User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
This drug works, but wait till you hear what’s in it
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.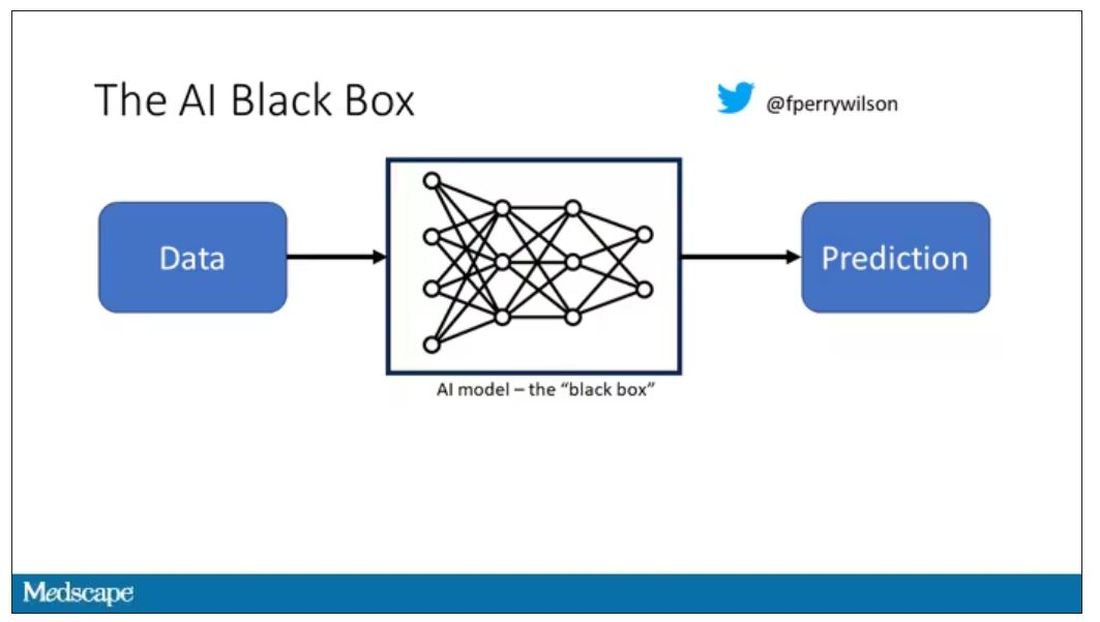
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.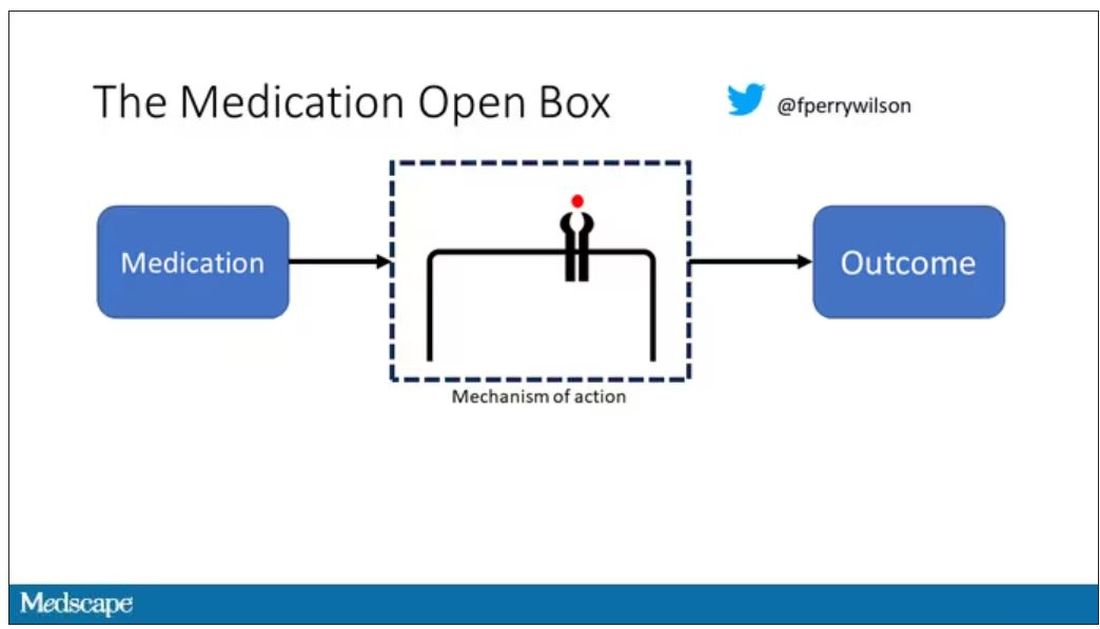
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.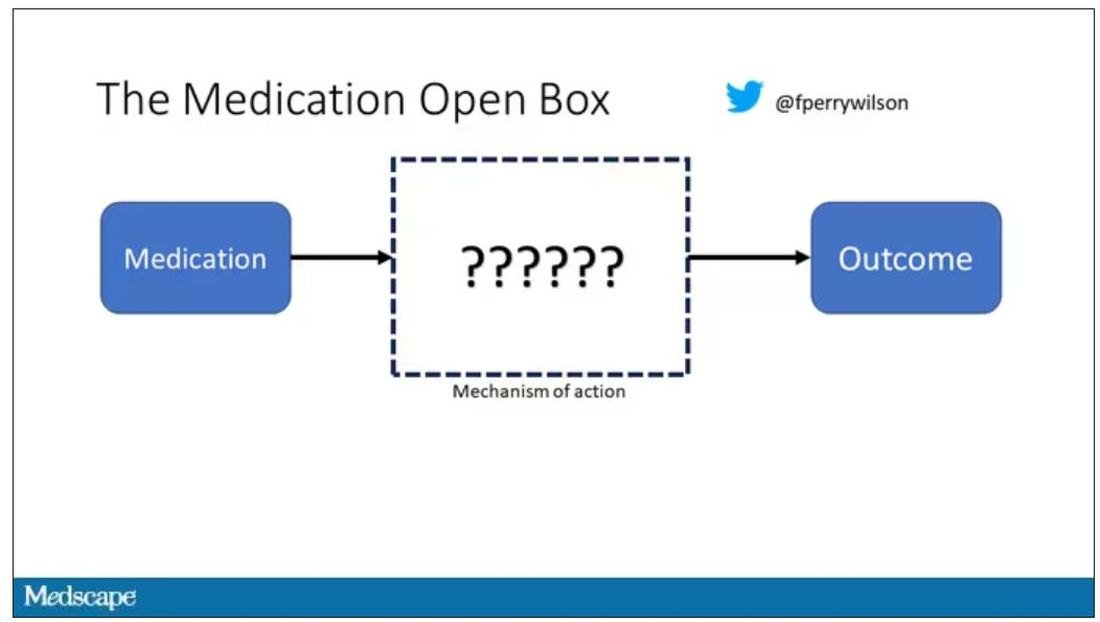
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.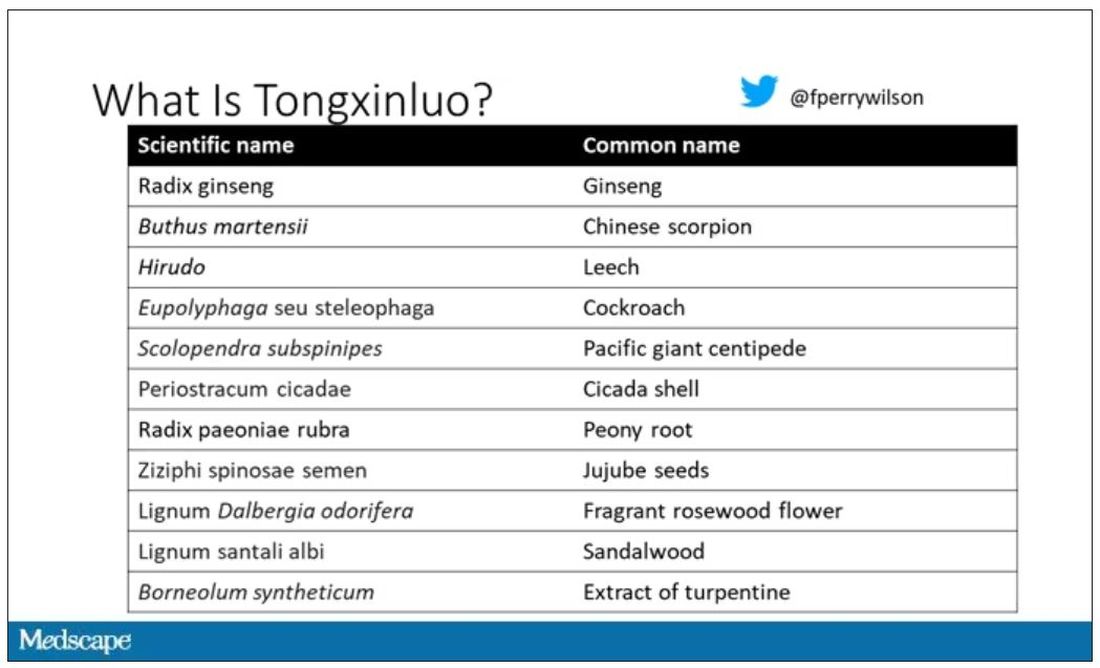
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.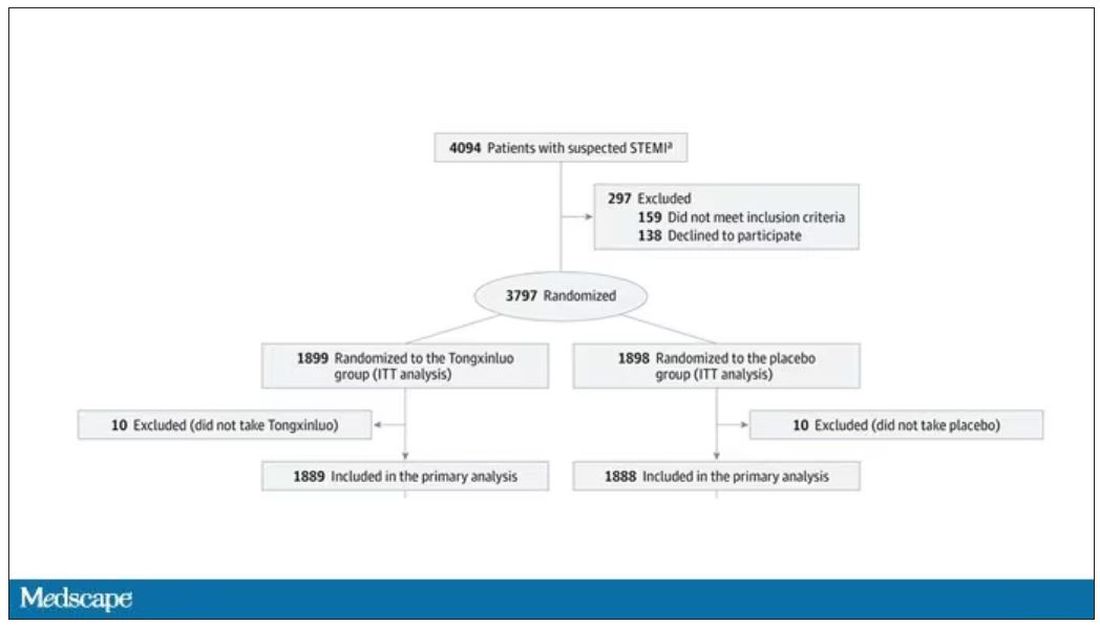
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.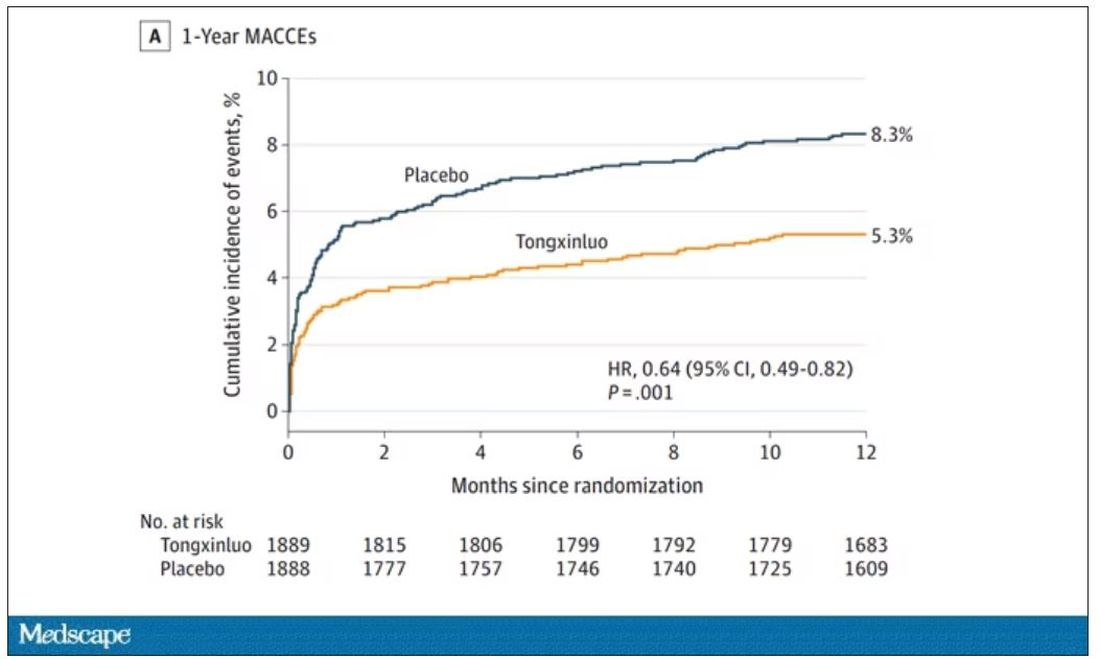
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.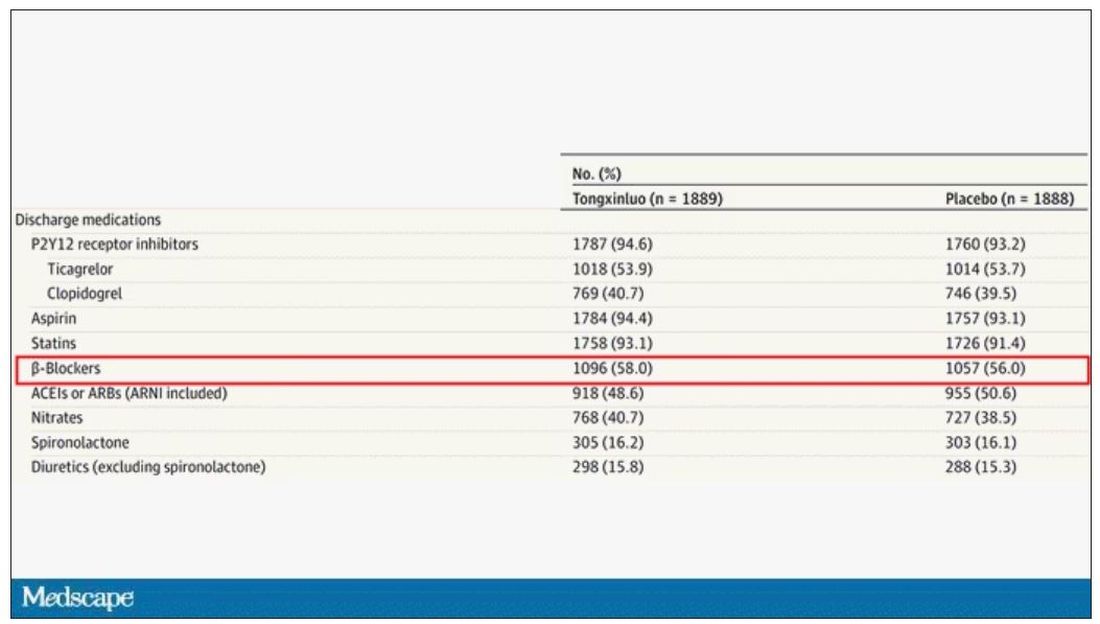
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
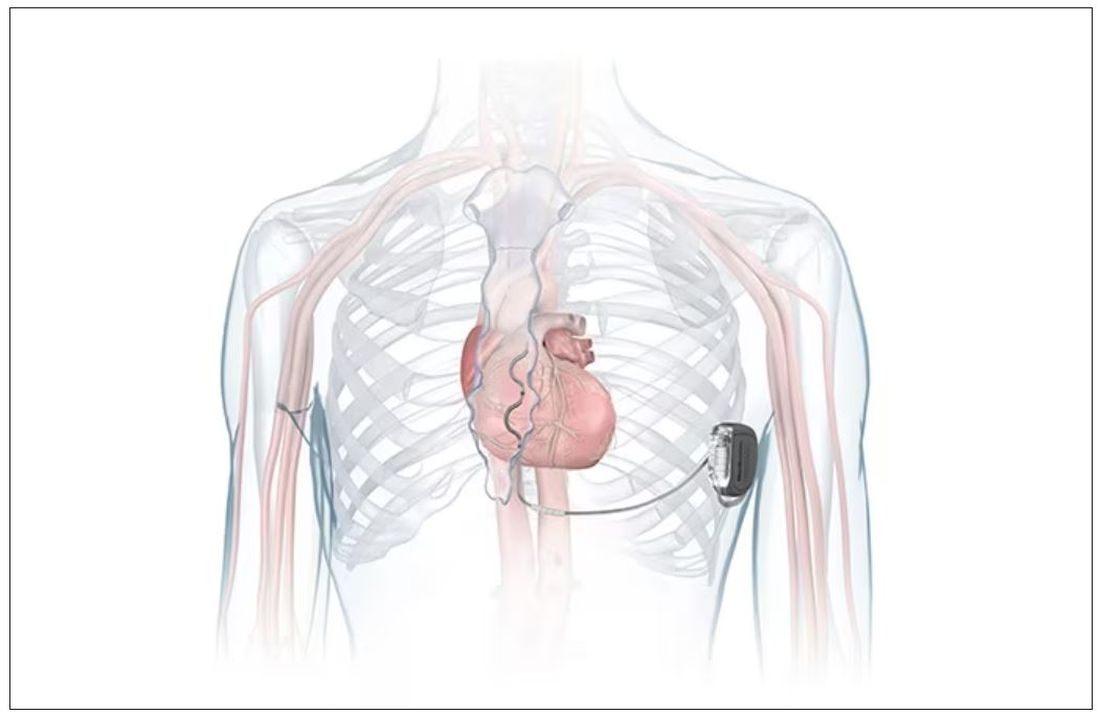
FDA okays first extravascular ICD system
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
COVID coronary plaque infection confirms CV risk
The findings may not only explain the link between COVID and the increased risk of cardiovascular events but mark a starting point for new therapeutic approaches.
“Our study shows there is persistence of viral debris in the artery,” senior investigator Chiara Giannarelli, MD, associate professor of medicine and pathology at NYU Langone Health, New York, said in an interview. “There is an important inflammatory response. We can now look at ways to control this inflammation,” she said.
Dr. Giannarelli says COVID is more than a respiratory virus and that it can affect the whole body. “Our study shows a remarkable ability of the virus to hijack the immune system,” she points out. “Our findings may explain how that happens.”
Dr. Giannarelli says it’s important for doctors and patients to be aware of an increased cardiovascular risk after a SARS-CoV-2 infection and to pay extra attention to traditional risk factors, such as blood pressure and cholesterol.
“This study showing that severe acute respiratory syndrome coronavirus directly infects coronary artery plaques, producing inflammatory substances, really joins the dots and helps our understanding on why we’re seeing so much heart disease in COVID patients,” Peter Hotez, MD, professor of molecular virology and microbiology at Baylor College of Medicine, Houston, said in an interview.
Asked whether this direct infection of vascular plaques was unique to SARS-CoV-2 or whether this may also occur with other viruses, both Dr. Giannarelli and Dr. Hotez said they believe this may be a specific COVID effect.
“I wouldn’t say it is likely that other viruses infect coronary arteries in this way, but I suppose it is possible,” Dr. Giannarelli said.
Dr. Hotez pointed out that other viruses can cause inflammation in the heart, such as myocarditis. “But I can’t think of another virus that stimulates the sequence of events in coronary artery inflammation like we’re seeing here.”
Dr. Giannarelli noted that influenza is also associated with an increased risk of cardiovascular events, but there has been no evidence to date that it directly affects coronary arteries.
Dr. Hotez added that the increased risk of cardiovascular events with influenza has also been reported to be prolonged after the acute infection. “These new findings with SARS-CoV-2 could stimulate a redoubling of efforts to look at this possibility with influenza,” he suggested.
Heart disease after COVID
In a recent article published online in Nature Cardiovascular Research, Dr. Giannarelli and colleagues analyzed human autopsy tissue samples from coronary arterial walls of patients who had died from COVID in the early stages of the pandemic in New York.
They found an accumulation of viral RNA in atherosclerotic plaques in the coronary arteries, which was particularly concentrated in lipid-rich macrophage foam cells present within the plaques.
“Our data conclusively demonstrate that severe acute respiratory syndrome coronavirus is capable of infecting and replicating in macrophages within the coronary vasculature,” the researchers report.
The virus preferentially replicates in foam cells, in comparison with other macrophages, they add, suggesting that these cells might act as a reservoir of viral debris in atherosclerotic plaque.
“We have shown that the virus is targeting lipid-rich macrophages in atherosclerotic lesions. This is the first time this has been shown, and we think this is a very important finding,” Dr. Giannarelli said in an interview.
“We also found that the virus persists in these foam cells that could be responsible for long-term, low-grade inflammation in the vasculature that could contribute to the long-term cardiovascular manifestations in patients who have recovered from COVID,” she said.
Viral reservoirs
Macrophages residing in vascular tissue can undergo self-renewal and can remain in the tissue for many years, the investigators point out. They suggest that these macrophages may act as viral reservoirs of SARS-CoV-2 RNA in atherosclerotic plaques.
Using an ex vivo model, the researchers also found that atherosclerotic tissue could be directly infected by the virus. And just as was seen in cultured macrophages and foam cells, infection of vascular tissue triggered an inflammatory response. That response induced the secretion of key proatherogenic cytokines, such as interleukin-6 and interleukin-1 beta, which have been implicated in the pathogenesis of atherosclerosis and in an increased risk of cardiovascular events.
“Considering that plaque inflammation promotes disease progression and contributes to plaque rupture, our results provide a molecular basis for how infection of coronary lesions can contribute to the acute cardiovascular manifestations of COVID-19, such as myocardial infarction,” the researchers report.
Another interesting finding was a higher accumulation of viral RNA in the coronary vasculature of the three patients with acute ischemic cardiovascular manifestations, which they say adds to evidence that infection may increase cardiovascular risk.
Dr. Giannarelli points out that the patients in their study died in New York early in the pandemic, before vaccines were available. “They were unvaccinated and likely had little immunity against initial viral strains.”
Dr. Hotez says that when COVID-19 first emerged, many in the medical and scientific communities thought it would closely resemble the original SARS viral infection, which was primarily a respiratory pathogen.
“But it became pretty clear early on this virus was causing a lot of cardiovascular and thromboembolic disease,” he says. “This study provides an insight into the mechanisms involved here.”
Affecting more than lungs
Dr. Hotez pointed out that a recent study reported a 5% increase in cardiovascular deaths during the years 2020-2022, compared with before the pandemic.
“Those peaks of cardiovascular deaths corresponded with specific waves of COVID – the first happening at the time of the initial wave with the original virus and second during the Delta wave. So, there’s no question that this virus is contributing to excess cardiovascular mortality, and this paper appears to explain the mechanism.”
Dr. Hotez pointed out that the new findings suggest the cardiovascular risk may be prolonged well after the acute infection resolves.
“In long COVID, a lot of people focus on the neurological effects – brain fog and depression. But cardiac insufficiency and other cardiovascular events can also be considered another element of long COVID,” he said.
Dr. Giannarelli says her group is now studying whether patients with long COVID have virus in their coronary arteries. She points out that the current studies were a result of a team effort between experts in cardiovascular disease and virology and infectious disease. “We need to collaborate more like this to understand better the impact of viral infection in patients and the clinical manifestations,” she said.
Dr. Hotez says he believes these new findings will have implications for the future.
“COVID hasn’t gone away. The numbers have been going up again steadily in the U.S. in the last few months. There are still a significant number of hospitalizations,” he said.
While it would be unwieldy to ask for a cardiology consult for every COVID patient, he acknowledged, “there is probably a subset of people – possibly those of older age and who have had a severe case of COVID – who we suspect are now going to be more prone to cardiovascular disease because of having COVID.
“We should be vigilant in looking for cardiovascular disease in these patients,” Dr. Hotez said, “and perhaps be a bit more aggressive about controlling their cardiovascular risk factors.”
The study was funded by the U.S. National Institutes of Health, the American Heart Association, and the Chan Zuckerberg Initiative.
A version of this article first appeared on Medscape.com .
The findings may not only explain the link between COVID and the increased risk of cardiovascular events but mark a starting point for new therapeutic approaches.
“Our study shows there is persistence of viral debris in the artery,” senior investigator Chiara Giannarelli, MD, associate professor of medicine and pathology at NYU Langone Health, New York, said in an interview. “There is an important inflammatory response. We can now look at ways to control this inflammation,” she said.
Dr. Giannarelli says COVID is more than a respiratory virus and that it can affect the whole body. “Our study shows a remarkable ability of the virus to hijack the immune system,” she points out. “Our findings may explain how that happens.”
Dr. Giannarelli says it’s important for doctors and patients to be aware of an increased cardiovascular risk after a SARS-CoV-2 infection and to pay extra attention to traditional risk factors, such as blood pressure and cholesterol.
“This study showing that severe acute respiratory syndrome coronavirus directly infects coronary artery plaques, producing inflammatory substances, really joins the dots and helps our understanding on why we’re seeing so much heart disease in COVID patients,” Peter Hotez, MD, professor of molecular virology and microbiology at Baylor College of Medicine, Houston, said in an interview.
Asked whether this direct infection of vascular plaques was unique to SARS-CoV-2 or whether this may also occur with other viruses, both Dr. Giannarelli and Dr. Hotez said they believe this may be a specific COVID effect.
“I wouldn’t say it is likely that other viruses infect coronary arteries in this way, but I suppose it is possible,” Dr. Giannarelli said.
Dr. Hotez pointed out that other viruses can cause inflammation in the heart, such as myocarditis. “But I can’t think of another virus that stimulates the sequence of events in coronary artery inflammation like we’re seeing here.”
Dr. Giannarelli noted that influenza is also associated with an increased risk of cardiovascular events, but there has been no evidence to date that it directly affects coronary arteries.
Dr. Hotez added that the increased risk of cardiovascular events with influenza has also been reported to be prolonged after the acute infection. “These new findings with SARS-CoV-2 could stimulate a redoubling of efforts to look at this possibility with influenza,” he suggested.
Heart disease after COVID
In a recent article published online in Nature Cardiovascular Research, Dr. Giannarelli and colleagues analyzed human autopsy tissue samples from coronary arterial walls of patients who had died from COVID in the early stages of the pandemic in New York.
They found an accumulation of viral RNA in atherosclerotic plaques in the coronary arteries, which was particularly concentrated in lipid-rich macrophage foam cells present within the plaques.
“Our data conclusively demonstrate that severe acute respiratory syndrome coronavirus is capable of infecting and replicating in macrophages within the coronary vasculature,” the researchers report.
The virus preferentially replicates in foam cells, in comparison with other macrophages, they add, suggesting that these cells might act as a reservoir of viral debris in atherosclerotic plaque.
“We have shown that the virus is targeting lipid-rich macrophages in atherosclerotic lesions. This is the first time this has been shown, and we think this is a very important finding,” Dr. Giannarelli said in an interview.
“We also found that the virus persists in these foam cells that could be responsible for long-term, low-grade inflammation in the vasculature that could contribute to the long-term cardiovascular manifestations in patients who have recovered from COVID,” she said.
Viral reservoirs
Macrophages residing in vascular tissue can undergo self-renewal and can remain in the tissue for many years, the investigators point out. They suggest that these macrophages may act as viral reservoirs of SARS-CoV-2 RNA in atherosclerotic plaques.
Using an ex vivo model, the researchers also found that atherosclerotic tissue could be directly infected by the virus. And just as was seen in cultured macrophages and foam cells, infection of vascular tissue triggered an inflammatory response. That response induced the secretion of key proatherogenic cytokines, such as interleukin-6 and interleukin-1 beta, which have been implicated in the pathogenesis of atherosclerosis and in an increased risk of cardiovascular events.
“Considering that plaque inflammation promotes disease progression and contributes to plaque rupture, our results provide a molecular basis for how infection of coronary lesions can contribute to the acute cardiovascular manifestations of COVID-19, such as myocardial infarction,” the researchers report.
Another interesting finding was a higher accumulation of viral RNA in the coronary vasculature of the three patients with acute ischemic cardiovascular manifestations, which they say adds to evidence that infection may increase cardiovascular risk.
Dr. Giannarelli points out that the patients in their study died in New York early in the pandemic, before vaccines were available. “They were unvaccinated and likely had little immunity against initial viral strains.”
Dr. Hotez says that when COVID-19 first emerged, many in the medical and scientific communities thought it would closely resemble the original SARS viral infection, which was primarily a respiratory pathogen.
“But it became pretty clear early on this virus was causing a lot of cardiovascular and thromboembolic disease,” he says. “This study provides an insight into the mechanisms involved here.”
Affecting more than lungs
Dr. Hotez pointed out that a recent study reported a 5% increase in cardiovascular deaths during the years 2020-2022, compared with before the pandemic.
“Those peaks of cardiovascular deaths corresponded with specific waves of COVID – the first happening at the time of the initial wave with the original virus and second during the Delta wave. So, there’s no question that this virus is contributing to excess cardiovascular mortality, and this paper appears to explain the mechanism.”
Dr. Hotez pointed out that the new findings suggest the cardiovascular risk may be prolonged well after the acute infection resolves.
“In long COVID, a lot of people focus on the neurological effects – brain fog and depression. But cardiac insufficiency and other cardiovascular events can also be considered another element of long COVID,” he said.
Dr. Giannarelli says her group is now studying whether patients with long COVID have virus in their coronary arteries. She points out that the current studies were a result of a team effort between experts in cardiovascular disease and virology and infectious disease. “We need to collaborate more like this to understand better the impact of viral infection in patients and the clinical manifestations,” she said.
Dr. Hotez says he believes these new findings will have implications for the future.
“COVID hasn’t gone away. The numbers have been going up again steadily in the U.S. in the last few months. There are still a significant number of hospitalizations,” he said.
While it would be unwieldy to ask for a cardiology consult for every COVID patient, he acknowledged, “there is probably a subset of people – possibly those of older age and who have had a severe case of COVID – who we suspect are now going to be more prone to cardiovascular disease because of having COVID.
“We should be vigilant in looking for cardiovascular disease in these patients,” Dr. Hotez said, “and perhaps be a bit more aggressive about controlling their cardiovascular risk factors.”
The study was funded by the U.S. National Institutes of Health, the American Heart Association, and the Chan Zuckerberg Initiative.
A version of this article first appeared on Medscape.com .
The findings may not only explain the link between COVID and the increased risk of cardiovascular events but mark a starting point for new therapeutic approaches.
“Our study shows there is persistence of viral debris in the artery,” senior investigator Chiara Giannarelli, MD, associate professor of medicine and pathology at NYU Langone Health, New York, said in an interview. “There is an important inflammatory response. We can now look at ways to control this inflammation,” she said.
Dr. Giannarelli says COVID is more than a respiratory virus and that it can affect the whole body. “Our study shows a remarkable ability of the virus to hijack the immune system,” she points out. “Our findings may explain how that happens.”
Dr. Giannarelli says it’s important for doctors and patients to be aware of an increased cardiovascular risk after a SARS-CoV-2 infection and to pay extra attention to traditional risk factors, such as blood pressure and cholesterol.
“This study showing that severe acute respiratory syndrome coronavirus directly infects coronary artery plaques, producing inflammatory substances, really joins the dots and helps our understanding on why we’re seeing so much heart disease in COVID patients,” Peter Hotez, MD, professor of molecular virology and microbiology at Baylor College of Medicine, Houston, said in an interview.
Asked whether this direct infection of vascular plaques was unique to SARS-CoV-2 or whether this may also occur with other viruses, both Dr. Giannarelli and Dr. Hotez said they believe this may be a specific COVID effect.
“I wouldn’t say it is likely that other viruses infect coronary arteries in this way, but I suppose it is possible,” Dr. Giannarelli said.
Dr. Hotez pointed out that other viruses can cause inflammation in the heart, such as myocarditis. “But I can’t think of another virus that stimulates the sequence of events in coronary artery inflammation like we’re seeing here.”
Dr. Giannarelli noted that influenza is also associated with an increased risk of cardiovascular events, but there has been no evidence to date that it directly affects coronary arteries.
Dr. Hotez added that the increased risk of cardiovascular events with influenza has also been reported to be prolonged after the acute infection. “These new findings with SARS-CoV-2 could stimulate a redoubling of efforts to look at this possibility with influenza,” he suggested.
Heart disease after COVID
In a recent article published online in Nature Cardiovascular Research, Dr. Giannarelli and colleagues analyzed human autopsy tissue samples from coronary arterial walls of patients who had died from COVID in the early stages of the pandemic in New York.
They found an accumulation of viral RNA in atherosclerotic plaques in the coronary arteries, which was particularly concentrated in lipid-rich macrophage foam cells present within the plaques.
“Our data conclusively demonstrate that severe acute respiratory syndrome coronavirus is capable of infecting and replicating in macrophages within the coronary vasculature,” the researchers report.
The virus preferentially replicates in foam cells, in comparison with other macrophages, they add, suggesting that these cells might act as a reservoir of viral debris in atherosclerotic plaque.
“We have shown that the virus is targeting lipid-rich macrophages in atherosclerotic lesions. This is the first time this has been shown, and we think this is a very important finding,” Dr. Giannarelli said in an interview.
“We also found that the virus persists in these foam cells that could be responsible for long-term, low-grade inflammation in the vasculature that could contribute to the long-term cardiovascular manifestations in patients who have recovered from COVID,” she said.
Viral reservoirs
Macrophages residing in vascular tissue can undergo self-renewal and can remain in the tissue for many years, the investigators point out. They suggest that these macrophages may act as viral reservoirs of SARS-CoV-2 RNA in atherosclerotic plaques.
Using an ex vivo model, the researchers also found that atherosclerotic tissue could be directly infected by the virus. And just as was seen in cultured macrophages and foam cells, infection of vascular tissue triggered an inflammatory response. That response induced the secretion of key proatherogenic cytokines, such as interleukin-6 and interleukin-1 beta, which have been implicated in the pathogenesis of atherosclerosis and in an increased risk of cardiovascular events.
“Considering that plaque inflammation promotes disease progression and contributes to plaque rupture, our results provide a molecular basis for how infection of coronary lesions can contribute to the acute cardiovascular manifestations of COVID-19, such as myocardial infarction,” the researchers report.
Another interesting finding was a higher accumulation of viral RNA in the coronary vasculature of the three patients with acute ischemic cardiovascular manifestations, which they say adds to evidence that infection may increase cardiovascular risk.
Dr. Giannarelli points out that the patients in their study died in New York early in the pandemic, before vaccines were available. “They were unvaccinated and likely had little immunity against initial viral strains.”
Dr. Hotez says that when COVID-19 first emerged, many in the medical and scientific communities thought it would closely resemble the original SARS viral infection, which was primarily a respiratory pathogen.
“But it became pretty clear early on this virus was causing a lot of cardiovascular and thromboembolic disease,” he says. “This study provides an insight into the mechanisms involved here.”
Affecting more than lungs
Dr. Hotez pointed out that a recent study reported a 5% increase in cardiovascular deaths during the years 2020-2022, compared with before the pandemic.
“Those peaks of cardiovascular deaths corresponded with specific waves of COVID – the first happening at the time of the initial wave with the original virus and second during the Delta wave. So, there’s no question that this virus is contributing to excess cardiovascular mortality, and this paper appears to explain the mechanism.”
Dr. Hotez pointed out that the new findings suggest the cardiovascular risk may be prolonged well after the acute infection resolves.
“In long COVID, a lot of people focus on the neurological effects – brain fog and depression. But cardiac insufficiency and other cardiovascular events can also be considered another element of long COVID,” he said.
Dr. Giannarelli says her group is now studying whether patients with long COVID have virus in their coronary arteries. She points out that the current studies were a result of a team effort between experts in cardiovascular disease and virology and infectious disease. “We need to collaborate more like this to understand better the impact of viral infection in patients and the clinical manifestations,” she said.
Dr. Hotez says he believes these new findings will have implications for the future.
“COVID hasn’t gone away. The numbers have been going up again steadily in the U.S. in the last few months. There are still a significant number of hospitalizations,” he said.
While it would be unwieldy to ask for a cardiology consult for every COVID patient, he acknowledged, “there is probably a subset of people – possibly those of older age and who have had a severe case of COVID – who we suspect are now going to be more prone to cardiovascular disease because of having COVID.
“We should be vigilant in looking for cardiovascular disease in these patients,” Dr. Hotez said, “and perhaps be a bit more aggressive about controlling their cardiovascular risk factors.”
The study was funded by the U.S. National Institutes of Health, the American Heart Association, and the Chan Zuckerberg Initiative.
A version of this article first appeared on Medscape.com .
FROM NATURE CARDIOVASCULAR RESEARCH
Common meds link to sudden cardiac arrest in type 2 diabetes
HAMBURG, Germany – , shows the first such analysis of real-world, primary care data.
