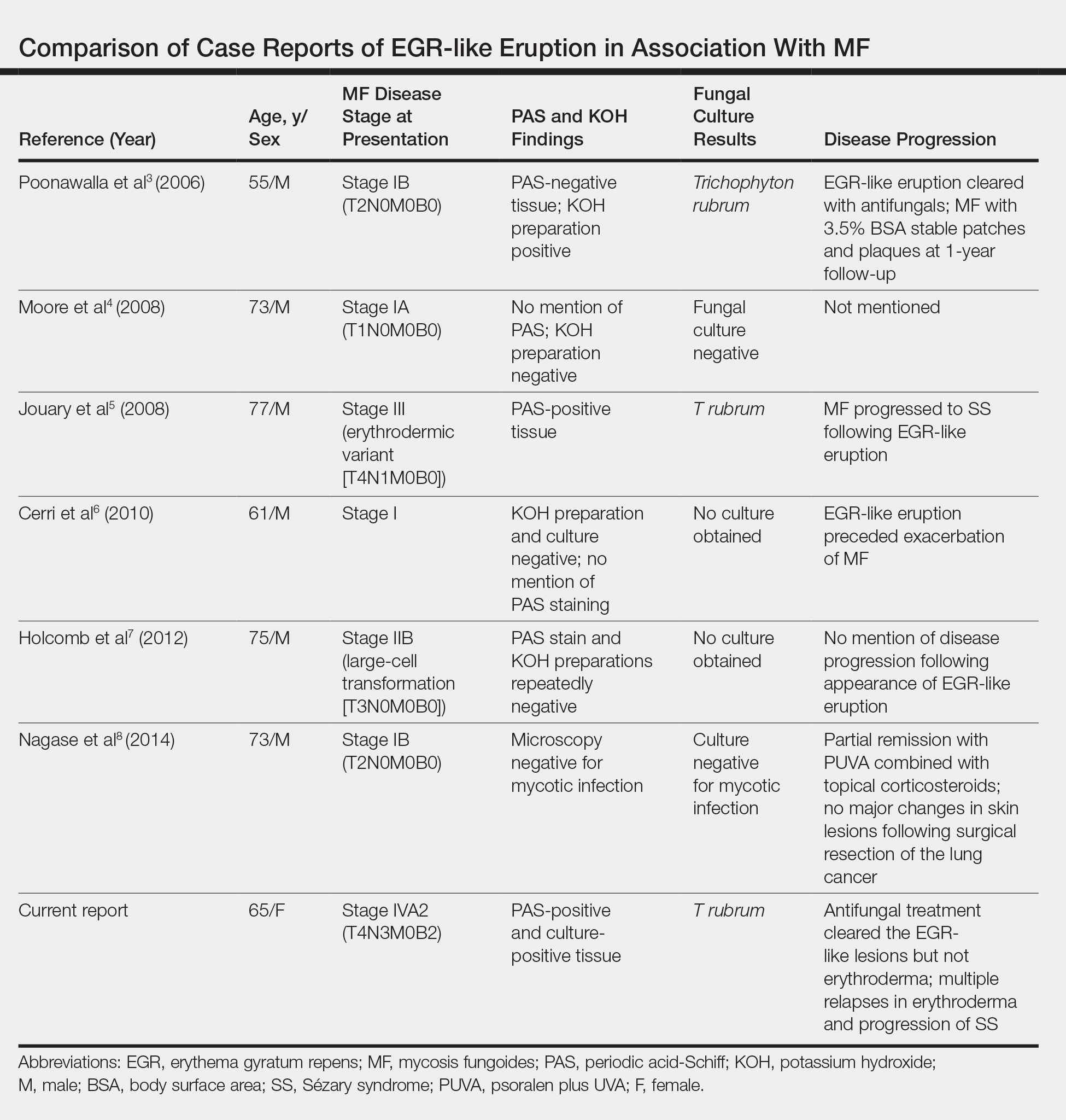
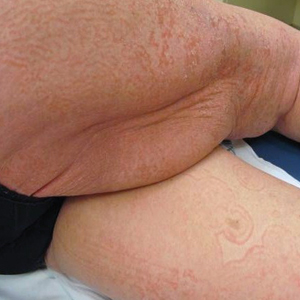
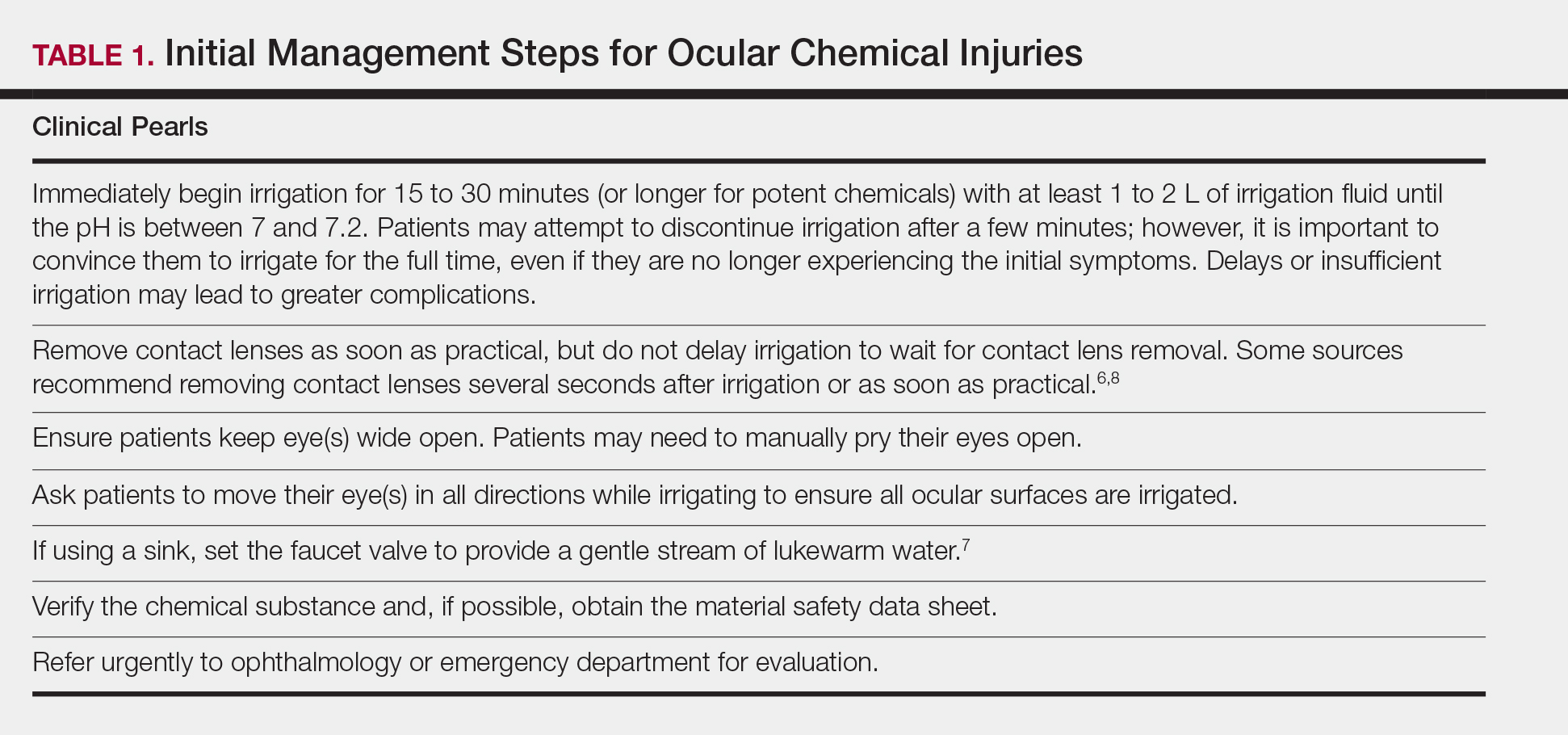
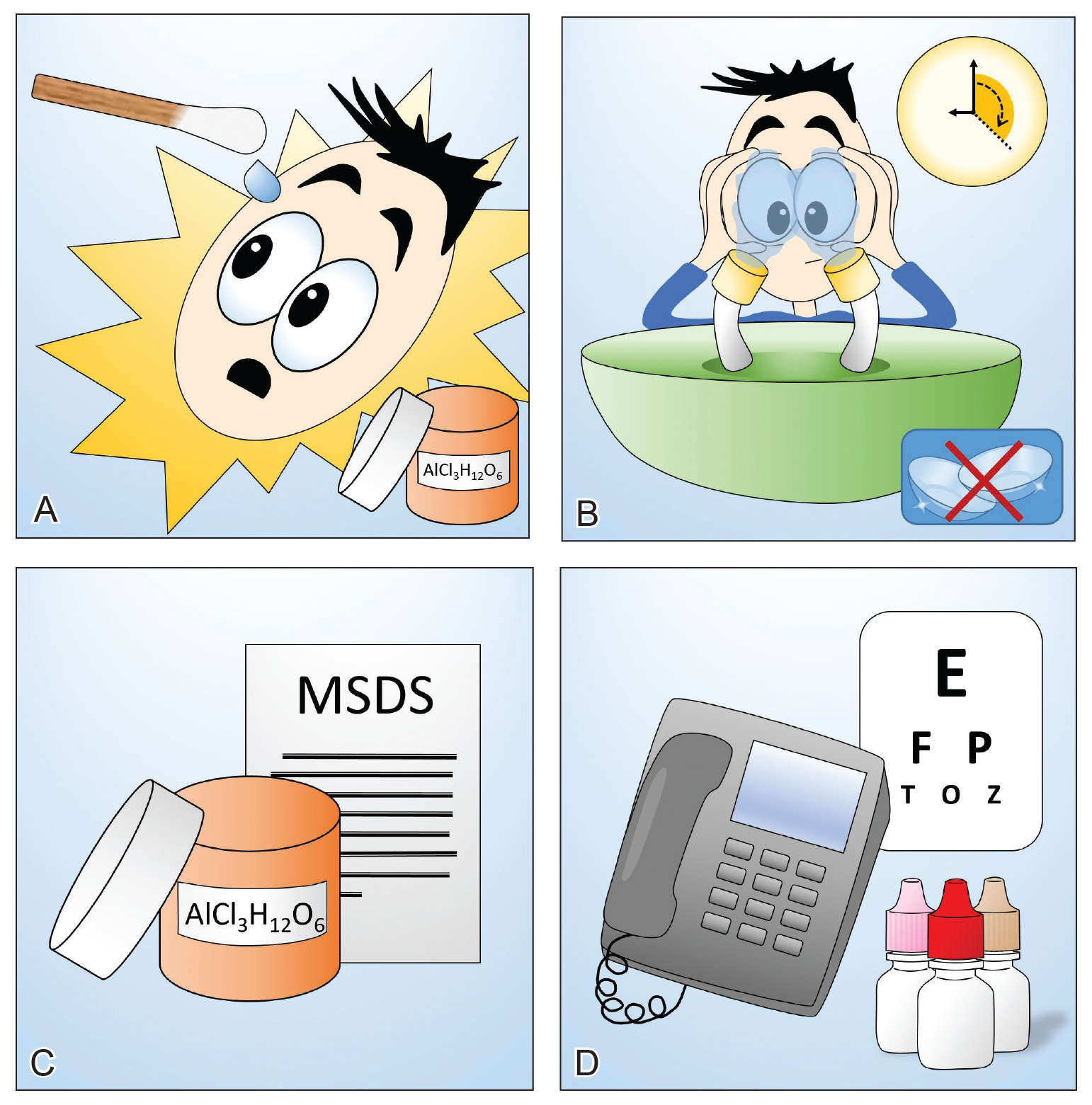
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
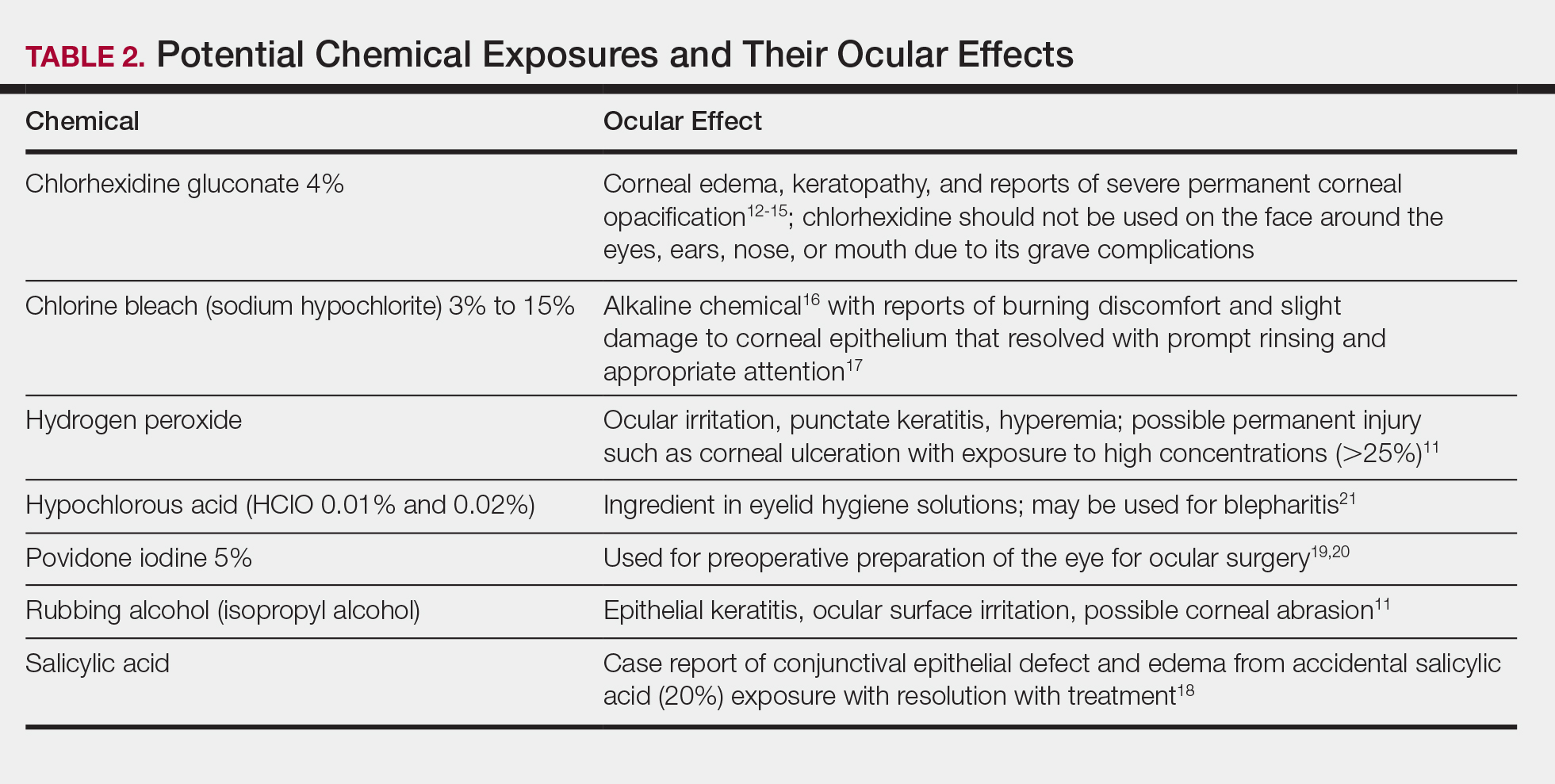
bleach
Boko Haram
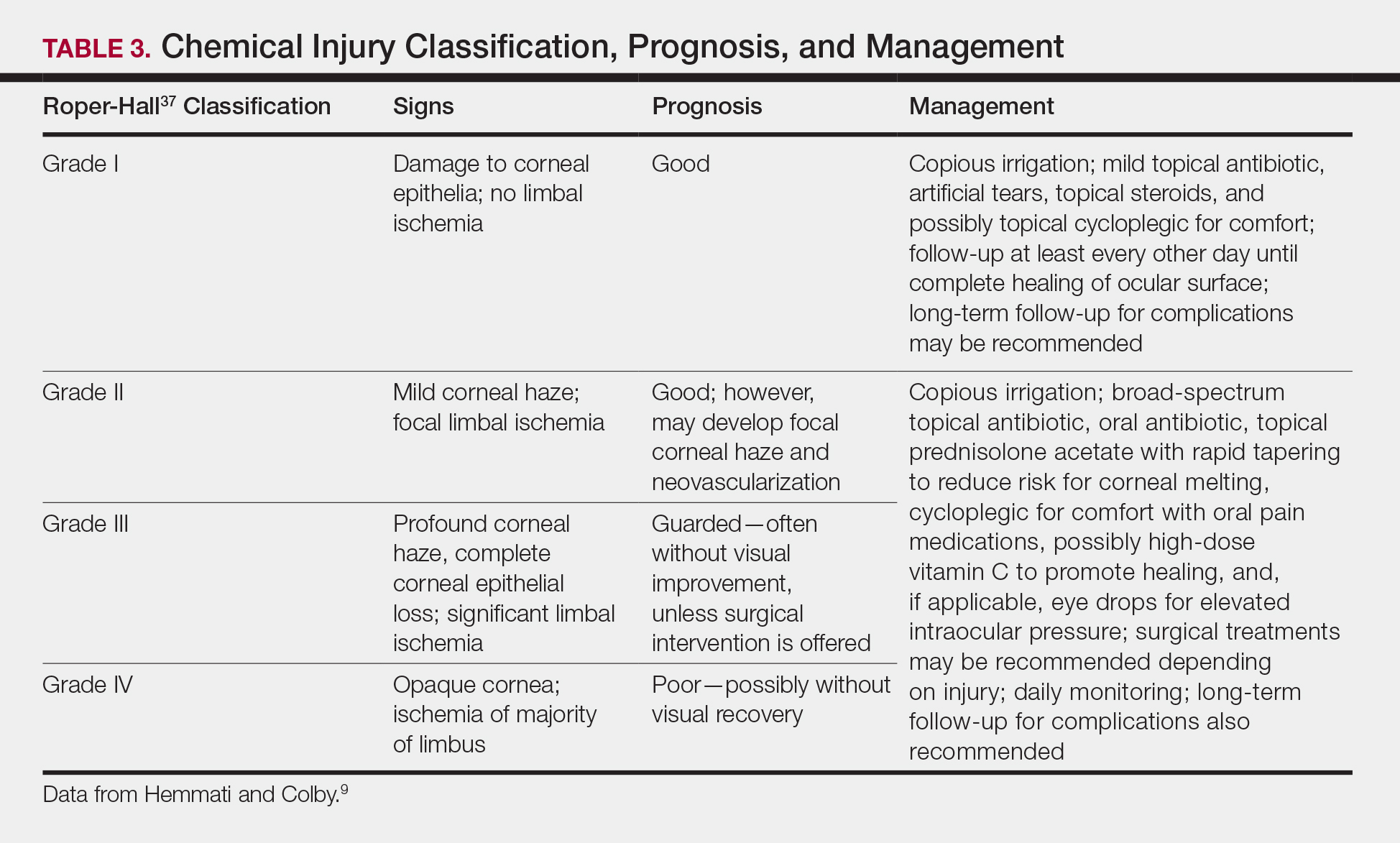
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
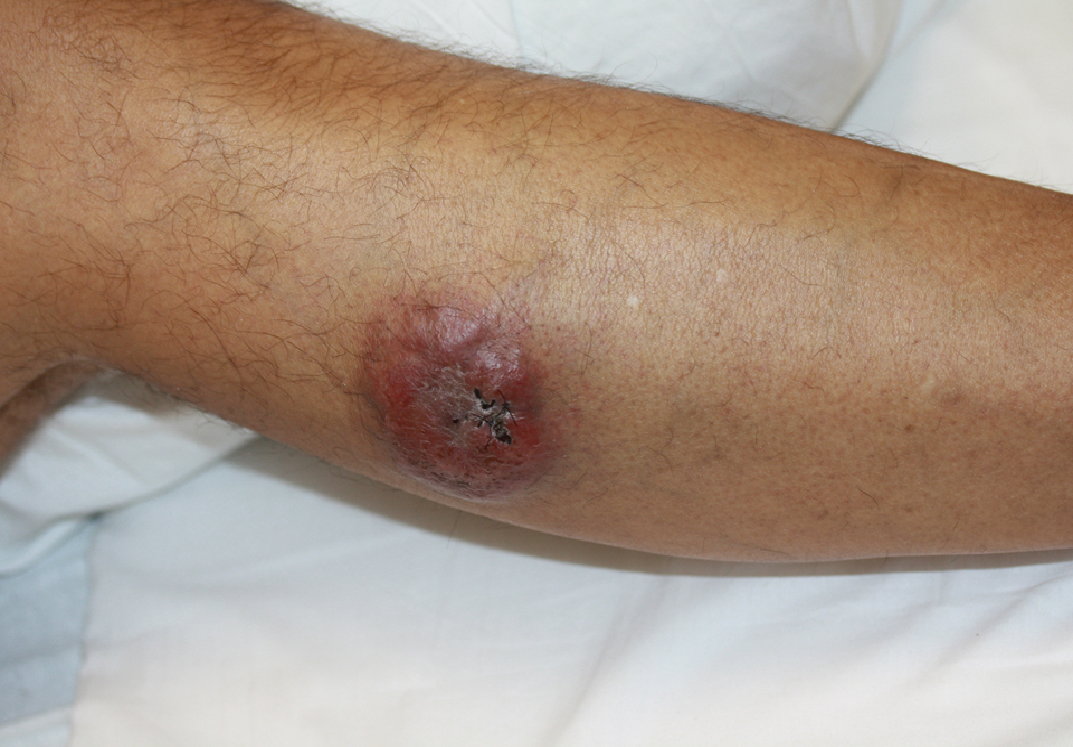
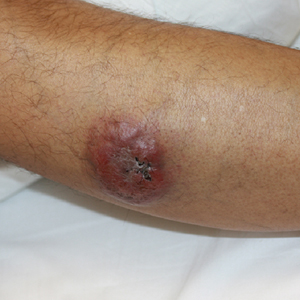
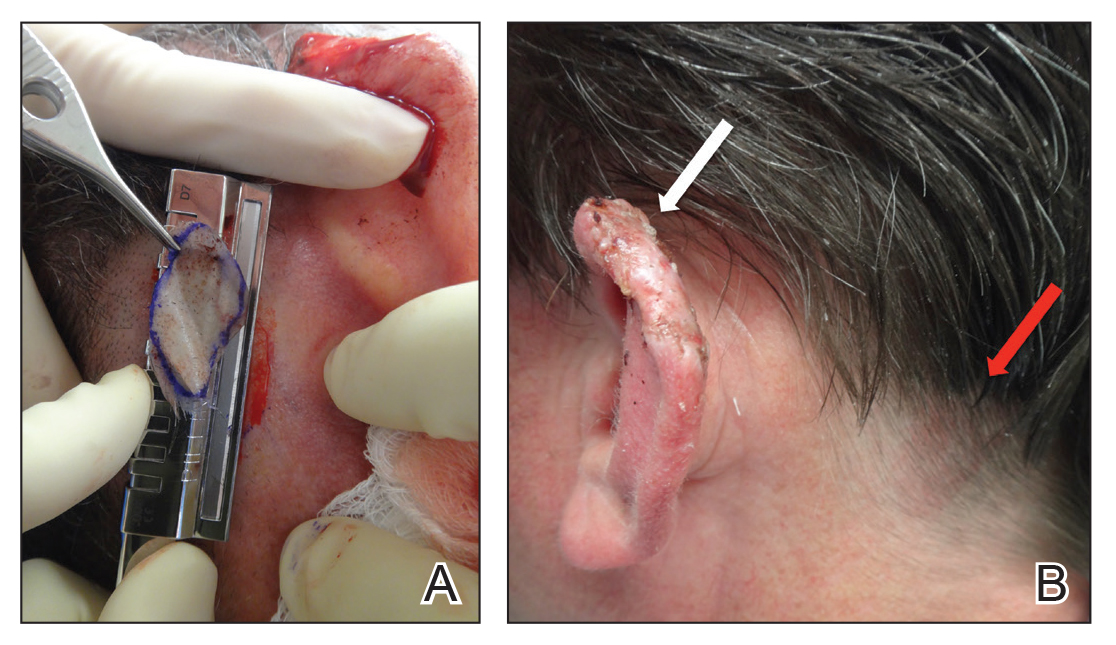
Painless Nodule on the Leg
The Diagnosis: Plasmablastic Lymphoma
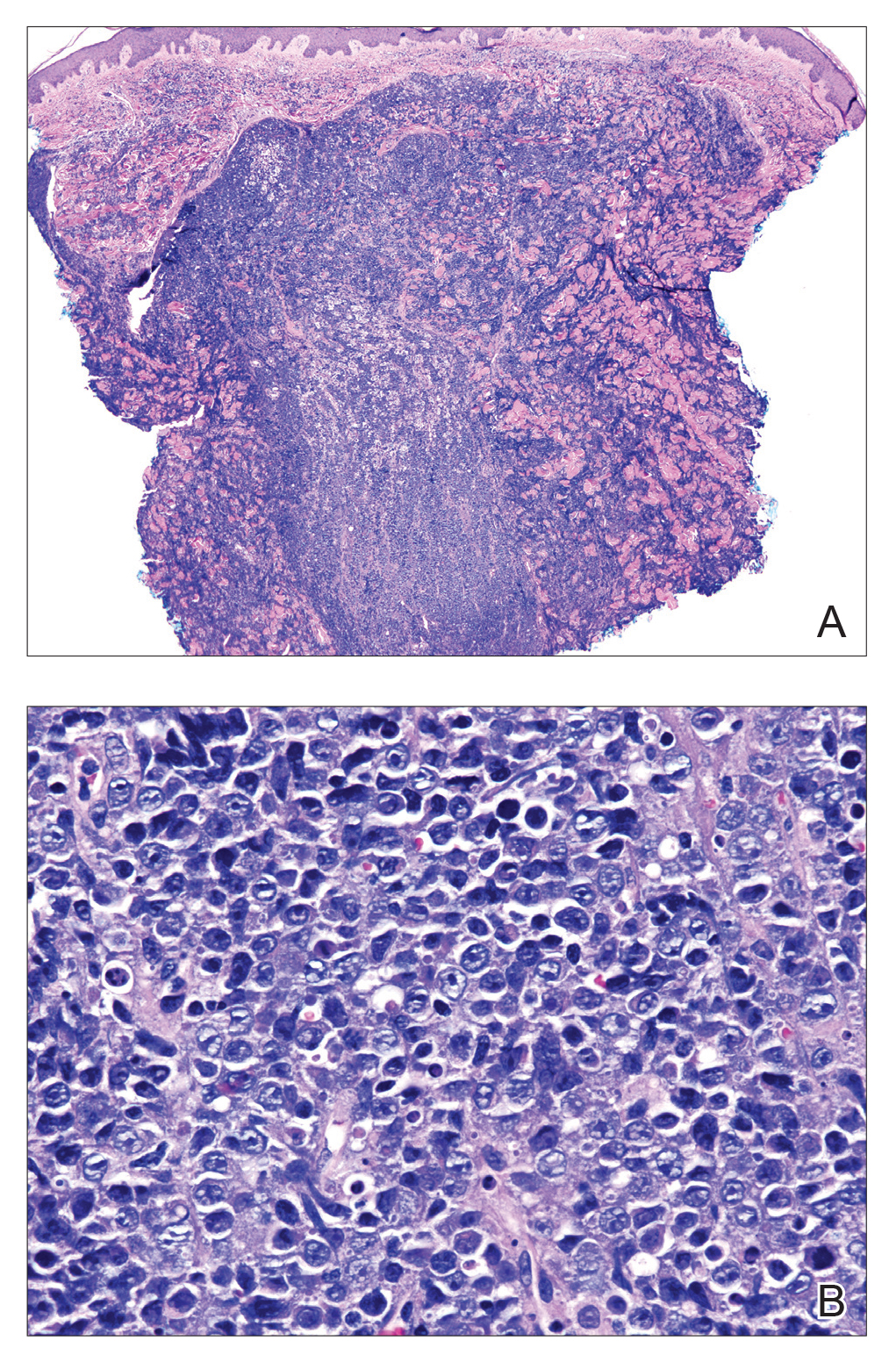
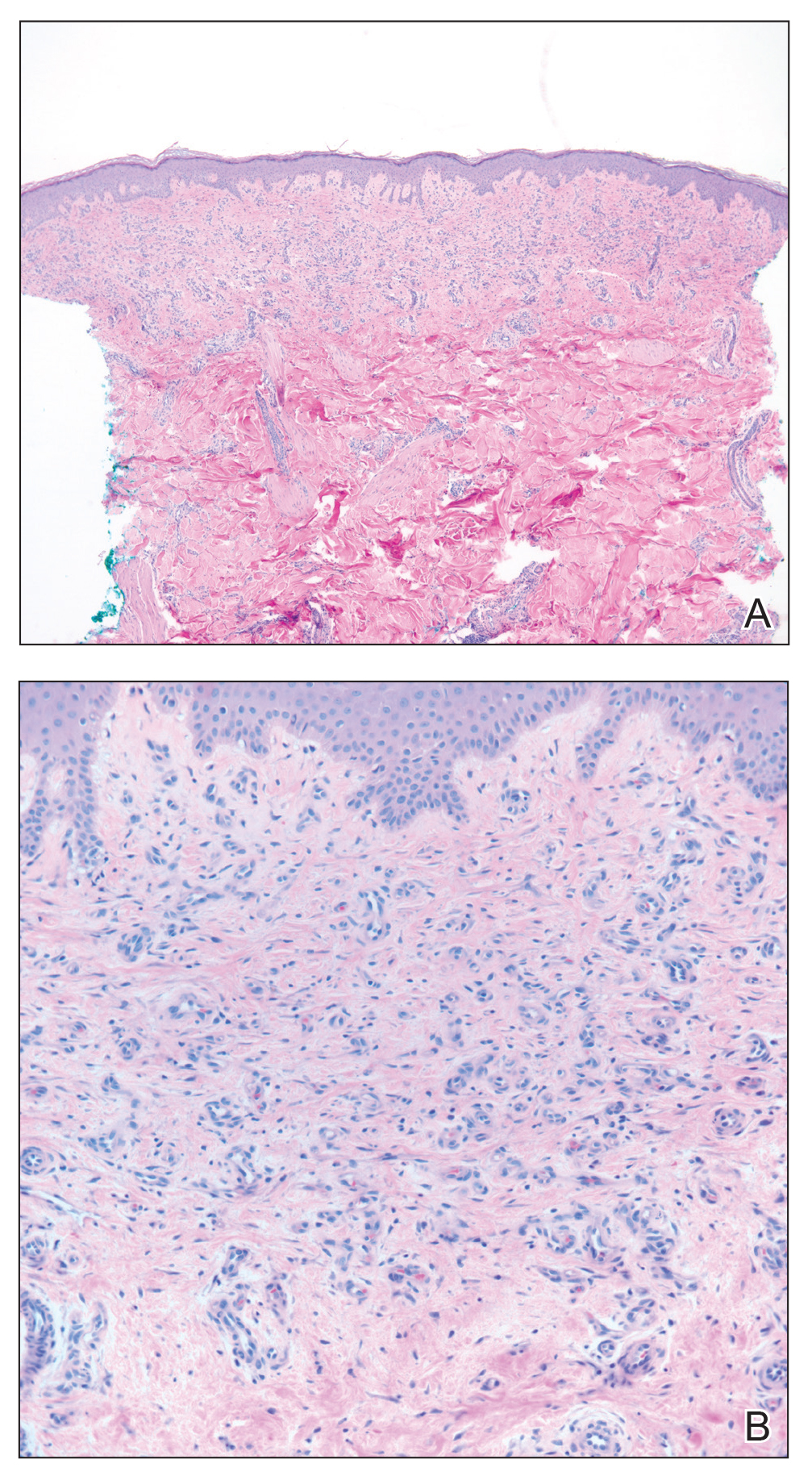
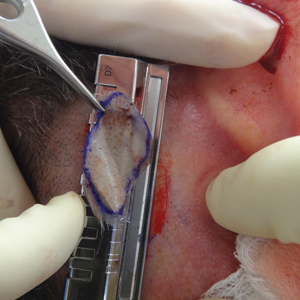
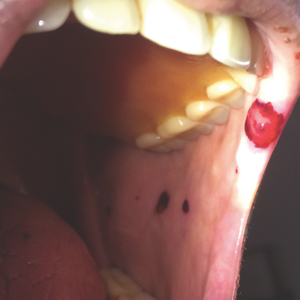
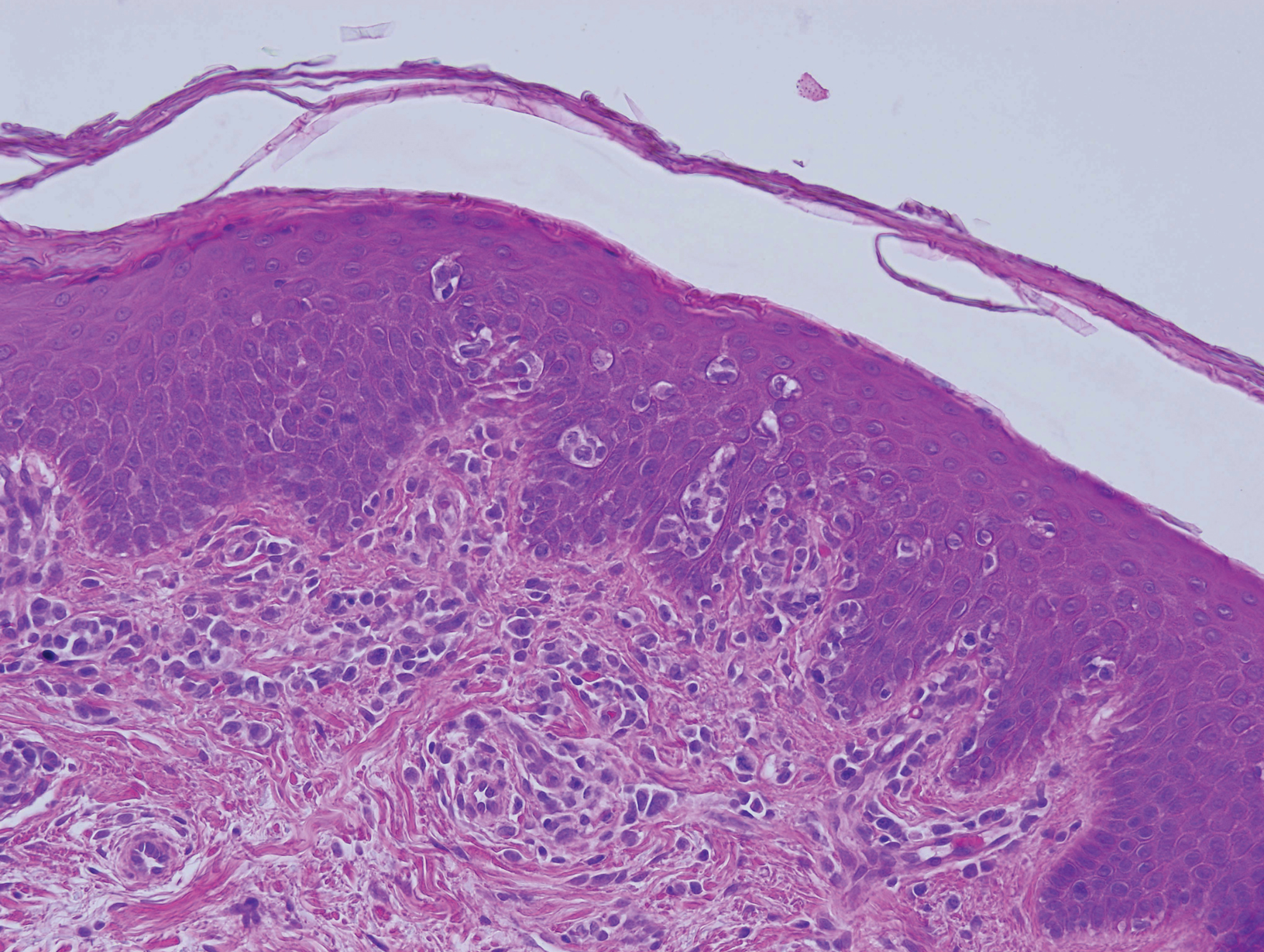
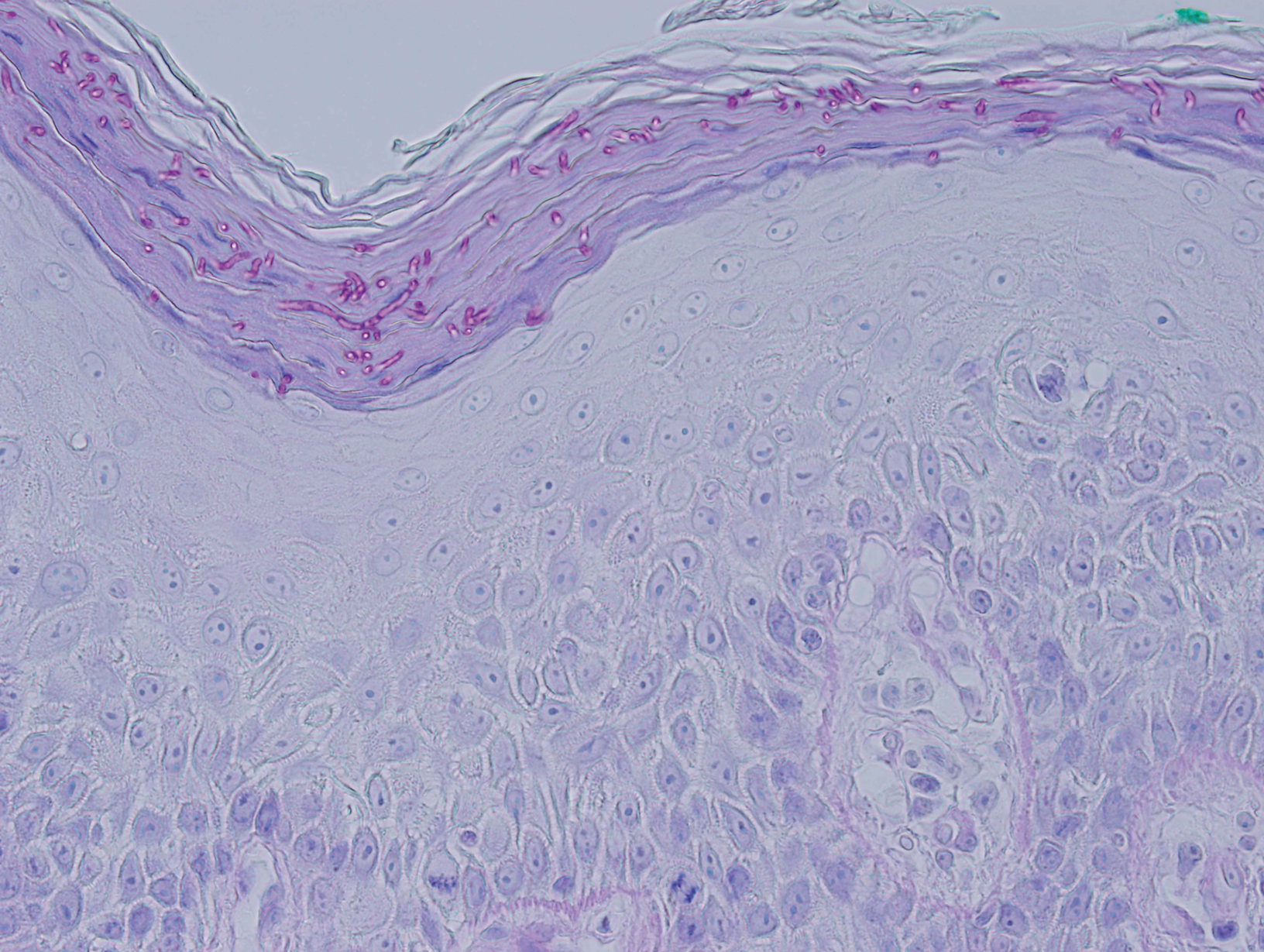
Histopathologic examination revealed a diffuse dense proliferation of large, atypical, and pleomorphic mononuclear cells with prominent nucleoli and many mitotic figures representing plasmacytoid cells in the dermis (Figure). Immunostaining was positive for MUM-1 (marker of late-stage plasma cells and activated T cells) and BCL-2 (antiapoptotic marker). Fluorescent polymerase chain reaction was positive for clonal IgH gene arrangement, and fluorescence in situ hybridization was positive for C-MYC rearrangement in 94% of cells. Epstein-Barr encoding region in situ hybridization also was positive. Rare cells stained positive for T-cell markers. CD20, BCL-6, and CD30 immunostains were negative, suggesting that these cells were not B or T cells, though terminally differentiated B cells also can lack these markers. Bone marrow biopsy showed a similar staining pattern to the skin with 10% atypical plasmacytoid cells. Computed tomography of the left leg showed an enlargement of the semimembranosus muscle with internal areas of high density and heterogeneous enhancement. The patient underwent decompression of the left peroneal nerve. Biopsy showed a staining pattern similar to the right skin nodule and bone marrow, consistent with lymphoma.
