User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
COVID surge in Western Europe puts U.S. health experts on alert
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
Mercury and other risks of cosmetic skin lighteners
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Skin hyperpigmentation – whether it is caused by postinflammatory hyperpigmentation from acne or trauma to the skin, melasma, autoimmune disorders, or disorders of pigmentation – is a condition where treatment is commonly sought after in dermatology offices. Topical products used to fade hyperpigmented areas of the skin have long been used around the world, and because of safety concerns, regulations aimed at reducing potential harm or adverse effects caused by certain ingredients in these products are increasing in different countries.
For example, while extremely effective at treating most forms of hyperpigmentation, hydroquinone has been definitively linked to ochronosis, kojic acid has been linked to contact dermatitis in humans, and acid peels and retinoids are associated with irritant dermatitis, disruption of the skin barrier, and photosensitivity. In animal studies, licorice root extract has been linked to endocrine and other organ system irregularities.
Kojic acid was banned in Japan in 2003, and subsequently in South Korea and Switzerland because of concerns over animal studies indicating that its fungal metabolite might be carcinogenic (. Hydroquinone is classified as a drug and has been banned for use in cosmetic products in Japan, the European Union, Australia, and several African nations since at least 2006 because of concerns over adrenal gland dysregulation and high levels of mercury in hydroquinone products in those countries. In Africa specifically, South Africa banned all but 2% hydroquinone in 1983, the Ivory Coast banned all skin whitening creams in 2015, and in 2016, Ghana initiated a ban on certain skin products containing hydroquinone.
The United States followed suit in February 2020 with the Food and Drug Administration introducing a ban on all OTC hydroquinone-containing products because of concerns over carcinogenicity in animal studies (which has not been shown in human studies to date). The “Coronavirus Aid, Relief, and Economic Security” (CARES) Act signed in March 2020 then made the changes effective by halting the sale of OTC hydroquinone products in the United States as of September 2020.
Mercury concerns
Despite these bans, hydroquinone continues to be sold in cosmetics and OTC products around the world and online. And despite being banned or limited in these products, in particular. Mercury has been used in cosmetic products as a skin lightening agent (on its own) and as a preservative.
Mercury has been shown to be carcinogenic, neurotoxic, as well as cytotoxic to the renal and endocrine systems, causes reproductive toxicity, and may be bioaccumulative in wildlife and humans. There is particular concern regarding the risks of exposure in pregnant women and babies because of potential harm to the developing brain and nervous system. Initial signs and symptoms of mercury poisoning include irritability, shyness, tremors, changes in vision or hearing, memory problems, depression, numbness and tingling in the hands, feet, or around the mouth.
Organizations such as the Zero Mercury Working Group (ZMWG) – an international coalition of public interest environmental and health nongovernmental organizations from more than 55 countries, focused on eliminating the use, release, and exposure to mercury – have been working to help ensure safety and mercury levels are below the threshold deemed allowable in hydroquinone-containing products.
On March 10, the ZMWG published the results of a new study demonstrating that skin lighteners containing mercury are still being sold online, despite bans and safety concerns. Ebay, Amazon, Shopee, Jiji, and Flipkart are among the websites still selling high mercury–containing skin lightener products. Some of them were the same offenders selling the banned products in 2019. Of the 271 online products tested from 17 countries, nearly half contained over 1 ppm of mercury, which is the legal limit that has been established by most governments and the Minamata Convention on Mercury. Based on their packaging, the majority of these products were manufactured in Asia, most often in Pakistan (43%), Thailand (8%), China (6%), and Taiwan (4%), according to the report.
In ZMWG’s prior publications, mercury concentrations reported in some of these products ranged from 93 ppm to over 16,000 ppm. Even higher concentrations have been reported by other entities. And according to a World Health Organization November 2019 report, mercury-containing skin lightening products have been manufactured in many countries and areas, including Bangladesh, China, Dominican Republic Hong Kong SAR (China), Jamaica, Lebanon, Malaysia, Mexico, Pakistan, Philippines, Republic of Korea, Thailand, and the United States. According to the ZMWG, 137 countries have committed to the Minamata Convention to phase out and limit mercury, including in cosmetics.
Despite bans on some of these products, consumers in the United States and other countries with bans and restrictions are still at risk of exposure to mercury-containing skin lighteners because of online sales. Hopefully, the work of the ZMWG and similar entities will continue to help limit potentially harmful exposures to mercury, while maintaining access to safe and effective methods to treat hyperpigmentation.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
New ACC guidance on cardiovascular consequences of COVID-19
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
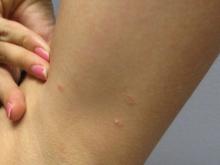
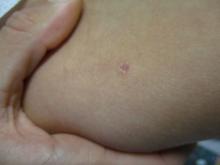
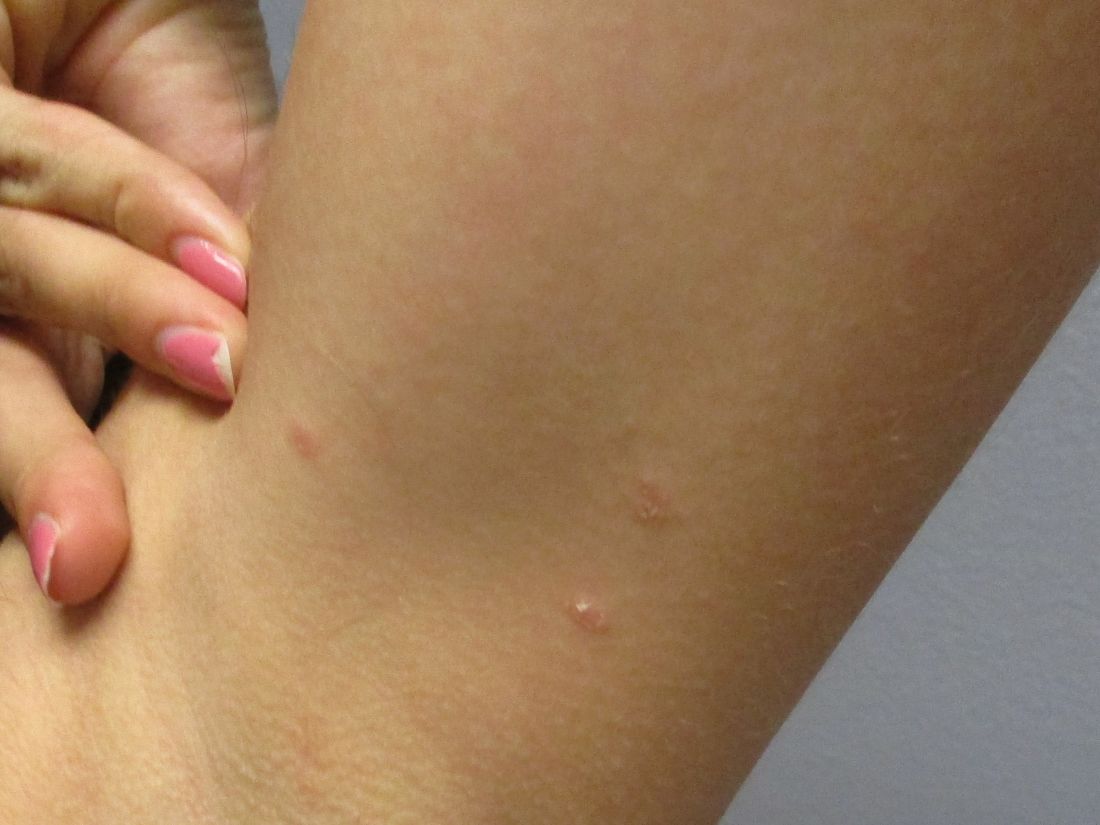
A 31-year-old female presented with a burning rash on upper arms, groin, and axillae
The exact cause is unknown, but possible causes include medications, dental amalgam fillings, or an autoimmune reaction. Drugs implicated in causing LP include beta-blockers, methyldopa, penicillamine, quinidine, and quinine. A meta-analysis of case-control studies show a statistically significant association between hepatitis C infection and LP patients; thus, all patients presenting with LP should be screened for hepatitis.1 Individuals of all age groups and races can be affected by LP, but it is predominantly observed in middle-aged adults. Women are also twice as likely to get oral lichen planus.2
Atrophic lichen planus, the least common form of LP, presents as flat, violaceous papules with an atrophic, pale center. Although these papules can be found anywhere on the body, they most commonly affect the trunk and/or legs on areas of the skin previously affected by classical lichen planus.3 In most cases, LP is diagnosed by observing its clinical features. A biopsy is recommended to confirm the diagnosis for more atypical cases.
