Theory Pract. 2016;21:5-17.
- Hojat M, Vergare MJ, Maxwell K, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. 2009;84:1182-1191.
- Neumann M, Edelhäuser F, Tauschel D, et al. Empathy decline and its reasons: a systematic review of studies with medical students and residents. Acad Med. 2011;86:996-1009.
- Chou CL, Hirschmann K, Fortin AHT, et al. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89:1051-1056.
- Rider EA. Advanced communication strategies for relationship-centered care. Pediatr Ann. 2011;40:447-453.
- Reichman JAH. Narrative competence, mindfulness,and relationship-centered care in medical education: an innovative approach to teaching medical interviewing. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2015;75(8-A(E)).
- Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31:755-761.
- Hatem DS, Barrett SV, Hewson M, et al. Teaching the medical interview: methods and key learning issues in a faculty development course. J Gen Intern Med. 2007;22:1718-1724.
- Gilligan TD, Baile WF. ASCO patient-clinician communication guideline: fostering relationship-centered care. ASCO Connection. November 20, 2017. Accessed March 5, 2021. https://connection.asco.org/blogs/asco-patient-clinician-communication-guideline-fostering-relationship-centered-care
- Haidet P, Stein HF. The role of the student-teacher relationship in the formation of physicians. The hidden curriculum as process. J Gen Intern Med. 2006;(suppl 1):S16-S20.
- Puchalski CM, Guenther M. Restoration and re-creation: spirituality in the lives of healthcare professionals. Curr Opin Support Palliat Care. 2012;6:254-258.
- Williams SW, Hanson LC, Boyd C, et al. Communication, decision making, and cancer: what African Americans want physicians to know. J Palliative Med. 2008;11:1221-1226.
- Lindsley I, Woodhead S, Micallef C, et al. The concept of body language in the medical consultation. Psychiatr Danub. 2015;27(suppl 1):S41-S47.
- Hall JA, Harrigan JA, Rosenthal R. Nonverbal behavior in clinician-patient interaction. Appl Prev Psychol. 1995;4:21-37.
- Ness DE, Kiesling SF. Language and connectedness in the medical and psychiatric interview. Patient Educ Couns. 2007;68:139-144.
- Miller WL. The clinical hand: a curricular map for relationship-centered care. Fam Med. 2004;36:330-335.
- Wald HS, George P, Reis SP, et al. Electronic health record training in undergraduate medical education: bridging theory to practice with curricula for empowering patient- and relationship-centered care in the computerized setting. Acad Med. 2014;89:380-386.
- Silverman H, Ho YX, Kaib S, et al. A novel approach to supporting relationship-centered care through electronic health record ergonomic training in preclerkship medical education. Acad Med. 2014;89:1230-1234.
- Weiss T, Swede MJ. Transforming preprofessional health education through relationship-centered care and narrative medicine. Teach Learn Med. 2019;31:222-233.
- Blanch-Hartigan D. An effective training to increase accurate recognition of patient emotion cues. Patient Educ Couns. 2012;89:274-280.
- White J, Levinson W, Roter D. “Oh, by the way ...”: the closing moments of the medical visit. J Gen Intern Med. 1994;9:24-28.
- Suchman AL, Williamson PR, Litzelman DK, et al. Toward an informal curriculum that teaches professionalism. Transforming the social environment of a medical school. J Gen Intern Med. 2004;19:501-504.
- Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998-1009.
- Little P, White P, Kelly J, et al. Randomised controlled trial of a brief intervention targeting predominantly non-verbal communication in general practice consultations. Br J Gen Pract. 2015;65:E351-E356.
Practice Points
- Relationship-centered care emphasizes that all relationships in health care are important, including not only relationships between physicians and patients but also among physicians and colleagues, staff, students, community, and self.
- The physician-patient relationship can be complex, and metacognition can lead to habitual practice of simple techniques to optimize the interaction
Phacomatosis Pigmentokeratotica Associated With Raynaud Phenomenon, Segmental Nevi, Hyperhidrosis, and Scoliosis
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
Practice Points
- Phacomatosis pigmentokeratotica (PPK) is characterized by the coexistence of speckled lentiginous nevus and nevus sebaceous.
- Raynaud phenomenon may be an unreported association with PPK.
The Power of a Multidisciplinary Tumor Board: Managing Unresectable and/or High-Risk Skin Cancers
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
Resident Pearl
- Participating in a multidisciplinary tumor board allows residents to learn more about how to manage and treat high-risk skin cancers. The multidisciplinary team approach provides high-quality care for challenging patients.
Urticarial Vasculitis Successfully Treated With Omalizumab
To the Editor:
Urticarial vasculitis (UV) is a clinicopathologic entity. It manifests as an eruption of erythematous wheals that clinically resemble urticaria, but the lesions of UV last longer, may leave residual hyperpigmentation, and may or may not be pruritic.1 Therapies most often employed include oral antihistamines and systemic immunosuppressant drugs such as corticosteroids, dapsone, colchicine, or hydroxychloroquine.2 We present a woman with UV who successfully was treated with omalizumab.
A 49-year-old woman presented to our outpatient clinic with generalized pruritic skin rashes of 2 years’ duration. She also described swelling on the upper eyelids 2 times monthly. She used several antihistamines (up to 4 times daily) and was taking systemic corticosteroids and antidepressants. Physical examination revealed generalized erythematous and edematous papules and plaques on the trunk and extremities (Figure 1). At follow-up a few days later, we observed that the lesions were lasting for more than 24 hours, but there was no residual pigmentation. According to clinical concerns and the association with angioedema, we initially thought the diagnosis was chronic urticaria and angioedema. The patient had no extracutaneous manifestations such as fever, arthralgia, or lymphadenopathy. Routine laboratory examinations including antinuclear antibodies were within reference range. She had normal C3 and C4 levels and an elevated total IgE level (344 IU/mL [reference range, 0–170 IU/mL]). Because the IgE level was elevated and she had no response to the highest dosages of antihistamines, we decided to start omalizumab therapy. Prior to starting omalizumab, we performed a skin biopsy for histopathologic and direct immunofluorescence examinations for UV, as the duration of the lesions was more than 24 hours. Histopathologic examination revealed lymphocytes within the vessel wall and perivascular lymphocytic infiltration with eosinophils (Figure 2). On direct immunofluorescence, perivascular IgA deposition was observed (Figure 3). Histopathologic findings were associated with lymphocytic vasculitis. Systemic involvement was not detected on detailed laboratory and radiologic examinations.
