User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Adverse reactions to immunotherapy can appear after a year
Clinicians should be on the lookout for immune-related adverse events (irAEs) even after patients have been receiving anti-PD-1 immunotherapy for a year or longer, according to team of international investigators.
They reported that, among melanoma patients, the incidence of new-onset reactions that occurred 1 year or longer after anti-PD-1 treatment was 5.3%.
In a review of 118 patients, the investigators found that irAEs are often “high grade, difficult to manage, and can lead to death.”
Reactions are more likely to occur in those for whom treatment with an anti-PD-1 checkpoint inhibitor – primarily pembrolizumab and nivolumab – continued for longer than a year, and patients can present “long after stopping” the treatment, the investigators noted.
The findings were published online in Annals of Oncology.
“We do not yet understand why some patients have no side effects for months or years, then develop toxicities so late in their course,” said one of the coauthors, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn.
“Physicians should continue to monitor patients for side effects, even if they have been on anti-PD-1 therapy for some time, since delayed side effects may cause morbidity and even death,” Dr. Johnson said.
Patients and clinicians need “to be aware of these risks when making decisions regarding therapy continuation” and need “to consider irAE as a possible diagnosis in any presentation where there is a history of checkpoint inhibitor treatment, regardless of the time frame, to enable early recognition and appropriate treatment,” Dr. Johnson and colleagues concluded.
Largest series to document delayed reactions
Immunotherapies have revolutionized cancer treatment of many types of tumors, but they carry a well known risk for autoimmune toxicity, which typically occurs within the first 4-6 months, the authors wrote.
Delayed reactions have been reported but are not as well described. The new study is the largest to date on this question, and Dr. Johnson said the findings likely apply across indications, not simply in regard to melanoma patients.
An expert not involved in the study agrees.
“We are definitely seeing delayed reactions to immunotherapy in our practice” in several organ systems, including the skin, said Jennifer Choi, MD, chief of oncodermatology at Northwestern University’s Comprehensive Cancer Center, Chicago.
“Some of these side effects can take months to resolve and may require systemic treatment, such as steroids, nonsteroidal immunosuppressants, or biologics. Clinicians must be on high alert of any possible side effect for a patient on immunotherapy throughout their entire course, and even after they have completed treatment,” Dr. Choi said in an interview.
Anti-PD-1 therapy doesn’t “follow the typical drug hypersensitivity laws and rules with respect to timing,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
Median onset was 16 months
The investigators reported in detail on 118 patients. A total of 140 delayed irAEs that occurred 1 year or longer after treatment were identified in 20 centers around the world.
The median onset of delayed irAE was 16 months after start of treatment. Most occurred in conjunction with stand-alone anti-PD-1 therapy, but in the case of 20 patients, a combination of an anti-PD-1 drug and the anti-CTLA-4 drug ipilimumab was used.
In 39% of patients (n = 55), the adverse reaction was of grade 3 or worse. These included two deaths: one case of fatal encephalitis with concurrent anti-PD-1 use, and a death from immune-related multiple organ failure 11 months after anti-PD-1 discontinuation.
Most of the patients (n = 87; 74%) were receiving anti-PD-1 therapy at the time of onset of the adverse reaction; 15 patients (12%) were within 3 months of their last dose, and 16 (14%) were 3 months past their last dose.
Among the subgroup who developed an irAE after discontinuation of treatment was a patient with grade 4 colitis that required colectomy 26 months afterward, although Dr. Johnson noted it’s difficult to be sure that the colitis was related to the immunotherapy, because it occurred so long after treatment had ended.
An early warning system
The most common reactions were colitis, pneumonitis, and rash.
The reactions were often tough to manage, the authors reported. Eighty patients (68%) required steroids, and 27 (23%) required steroids plus additional immunosuppressives, such as tumor necrosis factor blockers, particularly for colitis and renal, rheumatologic, and neurologic complications. Rheumatologic events required a median corticosteroid course of 15 months plus additional immunosuppression in half of cases and often left patients with ongoing morbidity.
“Often, the skin is one of the first and most easily visible immune-related adverse event that develops,” said Bernice Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, who was not involved in the study and was approached for comment.
Presentations can range from small itchy plaques to total body dermatitis. It is something to be aware of, because the skin can act as an early warning system to catch internal organ damage earlier, she said.
On a positive note, the investigators found no indication that the effect of immunotherapy was diminished by delayed reactions and their treatment.
Managing events “gets a little complicated” when anti-PD-1 drugs are still being administered, but “we have successfully utilized systemic steroid pulses for several weeks without impeding the efficacy of the therapy. For the lichenoid and psoriasiform dermatitis, topical steroids and oral retinoids have been useful and can be used concurrently with immunotherapy,” Dr. Friedman said.