Histopathology reveals thinning of the epidermis with flattening of the rete ridges, vacuolar degeneration of the basal layer, and a lichenoid mononuclear infiltrate in the papillary dermis.
If the patient is diagnosed with LP but experiences no symptoms, treatment is not needed as LP may resolve spontaneously within 1-2 years. Recurrences are common, however. Lesions may heal with hyperpigmentation. Possible treatments that can help relieve symptoms of pruritus are high potency topical corticosteroids, calcineurin inhibitors, and antihistamines. In more severe and widespread cases, lesions may respond well to systemic corticosteroids or intralesional steroid injections.4 Phototherapy is reported to be effective as well. Acitretin, isotretinoin, methotrexate, hydroxychloroquine, and mycophenolate mofetil are all described in the literature. It is important to note that LP on mucous membranes may be more persistent and resistant to treatment.1
In this patient, a punch biopsy was performed, confirming the diagnosis. The patient was treated with topical and intralesional steroids, as well as a course of prednisone, and her lesions improved with treatment. Hepatitis serologies were negative.
This case and photo were submitted by Ms. Erras of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology, and edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Usatine R, Tinitigan M. Am Fam Physician. 2011 Jul 1;84(1):53-602.
2. Lichen planus, Johns Hopkins Medicine. [Cited 2022 Mar 13.]
3. Atrophic lichen planus, Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [Cited 2022 Mar 13.]
4. ”Atrophic lichen planus,” Medscape, 2004 Feb 1. [Cited 2022 Mar 13.]
The exact cause is unknown, but possible causes include medications, dental amalgam fillings, or an autoimmune reaction. Drugs implicated in causing LP include beta-blockers, methyldopa, penicillamine, quinidine, and quinine. A meta-analysis of case-control studies show a statistically significant association between hepatitis C infection and LP patients; thus, all patients presenting with LP should be screened for hepatitis.1 Individuals of all age groups and races can be affected by LP, but it is predominantly observed in middle-aged adults. Women are also twice as likely to get oral lichen planus.2
Atrophic lichen planus, the least common form of LP, presents as flat, violaceous papules with an atrophic, pale center. Although these papules can be found anywhere on the body, they most commonly affect the trunk and/or legs on areas of the skin previously affected by classical lichen planus.3 In most cases, LP is diagnosed by observing its clinical features. A biopsy is recommended to confirm the diagnosis for more atypical cases.
Histopathology reveals thinning of the epidermis with flattening of the rete ridges, vacuolar degeneration of the basal layer, and a lichenoid mononuclear infiltrate in the papillary dermis.
If the patient is diagnosed with LP but experiences no symptoms, treatment is not needed as LP may resolve spontaneously within 1-2 years. Recurrences are common, however. Lesions may heal with hyperpigmentation. Possible treatments that can help relieve symptoms of pruritus are high potency topical corticosteroids, calcineurin inhibitors, and antihistamines. In more severe and widespread cases, lesions may respond well to systemic corticosteroids or intralesional steroid injections.4 Phototherapy is reported to be effective as well. Acitretin, isotretinoin, methotrexate, hydroxychloroquine, and mycophenolate mofetil are all described in the literature. It is important to note that LP on mucous membranes may be more persistent and resistant to treatment.1
In this patient, a punch biopsy was performed, confirming the diagnosis. The patient was treated with topical and intralesional steroids, as well as a course of prednisone, and her lesions improved with treatment. Hepatitis serologies were negative.
This case and photo were submitted by Ms. Erras of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology, and edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Usatine R, Tinitigan M. Am Fam Physician. 2011 Jul 1;84(1):53-602.
2. Lichen planus, Johns Hopkins Medicine. [Cited 2022 Mar 13.]
3. Atrophic lichen planus, Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [Cited 2022 Mar 13.]
4. ”Atrophic lichen planus,” Medscape, 2004 Feb 1. [Cited 2022 Mar 13.]
The exact cause is unknown, but possible causes include medications, dental amalgam fillings, or an autoimmune reaction. Drugs implicated in causing LP include beta-blockers, methyldopa, penicillamine, quinidine, and quinine. A meta-analysis of case-control studies show a statistically significant association between hepatitis C infection and LP patients; thus, all patients presenting with LP should be screened for hepatitis.1 Individuals of all age groups and races can be affected by LP, but it is predominantly observed in middle-aged adults. Women are also twice as likely to get oral lichen planus.2
Atrophic lichen planus, the least common form of LP, presents as flat, violaceous papules with an atrophic, pale center. Although these papules can be found anywhere on the body, they most commonly affect the trunk and/or legs on areas of the skin previously affected by classical lichen planus.3 In most cases, LP is diagnosed by observing its clinical features. A biopsy is recommended to confirm the diagnosis for more atypical cases.
Histopathology reveals thinning of the epidermis with flattening of the rete ridges, vacuolar degeneration of the basal layer, and a lichenoid mononuclear infiltrate in the papillary dermis.
If the patient is diagnosed with LP but experiences no symptoms, treatment is not needed as LP may resolve spontaneously within 1-2 years. Recurrences are common, however. Lesions may heal with hyperpigmentation. Possible treatments that can help relieve symptoms of pruritus are high potency topical corticosteroids, calcineurin inhibitors, and antihistamines. In more severe and widespread cases, lesions may respond well to systemic corticosteroids or intralesional steroid injections.4 Phototherapy is reported to be effective as well. Acitretin, isotretinoin, methotrexate, hydroxychloroquine, and mycophenolate mofetil are all described in the literature. It is important to note that LP on mucous membranes may be more persistent and resistant to treatment.1
In this patient, a punch biopsy was performed, confirming the diagnosis. The patient was treated with topical and intralesional steroids, as well as a course of prednisone, and her lesions improved with treatment. Hepatitis serologies were negative.
This case and photo were submitted by Ms. Erras of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology, and edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Usatine R, Tinitigan M. Am Fam Physician. 2011 Jul 1;84(1):53-602.
2. Lichen planus, Johns Hopkins Medicine. [Cited 2022 Mar 13.]
3. Atrophic lichen planus, Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [Cited 2022 Mar 13.]
4. ”Atrophic lichen planus,” Medscape, 2004 Feb 1. [Cited 2022 Mar 13.]
Betamethasone cream did not alleviate symptoms.
Physician loses right leg, sues podiatrist; more
, as a story in the Pennsylvania Record, among other news sites, reports.
In December 2020, Mario Adajar, MD, 59, an internist in Wyoming, Penn., sought treatment for his foot calluses and the chronic ulceration of his right foot.
Dr. Adajar consulted a podiatrist, who has surgical privileges at Wilkes-Barre Commonwealth Hospital. According to his complaint, Dr. Adajar made the podiatrist aware that he had type 2 diabetes and had recently undergone a kidney transplant.
Over the next several months, Adajar continued to be treated by the podiatrist who, among other things, debrided and cleaned his patient’s ulcerated right foot on multiple occasions. In June 2021, working out of the hospital’s Wound Healing Center, the podiatrist placed Dr. Adajar’s right leg in a total contact cast.
By the following day, the patient experienced what he later described as “excruciating” pain around the cast. He was also running a fever of 102.3. Taken to a local emergency department, Dr. Adajar soon went into septic shock, accompanied by both atrial fibrillation and acute hypoxic respiratory failure.
Doctors soon had a diagnosis: a gram-negative bacilli infection. Meanwhile, his right leg had become severely gangrenous, of the gas gangrene type. Nevertheless, after treatment, Dr. Adajar was discharged on June 15, 2021, and advised to continue with his follow-up, which included a referral to physical therapy. However, on July 27, 2021, doctors at Wilkes-Barre Commonwealth were forced to amputate Dr. Adajar’s right leg through the fibula and tibia.
In his suit, Dr. Adajar claims that the decision by the podiatrist and his associates to place him in a total contact cast was the direct and immediate cause of his injuries, most catastrophically the amputation of his right leg. He and his legal team are seeking damages “in excess of $50,000,” the standard language in Pennsylvania for cases likely to involve much larger awards.
Dr. Adajar, despite the loss of his right leg, continues to practice internal medicine.
Doctor wins forceps-delivery suit
Last month, a Virginia jury decided in favor of a physician accused of damaging a baby’s eye during delivery, a story in The Winchester Star reports.
In December 2015, Melissa Clements went to Winchester Medical Center, part of Valley Health, to have her baby delivered. Her doctor was ob.gyn. George F. Craft II, at the time a member of Winchester Women’s Specialists. At one point during the roughly 30-minute delivery, Dr. Craft used forceps to remove Ms. Clements’s baby, who in the process sustained facial fractures and left-eye damage.
At trial, Craft argued that a forceps delivery was justified because the baby was stuck and his patient had refused a C-section.
The attorney for the plaintiffs — which included Ms. Clements’s husband — claimed that the use of forceps was premature, as professional guidelines require that a woman in labor be allowed at least 3 hours to push on her own before forceps are employed. (The suit, initially filed in 2019, also accused Dr. Craft of failing to properly inform his patient about the risks of, and alternatives to, this form of delivery. That part of the complaint was dropped, however, prior to the recent trial.)
The jury debated just 50 minutes before deciding Dr. Craft wasn’t medically negligent in the birth of William, Ms. Clements’s now 6-year-old son, who will be forced to wear contact lenses or glasses for life, or undergo corrective surgery.
As Dr. Craft’s attorney explained at trial: “He [Dr. Craft] hoped to give her [Ms. Clements] what she wanted: a vaginal delivery. But forceps techniques can and will cause injuries, even when properly placed.”
Unsupervised PAs subject to med-mal cap, state says
The California Supreme Court ruled late last month that even unsupervised physician assistants (PAs) are protected under the state’s $250,000 cap on noneconomic damages, according to a posting on the website of the Claims Journal, among other news sites.
The ruling stems from a 2013 suit filed by Marisol Lopez, who claimed that a dermatologist, a plastic surgeon, and two PAs had misdiagnosed her child’s skin cancer. Ms. Lopez’s child, Olivia Sarinana, died in February 2014, causing her mother to amend her original claim to a wrongful-death suit.
A trial court found both the doctors and the PAs liable for negligence, awarding the plaintiff $11,200 in economic damages and $4.25 million in noneconomic damages. The court subsequently reduced that amount, however, referencing the state’s $250,000 limit on noneconomic damages, which is part of the Medical Injury Compensation Reform Act of 1975, known as MICRA.
Ms. Lopez appealed the decision, arguing that the cap shouldn’t apply to the two PAs, because neither was under a physician’s direct supervision and therefore not acting within the proper scope of practice, as defined by state law. Despite agreeing with the factual basis of Ms. Lopez’s claim — that neither PA was being supervised during the period in question — the trial court refused to wave the state cap. Ms. Lopez again appealed, and, in a split decision, the Second District Court of Appeal upheld the trial court’s decision.
At this point, attorneys for Ms. Lopez applied for, and obtained, a review before the state’s highest court. Last month, the justices weighed in, ruling that the PAs were still entitled to protection under MICRA because they “had valid delegation-of-service agreements in place.” In other words, while the two PAs had not been directly supervised by a physician, their services had been properly delegated by one.
Said Associate Justice Goodwin Liu, who wrote the opinion: “To be sure, there are reasonable policy arguments for excluding physician assistants who perform medical services without actual supervision from a cap on non-economic damages, and the Legislature is well equipped to weigh and reweigh the competing policy considerations. But our role is confined to interpreting the statute before us in the manner that comports most closely with the Legislature’s purpose in enacting MICRA.”
Despite the high-court ruling, voters may soon get a chance to amend the nearly 5-decades-old MICRA legislation. A November ballot initiative would not only adjust the cap for inflation, raising it to more than $1.2 million, but would also permit “judges and juries to waive the cap entirely for cases involving death and permanent disability.”
Medical groups have said that if either or both of these changes happen the cost of healthcare in the Golden State will surely go up.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
, as a story in the Pennsylvania Record, among other news sites, reports.
In December 2020, Mario Adajar, MD, 59, an internist in Wyoming, Penn., sought treatment for his foot calluses and the chronic ulceration of his right foot.
Dr. Adajar consulted a podiatrist, who has surgical privileges at Wilkes-Barre Commonwealth Hospital. According to his complaint, Dr. Adajar made the podiatrist aware that he had type 2 diabetes and had recently undergone a kidney transplant.
Over the next several months, Adajar continued to be treated by the podiatrist who, among other things, debrided and cleaned his patient’s ulcerated right foot on multiple occasions. In June 2021, working out of the hospital’s Wound Healing Center, the podiatrist placed Dr. Adajar’s right leg in a total contact cast.
By the following day, the patient experienced what he later described as “excruciating” pain around the cast. He was also running a fever of 102.3. Taken to a local emergency department, Dr. Adajar soon went into septic shock, accompanied by both atrial fibrillation and acute hypoxic respiratory failure.
Doctors soon had a diagnosis: a gram-negative bacilli infection. Meanwhile, his right leg had become severely gangrenous, of the gas gangrene type. Nevertheless, after treatment, Dr. Adajar was discharged on June 15, 2021, and advised to continue with his follow-up, which included a referral to physical therapy. However, on July 27, 2021, doctors at Wilkes-Barre Commonwealth were forced to amputate Dr. Adajar’s right leg through the fibula and tibia.
In his suit, Dr. Adajar claims that the decision by the podiatrist and his associates to place him in a total contact cast was the direct and immediate cause of his injuries, most catastrophically the amputation of his right leg. He and his legal team are seeking damages “in excess of $50,000,” the standard language in Pennsylvania for cases likely to involve much larger awards.
Dr. Adajar, despite the loss of his right leg, continues to practice internal medicine.
Doctor wins forceps-delivery suit
Last month, a Virginia jury decided in favor of a physician accused of damaging a baby’s eye during delivery, a story in The Winchester Star reports.
In December 2015, Melissa Clements went to Winchester Medical Center, part of Valley Health, to have her baby delivered. Her doctor was ob.gyn. George F. Craft II, at the time a member of Winchester Women’s Specialists. At one point during the roughly 30-minute delivery, Dr. Craft used forceps to remove Ms. Clements’s baby, who in the process sustained facial fractures and left-eye damage.
At trial, Craft argued that a forceps delivery was justified because the baby was stuck and his patient had refused a C-section.
The attorney for the plaintiffs — which included Ms. Clements’s husband — claimed that the use of forceps was premature, as professional guidelines require that a woman in labor be allowed at least 3 hours to push on her own before forceps are employed. (The suit, initially filed in 2019, also accused Dr. Craft of failing to properly inform his patient about the risks of, and alternatives to, this form of delivery. That part of the complaint was dropped, however, prior to the recent trial.)
The jury debated just 50 minutes before deciding Dr. Craft wasn’t medically negligent in the birth of William, Ms. Clements’s now 6-year-old son, who will be forced to wear contact lenses or glasses for life, or undergo corrective surgery.
As Dr. Craft’s attorney explained at trial: “He [Dr. Craft] hoped to give her [Ms. Clements] what she wanted: a vaginal delivery. But forceps techniques can and will cause injuries, even when properly placed.”
Unsupervised PAs subject to med-mal cap, state says
The California Supreme Court ruled late last month that even unsupervised physician assistants (PAs) are protected under the state’s $250,000 cap on noneconomic damages, according to a posting on the website of the Claims Journal, among other news sites.
The ruling stems from a 2013 suit filed by Marisol Lopez, who claimed that a dermatologist, a plastic surgeon, and two PAs had misdiagnosed her child’s skin cancer. Ms. Lopez’s child, Olivia Sarinana, died in February 2014, causing her mother to amend her original claim to a wrongful-death suit.
A trial court found both the doctors and the PAs liable for negligence, awarding the plaintiff $11,200 in economic damages and $4.25 million in noneconomic damages. The court subsequently reduced that amount, however, referencing the state’s $250,000 limit on noneconomic damages, which is part of the Medical Injury Compensation Reform Act of 1975, known as MICRA.
Ms. Lopez appealed the decision, arguing that the cap shouldn’t apply to the two PAs, because neither was under a physician’s direct supervision and therefore not acting within the proper scope of practice, as defined by state law. Despite agreeing with the factual basis of Ms. Lopez’s claim — that neither PA was being supervised during the period in question — the trial court refused to wave the state cap. Ms. Lopez again appealed, and, in a split decision, the Second District Court of Appeal upheld the trial court’s decision.
At this point, attorneys for Ms. Lopez applied for, and obtained, a review before the state’s highest court. Last month, the justices weighed in, ruling that the PAs were still entitled to protection under MICRA because they “had valid delegation-of-service agreements in place.” In other words, while the two PAs had not been directly supervised by a physician, their services had been properly delegated by one.
Said Associate Justice Goodwin Liu, who wrote the opinion: “To be sure, there are reasonable policy arguments for excluding physician assistants who perform medical services without actual supervision from a cap on non-economic damages, and the Legislature is well equipped to weigh and reweigh the competing policy considerations. But our role is confined to interpreting the statute before us in the manner that comports most closely with the Legislature’s purpose in enacting MICRA.”
Despite the high-court ruling, voters may soon get a chance to amend the nearly 5-decades-old MICRA legislation. A November ballot initiative would not only adjust the cap for inflation, raising it to more than $1.2 million, but would also permit “judges and juries to waive the cap entirely for cases involving death and permanent disability.”
Medical groups have said that if either or both of these changes happen the cost of healthcare in the Golden State will surely go up.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
, as a story in the Pennsylvania Record, among other news sites, reports.
In December 2020, Mario Adajar, MD, 59, an internist in Wyoming, Penn., sought treatment for his foot calluses and the chronic ulceration of his right foot.
Dr. Adajar consulted a podiatrist, who has surgical privileges at Wilkes-Barre Commonwealth Hospital. According to his complaint, Dr. Adajar made the podiatrist aware that he had type 2 diabetes and had recently undergone a kidney transplant.
Over the next several months, Adajar continued to be treated by the podiatrist who, among other things, debrided and cleaned his patient’s ulcerated right foot on multiple occasions. In June 2021, working out of the hospital’s Wound Healing Center, the podiatrist placed Dr. Adajar’s right leg in a total contact cast.
By the following day, the patient experienced what he later described as “excruciating” pain around the cast. He was also running a fever of 102.3. Taken to a local emergency department, Dr. Adajar soon went into septic shock, accompanied by both atrial fibrillation and acute hypoxic respiratory failure.
Doctors soon had a diagnosis: a gram-negative bacilli infection. Meanwhile, his right leg had become severely gangrenous, of the gas gangrene type. Nevertheless, after treatment, Dr. Adajar was discharged on June 15, 2021, and advised to continue with his follow-up, which included a referral to physical therapy. However, on July 27, 2021, doctors at Wilkes-Barre Commonwealth were forced to amputate Dr. Adajar’s right leg through the fibula and tibia.
In his suit, Dr. Adajar claims that the decision by the podiatrist and his associates to place him in a total contact cast was the direct and immediate cause of his injuries, most catastrophically the amputation of his right leg. He and his legal team are seeking damages “in excess of $50,000,” the standard language in Pennsylvania for cases likely to involve much larger awards.
Dr. Adajar, despite the loss of his right leg, continues to practice internal medicine.
Doctor wins forceps-delivery suit
Last month, a Virginia jury decided in favor of a physician accused of damaging a baby’s eye during delivery, a story in The Winchester Star reports.
In December 2015, Melissa Clements went to Winchester Medical Center, part of Valley Health, to have her baby delivered. Her doctor was ob.gyn. George F. Craft II, at the time a member of Winchester Women’s Specialists. At one point during the roughly 30-minute delivery, Dr. Craft used forceps to remove Ms. Clements’s baby, who in the process sustained facial fractures and left-eye damage.
At trial, Craft argued that a forceps delivery was justified because the baby was stuck and his patient had refused a C-section.
The attorney for the plaintiffs — which included Ms. Clements’s husband — claimed that the use of forceps was premature, as professional guidelines require that a woman in labor be allowed at least 3 hours to push on her own before forceps are employed. (The suit, initially filed in 2019, also accused Dr. Craft of failing to properly inform his patient about the risks of, and alternatives to, this form of delivery. That part of the complaint was dropped, however, prior to the recent trial.)
The jury debated just 50 minutes before deciding Dr. Craft wasn’t medically negligent in the birth of William, Ms. Clements’s now 6-year-old son, who will be forced to wear contact lenses or glasses for life, or undergo corrective surgery.
As Dr. Craft’s attorney explained at trial: “He [Dr. Craft] hoped to give her [Ms. Clements] what she wanted: a vaginal delivery. But forceps techniques can and will cause injuries, even when properly placed.”
Unsupervised PAs subject to med-mal cap, state says
The California Supreme Court ruled late last month that even unsupervised physician assistants (PAs) are protected under the state’s $250,000 cap on noneconomic damages, according to a posting on the website of the Claims Journal, among other news sites.
The ruling stems from a 2013 suit filed by Marisol Lopez, who claimed that a dermatologist, a plastic surgeon, and two PAs had misdiagnosed her child’s skin cancer. Ms. Lopez’s child, Olivia Sarinana, died in February 2014, causing her mother to amend her original claim to a wrongful-death suit.
A trial court found both the doctors and the PAs liable for negligence, awarding the plaintiff $11,200 in economic damages and $4.25 million in noneconomic damages. The court subsequently reduced that amount, however, referencing the state’s $250,000 limit on noneconomic damages, which is part of the Medical Injury Compensation Reform Act of 1975, known as MICRA.
Ms. Lopez appealed the decision, arguing that the cap shouldn’t apply to the two PAs, because neither was under a physician’s direct supervision and therefore not acting within the proper scope of practice, as defined by state law. Despite agreeing with the factual basis of Ms. Lopez’s claim — that neither PA was being supervised during the period in question — the trial court refused to wave the state cap. Ms. Lopez again appealed, and, in a split decision, the Second District Court of Appeal upheld the trial court’s decision.
At this point, attorneys for Ms. Lopez applied for, and obtained, a review before the state’s highest court. Last month, the justices weighed in, ruling that the PAs were still entitled to protection under MICRA because they “had valid delegation-of-service agreements in place.” In other words, while the two PAs had not been directly supervised by a physician, their services had been properly delegated by one.
Said Associate Justice Goodwin Liu, who wrote the opinion: “To be sure, there are reasonable policy arguments for excluding physician assistants who perform medical services without actual supervision from a cap on non-economic damages, and the Legislature is well equipped to weigh and reweigh the competing policy considerations. But our role is confined to interpreting the statute before us in the manner that comports most closely with the Legislature’s purpose in enacting MICRA.”
Despite the high-court ruling, voters may soon get a chance to amend the nearly 5-decades-old MICRA legislation. A November ballot initiative would not only adjust the cap for inflation, raising it to more than $1.2 million, but would also permit “judges and juries to waive the cap entirely for cases involving death and permanent disability.”
Medical groups have said that if either or both of these changes happen the cost of healthcare in the Golden State will surely go up.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Is cancer testing going to the dogs? Nope, ants
The oncologist’s new best friend
We know that dogs have very sensitive noses. They can track criminals and missing persons and sniff out drugs and bombs. They can even detect cancer cells … after months of training.
And then there are ants.
Cancer cells produce volatile organic compounds (VOCs), which can be sniffed out by dogs and other animals with sufficiently sophisticated olfactory senses. A group of French investigators decided to find out if Formica fusca is such an animal.
First, they placed breast cancer cells and healthy cells in a petri dish. The sample of cancer cells, however, included a sugary treat. “Over successive trials, the ants got quicker and quicker at finding the treat, indicating that they had learned to recognize the VOCs produced by the cancerous cells, using these as a beacon to guide their way to the sugary delight,” according to IFL Science.
When the researchers removed the treat, the ants still went straight for the cancer cells. Then they removed the healthy cells and substituted another type of breast cancer cell, with just one type getting the treat. They went for the cancer cells with the treat, “indicating that they were capable of distinguishing between the different cancer types based on the unique pattern of VOCs emitted by each one,” IFL Science explained.
It’s just another chapter in the eternal struggle between dogs and ants. Dogs need months of training to learn to detect cancer cells; ants can do it in 30 minutes. Over the course of a dog’s training, Fido eats more food than 10,000 ants combined. (Okay, we’re guessing here, but it’s got to be a pretty big number, right?)
Then there’s the warm and fuzzy factor. Just look at that picture. Who wouldn’t want a cutie like that curling up in the bed next to you?
Console War II: Battle of the Twitter users
Video games can be a lot of fun, provided you’re not playing something like Rock Simulator. Or Surgeon Simulator. Or Surgeon Simulator 2. Yes, those are all real games. But calling yourself a video gamer invites a certain negative connotation, and nowhere can that be better exemplified than the increasingly ridiculous console war.
For those who don’t know their video game history, back in the early 90s Nintendo and Sega were the main video game console makers. Nintendo had Mario, Sega had Sonic, and everyone had an opinion on which was best. With Sega now but a shell of its former self and Nintendo viewed as too “casual” for the true gaming connoisseur, today’s battle pits Playstation against Xbox, and fans of both consoles spend their time trying to one-up each other in increasingly silly online arguments.
That brings us nicely to a Twitter user named “Shreeveera,” who is very vocal about his love of Playstation and hatred of the Xbox. Importantly, for LOTME purposes, Shreeveera identified himself as a doctor on his profile, and in the middle of an argument, Xbox enthusiasts called his credentials into question.
At this point, most people would recognize that there are very few noteworthy console-exclusive video games in today’s world and that any argument about consoles essentially comes down to which console design you like or which company you find less distasteful, and they would step away from the Twitter argument. Shreeveera is not most people, and he decided the next logical move was to post a video of himself and an anesthetized patient about to undergo a laparoscopic cholecystectomy.
This move did prove that he was indeed a doctor, but the ethics of posting such a video with a patient in the room is a bit dubious at best. Since Shreeveera also listed the hospital he worked at, numerous Twitter users review bombed the hospital with one-star reviews. Shreeveera’s fate is unknown, but he did take down the video and removed “doctor by profession” from his profile. He also made a second video asking Twitter to stop trying to ruin his life. We’re sure that’ll go well. Twitter is known for being completely fair and reasonable.
Use your words to gain power
We live in the age of the emoji. The use of emojis in texts and emails is basically the new shorthand. It’s a fun and easy way to chat with people close to us, but a new study shows that it doesn’t help in a business setting. In fact, it may do a little damage.
The use of images such as emojis in communication or logos can make a person seem less powerful than someone who opts for written words, according to Elinor Amit, PhD, of Tel Aviv University and associates.
Participants in their study were asked to imagine shopping with a person wearing a T-shirt. Half were then shown the logo of the Red Sox baseball team and half saw the words “Red Sox.” In another scenario, they were asked to imagine attending a retreat of a company called Lotus. Then half were shown an employee wearing a shirt with an image of lotus flower and half saw the verbal logo “Lotus.” In both scenarios, the individuals wearing shirts with images were seen as less powerful than the people who wore shirts with words on them.
Why is that? In a Eurekalert statement, Dr. Amit said that “visual messages are often interpreted as a signal for desire for social proximity.” In a world with COVID-19, that could give anyone pause.
That desire for more social proximity, in turn, equals a suggested loss of power because research shows that people who want to be around other people more are less powerful than people who don’t.
With the reduced social proximity we have these days, we may want to keep things cool and lighthearted, especially in work emails with people who we’ve never met. It may be, however, that using your words to say thank you in the multitude of emails you respond to on a regular basis is better than that thumbs-up emoji. Nobody will think less of you.
Should Daylight Savings Time still be a thing?
This past week, we just experienced the spring-forward portion of Daylight Savings Time, which took an hour of sleep away from us all. Some of us may still be struggling to find our footing with the time change, but at least it’s still sunny out at 7 pm. For those who don’t really see the point of changing the clocks twice a year, there are actually some good reasons to do so.
Sen. Marco Rubio, sponsor of a bill to make the time change permanent, put it simply: “If we can get this passed, we don’t have to do this stupidity anymore.” Message received, apparently, since the measure just passed unanimously in the Senate.
It’s not clear if President Biden will approve it, though, because there’s a lot that comes into play: economic needs, seasonal depression, and safety.
“I know this is not the most important issue confronting America, but it’s one of those issues where there’s a lot of agreement,” Sen. Rubio said.
Not total agreement, though. The National Association of Convenience Stores is opposed to the bill, and Reuters noted that one witness at a recent hearing said the time change “is like living in the wrong time zone for almost eight months out of the year.”
Many people, however, seem to be leaning toward the permanent spring-forward as it gives businesses a longer window to provide entertainment in the evenings and kids are able to play outside longer after school.
Honestly, we’re leaning toward whichever one can reduce seasonal depression.
The oncologist’s new best friend
We know that dogs have very sensitive noses. They can track criminals and missing persons and sniff out drugs and bombs. They can even detect cancer cells … after months of training.
And then there are ants.
Cancer cells produce volatile organic compounds (VOCs), which can be sniffed out by dogs and other animals with sufficiently sophisticated olfactory senses. A group of French investigators decided to find out if Formica fusca is such an animal.
First, they placed breast cancer cells and healthy cells in a petri dish. The sample of cancer cells, however, included a sugary treat. “Over successive trials, the ants got quicker and quicker at finding the treat, indicating that they had learned to recognize the VOCs produced by the cancerous cells, using these as a beacon to guide their way to the sugary delight,” according to IFL Science.
When the researchers removed the treat, the ants still went straight for the cancer cells. Then they removed the healthy cells and substituted another type of breast cancer cell, with just one type getting the treat. They went for the cancer cells with the treat, “indicating that they were capable of distinguishing between the different cancer types based on the unique pattern of VOCs emitted by each one,” IFL Science explained.
It’s just another chapter in the eternal struggle between dogs and ants. Dogs need months of training to learn to detect cancer cells; ants can do it in 30 minutes. Over the course of a dog’s training, Fido eats more food than 10,000 ants combined. (Okay, we’re guessing here, but it’s got to be a pretty big number, right?)
Then there’s the warm and fuzzy factor. Just look at that picture. Who wouldn’t want a cutie like that curling up in the bed next to you?
Console War II: Battle of the Twitter users
Video games can be a lot of fun, provided you’re not playing something like Rock Simulator. Or Surgeon Simulator. Or Surgeon Simulator 2. Yes, those are all real games. But calling yourself a video gamer invites a certain negative connotation, and nowhere can that be better exemplified than the increasingly ridiculous console war.
For those who don’t know their video game history, back in the early 90s Nintendo and Sega were the main video game console makers. Nintendo had Mario, Sega had Sonic, and everyone had an opinion on which was best. With Sega now but a shell of its former self and Nintendo viewed as too “casual” for the true gaming connoisseur, today’s battle pits Playstation against Xbox, and fans of both consoles spend their time trying to one-up each other in increasingly silly online arguments.
That brings us nicely to a Twitter user named “Shreeveera,” who is very vocal about his love of Playstation and hatred of the Xbox. Importantly, for LOTME purposes, Shreeveera identified himself as a doctor on his profile, and in the middle of an argument, Xbox enthusiasts called his credentials into question.
At this point, most people would recognize that there are very few noteworthy console-exclusive video games in today’s world and that any argument about consoles essentially comes down to which console design you like or which company you find less distasteful, and they would step away from the Twitter argument. Shreeveera is not most people, and he decided the next logical move was to post a video of himself and an anesthetized patient about to undergo a laparoscopic cholecystectomy.
This move did prove that he was indeed a doctor, but the ethics of posting such a video with a patient in the room is a bit dubious at best. Since Shreeveera also listed the hospital he worked at, numerous Twitter users review bombed the hospital with one-star reviews. Shreeveera’s fate is unknown, but he did take down the video and removed “doctor by profession” from his profile. He also made a second video asking Twitter to stop trying to ruin his life. We’re sure that’ll go well. Twitter is known for being completely fair and reasonable.
Use your words to gain power
We live in the age of the emoji. The use of emojis in texts and emails is basically the new shorthand. It’s a fun and easy way to chat with people close to us, but a new study shows that it doesn’t help in a business setting. In fact, it may do a little damage.
The use of images such as emojis in communication or logos can make a person seem less powerful than someone who opts for written words, according to Elinor Amit, PhD, of Tel Aviv University and associates.
Participants in their study were asked to imagine shopping with a person wearing a T-shirt. Half were then shown the logo of the Red Sox baseball team and half saw the words “Red Sox.” In another scenario, they were asked to imagine attending a retreat of a company called Lotus. Then half were shown an employee wearing a shirt with an image of lotus flower and half saw the verbal logo “Lotus.” In both scenarios, the individuals wearing shirts with images were seen as less powerful than the people who wore shirts with words on them.
Why is that? In a Eurekalert statement, Dr. Amit said that “visual messages are often interpreted as a signal for desire for social proximity.” In a world with COVID-19, that could give anyone pause.
That desire for more social proximity, in turn, equals a suggested loss of power because research shows that people who want to be around other people more are less powerful than people who don’t.
With the reduced social proximity we have these days, we may want to keep things cool and lighthearted, especially in work emails with people who we’ve never met. It may be, however, that using your words to say thank you in the multitude of emails you respond to on a regular basis is better than that thumbs-up emoji. Nobody will think less of you.
Should Daylight Savings Time still be a thing?
This past week, we just experienced the spring-forward portion of Daylight Savings Time, which took an hour of sleep away from us all. Some of us may still be struggling to find our footing with the time change, but at least it’s still sunny out at 7 pm. For those who don’t really see the point of changing the clocks twice a year, there are actually some good reasons to do so.
Sen. Marco Rubio, sponsor of a bill to make the time change permanent, put it simply: “If we can get this passed, we don’t have to do this stupidity anymore.” Message received, apparently, since the measure just passed unanimously in the Senate.
It’s not clear if President Biden will approve it, though, because there’s a lot that comes into play: economic needs, seasonal depression, and safety.
“I know this is not the most important issue confronting America, but it’s one of those issues where there’s a lot of agreement,” Sen. Rubio said.
Not total agreement, though. The National Association of Convenience Stores is opposed to the bill, and Reuters noted that one witness at a recent hearing said the time change “is like living in the wrong time zone for almost eight months out of the year.”
Many people, however, seem to be leaning toward the permanent spring-forward as it gives businesses a longer window to provide entertainment in the evenings and kids are able to play outside longer after school.
Honestly, we’re leaning toward whichever one can reduce seasonal depression.
The oncologist’s new best friend
We know that dogs have very sensitive noses. They can track criminals and missing persons and sniff out drugs and bombs. They can even detect cancer cells … after months of training.
And then there are ants.
Cancer cells produce volatile organic compounds (VOCs), which can be sniffed out by dogs and other animals with sufficiently sophisticated olfactory senses. A group of French investigators decided to find out if Formica fusca is such an animal.
First, they placed breast cancer cells and healthy cells in a petri dish. The sample of cancer cells, however, included a sugary treat. “Over successive trials, the ants got quicker and quicker at finding the treat, indicating that they had learned to recognize the VOCs produced by the cancerous cells, using these as a beacon to guide their way to the sugary delight,” according to IFL Science.
When the researchers removed the treat, the ants still went straight for the cancer cells. Then they removed the healthy cells and substituted another type of breast cancer cell, with just one type getting the treat. They went for the cancer cells with the treat, “indicating that they were capable of distinguishing between the different cancer types based on the unique pattern of VOCs emitted by each one,” IFL Science explained.
It’s just another chapter in the eternal struggle between dogs and ants. Dogs need months of training to learn to detect cancer cells; ants can do it in 30 minutes. Over the course of a dog’s training, Fido eats more food than 10,000 ants combined. (Okay, we’re guessing here, but it’s got to be a pretty big number, right?)
Then there’s the warm and fuzzy factor. Just look at that picture. Who wouldn’t want a cutie like that curling up in the bed next to you?
Console War II: Battle of the Twitter users
Video games can be a lot of fun, provided you’re not playing something like Rock Simulator. Or Surgeon Simulator. Or Surgeon Simulator 2. Yes, those are all real games. But calling yourself a video gamer invites a certain negative connotation, and nowhere can that be better exemplified than the increasingly ridiculous console war.
For those who don’t know their video game history, back in the early 90s Nintendo and Sega were the main video game console makers. Nintendo had Mario, Sega had Sonic, and everyone had an opinion on which was best. With Sega now but a shell of its former self and Nintendo viewed as too “casual” for the true gaming connoisseur, today’s battle pits Playstation against Xbox, and fans of both consoles spend their time trying to one-up each other in increasingly silly online arguments.
That brings us nicely to a Twitter user named “Shreeveera,” who is very vocal about his love of Playstation and hatred of the Xbox. Importantly, for LOTME purposes, Shreeveera identified himself as a doctor on his profile, and in the middle of an argument, Xbox enthusiasts called his credentials into question.
At this point, most people would recognize that there are very few noteworthy console-exclusive video games in today’s world and that any argument about consoles essentially comes down to which console design you like or which company you find less distasteful, and they would step away from the Twitter argument. Shreeveera is not most people, and he decided the next logical move was to post a video of himself and an anesthetized patient about to undergo a laparoscopic cholecystectomy.
This move did prove that he was indeed a doctor, but the ethics of posting such a video with a patient in the room is a bit dubious at best. Since Shreeveera also listed the hospital he worked at, numerous Twitter users review bombed the hospital with one-star reviews. Shreeveera’s fate is unknown, but he did take down the video and removed “doctor by profession” from his profile. He also made a second video asking Twitter to stop trying to ruin his life. We’re sure that’ll go well. Twitter is known for being completely fair and reasonable.
Use your words to gain power
We live in the age of the emoji. The use of emojis in texts and emails is basically the new shorthand. It’s a fun and easy way to chat with people close to us, but a new study shows that it doesn’t help in a business setting. In fact, it may do a little damage.
The use of images such as emojis in communication or logos can make a person seem less powerful than someone who opts for written words, according to Elinor Amit, PhD, of Tel Aviv University and associates.
Participants in their study were asked to imagine shopping with a person wearing a T-shirt. Half were then shown the logo of the Red Sox baseball team and half saw the words “Red Sox.” In another scenario, they were asked to imagine attending a retreat of a company called Lotus. Then half were shown an employee wearing a shirt with an image of lotus flower and half saw the verbal logo “Lotus.” In both scenarios, the individuals wearing shirts with images were seen as less powerful than the people who wore shirts with words on them.
Why is that? In a Eurekalert statement, Dr. Amit said that “visual messages are often interpreted as a signal for desire for social proximity.” In a world with COVID-19, that could give anyone pause.
That desire for more social proximity, in turn, equals a suggested loss of power because research shows that people who want to be around other people more are less powerful than people who don’t.
With the reduced social proximity we have these days, we may want to keep things cool and lighthearted, especially in work emails with people who we’ve never met. It may be, however, that using your words to say thank you in the multitude of emails you respond to on a regular basis is better than that thumbs-up emoji. Nobody will think less of you.
Should Daylight Savings Time still be a thing?
This past week, we just experienced the spring-forward portion of Daylight Savings Time, which took an hour of sleep away from us all. Some of us may still be struggling to find our footing with the time change, but at least it’s still sunny out at 7 pm. For those who don’t really see the point of changing the clocks twice a year, there are actually some good reasons to do so.
Sen. Marco Rubio, sponsor of a bill to make the time change permanent, put it simply: “If we can get this passed, we don’t have to do this stupidity anymore.” Message received, apparently, since the measure just passed unanimously in the Senate.
It’s not clear if President Biden will approve it, though, because there’s a lot that comes into play: economic needs, seasonal depression, and safety.
“I know this is not the most important issue confronting America, but it’s one of those issues where there’s a lot of agreement,” Sen. Rubio said.
Not total agreement, though. The National Association of Convenience Stores is opposed to the bill, and Reuters noted that one witness at a recent hearing said the time change “is like living in the wrong time zone for almost eight months out of the year.”
Many people, however, seem to be leaning toward the permanent spring-forward as it gives businesses a longer window to provide entertainment in the evenings and kids are able to play outside longer after school.
Honestly, we’re leaning toward whichever one can reduce seasonal depression.
Study: Majority of research on homeopathic remedies unpublished or unregistered
Homeopathy is a form of alternative medicine based on the concept that increasing dilution of a substance leads to a stronger treatment effect.
The authors of the new paper, published in BMJ Evidence-Based Medicine, also found that a quarter of the 90 randomized published trials on homeopathic remedies they analyzed changed their results before publication.
The benefits of homeopathy touted in studies may be greatly exaggerated, suggest the authors, Gerald Gartlehner, MD, of Danube University, Krems, Austria, and colleagues.
The results raise awareness that published homeopathy trials represent a limited proportion of research, skewed toward favorable results, they wrote.
“This likely affects the validity of the body of evidence of homeopathic literature and may substantially overestimate the true treatment effect of homeopathic remedies,” they concluded.
Homeopathy as practiced today was developed approximately 200 years ago in Germany, and despite ongoing debate about its effectiveness, it remains a popular alternative to conventional medicine in many developed countries, the authors noted.
According to the National Institutes of Health, homeopathy is based on the idea of “like cures like,” meaning that a disease can be cured with a substance that produces similar symptoms in healthy people, and the “law of minimum dose,” meaning that a lower dose of medication will be more effective. “Many homeopathic products are so diluted that no molecules of the original substance remain,” according to the NIH.
Homeopathy is not subject to most regulatory requirements, so assessment of effectiveness of homeopathic remedies is limited to published data, the researchers said. “When no information is publicly available about the majority of homeopathic trials, sound conclusions about the efficacy and the risks of using homeopathic medicinal products for treating health conditions are impossible,” they wrote.
Study methods and findings
The researchers examined 17 trial registries for studies involving homeopathic remedies conducted since 2002.
The registries included clinicaltrials.gov, the EU Clinical Trials Register, and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials.
To determine whether registered trials were published and to identify trials that were published but unregistered, the researchers examined PubMed, the Allied and Complementary Medicine Database, Embase, and Google Scholar up to April 2021.
They found that approximately 38% of registered trials of homeopathy were never published, and 53% of the published randomized, controlled trials (RCTs) were not registered. Notably, 25% of the trials that were registered and published showed primary outcomes that were changed compared with the registry.
The number of registered homeopathy trials increased significantly over the past 5 years, but approximately one-third (30%) of trials published during the last 5 years were not registered, they said. In a meta-analysis, unregistered RCTs showed significantly greater treatment effects than registered RCTs, with standardized mean differences of –0.53 and –0.14, respectively.
The study findings were limited by several factors including the potential for missed records of studies not covered by the registries searched. Other limitations include the analysis of pooled data from homeopathic treatments that may not generalize to personalized homeopathy, and the exclusion of trials labeled as terminated or suspended.
Proceed with caution before recommending use of homeopathic remedies, says expert
Linda Girgis, MD, noted that prior to reading this report she had known that most homeopathic remedies didn’t have any evidence of being effective, and that, therefore, the results validated her understanding of the findings of studies of homeopathy.
The study is especially important at this time in the wake of the COVID-19 pandemic, Dr. Girgis, a family physician in private practice in South River, N.J., said in an interview.
“Many people are promoting treatments that don’t have any evidence that they are effective, and more people are turning to homeopathic treatments not knowing the risks and assuming they are safe,” she continued. “Many people are taking advantage of this and trying to cash in on this with ill-proven remedies.”
Homeopathic remedies become especially harmful when patients think they can use them instead of traditional medicine, she added.
Noting that some homeopathic remedies have been studied and show some evidence that they work, Dr. Girgis said there may be a role for certain ones in primary care.
“An example would be black cohosh or primrose oil for perimenopausal hot flashes. This could be a good alternative when you want to avoid hormonal supplements,” she said.
At the same time, Dr. Girgis advised clinicians to be cautious about suggesting homeopathic remedies to patients.
“Homeopathy seems to be a good money maker if you sell these products. However, you are not protected from liability and can be found more liable for prescribing off-label treatments or those not [Food and Drug Administration] approved,” Dr. Girgis said. Her general message to clinicians: Stick with evidence-based medicine.
Her message to patients who might want to pursue homeopathic remedies is that just because something is “homeopathic” or natural doesn’t mean that it is safe.
“There are some [homeopathic] products that have caused liver damage or other problems,” she explained. “Also, these remedies can interact with other medications.”
The study received no outside funding. The researchers and Dr. Girgis had no financial conflicts to disclose.
Homeopathy is a form of alternative medicine based on the concept that increasing dilution of a substance leads to a stronger treatment effect.
The authors of the new paper, published in BMJ Evidence-Based Medicine, also found that a quarter of the 90 randomized published trials on homeopathic remedies they analyzed changed their results before publication.
The benefits of homeopathy touted in studies may be greatly exaggerated, suggest the authors, Gerald Gartlehner, MD, of Danube University, Krems, Austria, and colleagues.
The results raise awareness that published homeopathy trials represent a limited proportion of research, skewed toward favorable results, they wrote.
“This likely affects the validity of the body of evidence of homeopathic literature and may substantially overestimate the true treatment effect of homeopathic remedies,” they concluded.
Homeopathy as practiced today was developed approximately 200 years ago in Germany, and despite ongoing debate about its effectiveness, it remains a popular alternative to conventional medicine in many developed countries, the authors noted.
According to the National Institutes of Health, homeopathy is based on the idea of “like cures like,” meaning that a disease can be cured with a substance that produces similar symptoms in healthy people, and the “law of minimum dose,” meaning that a lower dose of medication will be more effective. “Many homeopathic products are so diluted that no molecules of the original substance remain,” according to the NIH.
Homeopathy is not subject to most regulatory requirements, so assessment of effectiveness of homeopathic remedies is limited to published data, the researchers said. “When no information is publicly available about the majority of homeopathic trials, sound conclusions about the efficacy and the risks of using homeopathic medicinal products for treating health conditions are impossible,” they wrote.
Study methods and findings
The researchers examined 17 trial registries for studies involving homeopathic remedies conducted since 2002.
The registries included clinicaltrials.gov, the EU Clinical Trials Register, and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials.
To determine whether registered trials were published and to identify trials that were published but unregistered, the researchers examined PubMed, the Allied and Complementary Medicine Database, Embase, and Google Scholar up to April 2021.
They found that approximately 38% of registered trials of homeopathy were never published, and 53% of the published randomized, controlled trials (RCTs) were not registered. Notably, 25% of the trials that were registered and published showed primary outcomes that were changed compared with the registry.
The number of registered homeopathy trials increased significantly over the past 5 years, but approximately one-third (30%) of trials published during the last 5 years were not registered, they said. In a meta-analysis, unregistered RCTs showed significantly greater treatment effects than registered RCTs, with standardized mean differences of –0.53 and –0.14, respectively.
The study findings were limited by several factors including the potential for missed records of studies not covered by the registries searched. Other limitations include the analysis of pooled data from homeopathic treatments that may not generalize to personalized homeopathy, and the exclusion of trials labeled as terminated or suspended.
Proceed with caution before recommending use of homeopathic remedies, says expert
Linda Girgis, MD, noted that prior to reading this report she had known that most homeopathic remedies didn’t have any evidence of being effective, and that, therefore, the results validated her understanding of the findings of studies of homeopathy.
The study is especially important at this time in the wake of the COVID-19 pandemic, Dr. Girgis, a family physician in private practice in South River, N.J., said in an interview.
“Many people are promoting treatments that don’t have any evidence that they are effective, and more people are turning to homeopathic treatments not knowing the risks and assuming they are safe,” she continued. “Many people are taking advantage of this and trying to cash in on this with ill-proven remedies.”
Homeopathic remedies become especially harmful when patients think they can use them instead of traditional medicine, she added.
Noting that some homeopathic remedies have been studied and show some evidence that they work, Dr. Girgis said there may be a role for certain ones in primary care.
“An example would be black cohosh or primrose oil for perimenopausal hot flashes. This could be a good alternative when you want to avoid hormonal supplements,” she said.
At the same time, Dr. Girgis advised clinicians to be cautious about suggesting homeopathic remedies to patients.
“Homeopathy seems to be a good money maker if you sell these products. However, you are not protected from liability and can be found more liable for prescribing off-label treatments or those not [Food and Drug Administration] approved,” Dr. Girgis said. Her general message to clinicians: Stick with evidence-based medicine.
Her message to patients who might want to pursue homeopathic remedies is that just because something is “homeopathic” or natural doesn’t mean that it is safe.
“There are some [homeopathic] products that have caused liver damage or other problems,” she explained. “Also, these remedies can interact with other medications.”
The study received no outside funding. The researchers and Dr. Girgis had no financial conflicts to disclose.
Homeopathy is a form of alternative medicine based on the concept that increasing dilution of a substance leads to a stronger treatment effect.
The authors of the new paper, published in BMJ Evidence-Based Medicine, also found that a quarter of the 90 randomized published trials on homeopathic remedies they analyzed changed their results before publication.
The benefits of homeopathy touted in studies may be greatly exaggerated, suggest the authors, Gerald Gartlehner, MD, of Danube University, Krems, Austria, and colleagues.
The results raise awareness that published homeopathy trials represent a limited proportion of research, skewed toward favorable results, they wrote.
“This likely affects the validity of the body of evidence of homeopathic literature and may substantially overestimate the true treatment effect of homeopathic remedies,” they concluded.
Homeopathy as practiced today was developed approximately 200 years ago in Germany, and despite ongoing debate about its effectiveness, it remains a popular alternative to conventional medicine in many developed countries, the authors noted.
According to the National Institutes of Health, homeopathy is based on the idea of “like cures like,” meaning that a disease can be cured with a substance that produces similar symptoms in healthy people, and the “law of minimum dose,” meaning that a lower dose of medication will be more effective. “Many homeopathic products are so diluted that no molecules of the original substance remain,” according to the NIH.
Homeopathy is not subject to most regulatory requirements, so assessment of effectiveness of homeopathic remedies is limited to published data, the researchers said. “When no information is publicly available about the majority of homeopathic trials, sound conclusions about the efficacy and the risks of using homeopathic medicinal products for treating health conditions are impossible,” they wrote.
Study methods and findings
The researchers examined 17 trial registries for studies involving homeopathic remedies conducted since 2002.
The registries included clinicaltrials.gov, the EU Clinical Trials Register, and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials.
To determine whether registered trials were published and to identify trials that were published but unregistered, the researchers examined PubMed, the Allied and Complementary Medicine Database, Embase, and Google Scholar up to April 2021.
They found that approximately 38% of registered trials of homeopathy were never published, and 53% of the published randomized, controlled trials (RCTs) were not registered. Notably, 25% of the trials that were registered and published showed primary outcomes that were changed compared with the registry.
The number of registered homeopathy trials increased significantly over the past 5 years, but approximately one-third (30%) of trials published during the last 5 years were not registered, they said. In a meta-analysis, unregistered RCTs showed significantly greater treatment effects than registered RCTs, with standardized mean differences of –0.53 and –0.14, respectively.
The study findings were limited by several factors including the potential for missed records of studies not covered by the registries searched. Other limitations include the analysis of pooled data from homeopathic treatments that may not generalize to personalized homeopathy, and the exclusion of trials labeled as terminated or suspended.
Proceed with caution before recommending use of homeopathic remedies, says expert
Linda Girgis, MD, noted that prior to reading this report she had known that most homeopathic remedies didn’t have any evidence of being effective, and that, therefore, the results validated her understanding of the findings of studies of homeopathy.
The study is especially important at this time in the wake of the COVID-19 pandemic, Dr. Girgis, a family physician in private practice in South River, N.J., said in an interview.
“Many people are promoting treatments that don’t have any evidence that they are effective, and more people are turning to homeopathic treatments not knowing the risks and assuming they are safe,” she continued. “Many people are taking advantage of this and trying to cash in on this with ill-proven remedies.”
Homeopathic remedies become especially harmful when patients think they can use them instead of traditional medicine, she added.
Noting that some homeopathic remedies have been studied and show some evidence that they work, Dr. Girgis said there may be a role for certain ones in primary care.
“An example would be black cohosh or primrose oil for perimenopausal hot flashes. This could be a good alternative when you want to avoid hormonal supplements,” she said.
At the same time, Dr. Girgis advised clinicians to be cautious about suggesting homeopathic remedies to patients.
“Homeopathy seems to be a good money maker if you sell these products. However, you are not protected from liability and can be found more liable for prescribing off-label treatments or those not [Food and Drug Administration] approved,” Dr. Girgis said. Her general message to clinicians: Stick with evidence-based medicine.
Her message to patients who might want to pursue homeopathic remedies is that just because something is “homeopathic” or natural doesn’t mean that it is safe.
“There are some [homeopathic] products that have caused liver damage or other problems,” she explained. “Also, these remedies can interact with other medications.”
The study received no outside funding. The researchers and Dr. Girgis had no financial conflicts to disclose.
FROM BMJ EVIDENCE BASED MEDICINE
Selling your practice
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
The science of clean skin care and the clean beauty movement
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).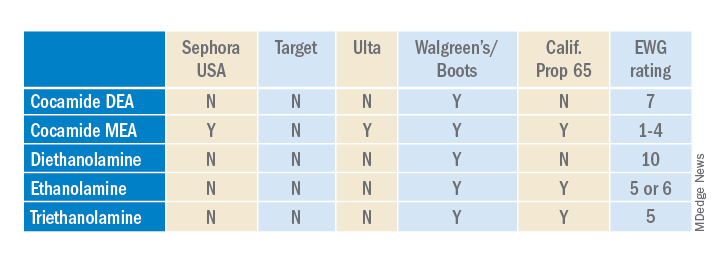
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
FDA warns about off-label use of laparoscopic device for aesthetic procedures
The .
The device is cleared by the FDA for “general use of cutting, coagulation, and ablation of soft tissue during open and laparoscopic surgical procedures” but it “has not been determined to be safe or effective for any procedure intended to improve the appearance of the skin,” according to the March 14 statement from the FDA. The statement adds that the agency has received reports describing “serious and potentially life-threatening adverse events with use of this device for certain aesthetic procedures,” including some that have required treatment in an intensive care unit. The statement does not mention whether any cases were fatal.
Adverse events that have been reported include second- and third-degree burns, infections, changes in skin color, scars, nerve damage, “significant bleeding,” and “air or gas accumulation under the skin, in body cavities, and in blood vessels.”
Manufactured by Apyx medical, the device includes a hand piece and generator and uses radiofrequency energy and helium to generate plasma, which is used to “cut, coagulate ... and eliminate soft tissue with heat during surgery,” according to the FDA.
The FDA is advising health care providers not to use the device for dermal resurfacing or skin contraction “alone or in combination with liposuction.”
The statement also advises consumers who are considering an aesthetic skin treatment with this device to consult their health care providers regarding its use – and if they have any problems or are concerned after being treated with this device, to “seek care from a licensed health care provider.”
The FDA is working with Apyx to evaluate information about the use of the device for aesthetic skin procedures and to inform consumers and health care providers about the warning.
Health care providers and consumers should report problems or complications associated with the Renuvion/J-Plasma device to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
The .
The device is cleared by the FDA for “general use of cutting, coagulation, and ablation of soft tissue during open and laparoscopic surgical procedures” but it “has not been determined to be safe or effective for any procedure intended to improve the appearance of the skin,” according to the March 14 statement from the FDA. The statement adds that the agency has received reports describing “serious and potentially life-threatening adverse events with use of this device for certain aesthetic procedures,” including some that have required treatment in an intensive care unit. The statement does not mention whether any cases were fatal.
Adverse events that have been reported include second- and third-degree burns, infections, changes in skin color, scars, nerve damage, “significant bleeding,” and “air or gas accumulation under the skin, in body cavities, and in blood vessels.”
Manufactured by Apyx medical, the device includes a hand piece and generator and uses radiofrequency energy and helium to generate plasma, which is used to “cut, coagulate ... and eliminate soft tissue with heat during surgery,” according to the FDA.
The FDA is advising health care providers not to use the device for dermal resurfacing or skin contraction “alone or in combination with liposuction.”
The statement also advises consumers who are considering an aesthetic skin treatment with this device to consult their health care providers regarding its use – and if they have any problems or are concerned after being treated with this device, to “seek care from a licensed health care provider.”
The FDA is working with Apyx to evaluate information about the use of the device for aesthetic skin procedures and to inform consumers and health care providers about the warning.
Health care providers and consumers should report problems or complications associated with the Renuvion/J-Plasma device to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
The .
The device is cleared by the FDA for “general use of cutting, coagulation, and ablation of soft tissue during open and laparoscopic surgical procedures” but it “has not been determined to be safe or effective for any procedure intended to improve the appearance of the skin,” according to the March 14 statement from the FDA. The statement adds that the agency has received reports describing “serious and potentially life-threatening adverse events with use of this device for certain aesthetic procedures,” including some that have required treatment in an intensive care unit. The statement does not mention whether any cases were fatal.
Adverse events that have been reported include second- and third-degree burns, infections, changes in skin color, scars, nerve damage, “significant bleeding,” and “air or gas accumulation under the skin, in body cavities, and in blood vessels.”
Manufactured by Apyx medical, the device includes a hand piece and generator and uses radiofrequency energy and helium to generate plasma, which is used to “cut, coagulate ... and eliminate soft tissue with heat during surgery,” according to the FDA.
The FDA is advising health care providers not to use the device for dermal resurfacing or skin contraction “alone or in combination with liposuction.”
The statement also advises consumers who are considering an aesthetic skin treatment with this device to consult their health care providers regarding its use – and if they have any problems or are concerned after being treated with this device, to “seek care from a licensed health care provider.”
The FDA is working with Apyx to evaluate information about the use of the device for aesthetic skin procedures and to inform consumers and health care providers about the warning.
Health care providers and consumers should report problems or complications associated with the Renuvion/J-Plasma device to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.