User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
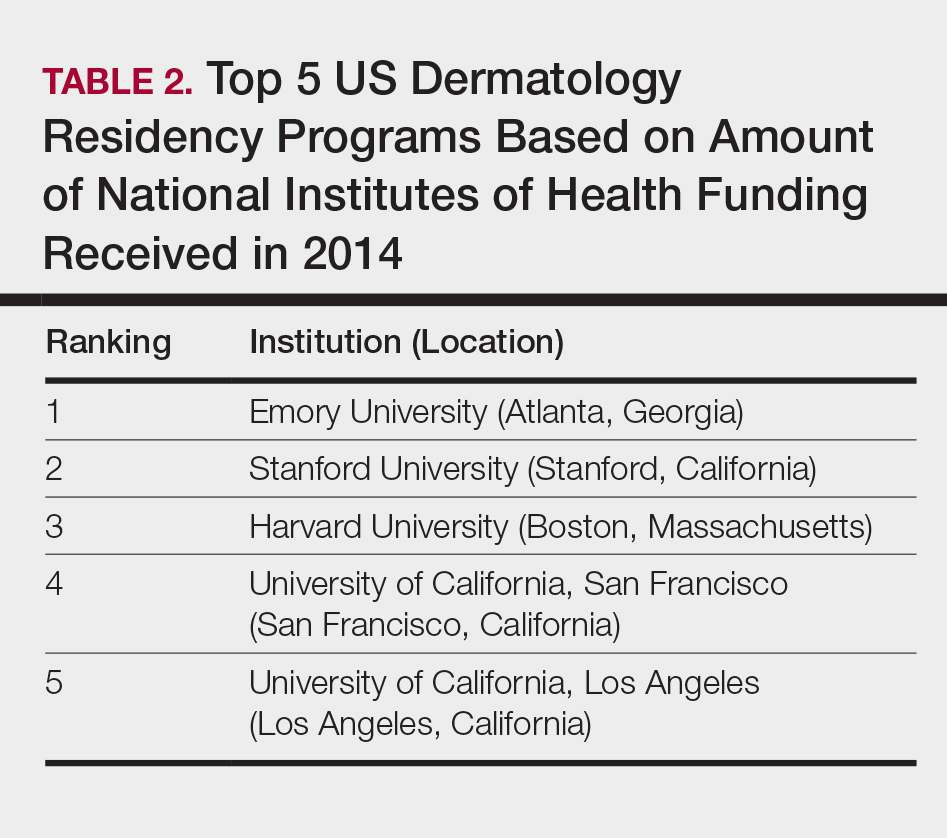
US Dermatology Residency Program Rankings Based on Academic Achievement
Rankings of US residency programs based on academic achievement are a resource for fourth-year medical students applying for residency through the National Resident Matching Program. They also highlight the leading academic training programs in each medical specialty. Currently, the Doximity Residency Navigator (https://residency.doximity.com) provides rankings of US residency programs based on either subjective or objective criteria. The subjective rankings utilize current resident and recent alumni satisfaction surveys as well as nominations from board-certified Doximity members who were asked to nominate up to 5 residency programs in their specialty that offer the best clinical training. The objective rankings are based on measurement of research output, which is calculated from the collective h-index of publications authored by graduating alumni within the last 15 years as well as the amount of research funding awarded.1
Aquino et al2 provided a ranking of US dermatology residency programs using alternative objective data measures (as of December 31, 2008) from the Doximity algorithm, including National Institutes of Health (NIH) and Dermatology Foundation (DF) funding, number of publications by full-time faculty members, number of faculty lectures given at annual meetings of 5 societies, and number of full-time faculty members serving on the editorial boards of 6 dermatology journals. The current study is an update to those rankings utilizing data from 2014.
Methods
The following data for each dermatology residency program were obtained to formulate the rankings: number of full-time faculty members, amount of NIH funding received in 2014 (https://report.nih.gov/), number of publications by full-time faculty members in 2014 (http://www.ncbi.nlm.nih.gov/pubmed/), and the number of faculty lectures given at annual meetings of 5 societies in 2014 (American Academy of Dermatology, the Society for Investigative Dermatology, the American Society of Dermatopathology, the Society for Pediatric Dermatology, and the American Society for Dermatologic Surgery). This study was approved by the institutional review board at Kaiser Permanente Southern California.
The names of all US dermatology residency programs were obtained as of December 31, 2014, from FREIDA Online using the search term dermatology. An email was sent to a representative from each residency program (eg, residency program coordinator, program director, full-time faculty member) requesting confirmation of a list of full-time faculty members in the program, excluding part-time and volunteer faculty. If a response was not obtained or the representative declined to participate, a list was compiled using available information from that residency program’s website.
National Institutes of Health funding for 2014 was obtained for individual faculty members from the NIH Research Portfolio Online Reporting Tools expenditures and reports (https://projectreporter.nih.gov/reporter.cfm) by searching the first and last name of each full-time faculty member along with their affiliated institution. The search results were filtered to only include NIH funding for full-time faculty members listed as principal investigators rather than as coinvestigators. The fiscal year total cost by institute/center for each full-time faculty member’s projects was summated to obtain the total NIH funding for the program.
The total number of publications by full-time faculty members in 2014 was obtained utilizing a PubMed search of articles indexed for MEDLINE using each faculty member’s first and last name. The authors’ affiliations were verified for each publication, and the number of publications was summed for all full-time faculty members at each residency program. If multiple authors from the same program coauthored an article, it was only counted once toward the total number of faculty publications from that program.
Program brochures for the 2014 meetings of the 5 societies were reviewed to quantify the number of lectures given by full-time faculty members in each program.
Each residency program was assigned a score from 0 to 1.0 for each of the 4 factors of academic achievement analyzed. The program with the highest number of faculty publications was assigned a score of 1.0 and the program with the lowest number of publications was assigned a score of 0. The programs in between were subsequently assigned scores from 0 to 1.0 based on the number of publications as a percentage of the number of publications from the program with the most publications.
A weighted ranking scheme was used to rank residency programs based on the relative importance of each factor. There were 3 factors that were deemed to be the most reflective of academic achievement among dermatology residency programs: amount of NIH funding received in 2014, number of publications by full-time faculty members in 2014, and number of faculty lectures given at society meetings in 2014; thus, these factors were given a weight of 1.0. The remaining factor— total number of full-time faculty members—was given a weight of 0.5. Values were totaled and programs were ranked based on the sum of these values. All quantitative analyses were performed using an electronic spreadsheet program.
Results
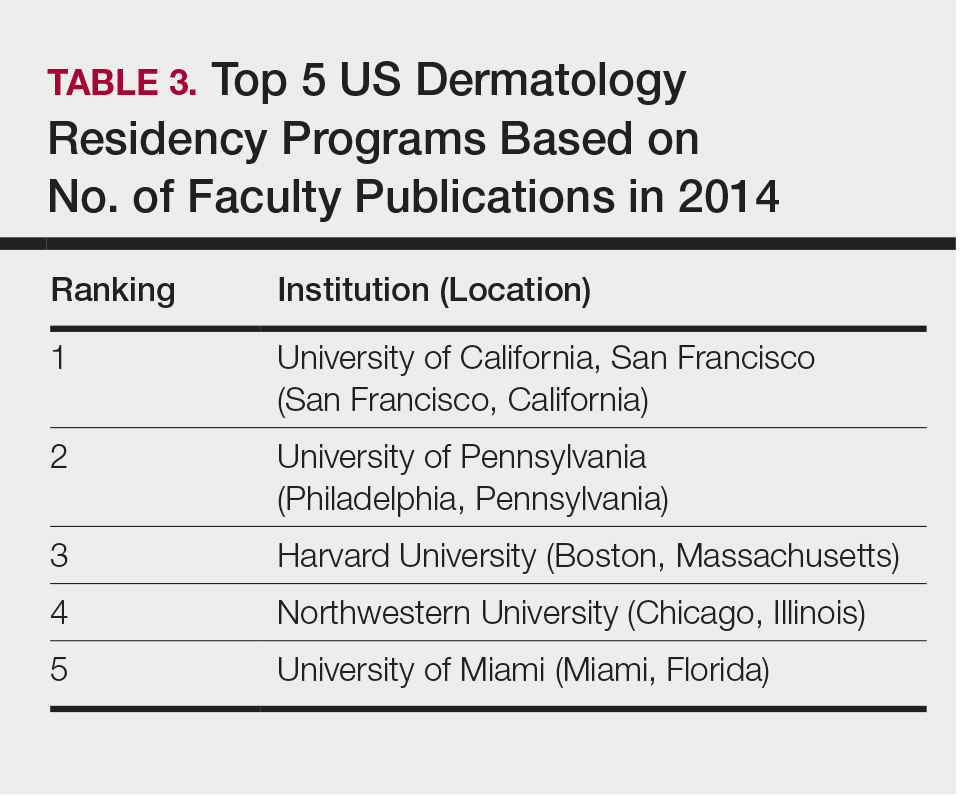
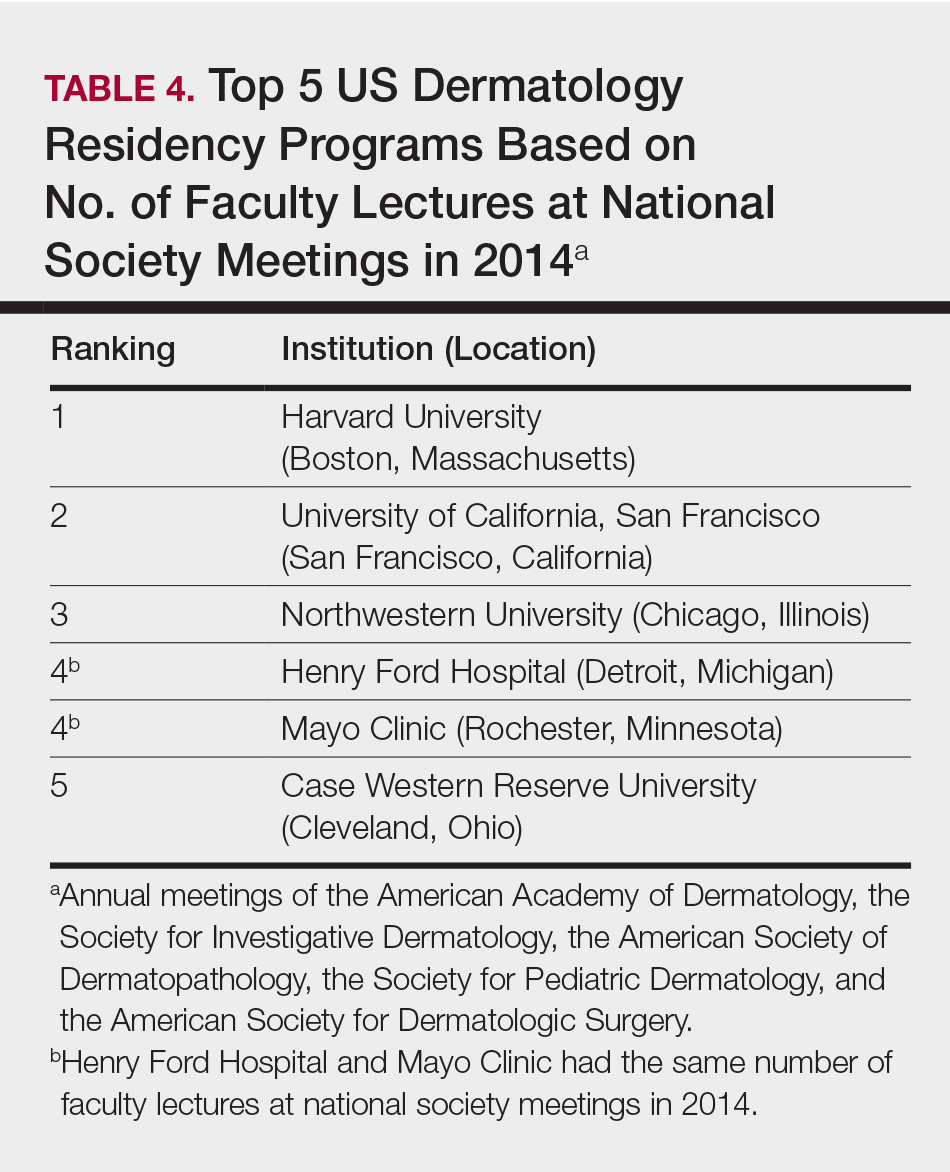
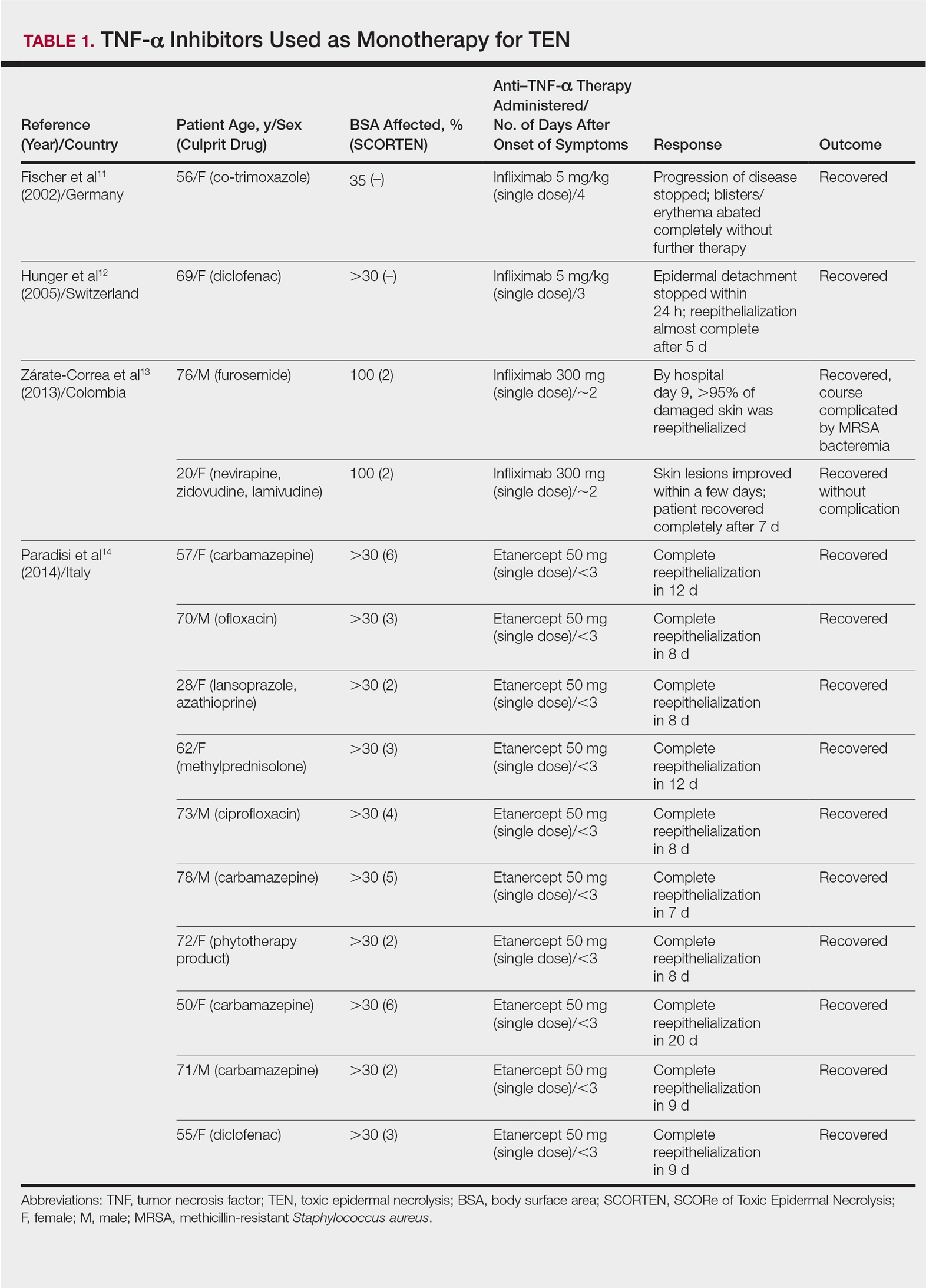
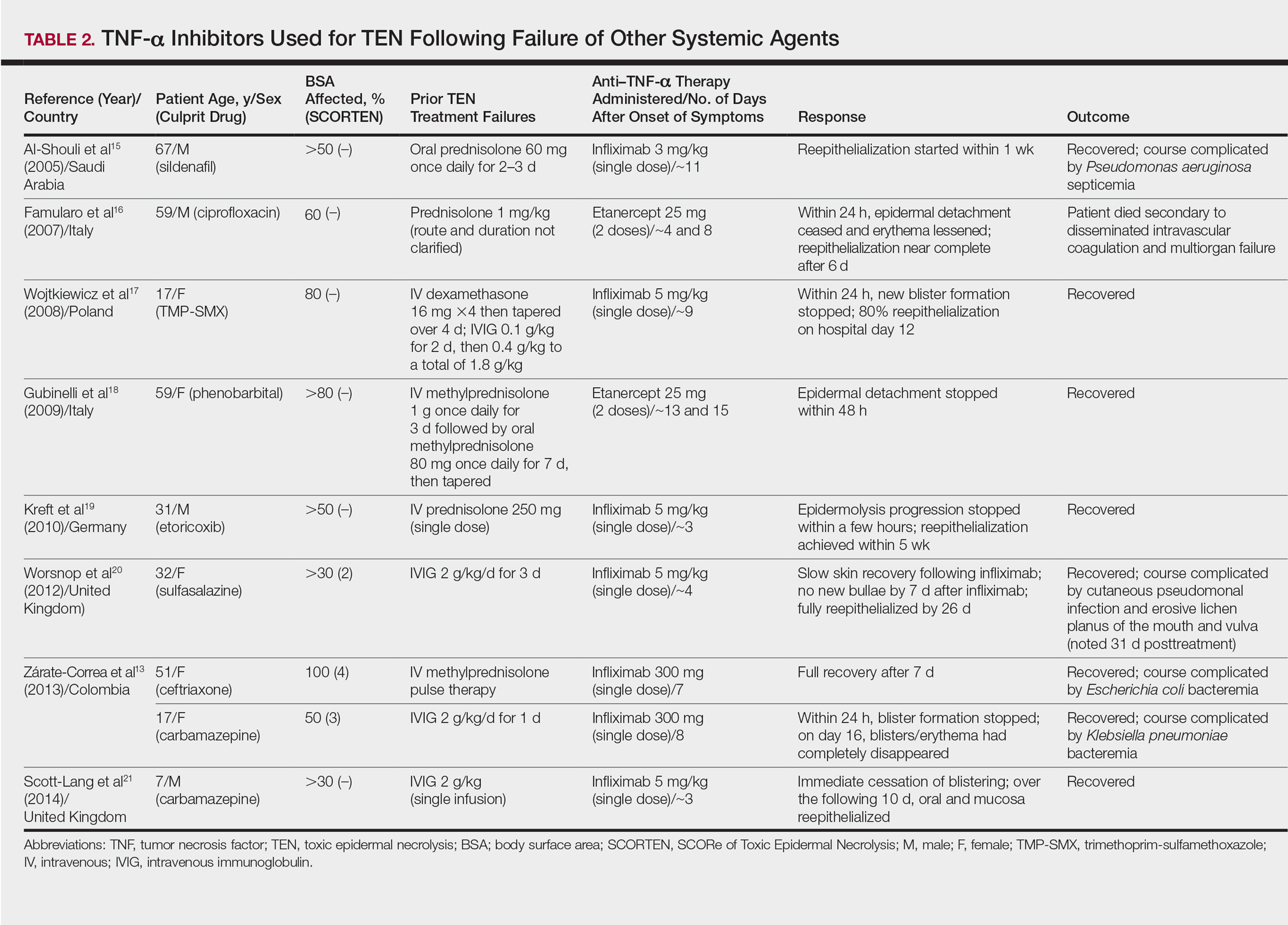
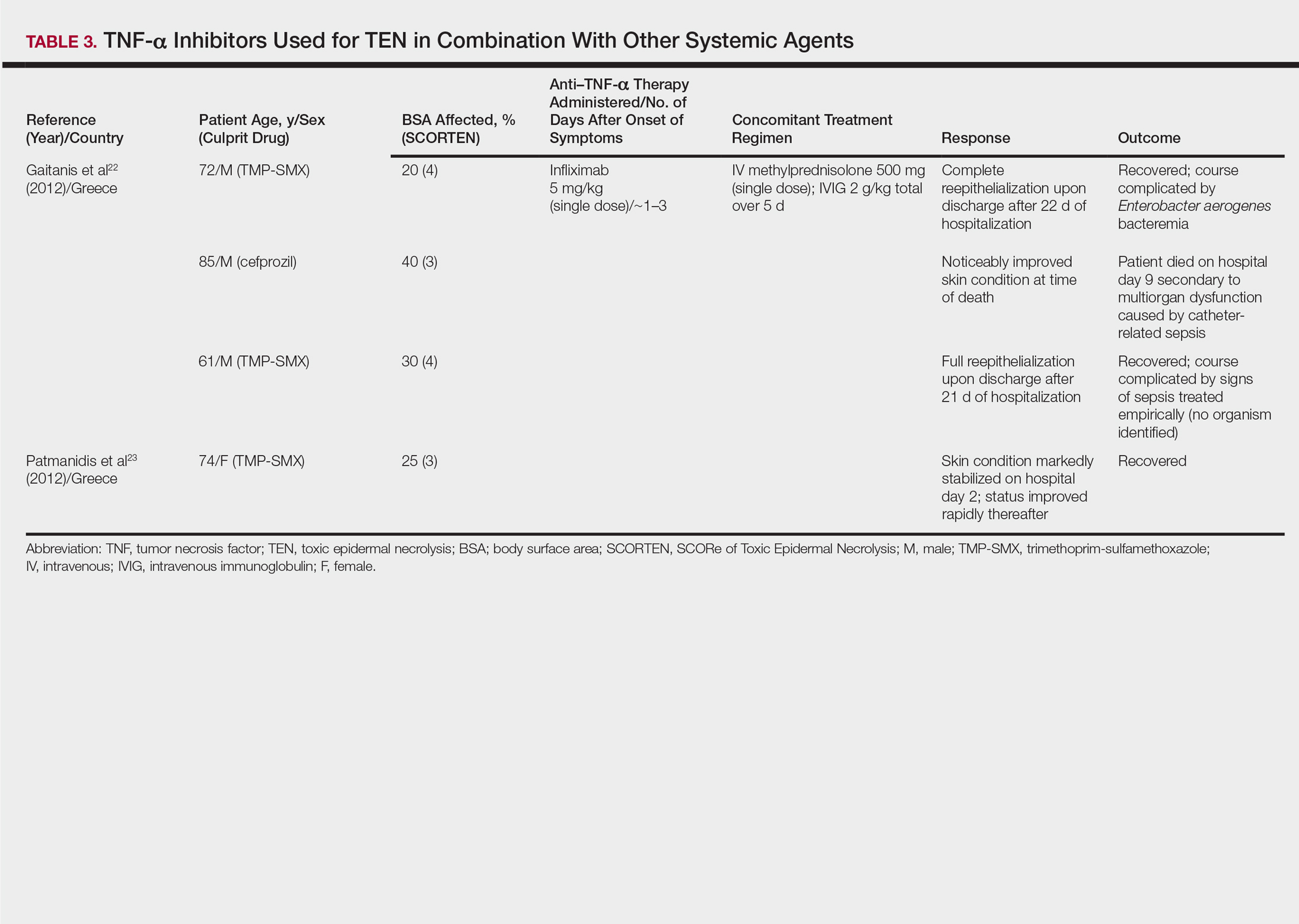
The overall ranking of the top 20 US dermatology residency programs in 2014 is presented in Table 1. The top 5 programs based on each of the 3 factors most reflective of academic achievement used in the weighted ranking algorithm are presented in Tables 2 through 4.
Comment
The ranking of US residency programs involves using data in an unbiased manner while also accounting for important subjective measures. In a 2015 survey of residency applicants (n=6285), the 5 most important factors for applicants in selecting a program were the program’s ability to prepare residents for future training or position, resident esprit de corps, faculty availability and involvement in teaching, depth and breadth of faculty, and variety of patients and clinical resources.3 However, these subjective measures are difficult to quantify in a standardized fashion. In its ranking of residency programs, the Doximity Residency Navigator utilizes surveys of current residents and recent alumni as well as nominations from board-certified Doximity members.1
One of the main issues in utilizing survey data to rank residency programs is the inherent bias that most residents and alumni possess toward their own program. Moreover, the question arises whether most residents, faculty members, or recent alumni of residency programs have sufficient knowledge of other programs to rank them in a well-informed manner.