People with type 2 diabetes who do not have a history of CVD have almost three times the risk of SCA if they take antipsychotic medications and nearly double the risk if they take certain antibiotics that prolong the QT interval, notably, macrolides and fluoroquinolones.
“These data show that commonly prescribed drugs - antipsychotic medications, used by about 3% of people with type 2 diabetes, and antibiotics, taken by 5% to 10%, convey an increased risk of sudden cardiac arrest in those without a history of cardiovascular disease,” said Peter Harms, MSc, who presented the study at the annual meeting of the European Association for the Study of Diabetes. Another drug associated with an increase in SCA among patients with diabetes was domperidone, an antinausea medication.
“Perhaps these drugs could be avoided in some cases, and GPs should be more aware of the possible consequences of their use,” he added. “If the patient has type 2 diabetes, then maybe it’s better to avoid some of these medications and try and cope without them, or at least find an alternative antibiotic.”
Mr. Harms, an epidemiologist from Amsterdam University Medical Centers, highlighted that their study was unique because the investigators drew upon primary care data. “These data are extensive, and we find a lot of associations which are very real.”
SCA is associated with 50% of all cardiac deaths and accounts for 20% of all mortality in high-income countries. Of those people who experience SCA, 80% of cases prove fatal.
“As the name suggests, it is difficult to predict because it is sudden, especially in people without a cardiovascular disease history,” Mr. Harms pointed out in an interview with this news organization. He highlighted that “around half of those who experience SCA, often between the ages of 40 and 60 years, have never seen a cardiologist, but many do have type 2 diabetes.
“We need to better understand how to recognize people at risk of SCA, know who to watch and how to prevent these events,” he emphasized.
Vladimira Fejfarova, MD, comoderated the session and commented on the study. “From the clinical point of view, it’s necessary to evaluate risk factors that can contribute to sudden cardiac arrest.”
Overall, the researchers found that, among people with type 2 diabetes who do not have a history of CVD, hypoglycemia, severe hypertension, dyslipidemia, and use of QTc-prolonging medications are associated with SCA risk. Among people with type 2 diabetes and CVD, albuminuria and heart failure are associated with SCA risk.
Dr. Fejfarova added: “With type 2 diabetes and also type 1, we need to look more at adverse events, especially when treating infections with macrolides, but also mycotic infections, because antimycotic drugs are known to influence QT intervals that could contribute to sudden cardiac arrest.
“We need to be more cautious with prescribing certain antibiotics that have these side effects in our patients with diabetes,” asserted Dr. Fejfarova, from the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Type 2 diabetes doubles the risk of SCA
The researcher decided to investigate the population of people with type 2 diabetes because their risk of SCD is around twice that of those without type 2 diabetes. Because these patients have relatively frequent checkups with general practitioners, Mr. Harms turned to primary care databases that contained comprehensive and relatively routine information on risk indicators.
Longitudinal associations between clinical characteristics of 3,919 patients with type 2 diabetes – both those with and those without a history of CVD – and SCA (a total of 689 patients) were determined.
Cases were found in the AmsteRdam REsuscitation STtudies (ARREST) registry of out-of-hospital resuscitation attempts by emergency medical services in the Dutch region of Noord-Holland from 2010 to 2019. Case patients were matched with up to five control patients. The control group comprised people with type 2 diabetes who had not experienced an SCA. Control patients were sourced from the same primary care practices who were of similar age and sex. Clinical measurements, including blood pressure and blood glucose readings, medication use, and medical history for the 5 years leading up to an SCA, were obtained from general practice records. A multivariable analysis was performed, and results were stratified for people with and for those without a history of CVD.
Of particular interest were drugs that interfere with cardiac function, including some prokinetic, antibiotic, and antipsychotic medications. All of the drugs are known to be associated with a change in QTc prolongation. Examples include domperidone (QTc-prolonging prokinetic), macrolides and fluoroquinolones (QTc-prolonging antibiotics), and haloperidol (a QTc-prolonging antipsychotic).
Antibiotic and antipsychotic use might contribute to SCA in T2D
Case patients and control patients were similar in age, hemoglobin A1c level, and other characteristics with the exception that more patients with SCA had a history of CVD (40.0% vs. 29.4%).
“Looking at the associations in the overall population, insulin use was strongly associated with SCA risk [hazard ratio, 2.38] and perhaps this was an indicator of severity of type 2 diabetes,” remarked Mr. Harms. “Also, unsurprisingly, a history of arrhythmia [HR, 1.68] and, more surprisingly, prokinetic drug use [HR, 1.66; 95% confidence interval, 1.20-2.31], specifically those known for QTc-prolongation, were associated with SCA.”
Among people who had experienced an SCA and who did not have a history of CVD (337 case patients/2,023 control patients), QTc-prolonging antipsychotic medication use was associated with SCA at an HR of 2.87, and antibiotic medication use was associated with SCA at an HR of 1.66. A low fasting glucose level (< 4.5 mmol/mol) was associated with SCA at an HR of 2.5; severely high systolic blood pressure (> 180 mm Hg) was associated with SCA at an HR of 2.21; low HDL cholesterol level, with an HR of 1.35; and high LDL cholesterol level (> 2.6 mmol/L), with an HR of 1.64.
Among people with a history of CVD (352 case patients/1,207 control patients), associations between albuminuria and SCA were moderate (HR, 1.54) and severe (HR, 1.55); heart failure was associated with SCA at an HR of 1.85 (95% CI, 1.50-2.29).
Comoderator Dr. Fejfarova added that, in addition to the findings from Dr. Harms’ study, other research presented in the same session highlighted the importance of checking patients for the presence of arrhythmias that could lead to the development of atrioventricular blocks, sinus node diseases, and SCA.
Mr. Harms and Dr. Fejfarova have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HAMBURG, Germany – , shows the first such analysis of real-world, primary care data.
People with type 2 diabetes who do not have a history of CVD have almost three times the risk of SCA if they take antipsychotic medications and nearly double the risk if they take certain antibiotics that prolong the QT interval, notably, macrolides and fluoroquinolones.
“These data show that commonly prescribed drugs - antipsychotic medications, used by about 3% of people with type 2 diabetes, and antibiotics, taken by 5% to 10%, convey an increased risk of sudden cardiac arrest in those without a history of cardiovascular disease,” said Peter Harms, MSc, who presented the study at the annual meeting of the European Association for the Study of Diabetes. Another drug associated with an increase in SCA among patients with diabetes was domperidone, an antinausea medication.
“Perhaps these drugs could be avoided in some cases, and GPs should be more aware of the possible consequences of their use,” he added. “If the patient has type 2 diabetes, then maybe it’s better to avoid some of these medications and try and cope without them, or at least find an alternative antibiotic.”
Mr. Harms, an epidemiologist from Amsterdam University Medical Centers, highlighted that their study was unique because the investigators drew upon primary care data. “These data are extensive, and we find a lot of associations which are very real.”
SCA is associated with 50% of all cardiac deaths and accounts for 20% of all mortality in high-income countries. Of those people who experience SCA, 80% of cases prove fatal.
“As the name suggests, it is difficult to predict because it is sudden, especially in people without a cardiovascular disease history,” Mr. Harms pointed out in an interview with this news organization. He highlighted that “around half of those who experience SCA, often between the ages of 40 and 60 years, have never seen a cardiologist, but many do have type 2 diabetes.
“We need to better understand how to recognize people at risk of SCA, know who to watch and how to prevent these events,” he emphasized.
Vladimira Fejfarova, MD, comoderated the session and commented on the study. “From the clinical point of view, it’s necessary to evaluate risk factors that can contribute to sudden cardiac arrest.”
Overall, the researchers found that, among people with type 2 diabetes who do not have a history of CVD, hypoglycemia, severe hypertension, dyslipidemia, and use of QTc-prolonging medications are associated with SCA risk. Among people with type 2 diabetes and CVD, albuminuria and heart failure are associated with SCA risk.
Dr. Fejfarova added: “With type 2 diabetes and also type 1, we need to look more at adverse events, especially when treating infections with macrolides, but also mycotic infections, because antimycotic drugs are known to influence QT intervals that could contribute to sudden cardiac arrest.
“We need to be more cautious with prescribing certain antibiotics that have these side effects in our patients with diabetes,” asserted Dr. Fejfarova, from the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Type 2 diabetes doubles the risk of SCA
The researcher decided to investigate the population of people with type 2 diabetes because their risk of SCD is around twice that of those without type 2 diabetes. Because these patients have relatively frequent checkups with general practitioners, Mr. Harms turned to primary care databases that contained comprehensive and relatively routine information on risk indicators.
Longitudinal associations between clinical characteristics of 3,919 patients with type 2 diabetes – both those with and those without a history of CVD – and SCA (a total of 689 patients) were determined.
Cases were found in the AmsteRdam REsuscitation STtudies (ARREST) registry of out-of-hospital resuscitation attempts by emergency medical services in the Dutch region of Noord-Holland from 2010 to 2019. Case patients were matched with up to five control patients. The control group comprised people with type 2 diabetes who had not experienced an SCA. Control patients were sourced from the same primary care practices who were of similar age and sex. Clinical measurements, including blood pressure and blood glucose readings, medication use, and medical history for the 5 years leading up to an SCA, were obtained from general practice records. A multivariable analysis was performed, and results were stratified for people with and for those without a history of CVD.
Of particular interest were drugs that interfere with cardiac function, including some prokinetic, antibiotic, and antipsychotic medications. All of the drugs are known to be associated with a change in QTc prolongation. Examples include domperidone (QTc-prolonging prokinetic), macrolides and fluoroquinolones (QTc-prolonging antibiotics), and haloperidol (a QTc-prolonging antipsychotic).