He was diagnosed with stage IV human immunodeficiency virus (HIV)-associated plasmablastic lymphoma (PBL) and received 6 cycles of R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) without vincristine with intrathecal methotrexate, followed by 3 cycles of DHAP (dexamethasone, high dose Ara C, cisplatin) with bortezomib and daratumumab after relapse. Ultimately, he underwent autologous stem cell transplantation and was alive 13 months after diagnosis.
Plasmablastic lymphoma is a rare subtype of non-Hodgkin lymphoma that most commonly arises in the oral cavity of individuals with HIV.1 In addition to HIV infection, PBL also is seen in patients with other causes of immunodeficiency such as iatrogenic immunosuppression following solid organ transplantation.1 The typical disease presentation is an expanding mass in the oral cavity; however, 34% (52/151) of reported cases arose at extraoral primary sites, with a minority of cases confined to cutaneous sites with no systemic involvement.2 Cutaneous PBL presentations may include flesh-colored or purple, grouped or solitary nodules; an erythematous infiltrated plaque; or purple-red ulcerated nodules. The lesions usually are asymptomatic and located on the arms and legs.3
On histologic examination, PBL is characterized by a diffuse monomorphic lymphoid infiltrate that sometimes invades the surrounding soft tissue.4-6 The neoplastic cells have eccentric round nucleoli. Plasmablastic lymphoma characteristically displays a high proliferation index with many mitotic figures and signs of apoptosis.4-6 Definitive diagnosis requires immunohistochemical staining. Typical B-cell antigens (CD20) as well as CD45 are negative, while plasma cell markers such as CD38 are positive. Other B- and T-cell markers usually are negative.5,7 The pathogenesis of PBL is thought to be related to Epstein-Barr virus or human herpesvirus 8 infection. In a series of PBL cases, Epstein-Barr virus and human herpesvirus 8 was positive in 75% (97/129) and 17% (13/75) of tested cases, respectively.1
The prognosis for PBL is poor, with a median overall survival of 15 months and a 3-year survival rate of 25% in HIV-infected individuals.8 However, cutaneous PBL without systemic involvement has a considerably better prognosis, with only 1 of 12 cases resulting in death.2,3,9 Treatment of PBL depends on the extent of the disease. Cutaneous PBL can be treated with surgery and adjuvant radiation.3 Chemotherapy is required for patients with multiple lesions or systemic involvement. Current treatment regimens are similar to those used for other aggressive lymphomas such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).1 Transplant recipients should have their immunosuppression reduced, and HIV-infected patients should have their highly active antiretroviral therapy regimens optimized. Patients presenting with PBL without HIV should be tested for HIV, as PBL has previously been reported to be the presenting manifestation of HIV infection.10
The differential diagnosis for a rapidly expanding, vascular-appearing, red mass on the legs in an immunosuppressed individual includes abscess, malignancy, Kaposi sarcoma, Sweet syndrome, and tertiary syphilis.
Acknowledgment
We thank Sameera Husain, MD (New York, New York), for her assistance with histopathologic photographs and interpretation.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267.
- Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol. 2012;67:E202-E205.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420.
- Gaidano G, Cerri M, Capello D, et al. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622-628.
- Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809.
- Horna P, Hamill JR, Sokol L, et al. Primary cutaneous plasmablastic lymphoma in an immunocompetent patient. J Am Acad Dermatol. 2013;69:E274-E276.
- Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358-1361.
The Diagnosis: Plasmablastic Lymphoma
Histopathologic examination revealed a diffuse dense proliferation of large, atypical, and pleomorphic mononuclear cells with prominent nucleoli and many mitotic figures representing plasmacytoid cells in the dermis (Figure). Immunostaining was positive for MUM-1 (marker of late-stage plasma cells and activated T cells) and BCL-2 (antiapoptotic marker). Fluorescent polymerase chain reaction was positive for clonal IgH gene arrangement, and fluorescence in situ hybridization was positive for C-MYC rearrangement in 94% of cells. Epstein-Barr encoding region in situ hybridization also was positive. Rare cells stained positive for T-cell markers. CD20, BCL-6, and CD30 immunostains were negative, suggesting that these cells were not B or T cells, though terminally differentiated B cells also can lack these markers. Bone marrow biopsy showed a similar staining pattern to the skin with 10% atypical plasmacytoid cells. Computed tomography of the left leg showed an enlargement of the semimembranosus muscle with internal areas of high density and heterogeneous enhancement. The patient underwent decompression of the left peroneal nerve. Biopsy showed a staining pattern similar to the right skin nodule and bone marrow, consistent with lymphoma.
He was diagnosed with stage IV human immunodeficiency virus (HIV)-associated plasmablastic lymphoma (PBL) and received 6 cycles of R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) without vincristine with intrathecal methotrexate, followed by 3 cycles of DHAP (dexamethasone, high dose Ara C, cisplatin) with bortezomib and daratumumab after relapse. Ultimately, he underwent autologous stem cell transplantation and was alive 13 months after diagnosis.
Plasmablastic lymphoma is a rare subtype of non-Hodgkin lymphoma that most commonly arises in the oral cavity of individuals with HIV.1 In addition to HIV infection, PBL also is seen in patients with other causes of immunodeficiency such as iatrogenic immunosuppression following solid organ transplantation.1 The typical disease presentation is an expanding mass in the oral cavity; however, 34% (52/151) of reported cases arose at extraoral primary sites, with a minority of cases confined to cutaneous sites with no systemic involvement.2 Cutaneous PBL presentations may include flesh-colored or purple, grouped or solitary nodules; an erythematous infiltrated plaque; or purple-red ulcerated nodules. The lesions usually are asymptomatic and located on the arms and legs.3
On histologic examination, PBL is characterized by a diffuse monomorphic lymphoid infiltrate that sometimes invades the surrounding soft tissue.4-6 The neoplastic cells have eccentric round nucleoli. Plasmablastic lymphoma characteristically displays a high proliferation index with many mitotic figures and signs of apoptosis.4-6 Definitive diagnosis requires immunohistochemical staining. Typical B-cell antigens (CD20) as well as CD45 are negative, while plasma cell markers such as CD38 are positive. Other B- and T-cell markers usually are negative.5,7 The pathogenesis of PBL is thought to be related to Epstein-Barr virus or human herpesvirus 8 infection. In a series of PBL cases, Epstein-Barr virus and human herpesvirus 8 was positive in 75% (97/129) and 17% (13/75) of tested cases, respectively.1
The prognosis for PBL is poor, with a median overall survival of 15 months and a 3-year survival rate of 25% in HIV-infected individuals.8 However, cutaneous PBL without systemic involvement has a considerably better prognosis, with only 1 of 12 cases resulting in death.2,3,9 Treatment of PBL depends on the extent of the disease. Cutaneous PBL can be treated with surgery and adjuvant radiation.3 Chemotherapy is required for patients with multiple lesions or systemic involvement. Current treatment regimens are similar to those used for other aggressive lymphomas such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).1 Transplant recipients should have their immunosuppression reduced, and HIV-infected patients should have their highly active antiretroviral therapy regimens optimized. Patients presenting with PBL without HIV should be tested for HIV, as PBL has previously been reported to be the presenting manifestation of HIV infection.10
The differential diagnosis for a rapidly expanding, vascular-appearing, red mass on the legs in an immunosuppressed individual includes abscess, malignancy, Kaposi sarcoma, Sweet syndrome, and tertiary syphilis.
Acknowledgment
We thank Sameera Husain, MD (New York, New York), for her assistance with histopathologic photographs and interpretation.
The Diagnosis: Plasmablastic Lymphoma
Histopathologic examination revealed a diffuse dense proliferation of large, atypical, and pleomorphic mononuclear cells with prominent nucleoli and many mitotic figures representing plasmacytoid cells in the dermis (Figure). Immunostaining was positive for MUM-1 (marker of late-stage plasma cells and activated T cells) and BCL-2 (antiapoptotic marker). Fluorescent polymerase chain reaction was positive for clonal IgH gene arrangement, and fluorescence in situ hybridization was positive for C-MYC rearrangement in 94% of cells. Epstein-Barr encoding region in situ hybridization also was positive. Rare cells stained positive for T-cell markers. CD20, BCL-6, and CD30 immunostains were negative, suggesting that these cells were not B or T cells, though terminally differentiated B cells also can lack these markers. Bone marrow biopsy showed a similar staining pattern to the skin with 10% atypical plasmacytoid cells. Computed tomography of the left leg showed an enlargement of the semimembranosus muscle with internal areas of high density and heterogeneous enhancement. The patient underwent decompression of the left peroneal nerve. Biopsy showed a staining pattern similar to the right skin nodule and bone marrow, consistent with lymphoma.
He was diagnosed with stage IV human immunodeficiency virus (HIV)-associated plasmablastic lymphoma (PBL) and received 6 cycles of R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) without vincristine with intrathecal methotrexate, followed by 3 cycles of DHAP (dexamethasone, high dose Ara C, cisplatin) with bortezomib and daratumumab after relapse. Ultimately, he underwent autologous stem cell transplantation and was alive 13 months after diagnosis.
Plasmablastic lymphoma is a rare subtype of non-Hodgkin lymphoma that most commonly arises in the oral cavity of individuals with HIV.1 In addition to HIV infection, PBL also is seen in patients with other causes of immunodeficiency such as iatrogenic immunosuppression following solid organ transplantation.1 The typical disease presentation is an expanding mass in the oral cavity; however, 34% (52/151) of reported cases arose at extraoral primary sites, with a minority of cases confined to cutaneous sites with no systemic involvement.2 Cutaneous PBL presentations may include flesh-colored or purple, grouped or solitary nodules; an erythematous infiltrated plaque; or purple-red ulcerated nodules. The lesions usually are asymptomatic and located on the arms and legs.3
On histologic examination, PBL is characterized by a diffuse monomorphic lymphoid infiltrate that sometimes invades the surrounding soft tissue.4-6 The neoplastic cells have eccentric round nucleoli. Plasmablastic lymphoma characteristically displays a high proliferation index with many mitotic figures and signs of apoptosis.4-6 Definitive diagnosis requires immunohistochemical staining. Typical B-cell antigens (CD20) as well as CD45 are negative, while plasma cell markers such as CD38 are positive. Other B- and T-cell markers usually are negative.5,7 The pathogenesis of PBL is thought to be related to Epstein-Barr virus or human herpesvirus 8 infection. In a series of PBL cases, Epstein-Barr virus and human herpesvirus 8 was positive in 75% (97/129) and 17% (13/75) of tested cases, respectively.1
The prognosis for PBL is poor, with a median overall survival of 15 months and a 3-year survival rate of 25% in HIV-infected individuals.8 However, cutaneous PBL without systemic involvement has a considerably better prognosis, with only 1 of 12 cases resulting in death.2,3,9 Treatment of PBL depends on the extent of the disease. Cutaneous PBL can be treated with surgery and adjuvant radiation.3 Chemotherapy is required for patients with multiple lesions or systemic involvement. Current treatment regimens are similar to those used for other aggressive lymphomas such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).1 Transplant recipients should have their immunosuppression reduced, and HIV-infected patients should have their highly active antiretroviral therapy regimens optimized. Patients presenting with PBL without HIV should be tested for HIV, as PBL has previously been reported to be the presenting manifestation of HIV infection.10
The differential diagnosis for a rapidly expanding, vascular-appearing, red mass on the legs in an immunosuppressed individual includes abscess, malignancy, Kaposi sarcoma, Sweet syndrome, and tertiary syphilis.
Acknowledgment
We thank Sameera Husain, MD (New York, New York), for her assistance with histopathologic photographs and interpretation.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267.
- Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol. 2012;67:E202-E205.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420.
- Gaidano G, Cerri M, Capello D, et al. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622-628.
- Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809.
- Horna P, Hamill JR, Sokol L, et al. Primary cutaneous plasmablastic lymphoma in an immunocompetent patient. J Am Acad Dermatol. 2013;69:E274-E276.
- Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358-1361.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267.
- Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol. 2012;67:E202-E205.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420.
- Gaidano G, Cerri M, Capello D, et al. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622-628.
- Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809.
- Horna P, Hamill JR, Sokol L, et al. Primary cutaneous plasmablastic lymphoma in an immunocompetent patient. J Am Acad Dermatol. 2013;69:E274-E276.
- Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358-1361.
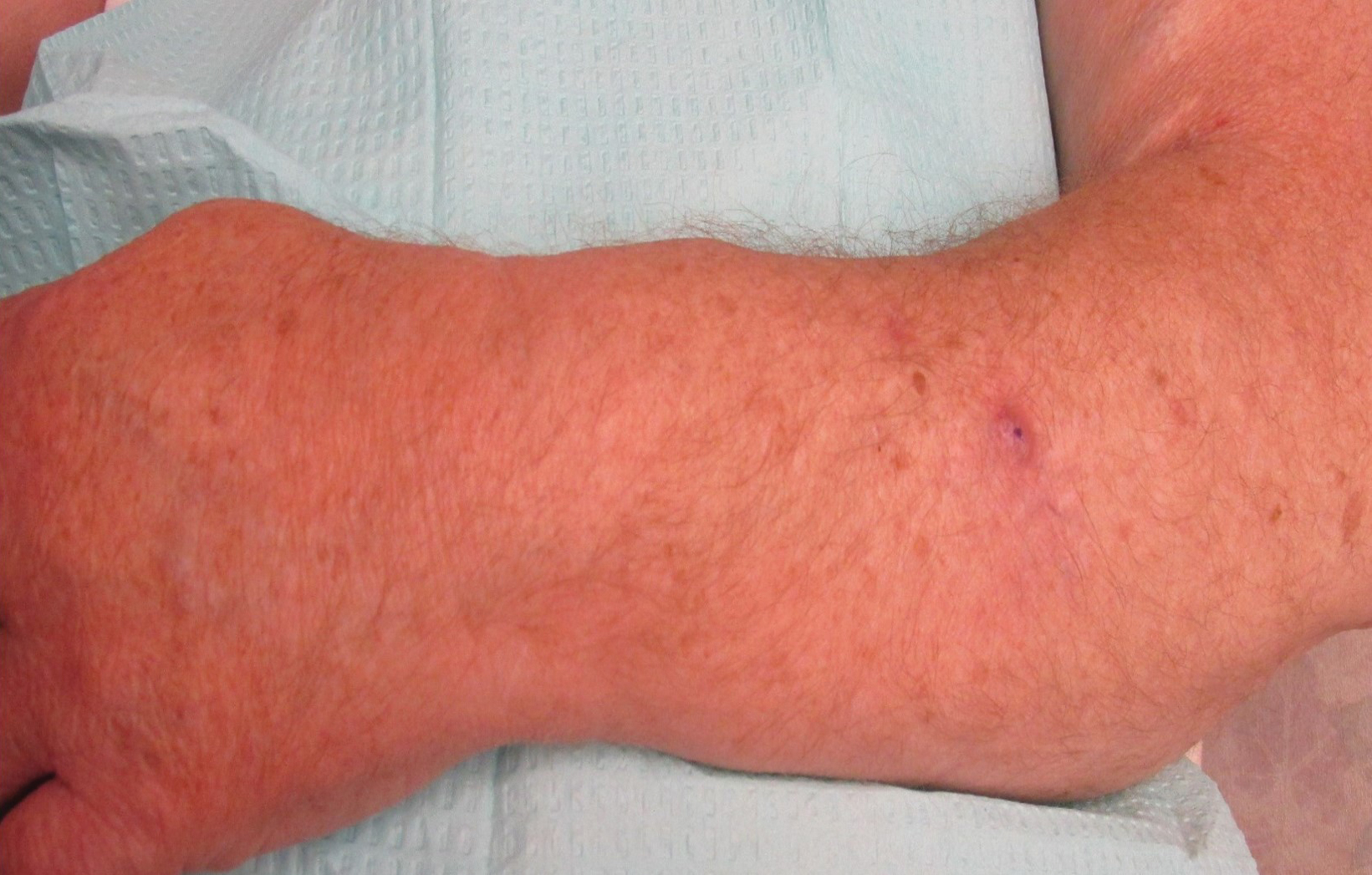
A 44-year-old man presented with numbness and a burning sensation of the left lateral leg and dorsal foot of 3 days' duration as well as a left foot drop of 1 day's duration. A painless red nodule on the right shin also developed over a 10-day period. He had been diagnosed with human immunodeficiency virus a year prior and reported compliance with antiretroviral therapy. There was a newly identified, well-demarcated, 6-cm, round, red-purple, flat-topped, nodular tumor with central depression on the right lateral shin. Ultrasonography of the nodule revealed a heterogeneous septate structure with increased vascularity. There was no regional or generalized lymphadenopathy. Laboratory values were notable for microcytic anemia. The white blood cell count was within reference range. Human immunodeficiency virus RNA viral load was elevated (3183 viral copies/mL [reference range, <20 viral copies/mL]). Two punch biopsies of the nodule were performed.