Wu et al4 used data from 2004 to perform the first algorithmic ranking of US dermatology programs, which was based on publications in 2001 to 2004, the amount of NIH funding in 2004, DF grants in 2001 to 2004, faculty lectures delivered at national conferences in 2004, and number of full-time faculty members on the editorial boards of the top 3 US dermatology journals and the top 4 subspecialty journals. Aquino et al2 provided updated rankings that utilized a weighted algorithm to collect data from 2008 related to a number of factors, including annual amount of NIH and DF funding received, number of publications by full-time faculty members, number of faculty lectures given at 5 annual society meetings, and number of full-time faculty members who were on the editorial boards of 6 dermatology journals with the highest impact factors. The top 5 ranked programs based on the 2008 data were the University of California, San Francisco (San Francisco, California); Northwestern University (Chicago, Illinois); University of Pennsylvania (Philadelphia, Pennsylvania); Yale University (New Haven, Connecticut); and Stanford University (Stanford, California).2
The current ranking algorithm is more indicative of a residency program’s commitment to research and scholarship, with an assumption that successful clinical training is offered. Leading researchers in the field also are usually known to be clinical experts, but the current data does not take into account the frequency, quality, or methodology of teaching provided to residents. Perhaps the most objective measure reflecting the quality of resident education would be American Board of Dermatology examination scores, but these data are not publically available. Additional factors such as the percentage of residents who received fellowship positions; diversity of the patient population; and number and extent of surgical, cosmetic, or laser procedures performed also are not readily available. Doximity provides board pass rates for each residency program, but these data are self-reported and are not taken into account in their rankings.1
The current study aimed to utilize publicly available data to rank US dermatology residency programs based on objective measures of academic achievement. A recent study showed that 531 of 793 applicants (67%) to emergency medicine residency programs were aware of the Doximity residency rankings.One-quarter of these applicants made changes to their rank list based on this data, demonstrating that residency rankings may impact applicant decision-making.5 In the future, the most accurate and unbiased rankings may be performed if each residency program joins a cooperative effort to provide more objective data about the training they provide and utilizes a standardized survey system for current residents and recent graduates to evaluate important subjective measures.
Conclusion
Based on our weighted ranking algorithm, the top 5 dermatology residency programs in 2014 were Harvard University (Boston, Massachusetts); University of California, San Francisco (San Francisco, California); Stanford University (Stanford, California); University of Pennsylvania (Philadelphia, Pennsylvania); and Emory University (Atlanta, Georgia).
Acknowledgments
We thank all of the program coordinators, full-time faculty members, program directors, and chairs who provided responses to our inquiries for additional information about their residency programs.
- Residency navigator 2017-2018. Doximity website. https://residency.doximity.com. Accessed January 19, 2018.
- Aquino LL, Wen G, Wu JJ. US dermatology residency program rankings. Cutis. 2014;94:189-194.
- Phitayakorn R, Macklin EA, Goldsmith J, et al. Applicants’ self-reported priorities in selecting a residency program. J Grad Med Educ. 2015;7:21-26.
- Wu JJ, Ramirez CC, Alonso CA, et al. Ranking the dermatology programs based on measurements of academic achievement. Dermatol Online J. 2007;13:3.
- Peterson WJ, Hopson LR, Khandelwal S. Impact of Doximity residency rankings on emergency medicine applicant rank lists [published online May 5, 2016]. West J Emerg Med. 2016;17:350-354.
Rankings of US residency programs based on academic achievement are a resource for fourth-year medical students applying for residency through the National Resident Matching Program. They also highlight the leading academic training programs in each medical specialty. Currently, the Doximity Residency Navigator (https://residency.doximity.com) provides rankings of US residency programs based on either subjective or objective criteria. The subjective rankings utilize current resident and recent alumni satisfaction surveys as well as nominations from board-certified Doximity members who were asked to nominate up to 5 residency programs in their specialty that offer the best clinical training. The objective rankings are based on measurement of research output, which is calculated from the collective h-index of publications authored by graduating alumni within the last 15 years as well as the amount of research funding awarded.1
Aquino et al2 provided a ranking of US dermatology residency programs using alternative objective data measures (as of December 31, 2008) from the Doximity algorithm, including National Institutes of Health (NIH) and Dermatology Foundation (DF) funding, number of publications by full-time faculty members, number of faculty lectures given at annual meetings of 5 societies, and number of full-time faculty members serving on the editorial boards of 6 dermatology journals. The current study is an update to those rankings utilizing data from 2014.
Methods
The following data for each dermatology residency program were obtained to formulate the rankings: number of full-time faculty members, amount of NIH funding received in 2014 (https://report.nih.gov/), number of publications by full-time faculty members in 2014 (http://www.ncbi.nlm.nih.gov/pubmed/), and the number of faculty lectures given at annual meetings of 5 societies in 2014 (American Academy of Dermatology, the Society for Investigative Dermatology, the American Society of Dermatopathology, the Society for Pediatric Dermatology, and the American Society for Dermatologic Surgery). This study was approved by the institutional review board at Kaiser Permanente Southern California.
The names of all US dermatology residency programs were obtained as of December 31, 2014, from FREIDA Online using the search term dermatology. An email was sent to a representative from each residency program (eg, residency program coordinator, program director, full-time faculty member) requesting confirmation of a list of full-time faculty members in the program, excluding part-time and volunteer faculty. If a response was not obtained or the representative declined to participate, a list was compiled using available information from that residency program’s website.
National Institutes of Health funding for 2014 was obtained for individual faculty members from the NIH Research Portfolio Online Reporting Tools expenditures and reports (https://projectreporter.nih.gov/reporter.cfm) by searching the first and last name of each full-time faculty member along with their affiliated institution. The search results were filtered to only include NIH funding for full-time faculty members listed as principal investigators rather than as coinvestigators. The fiscal year total cost by institute/center for each full-time faculty member’s projects was summated to obtain the total NIH funding for the program.