Question on treatment duration
No obvious factors were predictive of delayed events, including previous autoimmune disease or earlier reactions, which usually affected different organs, the authors said.
The findings raise a question about the appropriate duration of anti-PD-1 therapy, at least for melanoma.
The standard duration of adjuvant therapy was empirically determined to be 1 year for melanoma, and trials support anti-PD-1 therapy for up to 2 years for metastatic disease.
However, the authors suggest that “shorter treatment duration may reduce the risk of delayed irAE” and may be sufficient for patients who have a complete response.
“This should be considered when making decisions regarding therapy continuation in responding patients,” they wrote.
Ongoing clinical trials are investigating the optimal duration of therapy, they wrote.
No outside funding was reported. Dr. Johnson has been an adviser for Array Biopharma, BMS, Iovance, Jansen, Merck, and Novartis and has received research funding from BMS and Incyte. Other investigators reported similar ties. Dr. Choi, Dr. Kwong, and Dr. Friedman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should be on the lookout for immune-related adverse events (irAEs) even after patients have been receiving anti-PD-1 immunotherapy for a year or longer, according to team of international investigators.
They reported that, among melanoma patients, the incidence of new-onset reactions that occurred 1 year or longer after anti-PD-1 treatment was 5.3%.
In a review of 118 patients, the investigators found that irAEs are often “high grade, difficult to manage, and can lead to death.”
Reactions are more likely to occur in those for whom treatment with an anti-PD-1 checkpoint inhibitor – primarily pembrolizumab and nivolumab – continued for longer than a year, and patients can present “long after stopping” the treatment, the investigators noted.
The findings were published online in Annals of Oncology.
“We do not yet understand why some patients have no side effects for months or years, then develop toxicities so late in their course,” said one of the coauthors, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn.
“Physicians should continue to monitor patients for side effects, even if they have been on anti-PD-1 therapy for some time, since delayed side effects may cause morbidity and even death,” Dr. Johnson said.
Patients and clinicians need “to be aware of these risks when making decisions regarding therapy continuation” and need “to consider irAE as a possible diagnosis in any presentation where there is a history of checkpoint inhibitor treatment, regardless of the time frame, to enable early recognition and appropriate treatment,” Dr. Johnson and colleagues concluded.
Largest series to document delayed reactions
Immunotherapies have revolutionized cancer treatment of many types of tumors, but they carry a well known risk for autoimmune toxicity, which typically occurs within the first 4-6 months, the authors wrote.
Delayed reactions have been reported but are not as well described. The new study is the largest to date on this question, and Dr. Johnson said the findings likely apply across indications, not simply in regard to melanoma patients.
An expert not involved in the study agrees.
“We are definitely seeing delayed reactions to immunotherapy in our practice” in several organ systems, including the skin, said Jennifer Choi, MD, chief of oncodermatology at Northwestern University’s Comprehensive Cancer Center, Chicago.
“Some of these side effects can take months to resolve and may require systemic treatment, such as steroids, nonsteroidal immunosuppressants, or biologics. Clinicians must be on high alert of any possible side effect for a patient on immunotherapy throughout their entire course, and even after they have completed treatment,” Dr. Choi said in an interview.
Anti-PD-1 therapy doesn’t “follow the typical drug hypersensitivity laws and rules with respect to timing,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
Median onset was 16 months
The investigators reported in detail on 118 patients. A total of 140 delayed irAEs that occurred 1 year or longer after treatment were identified in 20 centers around the world.
The median onset of delayed irAE was 16 months after start of treatment. Most occurred in conjunction with stand-alone anti-PD-1 therapy, but in the case of 20 patients, a combination of an anti-PD-1 drug and the anti-CTLA-4 drug ipilimumab was used.
In 39% of patients (n = 55), the adverse reaction was of grade 3 or worse. These included two deaths: one case of fatal encephalitis with concurrent anti-PD-1 use, and a death from immune-related multiple organ failure 11 months after anti-PD-1 discontinuation.
Most of the patients (n = 87; 74%) were receiving anti-PD-1 therapy at the time of onset of the adverse reaction; 15 patients (12%) were within 3 months of their last dose, and 16 (14%) were 3 months past their last dose.
Among the subgroup who developed an irAE after discontinuation of treatment was a patient with grade 4 colitis that required colectomy 26 months afterward, although Dr. Johnson noted it’s difficult to be sure that the colitis was related to the immunotherapy, because it occurred so long after treatment had ended.
An early warning system
The most common reactions were colitis, pneumonitis, and rash.
The reactions were often tough to manage, the authors reported. Eighty patients (68%) required steroids, and 27 (23%) required steroids plus additional immunosuppressives, such as tumor necrosis factor blockers, particularly for colitis and renal, rheumatologic, and neurologic complications. Rheumatologic events required a median corticosteroid course of 15 months plus additional immunosuppression in half of cases and often left patients with ongoing morbidity.