Acroangiodermatitis of Mali and Stewart-Bluefarb Syndrome
Case Reports
Patient 1
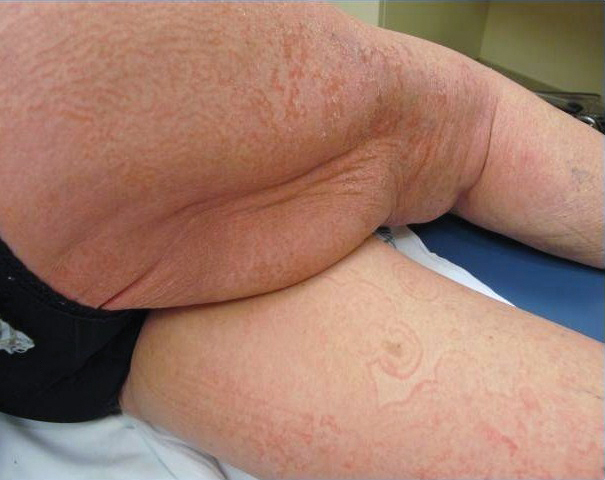
A 56-year-old white man with a history of hypertension, hyperlipidemia, sleep apnea, bilateral knee replacement, and cataract removal presented to the emergency department with a worsening rash on the left posterior medial leg of 6 months’ duration. He reported associated redness and tenderness with the plaques as well as increased swelling and firmness of the leg. He was admitted to the hospital where the infectious disease team treated him with cefazolin for presumed cellulitis. His condition did not improve, and another course of cefazolin was started in addition to oral fluconazole and clotrimazole–betamethasone dipropionate lotion for a possible fungal cause. Again, treatment provided no improvement.
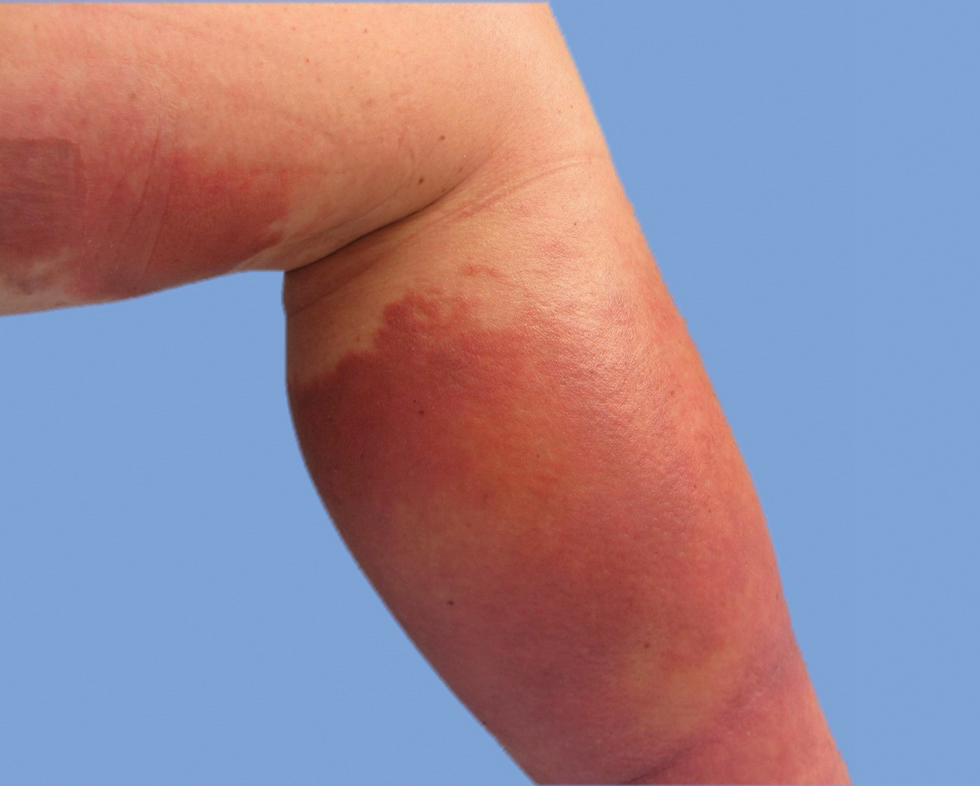
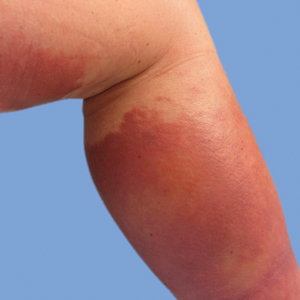
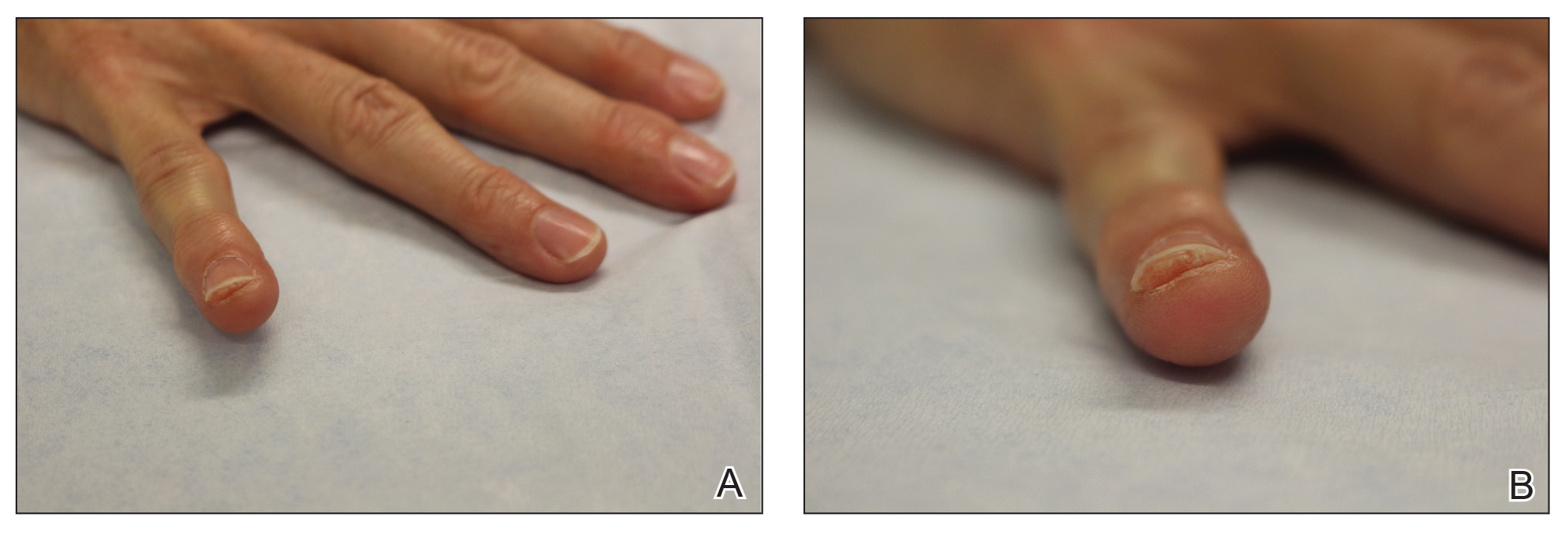
He was then evaluated by dermatology. On physical examination, the patient had edema, warmth, and induration of the left lower leg. There also was an annular and serpiginous indurated plaque with minimal scale on the left lower leg (Figure 1). A firm, dark red to purple plaque on the left medial thigh with mild scale was present. There also was scaling of the right plantar foot.
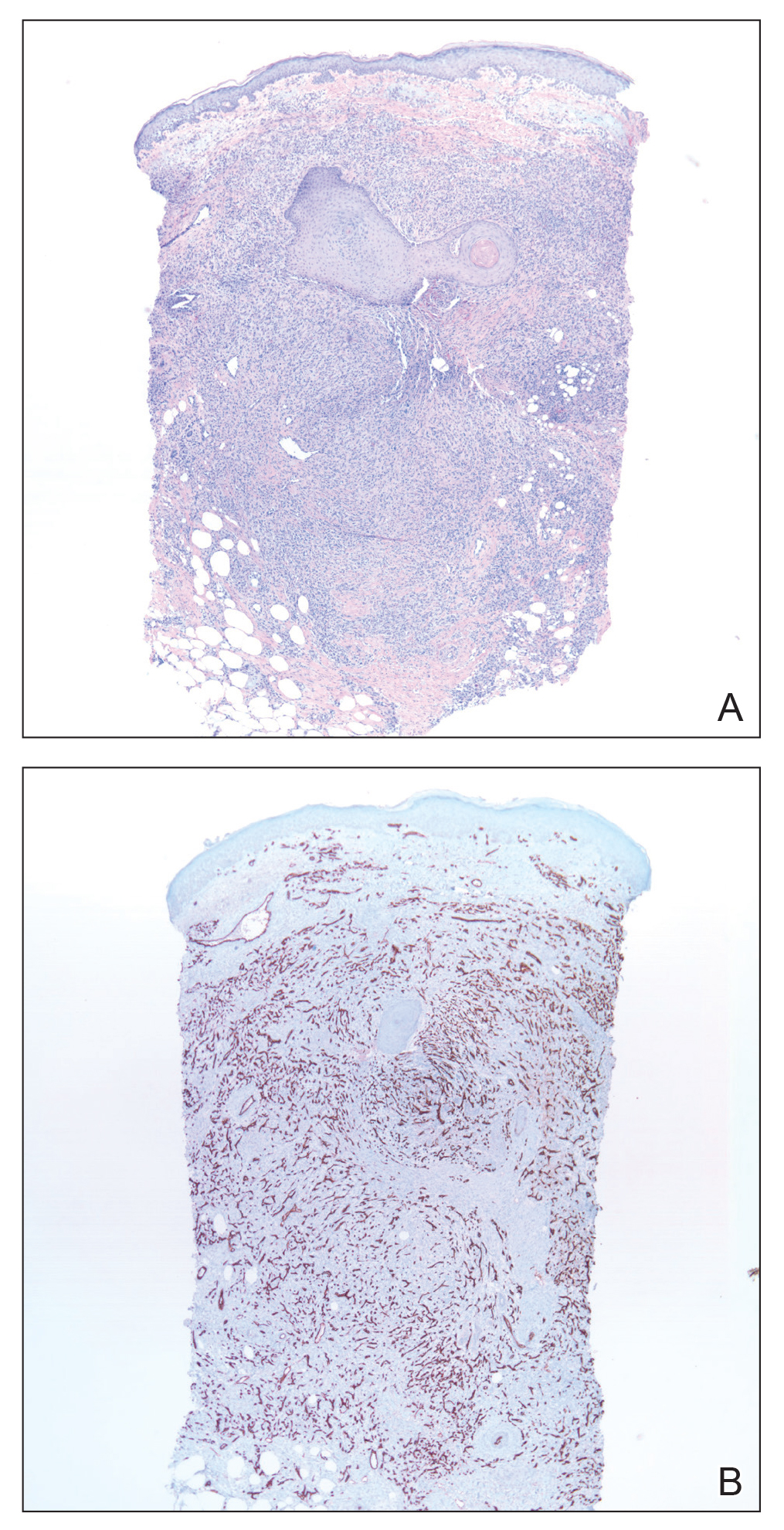
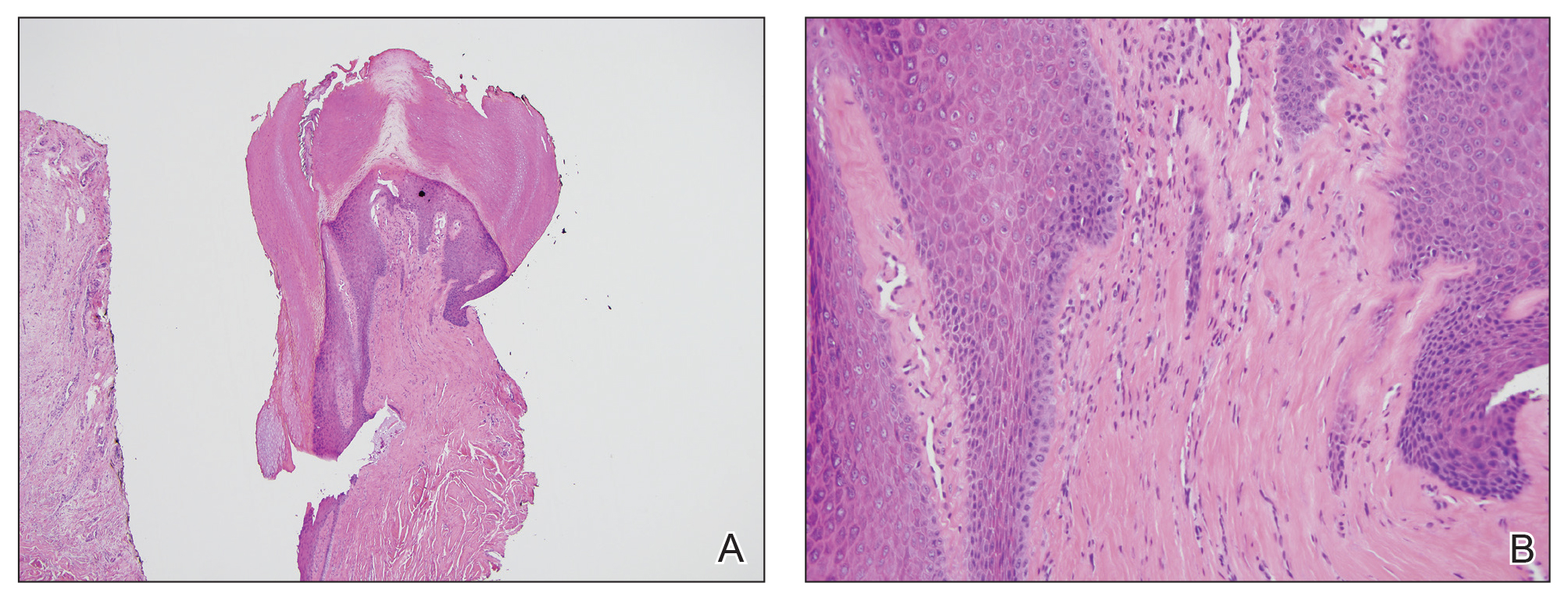
Skin biopsy revealed a dermal capillary proliferation with a scattering of inflammatory cells including eosinophils as well as dermal fibrosis (Figure 2). Periodic acid–Schiff and human herpesvirus 8 (HHV-8) immunostains were negative. Considering the degree and depth of vascular proliferation, Mali-type acroangiodermatitis (AAD) was the favored diagnosis.
Patient 2
A 72-year-old white man presented with a firm asymptomatic growth on the left dorsal forearm of 3 months’ duration. It was located near the site of a prior squamous cell carcinoma that was excised 1 year prior to presentation. The patient had no treatment or biopsy of the presenting lesion. His medical and surgical history included polycystic kidney disease and renal transplantation 4 years prior to presentation. He also had an arteriovenous fistula of the left arm. His other chronic diseases included chronic obstructive lung disease, congestive heart failure, hypertension, type 2 diabetes mellitus, and obstructive sleep apnea.
On physical examination, the patient had a 1-cm violaceous nodule on the extensor surface of the left mid forearm. An arteriovenous fistula was present proximal to the lesion on the left arm (Figure 3).
Skin biopsy revealed a tightly packed proliferation of small vascular channels that tested negative for HHV-8, tumor protein p63, and cytokeratin 5/6. Erythrocytes were noted in the lumen of some of these vessels. Neutrophils were scattered and clustered throughout the specimen (Figure 4A). Blood vessels were highlighted with CD34 (Figure 4B). Grocott-Gomori methenamine-silver stain was negative for infectious agents. These findings favored AAD secondary to an arteriovenous malformation, consistent with Stewart-Bluefarb syndrome (SBS).
Comment
Presentation of AAD
Acroangiodermatitis is a rare chronic inflammatory skin process involving a reactive proliferation of capillaries and fibrosis of the skin that resembles Kaposi sarcoma both clinically and histopathologically. The condition has been reported in patients with chronic venous insufficiency,1 congenital arteriovenous malformation,2 acquired iatrogenic arteriovenous fistula,3 paralyzed extremity,4 suction socket lower limb prosthesis (amputees),5 and minor trauma.6-8 The lesions of AAD tend to be circumscribed, slowly evolving, red-violaceous (or brown or dusky) macules, papules, or plaques that may become verrucous or develop into painful ulcerations. They generally occur on the distal dorsal aspects of the lower legs and feet.110
Variants of AAD
Mali et al9 first reported cutaneous manifestations resembling Kaposi sarcoma in 18 patients with chronic venous insufficiency in 1965. Two years later, Bluefarb and Adams10 described kaposiform skin lesions in one patient with a congenital arteriovenous malformation without chronic venous insufficiency. It was not until 1974, however, that Earhart et al11 proposed the term pseudo-Kaposi sarcoma.10,11 Based on these findings, AAD is described as 2 variants: Mali type and SBS.
Mali-type AAD is more common and typically occurs in elderly men. It classically presents bilaterally on the lower extremities in association with severe chronic venous insufficiency.5 Skin lesions usually occur on the medial aspect of the lower legs (as in patient 1), dorsum of the heel, hallux, or second toe.12
The etiology of Mali-type AAD is poorly understood. The leading theory is that the condition involves reduced perfusion due to chronic edema, resulting in neovascularization, fibroblast proliferation, hypertrophy, and inflammatory skin changes. When AAD occurs in the setting of a suction socket prosthesis, the negative pressure of the stump-socket environment is thought to alter local circulation, leading to proliferation of small blood vessels.5,13
Stewart-Bluefarb syndrome usually involves a single extremity in young adults with congenital arteriovenous malformations, amputees, and individuals with hemiplegia or iatrogenic arteriovenous fistulae (as in patient 2).1 It was once thought to occur secondary to Klippel-Trenaunay-Weber syndrome; however, SBS rarely is accompanied by limb hypertrophy.9 Pathogenesis is thought to involve an angiogenic response to a high perfusion rate and high oxygen saturation, which leads to fibroblast proliferation and reactive endothelial hyperplasia.1,14
Diagnosis and Differential Diagnosis
Prompt identification of an underlying arteriovenous anomaly is critical, given the sequelae of high-flow shunts, which may result in skin ulceration, limb length discrepancy, cortical thinning of bone with regional osteoporosis, and congestive heart failure.1,5 Duplex ultrasonography is the first-line diagnostic modality because it is noninvasive and widely available. The key doppler feature of an arteriovenous malformation is low resistance and high diastolic pulsatile flow,1 which should be confirmed with magnetic resonance angiography or computed tomography angiography if present on ultrasonography.
The differential diagnosis of AAD includes Kaposi sarcoma, reactive angioendotheliomatosis, diffuse dermal angiomatosis, intravascular histiocytosis, glomeruloid angioendotheliomatosis, and angiopericytomatosis.15,16 These entities present as multiple erythematous, violaceous, purpuric patches and plaques generally on the extremities but can have a widely varied distribution. Some lesions evolve to necrosis or ulceration. Histopathologic analysis is useful to differentiate these entities.
Histopathology
The histopathologic features of AAD can be nonspecific; clinicopathologic correlation often is necessary to establish the diagnosis. Features include a proliferation of small thick-walled vessels, often in a lobular arrangement, in an edematous papillary dermis. Small thrombi may be observed. There may be increased fibroblasts; plump endothelial cells; a superficial mixed infiltrate comprised of lymphocytes, histiocytes, and eosinophils; and deposition of hemosiderin.2,5 These characteristics overlap with features of Kaposi sarcoma; AAD, however, lacks slitlike vascular spaces, perivascular CD34+ expression, and nuclear atypia. A negative HHV-8 stain will assist in ruling out Kaposi sarcoma.1,17
Management
Treatment reports are anecdotal. The goal is to correct underlying venous hypertension. Conservative measures with compression garments, intermittent pneumatic compression, and limb elevation are first line.18 Oral antibiotics and local wound care with topical emollients and corticosteroids have been shown to be effective treatments.19-21
Oral erythromycin 500 mg 4 times daily for 3 weeks and clobetasol propionate cream 0.05% healed a lower extremity ulcer in a patient with Mali-type AAD.21 In another patient, conservative treatment of Mali-type AAD failed, but rapid improvement of 2 lower extremity ulcers resulted after 3 weeks of oral dapsone 50 mg twice daily.22
Conclusion
Acroangiodermatitis is a rare entity that is characterized by erythematous violaceous papules and plaques of the extremities, commonly in the setting of chronic venous insufficiency or an arteriovenous shunt. Histopathologic analysis shows proliferation of capillaries with fibrosis, extravasation of erythrocytes, and deposition of hemosiderin without the spindle cells and slitlike vascular spaces characteristic of Kaposi sarcoma. Detection of an underlying arteriovenous malformation is essential, as the disease can have local and systemic consequences, such as skin ulceration and congestive heart failure.1 Treatment options are conservative, directed toward local wound care, compression, and management of complications, such as ulceration and infection, as well as obliterating any underlying arteriovenous malformation.
- Parsi K, O’Connor AA, Bester L. Stewart-Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514.
- Larralde M, Gonzalez V, Marietti R, et al. Pseudo-Kaposi sarcoma with arteriovenous malformation. Pediatr Dermatol. 2001;18:325-327.
- Nakanishi G, Tachibana T, Soga H, et al. Pseudo-Kaposi’s sarcoma of the hand associated with acquired iatrogenic arteriovenous fistula. Indian J Dermatol. 2014;59:415-416.
- Landthaler M, Langehenke H, Holzmann H, et al. Mali’s acroangiodermatitis (pseudo-Kaposi) in paralyzed legs. Hautarzt. 1988;39:304-307.
- Trindade F, Requena L. Pseudo-Kaposi’s sarcoma because of suction socket lower limb prosthesis. J Cutan Pathol. 2009;36:482-485.
- Yu-Lu W, Tao Q, Hong-Zhong J, et al. Non-tender pedal plaques and nodules: pseudo-Kaposi’s sarcoma (Stewart-Bluefarb type) induced by trauma. J Dtsch Dermatol Ges. 2015;13:927-930.
- Del-Río E, Aguilar A, Ambrojo P, et al. Pseudo-Kaposi sarcoma induced by minor trauma in a patient with Klippel-Trenaunay-Weber syndrome. Clin Exp Dermatol. 1993;18:151-153.
- Archie M, Khademi S, Aungst D, et al. A rare case of acroangiodermatitis associated with a congenital arteriovenous malformation (Stewart-Bluefarb Syndrome) in a young veteran: case report and review of the literature. Ann Vasc Surg. 2015;29:1448.e5-1448.e10.
- Mali JW, Kuiper JP, Hamers AA. Acro-angiodermatitis of the foot. Arch Dermatol. 1965;92:515-518.
- Bluefarb SM, Adams LA. Arteriovenous malformation with angiodermatitis. stasis dermatitis simulating Kaposi’s disease. Arch Dermatol. 1967;96:176-181.
- Earhart RN, Aeling JA, Nuss DD, et al. Pseudo-Kaposi sarcoma. A patient with arteriovenous malformation and skin lesions simulating Kaposi sarcoma. Arch Dermatol. 1974;110:907-910.
- Lugovic´ L, Pusic´ J, Situm M, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma): three case reports. Acta Dermatovenerol Croat. 2007;15:152-157.
- Horiguchi Y, Takahashi K, Tanizaki H, et al. Case of bilateral acroangiodermatitis due to symmetrical arteriovenous fistulas of the soles. J Dermatol. 2015;42:989-991.
- Dog˘an S, Boztepe G, Karaduman A. Pseudo-Kaposi sarcoma: a challenging vascular phenomenon. Dermatol Online J. 2007;13:22.
- Mazloom SE, Stallings A, Kyei A. Differentiating intralymphatic histiocytosis, intravascular histiocytosis, and subtypes of reactive angioendotheliomatosis: review of clinical and histologic features of all cases reported to date. Am J Dermatopathol. 2017;39:33-39.
- Rongioletti F, Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Kanitakis J, Narvaez D, Claudy A. Expression of the CD34 antigen distinguishes Kaposi’s sarcoma from pseudo-Kaposi’s sarcoma (acroangiodermatitis). Br J Dermatol. 1996;134:44-46.
- Pires A, Depairon M, Ricci C, et al. Effect of compression therapy on a pseudo-Kaposi sarcoma. Dermatology. 1999;198:439-441.
- Hayek S, Atiyeh B, Zgheib E. Stewart-Bluefarb syndrome: review of the literature and case report of chronic ulcer treatment with heparan sulphate (Cacipliq20®). Int Wound J. 2015;12:169-172.
- Varyani N, Thukral A, Kumar N, et al. Nonhealing ulcer: acroangiodermatitis of Mali. Case Rep Dermatol Med. 2011;2011:909383.
- Mehta AA, Pereira RR, Nayak C, et al. Acroangiodermatitis of Mali: a rare vascular phenomenon. Indian J Dermatol Venereol Leprol. 2010;76:553-556.
- Rashkovsky I, Gilead L, Schamroth J, et al. Acro-angiodermatitis: review of the literature and report of a case. Acta Derm Venereol. 1995;75:475-478.
Case Reports
Patient 1
A 56-year-old white man with a history of hypertension, hyperlipidemia, sleep apnea, bilateral knee replacement, and cataract removal presented to the emergency department with a worsening rash on the left posterior medial leg of 6 months’ duration. He reported associated redness and tenderness with the plaques as well as increased swelling and firmness of the leg. He was admitted to the hospital where the infectious disease team treated him with cefazolin for presumed cellulitis. His condition did not improve, and another course of cefazolin was started in addition to oral fluconazole and clotrimazole–betamethasone dipropionate lotion for a possible fungal cause. Again, treatment provided no improvement.
He was then evaluated by dermatology. On physical examination, the patient had edema, warmth, and induration of the left lower leg. There also was an annular and serpiginous indurated plaque with minimal scale on the left lower leg (Figure 1). A firm, dark red to purple plaque on the left medial thigh with mild scale was present. There also was scaling of the right plantar foot.
Skin biopsy revealed a dermal capillary proliferation with a scattering of inflammatory cells including eosinophils as well as dermal fibrosis (Figure 2). Periodic acid–Schiff and human herpesvirus 8 (HHV-8) immunostains were negative. Considering the degree and depth of vascular proliferation, Mali-type acroangiodermatitis (AAD) was the favored diagnosis.
Patient 2
A 72-year-old white man presented with a firm asymptomatic growth on the left dorsal forearm of 3 months’ duration. It was located near the site of a prior squamous cell carcinoma that was excised 1 year prior to presentation. The patient had no treatment or biopsy of the presenting lesion. His medical and surgical history included polycystic kidney disease and renal transplantation 4 years prior to presentation. He also had an arteriovenous fistula of the left arm. His other chronic diseases included chronic obstructive lung disease, congestive heart failure, hypertension, type 2 diabetes mellitus, and obstructive sleep apnea.
On physical examination, the patient had a 1-cm violaceous nodule on the extensor surface of the left mid forearm. An arteriovenous fistula was present proximal to the lesion on the left arm (Figure 3).
Skin biopsy revealed a tightly packed proliferation of small vascular channels that tested negative for HHV-8, tumor protein p63, and cytokeratin 5/6. Erythrocytes were noted in the lumen of some of these vessels. Neutrophils were scattered and clustered throughout the specimen (Figure 4A). Blood vessels were highlighted with CD34 (Figure 4B). Grocott-Gomori methenamine-silver stain was negative for infectious agents. These findings favored AAD secondary to an arteriovenous malformation, consistent with Stewart-Bluefarb syndrome (SBS).
Comment
Presentation of AAD
Acroangiodermatitis is a rare chronic inflammatory skin process involving a reactive proliferation of capillaries and fibrosis of the skin that resembles Kaposi sarcoma both clinically and histopathologically. The condition has been reported in patients with chronic venous insufficiency,1 congenital arteriovenous malformation,2 acquired iatrogenic arteriovenous fistula,3 paralyzed extremity,4 suction socket lower limb prosthesis (amputees),5 and minor trauma.6-8 The lesions of AAD tend to be circumscribed, slowly evolving, red-violaceous (or brown or dusky) macules, papules, or plaques that may become verrucous or develop into painful ulcerations. They generally occur on the distal dorsal aspects of the lower legs and feet.110
Variants of AAD
Mali et al9 first reported cutaneous manifestations resembling Kaposi sarcoma in 18 patients with chronic venous insufficiency in 1965. Two years later, Bluefarb and Adams10 described kaposiform skin lesions in one patient with a congenital arteriovenous malformation without chronic venous insufficiency. It was not until 1974, however, that Earhart et al11 proposed the term pseudo-Kaposi sarcoma.10,11 Based on these findings, AAD is described as 2 variants: Mali type and SBS.
Mali-type AAD is more common and typically occurs in elderly men. It classically presents bilaterally on the lower extremities in association with severe chronic venous insufficiency.5 Skin lesions usually occur on the medial aspect of the lower legs (as in patient 1), dorsum of the heel, hallux, or second toe.12
The etiology of Mali-type AAD is poorly understood. The leading theory is that the condition involves reduced perfusion due to chronic edema, resulting in neovascularization, fibroblast proliferation, hypertrophy, and inflammatory skin changes. When AAD occurs in the setting of a suction socket prosthesis, the negative pressure of the stump-socket environment is thought to alter local circulation, leading to proliferation of small blood vessels.5,13
Stewart-Bluefarb syndrome usually involves a single extremity in young adults with congenital arteriovenous malformations, amputees, and individuals with hemiplegia or iatrogenic arteriovenous fistulae (as in patient 2).1 It was once thought to occur secondary to Klippel-Trenaunay-Weber syndrome; however, SBS rarely is accompanied by limb hypertrophy.9 Pathogenesis is thought to involve an angiogenic response to a high perfusion rate and high oxygen saturation, which leads to fibroblast proliferation and reactive endothelial hyperplasia.1,14
Diagnosis and Differential Diagnosis
Prompt identification of an underlying arteriovenous anomaly is critical, given the sequelae of high-flow shunts, which may result in skin ulceration, limb length discrepancy, cortical thinning of bone with regional osteoporosis, and congestive heart failure.1,5 Duplex ultrasonography is the first-line diagnostic modality because it is noninvasive and widely available. The key doppler feature of an arteriovenous malformation is low resistance and high diastolic pulsatile flow,1 which should be confirmed with magnetic resonance angiography or computed tomography angiography if present on ultrasonography.
The differential diagnosis of AAD includes Kaposi sarcoma, reactive angioendotheliomatosis, diffuse dermal angiomatosis, intravascular histiocytosis, glomeruloid angioendotheliomatosis, and angiopericytomatosis.15,16 These entities present as multiple erythematous, violaceous, purpuric patches and plaques generally on the extremities but can have a widely varied distribution. Some lesions evolve to necrosis or ulceration. Histopathologic analysis is useful to differentiate these entities.
Histopathology
The histopathologic features of AAD can be nonspecific; clinicopathologic correlation often is necessary to establish the diagnosis. Features include a proliferation of small thick-walled vessels, often in a lobular arrangement, in an edematous papillary dermis. Small thrombi may be observed. There may be increased fibroblasts; plump endothelial cells; a superficial mixed infiltrate comprised of lymphocytes, histiocytes, and eosinophils; and deposition of hemosiderin.2,5 These characteristics overlap with features of Kaposi sarcoma; AAD, however, lacks slitlike vascular spaces, perivascular CD34+ expression, and nuclear atypia. A negative HHV-8 stain will assist in ruling out Kaposi sarcoma.1,17
Management
Treatment reports are anecdotal. The goal is to correct underlying venous hypertension. Conservative measures with compression garments, intermittent pneumatic compression, and limb elevation are first line.18 Oral antibiotics and local wound care with topical emollients and corticosteroids have been shown to be effective treatments.19-21
Oral erythromycin 500 mg 4 times daily for 3 weeks and clobetasol propionate cream 0.05% healed a lower extremity ulcer in a patient with Mali-type AAD.21 In another patient, conservative treatment of Mali-type AAD failed, but rapid improvement of 2 lower extremity ulcers resulted after 3 weeks of oral dapsone 50 mg twice daily.22
Conclusion
Acroangiodermatitis is a rare entity that is characterized by erythematous violaceous papules and plaques of the extremities, commonly in the setting of chronic venous insufficiency or an arteriovenous shunt. Histopathologic analysis shows proliferation of capillaries with fibrosis, extravasation of erythrocytes, and deposition of hemosiderin without the spindle cells and slitlike vascular spaces characteristic of Kaposi sarcoma. Detection of an underlying arteriovenous malformation is essential, as the disease can have local and systemic consequences, such as skin ulceration and congestive heart failure.1 Treatment options are conservative, directed toward local wound care, compression, and management of complications, such as ulceration and infection, as well as obliterating any underlying arteriovenous malformation.
Case Reports
Patient 1
A 56-year-old white man with a history of hypertension, hyperlipidemia, sleep apnea, bilateral knee replacement, and cataract removal presented to the emergency department with a worsening rash on the left posterior medial leg of 6 months’ duration. He reported associated redness and tenderness with the plaques as well as increased swelling and firmness of the leg. He was admitted to the hospital where the infectious disease team treated him with cefazolin for presumed cellulitis. His condition did not improve, and another course of cefazolin was started in addition to oral fluconazole and clotrimazole–betamethasone dipropionate lotion for a possible fungal cause. Again, treatment provided no improvement.
He was then evaluated by dermatology. On physical examination, the patient had edema, warmth, and induration of the left lower leg. There also was an annular and serpiginous indurated plaque with minimal scale on the left lower leg (Figure 1). A firm, dark red to purple plaque on the left medial thigh with mild scale was present. There also was scaling of the right plantar foot.
Skin biopsy revealed a dermal capillary proliferation with a scattering of inflammatory cells including eosinophils as well as dermal fibrosis (Figure 2). Periodic acid–Schiff and human herpesvirus 8 (HHV-8) immunostains were negative. Considering the degree and depth of vascular proliferation, Mali-type acroangiodermatitis (AAD) was the favored diagnosis.
Patient 2
A 72-year-old white man presented with a firm asymptomatic growth on the left dorsal forearm of 3 months’ duration. It was located near the site of a prior squamous cell carcinoma that was excised 1 year prior to presentation. The patient had no treatment or biopsy of the presenting lesion. His medical and surgical history included polycystic kidney disease and renal transplantation 4 years prior to presentation. He also had an arteriovenous fistula of the left arm. His other chronic diseases included chronic obstructive lung disease, congestive heart failure, hypertension, type 2 diabetes mellitus, and obstructive sleep apnea.
On physical examination, the patient had a 1-cm violaceous nodule on the extensor surface of the left mid forearm. An arteriovenous fistula was present proximal to the lesion on the left arm (Figure 3).
Skin biopsy revealed a tightly packed proliferation of small vascular channels that tested negative for HHV-8, tumor protein p63, and cytokeratin 5/6. Erythrocytes were noted in the lumen of some of these vessels. Neutrophils were scattered and clustered throughout the specimen (Figure 4A). Blood vessels were highlighted with CD34 (Figure 4B). Grocott-Gomori methenamine-silver stain was negative for infectious agents. These findings favored AAD secondary to an arteriovenous malformation, consistent with Stewart-Bluefarb syndrome (SBS).
Comment
Presentation of AAD
Acroangiodermatitis is a rare chronic inflammatory skin process involving a reactive proliferation of capillaries and fibrosis of the skin that resembles Kaposi sarcoma both clinically and histopathologically. The condition has been reported in patients with chronic venous insufficiency,1 congenital arteriovenous malformation,2 acquired iatrogenic arteriovenous fistula,3 paralyzed extremity,4 suction socket lower limb prosthesis (amputees),5 and minor trauma.6-8 The lesions of AAD tend to be circumscribed, slowly evolving, red-violaceous (or brown or dusky) macules, papules, or plaques that may become verrucous or develop into painful ulcerations. They generally occur on the distal dorsal aspects of the lower legs and feet.110
Variants of AAD
Mali et al9 first reported cutaneous manifestations resembling Kaposi sarcoma in 18 patients with chronic venous insufficiency in 1965. Two years later, Bluefarb and Adams10 described kaposiform skin lesions in one patient with a congenital arteriovenous malformation without chronic venous insufficiency. It was not until 1974, however, that Earhart et al11 proposed the term pseudo-Kaposi sarcoma.10,11 Based on these findings, AAD is described as 2 variants: Mali type and SBS.