The total number of publications by full-time faculty members in 2014 was obtained utilizing a PubMed search of articles indexed for MEDLINE using each faculty member’s first and last name. The authors’ affiliations were verified for each publication, and the number of publications was summed for all full-time faculty members at each residency program. If multiple authors from the same program coauthored an article, it was only counted once toward the total number of faculty publications from that program.
Program brochures for the 2014 meetings of the 5 societies were reviewed to quantify the number of lectures given by full-time faculty members in each program.
Each residency program was assigned a score from 0 to 1.0 for each of the 4 factors of academic achievement analyzed. The program with the highest number of faculty publications was assigned a score of 1.0 and the program with the lowest number of publications was assigned a score of 0. The programs in between were subsequently assigned scores from 0 to 1.0 based on the number of publications as a percentage of the number of publications from the program with the most publications.
A weighted ranking scheme was used to rank residency programs based on the relative importance of each factor. There were 3 factors that were deemed to be the most reflective of academic achievement among dermatology residency programs: amount of NIH funding received in 2014, number of publications by full-time faculty members in 2014, and number of faculty lectures given at society meetings in 2014; thus, these factors were given a weight of 1.0. The remaining factor— total number of full-time faculty members—was given a weight of 0.5. Values were totaled and programs were ranked based on the sum of these values. All quantitative analyses were performed using an electronic spreadsheet program.
Results
The overall ranking of the top 20 US dermatology residency programs in 2014 is presented in Table 1. The top 5 programs based on each of the 3 factors most reflective of academic achievement used in the weighted ranking algorithm are presented in Tables 2 through 4.
Comment
The ranking of US residency programs involves using data in an unbiased manner while also accounting for important subjective measures. In a 2015 survey of residency applicants (n=6285), the 5 most important factors for applicants in selecting a program were the program’s ability to prepare residents for future training or position, resident esprit de corps, faculty availability and involvement in teaching, depth and breadth of faculty, and variety of patients and clinical resources.3 However, these subjective measures are difficult to quantify in a standardized fashion. In its ranking of residency programs, the Doximity Residency Navigator utilizes surveys of current residents and recent alumni as well as nominations from board-certified Doximity members.1
One of the main issues in utilizing survey data to rank residency programs is the inherent bias that most residents and alumni possess toward their own program. Moreover, the question arises whether most residents, faculty members, or recent alumni of residency programs have sufficient knowledge of other programs to rank them in a well-informed manner.
Wu et al4 used data from 2004 to perform the first algorithmic ranking of US dermatology programs, which was based on publications in 2001 to 2004, the amount of NIH funding in 2004, DF grants in 2001 to 2004, faculty lectures delivered at national conferences in 2004, and number of full-time faculty members on the editorial boards of the top 3 US dermatology journals and the top 4 subspecialty journals. Aquino et al2 provided updated rankings that utilized a weighted algorithm to collect data from 2008 related to a number of factors, including annual amount of NIH and DF funding received, number of publications by full-time faculty members, number of faculty lectures given at 5 annual society meetings, and number of full-time faculty members who were on the editorial boards of 6 dermatology journals with the highest impact factors. The top 5 ranked programs based on the 2008 data were the University of California, San Francisco (San Francisco, California); Northwestern University (Chicago, Illinois); University of Pennsylvania (Philadelphia, Pennsylvania); Yale University (New Haven, Connecticut); and Stanford University (Stanford, California).2
The current ranking algorithm is more indicative of a residency program’s commitment to research and scholarship, with an assumption that successful clinical training is offered. Leading researchers in the field also are usually known to be clinical experts, but the current data does not take into account the frequency, quality, or methodology of teaching provided to residents. Perhaps the most objective measure reflecting the quality of resident education would be American Board of Dermatology examination scores, but these data are not publically available. Additional factors such as the percentage of residents who received fellowship positions; diversity of the patient population; and number and extent of surgical, cosmetic, or laser procedures performed also are not readily available. Doximity provides board pass rates for each residency program, but these data are self-reported and are not taken into account in their rankings.1
The current study aimed to utilize publicly available data to rank US dermatology residency programs based on objective measures of academic achievement. A recent study showed that 531 of 793 applicants (67%) to emergency medicine residency programs were aware of the Doximity residency rankings.One-quarter of these applicants made changes to their rank list based on this data, demonstrating that residency rankings may impact applicant decision-making.5 In the future, the most accurate and unbiased rankings may be performed if each residency program joins a cooperative effort to provide more objective data about the training they provide and utilizes a standardized survey system for current residents and recent graduates to evaluate important subjective measures.
Conclusion
Based on our weighted ranking algorithm, the top 5 dermatology residency programs in 2014 were Harvard University (Boston, Massachusetts); University of California, San Francisco (San Francisco, California); Stanford University (Stanford, California); University of Pennsylvania (Philadelphia, Pennsylvania); and Emory University (Atlanta, Georgia).
Acknowledgments
We thank all of the program coordinators, full-time faculty members, program directors, and chairs who provided responses to our inquiries for additional information about their residency programs.
Rankings of US residency programs based on academic achievement are a resource for fourth-year medical students applying for residency through the National Resident Matching Program. They also highlight the leading academic training programs in each medical specialty. Currently, the Doximity Residency Navigator (https://residency.doximity.com) provides rankings of US residency programs based on either subjective or objective criteria. The subjective rankings utilize current resident and recent alumni satisfaction surveys as well as nominations from board-certified Doximity members who were asked to nominate up to 5 residency programs in their specialty that offer the best clinical training. The objective rankings are based on measurement of research output, which is calculated from the collective h-index of publications authored by graduating alumni within the last 15 years as well as the amount of research funding awarded.1
Aquino et al2 provided a ranking of US dermatology residency programs using alternative objective data measures (as of December 31, 2008) from the Doximity algorithm, including National Institutes of Health (NIH) and Dermatology Foundation (DF) funding, number of publications by full-time faculty members, number of faculty lectures given at annual meetings of 5 societies, and number of full-time faculty members serving on the editorial boards of 6 dermatology journals. The current study is an update to those rankings utilizing data from 2014.
Methods
The following data for each dermatology residency program were obtained to formulate the rankings: number of full-time faculty members, amount of NIH funding received in 2014 (https://report.nih.gov/), number of publications by full-time faculty members in 2014 (http://www.ncbi.nlm.nih.gov/pubmed/), and the number of faculty lectures given at annual meetings of 5 societies in 2014 (American Academy of Dermatology, the Society for Investigative Dermatology, the American Society of Dermatopathology, the Society for Pediatric Dermatology, and the American Society for Dermatologic Surgery). This study was approved by the institutional review board at Kaiser Permanente Southern California.
The names of all US dermatology residency programs were obtained as of December 31, 2014, from FREIDA Online using the search term dermatology. An email was sent to a representative from each residency program (eg, residency program coordinator, program director, full-time faculty member) requesting confirmation of a list of full-time faculty members in the program, excluding part-time and volunteer faculty. If a response was not obtained or the representative declined to participate, a list was compiled using available information from that residency program’s website.
National Institutes of Health funding for 2014 was obtained for individual faculty members from the NIH Research Portfolio Online Reporting Tools expenditures and reports (https://projectreporter.nih.gov/reporter.cfm) by searching the first and last name of each full-time faculty member along with their affiliated institution. The search results were filtered to only include NIH funding for full-time faculty members listed as principal investigators rather than as coinvestigators. The fiscal year total cost by institute/center for each full-time faculty member’s projects was summated to obtain the total NIH funding for the program.
The total number of publications by full-time faculty members in 2014 was obtained utilizing a PubMed search of articles indexed for MEDLINE using each faculty member’s first and last name. The authors’ affiliations were verified for each publication, and the number of publications was summed for all full-time faculty members at each residency program. If multiple authors from the same program coauthored an article, it was only counted once toward the total number of faculty publications from that program.
Program brochures for the 2014 meetings of the 5 societies were reviewed to quantify the number of lectures given by full-time faculty members in each program.
Each residency program was assigned a score from 0 to 1.0 for each of the 4 factors of academic achievement analyzed. The program with the highest number of faculty publications was assigned a score of 1.0 and the program with the lowest number of publications was assigned a score of 0. The programs in between were subsequently assigned scores from 0 to 1.0 based on the number of publications as a percentage of the number of publications from the program with the most publications.
A weighted ranking scheme was used to rank residency programs based on the relative importance of each factor. There were 3 factors that were deemed to be the most reflective of academic achievement among dermatology residency programs: amount of NIH funding received in 2014, number of publications by full-time faculty members in 2014, and number of faculty lectures given at society meetings in 2014; thus, these factors were given a weight of 1.0. The remaining factor— total number of full-time faculty members—was given a weight of 0.5. Values were totaled and programs were ranked based on the sum of these values. All quantitative analyses were performed using an electronic spreadsheet program.
Results
The overall ranking of the top 20 US dermatology residency programs in 2014 is presented in Table 1. The top 5 programs based on each of the 3 factors most reflective of academic achievement used in the weighted ranking algorithm are presented in Tables 2 through 4.
Comment
The ranking of US residency programs involves using data in an unbiased manner while also accounting for important subjective measures. In a 2015 survey of residency applicants (n=6285), the 5 most important factors for applicants in selecting a program were the program’s ability to prepare residents for future training or position, resident esprit de corps, faculty availability and involvement in teaching, depth and breadth of faculty, and variety of patients and clinical resources.3 However, these subjective measures are difficult to quantify in a standardized fashion. In its ranking of residency programs, the Doximity Residency Navigator utilizes surveys of current residents and recent alumni as well as nominations from board-certified Doximity members.1
One of the main issues in utilizing survey data to rank residency programs is the inherent bias that most residents and alumni possess toward their own program. Moreover, the question arises whether most residents, faculty members, or recent alumni of residency programs have sufficient knowledge of other programs to rank them in a well-informed manner.
Wu et al4 used data from 2004 to perform the first algorithmic ranking of US dermatology programs, which was based on publications in 2001 to 2004, the amount of NIH funding in 2004, DF grants in 2001 to 2004, faculty lectures delivered at national conferences in 2004, and number of full-time faculty members on the editorial boards of the top 3 US dermatology journals and the top 4 subspecialty journals. Aquino et al2 provided updated rankings that utilized a weighted algorithm to collect data from 2008 related to a number of factors, including annual amount of NIH and DF funding received, number of publications by full-time faculty members, number of faculty lectures given at 5 annual society meetings, and number of full-time faculty members who were on the editorial boards of 6 dermatology journals with the highest impact factors. The top 5 ranked programs based on the 2008 data were the University of California, San Francisco (San Francisco, California); Northwestern University (Chicago, Illinois); University of Pennsylvania (Philadelphia, Pennsylvania); Yale University (New Haven, Connecticut); and Stanford University (Stanford, California).2
The current ranking algorithm is more indicative of a residency program’s commitment to research and scholarship, with an assumption that successful clinical training is offered. Leading researchers in the field also are usually known to be clinical experts, but the current data does not take into account the frequency, quality, or methodology of teaching provided to residents. Perhaps the most objective measure reflecting the quality of resident education would be American Board of Dermatology examination scores, but these data are not publically available. Additional factors such as the percentage of residents who received fellowship positions; diversity of the patient population; and number and extent of surgical, cosmetic, or laser procedures performed also are not readily available. Doximity provides board pass rates for each residency program, but these data are self-reported and are not taken into account in their rankings.1
The current study aimed to utilize publicly available data to rank US dermatology residency programs based on objective measures of academic achievement. A recent study showed that 531 of 793 applicants (67%) to emergency medicine residency programs were aware of the Doximity residency rankings.One-quarter of these applicants made changes to their rank list based on this data, demonstrating that residency rankings may impact applicant decision-making.5 In the future, the most accurate and unbiased rankings may be performed if each residency program joins a cooperative effort to provide more objective data about the training they provide and utilizes a standardized survey system for current residents and recent graduates to evaluate important subjective measures.
Conclusion
Based on our weighted ranking algorithm, the top 5 dermatology residency programs in 2014 were Harvard University (Boston, Massachusetts); University of California, San Francisco (San Francisco, California); Stanford University (Stanford, California); University of Pennsylvania (Philadelphia, Pennsylvania); and Emory University (Atlanta, Georgia).
Acknowledgments
We thank all of the program coordinators, full-time faculty members, program directors, and chairs who provided responses to our inquiries for additional information about their residency programs.
- Residency navigator 2017-2018. Doximity website. https://residency.doximity.com. Accessed January 19, 2018.
- Aquino LL, Wen G, Wu JJ. US dermatology residency program rankings. Cutis. 2014;94:189-194.
- Phitayakorn R, Macklin EA, Goldsmith J, et al. Applicants’ self-reported priorities in selecting a residency program. J Grad Med Educ. 2015;7:21-26.
- Wu JJ, Ramirez CC, Alonso CA, et al. Ranking the dermatology programs based on measurements of academic achievement. Dermatol Online J. 2007;13:3.
- Peterson WJ, Hopson LR, Khandelwal S. Impact of Doximity residency rankings on emergency medicine applicant rank lists [published online May 5, 2016]. West J Emerg Med. 2016;17:350-354.
- Residency navigator 2017-2018. Doximity website. https://residency.doximity.com. Accessed January 19, 2018.
- Aquino LL, Wen G, Wu JJ. US dermatology residency program rankings. Cutis. 2014;94:189-194.
- Phitayakorn R, Macklin EA, Goldsmith J, et al. Applicants’ self-reported priorities in selecting a residency program. J Grad Med Educ. 2015;7:21-26.
- Wu JJ, Ramirez CC, Alonso CA, et al. Ranking the dermatology programs based on measurements of academic achievement. Dermatol Online J. 2007;13:3.
- Peterson WJ, Hopson LR, Khandelwal S. Impact of Doximity residency rankings on emergency medicine applicant rank lists [published online May 5, 2016]. West J Emerg Med. 2016;17:350-354.
Practice Points
- Dermatology is not among the many hospital-based adult specialties that are routinely ranked annually by US News & World Report.
- In the current study, US dermatology residency programs were ranked based on various academic factors, including the number of full-time faculty members, amount of National Institutes of Health funding received in 2014, number of publications by full-time faculty members in 2014, and the number of faculty lectures given at annual meetings of 5 societies in 2014.
