User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Real medical news: Many teens trust fake medical news
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
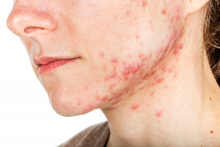
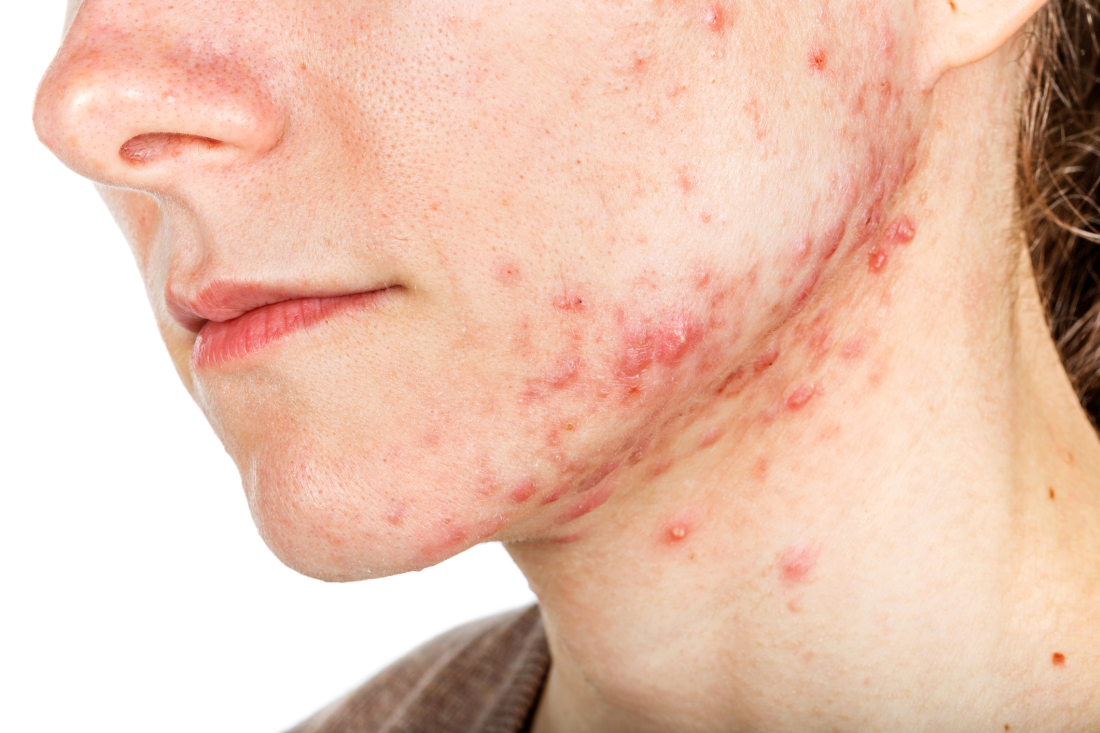
Hormonal therapy a safe, long term option for older women with recalcitrant acne
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
AT PDA 2022
Inhaled, systemic steroids linked to changes in brain structure
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
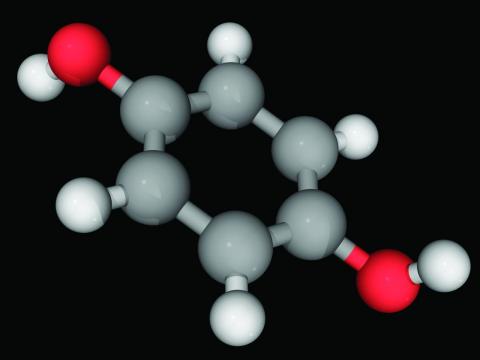
Hydroquinone, found in skin-lightening agents worldwide, linked with increased skin cancer risk
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Consider the ‘long game’ in tumor management following Mohs surgery
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
AT PDA 2022
How do you live with COVID? One doctor’s personal experience
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
VTE risk not elevated in AD patients on JAK inhibitors: Study
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
FROM JAMA DERMATOLOGY
Autoimmune disease patients’ waxing, waning response to COVID vaccination studied in-depth
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Infographic: Is physician behavior on social media really so bad?
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
A version of this article first appeared on Medscape.com.