After the first application of omalizumab, the lesions disappeared within a few days. She was treated with subcutaneous omalizumab 300 mg every 4 weeks for 6 months, and we did not observe any adverse effects related to the drug. There was no relapse after therapy cessation.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody that is approved by the US Food and Drug Administration for treatment of chronic idiopathic urticaria.3-5 Studies have suggested that omalizumab might play an important role in the treatment of other potentially IgE-mediated disease processes including allergic asthma, atopic dermatitis, allergic rhinitis, nasal polyposis, and severe ocular allergies.6 The proposed mechanism of action of omalizumab includes reduction of free IgE through the reversible formation of tiny, biologically inert complexes; targeting IgE-expressing B cells; and inhibiting production of IgE. Because it reduces free IgE, omalizumab has been used in normal IgE or hyper-IgE situations. Omalizumab also induces eosinophil apoptosis; increases IL-2, IL-3, tumor necrosis factor α, and IFN-γ; and reduces IL-4.7 A number of off-label uses have been described such as atopic dermatitis, bullous pemphigoid, hyper-IgE syndrome, cutaneous mastocytosis, toxic epidermal necrolysis, and eosinophilic granulomatosis with polyangitis.8 There are no clinical studies of omalizumab for UV, and only a few case reports have shown that omalizumab also might be beneficial for this condition.2-4 Diez et al4 reported 3 cases of women aged 28, 51, and 54 years with spontaneous chronic urticaria with autoimmune and pressure components as well as vasculitis whose symptoms completely improved after starting omalizumab. Kai et al3 successfully treated a patient with normocomplementemic UV with omalizumab and suggested that omalizumab markedly improved the patient’s quality of life with chronic urticaria and UV. Ghazanfar and Thomsen2 reported the case of a 68-year-old man diagnosed with histopathologically confirmed leukocytoclastic vasculitis. He had used systemic corticosteroid therapy and dapsone without notable improvement. The patient was switched to subcutaneous omalizumab 300 mg once every 4 weeks; after 1 month, he observed complete remission of the UV and symptoms.2
Our case suggests that omalizumab has a beneficial effect on patients with UV. Omalizumab may be effective in UV through its reduction of IgE, as in chronic urticaria, and through downstream effects on cellular activation mechanisms (possibly a reduction in chemotaxis or immune complex formation). However, the mechanism of action of omalizumab for UV remains, in part, unresolved. It is not known whether omalizumab is efficacious against both normocomplementemic and hypocomplementemic UV. Further studies with a greater number of patients are needed to confirm the effects of omalizumab for vasculitic patients.
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc. 2007;28:97-100.
- Ghazanfar MN, Thomsen SF. Omalizumab for urticarial vasculitis: case report and review of the literature. Case Rep Dermatol Med. 2015:576893.
- Kai AC, Flohr C, Grattan CE. Improvement in quality of life impairment followed by relapse with 6-monthly periodic administration of omalizumab for severe treatment-refractory chronic urticaria and urticarial vasculitis. Clin Exp Dermatol. 2014;39:651-652.
- Diez LS, Tamayo LM, Cardona R. Omalizumab: therapeutic option in chronic spontaneous urticaria difficult to control with associated vasculitis, report of three cases. Biomedica. 2013;33:503-512.
- Maurer M, Rosen K, Hsieh HJ. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2530.
- Ben Shoshan M. Omalizumab: not only for asthma. Recent Pat Inflamm Allergy Drug Discov. 2008;2:191-201.
- Fueyo-Casado A, Campos-Munoz L, Gonzalez-Guerra E, et al. Effectiveness of omalizumab in a case of urticarial vasculitis. Clin Exp Dermatol. Published March 1, 2017. doi:10.1111/ced.13076
- Chia JC, Mydlarski PR. Dermatologic uses of omalizumab. J Dermatol Treat. Published November 7, 2016. doi:10.1080/09546634.2016.1249819
To the Editor:
Urticarial vasculitis (UV) is a clinicopathologic entity. It manifests as an eruption of erythematous wheals that clinically resemble urticaria, but the lesions of UV last longer, may leave residual hyperpigmentation, and may or may not be pruritic.1 Therapies most often employed include oral antihistamines and systemic immunosuppressant drugs such as corticosteroids, dapsone, colchicine, or hydroxychloroquine.2 We present a woman with UV who successfully was treated with omalizumab.
A 49-year-old woman presented to our outpatient clinic with generalized pruritic skin rashes of 2 years’ duration. She also described swelling on the upper eyelids 2 times monthly. She used several antihistamines (up to 4 times daily) and was taking systemic corticosteroids and antidepressants. Physical examination revealed generalized erythematous and edematous papules and plaques on the trunk and extremities (Figure 1). At follow-up a few days later, we observed that the lesions were lasting for more than 24 hours, but there was no residual pigmentation. According to clinical concerns and the association with angioedema, we initially thought the diagnosis was chronic urticaria and angioedema. The patient had no extracutaneous manifestations such as fever, arthralgia, or lymphadenopathy. Routine laboratory examinations including antinuclear antibodies were within reference range. She had normal C3 and C4 levels and an elevated total IgE level (344 IU/mL [reference range, 0–170 IU/mL]). Because the IgE level was elevated and she had no response to the highest dosages of antihistamines, we decided to start omalizumab therapy. Prior to starting omalizumab, we performed a skin biopsy for histopathologic and direct immunofluorescence examinations for UV, as the duration of the lesions was more than 24 hours. Histopathologic examination revealed lymphocytes within the vessel wall and perivascular lymphocytic infiltration with eosinophils (Figure 2). On direct immunofluorescence, perivascular IgA deposition was observed (Figure 3). Histopathologic findings were associated with lymphocytic vasculitis. Systemic involvement was not detected on detailed laboratory and radiologic examinations.