Pain-Minimizing Strategies for Nail Surgery
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
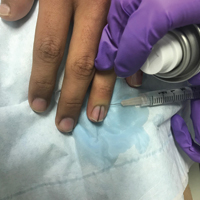
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11
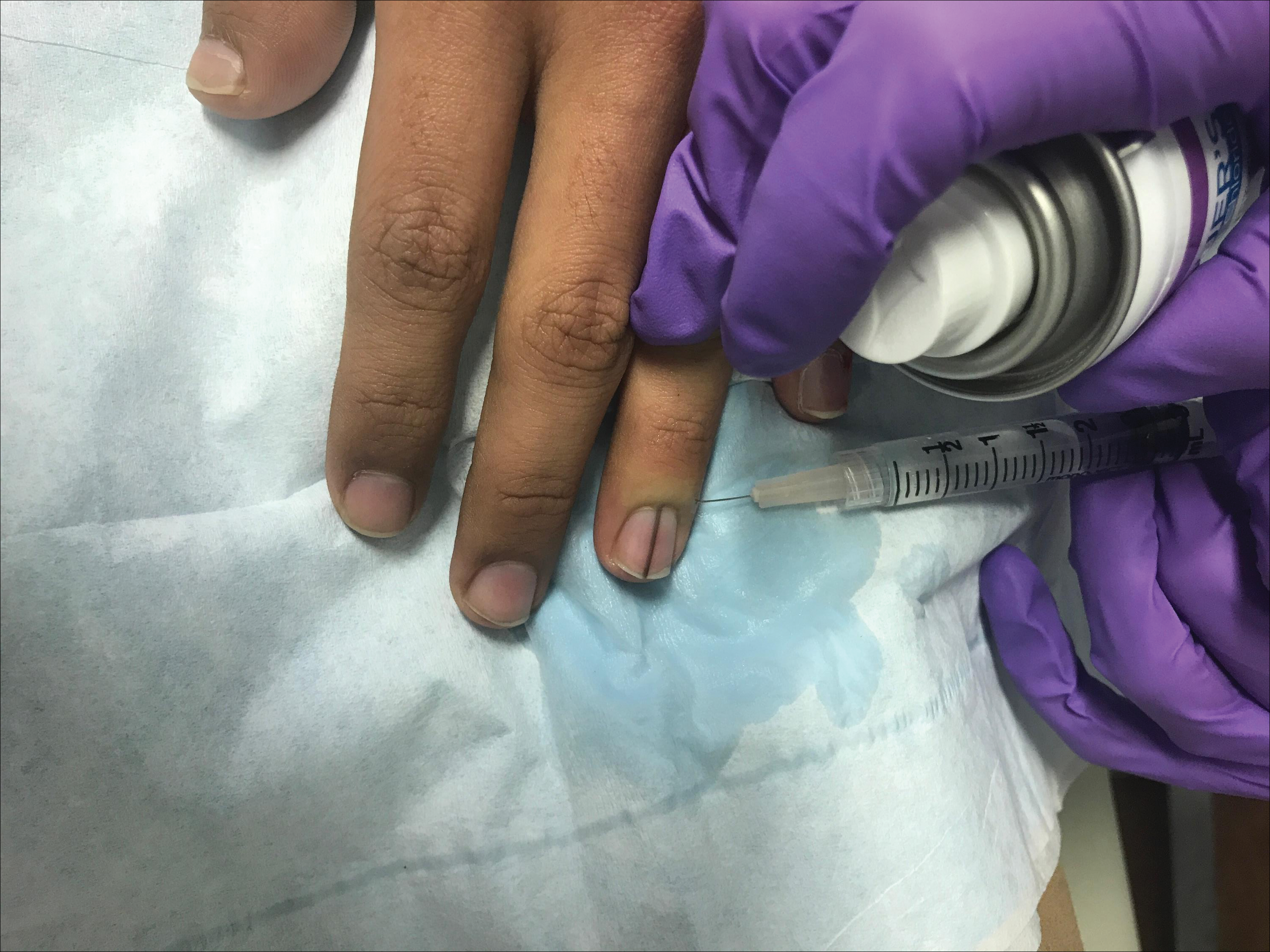
Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Smallpox Vaccine Complications: The Dermatologist’s Role in Diagnosis and Management
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
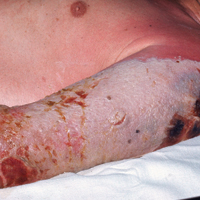
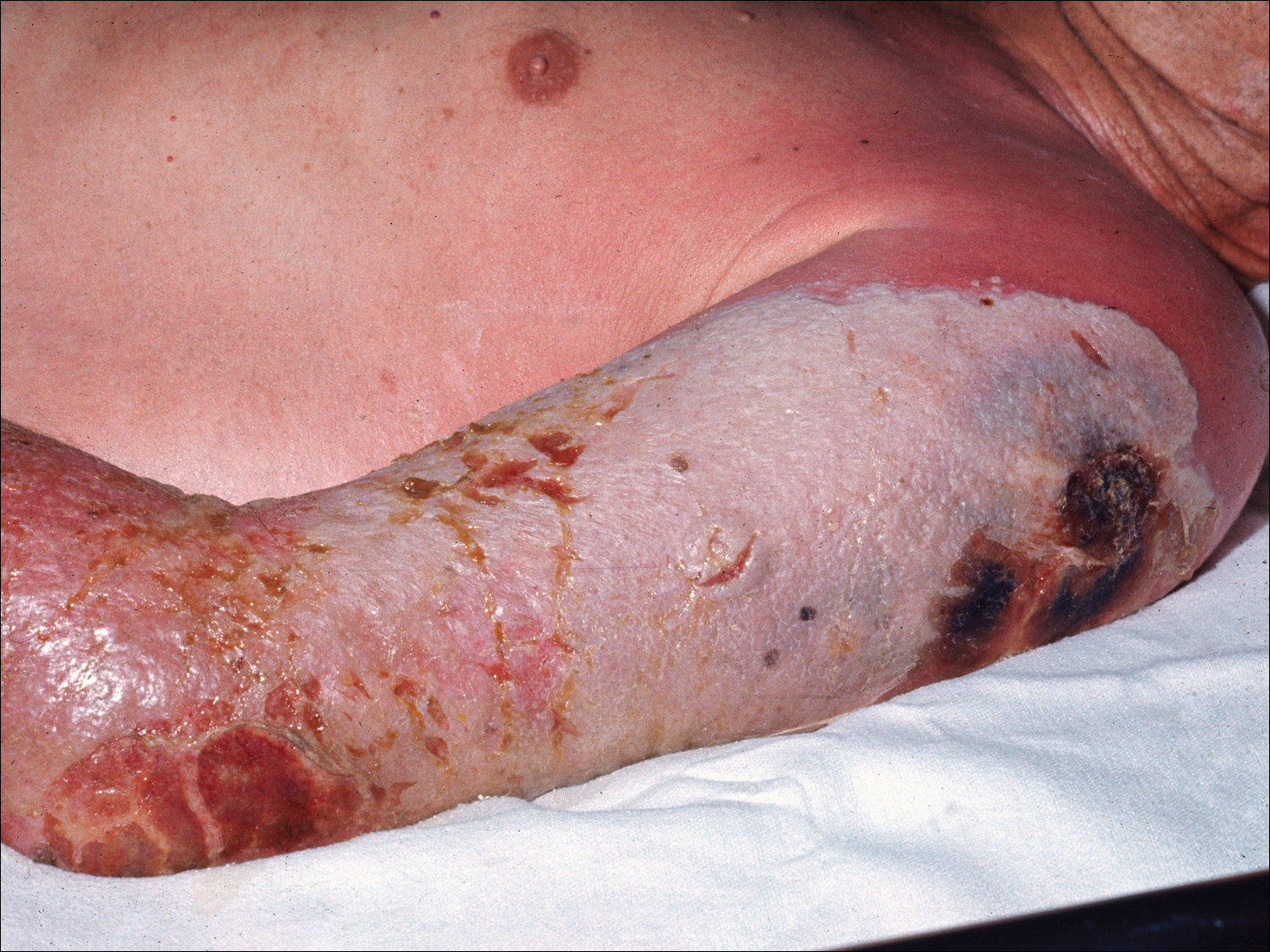
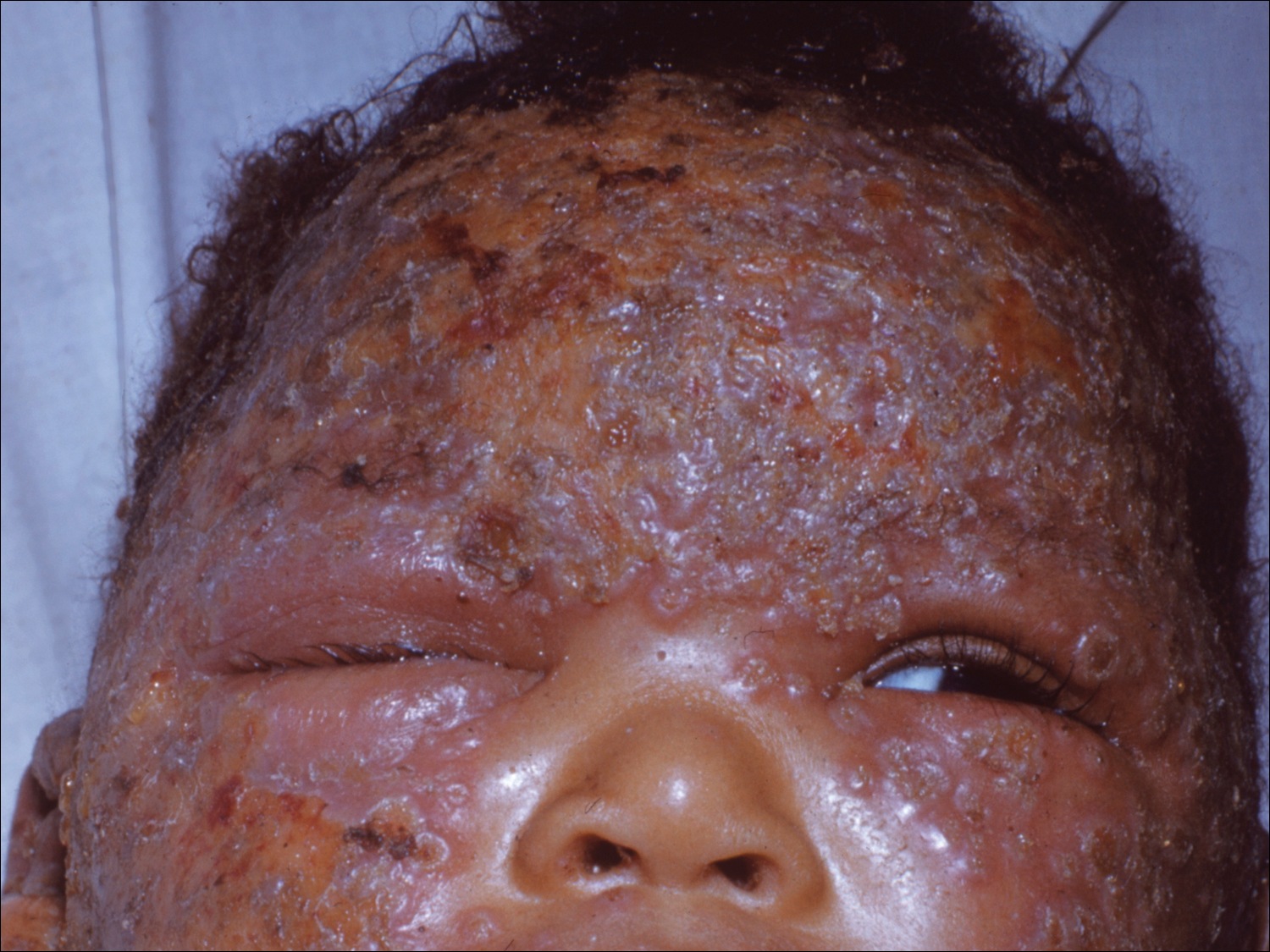
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.
Progressive Vaccinia
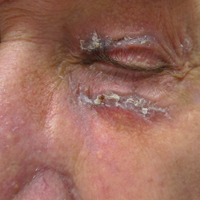
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.
The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
Practice Points
- Dermatologists should be aware that smallpox vaccinations are being administered to patients and may present with a myriad of cutaneous complications.
- Progressive vaccinia should be suspected if a smallpox inoculation has not healed after 14 days and, most specifically, if there is no inflammation surrounding the site.
- Generalized vaccinia generally is a benign condition seen in otherwise healthy patients and usually requires no treatment.
Atopic patients should be educated to avoid receiving routine smallpox vaccinations if they would be considered at risk for requiring the inoculation.
What’s Eating You? Sand Flies
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
Practice Points
- Sand flies cause a wide array of cutaneous and systemic diseases worldwide.
- Identification and treatment of leishmaniasis and other diseases transmitted by sand flies requires a high degree of clinical suspicion.
- With the increase in global travel and the rise of autochthonous disease in the United States, American physicians must increase their awareness of diseases for which sand flies serve as vectors.
Primary Cutaneous Follicle Center Lymphoma Mimicking Folliculitis
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.
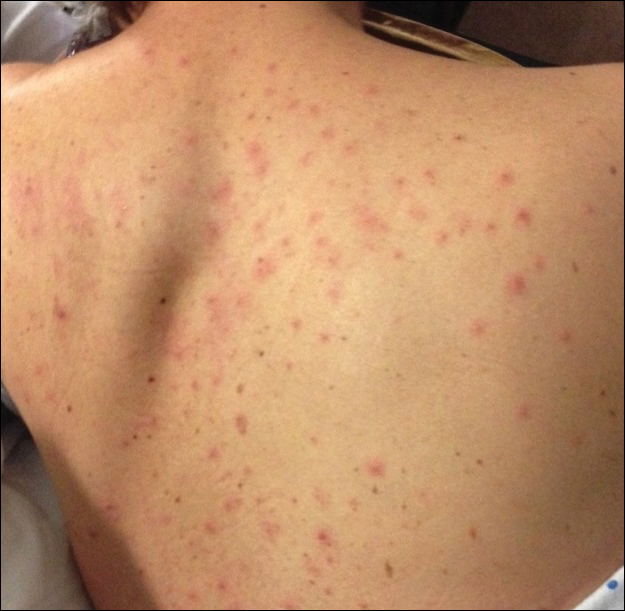
Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
Practice Points
- Atypical or unresponsive folliculitis should be biopsied.
- Primary cutaneous follicle center lymphoma can mimic folliculitis or Grover disease.
Periorbital Lupuslike Presentation of Graft-versus-host Disease
To the Editor:
A 79-year-old man presented with a scaling eruption in the periorbital area, on the bilateral forearms, and on the chest of 4 weeks’ duration. The patient denied systemic symptoms including lethargy, muscle weakness, and fevers. His medical history was notable for blastic plasmacytoid dendritic cell neoplasm, a form of acute myeloid leukemia, diagnosed 3 years prior to presentation. The patient received an allogeneic hematopoietic stem cell transplant 8 months later. His posttransplant course was complicated by gastrointestinal graft-versus-host disease (GVHD); progressive graft loss requiring a donor lymphocyte infusion after 1 month; and leukemia cutis, which spontaneously resolved after 1 month. The patient was taken off all immunosuppressive therapy 5 months after the transplant and had been doing well for 2 years with only mild mucosal GVHD affecting the oral mucosa and the head of the penis.
Physical examination at the current presentation revealed linear, atrophic, scaling, purplish plaques with adherent white scale on the upper and lower eyelids (Figure 1). The patient also had scattered purple scaling patches on the bilateral forearms and chest. Laboratory tests including complete blood cell count, comprehensive metabolic panel, and lactate dehydrogenase demonstrated no gross abnormalities. Two shave biopsies of the right lower eyelid (Figure 2) and left arm (Figure 3) were performed for histologic examination and revealed basket weave hyperkeratosis, irregular acanthosis, sawtooth rete ridges, and scattered dyskeratotic cells. Vacuolar changes and smudging of the basement membrane zone along with a bandlike lymphocytic infiltrate in the upper dermis also were noted in both biopsies. A diagnosis of lupuslike grade 1 GVHD was made.