Mali-type AAD is more common and typically occurs in elderly men. It classically presents bilaterally on the lower extremities in association with severe chronic venous insufficiency.5 Skin lesions usually occur on the medial aspect of the lower legs (as in patient 1), dorsum of the heel, hallux, or second toe.12
The etiology of Mali-type AAD is poorly understood. The leading theory is that the condition involves reduced perfusion due to chronic edema, resulting in neovascularization, fibroblast proliferation, hypertrophy, and inflammatory skin changes. When AAD occurs in the setting of a suction socket prosthesis, the negative pressure of the stump-socket environment is thought to alter local circulation, leading to proliferation of small blood vessels.5,13
Stewart-Bluefarb syndrome usually involves a single extremity in young adults with congenital arteriovenous malformations, amputees, and individuals with hemiplegia or iatrogenic arteriovenous fistulae (as in patient 2).1 It was once thought to occur secondary to Klippel-Trenaunay-Weber syndrome; however, SBS rarely is accompanied by limb hypertrophy.9 Pathogenesis is thought to involve an angiogenic response to a high perfusion rate and high oxygen saturation, which leads to fibroblast proliferation and reactive endothelial hyperplasia.1,14
Diagnosis and Differential Diagnosis
Prompt identification of an underlying arteriovenous anomaly is critical, given the sequelae of high-flow shunts, which may result in skin ulceration, limb length discrepancy, cortical thinning of bone with regional osteoporosis, and congestive heart failure.1,5 Duplex ultrasonography is the first-line diagnostic modality because it is noninvasive and widely available. The key doppler feature of an arteriovenous malformation is low resistance and high diastolic pulsatile flow,1 which should be confirmed with magnetic resonance angiography or computed tomography angiography if present on ultrasonography.
The differential diagnosis of AAD includes Kaposi sarcoma, reactive angioendotheliomatosis, diffuse dermal angiomatosis, intravascular histiocytosis, glomeruloid angioendotheliomatosis, and angiopericytomatosis.15,16 These entities present as multiple erythematous, violaceous, purpuric patches and plaques generally on the extremities but can have a widely varied distribution. Some lesions evolve to necrosis or ulceration. Histopathologic analysis is useful to differentiate these entities.
Histopathology
The histopathologic features of AAD can be nonspecific; clinicopathologic correlation often is necessary to establish the diagnosis. Features include a proliferation of small thick-walled vessels, often in a lobular arrangement, in an edematous papillary dermis. Small thrombi may be observed. There may be increased fibroblasts; plump endothelial cells; a superficial mixed infiltrate comprised of lymphocytes, histiocytes, and eosinophils; and deposition of hemosiderin.2,5 These characteristics overlap with features of Kaposi sarcoma; AAD, however, lacks slitlike vascular spaces, perivascular CD34+ expression, and nuclear atypia. A negative HHV-8 stain will assist in ruling out Kaposi sarcoma.1,17
Management
Treatment reports are anecdotal. The goal is to correct underlying venous hypertension. Conservative measures with compression garments, intermittent pneumatic compression, and limb elevation are first line.18 Oral antibiotics and local wound care with topical emollients and corticosteroids have been shown to be effective treatments.19-21
Oral erythromycin 500 mg 4 times daily for 3 weeks and clobetasol propionate cream 0.05% healed a lower extremity ulcer in a patient with Mali-type AAD.21 In another patient, conservative treatment of Mali-type AAD failed, but rapid improvement of 2 lower extremity ulcers resulted after 3 weeks of oral dapsone 50 mg twice daily.22
Conclusion
Acroangiodermatitis is a rare entity that is characterized by erythematous violaceous papules and plaques of the extremities, commonly in the setting of chronic venous insufficiency or an arteriovenous shunt. Histopathologic analysis shows proliferation of capillaries with fibrosis, extravasation of erythrocytes, and deposition of hemosiderin without the spindle cells and slitlike vascular spaces characteristic of Kaposi sarcoma. Detection of an underlying arteriovenous malformation is essential, as the disease can have local and systemic consequences, such as skin ulceration and congestive heart failure.1 Treatment options are conservative, directed toward local wound care, compression, and management of complications, such as ulceration and infection, as well as obliterating any underlying arteriovenous malformation.
- Parsi K, O’Connor AA, Bester L. Stewart-Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514.
- Larralde M, Gonzalez V, Marietti R, et al. Pseudo-Kaposi sarcoma with arteriovenous malformation. Pediatr Dermatol. 2001;18:325-327.
- Nakanishi G, Tachibana T, Soga H, et al. Pseudo-Kaposi’s sarcoma of the hand associated with acquired iatrogenic arteriovenous fistula. Indian J Dermatol. 2014;59:415-416.
- Landthaler M, Langehenke H, Holzmann H, et al. Mali’s acroangiodermatitis (pseudo-Kaposi) in paralyzed legs. Hautarzt. 1988;39:304-307.
- Trindade F, Requena L. Pseudo-Kaposi’s sarcoma because of suction socket lower limb prosthesis. J Cutan Pathol. 2009;36:482-485.
- Yu-Lu W, Tao Q, Hong-Zhong J, et al. Non-tender pedal plaques and nodules: pseudo-Kaposi’s sarcoma (Stewart-Bluefarb type) induced by trauma. J Dtsch Dermatol Ges. 2015;13:927-930.
- Del-Río E, Aguilar A, Ambrojo P, et al. Pseudo-Kaposi sarcoma induced by minor trauma in a patient with Klippel-Trenaunay-Weber syndrome. Clin Exp Dermatol. 1993;18:151-153.
- Archie M, Khademi S, Aungst D, et al. A rare case of acroangiodermatitis associated with a congenital arteriovenous malformation (Stewart-Bluefarb Syndrome) in a young veteran: case report and review of the literature. Ann Vasc Surg. 2015;29:1448.e5-1448.e10.
- Mali JW, Kuiper JP, Hamers AA. Acro-angiodermatitis of the foot. Arch Dermatol. 1965;92:515-518.
- Bluefarb SM, Adams LA. Arteriovenous malformation with angiodermatitis. stasis dermatitis simulating Kaposi’s disease. Arch Dermatol. 1967;96:176-181.
- Earhart RN, Aeling JA, Nuss DD, et al. Pseudo-Kaposi sarcoma. A patient with arteriovenous malformation and skin lesions simulating Kaposi sarcoma. Arch Dermatol. 1974;110:907-910.
- Lugovic´ L, Pusic´ J, Situm M, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma): three case reports. Acta Dermatovenerol Croat. 2007;15:152-157.
- Horiguchi Y, Takahashi K, Tanizaki H, et al. Case of bilateral acroangiodermatitis due to symmetrical arteriovenous fistulas of the soles. J Dermatol. 2015;42:989-991.
- Dog˘an S, Boztepe G, Karaduman A. Pseudo-Kaposi sarcoma: a challenging vascular phenomenon. Dermatol Online J. 2007;13:22.
- Mazloom SE, Stallings A, Kyei A. Differentiating intralymphatic histiocytosis, intravascular histiocytosis, and subtypes of reactive angioendotheliomatosis: review of clinical and histologic features of all cases reported to date. Am J Dermatopathol. 2017;39:33-39.
- Rongioletti F, Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Kanitakis J, Narvaez D, Claudy A. Expression of the CD34 antigen distinguishes Kaposi’s sarcoma from pseudo-Kaposi’s sarcoma (acroangiodermatitis). Br J Dermatol. 1996;134:44-46.
- Pires A, Depairon M, Ricci C, et al. Effect of compression therapy on a pseudo-Kaposi sarcoma. Dermatology. 1999;198:439-441.
- Hayek S, Atiyeh B, Zgheib E. Stewart-Bluefarb syndrome: review of the literature and case report of chronic ulcer treatment with heparan sulphate (Cacipliq20®). Int Wound J. 2015;12:169-172.
- Varyani N, Thukral A, Kumar N, et al. Nonhealing ulcer: acroangiodermatitis of Mali. Case Rep Dermatol Med. 2011;2011:909383.
- Mehta AA, Pereira RR, Nayak C, et al. Acroangiodermatitis of Mali: a rare vascular phenomenon. Indian J Dermatol Venereol Leprol. 2010;76:553-556.
- Rashkovsky I, Gilead L, Schamroth J, et al. Acro-angiodermatitis: review of the literature and report of a case. Acta Derm Venereol. 1995;75:475-478.
- Parsi K, O’Connor AA, Bester L. Stewart-Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514.
- Larralde M, Gonzalez V, Marietti R, et al. Pseudo-Kaposi sarcoma with arteriovenous malformation. Pediatr Dermatol. 2001;18:325-327.
- Nakanishi G, Tachibana T, Soga H, et al. Pseudo-Kaposi’s sarcoma of the hand associated with acquired iatrogenic arteriovenous fistula. Indian J Dermatol. 2014;59:415-416.
- Landthaler M, Langehenke H, Holzmann H, et al. Mali’s acroangiodermatitis (pseudo-Kaposi) in paralyzed legs. Hautarzt. 1988;39:304-307.
- Trindade F, Requena L. Pseudo-Kaposi’s sarcoma because of suction socket lower limb prosthesis. J Cutan Pathol. 2009;36:482-485.
- Yu-Lu W, Tao Q, Hong-Zhong J, et al. Non-tender pedal plaques and nodules: pseudo-Kaposi’s sarcoma (Stewart-Bluefarb type) induced by trauma. J Dtsch Dermatol Ges. 2015;13:927-930.
- Del-Río E, Aguilar A, Ambrojo P, et al. Pseudo-Kaposi sarcoma induced by minor trauma in a patient with Klippel-Trenaunay-Weber syndrome. Clin Exp Dermatol. 1993;18:151-153.
- Archie M, Khademi S, Aungst D, et al. A rare case of acroangiodermatitis associated with a congenital arteriovenous malformation (Stewart-Bluefarb Syndrome) in a young veteran: case report and review of the literature. Ann Vasc Surg. 2015;29:1448.e5-1448.e10.
- Mali JW, Kuiper JP, Hamers AA. Acro-angiodermatitis of the foot. Arch Dermatol. 1965;92:515-518.
- Bluefarb SM, Adams LA. Arteriovenous malformation with angiodermatitis. stasis dermatitis simulating Kaposi’s disease. Arch Dermatol. 1967;96:176-181.
- Earhart RN, Aeling JA, Nuss DD, et al. Pseudo-Kaposi sarcoma. A patient with arteriovenous malformation and skin lesions simulating Kaposi sarcoma. Arch Dermatol. 1974;110:907-910.
- Lugovic´ L, Pusic´ J, Situm M, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma): three case reports. Acta Dermatovenerol Croat. 2007;15:152-157.
- Horiguchi Y, Takahashi K, Tanizaki H, et al. Case of bilateral acroangiodermatitis due to symmetrical arteriovenous fistulas of the soles. J Dermatol. 2015;42:989-991.
- Dog˘an S, Boztepe G, Karaduman A. Pseudo-Kaposi sarcoma: a challenging vascular phenomenon. Dermatol Online J. 2007;13:22.
- Mazloom SE, Stallings A, Kyei A. Differentiating intralymphatic histiocytosis, intravascular histiocytosis, and subtypes of reactive angioendotheliomatosis: review of clinical and histologic features of all cases reported to date. Am J Dermatopathol. 2017;39:33-39.
- Rongioletti F, Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Kanitakis J, Narvaez D, Claudy A. Expression of the CD34 antigen distinguishes Kaposi’s sarcoma from pseudo-Kaposi’s sarcoma (acroangiodermatitis). Br J Dermatol. 1996;134:44-46.
- Pires A, Depairon M, Ricci C, et al. Effect of compression therapy on a pseudo-Kaposi sarcoma. Dermatology. 1999;198:439-441.
- Hayek S, Atiyeh B, Zgheib E. Stewart-Bluefarb syndrome: review of the literature and case report of chronic ulcer treatment with heparan sulphate (Cacipliq20®). Int Wound J. 2015;12:169-172.
- Varyani N, Thukral A, Kumar N, et al. Nonhealing ulcer: acroangiodermatitis of Mali. Case Rep Dermatol Med. 2011;2011:909383.
- Mehta AA, Pereira RR, Nayak C, et al. Acroangiodermatitis of Mali: a rare vascular phenomenon. Indian J Dermatol Venereol Leprol. 2010;76:553-556.
- Rashkovsky I, Gilead L, Schamroth J, et al. Acro-angiodermatitis: review of the literature and report of a case. Acta Derm Venereol. 1995;75:475-478.
Practice Points
- Acroangiodermatitis (AAD) may mimic Kaposi sarcoma clinically and histopathologically. A human herpesvirus 8 stain is helpful to differentiate these two entities.
- Diagnosis of AAD should prompt investigation of an underlying arteriovenous malformation, as the disease may have systemic consequences such as congestive heart failure.
Phototherapy: Is It Still Important?
Phototherapy has been used to treat skin diseases for millennia. From the Incas to the ancient Greeks and Egyptians, nearly every major civilization has attempted to harness the sun, with some even worshipping it for its healing powers.1 Today, phototherapy remains as important as ever. Despite the technological advances that have brought about biologic medications, small molecule inhibitors, and elegant vehicle delivery systems, phototherapy continues to be a valuable tool in the dermatologist’s armamentarium.
Patient Access to Phototherapy
An important step in successfully managing any disease is access to treatment. In today’s health care landscape, therapeutic decisions frequently are dictated by a patient’s financial situation as well as by the discretion of payers. Costly medications such as biologics often are not accessible to patients on government insurance who fall into the Medicare “donut hole” and may be denied by insurance companies for a myriad of reasons. Luckily, phototherapy typically is well covered and is even a first-line treatment option for some conditions, such as mycosis fungoides.
Nevertheless, phototherapy also has its own unique accessibility hurdles. The time-consuming nature of office-based phototherapy treatment is the main barrier, and many patients find it difficult to incorporate treatments into their daily lives. Additionally, office-based phototherapy units often are clustered in major cities, making access more difficult for rural patients. Because light-responsive conditions often are chronic and may require a lifetime of treatment, home phototherapy units are now being recognized as cost-effective treatment options and are increasingly covered by insurance. In fact, one study comparing psoriasis patients treated with home narrowband UVB (NB-UVB) vs outpatient NB-UVB found that in-home treatment was equally as effective as office-based treatment at a similar cost.2 Because studies comparing the effectiveness of office-based vs home-based phototherapy treatment are underway for various other diseases, hopefully more patients will be able to receive home units, thus increasing access to safe and effective treatment.
Wide Range of Treatment Indications
Another merit of phototherapy is its ability to be used in almost all patient populations. It is one of the few modalities whose indications span the entire length of the human lifetime—from pediatric atopic dermatitis to chronic pruritus in elderly patients. Phototherapy also is one of the few treatment options that is safe to use in patients with an active malignancy or in patients who have multiple other medical conditions. Comorbidities including congestive heart failure, chronic infections, and demyelinating disorders often prevent the use of oral and injectable medications for immune-mediated disorders such as psoriasis or atopic dermatitis. In patients with multiple comorbidities whose disease remains uncontrolled despite an adequate topical regimen, phototherapy is one of the few effective treatment options that remain. Additionally, there is a considerable number of patients who prefer external treatments for cutaneous diseases. For these patients, phototherapy offers the opportunity to control skin conditions without the use of an internal medication.
Favorable Safety Profile
Phototherapy is a largely benign intervention with an excellent safety profile. Its main potential adverse events include erythema, pruritus, xerosis, recurrence of herpes simplex virus infection, and premature skin aging. The effects of phototherapy on skin carcinogenesis have long been controversial; however, data suggest a clear distinction in risk between treatment with NB-UVB and psoralen plus UVA (PUVA). A systematic review of psoriasis patients treated with phototherapy found no evidence to suggest an increased risk of melanoma or nonmelanoma skin cancer with NB-UVB treatment.3 The same cannot be said for psoriasis patients treated with PUVA, who were noted to have a higher incidence of nonmelanoma skin cancer than the general population. This increased risk was more substantial in American cohorts than in European cohorts, likely due to multiple factors including variable skin types and treatment regimens. Increased rates of melanoma also were noted in American PUVA cohorts, with no similar increase seen in their European counterparts.3
Broad vs Targeted Therapies
Targeted therapies have dominated the health care landscape over the last few years, with the majority of new medications being highly focused and only efficacious in a few conditions. One of phototherapy’s greatest strengths is its lack of specificity. Because the field of dermatology is filled with rare, overlapping, and often poorly understood diseases, nonspecific treatment options are needed to fill the gaps. Many generalized skin conditions may lack treatment options indicated by the US Food and Drug Administration. Phototherapy is the ultimate untargeted intervention and may be broadly used for a wide range of cutaneous conditions. Although classically utilized for atopic dermatitis and psoriasis, NB-UVB also can effectively treat generalized pruritus, vitiligo, urticaria, and seborrheic dermatitis.4 Not to be outdone, PUVA has shown success in treating more than 50 different dermatologic conditions including lichen planus, alopecia areata, and mycosis fungoides.
Final Thoughts
Phototherapy is a safe, accessible, and widely applicable treatment for a range of cutaneous disorders. Although more precisely engineered internal therapies have begun to replace UV light in psoriasis and atopic dermatitis, phototherapy likely will always remain an ideal treatment for a wide cohort of patients. Between increased access to home units and the continued validation of its excellent safety record, the future of phototherapy is looking bright.
- Grzybowski A, Sak J, Pawlikowski J. A brief report on the history of phototherapy. Clin Dermatol. 2016;34:532-537.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ledo E, Ledo A. Phototherapy, photochemotherapy, and photodynamic therapy: unapproved uses or indications. Clin Dermatol. 2000;18:77-86.
Phototherapy has been used to treat skin diseases for millennia. From the Incas to the ancient Greeks and Egyptians, nearly every major civilization has attempted to harness the sun, with some even worshipping it for its healing powers.1 Today, phototherapy remains as important as ever. Despite the technological advances that have brought about biologic medications, small molecule inhibitors, and elegant vehicle delivery systems, phototherapy continues to be a valuable tool in the dermatologist’s armamentarium.
Patient Access to Phototherapy
An important step in successfully managing any disease is access to treatment. In today’s health care landscape, therapeutic decisions frequently are dictated by a patient’s financial situation as well as by the discretion of payers. Costly medications such as biologics often are not accessible to patients on government insurance who fall into the Medicare “donut hole” and may be denied by insurance companies for a myriad of reasons. Luckily, phototherapy typically is well covered and is even a first-line treatment option for some conditions, such as mycosis fungoides.
Nevertheless, phototherapy also has its own unique accessibility hurdles. The time-consuming nature of office-based phototherapy treatment is the main barrier, and many patients find it difficult to incorporate treatments into their daily lives. Additionally, office-based phototherapy units often are clustered in major cities, making access more difficult for rural patients. Because light-responsive conditions often are chronic and may require a lifetime of treatment, home phototherapy units are now being recognized as cost-effective treatment options and are increasingly covered by insurance. In fact, one study comparing psoriasis patients treated with home narrowband UVB (NB-UVB) vs outpatient NB-UVB found that in-home treatment was equally as effective as office-based treatment at a similar cost.2 Because studies comparing the effectiveness of office-based vs home-based phototherapy treatment are underway for various other diseases, hopefully more patients will be able to receive home units, thus increasing access to safe and effective treatment.