After the first application of omalizumab, the lesions disappeared within a few days. She was treated with subcutaneous omalizumab 300 mg every 4 weeks for 6 months, and we did not observe any adverse effects related to the drug. There was no relapse after therapy cessation.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody that is approved by the US Food and Drug Administration for treatment of chronic idiopathic urticaria.3-5 Studies have suggested that omalizumab might play an important role in the treatment of other potentially IgE-mediated disease processes including allergic asthma, atopic dermatitis, allergic rhinitis, nasal polyposis, and severe ocular allergies.6 The proposed mechanism of action of omalizumab includes reduction of free IgE through the reversible formation of tiny, biologically inert complexes; targeting IgE-expressing B cells; and inhibiting production of IgE. Because it reduces free IgE, omalizumab has been used in normal IgE or hyper-IgE situations. Omalizumab also induces eosinophil apoptosis; increases IL-2, IL-3, tumor necrosis factor α, and IFN-γ; and reduces IL-4.7 A number of off-label uses have been described such as atopic dermatitis, bullous pemphigoid, hyper-IgE syndrome, cutaneous mastocytosis, toxic epidermal necrolysis, and eosinophilic granulomatosis with polyangitis.8 There are no clinical studies of omalizumab for UV, and only a few case reports have shown that omalizumab also might be beneficial for this condition.2-4 Diez et al4 reported 3 cases of women aged 28, 51, and 54 years with spontaneous chronic urticaria with autoimmune and pressure components as well as vasculitis whose symptoms completely improved after starting omalizumab. Kai et al3 successfully treated a patient with normocomplementemic UV with omalizumab and suggested that omalizumab markedly improved the patient’s quality of life with chronic urticaria and UV. Ghazanfar and Thomsen2 reported the case of a 68-year-old man diagnosed with histopathologically confirmed leukocytoclastic vasculitis. He had used systemic corticosteroid therapy and dapsone without notable improvement. The patient was switched to subcutaneous omalizumab 300 mg once every 4 weeks; after 1 month, he observed complete remission of the UV and symptoms.2
Our case suggests that omalizumab has a beneficial effect on patients with UV. Omalizumab may be effective in UV through its reduction of IgE, as in chronic urticaria, and through downstream effects on cellular activation mechanisms (possibly a reduction in chemotaxis or immune complex formation). However, the mechanism of action of omalizumab for UV remains, in part, unresolved. It is not known whether omalizumab is efficacious against both normocomplementemic and hypocomplementemic UV. Further studies with a greater number of patients are needed to confirm the effects of omalizumab for vasculitic patients.
To the Editor:
Urticarial vasculitis (UV) is a clinicopathologic entity. It manifests as an eruption of erythematous wheals that clinically resemble urticaria, but the lesions of UV last longer, may leave residual hyperpigmentation, and may or may not be pruritic.1 Therapies most often employed include oral antihistamines and systemic immunosuppressant drugs such as corticosteroids, dapsone, colchicine, or hydroxychloroquine.2 We present a woman with UV who successfully was treated with omalizumab.
A 49-year-old woman presented to our outpatient clinic with generalized pruritic skin rashes of 2 years’ duration. She also described swelling on the upper eyelids 2 times monthly. She used several antihistamines (up to 4 times daily) and was taking systemic corticosteroids and antidepressants. Physical examination revealed generalized erythematous and edematous papules and plaques on the trunk and extremities (Figure 1). At follow-up a few days later, we observed that the lesions were lasting for more than 24 hours, but there was no residual pigmentation. According to clinical concerns and the association with angioedema, we initially thought the diagnosis was chronic urticaria and angioedema. The patient had no extracutaneous manifestations such as fever, arthralgia, or lymphadenopathy. Routine laboratory examinations including antinuclear antibodies were within reference range. She had normal C3 and C4 levels and an elevated total IgE level (344 IU/mL [reference range, 0–170 IU/mL]). Because the IgE level was elevated and she had no response to the highest dosages of antihistamines, we decided to start omalizumab therapy. Prior to starting omalizumab, we performed a skin biopsy for histopathologic and direct immunofluorescence examinations for UV, as the duration of the lesions was more than 24 hours. Histopathologic examination revealed lymphocytes within the vessel wall and perivascular lymphocytic infiltration with eosinophils (Figure 2). On direct immunofluorescence, perivascular IgA deposition was observed (Figure 3). Histopathologic findings were associated with lymphocytic vasculitis. Systemic involvement was not detected on detailed laboratory and radiologic examinations.
After the first application of omalizumab, the lesions disappeared within a few days. She was treated with subcutaneous omalizumab 300 mg every 4 weeks for 6 months, and we did not observe any adverse effects related to the drug. There was no relapse after therapy cessation.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody that is approved by the US Food and Drug Administration for treatment of chronic idiopathic urticaria.3-5 Studies have suggested that omalizumab might play an important role in the treatment of other potentially IgE-mediated disease processes including allergic asthma, atopic dermatitis, allergic rhinitis, nasal polyposis, and severe ocular allergies.6 The proposed mechanism of action of omalizumab includes reduction of free IgE through the reversible formation of tiny, biologically inert complexes; targeting IgE-expressing B cells; and inhibiting production of IgE. Because it reduces free IgE, omalizumab has been used in normal IgE or hyper-IgE situations. Omalizumab also induces eosinophil apoptosis; increases IL-2, IL-3, tumor necrosis factor α, and IFN-γ; and reduces IL-4.7 A number of off-label uses have been described such as atopic dermatitis, bullous pemphigoid, hyper-IgE syndrome, cutaneous mastocytosis, toxic epidermal necrolysis, and eosinophilic granulomatosis with polyangitis.8 There are no clinical studies of omalizumab for UV, and only a few case reports have shown that omalizumab also might be beneficial for this condition.2-4 Diez et al4 reported 3 cases of women aged 28, 51, and 54 years with spontaneous chronic urticaria with autoimmune and pressure components as well as vasculitis whose symptoms completely improved after starting omalizumab. Kai et al3 successfully treated a patient with normocomplementemic UV with omalizumab and suggested that omalizumab markedly improved the patient’s quality of life with chronic urticaria and UV. Ghazanfar and Thomsen2 reported the case of a 68-year-old man diagnosed with histopathologically confirmed leukocytoclastic vasculitis. He had used systemic corticosteroid therapy and dapsone without notable improvement. The patient was switched to subcutaneous omalizumab 300 mg once every 4 weeks; after 1 month, he observed complete remission of the UV and symptoms.2
Our case suggests that omalizumab has a beneficial effect on patients with UV. Omalizumab may be effective in UV through its reduction of IgE, as in chronic urticaria, and through downstream effects on cellular activation mechanisms (possibly a reduction in chemotaxis or immune complex formation). However, the mechanism of action of omalizumab for UV remains, in part, unresolved. It is not known whether omalizumab is efficacious against both normocomplementemic and hypocomplementemic UV. Further studies with a greater number of patients are needed to confirm the effects of omalizumab for vasculitic patients.