“Often, the skin is one of the first and most easily visible immune-related adverse event that develops,” said Bernice Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, who was not involved in the study and was approached for comment.
Presentations can range from small itchy plaques to total body dermatitis. It is something to be aware of, because the skin can act as an early warning system to catch internal organ damage earlier, she said.
On a positive note, the investigators found no indication that the effect of immunotherapy was diminished by delayed reactions and their treatment.
Managing events “gets a little complicated” when anti-PD-1 drugs are still being administered, but “we have successfully utilized systemic steroid pulses for several weeks without impeding the efficacy of the therapy. For the lichenoid and psoriasiform dermatitis, topical steroids and oral retinoids have been useful and can be used concurrently with immunotherapy,” Dr. Friedman said.
Question on treatment duration
No obvious factors were predictive of delayed events, including previous autoimmune disease or earlier reactions, which usually affected different organs, the authors said.
The findings raise a question about the appropriate duration of anti-PD-1 therapy, at least for melanoma.
The standard duration of adjuvant therapy was empirically determined to be 1 year for melanoma, and trials support anti-PD-1 therapy for up to 2 years for metastatic disease.
However, the authors suggest that “shorter treatment duration may reduce the risk of delayed irAE” and may be sufficient for patients who have a complete response.
“This should be considered when making decisions regarding therapy continuation in responding patients,” they wrote.
Ongoing clinical trials are investigating the optimal duration of therapy, they wrote.
No outside funding was reported. Dr. Johnson has been an adviser for Array Biopharma, BMS, Iovance, Jansen, Merck, and Novartis and has received research funding from BMS and Incyte. Other investigators reported similar ties. Dr. Choi, Dr. Kwong, and Dr. Friedman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should be on the lookout for immune-related adverse events (irAEs) even after patients have been receiving anti-PD-1 immunotherapy for a year or longer, according to team of international investigators.
They reported that, among melanoma patients, the incidence of new-onset reactions that occurred 1 year or longer after anti-PD-1 treatment was 5.3%.
In a review of 118 patients, the investigators found that irAEs are often “high grade, difficult to manage, and can lead to death.”
Reactions are more likely to occur in those for whom treatment with an anti-PD-1 checkpoint inhibitor – primarily pembrolizumab and nivolumab – continued for longer than a year, and patients can present “long after stopping” the treatment, the investigators noted.
The findings were published online in Annals of Oncology.
“We do not yet understand why some patients have no side effects for months or years, then develop toxicities so late in their course,” said one of the coauthors, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn.
“Physicians should continue to monitor patients for side effects, even if they have been on anti-PD-1 therapy for some time, since delayed side effects may cause morbidity and even death,” Dr. Johnson said.
Patients and clinicians need “to be aware of these risks when making decisions regarding therapy continuation” and need “to consider irAE as a possible diagnosis in any presentation where there is a history of checkpoint inhibitor treatment, regardless of the time frame, to enable early recognition and appropriate treatment,” Dr. Johnson and colleagues concluded.
Largest series to document delayed reactions
Immunotherapies have revolutionized cancer treatment of many types of tumors, but they carry a well known risk for autoimmune toxicity, which typically occurs within the first 4-6 months, the authors wrote.
Delayed reactions have been reported but are not as well described. The new study is the largest to date on this question, and Dr. Johnson said the findings likely apply across indications, not simply in regard to melanoma patients.
An expert not involved in the study agrees.
“We are definitely seeing delayed reactions to immunotherapy in our practice” in several organ systems, including the skin, said Jennifer Choi, MD, chief of oncodermatology at Northwestern University’s Comprehensive Cancer Center, Chicago.
“Some of these side effects can take months to resolve and may require systemic treatment, such as steroids, nonsteroidal immunosuppressants, or biologics. Clinicians must be on high alert of any possible side effect for a patient on immunotherapy throughout their entire course, and even after they have completed treatment,” Dr. Choi said in an interview.
Anti-PD-1 therapy doesn’t “follow the typical drug hypersensitivity laws and rules with respect to timing,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
Median onset was 16 months
The investigators reported in detail on 118 patients. A total of 140 delayed irAEs that occurred 1 year or longer after treatment were identified in 20 centers around the world.
The median onset of delayed irAE was 16 months after start of treatment. Most occurred in conjunction with stand-alone anti-PD-1 therapy, but in the case of 20 patients, a combination of an anti-PD-1 drug and the anti-CTLA-4 drug ipilimumab was used.
In 39% of patients (n = 55), the adverse reaction was of grade 3 or worse. These included two deaths: one case of fatal encephalitis with concurrent anti-PD-1 use, and a death from immune-related multiple organ failure 11 months after anti-PD-1 discontinuation.