Wide Range of Treatment Indications
Another merit of phototherapy is its ability to be used in almost all patient populations. It is one of the few modalities whose indications span the entire length of the human lifetime—from pediatric atopic dermatitis to chronic pruritus in elderly patients. Phototherapy also is one of the few treatment options that is safe to use in patients with an active malignancy or in patients who have multiple other medical conditions. Comorbidities including congestive heart failure, chronic infections, and demyelinating disorders often prevent the use of oral and injectable medications for immune-mediated disorders such as psoriasis or atopic dermatitis. In patients with multiple comorbidities whose disease remains uncontrolled despite an adequate topical regimen, phototherapy is one of the few effective treatment options that remain. Additionally, there is a considerable number of patients who prefer external treatments for cutaneous diseases. For these patients, phototherapy offers the opportunity to control skin conditions without the use of an internal medication.
Favorable Safety Profile
Phototherapy is a largely benign intervention with an excellent safety profile. Its main potential adverse events include erythema, pruritus, xerosis, recurrence of herpes simplex virus infection, and premature skin aging. The effects of phototherapy on skin carcinogenesis have long been controversial; however, data suggest a clear distinction in risk between treatment with NB-UVB and psoralen plus UVA (PUVA). A systematic review of psoriasis patients treated with phototherapy found no evidence to suggest an increased risk of melanoma or nonmelanoma skin cancer with NB-UVB treatment.3 The same cannot be said for psoriasis patients treated with PUVA, who were noted to have a higher incidence of nonmelanoma skin cancer than the general population. This increased risk was more substantial in American cohorts than in European cohorts, likely due to multiple factors including variable skin types and treatment regimens. Increased rates of melanoma also were noted in American PUVA cohorts, with no similar increase seen in their European counterparts.3
Broad vs Targeted Therapies
Targeted therapies have dominated the health care landscape over the last few years, with the majority of new medications being highly focused and only efficacious in a few conditions. One of phototherapy’s greatest strengths is its lack of specificity. Because the field of dermatology is filled with rare, overlapping, and often poorly understood diseases, nonspecific treatment options are needed to fill the gaps. Many generalized skin conditions may lack treatment options indicated by the US Food and Drug Administration. Phototherapy is the ultimate untargeted intervention and may be broadly used for a wide range of cutaneous conditions. Although classically utilized for atopic dermatitis and psoriasis, NB-UVB also can effectively treat generalized pruritus, vitiligo, urticaria, and seborrheic dermatitis.4 Not to be outdone, PUVA has shown success in treating more than 50 different dermatologic conditions including lichen planus, alopecia areata, and mycosis fungoides.
Final Thoughts
Phototherapy is a safe, accessible, and widely applicable treatment for a range of cutaneous disorders. Although more precisely engineered internal therapies have begun to replace UV light in psoriasis and atopic dermatitis, phototherapy likely will always remain an ideal treatment for a wide cohort of patients. Between increased access to home units and the continued validation of its excellent safety record, the future of phototherapy is looking bright.
Phototherapy has been used to treat skin diseases for millennia. From the Incas to the ancient Greeks and Egyptians, nearly every major civilization has attempted to harness the sun, with some even worshipping it for its healing powers.1 Today, phototherapy remains as important as ever. Despite the technological advances that have brought about biologic medications, small molecule inhibitors, and elegant vehicle delivery systems, phototherapy continues to be a valuable tool in the dermatologist’s armamentarium.
Patient Access to Phototherapy
An important step in successfully managing any disease is access to treatment. In today’s health care landscape, therapeutic decisions frequently are dictated by a patient’s financial situation as well as by the discretion of payers. Costly medications such as biologics often are not accessible to patients on government insurance who fall into the Medicare “donut hole” and may be denied by insurance companies for a myriad of reasons. Luckily, phototherapy typically is well covered and is even a first-line treatment option for some conditions, such as mycosis fungoides.
Nevertheless, phototherapy also has its own unique accessibility hurdles. The time-consuming nature of office-based phototherapy treatment is the main barrier, and many patients find it difficult to incorporate treatments into their daily lives. Additionally, office-based phototherapy units often are clustered in major cities, making access more difficult for rural patients. Because light-responsive conditions often are chronic and may require a lifetime of treatment, home phototherapy units are now being recognized as cost-effective treatment options and are increasingly covered by insurance. In fact, one study comparing psoriasis patients treated with home narrowband UVB (NB-UVB) vs outpatient NB-UVB found that in-home treatment was equally as effective as office-based treatment at a similar cost.2 Because studies comparing the effectiveness of office-based vs home-based phototherapy treatment are underway for various other diseases, hopefully more patients will be able to receive home units, thus increasing access to safe and effective treatment.
Wide Range of Treatment Indications
Another merit of phototherapy is its ability to be used in almost all patient populations. It is one of the few modalities whose indications span the entire length of the human lifetime—from pediatric atopic dermatitis to chronic pruritus in elderly patients. Phototherapy also is one of the few treatment options that is safe to use in patients with an active malignancy or in patients who have multiple other medical conditions. Comorbidities including congestive heart failure, chronic infections, and demyelinating disorders often prevent the use of oral and injectable medications for immune-mediated disorders such as psoriasis or atopic dermatitis. In patients with multiple comorbidities whose disease remains uncontrolled despite an adequate topical regimen, phototherapy is one of the few effective treatment options that remain. Additionally, there is a considerable number of patients who prefer external treatments for cutaneous diseases. For these patients, phototherapy offers the opportunity to control skin conditions without the use of an internal medication.
Favorable Safety Profile
Phototherapy is a largely benign intervention with an excellent safety profile. Its main potential adverse events include erythema, pruritus, xerosis, recurrence of herpes simplex virus infection, and premature skin aging. The effects of phototherapy on skin carcinogenesis have long been controversial; however, data suggest a clear distinction in risk between treatment with NB-UVB and psoralen plus UVA (PUVA). A systematic review of psoriasis patients treated with phototherapy found no evidence to suggest an increased risk of melanoma or nonmelanoma skin cancer with NB-UVB treatment.3 The same cannot be said for psoriasis patients treated with PUVA, who were noted to have a higher incidence of nonmelanoma skin cancer than the general population. This increased risk was more substantial in American cohorts than in European cohorts, likely due to multiple factors including variable skin types and treatment regimens. Increased rates of melanoma also were noted in American PUVA cohorts, with no similar increase seen in their European counterparts.3
Broad vs Targeted Therapies
Targeted therapies have dominated the health care landscape over the last few years, with the majority of new medications being highly focused and only efficacious in a few conditions. One of phototherapy’s greatest strengths is its lack of specificity. Because the field of dermatology is filled with rare, overlapping, and often poorly understood diseases, nonspecific treatment options are needed to fill the gaps. Many generalized skin conditions may lack treatment options indicated by the US Food and Drug Administration. Phototherapy is the ultimate untargeted intervention and may be broadly used for a wide range of cutaneous conditions. Although classically utilized for atopic dermatitis and psoriasis, NB-UVB also can effectively treat generalized pruritus, vitiligo, urticaria, and seborrheic dermatitis.4 Not to be outdone, PUVA has shown success in treating more than 50 different dermatologic conditions including lichen planus, alopecia areata, and mycosis fungoides.
Final Thoughts
Phototherapy is a safe, accessible, and widely applicable treatment for a range of cutaneous disorders. Although more precisely engineered internal therapies have begun to replace UV light in psoriasis and atopic dermatitis, phototherapy likely will always remain an ideal treatment for a wide cohort of patients. Between increased access to home units and the continued validation of its excellent safety record, the future of phototherapy is looking bright.
- Grzybowski A, Sak J, Pawlikowski J. A brief report on the history of phototherapy. Clin Dermatol. 2016;34:532-537.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ledo E, Ledo A. Phototherapy, photochemotherapy, and photodynamic therapy: unapproved uses or indications. Clin Dermatol. 2000;18:77-86.
- Grzybowski A, Sak J, Pawlikowski J. A brief report on the history of phototherapy. Clin Dermatol. 2016;34:532-537.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ledo E, Ledo A. Phototherapy, photochemotherapy, and photodynamic therapy: unapproved uses or indications. Clin Dermatol. 2000;18:77-86.
Clinical Pearl: Advantages of the Scalp as a Split-Thickness Skin Graft Donor Site
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
Heparin-Induced Bullous Hemorrhagic Dermatosis Confined to the Oral Mucosa
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
Practice Points
- It is important for physicians to recognize the clinical appearance of cutaneous adverse reactions to heparin, including bullous hemorrhagic dermatosis.
- Heparin-induced bullous hemorrhagic dermatosis tends to self-resolve, even with continuation of unfractionated heparin.
Erythema Gyratum Repens–like Eruption in Sézary Syndrome: Evidence for the Role of a Dermatophyte
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
Practice Points
- Erythema gyratum repens (EGR) presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with fine trailing scale.
- Although EGR typically is associated with underlying malignancy, it is not an obligate paraneoplastic syndrome. There are numerous cases that are not associated with underlying neoplasms.
- An EGR-like eruption may be observed in Sézary syndrome, and an overlying superficial dermatophyte infection may play a role.
Addressing Health Literacy for Miscommunication in Dermatology
To the Editor:
We read with interest the Cutis Resident Corner column by Tracey1 on miscommunication with dermatology patients in which the author highlighted how seemingly straightforward language can deceivingly complicate effective communication between dermatologists and their patients. The examples she provided, including subtleties in describing what constitutes the “affected area” for proper application of a topical treatment or the inconsistent use of trade names for medications, underscore how misperceptions of verbal instruction can lead to poor treatment adherence and unintended health outcomes.1
In addition to how dermatologists deliver treatment information to their patients, a broader aspect of physician-patient communication is health literacy, which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”2 Health literacy involves reading, listening, numeracy, decision-making, and health knowledge; patients who are potentially at risk for having limited skills in these areas include the elderly, those with poor English language proficiency, and those of lower socioeconomic status.3
In 2003, the National Assessment of Adult Literacy found that only 12% of individuals older than 16 years had a proficient level of health literacy.4 In an effort to address gaps in communication between health care providers and patients, the American Medical Association, National Institutes of Health, and the US Department of Health & Human Services recommend that educational materials be written at no higher than a 6th grade reading level.5,6 Currently, only 2% of dermatology educational materials meet this recommendation; the average reading level of patient dermatology materials is at a 12th grade level, despite the average American adult reading at an 8th grade level.7
It is imperative that dermatologists seek to improve both their verbal and nonverbal communication skills to effectively reach a broader patient population. Visual cues, such as pamphlets to illustrate what is meant by a “pea-sized” amount of adapalene or a photograph demonstrating “border asymmetry” in a melanoma, may be more effective than verbal or written communication alone. In addition, when certain drugs or treatments may be called by various names or when different drug names sound similar
The visual nature of dermatology creates unique psychosocial scenarios that may inherently motivate patients to understand their cutaneous disease; for example, providing photographs that depict acne improvement at different time points throughout isotretinoin treatment allows for more realistic expectations during therapy. Therefore, it is only fitting that instructive imagery would serve to benefit patient education.
In conclusion, communication between dermatologists and their patients involves multiple variables that can contribute to successful patient instruction for the management of dermatologic disease. Indeed, successful interaction not only includes mutual awareness of words or phrases that can otherwise be misconstrued but also attention to the readability of written materials and the benefits of visual instruction in the clinic setting. Integrating these aspects of health literacy can optimize rapport, treatment adherence, and health outcomes.
- Tracey E. Miscommunication with dermatology patients: are we speaking the same language? Cutis. 2018;102:E27-E28.
- Selden CR, Zorn M, Ratzan SC, et al, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, US Department of Health and Human Services; 2000.
- Institute of Medicine (US) Committee on Health Literacy; Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
- Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Jessup, MD: National Center for Education Statistics, US Department of Education, Institute of Education Sciences; 2006. http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2006470. Published December 15, 2005. Accessed May 21, 2019.
- Weiss BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Association Foundation and American Medical Association; 2003.
- How to write easy-to-read health materials. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/etr.html. Accessed May 21, 2019.
- Prabhu AV, Gupta R, Kim C, et al. Patient education materials in dermatology: addressing the health literacy needs of patients. JAMA Dermatol. 2016;152:946-947.
To the Editor:
We read with interest the Cutis Resident Corner column by Tracey1 on miscommunication with dermatology patients in which the author highlighted how seemingly straightforward language can deceivingly complicate effective communication between dermatologists and their patients. The examples she provided, including subtleties in describing what constitutes the “affected area” for proper application of a topical treatment or the inconsistent use of trade names for medications, underscore how misperceptions of verbal instruction can lead to poor treatment adherence and unintended health outcomes.1
In addition to how dermatologists deliver treatment information to their patients, a broader aspect of physician-patient communication is health literacy, which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”2 Health literacy involves reading, listening, numeracy, decision-making, and health knowledge; patients who are potentially at risk for having limited skills in these areas include the elderly, those with poor English language proficiency, and those of lower socioeconomic status.3
In 2003, the National Assessment of Adult Literacy found that only 12% of individuals older than 16 years had a proficient level of health literacy.4 In an effort to address gaps in communication between health care providers and patients, the American Medical Association, National Institutes of Health, and the US Department of Health & Human Services recommend that educational materials be written at no higher than a 6th grade reading level.5,6 Currently, only 2% of dermatology educational materials meet this recommendation; the average reading level of patient dermatology materials is at a 12th grade level, despite the average American adult reading at an 8th grade level.7
It is imperative that dermatologists seek to improve both their verbal and nonverbal communication skills to effectively reach a broader patient population. Visual cues, such as pamphlets to illustrate what is meant by a “pea-sized” amount of adapalene or a photograph demonstrating “border asymmetry” in a melanoma, may be more effective than verbal or written communication alone. In addition, when certain drugs or treatments may be called by various names or when different drug names sound similar
The visual nature of dermatology creates unique psychosocial scenarios that may inherently motivate patients to understand their cutaneous disease; for example, providing photographs that depict acne improvement at different time points throughout isotretinoin treatment allows for more realistic expectations during therapy. Therefore, it is only fitting that instructive imagery would serve to benefit patient education.
In conclusion, communication between dermatologists and their patients involves multiple variables that can contribute to successful patient instruction for the management of dermatologic disease. Indeed, successful interaction not only includes mutual awareness of words or phrases that can otherwise be misconstrued but also attention to the readability of written materials and the benefits of visual instruction in the clinic setting. Integrating these aspects of health literacy can optimize rapport, treatment adherence, and health outcomes.
To the Editor:
We read with interest the Cutis Resident Corner column by Tracey1 on miscommunication with dermatology patients in which the author highlighted how seemingly straightforward language can deceivingly complicate effective communication between dermatologists and their patients. The examples she provided, including subtleties in describing what constitutes the “affected area” for proper application of a topical treatment or the inconsistent use of trade names for medications, underscore how misperceptions of verbal instruction can lead to poor treatment adherence and unintended health outcomes.1
In addition to how dermatologists deliver treatment information to their patients, a broader aspect of physician-patient communication is health literacy, which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”2 Health literacy involves reading, listening, numeracy, decision-making, and health knowledge; patients who are potentially at risk for having limited skills in these areas include the elderly, those with poor English language proficiency, and those of lower socioeconomic status.3
In 2003, the National Assessment of Adult Literacy found that only 12% of individuals older than 16 years had a proficient level of health literacy.4 In an effort to address gaps in communication between health care providers and patients, the American Medical Association, National Institutes of Health, and the US Department of Health & Human Services recommend that educational materials be written at no higher than a 6th grade reading level.5,6 Currently, only 2% of dermatology educational materials meet this recommendation; the average reading level of patient dermatology materials is at a 12th grade level, despite the average American adult reading at an 8th grade level.7
It is imperative that dermatologists seek to improve both their verbal and nonverbal communication skills to effectively reach a broader patient population. Visual cues, such as pamphlets to illustrate what is meant by a “pea-sized” amount of adapalene or a photograph demonstrating “border asymmetry” in a melanoma, may be more effective than verbal or written communication alone. In addition, when certain drugs or treatments may be called by various names or when different drug names sound similar
The visual nature of dermatology creates unique psychosocial scenarios that may inherently motivate patients to understand their cutaneous disease; for example, providing photographs that depict acne improvement at different time points throughout isotretinoin treatment allows for more realistic expectations during therapy. Therefore, it is only fitting that instructive imagery would serve to benefit patient education.
In conclusion, communication between dermatologists and their patients involves multiple variables that can contribute to successful patient instruction for the management of dermatologic disease. Indeed, successful interaction not only includes mutual awareness of words or phrases that can otherwise be misconstrued but also attention to the readability of written materials and the benefits of visual instruction in the clinic setting. Integrating these aspects of health literacy can optimize rapport, treatment adherence, and health outcomes.
- Tracey E. Miscommunication with dermatology patients: are we speaking the same language? Cutis. 2018;102:E27-E28.
- Selden CR, Zorn M, Ratzan SC, et al, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, US Department of Health and Human Services; 2000.
- Institute of Medicine (US) Committee on Health Literacy; Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
- Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Jessup, MD: National Center for Education Statistics, US Department of Education, Institute of Education Sciences; 2006. http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2006470. Published December 15, 2005. Accessed May 21, 2019.
- Weiss BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Association Foundation and American Medical Association; 2003.
- How to write easy-to-read health materials. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/etr.html. Accessed May 21, 2019.
- Prabhu AV, Gupta R, Kim C, et al. Patient education materials in dermatology: addressing the health literacy needs of patients. JAMA Dermatol. 2016;152:946-947.
- Tracey E. Miscommunication with dermatology patients: are we speaking the same language? Cutis. 2018;102:E27-E28.
- Selden CR, Zorn M, Ratzan SC, et al, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: National Institutes of Health, US Department of Health and Human Services; 2000.
- Institute of Medicine (US) Committee on Health Literacy; Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
- Kutner M, Greenberg E, Baer J. A First Look at the Literacy of America’s Adults in the 21st Century. Jessup, MD: National Center for Education Statistics, US Department of Education, Institute of Education Sciences; 2006. http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2006470. Published December 15, 2005. Accessed May 21, 2019.
- Weiss BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Association Foundation and American Medical Association; 2003.
- How to write easy-to-read health materials. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/etr.html. Accessed May 21, 2019.
- Prabhu AV, Gupta R, Kim C, et al. Patient education materials in dermatology: addressing the health literacy needs of patients. JAMA Dermatol. 2016;152:946-947.
Ocular Chemical Burns in the Dermatology Office: A Practical Approach to Managing Safety Precautions
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
Practice Points
- Dermatologists should be cognizant of potential hazards to the eyes during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- If a patient’s eye(s) becomes exposed to a chemical during a dermatologic procedure, immediate copious irrigation for at least 15 to 30 minutes (longer for alkaline burns) is crucial, followed by prompt evaluation by an ophthalmologist.
- The patient should be instructed to manually hold open the eye and move the eyeball in all directions to achieve the most effective irrigation of the chemical.
- If the patient is wearing contact lenses, they should be removed promptly, but do not delay the irrigation to do so. Lenses should be removed once irrigation is underway.
Acquired Digital Fibrokeratoma Presenting as a Painless Nodule on the Right Fifth Fingernail
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
Case Report
A 53-year-old woman presented for an initial visit to the dermatology clinic for a growth under the right fifth fingernail of 1 year’s duration. She had no history of trauma to the digit or pain or bleeding. She self-treated with over-the-counter wart remover for several months without improvement. She reported no other skin concerns. She had a medical history of rheumatoid arthritis (RA) and basal cell carcinoma of the nose; she was taking methotrexate and adalimumab for the RA. She had a family history of melanoma in her father.