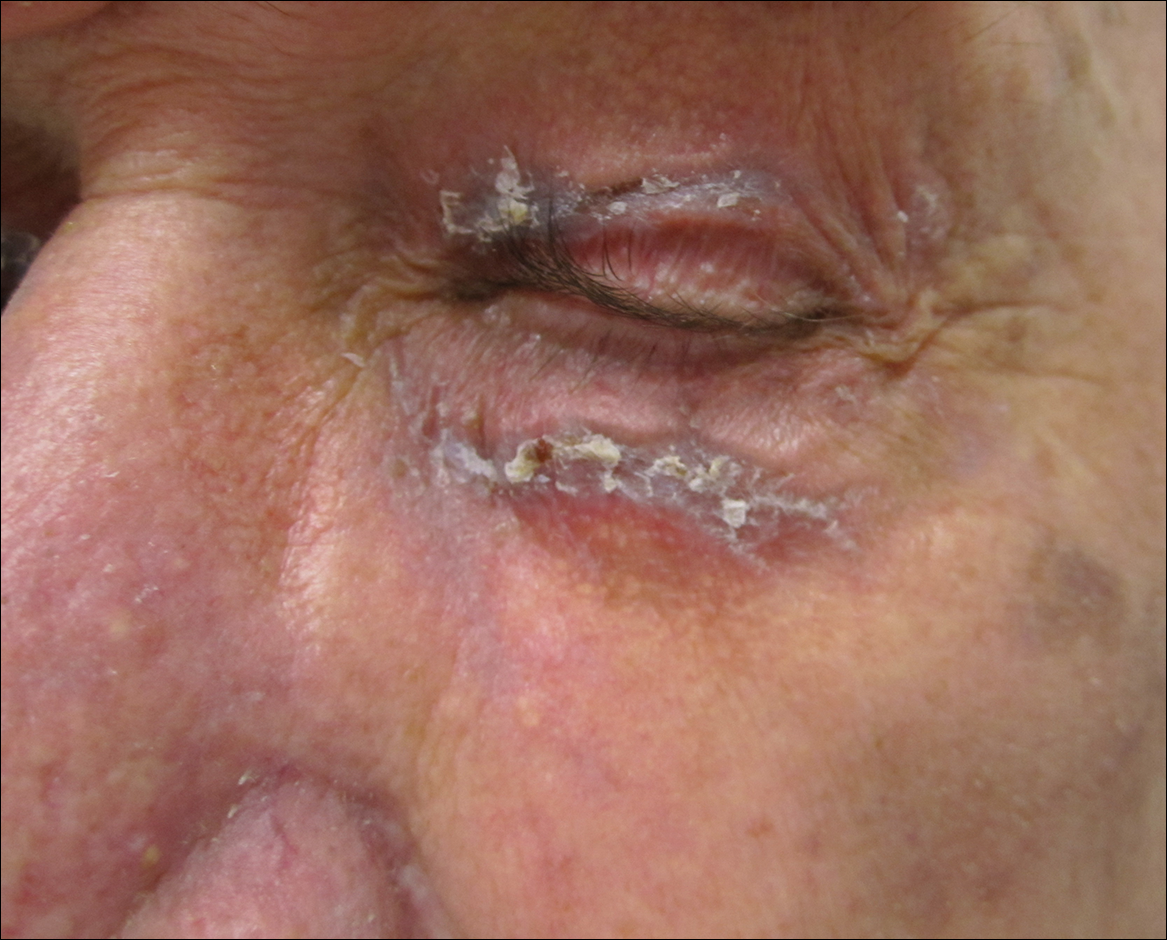
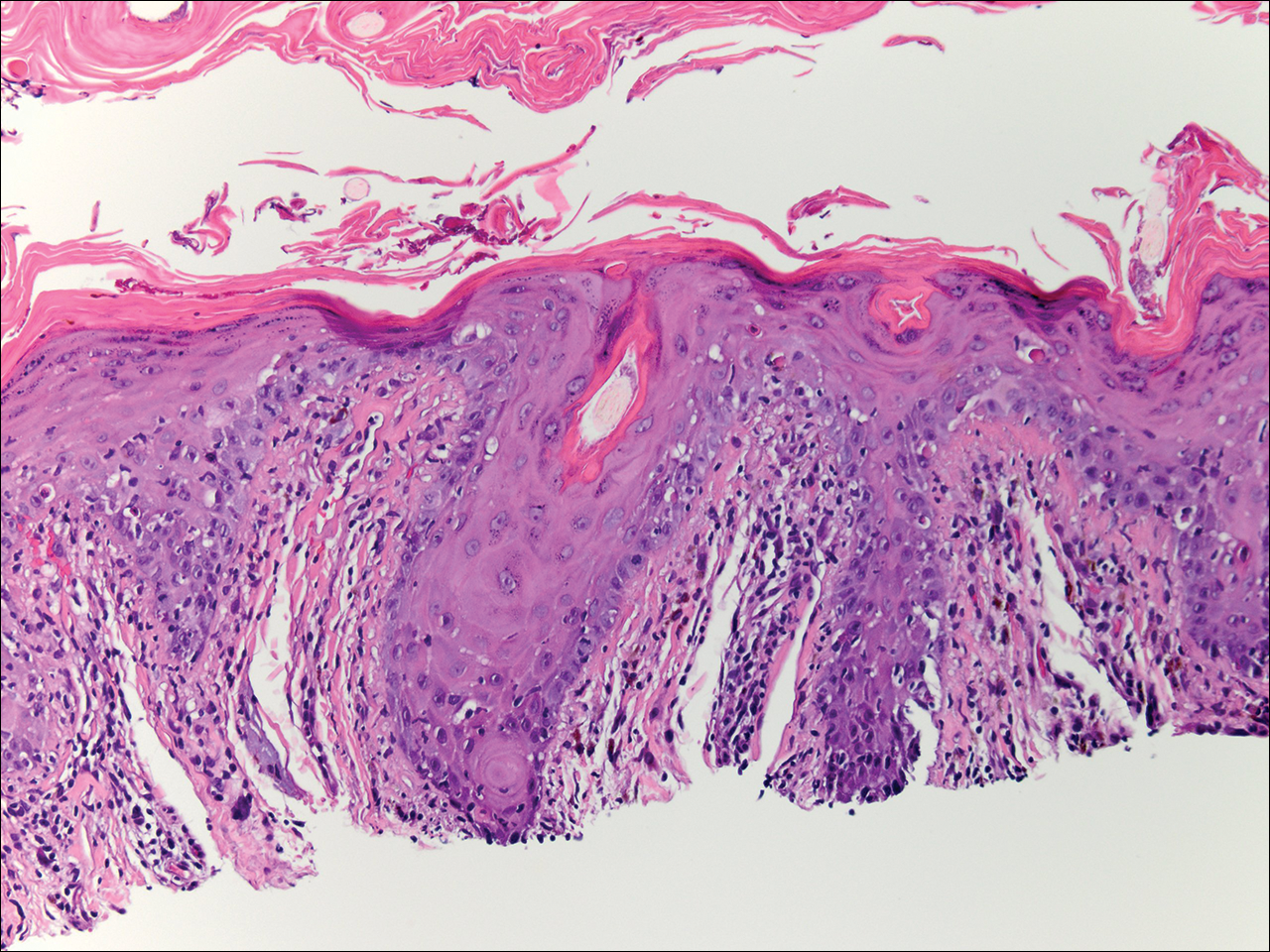
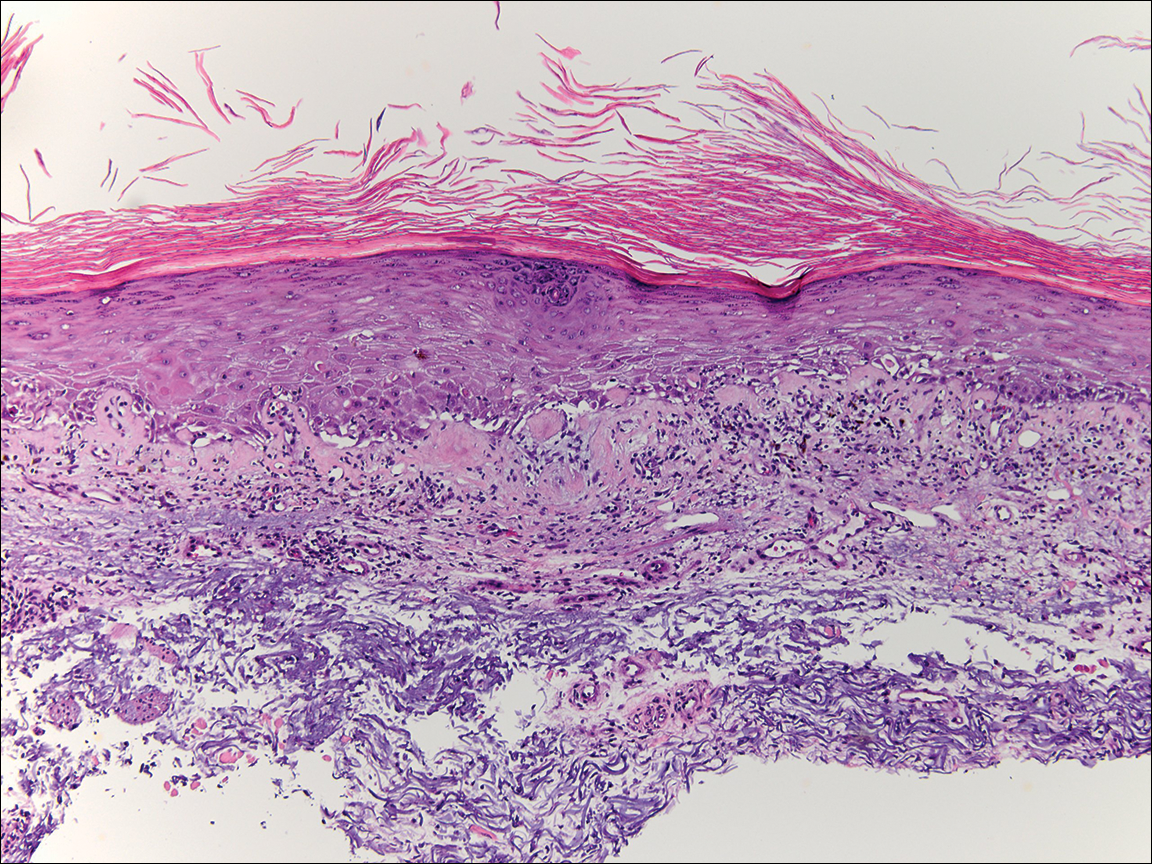
Graft-versus-host disease remains a notable cause of morbidity and mortality in allogenic hematopoietic stem cell transplant patients.1 Skin manifestations represent the most common manifestation of GVHD and have been reclassified as acute or chronic disease based on clinical and histologic findings rather than time of onset. Although acute GVHD classically presents as diffuse morbilliform papules and macules, chronic GVHD has a large range of clinical presentations most commonly mimicking the skin findings of lichen planus, morphea, scleroderma, or lichen sclerosus.1
Lupuslike GVHD is a rarely reported manifestation of chronic GVHD that predominantly affects the lower eyelids and malar regions.2,3 Our case documents extensive involvement of both the upper and lower eyelids. A lupuslike manifestation of GVHD may portend a poor prognosis. In a case series of 5 patients with chronic GVHD presenting as facial lupuslike plaques, 1 patient died from a relapse of leukemia and 3 patients developed sclerodermatous GVHD. The fifth patient was lost to follow-up.2 In another case series, a retrospective analysis discovered that 3 of 7 patients with sclerodermatous GVHD initially presented with hyperpigmented periorbital plaques.4 Resolution of skin findings with topical steroids and oral tacrolimus was reported in a case of GVHD presenting with periorbital lupuslike plaques.3 Although further reports are needed to validate the relationship, a lupuslike presentation of chronic GVHD may be an important harbinger for the development of extensive sclerodermatous GVHD.
A diagnosis of lupuslike GVHD is made based on the correlation of a comprehensive medical history, clinical examination, and histopathologic findings. Although other cases of chronic GVHD resembling dermatomyositis presented with purple periorbital plaques, these patients demonstrated dermatomyositislike systemic symptoms including muscle weakness and fatigue, which were not present in our patient.5,6 Antinuclear antibody (ANA) testing is unlikely to be helpful in the diagnosis of this uncommon presentation, as 65% (41/63) of chronic GVHD patients developed ANA antibodies in one study.7 Also, other patients with lupuslike GVHD who progressed to sclerodermatous GVHD have had both positive and negative ANA serology.2 The histopathology of GVHD and lupus erythematosus can exhibit overlapping features, such as lymphocytic infiltrate with interface changes; however, in lupus erythematosus, mucin usually is present, the infiltrate usually is denser and deeper, and a thickened basement membrane zone may be present. Necrotic keratinocytes also usually are not seen in lupus erythematosus unless the patient’s photosensitivity has led to a sunburn reaction.
After his initial presentation, our patient’s mucosal GVHD flared in the mouth and on the penis, and he was started on prednisone 50 mg once daily and mycophenolate mofetil 1 g twice daily. With this treatment, our patient’s periorbital scaling plaques resolved to residual hyperpigmentation along with remarkable improvement of the mucosal GVHD. He has not manifested any signs of leukemia relapse or sclerodermatous GVHD; however, he remains under close clinical evaluation.
This case highlights an unusual presentation of GVHD with periorbital plaques mimicking hypertrophic lupus erythematous. A greater recognition of this rare entity is essential to further elucidate its prognosis and its relationship with sclerodermatous GVHD.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-5.15e18; quiz 533-534.
- Goiriz R, Peñas PF, Delgado-Jiménez Y, et al. Cutaneous lichenoid graft-versus-host disease mimicking lupus erythematosus. Lupus. 2008;17:591-595.
- Hu SW, Myskowski PL, Papadopoulos EB, et al. Chronic cutaneous graft-versus host disease simulating hypertrophic lupus erythematosus—a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34:E81-E83.
- Chosidow O, Bagot M, Vernant JP, et al. Sclerodermatous chronic graft-versus-host disease. J Am Acad Dermatol. 1992;26:49-55.
- Ollivier I, Wolkenstein P, Gherardi R, et al. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558-559.
- Arin MJ, Scheid C, Hübel K, et al. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31:141-143.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
To the Editor:
A 79-year-old man presented with a scaling eruption in the periorbital area, on the bilateral forearms, and on the chest of 4 weeks’ duration. The patient denied systemic symptoms including lethargy, muscle weakness, and fevers. His medical history was notable for blastic plasmacytoid dendritic cell neoplasm, a form of acute myeloid leukemia, diagnosed 3 years prior to presentation. The patient received an allogeneic hematopoietic stem cell transplant 8 months later. His posttransplant course was complicated by gastrointestinal graft-versus-host disease (GVHD); progressive graft loss requiring a donor lymphocyte infusion after 1 month; and leukemia cutis, which spontaneously resolved after 1 month. The patient was taken off all immunosuppressive therapy 5 months after the transplant and had been doing well for 2 years with only mild mucosal GVHD affecting the oral mucosa and the head of the penis.