Most of the patients (n = 87; 74%) were receiving anti-PD-1 therapy at the time of onset of the adverse reaction; 15 patients (12%) were within 3 months of their last dose, and 16 (14%) were 3 months past their last dose.
Among the subgroup who developed an irAE after discontinuation of treatment was a patient with grade 4 colitis that required colectomy 26 months afterward, although Dr. Johnson noted it’s difficult to be sure that the colitis was related to the immunotherapy, because it occurred so long after treatment had ended.
An early warning system
The most common reactions were colitis, pneumonitis, and rash.
The reactions were often tough to manage, the authors reported. Eighty patients (68%) required steroids, and 27 (23%) required steroids plus additional immunosuppressives, such as tumor necrosis factor blockers, particularly for colitis and renal, rheumatologic, and neurologic complications. Rheumatologic events required a median corticosteroid course of 15 months plus additional immunosuppression in half of cases and often left patients with ongoing morbidity.
“Often, the skin is one of the first and most easily visible immune-related adverse event that develops,” said Bernice Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, who was not involved in the study and was approached for comment.
Presentations can range from small itchy plaques to total body dermatitis. It is something to be aware of, because the skin can act as an early warning system to catch internal organ damage earlier, she said.
On a positive note, the investigators found no indication that the effect of immunotherapy was diminished by delayed reactions and their treatment.
Managing events “gets a little complicated” when anti-PD-1 drugs are still being administered, but “we have successfully utilized systemic steroid pulses for several weeks without impeding the efficacy of the therapy. For the lichenoid and psoriasiform dermatitis, topical steroids and oral retinoids have been useful and can be used concurrently with immunotherapy,” Dr. Friedman said.
Question on treatment duration
No obvious factors were predictive of delayed events, including previous autoimmune disease or earlier reactions, which usually affected different organs, the authors said.
The findings raise a question about the appropriate duration of anti-PD-1 therapy, at least for melanoma.
The standard duration of adjuvant therapy was empirically determined to be 1 year for melanoma, and trials support anti-PD-1 therapy for up to 2 years for metastatic disease.
However, the authors suggest that “shorter treatment duration may reduce the risk of delayed irAE” and may be sufficient for patients who have a complete response.
“This should be considered when making decisions regarding therapy continuation in responding patients,” they wrote.
Ongoing clinical trials are investigating the optimal duration of therapy, they wrote.
No outside funding was reported. Dr. Johnson has been an adviser for Array Biopharma, BMS, Iovance, Jansen, Merck, and Novartis and has received research funding from BMS and Incyte. Other investigators reported similar ties. Dr. Choi, Dr. Kwong, and Dr. Friedman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Goodbye, OTC hydroquinone
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
How to counsel worried patients about the J&J vaccine news
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Rankings of most common cancers to shift over next 20 years
The next 20 years will see a big shift in cancer type rankings, researchers predict.
At the moment, the most common cancers in the United States are breast, lung, prostate, colorectal, and melanoma.
the study authors predicted. Breast cancer will remain the top cancer to be diagnosed, lung cancer will drop from second to third, and colorectal cancer will remain at fourth.
These predicted rankings of cancer types by their total number of annual cases were published online April 7, 2021, in JAMA Network Open.
The authors also rank cancer type by mortality. Currently, most cancer deaths are caused by lung cancer, followed by colorectal, pancreatic, and breast. By 2040, the most notable change in cancer deaths is that liver and intrahepatic bile duct cancer, currently at sixth, will jump up to third.
Two decades from now, the ranking in terms of cancer deaths will be lung, pancreatic, liver and intrahepatic bile duct, and colorectal.
“Our findings reflect the shifting dynamics of cancer screening and treatment,” lead author Lola Rahib, PhD, a pancreatic cancer scientist at Cancer Commons, the advocacy nonprofit, commented in a press statement.
The new analysis used population-growth projections (based on 2010 U.S. Census data) and current population-based cancer incidence and death rates (from Surveillance, Epidemiology, and End Results 2014-2016) to calculate the changes in incidences and deaths to the year 2040.
The projected, estimated numbers are not ironclad, the researchers acknowledged.
“Our projections assume that the observed rates and trends [from recent years] don’t change over time,” Dr. Rahib said in an interview, but she pointed out that change may indeed happen.
“Any long-term projections should be considered with a grain of salt,” said Kim Miller, MPH, a surveillance research scientist at the American Cancer Society, who was approached for comment.
Dr. Miller explained that “cancer trends can sometimes rapidly change within a few years.” Projections just 2-4 years ahead are “extremely difficult” and those 20 years ahead are even more so, she added in an interview.