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc. 2007;28:97-100.
- Ghazanfar MN, Thomsen SF. Omalizumab for urticarial vasculitis: case report and review of the literature. Case Rep Dermatol Med. 2015:576893.
- Kai AC, Flohr C, Grattan CE. Improvement in quality of life impairment followed by relapse with 6-monthly periodic administration of omalizumab for severe treatment-refractory chronic urticaria and urticarial vasculitis. Clin Exp Dermatol. 2014;39:651-652.
- Diez LS, Tamayo LM, Cardona R. Omalizumab: therapeutic option in chronic spontaneous urticaria difficult to control with associated vasculitis, report of three cases. Biomedica. 2013;33:503-512.
- Maurer M, Rosen K, Hsieh HJ. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2530.
- Ben Shoshan M. Omalizumab: not only for asthma. Recent Pat Inflamm Allergy Drug Discov. 2008;2:191-201.
- Fueyo-Casado A, Campos-Munoz L, Gonzalez-Guerra E, et al. Effectiveness of omalizumab in a case of urticarial vasculitis. Clin Exp Dermatol. Published March 1, 2017. doi:10.1111/ced.13076
- Chia JC, Mydlarski PR. Dermatologic uses of omalizumab. J Dermatol Treat. Published November 7, 2016. doi:10.1080/09546634.2016.1249819
- Chang S, Carr W. Urticarial vasculitis. Allergy Asthma Proc. 2007;28:97-100.
- Ghazanfar MN, Thomsen SF. Omalizumab for urticarial vasculitis: case report and review of the literature. Case Rep Dermatol Med. 2015:576893.
- Kai AC, Flohr C, Grattan CE. Improvement in quality of life impairment followed by relapse with 6-monthly periodic administration of omalizumab for severe treatment-refractory chronic urticaria and urticarial vasculitis. Clin Exp Dermatol. 2014;39:651-652.
- Diez LS, Tamayo LM, Cardona R. Omalizumab: therapeutic option in chronic spontaneous urticaria difficult to control with associated vasculitis, report of three cases. Biomedica. 2013;33:503-512.
- Maurer M, Rosen K, Hsieh HJ. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2530.
- Ben Shoshan M. Omalizumab: not only for asthma. Recent Pat Inflamm Allergy Drug Discov. 2008;2:191-201.
- Fueyo-Casado A, Campos-Munoz L, Gonzalez-Guerra E, et al. Effectiveness of omalizumab in a case of urticarial vasculitis. Clin Exp Dermatol. Published March 1, 2017. doi:10.1111/ced.13076
- Chia JC, Mydlarski PR. Dermatologic uses of omalizumab. J Dermatol Treat. Published November 7, 2016. doi:10.1080/09546634.2016.1249819
Practice Points
- The differential diagnosis of urticaria and urticarial vasculitis may be complicated.
- Omalizumab is an effective urticaria treatment and also can be an alternative treatment choice in resistant urticarial vasculitis.
Multiple Crusted Swellings on the Chin
The Diagnosis: Cutaneous Cryptococcosis
Histologic examination revealed infiltration of the dermis and subcutaneous tissue with rounded basophilic cells on low magnification (Figure 1A). On higher magnification, encapsulated yeast cells (cryptococci) of varying size accompanied by chronic granulomatous inflammatory infiltration with occasional giant cells were seen (Figure 1B). Alcian blue stain showed mucinous capsular material (Figure 1C). There was no history of diabetes mellitus, tuberculosis, steroid therapy, or immunosuppression. Moreover, systemic involvement or systemic focus of infection was ruled out after computed tomography of the head, chest, and abdomen. Therefore, the diagnosis of primary cutaneous cryptococcosis (PCC) was established. The patient was started on oral itraconazole 100 mg twice daily along with 5 drops of a saturated solution of potassium iodide 3 times daily that later was increased to 20 drops 3 times daily at a weekly interval. The lesions started improving after 1 month and healed completely after 9 months of treatment (Figure 2).
Primary cutaneous cryptococcosis is the identification of Cryptococcus neoformans in a skin lesion without evidence of simultaneous disseminated disease. Neuville et al1 observed that skin lesions resemble cellulitis, ulcerations, or whitlows and were located on unclothed areas. In contrast, lesions from disseminated disease presented as scattered umbilicated papules resembling molluscum contagiosum. Diagnosis of PCC is based on the observation of encapsulated yeasts by direct microscopic examination, isolation of C neoformans or Cryptococcus gattii in culture, and by the demonstration of capsular antigen in various fluids, including serum and cerebrospinal fluid by latex particle agglutination or enzyme-linked immunosorbent assay. Histologically, Cryptococcus species produce a proliferative inflammatory reaction in immunocompetent hosts with the formation of compact epithelioid granulomas, with giant cells and a peripheral layer of lymphocytes. Treatment options for PCC infection range from antifungal medications and surgical debridement to observation.
The differential diagnosis may include cutaneous leishmaniasis, cutaneous tuberculosis, cutaneous histoplasmosis, and basal cell carcinoma. These entities may have similar presentations and can only be confidently differentiated on direct microscopy and histopathologic examination. The characteristic Leishmania donovani bodies on microscopy in cutaneous leishmaniasis and tubercular granuloma with central necrosis on histology in cutaneous tuberculosis can differentiate these conditions from cryptococcosis. In some patients with cryptococcosis, the yeast may produce a less characteristic polysaccharide capsule and thus may be confused with histoplasmosis. Fontana-Masson staining may show melanin-producing yeast, which is characteristic of cryptococci.2 Ulcerated basal cell carcinoma may present similar clinically; however, histopathology will rule it out.
Cutaneous cryptococcal infection should be presumed to be disseminated until proven otherwise, and a search for other sites of involvement must immediately be undertaken. Cutaneous signs may be the first indication of infection, preceding the diagnosis of disseminated disease by 2 to 8 months, making its recognition crucial to early treatment. It is not possible to diagnose PCC on a specific clinical manifestation because a diverse range of skin lesions may be present. Therefore, culture and histology are the gold standards for diagnosis of cryptococcosis.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
The Diagnosis: Cutaneous Cryptococcosis
Histologic examination revealed infiltration of the dermis and subcutaneous tissue with rounded basophilic cells on low magnification (Figure 1A). On higher magnification, encapsulated yeast cells (cryptococci) of varying size accompanied by chronic granulomatous inflammatory infiltration with occasional giant cells were seen (Figure 1B). Alcian blue stain showed mucinous capsular material (Figure 1C). There was no history of diabetes mellitus, tuberculosis, steroid therapy, or immunosuppression. Moreover, systemic involvement or systemic focus of infection was ruled out after computed tomography of the head, chest, and abdomen. Therefore, the diagnosis of primary cutaneous cryptococcosis (PCC) was established. The patient was started on oral itraconazole 100 mg twice daily along with 5 drops of a saturated solution of potassium iodide 3 times daily that later was increased to 20 drops 3 times daily at a weekly interval. The lesions started improving after 1 month and healed completely after 9 months of treatment (Figure 2).
Primary cutaneous cryptococcosis is the identification of Cryptococcus neoformans in a skin lesion without evidence of simultaneous disseminated disease. Neuville et al1 observed that skin lesions resemble cellulitis, ulcerations, or whitlows and were located on unclothed areas. In contrast, lesions from disseminated disease presented as scattered umbilicated papules resembling molluscum contagiosum. Diagnosis of PCC is based on the observation of encapsulated yeasts by direct microscopic examination, isolation of C neoformans or Cryptococcus gattii in culture, and by the demonstration of capsular antigen in various fluids, including serum and cerebrospinal fluid by latex particle agglutination or enzyme-linked immunosorbent assay. Histologically, Cryptococcus species produce a proliferative inflammatory reaction in immunocompetent hosts with the formation of compact epithelioid granulomas, with giant cells and a peripheral layer of lymphocytes. Treatment options for PCC infection range from antifungal medications and surgical debridement to observation.
The differential diagnosis may include cutaneous leishmaniasis, cutaneous tuberculosis, cutaneous histoplasmosis, and basal cell carcinoma. These entities may have similar presentations and can only be confidently differentiated on direct microscopy and histopathologic examination. The characteristic Leishmania donovani bodies on microscopy in cutaneous leishmaniasis and tubercular granuloma with central necrosis on histology in cutaneous tuberculosis can differentiate these conditions from cryptococcosis. In some patients with cryptococcosis, the yeast may produce a less characteristic polysaccharide capsule and thus may be confused with histoplasmosis. Fontana-Masson staining may show melanin-producing yeast, which is characteristic of cryptococci.2 Ulcerated basal cell carcinoma may present similar clinically; however, histopathology will rule it out.
Cutaneous cryptococcal infection should be presumed to be disseminated until proven otherwise, and a search for other sites of involvement must immediately be undertaken. Cutaneous signs may be the first indication of infection, preceding the diagnosis of disseminated disease by 2 to 8 months, making its recognition crucial to early treatment. It is not possible to diagnose PCC on a specific clinical manifestation because a diverse range of skin lesions may be present. Therefore, culture and histology are the gold standards for diagnosis of cryptococcosis.
The Diagnosis: Cutaneous Cryptococcosis
Histologic examination revealed infiltration of the dermis and subcutaneous tissue with rounded basophilic cells on low magnification (Figure 1A). On higher magnification, encapsulated yeast cells (cryptococci) of varying size accompanied by chronic granulomatous inflammatory infiltration with occasional giant cells were seen (Figure 1B). Alcian blue stain showed mucinous capsular material (Figure 1C). There was no history of diabetes mellitus, tuberculosis, steroid therapy, or immunosuppression. Moreover, systemic involvement or systemic focus of infection was ruled out after computed tomography of the head, chest, and abdomen. Therefore, the diagnosis of primary cutaneous cryptococcosis (PCC) was established. The patient was started on oral itraconazole 100 mg twice daily along with 5 drops of a saturated solution of potassium iodide 3 times daily that later was increased to 20 drops 3 times daily at a weekly interval. The lesions started improving after 1 month and healed completely after 9 months of treatment (Figure 2).
Primary cutaneous cryptococcosis is the identification of Cryptococcus neoformans in a skin lesion without evidence of simultaneous disseminated disease. Neuville et al1 observed that skin lesions resemble cellulitis, ulcerations, or whitlows and were located on unclothed areas. In contrast, lesions from disseminated disease presented as scattered umbilicated papules resembling molluscum contagiosum. Diagnosis of PCC is based on the observation of encapsulated yeasts by direct microscopic examination, isolation of C neoformans or Cryptococcus gattii in culture, and by the demonstration of capsular antigen in various fluids, including serum and cerebrospinal fluid by latex particle agglutination or enzyme-linked immunosorbent assay. Histologically, Cryptococcus species produce a proliferative inflammatory reaction in immunocompetent hosts with the formation of compact epithelioid granulomas, with giant cells and a peripheral layer of lymphocytes. Treatment options for PCC infection range from antifungal medications and surgical debridement to observation.
The differential diagnosis may include cutaneous leishmaniasis, cutaneous tuberculosis, cutaneous histoplasmosis, and basal cell carcinoma. These entities may have similar presentations and can only be confidently differentiated on direct microscopy and histopathologic examination. The characteristic Leishmania donovani bodies on microscopy in cutaneous leishmaniasis and tubercular granuloma with central necrosis on histology in cutaneous tuberculosis can differentiate these conditions from cryptococcosis. In some patients with cryptococcosis, the yeast may produce a less characteristic polysaccharide capsule and thus may be confused with histoplasmosis. Fontana-Masson staining may show melanin-producing yeast, which is characteristic of cryptococci.2 Ulcerated basal cell carcinoma may present similar clinically; however, histopathology will rule it out.
Cutaneous cryptococcal infection should be presumed to be disseminated until proven otherwise, and a search for other sites of involvement must immediately be undertaken. Cutaneous signs may be the first indication of infection, preceding the diagnosis of disseminated disease by 2 to 8 months, making its recognition crucial to early treatment. It is not possible to diagnose PCC on a specific clinical manifestation because a diverse range of skin lesions may be present. Therefore, culture and histology are the gold standards for diagnosis of cryptococcosis.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
A 54-year-old man with no comorbidities presented with multiple painless swellings on the left side of the chin of 1 month’s duration that progressively were increasing, both in size and number. He denied any discharge of pus or grains from the lesion, facial trauma, insect bites, or dental procedures. The patient was treated with oral antibiotics for 15 days with no relief at an outside hospital. All routine blood and serologic investigations including viral markers and chest radiography were normal. Bacterial and fungal cultures as well as an acid-fast bacilli culture were negative. Systemic examination was normal, and vitals were within reference range. Mucocutaneous examination revealed multiple nontender small nodules and plaques with yellow-brown to dark brown hemorrhagic crusts with mild perilesional erythema on the left side of the chin extending to the adjacent neck. All mucosal sites were normal, and a biopsy was performed.
Bullous Pemphigoid Triggered by Liraglutide
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease that typically affects the elderly, with an incidence of approximately 7 new cases per million.1 The pathogenesis of BP involves autoantibodies to BP antigens 180 and 230 at the dermoepidermal junction. Bullous pemphigoid has been associated with the use of multiple medications; vaccines; and physical damage to the skin, including trauma, radiation, and surgery.2
Several classes of medications may cause BP; one study described an association of BP with loop diuretics,3 while others found higher incidences of BP in patients taking aldosterone antagonists and neuroleptics.4 We describe a case of drug-triggered BP to liraglutide, a glucagonlike peptide 1 (GLP-1) receptor agonist.
A 75-year-old man presented to dermatology for evaluation of a vesicular eruption on the head, neck, trunk, and arms of 6 months’ duration. The eruption developed 2 weeks after starting liraglutide 1.2 mg subcutaneously daily for diabetes mellitus. The patient had a medical history of type 2 diabetes mellitus, hypertension, stroke, and prostate cancer treated with prostatectomy, and he also was taking insulin. Liraglutide was discontinued shortly after the onset of the eruption.
Physical examination revealed annular plaques on the head, neck, trunk, and arms with central hypopigmentation and hyperpigmented borders (Figure 1). Two tense bullae were evident on the left flank (Figure 2). Histopathology revealed a subepidermal blister, mixed perivascular infiltrate with numerous eosinophils, and pigment incontinence (Figure 3). Direct immunofluorescence showed linear deposition of IgG and C3 along the basement membrane zone that was localized to the roof of the blister on salt-split analysis. No microorganisms were identified on periodic acid–Schiff, Grocott-Gomori methenamine-silver, acid-fast bacilli, and Fite stains. The patient initially was treated with clobetasol ointment 0.05%, leading to marginal improvement. He declined treatment with prednisone or dapsone, and he was started on doxycycline. Seven months after stopping liraglutide and starting doxycycline, the patient had no blisters, but residual pigmentary changes remained.
Two types of BP have been described in response to medications: drug-induced BP and drug-triggered BP. Drug-induced BP presents as an acute, self-limited eruption that typically resolves after withdrawal of the offending agent. It tends to involve a younger population and may present with mucosal involvement and target lesions on the palms and soles. Direct immunofluorescence shows linear IgG and C3 deposition at the basement membrane zone. Patients tend to respond quickly to systemic corticosteroids and have low recurrence rates. Drug-triggered BP is a chronic form of BP that is caused by a medication and is not resolved with removal of the offending agent.5 Therefore, drug-triggered BP is more difficult to detect, especially in patients taking multiple medications.
Our patient represents a case of drug-triggered BP to liraglutide. Liraglutide is a GLP-1 receptor agonist that is US Food and Drug Administration approved for the treatment of type 2 diabetes mellitus. Glucagonlike peptide 1 is an incretin hormone that is secreted by the intestine during digestion. It binds to the GLP-1 receptor leading to an increase in glucose-dependent insulin secretion and a decrease in glucagon secretion.6 Glucagonlike peptide 1 agonists also affect the immune system; liraglutide has been shown to modestly improve psoriasis, reduce the number of dermal gamma delta T cells, and decrease IL-17 expression.7 Glucagonlike peptide 1 agonists also produce anti-inflammatory effects on multiple organs including the liver, brain, vasculature, kidney, and skin.8
Dipeptidyl peptidase 4 (DPP-4) inhibitors that function to inhibit the degradation of GLP-1 and other peptides also have been reported to cause BP. In several patients, the DPP-4 inhibitors vildagliptin and sitagliptin caused drug-induced BP that resolved with discontinuation of the medication.9 Dipeptidyl peptidase 4 is expressed in various organ systems including the skin, and inhibition of DPP-4 enhances eosinophil mobilization in the blood and recruitment to the skin in animal models.10
Although the pathogenesis of BP involves autoantibodies to BP antigens 180 and 230, these antibodies are not sufficient to cause disease, as antibasement antibodies have been detected in patients without clinically evident BP. These patients, however, may be more susceptible to developing medication-induced BP. Several hypotheses regarding the pathogenesis of medication-induced BP have been proposed, including immune dysregulation, molecular mimicry, and cross-reactivity to a prior sensitizing agent.5 Liraglutide and the DPP-4 inhibitors affect the immune system, supporting the hypothesis of immune dysregulation; however, the exact mechanism of how immune modulating medications such as GLP-1 agonists and DPP-4 inhibitors cause BP remains unclear.
The effects of liraglutide and the DPP-4 inhibitors on the immune system may play a role in the pathogenesis of drug-triggered BP and drug-induced BP, respectively. Additional studies of the immunomodulatory effects of GLP-1 agonists and DPP-4 inhibitors may help elucidate the pathogenesis of drug-triggered or drug-induced BP.
- Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol. 2014;53:E432-E437.
- Danescu S, Chiorean R, Macovei V, et al. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134-130.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, et al. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58-62.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ. 2015;41(suppl 1):32S-46S.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal gammadelta T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161.
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642.
- Skandalis K, Spirova M, Gaitanis G, et al Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249-253.
- Forssmann U, Stoetzer C, Stephan M, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120-1127.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease that typically affects the elderly, with an incidence of approximately 7 new cases per million.1 The pathogenesis of BP involves autoantibodies to BP antigens 180 and 230 at the dermoepidermal junction. Bullous pemphigoid has been associated with the use of multiple medications; vaccines; and physical damage to the skin, including trauma, radiation, and surgery.2
Several classes of medications may cause BP; one study described an association of BP with loop diuretics,3 while others found higher incidences of BP in patients taking aldosterone antagonists and neuroleptics.4 We describe a case of drug-triggered BP to liraglutide, a glucagonlike peptide 1 (GLP-1) receptor agonist.
A 75-year-old man presented to dermatology for evaluation of a vesicular eruption on the head, neck, trunk, and arms of 6 months’ duration. The eruption developed 2 weeks after starting liraglutide 1.2 mg subcutaneously daily for diabetes mellitus. The patient had a medical history of type 2 diabetes mellitus, hypertension, stroke, and prostate cancer treated with prostatectomy, and he also was taking insulin. Liraglutide was discontinued shortly after the onset of the eruption.
Physical examination revealed annular plaques on the head, neck, trunk, and arms with central hypopigmentation and hyperpigmented borders (Figure 1). Two tense bullae were evident on the left flank (Figure 2). Histopathology revealed a subepidermal blister, mixed perivascular infiltrate with numerous eosinophils, and pigment incontinence (Figure 3). Direct immunofluorescence showed linear deposition of IgG and C3 along the basement membrane zone that was localized to the roof of the blister on salt-split analysis. No microorganisms were identified on periodic acid–Schiff, Grocott-Gomori methenamine-silver, acid-fast bacilli, and Fite stains. The patient initially was treated with clobetasol ointment 0.05%, leading to marginal improvement. He declined treatment with prednisone or dapsone, and he was started on doxycycline. Seven months after stopping liraglutide and starting doxycycline, the patient had no blisters, but residual pigmentary changes remained.
Two types of BP have been described in response to medications: drug-induced BP and drug-triggered BP. Drug-induced BP presents as an acute, self-limited eruption that typically resolves after withdrawal of the offending agent. It tends to involve a younger population and may present with mucosal involvement and target lesions on the palms and soles. Direct immunofluorescence shows linear IgG and C3 deposition at the basement membrane zone. Patients tend to respond quickly to systemic corticosteroids and have low recurrence rates. Drug-triggered BP is a chronic form of BP that is caused by a medication and is not resolved with removal of the offending agent.5 Therefore, drug-triggered BP is more difficult to detect, especially in patients taking multiple medications.
Our patient represents a case of drug-triggered BP to liraglutide. Liraglutide is a GLP-1 receptor agonist that is US Food and Drug Administration approved for the treatment of type 2 diabetes mellitus. Glucagonlike peptide 1 is an incretin hormone that is secreted by the intestine during digestion. It binds to the GLP-1 receptor leading to an increase in glucose-dependent insulin secretion and a decrease in glucagon secretion.6 Glucagonlike peptide 1 agonists also affect the immune system; liraglutide has been shown to modestly improve psoriasis, reduce the number of dermal gamma delta T cells, and decrease IL-17 expression.7 Glucagonlike peptide 1 agonists also produce anti-inflammatory effects on multiple organs including the liver, brain, vasculature, kidney, and skin.8
Dipeptidyl peptidase 4 (DPP-4) inhibitors that function to inhibit the degradation of GLP-1 and other peptides also have been reported to cause BP. In several patients, the DPP-4 inhibitors vildagliptin and sitagliptin caused drug-induced BP that resolved with discontinuation of the medication.9 Dipeptidyl peptidase 4 is expressed in various organ systems including the skin, and inhibition of DPP-4 enhances eosinophil mobilization in the blood and recruitment to the skin in animal models.10
Although the pathogenesis of BP involves autoantibodies to BP antigens 180 and 230, these antibodies are not sufficient to cause disease, as antibasement antibodies have been detected in patients without clinically evident BP. These patients, however, may be more susceptible to developing medication-induced BP. Several hypotheses regarding the pathogenesis of medication-induced BP have been proposed, including immune dysregulation, molecular mimicry, and cross-reactivity to a prior sensitizing agent.5 Liraglutide and the DPP-4 inhibitors affect the immune system, supporting the hypothesis of immune dysregulation; however, the exact mechanism of how immune modulating medications such as GLP-1 agonists and DPP-4 inhibitors cause BP remains unclear.