Physical examination at the current presentation revealed linear, atrophic, scaling, purplish plaques with adherent white scale on the upper and lower eyelids (Figure 1). The patient also had scattered purple scaling patches on the bilateral forearms and chest. Laboratory tests including complete blood cell count, comprehensive metabolic panel, and lactate dehydrogenase demonstrated no gross abnormalities. Two shave biopsies of the right lower eyelid (Figure 2) and left arm (Figure 3) were performed for histologic examination and revealed basket weave hyperkeratosis, irregular acanthosis, sawtooth rete ridges, and scattered dyskeratotic cells. Vacuolar changes and smudging of the basement membrane zone along with a bandlike lymphocytic infiltrate in the upper dermis also were noted in both biopsies. A diagnosis of lupuslike grade 1 GVHD was made.



Graft-versus-host disease remains a notable cause of morbidity and mortality in allogenic hematopoietic stem cell transplant patients.1 Skin manifestations represent the most common manifestation of GVHD and have been reclassified as acute or chronic disease based on clinical and histologic findings rather than time of onset. Although acute GVHD classically presents as diffuse morbilliform papules and macules, chronic GVHD has a large range of clinical presentations most commonly mimicking the skin findings of lichen planus, morphea, scleroderma, or lichen sclerosus.1
Lupuslike GVHD is a rarely reported manifestation of chronic GVHD that predominantly affects the lower eyelids and malar regions.2,3 Our case documents extensive involvement of both the upper and lower eyelids. A lupuslike manifestation of GVHD may portend a poor prognosis. In a case series of 5 patients with chronic GVHD presenting as facial lupuslike plaques, 1 patient died from a relapse of leukemia and 3 patients developed sclerodermatous GVHD. The fifth patient was lost to follow-up.2 In another case series, a retrospective analysis discovered that 3 of 7 patients with sclerodermatous GVHD initially presented with hyperpigmented periorbital plaques.4 Resolution of skin findings with topical steroids and oral tacrolimus was reported in a case of GVHD presenting with periorbital lupuslike plaques.3 Although further reports are needed to validate the relationship, a lupuslike presentation of chronic GVHD may be an important harbinger for the development of extensive sclerodermatous GVHD.
A diagnosis of lupuslike GVHD is made based on the correlation of a comprehensive medical history, clinical examination, and histopathologic findings. Although other cases of chronic GVHD resembling dermatomyositis presented with purple periorbital plaques, these patients demonstrated dermatomyositislike systemic symptoms including muscle weakness and fatigue, which were not present in our patient.5,6 Antinuclear antibody (ANA) testing is unlikely to be helpful in the diagnosis of this uncommon presentation, as 65% (41/63) of chronic GVHD patients developed ANA antibodies in one study.7 Also, other patients with lupuslike GVHD who progressed to sclerodermatous GVHD have had both positive and negative ANA serology.2 The histopathology of GVHD and lupus erythematosus can exhibit overlapping features, such as lymphocytic infiltrate with interface changes; however, in lupus erythematosus, mucin usually is present, the infiltrate usually is denser and deeper, and a thickened basement membrane zone may be present. Necrotic keratinocytes also usually are not seen in lupus erythematosus unless the patient’s photosensitivity has led to a sunburn reaction.
After his initial presentation, our patient’s mucosal GVHD flared in the mouth and on the penis, and he was started on prednisone 50 mg once daily and mycophenolate mofetil 1 g twice daily. With this treatment, our patient’s periorbital scaling plaques resolved to residual hyperpigmentation along with remarkable improvement of the mucosal GVHD. He has not manifested any signs of leukemia relapse or sclerodermatous GVHD; however, he remains under close clinical evaluation.
This case highlights an unusual presentation of GVHD with periorbital plaques mimicking hypertrophic lupus erythematous. A greater recognition of this rare entity is essential to further elucidate its prognosis and its relationship with sclerodermatous GVHD.
To the Editor:
A 79-year-old man presented with a scaling eruption in the periorbital area, on the bilateral forearms, and on the chest of 4 weeks’ duration. The patient denied systemic symptoms including lethargy, muscle weakness, and fevers. His medical history was notable for blastic plasmacytoid dendritic cell neoplasm, a form of acute myeloid leukemia, diagnosed 3 years prior to presentation. The patient received an allogeneic hematopoietic stem cell transplant 8 months later. His posttransplant course was complicated by gastrointestinal graft-versus-host disease (GVHD); progressive graft loss requiring a donor lymphocyte infusion after 1 month; and leukemia cutis, which spontaneously resolved after 1 month. The patient was taken off all immunosuppressive therapy 5 months after the transplant and had been doing well for 2 years with only mild mucosal GVHD affecting the oral mucosa and the head of the penis.
Physical examination at the current presentation revealed linear, atrophic, scaling, purplish plaques with adherent white scale on the upper and lower eyelids (Figure 1). The patient also had scattered purple scaling patches on the bilateral forearms and chest. Laboratory tests including complete blood cell count, comprehensive metabolic panel, and lactate dehydrogenase demonstrated no gross abnormalities. Two shave biopsies of the right lower eyelid (Figure 2) and left arm (Figure 3) were performed for histologic examination and revealed basket weave hyperkeratosis, irregular acanthosis, sawtooth rete ridges, and scattered dyskeratotic cells. Vacuolar changes and smudging of the basement membrane zone along with a bandlike lymphocytic infiltrate in the upper dermis also were noted in both biopsies. A diagnosis of lupuslike grade 1 GVHD was made.



Graft-versus-host disease remains a notable cause of morbidity and mortality in allogenic hematopoietic stem cell transplant patients.1 Skin manifestations represent the most common manifestation of GVHD and have been reclassified as acute or chronic disease based on clinical and histologic findings rather than time of onset. Although acute GVHD classically presents as diffuse morbilliform papules and macules, chronic GVHD has a large range of clinical presentations most commonly mimicking the skin findings of lichen planus, morphea, scleroderma, or lichen sclerosus.1
Lupuslike GVHD is a rarely reported manifestation of chronic GVHD that predominantly affects the lower eyelids and malar regions.2,3 Our case documents extensive involvement of both the upper and lower eyelids. A lupuslike manifestation of GVHD may portend a poor prognosis. In a case series of 5 patients with chronic GVHD presenting as facial lupuslike plaques, 1 patient died from a relapse of leukemia and 3 patients developed sclerodermatous GVHD. The fifth patient was lost to follow-up.2 In another case series, a retrospective analysis discovered that 3 of 7 patients with sclerodermatous GVHD initially presented with hyperpigmented periorbital plaques.4 Resolution of skin findings with topical steroids and oral tacrolimus was reported in a case of GVHD presenting with periorbital lupuslike plaques.3 Although further reports are needed to validate the relationship, a lupuslike presentation of chronic GVHD may be an important harbinger for the development of extensive sclerodermatous GVHD.
A diagnosis of lupuslike GVHD is made based on the correlation of a comprehensive medical history, clinical examination, and histopathologic findings. Although other cases of chronic GVHD resembling dermatomyositis presented with purple periorbital plaques, these patients demonstrated dermatomyositislike systemic symptoms including muscle weakness and fatigue, which were not present in our patient.5,6 Antinuclear antibody (ANA) testing is unlikely to be helpful in the diagnosis of this uncommon presentation, as 65% (41/63) of chronic GVHD patients developed ANA antibodies in one study.7 Also, other patients with lupuslike GVHD who progressed to sclerodermatous GVHD have had both positive and negative ANA serology.2 The histopathology of GVHD and lupus erythematosus can exhibit overlapping features, such as lymphocytic infiltrate with interface changes; however, in lupus erythematosus, mucin usually is present, the infiltrate usually is denser and deeper, and a thickened basement membrane zone may be present. Necrotic keratinocytes also usually are not seen in lupus erythematosus unless the patient’s photosensitivity has led to a sunburn reaction.
After his initial presentation, our patient’s mucosal GVHD flared in the mouth and on the penis, and he was started on prednisone 50 mg once daily and mycophenolate mofetil 1 g twice daily. With this treatment, our patient’s periorbital scaling plaques resolved to residual hyperpigmentation along with remarkable improvement of the mucosal GVHD. He has not manifested any signs of leukemia relapse or sclerodermatous GVHD; however, he remains under close clinical evaluation.
This case highlights an unusual presentation of GVHD with periorbital plaques mimicking hypertrophic lupus erythematous. A greater recognition of this rare entity is essential to further elucidate its prognosis and its relationship with sclerodermatous GVHD.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-5.15e18; quiz 533-534.
- Goiriz R, Peñas PF, Delgado-Jiménez Y, et al. Cutaneous lichenoid graft-versus-host disease mimicking lupus erythematosus. Lupus. 2008;17:591-595.
- Hu SW, Myskowski PL, Papadopoulos EB, et al. Chronic cutaneous graft-versus host disease simulating hypertrophic lupus erythematosus—a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34:E81-E83.
- Chosidow O, Bagot M, Vernant JP, et al. Sclerodermatous chronic graft-versus-host disease. J Am Acad Dermatol. 1992;26:49-55.
- Ollivier I, Wolkenstein P, Gherardi R, et al. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558-559.
- Arin MJ, Scheid C, Hübel K, et al. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31:141-143.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-5.15e18; quiz 533-534.
- Goiriz R, Peñas PF, Delgado-Jiménez Y, et al. Cutaneous lichenoid graft-versus-host disease mimicking lupus erythematosus. Lupus. 2008;17:591-595.
- Hu SW, Myskowski PL, Papadopoulos EB, et al. Chronic cutaneous graft-versus host disease simulating hypertrophic lupus erythematosus—a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34:E81-E83.
- Chosidow O, Bagot M, Vernant JP, et al. Sclerodermatous chronic graft-versus-host disease. J Am Acad Dermatol. 1992;26:49-55.
- Ollivier I, Wolkenstein P, Gherardi R, et al. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558-559.
- Arin MJ, Scheid C, Hübel K, et al. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31:141-143.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
Complete Remission of Metastatic Merkel Cell Carcinoma in a Patient With Severe Psoriasis
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.
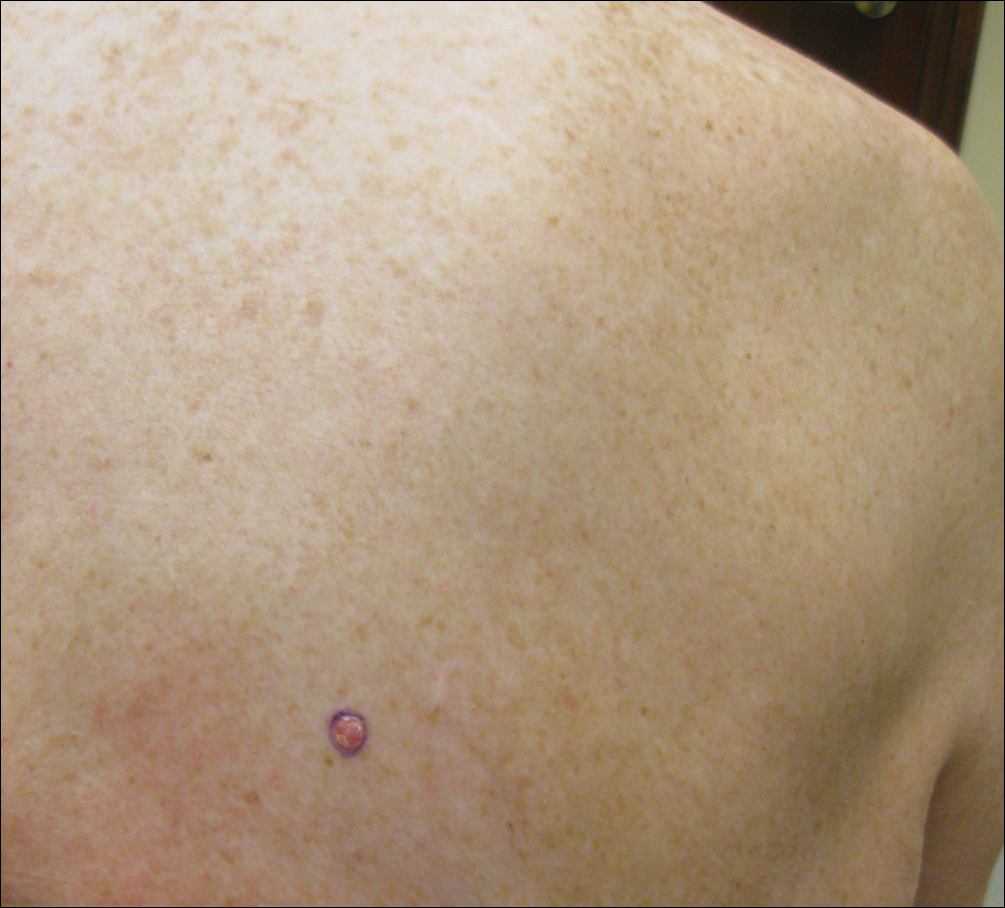
A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.
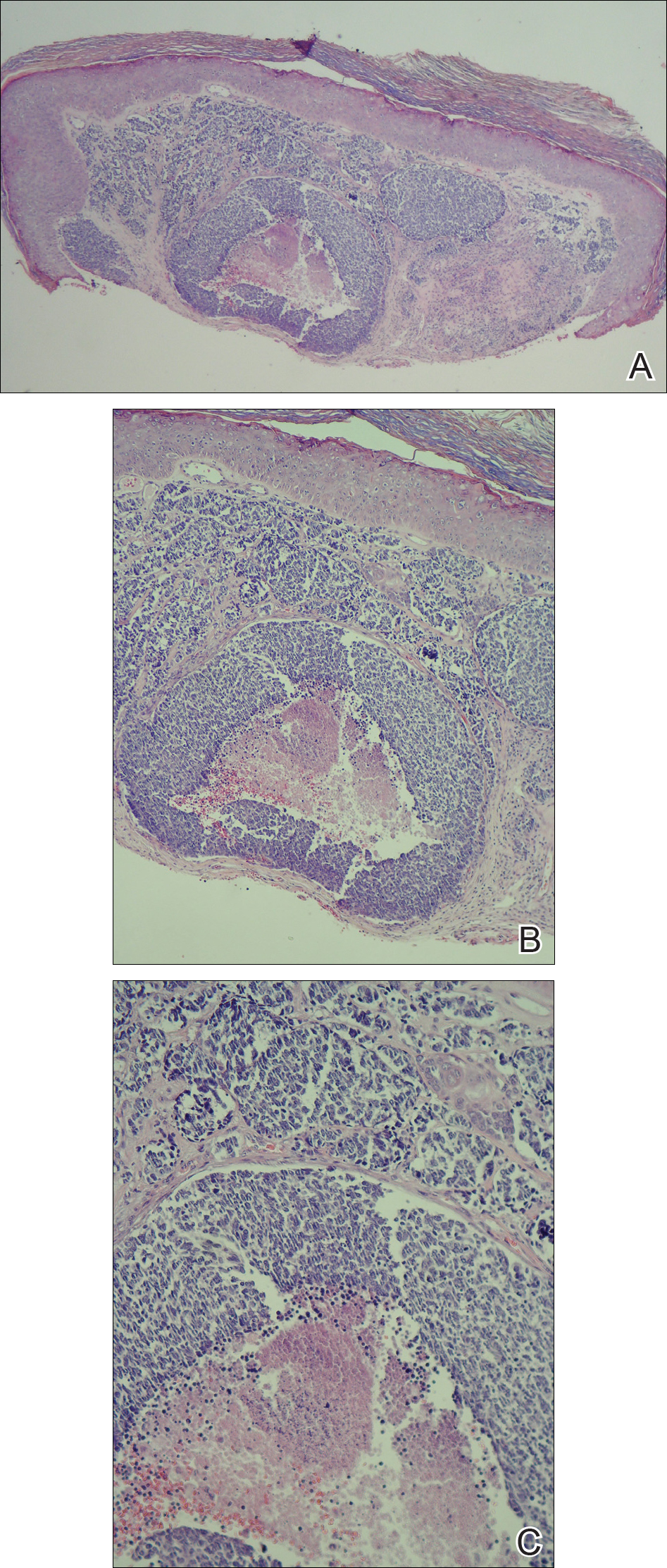
The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
Practice Points
- Merkel cell carcinoma (MCC) has remarkable metastatic potential.