“We’re encouraged to see the projected decreases in deaths from lung, colorectal, and breast cancer in the coming years,” said coauthor Lynn Matrisian, PhD, MBA, chief science officer at the Pancreatic Cancer Action Network. “It’s time to shift focus to some of the less commonly diagnosed cancers with the lowest survival rates, like pancreatic and liver cancer.”
Difference in opinion on prostate cancer
The huge fall in the incidence of prostate cancer that the authors predict will come about as a result of changes in prostate-specific antigen (PSA)–screening recommendations over the last 15 years, they suggested.
“The most recent change in 2018 recommends that men aged 55-69 can make their own decisions regarding screening, but previous changes recommended against PSA screening,” said Dr. Rahib.
“These changes in screening guidelines have influenced the number of diagnoses of prostate cancer in recent years and will continue to do so to 2040,” Dr. Rahib commented.
Dr. Miller casts doubt on this prediction.
Using data through 2017, “we have seen that the patterns in prostate cancer incidence are already shifting from the steep declines we saw in the early 2010s,” she said. “I would use caution when interpreting the overall trends for prostate, because this cancer in particular is dramatically affected by changes in recommendations for screening with the PSA test.”
Screening has also influenced colorectal cancer incidence, the authors pointed out, saying that the uptake of colorectal cancer screening is associated with a decrease in the number of colorectal cancers and deaths out to 2040, as a result of effectiveness of screening.
For breast cancer, the authors highlighted the fact that, although the number of breast cancers will continue to increase, the number of breast cancer deaths will decrease. That ongoing trend is most likely attributable to increased screening and advancements in treatment.
The study was supported by the National Institutes of Health, National Cancer Institute, the Cancer Prevention and Research Institute of Texas, Cancer Commons and the Pancreatic Cancer Action Network. The study authors and Dr. Miller disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The next 20 years will see a big shift in cancer type rankings, researchers predict.
At the moment, the most common cancers in the United States are breast, lung, prostate, colorectal, and melanoma.
the study authors predicted. Breast cancer will remain the top cancer to be diagnosed, lung cancer will drop from second to third, and colorectal cancer will remain at fourth.
These predicted rankings of cancer types by their total number of annual cases were published online April 7, 2021, in JAMA Network Open.
The authors also rank cancer type by mortality. Currently, most cancer deaths are caused by lung cancer, followed by colorectal, pancreatic, and breast. By 2040, the most notable change in cancer deaths is that liver and intrahepatic bile duct cancer, currently at sixth, will jump up to third.
Two decades from now, the ranking in terms of cancer deaths will be lung, pancreatic, liver and intrahepatic bile duct, and colorectal.
“Our findings reflect the shifting dynamics of cancer screening and treatment,” lead author Lola Rahib, PhD, a pancreatic cancer scientist at Cancer Commons, the advocacy nonprofit, commented in a press statement.
The new analysis used population-growth projections (based on 2010 U.S. Census data) and current population-based cancer incidence and death rates (from Surveillance, Epidemiology, and End Results 2014-2016) to calculate the changes in incidences and deaths to the year 2040.
The projected, estimated numbers are not ironclad, the researchers acknowledged.
“Our projections assume that the observed rates and trends [from recent years] don’t change over time,” Dr. Rahib said in an interview, but she pointed out that change may indeed happen.
“Any long-term projections should be considered with a grain of salt,” said Kim Miller, MPH, a surveillance research scientist at the American Cancer Society, who was approached for comment.
Dr. Miller explained that “cancer trends can sometimes rapidly change within a few years.” Projections just 2-4 years ahead are “extremely difficult” and those 20 years ahead are even more so, she added in an interview.
“We’re encouraged to see the projected decreases in deaths from lung, colorectal, and breast cancer in the coming years,” said coauthor Lynn Matrisian, PhD, MBA, chief science officer at the Pancreatic Cancer Action Network. “It’s time to shift focus to some of the less commonly diagnosed cancers with the lowest survival rates, like pancreatic and liver cancer.”
Difference in opinion on prostate cancer
The huge fall in the incidence of prostate cancer that the authors predict will come about as a result of changes in prostate-specific antigen (PSA)–screening recommendations over the last 15 years, they suggested.
“The most recent change in 2018 recommends that men aged 55-69 can make their own decisions regarding screening, but previous changes recommended against PSA screening,” said Dr. Rahib.
“These changes in screening guidelines have influenced the number of diagnoses of prostate cancer in recent years and will continue to do so to 2040,” Dr. Rahib commented.
Dr. Miller casts doubt on this prediction.
Using data through 2017, “we have seen that the patterns in prostate cancer incidence are already shifting from the steep declines we saw in the early 2010s,” she said. “I would use caution when interpreting the overall trends for prostate, because this cancer in particular is dramatically affected by changes in recommendations for screening with the PSA test.”
Screening has also influenced colorectal cancer incidence, the authors pointed out, saying that the uptake of colorectal cancer screening is associated with a decrease in the number of colorectal cancers and deaths out to 2040, as a result of effectiveness of screening.
For breast cancer, the authors highlighted the fact that, although the number of breast cancers will continue to increase, the number of breast cancer deaths will decrease. That ongoing trend is most likely attributable to increased screening and advancements in treatment.
The study was supported by the National Institutes of Health, National Cancer Institute, the Cancer Prevention and Research Institute of Texas, Cancer Commons and the Pancreatic Cancer Action Network. The study authors and Dr. Miller disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The next 20 years will see a big shift in cancer type rankings, researchers predict.
At the moment, the most common cancers in the United States are breast, lung, prostate, colorectal, and melanoma.
the study authors predicted. Breast cancer will remain the top cancer to be diagnosed, lung cancer will drop from second to third, and colorectal cancer will remain at fourth.
These predicted rankings of cancer types by their total number of annual cases were published online April 7, 2021, in JAMA Network Open.
The authors also rank cancer type by mortality. Currently, most cancer deaths are caused by lung cancer, followed by colorectal, pancreatic, and breast. By 2040, the most notable change in cancer deaths is that liver and intrahepatic bile duct cancer, currently at sixth, will jump up to third.
Two decades from now, the ranking in terms of cancer deaths will be lung, pancreatic, liver and intrahepatic bile duct, and colorectal.
“Our findings reflect the shifting dynamics of cancer screening and treatment,” lead author Lola Rahib, PhD, a pancreatic cancer scientist at Cancer Commons, the advocacy nonprofit, commented in a press statement.
The new analysis used population-growth projections (based on 2010 U.S. Census data) and current population-based cancer incidence and death rates (from Surveillance, Epidemiology, and End Results 2014-2016) to calculate the changes in incidences and deaths to the year 2040.
The projected, estimated numbers are not ironclad, the researchers acknowledged.
“Our projections assume that the observed rates and trends [from recent years] don’t change over time,” Dr. Rahib said in an interview, but she pointed out that change may indeed happen.
“Any long-term projections should be considered with a grain of salt,” said Kim Miller, MPH, a surveillance research scientist at the American Cancer Society, who was approached for comment.
Dr. Miller explained that “cancer trends can sometimes rapidly change within a few years.” Projections just 2-4 years ahead are “extremely difficult” and those 20 years ahead are even more so, she added in an interview.
“We’re encouraged to see the projected decreases in deaths from lung, colorectal, and breast cancer in the coming years,” said coauthor Lynn Matrisian, PhD, MBA, chief science officer at the Pancreatic Cancer Action Network. “It’s time to shift focus to some of the less commonly diagnosed cancers with the lowest survival rates, like pancreatic and liver cancer.”
Difference in opinion on prostate cancer
The huge fall in the incidence of prostate cancer that the authors predict will come about as a result of changes in prostate-specific antigen (PSA)–screening recommendations over the last 15 years, they suggested.
“The most recent change in 2018 recommends that men aged 55-69 can make their own decisions regarding screening, but previous changes recommended against PSA screening,” said Dr. Rahib.
“These changes in screening guidelines have influenced the number of diagnoses of prostate cancer in recent years and will continue to do so to 2040,” Dr. Rahib commented.
Dr. Miller casts doubt on this prediction.
Using data through 2017, “we have seen that the patterns in prostate cancer incidence are already shifting from the steep declines we saw in the early 2010s,” she said. “I would use caution when interpreting the overall trends for prostate, because this cancer in particular is dramatically affected by changes in recommendations for screening with the PSA test.”
Screening has also influenced colorectal cancer incidence, the authors pointed out, saying that the uptake of colorectal cancer screening is associated with a decrease in the number of colorectal cancers and deaths out to 2040, as a result of effectiveness of screening.
For breast cancer, the authors highlighted the fact that, although the number of breast cancers will continue to increase, the number of breast cancer deaths will decrease. That ongoing trend is most likely attributable to increased screening and advancements in treatment.
The study was supported by the National Institutes of Health, National Cancer Institute, the Cancer Prevention and Research Institute of Texas, Cancer Commons and the Pancreatic Cancer Action Network. The study authors and Dr. Miller disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seaweed and other marine-derived products in skin care, part 1: Current indications
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Don’t screen for vitamin D in general population, says USPSTF
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Melanoma presents at later stages, but at an earlier age in Asian Americans
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
FROM SOC SOCIETY 2021
Survey shines light on pediatric dermatologists’ earnings
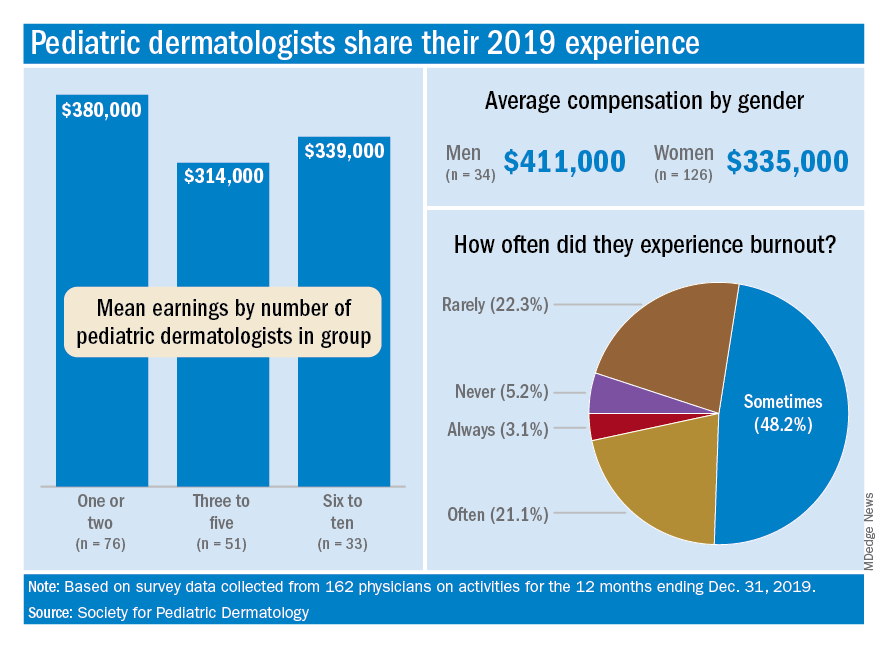
For one thing, the median total compensation for the 162 pediatric dermatologists whose survey responses were included in the final data set was a somewhat lower $335,000, the SPD said in its 2020 Pediatric Dermatology Physician Compensation Report.
Getting back to the mean, average earnings were highest, over $505,000, among those working in hospitals/health systems, followed by independent group practices at $436,000, while those working in academic hospitals/health systems – the most popular type of ownership entity (69% of all respondents) – had a mean compensation of $323,000, the SPD said in the report.
At a more basic level, average earnings tilted toward men over women, $411,000-$335,000, although a majority of the respondents (78%) were female, according to the SPD.
Patient mix produced a strong trend of increasing earnings with decreasing pediatric case load. Average compensation was lowest among those who saw 98%-100% pediatric patients ($330,000), rose for physicians who saw 80%-97% ($345,000) and 50%-79% children ($398,000), and topped out at $444,000 for those who saw fewer than 50% children, the SPD data show.
The number of pediatric dermatologists working in a practice also had an effect: Average compensation in practices with 1-2 such specialists was almost $380,000 in 2019, compared with $340,000 in groups with 6-10 pediatric dermatologists and $314,000 for those with 3-5. There were too few groups with more than 10 to meet the sample-size criteria, the SPD noted.
Average starting salary was $286,000 for the 17 respondents who reported that they were newly hired for full-time positions, with a median of $262,500, which was “22% lower than the median clinical compensation reported by pediatric dermatology physicians hired prior to 2019,” the report indicated.
Respondents also were asked about issues of satisfaction and burnout, and these data include responses from additional physicians (for a total of 193) not included in the compensation data set.
The largest share, 79%, said that patient relationships were most satisfying factor of their profession, with intellectual stimulation next at 59% and interaction with colleagues third at 42%. The least satisfying elements were regulatory/paperwork burdens (80%), inefficient EHR design/interoperability (37%), and the commoditization of medicine (21%), the SPD said.
Feelings of burnout were common among almost a quarter of pediatric dermatologists, with 3.1% saying they always have such feelings and 21.2% disclosing that they often feel burned out. Only 5.2% said that they never have feelings of burnout, the SPD reported.
Demographically speaking, 71% of those surveyed identified as White, 22% as Asian, 8.5% as Hispanic/Latino/Spanish, 2.5% as Middle Eastern or North African, and 2.5% as Black or African American. The largest age group, with 61% of all respondents, was 36-50 years, and geography gave the East a slight edge over the West, 30% to 28%, although California had the largest share by state, 17.4%, the report said.

For one thing, the median total compensation for the 162 pediatric dermatologists whose survey responses were included in the final data set was a somewhat lower $335,000, the SPD said in its 2020 Pediatric Dermatology Physician Compensation Report.
Getting back to the mean, average earnings were highest, over $505,000, among those working in hospitals/health systems, followed by independent group practices at $436,000, while those working in academic hospitals/health systems – the most popular type of ownership entity (69% of all respondents) – had a mean compensation of $323,000, the SPD said in the report.