Antibiotic and antipsychotic use might contribute to SCA in T2D
Case patients and control patients were similar in age, hemoglobin A1c level, and other characteristics with the exception that more patients with SCA had a history of CVD (40.0% vs. 29.4%).
“Looking at the associations in the overall population, insulin use was strongly associated with SCA risk [hazard ratio, 2.38] and perhaps this was an indicator of severity of type 2 diabetes,” remarked Mr. Harms. “Also, unsurprisingly, a history of arrhythmia [HR, 1.68] and, more surprisingly, prokinetic drug use [HR, 1.66; 95% confidence interval, 1.20-2.31], specifically those known for QTc-prolongation, were associated with SCA.”
Among people who had experienced an SCA and who did not have a history of CVD (337 case patients/2,023 control patients), QTc-prolonging antipsychotic medication use was associated with SCA at an HR of 2.87, and antibiotic medication use was associated with SCA at an HR of 1.66. A low fasting glucose level (< 4.5 mmol/mol) was associated with SCA at an HR of 2.5; severely high systolic blood pressure (> 180 mm Hg) was associated with SCA at an HR of 2.21; low HDL cholesterol level, with an HR of 1.35; and high LDL cholesterol level (> 2.6 mmol/L), with an HR of 1.64.
Among people with a history of CVD (352 case patients/1,207 control patients), associations between albuminuria and SCA were moderate (HR, 1.54) and severe (HR, 1.55); heart failure was associated with SCA at an HR of 1.85 (95% CI, 1.50-2.29).
Comoderator Dr. Fejfarova added that, in addition to the findings from Dr. Harms’ study, other research presented in the same session highlighted the importance of checking patients for the presence of arrhythmias that could lead to the development of atrioventricular blocks, sinus node diseases, and SCA.
Mr. Harms and Dr. Fejfarova have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HAMBURG, Germany – , shows the first such analysis of real-world, primary care data.
People with type 2 diabetes who do not have a history of CVD have almost three times the risk of SCA if they take antipsychotic medications and nearly double the risk if they take certain antibiotics that prolong the QT interval, notably, macrolides and fluoroquinolones.
“These data show that commonly prescribed drugs - antipsychotic medications, used by about 3% of people with type 2 diabetes, and antibiotics, taken by 5% to 10%, convey an increased risk of sudden cardiac arrest in those without a history of cardiovascular disease,” said Peter Harms, MSc, who presented the study at the annual meeting of the European Association for the Study of Diabetes. Another drug associated with an increase in SCA among patients with diabetes was domperidone, an antinausea medication.
“Perhaps these drugs could be avoided in some cases, and GPs should be more aware of the possible consequences of their use,” he added. “If the patient has type 2 diabetes, then maybe it’s better to avoid some of these medications and try and cope without them, or at least find an alternative antibiotic.”
Mr. Harms, an epidemiologist from Amsterdam University Medical Centers, highlighted that their study was unique because the investigators drew upon primary care data. “These data are extensive, and we find a lot of associations which are very real.”
SCA is associated with 50% of all cardiac deaths and accounts for 20% of all mortality in high-income countries. Of those people who experience SCA, 80% of cases prove fatal.
“As the name suggests, it is difficult to predict because it is sudden, especially in people without a cardiovascular disease history,” Mr. Harms pointed out in an interview with this news organization. He highlighted that “around half of those who experience SCA, often between the ages of 40 and 60 years, have never seen a cardiologist, but many do have type 2 diabetes.
“We need to better understand how to recognize people at risk of SCA, know who to watch and how to prevent these events,” he emphasized.
Vladimira Fejfarova, MD, comoderated the session and commented on the study. “From the clinical point of view, it’s necessary to evaluate risk factors that can contribute to sudden cardiac arrest.”
Overall, the researchers found that, among people with type 2 diabetes who do not have a history of CVD, hypoglycemia, severe hypertension, dyslipidemia, and use of QTc-prolonging medications are associated with SCA risk. Among people with type 2 diabetes and CVD, albuminuria and heart failure are associated with SCA risk.
Dr. Fejfarova added: “With type 2 diabetes and also type 1, we need to look more at adverse events, especially when treating infections with macrolides, but also mycotic infections, because antimycotic drugs are known to influence QT intervals that could contribute to sudden cardiac arrest.
“We need to be more cautious with prescribing certain antibiotics that have these side effects in our patients with diabetes,” asserted Dr. Fejfarova, from the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Type 2 diabetes doubles the risk of SCA
The researcher decided to investigate the population of people with type 2 diabetes because their risk of SCD is around twice that of those without type 2 diabetes. Because these patients have relatively frequent checkups with general practitioners, Mr. Harms turned to primary care databases that contained comprehensive and relatively routine information on risk indicators.
Longitudinal associations between clinical characteristics of 3,919 patients with type 2 diabetes – both those with and those without a history of CVD – and SCA (a total of 689 patients) were determined.
Cases were found in the AmsteRdam REsuscitation STtudies (ARREST) registry of out-of-hospital resuscitation attempts by emergency medical services in the Dutch region of Noord-Holland from 2010 to 2019. Case patients were matched with up to five control patients. The control group comprised people with type 2 diabetes who had not experienced an SCA. Control patients were sourced from the same primary care practices who were of similar age and sex. Clinical measurements, including blood pressure and blood glucose readings, medication use, and medical history for the 5 years leading up to an SCA, were obtained from general practice records. A multivariable analysis was performed, and results were stratified for people with and for those without a history of CVD.
Of particular interest were drugs that interfere with cardiac function, including some prokinetic, antibiotic, and antipsychotic medications. All of the drugs are known to be associated with a change in QTc prolongation. Examples include domperidone (QTc-prolonging prokinetic), macrolides and fluoroquinolones (QTc-prolonging antibiotics), and haloperidol (a QTc-prolonging antipsychotic).
Antibiotic and antipsychotic use might contribute to SCA in T2D
Case patients and control patients were similar in age, hemoglobin A1c level, and other characteristics with the exception that more patients with SCA had a history of CVD (40.0% vs. 29.4%).
“Looking at the associations in the overall population, insulin use was strongly associated with SCA risk [hazard ratio, 2.38] and perhaps this was an indicator of severity of type 2 diabetes,” remarked Mr. Harms. “Also, unsurprisingly, a history of arrhythmia [HR, 1.68] and, more surprisingly, prokinetic drug use [HR, 1.66; 95% confidence interval, 1.20-2.31], specifically those known for QTc-prolongation, were associated with SCA.”
Among people who had experienced an SCA and who did not have a history of CVD (337 case patients/2,023 control patients), QTc-prolonging antipsychotic medication use was associated with SCA at an HR of 2.87, and antibiotic medication use was associated with SCA at an HR of 1.66. A low fasting glucose level (< 4.5 mmol/mol) was associated with SCA at an HR of 2.5; severely high systolic blood pressure (> 180 mm Hg) was associated with SCA at an HR of 2.21; low HDL cholesterol level, with an HR of 1.35; and high LDL cholesterol level (> 2.6 mmol/L), with an HR of 1.64.
Among people with a history of CVD (352 case patients/1,207 control patients), associations between albuminuria and SCA were moderate (HR, 1.54) and severe (HR, 1.55); heart failure was associated with SCA at an HR of 1.85 (95% CI, 1.50-2.29).
Comoderator Dr. Fejfarova added that, in addition to the findings from Dr. Harms’ study, other research presented in the same session highlighted the importance of checking patients for the presence of arrhythmias that could lead to the development of atrioventricular blocks, sinus node diseases, and SCA.
Mr. Harms and Dr. Fejfarova have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2023
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Still, employed physicians also had plenty to say about the downsides of their jobs.
Many pointed to “feeling like a cog in the machine,” and one doctor pointed to the hassle of dealing with bureaucracy. Others complained about the fact that nonphysicians ran the business and lacked an understanding of what physicians really need from their jobs. When asked whether administrative rules made sense, 63% of physician respondents said that yes, the rules make sense for the business; but, only 52% said that the rules make sense for the doctors themselves.
Other complaints included the requirement to reach high productivity targets and too low an income potential. In the 9 years since Medscape’s 2104 Employed Physicians Report, the share of employed doctors paid on a straight salary has declined from 46% to 31%. Those compensated on a base salary plus productivity targets and other performance metrics rose from 13% in 2014 to 32% now.
“Many doctors go into private practice because of the freedom it brings and the potential financial incentives,” added Dr. Boduch. “I know that many doctors have a dream of working for themselves, and in many cases, that works out great for them.”
Dr. Boduch noted that in her job as chief medical officer at PsychPlus, she still has flexibility plus the perks of working with a bigger practice. In this scenario, Dr. Boduch said, the company can negotiate with insurance companies, allowing her the financial rewards of private practice.
What’s right for you?
“I think it might be somewhat generational,” said Cody Futch, senior recruiting executive at AMN Healthcare. “It used to be that fewer hospitals offered employment, so private practice was the way to go. Now, there are fewer privates because hospitals and corporations are buying them up.”
This reality has potentially shaped the way younger generations approach their workplace. Also, Gen Z tends to have less intention to stay with a current employer for the long term than did their parents. “Older physicians were trained to expect they’d run their own business and build it over the years,” said Mr. Futch. “The younger generations look at it as a job, something they may want to switch in a few years. It’s a combination of candidates wanting more options, and also the fact that there are more options to be employed.”
Along those lines, younger generations in general tend to place work-life balance as a higher priority than do older generations, and employed physicians place this equation high on the list as well. In the Employed Physicians Report 2023, 54% said that they are satisfied or better with their work-life balance, up from 51% in the 2022 report.
With that in mind, Dr. Kharazi noted that flexibility is one of the chief reasons why she likes private practice. “If my kid has an event I want to attend, I don’t have to adhere to a strict schedule,” she said.
Satisfaction as an employee vs. employed doctor sometimes changes based on the type of medicine you practice too. With specialties that tend to be primarily outpatient, such as dermatology and allergy, private practice may be the best option regardless. “Hospitals don’t seek out those specialists as much and the specialists can operate successfully without a hospital,” said Mr. Futch.
Hospitals try to incentivize doctors with perks like hefty sign-on bonuses, student loan forgiveness, plenty of vacation time, and more. They also put money into marketing their doctors, a time-consuming and expensive aspect that is tough to shoulder in private practice, especially in the early years. Mr. Futch adds that many doctors view employment as a more stable option. “As the government changes reimbursement policies, the income from private practice fluctuates,” he said. “So many doctors worry that if they buy into a private practice, it is a risky endeavor.”
Hospitals aren’t always a sure bet in that regard, either: They go through tough financial times, lay off staff, or make salary cuts. Historically, however, employment tends to be the safer route, which can make it an attractive option.
Ultimately, the pros and cons of each scenario are individual. It’s up to physicians to do their own math and balance sheet before making a decision.
A version of this article first appeared on Medscape.com.
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Still, employed physicians also had plenty to say about the downsides of their jobs.
Many pointed to “feeling like a cog in the machine,” and one doctor pointed to the hassle of dealing with bureaucracy. Others complained about the fact that nonphysicians ran the business and lacked an understanding of what physicians really need from their jobs. When asked whether administrative rules made sense, 63% of physician respondents said that yes, the rules make sense for the business; but, only 52% said that the rules make sense for the doctors themselves.
Other complaints included the requirement to reach high productivity targets and too low an income potential. In the 9 years since Medscape’s 2104 Employed Physicians Report, the share of employed doctors paid on a straight salary has declined from 46% to 31%. Those compensated on a base salary plus productivity targets and other performance metrics rose from 13% in 2014 to 32% now.
“Many doctors go into private practice because of the freedom it brings and the potential financial incentives,” added Dr. Boduch. “I know that many doctors have a dream of working for themselves, and in many cases, that works out great for them.”
Dr. Boduch noted that in her job as chief medical officer at PsychPlus, she still has flexibility plus the perks of working with a bigger practice. In this scenario, Dr. Boduch said, the company can negotiate with insurance companies, allowing her the financial rewards of private practice.
What’s right for you?
“I think it might be somewhat generational,” said Cody Futch, senior recruiting executive at AMN Healthcare. “It used to be that fewer hospitals offered employment, so private practice was the way to go. Now, there are fewer privates because hospitals and corporations are buying them up.”
This reality has potentially shaped the way younger generations approach their workplace. Also, Gen Z tends to have less intention to stay with a current employer for the long term than did their parents. “Older physicians were trained to expect they’d run their own business and build it over the years,” said Mr. Futch. “The younger generations look at it as a job, something they may want to switch in a few years. It’s a combination of candidates wanting more options, and also the fact that there are more options to be employed.”
Along those lines, younger generations in general tend to place work-life balance as a higher priority than do older generations, and employed physicians place this equation high on the list as well. In the Employed Physicians Report 2023, 54% said that they are satisfied or better with their work-life balance, up from 51% in the 2022 report.
With that in mind, Dr. Kharazi noted that flexibility is one of the chief reasons why she likes private practice. “If my kid has an event I want to attend, I don’t have to adhere to a strict schedule,” she said.
Satisfaction as an employee vs. employed doctor sometimes changes based on the type of medicine you practice too. With specialties that tend to be primarily outpatient, such as dermatology and allergy, private practice may be the best option regardless. “Hospitals don’t seek out those specialists as much and the specialists can operate successfully without a hospital,” said Mr. Futch.
Hospitals try to incentivize doctors with perks like hefty sign-on bonuses, student loan forgiveness, plenty of vacation time, and more. They also put money into marketing their doctors, a time-consuming and expensive aspect that is tough to shoulder in private practice, especially in the early years. Mr. Futch adds that many doctors view employment as a more stable option. “As the government changes reimbursement policies, the income from private practice fluctuates,” he said. “So many doctors worry that if they buy into a private practice, it is a risky endeavor.”
Hospitals aren’t always a sure bet in that regard, either: They go through tough financial times, lay off staff, or make salary cuts. Historically, however, employment tends to be the safer route, which can make it an attractive option.
Ultimately, the pros and cons of each scenario are individual. It’s up to physicians to do their own math and balance sheet before making a decision.
A version of this article first appeared on Medscape.com.
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Still, employed physicians also had plenty to say about the downsides of their jobs.
Many pointed to “feeling like a cog in the machine,” and one doctor pointed to the hassle of dealing with bureaucracy. Others complained about the fact that nonphysicians ran the business and lacked an understanding of what physicians really need from their jobs. When asked whether administrative rules made sense, 63% of physician respondents said that yes, the rules make sense for the business; but, only 52% said that the rules make sense for the doctors themselves.
Other complaints included the requirement to reach high productivity targets and too low an income potential. In the 9 years since Medscape’s 2104 Employed Physicians Report, the share of employed doctors paid on a straight salary has declined from 46% to 31%. Those compensated on a base salary plus productivity targets and other performance metrics rose from 13% in 2014 to 32% now.
“Many doctors go into private practice because of the freedom it brings and the potential financial incentives,” added Dr. Boduch. “I know that many doctors have a dream of working for themselves, and in many cases, that works out great for them.”
Dr. Boduch noted that in her job as chief medical officer at PsychPlus, she still has flexibility plus the perks of working with a bigger practice. In this scenario, Dr. Boduch said, the company can negotiate with insurance companies, allowing her the financial rewards of private practice.
What’s right for you?
“I think it might be somewhat generational,” said Cody Futch, senior recruiting executive at AMN Healthcare. “It used to be that fewer hospitals offered employment, so private practice was the way to go. Now, there are fewer privates because hospitals and corporations are buying them up.”
This reality has potentially shaped the way younger generations approach their workplace. Also, Gen Z tends to have less intention to stay with a current employer for the long term than did their parents. “Older physicians were trained to expect they’d run their own business and build it over the years,” said Mr. Futch. “The younger generations look at it as a job, something they may want to switch in a few years. It’s a combination of candidates wanting more options, and also the fact that there are more options to be employed.”
Along those lines, younger generations in general tend to place work-life balance as a higher priority than do older generations, and employed physicians place this equation high on the list as well. In the Employed Physicians Report 2023, 54% said that they are satisfied or better with their work-life balance, up from 51% in the 2022 report.
With that in mind, Dr. Kharazi noted that flexibility is one of the chief reasons why she likes private practice. “If my kid has an event I want to attend, I don’t have to adhere to a strict schedule,” she said.
Satisfaction as an employee vs. employed doctor sometimes changes based on the type of medicine you practice too. With specialties that tend to be primarily outpatient, such as dermatology and allergy, private practice may be the best option regardless. “Hospitals don’t seek out those specialists as much and the specialists can operate successfully without a hospital,” said Mr. Futch.
Hospitals try to incentivize doctors with perks like hefty sign-on bonuses, student loan forgiveness, plenty of vacation time, and more. They also put money into marketing their doctors, a time-consuming and expensive aspect that is tough to shoulder in private practice, especially in the early years. Mr. Futch adds that many doctors view employment as a more stable option. “As the government changes reimbursement policies, the income from private practice fluctuates,” he said. “So many doctors worry that if they buy into a private practice, it is a risky endeavor.”
Hospitals aren’t always a sure bet in that regard, either: They go through tough financial times, lay off staff, or make salary cuts. Historically, however, employment tends to be the safer route, which can make it an attractive option.
Ultimately, the pros and cons of each scenario are individual. It’s up to physicians to do their own math and balance sheet before making a decision.
A version of this article first appeared on Medscape.com.
CMS ‘million hearts’ CVD risk reduction model works
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Durable LVAD for advanced HF still underutilized
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JACC
Novel PET tracer for perfusion imaging: What’s the potential?
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emerging advantages of PET myocardial perfusion imaging (MPI) for coronary artery disease (CAD) diagnosis and assessment of cardiovascular event risk has prompted growing use of this technology as an alternative to the more commonly used single photon–emission CT (SPECT) MPI.
The advantages of PET MPI include better diagnostic performance and shorter acquisition times. , including consistent, high-quality images and low radiation exposure. It also allows quantification of myocardial blood flow, and it has “strong prognostic power.”
Tracer availability
Despite these advantages, that position paper and subsequent studies note that PET MPI has been underutilized in the United States, largely owing to issues with the available tracers, which have characteristics that limit widespread use in the clinic.
Rubidium, arguably the most commonly used tracer for PET MPI, is not available in unit dosing and so can be expensive for low-volume centers, plus it also requires an on-site generator, Michael Salerno, MD, PhD, a member of the American College of Cardiology’s Imaging Council and section chief of cardiovascular imaging, Stanford (Calif.) University, told this news organization.
N-ammonia, the other U.S. Food and Drug Administration–approved tracer, is available in unit dosing, but its short half-life means that centers need an onsite cyclotron, Dr. Salerno said.
For cardiac perfusion imaging and myocardial blood flow (MBF) quantification, 15O-water is considered the gold standard, although it’s not approved by the FDA. This tracer also requires an on-site cyclotron and “is challenging to use,” Dr. Salerno said. Use has been largely restricted to research purposes, though efforts are underway to widen its availability.