The effects of liraglutide and the DPP-4 inhibitors on the immune system may play a role in the pathogenesis of drug-triggered BP and drug-induced BP, respectively. Additional studies of the immunomodulatory effects of GLP-1 agonists and DPP-4 inhibitors may help elucidate the pathogenesis of drug-triggered or drug-induced BP.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease that typically affects the elderly, with an incidence of approximately 7 new cases per million.1 The pathogenesis of BP involves autoantibodies to BP antigens 180 and 230 at the dermoepidermal junction. Bullous pemphigoid has been associated with the use of multiple medications; vaccines; and physical damage to the skin, including trauma, radiation, and surgery.2
Several classes of medications may cause BP; one study described an association of BP with loop diuretics,3 while others found higher incidences of BP in patients taking aldosterone antagonists and neuroleptics.4 We describe a case of drug-triggered BP to liraglutide, a glucagonlike peptide 1 (GLP-1) receptor agonist.
A 75-year-old man presented to dermatology for evaluation of a vesicular eruption on the head, neck, trunk, and arms of 6 months’ duration. The eruption developed 2 weeks after starting liraglutide 1.2 mg subcutaneously daily for diabetes mellitus. The patient had a medical history of type 2 diabetes mellitus, hypertension, stroke, and prostate cancer treated with prostatectomy, and he also was taking insulin. Liraglutide was discontinued shortly after the onset of the eruption.
Physical examination revealed annular plaques on the head, neck, trunk, and arms with central hypopigmentation and hyperpigmented borders (Figure 1). Two tense bullae were evident on the left flank (Figure 2). Histopathology revealed a subepidermal blister, mixed perivascular infiltrate with numerous eosinophils, and pigment incontinence (Figure 3). Direct immunofluorescence showed linear deposition of IgG and C3 along the basement membrane zone that was localized to the roof of the blister on salt-split analysis. No microorganisms were identified on periodic acid–Schiff, Grocott-Gomori methenamine-silver, acid-fast bacilli, and Fite stains. The patient initially was treated with clobetasol ointment 0.05%, leading to marginal improvement. He declined treatment with prednisone or dapsone, and he was started on doxycycline. Seven months after stopping liraglutide and starting doxycycline, the patient had no blisters, but residual pigmentary changes remained.
Two types of BP have been described in response to medications: drug-induced BP and drug-triggered BP. Drug-induced BP presents as an acute, self-limited eruption that typically resolves after withdrawal of the offending agent. It tends to involve a younger population and may present with mucosal involvement and target lesions on the palms and soles. Direct immunofluorescence shows linear IgG and C3 deposition at the basement membrane zone. Patients tend to respond quickly to systemic corticosteroids and have low recurrence rates. Drug-triggered BP is a chronic form of BP that is caused by a medication and is not resolved with removal of the offending agent.5 Therefore, drug-triggered BP is more difficult to detect, especially in patients taking multiple medications.
Our patient represents a case of drug-triggered BP to liraglutide. Liraglutide is a GLP-1 receptor agonist that is US Food and Drug Administration approved for the treatment of type 2 diabetes mellitus. Glucagonlike peptide 1 is an incretin hormone that is secreted by the intestine during digestion. It binds to the GLP-1 receptor leading to an increase in glucose-dependent insulin secretion and a decrease in glucagon secretion.6 Glucagonlike peptide 1 agonists also affect the immune system; liraglutide has been shown to modestly improve psoriasis, reduce the number of dermal gamma delta T cells, and decrease IL-17 expression.7 Glucagonlike peptide 1 agonists also produce anti-inflammatory effects on multiple organs including the liver, brain, vasculature, kidney, and skin.8
Dipeptidyl peptidase 4 (DPP-4) inhibitors that function to inhibit the degradation of GLP-1 and other peptides also have been reported to cause BP. In several patients, the DPP-4 inhibitors vildagliptin and sitagliptin caused drug-induced BP that resolved with discontinuation of the medication.9 Dipeptidyl peptidase 4 is expressed in various organ systems including the skin, and inhibition of DPP-4 enhances eosinophil mobilization in the blood and recruitment to the skin in animal models.10
Although the pathogenesis of BP involves autoantibodies to BP antigens 180 and 230, these antibodies are not sufficient to cause disease, as antibasement antibodies have been detected in patients without clinically evident BP. These patients, however, may be more susceptible to developing medication-induced BP. Several hypotheses regarding the pathogenesis of medication-induced BP have been proposed, including immune dysregulation, molecular mimicry, and cross-reactivity to a prior sensitizing agent.5 Liraglutide and the DPP-4 inhibitors affect the immune system, supporting the hypothesis of immune dysregulation; however, the exact mechanism of how immune modulating medications such as GLP-1 agonists and DPP-4 inhibitors cause BP remains unclear.
The effects of liraglutide and the DPP-4 inhibitors on the immune system may play a role in the pathogenesis of drug-triggered BP and drug-induced BP, respectively. Additional studies of the immunomodulatory effects of GLP-1 agonists and DPP-4 inhibitors may help elucidate the pathogenesis of drug-triggered or drug-induced BP.
- Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol. 2014;53:E432-E437.
- Danescu S, Chiorean R, Macovei V, et al. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134-130.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, et al. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58-62.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ. 2015;41(suppl 1):32S-46S.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal gammadelta T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161.
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642.
- Skandalis K, Spirova M, Gaitanis G, et al Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249-253.
- Forssmann U, Stoetzer C, Stephan M, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120-1127.
- Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol. 2014;53:E432-E437.
- Danescu S, Chiorean R, Macovei V, et al. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134-130.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, et al. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58-62.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ. 2015;41(suppl 1):32S-46S.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal gammadelta T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161.
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642.
- Skandalis K, Spirova M, Gaitanis G, et al Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249-253.
- Forssmann U, Stoetzer C, Stephan M, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120-1127.
Practice Points
- Liraglutide and dipeptidyl peptidase 4 inhibitors, medications used in the treatment of diabetes mellitus, may be linked to the development of bullous pemphigoid (BP).
- Further study of the mechanism of action of these medications may lead to improved understanding of the pathogenesis of BP.