At a more basic level, average earnings tilted toward men over women, $411,000-$335,000, although a majority of the respondents (78%) were female, according to the SPD.
Patient mix produced a strong trend of increasing earnings with decreasing pediatric case load. Average compensation was lowest among those who saw 98%-100% pediatric patients ($330,000), rose for physicians who saw 80%-97% ($345,000) and 50%-79% children ($398,000), and topped out at $444,000 for those who saw fewer than 50% children, the SPD data show.
The number of pediatric dermatologists working in a practice also had an effect: Average compensation in practices with 1-2 such specialists was almost $380,000 in 2019, compared with $340,000 in groups with 6-10 pediatric dermatologists and $314,000 for those with 3-5. There were too few groups with more than 10 to meet the sample-size criteria, the SPD noted.
Average starting salary was $286,000 for the 17 respondents who reported that they were newly hired for full-time positions, with a median of $262,500, which was “22% lower than the median clinical compensation reported by pediatric dermatology physicians hired prior to 2019,” the report indicated.
Respondents also were asked about issues of satisfaction and burnout, and these data include responses from additional physicians (for a total of 193) not included in the compensation data set.
The largest share, 79%, said that patient relationships were most satisfying factor of their profession, with intellectual stimulation next at 59% and interaction with colleagues third at 42%. The least satisfying elements were regulatory/paperwork burdens (80%), inefficient EHR design/interoperability (37%), and the commoditization of medicine (21%), the SPD said.
Feelings of burnout were common among almost a quarter of pediatric dermatologists, with 3.1% saying they always have such feelings and 21.2% disclosing that they often feel burned out. Only 5.2% said that they never have feelings of burnout, the SPD reported.
Demographically speaking, 71% of those surveyed identified as White, 22% as Asian, 8.5% as Hispanic/Latino/Spanish, 2.5% as Middle Eastern or North African, and 2.5% as Black or African American. The largest age group, with 61% of all respondents, was 36-50 years, and geography gave the East a slight edge over the West, 30% to 28%, although California had the largest share by state, 17.4%, the report said.

For one thing, the median total compensation for the 162 pediatric dermatologists whose survey responses were included in the final data set was a somewhat lower $335,000, the SPD said in its 2020 Pediatric Dermatology Physician Compensation Report.
Getting back to the mean, average earnings were highest, over $505,000, among those working in hospitals/health systems, followed by independent group practices at $436,000, while those working in academic hospitals/health systems – the most popular type of ownership entity (69% of all respondents) – had a mean compensation of $323,000, the SPD said in the report.
At a more basic level, average earnings tilted toward men over women, $411,000-$335,000, although a majority of the respondents (78%) were female, according to the SPD.
Patient mix produced a strong trend of increasing earnings with decreasing pediatric case load. Average compensation was lowest among those who saw 98%-100% pediatric patients ($330,000), rose for physicians who saw 80%-97% ($345,000) and 50%-79% children ($398,000), and topped out at $444,000 for those who saw fewer than 50% children, the SPD data show.
The number of pediatric dermatologists working in a practice also had an effect: Average compensation in practices with 1-2 such specialists was almost $380,000 in 2019, compared with $340,000 in groups with 6-10 pediatric dermatologists and $314,000 for those with 3-5. There were too few groups with more than 10 to meet the sample-size criteria, the SPD noted.
Average starting salary was $286,000 for the 17 respondents who reported that they were newly hired for full-time positions, with a median of $262,500, which was “22% lower than the median clinical compensation reported by pediatric dermatology physicians hired prior to 2019,” the report indicated.
Respondents also were asked about issues of satisfaction and burnout, and these data include responses from additional physicians (for a total of 193) not included in the compensation data set.
The largest share, 79%, said that patient relationships were most satisfying factor of their profession, with intellectual stimulation next at 59% and interaction with colleagues third at 42%. The least satisfying elements were regulatory/paperwork burdens (80%), inefficient EHR design/interoperability (37%), and the commoditization of medicine (21%), the SPD said.
Feelings of burnout were common among almost a quarter of pediatric dermatologists, with 3.1% saying they always have such feelings and 21.2% disclosing that they often feel burned out. Only 5.2% said that they never have feelings of burnout, the SPD reported.
Demographically speaking, 71% of those surveyed identified as White, 22% as Asian, 8.5% as Hispanic/Latino/Spanish, 2.5% as Middle Eastern or North African, and 2.5% as Black or African American. The largest age group, with 61% of all respondents, was 36-50 years, and geography gave the East a slight edge over the West, 30% to 28%, although California had the largest share by state, 17.4%, the report said.