Enter flurpiridaz F-18 (GE Healthcare), a novel PET MPI tracer labeled with fluorine-18. Its longer half-life – similar to that of fluorodeoxyglucose, a tracer used to detect various cancers – could broaden the number of sites that could perform perfusion PET studies, Dr. Salerno said.
“Flurpiridaz also is supposed to have a more linear relationship between flow and tracer uptake, which could improve the ability to perform quantification of perfusion,” he noted. “It also offers the ability to do exercise PET, which is impossible for rubidium and challenging for ammonia, given its 11-minute half-life.”
Flurpiridaz status
The FDA requires two phase-3 studies that show safety and sufficient diagnostic performance before it will approve a new tracer. The first required study, published in the Journal of the American College of Cardiology, showed that the tracer’s sensitivity for detection of greater than or equal to 50% stenosis by ICA was significantly higher than SPECT; however, the specificity did not meet the prespecified noninferiority criterion.
The second FDA-required study, published online recently, also in the Journal of the American College of Cardiology, was designed differently from the first in that only patients with suspected – not known – CAD were enrolled. The primary efficacy endpoint was sensitivity and specificity of flurpiridaz PET for overall detection of CAD, rather than comparing it to SPECT MPI (which became a secondary endpoint). PET and SPECT studies were both performed before invasive coronary angiography to minimize referral bias; SPECT studies included cadmium zinc telluride cameras.
In that study, which included 578 patients (mean age, 64; 32.5% women) from 48 centers in the United States, Canada, and Europe, flurpiridaz met the efficacy endpoints: Its sensitivity and specificity were significantly higher than the prespecified threshold value by two of the three readers; its sensitivity was higher than SPECT (80.3% vs. 68.7%); and its specificity was noninferior (63.8% vs. 61.7%).
PET areas under the receiver-operating characteristic curves were higher than SPECT in the overall population and in women and obese patients, at half the radiation dose of SPECT.
“Cardiac PET MPI is positioned to serve as the leading modality for the functional evaluation of suspected and known CAD,” Jamieson M. Bourque, MD, MHS, medical director of nuclear cardiology, echocardiography, and the Stress Laboratory, University of Virginia, Charlottesville, wrote in an editorial accompanying the second study . “18F-flurpiridaz will facilitate this upward progression with beneficial tracer characteristics that will increase access and availability, enable exercise stress, and optimize MBF quantification.”
At this point, FDA approval of flurpiridaz is expected sometime in 2024, said James E. Udelson, MD, principal investigator of the recent study, chief of the division of cardiology, and director of the Nuclear Cardiology Laboratory at Tufts University School of Medicine, Boston.
Learning curve
Flurpiridaz comes with “a really interesting and important” learning curve, Dr. Udelson said. “The images are really crisp, and they look very different from what most people are used to. The GE folks are going to have to make sure that the American Society of Nuclear Cardiology and other professional societies are tuned in to help in the education part, because it’s not an easy, automatic switch. Very good image readers can adapt, but it’s not just one day you do one, then switch to the other.”
A “somewhat apt” analogy would be the difference between an echocardiogram and an MRI, he explained. “The MRI is much crisper. You’re seeing edges more crisply. You’re seeing the difference between a thicker and a thinner segment of the wall more crisply, and that’s actually real. You can’t say the thinner segment is abnormal; it’s just that you’re seeing it better. So, with this tracer, normal differences in the thickness of a wall can almost look like a defect if you’re not used to knowing that’s the new normal.”
The expected approval of flurpiridaz “will be a win for cardiac PET, broadening the range of sites that could perform PET,” Dr. Salerno commented. “However, it is worth cautioning that all of the prior data with PET using different agents does not necessarily equate to the same performance with the new agent, given that the performance seems to be lower than that shown in prior PET studies using other agents.”
Dr. Salerno would like to see additional studies comparing flurpiridaz with rubidium or ammonia, as well as studies performing quantification with flurpiridaz, “which theoretically should have some advantages,” he said.
Dr. Udelson noted that MedTrace, a company in Denmark, is working on a radiolabeled water tracer based on 15-O-water that is just starting a pivotal trial. Dr. Udelson is a consultant to the company and is a steering committee member for the pivotal trial.
For now, “the big take-home is that there are a lot of ways these days to test people for CAD,” he said. “As the types of things we can do to test people expand, individuals and centers need to make sure they focus on providing any new service, however they do it, with really superb quality and experience.”
“You don’t just do something new because it’s new,” he added. “It has to be done really well. If you do the new thing badly, you’re not going to get better information.”
Dr. Udelson is a consultant and advisory board member for GE Healthcare, a consultant to MedTrace, and a steering committee member for MedTrace’s pivotal trial. Dr. Bourque has served on a GE Healthcare advisory board for amyloid imaging. Dr. Salerno reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Stair climbing tied to reduced risk for heart disease
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antidepressants ‘don’t blunt’ semaglutide and weight loss
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
in a post hoc analysis of the Semaglutide Treatment Effect in People with Obesity (STEP) program.
Adverse events, including psychiatric events, were slightly more usual in the patients on antidepressants, Robert Kushner, MD, noted, in an oral session at the annual meeting of the Obesity Society.
“It is very common that patients who present for weight management are taking antidepressants for various reasons, including depression, anxiety, insomnia, or chronic pain,”Dr. Kushner, from Northwestern University in Chicago, said in an email. “We wanted to see if these participants responded differently to semaglutide, compared to those not on antidepressants.”
“We found that antidepressants do not blunt the effect of semaglutide for weight loss,” he said. “However, there is a slight increase in reported adverse effects.”
“Semaglutide 2.4 mg provides an effective treatment option for weight management, regardless of antidepressant use at baseline,” Dr. Kushner summarized. “Clinicians should be assured that we can use semaglutide in this population of patients.”
Jack Yanovski, MD, PhD, said this was a “great presentation,” noting that “it’s really important that we understand what goes on in patients with depression.”
“Of course, all these trials still had rules that prevent the folks with the most severe depressive symptoms or past suicidality to participate,” added Dr. Yanovski, chief of the Growth and Obesity Section, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md. “We need specific trials to know exactly how well we do.”
Dr. Kushner agreed, but also noted that, ever since some earlier antidepressants were associated with risk for suicidal ideation and death, strict guidelines were put in place that exclude certain patients from participating in clinical trials.
Dr. Yanovski suggested that now that the drugs are approved, it would be possible to study this, and the information would be important for clinicians.
Dr. Kushner said he hopes that such studies are forthcoming. In the meantime, “data like this will add some support and understanding,” he suggested.
36,000 Patients with obesity, 500 on antidepressants
Many people living with obesity report taking antidepressants for depression, anxiety, chronic pain, obsessive-compulsive disorder, sleep disturbance, neuropathy, panic disorder, or posttraumatic stress disorder, Dr. Kushner noted.
However, some of these medications can cause weight gain, and little is known about treatment outcomes for people with obesity who are on antidepressants, since most weight-loss studies exclude people with active major depressive disorder.
The researchers analyzed data from 1,961 patients in STEP 1 and 807 patients in STEP 2 as well as 611 patients in STEP 3 and 304 patients in STEP 5 – 3,683 participants in total, of which 539 were on antidepressants at baseline.
The patients were randomly assigned to 2.4 mg semaglutide vs. placebo plus a lifestyle intervention (STEP 1, 2, and 5) or intensive behavioral therapy (STEP 3 only), for 68 weeks, except STEP 5, which was 104 weeks.
Patients were included if they were aged 18 or older with a body mass index ≥30 kg/m2, or ≥27 kg/m2 with more than one weight-related complication (STEP 1, 3, and 5) or BMI ≥27 kg/m2 with type 2 diabetes (STEP 2 only), and at least one self-reported unsuccessful effort to lose weight by diet.
They were excluded if they had active major depressive disorder within 2 years prior to screening (or other severe psychiatric disorders such as schizophrenia or bipolar disorder) or a Patient Health Questionnaire-9 score of 15 or higher (indicating moderately severe or severe depression), or suicide ideation (type 4 or 5 on the Columbia Suicide Severity Rating Scale) or suicide behavior, within 30 days of screening.
From baseline to week 68, patients on semaglutide (with/without baseline antidepressant use) had a significantly greater change in weight vs. patients on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: –15.7% / –14.7% vs. –0.2% / –2.8%
- STEP 2: –10.7% / –9.5% vs. –3.3% / –3.4%
- STEP 3: –16.2% / –15.9% vs. –5.0% / –5.9%
- STEP 5: –19.0% / –14.1% vs. +1.6% / – 4.0%.
The proportion of reported adverse events was generally slightly greater in patients receiving semaglutide (with/without baseline antidepressant use) than those on placebo (with/without baseline antidepressant use), respectively:
- STEP 1: 97.7% vs 88.6% and 92.9% vs. 86%
- STEP 2: 97.6% vs 86.5% and 88.6% vs. 77.2%
- STEP 3: 97.6% vs 95.3% and 100% vs. 95.8%
- STEP 5: 100% vs 94.8% and 95.5% vs. 89.2%.
Gastrointestinal adverse events were more frequently reported in the semaglutide group and in patients on antidepressants at baseline. The proportion of patients with psychiatric adverse events was greater in participants on antidepressants at baseline. There were no differences in suicidal ideation/behavior in patients with/without antidepressant use at baseline.
The STEP trials were funded by Novo Nordisk. Dr. Kushner discloses that he served as a consultant for Novo Nordisk, WeightWatchers, Eli Lilly, and Pfizer, and received a research grant from Epitomee.
A version of this article appeared on Medscape.com.
FROM OBESITYWEEK® 2023