On physical examination, a firm nontender nodule was noted on the distal nail bed of the right fifth fingernail with onycholysis; the nail plate was otherwise intact (Figure 1). All other nails were normal. A plain radiograph of the involved digit showed no bony abnormality. Excisional biopsy of the nodule was performed and analyzed by histopathology (Figure 2). The biopsy specimen showed a benign epidermis that was acanthotic and surmounted by hyperkeratotic scale. The dermis was fibrotic with collagen bundles assuming a vertical orientation to the long axis of the epidermis, typical of a fibrokeratoma. There were no atypical features in the dermal component or epidermis (Figure 2). These findings were consistent with the diagnosis of acquired digital fibrokeratoma (ADF). The patient tolerated excisional biopsy well and had no evidence of recurrence 4 months following excision.
Comment
History and Clinical Presentation
First described by Bart et al1 in 1968, ADF is a rare benign fibrous tumor localized to the nail bed or periungual area.1 Typically, it presents as a solitary flesh-colored papule measuring 3 to 5 mm in diameter. It can be keratotic with a surrounding collarette of elevated skin. Acquired digital fibrokeratoma usually is localized to the digits of the hands or feet; when presenting subungually, it is more commonly found arising from the proximal matrix or nail bed of the great toe. Observed nail changes include longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.2 The affected nail can be painful, depending on the size and location of the tumor.
Acquired digital fibrokeratoma is more commonly found in middle-aged men; however, it has been reported among patients of various ages and in both sexes.1,3 In a study of 20 cases, the average duration before presenting for medical advice was 28 months.2 Acquired digital fibrokeratoma arises sporadically; some patients report prior local trauma. Lesions typically do not self-resolve.
Diagnosis
The diagnosis of ADF is made using a combination of clinical and histopathological findings. Dermoscopy is helpful and may show homogenous white or milky white structures, likely representing hyperkeratosis, proliferation of capillaries, and an increase in collagen bundles with a surrounding collarette of scale.4,5 Histopathology shows acanthosis and hyperkeratosis of the epidermis. Collagen bundles assume a characteristic vertical orientation to the long axis of the epidermis.
Two other histomorphologic subtypes, less common than the type I variant, are the type II variant, in which the number of fibroblasts is increased and the number of elastic fibers is decreased, and the type III variant, in which the stroma are edematous and cell poor. There is an even greater reduction in elastic tissue content in the type III variant than in the type I variant. There is evidence that type II ADFs exhibit more hyperkeratosis clinically than the other 2 subtypes, but from a practical perspective, this subclassification is not conducted in routine practice because it does not have clinical significance.5
Differential Diagnosis
The clinical differential diagnosis of ADF is broad and includes squamous cell carcinoma, onychomatricoma, onychopapilloma, verruca vulgaris, supernumerary digit, neurofibroma, cellular digital fibroma, and Koenen tumor (periungual fibroma). Almost all of these entities are easily differentiated from ADF on biopsy. A fibrokeratoma does not exhibit the atypia seen in squamous cell carcinoma. The multiple fibroepithelial projections and nail plate perforations characteristic of onychomatricoma are not observed in ADF. Onychopapilloma shows acanthosis and papillomatosis, similar to ADF; however, onychopapilloma lacks the characteristic vertical orientation of collagen in ADF. Verruca vulgaris classically shows koilocytosis, dilated blood vessels in papillae, and hypergranulosis. A supernumerary digit clinically lacks a collarette of scale and often presents in a bilateral fashion on the lateral fifth digits in children; histopathologically, a supernumerary digit is distinct from an ADF in that nerve bundles are abundant in the dermis, defining a form of amputation neuroma. Neurofibroma exhibits a spindle cell proliferation that assumes a patternless disposition in the dermis, accompanied by mucin, mast cells, and delicate collagen. The defining cell populace has a typical serpiginous nuclear outline that is characteristic of a Schwann cell. Cellular digital fibroma can present similar to ADF; it is considered by some to be a mucin-poor variant of superficial acral fibromyxoma. Its morphology is distinct: a proliferation of bland-appearing spindled cells exhibiting a storiform or fascicular growth pattern and CD34 positivity.
The differential diagnosis to consider when ADF is suspected is a Koenen tumor, which resembles a fibrokeratoma clinically and also is localized to the digits. Koenen tumors can be differentiated from fibrokeratoma by its association with tuberous sclerosis; a multiple, rather than solitary, presentation; a distinctive clove-shaped gross appearance; and an appearance on histopathology of stellate-shaped fibroblasts with occasional giant cells. Despite these important differences, Koenen tumor does exhibit a striking morphologic similarity to ADF, given that the vertical orientation of collagen bundles in Koenen tumor is virtually identical to ADF.6
Management
There are no known associations between ADF and medication use, including methotrexate and adalimumab, which our patient was taking; additionally, no association with RA or other systemic disorder has been reported.2 The preferred treatment of ADF is complete excision to the basal attachment of the tumor; recurrence is uncommon. Alternative therapies include destructive methods, such as cryotherapy, CO2 laser ablation, and electrodesiccation.2
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
- Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;2:120-129.
- Hwang S, Kim M, Cho BK, et al. Clinical characteristics of acquired ungual fibrokeratoma. Indian J Dermatol Venereol Leprol. 2017;83:337-343.
- Yu D, Morgan RF. Acquired digital fibrokeratoma: a case report. Ann Plast Surg. 2015;74:304-305.
- Ehara Y, Yoshida Y, Ishizu S, et al. Acquired subungual fibrokeratoma. J Dermatol. 2017;44:e140-e141.
- Rubegni P, Poggiali S, Lamberti A, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol. 2012:53:47-48.
- Kint A, Baran R, De Keyser H. Acquired (digital) fibrokeratoma. J Am Acad Dermatol. 1985;12:816-821.
Practice Points
- Acquired digital fibrokeratoma is a benign tumor of the nail bed and periungual area.
- Histopathology shows epidermal acanthosis and hyperkeratosis, and collagen bundles are arranged in a vertical orientation to the long axis of the epidermis.
- Acquired digital fibrokeratoma should be considered in the differential diagnosis of flesh-colored papules on the nail unit associated with longitudinal grooves, trachyonychia, subungual hyperkeratosis, and onycholysis.
Treatment Consideration for US Military Members With Skin Disease
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
The National Defense Authorization Act for Fiscal Year 20171 has changed military medicine, including substantial reduction in military medical personnel as positions are converted to combat functions. As a result, there will be fewer military dermatologists, which means many US soldiers, sailors, airmen, and marines will seek medical care outside of military treatment facilities. This article highlights some unique treatment considerations in this patient population for our civilian dermatology colleagues.
Medical Readiness
In 2015, General Joseph F. Dunford Jr, 19th Chairman of the Joint Chiefs of Staff, made readiness his top priority for the US Armed Forces.2 Readiness refers to service members’ ability to deploy to locations across the globe and perform their military duties with little advanced notice, which requires personnel to be medically prepared at all times to leave home and perform their duties in locations with limited medical support.
Medical readiness is maintaining a unit that is medically able to perform its military function both at home and in a deployed environment. Military members’ medical readiness status is carefully tracked and determined via annual physical, dental, hearing, and vision examinations, as well as human immunodeficiency virus status and immunizations. The readiness status of the unit (ie, the number of troops ready to deploy at any given time) is available to commanders at all levels at any time. Each military branch has tracking systems that allow commanders to know when a member is past due for an examination or if a member’s medical status has changed, making them nondeployable. When a member is nondeployable, it affects the unit’s ability to perform its mission and degrades its readiness. If readiness is suboptimal, the military cannot deploy and complete its missions, which is why readiness is a top priority. The primary function of military medicine is to support the medical readiness of the force.
Deployment Eligibility
A unique aspect of military medicine that can be foreign to civilian physicians is the unit commanders’ authority to request and receive information on military members’ medical conditions as they relate to readiness. Under most circumstances, an individual’s medical information is his/her private information; however, that is not always the case in the military. If a member’s medical status changes and he/she becomes nondeployable, by regulation the commander can be privy to pertinent aspects of that member’s medical condition as it affects unit readiness, including the diagnosis, treatment plan, and prognosis. Commanders need this information to aid in the member’s recovery, ensure training does not impact his/her care, and identify possible need of replacement.
Published accession guidelines are used to determine medical eligibility for service.3 These instructions are organized by major organ systems and broad disease categories. They provide guidance on medically disqualifying conditions. The Table outlines those conditions that apply to the skin.3 Individual military branches may have additional regulations with guidance on medically disqualifying conditions that are job specific. Additional regulations also are available based on an area of military operation that can be more restrictive and specific to those locations.4
Similarly, each military branch has its own retention standards.5,6 Previously healthy individuals can develop new medical conditions, and commanders are notified if a service member becomes medically nondeployable. If a medical condition limits a service member’s ability to deploy, he/she will be evaluated for retention by a medical evaluation board (MEB). Three outcomes are possible: return in current function, retain the service member but retrain in another military occupation, or separate from military service.7 Rarely, waivers are provided so that the service member can return to duty.
Readiness and Patient Care
Importantly, readiness should not be seen as a roadblock to appropriate patient care. Patients should receive treatment that is appropriate for their medical condition. Much of the difficulty within military medicine is understanding and communicating how the natural disease history, prognosis, and treatment of their respective medical conditions will impact members’ service.
In some cases, the condition and/or treatment is incompatible with military service. Consider the following scenario: A 23-year-old active-duty soldier with a history of psoriasis developed widespread disease of 1 year’s duration and was referred to a civilian dermatologist due to nonavailability of a military dermatologist. After topical and light-based therapies failed, he was started on ustekinumab, which cleared the psoriasis. He wanted to continue on ustekinumab due to its good efficacy, but his unit was set to deploy in the coming year, and the drug made him medically nondeployable due to its immunosuppressive nature.
This real-life example was a difficult case to disposition. The service member was unsure if he could perform his military duties and deploy without continuing treatment with ustekinumab. His prior dermatology notes were requested to better assess the severity of his baseline disease, followed by a candid discussion between the military dermatologist and the patient about treatment options and their respective ramifications to his military career. One option included continuing ustekinumab, which would initiate an MEB evaluation and likely result in separation. Another option was UV therapy, which would not prompt an MEB evaluation but would not be available in deployed environments. Apremilast was offered as a third treatment option and could be used in place of UV therapy during deployment along with topical medications. This patient opted to continue treatment with ustekinumab, resulting in MEB review and separation from military service.
Dermatology Treatment Considerations
Civilian dermatologists should be aware of specific considerations when treating active US service members with common cutaneous diagnoses such as acne, atopic dermatitis (AD), psoriasis, dissecting cellulitis of the scalp (DCS), and lupus erythematosus (LE). This discussion is not meant to be all-inclusive but provides information and examples related to common treatment challenges in this patient population.
Acne
Acne is common in the active-duty military population. Typically, acne should be treated per recommended guidelines based on type and severity.8 Medical evaluation board review is warranted in cases of severe acne that is unresponsive to treatment and interferes with a service member’s performance.5,6 Unique situations in the active-duty military population include the following:
• Use of minocycline. Aircrew members have unique restrictions on many medications,6 including minocycline, which is restricted in this population due to vestibular side effects. Doxycycline is an acceptable alternative for aircrew members; however, even this medication may require a ground trial to ensure there are no idiosyncratic effects.
• Use of isotretinoin, which is not permitted in aircrew members, submariners, or divers. If they take this medication, they will be temporarily removed from duty for the duration of treatment and for a period of time after completion (1–3 months, depending on service). Isotretinoin also is not used during deployment due to potential side effects, the need for laboratory monitoring, and iPLEDGE system requirements.
Atopic Dermatitis
A history of AD after the 12th birthday is considered a disqualifying condition with regard to military service,3 though mild and well-controlled disease can easily be overlooked during entrance physical examinations. Members frequently present with eczema flares following field training exercises where they are outdoors for many hours and have been exposed to grass or other environmental triggers while wearing military gear that is heavy and occlusive, which is further exacerbated by being unable to bathe or care for their skin as they would at home.
Separation from the military is considered when AD is moderate to severe, is unresponsive to treatment, and/or interferes with performance of duty. Severity often can be evaluated based on the impact of AD on performance of duties in addition to clinical appearance. A pilot who is distracted by itching presents a potentially dangerous situation. A soldier whose AD flares every time he/she goes to the field, requiring him/her to return home early to control symptoms, can be considered moderate to severe due to lack of ability to do his/her job away from home base.
Response to treatment is more often where trouble lies for military members with AD, as patients are only permitted to take emollients, preferred cleansers, and topical medications to field training exercises and deployments. UV therapy is used to control disease in the military population but is not an option in deployed environments. Classic immunosuppressants (eg, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine) may result in a good response to treatment; however, due to their side-effect profiles, need for laboratory monitoring, and immunosuppressive nature, long-term use of those medications will result in a nondeployable status. Dupilumab does not appear to have the immunosuppressive effects of other biologics; however, the medication requires refrigeration,9 which currently precludes its use in the deployed environment, as it would be difficult to ensure supply and storage in remote areas.
Service members with a history of AD are exempt from the smallpox vaccine due to concerns about eczema vaccinatum.10
Psoriasis
Psoriasis is another dermatologic condition that does not meet military admission standards,3 and mild undiagnosed cases may be overlooked during the entrance physical examination. Because psoriasis commonly affects young adults, it may manifest in service members after entering service. If psoriasis is extensive or refractory to treatment, an MEB evaluation may be required.5,6 Widespread psoriasis can result in considerable discomfort when wearing body armor and other military gear. Severe localized disease can have duty implications; service members with treatment-resistant scalp psoriasis or pustular psoriasis of the feet may have difficulty wearing helmets or military boots, respectively.
Most service members with limited psoriasis vulgaris can be managed with topical steroids and steroid-sparing agents such as calcipotriene. Some service members opt not to aggressively treat their psoriasis if it is limited in nature and not symptomatic.
When discussing systemic treatments beyond light therapy in those with refractory disease, apremilast can be a good first-line treatment option.11 It is an oral medication, has minimal monitoring requirements, and lacks immunosuppressive side effects; therefore, it does not adversely impact deployability. If patients do not improve in 4 months with apremilast, biologics should then be considered; however, biologics have service implications, the most important being inability to deploy while taking the medication. In rare circumstances, military dermatologists may discuss utilizing biologic therapy only in the nondeployed setting. In these cases, service members are counseled that biologic therapy will be discontinued if they deploy in the future and treatment will be sustained with topicals and/or apremilast through the deployment. The treatment plan also should be communicated to the patient’s primary care provider to ensure that he/she is in agreement.
Dissecting Cellulitis of the Scalp
Dissecting cellulitis of the scalp may result in separation if the condition is unresponsive to treatment and/or interferes with satisfactory performance of duty.5 In addition to causing considerable pain, this condition can prevent service members from wearing combat helmets, which limits their ability to train and deploy. One of the authors (S.C.) has had more service members undergo an MEB evaluation for DCS than any of the other conditions mentioned.
Topical tretinoin and topical antibiotics can be used in conjunction with either doxycycline or minocycline to treat DCS, with the addition of intralesional corticosteroids for painful nodules. Fluctuant lesions are treated with incision and drainage. If there is inadequate response to treatment after 2 to 3 months, oral clindamycin and rifampin can be tried for 3 months. As an alternative measure or if the condition is refractory to oral clindamycin and rifampin, isotretinoin can then be used. One of the authors (S.C.) typically recommends a temporary no-helmet profile to the patient’s primary care provider until his/her next dermatology appointment. If the patient still has substantial disease despite these treatment options, it is recommended that the patient be issued a permanent profile for no helmet wear, which will prompt an MEB evaluation. Although tumor necrosis factor α inhibitors can work well in patients with DCS, the use of biologics is not conducive to continued service.
Lupus Erythematosus
A history of LE is disqualifying from military service. Patients who develop LE while on active duty will be referred for MEB evaluation if their disease is unresponsive to treatment and/or interferes with the satisfactory performance of duty.5,6 In general, connective tissue diseases have an array of physical implications that can affect military service, including photosensitivity, joint inflammation, and internal organ involvement. Similar to the other dermatologic conditions described, treatment of connective tissue diseases also can present challenges to continued military service. Considerations in the case of LE that are unique to military service members include the following:
• Sun exposure. Most military service members are required to work outside in all manners of conditions, which include hot, sunny, humid, and/or dry climates. Often physicians might counsel sun-sensitive patients with LE to avoid being outside during daylight hours, limit window exposure at work, and avoid daytime driving when possible; however, these recommendations are not possible for many, if not most, service members.
• Immunosuppressive therapies are incompatible with military deployment; therefore, prescribing methotrexate, cyclosporine, mycophenolate mofetil, rituximab, or belimumab for treatment of LE would prompt an MEB evaluation if the treatment is necessary to control the disease.
Final Thoughts
The recent changes to military medicine are needed to meet our country’s defense requirements and will ultimately result in civilian specialists playing a larger role in the care of our military population. This article highlights unique factors civilian dermatologists must consider when treating active-duty military patients to ensure they remain deployable during treatment.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
- National Defense Authorization Act for Fiscal Year 2017, S 2943, 114th Congress, 2nd Sess (2016).
- Garamone J. Dunford sends message to joint force, stresses readiness, warfighting, education [news release]. Washington, DC: US Department of Defense; October 2, 2015. https://dod.defense.gov/News/Article/Article/621725/dunford-sends-message-to-joint-force-stresses-readiness-warfighting-education/. Accessed May 17, 2019.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; March 30, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 17, 2019.
- Force health protection guidance for deployment in USSOUTHCOM as of 7 December 2017. US Southern Command website. https://www.southcom.mil/Portals/7/Documents/Operational%20Contract%20Support/USSOUTHCOM_Force_Health_Protection_Guidance_AS_OF_7_DEC_2017.pdf?ver=2018-01-29-100603-957. Published December 7, 2017. Accessed May 28, 2019.
- US Department of the Army. Standards of medical fitness. http://www.au.af.mil/au/awc/awcgate/army/r40_501.pdf. Published August 26, 2003. Accessed May 17, 2019.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed May 17, 2019.
- Medical and physical evaluation boards (MEB/PEB). US Army Warrior Care and Transition website. https://wct.army.mil/modules/soldier/s6-medicalBoards.html. Accessed May 28, 2019.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Dupixent [package insert]. Tarrytown, NY: Regeneron, Inc; 2017.
- Departments of the Army, the Navy, the Air Force, and the Coast Guard. Immunizations and chemoprophylaxis for the prevention of infectious diseases. https://health.mil/Reference-Center/Policies/2013/10/07/Immunizations-and-Chemoprophylaxis-for-the-Prevention-of-Infectious-Diseases. Published October 7, 2013. Accessed May 28, 2019.
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631.
Practice Points
- Certain conditions and treatments are incompatible with military service and may result in separation.
- Dermatologists must consider a patient’s profession when choosing a treatment modality.