- Initial evaluation of patients with MCC should include clinical examination to detect cutaneous, lymph node, and distant metastasis.
- Risk factors associated with the development of MCC include immunosuppression, older age, and UV-exposed fair skin.
Cosmetic Corner: Dermatologists Weigh in on Bar Soaps
To improve patient care and outcomes, leading dermatologists offered their recommendations on bar soaps. Consideration must be given to:
- Avène Cold Cream Ultra-Rich Cleansing Bar
Pierre Fabre Dermo-Cosmetique USA
“This gentle cleansing bar is not only hypoallergenic, soap free, and lanolin free, it also has Avène’s soothing Thermal Spring Water, plus white beeswax and a noncomedogenic, pharmaceutical-grade paraffin oil to protect the skin.”—Jeannette Graf, MD, Great Neck, New York
- Hydrating Cleanser Bar
CeraVe
Recommended by Shari Lipner, MD, PhD, New York, New York
- Vanicream Cleansing Bar
Pharmaceutical Specialties, Inc
“This is a great option for patients with eczema or dry, sensitive skin.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Lip plumpers, shaving lotions for men, and night creams will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on bar soaps. Consideration must be given to:
- Avène Cold Cream Ultra-Rich Cleansing Bar
Pierre Fabre Dermo-Cosmetique USA
“This gentle cleansing bar is not only hypoallergenic, soap free, and lanolin free, it also has Avène’s soothing Thermal Spring Water, plus white beeswax and a noncomedogenic, pharmaceutical-grade paraffin oil to protect the skin.”—Jeannette Graf, MD, Great Neck, New York
- Hydrating Cleanser Bar
CeraVe
Recommended by Shari Lipner, MD, PhD, New York, New York
- Vanicream Cleansing Bar
Pharmaceutical Specialties, Inc
“This is a great option for patients with eczema or dry, sensitive skin.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Lip plumpers, shaving lotions for men, and night creams will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on bar soaps. Consideration must be given to:
- Avène Cold Cream Ultra-Rich Cleansing Bar
Pierre Fabre Dermo-Cosmetique USA
“This gentle cleansing bar is not only hypoallergenic, soap free, and lanolin free, it also has Avène’s soothing Thermal Spring Water, plus white beeswax and a noncomedogenic, pharmaceutical-grade paraffin oil to protect the skin.”—Jeannette Graf, MD, Great Neck, New York
- Hydrating Cleanser Bar
CeraVe
Recommended by Shari Lipner, MD, PhD, New York, New York
- Vanicream Cleansing Bar
Pharmaceutical Specialties, Inc
“This is a great option for patients with eczema or dry, sensitive skin.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Lip plumpers, shaving lotions for men, and night creams will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis
Toxic epidermal necrolysis (TEN) is a rare, life-threatening adverse drug reaction with an estimated incidence of 0.4 to 1.9 cases per million persons per year worldwide and an estimated mortality rate of 25% to 35%.1,2 This dermatologic emergency is characterized by extensive detachment of the epidermis and erosions of the mucous membranes secondary to massive keratinocyte cell death via apoptosis, evolving quickly into full-thickness epidermal necrosis.
Primary treatment of TEN includes (1) prompt discontinuation of the suspected medication; (2) rapid transfer to an intensive care unit, burn center, or other specialty unit; and (3) supportive care, including wound care, fluid and electrolyte maintenance, and treatment of infections. Aside from the primary treatment, controversy remains over the most effective adjunctive therapy for TEN, as none has proven consistent superiority over well-conducted primary treatment alone. Therefore, established therapeutic guidelines do not exist.1-3
The use of adjunctive systemic therapy in TEN (eg, corticosteroids, intravenous immunoglobulin [IVIG], cyclosporine, plasmapheresis, granulocyte-colony stimulating factor) is based primarily on theories of pathogenesis, which unfortunately remain unclear. Activated CD8+ T cells are thought to increase the expression and production of granulysin, granzyme B, and perforins, leading to keratinocyte apoptosis. Fas ligand and tumor necrosis factor α (TNF-α) also are implicated as secondary mediators of cell death via the inducible nitric oxide synthase pathway.1,4-6
Since TNF-α was found to be elevated in serum and blister fluid in patients with TEN,7,8 medications aimed at decreasing the TNF-α concentration, such as pentoxifylline (PTX) and thalidomide, have been attempted for treatment.9,10 Biologic inhibitors of TNF-α, such as infliximab and etanercept, are novel therapeutic options in the treatment of TEN, as numerous reports document their successful use in the treatment of this disease.11-24 The purpose of this study is to systematically review the current literature on the use of TNF-α antagonists in the treatment of TEN.
METHODS
A PubMed search of all available articles indexed for MEDLINE using the terms toxic epidermal necrolysis and TNF-alpha and pentoxifylline or thalidomide or infliximab or etanercept or adalimumab was conducted.
RESULTS
Sixteen articles published between 1994 and 2014 were retrieved from PubMed and reviewed.9-24 Fourteen articles were case reports and case series involving the use of TNF-α inhibitors as either monotherapy, second-line agents, or in combination with other medications in the treatment of TEN, providing a total of 28 patients.9,11-23 Two articles were prospective trials, one evaluating the efficacy of thalidomide10 and the other infliximab24 in treating TEN. All studies implemented primary treatment (ie, prompt discontinuation of the suspected medication and aggressive supportive care) in addition to TNF-α inhibition.
Pentoxifylline
The first case report describing the use of an anti–TNF-α inhibitor for TEN was with PTX in 1994.9 Pentoxifylline, a vasoactive drug with immunomodulatory properties including the downregulation of TNF-α synthesis, was used to treat a 26-year-old woman with TEN on phenylhydantoin 15 days following resection of a grade II astrocytoma. The patient initially received intravenous N-acetylcysteine (NAC) (9 g once daily) and S-adenosyl-L-methionine (100 mg once daily) for antioxidant effects. On the second day of treatment, intravenous PTX (900 mg once daily) was added for TNF-α inhibition. Following PTX administration, the investigators reported quick stabilization of the eruption and achievement of reepithelialization after 7 days of therapy. Upon cessation of PTX therapy, a recurrence of generalized erythema occurred, suggesting a relapse of TEN; therefore, PTX was reinitiated for an additional 3 days, and the patient’s skin remained clear.9
Thalidomide
The earliest prospective trial we reviewed using anti–TNF-α therapy in TEN occurred in 1998 with thalidomide, a moderate inhibitor of TNF-α.10 In this randomized controlled trial, 22 TEN patients received either a 5-day course of thalidomide (400 mg once daily) or placebo. There was increased mortality in the thalidomide group (10/12 [83.3%]) versus the placebo group (3/10 [30.0%]). Additionally, the plasma TNF-α concentrations in the thalidomide group were higher than the control group. This study was stopped prematurely due to the excess mortality in the thalidomide group.10
Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14
TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.
Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.
N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24
COMMENT
Tumor necrosis factor α inhibition in the treatment of TEN was first utilized in the 1990s with PTX and thalidomide.9,10 In 1994, PTX in addition to antioxidant therapy was found to successfully treat a 26-year-old woman with TEN attributed to anticonvulsant therapy.9 Other reports of PTX in the treatment of TEN were not found; however, there is a case series describing the successful treatment of 2 pediatric patients with Stevens-Johnson syndrome (SJS) and SJS-TEN overlap with PTX.25 Thalidomide, however, proved detrimental to patients with TEN as evidenced by an increased mortality in the 1998 trial.10 Paradoxically, the treatment group was found to have increased rather than decreased TNF-α concentrations, which was hypothesized to be the cause of increased mortality. This finding furthered the theory that TNF-α is an important mediator in TEN pathogenesis and a potential novel target in disease management.10
Since the PTX case report and the thalidomide trial, many physicians have reported the beneficial effects of biologic TNF-α inhibitors in the course of TEN; however, most of the literature is composed of case reports and case series describing a small number of patients. Therefore, the beneficial effects of anti–TNF-α therapy in TEN cannot be conclusively derived. Furthermore, cases using TNF-α inhibitors in combination with or after other systemic agents complicate the effects of TNF-α inhibitors themselves. Most of these case reports and case series describe the beneficial effects of TNF-α inhibitors in TEN; however, it is important to remember that cases in which these agents were ineffective are less likely to be published. The strongest evidence for TNF-α inhibitor use in the treatment TEN comes from the Paradisi et al14 case series, which showed a decrease in expected mortality with etanercept monotherapy in a relatively large cohort of patients. However, when evaluated prospectively by Paquet et al,24 there was no benefit seen by adding infliximab to NAC therapy and possibly an increased mortality in the group treated with both agents.
In the cases reviewed, a total of 32 patients were treated with infliximab or etanercept, and of these patients there were 4 deaths (12.5%).16,22,24 Three deaths were attributed to infection and 1 was attributed to disseminated intravascular coagulation. Furthermore, infection complicated the hospital course of 9 (28.1%) patients.13,15,22,24 The bacteria cultured from these patients included methicillin-resistant S aureus, P aeruginosa, E coli, Enterobacter aerogenes, and K pneumoniae. Patients who received TNF-α antagonists in combination with or after other systemic immunosuppressants appeared to have a higher incidence of infections. All patients treated with TNF-α antagonists in TEN should undergo careful evaluation and monitoring for infections due to the immunosuppressant effect of these drugs.
In our review, a total of 3 pediatric/adolescent patients received a TNF-α inhibitor for the treatment of TEN.13,17,21 Two patients received infliximab as a second-line medication after failure of IVIG to arrest progression of disease13,17 and one patient received infliximab as a second-line medication after dexamethasone.21 Each of these patients recovered without any reported infections or long-term complications.
Although excluded from this review, both infliximab and etanercept have been reported to show benefit in acute generalized exanthematous pustulosis/TEN overlap.26,27 Interestingly, in postmarketing surveillance, rare reports have implicated both infliximab and etanercept in causing both SJS and TEN.28 Also, there have been case reports of adalimumab causing SJS, but no cases of it causing TEN were identified.29,30
CONCLUSION
Rapid discontinuation of the culprit drug and aggressive supportive care remain the primary treatment of TEN. Tumor necrosis factor α inhibitors as monotherapy or as second-line agents show promise in the treatment of this complex disease state in both the adult and pediatric populations. The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections. Additional studies are needed to further characterize the role of TNF-α inhibition in the treatment of TEN.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173-186.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187-203.
- Fernando S. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;55:165-171.
- Paquet P, Paquet F, Saleh W, et al. Immunoregulatory effector cells in drug-induced toxic epidermal necrolysis. Am J Dermatopathol. 2000;22:413-417.
- Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850-855.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and INF-γ are potential inducers of Fas-mediated keratinocyte apoptosis thought activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Paquet P, Pierard G. Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med. 1998;1:459-462.
- Correia O, Delgado L, Barbosa I, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- Redondo P, Rutz de Erenchun F, Iglesias M, et al. Toxic epidermal necrolysis. treatment with pentoxifylline. Br J Dermatol. 1994;130:688-689.
- Wolkenstein P, Latarjet J, Roujeau J, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch W, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-708.
- Hunger R, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Al-Shouli S, Bogusz M, Al Tufail M, et al. Toxic epidermal necrosis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-553.
- Famularo G, Di Dona B, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Worsnop F, Wee J, Moosa Y, et al. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37:879-881.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulins. Dermatology. 2012;224:134-139.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Paquet P, Jennes S, Rousseua A, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;1:1-6.
- Sanclemente G, De le Rouche C, Escobar C, et al. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 1998;38:878-879.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumor necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South Med J. 2009;102:1133-1140.
- Muna S, Lawrance I. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Mounach A, Rezgi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
Toxic epidermal necrolysis (TEN) is a rare, life-threatening adverse drug reaction with an estimated incidence of 0.4 to 1.9 cases per million persons per year worldwide and an estimated mortality rate of 25% to 35%.1,2 This dermatologic emergency is characterized by extensive detachment of the epidermis and erosions of the mucous membranes secondary to massive keratinocyte cell death via apoptosis, evolving quickly into full-thickness epidermal necrosis.
Primary treatment of TEN includes (1) prompt discontinuation of the suspected medication; (2) rapid transfer to an intensive care unit, burn center, or other specialty unit; and (3) supportive care, including wound care, fluid and electrolyte maintenance, and treatment of infections. Aside from the primary treatment, controversy remains over the most effective adjunctive therapy for TEN, as none has proven consistent superiority over well-conducted primary treatment alone. Therefore, established therapeutic guidelines do not exist.1-3
The use of adjunctive systemic therapy in TEN (eg, corticosteroids, intravenous immunoglobulin [IVIG], cyclosporine, plasmapheresis, granulocyte-colony stimulating factor) is based primarily on theories of pathogenesis, which unfortunately remain unclear. Activated CD8+ T cells are thought to increase the expression and production of granulysin, granzyme B, and perforins, leading to keratinocyte apoptosis. Fas ligand and tumor necrosis factor α (TNF-α) also are implicated as secondary mediators of cell death via the inducible nitric oxide synthase pathway.1,4-6
Since TNF-α was found to be elevated in serum and blister fluid in patients with TEN,7,8 medications aimed at decreasing the TNF-α concentration, such as pentoxifylline (PTX) and thalidomide, have been attempted for treatment.9,10 Biologic inhibitors of TNF-α, such as infliximab and etanercept, are novel therapeutic options in the treatment of TEN, as numerous reports document their successful use in the treatment of this disease.11-24 The purpose of this study is to systematically review the current literature on the use of TNF-α antagonists in the treatment of TEN.
METHODS
A PubMed search of all available articles indexed for MEDLINE using the terms toxic epidermal necrolysis and TNF-alpha and pentoxifylline or thalidomide or infliximab or etanercept or adalimumab was conducted.
RESULTS
Sixteen articles published between 1994 and 2014 were retrieved from PubMed and reviewed.9-24 Fourteen articles were case reports and case series involving the use of TNF-α inhibitors as either monotherapy, second-line agents, or in combination with other medications in the treatment of TEN, providing a total of 28 patients.9,11-23 Two articles were prospective trials, one evaluating the efficacy of thalidomide10 and the other infliximab24 in treating TEN. All studies implemented primary treatment (ie, prompt discontinuation of the suspected medication and aggressive supportive care) in addition to TNF-α inhibition.
Pentoxifylline
The first case report describing the use of an anti–TNF-α inhibitor for TEN was with PTX in 1994.9 Pentoxifylline, a vasoactive drug with immunomodulatory properties including the downregulation of TNF-α synthesis, was used to treat a 26-year-old woman with TEN on phenylhydantoin 15 days following resection of a grade II astrocytoma. The patient initially received intravenous N-acetylcysteine (NAC) (9 g once daily) and S-adenosyl-L-methionine (100 mg once daily) for antioxidant effects. On the second day of treatment, intravenous PTX (900 mg once daily) was added for TNF-α inhibition. Following PTX administration, the investigators reported quick stabilization of the eruption and achievement of reepithelialization after 7 days of therapy. Upon cessation of PTX therapy, a recurrence of generalized erythema occurred, suggesting a relapse of TEN; therefore, PTX was reinitiated for an additional 3 days, and the patient’s skin remained clear.9
Thalidomide
The earliest prospective trial we reviewed using anti–TNF-α therapy in TEN occurred in 1998 with thalidomide, a moderate inhibitor of TNF-α.10 In this randomized controlled trial, 22 TEN patients received either a 5-day course of thalidomide (400 mg once daily) or placebo. There was increased mortality in the thalidomide group (10/12 [83.3%]) versus the placebo group (3/10 [30.0%]). Additionally, the plasma TNF-α concentrations in the thalidomide group were higher than the control group. This study was stopped prematurely due to the excess mortality in the thalidomide group.10
Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14
TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.
Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.
N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24
COMMENT
Tumor necrosis factor α inhibition in the treatment of TEN was first utilized in the 1990s with PTX and thalidomide.9,10 In 1994, PTX in addition to antioxidant therapy was found to successfully treat a 26-year-old woman with TEN attributed to anticonvulsant therapy.9 Other reports of PTX in the treatment of TEN were not found; however, there is a case series describing the successful treatment of 2 pediatric patients with Stevens-Johnson syndrome (SJS) and SJS-TEN overlap with PTX.25 Thalidomide, however, proved detrimental to patients with TEN as evidenced by an increased mortality in the 1998 trial.10 Paradoxically, the treatment group was found to have increased rather than decreased TNF-α concentrations, which was hypothesized to be the cause of increased mortality. This finding furthered the theory that TNF-α is an important mediator in TEN pathogenesis and a potential novel target in disease management.10
Since the PTX case report and the thalidomide trial, many physicians have reported the beneficial effects of biologic TNF-α inhibitors in the course of TEN; however, most of the literature is composed of case reports and case series describing a small number of patients. Therefore, the beneficial effects of anti–TNF-α therapy in TEN cannot be conclusively derived. Furthermore, cases using TNF-α inhibitors in combination with or after other systemic agents complicate the effects of TNF-α inhibitors themselves. Most of these case reports and case series describe the beneficial effects of TNF-α inhibitors in TEN; however, it is important to remember that cases in which these agents were ineffective are less likely to be published. The strongest evidence for TNF-α inhibitor use in the treatment TEN comes from the Paradisi et al14 case series, which showed a decrease in expected mortality with etanercept monotherapy in a relatively large cohort of patients. However, when evaluated prospectively by Paquet et al,24 there was no benefit seen by adding infliximab to NAC therapy and possibly an increased mortality in the group treated with both agents.
In the cases reviewed, a total of 32 patients were treated with infliximab or etanercept, and of these patients there were 4 deaths (12.5%).16,22,24 Three deaths were attributed to infection and 1 was attributed to disseminated intravascular coagulation. Furthermore, infection complicated the hospital course of 9 (28.1%) patients.13,15,22,24 The bacteria cultured from these patients included methicillin-resistant S aureus, P aeruginosa, E coli, Enterobacter aerogenes, and K pneumoniae. Patients who received TNF-α antagonists in combination with or after other systemic immunosuppressants appeared to have a higher incidence of infections. All patients treated with TNF-α antagonists in TEN should undergo careful evaluation and monitoring for infections due to the immunosuppressant effect of these drugs.
In our review, a total of 3 pediatric/adolescent patients received a TNF-α inhibitor for the treatment of TEN.13,17,21 Two patients received infliximab as a second-line medication after failure of IVIG to arrest progression of disease13,17 and one patient received infliximab as a second-line medication after dexamethasone.21 Each of these patients recovered without any reported infections or long-term complications.
Although excluded from this review, both infliximab and etanercept have been reported to show benefit in acute generalized exanthematous pustulosis/TEN overlap.26,27 Interestingly, in postmarketing surveillance, rare reports have implicated both infliximab and etanercept in causing both SJS and TEN.28 Also, there have been case reports of adalimumab causing SJS, but no cases of it causing TEN were identified.29,30
CONCLUSION
Rapid discontinuation of the culprit drug and aggressive supportive care remain the primary treatment of TEN. Tumor necrosis factor α inhibitors as monotherapy or as second-line agents show promise in the treatment of this complex disease state in both the adult and pediatric populations. The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections. Additional studies are needed to further characterize the role of TNF-α inhibition in the treatment of TEN.
Toxic epidermal necrolysis (TEN) is a rare, life-threatening adverse drug reaction with an estimated incidence of 0.4 to 1.9 cases per million persons per year worldwide and an estimated mortality rate of 25% to 35%.1,2 This dermatologic emergency is characterized by extensive detachment of the epidermis and erosions of the mucous membranes secondary to massive keratinocyte cell death via apoptosis, evolving quickly into full-thickness epidermal necrosis.
Primary treatment of TEN includes (1) prompt discontinuation of the suspected medication; (2) rapid transfer to an intensive care unit, burn center, or other specialty unit; and (3) supportive care, including wound care, fluid and electrolyte maintenance, and treatment of infections. Aside from the primary treatment, controversy remains over the most effective adjunctive therapy for TEN, as none has proven consistent superiority over well-conducted primary treatment alone. Therefore, established therapeutic guidelines do not exist.1-3
The use of adjunctive systemic therapy in TEN (eg, corticosteroids, intravenous immunoglobulin [IVIG], cyclosporine, plasmapheresis, granulocyte-colony stimulating factor) is based primarily on theories of pathogenesis, which unfortunately remain unclear. Activated CD8+ T cells are thought to increase the expression and production of granulysin, granzyme B, and perforins, leading to keratinocyte apoptosis. Fas ligand and tumor necrosis factor α (TNF-α) also are implicated as secondary mediators of cell death via the inducible nitric oxide synthase pathway.1,4-6
Since TNF-α was found to be elevated in serum and blister fluid in patients with TEN,7,8 medications aimed at decreasing the TNF-α concentration, such as pentoxifylline (PTX) and thalidomide, have been attempted for treatment.9,10 Biologic inhibitors of TNF-α, such as infliximab and etanercept, are novel therapeutic options in the treatment of TEN, as numerous reports document their successful use in the treatment of this disease.11-24 The purpose of this study is to systematically review the current literature on the use of TNF-α antagonists in the treatment of TEN.
METHODS
A PubMed search of all available articles indexed for MEDLINE using the terms toxic epidermal necrolysis and TNF-alpha and pentoxifylline or thalidomide or infliximab or etanercept or adalimumab was conducted.
RESULTS
Sixteen articles published between 1994 and 2014 were retrieved from PubMed and reviewed.9-24 Fourteen articles were case reports and case series involving the use of TNF-α inhibitors as either monotherapy, second-line agents, or in combination with other medications in the treatment of TEN, providing a total of 28 patients.9,11-23 Two articles were prospective trials, one evaluating the efficacy of thalidomide10 and the other infliximab24 in treating TEN. All studies implemented primary treatment (ie, prompt discontinuation of the suspected medication and aggressive supportive care) in addition to TNF-α inhibition.
Pentoxifylline
The first case report describing the use of an anti–TNF-α inhibitor for TEN was with PTX in 1994.9 Pentoxifylline, a vasoactive drug with immunomodulatory properties including the downregulation of TNF-α synthesis, was used to treat a 26-year-old woman with TEN on phenylhydantoin 15 days following resection of a grade II astrocytoma. The patient initially received intravenous N-acetylcysteine (NAC) (9 g once daily) and S-adenosyl-L-methionine (100 mg once daily) for antioxidant effects. On the second day of treatment, intravenous PTX (900 mg once daily) was added for TNF-α inhibition. Following PTX administration, the investigators reported quick stabilization of the eruption and achievement of reepithelialization after 7 days of therapy. Upon cessation of PTX therapy, a recurrence of generalized erythema occurred, suggesting a relapse of TEN; therefore, PTX was reinitiated for an additional 3 days, and the patient’s skin remained clear.9
Thalidomide
The earliest prospective trial we reviewed using anti–TNF-α therapy in TEN occurred in 1998 with thalidomide, a moderate inhibitor of TNF-α.10 In this randomized controlled trial, 22 TEN patients received either a 5-day course of thalidomide (400 mg once daily) or placebo. There was increased mortality in the thalidomide group (10/12 [83.3%]) versus the placebo group (3/10 [30.0%]). Additionally, the plasma TNF-α concentrations in the thalidomide group were higher than the control group. This study was stopped prematurely due to the excess mortality in the thalidomide group.10
Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14
TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.
Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.
N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24
COMMENT
Tumor necrosis factor α inhibition in the treatment of TEN was first utilized in the 1990s with PTX and thalidomide.9,10 In 1994, PTX in addition to antioxidant therapy was found to successfully treat a 26-year-old woman with TEN attributed to anticonvulsant therapy.9 Other reports of PTX in the treatment of TEN were not found; however, there is a case series describing the successful treatment of 2 pediatric patients with Stevens-Johnson syndrome (SJS) and SJS-TEN overlap with PTX.25 Thalidomide, however, proved detrimental to patients with TEN as evidenced by an increased mortality in the 1998 trial.10 Paradoxically, the treatment group was found to have increased rather than decreased TNF-α concentrations, which was hypothesized to be the cause of increased mortality. This finding furthered the theory that TNF-α is an important mediator in TEN pathogenesis and a potential novel target in disease management.10
Since the PTX case report and the thalidomide trial, many physicians have reported the beneficial effects of biologic TNF-α inhibitors in the course of TEN; however, most of the literature is composed of case reports and case series describing a small number of patients. Therefore, the beneficial effects of anti–TNF-α therapy in TEN cannot be conclusively derived. Furthermore, cases using TNF-α inhibitors in combination with or after other systemic agents complicate the effects of TNF-α inhibitors themselves. Most of these case reports and case series describe the beneficial effects of TNF-α inhibitors in TEN; however, it is important to remember that cases in which these agents were ineffective are less likely to be published. The strongest evidence for TNF-α inhibitor use in the treatment TEN comes from the Paradisi et al14 case series, which showed a decrease in expected mortality with etanercept monotherapy in a relatively large cohort of patients. However, when evaluated prospectively by Paquet et al,24 there was no benefit seen by adding infliximab to NAC therapy and possibly an increased mortality in the group treated with both agents.
In the cases reviewed, a total of 32 patients were treated with infliximab or etanercept, and of these patients there were 4 deaths (12.5%).16,22,24 Three deaths were attributed to infection and 1 was attributed to disseminated intravascular coagulation. Furthermore, infection complicated the hospital course of 9 (28.1%) patients.13,15,22,24 The bacteria cultured from these patients included methicillin-resistant S aureus, P aeruginosa, E coli, Enterobacter aerogenes, and K pneumoniae. Patients who received TNF-α antagonists in combination with or after other systemic immunosuppressants appeared to have a higher incidence of infections. All patients treated with TNF-α antagonists in TEN should undergo careful evaluation and monitoring for infections due to the immunosuppressant effect of these drugs.
In our review, a total of 3 pediatric/adolescent patients received a TNF-α inhibitor for the treatment of TEN.13,17,21 Two patients received infliximab as a second-line medication after failure of IVIG to arrest progression of disease13,17 and one patient received infliximab as a second-line medication after dexamethasone.21 Each of these patients recovered without any reported infections or long-term complications.
Although excluded from this review, both infliximab and etanercept have been reported to show benefit in acute generalized exanthematous pustulosis/TEN overlap.26,27 Interestingly, in postmarketing surveillance, rare reports have implicated both infliximab and etanercept in causing both SJS and TEN.28 Also, there have been case reports of adalimumab causing SJS, but no cases of it causing TEN were identified.29,30
CONCLUSION
Rapid discontinuation of the culprit drug and aggressive supportive care remain the primary treatment of TEN. Tumor necrosis factor α inhibitors as monotherapy or as second-line agents show promise in the treatment of this complex disease state in both the adult and pediatric populations. The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections. Additional studies are needed to further characterize the role of TNF-α inhibition in the treatment of TEN.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173-186.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187-203.
- Fernando S. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;55:165-171.
- Paquet P, Paquet F, Saleh W, et al. Immunoregulatory effector cells in drug-induced toxic epidermal necrolysis. Am J Dermatopathol. 2000;22:413-417.
- Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850-855.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and INF-γ are potential inducers of Fas-mediated keratinocyte apoptosis thought activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Paquet P, Pierard G. Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med. 1998;1:459-462.
- Correia O, Delgado L, Barbosa I, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- Redondo P, Rutz de Erenchun F, Iglesias M, et al. Toxic epidermal necrolysis. treatment with pentoxifylline. Br J Dermatol. 1994;130:688-689.
- Wolkenstein P, Latarjet J, Roujeau J, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch W, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-708.
- Hunger R, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Al-Shouli S, Bogusz M, Al Tufail M, et al. Toxic epidermal necrosis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-553.
- Famularo G, Di Dona B, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Worsnop F, Wee J, Moosa Y, et al. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37:879-881.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulins. Dermatology. 2012;224:134-139.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Paquet P, Jennes S, Rousseua A, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;1:1-6.
- Sanclemente G, De le Rouche C, Escobar C, et al. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 1998;38:878-879.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumor necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South Med J. 2009;102:1133-1140.
- Muna S, Lawrance I. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Mounach A, Rezgi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173-186.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187-203.
- Fernando S. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;55:165-171.
- Paquet P, Paquet F, Saleh W, et al. Immunoregulatory effector cells in drug-induced toxic epidermal necrolysis. Am J Dermatopathol. 2000;22:413-417.
- Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850-855.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and INF-γ are potential inducers of Fas-mediated keratinocyte apoptosis thought activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Paquet P, Pierard G. Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med. 1998;1:459-462.
- Correia O, Delgado L, Barbosa I, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- Redondo P, Rutz de Erenchun F, Iglesias M, et al. Toxic epidermal necrolysis. treatment with pentoxifylline. Br J Dermatol. 1994;130:688-689.
- Wolkenstein P, Latarjet J, Roujeau J, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch W, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-708.
- Hunger R, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Al-Shouli S, Bogusz M, Al Tufail M, et al. Toxic epidermal necrosis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-553.
- Famularo G, Di Dona B, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Worsnop F, Wee J, Moosa Y, et al. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37:879-881.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulins. Dermatology. 2012;224:134-139.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Paquet P, Jennes S, Rousseua A, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;1:1-6.
- Sanclemente G, De le Rouche C, Escobar C, et al. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 1998;38:878-879.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumor necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South Med J. 2009;102:1133-1140.
- Muna S, Lawrance I. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Mounach A, Rezgi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
Practice Points
- Controversy remains over the most effective adjunctive therapy for toxic epidermal necrolysis (TEN), as none have consistently displayed superiority over rapid discontinuation of the culprit drug and aggressive supportive care alone.
- Since tumor necrosis factor α (TNF-α) was implicated in the pathogenesis of TEN, TNF-α inhibition has been attempted in treatment of the disease. These medications have shown positive outcomes.
- The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections.